13.3
Impact Factor
Theranostics 2022; 12(17):7586-7602. doi:10.7150/thno.78616 This issue Cite
Research Paper
TIMM44 is a potential therapeutic target of human glioma
1. Institute of Neuroscience, Soochow University, Suzhou, and Department of Neurosurgery, The affiliated Changzhou No. 2 People's Hospital of Nanjing Medical University, Changzhou, China.
2. Department of Neurosurgery, the First Affiliated Hospital of Soochow University, Suzhou, China.
3. Department of Oncology, Dushu Lake Hospital Affiliated of Soochow University, Suzhou, China.
4. Department of Radiotherapy and Oncology, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, China.
5. The Fourth School of Clinical Medicine, The Affiliated Eye Hospital, Nanjing Medical University, Nanjing, China.
6. Jiangsu Key Laboratory of Neuropsychiatric Diseases, Clinical Research Center of Neurological Disease, The Second Affiliated Hospital of Soochow University, Suzhou, China.
#Co-first authors.
Abstract
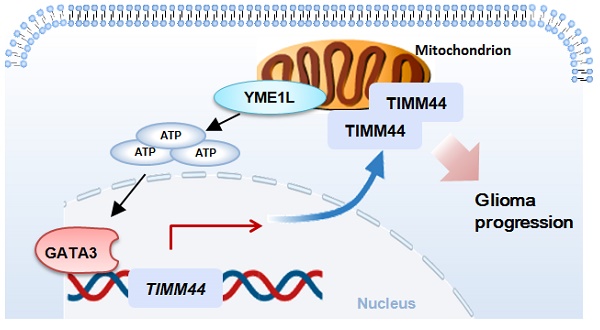
TIMM44 (translocase of inner mitochondrial membrane 44) is essential for the maintenance of mitochondrial functions. Bioinformatics studies and results from the local high-grade glioma tissues showed that TIMM44 mRNA and protein levels are elevated in glioma, correlating with poor overall survival. Mitochondrial TIMM44 upregulation was also detected in patient-derived primary glioma cells and immortalized cell line. In primary and established glioma cells, TIMM44 depletion, using the lentiviral shRNA strategy or the CRISPR/Cas9 knockout (KO) method, robustly inhibited cell viability, proliferation and migration. Moreover, TIMM44 silencing/KO resulted in mitochondrial complex I inhibition, ATP depletion, mitochondrial membrane potential reduction, oxidative stress and DNA damage, and eventually provoked apoptosis. Conversely, ectopic overexpression of TIMM44 augmented glioma cell proliferation and migration. TIMM44 upregulation in glioma is possibly due to increased TIMM44 transcriptional machinery by the transcription factor GATA3 in a YME1L (YME1 Like 1 ATPase)-dependent manner. In vivo, the growth of subcutaneous glioma xenografts was suppressed after intratumoral injection of TIMM44 shRNA adeno-associated virus (AAV). TIMM44 depletion, ATP reduction, oxidative injury and apoptosis were detected in TIMM44 shRNA AAV-injected glioma xenografts. Moreover, the intracranial growth of TIMM44 KO glioma cells in the mouse brain was largely inhibited. Together, overexpressed TIMM44 could be a novel and promising therapeutic target of human glioma.
Keywords: Glioma, TIMM44, Mitochondria, Therapeutic target
 Global reach, higher impact
Global reach, higher impact