13.3
Impact Factor
Theranostics 2021; 11(16):7911-7947. doi:10.7150/thno.56639 This issue Cite
Review
A perspective on the radiopharmaceutical requirements for imaging and therapy of glioblastoma
1. Radiobiology, Radiation Biophysics Division, Nuclear Medicine Department, iThemba LABS, Cape Town, South Africa.
2. Nuclear Medicine Research Infrastructure NPC, Pretoria, South Africa.
3. Nuclear Medicine Department, University of Pretoria and Steve Biko Academic Hospital, Pretoria, South Africa.
4. Laboratory of Radiopharmacy, Ghent University, Ghent, Belgium.
5. Ghent University Hospital, Department of Nuclear Medicine, Ghent, Belgium.
6. Nuclear Medicine Department, University of Pretoria, Pretoria, South Africa.
Abstract
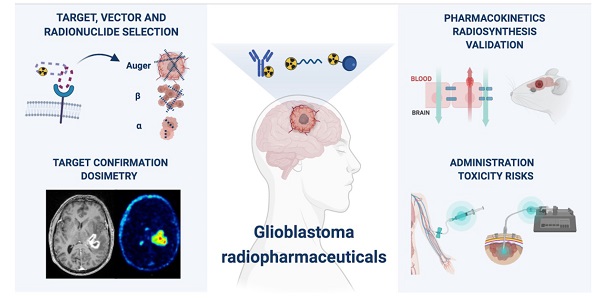
Despite numerous clinical trials and pre-clinical developments, the treatment of glioblastoma (GB) remains a challenge. The current survival rate of GB averages one year, even with an optimal standard of care. However, the future promises efficient patient-tailored treatments, including targeted radionuclide therapy (TRT). Advances in radiopharmaceutical development have unlocked the possibility to assess disease at the molecular level allowing individual diagnosis. This leads to the possibility of choosing a tailored, targeted approach for therapeutic modalities. Therapeutic modalities based on radiopharmaceuticals are an exciting development with great potential to promote a personalised approach to medicine. However, an effective targeted radionuclide therapy (TRT) for the treatment of GB entails caveats and requisites. This review provides an overview of existing nuclear imaging and TRT strategies for GB. A critical discussion of the optimal characteristics for new GB targeting therapeutic radiopharmaceuticals and clinical indications are provided. Considerations for target selection are discussed, i.e. specific presence of the target, expression level and pharmacological access to the target, with particular attention to blood-brain barrier crossing. An overview of the most promising radionuclides is given along with a validation of the relevant radiopharmaceuticals and theranostic agents (based on small molecules, peptides and monoclonal antibodies). Moreover, toxicity issues and safety pharmacology aspects will be presented, both in general and for the brain in particular.
Keywords: targeted radionuclide therapy, radiochemistry, glioblastoma, theranostics, PET SPECT imaging
 Global reach, higher impact
Global reach, higher impact