13.3
Impact Factor
Theranostics 2021; 11(16):7896-7910. doi:10.7150/thno.61337 This issue Cite
Research Paper
Probing the fluorination effect on the self-assembly characteristics, in vivo fate and antitumor efficacy of paclitaxel prodrug nanoassemblies
1. Department of Pharmaceutics, Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang 110016, China.
2. School of Pharmacy, China Medical University, 77 Puhe Road, Shenyang 110122, China.
3. Department of Pharmacy, the First Hospital of China Medical University, 155 Nanjing North Street, Shenyang 110001, China.
4. School of Pharmacy, Shenyang Pharmaceutical University, Shenyang, 110016, China.
*Both authors contributed equally to this work.
Abstract
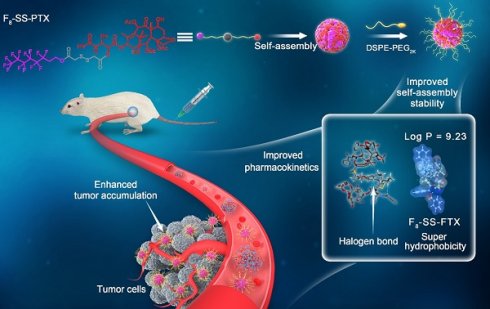
Rationale: Small-molecule prodrug nanoassembly is emerging as an efficient platform for chemotherapy. The self-assembly stability plays a vital role on the drug delivery efficiency of prodrug nanoassembly. It is reported that fluoroalkylation could improve the self-assembly stability of amphiphilic polymers by utilizing the unique fluorination effect. But the application of fluoroalkylation on small-molecule prodrug nanoassembly has never been reported.
Methods: Here, fluoro-modified prodrug was developed by conjugating paclitaxel with perfluorooctanol (F8-SS-PTX), and the paclitaxel-octanol prodrug (C8-SS-PTX) was used as control. The fluoro-mediated self-assembly mechanisms were illustrated using molecular dynamics simulation. In addition, the impacts of fluoroalkylation on the pharmacy characters, in vivo fate and antitumor effect of small-molecule prodrug nanoassembly were investigated in details.
Results: Fluoroalkylation significantly improved the self-assembly stability of F8-SS-PTX NPs both in vitro and in vivo, which could be attributed to the fluoro-mediated hydrophobic force and halogen bonds. The AUC0-24h and tumor accumulation of F8-SS-PTX NPs was 6-fold and 2-fold higher than that of C8-SS-PTX NPs, respectively. As a result, F8-SS-PTX NPs exhibited much better antitumor effect than C8-SS-PTX NPs and Abraxane.
Conclusion: Fluoroalkylation could improve the self-assembly stability, in vivo fate, and antitumor efficacy of small-molecule prodrug nanoassemblies, which could be an effective strategy for the rational design of advanced nanomedicines.
Keywords: prodrug self-assembly, fluorination effect, paclitaxel, redox responsive, tumor accumulation
 Global reach, higher impact
Global reach, higher impact