13.3
Impact Factor
Theranostics 2023; 13(11):3524-3549. doi:10.7150/thno.83383 This issue Cite
Review
Intrinsic mechanism and pharmacologic treatments of noise-induced hearing loss
1. Materdicine Lab, School of Life Sciences, Shanghai University, Shanghai, China.
2. Department of Otolaryngology-Head and Neck Surgery, Shanghai Ninth People's Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
3. Ear Institute, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
4. Shanghai Key Laboratory of Translational Medicine on Ear and Nose Diseases, Shanghai, China.
Received 2023-2-9; Accepted 2023-6-12; Published 2023-6-19
Abstract
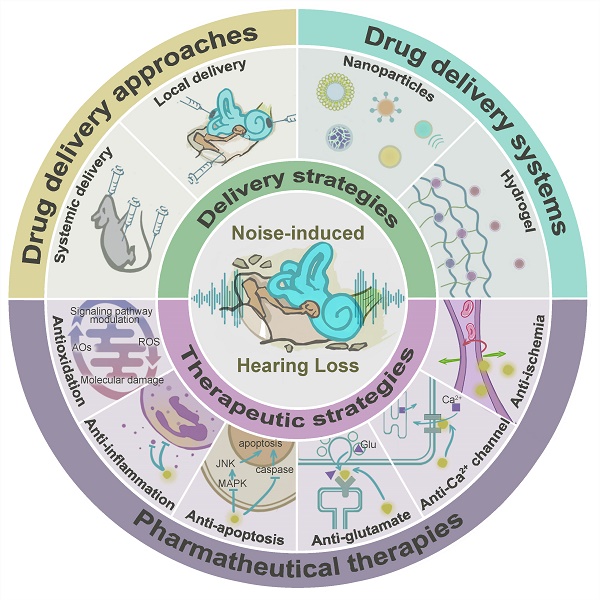
Noise accounts for one-third of hearing loss worldwide. Regretfully, noise-induced hearing loss (NIHL) is deemed to be irreversible due to the elusive pathogenic mechanisms that have not been fully elucidated. The complex interaction between genetic and environmental factors, which influences numerous downstream molecular and cellular events, contributes to the NIHL. In clinical settings, there are no effective therapeutic drugs other than steroids, which are the only treatment option for patients with NIHL. Therefore, the need for treatment of NIHL that is currently unmet, along with recent progress in our understanding of the underlying regulatory mechanisms, has led to a lot of new literatures focusing on this therapeutic field. The emergence of novel technologies that modify local drug delivery to the inner ear has led to the development of promising therapeutic approaches, which are currently under clinical investigation. In this comprehensive review, we focus on outlining and analyzing the basics and potential therapeutics of NIHL, as well as the application of biomaterials and nanomedicines in inner ear drug delivery. The objective of this review is to provide an incentive for NIHL's fundamental research and future clinical translation.
Keywords: noise-induced hearing loss, pharmacologic strategies, drug delivery systems, nanoparticles
Introduction
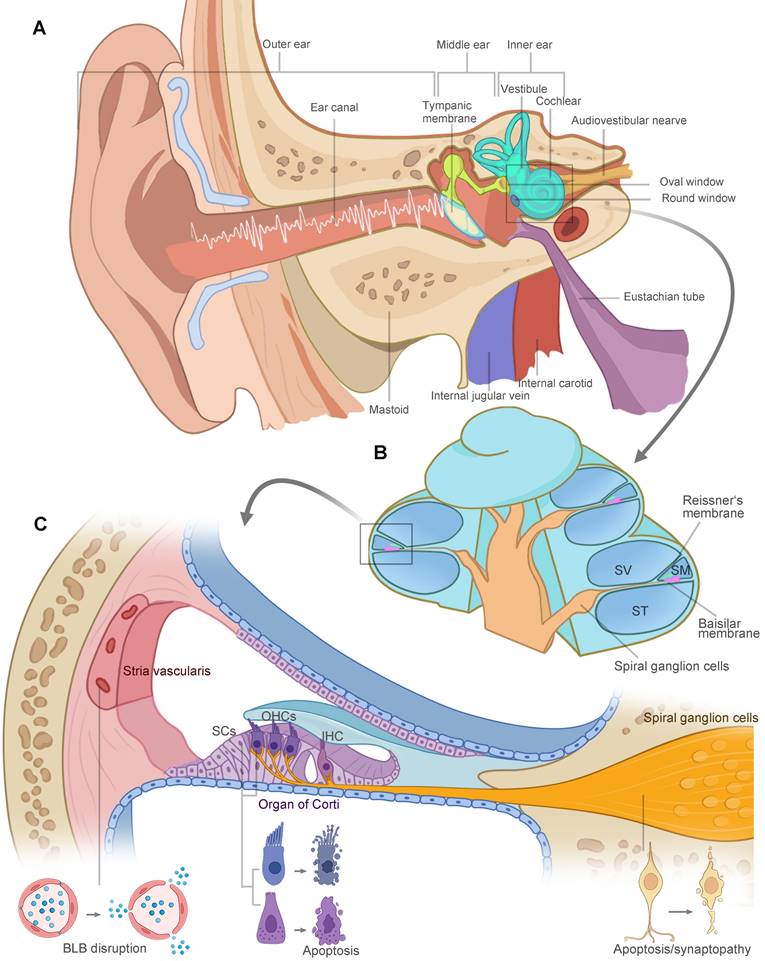
The vertebrate inner ear is an exquisite design and sensitive sensory organ of serving both the hearing (cochlea) and balance (vestibular) functions. Anatomically, the structures of the ear are divided into three parts: the outer, middle, and inner ear, illustrated in Figure 1A. Sound waves enter the ear canal and travel through the tympanic membrane and the ossicular chain into the fluid-filled cochlea. As shown in Figure 1B, the cochlea is divided into three fluid-filled membranous tubes that coil around the modiolus: the scala tympani, scala vestibuli, and scala media. The sound creates a traveling wave along the basilar membrane, which houses the mechanosensitive hair cells, including three rows of outer hair cells (OHCs) and one row of inner hair cells (IHCs) located in the organ of Corti. OHCs function as a cochlear amplifier and IHCs are responsible for electrical signal transmission to the spiral ganglion neurons (SGNs). Long-term or intense noise exposures beyond safe limits lead to irreversible loss of hair cells, SGNs and other cochlear cell types in the lateral wall, damaging three functional portions of the cochlea (Figure 1C): (1) the organ of Corti for mechanotransduction, (2) the SGNs for electrical signal transmission, and (3) the stria vascularis in charge of the inner ear homeostasis. The noise-related damage to the auditory system and the resulting sensory deafness is termed noise-induced hearing loss (NIHL).
In the last few years, several facts have drawn wide attention, which are also key points that make NIHL different from other sensorineural hearing loss (SNHL). First, we live together with sound or noise in various social and recreational activities. The increasing prevalence of hearing loss is attributed to the audio devices, venues, and events that emit loud music or recreational sounds. According to the World Health Organization, teenagers or young people are the most likely to develop NIHL, with one billion people aged 12 to 35 years at risk [1]. The escalating trend in NIHL should prompt changes in the age structure and risk factors. It also encourages personal awareness of safe listening behavior and the need for a new standard for recreational places and events. Second, NIHL has unexpected auditory and non-auditory consequences, which pose an elusive threat to our overall health. Light noise may damage synaptic connections between IHCs and type I SGNs without hair cell loss [2]. This is speculated to be connected with “hidden” hearing loss, which is shown by tinnitus, loudness intolerance, or a deficit in hearing under background noise [3]. In addition, chronic and repeated exposures activate the body's stress response system and are linked with a higher risk of developing cardiovascular diseases [4] and cognitive issues [5]. Recent studies have highlighted the potential role of chronic noise exposure in the development of Alzheimer's disease [6]. Third, excitotoxicity to hair cells and neurocytes which involves calcium overload and glutamate neurotoxicity is likely to be one distinct pathophysiology of NIHL as compared to age or drug-related hearing loss. Therefore, current studies of pharmacotherapy with the majority merely focusing on antioxidants should benefit from multiple therapeutic targets.
Currently, the primary methods of prevention of NIHL rely on noise control and hearing protection devices. Hearing aids are the most effective means of restoring auditory perception in patients with mild to moderate hearing loss [7], and cochlear implants are the most effective in patients with severe to profound hearing loss [8]. However, it should be noted that hearing protection devices are not suitable for all occasions, and are sometimes not carefully chosen and used in accordance with personal needs. Varied patient outcomes and difficulty in speech perception in noise are typical challenges for cochlear implants [8]. Numerous researchers are exploring pharmacologic therapies to prevent, interrupt, or treat NIHL. It is known that cochlear sensory cells in mammals do not possess regeneration ability, making hair cell loss irreversible. However, there is a complex interplay between the cellular and molecular mechanisms of damage and repair, before permanent sensorineural damage sets in. Theoretically, it provides not only a precious therapeutic window for intervention but also a variety of molecular targets against NIHL.
Moderate progress has been achieved in this field. Yet there are no FDA-approved drugs for the treatment of NIHL. The only therapeutic drug option in clinical settings is the off-label use of steroids, either through oral administration or intratympanic injection, due to their known anti-inflammatory effect. Unfortunately, the reported efficacy of steroid therapy is not ideal. This situation has led to numerous of preclinical studies regarding novel drugs and strategies for alleviating hearing impairment. Studies have revealed the highly sophisticated and intertwined cellular and molecular mechanisms underlying the pathogenesis of NIHL. What factors contribute to the pathogenesis of cochlear noise damage? How do various biochemical events, like oxidative stress, inflammation, metabolic damage, Ca2+ overload and glutamate neurotoxicity, work together? We have been constantly evolving our understanding, and answers to these questions have become foundations for pharmacological interventions. More and more molecular agents have shown reliable therapeutic efficacy, and some have been tested in clinical trials [9].
The unique isolated anatomical position of the inner ear and the presence of the blood-labyrinth barrier (BLB) make inner ear therapy particularly challenging. Different ways to deliver inner ear drugs, such as systemic and local administration, have varied advantages and challenges. Multiple strategies have already been used to maintain the high permeation of drug molecules through the round window membrane (RWM), but researchers are having difficulty opening the BLB in a systemic delivery. Among them, targeted and responsive drug delivery systems have been highlighted with their current utility and future potential in NIHL therapy.
In this review, we aim to summarize and discuss recent comprehension of the pathological mechanisms underlying NIHL, based on which multiple pharmaceutical explorations have been performed in rodents. The advancing understanding of drug pharmacokinetics across different delivery routes, and the progress in drug delivery systems with targeted and responsive drug delivery capabilities will be analyzed, therefore advancing our understanding of NIHL prevention and treatment. In the end, challenges and opportunities in some critical aspects of NIHL drug therapy are suggested.
The anatomy of the human ear. (A) Schematic representation of ear anatomy. The ear consists of three parts: the outer, middle, and inner ear. The outer and middle ear are separated by the tympanic membrane. Sound waves are transduced through tympanic membrane to the chain of three tiny bones (the ossicles) in middle ear cavity, which is attached to the oval window membrane, leading to fluid vibrations in the inner ear. The inner ear is responsible for hearing (cochlea) and balance (vestibule). (B) The cochlea is divided into three fluid-filled membranous tube coiling around the modiolus: the scala tympani, scala vestibuli, and scala media. The fluid vibrations create a traveling wave along the basilar membrane, in which hair cells generate the electrical signals and pass them to the spiral ganglion cells. (C) High-intensity sound waves travel through the cochlear duct, causing damage to three key functional areas: the organ of Corti, the SGNs and the stria vascularis. Hair cells, supporting cells and SGNs go through morphological changes and eventual apoptosis in extreme cases. Loss of tight junctions, malfunction of endothelial cells and surrounding cell types contribute to the BLB disruption. Abbreviations: SV: scala vestibuli; SM: scala media; ST: scala tympani; SCs: supporting cells; OHCs: outer hair cells; IHC: inner hair cell; BLB: blood-labyrinth barrier. Created with BioRender (www.biorender.com).
Underlying mechanisms and therapeutic strategies
The auditory system is impacted by two distinct types of acoustic damage: (1) The bioelectrical signal transmission pathway is damaged by acoustic noise. The tympanic membrane, ossicular chain and basement membrane that conduct sound waves can be torn by direct mechanical trauma, often seen in a blast exposure. But in most cases, the hearing loss is rather long-term and progressive, characterized by impairment to the organ of Corti and auditory nerve. They are critical for the mechanotransduction of sound signals and the subsequent transmission of those signals to our brain to be ultimately recognized and understood [10]. Moderate-intensity (85 to 96 dB SPL) exposures may lead to temporary threshold shift (TTS) that resolves over days or weeks. However, if the ascending auditory pathway is cut off by the massive loss of hair cells and SGNs after high-intensity noise exposures, patients develop permanent threshold shift (PTS), which is an irreversible hearing impairment. (2) The inner ear homeostasis is disturbed. The chemical and electrical homeostasis in the cochlea is largely maintained by stria vascularis. The unique structure rich in blood vessels serves not only as a gatekeeper for the substance exchanges between the circulation and the inner ear but also as a battery to promote endocochlear potential (EP) formation. Both are essential for normal hair cell function [11]. Prier studies have demonstrated significant noise-related abnormalities in EP maintenance, cochlear microcirculation, and integrity of the BLB [12]. More detailed structural investigations into stria vascularis revealed tight junction loss between endothelial cells and morphological changes of pericytes and other cell types [13].
As shown in Figure 2, those abnormalities in the auditory system stem from a complex interplay between a number of biochemical events. The reactive oxygen species (ROS) overproduction can be initiated by ischemia reperfusion injury, and mediate a series of signaling pathways involving inflammation and metabolic damage. Some events such as Ca2+ overload and glutamate neurotoxicity are pathogenic contributors independent from ROS generation, and they usually prefer certain cell types including hair cells and SGNs. After the initial burst of signal cascade is the subsequent DNA and organelle damages, triggering ultimate cell necrosis or programmed cell death. Therefore, the dominant pathogenic events depend on disease stages and cellular types, which is worth additional consideration during therapy design.
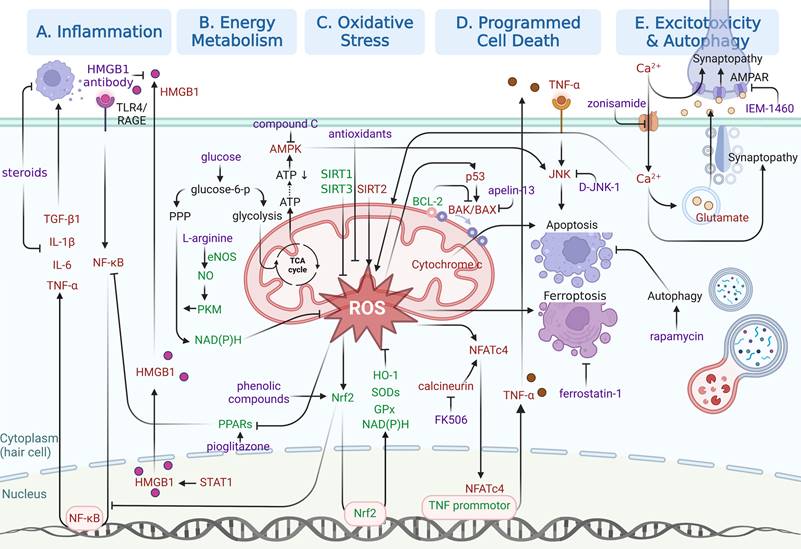
In this section, emphasis will be laid on molecular pathways responsible for NIHL pathogenesis, especially those identified in the last five years. As shown in Figure 3, specific molecular pathways as potential pharmacological targets are highlighted in an attempt to inspire NIHL preclinical drug development. Three kinds of drugs for pharmacological interventions are included in our discussion, including small molecule drugs, biomacromolecules and functional oligonucleotides.
Illustration of the contributing factors to NIHL and their interactions with each other. Noise-related disfunction of the stria vascularis leads to ischemia reperfusion injury, which increases the level of ROS in the cochlea. The overproduction of ROS and cochlear inflammation are two biochemical events that promote each other. With excessive calcium influx triggering the glutamate neurotoxicity at the ribbon synapses, both hair cells and the synaptic structures are damaged. Intrinsic or extrinsic pathways of apoptosis are activated by the resulting injuries. Abbreviations: ROS: reactive oxygen species; GluR: glutamate receptor.
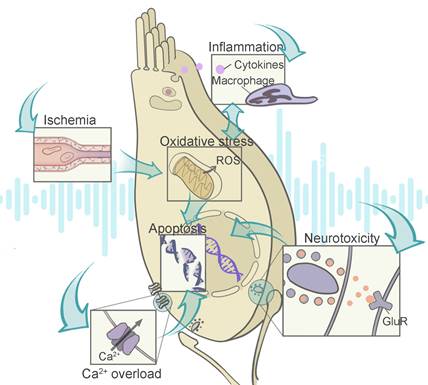
Illustration of relatively recent findings on molecular pathways underlying NIHL as potential therapeutic opportunities. Signaling pathways involved in (A) inflammation, (B) energy metabolism, (C) oxidative stress, (D) programmed cell death and (E) excitotoxicity and autophagy are illustrated in separated regions. Protective responses are shown in green. Adverse cellular events are shown in red. Pharmacological interventions are shown in purple. Created with BioRender (www.biorender.com).
Oxidative stress and antioxidative therapies
Following PTS-producing noise, ROS overproduction took place right after the insult, reached a maximum at 7 to 10 days and then declined, indicating a ROS accumulating stage for potential therapeutic intervention [14]. There is no sufficient evidence that oxidative stress is involved in the late stage of NIHL. The overproduction of ROS originates from multiple sources. Intracellular calcium overload, stimulated by noise exposure, leads to loss of mitochondrial membrane potential and leakage of ROS into the cytoplasm [15]. Upregulation of NADPH oxidase, reduced cochlear blood flow and ischemic/reperfusion damage all give rise to initial ROS production [16-18]. Numerous downstream factors in ROS signaling mediating inflammation or apoptosis further exacerbate ROS generation in a vicious cycle. For example, BAX and BAD are pro-apoptotic proteins that decrease the mitochondrial potential and cause more ROS to be released [19]. When excessive ROS generation is far beyond what the antioxidant system can neutralize, it can lead to morphological nuclear changes [20], DNA damage [21], lipid peroxidation [22], and ultimately necrosis or apoptosis. Furthermore, it has been demonstrated to directly induce apoptosis through the MAPK-c-JNK pathway [23].
Since ROS generation is a significant contributor to noise-induced cochlear damage, a typical therapeutic intervention involves neutralizing or suppressing its generation through antioxidant supplements. There are some intrinsic antioxidant molecules with in vivo production (e.g., glutathione [24], N-acetylcysteine(NAC) [25], and ebselen [26]). Others are exogeneous antioxidant supplements including resveratrol [27], d-methionine [28,29], vitamin C [30] and coenzyme Q10 [31]. NAC, which is a precursor of glutathione, is the most studied antioxidant to reduce acoustic damage in both rodent models and human studies [25,32-34]. The efficacy of this drug varies among studies, but it is still the only studied molecule that has entered a phase 3 clinical trial [35]. Herbal products from traditional medicine are one of the main sources of drug discovery. Several herbal-derived compounds (e.g., resveratrol [27], curcumin [36], and baicalein [37]) have been investigated for their antioxidant capability in NIHL animal models. Notably, many of those herbal-derived compounds are reported to have multiple targets simultaneously including oxidative stress, inflammation, apoptosis and neurodegeneration [38], but the details of their mechanisms are often not fully elucidated. For other concerns, bioavailability differs among antioxidants, and some antioxidants react with only a few forms of free radicals, or may throw innate stress-protective response out of balance [39].
Another approach is to start by revealing ROS signaling pathways involved in the pathogenesis of NIHL, either protective or devastating. Drugs or functional oligonucleotides targeting those pathways are then investigated (Figure 3C). ROS are essential for maintaining normal cell homeostasis. They function as critical modulators of stress-protective response after a mild noise insult, trying to restore reducing equivalents. Sound conditioning is a great example of using mild exposures to prepare innate protective systems for more severe stress [40]. Only when noise exposure has reached a damaging level does ROS trigger a down-stream cascade of oxidative stress and other adverse cellular events. In those cases, the devastating consequences are more likely the result of an overwhelming oxidative stress over stress-protective response. It is difficult to determine which strategy performs better, motivating the innate protective mechanism of ROS, or suppressing pathways to adverse cellular events. NRF2/HO1 pathway was thought to be a major part of the defense system against NIHL [41]. In response to oxidative stress, nuclear factor erythroid2-related factor 2 (NRF2) transcriptionally upregulates the expression of innate antioxidants (heme oxygenase-1 (HO-1), Superoxide dismutase (SOD) and glutathione peroxidase (GPx)), increases reducing equivalents (NAD(P)H), and interferes with inflammatory signaling pathways [42]. Recently, activating transcription factor (ATF), a basic leucine zipper transcription factor, has been shown to be a key therapeutic target for modulating NRF2 and cellular defense system against NIHL [43]. Yang's group further explored the avenue by revealing the protective role of eNOS-NO-PKM2 in glucose metabolism against NIHL [44]. Endothelial nitric oxide synthases (eNOS) and nitric oxide (NO) increased in noise-exposed cochlea, directing glucose metabolism to pentose phosphate pathway with more reducing equivalent produced by pyruvate kinase M2 (PKM2). L-arginine, which is a precursor of NO, was found to be effective, and more future therapies modulating NO or glucose metabolism possess great potential. Furthermore, sirtuin (SIRT) proteins, a group of NAD-dependent deacetylase, have been focused in recent years. Unlike the pathways mentioned above, there is a diversity in the functions of those proteins. Studies revealed that SIRT2 pathways cause mitochondrial oxidative stress and apoptosis [45], but SIRT1 and SIRT3 stabilize the mitochondrial function and attenuate oxidative stress and apoptosis in animal models [46-48]. So far, the therapeutic value of modulating SIRTs in NIHL models has been proved [45,47,48], but the details of their role in cochlea after noise exposure: the upstream modulators and downstream effectors have not been fully elucidated. More in-depth mechanism and in vivo drug investigations deserve consideration.
It is important to note that some factors hampered the therapeutic applications in modulating ROS signaling pathways. The target molecule may possess multiple biological functions, or may be involved in multiple analogues. Additionally, some regimes lack specificity for their targets, which might lead to off-target effects [49].
Inflammation and anti-inflammatory therapies
Robust cochlear inflammation occurs almost immediately in response to acoustic trauma, which can be characterized by recruitment of inflammatory cells, as well as generation of inflammatory mediators in the cochlea [50]. For unknown reasons, neutrophils do not participate in this response. The primary mediators of this process are bone marrow-derived monocytes and macrophages [51]. Monocytes from the blood stream are recruited by chemokines, infiltrate through venules in the lateral wall within 2 days and then subsequently transform into macrophages upon entering the inner ear [50]. Furthermore, there are abundant resident macrophages present in the spiral ligament, which experience activation and rapid proliferation. It appears that systemic depletion of macrophages is protective for acute acoustic damage, and therefore it seems that the macrophage activities are generally harmful in NIHL [52]. However, the study did not classify macrophages or monocytes because the role of those cells in the cochlear immune response can be complicated and likely depends on the stage of the disease. When noise insult upsets the balance of dynamic equilibrium, they secrete a lot of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), and interleukin-6 (IL-6) [53,54]. After the initial burst of inflammation is over, cell debris after apoptosis or necrosis is phagocytosed by the infiltrated and resident macrophages [55]. Intriguingly, a novel function of those macrophages was reported in promoting IHC ribbon synaptic repair [56]. Overall, multiple factors govern macrophage dynamics and transformation of subtypes. With more explicit knowledge, we might be able to direct infiltration and other biological activities of monocytes and macrophages, or mediate the status of macrophage subtypes in the inner ear. The potential of those strategies in treating NIHL has not been fully investigated yet.
From another perspective, the regulation of inflammatory and immune response is based on intricate biological processes that involve both immune cells and non-immune cells (Figure 3A). The generation and signaling of cytokines and chemokines in those cells are key targets for some medications. Preserving hearing function and cochlear morphology before and after noise exposure can be achieved by inhibiting transforming growth factor beta 1 (TGF-β1) signaling with the blocking peptides [57]. High mobility group box 1 (HMGB1) is another extracellular mediator of inflammatory response, and is an extensively explored damage-associated molecular pattern [58]. HMGB1 acts as an indicator of cell damage, which accelerates inflammation in neighbor cells and stimulates professional scavengers. Its direct binding to the toll-like receptor 4 (TLR4) initiates NIHL via the NF-κB and MAPK signaling pathways [59]. Translocation of HMGB1 from the nucleus to the cytosol is the prerequisite for its intracellular function and subsequent cellular release. Any contributors to HMGB1 acetylation (e.g., SIRT1) were speculated to manipulate the process and hold promises as future therapeutic targets [60,61]. The existing literatures have described successful applications of HMGB1-neutralizing antibodies for the precise blocking of extracellular HMGB1 functions [60,62]. It is suggested that the early development of inflammation involves a crosstalk with ROS signaling, and that targeting their interplay might cut off the progression of NIHL. Researchers found peroxisome proliferators-activated receptors (PPARs), a family of three ligand-regulated transcription factors, have critical role in regulating oxidative stress/inflammatory interplay in the NIHL [63]. Oxidative stress suppresses the expression of PPARs, which in turn facilitates inflammation by preventing negative interactions with NF-κB. The same research group demonstrated the therapeutic potential of targeting PPARs by the local delivery of pioglitazone, a PPARγ agonist, for NIHL treatment [64].
Traditionally, corticosteroids (e.g., prednisolone, hydrocortisone, and dexamethasone) have been extensively evaluated in NIHL treatment [65-69]. It is suggested that the steroid action in the cochlea extends far beyond anti-inflammation [70], but heterogeneity in treatment outcomes and side effects due to broad therapeutic targets hinder their application. Nevertheless, the use of steroids enlightened the exploration of unknown mechanisms underlying the progression of NIHL. Shih et al. showed that dexamethasone considerately suppressed the upregulation of HMGB1 in the spiral ligament and ameliorated cochlear and hearing impairments [71]. To our knowledge, it is the first study to show that HMGB1 is involved in noise-induced cochlear inflammation. Nelson et al. conducted a comparative analysis of steroid-responsive genes in specific cochlear cell types using published transcriptome and single-cell RNA datasets [70]. The research identifies differentially expressed steroid-responsive genes and pathways as potential drug targets, particularly for specific cell types. The group also utilized gene target databases to identify existing FDA-approved drugs, which may be repurposed for NIHL treatment [72].
Energy metabolism dysfunction and related therapies
The role of the cochlear lateral wall involved in the disease pathogenesis probably has been underestimated. It is well known that extensive noise diminishes normal cochlear blood flow, inducing ischemia/reperfusion damage and a surge of oxidative stress [73]. Studies have revealed elevated vasoactive gene expression and an accumulation of vasoconstrictor in the cochlear lateral wall [18,73]. The overwhelming vasoconstriction factors are speculated to originate from damages to fibrocytes or pericytes in the cochlea, which is highly responsible for orchestrating the cochlear capillary system [74].
Magnesium supplements have been suggested as a treatment for NIHL for a considerable period of time [75-77]. As a vasodilator, magnesium is also known for a variety of pharmacological properties, such as blocking NMDA receptor, attenuating glutamate overproduction, and reducing calcium influx into hair cells [78]. Some vasodilators used in cardiovascular diseases have a long history of clinical application and a well-established safety profile. Among them, milrinone, a PDE III inhibitor, and papaverine, a direct-acting vasodilator, have received preclinical investigations in rodent animals and shown protective effects for hearing impairment and hair cell loss [79,80].
Hypoxia and energy depletion escalate the metabolic dilemma in noise induced cochlear damage (Figure 3B). The oxidation defense system is also energy-consuming. Transient ATP depletion was reported following acoustic damage due to increased mitochondrial aerobic respiration [17]. However, a glucose supplement partially restored the ATP level and enhanced antioxidant defense by a single dose injection shortly before noise exposure [81]. The details of how glucose metabolism works in NIHL are still unclear. And the study raised the question of whether a high-sugar diet enhance cochlear tolerance to acoustic damage. Furthermore, the ATP depletion exhibits downstream effect on adenosine monophosphate-activated protein kinase (AMPK). It is a critical energy modulator sensitive to AMP/ATP increase and upregulate energy generation [82]. Moderate activation of AMPK boosts energy supply, which might be protective against NIHL. By sound conditioning using low-intensity noise, an innate causality was implied between activated AMPK and reduced hearing loss or synapse damage [83]. However, excessive and sustained activation of AMPKα signaling is responsible for subsequent c-Jun N-terminal kinase (JNK) mediated apoptosis of hair cells [84]. Studies showed that inhibition of AMPK or its upstream AMPK kinase liver kinase B1 (LKB1) significantly reduced the loss of OHCs and synaptopathy [85]. Those findings also indicated that AMPK signaling plays a critical role in cochlear synaptic disorders.
Autophagy and anti-autophagic therapies
Recently, it has been thought that autophagy is involved in the development of SNHL including hearing loss originated from noise exposures, ototoxic drugs and aging [86]. In response to multiple stress conditions, abnormal intracellular substances or organelles undergo certain trafficking and degradation processes, providing extra energy or maintaining cellular homeostasis [87]. However, limited studies have focused on the role of autophagy in the defense system against NIHL. Yuan et al. reported that increasing the autophagy level protected against noise-induced hair cell loss, while suppressing it exacerbated oxidative stress and noise damage. All of these effects were achieved using pharmacological or siRNA tools (Figure 3E) [88]. Apart from its pro-survival effect, autophagy is known as the inducer of cell apoptosis in many diseases [89], but research data did not support a pro-apoptotic role of autophagy in NIHL since the autophagy marker was found significantly elevated after a mild noise exposure but only slightly increased in more severe noise damages [88]. Further complementary researches, based on phenotyping genetically modified animal models, are in critical need because pharmacological inhibitors and activators target other cellular pathways as well. It seemed that activation of autophagy alone yielded only minimal hearing and morphological improvements, but synergistic action with other targeted pathways achieved more efficient protective effects [90].
Calcium-induced glutamate neurotoxicity and related therapies
A series of innate intracellular mechanisms are responsible for signal processing between IHCs and type I SGNs, including the calcium-triggered glutamate release from IHCs, glutamate transmission and Ca2+ influx into the postsynaptic terminals (Figure 3E) [91]. Those areas are extremely vulnerable to noise-induced excitotoxicity, leading to hair cell loss and synaptopathy. Specifically, the damaging effect of noise on synapses has been associated with noise-induced hidden hearing loss [3].
Preventing the damage at first place and promoting synaptic recovery are two ways to diminish the harm of noise-induced cochlear excitotoxicity. Voltage gated calcium channel (VGCC) blockers have been evaluated considering that the calcium influx into overstimulated hair cells via VGCCs is the initial step towards excitotoxicity [92]. Their protective effect was evident, especially in preventing hair cell loss, but which type of VGCCs, T-, or L-type, that is most responsible for the calcium overload in hair cells remains controversial [93,94]. Although Ca2+ influx is considered to be a key mediator of synaptic trauma, there has been no observed improvement in synaptopathy by using VGCC blockers up to now, presumably because postsynaptic calcium influx is not mainly driven by VGCCs, but calcium permeable glutamate receptors (GluRs) instead [95]. Among the various GluR types, AMPARs, NMDARs, and kainite-Rs, NMDARs have a major role in Ca2+ influx in the brain [96], but new evidence suggested that AMPARs are major contributors to Ca2+ influx and synaptic excitotoxicity in the cochlea [95]. By selectively blocking the Ca2+-permeable AMPARs via chronic intracochlear delivery of IEM-1460, both the synaptic trauma and the reduction of cochlear nerve activity in response to sound were significantly attenuated [97]. Indeed, the surface Ca2+-permeable AMPARs is in dynamic changes with constant endocytosis and replenishment [98]. Another therapeutic approach may be down regulating the membrane AMPARs by facilitating their internalization [99].
The degree of recovery in synapse number and function may vary in different settings of noise exposure or animal species and strains [100]. In order to promote the synapse recovery, exogenous application of trophic factors, including neurotrophin-3 (NT-3) and brain-derived neurotrophic factor (BDNF), was probably the most evaluated medication. Studies have demonstrated that both exogenous supplementation and genetic upregulation of NT-3 significantly enhanced synaptic regeneration following noise-induced synaptopathy [101,102]. In recent years, other approaches have been pursued to better harness synaptic regeneration. Manickam et al. reported that cochlear resident macrophages were necessary and sufficient for the recovery of noise-induced synaptopathy [56]. It should be a recovery mechanism parallel to the action of neurotrophins because neurotrophins in cochlea were expressed by hair cells and supporting cells, not macrophages. Another study by Kim et al. showed the unexpected role of glutamate release in synapse regeneration after noise exposure, indicating that the regeneration may require normal postsynaptic Ca2+ influx [103]. These findings revealed a complex network of signaling pathways involving multiple cell types, which may serve as therapeutic targets.
Programmed cell death and related therapies
The progress of programmed cell death is mediated by the activation of multiple cell death pathways described in the preceding sections. The higher the noise level, the greater the difficulty in achieving meaningful physiological protection through intervention in the downstream cascades. Mostly evaluated and targeted are ROS-mediated and inflammation-mediated hair cell apoptosis, realized by the sequential action of caspases (Figure 3D) [104]. However, direct inhibition of caspases was recognized to shift OHC death to necrosis [84]. Over the past decade, researchers have been seeking anti-apoptotic and pro-apoptotic regulators in the development of apoptosis. There are two different but interconnected pathways. In extrinsic pathway involving inflammation and cytokines, MAPK-c-JNK pathway has a crucial role mediating the activation of apoptosis. Synthetic JNK blocking ligand (D-JNK-1) attenuated noise trauma with intratympanic administration in guinea pigs, and different formulations based on mini pump or hydrogels have been developed [105-107]. In intrinsic pathway modulated by the mitochondrial and oxidative stress, mitochondrial-related apoptosis is mediated by Bax/Bcl2 balance. Upregulation of Bax/Bcl2 ratio induces the release of pro-apoptotic factors like cytochrome C from the mitochondrial intermembrane space, triggering the subsequent activation of caspase9 and caspase3 [108]. A recent study showed that apelin-13, a neuropeptide, had strong anti-apoptotic effects. This effect was linked to a change in the Bax/Bcl2 balance and an increase in SIRT1 in the mitochondrial-related apoptotic pathway [48]. Additionally, the nuclear factor of activated T cells (NFAT) protein family was found to be a critical component in the crosstalk of ROS signaling and TNF generation. The expression and function of NFATc4, specifically, was found in cochlear hair cells [109]. When activated by ROS or calcineurin, they translocate to the nucleus and upregulate the expression of TNF and TNF-mediated hair cell apoptosis. The calcineurin inhibitor FK506 attenuated noise-induced hearing loss and OHC loss, but the protective effect diminished greatly for aminoglycoside-related ototoxicity [90,109], indicating different roles of calcineurin/NFAT signaling in the pathophysiology of the two diseases.
It is likely that not only apoptosis, but also several types of programmed cell death, including necroptosis, pyroptosis ferroptosis and cuproptosis, are triggered by noise stimuli [110]. Those critical modulators or crosstalk molecules within the signaling network provide new avenues for NIHL treatment. But supporting evidence for this assumption is lacking in the scenario of NIHL. The first breakthrough comes from a recent study that showed that ferrostatin-1 (Fer-1), a well-known ferroptosis inhibitor, protects against NIHL. This shows that ferroptosis is involved [111].
Translational dilemma from animals to human
We have emphasized the diversity of cellular pathways that are involved in the pathogenesis of NIHL, hoping to bring inspiration to new medications for the prevention or the treatment of the disease. We wonder if there is some sort of 'command center' that controls how cells react to stress. Since there is no clue, combining different drugs to have synergistic effects or using drug that has multiple actions has proved to be highly successful [90,112]. There are concerns that excessive activation or suppression of certain pathways may disturb the balance of innate defense system [113].
With new inspirations and strategies, a broad range of potential medications have demonstrated therapeutic values in animal studies, which has been reviewed by Isabel's group [1]. However, a limited number of them have been subjected to clinical testing (Figure 4). The database of the ClinicalTrials.gov, which was searched on April 10, 2023, currently contains 12 registered clinical studies exclusively focusing on NIHL pharmacologic treatment, including six completed clinical trials. The antioxidant activities of N-acetylcysteine have been identified [114], but a combination therapy of antioxidant vitamins and the mineral magnesium fail to prevent temporary threshold shift [115]. Notably, ebselen showed significant efficacy in alleviating a temporary threshold shift in a phase 2 trial [116]. A phase 2b study of FX-322, a promising drug aiming to restore hearing by hair cell regeneration, was recently completed with a disappointing result. Overall, there were setbacks, which might be attributed to the weaknesses in the study designs or perhaps, in the drug delivery approaches.
There are some limitations and haunting questions in a reexamination of the development of pharmacological interventions of NIHL: (1) Most studies do not distinguish between noise types. A representative noise design in an experimental model is a narrowband or broadband noise with constant power spectral density at a constant sound pressure level for a few hours [1]. However, the noise in the real world is a mixture of various patterns. For example, the temporal characteristics of noise. In fact, many people develop NIHL from moderate and repeated exposures lasting probably for years rather than from acute ones, which has been given limited attention when designing preclinical animal models. (2) The therapeutic window for treatment after noise is yet unknown. It might depend on the drug and the time course in which the targeted pathway was involved. It was found that ROS was generated immediately after the insult and reached a maximum of 7 to 10 days [14]. According to it, maximal effectiveness of an antioxidant therapy was achieved when the therapy was initiated within 24 h and extended for 10 days after noise exposure [117]. But supporting evidence for the therapeutic window of other drug types is scarce. (3) Drug delivery to the inner ear is a challenging task in itself. The pharmacokinetics of numerous drugs in the inner ear are indeterminate, posing challenges in maintaining effective drug concentration in this difficult-to-reach sensory organ. It is a dynamically evolving research field that we will discuss in the following sections.
Improving inner ear drug delivery efficiency across biological barriers
Pharmacological interventions with physiologically meaningful protection against NIHL are complicated by the fact that the drug has to be delivered to the inner ear across multiple biological barriers. In fact, it is a common challenge facing all SNHL therapies. Approaches to the inner ear can be categorized into two groups: systemic delivery and local delivery (Figure 5). Systemic delivery must overcome the BLB, and local delivery is hindered by the isolated anatomical position and tissue barriers. For achieving efficacy, several key design principles were introduced for both classes of delivery approaches.
Molecular structures of the clinical candidates for NIHL therapy. A non-exhaustive list of evaluated drugs in preclinical studies is illustrated in the blue region. Drugs that have entered clinical trials are illustrated in separated regions according to the stage of clinical trials they are in.
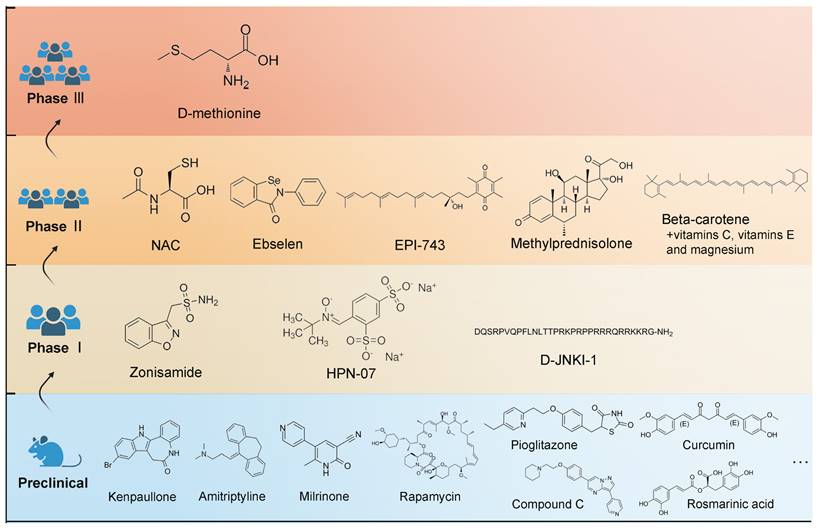
Summary of different drug delivery routes to the inner ear. Abbreviations: CCA: common cochlear artery; CNS: central neural system; IHCs: inner hair cells; OC: organ of Corti; OHCs: outer hair cells; OW: oval window; RW: round window; SM: scala media; SP: spiral prominence; ST: scala tympani; SV: scala vestibuli.
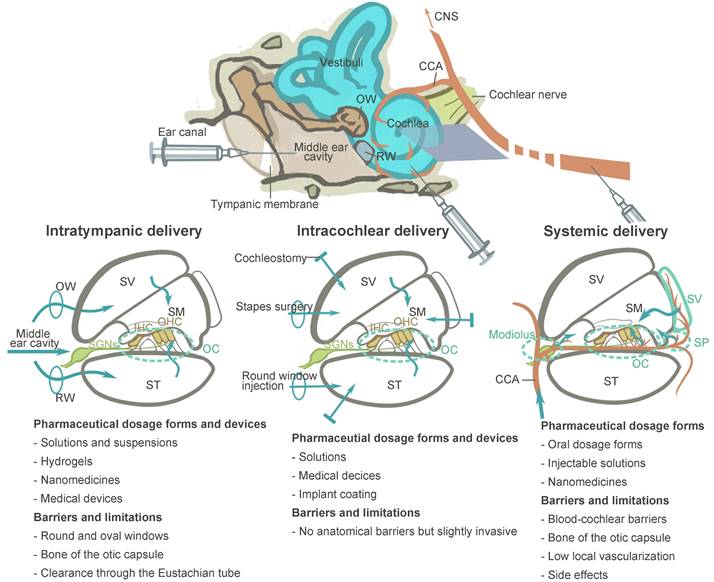
Systemic administration across the blood-labyrinth barrier
Systemic administration used to have a dominant role in clinical studies and clinical practices of NIHL treatment, due to its feasible and non-invasive nature (Figure 5). It is cost-effective when compared to intratympanic delivery because it does not require a repeated visit to professional healthcare facility and an experienced otolaryngologist to perform the whole procedure which include otoscopy, local anesthesia, administration and the following resting time [118]. Although the outcomes varied, several orally administered regimes, mostly corticosteroid therapy, have shown overall improvement in audiometric results and clinical outcomes [119-121].
However, the presence of the BLB casts a shadow on the theoretical basis of this delivery approach. Several studies suggested that drug passage from the bloodstream into the inner ear fluid is scarce (around 0.000005% for methylprednisolone) [122]. A human study showed that the concentration of dexamethasone in the inner ear after systemic administration was about 88 times lower than that after intratympanic injection [123]. The difficulty of obtaining appropriate drug concentrations within the therapeutic window also poses a great danger of significant adverse effects on other organs.
BLB is a functional barrier that controls substance exchange between the systemic vascular system and the inner ear fluid. In the inner ear, regions with blood vessel distributions could be part of the BBB, such as the modiolus, the spiral prominence, the organ of Corti and most importantly, the stria vascularis [124]. As shown in Figure 6, the intrastrial space contains a complex branching system of capillaries, together with pericytes and perivascular-resident macrophage-like melanocytes (PVM/Ms) [13]. On the medial surface of the stria vascularis is a layer of tight junction-coupled marginal cells that separate it from the endolymph in the scala media. Abundant publications have suggested that pericytes and PVM/Ms indirectly maintain the barrier integrity [13,125], while transcellular or paracellular transport is directly limited by two layers of junction-coupled cells: the endothelial cells of the capillaries and the marginal cells [124].
Despite this, there are opportunities for drug delivery across the BLB (Figure 6). The majority of the advanced strategies in this review are still in the early stages of development. To begin with, substances with low molecular weight are basically favored for the passage through the BLB, but there is a selectivity for certain small molecules such as ototoxic drugs. For example, Gentamicin was found to enter the inner ear via an endocytic transporter, megalin (LRP-2), expressed in the marginal cells of stria vascularis [126]. Although the idea of utilizing the innate biological transport mechanisms to cross the BLB for drug delivery is still in its infancy, it could be facilitated in the near future. One recent study found that the low-density lipoprotein receptor-related protein 1 (LRP-1) on the BLB can be a potential target for shuttling therapeutics across this barrier [127], and its therapeutic value in facilitating the NIHL treatment has not been testified yet. Another approach to be explored in this field is cell-mediated drug delivery. Based on the observation that circulating macrophages and monocytes actively migrate into the inner ear under both physiological and stress conditions [50,128], they could be engineered for therapeutic purposes. Secondly, nanoparticulate systems have been widely investigated as drug delivery platforms for central nervous system (CNS) disorders across the blood-brain barrier [129]. Although non-targeted nanoparticles are generally considered less permeable to biological barriers, it was reported that a zeolitic imidazolate framework-based nano system loaded with steroids efficiently accumulated in the inner ear, and protected the cochlea from noise damage [130]. The research and subsequent investigations have been unsuccessful in elucidating the precise mechanism of nanostructures passing through the BLB [131]. Advanced technologies, like the surface decoration of peptides that target or penetrate, may further improve the drug delivery efficiency. Lastly, certain conditions were reported to improve the barrier permeability, including inflammation [132], diuretics [133], and osmotic agents [134], which provide the possibility for drug entry theoretically. An animal model of drug-induced hearing loss showed that a combination of antioxidants with mannitol, which is a diuretic medication, improved the otoprotective effect of the antioxidative therapy [135]. It shows that this therapeutic strategy has a lot of potential in NIHL research.
Potential future strategies for drug delivery across the BLB that have been discussed in this review. Multiple strategies including receptor-mediated transcytosis, cell-mediated drug delivery, nanoparticle-facilitated drug delivery and exploiting certain conditions to open the barrier are illustrated. The image of cochlear blood supply by ink perfusion was reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/) [11]. Copyright 2018, the authors, published by Elsevier. Abbreviations: EC: endothelial cells; LRP-1: low-density lipoprotein receptor-related protein 1; NPs: nanoparticles; PC: pericyte; PVM/M: perivascular resident macrophage type-melanocyte; TJ: tight junction; SM: scala media; ST: scala tympani; SV: scala vestibuli. Created with BioRender (www.biorender.com).
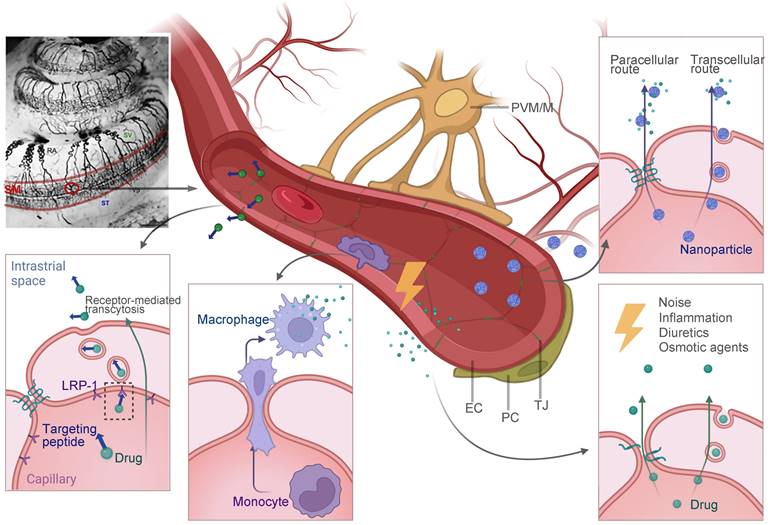
Local administration across the tissue barrier
Considering the limitations of the traditional drug delivery routes, there has been an increasing interest in local administration, which bypasses the vascular systems and the BLB to reach the inner ear more direct and efficiently. Local administration is represented by two basic approaches: intratympanic and intracochlear approaches (Figure 5).
Intratympanic delivery
Intratympanic drug delivery is nowadays the mainstream delivery route in preclinical animal studies of NIHL, and is extending its applications to clinical trials. There are two biological barriers including the tympanic membrane (TM) and the RWM before reaching the inner ear [11]. The TM separates the outer and the middle ear. Within the tympanic cavity, the ossicular chain is vital for sound conduction and treatment-related trauma should be avoided. The eustachian tube, leading from the middle ear to the throat, would serve to clear drug formulations delivered in the middle ear [136]. The last barrier separating the middle ear from the inner ear fluid is the RWM or oval window (OW) [137].
Since the TM is much more impermeable than the RWM, a direct injection through TM into the tympanic cavity is usually involved [138]. Ultimately, the RWM becomes the main challenge of drug diffusion into the inner ear, considering that the OW is partly occluded by the stapes [139,140]. The RWM is semi-permeable and the drug diffusion through RWM bears some resemblance to that through skin. To sum up, the drug delivery involves two general principles: (1) maintaining close contact with the RWM for the longest possible period, and (2) improving the transport efficiency through the RWM (Figure 7).
Biocompatible materials such as hydrogels can retain drug solution or drug loading nanoparticles in its polymer structures. When injected into the tympanic cavity or placed on the RWM, the gels with their high viscosity ensure the longest drug retention, and minimize the drug clearance from the eustachian tube [141]. Based on different polymer compositions, gels can have sheared thinning behavior or finely tuned thermosensitivity, which means they can be easily applied by a syringe or gel quickly at body temperature. One tricky problem is that, in clinical researches, placing the gel precisely into the round window niche is challenging during an intratympanic injection. While in most NIHL animal studies, the gels were surgically placed on the RWM after opening the temporal bone. This detail should be noted in future NIHL translational researches. Besides, bulk hydrogels can induce conductive hearing impairment due to their large volume [142]. Microgels, which are basically micro-scale hydrogels, also possess adhesion ability but will preserve the normal acoustic wave transmission [143].
To improve the transport efficiency through the RWM, advanced drug delivery systems including nanostructures have intensively been applied for inner ear drug delivery. For one thing, nanocarriers improve the drug bioavailability by controlled release and higher drug stability [144]. For another, PLGA nanoparticles as an example, were found to be actively transported within the RWM via multiple mechanisms of cell endocytosis and exocytosis [145]. Our research group has designed an inner ear drug delivery system based on tetrahedral DNA nanostructures (TDNs) [146]. The TDNs rapidly penetrated the RWM 0.5 h after administration and delivered epigallocatechin gallate for hearing protection in the NIHL model. It is supposed that TDNs can penetrate the tissue barrier via both paracellular and transcellular pathways [147]. This field of research has been propelled by engineering of intelligent drug delivery systems [144]. Beyond that, the permeability of the membrane can be improved by pre- or cotreatment with chemical permeation enhancers (CPEs). For example, Jeong et al. attempted to modulate the tight junctions of the RWM using a medium chain fatty acid, caprate. The co-treatment of caprate and dexamethasone led to enhanced drug diffusion and significantly relieved noise-induced hearing loss [148]. Other CPEs, including bupivacaine, limonene and sodium dodecyl sulfate, or certain biomaterials such as chitosan nanoparticles have great potential to facilitate the paracellular transport pathway across the barrier in future NIHL treatment [149,150].
Intracochlear delivery
This delivery approach provides direct access to the inner ear, without the drug bioavailability being affected by multiple biological barriers. For intracochlear delivery, drugs can be injected with a fine needle through the RWM or by a cochleostomy [151,152]. However, the overall application of intracochlear delivery in this field is restrained by its invasive nature. The volume and pressure changes induced by the injection or efflux of perilymph from the opening can damage the fragile sensory epithelium of the cochlea [153,154].
To diminish the harm, a microcatheter connected to an osmotic or infusion pump enables long-term, ultra-low and highly controlled delivery rate [155]. Due to its advantage in allowing application of a precisely designed drug concentration bypassing biological barriers, it is a great tool for evaluating drug efficacy in basic research. Malfeld et al. developed a NIHL animal model with an osmotic pump-based system for evaluation of preventive drug effects [156,157]. The current pump- and catheter-based intracochlear delivery system is still too traumatic for NIHL therapies in human patients. To maintain fluid volume, an infuse-withdraw delivery pattern that mixes drugs into perilymph has been developed [158,159]. The administration of BDNF can, in part, temporarily ameliorate the hearing impairment caused by the chronic intracochlear delivery devices [160].
Targeted and responsive drug delivery strategies in NIHL
For the last decades, drug delivery systems have been increasingly utilized in both systemic and intratympanic routes to improve the therapeutic outcomes of NIHL treatments (Table 1). Untargeted formulations which largely depend on passive permeation through barriers do not meet the demand. Conversely, functional engineering of the drug delivery systems is the key future research trend for better delivery efficiency. Active targeting strategy can not only diminish the toxic or side effects due to the large dose, but also improve the drug concentration and extend the period of effective drug concentration at the targeted site, which is basically the organ of Corti. By exploring smart drug delivery systems with responsiveness to internal or external stimuli, researchers enable more precisely controlled drug release profile, or more robust drug entry propelled by those systems. We will next introduce those two major categories of advanced drug delivery strategy in NIHL therapies: targeted and responsive drug delivery, which are technically facilitated by biocompatible materials in their various forms.
Advanced strategies for intratympanic drug delivery across the RWM that have been discussed in this review. Nanoparticulate systems can penetrate the tissue barrier via both paracellular and transcellular pathways. CPEs can be used to decrease the tight junctions to improve drug penetration. Gelling systems maintain close attachment to the RWM for prolonged drug delivery. Abbreviations: CPE: chemical permeation enhancers; CT: connective tissue; OE: outer epithelium; RWM: round window membrane; SE: squamous epithelium; TDNs: tetrahedral DNA nanostructures. Created with BioRender (www.biorender.com).
Drug delivery systems in NIHL animal models
| Drug delivery systems | Characteristics | Drug | Pharmacological action | Animals | Noise | Administration | Therapeutic outcomes | Ref. |
|---|---|---|---|---|---|---|---|---|
| PEGylated PLA nanoparticles | 116 nm | Betamethasone phosphate | Anti-inflammatory | CBA/N mice4-6 weeks | OBN 120 dB SPL 8 kHz 2 h | IVImmediately after noise | GR translocation into the nuclei in OHCs↓ABR TS at 8 kHz on day 14, 32 kHz on days 7 and 14↓OHC loss in the apical turns; ↓IHC loss in the apical and basal turns | [131] |
| Solid lipid nanoparticles | Not described | Edaravone | Antioxidant | Guinea pigsAdult | SN 110 dB SPL 0.25-4 kHz 2 h/d, 4 d | ITI1 d after noise | ↓ABR TS on day 4↓ROS in cochlea | [161] |
| Zeolitic imidazolate framework-based nanoparticles | 105 nm PDI = 0.22 Zeta potential = -6.9 mV | Methylprednisolone | Anti-inflammatory | C57BL/6J mice4-5 weeks | WN 100 dB SPL 3 h/d, 3 d | IVBefore noise | ↓ABR TS at 16 kHz, 24 kHz, and 32 kHz↓HC loss at basal and middle turns | [130] |
| Solid lipid nanoparticles | 203 nmPDI=0.361 | Clozapine | Antioxidant | Guinea pigsAdult | SN 110 dB SPL 0.25 - 4 kHz 2 h/d, 4 d | ITIImmediately after noise | ↓ABR TS on day 6↓ROS in cochlea | [162] |
| Cross-linked hyaluronic acid with polylactide-co-glycolide microcapsules | 50 μm | Dexamethasone;Insulin-like growth factor 1 | Anti-inflammatory;Anti-apoptosis; Inducing the proliferation of supporting cells | SD Rats6 weeks | WN 3.7 h | ITI 3 h after noise | ↓ABR TS | [163] |
| ROS-responsive and OHC-targeting liposomes | 219.08 ± 8.86 nmZeta potential = -6.70 ± 0.13 mV | Poly(propylenesulfide)120;Berberine | Anti-inflammatory and antioxidant | Guinea pigs10-12 weeks | WN 120 dB SPL 6.3-20 kHz 4 h | RWM application 12 h after noise | ↓MDA and ↑SOD ↓TNF-α, IL-6 and IL-1↓OHC loss in four turns and better morphology of OHCs↓ABR TS at 8, 16 and 24 kHz on days 4, 7 and 14 | [164] |
| OHC-targeting liposomes | 116.6 nmzeta potential = - 18.58 mV | forskolin | Antioxidant | C57BL/6J mice | WN 115 dB SPL 2-20 kHz 2 h | RWM application3 d before noise | ↓ABR TS at 4, 8 and 16 kHz on days 1, 7 and 14; 32 kHz on day 28↓OHC loss in the mid-basal turns | [165] |
| Superparamagnetic iron oxide nanoparticles | magnetically driven | AAV2-BDNF vectors | Promoting the SGNs survival | Long-Evans rats6 - 8 weeks | OBN110 dB SPL8-16 kHz 2 h | RWM applicationMagnet placed on the contralateral ear for 30 min3 d after noise | ↓ABR TS at 16, 24 and 32 kHz by 2 weeks↓Decrease of ABR wave I amplitude↓Synaptic ribbon loss in the basal and mid turns ↑BDNF expression | [166] |
| Gelatin methacryloyl microgel particles | 32.81 μmCoefficient of variation = 15% | Dexamethasone sodium phosphate | Anti-inflammatory | Guinea pigsAdult | WN 120 dB SPL 6 h | ITI1 d before noise | ↓ABR TS at 4, 16, 24, 32 kHz↓OHC loss↓Synaptic ribbon loss↓Decrease of ABR wave I amplitude↓Increase of ABR wave I latency | [143] |
| Gelatin hydrogel | Biodegradable | Insulin-like growth factor 1 | Anti-apoptosis; Inducing the proliferation of supporting cells | Guinea pigsAdult | OBN 120 dB SPL 4 kHz 5 h | RWM application 5 h after noise | ↓ABR TS at 4 kHz on days 14 and 21; 8 kHz on day 14↓OHC loss in basal and middle turns | [167] |
| Gelatin hydrogel | Biodegradable | Hepatocyte growth factor | Anti-apoptosis | Guinea pigs4 weeks | OBN 120 dB SPL 4 kHz 3 h | RWM application 1 h after noise | ↓ABR TS at 16 kHz on day 21↓OHC loss in the 60-80% distance from the apex | [168] |
| Chitosan glycerophosphate hydrogel containing PEGylated OHC-targeting liposomes | Liposomes: OHCs targeting 87 ± 5 nmGel: Chitosan glycerophosphate-based Thermosensitive | D-JNKi-1 | Anti-apoptosis | CBA/J mice6-10 weeks | WN 115-120 dB SPL 6.3-20 kHz 4 h | RWM application 2 d before noise | ↓ABR TS in 4, 8, 16, 24, 32 kHz in 1-14 days | [105] |
| Hyaluronic acid gel containing liposomes | Liposomes: 140 nmGel: Hyaluronic acid-based | Dexamethasone | Anti-inflammatory | Guinea pigs | PT 100 dB SPL 5 kHz 1 h | RWM application 2 d after noise | No additional hearing recovery at 4, 16, 24 and 32 kHz compared to spontaneous recovery | [169] |
| Thermosensative hydrogel containing sodium phosphate multivesicular liposomes | Liposomes: 14.7 μmGel: Poloxamer-based; Gelling temperature at 34.9 °C | Dexamethasone | Anti-inflammatory | Guinea pigs Adult | WN 112 dB SPL 20-20000 Hz 6 h | ITI1 d before noise | ↓ABR TS at 4, 8, 16 and 24 kHz on day 7↓OHC loss in the basal and middle turns | [170] |
| Monodisperse droplets of water | 6.96 μmFlow rate = 20 μL/min | Methylprednisolone | Anti-inflammatory | Guinea pigsAdult | WN 106 dB SPL 15-125 kHz 48 h | ITIImmediately after noise | ↓ABR TS after 2 weeks↓OHC loss in 5 and 8 kHz frequency range | [171] |
| Ultrasound-aided microbubbles | 7.09 μm10^8 bubbles/mL | Dexamethasone | Anti-inflammatory | Guinea pigs | WN 118 dB SLP 5 h | RWM application followed by ultrasound2 h before noise | ↓ABR TS at 8, 16, and 32 kHz on days 7, 14, and 28↓OHC loss in the basal and second turns↓HMGB1 and ICAM-1 expression in spiral ligament | [71] |
| Ultrasound-aided microbubbles | 0.7-10 μm 1-5 × 105 bubbles/mL | Insulin-like growth factor 1 | Anti-apoptosis; Inducing the proliferation of supporting cells | Guinea pigs | NBN 118 dB SPL 8 kHz 5 h | RWM application or ITI followed by ultrasound 24 h after noise | ↓ABR TS at 8, 16 and 24 kHz on days 14 and 28; 32 kHz on day 28↓OHC loss in the basal and second turns ↓Synaptic ribbon loss in the basal and second turns ↑Akt1 and Mapk3 expression | [172] |
Abbreviations: ABR: auditory brainstem response; AN: after noise; BN: before noise; GR: glucocorticoid receptor; HC: hair cell; HGF: hepatocyte growth factor; HMGB1: high mobility group box 1; ICAM-1: intercellular adhesion molecule-; IHC: inner hair cell; IL-1: interleukin 1; IL-6: interleukin 6; IP: intraperitoneal; IT: intratympanic; IV: intravenous; MDA: malondialdehyde; NBN: narrowband noise; NP: nanoparticle; OBN: octave band noise; OHC: outer hair cell; PDI: polydispersity index; PEG: polyethylene glycol-coated; PLA: polylactic acid; PT: pure tone; rhIGF-1: recombinant human insulin-like growth factor 1; ROS: reactive oxygen species; RWM: round window membrane; SN: stationary noise; SPL: sound pressure level; SOD: superoxide dismutase; TNF-α: tumor necrosis factor; TS: threshold shift; WN: white noise
Targeted drug delivery strategies
After a typical non-specific intratympanic drug delivery, there will be a decreasing basal-apical gradient of drugs or nanocarriers due to their slow diffusion within the fluid of the inner ear [105]. It should be noted that with cochlear aqueduct connecting perilymph and cerebrospinal fluid [137], drugs or nanocarriers after intratympanic administration can be present in cerebrospinal fluid, posing potential off-target threats to the central nervous system [173]. To minimize off-target effect and improve therapeutic outcomes, recognition units, such as ligands, aptamers and antibodies, were conjugated with drugs or nanocarriers to actively target certain cell types (Figure 8).
Targeting outer hair cells
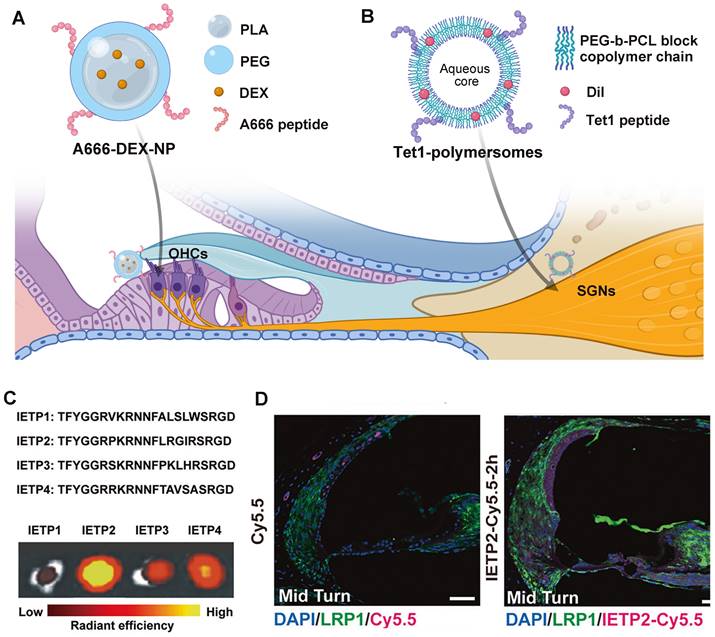
Current targeted drug delivery for NIHL is focusing on prestin-binding peptides for OHC targeting. This is because prestin is the only recognized biomarker exclusively expressed in one single cochlear cell type [174], and hair cell damage is believed to play a critical role in the pathology of NIHL. After the identification of several prestin-binding peptides from phage display experiments [175], Kayyali et al. used one of the peptides to decorate liposomes, which improved the cochlear distribution of the nanocarriers to apical turns. With the payload of JNK inhibitor, the system produced a dramatic improvement in therapeutic outcomes than untargeted ones after an acute noise insult [105]. Another two researches utilized the same peptide with appropriate modifications of the amino acid sequence in the construction of targeting nanocarriers against NIHL [164,165]. The presence of peptide modification in functionalized nanocarriers significantly improved their protective effect against noise-induced hair cell apoptosis and hearing threshold elevation. Previously, our research group used another peptide designated, A666, to endow PEG6K-b-PCL19K polymersomes with OHC targeting ability (Figure 8A), which significantly improved the efficacy of DEX in a drug-induced ototoxicity model [176]. So far, researchers have verified the selective accumulation of prestin-targeting nanoparticles in the cochlea of guinea pigs and C57BL/6J mice, and in the lateral line neuromasts of zebrafish. The major concern of this targeting system is that the expression level of prestin will drop sharply when the apoptosis of OHCs is set off, and therefore application within the therapeutic window is of critical importance.
Targeting spiral ganglion neurons
Besides hair cell loss, acoustic damage to neurons can also take part in the permanent hearing impairment. Specifically, synaptic trauma may lead to temporary loss of hearing, or more elusive perceptual disfunction [15]. However, targeted drug delivery to the neural element of the cochlea has not been achieved in the management of NIHL, nor in that of other acquired cochlear disorders. Some neuronal protein biomarkers have shown significant potential among others, including the trisialoganglioside clostridial toxin receptor (GT1b) and the BDNF/NT-3 growth factors receptor (TrkB). Although their expression extends beyond the cochlear SGNs and throughout the central and peripheral nervous system [178,179], the systemic side effect can be minimized via local administration. A notable difference between the two candidates is that GT1b binding does not alter cell signaling, but TrkB activation contributes to a series of neuronal survival signaling. Targeting TrkB via peptide ligands or agonistic antibodies might at the same time enhance neurite survival [180]. Whereas, the challenge of this system is that the specific binding with TrkB receptor does not necessarily lead to enhanced cellular uptake [181]. The utility of GT1b and TrkB targeting strategies have been investigated in the development of inner ear drug delivery vehicles (Figure 8B) [177,181], showing translational potential in treating noise-induced neuropathy.
Targeting the blood-labyrinth barrier
For systemic drug delivery to the inner ear, targeting the BLB barrier is an emerging strategy. It does not aim to deliver therapeutic agents directly to the site of action, instead it improves the drug accumulation and transportation at the biological barrier. This is well described in delivering therapeutics to the CNS across the blood-brain barrier, but is still in its infancy in inner ear drug delivery [129]. The utility of low-density lipoprotein receptor-related protein 1 (LRP-1) was initially explored in the drug delivery into the brain [182]. Shi et al. first described the wide expression of LRP-1 on the BLB, as a receptor able to transport its multiple binding peptides across the BLB via endocytosis (Figure 8C and 8D) [127]. By utilizing LRP-1 binding peptide, the study validated the efficient delivery of small-molecule compounds including potential drugs and imaging agents into the inner ear.
Responsive drug delivery strategies
Researchers have attempted to design or make use of various types of internal (e.g., pH, ROS, enzyme) and external (e.g., light, heat, magnetic field) stimuli to manipulate the in vivo behavior of drug delivery systems. These stimuli act either to promote RWM retention and penetration, or as a trigger for precise drug release at the site of action in the cochlea. Due to their versatility, responsive drug delivery systems have been intensively developed and employed mainly via an intratympanic delivery route in the research field of NIHL.
Targeted drug delivery strategies for NIHL treatment. (A) Schematic of the A666 peptide-conjugated nanodelivery system targeting prestin on OHCs [176]. (B) Schematic of the Tet1 functionalized polymersome targeting the trisialoganglioside clostridial toxin receptors on SGNs [177]. Created with BioRender (www.biorender.com). (C) Four candidate BLB targeting peptides and their uptake in mouse cochleae by ex vivo fluorescence imaging. Reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/) [127]. Copyright 2022, The authors, published by Springer Nature. (D) The ability of IETP2 to cross the BLB in vivo. Reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/) [127]. Copyright 2022, The Authors, published by Springer Nature. Abbreviations: OHCs: outer hair cells; SGNs: spiral ganglion cells; LRP1: low-density lipoprotein receptor-related protein 1
Thermal- and photosensitive gelling system
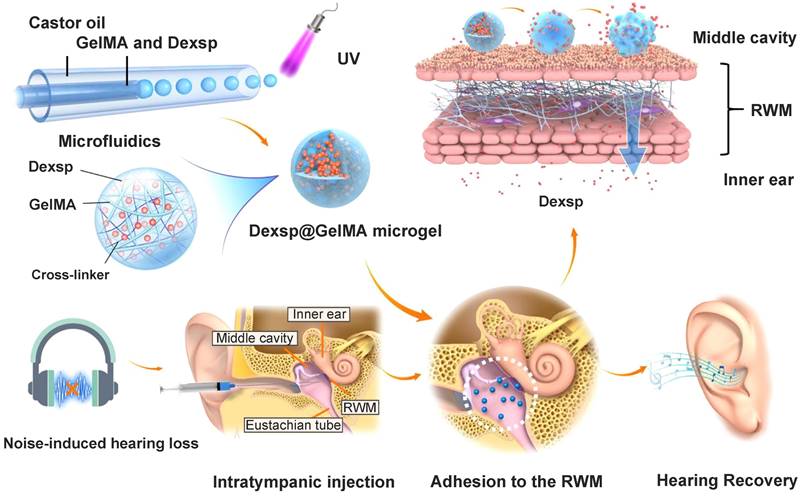
The stable and prolonged drug delivery on the RWM is always the essential purpose of using responsive gelling systems. They are more easily administered as a liquid, and then experience a sharp increase in their viscosity in response to the temperature change or ultraviolet (UV) light exposure. Poloxamer and chitosan are the most investigated thermosensitive biomaterials in this field [183]. Poloxamer 407 is a triblock copolymer composed of both hydrophilic and hydrophobic segments, which endow it with temperature-dependent self-assembling ability [184]. As reported by Li et al., poloxamer 188 with a higher hydrophilic content was supplemented to poloxamer 407 to obtain a finely tuned gelling temperature closest to that of the middle ear [170]. Their utility has been well described in many NIHL preclinical researches, and innovative formulations such as gelatin methacryloyl (GelMA) hydrogel have great potential for future research. GelMA solution can be applied on the TM or RWM, and a UV light exposure triggers the covalent crosslinking process to form bulk GelMA hydrogels [185]. Contrary to the bulk hydrogels, GelMA microgels do not impede the hearing with a micro-scale diameter, and they have been investigated recently as an otic drug delivery vehicle for NIHL therapy (Figure 9) [143]. Researchers fabricated the microgels with an average diameter of 32.81 μm using a microfluidic method, and polymerized them by UV irradiation before adhesion to the RWM. Over 80% of the GelMA microgels remained closely attached to the RWM surface for one week, allowing the drug to be released in a sustained manner.
Ultrasound-aided microsystem
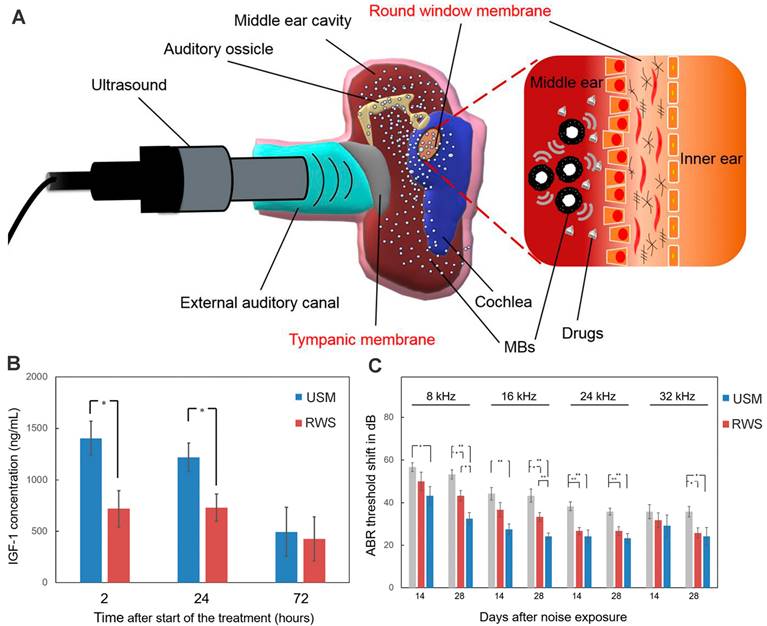
Ultrasound-driven microbubbles were applied to facilitate drug entry through the RWM (Figure 10A). Its theoretical basis is that the ultrasound-irradiated microbubbles can generate transient and reversible changes in the permeability of cell membrane via cavitation or sonoporation effect [186]. After the RWM administration of a microbubble/DEX mixture followed by ultrasound irradiation, the DEX accumulation in perilymph was 2.4 to 11.2 times higher than that of round window soaking [71]. Even if the drug was administered after the microbubble irradiation was finished, the inner ear delivery of rhIGF-1 was enhanced by 1.95 folds (Figure 10B). The two different therapies both resulted in significantly improved therapeutic effects for NIHL prevention and protection in guinea pigs [71,172].
Schematics of the ultraviolet polymerized GelMA microgels for prolonged drug delivery of dexamethasone sodium phosphate for NIHL therapy. Reproduced with permission [143]. Copyright 2022, American Chemical Society.
(A) Schematic of ultrasound-induced microbubble cavitation to increase the permeability of the RWM. Reproduced under the terms of the CC-BY Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/) [187]. Copyright 2020, The Authors, published by The American Society for Clinical Investigation. (B) Concentration of IGF-1 in the cochleae after the IGF-1 treatment with and without microbubble irradiation. Reproduced under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/) [172]. Copyright 2021, by The Authors, published by MDPI. (C) ABR threshold shift of guinea pigs after the IGF-1 treatment with and without microbubble irradiation. Reproduced under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/) [172]. Copyright 2021, by The Authors, published by MDPI. Abbreviations: MBs: microbubbles; RWS, round window soaking; USM, ultrasound microbubble treatment.
Magnetically Driven nanoparticles
Magnetic particle-based approaches are naturally suitable for active and directional delivery of drugs from the tympanic cavity into the inner ear. After being deposited onto the RWM or injected into the middle ear, the magnetic particles can be pushed from the identical side or be pulled from the contralateral side into the inner ear with a powerful magnet [188,189]. For example, superparamagnetic iron oxide nanoparticles (SPION) were attached to the AAV2 virus vector for the magnetic delivery of BDNF-gene therapy in a NIHL rat model. An additional advantage of SPION-based system is that their dynamic distribution in the cochlea can be visualized using MRI. Local application of this system substantially enhanced BDNF gene expression in the inner ear and reduced cochlear synaptopathy, with no ototoxicity to the inner ear [166].
Aside from the magnetic force, other kinds of physical fuels, or biofuels and chemical fuels, can be taken advantage of [190]. For instance, Liang, et al. constructed a "slim waist" shaped microshotgun drug delivery system loaded with dry chemical propellants and nanoparticles for TM penetration [191]. Here, water was the external stimuli added to ignite the chemical reaction inside the microshotgun, generating gas that ejected the nanoparticles deep into the TM. With Fe3O4 loaded in both the microshotgun wall and nanoparticles, a magnetic field can be applied to guide the microshotgun and provide the second penetration power directionally. Contrary to a usual tympanic injection, the system is a non-invasive intratympanic approach with minimum TM damage.
ROS-responsive nanoparticles
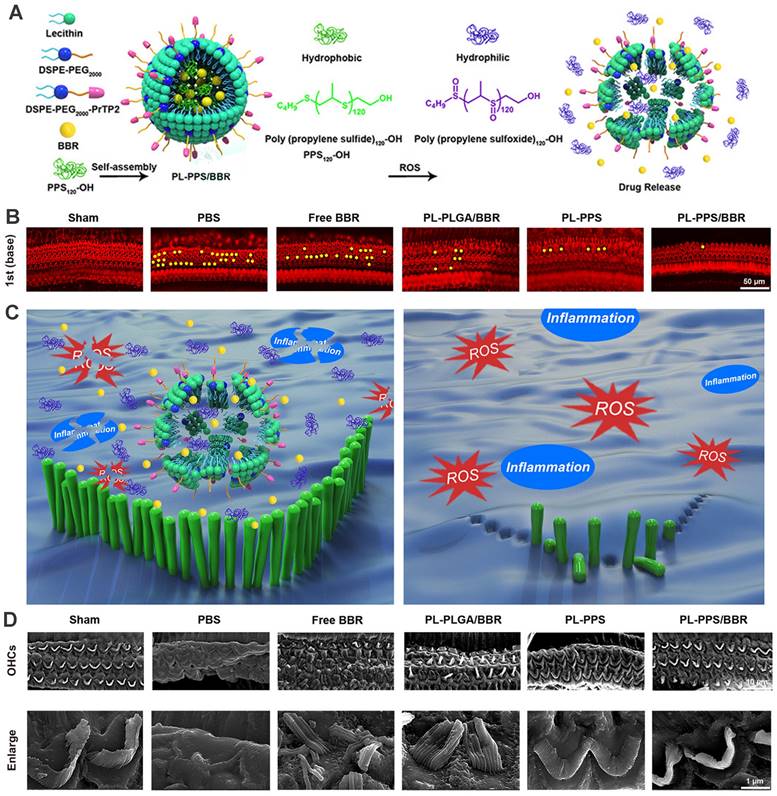
Considering that overproduction of ROS in various cochlear cell types was recognized as a crucial contributing factor to NIHL, the need for controllable and 'on demand' payload release at the ROS-rich sites was fueled. Poly(propylene sulfide) (PPS) is a ROS-receptor and a ROS-consuming material, which can be exploited to deliver drugs precisely and exert a scavenging effect on ROS [192]. PPS can convert into more hydrophilic poly (propylene sulfoxide) and poly (propylene sulfone) and provoke the disintegration of the nanocarrier with rapid release of encapsulated cargo [193]. Our group previously has developed a novel ROS-responsive/consuming nanoparticle system based on PPS, which combined the ROS scavenging effect of PPS with the antioxidant action of astaxanthin (ATX). The results of the study showed superior protective efficacy of the ATX-loaded PPS-NP than that of equivalent ATX in HEI-OC1 cell line, cochlear explants and guinea pigs [194]. Zhao et al. integrated poly(propylene sulfide)120 (PPS120) into the OHC-targeting liposomes for ROS-responsive drug release in NIHL treatment (Figure 11) [164]. In vitro H2O2-responsive drug releasing study suggested that PPS120 promoted berberine release once in the ROS environment, and exhibited greater ROS scavenging ability itself than berberine, leading to OHC morphological resilience and attenuated hearing loss to noise impairment in vivo [164].
(A) Schematics of the ROS-Responsive nanoparticle (PL-PPS/BBR) delivery system which disassembled in a ROS-rich lymph for berberine release. (B) Comparison of OHC loss at basal turn in each group. (C) Schematics of the anti-inflammatory and antioxidant effects of PL-PPS/BBR on OHCs. (D) SEM images of OHCs and their stereocilia in each group. Reproduced with permission [164]. Copyright 2021, American Chemical Society.
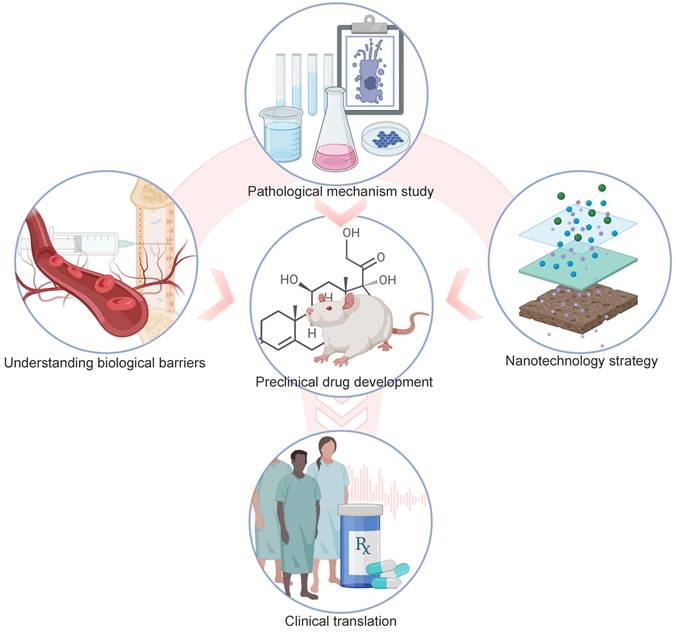
Summary of drug development and drug delivery for NIHL. Pathological mechanism studies of NIHL are the basics of preclinical drug development. More efficient drug delivery into the inner ear will be propelled by an advanced understanding of the main patterns of substance transport across the biological barriers, with the combined efforts of fast-developing nanotechnology. Those factors also contribute to the clinical translation of drug candidates. Created with BioRender (www.biorender.com).
Challenges and future opportunities
Here, we provide a comprehensive and systematic overview of pathogenic mechanisms and therapeutic interventions regarding NIHL. Our understanding of the disease and means for intervention have been rapidly evolving, but several scientific issues related to NIHL design and clinical translation still require elucidation.
To begin with, traditional diagnostic methods are unmet for the expansion of NIHL, including noise-induced synaptopathy, noise-induced hidden hearing loss, and coding-in-noise deficit. The therapeutic interventions for noise-induced synaptopathy have been explored, but those evidences are primarily derived from morphological observations in animal models. There is no reliable method for evaluating the functional outcomes of these synaptic deficits in animals. Due to ethical limitations, it is nearly impossible to have direct histological observation in human subjects. The current diagnostic tools, which were mostly made to deal with NIHL caused by steady broadband noise, are inadequate to diagnose NIHL caused by different types of exposure. Therefore, it is urgent to provide guidelines for the methods that are recommended for assessment.
The development of novel drug targets and therapeutic molecules are in great need. Although progresses in understanding the mechanisms underlying NIHL have been made, little is known about the protective responses against noise and the cochlear repair after damage. It is speculated that the repair process may involve multiple cell types, including hair cells, supporting cells, fibrocytes in the lateral wall and migrated macrocytes. Further in-depth researches are needed to fully unleash the power of the innate defense and repair systems. Drug evaluations in animal models and later in clinical trials are challenging yet critical work because of the biological differences between in vivo and in vitro environments, and between different species.
It is well known that an appropriate drug delivery strategy can improve therapy outcomes and minimize side effects. The BLB or the RWM is the major challenge of drug delivery, depending on the route of administration chosen. We have discussed several potential strategies to open new frontiers in systemic delivery, but indeed there is a lack of clear, robust evidence to reveal the underlying mechanisms of the substance transport through the BLB, leaving very limited approaches available for opening the barrier. The intratympanic delivery, which has demonstrated superior efficacy in animal studies, relies on the use of advanced delivery systems including nanoparticles and gelling systems. Many of them have proven their utility as potential delivery platforms across barriers, but have not yet been applied in NIHL studies, such as the microshotgun drug delivery system. For more accurate delivery, it is essential to identify cell-specific protein biomarkers. Those biomarkers have to be expressed on the cell membrane and induce endocytosis upon ligand coupling. Only a few of them, including prestin, are considered as the most acknowledged candidates to meet such criteria. It is necessary to better our understanding of the major physicochemical parameters that mediate nanoparticle passage, biodistribution, and safety in cochlear fluids for drug delivery that based on biomaterials. In many NIHL researches, the assessment of the drug concentration and drug distribution once they enter the cochlea is omitted. Whether they have successfully overcome the tissue barrier is unclear and can only be speculated from their therapeutic effect. With prudence, the immunogenicity of biomaterials and the potential effects of advanced strategies such as targeting peptides should be evaluated.
Data acquired from animal models may have difficulty translating to human beings. There are huge differences in noise exposures experienced by preclinical animals and human subjects. The noise used in animal research, usually being wide or narrow band noise at a fixed sound pressure, is different from what people experience in real life, which is more complicated and flexible in pitch and intensity. It may have occurred in an intermittent pattern for years. As shown in Figure 12, future development of NIHL therapy relies on novel drug targets identified in NIHL mechanism studies, approaches to overcome biological barriers, and construction of advanced drug delivery systems. Distinguishing between different noise types and optimizing drug dose and time may better facilitate the translation of basic NIHL research to the clinical setting.
Abbreviations
AMPK: adenosine monophosphate-activated protein kinase; BLB: blood-labyrinth barrier; CPEs: chemical permeation enhancers; eNOS: endothelial nitric oxide synthases; GelMA: gelatin methacryloyl; GT1b: trisialoganglioside clostridial toxin receptor; Glu: glutamate; GluRs: glutamate receptors; HMGB1: high mobility group box 1; IHCs: inner hair cells; IL-1β: interleukin-1beta; IL-6: interleukin-6; JNK: c-Jun N-terminal kinase; LRP-1: low-density lipoprotein receptor-related protein 1; MAPK: mitogen-activated protein kinase; NAC: N-acetylcysteine; NIHL: noise-induced hearing loss; NO: nitric oxide; NPs: nanoparticles; NRF2: nuclear factor erythroid2-related factor 2; OHCs: outer hair cells; PKM2: pyruvate kinase M2; PPS: poly(propylene sulfide); RWM: round window membrane; ROS: reactive oxygen species; SGNs: type I spiral ganglion neurons; SIRT: sirtuin; SPION: superparamagnetic iron oxide nanoparticles; TDNs: tetrahedral DNA nanostructures; TM: tympanic membrane; TNF-α: tumor necrosis factor-alpha; VGCCs: voltage-gated Ca2+ channels.
Acknowledgements
The research was supported by the National Science Foundations of Chins (Grant No. 81970874), Shanghai Science and Technology Program (Grant No. 21010500100, 22140901700, 22DZ2204700, 22142202200), Basic Research Program of Shanghai Municipal Government (Grant No. 21JC1406002), Shanghai Shuguang Program (Grant No. 21SG39), and Program of Shanghai Subject Chief Scientist (Grant No. 18XD1404300).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Varela-Nieto I, Murillo-Cuesta S, Calvino M, Cediel R, Lassaletta L. Drug development for noise-induced hearing loss. Expert Opin Drug Discov. 2020;15:1457-71
2. Song Q, Shen P, Li X, Shi L, Liu L, Wang J. et al. Coding deficits in hidden hearing loss induced by noise: the nature and impacts. Sci Rep. 2016;6:25200
3. Chen H, Shi L, Liu L, Yin S, Aiken S, Wang J. Noise-induced cochlear synaptopathy and signal processing disorders. Neuroscience. 2019;407:41-52
4. Virkkunen H, Kauppinen T, Tenkanen L. Long-term effect of occupational noise on the risk of coronary heart disease. Scand J Work Environ Health. 2005;31:291-9
5. Manohar S, Chen GD, Ding D, Liu L, Wang J, Chen YC. et al. Unexpected consequences of noise-induced hearing loss: impaired hippocampal neurogenesis, memory, and stress. Front Integr Neurosci. 2022;16:871223
6. Paciello F, Rinaudo M, Longo V, Cocco S, Conforto G, Pisani A. et al. Auditory sensory deprivation induced by noise exposure exacerbates cognitive decline in a mouse model of Alzheimer's disease. Elife. 2021;10:e70908
7. Ferguson MA, Kitterick PT, Chong LY, Edmondson-Jones M, Barker F, Hoare DJ. Hearing aids for mild to moderate hearing loss in adults. Cochrane Database Syst Rev. 2017;9:CD12023
8. Boisvert I, Reis M, Au A, Cowan R, Dowell RC. Cochlear implantation outcomes in adults: a scoping review. PloS One. 2020;15:e232421
9. Le Prell CG. Investigational medicinal products for the inner ear: review of clinical trial characteristics in ClinicalTrials.gov. J Am Acad Audiol. 2021;32:670-94
10. O MD, Ricci AJ. A bundle of mechanisms: inner-ear hair-cell mechanotransduction. Trends Neurosci. 2019;42:221-36
11. Glueckert R, Johnson CL, Rask-Andersen H, Liu W, Handschuh S, Schrott-Fischer A. Anatomical basis of drug delivery to the inner ear. Hear Res. 2018;368:10-27
12. Wu YX, Zhu GX, Liu XQ, Sun F, Zhou K, Wang S. et al. Noise alters guinea pig's blood-labyrinth barrier ultrastructure and permeability along with a decrease of cochlear claudin-5 and occludin. Bmc Neurosci. 2014;15:136
13. Shi X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear Res. 2016;338:52-63
14. Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019:201-9
15. Kurabi A, Keithley EM, Housley GD, Ryan AF, Wong AC. Cellular mechanisms of noise-induced hearing loss. Hear Res. 2017;349:129-37
16. Ohinata Y, Miller JM, Altschuler RA, Schacht J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000;878:163-73
17. Vlajkovic SM, Housley GD, Munoz DJ, Robson SC, Sevigny J, Wang CJ. et al. Noise exposure induces up-regulation of ecto-nucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Neuroscience. 2004;126:763-73
18. Miller JM, Brown JN, Schacht J. 8-iso-prostaglandin F(2alpha), a product of noise exposure, reduces inner ear blood flow. Audiol Neurootol. 2003;8:207-21
19. Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47-59
20. Hu BH, Henderson D, Nicotera TM. F-actin cleavage in apoptotic outer hair cells in chinchilla cochleas exposed to intense noise. Hear Res. 2002;172:1-9
21. Kamio T, Watanabe K, Okubo K. Acoustic stimulation promotes DNA fragmentation in the guinea pig cochlea. J Nippon Med Sch. 2012;79:349-56
22. Jacob RF, Mason RP. Lipid peroxidation induces cholesterol domain formation in model membranes. J Biol Chem. 2005;280:39380-7
23. Wang J, Ruel J, Ladrech S, Bonny C, van de Water TR, Puel JL. Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol Pharmacol. 2007;71:654-66
24. Ohinata Y, Yamasoba T, Schacht J, Miller JM. Glutathione limits noise-induced hearing loss. Hear Res. 2000;146:28-34
25. Lu J, Li W, Du X, Ewert DL, West MB, Stewart C. et al. Antioxidants reduce cellular and functional changes induced by intense noise in the inner ear and cochlear nucleus. J Assoc Res Otolaryngol. 2014;15:353-72
26. Pourbakht A, Yamasoba T. Ebselen attenuates cochlear damage caused by acoustic trauma. Hear Res. 2003;181:100-8
27. Seidman M, Babu S, Tang W, Naem E, Quirk WS. Effects of resveratrol on acoustic trauma. Otolaryngol Head Neck Surg. 2003;129:463-70
28. Campbell KC, Meech RP, Klemens JJ, Gerberi MT, Dyrstad SS, Larsen DL. et al. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007;226:92-103
29. Rewerska A, Pawelczyk M, Rajkowska E, Politanski P, Sliwinska-Kowalska M. Evaluating D-methionine dose to attenuate oxidative stress-mediated hearing loss following overexposure to noise. Eur Arch Otorhinolaryngol. 2013;270:1513-20
30. McFadden SL, Woo JM, Michalak N, Ding D. Dietary vitamin C supplementation reduces noise-induced hearing loss in guinea pigs. Hear Res. 2005;202:200-8
31. Fetoni AR, De Bartolo P, Eramo SL, Rolesi R, Paciello F, Bergamini C. et al. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase in antioxidant defense. J Neurosci. 2013;33:4011-23
32. Ewert DL, Lu J, Li W, Du X, Floyd R, Kopke R. Antioxidant treatment reduces blast-induced cochlear damage and hearing loss. Hear Res. 2012;285:29-39
33. Bielefeld EC, Kopke RD, Jackson RL, Coleman JK, Liu J, Henderson D. Noise protection with N-acetyl-l-cysteine (NAC) using a variety of noise exposures, NAC doses, and routes of administration. Acta Otolaryngol. 2007;127:914-9
34. Rosenhall U, Skoog B, Muhr P. Treatment of military acoustic accidents with N-Acetyl-L-cysteine (NAC). Int J Audiol. 2019;58:151-7
35. Kramer S, Dreisbach L, Lockwood J, Baldwin K, Kopke R, Scranton S. et al. Efficacy of the antioxidant N-acetylcysteine (NAC) in protecting ears exposed to loud music. J Am Acad Audiol. 2006;17:265-78
36. Soyalıç H, Gevrek F, Karaman S. Curcumin protects against acoustic trauma in the rat cochlea. Int J Pediatr Otorhinolaryngol. 2017;99:100-6
37. Rodriguez I, Hong BN, Nam YH, Kim EY, Park GH, Ji MG. et al. Bioconversion of Scutellaria baicalensis extract can increase recovery of auditory function in a mouse model of noise-induced hearing loss. Biomed Pharmacother. 2017;93:1303-9
38. Castaneda R, Natarajan S, Jeong SY, Hong BN, Kang TH. Traditional oriental medicine for sensorineural hearing loss: Can ethnopharmacology contribute to potential drug discovery? J Ethnopharmacol. 2019;231:409-28
39. Ohlemiller KK. Recent findings and emerging questions in cochlear noise injury. Hear Res. 2008;245:5-17
40. Yoshida N, Liberman MC. Sound conditioning reduces noise-induced permanent threshold shift in mice. Hear Res. 2000;148:213-9
41. Honkura Y, Matsuo H, Murakami S, Sakiyama M, Mizutari K, Shiotani A. et al. NRF2 is a key target for prevention of noise-induced hearing loss by reducing oxidative damage of cochlea. Sci Rep. 2016;6:19329
42. Fetoni AR, Paciello F, Rolesi R, Paludetti G, Troiani D. Targeting dysregulation of redox homeostasis in noise-induced hearing loss: oxidative stress and ROS signaling. Free Radic Biol Med. 2019;135:46-59
43. Wang X, Zeng C, Lai Y, Su B, Chen F, Zhong J. et al. NRF2/HO-1 pathway activation by ATF3 in a noise-induced hearing loss murine model. Arch Biochem Biophys. 2022;721:109190
44. Yang H, Zhu Y, Ye Y, Guan J, Min X, Xiong H. Nitric oxide protects against cochlear hair cell damage and noise-induced hearing loss through glucose metabolic reprogramming. Free Radic Biol Med. 2022;179:229-41
45. Liu Y, Ao L, Li Y, Zhao Y, Wen Y, Ding H. The SIRT2 inhibitor AK-7 decreases cochlear cell apoptosis and attenuates noise-induced hearing loss. Biochem Biophys Res Commun. 2019;509:641-6
46. Zhao C, Liang W, Yang Z, Chen Z, Du Z, Gong S. SIRT3-mediated deacetylation protects inner hair cell synapses in a H(2)O(2)-induced oxidative stress model in vitro. Exp Cell Res. 2022;418:113280
47. Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W. et al. Activation of SIRT3 by the NAD(+) precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 2014;20:1059-68
48. Khoshsirat S, Abbaszadeh HA, Peyvandi AA, Heidari F, Peyvandi M, Simani L. et al. Apelin-13 prevents apoptosis in the cochlear tissue of noise-exposed rat via Sirt-1 regulation. J Chem Neuroanat. 2021;114:101956
49. Hati S, Zallocchi M, Hazlitt R, Li Y, Vijayakumar S, Min J. et al. AZD5438-PROTAC: a selective CDK2 degrader that protects against cisplatin- and noise-induced hearing loss. Eur J Med Chem. 2021;226:113849
50. Shin SH, Jung J, Park HR, Sim NS, Choi JY, Bae SH. The time course of monocytes infiltration after acoustic overstimulation. Front Cell Neurosci. 2022;16:844480
51. Fujioka M, Okano H, Ogawa K. Inflammatory and immune responses in the cochlea: potential therapeutic targets for sensorineural hearing loss. Front Pharmacol. 2014;5:287
52. Mizushima Y, Fujimoto C, Kashio A, Kondo K, Yamasoba T. Macrophage recruitment, but not interleukin 1 beta activation, enhances noise-induced hearing damage. Biochem Biophys Res Commun. 2017;493:894-900
53. Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res. 2006;83:575-83
54. Yang S, Cai Q, Vethanayagam RR, Wang J, Yang W, Hu BH. Immune defense is the primary function associated with the differentially expressed genes in the cochlea following acoustic trauma. Hear Res. 2016;333:283-94
55. Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol. 2003;4:339-52
56. Manickam V, Gawande DY, Stothert AR, Clayman AC, Batalkina L, Warchol ME. et al. Macrophages promote repair of inner hair cell ribbon synapses following noise-induced cochlear synaptopathy. J Neurosci. 2023;43:2075-89
57. Murillo-Cuesta S, Rodriguez-de LRL, Contreras J, Celaya AM, Camarero G, Rivera T. et al. Transforming growth factor beta1 inhibition protects from noise-induced hearing loss. Front Aging Neurosci. 2015;7:32
58. Bianchi ME, Crippa MP, Manfredi AA, Mezzapelle R, Rovere QP, Venereau E. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol Rev. 2017;280:74-82
59. Xiao L, Sun Y, Liu C, Zheng Z, Shen Y, Xia L. et al. Molecular behavior of hmgb1 in the cochlea following noise exposure and in vitro. Front Cell Dev Biol. 2021;9:642946
60. Xiao L, Zhang Z, Liu J, Zheng Z, Xiong Y, Li C. et al. HMGB1 accumulation in cytoplasm mediates noise-induced cochlear damage. Cell Tissue Res. 2023;391:43-54
61. Wei L, Zhang W, Li Y, Zhai J. The SIRT1-HMGB1 axis: therapeutic potential to ameliorate inflammatory responses and tumor occurrence. Front Cell Dev Biol. 2022;10:986511
62. Shih CP, Kuo CY, Lin YY, Lin YC, Chen HK, Wang H. et al. Inhibition of cochlear HMGB1 expression attenuates oxidative stress and inflammation in an experimental murine model of noise-induced hearing loss. Cells. 2021;10(Suppl 4):810
63. Paciello F, Pisani A, Rolesi R, Escarrat V, Galli J, Paludetti G. et al. Noise-induced cochlear damage involves PPAR down-regulation through the interplay between oxidative stress and inflammation. Antioxidants (Basel). 2021;10(Suppl 8):1188
64. Paciello F, Fetoni AR, Rolesi R, Wright MB, Grassi C, Troiani D. et al. Pioglitazone represents an effective therapeutic target in preventing oxidative/inflammatory cochlear damage induced by noise exposure. Front Pharmacol. 2018;9:1103
65. Takemura K, Komeda M, Yagi M, Himeno C, Izumikawa M, Doi T. et al. Direct inner ear infusion of dexamethasone attenuates noise-induced trauma in guinea pig. Hear Res. 2004;196:58-68
66. Han MA, Back SA, Kim HL, Park SY, Yeo SW, Park SN. Therapeutic effect of dexamethasone for noise-induced hearing loss: systemic versus intratympanic injection in mice. Otol Neurotol. 2015;36:755-62
67. Harrop-Jones A, Wang X, Fernandez R, Dellamary L, Ryan AF, LeBel C. et al. The sustained-exposure dexamethasone formulation OTO-104 offers effective protection against noise-induced hearing loss. Audiol Neurootol. 2016;21:12-21
68. Heinrich UR, Strieth S, Schmidtmann I, Stauber R, Helling K. Dexamethasone prevents hearing loss by restoring glucocorticoid receptor expression in the guinea pig cochlea. Laryngoscope. 2016;126:E29-34
69. Muller M, Tisch M, Maier H, Lowenheim H. Reduction of permanent hearing loss by local glucocorticoid application: guinea pigs with acute acoustic trauma. HNO. 2017;65:59-67
70. Nelson L, Lovett B, Johns JD, Gu S, Choi D, Trune D. et al. In silico single-cell analysis of steroid-responsive gene targets in the mammalian cochlea. Front Neurol. 2021;12:818157
71. Shih CP, Chen HC, Lin YC, Chen HK, Wang H, Kuo CY. et al. Middle-ear dexamethasone delivery via ultrasound microbubbles attenuates noise-induced hearing loss. Laryngoscope. 2019;129:1907-14
72. Missner AA, Johns JD, Gu S, Hoa M. Repurposable drugs that interact with steroid responsive gene targets for inner ear disease. Biomolecules. 2022;12(Suppl 11):1641
73. Shin S, Lyu A, Jeong S, Kim TH, Park MJ, Park Y. Acoustic trauma modulates cochlear blood flow and vasoactive factors in a rodent model of noise-induced hearing loss. Int J Mol Sci. 2019;20:5316
74. Dai M, Shi X. Fibro-vascular coupling in the control of cochlear blood flow. PloS One. 2011;6:e20652
75. Abaamrane L, Raffin F, Gal M, Avan P, Sendowski I. Long-term administration of magnesium after acoustic trauma caused by gunshot noise in guinea pigs. Hear Res. 2009;247:137-45
76. Attias J, Sapir S, Bresloff I, Reshef-Haran I, Ising H. Reduction in noise-induced temporary threshold shift in humans following oral magnesium intake. Clin Otolaryngol Allied Sci. 2004;29:635-41
77. Sendowski I, Raffin F, Braillon-Cros A. Therapeutic efficacy of magnesium after acoustic trauma caused by gunshot noise in guinea pigs. Acta Otolaryngol. 2006;126:122-9
78. Alvarado JC, Fuentes-Santamaria V, Juiz JM. Antioxidants and vasodilators for the treatment of noise-induced hearing loss: Are they really effective? Front Cell Neurosci. 2020;14:226
79. Kum NY, Yilmaz YF, Gurgen SG, Kum RO, Ozcan M, Unal A. Effects of parenteral papaverine and piracetam administration on cochlea following acoustic trauma. Noise Health. 2018;20:47-52
80. Ceylan SM, Uysal E, Altinay S, Sezgin E, Bilal N, Petekkaya E. et al. Protective and therapeutic effects of milrinone on acoustic trauma in rat cochlea. Eur Arch Otorhinolaryngol. 2019;276:1921-31
81. Xiong H, Lai L, Ye Y, Zheng Y. Glucose protects cochlear hair cells against oxidative stress and attenuates noise-induced hearing loss in mice. Neurosci Bull. 2021;37:657-68
82. Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179-83
83. Zhao R, Ma C, Wang M, Li X, Liu W, Shi L. et al. Killer or helper? The mechanism underlying the role of adenylate activated kinase in sound conditioning. Front Synaptic Neurosci. 2022;14:940788
84. Zheng HW, Chen J, Sha SH. Receptor-interacting protein kinases modulate noise-induced sensory hair cell death. Cell Death Dis. 2014;5:e1262
85. Hill K, Yuan H, Wang X, Sha SH. Noise-induced loss of hair cells and cochlear synaptopathy are mediated by the activation of AMPK. J Neurosci. 2016;36:7497-510
86. Ye B, Fan C, Shen Y, Wang Q, Hu H, Xiang M. The antioxidative role of autophagy in hearing loss. Front Neurosci. 2018;12:1010
87. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069-75
88. Yuan H, Wang X, Hill K, Chen J, Lemasters J, Yang SM. et al. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid Redox Signal. 2015;22:1308-24
89. Singh SS, Vats S, Chia AY, Tan TZ, Deng S, Ong MS. et al. Dual role of autophagy in hallmarks of cancer. Oncogene. 2018;37:1142-58
90. He ZH, Pan S, Zheng HW, Fang QJ, Hill K, Sha SH. Treatment with calcineurin inhibitor FK506 attenuates noise-induced hearing loss. Front Cell Dev Biol. 2021;9:648461
91. Castellano-Muñoz M, Ricci AJ. Role of intracellular calcium stores in hair-cell ribbon synapse. Front Cell Neurosci. 2014;8:162
92. Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122-9
93. Uemaetomari I, Tabuchi K, Nakamagoe M, Tanaka S, Murashita H, Hara A. L-type voltage-gated calcium channel is involved in the pathogenesis of acoustic injury in the cochlea. Tohoku J Exp Med. 2009;218:41-7
94. Shen H, Zhang B, Shin JH, Lei D, Du Y, Gao X. et al. Prophylactic and therapeutic functions of T-type calcium blockers against noise-induced hearing loss. Hear Res. 2007;226:52-60
95. Sebe JY, Cho S, Sheets L, Rutherford MA, von Gersdorff H, Raible DW. Ca(2+)-permeable AMPARs mediate glutamatergic transmission and excitotoxic damage at the hair cell ribbon synapse. J Neurosci. 2017;37:6162-75
96. Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325-37
97. Hu N, Rutherford MA, Green SH. Protection of cochlear synapses from noise-induced excitotoxic trauma by blockade of Ca(2+)-permeable AMPA receptors. Proc Natl Acad Sci U S A. 2020;117:3828-38
98. Diering GH, Huganir RL. The AMPA receptor code of synaptic plasticity. Neuron. 2018;100:314-29
99. Zhu J, Yang Y, Ma W, Wang Y, Chen L, Xiong H. et al. Antiepilepticus effects of tetrahedral framework nucleic acid via inhibition of gliosis-induced downregulation of glutamine synthetase and increased AMPAR internalization in the postsynaptic membrane. Nano Lett. 2022;22:2381-90
100. Wang J, Yin S, Chen H, Shi L. Noise-induced cochlear synaptopathy and ribbon synapse regeneration: repair process and therapeutic target. Adv Exp Med Biol. 2019;1130:37-57
101. Suzuki J, Corfas G, Liberman MC. Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci Rep. 2016;6:24907
102. Wan G, Gomez-Casati ME, Gigliello AR, Liberman MC, Corfas G. Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. Elife. 2014;3:e03564
103. Kim KX, Payne S, Yang-Hood A, Li SZ, Davis B, Carlquist J. et al. Vesicular glutamatergic transmission in noise-induced loss and repair of cochlear ribbon synapses. J Neurosci. 2019;39:4434-47
104. Yang WP, Henderson D, Hu BH, Nicotera TM. Quantitative analysis of apoptotic and necrotic outer hair cells after exposure to different levels of continuous noise. Hear Res. 2004;196:69-76
105. Kayyali MN, Wooltorton J, Ramsey AJ, Lin M, Chao TN, Tsourkas A. et al. A novel nanoparticle delivery system for targeted therapy of noise-induced hearing loss. J Control Release. 2018;279:243-50
106. Wang J, Ruel J, Ladrech S, Bonny C, van de Water TR, Puel JL. Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol Pharmacol. 2007;71:654-66
107. Eshraghi AA, Aranke M, Salvi R, Ding D, Coleman JJ, Ocak E. et al. Preclinical and clinical otoprotective applications of cell-penetrating peptide D-JNKI-1 (AM-111). Hear Res. 2018;368:86-91
108. Coffin AB, Rubel EW, Raible DW. Bax, Bcl2, and p53 differentially regulate neomycin- and gentamicin-induced hair cell death in the zebrafish lateral line. J Assoc Res Otolaryngol. 2013;14:645-59
109. Zhang Y, Chen D, Zhao L, Li W, Ni Y, Chen Y. et al. Nfatc4 deficiency attenuates ototoxicity by suppressing Tnf-mediated hair cell apoptosis in the mouse cochlea. Front Immunol. 2019;10:1660
110. Kopeina GS, Zhivotovsky B. Programmed cell death: past, present and future. Biochem Biophys Res Commun. 2022;633:55-8
111. Ma PW, Wang WL, Chen JW, Yuan H, Lu PH, Gao W. et al. Treatment with the ferroptosis inhibitor ferrostatin-1 attenuates noise-induced hearing loss by suppressing ferroptosis and apoptosis. Oxid Med Cell Longev. 2022;2022:3373828
112. Bao J, Hungerford M, Luxmore R, Ding D, Qiu Z, Lei D. et al. Prophylactic and therapeutic functions of drug combinations against noise-induced hearing loss. Hear Res. 2013;304:33-40
113. Kishimoto-Urata M, Urata S, Fujimoto C, Yamasoba T. Role of oxidative stress and antioxidants in acquired inner ear disorders. Antioxidants (Basel). 2022;11(Suppl 8):1469
114. Lin CY, Wu JL, Shih TS, Tsai PJ, Sun YM, Ma MC. et al. N-Acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear Res. 2010;269:42-7
115. Le Prell CG, Johnson AC, Lindblad AC, Skjonsberg A, Ulfendahl M, Guire K. et al. Increased vitamin plasma levels in Swedish military personnel treated with nutrients prior to automatic weapon training. Noise Health. 2011;13:432-43
116. Kil J, Lobarinas E, Spankovich C, Griffiths SK, Antonelli PJ, Lynch ED. et al. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2017;390:969-79
117. Choi CH, Chen K, Du X, Floyd RA, Kopke RD. Effects of delayed and extended antioxidant treatment on acute acoustic trauma. Free Radic Res. 2011;45:1162-72
118. Meyer T. Intratympanic treatment for tinnitus: a review. Noise Health. 2013;15:83-90
119. Choi N, Kim JS, Chang YS. Comparison of oral steroid regimens for acute acoustic trauma caused by gunshot noise exposure. J Laryngol Otol. 2019;133:566-70
120. Bayoumy AB, van der Veen EL, van Ooij P, Besseling-Hansen FS, Koch D, Stegeman I. et al. Effect of hyperbaric oxygen therapy and corticosteroid therapy in military personnel with acute acoustic trauma. Bmj Mil Health. 2020;166:243-8
121. Mora R, Mora E, Salzano FA, Guastini L. Audiometric characteristics in patients with noise-induced hearing loss after sodium enoxaparin treatment. Ann Otol Rhinol Laryngol. 2012;121:85-90
122. Bird PA, Begg EJ, Zhang M, Keast AT, Murray DP, Balkany TJ. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol Neurotol. 2007;28:1124-30
123. Bird PA, Murray DP, Zhang M, Begg EJ. Intratympanic versus intravenous delivery of dexamethasone and dexamethasone sodium phosphate to cochlear perilymph. Otol Neurotol. 2011;32:933-6
124. Nyberg S, Abbott NJ, Shi X, Steyger PS, Dabdoub A. Delivery of therapeutics to the inner ear: the challenge of the blood-labyrinth barrier. Sci Transl Med. 2019;11(Suppl 482):eaao0935
125. Zhang W, Dai M, Fridberger A, Hassan A, Degagne J, Neng L. et al. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc Natl Acad Sci U S A. 2012;109:10388-93
126. Kim J, Ricci AJ. In vivo real-time imaging reveals megalin as the aminoglycoside gentamicin transporter into cochlea whose inhibition is otoprotective. Proc Natl Acad Sci U S A. 2022;119(Suppl 9):e2117946119
127. Shi X, Wang Z, Ren W, Chen L, Xu C, Li M. et al. LDL receptor-related protein 1 (LRP1), a novel target for opening the blood-labyrinth barrier (BLB). Signal Transduct Target Ther. 2022;7:175
128. Shi X. Resident macrophages in the cochlear blood-labyrinth barrier and their renewal via migration of bone-marrow-derived cells. Cell Tissue Res. 2010;342:21-30
129. Terstappen GC, Meyer AH, Bell RD, Zhang W. Strategies for delivering therapeutics across the blood-brain barrier. Nat Rev Drug Discov. 2021;20:362-83
130. Xu X, Lin K, Wang Y, Xu K, Sun Y, Yang X. et al. A metal-organic framework based inner ear delivery system for the treatment of noise-induced hearing loss. Nanoscale. 2020;12:16359-65
131. Horie RT, Sakamoto T, Nakagawa T, Ishihara T, Higaki M, Ito J. Stealth-nanoparticle strategy for enhancing the efficacy of steroids in mice with noise-induced hearing loss. Nanomedicine (Lond). 2010;5:1331-40
132. Hirose K, Hartsock JJ, Johnson S, Santi P, Salt AN. Systemic lipopolysaccharide compromises the blood-labyrinth barrier and increases entry of serum fluorescein into the perilymph. J Assoc Res Otolaryngol. 2014;15:707-19
133. Liu H, Ding DL, Jiang HY, Wu XW, Salvi R, Sun H. Ototoxic destruction by co-administration of kanamycin and ethacrynic acid in rats. J Zhejiang Univ Sci B. 2011;12:853-61
134. Le TN, Blakley BW. Mannitol and the blood-labyrinth barrier. J Otolaryngol Head Neck Surg. 2017;46:66
135. Noman A, Mukherjee S, Le TN. Manipulating the blood labyrinth barrier with mannitol to prevent cisplatin-induced hearing loss. Hear Res. 2022;426:108646
136. Mittal R, Pena SA, Zhu A, Eshraghi N, Fesharaki A, Horesh EJ. et al. Nanoparticle-based drug delivery in the inner ear: current challenges, limitations and opportunities. Artif Cells Nanomed Biotechnol. 2019;47:1312-20
137. Salt AN, Hirose K. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear Res. 2018;362:25-37
138. Yang R, Wei T, Goldberg H, Wang W, Cullion K, Kohane DS. Getting drugs across biological barriers. Adv Mater. 2017;29(Suppl 37):10.1002
139. Suzuki J, Corfas G, Liberman MC. Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci Rep. 2016;6:24907
140. Paciello F, Fetoni AR, Rolesi R, Wright MB, Grassi C, Troiani D. et al. Pioglitazone represents an effective therapeutic target in preventing oxidative/inflammatory cochlear damage induced by noise exposure. Front Pharmacol. 2018;9:1103
141. Zhang Z, Li X, Zhang W, Kohane DS. Drug delivery across barriers to the middle and inner ear. Adv Funct Mater. 2021;31(Suppl 44):2008701
142. Yang R, Sabharwal V, Okonkwo OS, Shlykova N, Tong R, Lin LY. et al. Treatment of otitis media by transtympanic delivery of antibiotics. Sci Transl Med. 2016;8:356ra120
143. Wang J, Wang C, Wang Q, Zhang Z, Wang H, Wang S. et al. Microfluidic preparation of gelatin methacryloyl microgels as local drug delivery vehicles for hearing loss therapy. ACS Appl Mater Interfaces. 2022;14(Suppl 41):46212-46223
144. Mittal R, Pena SA, Zhu A, Eshraghi N, Fesharaki A, Horesh EJ. et al. Nanoparticle-based drug delivery in the inner ear: current challenges, limitations and opportunities. Artif Cells Nanomed Biotechnol. 2019;47:1312-20
145. Zhang L, Xu Y, Cao W, Xie S, Wen L, Chen G. Understanding the translocation mechanism of PLGA nanoparticles across round window membrane into the inner ear: a guideline for inner ear drug delivery based on nanomedicine. Int J Nanomedicine. 2018;13:479-92
146. Chen Y, Gu J, Liu Y, Xu K, Song J, Wang X. et al. Epigallocatechin gallate-loaded tetrahedral DNA nanostructures as a novel inner ear drug delivery system. Nanoscale. 2022;14:8000-11
147. Wiraja C, Zhu Y, Lio DCS, Yeo DC, Xie M, Fang W. et al. Framework nucleic acids as programmable carrier for transdermal drug delivery. Nat Commun. 2019;10(Suppl 1):1147
148. Jeong S, Kim Y, Lyu A, Shin S, Kim TH, Huh YH. et al. Junctional modulation of round window membrane enhances dexamethasone uptake into the inner ear and recovery after NIHL. Int J Mol Sci. 2021;22:10061
149. Liu M, Zhang J, Zhu X, Shan W, Li L, Zhong J. et al. Efficient mucus permeation and tight junction opening by dissociable "mucus-inert" agent coated trimethyl chitosan nanoparticles for oral insulin delivery. J Control Release. 2016;222:67-77
150. Khoo X, Simons EJ, Chiang HH, Hickey JM, Sabharwal V, Pelton SI. et al. Formulations for trans-tympanic antibiotic delivery. Biomaterials. 2013;34:1281-8
151. Braun S, Ye Q, Radeloff A, Kiefer J, Gstoettner W, Tillein J. Protection of inner ear function after cochlear implantation: compound action potential measurements after local application of glucocorticoids in the guinea pig cochlea. Orl J Otorhinolaryngol Relat Spec. 2011;73:219-28
152. Dai C, Lehar M, Sun DQ, Rvt LS, Carey JP, MacLachlan T. et al. Rhesus cochlear and vestibular functions are preserved after inner ear injection of saline volume sufficient for gene therapy delivery. J Assoc Res Otolaryngol. 2017;18:601-17
153. El KN, Agnely F, Mamelle E, Nguyen Y, Ferrary E, Bochot A. Recent advances in local drug delivery to the inner ear. Int J Pharm. 2015;494:83-101
154. Plontke SK, Lowenheim H, Mertens J, Engel C, Meisner C, Weidner A. et al. Randomized, double blind, placebo controlled trial on the safety and efficacy of continuous intratympanic dexamethasone delivered via a round window catheter for severe to profound sudden idiopathic sensorineural hearing loss after failure of systemic therapy. Laryngoscope. 2009;119:359-69
155. Peppi M, Marie A, Belline C, Borenstein JT. Intracochlear drug delivery systems: a novel approach whose time has come. Expert Opin Drug Deliv. 2018;15:319-24
156. Malfeld K, Armbrecht N, Pich A, Volk HA, Lenarz T, Scheper V. Prevention of noise-induced hearing loss in vivo: continuous application of insulin-like growth factor 1 and its effect on inner ear synapses, auditory function and perilymph proteins. Int J Mol Sci. 2022;24(Suppl 1):291
157. Malfeld K, Baumhoff P, Volk HA, Lenarz T, Scheper V. Local long-term inner ear drug delivery in normal hearing guinea pig-an animal model to develop preventive treatment for noise-induced hearing loss. Biomolecules. 2022;12(Suppl 10):1427
158. Tandon V, Kang WS, Spencer AJ, Kim ES, Pararas EE, McKenna MJ. et al. Microfabricated infuse-withdraw micropump component for an integrated inner-ear drug-delivery platform. Biomed Microdevices. 2015;17:37
159. Tandon V, Kang WS, Robbins TA, Spencer AJ, Kim ES, McKenna MJ. et al. Microfabricated reciprocating micropump for intracochlear drug delivery with integrated drug/fluid storage and electronically controlled dosing. Lab Chip. 2016;16:829-46
160. Sale P, Uschakov A, Saief T, Rowe DP, Abbott CJ, Luu CD. et al. Cannula-based drug delivery to the guinea pig round window causes a lasting hearing loss that may be temporarily mitigated by BDNF. Hear Res. 2017;356:104-15
161. Gao G, Liu Y, Zhou CH, Jiang P, Sun JJ. Solid lipid nanoparticles loaded with edaravone for inner ear protection after noise exposure. Chin Med J (Engl). 2015;128:203-9
162. Wang N, Gao X, Li M, Li Y, Sun M. Use of solid lipid nanoparticles for the treatment of acute acoustic stress-induced cochlea damage. J Nanosci Nanotechnol. 2020;20:7412-8
163. Cho J, Kim BJ, Hwang Y, Woo S, Noh T, Suh M. Effect and biocompatibility of a cross-linked hyaluronic acid and polylactide-co-glycolide microcapsule vehicle in intratympanic drug delivery for treating acute acoustic trauma. Int J Mol Sci. 2021;22:5720
164. Zhao Z, Han Z, Naveena K, Lei G, Qiu S, Li X. et al. ROS-responsive nanoparticle as a berberine carrier for OHC-targeted therapy of noise-induced hearing loss. ACS Appl Mater Interfaces. 2021;13:7102-14
165. An X, Wang R, Chen E, Yang Y, Fan B, Li Y. et al. A forskolin-loaded nanodelivery system prevents noise-induced hearing loss. J Control Release. 2022;348:148-57
166. Mukherjee S, Kuroiwa M, Oakden W, Paul BT, Noman A, Chen J. et al. Local magnetic delivery of adeno-associated virus AAV2(quad Y-F)-mediated BDNF gene therapy restores hearing after noise injury. Mol Ther. 2022;30:519-33
167. Lee KY, Nakagawa T, Okano T, Hori R, Ono K, Tabata Y. et al. Novel therapy for hearing loss: delivery of insulin-like growth factor 1 to the cochlea using gelatin hydrogel. Otol Neurotol. 2007;28:976-81
168. Inaoka T, Nakagawa T, Kikkawa YS, Tabata Y, Ono K, Yoshida M. et al. Local application of hepatocyte growth factor using gelatin hydrogels attenuates noise-induced hearing loss in guinea pigs. Acta Otolaryngol. 2009;129:453-7
169. Mamelle E, El KN, Adenis V, Nguyen Y, Sterkers O, Agnely F. et al. Assessment of the efficacy of a local steroid rescue treatment administered 2 days after a moderate noise-induced trauma in guinea pig. Acta Otolaryngol. 2018;138:610-6
170. Li Y, Zhang R, Li X, Li W, Lu Y, Dai C. The preparation of dexamethasone sodium phosphate multivesicular liposomes thermosensative hydrogel and its impact on noise-induced hearing loss in the guinea pigs. Exp Cell Res. 2020;387:111755
171. Li ML, Lee LC, Cheng YR, Kuo CH, Chou YF, Chen YS. et al. A novel aerosol-mediated drug delivery system for inner ear therapy: intratympanic aerosol methylprednisolone can attenuate acoustic trauma. IEEE Trans Biomed Eng. 2013;60:2450-60
172. Lin Y, Lin Y, Chen H, Kuo C, Liao A, Chou Y. et al. Ultrasound microbubbles enhance the efficacy of insulin-like growth factor-1 therapy for the treatment of noise-induced hearing loss. Molecules. 2021;26:3626
173. Zhang X, Chen G, Wen L, Yang F, Shao AL, Li X. et al. Novel multiple agents loaded PLGA nanoparticles for brain delivery via inner ear administration: in vitro and in vivo evaluation. Eur J Pharm Sci. 2013;48:595-603
174. Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149-55
175. Surovtseva EV, Johnston AH, Zhang W, Zhang Y, Kim A, Murakoshi M. et al. Prestin binding peptides as ligands for targeted polymersome mediated drug delivery to outer hair cells in the inner ear. Int J Pharm. 2012;424:121-7
176. Wang X, Chen Y, Tao Y, Gao Y, Yu D, Wu H. A666-conjugated nanoparticles target prestin of outer hair cells preventing cisplatin-induced hearing loss. Int J Nanomedicine. 2018;13:7517-31
177. Zhang Y, Zhang W, Johnston AH, Newman TA, Pyykko I, Zou J. Targeted delivery of Tet1 peptide functionalized polymersomes to the rat cochlear nerve. Int J Nanomedicine. 2012;7:1015-22
178. Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272-80
179. Santi PA, Mancini P, Barnes C. Identification and localization of the GM1 ganglioside in the cochlea using thin-layer chromatography and cholera toxin. J Histochem Cytochem. 1994;42:705-16
180. Kempfle JS, Nguyen K, Hamadani C, Koen N, Edge AS, Kashemirov BA. et al. Bisphosphonate-linked TrkB agonist: cochlea-targeted delivery of a neurotrophic agent as a strategy for the treatment of hearing loss. Bioconjug Chem. 2018;29:1240-50
181. Glueckert R, Pritz CO, Roy S, Dudas J, Schrott-Fischer A. Nanoparticle mediated drug delivery of rolipram to tyrosine kinase B positive cells in the inner ear with targeting peptides and agonistic antibodies. Front Aging Neurosci. 2015;7:71
182. Tian X, Nyberg S, S SP, Madsen J, Daneshpour N, Armes SP. et al. LRP-1-mediated intracellular antibody delivery to the central nervous system. Sci Rep. 2015;5:11990
183. Mäder K, Lehner E, Liebau A, Plontke SK. Controlled drug release to the inner ear: concepts, materials, mechanisms, and performance. Hear Res. 2018;368:49-66
184. Zarrintaj P, Ramsey JD, Samadi A, Atoufi Z, Yazdi MK, Ganjali MR. et al. Poloxamer: a versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020;110:37-67
185. Li X, Wang Y, Xu F, Zhang F, Xu Y, Tang L. et al. Artemisinin loaded mPEG-PCL nanoparticle based photosensitive gelatin methacrylate hydrogels for the treatment of gentamicin induced hearing loss. Int J Nanomedicine. 2020;15:4591-606
186. Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery-a general review. Expert Opin Drug Deliv. 2004;1:37-56
187. Liao AH, Wang CH, Weng PY, Lin YC, Wang H, Chen HK. et al. Ultrasound-induced microbubble cavitation via a transcanal or transcranial approach facilitates inner ear drug delivery. JCI Insight. 2020;5(Suppl 3):e132880
188. Du X, Chen K, Kuriyavar S, Kopke RD, Grady BP, Bourne DH. et al. Magnetic targeted delivery of dexamethasone acetate across the round window membrane in guinea pigs. Otol Neurotol. 2013;34:41-7
189. Mondalek FG, Zhang YY, Kropp B, Kopke RD, Ge X, Jackson RL. et al. The permeability of SPION over an artificial three-layer membrane is enhanced by external magnetic field. J Nanobiotechnology. 2006;4:4
190. Doot RK, Hess H, Vogel V. Engineered networks of oriented microtubule filaments for directed cargo transport. Soft Matter. 2007;3:349-56
191. Liang Z, Yu H, Lai J, Wen L, Chen G. An easy-to-prepare microshotgun for efficient transmembrane delivery by powering nanoparticles. J Control Release. 2020;321:119-31
192. Poole KM, Nelson CE, Joshi RV, Martin JR, Gupta MK, Haws SC. et al. ROS-responsive microspheres for on demand antioxidant therapy in a model of diabetic peripheral arterial disease. Biomaterials. 2015;41:166-75
193. Pu HL, Chiang WL, Maiti B, Liao ZX, Ho YC, Shim MS. et al. Nanoparticles with dual responses to oxidative stress and reduced ph for drug release and anti-inflammatory applications. ACS Nano. 2014;8:1213-21
194. Gu J, Wang X, Chen Y, Xu K, Yu D, Wu H. An enhanced antioxidant strategy of astaxanthin encapsulated in ROS-responsive nanoparticles for combating cisplatin-induced ototoxicity. J Nanobiotechnology. 2022;20:268
Author contact
![]() Corresponding authors: Materdicine Lab, School of Life Sciences, Shanghai University, Shanghai, China. E-mail address: chenyueduedu.cn; dehongyuedu.cn.
Corresponding authors: Materdicine Lab, School of Life Sciences, Shanghai University, Shanghai, China. E-mail address: chenyueduedu.cn; dehongyuedu.cn.
 Global reach, higher impact
Global reach, higher impact