13.3
Impact Factor
Theranostics 2025; 15(5):1949-1965. doi:10.7150/thno.102682 This issue Cite
Research Paper
Betaine inhibits the stem cell-like properties of hepatocellular carcinoma by activating autophagy via SAM/m6A/YTHDF1-mediated enhancement on ATG3 stability
1. Department of Nutrition, School of Public Health, Sun Yat-sen University, Guangzhou, 510080, China.
2. Guangdong Provincial Key Laboratory of Food, Nutrition and Health, School of Public Health, Sun Yat-sen University, Guangzhou, 510080, China.
3. Center of Experimental Animals, Sun Yat-sen University, Guangzhou, 510080, China.
Received 2024-8-22; Accepted 2024-12-24; Published 2025-1-6
Abstract
Background: Stem cell-like properties are known to promote the recurrence and metastasis of hepatocellular carcinoma (HCC), contributing to a poor prognosis for HCC patients. Betaine, an important phytochemical and a methyl-donor related substance, has shown protective effects against liver diseases. However, its effect on HCC stem cell-like properties and the underlying mechanisms remains uninvestigated.
Methods: We measured the effects of betaine on the stem cell-like properties and malignant progression of HCC using patient-derived xenografts, cell-derived xenografts, tail vein-lung metastasis models, in vitro limiting dilution, tumor sphere formation, colony formation, and transwell assays. Mechanistic exploration was conducted using western blots, dot blots, methylated RNA immunoprecipitation-qPCR, RNA stability assays, RNA immunoprecipitation-qPCR, RNA pull-down, and gene mutation assays.
Results: A cohort study of HCC found that a higher serum concentration of betaine was associated with decreased levels of stemness-related markers. Furthermore, in HCC cells and xenograft mice, betaine suppressed the stem cell-like properties of HCC by activating autophagy. Mechanistically, betaine increased the m6A modification in HCC by producing S-adenosylmethionine (SAM) via betaine-homocysteine S-methyltransferase (BHMT). This increase in SAM subsequently triggered autophagy by enhancing the stability of autophagy-related protein 3 (ATG3) via YTHDF1 in an m6A-dependent manner, thereby inhibiting the stem cell-like properties of HCC cells.
Conclusions: These findings indicate that betaine inhibits the stem cell-like properties of HCC via the SAM/m6A/YTHDF1/ATG3 pathway. This study underscores the potential anti-tumor effects of betaine on HCC and offers novel therapeutic prospects for HCC patients.
Keywords: betaine, N6-methyladenosine, hepatocellular carcinoma, stem cell-like properties, autophagy
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality globally and imposes a substantial health burden [1, 2]. The main contributors to this poor prognosis are tumor recurrence and metastasis [3, 4]. Previous studies have revealed that, the stem cell-like properties endow HCC cells with self-renewal and metastatic capabilities [5, 6]. Furthermore, HCC patients with higher stemness indexes consistently experience a shorter survival duration [7-9]. Thus, investigating the mechanisms underlying these stem cell-like properties and developing novel therapeutic strategies may improve the prognosis for HCC patients.
Autophagy is a fundamental and conserved cellular process that is regulated by a series of autophagy-related genes (ATGs), and plays a crucial role in modulating the stem cell-like properties of cancer [10, 11]. Recent studies have reported that activating autophagy with natural products can impede the stem cell-like properties of cancer cells, including capsaicin, curcumin, and resveratrol [12, 13]. Betaine, an important phytochemical in sugar beet and spinach [14], carries significant protective roles against several diseases, including mental disorders [15], muscle loss [16], reproductive disorders [17, 18], and liver-associated diseases [19-23]. Experimental investigations found that these beneficial effects are associated with several mechanisms, such as modulating microglial M1/M2 differentiation [15], stimulating METTL21C/p97/VCP-dependent autophagy in myoblast [16], and mitigating senescence and apoptosis in reproductive cells [17, 18]. More importantly, recent studies demonstrated that the protective effects of betaine on liver diseases are associated with activated autophagy [24, 25]. However, whether betaine-activated autophagy could inhibit the stem cell-like properties of HCC cells, and the mechanisms behind betaine's activation of autophagy remain unclear.
N6-methyladenosine (m6A) is an epi-transcriptomic modification on RNAs [26, 27] and is instrumental in the activation of autophagy [28-30]. Mechanically, m6A "writers" transfer a methyl group from S-adenosylmethionine (SAM) to RNAs, whereas "erasers" remove it. The "readers" like YTHDFs, YTHDCs, IGF2BPs, and HNRNPs recognize m6A in RNAs and regulate RNA processes including maturation, splicing, stability, and translation [31-33]. Notably, betaine serves as a crucial donor of methyl groups by generating SAM, and plays an important role in modulating m6A modification [14, 34, 35]. Hence, we hypothesize that betaine can inhibit the stem cell-like properties of HCC cells by activating autophagy via SAM-dependent m6A modification on ATGs.
In the present study, we aimed to investigate the inhibitory effects of betaine on the stem cell-like properties of HCC and to elucidate the underlying mechanisms. Our findings may provide further insights into the antitumor effects of betaine, and expand the range of therapeutic options for cancer patients.
Materials and Methods
Serum and tissue collection
The serum and patient tissues utilized in this study were derived from the on-going "Guangdong Liver Cancer Cohort (GLCC)" (NCT03297255), which was established in 2013 [36]. The criteria for patient recruitment were as follows: 1) newly diagnosed within the past month, 2) no history of other cancers, 3) no previous anticancer treatment, and 4) availability of fasting blood samples and surgical HCC tissues.
Serum betaine measurement
Serum betaine was measured by high-performance liquid chromatography with online electro-spray ionization tandem mass spectrometry, following the methods described previously [37].
Tumor sphere formation
To evaluate the stem cell-like properties of HCC cells, a tumor sphere formation assay was undertaken. Briefly, around 400 cells were seeded into a 6-well ultra-low plate containing 4 mL of serum-free medium. This medium was primarily constituted of DMEM-F/12 medium, supplemented with 1% B27, 1% N2, 25 ng/mL EGF, and 25 ng/mL FGF. The cells were then incubated for a period of 14 days. Afterwards, the formed tumor spheres were visualized and imaged using an inverted microscope.
In vitro limiting dilution assay
After being diluted, HCC cells were added into a 96-well ultra-low plate at the density of 2, 5, 10, 25, 50 cells/well (n = 18 wells) and incubated with serum-free medium for 14 days. Afterwards, the number of wells with sphere were counted. The ability of sphere formation was calculated using online ELDA software in accordance with the instructions.
Measurement of autophagosomes
After HCC cells were treated with betaine for 24 h, cells were washed with ice-cold PBS and harvested. Then, cells were fixed with 2.5% glutaraldehyde for 24 h and stained with phosphotungstic acid. Finally, cellular autophagosomes were captured and counted using a transmission electron microscope (TEM).
Assessment of autophagic flux
HCC cells were planted into a six-well plate to reach a 40% confluence. Then, Ad-mCherry-GFP-LC3 (Beyotime, Shanghai, China) was added into each well and incubated for 48 h. Subsequently, the cells were planted into a confocal dish and cultured for 24 h. Afterwards, the cells were subjected to betaine treatment for 24 h and the LC3 puncta was captured using a con-focal microscope. The number of cellular LC3 puncta were analyzed by ImageJ software.
Dot blots assay
After extraction, total RNA was diluted to a final concentration of 25 ng/μL and denatured at 95 ℃ for 6 min. Then, different concentrations of denatured RNA (50, 100, 200 ng) were added to a nitrocellulose membrane and cross-linked under an ultraviolet lamp for 16 min. Subsequently, the membrane was washed with TBST and incubated with 5% skim milk for 30 min. The remaining procedures followed the Western blots (WB) assay. Methylene blue (MB) staining was used as a loading control.
m6A RNA methylation quantification
The level of RNA containing m6A modification in HCC cells was detected using EpiQuik m6A methylation quantification kit (Epigentek, Farmingdale, USA). After total RNA was extracted and mRNA was purified, the level of m6A in 100 ng of mRNA was measured according to the instruction of manufacturer.
Methylated RNA immunoprecipitation-qPCR
The level of m6A-modified ATG3 in HCC cells was detected using the Methylated RNA Immunoprecipitation-qPCR (MeRIP-qPCR) method. After total RNA was extracted and purified, the extraction of m6A-modified RNA was performed using MeRIP kit (BersinBio, Guangzhou, China) according to the manufacturer's guideline. Then, isolated RNA was subjected to qPCR assay to detect the level of m6A-modified ATG3.
RNA stability assay
HCC cells were planted into a 6-well plate and subjected to betaine treatment for 24 h. Then, the medium was removed, fresh medium containing 5 μg/mL Actinomycin D (MCE, Shanghai, China) was added into each well. Cells were harvested after different time of culture (0, 3, 6 h). The remaining procedures followed the RNA isolation and qPCR assay.
RNA immunoprecipitation-qPCR
The RNA immunoprecipitation-qPCR (RIP-qPCR) kit (BersinBio) was used to detect the interaction between ATG3 mRNA and YTHDF1 protein. Briefly, HCC cells were lysed by RIP lysis buffer at 4 ℃ for 30 min. Meanwhile, 7.5 μg of YTHDF1 primary antibody was pre-bound to Protein A/G magnetic beads in immunoprecipitation buffer for 1.5 h. Then the cell lysates were added and rotated at 4 ℃ overnight. Afterward, RNA was eluted with elution buffer and extracted with TransZOL reagent. Enrichment of ATG3 mRNA was detected by qPCR assay. The IgG antibody was used as a negative control.
RNA pull-down assay
RNA pull-down kit (BersinBio) was used to validate the interaction between ATG3 mRNA and YTHDF1 protein. The biotin-labelled ATG3 sense probe and the antisense probe were designed and synthesized by GenePharm. Briefly, the biotin-labelled ATG3 probe was added to streptavidin magnetic beads and precoated for 60 min. Then, cell lysates were mixed with the pre-coated magnetic bead and incubated at 4 °C for 150 min. Finally, proteins were eluted and subjected to WB assay.
Construction of wild type and mutant cell with m6A-recognition site of YTHDF1
To validate that the binding of YTHDF1 protein and ATG3 mRNA was m6A dependent, the YTHDF1 specific m6A-recognition site mutant lentivirus was constructed by GenePharm (YTHDF1-MUT), and the wild type of YTHDF1 (YTHDF1-WT) was used as control (Table S1). After construction, the following procedures follow the process described in supplementary method section regarding the construction of stable transfected cell line.
Animal purchase and feeding
Four-week BALB/c nude and NCG mice (male) were purchased from the Laboratory Animal Center of Sun Yat-sen University and Gempharmatech (Jiangsu, China), respectively. Mice were kept in an individual ventilated cage at the specific pathogen free (SPF) animal facility. All the animal studies have received permission from the animal experiments ethics committee (No. 2019-020), and conducted according to the institutional guidelines for the care and use of animals. After one week of acclimation, the mice were randomly assigned to different groups to carry out the following animal assays.
In vivo limiting dilution assay
To detect the stem cell-like properties of HCC cells, in vivo limiting dilution assay was performed. Briefly, HCC spheres with different treatments were dissociated using accutase and suspended in PBS at a final density of 2×105, 2×106, 2×107 cells/mL, respectively. Then, different concentrations of HCC cells (100 μL) were subcutaneous injected into mice (n = 5). After 30 days, the mice were humanely euthanized and tumors were extracted. The ability of tumor formation was calculated using online ELDA software in accordance with the instructions.
Tail vein-lung metastasis assay
To investigate the metastatic ability, tail vein-lung metastasis assay was conducted. Briefly, HCC cells were subjected to different treatments for the generation of sphere cells. Then, sphere cells in different groups were dissociated with accuatse and diluted in PBS to reach a final density of 5×107 cells/mL. Subsequently, 100 μL of cell mixture was injected into the lateral tail vein of mice (n = 8). The survival time of each mouse was recorded. Euthanasia was performed 66 days post-injection, and the lung tissue samples were obtained. Survival analysis was carried out to compare the difference of the survival time between different groups.
Establishment of patient derived xenograft model
Patient derived xenograft (PDX) model was established to detect the effect of betaine on the stem cell-like properties, autophagy, and m6A modification in HCC tissues. Briefly, after surgery, the fresh HCC tissues were preserved in ice-cold Hanks Balanced salt Solution containing 5% antibiotic-antimycotic and delivered to the animal laboratory on ice within 2 h. Then, the HCC tissues were sectioned into a 1 mm3 fragments, and transplanted into the NCG mice on the subcutaneous side. After 90 days of first generation (G1) growth, the HCC tumor tissues from G1 mice were extracted and transplanted into NCG mice for second generation (G2) growth. After a further 60 days of growth, the G2 tumor tissues were isolated for the following intervention experiment.
In short, NCG mice were subjected to normal and 3% betaine containing drinking water for 7 days before the third generation (G3) transplantation. After G3 transplantation, mice in the normal and 3% betaine drinking group were randomly divided into 4 groups: 1) Control group: received normal drinking water and intraperitoneally injected with 100 µL of water every four days; 2) HCQ (Hydroxychloroquine) group: intraperitoneally injected with 100 µL of 60 mg/kg HCQ every four days; 3) Betaine group: received drinking water containing 3% betaine, and intraperitoneally injected with 100 µL of water every four days; 4) Betaine + HCQ group: received drinking water containing 3% betaine, and intraperitoneally injected with 100 µL of 60 mg/kg HCQ every four days. Mice were continually fed for 60 days. Subsequently, the mice were humanely euthanized and the HCC tumor tissues from G3 mice were collected for following assays.
Statistical analysis
All data are presented as mean ± SD. The difference between two groups was calculated by the Student's t-test using SPSS 22.0 software (IBM, Armonk, USA). Survival analysis was performed using the Log-rank test. All statistical graphs and figures were generated using GraphPad Prism 8.0 (San Diego, USA) and Adobe Illustrator CS6 (Adobe, San Jose, USA) software, respectively. All experiments were repeated at least 3 times. A P value less than 0.05 was regarded as statistically significant.
Results
Betaine inhibits the stem-cell like properties of HCC cells
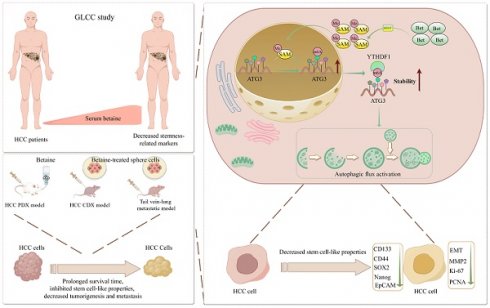
To obtain a comprehensive understanding of betaine's impact on the stem cell-like properties of HCC, both the serum betaine levels and the expression of stemness-related markers in 70 HCC patients were assessed. The results indicated that patients with higher serum betaine levels exhibited reduced expression of stemness-related markers (Figure 1A-E and Table S2), suggesting that betaine may suppress the stem cell-like properties of HCC cells. Then, a PDX model of HCC was established and treated with betaine (Figure S1A). The findings revealed that betaine significantly inhibited tumor growth, as well as the volume and weight of HCC tumors (Figure 1F-H). Histologically, compared with the control (Ctrl) group, there was a reduction in nuclear atypia, mitotic figures, and the nuclear-to-cytoplasmic ratio after betaine (Bet) intervention. In addition, increased tumor cell pyknosis and dissolution of nuclei were observed (Figure 1I). This was accompanied by a downregulation of stemness-related markers in the betaine-treated group (Figure 1J and Figure S1B).
Subsequently, HCC cells were treated with 200 mM of betaine to investigate its impact on their stem cell-like properties (Figure S1C-F). In vitro assays demonstrated that betaine treatment significantly attenuated the stem cell-like properties of HCC cells, manifested by the downregulation of stemness-related markers, a decrease in sphere number, and a reduction in sphere formation capacity (Figure 1K-L and Figure S1G). Moreover, in vivo limiting dilution assay confirmed that betaine markedly suppressed the tumorigenic abilities of HCC sphere cells (Figure 1M). In summary, these results collectively demonstrate that betaine effectively inhibits the stem cell-like properties of HCC.
Betaine inhibits the stemness of HCC cells. (A-D) The correlation between serum betaine concentrations and the expression levels of stemness-related markers in HCC tissues from the GLCC cohort (n = 70) was analyzed using Pearson correlation coefficient. (E) HCC tissues were divided into four groups based on their serum betaine levels using the quartile method, seven HCC tissues were randomly chosen from the lowest quartile group to represent the low level of serum betaine group, and another seven HCC tissues were randomly selected from the highest quartile group to represent the high level of serum betaine group. The expression levels of CD133, CD44, SOX2, Nanog and EpCAM proteins were analyzed by WB assay. (F) Effects of betaine (Bet), HCQ, and Bet+HCQ on the growth of HCC were evaluated via an PDX model (n = 5/group). Tumor entity view. (G-H) Statistical analysis of tumor volume and tumor weight in PDX model among different groups. (I) HE staining of PDX tumor tissues (scale bars = 100 μm for left panel, and 40 μm for right panel). (J) IHC analysis of CD133, CD44, and Nanog in PDX tumor tissues with or without betaine treatment (scale bars = 100 μm). (K) Effects of betaine on the sphere formation ability of HCC cells were evaluated (scale bars = 100 μm). A statistical graph is shown. (L) Effects of betaine on the stemness of HCC cells were measured by in vitro limiting dilution assay (n = 18/group). (M) HCC cells were treated with betaine and subjected to sphere cells formation. Then, sphere cells were diluted in a gradient and injected subcutaneously into nude mice. The number of tumors in each group was counted after 30 days. The injected cell quantity and tumor formation frequency (n = 5 per group) were shown. *P < 0.05, **P < 0.01, ***P < 0.001.
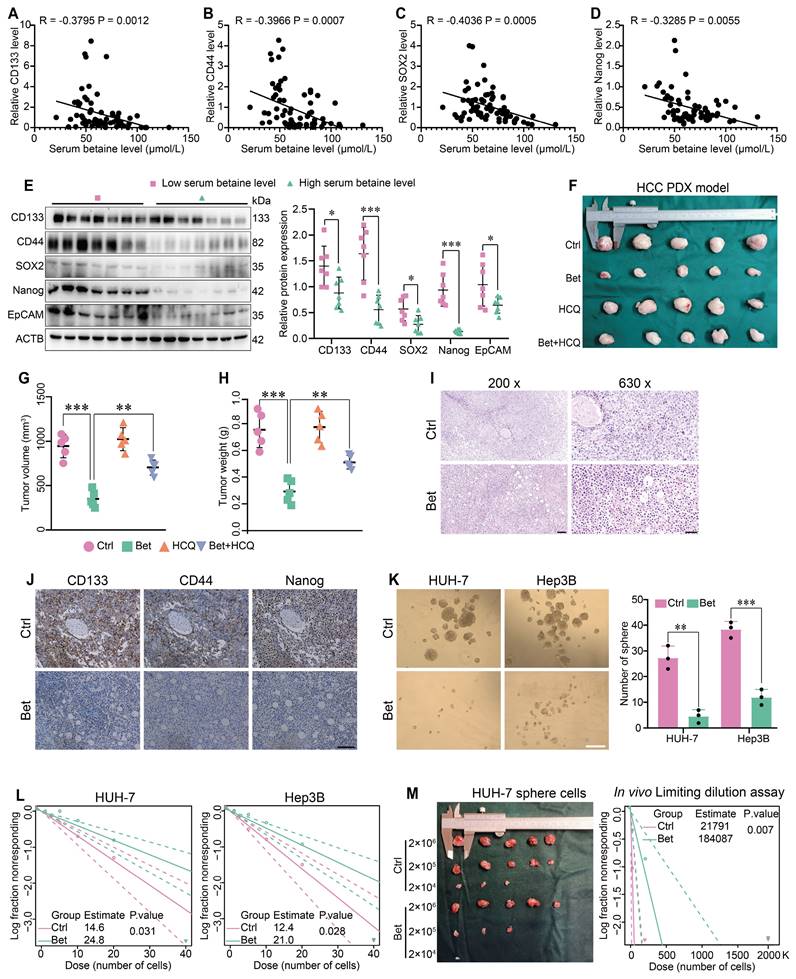
Betaine inhibits the malignant progression of HCC. (A) IHC analysis of E-cadherin, N-cadherin, and Ki-67 in PDX tumor tissues with or without betaine treatment (scale bars = 100 μm). (B-D) HCC cells were treated with betaine and subjected to sphere cells formation, then sphere cells were dissociated and subjected to colony formation and transwell assays to detect their clonogenic, migrative and invasive capabilities. Statistical graphs are shown. (E) HCC cells were treated with betaine and subjected to sphere cells formation, then sphere cells were dissociated and 5×106 of cells were injected into the tail vein of nude mice. After 66 days of injection, mice were humanely euthanized. Images of lung metastatic nodules and H&E staining are shown (scale bar = 1 mm for left panel, and 200 μm for right panel). (F) Quantitative results of lung metastatic nodules for the Bet and Ctrl groups. (G) Kaplan-Meier survival curve analysis of nude mice injected with HCC sphere cells (n = 8/group). HR: Hazard Ratio. **P < 0.01, ***P < 0.001.
Amelioration of the malignant progression of HCC following suppression of stem cell-like properties by betaine
The stem cell-like properties of cancer cells are associated with malignant cellular behaviors, including increased growth, metastasis, and epithelial-mesenchymal transition (EMT). Therefore, we evaluated the change of growth and metastasis of HCC cells after the inhibition of stem cell-like properties by betaine. WB and IHC assays indicated that betaine notably suppressed the expression of the protein markers associated with growth and metastasis and inhibited EMT in PDX tissues (Figure 2A and Figure S2A). In addition, sphere cells derived from betaine-treated HCC cells showed decreased viability and motility, as evidenced by inhibited colony formation, migration, and invasion (Figure 2B-D and Figure S2B-C). Similarly, the expression of protein markers associated with growth, metastasis, and EMT was downregulated in betaine-treated sphere cells (Figure S2D). Lastly, in a mouse model of tail vein metastasis, mice injected with betaine-treated sphere cells exhibited decreased metastasis and extended survival times (Figure 2E-G and Figure S2E). Thus, our data demonstrate that betaine ameliorates the malignant progression of HCC.
Betaine inhibits the stem cell-like properties of HCC by activating autophagy
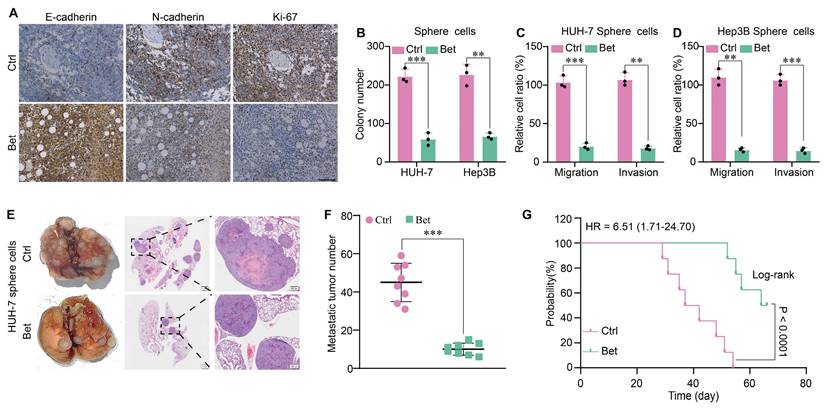
Previous study has indicated that the protective effect of betaine on liver disease is linked to increased autophagy [25]. Therefore, we investigated the impact of betaine on autophagy in HCC cells. Our findings revealed that betaine treatment significantly elevated the levels of LC3 II in HCC cells and PDX tissues (Figure 3A, and Figure S3A-B). The observed increase in LC3 II levels could be due to either the activation of autophagy or the inhibition of autophagosome degradation. To clarify this, we examined the autophagic flux in HCC cells treated with betaine. TEM and mCherry-GFP-LC3 transfection results demonstrated that betaine treatment significantly promoted autophagosome formation and contributed to a higher red/yellow puncta ratio (Figure 3B-C and Figure S3C-D), suggesting the activation of autophagy by betaine in HCC cells.
To investigate whether the inhibitory effects of betaine on the stem cell-like properties of HCC cells are linked to the activation of autophagy, we first examined the change in stemness- and autophagy-related markers in HCC cells with betaine treatment. IF assay showed that the expression of Nanog was downregulated while LC3B was upregulated in HCC cells after betaine treatment (Figure 3D and Figure S3E), indicating the negative correlation of stem cell-like properties and autophagy in HCC cells under betaine treatment. Then, we utilized HCQ (a terminal autophagy inhibitor) to inhibit autophagy in PDX tumors and found that inhibition of autophagy impaired the antitumor effects of betaine on HCC (Figure 1F-H). Histologically, compared with the betaine group, there was an increase in nuclear atypia, mitotic figures, nuclear-to-cytoplasmic ratio, and reduced tumor cell pyknosis and dissolution of nuclei were observed in Bet+HCQ group (Figure 3E). In addition, a significant elevated stemness-related marker was detected in Bet+HCQ group compared to Bet group (Figure 3F and Figure S3F-H). Subsequently, autophagy was inhibited in HCC cells using HCQ and ATG5 shRNA/siRNA treatments, and the changes in stem cell-like properties were observed (Figure S4A-B, S4G-H, and S5A-H). Results revealed that autophagy inhibition significantly reduced the inhibitory effects of betaine on the stem cell-like properties of HCC cells, as demonstrated by upregulated stemness-related markers and sphere formation ability (Figure 3G-H and Figure S6A-D).
Subsequently, we measured the change of malignant cellular phenotypes of HCC following upregulation of stemness. Results showed that the inhibition of EMT, metastasis, and growth-related markers by betaine treatment was reversed when autophagy was suppressed in PDX tumors and HCC cells (Figure 4A-C and Figure S7A-C). Meanwhile, sphere formation, colony formation, transwell, and in vivo tumor growth assays showed that both HCQ treatment and ATG5 silencing significantly mitigated the inhibitory effects of betaine on these cellular phenotypes (Figure 4D-K and Figure S7D-M). Furthermore, an in vivo metastasis assay showed that the inhibition of autophagy markedly increased the metastatic ability and shortened the survival time of mice compared to the Bet group (Figure 4L-N). Hence, our findings demonstrate that the inhibition of autophagy attenuates the effects of betaine on the stem cell-like properties and malignant phenotypes of HCC.
Betaine inhibits the stem cell-like properties of HCC by activating autophagy. (A) After betaine treatment, the expression levels of LC3 I/II protein in HUH-7 cells and PDX tumor tissues were analyzed by WB assay. (B) HUH-7 cells were treated with betaine, and the intracellular autophagosomes were captured via a TEM (scale bar = 2 μm for left panel and 1 μm for right panel). A statistical graph is shown. (C) HUH-7 cells were pre-transfected with mCherry-GFP-LC3B, and then subjected to betaine treatment. The red and yellow puncta were captured by a confocal microscope and quantitated (scale bar = 20 μm). A statistical graph is shown. (D) HUH-7 cells were treated with betaine, the expression levels of Nanog and LC3B proteins in the same cells were detected by IF assay (scale bar = 20 μm). (E) HE staining of PDX tumor tissues (scale bar = 100 μm for upper panel, 40 μm for lower panel). (F) After being exposed to betaine, HCQ, or combined betaine and HCQ treatments, IHC analysis of CD133, CD44, Nanog, and LC3B in PDX tumors (scale bars = 100 μm). (G) HUH-7 cells were pre-transfected with shATG5, and then subjected to betaine treatment. The expression levels of CD133, CD44, SOX2, Nanog, EpCAM, and LC3B proteins were analyzed by WB assay. (H) HUH-7 cells were subjected to betaine, HCQ or combined betaine and HCQ treatments, and the expression levels of CD133, CD44, SOX2, Nanog, EpCAM, and LC3B proteins were analyzed by WB assay. *P < 0.05, **P < 0.01, ***P < 0.001.
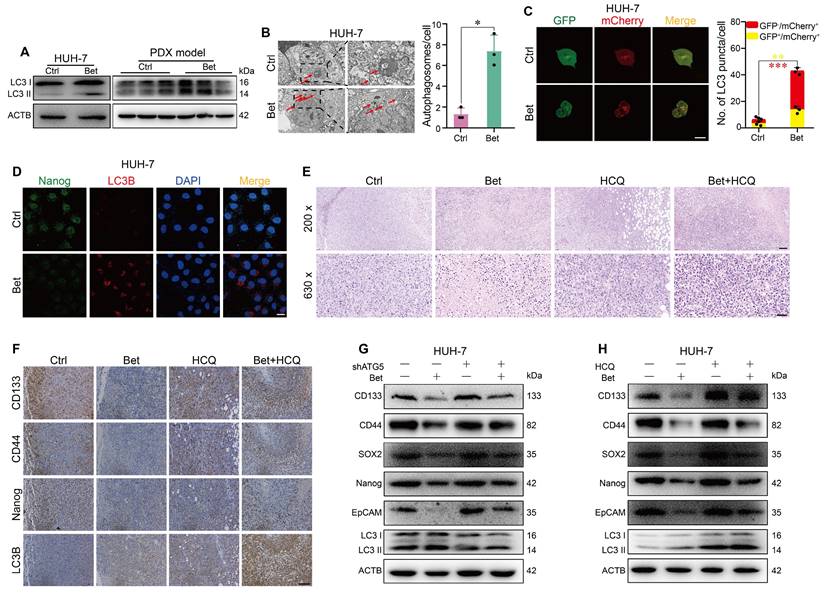
Inhibition of autophagy impairs the antitumor effects of betaine on HCC. (A) IHC results of E-cadherin, N-cadherin, and Ki-67 in PDX tumor tissues (scale bars = 100 μm). (B-C) WB analysis of the expression levels of E-cadherin, N-cadherin, Vimentin, MMP2, Ki-67, and PCNA proteins in HUH-7 sphere cells. (D) HUH-7 cells were pre-transfected with shATG5, and then were subjected to betaine treatment. The tumor sphere formation ability of HUH-7 cells under different treatments was measured (scale bars = 100 μm). (E) HUH-7 cells were subjected to betaine, HCQ, or combined betaine and HCQ treatments, and the tumor sphere formation ability of HUH-7 cells under different treatments was measured (scale bars = 100 μm). (F-G) HUH-7 cells were pre-transfected with shATG5 and subjected to betaine treatment for generation of sphere cells. Then, HUH-7 sphere cells were diluted in a gradient and injected subcutaneously into nude mice. The number of tumors in each group was counted after 30 days. The injected cell quantity and tumor formation frequency (n = 5 per group) were shown. (H-K) HUH-7 cells were pre-transfected with shATG5 or treated with HCQ, then subjected to betaine treatment for generation of sphere cells. After being dissociated and diluted, sphere cells were subjected to colony formation, and transwell migration and invasion assays. Statistical graphs are shown. (L) HUH-7 cells were pre-transfected with shATG5 and subjected to betaine treatment for generation of sphere cells. After being dissociated and diluted, 5×106 of cells were injected into the tail vein of nude mice. After 66 days of injection, mice were humanely euthanized. Images of lung metastatic nodules and H&E staining were shown (scale bar = 1 mm for upper panel, and 200 μm for lower panel). (M) Quantitative results of lung metastatic nodules in different groups. (N) Kaplan-Meier survival curve analysis of nude mice injected with HCC cells in different groups (n = 8/group). HR: Hazard Ratio. *P < 0.05, **P < 0.01, ***P < 0.001.
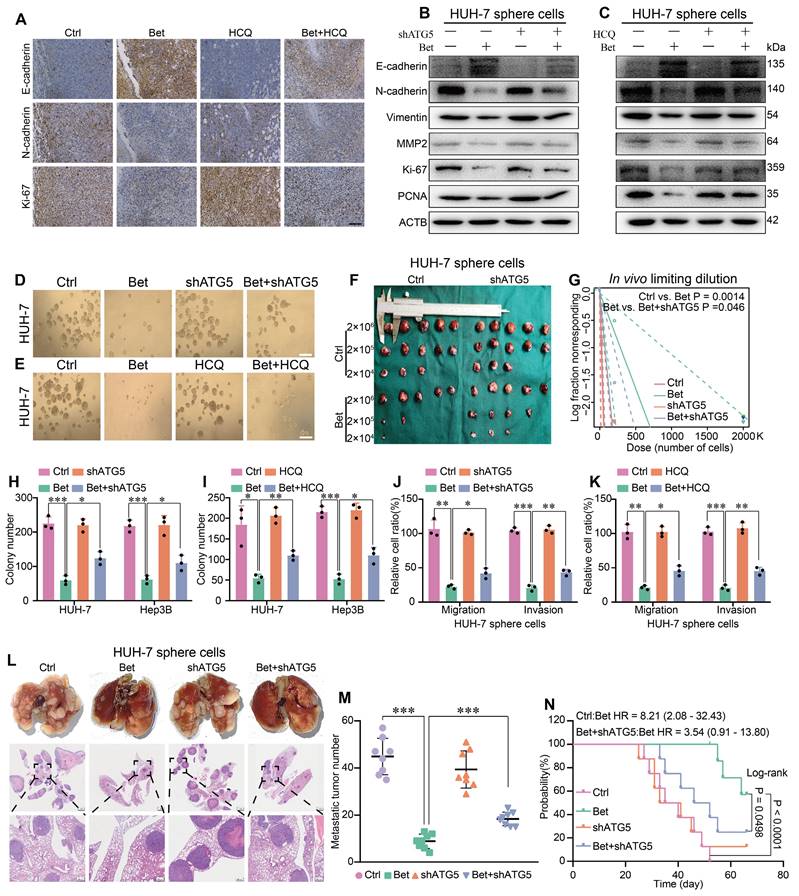
Betaine activates autophagy via SAM-mediated m6A modification. (A) HCC tissues from GLCC cohort (n = 70) were divided into four groups based on their serum betaine levels using the quartile method, and the levels of m6A modification in HCC tissues of these four groups (Q1 for quartile 1 group, Q2 for quartile 2 group, Q3 for quartile 3 group, Q4 for quartile 4 group) were detected. (B-E) The effects of betaine exposure on the m6A modification in HCC cells and PDX tumor tissues were detected using dot blot and quantified using the EpiQuik m6A methylation quantification kit, respectively. (F-G) After being exposed to betaine, the concentration of SAM in HCC cells and PDX tumor tissues was detected by a SAM ELISA kit. (H) HCC cells were treated with various concentrations of SAM (0, 25, 50, and 100 μM), and the concentration of SAM in HCC cells was detected by a SAM ELISA kit. (I-J) HCC cells were treated with various concentrations of SAM, and the m6A modification in HCC cells was detected using dot blot and quantified using the EpiQuik m6A methylation quantification kit, respectively. (K-N) HCC cells were pre-transfected with siBHMT, and then subjected to betaine or combined betaine and SAM treatments. The SAM levels, m6A modification, and LC3 I/II protein levels in HCC cells were detected via SAM ELISA kit, dot blot, EpiQuik m6A methylation quantification kit, and WB assay, respectively. *P < 0.05, **P < 0.01, ***P < 0.001.
Betaine promotes autophagy via SAM-mediated m6A modification
As one of the pivotal epigenetic modifications in eukaryotes, m6A methylation has been reported to play a role in the activation of autophagy[29, 38]. Interestingly, betaine has been shown to act as an important methyl donor source for the generation of SAM. Consequently, we further explored whether betaine could promote autophagy in HCC cells via SAM-mediated m6A modification. In GLCC cohort, we found that HCC patients with a high level of serum betaine had an increased m6A content in HCC tissues (Figure 5A). Similarly, betaine treatment significantly elevated m6A content in HCC cells and PDX tumors (Figure 5B-E), while inhibition of autophagy, either through treatment with HCQ or transfection with shATG5, has no effect on m6A modification (Figure S8A-F).
Subsequently, we investigated the role of SAM in betaine-induced m6A modification in HCC. We found that betaine treatment markedly increased SAM levels in HCC cells and PDX tissues (Figure 5F-G), but no changes were observed when suppressing autophagy (Figure S8G-I). Moreover, SAM promoted m6A modification in a dose-dependent manner in HCC cells (Figure 5H-J). However, silencing of BHMT not only decreased the production of SAM but also inhibited the m6A modification in HCC cells, while additional SAM supplementation counteracted the downregulation effect of BHMT (Figure 5K-M, Figure S4C and S4I). Furthermore, our results demonstrated that knockdown of BHMT inhibited the expression of LC3 II, reduced the number of autophagosomes, and decreased LC3 puncta, whereas SAM treatment significantly reversed these effects (Figure 5N and Figure S9A -D). Therefore, our results imply that betaine activates autophagy in HCC via SAM-mediated m6A modification.
Betaine activates autophagy by promoting the expression of ATG3 in a SAM/m6A-dependent manner
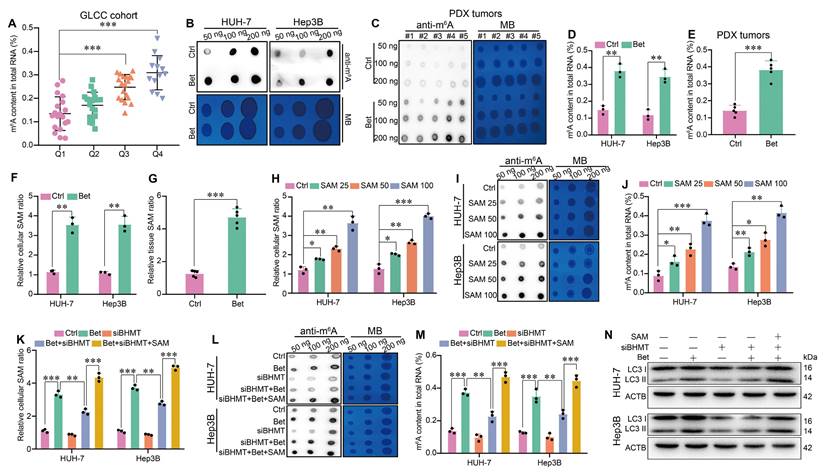
The process of autophagy activation is tightly regulated by a class of autophagy- related genes (ATGs). To clarify the target genes of betaine, we measured the m6A modification of ATGs in betaine-treated HCC cells. Initially, qPCR results indicated that among 13 key ATGs, only ATG3 and ATG5 mRNA were consistently upregulated, while SQSTM1 and BECN 1 were downregulated in HUH-7 and Hep3B cell lines after betaine exposure (Figure 6A). MeRIP-qPCR results demonstrated that betaine treatment increased the m6A modification on ATG3 and BECN 1 mRNA in two cell lines (Figure 6B), suggesting that betaine-mediated m6A modification may activate autophagy via ATG3. Meanwhile, MeRIP-qPCR further confirmed that the m6A modification at the predicted site 2 of ATG3 mRNA was significantly elevated in Bet group, while no significant change was observed in site 1(Figure 6C-D). In addition, WB and IHC assays illustrated that betaine exposure markedly enhanced the expression of ATG3 protein (Figure 6E-F).
Betaine activates autophagy via SAM-mediated m6A modification on ATG3 mRNA. (A) The effects of betaine exposure on the expression levels of autophagy-related genes in HCC cells were detected by qPCR analysis. (B) After treatment with betaine, MeRIP-qPCR assay was performed to measure the level of m6A-modified ATGs in HCC cells. (C) m6A modification sites in ATG3 mRNA were predicted via SRAMP database and those with high confidence were selected for further investigation. (D) After treatment with betaine, the level of m6A modification in these two sites of ATG3 mRNA in HCC cells was detected using MeRIP-qPCR assay. (E-F) After treatment with betaine, the expression levels of ATG3 protein in HCC cells and PDX tumor tissues were measured using WB and IHC assays (Scale bar = 100 μm), respectively. (G-I) HCC cells were pre-transfected with siBHMT, and then were subjected to betaine or combined betaine and SAM treatments. The level of m6A modification in ATG3 mRNA, as well as the expression levels of ATG3 mRNA and protein, were detected using MeRIP-qPCR, qPCR, and WB assays, respectively. (J) HCC cells were pre-transfected with shATG3, and then were subjected to betaine treatment, the expression levels of LC3 I/II proteins were detected using WB assay. *P < 0.05, **P < 0.01, ***P < 0.001.
Then, we explored whether the increase in ATG3 expression induced by betaine was attributed to SAM-mediated m6A modification. Results found that BHMT silencing not only inhibited m6A modification on ATG3 mRNA but also attenuated the expression of ATG3 mRNA and protein in HCC cells, while SAM supplementation reversed those effects (Figure 6G-I). Furthermore, silencing of ATG3 impaired the activation of autophagy by betaine in HCC cells (Figure 6J, Figure S4D-E, S4J-K, and S10A-D). Thus, our results demonstrate that betaine activates autophagy by upregulating ATG3 via SAM-mediated m6A modification.
Betaine enhances the stability of ATG3 mRNA in a SAM/m6A/YTHDF1-dependent manner
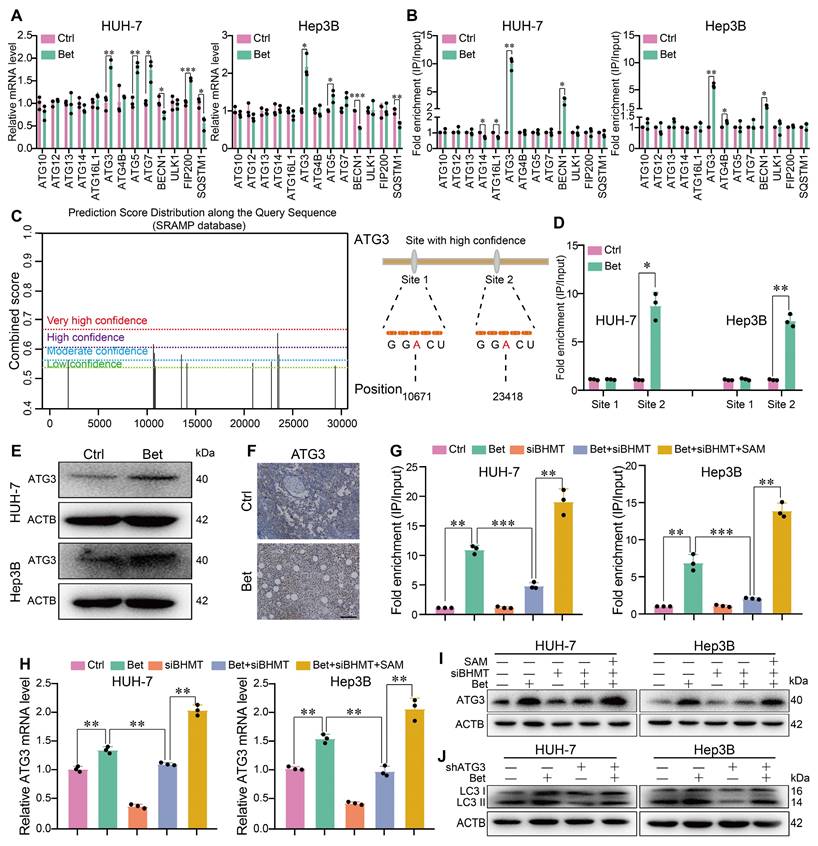
It has been reported that the m6A modification can influence the stability, translation, splicing, decay, and transport of RNAs through specific reader proteins [39]. To elucidate which reader mediates the upregulation of ATG3 under betaine treatment via m6A-dependent manner, we initially focused on the stability of ATG3 mRNA, given the observed elevation of ATG3 mRNA levels under betaine treatment. qPCR assays revealed that betaine significantly restored the level of ATG3 mRNA after Act D treatment compared to the control (Ctrl) group (Figure 7A), and silencing of BHMT attenuated the upregulation of ATG3 mRNA level under betaine treatment (Figure 7B). However, additional SAM supplementation significantly reversed the effect of BHMT knockdown on betaine-increased ATG3 mRNA stability (Figure 7B), suggesting that betaine could enhance the stability of ATG3 mRNA via SAM-mediated m6A modification. Next, bioinformatic analysis found that, among the m6A-related readers that mediates the stability of RNAs, YTHDF1 was significantly positively correlated with ATG3 expression in HCC tissues (Figure 7C). Therefore, we further investigated the role of YTHDF1 in mediating ATG3 mRNA stability. RIP-qPCR and RNA pull-down results demonstrated that YTHDF1 could directly interact with ATG3 mRNA (Figure 7D-E).
To confirm that the interaction between YTHDF1 protein and ATG3 mRNA was m6A-dependent, we genetically impaired the m6A-binding pockets of YTHDF1 by mutating the K395 and Y397 residues within the YTH domain (Figure 7F). RIP-qPCR results indicated that ATG3 mRNA was significantly immunoprecipitated from HCC cells transfected with YTHDF1-WT, while the interaction between YTHDF1-MUT and ATG3 mRNA was significantly decreased, implying the pivotal role of m6A-binding pocket of YTHDF1 in its interaction with ATG3 mRNA (Figure 7G). Moreover, we found that YTHDF1-WT, but not YTHDF1-MUT, significantly upregulated the levels of ATG3 mRNA and protein (Figure 7H-I). Finally, we found that knockdown of YTHDF1 not only decreased ATG3 mRNA and protein levels, but also impaired the enhancing effect of betaine on ATG3 mRNA and protein levels as well as ATG3 mRNA stability (Figure 7J-N and Figure S4F). Thus, our data demonstrate that betaine could promote ATG3 mRNA stability via a SAM/m6A/YTHDF1-dependent mechanism.
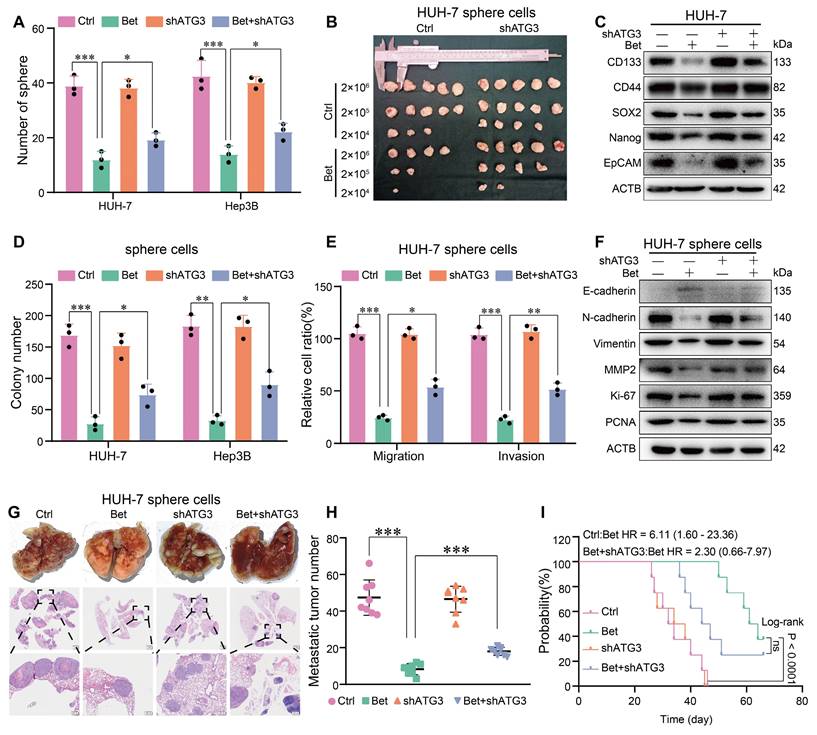
Silencing of ATG3 impairs the effects of betaine on the stem cell-like properties of HCC cells
Given the crucial role of ATG3 in betaine-activated autophagy, we conducted rescue assays to investigate the effect of ATG3 on the suppression of stem cell-like properties in HCC cells induced by betaine. Results from sphere formation, in vitro, and in vivo limiting dilution assays revealed that silencing of ATG3 significantly impaired the inhibitory effects of betaine on the stem cell-like properties of HCC cells (Figure 8A-B and Figure S11A-D). Additionally, the knockdown of ATG3 markedly attenuated the downregulation of stemness-related markers induced by betaine treatment (Figure 8C and Figure S11E).
Furthermore, colony formation, transwell, WB, and in vivo metastasis assays demonstrated that ATG3 silencing mitigated the effects of betaine on malignant cellular phenotypes, as evidenced by increased colony formation, enhanced migration and invasion, upregulated protein markers, and elevated metastatic nodules (Figure 8D-H and Figure S11F-I). Although there was no significant difference in the survival duration between betaine group and betaine+shATG3 group, we can still observe a decreased trend of survival time in betaine+shATG3 group (Figure 8I). In summary, our findings illustrate that betaine inhibits the stem cell-like properties of HCC cells through the upregulation of ATG3 in SAM/m6A/YTHDF1-dependent manner.
Discussion
Although a few studies have reported the protective effects of betaine on liver diseases, its roles in HCC progression, particularly in the context of stem cell-like properties, remain unknown. Furthermore, the mechanism underlying how betaine exhibits its anti-tumor effects has been poorly investigated. In this study, we found that betaine significantly inhibited the stem cell-like properties of HCC cells, as well as suppressed the growth and metastasis of HCC sphere cells both in vitro and in vivo. Moreover, the anti-tumor effects of betaine were markedly attenuated by inhibiting autophagy. Mechanistically, betaine promoted m6A modification on ATG3 mRNA by generating SAM, while YTHDF1 recognized and bound to ATG3 mRNA to enhance its stability. Collectively, betaine inhibited the stem cell-like properties of HCC by activating autophagy via SAM/m6A/YTHDF1-mediated enhancement on ATG3 stability.
Betaine promotes ATG3 mRNA stability via SAM/m6A/YTHDF1-dependent manner. (A) After treatment with betaine, HCC cells were treated with 5 μg/mL of Act D for 0, 3, and 6 h, respectively. The expression levels of ATG3 mRNA at these different time points were detected using qPCR assay. (B) After pre-transfection with siBHMT, HCC cells were subjected to betaine or combined betaine and SAM treatments. Then, 5 μg/mL of Act D was added for further treatment for 0, 3, and 6 h, respectively. The expression levels of ATG3 mRNA at these different time points were detected using qPCR assay. (C) The correlation between ATG3 and YTHDF1 in HCC tissues was assessed using online GEPIA 2 database. (D) The interaction between ATG3 mRNA and YTHDF1 protein was analyzed using RIP-qPCR assay, and the RIP-derived protein and ATG3 mRNA in HCC cells were detected by WB and qPCR assays, respectively. (E) The interaction between ATG3 mRNA and YTHDF1 protein was confirmed by RNA pull-down assay, and the protein derived from the RNA pull-down assay in HCC cells was detected using WB assay. (F) Schematic graph of the YTHDF1 wild-type (YTHDF1-WT) and the mutant of YTHDF1 m6A-binding pocket (YTHDF1-MUT) construction. (G) HCC cells were pre-transfected with YTHDF1-WT and YTHDF1-MUT overexpressed plasmids, and the interaction between ATG3 mRNA and YTHDF1 was analyzed via RIP-qPCR assay. (H and I) HCC cells were transfected with YTHDF1-WT and YTHDF1-MUT overexpressed plasmids, and the expression levels of ATG3 mRNA as well as the levels ATG3 and YTHDF1 proteins were detected using qPCR and WB assays, respectively. (J and K) HCC cells were transfected with siYTHDF1, and the expression levels of ATG3 mRNA as well as the levels of ATG3 and YTHDF1 proteins were detected using qPCR and WB assays, respectively. (L-M) HCC cells were pre-transfected with siYTHDF1, and then were subjected to betaine treatment. The expression levels of ATG3 mRNA and protein were detected using qPCR and WB assays, respectively. (N) After pre-transfection with siYTHDF1, HCC cells were subjected to betaine treatment. Then, 5 μg/mL of Act D was added for further treatment for 0, 3, and 6 h, respectively. The expression levels of ATG3 mRNA at these different time points were detected using qPCR assay. **P < 0.01, ***P < 0.001.
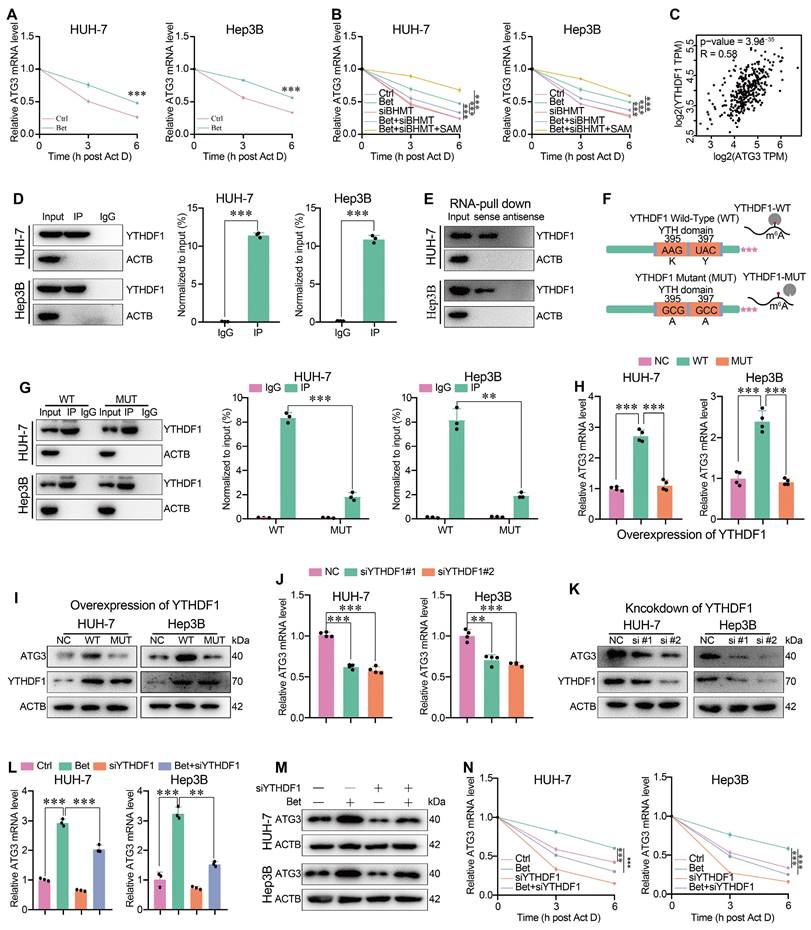
betaine inhibits the stem cell-like properties of HCC via ATG3. (A) HUH-7 cells were pre-transfected with shATG3 and subjected to betaine treatment. The tumor sphere formation ability of HUH-7 cells under different treatments was measured. A statistical graph is shown. (B) HUH-7 cells were pre-transfected with shATG3 and subjected to betaine treatment for generation of sphere cells. Then, HUH-7 sphere cells were diluted in a gradient and injected subcutaneously into nude mice. The number of tumors in each group was counted after 30 days. The injected cell quantity and tumor formation frequency (n = 5 per group) were shown. (C) HUH-7 cells were pre-transfected with shATG3, and then subjected to betaine treatment. The expression levels of CD133, CD44, SOX2, Nanog, and EpCAM proteins in HUH-7 cells were detected using WB assay. (D-E) HUH-7 cells were pre-transfected with shATG3 and subjected to betaine treatment for generation of sphere cells. After being dissociated and diluted, sphere cells were subjected to colony formation, and transwell migration and invasion assays. Statistical graphs are shown. (F) WB analysis of the expression levels of E-cadherin, N-cadherin, Vimentin, MMP2, Ki-67, and PCNA proteins in HUH-7 sphere cells under different treatments. (G) HUH-7 cells were pre-transfected with shATG3 and subjected to betaine treatment for generation of sphere cells. After being dissociated and diluted, 5×106 of cells were injected into the tail vein of nude mice. After 66 days of injection, mice were humanely euthanized. Images of lung metastatic nodules and H&E staining were shown (scale bar = 1 mm for upper panel, and 200 μm for lower panel). (H) Quantitative results of lung metastatic nodules in different groups. (I) Kaplan-Meier survival curve analysis of nude mice injected with HCC cells in different groups (n = 8/group). HR: Hazard Ratio; ns: not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
Despite great advancements in diagnosis and treatment, HCC remains the third leading cause of cancer-associated death worldwide. Recent studies have consistently shown that the primary contributors to poor prognosis in HCC patients are recurrence and distant metastasis, resulting in a significant reduction in overall and cancer-specific survival times [40-42]. Hence, targeting the risk factors associated with the recurrence and metastasis provides an opportunity for improving the prognosis of HCC. Cancer stem cells (CSCs) are a small population of cancer cells with high capacities of self-renewal, differentiation, and carcinogenic potential. They have been implicated in cancer recurrence and metastasis [43, 44]. Patients with elevated levels of CSCs-related genes tend to experience a worse prognosis, whereas suppression of stem cell-like properties can inhibit recurrence and metastasis. Recently, a few phytochemicals have been reported to possess the ability to hinder the stem cell-like properties of cancer cells. For example, curcumin has demonstrated its ability to inhibit breast cancer cell metastasis by suppressing stem cell-like properties [45]. Similarly, pharmacological ascorbate has been shown to reduce these properties, thereby preventing recurrence and systemic metastasis after surgery [46]. Betaine, a natural compound in many foods, has shown protective roles against liver cancer. For example, our previous studies found that high dietary betaine intake is associated with a reduced risk of primary liver cancer, and betaine treatment alleviated DEN-induced liver cancer in rats [20, 23]. Meanwhile, Oliva et al. reported that betaine administration inhibited the growth of murine HCC cells [47]. However, the impact of betaine on the late-stage-related phenotype of HCC, which significantly influences the prognosis of HCC patients, and the mechanisms behind its anti-tumor effects remain uninvestigated. In this study, we found that HCC patients with higher serum betaine levels exhibited lower expression of stemness-related genes. In vitro and in vivo experiments further demonstrated that betaine treatment significantly attenuated the stem cell-like properties of HCC cells. Moreover, mice treated with betaine exhibited reduced lung metastatic nodules and prolonged survival times.
Autophagy is a highly conserved catabolic process in eukaryotes that is crucial for maintaining cellular homeostasis [48]. The process, known as autophagic flux, involves a series of ATGs orchestrating the formation of double-membrane autophagosomes to sequester cellular components. These autophagosomes then fuse with lysosomes to degrade and recycle cargo, thereby supporting cancer cell survival [10]. Numerous studies have shown that the activation of autophagy contributes to the acquisition of stem cell-like properties in cancer cells through regulation of stemness-related genes or modulation of tumor microenvironments [49, 50]. Consequently, autophagy plays a significant role in the stem cell-like properties of cancer cells. However, recent research has unveiled a contrasting role for autophagy activated by phytochemicals in stem cell-like properties. For example, curcumin inhibits colorectal cancer stem cells by activating autophagy via suppressing TFAP2A/ECM pathway [51]. Similarly, posaconazole attenuates the stem cell-like properties by inducing autophagy in glioblastoma [44]. Although betaine has been reported to exert protective effects against liver diseases by activating autophagy [24, 25], its impact on the stem cell-like properties of HCC cells remains unknown. In the present study, we demonstrate that betaine activates autophagic flux to inhibit the stem cell-like properties of HCC cells both in vitro and in vivo, while inhibition of autophagy not only accelerates the growth and metastasis of HCC cells but also shortens the survival time of mice. Overall, our findings indicate that betaine inhibits the stem cell-like properties of HCC cells by activating autophagic flux.
As an important phytochemical, the protective roles of betaine have been well demonstrated in several liver diseases, but the underlying mechanisms remain poorly understood. Betaine serves two primary biological functions. Firstly, as an effective osmolyte, it regulates intercellular osmotic pressure akin to other electrolytes [34]. Secondly, betaine acts as a crucial methyl donor, producing SAM via BHMT, thereby regulating genomic methylation [34]. For example, several studies have linked betaine's protective effects to its modulation of DNA methylation in rat models [52-55]. In addition, recent attention has focused on betaine's regulation of RNA methylation, particularly m6A modification [56-58]. m6A modification is a prevalent epi-transcriptional alteration in mRNA that governs various mRNA processes, including splicing, nuclear transport, decay, stability, and translation, via specific readers. Increasing evidence highlights the crucial role of m6A modulation in autophagy activation, particularly its direct regulation of ATGs [28]. For example, YTHDF1 activates autophagy by stabilizing BECN1, leading to hepatic stellate cell ferroptosis and ameliorating liver fibrosis in murine models [59]. METTL14 suppresses the development of oral squamous cell carcinoma by activating autophagy via m6A/IGF2BP2-dependent modification on FIP200 stability [60]. Our study demonstrates that betaine treatment enhances total m6A modification in HCC cells in a BHMT/SAM-dependent manner, while inhibition of SAM by BHMT silencing significantly attenuates betaine-induced autophagic flux. Further investigation unveiled that betaine activates autophagic flux by promoting m6A modification on ATG3 mRNA, thereby upregulating its mRNA and protein expression. Conversely, ATG3 silencing impairs betaine's inhibitory effects on the stem cell-like properties of HCC cells. These data indicate that betaine inhibits the stem cell-like properties of HCC cells by activating ATG3-dependent autophagy via m6A modification.
After m6A modification, specific readers govern the fate of mRNAs. Given the increased m6A modification on ATG3 mRNA and the upregulation of its mRNA level, we investigated the mRNA stability under betaine treatment. RNA stability assay found that betaine significantly enhanced the stability of ATG3 mRNA. Silence of BHMT attenuated the effect of betaine on ATG3 mRNA stability, while additional SAM treatment reversed this effect, indicating that betaine promoted ATG3 expression by enhancing its stability via m6A modification. To date, several m6A-related readers, such as YTHDF1 and IGF2BP1/2/3, are known to enhance the stability of mRNAs. In the present study, we found that YTHDF1 was positively correlated with ATG3 expression in HCC. RIP-qPCR and RNA pull-down confirmed the interaction between YTHDF1 protein and ATG3 mRNA. Previous study has demonstrated that YTHDF1 recognizes and binds to the m6A site of targeted mRNA via m6A-binding pockets [28]. To further investigate whether the interaction between YTHDF1 and ATG3 was m6A dependent, we genetically mutated YTHDF1 at residues K395 and Y397. In line with previous findings, we found that YTHDF1 enhanced the stability of ATG3 mRNA in an m6A-dependent manner.
This study utilized different HCC line lines and animal models to investigate the effects of betaine on the stem cell-like properties of HCC. The consistency of the results provided a reliable, generalized, and comprehensive understanding of our study. However, there is a limitation in this study. While our study examines the effects of betaine treatment on the growth of PDX tumors and the changes of specific histological features such as nuclear atypia, mitosis, and the nuclear-to-cytoplasmic ratio, we did not observe significant differences in tumor grading, categorizing tumors into well, moderately, and poorly differentiated grades.
Conclusion
In this study, we found that betaine increased the m6A modification on ATG3 mRNA by producing SAM via BHMT. Subsequently, YTHDF1 was found to recognize and bind to ATG3 mRNA, enhancing its stability in an m6A-dependent manner. Consequently, the upregulated ATG3 activated autophagy, thereby inhibiting the stem cell-like properties of HCC cells. Our findings provide a novel understanding of the anti-tumor effects of betaine on HCC and its underlying mechanism, potentially expanding therapeutic options for HCC patients.
Abbreviations
ATGs: autophagy related genes; BHMT: betaine-homocysteine methyltransferase; CCK-8: cell counting kit-8; ELISA: enzyme-linked immunosorbent; EMT: epithelial-mesenchymal transition; GLCC: Guangdong liver cancer cohort; HCC: hepatocellular carcinoma; HE: hematoxylin & eosin; IF: immunofluorescence; IHC: immunohistochemistry; MeRIP-qPCR: Methylated RNA Immunoprecipitation-qPCR; m6A: N6-methyladenosine; PDX: patient derived xenografts; RIP-qPCR: RNA immunoprecipitation-qPCR; SAM: S-adenosylmethionine; TEM: transmission electronic microscopy.
Supplementary Material
Supplementary methods, figures and tables.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant numbers 81973016, 82103825], the Basic and Applied Basic Research Foundation of Guangdong Province, China [grant number 2020A1515110682], Guangdong Provincial Science and Technology Project [grant number 2017A040406008].
Author contributions
HL. Zhu conceived and supervised the study. C. Wang and ZY. Liu wrote the original draft of the manuscript. MC. Li, WG. Huang, SY. Huang, and M. Wusiman revised the manuscript. C. Wang, MC. Li, and ZY. Liu performed the experiments and analyzed the data.
Data availability
All data used are available in the main text and supporting information.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the School of Public Health at Sun Yat-sen University approved the protocol of GLCC (NCT03297255). Written informed consent was obtained from each participant. Animal studies were approved by the animal experiments ethics committee of Sun Yat-sen University (No. 2019-020), in compliance with institutional guidelines for the care and use of animals.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
2. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-62
3. Zhan H, Zhao X, Lu Z, Yao Y, Zhang X. Correlation and Survival Analysis of Distant Metastasis Site and Prognosis in Patients With Hepatocellular Carcinoma. Front Oncol. 2021;11:652768
4. Yoon JH, Choi SK, Cho SB, Kim HJ, Ko YS, Jun CH. Early extrahepatic recurrence as a pivotal factor for survival after hepatocellular carcinoma resection: A 15-year observational study. World J Gastroenterol. 2022;28:5351-63
5. Niu Q, Ye S, Zhao L, Qian Y, Liu F. The role of liver cancer stem cells in hepatocellular carcinoma metastasis. Cancer Biol Ther. 2024;25:2321768
6. Li J, Tang M, Wu J, Qu H, Tu M, Pan Z. et al. NUSAP1, a novel stemness-related protein, promotes early recurrence of hepatocellular carcinoma. Cancer Sci. 2022;113:4165-80
7. Liang R, Hong W, Zhang Y, Ma D, Li J, Shi Y. et al. Deep dissection of stemness-related hierarchies in hepatocellular carcinoma. J Transl Med. 2023;21:631
8. Wang X, Chen X, Zhao M, Li G, Cai D, Yan F. et al. Integration of scRNA-seq and bulk RNA-seq constructs a stemness-related signature for predicting prognosis and immunotherapy responses in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2023;149:13823-39
9. Zhang Y, Zhang R, Zeng L, Wang H, Peng R, Zhang M. et al. Identification and Validation of a Potential Stemness-Associated Biomarker in Hepatocellular Carcinoma. Stem Cells Int. 2022;2022:1534593
10. Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24:560-75
11. Yamamoto H, Zhang S, Mizushima N. Autophagy genes in biology and disease. Nat Rev Genet. 2023;24:382-400
12. Rahman MA, Saha SK, Rahman MS, Uddin MJ, Uddin MS, Pang MG. et al. Molecular Insights Into Therapeutic Potential of Autophagy Modulation by Natural Products for Cancer Stem Cells. Front Cell Dev Biol. 2020;8:283
13. Wu L, Xu S, Cheng X, Zhang L, Wang Y, Wu J. et al. Capsaicin inhibits the stemness of anaplastic thyroid carcinoma cells by triggering autophagy-lysosome mediated OCT4A degradation. Phytother Res. 2022;36:938-50
14. Dobrijevic D, Pastor K, Nastic N, Ozogul F, Krulj J, Kokic B. et al. Betaine as a Functional Ingredient: Metabolism, Health-Promoting Attributes, Food Sources, Applications and Analysis Methods. Molecules. 2023;28:4824
15. Zhang M, Wang XL, Shi H, Meng LQ, Quan HF, Yan L. et al. Betaine Inhibits NLRP3 Inflammasome Hyperactivation and Regulates Microglial M1/M2 Phenotypic Differentiation, Thereby Attenuating Lipopolysaccharide-Induced Depression-Like Behavior. J Immunol Res. 2022;2022:9313436
16. Chen S, Chen J, Wang C, He T, Yang Z, Huang W. et al. Betaine attenuates age-related suppression in autophagy via Mettl21c/p97/VCP axis to delay muscle loss. J Nutr Biochem. 2024;125:109555
17. Mohammadi N, Hemmati M, Motlagh B, Biyabani A. Betaine postpones hyperglycemia-related senescence in ovarian and testicular cells: Involvement of RAGE and beta-galactosidase. Cell Biochem Funct. 2024;42:e3973
18. Lin Q, Ge X, Gao L, Chen Y, Su T, Ma M. et al. Betaine alleviates spermatogenic cells apoptosis of oligoasthenozoospermia rat model by up-regulating methyltransferases and affecting DNA methylation. Phytomedicine. 2024;129:155713
19. Chen W, Xu M, Xu M, Wang Y, Zou Q, Xie S. et al. Effects of betaine on non-alcoholic liver disease. Nutr Res Rev. 2022;35:28-38
20. Zhou RF, Chen XL, Zhou ZG, Zhang YJ, Lan QY, Liao GC. et al. Higher dietary intakes of choline and betaine are associated with a lower risk of primary liver cancer: a case-control study. Sci Rep. 2017;7:679
21. Arumugam MK, Chava S, Perumal SK, Paal MC, Rasineni K, Ganesan M. et al. Acute ethanol-induced liver injury is prevented by betaine administration. Front Physiol. 2022;13:940148
22. Bingul I, Aydin AF, Basaran-Kucukgergin C, Dogan-Ekici I, Coban J, Dogru-Abbasoglu S. et al. High-fat diet plus carbon tetrachloride-induced liver fibrosis is alleviated by betaine treatment in rats. Int Immunopharmacol. 2016;39:199-207
23. Du YP, Peng JS, Sun A, Tang ZH, Ling WH, Zhu HL. Assessment of the effect of betaine on p16 and c-myc DNA methylation and mRNA expression in a chemical induced rat liver cancer model. BMC Cancer. 2009;9:261
24. Seo J, Kwon D, Kim SH, Byun MR, Lee YH, Jung YS. Role of autophagy in betaine-promoted hepatoprotection against non-alcoholic fatty liver disease in mice. Curr Res Food Sci. 2024;8:100663
25. Veskovic M, Mladenovic D, Milenkovic M, Tosic J, Borozan S, Gopcevic K. et al. Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur J Pharmacol. 2019;848:39-48
26. Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat Rev Genet. 2014;15:293-306
27. Lee M, Kim B, Kim VN. Emerging roles of RNA modification: m(6)A and U-tail. Cell. 2014;158:980-7
28. Li Q, Ni Y, Zhang L, Jiang R, Xu J, Yang H. et al. HIF-1alpha-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Target Ther. 2021;6:76
29. Hao W, Dian M, Zhou Y, Zhong Q, Pang W, Li Z. et al. Autophagy induction promoted by m(6)A reader YTHDF3 through translation upregulation of FOXO3 mRNA. Nat Commun. 2022;13:5845
30. He M, Lei H, He X, Liu Y, Wang A, Ren Z. et al. METTL14 Regulates Osteogenesis of Bone Marrow Mesenchymal Stem Cells via Inducing Autophagy Through m6A/IGF2BPs/Beclin-1 Signal Axis. Stem Cells Transl Med. 2022;11:987-1001
31. Sendinc E, Shi Y. RNA m6A methylation across the transcriptome. Mol Cell. 2023;83:428-41
32. Deng X, Qing Y, Horne D, Huang H, Chen J. The roles and implications of RNA m(6)A modification in cancer. Nat Rev Clin Oncol. 2023;20:507-26
33. Kumari S, Kumar S, Muthuswamy S. RNA N6-methyladenosine modification in regulating cancer stem cells and tumor immune microenvironment and its implication for cancer therapy. J Cancer Res Clin Oncol. 2023;149:1621-33
34. Arumugam MK, Paal MC, Donohue TM Jr, Ganesan M, Osna NA, Kharbanda KK. Beneficial Effects of Betaine: A Comprehensive Review. Biology (Basel). 2021;10:456
35. Chen J, Zhou X, Wu W, Wang X, Wang Y. FTO-dependent function of N6-methyladenosine is involved in the hepatoprotective effects of betaine on adolescent mice. J Physiol Biochem. 2015;71:405-13
36. Fang AP, Long JA, Zhang YJ, Liu ZY, Li QJ, Zhang DM. et al. Serum Bioavailable, Rather Than Total, 25-hydroxyvitamin D Levels Are Associated With Hepatocellular Carcinoma Survival. Hepatology. 2020;72:169-82
37. Liu ZY, Yishake D, Fang AP, Zhang DM, Liao GC, Tan XY. et al. Serum choline is associated with hepatocellular carcinoma survival: a prospective cohort study. Nutr Metab (Lond). 2020;17:25
38. Gao X, Wang J, Wang Y, Li W, Pan Z. The m(6)A Reader YTHDF1 Accelerates the Osteogenesis of Bone Marrow Mesenchymal Stem Cells Partly via Activation of the Autophagy Signaling Pathway. Stem Cells Int. 2023;2023:5563568
39. Flamand MN, Tegowski M, Meyer KD. The Proteins of mRNA Modification: Writers, Readers, and Erasers. Annu Rev Biochem. 2023;92:145-73
40. Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE. et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534-40
41. Chen ZH, Zhang XP, Feng JK, Li LQ, Zhang F, Hu YR. et al. Patterns, treatments, and prognosis of tumor recurrence after resection for hepatocellular carcinoma with microvascular invasion: a multicenter study from China. HPB (Oxford). 2022;24:1063-73
42. Bu Q, Wu D, Xia J, Wu M, Liu X, Cao Z. et al. Polybrominated diphenyl ethers and novel brominated flame retardants in indoor dust of different microenvironments in Beijing, China. Environ Int. 2019;122:159-67
43. Martinez-Outschoorn UE, Prisco M, Ertel A, Tsirigos A, Lin Z, Pavlides S. et al. Ketones and lactate increase cancer cell "stemness," driving recurrence, metastasis and poor clinical outcome in breast cancer: achieving personalized medicine via Metabolo-Genomics. Cell Cycle. 2011;10:1271-86
44. Wang H, Tan Y, Jia H, Liu D, Liu R. Posaconazole inhibits the stemness of cancer stem-like cells by inducing autophagy and suppressing the Wnt/beta-catenin/survivin signaling pathway in glioblastoma. Front Pharmacol. 2022;13:905082
45. Hu C, Li M, Guo T, Wang S, Huang W, Yang K. et al. Anti-metastasis activity of curcumin against breast cancer via the inhibition of stem cell-like properties and EMT. Phytomedicine. 2019;58:152740
46. Jiang X, Liu J, Mao J, Han W, Fan Y, Luo T. et al. Pharmacological ascorbate potentiates combination nanomedicines and reduces cancer cell stemness to prevent post-surgery recurrence and systemic metastasis. Biomaterials. 2023;295:122037
47. Oliva J, Zhong J, Buslon VS, French SW. The effect of SAMe and betaine on Hepa 1-6, C34 and E47 liver cell survival in vitro. Exp Mol Pathol. 2012;92:126-30
48. Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy. 2021;17:1-382
49. Chen Y, Zhao H, Liang W, Jiang E, Zhou X, Shao Z. et al. Autophagy regulates the cancer stem cell phenotype of head and neck squamous cell carcinoma through the noncanonical FOXO3/SOX2 axis. Oncogene. 2022;41:634-46
50. Nazio F, Bordi M, Cianfanelli V, Locatelli F, Cecconi F. Autophagy and cancer stem cells: molecular mechanisms and therapeutic applications. Cell Death Differ. 2019;26:690-702
51. Mao X, Zhang X, Zheng X, Chen Y, Xuan Z, Huang P. Curcumin suppresses LGR5(+) colorectal cancer stem cells by inducing autophagy and via repressing TFAP2A-mediated ECM pathway. J Nat Med. 2021;75:590-601
52. Zhao N, Yang S, Sun B, Feng Y, Zhao R. Maternal betaine protects rat offspring from glucocorticoid-induced activation of lipolytic genes in adipose tissue through modification of DNA methylation. Eur J Nutr. 2020;59:1707-16
53. Zhao N, Yang S, Hu Y, Dong H, Zhao R. Maternal betaine supplementation in rats induces intergenerational changes in hepatic IGF-1 expression and DNA methylation. Mol Nutr Food Res. 2017 61
54. Yang S, Zhao N, Sun B, Yang Y, Hu Y, Zhao R. Grandmaternal betaine supplementation enhances hepatic IGF2 expression in F2 rat offspring through modification of promoter DNA methylation. J Sci Food Agric. 2020;100:1486-94
55. Yang S, Zhao N, Yang Y, Hu Y, Dong H, Zhao R. Mitotically Stable Modification of DNA Methylation in IGF2/H19 Imprinting Control Region Is Associated with Activated Hepatic IGF2 Expression in Offspring Rats from Betaine-Supplemented Dams. J Agric Food Chem. 2018;66:2704-13
56. Ji C, Tao Y, Li X, Wang J, Chen J, Aniagu S. et al. AHR-mediated m(6)A RNA methylation contributes to PM(2.5)-induced cardiac malformations in zebrafish larvae. J Hazard Mater. 2023;457:131749
57. Li K, Huang W, Wang Z, Nie Q. m(6)A demethylase FTO regulate CTNNB1 to promote adipogenesis of chicken preadipocyte. J Anim Sci Biotechnol. 2022;13:147
58. Sun L, Gao M, Qian Q, Guo Z, Zhu P, Wang X. et al. Triclosan-induced abnormal expression of miR-30b regulates fto-mediated m(6)A methylation level to cause lipid metabolism disorder in zebrafish. Sci Total Environ. 2021;770:145285
59. Shen M, Li Y, Wang Y, Shao J, Zhang F, Yin G. et al. N(6)-methyladenosine modification regulates ferroptosis through autophagy signaling pathway in hepatic stellate cells. Redox Biol. 2021;47:102151
60. Liang J, Cai H, Hou C, Song F, Jiang Y, Wang Z. et al. METTL14 inhibits malignant progression of oral squamous cell carcinoma by targeting the autophagy-related gene RB1CC1 in an m6A-IGF2BP2-dependent manner. Clin Sci (Lond). 2023;137:1373-89
Author contact
![]() Corresponding authors: Hui-lian Zhu, E-mail: zhuhlsysu.edu.cn; Zhao-yan Liu, E-mail: liuzhy235sysu.edu.cn.
Corresponding authors: Hui-lian Zhu, E-mail: zhuhlsysu.edu.cn; Zhao-yan Liu, E-mail: liuzhy235sysu.edu.cn.
 Global reach, higher impact
Global reach, higher impact