13.3
Impact Factor
Theranostics 2019; 9(14):4006-4018. doi:10.7150/thno.32720 This issue Cite
Research Paper
Alarmin augments the antitumor immunity of lentiviral vaccine in ectopic, orthotopic and autochthonous hepatocellular carcinoma mice
1. Department of Cell Biology, Key Laboratory of Immune Microenvironment and Disease (Ministry of Education), Tianjin Medical University, Qixiangtai Road, Heping District, Tianjin, 300070, China
2. School of Medical Laboratory, Tianjin Medical University, Guangdong Road, Tianjin, 300203, China
* These authors contributed equally as joint first authors.
Received 2019-1-1; Accepted 2019-4-30; Published 2019-5-31
Abstract
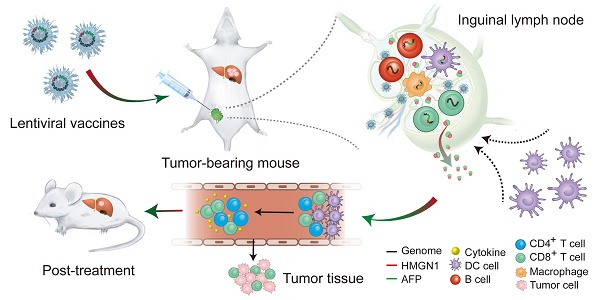
It is a daunting therapeutic challenge to completely eradicate hepatocellular carcinoma (HCC) from patients. Alpha-fetoprotein (AFP) -based vaccines appear promising, however the efficacy needs to be improved.
Methods: Here, we explore if fusing high-mobility group nucleosome binding protein 1 (HMGN1), a potent immunoadjuvant, to AFP (lenti-HA) can augment the antitumor immunity of AFP-expressing lentiviral vector (lenti-AFP), a vehicle extensively employed for genetic immunization with high transduction efficacy and good safety profiles. The antitumor immunity of Lenti-HA was systemically assessed in ectopic, orthotopic and autochthonous HCC models.
Results: Lenti-HA elicited strong anti-HCC effects in mice and amplified the antitumor immunity of lenti-AFP by reducing effective dose 6-fold. Importantly, lenti-HA induced a robust antitumor immune response with prolonged survival rate and improved the immune and tumor microenvironment in mice with carcinogen-induced autochthonous HCC. Lenti-HA localized primarily to lymphoid organs with no preference for specific immune cell types. Activated dendritic cells (DCs), particularly CD103+CD11b- DCs, were also actively recruited to lymph nodes in lenti-HA-treated HCC mice. Moreover, lenti-HA-transduced human DCs elicited stronger immune response than lenti-AFP against HCC cells in vitro.
Conclusion: Our study demonstrates that HMGN1 augments the antitumor immunity of AFP-expressing lentiviral vaccines in HCC mice and human cells in vitro and thus provides a new therapeutic strategy for HCC.
Keywords: HMGN1, lentivirus, immunotherapy, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) remains one of the most refractory malignancies worldwide without effective non-surgical treatment in the clinic [1]. While resection is applicable to a subpopulation of early stage HCC patients, it frequently recurs [1-3]. Chemotherapy has advanced rapidly, but drug resistance and associated adverse effects have limited improvements on survival rate [2]. Alpha-fetoprotein (AFP) is elevated in 50-80% of HCC patients [4-6] and thus is a primary target for HCC immunotherapy. However, different AFP-based vaccines under investigation have only achieved modest levels of immune response with disappointing clinical outcomes [7-9]. Therefore, approaches to amplify the antitumor immunity of AFP-based vaccines are urgently required.
Lentivirus is superior to adenovirus and adenovirus-associated virus in transducing non-replicating cells such as dendritic cells (DCs) [10-13]. Thus, lentivirus has been widely employed in gene therapy and immunotherapy for a myriad of diseases with the first lentivirus-transduced cell therapy approved in 2017 [14-18]. Lentivirus expressing optimized AFP protected mice against tumor challenge [19] and lentivirus-transduced DCs activated AFP-specific T cells in HCC mice [13], indicating the potential of lentivirus as a viral vector in immunotherapy for HCC.
However, the tenacity of HCC necessitates enhancement of AFP-based lentiviral vaccine immunogenicity which is critical for the clinical use and success. Recently, immunoadjuvants such as alarmin, which are a group of damage-associated molecular patterns showing the capacity to recruit and activate DCs [20], have been under close scrutiny. The high-mobility group nucleosome binding protein 1 (HMGN1) is particularly appealing as it acts on the toll-like receptor 4 (TLR4) ligand which preferentially promotes Th1 polarizing immune response critical for the generation of antitumor immunity [21]. HMGN1 has been shown to be effective in melanoma, thymoma and colon cancer immunotherapy [22-24]. However, the potential of HMGN1 in augmenting AFP-specific antitumor immune response in HCC remains elusive. Here, we investigated whether direct fusion of HMGN1 and AFP with the insulin signal peptide for extracellular secretion packaged into lentivirus (lenti-HA) can trigger stronger antitumor immunity in different HCC mouse models as covalent linkage of HMGN1 to a tumor-associated antigen was shown to improve antigen-specific immune responses and immunoprotection [22, 25]. Also to further validate its clinical applicability, we tested the antitumor effect of lenti-HA in human cells in vitro.
Results
HMGN1 promotes the activation and immunogenicity of DCs in vitro
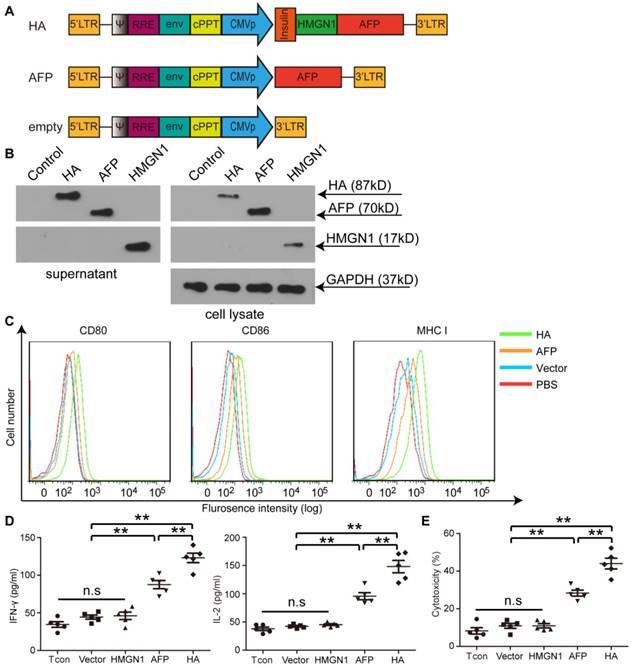
To investigate the potency of HMGN1 to enhance antigen-specific immune responses in HCC, we constructed a lentiviral vector expressing a fusion protein of HMGN1 and AFP (HA) with an insulin signal peptide for extracellular secretion (Figure 1A) [22]. Extracellular expression of HA was confirmed as a strong HA band was detected in HA-expressing cells' culture medium via Western blot (Figure 1B). Major histocompatibility complex I (MHC I) and other co-stimulatory molecules such as CD80 and CD86 were predictably up-regulated in bone marrow-derived DCs (BMDCs) transduced with lenti-HA (2.5x107 copies) compared to AFP-expressing lentivirus (lenti-AFP) under identical conditions (Figure 1C), indicating that secreted HMGN1 promotes DC maturation. Consistently, significantly higher levels of interleukin-2 (IL-2) and interferon-γ (IFN-γ) were detected in the supernatant of splenic T lymphocytes primed by DCs transduced with lenti-HA compared to DCs transduced by lenti-AFP, HMGN1-expressing lentivirus (lenti-HMGN1), empty lentivirus (containing empty vector) or unstimulated T cell controls (Figure 1D). This, in turns, induced greater antigen-specific cytolysis against Hepa1-6 cells (H-2b) at effector T: target cell (E: T) ratio of 10:1 (Figure 1E). To confirm the antigen-specific effect, we subcutaneously administered lenti-HA, lenti-AFP and empty lentivirus (3x107 copies) into day-7 ectopic HCC mice for 3 weeks at weekly interval and harvested splenic T lymphocytes 3 days after last injection. Re-stimulation of splenic T lymphocytes from different treatment groups with AFP212 and AFP499, identified immunodominant HCC epitopes [19], revealed significantly higher levels of IL-2 and IFN-γ (Figure S1A) and cytolysis rates against Hepa1-6 cells (Figure S1B) in lenti-HA and lenti-AFP groups compared to controls. In contrast, there was no stimulatory effect with the random control peptide consisting of the same amino acid composition but in different orders (Figure S1), indicating that lenti-HA elicits an antigen-specific immune response.
HMGN1 amplifies lenti-AFP-specific immune response in ectopic HCC mice by promoting DC's homing capacity
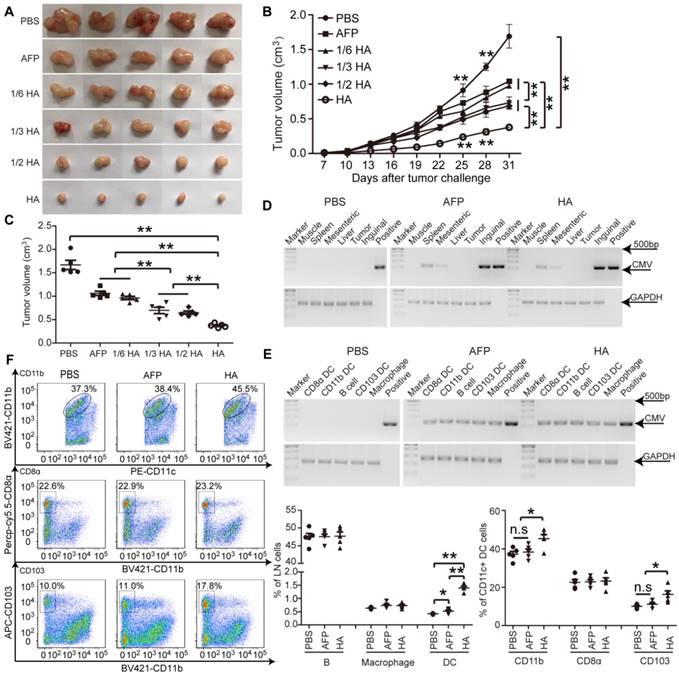
To determine the enhanced effect of HMGN1 on AFP-mediated antitumor immunity, we titrated lenti-HA (5x106, 1x107, 1.5x107 and 3x107copies) and administered in day-7 established ectopic C57BL/6 HCC mice subcutaneously once per week for 3 weeks, a schedule of administration adopted from previous studies [5, 14, 26]. We reasoned that a low but effective dose would allow differentiating the enhancement conferred by HMGN1. A dose-dependent cumulative antitumor effect was established in lenti-HA, with 1/6 dose of lenti-HA (5x106 copies) showing comparable antitumor efficacy to 3x107 copies of lenti-AFP (Figure 2A-C), indicating that HMGN1 amplifies the AFP-specific antitumor immunity 6-fold on a dose basis. Concordantly, IL-2 and IFN-γ levels were significantly elevated (Figure S2A), whereas immunosuppressive cytokines including interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) (Figure S2B) significantly declined in tumor lysates from lenti-HA-treated mice compared to other groups. To assess whether HMGN1 affects lentiviral distribution, we quantified the lentivirus in different tissues with semi-quantitative PCR. Consistent with previous reports [10], lentivirus preferentially localized in lymphoid organs namely inguinal, mesenteric lymph nodes and spleen with lenti-HA and lenti-AFP showing similar distribution (Figure 2D). Likewise, similar amounts of lentivirus were found in different immune cells from inguinal lymph nodes of lenti-HA- and lenti-AFP-treated mice (Figure 2E), demonstrating that HMGN1 does not alter the lentiviral distribution. As HMGN1 can actively recruit and activate CD11c+ DCs [21], we analyzed immune cell populations in inguinal lymph nodes of treated ectopic HCC mice. Strikingly, DCs were significantly more abundant in lenti-HA-treated lymph nodes when compared to other groups, whereas B cells and macrophages were unchanged (Figure 2F). Similarly, migratory CD103+CD11b- DCs in inguinal lymph nodes from lenti-HA-treated mice were significantly increased, though to a lesser extent for CD11b+CD11c+ DCs, and no increase was observed in CD8α+CD11b- resident DCs compared to other groups (Figure 2F) [27, 28], suggesting that HMGN1 promotes the homing capacity of DCs to lymph nodes, particularly migratory CD103+CD11b- DCs. These data demonstrate that HMGN1 likely enhances the antitumor immunity of lenti-AFP by promoting the homing capacity of DCs, particularly migratory DCs.
Illustration of lentiviral constructs and characterization of HMGN1-AFP-expressing lentivirus (lenti-HA) in vitro. (A) Illustration of HMGN1-AFP (HA) -expressing constructs. CMVp refers to CMV promoter. Insulin represents Insulin signal peptide. (B) Western blot analysis to show the expression of HA, AFP and HMGN1 in the supernatant and cell lysates. HMGN1 was used as a control. Total protein (20 μg) from supernatants or cell lysates was loaded and GAPDH was used as a loading control. (C) Flow cytrometric analysis of surface markers and co-stimulatory molecules on bone marrow-derived DCs (BMDCs) transduced with lenti-HA, lenti-AFP or lenti-vector (empty vector used as a negative control). (D) Measurement of IL-2 and IFN-γ in supernatants of splenic T lymphocytes primed by lenti-HA, lenti-AFP, lenti-HMGN1 or lenti-vector-transduced DCs with ELISA. The comparison was conducted between lenti-HA and other groups or lenti-AFP and other groups (n=5, **P<0.01). (E) Cytolysis assay for murine hepa1-6 cells with effector T cells at the E: T (Effector: Target) ratio of 10:1. The comparison was conducted between lenti-HA and other groups or lenti-AFP and other groups (n=5, **P<0.01). N.s refers to not significant. Two-tailed t test was used for statistical analysis and all experiments were repeated twice (two repeated experiments yielded similar results and thus one representative result was shown).
Evaluation of efficacy and homing capacity of lenti-HA in ectopic HCC mice. Different amounts of lentivirus were subcutaneously administered into day-7 ectopic HCC mice for 3 weeks at weekly interval. (A) Representative tumor images for treated mice. (B-C) Analysis of tumor volume from ectopic HCC mice treated with different doses of lenti-HA or lenti-AFP at different time-points (n=5, **P<0.01). Tumors were harvested at 31 days after tumor challenge in (C). Semi-quantitative PCR results for showing the tissue distribution (D) or cellular localization (E) of lentivirus in ectopic HCC mice 3 days after last injection. The plasmid was used as a positive control. (F) Flow cytometric analysis of immune cells in lymph nodes (n=5, *P<0.05; **P<0.01). N.s refers to not significant. Two-tailed t test was used for statistical analysis and all experiments were repeated twice (two repeated experiments yielded similar results and thus one representative result was shown).
HMGN1 enhances lentiviral vaccine antitumor immunity in orthotopic HCC mice
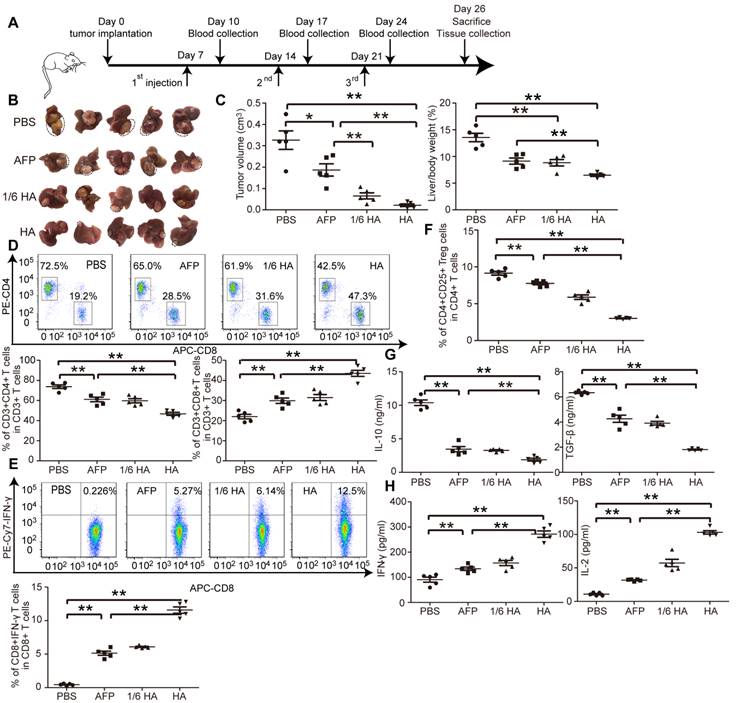
The microenvironment plays a critical role on HCC progression and prognosis, and the unique immunotolerogenic nature of HCC makes it one of the most difficult-to -treat malignancies [29]. In order to test lenti-HA potency in a clinically relevant mouse model, we subcutaneously administered 3x107 copies or 5x106 copies (1/6 dose) of lentiviral vaccines into day 7-established orthotopic C57BL/6 HCC mice once per week for 3 weeks (Figure 3A). Significant tumor retardation was elicited in lenti-HA-treated mice compared to lenti-AFP and untreated controls (Figure 3B and 3C). Importantly, comparable antitumor efficacy was achieved with 1/6 dose of lenti-HA and lenti-AFP, demonstrating that HMGN1 improves the antitumor immunity of lenti-AFP. Concordantly, a significant rise of circulatory CD8+ T cells, particularly IFN-γ-expressing functional CD8+ T cells, in lenti-HA-treated mice compared to other groups implies that CD8+ T cells largely account for the observed antitumor immune response (Figure 3D and 3E); in contrast, circulatory CD4+ T cells, particularly CD4+CD25+ regulatory T cells (Tregs), were significantly decreased in lenti-HA-treated mice compared to other groups (Figure 3D and 3F). Circulating IL-10 and TGF-β secretion significantly declined (Figure 3G), whereas levels of immunostimulatory cytokines IL-2 and IFN-γ were significantly increased in lenti-HA-treated mice compared to other groups (Figure 3H), showing that lenti-HA improves the immune environment in orthotopic HCC mice. The tumor microenvironment also consistently improved as levels of IL-2 and IFN-γ were increased (Figure S3A) and IL-10 and TGF-β were decreased (Figure S3B) in tumor lysates. To monitor the dynamic change of mounted immune responses, we examined circulatory cytokines in a real-time manner. The results showed accumulated immune responses as IL-2 and IFN-γ levels were gradually increased (Figure S3C) and IL-10 and TGF-β declined (Figure S3D) in blood from lenti-HA-treated orthotopic HCC mice at different time-points following repeated administrations. Corroborating with the ectopic data, CD103+CD11b- migratory DCs in inguinal lymph nodes were significantly increased in lenti-HA-treated mice when compared to other groups (Figure S3E). These data show that HMGN1 enhances the antitumor immunity of lenti-AFP and improves immune microenvironment in a clinically relevant model.
Systemic investigation of lenti-HA in orthotopic C57BL/6 HCC mice. Lenti-HA (3x107 or 5x106 copies) or lenti-AFP (3x107 copies) was subcutaneously administered into day-7 established orthotopic HCC mice for 3 weeks at weekly interval. (A) Schematic diagram for the dosing regimen of lentiviral vaccines in day-7 orthotopic C57BL/6 HCC mice. (B) Representative tumor images for treated orthotopic HCC mice. (C) Analysis of tumor volume and weight from orthotopic HCC mice treated with lenti-HA or lenti-AFP (n=5, *P<0.05; **P<0.01). Flow cytometric analysis of CD4+ or CD8+ T lymphocytes (D) or IFN-γ-expressing CD8+ T lymphocytes (E) or CD4+CD25+ Tregs (F) in blood from orthotopic C57BL/6 HCC mice treated with lenti-AFP or lenti-HA 3 days after last injection (n=5, **P<0.01). Measurement of IL-10 and TGF-β (G) or IFN-γ and IL-2 (H) in blood from orthotopic HCC mice treated with lenti-HA or lenti-AFP (n=5, **P<0.01). Two-tailed t test was used for statistical analysis and all experiments were repeated twice (two repeated experiments yielded similar results and thus one representative result was shown).
HMGN1 augments lenti-AFP antitumor immunity in autochthonous HCC mice
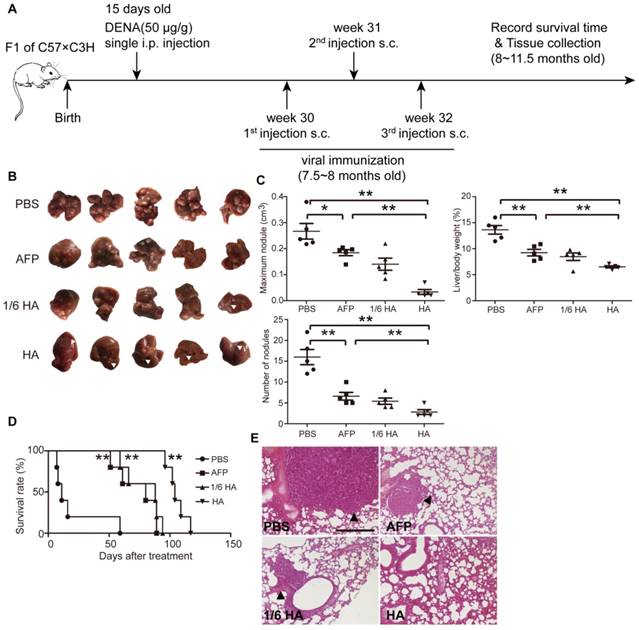
To better simulate the pathological complexity and heterogeneity of HCC patients [30], we established a diethylnitrosamine (DENA)- induced autochthonous HCC model [5], which showed HCC micronodules and the expression of AFP at 7.5 months after induction (Figure S4A and S4B). Lenti-HA and lenti-AFP (3x107 copies) were subcutaneously injected into DENA-induced autochthonous HCC mice for 3 weeks at weekly interval (Figure 4A). Decreased number of nodules, volume of maximal tumor nodules and liver /bodyweight ratio (Figure 4B and 4C) in lenti-HA-treated mice compared to other groups demonstrates significant tumor suppression. As expected, comparable or even greater antitumor efficacy was obtained with 1/6 dose of lenti-HA (5x106 copies) when compared to lenti-AFP (Figure 4B and 4C). Strikingly, lenti-HA significantly prolonged the lifespan of HCC mice with a 100% survival rate at 96 days after treatment whereas no mouse survived in lenti-AFP, 1/6 dose of lenti-HA and PBS treatment (Figure 4D). Concordantly, no lung metastasis was observed in lenti-HA-treated mice, whereas visible tumor nodules were found in lungs from other treatment groups or untreated controls (Figure 4E). These findings indicate that HMGN1 significantly augments the antitumor effect of lenti-AFP in autochthonous HCC mice and effectively prolongs lifespan.
Systemic assessment of lenti-HA in DENA-induced autochthonous HCC mice. Lenti-HA (3x107 or 5x106 copies) or lenti-AFP (3x107 copies) was subcutaneously administered into DENA-induced autochthonous HCC mice for 3 weeks at weekly interval. (A) Schematic diagram for the dosing regimen of lentiviral vaccines in DENA-induced autochthonous HCC mice. (B) Representative tumor images for treated autochthonous HCC mice. Arrowheads point to HCC nodules. (C) Analysis of tumor volume, weight and number of nodules from autochthonous HCC mice treated with lenti-HA or lenti-AFP (n=5, *P<0.05; **P<0.01). (D) Survival rate of autochthonous HCC mice treated with lenti-AFP or lenti-HA (n=5, **P<0.01). (E) Histological examination of pulmonary metastasis of HCC in lungs from autochthonous HCC mice treated with lenti-AFP or lenti-HA (scale bar=200 μm). Arrowheads point to HCC nodules.
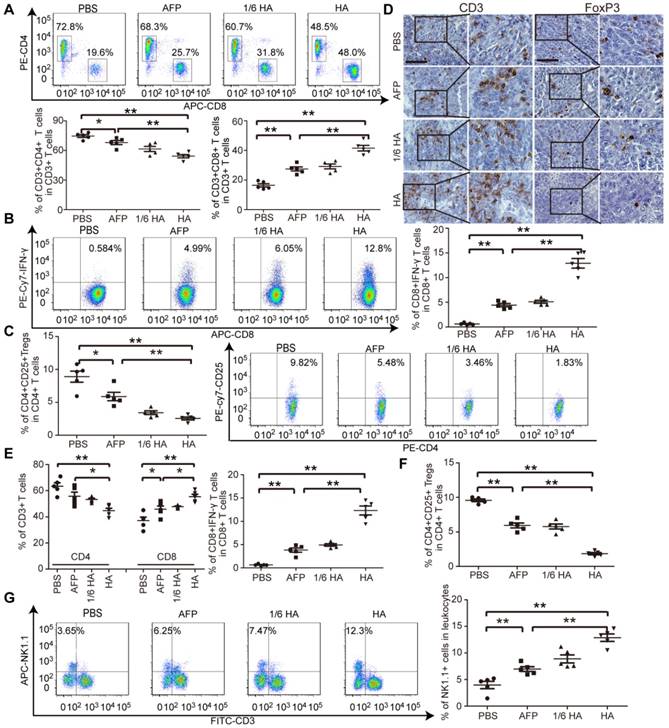
Lenti-HA improves immune state in autochthonous HCC mice
As demonstrated in our earlier study, an immunosuppressive tumor milieu was formed in DENA-induced autochthonous HCC mice [5]. To evaluate whether lenti-HA can alter the immunosuppressive immune state in autochthonous HCC mice, circulatory and tumor immune cells and cytokines were measured. The percentage of CD8+ T cells, particularly IFN-γ-expressing CD8+ T cells, significantly rose (Figure 5A and 5B), and the percentage of CD4+ T cells and CD4+CD25+ Tregs declined (Figure 5A and 5C) in blood from lenti-HA-treated mice compared to other groups, suggesting that CD8+ T cells mediates antitumor immunity elicited by lenti-HA. Correspondingly, significantly higher levels of IL-2 and IFN-γ (Figure S5A) and lower levels of IL-10 and TGF-β (Figure S5B) were found in blood from lenti-HA-treated HCC mice. T cell infiltration into tumor tissues was examined to determine if effector T cell recruitment to primary tumor sites was responsible for the antitumor effect. Immunohistochemical staining of CD3+ T cells and FoxP3+ Tregs revealed more CD3+ T cells and fewer FoxP3+ Tregs in tumor tissues from lenti-HA-treated mice than other groups (Figure 5D). A significant rise in CD8+ T cells, particularly IFN-γ-expressing CD8+ T cells (Figure 5E), and reduction in Tregs (Figure 5F) were also observed. Intratumoral IL-2 and IFN-γ levels were consistently elevated (Figure S5C) and IL-10 and TGF-β (Figure S5D) significantly declined in lenti-HA-treated mice compared to other groups, showing that CD8+ T cell activation and infiltration contribute substantially to lenti-HA efficacy. Concordant with ectopic and orthotopic data, the percentage of CD11c+ and CD103+CD11b- DCs was significantly increased in inguinal lymph nodes from lenti-HA-treated autochthonous HCC mice compared to other groups (Figure S6A). Moreover, MHC I and CD86 were up-regulated in CD103+CD11b- DCs from lenti-HA-treated mice (Figure S6B), resulting in significantly enhanced cytolysis rates against murine Hepa1-6 cells compared to other groups at the E: T ratio of 10:1 (Figure S6C). Strikingly, natural killer cells (NKs), effector lymphocytes of innate immunity [31, 32], were significantly elevated in blood from lenti-HA-treated HCC mice compared to other groups (Figure 5G). These findings demonstrate that lenti-HA can improve the immune state and tumor microenvironment in autochthonous HCC mice and CD8+ T cells are largely responsible for the antitumor immunity elicited by lenti-HA.
Measurement of immune microenvironment in treated autochthonous HCC mice. Flow cytometric analysis of CD4+ or CD8+ T lymphocytes (A) or IFN-γ-expressing CD8+ T lymphocytes (B) or CD4+CD25+ Tregs (C) in blood from autochthonous C57BL/6 HCC mice treated with PBS, lenti-AFP or lenti-HA (n=5, *P<0.05;**P<0.01). (D) Immunohistochemistry of CD3+ T cells and Foxp3+ Tregs in tumor sections from different treatment samples to determine the extent of T cell infiltration (scale bar = 100 μm). Flow cytometric analysis of CD4+ or CD8+ T lymphocytes and IFN-γ-expressing CD8+ T lymphocytes (E) or CD4+CD25+ Tregs (F) in tumor tissues from autochthonous C57BL/6 HCC mice treated with PBS, lenti-AFP or lenti-HA (n=5, *P<0.05; **P<0.01). (G) Flow cytometric analysis of natural killer (NK) cells in blood from autochthonous HCC mice treated with lenti-HA or lenti-AFP (n=5, **P<0.01). Two-tailed t test was used for statistical analysis.
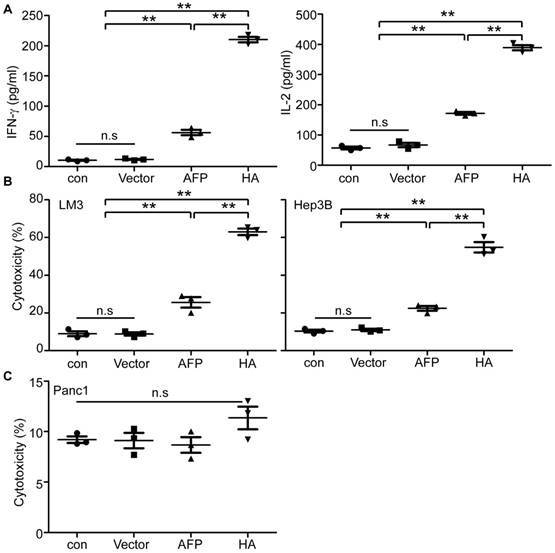
In vitro evaluation of lenti-HA in human HCC cells. (A) Measurement of IFN-γ and IL-2 in supernatant of human lymphocytes primed by lentivirus-transduced DCs with ELISA (n=3, **P<0.01). (B) Cytolysis rate against different human HCC cells with effector T cells at the E: T ratio of 10:1. A LDH-releasing cytotoxic assay was performed to measure the cytolysis efficiency of effector T cells activated by lentivirus-transduced peripheral monocyte-derived DCs (n=3, **P<0.01). (C) Cytolysis rate against human pancreatic cells with effector T cells (E: T =10:1). N.s refers to not significant. Two-tailed t test was used for statistical analysis and all the experiments were repeated twice (two repeated experiments yielded similar results and thus one representative result was shown).
Lenti-HA augments antitumor effect in human HCC cells in vitro
To establish if lenti-HA's antitumor effect is applicable to human cells, we transduced peripheral monocytes derived DCs (PMDCs) derived from healthy volunteers' peripheral blood mononuclear cells (PBMCs) with lenti-HA and lenti-AFP (3x107 copies). Levels of IL-2 and IFN-γ in the supernatant of lymphocytes primed by lenti-HA-transduced PMDCs were significantly elevated compared to lymphocytes primed by PMDCs transduced with lenti-AFP, empty lentivirus and unprimed lymphocyte controls (Figure 6A). Co-incubation of activated effector lymphocytes with AFP-expressing human HCC cells including LM3 and Hep3B cells (Figure S7) at the E: T ratio of 10:1 resulted in a significantly increased tumor-specific cytolysis rate against human HCC cells in lenti-HA compared to other groups (Figure 6B). In clear contrast, no cytolysis was observed when activated effector lymphocytes were incubated with pancreatic tumor cells (Panc1), which do not express AFP (Figure S7), under identical conditions (Figure 6C). To further confirm the antigen-specific effect in human in vitro, we incubated lymphocytes primed by lenti-HA-transduced PMDCs with recombinant AFP-loaded autologous PMDCs and the results showed significantly elevated levels of IL-2 and IFN-γ in supernatant of lymphocytes primed by lenti-HA- and lenti-AFP-transduced PMDCs after co-incubation with recombinant AFP -loaded autologous PMDCs compared to unpulsed PMDCs (Figure S8A), though only a marginal increase in cytolysis rate was found in recombinant AFP-loaded PMDCs compared to unpulsed PMDCs (Figure S8B). These results demonstrate that HMGN1 enables the enhancement of lentiviral vaccines in human HCC cells.
Discussion
HCC represents one of the most difficult-to-treat malignancies worldwide without effective treatment available. Lentivirus is emerging as a new treatment modality for tumors because it can transduce non-dividing DCs [33, 34]. Here, we investigated the feasibility of using lentivirus to deliver HMGN1, an endogenous immunoadjuvant [21], and as a vaccine in treating HCC. The results demonstrated that lentivirus encoding the fusion protein of HMGN1 and AFP enables augmentation of the antigen-specific antitumor immunity in different HCC mice and human cells in vitro. Importantly, significantly higher antitumor activities were achieved by incorporating HMGN1 into lentiviral vaccines across different HCC mice, particularly in autochthonous HCC mice, a model commonly used for closely mimicking patient HCC. Lenti-HA significantly improved the immune state and tumor microenvironment in autochthonous HCC mice and the enhanced antitumor immunity is likely due to greater homing capacity and activation of DCs conferred by HMGN1.
Detailed calibration of different doses of lenti-HA in comparison with lenti-AFP revealed that HMGN1 can significantly reduce the effective dose required for lenti-AFP by at least 6-fold. This enhancement can have a significant impact on the manufacture and cost of lentiviral vaccines during clinical translation, and thus ease patients' economic burden. Although significant tumor retardation was achieved with lenti-HA at the dose of 3x107 copies across different HCC mice, all mice eventually succumbed to the cancer, further confirming the tenacity of HCC and difficulty in treating poorly immunogenic HCC. As treatment was begun when tumors were palpable, the tumor has already progressed to an advanced stage; similar to the stage human HCC patients are typically diagnosed. As such, the vaccine only offers limited improvements in mice. Nevertheless, this improvement may be able to be further enhanced with “booster” shots to mount a sustained immune attack on the tumor cells. Furthermore, targeting multiple tumor targets enhanced by HMGN1 for immunotherapy may also improve the efficacy of the therapy. Lastly, this enhancement could conceivably be combined with other treatment modalities, including chemotherapy and resection, to improve patient survival in the clinic, which is likely to be the case for clinical deployment as the vaccine will not be the sole treatment utilized in the clinic. These improvements could be investigated further in the future.
Besides CD8+ T cells, NKs were also shown to be elevated in blood from lenti-HA-treated autochthonous HCC mice, implying that other effector cells also contribute to the enhanced antitumor effect elicited by lenti-HA. Although no difference in tissue and cellular distribution was observed between lenti-HA and lenti-AFP when lentivirus was administered subcutaneously into ectopic HCC mice as demonstrated in Figure 2D and 2E, a significantly higher number of CD11c+ DCs was found in inguinal lymph nodes from lenti-HA-treated mice than lenti-AFP, which coined with the previous report [21], supporting the notion that HMGN1 can enhance the homing capacity and activation of DCs. Further examination on the subpopulations of CD11c+ DCs in inguinal lymph nodes revealed significant rises in CD11b+ and CD103+CD11b- DCs, with CD103+CD11b- showing the most dramatic increase, in lenti-HA-treated mice compared to lenti-AFP and untreated controls, suggesting that HMGN1 possibly has a more profound impact on migratory CD103+ CD11b- DCs than other cells. Further detailed elucidation on the mechanism is warranted in future studies.
In summary, our study demonstrates that HMGN1 is an effective immunoadjuvant and can significantly augment AFP-specific antitumor immune response in different HCC mice and in human cells in vitro, and thus provides a new treatment option for HCC.
Materials and Methods
Animals
C57BL/6 wild-type mice (H-2b) (6-8 weeks old, no gender preference) were used in the experiments (5 mice were used in each group for ectopic or orthotopic studies, respectively, the experiments were repeated twice unless otherwise specified). To increase the tumor take rates, we cross-bred C57BL/6 female (H-2b) with C3H male (H-2k) mice as described previously [5]. All animal experiments were carried out in the animal unit of Tianjin Medical University (Tianjin, China) according to procedures authorized and specifically approved by the institutional ethical committee (permit No. SYXK 2009-0001). Mice were sacrificed by CO2 inhalation or cervical dislocation at desired time-points; and tissues were either collected for cell isolation or fixed with Bouin's solution (Sigma) and embedded with paraffin for histological study.
Cell lines
The murine HCC cell line (hepa1-6, derived from C57L mice, H-2b) was purchased from Boster Biological Technology Ltd. (Wuhan, China). Murine dendritic cell line (DC2.4, kindly provided by Dr De Yang, Center for Cancer Research, National Institutes of Health, US) and DCAFP were kept in house and cultured as described previously [5]. Human HCC cell lines, including Hep3B, LM3 and human pancreatic cancer cell (Panc1) were purchased from the American Type Culture Collection biobank and cultured per the manufacturer's instructions [35]. Human 293FT cell line used for lentivirus packaging was kept in house and cells were grown at 37 °C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% PS (100 U/ml penicillin and 100 μg/ml streptomycin). All the cell lines used were tested to rule out the presence of mycoplasma contamination.
Construction of lentivirus expressing murine and human HA or AFP
The murine or human AFP full-length coding sequence (1.8 kb) was cloned from total RNA isolated from fetal liver of C57BL/6 mice or human HCC cells (LM3) and cloned into the lentivirus expression vector pCDH-CMV-puro (System Biosciences, CA, US) or the lentivirus expression vector pCDH-CMV-puro-insulin-HMGN1 (kindly provided by Dr. De Yang, Center for Cancer Research, National Institutes of Health, US) [22]. The primers used for murine AFP gene RT-PCR were (F) 5'-CGGAATTCGGAGGAGGAGGATCAGGAGGAGGAGGATCAATGAAGTGGATCACACCCGCTTCCCTCA-3' (underlined bold letters are EcoR I restriction site) and (R) 5'-CGCGGATCCTTAAACGCCCAAAGCATCACG-3' (underlined bold letters are BamH I restriction site). The primers used for human AFP gene RT-PCR were (F) CGGAATTCGGAGGAGGAGGATCAGGAGGAGGAGGATCAATGAAGTGGGTGGAATCAA-3' (underlined bold letters are EcoR I restriction site; italicized letters are linker) and (R) 5'-AAAAGGAAAAGCGGCCGCTTAAACTCCCAAAGCAGCA-3' (underlined bold letters are Not I restriction site). Human 293FT cells (3x106) were seeded in a 10-cm Petri dish for 24 hrs followed by co-transfection of pCDH-CMV-puro-AFP, pCDH-CMV-puro-HMGN1 or pCDH-CMV-puro-HMGN1 -AFP (HA), psPAX2 and pM2D.G plasmids (gifted with pCDH-CMV-puro) in a ratio of 20:15:5 (mass ratio) by Polyethylenimine (PEI, Polysciences, US). Viruses were harvested and titred 48 hrs later with Lenti-PacTM qRT-PCR Titration Kit (GeneCopoeiaTM, Maryland, US) per the manufacturer's instructions. Functional titres (Transducing Unit-TU/ml) were measured as previously reported [17, 36]. Briefly, 293FT cells (1x106 cells per well for 6-well plate) were seeded overnight and counted before transduction with serial dilutions of viral vector. Genomic DNA was extracted with DNeasy Blood & Tissue Kits (Qiagen) and viral DNA genomes were quantified with Lenti-PacTM qRT-PCR Titration Kit (GeneCopoeiaTM, Maryland, US) per the manufacturer's instructions. Based on the calculation, 3x107 copies generated 2.4x106 TU, a conversion coefficient of 0.08.
Isolation of mouse bone marrow-derived DC
Mouse BMDCs were generated as previously reported [5]. Briefly, BM progenitors isolated from bone marrows of C57BL/6 mice were incubated at 1×106 cells per well for 6-well plates and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium plus 10% FBS, 1% P/S, granulocyte-macrophage colony-stimulating factor (GMCSF, 200 U/ml) (PeproTech, US) and interleukin-4 (IL-4, 100 U/ml) (Peprotech, US) and 50 μmol/l 2-mercaptoethanol at 37 °C in a humidified incubator with 5% CO2 for 5 days to generate immature DCs. Subsequently, immature DCs were incubated in fresh culture medium at 1×106 cells per well for 6-well plates and transduced with AFP-, HA-, HMGN1-expressing lentivirus or lentivirus expressing empty vector (2.5x107 copies) containing 1/1000 polybrene (10 mg/ml) for 3 times every 12 hrs.
Establishment of HCC mouse models
Ectopic HCC mouse models were established by subcutaneous injection of hepa1-6 cells (3x106) into left axilla of C57BL/6 mice. Then tumors with a longitudinal diameter of 1 cm were peeled from ectopic HCC mice after schedule 1 killing. Tumor tissues were washed in D-hanks buffer. Necrotic tissues were removed from tumors and tumor tissues were cut into about 1 mm3 pieces. 2-3 tumor pieces were implanted in the left lobe of liver in the recipient mice under anesthesia as described previously [26]. For DENA-induced autochthonous HCC mice, the procedure was the same as described previously [5]. Briefly, 15-day old neonatal heterozygotes (H-2b/k) were injected intraperitoneally with 50 μg/g DENA for once and monitored over the experimental period. Tumor was measured for longitudinal(a) and lateral (b) diameters with a vernier calliper and tumor volume (cm3) was calculated with the equation of Tumor volume = 1/2ab2. Lenti-AFP (3x107 copies), 1/2 (1.5x107 copies), 1/3 (1x107 copies) and 1/6 dose of Lenti-HA (5x106 copies) and Lenti-HA (3x107 copies) in phosphate buffered saline (PBS) (200 μl) were subcutaneously injected into ectopic, orthotopic or autochthonous HCC mice per week for 3 weeks. For other doses of lenti-HA, the amount of lenti-HA was adjusted accordingly.
Isolation of human PBMC derived DCs and lymphocytes
For human in vitro studies, human peripheral blood was obtained from healthy volunteers (provided by Tianjin Blood Center, Tianjin, China). Peripheral blood mononuclear cells were isolated with human Lymphoprep solution (Axis-shield PoC AS, Oslo, Norway) per the manufacturer' s instruction as described previously [26]. Briefly, cells were seeded in a 10-cm Petri dish with 10 ml RPMI 1640 medium plus 10% FBS and incubated for 2 hrs to allow them to adhere to the surface. Adherent cells were harvested and induced to form immature DCs by culturing in RPMI 1640 medium containing 20% FBS, 120 ng/ml recombinant human GMCSF (PeproTech, US) and 60 ng/ml recombinant human IL-4 (PeproTech, US) for 5 days. Subsequently, immature DCs were incubated in fresh culture medium at 3×106 cells per well for 6-well pates and infected with 3x107 (copies) of AFP-or HA-expressing lentivirus containing 1/1000 polybrene (10 mg/ml) for 3 times every 12 hrs. Nonadherent cells were recovered and cultured in DMEM/F-12 medium containing 10% FBS for 7 days, and used as human lymphocytes.
Cytolysis assay
The cytotoxicity detection kit (R&D systems, US) was used to measure the cytolysis rate elicited by effector T cells against different tumor cells, in which effector T cells were referred to murine splenic T lymphocytes or mixed human lymphocytes primed by DCs transduced with lenti-HA or lenti-AFP for 72 hrs. For the cytolysis assay, the same procedure was performed as described previously [5].
In vitro antigen-specific assay
Lenti-HA, lenti-AFP and control lentivirus expressing the empty vector (3x107 copies) were subcutaneously administered into day-7 established ectopic HCC mice for 3 weeks at weekly interval. Splenic T lymphocytes were harvested from treated mice 3 days after last injection and re-stimulated with a random control peptide (EMYHRSLGNV), AFP212 and AFP499 (20 μg/ml) for 72 hrs [19], followed by analysis of IFN-γ (R&D systems, US) and IL-2 (eBioscience, US) in supernatants and the cytolysis assay against murine Hepa1-6 cells as described above. The peptides were synthesized and provided with >98% purity by Chinapeptide Co. Ltd. For human in vitro stimulation assay, recombinant human AFP protein (10 μg/ml; Sino Biological Ltd, Beijing, China) was incubated with human PMDCs (2x106/ well for 6-well plate) for 48 hrs [37], followed by co-incubation with activated autologous lymphocytes (1x105) primed by lentivirus transduced PMDCs for 72 hrs. Levels of IFN-γ and IL-2 in supernatant were detected as per the manufacturer's instructions. For cytolysis assay, recombinant AFP-loaded PMDCs were co-incubated with activated autologous lymphocytes primed by lentivirus transduced PMDCs for 12 hrs, followed by LDH assay as describe previously [5].
Immunohistochemistry
To examine the presence of T lymphocytes and regulatory T cells in tumor sections from autochthonous HCC mice treated with Lenti-AFP, 1/6 dose of Lenti-HA or Lenti-HA, immunohistochemical staining was conducted as described previously [5].
Histology
Routine haematoxylin and eosin (H&E) staining was applied. Paraffin lung sections (5-7 μm thick) from treated autochthonous HCC mice were prepared and slides were stained with Sirius red solution (Sigma, US) for 20 min, and then washed with PBS for 3 times, followed by staining with haematoxylin for 10 min. Slides were observed with conventional microscopy (Olympus FV1000, Olympus, Japan) for pathological evaluation.
Flow cytometry and Intracellular staining
For the detection of mouse DC surface markers, cells were washed in FACS buffer (PBS containing 0.5% BSA and 0.05% NaN3) and stained with a combination of FITC-anti-mouse MHC I, APC-anti-mouse CD86 and PE- anti-mouse CD11c (eBioscience, US); FITC-anti-mouse CD80 (Abcam, UK) on ice for 45 min prior to flow cytometric analysis. For detecting various subsets of leukocytes in peripheral blood, lymphoid organs or tumor tissues, single cell suspensions of different tissues were stained with FITC-anti-mouse CD3e, PE-anti-mouse CD4, APC-anti-mouse-B220, Percp-cy5.5 (FITC) -anti mouse- CD45, APC-anti-mouse-NK1.1, PE-cy7-anti-mouse-CD25 (eBioscience, US); APC (Percp-cy5.5)-anti-mouse-CD8α, APC-anti-mouse-CD103, PE-anti-mouse-CD11c, BV421-anti-mouse-CD11b, PE-cy7-anti-mouse-CD19 and PE-anti-mouse-F4/80 (Biolegend, US) on ice for 45 min. The samples were washed 3 times with the FACS buffer and analyzed with a BD Fortessa multichannel flow cytometer (BD Biosciences, US). All data analyses were performed using the software FlowJo (Tree Star Inc., Ashland, OR, US). Intracellular staining of IFN-γ was performed per manufacturer's instructions (BD Biosciences, US) [5]. Briefly, splenic T lymphocytes derived from different treated mice were incubated in DMEM/F-12 medium supplemented with 10% FBS, 200 nM L-glutamine, 1% P/S and 0.05 mM β-mercaptoethanol at 37 °C for 6 hrs, followed by the addition of Golgi stop (1.5 μg/ml, BD Biosciences, US) for the final 6 hrs to block cytokine secretion. Splenic T lymphocytes were harvested, washed and stained with 0.4 μg/ml APC-labelled CD8α, PE-labelled CD4 mouse Abs or FITC-labelled CD3 mouse mAb (ebioscience, US) for 60 min on ice. Then cells were fixed with 2% paraformaldehyde in PBS for 15 min at room temperature and permeabilized with permeabilization solution (BD Bioscences, US) for another 20 min at 4 °C. Finally, splenic T cells were further stained with PE-cy7 anti-mouse-IFN-γ (eBioscience, US) Ab (0.4 μg/ml) and analyzed by flow cytometry.
Western blot
To recover secreted protein from the cell culture medium, the medium was mixed with Trichloroacetic acid (TCA) at the ratio of 4:1 (volume ratio) and incubated at 4 °C for 30 min, followed by centrifugation at 16873 g for 30 min. The protein pellets were washed with 500 μl pre-cooled acetone for 3 times and the acetone was removed by heating at 95 °C for 8 min. Cell pellets were lysed in lysis buffer (125 mM Tris-HCl, pH6.8, 10% sodium dodecyl sulfate (SDS), 2 M urea, 20% glycerol and 5% 2-mercaptoethanol) and subjected to 10% SDS-polyacrylamide gel electrophoresis and gels were transferred to a PVDF membrane. Membranes were blocked in 5% skimmed milk and probed with primary antibodies including rabbit polyclonal antibodies for HMGN1 (1:1000) or AFP (1:1000; Proteintech, US), overnight at 4 °C. GAPDH (1:1000, Cell Signaling Technology, US) was used as a loading control. The bound primary antibody was detected by horseradish peroxidase-conjugated goat anti-mouse IgG (Sigma, US), goat anti-rabbit IgG (Sigma, US) respectively. The ECL western blotting analysis system (Millipore, Billerica, MA) was applied.
Isolation of lymphocytes in peripheral blood/ lymphoid organs/tumor tissue
Peripheral blood from treated HCC mice was collected with 1% heparin and mononuclear cells were isolated by adding the equal volume to Lymphoprep™ (Stem cell, Canada) and centrifuged at 3000 g for 5 min, followed by lysis with the ammonium chloride-potassium (ACK) lysis buffer for 5 min at room temperature to generate lymphocyte suspensions. Isolation of lymphocytes from tumor tissues was the same as described previously [5]. Mixed lymphocytes were stained with different antibodies on ice for 45 min as described above, followed by flow cytometry (BD, US) detection.
Cytokine release assay
Blood (centrifuged at 3000 g for 30 min at room temperature) and tumor homogenates (150 mg tumor tissue) were harvested from Lenti-AFP-, Lenti-HA-treated or control HCC mice and followed by analysis of IFN-γ (R&D systems, US), IL-2 (eBioscience, US), IL-10 (MultiSciences Biotech Co., Ltd., China) and TGF-β kits (MultiSciences Biotech Co., Ltd., China), respectively. To examine the immune response in orthtopic HCC mice treated with repeated administrations of lentiviral vaccines in a real-time manner, we collected blood 3 days after each injection via retro-orbital bleeding. For murine in vitro study, IFN-γ and IL-2 ELISA kits were purchased from R&D systems and eBioscience (US). For human in vitro cytokine assay, IFN-γ and IL-2 ELISA kits were purchased from Dakewe Biotech Co., Ltd (China). Briefly, 1x105 activated BMDCs (transduced with lentivirus), inguinal lymph node CD11c+DC or peripheral monocyte-derived DCs (PMDCs) were incubated with 1x106 mixed splenocytes from naïve mice or human lymphocytes for 72 hrs. Levels of IFN-γ and IL-2 in supernatant were detected as per the manufacturer's instructions.
Semi-quantitative PCR analysis
Semi-quantitative PCR was performed for detecting the integration of lentivirus into different tissues. Genomic DNA was extracted from lymph nodes, spleen, muscle, liver, and tumor and was used as template (1 μg for 20 μl reaction). The primer sequences for CMV promoter were (F) 5'-GCGTGGATAGCGGTTTGACT-3' and (R) 5'-GGCGGAGTTGTTACGACATTTT-3' and the primer sequences for GAPDH were 5′-CCATGTTTGTGATGGGTGTGAACCA-3′ (F) and 5′-ACCAGTGGATGCAGGGATGATGTTC-3′ (R). The cycling conditions were 95 °C for 5 min, 95 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s for 25 cycles. All semi-quantitative PCR data were performed with at least 3 repeats. The PCR products were confirmed by an agarose-gel electrophoresis.
Statistical analysis
All data are reported as mean values ± SEM. Statistical differences between treatment and control groups were evaluated by SigmaStat (Systat Software, London, UK). Both parametric and non-parametric analyses were applied, in which the Mann-Whitney Rank Sum Test (Mann-Whitney U test) was used for samples on a non-normal distribution whereas a two-tailed t test was performed for samples with a normal distribution, respectively. P <0.05 was considered statistically significant.
Supplementary Material
Supplementary figures.
Acknowledgements
The authors thank Dr Yiqi Seow (Biomedical Sciences Institutes, A*STAR, Singapore) for critical review of the manuscript and Dr De Yang (Center for Cancer Research, National Institutes of Health, US) for providing the HMGN1 plasmid.
Financial Support
This research was supported by National Key R&D Program of China (no.2017YFC1001902), National Natural Science Foundation of China (no. 8180346, 81672124, 81501531, 81671528 and 81273420), the Science & Technology Development Fund of Tianjin Education Commission for Higher Education (Grant no. 2017KJ222) and Tianjin Municipal 13th five-year plan (Tianjin Medical University Talent Project).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chawla A, Ferrone C. Hepatocellular carcinoma surgical therapy: perspectives on the current limits to resection. Chin Clin Oncol. 2018;7:48
2. Boland P, Wu J. Systemic therapy for hepatocellular carcinoma: beyond sorafenib. Chin Clin Oncol. 2018;7:50
3. Ikeda K. Recent advances in medical management of hepatocellular carcinoma. Hepatol Res. 2018
4. Cany J, Barteau B, Tran L, Gauttier V, Archambeaud I, Couty JP. et al. AFP-specific immunotherapy impairs growth of autochthonous hepatocellular carcinoma in mice. J Hepatol. 2011;54:115-21
5. Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z. et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67:739-48
6. Vollmer CM Jr, Eilber FC, Butterfield LH, Ribas A, Dissette VB, Koh A. et al. Alpha-fetoprotein-specific genetic immunotherapy for hepatocellular carcinoma. Cancer Res. 1999;59:3064-7
7. Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P. et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12:2817-25
8. Ritter M, Ali MY, Grimm CF, Weth R, Mohr L, Bocher WO. et al. Immunoregulation of dendritic and T cells by alpha-fetoprotein in patients with hepatocellular carcinoma. J Hepatol. 2004;41:999-1007
9. Behboudi S, Pereira SP. Alpha-fetoprotein specific CD4 and CD8 T cell responses in patients with hepatocellular carcinoma. World J Hepatol. 2010;2:256-60
10. Esslinger C, Chapatte L, Finke D, Miconnet I, Guillaume P, Levy F. et al. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses. J Clin Invest. 2003;111:1673-81
11. Lopes L, Dewannieux M, Gileadi U, Bailey R, Ikeda Y, Whittaker C. et al. Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses. J Virol. 2008;82:86-95
12. Kung SK, Bonifacino A, Metzger ME, Ringpis GE, Donahue RE, Chen IS. Lentiviral vector-transduced dendritic cells induce specific T cell response in a nonhuman primate model. Hum Gene Ther. 2005;16:527-32
13. Zarei S, Abraham S, Arrighi JF, Haller O, Calzascia T, Walker PR. et al. Lentiviral transduction of dendritic cells confers protective antiviral immunity in vivo. J Virol. 2004;78:7843-5
14. Dullaers M, Van Meirvenne S, Heirman C, Straetman L, Bonehill A, Aerts JL. et al. Induction of effective therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors. Gene Ther. 2006;13:630-40
15. Liechtenstein T, Perez-Janices N, Escors D. Lentiviral vectors for cancer immunotherapy and clinical applications. Cancers (Basel). 2013;5:815-37
16. He Y, Zhang J, Donahue C, Falo LD Jr. Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity. 2006;24:643-56
17. Alton EW, Beekman JM, Boyd AC, Brand J, Carlon MS, Connolly MM. et al. Preparation for a first-in-man lentivirus trial in patients with cystic fibrosis. Thorax. 2017;72:137-47
18. Arce F, Breckpot K, Collins M, Escors D. Targeting lentiviral vectors for cancer immunotherapy. Curr Cancer Ther Rev. 2011;7:248-60
19. Hong Y, Peng Y, Guo ZS, Guevara-Patino J, Pang J, Butterfield LH. et al. Epitope-optimized alpha-fetoprotein genetic vaccines prevent carcinogen-induced murine autochthonous hepatocellular carcinoma. Hepatology. 2014;59:1448-58
20. Nie Y, Yang D, Oppenheim JJ. Alarmins and Antitumor Immunity. Clin Ther. 2016;38:1042-53
21. Yang D, Postnikov YV, Li Y, Tewary P, de la Rosa G, Wei F. et al. High-mobility group nucleosome-binding protein 1 acts as an alarmin and is critical for lipopolysaccharide-induced immune responses. J Exp Med. 2012;209:157-71
22. Wei F, Yang D, Tewary P, Li Y, Li S, Chen X. et al. The Alarmin HMGN1 contributes to antitumor immunity and is a potent immunoadjuvant. Cancer Res. 2014;74:5989-98
23. Nie Y, Yang, Trivett A, Han Z, Xin H, Chen X. et al. Development of a Curative Therapeutic Vaccine (TheraVac) for the Treatment of Large Established Tumors. Sci Rep. 2017;7:14186
24. Yang D, Bustin M, Oppenheim JJ. Harnessing the alarmin HMGN1 for anticancer therapy. Immunotherapy. 2015;7:1129-31
25. Biragyn A, Surenhu M, Yang D, Ruffini PA, Haines BA, Klyushnenkova E. et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167:6644-53
26. Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C. et al. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64:456-72
27. Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM. et al. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell. 2016;30:324-36
28. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563-604
29. Pardee AD, Butterfield LH. Immunotherapy of hepatocellular carcinoma: Unique challenges and clinical opportunities. Oncoimmunology. 2012;1:48-55
30. Reig M, da Fonseca LG, Faivre S. New trials and results in systemic treatment of HCC. J Hepatol. 2018;69:525-33
31. Liu P, Chen L, Zhang H. Natural Killer Cells in Liver Disease and Hepatocellular Carcinoma and the NK Cell-Based Immunotherapy. J Immunol Res. 2018;2018:1206737
32. Yang D, de la Rosa G, Tewary P, Oppenheim JJ. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009;30:531-7
33. Follenzi A, Gupta S. The promise of lentiviral gene therapy for liver cancer. J Hepatol. 2004;40:337-40
34. Sundarasetty BS, Chan L, Darling D, Giunti G, Farzaneh F, Schenck F. et al. Lentivirus-induced 'Smart' dendritic cells: Pharmacodynamics and GMP-compliant production for immunotherapy against TRP2-positive melanoma. Gene Ther. 2015;22:707-20
35. Yalcin A, Solakoglu TH, Ozcan SC, Guzel S, Peker S, Celikler S. et al. 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase-3 is required for transforming growth factor beta1-enhanced invasion of Panc1 cells in vitro. Biochem Biophys Res Commun. 2017;484:687-93
36. Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc. 2009;4:495-505
37. Yu W, Qu H, Cao G, Liu C, Deng H, Zhang Z. MtHsp70-CLIC1-pulsed dendritic cells enhance the immune response against ovarian cancer. Biochem Biophys Res Commun. 2017;494:13-9
Author contact
![]() Corresponding author: HaiFang Yin or Yunde Liu, Email: haifangyinedu.cn or yundeliucom; Tel: +86 (0)22 83336537; Fax: +86 (0)22 83336537. Address: Tianjin Medical University, Qixiangtai Road, Heping District, Tianjin, 300070, China
Corresponding author: HaiFang Yin or Yunde Liu, Email: haifangyinedu.cn or yundeliucom; Tel: +86 (0)22 83336537; Fax: +86 (0)22 83336537. Address: Tianjin Medical University, Qixiangtai Road, Heping District, Tianjin, 300070, China
 Global reach, higher impact
Global reach, higher impact