13.3
Impact Factor
Theranostics 2015; 5(10):1068-1082. doi:10.7150/thno.11517 This issue Cite
Research Paper
Photoluminescent Mesoporous Silicon Nanoparticles with siCCR2 Improve the Effects of Mesenchymal Stromal Cell Transplantation after Acute Myocardial Infarction
1. Department of Cardiology, ZhongDa Hospital affiliated with Southeast University, China;
2. Department of Cardiology, the Second Hospital affiliated with Southeast University, China;
3. State Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, Southeast University, China.
*These authors contributed equally to this work.
Abstract
Background: Despite the benefits of mesenchymal stromal cell (MSC) transplantation in cardiac tissue, detailed in vivo observations have shown that MSCs only survive for a brief period after transplantation due to harsh microenvironmental conditions, including ischemia, inflammation and anoikis, in the infarcted myocardium. Thus, new strategies are needed to enhance MSC survival and inhibit cardiac remodeling. Studies have now demonstrated that chemokine [C-C motif] ligand 2 (CCL2) and its cognate receptor C-C chemokine receptor 2 (CCR2) promote excessive Ly6Chigh inflammatory monocyte infiltration at the infarct in response to ischemic myocardial injury. Therefore, decreasing the activities of these monocytes immediately after acute myocardial infarction (AMI) could be beneficial for AMI patients. Objectives: This study tested the hypothesis that therapeutic siRNA-loaded photoluminescent mesoporous silicon nanoparticles (PMSNs) targeting CCR2 expression in Ly6Chigh inflammatory monocytes decrease the accumulation of these cells in the infarct, improve the efficacy of MSC transplantation and attenuate myocardial remodeling.
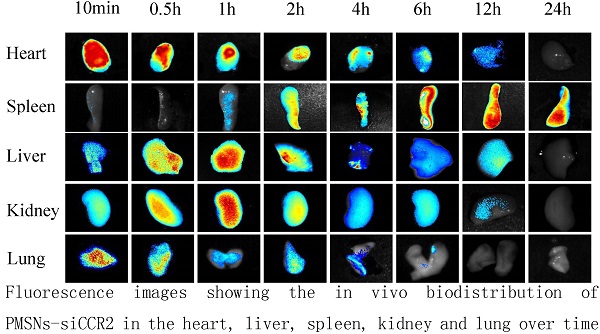
Methods: PMSNs carrying therapeutic siCCR2 were first synthesized without the inclusion of fluorescent materials or dyes. After AMI BALB/c mice were established, 105 5-ethynyl-2'- deoxyuridine (EdU)-labeled MSCs suspended in 100 µl of phosphate buffered saline (PBS) were injected into the border zone of the infarct of each mouse. PMSNs-siCCR2 (25 µg/g) were also intravenously injected via the tail vein immediately following AMI induction. Control mice were injected with an equal amount of PMSNs without siCCR2. PMSNs-siCCR2 were examined in vivo using near-infrared imaging technology. The therapeutic effects of PMSNs-siCCR2 for MSC transplantation were determined at the mRNA, protein and functional levels.
Results: PMSNs-siCCR2 circulated freely in vivo and were cleared in a relatively short period of time (t½=37 min) with no evidence of toxicity. The therapeutic PMSNs-siCCR2 showed higher levels of cellular accumulation in Ly6Chigh monocytes in the spleen and more efficient degradation of CCR2 compared with the control (8.04%±2.17% vs. 20.02%±4.55%, p<0.001). Subsequently, the PMSNs-siCCR2 decreased the accumulation of CD11b-positive monocytes at the infarct (49.3%±17.34% vs. 61.32%±22.43%, p<0.001) on day 1. Increased survival of transplanted MSCs (13±3/mm2 vs. 4±1/mm2, p<0.001) and significantly decreased TdT-mediated dUTP nick end labeling (TUNEL)+ cardiac myocytes (17.44%±6.26% vs. 39.49%±13.28%, p<0.001) were then identified in the infarct zone three days after AMI induction in the PMSNs-siCCR2 group. Three weeks after MSC injection, significant increases were observed in the vascular density (235.5±39.6/mm2 vs. 147.4±20.3/mm2, p<0.001) and the cardiac myosin-positive area (21.7%±8.4% vs. 13.2%±4.4%, p<0.001) of the infarct border zone. In addition, significant amelioration of left ventricular (LV) remodeling (thickness of the LV posterior walls) (0.84±0.11 mm vs. 0.61±0.08 mm, p<0.001) was also observed at the same time compared with the control group.
Conclusions: PMSNs-siCCR2-mediated CCR2 gene silencing in Ly6Chigh monocytes improved the effectiveness of MSC transplantation and selectively ameliorated myocardial remodeling after AMI. These results suggest that PMSNs-siCCR2 could potentially be used to develop an anti-inflammatory therapy for post-AMI MSC transplantation.
Keywords: AMI, PMSNs, siCCR2, MSCs, Ly6Chigh monocytes
 Global reach, higher impact
Global reach, higher impact