13.3
Impact Factor
Theranostics 2025; 15(6):2510-2522. doi:10.7150/thno.94521 This issue Cite
Research Paper
First-in-human study of an optimized, potential kit-type, SSTR antagonist 68Ga-DATA5m-LM4 in patients with metastatic neuroendocrine tumors
1. Department of Diagnostic Radiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
2. Theranostics Center of Excellence, Yong Loo Lin School of Medicine, National University of Singapore, 11 Biopolis Way, Helios, Singapore 138667, Singapore.
3. Clinical Imaging Research Centre, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
4. Nanomedicine Translational Research Program, NUS Center for Nanomedicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
5. Curanosticum Wiesbaden-Frankfurt, Center for Advanced Radiomolecular Precision Oncology, Wiesbaden, Germany.
6. Academy for Precision Oncology, International Centers for Precision Oncology (ICPO), Wiesbaden, Germany.
7. Department of Radiology, New York University Langone Medical Center, New York, NY, USA.
8. Department of Chemistry, TRIGA, Johannes Gutenberg University, Mainz, Germany.
*Contributed equally to this work
Received 2024-1-21; Accepted 2024-12-18; Published 2025-1-20
Abstract
Radiolabeled somatostatin receptor (SSTR) agonists 68Ga-DOTA-TATE and 68Ga-DOTA-TOC are widely applied for imaging of patients with neuroendocrine tumors (NETs). Preclinical and preliminary clinical evidence has indicated that SSTR antagonists perform better for NET imaging. In this study, we assessed the feasibility of using a new hybrid chelator DATA5m ((6-pentanoic acid)-6-(amino)methyl-1,4-diazepinetriacetate))-conjugated kit-type SSTR antagonist 68Ga-DATA5m-LM4 for PET and evaluated the safety, biodistribution, and preliminary diagnostic efficacy of 68Ga-DATA5m-LM4 in patients with metastatic NETs.
Methods: The DATA5m-conjugated form of LM4, was labeled with 68Ga. A total of 27 patients (19 men/8 women; mean age 61 years) with histopathologically confirmed well-differentiated NETs underwent 68Ga-DATA5m-LM4 PET/CT for the staging and restaging or patient selection for PRRT. All the patients underwent PET/CT scans 60 min after intravenous bolus injection of 1.85 MBq (0.05 mCi) per kilogram of body weight (151 ± 54 MBq mean ± SD) of 68Ga-DATA5m-LM4.
Results: DATA5m-LM4 was successfully labeled with 68Ga, achieving high yield and purity. After decay correction, radiochemical yields (RCYs) of 80-95% and radiochemical purities (RCP) greater than 98% were obtained. 68Ga -DATA5m-LM4 was well tolerated in all patients, without clinically relevant adverse effects. A significantly lower uptake in normal liver parenchyma was observed with 68Ga-DATA5m-LM4 compared to 68Ga-DOTA-TATE PET/CT (3.90 ± 0.88 vs. 9.12 ± 3.64, P < 0.000001). Additionally, uptake in the thyroid gland, pancreas, and spleen was also lower (P < 0.05). 14 patients underwent 68Ga-DOTA-TOC PET/CT. 68Ga-DATA5m-LM4 uptakes in the liver and spleen were significantly lower than those of 68Ga-DOTA-TOC uptake (3.70 ± 0.79 vs. 5.33 ± 2.43, P = 0.0397; 11.88 ± 6.88 vs. 26.55 ± 16.07, P = 0.0022). Tumor lesions showed high uptake intensity on 68Ga-DATA5m-LM4 PET/CT, with the highest SUVmax up to 167.93 (mean ± SD, 44.47 ± 36.22). With SUVmean of healthy liver, kidneys, and blood pool as background to normalize the SUVmax of the single most intense lesion, tumor-to-background ratios were 20.32 ± 19.97 (range, 3.40 - 98.78) and 4.30 ± 3.03 (range, 0.65 - 14.70), 38.63 ± 35.97 (range, 4.1 - 173.12), respectively.
Conclusion: This study demonstrated that the novel SSTR antagonist 68Ga-DATA5m-LM4 can be efficiently labeled with high radiochemical yield and purity, supported by a highly convenient production process. The tracer exhibited excellent imaging performance, with a highly favorable biodistribution characterized by high tumor contrast and minimal uptake in normal organs, particularly the liver, enabling superior lesion detection. The practical advantages of this straightforward labeling process, achieved without any apparent loss in diagnostic efficacy, offer a significant benefit over other competing antagonists. The ease of production, including the potential for a “kit-type” labeling method, makes 68Ga-DATA5m-LM4 an overall extraordinarily promising radiopharmaceutical for the staging and restaging of NET patients.
Keywords: SSTR antagonist, 68Ga-DATA5m-LM4, first-in-human, neuroendocrine tumors (NETs), somatostatin receptor, PET/CT
Introduction
Neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms arising with predominantly neural and endocrine differentiation that are often able to produce hormones and other biologically active substances (1, 2), arising from any organ where endocrine cells are present and are most commonly found in the gastrointestinal tract, pancreas, and lung (1, 3, 4). To reflect prognosis, NETs are also divided into histologically well- or poorly differentiated (5).
Neuroendocrine tumors are characterized by overexpression of the somatostatin receptor (SSTR), especially SSTR subtype 2 (SSTR2), making them accessible for radiodiagnostic and therapeutic strategies. Currently, Gallium-68 (68Ga) labeled SSTR-targeted somatostatin analogs have been used for positron emission tomography-computed tomography (PET/CT) in routine clinical practice for the diagnosis and management of NETs. The U.S. Food and Drug Administration, as well as the European Medicines Agency, have approved 68Ga-labeled [68Ga]Ga-DOTA-TATE and [68Ga]Ga-DOTA-TOC for imaging of SSTR positive neuroendocrine tumors.
DOTA-TOC and DOTA-TATE are comprised of the DOTA-chelator conjugated to 8 cyclized amino acids with high affinity for SSTR2, for which they act as agonists. SSTR agonists readily internalize into tumor cells, allowing tracer accumulation in the target cells. Recent studies have shown that SSTR antagonists are likely to perform better in certain patients, especially with low SSTR2 expression (6, 7). Accordingly, compared to SSTR2 agonist radiotracers complementary SSTR2 antagonist radiotracers such as 68Ga labeled, DOTA or NODAGA chelator conjugated to JR11 and LM3 (JR11 = p-Cl-Phe-cyclo(D-Cys-Aph(Hor)-D-Aph(cbm)-Lys-Thr-Cys)D-Tyr-NH2; NODAGA = 1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid; LM3 = p-Cl-Phe-cyclo[D-Cys-Tyr-D-4-amino-Phe(carbamoyl)-Lys-Thr-Cys]D-Tyr-NH2 have shown higher uptake in preclinical and clinical settings (6, 8-12).
However, there might be options for better-performing SSTR2-antagonists. The chelator and radioisotope greatly influence the affinity and pharmacokinetics of SSTR radiotracers, for example, by lipophilicity and charge of the molecule. The chelator DOTA, for example, is not well suited for complexing the relatively small (radio) metal Gallium and necessitates elevated reaction temperature which is not only detrimental for many antibodies but also small and heat-sensitive biomolecules. Furthermore, often longer reactions are necessary, and additionally, time is required for cooling before intravenous injection, thereby imposing limitations for clinical use due to the short half-life of 67.7 min. DATA (6-amino-1,4-diazepine-triacetic acid) is a novel type of chelator exhibiting cyclic, acyclic, and inter-mediate properties, which have advantageous properties for 68Ga-labeling compared with established chelators. In particular, DATA can afford a more rapid quantitative radiolabeling with 68Ga at ambient temperature in a less acidic pH range (13). Furthermore, the related chelate AAZTA provides the option for labeling with therapeutic isotopes such as 177Lu (14).
In this study, we present another promising candidate for SSTR-antagonist imaging, a novel DATA conjugated SSTR antagonist PET tracer LM4, designated as 68Ga-DATA5m-LM4. This first-in-human study aimed to establish proof-of-concept and evaluate the feasibility of using this novel SSTR antagonist 68Ga-DATA5m-LM4 for PET/CT in clinical use. We assessed the production, safety, biodistribution and preliminary diagnostic efficacy of 68Ga-DATA5m-LM4 in patients with metastatic NETs.
Materials and Methods
Patients
From September 2020 to January 2022, twenty-seven patients (19 men /8 women; mean age, 61.0 ± 12.1 years; age range, 38-82 y) with histopathologically confirmed well-differentiated metastatic NETs, who underwent 68Ga-DATA5m-LM4 PET/CT for disease staging and restaging, were included in this study. All procedures involving human participants were conducted in compliance with the German Medicinal Products Act (section 13, subsection 2b), the 1964 Declaration of Helsinki, and the responsible regulatory body. The study was performed in accordance with German regulations (Federal Agency for Radiation Protection) concerning radiation safety and was approved by the responsible ethical committee for data collection and analysis (Curanosticum Wiesbaden-Frankfurt, Germany). All patients signed a detailed informed consent form and consented to the use of their anonymized clinical data for scientific purposes. To avoid the influence of cold somatostatin analog treatment on imaging, PET scans were scheduled 3-4 weeks after the last dose of long-acting somatostatin analogs. The baseline demographics of the patients are shown in Tables 1 and 2.
Demographic and Characteristics of Patients with NETs (n = 27)
| Characteristic | Number (n) | Percentage (%) |
|---|---|---|
| Age at scanning (y) | ||
| Mean ± SD | 61.0 ± 12.1 | |
| Range | 38 - 82 | |
| Sex | ||
| Male | 19 | |
| Female | 8 | |
| Primary tumor site | ||
| Pancreas | 12 | 44.4 |
| Ileum | 9 | 33.3 |
| Duodenum | 1 | 3.7 |
| Midgut | 1 | 3.7 |
| Others | 4 | 14.8 |
| Ki-67 grading | ||
| G1 | 6 | 22.2 |
| G2 | 15 | 55.6 |
| G3 | 3 | 11.1 |
| Ki-67 not available | 3 | 11.1 |
| 68Ga-DATA5m-LM4 PET imaging | ||
| Liver mts | 22 | 81.5 |
| LN mts | 15 | 55.6 |
| Bone mts | 11 | 40.7 |
| Other mts | 13 | 48.1 |
Synthesis and radiolabeling
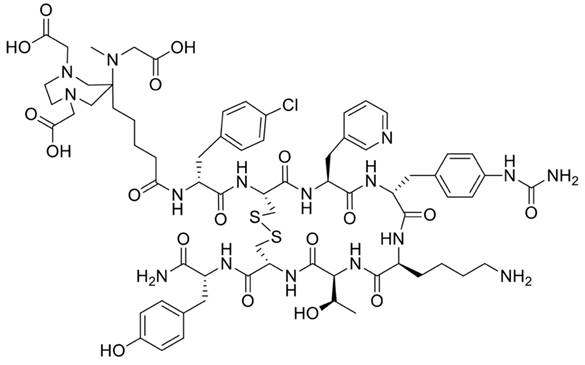
The precursor DATA5m-LM4, designated for labeling of 68Ga and targeting SSTR2, consists of the chelate ((6-pentanoic acid)-6-(amino)methyl-1,4-diazepinetriacetate)) and is conjugated to the SSTR2 ligand LM4 = p-Cl-Phe-cyclo[DCys-Pal-Daph(Cbm)-Lys-Thr-Cys]DTyr-NH2 with Pal = Pyridylalanine using a peptide bond (Figure 1). Synthesis was performed as previously described (15, 16).
68Ga was eluted from a 68Ge/68Ga generator (Eckhardt und Ziegler) using 0.1M HCl and mixed with sodium acetate buffer to adjust the pH value. 50 µg of the precursor DATA5m‑LM4 was added to 1000 µL sodium acetate buffer (pH 5.0), which was then added to the generator eluate. Quality control was performed using analytical high-performance liquid chromatography and thin-layer chromatography.
PET/CT imaging and evaluation
All the patients underwent PET/CT scans 60 min after intravenous bolus injection of 1.85 MBq (0.05 mCi) per kilogram of body weight (151 ± 54 MBq mean ± SD) of 68Ga-DATA5m-LM4. Patients received between 0.08 and 0.20 µg/MBq of the peptide which leads to values between 0.14 µg/kg and 0.44 µg/kg. One hour after intravenous administration, PET/CT images were acquired from the vertex to the proximal femora on a Biograph Vision 600 Edge (Siemens Healthineers). A low-dose CT scan was acquired with an automatic tube voltage selection and current modulation using CARE kV and CARE Dose4D (Siemens Healthcare). PET scanning was performed using continuous bed motion, with an average imaging speed of 0.8 mm/sec in the caudocranial direction, resulting in a scan time of approx. 20 min from the vertex to the proximal thighs. PET image reconstruction was performed using point spread function (TrueX) and time of flight modeling, four iterations with five subsets, no filtering was applied (“all-pass”). The images were transferred to an MMWP workstation (Siemens) for analysis. The vital parameters of the patients were measured, and routine blood tests, liver function, and renal function were examined. Any possible side effects during 68Ga-DATA5m-LM4 PET/CT scanning and within 1 week after the examination were collected and analyzed.
Patients received another 68Ga-SSTR PET/CT with 68Ga-DOTA-TOC/TATE and/or 68Ga-NODAGA-LM3 within 9 months, with PET/CT imaging protocols the same as those for 68Ga-DATA5m-LM4. Doses were calculated according to the participant's body weight (1.8-2.2 MBq/kg). All PET images were evaluated by two board-certified and experienced nuclear medicine physicians, without being blinded to the medical history of the patients, on the reconstructed images (Affinity Viewer, Hermes Medical Solutions). Disagreements were resolved via consensus.
Visual analysis was used to determine the general biodistribution and the temporal and inter-subject stability. The volume of interest of normal organs/tissues and concerned lesions were drawn on the serial images. The radioactivity concentration and standardized uptake value (SUV) in the volumes of interest were obtained through the software. The physiologic uptake of 68Ga-DATA5m-LM4 was evaluated in the brain, pituitary gland, parotid gland, thyroid gland, lungs, blood pool, liver, spleen, pancreas, stomach, small intestine, kidneys, adrenal glands, red marrow (vertebrae), and muscle.
Regions of interest were drawn manually on the site of lesions using a 3-dimensional ellipsoid iso-contour on each image with the assistance of the corresponding CT images. Any reasonably non-physiologic focal accumulations were interpreted as tumor lesions. Lesion uptake was measured using SUVmax. Targeted lesions were chosen in each patient, including the lesion with the highest uptake and the second-highest uptake of the tumors. Tumor-to-background ratio (TBR) was quantified using the SUVmean of healthy liver parenchyma, blood pool, and kidney as background reference tissues.
Statistical analysis
Statistical analysis was performed with Prism 5.0 software (Graph-Pad). Continuous variables were summarized as mean ± SD. Student's t-test was used to compare the SUVs of normal tissues between 68Ga-DATA5m-LM4 PET and other 68Ga-SSTR PET images. All tests were 2-tailed, with the statistical significance defined as P < 0.05.
Patient Characteristics
| No. | Sex | Age | Primary tumor | Differentiation | Functional | Grade | Ki-67 | Liver mts | LN mts | Bone mts | Other mts | Chromogranin A | Synaptophysin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 39 | Pancreas | Well | No | G 2 | 10% | yes | yes | yes | Peritoneal | / | / |
| 2 | F | 38 | Kidney | Well | No | / | / | yes | yes | no | / | / | |
| 3 | M | 69 | Ileum | Well | Yes | G 1 | <1 % | yes | yes | no | positive | positive | |
| 4 | F | 78 | Thyroid | / | / | / | / | no | yes | yes | / | / | |
| 5 | F | 76 | Ileum | Well | Yes | G 1 | 2% | yes | no | no | Peritoneal | positive | positive |
| 6 | F | 71 | Lung | Well | No | G 2 | 20% | yes | no | yes | positive | positive | |
| 7 | M | 59 | Pancreas | Well | No | G 3 | 47% | yes | no | yes | positive | positive | |
| 8 | M | 74 | Ileum | Well | Yes | G 1 | 1% | yes | yes | no | Peritoneal | / | positive |
| 9 | M | 65 | Midgut | Well | Yes | G 1 | <1% | yes | yes | no | Pleural | / | / |
| 10 | M | 54 | Ileum | Well | No | G 2 | 5% | yes | yes | no | positive | / | |
| 11 | M | 82 | Pancreas | Well | No | G 2 | 4% | yes | yes | yes | Peritoneal | positive | positive |
| 12 | M | 60 | Paraganglioma | / | No | G 2 | 10-20 % | no | no | yes | Adrenal glands | positive | positive |
| 13 | M | 60 | Pancreas | Well | Yes | G 2 | 10% | yes | yes | no | / | / | |
| 14 | M | 52 | Pancreas | Well | Yes | G 2 | 5% | no | yes | no | Stomach, spleen, adrenal gland | positive | positive |
| 15 | M | 49 | Pancreas | Well | No | G 2 | 10% | yes | yes | no | positive | positive | |
| 16 | M | 78 | Pancreas | Well | No | / | / | no | yes | no | / | / | |
| 17 | F | 62 | Ileum | Well | Yes | G 2 | 10% | yes | no | no | Ovaries, small intestinal mesentery, peritoneal | positive | positive |
| 18 | M | 73 | Ileum | Well | Yes | G 2 | 15% | yes | yes | yes | Lung, peritoneal | positive | positive |
| 19 | M | 53 | Ileum | Well | Yes | G 2 | 15% | yes | no | no | Peritoneal | / | / |
| 20 | M | 59 | Pancreas | Well | Yes | G 3 | 52% | yes | no | no | Peritoneal | / | / |
| 21 | M | 38 | Duodenum | Well | No | G 3 | 50% | yes | no | yes | positive | positive | |
| 22 | M | 69 | Ileum | Well | Yes | G 2 | 4% | yes | no | yes | Peritoneal, lateral abdominal wall, small intestinal mesentery, mediastinal LK metastasis | / | / |
| 23 | F | 60 | Ileum | Well | Yes | G 2 | 12% | no | no | no | Breasts, omentum, intramuscular | / | / |
| 24 | F | 60 | Pancreas | Well | No | G 1 | 1-2 % | yes | no | no | positive | positive | |
| 25 | F | 52 | Pancreas | Well | No | G 1 | 1-2 % | yes | no | no | / | / | |
| 26 | M | 57 | Pancreas | Well | No | G 2 | 13% | yes | yes | yes | positive | positive | |
| 27 | M | 62 | Pancreas | Well | Yes | G 1 | <2 % | yes | yes | yes | positive | positive |
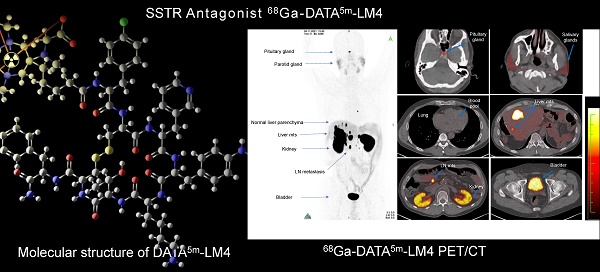
Structure of DATA5m-LM4.
Results
Chemistry and radiochemistry
The structure of DATA5m-LM4 is presented in Figure 1. Radiochemical yields (RCY) of 80-95% were achieved within 15 min at 50 °C, after decay correction. The entire labeling process of 68Ga-DATA5m-LM4 took approximately 20 min, including heating, purification, filtration, and formulation. Subsequent purification, formulation, and sterile filtration were carried out. Quality control was performed using analytical high-performance liquid chromatography and thin-layer chromatography, both of which demonstrated radiochemical purities (RCP) exceeding 98%. The specific activity was between 12 and 17 MBq/nmol.
Safety and biodistribution in normal organs
68Ga-DATA5m-LM4 was well tolerated in all patients, with no clinically significant adverse effects. All observed vital signs (including blood pressure, temperature, and heart rate) were normal at the 4-h follow-up.
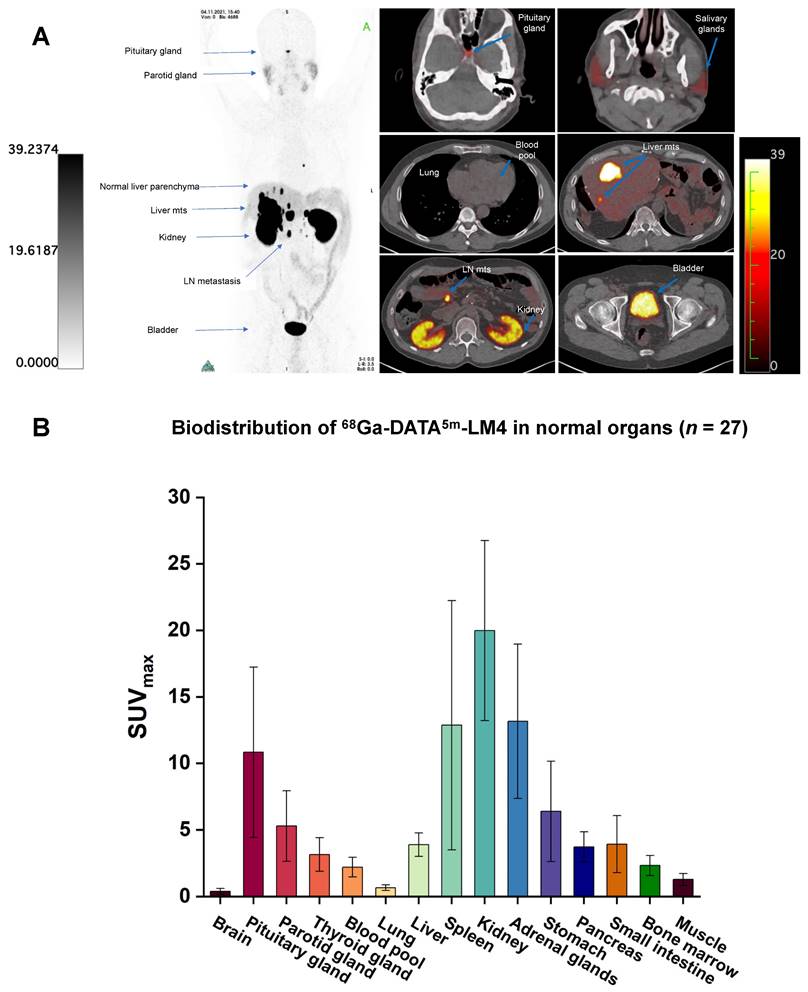
The biodistribution of 68Ga-DATA5m-LM4 is shown in Figure 2. 68Ga-DATA5m-LM4 was cleared efficiently after 1 h from the circulation and was excreted mainly through the kidneys and urinary tract. The highest normal organ uptake of 68Ga-DATA5m-LM4 (except for the kidneys and urinary bladder) was noted in the adrenal glands, followed by the spleen and pituitary gland, with SUVmax of 13.16 ± 5.80, 12.87 ± 9.37, and 10.84 ± 6.40 at 60 min after injection, respectively. The parotid gland, thyroid gland, liver, and pancreas showed moderate uptake, with SUVmax of 5.29 ± 2.64, 3.16 ± 1.26, 3.90 ± 0.88, and 3.73 ± 1.13, respectively. Uptake in the skeletal system, brain, lungs, blood pool, mediastinum, red marrow, and muscle were at background level.
Comparative biodistribution between 68Ga-DATA5m-LM4 and other 68Ga-labeled somatostatin analogs
The comparison of the in vivo distribution pattern between 68Ga-DATA5m-LM4 and that of 68Ga labeled other SSTR analogs, including 68Ga-DOTA-TATE, 68Ga-DOTA-TOC, 68Ga-NODAGA-JR11, 68Ga-NODAGA-LM3 are shown in Table 3. A significantly lower uptake in normal liver parenchyma was observed with 68Ga-DATA5m-LM4 compared to 68Ga-DOTA-TATE PET/CT (3.90 ± 0.88 vs. 9.12 ± 3.64, P < 0.000001). Additionally, uptake in the thyroid gland, pancreas, and spleen was also lower (P < 0.05).
Among the 27 patients, 14 also underwent 68Ga-DOTA-TOC PET/CT, 1 underwent 68Ga-DOTA-TATE PET/CT, and 4 underwent 68Ga-NODAGA-LM3 PET/CT (Table 4 and Table S1). The semiquantitative analysis demonstrated that 68Ga-DATA5m-LM4 uptake in the liver and spleen was significantly lower than the 68Ga-DOTA-TOC uptake (3.70 ± 0.79 vs. 5.33 ± 2.43, P = 0.0397; 11.88 ± 6.88 vs. 26.55 ± 16.07, P = 0.0022, respectively). In contrast, the uptake of 68Ga-DATA5m-LM4 in the kidneys was higher than that of 68Ga-DOTA-TOC (19.40 ± 7.12 vs. 16.44 ± 6.04, P = 0.0059). 68Ga-DATA5m-LM4 uptake in the pituitary gland was significantly higher than the 68Ga-NODAGA-LM3 uptake (8.87 vs. 4.79, P = 0.0183). Representative images are shown in Figures 3 and 4.
(A) Representative maximum-intensity-projection images fused axial PET/CT images of a 48-y-old patient with well-differentiated, non-functioning neuroendocrine neoplasm of the pancreas with extensive hepatic and lymph node metastases at 60 min after intravenous injection of 68Ga-DATA5m-LM4. The main regions with prominent 68Ga-DATA5m-LM4 uptake are the pituitary gland, kidneys, and bladder, as well as the liver metastases and lymph nodes metastases (arrows). There is low background activity in the normal liver parenchyma. (B) PET-based biodistribution analysis of the 27 patients imaged at 1 h after injection.
Tumor uptake
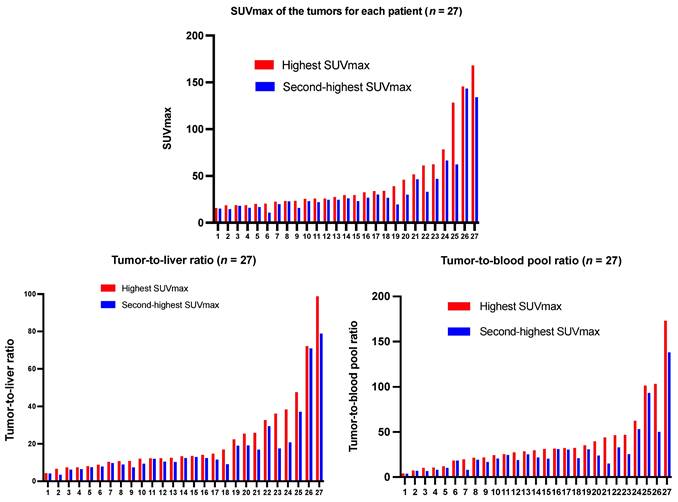
68Ga-DATA5m-LM4 PET/CT effectively detected tumor lesions with high uptake, including small lesions not visible on CT. Representative images are shown in Figure 5. The highest uptake and the second highest uptake of the tumor lesions in each patient ranged from 12.30 - 167.93 (mean ± SD, 44.47 ± 36.22) and 10.4 - 133.99 (mean ± SD, 34.00 ± 27.43), respectively (Figure 6).
Liver metastases, lymph node metastases, bone metastases, and other metastases were detected in 22 (81.5%), 16 (59.3%), 13 (48.1%), and 16 (59.3%) patients. The SUVmax of lesions in the liver, lymph nodes, bone, and other sites (pancreas, lung, peritoneum, spleen, subcutaneous pelvis, breast, skull base or glomus tympanicum, duodenum, stomach, cardia, intestines, abdominal wall, glomus caroticum, paratracheal, mesenterium, infradiaphragmal area, soft tissue dorsal to uterus, peritoneum or ovary) was 36.17 ± 30.39 (range, 4.30 - 128.30), 29.19 ± 18.95 (range, 3.80 - 62.30), 32.83 ± 45.81 (range, 2.2 - 167.93), 22.92 ± 21.79 (range, 4.30 - 93.4), respectively. Tumor-to-background ratios relative to the liver and blood pool were 20.32 ± 19.97 (range, 3.40 - 98.78) and 38.63 ± 35.97 (range, 4.1 - 173.12), respectively.
Discussion
In this study, we conducted the first clinical investigation using the novel SSTR antagonist 68Ga-DATA5m-LM4 in patients with metastatic NETs. We demonstrated that 68Ga-DATA5m-LM4 could be synthesized with high yield and fast radiolabeling with 68Ga. 68Ga-DATA5m-LM4 was safe and well-tolerated in all subjects. In patients with disseminated metastases NETs, it showed a superior biodistribution with very high tumor contrast and low uptake in the spleen, pancreas, and especially in the liver as compared to the agonistic SSTR ligands, such as 68Ga-DOTA-TATE, which facilitates favorable lesion detection with 68Ga-DATA5m-LM4.
Biodistribution of 68Ga-DATA5m-LM4 in patients with NETs (n = 27) and the comparison with other 68Ga -SSTR PET
| SUVmax | ||||||||
|---|---|---|---|---|---|---|---|---|
| 68Ga-DATA5m-LM4 (n = 27) | 68Ga-DOTA-TATE (n = 120) (26) | 68Ga-DOTA-TATE (n = 31) (22) | 68Ga-DOTA-TOC (n = 14) (35) | 68Ga-NODAGA-JR11 (n = 12) (9) | 68Ga-NODAGA-LM3 (n = 8) (11) | 68Ga-DOTA-NOC (n = 89) (25) | ||
| Brain | 0.39 ± 0.22 | <1 | ||||||
| Pituitary gland | 10.84 ± 6.4 | 9.74 ± 3.86 | P=0.245004 | 7.7 ± 3.2 | 5.8 ± 1.8 | 9.6 ± 3.5 | 2.6 ± 1.3 | |
| Parotid gland | 5.29 ± 2.64 | 3.17 ± 1.47 | P<0.000001 | 3.7 ± 1.9 | 2.4 ± 0.9 | |||
| Thyroid gland | 3.16 ± 1.26 | 4.18 ± 1.9 | P=0.008759 | 4.2 ± 0.9 | 2.3 ± 1.2 | 1.9 ± 0.6 | 3.4 ± 1.4 | |
| Blood pool | 2.21 ± 0.73 | 1.3 ± 0.5 | 2.6 ± 1.2 | |||||
| Lung | 0.66 ± 0.21 | 0.59 ± 0.28 | P=0.223454 | 1.7 ± 0.6 | 1.0 ± 0.3 | 0.9 ± 0.8 | ||
| Liver | 3.90 ± 0.88 | 9.12 ± 3.64 | P<0.000001 | 9.7 ± 3.0 | 6.0 ± 1.6 | 3.2 ± 0.8 | 6.4 ± 1.8 | 6.9 ± 2.0 |
| Spleen | 12.87 ± 9.37 | 24.67 ± 8.05 | P<0.000001 | 22.5 ± 8.0 | 5.1 ±1.3 | 11.7 ± 4.2 | 17.5 ± 7.7 | 22 ± 10 |
| Kidney | 19.98 ± 6.77 | 14.30 ± 4.55 | P<0.000001 | 14.6 ± 3.8 | 15.8 ± 7.7 | 17.9 ± 2.7 | 12.9 ± 3.8 | |
| Adrenal glands | 13.16 ± 5.80 | 13.73 ± 5.16 | P=0.613082 | 11.3 ± 4.4 | 10.1 ± 6.4 | 8.3 ± 3.9 | 11.2 ± 4.8 | 6.0 ± 2.5 |
| Stomach | 6.39 ± 3.77 | 7.1 ± 4.2 | 4.2 ± 2.3 | 3.0 ± 0.9 | ||||
| Pancreas | 3.73 ± 1.13 | 4.69 ± 1.86 | P=0.011086 | 4.3 ± 1.9 | 3.2 ± 2.0 | 3.7 ± 1.6 | 5.8 ± 2.0 | |
| Small intestine | 3.93 ± 2.14 | 6.1 ± 1.8 | 3.5 ± 1.3 | 3.2 ± 0.7 | 2.3 ± 1.0 | |||
| Bone marrow | 2.33 ± 0.76 | <1 | 1.6 ± 0.6 | 0.8 ± 0.3 | ||||
| Muscle | 1.28 ± 0.45 | <1 | ||||||
Uptake of normal organs in patients with a head-to-head comparison between 68Ga-DATA5m-LM4 PET/CT and 68Ga-DOTA-TOC PET/CT (n = 14)
| SUVmax | |||
|---|---|---|---|
| 68Ga-DATA5m-LM4 | 68Ga-DOTA-TOC | P | |
| Brain | 0.41 ± 0.30 | 0.38 ± 0.20 | 0.6678 |
| Pituitary gland | 8.86 ± 5.29 | 8.85 ± 4.84 | 0.9985 |
| Parotid gland | 5.19 ± 2.86 | 2.34 ± 0.97 | 0.0035 |
| Thyroid gland | 2.73 ± 1.03 | 4.11 ± 3.16 | 01952 |
| Blood pool (left ventricle) | 2.15 ± 0.82 | 1.65 ± 0.68 | 0.0038 |
| Lung | 0.58 ± 0.22 | 0.53 ± 0.24 | 0.6683 |
| Liver | 3.70 ± 0.79 | 5.33 ± 2.43 | 0.0397 |
| Spleen | 11.88 ± 6.88 | 26.55 ± 16.07 | 0.0022 |
| Kidney | 19.40 ± 7.12 | 16.44 ± 6.04 | 0.0059 |
| Adrenal glands | 13.13 ± 5.42 | 9.44 ± 3.14 | 0.1177 |
| Stomach | 6.77 ± 3.16 | 3.23 ± 1.35 | 0.0006 |
| Pancreas | 3.04 ± 0.74 | 2.93 ± 0.72 | 0.3368 |
| Small intestine | 4.03 ± 2.20 | 1.98 ± 0.70 | 0.0116 |
| Bone marrow (L4-5 vertebra) | 2.03 ± 0.63 | 1.50 ± 0.38 | 0.0112 |
| Muscle | 1.18 ± 0.37 | 1.27 ± 0.71 | 0.6563 |
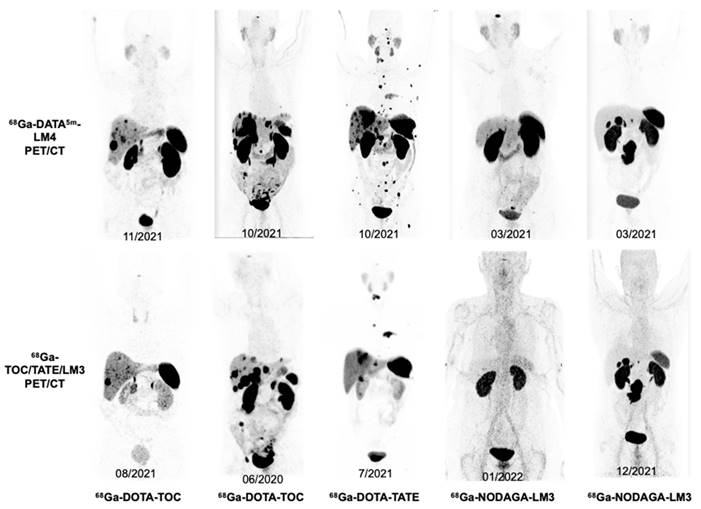
Illustration of image quality of whole-body maximum-intensity projections in 5 representative NET patients (Patients 3, 8, 21, 17, 10) who underwent both 68Ga-DATA5m-LM4 PET/CT (upper) and other SSTR-targeting 68Ga-TOC/TATE/LM3 PET/CT (lower). Patients 3 and 17 underwent 2 cycles PRRT between scans, Patient 21 received treatment with Everolimus, Patients 8 and 10 did not receive any treatments between scans.
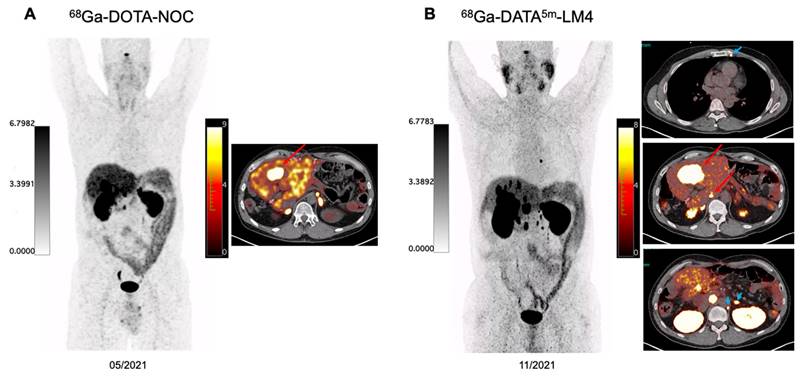
A 48-year-old patient with well-differentiated non-functioning serotonin-producing NET of the pancreas with extensive hepatic and lymph node metastases, G2 with Ki-67 index of 10%, immunohistochemical staining strongly positive for Chromogranin A and Synaptophysin. The patient has previously received treatment with duodenopancreatectomy, splenectomy, segmental liver resection and several hepatic metastectomies, lanreotide, right hemihepatectomy, CAPTEM chemotherapy, and 5 cycles of PRRT since 2017 using 90Y- and 177Lu-DOTA-TOC, 177Lu-DOTA-LM3 by May 2021. The patient received chemotherapy between May 2021 and November 2021. The intraindividual comparison between 68Ga-DOTA-NOC and 68Ga-DATA5m-LM4 PET/CT demonstrated significantly lower uptake in normal liver parenchyma on 68Ga-DATA5m-LM4 PET/CT resulting in a higher TBR of 10.3 allowing even the detection of very small hepatic metastases (>10 tumor lesions) as compared to 68Ga-DOTA-NOC PET/CT (green arrows). Additionally, a tiny left internal mammary lymph node metastasis was clearly visualized on 68Ga-DATA5m-LM4 PET/CT as well as para-aortic lymph node lesions left to the adrenal gland (blue arrowheads).
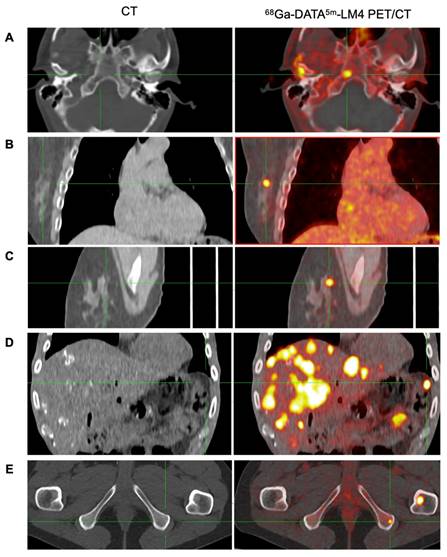
Representative images of 68Ga-DATA5m-LM4 PET/CT demonstrating the detection of tumor metastases that are undetectable on conventional CT, located in: skull base (A), right breast (B and C), peritoneum (D), and ischial bone (E).
List of SUVmax of tumors, tumor-to-liver ratio, and tumor-to-blood pool ratio in the 27 NET patients in ascending order. Red Bar: Highest SUVmax and TBR according to highest tumor uptake detected on 68Ga-DATA5m-LM4 PET/CT; Blue bar: The second-highest SUVmax and TBR in each patient.
Several preclinical studies have shown that radiolabeled SSTR antagonists exhibit higher uptake in SSTR-expressing tumors compared to SSTR agonists (6, 17). The first clinical evaluation of SSTR antagonists confirmed these findings, demonstrating higher tumor uptake and better tumor-to-background ratios with the antagonist 111In-DOTA-BASS compared to the SSTR agonist 111In-DTPA-octreotide (8). Clinically, SSTR antagonists have demonstrated higher sensitivity and better image contrast. 68Ga-NODAGA-JR11 has in regard to this exhibited superiority compared with the agonist 68Ga-DOTA-TOC in patients with GEP-NETs (9, 18), and similarly, the diagnostic efficacy superiority of the antagonist 68Ga-NODAGA-LM3 was shown when compared to agonist 68Ga-DOTA-TATE in NETs (11). More recently, 68Ga-NODAGA-JR11 (also known as 68Ga-SSO-120) has shown high diagnostic value in small cell lung cancer (SCLC) (19, 20), a tumor type with lower SSTR expression than NETs, highlighting its potential for theranostics applications.
The chelator DOTA can severely affect the affinity and pharmacokinetics of the SSTR ligand, which is not well suited for complexing the relatively small (radio) metal 68Ga and also overcompensates the charge of the metal leaving the metal with an overall charge of minus one. For SSTR antagonists, Fani et al. have demonstrated in preclinical experiments that the affinity and pharmacokinetics of the radiolabeled somatostatin-based antagonists strongly depend on the chelator and radiometal and reported 68Ga-NODAGA-LM3 has a 10-fold higher SSTR2 affinity than 68Ga-DOTA-LM3 (21). Zhu et al. reported in a head-to-head comparison that 68Ga-DOTA-JR11 was superior to 68Ga-DOTA-TATE in the detection of liver metastases but much less sensitive for bone metastases, and probably limited its role as a diagnostic pair for the theranostic approach due to the lower somatostatin receptor subtype 2 affinity (22). These findings emphasize the importance of developing novel chelates to further improve image contrast, and the search for novel tracers with even higher affinity and practicality is thus forthgoing and forthcoming.
DATA is a novel type of chelator exhibiting cyclic, acyclic, and intermediate properties, which have advantageous properties for 68Ga-labeling compared to established chelators. 68Ga-DATA chelates are immune against trans-chelation (DTPA and apo-transferrin) and trans-metalation (FeIII) and show high stability against human serum and different buffers (23). Furthermore, in contrast to 68Ga-DOTA radiotracers, labeling of DATA can be realized at ambient temperature, and therefore, labeling of heat-sensitive molecules is possible. Additionally, no time for cooling before the intravenous injection is needed, thereby overcoming the limitations for clinical use due to the short half-life of 68Ga. DATA can afford rapid quantitative radiolabeling with 68Ga at ambient temperature in a wide pH range, which greatly promotes convenience for clinical use. Furthermore, it only has three attached carboxylic acids to neutralize the charge of Ga(III), adding no additional charge to the molecule like DOTA and therefore acts similarly to the NODAGA derivatives.
The biodistribution of 68Ga-DATA5m-LM4 was similar to that of SSTR agonists and antagonists in SSTR2-rich organs (9, 11, 22, 24-26). As compared to SSTR agonists 68Ga-DOTA-TATE and 68Ga-DOTA-TOC, the background uptake of 68Ga-DATA5m-LM4 in the liver was significantly lower, resulting in high tumor contrast for the liver metastases and significantly higher tumor-to-liver ratios. Lower organ uptake was also observed in the spleen of 68Ga-DATA5m-LM4 as compared to both 68Ga-DOTA-TATE in the literature and 68Ga-DOTA-TOC in the head-to-head comparison of the present study. The bone marrow uptake and kidney uptake of 68Ga-DATA5m-LM4 were higher than those of the agonist 68Ga-DOTA-TATE. This suggests the need for dosimetry studies, as bone marrow and kidneys are critical dose-limiting organs in subsequent PRRT, particularly given that bone marrow toxicity is a limiting factor in the application of SSTR antagonist radionuclide therapy. Among SSTR antagonists, studies have indicated that 68Ga-NODAGA chelates, for example, 68Ga-NODAGA-LM3, achieve higher tumor uptake and greater uptake in SSTR2-positive organs compared to 68Ga-DOTA-LM3 (27). Therefore, we directly compared 68Ga-DATA5m-LM4 with a 68Ga-NODAGA chelated SSTR antagonist. As compared to other SSTR antagonists like 68Ga-NODAGA-JR11 and 68Ga-NODAGA-LM3, 68Ga-DATA5m-LM4 demonstrated a similar biodistribution but showed higher uptake in representative SSTR2-rich organs such as the pituitary and adrenal glands. The reported liver biodistribution for SSTR antagonists has shown variability in the literature (7, 9, 11, 19, 28-30). In our study, 68Ga-DATA5m-LM4 demonstrated lower liver uptake than previously reported but showed a higher background uptake in the liver, spleen, and kidneys in a direct head-to-head comparison with 68Ga-NODAGA-LM3 PET/CT conducted at our center, involving four patients. Future prospective studies with larger cohorts of NET patients are warranted to validate these findings and facilitate a more rigorous head-to-head comparison of these promising radiopharmaceuticals.
Our previous study on 177Lu chelated by an antagonist, 177Lu-DOTA-LM3, as a treatment showed promising results. It was well-tolerated by the majority of patients, even at a higher radiation dose than that used with SSTR agonists (31). Moreover, it was effective in treating metastatic NETs in patients with low or no tumor uptake of an SSTR agonist. In the present study, 68Ga-DATA5m-LM4 showed high tumor uptake, with the highest SUVmax up to 167.93 (44.47 ± 36.22) and the second highest SUVmax up to 133.99 (34.00 ± 27.43), comparable to those for SSTR agonists and other SSTR antagonists (8, 9, 11, 32-34). The high tumor uptake also provides the basis for the application of comparable ligands coupled to, e.g., AAZTA (1,4-bis (carboxymethyl)-6-[bis (carboxymethyl)]amino-6-methylperhydro-1,4-diazepine) to be used as a therapeutic agent for tumor-targeted peptide receptor radionuclide therapy of NETs after being labeled with β- emitters. Preliminary observations indicated that 68Ga-DATA5m-LM4 PET/CT efficiently detected tumor lesions, including very small metastases and CT-invisible tumors. The practical advantages of this straightforward labeling process, achieved without any apparent loss in diagnostic efficacy, distinguish 68Ga-DATA5m-LM4 from competing antagonists. The capability to label DATA at ambient temperature in a "kit-type" manner offers a significant advantage over other competing antagonists like DOTA- or NODAGA- labeled JR11/LM3. The advantages, along with the very convenient production and the option for a “kit-type” labeling, make 68Ga-DATA5m-LM4 an overall extraordinarily promising radiopharmaceutical for the staging and restaging of NET patients.
Compared to macrocyclic chelators based on the cyclen scaffold (e.g., DOTA), DATA chelators facilitate quantitative radiolabeling more rapidly and under milder conditions. Several studies have demonstrated the excellent labeling properties of DATA-conjugated derivatives. For instance, each of the DATAX chelators shows remarkable radiolabeling characteristics, achieving > 95% RCYs over a pH range of 4-7 within 3 minutes using just 15 nmol (10.7 mm) of the chelator at room temperature. In contrast, the current industry standard, DOTA (5-10 mm), requires 5-15 minutes at 80-95 °C to achieve similar RCYs. This combination of superior reaction rate and room-temperature labeling is particularly attractive for a kit-type approach to radiolabeling. The ability to label over a wide pH range is advantageous, as it offers a more robust labeling reaction at a pH suitable for in vivo administration, thus saving time, and may also enable the synthesis of previously inaccessible pH-sensitive biomolecules (13). Additionally, the benefit of this molecule is that, in general, it allows for labeling without heat in a single vial with a high formulation pH - an advantage over the SOMAKIT, although we did not demonstrate this in the present first-in-human study due to regulatory constraints.
This study suffers from a few limitations. The patient number is relatively small for definite assessment of this novel radiotracer, and only a few patients underwent paired 68Ga-DATA5m-LM4 and 68Ga-DOTA-TOC PET/CT imaging or paired 68Ga-DATA5m-LM4 and 68Ga-NODAGA-LM3 PET/CT imaging. For the patients who underwent paired PET/CT imaging for biodistribution, the results of the statistical analysis are questionable, due to the small number of patients investigated and the potential influence of the tumor uptakes. Another limitation is the lack of histologic confirmation of the detected metastases or other imaging modalities, although 68Ga-SSTR PET has been clinically established as superior for NET diagnosis. Furthermore, potential disease progression or remission between scans, particularly with longer intervals, could influence the results, thereby limiting the ability to draw definitive conclusions about the sensitivity or superiority of lesion detection. Prospective studies with larger patient populations and head-to-head comparisons are warranted in the future to better explore the role of 68Ga-DATA5m-LM4 and the potential superiority of 68Ga-DATA5m-LM4 in tumor diagnosis for NET patients.
Conclusion
This study indicated that the novel SSTR antagonist 68Ga-DATA5m-LM4 can be efficiently labeled with high radiochemical yield and purity, supported by a highly convenient production process. The tracer exhibited excellent imaging performance, with a highly favorable biodistribution characterized by very high tumor contrast and minimal uptake in normal organs, particularly the liver, enabling superior lesion detection. The practical advantages of this straightforward labeling process, achieved without any apparent loss in diagnostic efficacy, distinguish 68Ga-DATA5m-LM4 from other competing antagonists. Prospective studies with a head-to-head comparison between 68Ga-DATA5m-LM4 and other compounds are warranted to better explore the role of 68Ga-DATA5m-LM4 for NET patients.
Abbreviations
SSTR: somatostatin receptor; NET: neuroendocrine tumors; PET/CT: positron emission tomography combined with computed tomography; GEP-NETs: gastroenteropancreatic neuroendocrine tumors; SSTR2: somatostatin receptor subtype-2; DATA: (6-amino-1,4-diazepine-triacetic acid); DOTA: 1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid; LM4: p-Cl-Phe-cyclo[DCys-Pal-Daph(Cbm)-Lys-Thr-Cys]DTyr-NH2; JR11: p-Cl-Phe-cyclo(D-Cys-Aph(Hor)-D-Aph(cbm)-Lys-Thr-Cys)D-Tyr-NH2; NODAGA: 1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid; LM3: p-Cl-Phe-cyclo[D-Cys-Tyr-D-4-amino-Phe(carbamoyl)-Lys-Thr-Cys]D-Tyr-NH2; RCY: radiochemical yields; RCP: radiochemical purities; SUV: standardized uptake value; TBR: tumor-to-background ratio.
Supplementary Material
Supplementary table.
Acknowledgements
We thank the patients who participated in this study and all research support staff for their support, the physician colleagues, and nuclear medicine technologists for patient care at Curanosticum Wiesbaden-Frankfurt. This study was supported by the International Centers for Precision Oncology (ICPO) Foundation, the National University of Singapore Start-up Grant (NUHSRO/2021/097/Startup/13, NUHSRO/2020/133/Startup/08, NUHSRO/2023/008/NUSMed/TCE/LOA, NUHSRO/2021/034/TRP/09/Nanomedicine), National Medical Research Council (MOH-001483-00, MOH-001334-00, MOH-001388-00, MOH-001254-01, CG21APR1005), Singapore Ministry of Education (MOE-000387-00), Singapore Ministry of Education (FY2022) Tier 1 Grant (NUHSRO/2022/093/T1/Seed-Sep/06), and National Research Foundation (NRF-000352-00).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV. et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9(1):61-72
2. Tella SH, Starr JS, Kommalapati A, Sonbol MB, Halfdanarson TR. Management of well-differentiated neuroendocrine tumors. Clin Adv Hematol Oncol. 2021;19(9):582-93
3. Huguet I, Grossman AB, O'Toole D. Changes in the Epidemiology of Neuroendocrine Tumours. Neuroendocrinology. 2017;104(2):105-11
4. Ramírez-Rentería C, Ferreira-Hermosillo A, Marrero-Rodríguez D, Taniguchi-Ponciano K, Melgar-Manzanilla V, Mercado M. An Update on Gastroenteropancreatic Neuroendocrine Neoplasms: From Mysteries to Paradigm Shifts. Arch Med Res. 2020;51(8):765-76
5. Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-6
6. Ginj M, Zhang H, Waser B, Cescato R, Wild D, Wang X. et al. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci U S A. 2006;103(44):16436-41
7. Fani M, Nicolas GP, Wild D. Somatostatin Receptor Antagonists for Imaging and Therapy. J Nucl Med. 2017;58(Suppl 2):61S-6S
8. Wild D, Fani M, Behe M, Brink I, Rivier JE, Reubi JC. et al. First clinical evidence that imaging with somatostatin receptor antagonists is feasible. J Nucl Med. 2011;52(9):1412-7
9. Nicolas GP, Beykan S, Bouterfa H, Kaufmann J, Bauman A, Lassmann M. et al. Safety, Biodistribution, and Radiation Dosimetry of (68)Ga-OPS202 in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase I Imaging Study. J Nucl Med. 2018;59(6):909-14
10. Zhang J, Kulkarni HR, Singh A, Baum RP. Successful Intra-arterial Peptide Receptor Radionuclide Therapy of DOTATOC-Negative High-Grade Liver Metastases of a Pancreatic Neuroendocrine Neoplasm Using 177Lu-DOTA-LM3: A Somatostatin Receptor Antagonist. Clin Nucl Med. 2020;45(3):e165-e8
11. Zhu W, Jia R, Yang Q, Cheng Y, Zhao H, Bai C. et al. A prospective randomized, double-blind study to evaluate the diagnostic efficacy of (68)Ga-NODAGA-LM3 and (68)Ga-DOTA-LM3 in patients with well-differentiated neuroendocrine tumors: compared with (68)Ga-DOTATATE. Eur J Nucl Med Mol Imaging. 2022;49(5):1613-22
12. Baum RP, Zhang J, Schuchardt C, Muller D, Macke H. First-in-Humans Study of the SSTR Antagonist (177)Lu-DOTA-LM3 for Peptide Receptor Radionuclide Therapy in Patients with Metastatic Neuroendocrine Neoplasms: Dosimetry, Safety, and Efficacy. J Nucl Med. 2021;62(11):1571-81
13. Seemann J, Waldron BP, Roesch F, Parker D. Approaching 'Kit-Type' Labelling with (68)Ga: The DATA Chelators. ChemMedChem. 2015;10(6):1019-26
14. Greifenstein L, Grus T, Nagel J, Sinnes JP, Rosch F. Synthesis and labeling of a squaric acid containing PSMA-inhibitor coupled to AAZTA(5) for versatile labeling with (44)Sc, (64)Cu, (68)Ga and (177)Lu. Appl Radiat Isot. 2020;156:108867
15. Seemann J, Waldron B, Parker D, Roesch F. DATATOC: a novel conjugate for kit-type (68)Ga labelling of TOC at ambient temperature. EJNMMI Radiopharm Chem. 2017;1(1):4
16. Kanellopoulos P, Nock BA, Greifenstein L, Baum RP, Roesch F, Maina T. [(68)Ga]Ga-DATA(5m)-LM4, a PET Radiotracer in the Diagnosis of SST(2)R-Positive Tumors: Preclinical and First Clinical Results. Int J Mol Sci. 2022;23(23):14590
17. Cescato R, Waser B, Fani M, Reubi JC. Evaluation of 177Lu-DOTA-sst2 antagonist versus 177Lu-DOTA-sst2 agonist binding in human cancers in vitro. J Nucl Med. 2011;52(12):1886-90
18. Nicolas GP, Schreiter N, Kaul F, Uiters J, Bouterfa H, Kaufmann J. et al. Sensitivity Comparison of (68)Ga-OPS202 and (68)Ga-DOTATOC PET/CT in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase II Imaging Study. J Nucl Med. 2018;59(6):915-21
19. Kersting D, Sandach P, Sraieb M, Wiesweg M, Metzenmacher M, Darwiche K. et al. (68)Ga-SSO-120 PET for Initial Staging of Small Cell Lung Cancer Patients: A Single-Center Retrospective Study. J Nucl Med. 2023;64(10):1540-9
20. Mavroeidi IA, Romanowicz A, Haake T, Wienker J, Metzenmacher M, Darwiche K. et al. Theranostics with somatostatin receptor antagonists in SCLC: Correlation of (68)Ga-SSO120 PET with immunohistochemistry and survival. Theranostics. 2024;14(14):5400-12
21. Fani M, Braun F, Waser B, Beetschen K, Cescato R, Erchegyi J. et al. Unexpected sensitivity of sst2 antagonists to N-terminal radiometal modifications. J Nucl Med. 2012;53(9):1481-9
22. Zhu W, Cheng Y, Wang X, Yao S, Bai C, Zhao H. et al. Head-to-Head Comparison of (68)Ga-DOTA-JR11 and (68)Ga-DOTATATE PET/CT in Patients with Metastatic, Well-Differentiated Neuroendocrine Tumors: A Prospective Study. J Nucl Med. 2020;61(6):897-903
23. Moon ES, Elvas F, Vliegen G, De Lombaerde S, Vangestel C, De Bruycker S. et al. Targeting fibroblast activation protein (FAP): next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA(5m) chelators. EJNMMI Radiopharm Chem. 2020;5(1):19
24. Bodei L, Ambrosini V, Herrmann K, Modlin I. Current Concepts in (68)Ga-DOTATATE Imaging of Neuroendocrine Neoplasms: Interpretation, Biodistribution, Dosimetry, and Molecular Strategies. J Nucl Med. 2017;58(11):1718-26
25. Prasad V, Baum RP. Biodistribution of the Ga-68 labeled somatostatin analogue DOTA-NOC in patients with neuroendocrine tumors: characterization of uptake in normal organs and tumor lesions. Q J Nucl Med Mol Imaging. 2010;54(1):61-7
26. Kuyumcu S, Özkan ZG, Sanli Y, Yilmaz E, Mudun A, Adalet I. et al. Physiological and tumoral uptake of (68)Ga-DOTATATE: standardized uptake values and challenges in interpretation. Ann Nucl Med. 2013;27(6):538-45
27. Fani M, Del Pozzo L, Abiraj K, Mansi R, Tamma ML, Cescato R. et al. PET of somatostatin receptor-positive tumors using 64Cu- and 68Ga-somatostatin antagonists: the chelate makes the difference. J Nucl Med. 2011;52(7):1110-8
28. Nazar AK, Basu S. Radiolabeled Somatostatin Analogs for Cancer Imaging. Semin Nucl Med. 2024;54(6):914-40
29. Lin Z, Zhu W, Zhang J, Miao W, Yao S, Huo L. Head-to-Head Comparison of (68)Ga-NODAGA-JR11 and (68)Ga-DOTATATE PET/CT in Patients with Metastatic, Well-Differentiated Neuroendocrine Tumors: Interim Analysis of a Prospective Bicenter Study. J Nucl Med. 2023;64(9):1406-11
30. Lapi SE, Scott PJH, Scott AM, Windhorst AD, Zeglis BM, Abdel-Wahab M. et al. Recent advances and impending challenges for the radiopharmaceutical sciences in oncology. Lancet Oncol. 2024;25(6):e236-e49
31. Baum RP, Zhang J, Schuchardt C, Müller D, Mäcke H. First-in-Humans Study of the SSTR Antagonist. J Nucl Med. 2021;62(11):1571-81
32. Brogsitter C, Zophel K, Hartmann H, Schottelius M, Wester HJ, Kotzerke J. Twins in spirit part II: DOTATATE and high-affinity DOTATATE-the clinical experience. Eur J Nucl Med Mol Imaging. 2014;41(6):1158-65
33. Velikyan I, Sundin A, Sorensen J, Lubberink M, Sandstrom M, Garske-Roman U. et al. Quantitative and qualitative intrapatient comparison of 68Ga-DOTATOC and 68Ga-DOTATATE: net uptake rate for accurate quantification. J Nucl Med. 2014;55(2):204-10
34. Krebs S, Pandit-Taskar N, Reidy D, Beattie BJ, Lyashchenko SK, Lewis JS. et al. Biodistribution and radiation dose estimates for (68)Ga-DOTA-JR11 in patients with metastatic neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2019;46(3):677-85
35. Hartmann H, Zöphel K, Freudenberg R, Oehme L, Andreeff M, Wunderlich G. et al. [Radiation exposure of patients during 68Ga-DOTATOC PET/CT examinations]. Nuklearmedizin. 2009;48(5):201-7
Author contact
![]() Corresponding authors: Jingjing Zhang, MD, PhD. Department of Diagnostic Radiology, National University of Singapore. National University Hospital, Main Building, Lobby F, #04-398, 5 Lower Kent Ridge Road, Singapore 119074, Singapore. Phone: +65 84353534. E-mail: j.zhangedu.sg. Richard P. Baum, MD, PhD. Curanosticum Wiesbaden-Frankfurt, Center for Advanced Radiomolecular Precision Oncology, DKD HELIOS Klinik Wiesbaden Aukammallee 33, 65191 Wiesbaden, Germany. Email: baumrpcom.
Corresponding authors: Jingjing Zhang, MD, PhD. Department of Diagnostic Radiology, National University of Singapore. National University Hospital, Main Building, Lobby F, #04-398, 5 Lower Kent Ridge Road, Singapore 119074, Singapore. Phone: +65 84353534. E-mail: j.zhangedu.sg. Richard P. Baum, MD, PhD. Curanosticum Wiesbaden-Frankfurt, Center for Advanced Radiomolecular Precision Oncology, DKD HELIOS Klinik Wiesbaden Aukammallee 33, 65191 Wiesbaden, Germany. Email: baumrpcom.
 Global reach, higher impact
Global reach, higher impact