13.3
Impact Factor
Theranostics 2023; 13(10):3245-3275. doi:10.7150/thno.84759 This issue Cite
Review
Integrating physicomechanical and biological strategies for BTE: biomaterials-induced osteogenic differentiation of MSCs
1. Department of Plastic Surgery, The First Hospital of China Medical University, Shenyang 110001, China.
2. Liaoning Provincial Key Laboratory of Oral Diseases, School and Hospital of Stomatology, China Medical University, Shenyang 110001, China.
3. Department of Plastic Surgery, The First Affiliated Hospital of Medical College of Shihezi University, Shihezi, Xinjiang 832008, China.
Abstract
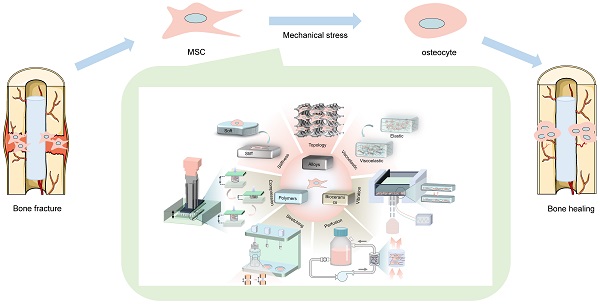
Large bone defects are a major global health concern. Bone tissue engineering (BTE) is the most promising alternative to avoid the drawbacks of autograft and allograft bone. Nevertheless, how to precisely control stem cell osteogenic differentiation has been a long-standing puzzle. Compared with biochemical cues, physicomechanical stimuli have been widely studied for their biosafety and stability. The mechanical properties of various biomaterials (polymers, bioceramics, metal and alloys) become the main source of physicomechanical stimuli. By altering the stiffness, viscoelasticity, and topography of materials, mechanical stimuli with different strengths transmit into precise signals that mediate osteogenic differentiation. In addition, externally mechanical forces also play a critical role in promoting osteogenesis, such as compression stress, tensile stress, fluid shear stress and vibration, etc. When exposed to mechanical forces, mesenchymal stem cells (MSCs) differentiate into osteogenic lineages by sensing mechanical stimuli through mechanical sensors, including integrin and focal adhesions (FAs), cytoskeleton, primary cilium, ions channels, gap junction, and activating osteogenic-related mechanotransduction pathways, such as yes associated proteins (YAP)/TAZ, MAPK, Rho-GTPases, Wnt/β-catenin, TGFβ superfamily, Notch signaling. This review summarizes various biomaterials that transmit mechanical signals, physicomechanical stimuli that directly regulate MSCs differentiation, and the mechanical transduction mechanisms of MSCs. This review provides a deep and broad understanding of mechanical transduction mechanisms and discusses the challenges that remained in clinical translocation as well as the outlook for the future improvements.
Keywords: osteogenesis, physicomechanical stimuli, biomaterials, mesenchymal stem cells, mechanisms
 Global reach, higher impact
Global reach, higher impact