13.3
Impact Factor
Theranostics 2022; 12(18):7760-7774. doi:10.7150/thno.76852 This issue Cite
Research Paper
m6A reader hnRNPA2B1 drives multiple myeloma osteolytic bone disease
1. Cancer Research Center, School of Medicine, Xiamen University, Xiamen, 361102, China.
2. Department of Hematology, Qingdao Municipal Hospital, School of Medicine, Qingdao University, Qingdao, 266011, China.
3. Eye Institute of Xiamen University, Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, School of Medicine, Xiamen University, Xiamen 361102, China.
4. State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics & Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen 361102, China.
5. Department of Hematology, The First Affiliated Hospital of Xiamen University and Institute of Hematology, School of Medicine, Xiamen University, Xiamen, 361102, China.
6. Department of Hematology, Key Laboratory of Xiamen for Diagnosis and Treatment of Hematological Malignancy, Xiamen, 361102, China.
7. Fujian Provincial Key Laboratory of Organ and Tissue Regeneration, Xiamen Key Laboratory of Regeneration Medicine, Organ Transplantation Institute of Xiamen University, School of Medicine, Xiamen University, Xiamen, 361102, China.
8. Shenzhen Research Institute of Xiamen University, Shenzhen, Guangdong 518057, China.
*Lead contact.
Abstract
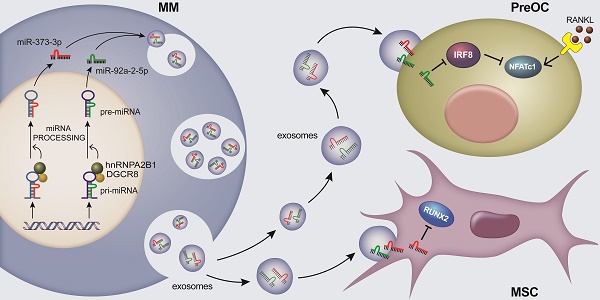
Rationale: Bone destruction is a hallmark of multiple myeloma (MM) and affects more than 80% of patients. Although previous works revealed the roles of N6-methyladenosine (m6A) reader hnRNPA2B1 in the development of tumors, whether hnRNPA2B1 regulates bone destruction in MM is still unknown.
Methods: Alizarin red S staining, TRAP staining, ELISA and quantitative real-time PCR assays were used to evaluate osteogenesis and osteoclastogenesis in vitro. X ray and bone histomorphometric analysis were preformed to identify bone resorption and bone formation in vivo. Exosome isolation and characterization were demonstrated by transmission electron microscopy, dynamic light scattering, immunofluorescence and flow cytometry assays. The interactions between hnRNPA2B1 and primary microRNAs were examined using RNA pull-down and RIP assays. Coimmunoprecipitation assay was used to test the interaction between hnRNPA2B1 and DGCR8 proteins. Luciferase assay was established to assess miRNAs target genes.
Results: Here we show that myeloma cells hnRNPA2B1 mediates microRNAs processing and upregulates miR-92a-2-5p and miR-373-3p expression. These two microRNAs are transported to recipient monocytes or mesenchymal stem cells (MSCs) through exosomes, leading to activation of osteoclastogenesis and suppression of osteoblastogenesis by inhibiting IRF8 or RUNX2. Furthermore, clinical studies revealed a highly positive correlation between the level of myeloma cells hnRNPA2B1 and the number of osteolytic bone lesions in myeloma patients.
Conclusions: This study elucidates an important mechanism by which myeloma-induced bone lesions, suggesting that hnRNPA2B1 may be targeted to prevent myeloma-associated bone disease.
Keywords: Multiple myeloma, bone lesion, hnRNPA2B1, exosome, microRNA
 Global reach, higher impact
Global reach, higher impact