13.3
Impact Factor
Theranostics 2025; 15(8):3551-3570. doi:10.7150/thno.108691 This issue Cite
Review
Recent advances and prospects of nanoparticle-based drug delivery for diabetic ocular complications
Department of Pharmaceutics, School of Pharmacy, Shenyang Pharmaceutical University, No. 103, Wenhua Road, Shenyang, 110016, P. R. China.
# These authors contributed to the work equally.
Received 2024-12-12; Accepted 2025-1-24; Published 2025-2-25
Abstract
Diabetes mellitus (DM) is a chronic metabolic disorder that significantly affects various organ systems. The systemic effects of DM lead to numerous complications, with ocular manifestations being of particular concern due to their severity and impact on quality of life. Hyperglycemia-induced ocular damage often results in a range of lesions, including diabetic retinopathy (DR), keratopathy, cataracts, and glaucoma. These conditions impose considerable physical discomfort on patients and place a substantial economic burden on healthcare systems. The advent of nanotechnology has facilitated the development of innovative therapeutic strategies for managing diabetic ocular complications. This review highlights several common ocular complications associated with DM, focusing on their pathogenesis and treatment strategies. Emphasis is placed on the innovative applications and potential of nanotechnology in treating diabetic ocular complications.
Keywords: diabetes eye complications, nanoparticles, therapeutic approaches, drug delivery, nanotechnology, ophthalmic vehicles
1. Introduction
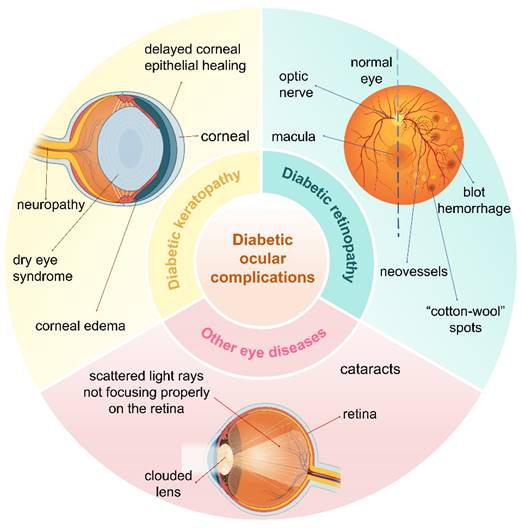
The eye is a vital organ in the human body. Diabetic eye complications primarily result from chronic hyperglycemia, which affects multiple ocular structures and contributes significantly to various eye diseases [1]. The increasing prevalence of diabetes mellitus (DM) has led to a rise in the incidence of ocular complications. Prominent among these complications are diabetic keratopathy, diabetic retinopathy (DR), cataracts, and glaucoma. These ocular manifestations cause considerable physical discomfort for affected individuals and impose a substantial economic burden on patients and healthcare systems (Figure 1).
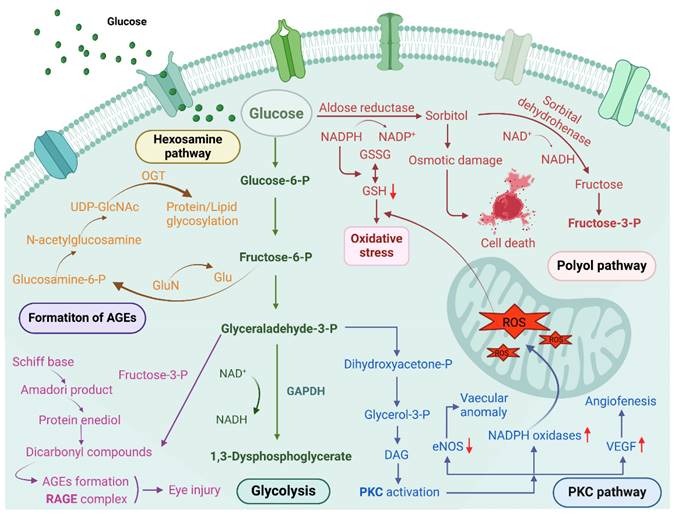
Following extensive research over the years, significant progress has been made in understanding the pathogenesis of diabetic ocular diseases; however, the mechanisms underlying diabetic dry eye and other ocular lesions remain incompletely understood, which hampers the development of effective clinical treatment strategies. Several common pathogenic mechanisms of hyperglycemia have been proposed, such as increased polyol pathway flux, overactivation of the hexosamine pathway, accumulation of intracellular advanced glycation end products (AGEs), activation of the protein kinase C (PKC) pathway, inflammatory responses, and oxidative stress [2, 3]. These mechanisms are summarized below. Since the proposal of a unified mechanism for diabetic complications, growing evidence indicates that reactive oxygen species (ROS) activate multiple signaling pathways, with oxidative stress induced by ROS being a key pathogenic factor in DM and its complications [4] (Figure 2).
The most commonly employed treatment approaches can be categorized into three types [5, 6]: (1) Topical administration, primarily for anterior segment diseases; (2) Intraocular administration, which provides superior efficacy compared to topical treatments; and (3) Oral administration, notable for its high patient compliance. These traditional drug delivery systems are cost-effective, convenient, and generally safe. However, the eye's complex physiological barriers and anatomical structures hinder the entry and penetration of drugs into intraocular tissues, often resulting in suboptimal therapeutic outcomes for ocular drug delivery. Tear turnover, blinking, and nasolacrimal drainage rapidly eliminate many topical eye drops [7]. Additionally, both static and dynamic barriers restrict the delivery of intraocular medications [8]. Oral administration is limited by the blood-retinal barrier, which prevents the influx of molecules towards the vitreous from the bloodstream, regulated by the retinal pigment epithelium [9]. Systemic administration typically requires high doses to achieve therapeutic efficacy in the eye [10].
In recent years, nano-delivery systems have made significant progress and hold promise for addressing the challenges of ocular disease treatment. Various carriers, such as liposomes, micelles, lipid nanoparticles, and dendritic macromolecules, have been extensively studied, providing several advantages for ocular drug delivery. These systems extend the residence time and reduce dosing frequency, allowing therapeutic agents to remain in the eye for longer periods. They also enhance drug solubility, thereby improving the bioavailability of therapeutic agents [11]. Additionally, these systems promote adhesion and rapid internalization, enhancing their interaction with the ocular surface. In gene therapy, these systems improve both the efficiency and duration of gene transfection, offering a promising approach for treating genetic disorders of the eye and potentially resulting in long-lasting therapeutic effects and improved patient outcomes. Beyond therapy, these systems also enhance ocular diagnosis and imaging, improving diagnostic accuracy and effectiveness. Finally, they can facilitate retinal repair, thereby providing a pivotal benefit in the management of ocular diseases [12].
This paper seeks to increase awareness of the detrimental effects of DM on ocular health and to highlight the ophthalmic complications that arise from the disease, simultaneously elucidating the potential role of nanotechnological interventions in the therapeutic management and monitoring of these conditions. The impact of various ocular complications on the patient and the advantages and limitations of currently available treatments are analyzed. The paper concludes with an exploration of recent advancements in nanotechnology as applied to ocular drug delivery, gene therapy, and other key areas, including diagnostics, sensing, and imaging.
Main types of ocular complications of DM, including diabetic keratopathy, DR, and cataracts. Created with BioRender.com. (http://biorender.com).
The main pathways causing hyperglycemia include the polyol pathway, hexosamine pathway, PKC activation, and formation of AGEs, etc. Created with BioRender.com. (http://biorender.com).
2. Diabetic eye complications: addressing major vision risks
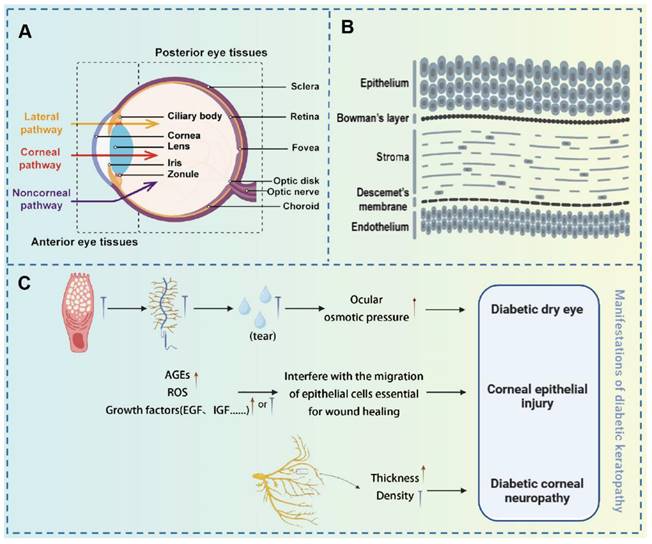
The human eye is a complex and vital organ, essential for visual perception. Structurally, the eye is divided into two primary segments: the anterior and posterior segments, each serving distinct functions related to visual processing and ocular protection [13]. The anterior segment, consisting of the cornea, iris, lens, and aqueous humor, plays a pivotal role in the eye's anterior functions. The cornea, a refractive tissue composed of five distinct layers (epithelium, Bowman's membrane, stroma, Descemet's membrane, and endothelium), contains an epithelial layer that serves as a formidable barrier through tight junctions, preventing foreign substances from penetrating the eye [14]. The posterior segment, comprising the vitreous body, retina, choroid, and posterior sclera, is equally crucial for visual function. The retina, a neural layer, is responsible for converting light signals into electrical impulses. The choroid, abundant in the vasculature, supplies the outer retina and plays a crucial role in regulating the intraocular environment. The sclera provides structural support and is integral to ocular defense mechanisms. However, DM induces progressive, vision-threatening changes in various ocular tissues. Therefore, timely recognition and management of diabetic ocular complications are essential for preserving vision and reducing long-term morbidity. The therapeutic strategies for several common ocular complications are discussed in the following sections.
2.1 Approaches for managing diabetic keratopathy
Approximately 47-64% of diabetic patients are affected by diabetic keratopathy [15]. Diabetic keratopathy is a prevalent complication in both type 1 and type 2 diabetes mellitus (T1DM and T2DM). As our understanding of diabetic ocular complications improves, and with the rising number of cases involving vision impairment due to corneal lesions, diabetic corneal complications are receiving greater attention in clinical practice [16]. Studies have demonstrated that diabetic corneas are exhibit greater susceptibility to bacterial and viral infections than healthy corneas, due to an imbalance in the ocular surface environment [17]. DM can impair the corneal structure, including the epithelium, stroma, and endothelium [18].
Hyperglycemia-induced oxidative stress can damage the mitochondria of corneal epithelial cells, reducing available cellular energy, which in turn impairs the cells' proliferation and migration abilities, ultimately leading to delayed corneal epithelial healing [19]. Additionally, the accumulation of AGEs can disrupt the homeostasis necessary for corneal epithelial wound healing, further exacerbating corneal epithelial damage [20]. Corneal neuropathy is another significant manifestation of diabetic corneal complications. In a hyperglycemic environment, corneal nerve fiber bundles thicken and undergo morphological changes, nerve conduction is impaired, and the concentration of neurotrophic factors decreases, leading to reduced corneal sensation and symptoms such as dry eye disease (DED) [21]. These pathological changes not only impair corneal function but also increase the risk of corneal infections and other complications, thereby posing a significant threat to patients' vision (Figure 3).
The previously mentioned symptoms of corneal lesions significantly affect ocular health and present a considerable threat to patients' quality of life. Furthermore, structural, metabolic, and functional changes in the cornea induced by DM pose substantial challenges in management and treatment. Glycemic control remains the primary strategy for preventing the rapid progression of ocular structural damage. Currently, numerous pharmacological agents, including oral medications (e.g., metformin) and injectable therapies (e.g., insulin), are utilized to manage glycemia. However, due to the delay between the onset of DM symptoms and the achievement of effective glycemic control, ocular damage may already be evident by the time treatment begins. This highlights the importance of early diagnosis in preventing the onset of DM-associated ocular complications [5].
Therefore, to prevent or delay ocular damage induced by hyperglycemia, considerable research has focused on developing more effective ophthalmic drugs and optimized drug delivery systems for managing diabetic ocular surface diseases. Current therapeutic strategies include conventional approaches (e.g., topical lubricants, soft bandage contact lenses, and corneal transplantation), antioxidants, growth factors, gene therapy, and novel interventions such as naltrexone therapy. The following table provides a comprehensive analysis of current clinical and experimental treatments for diabetic keratopathy, offering insights into their mechanisms of action, therapeutic efficacy, and potential limitations (Table 1).
(A) Diagram of ocular anatomical components; (B) Schematic representation of the corneal layers; (C) Manifestations of diabetic keratopathy. (A-B) Adapted with permission from [22], copyright 2024, Elsevier. (C) Original figure by Siqi Wang.
Clinical and experimental treatment strategies for DM-related corneal diseases.
| Treatment strategy | Therapeutic agent | Advantages | Limitation | Ref. |
|---|---|---|---|---|
| Blood glucose control | Nateglinide; Glibenclamide; Exenatide; Pioglitazone | Increase epithelial wound healing; Inhibit changes in Descemet | Prone to hypoglycemia | [23, 24] |
| Insulin eye drops/ implants | Accelerate corneal wound healing; prevent subbasal corneal nerve loss | Increase levels of VEGF and worsening DR | [25-27] | |
| Lipid control | Fibrates | Anti-inflammatory action; Enhance corneal innervation and sensitivity | Side effects of T2D patients | [28] |
| Statins | Significantly lower lipid peroxidation in diabetic neuropathy patients | Controversial effects | [29, 30] | |
| Opioid antagonists | Naltrexone | Corneal epithelial wound healing | Autoxidation | [31, 32] |
| Ergoline derivatives | Megerin | Promote corneal wound healing and improve corneal sensitivity | Current research has limitations | [33, 34] |
| Antioxidant | β-carotene, α-lipoic acid | Promote corneal epithelial regeneration | Eye transport difficulties | [35] |
| Aldose reductase inhibitor | Ranistat; CT-112; Kinostat® | Faster epithelial wound healing | the clinical effect is controversial | [36, 37] |
| Surgery | Corneal Transplantation | One-time treatment of corneal defect | Low success rate; Prone to corneal infection; Possible rejection | [38] |
| Treatment strategy | Therapeutic agent | Advantages | Limitation | Ref |
| Lubricant | Artificial tears | Relieve eye dryness | Unable to boost tear production | [39] |
| Anti-inflammatory | Corticosteroid | Reduce inflammation levels of dry eye; Prevention of corneal epithelial injury | Cause infections; Induce cataracts; Cannot long-term use | [40-42] |
| Nonsteroidal Anti-inflammatory Drugs (NSAIDs) | Avoid steroid side effects, relieve dryness, heal corneas, protect goblet cell | Cannot improve the production of tears; Reduce corneal sensitivity | [43, 44] | |
| Cyclosporine A | Improve tear production | Poor ocular drug availability | [45] | |
| Tacrolimus | Anti-inflammatory; Restore tear production; Reduce dry eye symptoms | Ocular toxicity; Dose dependence | [46] | |
| Autoserum | Safe and effective; Facilitates wound healing in diabetic patients | Induce secondary infections; specific causative factors are doubt | [47-49] | |
| Growth factor | NGF; SP; C-peptide | Increase neurite growth and subbasal nerve density; Promote epithelial wound healing; Restore corneal sensitivity | Limited sources; High cost; Serious side effects; Short biological half-life; The mechanism is not clear; | [50] |
| Gene therapy | mRNA; siRNA | Improve corneal wound healing rate; Promote corneal nerve regeneration | Too much target | [51] |
| Fatty acids | Docosahexaenoic acid (DHA) | Antioxidative stress | High dose of toxic side effects | [52, 53] |
2.2 Therapeutic strategies for DR
Under the prolonged effects of DM, individuals with either T1DM or T2DM are at significant risk of developing DR, a debilitating eye condition that poses a major threat to vision [54]. Angiogenesis plays a detrimental role during the progression of DR. Abnormal angiogenesis promotes the progression of non-proliferative diabetic retinopathy (NPDR) to more advanced stages. Although the initial stage of NPDR may be asymptomatic, angiogenesis-related changes are already occurring. If untreated, visual impairment can rapidly progress to severe consequences. In the proliferative diabetic retinopathy (PDR) stage, angiogenesis becomes more pronounced, and its harmful effects become more apparent [55]. The newly formed blood vessels are fragile and prone to rupture and hemorrhage. This also leads to retinal edema, which exacerbates retinal ischemia and can result in severe outcomes, including substantial vision loss or complete blindness.
Diabetic macular edema (DME) is a severe complication of DR, impacting all stages and forms of the disease; angiogenesis contributes significantly to its progression [56]. Optical coherence tomography (OCT) is used to classify DME into distinct patterns, such as diffuse macular edema, cystoid macular degeneration, and serous retinal detachment [57]. Despite significant advancements in understanding DR, the precise mechanisms underlying its development remain multifaceted and complex, involving various disrupted metabolic processes and immune responses [58]. The intricate interplay between these complex mechanisms and the detrimental effects of angiogenesis presents considerable challenges in the clinical management of DR.
Current therapeutic approaches for DR encompass glycemic control, topical administration, laser therapy, intravitreal medications, and vitrectomy. In recent years, researchers have made significant advances in understanding the molecular mechanisms of abnormal angiogenesis in DR, as well as potential therapeutic targets. Previous studies have shown that vascular endothelial growth factor (VEGF) plays a crucial role in promoting angiogenesis, while matrix metalloproteinase X1 (MMP-X1) is involved in early-stage vascular destruction and late-stage neovascularization in DR. Xinsheng Li et al. [59] have shown that the exosome lncRNA-MIAT/miR133a-3p/MMP-X1 axis may be a potential intervention for DR. Based on previous research and the correlation analysis in this study, exosome-delivered lncRNA-MIAT could jointly inhibit the expression of VEGF and MMP-X1 via miR-133a-3p, potentially suppressing abnormal angiogenesis in DR. Moreover, lncRNA-MIAT could modulate not only VEGF but also the expression of MMP-X1 through miR-133a-3p. Such findings deeply corroborate the role of lncRNA-MIAT in enhancing angiogenesis and suggest a new interventional and remedial target for DR.
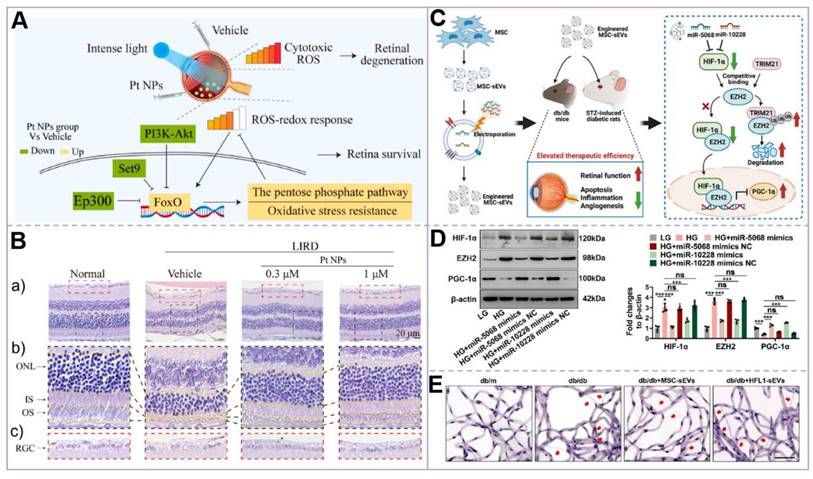
Similarly, Shaika Shanbagh et al. [60] found that the miR182-5p level in the eyes of PDR patients was relatively high. After transfecting retinal pigment epithelial (RPE) cells with the miR-182-5p mimic, they observed phenomena such as enhanced glycolysis and increased VEGF secretion, similar to those under high-glucose conditions. Therefore, regulating the expression of miR182-5p represents a potential method for achieving DR treatment. In addition, Tingyu Qin et al. [61] suggest that increasing the expression of polo-like kinase-3 (PLK-3) can also inhibit VEGF levels, thereby alleviating retinal vascular dysfunction in DR. In addition, researchers have made important breakthroughs in molecular mechanisms and cell therapy. On the one hand, the study by Lin Su et al. [62] demonstrated that platinum nanoparticles (Pt NPs) effectively reduce intracellular and retinal ROS and oxidative stress levels. Additionally, Pt NPs were shown to reduce photoreceptor apoptosis and maintain retinal structure and function in a DR-related light-induced retinal degeneration model. These findings provide a novel direction for DR intervention through the use of nanomaterials. On the other hand, Fengtian Sun et al. [63] successfully constructed engineered mesenchymal stem cell-derived small extracellular vesicles (MSC-sEVs), which enhanced their therapeutic efficiency in improving retinal function and reducing damage by increasing the levels of miR-5068 and miR-10228. These engineered vesicles exhibited a stronger therapeutic effect compared to natural MSC-sEVs, thereby opening a new avenue for the cell-free treatment of DR. These studies not only elucidate the complex pathological mechanisms of DR but also provide a scientific foundation for the development of novel therapeutic targets and strategies. In the future, therapeutic strategies integrating nanotechnology and extracellular vesicles are anticipated to emerge as a significant complementary approach for treating DR (Figure 4).
(A) Mechanism of Pt NPs in reducing ROS and protecting the retina from oxidative damage. In the Pt NPs-treated group, Pt NPs effectively reduced cytotoxic ROS levels and activated the FoxO signaling pathway, thereby regulating downstream protective mechanisms to mitigate oxidative damage and preserve retinal integrity; (B) H&E staining of the retina confirmed that Pt NPs effectively protected RPs from oxidative damage. (a) H&E staining images of each group of retinas. (b) An enlarged view of the area is in the black box in (a). (c) Enlarged view of the area in the red box in (a); (C) Mechanism of MSC-sEVs in treating diabetic retinopathy via the HIF-1α/EZH2/PGC-1α pathway. (D) Western blot analysis of HIF-1α, EZH2, and PGC-1α expression in retinal microvascular endothelial cells (RMECs) post-transfection; (E) Retinal trypsin digestion assay. Representative images of retinal vasculature following trypsin digestion, illustrating the structural changes in the retinal microvasculature. Scale bar: 50 μm. (A-B) Adapted with permission from [62], copyright 2023, Elsevier. (C-E) Adapted with permission from [63], copyright 2024, Ke Ai Publishing.
2.3 Treatments for other common diabetic eye diseases
Among the various ophthalmic complications associated with DM, cataracts and glaucoma are also significant contributors to visual impairment. This section provides a concise overview of the treatment strategies for diabetic cataract (DC) and glaucoma.
2.3.1 Therapeutic interventions for DC
DC is characterized by lens opacification, which not only threatens vision but may also exacerbate DR. Individuals with DM tend to develop cataracts earlier than those without DM [64]. Surgical intervention remains the primary therapeutic approach for DC, though it may induce secondary complications such as diminished vision, vitreous hemorrhage, and choroidal detachment. Pharmacological strategies, including the use of aldose reductase inhibitors (e.g., Fadaprestat, Ranitidine), antioxidants (e.g., curcumin, vitamin C), and glycation inhibitors (e.g., ibuprofen, aspirin), are being explored [65]. However, the efficacy of these treatments is often limited by the lipophilicity of certain drugs, which restricts their topical use. Clinical practice emphasizes strict glycemic control and continuous ophthalmic surveillance throughout the disease. Preoperative administration of NSAIDs in DC patients with NPDR or PDR may help mitigate postsurgical inflammatory responses [66].
2.3.2 Glaucoma management in diabetic patients
Primary open-angle glaucoma (POAG) is the main form of glaucomatous neuropathy, marked by chronic, progressive loss of retinal ganglion cells and their axons, leading to irreversible visual field defects and potential blindness [67]. Elevated intraocular pressure (IOP) and its fluctuations are major risk factors in the development and progression of POAG. Additional risk factors include advanced age, myopia, and variations in central corneal thickness. The association between DM and POAG is increasingly recognized, with epidemiological studies showing a higher incidence of POAG in individuals with DM compared to those without DM [68]. DM and glaucoma share several common pathophysiological mechanisms, with DM known to induce hypertension. The primary treatment goal for POAG is to reduce IOP through pharmacological, laser, or surgical interventions. Furthermore, DM can exacerbate retinal injury in POAG through oxidative stress and the accumulation of AGEs, contributing to vascular dysfunction and retinal ischemia. A deeper understanding of these mechanisms is crucial for developing targeted therapies to address concurrent DM and POAG, potentially halting or slowing the progression of vision loss. The integration of advanced diagnostic tools and personalized approaches is critical for optimizing outcomes, given the complex interplay between DM and POAG [69].
3. Innovative nanotechnology: revolutionizing eye disease treatment
Nanotechnology is an emerging discipline focusing on the properties and applications of materials with structural dimensions ranging from 0.1 to 100 nm. It has been extensively applied across diverse fields, including environmental science and chemistry [70, 71]. Nanoparticles address conventional drugs' limitations and offer novel disease surveillance strategies [72]. Compared to traditional formulations, nano-preparations offer several advantages: their small particle size enables them to cross barriers inaccessible to conventional treatments; they exhibit targeted delivery, directing drugs precisely to the site of action; their sustained-release properties extend the drug's therapeutic duration; and they minimize drug degradation by enzymes in the body. Nanotechnology is currently employed in diverse routes of administration, including intravenous injection, oral, and topical administration, for treating a broad range of diseases including cancer, ocular inflammation, and diabetic complications [73] (Figure 5).
The advent of nanotechnology has had a profound impact on various sectors of medicine, with ocular treatments experiencing notable advancements. Several marketed and approved nano-preparations for ocular treatments are listed in Table 2. The introduction of these products not only expands the range of ophthalmic therapies but also demonstrates the feasibility and potential of nanosystems in ocular applications, promising improved efficacy and safety in the treatment of eye diseases.
3.1 Core Functions of Nanotechnology in Eye Disease Therapy
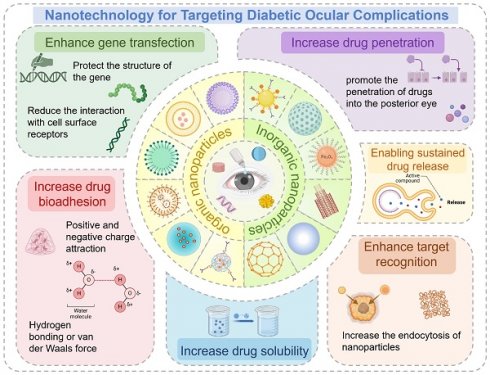
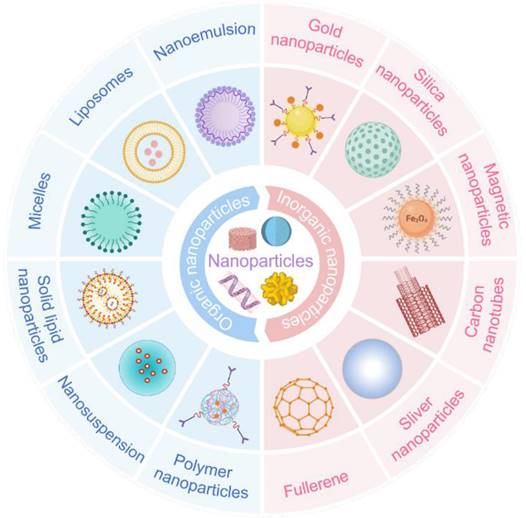
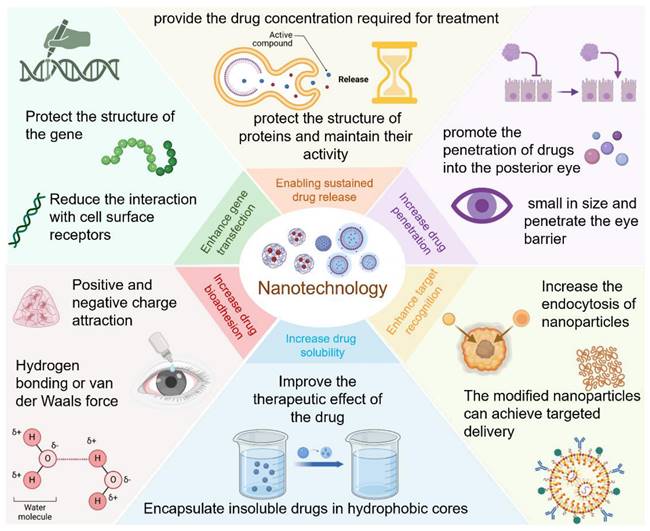
The drug delivery systems for diabetic eye therapies face inherent limitations, particularly in drug solubility and ocular retention. Nanotechnology can address these issues, thereby enhancing treatment efficacy. In the exploration of novel treatments for eye diseases, the application of nanotechnology has significantly improved the delivery of macromolecules, including genes, to ocular tissues. Beyond its essential role in therapy, nanotechnology has also been employed in the detection and monitoring of diabetic eye conditions (Figure 6).
Some common nanoparticles, which is divided into two categories: organic nanoparticles and inorganic nanoparticles. Each type has its characteristics that make it suitable for specific applications. Created with BioRender.com. (http://biorender.com).
Summary of the applications of nanotechnology in eye treatment. Created with BioRender.com. (http://biorender.com).
Some marketed/approved nano-preparations for the treatment of ocular diseases.
| Formulation | Active Ingredient | Product | Main composition | Administration Method | Adaptive Disease | Ref. |
|---|---|---|---|---|---|---|
| Nanoemulsion | Cyclosporin A | Restasis® | Castor oil; Polysorbate 80; Carbomer | Eye drop | DED | [74, 75] |
| Difluprednate | Durezol® | Castor oil; Polysorbate 80 | Eye drop | Eye inflammation | ||
| Cyclosporin A | Ikervis® | Medium chain triglycerides; Poloxamer 188 | Eye drop | DED | [76] | |
| Micelle | Cyclosporin A | Cequa® | HCO-40; OC-40 | Eye drop | DED/ KCS | [77, 78] |
| Liposome | Vitamin A palmitate; Vitamin E | Lacrisek® | Hydrogenated phospholipids | Eye drop | DED | [79] |
| Hyaluronic acid | Tears Again® | Liposomal soy lecithin; Phenoxyethanol | Eye spray | DED | ||
| Verteporfin | Visudyne® | Unsaturated EPG; DMPC | Intravitreal injection | AMD | [80, 81] | |
| Nanoparticle | Pegaptanib sodium | Macugen® | PLGA | Intravitreal injection | Neovascularization; AMD | [82, 83] |
| Implant | Fluocinolone acetonide | Retisert | PVA | Intravitreal implant | Uveitis | [84] |
| Dexamethasone | Ozurdex | PLGA | Intravitreal implant | Uveitis | ||
| Ganciclovir | Vitrasert® | PVA; EVA | Intravitreal injection | AIDS-associated cytomegalovirus retinitis | [85] | |
| Fluocinolone acetonide | Iluvien® | Polyimide | Intravitreal injection | DME | ||
| Yutiq® | Polyimide | Intravitreal injection | Chronic non-infectious uveitis | |||
| Suspension | Triamcinolone acetonide | Trivaris | Sodium hyaluronate; Sodium phosphate | Intravitreal injection | Uveitis | [86, 87] |
| Triesence | Sodium carboxymethyl cellulose; Polysorbate 80 | Intravitreal injection | Diabetic macular edema | |||
| Dexycu® | Acetyl triethyl citrate | Intravitreal injection | Inflammation | [85] | ||
| Bromfenac | Bromsite® | Bromfenac durasite; Synthetic polymer of cross-linked polyacrylic acid | Topical administration | Inflammation; Pain after cataract surgery | [88] | |
| Betaxolol | Betoptic S® | Ion exchange resins | Topical administration | Glaucoma | [89] | |
| Indomethacin | Indocollirio® | Hydroxypropyl-b-cyclodextrin | Topical administration | Eye inflammation | [90] | |
| Besifloxacin | Besivance® | Polycarbophil | Eye drop | Infection | [91] | |
| Hydrogel | Timolol maleate | TIMOPTIC-XE® | Gelrite | Eye drop | Glaucoma | [92] |
Dry eye disease (DED); Polyoxy hydrogenated castor oil (HCO-40); Octoxynol-40 (OC-40); Keratoconjunctivitis sicca (KCS); Age-related macular degeneration (AMD); Unsaturated Egg Phosphatidylglycerol (EPG); Dimyristoyl phosphatidylcholine (DMPC); Poly (lactic-co-glycolic acid) (PLGA); Polyvinyl alcohol (PVA); Ethylene vinyl acetate (EVA); Diabetic Macular Edema (DME).
3.1.1 Enhancing drug solubility
The limited solubility of some approved drugs and most investigational agents restricts their clinical use [93]. Many medications are formulated as suspensions due to poor solubility, but this form often leads to foreign body sensations and can obstruct lacrimal glands. Nanocarriers such as micelles can significantly enhance drug solubility. Micelles utilize their amphiphilic properties to encapsulate insoluble drugs in hydrophobic cores, forming transparent aqueous solutions. Triamcinolone acetonide, a widely used corticosteroid with anti-inflammatory effects, has been employed to treat diabetic eye diseases like DED, DR, and DME. Loading it into poly(ethylene glycol)-block-poly(ε-caprolactone) (PEG-b-PCL) micelles increased its solubility fivefold, while using poly(ethylene glycol)-block-poly(lactic acid) (PEG-b-PLA) micelles enhanced solubility tenfold [94]. Alpha-lipoic acid (ALA), known for its antioxidant properties, has been investigated for diabetic keratopathy and retinopathy [95]. In micelle preparations, its stability and solubility were significantly improved, with Soluplus® polymeric micelles increasing its solubility tenfold [96, 97].
Dapagliflozin (Dapa), a sodium-glucose cotransporter 2 (SGLT2) inhibitor used to therapy DM, has shown promise in targeting Epithelial-Mesenchymal Transition (EMT) biomarkers. This approach may reduce SGLT2 activation and related pathways in DC, while nanotechnology-based Dapa eye drops significantly improve Dapa's aqueous solubility, offering great potential for treating DC [98]. Albumin's unique three-dimensional network structure offers significant advantages in drug delivery by efficiently encapsulating hydrophobic drugs and improving their solubility [99, 100]. Nanoparticles formulated with albumin have been particularly effective in increasing the solubility and ocular bioavailability of curcumin, a treatment for DR, demonstrating that improved solubility contributes positively to therapeutic efficacy [101].
Furthermore, Siyu Gui et al. [102] successfully synthesized ultrasmall Fe-Quer nanozyme by coupling quercetin with low-toxicity iron ions. This innovative method not only effectively addressed the low solubility of quercetin but also endowed it with the ability to mimic three important antioxidant enzymes (superoxide dismutase, catalase, and peroxidase), thereby significantly enhancing its capacity to scavenge ROS. This dual enhancement in structure and function has endowed Fe-Quer nanozyme with significant potential in preventing and delaying the development and progression of DR. Strategies to enhance drug solubility, particularly through the development and optimization of nanoemulsions, have also demonstrated considerable potential. For the treatment of DED, ibuprofen has been formulated using chitosan-coated nanoemulsions to improve its solubility and stability [103]. The incorporation of chitosan imparts a positive charge to the nanoemulsions, thereby enhancing their interaction with the negatively charged ocular surface mucins, prolonging the residence time of the drug on the ocular surface, and improving its bioavailability.
3.1.2 Improving drug permeability
One of the major challenges in ocular drug delivery systems is the low bioavailability of drugs. This is mainly because most ophthalmic drugs are administered on the eye's surface, and special barriers and structural features limit drug absorption and distribution [104]. Ocular barriers include the dilution and rapid turnover of tears, the barrier effect of the corneal epithelium, and the blood-retinal barrier [105]. Therefore, the development of novel drugs capable of entering the eye's posterior segment is crucial for the effective treatment of ocular diseases.
Pioglitazone, a thiazolidinedione antidiabetic drug for T2DM treatment, increases the body's insulin sensitivity by activating peroxisome proliferator-activated receptor γ (PPAR-γ), thus helping to lower blood glucose levels. Moreover, PPAR-γ receptors are also implicated in DR [106, 107]. Umesh D. Laddha et al. prepared pioglitazone-loaded PLGA nanoparticles for ocular drug delivery via a single-emulsion solvent evaporation method. The surface modification was achieved by enhancing the interaction with the eye using the non-ionic surfactant sorbate 80, initially attaining the goal of drug delivery to the eye's posterior segment [108].
A research team from Tianjin Medical University has developed a novel liposome-based permeable eyedrop formulation (pDrops) utilizing liposomal technology [109]. This innovative delivery system consists of a core-shell structure, with the therapeutic agent encapsulated in the liposome core and chitosan forming the outer shell. The chitosan coating improves the adhesion of pDrops to mucin in tears, thereby extending the drug residence time on the ocular surface. Upon contact with the anterior ocular surface, pDrops transiently open tight junctions between corneal and conjunctival epithelial cells, facilitating drug penetration into the posterior segment and overcoming ocular barriers. Notably, pDrops can simultaneously deliver both hydrophobic curcumin and hydrophilic ganciclovir to the posterior segment of the eye.
As research progresses, nanozymes have drawn increasing attention. Nanozymes are nanomaterials with inherent enzyme-like properties and can achieve self-cascade catalysis that is difficult for enzymes or small molecules to achieve. Min Tian et al. noted the important role of metal nanoparticles in DR treatment and designed an eye drop based on ultra-small copper nanodots nanozymes capable of penetrating into the fundus [110, 111]. This eye drop adopts a comprehensive microenvironment regulation mode based on the nanoenzyme cascade, which has multiple functions, including alleviating hypoxia, scavenging free radicals, and anti-inflammation.
3.1.3 Augmenting pharmaceutical compound bioadhesion
Local administration, typically via eye drops, has remained the primary method for treating eye diseases. However, the rapid turnover of the tear film and nasolacrimal drainage significantly reduces the retention of eye drops, with only a small portion reaching the posterior segment of the eye. Nanotechnology offers a solution by promoting the rapid adhesion of drugs to the ocular epidermis tissue, thereby extending their retention time. The primary mechanisms underlying nanoparticle-mucosal adhesion include (1) Diffusion, where polymer chains penetrate the mucosal layer; (2) Adsorption, involving interactions between the carrier's functional groups and mucin through hydrogen bonding or van der Waals forces; and (3) Electrostatic attraction between positively and negatively charged components of the carrier and mucin [112-114].
Although the complete pathogenesis of DED remains unclear, ocular surface inflammation caused by bacteria and fungi, as well as hyperosmotic stress of the tear film, which leads to the excessive production of ROS, are considered to be the basic pathological changes of DED. Excessive accumulation of ROS can lead to not only oxidative stress on the ocular surface but also pathological changes in ocular tissues, such as reduced mucin production and immune dysregulation. Compared with traditional ocular antioxidants, nanozymes possess certain advantages [115]. Haoyu Zou et al. [116] developed a cerium oxide nanoenzyme, capable of effectively scavenging excessive ROS produced by oxidative damage and hypertonic stimulation. They exploited the affinity between phenylboronic acid (PBA) and o-diols to dynamically bind the tear film mucin layer, which contains many neighboring diol structures, to PBA fragments, thereby greatly improving the ocular surface adhesion force of the nanoenzyme.
To enhance the therapeutic effect in glaucoma, Yingshan Zhao et al. [117] developed a novel nano-delivery system capable of penetrating the mucin layer and delivering drugs to the cornea through electrostatic interactions between cationic carriers and anionic mucin in the tear film. Unlike simple drug molecular solutions, the nanosystem rapidly interacts with mucin upon contact with the ocular surface. This interaction not only prevents metabolism by tear and corneal enzymes but also enables controlled drug release, thereby prolonging the retention time on the ocular surface. Furthermore, nano-micelles have shown significant potential in ophthalmology. Sun Xingchen et al. [118] designed and synthesized a phenylboronic acid-conjugated chitosan-vitamin E copolymer for loading voriconazole, which treats fungal keratitis, to form mucin-adhesive nanomicelles. They also demonstrated these nanomicelles' strong permeability and ocular surface retention capabilities.
Similarly, Haijie Han et al. [119] developed a cabozantinib-loaded nanoparticle carrier, Cabo-NPs, based on cationic peptides. This carrier achieves mucoadhesion through the interaction between cationic peptides and mucosa, thereby prolonging the drug retention time on the cornea and enhancing bioavailability. It effectively inhibits corneal neovascularization (CNV), with efficacy comparable to dexamethasone but without significant side effects, offering a safer alternative for CNV treatment. The bioadhesive glycoconjugated nanoplatform developed by Yanlong Zhang et al. [120] has also advanced the treatment of CNV. This nanoplatform is self-assembled from amphiphilic boronic acid-based copolymers, demonstrating good stability and biocompatibility. It specifically binds to corneal epithelial cells via the boronic acid module, achieving long-term retention on the corneal surface. Furthermore, it releases dexamethasone (DEX) in a controllable manner, enabling efficient transcorneal drug delivery.
3.1.4 Optimizing nanomedicine surface for better targeting and uptake
Interactions between specific receptors and nanoparticles can lead to enhanced endocytosis, thereby increasing cellular uptake and drug efficacy [121]. This principle has been effectively utilized in the development of targeted drug delivery systems [122]. For instance, hyaluronic acid (HA), a negatively charged polysaccharide, binds to CD44 receptors on various retinal cells, facilitating the internalization of nanoparticles through HA-mediated endocytosis. Research demonstrates that HA-coated nanoparticles significantly improve retinal targeting compared to uncoated counterparts, emphasizing the potential of HA coatings for the localized therapy of posterior segment ocular disorders [123]. Folic acid (FA) receptors are expressed in the cell layer of the RPE [124], prompting studies to explore FA-modified nanoparticles for targeted drug delivery to the retina [125]. Dave V. and colleagues modified gold nanoparticles with synthetic FA-b-PEG block copolymer to facilitate the delivery of sorafenib tosylate for treating DR and reducing neovascularization. The observed reduction in retinal tortuosity and vascular dilation post-treatment with nanoparticles demonstrated the efficacy of FA-b-PEG-modified nanoparticles in targeted delivery [126]. These strategies demonstrate the potential of receptor-targeted nanoparticles in enhancing the delivery of drugs to ocular tissues, offering new avenues for the management of retinal disorders.
Liyang Zhou et al. [127] constructed a novel photosynthetic hybrid system (Cyano@Au@Ir) by utilizing cyanobacteria as a nanozyme carrier loaded with gold and iridium nanoparticles, thereby optimizing the surface of nano-drug formulations. In contrast to traditional nanoparticles modified with polysaccharides or small molecules, cyanobacteria leverage the light-transmitting properties of the eye to continuously produce oxygen in the retinal region under illumination. This process creates a favorable metabolic environment and enhances targeting specificity. The micrometer-sized Cyano@Au@Ir, when administered via subretinal injection, is distributed precisely between the RPE and photoreceptor cells. This delivery method circumvents rapid metabolism in the eye, enabling the system to exert its effects over an extended period in the retinal area. Li-Jyuan Luo et al. [128] functionalized chitosan and ZM241385 (a non-xanthine adenosine receptor antagonist) on the surface of hollow cerium nanoparticles (hCe NPs), enabling them to open tight junctions in the corneal epithelium and target the ciliary body. Additionally, they utilized the antioxidant and anti-inflammatory properties of hCe NPs to achieve multi-target treatment for glaucoma.
3.1.5 Sustained drug release mechanisms
The primary treatment for posterior segment diseases caused by DM is intravitreal injection, but frequent injections elevate the risk and reduce patient compliance. To maintain therapeutic concentration while minimizing the number of invasive administrations, a drug delivery system capable of sustained drug release in the posterior eye is essential. Nano-drug delivery systems for curing posterior eye disorders have been extensively studied, as nanomedicines can remain in the vitreous for extended periods, ensuring the required drug concentration for treatment.
Research has shown that cerium (Ce) effectively eliminates free radicals and peroxides, reducing lipid peroxidation. Mesoporous silica nanoparticles containing cerium (CeCl3@mSiO2) have demonstrated efficacy in reducing oxidative stress in lens epithelial cells [129]. In further studies, these nanoparticles exhibited sustained and controlled drug release for at least 60 hours. When injected intraperitoneally into a DC rat model, results indicated that CeCl3@mSiO2 nanoparticles improved disease progression by reducing oxidative damage [130].
Maintaining effective doses of protein drugs for posterior ocular diseases is more challenging due to their structural instability, susceptibility to protease degradation, and short half-lives. Nanocarriers not only delay drug release but also protect protein structure and maintain activity. Anti-VEGF drugs, which are high-molecular-weight proteins, prevent new blood vessel formation by blocking VEGF from binding to its receptor [131]. Bevacizumab is approved for treating ocular diseases caused by neovascularization [132], but its short half-life and the requirement for continuous injections result in side effects and financial burdens [133].
Encapsulating bevacizumab in mesoporous silica nanoparticles extends its release up to 28 days in vitro, and results in a longer mean retention time (MRT) in vivo, compared to the free drug. This approach prolongs the drug's half-life while maintaining its biological activity, as confirmed by its anti-angiogenic efficacy [134]. Rong X et al. developed a dual-controlled sustained-release system by incorporating insulin-loaded chitosan nanoparticles into a thermo-responsive hydrogel. Insulin release from individual nanoparticles lasted only 1 day, but the sustained release from nanoparticles within the hydrogel extended beyond 60 days [135]. A mucoadhesive and responsive nanogel serves as a carrier for the sustained delivery of timolol in glaucoma therapy. Animal experiments demonstrated that a single administration can reduce and maintain IOP in rabbits at normal levels for over 48 hours [136]. To enhance the therapeutic efficacy of the hydrophobic drug lutein, researchers developed a chitosan-sodium alginate-fatty acid nano-carrier system. This system exhibits sustained-release properties, which significantly improve lutein bioavailability in conditions like DR and DC [137].
3.1.6 Boosting transfection efficiency and prolonging expression duration
Compared to conventional drugs, gene therapy has the potential to address the underlying causes of diseases, thereby eliminating the need for repeated administration [138]. The cornea and retina are considered ideal targets for ocular gene therapy [139]. Ocular gene therapy's transfection efficiency is hindered by poor gene permeability, nuclease degradation, and cytoskeletal barriers. Following the approval of Luxturna, the first FDA-approved recombinant adeno-associated virus gene therapy, research into ocular gene therapy, particularly for diabetic eye diseases, has gained significant momentum [140]. However, the limited encapsulation capacity of viral vectors often raises safety concerns, particularly regarding their inherent immunogenicity. In contrast, non-viral vector nanoparticles provide a safer, more cost-effective, and non-toxic alternative, with simpler production processes. In response to elevated ROS levels in CNV, Anqi Liu et al. [141] developed lipid nanoparticles containing TK bonds. These nanoparticles degrade in response to the intracellular ROS environment, enabling the effective release of siRNA that silences VEGF expression, offering a novel therapeutic strategy for CNV.
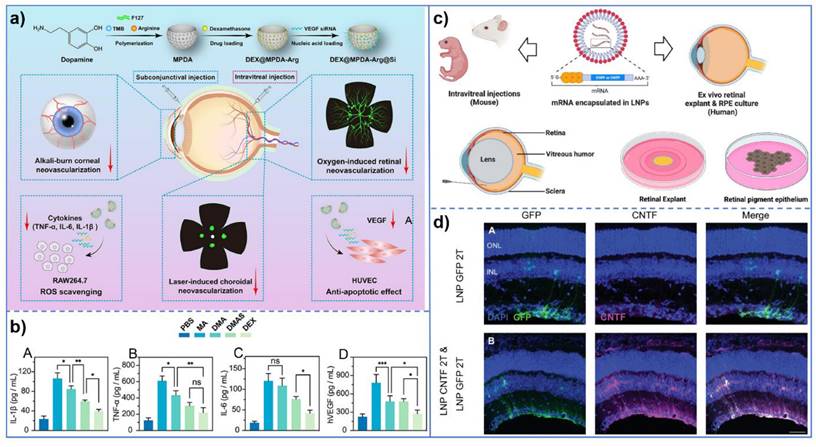
In the realm of retinopathy, retinopathy of prematurity (ROP) presents its distinct pathological features, with pathological retinal neovascularization and inflammatory reaction being the predominant aspects. In light of this pathological characteristic of ROP, Keke Huang et al. [142] developed folate-chitosan-modified nanomaterials for the delivery of miR-223. These nanomaterials can induce the transformation of retinal microglia from a pro-inflammatory phenotype to an anti-inflammatory phenotype, thereby achieving the treatment of ROP via anti-inflammatory and anti-angiogenesis pathways. It is noteworthy that the findings of this study are not confined to the treatment of ROP; rather, its broader significance lies in opening up a new avenue for the treatment of ocular inflammation-neovascularization-related diseases and providing a novel and potential treatment strategy. Furthermore, Xiaochen Ma et al. [143] developed a nanomedicine system by integrating VEGF-siRNA, dexamethasone, and bioactive mesoporous polydopamine (MPDA) to target multiple pathological mechanisms of ocular neovascular diseases through synergistic drug actions. This nanomedicine system not only inhibits angiogenesis but also exerts anti-inflammatory effects via dexamethasone and leverages the antioxidant properties of MPDA. In the context of gene delivery, Cheri Z. Chambers et al. [144] employed lipid nanoparticles (LNPs) as non-viral vectors for mRNA delivery. Compared to traditional adeno-associated virus (AAV) vectors, LNPs exhibit lower immunogenicity and can deliver larger transgenes, offering a novel approach for ocular gene therapy. These studies not only enhance the transfection efficiency of gene therapy but also prolong gene expression duration, thereby advancing novel strategies and methodologies for ocular gene therapy (Figure 7).
In general, nanoparticles offer several key advantages in gene delivery, including (1) protection of gene structure from enzymatic degradation; (2) enhancing gene delivery and increasing cellular uptake compared to single gene therapies; (3) minimizing interactions with cell surface receptors and reducing off-target effects of siRNA; and (4) sustaining intracellular gene delivery. Nanocarriers enhance drug solubility, penetration efficiency, and cellular internalization while sustaining gene delivery, offering more effective treatments for diabetic ocular complications. The nanoparticles developed in one study exhibit the synergistic advantages described above, leading to improved therapeutic outcomes. Table 3 presents an overview of the nanoparticles referenced in the text.
3.2 Broader impacts of nanotechnology on eye disease management
Nanotechnology not only addresses challenges in drug delivery for diabetic ocular complications but also enables early monitoring and non-invasive detection. Persistent hyperglycemia in eye tissue leads to severe complications, making early glucose monitoring essential to prevent these outcomes. Portable glucose monitoring devices require finger-pricking, causing discomfort and low compliance [157]. As an alternative, Google and Novartis developed glucose-sensing contact lenses for DM diagnosis, though these lenses require power, limiting their convenience [158]. Park S et al. [159] developed colorimetric contact lenses that detect glucose based on the color shift between cerium's oxidation states (Ce3+ to Ce4+). Cerium oxide nanoparticles (CNP) were conjugated with glucose oxidase (GOx) using PEG, forming CNP-PEG-GOx nanocomposites, which were integrated into a (hydroxyethyl) methacrylate solution to create the lenses. The hydrogen peroxide generated from glucose oxidation by GOx converts colorless Ce3+ to yellow Ce4+, and a smartphone-based algorithm is used to analyze the color intensity for glucose quantification.
(a) Schematic illustration of DEX@MPDA-Arg@Si (DMAS) for ocular neovascular disease (OND) therapy. The diagram outlines the preparation process of MPDA-based nanomedicine, including polymerization, drug loading, and functionalization. Additionally, it demonstrates the therapeutic efficacy of DMAS in multiple OND models, such as alkali burn-induced corneal neovascularization (CoNV), oxygen-induced retinopathy (OIR), and laser-induced CNV; (b) Expression levels of pro-inflammatory cytokines in RAW264.7 cell supernatant. Quantification of IL-1β (A), TNF-α (B), and IL-6 (C) levels in the supernatant of RAW264.7 cells. Data are presented as mean±SD (n=3); (c) Lipid nanoparticle delivery of mRNA into the mouse or human retina; (d) Transfection of CNTF mRNA into mouse Müller glial cells using 2T LNP. CNTF mRNA encapsulated in 2T lipid nanoparticles was successfully transfected into mouse Müller glial cells, as evidenced by the specific expression of CNTF in these cells, confirming efficient delivery into retinal tissue. (a-b) Adapted with permission from [143], copyright 2024, DOVE Medical Press. (c-d) Adapted with permission from [144], copyright 2024, Association for Research in Vision and Ophthalmology.
The above-mentioned nanoparticles for drug and gene delivery.
| Therapeutic Agent | Composition Of Recipe Type | Disease Type | Experimental Model | Size | Mechanism Or Principle | Ref. |
|---|---|---|---|---|---|---|
| Glycyrrhizin; Genistein | Micelles | Diabetic Corneal Wound Healing | C57BL/ 6J Mice | 29.50±2.05 Nm | Small Size Enhances Corneal Penetration | [145] |
| Triamcinolone Acetonide | Micelles | Inflammation | New Zealand Albino Rabbits | 146.90±4.29 Nm | Mucus Adhesion | [94] |
| Nanoparticle | DR | Sprague-Dawley Rats | 184±2 Nm | Mucosal Adhesive | [146] | |
| Pt Nanocluster | Nanoparticle | DC | Sprague-Dawley Rats | 160 Nm | Mucosal Adhesion; Penetration Enhancement | [147] |
| Pioglitazone | Nanoparticle | DR | Wistar Rats | 171.7 Nm (PLGA 50:50) | Penetration Of Nanoparticles | [147] |
| Apatinib | Nanoparticle | DR | Wistar Rats | 222.2±3.56 Nm | Enhance Target Recognition; Mucus Adhesion | [123] |
| Cyclosporin-A | Nanogel | Dry Eye | New Zealand Albino Rabbits | 15.8±0.26 Nm | Mucosal Adhesive | [148] |
| Curcumin | Nanogel | Multiple Eye Diseases | New Zealand White Rabbits | 221.2 Nm | Increased Solubility; Extended Residence Time | [149] |
| Cecl3 | Nanoparticle | DC | Wistar Rats | 87.6±8.9nm | Sustained Release; Enable Controlled Release of Drugs | [150] |
| Gold Nanoparticles; Sorafenib Tosylate | Nanoparticle | DR | Chinchilla Rabbits | 50.93 Nm | Sustained Release | [126] |
| Sirna | Lipoplexes | DR | Sprague⿿Dawley Rats | \ | Enhancement Of Gene Transfection Ability | [151] |
| Sirna | Nanoparticle | Choroidal Neovascularization | Pigmented Rats | 186±4.3 Nm | Reduce The Enzymatic Degradation | [152] |
| Insulin | Nanogel | DR | Sprague-Dawley Rats | 137.5 Nm | Controlled Release | [135] |
| ALA | Micelle | DM-Related Corneal Diseases | Bovine Cornea | 84.7 Nm | Increase Solubility | [153] |
| Corticosteroid Drugs | Micelle | Retinal Degenerative Diseases | Human RPE Cells | <500 Nm | Mucosal Adhesion Properties; Permeability Enhancement | [154] |
| Aons | Nanoparticle | Diabetic Corneal Wound Healing | Organ Cultured Human Diabetic Cornea; Lecs | \ | Enhance Gene Transfection | [155] |
| Curcumin | Nanogel | Multiple Eye Disease | Hcecs | 15.51±0.15 Nm | Increased Penetration | [156] |
The method detected glucose in tears from rabbit and human eyes, providing a reliable, simple, non-invasive way to monitor glucose levels. Schauval et al. encapsulated anti-VEGF drugs with VEGF aptamer-functionalized carbon dots, using the inherent fluorescence of carbon dots to achieve non-invasive detection of intraocular drug concentrations [160]. In addition, Dong Yun Lee et al. [161] developed a clinically viable suction cup (SD) strip biosensor, which incorporates a sensing paper coated with CNP and GOx. The sensor collects tear fluid through non-invasive contact with the lower eyelid conjunctiva (IPC), thereby avoiding eye irritation. It is designed for single-use and short-term application, enabling convenient self-monitoring of blood glucose levels.
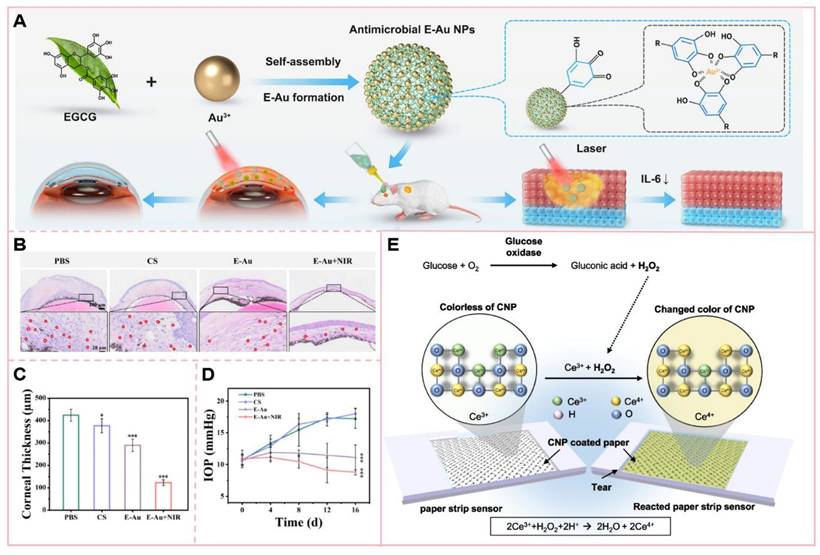
The application of nanotechnology in the management of eye diseases has demonstrated significant potential in enhancing drug delivery and therapeutic efficacy. For instance, epigallocatechin gallate-conjugated gold nanoparticles (E-Au NPs) can convert light energy into thermal energy under near-infrared (NIR) irradiation, thereby achieving mild photothermal therapeutic effects [162]. This photothermal transformation property allows E-Au NPs to elevate the temperature at the infection site, disrupt bacterial cell structures, interfere with their physiological processes, and ultimately enhance bacterial lethality. Notably, E-Au NPs exhibit a pronounced inhibitory effect on drug-resistant bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). In contrast to traditional high-temperature photothermal therapy, this mild photothermal approach minimizes damage to surrounding normal tissues and is particularly suitable for temperature-sensitive regions such as the eyes. In the treatment of ocular infections, such as keratitis, the combination of E-Au NPs and near-infrared irradiation not only effectively controls infection but also reduces inflammatory responses and promotes corneal tissue repair. This highlights the significant potential of integrating nanotechnology with photothermal therapy in ocular therapeutics.
Nanotechnology has also advanced the field of molecular imaging, particularly for tracking Vascular Cell Adhesion Molecule-1 (VCAM-1), a key inflammatory marker in DR progression. Although several imaging methods have been developed to visualize VCAM-1, many still suffer from limitations such as insufficient sensitivity and invasiveness [163, 164]. Uddin M D I et al. [165] developed gold nanoparticles functionalized with VCAM-1-targeted antisense hairpin DNA. These nanoparticles can hybridize with VCAM-1 mRNA in cells and induce fluorescence. The nanoprobe allows for the specific imaging of VCAM-1 mRNA in TNF-α-activated mouse retinal microvascular endothelial cells (MRMECs), without the need for transfection reagents or cell permeabilization. This innovation represents a significant advancement in non-invasive monitoring techniques for DR.
(A) Epigallocatechin gallate (EGCG)-functionalized gold nanoparticles (E-Au NPs) were synthesized via a facile one-step self-assembly method, demonstrating potent antibacterial and antibiofilm properties against [specific pathogens, e.g., Gram-positive and Gram-negative bacteria; (B) H&E staining of the cornea and (C) quantitative analysis of corneal thickness; (D) Intraocular pressure measurement; (E) Schematic illustration of the reaction mechanism on the sensing paper immobilized with CNP and GOx for glucose detection. The colorimetric detection is based on the oxidation of Ce³⁺ (colorless) to Ce⁴⁺ (yellow) induced by glucose concentration in tears, resulting in visible color changes on the indicator paper. (A-D) Adapted with permission from [162], copyright 2024, BioMed Central. (E) Adapted with permission from [161], copyright 2023, American Association for the Advancement of Science.
Early detection, early intervention, and early treatment have always been the key criteria for the treatment of ocular complications. Therefore, its early diagnosis, especially that of DR, is of great significance in clinical practice. Long-term hyperglycemia in diabetic patients gradually causes retinal microvascular damage, and the early lesions may not present obvious symptoms. If DR can be diagnosed early, timely intervention measures, such as controlling blood glucose, blood pressure, and blood lipids and other risk factors, can be taken to delay the development of lesions. Moreover, early diagnosis provides the best opportunity for more targeted treatment, such as laser therapy and anti-VEGF therapy. These treatments can achieve better therapeutic effects and reduce the risk of serious consequences, such as vision loss. Therefore, to achieve efficient DR detection, many researchers have explored from different perspectives. Among them, Vadanasundari et al. developed vanadium core-shell nanorods loaded with vanadium oxide on carbon dioxide. These nanorods can detect metabolic changes related to DR and effectively evaluate disease progression, and the related research has been advanced to the clinical trial stage [165]. In addition, Hainsworth D P et al. [166] developed a colorimetric nanotechnology-based paper sensor for measuring 8-Hydroxy-2'-Deoxyguanosine, a biomarker of DR, in urine, which facilitates convenient and accurate home-based testing.
In recent years, the rapid development of nanoprobe technology has enabled a further improvement in the accuracy and sensitivity of DR detection. Nanoprobes possess unique physical and chemical properties, such as extremely small size and customizable surface functionalization. These properties enable the nanoprobes to specifically identify biomarkers or cellular changes related to DR. For example, Yuanlin Zhang et al. [167] used vascular endothelial growth factor receptor 2 (VEGFR-2) as a biomarker for early DR diagnosis and developed a high-brightness adhesive fluorescent nanoprobe with biodegradable materials. This nanoprobe not only overcomes the obstacle of insufficient brightness but also promotes cellular immune response research in DM. Similarly, Linjie Wang et al. [168] designed a multifunctional nanoreactor based on Ru nanoparticles, which integrates biological and nanoenzymes and serves as a nanoprobe, thereby developing a new colorimetric/smartphone integrated sensing platform. Unlike the previous nanoprobe studies for DR, this nanoreactor primarily focuses on the critical link of blood glucose detection. By integrating the characteristics of biological enzymes and nanoenzymes, the nanoprobe can efficiently achieve rapid and accurate detection of blood glucose under near-neutral pH conditions. With the assistance of the smartphone integrated sensing platform, this detection method offers the advantages of convenience, low cost, and ease of promotion, providing a new technical means for the daily monitoring of DM. Simultaneously, this also further showcases the versatility and immense potential of nanotechnology in DM-related research. Whether for the diagnosis of diabetic complications or the direct detection of blood glucose, nanotechnology offers diversified solutions to enhance the health management of patients with diabetic eye complications.
4. Conclusion and Future Perspectives
The rising incidence of DM has increasingly focused attention on the management and prevention of its associated complications. Among these complications, ocular issues arising from chronic hyperglycemia are the leading causes of vision loss and blindness in individuals with DM. Current treatments for DR and other ocular conditions often require frequent dosing or invasive procedures such as intravitreal injections. This regimen not only burdens patients but also leads to suboptimal ocular bioavailability due to the eye's protective barriers. Consequently, there is a pressing need for advanced drug delivery systems capable of enhancing the efficacy and safety of ophthalmic therapies for DM.
Nanotechnology has emerged as a promising solution to these challenges. Nanocarriers offer significant advantages in ocular drug delivery, including improved solubility of poorly water-soluble drugs, extended ocular epidermis residence time, and improved drug permeability across eye tissues. These properties make them especially suitable for targeting the posterior segment of the eye, where many diabetic complications originate. Additionally, the ability of nanocarriers to encapsulate macromolecules, such as antibodies or genetic material, presents new opportunities for gene therapy in treating DM-related ocular diseases.
The purpose of gene therapy is to correct genetic defects or modify abnormal physiological processes, thereby providing longer-lasting benefits than conventional drug treatments. In the context of diabetic eye disorders, these therapies hold substantial promise due to their ability to target specific sites and provide sustained therapeutic effects. Nanocarriers can deliver therapeutic genes directly to diseased ocular tissues, minimizing systemic exposure and reducing side effects. This precise delivery method is expected to significantly benefit patients by reducing treatment frequency and improving adherence to therapy.
Despite the promising potential of nanotechnology in ocular treatments, several specific challenges remain. A major concern is the safety profile of nanoparticles, particularly their potential toxicity and immune responses in ocular tissues. Improved methods for tracking and monitoring nanoparticle distribution are also needed to ensure targeted delivery without damaging healthy tissues. In addition, the scalability and cost-effectiveness of nanoparticle-based formulations must be improved to facilitate widespread adoption. Current regulatory frameworks may not fully accommodate the unique properties of nanomedicines, presenting challenges for product approval and commercialization. Future research should focus on developing safe and efficient nanoparticles, establishing standardized preclinical assessment protocols, and collaborating with regulatory authorities to develop appropriate regulations for nanomedicine.
In conclusion, the future of nanotechnology in ophthalmology is promising. Advances in materials science have enabled the development of biocompatible and degradable nanoparticles that reduce toxicity and enhance drug delivery efficiency. These smart delivery systems can be engineered to respond to environmental triggers, such as pH or temperature, releasing their therapeutic payload precisely where needed. Additionally, nanotechnology offers the potential for combination therapies, where multiple drugs are loaded onto a single nanocarrier, enhancing treatment outcomes for complex conditions like age-related macular degeneration or glaucoma. As ongoing research addresses current limitations and regulatory agencies establish guidelines for the safe use of nanomedicines, nanotechnology is poised to play a transformative role in ophthalmology, offering patients more effective treatments with fewer side effects.
Acknowledgements
This work was grateful to the financial support from the National Natural Science Foundation of China (82304425), the project of Liaoning Province Department of Education (JYTQN2023321, LJ212410163025), the project 202410163017 supported by the National Training Program of Innovation and Entrepreneurship for Undergraduates and the Doctoral Start-up Foundation of Liaoning Province (2024-BS-071).
Author contributions
Siqi Wang: writing—original draft, visualization, writing—review and editing. Hongyu Yang: investigation, writing—review and editing. Jiaying Zheng: investigation, visualization. Aiyang Tong: investigation. Sen Mu: visualization. Dongkai Wang: conceptualization. Ming Zhao: writing—review and editing, conceptualization, project administration. Ji Li: conceptualization, project administration, writing—review and editing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cheloni R, Gandolfi SA, Signorelli C, Odone A. Global prevalence of diabetic retinopathy: protocol for a systematic review and meta-analysis. BMJ Open. 2019;9:e022188
2. Nedosugova LV, Markina YV, Bochkareva LA, Kuzina IA, Petunina NA, Yudina IY. et al. Inflammatory Mechanisms of Diabetes and Its Vascular Complications. Biomedicines. 2022;10:1168
3. González P, Lozano P, Ros G, Solano F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int J Mol Sci. 2023;24:9352
4. Wang H, Gong J, Chen W, Sun Q, Zhang T, Lin Y. et al. Tetrahedral framework nucleic acids' role in facilitating chronic diabetic wound repair via the endoplasmic reticulum-mitochondrial pathway. Nano Today. 2024;56:102252
5. Kattar A, Concheiro A, Alvarez-Lorenzo C. Diabetic eye: associated diseases, drugs in clinic, and role of self-assembled carriers in topical treatment. Expert Opin Drug Deliv. 2021;18:1589-1607
6. He W, Li H, Xu X, Zhang X, Chen J, Lv C. et al. An injectable and retrievable bioartificial pancreas fabricated by time-sequentially assembling islets-laden microgels for minimally invasive treatment of type 1 diabetes. Chem Eng J. 2024;492:152261
7. Han Y, Xu C, Shi H, Yu F, Zhong Y, Liu Z. et al. Engineered bio-adhesive polyhedral oligomeric silsesquioxane hybrid nanoformulation of amphotericin B for prolonged therapy of fungal keratitis. Chem Eng J. 2021;421:129734
8. Ma Z, Wang Y, He H, Liu T, Jiang Q, Hou X. Advancing ophthalmic delivery of flurbiprofen via synergistic chiral resolution and ion-pairing strategies. Asian J Pharm Sci. 2024;19:100928
9. Ramsay E, Hagström M, Vellonen K-S, Boman S, Toropainen E, Del Amo EM. et al. Role of retinal pigment epithelium permeability in drug transfer between posterior eye segment and systemic blood circulation. Eur J Pharm Biopharm. 2019;143:18-23
10. Peng T, Shao X, Long L, Liu H, Song W, Hou J. et al. Rational design of oral delivery nanosystems for hypoglycemic peptides. Nano Today. 2023;53:102031
11. Huang Y, Fan H, Ti H. Tumor microenvironment reprogramming by nanomedicine to enhance the effect of tumor immunotherapy. Asian J Pharm Sci. 2024;19:100902
12. Lohia A, Sahel DK, Salman M, Singh V, Mariappan I, Mittal A. et al. Delivery strategies for CRISPR/Cas genome editing tool for retinal dystrophies: challenges and opportunities. Asian J Pharm Sci. 2022;17:153-176
13. Han H, Li S, Xu M, Zhong Y, Fan W, Xu J. et al. Polymer- and lipid-based nanocarriers for ocular drug delivery: Current status and future perspectives. Adv Drug Deliv Rev. 2023;196:114770
14. Suanno G, Genna VG, Maurizi E, Dieh AA, Griffith M, Ferrari G. Cell therapy in the cornea: The emerging role of microenvironment. Prog Retin Eye Res. 2024;102:101275
15. Gezer A, Özkaraca M, Üstündağ H, Soydan M, Alkanoğlu Ö, Bedir G. Docosahexaenoic acid eliminates endoplasmic reticulum stress and inflammatory pathways in diabetic rat keratopathy. Int Immunopharmacol. 2024;140:112871
16. Cui W, Wang Y, Luo C, Xu J, Wang K, Han H. et al. Nanoceria for ocular diseases: recent advances and future prospects. Mater Today Nano. 2022;18:100218
17. Ge J, Li X, Xia Y, Chen Z, Xie C, Zhao Y. et al. Recent advances in NLRP3 inflammasome in corneal diseases: Preclinical insights and therapeutic implications. Ocul Surf. 2024;34:392-405
18. Qi Q, Wei Y, Zhang X, Guan J, Mao S. Challenges and strategies for ocular posterior diseases therapy via non-invasive advanced drug delivery. J Control Release. 2023;361:191-211
19. Ghezzi M, Ferraboschi I, Delledonne A, Pescina S, Padula C, Santi P. et al. Cyclosporine-loaded micelles for ocular delivery: Investigating the penetration mechanisms. J Control Release. 2022;349:744-755
20. Ladea L, Zemba M, Calancea MI, Călțaru MV, Dragosloveanu CD, Coroleucă R. et al. Corneal Epithelial Changes in Diabetic Patients: A Review. Int J Mol Sci. 2024;25:3471
21. Tang J, Lin Z, Liu X, Li B, Wu X, Lv J. et al. Analyzing the changing trend of corneal biomechanical properties under different influencing factors in T2DM patients. Sci Rep. 2024;14:8160
22. Wang T, Yu T, Liu Q, Sung T-C, Higuchi A. Lipid nanoparticle technology-mediated therapeutic gene manipulation in the eyes. Mol Ther Nucleic Acids. 2024;35:102236
23. Luo K, Huang W, Qiao L, Zhang X, Yan D, Ning Z. et al. Dendrocalamus latiflorus and its component rutin exhibit glucose-lowering activities by inhibiting hepatic glucose production via AKT activation. Acta Pharm Sin B. 2022;12:2239-2251
24. Ponirakis G, Abdul-Ghani MA, Jayyousi A, Almuhannadi H, Petropoulos IN, Khan A. et al. Effect of treatment with exenatide and pioglitazone or basal-bolus insulin on diabetic neuropathy: a substudy of the Qatar Study. BMJ Open Diabetes Res Care. 2020;8:e001420
25. Wang X, Luan F, Yue H, Song C, Wang S, Feng J. et al. Recent advances of smart materials for ocular drug delivery. Adv Drug Deliv Rev. 2023;200:115006
26. Wróblewska KB, Jadach B, Muszalska-Kolos I. Progress in drug formulation design and delivery of medicinal substances used in ophthalmology. Int J Pharm. 2021;607:121012
27. Khan N, Paterson AD, Roshandel D, Raza A, Ajmal M, Waheed NK. et al. Association of IGF1 and VEGFA polymorphisms with diabetic retinopathy in Pakistani population. Acta Diabetol. 2019;57:237-245
28. Matlock HG, Qiu F, Malechka V, Zhou K, Cheng R, Benyajati S. et al. Pathogenic role of PPARα downregulation in corneal nerve degeneration and impaired corneal sensitivity in diabetes. Diabetes. 2020;69:1279-1291
29. Abd El-Alim SH, Salama A, Darwish AB. Provesicular elastic carriers of Simvastatin for enhanced wound healing activity: An in-vitro/in-vivo study. Int J Pharm. 2020;585:119470
30. Monteiro RT, Da Silva TF, Filho RFM, Vasconcelos NF, Nogueira KB, Petrilli R. et al. Simvastatin-loaded alginate bilayer membranes for wound dressing applications. J Drug Deliv Sci Technol. 2023;86:104701
31. Benéitez García MC, Girón Moreno R, Colmena Crespo I, Díez-Orejas RM, Martín Fontelles MI, Goicoechea García C. et al. Promising biomedical subcutaneous delivery system in opioid disaccustom process: In vitro/in vivo evaluation of naloxone microparticles on antagonist effect of morphine. Int J Pharm. 2023;635:122766
32. Grilo CM, Lydecker JA, Jastreboff AM, Pittman B, McKee SA. Naltrexone/bupropion for binge-eating disorder: A randomized, double-blind, placebo-controlled trial. Obesity. 2023;31:2762-2773
33. Huang X-F, Li Z, De Guzman E, Robinson P, Gensler L, Ward MM. et al. Genomewide association study of acute anterior uveitis identifies new susceptibility loci. Invest Ophthalmol Vis Sci. 2020;61:146-152
34. Portela ALBM, Moreno RN, Ribeiro MHML, de Andrade FM, Alves YV, Alves M. et al. Role of nicergoline in corneal wound healing in diabetic rats. BMC Ophthalmol. 2021;21:1-6
35. Feng J, Zheng Y, Guo M, Ares I, Martínez M, Lopez-Torres B. et al. Oxidative stress, the blood-brain barrier and neurodegenerative diseases: The critical beneficial role of dietary antioxidants. Acta Pharm Sin B. 2023;13:3988-4024
36. Gao M-j, Cui N-h, Liu Xn, Wang X-b. Inhibition of mitochondrial complex I leading to NAD+/NADH imbalance in type 2 diabetic patients who developed late stent thrombosis: Evidence from an integrative analysis of platelet bioenergetics and metabolomics. Redox Biol. 2022;57:102507
37. Toriumi K, Iino K, Ozawa A, Miyashita M, Yamasaki S, Suzuki K. et al. Glucuronic acid is a novel source of pentosidine, associated with schizophrenia. Redox Biol. 2023;67:102876
38. Goldstein AS, Janson BJ, Skeie JM, Ling JJ, Greiner MA. The effects of diabetes mellitus on the corneal endothelium: A review. Surv Ophthalmol. 2020;65:438-450
39. López-Cano JJ, González-Cela-Casamayor MA, Andrés-Guerrero V, Vicario-de-la-Torre M, Del Castillo JMB, Herrero-Vanrell R. et al. Development of an osmoprotective microemulsion as a therapeutic platform for ocular surface protection. Int J Pharm. 2022;623:121-152
40. Barnes PJ. Oxidative stress-based therapeutics in COPD. Redox Biol. 2020;33:101544
41. Han R, Xiao Y, Bai Q, Choi CHJ. Self-therapeutic metal-based nanoparticles for treating inflammatory diseases. Acta Pharm Sin B. 2023;13:1847-1865
42. Shen L, Fang G, Tang B, Zhu Q. Enhanced topical corticosteroids delivery to the eye: A trade-off in strategy choice. J Control Release. 2021;339:91-113
43. Ma Y, Zhang Z, Yu Y, Wang X, Liu S, Sun B. et al. Nanomaterials in the diagnosis and treatment of ophthalmic diseases. Nano Today. 2024;54:102117
44. Magierowska K, Wójcik-Grzybek D, Korbut E, Bakalarz D, Ginter G, Danielak A. et al. The mitochondria-targeted sulfide delivery molecule attenuates drugs-induced gastropathy. Involvement of heme oxygenase pathway. Redox Biol. 2023;66:102847
45. Liu Y, Wang Y, Yang J, Zhang H, Gan L. Cationized hyaluronic acid coated spanlastics for cyclosporine A ocular delivery: Prolonged ocular retention, enhanced corneal permeation and improved tear production. Int J Pharm. 2019;565:133-142
46. Kailasam V, Cheruvu SS, Malani M, Kameswari SMS, Kesharwani P, Nirmal J. Recent advances in novel formulation approaches for tacrolimus delivery in treatment of various ocular diseases. J Drug Deliv Sci Technol. 2022;78:103-142
47. Wang Q, Cheng Q, Yao G, Wang Z, Zhu L, Zeng Z. et al. A cationic hydrogel with anti-IL-17A-specific nanobodies for rheumatoid arthritis treatment via inhibition of inflammatory activities of neutrophils. Nano Today. 2024;59:102507
48. Yu Z, Li M, Yang L, Liu H, Ding G, Ma S. et al. Enhancing diabetic wound healing: A two-pronged approach with ROS scavenging and ROS-independent antibacterial properties. Nano Today. 2024;57:102358
49. Luna-Marco C, de Marañon AM, Hermo-Argibay A, Rodriguez-Hernandez Y, Hermenejildo J, Fernandez-Reyes M. et al. Effects of GLP-1 receptor agonists on mitochondrial function, inflammatory markers and leukocyte-endothelium interactions in type 2 diabetes. Redox Biol. 2023;66:102849
50. Dakroub A, Dbouk A, Asfour A, Nasser SA, El-Yazbi AF, Sahebkar A. et al. C-peptide in diabetes: A player in a dual hormone disorder? J Cell Physiol. 2024;239:e31212
51. Meng Q, Wang J, Jiang B, Zhang X, Xu J, Cao Y. et al. SiRNA-based delivery nanoplatform attenuates the CRC progression via HIF1α-AS2. Nano Today. 2022;47:101667
52. Li J, Xiang Q, Xia T, Meng H, Liu X. Design of nanoformulation of specialized pro-resolving mediators to facilitate inflammation resolution and disease treatment. Nano Today. 2023;52:101978
53. Coppey L, Davidson E, Shevalye H, Obrosov A, Torres M, Yorek MA. Progressive loss of corneal nerve fibers and sensitivity in rats modeling obesity and type 2 diabetes is reversible with omega-3 fatty acid intervention: supporting cornea analyses as a marker for peripheral neuropathy and treatment. Diabetes Metab Syndr Obes. 2020;13:1367-1384
54. Liu K, Gao X, Hu C, Gui Y, Gui S, Ni Q. et al. Capsaicin ameliorates diabetic retinopathy by inhibiting poldip2-induced oxidative stress. Redox Biol. 2022;56:102460
55. Fernandes Silva L, Hokkanen J, Vangipurapu J, Oravilahti A, Laakso M. Metabolites as Risk Factors for Diabetic Retinopathy in Patients With Type 2 Diabetes: A 12-Year Follow-up Study. J Clin Endocrinol Metab. 2024;109:100-106
56. Crespo-Garcia S, Fournier F, Diaz-Marin R, Klier S, Ragusa D, Masaki L. et al. Therapeutic targeting of cellular senescence in diabetic macular edema: preclinical and phase 1 trial results. Nat Med. 2024;30:443-454
57. Arf S, Sayman Muslubas I, Hocaoglu M, Ersoz MG, Ozdemir H, Karacorlu M. Spectral domain optical coherence tomography classification of diabetic macular edema: a new proposal to clinical practice. Graefes Arch Clin Exp Ophthalmol. 2020;258:1165-1172
58. Zubair M, Umair M, Ali Naqvi R, Hussain D, Owais M, Werghi N. A comprehensive computer-aided system for an early-stage diagnosis and classification of diabetic macular edema. J King Saud Univ Sci. 2023;35:101719
59. Li X, Cao Q, Xu C, Wang J, Pan T, Liu Q. et al. Exosomal lncRNA-MIAT promotes neovascularization via the miR-133a-3p/MMP-X1 axis in diabetic retinopathy. Exp Eye Res. 2024;243:109912
60. Shanbagh S, Gadde SG, Shetty R, Heymans S, Abilash VG, Chaurasia SS. et al. Hyperglycemia-induced miR182-5p drives glycolytic and angiogenic response in Proliferative Diabetic Retinopathy and RPE cells via depleting FoxO1. Exp Eye Res. 2024;238:109713
61. Qin T, Lv Y, Xi X, Wu Z. PLK-3-mediated phosphorylation of BAP1 prevents diabetic retinopathy. Biochem Pharmacol. 2024;226:116374
62. Su L, Gong X, Fan R, Ni T, Yang F, Zhang X. et al. Mechanism of action of platinum nanoparticles implying from antioxidant to metabolic programming in light-induced retinal degeneration model. Redox Biol. 2023;65:102836
63. Sun F, Sun Y, Wang X, Zhu J, Chen S, Yu Y. et al. Engineered mesenchymal stem cell-derived small extracellular vesicles for diabetic retinopathy therapy through HIF-1α/EZH2/PGC-1α pathway. Bioact Mater. 2024;33:444-459
64. Guo Z, Ma X, Zhang RX, Yan H. Oxidative stress, epigenetic regulation and pathological processes of lens epithelial cells underlying diabetic cataract. Adv Ophthalmol Pract Res. 2023;3:180-186
65. Li X, Chen D, Ouyang B, Wang S, Li Y, Li L. et al. KLF5/MDM2 Axis Modulates Oxidative Stress and Epithelial-Mesenchymal Transition in Human Lens Epithelial Cells: The Role in Diabetic Cataract. Lab Invest. 2023;103:100226
66. Guo S, Li C, Lian L, Le Z, Ren Y, Liao Y-X. et al. Fluorescence Imaging of Diabetic Cataract-Associated Lipid Droplets in Living Cells and Patient-Derived Tissues. ACS Sens. 2023;8:3882-3891
67. Kuang G, Halimitabrizi M, Edziah A-A, Salowe R, O'Brien JM. The potential for mitochondrial therapeutics in the treatment of primary open-angle glaucoma: a review. Front Physiol. 2023;14:1184060
68. Su Y, Ge QS, Li ZY, Bu QW, Hu D, Zhou LF. et al. Assessment of iris volume in glaucoma patients with type 2 diabetes mellitus by AS-OCT. Int J Ophthalmol. 2023;16:743-747
69. Liu WW, Kinzy TG, Cooke Bailey JN, Xu Z, Hysi P, Wiggs JL. et al. Mechanosensitive ion channel gene survey suggests potential roles in primary open angle glaucoma. Sci Rep. 2023;13:15871
70. Niu G, Wang H, Zhai Y, Zhou B, Kang Y, Pei Z. et al. Nanotechnology-based in situ cancer vaccines: Mechanisms, design, and recent advances. Nano Today. 2024;56:102286
71. Pang Q, Xu Z, Sun T, Yue S, Yu Y, Lu H. et al. Strategic chemical synthesis and application of nanocarriers responsive to the tumor microenvironment. Nano Today. 2024;58:102421
72. De Matteis V, Cascione M, Pellegrino P, Di Corato R, Catalano M, Miraglia A. et al. Multishaped bio-gold polyphenols bearing nanoparticles to promote inflammatory suppression. Nano Today. 2024;57:102329
73. Veneti S, Grammatikopoulou MG, Kintiraki E, Mintziori G, Goulis DG. Ketone Bodies in Diabetes Mellitus: Friend or Foe? Nutrients. 2023;15:4383
74. Lu Y, Shi Y, Luo Z, Guo X, Jiang M, Li X. et al. Reactivation of dysfunctional dendritic cells by a stress-relieving nanosystem resets anti-tumor immune landscape. Nano Today. 2022;43:101416
75. Zhang C, Qin W-J, Bai X-F, Zhang X-Z. Nanomaterials to relieve tumor hypoxia for enhanced photodynamic therapy. Nano Today. 2020;35:100960
76. Koca DS, Dietrich-Ntoukas T. Frequency of Topical Immunomodulatory and Immunosuppressive Therapies for Ocular Chronic Graft-versus-Host Disease. J Clin Med. 2024;13:4728
77. Mandal A, Gote V, Pal D, Ogundele A, Mitra AK. Ocular pharmacokinetics of a topical ophthalmic nanomicellar solution of cyclosporine (Cequa®) for dry eye disease. Pharm Res. 2019;36:1-21
78. Karpecki P, Barghout V, Schenkel B, Huynh L, Khanal A, Mitchell B. et al. PCR136 A Retrospective Analysis of Real-World Treatment Patterns in Patients over Age 64 with Dry Eye Disease Receiving OTX-101 Ophthalmic Solution 0.09%, Cyclosporine Ophthalmic Emulsion 0.05%, or Lifitegrast Ophthalmic Solution 5%. Value Health. 2023;26:S337
79. Coco G, Buffon G, Taloni A, Giannaccare G. Recent Advances in Nanotechnology for the Treatment of Dry Eye Disease. Nanomaterials (Basel). 2024;14:669
80. Mahalingam SM, Arumugam N, Shing Wong L, Cordelia Tanislaus Antony Dhanapal A, Djearamane S. Mechanistic investigation of photodynamic therapy using Visudyne in human KB carcinoma cells. J King Saud Univ Sci. 2023;35:102871
81. Siriwardane DA, Jiang W, Mudalige T. Profiling in-vitro release of verteporfin from VISUDYNE® liposomal formulation and investigating verteporfin binding to human serum albumin. Int J Pharm. 2023;646:123449
82. Santarpia G, Carnes E. Therapeutic Applications of Aptamers. Int J Mol Sci. 2024;25:6742
83. Mignani S, Shi X, Ceña V, Majoral J-P. Dendrimer-and polymeric nanoparticle-aptamer bioconjugates as nonviral delivery systems: A new approach in medicine. Drug Discov Today. 2020;25:1065-1073
84. Kang-Mieler JJ, Rudeen KM, Liu W, Mieler WF. Advances in ocular drug delivery systems. Eye (Lond). 2020;34:1371-1379
85. Cao Y, Samy KE, Bernards DA, Desai TA. Recent advances in intraocular sustained-release drug delivery devices. Drug Discov Today. 2019;24:1694-1700
86. Aceves-Franco LA, Sanchez-Aguilar OE, Barragan-Arias AR, Ponce-Gallegos MA, Navarro-Partida J, Santos A. The Evolution of Triamcinolone Acetonide Therapeutic Use in Retinal Diseases: From Off-Label Intravitreal Injection to Advanced Nano-Drug Delivery Systems. Biomedicines. 2023;11:1901
87. Gaballa SA, Kompella UB, Elgarhy O, Alqahtani AM, Pierscionek B, Alany RG. et al. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv Transl Res. 2021;11:866-893
88. Kulikov AN, Vasiliev AS, Kalinicheva YA, Maltsev DS. Topical bromfenac in VEGF-driven maculopathies: topical review and meta-analysis. BMC Ophthalmol. 2024;24:369
89. Wei D, Yang H, Zhang Y, Zhang X, Wang J, Wu X. et al. Nano-traditional Chinese medicine: a promising strategy and its recent advances. J Mater Chem B. 2022;10:2973-2994
90. Gonçalves MdS, Cabral LM, de Sousa VP, do Carmo FA. Development of ocular delivery systems for macitentan and ex vivo study of intraocular permeation. J Drug Deliv Sci Technol. 2024;100:106023
91. Dos Santos GA, Ferreira-Nunes R, Dalmolin LF, dos Santos Ré AC, Anjos JLV, Mendanha SA. et al. Besifloxacin liposomes with positively charged additives for an improved topical ocular delivery. Sci Rep. 2020;10:192-210
92. Fang G, Yang X, Wang Q, Zhang A, Tang B. Hydrogels-based ophthalmic drug delivery systems for treatment of ocular diseases. Mater Sci Eng C Mater Biol Appl. 2021;127:112-151
93. Stielow M, Witczyńska A, Kubryń N, Fijałkowski Ł, Nowaczyk J, Nowaczyk A. The Bioavailability of Drugs—The Current State of Knowledge. Molecules. 2023;28:8038
94. Safwat MA, Mansour HF, Hussein AK, Abdelwahab S, Soliman GM. Polymeric micelles for the ocular delivery of triamcinolone acetonide: preparation and in vivo evaluation in a rabbit ocular inflammatory model. Drug Deliv. 2020;27:1115-1124
95. Ajith TA. Alpha-lipoic acid: A possible pharmacological agent for treating dry eye disease and retinopathy in diabetes. Clin Exp Pharmacol Physiol. 2020;47:1883-1890
96. Sipos B, Földes F, Budai-Szűcs M, Katona G, Csóka I. Comparative Study of TPGS and Soluplus Polymeric Micelles Embedded in Poloxamer 407 In Situ Gels for Intranasal Administration. Gels. 2024;10:521
97. Ali R, Qamar W, Kalam MA, Binkhathlan Z. Soluplus-TPGS Mixed Micelles as a Delivery System for Brigatinib: Characterization and In Vitro Evaluation. ACS Omega. 2024;9:41830-41840
98. Chen Y-Y, Chen C-K, Wu T-T, Ho C-Y, Yeh T-C, Sun G-C. et al. Attenuation of epithelial-mesenchymal transition via SGLT2 inhibition and diabetic cataract suppression by dapagliflozin nanoparticles treatment. Life Sci. 2023;330:122005
99. Jin M, Zhu S, Hou Y. Insight on Serum Albumin: From Structure and Biological Properties to Functional Biomaterials for Bone Repair. ACS Biomater Sci Eng. 2023;9:2235-2250
100. Niu X, Lin M, Lee BH. An Engineered Protein-Based Building Block (Albumin Methacryloyl) for Fabrication of a 3D In Vitro Cryogel Model. Gels. 2022;8:404
101. Ungor D, Juhász Á, Varga N, Csapó E. Evaluation of noble metal nanostructure-serum albumin interactions in 2D and 3D systems: Thermodynamics and possible mechanisms. Adv Colloid Interface Sci. 2022;301:102616
102. Gui S, Tang W, Huang Z, Wang X, Gui S, Gao X. et al. Ultrasmall Coordination Polymer Nanodots Fe-Quer Nanozymes for Preventing and Delaying the Development and Progression of Diabetic Retinopathy. Adv Funct Mater. 2023;33:2300261
103. Jurišić Dukovski B, Juretić M, Bračko D, Randjelović D, Savić S, Crespo Moral M. et al. Functional ibuprofen-loaded cationic nanoemulsion: Development and optimization for dry eye disease treatment. Int J Pharm. 2020;576:118979
104. Wang Y, Wang C. Novel eye drop delivery systems: advance on formulation design strategies targeting anterior and posterior segments of the eye. Pharmaceutics. 2022;14:1150
105. Valtari A, Posio S, Toropainen E, Balla A, Puranen J, Sadeghi A. et al. Comprehensive ocular and systemic pharmacokinetics of dexamethasone after subconjunctival and intravenous injections in rabbits. Eur J Pharm Biopharm. 2024;198:114260
106. Teixeira C, Sousa AP, Santos I, Rocha AC, Alencastre I, Pereira AC. et al. Enhanced 3T3-L1 Differentiation into Adipocytes by Pioglitazone Pharmacological Activation of Peroxisome Proliferator Activated Receptor-Gamma (PPAR-γ). Biology (Basel). 2022;11:806
107. Haddadi R, Cheraghi-poor M. Peroxisome proliferator activated receptor-gamma (PPAR-γ) ligand, pioglitazone, increases analgesic and anti-inflammatory effects of naproxen. Naunyn Schmiedebergs Arch Pharmacol. 2024;397:1633-1646
108. Laddha UD, Kshirsagar SJ. Formulation of PPAR-gamma agonist as surface modified PLGA nanoparticles for non-invasive treatment of diabetic retinopathy: In vitro and in vivo evidences. Heliyon. 2020;6:2405-2488
109. Wang Y, Hu Y, An J, Zhang H, Liu X, Li X. et al. Liposome-Based Permeable Eyedrops for Effective Posterior Segment Drug Delivery. Adv Funct Mater. 2024;34:2403142
110. Gui S-Y, Wang X-C, Huang Z-H, Li M-M, Wang J-H, Gui S-Y. et al. Nanoscale coordination polymer Fe-DMY downregulating Poldip2-Nox4-H2O2 pathway and alleviating diabetic retinopathy. J Pharm Anal. 2023;13:1326-1345
111. Tian M, Li Z, Liu S, Wang Z, Deng B, Cao Y. et al. Ultrasmall copper nanodots eye drops for microenvironment regulation of diabetes retinopathy by relieving hypoxia and inhibiting HIF-1α/VEGF signaling pathway. Chem Eng J. 2024;491:1385-1408
112. Zheng Y, Luo S, Xu M, He Q, Xie J, Wu J. et al. Transepithelial transport of nanoparticles in oral drug delivery: From the perspective of surface and holistic property modulation. Acta Pharm Sin B. 2024;14:3876-3900
113. Wright L, Wignall A, Jõemetsa S, Joyce P, Prestidge CA. A membrane-free microfluidic approach to mucus permeation for efficient differentiation of mucoadhesive and mucopermeating nanoparticulate systems. Drug Deliv Transl Res. 2023;13:1088-1101
114. Dave RS, Goostrey TC, Ziolkowska M, Czerny-Holownia S, Hoare T, Sheardown H. Ocular drug delivery to the anterior segment using nanocarriers: A mucoadhesive/mucopenetrative perspective. J Control Release. 2021;336:71-88
115. Xue B, Lu Y, Wang S, Xiao Q, Luo X, Wang Y. et al. The emerging role of nanozymes in ocular antioxidant therapy. Nano Today. 2024;58:102448
116. Zou H, Hong Y, Xu B, Wang M, Xie H, Lin Q. Multifunctional cerium oxide nanozymes with high ocular surface retention for dry eye disease treatment achieved by restoring redox balance. Acta Biomater. 2024;185:441-455
117. Zhao Y, Hu J, Ke Y, Long Q, Mao J, Li H. et al. Micro-interaction of montmorillonite-loaded nanoparticles with mucin promotes retention of betaxolol hydrochloride on the ocular surface and the tear film microenvironment. Appl Clay Sci. 2024;247:169-187
118. Sun X, Sheng Y, Li K, Sai S, Feng J, Li Y. et al. Mucoadhesive phenylboronic acid conjugated chitosan oligosaccharide-vitamin E copolymer for topical ocular delivery of voriconazole: Synthesis, in vitro/vivo evaluation, and mechanism. Acta Biomater. 2022;138:193-207
119. Han H, Yin Q, Tang X, Yu X, Gao Q, Tang Y. et al. Development of mucoadhesive cationic polypeptide micelles for sustained cabozantinib release and inhibition of corneal neovascularization. J Mater Chem B. 2020;8:5143-5154
120. Zhang Y, Yu Y, Li G, Zhang X, Wu Z, Lin L. Bioadhesive glycosylated nanoformulations for extended trans-corneal drug delivery to suppress corneal neovascularization. J Mater Chem B. 2021;9:4190-4200
121. Sabourian P, Yazdani G, Ashraf SS, Frounchi M, Mashayekhan S, Kiani S. et al. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int J Mol Sci. 2020;21:E8019
122. Manzari-Tavakoli A, Babajani A, Tavakoli MM, Safaeinejad F, Jafari A. Integrating natural compounds and nanoparticle-based drug delivery systems: A novel strategy for enhanced efficacy and selectivity in cancer therapy. Cancer Med. 2024;13:e7010
123. Radwan SE-S, El-Kamel A, Zaki EI, Burgalassi S, Zucchetti E, El-Moslemany RM. Hyaluronic-coated albumin nanoparticles for the non-invasive delivery of apatinib in diabetic retinopathy. Int J Nanomedicine. 2021;16:4481-4494
124. Shakeri-Zadeh A, Rezaeyan A, Sarikhani A, Ghaffari H, Samadian H, Khademi S. et al. Folate receptor-targeted nanoprobes for molecular imaging of cancer: Friend or foe? Nano Today. 2021;39:101173
125. Wu KY, Wang XC, Anderson M, Tran SD. Advancements in Nanosystems for Ocular Drug Delivery: A Focus on Pediatric Retinoblastoma. Molecules. 2024;29:2263
126. Dave V, Sharma R, Gupta C, Sur S. Folic acid modified gold nanoparticle for targeted delivery of Sorafenib tosylate towards the treatment of diabetic retinopathy. Colloids Surf B Biointerfaces. 2020;194:111-151
127. Zhou L, Lan K, Huang X, Huang Y, Jin Y, Lu S. et al. A Novel Photosynthetic Biohybrid System for Microenvironment Regulation of Diabetes Retinopathy through Continuous Oxygen Supply and Nanozyme Cascade Reaction. Adv Funct Mater. 2023;33:2302493
128. Luo L-J, Nguyen DD, Lai J-Y. Dually functional hollow ceria nanoparticle platform for intraocular drug delivery: A push beyond the limits of static and dynamic ocular barriers toward glaucoma therapy. Biomaterials. 2020;243:119961
129. Byun S-Y, Han AR, Kim K-M, Kwon J-S. Antibacterial properties of mesoporous silica coated with cerium oxide nanoparticles in dental resin composite. Sci Rep. 2024;14:18014
130. Chaki Borrás ML, Das RC, Barker PJ, Sluyter R, Konstantinov K. Silica Nanoparticles Decorated with Ceria Quantum Dots Modulate Intra- and Extracellular Reactive Oxygen Species Formation and Selectively Reduce Human A375 Melanoma Cell Proliferation. ACS Appl Mater Interfaces. 2024;16:50430-50441
131. Li J, Tian Q, Sun H, Zhang Y, Yang X, Kaur P. et al. A novel, liposome-loaded, injectable hydrogel for enhanced treatment of choroidal neovascularization by sub-tenon's injection. Mater Today Nano. 2022;20:100264
132. Śpiewak D, Drzyzga Ł, Dorecka M, Wyględowska-Promieńska D. Summary of the Therapeutic Options for Patients with Dry and Neovascular AMD. J Clin Med. 2024;13:4227
133. Dohlman TH, Singh RB, Amparo F, Carreno-Galeano T, Dastjerdi M, Coco G. et al. Suppression of Neovascularization by Topical and Subconjunctival Bevacizumab After High-Risk Corneal Transplantation. Ophthalmol Sci. 2024;4:100492
134. Sun J-G, Jiang Q, Zhang X-P, Shan K, Liu B-H, Zhao C. et al. Mesoporous silica nanoparticles as a delivery system for improving antiangiogenic therapy. Int J Nanomedicine. 2019;14:1489-1501
135. Rong X, Yang J, Ji Y, Zhu X, Lu Y, Mo X. Biocompatibility and safety of insulin-loaded chitosan nanoparticles/PLGA-PEG-PLGA hydrogel (ICNPH) delivered by subconjunctival injection in rats. J Drug Deliv Sci Technol. 2019;49:556-562
136. Cuggino JC, Tártara LI, Gugliotta LM, Palma SD, Alvarez Igarzabal CI. Mucoadhesive and responsive nanogels as carriers for sustainable delivery of timolol for glaucoma therapy. Mater Sci Eng C Mater Biol Appl. 2021;118:111383
137. Toragall V, Baskaran V. Chitosan-sodium alginate-fatty acid nanocarrier system: Lutein bioavailability, absorption pharmacokinetics in diabetic rat and protection of retinal cells against H2O2 induced oxidative stress in vitro. Carbohydr Polym. 2021;254:117409
138. Kumar S, Fry LE, Wang J-H, Martin KR, Hewitt AW, Chen FK. et al. RNA-targeting strategies as a platform for ocular gene therapy. Prog Retin Eye Res. 2023;92:101110
139. Leroy BP, Fischer MD, Flannery JG, MacLaren RE, Dalkara D, Scholl HPN. et al. Gene Therapy for Inherited Retinal Disease: Long-Term Durability of Effect. Ophthalmic Res. 2022;66:179-196
140. Keeler AM, Flotte TR. Recombinant adeno-associated virus gene therapy in light of Luxturna (and Zolgensma and Glybera): where are we, and how did we get here? Annu Rev Virol. 2019;6:601-621
141. Liu A, Liang C, Liu J, Huang Y, Wang M, Wang L. Reactive Oxygen Species─Responsive Lipid Nanoparticles for Effective RNAi and Corneal Neovascularization Therapy. ACS Appl Mater Interfaces. 2022;14:17022-17031
142. Huang K, Lin Z, Ge Y, Chen X, Pan Y, Lv Z. et al. Immunomodulation of MiRNA-223-based nanoplatform for targeted therapy in retinopathy of prematurity. J Control Release. 2022;350:789-802
143. Ma X, Cui Y, Zhang M, Lyu Q, Zhao J. A Multifunctional Nanodrug Co-Delivering VEGF-siRNA and Dexamethasone for Synergistic Therapy in Ocular Neovascular Diseases. Int J Nanomedicine. 2024;19:12369-12387
144. Chambers CZ, Soo GL, Engel AL, Glass IA, the Birth Defects Research L, Frassetto A. et al. Lipid Nanoparticle-Mediated Delivery of mRNA Into the Mouse and Human Retina and Other Ocular Tissues. Transl Vis Sci Technol. 2024;13:7
145. Hou Y, Xin M, Li Q, Wu X. Glycyrrhizin micelle as a genistein nanocarrier: Synergistically promoting corneal epithelial wound healing through blockage of the HMGB1 signaling pathway in diabetic mice. Exp Eye Res. 2021;204:108-154
146. Bridges CC, El-Sherbeny A, Ola MS, Ganapathy V, Smith SB. Transcellular transfer of folate across the retinal pigment epithelium. Curr Eye Res. 2002;24:129-138
147. Liu B, Song W, Wu H, Liu Z, Teng Y, Sun Y. et al. Degradation of norfloxacin with peroxymonosulfate activated by nanoconfinement Co3O4@ CNT nanocomposite. Chem Eng J. 2020;398:125-135
148. Terreni E, Zucchetti E, Tampucci S, Burgalassi S, Monti D, Chetoni P. Combination of nanomicellar technology and in situ gelling polymer as ocular drug delivery system (ODDS) for cyclosporine-A. Pharmaceutics. 2021;13:192-202
149. Lou J, Hu W, Tian R, Zhang H, Jia Y, Zhang J. et al. Optimization and evaluation of a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles. Int J Nanomedicine. 2014;9:2517-2525
150. Yang J, Gong X, Fang L, Fan Q, Cai L, Qiu X. et al. Potential of CeCl3@mSiO2 nanoparticles in alleviating diabetic cataract development and progression. Nanomedicine. 2017;13:1147-1155
151. Supe S, Upadhya A, Tripathi S, Dighe V, Singh K. Liposome-polyethylenimine complexes for the effective delivery of HuR siRNA in the treatment of diabetic retinopathy. Drug Deliv Transl Res. 2023;13:1675-1698
152. Chaharband F, Daftarian N, Kanavi MR, Varshochian R, Hajiramezanali M, Norouzi P. et al. Trimethyl chitosan-hyaluronic acid nano-polyplexes for intravitreal VEGFR-2 siRNA delivery: formulation and in vivo efficacy evaluation. Nanomedicine. 2020;26:102-132
153. Alvarez-Rivera F, Fernández-Villanueva D, Concheiro A, Alvarez-Lorenzo C. α-Lipoic acid in Soluplus® polymeric nanomicelles for ocular treatment of diabetes-associated corneal diseases. J Pharm Sci. 2016;105:2855-2863
154. Ilochonwu BC, van der Lugt SA, Annala A, Di Marco G, Sampon T, Siepmann J. et al. Thermo-responsive Diels-Alder stabilized hydrogels for ocular drug delivery of a corticosteroid and an anti-VEGF fab fragment. J Control Release. 2023;361:334-349
155. Patil R, Sun T, Rashid MH, Israel LL, Ramesh A, Davani S. et al. Multifunctional nanopolymers for blood-brain barrier delivery and inhibition of glioblastoma growth through EGFR/EGFRvIII, c-Myc, and PD-1. Nanomaterials (Basel). 2021;11:2892-2900
156. Hu D, Huang G, Tang D, Zhao S, Zheng H. Joint task offloading and computation in cooperative multicarrier relaying-based mobile-edge computing systems. IEEE Internet Things J. 2021;8:11487-11502
157. Moses JC, Adibi S, Wickramasinghe N, Nguyen L, Angelova M, Islam SMS. Non-invasive blood glucose monitoring technology in diabetes management: review. JMIR Mhealth Uhealth. 2023;10:1-16
158. Kazanskiy NL, Khonina SN, Butt MA. Smart Contact Lenses—A Step towards Non-Invasive Continuous Eye Health Monitoring. Biosensors (Basel). 2023;13:933
159. Park S, Hwang J, Jeon H-J, Bae WR, Jeong I-K, Kim TG. et al. Cerium oxide nanoparticle-containing colorimetric contact lenses for noninvasively monitoring human tear glucose. ACS Appl Nano Mater. 2021;4:5198-5210
160. Shoval A, Markus A, Zhou Z, Liu X, Cazelles R, Willner I. et al. Anti-VEGF-Aptamer modified C-Dots—A hybrid nanocomposite for topical treatment of ocular vascular disorders. Small. 2019;15:1902776
161. Park S, Nam DY, Jeon H-J, Han JH, Jang D, Hwang J. et al. Chromophoric cerium oxide nanoparticle-loaded sucking disk-type strip sensor for optical measurement of glucose in tear fluid. Biomater Res. 2023;27:135
162. Ye Y, Zheng Q, Wang Z, Wang S, Lu Z, Chu Q. et al. Metal-phenolic nanoparticles enhance low temperature photothermal therapy for bacterial biofilm in superficial infections. J Nanobiotechnology. 2024;22:713
163. Johanssen VA, Ruan JL, Vince O, Thomas A, Peeters S, Soto MS. et al. Targeted opening of the blood-brain barrier using VCAM-1 functionalised microbubbles and "whole brain" ultrasound. Theranostics. 2024;14:4076-4089
164. Kou H, Yang H. Molecular imaging nanoprobes and their applications in atherosclerosis diagnosis. Theranostics. 2024;14:4747-4772
165. Vedarethinam V, Huang L, Zhang M, Su H, Hu H, Xia H. et al. Vanadium Core-Shell Nanorods Inspect Metabolic Changes of Diabetic Retinopathy. Adv Funct Mater. 2020;30:2002791
166. Hainsworth DP, Gangula A, Ghoshdastidar S, Kannan R, Upendran A. Diabetic retinopathy screening using a gold nanoparticle-based paper strip assay for the at-home detection of the urinary biomarker 8-hydroxy-2′-deoxyguanosine. Am J Ophthalmol. 2020;213:306-319
167. Zhang Y, Ranaei Pirmardan E, Jiang H, Barakat A, Hafezi-Moghadam A. VEGFR-2 adhesive nanoprobes reveal early diabetic retinopathy in vivo. Biosens Bioelectron. 2023;237:115476
168. Wang L, Zheng S, Lin X, Chen Y, Li J, Liu X. et al. A RuNPs-based multifunctional nanoplatform with excellent dual enzyme-mimic activities for diabetes diagnosis, cancer cell elimination, and in vitro antibacterial. Talanta. 2025;283:127121
Author contact
![]() Corresponding authors: Ji Li (syphulijicom); Ming Zhao (syphuzmcom).
Corresponding authors: Ji Li (syphulijicom); Ming Zhao (syphuzmcom).
 Global reach, higher impact
Global reach, higher impact