13.3
Impact Factor
Theranostics 2022; 12(17):7550-7566. doi:10.7150/thno.77630 This issue Cite
Research Paper
circHIPK3 prevents cardiac senescence by acting as a scaffold to recruit ubiquitin ligase to degrade HuR
1. Institute for Cardiovascular Science and Department of Cardiovascular Surgery, First Affiliated Hospital and Medical College of Soochow University, Collaborative Innovation Center of Hematology, Soochow University, Suzhou, Jiangsu 215123, P. R. China.
2. Department of Structural Heart Disease, National Center for Cardiovascular Disease, China & Fuwai Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, Key Laboratory of Cardiovascular Appratus Innovation, Beijing 100037, P.R. China.
3. Department of Cardiology, the Second Hospital of Jilin University, Changchun, Jilin 130041, P. R. China.
4. Key Laboratory of Molecular Target & Clinical Pharmacology and the NMPA & State Key Laboratory of Respiratory Disease, Guangzhou Medical University, Guangzhou, Guangdong 511436, P. R. China.
*These authors contributed equally to this work.
Abstract
Rational: Senescence is a major aging process that contributes to the development of cardiovascular diseases, but the underlying molecular mechanisms remain largely unknown. One reason is due to the lack of suitable animal models. We aimed to generate a cardiomyocyte (CM)-specific senescent animal model, uncover the underlying mechanisms, and develop new therapies for aging associated cardiac dysfunction.
Methods: The gain/loss of circHIPK3 approach was used to explore the role of circHIPK3 in cardiomyocyte (CM) senescence. To investigate the mechanisms of circHIPK3 function in cardiac senescence, we generated CM-specific tamoxifen-induced circHIPK3 knockout (CKO) mice. We also applied various analyses including PCR, Western blot, nuclear and cytoplasmic protein extraction, immunofluorescence, echocardiography, RNA immunoprecipitation assay, RNA-pulldown assay, and co-immunoprecipitation.
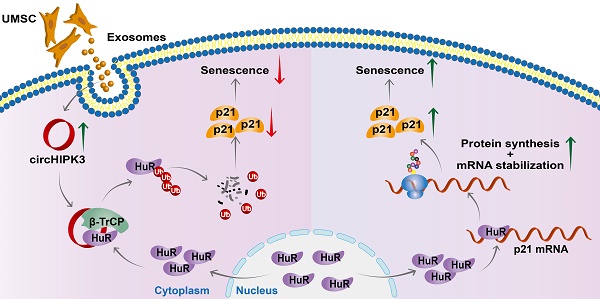
Results: Our novel CKO mice exhibited worse cardiac function, decreased circHIPK3 expression and telomere length shortening in the heart. The level of the senescence-inducer p21 in the hearts of CKO mice was significantly increased and survival was poor compared with control mice. In vitro, the level of p21 in CMs was significantly decreased by circHIPK3 overexpression, but increased by circHIPK3 silencing. We showed that circHIPK3 was a scaffold for p21 mRNA-binding protein HuR and E3 ubiquitin ligase β-TrCP. circHIPK3 silencing weakened the interaction between HuR and β-TrCP, reduced HuR ubiquitination, and enhanced the interaction between HuR and p21 mRNA. Moreover, we found that mice injected with human umbilical cord mesenchymal stem cell-derived exosomes (UMSC-Exos) showed increased circHIPK3 levels, decreased levels of p21, longer telomere length, and good cardiac function. However, these beneficial effects exerted by UMSC-Exos were inhibited by silencing circHIPK3.
Conclusions: We successfully generated CM-specific CKO mice for aging research. Our results showed that deletion of circHIPK3 led to exaggerated CM senescence and decreased cardiac function. As a scaffold, circHIPK3 enhanced the binding of E3 ubiquitin ligase β-TrCP and HuR in the cytoplasm, leading to the ubiquitination and degradation of HuR and reduced p21 activity. In addition, UMSC-Exos exerted an anti-senescence and cardio-protective effect by delivering circHIPK3. These findings pave the way to the development of new therapies for aging associated cardiac dysfunction.
Keywords: exosome, circHIPK3, RNA-binding protein, senescence, ubiquitin ligase, aging
 Global reach, higher impact
Global reach, higher impact