13.3
Impact Factor
Theranostics 2025; 15(11):5440-5480. doi:10.7150/thno.112475 This issue Cite
Review
Nano drug delivery systems for advanced immune checkpoint blockade therapy
1. Department of Radiology, Huaxi MR Research Center (HMRRC), Institution of Radiology and Medical Imaging, Rehabilitation Therapy, Breast Center, Institute of Breast Health Medicine, Frontiers Science Center for Disease-Related Molecular Network, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu 610041, China.
2. West China School of Medicine, Chengdu 610041, China.
3. Functional and molecular imaging Key Laboratory of Sichuan Province, Key Laboratory of Transplant Engineering and Immunology, NHC, and Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, China.
4. Xiamen Key Lab of Psychoradiology and Neuromodulation, Department of Radiology, West China Xiamen Hospital of Sichuan University, Xiamen 361021, China.
#These authors contributed equally: Chenqi Guo, and Ling Lin.
Received 2025-2-19; Accepted 2025-4-4; Published 2025-4-13
Abstract
Immune checkpoint inhibitors (ICIs) have been widely utilized in the first-line therapy of various types of cancer. However, immune-related adverse events (irAEs) and resistance to ICIs remain intractable challenges for immune checkpoint blockade (ICB) therapy during clinic treatment. Nano drug delivery systems (NDDSs) have shown promising potential to improve anticancer efficacy and reduce side effects of small molecular drugs. The combination of nanotechnology and ICB provides new opportunities to overcome the challenges of immunotherapy. Nanoplatforms have been employed for direct delivery of ICIs, and they are preferred vehicles for combination therapy of ICIs and other therapeutic agents. In this review, the strategies of using NDDSs for advancing ICB therapy in recent years are surveyed, emphasizing the employment of NDDSs for combination treatment by ICIs and other agents to manipulate antitumor immunity. Analysis of current strategies for applying NDDSs for ICB leads to future research directions and development trends.
1. Introduction
Immunotherapy is the most attractive cancer treatment method. Especially, the emergence of immune checkpoint blockade (ICB) therapy has brought unprecedented benefits to cancer patients. Immunosuppressive signals in tumors can be manipulated through immune checkpoint pathways, thereby impairing antitumor immune responses and leading to immune escape. Cytotoxic T lymphocyte-associated protein 4 (CTLA-4) is the first discovered immune checkpoint (ICP), while programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) are the most studied ICPs [1-3]. Other ICPs, such as lymphocyte activation gene-3 (LAG-3), T cell immunoglobulin and mucin-domain containing-3 (TIM-3), T cell immunoglobin and ITIM domain (TIGIT), V-domain Ig suppressor of T cell activation (VISTA), have been revealed in recent years [4-6]. So far, immune checkpoint inhibitors (ICIs) approved by FDA are monoclonal antibodies (mAbs), including anti-PD-1 mAbs (aPD-1) (nivolumab, pembrolizumab, cemiplimab, dostarlimab and retifanlimab), anti-PD-L1 mAbs (aPD-L1) (durvalumab, atezolizumab and avelumab), anti-CTLA-4 mAbs (aCTLA-4) (ipilimumab and tremelimumab), and anti-LAG-3 mAb (relatlimab), and these ICIs block the interaction between ICPs and their ligands [7]. In addition, nucleic acids (such as small interfering RNA (siRNA), short hairpin RNA (shRNA), clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated proteins (CRISPR/Cas), plasmid DNA, and aptamers), peptides (such as DPPA-1, TPP-1, OPBP-1, and AUNP-12), small molecular drugs (such as CA-170, BMS-202, JQ1, and metformin), and DNAzyme have been used as ICIs in clinical and preclinical studies [8-10]. These drugs acting as ICIs could be through the mechanism of inhibiting the expression, promoting the degradation of ICPs, or blocking their interaction with their ligands [11].
ICB has been employed to treat some types of cancer in the clinic. However, immune-related adverse events (irAEs) and resistance to ICB are two major challenges of ICIs for their clinical application [7, 12, 13]. According to variations in the kinds and stages of cancer and the targets of ICIs, distinct irAEs, including more than 70 diverse pathologies, are found in all organs of the entire body, such as leukoderma, colitis, hypophysitis, pneumonia, arthritis, and myocarditis [14, 15]. These irAEs can be graded from 1 to 5 based on the severity according to the Common Terminology Criteria for Adverse Events (CTCAE) [16]. Low-grade irAEs occur in more than 90% of patients, while more severe irAEs, some of which may be potentially fatal, have been observed in 20%-60% of patients [17]. Overactivation of the immune system and indiscriminate attack of T cells on both tumor and normal tissues are two predominant causes of irAEs [18-20]. Stronger side effects are often ascribed to more effective activation of the immune system. Thus, irAEs are correlated with positive responses to ICIs [17]. The scope of the immune activity can be broadened due to the widespread of ICIs in peripheral tissues outside of tumors. Therefore, blocking drug diffusion into peripheral tissues and elevating the level of ICI accumulation within tumors are crucial to relieving irAEs for ICB therapy. Meanwhile, ICI resistance, including primary resistance and acquired resistance, is another challenge for ICB. The degree of ICI resistance in diverse types of cancer is distinctly different. The response rate to single-agent aPD-1 ranges from about 40% to 70% in some types of cancer (such as melanoma, Merkel cells, Hodgkin's lymphoma), compared to only 10%-25% in other indications. Among those patients who initially respond to ICIs, a large proportion of them experience disease progression, which is considered as acquired resistance. The occurrence rate of acquired resistance varies in different types of cancer, ranging from approximately 10%-70% [21]. The precise mechanisms underlying ICI resistance remain to be fully elucidated. However, it is known that complex interactions among tumor cells, the tumor microenvironment (TME), and host factors contribute to ICI resistance. These include the loss or reduction of tumor immunogenicity, dysfunction of antigen presenting cells (APCs), blockade of the antigen presentation process, metabolic adaptability of tumor cells, and excessive infiltration of immunosuppressive cells [17]. Harnessing these interactions could improve the response rate and therapeutic efficacy of ICIs.
Nanotechnology has been proven to improve the physiochemical properties of therapeutic drugs. Nano drug delivery systems (NDDSs) based on nanomaterials, including nanoparticles, liposomes, micelles, and vesicles, have been utilized to incorporate therapeutic drugs to formulate nanomedicines for treating various diseases in the past few decades. Compared with therapeutic drugs without NDDSs, nanomedicines have remarkably bolstered the efficacy and compliance of antitumor agents by increasing their physic-chemical stability, improving their pharmacokinetic properties, enhancing their target tissue accumulation, and regulating their release behaviors [22-24]. An array of nanomedicines have been approved for clinical application [25]. In addition, many of them are in preclinical and clinical trials, demonstrating promising application prospects of nanomedicines. In the field of ICB therapy, rationally designed drug delivery systems are crucial to maximize the therapeutic efficacy of multifarious ICIs since NDDSs can directly optimize the physiochemical properties of ICIs, and they can be used for combination treatment to overcome ICI resistance [26, 27].
In this review, we survey recent strategies for enhancing the therapeutic effect of ICIs through NDDSs (Figure 1). NDDSs are employed to deliver ICIs to specifically reach target cells or improve their accumulation at the tumor site; alternatively, NDDSs are harnessed to accommodate multiple therapeutic drugs for combination therapy. We classify these nanomedicines based on their intrinsic therapeutic mechanisms. Design strategies for these nanomedicines to improve ICI efficacy and mitigate irAEs and resistance are elaborated. Moreover, future trends and challenges in developing nanotechnology-enabled ICB therapy are discussed.
2. NDDSs for different types of ICIs
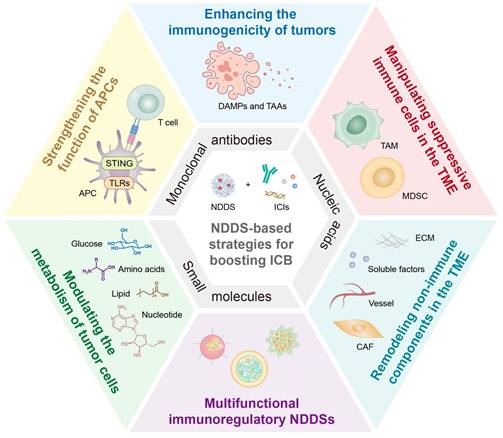
NDDSs have been developed for targeted delivery of various ICIs, most of which are devoted to PD-1/PD-L1 inhibitors. The PD-1/PD-L1 axis is involved in inhibiting the function of cytotoxic T cells. PD-1 is one of the co-inhibitory receptors of the CD28 superfamily, and it can be induced by an inflammatory response in T cells, especially active cytotoxic T lymphocytes (CTLs). PD-L1, an immune checkpoint protein expressed on the many normal tissue cell and tumor cell surfaces, binds to PD-1 on active T cells, thereby preventing cell recognition by CTLs, diminishing immune responses, and inhibiting CTL activities, including cytokine secretion, lymphocyte proliferation, and activation. The interaction between PD1 and PD-L1 leads to the immune evasion of tumor cells to T cells [28, 29]. ICIs could restore T-cell activities by blocking the interaction between PD-1 and PD-L1, thereby enhancing their cytotoxic effects on tumor cells [30, 31]. Apart from blocking interactions between immune checkpoints, the mechanisms of these ICIs have been revealed, primarily including inhibiting transcription and translation of checkpoint proteins, promoting their degradation, and inducing functional impairment (Figure 2). Different types of ICIs, including antibodies, nucleic acids, and small molecule inhibitors, can be specifically delivered to tumor tissues via NDDS to enhance their antitumor efficacy. NDDSs have also been designed for dual ICI delivery, for example, simultaneous delivery of PD-1/PD-L1 and CTLA-4 inhibitors. The examples will be discussed in the Section on antibodies-related NDDSs.
NDDS-based strategies for boosting ICB therapeutic effects. ICIs include monoclonal antibodies, nucleic acids, and small molecules. The combination therapy strategies include enhancing the immunogenicity of tumors, strengthening the function of APCs, manipulating suppressive immune cells in the TME, modulating the metabolism of tumor cells, remodeling non-immune components in the TME, and employing multifunctional immunoregulatory NDDSs. NDDS, nano drug delivery system; ICB, immune checkpoint blockade; ICIs, immune checkpoint inhibitors; APCs, antigen presenting cells; TME, tumor microenvironment.
2.1. Antibodies-related NDDSs
2.1.1. aPD-1 and aPD-L1
Monoclonal antibodies are the earliest developed ICIs, and they have been widely used in clinical practice. They function by disrupting the interaction between the antibody and its ligand. Monoclonal antibodies as large-molecule drugs exhibit inherent limitations, such as poor permeability and suboptimal tumor targeting, resulting in "on-target but off-tumor" effects with systemic administration of aPD-L1 antibodies. This severely hampers therapeutic efficiency and causes irAEs. In order to address these challenges, NDDSs have been developed to improve their in vivo distribution, enhance tumor targeting ability, and increase permeability behavior [12, 17]. Antibodies could be simply encapsulated in nanomaterials, such as liposomes, polymeric micelles, gold nanoparticles, and dendrimers, to reduce off-target distribution [32-35]. NDDSs have been explored to encapsulate aPD-1 for targeting tumor cells and aPD-L1 for targeting naive T cells [36]. Furthermore, specific tissue-targeting has been explored by modification of functional binding groups on NDDSs. For example, a glycosylated PEG-based NDDS (glucs-aPD-L1) was designed for targeted drug release in brain tumors by utilizing glucose transporter 1 (GLUT1), a highly expressed transporter in brain capillaries. The NDDS was constructed with multiple detachable PEG chains that are cleavable in the reductive TME, enabling selective drug release within the brain tissue [37]. Alternative mechanisms have been employed to facilitate drug traversal across the blood-brain barrier in other studies.
Nano drug delivery systems (NDDSs)-based strategies for different types of ICIs. (A) Schematic illustration for tumor cells attacked by T cells after blockage of the PD-1/PD-L1 axis. (B) Blocking the PD-1/PD-L1 axis by NDDS-delivered antibodies. (C) Downregulating PD-L1 by NDDS-delivered nucleic acids. (D) Blocking the PD-1/PD-L1 axis by NDDS-delivered small molecular inhibitors.
2.1.2. Dual ICIs
NDDSs have also been reported for dual-ICI delivery. In addition to the encapsulation of previously discussed PD-1/PD-L1 inhibitors into an NDDS, CTL-4 could be simultaneously encapsulated into the same NDDS. The primary mechanism of CTLA-4 is to block the interaction between CTL-4 on T cells and B7 molecules (CD80/CD86) on antigen-presenting cells. The binding of CTLA-4 and B7 activates inhibitory signals to inhibit excessive T cell activation and proliferation and avoid overactivation of the immune system. CTLA-4 inhibitors enhance T cell activation by disrupting the binding of CTLA-4 to its ligands, leading to enhanced antitumor immune responses. The concurrent use of PD-1/PD-L1 and CTLA-4 inhibitors may effectively boost the immune response by simultaneously increasing the T cell number and restoring their functionality, and the strategy has been applied in clinics for certain indications, and it is currently undergoing clinical studies for other indications. The use of NDDSs for dual-inhibitor delivery aims to improve targeting specificity and reduce non-targeted distribution [38, 39]. They are also used for the delivery of dual inhibitors to specific tissues, such as the brain. For example, it was reported that a nanoscale immunoconjugate (NIC), P/a-CTLA-4 + P/a-PD-1, for trans-blood-brain barrier (BBB) delivery of CTLA-4 and PD-1 antibodies. Compared to the single checkpoint inhibitor-loaded NIC or free aCTLA-4 and aPD-1, P/a-CTLA-4 + P/a-PD-1 treatment resulted in a remarkable increase in the abundance of CD8+ T cells, NK cells, and macrophages and a significant reduction in the number of regulatory T cells (Tregs) in mice GL261 glioblastomas. The improvement in intratumoral antitumor immune responses prolonged the survival of glioblastoma (GBM)-bearing mice [40]. Although a great antitumor efficacy was achieved in this study, the dual-ICIs delivery strategy has not been extensively explored, and there are very few reports on this strategy.
2.2. Nucleic acids-related NDDSs
2.2.1. Strategies at post-transcriptional level
Nucleic acid ICIs, including siRNA, shRNA, CRISPR/Cas, plasmid DNA, and aptamers, have garnered extensive research attention. The inherent instability and susceptibility to degradation of nucleic acids offer great opportunities for the use of NDDSs. siRNA is the most investigated type of nucleic acid ICI. It suppresses the PD-1/PD-L1 axis by down-regulating PD-L1 or PD-1 expression at the post-transcriptional level. Various NDDS platforms have been developed for siRNA delivery, such as polymers, lipids, and biomolecules [27, 41, 42]. siRNA for PD-1 (siPD-1) was reported to be encapsulated in noncationic soft polyphenol nanocapsules, amphiphilic triblock polymers, and dendrimer-entrapped gold nanoparticles to increase its tumor tissue penetration [43, 44] and avoid its degradation through facilitating endosomal escape of internalized siPD-1 [45]. A dual reactive oxygen species (ROS)/pH-sensitive siPD-L1 delivery system was attached to erythrocytes to construct a FEGCG/Zn/siPD-L1/erythrocyte system, which could enhance the stability of siPD-L1 via erythrocytes and promote tumor targeting accumulation via stimuli response [46]. siPD-1-loaded liposomes were employed to deliver siPD-1 to T cells [47]. Apart from direct encapsulation of siRNA, studies have explored indirect methods to generate nucleic acids. For instance, engineered bacteria that can produce RNA were developed to serve as in vivo "cell factories". This strategy can sustainably produce siRNA of PD-L1 within tumor cells, simplifying its manufacturing process and eliminating stringent shipping requirements [48]. Moreover, a limited number of studies have investigated the structure-activity relationship. One study evaluating the delivery efficacy of several inorganic NPs for siRNA delivery demonstrated that layered double hydroxide and lipid-coated calcium phosphate had an equivalent delivery efficiency [49]. Systematic investigations and meta-analyses involved in the structure-activity relationships across diverse types of NDDS for nucleic acid drug delivery remain scarce. This reveals a significant gap between the current laboratory research of nucleic acid-related NDDSs and their clinical application. In summary, various NDDSs have been extensively applied in siRNA delivery research. These NDDSs pronouncedly enhance the stability of siRNA through direct encapsulation or bypass of the endocytosis pathway to prevent degradation, resulting in reducing systemic clearance, improving tissue permeability, and enhancing tumor targeting.
2.2.2. Strategies for gene editing
CRISPR/Cas is a highly efficient gene-editing tool that can be utilized to knock out PD-1/PD-L1 genes. In order to enhance the stability and delivery efficiency of nucleic acids, NDDSs have been introduced to protect them from degradation. The rational design of NDDSs for CRISPR/Cas delivery should consider multiple factors, including the choice of nanomaterials to minimize non-specific in vivo distribution and enhance the targeted release and an appropriate gene editing system to improve gene editing efficiency while reducing off-target effects [50]. For example, a silk fiber-derived hydrogel was constructed with genetically engineered adenoviruses, which were employed to deliver CRISPR/Cas9 for PD-L1 gene editing in Hepa 1-6 liver cancer cells. The NDDS was found to promote local retention of CRISPR/Cas9 and obtain effective gene transduction over 9 days [51]. Stimuli-responsive NDDSs were designed to mitigate the side effects of the CRISPR/Cas 9 system by responding to specific tumor environment characteristics [52, 53]. A dual-locking NP (DLNP) was used to load CRISPR/Cas13a inside the core, and the outer shell was a dual-responsive polymer layer. The shell could promote the stability of DLNP in the circulation system to improve its biosafety, and achieve responsive release of CRISPR/Cas13a from its core in the tumor tissue [54]. A remarkable efficiency in knocking out the PD-L1 gene has been achieved by optimizing of gene editing systems and nanomaterials. Gene editing systems, including CRISPR/Cas9, and CRISPR/Cas13a, have been studied for gene editing in tumor cells or T cells. Among nanomaterials, polymer nanoparticles, lipid-based nanoparticles, hydrogels, gold nanoparticles, and exosomes have been demonstrated to be promising for constructing effective NDDSs for transporting these gene editing systems.
2.2.3. Other strategies
In addition to directly downregulating PD-1/PD-L1 expression, other strategies for regulating gene transcription, translation, and post-translational modification have also been developed. For instance, a lipid-protamine-DNA nanoparticle was loaded with a plasmid DNA encoding a trap protein targeting PD-L1 (PD-L1 trap). The translation of the PD-L1 trap gene generated a trivalent trap protein that had a significantly stronger degree of affinity for mouse PD-L1 than endogenous PD-1; thus, this trap protein could be used as an antagonist to inhibit the PD-1/PD-L1 axis [55]. Aptamers have also been employed because of their similar functions to antibodies but better stability and modifiability. A hydrogel was reported to encapsulate aptamers. The hydrogel NDDS encapsulated an aptamer and a targeting sgRNA sequence. The PD-1 aptamer sequence could be cut by Cas9/sgRNA after the hydrogel lost its gel property for programmed release [56].
2.3. NDDSs for small molecular inhibitors
2.3.1. Peptides
The remarkable anti-cancer efficacy of large-molecule ICIs in clinical practice with intrinsic limitations of large-molecule drugs has driven the development of small-molecule ICIs. There are two main types of small-molecule ICIs: peptides and chemical ICIs. Although these ICIs exhibit superior tissue penetration compared to large molecular ICIs, the non-specific distribution of small molecular ICIs may result in systemic toxicity, which could be addressed by employing NDDSs. Below are a few examples of NDDSs utilized in peptide ICI delivery. P-F4, a peptide identified by screening a phage display peptide library, could block the PD-1/PD-L1 axis. It was encapsulated with mPEG-PLA to improve the solubility of the peptide and avoid its rapid enzymatic degradation [57]. It was also reported that an anti-PD-L1 peptide was encapsulated in a photo-induced crosslinked liposome and then conjugated on the terminal of DSPE-PEG2k for PD-L1 tumor targeting. The peptide could intervene in the PD-L1 recycling mechanism, and the NDDS could achieve controlled release by promoting the intermolecular crosslinking of the liposomal bilayers to form a multivalent binding complex. The complex was transported into lysosomes instead of endosomes. Thus, the recycling of PD-L1 onto tumor cell surfaces was prevented [58].
2.3.2. Chemical ICIs
Chemical ICIs are also widely studied for immunotherapy through various inhibitory pathways, including interfering with the transcription or translation of PD-1/PD-L1, reducing protein expression, promoting degradation, or competitively binding to the immune checkpoint to prevent the interaction between PD-1 and PD-L1 [59]. NDDSs have been explored to deliver chemical ICIs. For instance, a chemically synthesized mimicking antibody, molecularly imprinted polymer (MIP), was reported to act as a nano-sized PD-1 inhibitor. It was designed to construct the anti-PD-1 nanoMIP by epitope imprinting using the N-terminal epitope of PD-1 as the binding site [60]. Notably, most peptides and chemically synthesized ICIs are currently in the laboratory/preclinical research stage. Their application requires extensive experimental validation, including druggability studies and safety investigations.
In summary, antibodies, nucleic acids, and small molecular inhibitors have been loaded into various NDDSs to improve the antitumor efficacy of these ICIs by improving their tumor targetability and accumulation. NDDSs have endowed ICIs with tumor-targeting biodistribution and strengthened their function specificity, which could contribute to reducing the incidence and alleviating the degree of irAEs [17]. However, experimental/clinical evidence of relieving irAEs via NDDSs remains to be seen because animal models used in these studies have not shown clinically relevant adverse events, which may be due to species differences between mice and humans, the tolerance of certain strains of mice, or short-term treatment interventions in the currently available preclinical models [61]. In addition, to elicit robust antitumor immune responses and diminish general toxicity, ICIs are usually combined with other therapeutic agents, which will be discussed in Section 3.
3. NDDSs for combination immunotherapy with ICIs
Monotherapy with ICIs is often insufficient to restore the tumoricidal function of T cells, and immune resistance is easily developed because the initiation of the antitumor immunity is strongly influenced by complicated interplays between cancer cells, the TME, and the immune system. Tumor intrinsic factors, such as low immunogenicity, lead to insufficient tumor antigen presentation. Thus, tumor cells are invisible to the immune system [62, 63]. Besides, anergic lymphocytes, infiltrated immunosuppressive cells, cancer-associated fibroblasts (CAFs), dense extracellular matrix (ECM), and immunosuppressive signal/chemotaxis factors in tumor tissues constitute an immunosuppressive TME. The suppressive immune cells, including Tregs, MDSCs, and tumor-associated macrophages (TAMs) in the TME play crucial roles in tumor progression. Immunosuppressive signal factors, including vascular endothelial growth factor (VEGF) and transforming growth factor-β (TGF-β), can be secreted by neoplastic cells and other stromal cells in the TME to suppress antitumor immune responses [17, 64]. In addition, unique metabolic characteristics of tumor cells contribute to the formation of an immunosuppressive TME. Hypoxia in solid tumors, caused by insufficient oxygen supply by the abnormal vasculatures and a high level of oxygen consumption by rapidly growing tumor cells, leads to the recruitment of inhibitory immune cells at tumor sites to secrete adenosine and enhance PD-L1 expression for suppression of immune responses. Low pH in the TME resulting from aerobic glycolysis and lactic acid generation of tumor cells could also inhibit the infiltration and activation of antitumor effector cells. T cell receptor expression and antigen-specific T cell response can be restrained by arginase-mediated arginine depletion, which eventually hampers immune cell infiltration and reduces their antitumor activity [65]. Interaction between tumor cells and immunosuppressive TME constituents jointly induces immune escape of tumor cells, ultimately leading to uncontrolled tumor growth and an unsatisfactory antitumor effect of ICIs [13]. Various NDDS-based strategies for combination therapy illustrated in Figure 1 have been developed to boost the antitumor efficacy of ICIs, reshape the immunosuppressive TME during ICI treatment, mitigate immune resistance, and reduce irAEs after ICB treatment.
3.1. Enhancing the immunogenicity of tumors
3.1.1. Multimodal therapy
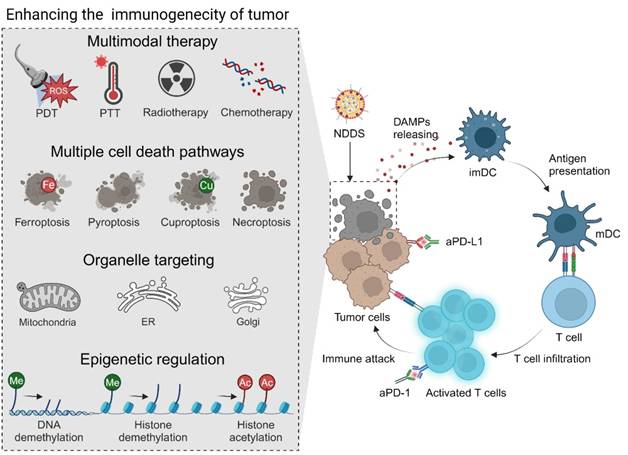
It is well known that neoplastic cells lose their immunogenicity through multiple mechanisms. A low level of immunogenicity of tumor cells allows them to avoid being recognized and captured by the immune system, which is one of the primary mechanisms of tumor immune escape and ICI resistance. Therefore, enhancing the immunogenicity of tumors appears to be an effective strategy to improve the ICB response, and it can be realized through multiple approaches (Figure 3). Typically, immunogenic cell death (ICD), characterized by exposure and release of damage-associated molecular patterns (DAMPs), including calreticulin (CRT), high mobility group protein B1 (HMGB1), and adenosine triphosphate (ATP), has been regarded as a crucial event in stimulating the immune system and triggering the antitumor immunity [66, 67]. Chemotherapy, radiotherapy, chemodynamic therapy (CDT), photodynamic therapy (PDT), photothermal therapy (PTT), sonodynamic therapy (SDT), and two or more combinations have been extensively used to induce ICD of tumors to improve their immunogenicity, initiate the antitumor immunity, and strengthen the therapeutic effect of ICIs [68-76]. In this field, nanomaterials are usually employed to load therapeutic drugs for the above therapeutic modalities to improve their tumor-targeting ability, enhance their bioavailability, reduce their side effects, and optimize their release properties [77-80]. A glutathione (GSH)-sensitive polyethylene glycol-poly-L-lysine (PEG-PLL) micelle, which was decorated with angiopep-2 peptide on the surface to allow efficient BBB penetration, was used to co-encapsulate aPD-L1 and paclitaxel (PTX) to realize effective chemo-immunotherapy of GBM [81]. In another study, hollow mesoporous organosilica nanoparticles coated with the Fe metal-organic framework (MOF) that were dual-responsive to the TME were exploited to deliver doxorubicin (DOX). This nanosuspension exhibited strong tissue adhesion, killed residual tumor cells, and induced ICD via locally sustained release of DOX after perioperative treatment. When the DOX-loaded nanoparticles were combined with aPD-1, postoperative recurrence and brain metastasis of 4T1 breast cancer were effectively inhibited [82]. In addition to commonly used cytotoxic agents, naturally-derived bioactive compounds, such as ginsenoside Rg3, quercetin [83], artesunate [84], celastrol [85], and shikonin [86], have been explored for inducing ICD and synergizing PD-1/PD-L1 blockade.
3.1.2. Multiple cell death pathways
In recent years, many efforts have been devoted to inducing potent ICD through specific cell death modes, especially ferroptosis, pyroptosis, and cuproptosis. Ferroptosis is an iron-dependent cell death mode caused by GSH depletion, excessive accumulation of ROS, and lipid peroxidation in cells [87, 88]. When ferroptosis induction is combined with PD-1/PD-L1 blockade, interferon-gamma (IFN-γ) released by activated CD8+ T cells can promote ferroptosis of tumor cells by inhibiting system xc-, establishing a positive feedback loop between ferroptosis and immune responses to enhance the synergistic effect on curbing tumor progression and metastasis [89]. Therefore, the combination of ferroptosis induction and ICB may have great therapeutic potential. Although iron-based nanoparticles, such as Fe3O4 nanoparticles, zero-valent-iron nanoparticles, and ultrasmall single-crystal Fe NPs with an Fe core and an Fe3O4 shell, have been reported as nano inducers of ferroptosis in combination with ICIs, these nanoparticles exhibit an inadequate efficiency in ferroptosis induction [90-92]. DOX and glycyrrhetinic acid have been reported to support iron oxide nanoparticles for enhanced ferroptosis, which in turn sensitizes the immune system to PD-1/PD-L1 blockage [93, 94]. Furthermore, biocompatible iron-based metal-phenolic networks (MPNs) can serve as iron sources for triggering ferroptosis, as well as nanocarriers to deliver other drugs for promoting ferroptosis. In this context, sonodynamic, photodynamic, and photothermal therapeutic modalities, as well as glucose oxidase (GOx) and CO prodrugs, have been used to enhance iron-triggered ferroptosis of tumor cells by elevating the production of ROS, thus improving the antitumor effect of ICIs [95-98]. Other metals have been explored to replace iron and induce ferroptosis. For example, a PEGylated Cu2WS4 nanozyme (CWP) was synthesized to trigger ferroptosis via the KEAP1/NRF2/HMOX1/GPX4 pathway and simultaneously assisted in radiation dose deposition in 4T1 cells, and CWP was applied in combination with a PD-L1 antibody [99]. in another study, arsenic trioxide was exploited to induce ferroptosis. After integrating the photothermal effect of AuNPs, the release of tumor-associated antigens (TAAs) was significantly enhanced, thus increasing the efficacy of aPD-L1 [100]. In addition to metal-based inducers, small molecular ferroptosis inducers, such as erastin and (1S,3R)-RSL-3 (RSL-3), have been used in NDDS-based combination therapy with ICIs [101, 102]. Moreover, ferroptosis resistance mechanisms have been harnessed to optimize ICD induction and sensitize ICB. A biomimetic nanoplatform (PMVL) was constructed through the self-assembly of tannic acid and vanadium oxides, encapsulation of lonidamine (LND), and coating with B16F10 cell membranes. The nanoplatform was then modified with an anti-PD-L1 peptide (PPA). In this nanosystem, LND-sensitized vanadium (VIV and VV)-induced ferroptosis by reduced nicotinamide adenine dinucleotide phosphate (NADPH) and ATP in cells through glycolysis inhibition, and PPA was released in response to matrix metalloproteinase-2 (MMP-2) in the TME to block PD-1/PD-L1 recognition. Eventually, PMVL exhibited a pronounced antitumor efficacy in B16F10 tumor-bearing mice [103]. The resistance to ferroptosis induction can be circumvented by inhibiting ferroptosis suppressor protein 1 (FSP1) [104]. Remarkably, spontaneous ferroptosis of neutrophils in the TME has been reported to impair T cell activity via releasing oxidized lipids, revealing diverse roles of ferroptosis in the TME [105]. A liposome was prepared to co-encapsulate a di-iodinated photosensitizer IR780 (Icy7) and a ferroptosis inhibitor, liprostatin-1, to trigger ICD of tumor cells through PDT but suppress ferroptosis of neutrophils, and this synergistic strategy enhanced the therapeutic effect of aPD-1 in gastric cancer [106].
Strategies for improving the efficacy of ICIs by enhancing the immunogenicity of tumors, including applying PDT, PTT, radiotherapy, and/or chemotherapy to induce immunogenic cell death through ferroptosis, pyroptosis, cuproptosis or necroptosis, targeting specific organelles, such as the ER and mitochondria, and performing epigenetic regulation by DNA demethylation and histone demethylation and acetylation. ER, endoplasmic reticulum; imDC, immature dendritic cell; mDC, mature dendritic cell.
Pyroptosis is a programmed cell death mode mediated by proteolytic cleavage and N-terminal domain exposure of gasdermin (GSDM). It is characterized by pore formation on the plasma membrane and release of proinflammatory substances, which can initiate strong immune responses. Thus, it can be combined with ICIs to achieve potent antitumor immunotherapeutic effects [107, 108]. Recently, nanoengineered DOX (a chemotherapeutic agent) [109], indocyanine green (ICG, a photothermal photosensitizer) [110], YBS (a photodynamic photosensitizer) [111], phthalocyanine (a sonosensitizer) [112], Cinobufagin (an active ingredient of traditional Chinese medicine) [113], Fe- and Cu-incorporated hollow carbon spheres (a nanoenzyme) [114], have been reported to escalate inflammatory responses by inducing pyroptosis of tumor cells, effectively improving the efficacy of immune checkpoint inhibition. Considering an increased expression level of cyclooxygenase-2 (COX-2) in cancer cells after pyroptosis induced by platinum-based drugs, an amphiphilic polymer nanoparticle (Pt-In NP) was developed after co-loading a platinum prodrug and indomethacin, a COX-2 inhibitor. The Pt-In NP combined with aPD-L1 effectively restrained primary and distant tumor growth in pancreatic cancer [115]. Moreover, direct expression of the N-terminal domain of GSDM (GSDMNT) through recombinant adeno-associated virus (rAAV) or mRNA lipid nanoparticles were reported to induce pyroptosis and sensitize tumors to checkpoint immunotherapy [116, 117].
Cuproptosis is a recently discovered mode of cell death in 2022. The main process of cuproptosis includes binding of excess copper to lipoylated components in the mitochondrial tricarboxylic acid (TCA) cycle, lipoylated protein aggregation, and iron-sulfur cluster protein reduction, ultimately resulting in proteotoxic stress and cell death [118]. Similar to ferroptosis, cuproptosis can be recognized as ICD that promotes the release of TAAs and initiates antitumor immune responses [119, 120]. Elesclomol (ES), a copper ionophore, can specifically transport copper to the mitochondria. It has been reported for mitochondrion-targeting delivery of Cu(II) to induce cuproptosis in neoplastic cells. Its combination therapy with checkpoint antibodies was applied to treat melanoma and bladder cancer [121, 122]. In addition, cuproptosis is induced principally after interference with the TCA cycle, while tumor cells are insensitive to cuproptosis induction in a hypoxic TME since they preferentially rely on glycolysis rather than oxidative phosphorylation (OXPHOS). Therefore, siRNA for catalase and pyruvate dehydrogenase kinase 1 were incorporated into cuproptosis nanoinducers to sensitize cuproptosis by alleviating hypoxia or inhibiting glycolysis, respectively. As a result, effective anabatic cuproptosis was realized, and the antitumor efficacy of aPD-L1 was also enhanced [123, 124]. Promising results of triggering antitumor immune responses after cuproptosis warrant future efforts into exploring cuproptosis inducers and their combination with ICIs in cancer immunotherapy. It is worth noting that randomly distributed copper ions in the body can cause side effects. It is crucial to improve the efficiency of targeted delivery of copper ions to tumor cells. In addition, the efficiency of cuproptosis induction in tumor cells can be compromised by a high concentration of GSH that chelates copper ions and the copper efflux mediated by ATPase copper transporting alpha/beta (ATP7A/B) [125, 126]. NDDSs could play a role in mitigating these issues, paving the way for the application of cuproptosis for cancer immunotherapy.
3.1.3. Organelle-targeting NDDSs
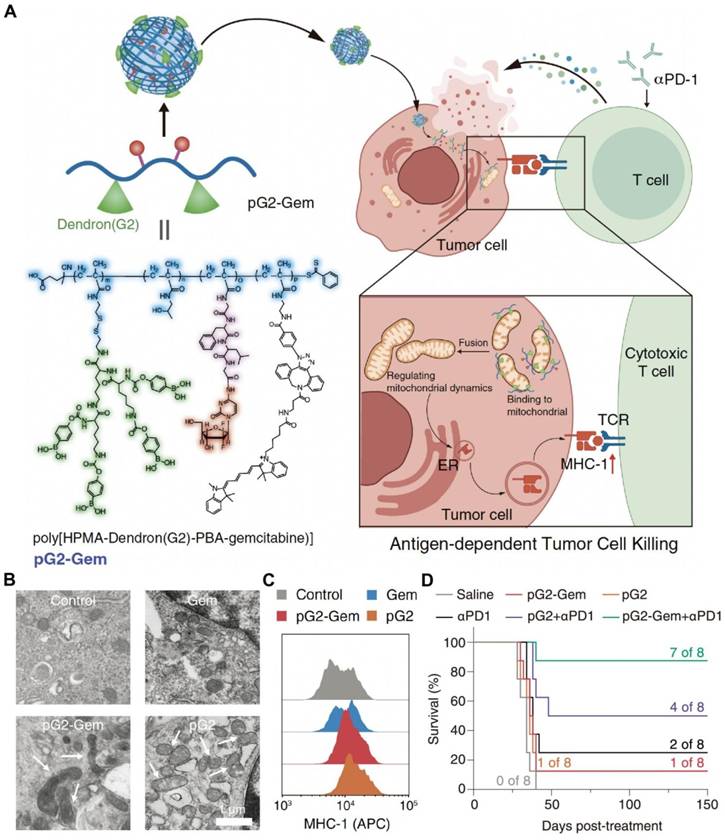
Compared with traditional tumor-targeting drug delivery systems that recognize and bind to surface receptors, organelle-targeting NDDSs are designed to deliver drugs to specific organelles and accurately regulate their biological functions within the organelles, which is conducive to improving efficacy and reducing toxicity [127-129]. Moreover, the dysfunction of specific organelles contributes to cell death and DAMP release. It is well known that endoplasmic reticulum (ER) stress and ROS production are essential for ICD induction. ER-targeting drug delivery systems have been developed to efficiently induce ER stress. Drugs have been delivered to the ER for ICD induction and combination therapy with ICIs, including chemotherapy agents, sonosensitizers, and AIE photosensitizers [130-132]. Meanwhile, the mitochondrion is an important organelle to produce intracellular ROS. Thus, mitochondrion-targeting nanodrugs have also been widely studied. To date, DOX [133], fenofibric acid [134], dichloroacetate [135], LND [136], self-assembling peptides [137], and AIE photosensitizers [138] have been conjugated with mitochondrion-targeting moieties or encapsulated into mitochondrion-targeting nanoplatforms for advancing ICB treatment. In our group, we designed and prepared a mitochondrion-targeting drug-free dendronized polymer (pG2) (Figure 4). The polymer promoted mitochondrial fusion, resulting in enhanced expression of major histocompatibility complex (MHC)-I. The treatment by the dendronized polymer conjugated with gemcitabine (pG2-Gem) and aPD-1 exhibited a potent antitumor efficacy in a 4T1 mouse model [139].
An NDDS derived from a mitochondrion-targeting dendronized polymer for synergic therapy with aPD-1 by promoting mitochondrial fusion and enhancing the expression of major histocompatibility complex (MHC)-I. (A) pG2-Gem released gemcitabine to exert its cytotoxic effects and the drug-free polymer, pG2, to regulate mitochondrial dynamics and promote mitochondrial fusion, thereby mediating MHC-I antigen presentation and leading to the activation of cytotoxic T cells for effective synergic therapy with aPD-1. (B) TEM images of mitochondrial fusion in 4T1 tumor cells treated with pG2 and pG2-Gem. Free Gem did not impact the mitochondrial morphology (Scale bar = 1 μm). (C) Expression levels of MHC-I in 4T1 cells after treatment with Gem, pG2, and pG2-Gem. (D) Kaplan-Meier survival curves of the 4T1 tumor model after various therapies. Reproduced with permission from ref [139]. Copyright 2024, Wiley.
3.1.4. Epigenetic regulation
Epigenetic alterations play a critical role in tumorigenesis and progression, and epigenetic modifiers, such as DNA methyltransferase (DNMT) inhibitors, histone deacetylase (HDAC) inhibitors, and histone methyltransferase (HMT) inhibitors, could be promising antitumor therapeutic agents. They have been explored to directly kill tumor cells, dampen hypermethylation of tumor suppressor gene promoters, enhance expression of TAAs and MHC class I and II molecules, and alleviate T cell exhaustion. The revealed antitumor mechanisms of these drugs imply their potential to improve ICB therapeutic efficacy [140, 141]. Zebularine [142, 143], Panobinostat [144], chidamide [145] and SAHA [146] have been incorporated in NDDSs for combination therapy with PD-1/PD-L1 blockade. Decitabine [147], olsalazine [148], 5-azacytidine [149], and CM-272 [150] have been applied to sensitize nanoplatform-based chemotherapy or SDT for amplified ICD and effective synergistic influence with PD-1/PD-L1 antibodies.
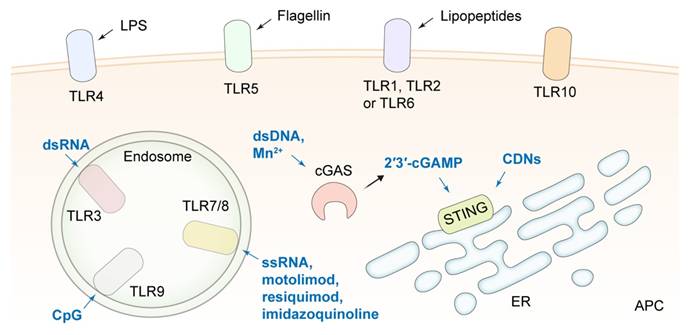
TLR and STING agonists delivered by NDDSs for strengthening the function of APCs and improving the efficiency of ICB. TLR, toll-like receptor; cGAS, cyclic guanosine monophosphate-adenosine monophosphate synthase; STING, stimulator of interferon genes; 2′3′-cGAMP, 2′3′-cyclic guanosine monophosphate-adenosine monophosphate; CDNs, cyclic dinucleotides; dsRNA, double-stranded RNA; dsDNA, double-stranded DNA; LPS, lipopolysaccharide; ER, endoplasmic reticulum; APC, antigen presenting cell.
3.2. Strengthening the function of APCs
APCs, predominantly DCs and macrophages, play a crucial role in the process from the release of tumor antigens to the induction of antitumor immune responses, including antigen processing and presentation and T cell recruitment and activation [151, 152]. The function of APCs is significantly impacted by their surrounding environmental factors. The immaturity of DCs in an immunosuppressive TME severely curtails specific antitumor immunity [153]. Therefore, various immune stimulants, such as toll-like receptor (TLR) agonists, cytokines, chemokines, and STING agonists, have been applied to improve the therapeutic effect of ICB by strengthening the function of APCs (Figure 5).
CpG oligonucleotides [154, 155], imiquimod (R837) as a TLR7 agonist [156, 157], resiquimod (R848) [158-161], imidazoquinoline [162] and motolimod [163] as TLRs 7/8 agonists have been delivered by DNA tetrahedrons, polymer-based nanoparticles, liposomes, nanogels and MOFs, respectively, for APC activation in tumor tissues and combination therapy with ICIs. For example, a bispecific nano-immunoengager (NIE) that was assembled from two transformable peptide monomers was reported. One monomer consisted of a tumor-targeting LXY30 cyclic peptide, a β-sheet-forming peptide, and a hydrophobic pheophorbide a (Pa) moiety. Another monomer was composed of a "pro-ligand" form of LLP2A that targeted the activated α4β1 integrin on lymphocytes, a β-sheet-forming peptide, and R848. The two monomers could co-assemble into NPs with a diameter of about 28 nm (NIE-NPs) when they were mixed at a ratio of 1:1. After binding to the α3β1 integrin of tumor cells, NIE-NPs formed a nanofibrillar structural network on cancer cells. Under the action of esterase in the TME, LLP2A converted from proLLP2A captured CD8+ T cells by recognizing the activated α4β1 integrin, and R848 released from NIE stimulated the presentation of antigens, the release of antitumor response factors, and the transformation of the macrophage phenotype from M2 to M1. The NIE significantly improved the efficacy of PD-1/PD-L1 ICB therapy in two different cancer models in mice [158]. A tumor-colonized attenuated Salmonella typhimurium VNP20009 strain was engineered to synthesize granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 7 (IL-7), which helped recruiting macrophages and DCs and enhancing T cell antitumor responses. The GM-CSF-IL-7-VNP20009 strain combined with a PD-1 antibody synergistically suppressed the progression and metastasis of B16F10 melanoma [164]. In addition, synthetic materials may possess immunostimulating functions that assist in ICB therapy. A phosphorus dendrimer (AK128) was reported to promote the proliferation of NK cells. The nanocomplexes were comprised of AK128 and aPD-1 and camouflaged with M1-type macrophage cell membranes. The encouraging therapeutic effect after applying the nanocomplexes to treat glioma suggested the polymers could be novel immunostimulants [165].
The cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS)-stimulator of interferon genes (STING) pathway, another innate immune pathway, has received great attention for improving the therapeutic effect of ICB. After cGAS recognizes DNA fragments in the cytoplasm, 2′3′-cyclic guanosine monophosphate-adenosine monophosphate (2′3′-cGAMP) is synthesized to activate STING, promoting the production of type I interferon (IFN-I) and other proinflammatory cytokines [166-168]. Activation of the STING pathway in the APCs is essential to initiate the anticancer effect of CD8+ T cells. Cyclic dinucleotides (CDNs) are the most widely studied STING agonists. There is a very low level of internalization of CDNs by tumor cells due to their hydrophilicity and electronegativity, and they could induce T cell apoptosis and enhance tumor cell tolerance to ICB [169]. A myriad of lipid-based nanoplatforms have been developed for the efficient delivery of CDNs. For example, a CDN-loaded and phosphatidylserine (PS)-coated liposome (LNP-CDN) was reported [170]. In LNP-CDN, CDN was complexed with calcium phosphate to ensure the release of CDN from endosomes to the cytosol. LNP-CDN was demonstrated to be primarily uptaken by phagocytes, i.e., macrophages, CD103+ DCs, and CD11b+ DCs, in both malignant pleural effusion (MPE) and pleural tumors in the pleural cavity. By remodeling the phenotype of myeloid cells and enhancing the cytotoxicity mediated by CD8+ T cells and NK cells, LNP-CDN significantly boosted the antitumor immune response to anti-PD-L1 therapy in both mouse and human MPE. Moreover, liposomal CDN decorated with a Clec9a targeting peptide for specifically activating CD103+ DCs was reported to amplify the efficacy of aPD-L1 [171]. In another study, a CD44×PD-L1/CD3 trispecific T-cell nanoengager loaded with c-di-AMP (CDA) was designed to form an immunological cytolytic synapse between triple-negative breast cancer (TNBC) cells and T cells, leading to TNBC cell lysis, therefore, the nanoengager exhibited an outstanding antitumor efficacy [172].
In addition to CDNs, double-stranded DNA (dsDNA) can be nanoengineered to activate cGAS-STING signaling during combination therapy with ICIs [173, 174]. Interestingly, nanoscale vesicles containing plant-derived mitochondrial DNA (mtDNA) isolated from Artemisia annua were shown to activate cGAS-STING and reshape TAMs to their proinflammatory phenotype, thereby enhancing ICB therapeutic effects [175]. Nanoplatforms have been developed to selectively trigger DNA double-strand breaks (DSBs) or mtDNA leakage to stimulate the cGAS-STING pathway for combination therapy with ICIs. The reported nanoplatforms include β-lapachone and tirapazamine co-loaded liposomes anchored with a TIGIT block peptide and coated with erythrocyte membranes [176], CCR2 antagonist-incorporated ultra-small-sized micelles based on gemcitabine-conjugated polymers [177], cisplatin prodrug (Pt(IV))/WEE1 inhibitor (MK1775) co-encapsulated nanoparticles [178], and peptide nanodrugs composed of poly(2-(diisopropyl amino) ethylmethacrylate) (PDPA) conjugated with an antimicrobial peptide (AMP, KLAKLAK2) and a PD-L1 antagonist peptide (CVRARTR) [179].
Manganese (Mn) has been reported to play a critical role in innate immune activation via the cGAS-STING pathway [180]. It could also catalyze ROS production through a Fenton-like reaction for cancer CDT and act as a contrast agent to enhance magnetic resonance imaging (MRI) [181]. Hence, nanoformulations containing Mn2+ have been extensively explored in recent years. In order to reduce toxicity and increase tumor accumulation of free Mn2+, iRGD-modified hollow mesoporous silica [182], hollow rough MnO2 [183], silk sericin and pentapeptide CREKA [184], zeolite imidazole framework (ZIF-8) [185] and bioactive glass [186] have been employed to construct Mn2+ delivery systems for synergistic therapy with ICIs. Furthermore, mutant p53 (mutp53) proteins inhibit the STING downstream pathway by binding to TANK-binding kinase 1 (TBK1), while mutp53 can be degraded by Zn2+ through proteasome ubiquitination, thus mitigating the inhibitive effect by mutp53. A ZIF-8@MnO2 nanoparticle was proposed to deliver Mn2+ and Zn2+, and the immunotherapeutic efficacy of ZIF-8@MnO2 combined with aPD-L1 in tumors with mutated p53 was verified [187]. For synergetic STING activation, Mn2+ collaborated with CDNs in nanoplatforms to enhance the potency of ICIs [188, 189]. In addition, an ultra-pH-sensitive and TME-targeting NDDS was designed by encapsulating a TLR4 agonist MPLA and manganese tetroxide nanoparticles into polyethylene glycol-poly(ethylpropylaminoethyl methacrylate). Since nuclear factor-κB (NF-κB) activation mediated by the TLR4 agonist amplified the magnitude of STING activation, this NDDS synergizing with aPD-1 induced tumor regression and established systemic antitumor memory [190].
Cancer vaccines, which directly activate APCs to trigger tumor-specific immune responses, have exhibited great potential in immunotherapy [191]. Especially, nanovaccines can efficiently deliver antigens to APCs, improve cytosolic retention of antigens, protect antigens from degradation during the delivery process, and manipulate spatiotemporal codelivery of antigens and adjuvants compared with traditional vaccines. Therefore, they can be employed to improve the efficacy of ICB therapy [192, 193]. Nanovaccines often consist of antigens, adjuvants, and carriers. Ovalbumin (OVA) and its derived antigenic peptides are the most commonly used model antigens in nanovaccines for combination therapy with ICIs (Table 1) [194-202]. In addition, antigenic peptides identified from cancer cell lines, as well as tumor cell membranes or tumor cell lysates, have been utilized as antigens to construct nanovaccines for preclinical and clinical studies [203-206]. Importantly, due to the heterogeneity and intricacy of cancer, antigens with a high degree of universality and immunogenicity remain to be discovered. Adjuvants in vaccines are essential for bolstering the immune response triggered by antigen stimulation. Many adjuvants employed in antitumor nanovaccines, such as CpG, R848, dsDNA, CDNs, and Mn2+, have been discussed in the previous section. Currently, very few adjuvants are approved for clinical application, and novel translatable adjuvants are actively sought for antitumor vaccines. Various carriers used in nanomedicines are also exploited for nanovaccines, such as proteins [207, 208], lipids [194, 199, 209], polymers [195, 200, 203, 210, 211], inorganic materials [204, 212, 213], cell membranes [214-217], and extracellular vesicles (EVs) [197, 218-220]. It is feasible to prepare carrier-free nanovaccines by harnessing the characteristics of antigens and adjuvants through a self-assembly process [221]. Particularly, a few delivery vehicles have been reported to have similar properties as adjuvants, and they can assemble with antigens into nanovaccines that initiate potent immune activation and boost ICB efficacy in cancer models [198, 222-225]. It is worth noting that the efficiency of antigen cross-presentation is crucial for the efficacy of nanovaccines. The efficiency, generally characterized by examining the SIINFEKL-H-2Kb expression level on APCs when OVA is used as a model antigen or the T cell activation level for non-OVA antigens, is highly dependent on the lysosomal escape of the antigen or circumvention of the lysosomal endocytosis pathway. In addition to detecting immune cell activation in lymph nodes, the infiltration level of immune cells in tumor tissues is extremely important for assessing the effect of antitumor immunity.
Nanovaccines for combination therapy with ICIs to activate APCs.
| Nanovaccine | Antigen | Adjuvant | Carrier | ICI | Cancer model | Refs |
|---|---|---|---|---|---|---|
| OVA@MM | OVA | Mn2+ | Mn2++2-methylimidazole | aPD-1 | B16-OVA melanoma | [226] |
| Man-PLL-RT/OVA/CpG | OVA | CpG | Man-PLL-RT (polymer) | shPD-L1 | B16-OVA melanoma | [227] |
| DGBA-OVA-CpG | OVA | CpG | dendrimer grafted with guanidinobenzoic acid | aPD-1 | B16-OVA melanoma | [228] |
| PCO | OVA | CpG | protamine | aPD-1 | B16-OVA melanoma | [207] |
| MALO@HBNS | OVA | STAT3 siRNA | polyester, lipid | aPD-L1 | B16-OVA melanoma | [199] |
| DC-sEVs-CpG | OVA | CpG | DC-derived small EVs | aPD-1 | B16-OVA melanoma | [197] |
| DoriVac | OVA | CpG | square-block DNA origami | aPD-L1 | B16-OVA melanoma | [196] |
| nChap@OVA | OVA | R848 | mannose-PEG-b-PCL, PAE-b-PCL | aPD-1 | B16-OVA melanoma | [229] |
| PP-SS-OVA/CpG | OVA | CpG | PCL-PEG-PDS, PCL-PEI | aPD-1 | B16-OVA melanoma | [195] |
| NL(pro-TLR7/8a) | OVA | liposomal prodrug-like TLR7/8 agonist (TLR7/8a) | liposome | aCTLA-4+aPD-1 | B16-OVA melanoma | [201] |
| PD-K-OVA | OVA | poly(L-phenylalanine)-block-poly(D-lysine) | poly(L-phenylalanine)-block-poly(D-lysine) | aPD-1 | B16-OVA melanoma | [198] |
| KK2DP7/OVA | OVA | dendrimer polypeptide | dendrimer polypeptide | aPD-1 | E.G7-OVA lymphoma | [224] |
| SMONV | OVA | / | soft mesoporous organosilica | aPD-L1 | E.G7-OVA lymphoma | [213] |
| 4RDP(F5)-OVA | OVA | fluorinated supramolecular peptide adjuvant | fluorinated supramolecular peptide adjuvant | aPD-L1 | E.G7-OVA lymphoma | [223] |
| PoIC/OVA-R8L | OVA | poly I:C | liposome | aPD-L1 | MO5 melanoma | [194] |
| OVAPEP-SLNP@CpG | SIINFEKL | CpG | lipid nanoparticle | aPD-1 | E.G7-OVA lymphoma | [202] |
| RNA-OG-pOVA | SIINFEKL | RNA origami | RNA origami | aPD-1 | B16-OVA melanoma | [225] |
| C-25/OVA257-280 | peptide antigen OVA257-280 | PEGMA-co-BMA-co-C7AMA | PEGMA-co-BMA-co-C7AMA | aPD-L1 | B16-OVA melanoma | [222] |
| R848@M2pep-MPsAFP | alpha-fetoprotein (AFP), OVA | R848 | engineered microparticles derived from AFP/OVA-overexpressing macrophages | aPD-1 | B16-OVA melanoma, Hepa1-6 hepatocellular carcinoma | [218] |
| NV, PNV | OVA, supernatant of tumor abrasive fluid | CpG+Mn2+ | Mn2++2-methylimidazole | aPD-L1 | B16-OVA, B16F10 melanoma | [230] |
| CpG&Ag | SIINFEKL, Adpgk | CpG | / | aPD-1 | B16-OVA melanoma, MC-38 colorectal cancer | [221] |
| sHDL-Ag+PolyICLC | Adpgk neoantigen | polyICLC | synthetic high-density protein nanodiscs | aPD-1 | MC-38 colorectal cancer | [208] |
| banNV | Adpgk neoantigen | R848+CpG | PEG-PLA, PPT-g-PEG | aPD-1 | MC-38 colorectal cancer | [231] |
| Nanovaccine | Adpgk neoantigen, MUT30 | CpG+poly(I:C) | PLGA nanoparticles | aPD-1 | MC-38, CT26 colorectal cancer, B16F10 melanoma | [203] |
| RGO(CpG)-PEG-(M27+M30) | M27 + M30 peptides | CpG | PEGylated reduced graphene oxide nanosheet | aPD-1 | B16F10 melanoma | [232] |
| Gel(Vaccine NPs) | M27 + M30 peptides | Bacille Calmette-Guérin (BCG) bacterial cell wall skeleton | PLGA | aPD-L1 | B16F10 melanoma | [211] |
| Nanovaccine | Tyrp1+M20+M27 peptides | MontanideTM ISA 51 | DSPE-PEG2000 | aPD-1 | B16F10 melanoma | [209] |
| NTV2 | M27+M30+M47+M48 | / | polymer-peptide | aPD-L1 | B16F10 melanoma | [210] |
| Mutation-M33-M47 BDVs | M33+M47 | bacteria derived vesicles | bacteria derived vesicles, GM-CSF | aPD-1 | B16F10 melanoma | [220] |
| 8FNs@Trp2 | TRP2181-188 | antimicrobial peptide | L-phenylalanine-based poly(ester amide) polymers | aPD-1 | B16F10 melanoma | [200] |
| TCL@CaCO3 | tumor cell lysates | dsDNA | CaCO3 | aPD-1 | B16F10 melanoma | [233] |
| MSNs@cGAMP@CM-SN21 | B16F10 cell membranes | cGAMP | mesoporous silica nanoparticles (MSNs) coated with cell membranes | aPD-1 | B16F10 melanoma | [214] |
| MSN-CpG@CM | B16F10 cell membranes | CpG | MSNs coated with cell membranes | aCTLA-4 | B16F10 melanoma | [215] |
| DBE@CCNPs | CD47KO/CRT dual-bioengineered B16F10 cell membranes | CpG | PEI + bioengineered B16F10 cell membranes | aPD-L1 | B16F10 melanoma | [217] |
| LMP | B16F10 cells | Mn2+ + LPS | Mn2+ + TA | aPD-L1 | B16F10 melanoma | [234] |
| Vaccine A + vaccine B | water-insoluble and water-soluble components of tumor tissues/cells | poly(I:C) | PLGA | aPD-1 | B16F10 melanoma, 4T1 breast cancer | [205] |
| MPE-C | MUCI-derived peptide | CpG | mannosylated Pickering emulsion | aPD-1 | B16-MUCI melanoma | [235] |
| Neo NV | Zfp142 peptide | / | norovirus S protein nanoparticles | aPD-1 | 4T1 breast cancer | [236] |
| PD1/CD40L-NVs | PD-1/CD40L-overexpressed 4T1 cytomembranes | CD40L | PD-1/CD40L-overexpressed 4T1 cytomembranes | PD-1 on nanovaccines | 4T1 breast cancer | [237] |
| K-nanoadjuvant | E7-long peptide | timely activating TLR7/8a+poly (I:C) | liposome | aPD-L1 | TC-1 lung cancer | [238] |
| LDHs-cGAMP | TAAs | cGAMP | layered double hydroxides | aPD-L1 | Hepa1-6 hepatocellular carcinoma | [204] |
| DIA-NPs | chemotherapy-induced antigens | / | / | aPD-1 | CT26 colorectal cancer | [206] |
| MON@LA-PDE5i@M | LLC cell membranes | phosphodiesterase-5 inhibitor + NO | MSN coated with LLC cell membranes | aPD-L1 | LLC lung cancer | [216] |
| NA1C | EBNA1ΔGA92-327 | CpG | tannic acid (TA) | aPD-L1 | EBNA1-TC1 lung cancer | [239] |
| cBEV | bacteria-derived EVs | Mn2+ | bacteria-derived EVs | aPD-L1 | MCF-7 breast cancer | [219] |
Nanomedicines containing ICD inducers and immune adjuvants can be promising candidates for enhancing the therapeutic efficacy of ICIs since they share a mechanism similar to that of nanovaccines. The drugs discussed in Section 3.1 can be used as ICD inducers in the nanomedicines to induce ICD, promote TAA release, and enhance tumor immunogenicity. The immune stimulants mentioned above can be used as adjuvants in nanomedicines. Compared with specific antigens used in nanovaccines, the nanomedicines can trigger ICDs to generate a broader spectrum of tumor antigens, which may be beneficial to stimulate stronger immune responses [240]. For example, a nanosystem (mB4S) was constructed to deliver epirubicin (EPI) as an ICD inducer and diABZI as a STING agonist, which potentiated the potency of aPD-L1 in both 4T1 and CT26 tumor-bearing mice [241]. A CpG-loaded liposome was designed to incorporate 2,3-bis(((5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoyl)oxy)propyl (2-(trimethylammonio)ethyl) phosphate (DAPC), a tailored phospholipid that acted as a ferroptosis inducer, and this liposome was administrated in combination with aPD-L1 [242]. PARE NPs were reported to be assembled from a prodrug synthesized by conjugating R848 with a photosensitizer pyropheophorbide-a, and the nanoparticles markedly inhibited the progression of distant tumors in a subcutaneous HNSCC tumor mouse model when they were combined with aPD-1 [243].
3.3. Manipulating suppressive immune cells in the TME
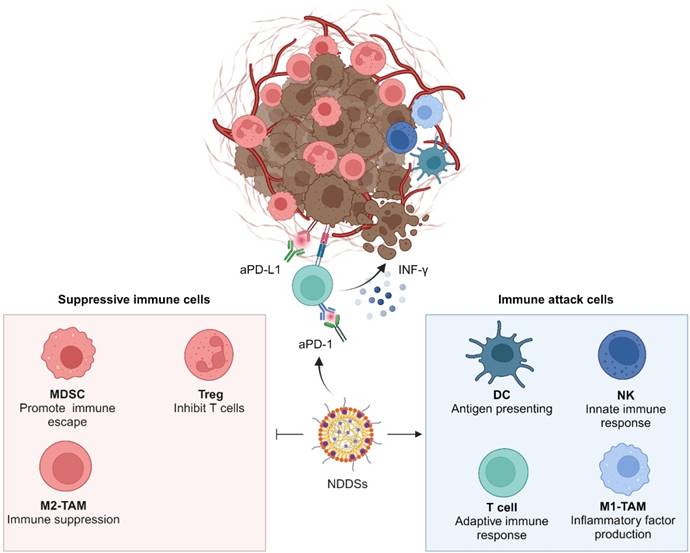
Suppressive immune cells in tumor tissues, including Tregs, TAMs, and MDSCs, are key players in maintaining an inhibitory TME. These cells impair the function of effector T cells (Teffs) and severely diminish immune responses to ICB (Figure 6). Currently, NDDSs for sensitizing ICIs by regulating immunosuppressive cells predominantly target TAMs and MDSCs.
Suppressive immune cells in tumor tissues, including Tregs, M1-TAMs, and MDSCs, abate synergetic immune attacks of DCs, T cells, NK cells, and M1-TAMs.
TAMs, the most abundant immune cells in TME, exhibit an anti-inflammatory M2 phenotype, which promotes tumor progression and maintains an immunosuppressive TME by secreting anti-inflammatory factors, such as TGF-β, and IL-10 [244]. Therefore, efforts have been made to strengthen the efficacy of ICB by repolarizing TAMs to their M1 phenotype through regulators in NDDSs. A supramolecular peptide amphiphile drug-delivery system (SPADS) was reported. In SPADS, mannose was modified to target M2-TAMs, and toyocamycin and α-tocopherol were employed to reprogram M2-TAMs by inhibiting ER stress and oxidative stress [245]. Similarly, a KIRA6 (an ER stress inhibitor) and α-tocopherol (an oxidative stress inhibitor) co-loaded nanoemulsion was developed [246]. Both of the nano-formulations improved the efficacy of aPD-1 via repolarizing TAMs. Mannose-containing precursor glycopeptides that multivalently bound to mannose receptors on M2-TAMs and gold nanoparticles (Au NPs) coated with a polyaniline-based glyco structure were revealed to promote TAM M1-polarization and boost aPD-1 treatment effects, respectively [247, 248]. In addition, TLR agonists, proinflammatory cytokines, and kinase inhibitors have been reported to reprogram the antitumor activity of macrophages for combination therapy with ICIs [249-252]. M2-TAMs as APCs exhibit rather weak antigen cross-presentation due to an elevation in the lysosomal cysteine protease activity. To strengthen antigen cross-presentation of M2-TAMs, MSNs loaded with E64, a cysteine protease inhibitor, were developed. The E64-loaded MSNs were coated with surgical tumor-derived cancer cell membranes decorated with galactose ligands to target M2-TAMs by binding their CD302 receptors. Treatment with ME@C significantly improved antigen cross-presentation of M2-TAMs by inhibiting the activity of cysteine proteases and delivering abundant TAAs from the cancer cell membranes, effectively suppressing the progression and recurrence of tumors [253].
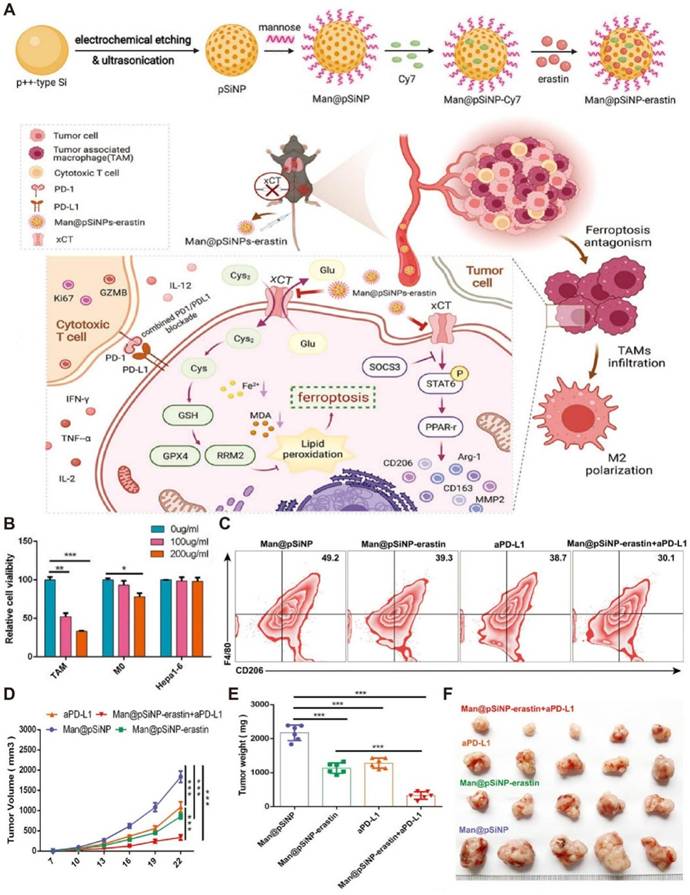
Although ferroptosis in tumor cells has been widely studied, ferroptosis of TAMs is rarely reported. It has been revealed that overexpressed xCT in macrophages encoded by SLC7A11 regulated M2 polarization of TAMs mediated by IL-4 and participated in activating the SOCS3-STAT6-PPAR-γ pathway. Ferroptosis induction by xCT knockout in macrophages was associated with GPX4/RRM2 signaling. Encouraged by these findings, mannose-functionalized porous silicon nanoparticles were loaded with erastin, a ferroptosis inducer, to form Man@pSiNPs-erastin. The resulting nanodrug achieved specific ferroptosis activation in M2-TAMs (Figure 7), which dramatically impeded tumor progression in hepatocellular carcinoma (HCC) when it was combined with aPD-L1 [254].
In addition to TAMs, MDSCs are another major type of inhibitory immune cells in the TME, and they collaborate with TAMs to maintain an immunosuppressive TME [255]. Au NPs at a size of 30 nm were reported to block NLRP3-NEK7 interaction by scavenging ROS, suppressing the activity of NLRP3 inflammasomes, and blocking the release of IL-1β in myeloid cells. The authors coupled Au NPs with H6, an MDSC-targeting peptide, to improve the efficacy of aPD-1-mediated immunotherapy. The combination of Au-H6-NPs and aPD-1 significantly diminished the release of IL-1β, reduced the population of MDSCs in the TME, and promoted tumor infiltration of CD8+ T cells in both aPD-1 sensitive and insensitive cancer models [256]. In addition, MDSCs have been demonstrated to be populated in residual tumor tissues of HCC after insufficient radiofrequency ablation (iRFA), and compensatory upregulation of PD-L1 on residual MDSCs could be achieved during combination therapy with iRFA and MDSC inhibition. A size-tunable acid-sensitive perfluorohexane-cored liposome (LPIP), which released IPI549 and aPD-L1 in response to the acidity in the TME and mild heat under iRFA, was designed. Due to a high expression level of the gamma isoform of phosphoinositide 3-kinase (PI3Kγ) in MDSCs, IPI549, a PI3Kγ inhibitor, selectively mitigated immune suppression of MDSCs. Finally, the combination of IPI549 and aPD-L1 effectively mitigated post-iRFA relapse and hindered the progression of HCC [257].
Since both MDSCs and TAMs play important roles in the formation and maintenance of an immunosuppressive TME, and they directly interact with each other in a complex way to jointly promote the immune escape of cancer cells, the simultaneous intervention of both types of immune cells has been proposed. An entinostat (ENT) and BSM-1 co-loaded micelle coated with dextran sulfate (DXS) was prepared. ENT acted as an MDSC inhibitor, BSM-1 was an ICI, and DXS could reshape M2-TAMs to a proinflammatory M1 phenotype by blocking scavenger receptor A [258]. Another ROS scavenger, a Zr-CeO nanozyme, was reported to dampen MDSCs by downregulating the unfolded protein response (UPR) and restraining the M2 polarization of TAMs via hindering the ERK and STAT3 pathways. This nanozyme boosted the therapeutic efficacy of PD-1 inhibition in both renal and breast cancer [259].
Manipulating suppressive immune cells in the TME by NDDS-based nanomedicines to boost immunotherapeutic effects of ICIs. (A) Schematic illustration of the preparation of Man@pSiNP-erastin and the mechanism of boosting immunotherapeutic effects using Man@pSiNP-erastin+aPD-L1. Man@pSiNP-erastin enhanced the antitumor efficacy of aPD-L1 by inducing TAM ferroptosis and reducing M2-like transformation. (B) Man@pSiNPs-erastin exhibited higher cytotoxicity on TAMs than M0 cells and Hepa1-6 cells in a dose-dependent manner (n = 3). (C) FCM analysis revealed a significantly decreased proportion of infiltrating M2-like cells in tumor tissues after treatment with Man@pSiNP-erastin+aPD-L1. (D) Growth curves, (E) tumor weights, and (F) photos of Hepa1-6 tumors after different treatments (n = 6). Reproduced with permission from ref [254]. Copyright 2023, Wiley.
3.4. Modulating the metabolism of tumor cells
Metabolic reprogramming represents a hallmark of tumors. Rapidly proliferating tumor cells consume a large amount of nutrients, such as glucose and glutamine, which leads to nutrient depletion for immune cells in the TME and aggravation of immunosuppression of the TME. In addition, abnormal metabolism of amino acids, lipids, and nucleotides contributes to the formation of a suppressive immune microenvironment in tumor tissues [260]. Recently, an array of nanomedicines have been designed to sensitize ICB by regulating tumor metabolism, and the strategies are summarized in Figure 8 and will be discussed in this section.
Improving the efficiency of ICB by regulating the metabolism of cells in the TME using drugs delivered by NDDSs. Pink for tumor cells, and yellow for T cells. (A) Intervening glucose metabolism of tumor cells by GLUT1 inhibitors, hexokinase inhibitors, glucose oxidase, PDK1 inhibitors, or LDHA inhibitors. (B) Intervening glutamine metabolism of tumor cells by glutamine transporter inhibitors or glutaminase inhibitors. (C) Intervening tryptophan metabolism of tumor cells by IDO inhibitors or kynureninase. (D) Intervening arginine metabolism of tumor cells by CAT-2 inhibition and supplying arginine to T cells via L-arginine delivery systems. (E) Intervening lipid metabolism of tumor cells by COX-2 inhibitors or FAO enhancers. (F) Intervening adenosine metabolism of tumor cells by adenosine deaminase and CD39 and CD73 inhibitors or blocking adenosine receptors on T cells. GLUT1, glucose transporter 1; PDK1, 3-phosphoinositide-dependent protein kinase-1; 2-DG, 2-deoxy-D-glucose; LDHA, lactate dehydrogenase A; DON, 6-Diazo-5-oxo-L-norleucine; Kyn, kynurenine; Trp, tryptophan; IDO, indoleamine 2,3-dioxygenase; 1-MT, as 1-methyl-DL-tryptophan; CAT, cationic amino acid transporter; COX-2, cyclooxygenase-2; AA, arachidonic acid; PGE2, prostaglandin E2; FAO, fatty acid oxidation; eATP, extracellular ATP; POM1, sodium polyoxotungstate; AMPCP, α, β-methylene adenosine 5′ diphosphate; DPCPX, 8-Cyclopentyl-1, 3-dipropylxanthine.
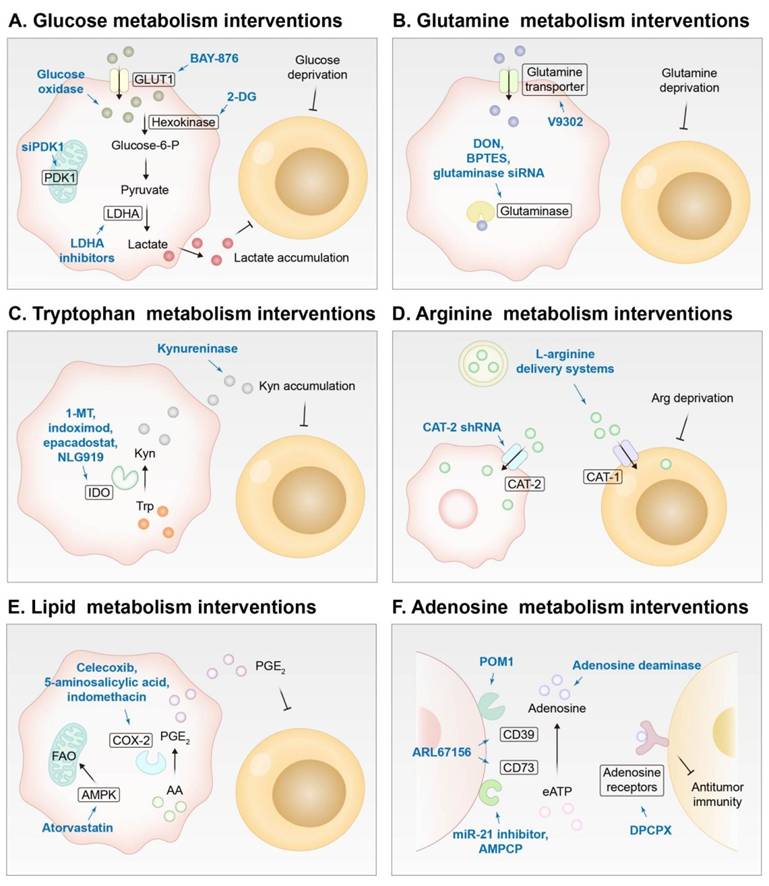
As a primary energy supply mode in cancer cells, glycolysis plays a crucial part in maintaining the energy supply of neoplastic cells, leading to the deprivation of glucose in the TME and the creation of an acidic immunosuppressive TME [261]. Inhibition of glycolysis has been shown to enhance ICB potency by restoring glucose supply to immune cells and reshaping the acidic immunosuppressive TME. A nano-assembly derived from poly β-amino ester (PAE) was prepared. This nano-assembly was loaded with BAY-876, a GLUT1 inhibitor, and the PAE moiety was conjugated with PD-L1 and CTLA-4-antagonizing aptamers, which could be released when the PAE moieties were protonated and transformed from hydrophobic to hydrophilic in an acidic TME [38]. In another study, siRNA targeting 3-phosphoinositide-dependent protein kinase-1 (PDK1) was delivered to interfere with glycolysis, thus achieving metabolic reprogramming and metastasis modulation [262]. Amphiphilic dendrimers were employed as vectors for siPDK1, and PD-L1 antibodies were decorated on the hydrophilic terminal of the dendrimers for targeting tumors and performing ICB. 2-deoxy-D-glucose (2-DG), an antiglycolytic agent, was also reported to be incorporated into nanoplatforms for combination with ICIs [263, 264]. A synergetic metabolic nanoregulator was designed by enveloping 2-DG, BAY-876, and chloroquine (an autophagy inhibitor) into ZIF-8 to boost aCTLA-4 immunotherapy [265]. A nanosonosensitizer modified with a metabolic regulation peptide R7, which restrained glycolysis of tumor cells but exerted negligible effects on normal cells, was proposed to synergize with aPD-L1 for treating challenging spinal metastasized and distant tumors [266]. In addition, due to the positive effect of OXPHOS on antitumor immunity, mPEG-PLA-PHis-ss-PEI polyplexes were developed to co-deliver siPD-L1 and resveratrol, which upregulated OXPHOS and suppressed glycolysis [267]. Nanoengineered glucose oxidase has also been reported to enhance the antitumor effect of PD-1/PD-L1 blockade by regulating glucose metabolism [268-271]. Moreover, CRISPR/Cas9 and siRNA targeting lactate dehydrogenase A (LDHA) and inhibitors of LDHA such as GSK2837808A were delivered by NDDSs to downregulate lactate production for sensitizing checkpoint blockade [272-274].
Similar to glucose, glutamine in the TME is also overconsumed by tumor cells, which severely weakens the activation of immune cells and impairs their function [275]. For example, PLL-modified lamellar molybdenum disulfide (MoS2) was constructed for co-delivering aPD-L1 and V9302, an inhibitor of the glutamine transporter that blocks glutamine internalization by tumor cells but not T cells [276]. Moreover, V9302 could drive the distribution of tumor-infiltrating lymphocytes from the periphery to the core of carcinoma tissues. This nanosystem significantly increased the glutamine concentration in the TME and evoked a potent antitumor immune response in TNBC. In another report, V9302 blocked GSH synthesis by hindering glutamine uptake and synergized with an HDAC inhibitor MS-275 that promoted the generation of a ROS storm, leading to pyroptosis of tumor cells and boosting aPD-1 efficacy [277]. Glutamine antagonists can also be used to reshape the metabolism of tumors and immune cells to benefit their combination therapy with ICIs [278, 279]. It is worth mentioning that inhibition of glutamine uptake may lead to a switch of compensatory glycolysis, which exacerbates lactate accumulation, deteriorates the immunosuppressive TME, and even enhances PD-L1 expression on tumor cells. In this context, a PD-L1-targeting metabolism and immune regulator (PMIR) was prepared. It was derived from a glutaminase inhibitor (BPTES)-loaded ZIF that was encapsulated by a liposome and modified with a PD-L1-targeting peptide on the surface [280]. ZIF not only acted as a carrier for BPTES, but also released Zn2+ in the tumor cells that could reduce NAD+ and inhibit glycolysis to counteract glycolytic compensation induced by glutamine metabolism inhibition. In addition, a mesoporous silica nanoplatform was developed to co-encapsulate lonidamine and siRNA for antiglutaminase for combating anti-PD-1 resistant tumors [281].
Tryptophan (Trp) in tumor cells can be catabolized to kynurenine (Kyn) by upregulated indoleamine 2,3-dioxygenase (IDO). The accumulation of Kyn contributes to the loss of the tumoricidal function of T cells and NK cells and the immune escape of cancer cells [282]. Nanoparticles, nanosheets, nanovesicles, and liposomes have been exploited to deliver IDO inhibitors, such as 1-methyl-DL-tryptophan, indoximod, epacadostat, and NLG919, for combination therapy of IDO inhibition and checkpoint blockade [283-292]. NLG919 and OTX015 (a bromodomain extra-terminal inhibitor) were co-loaded into mesoporous polydopamine nanoparticles for synergistic therapy of IDO inhibition, PTT, and dual immune checkpoints (PD-L1 and CD47) blockade [293]. Recently, porous silica nanoparticles (PSNs) were employed to encapsulate kynureninase (KYNase), which hydrolyzed Kyn into anthranilic acid and alanine. By reducing the level of Kyn in the TME, tumor immunosuppression was alleviated, and the therapeutic effect of aPD-1 was improved in CT26, 4T1, and B16F10-bearing mice [294].
L-arginine is essential for the proliferation, differentiation, activation, and function of T cells [295], but its delivery to a tumor site is still challenging due to its hydrophilicity. Fortunately, L-arginine was reported to be decorated with terephthalaldehyde (Ter) via an acid-responsive imine bond to allow the formation of ArgNPs (~104 nm) via a self-assembly process and acid-triggered release of L-arginine in the TME. ArgNPs, in combination with aPD-L1, dramatically increased the number of tumor-infiltrating T cells and induced the formation of memory T cells [296]. Moreover, considering that L-arginine in the TME could promote cancer proliferation when internalized by tumor cells, L-arginine-loaded multivesicular liposomes (L-arg@MVLs) were developed to simultaneously achieve L-arginine supplementation to immune cells and deprivation in tumor cells [297]. The authors employed shRNA to downregulate cationic amino acid transporter-2 (CAT-2), which is involved in transmembrane transport of L-arginine into tumor cells, while this shRNA did not impact amino acid transporter-1 (CAT-1) on the surface of CD8+ T cells and macrophages. L-arg@MVLs provided the supply of L-arginine to immune cells, and they also neutralized the acidic TME as an alkali, which effectively inhibited the growth of B16 melanoma in the mice when combined with aPD-1.
Lipid metabolic dysregulation contributes to the proliferation of cancer cells and the creation of an immunosuppressive TME [298]. Arachidonic acid (AA), an omega-6 (ω-6) polyunsaturated fatty acid, can be catalyzed into prostaglandin E2 (PGE2), a protumor inflammation mediator, by overexpressed COX-2 in neoplastic tissues [299]. Therefore, COX-2 inhibitors have been used to block PGE2 production to mitigate immunosuppression of the TME. In combination with antibodies against PD-1/PD-L1, bionic nanoparticles or polymer micelles have been employed to deliver celecoxib, 5-aminosalicylic acid, or indomethacin for COX-2 inhibition, thus reducing the presence of immunosuppressive MDSCs and TAMs while enhancing the antitumor activity of Teffs [115, 300, 301]. In addition, fatty acid oxidation (FAO) is usually downregulated in tumor cells to avoid oxidative damage caused by excess ROS production. A lipopeptide nanoplex loaded with atorvastatin (ATO) and siPD-L1 was reported to treat melanoma and colorectal cancer. Adenosine monophosphate (AMP)-activated protein kinase (AMPK) stimulated by ATO restored the downregulated FAO and promoted ROS production, and continuous production of ROS led to tumor cell killing. Moreover, ATO could inhibit triglyceride synthesis to ensure intracellular fatty acid availability. The ICD effect caused by excess ROS combined with PD-L1 silencing resulted in a favorable antitumor efficacy [302].
In addition, nucleotide metabolism may influence the formation of an immunosuppressive TME. Under the action of overexpressed ectonucleotidases, including CD39 and CD73, extracellular ATP in the TME can be converted into adenosine, which promotes the infiltration of immunosuppressive cells and inhibits the proliferation and impairs the function of immune effector cells by binding to adenosine receptors [303]. Hence, inhibiting ectonucleotidases and eliminating adenosine in the TME, or blocking adenosine receptors on immune effector cells, have been studied to alleviate tumor immunosuppression and enhance the effect of ICIs. Glioma-associated mesenchymal stem cells (GA-MSCs) were revealed to promote CD73 expression on MDSCs via exosomal miR-21 signaling. DC-derived exosomes were decorated with angiopep-2, a BBB-penetrating peptide, for GA-MSC-targeting delivery of a miR-21 inhibitor. The resulting nanomedicine, Dex-miR-21 inhibitor, downregulated CD73 expression on MDSCs. The combination of the Dex-miR-21 inhibitor and aPD-1 significantly extended the survival of GL261 glioma-bearing mice [304]. Besides, sodium polyoxotungstate (POM1, a CD39 inhibitor) [305], α, β-methylene adenosine 5′ diphosphate (AMPCP, a CD73 inhibitor) [306, 307], and ARL67156 (a dual CD39/CD73 ectonucleotidase inhibitor) [308] have been employed to restrain the ectonucleotidases activity for sensitizing ICB. In another report, 8-cyclopentyl-1, 3-dipropylxanthine (DPCPX), an adenosine A1 receptor inhibitor, and anti-PD-L1 DNAzyme were co-delivered by a core-shell nanoparticle for melanoma treatment [309]. Moreover, a nano-immunocomplex comprised of adenosine deaminase, a sonosensitizer, and aPD-L1 was designed for sono-metabolic trimodal cancer therapy, which exhibited a distinguished antitumor effect in 4T1-bearing mice [310].
3.5. Remodeling non-immune components in the TME
In addition to immune cells, non-immune stromal components in the TME, including stromal cells, ECM, vasculature, and soluble factors, interact with tumor and immune cells synchronously, and they play a pivotal regulatory role in cancer progression and immune response [311, 312]. These components have been targeted to enhance the efficacy of ICIs. The reported NDDSs for targeting non-immune stromal constituents in the TME to sensitize ICB are elaborated in Figure 9.
Strategies of reprograming non-immune components in the TME for superior antitumor effects of ICIs include alleviating hypoxia by delivering or producing O2 or inhibiting HIF-1, normalizing vasculature by antiangiogenic drugs or multikinase inhibitors, regulating CAFs by killing CAFs, suppressing CAF activation or engineering CAFs into APCs, eliminating ECM by methanotherapeutics, neutrophil elastase, hyaluronidase or collagenase, and inhibiting TGF-β using siRNA or small molecule inhibitors. HIF-1, hypoxia inducible factor-1; CAF, cancer-associated fibroblast.
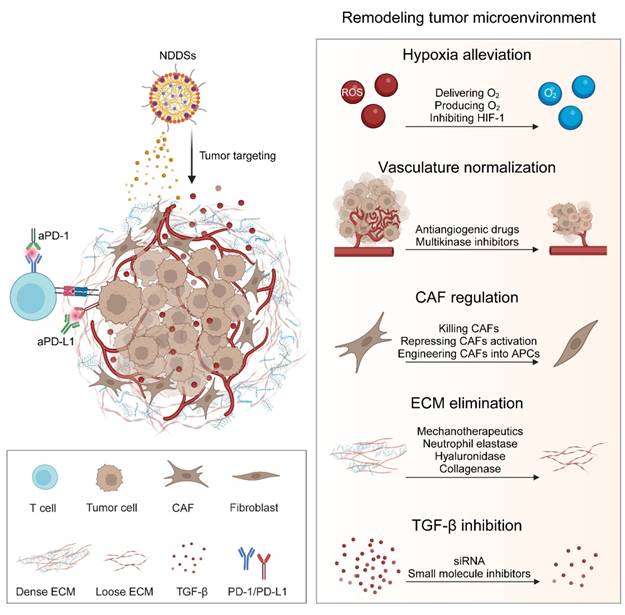
Hypoxia is one of the prominent features of the TME, and it results from excessive oxygen consumption by rapidly proliferating tumor cells and abnormal angiogenesis in the neoplasm. Hypoxia and aberrant vasculature not only seriously hinder the penetration of drugs and diminish their efficacy but also strengthen immune suppression of the TME [313]. Hence, various strategies have been formulated to alleviate tumor hypoxia. Three hypoxia alleviation strategies, including liposomal perfluorocarbon (PFC@lipo), liposomal hemoglobin (Hb@lipo), and PX-478, a commercial inhibitor for hypoxia-inducible factor-1α (HIF-1α, a hypoxia-activated transcription factor that encodes a variety of proteins responsible for cancer progression), have been compared and Hb@lipo has been identified to possess the best tolerance and safety and improve the effect of aPD-1 [314]. Interestingly, photosynthesis microcapsules were constructed with acquired cyanobacteria and upconversion nanoparticles, in which long-lasting oxygen supply at a tumor site could provide an oxygen-rich microenvironment, resulting in an enhancement of synergistic therapeutic effect with aPD-1 [315]. More recently, oxygen-encapsulated polydopamine nanoparticles were also synthesized to enhance the efficacy of aPD-1 [316]. Moreover, hypoxia is the culprit of inefficacious PDT and resistance to chemotherapy and radiotherapy. MnO2 nanoparticles have exhibited a higher catalase-like activity than catalase and other nanozymes. MnO2 has been utilized to mitigate hypoxia and boost combination immunotherapeutic effects with ICB [317-322]. For example, ICG-loaded hollow MnO2 nanospheres coated with aPD-L1-modified exosomes were prepared. They bolstered the efficacy of PDT and ICB by elevating the O2 level in the TME of non-small cell lung cancer (NSCLC) [317]. In addition, other nanoplatform-based agents to alleviate hypoxia in combination with ICIs include platinum nanoparticles [323, 324], metformin [325, 326], and Prussian blue [327]. Anti-angiogenesis represents another strategy to cope with hypoxia and enhance immunotherapy [328]. Apatinib [329], sorafenib [330], DC101 [331], and antiangiogenic peptides [332] have been incorporated into NDDSs for combined treatment with ICB.
Many types of cancer, especially pancreatic and breast cancer, are characterized by dense stroma, which contributes to tumor stiffness, a high interstitial fluid pressure (IFP), compressed vessels, and low perfusion, resulting in poor penetration of therapeutic agents and deficient infiltration of immune cells [333, 334]. Therefore, dense stroma often accounts for the dismal efficacy of ICIs in desmoplastic tumors. Blocking the production and deposition of stroma appears as an appealing approach to sensitizing ICIs. CAFs are activated and stimulated by TGF-β in tumor tissues. They not only secrete abundant extracellular matrix proteins but also attract suppressive immune cells and repel CD8+ T cells [335]. Hence, targeting CAFs could facilitate the delivery of immunotherapeutic agents and the infiltration of cytotoxic T lymphocytes for cancer immunotherapy. An oxymatrine (Om) and astragaloside IV (As) co-loaded MOF-based nanoparticle camouflaged with the platelet membrane was reported to suppress the activation of CAFs and improve the activity of tumor-infiltrating T lymphocytes (TILs) by promoting the mitochondrial function, which boosted the antitumor efficacy of aPD-L1 [336]. Dendritic poly[OEGMA-Dendron(G2)-Gly-Phe-Leu-Gly-DAS] (P-DAS, a prodrug of dasatinib) was synthesized to reprogram CAFs for reducing collagen anabolism and energy metabolism, which led to deeper penetration and superior efficacy of poly[OEGMA-Dendron(G2)- hydrazone-Epi] (P-Epi, a prodrug of epirubicin) and aPD-1 [337]. In another study, a molecular pro-theranostic probe was employed to simultaneously kill CAFs and cancer cells [338]. Melittin was also reported to induce ICD and transform activated CAFs into quiescent cells, and it collaborated with nitric oxide (NO) to enhance the potency of aCTLA-4 [339]. In addition, inspired by similar characteristics of CAFs as APCs, a photoactivatable thermochromic gene expression nanoplatform was developed by assembling a molten eutectic mixture, chitosan, and a fusion plasmid encoding CD86 and a PD-L1 trap. This nanoplatform was demonstrated to convert CAFs into APCs and block PD-1/PD-L1 signaling. The expression of CD86, a co-stimulatory molecule, resulted in the conversion of CAFs ("foe") to engineered CAFs ("friend") for processing and presenting tumor-associated antigens, which dramatically suppressed the progression of highly fibrotic 4T1 breast cancer in conjunction with PD-L1 blockade [340].
Mechanotherapeutics, such as antihypertensives, antihistamines, antifibrotic agents, and corticosteroids, have been demonstrated to normalize the TME components. Mechanotherapeutics-containing NDDSs for delivering chemotherapeutic or immunotherapeutic drugs could target specific extracellular matrix components or CAFs to remodel the TME [341]. Meanwhile, NDDSs can be designed to tailor the dose requirement for mechanotherapeutics, broaden their treatment window, augment their tumor accumulation, and relieve their side effects beyond TME normalization when administrated systemically. A micelle of [BzMA-co-DEAEMA]-b-HEGMA was prepared, and pirfenidone, an antifibrotic drug, was encapsulated into the micelle. The application of the pirfenidone-containing micelle (Pirfenidone/m) reduced the hyaluronan and collagen content in the TME at a pirfenidone dose 100-fold lower than that of its free form, resulting in a reduction in the stiffness of the stroma and the IFP within the tumor tissue, but an enhancement in the accumulation and penetration of delivered ICIs at the tumor site, thereby significantly improving the therapeutic effect of aPD-1+aCTLA-4 (Figure 10) [342]. In addition, neutrophil elastase-decorated biomimetic liposomes were prepared to degrade elastin and type I collagen in the TME and improve the accumulation of PTX and aPD-1 at the tumor site [343]. In another study, CPT prodrug-derived nanofibers were constructed to co-deliver two DNA plasmids expressing shRNA of PD-L1 (shPD-L1) and hyaluronidase (HAase). The fiberlike morphology of the nanocarrier and HA degradation by HAase contributed to enhanced penetration of shPD-L1 and boosted its efficacy [344]. Furthermore, multi-targeted tyrosine kinase inhibitors can be used to remediate collagen deposition and abnormal vasculature for effective synergetic therapy with ICIs [345-347].
In addition to the type I collagen (Col1) heterotrimer (α1/α2/α1) secreted by fibroblasts, the oncogenic collagen, the Col1 homotrimer (α1/α1/α1), has been revealed to play a role in the formation of an immunosuppressive TME in pancreatic ductal adenocarcinoma (PDAC). The Col1 homotrimer is induced in cancer cells due to epigenetic silencing of the COL1A2 gene, and it upregulates the FAK/EGFR/MAPK/MYC signaling network by interacting with integrin α3β1, promoting the proliferation of neoplastic cells and development of their therapeutic resistance as well as the recruitment of immunosuppressive cells. A ROS-sensitive protein cage was designed to accommodate collagenase and aPD-L1, and the cage was attached to the surface of a probiotic Escherichia coli strain Nissle 1917 (ECN). The hypoxia tropism of ECN and its outstanding motility facilitated efficient penetration of collagenase into deep tumor tissues, where the oncogenic collagen is deposited. This nanodrug-bacteria conjugate markedly strengthened the immunotherapeutic effect against PDAC [348].
Notably, TGF-β is a prominent immunosuppressive cytokine secreted by neoplastic cells, CAFs, and TAMs, and it plays a pivotal role in angiogenesis, ECM deposition, and immunosuppression in the TME [349, 350]. Therefore, enhancing the therapeutic effect of ICIs by targeting TGF-β has been extensively explored. For example, an HAase and GSH dual-responsive nanosystem with a coating layer of hyaluronic acid (HA) was constructed to deliver TGF-β siRNA to tumor cells and CAFs, which inhibited both primary and distant tumors when combined with aPD-L1 in a 4T1 breast cancer model [351]. In addition, a multifunctional nanodrug was reported to exert a significantly synergetic effect with aPD-L1. In this nanodrug, salvianolic acid B was exploited to inhibit TGF-β signaling and upregulate PD-L1 expression, and a photothermal agent was used to trigger ICD [352]. Furthermore, small-molecular inhibitors, such as galunisertib [353, 354], SB-505124 [355, 356], LY3200882 [357], and LY2157299 [358], have been utilized to block the TGF-β signal pathway for improving ICB-based immunotherapeutic effects.
Mechanotherapeutics delivered by an NDDS for enhancing the efficacy of ICIs by reducing the stiffness of tumor stroma, decreasing the IFP, and increasing drug perfusion. (A) Schematic of the formation of a pirfenidone-loaded micelle (Pirfenidone/m). (B) Size distribution of the micelles. Representative images of (C) shear wave elastography (SWE) and (D) contrast enhanced ultrasound (CEUS) to support a significantly reduced stiffness and an increased perfusion rate after treatment with Pirfenidone/m. The lines delineate the tumor margins. (E) Growth curves of E0771 tumors (n = 9 or 10 mice). (F) Kaplan-Meier survival curves of the E0771 tumor model after various treatments. Reproduced with permission from ref [342]. Copyright 2023, American Chemical Society.
3.6. Multifunctional immunoregulatory NDDSs
Immune system activation is a multifaceted process involving several steps and multiple factors. Manipulating a solitary factor may provide inadequate immune response activation, resulting in immune evasion and resistance to ICB. NDDSs with multiple modulations can theoretically activate effective immune responses and achieve a synergistic antitumor effect. These nanomedicines have the capacity to enhance immunogenicity, regulate the metabolism of both tumor cells and immune cells, activate immune cells, and reshape the tumor microenvironment (TME). For example, a biomimetic nanoplatform (SS@Lipo/Hb/GOx/JQ1) was constructed by modifying a Hb, GOx, and JQ1 co-loaded liposome with spore shells (SSs) of Bacillus coagulans. In this system, SSs with an adjuvant activity could activate immune cells, and GOx catalyzed glucose into ROS, which triggered ICD under the action of Fe2+ in Hb. Meanwhile, Hb relieved hypoxia in the TME, and JQ1 downregulated the expression of PD-L1 [359]. A radio-immunostimulant nanomedicine (IPI549@HMP) was engineered, in which hollow MnO2 was employed as a drug delivery carrier and a catalyst of hydrolyzing H2O2 to alleviate hypoxia and potentiate radiotherapy, and IPI549 was applied to eliminate immunosuppressive myeloid cells. IPI549@HMP-mediated radiotherapy in synergy with aPD-L1 could establish immune memory to combat tumor rechallenge, leading to inhibition of residual and distant tumors [360]. In another study, a multifunctional nanosystem composed of CpG ODNs, an immunostimulating adjuvant, and siRNA cocktails, including STAT3 siRNA to induce ICD, CCR2 siRNA to trigger MDSCs, and TGF-β siRNA to alleviate immune suppression was designed. Moreover, cholesterol-modified AMP DP7 (DP7-C) with an adjuvant activity was exploited as the nanocarrier for the multifunctional nanosystem. The constructed DP7-C/siRNAs/CpG ODNs resulted in a pronounced antitumor effect in both CT26 and B16F10 tumor models when combined with aPD-1 [361]. Many studies have shown that multifunctional immunoregulatory NDDSs can exhibit excellent antitumor efficacy, generate abscopal effects to prevent distant metastasis, and provide long-term immune memory against tumor recurrence.
Multifunctional NDDSs could also be employed for integrated theranostic design. The activation status of the immune system can be assessed through non-invasive visualization of immune responses via an imaging probe in an NDDS, and ICB can be implemented by delivering ICIs through the NDDS in a controllable manner. In this context, precise imaging-guided ICB could be achieved [362]. Conventional clinical detection methods, such as ultrasound, PET/SPECT, MRI, and CT, can be employed to monitor immune responses [363, 364]. An array of NDDS platforms have been utilized for tumor imaging, for example, microbubbles for ultrasound imaging, liposomes, dendritic macromolecules, or polymer nanoparticles loaded with MRI contrast agents (e.g., iron oxide, gadolinium ions, or manganese ions), gold nanoparticles as inorganic carriers for CT imaging, and tracers containing radioactive isotopes or fluorescent groups for PET and SPECT [365, 366]. In order to enable real-time and dynamic monitoring, NDDSs are often armed with TME stimuli-responsive groups. Optical imaging is the most extensively studied method, particularly when activatable molecular probes are used. Biomarkers associated with immune activation can be harnessed for the development of optical imaging methods [367, 368]. These probes generate signals exclusively in the presence of specific biomarkers (e.g., PD-L1/PD-1, granzyme B, and ROS), thereby significantly enhancing detection specificity and minimizing background noises. This technique allows non-invasive, real-time visualization of imaging probes distributed in the body and biomarkers that are targeted by these imaging probes. Other imaging strategies have also made progress. Silica-coated iron oxide nanoparticles for MRI and micro-computed tomography (μCT) imaging were employed for non-invasive diagnosis of solid tumors. Additionally, a system responsive to MMP-2 in the tumor microenvironment to realize controlled release of drugs was developed for targeted delivery of PD-L1 inhibitors [369]. 68Ga-NOTA-Nb109, a single-domain antibody with a special structure and a small molecular weight, could preferably accumulate into the A375-human PD-L1 tumor and could realize non-invasive and dynamic imaging of PD-L1 in vivo [370]. Similar nanoprobes labeled with other contrasts have been constructed to target PD-L1 [371].
In summary, NDDSs with multifunctional immune-modulating ability can improve the permeability and distribution of therapeutic agents in tumor tissues and have been widely applied in combination immunotherapy. NDDSs can be constructed with therapeutic and diagnostic agents. They could also provide real-time monitoring of immunotherapy efficacy, reducing irAEs and resistance to ICB. This approach demonstrates great potential in enhancing cancer theranostics and promoting treatment effectiveness.
4. Emerging Strategies for ICB
In addition to conventional ICIs, emerging ICB strategies based on NDDSs to induce checkpoint protein degradation or indirectly inhibit checkpoint expression using therapeutic drugs have been extensively explored. Instead of using antibodies, nucleic acids, or small molecular ICIs, the ICP pathway can be effectively blocked by targeting the degradation of PD-L1, inhibiting PD-L1 palmitoylation, activating AMPK by suppressing OXPHOS, alleviating hypoxia or other mechanisms. These emerging strategies could open a new avenue for ICB.
Proteolysis targeting chimera (PROTAC), a protein degradation technology, has been employed in disease treatment by inducing targeted protein degradation via the ubiquitin-proteasome system (UPS). Recently, a checkpoint nano-PROTAC (NPRO) for blocking CD47/SIRPα and PD-1/PD-L1 by targeting the Src homology 2 domain-containing phosphatase 2 (SHP2) was reported [372]. The checkpoint NPRO self-assembled from the blocked PROTAC peptide (bPRO) composed of protoporphyrin IX, a caspase 3-cleavable segment (DEVD), a UBR E3 ligase-targeting unit, and a SHP2-targeting peptide. Upon ICD induced by PDT, DEVD was cleaved by caspase 3, and the activated PROTAC (aPRO) was subsequently released for the depletion of SHP2 (Figure 11). Similarly, a PROTAC of bromodomain and extraterminal protein 4 (BRD4) for epigenetic regulation of PD-L1 and c-Myc [373] and a carbon-dot-based PROTAC of the PD-L1 protein have been developed for cancer immunotherapy [374]. PROTAC has been explored for the degradation of pathogenic proteins since the discovery of small molecular inhibitors for these proteins is challenging. It has shown great potential in treating many diseases, including cancer. Complex chemical synthesis steps, risks of immunogenicity and off-target, and high development costs are critical obstacles to future application of the PROTAC technology [375-377].
NDDS-based proteolysis targeting chimera (PROTAC) for immune checkpoint blockade. (A) Construction of a checkpoint nano-PROTAC (NPRO) and its chemical structure. Schematic illustration of NPRO-mediated cancer photo-immunotherapy: degradation of SHP2 by caspase 3-specifically activated NPRO to inhibit CD47/SIRPα signaling in macrophages (Macs) and the PD-1/PD-L1 axis in T cells, leading to enhanced phagocytosis effects and antitumor T-cell immunity. (B) TEM image of NPRO. (C) SHP2 immunofluorescence staining images and MFIs of 4T1 tumor tissues after treatment with saline, NPRO, and NPRO + L. Blue for DAPI and green for SHP2. (D) GranB+CD8+ T cells in 4T1 tumors after different treatments (n = 3). Reproduced with permission from ref [372]. Copyright 2023, Wiley.
The palmitoylation of PD-L1 by palmitoyltransferase ZDHHC3 (DHHC3) has been demonstrated to block PD-L1 ubiquitination and degradation, leading to the redistribution of PD-L1 on the plasma membrane and a reduction in therapeutic benefits of ICB [378]. Encouraged by this finding, 2-bromopalmitate, a palmitoylated inhibitor, was incorporated into polymer-lipid hybrid nanoparticles for ICB treatment [379]. Harnessing superior targeting specificity of competitive peptides, another team designed a nano-inhibitor (FRS) for PD-L1 palmitoylation. The nano-inhibitor was composed of a fluoroalkylated competitive peptide (RMMDVKKCGIQDTNS, termed "RS"). The fluoroalkylated peptide and DOX self-assembled into a nanoparticle [380]. After the nanoparticle was transported into tumor cells, the disulfide bond between RS and the fluorous tag could be cleaved to release RS and DOX. RS competed with PD-L1 in palmitoylation by DHHC3. Lysosomal clearance of PD-L1 combined with the released chemotherapeutic DOX triggered a powerful antitumor immune response in a CT26 mouse tumor model.
Elevated glycolysis and OXPHOS levels in tumor cells lead to a decrease in intracellular adenosine diphosphate (ADP)/ATP and AMP/ATP ratios, which impedes the activation of AMPK. Activating AMPK by inhibiting OXPHOS can phosphorylate PD-L1 and promote its ER accumulation and ER-associated protein degradation (ERAD) [381, 382]. Inspired by this, a series of nanosystems derived from albumin nanoparticles or liposomes were developed to deliver mitochondria-targeting OXPHOS suppressors [383-387]. Mitochondria-targeting triphenylphosphonium cation (TPP+) or heptamethine cyanine dyes (MHI or IR-68) have been chemically conjugated with an OXPHOS disruption agent (metformin, lonidamine, or tamoxifen) to improve the OXPHOS inhibition efficiency and reduce the therapeutic dose of OXPHOS suppressors.
It is well known that PD-L1 is a downstream target gene of HIF-1. In this context, suppressing HIF-1 by alleviating hypoxia or applying HIF-1 inhibitors has been widely used to downregulate PD-L1. Hemoglobin [388], catalase [389], hyaluronidase [390], γ-Fe2O3 [391], manganese oxide nanoparticles [392-394], and mesoporous platinum nanoparticles [395] are reported to decrease PD-L1 by mitigating hypoxia and improve antitumor immune responses. HIF-1 inhibitors, including acriflavine [396] and BAY87-2243 [397], are also utilized to curtail the expression of PD-L1 in nanoplatform-based immunotherapy. Moreover, glucosamine-labeled liposomal ceramide was reported to block HIF-1-mediated PD-L1 expression [398]. Other strategies for downregulating immune checkpoints are listed in Table 2.
List of novel nanoplatform-based strategies for immune checkpoint blockade.
| Nanoplatform | Therapeutic agents | NDDS types | Mechanism of ICB | Cancer model | Refs |
|---|---|---|---|---|---|
| NPRO | SHP2-targeting PROTAC, protoporphyrin IX | peptide nanoparticle | targeted degradation of SHP2 | 4T1 breast cancer | [372] |
| DdLD NPs | PROTAC of BRD4, DOX | self-assembled nanoparticle | targeted degradation of BRD4 | CT26 colorectal cancer | [373] |
| CDTACs | PROTAC of PD-L1 | carbon-dot | targeted degradation of PD-L1 | CT26 colorectal cancer, B16-F10 melanoma | [374] |
| 2-BP/CPT-PLNs | 2-bromopalmitate, CPT | polymer-lipid hybrid nanoparticle | inhibiting PD-L1 palmitoylation inhibiting PD-L1 palmitoylation | B16F10 melanoma | [379] |
| FRS/DOX | PD-L1 competitive inhibitor peptide, DOX | peptide nanoparticle | inhibiting PD-L1 palmitoylation inhibiting PD-L1 palmitoylation | CT26 colorectal cancer | [380] |
| MHI-TMX@ALB | tamoxifen, MHI | albumin nanoparticle | activating AMPK by inhibiting OXPHOS | 4T1 breast cancer, MB49 bladder cancer | [383] |
| IR-LND@Lip | lonidamine, radiotherapy | liposome | activating AMPK by inhibiting OXPHOS | 4T1 breast cancer | [384] |
| IR-TAM@Alb | tamoxifen, radiotherapy | albumin nanoparticle | activating AMPK by inhibiting OXPHOS | 4T1 breast cancer, MB49 bladder cancer | [385] |
| IR-LND@Alb | lonidamine, IR-68 | albumin nanoparticle | activating AMPK by inhibiting OXPHOS | CT26 colorectal cancer, MB49 bladder cancer | [386] |
| TPP-LND@Lip | lonidamine, radiotherapy | liposome | activating AMPK by inhibiting OXPHOS | LLC lung cancer | [387] |
| V(Hb)@DOX | hemoglobin, DOX | biomimetic nanovesicle | alleviating hypoxia | 4T1 breast cancer, CT26 colorectal cancer | [388] |
| p-Adv-CAT-KR | catalase, KillerRed, adenovirus | adenovirus system | alleviating hypoxia | AKT/YapS127A cholangiocarcinoma | [389] |
| mPDAase | hyaluronidase, mesoporous polydopamine | polydopamine nanoparticle | alleviating hypoxia | 4T1 breast cancer | [390] |
| γ-Fe2O3/ISDN@(NBCF-CMCS) | γ-Fe2O3, isosorbide dinitrate | polysaccharide micelle | alleviating hypoxia | H22 hepatoma | [391] |
| BMIP2N NPs | MnO2, IR780, NLG919, PTX | manganese dioxide albumin nanoparticle | alleviating hypoxia | 4T1 breast cancer | [392] |
| BMI | MnO2, IPI549 | albumin nanoparticle | alleviating hypoxia | 4T1 breast cancer | [393] |
| Melanin/MnOx nanohybrids | melanin, MnOx | hybride nanoparticle | alleviating hypoxia | 4T1 breast cancer | [394] |
| Pt@PEG-Ce6 | mesoporous Pt, Ce6 | Pt nanoparticle | alleviating hypoxia | 4T1 breast cancer | [395] |
| LipoCu-OA/ACF | acriflavine, copper oleate | liposome | HIF-1 inhibition | 4T1 breast cancer | [396] |
| PNEs | BAY87-2243, neutrophils | polysaccharide nanoparticle | HIF-1 inhibition | 4T1 breast cancer | [397] |
| G5C3 | ceramide, paclitaxel/carboplatin | liposome | HIF-1 inhibition | LLC-1, H1299 lung cancer | [398] |
| QFN | quercetin, Ferrum ion | phenolic-metal nanoparticle | quercetin reduced PD-L1 | B16F10 melanoma | [399] |
| MB@Bu@MnO2 | methylene blue (MB), Butformin (Bu) | manganese dioxide albumin nanoparticle | MB inhibited PD-1 signaling and Bu enhanced AMPK phosphorylation | MB49 bladder cancer, 4T1 breast cancer | [400] |
| sAZD1080 | AZD1080 | silicasome | downregulating PD-1 expression by inhibiting glycogen synthase kinase 3 | MC38, CT26 colorectal cancer, LLC lung cancer, KPC pancreatic cancer | [401] |
| LMDFP | Cu2+, D-penicillamine, lanthanide-doped nanocrystals | MSN | Cu2+ inhibited PD-L1 expression | PC-3 prostatic cancer, 4T1 breast cancer | [402] |
| OA@CuMnCs | Cu2+, Mn2+, oncolytic adenovirus | biomineralized adenovirus | Cu2+ inhibited PD-L1 expression | CT26 colorectal cancer | [403] |
| RCH NPs | roscovitine, hemin, celecoxib | albumin nanoparticle | roscovitine suppressed IFN-γ-dependent PD-L1 gene transcription by inhibiting the Cdk5 pathway | B16F10 melanoma | [404] |
| CMArg@Lip | ML385, L-arginine, Ce6 | liposome | ML385 inhibited the nuclear factor erythroid 2-related factor 2 (NRF2) and NO reduced PD-L1 expression | QBC-939 cholangiocarcinoma | [405] |
| MPB-NO@DOX+ATRA | NO, DOX, Mn2+, all-trans retinoic acid | prussian blue nanoparticle | NO restrained PD-L1 expression | 4T1 breast cancer | [406] |
| mCGYL-LOx | lactate oxidase, CuO, Gd2O3 | metal nanoparticle | lactate exhaustion reduced PD-L1 expression | Renca renal cell carcinoma | [407] |
| Cu-DBCO/CL | Cu-DBCO nanozyme, cholesterol, lysyl oxidase inhibitor | MOF | cholesterol depletion downregulated the expression of PD-1 and TIM-3 | 4T1 breast cancer | [408] |
| CCP@DA | the cell-membrane targeting chimeric peptide C16-Cypate-RRKK-PEG8-COOH, diclofenac | peptide nanoparticle | downregulating the PD-L1 expression by destroying the cell membrane and blocking the COX-2/PGE2 pathway | 4T1 breast cancer | [409] |
| a-LDH@YW3-56-PEG | YW3-56, CoW-layered double hydroxide | nanosheet | inhibiting peptidyl arginine deiminase 4 | 4T1 breast cancer | [410] |
5. Perspectives and challenges
Numerous studies have utilized NDDSs to address irAEs and drug resistance associated with ICIs and improve immunotherapeutic efficacy. Strategies have been developed to prolong the drug circulation time, minimize effective doses, improve tumor targeting specificity, reduce non-specific uptake, and promote remodeling of the TME. However, very few NDDS-based therapeutic nanomedicines have been translated into clinical application. Extensive studies on intricate mechanisms of ICI resistance and irAEs, NDDS construction strategies, treatment effect monitoring technologies, and combinational optimization of therapeutic agents are required to successfully translate these NDDS-based therapeutic nanomedicines into clinical trials (Figure 12).
5.1. Mechanism investigation
It is crucial to recognize that conventionally used strategies may be insufficient to address complex and multifaceted challenges of response and resistance to ICIs [17]. It has been found that the mechanisms of immune escape vary in different types of tumors, and even within a specific tumor type, immune evasion mechanisms can exhibit significant variations [411]. Although combinational drugs to achieve direct tumor cell elimination and TME modulation have shown enhanced immunotherapeutic efficacy in certain animal models, the effectiveness of such combinations may be compromised by intricate interactions among drugs, tumor cells, immune cells, TME components, and signaling/chemotactic factors [412]. Therefore, an in-depth revelation of these interactions and the underlying mechanisms of irAEs and ICI resistance can tailor immunomodulation strategies and facilitate optimal antitumor efficacy.
The underlying mechanisms of ICI resistance are related to tumor cells, their TME, and the host. (1) Tumors can inhibit the immune response by downregulating presentation antigens, suppressing APC functions, increasing inflammatory cytokine accumulation, and restraining T cell activation and infiltration. The processes by which cancer neoantigens are internalized by APCs and subsequently cross-presented to naive CD8+ T cells, and the factors that modulate the activation and function of both naive and primed CD8+ T cells are critical for eliciting effective antitumor immune responses; (2) Tumor cells may adapt compensatory metabolic pathways upon introducing metabolic interventions, leading to acquired resistance [65]. Identifying key metabolic pathways in different types of cancer cells could facilitate the development of specific and effective metabolic intervention strategies to suppress tumor proliferation without inducing resistance. Since tumor cells in different regions of a tumor site exhibit distinct metabolic characteristics, which may dynamically change with tumor progression, metabolic intervention strategies must account for the spatial-temporal heterogeneity of tumor cells within the TME; (3) It has been shown that only less than 20% of patients exhibit an immune-responsive TME. In contrast, the majority of patients have an immunosuppressive TME, resulting in poor responses and prognoses to ICB therapy. The TME is a complex system constituted of various types of immune cells, stromal cells, dense ECM, and abnormal vasculature. All these components are dynamically altered, interdependently interactive with each other, and synchronously coordinated to establish a favorable environment for tumor progression and metastasis [17, 413]. The current understanding of these components, especially interactions between these components, remains the tip of the iceberg, and profiling the TME helps the development of therapeutic drugs to destroy tumor physical barriers, modulate the TME composition, and activate effector T cells in the TME; (4) Host-derived non-immune factors, such as microbiomes, hormones, and neuronal signaling molecules, have been shown to play a role in ICI response and resistance [12, 414]. Correlations between the clinical efficacy of ICIs and race, age, gender, lifestyle, diet, and other factors of patients remain to be discovered through advanced data analytics.
Nanomedicine development directions for advanced ICB therapy. CT, computed tomography; PET, positron emission tomography; SPECT, single-photon emission computerized tomography; MRI, magnetic resonance imaging.
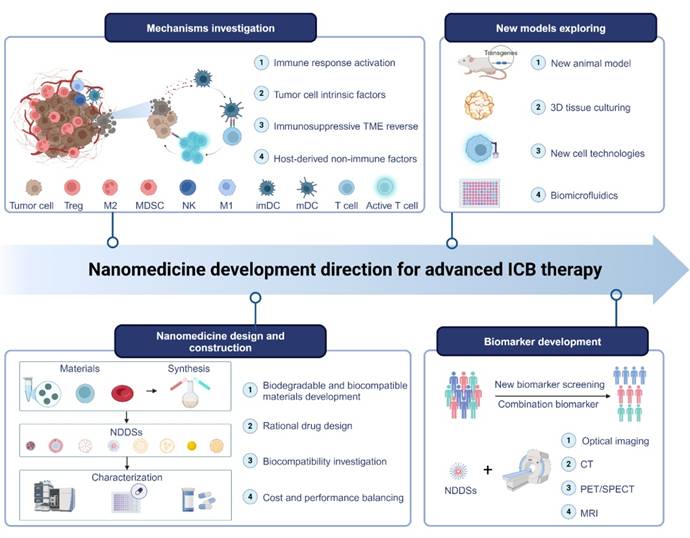
5.2. Safety issues stemmed from nanomedicine construction
Despite the demonstrated superior efficacy of many nanodrugs, there are challenging issues associated with NDDSs for ICB therapy. To improve the therapeutic efficacy of NDDS-based ICIs and ensure their biosafety, a variety of factors should be taken into consideration for NDDS design and preparation: (1) Material chemistry is the core of NDDSs, and novel biodegradable and biocompatible materials should be developed to meet safety needs for ICB therapy; (2) Different properties of drugs and their dosage ratios should be considered during design and preparation of NDDSs because pharmacokinetic (PK) and pharmacodynamic (PD) performances of these drugs can be pronouncedly improved by NDDSs. Therefore, more studies should be implemented on studying PK/PD of combination treatments with diverse kinds of drugs and investigating their application in different types of tumors; (3) Smart design endows NDDS-derived nanomedicines with the capability of targeting specific tissues, cells, and subcellular organelles, which may dramatically boost therapeutic effects of drugs. For example, stimulus-responsive nanomedicines can release drugs controllably by designing and constructing specific structures to respond to endogenous/exogenous stimuli. Studies should be conducted to investigate the stimuli-responsiveness of these structures and fine-tune the balance between the efficacy and the safety of ICIs; (4) Too complex drug delivery systems may not be conducive to large-scale production, quality control, and clinical translation of nanomedicines. The lack of efficient purification methods, a high cost of large-scale preparation, a low production yield, and challenging issues in quality control have hampered large-scale NDDS production. Collaborative efforts among material chemists, engineers, and clinicians could be essential to overcome obstacles to NDDS production in a good manufacturing practice (GMP) facility.
5.3. Biomarker development
Biomarkers could be used to predict/monitor/assess the therapeutic effects of ICB therapy. The discovery of reliable and specific biomarkers is essential for accelerating the transformation of ICB therapy into clinical application. Ideal biomarkers are expected to assist ICB therapy from multiple perspectives, including identifying patients who may positively respond to ICIs, assisting clinicians with decision-making processes via prognosis assessment regarding disease progression and patient survival duration, or predicting potential adverse events associated with the therapy via evaluating the effects of ICIs on the immune system. To date, PD-L1 and MSI-H/dMMR have been officially approved for clinical use as biomarkers, and they have shown great promise in certain indications [415, 416]. Additional biomarker candidates are actively sought. Based on their sources, these biomarkers can be categorized into three types: immune biomarkers, genetic biomarkers, and biomarkers in peripheral blood. Among all these biomarkers, PD-L1 expression, microsatellite instability (MSI), tumor mutation burden (TMB), and mismatch repair deficiency (dMMR) have been well-established to determine whether patients can benefit from ICIs [417, 418]. An elevation in the PD-L1 expression is associated with a preferable prognosis [11, 419]. A high level of MSI indicates a high mutation burden and great sensitivity to ICIs [420]. A greater level of TMB is often correlated with more neoantigens on tumor cells, which could promote attacks by the immune system [421]. The majority of these biomarkers are from tumor tissues, which may hamper their application due to the high demand for adequate tissue samples and the invasive nature of the procedure of obtaining tissue samples. Therefore, blood biomarkers are preferable in future clinical applications. Apart from discovering novel biomarkers, strategies for simultaneously harnessing two or more biomarkers have yielded comprehensive benefits. For example, combining TMB with PD-L1 expression exhibited better prediction than one single biomarker [422].
NDDSs have been used to combine ICIs with imaging probes for one or multiple biomarkers, enabling monitoring of immune responses and disease progression by detecting specific immune cell types, metabolites during/after immune activation, or biomarkers for indicating the immune cell status, therefore, precise prediction, real-time monitoring, and accurate assessment of the therapeutic effect of ICIs can be achieved. However, challenges to the clinical translation of NDDS-aided imaging techniques remain in many aspects. Optical imaging, the widely investigated method, has drawbacks, including poor tissue penetration and insufficient depth of examination [367]. On the contrary, clinical routine imaging techniques, such as CT, PET, and MRI, have not been extensively used for biomarker detection. Moreover, a comprehensive evaluation of these imaging techniques, including in vivo stability, biosafety, and diagnostic accuracy, is required for their clinical application. Nevertheless, the development of imaging probes for multiple biomarkers is a promising research direction for their clinical application in precise prediction, real-time monitoring, and accurate assessment of therapeutic outcomes of NDDS-aided ICI therapy.
5.4. Exploring new models
In vitro and in vivo models are indispensable tools for evaluating the antitumor efficacy, elucidating the mechanisms, and assessing the safety of ICIs. It has been noticed that the results of preclinical studies are often not replicable in clinical trials. This discrepancy can be ascribed to intrinsic differences between preclinical models and human patients. In order to bridge this gap, advanced models are developed to accurately replicate pathological conditions and tumor progression processes observed in human patients. For in vitro studies, 3D in vitro systems have been developed to model immune responses to solid tumors. Thus, they have been used to screen drugs, probe mechanisms, and examine interactions between immune cells and tumor cells since the conventional two-dimensional cell culture method fails to mimic interactions between cells and the ECM, between different types of cells, and/or between the same type of cells [423]. These interactions are crucial for regulating immune cell polarization, maintaining phenotypic stability, and influencing tumor cell proliferation and metabolic activity. Immune-oncology models developed with the assistance of biomaterials, such as scaffolds, organoids, and microfluidics, are more sophisticated in vitro platforms that can mimic the structural complexity of tumors and preserve tumor heterogeneity [424, 425]. These in vitro models have been exploited to replicate immune interactions in irAEs-related organs, which can help in the understanding of irAEs and assessing them induced from NDDS-based immunotherapeutic formulations.
Preclinical animal models have also been constructed to bridge the gaps between animal models and human patients. It has been observed that ICIs that are efficacious in animal models may fail in humans, and the incidence of irAEs in animal models is often lower than that in patients [426]. To address the challenge of human immune system homeostasis and the interactions between immune cells and tumor cells, a myriad of in vivo animal models have been developed to simulate tumor growth, examine immune responses, evaluate drug efficacy, and assess irAEs. Among them, xenograft mice models resemble clinical patients more closely than traditional syngeneic tumor models. The patient-derived xenograft (PDX) model developed by transplanting human tumor tissues into immunodeficient mice preserves the heterogeneity and complexity of the original tumor. The PDX model can be employed for personalized treatment evaluation [427]. Humanized mice models are in the development stage to accurately mimic the intricate interaction between the immune system and the tumor [428]. Engineered transgenic mice are often constructed with specific gene phenotypes. Autoimmune-prone mice, such as non-obese diabetic (NOD) mice and the Murphy Roths Large (MRL) mice with the lymphoproliferation spontaneous mutation (Faslpr), have been utilized as model animals to assess irAEs of immunotherapy. Depletion of immunosuppressive cells, represented by Tregs, in C57BL/6 and BALB/c mice, has been harnessed to predict the occurrence of irAEs under the administration of ICIs [429, 430]. Other experimental animals, such as dogs and cynomolgus monkeys, are also important research tools for accelerating the translation of preclinical outcomes of NDDS-based Immunotherapeutics into their clinical benefits. However, these animal models can only partially replicate the dynamic, complex system in patients. Efforts have been driven to reduce the cost and expand their versatility as regular evaluation methods. Moreover, the collection and analysis of clinical samples and processing of large-scale clinical data may provide a better understanding of human pathology, which helps guide the direction of preclinical studies [431]. The refinement of current preclinical models and the development of novel models will facilitate successful clinical translation of NDDS-based ICI Immunotherapeutics.
6. Conclusion
This review briefly elaborated on the predominant mechanisms for irAEs and ICI resistance. Strategies for designing and formulating nanoplatforms for ICB therapy to improve immunotherapeutic effects and address irAEs and ICI resistance were reviewed. Different types of ICIs that have been encapsulated in NDDSs were summarized, and nanomedicines derived from NDDSs have been demonstrated to enhance the immunogenicity of tumors, strengthen the function of APCs, manipulate suppressive immune cells in the TME, modulate the metabolism of tumor cells, and remodel non-immune components in the TME. Although advanced NDDS-based ICI treatment strategies have shown their effectiveness in preclinical models, very few of them have reached clinical translation. Based on a comprehensive summary of current progress, we analyzed unmet clinical needs and existing challenges in several key areas, including irAEs and drug resistance mechanisms, safety issues stemming from nanomedicine construction, NDDS-based integrated diagnosis and treatment, and in vitro/in vivo new models. Our insights into the future direction of research and development of NDDS-based ICB therapy could help accelerate its clinical application and benefit more cancer patients.
Abbreviations
ICIs: Immune checkpoint inhibitors; irAEs: immune-related adverse events; ICB: immune checkpoint blockade; NDDSs: Nano drug delivery systems; CTLA-4: cytotoxic T lymphocyte-associated protein 4; PD-1: programmed cell death protein 1; PD-L1: programmed death-ligand 1; ICPs: immune checkpoints; LAG-3: lymphocyte activation gene-3; TIM-3: T cell immunoglobulin and mucin-domain containing-3; TIGIT: T cell immunoglobin and ITIM domain; VISTA: V-domain Ig suppressor of T cell activation; APCs: antigen presenting cells; CTLs: cytotoxic T lymphocytes; GLUT1: glucose transporter 1; BBB: blood-brain barrier; Tregs: regulatory T cells; GBM: glioblastoma; NPs: nanoparticles; ROS: reactive oxygen species; TAMs: tumor-associated macrophages; MDSCs: myeloid-derived suppressor cells; CAFs: cancer-associated fibroblasts; ECM: extracellular matrix; VEGF: vascular endothelial growth factor; TGF-β: transforming growth factor-β; ICD: immunogenic cell death; DAMPs: damage-associated molecular patterns; HMGB1: high mobility group protein B1; CRT: calreticulin; ATP: adenosine triphosphate; PTT: photothermal therapy; PDT: photodynamic therapy; SDT: sonodynamic therapy; CDT: chemodynamic therapy; GSH: glutathione; PTX: paclitaxel; IFN-γ: interferon gamma; DOX: doxorubicin; TAAs: tumor-associated antigens; LND: lonidamine; GSDM: gasdermin; TCA: tricarboxylic acid; OXPHOS: oxidative phosphorylation; ER: endoplasmic reticulum; MHC: major histocompatibility complex; DNMT: DNA methyltransferase; HDAC: histone deacetylase; HMT: histone methyltransferase; TLR: toll-like receptor; cGAS: cyclic guanosine monophosphate-adenosine monophosphate synthase; STING: stimulator of interferon genes; CDNs: cyclic dinucleotides; TNBC: triple-negative breast cancer; dsDNA: double-stranded DNA; mtDNA: mitochondrial DNA; OVA: Ovalbumin; Teffs: effector T cells; HCC: hepatocellular carcinoma; LDHA: lactate dehydrogenase A; IDO: indoleamine 2,3-dioxygenase; AA: arachidonic acid; PGE2: prostaglandin E2; COX-2: cyclooxygenase-2; NSCLC: non-small cell lung cancer; IFP: interstitial fluid pressure; HAase: hyaluronidase; PROTAC: proteolysis targeting chimera; AMPK: adenosine monophosphate-activated protein kinase; MSI: microsatellite instability; TMB: tumor mutation burden; dMMR: mismatch repair deficiency.
Acknowledgements
Illustrations were created with BioRender.com and/or Adobe Illustrator. This work was financially supported by the National Natural Science Foundation of China (32271445), National Key Research and Development Program of China (2023YFB3810004, 2023ZD0502300, 2022YFC2009900), Department of Science and Technology of Sichuan Province (2024NSFJQ0050, 20233ZYD0167), 1‧3‧5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYGD23026), China Postdoctoral Science Foundation (2024M752275).
Author contributions
C.-Q. Guo, L. Lin, and K. Luo conceived the outline of this review. C.-Q. Guo, L. Lin and Y.-H. Wang collected papers for writing, drafted the original manuscript, and performed the creation of figures/tables. J. Jing and Q.-Y. Gong helped revise the manuscript and discuss the content. K. Luo supervised this project and finalized this manuscript. All authors have read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG. et al. A new member of the immunoglobulin superfamily-CTLA-4. Nature. 1987;328:267-70
2. Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131:58-67
3. Lin X, Kang K, Chen P, Zeng Z, Li G, Xiong W. et al. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol Cancer. 2024;23:108
4. Cai L, Li Y, Tan J, Xu L, Li Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J Hematol Oncol. 2023;16:101
5. Borgeaud M, Sandoval J, Obeid M, Banna G, Michielin O, Addeo A. et al. Novel targets for immune-checkpoint inhibition in cancer. Cancer Treat Rev. 2023;120:102614
6. Aggarwal V, Workman CJ, Vignali DAA. LAG-3 as the third checkpoint inhibitor. Nat Immunol. 2023;24:1415-22
7. Sun Q, Hong Z, Zhang C, Wang L, Han Z, Ma D. Immune checkpoint therapy for solid tumours: clinical dilemmas and future trends. Signal Transduct Target Ther. 2023;8:320
8. Wu Y, Yang Z, Cheng K, Bi H, Chen J. Small molecule-based immunomodulators for cancer therapy. Acta Pharm Sin B. 2022;12:4287-308
9. Yin S, Chen Z, Chen D, Yan D. Strategies targeting PD-L1 expression and associated opportunities for cancer combination therapy. Theranostics. 2023;13:1520-44
10. Wu Q, Jiang L, Li SC, He QJ, Yang B, Cao J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol Sin. 2021;42:1-9
11. Yamaguchi H, Hsu JM, Yang WH, Hung MC. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat Rev Clin Oncol. 2022;19:287-305
12. Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A. et al. Immune checkpoint therapy-current perspectives and future directions. Cell. 2023;186:1652-69
13. Pang K, Shi ZD, Wei LY, Dong Y, Ma YY, Wang W. et al. Research progress of therapeutic effects and drug resistance of immunotherapy based on PD-1/PD-L1 blockade. Drug Resist Updat. 2023;66:100907
14. Goswami S, Siddiqui BA, Subudhi SK, Basu S, Yadav SS, Diab A. et al. A composite T cell biomarker in pre-treatment blood samples correlates with detection of immune-related adverse events. Cancer Cell. 2022;40:249-51
15. Fletcher K, Johnson DB. Chronic immune-related adverse events arising from immune checkpoint inhibitors: an update. J Immunother Cancer. 2024;12:e008591
16. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C. et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Annals of Oncology. 2022;33:1217-38
17. Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184:5309-37
18. Berner F, Bomze D, Diem S, Ali OH, Fässler M, Ring S. et al. Association of Checkpoint Inhibitor-Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol. 2019;5:1043-7
19. Wang SJ, Dougan SK, Dougan M. Immune mechanisms of toxicity from checkpoint inhibitors. Trends Cancer. 2023;9:543-53
20. Suijkerbuijk KPM, van Eijs MJM, van Wijk F, Eggermont AMM. Clinical and translational attributes of immune-related adverse events. Nat Cancer. 2024;5:557-71
21. Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell. 2020;37:443-55
22. Peng X, Fang J, Lou C, Yang L, Shan S, Wang Z. et al. Engineered nanoparticles for precise targeted drug delivery and enhanced therapeutic efficacy in cancer immunotherapy. Acta Pharm Sin B. 2024;14:3432-56
23. Wang B, Hu S, Teng Y, Chen J, Wang H, Xu Y. et al. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct Target Ther. 2024;9:200
24. Zhang Q, Hu C, Feng J, Long H, Wang Y, Wang P. et al. Anti-inflammatory mechanisms of neutrophil membrane-coated nanoparticles without drug loading. J Control Release. 2024;369:12-24
25. Shan X, Gong X, Li J, Wen J, Li Y, Zhang Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm Sin B. 2022;12:3028-48
26. Chen W, Tang C, Chen G, Li J, Li N, Zhang H. et al. Boosting Checkpoint Immunotherapy with Biomimetic Nanodrug Delivery Systems. Adv Healthc Mater. 2024;13:e2304284
27. Kiaie SH, Salehi-Shadkami H, Sanaei MJ, Azizi M, Shokrollahi Barough M, Nasr MS. et al. Nano-immunotherapy: overcoming delivery challenge of immune checkpoint therapy. J Nanobiotechnology. 2023;21:339
28. Buchbinder EI, Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39:98-106
29. Granier C, De Guillebon E, Blanc C, Roussel H, Badoual C, Colin E. et al. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open. 2017;2:e000213
30. Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069-86
31. Zhang H, Dai Z, Wu W, Wang Z, Zhang N, Zhang L. et al. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J Exp Clin Cancer Res. 2021;40:184
32. Ordikhani F, Uehara M, Kasinath V, Dai L, Eskandari SK, Bahmani B. et al. Targeting antigen-presenting cells by anti-PD-1 nanoparticles augments antitumor immunity. JCI Insight. 2018;3:e122700
33. Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37
34. Zhong XF, Sun X. Nanomedicines based on nanoscale metal-organic frameworks for cancer immunotherapy. Acta Pharmacol Sin. 2020;41:928-35
35. Yang F, Shi K, Jia YP, Hao Y, Peng JR, Qian ZY. Advanced biomaterials for cancer immunotherapy. Acta Pharmacol Sin. 2020;41:911-27
36. Li JH, Huang LJ, Zhou HL, Shan YM, Chen FM, Lehto VP. et al. Engineered nanomedicines block the PD-1/PD-L1 axis for potentiated cancer immunotherapy. Acta Pharmacol Sin. 2022;43:2749-58
37. Yang T, Mochida Y, Liu X, Zhou H, Xie J, Anraku Y. et al. Conjugation of glucosylated polymer chains to checkpoint blockade antibodies augments their efficacy and specificity for glioblastoma. Nat Biomed Eng. 2021;5:1274-87
38. Ren X, Cheng Z, He J, Yao X, Liu Y, Cai K. et al. Inhibition of glycolysis-driven immunosuppression with a nano-assembly enhances response to immune checkpoint blockade therapy in triple negative breast cancer. Nat Commun. 2023;14:7021
39. Xu Z, Tsai HI, Xiao Y, Wu Y, Su D, Yang M. et al. Engineering Programmed Death Ligand-1/Cytotoxic T-Lymphocyte-Associated Antigen-4 Dual-Targeting Nanovesicles for Immunosuppressive Therapy in Transplantation. ACS Nano. 2020;14:7959-69
40. Galstyan A, Markman JL, Shatalova ES, Chiechi A, Korman AJ, Patil R. et al. Blood-brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat Commun. 2019;10:3850
41. Liu S, Wang H, Shao X, Chen H, Chao S, Zhang Y. et al. Advances in PD-1 signaling inhibition-based nano-delivery systems for tumor therapy. J Nanobiotechnology. 2023;21:207
42. Ding Y, Wang L, Li H, Miao F, Zhang Z, Hu C. et al. Application of lipid nanovesicle drug delivery system in cancer immunotherapy. J Nanobiotechnology. 2022;20:214
43. Kim H, Yuk SA, Dieterly AM, Kwon S, Park J, Meng F. et al. Nanosac, a Noncationic and Soft Polyphenol Nanocapsule, Enables Systemic Delivery of siRNA to Solid Tumors. ACS Nano. 2021;15:4576-93
44. Gao Y, Ouyang Z, Yang C, Song C, Jiang C, Song S. et al. Overcoming T Cell Exhaustion via Immune Checkpoint Modulation with a Dendrimer-Based Hybrid Nanocomplex. Adv Healthc Mater. 2021;10:e2100833
45. Li C, Zhou J, Wu Y, Dong Y, Du L, Yang T. et al. Core Role of Hydrophobic Core of Polymeric Nanomicelle in Endosomal Escape of siRNA. Nano Lett. 2021;21:3680-9
46. Wu P, Zhang H, Yin Y, Sun M, Mao S, Chen H. et al. Engineered EGCG-Containing Biomimetic Nanoassemblies as Effective Delivery Platform for Enhanced Cancer Therapy. Adv Sci (Weinh). 2022;9:e2105894
47. Barati M, Mirzavi F, Nikpoor AR, Sankian M, Namdar Ahmadabad H, Soleimani A. et al. Enhanced antitumor immune response in melanoma tumor model by anti-PD-1 small interference RNA encapsulated in nanoliposomes. Cancer Gene Ther. 2022;29:814-24
48. Sun D, Li Y, Yin X, Fan Y, Liu J, Wang Y. et al. Utilizing Engineered Bacteria as "Cell Factories" In Vivo for Intracellular RNA-Loaded Outer Membrane Vesicles' Self-Assembly in Tumor Treatment. ACS Nano. 2024;52:35296-35309
49. Wu Y, Gu W, Li L, Chen C, Xu ZP. Enhancing PD-1 Gene Silence in T Lymphocytes by Comparing the Delivery Performance of Two Inorganic Nanoparticle Platforms. Nanomaterials (Basel). 2019;9:159
50. Liu X, Yang S, Wang L, Wu X, Wang X, Ou C. et al. Hierarchically tumor-activated nanoCRISPR-Cas13a facilitates efficient microRNA disruption for multi-pathway-mediated tumor suppression. Theranostics. 2023;13:2774-86
51. Wu M, Li H, Zhang C, Wang Y, Zhang C, Zhang Y. et al. Silk-Gel Powered Adenoviral Vector Enables Robust Genome Editing of PD-L1 to Augment Immunotherapy across Multiple Tumor Models. Adv Sci (Weinh). 2023;10:e2206399
52. Liu J, Li G, Guo H, Ni C, Gao Y, Cao X. et al. Dual-Responsive Core-Shell Tecto Dendrimers Enable Efficient Gene Editing of Cancer Cells to Boost Immune Checkpoint Blockade Therapy. ACS Appl Mater Interfaces. 2023;15:12809-21
53. Zhao L, Luo Y, Huang Q, Cao Z, Yang X. Photo-Enhanced CRISPR/Cas9 System Enables Robust PD-L1 Gene Disruption in Cancer Cells and Cancer Stem-Like Cells for Efficient Cancer Immunotherapy. Small. 2020: e2004879.
54. Zhang Z, Wang Q, Liu Q, Zheng Y, Zheng C, Yi K. et al. Dual-Locking Nanoparticles Disrupt the PD-1/PD-L1 Pathway for Efficient Cancer Immunotherapy. Adv Mater. 2019;31:e1905751
55. Liu X, Zhou J, Wu H, Chen S, Zhang L, Tang W. et al. Fibrotic immune microenvironment remodeling mediates superior anti-tumor efficacy of a nano-PD-L1 trap in hepatocellular carcinoma. Mol Ther. 2023;31:119-33
56. Lee J, Le QV, Yang G, Oh YK. Cas9-edited immune checkpoint blockade PD-1 DNA polyaptamer hydrogel for cancer immunotherapy. Biomaterials. 2019;218:119359
57. Tao H, Cheng L, Liu L, Wang H, Jiang Z, Qiang X. et al. A PD-1 peptide antagonist exhibits potent anti-tumor and immune regulatory activity. Cancer Lett. 2020;493:91-101
58. Lee Y, Song S, Yang S, Kim J, Moon Y, Shim N. et al. Photo-induced crosslinked and anti-PD-L1 peptide incorporated liposomes to promote PD-L1 multivalent binding for effective immune checkpoint blockade therapy. Acta Pharm Sin B. 2024;14:1428-40
59. Chen L, Zhao X, Liu X, Ouyang Y, Xu C, Shi Y. Development of small molecule drugs targeting immune checkpoints. Cancer Biol Med. 2024;21:382-99
60. Gu Z, Xu S, Guo Z, Liu Z. Rational development of molecularly imprinted nanoparticles for blocking PD-1/PD-L1 axis. Chem Sci. 2022;13:10897-903
61. Bayless NL, Bluestone JA, Bucktrout S, Butterfield LH, Jaffee EM, Koch CA. et al. Development of preclinical and clinical models for immune-related adverse events following checkpoint immunotherapy: a perspective from SITC and AACR. Journal for ImmunoTherapy of Cancer. 2021;9:e002627
62. Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. 2021;16:223-49
63. Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20:25-39
64. Zhang Q, Shen Y, Zhang C, Zhang H, Li X, Yang S. et al. Immunoengineered mitochondria for efficient therapy of acute organ injuries via modulation of inflammation and cell repair. Sci Adv. 2025;11:eadj1896
65. Bell HN, Zou W. Beyond the Barrier: Unraveling the Mechanisms of Immunotherapy Resistance. Annu Rev Immunol. 2024;42:521-50
66. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860-75
67. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51-72
68. He S, Wang L, Wu D, Tong F, Zhao H, Li H. et al. Dual-responsive supramolecular photodynamic nanomedicine with activatable immunomodulation for enhanced antitumor therapy. Acta Pharm Sin B. 2024;14:765-80
69. Liu D, Cheng Y, Qiao S, Liu M, Ji Q, Zhang BL. et al. Nano-Codelivery of Temozolomide and siPD-L1 to Reprogram the Drug-Resistant and Immunosuppressive Microenvironment in Orthotopic Glioblastoma. ACS Nano. 2022;16:7409-27
70. Li T, Gao M, Wu Z, Yang J, Mo B, Yu S. et al. Tantalum-Zirconium Co-Doped Metal-Organic Frameworks Sequentially Sensitize Radio-Radiodynamic-Immunotherapy for Metastatic Osteosarcoma. Adv Sci (Weinh). 2023;10:e2206779
71. Wang Y, Lv B, Wang H, Ren T, Jiang Q, Qu X. et al. Ultrasound-Triggered Azo Free Radicals for Cervical Cancer Immunotherapy. ACS Nano. 2024;18:11042-57
72. Cao Y, Wei D, Yang L, Luo Z, Yu P, Li H. et al. Nanoplatform Self-Assembly from Small Molecules of Porphyrin Derivatives for NIR-II Fluorescence Imaging Guided Photothermal-Immunotherapy. Adv Healthc Mater. 2022;11:e2102526
73. Ding J, Lu Y, Zhao X, Long S, Du J, Sun W. et al. Activating Iterative Revolutions of the Cancer-Immunity Cycle in Hypoxic Tumors with a Smart Nano-Regulator. Adv Mater. 2024;36:e2400196
74. Li G, Bao Y, Zhang H, Wang J, Wu X, Yan R. et al. Enhanced catalytic activity of Fe(3)O(4)-carbon dots complex in the Fenton reaction for enhanced immunotherapeutic and oxygenation effects. J Colloid Interface Sci. 2024;668:618-33
75. Cao Y, Tang L, Fu C, Yin Y, Liu H, Feng J. et al. Black Phosphorus Quantum Dot Loaded Bioinspired Nanoplatform Synergized with aPD-L1 for Multimode Cancer Immunotherapy. Nano Letters. 2024;24:6767-77
76. Tang L, Cao Y, Yin Y, Liu H, Feng J, Fu C. et al. Dual-targeting nanozyme combined with aPD-L1-based immunotherapy for combating cancer recurrence and metastasis. Materials Today. 2024;73:79-95
77. Tang L, Xiao Q, Yin Y, Mei Y, Li J, Xu L. et al. An enzyme-responsive and NIR-triggered lipid-polymer hybrid nanoplatform for synergistic photothermal/chemo cancer therapy. Biomater Sci. 2022;10:2370-83
78. Tang L, Xie M, Li J, Mei Y, Cao Y, Xiao Q. et al. Leveraging nano-engineered mesenchymal stem cells for intramedullary spinal cord tumor treatment. Chinese Chemical Letters. 2023;34:107801
79. Tang L, Liu H, Yin Y, Pan T, Fu C, Cao Y. et al. Micro/nano system-mediated local treatment in conjunction with immune checkpoint inhibitor against advanced-Stage malignant melanoma. Chemical Engineering Journal. 2024;497:154499
80. Yin Y, Tang L, Cao Y, Liu H, Fu C, Feng J. et al. Microneedle patch-involved local therapy synergized with immune checkpoint inhibitor for pre- and post-operative cancer treatment. J Control Release. 2025;379:678-95
81. Zhang Z, Xu X, Du J, Chen X, Xue Y, Zhang J. et al. Redox-responsive polymer micelles co-encapsulating immune checkpoint inhibitors and chemotherapeutic agents for glioblastoma therapy. Nat Commun. 2024;15:1118
82. Qian M, Jiang G, Guo W, Huang R. A Biodegradable Nanosuspension Locally Used for Inhibiting Postoperative Recurrence and Brain Metastasis of Breast Cancer. Nano Letters. 2024;24:3165-75
83. Sun D, Zou Y, Song L, Han S, Yang H, Chu D. et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm Sin B. 2022;12:378-93
84. Gao Y, Zhang H, Tang L, Li F, Yang L, Xiao H. et al. Cancer Nanobombs Delivering Artoxplatin with a Polyigniter Bearing Hydrophobic Ferrocene Units Upregulate PD-L1 Expression and Stimulate Stronger Anticancer Immunity. Adv Sci (Weinh). 2024;11:e2300806
85. Chen W, Sheng P, Chen Y, Liang Y, Wu S, Jia L. et al. Hypoxia-responsive Immunostimulatory Nanomedicines Synergize with Checkpoint Blockade Immunotherapy for Potentiating Cancer Immunotherapy. Chem Eng J. 2023;451:138781
86. Li J, Zhao M, Sun M, Wu S, Zhang H, Dai Y. et al. Multifunctional Nanoparticles Boost Cancer Immunotherapy Based on Modulating the Immunosuppressive Tumor Microenvironment. ACS Appl Mater Interfaces. 2020;12:50734-47
87. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-72
88. Dixon SJ, Olzmann JA. The cell biology of ferroptosis. Nat Rev Mol Cell Biol. 2024;25:424-42
89. Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK. et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270-4
90. Liu B, Ji Q, Cheng Y, Liu M, Zhang B, Mei Q. et al. Biomimetic GBM-targeted drug delivery system boosting ferroptosis for immunotherapy of orthotopic drug-resistant GBM. J Nanobiotechnology. 2022;20:161
91. Hsieh CH, Hsieh HC, Shih FS, Wang PW, Yang LX, Shieh DB. et al. An innovative NRF2 nano-modulator induces lung cancer ferroptosis and elicits an immunostimulatory tumor microenvironment. Theranostics. 2021;11:7072-91
92. Liang H, Wu X, Zhao G, Feng K, Ni K, Sun X. Renal Clearable Ultrasmall Single-Crystal Fe Nanoparticles for Highly Selective and Effective Ferroptosis Therapy and Immunotherapy. J Am Chem Soc. 2021;143:15812-23
93. Li Y, Cao Y, Ma K, Ma R, Zhang M, Guo Y. et al. A Triple-Responsive Polymeric Prodrug Nanoplatform with Extracellular ROS Consumption and Intracellular H(2)O(2) Self-Generation for Imaging-Guided Tumor Chemo-Ferroptosis-Immunotherapy. Adv Healthc Mater. 2024;13:e2303568
94. Li Q, Su R, Bao X, Cao K, Du Y, Wang N. et al. Glycyrrhetinic acid nanoparticles combined with ferrotherapy for improved cancer immunotherapy. Acta Biomater. 2022;144:109-20
95. Ma J, Yuan H, Zhang J, Sun X, Yi L, Li W. et al. An ultrasound-activated nanoplatform remodels tumor microenvironment through diverse cell death induction for improved immunotherapy. J Control Release. 2024;370:501-15
96. Liu P, Shi X, Peng Y, Hu J, Ding J, Zhou W. Anti-PD-L1 DNAzyme Loaded Photothermal Mn(2+) /Fe(3+) Hybrid Metal-Phenolic Networks for Cyclically Amplified Tumor Ferroptosis-Immunotherapy. Adv Healthc Mater. 2022;11:e2102315
97. Zhang K, Ma Z, Li S, Wu Y, Zhang J, Zhang W. et al. Disruption of dual homeostasis by a metal-organic framework nanoreactor for ferroptosis-based immunotherapy of tumor. Biomaterials. 2022;284:121502
98. Li J, Zhou Y, Liu J, Yang X, Zhang K, Lei L. et al. Metal-phenolic networks with ferroptosis to deliver NIR-responsive CO for synergistic therapy. J Control Release. 2022;352:313-27
99. Fu S, Li Y, Shen L, Chen Y, Lu J, Ran Y. et al. Cu(2)WS(4)-PEG Nanozyme as Multifunctional Sensitizers for Enhancing Immuno-Radiotherapy by Inducing Ferroptosis. Small. 2024;20:e2309537
100. Qiu Y, Wu Z, Chen Y, Liao J, Zhang Q, Wang Q. et al. Nano Ultrasound Contrast Agent for Synergistic Chemo-photothermal Therapy and Enhanced Immunotherapy Against Liver Cancer and Metastasis. Adv Sci (Weinh). 2023;10:e2300878
101. Cao W, Zhang X, Feng Y, Li R, Lu A, Li Z. et al. Lipid Nanoparticular Codelivery System for Enhanced Antitumor Effects by Ferroptosis-Apoptosis Synergistic with Programmed Cell Death-Ligand 1 Downregulation. ACS Nano. 2024;18:17267-17281
102. Dai X, Zhang J, Bao X, Guo Y, Jin Y, Yang C. et al. Induction of Tumor Ferroptosis-Dependent Immunity via an Injectable Attractive Pickering Emulsion Gel. Adv Mater. 2023;35:e2303542
103. Zhang Y, Du X, He Z, Gao S, Ye L, Ji J. et al. A Vanadium-Based Nanoplatform Synergizing Ferroptotic-like Therapy with Glucose Metabolism Intervention for Enhanced Cancer Cell Death and Antitumor Immunity. ACS Nano. 2023;17:11537-56
104. Zhou Y, Chen K, Lin WK, Liu J, Kang W, Zhang Y. et al. Photo-Enhanced Synergistic Induction of Ferroptosis for Anti-Cancer Immunotherapy. Adv Healthc Mater. 2023;12:e2300994
105. Kim R, Hashimoto A, Markosyan N, Tyurin VA, Tyurina YY, Kar G. et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022;612:338-46
106. Zhu X, Zheng W, Wang X, Li Z, Shen X, Chen Q. et al. Enhanced Photodynamic Therapy Synergizing with Inhibition of Tumor Neutrophil Ferroptosis Boosts Anti-PD-1 Therapy of Gastric Cancer. Adv Sci (Weinh). 2024;11:e2307870
107. Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128
108. Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T. et al. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. 2022;19:971-92
109. Liang MY, Zhang MJ, Qiu W, Xiao Y, Ye MJ, Xue P. et al. Stepwise Size Shrinkage Cascade-Activated Supramolecular Prodrug Boosts Antitumor Immunity by Eliciting Pyroptosis. Adv Sci (Weinh). 2022;9:e2203353
110. Xu J, Qiu W, Liang M, Ye M, Hu J, Ma X. et al. Dual-stimulus phototherapeutic nanogel for triggering pyroptosis to promote cancer immunotherapy. J Control Release. 2023;358:219-31
111. Wang H, He Z, Gao Y, Feng D, Wei X, Huang Y. et al. Dual-Pronged Attack: pH-Driven Membrane-Anchored NIR Dual-Type Nano-Photosensitizer Excites Immunogenic Pyroptosis and Sequester Immune Checkpoint for Enhanced Prostate Cancer Photo-Immunotherapy. Adv Sci (Weinh). 2023;10:e2302422
112. Zhang N, Zeng W, Xu Y, Li R, Wang M, Liu Y. et al. Pyroptosis Induction with Nanosonosensitizer-Augmented Sonodynamic Therapy Combined with PD-L1 Blockade Boosts Efficacy against Liver Cancer. Adv Healthc Mater. 2024;13:e2302606
113. Long Y, Fan J, Zhou N, Liang J, Xiao C, Tong C. et al. Biomimetic Prussian blue nanocomplexes for chemo-photothermal treatment of triple-negative breast cancer by enhancing ICD. Biomaterials. 2023;303:122369
114. Tao N, Jiao L, Li H, Deng L, Wang W, Zhao S. et al. A Mild Hyperthermia Hollow Carbon Nanozyme as Pyroptosis Inducer for Boosted Antitumor Immunity. ACS Nano. 2023;17:22844-58
115. Yu B, Wang Y, Bing T, Tang Y, Huang J, Xiao H. et al. Platinum Prodrug Nanoparticles with COX-2 Inhibition Amplify Pyroptosis for Enhanced Chemotherapy and Immune Activation of Pancreatic Cancer. Adv Mater. 2024;36:e2310456
116. Lu Y, He W, Huang X, He Y, Gou X, Liu X. et al. Strategies to package recombinant Adeno-Associated Virus expressing the N-terminal gasdermin domain for tumor treatment. Nat Commun. 2021;12:7155
117. Li F, Zhang XQ, Ho W, Tang M, Li Z, Bu L. et al. mRNA lipid nanoparticle-mediated pyroptosis sensitizes immunologically cold tumors to checkpoint immunotherapy. Nat Commun. 2023;14:4223
118. Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M. et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254-61
119. Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B. et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15:174
120. Xie J, Yang Y, Gao Y, He J. Cuproptosis: mechanisms and links with cancers. Mol Cancer. 2023;22:46
121. Lu X, Chen X, Lin C, Yi Y, Zhao S, Zhu B. et al. Elesclomol Loaded Copper Oxide Nanoplatform Triggers Cuproptosis to Enhance Antitumor Immunotherapy. Adv Sci (Weinh). 2024;11:e2309984
122. Guo B, Yang F, Zhang L, Zhao Q, Wang W, Yin L. et al. Cuproptosis Induced by ROS Responsive Nanoparticles with Elesclomol and Copper Combined with αPD-L1 for Enhanced Cancer Immunotherapy. Adv Mater. 2023;35:e2212267
123. Huang Y, Liu X, Zhu J, Chen Z, Yu L, Huang X. et al. Enzyme Core Spherical Nucleic Acid That Enables Enhanced Cuproptosis and Antitumor Immune Response through Alleviating Tumor Hypoxia. J Am Chem Soc. 2024;146:13805-16
124. Yan C, Liu Y, Zhao G, Yang H, Lv H, Li G. et al. Inhalable metal-organic framework-mediated cuproptosis combined with PD-L1 checkpoint blockade for lung metastasis synergistic immunotherapy. Acta Pharm Sin B. 2024;14:2281-97
125. Zhang N, Ping W, Rao K, Zhang Z, Huang R, Zhu D. et al. Biomimetic copper-doped polypyrrole nanoparticles induce glutamine metabolism inhibition to enhance breast cancer cuproptosis and immunotherapy. J Control Release. 2024;371:204-15
126. Guan M, Cheng K, Xie XT, Li Y, Ma MW, Zhang B. et al. Regulating copper homeostasis of tumor cells to promote cuproptosis for enhancing breast cancer immunotherapy. Nat Commun. 2024;15:10060
127. Fu X, Shi Y, Qi T, Qiu S, Huang Y, Zhao X. et al. Precise design strategies of nanomedicine for improving cancer therapeutic efficacy using subcellular targeting. Signal Transduct Target Ther. 2020;5:262
128. Li Z, Zou J, Chen X. In Response to Precision Medicine: Current Subcellular Targeting Strategies for Cancer Therapy. Adv Mater. 2023;35:e2209529
129. Yang J, Song L, Shen M, Gou X, Bai L, Wang L. et al. Hierarchically Responsive Tumor-Microenvironment-Activated Nano-Artificial Virus for Precise Exogenous and Endogenous Apoptosis Coactivation. Advanced Functional Materials. 2021;31:2104423
130. Xiang Y, Chen L, Liu C, Yi X, Li L, Huang Y. Redirecting Chemotherapeutics to the Endoplasmic Reticulum Increases Tumor Immunogenicity and Potentiates Anti-PD-L1 Therapy. Small. 2022;18:e2104591
131. Chen J, Feng L, Jin P, Shen J, Lu J, Song Y. et al. Cavitation assisted endoplasmic reticulum targeted sonodynamic droplets to enhanced anti-PD-L1 immunotherapy in pancreatic cancer. J Nanobiotechnology. 2022;20:283
132. Li J, Dai J, Zhuang Z, Meng Z, Hu JJ, Lou X. et al. Combining PD-L1 blockade with immunogenic cell death induced by AIE photosensitizer to improve antitumor immunity. Biomaterials. 2022;291:121899
133. Li Q, Chen C, Kong J, Li L, Li J, Huang Y. Stimuli-responsive nano vehicle enhances cancer immunotherapy by coordinating mitochondria-targeted immunogenic cell death and PD-L1 blockade. Acta Pharm Sin B. 2022;12:2533-49
134. Wang Y, Wang W, Gu R, Chen J, Chen Q, Lin T. et al. In Situ Vaccination with Mitochondria-Targeting Immunogenic Death Inducer Elicits CD8(+) T Cell-Dependent Antitumor Immunity to Boost Tumor Immunotherapy. Adv Sci (Weinh). 2023;10:e2300286
135. Jin J, Yuan P, Yu W, Lin J, Xu A, Xu X. et al. Mitochondria-Targeting Polymer Micelle of Dichloroacetate Induced Pyroptosis to Enhance Osteosarcoma Immunotherapy. ACS Nano. 2022;16:10327-40
136. He Y, Lei L, Cao J, Yang X, Cai S, Tong F. et al. A combinational chemo-immune therapy using an enzyme-sensitive nanoplatform for dual-drug delivery to specific sites by cascade targeting. Sci Adv. 2021;7:eaba0776
137. Zheng D, Liu J, Xie L, Wang Y, Ding Y, Peng R. et al. Enzyme-instructed and mitochondria-targeting peptide self-assembly to efficiently induce immunogenic cell death. Acta Pharm Sin B. 2022;12:2740-50
138. Sun Z, Wen H, Zhang Z, Xu W, Bao M, Mo H. et al. Acceptor engineering-facilitated versatile AIEgen for mitochondria-targeted multimodal imaging-guided cancer photoimmunotherapy. Biomaterials. 2023;301:122276
139. Hou X, Pan D, Zhong D, Gong Q, Luo K. Dendronized Polymer-Derived Nanomedicines for Mitochondrial Dynamics Regulation and Immune Modulation. Adv Mater. 2024;36:e2400582
140. Dai E, Zhu Z, Wahed S, Qu Z, Storkus WJ, Guo ZS. Epigenetic modulation of antitumor immunity for improved cancer immunotherapy. Mol Cancer. 2021;20:171
141. Davalos V, Esteller M. Cancer epigenetics in clinical practice. CA Cancer J Clin. 2023;73:376-424
142. Ruan H, Hu Q, Wen D, Chen Q, Chen G, Lu Y. et al. A Dual-Bioresponsive Drug-Delivery Depot for Combination of Epigenetic Modulation and Immune Checkpoint Blockade. Adv Mater. 2019;31:e1806957
143. Fang H, Guo Z, Chen J, Lin L, Hu Y, Li Y. et al. Combination of epigenetic regulation with gene therapy-mediated immune checkpoint blockade induces anti-tumour effects and immune response in vivo. Nat Commun. 2021;12:6742
144. He Y, Fang Y, Zhang M, Zhao Y, Tu B, Shi M. et al. Remodeling "cold" tumor immune microenvironment via epigenetic-based therapy using targeted liposomes with in situ formed albumin corona. Acta Pharm Sin B. 2022;12:2057-73
145. Tu K, Yu Y, Wang Y, Yang T, Hu Q, Qin X. et al. Combination of Chidamide-Mediated Epigenetic Modulation with Immunotherapy: Boosting Tumor Immunogenicity and Response to PD-1/PD-L1 Blockade. ACS Appl Mater Interfaces. 2021;13:39003-17
146. Lu F, Hou L, Wang S, Yu Y, Zhang Y, Sun L. et al. Lysosome activable polymeric vorinostat encapsulating PD-L1KD for a combination of HDACi and immunotherapy. Drug Deliv. 2021;28:963-72
147. Gao T, Sang X, Huang X, Gu P, Liu J, Liu Y. et al. Macrophage-camouflaged epigenetic nanoinducers enhance chemoimmunotherapy in triple negative breast cancer. Acta Pharm Sin B. 2023;13:4305-17
148. Li J, Zhao Q, Zhang N, Wu L, Wang Q, Li J. et al. Triune Nanomodulator Enables Exhausted Cytotoxic T Lymphocyte Rejuvenation for Cancer Epigenetic Immunotherapy. ACS Nano. 2024;18:13226-40
149. Luo Z, Wan Z, Ren P, Zhang B, Huang Y, West RE 3rd. et al. In Situ Formation of Fibronectin-Enriched Protein Corona on Epigenetic Nanocarrier for Enhanced Synthetic Lethal Therapy. Adv Sci (Weinh). 2024;11:e2307940
150. Liu R, Yang J, Du Y, Yu X, Liao Y, Wang B. et al. A "One Arrow Three Eagle" Strategy to Improve CM-272 Primed Bladder Cancer Immunotherapy. Adv Mater. 2024;36:e2310522
151. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014-22
152. Maiorino L, Daßler-Plenker J, Sun L, Egeblad M. Innate Immunity and Cancer Pathophysiology. Annu Rev Pathol. 2022;17:425-57
153. Khosravi GR, Mostafavi S, Bastan S, Ebrahimi N, Gharibvand RS, Eskandari N. Immunologic tumor microenvironment modulators for turning cold tumors hot. Cancer Commun (Lond). 2024;44:521-53
154. Fan Q, Li Z, Yin J, Xie M, Cui M, Fan C. et al. Inhalable pH-responsive DNA tetrahedron nanoplatform for boosting anti-tumor immune responses against metastatic lung cancer. Biomaterials. 2023;301:122283
155. Lin L, Hu Y, Guo Z, Chen J, Sun P, Tian H. et al. Gene-guided OX40L anchoring to tumor cells for synergetic tumor "self-killing" immunotherapy. Bioact Mater. 2023;25:689-700
156. Ou W, Jiang L, Gu Y, Soe ZC, Kim BK, Gautam M. et al. Regulatory T Cells Tailored with pH-Responsive Liposomes Shape an Immuno-Antitumor Milieu against Tumors. ACS Appl Mater Interfaces. 2019;11:36333-46
157. Wei Y, Qin G, Wang Z, Zhao C, Ren J, Qu X. Bioorthogonal Activation of TLR7 Agonists Provokes Innate Immunity to Reinforce Aptamer-Based Checkpoint Blockade. ACS Nano. 2023;17:5808-20
158. Zhang L, Bo R, Wu Y, Li L, Zhu Z, Ma AH. et al. Programmable Bispecific Nano-immunoengager That Captures T Cells and Reprograms Tumor Microenvironment. Nano Lett. 2022;22:6866-76
159. Duwa R, Pokhrel RH, Banstola A, Pandit M, Shrestha P, Jeong JH. et al. T-cell engaging poly(lactic-co-glycolic acid) nanoparticles as a modular platform to induce a potent cytotoxic immunogenic response against PD-L1 overexpressing cancer. Biomaterials. 2022;291:121911
160. Huang S, Wen T, Wang J, Wei H, Xiao Z, Li B. et al. Nanoparticle-integrated dissolving microneedles for the co-delivery of R848/aPD-1 to synergistically reverse the immunosuppressive microenvironment of triple-negative breast cancer. Acta Biomater. 2024;176:344-55
161. Song Q, Zhang G, Wang B, Cao G, Li D, Wang Y. et al. Reinforcing the Combinational Immuno-Oncotherapy of Switching "Cold" Tumor to "Hot" by Responsive Penetrating Nanogels. ACS Appl Mater Interfaces. 2021;13:36824-38
162. Hao Y, Li H, Ge X, Liu Y, Yin J, Li X. et al. Site-specific nanoswitch circumventing immune resistance via activating TLR and inhibiting PD-L1/PD-1 axis. J Control Release. 2023;361:64-76
163. Zhang W, Liu X, Cao S, Zhang Q, Chen X, Luo W. et al. Multifunctional Redox-Responsive Nanoplatform with Dual Activation of Macrophages and T Cells for Antitumor Immunotherapy. ACS Nano. 2023;17:14424-41
164. Lin Z, Meng F, Ma Y, Zhang C, Zhang Z, Yang Z. et al. In situ immunomodulation of tumors with biosynthetic bacteria promote anti-tumor immunity. Bioact Mater. 2024;32:12-27
165. Peng Y, Zhan M, Karpus A, Zou Y, Mignani S, Majoral JP. et al. Brain Delivery of Biomimetic Phosphorus Dendrimer/Antibody Nanocomplexes for Enhanced Glioma Immunotherapy via Immune Modulation of T Cells and Natural Killer Cells. ACS Nano. 2024;18:10142-55
166. Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786-91
167. Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Röhl I. et al. cGAS produces a 2'-5'-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498:380-4
168. Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788-92
169. Gulen MF, Koch U, Haag SM, Schuler F, Apetoh L, Villunger A. et al. Signalling strength determines proapoptotic functions of STING. Nat Commun. 2017;8:427
170. Liu Y, Wang L, Song Q, Ali M, Crowe WN, Kucera GL. et al. Intrapleural nano-immunotherapy promotes innate and adaptive immune responses to enhance anti-PD-L1 therapy for malignant pleural effusion. Nat Nanotechnol. 2022;17:206-16
171. Doshi AS, Cantin S, Prickett LB, Mele DA, Amiji M. Systemic nano-delivery of low-dose STING agonist targeted to CD103+ dendritic cells for cancer immunotherapy. J Control Release. 2022;345:721-33
172. Shen M, Chen C, Guo Q, Wang Q, Liao J, Wang L. et al. Systemic Delivery of mPEG-Masked Trispecific T-Cell Nanoengagers in Synergy with STING Agonists Overcomes Immunotherapy Resistance in TNBC and Generates a Vaccination Effect. Adv Sci (Weinh). 2022;9:e2203523
173. Lin M, Cai Y, Chen G, Zhong H, Li B, Li T. et al. A hierarchical tumor-targeting strategy for eliciting potent antitumor immunity against triple negative breast cancer. Biomaterials. 2023;296:122067
174. Li J, Han X, Gao S, Yan Y, Li X, Wang H. Tumor microenvironment-responsive DNA-based nanomedicine triggers innate sensing for enhanced immunotherapy. J Nanobiotechnology. 2023;21:382
175. Liu J, Xiang J, Jin C, Ye L, Wang L, Gao Y. et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J Nanobiotechnology. 2023;21:78
176. Zhang B, Zhang J, Li Y, Li N, Wang Y, Jang R. et al. In Situ STING-Activating Nanovaccination with TIGIT Blockade for Enhanced Immunotherapy of Anti-PD-1-Resistant Tumors. Adv Mater. 2023;35:e2300171
177. Wan Z, Huang H, West RE 3rd, Zhang M, Zhang B, Cai X. et al. Overcoming pancreatic cancer immune resistance by codelivery of CCR2 antagonist using a STING-activating gemcitabine-based nanocarrier. Mater Today (Kidlington). 2023;62:33-50
178. Wang W, Yang F, Zhang L, Wang M, Yin L, Dong X. et al. Targeting DNA Damage and Repair Machinery via Delivering WEE1 Inhibitor and Platinum (IV) Prodrugs to Stimulate STING Pathway for Maximizing Chemo-Immunotherapy in Bladder Cancer. Adv Mater. 2024;36:e2308762
179. Xing Y, Peng A, Yang J, Cheng Z, Yue Y, Liu F. et al. Precisely Activating cGAS-STING Pathway with a Novel Peptide-Based Nanoagonist to Potentiate Immune Checkpoint Blockade Cancer Immunotherapy. Adv Sci (Weinh). 2024;11:e2309583
180. Sun X, Zhang Y, Li J, Park KS, Han K, Zhou X. et al. Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat Nanotechnol. 2021;16:1260-70
181. Zhang K, Qi C, Cai K. Manganese-Based Tumor Immunotherapy. Adv Mater. 2023;35:e2205409
182. Sun Z, Wang Z, Wang T, Wang J, Zhang H, Li Z. et al. Biodegradable MnO-Based Nanoparticles with Engineering Surface for Tumor Therapy: Simultaneous Fenton-Like Ion Delivery and Immune Activation. ACS Nano. 2022;16:11862-75
183. Chen B, Guo K, Zhao X, Liu Z, Xu C, Zhao N. et al. Tumor microenvironment-responsive delivery nanosystems reverse immunosuppression for enhanced CO gas/immunotherapy. Exploration. 2023;3:20220140
184. Huang Z, Wang Y, Su C, Li W, Wu M, Li W. et al. Mn-Anti-CTLA4-CREKA-Sericin Nanotheragnostics for Enhanced Magnetic Resonance Imaging and Tumor Immunotherapy. Small. 2024;20:e2306912
185. Qiao W, Chen J, Zhou H, Hu C, Dalangood S, Li H. et al. A Single-Atom Manganese Nanozyme Mn-N/C Promotes Anti-Tumor Immune Response via Eliciting Type I Interferon Signaling. Adv Sci (Weinh). 2024;11:e2305979
186. Liu X, Shen M, Bing T, Zhang X, Li Y, Cai Q. et al. A Bioactive Injectable Hydrogel Regulates Tumor Metastasis and Wound Healing for Melanoma via NIR-Light Triggered Hyperthermia. Adv Sci (Weinh). 2024;11:e2402208
187. Sun L, Gao H, Wang H, Zhou J, Ji X, Jiao Y. et al. Nanoscale Metal-Organic Frameworks-Mediated Degradation of Mutant p53 Proteins and Activation of cGAS-STING Pathway for Enhanced Cancer Immunotherapy. Adv Sci (Weinh). 2024;11:e2307278
188. Chen C, Tong Y, Zheng Y, Shi Y, Chen Z, Li J. et al. Cytosolic Delivery of Thiolated Mn-cGAMP Nanovaccine to Enhance the Antitumor Immune Responses. Small. 2021;17:e2006970
189. Aikins ME, Sun X, Dobson H, Zhou X, Xu Y, Lei YL. et al. STING-activating cyclic dinucleotide-manganese nanoparticles evoke robust immunity against acute myeloid leukemia. J Control Release. 2024;368:768-79
190. Liu D, Liang S, Ma K, Meng QF, Li X, Wei J. et al. Tumor Microenvironment-Responsive Nanoparticles Amplifying STING Signaling Pathway for Cancer Immunotherapy. Adv Mater. 2024;36:e2304845
191. Oladejo M, Paulishak W, Wood L. Synergistic potential of immune checkpoint inhibitors and therapeutic cancer vaccines. Semin Cancer Biol. 2023;88:81-95
192. Zhu G, Zhang F, Ni Q, Niu G, Chen X. Efficient Nanovaccine Delivery in Cancer Immunotherapy. ACS Nano. 2017;11:2387-92
193. Desai N, Chavda V, Singh TRR, Thorat ND, Vora LK. Cancer Nanovaccines: Nanomaterials and Clinical Perspectives. Small. 2024;20:e2401631
194. Nakamura T, Haloho SEE, Harashima H. Intravenous liposomal vaccine enhances CTL generation, but not until antigen presentation. J Control Release. 2022;343:1-12
195. Sui Y, Li J, Qu J, Fang T, Zhang H, Zhang J. et al. Dual-responsive nanovaccine for cytosolic delivery of antigens to boost cellular immune responses and cancer immunotherapy. Asian J Pharm Sci. 2022;17:583-95
196. Zeng YC, Young OJ, Wintersinger CM, Anastassacos FM, MacDonald JI, Isinelli G. et al. Fine tuning of CpG spatial distribution with DNA origami for improved cancer vaccination. Nat Nanotechnol. 2024;19:1055-65
197. Lu Y, Ye H, Zhao J, Wang K, Fan X, Lu Q. et al. Small EV-based delivery of CpG ODNs for melanoma postsurgical immunotherapy. J Control Release. 2023;363:484-95
198. Su Y, Xu W, Wei Q, Ma Y, Ding J, Chen X. Chiral polypeptide nanoparticles as nanoadjuvants of nanovaccines for efficient cancer prevention and therapy. Sci Bull (Beijing). 2023;68:284-94
199. Liu Z, Liu B, Feng Y, Zhao L, Wang Q, He H. et al. Dual-Targeted Self-Adjuvant Heterocyclic Lipidoid@Polyester Hybrid Nanovaccines for Boosting Cancer Immunotherapy. ACS Nano. 2024;18:15557-75
200. Xie C, You X, Zhang H, Li J, Wang L, Liu Y. et al. A Nanovaccine Based on Adjuvant Peptide FK-13 and l-Phenylalanine Poly(ester amide) Enhances CD8(+) T Cell-Mediated Antitumor Immunity. Adv Sci (Weinh). 2023;10:e2300418
201. Shin HS, Kim S, Jin SM, Yoo YJ, Heo JH, Lim YT. Molecular Masking of Synthetic Immunomodulator Evokes Antitumor Immunity With Reduced Immune Tolerance and Systemic Toxicity by Temporal Activity Recovery and Sustained Stimulation. Adv Mater. 2024;36:e2309039
202. Kim Y, Kang S, Shin H, Kim T, Yu B, Kim J. et al. Sequential and Timely Combination of a Cancer Nanovaccine with Immune Checkpoint Blockade Effectively Inhibits Tumor Growth and Relapse. Angew Chem Int Ed Engl. 2020;59:14628-38
203. Matos AI, Peres C, Carreira B, Moura LIF, Acúrcio RC, Vogel T. et al. Polyoxazoline-Based Nanovaccine Synergizes with Tumor-Associated Macrophage Targeting and Anti-PD-1 Immunotherapy against Solid Tumors. Adv Sci (Weinh). 2023;10:e2300299
204. Tian Z, Hu Q, Sun Z, Wang N, He H, Tang Z. et al. A Booster for Radiofrequency Ablation: Advanced Adjuvant Therapy via In Situ Nanovaccine Synergized with Anti-programmed Death Ligand 1 Immunotherapy for Systemically Constraining Hepatocellular Carcinoma. ACS Nano. 2023;17:19441-58
205. Ma L, Diao L, Peng Z, Jia Y, Xie H, Li B. et al. Immunotherapy and Prevention of Cancer by Nanovaccines Loaded with Whole-Cell Components of Tumor Tissues or Cells. Adv Mater. 2021;33:e2104849
206. Li R, Hao Y, Roche K, Chen G, Pan W, Wang AZ. et al. Chemotherapy-induced nanovaccines implement immunogenicity equivalence for improving cancer chemoimmunotherapy. Biomaterials. 2023;301:122290
207. Jiang M, Zhao L, Cui X, Wu X, Zhang Y, Guan X. et al. Cooperating minimalist nanovaccine with PD-1 blockade for effective and feasible cancer immunotherapy. J Adv Res. 2022;35:49-60
208. Najafabadi AH, Abadi ZIN, Aikins ME, Foulds KE, Donaldson MM, Yuan W. et al. Vaccine nanodiscs plus polyICLC elicit robust CD8+ T cell responses in mice and non-human primates. J Control Release. 2021;337:168-78
209. Chu Y, Qian L, Ke Y, Feng X, Chen X, Liu F. et al. Lymph node-targeted neoantigen nanovaccines potentiate anti-tumor immune responses of post-surgical melanoma. J Nanobiotechnology. 2022;20:190
210. Gong N, Zhang Y, Teng X, Wang Y, Huo S, Qing G. et al. Proton-driven transformable nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2020;15:1053-64
211. Liu K, Peng J, Guo Y, Li Y, Qi X, Duan D. et al. Expanding the Potential of Neoantigen Vaccines: Harnessing Bacille Calmette-Guérin Cell-Wall-Based Nanoscale Adjuvants for Enhanced Cancer Immunotherapy. ACS Nano. 2024;18:11910-20
212. Huang Y, Nahar S, Alam MM, Hu S, McVicar DW, Yang D. Reactive Oxygen Species-Sensitive Biodegradable Mesoporous Silica Nanoparticles Harboring TheraVac Elicit Tumor-Specific Immunity for Colon Tumor Treatment. ACS Nano. 2023;17:19740-52
213. Li Q, Teng Z, Tao J, Shi W, Yang G, Zhang Y. et al. Elastic Nanovaccine Enhances Dendritic Cell-Mediated Tumor Immunotherapy. Small. 2022;18:e2201108
214. Yang C, Zhang F, Chen F, Chang Z, Zhao Y, Shao D. et al. Biomimetic Nanovaccines Potentiating Dendritic Cell Internalization via CXCR4-Mediated Macropinocytosis. Adv Healthc Mater. 2023;12:e2202064
215. Hu H, Yang C, Zhang F, Li M, Tu Z, Mu L. et al. A Versatile and Robust Platform for the Scalable Manufacture of Biomimetic Nanovaccines. Adv Sci (Weinh). 2021;8:2002020
216. Wu H, Li H, Liu Y, Liang J, Liu Q, Xu Z. et al. Blockading a new NSCLC immunosuppressive target by pluripotent autologous tumor vaccines magnifies sequential immunotherapy. Bioact Mater. 2022;13:223-38
217. Liu S, Wu J, Feng Y, Guo X, Li T, Meng M. et al. CD47KO/CRT dual-bioengineered cell membrane-coated nanovaccine combined with anti-PD-L1 antibody for boosting tumor immunotherapy. Bioact Mater. 2023;22:211-24
218. Zhang X, Wei Z, Yong T, Li S, Bie N, Li J. et al. Cell microparticles loaded with tumor antigen and resiquimod reprogram tumor-associated macrophages and promote stem-like CD8(+) T cells to boost anti-PD-1 therapy. Nat Commun. 2023;14:5653
219. Zhang J, Wan S, Zhou H, Du J, Li Y, Zhu H. et al. Programmed Nanocloak of Commensal Bacteria-Derived Nanovesicles Amplify Strong Immunoreactivity against Tumor Growth and Metastatic Progression. ACS Nano. 2024;18:9613-26
220. Meng F, Li L, Zhang Z, Lin Z, Zhang J, Song X. et al. Biosynthetic neoantigen displayed on bacteria derived vesicles elicit systemic antitumour immunity. J Extracell Vesicles. 2022;11:e12289
221. Xiang Z, Lu J, Rao S, Fu C, Yao Y, Yi Y. et al. Programming peptide-oligonucleotide nano-assembly for engineering of neoantigen vaccine with potent immunogenicity. Theranostics. 2024;14:2290-303
222. Niu L, Miao Y, Cao Z, Wei T, Zhu J, Li M. et al. Minimalist Nanovaccine with Optimized Amphiphilic Copolymers for Cancer Immunotherapy. ACS Nano. 2024;18:3349-61
223. Jia S, Ji S, Zhao J, Lv Y, Wang J, Sun D. et al. A Fluorinated Supramolecular Self-Assembled Peptide as Nanovaccine Adjuvant for Enhanced Cancer Vaccine Therapy. Small Methods. 2023;7:e2201409
224. Zhang R, Tang L, Wang Y, Tian Y, Wu S, Zhou B. et al. A Dendrimer Peptide (KK2DP7) Delivery System with Dual Functions of Lymph Node Targeting and Immune Adjuvants as a General Strategy for Cancer Immunotherapy. Adv Sci (Weinh). 2023;10:e2300116
225. Yip T, Qi X, Yan H, Chang Y. RNA Origami Functions as a Self-Adjuvanted Nanovaccine Platform for Cancer Immunotherapy. ACS Nano. 2024;18:4056-67
226. Lv X, Huang J, Min J, Wang H, Xu Y, Zhang Z. et al. Multi-signaling pathway activation by pH responsive manganese particles for enhanced vaccination. J Control Release. 2023;357:109-19
227. Chen J, Fang H, Hu Y, Wu J, Zhang S, Feng Y. et al. Combining mannose receptor mediated nanovaccines and gene regulated PD-L1 blockade for boosting cancer immunotherapy. Bioact Mater. 2022;7:167-80
228. Xu J, Wang H, Xu L, Chao Y, Wang C, Han X. et al. Nanovaccine based on a protein-delivering dendrimer for effective antigen cross-presentation and cancer immunotherapy. Biomaterials. 2019;207:1-9
229. Li X, Cai X, Zhang Z, Ding Y, Ma R, Huang F. et al. Mimetic Heat Shock Protein Mediated Immune Process to Enhance Cancer Immunotherapy. Nano Lett. 2020;20:4454-63
230. Zhang S, Feng Y, Meng M, Li Z, Li H, Lin L. et al. A generally minimalist strategy of constructing biomineralized high-efficiency personalized nanovaccine combined with immune checkpoint blockade for cancer immunotherapy. Biomaterials. 2022;289:121794
231. Ni Q, Zhang F, Liu Y, Wang Z, Yu G, Liang B. et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci Adv. 2020;6:eaaw6071
232. Xu C, Hong H, Lee Y, Park KS, Sun M, Wang T. et al. Efficient Lymph Node-Targeted Delivery of Personalized Cancer Vaccines with Reactive Oxygen Species-Inducing Reduced Graphene Oxide Nanosheets. ACS Nano. 2020;14:13268-78
233. Li Q, Dong Z, Cao Z, Lei H, Wang C, Hao Y. et al. A General Biomineralization Strategy to Synthesize Autologous Cancer Vaccines with cGAS-STING Activating Capacity for Postsurgical Immunotherapy. ACS Nano. 2023;17:10496-510
234. He X, Gong G, Chen M, Zhang H, Zhang Y, Richardson JJ. et al. Metal-Phenolic Nanocloaks on Cancer Cells Potentiate STING Pathway Activation for Synergistic Cancer Immunotherapy. Angew Chem Int Ed Engl. 2024;63:e202314501
235. Du Y, Song T, Wu J, Gao XD, Ma G, Liu Y. et al. Engineering mannosylated pickering emulsions for the targeted delivery of multicomponent vaccines. Biomaterials. 2022;280:121313
236. Zheng P, He J, Yang Z, Fu Y, Yang Y, Li W. et al. Neoantigen-Based Nanovaccine In Combination with Immune Checkpoint Inhibitors Abolish Postsurgical Tumor Recurrence and Metastasis. Small. 2023;19:e2302922
237. Pan Y, Wu X, Liu L, Zhao C, Zhang J, Yang S. et al. Genetically Engineered Cytomembrane Nanovaccines for Cancer Immunotherapy. Adv Healthc Mater. 2024;13:e2400068
238. Jin SM, Yoo YJ, Shin HS, Kim S, Lee SN, Lee CH. et al. A nanoadjuvant that dynamically coordinates innate immune stimuli activation enhances cancer immunotherapy and reduces immune cell exhaustion. Nat Nanotechnol. 2023;18:390-402
239. Liu H, Chen H, Liu Z, Le Z, Nie T, Qiao D. et al. Therapeutic nanovaccines sensitize EBV-associated tumors to checkpoint blockade therapy. Biomaterials. 2020;255:120158
240. Li Y, Tong F, Wang Y, Wang J, Wu M, Li H. et al. I n situ tumor vaccine with optimized nanoadjuvants and lymph node targeting capacity to treat ovarian cancer and metastases. Acta Pharm Sin B. 2024;14:4102-17
241. Li Z, Zhang Q, Li Z, Ren L, Pan D, Gong Q. et al. Branched glycopolymer prodrug-derived nanoassembly combined with a STING agonist activates an immuno-supportive status to boost anti-PD-L1 antibody therapy. Acta Pharm Sin B. 2024;14:2194-209
242. Gao Z, Zhang J, Hou Y, Lu J, Liang J, Gao Y. et al. Boosting the synergism between cancer ferroptosis and immunotherapy via targeted stimuli-responsive liposomes. Biomaterials. 2024;305:122442
243. Qu H, Li L, Chen H, Tang M, Cheng W, Lin TY. et al. Drug-drug conjugates self-assembled nanomedicines triggered photo-/immuno- therapy for synergistic cancer treatments. J Control Release. 2023;363:361-75
244. Zhang H, Liu L, Liu J, Dang P, Hu S, Yuan W. et al. Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol Cancer. 2023;22:58
245. Xiao Q, Huang J, Wang X, Chen Z, Zhang W, Liu F. et al. Supramolecular Peptide Amphiphile Nanospheres Reprogram Tumor-associated Macrophage to Reshape the Immune Microenvironment for Enhanced Breast Cancer Immunotherapy. Small. 2024;20:e2307390
246. Jiang M, Li X, Zhang J, Lu Y, Shi Y, Zhu C. et al. Dual Inhibition of Endoplasmic Reticulum Stress and Oxidation Stress Manipulates the Polarization of Macrophages under Hypoxia to Sensitize Immunotherapy. ACS Nano. 2021;15:14522-34
247. Zhao YD, An HW, Mamuti M, Zeng XZ, Zheng R, Yang J. et al. Reprogramming Hypoxic Tumor-Associated Macrophages by Nanoglycoclusters for Boosted Cancer Immunotherapy. Adv Mater. 2023;35:e2211332
248. Su WP, Chang LC, Song WH, Yang LX, Wang LC, Chia ZC. et al. Polyaniline-Based Glyco-Condensation on Au Nanoparticles Enhances Immunotherapy in Lung Cancer. ACS Appl Mater Interfaces. 2022;14:24144-59
249. Wang S, Liu X, Yang M, Ouyang L, Ding J, Wang S. et al. Non-cytotoxic nanoparticles re-educating macrophages achieving both innate and adaptive immune responses for tumor therapy. Asian J Pharm Sci. 2022;17:557-70
250. Zheng P, He J, Fu Y, Yang Y, Li S, Duan B. et al. Engineered Bacterial Biomimetic Vesicles Reprogram Tumor-Associated Macrophages and Remodel Tumor Microenvironment to Promote Innate and Adaptive Antitumor Immune Responses. ACS Nano. 2024;18:6863-86
251. Kim J, Kim M, Yong SB, Han H, Kang S, Lahiji SF. et al. Engineering TGF-β inhibitor-encapsulated macrophage-inspired multi-functional nanoparticles for combination cancer immunotherapy. Biomater Res. 2023;27:136
252. Liu C, Zhou Y, Guo D, Huang Y, Ji X, Li Q. et al. Reshaping Intratumoral Mononuclear Phagocytes with Antibody-Opsonized Immunometabolic Nanoparticles. Adv Sci (Weinh). 2023;10:e2303298
253. Qiao G, Li S, Pan X, Xie P, Peng R, Huang X. et al. Surgical tumor-derived nanoplatform targets tumor-associated macrophage for personalized postsurgical cancer immunotherapy. Sci Adv. 2024;10:eadk7955
254. Tang B, Zhu J, Wang Y, Chen W, Fang S, Mao W. et al. Targeted xCT-mediated Ferroptosis and Protumoral Polarization of Macrophages Is Effective against HCC and Enhances the Efficacy of the Anti-PD-1/L1 Response. Adv Sci (Weinh). 2023;10:e2203973
255. Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016;37:208-20
256. Zhu Y, Chen P, Hu B, Zhong S, Yan K, Wu Y. et al. MDSC-targeting gold nanoparticles enhance PD-1 tumor immunotherapy by inhibiting NLRP3 inflammasomes. Biomaterials. 2024;307:122533
257. Tang Y, Shu Z, Zhu M, Li S, Ling Y, Fu Y. et al. Size-Tunable Nanoregulator-Based Radiofrequency Ablation Suppresses MDSCs and Their Compensatory Immune Evasion in Hepatocellular Carcinoma. Adv Healthc Mater. 2023;12:e2302013
258. Yan W, Li Y, Zou Y, Zhu R, Wu T, Sun X. et al. Breaking Tumor Immunosuppressive Network by Regulating Multiple Nodes with Triadic Drug Delivery Nanoparticles. ACS Nano. 2023;17:17826-44
259. Mo W, Liu S, Zhao X, Wei F, Li Y, Sheng X. et al. ROS Scavenging Nanozyme Modulates Immunosuppression for Sensitized Cancer Immunotherapy. Adv Healthc Mater. 2023;12:e2300191
260. Liu J, Bai Y, Li Y, Li X, Luo K. Reprogramming the immunosuppressive tumor microenvironment through nanomedicine: an immunometabolism perspective. EBioMedicine. 2024;107:105301
261. Qiao Q, Hu S, Wang X. The regulatory roles and clinical significance of glycolysis in tumor. Cancer Commun (Lond). 2024;44:761-86
262. Zhang P, Li Z, Cao W, Tang J, Xia Y, Peng L. et al. A PD-L1 Antibody-Conjugated PAMAM Dendrimer Nanosystem for Simultaneously Inhibiting Glycolysis and Promoting Immune Response in Fighting Breast Cancer. Adv Mater. 2023;35:e2305215
263. Lin B, Wang Y, Zhao K, Lü WD, Hui X, Ma Y. et al. Exosome-based rare earth nanoparticles for targeted in situ and metastatic tumor imaging with chemo-assisted immunotherapy. Biomater Sci. 2022;10:744-52
264. Li B, Yang T, Liu J, Yu X, Li X, Qin F. et al. Genetically engineered PD-1 displaying nanovesicles for synergistic checkpoint blockades and chemo-metabolic therapy against non-small cell lung cancer. Acta Biomater. 2023;161:184-200
265. Luo Y, Li Y, Huang Z, Li X, Wang Y, Hou J. et al. A Nanounit Strategy Disrupts Energy Metabolism and Alleviates Immunosuppression for Cancer Therapy. Nano Lett. 2022;22:6418-27
266. Chen Z, Chen L, Ma Y, Liu Y, Zhang Q, Qin H. et al. Peptide-Appended Nanosonosensitizers Targeting Tumor Glycolysis for Synergistic Sonodynamic-Immunometabolic Therapy of Spinal-Metastasized Tumors. Adv Mater. 2023;35:e2304246
267. Jia L, Gao Y, Zhou T, Zhao XL, Hu HY, Chen DW. et al. Enhanced response to PD-L1 silencing by modulation of TME via balancing glucose metabolism and robust co-delivery of siRNA/Resveratrol with dual-responsive polyplexes. Biomaterials. 2021;271:120711
268. Xie W, Deng WW, Zan M, Rao L, Yu GT, Zhu DM. et al. Cancer Cell Membrane Camouflaged Nanoparticles to Realize Starvation Therapy Together with Checkpoint Blockades for Enhancing Cancer Therapy. ACS Nano. 2019;13:2849-57
269. Zhang S, Zhang Y, Feng Y, Wu J, Hu Y, Lin L. et al. Biomineralized Two-Enzyme Nanoparticles Regulate Tumor Glycometabolism Inducing Tumor Cell Pyroptosis and Robust Antitumor Immunotherapy. Adv Mater. 2022;34:e2206851
270. Ren H, Yong J, Yang Q, Yang Z, Liu Z, Xu Y. et al. Self-assembled FeS-based cascade bioreactor with enhanced tumor penetration and synergistic treatments to trigger robust cancer immunotherapy. Acta Pharm Sin B. 2021;11:3244-61
271. Wang M, Chang M, Zheng P, Sun Q, Wang G, Lin J. et al. A Noble AuPtAg-GOx Nanozyme for Synergistic Tumor Immunotherapy Induced by Starvation Therapy-Augmented Mild Photothermal Therapy. Adv Sci (Weinh). 2022;9:e2202332
272. Ju H, Kim D, Oh YK. Lipid nanoparticle-mediated CRISPR/Cas9 gene editing and metabolic engineering for anticancer immunotherapy. Asian J Pharm Sci. 2022;17:641-52
273. Zhang YX, Zhao YY, Shen J, Sun X, Liu Y, Liu H. et al. Nanoenabled Modulation of Acidic Tumor Microenvironment Reverses Anergy of Infiltrating T Cells and Potentiates Anti-PD-1 Therapy. Nano Lett. 2019;19:2774-83
274. Yan J, Li W, Tian H, Li B, Yu X, Wang G. et al. Metal-Phenolic Nanomedicines Regulate T-Cell Antitumor Function for Sono-Metabolic Cancer Therapy. ACS Nano. 2023;17:14667-77
275. Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer. 2017;3:169-80
276. Tang Y, Wang S, Li Y, Yuan C, Zhang J, Xu Z. et al. Simultaneous glutamine metabolism and PD-L1 inhibition to enhance suppression of triple-negative breast cancer. J Nanobiotechnology. 2022;20:216
277. Ren H, Wu Z, Tan J, Tao H, Zou W, Cao Z. et al. Co-delivery Nano System of MS-275 and V-9302 Induces Pyroptosis and Enhances Anti-Tumor Immunity Against Uveal Melanoma. Adv Sci (Weinh). 2024;11:e2404375
278. Li X, Zhu T, Wang R, Chen J, Tang L, Huo W. et al. Genetically Programmable Vesicles for Enhancing CAR-T Therapy against Solid Tumors. Adv Mater. 2023;35:e2211138
279. Xie W, Chen B, Wen H, Xiao P, Wang L, Liu W. et al. Biomimetic Nanoplatform Loading Type I Aggregation-Induced Emission Photosensitizer and Glutamine Blockade to Regulate Nutrient Partitioning for Enhancing Antitumor Immunotherapy. ACS Nano. 2022;16:10742-53
280. Jin XK, Zhang SM, Liang JL, Zhang SK, Qin YT, Huang QX. et al. A PD-L1-targeting Regulator for Metabolic Reprogramming to Enhance Glutamine Inhibition-Mediated Synergistic Antitumor Metabolic and Immune Therapy. Adv Mater. 2024;36:e2309094
281. Zhang R, Li R, Zhang L, Chen G, Mo L, Jiang R. et al. A Dual-Mechanism Based Nutrient Partitioning Nanoregulator for Enhanced Immunotherapy against Anti-PD-1 Resistant Tumors. ACS Nano. 2023;17:13461-73
282. Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang S. et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol. 2018;11:100
283. Li G, Gao Y, Gong C, Han Z, Qiang L, Tai Z. et al. Dual-Blockade Immune Checkpoint for Breast Cancer Treatment Based on a Tumor-Penetrating Peptide Assembling Nanoparticle. ACS Appl Mater Interfaces. 2019;11:39513-24
284. Han X, Cheng K, Xu Y, Wang Y, Min H, Zhang Y. et al. Modularly Designed Peptide Nanoprodrug Augments Antitumor Immunity of PD-L1 Checkpoint Blockade by Targeting Indoleamine 2,3-Dioxygenase. J Am Chem Soc. 2020;142:2490-6
285. Hu C, Song Y, Zhang Y, He S, Liu X, Yang X. et al. Sequential delivery of PD-1/PD-L1 blockade peptide and IDO inhibitor for immunosuppressive microenvironment remodeling via an MMP-2 responsive dual-targeting liposome. Acta Pharm Sin B. 2023;13:2176-87
286. Huai M, Wang Y, Li J, Pan J, Sun F, Zhang F. et al. Intelligent nanovesicle for remodeling tumor microenvironment and circulating tumor chemoimmunotherapy amplification. J Nanobiotechnology. 2024;22:257
287. Wang Z, Little N, Chen J, Lambesis KT, Le KT, Han W. et al. Immunogenic camptothesome nanovesicles comprising sphingomyelin-derived camptothecin bilayers for safe and synergistic cancer immunochemotherapy. Nat Nanotechnol. 2021;16:1130-40
288. Kim M, Lee JS, Kim W, Lee JH, Jun BH, Kim KS. et al. Aptamer-conjugated nano-liposome for immunogenic chemotherapy with reversal of immunosuppression. J Control Release. 2022;348:893-910
289. Liu Y, Lu Y, Zhu X, Li C, Yan M, Pan J. et al. Tumor microenvironment-responsive prodrug nanoplatform via co-self-assembly of photothermal agent and IDO inhibitor for enhanced tumor penetration and cancer immunotherapy. Biomaterials. 2020;242:119933
290. Yan M, Liu Y, Zhu X, Wang X, Liu L, Sun H. et al. Nanoscale Reduced Graphene Oxide-Mediated Photothermal Therapy Together with IDO Inhibition and PD-L1 Blockade Synergistically Promote Antitumor Immunity. ACS Appl Mater Interfaces. 2019;11:1876-85
291. Zhang N, Sun Q, Li J, Li J, Tang L, Zhao Q. et al. A lipid/PLGA nanocomplex to reshape tumor immune microenvironment for colon cancer therapy. Regen Biomater. 2024;11:rbae036
292. Xiao B, Xu H, Xu X, Pan Y, Shi X, Yuan P. et al. Multifunctional Nanoassembly for MRI-Trackable Dendritic Cell Dependent and Independent Photoimmunotherapy. Nano Lett. 2023;23:9133-42
293. Tian Y, Younis MR, Zhao Y, Guo K, Wu J, Zhang L. et al. Precision Delivery of Dual Immune Inhibitors Loaded Nanomodulator to Reverse Immune Suppression for Combinational Photothermal-Immunotherapy. Small. 2023;19:e2206441
294. Chae SY, Shin H, Woo J, Kang S, Lee SM, Min DH. Metabolic Modulation of Kynurenine Based on Kynureninase-Loaded Nanoparticle Depot Overcomes Tumor Immune Evasion in Cancer Immunotherapy. ACS Appl Mater Interfaces. 2024;16:18490-502
295. Kelly B, Pearce EL. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020;32:154-75
296. Zang J, Yang Y, Zheng X, Yang Y, Zhao Y, Miao Z. et al. Dynamic tagging to drive arginine nano-assembly to metabolically potentiate immune checkpoint blockade therapy. Biomaterials. 2023;292:121938
297. Zhang J, Wang S, Guo X, Lu Y, Liu X, Jiang M. et al. Arginine Supplementation Targeting Tumor-Killing Immune Cells Reconstructs the Tumor Microenvironment and Enhances the Antitumor Immune Response. ACS Nano. 2022;16:12964-78
298. Luo X, Cheng C, Tan Z, Li N, Tang M, Yang L. et al. Emerging roles of lipid metabolism in cancer metastasis. Mol Cancer. 2017;16:76
299. Bell CR, Pelly VS, Moeini A, Chiang SC, Flanagan E, Bromley CP. et al. Chemotherapy-induced COX-2 upregulation by cancer cells defines their inflammatory properties and limits the efficacy of chemoimmunotherapy combinations. Nat Commun. 2022;13:2063
300. Liu Y, He J, Li M, Ren K, Zhao Z. Inflammation-Driven Nanohitchhiker Enhances Postoperative Immunotherapy by Alleviating Prostaglandin E2-Mediated Immunosuppression. ACS Appl Mater Interfaces. 2024;16:6879-93
301. Huang H, Huang Y, Chen Y, Luo Z, Zhang Z, Sun R. et al. A novel immunochemotherapy based on targeting of cyclooxygenase and induction of immunogenic cell death. Biomaterials. 2021;270:120708
302. Gao Y, Song Z, Jia L, Tang Y, Wang C, Zhao X. et al. Self-amplified ROS production from fatty acid oxidation enhanced tumor immunotherapy by atorvastatin/PD-L1 siRNA lipopeptide nanoplexes. Biomaterials. 2022;291:121902
303. Xia C, Yin S, To KKW, Fu L. CD39/CD73/A2AR pathway and cancer immunotherapy. Mol Cancer. 2023;22:44
304. Qiu W, Guo Q, Guo X, Wang C, Li B, Qi Y. et al. Mesenchymal stem cells, as glioma exosomal immunosuppressive signal multipliers, enhance MDSCs immunosuppressive activity through the miR-21/SP1/DNMT1 positive feedback loop. J Nanobiotechnology. 2023;21:233
305. Wu L, Xie W, Li Y, Ni Q, Timashev P, Lyu M. et al. Biomimetic Nanocarriers Guide Extracellular ATP Homeostasis to Remodel Energy Metabolism for Activating Innate and Adaptive Immunity System. Adv Sci (Weinh). 2022;9:e2105376
306. Xiong H, Ma X, Wang X, Su W, Wu L, Zhang T. et al. Inspired Epigenetic Modulation Synergy with Adenosine Inhibition Elicits Pyroptosis and Potentiates Cancer Immunotherapy. Advanced Functional Materials. 2021;31:2100007
307. Liu P, Guo J, Xie Z, Pan Y, Wei B, Peng Y. et al. Co-Delivery of aPD-L1 and CD73 Inhibitor Using Calcium Phosphate Nanoparticles for Enhanced Melanoma Immunotherapy with Reduced Toxicity. Adv Sci (Weinh). 2025;12:e2410545
308. Mao C, Yeh S, Fu J, Porosnicu M, Thomas A, Kucera GL. et al. Delivery of an ectonucleotidase inhibitor with ROS-responsive nanoparticles overcomes adenosine-mediated cancer immunosuppression. Sci Transl Med. 2022;14:eabh1261
309. Guo J, Liu P, Wei BL, Peng Y, Ding JS, Zhang HL. et al. Reversing the negative effect of adenosine A1 receptor-targeted immunometabolism modulation on melanoma by a co-delivery nanomedicine for self-activation of anti-PD-L1 DNAzyme. Nano Today. 2023;48:18
310. Zhang C, Huang J, Zeng Z, He S, Cheng P, Li J. et al. Catalytical nano-immunocomplexes for remote-controlled sono-metabolic checkpoint trimodal cancer therapy. Nat Commun. 2022;13:3468
311. Sun X-S, Liu L-T, Yuan L, Du C-C, Sun R, Luo D-H. et al. High extracellular matrix stiffness promotes the metastasis of nasopharyngeal carcinoma through STAT3/FGF1 positive feedback regulation activated by JAK2. Precision Medicine and Engineering. 2024;1:100005
312. Huang X, Li L, Ou C, Shen M, Li X, Zhang M. et al. Tumor Environment Regression Therapy Implemented by Switchable Prune-to-Essence Nanoplatform Unleashed Systemic Immune Responses. Adv Sci (Weinh). 2023;10:e2303715
313. Chen Z, Han F, Du Y, Shi H, Zhou W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8:70
314. Jiang M, Qin B, Luo L, Li X, Shi Y, Zhang J. et al. A clinically acceptable strategy for sensitizing anti-PD-1 treatment by hypoxia relief. J Control Release. 2021;335:408-19
315. Wang W, Zheng H, Jiang J, Li Z, Jiang D, Shi X. et al. Engineering micro oxygen factories to slow tumour progression via hyperoxic microenvironments. Nat Commun. 2022;13:4495
316. Wu J, Wang X, Chen L, Wang J, Zhang J, Tang J. et al. Oxygen microcapsules improve immune checkpoint blockade by ameliorating hypoxia condition in pancreatic ductal adenocarcinoma. Bioact Mater. 2023;20:259-70
317. Guo J, Zhao W, Xiao X, Liu S, Liu L, Zhang L. et al. Reprogramming exosomes for immunity-remodeled photodynamic therapy against non-small cell lung cancer. Bioact Mater. 2024;39:206-23
318. Zhang J, Yang M, Fan X, Zhu M, Yin Y, Li H. et al. Biomimetic radiosensitizers unlock radiogenetics for local interstitial radiotherapy to activate systematic immune responses and resist tumor metastasis. J Nanobiotechnology. 2022;20:103
319. Liu Y, Pan Y, Cao W, Xia F, Liu B, Niu J. et al. A tumor microenvironment responsive biodegradable CaCO(3)/MnO(2)- based nanoplatform for the enhanced photodynamic therapy and improved PD-L1 immunotherapy. Theranostics. 2019;9:6867-84
320. Meng L, Wang C, Lu Y, Sheng G, Yang L, Wu Z. et al. Targeted Regulation of Blood-Brain Barrier for Enhanced Therapeutic Efficiency of Hypoxia-Modifier Nanoparticles and Immune Checkpoint Blockade Antibodies for Glioblastoma. ACS Appl Mater Interfaces. 2021;13:11657-71
321. Chang CC, Dinh TK, Lee YA, Wang FN, Sung YC, Yu PL. et al. Nanoparticle Delivery of MnO(2) and Antiangiogenic Therapy to Overcome Hypoxia-Driven Tumor Escape and Suppress Hepatocellular Carcinoma. ACS Appl Mater Interfaces. 2020;12:44407-19
322. Hou G, Qian J, Guo M, Xu W, Wang J, Wang Y. et al. Hydrazide-manganese coordinated multifunctional nanoplatform for potentiating immunotherapy in hepatocellular carcinoma. J Colloid Interface Sci. 2022;628:968-83
323. Sun X, Zhang J, Xiu J, Zhao X, Yang C, Li D. et al. A phenolic based tumor-permeated nano-framework for immunogenic cell death induction combined with PD-L1 immune checkpoint blockade. Biomater Sci. 2022;10:3808-22
324. Li X, Jiang C, Jia X, Cao Y, Mao Y, Hao JN. et al. Dual "Unlocking" Strategy to Overcome Inefficient Nanomedicine Delivery and Tumor Hypoxia for Enhanced Photodynamic-Immunotherapy. Adv Healthc Mater. 2023;12:e2202467
325. Zhao M, Yang X, Fu H, Chen C, Zhang Y, Wu Z. et al. Immune/Hypoxic Tumor Microenvironment Regulation-Enhanced Photodynamic Treatment Realized by pH-Responsive Phase Transition-Targeting Nanobubbles. ACS Appl Mater Interfaces. 2021;13:32763-79
326. Xiong W, Qi L, Jiang N, Zhao Q, Chen L, Jiang X. et al. Metformin Liposome-Mediated PD-L1 Downregulation for Amplifying the Photodynamic Immunotherapy Efficacy. ACS Appl Mater Interfaces. 2021;13:8026-41
327. Zhou T, Liang X, Wang P, Hu Y, Qi Y, Jin Y. et al. A Hepatocellular Carcinoma Targeting Nanostrategy with Hypoxia-Ameliorating and Photothermal Abilities that, Combined with Immunotherapy, Inhibits Metastasis and Recurrence. ACS Nano. 2020;14:12679-96
328. Ye D, Chen Y, Xu H, Zheng M, Liu Z, Tang Z. Tumor-targeting drug delivery regimens based on vascular disrupting agents. Precision Medicine and Engineering. 2024;1:100003
329. Zhang Y, Zhong A, Min J, Tu H, Cao Y, Fu J. et al. Biomimetic Responsive Nanoconverters with Immune Checkpoint Blockade Plus Antiangiogenesis for Advanced Hepatocellular Carcinoma Treatment. ACS Appl Mater Interfaces. 2024;16:6894-907
330. Li B, Zhang X, Wu Z, Chu T, Yang Z, Xu S. et al. Reducing Postoperative Recurrence of Early-Stage Hepatocellular Carcinoma by a Wound-Targeted Nanodrug. Adv Sci (Weinh). 2022;9:e2200477
331. Bao X, Shen N, Lou Y, Yu H, Wang Y, Liu L. et al. Enhanced anti-PD-1 therapy in hepatocellular carcinoma by tumor vascular disruption and normalization dependent on combretastatin A4 nanoparticles and DC101. Theranostics. 2021;11:5955-69
332. Taleb M, Atabakhshi-Kashi M, Wang Y, Rezvani Alanagh H, Farhadi Sabet Z, Li F. et al. Bifunctional Therapeutic Peptide Assembled Nanoparticles Exerting Improved Activities of Tumor Vessel Normalization and Immune Checkpoint Inhibition. Adv Healthc Mater. 2021;10:e2100051
333. Valkenburg KC, de Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018;15:366-81
334. Xu M, Zhang T, Xia R, Wei Y, Wei X. Targeting the tumor stroma for cancer therapy. Mol Cancer. 2022;21:208
335. Pei L, Liu Y, Liu L, Gao S, Gao X, Feng Y. et al. Roles of cancer-associated fibroblasts (CAFs) in anti- PD-1/PD-L1 immunotherapy for solid cancers. Mol Cancer. 2023;22:29
336. Guo H, Liu Y, Li X, Wang H, Mao D, Wei L. et al. Magnetic Metal-Organic Framework-Based Nanoplatform with Platelet Membrane Coating as a Synergistic Programmed Cell Death Protein 1 Inhibitor against Hepatocellular Carcinoma. ACS Nano. 2023;17:23829-49
337. Zhang Y, Fang Z, Pan D, Li Y, Zhou J, Chen H. et al. Dendritic Polymer-Based Nanomedicines Remodel the Tumor Stroma: Improve Drug Penetration and Enhance Antitumor Immune Response. Adv Mater. 2024;36:e2401304
338. Zhou H, Zhang Y, Zhang R, Zhao M, Chen W, Liu Y. et al. A Tumor-Microenvironment-Activatable Molecular Pro-Theranostic Agent for Photodynamic and Immunotherapy of Cancer. Adv Mater. 2023;35:e2211485
339. Shen W, Li Y, Yang Z, Li W, Cao Y, Liu Y. et al. Tumor microenvironment reprogramming combined with immunogenic enhancement by nanoemulsions potentiates immunotherapy. J Nanobiotechnology. 2024;22:154
340. Geng S, Xiang T, Zhang Y, Guo P, Zhang H, Zhang Z. et al. Safe engineering of cancer-associated fibroblasts enhances checkpoint blockade immunotherapy. J Control Release. 2023;356:272-87
341. Panagi M, Mpekris F, Chen P, Voutouri C, Nakagawa Y, Martin JD. et al. Polymeric micelles effectively reprogram the tumor microenvironment to potentiate nano-immunotherapy in mouse breast cancer models. Nat Commun. 2022;13:7165
342. Mpekris F, Papaphilippou PC, Panagi M, Voutouri C, Michael C, Charalambous A. et al. Pirfenidone-Loaded Polymeric Micelles as an Effective Mechanotherapeutic to Potentiate Immunotherapy in Mouse Tumor Models. ACS Nano. 2023;17:24654-67
343. Li YJ, Wu JY, Hu XB, Ding T, Tang T, Xiang DX. Biomimetic Liposome with Surface-Bound Elastase for Enhanced Tumor Penetration and Chemo-Immumotherapy. Adv Healthc Mater. 2021;10:e2100794
344. Guo Z, Hu Y, Zhao M, Hao K, He P, Tian H. et al. Prodrug-Based Versatile Nanomedicine with Simultaneous Physical and Physiological Tumor Penetration for Enhanced Cancer Chemo-Immunotherapy. Nano Lett. 2021;21:3721-30
345. Luo L, Wang X, Liao YP, Xu X, Chang CH, Nel AE. Reprogramming the pancreatic cancer stroma and immune landscape by a silicasome nanocarrier delivering nintedanib, a protein tyrosine kinase inhibitor. Nano Today. 2024;54:102058
346. Zheng X, Shi Y, Tang D, Xiao H, Shang K, Zhou X. et al. Near-Infrared-II Nanoparticles for Vascular Normalization Combined with Immune Checkpoint Blockade via Photodynamic Immunotherapy Inhibit Uveal Melanoma Growth and Metastasis. Adv Sci (Weinh). 2023;10:e2206932
347. Yang H, Mu W, Yuan S, Yang H, Chang L, Sang X. et al. Self-delivery photothermal-boosted-nanobike multi-overcoming immune escape by photothermal/chemical/immune synergistic therapy against HCC. J Nanobiotechnology. 2024;22:137
348. Li Z, Mo F, Guo K, Ren S, Wang Y, Chen Y. et al. Nanodrug-bacteria conjugates-mediated oncogenic collagen depletion enhances immune checkpoint blockade therapy against pancreatic cancer. Med. 2024;5:348-67.e7
349. Colak S, Ten Dijke P. Targeting TGF-β Signaling in Cancer. Trends Cancer. 2017;3:56-71
350. Massagué J, Sheppard D. TGF-β signaling in health and disease. Cell. 2023;186:4007-37
351. Yang M, Qin C, Tao L, Cheng G, Li J, Lv F. et al. Synchronous targeted delivery of TGF-β siRNA to stromal and tumor cells elicits robust antitumor immunity against triple-negative breast cancer by comprehensively remodeling the tumor microenvironment. Biomaterials. 2023;301:122253
352. Meng M, Wu J, Feng Y, Lin L, Chen J, Pang X. et al. A Comprehensive Strategy Based on High Clinical Translational Nanosystem for Programmable Immunotherapy of Triple Negative Breast Cancer. Adv Mater. 2024;36:e2314309
353. Huang S, Ding D, Lan T, He G, Ren J, Liang R. et al. Multifunctional nanodrug performs sonodynamic therapy and inhibits TGF-β to boost immune response against colorectal cancer and liver metastasis. Acta Biomater. 2023;164:538-52
354. Wang S, Song Y, Cao K, Zhang L, Fang X, Chen F. et al. Photothermal therapy mediated by gold nanocages composed of anti-PDL1 and galunisertib for improved synergistic immunotherapy in colorectal cancer. Acta Biomater. 2021;134:621-32
355. Wang B, Bai J, Tian B, Chen H, Yang Q, Chen Y. et al. Genetically Engineered Hematopoietic Stem Cells Deliver TGF-β Inhibitor to Enhance Bone Metastases Immunotherapy. Adv Sci (Weinh). 2022;9:e2201451
356. Huang J, Zhang L, Zhou W, Wang J, Zhang R, Wang Z. et al. Dual mitigation of immunosuppression combined with photothermal inhibition for highly effective primary tumor and metastases therapy. Biomaterials. 2021;274:120856
357. Zhang P, Qin C, Liu N, Zhou X, Chu X, Lv F. et al. The programmed site-specific delivery of LY3200882 and PD-L1 siRNA boosts immunotherapy for triple-negative breast cancer by remodeling tumor microenvironment. Biomaterials. 2022;284:121518
358. Wang Y, Gao Z, Du X, Chen S, Zhang W, Wang J. et al. Co-inhibition of the TGF-β pathway and the PD-L1 checkpoint by pH-responsive clustered nanoparticles for pancreatic cancer microenvironment regulation and anti-tumor immunotherapy. Biomater Sci. 2020;8:5121-32
359. Zheng C, Sun L, Zhao H, Niu M, Zhang D, Liu X. et al. A biomimetic spore nanoplatform for boosting chemodynamic therapy and bacteria-mediated antitumor immunity for synergistic cancer treatment. Asian J Pharm Sci. 2024;19:100912
360. Guan X, Sun L, Shen Y, Jin F, Bo X, Zhu C. et al. Nanoparticle-enhanced radiotherapy synergizes with PD-L1 blockade to limit post-surgical cancer recurrence and metastasis. Nat Commun. 2022;13:2834
361. Liu M, Xie D, Hu D, Zhang R, Wang Y, Tang L. et al. In Situ Cocktail Nanovaccine for Cancer Immunotherapy. Adv Sci (Weinh). 2023;10:e2207697
362. Li H, Gong Q, Luo K. Biomarker-driven molecular imaging probes in radiotherapy. Theranostics. 2024;14:4127-46
363. Lynch C, Pitroda SP, Weichselbaum RR. Radiotherapy, immunity, and immune checkpoint inhibitors. Lancet Oncol. 2024;25:e352-e62
364. Pei Z, Lei H, Cheng L. Bioactive inorganic nanomaterials for cancer theranostics. Chem Soc Rev. 2023;52:2031-81
365. Li H, Feng Y, Luo Q, Li Z, Li X, Gan H. et al. Stimuli-activatable nanomedicine meets cancer theranostics. Theranostics. 2023;13:5386-417
366. Li H, Sun J, Zhu H, Wu H, Zhang H, Gu Z. et al. Recent advances in development of dendritic polymer-based nanomedicines for cancer diagnosis. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13:e1670
367. Zhou M, Liang S, Liu D, Ma K, Peng Y, Wang Z. Engineered Nanoprobes for Immune Activation Monitoring. ACS Nano. 2022;16:19940-58
368. Yu S, Xiong G, Zhao S, Tang Y, Tang H, Wang K. et al. Nanobodies targeting immune checkpoint molecules for tumor immunotherapy and immunoimaging (Review). Int J Mol Med. 2021;47:444-54
369. Feng Y, Xie X, Zhang H, Su Q, Yang G, Wei X. et al. Multistage-responsive nanovehicle to improve tumor penetration for dual-modality imaging-guided photodynamic-immunotherapy. Biomaterials. 2021;275:120990
370. Lv G, Sun X, Qiu L, Sun Y, Li K, Liu Q. et al. PET Imaging of Tumor PD-L1 Expression with a Highly Specific Nonblocking Single-Domain Antibody. J Nucl Med. 2020;61:117-22
371. Li X, Liu Y, Qi X, Xiao S, Xu Z, Yuan Z. et al. Sensitive Activatable Nanoprobes for Real-Time Ratiometric Magnetic Resonance Imaging of Reactive Oxygen Species and Ameliorating Inflammation In Vivo. Adv Mater. 2022;34:e2109004
372. Zhang C, Xu M, He S, Huang J, Xu C, Pu K. Checkpoint Nano-PROTACs for Activatable Cancer Photo-Immunotherapy. Adv Mater. 2023;35:e2208553
373. Zhao LP, Zheng RR, Rao XN, Huang CY, Zhou HY, Yu XY. et al. Chemotherapy-Enabled Colorectal Cancer Immunotherapy of Self-Delivery Nano-PROTACs by Inhibiting Tumor Glycolysis and Avoiding Adaptive Immune Resistance. Adv Sci (Weinh). 2024;11:e2309204
374. Su W, Tan M, Wang Z, Zhang J, Huang W, Song H. et al. Targeted Degradation of PD-L1 and Activation of the STING Pathway by Carbon-Dot-Based PROTACs for Cancer Immunotherapy. Angew Chem Int Ed Engl. 2023;62:e202218128
375. Chirnomas D, Hornberger KR, Crews CM. Protein degraders enter the clinic - a new approach to cancer therapy. Nat Rev Clin Oncol. 2023;20:265-78
376. Békés M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21:181-200
377. Tong F, Wang Y, Xu Y, Zhou Y, He S, Du Y. et al. MMP-2-triggered, mitochondria-targeted PROTAC-PDT therapy of breast cancer and brain metastases inhibition. Nat Commun. 2024;15:10382
378. Yao H, Lan J, Li C, Shi H, Brosseau JP, Wang H. et al. Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nat Biomed Eng. 2019;3:306-17
379. Tan X, Wang C, Zhou H, Zhang S, Liu X, Yang X. et al. Bioactive fatty acid analog-derived hybrid nanoparticles confer antibody-independent chemo-immunotherapy against carcinoma. J Nanobiotechnology. 2023;21:183
380. Xie F, Tang S, Zhang Y, Zhao Y, Lin Y, Yao Y. et al. Designing Peptide-Based Nanoinhibitors of Programmed Cell Death Ligand 1 (PD-L1) for Enhanced Chemo-immunotherapy. ACS Nano. 2024;18:1690-701
381. Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO. et al. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Mol Cell. 2018;71:606-20.e7
382. Liu J, Li X, Li Y, Gong Q, Luo K. Metformin-based nanomedicines for reprogramming tumor immune microenvironment. Theranostics. 2025;15:993-1016
383. Jiang X, Yi L, Li C, Wang H, Xiong W, Li Y. et al. Mitochondrial Disruption Nanosystem Simultaneously Depressed Programmed Death Ligand-1 and Transforming Growth Factor-β to Overcome Photodynamic Immunotherapy Resistance. ACS Nano. 2024;18:3331-48
384. Zhou Z, Li C, Li C, Zhou L, Tan S, Hou W. et al. Mitochondria-Targeted Nanoadjuvants Induced Multi-Functional Immune-Microenvironment Remodeling to Sensitize Tumor Radio-Immunotherapy. Adv Sci (Weinh). 2024;11:e2400297
385. Zhou Z, Jiang X, Yi L, Li C, Wang H, Xiong W. et al. Mitochondria Energy Metabolism Depression as Novel Adjuvant to Sensitize Radiotherapy and Inhibit Radiation Induced-Pulmonary Fibrosis. Adv Sci (Weinh). 2024;11:e2401394
386. Liu Y, Zhou Z, Hou J, Xiong W, Kim H, Chen J. et al. Tumor Selective Metabolic Reprogramming as a Prospective PD-L1 Depression Strategy to Reactivate Immunotherapy. Adv Mater. 2022;34:e2206121
387. Wang S, Zhou Z, Hu R, Dong M, Zhou X, Ren S. et al. Metabolic Intervention Liposome Boosted Lung Cancer Radio-Immunotherapy via Hypoxia Amelioration and PD-L1 Restraint. Adv Sci (Weinh). 2023;10:e2207608
388. Wang Y, Yu J, Luo Z, Shi Q, Liu G, Wu F. et al. Engineering Endogenous Tumor-Associated Macrophage-Targeted Biomimetic Nano-RBC to Reprogram Tumor Immunosuppressive Microenvironment for Enhanced Chemo-Immunotherapy. Adv Mater. 2021;33:e2103497
389. Wang J, Zhu Y, Chen Y, Huang Y, Guo Q, Wang Y. et al. Three-in-One Oncolytic Adenovirus System Initiates a Synergetic Photodynamic Immunotherapy in Immune-Suppressive Cholangiocarcinoma. Small. 2023;19:e2207668
390. Liu Y, Xu D, Liu Y, Zheng X, Zang J, Ye W. et al. Remotely boosting hyaluronidase activity to normalize the hypoxic immunosuppressive tumor microenvironment for photothermal immunotherapy. Biomaterials. 2022;284:121516
391. Jiang L, Zhang M, Bai Y, Cui F, Zhang C, Wang Z. et al. O-carboxymethyl chitosan based pH/hypoxia-responsive micelles relieve hypoxia and induce ROS in tumor microenvironment. Carbohydr Polym. 2022;275:118611
392. He M, Zhang M, Xu T, Xue S, Li D, Zhao Y. et al. Enhancing photodynamic immunotherapy by reprograming the immunosuppressive tumor microenvironment with hypoxia relief. J Control Release. 2024;368:233-50
393. Yu M, Duan X, Cai Y, Zhang F, Jiang S, Han S. et al. Multifunctional Nanoregulator Reshapes Immune Microenvironment and Enhances Immune Memory for Tumor Immunotherapy. Adv Sci (Weinh). 2019;6:1900037
394. Guo K, Jiao Z, Zhao X, Hu Y, Zhao N, Xu FJ. Melanin-Based Immunoregulatory Nanohybrids Enhance Antitumor Immune Responses in Breast Cancer Mouse Model. ACS Nano. 2023;17:10792-805
395. Zhang L, Li M, Zhou Q, Dang M, Tang Y, Wang S. et al. Computed tomography and photoacoustic imaging guided photodynamic therapy against breast cancer based on mesoporous platinum with insitu oxygen generation ability. Acta Pharm Sin B. 2020;10:1719-29
396. Zhang X, He C, He X, Fan S, Ding B, Lu Y. et al. HIF-1 inhibitor-based one-stone-two-birds strategy for enhanced cancer chemodynamic-immunotherapy. J Control Release. 2023;356:649-62
397. Du J, Wang C, Chen Y, Zhong L, Liu X, Xue L. et al. Targeted downregulation of HIF-1α for restraining circulating tumor microemboli mediated metastasis. J Control Release. 2022;343:457-68
398. Yu LY, Shueng PW, Chiu HC, Yen YW, Kuo TY, Li CR. et al. Glucose Transporter 1-Mediated Transcytosis of Glucosamine-Labeled Liposomal Ceramide Targets Hypoxia Niches and Cancer Stem Cells to Enhance Therapeutic Efficacy. ACS Nano. 2023;17:13158-75
399. Li L, Zhang M, Liu T, Li J, Sun S, Chen J. et al. Quercetin-ferrum nanoparticles enhance photothermal therapy by modulating the tumor immunosuppressive microenvironment. Acta Biomater. 2022;154:454-66
400. Zhou Z, Liu Y, Song W, Jiang X, Deng Z, Xiong W. et al. Metabolic reprogramming mediated PD-L1 depression and hypoxia reversion to reactivate tumor therapy. J Control Release. 2022;352:793-812
401. Allen SD, Liu X, Jiang J, Liao YP, Chang CH, Nel AE. et al. Immune checkpoint inhibition in syngeneic mouse cancer models by a silicasome nanocarrier delivering a GSK3 inhibitor. Biomaterials. 2021;269:120635
402. Li W, Xin H, Gao W, Yuan P, Ni F, Ma J. et al. NIR-IIb fluorescence antiangiogenesis copper nano-reaper for enhanced synergistic cancer therapy. J Nanobiotechnology. 2024;22:73
403. Li YS, Ye LY, Luo YX, Zheng WJ, Si JX, Yang X. et al. Tumor-targeted delivery of copper-manganese biomineralized oncolytic adenovirus for colorectal cancer immunotherapy. Acta Biomater. 2024;179:243-55
404. Zhang M, Qin X, Zhao Z, Du Q, Li Q, Jiang Y. et al. A self-amplifying nanodrug to manipulate the Janus-faced nature of ferroptosis for tumor therapy. Nanoscale Horiz. 2022;7:198-210
405. Wang W, Gao Y, Xu J, Zou T, Yang B, Hu S. et al. A NRF2 Regulated and the Immunosuppressive Microenvironment Reversed Nanoplatform for Cholangiocarcinoma Photodynamic-Gas Therapy. Adv Sci (Weinh). 2024;11:e2307143
406. Huang S, Zhou C, Song C, Zhu X, Miao M, Li C. et al. In situ injectable hydrogel encapsulating Mn/NO-based immune nano-activator for prevention of postoperative tumor recurrence. Asian J Pharm Sci. 2024;19:100901
407. Xu X, Li H, Tong B, Zhang W, Wang X, Wang Y. et al. Biomimetic Nano-Regulator that Induces Cuproptosis and Lactate-Depletion Mediated ROS Storm for Metalloimmunotherapy of Clear Cell Renal Cell Carcinoma. Adv Healthc Mater. 2024;13:e2400204
408. Liu Y, Niu R, Zhao H, Wang Y, Song S, Zhang H. et al. Single-Site Nanozymes with a Highly Conjugated Coordination Structure for Antitumor Immunotherapy via Cuproptosis and Cascade-Enhanced T Lymphocyte Activity. J Am Chem Soc. 2024;146:3675-88
409. Lai JM, Chen PL, Shi QY, Xie YQ, Jiaerheng G, Liu LH. A Self-Delivery Nanodrug Simultaneously Inhibits COX-2/PGE(2) Mediated Inflammation and Downregulates PD-L1 to Boost Photoimmunotherapy. Adv Healthc Mater. 2024;13:e2400367
410. Zhu D, Lu Y, Yang S, Hu T, Tan C, Liang R. et al. PAD4 Inhibitor-Functionalized Layered Double Hydroxide Nanosheets for Synergistic Sonodynamic Therapy/Immunotherapy Of Tumor Metastasis. Adv Sci (Weinh). 2024;11:e2401064
411. Gu T-Q, Xiao Y-L, Shao Z-M. Intratumor heterogeneity in breast cancer: Tracing its origins and translating findings into clinical practice. Precision Medicine and Engineering. 2024;1:100006
412. Vafaei S, Zekiy AO, Khanamir RA, Zaman BA, Ghayourvahdat A, Azimizonuzi H. et al. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 2022;22:2
413. Zeng W, Wang Y, Zhang Q, Hu C, Li J, Feng J. et al. Neutrophil Nanodecoys Inhibit Tumor Metastasis by Blocking the Interaction between Tumor Cells and Neutrophils. ACS Nano. 2024;18:7363-78
414. Xie J, Liu M, Deng X, Tang Y, Zheng S, Ou X. et al. Gut microbiota reshapes cancer immunotherapy efficacy: Mechanisms and therapeutic strategies. Imeta. 2024;3:e156
415. Galassi C, Chan TA, Vitale I, Galluzzi L. The hallmarks of cancer immune evasion. Cancer Cell. 2024;42:1825-63
416. Holder AM, Dedeilia A, Sierra-Davidson K, Cohen S, Liu D, Parikh A. et al. Defining clinically useful biomarkers of immune checkpoint inhibitors in solid tumours. Nat Rev Cancer. 2024;24:498-512
417. Budczies J, Kazdal D, Menzel M, Beck S, Kluck K, Altbürger C. et al. Tumour mutational burden: clinical utility, challenges and emerging improvements. Nat Rev Clin Oncol. 2024;21:725-742
418. Yamaguchi H, Hsu JM, Sun L, Wang SC, Hung MC. Advances and prospects of biomarkers for immune checkpoint inhibitors. Cell Rep Med. 2024;5:101621
419. Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S. et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18:345-62
420. Qin Y, Huo M, Liu X, Li SC. Biomarkers and computational models for predicting efficacy to tumor ICI immunotherapy. Front Immunol. 2024;15:1368749
421. Addeo A, Friedlaender A, Banna GL, Weiss GJ. TMB or not TMB as a biomarker: That is the question. Crit Rev Oncol Hematol. 2021;163:103374
422. Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J. et al. The Next Decade of Immune Checkpoint Therapy. Cancer Discov. 2021;11:838-57
423. Skirzynska A, Xue C, Shoichet MS. Engineering Biomaterials to Model Immune-Tumor Interactions In Vitro. Adv Mater. 2024;36:e2310637
424. Zhou Z, Pang Y, Ji J, He J, Liu T, Ouyang L. et al. Harnessing 3D in vitro systems to model immune responses to solid tumours: a step towards improving and creating personalized immunotherapies. Nat Rev Immunol. 2024;24:18-32
425. Elhanani O, Ben-Uri R, Keren L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell. 2023;41:404-20
426. Wei SC, Meijers WC, Axelrod ML, Anang NAS, Screever EM, Wescott EC. et al. A Genetic Mouse Model Recapitulates Immune Checkpoint Inhibitor-Associated Myocarditis and Supports a Mechanism-Based Therapeutic Intervention. Cancer Discov. 2021;11:614-25
427. Huang YV, Sun Y, Chou H, Wagner N, Vitale MR, Bayer AL. et al. Novel Therapeutic Approach Targeting CXCR3 to Treat Immunotherapy Myocarditis. Circ Res. 2025;136:473-90
428. Chuprin J, Buettner H, Seedhom MO, Greiner DL, Keck JG, Ishikawa F. et al. Humanized mouse models for immuno-oncology research. Nat Rev Clin Oncol. 2023;20:192-206
429. Cina ML, Venegas J, Young A. Stocking the toolbox-Using preclinical models to understand the development and treatment of immune checkpoint inhibitor-induced immune-related adverse events. Immunological Reviews. 2023;318:110-37
430. Bayless NL, Bluestone JA, Bucktrout S, Butterfield LH, Jaffee EM, Koch CA. et al. Development of preclinical and clinical models for immune-related adverse events following checkpoint immunotherapy: a perspective from SITC and AACR. J Immunother Cancer. 2021;9:e002627
431. Tan P, Chen X, Zhang H, Wei Q, Luo K. Artificial intelligence aids in development of nanomedicines for cancer management. Semin Cancer Biol. 2023;89:61-75
Author contact
![]() Corresponding author: Prof. Kui Luo, E-mail: luokuiedu.cn.
Corresponding author: Prof. Kui Luo, E-mail: luokuiedu.cn.
 Global reach, higher impact
Global reach, higher impact