13.3
Impact Factor
Theranostics 2023; 13(10):3245-3275. doi:10.7150/thno.84759 This issue Cite
Review
Integrating physicomechanical and biological strategies for BTE: biomaterials-induced osteogenic differentiation of MSCs
1. Department of Plastic Surgery, The First Hospital of China Medical University, Shenyang 110001, China.
2. Liaoning Provincial Key Laboratory of Oral Diseases, School and Hospital of Stomatology, China Medical University, Shenyang 110001, China.
3. Department of Plastic Surgery, The First Affiliated Hospital of Medical College of Shihezi University, Shihezi, Xinjiang 832008, China.
Received 2023-3-28; Accepted 2023-5-12; Published 2023-5-21
Abstract
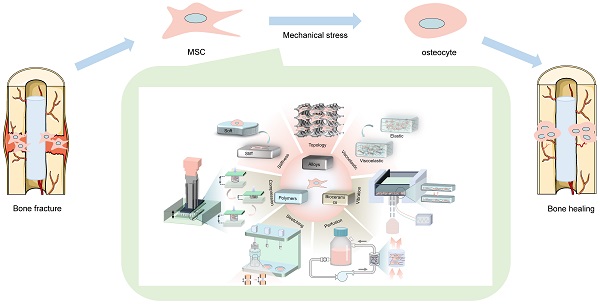
Large bone defects are a major global health concern. Bone tissue engineering (BTE) is the most promising alternative to avoid the drawbacks of autograft and allograft bone. Nevertheless, how to precisely control stem cell osteogenic differentiation has been a long-standing puzzle. Compared with biochemical cues, physicomechanical stimuli have been widely studied for their biosafety and stability. The mechanical properties of various biomaterials (polymers, bioceramics, metal and alloys) become the main source of physicomechanical stimuli. By altering the stiffness, viscoelasticity, and topography of materials, mechanical stimuli with different strengths transmit into precise signals that mediate osteogenic differentiation. In addition, externally mechanical forces also play a critical role in promoting osteogenesis, such as compression stress, tensile stress, fluid shear stress and vibration, etc. When exposed to mechanical forces, mesenchymal stem cells (MSCs) differentiate into osteogenic lineages by sensing mechanical stimuli through mechanical sensors, including integrin and focal adhesions (FAs), cytoskeleton, primary cilium, ions channels, gap junction, and activating osteogenic-related mechanotransduction pathways, such as yes associated proteins (YAP)/TAZ, MAPK, Rho-GTPases, Wnt/β-catenin, TGFβ superfamily, Notch signaling. This review summarizes various biomaterials that transmit mechanical signals, physicomechanical stimuli that directly regulate MSCs differentiation, and the mechanical transduction mechanisms of MSCs. This review provides a deep and broad understanding of mechanical transduction mechanisms and discusses the challenges that remained in clinical translocation as well as the outlook for the future improvements.
Keywords: osteogenesis, physicomechanical stimuli, biomaterials, mesenchymal stem cells, mechanisms
1. Introduction
Bones have remarkable healing potential and are able to regenerate themselves upon injury or defect. Small bone defects achieve self-healing with the formation of new bone. However, large bone defects caused by trauma, tumor or infection, such as osteoporosis and osteonecrosis, are far beyond their self-healing capability, thereby requiring grafts to promote defect repair and bone regeneration [1, 2]. Although autologous bone transplantation is considered to be an optimal strategy for treating bone defects, its clinical application is limited by the insufficiency of autologous bone transplantation and the morbidity of the donor site [3]. Bone allografts have a high risk of immune rejection and are also abandoned [3]. Thus, tissue engineered bone seems to be a promising alternative [3, 4].
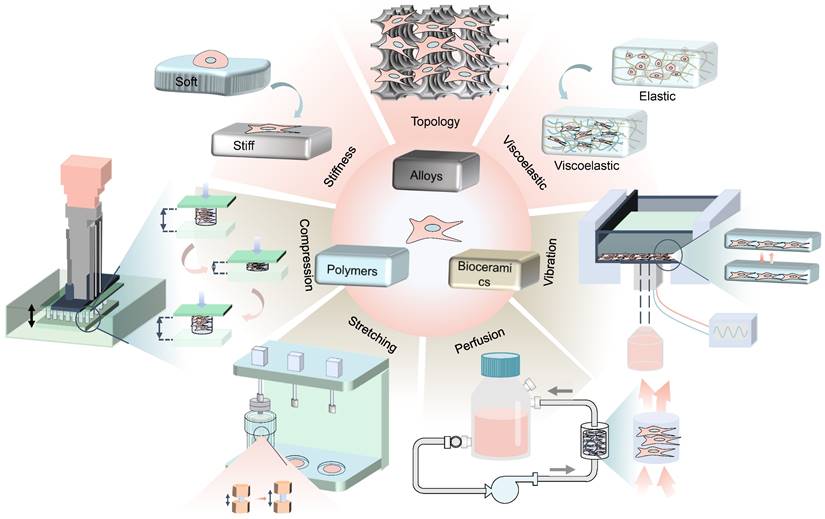
Schematic illustration of physicomechanical stimuli based on biomaterials to induce osteogenic differentiation of MSCs.
In recent years, BTE based on MSCs has aroused much interest [4]. These cells are not only easy to obtain, but also have the potential to differentiate into lineages, including osteoblasts, chondrocytes, adipocytes, and muscle cells [3, 5]. However, how to precisely control the fate of MSCs is still an important subject for investigation in BTE. The conventional approach induces stem cells to differentiate into various lineages by transmitting biochemical signaling molecules [6]. Nonetheless, the biosafety of these biochemical factors still needs to be evaluated. And how to achieve temporally and spatially controlled release has not been solved [3, 7]. Therefore, the regulation of MSCs osteogenic differentiation by physical and mechanical strategies is considered to be a safer and more stable approach.
Physicomechanical stimuli is divided into internal forces generated by the cell-laden biomaterials (such as stiffness, viscoelasticity, and topography) and externally mechanical forces (such as compression stress, tensile stress, fluid shear stress and vibration) [6], which has substantial effects on stem cell differentiation through different mechanisms (Figure 1). For instance, high stiffness of biomaterials drives MSCs into the osteogenic lineage, while the low stiffness promotes adipogenic differentiation [8]. Various rough topographies, such as groove or ridge structures [9], have been demonstrated to promote osteogenic differentiation as well. However, static culture only allows oxygen and nutrients to slowly diffuse to the center of the scaffold, which causes some cells to undergo apoptosis due to insufficient supply of nutrients and oxygen [2]. In contrast, dynamic culture with bioreactors that provide mechanical loads not only allows for more uniform cell distribution and adequate nutrition, but also has been shown to better promote osteogenic differentiation of MSCs [2]. Therefore, dynamic cultivation by applying external mechanical force is also widely concerned in the field of BTE recently.
Mechanobiology is an emerging field, which integrates both physicomechanical and biological strategies, including receiving mechanical signals and transforming extracellular mechanical signals into intracellular biological ones [3]. Mechanoreceptors on cell surface sense mechanical cues and subsequently transmit signals to the nucleus through dynamic regulation of cytoskeletal integrity and tension. The nucleus responds to the signals by up-regulating or down-regulating the expression of genes associated with mechanical stimulation [3, 5, 10]. In this review, we first listed different biomaterials and the approaches to alter their mechanical properties, in order to dictate MSCs differentiation towards osteogenic lineage. Then, we summarized physicomechanical stimuli that drove osteogenesis, including stiffness, viscoelasticity, and topological structure of materials, as well as external mechanical forces. Subsequently, we illustrated how MSCs converted mechanical stimuli into biochemical signals, and several mechanotransduction-associated signaling pathways during osteogenesis. Finally, we discussed the major challenges that might encounter in the future transformation of MSCs-laden biomaterials based on mechanical conduction in BTE.
2. Biomaterials-induced physicomechanical stimuli towards MSCs
2.1 Internal mechanical stimulation on MSCs-laden biomaterials
Biomaterials regulate cell behavior by mimicking the natural ECM [11], and their physicomechanical properties are regarded as the major stimuli that governs fate decisions of MSCs. This section will focus on the processing methods of biomaterials for inducing osteogenesis of MSCs including hydrogels and other polymers, bioceramics, metal and alloys. Furthermore, as the primary means for directing osteogenesis, the modulation on their stiffness, viscoelasticity, and topography will be detailed below.
2.1.1 Biomaterials processing method
2.1.1.1 Hydrogels and other polymers
Hydrogels are widely used as matrix material in BTE and regenerative medicine, especially in three-dimensional (3D) microenvironment [12]. Compared with bioceramic and metal-based materials, hydrogels have become the mainstream matrix materials for inducing osteogenic differentiation of MSCs due to their adjustable stiffness [13], ease of altering morphology [14], and unique viscoelastic properties [15-17].
As is well-known, cell proliferation, migration or differentiation can be easily modulated by changing the stiffness of the hydrogel. Cells tend to differentiate into osteogenic lineage on a stiff matrix [18], while soft matrix enhances cell proliferation and migration [19]. Therefore, hydrogels play a critical role in BTE for their adjustability of stiffness [17]. In recent years, the fabrication of hydrogels has been extensively explored, with most attention on how to precisely regulate the physicomechanical properties of hydrogels in a simple way. The traditional method is to adjust the proportion of each component of the hydrogel. For example, polyacrylamide (PA) hydrogels' physiological stiffness can be adjusted by controlling the ratio of acrylamide to bis-acrylamide [20]. Hadden WJ et al. developed an approach of polymerization control to synthesize linear stiffness gradient PA hydrogels, which was simpler and cheaper than other synthesis methods [21]. By adjusting the concentration of hydroxyapatite (HAp) in the methacrylated hyaluronic acid hydrogel, a matrix material with tunable stiffness can be formed, which alters the cell volume, differentiation and cell fate decisions [22]. Furthermore, some emerging technologies have aroused increasing attention nowadays. Wet spinning method allows the fabrication of gelatin-based microstrip hydrogels with various stiffness [23]. Photoresponsive hydrogels change photoswitchable stiffness in the presence of cells through rapid cytocompatible light-based chemistries [11], this allows MSCs stiffness regulation to be investigated independently without the interference of other reagents. In later studies, soft lithography has been used to precisely control the surface morphology of hydrogels, achieving linear surface roughness variation from nanometer to micrometer on a stiffness controllable matrix [14, 20]. Ultrahigh strength and high stiffness of hydrogels can also be developed through a brick-mortar-like network that composed of bacterial cellulose nanofibers and alginate-Ca2+ [24], or forming a hybrid scaffold with 3D PCL/nano-hydroxyapatite (nHA) scaffold [25]. Macro-porous recombinant elastin-like protein substrates [4] and the combination of alginate and gelatin for bioprinting [26] are promising for the optimization of stiffness as well.
Tunable viscoelasticity and stress-relaxation properties is another major strength of degradable hydrogels. By altering the molecular weight and density, hydrogel can mimic some of the dynamic mechanical properties of natural tissues under physiological conditions [15]. For instance, hydrogels with adjustable stress-relaxation properties are developed by changing molecular weights to combine different calcium crosslinking densities, which crosslinks alginate ionically [27], or altering the molecular weight and density of polyethylene glycol (PEG) which is independent of the initial elastic modulus of the material [28]. More novel preparation methods have been developed recently. In order to mimic the viscoelastic characteristics of bone ECM, Chen J et al. developed photocurable liquid crystal hydrogels based on chitin whiskers, and found negatively charged maleic anhydride chitin whiskers hydrogels were more conducive to the formation of bone than hydrogels based on positively charged chitin whiskers [16]. Zhang J and his colleagues fabricated a kind of thermosensitive hydrogels, whose reversible mechanical deformation could be easily achieved through adjusting temperature from 25℃ to 37℃ [29]. Upon sensing relaxation in the mechanical response, stem-cell spheroids promoted osteogenic differentiation by increasing the maturity of the FAs and the rate of F-actin polymerization [29]. Moreover, the viscoelasticity of the hydrogel can also be dynamically changed by ionic cross-linking [30], improving hydrogen bond interactions [31] and hydrophobic interactions [32, 33].
A wide variety of other synthetic materials with excellent physicomechanical properties has also been explored. In contrast to the viscoelastic properties of hydrogels, studies on other synthetic matrix materials such as polymers mainly focus on the stiffness and surface nano-patterns design of materials, which are both valid parameters for regulating cell behavior [34].
Polydimethylsilane (PDMS) is a common polymer matrix material that can be easily fabricated into different stiffness [35, 36], which benefits the investigation on the specific mechanism of ECM stiffness to stem cell behavior. Changing the ratio of curing agent vs oligomeric base during substrate preparation is a common method to modify the stiffness of PDMS [35]. Furthermore, it is also feasible to use temperature gradients to synthesize PDMS with stiffness gradients [36], or air plasma treatment to produce the desired wavy surface topology at different pressures and oxidation times [37].
PCL can also be synthesized with different stiffness [38]. To explore osteogenic differentiation of MSCs on PCL scaffolds, multiwall carbon nanotubes (MNNTs) [34] and nano-HAp [39] are incorporated into PCL nanofibers to form a composite scaffold to enhance the material stiffness. The addition of functionalized MNNTs to PCL nanofibers independently changed the nanoroughness of PCL while adjusting its stiffness [34]. In addition, the nacreous topology characteristic of the shell of invertebrates induced osseointegration and has been incorporated into the design of biomaterials [40, 41]
Other methods of processing polymers to create micropatterns are usually based on reactive ion etching or multi beam laser interference on polyimide (PI) materials [9], and the microphase separation between poly(desaminotyrosyl-tyrosine carbonate) (PDTEC) and polystyrene (PS) [42]. A poly(urea-urethane) nanohybrid scaffolds fabricated by 3D printing-guided thermally induced phase separation technique has the property of stiffness memory [43], and can be self-softening in a body temperature environment [44]. In addition, superior physicomechanical properties have also been confirmed when natural biomaterials are combined with polymers, such as tissue engineering scaffolds with chitosan and gelatin combination [45] and silk fibroin combined with graphene oxide hydrogel matrix [46].
2.1.1.2 Bioceramics
Bioceramic are widely used in BTE [47-49]. Unlike hydrogels, bioceramic composites are poor in viscoelastic or stiffness tunable properties. Instead, they can mimic both physical architecture and chemical composition of nature bone [50], and be fabricated into different nanotopologies to regulate cell behavior.
As one of the most frequently used bioceramic materials in BTE [51], HAp can be processed in various forms and combined with a variety of other composite materials. The Ca(NO3)2·4H2O and (NH4)2HPO4 aqueous solutions can be treated by simple chemical precipitation to prepare nanosized HAp samples, and HAp nanorods of different shapes can be obtained by changing the reaction temperature and time, which show stronger osteo-inductive ability than traditional nano-HAp [52]. Using this method, HAp nanorods which are similar to natural bone nanocrystals can be fabricated without organic solvents. The HAp micro-nanorod structure can also be loaded on the composite ceramic (β-TCP/CaSiO3) scaffold as a surface layer, and the process requires 3D printing technology [53]. The strontium substituted HAp scaffold developed by Prabha RD et al. can be used to enhance alkaline phosphatase activity [54], an alternative processing modality for HAp. In recent years, researchers focus on the fabrication of surface topology. Ramaswamy Y et al. fabricated HAp surfaces with honeycomb, pillars and isolated islands topologies by microcasting with molds made of plant petals [55], which avoided the need for expensive micro-contact printing or lithographic devices and increases osteogenesis.
Recently, the composite scaffolds of bioceramics and other materials have also been extensively studied. Poly-l-lactic acid (PLLA)/HAp bone scaffold is prepared by enhancing the interfacial bonding between HAp and PLLA via nano-modifying HAp surface with a phosphonic acid coupling agent(2-Carboxyethylphosphonic acid) [50]. In the latest study, PLLA coated with nanocomposite (NiFe2O4/ZnO) accelerated the osteogenic differentiation of MSCs [56]. Composite scaffolds made of calcium-deficient HAp with fibrillated collagen and human umbilical cord serum (hUCS) have also been reported [57].
The surface nanotopology of other bioceramics such as silicon [58] and TiO2 [59] can be tuned to nanorod arrays. Moreover, BMP-2 coating can be added on TiO2 nanotubes [60]. The behavior of the cells cultured on the surface of bioactive glass substrates nanorods was also similar to that of the cells on the hydrogel [61].
2.1.1.3 Metal and alloys
Ti and Ti-based alloys have superior biocompatibility and osseointegration capability, playing an important role in the long-term survival of implants. Generally, bioactivity of the alloys is enhanced with the addition of bioactive elements, such as magnesium [62], cobalt-chrome-molybdenum [63], etc. Recently, surface modification has become a novel approach to accelerate the osteogenesis by improving the mechanical properties. The surface modification processes of Ti-based materials include sandblasting to change the roughness [64, 65], hydrofluoric acid etching to form micropitted topography [66] and hot solution of HCl/H2SO4 acid etching [67]. In addition, the surface topology of pure Ti treated with hydrogen peroxide after acid etching was also shown to be favorable for bone integration [68].
Nanotopology are commonly fabricated in Ti alloy implants to drive osteogenesis. Ti-6Al-4V alloy with highly-ordered TiO2 nanotube structure stimulates the capacity of MSCs osteogenic differentiation. It is developed via electrochemical anodization, and the diameter of the nanotube can be adjusted by changing the voltage [69, 70],which is a processing method similar to that previously used for Ti [71]. Pulsed laser remelting [72], femtosecond laser texturing [73], electron beam technique [74], and acid etching [75] have also been used to prepare Ti-6Al-4V alloy surface nanostructures. All of the above surface modification strategies enhanced the osteoinductive capability of alloy materials. In another study of other Ti-based alloys, the surface of Ti-25Nb-3Mo-2Sn-3Zr alloy treated by mechanical attrition treatment formed nanograined with osteogenic effect [76].
Tantalum (Ta) has unique advantages in promoting bone integration due to its good biocompatibility and mechanical properties [77]. Chemical vapour deposition [78] combined with 3D-printing (selective laser melting) [79] can be used to process porous Ta, which has shown higher bone-induction ability than Ti-6Al-4V [79, 80]. Ta alloys such as Ta-Ti gyroid scaffold [81] and Ti-Ta-Nb-Zr alloy [82] are also shown to upregulate the expression of osteogenic genes.
2.1.2 Stiffness
Effects of substrate stiffness on regulating stem cell behavior has attracted significant attention in recent years [83] (Table 1). Engler AJ et al. demonstrated for the first time that matrix stiffness was a promising mechanical target to modify MSCs fate. In their study, MSCs were seeded onto collagen-coated PA substrates with three levels of stiffness. It was revealed that MSCs showed markers for neurogenic lineages on the softest gels (1 kPa), myogenic lineages at moderately stiff matrices (11 kPa) and osteogenic lineages at the stiffest matrices (34 kPa) [8]. Interestingly, after several weeks of stiffness-directed differentiation, reprogramming of these lineages seemed to be impossible, even with addition of soluble induction factors. The stiffness-dependent differentiation has also been demonstrated in the study on human adipose-derived stem cells (hASCs). Hadden WJ et al. fabricated planar PA hydrogels with different stiffness gradients and analyzed stiffness-dependent hASC differentiation. Similarly, the expression of the adipogenic marker PPARγ peaked at low stiffnesses (E<3 kPa) after 6 days, MyoD, myogenic transcription factor, was highest around E∼12 kPa, and CBFA1, an osteogenic marker, was peak at E∼36 kPa [21].
Given that substrate stiffness exerts a significant influence on stem cell differentiation, researchers have started to perform a series of experiments to gain insight into its specific mechanism, and focus on exploring the optimal stiffness of biomaterials, to draw a feasible strategy for promoting osteogenic differentiation in BTE. Recently, numerous studies have confirmed that increased stiffness of biomaterials favorably drives stem cells into the osteogenic lineage [83-87]. In the study of Liu Y et al., higher expression of differentiation markers in stiffer matrices demonstrated a more significant response of MSCs towards stiffer hydrogels. In contrast, the differentiation of MSCs in softer matrix appeared to be slower and more limited [86]. This is because rigid substrates are more likely to induce F-actin polymerization and actomyosin cytoskeleton contraction, thus promoting nuclear translocation of YAP/TAZ and osteogenic differentiation of MSCs [88]. Similarly, Zhang T et al. delivered straightforward evidence that rigid matrices allowed broader cell spreading, faster cell growth and stronger expression of vinculin in ADSCs [85]. This might because viscoelastic behavior presented by low stiffness influences cell spreading and stromal cells fate [85].
It has previously been shown that osteogenic differentiation of MSCs mainly occurs at 25-40 kPa [8]. Interestingly, MSCs can also respond to stiffness beyond this range. Yang Y et al. manufactured polyethylene glycol/silk fibroin/hydroxyapatite (PEG/SF/HAp) scaffolds with different proportions of HAp (25, 50, 75, and 100 mg), and the stiffness ranged from 80.98 to 190.51 kPa. The results showed that scaffolds with 50mg HAp (nearly 130 kPa) significantly enhanced the effect of osteogenesis, compared with the stiffer or the softer ones [89]. However, when the stiffness reaches 600-700 kPa, cellular growth and osteogenic differentiation was more obvious [90]. And lower stiffness presents better osteogenesis when stiffness lies outside of this optimal range [26]. Hu Q et al. manufactured demineralized bone matrix (DBM) scaffolds with various compressive modulus (66.06 ± 27.83 MPa, 26.90 ± 13.16 MPa and 0.67 ± 0.14 MPa). In contrast to the two former ones, DBM scaffolds with a stiffness of 0.67 ± 0.14 MPa promoted osteogenesis, and significantly enhanced bone integration [91]. Similarly, Maggi et al. constructed 3D nano-structured scaffolds with stiffness ranging from 0.69 ± 0.2 MPa to 60.2 ± 7.4 MPa. They found that the nanolattice with lowest stiffness (0.7 MPa) exhibited 20% more F-actin than others [92].
There are several potential mechanisms that may explain why there are some biomaterials with less stiffness perform better in supporting cell proliferation and enhancing osteoblastic differentiation. On one hand, integrins bond formation between MSCs and soft matrix is higher than in the stiff one, which in return promote MSCs osteogenic differentiation [26]. On the other hand, degradation-mediated cellular traction is another essential element to regulate the differentiation of MSCs [26]. In stiff scaffolds with high alginate concentrations, cell-mediated degradation may be slow, resulting in low traction between the cell and substrate, thereby inhibiting osteogenesis. Conversely, the cell-mediated degradation in soft substates exhibits a high degree of cell diffusion and high traction, which favors osteogenesis [26]. Additionally, for high substrate stiffness, the cell's ability to sense biophysical cues in the microenvironment is reduced, preventing excessive mechanical signals from being transmitted to related proteins on the cell membrane, resulting in reduced osteogenic differentiation ability [89].
2.1.3 Viscoelasticity
How does matrix elastic modulus/stiffness affect cell-matrix mechanical interactions and MSCs differentiation has been extensively studied through researches on elastic biomaterials [83, 93]. It should be noted, however, that the ECM of bone tissue is not purely elastic, but viscoelastic [16]. The resident cells sense and respond to the mechanical deformation caused by viscoelasticity in a time-dependent manner [96]. Recently, it has been demonstrated that the viscoelasticity of bone ECM plays an essential role in regulating cell behaviors and osteogenic differentiation [16]. Therefore, how to better simulate the viscoelasticity of bone ECM is crucial for the design of scaffolds in BTE [16].
Beyond the characteristics of elastic solids, more importantly, biomaterials with viscoelastic properties need to contain the characteristics of viscous fluids [7, 97]. The elastic properties determine its elasticity as well as the initial resistance to applied forces [5]. Nonetheless, biomaterials with elasticity solely restrict cell adhesion, proliferation, diffusion and differentiation to a large extent because of their inability to relax forces effectively [98, 99]. In contrast, the viscous properties dissipate the applied load and lead to extinction of drag force as well as permanent deformation over time.
Effects of cell-laden biomaterials on MSCs osteogenic differentiation induced by matrix stiffness
| Stem cell source | Biomaterial | Stiffness range | Functional activities | Ref |
|---|---|---|---|---|
| hMSCs | Macro-porous recombinant elastin-like protein (ELP) substrates | 0.5-50 kPa | Increase adipogenic and osteogenic differentiation markers with increasing stiffness. | [4] |
| PA hydrogels | 3, 14, 38 kPa | Stiffness-induced YAP nuclear translocation was only observed when hMSCs were cultured on hydrogels coated with intermediate concentration of fibronectin. | [93] | |
| Methacrylate gelatin (GelMA) hydrogels | 3.8, 31.3 kPa | Osteogenesis were enhanced on very soft hydrogels with high surface roughness. | [14] | |
| Electrospun PLLA ultrafine fibers | 77.4, 729,1124 MPa (Young's modulus) | A stiff substrate downregulates the stemness property of hMSCs and directs the cells toward the osteogenic lineage. | [83] | |
| 3D bioprinted cell-laden scaffolds | 0.66, 5.4 kPa | Soft scaffolds had enhanced ALP activity and stimulated osteogenic differentiation than stiff ones. | [26] | |
| Methacrylated hyaluronic acid (MeHA) hydrogels | 5, 12, 23 kPa | When cells had an optimal volume, cells could form clear stress fibers and FAs on soft, intermediate, or stiff matrix. | [22] | |
| rat MSCs | 3D DBM scaffold | 66.06, 26.90, 0.67 MPa (compressive modulus) | Low scaffolds could promote the osteogenic differentiation of MSCs. | [91] |
| GelMA hydrogels | 6, 10, 25 kPa | Osteogenic differentiation was increased with the elevation of 3D ECM stiffness. | [84] | |
| Magnetic liquid metal (MLM) scaffold | 3.58-14.32 MPa | MLM scaffold has good biocompatibility and can promote the osteogenic differentiation of MSCs. | [87] | |
| PEG/SF/HAp scaffolds | 80.98-190.51 kPa | The scaffolds fabricated with HAp (50 mg) increased cell adhesion and viability as well as the expression of all the osteogenesis-related markers. | [89] | |
| mouse MSCs | Alginate-gelatin (Alg-Gel) composite hydrogels | 50 kPa, 225 kPa (Young's modulus) | Higher expression of adipogenic and osteogenic markers were shown in stiffer 3D-bioprinted matrices. | [86] |
| DBM scaffolds | 0.67 MPa | Low matrix stiffness could polarize macrophages into an anti-inflammatory phenotype, and specialized pro-resolving lipid mediators (SPMs) biosynthesis beneficial for the osteogenesis of MSCs. | [94] | |
| Transglutaminase cross-linked gelatin (TG-gel) | 60.54, 1.58 kPa (yield strength) | Low-stiffness TG-gels promoted BMSC proliferation, whereas high-stiffness TG-gels supported cell osteogenic differentiation. | [95] |
Effects of cell-laden biomaterials on MSCs osteogenic differentiation induced by viscoelasticity
| Stem cell source | Biomaterial | Initial elastic modulus | Half stress relaxation time (τ1/2) | Functional activities | Ref |
|---|---|---|---|---|---|
| hMSCs | Alginate hydrogels | - | 20 s | Significant increases were observed in calcium deposition by MSC spheroids loaded with BMP-2-HA in viscoelastic gels. | [30] |
| - | 14.4±1.0 s | Modulating viscoelastic properties of biomaterials, in conjunction with dual peptide functionalization, can simultaneously enhance multiple aspects of MSC regenerative potential. | [97] | ||
| Boronate-Based Hydrogels | 14.1±2.7 kPa | - | The fast relaxation matrix mechanics are found to promote cell-matrix interactions, leading to spreading and an increase in nuclear volume, and induce yes-associated protein/PDZ binding domain nuclear localization at longer times. | [15] | |
| Hyaluronic acid-collagen hydrogels | - | 560-2200 s | Faster relaxation in the interpenetrating network hydrogels promotes cell spreading, fiber remodeling, and FA formation. | [107] | |
| mouse MSCs | RGD-coupled alginate-PEG hydrogels | 3 kPa | A few hours to a few minutes | Faster relaxation in RGD-coupled alginate-PEG hydrogels led to increased spreading and proliferation of fibroblasts, and enhanced osteogenic differentiation of MSCs. | [28] |
| RGD coupled alginate hydrogels | 17kPa | 1 min | Cell spreading, proliferation, and osteogenic differentiation of MSCs are all enhanced in cells cultured in gels with faster relaxation. | [27] | |
| Alginate hydrogels | 20kPa | - | MSCs in viscoelastic hydrogels exhibit volume expansion during cell spreading, and greater volume expansion is associated with enhanced osteogenesis. | [105] |
During this process, the stored energy is fully released through stress relaxation. This stress release not only guides cells to reshape the matrix, but also transforms a dynamic signaling within stem cells that regulates its spreading, polarization and differentiation in turn [5, 100, 101].
In recent years, a growing effort has been devoted to developing viscoelastic substrates with stress relaxation to regulate osteogenic differentiation by simulating the mechanical microenvironment of bone tissue [7, 99, 102] (Table 2). Hydrogels are considered to be the promising candidates for simulating bone ECM due to their highly adjustable biophysical properties [103]. Chaudhuri O et al. developed a synthetic hydrogel system for the first time to simulate the stress relaxation behavior of viscoelastic tissues. It was demonstrated that osteogenic differentiation of MSCs changed with alterations to the matrix viscoelasticity, and significantly increased when cultured in a substrate with faster relaxation kinetics, compared with a static substrate [27]. It is possibly due to rapid stress relaxation regulates intracellular integrin adhesion and actomyosin contraction, as well as nuclear localization of mechanosensitive transcriptional regulator YAP, thereby promoting osteogenic differentiation of MSCs [27]. It has been further demonstrated in follow-up studies that, except for the direct osteogenic action on stem cells, fast relaxing matrices facilitates bone matrix formation by stimulating cell volume expansion, adhesion, spreading and proliferation as well [28, 97, 102, 104, 105]. These results suggest that bone formation capacity of biomaterials can be optimized by adjusting the stress relaxation timescale and thereby changing the viscoelasticity of the matrix [7, 104].
Although several researches on regulating matrix viscoelasticity have been reported, these methods usually require complex physical crosslinking methods and chemical treatments [99, 106-108], and they merely focused on improving the viscoelastic properties of the material itself to achieve a high level of bone regeneration. Future directions need to focus on the new possibilities of combining with strategies that facilitate bone regeneration, such as stem-cell spheroids [29, 30], the addition of natural ECM [109], etc. Moreover, additional in vivo analytical models are required to investigate changes in viscoelastic properties at the bone-implant interface, in order to accurately predict the degree of bone integration [110]. Only in this way can appropriate biomaterial systems be constructed to better simulate the viscoelasticity of bone tissue ECM as well as guide the function and fate of stem cells.
2.1.4 Topography
As is well-known, superior mechanical properties of biomaterials is regarded as one of the evaluation criteria of medical implants [55]. Different from the modulation of stiffness and viscoelasticity, the topological structure printed on the substrate surface has greater clinical translational value due to its negligible effect on the overall mechanical properties of the material [55]. Moreover, altering surface topography gains popularity for offering not only the advantage of long-term stability, but also cost-effective fabrication methods [111]. Ever since Harrison RG et al. first confirmed in 1911 that stem cell differentiation could be modulated by topographic cues from underlying substrates [112], considerable effort has been devoted to guiding the MSC lineage determination by adjusting the surface topology of materials (Table 3).
As an important feature of surface topography, the roughness of biomaterials can directly regulate the migration and proliferation of cells on the surface [113]. More importantly, compared with smooth surface, rough surface topology enables stem cells with better osteogenic capability [114]. For instance, Yang W and his colleagues fabricated HAp-based scaffolds with different surface roughness. It was found that scaffolds with average roughness (Ra) (0.77 -1.09 μm) and mean distance between peaks (RSm) (53.9 - 39.3 μm) achieved optimal osteogenic differentiation by influencing cell attachment and cytoskeletal tension [115]. However, it should be noted that, surface topologies with different roughness can also induce the adipogenic differentiation of stem cells. Abagnale G et al. discovered that 2μm ridge enhanced osteogenic differentiation, while 15μm ridge supported adipose differentiation. This may be attributed to the direct effect of their physical size on cell morphology, with elongated morphology promoting cell progression toward osteoblastic lineages and rounded morphology promoting lipogenesis [9]. Recently, various rough topographies (such as ribbon structures [42], wavelike structures [37, 116], groove or ridge structures [9], microchannels [117], isolated islands [55], etc) have been successfully fabricated to promote osteogenic differentiation, among which the ribbon structure is the most widely used [6]. Vega SL et al. fabricated substrates with co-continuous (ribbons) or discontinuous (islands and pits) regions. The findings show that ribbon topographies (spacing: 48±5μm) favor cytoskeletal anisotropy and FA maturation, which promoted long-term expression of osteogenic differentiation markers [42].
Aside from the micron-structured biomaterials mentioned above, the interaction between cells and nano-morphology is also considered to be an effective approach to control stem cell differentiation in BTE [59]. This is because bone itself has the unique hierarchical nanostructure structure [118]. TiO2 nanotube arrays, manufactured by anodizing on a Ti substrate, are most commonly used to investigate the effects of nanoscale geometry on stem cell behavior [118, 119].
Effects of cell-laden biomaterials on MSCs osteogenic differentiation induced by topography
| Cell type | Material | Surface patterns | Result | Ref |
|---|---|---|---|---|
| hMSCs | PI | Micro-patterns Width: 2-15μm Depth: 2μm | 15 μm ridges increased adipogenic differentiation whereas 2 μm ridges enhanced osteogenic differentiation. | [9] |
| Nano-patterns Diameter: 600μm Depth: 200nm Periodicity: 650nm | Nano-patterns increased differentiation towards both osteogenic and adipogenic lineages. | [9] | ||
| PDTEC PS | Co-continuous ribbons Spacing: 48±5μm Height: 200nm | Co-continuous topographies favor cytoskeletal anisotropy, FA maturation and osteogenic differentiation. | [42] | |
| HA | Micro/nano hybrid structure Width: 28μm Space: 24μm Diameter:70-100nm | The micro/nano hybrid structure significantly enhanced the cell behavior including the adhesion, proliferation and osteogenic gene expression. | [127] | |
| Quartz | Chiral geometry Linewidth: 2μm Spacing: 2μm Depth: 3μm | Cell adhesion, proliferation, and differentiation are greatly enhanced for cells cultured on dextral geometry than those on sinistral geometry. | [131] | |
| Silicone | Periodic nanopillar arrays Diameter: 54-105nm Periodicity:70-201nm Height: 39-85nm | The nanopillar arrays enhance osteogenic differentiation of hMSCs, dependent on the age of the donor. | [58] | |
| Multiscale hierarchical topography | The 0.5⊥3∥25 substrate, resembling collagen topography the most, exhibits the highest osteogenesis. | [132] | ||
| CDMs PDMS | Wave-like structure Amplitude: 0.4, 2.2μm | CDMs and topography synergistically enhances osteogenic differentiation. | [116] | |
| PDMS | Wave-like topographies Wavelength: 0.5,3,10,27μm | Compared to W27, W3 showed the enhanced stiffness of stem cell, promoting higher degree of osteogenic differentiation. | [37] | |
| TiO2 nanotubes | TiO2 nanograin with the nanopore surface Width: 50-60nm Diameter: 30-40 nm | The expression of p-ERK and p-CREB increased in the TiO2 nanograin with the nanopore surface compared to the micro rough and nanotube surfaces. | [119] | |
| Rat MSCs | HAp | Micropatterns Height: 11.38±0.58μm Length: 63.87±3.41μm Width: 43.31±2.55μm | The micro-patterned topography and Sr-doping had a synergetic effect on the adhesion, growth and osteogenic differentiation of BMSCs. | [111] |
| BaTiO3/ poly-(l-lactic acid) fibrous scaffolds | Randomly oriented electrospun | The topographical structure and electrical activity have combining effects on cell attachment, growth, and osteogenic response. | [130] | |
| TiO2 nanorod array | Nanoscale geometry Length: 1.5μm Diameter: 100nm | A TiO2 nanorod array promotes the osteogenic differentiation of MSCs, while a TiO2 ceramic with a smooth surface suppresses it. | [59] | |
| MSCs | PCL | Micro-grooves Width: 16μm Height: 6μm | The space constraint inhibits the extension of actomyosin cytoskeleton, instead, pseudopodia lead to cell polarization. | [126] |
| Nano-grooves Width: 400nm Height: 500nm | The adhesion induction leads to the formation of FAs, promoting the osteogenic differentiation of stem cells. | [126] |
The early experiments proposed that the difference of diameter gave rise to different mechanisms responsible for osteogenic differentiation of stem cells [120]. Small diameter (approximately 30 nm) nanotubes promoting cell adhesion, conversely, larger diameter (70-100 nm) ones benefited cell elongation, which might lead to a change in cytoskeletal stress [120]. Recently, Lv L et al. found that TiO2 nanotubes, whose diameter were 70nm, were the optimal size for osteogenic differentiation of hASCs, compared with that of 50nm and 100nm [118]. Similarly, the optimal osteogenic diameter of nanorods was also confirmed to be 70nm [121]. In addition to the diameter, the distance between nanotubes also has implications on cell differentiation. Smaller pitch promoted MSCs differentiation from a young donor, while a larger pitch promoted that from an old one. This suggests that the nanotube spacing can be adjusted according to the age of the patient to prepare novel implants with the best osteogenic effect [58]. Nanogrooves and nanofibers are also proved to be powerful for material-driven osteogenesis. Yang L et al. combined substrates with nanogrooves and cell-derived matrices (CDM), which dramatically enhanced osteogenesis. However, CDM itself displayed only a minor contribution without nanogrooves. This suggests the strong synergistic effect on MSC osteogenesis [116]. Another combinatorial scaffold system was established by utilizing nanofiber scaffolds and polymeric microspheres. The nanoscale fibers not only mimic natural ECM, but also evoke directed response, especially osteogenesis [122].
Nevertheless, Li X et al. found that nanostructures alone might not be the optimal structure for osseointegration. Compared with flat quartz, nano-morphology significantly abated the osteogenic capacity [61]. Furthermore, the structural size of stem cells and natural ECMs is usually at the microscopic level. Stem cells may fail to stimulate osteogenic developmental signaling pathways due to their inability to perceive nanotopology [6]. Therefore, increasing researchers have recently devoted themselves to developing biomaterials with micro/nano-scale hybrid topologies, which show excellent osteogenic effects [123-125]. In fact, the pro-osteogenic mechanism of micron and nano-structure is different [126, 127]. Micro-groove promotes stem cell differentiation by activating the formation of pseudopodia. In contrast, nano-groove stimulate the formation of FAs and activates the RhoA/ROCK pathway, which shows stronger effects on osteogenesis [126]. It was further confirmed that the two structures have different activation mechanisms for integrins [127]. Therefore, the combination of microstructures and nanostructures has a synergistic activation effect [127].
To faithfully represent the in vivo-like microenvironment with complex topological structure, nacre topography with better osteoinductivity was fabricated via biomimetic approaches [128, 129]. It was shown that bone tissue that formed in response to nacre topography exhibited a higher crystallinity than those to chemical cues [128]. Furthermore, other biomaterials with novel topologies have been shown to be osteoinductive, such as randomly oriented fiber scaffolds [130], quartz with chiral geometry [131], multiscale hierarchical topography [132], etc. Nonetheless, the most current surface morphologies are designed on plane models. The construction of biomaterials with 3D topological structures is an urgent issue in the process of clinical transformation [59].
2.1.5 Dimensionalities of internal mechanical stimulation
Recently, dimensionality has been demonstrated to be a major contributor to affect cellular responses to mechanical stimulation. Vastly different outcomes have been shown when cells are cultured in 2D versus 3D microenvironment [133]. Generally, dimensionalities alter cellular shapes, thus affecting cell proliferation, migration and differentiation. It was shown that cell shape was flatter in 2D than in 3D, which might be related to whether integrin-mediated cell adhesion occurs on one side or around the cell, thereby influencing F-actin arrangement and expression [134].
Although researches on 2D culture are well established, 3D cell culture platforms have attracted attention recently, for mimicking more closely the geometrically complicated environment in vivo. Since the cells in 3D microenvrionnment may be affected by material stiffness, topography, permeability, oxygen, and other factors, a separate study on dimensionality appears to be unrealistic. Hsieh W-T et al. investigated the influence of dimensionality and stiffness on osteogenic differentiation of MSCs [135]. The results showed that the cell differentiation capability of 3D scaffolds was significantly enhanced with the increase of stiffness, compared with 2D substrates. This is due to the increased abundance and good alignment of actin stress fibers in a 3D environment with high stiffness. However, Major L G et al. held the opposite viewpoints [133]. They maintained that the cells responded to stiffness in a totally different way in 2D and 3D environments. With the increase of stiffness, cell volume increased in 2D environment, while, an opposite trend was observed in the 3D environment, along with decreased expression of the osteogenic gene RUNX2. This might due to the physically restriction to cell volume in 3D microenvironment [133]. To further explore the effect of cell volume on osteogenic differentiation, Bao M et al. developed a way to change cell volume alone, instead of depending on stiffness in a 3D microniche. It was shown that in small cells, stress fiber formation and YAP/TAZ localization could be observed on both soft and stiff matrix, showing that the osteogenic differentiation of cells was not affected by stiffness in cells with small volume. Conversely, stiffness was the major determinant for stress fiber formation in the largest cells [22]. This finding suggests that the difference brought by dimension (to be more specifically, physically restriction to cells) should be taken into account when designing biomaterials with various stiffness in the future.
In addition, dimensionality can also affect the optimal oxygen content of MSCs in scaffolds, thus affecting their osteogenic differentiation. It was shown that the expression of RUNX2 and VEGFA reached the highest when O2 concentration was 5% in 2D environment, while in 3D environment, O2 concentration needed to achieve up to 21% [136]. However, the reasons for this difference remain to be studied. In conclusion, dimensionality alters cellular response to biomaterial properties to some extent, thus affecting osteogenic differentiation, but the underlying mechanism by which mechanical stimulation regulates cell fate in different dimensionalities requires further exploration.
2.2 External mechanical stimulation on MSCs-laden biomaterials
Numerous studies have demonstrated that mechanical properties of biomaterials promote the osteogenic differentiation of MSCs. However, static culture may lead to insufficient supply of nutrients and oxygen [2]. Conversely, dynamic culture allows for more adequate nutrition and is closer to physiological systems in vivo, thus showing better osteogenesis [2]. Therefore, the application of various mechanical stimuli by bioreactors, such as shear stress [137], and micromechanical strain induced by compression, tension and vibration [138-140], becomes a promising approach to induce osteogenic differentiation of MSCs in vitro [141] (Table 4).
2.2.1 Compressive stress
Physiologically, the bone matrix is subjected to compressive or tensile loading due to gravity and muscle contraction [147]. This mechanical stimulation acts on the cells in the bone tissue and plays an important role in bone remodeling, such as early bone healing when fractures or bone defects occur [2]. Therefore, in order to investigate the optimal compressive stress on osteogenic differentiation of MSCs in vitro, large numbers of studies have been conducted by using compression bioreactors [158, 159].
Effects of cell-laden biomaterials on MSCs osteogenic differentiation induced by external mechanical stimulation
| Loading | Loading Regime | Scaffold | Osteogenesis | Ref |
|---|---|---|---|---|
| Compression | 42%, 0.3Hz, 3h/day for 21 days | GelMA | ALP, RUNX2, OCN, OPN, Mineral deposition (+) | [140] |
| 5-10%, 1Hz, 8h/day for 6 days | Collagen | BMP-2 (+) RUNX2, Col-1 (-) | [142] | |
| 5-20%, 1Hz, 2h/day for 28 days | Poly(ε-caprolactone) | RUNX2 (+) COL1A1 (-) | [139] | |
| 20-60%, 0.75Hz, 4h/day for 7 days | Octacalcium phosphate-gelatin | OCN, OPN, Col-1 (+) | [143] | |
| Stretching | 10%, 1Hz, 4h/day for 21 days | Fibrin hydrogel | ACAN, SOX9, BMP-2, RUNX2, OPN, COL1A1 (+) ALP (-) | [144] |
| Perfusion | 3ml/min (0.2 dynes/cm2) for 14 days | HA-PLGA | IBSP (+) | [145] |
| 7ml/min for 6 weeks | Alginate and gelatin-based hydrogel | Mineral deposition (+) | [146] | |
| 1ml/min, 30min/day for 3 weeks | Collagen-HA | OCN, OPN, Collagen, Mineral deposition (+) | [147] | |
| 0.8ml/min, 8h/day for 21 days | LTMC | Collagen, ALP (+) | [148] | |
| 6.3 cm3 min-1 for 0-2 weeks in the standard medium and 0-2 weeks in a differentiation medium | Apatite-Fiber | ALP, Calcification (+) | [149] | |
| 1.7ml/min, 5min every 15 min/day for 21 days | RCP, MgAp | Cell viability (+) | [150] | |
| 116μm/s for 21 days | Collagen coated with Mg -doped HA | ALP, OCN, OPN, BMP-2 (+) | [151] | |
| 3μl/min, 6h /day for 7 days | HA (750-900μm) | ALP (+) | [51] | |
| 10ml/min for 14 days | Fibrin breads | OPN, RUNX2, VEGF (+) | [152] | |
| 1.7ml/min for 21 days | Chitsan/HA | Collagen, Osteocalcin, Calcium deposition (+) | [153] | |
| 2ml/min for 14 days | HA-PCL | ALP, RUNX2 (+) | [154] | |
| Rotating, perfusion and compression | 0.22%, 1Hz, 5rpm /min, 4h/day for 2 weeks | PCL/TCP | RUNX2, COL1A1 (+) | [155] |
| Vibration | 30nm amplitude, 1000Hz for 21 days | Collagen | RUNX2, Collagen, ALP, OCN, OPN, BMP-2 (+) | [156] |
| 90nm amplitude, 1000Hz for 9 days | Collagen | RUNX2, OSX, ALP, OCN, OPN, ON (+) | [157] | |
| 30nm amplitude, 1000Hz for 3 weeks | Collagen | RUNX2, OSX, OPN, OCN, ALP (+) | [138] |
Compared with 2D environment, MSCs loaded on 3D scaffolds are studied more extensively in recent years, which is more closely to the physiological conditions in vivo [2]. It was found that compressive stress could promote osteogenic differentiation of MSCs in octacalcium phosphate-gelatin scaffold under a certain stress amplitude (20%) [143]. However, excessive stress amplitude (40%, 60%) inhibited the differentiation of MSCs. These results indicated that stress amplitude had significant effect on MSCs differentiation. In addition, compressive stress can also promote the differentiation of MSCs indirectly by altering the stiffness of scaffolds. In the study of Baumgartner W et al., it was found that under the condition of 5% cyclic compression, the stiffness of PLGA/aCaP scaffolds increased by about 2 times, and osteogenic markers RUNX2 and type I collagen were significantly up-regulated [160].
Compressive stress acts on the scaffold material and is then delivered to the cell [161]. Therefore, the mechanical stimulation of compression sensed by cells is related to the scaffold material. Hydrogels are often used as cell-loaded scaffolds in compression bioreactors because of their low elastic modulus and no noticeable deformation even in the setting of repeat compression forces [159]. The effects of different concentrations of GelMA hydrogel (5%, 7.5%, 10%) and dynamic compression (0, 10, 27 and 42%) on cell differentiation were studied by Seo J et al [140]. The results showed that 5% GelMA hydrogel and 42% dynamic compression had the best effect on cell diffusion and osteogenic differentiation, with the overexpression of ALP, OCN, OPN and mineral deposition. The reason may be that the degree of crosslinking of hydrogels affects the size of pores in the polymer network. The higher the degree of crosslinking, the smaller the pores, resulting in reduced cell diffusion and growth, which affects the transmission of compressive force [140]. Therefore, hydrogels with lower degree of crosslinking provide larger pores and promote cell migration, which is more recommended.
However, counter to the view as mentioned above, some experts hold that dynamic compression stimulation could not significantly promote the osteogenic differentiation of MSCs. It was found that cyclic compression stimulation (5%, 10%) reduced MSCs migration, but did not stimulate osteogenic differentiation. Meanwhile, the up-regulation of transcription factor RUNX2 should be followed by the up-regulation of BMP-2 [142]. It was also found that dynamic compression (15%) was more conducive to chondrogenic differentiation of MSCs [139]. Therefore, the magnitude and mechanism of appropriate compressive stress promoting osteogenic differentiation of MSC need further investigation.
2.2.2 Tensile stress
Distraction osteogenesis is a treatment modality applied to the healing of bone defects [162]. It stimulates new bone production by stretching the fractured end toward the other end. Therefore, it is suggested that loading cells with tensile stress using a tensile strain bioreactor promotes osteogenic differentiation of MSCs. Qi et al. investigated the effect of short-term tensile stress (0.5 Hz, 2,000 με) on the proliferation and osteogenic differentiation of MSCs [163]. The expression of growth factors TGF-β, bFGF and IGF-II and transcription factors RUNX2 and Ets-1 were upregulated under the stress. Wu et al. also found that short-term tensile stress (10%, 0.5Hz,6h/day) promote the expression of OPN, RUNX2, and OCN [164]. And this study further found that long non-coding RNA H19 was a positive regulator in osteogenesis of MSCs. It indicates that tensile stress has a critical role in promoting osteogenic differentiation in MSCs.
In order to fully simulate and investigate the effect of tensile stress on MSCs in vivo, cell-laden 3D biological scaffolds are fabricated recently. The hydrogel-coated MSCs, stimulated by uniaxial cyclic stretching, was found to promote osteogenesis and the expression of TNC markers [144]. Meanwhile, MSCs differentiation is dependent on the frequency and amplitude of strain in the endochondral osteogenic pathway. The expressions of osteogenic markers BMP-2, RUNX2, OPN and COL3A1, and chondrogenic genes ACAN and SOX9 were more strongly expressed at high amplitude and frequency (10%, 1 Hz) than at low amplitude (5%) [144]. It shows that the osteogenic pathway can be activated by adjusting the frequency and amplitude of tensile stress. However, studies on the effect of tensile stress on osteogenic differentiation of MSCs in 3D scaffolds are still relatively few, and more studies are needed in the future to explore the optimal tensile stress and the involved mechanic pathways.
2.2.3 Fluid shear stress
Many studies have shown that pretreatment of MSCs in a bioreactor promotes new bone formation in vivo [154]. Fluid shear stress in vivo can be simulated through a perfusion bioreactor, which facilitates osteogenic differentiation. Fluid shear stress enables cells seeding in a dynamic fashion, allowing them to be more uniformly distributed inside the scaffold, rather than just being located on the surface of the scaffold [141]. Cells located on the surface of the scaffold are easily washed away under high shear stress, which greatly reduce cell viability. At the same time, compared to static cell culture, the perfusion bioreactor drives the flow of medium at a certain rate, which facilitates the provision of more adequate nutrients and oxygen to the cells inside the scaffold, transports metabolic wastes, and maintains cell viability [150].
Based on the above-mentioned advantages, fluid shear stress has been extensively studied in terms of promoting cellular osteogenic differentiation [146]. Since the fluid shear stress to which the cells are subjected is generated by the perfusion device and transmitted through the scaffold, the effect of MSCs osteogenic differentiation is closely related to the appropriate perfusion conditions and culture medium, as well as the physicomechanical properties of scaffolds [165]. In recent years, an extensive investigation has been conducted into the effects of flow rate and incubation time on MSCs osteogenic differentiation under laminar, radial, and oscillatory fluid flow (OFF) through various perfusion bioreactors [154]. Laminar flow, which is unidirectional perfusion, provides a mild culture environment for cells and has been shown to promote MSCs osteogenic differentiation when cultured dynamically at low flow rates in normal medium [148]. In the study of Yamada S et al., it was confirmed that even in the absence of chemical stimulation, fluid stimuli in appropriate level significantly promoted osteoblastic differentiation of MSCs on 3D scaffolds [148]. Oscillatory perfusion helps to distribute the cells more uniformly within the scaffold [147]. According to recent studies, MSCs were cultured in osteogenic induction medium supplemented with dexamethasone, β-glycerophosphate and ascorbic acid 2-phosphate to further enhance osteogenic differentiation [149]. However, there are certain differences in the appropriate perfusion velocity and culture time required by different devices and scaffolds. Generally, the fluid shear stress that between 0.1 and 10 MPa is considered to promote bone tissue regeneration [51]. Excessive shear stress has a damaging effect on cells [148]. However, in the study of Mainardi VL et al. [146], the fluid shear stress provided by the high perfusion flow rate of 7ml/min (56.09 MPa) was slightly higher than this range, but compared with the low perfusion flow rate of 0.7ml/min (5.59MPa), the effect of alginate-gelatin hydrogel scaffold on promoting the deposition of mineralized matrix was more obvious. The reason might be that cells were embedded inside the scaffold by 3D bioprinting technology, and the fluid shear stresses that received were converted more into compressive and tensile mechanical stimuli, thus promoting the deposition of mineralized matrix. Similarly, in the study by Yaghoobi M et al. [154], high flow rate (4.5 ml/min), compared to low flow rate (2 ml/min), promoted the upregulation of RUNX2 expression in MSCs in nHA-PCL multilayer electrospun silk scaffolds under the conditions of combined mechanical pressure. Thus, higher perfusion flow rate shows better osteogenic potential.
In terms of culture duration, continuous perfusion culture tends to reduce the cellular response to stress stimuli and make cells adaptive [166]. Therefore, intermittent perfusion culture is more favorable. A culture time from 5 days to 5 weeks is the most widely-applicable [167]. However, some studies have shown that longer culture time does not necessarily imply better osteogenesis. MSCs produced the highest angiogenic markers at 7 days under perfusion culture at a flow rate of 3 mL/min, but the DNA content and osteogenic differentiation markers stabilized at 14 days [168]. Suzuki K et al. used a different culture protocol, using standard medium for one week and then osteogenic differentiation medium for one week [149]. The highest ALP activity was observed at this time point, however, the activity of ALP decreased at 2 weeks of osteogenic differentiation medium culture. It indicates that MSCs may promote early bone differentiation. Therefore, it is important to find the appropriate culture time for different materials.
In addition, the fluid shear stress is closely related to the pore size of the scaffold [51]. It is generally believed that a pore size of over 300 μm is favorable for cell migration, proliferation, and the growth of blood vessels and bone tissue into the scaffold [169]. It was further demonstrated that the shear stress provided by the medium pore size (750-900 μm) of the HAp scaffold (2.65 MPa) was more suitable for osteogenic differentiation compared to the large pore size (1.55 MPa), and the small one (5.78 MPa) [51]. This suggests that when exploring the optimal scaffold pore size, the effect of shear stress also need to be considered. Moreover, in the study by Rogina A et al., chitosan scaffolds containing 30% HA showed significantly higher deposition of collagen and calcium after 21 days of perfusion culture compared to 50% HA versus chitosan scaffolds alone. This indicates that besides pore size of scaffolds, the chemical composition also influences cell adhesion, growth, proliferation and differentiation [153].
2.2.4 Vibration
Low-magnitude, high-frequency vibration plays a critical role in maintaining bone homeostasis and promoting bone metabolism, and was recently introduced to induce MSCs osteogenesis [170, 171]. Prè D et al. studied the effects of high frequency vibration (30 Hz, 0.59 × g, 45 min/day) on the proliferation and osteogenic differentiation of MSCs and found that calcium deposition, type I collagen deposition, and RUNX2 expression were significantly increased after 21 days of culture [170]. Another study showed that vibratory stimulation (50 Hz, 0.05-0.9 × g, 30 min/day) promoted osteogenic differentiation of periodontal stem cells [171]. These results suggest that MSCs respond to the mechanical effects of high-frequency vibration and can be induced to osteogenic differentiation by loading cells with high-frequency vibration.
Vibrational bioreactor, an in vitro device that generates high frequency vibrations, is extensively studied in recent years, especially nanovibrational bioreactor. The nanovibrational bioreactor allows the culture to produce nanoscale displacement at a certain frequency to induce osteogenic differentiation of MSCs [138]. However, in 3D scaffolds, nanoscale vibrations need to be transmitted through the scaffold to the cells, thereby making stable transmission of vibrations more difficult. Because of the good viscoelastic characteristics, type I collagen gel can adhere to the sidewalls and bottom of the culture dish, forming a monolith with the culture vessel and good delivery of vibrational stimuli [156], which makes it more commonly used in the study of nano-vibrational bioreactors [172]. It was found that the expression of osteogenesis-related genes such as ALP, OCN and OPN were significantly increased in collagen gels under nanoscale vibration (30 nm, 1 kHz) using the principle of reverse piezoelectricity [156]. It indicates that nano-vibration stimulation has a positive effect on MSCs osteogenic differentiation. Meanwhile, it was further revealed that nano-vibration stimulation could be delivered to cells via mechanoreceptors such as Piezo, TRP and potassium channel subfamily K member (KCNK), affecting cytoskeletal tension and adhesion. However, the mechanism of nano-vibration stimulation on cells in 3D scaffolds is still unclear. Therefore, the osteogenic differentiation of MSC under 90 nm amplitude conditions was further investigated [157]. It was found that the expression of mechanoreceptors TRPA1, Piezo1/2 and KCNK2 were upregulated in cells at 90 nm amplitude compared to 30 nm amplitude. Thus, higher nano-amplitude showed a greater advantage in 3D scaffolds. However, higher amplitudes are associated with higher levels of reactive oxygen species and inflammation, which inhibit osteogenic differentiation of cells [173]. Therefore, it is important to balance both osteogenic differentiation and inflammation levels when designing the amplitude.
2.3 Combined effects on MSCs-laden biomaterials
It is well known that cell behaviors are profoundly affected by the variable 3D surrounding microenvironment. Hydrostatic pressure (HP), fluid shear stress (FSS), compression, and stretching mechanical stimulation work together with the complex structure of ECM to affect cell fate [174]. Therefore, it is believed that researches on the combined effects of multiple physical cues can better simulate the microenvironment in vivo, thus promoting the osteogenic differentiation of cells [175]. Reinwald Y et al. confirmed that intermittent hydrostatic pressure (IHP) (270 kPa) in combination with topographical cues (fiber alignment) could direct the fate of MSCs, and enhanced the effect of osteogenesis [174]. Moreover, cells on random fiber substrates were more responsive to the IHP, compared with those on aligned substrates [174]. Two types of FSS, uniaxial rotation and perfusion were combined to investigate the effect of MSCs osteogenic differentiation in 3D β-TCP scaffolds [176]. The results showed that the rotated and perfused group significantly up-regulated ALP activity and the expression of OCN, RUNX2, and COLI, compared to perfusion alone. The combination of FSS and compression was also explored. Ravichandran A et al. invented a biaxial rotation bioreactor that rotated along the X and Z axes, similar to the gyroscopic motion of a fetus in utero, and is simultaneously loaded with cyclic compression stimuli to mimic the biomechanical stimulation to which bone is physiologically subjected [155]. It was shown that the biaxial rotation approach increased the rotational velocity component compared to the uniaxial rotation, thereby improving fluid transport within the scaffold. The expression of RUNX2 and COL1A1, as well as mineral matrix deposition, were significantly elevated after simultaneous loading of cyclic compression stimuli.
As additional insights into the interaction between cells and ECM in recent years, it was found that the combination of matrix stiffness and nano- topography significantly affected the fate of cells as well [175]. After attaching to the random nanofibers, cells presented apparent stretching morphology and transformed mechanical signals into intracellular signals through cytoskeletal rearrangement, thus promoting osteogenic differentiation [174]. And this effect could be amplified by the combination with matrix stiffness [175]. Nevertheless, it is difficult to obtain the optimal stiffness and topography for osteogenesis simultaneously. Jahanmard F et al. attempted to investigate the balance between stiffness and topography by adding carbon nanotubes to the substrate [34]. It was found that low concentration of carbon nanotubes (0.5wt %, 1wt %) significantly improved the stiffness of electrospun nanofibers, while relatively high concentration (2wt %, 3wt %) showed obvious nano roughness. Moreover, the two above-mentioned mechanical forces show distinct effects on MSCs. High stiffness promotes cell proliferation and osteogenic differentiation, while roughness affects cell morphology and cell adhesion [19]. Seo J et al. further demonstrated that stiffness increased the number of adhesion sites on MSCs, but for mature adhesion sites, it was only determined by the roughness of surface topography [140]. At present, although the influence of matrix stiffness and surface topography on osteogenic differentiation of MSCs has been understood to a certain extent, how to balance the relationship of two for enhancing the synergistic effect and better promoting osteogenic differentiation remains to be further studied.
3. Mechanisms involved in biomaterials-induced osteogenic differentiation of MSCs
3.1 Mechanosensors
The response of MSCs to mechanical stimulation comprises two major phases: mechanoreception and mechanotransduction [3]. Mechanoreception is the process that cells sense physicomechanical signals from the ECM through mechanoreceptors. This further leads to cell differentiates into specific lineage through signaling pathways, which is known as mechanotransduction [3]. Mechanoreception is crucial for transforming physical signals into biochemical ones by adjusting cytoskeletal arrangement, cell and nucleus morphology [177]. Herein, the major mechanosensors, including integrin and FAs, cytoskeleton, primary cilium, ion channels, and gap junction, will be discussed in detail (Figure 2).
Mechanosensors involved in biomaterials-induced osteogenic differentiation of MSCs. Mechanical stimulation is sensed by different mechanosensors on MSCs, including integrin and FAs, cytoskeleton, primary cilium, ion channels and gap junction. FAs function by transmitting mechanical signals to the cytoskeleton, thereby affecting cytoskeletal arrangement. Primary cilium alters the length in response to mechanical signals. Ion channels permit Ca2+ influx to modulate downstream pathways. Gap junction mediates cell-cell interactions by upregulating the expression of osteogenic-related genes.
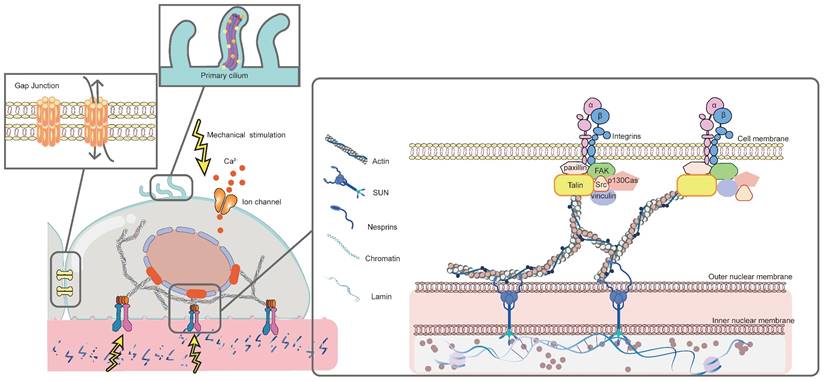
3.1.1 Integrin and focal adhesions (FAs)
Integrins, widely known as mechanical sensors, are ubiquitous in thin cell membrane projections and filopodia, mediating adhesion between cells and ECM and transmitting mechanical signals [55, 178]. As a transmembrane protein, one end of the integrin is attached to a protein ligand in ECM, the other connects to the intracellular actin fibers via an adaptor protein [3]. This establishes an integrin-dependent bidirectional signaling, that is, not only transmiting cellular signals to the ECM, but also conveying signals from the ECM intracellularly can be achieved [179], which triggers intracellular signaling pathways that lead to cell migration, proliferation, and differentiation [127].
Integrins are heterodimers, consisting of non-covalent binding of α and β subunits. Compared to β-subunit integrins promoting intracellular signaling and cytoskeletal linkage, the α-subunits induce ECM ligand specificity [13]. In vertebrates, the 18 α and 8 β subunits assemble 24 complexes with diverse functions [180]. Different subunits play distinct roles in regulating stem cell responses to the physicomechanical properties of the microenvironment. During the osteogenic differentiation of MSCs, the expression of integrins α1, α2, α5, αv, and β1 are upregulated [125, 181-184], and α3, α4, α6, β2, β3, β4 are downregulated [185, 186]. Interestingly, the expression of integrin on cell surface fluctuates with different types of mechanical stimulation. For example, more α5β2 is expressed on microstructures than on nanostructured materials, while there is no significant difference in the expression of αvβ2 on microstructures and nanostructures [127]. High matrix stiffness promotes α2 integrin expression [183]. Furthermore, when binding to various ECM proteins, subunits show different affinity and specificity. For instance, mediated mechanical transduction mediated by fibronectin requires the synergistic action of multiple integrin subunits such as αvβ3, α2β1 and α5β1, whereas type I and type IV collagen require only α5 integrins [187]. This may be due to the fact that the arginine-glycine-aspartic acid (RGD) motif of fibronectin needs to recognize the epitopes of two subunits, whereas the binding site of collagen only needs to bind to the specific domain of the α subunit [177].
In response to mechanical stimulation, integrins with higher affinity are activated, enabling the recruitment of a wide range of intracellular proteins, which are termed as integrin adhesion complexes (IACs). They mediate mechanical signals between the integrin and actin cytoskeleton, which further directs stem cell differentiation [188]. Three protein layers assemble the IACs. The outermost signaling layer contains highly phosphorylated proteins FAK and paxillin. The intermediate force transduction layer consists of two adaptor proteins, talin and vinculin. And the innermost actin regulatory layer is dominated by α-actinin [189, 190]. It is worth noting that talin plays a major role in IACs. This encoded protein forms the integrin-protein-actin axis complex by coupling integrin to F-actin [177]. Following the transfer of mechanical forces from the integrin, talin transitions into an unfolded conformation and exposes its binding site [190], allowing IACs to rapidly aggregate into focal complexes and further mature into supramolecular complexes known as FAs in a brief period of time [177].
The physicomechanical properties of different biomaterials directly influence the number and size of FAs, which significantly affect the osteogenic differentiation of MSCs [13]. For example, enhanced matrix stiffness increases the expression of specific integrins (αv, α5, and β1), inducing the formation of FAs and the further activation of downstream osteogenic signaling pathways [184]. Additionally, the area of FAs increases with the roughening of material surface [14]. Compared with a flat substrate, the 100μm groove/ridge promotes mature FAs formation, leading to osteogenic differentiation of MSCs. Conversely, the 10μm groove/ridge array formed fewer FAs and promoted adipogenic differentiation [191]. Two potential reasons can explain why mature FAs drive osteogenesis. On one hand, they can directly transmit mechanical signals to sensors for nuclear mechanics (lamin A/C) through actin stress fibers [192]. Specifically, FAs modulate the spatial organization of radial and transverse fibers in actin cytoskeletons. In this way, the FA-nuclear mechanical coupling is established, and physical signals are translated into biological activities controlling MSC fate commitment [193]. On the other hand, FAs induce downstream cell responses through chemical signals, involving the recruitment and activation of signal proteins, dominated by FAK [14]. Under mechanical stimulation, the conformation of FAK changes, exposing phosphorylation sites and activating intracellular osteogenic-related pathways [14]. Moreover, other key signals related to osteogenic differentiation are also activated, such as BMP [127], RhoA [55], extracellular-signal-regulated kinase (ERK) [181], etc. It is thus clear that abundant and tightly packed FAs are essential for subsequent cytoskeletal changes and the triggering of intracellular signaling pathways during physicomechanical stimulation-induced osteogenesis.
3.1.2 Cytoskeleton
The cytoskeleton, including the cytoplasmic and nuclear skeleton, is responsible for maintaining cell shape, motility, contractility, etc. More importantly, they also act as mechanosensors of ECM [13, 194]. The cytoplasmic skeleton senses mechanical stimulation in ECM and then transmits signals to the nucleus, ultimately alterating the gene expression [10]. The structural elements of the cytoplasmic skeleton are composed of microfilaments, microtubules, and intermediate filaments (IFs) [195]. Among them, the actin microfilaments play a critical role in transmission of mechanical signaliing, which are tightly connected with FAs and nucleus [195].
Acting as a highly dynamic network, actin cytoskeleton realizes the transmission of mechanical signals by reshaping its own microstructure [13, 196]. Specifically, under mechanical stimulation (such as cyclic strain [197], fluid flow shear stress [198], oscillatory shear stress [199], vibration [156], specific substrate topography [200, 201], etc.), FAs are formed and FAK is subsequently phosphorylated. This stimulates G-actin to assemble into F-actin, which forms stress fibers together with myosin-2. One end of the stress fibers binds to actin-binding proteins (vinculin and talin) on FAs, and the other connects to the nucleus, thereby conveying signals from FAs to the nucleus [13]. During mechanotransduction, myosin-2 acts as a crosslinking agent to harden or soften the actin network by regulating the slip and rearrangement of actin filaments [200, 202]. Thus, through regulating myosin-2 activity, many kinases enhance cytoskeletal tension and then participate in mechanosensitive signaling pathways, such as the Rho GTPase protein family: RhoA, Rac1, and cell division control protein 42 homolog (cdc42) [55, 203-206]. Conversely, any disruption to myosin-2 hinders the actomyosin from contracting, leading to the alteration of the mechanics inside the nucleus. Subsequently, the activity of osteogenic-related signals, such as ERK and Yes-associated protein (YAP) pathways, is decreased [207]. According to this, high levels of actin polymerization and high density of stress fibers is crucial for driving osteogenesis. In addition to acting directly on the nucleus, actin filaments can also transfer mechanical forces to ion channels, such as TRPM7, triggering plasma membrane Ca2+ influx [208]. It was shown that the disruption of cytoskeletal actin filaments by cytochalasin D (Cyto D), ML-7 or blebbistatin, completely eliminated the force-induced Ca2+ oscillations through TRPM7 [208, 209]. Interstingly, TRPM7-induced Ca2+ influx can in turn promoting actin polymerization by increasing intracellular Ca2+ concentration [209]. This suggests that the interaction between actin microfilaments and TRPM7 during mechanical transduction further enhance the osteogenic effects [209].
In addition to actin, the microtubule dynamics is also proved to be involved in the mechanotransduction pathways underlying MSCs osteogenic differentiation. Although the microtubule cytoskeleton also acts through maintaining the shape of cells and nuclei, it acts passively in the periphery of cells and serves as a “pillar” in the cell structure to support the core cells stably. This is totally different from active stress generated by actin contraction [5, 208]. This discrepancy leads to their different ways of altering cell morphology, especially in different microenvironments. In 2D environments, MSCs sense the microenvironment through FAs and the reorganization of actin cytoskeleton, while the microtubule cytoskeleton remains relatively stable [5]. The actin cytoskeleton pulls the nucleus on its two separate sides, while the stress fibers push the nucleus downward, flattening the nucleus and allowing MSCs to adjust the morphology freely [210]. On the contrast, the 3D environment may limit cell extension, and the overall perceived tension is mainly transmitted through the microtubule cytoskeleton. Microtubule exerts a force opposite to actin, acting on the nucleus. This in turn alters cell and nuclear morphology, accompanied by changes in the heterochromatin in nucleus, thus affecting the gene expression profile of MSC [210]. The precise regulation of the microtubule dynamics (polymerization and de polymerization) was confirmed to be important for controlling MSCs fate [211]. This is mainly because a complementary force balance is formed between contractile actomyosin filaments and compression-supporting microtubules, supporting the modulation of cell morphorlogy [200]. Interestingly, however, microtubule depolymerization, rather than polymerization, appears to favor osteogenic differentiation of MSCs. After microtubule depolymerization, myosin alter its mechanochemical activity by regulating side chain phosphorylation, resulting in an increase in myosin contraction [211]. The enhanced contractile force not only directly induces osteogenesis, but also counteracts the traction exerted by the matrix and achieves tensile equilibrium, which in turn further reduces microtubule polymerization and accelerates osteogenesis [5]. Moreover, it was confirmed that the passive cytoskeletal support also played an important role in the mechanoactivation of TRPM7 channels and Ca2+ influx across the plasma membrane [208].
Compared to actin filaments and microtubules, few studies have been reported on the role of IFs in the osteogenic differentiation of MSCs. A deficient vimentin IF network was shown to decrease the deformability of MSCs, thus affecting osteogenesis [212]. Similarly, Stavenschi E et al. demonstrated for the first time that under cyclic hydrostatic pressure (CHP), the remodeling of IFs was required for loading-induced osteogenesis of stem cells [213]. To be more specific, under the mechanical pressure, vimentin-based IFs remodel and recoil toward the perinuclear region, inducing downstream osteogenesis [213]. These results suggest the potential role of IFs during osteogenesis. Nevertheless, further experiments are required for exploring the specific mechanisms of IFs on loading-induced MSCs osteogenesis.
After sensing mechanical stimulation, the above-mentioned three cytoplasmic cytoskeletons deliver the signal to internal nuclear receptors lamin A/C via Linker of Nucleoskeleton and Cytoskeleton (LINC) complex [214]. This complex consists of SUN proteins anchored in the inner nuclear membrane and nesprins anchored in the outer nuclear membrane. In this way , the LINC complex links the cytoplasmic cytoskeleton with the nucleoskeletal lamin A/C. The reorganization of lamins is then achieved in response to mechanical stress [215]. Lamin A/C is a kind of intermediate filament proteins, forming a protein meshwork under the nuclear membrane, on which the chromatin is arranged [216]. Therefore, mechanical forces lead to alterations in the nuclear envelope structure via lamin A/C mechanotransduction. Subsequently, this structural deformation changes chromatin arrangement and gene expression [215, 216]. It was shown that the increased lamin A/C enhaces the stiffness of nucleus, inducing MSCs osteogenic differentiation [214]. In contrast, the depletion of lamin A/C contributes to nuclei with irregular shape and severely reduced stiffness [215]. Enhanced matrix stiffness [216, 217], hyperboloidal topography [218], convex substrates [219] are demonstrated to improve laminA/C level as well as cell stiffness. For example, lamin A/C levels were 2.5 times higher on convex substrates compared to concave surfaces, and 1.4 times higher compared to flat surfaces [219]. Furthermore, the upreguulated lamin A/C may interact with histone deacetylases (HDACs), affecting osteognic gene expression via epigenetic alterations [216]. When MSCs were cultured in rigid hydrogel microenvironments, HDAC activity decreased significantly with increased lamin A/C expression, which initiated RUNX2 transcription and promoted osteogenesis [216]. Conversely, the disruption of nuclear mechanosensing up-regulated HDACs, preventing epigenetic response as well as osteogenic fate determination. Therefore, lamin A/C plays a determining role during stem cell differentiation.
In short, as the major components of MSCs, the cytoskeleton works together to maintain normal cell morphology and regulate cellular response to mechanical stimuli. Additionally, some specialized structures produced by the cytoskeleton, such as primary cilia composed of microtubules, have been widely recognized as major mechanoreceptors in MSCs [202]. Future studies should be dedicated to exploring more on IFs, as well as the interactions between different types of cytoskeletons.
3.1.3 Primary cilium
As a solitary and unfixed mechanoreceptor [220], primary cilium which extends from the cell membrane exist in various tissues, including bone, cartilage, and cardiovascular tissues, etc [202]. It consists of nine concentric microtubule filaments, which form the core of the cilium, also known as axoneme [202, 221]. Primary cilium senses the mechanical environment through this unique structure and transmits extracellular mechanical signals [202]. Recent studies have shown that primary cilium has a considerable role on MSCs after sensing biomaterial-induced physicomechanical stimuli (such as topography [222, 223], fluid shear stress [224], cyclic tensile strain [221], etc.) during driving osteogenesis.
Hoey DA and his colleagues demonstrated for the first time that the primary cilia of stem cells were essential for mechanically-mediated osteogenesis. They stimulated MSCs with OFF in vitro to simulate FSS in physiological environments. The results showed that OFF promoted the proliferation of MSCs and upregulated the expression of osteogenic genes, which was proven to be mediated by primary cilium. On the contrary, hMSCs without primary cilium significantly inhibited osteogenesis in response to mechanical stimulation [224]. Similar results were obtained in the study of Chen JC et al [225]. Several studies have shown that this mechanically-mediated osteogenic effect is related to the length of cilium [221-223]. McMurray RJ et al. found that on the grooved topography, MSCs extended into an elongated morphology toward the groove, and had primary cilia with lengths greater than 3 μm. Nonetheless, such long primary cilia decreased the expression of osteogenic factors [223]. Bodle J et al. observed a similar trend. The cells cultured on a hard substrate showed reduced cilia lengths but with stronger osteogenic differentiation ability, compared to those on the softer silicone membrane substrates [221]. Additionally, a decreasing trend in the length of cilium was observed as well under cyclic tensile strain [221]. Apparently, primary cilium is mechanically sensitive. There are currently two theories to explain this change in cilia length. On one hand, an extended cilium is more likely to detect smaller magnitude changes in the surroundings. On the contrary, the cilium no longer needs such a large “lever arm” to sense mechanical signals when there are larger magnitude mechanical stimuli [221]. On the other hand, after exposed to mechanical stimulation, stem cells alter cilia length by reducing actins to regulate downstream osteogenic signaling pathways [223], such as Hedgehog signaling [221], TGF-β signaling [222], Wnt signaling [223], etc.
Additionally, TRPV4 ion channels [226, 227] and G protein coupled receptors (GPCR) [228] are widespread on the primary cilium to mediate mechanical signal transduction and osteogenesis of MSCs. TRPV4 upregulates early osteogenic gene expression through mediating calcium signaling induced by oscillating fluid shear [227]. Gpr161, a mechanoreactive GPCR localized in cilia, modulates cAMP and MSC osteogenesis by activating adenylyl cyclase 6 (AC6) [220, 228]. Nevertheless, the present study cannot fully explain the specific mechanism of primary cilia promoting osteogenesis in stem cells after sensing biomaterial-induced physical mechanical stimulation, which needs to be further elucidated in further studies.
3.1.4 Ion channels
Recent studies have found that under the mechanical stimulation (such as HP [229], FSS [230], vibration [156], stiffness [230], etc.), MSCs show a rapid increase in the intracellular Ca2+ concentration, which drives osteogenesis. This is attributed to the activation of mechanically sensitive Ca2+ channels on MSCs, such as Piezo and transient receptor potential (TRP) ion channels.
In 2010, the discovery of Piezo opened a new era of researches on mechanotransduction [231]. As one of the most widely studied mechanosensitive cation channels to date, Piezo proteins (Piezo 1 and Piezo 2) are universally localized on the plasma membrane, particularly the lamellipodia and flopodial tips [229]. In the presence of membrane tension, Piezo can be activated directly without any additional components [232]. The refined structure is the principle reason of its sensitivity to mechanical stimulation. Piezo has a homotrimer structure, similar to a three-bladed propeller, consisting of the peripheral mechanotransduction module and the central ion conduction pore module [233, 234]. There are several hypotheses upon the gating mode of Piezo. The force-from-lipids hypothesis states that tension affects lipid-protein interactions between the membrane and ion channel. The protein conformation is subsequently altered, directly activating the channel [235]. According to the force-from-filaments hypothesis, interacting cytoskeletal components or ECM is the major cause of the change on Piezo conformation [236]. Geng J et al. presented a plug-and-latch hypothesis. More precisely, a plug and a latch exist on each monomer of Piezo. The plug is removed to open ion channels under the pulling of the latch [237].
HP was demonstrated for the first time to transmit mechanical signals via Piezo [229]. The Piezo inhibitor GsMTx4 inhibited osteogenic differentiation induced by 0.01 MPa HP loading. Conversely, the Piezo activator Yoda1 drove osteogenesis by upregulating BMP-2, thus enhancing MSC osteogenic differentiation [229]. Besides HP, FSS is also involved in signaling through Piezo channels [230]. To be more specific, Piezo channels relayed FSS signals to activate Ca2+ influx to stimulate Calcineurin. The nuclear factor of activated T cells c1 (NFATc1), YAP1 and β-catenin transcription factors were further activated, to form NFAT/YAP1/β-catenin complex which enhanced osteogenesis. Furthermore, when plated on stiff (40 kPa) hydrogels, MSCs spread to much larger areas with strong nuclear Yap1 localization [230]. This suggests that Piezo can sense mechnical stimulus brought by stiffness as well.
TRP is another ubiquitous mechanosensitive channel, consisting of intact membrane proteins with permeable Ca2+ [202]. The mechanical force is transformed to the channel by surface tension or bending of the lipid bilayer, which leads to a hydrophobic mismatch that opens the channel [238]. TRP channels are grouped into seven major subfamilies in mammals according to the nucleotide sequence homology: TRPV (vanilloid), TRPM (melastatin), TRPC (canonical), TRPA (ankyrin), TRPP (polycystin), TRPML (mucolipin), and TRPN (Drosophila NOMPC) [239]. Several TRP channels, including TRPV1 [156], TRPV4 [226, 227], TRPM7 [208, 240, 241], have been found to be involved in the mechanical signal transduction of stem cells.
TRPV4 is an extensively investigated TRP channel located in the high strain region, especially in the basal bodies of primary cilia [227]. It mainly induces the intracellular Ca2+ influx under the stimulus of FSS, as well as the subsequent upregulation of osteogenic genes [226, 227]. This is mainly because TRPV4 channels mediate FSS-induced NFATc1 nuclear translocation. Then, NFATc1 and osterix (Osx) form complex to induce the transcription of osteogenic genes of MSCs [226]. Moreover, a unique reciprocal feedback loop exists in MSCs between TRPV4 and cell volume expansion, which results in enhanced osteogenic differentiation [105]. Under rapid stress relaxation hydrogels or low osmotic pressure, TRPV4 ion channels on the cell membrane increase as cell volume expands. The overexpressed TRPV4 further accelerated cell volume expansion, promoting actomyosin contraction and actin polymerization, thereby driving MSC osteogenesis. In contrast, cells cultured in slowly relaxed hydrogels and high osmotic pressure were limited in volume, even if the TRPV4 was activated [105].
Different from TRPV4, TRPV1 channels sense mechanical forces via nanovibrational stimulation. The influxed Ca2+ through the TRPV1 channels triggered the activation of protein kinase C (PKC) and ERK, leading to the activation of downstream β-catenin. β-catenin then translocated into the nucleus and inducing the transcription of osteogenic genes [156].
Recently, TRPM7 is confirmed as one of key mechanical sensors involved in the osteogenesis of MSCs [240, 241]. Under different mechanical stimuli including shear stress [241], stretch [208] and pressure [240], TRPM7 channel is activated, resulting in Ca2+ release into the cytoplasm. In contrast, TRPM7 mutation can not only completely block the increase of intracellular Ca2+ and the nuclear localization of NFATc1 [240], but also reduce actin polymerization [209], which is detrimental to osteogenesis. Interestingly, after activated, TRPM7 channels tend to further amplify Ca2+ signaling by triggering endoplasmic reticulum (ER) Ca2+ release. The activated TRPM7 then interacted with cytophospholipase C (PLC), produced IP3 by hydrolyzing phosphatidylinositol 4,5-bisphosphate (PIP2). Subsequently, IP3 activates inositol trisphosphate receptor type 2 (IP3R2) on the ER conducting Ca2+ release [240].
Although the mechanosensitive channels described above can be activated in response to mechanical forces, it is important to note that different types of ions channels may differ in the optimal intensity and duration of stimuli. For instance, it was confirmed that the high-intensity mechanical loading in chondrocytes was mediated by Piezo channels, while the low one was mediated by TRPV4 [242]. Similar results were found in osteoblastic cells. TRPV4, rather than Piezo1, was sensitive to shear stress upon induction with fluid flow for 5 seconds [243]. Nonetheless, comprhensive and precise comparisons have not been performed between TRP and Piezo channels in MSCs.
3.1.5 Gap junction
In addition to the above-mentioned mechanosensors, MSCs communicate with neighbouring cells via gap junctions (GJs) formed by connexins [244, 245]. Six identical or different connexins constitute connexons, which exists in pairs to form GJs between adjacent cells as material exchange channels [245]. Among various kinds of connexins, Cx43 is the most highly expressed subtype [246]. As a communication hub, it functions through special C-termini, helping cells to sense and respond to mechanical stimuli from ECM [245].
Currently, only several kinds of mechanical stimuli have been demonstrated to drive osteogenesis by activating Cx43. Shear stress (0.5 Pa) was confirmed to enhance the osteogenic differentiation of stem cells through Cx43 and Erk1/2 signaling [247]. The highest expression of Cx43 and the greatest Erk1/2 activation was shown in the shear stress loading group compared to that in the static group [247]. In another study, micro/nano structure was found to promote osteogenic differentiation, related to the upregulated Cx43. To be more specific, on one hand, activated integrins interact directly with Cx43 and induce the opening of Cx43 semi-channels, thus activating cell-cell communication. On the other hand, the activated Cx43 regulates the BMP-2 signaling pathway by partially upregulating BMPR1 on the nanostructure. The overexpressed BMP-2 in turn further regulates Cx43-related intercellular communication [127]. Furthermore, micro/nano hybrid structures exhibited a higher stimulative effect on Cx43 expression than micropatterns or nanorods alone. This suggests that micro- and nano-topography play different roles in activating osteogenic signaling pathways. Compared with the micropatterns, nanostructures are more likely to induce stronger Cx43-mediated cell-cell interactions by upregulating BMP-2 expression, resulting in better osteogenesis [127]. Although the intercellular communication mediated by Cx43 channels has been demonstrated to play a central role in the osteogenic differentiation of MSCs [244], relatively little is known about Cx43 compared with other mechanosensors. More attention should be devoted to explore other types of mechanical stimuli that act on Cx43, the interaction between Cx43 and other mechanosensors (such as FAs), as well as the Cx43-mediated downstream signaling pathways during osteogenic differentiation [248].
3.2 Mechanotransduction pathways
As discussed in the previous section, after mediated by mechanoreceptor, MSCs differentiate into osteogenic lineage through multiple pathways. Several mechanotransduction-associated pathways recently reported in MSCs will be discussed in this section, including YAP/TAZ signaling, MAPK signaling, Rho-GTPases signaling, Wnt/β-catenin signaling, TGFβ superfamily signaling, Notch signaling, etc. (Figure 3).
3.2.1 YAP/TAZ signaling
The Hippo transcriptional coactivator YAP and TAZ are identified as a mechanical rheostat of MSCs, mediating osteogenic differentiation [177]. They are highly mechanosensitive to complex microenvironmental cues, resulting in rapid on-off mechanotransduction. When no stimulus is present, large tumor suppressor kinase (LATS)1/2 phosphorylates YAP/TAZ, which leads to cytoplasmic sequestration [177]. Conversely, mechanical stress triggers the activated YAP/TAZ to form a complex with Scalloped (Sd) to permit nuclear translocation. YAP/TAZ subsequently interacts with DNA-binding partner TEA domain family member (TEAD) to induce the expression of genes involved in osteogenic differentiation. YAP/TAZ nuclear translocation is driven by FAs formation and the subsequent activation of Rho-GTPase, which promotes actin polymerization and enhanced actin cytoskeleton tension [246, 249]. Then, nuclear pores enlarge and YAP translocation occurs due to the cytoskeletal remodeling [14, 249].
Matrix with higher stiffness is confirmed to be an initiator of YAP/TAZ signaling, for it can promote nuclear colocalization of YAP and RUNX2 by progressively organized actin filaments [29, 86, 184, 217]. Recently, it was further demonstrated that stiff substrates increased the expression of migration inhibitory factor (MIF) in MSCs, which in turn regulates AKT/YAP signaling to direct osteogenic differentiation [83]. Besides actin filaments, biochemical ligand density plays a critical role in stiffness-induced YAP nuclear translocation [187]. It was found that YAP translocation was dominated by stiffness only at intermediate ligand densities. However, the low or high ligand densities, rather than stiffness, dominates YAP location [207]. Different from the simple 2D culture, YAP/TAZ nuclear localization is less correlated with substrate stiffness in 3D environments [22]. Scott KE et al. constructed a 3D spatial model of YAP/ TAZ nuclear translocation in response to stiffness. The aim is to clarify the transfer functions that govern this mechanotransduction pathway [88]. It was found that when YAP/TAZ integrates signals from the cytoskeletons, upstream components responded to stiffness changes while dimensionality changes were sensed downstream [88]. These findings show the dynamic and complex processes when cells sense their mechanical environment [93].
Mechanotransduction pathway involved in biomaterials-induced osteogenic differentiation of MSCs. After integrin and FAs sense the mechanical stimulation, the conformation of FAK changes, exposing phosphorylation sites and activating intracellular osteogenic-related pathways. The activated FAK transmits mechanical signals to the downstream target Rho GTPase, leading to the stimulation of the myosin light chain kinase (MLCK), cooperating with actomyosin and actin filaments to generate the appropriate cytoskeleton tension, which facilitates the entry of YAP into the nucleus by inhibiting LATS1. The canonical Wnt pathway also plays a crucial role. It is activated after Wnt binding and complexing with Lrp5/6 and Fzd, causing Dvl. This destabilizes of the Axin-Apc complex, which facilitates phosphorylation of β-catenin by GSK-3β and Ck1. The unphosphorylated β-catenin then escapes from degradation, allowing it to accumulate in the cytoplasm and translocate to the nucleus. Subsequently, β-catenin initiates the osteogenic gene transcription, acting as a coactivator of with TCF/LEF family. Rho/ROCK is a potential upstream signaling of Wnt/β-catenin sign in stem cells. RhoA and cdc42 inhibit the activity of GSK-3β through phosphorylation, which in turn prevents β-catenin from being degraded. The feedback-regulation is formed between ROCK and Wnt5a. This not only enhances β-catenin transcriptional activity, but also upregulates Wnt signals. Subsequently, Wnt signals in turn improves the activity of ROCK to form a feedback loop. TRP channels are regarded as essential Ca2+ channels. TRPV channels sense mechanical forces and permit Ca2+ influx, resulting in the activation of downstream β-catenin. TRPM7 channels further amplify Ca2+ signaling by triggering ER Ca2+ release. The activated TRPM7 then interacted with PLC, produced IP3 by hydrolyzing PIP2. Subsequently, IP3 activates IP3R2 on the ER conducting Ca2+ release. MAPK signaling is the downstream of other osteogenic pathways. ERK or p38/MAPK signaling can be activated by FAK, Cx43, Rho-GTPases, etc.
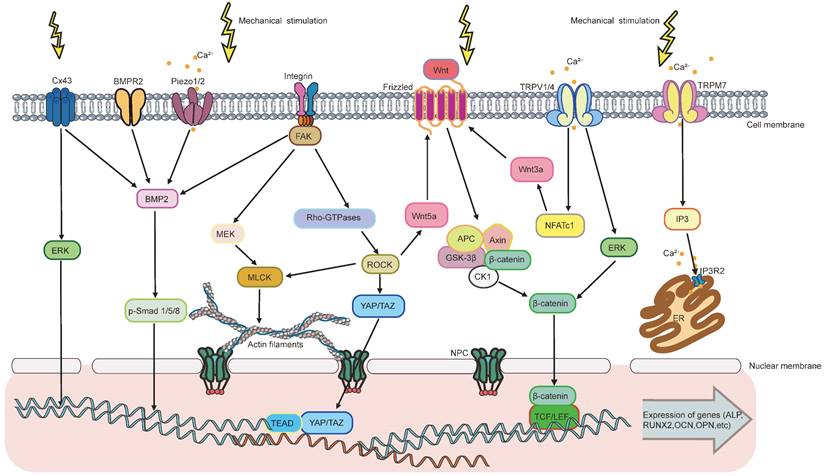
Topography (such as specific micro-/nano-topography [201, 250], increased surface roughness [14], biomimetic multiscale hierarchical structure [132], curvature [37, 251], etc.) drives osteogenesis through YAP/TAZ activation to a large extent as well. It was demonstrated that integrin clustering and FAs formation were closely related to YAP/TAZ signaling. Larger cell adhesion areas facilitated the activation of YAP/TAZ and subsequent bone formation [250].
3.2.2 MAPK signaling
MAPK signaling pathway consists of ERK, p38 and Jun aminoterminal kinases (JNK) [111]. Mechanical cues guide osteogenic differentiation in MSCs mainly through ERK/MAPK signaling [111, 119, 247]. For example, ERK1/2 was upregulated after the activation of FAK when mechanical strain was applied on the TiO2 nanotubes substrate [252]. Shear stress enhanced ERK1/2 phosphorylation, via regulating cell surface channels Cx43 [247]. Acting as the downstream signal of ERK, CREB is also found to play a critical role when MSCs sense TiO2 nanotopography [119]. The active ERK1/2 phosphorylates RUNX2, leading to an increase in RUNX2 binding to cofactors CREB, which upregulates the expression of target osteogenic genes [247]. Moreover, ERK1/2 and AKT phosphorylation signaling axis was found to be associated with PFKP-mediated-glycolysis, which directly regulated osteogenic differentiation [84]. When responding to stiffness cues, PCK2 enhances the rate-limiting metabolic enzyme pallet isoform phosphofructokinase (PFKP), which further activates AKT/ERK1/2 cascades and initiates osteogenesis [84]. p38/MAPK signaling can also regulates MSCs fate and activity [131]. A recent study revealed that p38 signaling, rather than ERK or JNK, was involved in the chirality-sensing of fate commitment [131]. Similar to ERK, p38 interacts with several mechanotransduction-associated signaling pathways as well. Phosphorylated-p38 together with its upstream TRPM7 were both upregulated under shear stress [247]. Moreover, it was further indicated in recent studies that ERK1/2 and p38 could both be regulated by Rac1 [205].
3.2.3 Rho-GTPases signaling
The Rho GTPase family belongs to the small G protein superfamily [253], which is critical for cell shape remodeling and cytoskeleton organization in response to mechanotranduction [204]. Mammalian genomes encode over 20 Rho family members, including RhoA, Rac1, cdc42, etc [253]. RhoA and its effector proteins ROCK are most widely studied for their roles in shifting the differentiation potential from adipogenic to osteogenic lineage [254]. The enhanced activity of RhoA stimulates the commitment of the osteoblast lineage. Conversely, the reduced activity promotes adipogenic commitment [254]. Matrix with high stiffness [85, 255], wavy microstructures [206], fluid flow [148] and nanovibration [156, 256], have been proved to promote osteogenesis of MSCs via the RhoA/ROCK pathway. After sensing mechanical forces, the upregulation of ROCK is observed [148], together with cellular morphological changes due to the increased adhesion-driven cytoskeletal tension [156, 256]. Then, osteogenic markers are significantly upregulated and RUNX2 is localized in the nuclei [148]. However, the inhibition of ROCK contributes to the disruption of cytoskeleton tension, with downregulated expression of mechanomarkers [133, 257].
The Rho GTPase family are considered as essential regulators of cytoskeleton formation, mainly because their critical roles in FAs-induced osteogenic-related pathways [253]. To be more specific, RhoA/ROCK acts as effectors of FAK signaling [253], especially when sensing the roughness and stiffness on matrix [14]. After regulated by GTP exchange factors or GTPase activating proteins (GAPs), RhoA is activated. This leads to the stimulation of MLCK, cooperating with actomyosin and actin filaments to generate the appropriate cytoskeleton tension [258]. Subsequently, activated RhoA transmits mechanical signals to the downstream target YAP [203], facilitating the entry of YAP into the nucleus by inhibiting LATS1 [253]. Moreover, it can also promote the phosphorylation of ERK [156], nuclear accumulation of β-catenin [85, 124], thereby activating RUNX2 to enhance osteogenesis. Except for RhoA, Rac1 and cdc42 also play a crucial role in cell differentiation regulation. Rac1 mediates the mechanosensing-dependent osteogenic differentiation of MSCs through regulating downstream ERK1/2 and p38 in MAPK pathway [205], while cdc42 regulates β-catenin signaling activity by phosphorylating GSK-3β [204]. This suggests that multiple points of crosstalk are likely to exist between Rho GTPase family and other signals.
Although numerous studies have demonstrated that RhoA/ROCK pathway drives osteogenesis, it is not the central driver compared to other signaling pathways [157]. Orapiriyakul W et al. found that reducing intracellular tension via ROCK inhibition lead to only a subtle loss of osteogenesis, which has no significant effect on the overall bone formation [157]. It was shown in another study that topography-induced differentiation did not strictly rely on the activation of RhoA. Cells on smooth surface exhibit increased sensitivity to activated RhoA, while those attach to micro/nanostructured surface relies less on it [259]. This suggests that other signal molecules might play a dominant role in participating in mechanosensitive regulation. Therefore, much more experimental and theorectical work needs to be done to explore the crosstalk between diverse signaling pathways.
3.2.4 Wnt/β-catenin signaling
The Wnt pathway plays a critical role in MSCs osteogenic differentiation initiated by mechanical forces. The canonical Wnt pathway is activated after Wnt binding and complexing with Lrp5/6 and Frizzled (Fzd), causing Dishevelled (Dvl) [249]. This destabilizes of the Axin-Apc complex, which facilitates phosphorylation of β-catenin by GSK-3β and Ck1 [260]. The unphosphorylated β-catenin then escape from degradation, allowing it to accumulate in the cytoplasm and translocate to the nucleus [260]. Subsequently, β-catenin initiates the osteogenic gene transcription, acting as a coactivator of with the transcription factor/lymphoid enhancer-binding factor (TCF/LEF) family [249].
Accumulated experimental studies have demonstrated the involvement of canonical Wnt pathways under mechanical stress. When MSCs sense mechanical stress (such as FSS [230], oscillatory shear stress (OS) [199], low-magnitude and high-frequency (LMHF) vibration [261], etc) or they are seeded on substrates with high stiffness [35, 85, 184, 262, 263] and specific micro/nanotopographies [121, 125, 204, 264, 265], Wnt/β-catenin signaling is initiated and osteogenesis differentiation occurs. Many critical regulators are confirmed to participate in mechanical-stress induced Wnt/β-catenin signaling in MSCs. Piezo1/2 upregulates Wnt/β-catenin and Yap1 activity, by activating Ca2+ influx to induce the formation of NFAT/YAP1/β-catenin complex [230]. TRPV channels induce the activation of protein kinase C (PKC) and ERK [156], or the nuclear translocation of NFATc1 [266], to mediate Wnt/β-catenin activity in MSCs. Rspo1 and its receptor of leucine-rich repeat containing G-protein-coupled receptor 4 (Lgr4) is another novel molecular signal in the upstream of Wnt/β-catenin signaling when transmitting mechanical stimuli to biological signal [267]. Rho/ROCK is also a potential upstream signaling of Wnt/β-catenin sign in stem cells [85]. Stiff matrices upregulate RhoA, followed by the activation of the Wnt/β-catenin and the promotion of osteogenic differentiation [85]. Similarly, cdc42, another member of Rho GTPases family, inhibits the activity of GSK-3β through phosphorylation, which in turn prevents β-catenin from being degraded [204]. After β-catenin accumulating in cytoplasm, FAK, paxilin, vinculin, integrin linked kinase (ILK) in FAs can also interact with β-catenin, triggering intracellular β-catenin signaling and promoting its nuclear translocation especially on the stiff substrate [35, 263, 268]. It is further shown that a feedback-regulation is formed between ROCK and Wnt5a. Specificially, after sensing the surface structure of the materials, ROCK-signaling pathway is activated, which not only enhances β-catenin transcriptional activity, but also upregulates Wnt5a. Subsequently, Wnt5a in turn improves the activity of ROCK to form a feedback loop [124].
Although the canonical Wnt pathways have been confirmed to exert osteogenic inductive effects under mechanical stress, there still remains some controversy. A recent study found that shear stress induced MSCs to sustain self-renewal capability, rather than differentiatie into osteocytes through inhibiting β-catenin/Wnt signaling and enhancing the expression of SOX2 [199]. Similar results were obtained in another study [137]. This discrepancy could be due to the difficulty in simulating shear stress signals in in vivo microenvironment [199]. Therefore, in vivo studies are required to mimic the physiological situation more closely on how mechanical signals alter cell fate choices and cell differentiation of MSCs. In addition, β-catenin was found to act as a negative regulator of osteogenic response. When β-catenin is knocked out, isolated canonical Wnt inhibition increased osteogenic differentiation [262]. This is mainly because β-catenin knockdown increases p-Smad1/5, RUNX2, and BMP-4 expression, especially on stiif matrix. Thus, β-catenin may play diverse roles (osteogenic or anti-osteogenic effects) depending on the cell types, species and microenvironment [262].
Except for the canonical Wnt pathway, non-canonical Wnt pathway is also critical for mechanically-induced differentiation [269]. It functions independently of β-catenin via two major pathways, the PCP (planar cell polarity) and calcium pathways [262]. In the PCP pathway, Fzd activates Rho, Rac, and Cdc42 after associated with Dvl and disheveled-associated activator of morphogenesis (Daam). In the calcium pathway, Ca2+ influx occurs through receptor coupled G proteins and phospholipase C [249]. Arnsdorf et al. investigated the role of non-canonical Wnt pathway in mechanical forces-induced MSCs for the first time. Exposure to OFF led to translocation of β-catenin and upregulation of Wnt5a. Nonetheless, inhibiting Wnt5a had no significant impact on β-catenin translocation, suggesting the upregulated Wnt5a might be involved in mediating non-canonical Wnt pathway, instead of the canonical one [270]. Different from the fluid flow stress, the micro/nano-textured topography down-regulates the ligands of the non-canonical Wnt pathway, including Wnt4, Wnt5a, and Wnt7a [204]. Additional research should explore the underlying mechanisms on how various mechanical stress regulates non-canonical Wnt pathway in the future.
3.2.5 TGFβ superfamily signaling
It has been well documented that TGFβ superfamily signaling is crucial for MSCs osteogenic differentiation. As the cytokines of the TGFβ superfamily, BMPs and TGFβ interact with receptors on membranes, then Smad1/5/8 and Smad2/3 are phosphorylated respectively, which are regarded as BMP signaling and TGFβ signaling [222].
BMPs have been extensively studied in recent years [271], they function through interacting with hetero-tetrameric complexes consisting of two dimers, leading to phosphorylation of Smad1/5/8, and the activation of downstream osteogenic-related signaling [271]. Nanostructured surfaces have been recently proved to favor cell adhesion and osteogenic differentiation of MSCs, mainly attributed to the activation of BMP/Smad signaling pathway [272]. This is because different from other adsorbed proteins that may be affected by the nano-topography, the amount and conformation of BMP-2 remains stable, which results in the excellent osteoinductivity [273]. Furthermore, BMP-2 signaling is demonstrated to affect gap junction-mediated intercellular communication in response to micro- or nano-structure of biomaterials [127]. To be more specific, osteogenic differentiation induced by surface topography activates intercellular communication, which regulates BMP-2 signaling. Meanwhile, BMP-2 can in turn modulate Cx43-related communication, further driving osteogenesis [127].
BMP signaling also functions in mechano-regulation and stem cell differentiation mediated by stiffness, although Rho GTPase and Wnt signaling may be the ones to play a dominant role [184]. Interestingly, when Rho GTPase or F-Actin polymerization is inhibited, a compensatory overexpression in p-Smad1/5 and BMP-2 is observed, instead of the active β-catenin [184]. It is further confirmed that BMP-2 might function via a PINCH-1-SMAD specific E3 ubiquitin protein ligase 1 (Smurf1) signaling axis. Generally, Smurf1 binds BMPR2 and controls its degradation in stem cells in response to mechanical signals. After sensing the stiffness in ECM, PINCH-1 is activated, binding directly to the Smurf1 C2 domain where BMPR2 binds. This leads to the inhibition of Smurf1-BMPR2 interaction as well as the degradation of BMPR2, resulting in the consequently augmented BMP signaling and osteogenic differentiation [271].
Different from BMP signaling, TGFβ signaling are poorly investigated. Although it was revealed that TGFβ signaling could be modulated by substrate stiffness and cytoskeletal tension, the underlying mechanisms remain elusive. A recent study showed that surface topography could initiate TGFβ signaling by regulating primary cilia length and TGFβ receptor localization in the cilium [222]. However, more exploration is still required on associated pathways in the future.
3.2.6 Notch signaling
Notch signaling has been recently explored as a mechano-transduction pathway in MSCs that regulates cell fate determination and differentiation [274]. Among the four Notch receptors (Notch1-4) and five ligands (Dll1, Dll3, Dll4, Jag1, and Jag2), Notch receptor (Notch1, Notch2) and Notch ligand (Dll4, Jag1) serve a dominant function in osteogenic differentiation, especially when MSCs are exposed to low fluid shear stress [275], specific nanostructures [276], cyclic stretching [274], etc. For instance, it was found that low FSS upregulated Dll4 mRNA expression of MSCs, indicating the involvement of Notch signaling in mechanoregulated osteogenic differentiation [275]. Notch1, Notch2 genes and their ligand Jag1 was commonly increased through mechanical strain, accompanied by the up-regulated mRNA expression of HES1, HEY1, HEY2, and HEYL, which are the crucial Notch pathway genes [274]. Moreover, the active NOTCH signaling has been recently demonstrated to link with the organization of the actin cytoskeleton, but the precise signal still remains elusive [274]. Further studies will be required to elucidate the mechanisms underlying the mechanosensitive role of Notch signaling in MSC osteogenic differentiation.
4. Perspectives on current understanding
Both physicomechanical properties of biomaterials and mechanical stimulation from external environment play an important role in bone regeneration. MSCs sense specific mechanical signals via mechanosensors on the cell membrane, thereby activating downstream osteogenic-related pathways [3].
Despite intensive research efforts, biomaterials loaded with MSCs have not yet been used in the clinical treatment of bone defects, via mechanical transduction. This is mainly attributed to the degradability of biomaterials after implantation. The physicomechanical properties vary dynamically during degradation [5]. Nonetheless, when exploring the optimal mechanical properties such as stiffness and viscoelasticity for osteogenesis in vitro, the degradability of biomaterials and its effects are often ignored. Whether the mechanical properties of materials remain stable during degradation has long been of interest, but less attention has been placed on the effect of mechanical properties on osteogenesis. Another reason that limits mechanical forces to guide the fate of MSCs in clinical practice is that most in vitro studies have been conducted using 2D substrates. On the contrary, the 3D environment in vivo brings different results [277]. It is well known that 3D culture is necessary for constructing tissue engineered bone. Compared with 2D culture systems, 3D biomaterials exhibit different cell morphology, cytoskeletal dynamics, and fate determination [5]. Therefore, the mechanism of MSCs response to mechanical signals in 3D environment may be the focus of future research.
As traditional static culture cannot provide adequate nutrition and oxygen supply to cells located in the center of the scaffold, the probability of osteolysis is greatly increased [2]. Therefore, dynamic culture method becomes the key to solve this issue [140]. However, dynamic cell culture through mechanical stimulation remains elusive for subjects in clinical trials. Although mechanical therapies have been moved into the clinic, including low-level vibrations, dynamic hydraulic stimulation (DHS) and extracorporeal shockwave therapy (ESWT), however, the efficiency of these mechanical therapies in bone repair remains controversial [278]. Therefore, future researches may focus on the development of more efficient mechanical therapies, as well as the combined treatment of mechanical properties of biomaterials and external mechanical stimulation on MSCs.
Although numerous studies have been conducted on promoting osteogenic differentiation of MSCs under mechanical stimulation, the interaction between MSCs and other cell types remain poorly understood, including vascular endothelial cells, osteocytes, osteoblasts, etc [95]. In contrast, further researches on macrophages are underway. It was previously reported that mechanical force exerted by orthodontic process induced the targeted activation of Smad1 by macrophages-derived ubiquitin carboxyl-terminal hydrolase isozyme L3 (an exosome) to promote osteogenesis of MSCs [279]. Moreover, the polarization of macrophages is of more concern recently. Generally, macrophages are divided into pro-inflammatory M1 phenotypes and anti-inflammatory M2 phenotypes [95], which function at different stages of bone defect healing cascades and regeneration. Several biomaterial characteristics have been shown to guide macrophage polarization, including stiffness [95], topological structure [280, 281] and cytokines [282, 283]. He et al. demonstrated that macrophages cultured under both 2D and 3D conditions exhibited M2 polarization at low stiffness and M1 polarization at high stiffness by using gelatin materials with adjustable stiffness [95]. It was further demonstrated that it was the altered lipid metabolism that led to the 12-lipoxygenase mediated change in macrophage phenotype [94]. However, different from the results of culturing MSCs alone [85], when macrophages were also co-cultured on a high-stiffness matrix, the pro-inflammatory phenotype of M1 macrophages impaired the osteogenesis process. Interestingly, the most recent developed materials show totally different results. The decellularized placental sponge with native biological structure can promote M2 macrophages polarization as well as the osteogenesis crosstalk between two cells [284]. Another similar material, decellularized cartilage matrix with appropriate IL-4 delivery, is also a good immunomodulatory strategy, which may be related to the regulation of macrophage polarization directed by collagen type Ⅵ [285-287].
Surface topography of biomaterials has also been shown to be one of the key factors affecting macrophage polarization and osseointegration efficiency [280, 288, 289]. Altering the roughness, surface modification, etc, can drive cellular migration and polarization, thus down-regulating the initiation of pro-inflammatory cascades [289]. Compared with the traditional Ti coating, the micropatterned Ti surface promotes the M2 polarization [280]. Furthermore, Wang et al. discovered that the alteration in the diameter of micropatterned nanotubes also affected the polarization direction: macrophages on small diameter(30nm) nanotubes were more inclined to M2 polarization, and those on large diameter(100nm) nanotubes had the opposite results [281]. Similar conclusion was obtained by using Ti nanotubes with diameters of 80-100nm [290]. And by blocking the secretion of MSCs exosomes, it was concluded that M1 polarization was regulated by the paracrine pathway of MSCs. Moreover, the nanofiber membrane can also serve as a promoting surface design. The membrane with lattice topology prepared by electrospinning method is proved to recruit macrophages [291], and the layered scaffold with nano-morphology fiber membrane combined with mineralized particles can induce M2 polarization [292].
5. Conclusion
MSCs-directed osteogenic differentiation by physicomechanical stimulation has become a growing area of research in recent years. By altering the stiffness, viscoelasticity, and topography, or exerting external loading, mechanical signals can influence the development of MSCs via distinct pathways, thereby controlling their fate more precisely. However, additional studies are needed to fully elucidate more detailed mechanotransduction mechanisms, including how mechanoreceptors and ion channels respond to mechanical stimuli, and downstream pathways that regulate osteogenic-related transcription factors. In addition, the development of novel biomaterials that provide more stable and independently regulated properties are still required for better guidance in BTE. In conclusion, it can be predicted that mechanically stimulated MSCs-laden materials will be an ideal source for BTE. This emerging research area warrants further study, which may offer substantial clinical benefits in the near future.
Abbreviations
AC6: adenylyl cyclase 6; BTE: Bone tissue engineering; CDM: cell-derived matrices; cdc42: control protein 42 homolog; CHP: cyclic hydrostatic pressure; Cyto D: cytochalasin D; PLC: cytophospholipase C; Daam: disheveled-associated activator of morphogenesis; Dvl: Dishevelled; DHS: dynamic hydraulic stimulation; ERK: extracellular-signal-regulated kinase; ESWT: extracorporeal shockwave therapy; FSS: fluid shear stress; FAs: focal adhesions; Fzd: Frizzled; GPCR: G protein coupled receptors; GJs: gap junctions; GAPs: GTPase activating proteins; HDACs: histone deacetylases; hASCs: human adipose-derived stem cells; hUCS: human umbilical cord serum; HP: hydrostatic pressure; HAp: hydroxyapatite; IP3R2: inositol trisphosphate receptor type 2; IACs: integrin adhesion complexes; ILK: integrin linked kinase; IFs: intermediate filaments; IHP: intermittent hydrostatic pressure; JNK: Jun aminoterminal kinases; LATS: large tumor suppressor kinase; Lgr4: leucine-rich repeat containing G-protein-coupled receptor 4; LINC: Linker of Nucleoskeleton and Cytoskeleton; LMHF: low-magnitude and high-frequency; MSCs: mesenchymal stem cells; MIF: migration inhibitory factor; MNNTs: multiwall carbon nanotubes; nHA: nano-hydroxyapatite; NFATc1: nuclear factor of activated T cells c1; OFF: oscillatory fluid flow; OS: oscillatory shear stress; Osx: osterix; PCP: planar cell polarity; PIP2: phosphatidylinositol 4,5-bisphosphate; PFKP: phosphofructokinase; PDTEC: poly(desaminotyrosyl-tyrosine carbonate); PA: polyacrylamide; PDMS: polydimethylsilane; PEG: polyethylene glycol; PEG/SF/HAp: polyethylene glycol/silk fibroin/hydroxyapatite; PI: polyimide; PLLA: poly-l-lactic acid; PS: polystyrene; PKC: protein kinase C; Sd: Scalloped; Ta: Tantalum; TEAD: TEA domain family member; 3D: three-dimensional; TCF/LEF: transcription factor/lymphoid enhancer-binding factor; TRP: transient receptor potential; YAP: yes associated proteins.
Acknowledgements
This study was financially supported by the Medical Engineering Intersection Joint Funds of the Natural Science Foundation of Liaoning Province of China (No. 2022-YGJC-01) and Key Research and Development Projects of Liaoning Province (2020020184- JH2/103).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dejob L, Toury B, Tadier S, Grémillard L, Gaillard C, Salles V. Electrospinning of in situ synthesized silica-based and calcium phosphate bioceramics for applications in bone tissue engineering: A review. Acta Biomater. 2021;123:123-53
2. Yong KW, Choi JR, Choi JY, Cowie AC. Recent Advances in Mechanically Loaded Human Mesenchymal Stem Cells for Bone Tissue Engineering. Int J Mol Sci. 2020;21(16):5816
3. Sun Y, Wan B, Wang R, Zhang B, Luo P, Wang D. et al. Mechanical Stimulation on Mesenchymal Stem Cells and Surrounding Microenvironments in Bone Regeneration: Regulations and Applications. Front Cell Dev Biol. 2022;10:808303
4. Haugh MG, Vaughan TJ, Madl CM, Raftery RM, McNamara LM, O'Brien FJ. et al. Investigating the interplay between substrate stiffness and ligand chemistry in directing mesenchymal stem cell differentiation within 3D macro-porous substrates. Biomaterials. 2018;171:23-33
5. Guo Y, Du S, Quan S, Jiang F, Yang C, Li J. Effects of biophysical cues of 3D hydrogels on mesenchymal stem cells differentiation. J Cell Physiol. 2021;236:2268-75
6. Huang J, Chen Y, Tang C, Fei Y, Wu H, Ruan D. et al. The relationship between substrate topography and stem cell differentiation in the musculoskeletal system. Cell Mol Life Sci. 2019;76:505-21
7. Kamguyan K, Katbab AA, Mahmoudi M, Thormann E, Zajforoushan Moghaddam S, Moradi L. et al. An engineered cell-imprinted substrate directs osteogenic differentiation in stem cells. Biomater Sci. 2017;6:189-99
8. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677-89
9. Abagnale G, Steger M, Nguyen VH, Hersch N, Sechi A, Joussen S. et al. Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials. 2015;61:316-26
10. Liu Y, Yang G, Ji H, Xiang T, Luo E, Zhou S. Synergetic effect of topological cue and periodic mechanical tension-stress on osteogenic differentiation of rat bone mesenchymal stem cells. Colloids Surf B Biointerfaces. 2017;154:1-9
11. Rosales AM, Anseth KS. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat Rev Mater. 2016;1:15012
12. Raeber GP, Lutolf MP, Hubbell JA. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J. 2005;89:1374-88
13. El-Rashidy AA, El Moshy S, Radwan IA, Rady D, Abbass MMS, Dörfer CE. et al. Effect of Polymeric Matrix Stiffness on Osteogenic Differentiation of Mesenchymal Stem/Progenitor Cells: Concise Review. Polymers (Basel). 2021;13(17):2950
14. Hou Y, Yu L, Xie W, Camacho LC, Zhang M, Chu Z. et al. Surface Roughness and Substrate Stiffness Synergize to Drive Cellular Mechanoresponse. Nano Letters. 2020;20:748-57
15. Tang S, Ma H, Tu H-C, Wang H-R, Lin P-C, Anseth KS. Adaptable Fast Relaxing Boronate-Based Hydrogels for Probing Cell-Matrix Interactions. Adv Sci. 2018;5:1800638
16. Chen J, Zhu Z, Chen J, Luo Y, Li L, Liu K. et al. Photocurable liquid crystal hydrogels with different chargeability and tunable viscoelasticity based on chitin whiskers. Carbohydr Polym. 2023;301:120299
17. Ayala-Ham A, López-Gutierrez J, Bermúdez M, Aguilar-Medina M, Sarmiento-Sánchez JI, López-Camarillo C. et al. Hydrogel-Based Scaffolds in Oral Tissue Engineering. Front Mater. 2021;8:708945
18. Xu J, Sun M, Tan Y, Wang H, Wang H, Li P. et al. Effect of matrix stiffness on the proliferation and differentiation of umbilical cord mesenchymal stem cells. Differentiation. 2017;96:30-9
19. Kozaniti FK, Deligianni DD, Georgiou MD, Portan DV. The Role of Substrate Topography and Stiffness on MSC Cells Functions: Key Material Properties for Biomimetic Bone Tissue Engineering. Biomimetics. 2022;7:7
20. Luo C, Lü D, Zheng L, Zhang F, Zhang X, Lü S. et al. Hepatic differentiation of human embryonic stem cells by coupling substrate stiffness and microtopography. Biomater Sci. 2021;9:3776-90
21. Hadden WJ, Young JL, Holle AW, McFetridge ML, Kim DY, Wijesinghe P. et al. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc Natl Acad Sci. 2017;114:5647-52
22. Bao M, Xie J, Katoele N, Hu X, Wang B, Piruska A. et al. Cellular Volume and Matrix Stiffness Direct Stem Cell Behavior in a 3D Microniche. ACS Appl Mater Interfaces. 2019;11:1754-9
23. Conrad B, Hayashi C, Yang F. Gelatin-Based Microribbon Hydrogels Support Robust MSC Osteogenesis across a Broad Range of Stiffness. ACS Biomater Sci Eng. 2020;6:3454-63
24. Wang L, Zhao W, Zhao Y, Li W, Wang G, Zhang Q. Enzymatically-mineralized double-network hydrogels with ultrahigh mechanical strength, toughness, and stiffness. Theranostics. 2023;13:673-84
25. Ji X, Yuan X, Ma L, Bi B, Zhu H, Lei Z. et al. Mesenchymal stem cell-loaded thermosensitive hydroxypropyl chitin hydrogel combined with a three-dimensional-printed poly(ε-caprolactone) /nano-hydroxyapatite scaffold to repair bone defects via osteogenesis, angiogenesis and immunomodulation. Theranostics. 2020;10:725-40
26. Zhang J, Wehrle E, Adamek P, Paul GR, Qin X-H, Rubert M. et al. Optimization of mechanical stiffness and cell density of 3D bioprinted cell-laden scaffolds improves extracellular matrix mineralization and cellular organization for bone tissue engineering. Acta Biomater. 2020;114:307-22
27. Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15:326-34
28. Nam S, Stowers R, Lou J, Xia Y, Chaudhuri O. Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials. 2019;200:15-24
29. Zhang J, Yang H, Abali BE, Li M, Xia Y, Haag R. Dynamic Mechanics-Modulated Hydrogels to Regulate the Differentiation of Stem-Cell Spheroids in Soft Microniches and Modeling of the Nonlinear Behavior. Small. 2019;15:e1901920
30. Whitehead J, Griffin KH, Gionet-Gonzales M, Vorwald CE, Cinque SE, Leach JK. Hydrogel mechanics are a key driver of bone formation by mesenchymal stromal cell spheroids. Biomaterials. 2021;269:120607
31. Teodorescu M, Morariu S, Bercea M, Săcărescu L. Viscoelastic and structural properties of poly(vinyl alcohol)/poly(vinylpyrrolidone) hydrogels. RSC Adv. 2016;6:39718-27
32. Kaewprasit K, Kobayashi T, Damrongsakkul S. Thai silk fibroin gelation process enhancing by monohydric and polyhydric alcohols. Int J of Biol Macromol. 2018;118:1726-35
33. Mihajlovic M, Wyss HM, Sijbesma RP. Effects of Surfactant and Urea on Dynamics and Viscoelastic Properties of Hydrophobically Assembled Supramolecular Hydrogel. Macromolecules. 2018;51:4813-20
34. Jahanmard F, Baghban Eslaminejad M, Amani-Tehran M, Zarei F, Rezaei N, Croes M. et al. Incorporation of F-MWCNTs into electrospun nanofibers regulates osteogenesis through stiffness and nanotopography. Mater Sci and Eng C. 2020;106:110163
35. Zhou C, Zhang D, Zou J, Li X, Zou S, Xie J. Substrate Compliance Directs the Osteogenic Lineages of Stem Cells from the Human Apical Papilla via the Processes of Mechanosensing and Mechanotransduction. ACS Appl Mater Interfaces. 2019;11:26448-59
36. Wang P-Y, Tsai W-B, Voelcker NH. Screening of rat mesenchymal stem cell behaviour on polydimethylsiloxane stiffness gradients. Acta Biomater. 2012;8:519-30
37. Yang L, Gao Q, Ge L, Zhou Q, Warszawik EM, Bron R. et al. Topography induced stiffness alteration of stem cells influences osteogenic differentiation. Biomater Sci. 2020;8:2638-52
38. Das A, Adhikary S, Chowdhury AR, Barui A. Leveraging Substrate Stiffness to Promote Stem Cell Asymmetric Division via Mechanotransduction-Polarity Protein Axis and Its Bayesian Regression Analysis. Rejuvenation Res. 2022;25:59-69
39. Huang B, Vyas C, Byun JJ, El-Newehy M, Huang Z, Bártolo P. Aligned multi-walled carbon nanotubes with nanohydroxyapatite in a 3D printed polycaprolactone scaffold stimulates osteogenic differentiation. Mater Sci Eng C. 2020;108:110374
40. Chen J-C, Kung J-C, Hsieh C-H, Hou M-J, Shih C-J, Hung C-C. Mineralization and Osteoblast Cells Response of Nanograde Pearl Powders. J Nanomater. 2013;2013:752863
41. Liu Y, Huang Q, Feng Q. 3D scaffold of PLLA/pearl and PLLA/nacre powder for bone regeneration. Biomed Mater. 2013;8:065001
42. Vega SL, Arvind V, Mishra P, Kohn J, Sanjeeva Murthy N, Moghe PV. Substrate micropatterns produced by polymer demixing regulate focal adhesions, actin anisotropy, and lineage differentiation of stem cells. Acta Biomater. 2018;76:21-8
43. Wu L, Virdee J, Maughan E, Darbyshire A, Jell G, Loizidou M. et al. Stiffness memory nanohybrid scaffolds generated by indirect 3D printing for biologically responsive soft implants. Acta Biomater. 2018;80:188-202
44. Wu L, Magaz A, Wang T, Liu C, Darbyshire A, Loizidou M. et al. Stiffness memory of indirectly 3D-printed elastomer nanohybrid regulates chondrogenesis and osteogenesis of human mesenchymal stem cells. Biomaterials. 2018;186:64-79
45. Papadogiannis F, Batsali A, Klontzas ME, Karabela M, Georgopoulou A, Mantalaris A. et al. Osteogenic differentiation of bone marrow mesenchymal stem cells on chitosan/gelatin scaffolds: gene expression profile and mechanical analysis. Biomed Mater. 2020;15:064101
46. Wang L, Lu R, Hou J, Nan X, Xia Y, Guo Y. et al. Application of injectable silk fibroin/graphene oxide hydrogel combined with bone marrow mesenchymal stem cells in bone tissue engineering. Colloids Surf A. 2020;604:125318
47. Zhang H, Zhang H, Xiong Y, Dong L, Li X. Development of hierarchical porous bioceramic scaffolds with controlled micro/nano surface topography for accelerating bone regeneration. Mater Sci Eng C. 2021;130:112437
48. Lee SS, Laganenka L, Du X, Hardt W-D, Ferguson SJ. Silicon Nitride, a Bioceramic for Bone Tissue Engineering: A Reinforced Cryogel System With Antibiofilm and Osteogenic Effects. Front Bioeng Biotechnol. 2021;9:794586
49. Uskokovic PS, Tang CY, Tsui CP, Ignjatovic N, Uskokovic DP. Micromechanical properties of a hydroxyapatite/poly-l-lactide biocomposite using nanoindentation and modulus mapping. J Eur Ceram Soc. 2007;27:1559-64
50. Shuai C, Yu L, Yang W, Peng S, Zhong Y, Feng P. Phosphonic Acid Coupling Agent Modification of HAP Nanoparticles: Interfacial Effects in PLLA/HAP Bone Scaffold. Polymers. 2020;12:199
51. Shi F, Xiao D, Zhang C, Zhi W, Liu Y, Weng J. The effect of macropore size of hydroxyapatite scaffold on the osteogenic differentiation of bone mesenchymal stem cells under perfusion culture. Regen Biomater. 2021;8:rbab050
52. Li Y, Wang Y, Li Y, Luo W, Jiang J, Zhao J. et al. Controllable Synthesis of Biomimetic Hydroxyapatite Nanorods with High Osteogenic Bioactivity. ACS Biomater Sci Eng. 2020;6:320-8
53. Liu X, Miao Y, Liang H, Diao J, Hao L, Shi Z. et al. 3D-printed bioactive ceramic scaffolds with biomimetic micro/nano-HAp surfaces mediated cell fate and promoted bone augmentation of the bone-implant interface in vivo. Bioact Mater. 2022;12:120-32
54. Prabha RD, Ding M, Bollen P, Ditzel N, Varma HK, Nair PD. et al. Strontium ion reinforced bioceramic scaffold for load bearing bone regeneration. Mater Sci Eng C. 2020;109:110427
55. Ramaswamy Y, Roohani I, No YJ, Madafiglio G, Chang F, Zhang F. et al. Nature-inspired topographies on hydroxyapatite surfaces regulate stem cells behaviour. Bioact Mater. 2021;6:1107-17
56. Shariati S, Seyedjafari E, Mahdavi FS, Maali A, Ferdosi-Shahandashti E. NiFe2O4/ZnO-coated Poly(L-Lactide) nanofibrous scaffold enhances osteogenic differentiation of human mesenchymal stem cells. Front Bioeng Biotechnol. 2022;10:1005028
57. Jang CH, Kim W, Kim G. Effects of fibrous collagen/CDHA/hUCS biocomposites on bone tissue regeneration. Int J Biol Macromol. 2021;176:479-89
58. Pedrosa CR, Arl D, Grysan P, Khan I, Durrieu S, Krishnamoorthy S. et al. Controlled Nanoscale Topographies for Osteogenic Differentiation of Mesenchymal Stem Cells. ACS Appl Mater Interfaces. 2019;11:8858-66
59. Qiu J, Li J, Wang S, Ma B, Zhang S, Guo W. et al. TiO2 Nanorod Array Constructed Nanotopography for Regulation of Mesenchymal Stem Cells Fate and the Realization of Location-Committed Stem Cell Differentiation. Small. 2016;12:1770-8
60. Park J, Bauer S, Pittrof A, Killian MS, Schmuki P, von der Mark K. Synergistic Control of Mesenchymal Stem Cell Differentiation by Nanoscale Surface Geometry and Immobilized Growth Factors on TiO2 Nanotubes. Small. 2012;8:98-107
61. Li X, Klausen LH, Zhang W, Jahed Z, Tsai C-T, Li TL. et al. Nanoscale Surface Topography Reduces Focal Adhesions and Cell Stiffness by Enhancing Integrin Endocytosis. Nano Lett. 2021;21:8518-26
62. Wang N, Yang S, Shi HX, Song YP, Sun H, Wang Q. et al. Magnesium alloys for orthopedic applications:A review on the mechanisms driving bone healing. J Magnesium Alloys. 2022;10:3327-53
63. Lohberger B, Eck N, Glaenzer D, Lichtenegger H, Ploszczanski L, Leithner A. Cobalt Chromium Molybdenum Surface Modifications Alter the Osteogenic Differentiation Potential of Human Mesenchymal Stem Cells. Mater (Basel). 2020;13(19):4292
64. Wang Y, Yu Z, Li K, Hu J. Effects of surface properties of titanium alloys modified by grinding, sandblasting and acidizing and nanosecond laser on cell proliferation and cytoskeleton. Appl Surf Sci. 2020;501:144279
65. Kieswetter K, Schwartz Z, Hummert TW, Cochran DL, Simpson J, Dean DD. et al. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J Biomed Mater Res. 1996;32:55-63
66. Shi M, Song W, Han T, Chang B, Li G, Jin J. et al. Role of the unfolded protein response in topography-induced osteogenic differentiation in rat bone marrow mesenchymal stem cells. Acta Biomater. 2017;54:175-185
67. An Y, Song Y, Wang Z, Wang J, Wu G, Zhu G. et al. Effect of low-intensity pulsed ultrasound on the biological behaviors of bone marrow mesenchymal stem cells on titanium with different surface topographies. Am J Transl Res. 2018;10:67-76
68. Zhang W, Li Z, Huang Q, Xu L, Li J, Jin Y. et al. Effects of a hybrid micro/nanorod topography-modified titanium implant on adhesion and osteogenic differentiation in rat bone marrow mesenchymal stem cells. Int J Nanomedicine. 2013;8:257-65
69. Li J, Mutreja I, Tredinnick S, Jermy M, Hooper GJ, Woodfield TBF. Hydrodynamic control of titania nanotube formation on Ti-6Al-4V alloys enhances osteogenic differentiation of human mesenchymal stromal cells. Mater Sci Eng C. 2020;109:110562
70. Maher S, Wijenayaka AR, Lima-Marques L, Yang D, Atkins GJ, Losic D. Advancing of Additive-Manufactured Titanium Implants with Bioinspired Micro- to Nanotopographies. ACS Biomater Sci Eng. 2021;7:441-50
71. Xu Z, Huang J, He Y, Su J, Xu L, Zeng X. Fabrication of an ordered micro-/nanotextured titanium surface to improve osseointegration. Colloids Surf B. 2022;214:112446
72. Mukherjee S, Dhara S, Saha P. Enhanced corrosion, tribocorrosion resistance and controllable osteogenic potential of stem cells on micro-rippled Ti6Al4V surfaces produced by pulsed laser remelting. J Manuf Process. 2021;65:119-33
73. Klos A, Sedao X, Itina TE, Helfenstein-Didier C, Donnet C, Peyroche S. et al. Ultrafast Laser Processing of Nanostructured Patterns for the Control of Cell Adhesion and Migration on Titanium Alloy. Nanomaterials (Basel). 2020;10(5):864
74. Janßen S, Gach S, Kant S, Aveic S, Rütten S, Olschok S. et al. Enhanced osteogenic differentiation of human mesenchymal stromal cells as response to periodical microstructured Ti6Al4V surfaces. J Biomed Mater Res B Appl Biomater. 2020;108:2218-26
75. Bloise N, Waldorff EI, Montagna G, Bruni G, Fassina L, Fang S. et al. Early Osteogenic Marker Expression in hMSCs Cultured onto Acid Etching-Derived Micro- and Nanotopography 3D-Printed Titanium Surfaces. Int J Mol Sci. 2022;23(13):7070
76. Huang R, Liu L, Li B, Qin L, Huang L, Yeung KWK. et al. Nanograins on Ti-25Nb-3Mo-2Sn-3Zr alloy facilitate fabricating biological surface through dual-ion implantation to concurrently modulate the osteogenic functions of mesenchymal stem cells and kill bacteria. J Mater Sci Technol. 2021;73:31-44
77. Additively Manufactured Tantalum Implants for Repairing Bone Defects. A Systematic Review. Tissue Eng Part B: Rev. 2021;27:166-80
78. Dou X, Wei X, Liu G, Wang S, Lv Y, Li J. et al. Effect of porous tantalum on promoting the osteogenic differentiation of bone marrow mesenchymal stem cells in vitro through the MAPK/ERK signal pathway. J Orthop Translat. 2019;19:81-93
79. Huang G, Pan S-T, Qiu J-X. The osteogenic effects of porous Tantalum and Titanium alloy scaffolds with different unit cell structure. Colloids Surf B. 2022;210:112229
80. Guo Y, Xie K, Jiang W, Wang L, Li G, Zhao S. et al. In Vitro and in Vivo Study of 3D-Printed Porous Tantalum Scaffolds for Repairing Bone Defects. ACS Biomater Sci Eng. 2019;5:1123-33
81. Zhao D, Liang H, Han C, Li J, Liu J, Zhou K. et al. 3D printing of a titanium-tantalum Gyroid scaffold with superb elastic admissible strain, bioactivity and in-situ bone regeneration capability. Addit Manuf. 2021;47:102223
82. Guo Y, Wu J, Xie K, Tan J, Yang Y, Zhao S. et al. Study of Bone Regeneration and Osteointegration Effect of a Novel Selective Laser-Melted Titanium-Tantalum-Niobium-Zirconium Alloy Scaffold. ACS Biomater Sci Eng. 2019;5:6463-73
83. Yuan H, Zhou Y, Lee MS, Zhang Y, Li WJ. A newly identified mechanism involved in regulation of human mesenchymal stem cells by fibrous substrate stiffness. Acta Biomater. 2016;42:247-57
84. Li Z, Yue M, Liu X, Liu Y, Lv L, Zhang P. et al. The PCK2-glycolysis axis assists three-dimensional-stiffness maintaining stem cell osteogenesis. Bioact Mater. 2022;18:492-506
85. Zhang T, Lin S, Shao X, Shi S, Zhang Q, Xue C. et al. Regulating osteogenesis and adipogenesis in adipose-derived stem cells by controlling underlying substrate stiffness. J Cell Physiol. 2018;233:3418-28
86. Liu Y, Li Z, Li J, Yang S, Zhang Y, Yao B. et al. Stiffness-mediated mesenchymal stem cell fate decision in 3D-bioprinted hydrogels. Burns Trauma. 2020;8:tkaa029
87. Li S, Dong C, Lv Y. Magnetic liquid metal scaffold with dynamically tunable stiffness for bone tissue engineering. Biomater Adv. 2022;139:212975
88. Scott KE, Fraley SI, Rangamani P. A spatial model of YAP/TAZ signaling reveals how stiffness, dimensionality, and shape contribute to emergent outcomes. Proc Natl Acad Sci U S A. 2021 118
89. Yang Y, Feng Y, Qu R, Li Q, Rong D, Fan T. et al. Synthesis of aligned porous polyethylene glycol/silk fibroin/hydroxyapatite scaffolds for osteoinduction in bone tissue engineering. Stem Cell Res Ther. 2020;11:522
90. Khoramgah MS, Ranjbari J, Abbaszadeh HA, Tabatabaei Mirakabad FS, Hatami S, Hosseinzadeh S. et al. Freeze-dried multiscale porous nanofibrous three dimensional scaffolds for bone regenerations. Bioimpacts. 2020;10:73-85
91. Hu Q, Liu M, Chen G, Xu Z, Lv Y. Demineralized Bone Scaffolds with Tunable Matrix Stiffness for Efficient Bone Integration. ACS Appl Mater Interfaces. 2018;10:27669-80
92. Maggi A, Li H, Greer JR. Three-dimensional nano-architected scaffolds with tunable stiffness for efficient bone tissue growth. Acta Biomater. 2017;63:294-305
93. Lee S, Stanton AE, Tong X, Yang F. Hydrogels with enhanced protein conjugation efficiency reveal stiffness-induced YAP localization in stem cells depends on biochemical cues. Biomaterials. 2019;202:26-34
94. Yao D, Qiao F, Song C, Lv Y. Matrix stiffness regulates bone repair by modulating 12-lipoxygenase-mediated early inflammation. Mater Sci Eng C Mater Biol Appl. 2021;128:112359
95. He XT, Wu RX, Xu XY, Wang J, Yin Y, Chen FM. Macrophage involvement affects matrix stiffness-related influences on cell osteogenesis under three-dimensional culture conditions. Acta Biomater. 2018;71:132-47
96. Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316-23
97. Hung BP, Gonzalez-Fernandez T, Lin JB, Campbell T, Lee YB, Panitch A. et al. Multi-peptide presentation and hydrogel mechanics jointly enhance therapeutic duo-potential of entrapped stromal cells. Biomaterials. 2020;245:119973
98. Lee HP, Gu L, Mooney DJ, Levenston ME, Chaudhuri O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat Mater. 2017;16:1243-51
99. Tan Y, Song J. Independent and Synergistic Modulations of Viscoelasticity and Stiffness of Dynamically Cross-Linked Cell-Encapsulating ClickGels by Covalently Tethered Polymer Brushes. Biomacromolecules. 2021;22:3408-15
100. Sommerfeld SD, Elisseeff JH. Time to Relax: Mechanical Stress Release Guides Stem Cell Responses. Cell Stem Cell. 2016;18:166-7
101. Lee K, Chen Y, Yoshitomi T, Kawazoe N, Yang Y, Chen G. Osteogenic and Adipogenic Differentiation of Mesenchymal Stem Cells in Gelatin Solutions of Different Viscosities. Adv Healthc Mater. 2020: e2000617.
102. Shahin-Shamsabadi A, Hashemi A, Tahriri M, Bastami F, Salehi M, Mashhadi Abbas F. Mechanical, material, and biological study of a PCL/bioactive glass bone scaffold: Importance of viscoelasticity. Mater Sci Eng C Mater Biol Appl. 2018;90:280-8
103. Leach JK, Whitehead J. Materials-Directed Differentiation of Mesenchymal Stem Cells for Tissue Engineering and Regeneration. ACS Biomater Sci Eng. 2018;4:1115-27
104. Darnell M, Young S, Gu L, Shah N, Lippens E, Weaver J. et al. Substrate Stress-Relaxation Regulates Scaffold Remodeling and Bone Formation In Vivo. Adv Healthc Mater. 2017;6(1):10.1002
105. Lee HP, Stowers R, Chaudhuri O. Volume expansion and TRPV4 activation regulate stem cell fate in three-dimensional microenvironments. Nat Commun. 2019;10:529
106. Dey K, Agnelli S, Sartore L. Dynamic freedom: substrate stress relaxation stimulates cell responses. Biomater Sci. 2019;7:836-42
107. Lou J, Stowers R, Nam S, Xia Y, Chaudhuri O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials. 2018;154:213-22
108. Niepel MS, Ekambaram BK, Schmelzer CEH, Groth T. Polyelectrolyte multilayers of poly (l-lysine) and hyaluronic acid on nanostructured surfaces affect stem cell response. Nanoscale. 2019;11:2878-91
109. Dennis SC, Whitlow J, Detamore MS, Kieweg SL, Berkland CJ. Hyaluronic-Acid-Hydroxyapatite Colloidal Gels Combined with Micronized Native ECM as Potential Bone Defect Fillers. Langmuir. 2017;33:206-18
110. Chen HH, Lai WY, Chee TJ, Chan YH, Feng SW. Monitoring the Changes of Material Properties at Bone-Implant Interface during the Healing Process In Vivo: A Viscoelastic Investigation. Biomed Res Int. 2017;2017:1945607
111. Zhang X, Li H, Lin C, Ning C, Lin K. Synergetic topography and chemistry cues guiding osteogenic differentiation in bone marrow stromal cells through ERK1/2 and p38 MAPK signaling pathway. Biomater Sci. 2018;6:418-30
112. Harrison RG. On the stereotropism of embryonic cells. Science. 1911;34:279-81
113. Bacakova L, Filova E, Parizek M, Ruml T, Svorcik V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol Adv. 2011;29:739-67
114. Xing F, Yin HM, Zhe M, Xie JC, Duan X, Xu JZ. et al. Nanotopographical 3D-Printed Poly(ε-caprolactone) Scaffolds Enhance Proliferation and Osteogenic Differentiation of Urine-Derived Stem Cells for Bone Regeneration. Pharmaceutics. 2022;14(7):1437
115. Yang W, Han W, He W, Li J, Wang J, Feng H. et al. Surface topography of hydroxyapatite promotes osteogenic differentiation of human bone marrow mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2016;60:45-53
116. Yang L, Ge L, van Rijn P. Synergistic Effect of Cell-Derived Extracellular Matrices and Topography on Osteogenesis of Mesenchymal Stem Cells. ACS Appl Mater Interfaces. 2020;12:25591-603
117. Pilia M, Guda T, Shiels SM, Appleford MR. Influence of substrate curvature on osteoblast orientation and extracellular matrix deposition. J Biol Eng. 2013;7:23
118. Lv L, Liu Y, Zhang P, Zhang X, Liu J, Chen T. et al. The nanoscale geometry of TiO2 nanotubes influences the osteogenic differentiation of human adipose-derived stem cells by modulating H3K4 trimethylation. Biomaterials. 2015;39:193-205
119. Ro HS, Park HJ, Seo YK. Fluorine-incorporated TiO(2) nanotopography enhances adhesion and differentiation through ERK/CREB pathway. J Biomed Mater Res A. 2021;109:1406-17
120. Brammer KS, Oh S, Cobb CJ, Bjursten LM, van der Heyde H, Jin S. Improved bone-forming functionality on diameter-controlled TiO(2) nanotube surface. Acta Biomater. 2009;5:3215-23
121. Zhou J, Zhao L, Li B, Han Y. Nanorod diameter modulated osteogenic activity of hierarchical micropore/nanorod-patterned coatings via a Wnt/β-catenin pathway. Nanomedicine. 2018;14:1719-31
122. Rahman SU, Ponnusamy S, Nagrath M, Arany PR. Precision-engineered niche for directed differentiation of MSCs to lineage-restricted mineralized tissues. J Tissue Eng. 2022;13:20417314211073934
123. Jiang N, Guo Z, Sun D, Li Y, Yang Y, Chen C. et al. Promoting Osseointegration of Ti Implants through Micro/Nanoscaled Hierarchical Ti Phosphate/Ti Oxide Hybrid Coating. ACS Nano. 2018;12:7883-91
124. Yu Y, Shen X, Liu J, Hu Y, Ran Q, Mu C. et al. Regulation of osteogenesis by micro/nano hierarchical titanium surfaces through a Rock-Wnt5a feedback loop. Colloids Surf B. 2018;170:1-10
125. Fu J, Liu X, Tan L, Cui Z, Liang Y, Li Z. et al. Modulation of the mechanosensing of mesenchymal stem cells by laser-induced patterning for the acceleration of tissue reconstruction through the Wnt/β-catenin signaling pathway activation. Acta Biomater. 2020;101:152-67
126. Liu W, Sun Q, Zheng ZL, Gao YT, Zhu GY, Wei Q. et al. Topographic Cues Guiding Cell Polarization via Distinct Cellular Mechanosensing Pathways. Small. 2022;18:e2104328
127. Zhao C, Wang X, Gao L, Jing L, Zhou Q, Chang J. The role of the micro-pattern and nano-topography of hydroxyapatite bioceramics on stimulating osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018;73:509-21
128. Alakpa EV, Burgess KEV, Chung P, Riehle MO, Gadegaard N, Dalby MJ. et al. Nacre Topography Produces Higher Crystallinity in Bone than Chemically Induced Osteogenesis. ACS Nano. 2017;11:6717-27
129. Waddell SJ, de Andrés MC, Tsimbouri PM, Alakpa EV, Cusack M, Dalby MJ. et al. Biomimetic oyster shell-replicated topography alters the behaviour of human skeletal stem cells. J Tissue Eng. 2018;9:2041731418794007
130. Li Y, Dai X, Bai Y, Liu Y, Wang Y, Liu O. et al. Electroactive BaTiO(3) nanoparticle-functionalized fibrous scaffolds enhance osteogenic differentiation of mesenchymal stem cells. Int J Nanomedicine. 2017;12:4007-18
131. Dong L, Gong J, Wang Y, He J, You D, Zhou Y. et al. Chiral geometry regulates stem cell fate and activity. Biomaterials. 2019;222:119456
132. Yang LL, Ge L, Zhou QH, Mokabber T, Pei YT, Bron R. et al. Biomimetic Multiscale Hierarchical Topography Enhances Osteogenic Differentiation of Human Mesenchymal Stem Cells. Adv Mater Interfaces. 2020;7:2000385
133. Major LG, Holle AW, Young JL, Hepburn MS, Jeong K, Chin IL. et al. Volume Adaptation Controls Stem Cell Mechanotransduction. ACS Appl Mater Interfaces. 2019;11:45520-30
134. Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015-24
135. Hsieh WT, Liu YS, Lee YH, Rimando MG, Lin KH, Lee OK. Matrix dimensionality and stiffness cooperatively regulate osteogenesis of mesenchymal stromal cells. Acta Biomater. 2016;32:210-22
136. Sayin E, Baran ET, Elsheikh A, Mudera V, Cheema U, Hasirci V. Evaluating Oxygen Tensions Related to Bone Marrow and Matrix for MSC Differentiation in 2D and 3D Biomimetic Lamellar Scaffolds. Int J Mol Sci. 2021 22
137. Nath SC, Day B, Harper L, Yee J, Hsu CY, Larijani L. et al. Fluid shear stress promotes embryonic stem cell pluripotency via interplay between β-catenin and vinculin in bioreactor culture. Stem Cells. 2021;39:1166-77
138. Campsie P, Childs PG, Robertson SN, Cameron K, Hough J, Salmeron-Sanchez M. et al. Design, construction and characterisation of a novel nanovibrational bioreactor and cultureware for osteogenesis. Sci Rep. 2019;9:12944
139. Horner CB, Hirota K, Liu J, Maldonado M, Hyle Park B, Nam J. Magnitude-dependent and inversely-related osteogenic/chondrogenic differentiation of human mesenchymal stem cells under dynamic compressive strain. J Tissue Eng Regen Med. 2018;12:e637-e47
140. Seo J, Shin JY, Leijten J, Jeon O, Bal Öztürk A, Rouwkema J. et al. Interconnectable Dynamic Compression Bioreactors for Combinatorial Screening of Cell Mechanobiology in Three Dimensions. ACS Appl Mater Interfaces. 2018;10:13293-303
141. Montorsi M, Genchi GG, De Pasquale D, De Simoni G, Sinibaldi E, Ciofani G. Design, fabrication, and characterization of a multimodal reconfigurable bioreactor for bone tissue engineering. Biotechnol Bioeng. 2022;119:1965-79
142. Schreivogel S, Kuchibhotla V, Knaus P, Duda GN, Petersen A. Load-induced osteogenic differentiation of mesenchymal stromal cells is caused by mechano-regulated autocrine signaling. J Tissue Eng Regen Med. 2019;13:1992-2008
143. Yamada M, Anada T, Masuda T, Takano-Yamamoto T, Suzuki O. Effect of mechanical stress on differentiation of mouse mesenchymal stem cells seeded into an octacalcium phosphate-gelatin scaffold. Sens Actuators B Chem. 2015;220:125-30
144. Carroll SF, Buckley CT, Kelly DJ. Cyclic Tensile Strain Can Play a Role in Directing both Intramembranous and Endochondral Ossification of Mesenchymal Stem Cells. Front Bioeng Biotechnol. 2017;5:73
145. Mitra D, Whitehead J, Yasui OW, Leach JK. Bioreactor culture duration of engineered constructs influences bone formation by mesenchymal stem cells. Biomaterials. 2017;146:29-39
146. Mainardi VL, Rubert M, Sabato C, de Leeuw A, Arrigoni C, Dubini G. et al. Culture of 3D bioprinted bone constructs requires an increased fluid dynamic stimulation. Acta Biomater. 2022;153:374-85
147. Chen G, Xu R, Zhang C, Lv Y. Responses of MSCs to 3D Scaffold Matrix Mechanical Properties under Oscillatory Perfusion Culture. ACS Appl Mater Interfaces. 2017;9:1207-18
148. Yamada S, Yassin MA, Schwarz T, Hansmann J, Mustafa K. Induction of osteogenic differentiation of bone marrow stromal cells on 3D polyester-based scaffolds solely by subphysiological fluidic stimulation in a laminar flow bioreactor. J Tissue Eng. 2021;12:20417314211019375
149. Suzuki K, Fukasawa J, Miura M, Lim PN, Honda M, Matsuura T. et al. Influence of Culture Period on Osteoblast Differentiation of Tissue-Engineered Bone Constructed by Apatite-Fiber Scaffolds Using Radial-Flow Bioreactor. Int J Mol Sci. 2021;22(23):13080
150. Ramírez-Rodríguez GB, Pereira AR, Herrmann M, Hansmann J, Delgado-López JM, Sprio S. et al. Biomimetic Mineralization Promotes Viability and Differentiation of Human Mesenchymal Stem Cells in a Perfusion Bioreactor. Int J Mol Sci. 2021;22(3):1447
151. Mondragón E, Cowdin M, Taraballi F, Minardi S, Tasciotti E, Gregory CA. et al. Mimicking the Organic and Inorganic Composition of Anabolic Bone Enhances Human Mesenchymal Stem Cell Osteoinduction and Scaffold Mechanical Properties. Front Bioeng Biotechnol. 2020;8:753
152. Gandhi JK, Kao SW, Roux BM, Rodriguez RA, Tang SJ, Fisher JP. et al. Perfusion Bioreactor Culture of Bone Marrow Stromal Cells Enhances Cranial Defect Regeneration. Plast Reconstr Surg. 2019;143:993e-1002e
153. Rogina A, Antunović M, Pribolšan L, Caput Mihalić K, Vukasović A, Ivković A. et al. Human Mesenchymal Stem Cells Differentiation Regulated by Hydroxyapatite Content within Chitosan-Based Scaffolds under Perfusion Conditions. Polymers (Basel). 2017;9(9):387
154. Yaghoobi M, Hashemi-Najafabadi S, Soleimani M, Vasheghani-Farahani E. Osteogenic induction of human mesenchymal stem cells in multilayered electrospun scaffolds at different flow rates and configurations in a perfusion bioreactor. J Biosci Bioeng. 2019;128:495-503
155. Ravichandran A, Wen F, Lim J, Chong MSK, Chan JKY, Teoh SH. Biomimetic fetal rotation bioreactor for engineering bone tissues-Effect of cyclic strains on upregulation of osteogenic gene expression. J Tissue Eng Regen Med. 2018;12:e2039-e50
156. Tsimbouri PM, Childs PG, Pemberton GD, Yang J, Jayawarna V, Orapiriyakul W. et al. Stimulation of 3D osteogenesis by mesenchymal stem cells using a nanovibrational bioreactor. Nat Biomed Eng. 2017;1:758-70
157. Orapiriyakul W, Tsimbouri MP, Childs P, Campsie P, Wells J, Fernandez-Yague MA. et al. Nanovibrational Stimulation of Mesenchymal Stem Cells Induces Therapeutic Reactive Oxygen Species and Inflammation for Three-Dimensional Bone Tissue Engineering. ACS Nano. 2020;14:10027-44
158. Hazenbiller O, Nasr S, Krawetz RJ, Duncan NA. Effect of mechanical strain on the pluripotency of murine embryonic stem cells seeded in a collagen-I scaffold. J Orthop Res. 2018;36:799-807
159. Prouvé E, Drouin B, Chevallier P, Rémy M, Durrieu MC, Laroche G. Evaluating Poly(Acrylamide-co-Acrylic Acid) Hydrogels Stress Relaxation to Direct the Osteogenic Differentiation of Mesenchymal Stem Cells. Macromol Biosci. 2021;21:e2100069
160. Baumgartner W, Schneider I, Hess SC, Stark WJ, Märsmann S, Brunelli M. et al. Cyclic uniaxial compression of human stem cells seeded on a bone biomimetic nanocomposite decreases anti-osteogenic commitment evoked by shear stress. J Mech Behav Biomed Mater. 2018;83:84-93
161. Gamez C, Schneider-Wald B, Bieback K, Schuette A, Büttner S, Hafner M. et al. Compression Bioreactor-Based Mechanical Loading Induces Mobilization of Human Bone Marrow-Derived Mesenchymal Stromal Cells into Collagen Scaffolds In Vitro. Int J Mol Sci. 2020;21(21):8249
162. Dahl MT, Morrison S. Segmental Bone Defects and the History of Bone Transport. J Orthop Trauma. 2021;35:S1-s7
163. Qi MC, Zou SJ, Han LC, Zhou HX, Hu J. Expression of bone-related genes in bone marrow MSCs after cyclic mechanical strain: implications for distraction osteogenesis. Int J Oral Sci. 2009;1:143-50
164. Wu J, Zhao J, Sun L, Pan Y, Wang H, Zhang WB. Long non-coding RNA H19 mediates mechanical tension-induced osteogenesis of bone marrow mesenchymal stem cells via FAK by sponging miR-138. Bone. 2018;108:62-70
165. Birru B, Mekala NK, Parcha SR. Improved osteogenic differentiation of umbilical cord blood MSCs using custom made perfusion bioreactor. Biomed J. 2018;41:290-7
166. Zhao LG, Chen SL, Teng YJ, An LP, Wang J, Ma JL. et al. The MEK5/ERK5 pathway mediates fluid shear stress promoted osteoblast differentiation. Connect Tissue Res. 2014;55:96-102
167. Grayson WL, Marolt D, Bhumiratana S, Fröhlich M, Guo XE, Vunjak-Novakovic G. Optimizing the medium perfusion rate in bone tissue engineering bioreactors. Biotechnol Bioeng. 2011;108:1159-70
168. Mitra D, Yasui OW, Harvestine JN, Link JM, Hu JC, Athanasiou KA. et al. Exogenous Lysyl Oxidase-Like 2 and Perfusion Culture Induce Collagen Crosslink Formation in Osteogenic Grafts. Biotechnol J. 2019;14:e1700763
169. Murphy CM, Haugh MG, O'Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31:461-6
170. Prè D, Ceccarelli G, Visai L, Benedetti L, Imbriani M, Cusella De Angelis MG. et al. High-Frequency Vibration Treatment of Human Bone Marrow Stromal Cells Increases Differentiation toward Bone Tissue. Bone Marrow Res. 2013;2013:803450
171. Zhang C, Lu Y, Zhang L, Liu Y, Zhou Y, Chen Y. et al. Influence of different intensities of vibration on proliferation and differentiation of human periodontal ligament stem cells. Arch Med Sci. 2015;11:638-46
172. Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H. et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater. 2014;13:979-87
173. Lin CH, Li NT, Cheng HS, Yen ML. Oxidative stress induces imbalance of adipogenic/osteoblastic lineage commitment in mesenchymal stem cells through decreasing SIRT1 functions. J Cell Mol Med. 2018;22:786-96
174. Reinwald Y, El Haj AJ. Hydrostatic pressure in combination with topographical cues affects the fate of bone marrow-derived human mesenchymal stem cells for bone tissue regeneration. J Biomed Mater Res A. 2018;106:629-40
175. Ribeiro S, Pugliese E, Korntner SH, Fernandes EM, Gomes ME, Reis RL. et al. Assessing the combined effect of surface topography and substrate rigidity in human bone marrow stem cell cultures. Eng Life Sci. 2022;22:619-33
176. Nokhbatolfoghahaei H, Bohlouli M, Paknejad Z, M RR, L MA, Salehi-Nik N. et al. Bioreactor cultivation condition for engineered bone tissue: Effect of various bioreactor designs on extra cellular matrix synthesis. J Biomed Mater Res A. 2020;108:1662-72
177. Luo J, Walker M, Xiao Y, Donnelly H, Dalby MJ, Salmeron-Sanchez M. The influence of nanotopography on cell behaviour through interactions with the extracellular matrix - A review. Bioact Mater. 2022;15:145-59
178. Kechagia JZ, Ivaska J, Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nat Rev Mol Cell Biol. 2019;20:457-73
179. Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720-5
180. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673-87
181. Sun M, Chi G, Xu J, Tan Y, Xu J, Lv S. et al. Extracellular matrix stiffness controls osteogenic differentiation of mesenchymal stem cells mediated by integrin α5. Stem Cell Res Ther. 2018;9:52
182. Saidova AA, Vorobjev IA. Lineage Commitment, Signaling Pathways, and the Cytoskeleton Systems in Mesenchymal Stem Cells. Tissue Eng Part B Rev. 2020;26:13-25
183. Shih YR, Tseng KF, Lai HY, Lin CH, Lee OK. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res. 2011;26:730-8
184. Zhou Q, Lyu S, Bertrand AA, Hu AC, Chan CH, Ren X. et al. Stiffness of Nanoparticulate Mineralized Collagen Scaffolds Triggers Osteogenesis via Mechanotransduction and Canonical Wnt Signaling. Macromol Biosci. 2021;21:e2000370
185. Guadarrama Bello D, Fouillen A, Badia A, Nanci A. A nanoporous titanium surface promotes the maturation of focal adhesions and formation of filopodia with distinctive nanoscale protrusions by osteogenic cells. Acta Biomater. 2017;60:339-49
186. Frith JE, Mills RJ, Hudson JE, Cooper-White JJ. Tailored integrin-extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem Cells Dev. 2012;21:2442-56
187. Stanton AE, Tong X, Yang F. Extracellular matrix type modulates mechanotransduction of stem cells. Acta Biomater. 2019;96:310-20
188. Horton ER, Astudillo P, Humphries MJ, Humphries JD. Mechanosensitivity of integrin adhesion complexes: role of the consensus adhesome. Exp Cell Res. 2016;343:7-13
189. Lee MS, Lee DH, Jeon J, Oh SH, Yang HS. Topographically Defined, Biodegradable Nanopatterned Patches to Regulate Cell Fate and Acceleration of Bone Regeneration. ACS Appl Mater Interfaces. 2018;10:38780-90
190. Liu J, Wang Y, Goh WI, Goh H, Baird MA, Ruehland S. et al. Talin determines the nanoscale architecture of focal adhesions. Proc Natl Acad Sci U S A. 2015;112:E4864-73
191. Cassidy JW, Roberts JN, Smith CA, Robertson M, White K, Biggs MJ. et al. Osteogenic lineage restriction by osteoprogenitors cultured on nanometric grooved surfaces: the role of focal adhesion maturation. Acta Biomater. 2014;10:651-60
192. Hou Y, Xie W, Yu L, Camacho LC, Nie C, Zhang M. et al. Surface Roughness Gradients Reveal Topography-Specific Mechanosensitive Responses in Human Mesenchymal Stem Cells. Small. 2020;16:e1905422
193. Wu MC, Yu HW, Chen YQ, Ou MH, Serrano R, Huang GL. et al. Early committed polarization of intracellular tension in response to cell shape determines the osteogenic differentiation of mesenchymal stromal cells. Acta Biomater. 2022: S1742-7061(22)00709-7.
194. Khan AU, Qu R, Fan T, Ouyang J, Dai J. A glance on the role of actin in osteogenic and adipogenic differentiation of mesenchymal stem cells. Stem Cell Res Ther. 2020;11:283
195. Liu P, Tu J, Wang W, Li Z, Li Y, Yu X. et al. Effects of Mechanical Stress Stimulation on Function and Expression Mechanism of Osteoblasts. Front Bioeng Biotechnol. 2022;10:830722
196. Xiao E, Chen C, Zhang Y. The mechanosensor of mesenchymal stem cells: mechanosensitive channel or cytoskeleton? Stem Cell Res Ther. 2016;7:140
197. Song Y, Tang Y, Song J, Lei M, Liang P, Fu T. et al. Cyclic mechanical stretch enhances BMP9-induced osteogenic differentiation of mesenchymal stem cells. Int Orthop. 2018;42:947-55
198. Lu J, Fan Y, Gong X, Zhou X, Yi C, Zhang Y. et al. The Lineage Specification of Mesenchymal Stem Cells Is Directed by the Rate of Fluid Shear Stress. J Cell Physiol. 2016;231:1752-60
199. Kuo YC, Chang TH, Hsu WT, Zhou J, Lee HH, Hui-Chun Ho J. et al. Oscillatory shear stress mediates directional reorganization of actin cytoskeleton and alters differentiation propensity of mesenchymal stem cells. Stem Cells. 2015;33:429-42
200. Carthew J, Abdelmaksoud HH, Hodgson-Garms M, Aslanoglou S, Ghavamian S, Elnathan R. et al. Precision Surface Microtopography Regulates Cell Fate via Changes to Actomyosin Contractility and Nuclear Architecture. Adv Sci (Weinh). 2021;8:2003186
201. Qian W, Gong L, Cui X, Zhang Z, Bajpai A, Liu C. et al. Nanotopographic Regulation of Human Mesenchymal Stem Cell Osteogenesis. ACS Appl Mater Interfaces. 2017;9:41794-806
202. Wang L, You X, Zhang L, Zhang C, Zou W. Mechanical regulation of bone remodeling. Bone Res. 2022;10:16
203. Xue X, Hong X, Li Z, Deng CX, Fu J. Acoustic tweezing cytometry enhances osteogenesis of human mesenchymal stem cells through cytoskeletal contractility and YAP activation. Biomaterials. 2017;134:22-30
204. Li G, Song Y, Shi M, Du Y, Wang W, Zhang Y. Mechanisms of Cdc42-mediated rat MSC differentiation on micro/nano-textured topography. Acta Biomater. 2017;49:235-46
205. Wang W, Li GW, Wang JJ, Song W, Luo J, Meng FH. et al. Involvement of Rac1 in the micro/nano-topography sensing and osteogenic differentiation in bone marrow mesenchymal stem cells. Mater Des. 2018;157:402-11
206. Zhang Q, Lin S, Zhang T, Tian T, Ma Q, Xie X. et al. Curved microstructures promote osteogenesis of mesenchymal stem cells via the RhoA/ROCK pathway. Cell Prolif. 2017;50(4):e12356
207. Stanton AE, Tong X, Lee S, Yang F. Biochemical Ligand Density Regulates Yes-Associated Protein Translocation in Stem Cells through Cytoskeletal Tension and Integrins. ACS Appl Mater Interfaces. 2019;11:8849-57
208. Kim TJ, Joo C, Seong J, Vafabakhsh R, Botvinick EL, Berns MW. et al. Distinct mechanisms regulating mechanical force-induced Ca²⁺ signals at the plasma membrane and the ER in human MSCs. Elife. 2015;4:e04876
209. Yao H, Zhang L, Yan S, He Y, Zhu H, Li Y. et al. Low-intensity pulsed ultrasound/nanomechanical force generators enhance osteogenesis of BMSCs through microfilaments and TRPM7. J Nanobiotechnol. 2022;20:378
210. Meka SRK, Chacko LA, Ravi A, Chatterjee K, Ananthanarayanan V. Role of Microtubules in Osteogenic Differentiation of Mesenchymal Stem Cells on 3D Nanofibrous Scaffolds. ACS Biomater Sci Eng. 2017;3:551-9
211. Das RK, Gocheva V, Hammink R, Zouani OF, Rowan AE. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat Mater. 2016;15:318-25
212. Sharma P, Bolten ZT, Wagner DR, Hsieh AH. Deformability of Human Mesenchymal Stem Cells Is Dependent on Vimentin Intermediate Filaments. Ann Biomed Eng. 2017;45:1365-74
213. Stavenschi E, Hoey DA. Pressure-induced mesenchymal stem cell osteogenesis is dependent on intermediate filament remodeling. FASEB J. 2019;33:4178-87
214. Zonderland J, Moldero IL, Anand S, Mota C, Moroni L. Dimensionality changes actin network through lamin A/C and zyxin. Biomaterials. 2020;240:119854
215. Alcorta-Sevillano N, Macías I, Rodríguez CI, Infante A. Crucial Role of Lamin A/C in the Migration and Differentiation of MSCs in Bone. Cells. 2020;9(6):1330
216. Killaars AR, Walker CJ, Anseth KS. Nuclear mechanosensing controls MSC osteogenic potential through HDAC epigenetic remodeling. Proc Natl Acad Sci U S A. 2020;117:21258-66
217. Chen L, Wu C, Wei D, Chen S, Xiao Z, Zhu H. et al. Biomimetic mineralized microenvironment stiffness regulated BMSCs osteogenic differentiation through cytoskeleton mediated mechanical signaling transduction. Mater Sci Eng C Mater Biol Appl. 2021;119:111613
218. Yang Y, Xu T, Bei HP, Zhang L, Tang CY, Zhang M. et al. Gaussian curvature-driven direction of cell fate toward osteogenesis with triply periodic minimal surface scaffolds. Proc Natl Acad Sci U S A. 2022;119:e2206684119
219. Werner M, Blanquer SB, Haimi SP, Korus G, Dunlop JW, Duda GN. et al. Surface Curvature Differentially Regulates Stem Cell Migration and Differentiation via Altered Attachment Morphology and Nuclear Deformation. Adv Sci (Weinh). 2017;4:1600347
220. Johnson GP, Stavenschi E, Eichholz KF, Corrigan MA, Fair S, Hoey DA. Mesenchymal stem cell mechanotransduction is cAMP dependent and regulated by adenylyl cyclase 6 and the primary cilium. J Cell Sci. 2018;131(21):jcs222737
221. Bodle J, Hamouda MS, Cai S, Williams RB, Bernacki SH, Loboa EG. Primary Cilia Exhibit Mechanosensitivity to Cyclic Tensile Strain and Lineage-Dependent Expression in Adipose-Derived Stem Cells. Sci Rep. 2019;9:8009
222. Zhang J, Dalbay MT, Luo X, Vrij E, Barbieri D, Moroni L. et al. Topography of calcium phosphate ceramics regulates primary cilia length and TGF receptor recruitment associated with osteogenesis. Acta Biomater. 2017;57:487-97
223. McMurray RJ, Wann AK, Thompson CL, Connelly JT, Knight MM. Surface topography regulates wnt signaling through control of primary cilia structure in mesenchymal stem cells. Sci Rep. 2013;3:3545
224. Hoey DA, Tormey S, Ramcharan S, O'Brien FJ, Jacobs CR. Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells. 2012;30:2561-70
225. Chen JC, Hoey DA, Chua M, Bellon R, Jacobs CR. Mechanical signals promote osteogenic fate through a primary cilia-mediated mechanism. FASEB J. 2016;30:1504-11
226. Hu K, Sun H, Gui B, Sui C. TRPV4 functions in flow shear stress induced early osteogenic differentiation of human bone marrow mesenchymal stem cells. Biomed Pharmacother. 2017;91:841-8
227. Corrigan MA, Johnson GP, Stavenschi E, Riffault M, Labour MN, Hoey DA. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci Rep. 2018;8:3824
228. Johnson GP, Fair S, Hoey DA. Primary cilium-mediated MSC mechanotransduction is dependent on Gpr161 regulation of hedgehog signalling. Bone. 2021;145:115846
229. Sugimoto A, Miyazaki A, Kawarabayashi K, Shono M, Akazawa Y, Hasegawa T. et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci Rep. 2017;7:17696
230. Zhou T, Gao B, Fan Y, Liu Y, Feng S, Cong Q. et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. Elife. 2020;9:e52779
231. Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ. et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55-60
232. He L, Ahmad M, Perrimon N. Mechanosensitive channels and their functions in stem cell differentiation. Exp Cell Res. 2019;374:259-65
233. Jiang Y, Yang X, Jiang J, Xiao B. Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels. Trends Biochem Sci. 2021;46:472-88
234. Zhao Q, Zhou H, Li X, Xiao B. The mechanosensitive Piezo1 channel: a three-bladed propeller-like structure and a lever-like mechanogating mechanism. FEBS J. 2019;286:2461-70
235. Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR. et al. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun. 2016;7:10366
236. Wang J, Jiang J, Yang X, Zhou G, Wang L, Xiao B. Tethering Piezo channels to the actin cytoskeleton for mechanogating via the cadherin-β-catenin mechanotransduction complex. Cell Rep. 2022;38:110342
237. Geng J, Liu W, Zhou H, Zhang T, Wang L, Zhang M. et al. A Plug-and-Latch Mechanism for Gating the Mechanosensitive Piezo Channel. Neuron. 2020;106:438-51.e6
238. Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci. 2007;8:510-21
239. Li H. TRP Channel Classification. Adv Exp Med Biol. 2017;976:1-8
240. Xiao E, Yang HQ, Gan YH, Duan DH, He LH, Guo Y. et al. Brief reports: TRPM7 Senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells. 2015;33:615-21
241. Liu YS, Liu YA, Huang CJ, Yen MH, Tseng CT, Chien S. et al. Mechanosensitive TRPM7 mediates shear stress and modulates osteogenic differentiation of mesenchymal stromal cells through Osterix pathway. Sci Rep. 2015;5:16522
242. Du G, Li L, Zhang X, Liu J, Hao J, Zhu J. et al. Roles of TRPV4 and piezo channels in stretch-evoked Ca(2+) response in chondrocytes. Exp Biol Med (Maywood). 2020;245:180-9
243. Yoneda M, Suzuki H, Hatano N, Nakano S, Muraki Y, Miyazawa K. et al. PIEZO1 and TRPV4, which Are Distinct Mechano-Sensors in the Osteoblastic MC3T3-E1 Cells, Modify Cell-Proliferation. Int J Mol Sci. 2019;20(19):4960
244. Talbot J, Brion R, Lamora A, Mullard M, Morice S, Heymann D. et al. Connexin43 intercellular communication drives the early differentiation of human bone marrow stromal cells into osteoblasts. J Cell Physiol. 2018;233:946-57
245. An S, Zheng S, Cai Z, Chen S, Wang C, Li Y. et al. Connexin43 in Musculoskeletal System: New Targets for Development and Disease Progression. Aging Dis. 2022;13:1715-32
246. Qin L, Liu W, Cao H, Xiao G. Molecular mechanosensors in osteocytes. Bone Res. 2020;8:23
247. Limraksasin P, Nattasit P, Manokawinchoke J, Tiskratok W, Vinaikosol N, Okawa H. et al. Application of shear stress for enhanced osteogenic differentiation of mouse induced pluripotent stem cells. Sci Rep. 2022;12:19021
248. Lin FX, Zheng GZ, Chang B, Chen RC, Zhang QH, Xie P. et al. Connexin 43 Modulates Osteogenic Differentiation of Bone Marrow Stromal Cells Through GSK-3beta/Beta-Catenin Signaling Pathways. Cell Physiol Biochem. 2018;47:161-75
249. Bertrand AA, Malapati SH, Yamaguchi DT, Lee JC. The Intersection of Mechanotransduction and Regenerative Osteogenic Materials. Adv Healthc Mater. 2020;9:e2000709
250. Wang X, Hu X, Dulińska-Molak I, Kawazoe N, Yang Y, Chen G. Discriminating the Independent Influence of Cell Adhesion and Spreading Area on Stem Cell Fate Determination Using Micropatterned Surfaces. Sci Rep. 2016;6:28708
251. Swanson WB, Omi M, Woodbury SM, Douglas LM, Eberle M, Ma PX. et al. Scaffold Pore Curvature Influences ΜSC Fate through Differential Cellular Organization and YAP/TAZ Activity. Int J Mol Sci. 2022 23
252. Chang Y, Shao Y, Liu Y, Xia R, Tong Z, Zhang J. et al. Mechanical strain promotes osteogenic differentiation of mesenchymal stem cells on TiO(2) nanotubes substrate. Biochem Biophys Res Commun. 2019;511:840-6
253. He J, You D, Li Q, Wang J, Ding S, He X. et al. Osteogenesis-Inducing Chemical Cues Enhance the Mechanosensitivity of Human Mesenchymal Stem Cells for Osteogenic Differentiation on a Microtopographically Patterned Surface. Adv Sci (Weinh). 2022;9:e2200053
254. Hoon JL, Tan MH, Koh CG. The Regulation of Cellular Responses to Mechanical Cues by Rho GTPases. Cells. 2016;5(2):17
255. Ji Y, Li J, Wei Y, Gao W, Fu X, Wang Y. Substrate stiffness affects the immunosuppressive and trophic function of hMSCs via modulating cytoskeletal polymerization and tension. Biomater Sci. 2019;7:5292-300
256. Nikukar H, Reid S, Tsimbouri PM, Riehle MO, Curtis AS, Dalby MJ. Osteogenesis of mesenchymal stem cells by nanoscale mechanotransduction. ACS Nano. 2013;7:2758-67
257. Kim C, Young JL, Holle AW, Jeong K, Major LG, Jeong JH. et al. Stem Cell Mechanosensation on Gelatin Methacryloyl (GelMA) Stiffness Gradient Hydrogels. Ann Biomed Eng. 2020;48:893-902
258. Lv H, Li L, Sun M, Zhang Y, Chen L, Rong Y. et al. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res Ther. 2015;6:103
259. Ogino Y, Liang R, Mendonça DB, Mendonça G, Nagasawa M, Koyano K. et al. RhoA-Mediated Functions in C3H10T1/2 Osteoprogenitors Are Substrate Topography Dependent. J Cell Physiol. 2016;231:568-75
260. Choi RB, Robling AG. The Wnt pathway: An important control mechanism in bone's response to mechanical loading. Bone. 2021;153:116087
261. Chen B, Lin T, Yang X, Li Y, Xie D, Zheng W. et al. Low-magnitude, high-frequency vibration promotes the adhesion and the osteogenic differentiation of bone marrow-derived mesenchymal stem cells cultured on a hydroxyapatite-coated surface: The direct role of Wnt/β-catenin signaling pathway activation. Int J Mol Med. 2016;38:1531-40
262. Zhou Q, Ren X, Oberoi MK, Bedar M, Caprini RM, Dewey MJ. et al. β-Catenin Limits Osteogenesis on Regenerative Materials in a Stiffness-Dependent Manner. Adv Healthc Mater. 2021;10:e2101467
263. Xie J, Zhang D, Zhou C, Yuan Q, Ye L, Zhou X. Substrate elasticity regulates adipose-derived stromal cell differentiation towards osteogenesis and adipogenesis through β-catenin transduction. Acta Biomater. 2018;79:83-95
264. Mao L, Liu J, Zhao J, Chang J, Xia L, Jiang L. et al. Effect of micro-nano-hybrid structured hydroxyapatite bioceramics on osteogenic and cementogenic differentiation of human periodontal ligament stem cell via Wnt signaling pathway. Int J Nanomedicine. 2015;10:7031-44
265. Shao XR, Lin SY, Peng Q, Shi SR, Li XL, Zhang T. et al. Effect of tetrahedral DNA nanostructures on osteogenic differentiation of mesenchymal stem cells via activation of the Wnt/β-catenin signaling pathway. Nanomedicine. 2017;13:1809-19
266. Hou W, Fu H, Liu X, Duan K, Lu X, Lu M. et al. Cation Channel Transient Receptor Potential Vanilloid 4 Mediates Topography-Induced Osteoblastic Differentiation of Bone Marrow Stem Cells. ACS Biomater Sci Eng. 2019;5:6520-9
267. Shi GX, Zheng XF, Zhu C, Li B, Wang YR, Jiang SD. et al. Evidence of the Role of R-Spondin 1 and Its Receptor Lgr4 in the Transmission of Mechanical Stimuli to Biological Signals for Bone Formation. Int J Mol Sci. 2017;18(3):564
268. Niu H, Lin D, Tang W, Ma Y, Duan B, Yuan Y. et al. Surface Topography Regulates Osteogenic Differentiation of MSCs via Crosstalk between FAK/MAPK and ILK/β-Catenin Pathways in a Hierarchically Porous Environment. ACS Biomater Sci Eng. 2017;3:3161-75
269. Chen JC, Jacobs CR. Mechanically induced osteogenic lineage commitment of stem cells. Stem Cell Res Ther. 2013;4:107
270. Arnsdorf EJ, Tummala P, Jacobs CR. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One. 2009;4:e5388
271. Guo L, Wang R, Zhang K, Yuan J, Wang J, Wang X. et al. A PINCH-1-Smurf1 signaling axis mediates mechano-regulation of BMPR2 and stem cell differentiation. J Cell Biol. 2019;218:3773-94
272. Wang J, Wang M, Chen F, Wei Y, Chen X, Zhou Y. et al. Nano-Hydroxyapatite Coating Promotes Porous Calcium Phosphate Ceramic-Induced Osteogenesis Via BMP/Smad Signaling Pathway. Int J Nanomedicine. 2019;14:7987-8000
273. Li X, Liu M, Chen F, Wang Y, Wang M, Chen X. et al. Design of hydroxyapatite bioceramics with micro-/nano-topographies to regulate the osteogenic activities of bone morphogenetic protein-2 and bone marrow stromal cells. Nanoscale. 2020;12:7284-300
274. Ziouti F, Ebert R, Rummler M, Krug M, Müller-Deubert S, Lüdemann M. et al. NOTCH Signaling Is Activated through Mechanical Strain in Human Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells Int. 2019;2019:5150634
275. Zhao Y, Richardson K, Yang R, Bousraou Z, Lee YK, Fasciano S. et al. Notch signaling and fluid shear stress in regulating osteogenic differentiation. Front Bioeng Biotechnol. 2022;10:1007430
276. Zhou M, Liu NX, Shi SR, Li Y, Zhang Q, Ma QQ. et al. Effect of tetrahedral DNA nanostructures on proliferation and osteo/odontogenic differentiation of dental pulp stem cells via activation of the notch signaling pathway. Nanomedicine. 2018;14:1227-36
277. Caliari SR, Vega SL, Kwon M, Soulas EM, Burdick JA. Dimensionality and spreading influence MSC YAP/TAZ signaling in hydrogel environments. Biomaterials. 2016;103:314-23
278. Ng JL, Kersh ME, Kilbreath S, Knothe Tate M. Establishing the Basis for Mechanobiology-Based Physical Therapy Protocols to Potentiate Cellular Healing and Tissue Regeneration. Front Physiol. 2017;8:303
279. Pu P, Wu S, Zhang K, Xu H, Guan J, Jin Z. et al. Mechanical force induces macrophage-derived exosomal UCHL3 promoting bone marrow mesenchymal stem cell osteogenesis by targeting SMAD1. J Nanobiotechnology. 2023;21:88
280. Zhang Z, Xie Y, Pan H, Huang L, Zheng X. Influence of patterned titanium coatings on polarization of macrophage and osteogenic differentiation of bone marrow stem cells. J Biomater Appl. 2018;32:977-86
281. Wang JJ, Meng FH, Song W, Jin JY, Ma QL, Fei DD. et al. Nanostructured titanium regulates osseointegration via influencing macrophage polarization in the osteogenic environment. Int J Nanomedicine. 2018;13:4029-43
282. Yin C, Zhao Q, Li W, Zhao Z, Wang J, Deng T. et al. Biomimetic anti-inflammatory nano-capsule serves as a cytokine blocker and M2 polarization inducer for bone tissue repair. Acta Biomater. 2020;102:416-26
283. He X-T, Li X, Xia Y, Yin Y, Wu R-X, Sun H-H. et al. Building capacity for macrophage modulation and stem cell recruitment in high-stiffness hydrogels for complex periodontal regeneration: Experimental studies in vitro and in rats. Acta Biomater. 2019;88:162-80
284. Khosrowpour Z, Hashemi SM, Mohammadi-Yeganeh S, Moghtadaei M, Brouki Milan P, Moroni L. et al. Coculture of adipose-derived mesenchymal stem cells/macrophages on decellularized placental sponge promotes differentiation into the osteogenic lineage. Artif Organs. 2023;47:47-61
285. Tian G, Jiang S, Li J, Wei F, Li X, Ding Y. et al. Cell-free decellularized cartilage extracellular matrix scaffolds combined with interleukin 4 promote osteochondral repair through immunomodulatory macrophages: In vitro and in vivo preclinical study. Acta Biomater. 2021;127:131-45
286. Liu XY, Su JY, Zhou H, Zeng ZY, Li ZH, Xiao Z. et al. Collagen VI antibody reduces atherosclerosis by activating monocyte/macrophage polarization in ApoE(-/-) mice. Int Immunopharmacol. 2022;111:109100
287. Chen P, Cescon M, Zuccolotto G, Nobbio L, Colombelli C, Filaferro M. et al. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol. 2015;129:97-113
288. Lian M, Sun B, Han Y, Yu B, Xin W, Xu R. et al. A low-temperature-printed hierarchical porous sponge-like scaffold that promotes cell-material interaction and modulates paracrine activity of MSCs for vascularized bone regeneration. Biomaterials. 2021;274:120841
289. Batool F, Özçelik H, Stutz C, Gegout PY, Benkirane-Jessel N, Petit C. et al. Modulation of immune-inflammatory responses through surface modifications of biomaterials to promote bone healing and regeneration. J Tissue Eng. 2021;12:20417314211041428
290. Wang J, Wang Y, Li Y, He Y, Song W, Wang Q. et al. Unique regulation of TiO2 nanoporous topography on macrophage polarization via MSC-derived exosomes. Regen Biomater. 2023;10:rbad012
291. Jin S, Yang R, Chu C, Hu C, Zou Q, Li Y. et al. Topological structure of electrospun membrane regulates immune response, angiogenesis and bone regeneration. Acta Biomater. 2021;129:148-58
292. He Y, Tian M, Li XL, Hou JW, Chen S, Yang G. et al. A Hierarchical-Structured Mineralized Nanofiber Scaffold with Osteoimmunomodulatory and Osteoinductive Functions for Enhanced Alveolar Bone Regeneration. Adv Healthc Mater. 2022;11(3):e2102236
Author contact
![]() Corresponding authors: Shu Guo: No. 115 Nanjing North Street, Department of Plastic Surgery, The First Hospital of China Medical University, Shenyang 110001, China. sguoedu.cn; Zhe Yi: No. 117 Nanjing North Street, School and Hospital of Stomatology, China Medical University, Liaoning Provincial Key Laboratory of Oral Diseases, Shenyang 110001, China. zheyiedu.cn; Shude Yang: No. 115 Nanjing North Street, Department of Plastic Surgery, The First Hospital of China Medical University, Shenyang 110001, China. sdyangedu.cn.
Corresponding authors: Shu Guo: No. 115 Nanjing North Street, Department of Plastic Surgery, The First Hospital of China Medical University, Shenyang 110001, China. sguoedu.cn; Zhe Yi: No. 117 Nanjing North Street, School and Hospital of Stomatology, China Medical University, Liaoning Provincial Key Laboratory of Oral Diseases, Shenyang 110001, China. zheyiedu.cn; Shude Yang: No. 115 Nanjing North Street, Department of Plastic Surgery, The First Hospital of China Medical University, Shenyang 110001, China. sdyangedu.cn.
 Global reach, higher impact
Global reach, higher impact