13.3
Impact Factor
Theranostics 2019; 9(7):1923-1951. doi:10.7150/thno.30787 This issue Cite
Review
Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics
1. The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, 230001, P.R. China
2. Research Group on Community Nutrition and Oxidative Stress, University of the Balearic Islands, Palma de Mallorca, Spain and CIBEROBN (Physiopathology of Obesity and Nutrition CB12/03/30038), Instituto de Salud Carlos III, Madrid, Spain.
3. Department of Pharmacognosy, School of Pharmacy, Zanjan University of Medical Sciences, Zanjan, Iran.
4. Traditional Medicine and History of Medical Sciences Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran
5. Department of Pharmacognosy, School of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab, India.
6. Department of Pharmacy, University of Naples Federico II, Naples, Italy.
7. Dipartimento di Medicina Clinica e Chirurgia, Sezione di Endocrinologia, Università Federico II di Napoli, Naples, Italy.
8. Department of Natural Drugs, Faculty of Pharmacy, University of Veterinary and Pharmaceutical Sciences Brno, Palackého tř. 1946/1, 612 42 Brno, Czech Republic.
9. Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina, 98168, Messina, Italy.
10. Institute of Pharmacology and Experimental Toxicology, Faculty of Medicine, University of Ljubljana, SI-1000 Ljubljana, Slovenia.
11. Department of Health Sciences, Faculty of Science, University of Mauritius, Réduit, Mauritius.
12. Department of Biology, Science Faculty, Selcuk University, Campus, Konya, Turkey.
13. Aab Cardiovascular Research Institute, Department of Medicine, University of Rochester, School of Medicine and Dentistry, Rochester, NY, USA.
14. Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran 14359-16471, Iran.
Received 2018-10-18; Accepted 2019-2-5; Published 2019-3-16
Abstract
Cardiovascular and metabolic diseases (CVMD) are the leading causes of death worldwide, underscoring the urgent necessity to develop new pharmacotherapies. Berberine (BBR) is an eminent component of traditional Chinese and Ayurvedic medicine for more than 2000 years. Recently, BBR has attracted much interest for its pharmacological actions in treating and/or managing CVMD. Recent discoveries of basic, translational and clinical studies have identified many novel molecular targets of BBR (such as AMPK, SIRT1, LDLR, PCSK9, and PTP1B) and provided novel evidences supporting the promising therapeutic potential of BBR to combat CVMD. Thus, this review provides a timely overview of the pharmacological properties and therapeutic application of BBR in CVMD, and underlines recent pharmacological advances which validate BBR as a promising lead drug against CVMD.
Keywords: berberine, cardiovascular diseases, metabolic diseases, targets, therapeutics
Introduction
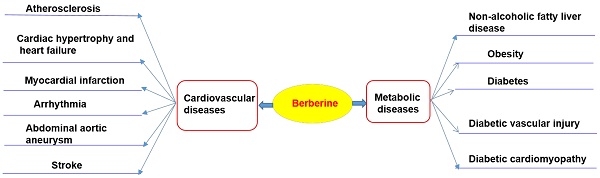
Cardiovascular diseases (including atherosclerosis, myocardial infarction, hypertension, cardiac hypertrophy and heart failure), and metabolic diseases (including diabesity, obesity, and non-alcoholic fatty liver disease), are the leading causes of death worldwide [1-8]. These diseases are caused by the combined effects of multiple pathological factors, and their pathogenesis has not been fully clarified yet [1-8]. Although the prevention and treatment of cardiovascular diseases and metabolic diseases (CVMD) have made great progress in the past 20 years, the morbidity and mortality arising from CVMD are still very high [1-8]. Western medicine is still the mainstream therapy of CVMD [9-12]. For example, hypoglycemic agents, statins, anticoagulants, beta receptor blockers, nitrates, and anti-thrombotic drugs are widely used in patients with CVMD [9-11, 13, 14]. Despite widespread evidences showing that these drugs are effective in a treatment of CVMD, potentially serious adverse consequences remain key challenges [9-14]. Therefore, there is an urgent need to identify alternative and complementary therapies to better manage CVMD. Berberine (BBR) is widely used in Asian countries (mainly in China) due to its good clinical and safety profile [15-17]. With the advances of pharmacological research, BBR is considered to be one of the most promising natural product-derived drug for the treatment of CVMD (Figure 1). To this end, we provide a timely and insightful overview of the therapeutic potential and molecular targets of BBR in treating CVMD.
BBR - basic characteristics and history of use
BBR is the principal bioactive ingredient of Rhizoma coptidis (also named 'Huang Lian' in Chinese), a common traditional Chinese medicinal herb used for the therapy of inflammatory disorders and diabetes mellitus (DM) [18, 19]. The earliest record of the use of Rhizoma coptidis as a medication is dated in A.D. 200 in the book of 'Shen Nong Ben Cao Jing' [18, 19]. For the first time, the anti-diabetic effect of Rhizoma coptidis was recorded in the book Note of Elite Physicians in about 1500 years ago by Hongjing Tao [20].
BBR (C20H18NO4) is a quaternary ammonium salt derived from isoquinoline alkaloid, with a molar weight of 336.36 g/mol [21, 22]. BBR is a yellow powder that is odorless and has characteristic alkaloid bitterness [21]. It is sparingly soluble in water, slightly soluble in ethanol or methanol; however, the salt form is relatively water-soluble [21, 22]. BBR can be easily obtained from medicinal plants or through total synthesis [23, 24].
Bioavailability and metabolism of BBR
Chloride or sulfate of BBR are commonly used for clinical purposes [15, 17]. Nevertheless, pharmacokinetic data in rodents and humans have revealed poor absorption from the gut and rapid metabolism in the body that caused its low oral bioavailability [21]. For example, BBR is converted to ionic form under physiological conditions and self-aggregates at low pH values [25-27]. Self-aggregation of BBR reduces its solubility in the gastrointestinal tract and its ability to permeate the gut wall [26, 27]. P-glycoprotein (P-gp) is located in the epithelial cell membrane and can efflux many drugs (including BBR), thereby limiting their oral bioavailability [26]. P-gp inhibitors, including D-tocopheryl polyethylene glycol 1000 succinate, are common adjuvants to increase the oral bioavailability of BBR [25]. In addition, penetration enhancers and lipid particle delivery systems can also increase the bioavailability of BBR [27]. BBR is metabolized by oxidative demethylation and glucuronidation to berberrubine, thalifendine, demethyleneberberine and jatrorrhizine and their corresponding glucuronides in the liver [21, 28] (Figure 2). CYP2D6 is the major cytochrome P450 (CYPs) for BBR metabolism, followed by CYP1A2, 3A4, 2E1 and CYP2C19. Finally, BBR metabolites are excreted through bile, feces, and urine [21, 28].
Although plasma concentration of BBR is low, the tissue concentrations of BBR and its metabolites are high [29]. BBR and its metabolites are widely distributed in the liver, kidney, muscle, lung, brain, heart, pancreas and adipose tissue [29-31]. BBR can also penetrate the blood-brain barrier [32]. Specifically, the rapid clearance of BBR from plasma as compared to the hippocampus indicates that BBR may have an important effect on hippocampal neurons [31]. Moreover, infusion of BBR (2 μg/h, 28d) by bilateral hypothalamic paraventricular nucleus (PVN) through an osmotic minipump can reduce hypertension and sympathoexcitation in two-kidney, one-clip (2K1C) renovascular hypertensive rats by ROS/ERK1/2 (extracellular-signal regulated kinase 1/2)/ inducible nitric oxide (iNOS) pathway [33].
Therapeutic potential of BBR in cardiometabolic diseases. Current researches support that BBR may play a therapeutic role in the treatment of cardiovascular disease (including atherosclerosis, heart failure, myocardial infarction, arrhythmia, abdominal aortic aneurysm, stroke) and metabolic diseases (including nonalcoholic fatty liver, obesity, diabetes and its cardiovascular complications).
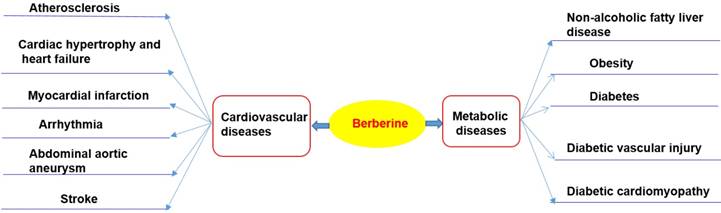
Selected metabolites of BBR in human. BBR is metabolized in the body by metabolic pathways (such as demethylation, glucuronidation etc) to thalifendin, berberrubine, jatrorrhizin, demethyleneberberin.
Emerging studies have shown that BBR is almost safe at conventional doses, with a relatively low incidence of adverse reactions, such as gastrointestinal discomfort, and transient increases in plasma bilirubin levels [27, 34]. Although the safety of BBR is relatively high, it should be taken carefully to avoid adverse reactions in specific cases. For example, BBR replaces bilirubin in binding to albumin (in nearly 10 times greater effect compared to phenylbutazone), so any BBR containing herbs should be avoided in jaundice in pregnant women and infants [35]. BBR interacts with macrolides and it may lead to potentially dangerous arrhythmias [36]. BBR in combination with statins increases cardiotoxicity by inhibiting CYP3A4 and human ether-a-go-go related genes (hERG) potassium channels [37]. On the other hand, BBR can prevent toxic reactions in different tissues caused by antitumor drugs such as cisplatin [38], cyclophosphamide [39], doxorubicin [40] and bleomycin [41] as well as side effects of analgesics (e.g. acetaminophen [42]).
BBR in the treatment of cardiovascular diseases
Atherosclerosis
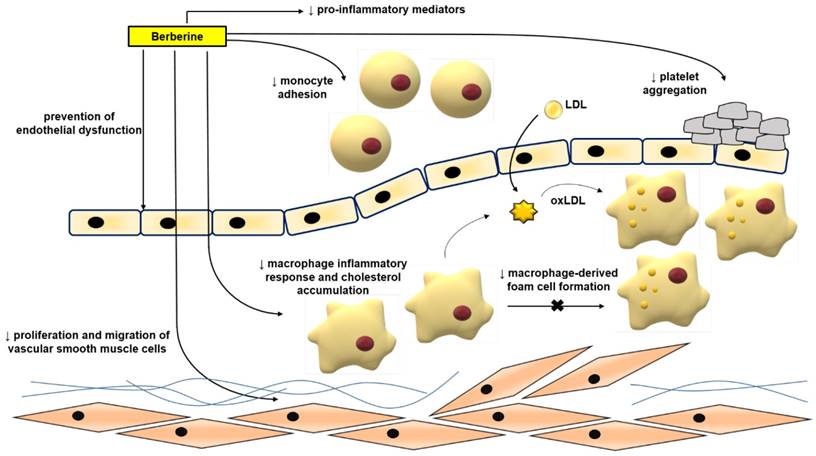
Atherosclerosis is mainly a lipid metabolic disorder which underlies multiple cardio- and cerebro-vascular diseases [43-46]. Atherosclerosis commences with endothelial dysfunction, followed by neointima formation, lipid accumulation, foam cell formation, and plaque rupture [43, 44, 46-48]. BBR exerts protective effects against atherosclerosis by modulating various pro-atherogenic cellular events (Figure 3).
Mechanism of BBR in protecting against atherosclerosis in vitro
Normalizing endothelial function
Endothelial dysfunction and its related complications are typically specified by reduced local bioavailability of nitric oxide (NO) and excessive oxidative stress; the increased NADH/NADPH and xanthine-oxidase activity are important factors for oxidative stress in endothelial cells [49-51]. Enhanced adhesion of leukocytes to the endothelium plays an initiating role in inflammation [3, 7, 52, 53]. Studies have proven the ameliorative effects of BBR in endothelial dysfunction via regulating reactive oxygen species (ROS)/NO balance [49, 52, 54]. BBR treatment is capable to reduce oxidized low density lipoprotein (oxLDL)-stimulated production of ROS in human umbilical vein endothelial cells (HUVECs) [54]. BBR could inhibit oxLDL‑stimulated monocyte adhesion to HUVECs via the mechanism associated with the suppression of vascular cell adhesion molecule-1 (VCAM‑1) and intercellular adhesion molecule-1 (ICAM‑1) [52]. BBR has been shown to reduce the tumor necrosis factor α (TNFα)‑stimulated expression of the pro-inflammatory monocyte chemotactic protein-1 (MCP-1) and ICAM‑1 in human amniotic epithelial cells (HAECs) and to suppress the activation of nuclear factor kappa B (NF‑κB) in HAECs involving AMP protein kinase (AMPK)‑dependent pathway [55]. The exposure of HUVECs to oxLDL or TNFα significantly increased the expression of lectin-like oxidized low density lipoprotein receptor-1 (LOX-1) and the production of intracellular ROS [56-58]. Treatment with BBR reduced ERK1/2 signaling pathway, the expression of nicotinamide adenine dinucleotide phosphate-oxidase 2 (NOX2) and the expression of VCAM-1 and ICAM-1 [56]. BBR protected against palmitate-induced endothelial dysfunction via increasing NO level, and endothelial nitric oxide synthase (eNOS) expression and down-regulating the expression of NOX4 in HUVECs [59]. BBR also contributed to the inhibition of the vascular inflammation via preventing angiotensin II (Ang II)-induced adhesion of monocytes to endothelial cells and reduced ROS and MCP-1 expression in HUVECs [60]. Berberrubine, the active metabolite of BBR, has recently been shown to decrease xanthine oxidase activity and reduce TNFα-induced ICAM-1 expression in HUVECs [61].
BBR also dose-dependently inhibited the proliferation of HUVECs induced by oxLDL; its anti-proliferative and anti-inflammatory properties are exerted through decreasing the expression of NF-кB, LOX-1, proliferating cell nuclear antigen (PCNA) and inhibiting the phosphorylation of the phosphoinositide 3-kinase (PI3K)/Akt, ERK1/2, p38 mitogen activated protein kinases (MAPKs) signaling pathways [62]. In HUVECs, BBR treatment showed protective effects also against oxLDL-induced endothelial injury and apoptosis through cytochrome c-mediated caspase activation pathway [54].
Inhibiting migration and proliferation of vascular smooth muscle cells (VSMCs)
The enhancement of VSMCs proliferation and migration is an important factor for causing of neointimal hyperplasia and luminal stenosis after vascular injury [63]. Components of extracellular matrix, cytokines, shear stress, ROS, and other factors can significantly reduce the expression of markers of the differentiation of VSMCs, increase VSMCs proliferation and migration, and also a capacity for the synthesis of the extracellular matrix and promotion of neointimal formation [64]. BBR had a beneficial effect on vascular remodeling [65, 66]. BBR inhibited fetal bovine serum-induced proliferation of VSMCs by up-regulation of peroxisome proliferator-activated receptor α (PPARα) expression and NO concentration [67]. BBR inhibited the migration of human aortic VSMCs by down-regulating matrix metalloproteinase (MMP)-2 and MMP-9, as well as urokinase-type plasminogen activator (u-PA) [68]. Similarly, BBR attenuated Chlamydia pneumoniae infection-induced VSMCs migration by inhibiting PI3K and down-regulating the expression of MMP-3 and MMP-9 [69].
Anti-atherosclerotic effects of BBR. The role of BBR in inhibiting atherosclerosis includes improving endothelial dysfunction; inhibiting smooth muscle cell proliferation and migration; reducing monocyte adhesion, macrophage inflammation and cholesterol aggregation, foam cell formation, and platelet aggregation. Abbreviations: low density lipoprotein (LDL); oxidized LDL (oxLDL).
In addition to its effect on the extracellular matrix, BBR reduced platelet-derived growth factor (PDGF)-induced proliferation of VSMCs by activating AMPK/p53/p21 signaling, while simultaneously inactivating Ras/Rac1/Cyclin D/Cdks, and attenuated PDGF-induced migration by inhibiting Rac1 and Cdc42 [70]. BBR limited the proliferation and migration of lysophosphatidylcholine (lysoPC)-stimulated VSMCs by decreasing ROS level and subsequent activation of the ERK1/2 pathway [71]. BBR also significantly decreased the proliferation and migration of VSMCs stimulated by Ang II and heparin binding epidermal growth factor (HB-EGF) by inhibiting the activation of Akt pathway [65]. In norepinephrine (NE)-induced pulmonary arterial VSMC proliferation and migration models, BBR can reverse the effect of NE by increasing protein phosphatase 2A (PP2A) signaling, suggesting that BBR may have a therapeutic effect in pulmonary hypertension [72]. Furthermore, BBR induced the cell cycle arrest of A7r5 cells by disrupting the combination of p27, p21 and Skp2, inhibited the proliferation of A7r5 induced by PDGF-BB and enhanced the anti-proliferative effect of adrenal steroid dehydroepiandrosterone sulfate on A7r5 [73]. BBR blocked the cell cycle progression of VSMCs at G(1) phase by down-regulating the expression of cyclin D1, without affecting G(2)/M phase [74].
Inhibiting of macrophage-derived foam cell formation, inflammation, and inflammasome activation
The uptake of oxLDL into macrophages is mediated by diverse scavenger receptors (SRs), such as scavenger receptor A (SR-A)I/II, scavenger receptor class B type I (SR-BI), LOX-1, and the cluster of differentiation 36 (CD36)[75]. When oxLDL is endocytosed into macrophages, they develop into foam cells that form the core of atherosclerotic lesion [76, 77]. BBR reduced oxLDL uptake by inhibiting CD36 and LOX-1, and promoted cholesterol efflux by suppressing the expression of adipocyte enhancer-binding protein 1 (AEBP1) in macrophages [78]. BBR also inhibited the effect of oxLDL on macrophage-derived foam cell formation by inhibiting LOX-1, while up-regulating SR-BI expression [79]. Moreover, it has been reported that BBR minimized the accumulation of lipids in human macrophages exposed to hypercholesterolemic serum by reducing the process of micropinocytosis and also decreased the secretion of MCP-1 [80]. Furthermore, BBR eliminated foam cells by enhancing liver X receptor α (LXRα) and ATP-binding membrane cassette transport protein A1 (ABCA1)-stimulated cholesterol efflux [81]. The combination of atorvastatin and BBR suppressed the foam cells formation more effectively than atorvastatin alone [82]. BBR suppressed the formation of foam cells from THP-1-derived macrophages by increasing the expression of AMPK and silent information regulator 1 (SIRT1), by decreasing the expression of PPARγ, and reducing the uptake of oxLDL [82]. In addition, BBR combined with atorvastatin diminished the levels of serum triglyceride (TG), TC, and low-density lipoprotein cholesterol (LDL-C) and inflammation and oxidative stress markers [83]. BBR combined with atorvastatin down-regulated the expression of LOX-1 through endothelin 1 receptor (ET-1R) in monocytes/macrophages [83], and in macrophages-derived foam cells, BBR-mediated sonodynamic therapy enhanced cholesterol efflux by promoting the production of ROS, and enhanced autophagy through the PI3K/Akt/mammalian target of rapamycin (mTOR) signaling pathway [84]. However, it was shown that BBR induced the foam cell formation and promoted atherosclerosis by inducing the expression of SR-A in macrophages and increasing the uptake of modified low-density lipoprotein (LDL) [85]. Although the vast majority of above mentioned studies show that BBR can suppress foam cell formation and the progression of atherosclerosis, this conclusion is still controversial.
BBR also exerted anti-inflammatory effects in macrophages stimulated with oxLDL. BBR down-regulated lipopolysaccharide (LPS)-induced IL-1β, IL-6, iNOS, MCP-1, cyclooxygenase (COX)-2 and MMP-9 expression, and p-MAPKs (including p38, c-Jun N-terminal kinase (JNK), and ERK) by activating AMPK [86]. BBR reduced the production of inflammatory markers and induced autophagy in oxLDL-exposed J774A.1 cells [87]. The mechanism of effect was associated with the activation of AMPK/mTOR signaling pathway [87]. Furthermore, BBR inhibited the activation of the Nod-like receptor family pyrin domain containing-3 (NLRP3) inflammasome: BBR exposure reduced ROS-dependent activation of NLRP3 inflammasome in macrophages and inhibited the expression and release of IL-1β by inhibition of NF-κB [88]. BBR can also reduce NLRP3 inflammatory expression in PMA-stimulated macrophages by inhibiting Toll-like receptor 4 (TLR4)/ myeloid differentiation factor 88 (MyD88)/NF-κB signaling pathway [89]. BBR decreased sodium uric acid crystal induced inflammasome activation by suppressing the expression of NLRP3 and IL-1β [90]. In addition, in adipose tissue-derived macrophages, BBR suppressed NLRP3 inflammasome activation and IL-1β production stimulated by saturated fatty acid (palmitate), by activating AMPK-dependent autophagy [91]. BBR also inhibited the activation of the NLRP3 inflammasome pathway, and then improved liver injury in mice by inhibiting the purinoreceptor P2X7 [92]. TNFα and LPS stimulated the expression of miR-23a in RAW 264.7 macrophages, resulting in TLR4/TNF receptor-associated factor 2 (TRAF2)-mediated activation of apoptosis-signaling kinase 1 (ASK1) and phosphorylation of p38, which mediate inflammatory reaction [93]. BBR treatment inhibited LPS and TNFα-induced inflammatory responses by up-regulating miR-23a, ameliorating TLR4, TRAF2, TNFα, IL-6 and IL-23 gene expression, and inhibiting TLR4/TRAF2-mediated ASK1/p38 phosphorylation [93]. After percutaneous coronary intervention, macrophage activation plays an essential role in neovascular atherosclerosis and in-stent restenosis [94]. Galectin-3, which was mainly expressed on macrophages, is an important mediator of inflammation. BBR down-regulated oxLDL-induced macrophage activation by down-regulating Galectin-3 via NF-κB and AMPK signaling pathways [94].
Inhibiting platelet activation
BBR has been shown to effectively prevent thrombus formation in arteries [95], and assisted thrombolysis induced by the plasminogen activators urokinase and streptokinase [96]. The anti-platelet aggregation effects of BBR have been demonstrated in platelets isolated from healthy subjects, patients with platelet hyperaggregability, and also patients with atherosclerotic cerebral infarction [95]. Chu et al. well reviewed the effect of BBR on platelets in 1996 [97]. BBR in vitro could inhibit platelet aggregation stimulated by adenosine diphosphate (ADP), collagen, adrenaline, arachidonic acid (AA), and calcium ionophore A23187 [97]. It also had inhibitory effect on clot retraction induced by ADP [98]. BBR inhibited platelet activation by modulating the effect on cAMP level in platelets, antagonistic activity against Ca2+, partial and competitive binding with α2-adrenoreceptors on platelet membrane, affecting AA metabolism and ADP release from platelets [97].
BBR chloride decreased plasmatic levels of thromboxane B2 and P-selectin in mice with dextran sulphate sodium-stimulated colitis [99]. BBR chloride also inhibited platelet activation via down-regulation of P-selectin expression and inhibiting fibrinogen binding to platelet GP IIb/IIIa integrin receptor on the surface of platelets [100]. Shah et al. [101] reported that BBR inhibited collagen-induced platelet aggregation without affecting the platelet responses to the other platelet agonists including platelet-activating factor (PAF), AA and adrenaline. Its anti-platelet activity has been suggested to be exerted via suppressing with the collagen-induced adhesion process without any effect on cAMP, or A23187-induced platelet aggregation [101]. While in one animal study investigating the effect of BBR on GPVI (glycoprotein receptor for collagen, expressed in platelets) BBR could not statistically inhibit the expression of GPVI [102], another study has proven the protective effects of BBR in combination with ligustrazine (alkylpyrazine from Ligusticum chuanxiong) in a rat model of coronary microembolization [103]. In this study, ADP-induced platelet activation, von Willebrand factor, ET-1, and levels of TNFα, IL-1β, and ICAM-1 in serum and heart tissues were decreased by BBR/ligustrazine combination [103]. Tissue factor (TF) is closely related to coagulation; its expression by vascular cells surrounding blood vessels is stimulated by pro-inflammatory substances such as LPS or TNFα [104]. In this regard, BBR attenuated LPS-stimulated TF protein expression by suppressing NF-κB, Akt and MAPK signaling pathways in THP-1 cells [105]. However, another study revealed that BBR could increase TNFα-stimulated TF expression in HAECs, and could decrease the expression of TF pathway inhibitor in HAECs and in arteries of apolipoprotein E-deficient mice (ApoE-/-) mice, what might promote thrombosis [104]. BBR therefore might act bidirectionally on TF expression in different cell types [105].
Anti-atherosclerotic effects of BBR in vivo
Diverse investigations have reported the therapeutic potential of BBR to inhibit atherosclerosis in mouse models of atherosclerosis. For example, BBR treatment (150 mg/kg/d, p.o., 12wk) obviously reduced the atherosclerotic plaque area and displayed suppression of inflammatory and oxidative markers [106]. Specifically, BBR diminished the serum levels of IL-1β and TNFα, and decreased the expression of ICAM-1, iNOS, and IL-6 in the aorta. Furthermore, the treatment with BBR reduced the translocation of NF-κB to the nucleus [106]. In homocysteine thiolactone (HTL)-fed ApoE-/- mice, BBR (1.0 g/kg/d, p.o., 8wk) increased atherosclerotic plaque stability in the carotid artery and normalized the redox state. These protective effects on vascular function might be ascribed to an activation of PPARγ, which leads to a down-regulation of oxidative stress [107]. BBR also abrogated the HTL-induced oxidative stress in HUVECs by activating PPARγ [107].
Visfatin is a pro-inflammatory adipokine, which is expressed in visceral fat and induces endothelial dysfunction by promoting the production of inflammatory and adhesion molecules [108]. Oral administration of BBR (5 mg/kg/d, p.o., 12wk) in ApoE-/- mice lowered the serum expression of visfatin and inflammatory cytokines (TNFα and IL-6), as well as it reduced the distribution of visfatin in the atherosclerotic plaques [108]. BBR pretreatment also reversed the visfatin-induced increase of IL-6 and TNFα in HUVECs [108]. The amelioration of visfatin-induced endothelial dysfunction was associated with the inhibition of MAPK signaling pathway (p38 and JNK) [108]. Moreover, BBR suppressed the increase of p-p38 MAPK, p-JNK and Bcl2- associated X protein (Bax) in visfatin-induced endothelial dysfunction in HUVECs [108]. Furthermore, it was suggested that the protective effects of BBR (in drinking water (0.5 g/L), 14wk) in ApoE-/- mice can be related to the modulation of gut microbiota, specifically an up-regulation in Akkermansia spp. abundance which has been known to regulate inflammation, metabolic endotoxemia, and gut barrier integrity [109]. A recent study showed that BBR (50 mg/kg, p.o., twice weekly, 12wk) inhibition of HFD-induced atherosclerosis in ApoE-/- mice is associated with changes in gut microbiota composition, such as BBR treatment altering an occurrence of Firmicutes and Verrucomicrobia [110].
Increased circulating endothelial microparticles in the peripheral circulation show the state of endothelial damage and endothelial cell activation, apoptosis and injury [111]. BBR treatment (1.2 g/d, p.o.) in healthy humans led to a decrease in serum malondialdehyde and circulating CD31+/CD4- microparticles (as markers of endothelial injury) and the improvement of flow-mediated vasodilation. BBR contributed to an improvement of endothelial function via up-regulating eNOS expression and down-regulating NOX4 expression, and ROS production in HUVECs [112-114].
The MyD88 dependent TLR4 signaling pathway plays a critical role in hypertension-induce endothelial damage. TLR4 activation could activate NF-κB, leading to endothelial cell injury via an increased production of inflammatory enzymes and mediators (including COX-2, IL-1, and IL-6) [115]. An increased level of TNFα initiates apoptosis and endothelial cell turnover, resulting in the formation of atherosclerotic plaques [115]. BBR treatment inhibited the apoptosis, decreased the expression of NF-κB as well as the levels of TNFα and IL-6 in aortic endothelial cells isolated from spontaneously hypertensive rats (SHR) by decreasing the expression of TLR4 and MyD88 [116]. Endothelium-dependent contractions (EDCs) are also involved in endothelial dysfunction, and EDCs are caused by contracting factors such as endothelial COX-produced prostanoids or augmented level of ROS [117]. EDCs of the carotid arteries of SHR were reduced by BBR administration [118]. This effect has been reported to be caused by triggering of activated AMPK, inhibition of endoplasmic reticulum (ER) stress, reduction of ROS and expression of COX-2 [118]. BBR (5, 10 mg/kg, i.p.) has also been reported to inhibit NF-κB activity and decrease VCAM-1 production in lung of LPS-stimulated rats [119].
Cardiac hypertrophy and heart failure
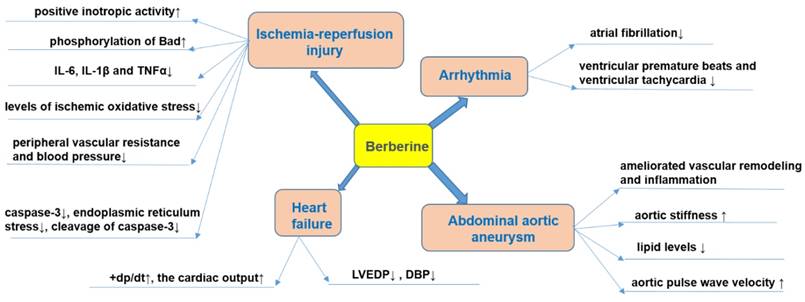
Cardiac remodeling is associated with progressive cardiac hypertrophy, fibrosis, and the eventual occurrence of heart failure (HF) [120-123]. Interventions in cardiac remodeling are important strategies for the treatment of HF [124]. The effect of BBR on HF is shown in Figure 4.
In animal models, BBR can reduce the degree of HF. For example, in ischemic left ventricular heart failure dogs, intravenous injection of BBR (1 mg/kg, within 3 min) and then repeated infusion (0.2 mg/kg/min, 30 min) for 10 consecutive days, increased the cardiac output and peak rate of rise of left ventricular pressure (+dp/dt) while left ventricular end-diastolic pressure (LVEDP), diastolic blood pressure (DBP), and systemic vascular resistance were decreased[125]. The total saponins of Panax ginseng (20 mg/kg/d) combined with BBR (20 mg/kg/d), and captopril were administered for 12 consecutive days to rats in a model of HF induced by injecting of high dose of isoproterenol (s.c.), and a similar anti-HF effect was observed in both groups [126]. BBR (63 mg/kg/d, p.o., 4wk) further proved to be a potential drug for improving HF symptoms by inhibiting the Ca2+ overload in cardiomyocytes [127].
Pathological cardiac hypertrophy is a critical intermediate step toward HF and other cardiac diseases [128]. BBR (10 mg/kg/d, p.o., 8wk) prevented the development of left ventricular hypertrophy in rats caused by pressure overload; +dp/dt increased, while the weight of the whole heart and left ventricle decreased, and the increase of left ventricular myocardial cell size was significantly reduced [129, 130]. Mechanistic studies have shown that BBR can up-regulate F-Box protein 32 (FBXO32), an E3 ubiquitin ligase, and down-regulated mTOR, ERK1/2, as well as p38 phosphorylation, which enhances autophagy and thus inhibits cardiac hypertrophy [128, 131]. Cardiac fibrosis is an important pathological change in the development of HF, arrhythmia and cardiac arrest [132]. BBR can restrict cardiac fibrosis by up-regulating the expression of relaxin in vitro [133]. BBR administration (5, 10 mg/kg/d, p.o., 4wk) also obviously reduced cardiac fibrosis in two-kidney, two-clip (2K2C) rats via increased levels of NO and cAMP in left ventricular tissue[134].
Abdominal aortic aneurysm
Abdominal aortic aneurysm is characterized by dilatation of the abdominal aorta with an etiology not completely understood [135]. The presence of aneurysm leads to the weakening of the aortic wall, followed by a progressive dilatation and potential risk of aortic rupture [136]. To date, only very limited studies investigating the effects of BBR against abdominal aortic aneurysm were published. One of the potential causes of formation of aneurysms is the increase in the ratio of collagen to elastin [137]. It has been evidenced that BBR significantly reduced collagen synthesis in cardiac fibroblasts subjected to Ang II [138]. The activation of MMPs is also a mechanism that participates in the etiology of aneurysms since it weakens the aortic media [139]. In this sense, it was reported that BBR obviously decreased the activity and expression of MMP-9 as well as the expression of extracellular MMP inducer (EMMPRIN) in stimulated macrophages [140]. In addition, BBR treatment ameliorated vascular remodeling in a rat model of metabolic syndrome by reducing the aortic levels of MMP-2 [141].
It is well established that the formation of abdominal aortic aneurysm is related to abnormal wall stiffness [142]. A clinical study investigated the effects of the two months consumption of 200 mg red yeast rice (RYR), 500 mg BBR and 10 mg policosanols in 70 hypercholesterolemic patients [143]. The intervention significantly improved aortic stiffness by reducing lipid levels and increasing aortic pulse wave velocity [143]. Furthermore, in aged mice, it has been evidenced that BBR induced the relaxation of VSMCs with decreasing of blood pressure and also reduced vascular stiffness via suppression of the activity of Ca2+ channel transient receptor potential vanilloid 4 (TRPV4) [144]. The effect of BBR on abdominal aortic aneurysm was summarized in Figure 4.
Ischemia-reperfusion injury
Ischemic heart disease (ISHD) is primarily caused by coronary atherosclerosis and its complications [145, 146]. Myocardial infarction is the most severe ISHD with high mortality [145]. Reperfusion is beneficial for ischemia, but it can also cause severe cardiac damage [145]. Thus, studying the underlying mechanisms of myocardial ischemia-reperfusion (I/R), contributes to the treatment of I/R injury in myocardial tissue [145, 146]. The effect of BBR on myocardial ischemia was shown in Figure 4.
Experiments on isolated cardiomyocytes in hypoxia-reoxygenation model showed that pretreatment with BBR resulted in reduction in lactate dehydrogenase (LDH) and malondialdehyde (MDA) release, which are biomarkers of the extent of I/R injury [147]. BBR inhibited I/R injury by the following mechanisms: i) direct and/or indirect antioxidant activity [148]; ii) post-ischemic anti-inflammatory activity [149]; iii) vasodilation of coronary arteries [150]; iv) anti‑apoptotic activity on cardiomyocytes [151]; v) suppressing an activation of autophagy during I/R[152]; vi) promoting angiogenesis in heart after I/R injury[153].
Antioxidant activity of BBR
BBR showed direct antioxidant activity in several cell-free systems, such as DPPH radical scavenging assay [154, 155]. Moreover, antioxidant activity was observed in cultured cells subject to oxidative stress [148, 156]. BBR inhibited oxidative stress by increasing super oxide dismutase (SOD), uncoupling protein 2 (UCP2) and decreasing NOX expression, via SIRT1/FoxO (forkhead box O) or AMPK signaling pathways [157].
Anti-inflammatory effects of BBR
BBR is anti-inflammatory active by decreasing the secretion of an array of pro-inflammatory cytokines/mediators (IL-6, IL-1β, and TNFα) in myocardial tissue and serum by suppressing PI3K/Akt signaling pathway [157-159]. BBR in cardiomyocytes in the mouse model of I/R injury inhibited inflammatory responses through the suppression of NF-κB signaling pathway [149]. Another study showed that BBR can also activate SIRT1 signaling, and thus further reduce myocardial inflammatory response [160].
Hypotensive effects of BBR
BBR has hypotensive effects by functioning as a vasodilator in isolated blood vessels [161, 162]. Vasodilation of coronary arteries and thus coronary flow is of key importance during the I/R injury of the heart [163]. In this context, BBR enhanced coronary blood flow in isolated guinea pig hearts with ventricular fibrillation [150]. Moreover, BBR can stimulate cardiac contractility (positive inotropic activity) by increasing intracellular calcium levels in addition to lowering peripheral vascular resistance and blood pressure [127]. Several mechanisms are proposed for vasodilation and/or hypotensive effect of BBR: i) the antagonism on α1-adrenoreceptors on VSMCs [164, 165]; ii) the enhancement of the hypotensive effect of acetylcholine (ACh) on nervus vagus, and thus the inhibition of carotid sinus pressor reflex [166]; iii) the endothelium-dependent release of NO [167, 168]; iv) via angiotensin-converting enzyme (ACE) inhibition of the NO-cGMP axis [169]; v) the direct activation of K+ channels in arterial VSMCs leading to hyperpolarization, thereby the inhibition of calcium influx leading to smooth muscle relaxation [168, 170]; vi) the inhibition of L- and T-type voltage-gated Ca2+ currents in ventricular myocytes [171].
Anti-apoptotic effects of BBR
One of the main mechanisms of BBR in protecting against I/R injury is the inhibition of apoptosis of cardiomyocytes. For example, in a mouse model of myocardial I/R, BBR inhibited the activation of caspase-3, caspase-9 and apoptotic protease-activating factor 1 (Apaf-1) in cardiomyocytes, while increased the expression of Bcl-2-like protein 1 and p53 [149]. In the rat hearts subjected to I/R injury, BBR treatment decreased AMPK and Bcl-2-like protein 1 in the myocardial risk areas, while increased AMPK in the non-ischemia areas compared to the control hearts [172]. In neonatal rat cardiomyocytes, BBR decreased hypoxia-reoxygenation-stimulated cardiomyocytes apoptosis, increased Bcl-2/Bax ratio, activated PI3K-Akt, AMPK and eNOS phosphorylation, while decreased caspase-3 expression [173, 174]. Recent studies on hypoxia-reoxygenation injury on H9C2 rat cardiac myoblasts proposed that BBR prevents apoptosis by modulating Notch1/Hes1-PTEN (phosphatase and tensin homolog)/Akt signaling pathway [175], as well as Smad7 (Mothers against decapentaplegic homolog 7) activation [151]. BBR-mediated activation of Smad7 pathway resulted in the suppression of caspase-3 expression and activity. Indeed, the Smad7 knockdown confirmed this hypothesis [151]. Importantly, BBR also activated the Janus kinase 2 (JAK2)/ signal transducer and activator of transcription 3 (STAT3) pathway, and thus decreased the endoplasmic reticulum (ER) stress [176].
Effect of BBR on autophagy and angiogenesis
Inhibition of activation of autophagy is an additional mechanism underlying the protective effects of BBR [152]. Incubation of the cells with BBR or treatment with BBR increased survival of cells, decreased the infarct zone in mice hearts, and suppressed autophagy, which was confirmed by the decreased expression levels of autophagy-related proteins such as SIRT1, BNIP3, and Beclin-1 [152]. BBR can also be useful after I/R injury, since BBR can promote angiogenesis of the small vascular beds in coronary arteries [153]. Post-ischemic application of BBR for 14 days reduced the infract zone, improved the heart function, as well as up-regulated miR-29b expression, and enhanced cell proliferation and migration in endothelial cells from left anterior descending (LAD) coronary artery [153].
Importantly, similar protective activities of BBR against I/R injury were observed also in non‑cardiac tissues [177]. In neuronal cells during ischemia, BBR reduced p53 and cyclin D1, enhanced phosphorylation of Bad and decreased cleavage of caspase-3, thereby inducing cell cycle arrest and inhibiting apoptosis [177]. During the reperfusion phase, BBR protected from cerebral ischemia injury via activating the PI3K/Akt signaling pathway [177]. A pretreatment with BBR significantly preserved cells in response to a strong oxidant (CoCl2) [178]. BBR injections were given prior to I/R surgery in rats and led to increased survival of neurons due to anti‑apoptotic effects mediated by PI3K/Akt signaling pathway [179]. Moreover, in a mouse model of cerebral ischemia, BBR decreased the neuronal apoptosis via activating PI3K/Akt signaling pathway [180].
Protective activity was not only BBR-specific but it can be related to the other members of structural class of isoquinoline alkaloids, as both coptisine [181] and palmatine [182] showed similar protective effects against I/R injury on hearts of rats.
Effects of BBR on heart failure, arrhythmia, myocardial ischemia and abdominal aortic aneurysm. Specifically, BBR prevents ischemia/reperfusion injury via its positive inotropic activity, increased phosphorylation of Bad, decreased production of pro-inflammatory mediators (IL-6, IL-1β, and TNFα), reducing oxidative stress, blood-pressure lowering, anti-apoptotic effects and protective effects against endoplasmic reticulum stress. BBR prevents heart failure by increasing cardiac output, and decreasing LVEDP and DBP. BBR prevents arrhythmia by reducing ventricular premature beats and tachycardia. BBR prevents abdominal aortic aneurysm by reducing vascular remodeling and pressure, reducing aortic stiffness, modulating lipid level, and increase aortic pulse wave velocity. ↑indicates increase or activation, and ↓indicates decrease or suppression. Abbreviations: Bad, Bcl-2-associated death promoter; diastolic blood pressure (DBP), interleukin-1β (IL-1β), interleukin-6 (IL-6), left ventricular end-diastolic pressure (LVEDP) , peak rate of rise of left ventricular pressure (+dp/dt), tumor necrosis factor α (TNFα).
Furthermore, administration of BBR (100 mg/ kg/d, p.o., 14d) in rats prior to I/R injury (a surgical occlusion of LAD coronary artery) resulted in a reduced size of infarction and protection from arrhythmias [172]. BBR prevented the occurrence and duration of isolated premature beats, ventricular fibrillation and ventricular tachycardia [172]. In another model of acute myocardial infarction (induction by isoproterenol injection), BBR (30, 60 mg/kg/d, p.o., 14d) decreased the ST elevation stimulated by acute myocardial ischemia, and also down-regulated serum levels of ischemic biomarkers muscle/brain (MB) isoenzyme of creatine kinase (CK-MB), IL-6, TNFα and LDH [183]. Moreover, ex vivo experiments evaluating I/R on isolated hearts showed that hearts pre-treated with BBR before ischemia had improved preservation of left ventricular developed pressure and LVEDP when compared to the non-treated group [172]. Importantly, BBR had no influence on these parameters per se (on hearts without I/R injury) [172]. In addition, a solid dispersion of BBR and sodium citrate (HGSD) improved BBR bioavailability and membrane permeability, and HGSD treatment (12.5, 25, 50 mg/kg/d, p.o., 7d) protected the rat heart from I/R injury by attenuating NF-κB and JNK signaling pathways [184].
Stroke
Similar to myocardial infarction, stroke also results from vascular or microvascular diseases which cause an interruption of cerebral blood supply and consequently brain dysfunction [185]. To date, the reperfusion is the merely approved treatment for acute ischemic stroke [186]. Consistently, thrombolytic/anti-platelet agents and surgery are the only available options [187]. However, due to the fact that multiple mechanisms are involved in stroke progression, the therapeutic effect of current regimens is limited and agents that possess multiple pharmacologic actions have attracted much attention. Inflammation, apoptosis, and oxidation are three major mechanisms for the pathogenic proceeding of stroke [188].
In addition to the routine treatment, the administration of BBR 300 mg (t.i.d., p.o.) significantly reduced serum levels of macrophage migration inhibitory factor (MIF) and IL-6 in patients with acute cerebral ischemic stroke and to a certain extent decreased the carotid atherosclerosis and neurological deficit [189].
The right carotid artery of rats was ligated (ischemic injury) and BBR solution (0.2‒2 mg/kg) was intraperitoneally injected. After 30 min, the rats were subjected to hypoxic conditions by breathing in air with 10% oxygen and 90% nitrogen (a model of hypoxic damage). BBR dose-dependently reduced cerebral ischemia-hypoxic injury [190]. BBR (0.2, 0.5, 1 or 2 mg/kg, i.p. or 0.2, 0.02, 0.002 mg/kg, i.v.) inhibited ischemia-induced apoptosis via enhancing the PI3K/Akt signaling pathway and exerted its neuroprotective effects in vivo and in vitro [179, 191]. Additional mechanisms of BBR in preventing ischemic brain damage include reducing intracellular ROS levels and subsequently inhibiting of the mitochondrial apoptotic pathway [192]. Moreover, BBR (10, 40 mg/kg) can reduce the ischemic brain injury in rats by promoting the activation of Akt/GSK3β (glycogen synthase kinase 3β) signaling pathway and claudin-5, while down-regulating the NF-κB expression [32]. A similar study showed that BBR (25, 50 mg/kg/d, p.o., 14d) pretreatment dose-dependently reduced infarct size, neurological deficits, and cerebral edema in model of cortical tissue ischemia performed by transient middle cerebral artery occlusion [193]. Its mechanism may depend on its anti-inflammatory effects, including an inhibition of nuclear translocation of high-mobility group box1 (HMGB1) and NF-κB, as well as TLR4 expression [193]. In addition, BBR can promote angiogenesis in rats suffering from cerebral ischemia-reperfusion injury, which may be related to increased expression of hypoxia-inducible factor 1α (HIF-1α) and its downstream gene-vascular endothelial growth factor (VEGF) [194].
BBR can protect neurons during focal cerebral ischemia by up-regulating anti-inflammatory cytokines and down-regulating pro-inflammatory cytokines [195]. The combination of BBR and other drugs has been tested to improve cerebral ischemia. For example, rats were reperfused after bilateral common carotid occlusion to induce a transient global cerebral ischemia model. Co-administration of BBR (5, 10, 20 mg/kg/d, 19d) and verapamil (2mg/kg/d, 19d) enhanced brain uptake of BBR and improved brain functions in rats [196]. Berberis koreana extract (containing 7.39±0.78 mg/g of BBR) significantly ameliorated nerve injury induced by ischemic stroke in gerbils, by inhibiting COX-2 expression and PGE2 production [197]. The main components of Huang-Lian-Jie-Du-Decoction (HLJDD) are BBR, baicalin and jasminoidin, which are commonly used in traditional Chinese medicine to treat ischemic stroke [198]. HLJDD administration ameliorated ischemic stroke in rats by improving abnormal metabolism and regulating oxidative stress, neuronal autophagy, and inflammatory responses [198]. A study evaluating the Yi-qi-jie-du formula (YJ) with the main constituents ginsenosides (G), BBR and jasminoidin (J) (in a ratio 3 (G): 2 (BBR): 0.5 (J)) showed the improvement of state in the focal cerebral ischemia in rats [199].
Arrhythmia
The anti-arrhythmic effect of BBR was firstly reported by Huang et al. in 1989 [200]. The authors induced ischemic ventricular arrhythmias in canines through occluding the LAD coronary artery and showed that BBR can prevent total ventricular premature beats and ventricular tachycardia [200]. Recently, BBR (2 mg/kg, i.v.) hindered ACh-induced atrial fibrillation in rabbits via extension of action potential (AP) and effective refractory period in atrial myocytes [201]. In addition, BBR (at a concentration of 300 mmol/L) prevented stretch-induced arrhythmia in isolated myocardial infracted hearts of Wistar rats [202].
Early studies proposed that BBR has class III anti-arrhythmic effects (by blocking K+ channel) [203]. However, further studies revealed that BBR targets several types of channels such as the cardiac slow (IKs) and rapid (IKr) delayed rectifier K+ channels, ATP-sensitive K+ channel (KATP), inwardly-rectifying K+ channel (IKl), L-type Ca2+ (ICa) [166] and human hyperpolarization-activated cyclic nucleotide-gated 4 (hHCN4) channels [204]. BBR (10, 20 mg/kg, p.o.) suppressed expression of IKr channel in rat ventricular tissues [205]. Moreover, BBR inhibited the expression of hERG channel in hERG-transfected HEK293 cells [205]. HERG channel encoded by hERG is an important subunit of Ikr channel [206]. It crucially contributes to cardiac repolarization and its defect leads to long QT syndrome with manifestations such as delayed repolarization and prolonged QT interval [206]. BBR (27.1 mg/kg, p.o..) induced extension of the AP duration and the QT interval in guinea pigs [207]. BBR caused mature hERG reduction through inducing defect in channel trafficking, which led to an immature hERG response (unfolded protein response), and defective hERG underwent degradation in lysosomes and proteasomes [207]. Fexofenadine and resveratrol, as two drugs preventing hERG trafficking defect, can reverse BBR-induced prolongation of AP duration [208]. BBR can also block hERG channel directly via interacting with residues V625, Y652, and F656, respectively, in the central cavity of the channel [209]. The effect of BBR on arrhythmia was summarized in Figure 4.
BBR in treating metabolic diseases
BBR in diabetes mellitus and its cardiovascular complications
Diabetes mellitus (DM) is linked to chronic hyperglycemia and abnormal metabolism of carbohydrates, lipids, and lipoproteins, which are caused by an inadequate production of insulin and/or insulin action [210, 211]. DM is increasingly being recognized as a significant cause of mortality and morbidity, and profoundly contributes to the global health burden and death toll [210, 211]. Based on the latest disease statistical report (The International Diabetes Federation, https://www.idf.org/): approximately 425 million people were diabetics in 2017 and this number is anticipated to increase to 629 million by 2045. There are two categories of DM: Type 1 diabetes (T1DM) and Type 2 diabetes (T2DM) [212]. T1DM is characterized by the absolute deficiency of insulin secretion, while T2DM, the most common one, has features of insulin resistance (IR) and an insufficient compensatory insulin secretory response [212]. Therapeutic effects of BBR on diabetes mellitus and its cardiovascular complications were summarized in Figure 5.
Effects of BBR on T1DM and T2DM
Physiologically, blood glucose level is regulated by the liver (gluconeogenesis and glycogenolysis) and glucose utilization by the peripheral tissues. An increase in the hepatic glucose production due to an inadequate insulin secretion/action is the major cause of hyperglycemia in DM patients [210, 211]. DM can lead to many complications such as hyperlipidemia, hypertension, atherosclerosis, hyperinsulinemia, retinopathy, nephropathy and peripheral neuropathy [213, 214]. Approved oral hyperglycemic drugs such as derivatives of sulfonylurea, metformin, and thiazolidinediones are reported to induce some side effects [9-11]. In addition to the adverse effects, the conventional medicines are expensive and not always satisfactory in maintaining normal level of blood glucose [215]. Many herbal medicines with potential of anti-diabetic effect have been widely used for treating DM in various traditional systems of medicine worldwide since the immemorial time [216]. Nowadays, the market for natural anti-diabetic drugs is booming as they are preferred for their effectiveness, lower occurrence of side effects and relative low costs [216].
Recently, a plethora of investigations has shown that BBR is a promising hypoglycemic, and anti-hyperlipidemic compound by modulating various signaling pathways [217-221]. It diminished body mass in DM patients [217-219]. The exact mechanism of action underlying the hypoglycemic effect of BBR is not fully elucidated. In vitro and in vivo studies have highlighted that BBR contributed to its therapeutic activities via variety of mechanisms and signaling pathways, including improving IR, activating AMPK, modulating gut microbiota, reducing gluconeogenesis in liver and enhancing glycolysis in peripheral tissues [217, 222-224]. Although many studies have shown that BBR is an AMPK activator [30, 225, 226], in HepG2 and C2C12 cells BBR promoted glucose metabolism by stimulating glycolysis, and this effect may be independent of AMPK activity [227]. A recent study has shown that BBR (150 mg/kg/d, p.o., 7d) promoted glucose uptake and restrict gluconeogenesis in rats by inhibiting SIRT3 and promoting ubiquitination of phosphoenol pyruvate carboxykinase 1 (PEPCK1) [228]. The entry of pyruvate into the mitochondria via the mitochondrial pyruvate carrier (MPC) is a central step in hepatic gluconeogenesis [229]. BBR limited the gluconeogenesis of mitochondrial pyruvate by inhibiting the deacetylation of MPC1 by SIRT3 [229]. This can be a therapeutic strategy to prevent excessive hepatic glucose production [229]. The hypoglycemic effect of BBR was also partially mediated by an anti-inflammatory and antioxidative mechanism [157, 230, 231]. It is challenging to justify the clinical efficacy of BBR due to its low oral bioavailability, although it is clinically active, and it exerted therapeutic effects through different mechanisms [232, 233].
Effects of BBR on insulin resistance (IR)
BBR has been shown to ameliorate IR, which is the main metabolic abnormality culminating not only to T2DM, but also to metabolic syndrome [234]. It can be defined as a state in which normal or increased insulin level produces an attenuated biological response [235]. Several researchers have reported that BBR is effective in mitigating the IR through different pathways. Kong et al. [236] have investigated the molecular mechanism of BBR against IR and BBR was found to reduce the fasting blood glucose (FBG) and fasting serum insulin (FSI), increase insulin receptor (InsR) mRNA and insulin sensitivity as well as protein kinase C (PKC) activity both in vitro and in T2DM rats. Mahmoud et al. [237] have demonstrated that treating rats, with IR syndrome, with 50 mg/kg/day of BBR for 2 weeks was effective against IR syndrome by improving IR, lipid profile, antioxidant enzymes, pro-inflammatory cytokines, and IFN-γ. Liu et al. [238, 239] have also revealed the effectiveness of BBR against IR. Increased branched-chain amino acids (BCAAs) are involved in obesity and IR as Yue et al. [240] have reported that BBR (200 mg/kg/d, p.o., 10wk) can improve IR in HFD-fed mice and diabetic patients by altering the intestinal microbiota of BCAAs biosynthesis and BCAAs catabolism in liver and adipose tissue.
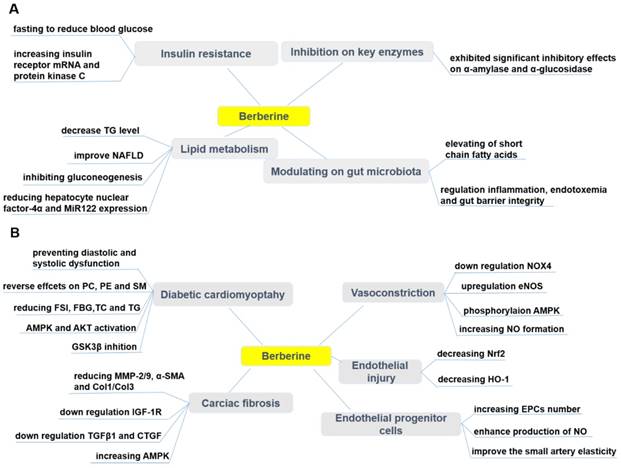
Pharmacological effects of berberine in treating diabetes (A) and its cardiovascular complications (B). BBR exerts protective effects in diabetes by ameliorating insulin resistance, modulating lipid metabolism and gut microbiota, inhibiting α-amylase and α-glucosidase activity. BBR also prevents cardiovascular complications associated with diabetes, such as diabetic cardiomyopathy, cardiac fibrosis, endothelial injury, endothelial progenitor cell dysfunction, and vasoconstriction. ↑indicates increase or activation, and ↓indicates decrease or suppression. Abbreviations: AMP protein kinase (AMPK), α-smooth muscle actin (α-SMA), cholesterol (TC), collagen (Col), connective tissue growth factor (CTGF), dinucleotide phosphate-oxidase (NOX), endothelial nitric oxide synthase (eNOS), endothelial progenitor cells (EPCs), fasting blood glucose (FBG), fasting serum insulin (FSI), glycogen synthase kinase 3β (GSK3β), heme oxygenase-1 (HO-1), insulin-like growth factor-1 receptor (IGF-1R), matrix metalloproteinase (MMP), non-alcoholic fatty liver disease (NAFLD), nitric oxide (NO), nuclear factor erythroid 2-related factor 2 (Nrf2), phosphatidylcholine (PC), phosphatidylethanolamine (PE), protein kinase B (Akt), sphingolipid (SM), triglyceride (TG), transforming growth factor β1 (TGFβ1).
Effects of BBR on lipid metabolism
BBR is well-known to improve glucose and lipid metabolic disorders. BBR was an approved component of nutraceuticals for treatment of hyperlipidemia in many countries [241]. Kong et al. [23] have described BBR as “a new lipid-lowering drug” after they observed the efficacy of BBR in lowering lipid level in vitro and in vivo, which was comparable to that of statins. Zhao et al. [242] have reported that BBR (150 mg/kg/d, p.o., 16wk) improved NAFLD, a critical hepatic manifestation of metabolic syndrome, by inhibiting gluconeogenesis and regulating lipid metabolism. BBR (40, 60 mg/kg/d, p.o., 4wk) regulated hepatic gluconeogenesis and lipid metabolism in T2DM mice via reducing the expression of hepatocyte nuclear factor-4α and miR122 [243]. The main metabolite of BBR in vivo, berberrubine (M3), showed the most potential hypolipidemic effect by up-regulating LDLR expression in HepG2 cells [244]. Nine berberrubine analogs modified at the C9 position were tested for their lipid-lowering activity. The results showed that berberrubine and hydroxypropyl-berberine can regulate the expression of liver LDLR and PCSK9 through ERK signaling pathway. Hydroxypropyl-berberine showing a greater effect, which may indicate it as a candidate drug for anti-hyperlipidemia [244].
Effect of BBR on modulating gut microbiota
Human gut microbiota plays a vital role in mediating obesity-related metabolic dysfunction, including T2DM [245]. For example, Fei et al. [246] have revealed that an endotoxin-producing Enterobacter cloacae B29, obtained from the gut of obese subjects, induced IR and obesity in germ-free mice. Another study has shown that endotoxin produced by the pathogen in the gut, such as Escherichia coli, caused obesity and IR when it was subcutaneously administered into mice [247]. As BBR has been known for treating intestinal infection-related diarrhea, Han et al. [248] hypothesized that gut microbiota regulation can be one of the anti-diabetic mechanisms of BBR. Recently, Zhu et al. [109] have reported that BBR (in drinking water (0.5 g/L), 14wk) treatment significantly reduced atherosclerosis in HFD treated mice by up-regulating the population of Akkermansia spp. which was confirmed to regulate inflammation, endotoxemia and gut barrier integrity. The study carried by Zhang et al. [233] showed that BBR (100 mg/kg/d, p.o., 18wk) modulated gut microbiota, and it is particularly important that the observed elevating of short chain fatty acids (SCFAs) levels in the intestine, may contribute to its therapeutic effect against DM, obesity and other metabolic diseases.
BBR as inhibitors of α-amylase and α-glucosidase
Inhibition of key carbohydrate hydrolyzing enzymes, in clinic relevant α-amylase and α-glucosidase, has been acknowledged as one of the most effective approaches for managing of DM and delaying postprandial hyperglycemia [249, 250]. Enzymes α-amylase and α-glucosidase are present in the small intestine and its brush border and are responsible for hydrolytic breakdown of complex oligosaccharides and disaccharides into glucose and further monosaccharides suitable for absorption [249, 250]. Inhibition of these enzymes delayed carbohydrate digestion, resulting in a down-regulation in the rate of intestinal glucose absorption and subsequent reduction of the post-prandial glucose levels [249, 250]. Interestingly, BBR has been found to exhibit significant inhibitory potency against both α-amylase and α-glucosidase [223, 249, 251, 252].
Effect of BBR on DM-induced cardiovascular disease and complications
Chronic exposure to hyperglycemic conditions (accompanying DM), can cause damages to various tissues, as well as induces vascular injury and cardiomyopathy [253, 254]. High glucose level plays an important role for endothelial injury. BBR (100 mg/kg/d, p.o., 8wk) ameliorated impaired endothelium-dependent vasorelaxation of aorta in T2DM rats by down-regulating NOX4 expression and up-regulating eNOS expression [255]. Normal vascular tone is sustained by various dilatory and constrictory agents where NO is the major vasodilator. A main feature of endothelial dysfunction is impaired endothelium-dependent relaxation [256]. An effect of BBR on vascular tone has been reported in different studies [168, 169, 257]. BBR caused vasorelaxation in isolated rat aorta cells via phosphorylation of AMPK and eNOS [257]. BBR has been evidenced to exert its protective effects on vascular endothelium via increasing NO formation [168, 169]. In addition, it has been shown that BBR (50, 100, 200 mg/kg/d, p.o., 4wk) might contribute to the protection against endothelial injuries in retinal tissue of DM rats via decreasing the expressions of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) [258]. Both the endothelium and the underlying VSMCs could be affected by BBR to induce relaxation [168]. BBR, as a sensitizer of the insulin signal in HUVECs, also improved insulin-mediated vasodilatation in diabetic rats involving PI3K/Akt and AMPK activation via the up-regulation of the InsR [259]. The vasodilator effect of BBR (200 mg/kg/d, p.o., 4wk) also improved ACh-induced mesenteric artery vasodilation in diabetic rats [259]. There are findings indicating that the decrease in UCP2 expression and mitochondrial ROS accumulation are related to vascular damage [260]. UCP2 has been found to reduce high glucose-induced apoptosis in HUVECs by increasing Bcl-2 while decreasing caspase-3 and cytochrome c [261]. Concurrently, BBR promoted the mitochondrial biogenesis and enhance UCP2 mRNA and protein expression in cultured HUVECs [262]. BBR exerted this pharmacological effect through AMPK-dependent manner, which led to reduction in oxidative stress and vascular inflammation [262]. Increased atherothrombotic events due to platelet activation and apoptosis are the leading cause of high mortality and morbidity during DM [263]. Platelet hyperresponsiveness and apoptosis during DM are ROS accumulation caused by activation of aldose reductase (AR) and NOX. BBR protects platelets by suppressing AR and NOX activity in high glucose treated platelets [263].
Endothelial progenitor cells (EPCs) play a significant role in endothelial function [264]. The reduced number and impaired function of circulating EPCs are markers of vascular diseases, and contribute to impaired arterial elasticity and endothelial dysfunction, particularly in cardiovascular diseases [264]. BBR (1.2 g/d, p.o., 30d) has been confirmed to increase the number of circulating EPCs [265, 266], enhanced the production of NO[265] and improved the small artery elasticity [266] in healthy volunteers. However, in one randomized trial there were no changes in circulating EPCs in individuals with dyslipidemia after treatment with a nutraceutical formulation containing BBR [267]. Oppositely, another trial concluded the improvement of endothelial function after treatment by combination of BBR, RYR and policosanols, through increasing the endothelial-dependent flow-mediated dilation (FMD) in patients with hypercholesterolemia [268].
Diabetic cardiomyopathy (DCM) is considered to be a clinical pathology independent of concomitant vascular diseases and can be primarily caused by disturbances in the energy substrate [269]. In the high-sucrose and HFD/streptozotocin (STZ)-induced rat DCM model, BBR (10, 30 mg/kg/d, p.o., 16wk) significantly prevented diastolic and systolic dysfunction, and cardiac hypertrophy [269]. Phosphatidylcholine (PC), phosphatidylethanolamine (PE) and sphingolipid (SM) are potential biomarkers of DCM, and the protective effect of BBR on DCM can be through reversal of PC, PE and SM metabolic disturbances [269]. BBR treatment (100 mg/kg/d, p.o., 16wk) of diabetic rats can partially improve cardiac function and significantly reduce FSI, FBG, total TC and TG levels. The underlying mechanism can be that BBR activated cardiac AMPK and Akt in diabetic rats and inhibited GSK3β activity [270]. Similarly, in the palmitate-induced H9C2 cell hypertrophy model, BBR up-regulated alpha-myosin heavy chain (α-MHC) expression, down-regulated beta-myosin heavy chain (β-MHC) expression and thus inhibited H9C2 cell hypertrophy [270]. Moreover, BBR also enhanced AMPK and Akt activation in H9C2 cells and inhibited GSK3β activity [270, 271]. Diabetic hearts are more sensitive to I/R injury, and BBR treatment protected the heart of diabetic rats from I/R damage [272]. This protective effect of BBR was achieved by activating AMPK and Akt activity, while inhibiting GSK3β activity in non-ischemic regions of the diabetic rat hearts [272].
Diabetic cardiac fibrosis causes ventricular stiffness and leads to diastolic dysfunction [273]. In a diabetic rat model, BBR treatment (100 mg/kg/d, p.o., 4wk) improved cardiac fibrosis and dysfunction by down-regulating insulin-like growth factor-1 receptor (IGF-1R) expression in cardiac fibroblasts, specifically by reducing expression of MMP-2/9, α-smooth muscle actin (α-SMA) and type 1 collagen (Col1) in diabetic hearts. BBR also exerted a similar effect in cardiac fibroblasts exposed to high levels of glucose [273]. Furthermore, BBR (50, 100, 150 mg/kg/d, p.o., 12wk) reduced cardiac fibrosis in DM rats by down-regulating the expression of transforming growth factor β1 (TGFβ1) and connective tissue growth factor (CTGF), reducing the expression of Col1 and Col3 [274]. In addition, BBR treatment promoted glucose depletion and glucose uptake as well as improved IR in H9C2 cells, at least partially by increasing AMPK activity [222]. Further, BBR (100 mg/kg/day, p.o., 7d) ameliorated arrhythmias induced by ischemia (LAD ligature) in diabetic rats [275]. Researchers have shown that BBR reversed the down-regulation of the transient outward K+ current (Ito) and ICa [275]. In a similar study, BBR (60 mg/kg/day, p.o., 14d) led to the restoration of diminished inwardly rectifying potassium channel Kir2.1 to nearly normal levels [276]. The effect of BBR on Kir2.1, the main subunit of IK1, was associated with recovering of resting membrane potential and alleviating of ischemia-induced arrhythmias in diabetic rats [276].
In conclusion, BBR is a promising candidate for therapy of T2DM and metabolic syndrome. However, further investigations of the mechanisms of BBR are required for its appropriate application in clinical settings.
Obesity
Obesity is a complex metabolic disease occurring in both developed and developing countries [277]. Obesity contributes to the development of several other health problems, such as cardiovascular diseases, DM, cancer, chronic obstructive pulmonary disease, and depression [277]. Etiology of obesity is multi-factorial and is associated with the energy input exceeding energy output [278]. Most commonly, it is induced by excessive food intake, imbalanced nutrition, physical inactivity, and genetic predisposeitions. Other causes of obesity include endocrine dysfunction, mental illnesses, or iatrogenic etiologies [278].
BBR reduced food intake in the mice fed with HFD, and decreased their weight gain [279]. Such an effect led to the question as to how BBR affects the appetite. BBR can improve lipid dysregulation by controlling both peripheral and central AMPK activity [280]. Although BBR cannot cross the cerebrovascular barrier, studies have shown that an application of BBR intraperitoneally caused a 47% up-regulation in serotonin levels in the brain [281]. The expression of glucagon-like peptide 1 receptor (GLP-1R), orexin A and neuropeptide Y in brain of tested animals were found to be up-regulated [282]. Together with changes observed in hypothalamus, this can be a factor causing the decrease of body weight, enhanced lipolysis, and decrease of IR in obese experimental animals and even humans.
It is clear, that anti-obesity effects of BBR are associated with its anti-diabetic activity [283]. Many studies identified BBR as an activator of AMPK, which is, besides other effects, responsible for triggering of the glucose uptake and fatty acid oxidation in skeletal muscles, and regulation of insulin secretion by pancreatic β-secretory cells [30, 225, 226]. Effects of BBR on an increase of glucagon-like peptide-1 (GLP-1) via enhancing GLP-1 secretion and GLP-1 biosynthesis in enteroendocrine L-cells and some neurons were also observed, and BBR stimulated GLP-1 secretion can trigger the feeling of satiety and decrease the food intake [284]. Mitochondrial stress responses (e.g., mitochondrial swelling and membrane rupture) and GLP-1 expression were decreased in the colon of diet-induced obese mice [285]. Administration of BBR (100 mg/kg/d, p.o., 8wk) up-regulated GLP-1 expression in mice and prevented mitochondrial stress response [285]. All these pharmacological effects of BBR can translate to improved outcomes in DM and obesity.
Several studies have shown altered intestinal architecture as a result of a shift in gut microbiota composition to the phyla Bacteroidetes and Firmicutes [286]. The pivotal barrier-protecting function conferred by BBR was mediated by elevated levels of SCFAs [233]. Gene profiling analysis showed that BBR can regulate gut microbiota diversity. In the group of BBR-treated rats (100 mg/kg/d, p.o., 18wk), the shift to bacteria producing SCFAs was shown to be significant [233]. On the other hand, fasting-induced adipose factor (FIAF), which inhibits circulating lipoprotein lipase (LPL), is a regulator as a putative mediator of microbial modulation of energy storage, and fat mobilization [287]. BBR (200 mg/kg/d, p.o., 6wk) up-regulated FIAF expression in adipose tissues and intestinal and also changed the abundance of fecal Bacteroidetes and Firmicutes in total bacteria in the gut of mice fed with HFD [288]. Additionally, Chae et al.[289] showed modulation of growth of gut microbiota, especially Lactobacillus and Bifidobacterium by BBR.
Non-alcoholic fatty liver disease (NAFLD)
NAFLD is manifested as an accumulation of TG in liver [290]. NAFLD is closely related to the obesity and related metabolic risk factors (T2DM and dyslipidemia). Untreated NAFLD can progress to non-alcoholic steatohepatitis (NASH), characterized by persistent hepatocyte inflammation and injury with or without fibrosis. NASH can progress to cirrhosis and liver cancer [291, 292].
According to the main characteristics of NAFLD, the first therapeutic option is the restoration of dysregulated lipid metabolism, which leads to the fat reduction and weight loss [290]. BBR (5 mg/kg/d, p.o., 3wk) improved fatty liver in obese mice by regulating neural signaling from the central nervous system and peripheral AMPK signaling [293]. Sterol regulatory element binding protein 1c (SREBP1c) can be regulated by AMPK via direct phosphorylation [294]. AMPK activation suppresses SREBP-1c activity and lowers hepatic and plasma levels of TG and TC [294]. BBR acted as a potent inhibitor of SREBP-1c, possibly through AMPK pathway [295, 296]. BBR (100 mg/kg) can down-regulate SREBP-1c and ameliorate lipid profile in rats [297]. The combination of BBR (50 mg/kg/d, p.o., 8wk) and curcumin (50 mg/kg/d, p.o., 8wk) is more effective than lovastatin (100 mg/kg/d, p.o., 8wk) [298]. BBR can reverse the disorder of lipid metabolism in 3T3-L1 cells induced by olanzapine, a second-generation antipsychotic drug, by inhibiting SREBP and activating AMPKα [299]. Overexpression of SREBP-1c leads to the down-regulation of insulin receptor substrate 2 (IRS-2) mRNA levels which is closely linked to the hepatic IR [300]. BBR reduced liver TC levels by down-regulation of sterol carrier protein 2 expression and inhibiting COX-2 induced prostaglandin production[301]. The improvement of IR by increasing IRS-2 mRNA expression is one of the possible mechanisms in the treatment of NAFLD with BBR [302].
Another transcription factor carbohydrate-responsive element binding protein (ChREBP) also participated in the modulation of lipogenic genes (e.g. L-PK) as it has been demonstrated in the liver of carbohydrate-fed rats [303]. BBR (380 mg/kg/d, p.o., 5wk) was able to suppress the expression of ChREBP and ameliorated fatty acid synthesis in the liver [304]. BBR has been reported as a potential PPARα activator in diabetic hamsters [305], although this finding is inconsistent with the results of the study conducted previously [306], where BBR did not influence the expression of PPARα, but directly up-regulated the expression of microsomal triglyceride transfer protein (MTTP) through reduction of methylation of MTTP promoter. MTTP regulated the assembly and secretion of ApoB-containing lipoproteins (including LDL and VLDL) [306]. Similarly, BBR (200 mg/kg/d, p.o., 16wk) can ameliorate the symptoms of NAFLD in rats by increasing the expression of LXRα/FAS [307], UCP2 [308] and MTTP [306] in hepatocytes. In addition, BBR (50mg/kg/d, p.o., 8wk) inhibited HFD-induced down-regulation of carnitine-palmitoyltransferase 1A (CPT 1A) and PPARα expression in fish [309]. BBR obviously reduced TC and LDL-C in hyperlipidemic rats while up-regulated high-density lipoprotein-cholesterol (HDL-C) and CPT 1A expression [310]. BBR (5 mg/kg/d, p.o., 4wk) maintained the energy balance and reduced hepatic steatosis in mice by enhancing autophagy and activating fibroblast growth factor 21 (FGF21), and this effect was dependent on SIRT1 [311], which is the inductor of the expression of PPARα. The depletion of SIRT1 attenuated PPARα signaling and fatty acid β-oxidation, and it can lead to the development of ER stress, hepatic inflammation, and hepatic steatosis [312]. Thus, BBR may be effective in reversing steatohepatitis and fibrosis similarly as fibrates and other PPARα agonists in mice, although there was no significant improvement in histological findings in human subjects [313]. C‑C motif ligand 19 (CCL19) was highly expressed in patients with NAFLD [314]. In a model of HFD-induced rat NAFLD, metformin and BBR improved NAFLD by activating AMPK signaling, and metformin and BBR significantly reduced high expression of CCL19 in NAFLD rats. It is postulated that inhibition of CCL19 may be an effective treatment for NAFLD [314].
Oxidative stress, lipid peroxidation, and inflammatory cytokines lead to the development of hepatic steatosis that can progress to devastating NASH, potentially leading to fibrosis and cirrhosis [315]. An increased inflammatory response (e.g. TNFα, IL-6 up-regulation) has been observed in patients with NAFLD [316]. TNFα is recognized as a critical factor in NAFLD and related liver diseases and an inhibition of pro-inflammatory cytokines production could be an important strategy to treat NAFLD [316]. BBR obviously reduced the p-JNK1 in mouse primary hepatocytes, whereas the level of p-AMPK was not significantly changed. These results showed, that the inhibition of inflammation by BBR could have AMPK-independent mechanisms [317], although Jeong et al. [86] have reported that BBR (5 mg/kg/d, i.p., 3wk) down-regulated the production of pro-inflammatory cytokines (IL-6, IL-1β and TNFα) in adipose tissue of obese db/db mice through AMPK activation. Additional mechanisms of attenuation of hepatic steatosis can be the modulation of gut microbiota [318]. HFD could cause the overgrowth of Gram negative bacteria in the intestine and subsequently the alternation of composition of intestinal microbiota, which leads to the increase of the intestinal permeability and inflammation [319]. BBR (150 mg/kg/d, p.o., 4 wk) could decrease the level of Faecalibacterium prausnitzii in rats [320] as well as the number of Firmicutes and Bacteroidetes in the feces of mice fed a HFD [288].
Taken together, BBR represents a very promising therapeutic drug in various metabolic diseases via its pleiotropic effects, including the regulation of lipid and glucose metabolism, oxidative stress, and inflammatory response. Since most studies are performed in pre-clinical animal models, more randomized large-scale clinical trials are needed to evaluate the metabolic effects of BBR in human patients.
Molecular targets of BBR
BBR modulates multiple cellular events involved in cardiometabolic diseases by modulating multiple disease-relevant targets (Figure 6). The major molecular targets involved in the cardioprotective and metabolic effects are AMPK, PPAR, LDL receptor (LDLR), hepatocyte nuclear factor 1α (HNF1α), IκB kinase β (IKKβ), silent information regulator (SIRT1), and gut microbiota. In this section, a detailed account on the molecular targets of BBR in various CVMD will be summarized.
Increased ratio of AMP/ATP promotes the activation of AMPK, which is a decisive regulator of energy metabolism and is responsible for controlling several features of cellular resistance [321, 322]. BBR was found to decrease ROS production by either up-regulation of SOD expression [323, 324] or negative regulation of NOXs, probably through AMPK activation [30, 225]. Pharmacological actions of BBR are similar to that of metformin, which acts through regulation of different effectors in lipid and energy metabolism, such as PKC, AMPK, and MAPK [236, 325]. The enhancement of adipose triglyceride lipase (ATGL) expression, which was mediated by AMPK, was mainly considered to be responsible for the long term body weight losing effect of BBR and this effect is also attributed to the enhancement of basal lipolysis of TG present in the adipocytes which is directly correlated with dyslipidemia and obesity [326]. However, the proliferation of preadipocytes was inhibited by BBR, via C/EBPα and PPARγ pathways [327, 328]. One of the risk factor of CVD, LDL-C, was also lowered by BBR and this effect was via increasing LDLR expression, which also upsurges the LDLR-mediated liver clearance and BBR-caused stabilization of LDLR mRNA was regulated through the ERK signaling pathway revealed the mechanism behind cholesterol lowering effect of BBR [23, 329]. The modulation of LDLR takes place at a post translational level where BBR degraded and ubiquitinized HNF1α [330-332]. Moreover, the up-regulation of LDLR expression is dependent on the ERK activation [23].
Molecular targets of BBR. BBR exerts its pharmacological effects by regulating the expression of genes/proteins responsible for transcription factors, enzymes, growth factors, cell survival/proliferative proteins, metastatic/invasion molecules, platelet activation, inflammatory cytokines, protein kinases, apoptotic proteins, receptors, and the others. By targeting these molecules, BBR prevents the development of multiple cardiovascular and metabolic diseases. ↑indicates increase or activation, and ↓indicates decrease or suppression. Abbreviations: arachidonic acid (AA), ATP-binding membrane cassette transport protein A1 (ABCA1), angiotensin-converting enzyme (ACE), adenosine diphosphate (ADP), adipocyte enhancer-binding protein 1 (AEBP1), apoptotic protease-activating factor 1 (Apaf-1), adipose triglyceride lipase (ATGL), aldose reductase (AR), calmodulin-dependent kinase II (CaMKK II), C‑C motif ligand 19 (CCL19), the cluster of differentiation 18 (CD18), the cluster of differentiation 36 (CD36), carbohydrate responsive element binding protein (ChREBP), MB isoenzyme of creatine kinase (CK-MB), type 1 collagen (Col1), C-reactive protein (CPR), connective tissue growth factor (CTGF), 1,2-diacyl-sn-glycerol (DAG), endoplasmic reticulum (ER), extracellular MMP inducer (EMMPRIN), endothelin 1 receptor(ET-1R), fatty acid synthase (FAS), fibroblast growth factor 21 (FGF21), fasting-induced adipose factor (FIAF), farnesoid X receptor (FXR), glucose-6-phosphatase (G6Pase), glucagon-like peptide 1 receptor (GLP-1R), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), hepatocyte nuclear factor-4α (HNF-4α), intercellular adhesion molecule-1 (ICAM-1), IκB kinase β (IKKβ), interleukin-1β (IL-1β), interleukin-6 (IL-6), insulin receptor (InsR), insulin receptor substrate 2 (IRS-2), N-terminal kinase (JNK), lactate dehydrogenase (LDH), LDL receptor (LDLR), lectin-like oxidized low density lipoprotein receptor-1 (LOX-1), monocyte chemotactic protein-1 (MCP-1), migration inhibitory factor (MIF), matrix metalloproteinases (MMPs), mitochondrial pyruvate carrier (MPC), microsomal triglyceride transfer protein (MTTP), nuclear factor-kappaB (NF-κB), Nod-like receptor family pyrin domain containing3 (NLRP3), nitric oxide (NO), nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX2), nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4), nuclear factor erythroid-2-related factor-2 (Nrf2), protein S6 kinase (p70S6K), proprotein convertase subtilisin/kexin type 9 (PCSK9), ribosomal proliferating cell nuclear antigen (PCNA), P-glycoprotein (P-gp), phosphoenol pyruvate carboxykinase (PEPCK), phosphatidylinositol 3 kinase (PI3K), peroxisome proliferator-activated receptor α (PPARα), peroxisome proliferator-activated receptor γ (PPARγ), protein tyrosine phosphatase1B (PTP1B), activate silent information regulator 1 (SIRT1), super oxide dismutase (SOD), scavenger receptor class B type I (SR-BI), sterol regulatory element binding protein (SREBP), 1,2,3-triacyl-sn-glycerol (TAG), tissue growth factor-β1(TGFβ1), Toll-like receptor 4 (TLR4), tumor necrosis factor α (TNFα), TNF receptor-associated factor 2 (TRAF2), transient receptor potential vanilloid 4 (TRPV4), uncoupling protein 2 (UCP2), urokinase-type plasminogen activator (u-PA), vascular cell adhesion molecule-1 (VCAM-1).
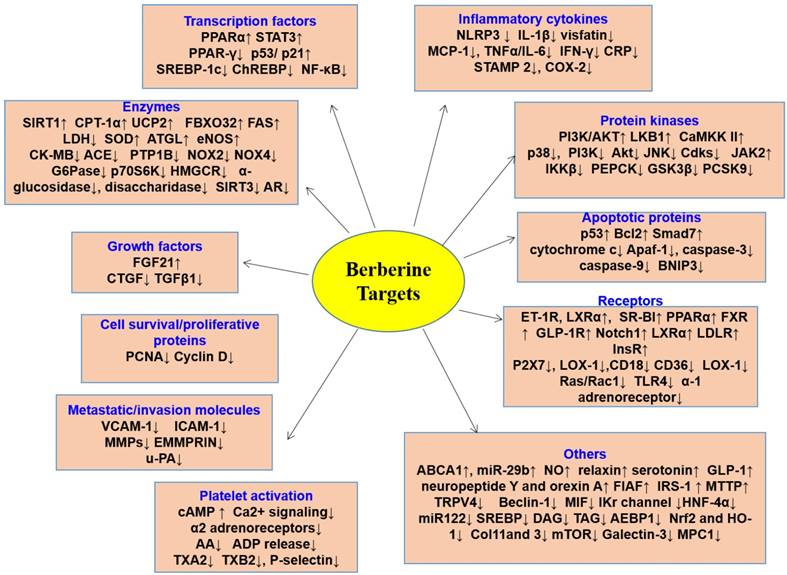
The up-regulation of AMP level and consequent activation of AMPK by BBR was also related to the inhibition of mitochondrial respiratory complex 1 which represents a prominent target for the compounds which improve entire body insulin sensitivity [333]. BBR blocked complex 1 and led to the enhancement of lactate release and glucose consumption. This process is independent of AMPK activation [331, 334]. Modulation of glycolipid metabolism by BBR occurred through enhancing PPARα/δ expression and down-regulated PPARγ expression in liver [335]. Lipid and glucose SOD homeostasis is also regulated by AMPK [336]. Zhao et al. [337] have demonstrated that in normal and Mac-CM-damaged adipocytes, nandinine, a BBR derivative, and BBR attenuated IR via the reduction of inflammation mediated by an effect on AMPK and also by targeting IKKβ. Apparent suppression of pro-inflammatory responses by AMPK activation in macrophages by BBR has also been reported [86, 337] Thus, the authors recommend the use of BBR and nandinine as dietary supplement in obesity.
The principal lipid-lowering mechanism of BBR was hepatic LDLR stabilization, which was mediated via intensification of the signal regulated kinase dependent pathway, along with the enhanced LDLR promoter transcriptional activity responsible for its cardioprotective effect against I/R injury [272]. A recent study has reported that BBR was beneficial for the maintenance of energy supply to diabetic hearts during I/R injury [272]. BBR inhibited Col1 and Col2 synthesis, promoted IL-10 secretion both in Ang II stimulated heart fibroblasts and suppressed TGFβ1 secretion [138]. The same study also revealed that AMPK phosphorylation was increased by BBR [138]. After Ang II stimulation in cardiac fibroblasts, protein S6 kinase (p70S6K) and mTOR phosphorylation was down-regulated by BBR. Moreover, myofibroblasts transformation could also be inhibited by BBR via decreasing the α-SMA expression [138]. BBR also exhibited inhibitory effects on protein tyrosine phosphatase 1B (PTP1B) which functions as a mediator of insulin signaling and catalyzes protein dephosphorylation by interacting InsR, and showed insulin mimicking effects in myocytes and adipocytes which suggested that BBR is a representative of a different class of anti-hyperglycemic agent [338].
Apart from the effect on AMPK, an effect of BBR on human health was plausible due to the stimulation of the SIRT1, which is also responsible for the potential effects against consequences of oxidative stress [339]. A recent study has shown that BBR (5 mg/kg/day, i.p., 3 wk) attenuated oxidative stress in diabetic mice via miR-106b/SIRT1 signaling pathway[340]. BBR also affected the islet function and thus was beneficial in plummeting cardiovascular risk factors in DM [340]. Another study has revealed that BBR has the potential not only to suppress formation of foam cells, but also the ability to adjust lipid accumulation [82]. This effect was mediated through activation of AMPK-SIRT1-PPARγ pathway [82]. Additionally, the same study has shown that the foam cell inhibitory effect was increased when both BBR and atorvastatin were used in combination rather than atorvastatin alone [82]. Another study performed in doxorubicin treated H9C2 cardiomyoblasts showed that pre-treatment with BBR up-regulated the protein levels of SIRT1 and SIRT3 in the presence of doxorubicin [341]. BBR also repressed the activation of caspase-3 and caspase-9 induced by doxorubicin. In doxorubicin- treated H9C2 cardiomyoblasts, BBR showed an autophagy modulating effect and increased mitochondrial biogenesis markers and hence BBR can be used as a modulator in decreasing doxorubicin induced cardiotoxicity [341]. The SIRT1/ER stress pathway represents a vital mechanism for the neuroprotective effects of BBR in diabetic encephalopathy [342]. BBR has been found to up-regulate SIRT1 protein expression and down-regulate the expression of proteins associated with ER stress, such as protein disulfide isomerase (PDI), inositol-requiring enzyme (IRE)1α, eukaryotic translation initiation factor 2 (eIF2), C/EBP homologous protein (CHOP) and PKR-like endoplasmic reticulum kinase (PERK) [342].
The regulation of gut microbiota is another important target of BBR in hyperglycemia, hyperlipidemia, IR, DM, and atherosclerosis [109, 233, 343-346]. Gut microbiota converted BBR to an absorbable form of dihydroberberine, which can be further oxidized to BBR after intestinal absorption [347]. The increase of gut Akkermansia may contribute to atheroprotective and metabolic protective effects of BBR [109]. BBR increases the amount of gut microbiota that produces SCFAs, contributing to the beneficial effects on the host [343]. Similar conclusions have appeared from several studies [233, 344-346]. Supplementation of BBR in broiler diet promoted intestinal colonization of healthy microbiota at high stocking densities [348]. By normalizing gut microbiota, BBR alleviated NASH and its predisposing factors in mice [349]. BBR also regulated energy metabolism as it could reduce lipids by regulating the turnover of bile acids, and subsequent ileal FXR signaling pathway [350]. Therefore, BBR acts through numerous signaling pathways which clearly suggests that it does not act via a ubiquitous mechanism and it acts through distinct mechanisms to combat cardiovascular and metabolic diseases.
Clinical trials of BBR in cardiometabolic diseases
Several clinical trials have been performed to study the effects of BBR in the therapy of cardiometabolic diseases and/or conditions related to them. These ongoing or completed clinical trials are summarized in Supplementary Table 1 (in supplemental material) [23, 112, 325, 351-364]. Trials testing the effects of BBR-based nutraceuticals combination were excluded, because the observed effects are not specifically attributable to BBR, but, probably, to the synergistic effects of all bioactive components. Generally, BBR is tested in dosage ranged from 0.5 to 2.0 g/d as well as treatment period ranged from 4 to 24 weeks. In these trials, BBR has been demonstrated to improve blood lipid profile [23, 112, 325, 351-360], glucose level [112, 325, 351, 357-361], blood pressure [112, 357, 360], IR [358, 360], endothelial function [112], body weight [357, 362], and systemic inflammation [356].
Selected clinical trials have been carried out on subjects with hypercholesterolemia [23, 112, 352, 353, 355], T2DM [325, 354, 360, 361, 363], obesity [362], congestive heart failure (CHF) [364], acute coronary syndrome (ACS) [356], metabolic syndrome [357], NAFLD [351, 359], and polycystic ovary syndrome (PCOS) [358]. Only one study was conducted on healthy subjects [112]. Particularly, 25 subjects were randomized into two groups: 1.2 g/d BBR treatment group and a control group without any treatment. After 4 weeks of treatment, TC, LDL-C, fasting blood glucose (FBG) and blood pressure were obviously decreased. In addition, improved endothelial function, and a reduction of levels of CD31+/CD42- microparticles were observed [112].
Clinical trial of metabolic diseases
The effect of BBR in patients with T2DM
In patients with T2DM, BBR ameliorated glucose metabolism and improved insulin sensitivity [361]. A pilot study was conducted in diabetic patients to investigate the efficacy of BBR. 36 diabetic patients were randomized into two groups: BBR or metformin (both 1.5 g/d, 12wk) [361]. After the treatment, in both groups, a statistically significant down-regulation in levels of glycosylated HbA1c (p<0.01), FPG and postprandial blood glucose (PBG) (p<0.01) were observed, suggesting that BBR exerted a hypoglycemic effect comparable to that of metformin. Further, BBR significantly reduced the levels of TG and TC [361]. Zhang et al. [325] have compared the effects of BBR (1.0 g/d) and metformin (1.5 g/d) or rosiglitazone (4.0 g/d) for 8 weeks in patients with T2DM. After the treatment period, BBR lowered the level of FPG and HbA1c. The effects of BBR were comparable to that of metformin and rosiglitazone. To evaluate the potential effects and mechanism of BBR in IR, the expression levels of InsR were assessed on peripheral blood lymphocytes (PBL) isolated from subjects before and after BBR treatment [325]. A significant up-regulation of InsR-expressing PBL was observed (p<0.01), suggesting that BBR can be effective in treating IR by up-regulation of InsR expression [325].
The effect of BBR in patients with T2DM and dyslipidemia
Zhang et al. [360] conducted a randomized, placebo-controlled, double-blinded study on 106 subjects with T2DM and dyslipidemia. After 12 weeks of treatment with BBR (1.0 g/d), a obvious improvement was observed in levels of FBG, post-load blood glucose (P-LBG), HbA1c, TG, TC, and LDL-C and insulin-sensitivity [360]. In addition, BBR also reduced systolic (SBP, p = 0.001) and DBP [360]. Similarly, Gu et al. [363] have shown that BBR (1.0 g/d, 12wk) improved lipid profile and insulin sensitivity. In addition, BBR treatment reduced serum concentration of 13 free fatty acids (FFAs), suggesting that BBR was effective in the T2DM-related pathological conditions reducing the level of FFAs [363].
The effect of BBR in patients with dyslipidemia
Kong et al. [23] have reported that BBR (1.0 g/d, 12wk) in a clinical study reduced the lipids (TC, TG and LDL-C) and increased LDLR expression. The proprotein convertase subtilisin/kexin type 9 (PCSK9) negatively regulates LDLR and has become a potential target for hypercholesterolemia [330, 365], and it was shown, that BBR can significantly improve dyslipidemia and LPS-induced inflammatory response in mice by down-regulation of the expression of PCSK9 in the liver and then up-regulating LDLR expression [365]. The specific mechanism by which BBR inhibited PCSK9 expression may be through a decrease in HNF1α expression [366] and attenuation of HNF1α and SREBP2 transcription of PCSK9 [332]. This increased LDLR mRNA is independent on HMGCR activity, suggesting that BBR is able to lower lipid level by a mechanism different from statins [23]. A follow-up study evaluated the hypolipidemic effects of BBR (1.0 g/d) and simvastatin (0.2 g/d) alone or in combination for 8 weeks, in subjects with hypercholesterolemia [355]. In the combination therapy, the serum levels of LDL-C, TC and TG were obviously reduced after the treatment. Interestingly, BBR monotherapy was more effective that simvastatin in reducing LDL-C, TC and TG [355].
Clinical trial of cardiovascular diseases
BBR also exerted beneficial effects in patients with CVD [356, 364]. In 12 patients with refractory CHF, an infusion (0.02 or 0.2 mg/kg/min, 30 min) of high doses of BBR could reduce systemic vascular resistance and enhanced cardiac index, while in the lower dosed group only reduced the patients' heart rate [367]. Reaching the plasma concentration of BBR in patients with CHF greater than 0.11 mg/L, significantly reduced the frequency and complexity of ventricular premature beats (VPBs) while left ventricular ejection fraction (LVEF) was increased [368]. 79 patients with CHF received BBR 1.2 to 2.0 g/day and routine anti-HF treatment for 8 consecutive weeks. BBR significantly increased exercise capacity and the dyspnea-fatigue index, while decreased frequency and complexity of VPBs [364]. Meng et al. [356] have evaluated the effects of BBR in 130 patients with ACS. Subjects were randomized into a treatment group (BBR 0.9 g/d as add-on therapy for 4 weeks) and a control group (only standard therapy). After treatment period, BBR reduced TC and LDL-C levels as well as markers of inflammation (including hypersensitive CRP, IL-6, MMP-9, VCAM-1, ICAM-1and MCP-1), suggesting that BBR is effective in the global management of CVD [356]. A recent clinical study evaluated the effect of an oral nutraceutical combination comprised of RYR and BBR on subclinical inflammation, lipid profile, PCSK9, and arterial stiffness in antiretroviral therapy treated HIV-infected patients [369]. The results of this study showed positive effect and reported that the nutraceutical combination substantially reduced PCSK9 and plasma cholesterol levels and improved arterial stiffness [369].
New development of BBR derivatives in treating cardiometabolic diseases
Many derivatives of BBR were synthesized with an aim to improve its relatively low bioavailability and to decrease the dosage necessary to trigger the desired therapeutic effects. The main positions of the BBR skeleton are depicted in Figure 7. Especially positions 7, 8, 9, 12 and 13 were targets of BBR modifications [370]. It has been observed that alkylation and acylation of BBR on the ring D can change bioavailability [370]. As example, pseudoberberine (10,11-dimethoxyderivative) IMB-Y53 in vivo increased the glucose lowering properties, probably due to diminished affinity to P-gp [371]. BBR has relatively low oral bioavailability due to extensive P-gp mediated drug efflux in gastrointestinal tract [372]. To address this issue, IMB‑Y53 was prepared with the increased gastrointestinal uptake and thus further anti-diabetic effects [371]. The potential of IMB-Y53 in CVMD remains to be investigated in future.
Similarly, dihydroberberine and its analog 8,8-dimethyldihydroberberine were synthesized and tested. Dihydroberberine and 8,8-dimethyldihydroberberine were more potent than BBR and appeared to have similar pharmacodynamic effects in treatment of type 2 diabetes at lower doses [373] . A study by Turner et al. [333] indicated that 560 mg/kg of BBR in a model of rats fed HFD had similar effects compared to 100 mg/kg of dihydroberberine in slowing the adiposity, tissue TG accumulation and IR. It represents a novel therapeutic agent for the therapy of T2DM [333]. Furthermore, BBR metabolites were studied, their activity was explored, and they were also used for synthesis of derivatives with more pronounced activity. As examples, we can mention derivatives of 12-aminomethyl berberrubine (e.g. 12-(N-methyl-piperazine-4-methyl)-berberrubine), which were synthetized as potential anti-diabetic substances, and tested on 3T3-L1 adipocytes and L6 myotubes to evaluate their effects on the insulin-resistant reversal activity and glucose transportation. Their effect was comparable to rosiglitazone [374]. Further in vitro assay analyzed effect of berberrubine derivatives (e.g. [2-oxo-2-[2-[(2,3,4-trihydroxyphenyl)methylene]hydrazinyl]ethoxy]-9-berberrubine) on the α-glucosidase, showing their ability to inhibit this enzyme and to be a potential anti-diabetic drug [370].
Derivatives of BBR in treating cardiovascular and metabolic diseases. Currently, there are several derivatives being developed based on BBR structural skeleton. These derivatives, such as dihydroberberine, 6-protoberberine, Raisanberine, IMB-Y53, and berberine-baicaline hybrids etc have therapeutic potential in treating cardiovascular and metabolic diseases.
BBR is an isoquinoline alkaloid that, and due to its quaternary ammonium ion, can form compounds with other molecules with negative charge, such as BBR-baicalin, BBR-wogonoside, and BBR-glycyrrhizin [375]. These complexes have been shown to possess improved bioavailability due to increased lipophilicity in comparison to parent drug BBR [375]. A good example of such as complex compound is berberrubine-magnolol, which was synthesized as anti-diabetic compound. When assayed, this derivative showed low toxicity, and improved absorption, metabolism time, and even greater effect on decreasing glycemia [376].
6-Protoberberine showed hypotensive activity, namely it decreased SBP and heart rate by a central sympatholytic effect [377]. Tetrahydroprotoberberine is a competitive antagonist at α1-adrenoceptors in rat aorta, and showed no endothelium-dependent vasorelaxatory activity [378]. Some protoberberine complexes, e.g. tetrahydroprotoberberine-aporphine dimer, were described in nature [379]. Novel tetrahydroprotoberberine derivatives are recently being developed as selective inhibitors of dopamine D1 receptor [380].
Raisanberine (p-chlorobenzyltetrahydroberberine chloride), named also CPU 86017, is a novel cardioprotective drug for the therapy of pulmonary arterial hypertension (PAH), HF and arrhythmia [381-384]. In a rat model of PAH, CPU 86017 reduced ET-1 in lung tissues, reversed an increase of iNOS mRNA level [381]. CPU 86017 also improved heart function and hemodynamic parameters in rats subject to development of artificial myocardial infarction [382]. The main mechanism of protective effects of CPU 86017 were attributed to the inhibitory effects on calcium influx, and stabilized expression of PLB, FKBP12.6, and SERCA2a [382].
Analysis of pharmacological activity in LPS-stimulated RAW264.7 macrophages and acute inflammation mouse models indicate that BBR and its natural derivatives have anti-inflammatory effects, with the order of oxyberberine (OBB) > BBR> reduced derivatives (two hydroquinone, DHBB) [385]. The mechanisms of BBR and its derivatives involve the inhibition of the NF-κB signaling pathway [385]. Further elucidation of the mechanism and therapeutic potential of BBR derivatives in cardiometabolic diseases are warranted.
Concluding remarks and future perspectives
Emerging studies over the past decade have convincingly shown that BBR has diverse therapeutic effects on various cardiometabolic diseases, including cardiac hypertrophy, HF, atherosclerosis, stroke, DM, and NAFLD. The efficacy of BBR for treating multiple diseases is mediated by its multi-target pharmacological profile, including amelioration of AMPK, PTP1B, SIRT1, PCSK9, LDLR, PPAR, NF-κB, and modulation of gut microbiota.
The safety of BBR may be based on the poor oral absorption. High doses of BBR can inhibit certain types of CYPs, such as CYP3A11 and CYP3A25 [386, 387] and repeated oral administration of BBR (0.3 g three times daily) can reduce the activity of CYP2D6, CYP2C9 and CYP3A4 in healthy subjects [388]. This may affect the metabolism of other drugs and could cause some herb-drug interactions; however, this should be further studied and supported by clinical investigations.
As mentioned, the low bioavailability of BBR due its poor oral absorption and extensive metabolism are main obstacles for practical application of BBR. Modern oral dosage forms offer a number of benefits including reduced frequency of dosing, better therapeutic control, and fewer side effects. Advances in polymer materials, particle engineering and nanotechnology could solve some of the problems described for BBR. Different types of nanocarriers (polymers, magnetic mesoporous silica, lipids, graphene, gold and silver nanoparticles) encapsulating BBR can also overcome its deficiencies [389]. In addition. BBR has been recently recommended by several groups of experts as an effective lipid-lowering nutraceutical both in low-risk patients and in statin-intolerant ones [390, 391].
Plants with content of BBR are commonly part of preparations used in Traditional Chinese Medicine and other traditional medicinal systems. Besides of this alkaloid, they contain other related compounds and derivatives of BBR. Preparations of TCM are commonly complex and also contain other, structurally unrelated plant secondary metabolites. This inspired research on combinations of BBR and other substances. As example, the oligomeric proanthocyanidins (OPCs) are the main anti-diabetic components in Cinnamon water extracts [392]. The combination of OPCs and BBR can significantly improve the pharmacokinetics of BBR in diabetic mice (by inhibiting the expression of P-gp in Caco-2 intestinal cells and thereby reducing the efflux of BBR) and hypoglycemic efficacy [392]. The combination of BBR and resveratrol has an enhanced hypolipidemic effect in mice fed with HFD [393]. Mechanistic studies have shown that the combination of BBR and resveratrol significantly increased the expression of LDLR in HepG2 cells to approximately one-fold compared to the two drugs alone [393].
Collectively, BBR represents a promising naturally-occurring lead compound for treating CVMD. Further structural modification of BBR may improve the oral bioavailability, efficacy, and reduces potential toxicity of BBR. However, study outcomes of BBR mixture with other nutraceuticals or compounds have to be analyzed carefully since not pure BBR was used for these studies. Furthermore, well-designed, large-scale, high-quality, and multi-center clinical trials are required to assess the safety, toxicology profile, clinical utility of BBR (as compared to traditional treatment regimens) on CVMD in human patients.
Acknowledgements
The work was supported by National Nature Science Foundation of China (81603339, 81602344) and Natural Science Foundation of Anhui Province (1708085QH175). S. X. is a recipient of Career Development Award from American Heart Association (18CDA34110359). L. Z. was supported by Slovenian Research Agency ARRS (J3-8209). A. S. was supported by the Institute of Health Carlos III (Project CIBEROBN CB12/03/30038).
Supplementary Material
Supplementary table 1.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chandirasegaran G, Elanchezhiyan C, Ghosh K. Effects of Berberine chloride on the liver of streptozotocin-induced diabetes in albino Wistar rats. Biomed Pharmacother. 2018;99:227-36
2. Xu S, Xu Y, Yin M, Zhang S, Liu P, Koroleva M. et al. Flow-dependent epigenetic regulation of IGFBP5 expression by H3K27me3 contributes to endothelial anti-inflammatory effects. Theranostics. 2018;8:3007-21
3. Xu S, Bai P, Little PJ, Liu P. Poly(ADP-ribose) polymerase 1 (PARP1) in atherosclerosis: from molecular mechanisms to therapeutic implications. Med Res Rev. 2014;34:644-75
4. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S. et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67-e492
5. Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M. et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur Heart J. 2018;39:508-79
6. Xu S, Pelisek J, Jin ZG. Atherosclerosis Is an Epigenetic Disease. Trends Endocrinol Metab. 2018
7. Fang J, Little PJ, Xu S. Atheroprotective Effects and Molecular Targets of Tanshinones Derived From Herbal Medicine Danshen. Med Res Rev. 2018;38:201-28
8. Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20:12
9. Bodmer M, Meier C, Krähenbühl S, Jick SS, Meier CR. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: a nested case-control analysis. Diabetes care. 2008;31:2086-91
10. Pillai GKG, Bharate SS, Awasthi A, Verma R, Mishra G, Singh AT. et al. Antidiabetic potential of polyherbal formulation DB14201: Preclinical development, safety and efficacy studies. J Ethnopharmacol. 2017;197:218-30
11. Stafford JM, Elasy T. Treatment update: thiazolidinediones in combination with metformin for the treatment of type 2 diabetes. Vasc Health Risk Manag. 2007;3:503
12. Zhao M, Klipstein-Grobusch K, Wang X, Reitsma JB, Zhao D, Grobbee DE. et al. Prevalence of cardiovascular medication on secondary prevention after myocardial infarction in China between 1995-2015: A systematic review and meta-analysis. PLoS One. 2017;12:e0175947
13. Squizzato A, Bellesini M, Takeda A, Middeldorp S, Donadini MP. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;12:CD005158
14. Blessberger H, Kammler J, Domanovits H, Schlager O, Wildner B, Azar D. et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity. Cochrane Database Syst Rev. 2018;3:CD004476
15. Jin Y, Khadka DB, Cho WJ. Pharmacological effects of berberine and its derivatives: a patent update. Expert Opin Ther Pat. 2016;26:229-43
16. Ju J, Li J, Lin Q, Xu H. Efficacy and safety of berberine for dyslipidaemias: A systematic review and meta-analysis of randomized clinical trials. Phytomedicine. 2018;50:25-34
17. Lin X, Zhang N. Berberine: Pathways to protect neurons. Phytother Res. 2018;32:1501-10
18. Pang B, Yu X-T, Zhou Q, Zhao T-Y, Wang H, Gu C-J. et al. Effect of Rhizoma coptidis (Huang Lian) on treating diabetes mellitus. Evid Based Complement Alternat Med. 2015;2015:921416
19. Tan H-L, Chan K-G, Pusparajah P, Duangjai A, Saokaew S, Mehmood Khan T. et al. Rhizoma coptidis: a potential cardiovascular protective agent. Front Pharmacol. 2016;7:362
20. Zhang Q, Xiao X, Li M, Li W, Yu M, Zhang H. et al. Berberine moderates glucose metabolism through the GnRH-GLP-1 and MAPK pathways in the intestine. BMC Complement Altern Med. 2014;14:188
21. Wang K, Feng X, Chai L, Cao S, Qiu F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab Rev. 2017;49:139-57
22. Battu SK, Repka MA, Maddineni S, Chittiboyina AG, Avery MA, Majumdar S. Physicochemical characterization of berberine chloride: a perspective in the development of a solution dosage form for oral delivery. AAPS PharmSciTech. 2010;11:1466-75
23. Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C. et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344-51
24. Huang ZJ, Zeng Y, Lan P, Sun PH, Chen WM. Advances in structural modifications and biological activities of berberine: an active compound in traditional Chinese medicine. Mini Rev Med Chem. 2011;11:1122-9
25. Chen W, Miao YQ, Fan DJ, Yang SS, Lin X, Meng LK. et al. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech. 2011;12:705-11
26. Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY. The involvement of P-glycoprotein in berberine absorption. Pharmacol Toxicol. 2002;91:193-7
27. Liu CS, Zheng YR, Zhang YF, Long XY. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia. 2016;109:274-82
28. Guo Y, Li F, Ma X, Cheng X, Zhou H, Klaassen CD. CYP2D plays a major role in berberine metabolism in liver of mice and humans. Xenobiotica. 2011;41:996-1005
29. Tan XS, Ma JY, Feng R, Ma C, Chen WJ, Sun YP. et al. Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS One. 2013;8:e77969
30. Kumar A, Ekavali, Chopra K, Mukherjee M, Pottabathini R, Dhull DK. Current knowledge and pharmacological profile of berberine: An update. Eur J Pharmacol. 2015;761:288-97
31. Wang X, Wang R, Xing D, Su H, Ma C, Ding Y. et al. Kinetic difference of berberine between hippocampus and plasma in rat after intravenous administration of Coptidis rhizoma extract. Life Sci. 2005;77:3058-67
32. Zhang X, Zhang X, Wang C, Li Y, Dong L, Cui L. et al. Neuroprotection of early and short-time applying berberine in the acute phase of cerebral ischemia: up-regulated pAkt, pGSK and pCREB, down-regulated NF-kappaB expression, ameliorated BBB permeability. Brain Res. 2012;1459:61-70
33. Tian H, Kang YM, Gao HL, Shi XL, Fu LY, Li Y. et al. Chronic infusion of berberine into the hypothalamic paraventricular nucleus attenuates hypertension and sympathoexcitation via the ROS/Erk1/2/iNOS pathway. Phytomedicine. 2019;52:216-24
34. Imenshahidi M, Hosseinzadeh H. Berberis Vulgaris and Berberine: An Update Review. Phytother Res. 2016;30:1745-64
35. Chan E. Displacement of bilirubin from albumin by berberine. Biol Neonate. 1993;63:201-8
36. Zhi D, Feng PF, Sun JL, Guo F, Zhang R, Zhao X. et al. The enhancement of cardiac toxicity by concomitant administration of Berberine and macrolides. Eur J Pharm Sci. 2015;76:149-55
37. Feng P, Zhao L, Guo F, Zhang B, Fang L, Zhan G. et al. The enhancement of cardiotoxicity that results from inhibiton of CYP 3A4 activity and hERG channel by berberine in combination with statins. Chem Biol Interact. 2018;293:115-23
38. Domitrovic R, Cvijanovic O, Pernjak-Pugel E, Skoda M, Mikelic L, Crncevic-Orlic Z. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem Toxicol. 2013;62:397-406
39. Germoush MO, Mahmoud AM. Berberine mitigates cyclophosphamide-induced hepatotoxicity by modulating antioxidant status and inflammatory cytokines. J Cancer Res Clin Oncol. 2014;140:1103-9
40. Hao G, Yu Y, Gu B, Xing Y, Xue M. Protective effects of berberine against doxorubicin-induced cardiotoxicity in rats by inhibiting metabolism of doxorubicin. Xenobiotica. 2015;45:1024-9
41. Chitra P, Saiprasad G, Manikandan R, Sudhandiran G. Berberine attenuates bleomycin induced pulmonary toxicity and fibrosis via suppressing NF-kappaB dependant TGF-beta activation: a biphasic experimental study. Toxicol Lett. 2013;219:178-93
42. Janbaz KH, Gilani AH. Studies on preventive and curative effects of berberine on chemical-induced hepatotoxicity in rodents. Fitoterapia. 2000;71:25-33
43. Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clin Sci (Lond). 2018;132:1243-52
44. Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852-66
45. Nowak WN, Deng J, Ruan XZ, Xu Q. Reactive Oxygen Species Generation and Atherosclerosis. Arterioscler Thromb Vasc Biol. 2017;37:e41-e52
46. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317-25
47. Escarcega RO, Lipinski MJ, Garcia-Carrasco M, Mendoza-Pinto C, Galvez-Romero JL, Cervera R. Inflammation and atherosclerosis: Cardiovascular evaluation in patients with autoimmune diseases. Autoimmun Rev. 2018;17:703-8
48. Raggi P, Genest J, Giles JT, Rayner KJ, Dwivedi G, Beanlands RS. et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. 2018;276:98-108
49. Halcox JPJ. Chapter 66 - Endothelial Dysfunction A2 - Robertson, David. In: (ed.) Biaggioni I, Burnstock G, Low PA, Paton JFR. Primer on the Autonomic Nervous System (Third Edition). San Diego: Academic Press. 2012 p. 319-24
50. Xu S, Kamato D, Little PJ, Nakagawa S, Pelisek J, Jin ZG. Targeting epigenetics and non-coding RNAs in atherosclerosis: from mechanisms to therapeutics. Pharmacol Ther. 2018 doi: 10.1016/j.pharmthera.2018.11.003
51. Nafisa A, Gray SG, Cao Y, Wang T, Xu S, Wattoo FH. et al. Endothelial function and dysfunction: Impact of metformin. Pharmacol Ther. 2018;192:150-62
52. Huang Z, Cai X, Li S, Zhou H, Chu M, Shan P. et al. Berberine-attenuated monocyte adhesion to endothelial cells induced by oxidized low-density lipoprotein via inhibition of adhesion molecule expression. Mol Med Rep. 2013;7:461-5
53. Xu S, Pelisek J, Jin ZG. Atherosclerosis Is an Epigenetic Disease. Trends Endocrinol Metab. 2018;29:739-42
54. Hsieh YS, Kuo WH, Lin TW, Chang HR, Lin TH, Chen PN. et al. Protective effects of berberine against low-density lipoprotein (LDL) oxidation and oxidized LDL-induced cytotoxicity on endothelial cells. J Agric Food Chem. 2007;55:10437-45
55. Liu SJ, Yin CX, Ding MC, Wang YZ, Wang H. Berberine inhibits tumor necrosis factor-alpha-induced expression of inflammatory molecules and activation of nuclear factor-kappaB via the activation of AMPK in vascular endothelial cells. Mol Med Rep. 2015;12:5580-6
56. Caliceti C, Rizzo P, Ferrari R, Fortini F, Aquila G, Leoncini E. et al. Novel role of the nutraceutical bioactive compound berberine in lectin-like OxLDL receptor 1-mediated endothelial dysfunction in comparison to lovastatin. Nutr Metab Cardiovasc Dis. 2017;27:552-63
57. Xu S, Ogura S, Chen J, Little PJ, Moss J, Liu P. LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cell Mol Life Sci. 2013;70:2859-72
58. Tian K, Ogura S, Little PJ, Xu SW, Sawamura T. Targeting LOX-1 in atherosclerosis and vasculopathy: current knowledge and future perspectives. Ann N Y Acad Sci. 2018 doi: 10.1111/nyas.13984
59. Zhang M, Wang C-M, Li J, Meng Z-J, Wei S-N, Li J. et al. Berberine protects against palmitate-induced endothelial dysfunction: involvements of upregulation of AMPK and eNOS and downregulation of NOX4. Mediators Inflamm. 2013;2013:260464
60. Ko YJ, Lee J-S, Park BC, Shin HM, Kim J-A. Inhibitory effects of Zoagumhwan water extract and berberine on angiotensin II-induced monocyte chemoattractant protein (MCP)-1 expression and monocyte adhesion to endothelial cells. Vascul Pharmacol. 2007;47:189-96
61. Porru E, Franco P, Calabria D, Spinozzi S, Roberti M, Caliceti C. et al. Combined analytical approaches to define biodistribution and biological activity of semi-synthetic berberrubine, the active metabolite of natural berberine. Anal Bioanal Chem. 2018;410:3533-45
62. Xu RX, Sun XC, Ma CY, Yao YH, Li XL, Guo YL. et al. Impacts of berberine on oxidized LDL-induced proliferation of human umbilical vein endothelial cells. Am J Transl Res. 2017;9:4375-89
63. Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13-40
64. Hayashi K, Takahashi M, Nishida W, Yoshida K, Ohkawa Y, Kitabatake A. et al. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ Res. 2001;89:251-8
65. Lee S, Lim HJ, Park HY, Lee KS, Park JH, Jang Y. Berberine inhibits rat vascular smooth muscle cell proliferation and migration in vitro and improves neointima formation after balloon injury in vivo. Berberine improves neointima formation in a rat model. Atherosclerosis. 2006;186:29-37
66. Liang KW, Ting CT, Yin SC, Chen YT, Lin SJ, Liao JK. et al. Berberine suppresses MEK/ERK-dependent Egr-1 signaling pathway and inhibits vascular smooth muscle cell regrowth after in vitro mechanical injury. Biochem Pharmacol. 2006;71:806-17
67. Qiu H, Wu Y, Wang Q, Liu C, Xue L, Wang H. et al. Effect of berberine on PPARalpha-NO signalling pathway in vascular smooth muscle cell proliferation induced by angiotensin IV. Pharm Biol. 2017;55:227-32
68. Liu SJ, Yin CX, Ding MC, Xia SY, Shen QM, Wu JD. Berberine suppresses in vitro migration of human aortic smooth muscle cells through the inhibitions of MMP-2/9, u-PA, AP-1, and NF-kappaB. BMB Rep. 2014;47:388-92
69. Ma L, Zhang L, Wang B, Wei J, Liu J, Zhang L. Berberine inhibits Chlamydia pneumoniae infection-induced vascular smooth muscle cell migration through downregulating MMP3 and MMP9 via PI3K. Eur J Pharmacol. 2015;755:102-9
70. Liang KW, Yin SC, Ting CT, Lin SJ, Hsueh CM, Chen CY. et al. Berberine inhibits platelet-derived growth factor-induced growth and migration partly through an AMPK-dependent pathway in vascular smooth muscle cells. Eur J Pharmacol. 2008;590:343-54
71. Cho BJ, Im EK, Kwon JH, Lee KH, Shin HJ, Oh J. et al. Berberine inhibits the production of lysophosphatidylcholine-induced reactive oxygen species and the ERK1/2 pathway in vascular smooth muscle cells. Mol Cells. 2005;20:429-34
72. Luo J, Gu Y, Liu P, Jiang X, Yu W, Ye P. et al. Berberine attenuates pulmonary arterial hypertension via protein phosphatase 2A signaling pathway both in vivo and in vitro. J Cell Physiol. 2018;233:9750-62
73. Liu J, Xiu J, Cao J, Gao Q, Ma D, Fu L. Berberine cooperates with adrenal androgen dehydroepiandrosterone sulfate to attenuate PDGF-induced proliferation of vascular smooth muscle cell A7r5 through Skp2 signaling pathway. Mol Cell Biochem. 2011;355:127-34
74. Tanabe H, Suzuki H, Mizukami H, Inoue M. Double blockade of cell cycle progression by coptisine in vascular smooth muscle cells. Biochem Pharmacol. 2005;70:1176-84
75. Levitan I, Volkov S, Subbaiah PV. Oxidized LDL: diversity, patterns of recognition, and pathophysiology. Antioxid Redox Signal. 2010;13:39-75
76. Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709-21
77. Xu S, Huang Y, Xie Y, Lan T, Le K, Chen J. et al. Evaluation of foam cell formation in cultured macrophages: an improved method with Oil Red O staining and DiI-oxLDL uptake. Cytotechnology. 2010;62:473-81
78. Huang Z, Dong F, Li S, Chu M, Zhou H, Lu Z. et al. Berberine-induced inhibition of adipocyte enhancer-binding protein 1 attenuates oxidized low-density lipoprotein accumulation and foam cell formation in phorbol 12-myristate 13-acetate-induced macrophages. Eur J Pharmacol. 2012;690:164-9
79. Guan S, Wang B, Li W, Guan J, Fang X. Effects of berberine on expression of LOX-1 and SR-BI in human macrophage-derived foam cells induced by ox-LDL. Am J Chin Med. 2010;38:1161-9
80. Zimetti F, Adorni MP, Ronda N, Gatti R, Bernini F, Favari E. The natural compound berberine positively affects macrophage functions involved in atherogenesis. Nutr Metab Cardiovasc Dis. 2015;25:195-201
81. Liang H, Wang Y. Berberine alleviates hepatic lipid accumulation by increasing ABCA1 through the protein kinase C delta pathway. Biochem Biophys Res Commun. 2018;498:473-80
82. Chi L, Peng L, Pan N, Hu X, Zhang Y. The anti-atherogenic effects of berberine on foam cell formation are mediated through the upregulation of sirtuin 1. Int J Mol Med. 2014;34:1087-93
83. Chi L, Peng L, Hu X, Pan N, Zhang Y. Berberine combined with atorvastatin downregulates LOX1 expression through the ET1 receptor in monocyte/macrophages. Int J Mol Med. 2014;34:283-90
84. Kou JY, Li Y, Zhong ZY, Jiang YQ, Li XS, Han XB. et al. Berberine-sonodynamic therapy induces autophagy and lipid unloading in macrophage. Cell Death Dis. 2017;8:e2558
85. Li K, Yao W, Zheng X, Liao K. Berberine promotes the development of atherosclerosis and foam cell formation by inducing scavenger receptor A expression in macrophage. Cell Res. 2009;19:1006-17
86. Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ. et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955-64
87. Fan X, Wang J, Hou J, Lin C, Bensoussan A, Chang D. et al. Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway. J Transl Med. 2015;13:92
88. Jiang Y, Huang K, Lin X, Chen Q, Lin S, Feng X. et al. Berberine Attenuates NLRP3 Inflammasome Activation in Macrophages to Reduce the Secretion of Interleukin-1beta. Ann Clin Lab Sci. 2017;47:720-8
89. Huang Z, Ye B, Han J, Kong F, Shan P, Lu Z. et al. NACHT, LRR and PYD domains-containing protein 3 inflammasome is activated and inhibited by berberine via toll-like receptor 4/myeloid differentiation primary response gene 88/nuclear factor-kappaB pathway, in phorbol 12-myristate 13-acetate-induced macrophages. Mol Med Rep. 2018;17:2673-80
90. Liu YF, Wen CY, Chen Z, Wang Y, Huang Y, Tu SH. Effects of Berberine on NLRP3 and IL-1beta Expressions in Monocytic THP-1 Cells with Monosodium Urate Crystals-Induced Inflammation. Biomed Res Int. 2016;2016:2503703
91. Zhou H, Feng L, Xu F, Sun Y, Ma Y, Zhang X. et al. Berberine inhibits palmitate-induced NLRP3 inflammasome activation by triggering autophagy in macrophages: A new mechanism linking berberine to insulin resistance improvement. Biomed Pharmacother. 2017;89:864-74
92. Vivoli E, Cappon A, Milani S, Piombanti B, Provenzano A, Novo E. et al. NLRP3 inflammasome as a target of berberine in experimental murine liver injury: interference with P2X7 signalling. Clin Sci (Lond). 2016;130:1793-806
93. Sujitha S, Dinesh P, Rasool M. Berberine modulates ASK1 signaling mediated through TLR4/TRAF2 via upregulation of miR-23a. Toxicol Appl Pharmacol. 2018;359:34-46
94. Pei C, Zhang Y, Wang P, Zhang B, Fang L, Liu B. et al. Berberine alleviates oxidized low-density lipoprotein-induced macrophage activation by downregulating galectin-3 via the NF-kappaB and AMPK signaling pathways. Phytother Res. 2019;33:294-308
95. Huang CG, Chu ZL, Wei SJ, Jiang H, Jiao BH. Effect of berberine on arachidonic acid metabolism in rabbit platelets and endothelial cells. Thromb Res. 2002;106:223-7
96. Zhao L CZ. The assisting effect of berberine on urokinase- and streptokinase-induced thrombolysis. Chin Pharmacol Bull. 1998;14:359-61
97. Chu Z-L, Huang C-G, Xu Z-P. Antiplatelet effects and mechanisms of berberine. Chinese Journal of Integrated Traditional and Western Medicine. 1996;2:75-7
98. Chu Z-L, Huang C-G, Lai F-S. The anti-platelet-rich-plasma-clot-retraction effect of berberine and its mechanism. Chin Pharmacol Bull. 1994;10:114-6
99. Gui-xian J, Bang-mao W, Wei-li F. The effects of berberine chloride on platelet activation of mice with dextransulphate sodium-induced colitis [J]. Journal of Tianjin Medical University. 2004;2:008
100. Allijn IE, Vaessen SF, Quarles van Ufford LC, Beukelman KJ, de Winther MP, Storm G. et al. Head-to-Head Comparison of Anti-Inflammatory Performance of Known Natural Products In Vitro. PLoS One. 2016;11:e0155325
101. Shah BH, Nawaz Z, Saeed SA, Gilani AHJPR. Agonist-dependent differential effects of berberine in human platelet aggregation. 2010; 12: S60-S2.
102. LU L, ZHANG H-q, CHEN G-r, WU J-b, CHEN Z-l, ZHOU X-y. et al. Effects of Berberine on the Expression of GPVI on Platelets of Diabetes Rats [J]. Herald of Medicine. 2007;11:012
103. Zhang Y, Ma XJ, Guo CY, Wang MM, Kou N, Qu H. et al. Pretreatment with a combination of ligustrazine and berberine improves cardiac function in rats with coronary microembolization. Acta Pharmacol Sin. 2016;37:463-72
104. Holy EW, Akhmedov A, Lüscher TF, Tanner FC. Berberine, a natural lipid-lowering drug, exerts prothrombotic effects on vascular cells. J Mol Cell Cardiol. 2009;46:234-40
105. Gao M-y, Chen L, Yang L, Yu X, Kou J-p, Yu B-y. Berberine inhibits LPS-induced TF procoagulant activity and expression through NF-κB/p65, Akt and MAPK pathway in THP-1 cells. Pharmacol Rep. 2014;66:480-4
106. Feng M, Zou Z, Zhou X, Hu Y, Ma H, Xiao Y. et al. Comparative effect of berberine and its derivative 8-cetylberberine on attenuating atherosclerosis in ApoE(-/-) mice. Int Immunopharmacol. 2017;43:195-202
107. Li H, He C, Wang J, Li X, Yang Z, Sun X. et al. Berberine activates peroxisome proliferator-activated receptor gamma to increase atherosclerotic plaque stability in Apoe(-/-) mice with hyperhomocysteinemia. J Diabetes Investig. 2016;7:824-32
108. Wan Q, Liu Z, Yang Y, Cui X. Suppressive effects of berberine on atherosclerosis via downregulating visfatin expression and attenuating visfatin-induced endothelial dysfunction. Int J Mol Med. 2018;41:1939-48
109. Zhu L, Zhang D, Zhu H, Zhu J, Weng S, Dong L. et al. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe(-/-) mice. Atherosclerosis. 2018;268:117-26
110. Shi Y, Hu J, Geng J, Hu T, Wang B, Yan W. et al. Berberine treatment reduces atherosclerosis by mediating gut microbiota in apoE-/- mice. Biomed Pharmacother. 2018;107:1556-63
111. Van Ierssel SH, Hoymans VY, Van Craenenbroeck EM, Van Tendeloo VF, Vrints CJ, Jorens PG. et al. Endothelial microparticles (EMP) for the assessment of endothelial function: an in vitro and in vivo study on possible interference of plasma lipids. PLoS One. 2012;7:e31496
112. Wang JM, Yang Z, Xu MG, Chen L, Wang Y, Su C. et al. Berberine-induced decline in circulating CD31+/CD42- microparticles is associated with improvement of endothelial function in humans. Eur J Pharmacol. 2009;614:77-83
113. Chen S. Inhibition of endothelial microparticles-mediated oxidative stress by berberine and its relation with endothelial function in humans. Heart. 2011;97:A90-A1
114. Cheng F, Wang Y, Li J, Su C, Wu F, Xia W-H. et al. Berberine improves endothelial function by reducing endothelial microparticles-mediated oxidative stress in humans. Int J Cardiol. 2013;167:936-42
115. Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-κB. J Appl Physiol. 2008;105:1333-41
116. Wang Y, Ding Y. Berberine protects vascular endothelial cells in hypertensive rats. Int J Clin Exp. 2015;8:14896-905
117. Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol. 2009;157:527-36
118. Liu L, Liu J, Huang Z, Yu X, Zhang X, Dou D. et al. Berberine improves endothelial function by inhibiting endoplasmic reticulum stress in the carotid arteries of spontaneously hypertensive rats. Biochem Biophys Res Commun. 2015;458:796-801
119. Wu Y, Chuang S, Hong W, Lai Y, Chang G, Pang JS. Berberine reduces leukocyte adhesion to LPS-stimulated endothelial cells and VCAM-1 expression both in vivo and in vitro. Int J Immunopathol Pharmacol. 2012;25:741-50
120. Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161-72
121. Rizzello V, Poldermans D, Biagini E, Schinkel AF, Boersma E, Boccanelli A. et al. Prognosis of patients with ischaemic cardiomyopathy after coronary revascularisation: relation to viability and improvement in left ventricular ejection fraction. Heart. 2009;95:1273-7
122. Le K, Li R, Xu S, Wu X, Huang H, Bao Y. et al. PPARalpha activation inhibits endothelin-1-induced cardiomyocyte hypertrophy by prevention of NFATc4 binding to GATA-4. Arch Biochem Biophys. 2012;518:71-8
123. Zou J, Le K, Xu S, Chen J, Liu Z, Chao X. et al. Fenofibrate ameliorates cardiac hypertrophy by activation of peroxisome proliferator-activated receptor-alpha partly via preventing p65-NFkappaB binding to NFATc4. Mol Cell Endocrinol. 2013;370:103-12
124. Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98-108
125. Huang WM, Yan H, Jin JM, Yu C, Zhang H. Beneficial effects of berberine on hemodynamics during acute ischemic left ventricular failure in dogs. Chin Med J (Engl). 1992;105:1014-9
126. Li Y, Chen X, Liu H, Luo F, Li G. [Effects of ginseng total saponins with berberine on plasma brain natriuretic peptide and Ca2+ concentration in experimental rats with chronic congestive heart failure]. Zhongguo Zhong Yao Za Zhi. 2009;34:324-7
127. Zhang XD, Ren HM, Liu L. [Effects of different dose berberine on hemodynamic parameters and [Ca2+]i of cardiac myocytes of diastolic heart failure rat model]. Zhongguo Zhong Yao Za Zhi. 2008;33:818-21
128. Tsui H, Zi M, Wang S, Chowdhury SK, Prehar S, Liang Q. et al. Smad3 Couples Pak1 With the Antihypertrophic Pathway Through the E3 Ubiquitin Ligase, Fbxo32. Hypertension. 2015;66:1176-83
129. Hong Y, Hui SC, Chan TY, Hou JY. Effect of berberine on regression of pressure-overload induced cardiac hypertrophy in rats. Am J Chin Med. 2002;30:589-99
130. Hong Y, Hui SS, Chan BT, Hou J. Effect of berberine on catecholamine levels in rats with experimental cardiac hypertrophy. Life Sci. 2003;72:2499-507
131. Li MH, Zhang YJ, Yu YH, Yang SH, Iqbal J, Mi QY. et al. Berberine improves pressure overload-induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur J Pharmacol. 2014;728:67-76
132. Daskalopoulos EP, Dufeys C, Bertrand L, Beauloye C, Horman S. AMPK in cardiac fibrosis and repair: Actions beyond metabolic regulation. J Mol Cell Cardiol. 2016;91:188-200
133. Gu HP, Lin S, Xu M, Yu HY, Du XJ, Zhang YY. et al. Up-regulating relaxin expression by G-quadruplex interactive ligand to achieve antifibrotic action. Endocrinology. 2012;153:3692-700
134. Zhao HP, Hong Y, Xie JD, Xie XR, Wang J, Fan JB. [Effect of berberine on left ventricular remodeling in renovascular hypertensive rats]. Yao Xue Xue Bao. 2007;42:336-41
135. Ailawadi G, Eliason JL, Upchurch GR Jr. Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg. 2003;38:584-8
136. Calero A, Illig KA. Overview of aortic aneurysm management in the endovascular era. Semin Vasc Surg. 2016;29:3-17
137. Wills A, Thompson MM, Crowther M, Sayers RD, Bell PR. Pathogenesis of abdominal aortic aneurysms-cellular and biochemical mechanisms. Eur J Vasc Endovasc Surg. 1996;12:391-400
138. Ai F, Chen M, Yu B, Yang Y, Xu G, Gui F. et al. Berberine regulates proliferation, collagen synthesis and cytokine secretion of cardiac fibroblasts via AMPK-mTOR-p70S6K signaling pathway. Int J Clin Exp Pathol. 2015;8:12509-16
139. van Vlijmen-van Keulen CJ, Pals G, Rauwerda JA. Familial abdominal aortic aneurysm: a systematic review of a genetic background. Eur J Vasc Endovasc Surg. 2002;24:105-16
140. Huang Z, Wang L, Meng S, Wang Y, Chen T, Wang C. Berberine reduces both MMP-9 and EMMPRIN expression through prevention of p38 pathway activation in PMA-induced macrophages. Int J Cardiol. 2011;146:153-8
141. Li XX, Li CB, Xiao J, Gao HQ, Wang HW, Zhang XY. et al. Berberine Attenuates Vascular Remodeling and Inflammation in a Rat Model of Metabolic Syndrome. Biol Pharm Bull. 2015;38:862-8
142. Raaz U, Zollner AM, Schellinger IN, Toh R, Nakagami F, Brandt M. et al. Segmental aortic stiffening contributes to experimental abdominal aortic aneurysm development. Circulation. 2015;131:1783-95
143. Pirro M, Lupattelli G, Del Giorno R, Schillaci G, Berisha S, Mannarino MR. et al. Nutraceutical combination (red yeast rice, berberine and policosanols) improves aortic stiffness in low-moderate risk hypercholesterolemic patients. PharmaNutrition. 2013;1:73-7
144. Wang J, Guo T, Peng QS, Yue SW, Wang SX. Berberine via suppression of transient receptor potential vanilloid 4 channel improves vascular stiffness in mice. J Cell Mol Med. 2015;19:2607-16
145. Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674-99
146. Heusch G. Cardioprotection Is Alive But Remains Enigmatic: The Nitric Oxide-Protein Kinases-Mitochondria Signaling Axis. Circulation. 2017;136:2356-8
147. Zheng L, Zhou Z, Tao D, Lan T. [Protective effect of berberine on cardiac myocyte injured by ischemia-reperfusion]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003;34:452-4
148. Tan Y, Tang Q, Hu BR, Xiang JZ. Antioxidant properties of berberine on cultured rabbit corpus cavernosum smooth muscle cells injured by hydrogen peroxide. Acta Pharmacol Sin. 2007;28:1914-8
149. Wang L, Ma H, Xue Y, Shi H, Ma T, Cui X. Berberine inhibits the ischemia-reperfusion injury induced inflammatory response and apoptosis of myocardial cells through the phosphoinositide 3-kinase/RAC-alpha serine/threonine-protein kinase and nuclear factor-kappaB signaling pathways. Exp Ther Med. 2018;15:1225-32
150. Huang W. [The role and mechanism of berberine on coronary arteries]. Zhonghua Xin Xue Guan Bing Za Zhi. 1990;18:231-4 54-5
151. Yao W, Wang X, Xiao K. Protective effect of berberine against cardiac ischemia/reperfusion injury by inhibiting apoptosis through the activation of Smad7. Mol Cell Probes. 2018;38:38-44
152. Huang Z, Han Z, Ye B, Dai Z, Shan P, Lu Z. et al. Berberine alleviates cardiac ischemia/reperfusion injury by inhibiting excessive autophagy in cardiomyocytes. Eur J Pharmacol. 2015;762:1-10
153. Zhu ML, Yin YL, Ping S, Yu HY, Wan GR, Jian X. et al. Berberine promotes ischemia-induced angiogenesis in mice heart via upregulation of microRNA-29b. Clin Exp Hypertens. 2017;39:672-9
154. Pongkittiphan V, Chavasiri W, Supabphol R. Antioxidant Effect of Berberine and its Phenolic Derivatives Against Human Fibrosarcoma Cells. Asian Pac J Cancer Prev. 2015;16:5371-6
155. Shirwaikar A, Shirwaikar A, Rajendran K, Punitha IS. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol Pharm Bull. 2006;29:1906-10
156. Chatuphonprasert W, Lao-Ong T, Jarukamjorn K. Improvement of superoxide dismutase and catalase in streptozotocin-nicotinamide-induced type 2-diabetes in mice by berberine and glibenclamide. Pharm Biol. 2013;52:419-27
157. Li Z, Geng YN, Jiang JD, Kong WJ. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid Based Complement Alternat Med. 2014;2014:289264
158. Shang W, Liu J, Yu X, Zhao J. [Effects of berberine on serum levels of inflammatory factors and inflammatory signaling pathway in obese mice induced by high fat diet]. Zhongguo Zhong Yao Za Zhi. 2010;35:1474-7
159. Qin-Wei Z, Yong-Guang LI. Berberine attenuates myocardial ischemia reperfusion injury by suppressing the activation of PI3K/AKT signaling. Exp Ther Med. 2016;11:978-84
160. Yu L, Li Q, Yu B, Yang Y, Jin Z, Duan W. et al. Berberine Attenuates Myocardial Ischemia/Reperfusion Injury by Reducing Oxidative Stress and Inflammation Response: Role of Silent Information Regulator 1. Oxid Med Cell Longev. 2016;2016:1689602
161. Chun YT, Yip TT, Lau KL, Kong YC, Sankawa U. A biochemical study on the hypotensive effect of berberine in rats. Gen Pharmacol. 1979;10:177-82
162. Fatehi-Hassanabad Z, Jafarzadeh M, Tarhini A, Fatehi M. The antihypertensive and vasodilator effects of aqueous extract from Berberis vulgaris fruit on hypertensive rats. Phytother Res. 2005;19:222-5
163. Heusch G. Myocardial Ischemia: Lack of Coronary Blood Flow or Myocardial Oxygen Supply/Demand Imbalance? Circ Res. 2016;119:194-6
164. Cheng B, Luo LY, Fang DC, Jiang MX. Cardiovascular aspects of pharmacology of berberine: I. Alpha-adrenoceptor blocking action of berberine in isolated rat anococcygeus muscle and rabbit aortic strip. J Tongji Med Univ. 1987;7:239-41
165. Olmez E, Ilhan M. Evaluation of the alpha-adrenoceptor antagonistic action of berberine in isolated organs. Arzneimittelforschung. 1992;42:1095-7
166. Lau CW, Yao XQ, Chen ZY, Ko WH, Huang Y. Cardiovascular actions of berberine. Cardiovasc Drug Rev. 2001;19:234-44
167. Wong KK. Mechanism of the aortic relaxation induced by low concentrations of berberine. Planta Med. 1998;64:756-7
168. Ko W-H, Yao X-Q, Lau C-W, Law W-I, Chen Z-Y, Kwok W. et al. Vasorelaxant and antiproliferative effects of berberine. Eur J Pharmacol. 2000;399:187-96
169. Kang DG, Sohn EJ, Kwon EK, Han JH, Oh H, Lee HS. Effects of berberine on angiotensin-converting enzyme and NO/cGMP system in vessels. Vascul Pharmacol. 2002;39:281-6
170. Chiou WF, Chen J, Chen CF. Relaxation of corpus cavernosum and raised intracavernous pressure by berberine in rabbit. Br J Pharmacol. 1998;125:1677-84
171. Xu SZ, Zhang Y, Ren JY, Zhou ZN. Effects of berberine of L- and T-type calcium channels in guinea pig ventricular myocytes. Zhongguo Yao Li Xue Bao. 1997;18:515-8
172. Chang W, Zhang M, Li J, Meng Z, Xiao D, Wei S. et al. Berberine attenuates ischemia-reperfusion injury via regulation of adenosine-5'-monophosphate kinase activity in both non-ischemic and ischemic areas of the rat heart. Cardiovasc Drugs Ther. 2012;26:467-78
173. Chen K, Li G, Geng F, Zhang Z, Li J, Yang M. et al. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K-Akt signaling in diabetic rats. Apoptosis. 2014;19:946-57
174. Wang Y, Liu J, Ma A, Chen Y. Cardioprotective effect of berberine against myocardial ischemia/reperfusion injury via attenuating mitochondrial dysfunction and apoptosis. Int J Clin Exp Med. 2015;8:14513-9
175. Yu L, Li F, Zhao G, Yang Y, Jin Z, Zhai M. et al. Protective effect of berberine against myocardial ischemia reperfusion injury: role of Notch1/Hes1-PTEN/Akt signaling. Apoptosis. 2015;20:796-810
176. Zhao GL, Yu LM, Gao WL, Duan WX, Jiang B, Liu XD. et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol Sin. 2016;37:354-67
177. Chai YS, Hu J, Lei F, Wang YG, Yuan ZY, Lu X. et al. Effect of berberine on cell cycle arrest and cell survival during cerebral ischemia and reperfusion and correlations with p53/cyclin D1 and PI3K/Akt. Eur J Pharmacol. 2013;708:44-55
178. Zhang Q, Qian Z, Pan L, Li H, Zhu H. Hypoxia-inducible factor 1 mediates the anti-apoptosis of berberine in neurons during hypoxia/ischemia. Acta Physiol Hung. 2012;99:311-23
179. Hu J, Chai Y, Wang Y, Kheir MM, Li H, Yuan Z. et al. PI3K p55gamma promoter activity enhancement is involved in the anti-apoptotic effect of berberine against cerebral ischemia-reperfusion. Eur J Pharmacol. 2012;674:132-42
180. Yang J, Yan H, Li S, Zhang M. Berberine Ameliorates MCAO Induced Cerebral Ischemia/Reperfusion Injury via Activation of the BDNF-TrkB-PI3K/Akt Signaling Pathway. Neurochem Res. 2018;43:702-10
181. Gong LL, Fang LH, Wang SB, Sun JL, Qin HL, Li XX. et al. Coptisine exert cardioprotective effect through anti-oxidative and inhibition of RhoA/Rho kinase pathway on isoproterenol-induced myocardial infarction in rats. Atherosclerosis. 2012;222:50-8
182. Kim YM, Ha YM, Jin YC, Shi LY, Lee YS, Kim HJ. et al. Palmatine from Coptidis rhizoma reduces ischemia-reperfusion-mediated acute myocardial injury in the rat. Food Chem Toxicol. 2009;47:2097-102
183. Zhang T, Yang S, Du J. Protective Effects of Berberine on Isoproterenol-Induced Acute Myocardial Ischemia in Rats through Regulating HMGB1-TLR4 Axis. Evid Based Complement Alternat Med. 2014;2014:849783
184. Yu Y, Zhang M, Hu Y, Zhao Y, Teng F, Lv X. et al. Increased Bioavailable Berberine Protects Against Myocardial Ischemia Reperfusion Injury Through Attenuation of NFkappaB and JNK Signaling Pathways. Int Heart J. 2018;59:1378-88
185. Kalani A, Kamat PK, Kalani K, Tyagi N. Epigenetic impact of curcumin on stroke prevention. Metab Brain Dis. 2015;30:427-35
186. Li W, Suwanwela NC, Patumraj S. Curcumin prevents reperfusion injury following ischemic stroke in rats via inhibition of NFkappaB, ICAM-1, MMP-9 and caspase-3 expression. Mol Med Rep. 2017;16:4710-20
187. Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A. et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115:e478-534
188. Li W, Suwanwela NC, Patumraj S. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc Res. 2016;106:117-27
189. Li Y, Wang P, Chai MJ, Yang F, Li HS, Zhao J. et al. [Effects of berberine on serum inflammatory factors and carotid atherosclerotic plaques in patients with acute cerebral ischemic stroke]. Zhongguo Zhong Yao Za Zhi. 2016;41:4066-71
190. Benaissa F, Mohseni-Rad H, Rahimi-Moghaddam P, Mahmoudian M. Berberine reduces the hypoxic-ischemic insult in rat pup brain. Acta Physiol Hung. 2009;96:213-20
191. Kim M, Shin MS, Lee JM, Cho HS, Kim CJ, Kim YJ. et al. Inhibitory Effects of Isoquinoline Alkaloid Berberine on Ischemia-Induced Apoptosis via Activation of Phosphoinositide 3-Kinase/Protein Kinase B Signaling Pathway. Int Neurourol J. 2014;18:115-25
192. Zhou XQ, Zeng XN, Kong H, Sun XL. Neuroprotective effects of berberine on stroke models in vitro and in vivo. Neurosci Lett. 2008;447:31-6
193. Zhu JR, Lu HD, Guo C, Fang WR, Zhao HD, Zhou JS. et al. Berberine attenuates ischemia-reperfusion injury through inhibiting HMGB1 release and NF-kappaB nuclear translocation. Acta Pharmacol Sin. 2018;39:1706-15
194. Liu H, Ren X, Ma C. Effect of Berberine on Angiogenesis and HIF-1α / VEGF Signal Transduction Pathway in Rats with Cerebral Ischemia - Reperfusion Injury. J Coll Physicians Surg Pak. 2018;28:753-7
195. Maleki SN, Aboutaleb N, Souri F. Berberine confers neuroprotection in coping with focal cerebral ischemia by targeting inflammatory cytokines. J Chem Neuroanat. 2018;87:54-9
196. Singh DP, Chopra K. Verapamil augments the neuroprotectant action of berberine in rat model of transient global cerebral ischemia. Eur J Pharmacol. 2013;720:98-106
197. Yoo KY, Hwang IK, Kim JD, Kang IJ, Park J, Yi JS. et al. Antiinflammatory effect of the ethanol extract of Berberis koreana in a gerbil model of cerebral ischemia/reperfusion. Phytother Res. 2008;22:1527-32
198. Zhang Q, Fu X, Wang J, Yang M, Kong L. Treatment Effects of Ischemic Stroke by Berberine, Baicalin, and Jasminoidin from Huang-Lian-Jie-Du-Decoction (HLJDD) Explored by an Integrated Metabolomics Approach. Oxid Med Cell Longev. 2017;2017:9848594
199. Li S, Wu C, Chen J, Lu P, Chen C, Fu M. et al. An effective solution to discover synergistic drugs for anti-cerebral ischemia from traditional Chinese medicinal formulae. PLoS One. 2013;8:e78902
200. Huang W, Wu Z, Gan Y. Effects of berberine on ischemic ventricular arrhythmia. Zhonghua Xin Xue Guan Bing Za Zhi. 1989;17:300-1 19
201. Zhou Z-W, Zheng H-C, Zhao L-F, Li W, Hou J-W, Yu Y. et al. Effect of berberine on acetylcholine-induced atrial fibrillation in rabbit. Am J Transl Res. 2015;7:1450-7
202. Jun-xian C, Lu F, Yu-hui D, Yan-li D, Jun-xian C. THE EFFECT OF BERBERINE ON ARRHYTHMIA CAUSED BY STRETCH OF ISOLATED MYOCARDIAL INFARCTED HEARTS IN RATS. Heart. 2012;98:E84
203. Neto FR. Electropharmacological effects of berberine on canine cardiac Purkinje fibres and ventricular muscle and atrial muscle of the rabbit. Br J Pharmacol. 1993;108:534-7
204. Chen H, Chen Y, Tang Y, Yang J, Wang D, Yu T. et al. Berberine attenuates spontaneous action potentials in sinoatrial node cells and the currents of human HCN4 channels expressed in Xenopus laevis oocytes. Mol Med Rep. 2014;10:1576-82
205. Wei T, Liang Z, Jin Y, Zhang L. [Effect of berberine, liensinine and neferine on HERG channel expression]. Zhongguo Zhong yao za zhi. 2013;38:239-44
206. Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463
207. Zhang K, Zhi D, Huang T, Gong Y, Yan M, Liu C. et al. Berberine Induces hERG Channel Deficiency through Trafficking Inhibition. Cell Physiol Biochem. 2014;34:691-702
208. Yan M, Zhang K, Shi Y, Feng L, Lv L, Li B. Mechanism and pharmacological rescue of berberine-induced hERG channel deficiency. Drug Des Devel Ther. 2015;9:5737-47
209. Rodriguez-Menchaca A, Ferrer-Villada T, Lara J, Fernandez D, Navarro-Polanco RA, Sanchez-Chapula JA. Block of hERG Channels by Berberine: Mechanisms of Voltage- and State-Dependence Probed With Site-Directed Mutant Channels. J Cardiovasc Pharmacol. 2006;47:21-9
210. Llorent-Martínez EJ, Zengin G, Lobine D, Molina-García L, Mollica A, Mahomoodally MF. Phytochemical characterization, in vitro and in silico approaches for three Hypericum species. New J Chem. 2018;42:5204-14
211. American Diabetets Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62-7
212. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes care. 2010;33:S62
213. Horr S, Nissen S. Managing hypertension in type 2 diabetes mellitus. Best Pract Res Clin Endocrinol Metab. 2016;30:445-54
214. Li J-w, Yuan K, Shang S-c, Guo Y. A safer hypoglycemic agent for type 2 diabetes—Berberine organic acid salt. J Funct Foods. 2017;38:399-408
215. Raman B, Krishna N, Rao N, Saradhi P, Rao B. Plants with antidiabetic activities and their medicinal values. Int Res J Pharm. 2012;3:11-5
216. Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81-100
217. Chang W, Chen L, Hatch GM. Berberine as a therapy for type 2 diabetes and its complications: From mechanism of action to clinical studies. Biochem Cell Biol. 2014;93:479-86
218. Dong H, Zhao Y, Zhao L, Lu F. The effects of berberine on blood lipids: a systemic review and meta-analysis of randomized controlled trials. Planta Medica. 2013;79:437-46
219. Gong J, Hu M, Huang Z, Fang K, Wang D, Chen Q. et al. Berberine attenuates intestinal mucosal barrier dysfunction in type 2 diabetic rats. Front Pharmacol. 2017;8:42
220. Lan T, Shen X, Liu P, Liu W, Xu S, Xie X. et al. Berberine ameliorates renal injury in diabetic C57BL/6 mice: Involvement of suppression of SphK-S1P signaling pathway. Arch Biochem Biophys. 2010;502:112-20
221. Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan T. et al. Berberine attenuates lipopolysaccharide-induced extracelluar matrix accumulation and inflammation in rat mesangial cells: involvement of NF-kappaB signaling pathway. Mol Cell Endocrinol. 2011;331:34-40
222. Chang W, Zhang M, Li J, Meng Z, Wei S, Du H. et al. Berberine improves insulin resistance in cardiomyocytes via activation of 5'-adenosine monophosphate-activated protein kinase. Metabolism. 2013;62:1159-67
223. Pan G, Wang G, Sun J, Huang Z, Zhao X, Gu Y. et al. Inhibitory action of berberine on glucose absorption. Yao xue xue bao. 2003;38:911-4
224. Vuddanda PR, Chakraborty S, Singh S. Berberine: a potential phytochemical with multispectrum therapeutic activities. Expert Opin Investig Drugs. 2010;19:1297-307
225. Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G. et al. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem. 2010;285:37503-12
226. Zhao L, Sun L, Nie H, Wang X, Guan G. Berberine improves kidney function in diabetic mice via AMPK activation. PLoS ONE. 2014;9:e113398
227. Xiao Y, Xu M, Alimujiang M, Bao Y, Wei L, Yin J. Bidirectional regulation of adenosine 5'-monophosphate-activated protein kinase activity by berberine and metformin in response to changes in ambient glucose concentration. J Cell Biochem. 2018;119:9910-20
228. Zhang B, Pan Y, Xu L, Tang D, Dorfman RG, Zhou Q. et al. Berberine promotes glucose uptake and inhibits gluconeogenesis by inhibiting deacetylase SIRT3. Endocrine. 2018;62:576-87
229. Li A, Liu Q, Li Q, Liu B, Yang Y, Zhang N. Berberine Reduces Pyruvate-driven Hepatic Glucose Production by Limiting Mitochondrial Import of Pyruvate through Mitochondrial Pyruvate Carrier 1. EBioMedicine. 2018;34:243-55
230. Xie W, Du L. Diabetes is an inflammatory disease: evidence from traditional Chinese medicines. Diabetes Obes Metab. 2011;13:289-301
231. Ma X, Chen Z, Wang L, Wang G, Wang Z, Dong X. et al. The Pathogenesis of Diabetes Mellitus by Oxidative Stress and Inflammation: Its Inhibition by Berberine. Front Pharmacol. 2018;9:782
232. Hua W, Ding L, Chen Y, Gong B, He J, Xu G. Determination of berberine in human plasma by liquid chromatography-electrospray ionization-mass spectrometry. J Pharm Biomed Anal. 2007;44:931-7
233. Zhang X, Zhao Y, Zhang M, Pang X, Xu J, Kang C. et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7:e42529
234. Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. 2013;3:1-58
235. Cefalu WT. Insulin resistance: cellular and clinical concepts. Exp Biol Med. 2001;226:13-26
236. Kong WJ, Zhang H, Song DQ, Xue R, Zhao W, Wei J. et al. Berberine reduces insulin resistance through protein kinase C-dependent up-regulation of insulin receptor expression. Metabolism. 2009;58:109-19
237. Mahmoud MA, Ghareeb DA, Sahyoun HA, Elshehawy AA, Elsayed MM. In Vivo Interrelationship between Insulin Resistance and Interferon Gamma Production: Protective and Therapeutic Effect of Berberine. Evid Based Complement Alternat Med. 2016;2016:2039897
238. Liu LZ, Cheung SC, Lan LL, Ho SK, Xu HX, Chan JC. et al. Berberine modulates insulin signaling transduction in insulin-resistant cells. Mol Cell Endocrinol. 2010;317:148-53
239. Liu D, Zhang Y, Liu Y, Hou L, Li S, Tian H. et al. Berberine Modulates Gut Microbiota and Reduces Insulin Resistance via the TLR4 Signaling Pathway. Exp Clin Endocrinol Diabetes. 2018;126:513-20
240. Yue SJ, Liu J, Wang AT, Meng XT, Yang ZR, Peng C. et al. Berberine alleviates insulin resistance by reducing peripheral branched-chain amino acids. Am J Physiol Endocrinol Metab. 2019;316:E73-E85
241. Affuso F, Mercurio V, Fazio V, Fazio S. Cardiovascular and metabolic effects of Berberine. World J Cardiol. 2010;2:71
242. Zhao L, Cang Z, Sun H, Nie X, Wang N, Lu Y. Berberine improves glucogenesis and lipid metabolism in nonalcoholic fatty liver disease. BMC Endocr Disord. 2017;17:13
243. Wei S, Zhang M, Yu Y, Lan X, Yao F, Yan X. et al. Berberine Attenuates Development of the Hepatic Gluconeogenesis and Lipid Metabolism Disorder in Type 2 Diabetic Mice and in Palmitate-Incubated HepG2 Cells through Suppression of the HNF-4alpha miR122 Pathway. PLoS One. 2016;11:e0152097
244. Cao S, Xu P, Yan J, Liu H, Liu L, Cheng L. et al. Berberrubine and its analog, hydroxypropyl-berberrubine, regulate LDLR and PCSK9 expression via the ERK signal pathway to exert cholesterol-lowering effects in human hepatoma HepG2 cells. J Cell Biochem. 2018
245. Han JL, Lin HL. Intestinal microbiota and type 2 diabetes: from mechanism insights to therapeutic perspective. World J Gastroenterol. 2014;20:17737-45
246. Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. The ISME journal. 2013;7:880
247. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-72
248. Han J, Lin H, Huang W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med Sci Monit. 2011;17:RA164
249. Jhong CH, Riyaphan J, Lin SH, Chia YC, Weng CF. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. Biofactors. 2015;41:242-51
250. Sales PM, Souza PM, Simeoni LA, Magalhães PO, Silveira D. α-Amylase inhibitors: a review of raw material and isolated compounds from plant source. J Pharm Pharm Sci. 2012;15:141-83
251. El-Wahab AEA, Ghareeb DA, Sarhan EE, Abu-Serie MM, El Demellawy MA. In vitro biological assessment of berberis vulgaris and its active constituent, berberine: antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement Altern Med. 2013;13:218
252. Xiao-Ping Y, Chun-Qing S, Ping Y, Ren-Gang M. α-Glucosidase and α-amylase inhibitory activity of common constituents from traditional Chinese medicine used for diabetes mellitus. Chinese Journal of Natural Medicines. 2010;8:349-52
253. Retnakaran R, Zinman B. Type 1 diabetes, hyperglycaemia, and the heart. Lancet. 2008;371:1790-9
254. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N. et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829-41
255. Wang C, Li J, Lv X, Zhang M, Song Y, Chen L. et al. Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur J Pharmacol. 2009;620:131-7
256. Wong SL, Wong WT, Tian XY, Lau CW, Huang Y. Chapter 3 - Prostaglandins in Action: Indispensable Roles of Cyclooxygenase-1 and -2 in Endothelium-Dependent Contractions. Adv Pharmacol. 2010;60:61-83
257. Wang Y, Huang Y, Lam KS, Li Y, Wong WT, Ye H. et al. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc Res. 2009;82:484-92
258. Tao K, Chen J, Wang L. Effects of berberine on the expressions of NRF2 and HO-1 in endothelial cells of diabetic rat. Biomedical Research. 2017;28:3860-64
259. Geng FH, Li GH, Zhang X, Zhang P, Dong MQ, Zhao ZJ. et al. Berberine improves mesenteric artery insulin sensitivity through up-regulating insulin receptor-mediated signalling in diabetic rats. Br J Pharmacol. 2016;173:1569-79
260. Pierelli G, Stanzione R, Forte M, Migliarino S, Perelli M, Volpe M. et al. Uncoupling Protein 2: A Key Player and a Potential Therapeutic Target in Vascular Diseases. Oxid Med Cell Longev. 2017;2017:7348372
261. He Y, Wang N, Shen Y, Zheng Z, Xu X. Inhibition of high glucose-induced apoptosis by uncoupling protein 2 in human umbilical vein endothelial cells. Int J Mol Med. 2014;33:1275-81
262. Wang Q, Zhang M, Liang B, Shirwany N, Zhu Y, Zou M-H. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: the role of uncoupling protein 2. PLoS One. 2011;6:e25436
263. Paul M, Hemshekhar M, Kemparaju K, Girish KS. Berberine mitigates high glucose-potentiated platelet aggregation and apoptosis by modulating aldose reductase and NADPH oxidase activity. Free Radic Biol Med. 2018;130:196-205
264. Sen S, McDonald SP, Coates PT, Bonder CS. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin Sci (Lond). 2011;120:263-83
265. Xu MG, Wang JM, Chen L, Wang Y, Yang Z, Tao J. Berberine-induced upregulation of circulating endothelial progenitor cells is related to nitric oxide production in healthy subjects. Cardiology. 2009;112:279-86
266. Xu MG, Wang JM, Chen L, Wang Y, Yang Z, Tao J. Berberine-induced mobilization of circulating endothelial progenitor cells improves human small artery elasticity. J Hum Hypertens. 2008;22:389-93
267. Spigoni V, Aldigeri R, Antonini M, Micheli MM, Fantuzzi F, Fratter A. et al. Effects of a New Nutraceutical Formulation (Berberine, Red Yeast Rice and Chitosan) on Non-HDL Cholesterol Levels in Individuals with Dyslipidemia: Results from a Randomized, Double Blind, Placebo-Controlled Study. Int J Mol Sci. 2017;18:1498
268. Affuso F, Ruvolo A, Micillo F, Sacca L, Fazio S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr Metab Cardiovasc Dis. 2010;20:656-61
269. Dong S, Zhang S, Chen Z, Zhang R, Tian L, Cheng L. et al. Berberine Could Ameliorate Cardiac Dysfunction via Interfering Myocardial Lipidomic Profiles in the Rat Model of Diabetic Cardiomyopathy. Front Physiol. 2018;9:1042
270. Chang W, Zhang M, Meng Z, Yu Y, Yao F, Hatch GM. et al. Berberine treatment prevents cardiac dysfunction and remodeling through activation of 5'-adenosine monophosphate-activated protein kinase in type 2 diabetic rats and in palmitate-induced hypertrophic H9c2 cells. Eur J Pharmacol. 2015;769:55-63
271. Hang W, He B, Chen J, Xia L, Wen B, Liang T. et al. Berberine Ameliorates High Glucose-Induced Cardiomyocyte Injury via AMPK Signaling Activation to Stimulate Mitochondrial Biogenesis and Restore Autophagic Flux. Front Pharmacol. 2018;9:1121
272. Chang W, Li K, Guan F, Yao F, Yu Y, Zhang M. et al. Berberine Pretreatment Confers Cardioprotection Against Ischemia-Reperfusion Injury in a Rat Model of Type 2 Diabetes. J Cardiovasc Pharmacol Ther. 2016;21:486-94
273. Li G, Xing W, Zhang M, Geng FH, Yang H, Zhang H. et al. Anti-fibrotic cardioprotection of berberine via down-regulating myocardial IGF-1 receptor-regulated MMP-2/9 expression in diabetic rats. Am J Physiol Heart Circ Physiol. 2018;315:H802-h13
274. Lu K, Shen Y, He J, Liu G, Song W. [Berberine inhibits cardiac fibrosis of diabetic rats]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016;32:1352-5
275. Wang LH, Li XL, Li Q, Fu Y, Yu HJ, Sun YQ. et al. Berberine alleviates ischemic arrhythmias via recovering depressed I(to) and I(Ca) currents in diabetic rats. Phytomedicine. 2012;19:206-10
276. Wang L-h, Yu C-h, Fu Y, Li Q, Sun Y-q. Berberine elicits anti-arrhythmic effects via IK1/Kir2.1 in the rat type 2 diabetic myocardial infarction model. Phytother Res. 2011;25:33-7
277. Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184-223
278. Wang FF, Wu Y, Zhu YH, Ding T, Batterham RL, Qu F. et al. Pharmacologic therapy to induce weight loss in women who have obesity/overweight with polycystic ovary syndrome: a systematic review and network meta-analysis. Obes Rev. 2018;19:1424-45
279. Hu Y, Davies GE. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia. 2010;81:358-66
280. Ma X, Egawa T, Kimura H, Karaike K, Masuda S, Iwanaka N. et al. Berberine-induced activation of 5'-adenosine monophosphate-activated protein kinase and glucose transport in rat skeletal muscles. Metabolism. 2010;59:1619-27
281. Kulkarni SK, Dhir A. On the mechanism of antidepressant-like action of berberine chloride. Eur J Pharmacol. 2008;589:163-72
282. Sun H, Wang N, Cang Z, Zhu C, Zhao L, Nie X. et al. Modulation of Microbiota-Gut-Brain Axis by Berberine Resulting in Improved Metabolic Status in High-Fat Diet-Fed Rats. Obes Facts. 2016;9:365-78
283. Yin J, Ye J, Jia W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharmaceutica Sinica B. 2012;2:327-34
284. Yu Y, Liu L, Wang X, Liu X, Liu X, Xie L. et al. Modulation of glucagon-like peptide-1 release by berberine: in vivo and in vitro studies. Biochem Pharmacol. 2010;79:1000-6
285. Sun Y, Jin C, Zhang X, Jia W, Le J, Ye J. Restoration of GLP-1 secretion by Berberine is associated with protection of colon enterocytes from mitochondrial overheating in diet-induced obese mice. Nutr Diabetes. 2018;8:53
286. Sommer F, Adam N, Johansson ME, Xia L, Hansson GC, Backhed F. Altered mucus glycosylation in core 1 O-glycan-deficient mice affects microbiota composition and intestinal architecture. PLoS One. 2014;9:e85254
287. Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979-84
288. Xie W, Gu D, Li J, Cui K, Zhang Y. Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PLoS One. 2011;6:e24520
289. Chae SH, Jeong IH, Choi DH, Oh JW, Ahn YJ. Growth-inhibiting effects of Coptis japonica root-derived isoquinoline alkaloids on human intestinal bacteria. J Agric Food Chem. 1999;47:934-8
290. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K. et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-23
291. Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656-65
292. Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519-23
293. Kim WS, Lee YS, Cha SH, Jeong HW, Choe SS, Lee MR. et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am J Physiol Endocrinol Metab. 2009;296:E812-9
294. Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B. et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376-88
295. Yuebin G, Yan Z, Rui L, Wei C, Yang L, Guoxun C. Berberine regulated Gck, G6pc, Pck1 and Srebp-1c expression and activated AMP-activated protein kinase in primary rat hepatocytes. Int J Biol Sci. 2011;7:673-84
296. Jang J, Jung Y, Seo S, Kim SM, Shim Y, Cho S. et al. Berberine activates AMPK to suppress proteolytic processing, nuclear translocation and target DNA binding of SREBP-1c in 3T3-L1 adipocytes. Mol Med Rep. 2017;15:4139-47
297. Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y. et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256-64
298. Feng WW, Kuang SY, Tu C, Ma ZJ, Pang JY, Wang YH. et al. Natural products berberine and curcumin exhibited better ameliorative effects on rats with non-alcohol fatty liver disease than lovastatin. Biomed Pharmacother. 2018;99:325-33
299. Li Y, Zhao X, Feng X, Liu X, Deng C, Hu CH. Berberine Alleviates Olanzapine-Induced Adipogenesis via the AMPKalpha-SREBP Pathway in 3T3-L1 Cells. Int J Mol Sci. 2016;17:1865
300. Ide T, Shimano H, Yahagi N, Matsuzaka T, Nakakuki M, Yamamoto T. et al. SREBPs suppress IRS-2-mediated insulin signalling in the liver. Nat Cell Biol. 2004;6:351-7
301. Sun H, Liu Q, Hu H, Jiang Y, Shao W, Wang Q. et al. Berberine ameliorates blockade of autophagic flux in the liver by regulating cholesterol metabolism and inhibiting COX2-prostaglandin synthesis. Cell Death Dis. 2018;9:824
302. Xing LJ, Zhang L, Liu T, Hua YQ, Zheng PY, Ji G. Berberine reducing insulin resistance by up-regulating IRS-2 mRNA expression in nonalcoholic fatty liver disease (NAFLD) rat liver. Eur J Pharmacol. 2011;668:467-71
303. Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107-10
304. Xia X, Yan J, Shen Y, Tang K, Yin J, Zhang Y. et al. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS One. 2011;6:e16556
305. Liu X, Li G, Zhu H, Huang L, Liu Y, Ma C. et al. Beneficial effect of berberine on hepatic insulin resistance in diabetic hamsters possibly involves in SREBPs, LXRalpha and PPARalpha transcriptional programs. Endocr J. 2010;57:881-93
306. Chang X, Yan H, Fei J, Jiang M, Zhu H, Lu D. et al. Berberine reduces methylation of the MTTP promoter and alleviates fatty liver induced by a high-fat diet in rats. J Lipid Res. 2010;51:2504-15
307. Han L, Yang QH, Zhang YP, Yan HZ, Zhu XF, Gong XW. et al. [Intervention of berberine on lipid deposition in liver cells of non-alcoholic fatty liver disease rats induced by high fat diet]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35:314-9
308. Yang QH, Hu SP, Zhang YP, Xie WN, Li N, Ji GY. et al. Effect of berberine on expressions of uncoupling protein-2 mRNA and protein in hepatic tissue of non-alcoholic fatty liver disease in rats. Chin J Integr Med. 2011;17:205-11
309. Lu KL, Zhang DD, Wang LN, Xu WN, Liu WB. Molecular characterization of carnitine palmitoyltransferase IA in Megalobrama amblycephala and effects on its expression of feeding status and dietary lipid and berberine. Comp Biochem Physiol B Biochem Mol Biol. 2016;191:20-5
310. Wang H, Shi L, Yin H, Zhou Q. [Study on effect of berberine on modulating lipid and CPT I A gene expression]. Zhongguo Zhong Yao Za Zhi. 2011;36:2715-8
311. Sun Y, Xia M, Yan H, Han Y, Zhang F, Hu Z. et al. Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21. Br J Pharmacol. 2018;175:374-87
312. Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327-38
313. Liss KH, Finck BN. PPARs and nonalcoholic fatty liver disease. Biochimie. 2017;136:65-74
314. Zhao J, Wang Y, Wu X, Tong P, Yue Y, Gao S. et al. Inhibition of CCL19 benefits nonalcoholic fatty liver disease by inhibiting TLR4/NFkappaBp65 signaling. Mol Med Rep. 2018;18:4635-42
315. Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7:251-64
316. Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371-9
317. Guo T, Woo SL, Guo X, Li H, Zheng J, Botchlett R. et al. Berberine Ameliorates Hepatic Steatosis and Suppresses Liver and Adipose Tissue Inflammation in Mice with Diet-induced Obesity. Sci Rep. 2016;6:22612
318. Feng W, Wang H, Zhang P, Gao C, Tao J, Ge Z. et al. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim Biophys Acta Gen Subj. 2017;1861:1801
319. Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M. et al. Intestinal permeability - a new target for disease prevention and therapy. Bmc Gastroenterology. 2014;14:189
320. Li D, Zheng J, Hu Y, Hou H, Hao S, Liu N. et al. Amelioration of Intestinal Barrier Dysfunction by Berberine in the Treatment of Nonalcoholic Fatty Liver Disease in Rats. Pharmacogn Mag. 2017;13:677-82
321. Abel ED, O'Shea KM, Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arterioscler Thromb Vasc Biol. 2012;32:2068-76
322. Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230-41
323. Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 2008;57:3222-30
324. Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M. et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120-7
325. Zhang H, Wei J, Xue R, Wu JD, Zhao W, Wang ZZ. et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59:285-92
326. Jiang D, Wang D, Zhuang X, Wang Z, Ni Y, Chen S. et al. Berberine increases adipose triglyceride lipase in 3T3-L1 adipocytes through the AMPK pathway. Lipids Health Dis. 2016;15:214
327. Evans R, Barish G, Wang Y. PPARs and the complex journey to obesity. Nat Med. 2004;10:355-61
328. Huang C, Zhang Y, Gong Z, Sheng X, Li Z, Zhang W. et al. Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARgamma pathway. Biochem Biophys Res Commun. 2006;348:571-8
329. Abidi P, Zhou Y, Jiang J, Liu J. Extracellular signal-regulated kinase-dependent stabilization of hepatic low-density lipoprotein receptor mRNA by herbal medicine berberine. Arterioscler Thromb Vasc Biol. 2005;25:2170-6
330. Cameron J, Ranheim T, Kulseth MA, Leren TP, Berge KE. Berberine decreases PCSK9 expression in HepG2 cells. Atherosclerosis. 2008;201:266-73
331. Wang H, Zhu C, Ying Y, Luo L, Huang D, Luo Z. Metformin and berberine, two versatile drugs in treatment of common metabolic diseases. Oncotarget. 2018;9:10135-46
332. Li H, Dong B, Park SW, Lee HS, Chen W, Liu J. Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J Biol Chem. 2009;284:28885-95
333. Turner N, Li JY, Gosby A, To SW, Cheng Z, Miyoshi H. et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414-8
334. Xu M, Xiao Y, Yin J, Hou W, Yu X, Shen L. et al. Berberine promotes glucose consumption independently of AMP-activated protein kinase activation. PLoS ONE. 2014;9:e103702
335. Xu Z, Feng W, Shen Q, Yu N, Yu K, Wang S. et al. Rhizoma Coptidis and Berberine as a Natural Drug to Combat Aging and Aging-Related Diseases via Anti-Oxidation and AMPK Activation. Aging Dis. 2017;8:760-77
336. Towler M, Hardie D. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328-41
337. Zhao W, Ge H, Liu K, Chen X, Zhang J, Liu B. Nandinine, a Derivative of Berberine, Inhibits Inflammation and Reduces Insulin Resistance in Adipocytes via Regulation of AMP-Kinase Activity. Planta Med. 2017;83:203-9
338. Chen C, Zhang Y, Huang C. Berberine inhibits PTP1B activity and mimics insulin action. Biochem Biophys Res Commun. 2010;397:543-7
339. Fusi J, Bianchi S, Daniele S, Pellegrini S, Martini C, Galetta F. et al. An in vitro comparative study of the antioxidant activity and SIRT1 modulation of natural compounds. Biomed Pharmacother. 2018;101:805
340. Chen DL, Yang KY. Berberine Alleviates Oxidative Stress in Islets of Diabetic Mice by Inhibiting miR-106b Expression and Up-Regulating SIRT1. J Cell Biochem. 2017;118:4349-57
341. Coelho AR, Martins TR, Couto R, Deus C, Pereira CV, Simoes RF. et al. Berberine-induced cardioprotection and Sirt3 modulation in doxorubicin-treated H9c2 cardiomyoblasts. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2904-23
342. Li HY, Wang XC, Xu YM, Luo NC, Luo S, Hao XY. et al. Berberine Improves Diabetic Encephalopathy Through the SIRT1/ER Stress Pathway in db/db Mice. Rejuvenation Res. 2018;21:200-9
343. Zhang X, Zhao Y, Xu J, Xue Z, Zhang M, Pang X. et al. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405
344. Qin C, Zhang H, Zhao L, Zeng M, Huang W, Fu G. et al. Microbiota transplantation reveals beneficial impact of berberine on hepatotoxicity by improving gut homeostasis. Sci China Life Sci. 2018;61:1537-44
345. Wang Y, Shou JW, Li XY, Zhao ZX, Fu J, He CY. et al. Berberine-induced bioactive metabolites of the gut microbiota improve energy metabolism. Metabolism. 2017;70:72-84
346. Li M, Shu X, Xu H, Zhang C, Yang L, Zhang L. et al. Integrative analysis of metabolome and gut microbiota in diet-induced hyperlipidemic rats treated with berberine compounds. J Transl Med. 2016;14:237
347. Feng R, Shou JW, Zhao ZX, He CY, Ma C, Huang M. et al. Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci Rep. 2015;5:12155
348. Zhang HY, Piao XS, Zhang Q, Li P, Yi JQ, Liu JD. et al. The effects of Forsythia suspensa extract and berberine on growth performance, immunity, antioxidant activities, and intestinal microbiota in broilers under high stocking density. Poult Sci. 2013;92:1981-8
349. Cao Y, Pan Q, Cai W, Shen F, Chen GY, Xu LM. et al. Modulation of Gut Microbiota by Berberine Improves Steatohepatitis in High-Fat Diet-Fed BALB/C Mice. Arch Iran Med. 2016;19:197-203
350. Sun R, Yang N, Kong B, Cao B, Feng D, Yu X. et al. Orally Administered Berberine Modulates Hepatic Lipid Metabolism by Altering Microbial Bile Acid Metabolism and the Intestinal FXR Signaling Pathway. Mol Pharmacol. 2017;91:110-22
351. Chang X, Wang Z, Zhang J, Yan H, Bian H, Xia M. et al. Lipid profiling of the therapeutic effects of berberine in patients with nonalcoholic fatty liver disease. J Transl Med. 2016;14:266
352. Cicero AF, Rovati LC, Setnikar I. Eulipidemic effects of berberine administered alone or in combination with other natural cholesterol-lowering agents. A single-blind clinical investigation. Arzneimittelforschung. 2007;57:26-30
353. Derosa G, D'Angelo A, Bonaventura A, Bianchi L, Romano D, Maffioli P. Effects of berberine on lipid profile in subjects with low cardiovascular risk. Expert Opin Biol Ther. 2013;13:475-82
354. Di Pierro F, Putignano P, Villanova N, Montesi L, Moscatiello S, Marchesini G. Preliminary study about the possible glycemic clinical advantage in using a fixed combination of Berberis aristata and Silybum marianum standardized extracts versus only Berberis aristata in patients with type 2 diabetes. Clin Pharmacol. 2013;5:167-74
355. Kong WJ, Wei J, Zuo ZY, Wang YM, Song DQ, You XF. et al. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism. 2008;57:1029-37
356. Meng S, Wang LS, Huang ZQ, Zhou Q, Sun YG, Cao JT. et al. Berberine ameliorates inflammation in patients with acute coronary syndrome following percutaneous coronary intervention. Clin Exp Pharmacol Physiol. 2012;39:406-11
357. Perez-Rubio KG, Gonzalez-Ortiz M, Martinez-Abundis E, Robles-Cervantes JA, Espinel-Bermudez MC. Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord. 2013;11:366-9
358. Wei W, Zhao H, Wang A, Sui M, Liang K, Deng H. et al. A clinical study on the short-term effect of berberine in comparison to metformin on the metabolic characteristics of women with polycystic ovary syndrome. Eur J Endocrinol. 2012;166:99-105
359. Yan HM, Xia MF, Wang Y, Chang XX, Yao XZ, Rao SX. et al. Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease. PLoS One. 2015;10:e0134172
360. Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N. et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559-65
361. Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712-7
362. Hu Y, Ehli EA, Kittelsrud J, Ronan PJ, Munger K, Downey T. et al. Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine. 2012;19:861-7
363. Gu Y, Zhang Y, Shi X, Li X, Hong J, Chen J. et al. Effect of traditional Chinese medicine berberine on type 2 diabetes based on comprehensive metabonomics. Talanta. 2010;81:766-72
364. Zeng XH, Zeng XJ, Li YY. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;92:173-6
365. Xiao HB, Sun ZL, Zhang HB, Zhang DS. Berberine inhibits dyslipidemia in C57BL/6 mice with lipopolysaccharide induced inflammation. Pharmacol Rep. 2012;64:889-95
366. Dong B, Li H, Singh AB, Cao A, Liu J. Inhibition of PCSK9 transcription by berberine involves down-regulation of hepatic HNF1alpha protein expression through the ubiquitin-proteasome degradation pathway. J Biol Chem. 2015;290:4047-58
367. Marin-Neto JA, Maciel BC, Secches AL, Gallo Junior L. Cardiovascular effects of berberine in patients with severe congestive heart failure. Clin Cardiol. 1988;11:253-60
368. Zeng X, Zeng X. Relationship between the clinical effects of berberine on severe congestive heart failure and its concentration in plasma studied by HPLC. Biomed Chromatogr. 1999;13:442-4
369. Pirro M, Francisci D, Bianconi V, Schiaroli E, Mannarino MR, Barsotti F. et al. NUtraceutical TReatment for hYpercholesterolemia in HIV-infected patients: The NU-TRY(HIV) randomized cross-over trial. Atherosclerosis. 2018;280:51-7
370. Xiao D, Liu Z, Zhang S, Zhou M, He F, Zou M. et al. Berberine Derivatives with Different Pharmacological Activities via Structural Modifications. Mini Rev Med Chem. 2018;18:1424-41
371. Shan YQ, Ren G, Wang YX, Pang J, Zhao ZY, Yao J. et al. Berberine analogue IMB-Y53 improves glucose-lowering efficacy by averting cellular efflux especially P-glycoprotein efflux. Metabolism. 2013;62:446-56
372. Maeng HJ, Yoo HJ, Kim IW, Song IS, Chung SJ, Shim CK. P-glycoprotein-mediated transport of berberine across Caco-2 cell monolayers. J Pharm Sci. 2002;91:2614-21
373. Cheng Z, Chen AF, Wu F, Sheng L, Zhang HK, Gu M. et al. 8,8-Dimethyldihydroberberine with improved bioavailability and oral efficacy on obese and diabetic mouse models. Bioorg Med Chem. 2010;18:5915-24
374. Li R, Wu J, He Y, Hai L, Wu Y. Synthesis and in vitro evaluation of 12-(substituted aminomethyl) berberrubine derivatives as anti-diabetics. Bioorg Med Chem Lett. 2014;24:1762-5
375. Wang JR, Tanaka T, Zhang H, Kouno I, Jiang ZH. Formation and conformation of baicalin-berberine and wogonoside-berberine complexes. Chem Pharm Bull (Tokyo). 2012;60:706-11
376. Yan L, Xiao Y, Rong X, Ying G, Qiu Z, Zhang Z. et al. Design, synthesis and biological evaluation of a hybrid compound berberine and magnolol for improvement of glucose and lipid metabolism. RSC Adv. 2016;6:81924-31
377. Liu JC, Chan P, Chen YJ, Tomlinson B, Hong SH, Cheng JT. The antihypertensive effect of the berberine derivative 6-protoberberine in spontaneously hypertensive rats. Pharmacology. 1999;59:283-9
378. Ko FN, Chang YL, Chen CM, Teng CM. (+/-)-Govadine and (+/-)-THP, two tetrahydroprotoberberine alkaloids, as selective alpha 1-adrenoceptor antagonists in vascular smooth muscle cells. J Pharm Pharmacol. 1996;48:629-34
379. Al-Howiriny TA, Zemaitis MA, Gao CY, Hadden CE, Martin GE, Lin FT. et al. Thalibealine, a novel tetrahydroprotoberberine-aporphine dimeric alkaloid from Thalictrum wangii. J Nat Prod. 2001;64:819-22
380. Qian W, Lu W, Sun H, Li Z, Zhu L, Zhao R. et al. Design, synthesis, and pharmacological evaluation of novel tetrahydroprotoberberine derivatives: selective inhibitors of dopamine D(1) receptor. Bioorg Med Chem. 2012;20:4862-71
381. Zhang TT, Cui B, Dai DZ, Su W. CPU 86017, p-chlorobenzyltetrahydroberberine chloride, attenuates monocrotaline- induced pulmonary hypertension by suppressing endothelin pathway. Acta Pharmacol Sin. 2005;26:1309-16
382. Qi MY, Feng Y, Dai DZ, Li N, Cheng YS, Dai Y. CPU86017, a berberine derivative, attenuates cardiac failure through normalizing calcium leakage and downregulated phospholamban and exerting antioxidant activity. Acta Pharmacol Sin. 2010;31:165-74
383. Gao J, Tang YQ, Dai DZ, Cheng YS, Zhang GL, Zhang C. et al. Raisanberine protected pulmonary arterial rings and cardiac myocytes of rats against hypoxia injury by suppressing NADPH oxidase and calcium influx. Acta Pharmacol Sin. 2012;33:625-34
384. Zhang GL, Dai DZ, Zhang C, Dai Y. Apocynin and raisanberine alleviate intermittent hypoxia induced abnormal StAR and 3beta-HSD and low testosterone by suppressing endoplasmic reticulum stress and activated p66Shc in rat testes. Reprod Toxicol. 2013;36:60-70
385. Li CL, Tan LH, Wang YF, Luo CD, Chen HB, Lu Q. et al. Comparison of anti-inflammatory effects of berberine, and its natural oxidative and reduced derivatives from Rhizoma Coptidis in vitro and in vivo. Phytomedicine. 2019;52:272-83
386. Hermann R, von Richter O. Clinical evidence of herbal drugs as perpetrators of pharmacokinetic drug interactions. Planta Med. 2012;78:1458-77
387. Guo Y, Pope C, Cheng X, Zhou H, Klaassen CD. Dose-response of berberine on hepatic cytochromes P450 mRNA expression and activities in mice. J Ethnopharmacol. 2011;138:111-8
388. Guo Y, Chen Y, Tan ZR, Klaassen CD, Zhou HH. Repeated administration of berberine inhibits cytochromes P450 in humans. Eur J Clin Pharmacol. 2012;68:213-7
389. Mirhadi E, Rezaee M, Malaekeh-Nikouei B. Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed Pharmacother. 2018;104:465-73
390. Banach M, Patti AM, Giglio RV, Cicero AFG, Atanasov AG, Bajraktari G. et al. The Role of Nutraceuticals in Statin Intolerant Patients. J Am Coll Cardiol. 2018;72:96-118
391. Patti AM, Al-Rasadi K, Giglio RV, Nikolic D, Mannina C, Castellino G. et al. Natural approaches in metabolic syndrome management. Arch Med Sci. 2018;14:422-41
392. Zhang H, Wang X, Wang T, Chen K, Wang H, Jia Q. et al. Enhancement of Berberine Hypoglycemic Activity by Oligomeric Proanthocyanidins. Molecules. 2018:23
393. Zhu X, Yang J, Zhu W, Yin X, Yang B, Wei Y. et al. Combination of Berberine with Resveratrol Improves the Lipid-Lowering Efficacy. Int J Mol Sci. 2018:19
Author contact
![]() Corresponding authors: Ai-Zong Shen (sazljlcom); Suowen Xu (suowen.xucom); Seyed Mohammad Nabavi (Nabavi208com)
Corresponding authors: Ai-Zong Shen (sazljlcom); Suowen Xu (suowen.xucom); Seyed Mohammad Nabavi (Nabavi208com)
 Global reach, higher impact
Global reach, higher impact