13.3
Impact Factor
Theranostics 2025; 15(8):3332-3344. doi:10.7150/thno.104900 This issue Cite
Review
Antigen-presenting fibroblasts: emerging players in immune modulation and therapeutic targets
1. Department of Dermatology, Hunan Key Laboratory of Medical Epigenomics, the Second Xiangya Hospital, Central South University, Changsha, 410011, China.
2. Institute of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, 210042, China.
3. Key Laboratory of Basic and Translational Research on Immune-Mediated Skin Diseases, Chinese Academy of Medical Sciences, Nanjing, China.
Received 2024-10-9; Accepted 2025-1-28; Published 2025-2-18
Abstract
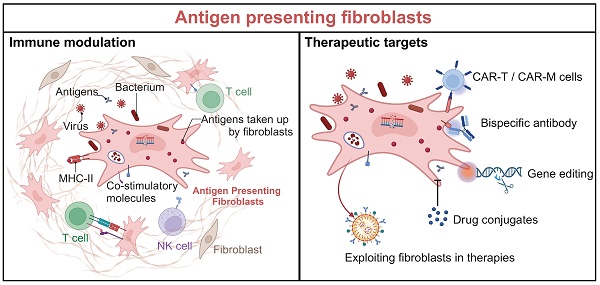
Antigen-presenting fibroblasts are a newly recognized subset that challenges the traditional view of these cells as mere structural components. Under pathological or environmental stimuli, fibroblasts acquire antigen-presenting capabilities through the expression of MHC-II molecules and co-stimulatory factors, enabling them to interact with T cells and modulate immune responses. These specialized fibroblasts have been identified across various tissues and diseases, where they play context-dependent roles, either amplifying immune dysregulation or contributing to immune homeostasis. This review synthesizes recent advances in understanding the origins, activation, and functions of antigen-presenting fibroblasts. It highlights their role in promoting pathogenic immune responses and offering therapeutic opportunities through targeted modulation. Advancing our understanding of antigen-presenting fibroblasts holds great promise for developing innovative approaches to immune modulation and therapy across a range of diseases.
Keywords: antigen presenting fibroblast, MHC-II, T cell, immune regulation, therapeutic potential
1. Introduction
Antigen presentation is a cornerstone of the immune system, driving adaptive immune responses through the activation of T cells [1, 2]. Classical antigen-presenting cells (APCs), such as dendritic cells, macrophages, and B cells, are central to this process [3]. Dendritic cells are the most potent initiators of primary T cell responses, while macrophages and B cells bridge innate and adaptive immunity by facilitating inflammation and humoral responses, respectively [4-7]. Professional APCs have long been seen as the primary mediators of antigen-driven immune activation.
Recent discoveries, however, challenge this established paradigm by revealing the antigen-presenting potential of fibroblasts [8]. Traditionally considered structural cells that maintain tissue integrity and regulate extracellular matrix composition, fibroblasts have now been shown to acquire antigen-presenting capabilities under pathological conditions, such as chronic inflammation, autoimmune diseases, and tumors [9]. This redefines fibroblasts as active players in immune regulation rather than passive structural components.
This review aims to consolidate recent advances in antigen-presenting fibroblasts research, with a focus on their tissue-specific distribution, mechanisms of activation, and functional roles in immune modulation. By offering a comprehensive overview, this review positions antigen-presenting fibroblasts as a promising focus for future research and therapeutic innovation [10, 11].
2. Discovery and distribution of antigen-presenting fibroblasts
In the early twentieth century, HLA-DR-positive fibroblasts were found to present tetanus toxoid (TT) to autologous TT-specific monoclonal helper T cells in an MHC-restricted manner, and hypothesis the antigen presentation function of human dermal fibroblasts [12]. However, this hypothesis remained largely unexplored due to the technological limitations of the time. The advent of advanced techniques, such as single-cell RNA sequencing and high-throughput molecular profiling, has dramatically transformed our understanding of fibroblast heterogeneity and their roles in immune regulation [13, 14]. This heterogeneity allows for the classification of fibroblasts into distinct subtypes, each with unique gene expression profiles that reflect their contributions to tissue homeostasis and diseases [15, 16]. Antigen-presenting fibroblasts are characterized by their ability to express MHC-II molecules (HLA-DR, HLA-DP, HLA-DQ) and CD74, which are traditionally associated with professional APCs [17]. Notably, these fibroblasts also upregulate co-stimulatory molecules (CD40, CD80, CD86) under certain pathological conditions, which are critical for effective T cell interaction and immune activation.
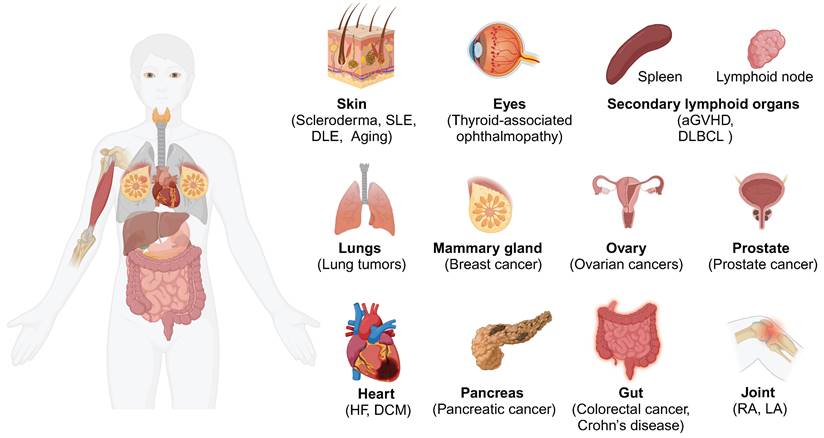
The presence of antigen-presenting fibroblasts has been confirmed across various human and murine tissues (Figure 1), including the skin [18], heart [19], lung [9], gut [20], pancreas [21], breast [22], joint [23], lymph nodes [24], and others [25]. Moreover, a recent integrative cross-disease analysis confirmed the widespread presence of CD74+ antigen-presenting fibroblasts in multiple organs and classified antigen-presenting fibroblasts as "shared" clusters [26]. While these antigen-presenting fibroblasts are present in low numbers under normal physiological conditions (e.g., in the fat pads of the pancreas and breast), their frequency and expression of inflammatory genes such as IL32 and CXCR4 increase markedly in inflammatory or malignant conditions [22]. This finding highlights the importance of considering fibroblasts within the broader immune landscape, particularly in disease settings where their antigen-presenting functions are amplified.
3. Origins and activation of antigen-presenting fibroblasts
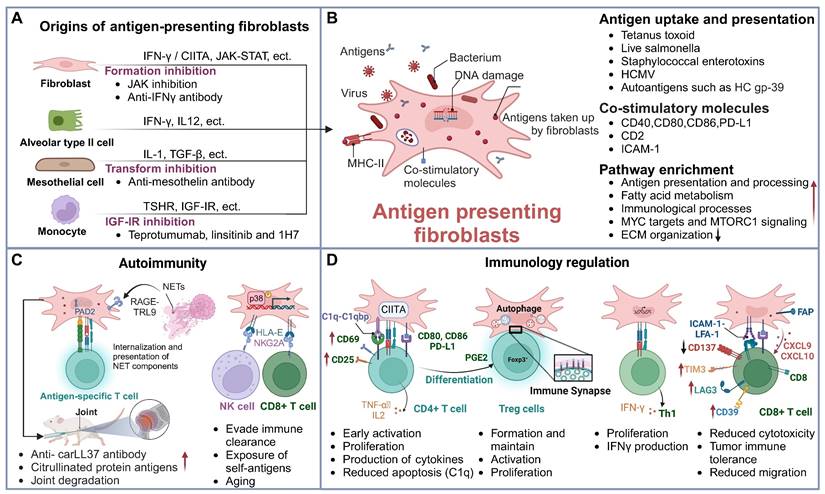
Fibroblasts exhibit considerable heterogeneity [27, 28], with IFN-γ being the critical driver for the formation of antigen-presenting fibroblasts. In addition to IFN-γ, other stimuli such as environmental factors and various signaling pathways can significantly modulate their antigen-presenting capabilities. Furthermore, a subset of antigen-presenting fibroblasts originates from the transformation of epithelial cells, mesothelial cells, and other cell types (Figure 2A). Understanding the mechanisms that trigger their activation is crucial to unraveling their roles in immune responses and disease progression.
Distribution of antigen-presenting fibroblasts. The distribution of antigen-presenting fibroblasts in different human tissues and organs is characterized by the expression of CD74 and MHC-II molecules such as HLA-DRA and HLA-DRB1. Abbreviations: SLE, systemic lupus erythematosus; DLE, discoid lupus erythematosus; aGvHD: Acute graft versus host disease; DLBCL: Diffuse Large B-Cell Lymphoma; HF, heart failure; DCM, dilated cardiomyopathy; RA, Rheumatoid arthritis; LA, Lyme arthritis.
3.1 IFN-γ: A key driver of antigen-presenting fibroblasts
In several tissues such as the heart and synovium, IFN-γ induces the expression of MHC-II molecules in fibroblasts, highlighting its potential role in antigen presentation [19]. Studies in IFN-γ or IFN-γ receptor-deficient mice have shown that MHC-II expression by fibroblasts is markedly reduced, further confirming the critical role of this cytokine [9]. At the molecular level, IFN-γ activates key transcription factors such as CIITA through the JAK-STAT pathway, which is essential for driving MHC-II expression [29-31]. Additionally, transcriptional regulators like CDK8 and CDK19 play an important role in the IFN-γ-mediated reprogramming of fibroblasts toward an antigen-presenting phenotype [32]. While IFN-γ is a key factor, the precise regulatory mechanisms underlying MHC-II expression in fibroblasts remain to be fully understood.
In disease contexts, IFN-γ plays a significant role in shaping fibroblasts behavior. For instance, in RA, IFN-γ secreted by natural killer (NK) cells stimulates synovial fibroblasts, leading to the formation of HLA-DR+CD90+ fibroblasts, which contribute to joint inflammation [33]. In the skin, IFN-γ produced by CD8+ T cells drives the upregulation of MHC-II and chemokines such as CXCL9 and CCL2, promoting immune cell recruitment [34]. Additionally, specific risk single nucleotide polymorphisms (SNPs) associated with RA, such as rs6074022, have been shown to influence the expression of CD40 induced by IFN-γ, further emphasizing the complexity of IFN-γ-mediated fibroblasts activation in various tissues [35].
3.2 Additional stimulations and signaling pathways
In addition to IFN-γ signaling, various stimuli and environmental cues play critical roles in enhancing the antigen-presenting capabilities of fibroblasts. These factors include inflammatory signals, tissue injury, and metabolic reprogramming, which collectively influence fibroblasts activation and their ability to engage in antigen presentation. For instance, DNA damage has been shown to enhance the expression of MHC-I and co-stimulatory molecules on fibroblasts, boosting their antigen-presenting capacity [36]. In the tumor microenvironment, antigen-presenting fibroblasts are closely associated with fatty acid metabolism [37]. IL-12 also plays a crucial role in promoting the development of antigen-presenting fibroblasts, with a reduction in these cells observed in IL-12-deficient mice [9].
In certain conditions, fibroblasts with antigen-presenting capabilities also appear to originate from epithelial cells or monocytes. For instance, in pancreatic cancer, tumor-derived paracrine signals trigger the transformation of mesothelial cells into antigen-presenting fibroblasts, which exhibits upregulation of MHC-II molecules alongside mesothelial markers such as Msln, Upk3b, and Ezr [38]. Similarly, IL-1 and TGF-β signaling pathways further enhance this transition, indicating that diverse pathways contribute to the formation of antigen-presenting fibroblasts in different disease contexts [21]. In conditions like thyroid ophthalmopathy, a distinct subset of CD34+ fibroblasts displays enhanced MHC-II and co-stimulatory molecule expression, primarily originating from peripheral blood mononuclear cells (PBMCs) [39]. Furthermore, in lung cancer, MHC-II+ fibroblasts are closely linked to alveolar epithelial cell gene signatures, suggesting that alveolar epithelial cells undergo EMT to become antigen-presenting fibroblasts.
4. Functional basis of antigen-presenting fibroblasts
Increasing evidence suggests that fibroblasts can assume the role of APCs under certain conditions. Their ability to present antigens relies on several key mechanisms, including antigen uptake and processing, as well as the expression of co-stimulatory molecules. Understanding these fundamental processes is crucial for revealing how fibroblasts contribute to immune regulation as important, non-professional APCs.
4.1 Antigen uptake and presentation
Antigen presentation begins with the recognition, phagocytosis, processing, and subsequent presentation of antigens. APCs possess sophisticated mechanisms for antigen uptake, including phagocytosis, receptor-mediated endocytosis, and macropinocytosis [40-42]. Fibroblasts, while typically exhibiting limited antigen uptake under normal physiological conditions, can enhance their antigen acquisition abilities in pathological states, such as inflammation and cancer.
Infections caused by viruses and bacteria represent common sources of antigens [43, 44]. Fibroblasts are capable of phagocytosing bacteria and processing antigens, and they can also be infected by viruses. For instance, dental pulp fibroblasts have been shown to produce C3b, facilitating the opsonization of bacteria and enhancing phagocytosis [45]. During inflammation, apoptotic pathways and exocytosis receptors are upregulated in fibroblasts and immune cells, allowing dermal fibroblasts to effectively engulf apoptotic endothelial cells, a process important for tissue repair and fibrosis [46, 47]. Moreover, fibroblasts can internalize polymeric nanocarriers through receptor-mediated endocytosis, influencing their functional responses and viruses can enter fibroblasts through pH- and kinetoprotein-dependent endocytosis [48, 49]. Additionally, macropinocytosis facilitates the uptake of extracellular antigens and other substances. For example, glutamine deprivation drives fibroblasts to enhance macropinocytosis, supporting cancer cell adaptation [50]. Notably, antigen-presenting fibroblasts can significantly increase their uptake capabilities upon stimulation, as demonstrated using ovalbumin (OVA) [21]. This flexibility suggests that their antigen uptake functions are influenced by their activation state, microenvironmental cues, and cytokine stimulation. These characteristics underscore the crucial role of fibroblasts in immune responses, particularly in the processes of antigen uptake, processing, and presentation [33, 45, 51].
4.2 Expression of co-stimulatory molecules
The effectiveness of antigen presentation is contingent upon the duration and intensity of TCR-peptide-MHC interactions, as well as the balance of co-stimulatory and co-inhibitory signals. Professional APCs provide essential co-stimulatory signals, such as CD80, CD86, and CD40, necessary for complete T cell activation and proliferation [52-54]. Under normal conditions, fibroblasts may lack these co-stimulatory molecules, which could limit their T cell activation capacity [55].
However, in pathological conditions, fibroblasts can upregulate co-stimulatory molecules, enhancing T cell activation. For example, in rheumatoid arthritis, synovial fibroblasts express CD80 and CD86, promoting the activation of autoreactive T cells and perpetuating inflammation [39]. Furthermore, fibroblasts can express additional co-stimulatory factors, such as CD2, which is vital for T cell activation, particularly in inflammatory contexts [56]. The expression of molecules like ICAM-1 on fibroblasts can further enhance T cell activation, as seen in psoriasis [57, 58]. Additionally, fibroblasts can express co-stimulatory molecules like 4-1BBL, OX-40L, and CD70, which are associated with the activation and maintenance of memory T cells [59]. While fibroblasts were traditionally thought to be less effective than professional APCs in antigen presentation, some studies have shown that their T cell-activating potential can be comparable to that of dendritic cells and B cells under certain conditions [20].
In summary, antigen-presenting fibroblasts are capable of both antigen uptake and presentation, as well as the expression of co-stimulatory molecules (Figure 2B). While their functional capacity may vary under different conditions, both professional APCs and fibroblasts are essential in modulating immune responses. Understanding the mechanisms underlying their interaction is a key area of ongoing research.
5. Antigen-presenting fibroblasts in different diseases
Recent research has unveiled multifaceted functions of fibroblasts in immune responses, extending beyond their traditional roles in tissue structure to actively modulate immune reactions through antigen presentation. Their interactions with immune cells, such as NK cells and T cells [60], significantly impact the local immune microenvironment in cancer, autoimmune diseases, infections, and tissue injuries (Figure 2C-D). Below, we discuss the specific functions of antigen-presenting fibroblasts in various disease contexts.
5.1 Cancer-associated antigen-presenting fibroblasts
In the tumor microenvironment, cancer-associated fibroblasts (CAFs) are key constituents of the stroma and contribute to tumor progression and immune evasion [38, 61]. A subset of these fibroblasts, termed antigen-presenting cancer-associated fibroblasts (apCAFs), express MHC-II molecules and interact with T cells in an antigen-specific manner. In PDAC, mesothelial cells can transition into antigen-presenting fibroblasts, which induce T cell expression of early activation markers CD25 and CD69 in an OVA-specific manner, while other CAF subtypes cannot [21]. In triple-negative breast cancer (TNBC) and pancreatic ductal adenocarcinoma (PDAC), apCAFs exhibit molecular signatures indicative of immunomodulatory functions [22]. In colorectal cancer, apCAFs can elicit T cell reactivity against mismatched fibroblast cell lines loaded with mutated SLP, promoting the activation of CD4+ T cells, as evidenced by increased expression of CD69 and CD25. However, this interaction also reduces the cytotoxicity and activation of CD8+ T cells, suggesting a dual role of apCAFs in modulating the immune response.
Additionally, apCAFs promote the formation of Tregs in colonic tissues, dependent on MHC-II and prostaglandin E2 (PGE2) [62]. In melanoma, α-SMA+ fibroblasts form an immunological synapse with Foxp3+ Tregs in the tumor microenvironment and instruct their activation and proliferation in an antigen-specific manner [63]. This interaction highlights the significant role of fibroblasts in facilitating tumor immune evasion by inducing the formation of Tregs and suppressing effector T cell functions, thus potentially promoting tumor progression [64]. Most importantly, the influence of a decrease in activating (CD137) and an increase in inhibitory (TIM3, LAG3, and CD39) checkpoint molecule expression on CD8+ T cells may lead to chemoresistance and decreased efficacy of chemotherapeutic drugs [20]. This illustrates the complex interplay by which apCAFs regulate immune responses via antigen presentation.
While apCAFs primarily exist in malignancy, their exact roles in metastatic cancers are not fully understood. It remains unclear whether their presence enhances tumor immunity or correlates with prognosis [65-68]. Despite their potential to promote immune suppression, CAFs can also enhance antitumor immunity by expressing molecules such as CD1d, which can activate NK cells and iNKT cells, leading to the destruction of tumor cells [69]. At the same time, apCAFs can also prevent T cells from apoptosis through the action of C1q and C1qbp, thus inhibiting tumor formation and exerting an anti-tumor effect [9]. This dual functionality suggests that the specific role of APFs depends on the cancer type and microenvironmental context.
Sources and functions of antigen-presenting fibroblasts. (A) Origins of antigen-presenting fibroblasts and intervention strategies. This panel illustrates the various cellular origins of antigen-presenting fibroblasts, including fibroblasts derived from mesothelial cells, monocytes, and epithelial cells. Each origin is associated with distinct therapeutic interventions, such as JAK inhibitors, anti-mesothelin antibodies, and IGF-IR inhibitors, which target specific pathways to prevent their pathogenic activation. (B) Antigen uptake and presentation by antigen-presenting fibroblasts. This schematic shows the antigen-presenting fibroblasts' ability to phagocytose bacteria, viruses, and self-antigens. These cells present antigens via MHC-II molecules and express co-stimulatory molecules, such as CD80 and CD86, to modulate T cell responses. Their primary functions include antigen presentation and immune regulation. (C) Antigen-presenting fibroblasts in autoimmunity. NETs are internalized by FLS via the RAGE-TLR9 axis, leading to increased inflammatory responses. This process upregulates MHC-II expression, allowing FLS to present citrullinated NET-derived antigens to CD4+ T cells. FLS releases membrane-bound PAD2, promoting the citrullination of cartilage fragments. Elevated anti-carLL37 antibody levels and ACPA generation in joint inflammation result in synovial cartilage degradation and arthritis. Additionally, the expression of HLA-E on fibroblasts helps them evade immune clearance, further exposing self-antigens, and linking fibroblasts antigen presentation to autoimmune responses. (D) Immunoregulatory functions of antigen-presenting fibroblasts. Antigen-presenting fibroblasts influence the activation and function of various T cell subsets. They enhance T cell activation, promote differentiation of Tregs and Th1 cells, and affect the cytotoxic function of CD8+ T cells, thereby playing a key role in immune regulation across different disease contexts. Abbreviations: PAD2, peptidylarginine deiminase 2; FLS, fibroblast-like synoviocytes; carLL37, carbamylated LL37; NETs, neutrophil extracellular traps.
5.2 Antigen-presenting fibroblasts in the skin
Dermal fibroblasts are essential for maintaining skin integrity and are primarily involved in synthesizing and renewing the extracellular matrix [70, 71]. Aging skin typically shows a decline in immune surveillance, leading to increased exposure to self-antigens and potential activation of autoimmune responses [72, 73]. In aged skin, the emergence of antigen-presenting fibroblasts is marked by a significant increase in HLA-II+ senescent fibroblasts. These cells are closely associated with heightened susceptibility to viral and bacterial infections, notably evidenced by the expression of HCMV RNA in aged dermal fibroblasts. Notably, these fibroblasts can present HCMV-gB to CD4+ T cells in an HLA-II-dependent manner, further activating T cells [74]. Senescent fibroblasts expressing HLA-E can evade immune clearance by binding to the inhibitory ligand NKG2A, which is recognized by CD8+ T cells and NK cells. Concurrently, this interaction also modulates the functionality of CD8+ T cells and NK cells [60, 75].
Scleroderma is a unique autoimmune skin disease. It was previously believed that the abnormal activation and proliferation of myofibroblasts were key factors in its pathogenesis [76-78]. When PBMCs are co-cultured with fibroblasts obtained from scleroderma patients, T cell expansion identical to that found in the skin of scleroderma patients and patient-derived PBMCs can be detected [79]. This suggests the presence of a subset of fibroblasts in scleroderma skin capable of activating T cells. Recent studies have found elevated levels of antigen-presenting fibroblasts in localized scleroderma which highly express HLA-related genes (HLA-DQB1, -DPA1, -DRB1, -DRA) and inflammatory gene expression (CXCR4, CD74, IL32). Although this is a small subset of fibroblasts, cell-cell communication indicates an enrichment of ligand-receptor pairs and significant interactions with immune cells [18]. Dermal HLA-DR-positive fibroblasts can present TT to autologous TT-specific monoclonal helper T cells [12]. In lupus skin lesions, a group of antigen-presenting fibroblasts characterized by CD74 and HLA-DRB1 has also been identified, significantly enriched in pathways related to antigen presentation and cytokine production, which highlights the importance of these cells in autoimmune-related skin diseases [80].
5.3 Antigen-presenting fibroblasts in joints
In RA, synovial fibroblasts are primary inflammatory effector cells that present autoantigens to T cells, directly contributing to the autoimmune response [81, 82]. They can present peptides from autoantigens such as HC gp-39 and human CII to antigen-specific MHC-restricted T cell hybridomas, functioning as antigen-presenting cells and directly interacting with infiltrating T cells in vitro [23]. Synovial fibroblasts can up-regulate the activation marker CD69 in CD4+ T cells. When infected with Borrelia burgdorferi, MHC-II+ synovial fibroblasts are inducible antigen-presenting cells that can induce CD4+ T cell activation in an antigen- and CD40-dependent manner [17]. Activated synovial fibroblasts can also present ECM-derived Lyme autoantigens, implicating them in amplifying tissue-localized autoimmunity in LA [17].
Proteins from neutrophil extracellular traps (NETs) constitute a significant portion of the autoantigen pool in RA [83]. Synovial fibroblasts can also internalize NETs, presenting citrullinated antigens to T cells, thus contributing to autoantibody production and joint degradation. NET-derived proteins, such as carLL37, are processed and presented by synovial fibroblasts, eliciting autoantibody responses that further promote inflammation and damage in RA. This antigen-presenting function of fibroblasts in the synovium links them closely to autoimmune responses and disease progression in RA [82, 84, 85].
5.4 Antigen-presenting fibroblasts in other tissues and organs
In cardiac tissue, fibroblasts play a crucial role in tissue repair after myocardial infarction [86, 87]. Recent studies have further confirmed the presence of a subset of cardiac fibroblasts characterized by the expression of MHC-II molecules [19]. These cells are significantly expanded in heart failure and dilated cardiomyopathy and can effectively present antigens, promoting T cell activation [88]. Notably, the specific deletion of MHC-II in fibroblasts can effectively alleviate cardiac remodeling and dysfunction induced by transverse aortic constriction (TAC). This is a significant breakthrough as it not only identifies this subset from the single-cell sequencing perspective but also validates the role of antigen-presenting fibroblasts in the development of cardiac diseases through in vivo experiments.
Fibroblasts also contribute to immune regulation in the lymph nodes, where fibroblastic reticular cells (FRCs) can present antigens to CD8+ T cells and inhibit their cytotoxic activity, promoting immune evasion in conditions like diffuse large B cell lymphoma (DLBCL). Intrinsic MHC-II expression in lymph node stromal cells promotes the transformation of MHC-I-restricted CD8+ T cell lineage into regulatory CD4+ T cells [89]. The activated LNSC acquires enhanced antigenic presentation and acts as an external brake system for CD4+ T cell response. FRCs located in the T cell zone of LN ectopically express and directly present model PTA to naive T cells, thereby inducing their proliferation [90, 91].
In addition to their direct antigen-presenting functions, HLA-DR expressed on fibroblasts can also act as a receptor molecule that transmits signals to fibroblasts based on DR-peptide-TCR interactions, resulting in the secretion of multiple cytokines [92]. This communication is often mediated through the secretion of cytokines and chemokines, which can significantly alter the immune landscape of a given tissue or organ. For example, cytokines produced by macrophages, such as IL-1β, IFN-γ, and TNF, can profoundly affect fibroblasts gene expression, leading to the production of CXCL and CCL ligands. These chemokines, in turn, can recruit and activate various immune cells, thereby shaping the immune response in a tissue-specific manner. Such interactions are especially relevant in chronic inflammatory conditions, where fibroblasts can either amplify or attenuate ongoing immune responses.
6. Diagnosis and therapeutic implications
The functional diversity of antigen-presenting fibroblasts highlights their significant roles in modulating immune responses across a spectrum of diseases (Table 1). This makes them attractive targets for therapeutic interventions aimed at either enhancing their immunostimulatory functions in cancer or inhibiting their pro-inflammatory roles in autoimmune diseases. Below, we explore their diagnostic potential and therapeutic strategies aimed at modulating their functions.
6.1 Diagnostic potential
The expression of MHC-II molecules has emerged as a robust prognostic marker for immune responses and a predictor of outcomes in patients undergoing immune checkpoint inhibitor therapy [98]. In prostate cancer, an increased presence of fibroblasts involved in antigen presentation and processing has been observed, with their antigen-presentation gene expression serving as a predictive marker of disease risk [65]. These antigen-presenting fibroblasts exhibit significant interaction with T cells and are strongly correlated with immune cell infiltration, highlighting their potential as biomarkers for disease progression in cancer and autoimmune disorders [65].
Moreover, imaging techniques targeting fibroblast activation protein (FAP) have shown significant promise in tumor diagnosis, particularly in metastatic cancers such as prostate cancer [99]. FAP is a marker that is frequently upregulated on activated fibroblasts, highly expressed in various malignancies, including cancer, fibrotic diseases, and inflammatory conditions [100, 101]. The use of selective tracers like Gallium 68 (68Ga)-labeled fibroblast activation protein inhibitor (FAPI) in PET imaging has demonstrated excellent diagnostic accuracy by visualizing activated fibroblasts [102, 103]. Integrating FAPI-PET imaging with MHC-II-targeted imaging techniques (e.g., MHC-II probes and molecular imaging) may offer a non-invasive method to assess antigen-presenting fibroblasts in diseases such as tumors, fibrosis, and chronic inflammation [104].
6.2 Therapeutic implications
Antigen-presenting fibroblasts play dual roles in diseases, serving as both mediators of immune activation and regulators of immune responses. This dual functionality renders them promising therapeutic targets for a range of diseases, including autoimmune disorders and cancers. Therapeutic strategies aimed at modulating antigen-presenting fibroblasts focus on inhibiting their formation, disrupting their antigen-presentation functions, or selectively eliminating them, depending on the disease context (Figure 3).
One approach to targeting antigen-presenting fibroblasts is to inhibit their differentiation and formation. For instance, JAK inhibitors have been primarily designed to suppress IFN-γ-induced activation of fibroblasts, thereby reducing their antigen-presenting capacity and mitigating autoreactive T cell activation in autoimmune diseases like RA [33]. Similarly, anti-mesothelin antibodies have been employed to block the transformation of mesothelial cells into antigen-presenting fibroblasts. This strategy has been demonstrated to enhance antitumor immunity, particularly in cancers such as pancreatic cancer [21]. Additionally, IGF-IR inhibitors reduce the generation of monocyte-derived antigen-presenting fibroblasts, effectively dampening pathological immune responses [39].
Beyond inhibiting their formation, therapies that disrupt the functional components of antigen-presenting fibroblasts provide another promising avenue. Strategies such as blocking MHC-II molecules using specific antibodies or nanotechnology-based systems have shown the potential to impair the antigen-presentation capabilities of these cells [105-107]. Gene-editing techniques, including those that inhibit autophagy or disrupt MHC-II expression, represent additional tools for reducing the immunopathological roles of antigen-presenting fibroblasts [108]. Such interventions offer therapeutic benefits in both autoimmune diseases and cancers, where aberrant antigen presentation drives disease progression.
Selective elimination of antigen-presenting fibroblasts has also emerged as an innovative therapeutic strategy, particularly in oncology. CAR-T cells engineered to target FAP, a marker widely expressed on pathogenic fibroblasts, have demonstrated significant potential in preclinical studies [109, 110]. Advanced CAR-T constructs, such as UniCAR modules and CAR-TEAM designs, enhance specificity while reducing the risk of off-target effects [111, 112]. Furthermore, CAR-macrophages, which possess superior tissue infiltration capabilities compared to CAR-T cells, have been employed to target fibroblasts in dense tumor microenvironments, broadening the scope of therapeutic options [100].
Antigen-Presenting fibroblasts in various tissues and organs
| Species | Diseases | Tissue | Name | Markers | Functions | References |
|---|---|---|---|---|---|---|
| Human | Ovarian cancers | Ovary | apCAFs | HLA-DRA, HLA-DRB | ApCAFs were found in various metastatic tumor foci. | [66] |
| Mouse | PDAC | Pancreas | apCAFs | Histocompatibility 2, class II antigen A, alpha (H2-Aa) and beta 1 (H2-Ab1), Serum Amyloid A3, Cd74, Slpi, stata1 | Antigen presentation and processing; Fatty acid metabolism; MYC targets and MTORC1 signaling. | [8] |
| Human | PDAC | Pancreas | apCAFs | HLA-DRA, HLA-DPA1 and HLA-DQA1, SLPI, CD74, XBP1 | ApCAFs isolated from the same orthotopic tumors demonstrated the capacity to induce CD25 and CD69 in co-culture 2d T cells in an OVA-specific manner. | [8] |
| Human,Mouse | Pancreatic cancer | Pancreas | apCAFs | MHC-II, Cd74, H2-Aa, H2-Ab1, H2-Dma, H2-Dmb1, H2-Eb1 | Expanded specifically in all late-stage GEMMs; ApCAFs induce naive CD4+ T cells into regulatory T cells, induce Tregs formation and expansion; Induced CD4+ T cell expression of early activation markers, CD25 and CD69. | [21] |
| Mouse | Pancreatic cancer | Pancreas | apCAFs | Cd74, H2-Ab1, Saa3 | ApCAFs clustered with mesothelial cells from normal pancreas. | [93] |
| Human | Colorectal cancer | Gut | apCAFs | HLA A3-B7-, A3+B7- or A3-B7+ | T cell activation, induced production of TNFα and IL-2; Impairs CD8+ T cell effector function Result in a decrease in activating (CD137) and increase in inhibitory (TIM3, LAG3 and CD39) checkpoint molecule. | [20] |
| Human | \ | Gut | Subepithelial myofibroblasts | αSMA+, CD90+, MHC-II, CD80, CD86 | Able to stimulate allogeneic CD4+ T cell proliferation. | [94] |
| Human | Staphylococcal enterotoxigenic disease | Gut | Subepithelial intestinal myofibroblasts | MHC-II | IMFs bind staphylococcal enterotoxins in an MHC-II-dependent fashion in vitro. | [95] |
| Human | Salmonella typhimurium infection | Gut | Intestinal stromal cells | CD90+ HLA-DR | Rapid internalization and sensing of live Salmonella; Capacity for phagocytosis and antigen processing. | [96] |
| Human | \ | Gut | Colonic myofibroblasts/fibroblasts | CD90+, αSMA+ MHC-II | Contribute to the maintenance of FoxP3+ phenotype of the nTregs; Induce generation of iTregs from naïve CD4+ T cells (MHC-II- and PGE2- dependent). | [62] |
| Mouse | Breast Cancer (Mammary Tumors) | Breast | apCAFs | H2-Aa, H2-Ab1, H2-Eb, Cd74, Krt7, Krt8 and Krt18 and Fsp1 (S100a4) | Immune modulatory role in the tumor microenvironment. | [22] |
| Human, Mouse | Lung tumors | Lungs | apCAFs | MHC-II and FAP, Lin- | ApCAFs activate adjacent CD4 T cells. | [9] |
| Human | Primary NSCLC with metastasis | Lungs | apCAFs | HLA-DRA, HLA-DPB1, HLA-DPA1, SP11, RUNX3, MAF | ApCAF may play a role in bone metastasis by activating signalling pathways associated with cancer stemness, such as SPP1-CD44 and SPP1-PTGER4. | [67] |
| Human | NTHi infection | Lungs | Fibroblasts | CD45-EpCAM-CD90+HLA-DR+ | Able to present Ag and activate bacteria-specific autologous Th cells when preconditioned with IFN-γ. | [59] |
| Human | Inflamed joint tissues | Joint | FLS | MHC-II | Present peptides from the autoantigens HC gp-39 and human CII to antigen-specific MHC-restricted T cell hybridomas. | [23] |
| Human | RA | Joint | FLS | HLA-DR+CD90+, HLA-DRB1, HLA-ABC, CD54 | Up-regulated the activation marker CD69 in CD4+ T cells. | [33] |
| Human | LA | Joint | FLS | HLA-DR | Induce CD4+ T cell activation in an antigen- and CD40-dependent manner; Present ECM-derived Lyme autoantigens, implicating FLS in amplifying tissue-localized autoimmunity in LA. | [17] |
| Human | Infection | Skin | HLA-DR+ fibroblasts | HLA-DR | Present tetanus toxoid (TT) to autologous TT-specific monoclonal helper T cells; Induce significant proliferation of cloned T cells. | [12] |
| Human | Localized Scleroderma | Skin | Cluster 11 (CD74/ DUSP2) | DUSP2, CD74, HLA-B, HLA-C, HLA-DRB1, HLADRA, CXCR4, CD74, IL32 | Unique fibroblast populations in LS compared to controls; Potential roles for fibroblasts through cell-cell communication and trajectory software, especially macrophage interaction. | [18] |
| Human | SLE | Skin | HLA+ fib | HLA-DRA, HLA-DRB1, CD74 | Function as nonclassical antigen-presenting cells. | [80] |
| Human | Aging | Skin | HLA-II + fib | HLA-II, HCMV-gB | Act as inducible antigen-presenting cells that can activate CD4 CTL from the human skin and participate in immune regulation. | [74] |
| Mouse | Melanoma | Skin | α-SMA+ CAFs | Cd74, H2-Aa, H2-DMa and H2DMb1 | Form an immunological synapse with Foxp3+ Tregs in the tumor microenvironment and instruct their activation and proliferation in an antigen-specific manner. | [63] |
| Human, Mouse | DLBCL | Lymph node | DLBCL activated FRCs | HLA genes, B2m and Cd74 | Inhibit CD8+ TIL cytotoxicity in an antigen-specific manner Reduced ability to promote T lymphocyte migration. | [64] |
| Mouse | HF | Heart | Cardiac fibroblasts | MHC-II | Induce naïve CD4+ T cell proliferation; Promote antigen-specific Th1 cell activation Be central to cardiac remodelling and dysfunction in response to TAC. | [19] |
| Mouse | aGvHD | Secondary lymphoid organs | CCL19+ FRCs | Cd74, Tap2, H2-Aa, H2-Eb1, H2-Eb2, and H2-Ab1, CCL19 | Promotes the expansion of antigen-specific Tregs and controls T cell alloreactivity in the effector phase of GvHD. | [97] |
Therapeutic interventions on antigen-presenting fibroblasts.
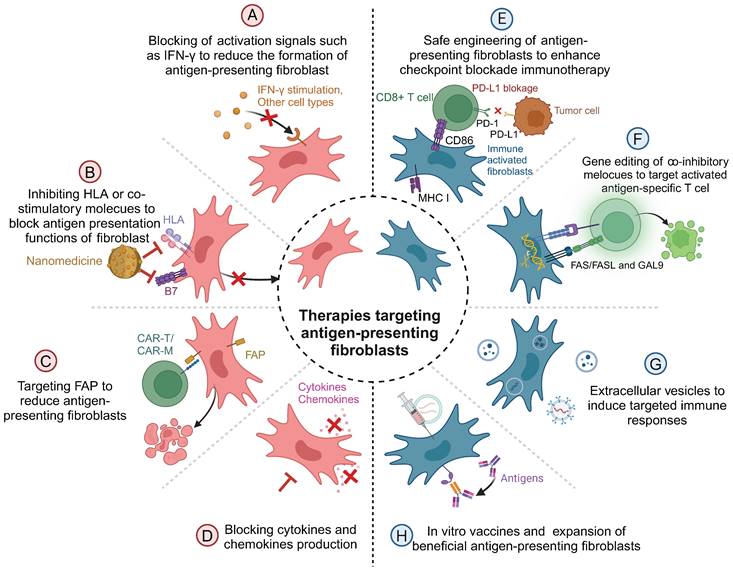
In addition to these direct interventions, the mediators secreted by antigen-presenting fibroblasts also present therapeutic targets. Neutralizing pro-inflammatory cytokines or blocking downstream signaling pathways has been shown to mitigate the pathological effects of antigen-presenting fibroblasts. Conversely, harnessing fibroblast-derived exosomes carrying therapeutic molecules, such as anti-inflammatory genes, offers novel opportunities for immunomodulation. For instance, tumor-associated fibroblast-derived exosomes carrying lncRNAs can influence immune evasion by downregulating HLA expression in pancreatic cancer, providing a potential therapeutic tool for both autoimmune diseases and cancers [113, 114].
Reprogramming antigen-presenting fibroblasts to enhance their immune-stimulatory properties represents another exciting frontier. Advances in genetic engineering have enabled the modification of fibroblasts to overexpress co-stimulatory molecules like CD86, thereby amplifying CD8+ T cell responses and improving the efficacy of immune checkpoint blockade therapies [10]. In vivo, vaccination strategies that deliver antigens directly to antigen-presenting fibroblasts or enhance their antigen-presentation capacity have been shown to promote local immune responses and systemic immune protection [115]. Additionally, inducing regulatory phenotypes in fibroblasts by stimulating the expression of molecules such as Gal-9 or Fas/FasL offers a novel approach for suppressing aberrant immune responses in autoimmune diseases [116].
The therapeutic potential of targeting antigen-presenting fibroblasts underscores the need for context-specific strategies. In cancers, the focus is on suppressing the immunosuppressive functions of antigen-presenting fibroblasts to enhance cytotoxic T cell activity, thereby promoting antitumor immunity [117-121]. Conversely, in autoimmune diseases, therapies aim to inhibit autoreactive T cell responses and restore immune tolerance [122-125]. These contrasting goals highlight the versatility of antigen-presenting fibroblasts-targeted therapies in addressing diverse pathological conditions.
As our understanding of antigen-presenting fibroblasts continues to evolve, advances in diagnostic tools to assess their activity in patient tissues will play a crucial role in guiding personalized treatment strategies. By precisely manipulating the biological functions of antigen-presenting fibroblasts, these cells can be transformed from disease mediators into therapeutic allies. Such progress holds significant promise for innovative treatments in cancer, autoimmune diseases, and beyond.
7. Conclusion
Fibroblasts have evolved from being regarded solely as structural components to key players in immune regulation, with their antigen-presenting capabilities redefining their role in pathology. Their dual functions underscore their significance in disease progression and therapeutic intervention. Understanding the molecular mechanisms and contextual cues that govern their antigen-presenting functions is essential for unlocking their full potential. This knowledge will not only deepen our comprehension of fibroblasts biology but also guide the development of innovative diagnostic tools and therapies, positioning fibroblasts at the forefront of immune modulation and disease management.
Abbreviations
MHC-II: major histocompatibility complex class II; APCs: antigen-presenting cells; SLE: systemic lupus erythematosus; DLE: discoid lupus erythematosus; HF: heart failure; DCM: dilated cardiomyopathy; RA: rheumatoid arthritis; NK: natural killer; Tregs: regulatory T cells; SNPs: single nucleotide polymorphisms; PBMCs: peripheral blood mononuclear cells; EMT: epithelial-mesenchymal transition; FRCs: fibroblastic reticular cells; OVA: ovalbumin; PAD2: peptidylarginine deiminase 2; FLS: fibroblast-like synoviocytes; CarLL37: carbamylated LL37; NETs: neutrophil extracellular traps; LA: lyme arthritis; GvHD: graft versus host disease; aGvHD: acute graft versus host disease; DLBCL: diffuse large B-cell lymphoma; CAFs: cancer-associated fibroblasts; ApCAFs: antigen-presenting cancer-associated fibroblasts; TAC: transverse aortic constriction; TNBC: triple-negative breast cancer; PDAC: pancreatic ductal adenocarcinoma; TT: tetanus toxoid; FAP: fibroblast activation protein; FAPI: fibroblast activation protein inhibitor; HCMV: human cytomegalovirus.
Acknowledgements
Figures were created using https://BioRender.com.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82473535, No. 82030097, No. 32141004), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2022-RC310-04), and the CAMS Innovation Fund for Medical Sciences (No. 2021-I2M-1-059).
Author contributions
Xiaoyun Chen wrote and revised the manuscript and completed the drawing of figures. Fangqi Chen wrote part of the manuscript and revised the manuscript. Sujie Jia and Qianjin Lu revised the manuscript and provided critical insights. Ming Zhao provided crucial advice, revised the manuscript, and oversaw the project. All authors read and approved the final paper.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Svensson-Arvelund J, Cuadrado-Castano S, Pantsulaia G, Kim K, Aleynick M, Hammerich L. et al. Expanding cross-presenting dendritic cells enhances oncolytic virotherapy and is critical for long-term anti-tumor immunity. Nat Commun. 2022;13:7149
2. Li Y, Zhang K, Wu Y, Yue Y, Cheng K, Feng Q. et al. Antigen capture and immune modulation by bacterial outer membrane vesicles as in situ vaccine for cancer immunotherapy post-photothermal therapy. Small. 2022;18:e2107461
3. Pishesha N, Harmand TJ, Ploegh HL. A guide to antigen processing and presentation. Nat Rev Immunol. 2022;22:751-764
4. Rastogi I, Jeon D, Moseman JE, Muralidhar A, Potluri HK, McNeel DG. Role of b cells as antigen presenting cells. Front Immunol. 2022;13:954936
5. Arbogast F, Arnold J, Hammann P, Kuhn L, Chicher J, Murera D. et al. Atg5 is required for b cell polarization and presentation of particulate antigens. Autophagy. 2019;15:280-294
6. Bowman-Kirigin JA, Desai R, Saunders BT, Wang AZ, Schaettler MO, Liu CJ. et al. The conventional dendritic cell 1 subset primes cd8+ t cells and traffics tumor antigen to drive antitumor immunity in the brain. Cancer Immunol Res. 2023;11:20-37
7. Bosteels C, Neyt K, Vanheerswynghels M, van Helden MJ, Sichien D, Debeuf N. et al. Inflammatory type 2 cdcs acquire features of cdc1s and macrophages to orchestrate immunity to respiratory virus infection. Immunity. 2020;52:1039-1056.e1039
8. Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA. et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9:1102-1123
9. Kerdidani D, Aerakis E, Verrou KM, Angelidis I, Douka K, Maniou MA. et al. Lung tumor mhcii immunity depends on in situ antigen presentation by fibroblasts. J Exp Med. 2022;219:e20210815
10. Geng S, Xiang T, Zhang Y, Guo P, Zhang H, Zhang Z. et al. Safe engineering of cancer-associated fibroblasts enhances checkpoint blockade immunotherapy. J Control Release. 2023;356:272-287
11. Wehrli M, Guinn S, Birocchi F, Kuo A, Sun Y, Larson RC. et al. Mesothelin car t cells secreting anti-fap/anti-cd3 molecules efficiently target pancreatic adenocarcinoma and its stroma. Clin Cancer Res. 2024;30:1859-1877
12. Umetsu DT, Pober JS, Jabara HH, Fiers W, Yunis EJ, Burakoff SJ. et al. Human dermal fibroblasts present tetanus toxoid antigen to antigen-specific t cell clones. J Clin Invest. 1985;76:254-260
13. Chen X, Wu Y, Jia S, Zhao M. Fibroblast: A novel target for autoimmune and inflammatory skin diseases therapeutics. Clin Rev Allergy Immunol. 2024;66:274-293
14. Zhou Y, Cao T, Li Z, Qiao H, Dang E, Shao S. et al. Fibroblasts in immune-mediated inflammatory diseases: The soil of inflammation. Clin Immunol. 2024;258:109849
15. Xu Z, Chen D, Hu Y, Jiang K, Huang H, Du Y. et al. Anatomically distinct fibroblast subsets determine skin autoimmune patterns. Nature. 2022;601:118-124
16. Cavagnero KJ, Li F, Dokoshi T, Nakatsuji T, O'Neill AM, Aguilera C. et al. Cxcl12+ dermal fibroblasts promote neutrophil recruitment and host defense by recognition of il-17. J Exp Med. 2024;221:e20231425
17. Rouse JR, Danner R, Wahhab A, Pereckas M, Nguyen C, McClune ME. et al. Hla-dr-expressing fibroblast-like synoviocytes are inducible antigen presenting cells that present autoantigens in lyme arthritis. ACR Open Rheumatol. 2024;6:678-689
18. Werner G, Sanyal A, Mirizio E, Hutchins T, Tabib T, Lafyatis R. et al. Single-cell transcriptome analysis identifies subclusters with inflammatory fibroblast responses in localized scleroderma. Int J Mol Sci. 2023;24:9796
19. Ngwenyama N, Kaur K, Bugg D, Theall B, Aronovitz M, Berland R. et al. Antigen presentation by cardiac fibroblasts promotes cardiac dysfunction. Nat Cardiovasc Res. 2022;1:761-774
20. Harryvan TJ, Visser M, de Bruin L, Plug L, Griffioen L, Mulder A. et al. Enhanced antigen cross-presentation in human colorectal cancer-associated fibroblasts through upregulation of the lysosomal protease cathepsin s. J Immunother Cancer. 2022;10:e003591
21. Huang H, Wang Z, Zhang Y, Pradhan RN, Ganguly D, Chandra R. et al. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory t cells in pancreatic cancer. Cancer Cell. 2022;40:656-673.e657
22. Sebastian A, Hum NR, Martin KA, Gilmore SF, Peran I, Byers SW. et al. Single-cell transcriptomic analysis of tumor-derived fibroblasts and normal tissue-resident fibroblasts reveals fibroblast heterogeneity in breast cancer. Cancers (Basel). 2020;12:1307
23. Tran CN, Davis MJ, Tesmer LA, Endres JL, Motyl CD, Smuda C. et al. Presentation of arthritogenic peptide to antigen-specific t cells by fibroblast-like synoviocytes. Arthritis Rheum. 2007;56:1497-1506
24. Kündig TM, Bachmann MF, DiPaolo C, Simard JJ, Battegay M, Lother H. et al. Fibroblasts as efficient antigen-presenting cells in lymphoid organs. Science. 1995;268:1343-1347
25. Chen J, Chen R, Huang J. A pan-cancer single-cell transcriptional analysis of antigen-presenting cancer-associated fibroblasts in the tumor microenvironment. Front Immunol. 2024;15:1372432
26. Gao Y, Li J, Cheng W, Diao T, Liu H, Bo Y. et al. Cross-tissue human fibroblast atlas reveals myofibroblast subtypes with distinct roles in immune modulation. Cancer Cell. 2024;42:1764-1783.e1710
27. Yin M, Sun L, Wu S, Ma J, Zhang W, Ji X. et al. Tgfβ-mediated inhibition of hypodermal adipocyte progenitor differentiation promotes wound-induced skin fibrosis. Cell Prolif. 2025;58:e13722
28. Sun L, Zhang X, Wu S, Liu Y, Guerrero-Juarez CF, Liu W. et al. Dynamic interplay between il-1 and wnt pathways in regulating dermal adipocyte lineage cells during skin development and wound regeneration. Cell Rep. 2023;42:112647
29. Miatello J, Lukaszewicz AC, Carter MJ, Faivre V, Hua S, Martinet KZ. et al. Ciita promoter polymorphism impairs monocytes hla-dr expression in patients with septic shock. iScience. 2022;25:105291
30. Zika E, Fauquier L, Vandel L, Ting JP. Interplay among coactivator-associated arginine methyltransferase 1, cbp, and ciita in ifn-gamma-inducible mhc-ii gene expression. Proc Natl Acad Sci U S A. 2005;102:16321-16326
31. Wijdeven RH, van Luijn MM, Wierenga-Wolf AF, Akkermans JJ, van den Elsen PJ, Hintzen RQ. et al. Chemical and genetic control of ifnγ-induced mhcii expression. EMBO Rep. 2018;19:e45553
32. Steinparzer I, Sedlyarov V, Rubin JD, Eislmayr K, Galbraith MD, Levandowski CB. et al. Transcriptional responses to ifn-γ require mediator kinase-dependent pause release and mechanistically distinct cdk8 and cdk19 functions. Mol Cell. 2019;76:485-499.e488
33. Zhao S, Grieshaber-Bouyer R, Rao DA, Kolb P, Chen H, Andreeva I. et al. Effect of jak inhibition on the induction of proinflammatory hla-dr+cd90+ rheumatoid arthritis synovial fibroblasts by interferon-γ. Arthritis Rheumatol. 2022;74:441-452
34. Xing E, Ma F, Wasikowski R, Billi AC, Gharaee-Kermani M, Fox J. et al. Pansclerotic morphea is characterized by ifn-γ responses priming dendritic cell fibroblast crosstalk to promote fibrosis. JCI Insight. 2023;8:e171307
35. Tsuchiya H, Ota M, Sumitomo S, Ishigaki K, Suzuki A, Sakata T. et al. Parsing multiomics landscape of activated synovial fibroblasts highlights drug targets linked to genetic risk of rheumatoid arthritis. Ann Rheum Dis. 2021;80:440-450
36. Tang ML, Khan MK, Croxford JL, Tan KW, Angeli V, Gasser S. The DNA damage response induces antigen presenting cell-like functions in fibroblasts. Eur J Immunol. 2014;44:1108-1118
37. Lyu L, Jiang Y, Ma W, Li H, Liu X, Li L. et al. Single-cell sequencing of pit1-positive pituitary adenoma highlights the pro-tumour microenvironment mediated by ifn-γ-induced tumour-associated fibroblasts remodelling. Br J Cancer. 2023;128:1117-1133
38. Affo S, Nair A, Brundu F, Ravichandra A, Bhattacharjee S, Matsuda M. et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell. 2021;39:866-882.e811
39. Fernando R, Caldera O, Smith TJ. Therapeutic igf-i receptor inhibition alters fibrocyte immune phenotype in thyroid-associated ophthalmopathy. Proc Natl Acad Sci U S A. 2021;118:e2114244118
40. Joffe AM, Bakalar MH, Fletcher DA. Macrophage phagocytosis assay with reconstituted target particles. Nat Protoc. 2020;15:2230-2246
41. Tufan T, Comertpay G, Villani A, Nelson GM, Terekhova M, Kelley S. et al. Rapid unleashing of macrophage efferocytic capacity via transcriptional pause release. Nature. 2024;628:408-415
42. Yang C, Zhang F, Chen F, Chang Z, Zhao Y, Shao D. et al. Biomimetic nanovaccines potentiating dendritic cell internalization via cxcr4-mediated macropinocytosis. Adv Healthc Mater. 2023;12:e2202064
43. Zhang B, Choi IK. Facts and hopes in the relationship of ebv with cancer immunity and immunotherapy. Clin Cancer Res. 2022;28:4363-4369
44. Vatti A, Monsalve DM, Pacheco Y, Chang C, Anaya JM, Gershwin ME. Original antigenic sin: A comprehensive review. J Autoimmun. 2017;83:12-21
45. Le Fournis C, Hadjichristou C, Jeanneau C, About I. Human pulp fibroblast implication in phagocytosis via complement activation. J Endod. 2019;45:584-590
46. Justynski O, Bridges K, Krause W, Forni MF, Phan QM, Sandoval-Schaefer T. et al. Apoptosis recognition receptors regulate skin tissue repair in mice. Elife. 2023 12
47. Romana-Souza B, Chen L, Leonardo TR, Chen Z, DiPietro LA. Dermal fibroblast phagocytosis of apoptotic cells: A novel pathway for wound resolution. Faseb j. 2021;35:e21443
48. Lee Y, Kim S, Seo J, Kim HK, Han YP, Park EJ. et al. Fibroblast-targeting polymeric nanovehicles to enhance topical wound healing through promotion of par-2 receptor-mediated endocytosis. Biomater Sci. 2023;11:450-460
49. Synowiec A, Dąbrowska A, Pachota M, Baouche M, Owczarek K, Niżański W. et al. Feline herpesvirus 1 (fhv-1) enters the cell by receptor-mediated endocytosis. J Virol. 2023;97:e0068123
50. Zhang Y, Recouvreux MV, Jung M, Galenkamp KMO, Li Y, Zagnitko O. et al. Macropinocytosis in cancer-associated fibroblasts is dependent on camkk2/arhgef2 signaling and functions to support tumor and stromal cell fitness. Cancer Discov. 2021;11:1808-1825
51. Farina A, Cirone M, York M, Lenna S, Padilla C, McLaughlin S. et al. Epstein-barr virus infection induces aberrant tlr activation pathway and fibroblast-myofibroblast conversion in scleroderma. J Invest Dermatol. 2014;134:954-964
52. Yang C, Luo Y, Shen H, Ge M, Tang J, Wang Q. et al. Inorganic nanosheets facilitate humoral immunity against medical implant infections by modulating immune co-stimulatory pathways. Nat Commun. 2022;13:4866
53. Jeannin P, Magistrelli G, Aubry JP, Caron G, Gauchat JF, Renno T. et al. Soluble cd86 is a costimulatory molecule for human t lymphocytes. Immunity. 2000;13:303-312
54. Peng S, Yan Y, Ogino K, Ma G, Xia Y. Spatiotemporal coordination of antigen presentation and co-stimulatory signal for enhanced anti-tumor vaccination. J Control Release. 2024;374:312-324
55. Corrigall VM, Solau-Gervais E, Panayi GS. Lack of cd80 expression by fibroblast-like synoviocytes leading to anergy in t lymphocytes. Arthritis Rheum. 2000;43:1606-1615
56. Orlik C, Deibel D, Küblbeck J, Balta E, Ganskih S, Habicht J. et al. Keratinocytes costimulate naive human t cells via cd2: A potential target to prevent the development of proinflammatory th1 cells in the skin. Cell Mol Immunol. 2020;17:380-394
57. Simmons D, Makgoba MW, Seed B. Icam, an adhesion ligand of lfa-1, is homologous to the neural cell adhesion molecule ncam. Nature. 1988;331:624-627
58. Li S, Wang H, Peng B, Zhang M, Zhang D, Hou S. et al. Efalizumab binding to the lfa-1 alphal i domain blocks icam-1 binding via steric hindrance. Proc Natl Acad Sci U S A. 2009;106:4349-4354
59. Hutton AJ, Polak ME, Spalluto CM, Wallington JC, Pickard C, Staples KJ. et al. Human lung fibroblasts present bacterial antigens to autologous lung th cells. J Immunol. 2017;198:110-118
60. Poggi A, Prevosto C, Zancolli M, Canevali P, Musso A, Zocchi MR. Nkg2d and natural cytotoxicity receptors are involved in natural killer cell interaction with self-antigen presenting cells and stromal cells. Ann N Y Acad Sci. 2007;1109:47-57
61. Li P, Lu M, Shi J, Gong Z, Hua L, Li Q. et al. Lung mesenchymal cells elicit lipid storage in neutrophils that fuel breast cancer lung metastasis. Nat Immunol. 2020;21:1444-1455
62. Pinchuk IV, Beswick EJ, Saada JI, Boya G, Schmitt D, Raju GS. et al. Human colonic myofibroblasts promote expansion of cd4+ cd25high foxp3+ regulatory t cells. Gastroenterology. 2011;140:2019-2030
63. Varveri A, Papadopoulou M, Papadovasilakis Z, Compeer EB, Legaki AI, Delis A. et al. Immunological synapse formation between t regulatory cells and cancer-associated fibroblasts promotes tumour development. Nat Commun. 2024;15:4988
64. Apollonio B, Spada F, Petrov N, Cozzetto D, Papazoglou D, Jarvis P. et al. Tumor-activated lymph node fibroblasts suppress t cell function in diffuse large b cell lymphoma. J Clin Invest. 2023;133:e166070
65. Wang W, Li T, Xie Z, Zhao J, Zhang Y, Ruan Y. et al. Integrating single-cell and bulk rna sequencing data unveils antigen presentation and process-related cafs and establishes a predictive signature in prostate cancer. J Transl Med. 2024;22:57
66. Chai C, Liang L, Mikkelsen NS, Wang W, Zhao W, Sun C. et al. Single-cell transcriptome analysis of epithelial, immune, and stromal signatures and interactions in human ovarian cancer. Commun Biol. 2024;7:131
67. Xu K, Wang H, Zou YX, Zhang HH, Wang YN, Ren XR. et al. Distinct fibroblast subpopulations associated with bone, brain or intrapulmonary metastasis in advanced non-small-cell lung cancer. Clin Transl Med. 2024;14:e1605
68. Corry SM, McCorry AM, Lannagan TR, Leonard NA, Fisher NC, Byrne RM. et al. Activation of innate-adaptive immune machinery by poly(i:C) exposes a therapeutic vulnerability to prevent relapse in stroma-rich colon cancer. Gut. 2022;71:2502-2517
69. Pei S, Sjölund J, Pan Y, Pietras K, Karlsson MCI. Cancer-associated fibroblasts express cd1d and activate invariant natural killer t cells under cellular stress. Cell Mol Immunol. 2024;21:91-94
70. Pal D, Ghatak S, Singh K, Abouhashem AS, Kumar M, El Masry MS. et al. Identification of a physiologic vasculogenic fibroblast state to achieve tissue repair. Nat Commun. 2023;14:1129
71. Plikus MV, Guerrero-Juarez CF, Ito M, Li YR, Dedhia PH, Zheng Y. et al. Regeneration of fat cells from myofibroblasts during wound healing. Science. 2017;355:748-752
72. Kaur A, Ecker BL, Douglass SM, Kugel CH 3rd, Webster MR, Almeida FV. et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 2019;9:64-81
73. Zhang J, Yu H, Man MQ, Hu L. Aging in the dermis: Fibroblast senescence and its significance. Aging Cell. 2024;23:e14054
74. Hasegawa T, Oka T, Son HG, Oliver-García VS, Azin M, Eisenhaure TM. et al. Cytotoxic cd4(+) t cells eliminate senescent cells by targeting cytomegalovirus antigen. Cell. 2023;186:1417-1431.e1420
75. Pereira BI, Devine OP, Vukmanovic-Stejic M, Chambers ES, Subramanian P, Patel N. et al. Senescent cells evade immune clearance via hla-e-mediated nk and cd8(+) t cell inhibition. Nat Commun. 2019;10:2387
76. Gur C, Wang SY, Sheban F, Zada M, Li B, Kharouf F. et al. Lgr5 expressing skin fibroblasts define a major cellular hub perturbed in scleroderma. Cell. 2022;185:1373-1388.e1320
77. Deng CC, Hu YF, Zhu DH, Cheng Q, Gu JJ, Feng QL. et al. Single-cell rna-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases. Nat Commun. 2021;12:3709
78. He Y, Tsou PS, Khanna D, Sawalha AH. Methyl-cpg-binding protein 2 mediates antifibrotic effects in scleroderma fibroblasts. Ann Rheum Dis. 2018;77:1208-1218
79. De Palma R, Del Galdo F, Lupoli S, Altucci P, Abbate G, Valentini G. Peripheral t lymphocytes from patients with early systemic sclerosis co-cultured with autologous fibroblasts undergo an oligoclonal expansion similar to that occurring in the skin. Clin Exp Immunol. 2006;144:169-176
80. Zheng M, Hu Z, Mei X, Ouyang L, Song Y, Zhou W. et al. Single-cell sequencing shows cellular heterogeneity of cutaneous lesions in lupus erythematosus. Nat Commun. 2022;13:7489
81. Smith MH, Gao VR, Periyakoil PK, Kochen A, DiCarlo EF, Goodman SM. et al. Drivers of heterogeneity in synovial fibroblasts in rheumatoid arthritis. Nat Immunol. 2023;24:1200-1210
82. Carmona-Rivera C, Carlucci PM, Moore E, Lingampalli N, Uchtenhagen H, James E. et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol. 2017;2:eaag3358
83. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS. et al. Nets are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5:178ra140
84. Carmona-Rivera C, Carlucci PM, Goel RR, James E, Brooks SR, Rims C. et al. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight. 2020;5:e139388
85. O'Neil LJ, Oliveira CB, Sandoval-Heglund D, Barrera-Vargas A, Merayo-Chalico J, Aguirre-Aguilar E. et al. Anti-carbamylated ll37 antibodies promote pathogenic bone resorption in rheumatoid arthritis. Front Immunol. 2021;12:715997
86. Liu X, Burke RM, Lighthouse JK, Baker CD, Dirkx RA Jr, Kang B. et al. P53 regulates the extent of fibroblast proliferation and fibrosis in left ventricle pressure overload. Circ Res. 2023;133:271-287
87. Wang Y, Li Q, Tao B, Angelini M, Ramadoss S, Sun B. et al. Fibroblasts in heart scar tissue directly regulate cardiac excitability and arrhythmogenesis. Science. 2023;381:1480-1487
88. Fan X, Huang K, Wu Y, Jin S, Pang L, Wang Y. et al. A specific inflammatory suppression fibroblast subpopulation characterized by mhcii expression in human dilated cardiomyopathy. Mol Cell Biochem. 2025;480:325-340
89. Honan AM, Vazquez EN, Chen Z. Lymph node stromal cell-intrinsic mhc class ii expression promotes mhc class i-restricted cd8 t cell lineage conversion to regulatory cd4 t cells. J Immunol. 2021;207:1530-1544
90. Abe J, Shichino S, Ueha S, Hashimoto S, Tomura M, Inagaki Y. et al. Lymph node stromal cells negatively regulate antigen-specific cd4+ t cell responses. J Immunol. 2014;193:1636-1644
91. Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS. et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207:689-697
92. Ohyama H, Nishimura F, Meguro M, Takashiba S, Murayama Y, Matsushita S. Counter-antigen presentation: Fibroblasts produce cytokines by signalling through hla class ii molecules without inducing t-cell proliferation. Cytokine. 2002;17:175-181
93. Dominguez CX, Müller S, Keerthivasan S, Koeppen H, Hung J, Gierke S. et al. Single-cell rna sequencing reveals stromal evolution into lrrc15(+) myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 2020;10:232-253
94. Saada JI, Pinchuk IV, Barrera CA, Adegboyega PA, Suarez G, Mifflin RC. et al. Subepithelial myofibroblasts are novel nonprofessional apcs in the human colonic mucosa. J Immunol. 2006;177:5968-5979
95. Pinchuk IV, Beswick EJ, Saada JI, Suarez G, Winston J, Mifflin RC. et al. Monocyte chemoattractant protein-1 production by intestinal myofibroblasts in response to staphylococcal enterotoxin a: Relevance to staphylococcal enterotoxigenic disease. J Immunol. 2007;178:8097-8106
96. Owens BM, Steevels TA, Dudek M, Walcott D, Sun MY, Mayer A. et al. Cd90(+) stromal cells are non-professional innate immune effectors of the human colonic mucosa. Front Immunol. 2013;4:307
97. Shaikh H, Pezoldt J, Mokhtari Z, Gamboa Vargas J, Le DD, Peña Mosca J. et al. Fibroblastic reticular cells mitigate acute gvhd via mhcii-dependent maintenance of regulatory t cells. JCI Insight. 2022;7:e154250
98. Johnson DB, Estrada MV, Salgado R, Sanchez V, Doxie DB, Opalenik SR. et al. Melanoma-specific mhc-ii expression represents a tumour-autonomous phenotype and predicts response to anti-pd-1/pd-l1 therapy. Nat Commun. 2016;7:10582
99. Hintz HM, Gallant JP, Vander Griend DJ, Coleman IM, Nelson PS, LeBeau AM. Imaging fibroblast activation protein alpha improves diagnosis of metastatic prostate cancer with positron emission tomography. Clin Cancer Res. 2020;26:4882-4891
100. Gao Z, Yan L, Meng J, Lu Z, Ge K, Jiang Z. et al. Targeting cardiac fibrosis with chimeric antigen receptor macrophages. Cell Discov. 2024;10:86
101. Zhao Y, Jia Y, Wang J, Chen X, Han J, Zhen S. et al. Circnox4 activates an inflammatory fibroblast niche to promote tumor growth and metastasis in nsclc via fap/il-6 axis. Mol Cancer. 2024;23:47
102. Peltier A, Seban RD, Buvat I, Bidard FC, Mechta-Grigoriou F. Fibroblast heterogeneity in solid tumors: From single cell analysis to whole-body imaging. Semin Cancer Biol. 2022;86:262-272
103. Mori Y, Dendl K, Cardinale J, Kratochwil C, Giesel FL, Haberkorn U. Fapi pet: Fibroblast activation protein inhibitor use in oncologic and nononcologic disease. Radiology. 2023;306:e220749
104. Venkatraman P, Nguyen TT, Sainlos M, Bilsel O, Chitta S, Imperiali B. et al. Fluorogenic probes for monitoring peptide binding to class ii mhc proteins in living cells. Nat Chem Biol. 2007;3:222-228
105. Xia Q, Zhang FF, Geng F, Liu CL, Xu P, Lu ZZ. et al. Anti-tumor effects of DNA vaccine targeting human fibroblast activation protein α by producing specific immune responses and altering tumor microenvironment in the 4t1 murine breast cancer model. Cancer Immunol Immunother. 2016;65:613-624
106. Duperret EK, Trautz A, Ammons D, Perales-Puchalt A, Wise MC, Yan J. et al. Alteration of the tumor stroma using a consensus DNA vaccine targeting fibroblast activation protein (fap) synergizes with antitumor vaccine therapy in mice. Clin Cancer Res. 2018;24:1190-1201
107. Tang L, Qiu H, Xu B, Su Y, Nyarige V, Li P. et al. Microparticle mediated delivery of apelin improves heart function in post myocardial infarction mice. Circ Res. 2024;135:777-798
108. Jenkins L, Jungwirth U, Avgustinova A, Iravani M, Mills A, Haider S. et al. Cancer-associated fibroblasts suppress cd8+ t-cell infiltration and confer resistance to immune-checkpoint blockade. Cancer Res. 2022;82:2904-2917
109. Millul J, Bassi G, Mock J, Elsayed A, Pellegrino C, Zana A. et al. An ultra-high-affinity small organic ligand of fibroblast activation protein for tumor-targeting applications. Proc Natl Acad Sci U S A. 2021;118:2101852118
110. Sakemura R, Hefazi M, Siegler EL, Cox MJ, Larson DP, Hansen MJ. et al. Targeting cancer-associated fibroblasts in the bone marrow prevents resistance to cart-cell therapy in multiple myeloma. Blood. 2022;139:3708-3721
111. Wang LC, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V. et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor t cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. 2014;2:154-166
112. Loureiro LR, Hoffmann L, Neuber C, Rupp L, Arndt C, Kegler A. et al. Immunotheranostic target modules for imaging and navigation of unicar t-cells to strike fap-expressing cells and the tumor microenvironment. J Exp Clin Cancer Res. 2023;42:341
113. Salazar-Puerta AI, Rincon-Benavides MA, Cuellar-Gaviria TZ, Aldana J, Vasquez Martinez G, Ortega-Pineda L. et al. Engineered extracellular vesicles derived from dermal fibroblasts attenuate inflammation in a murine model of acute lung injury. Adv Mater. 2023;35:e2210579
114. Yao H, Huang C, Zou J, Liang W, Zhao Y, Yang K. et al. Extracellular vesicle-packaged lncrna from cancer-associated fibroblasts promotes immune evasion by downregulating hla-a in pancreatic cancer. J Extracell Vesicles. 2024;13:e12484
115. Cupovic J, Ring SS, Onder L, Colston JM, Lütge M, Cheng HW. et al. Adenovirus vector vaccination reprograms pulmonary fibroblastic niches to support protective inflating memory cd8(+) t cells. Nat Immunol. 2021;22:1042-1051
116. Liu Y, Rao P, Qian H, Shi Y, Chen S, Lan J. et al. Regulatory fibroblast-like synoviocytes cell membrane coated nanoparticles: a novel targeted therapy for rheumatoid arthritis. Adv Sci (Weinh). 2023;10:e2204998
117. Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398:1002-1014
118. Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T. et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577-581
119. Park J, Hsueh PC, Li Z, Ho PC. Microenvironment-driven metabolic adaptations guiding cd8(+) t cell anti-tumor immunity. Immunity. 2023;56:32-42
120. Li H, Chen J, Li Z, Chen M, Ou Z, Mo M. et al. S100a5 attenuates efficiency of anti-pd-l1/pd-1 immunotherapy by inhibiting cd8(+) t cell-mediated anti-cancer immunity in bladder carcinoma. Adv Sci (Weinh). 2023;10:e2300110
121. Huang Y, Qin G, Cui T, Zhao C, Ren J, Qu X. A bimetallic nanoplatform for sting activation and crispr/cas mediated depletion of the methionine transporter in cancer cells restores anti-tumor immune responses. Nat Commun. 2023;14:4647
122. Michelson DA, Hase K, Kaisho T, Benoist C, Mathis D. Thymic epithelial cells co-opt lineage-defining transcription factors to eliminate autoreactive t cells. Cell. 2022;185:2542-2558.e2518
123. Zhang B, Wang Y, Yuan Y, Sun J, Liu L, Huang D. et al. In vitro elimination of autoreactive b cells from rheumatoid arthritis patients by universal chimeric antigen receptor t cells. Ann Rheum Dis. 2021;80:176-184
124. Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ. et al. Reengineering chimeric antigen receptor t cells for targeted therapy of autoimmune disease. Science. 2016;353:179-184
125. Kenison JE, Stevens NA, Quintana FJ. Therapeutic induction of antigen-specific immune tolerance. Nat Rev Immunol. 2024;24:338-357
Author contact
![]() Corresponding author: Ming Zhao; zhaoming301cams.cn.
Corresponding author: Ming Zhao; zhaoming301cams.cn.
 Global reach, higher impact
Global reach, higher impact