13.3
Impact Factor
Theranostics 2020; 10(6):2479-2494. doi:10.7150/thno.39560 This issue Cite
Research Paper
Probing and Enhancing Ligand-Mediated Active Targeting of Tumors Using Sub-5 nm Ultrafine Iron Oxide Nanoparticles
1. Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, Georgia, USA.
2. Boston Children's Hospital, Harvard Medical School, Boston, Massachusetts, USA.
3. Department of Surgery, Emory University School of Medicine, Atlanta, Georgia, USA.
4. Integrated Cellular Imaging Core, Emory University, Atlanta, Georgia, USA.
5. Ocean Nanotech, LLC, San Diego, California, USA.
Abstract
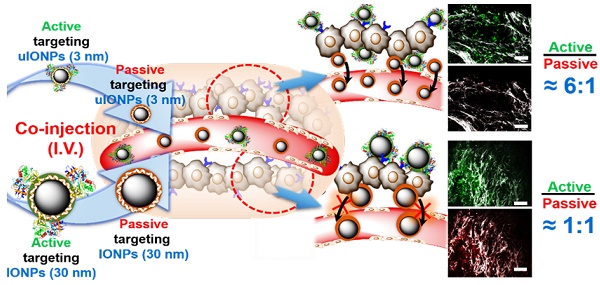
Rationale: “Active targeting” based on the ligand-target affinity is a common strategy to precisely deliver nanoparticle (NP) imaging probes or drug carriers to the diseased tissue. However, such ligand-mediated active targeting inevitably takes place with prerequisite “passive targeting”, driven by the enhanced permeability and retention (EPR) effect. Thus, the efficiency of active targeting in relation to off-targeted unbound NPs is of great importance in quantitative imaging of tumor biomarkers and delivery. With the notion that easy clearance of off-targeted uIONPs may lead to enhanced active targeting and tumor accumulation, we examined the NP size effect on “active targeting” of the transferrin receptor (TfR) using transferrin (Tf)-conjugated sub-5 nm (3 nm core) ultrafine iron oxide NPs (uIONPs) and larger IONPs (30 nm core).
Methods: Green fluorescent dye (FITC)-labeled active targeting uIONPs (FITC-Tf-uIONPs) and red fluorescent dye (TRITC)-labeled passive targeting uIONPs (TRITC-uIONPs) were prepared. FITC-Tf-IONPs and TRITC-IONPs were used as comparison for the NP size effect. Multiphoton imaging, confocal fluorescence imaging, histological staining and computational analysis were applied to track different types of NPs in tumors at 1, 3 and 24 hours after co-injection of equal amounts of paired NPs, e.g., active targeting FITC-Tf-uIONPs and non-targeting TRITC-uIONPs, or FITC-Tf-IONPs and TRITC-IONPs into the same mice bearing 4T1 mouse mammary tumors.
Results: Active targeting uIONPs exhibited an almost 6-fold higher level of tumor retention with deeper penetration comparing to non-targeting uIONPs at 24 hours after co-injection. However, accumulation of active targeting IONPs with a 30-nm core is only about 1.15-fold higher than non-targeting IONPs. The enhanced active targeting by uIONPs can be attributed to the size dependent clearance of unbound off-targeted NPs, as majority off-targeted uIONPs were readily cleared from the tumor by intravasation back into tumor blood vessels likely due to high interstitial pressure, even though they are not favorable for macrophage uptake.
Conclusion: Ligand-mediated active targeting improves the delivery and accumulation of the sub-5 nm NPs. The improvement on active targeting is size-dependent and facilitated by NPs with sub-5 nm core sizes. Thus, sub-5 nm NPs may serve as favorable platforms for development of NP-based molecular imaging probes and targeted drug carriers.
Keywords: Iron oxide nanoparticles, Active targeting, Enhanced permeability and retention, Molecular imaging, Drug delivery.
 Global reach, higher impact
Global reach, higher impact