13.3
Impact Factor
Theranostics 2025; 15(8):3223-3233. doi:10.7150/thno.100786 This issue Cite
Review
Artificial intelligence-enhanced retinal imaging as a biomarker for systemic diseases
1. School of Clinical Medicine, Tsinghua Medicine, Tsinghua University, Beijing, 100084, China.
2. Beijing Visual Science and Translational Eye Research Institute (BERI), Beijing Tsinghua Changgung Hospital, Tsinghua Medicine, Tsinghua University, Beijing, 100084, China.
3. Eye Center, Beijing Tsinghua Changgung Hospital, Beijing, 102218, China.
4. Center for Eye Research Australia, Royal Victorian Eye and Ear Hospital, Melbourne, VIC, Australia; Department of Surgery (Ophthalmology), The University of Melbourne, Melbourne, VIC, Australia.
5. College of Future Technology, Peking University, Beijing, China.
6. Key Laboratory for Biomechanics and Mechanobiology of Ministry of Education, Beijing Advanced Innovation Center for Biomedical Engineering, School of Biological Science and Medical Engineering, Beihang University, Beijing, China.
7. Shanghai International Joint Laboratory for Intelligent Prevention and Treatment of Metabolic Disorders, Department of Computer Science and Engineering, School of Electronic, Information, and Electrical Engineering, Shanghai Jiao Tong University, Department of Endocrinology and Metabolism, Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai Diabetes Institute, Shanghai Clinical Center for Diabetes, Shanghai, China.
8. MOE Key Laboratory of AI, School of Electronic, Information, and Electrical Engineering, Shanghai Jiao Tong University, Shanghai, China.
9. Department of Ophthalmology, University of Warmia and Mazury, Olsztyn, Poland.
10. Institute for Research in Ophthalmology, Foundation for Ophthalmology Development, Poznan, Poland.
11. Singapore Eye Research Institute, Singapore National Eye Center, Singapore.
Received 2024-7-10; Accepted 2024-9-18; Published 2025-2-18
Abstract
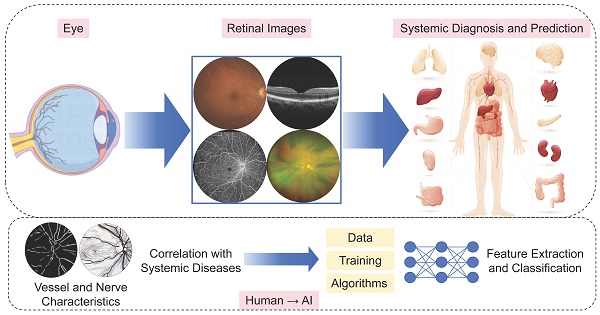
Retinal images provide a non-invasive and accessible means to directly visualize human blood vessels and nerve fibers. Growing studies have investigated the intricate microvascular and neural circuitry within the retina, its interactions with other systemic vascular and nervous systems, and the link between retinal biomarkers and various systemic diseases. Using the eye to study systemic health, based on these connections, has been given a term as oculomics. Advancements in artificial intelligence (AI) technologies, particularly deep learning, have further increased the potential impact of this study. Leveraging these technologies, retinal analysis has demonstrated potentials in detecting numerous diseases, including cardiovascular diseases, central nervous system diseases, chronic kidney diseases, metabolic diseases, endocrine disorders, and hepatobiliary diseases. AI-based retinal imaging, which incorporates established modalities such as digital color fundus photographs, optical coherence tomography (OCT) and OCT angiography, as well as emerging technologies like ultra-wide field imaging, shows great promises in predicting systemic diseases. This provides a valuable opportunity for systemic diseases screening, early detection, prediction, risk stratification, and personalized prognostication. As the AI and big data research field grows, with the mission of transforming healthcare, they also face numerous challenges and limitations both in data and technology. The application of natural language processing framework, large language model, and other generative AI techniques presents both opportunities and concerns that require careful consideration.
In this review, we not only summarize key studies on AI-enhanced retinal imaging for predicting systemic diseases but also underscore the significance of these advancements in transforming healthcare. By highlighting the remarkable progress made thus far, we provide a comprehensive overview of state-of-the-art techniques and explore the opportunities and challenges in this rapidly evolving field. This review aims to serve as a valuable resource for researchers and clinicians, guiding future studies and fostering the integration of AI in clinical practice.
Keywords: artificial intelligence, deep learning, retinal imaging, systemic prediction, color fundus photos.
Introduction
The retina offers a unique, noninvasive, in-vivo visualization of the human body's vasculature and neural tissues [1], serving as a window to the general health. The retinal nerve fibers are essentially an extension of the central nervous system (CNS) axons, and the retinal ganglion cells (RGCs) display typical properties of CNS neurons [2]. The retinal blood vessels, densely located in the fundus, not only mirror the features and regulatory mechanism of blood vessels throughout the body, but also act as indicators of general health. Given its vascularization and metabolic activities, the retina provides important clues on the presence of systemic diseases [3].
Over more than 100 years ago, Marcus Gunn described retinal vascular signs in patients with hypertension, kidney disease and stroke, marking the beginning of retinal examination as a source of important clues to systemic health [4]. At that time, Polish ophthalmologist Xavier Galezowski (1832-1907), one of the pioneers in the use of fundus examinations for the diagnosis of central nervous system disorders, published one of early textbooks on this subject and coined a term of cerebroscopy for this examination [5, 6]. In the last decades of 20th century, studies provided evidence of RGCs degeneration and retinal nerve fiber layer (RNFL) damages in Alzheimer's disease (AD) (neurodegenerative diseases) [7-11], and pathological findings in the retinal vasculature of stroke (cerebral hemorrhage and infarction) [10]. This evidence indicates that retinal neural and vascular changes likely exist in the early stage even during asymptomatic period, when they are not distinguishable or cannot be detected by conventional diagnostic methods. These changes can be detected and used as biomarkers for systemic disorders. Insights from many current studies have also proven the correlation between retinal images and the risks of dementia and stroke, which are associated with neural and vascular abnormality, respectively [12-14]. These studies laid the foundation for using retinal images to predict systemic diseases.
On the other hand, the rapid updates of technology brought the chance to boost the development of this area, making the previous evidences been taken into clinical practice. In the past decades, advances in information technology have made artificial intelligence (AI) come in quantum leaps in nearly every aspect of our lives, including the medical field. AI, as computer systems capable of performing complex tasks that historically only human could do [15]. Machine learning (ML), a subfield of AI uses algorithms trained on datasets to create self-learning models that can predict outcomes and classify information without human intervention [16]. ML has been widely used in medical AI. Deep learning (DL), a branch of ML, trains computers to process information in a way that mimics human neural processes and is composed of a neural network with three or more layers (input layer, hidden layers, output layer) [17, 18]. Recently, deep convolutional neural networks (CNN), a specialized type of DL technique optimized for images, have produced highly accurate algorithms capable of diagnosing diseases from medical images with accuracy comparable to human experts [19]. Leveraging these remarkable advancements, subtle extraction and analysis of retinal images have demonstrated good efficacy in detecting various diseases.
In 2012, Lambin et al. first proposed the concept of “radiomics”, the high-throughput extraction of image features from radiographic images. The combination of medical imaging and smart automated or semi-automated software offers the potential to revolutionize quantitative imaging. This approach has paved the way for the integration of AI and medical images in medical diagnosis, treatment and prediction, holding great promises for the future [20, 21].
Ophthalmology is now a field that relies heavily on advanced imaging techniques. Digital color fundus photograph (CFP) is the most common examination, providing detailed images of the retina, optic nerve head, and blood vessels. Optical coherence tomography (OCT) offers cross-sectional details of the retina and choroid, imaging structures at different depths within tissues. OCT angiography (OCTA), a relatively new technology, can show vasculature and structure in specific single layers, imaging and quantifying blood vessels at different depths within tissues. OCT images can serve as biomarkers for neuronal pathways, due to their detailed imaging of RGCs, the RNFL, the inner plexiform layer (IPL) and other neural layers structures [11], while CFP and OCTA can be accessed for vascular pathways [22]. Besides, emerging technologies such as ultra-wide field (UWF) imaging provide a comprehensive view of the fundus, capturing details beyond the macula area. As retinal visualization techniques continue to evolve, they will increasingly be applied in retinal biomarkers extraction using AI techniques.
Using the eye to study systemic health, based on these connections, has been given a term as “oculomics”. The term “oculomics” was first proposed in 2020 by Wagner et al. [23], indicating a comprehensive understanding of the macroscopic, microscopic, and molecular features associated with health and disease within the eye. With further insights and advancements, oculomics has expanded beyond mere observation, to integrate big data and AI.
Numerous studies have explored the applications of AI-enhanced retinal imaging in predicting different systemic diseases. Even though some of the studies tried to explain how AI works in this period, with interpretative heat map, we still have uncharted parts in this work. It is crucial to know how AI works in oculomics, and we need more investigation on the studies to explore more on the potential explanations.
Therefore, based and progressed on the methods of published reviews in this study area [3, 24-29], we conducted an extensive literature search using the databases “PubMed”, “Medline”, and “Embase” up to May 2024. Search terms include retinal/fundus imaging/image/photo/photograph, OCT, OCTA, UWF; AI, ML, DL, CNN; oculomics, systemic diseases/disorders, and various systemic diseases names. We have extracted the key information of key studies (See Table S1 in the electronic supplementary material for details). We provide a classified summary of key studies on AI-enhanced retinal imaging in predicting systemic diseases, with discussing the mechanisms underlying oculomics and highlighting the challenges and opportunities in this area.
Cardiovascular Diseases (CVD)
Recent studies have explored the potential of retinal imaging in predicting CVD risks with promising results, marking a significant advancement in medical diagnostics. Rim et al. [30] employed CFPs to assess coronary artery calcium (CAC) scores, demonstrating a notable correlation with the risk of fatal cardiovascular events. This approach not only mirrors the predictive capability of traditional computerized tomography (CT)-measured CAC but also enhance risk prediction accuracy when integrated with established cardiovascular risk models, particularly for patients at borderline or intermediate risk levels. Cheung et al. [31] developed a fully automated AI/DL-based retinal vessel software (SIVA-DLS), comparing its performance with SIVA-human (retinal-vessel calibre measured by expert human graders) to predict CVD risks. They found narrower arteriolar caliber was associated with higher blood pressure, while wider venular caliber was linked to higher body mass index (BMI), hemoglobin (Hb) A1c, and smoking. SIVA-DLS demonstrated similar associations with CVD risk factors as human measurements and other software's performance. Specifically, a narrower arteriolar diameter and a wider venular diameter were associated with a higher risk of incident CVD. Poplin et al. [19] predicted major adverse cardiovascular events (MACE) within 5 years using CFPs, achieving an area under the receiver operating characteristic (AUROC) of 0.73, and compared this model with the SCORE risk calculator. Additional research has applied retinal imaging to further understand the connection to CVD risk factors, traditional cardiovascular examination results or diagnosis standard, diseases detection and future incident risks.
Cerebrovascular Diseases (CeVD)
The CFPs combined with superficial and deep enface OCTA images [32] or CFP-originated vasculometry [33, 34] have been used to generate the AI algorithms for stroke events prediction. Different wavelength fundus photos have also been applied and compared in predictive work [34]. In addition to stroke prediction, Hong et al. [35] were the first to predict and stage moyamoya disease using a CFP-based algorithm. Notably, the retinal age gap, which was defined as the difference between predicted biological age based on CFP and chronological age, has been used to predict the hazard ratio (HR) for stroke events [36]. It has been shown that the highest stratified population by retinal age gap has an HR of 2.37 compared to the lowest. Another significant predictor is white matter lesions (WMLs) from magnetic resonance imaging (MRI) which reflects the severity of CeVD, used as the output prediction of CFP-based algorithm [37]. Retinal imaging can thus be used to output CeVD brain imaging results, directly detect CeVD, or predict future stroke incidents.
Neurodegenerative Diseases
Cheung et al. [38] used AI/DL algorithm to detect AD-dementia of 11 multi-ethnic, multi-country studies with 12949 retinal photos (648 AD-dementia cases vs 3240 controls). The algorithm used CFPs to detect AD with AUROC 0.93, whereas detect amyloid β-positive AD with AUROC 0.73-0.85. Another study [39] developed a model by CFPs and clinical dementia rating global scores. The model can automate estimate central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE), give the association with cognitive and incident dementia, which may be useful as a risk stratification tool. The study also showed narrower arteriolar diameter and wider venular diameter associated with higher risk of incident dementia. That proves the possibility AI/DL retinal vessel software predicts dementia. OCT images have been used to predict AD, Parkinson's disease (PD), multiple sclerosis (MS) [40-42] and long-term prediction of MS disability course [43]. OCTA images combined with OCT in predicting mild cognitive impairment (MCI) showed model efficacy with area under the curve (AUC)=0.693-0.960 [44]. Other studies combined CFPs and metadata or risk factors to generate the models with better task performance (Table S1). Various retinal imaging and the multi-modality imaging have been widely used in different neurological diseases based on the intense connection between eye and brain.
Schizophrenia and Other Psychiatric Diseases
Appaji et al. [45] developed a model to predict schizophrenia using CFPs. Their model was trained to distinguish individuals with schizophrenia from healthy subjects, achieving an AUC of 0.98. Although some literature mentioned that psychiatric diseases, such as bipolar disorder, have exhibit vascular abnormalities that can be detected in retinal images [46], further research and AI-based algorithms are still needed. Recent studies have proposed that changes detected by ML-based electroretinography (ERG) could serve as sensitive indicators of autism spectrum disorder [47]. It is hoped that more psychiatric diseases, including depression and anxiety that have seen a rise in prevalence in recent years, will be explored in AI-retinal imaging studies.
Renal Diseases
Kidney failure is typically defined by estimated glomerular filtration rate (eGFR) levels, several models have been developed to predict chronic kidney disease (CKD), diabetic kidney disease (DKD) or diabetic nephropathy (DN). Sabanayagam et al. [48] used CFPs and a combined model trained with risk factors to predict CKD, achieving AUCs of 0.911 and 0.938. Kang et al. [49] predicted early renal function impairment with an AUC of 0.81. Similar studies have been conducted to directly predict eGFR, early CKD, CKD, and DKD labels [50-54]. Liu et al. [55] used OCT images to predict DN, with an accuracy of 91.68%, a sensitivity of 89.99% and specificity of 92.18%. Zhang et al. [56] also studies retinal age, calculating the HR of incident kidney failure using cohort data. Similarly, Joo et al. [57], used the Reti-CKD score, derived from CFPs in a cohort, to calculate the HR of CKD incidence. When the population was stratified by the Reti-CKD score, the HR were 3.68 and 9.36 in 2 cohorts from the highest to the lowest scores. The retinal age gap and Reti-CKD score can successfully be applied as predictors of renal diseases incidence, showing great promise and inspiring more predictors contribution.
Metabolic Diseases
Algorithms based on CFPs have shown good performance in predicting hypertension, hyperglycemia, dyslipidemia with AUCs of 0.766, 0.880 and 0.703 respectively [58]. Benson et al., have demonstrated that diabetes and diabetic peripheral neuropathy (DPN) can be detected using AI-CFPs models [51, 59-61]. Xiang et al. [62] innovatively combined CFPs with traditional Chinese medicine characteristics (tongue and pulse conditions) to predict diabetes, which inspired more hybrid models related to metabolic factors to enhance the performance of standalone AI models on retinal imaging. Studies on retinal age gap studies have also revealed a relationship between retinal age gap and metabolic syndrome and inflammation. The stratified population shows different odds ratios (ORs) indicating that retinal age gap could become a promising variable [63].
Hepatobiliary Diseases
Xiao et al. [64] used CFPs to generate a screening model for hepatobiliary diseases and an identification model for 6 specific hepatobiliary diseases (including liver cancer, liver cirrhosis, chronic viral hepatitis, non-alcoholic fatty liver disease, cholelithiasis, hepatic cyst). The AUROCs for screening and identification were 0.68, 0.84, 0.83, 0.62, 0.70, 0.68 and 0.69 respectively. Xiao's model provided a non-invasive, convenient, and complementary method. In the same study, they also used slit-lamp photos to train models, which achieved good performance as well.
Anaemia
Anaemia is typically diagnosed by measuring Hb concentration through a blood assay test, which offered a quantitative variable as the ground truth. Some studies use anaemia diseases labels diagnosed by doctors. Haematocrit and red blood cell count have also been used as auxiliary variables in predicting anaemia. Mitani et al. [65] used CFPs, metadata (race or ethnicity, age, sex and blood pressure) and combined data to predict Hb concentration and diagnose anemia. The combined model showed better performance with Hb prediction (mean absolute error [MAE]=0.63g/dl), and achieved an AUC of 0.88 for detecting anemia and 0.95 for moderate anaemia. Rim et al. [1] also used CFPs to predict Hb, with an MAE of 0.79g/dl. UWF images have been used by Zhao et al. [66] to predict Hb and anaemia, achieving an MAE of 0.83g/dl and an AUC of 0.93 for anaemia. OCT images from anaemia patients and normal subjects were used by Chen et al. [67] and Wei et al. [68] to train classifiers, achieving accuracies of 0.8358 and 0.9865 respectively. Automated non-invasive anaemia screening based on retinal imaging represents a breakthrough, especially for individuals with conditions such as diabetes, where anaemia can increase morbidity and mortality risks [65, 69].
How AI Work with Retinal Imaging and Systemic Diseases Predicting?
Three Pathways Linking Retina and Body
AI-based retinal imaging has been intensively explored in predicting disorders across different systems or organs. To sum up, there are three pathways linking retina and body. First, when focusing on the onset of diseases such as etiology, pathogenesis and inducing factors, traditional risk factors like blood pressure, age, BMI, smoking, etc. can be used as predictors. Additionally, there are less understood or unidentified risk factors including genetic and inflammation factors. We can directly estimate the diseases risk factors, thereby linking these factors with different diseases. Second, we can predict established examination results that can be used to monitor or diagnose diseases, such as carotid ultrasound, cardiac computerized tomography, and MRI results. Retinal imaging offers a straightforward, non-invasive, and cost-effective alternative for replacing the biomarkers typically identified by these conventional methods. Third, we can directly estimate specific clinical diseases, distinguish between diseased and non-diseased individuals. Most notably, when adding metadata or risk factors into retinal imaging to develop hybrid algorithms, the predictive model showed improved effectiveness in predicting tasks.
New Parameters as a Bridge between Retina and Body
When we use AI-based retinal imaging to estimate risk factors or traditional biomarkers, we can also generate new variables derived from the predictive results. New variables originated from retinal images, such as Reti-Age, Reti-CKD, Reti-CAC, can be analyzed for their relationships with diseases outcomes. Another part of generating parameters is the direct retinal parameters, including caliber, geometry, fractals, bifurcation, tortuosity, and artery-vein nicking. While these parameters can be observed by ophthalmologists, they are better accessed and quantified by AI system for greater accuracy and subtlety, making these parameters quantitative and comparable. These direct and indirect variables extracted from retinal images using AI can be used to find correlations with clinical diseases through mathematical and statistical ways, rather than being limited to descriptive and qualitative analysis.
Current Retinal Imaging Predicting Future
With the availability of longitudinal data, it is possible to track the incidence of disease, mortality, and severe outcomes over time. By incorporating longitudinal data into AI model, we can predict the incidence and progression of diseases from baseline images. This enables risk stratification based on certain variables, which is particularly inspiring.
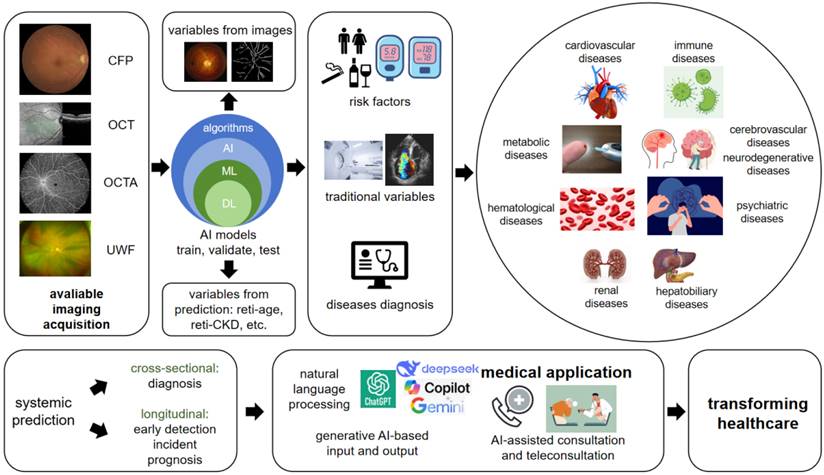
Figure 1 shows the ideas and workflow of current AI-based retinal imaging for systemic diseases prediction. Here we summarized how AI works in this study area, also provided thoughts on how to begin an AI-oculomics study.
Future Outlook and Considerations on AI-oculomics Studies
Challenges in Data
Despite remarkable advancements in this area, there are still many challenges and next steps to be determined. Firstly, the accuracy of AI predictions heavily relies on the quality and quantity of the available data. Limited or biased data may result in less accurate predictions. Additionally, AI models may struggle with rare or complex diseases that are underrepresented in the training sets. We need more real-world prospective studies. Another issue is, longitudinal data is much more limited compared to cross-sectional data. Large datasets containing ophthalmological imaging such as those from the UK biobank, Qatar biobank are still scarce. Local hospitals and health centers should be encouraged to enhance long-term and multi-modality data collection. This effort could significantly contribute to the global early detection and incidence and prognosis of systemic diseases. Khan et al. [70] reviewed the publicly available datasets for ophthalmological imaging, highlighting the need for more comprehensive datasets.
Currently, systemic biomarkers studied through CFPs are more common than those obtaied via OCT, OCTA and other novel modalities. There is a need for more algorithms to explore the feasibilities of these advanced imaging modalities. In addition, OCT can provide data on choroidal thickness, a crucial parameter reflecting choroidal circulation. However, this area is still an underexplored and requires more advanced imaging technologies like sweep source OCT (SS-OCT) for further investigation. We need to pay more attention to high-resolution instruments and reliable data.
The current study is mostly based on single image. It is still difficult to combine different modalities imaging into one model. In comparison with physicians' work, which typically use multi-modality imaging to achieve a comprehensive understanding of the disease, the AI's capability in this regard remains incomplete (Table 1).
The overview of how AI-enhanced retinal imaging predicting systemic diseases and the future considerations. Based on various modalities of available retinal imaging, through the pre-trained AI algorithms, we can predict risk factors, traditional variables and diseases diagnosis, which already applied in disorders from nearly all systems, including cardiovascular, metabolic, hematological, renal, hepatobiliary, psychiatric, cerebrovascular, neurodegenerative and immune diseases. The prediction includes not only diagnosis, but also early detection, incident and prognosis of the disorders based on the training on longitudinal data. The emerging new era of generative AI brings promising opportunities on medical application and healthcare transforming times. CFP: color fundus photo; OCT: optical coherence tomography; OCTA: optical coherence tomography angiography; UWF: ultra-wide field; AI: artificial intelligence; ML: machine learning; DL: deep learning.
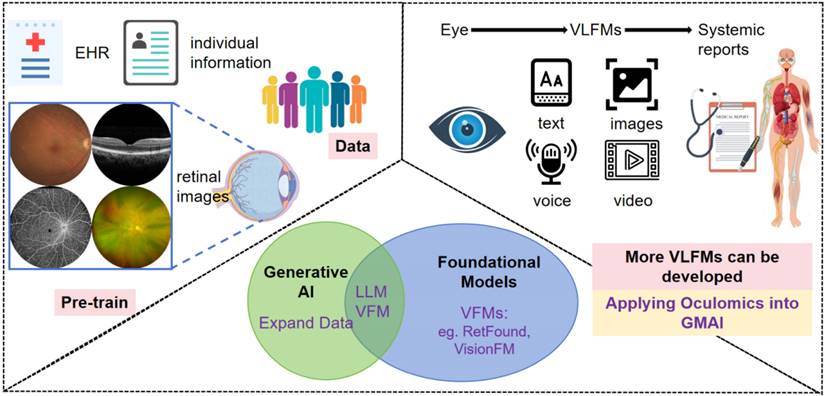
Systemic disorders diagnosed on eye images developed AI models. Previous AI algorithms trained on EHR, individual information, and retinal images of certain population, that sets good basics to the coming new AI models. With the development of generative AI, multimodality new VLFMs may contain text, voice, images and video, linking previous AI models into a friendly human-computer interaction mode, applying oculomics studies into the GMAI system, potentially transforming ophthalmology healthcare. EHR: electronic health record; AI: artificial intelligence; LLM: large language model; VFM: vision foundational models; VLFM: vision-language foundational models; GMAI: generalist medical artificial intelligence.
The current discussion and considerations on AI-oculomics studies. It is a double-edged sword, that the opportunities are in challenges, while challenges also imply the opportunities. We summarized the key points, giving a quick highlight of the discussion on the study.
| Challenges | Opportunities | |
|---|---|---|
| Data | Limited data, limited real-world studies in rare diseases, limited longitudinal cohort and comprehensive biobanks. | Call for more real-word studies in local hospitals and health centers. Physicians need the realization of biobank and data integration. Call for more consortium actions to combine data. |
| Limited modalities and we need more advanced imaging equipment involved. | Advanced imaging like SS-OCT, OCTA, UWF, Vis-OCT, PS-OCT, PD-OCT, AO-OCT, oximetry and etc. can be candidates in futural studies. Other functional examination also can be candidates in AI-based studies, such as microperimetry, visual acuity, intraocular pressure. | |
| Limited multi-modality algorithms. | The physicians' work are based on the comprehensive understanding of whole body. AI still has the potential to help with the process based on multi-modality work. | |
| Technology | Controversial results accuracy, interpretability, usability and reliability. | Call for more studies to strengthen oculomics concept through evidence, validation, mechanistic understanding and human-machine interaction testing. |
| No generative AI systems have been reviewed by FDA. | Call for more RCT clinical trials over the world. |
AI: artificial intelligence; SS-OCT: sweep source optical coherence tomography; OCTA: optical coherence tomography angiography; UWF: ultra-wide field; Vis-OCT: visible wavelength OCT; PS-OCT: polarization-sensitive OCT; PD-OCT: polarization-diversity OCT; AO-OCT: adaptive optics OCT; FDA: United States Food and Drug Administration; RCT: randomized controlled trial.
Challenges in Technology
Although AI can assist in detecting potential diseases or anomalies, it is crucial to involve human experts in reviewing and interpreting the results to ensure accurate diagnosis and appropriate treatment decisions. As we consider the future use of the AI technology, it is important to recognize that it will be used by a wide range of professionals, including ophthalmologists, optometrists, general physicians, neurologists, cardiologists, or patients themselves. The aim of this digital technology is to reduce the need for expensive complex examinations, providing an early detection method for diseases that is non-invasive, easy and cost-effective. However, the technology is still in the development stage, and more robust modeling is needed. The challenges of modeling also embody on integration of immense clinical and high throughput biological data, multi-task learning on disease diagnoses, therapy and outcome predictions and cost-effective computing capabilities. Hence, increasing the data by technological means, developing advanced multi-modal interpretative networks for prediction of multi-source ocular histology images, increasing the number of AI models for continuous learning and self-optimization, become much needed.
Future Directions with Generative AI and Foundation Models
Based on the previous findings in research, and numerous challenges and opportunities in data and technology. We propose that the novel technology in AI, including generative AI techniques and foundation models, can make up for the weaknesses in research and applications. These could give us inspirations in future directions and solve the existing problems. How these technologies apply into real medical situations and transform the healthcare? We explained in the following part and expressed our thoughts on how they could work in ophthalmology.
Recent years have witnessed significant advancements in the state of art AI technology. Generative AI includes a wide array of applications, including the generation of images, videos, text, sound, software codes, virtual environments, designs and even drug compound [71]. It is based on various techniques, including DL, generative adversarial networks (GAN), autoencoders, variational autoencoders and etc. These models can be utilized in retinal images research and provide robust support to address the limitations of current research methodologies. You et al. [72] conducted a survey on studies using GAN in ophthalmology image domain and introduced its performance on image synthesis and image-to-image translation. It helps extend datasets and modalities in research, particularly in scenarios with limited sample size and varying images quality and opens up new research possibilities through translational work.
Conversational large language model is a kind of generative AI, that makes sense of natural language. Large language model (LLM), including DeepSeek, GPT-o1 and 4o (OpenAI), LLaMA (Meta), PaLM (Google), BERT (Google), Gemini (Google), Copilot (Microsoft) and Claude (Anthropic) have recently drawn considerable interest from journalists, policymakers, and scholars across fields [73, 74]. By learning the probabilities of words sequences from extensive corpus of text, these models with billions of parameters, can respond to free-text queries without needing task-specific training [75].
LLMs have shown immense promise in the medicine field and are rapidly being integrated into clinical practice. In clinical situations, it can be applied to automatically communicate with patients, draft clinical notes, and even provide clinical suggestions. Due to their ability to handle free-text input, LLM-based tools can manage documents, recognize and extract information from text databases in clinical studies. Therefore, it is useful to build the relevant medical LLMs. The implements are not only in the human-machine interface, automatically interactions with data collection part and model/program users, but also in model/program developing procedures, being utilized to filter and administrate text parameters or labels and helping enhance and accelerate the multi-modality model inputting work.
Hence, we propose the clinical translation of generative AI-based input and output. 1) AI-assisted consultation: Nature language processing (NLP) framework combined with DL models, clinically relevant information and features can be extracted. Then, different AI-systemic prediction models can provide diagnostic systems for various systemic diseases and output medical recommendations. 2) AI-assisted teleconsultation. Currently, with AI-assisted consultation been used in different websites and APPs by chatbots, we have seen that there are already merchant products catering to the market. It is hoped that more generative AI approaches will make subjects more attractive and real experience. Recent SORA is also a breakthrough in video generation, which can be applied into human-computer interaction procedures (Figure 1).
RETFound, one of the first AI foundation models in healthcare, and the first in ophthalmology, was developed using millions of eye scans from the National Health Service (NHS) [76]. Zhou et al. [77] provided a generalizable solution that enables broad clinical AI applications from retinal imaging, predicting conditions such as ischaemic stroke, myocardial infarction, heart failure, PD, in addition to ophthalmic diseases. This foundation model has inspired more foundation models, AI-agents even artificial general intelligence developing in this area, especially vision-language foundational models (VLFM) that combine LLM, progressing the prediction work wider-use, more user friendly and competitive of usability [78]. The developed AI models will apply oculomics studies into the generalist medical artificial intelligence (GMAI) system [79], potentially transforming ophthalmology healthcare, which is much inspiring (Figure 2).
In addition, when we face formal clinical trials in following steps of AI models application in clinic, more ethical questions should be taken into consideration. Currently, no generative AI systems have been reviewed by the United States Food and Drug Administration (FDA) [71]. The problems contain data breach, privacy risks, medical responsibility, and supervision. Meanwhile, technically, it is not a totally correct system and still retains bias to some extent. The risks of misdiagnosis may bring more controversial problems (Table 1).
AI-oculomics Applications in Real World
We are thrilled to see emerging AI-related start-ups focusing on realizing the applications. It has been truly applying AI-oculomics studies' findings into practice. Singapore Eye Lesion Analyser (SELENA+) is a deep-learning AI software system that can detect potential threatening eye conditions accurately and efficiently [80]. It initially focused on diabetic retinopathy (DR) screening, then expanded to encompass the screening of multiple chronic diseases, especially in CVD. AIFUNDUS is another software that can detect DR and other retinal diseases. Combining with their health risk assessment system, it can provide health report related to CVD, metabolic diseases and nervous system diseases [81]. The EyeArt system [82] and LumineticsCore™ [83] are also AI diagnostic systems, maily focusing on DR.
There are still bunches of obstacles to face when the new start-ups make these technologies as products in our real life. Current bussiness modes are mostly business to business (B2B). The clients are mostly hospitals, pharmacy chains, optical chains, health screening outlets, and primary community healthcare settings. Apart from the lack of AI industrial standards and difficulty in registration and ethic review for AI products, whether doctors and patients can actually use the results report, and who will pay for the screening and detection under various coutries' healcare policies, are still difficulties that start-ups need to find ways to survive. Expanding the bussiness mode into the business to consumer (B2C) market with the launch of a dedicated app, offering personalized health insights and enhance customer engagement, still need a long way to explore.
However, as the global prevalence of chronic illnesses continues to rise, healthcare systems worldwide are increasingly challenged by escalating costs. Acting as an all-in-one (retina imaging) solution for the detection of multiple chronic diseases, we believe the existing and futural updating products will significantly streamline the screening process, eliminating the need for multiple tests. This not only boosts productivity and reduces costs but also expedites the delivery of critical results to patients.
Conclusion
Overall, while there are limitations, AI is continuously evolving and holds great potential in transforming healthcare. AI has the potential to accelerate existing forms of medical analysis, but its algorithms require further testing to be fully trusted. For now, the idea of an AI doctor independently making new diagnoses without human oversight remains a distant prospect - like decades away rather than years. The path toward a new AI-powered paradigm is needed.
With the emerging concept of “oculomics” and “retinomics” [84], more and more research are leveraging AI-derived methods to explore correlations between systemic biological factors and retina. The role of AI in retinal imaging for predicting systemic diseases is thus becoming more promising and sustainable.
Abbreviations
AD: Alzheimer's disease; AI: artificial intelligence; AUC: area under the curve; AUROC: area under the receiver operating characteristic; B2B: business to business; B2C: business to consumer; BMI: body mass index; CAC: coronary artery calcium; CeVD: cerebrovascular diseases; CFP: color fundus photograph; CKD: chronic kidney disease; CNN: convolutional neural networks; CNS: central nervous system; CRAE: central retinal arteriolar equivalent; CRVE: central retinal venular equivalent; CT: computerized tomography; CVD: cardiovascular diseases; DKD: diabetic kidney disease; DL: deep learning; DN: diabetic nephropathy; DPN: diabetic peripheral neuropathy; DR: diabetic retinopathy; eGFR: estimated glomerular filtration rate; ERG: electroretinography; FDA: Food and Drug Administration; GAN: generative adversarial networks; GMAI: generalist medical artificial intelligence; Hb: hemoglobin; HR: hazard ratio; IPL: inner plexiform layer; LLM: large language model; MACE: major adverse cardiovascular events; MAE: mean absolute error; MCI: mild cognitive impairment; ML: machine learning; MRI: magnetic resonance imaging; MS: multiple sclerosis; NHS: National Health Service; NLP: nature language processing; OCT: optical coherence tomography; OCTA: OCT angiography; ORs: odds ratios; PD: Parkinson's disease; RGCs: retinal ganglion cells; RNFL: retinal nerve fiber layer; SELENA+: Singapore Eye Lesion Analyser; SIVA-DLS: Singapore "I" vessel assessment-deep learning system; SS-OCT: sweep source OCT; UWF: ultra-wide field; VLFM: vision-language foundational models; WMLs: white matter lesions.
Acknowledgements
This study was funded by National Key R & D Program of China (2022YFC2502800), National Natural Science Fund of China (82388101) and Beijing Natural Science Foundation (IS23096).
Contributions
Conceptualization: Andrzej Grzybowski, Tien Yin Wong; Writing - original draft preparation: Jinyuan Wang, Yuchen Liu; Writing - review and editing: Ya Xing Wang, Dian Zeng, Zhuoting Zhu, Dawei Li, Bin Sheng, Andrzej Grzybowski, Tien Yin Wong; Funding acquisition: Tien Yin Wong; Supervision: Tien Yin Wong.
Supplementary Material
Supplementary table.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Rim TH, Lee G, Kim Y, Tham YC, Lee CJ, Baik SJ. et al. Prediction of systemic biomarkers from retinal photographs: development and validation of deep-learning algorithms. Lancet Digit Health. 2020;2:e526-e36
2. London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44-53
3. Wu JH, Liu TYA. Application of Deep Learning to Retinal-Image-Based Oculomics for Evaluation of Systemic Health: A Review. J Clin Med. 2022;12:152
4. Gunn R. Ophthalmoscopic evidence of (1) arterial changes associated with chronic renal disease, and (2) of increased arterial tension. Transactions of the Ophthalmological Society of the United Kingdom. 1892;12:124-5
5. Grzybowski A. Comment on: An eye on the brain: Adding insight to injury. Am J Ophthalmol. 2024;261:208
6. Galezowski X. Etude ophtalmoscopique sur les altérations du nerf optique et les maladies cérébrales dont elles dépendent. Paris: Librairie de L Leclerc. 1866.
7. Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer's disease. N Engl J Med. 1986;315:485-7
8. Tsai CS, Ritch R, Schwartz B, Lee SS, Miller NR, Chi T. et al. Optic nerve head and nerve fiber layer in Alzheimer's disease. Arch Ophthalmol. 1991;109:199-204
9. Curcio CA, Drucker DN. Retinal ganglion cells in Alzheimer's disease and aging. Ann Neurol. 1993;33:248-57
10. Goto I, Katsuki S, Ikui H, Kimoto K, Mimatsu T. Pathological studies on the intracerebral and retinal arteries in cerebrovascular and noncerebrovascular diseases. Stroke. 1975;6:263-9
11. Grzybowski A BP, editors. OCT and imaging in central nervous system diseases. Cham (Switzerland): Springer. 2020
12. Wong TY. Is retinal photography useful in the measurement of stroke risk? Lancet Neurol. 2004;3:179-83
13. Cheung CY, Mok V, Foster PJ, Trucco E, Chen C, Wong TY. Retinal imaging in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2021;92:983-94
14. Snyder PJ, Alber J, Alt C, Bain LJ, Bouma BE, Bouwman FH. et al. Retinal imaging in Alzheimer's and neurodegenerative diseases. Alzheimers Dement. 2021;17:103-11
15. https://www.coursera.org/articles/what-is-artificial-intelligence/
16. https://www.coursera.org/articles/what-is-machine-learning/
17. https://www.coursera.org/articles/what-is-deep-learning/
18. Ramesh AN, Kambhampati C, Monson JR, Drew PJ. Artificial intelligence in medicine. Ann R Coll Surg Engl. 2004;86:334-8
19. Poplin R, Varadarajan AV, Blumer K, Liu Y, McConnell MV, Corrado GS. et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat Biomed Eng. 2018;2:158-64
20. Dilsizian SE, Siegel EL. Artificial intelligence in medicine and cardiac imaging: harnessing big data and advanced computing to provide personalized medical diagnosis and treatment. Curr Cardiol Rep. 2014;16:441
21. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P. et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441-6
22. Arnould L, Meriaudeau F, Guenancia C, Germanese C, Delcourt C, Kawasaki R. et al. Using Artificial Intelligence to Analyse the Retinal Vascular Network: The Future of Cardiovascular Risk Assessment Based on Oculomics? A Narrative Review. Ophthalmol Ther. 2023;12:657-74
23. Wagner SK, Fu DJ, Faes L, Liu X, Huemer J, Khalid H. et al. Insights into Systemic Disease through Retinal Imaging-Based Oculomics. Transl Vis Sci Technol. 2020;9:6
24. Li H, Cao J, Grzybowski A, Jin K, Lou L, Ye J. Diagnosing Systemic Disorders with AI Algorithms Based on Ocular Images. Healthcare (Basel). 2023;11:1739
25. Ranchod TM. Systemic retinal biomarkers. Curr Opin Ophthalmol. 2021;32:439-44
26. Betzler BK, Rim TH, Sabanayagam C, Cheng CY. Artificial Intelligence in Predicting Systemic Parameters and Diseases From Ophthalmic Imaging. Front Digit Health. 2022;4:889445
27. Iao WC, Zhang W, Wang X, Wu Y, Lin D, Lin H. Deep Learning Algorithms for Screening and Diagnosis of Systemic Diseases Based on Ophthalmic Manifestations: A Systematic Review. Diagnostics (Basel). 2023;13:900
28. Al-Halafi AM. Applications of artificial intelligence-assisted retinal imaging in systemic diseases: A literature review. Saudi J Ophthalmol. 2023;37:185-92
29. Grzybowski A, Jin K, Zhou J, Pan X, Wang M, Ye J. et al. Retina Fundus Photograph-Based Artificial Intelligence Algorithms in Medicine: A Systematic Review. Ophthalmol Ther. 2024;13:2125-49
30. Rim TH, Lee CJ, Tham YC, Cheung N, Yu M, Lee G. et al. Deep-learning-based cardiovascular risk stratification using coronary artery calcium scores predicted from retinal photographs. Lancet Digit Health. 2021;3:e306-e16
31. Cheung CY, Xu D, Cheng CY, Sabanayagam C, Tham YC, Yu M. et al. A deep-learning system for the assessment of cardiovascular disease risk via the measurement of retinal-vessel calibre. Nat Biomed Eng. 2021;5:498-508
32. Pachade S, Coronado I, Abdelkhaleq R, Yan J, Salazar-Marioni S, Jagolino A. et al. Detection of Stroke with Retinal Microvascular Density and Self-Supervised Learning Using OCT-A and Fundus Imaging. J Clin Med. 2022;11:7408
33. Rudnicka AR, Welikala R, Barman S, Foster PJ, Luben R, Hayat S. et al. Artificial intelligence-enabled retinal vasculometry for prediction of circulatory mortality, myocardial infarction and stroke. Br J Ophthalmol. 2022;106:1722-9
34. Qu Y, Zhuo Y, Lee J, Huang X, Yang Z, Yu H. et al. Ischemic and haemorrhagic stroke risk estimation using a machine-learning-based retinal image analysis. Front Neurol. 2022;13:916966
35. Hong J, Yoon S, Shim KW, Park YR. Screening of Moyamoya Disease From Retinal Photographs: Development and Validation of Deep Learning Algorithms. Stroke. 2024;55:715-24
36. Zhu Z, Hu W, Chen R, Xiong R, Wang W, Shang X. et al. Retinal age gap as a predictive biomarker of stroke risk. BMC Med. 2022;20:466
37. Shu L, Zhong K, Chen N, Gu W, Shang W, Liang J. et al. Predicting the severity of white matter lesions among patients with cerebrovascular risk factors based on retinal images and clinical laboratory data: a deep learning study. Front Neurol. 2023;14:1168836
38. Cheung CY, Ran AR, Wang S, Chan VTT, Sham K, Hilal S. et al. A deep learning model for detection of Alzheimer's disease based on retinal photographs: a retrospective, multicentre case-control study. Lancet Digit Health. 2022;4:e806-e15
39. Cheung CY, Wong WLE, Hilal S, Kan CN, Gyanwali B, Tham YC. et al. Deep-learning retinal vessel calibre measurements and risk of cognitive decline and dementia. Brain Commun. 2022;4:fcac212
40. Nunes A, Silva G, Duque C, Januário C, Santana I, Ambrósio AF. et al. Retinal texture biomarkers may help to discriminate between Alzheimer's, Parkinson's, and healthy controls. PLoS One. 2019;14:e0218826
41. Cavaliere C, Vilades E, Alonso-Rodríguez MC, Rodrigo MJ, Pablo LE, Miguel JM. et al. Computer-Aided Diagnosis of Multiple Sclerosis Using a Support Vector Machine and Optical Coherence Tomography Features. Sensors (Basel). 2019;19:5323
42. Wang X, Jiao B, Liu H, Wang Y, Hao X, Zhu Y. et al. Machine learning based on Optical Coherence Tomography images as a diagnostic tool for Alzheimer's disease. CNS Neurosci Ther. 2022;28:2206-17
43. Montolío A, Martín-Gallego A, Cegoñino J, Orduna E, Vilades E, Garcia-Martin E. et al. Machine learning in diagnosis and disability prediction of multiple sclerosis using optical coherence tomography. Comput Biol Med. 2021;133:104416
44. Wisely CE, Richardson A, Henao R, Robbins CB, Ma JP, Wang D. et al. A Convolutional Neural Network Using Multimodal Retinal Imaging for Differentiation of Mild Cognitive Impairment from Normal Cognition. Ophthalmol Sci. 2024;4:100355
45. Appaji A, Harish V, Korann V, Devi P, Jacob A, Padmanabha A. et al. Deep learning model using retinal vascular images for classifying schizophrenia. Schizophr Res. 2022;241:238-43
46. Atagun MI, Sonugur G, Yusifova A, Celik I, Ugurlu N. Machine learning algorithms revealed distorted retinal vascular branching in individuals with bipolar disorder. J Affect Disord. 2022;315:35-41
47. Manjur SM, Hossain MB, Constable PA, Thompson DA, Marmolejo-Ramos F, Lee IO. et al. Detecting Autism Spectrum Disorder Using Spectral Analysis of Electroretinogram and Machine Learning: Preliminary results. Annu Int Conf IEEE Eng Med Biol Soc. 2022;2022:3435-8
48. Sabanayagam C, Xu D, Ting DSW, Nusinovici S, Banu R, Hamzah H. et al. A deep learning algorithm to detect chronic kidney disease from retinal photographs in community-based populations. Lancet Digit Health. 2020;2:e295-e302
49. Kang EY, Hsieh YT, Li CH, Huang YJ, Kuo CF, Kang JH. et al. Deep Learning-Based Detection of Early Renal Function Impairment Using Retinal Fundus Images: Model Development and Validation. JMIR Med Inform. 2020;8:e23472
50. James G, Mohankumar G, Cooper AJ. et al. Predicting Renal Disease and Associated Complications Through Deep Learning Using Retinal Fundus Images Linked to Clinical Data. SSRN Electronic Journal. 2021
51. Zhang K, Liu X, Xu J, Yuan J, Cai W, Chen T. et al. Deep-learning models for the detection and incidence prediction of chronic kidney disease and type 2 diabetes from retinal fundus images. Nat Biomed Eng. 2021;5:533-45
52. Shi S, Gao L, Zhang J, Zhang B, Xiao J, Xu W. et al. The automatic detection of diabetic kidney disease from retinal vascular parameters combined with clinical variables using artificial intelligence in type-2 diabetes patients. BMC Med Inform Decis Mak. 2023;23:241
53. Betzler BK, Chee EYL, He F, Lim CC, Ho J, Hamzah H. et al. Deep learning algorithms to detect diabetic kidney disease from retinal photographs in multiethnic populations with diabetes. J Am Med Inform Assoc. 2023;30:1904-14
54. An S, Vaghefi E, Yang S, Xie L, Squirrell D. Examination of alternative eGFR definitions on the performance of deep learning models for detection of chronic kidney disease from fundus photographs. PLoS One. 2023;18:e0295073
55. Liu Y, Zhang F, Gao X, Liu T, Dong J. Lesion-aware attention network for diabetic nephropathy diagnosis with optical coherence tomography images. Front Med (Lausanne). 2023;10:1259478
56. Zhang S, Chen R, Wang Y, Hu W, Kiburg KV, Zhang J. et al. Association of Retinal Age Gap and Risk of Kidney Failure: A UK Biobank Study. Am J Kidney Dis. 2023;81:537-44.e1
57. Joo YS, Rim TH, Koh HB, Yi J, Kim H, Lee G. et al. Non-invasive chronic kidney disease risk stratification tool derived from retina-based deep learning and clinical factors. NPJ Digit Med. 2023;6:114
58. Zhang L, Yuan M, An Z, Zhao X, Wu H, Li H. et al. Prediction of hypertension, hyperglycemia and dyslipidemia from retinal fundus photographs via deep learning: A cross-sectional study of chronic diseases in central China. PLoS One. 2020;15:e0233166
59. Benson J, Estrada T, Burge M, Soliz P. Diabetic Peripheral Neuropathy Risk Assessment using Digital Fundus Photographs and Machine Learning. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:1988-91
60. Al-Absi HRH, Pai A, Naeem U, Mohamed FK, Arya S, Sbeit RA. et al. DiaNet v2 deep learning based method for diabetes diagnosis using retinal images. Sci Rep. 2024;14:1595
61. Yun JS, Kim J, Jung SH, Cha SA, Ko SH, Ahn YB. et al. A deep learning model for screening type 2 diabetes from retinal photographs. Nutr Metab Cardiovasc Dis. 2022;32:1218-26
62. Xiang Y, Shujin L, Hongfang C, Yinping W, Dawei Y, Zhou D. et al. Artificial Intelligence-Based Diagnosis of Diabetes Mellitus: Combining Fundus Photography with Traditional Chinese Medicine Diagnostic Methodology. Biomed Res Int. 2021;2021:5556057
63. Zhu Z, Liu D, Chen R, Hu W, Liao H, Kiburg K. et al. The Association of Retinal age gap with metabolic syndrome and inflammation. J Diabetes. 2023;15:237-45
64. Xiao W, Huang X, Wang JH, Lin DR, Zhu Y, Chen C. et al. Screening and identifying hepatobiliary diseases through deep learning using ocular images: a prospective, multicentre study. Lancet Digit Health. 2021;3:e88-e97
65. Mitani A, Huang A, Venugopalan S, Corrado GS, Peng L, Webster DR. et al. Detection of anaemia from retinal fundus images via deep learning. Nat Biomed Eng. 2020;4:18-27
66. Zhao X, Meng L, Su H, Lv B, Lv C, Xie G. et al. Deep-Learning-Based Hemoglobin Concentration Prediction and Anemia Screening Using Ultra-Wide Field Fundus Images. Front Cell Dev Biol. 2022;10:888268
67. Chen Z, Mo Y, Ouyang P, Shen H, Li D, Zhao R. Retinal vessel optical coherence tomography images for anemia screening. Med Biol Eng Comput. 2019;57:953-66
68. Wei H, Shen H, Li J, Zhao R, Chen Z. AneNet: A lightweight network for the real-time anemia screening from retinal vessel optical coherence tomography images. Optics & Laser Technology. 2021;136:106773
69. Tham YC, Cheng CY, Wong TY. Detection of anaemia from retinal images. Nat Biomed Eng. 2020;4:2-3
70. Khan SM, Liu X, Nath S, Korot E, Faes L, Wagner SK. et al. A global review of publicly available datasets for ophthalmological imaging: barriers to access, usability, and generalisability. Lancet Digit Health. 2021;3:e51-e66
71. Duffourc M, Gerke S. Generative AI in Health Care and Liability Risks for Physicians and Safety Concerns for Patients. Jama. 2023;330:313-4
72. You A, Kim JK, Ryu IH, Yoo TK. Application of generative adversarial networks (GAN) for ophthalmology image domains: a survey. Eye Vis (Lond). 2022;9:6
73. Bowman S R. Eight Things to Know about Large Language Models. arXiv:2304.00612v1 [cs.CL]. 2023
74. Shah NH, Entwistle D, Pfeffer MA. Creation and Adoption of Large Language Models in Medicine. Jama. 2023;330:866-9
75. Thirunavukarasu AJ, Ting DSJ, Elangovan K, Gutierrez L, Tan TF, Ting DSW. Large language models in medicine. Nat Med. 2023;29:1930-40
77. Zhou Y, Chia MA, Wagner SK, Ayhan MS, Williamson DJ, Struyven RR. et al. A foundation model for generalizable disease detection from retinal images. Nature. 2023;622:156-63
78. He Y, Huang F, Jiang X. et al. Foundation Model for Advancing Healthcare: Challenges, Opportunities, and Future Directions. arXiv:2404.03264v1 [cs.CY]. 2024
79. Moor M, Banerjee O, Abad ZSH, Krumholz HM, Leskovec J, Topol EJ. et al. Foundation models for generalist medical artificial intelligence. Nature. 2023;616:259-65
80. https://www.synapxe.sg/healthtech/health-ai/selena/
82. https://www.eyenuk.com/en/products/eyeart/
83. https://www.digitaldiagnostics.com/products/eye-disease/lumineticscore/
84. https://www.medrxiv.org/content/10.1101/2023.07.31.23293176v1.full.pdf
Author contact
![]() Corresponding author: Tien Yin Wong, wongtienyinedu.cn.
Corresponding author: Tien Yin Wong, wongtienyinedu.cn.
 Global reach, higher impact
Global reach, higher impact