13.3
Impact Factor
Theranostics 2023; 13(3):1028-1041. doi:10.7150/thno.81494 This issue Cite
Review
When three is not a crowd: trispecific antibodies for enhanced cancer immunotherapy
1. Immuno-oncology and Immunotherapy Group, Biomedical Research Institute Hospital 12 de Octubre, Madrid, Spain.
2. Cancer Immunotherapy Unit (UNICA), Hospital Universitario 12 de Octubre, Madrid, Spain.
3. H12O-CNIO Cancer Immunotherapy Clinical Research Unit, Spanish National Cancer Research Centre (CNIO), Madrid, Spain.
4. Department of Antibody Engineering, Leadartis S.L., Madrid, Spain.
5. Molecular Immunology Unit, Biomedical Research Institute Hospital Universitario Puerta de Hierro Majadahonda, Madrid, Spain.
Abstract
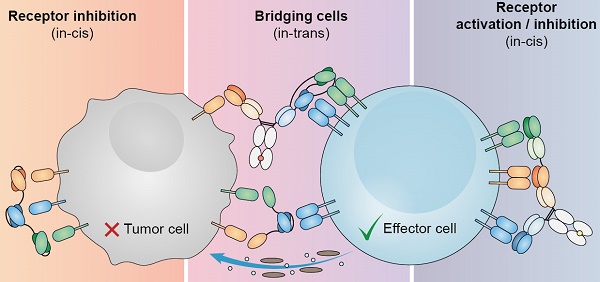
Despite the clinical success of the first bispecific antibody approved by the FDA against B cell malignancies (blinatumomab), many obstacles remain such as dosing, treatment resistance, and modest efficacy in solid tumors. To overcome these limitations, considerable efforts have been dedicated to the development of multispecific antibodies, opening up new avenues to address both the complex biology of cancer and the onset of anti-tumoral immune responses. Simultaneous targeting of two tumor-associated antigens is presumed to enhance cancer cell selectivity and reduce immune escape. Co-engagement of CD3, along with agonists of co-stimulatory molecules or antagonists of co-inhibitory immune checkpoint receptors in a single molecule, may revert T cell exhaustion. Similarly, targeting of two activating receptors in NK cells may improve their cytotoxic potency. And these are only examples of the potential of antibody-based molecular entities engaging three (or more) relevant targets. From the perspective of health care costs, multispecific antibodies are appealing, since a similar (or superior) therapeutic effect could be obtained with a single therapeutic agent as with a combination of different monoclonal antibodies. Despite challenges in production, multispecific antibodies are endowed with unprecedented properties, which may render them more potent biologics for cancer therapy.
Keywords: antibody engineering, cancer immunotherapy, trispecific antibody, T cell engager, NK cell engager
 Global reach, higher impact
Global reach, higher impact