13.3
Impact Factor
Theranostics 2025; 15(5):1787-1821. doi:10.7150/thno.105353 This issue Cite
Review
Lactylation in health and disease: physiological or pathological?
1. Key Laboratory of Holistic Integrative Management of Gastrointestinal Cancers and Department of Immunology, Fourth Military Medical University, Xi'an, Shanxi 710032, China.
2. Henan Key Laboratory of Immunology and Targeted Drugs, Xinxiang Key Laboratory of Tumor Microenvironment and Immunotherapy, School of Medical Technology, Xinxiang Medical University, Xinxiang, Henan, China.
*These authors contributed equally: Lijun Zhao, Haonan Qi.
#These authors jointly supervised this work and are co-corresponding authors.
Received 2024-10-17; Accepted 2024-12-11; Published 2025-1-2
Abstract
Lactate is an indispensable substance in various cellular physiological functions and plays regulatory roles in different aspects of energy metabolism and signal transduction. Lactylation (Kla), a key pathway through which lactate exerts its functions, has been identified as a novel posttranslational modification (PTM). Research indicates that Kla is an essential balancing mechanism in a variety of organisms and is involved in many key cellular biological processes through different pathways. Kla is closely related to disease development and represents a potential and important new drug target. In line with existing reports, we searched for newly discovered Kla sites on histone and nonhistone proteins; reviewed the regulatory mechanisms of Kla (particularly focusing on the enzymes directly involved in the reversible regulation of Kla, including “writers” (modifying enzymes), “readers” (modification-binding enzymes), and “erasers” (demodifying enzymes); and summarized the crosstalk between different PTMs to help researchers better understand the widespread distribution of Kla and its diverse functions. Furthermore, considering the "double-edged sword" role of Kla in both physiological and pathological contexts, this review highlights the "beneficial" biological functions of Kla in physiological states (energy metabolism, inflammatory responses, cell fate determination, development, etc.) and its "detrimental" pathogenic or inducive effects on pathological processes, particularly malignant tumors and complex nontumor diseases. We also clarify the molecular mechanisms of Kla in health and disease, and discuss its feasibility as a therapeutic target. Finally, we describe the detection technologies for Kla and their potential applications in diagnosis and clinical settings, aiming to provide new insights for the treatment of various diseases and to accelerate translation from laboratory research to clinical practice.
Keywords: Lactylation, Regulatory mechanism, Physiology, Pathology
Introduction
Lactate was traditionally considered a metabolic waste product of glycolysis under low-oxygen conditions. However, the lactate shuttle hypothesis has reshaped this perspective, emphasizing lactate's critical roles in cellular functions, energy metabolism, and signal transduction [1]. In recent years, increasing research has shown that lactate not only serves as an energy source for mitochondrial respiration but also plays vital roles in processes such as inflammation, wound healing, memory formation, neuroprotection, and pathological conditions like tumor growth and metastasis, influencing disease progression and prognosis [1, 2].
Lactylation (Kla) represents a key pathway for lactate's functions and was first identified on proteins several years ago. However, the biological significance of this modification remained unclear until 2019, when Zhao Yingming's team identified a mass shift of 72.021 Da on histone lysine residues. This matched the addition of a lactyl group to the ε-amino group of lysine residues, leading them to propose Kla as a novel type of protein post-translational modification (PTM) [3]. Recent research suggests that Kla is a vital regulatory mechanism in various organisms, playing critical roles in processes such as cellular energy metabolism, inflammatory responses, cell fate determination, and development. Moreover, Kla interacts with tumor-related genes, influencing the expression and function of oncogenes. It regulates the tumor immune microenvironment, tumor cell metabolism, drug resistance, and autophagy, thereby impacting the progression of tumor and non-tumor diseases [1, 2, 4].
Despite these advances, the precise regulatory mechanisms and biological functions of Kla remain poorly understood, and its role in physiological and pathological processes is still under investigation. This review summarizes recent preclinical in vivo and in vitro studies on lactylation, systematically examining its regulatory roles and molecular mechanisms in health and disease. Additionally, we highlight key findings, unresolved issues, and propose potential strategies to guide future clinical research and treatment development.
Lactylation modification sites
Histone lysine lactylation sites
Lactylation was first identified on lysine residues of human histones, with Professor Zhao discovering 28 Kla sites on core histones in 2019, mainly H3 and H4 [3]. Since then, an increasing number of histone Kla modification sites have been identified (Table 1). Research indicates that histone Kla mainly occurs on lysine in H2A, H2B, H3, and H4, particularly H3K18 [5]. H3K18 lactylation is typically enriched in gene promoter and enhancer regions, serving not only as an indicator of active gene expression but also being closely linked to various physiological and pathological processes, including gene transcription regulation, stem cell and embryonic development, macrophage polarization, and tumorigenesis [5]. In addition to H3K18, other histone lactylation sites with regulatory functions have been reported, including H2BK6, H3K9, H3K14, H3K23, H3K56, H4K5, H4K80, and H4K12. These histone Kla modifications have been shown to be involved in the regulation of various cancer types, including lung cancer, prostate cancer, kidney cancer, colon cancer, liver cancer, and melanoma [6]. However, most studies to date have focused on specific gene sets, and it remains unclear whether histone Kla generally suppresses or enhances gene expression. In summary, histone Kla modifications are widely present and conserved, closely associated with diseases, and holds potential as a therapeutic target.
Nonhistone lysine lactylation sites
In recent years, research has shown that Kla modifications are not limited to histones but are also widely present on nonhistone lysine residues (Table 2). Nonhistone Kla may have various effects, influencing protein stability, localization, structure, interactions, or functions by enhancing or inhibiting the original functions of these nonhistone proteins, and it is associated with multiple physiological and pathological contexts [4]. For instance, Miao et al. reported that hypoxia-induced glycolysis promotes the Kla of β-catenin, further increasing its protein stability and expression, which exacerbates the malignant behavior of colorectal cancer (CRC) cells [7]. Sun et al. discovered that copper ions regulate METTL16 K229 Kla by modulating lactylation and delactylation enzymes, such as SIRT2, thereby affecting its activity. Lactylated METTL16 leads to methylation at the FDX1 602 site, promoting FDX1 expression and resulting in copper-induced cell death [8]. Gu et al. demonstrated that lactate regulates the generation of Treg cells through the lactylation of Lys72 in MOESIN, thereby improving the interaction between MOESIN and transforming growth factor β (TGF-β) receptor I and downstream SMAD3 signaling [9]. Additionally, studies have shown that enzymes involved in metabolic processes, particularly glycolysis, are often subject to Kla modifications. For example, the key glycolytic enzyme pyruvate kinase M2 (PKM2) can undergo lactylation at the K62 site [10]. Fructose-bisphosphate aldolase A (ALDOA) is lactylated at the K147 residue. The glycolytic enzyme enolase is lactylated at the K343 site, affecting substrate-enzyme interactions [11]. Dehydrogenase reductase 7 can also be lactylated at K321, and this phenomenon is commonly observed in various human tissues, including the retina, spinal cord, liver, testis, ovary, and prostate [11]. In summary, the discovery of these modification sites indicates that Kla has a broader regulatory role in biological systems than previously thought, highlighting its significant research value in biological process regulation.
Regulatory mechanisms of lactylation
Lactate typically occurs in three stereoisomeric forms: D-lactic acid, L-lactic acid, and racemic DL-lactic acid (due to asymmetric carbon atoms). Lactylation encompasses three structurally similar but stereochemically distinct isomers: L-lactylation (KL-La), D-lactylation (KD-La) and N-ε-(carboxyethyl)-lysine (Kce) [12].
Histone lactylation sites identified in diverse species.
| Histone | Kla Site | Species | Function | Ref. |
|---|---|---|---|---|
| H2A | 11, 13, 115 | homo sapiens | unknown | PMID: 31645732 |
| 4, 9, 11, 115 | mice | unknown | PMID: 31645732, PMID: 35685793 | |
| 5, 21, 116 | Trypanosoma brucei | unknown | PMID: 34722503 | |
| 5, 137, 142 | Tachyzoite | unknown | PMID: 35610722 | |
| H2A. Z | 32, 36, 44, 165 | Trypanosoma brucei | unknown | PMID: 34722503 |
| 5, 9, 17, 23, 142, 150 | Tachyzoite | unknown | PMID: 36216028 | |
| H2A1 | 73 | Tachyzoite | unknown | PMID: 35610722 |
| H2AX | 127 | Tachyzoite | unknown | PMID: 35610722 |
| H2B | 5, 6, 11, 15, 16, 20, 23, 43, 85, 108, 116, 120 | homo sapiens | unknown | PMID: 31645732, PMID: 37919786 |
| 5, 11, 15, 16, 20, 85, 108 | mice | unknown | PMID: 31645732 | |
| 5, 97 | Trypanosoma brucei | unknown | PMID: 34722503 | |
| 37, 47, 70, 77, 99 | Tachyzoite | unknown | PMID: 35610722 | |
| 15, 48, 122 | Botrytis cinerea | unknown | PMID: 33193272 | |
| 41, 60, 66, 114, 136, 144 | Paddy | unknown | PMID: 34264677 | |
| H2Bv | 8, 20, 28 | Trypanosoma brucei | unknown | PMID: 34722503 |
| H2BZ | 3, 8, 18, 104 | Tachyzoite | unknown | PMID: 36216028 |
| H2Bb | 46, 98 | Tachyzoite | unknown | PMID: 36216028 |
| H3 | 9, 14, 18, 23, 24, 33, 56, 62, 79, 123 | homo sapiens | H3K9: promote muscle generated; temozolomide resistance in glioma; regulates CD8 T cell effector functions, including anti-tumor immunity; H3K9, H3K14, H3K56: Promote liver cancer cell proliferation and metabolic reprogramming; H3K18: Cell differentiation and metabolic reprogramming; Promote tumor progression; Promote bevacizumab/ cisplatin resistance; Potential biomarkers for diagnosing; Aggravate brain aging and Alzheimer's disease; Promote pulmonary fibrosis; Aggravating cerebral ischemia-reperfusion injury; Regulating effect of CD8 T cell functions; Cause inflammation and aggravate renal function damage; Activating transcription of TTK and BUB1B; Improves atherosclerosis; | PMID: 31645732, PMID: 35605812, PMID: 36192798, PMID: 37615625, PMID: 38670996, PMID: 38901791, PMID: 38854142, PMID: 38769664, PMID: 38767134, PMID: 38733581, PMID: 38711083, PMID: 38477507, PMID: 38443347, PMID: 37919243, PMID: 38295753 |
| 18 | Sheep | unknown | PMID: 34861240 | |
| 14, 18, 23, 27, 56 | mice | H3K18: Promote angiogenesis after myocardial infarction, improves cardiac function after myocardial infarction; Aggravate brain aging and Alzheimer's disease; H3K23: Participate in pre-implantation development | PMID: 31645732, PMID: 34930415, PMID: 38232735, PMID: 37697347 | |
| 24, 33, 62 | Trypanosoma brucei | unknown | PMID: 34722503 | |
| 14, 23, 27, 56, 122 | Tachyzoite | unknown | PMID: 35610722, PMID: 36216028 | |
| 123 | Botrytis cinerea | unknown | PMID: 33193272 | |
| 9, 14, 18, 56 | Paddy | unknown | PMID: 34264677 | |
| H4 | 5, 8, 12, 16, 31, 77, 80, 91 | homo sapiens | H4K5, H4K8: Regulation of telomerase activity; H4K5, H4K8, H4K12: Promote tumor progression and drug resistance; H4K12: Promote inflammation; A novel biomarker for Triple-negative breast cancer; Potential targets for the treatment of Alzheimer's disease; Promotes anaplastic thyroid carcinoma; Accelerate DNA replication and the cell cycle; | PMID: 31645732, PMID: 37919786, PMID: 34150616, PMID: 35959377, PMID: 35303422, PMID: 37777506, PMID: 38844063, PMID: 38779451, PMID: 37184950, PMID: 37587486 |
| 8, 12, 31, 91 | mice | glycolysis-related genes; Promotes inflammatory infiltration in the microenvironment; | PMID: 31645732, PMID: 35303422 PMID: 36430853, PMID: 38486199 | |
| 78 | Trypanosoma brucei | unknown | PMID: 34722503 | |
| 12, 31 | Tachyzoite | unknown | PMID: 35610722, PMID: 36216028 |
Under conditions of high glycolysis (e.g., hypoxia, cancer metabolism), elevated lactate production favors the formation of KL-La or KD-la. Among these, L-lactic acid is the predominant isomer in vivo, while KL-La serves as an important modification responsive to glycolysis [12]. KL-La involves attaching an L-lactyl group (which has polarity and hydroxyl groups) to lysine residues, enhancing their polarity and solubility. This modification can influence protein folding, receptor binding, structure, stability, and interactions with other molecules. Similarly, KD-La entails attaching a D-lactyl group, which differs structurally from L-lactyl, to lysine residues, affecting protein stability, interactions, and biological activity. However, due to the stereochemical properties of the D-lactyl group, KD-La may exhibit unique spatial selectivity. Currently, KD-La (produced through a nonenzymatic process involving lactylated glutathione) has been observed only when the glycolytic enzyme system is impaired, and it is associated with microbial metabolism or metabolic disorders [13]. In metabolically balanced states, Kce may dominate. Unlike KL-La, Kce involves adding a carboxyethyl group (acidic) to lysine's amino group, affecting enzyme activity, binding capacity, or intracellular localization of proteins. This modification alters lysine's charge state and polarity, impacting protein structure, active sites, and interactions, and is associated with oxidative stress, inflammation, and metabolic regulation [12]. In summary, these three lysine modifications alter the chemical properties and spatial configurations of lysine, potentially leading to different effects on protein function. The specific impact depends on the protein type, modification site, and cellular environment.
Nonhistone lactylation sites identified in diverse species.
| Species | Nonhistone | Kla Site | Function | Ref. |
|---|---|---|---|---|
| Homo sapiens | β-catenin | Unknown | Hypoxia-Wnt signaling and promote the protein stability; | PMID: 36464122 |
| PDHA1 | K336 | Inflow of acetyl-CoA that restricts pyruvate oxidation to inactivate enzymes and inhibit OXPHOS; | PMID: 38163844 | |
| PTBP1 | K436 | The proteasomal degradation of PTBP1 is inhibited by weakening its interaction with TRIM21; | PMID: 39570804 | |
| PD-L1 | unknown | It is associated with the mechanisms of immune evasion in cytotoxic T cell-mediated tumor immunity; | PMID: 39577415 | |
| NLRP3 | K245 | LDHA promotes myocardial I/R injury by enhancing the lactylation of NLRP3, thereby inducing cardiomyocyte pyroptosis. | PMID: 39548367 | |
| Hdac1 | unknown | Promote the removal of H3K27ac and the silencing of the 2-cell (2C) gene; | PMID: 39533309 | |
| Tufm | K286 | Tufm-mediated mitophagy is inhibited, while mitochondrial-induced neuronal apoptosis is increased; | PMID: 39496783 | |
| YTHDF1 | unknown | The virulence factor NSs of SFTSV can promote the liquid-liquid phase separation and degradation of YTHDF1, thereby enhancing the replication of SFTSV | PMID: 39496835 | |
| P4HB | K311 | Stabilize the kynurenine metabolism mediated by GOT2; | PMID: 39494721 | |
| TFEB | K91 | Emulsification of K91 prevents the interaction between TFEB and the E3 ubiquitin ligase WWP2, thereby inhibiting TFEB ubiquitination and proteasomal degradation; | PMID: 39196068 | |
| CREB | K136 | hCG-induced luteinization of GCs under hypoxic conditions. | PMID: 39322166 | |
| ACLY | K918/995 | It altered its affinity for metabolic substrates and consequently modulated its metabolic activity; | PMID: 39251781 | |
| TWIST1 | K150 | Promote Twist1 phosphorylation and nuclear translocation, thereby regulating the transcription of TGFB1 to induce a fibrotic phenotype. | PMID: 39474980 | |
| c-Myc | unknown | HSPA12A increases glycolysis-derived lactate production in a HIF1α-dependent manner, thereby promoting the lactylation of c-Myc; | PMID: 39277835 | |
| IGF2BP3 | unknown | Reprogram serine metabolism and enhance the antioxidant defense system; | PMID: 39450426 | |
| ALKBH5 | unknown | Lactylated ALKBH5 binds to interferon-β (IFN-β) messenger RNA (mRNA), leading to its m6A demethylation and promoting the biosynthesis of IFN-β mRNA; | PMID: 39413129 | |
| DHX9 | K146 | Alleviated the inhibitory effect of DHX9 on OSCC. | PMID: 39407253 | |
| α-tubulin | K40 | Enhanced microtubule dynamics, promoting the growth and branching of neurites; | PMID: 39333081 | |
| SNAP91 | unknown | By enhancing synaptic structural formation and neuronal activity in the medial prefrontal cortex (mPFC), it strengthened resilience to chronic restraint stress (CRS); | PMID: 39163863 | |
| CPT2 | 457/8 | Inflow of acetyl-CoA that limits fatty acid oxidation to inactivate enzymes and inhibit OXPHOS; | PMID: 38163844 | |
| YY1 | K183 | Upregulate FGF2 transcription and promotes angiogenesis; | PMID: 37085894 | |
| AK2 | K28 | Inhibition of enzyme activity; | PMID: 38750680 | |
| MOESIN | K72 | Enhance TGF- β signaling in Treg cells; | PMID: 35732125 | |
| eEF1A2 | K408 | Enhance translation elongation rate and protein synthesis, thereby promoting tumorigenesis; | PMID: 38359291 | |
| DCBLD1 | K172 | promote its expression, suppresses the autophagic degradation of G6PD in itself, and activates the pentose phosphate pathway (PPP) to promote cervical cancer progression; | PMID: 38291438 | |
| MRE11 | K673 | Promote its binding to DNA and promote DNA end resection and homologous recombination; | PMID: 38128537 | |
| AMPKα | unknown | Unknown; | PMID: 38486093 | |
| Snail1 | unknown | RHOF overexpression promotes the lactylation and nuclear translocation of Snail1. Silencing Snail1 reverses the promoting effects of RHOF and lactate on cell migration, invasion, and EMT; | PMID: 36735787 PMID: 9462429 | |
| AARS1 | unknown | Form a positive feedback loop with YAP-TEAD to promote gastric cancer (GC) cell proliferation; catalyze the formation of lactate-AMP and then transfer lactate to lytic receptor residues; | PMID: 38512451 PMID: 38653238 | |
| NCL | K447 | Bind to the primary transcript of the MAP kinase-activated death domain protein (MADD) and ensures efficient translation of MADD by circumventing alternative splicing that generates premature stop codons; | PMID: 38679071 | |
| ACSF2 | K182 | Result in mitochondrial dysfunction; | PMID: 38676722 | |
| Esrrb | K228/232 | In the absence of LIF and XEN differentiation, lactoylation of Esrrb enhances its activity to promote ESC self-renewal by increasing its binding at the target genes; | PMID: 38473939 | |
| ALDOA | K147 | Inhibition of protein activity; | PMID: 35761067 | |
| SHMT2 | unknown | Unknown; | PMID: 38175377 | |
| G6PD | K457 | Reexpression in the HPV16-positive SiHa cell line inhibited cell proliferation; | PMID: 38457903 | |
| NEDD4 | K33 | Inhibition the interaction with Caspase-11; | PMID: 38385085 | |
| NMNAT1 | K128 | Enhance its nuclear localization and maintained the enzymatic activity, thereby supporting the nuclear NAD rescue pathway and promoting cancer growth; | PMID: 38467179 | |
| PFKP | K668 | Inhibition of protein activity; | PMID: 38155775 | |
| METTL16 | K229 | Increase protein activity; | PMID: 37863889 | |
| P53 | unknown | Development of enhanced Enz resistance and progression of PCa; | PMID: 38880227 | |
| SLC16A1 | unknown | Promoting L-lactate transport through proton junctions in the plasma membrane; | PMID: 38817665 | |
| Ezrin | K263 | Activating the NF-κB pathway and enhance the protein level of GLUT1; | PMID: 38767134 | |
| kzf1 | 164 | Directly regulate the expression of TH17-related genes (Runx 1, Tlr 4, IL-2, and IL-4), promoting TH17 differentiation; | PMID: 37851814 | |
| ARF1 | K73 | Promote vesicle formation; | PMID: 38906140 | |
| ENO1 | K343 | Affect the substrate-enzyme interactions; | PMID: 35761067 | |
| DHRS7 | K321 | Unknown; | PMID: 35761067 | |
| Mice | METTL3 | K281/345 | Promote the capture of RNA capability; | PMID: 35320754 |
| tau | K677 | It may prevent AD by affecting ferroautophagy and ferroptosis through the MAPK signaling pathway. | PMID: 39307193 | |
| MDH2 | unknown | Lactylation of MDH2 can induce ferroptosis by impairing mitochondrial function, leading to MIRI (myocardial ischemia-reperfusion injury; | PMID: 39467114 | |
| CIRP | unknown | Leads to the release of CIRP; | PMID: 39465383 | |
| NCOA4 | K450 | Enhanced the stability of the NCOA4 protein; | PMID: 39166386 | |
| HMGB1 | unknown | Promote translocation of nucleus to cytoplasm and release through exosomes; | PMID: 34363018 | |
| PKM2 | K62 | Enhance enzyme activity and reduces nuclear distribution, and induces cell cycle arrest in S phase | PMID: 36439872 PMID: 38570082 | |
| LCP1 | unknown | Promote the protein stability; | PMID: 36574182 | |
| FASN | K673 | Inhibition of protein activity; | PMID: 36651176 | |
| IKZF1 | K164 | Promote the TH 17 differentiation; | PMID: 37851814 | |
| α-MHC | K1897 | Promote its interaction with myxin; | PMID: 37443257 | |
E. coli | PykF | K382 | Inhibition of protein activity; | PMID: 36333310 PMID: 37159428 |
| BGN | Unknown | Associated with the onset of tendinopathy; | ||
| TPM3 | Unknown | |||
| MYL3 | Unknown | |||
| COMP | Unknown | |||
| TNNC1 | Unknown | |||
| FGA | Unknown | |||
| POSTN | Unknown | |||
| LUM | Unknown | |||
| MMP3 | Unknown | |||
| DCN | Unknown | |||
| APOA1 | Unknown | Involved in cholesterol metabolism; | ||
| APOA4 | Unknown | |||
| APOC1 | Unknown | |||
| APOC3 | Unknown | |||
Toxoplasma planus | MIC1 | K157 | Invasion and survival; | PMID: 36216028 |
| MIC2 | K630 | |||
| RON6 | K1170 | |||
| ROP9 | K79/138/144/347 | |||
| ROP18 | K202 | |||
| GRA12 | K74 | |||
| CDPK1 | K50/59/80/93/341 | Invasion, intracellular development and propagation; | ||
| CDPK2A | K165/729/777 | |||
| CDPK9 | K5 |
The Kla process can be enzymatic or nonenzymatic, depending on the precursors (Fig. 1) [14]. Enzymatic Kla, particularly KL-La, is widely studied. In enzymatic Kla, the “writer” (modifying enzyme) transfers lactyl groups from lactyl-CoA to lysine residues on histones or non-histones, using endogenous or exogenous l-lactic acid, which alters protein structure and function. The “reader” (modification-binding enzymes, such as Dux [15] and TRIM33 [16]) recognizes Kla changes, influencing signaling pathways and triggering biological events. When signal transduction ends, “erasers” (demodifying enzymes) remove lactyl groups, halting the Kla cycle and reducing its lasting effects.
According to reports, enzyme-dependent lactylation is dynamically regulated by classical histone acetyltransferases [17, 18]. Acetyltransferase-mediated Kla modification typically depends on the enzyme's activity and substrate specificity. Under conditions of abundant nutrients and balanced energy metabolism (such as during cell proliferation, differentiation, or in the tumor microenvironment), acetyltransferase-mediated lactylation predominates. Acetyltransferases involved in Kla modifications can precisely target lactylation modifications to specific proteins [3]. For instance, p300, a classical histone acetyltransferase, serves as a “writer” for YY1 K-la, adding lactyl groups from lactyl-CoA to lysine residues, influencing cellular inflammation [17]. Additionally, p300 serves as a “writer” for Kla on the YTHDF2 promoter, promoting the degradation of PER1 and TP53 mRNA, accelerating ocular melanoma onset [18]. P300 also functions as a “writer” for the Kla of APCO2-K70, enhancing its Kla and promoting lipolysis [19]. TIP60, another acetyltransferase, catalyzes the Kla of NBS1 at K388, directly modifying the NBS1 protein, with enhanced effects under lactate and cisplatin treatment [20]. High lactate levels in tumors act as signaling molecules, mediating PIK3C3/VPS34 Kla via TIP60 at lysines 356 and 781, which enhances autophagy and promotes tumor progression [21]. Xie et al. discovered that KAT8, a lysine acetyltransferase, can act as a “writer” for panlactylation, installing Kla on numerous protein substrates involved in various biological processes [22]. Furthermore, recent studies identified ACSS2 and KAT2A as previously uncharacterized lactate coenzyme A synthetase and transferase, respectively. KAT2A facilitates lactate transfer to histone H3, activating Wnt/β-catenin, NF-κB, and PD-L1, promoting brain tumor growth and immune evasion. The interaction between ACSS2 and KAT2A, combined with peptide blockade and anti-PD-1 antibody treatment, produces an additive tumor-suppressive effect [23]. HDAC6, a deacetylase, also acts as a lactyltransferase, catalyzing the Kla of α-tubulin lysine 40 in soluble microtubule dimers. By competing with acetylation (Kac) at the same residue, it links cellular metabolism and cytoskeletal function through the regulation of microtubule dynamics. Interestingly, HDAC6 mediated lactylation is reversible, depending on lactate concentration. HDAC6 primarily lactylates α-tubulin under high lactate levels [24]. Moreover, deacetylases like HDACs and sirtuins can remove protein Kla [25, 26]. According to reports, HDAC3 is an effective “eraser” for K-la, interacting directly with NBS1 [20]and APCO2-K70 [19] to remove Kla and inhibit its expression. SIRT1 can eliminate Kla of CNPY3, promoting lysosomal rupture and triggering specific pyroptosis in prostate cancer cells [26]. SIRT3 can remove Kla from CCNE2, thereby regulating the cell cycle and hindering the progression of HCC [27]. p300 and SIRT1 function as lactyltransferase and delactylase for α-MHC, respectively. Reducing lactate production through chemical or genetic manipulation can decrease α-MHC Kla, impairing the interaction between α-MHC and titin, which exacerbates heart failure [28].
Regulatory mechanisms of of lactylation. Lysine l-lactylation (KL-la) is a novel protein posttranslational modification (PTM) driven by l-lactate. In addition to KL-la, it has two structurally similar but stereochemically distinct isomers: N-ε-(carboxyethyl)-lysine (Kce) and D-lactyl-lysine (KD-la). Depending on the different precursors, the Kla process can be enzymatic or nonenzymatic. Enzyme-dependent Kla, particularly Kl-la, has been widely studied. In enzyme-dependent Kla, the “writer” (modifying enzymes) uses endogenous or exogenous l-lactic acid as a substrate to transfer lactyl groups from lactyl-CoA to lysine residues on histones or non-histones, altering protein structure and function. The “reader” (modification-binding enzymes) recognizes Kla changes, influencing downstream signaling pathways and triggering biological events. When signal transduction ends, “erasers” (demodifying enzymes) remove lactyl groups from target proteins, halting the Kla cycle and mitigating the lasting effects of lysine Kla.
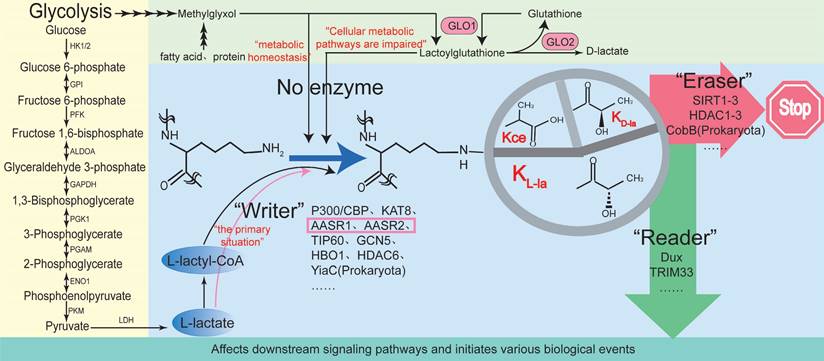
Notably, no acetyltransferase has been identified with strict specificity for Kla, making it difficult to define the precise conditions for efficient catalysis [29]. Specifically, the ability of acetyltransferases to distinguish between acetyl-CoA and lactyl-CoA primarily depends on enzyme structure, active site specificity, chemical environment affinity, and substrate kinetic differences. In the case of acetyl-CoA, the simple structure of the acetyl group (CH3CO-) facilitates the formation of specific hydrogen bonds and hydrophobic interactions, making it more adaptable to the enzyme's binding pocket. With respect to lactyl-CoA, the lactyl group (CH3CHOHCO-) is larger and more polar compared to the acetyl group, which may hinder its binding to certain acetyltransferases due to steric hindrance or insufficient affinity. The additional hydroxyl group may also interfere with this binding or require specific amino acid residues to stabilize its structure. Nevertheless, some studies suggest that certain acetyltransferases specialize in Kla or Kac through differences in active site residues, achieving functional differentiation. Furthermore, the catalytic selectivity of acetyltransferases for the two CoA derivatives is likely determined by intracellular concentrations and regulatory mechanisms. Acetyl-CoA generally exists at much higher intracellular concentrations, which may make it the preferred substrate. In contrast, endogenous lactyl-CoA levels in cancer cells are extremely low, around 0.011 pmol per 10⁶ cells, about 1,000 times lower than acetyl-CoA levels in mammalian cells [30]. This low concentration, combined with competitive inhibition, may hinder the ability of proposed acetyltransferases such as p300, CREB-binding protein (CBP) and TIP60 to effectively transfer lactyl groups to target proteins in vivo [29]. The low levels of lactylation also complicate distinguishing whether lactylation serves a primary regulatory function or is merely a secondary outcome of other metabolic processes [29]. Additionally, the concentrations of lactyl-CoA used in in vitro experiments are not well-documented in the literature, and key enzymatic parameters such as the dissociation constant (Kd) and Michaelis constant (Km) remain unavailable, raising concerns about whether these experiments accurately reflect in vivo conditions [30]. In summary, the crosstalk between Kla and Kac plays a key role in metabolism and epigenetics, warranting further investigation.
Besides acetyltransferases, mitochondrial alanyl-tRNA synthetase 1 (AARS1) and 2 (AARS2) also act as intracellular lactate sensors and lactate transferases [31]. AARS is a key enzyme class responsible for attaching amino acids to their tRNAs. Under stress (e.g., oxidative stress or changes in protein synthesis), AARS activity quickly adjusts to amino acid and lactate fluctuations, potentially leading to lactylation of proteins involved in translation regulation [31]. Recent evidence shows that, unlike acetyltransferases, AARS1 and AARS2 act as lactate sensors and lactyltransferases, using L-lactate, not lactyl-CoA, as a substrate, leading to lactylation of a wide range of proteins, including p53, Yap, and mitochondrial proteins [32-34]. This is the first report in over half a century of a non-CoA-dependent catalytic reaction since acetylation's discovery, where glucose-derived lactate + ATP is fully utilized and covalently added to proteins, altering the function of key proteins [31].
Furthermore, Wang et al. found that GCN5, a lactylation-modifying enzyme, regulates target gene transcription, providing new insights into the interaction between the metabolome, epigenome, and immune response after myocardial infarction [35]. YiaC, a member of the GCN5-related N-acetyltransferase (GNAT) family, is a lysine lactylase that catalyzes Kla in cells [36]. Niu et al. showed that HBO1 has Kla catalytic activity, regulating 95 Kla sites, especially H3K9la. Additionally, the scaffold proteins JADE1 and BRPF2 enhance HBO1's catalytic activity toward H3K9la [37].
Nonenzymatic lactylation is the reaction between lactate and substrates without enzyme catalysis. It depends on environmental factors like pH, temperature, and substrate concentration [13]. Under conditions of high glycolytic activity or vigorous lactate production, nonenzymatic Kla may become predominant. However, there is currently limited research on this topic, primarily focusing on lysine KD-la and the formation of Kce through reactions with lysine. KD-la forms through a nonenzymatic reaction between S-D-lactyl glutathione (LGSH), produced by the glyoxalase pathway, and proteins. Gaffney et al. showed that lactylation in cells depends on LGSH and GLO2 regulation at the K94 site of PGK1 [13]. Additionally, in the glyoxalase pathway, the high reactivity of the glycolytic byproduct methylglyoxal (MGO) allows it to react with various protein residues, including cysteine, arginine, and lysine. Among these, the Kce formed through reactions with lysine has been identified in cells, although its levels are lower than those of MGO-derived arginine residue modifications [13]. In summary, lactylation regulation varies under different cellular conditions. Exploring lactylation regulation holds promise as a therapeutic target.
Crosstalk between lactylation and other protein posttranslational modifications
PTMs are crucial epigenetic regulators in processes such as DNA replication, transcription, and cell differentiation, and play a key role in protein function [6]. The most common PTMs include methylation, acetylation, phosphorylation, ubiquitination, SUMOylation, glycosylation, butyrylation, succinylation, and propionylation. Recent studies have shown that the vast majority of PTMs do not exist independently. Instead, any two or more different PTMs can interact, where these combined PTM states can reinforce or inhibit each other (Fig. 2) [6].
Among them, Kla and Kac exhibit significant similarities and coordination, with their crosstalk serving as a crucial link between metabolism and epigenetics. For instance, Li et al. reported that Gli-similar transcription factor 1 (Glis1) enhances levels of acetyl-CoA and lactate, driving histone Kac and Kla by activating glycolytic genes and increasing glycolytic flux [38]. Yang et al. reported that in mixed bacterial sepsis, lactate simultaneously affects the Kla and Kac of macrophage HMGB1. Macrophages uptake extracellular lactate via monocarboxylate transporters, promoting HMGB1 Kla through a p300/CBP-dependent mechanism [39]. Lactate also stimulates HMGB1 Kac via GPR81 through Hippo/YAP-mediated SIRT1 inhibition and β-arrestin2-mediated p300/CBP recruitment to the nucleus [39]. Lu et al. reported that histone H3 lactylation synergistically promotes NF-κB and STAT6 transcription, influencing macrophage differentiation [40]. Li et al. also noted that classic acyltransferases such as p300 can catalyze both Kac and Kla of transcription factors, histones, and other nuclear proteins in macrophages and iPSCs [41]. However, some studies have pointed out the competitive relationship between Kla and Kac. For example, Sun et al. demonstrated that Kla and Kac can occur on the same residue within the self-modification domain of poly (ADP-ribose) polymerase 1(PARP1), where Kla may competitively inhibit Kac, restoring the ADP‒ribosyltransferase activity of PARP1 and promoting DNA repair while regulating pluripotency gene expression [42]. Notably, histones Kla and Kac exhibit different temporal dynamics, with varying cellular responses to different stimuli. For example, under hypoxic conditions, Kla levels increase in both human HeLa cells and mouse macrophages, but Kac levels decrease in HeLa cells while remaining unchanged in mouse macrophages [3].
Crosstalk between lactylation and other post-translational modifications. The vast majority of PTMs do not exist independently. Instead, any two or more different PTMs can interact, where these combined PTM states can reinforce or inhibit each other. Notably, there is a high degree of similarity and coordination between lactylation and acetylation, and their crosstalk is an important process connecting metabolism and epigenetics (A-E). Besides acetylation, there is considerable research on the crosstalk between lactylation and phosphorylation (F-H). Other PTMs, such as crotonylation, butyrylation and succinylation, have also been reported to interact with lactylation. For instance, it has been observed that crotonylation and lactylation can occur on all core histones and share the most common modification sites with histone lysine acetylation (G).
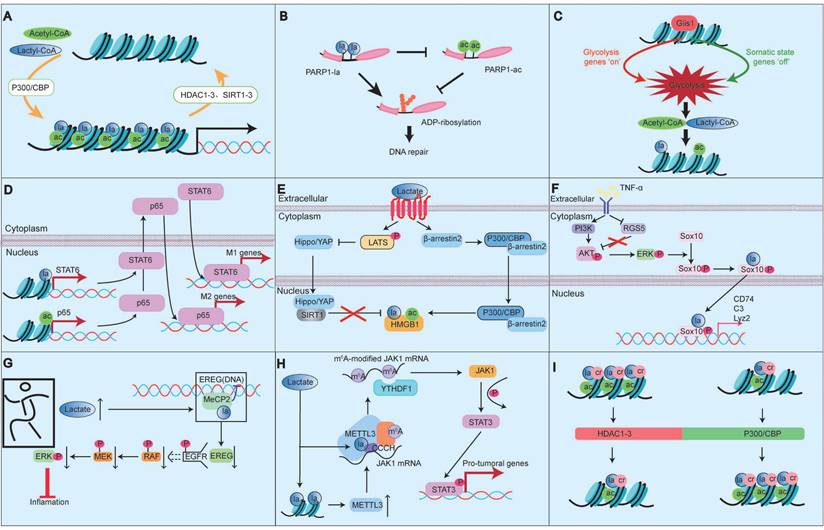
In addition to Kac, the crosstalk between Kla and phosphorylation has been studied extensively, often functioning synergistically. For example, Maschari et al. reported that sodium lactate treatment resulted in a dose-dependent relationship between protein Kla and IRS-1 serine 636 phosphorylation [43]. Xu et al. demonstrated that tumor necrosis factor α (TNF-α) induces Sox10 Kla through a phosphorylation-dependent mechanism involving the PI3K/AKT pathway, affecting vascular smooth muscle cell transdifferentiation [44]. Wang et al. reported that methyl-CpG binding protein 2 (Mecp2) k271la inhibits epithelial regulator protein expression by altering epidermal growth factor receptor phosphorylation levels, impacting the mitogen-activated protein kinase (MAPK) signaling pathway and improving atherosclerosis [45]. Ma et al. found that methylthio-methane enhances H3K18la-specific target gene expression during Staphylococcus aureus infection, promoting STAT3 phosphorylation [46]. Xiong et al. showed that lactate promotes METTL3 expression through H3K18la, increasing downstream signaling and increasing STAT3 phosphorylation levels [47].
Other PTMs, such as crotonylation, butyrylation and succinylation also interact with Kla. Studies indicate that crotonylation and Kla can occur on nearly all core histones and share modification sites with histone lysine Kac [6, 48]. Kla may be related to butyrate-mediated butyrylation [48]. In breast cancer treated with catalpol, Kac, 2-hydroxyisobutyrylation, and Kla were significantly increased, whereas succinylation, propionylation, and phosphorylation were significantly decreased, suggesting that catalpol may inhibit breast cancer progression by regulating different types of PTMs [49].
In summary, Kla is an important balancing mechanism in various organisms and has a complex relationship with other types of modifications. The crosstalk between Kla and other PTMs in different diseases, the potential synergistic or competitive relationships among various modifications in regulating protein function, and whether the ratio of different PTMs affects disease progression and prognosis, warrant further exploration.
"Beneficial" regulation of lactylation in biological processes
Recent research highlights Kla, mediated by lactate, as a potentially beneficial substance involved in various biological processes, including energy metabolism, inflammatory responses, cell fate determination, and development (Fig. 3). Hereafter, we review the roles and mechanisms of Kla in human health and physiology.
Energy metabolism
Metabolism is essential for life, as cells absorb nutrients to meet energy needs. Glucose is a key source of lactate in cells, and the lactate produced during glycolysis significantly influences various metabolic pathways, playing a vital role in cellular regulation [3]. For example, Wan et al. found that Kla, resulting from lactate accumulation, inhibits glycolytic feedback by covalently modifying upstream enzymes. When the glycolytic pathway is overactivated and produces excessive lactate, Kla at K147 reduces ALDOA activity, decreasing glycolytic flux via a “negative feedback loop” [11]. Gaffney et al. confirmed that Kla-modified proteins are enriched in the glycolytic pathway, and non-enzymatic Kla reactions using LGSH as a substrate also occurring on histones and non-histones [13]. Jia et al. found that under nutrient deprivation, ULK1 can directly interact with the activated glycolytic enzyme LDHA, phosphorylate serine 196, and promote lactate production. Lactate mediates Vps34 lactylation (lysine 356 and lysine 781) via TIP60, thereby enhancing autophagy and glycolysis [21]. In addition to mediating glycolysis, Zhao et al. proposed that hypoxia can induce mitochondrial protein lactylation to limit oxidative phosphorylation (OXPHOS). The study identified AARS2 as a lysine lactyltransferase, with its proteasomal degradation enhanced through proline 377 hydroxylation catalyzed by oxygen-sensing hydroxylase PHD2 [33]. Under hypoxia, AARS2 accumulation leads to lactylation of PDHA1 (lysine 336) and CPT2 (lysines 457/8), inactivating both enzymes and suppressing OXPHOS by limiting acetyl-CoA flux from pyruvate and fatty acid oxidation [33]. And this lactylation can be reversed by SIRT3, reactivating OXPHOS [33].
Inflammatory responses
Kla plays crucial roles in regulating immune cell activity, inflammatory responses, and interactions between immune cells [50, 51]. Modifications in Kla can influence specific inflammatory signaling pathways and modulate immune cell interactions, which in turn regulates inflammation intensity, immune cell cluster formation, and immune response coordination [3]. Macrophages, which are highly adaptable cells in the innate immune system, are essential in inflammatory responses. In the early stages of tissue damage, M1 macrophages initiate inflammation and eliminate external threats. Later, they polarize to the M2 phenotype to clear apoptotic cells and resolve inflammation [3]. Zhang et al. found that in the later stages of M1 macrophage polarization, histone Kla significantly increases on the promoters of M2-like genes, suggesting that histone Kla may act as a lactate clock to promote the transition of macrophages from an inflammatory phenotype to a homeostatic phenotype. This transition occurs in the later stages of inflammation, which may be related to wound healing [3]. Additionally, Zhang et al. observed that during bacterial infections, H3K18la enrichment at the promoters of M2-like genes, such as Arg1 and VEGFα, enhances gene expression, promoting M1-to-M2 macrophage conversion in the late polarization stage. This prevents further inflammation-induced damage [3]. Irizarry-Caro et al. found that BCAP deficiency disrupts FOXO1 and GSK3β inactivation, resulting in heightened inflammation, impaired aerobic glycolysis, and reduced lactate production, resulting in decreased histone lactylation. Adding exogenous lactate to BCAP-deficient bone marrow-derived macrophages (BMDMs) restored histone lactylation and promoted the transition from inflammatory to reparative macrophage characteristics [50]. Ma et al. reported that in macrophages from a peritonitis mouse model, MSM increased H3K18la levels, promoting the expression of M2 markers such as Arg1 [51]. This conferred protective effects against methicillin-resistant Staphylococcus aureus infections, suggesting a potential therapeutic strategy for addressing global drug-resistant infections [51].
The “Beneficial” regulation of lactylation in biological processes. Lactylation serves as a “beneficial” modification, playing a significant role in various biological processes. For instance, in eukaryotes, lactate produced during glycolysis can regulate multiple metabolic pathways through Kla. In addition to mediating glycolysis, hypoxia can trigger mitochondrial protein lactylation, limiting oxidative phosphorylation. Kla modification also influences specific inflammatory signaling pathways and immune cell interactions, thereby modulating the intensity of inflammatory responses, immune cell cluster formation, and coordination of immune responses. Lactylation links metabolism, transcription, and epigenetics, regulating gene expression at the chromosomal level and impacting cell fate. It enhances pre-implantation embryo development, promotes transcriptional elongation, and plays a critical role in muscle generation and bone formation. Additionally, lactylation participates in DNA damage repair, holds potential for skin rejuvenation, and contributes to optimizing immune function. While its roles are promising, further research is needed to fully understand these processes.
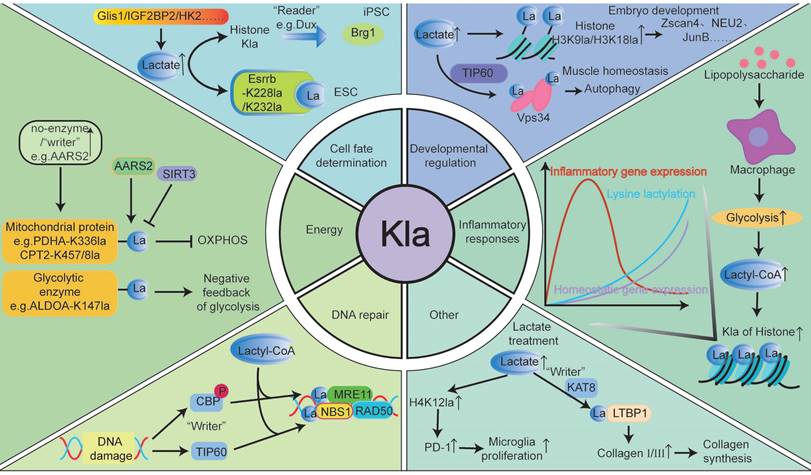
Cell fate determination
Kla links metabolism, transcription, and epigenetics, regulating gene expression at the chromosomal level and influencing cell fate. For instance, Dong et al. demonstrated that protein lactylation plays a key role in ESC self-renewal and extra-embryonic endoderm (XEN) differentiation. Their study showed that Esrrb, a nuclear receptor involved in pluripotency and XEN differentiation, is lactylated at K228 and K232. In the absence of LIF in ESCs or during XEN differentiation, Esrrb lactylation enhances its activity by increasing its binding to target genes, thereby promoting ESC self-renewal [52]. Hu et al. revealed that during the early stages of induced pluripotent stem cell (iPSC) reprogramming, Dux acts as a histone lactylation reader. It activates a metabolic-lactylation-mesenchymal-epithelial transition network via Brg1, improving reprogramming efficiency through metabolic switches and recruiting p300 via its C-terminal domain [15]. Li et al. found that histone lactylation significantly enhances stem cell survival, self-renewal, and reprogramming. In early reprogramming, Glis1 binds directly to and opens chromatin of glycolytic genes, increasing glycolysis. Elevated acetyl-CoA and lactate levels promote H3K27ac and H3K18la expression, facilitating senescent cell reprogramming and improving genomic stability [53]. Additionally, Zhou et al. identified IGF2BP2 as an m6A-binding protein regulating glycolysis by modulating ALDOA expression, mediating histone Kla, and enhancing hepatic stellate cell (HSC) activation [54]. Rho et al. found that lactate produced by activated HSCs induces hexokinase 2 (HK2) expression, determining HSC fate. HK2 deletion or inhibition of lactate production and histone Kla reduces HSC activation, while exogenous lactate (but not acetate) restores the activated phenotype and impacts HSC fate [55].
Developmental regulation
Lactylation enhances preimplantation embryonic development, promotes transcriptional elongation, and plays a crucial role in this process. Yang et al. demonstrated that appropriate lactate concentrations at the fetal-maternal interface act as embryo-derived signals, promoting lactylation of endometrial histones. This modification regulates redox homeostasis, apoptosis, cell proliferation, cell adhesion, and immune tolerance in the endometrium, transforming it into a receptive state and offering insights into improving implantation outcomes [56]. The study further highlighted that during pregnancy, increased histone H3K18la and lactate levels maintain glutathione-based redox homeostasis and apoptosis balance, both essential for successful embryo implantation [56]. Yang et al. also found that under hypoxic conditions, histone Kla levels in oocytes and preimplantation embryos are significantly reduced, impairing developmental potential [57]. Tian et al. confirmed that lactate upregulates cleavage-stage embryonic genes, such as the Zscan4 gene family, in embryonic stem cells. Lactate also promotes H3K18la in reproductive germline and cleavage-stage embryonic genes, enhancing transcriptional elongation [58]. Zhao et al. noted that in the absence of lactate, H3K18la modifications are significantly reduced, suggesting that lactate primarily affects early embryonic development via H3K18la rather than H3K27ac modifications [59]. Additionally, Yang et al. showed that inhibiting LDHA activity reduces lactate levels and histone Kla, thereby impairing preimplantation embryonic development [60]. Merkuri et al. reported that glycolysis-regulated histone Kla integrates the metabolic state of embryonic cells with chromatin organization and gene regulatory network activation [61]. Lactylation marks are enriched in glycolytic embryonic tissues, such as neural crest and precursor mesenchymal mesoderm [61]. In summary, these findings demonstrate how histone Kla links cellular metabolism with developmental GRNs, providing precise insights for clinical interventions to improve pregnancy outcomes in natural conception and assisted reproductive technologies.
Lactate promotes osteoblast differentiation and bone formation. Maschari et al. first identified Kla in human skeletal muscle, linking its levels to osteoblast differentiation and bone formation [43]. Later, Nian et al. observed that during osteoblast differentiation, levels of LDHA, lactate, and H3K18la progressively increased. Elevated H3K18la in the JunB promoter region of osteoblasts enhances its transcription, thereby promoting osteoblast differentiation [62]. Wu et al. reported that histone Kla and the expression of BMSC-related osteogenic genes are downregulated in osteoporosis patients. Enhanced glycolysis in endothelial cells can upregulate osteogenic-related genes through H3K18la, facilitating the differentiation of bone marrow mesenchymal stem cells into osteoblasts [63]. Additionally, Hao et al. found that the bone morphogenetic protein (BMP) signaling pathway in cranial neural crest cells (CNCC) is essential for producing glycolytic lactate and subsequent histone Kla, influencing craniofacial morphology development [64].
Lactylation is crucial for maintaining muscle cell homeostasis and promoting myogenesis. Lin et al. demonstrated that lactic acid produced during intense exercise acts as a signaling molecule, mediating Vps34 Kla to enhance autophagy in muscle tissue and maintain homeostasis. Moreover, autophagy, a conserved mechanism of cellular stability, promotes catabolism during exercise and clears damaged organelles and misfolded proteins, protecting skeletal muscles [21]. Huang et al. found that Kla protein levels in skeletal muscle and liver peak 24 hours after high-intensity interval training and stabilize within 72 hours [65]. Dai et al. confirmed that lactic acid enhances muscle generation by upregulating Neu2 expression via H3K9la activation [66]. Zhou et al. indicated that blocking lactic acid production or uptake impairs myocyte differentiation [67]. Desgeorges et al. discovered that histone Kla dynamics during muscle regeneration are critical for macrophage function. In macrophages, histone Kla predicts gene expression changes during ischemia-induced muscle regeneration [68]. In summary, Kla is vital for various developmental processes.
Others
Studies have shown that Kla is closely linked to DNA damage repair. For example, Sun et al. reported that Kla of PARP1 modulates its ADP-ribosylation activity, potentially aiding DNA repair [69]. Chen et al. reported that MRE11 is lactylated at the K673 site by CBP acetyltransferase, increasing protein binding to DNA and promoting DNA end excision and homologous recombination (HR) [70]. Chen et al. also found that lactate-driven lactation of NBS1 promotes HR-mediated DNA repair. NBS1 K388la is essential for forming the MRE11-RAD50-NBS1 complex and accumulating homologous recombination repair proteins at DNA double-strand breaks [20]. However, this “protective umbrella” effect appears detrimental in tumor cells. Specifically, lactate facilitates DNA break repair in cancer cells, maintaining genomic stability and contributing to chemotherapy resistance [20, 69, 70]. Disrupting the Kla process or inhibiting Kla modification with small-molecule polypeptides enhances tumor cell sensitivity to chemotherapy and improves drug efficacy.
Moreover, Zou et al. provided valuable insights into nonhistone Kla modifications for skin rejuvenation. The study showed that fibroblasts can take up extracellular lactate released by Poly-L-Lactic Acid via monocarboxylate transporter 1 (MCT1), which promotes Kla of lysine 752 on LTBP1 through a KAT8-dependent mechanism. This process increases type I and III collagen levels in fibroblasts, enhancing skin rejuvenation [71]. Hu et al. found that lactate and lactylation levels increased after spinal cord injury. Lactate-mediated upregulation of H4K12la promotes PD-1 transcription in microglia, which facilitates microglial proliferation, scar formation, axon regeneration, and motor function recovery after spinal cord injury [72]. This suggests that lactate and its mediated Kla play a crucial role in tissue repair via microglial activity, providing new therapeutic targets for spinal cord injury. Qiu et al. discovered that H3K18la regulates the transcriptional activation of the duox gene, leading to ROS production. ROS further promotes H3K18la, forming a positive feedback loop. This H3K18la-ROS-driven cycle contributes to light exposure-induced neutrophil recruitment in zebrafish [73]. The study emphasized the role of Kla mediated by light-dark cycles in optimizing immune function.
"Deleterious" regulation of lactylation in pathological processes
Kla is a double-edged sword for health, with elevated levels potentially causing pathogenic effects in diseases. This review will summarize Kla's roles and mechanisms in tumors and systemic non-tumor diseases, and evaluate its potential as a therapeutic target.
Tumor and lactylation regulation
Kla is commonly detected in various cancers and is associated with tumor occurrence, progression, and treatment response (Fig. 4, Table 3). While its role may differ depending on tumor type, stage, and individual factors, elevated Kla levels are often indicative of poor prognosis [74]. Recently, targeting lactate-lactylation and its associated metabolic pathways has emerged as a promising research direction in cancer treatment [74]. Next, we will discuss the current research status and future prospects of Kla in various cancers based on the latest 2022 global cancer burden data, aiming to offer new insights for cancer treatment and facilitate its clinical translation.
Lung cancer
Lung cancer (LC) is the most prevalent cancer worldwide and a major cause of cancer-related deaths [75]. Zhang et al. reported that IGF1R Kla is associated with lung cancer progression, as lactate-induced IGF1R Kla drives cell proliferation and metabolic reprogramming [75]. Non-small cell lung cancer (NSCLC) accounts for 80-85% of LC cases. Jiang et al. demonstrated that lactate is crucial for metabolic dysregulation in NSCLC. Lactate-mediated Kla downregulated glycolytic enzymes (HK-1, PKM) and upregulated TCA cycle enzymes (SDHA, IDH3G), reducing glycolysis and maintaining mitochondrial homeostasis in NSCLC cells [76]. Chen et al. revealed that lactate accumulation in NSCLC cells induces APOC2-K70 Kla, promoting extracellular lipolysis to produce FFA, enhancing metastasis, and contributing to immunotherapy resistance. Notably, the anti-APOC2-K70-lac antibody enhances tumor immunotherapy, suggesting potential for combinational approaches [19]. Zhang et al. reported elevated pan-Kla and H3K18la levels in NSCLC tissues, which were positively associated with poor patient prognosis [77]. H3K18la enhances immune evasion in NSCLC cells by activating the POM121/MYC/PD-L1 pathway [77]. Inhibiting glycolysis with 2-DG and oxalate, or silencing LDHA and LDHB, lowered H3K18la levels and reduced immune evasion in NSCLC cells by enhancing CD8+ T cell cytotoxicity [77]. Yan et al. found that hypoxia enhances sphere formation, migration, invasion, glucose consumption, lactate production, glycolysis, and global Kla. Hypoxia-induced SOX9 Kla promotes glycolysis, enhancing stemness, migration, and invasion in NSCLC cells [78]. These findings suggest that targeting hypoxia could be an effective therapeutic strategy for NSCLC. Additionally, KRAS gene mutations are common oncogenic drivers in NSCLC. Zhou et al. demonstrated that lactate-induced histone Kla from KRAS-mutated tumor cells activates circATXN7 transcription, driving tumor immune evasion by increasing activation-induced cell death sensitivity in tumor-specific T cells [79].
Lactylation modification sites, functions and Mechanism in malignant tumors.
| Tumor types | Site | Mechanisms and effects | Ref. | |
|---|---|---|---|---|
| Lung Cancer | NSCLC | IGF1R | Promotes the proliferation and metabolic reprogramming. | PMID: 38840891 |
| Histone Kla | Maintain mitochondrial homeostasis, Modulates cellular metabolism | PMID: 34150616 | ||
| APOC2 K70 | Induces tumor metastasis, and resistance to immunotherapy | PMID: 38981044 | ||
| H3K18la | Enhances immune evasion via the POM121/MYC/PD-L1 pathway | PMID: 39137401 | ||
| SOX9 | Promotes NSCLC stemness, migration, and invasion through glycolysis | PMID: 38226837 | ||
| H3 | H3la-PKM2-tumorigenesis | PMID: 38149461 | ||
| H4K12la | AKR1B10 promotes H4K12la and activates CCNB1 transcription contributing to PEM resistance | PMID: 37587486 | ||
| LUAD | H3K14la, H3K18la | Promotes the proliferation and migration of LUAD endothelial cells by downregulating SLC25A29 | PMID: 37775731 | |
| IDH3γ Kla | BZW2 promotes LUAD progression through glycolysis-mediated lactate production and IDH3γ Kla | PMID: 37955350 | ||
| H4K8 and H4K16 Kla | LKB1 inhibits H4K8 and H4K16 Kla reduces telomerase activity and promotes LUAD cell senescence | PMID: 38844063 | ||
| Colorectal cancer | H3K18la | METTL3-JaK1m6A-STAT3-Immunosuppression | PMID: 35320754 | |
| Promotes RUBCNL expression, exacerbating resistance to bevacizumab treatment | PMID: 37615625 | |||
| H3K18la- GPR37 -Hippopathway- liver metastases | PMID: 37749229 | |||
| Activates the transcription of NSUN2 and NSUN2 K356la, promoting CRC progression | PMID: 38769664 | |||
| Inhibits the transcription of the RAR γ gene and conferring macrophage protumor function | PMID: 38245869 | |||
| Increases the upregulation of CXCL1 and CXCL5 expression, ultimately promoting CRC liver metastasis. | PMID: 37749229 | |||
| H4K8la | Upregulates LINC00152 and promotes cancer cells invasion and migration | PMID: 35959377 | ||
| eEF1A2 K408la | KAT8 promotes CRC development through Kla of eEF1A2 | PMID: 38359291 | ||
| β-catenin | Hypoxia-induced β -catenin Kla promotes CRC proliferation through the Wnt signaling pathway | PMID: 36464122 | ||
| MRE11 K673 | Promotes DNA end resection and homologous repair | PMID: 38128537 | ||
| Protein Kla | Inhibiting M2 macrophage polarization to inhibit CRC progression | PMID: 36242053 | ||
| RIG-I Kla | affects the immunosuppressive activity of Tregs and the antitumor activity of CD8+ T cells | PMID: 38890429 | ||
| Liver cancer | HCC | H3K9la, H3K56la | Upregulates ESM 1 to promote the malignant phenotype, tumor growth, and metastasis of HCC cells | PMID: 39016629 |
| CCNE2 Kla | Promotes proliferation, migration, and invasion through lactylation | PMID: 36896611 | ||
| CENPA K124la | CENPA is cooperated with YY1 to drive CCND 1 and NRP2 expression to promote HCC progression | PMID: 37928273 | ||
| AK2 K28la | Inhibits AK2, leading to intracellular energy disorders, cell proliferation, invasion and metastasis | PMID:36593272 | ||
| PKM2 K505la | Enhanced the immunosuppressive microenvironment and HCC metastasis | PMID: 38471282 | ||
| MOESIN K72la | Regulates regulatory T cells and exerting an immunosuppressive function to promote HCC progression | PMID: 35732125 | ||
| IGF2BP3 | Reprogram serine metabolism and enhance the antioxidant defense system | PMID: 39450426 | ||
| GPC3 Kla | Promotes cell growth, stemness, and glycolysis | PMID: 37131292 | ||
| c-Myc | Reduced the stability of the c-myc protein; | PMID: 37131292 | ||
| H3K9la, H3K56la | Stimstimulates cell proliferation to promote HCC progression | PMID: 35605812 | ||
| H3K18la | PYCR1 regulates the transcriptional activity of IRS1 by affecting the lactylation of H3K18 in the IRS1 promoter region. | PMID: 39422696 | ||
| H3K56la, ALDOA K230/322 | Closely related to the stemness of LCSCs | PMID: 39099416 | ||
| iCCA | NCL K477la | Up-regulation of MADD enhances the pathogenesis of intrahepatic cholangiocarcinoma through the MAPK pathway | PMID: 38679071 | |
| Breast Cancer | H3K18la | Upregulates c-Myc and promote breast cancer progression | PMID: 37572497 | |
| p53 K120la, p53 K139la | Decreases transcriptional activity, promoting tumorigenesis | PMID: 38512451 | ||
| H4K12la | H4K12la is significantly upregulated in triple-negative breast cancer (TNBC) and is a novel biomarker. | PMID: 38779451 | ||
| Gastric Cancer | H3K9la H3K18la, H3K56la | Knockdown of GLUT3 significantly reduces the levels of LDHA, L-lactyl, H3K9, H3K18, and H3K56. | PMID: 38041125 | |
| H3K18la | Upregulates VCAM 1 transcription, activates AKT-mTOR signaling pathway and promotes tumor cell proliferation, EMT transformation, and tumor metastasis; Upregulate the expression of METTL14, which activates the WDR74/β-catenin axis by mediating the m6A modification of ATF5 mRNA, thereby promoting stemness in gastric cancer. | PMID: 39497511 PMID: 38512451 | ||
| CBX3 K10la | Promotes the tumor proliferation and growth | PMID: 39018247 | ||
| METTL16 K229la | Copper stress promotes METTL16 lactlation, and subsequently triggered copper death | PMID: 37863889 | ||
| Pancreatic cancer | PAAD | SLC16A1 Kla | Promotes tumor progression | PMID: 38817665 |
| NMNAT1 K128la | Supporting the nuclear NAD+ salvage pathway and promoting PAAD tumor growth | PMID: 38467179 | ||
| PDAC | H3K18la | Activates TTK and BUB1B transcription, and increased glycolysis aggravates PDAC dysfunction | PMID: 38711083 | |
| Snail1 | RHOF overexpression promotes the lactylation and nuclear translocation of Snail1. Silencing Snail1 reverses the promoting effects of RHOF and lactate on cell migration, invasion, and EMT; | PMID: 39462429 | ||
| CENPA | CENPA may become a promising therapeutic target for PDAC; | PMID: 39456925 | ||
| NUSAP1K34 | Forms a NUSAP1-LDHA-glycolytic-lactate feed-forward loop, thus accelerating the PDAC transfer | PMID: 37354982 | ||
| Esophageal cancer | SHMT2 Kla | Increasing MTHFD1L expression, accelerates the EC malignant progression | PMID: 38175377 | |
| Axin Kla | Promotes the ubiquitination of Axin 1, exerts its anti-glycolytic function | PMID: 38972426 | ||
| H3K9la | Enhances the transcription of LAMC 2 to promote the proliferation and invasion of ESCC | PMID: 38989468 | ||
| Prostate cancer | PCa | HIF1α Kla | Enhances the transcription of KIAA1199, and promote angiogenesis. | PMID: 36209908 |
| p53 Kla | p53 Kla via the NF-κB/STAT3/SLC4A4 axis, leading to Enz resistance and PCa progression | PMID: 38880227 | ||
| CNPY3 K215la and K224la | Inhibits lysosome-dependent CatB/caspase 1/GSDMD pyroptosis signaling pathway, promotes PCa progression | PMID: 38511243 | ||
| NEPC | Pan-Kla, H3K18la | Zeb1 drives histone lactylation modifications to regulates the development of neuroendocrine prostate cancer. | PMID: 38654072 | |
| Pan Kla and H3K18la | Numb/Parkin pathway Defects, accelerate metabolic reprogramming, accelerating NEPC progression | PMID: 36724072 | ||
| Cervical Cancer | G6PD K45la | HPV16 E6 inhibits the Kla of the G6PD dimer by activating PPP and promotes cell proliferation | PMID: 38457903 | |
| H3K18la | Promote the reprogramming of tumor-associated macrophages; | PMID: 39504115 | ||
| PPP1R14B K140la | Enhanced the proliferation and migration capabilities of cervical cancer | PMID: 39025375 | ||
| DCBLD1 K172la | Upregulates DCBLD1 expression, and then upregulates G6PD to stimulate PPP | PMID: 38291438 | ||
| Bladder cancer | H3K18 la | circXRN2 inhibited H3K18la-driven tumor progression in BCa by stabilizing LATS1 and activating the Hippo pathway | PMID: 37684641 | |
| H3K18la-driven YBX1 and YY1 promote BCa cisplatin resistance | PMID: 38295753 | |||
| Renal-cell carcinoma | H3K14la, H3K18la and H3K56la | Overactive Warburg effects, and modulates sensitivity to HIF 2 α blockade | PMID: 38233415 | |
| Pan Kla and H3K18la | High levels of Kla indicated poor prognosis. | PMID: 35637958 | ||
| H3K18la | Activates PDGFRβ gene transcription; PDGFRβ signaling further stimulates H3K18la to form an oncogenic positive feedback loop" | PMID: 35637958 | ||
| Glioblastoma | VEGFR2 and VE-cadherin Kla | LDHA binds and promotes the lactfication of VEGFR2 and VE-cadherin and promoting the development of angiogenic mimicry in glioblastoma | PMID: 37853052 | |
| Pan Kla and H3K18la | Driving NF- κ B-associated LINC01127 expression via the MAP4K4 / JNK / NF- κ B axis promotes self-renewal in GBM cells | PMID: 38084701 | ||
| PTBP1 K436la | Induced abnormal epigenetic modifications further stimulate glycolysis, leading to a vicious cycle that exacerbates tumorigenesis | PMID: 39570804 | ||
| c-Myc | Dexmedetomidine inhibits the lactylation of c-Myc and suppresses the stability of c-Myc; | PMID: 39193894 | ||
| H3K18la | Hypoxia can regulate TNFSF9 expression through the MCT-1/H3K18La signaling pathway, inducing M2 macrophage polarization and promoting the malignant progression of gliomas | PMID: 39010835 | ||
| XRCC1 K247la | Induces treatment resistance in glioblastoma | PMID: 39111285 | ||
| H3K9la | Retention of the MLH 1 intron 7 mediated by LUC7L2 confers resistance to TMZ in the GBM | PMID: 38477507 | ||
| Uveal melanoma | H3K18la | Induces YTHDF2 overexpression accelerates PER 1 and TP53 mRNA degradation and promoting the onset of UM | PMID: 33726814 | |
| Pan Kla, H3K18la | Histone lactlation enhances ALKBH3 expression through demethylation of m1A of SP100A | PMID: 38118002 | ||
| Other | Lymphadenoma | Kla | Elevated lactate levels in lymphoma patients influence the lactylation status, which in turn affects the prognosis, tumor immune function, and drug resistance of diffuse large B-cell lymphoma. | PMID: 39146596 |
| oral squamous cell carcinoma | DHX9 K146la | Alleviated the inhibitory effect of DHX9 on OSCC; | PMID: 39407253 | |
| Endometrial cancer | H3K9la, H3K18la, H3K28la, | Promotes USP39 expression, and targeting the PI3K/AKT/HIF-1α signaling pathway promotes the malignant progression of EC | PMID: 38459014 | |
| H3K18la | Both aerobic glycolysis and histone lactfication were increased | PMID: 38744310 | ||
| Anaplastic Carcinoma | H4K12la | Dysregulation of cell-cycle-related gene expression | PMID: 37184950 | |
| Ovarian cancer | H3K18la | Lactate activates CCL18 expression through H3K18la in macrophages, promoting the OC occurrence | PMID: 39010846 | |
NSCLC: Non-small cell lung cancer; LUAD: Lung adenocarcinoma; HCC: Hepatocellular carcinoma; iCCA: Intrahepatic cholangiocarcinoma; PAAD: Pancreatic cancer; PDAC: Pancreatic ductal adenocarcinoma; PCa: Prostate cancer; NEPC: Neuroendocrine prostate cancer.
Lung adenocarcinoma (LUAD) is the most common subtype of NSCLC, representing about 40% of lung cancer cases, with a poor prognosis and a 5-year survival rate of only 4-17% [80]. Zheng et al. found that SLC25A29 expression correlates with lactate levels, and that H3K14la and H3K18la modifications play key regulatory roles in the SLC25A29 promoter [80]. Wang et al. discovered that BZW2 promotes LUAD progression by enhancing lactate production through glycolysis and Kla of IDH3G. Inhibiting Kla suppresses LUAD progression, and combining BZW2 knockdown with 2-DG treatment significantly inhibits tumor growth in mice [81]. Liu et al. found that LKB1 suppresses Kla of histones H4 (Lys8) and H4 (Lys16), alters Sp1 activity, inhibits telomerase, and promotes senescence in LUAD cells [82]. In conclusion, these studies offer new possibilities for LUAD treatment and support targeting Kla in LUAD therapy.
Additionally, brain metastasis (BM) is a malignant event and a key factor in the poor prognosis of NSCLC patients [83]. Pemetrexed (PEM), a first-line chemotherapy drug capable of crossing the blood-brain barrier, faces limitations in treating lung cancer brain metastases due to drug resistance [83]. Wang et al. reported that AKR1B10 promotes glycolysis by upregulating LDHA expression and increasing lactate levels. This leads to H4K12la, which activates CCNB1 transcription, accelerates DNA replication, and drives the cell cycle, ultimately contributing to acquired PEM resistance in lung cancer bone marrow [83].
Colorectal cancer
Colorectal cancer (CRC) is the second leading cause of death worldwide and the third most common cancer [84]. Huang et al. found significantly elevated pan-Kla levels in CRC, especially in malignant tumors, suggesting that pan-Kla may serve as an independent prognostic factor for CRC. This suggests that risk models based on Kla-related genes could significantly improve the management and treatment outcomes of CRC patients [84]. Xiong et al. found that lactate accumulation in the tumor microenvironment (TME) enhances the transcription of methyltransferase METTL3 in tumor-infiltrating macrophages (TIMs) through H3K18la. METTL3 further mediates m6A modification of JAK1 mRNA, and the m6A-YTHDF1 axis ultimately promotes JAK1 protein translation and STAT3 phosphorylation, facilitating CRC immune evasion and tumor progression [47]. Xie et al. reported that KAT8 promotes CRC development by lactylating the lysine 408 site of eEF1A2, enhancing protein translation efficiency [22]. Chen et al. found that lactate accumulation in CRC cells activates NSUN2 transcription via H3K18la and induces NSUN2 K356la, promoting CRC progression [85]. Li et al. reported that tumor-derived lactate in CRC enhances H3K18la, suppresses RARγ transcription, elevates IL-6 levels in the TME, and activates STAT3 signaling, endowing macrophages with pro-tumor functions [86]. Li et al. also showed that tumor-derived lactate promotes RUBCNL expression via H3K18la in CRC, exacerbating resistance to bevacizumab therapy [87]. Sun et al. revealed that SMC4 downregulation induces abnormal glycolysis, lactate accumulation, and histone Kla, leading to increased ABC transporter expression and a dormancy-like CRC cell phenotype with low proliferation and chemoresistance [88]. Miao et al. found that hypoxia-induced β-catenin Kla promotes CRC cell proliferation and stemness via the Wnt pathway, exacerbating malignant behaviors [89]. Zhou et al. identified that GPR37 activates the Hippo pathway, upregulates LDHA expression and glycolysis, increases H3K18la levels, and enhances CXCL1 and CXCL5 expression, promoting CRC liver metastasis [90].
Given Kla's multifaceted roles in CRC, targeting Kla offers promising therapeutic potential. For example, Wang et al. found that silencing PCSK9 reduced levels of lactate, protein Kla, and macrophage migration inhibitory factor, promoting M1 macrophage polarization while inhibiting M2 polarization, ultimately suppressing CRC progression [91]. Similarly, Li et al. observed elevated histone Kla in CRC patients resistant to bevacizumab. Under hypoxic conditions, inhibiting histone Kla effectively suppressed CRC tumor formation, progression, and survival [87]. Combining drugs that inhibit lactylation and autophagy enhanced the efficacy of bevacizumab in CRC treatment [87]. Furthermore, Gu et al. showed that Escherichia coli could inhibit NF-κB recruitment to the NLRP3 promoter through RIG-I Kla in macrophages, affecting the immunosuppressive activity of Tregs and the antitumor function of CD8+ T cells [92]. These findings suggest that the tumor-resident microbiome may be a potential target for preventing and treating colorectal liver metastases. Collectively, these studies indicate that targeting the Kla process could provide new therapeutic strategies for CRC prevention and treatment.
Liver cancer
Hepatocellular carcinoma (HCC) is a common liver cancer closely linked to metabolic processes. Recent studies have identified widespread Kla modifications in HCC, affecting enzymes in various pathways, with Kla levels correlating to HCC aggressiveness and mutations [93]. Yang et al. were the first to map the landscape of Kla modifications in HCC. Through integrated lactyl-proteomic and proteomic analyses of tumor and adjacent liver tissues, they revealed that Kla is a widespread modification extending beyond histones and transcriptional regulation [93]. Notably, their analysis of Kla-modified substrates demonstrated significant impacts on enzymes in key metabolic pathways, including glycolysis, the TCA cycle, amino acid metabolism, fatty acid metabolism, and nucleotide metabolism. Higher Kla levels on these pathway proteins were closely associated with invasive clinical features and driver mutations in HCC [93]. Subsequently, Kla has been extensively studied in HCC. Cheng et al. developed an effective prognostic model and identified lactylation-related genes (LRGs) associated with HCC prognosis. They discovered that patients with low-risk LRG scores responded better to most targeted drugs and immunotherapies, while those with high-risk scores were more sensitive to chemotherapy and sorafenib, suggesting that LRG markers could serve as biomarkers for effective clinical treatment of HCC [94]. Jin et al. further confirmed that histone Kla in liver cancer is closely associated with tumor progression, lymph node metastasis, and staging. Collectively, these findings suggest that Kla may serve as a diagnostic and prognostic biomarker for HCC and that targeting lactate immunometabolism and Kla could offer a potential therapeutic strategy [27].
In recent years, increasing evidence has shown that Kla promotes the progression of HCC. For instance, Zhao et al. found that histone Kla levels, particularly H3K9la and H3K56la, are significantly elevated in HCC tissues and cells. This modification enhances the malignant phenotype, tumor growth, and metastasis of HCC cells by upregulating ESM1 expression [95]. Jin et al. discovered that nonhistone Kla of CCNE2 promotes the proliferation, migration, and invasion of liver cancer cells. In contrast, the NAD-dependent deacetylase SIRT3 removes Kla from CCNE2, thereby regulating the cell cycle and inhibiting HCC progression [27]. The study also suggested that andrographolide enhances SIRT3-mediated deacetylation of CCNE2, boosting its anti-HCC effect [27]. Liao et al. reported that centromere protein A (CENPA) can be lactylated at K124, which activates CENPA. This activation, in turn, drives the expression of CCND1 and NRP2, promoting HCC progression [96]. Yang et al. found that HCCpatients with the proliferative subtype had higher levels of AK2 Kla in tumor tissues, which was associated with poor prognosis. AK2 K28la inhibited its kinase activity, disrupting intracellular energy balance and promoting HCC cell proliferation, invasion, and metastasis [97]. Qian et al. observed that under high-glucose conditions, glycolysis in HCC cells increased, leading to elevated lactate levels. This, in turn, promoted PKM2 K505 Kla, inhibiting FBP binding to PKM2 and facilitating its transition from a tetramer to a dimer. PKM2 Kla also enhanced its shift from glycolytic function to gene transcription regulation, which reinforced the immunosuppressive microenvironment and promoted HCC metastasis [98]. Gu et al. found that effective anti-PD-1 treatment in HCC patients correlated with lower levels of MOESIN Kla in Treg cells compared to non-responders. The study showed that lactate regulated Treg cells through MOESIN K72la, enhancing the interaction between MOESIN, TGF-β receptor I, and downstream SMAD3 signaling. This process contributed to immunosuppression and facilitated HCC progression [99]. Cai et al. identified the SRSF10/MYB/glycolysis/lactate axis as a key mechanism in immune evasion and resistance to anti-PD-1 therapy. SRSF10 upregulated lactate production, creating a positive feedback loop that enhanced glycolysis and H3K18la in tumor cells. Increased lactate levels promoted macrophage polarization to the M2 phenotype, suppressing CD8+ T cell activity. These findings suggest that the SRSF10 inhibitor 1C8 could overcome HCC resistance to anti-PD-1 therapy [100]. Yao et al. found that knocking down GPC3 reduced overall Kla levels and c-myc Kla under hypoxic conditions, inhibiting HCC cell growth, stemness, and glycolysis. This suggests that GPC3-mediated Kla could be a promising therapeutic target for liver cancer [101]. Pan et al. showed that lactate induced histone H3K9 and H3K56 lactylation and increased the expression of cell cycle-related proteins in HCC stem cells, stimulating cell proliferation and promoting HCC progression [102]. Moreover, Feng et al. reported enhanced glycolytic metabolism, lactate accumulation, and elevated Kla levels in liver cancer stem cells (LCSCs) compared to HCC cells. H3K56la was closely related to tumorigenesis and LCSC stemness. Lactylation at ALDOA K230/322 played a crucial role in promoting LCSC stemness [103]. This research underscores the importance of Kla in regulating LCSC stemness and its impact on HCC progression, suggesting that targeting LCSC lactylation may offer a promising therapeutic strategy for HCC [103].
Intrahepatic cholangiocarcinoma (iCCA) is a highly aggressive malignant liver tumor [104]. Yang et al. reported that under hyperactive glycolysis, nucleolin (NCL) is primarily lactylated at lysine 477 by the acyltransferase P300, promoting iCCA cell proliferation and invasion. Further research showed that lactylated NCL binds to the primary transcript of MAP kinase-activating death domain protein (MADD) and facilitates efficient MADD translation by preventing premature stop codons through selective splicing. NCL Kla, MADD expression, and subsequent ERK activation drive xenograft tumor growth and correlate with overall survival in iCCA patients [104].
Additionally, studies suggest that certain natural compounds can inhibit lactate production and histone Kla, exerting anti-HCC effects. For example, demethylzeylone (DML) suppresses liver cancer stem cell tumorigenesis by inhibiting H3K9la and H3K56la [102]. Similarly, royal jelly acid disrupts lactate production and specifically inhibits Kla at H3K9 and K14, reducing liver cancer cell proliferation and metastasis [105]. Collectively, these studies highlight the role of Kla in HCC progression and propose that targeting Kla could provide novel therapeutic strategies for HCC.
Breast cancer
Breast cancer is the most common malignancy and a leading cause of death among female cancer patients [106]. Cui et al. found that H4K12la is significantly upregulated in triple-negative breast cancer (TNBC), serving as a novel biomarker [106]. Li et al. discovered that lactate-induced H4K12la specifically suppresses SLFN5 expression, thereby promoting TNBC progression, and proposed a key lactylation-dependent oncogenic pathway [107]. Hou et al. found that potassium two-pore domain channel subfamily K member 1 (KCNK1) accelerates glycolysis and lactate production by binding and activating LDHA. This process promotes Kla and induces the expression of downstream targets, including LDHA itself, leading to breast cancer proliferation, invasion, and metastasis [108]. Zong et al. revealed that AARS1 acts as a lactate sensor mediating global Kla in breast cancer cells. AARS1 catalyzes lactylation at K120 and K139 in the DNA binding domain of p53, suppressing its phase separation, DNA binding, and transcriptional activation, thereby driving breast cancer development [109]. Notably, this study employed an orthogonal translation system with pyrrolysyl-tRNA synthetase to enhance p53 Kla at specific sites, rather than adding exogenous lactate or overexpressing LDHA. The research also suggested using β-alanine to inhibit p53 Kla and restore its tumor-suppressor function, as β-alanine competes with lactate to bind AARS1, boosting chemotherapy effectiveness [109]. This groundbreaking study not only unveiled a novel regulatory mechanism linking the metabolite lactate to p53 function but also opened new avenues for cancer treatment strategies [109].
"Deleterious" regulation of lactylation in malignant tumors. Lactylation is widely present in various cancers and is closely related to the occurrence and progression of malignancies. It plays a crucial role in tumor metabolism, tumor microenvironment, tumor immunosuppression, tumor progression, metastasis, and treatment resistance through multiple mechanisms.
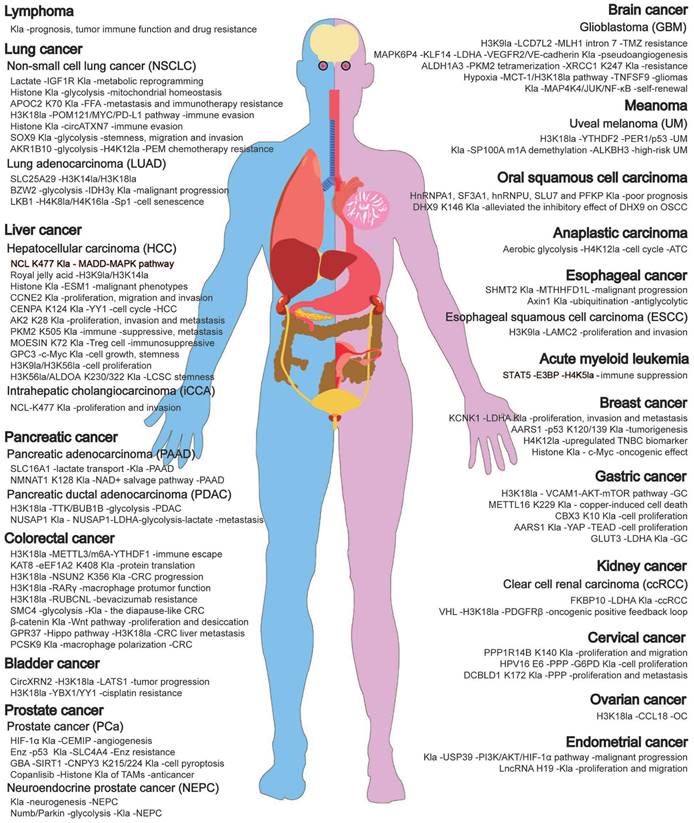
Additionally, Madhura et al. showed that lactate, a tumor metabolite, promotes breast cancer progression by regulating histone Kla-dependent c-Myc expression [110]. Liu et al. also found that asiatic acid enhances caspase-3 activity, inducing mitochondrial apoptosis, while modulating lactylation and 2-hydroxyisobutyrylation to inhibit breast cancer progression [111]. In summary, these studies suggest that lactylation and its related pathways could provide new therapeutic targets for breast cancer.
Gastric cancer
Gastric cancer (GC) is a malignant tumor from gastric mucosal cells, with high incidence and mortality, threatening human health [112]. Studies reveal that histone Kla is elevated in GC tissues compared to adjacent non-cancerous tissues and correlates with poor prognosis, suggesting its potential as a prognostic biomarker [112]. Moreover, bioinformatics analysis by Yang et al. identified six lactylation-related genes associated with GC prognosis, showing that lactylation scores are strongly correlated with overall survival and disease progression. Notably, patients with higher lactylation scores exhibit greater immune evasion and reduced response to immunotherapy [113]. This implies that lowering lactylation levels to improve tumor sensitivity to ICIs could be a potential therapeutic strategy.
In terms of molecular mechanisms, Yang et al. discovered that in gastric cancer, glucose transporter 3 (GLUT3) promotes lactylation modification by regulating LDHA. Knockdown of GLUT3 significantly reduced the levels of LDHA, L-lactyl, H3K9, H3K18, and H3K56 [114]. Zhao et al. found that lactate in the TME promotes dynamic H3K18la in gastric cancer cells, upregulating VCAM1 transcription. This activates the AKT-mTOR signaling pathway, promoting tumor cell proliferation, epithelial-mesenchymal transition (EMT), and tumor metastasis. Furthermore, VCAM1 upregulates CXCL1 expression via the AKT-mTOR pathway, facilitating the recruitment of hGC-MSCs and M2 macrophages [115]. Duan et al. revealed that chromobox protein homolog 3 (CBX3) K10la is significantly upregulated in various gastrointestinal tumors, including gastric cancer. This modification is closely linked to tumor cell proliferation and growth. Knockdown of CBX3 or blocking K10 Kla significantly inhibited tumor growth [116]. Sun et al. found that copper ions regulate METTL16-K229 Kla via lactylases and the delactylase SIRT2, modulating its activity. Lactylated METTL16 induces methylation at the FDX1 mRNA-602 site, promoting FDX1 expression and leading to ferroptosis. SIRT2 inhibits METTL16 Kla, and combining the SIRT2 inhibitor AGK2 with the ferroptosis inducer Eles significantly enhances the treatment of malignant gastric cancer, especially for mucinous adenocarcinomas with high copper content [117]. Ju et al. identified that AARS1 senses intracellular lactate and translocates to the nucleus, activating the YAP-TEAD complex and forming a positive feedback loop that promotes gastric cancer progression [32]. Given the competitive binding of lactate and alanine to AARS1, this explains why high lactate levels do not inhibit general mRNA translation, which is essential for cell proliferation and tumor growth. Moreover, AARS1 Kla may serve as an alternative regulatory mechanism for YAP activity, independent of the canonical Hippo pathway. Li et al. found that lactate accumulation leads to H3K18la, which is enriched in the PD-L1 promoter region, thereby promoting PD-L1 transcription. This suggests that cancer-associated fibroblasts (CAFs) may reduce the efficacy of PD-1/PD-L1 blockade immunotherapy through glycolysis and lactate accumulation induced by LOX [118]. In summary, lactylation plays a critical role in the development of gastric cancer. These findings not only expand the proteomic data on lactylation in gastric cancer but also suggest that lactylation could serve as a potential prognostic marker and a novel therapeutic target for gastric cancer.
Pancreatic cancer
Pancreatic cancer (PAAD) is known for its aggressiveness, high mortality, and poor prognosis [119]. Peng et al. identified 10 LRGs that are differentially expressed and have prognostic value, using RNA sequencing and clinical data analysis. These genes, including SLC16A1, HLA-DRB1, KCNN4, KIF23, and HPDL, were found to be strongly associated with overall survival in PAAD patients [119]. Furthermore, the research confirmed that LRG-SLC16A1 regulates lactylation in pancreatic cancer cells by facilitating lactate transport. Reducing SLC16A1 and its lactylation significantly slows tumor progression, suggesting that targeting the SLC16A1/Kla pathway could be a potential therapeutic approach for PAAD [119]. Huang et al. observed that the abundance of Pan-Kla is notably increased in PAAD patients and correlates with poor prognosis. The study also identified that lysine residue 128 of NMNAT1 plays a critical role in catalyzing lactylation. Lactylation of NMNAT1 enhances its nuclear localization and preserves its enzymatic activity, thus supporting the NAD+ salvage pathway and promoting tumor growth in PAAD [120]. Zhao et al. discovered that RHOF promotes the Kla of Snail1 by enhancing PKM2-mediated glycolysis, which drives EMT in pancreatic cancer cells [121].
Pancreatic ductal adenocarcinoma (PDAC) presents significant challenges, with a 5-year survival rate of approximately 9% [122]. Li et al. reported that histone Kla, especially H3K18la, is significantly increased in PDAC and associated with poor outcomes. H3K18la accumulates in promoter regions, activating transcription of mitotic checkpoint regulators TTK and BUB1B, which in turn increases P300 expression, intensifying PDAC glycolysis and dysfunction [122]. Chen et al. discovered that lactate inhibits the degradation of nucleolar and spindle-associated protein 1 (NUSAP1) through Kla modification, upregulating NUSAP1 expression and forming a feedback loop that accelerates PDAC metastasis [123]. Furthermore, Takata et al. proposed that Kla may play a role in the development of pancreatic epithelial tumors and could be a potential therapeutic target. Elevated Kla levels were observed in the nuclei of intraductal papillary mucinous neoplasms, non-invasive intraductal papillary mucinous carcinomas, and invasive cancers, along with increased hypoxia-inducible factor-1α levels, suggesting that hypoxia-related nuclear protein Kla could serve as a biochemical marker for pancreatic epithelial tumors [124]. Overall, these studies underscore the pivotal role of Kla in pancreatic cancer progression and highlight potential targets for new therapeutic approaches.
Esophageal cancer
Esophageal cancer (EC) is a prevalent and lethal gastrointestinal tumor. Recent research identifies the hypoxic microenvironment as a major driver of its rapid progression [125]. Hypoxia not only accelerates tumor growth but also causes lactic acid buildup, which induces histone lysine Kla, influencing gene transcription and regulation. For example, Qiao et al. revealed that hypoxia-induced Kla of SHMT2 enhances MTHFD1L expression, promoting malignant progression in EC cells [125]. Li et al. demonstrated that hypoxia increases Axin1 Kla, leading to its ubiquitination and degradation, which facilitates glycolysis and stemness in EC cells [126]. Zang et al. found that hypoxia elevates histone H3K9la levels, boosting LAMC2 transcription and driving proliferation and invasion in esophageal squamous cell carcinoma [127]. Additionally, Fu et al. reported that the long non-coding RNA AP001885.4 enhances esophageal squamous cancer cell proliferation through histone Kla, NF-κB transcriptional activation, and METTL3-mediated stabilization of c-Myc mRNA [128]. These findings provide new insights into EC pathogenesis and suggest targeting histone Kla as a potential therapeutic strategy.
Prostate cancer
Prostate cancer (PCa) is one of the most common malignancies in adult men [129]. Pan et al. showed that the LRG prognostic model effectively predicts disease-free survival and treatment response in PCa patients [129]. Luo et al. found that lactate enters PCa cells via MCT1, stabilizing HIF1α expression under normoxia through Kla, which promotes KIAA1199 transcription and angiogenesis [130]. Recent studies indicate that regulating lactate metabolism and Kla can reverse chemotherapy resistance and enhance treatment efficacy. For instance, the androgen receptor inhibitor enzalutamide (Enz) is effective in advanced PCa [131], but long-term use may lead to resistance [132]. Chen et al. found that long-term Enz treatment upregulates SLC4A4, which mediates P53 Kla via the NF-κB/STAT3/SLC4A4 axis, leading to Enz resistance and PCa progression [133]. This suggests that targeting SLC4A4 could be a promising strategy for overcoming Enz resistance. Zhang et al. found that gambogic acid forms hydrogen bonds with Glu171 and Thr100 of CNPY3, recruiting the SIRT1 protein to bind with CNPY3. This inhibits lactylation at the K215 and K224 sites of CNPY3 and promotes the lysosome-dependent CatB/caspase 1/GSDMD pyroptotic pathway, inducing pyroptosis in DU145 cells and suppressing prostate cancer progression [26]. These findings highlight the potential of inhibiting lactylation to induce cell pyroptosis and suppress tumor progression, offering strategies for developing innovative antitumor drugs. Kiranj Chaudagar et al. found that the PI3K inhibitor copanlisib reduced lactate production in tumor cells, inhibiting histone Kla in tumor-associated macrophages (TAMs) and enhancing their antitumor phagocytic activity. This activity was further enhanced by ADT/aPD-1 treatment but blocked by feedback activation of the Wnt/β-catenin pathway. This finding suggests an immunometabolic strategy combining lactate and PD-1-mediated TAM immunosuppression reversal with ADT treatment for patients with PTEN-deficient mCRPC [134]. Furthermore, Chaudagar et al. observed that, compared to copanlisib monotherapy (37.5%), combining lactate inhibition in the TME and H3K18la inhibition in TAMs achieved an 80% overall response rate. This suggests that in PTEN/p53-deficient AVPC, disrupting lactate-mediated crosstalk between cancer cells and TAMs can achieve durable tumor control independent of ADT [135].
Neuroendocrine prostate cancer (NEPC) is a rare tumor, and increasing evidence suggests that it is induced by prostate cancer treatment drugs [136]. Wang et al. revealed that during NEPC progression, prostate cancer cells enter an intermediate state marked by Zeb1 expression, characterized by EMT, stemness, and neuroendocrine traits. Zeb1 drives histone Kla, a process that influences NEPC development [136]. Additionally, cellular plasticity and neuroendocrine differentiation in prostate cancer and lung adenocarcinoma contribute significantly to resistance against targeted therapies [137]. He et al. identified the Numb/Parkin pathway as a key metabolic switch in cancer cell plasticity and a potential therapeutic target. Without this pathway, NEPC cells display numerous fragmented mitochondria with low membrane potential, leading to metabolic reprogramming [137]. This reprogramming boosts glycolysis, producing lactate that enhances Kla and the transcription of neuroendocrine-related genes, accelerating NEPC progression [137].
Cervical cancer
Cervical cancer is a prevalent gynecological malignancy among young women [138]. He et al. observed that the expression of the lactate-related gene PPP1R14B negatively correlates with CD8+ T cell infiltration and is linked to lower survival rates. The K140R mutation in PPP1R14B reduces Kla levels in cervical cancer cells, promoting their proliferation and migration [138]. Huang et al. reported that histone Kla driven by GPD2 induces M2 macrophage polarization, facilitating the malignant transformation and progression of cervical cancer [139]. Meng et al. demonstrated that the HPV16 E6 protein enhances cervical cancer cell proliferation by activating the pentose phosphate pathway (PPP) and suppressing the Kla of G6PD dimers [140]. In another study by Meng et al., lactylation at K172 of Discoidin, CUB, and LCCL domain-containing protein 1 (DCBLD1) upregulates DCBLD1 expression, which in turn increases G6PD expression and enzymatic activity. This activation of the PPP promotes the proliferation and metastasis of cervical cancer cells [141]. Overall, the research is limited, and the precise characterization and functional significance of the non-histone Kla in cervical cancer still require further exploration.
Bladder cancer
Bladder cancer (BCa) is one of the most common malignant tumors in the male urinary system, with a poor prognosis [142]. Xie et al. discovered that in BCa, circXRN2 (a negative regulator of glycolysis and lactate production) interacts with SPOP, inhibiting the ubiquitination and degradation of LATS1. This, in turn, activates the Hippo signaling pathway, which suppresses the transcription of lipocalin-2 by blocking H3K18la on its promoter. This process ultimately slows tumor growth and metastasis [142]. Furthermore, studies indicate that BCa patients often develop resistance to platinum-based chemotherapy, especially cisplatin [143]. Li et al. discovered that key transcription factors YBX1 and YY1, driven by H3K18la, contribute to cisplatin resistance in bladder cancer. Inhibiting H3K18la restores cisplatin sensitivity in resistant epithelial cells [143]. Although research is still limited, these findings reveal new therapeutic targets, offering promising strategies for clinical intervention in human bladder cancer.
Kidney cancer
Renal cell carcinoma is one of the three major malignant tumors of the urinary system, originating from the epithelial cells of the proximal renal tubules. Clear cell renal cell carcinoma (ccRCC) accounts for approximately 80% of renal cancer cases and is a metabolic disease driven by genetic mutations and epigenetic alterations [144]. In recent years, studies have revealed that lactylation modifications play a crucial role in ccRCC and may serve as prognostic diagnostic markers [145]. TIMP1, a key gene linked to histone Kla in ccRCC, holds substantial prognostic value, especially in a sex-dependent manner, either on its own or in combination with its receptor [144]. FKBP10 plays a critical role in hypoxia and glycolytic pathways during ccRCC progression. It binds to LDHA, a major glycolytic regulator, through its C-terminal domain, enhancing LDHA-Y10 phosphorylation. This promotes the Warburg effect, histone Kla, and ccRCC progression while affecting sensitivity to HIF2α blockade [146]. Additionally, mutations in the von Hippel-Lindau (VHL) tumor suppressor gene are common in ccRCC, resulting in metabolic changes and increased lactate production, creating a lactate-rich TME [147]. Inactive VHL correlates with high levels of histone Kla, which is linked to poor prognosis [147]. VHL inactivation activates H3K18la through an HIF-dependent mechanism, promoting PDGFRβ gene transcription. This PDGFRβ signaling further amplifies H3K18la, forming a carcinogenic feedback loop that worsens ccRCC [147]. These findings suggest that inhibiting lactylation may offer a promising therapeutic strategy for ccRCC.
Glioma
Gliomas are the most common and aggressive brain tumors, characterized by high proliferation, abnormal glycolysis, and poor prognosis. Glioblastoma (GBM) is the most malignant and treatment-resistant subtype [148]. Zhang et al. found that the pseudogene MAPK6P4 encodes a functional peptide, P4-135aa, which phosphorylates KLF15 at the S238 site, enhancing its stability and nuclear entry to activate LDHA transcription. LDHA binds to VEGFR2 and VE-cadherin, promoting their Kla and facilitating glioblastoma vascular mimicry [148]. Li et al. found that hypoxia-induced histone Kla activates NF-κB-related LINC01127 expression via the MAP4K4/JNK/NF-κB axis, promoting GBM cell self-renewal [149]. Hypoxia is a key factor in poor prognosis and treatment challenges in gliomas [150]. Li et al. found that hypoxia regulates TNFSF9 expression via the MCT-1/H3K18la pathway, inducing M2 macrophage polarization and promoting glioma progression [150]. Li et al. also found that ALDH1A3-mediated PKM2 tetramerization promotes lactate accumulation in GBM stem cells, inducing XRCC1 K247 Kla, which confers treatment resistance. D34-919 disrupts the ALDH1A3-PKM2 interaction, enhancing GBM cell sensitivity to radio-chemotherapy [151]. This suggests that ALDH1A3-mediated PKM2 tetramerization could be a potential therapeutic target to improve the response of ALDH1A3-high GBM to radio-chemotherapy [151]. Wang et al. found that lactate from patient-derived glioma stem cells and microglia/macrophages induces epigenetic reprogramming of tumor cells via histone Kla regulated by CBX3, promoting an immunosuppressive transcriptional program. This upregulates CD47 (a “don't eat me” signal) in GBM cells, inhibiting phagocytosis and promoting immune evasion [152]. This suggests that this mechanism can be leveraged to enhance immunotherapy efficacy.
Temozolomide (TMZ) resistance poses a significant challenge in GBM treatment [153]. Yue et al. reported that Kla is upregulated in recurrent GBM tissues and TMZ-resistant cells, especially H3K9la. H3K9la induces TMZ resistance by LUC7L2-mediated retention of MLH1 intron 7 [153]. Additionally, Leo et al. showed that pERK-driven glucose metabolism enhances MDM immunosuppressive activity via histone Kla, promoting T-cell accumulation and slowing tumor growth. This process can be combined with immunotherapy to stop GBM progression [154]. These findings provide new targets and strategies for GBM treatment.
Uveal melanoma
Uveal melanoma (UM), the most common intraocular malignancy in adults, is highly aggressive and prone to metastasis [155]. Yu et al. reported that histone pan-Kla is significantly elevated in UM tissues and cell lines, correlating with poor outcomes [155]. H3K18la promotes overexpression of the m6A reader YTHDF2, which binds to m6A sites on tumor-suppressor mRNAs PER1 and TP53, leading to their degradation and driving UM progression [155]. Targeting H3K18la can effectively block tumor growth, offering a promising new therapeutic approach. Gu et al. also found a strong positive correlation between UM and intracellular histone Kla. Kla increases ALKBH3 expression by demethylating m1A on SP100A, which decreases PML body formation and promotes UM malignancy [156]. These findings offer new perspectives for treating UM.
Other malignancies
The high global incidence and mortality rates of these cancers highlight the critical roles of lactate and Kla in tumor metabolism, microenvironment, immune suppression, growth, metastasis, and treatment resistance. For example, Song et al. identified 1,011 and 1,197 Kla sites in oral squamous cell carcinoma cells, respectively, observing enrichment in the spliceosome, ribosome, and glycolysis/gluconeogenesis pathways. Lactylation modifications were detected in spliceosomal proteins such as hnRNPA1, SF3A1, hnRNPU, and SLU7, as well as in the glycolytic enzyme PFKP. Furthermore, lactylation levels were negatively correlated with patient prognosis [157]. Sun et al. found that LRGs effectively identify high-risk patients and predict outcomes in multiple myeloma [158]. Zhu et al. reported elevated lactate levels in lymphoma patients, where Kla influences prognosis and immune function in diffuse large B-cell lymphoma [159]. Similarly, Yu et al. linked LRGs to tumor classification and immunity in ovarian cancer (OC), predicting patient outcomes [160]. Sun's research showed that lactate activates CCL18 expression in macrophages via H3K18la, promoting OC progression [161]. Wei et al. observed significantly increased Kla in endometrial cancer tissues, where histone Kla enhances USP39 expression, driving the PI3K/AKT/HIF-1α pathway to promote malignancy [162]. Treatment with 2-deoxy-D-glucose and sodium acetate reduced Kla levels, inhibiting cancer cell proliferation and migration [162]. Wen et al. reported elevated aerobic glycolysis and histone Kla in endometriosis, with lncRNA H19 overexpression exacerbating metabolic abnormalities and promoting proliferation and migration [163]. Huang et al. demonstrated that high STAT5 expression in acute myeloid leukemia leads to lactate accumulation, which promotes E3 ligase translocation and H4K5la, inducing PD-L1 transcription and immune suppression, suggesting the potential for PD-1-based immunotherapy [164]. Wang et al. noted that FOXP3+ NKT-like cells utilize lactate via high MCT1 and lactate dehydrogenase B expression, maintaining immune suppression in malignant pleural effusions [165]. Jia et al. found significantly higher lactate levels in lung and gastric cancer tissues compared to adjacent tissues, with lactate acting as a signaling molecule to enhance autophagy and tumor progression via KAT5/TIP60-mediated Kla at specific lysine residues [21]. Wang et al. observed that anaplastic thyroid carcinoma (ATC) exhibits high histone Kla levels, with aerobic glycolysis increasing intracellular lactate utilization and disrupting cell cycle gene expression. Blocking Kla mechanisms, combined with BRAFV600E inhibitors, suppressed ATC progression [166]. In summary, Kla significantly influences tumor epigenetics and gene expression, offering new therapeutic perspectives. Targeting tumor Kla, particularly in combination with immunotherapy, holds significant research potential.
Nontumor-related diseases and lactylation regulation
Recent evidence shows that Kla occurs in many nontumor cells and a variety of noncancerous diseases. We then classify and summarize the functions, molecular mechanisms, and potential applications of Kla in nontumor diseases (Fig. 5).
Lactylation in nontumor related diseases. Lactylation occurs in various non-tumor cells and is associated with numerous non-cancerous diseases. It significantly impacts the immune, respiratory, circulatory, nervous, skeletal, digestive, and endocrine systems, influencing the onset and regulation of these conditions. Note: Red indicates disease progression, while green signifies disease remission. Created in https://BioRender.com.
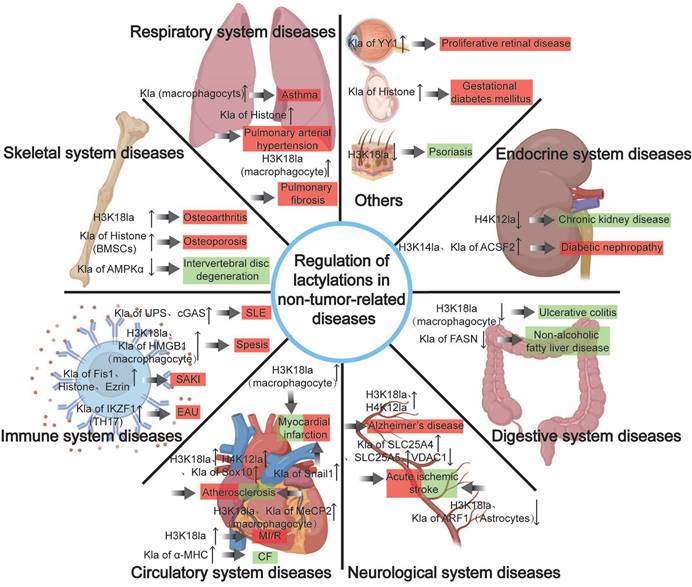
Immune system diseases
Sepsis occurs when the host immune system cannot control infections, causing systemic inflammation and multiple organ failure [167]. Chu et al. observed significantly elevated H3K18la levels in septic shock patients, with its expression positively correlated to APACHE II scores, mechanical ventilation duration, serum lactate, and procalcitonin levels [167]. These findings suggest that H3K18la could be a biomarker for diagnosing and predicting septic shock severity, reflecting critical illness and infection [167]. Yang et al. showed that serum lactate and high-mobility group box 1 (HMGB1) are positively linked to sepsis-related mortality. In sepsis, macrophage lactate promotes HMGB1 Kla via p300/CBP. Lactylated/acetylated HMGB1 is secreted through the exosome pathway, reducing VE-cadherin and claudin-5 levels, increasing ICAM1 levels, disrupting endothelial integrity, and increasing vascular permeability [39]. This causes endothelial barrier dysfunction, accelerating sepsis progression [39]. An et al. found that elevated lactate levels independently increase the risk of sepsis-associated acute kidney injury (SAKI). SIRT3 downregulation leads to hyperacetylation and inactivation of PDHA1, causing excessive lactate production. This, in turn, mediates Fis1 Kla, worsening SAKI. Lowering lactate levels and Fis1 secretion alleviates SAKI [168]. Qiao et al. identified histone Kla as a contributor to renal impairment in SAKI. They found lactate-dependent histone modifications enriched at the RhoA promoter region. Histone Kla activates the RhoA/ROCK/Ezrin pathway, triggering NF-κB, inflammation, apoptosis, and kidney dysfunction. Multiple Kla sites were identified on Ezrin, with K263 Kla playing a key role in inflammatory metabolic adaptation in renal proximal tubular epithelial cells [169]. Wu et al. found that METTL3, regulated by histone Kla, facilitates ferroptosis in sepsis-associated acute lung injury via m6A modification of ACSL4 [170].
Systemic lupus erythematosus (SLE) is an autoimmune inflammatory connective tissue disease and a classic example of an “interferon type I disease [171]. Zhang et al. found that mtDNA in SLE patients drives glycolysis to produce lactate, which promotes cGAS Kla. This process inhibits its binding to the E3 ubiquitin ligase MARCHF5, blocking cGAS degradation and triggering a strong IFN-I response [171]. Developmental defects in erythrocytes (RBCs) have also been implicated as triggers for SLE [172]. Caielli et al. reported that during RBC maturation, HIF activates the UPS and mediates UPS Kla, leading to an abnormal increase in mature RBCs. When engulfed by macrophages, mtDNA from these RBCs stimulates the cGAS/STING pathway, driving type I IFN production and SLE [173]. Lupus nephritis (LN), a significant risk factor for SLE morbidity and mortality, has been linked to lactate metabolism. Sun et al. identified lactate-related biomarkers COQ2, COQ4, and NDUFV1 as being associated with LN, suggesting a role for lactate metabolism and protein Kla in its onset [174].
Experimental autoimmune uveoretinitis (EAU) is a T-cell-mediated, organ-specific autoimmune disease. Fan et al. demonstrated that lactylation from lactic acid regulates CD4+ T cell differentiation. Hyper-lactylation at the Lys164 site of Ikzf1 directly influences the expression of TH17-related genes, such as Runx1, Tlr4, IL-2, and IL-4, promoting TH17 differentiation. Suppressing lactylation inhibits TH17 cell differentiation and reduces EAU inflammation [175]. These findings show how glycolysis-driven protein lactylation promotes TH17 differentiation, suggesting Ikzf1 Kla as a potential therapeutic target for autoimmune diseases.
Crohn's disease (CD) involves immune dysregulation in the gut, often linked to metabolic abnormalities. Wu et al. analyzed lactylation levels in immune cells using single-cell data and observed significant variations among cell types [176]. Notably, Lactylation levels were higher in immune cells from inflamed areas. This highlights the close relationship between lactylation-related genes and inflammatory cell changes in CD patients.
Respiratory system diseases
Pulmonary fibrosis is a chronic respiratory disease characterized by the gradual replacement of lung tissue with fibrotic tissue. Recent studies increasingly show that elevated lactate levels in human alveolar macrophages lead to increased histone Kla [177]. Histone Kla induces high expression of pro-fibrotic-related genes, contributing to disease progression [177]. For instance, Cui et al. found that lactate produced by myofibroblasts enriches H3K18la in the promoter regions of pro-fibrotic genes, such as ARG1, PDGFA, VEGFA, and THBS1, promoting their expression. This enhances the pro-fibrotic activity of alveolar macrophages, exacerbating pulmonary fibrosis [177]. Similarly, Wang et al. reported that extracellular lactate from myofibroblasts increases overall Kla and H3K18la levels through MCT1. H3K18la facilitates the progression of arsenic-related idiopathic pulmonary fibrosis via the YTHDF1/m6A/NREP pathway [178].
Pulmonary arterial hypertension (PAH) is a severe condition caused by increased pulmonary vascular resistance and remodeling, ultimately leading to right heart failure or death. Lactate plays a critical role in PAH development by influencing disease progression through lactylation [179]. Chen et al. discovered that hypoxia-induced mitochondrial reactive oxygen species (mROS) inhibit the hydroxylation of HIF-1α, triggering a glycolytic shift in pulmonary arterial smooth muscle cells (PASMCs) via the upregulation of the HIF-1α/PDK1&2/p-PDH-E1α axis, resulting in lactate accumulation and histone Kla [179]. Enhanced Kla of HIF-1α targets, such as Bmp5, Trpc5, and Kit, promotes PASMC proliferation. PDK1&2 knockout reduced lactate production, histone Kla, and PASMC proliferation. Additionally, pharmacological inhibition of lactate dehydrogenase reduced histone Kla, improved PASMC proliferation, and alleviated vascular remodeling in hypoxia-induced PH rat [179]. These findings highlight the potential of targeting lactate as a therapeutic strategy against vascular remodeling.
Asthma, a chronic lung disease caused by airway inflammation and constriction, leads to difficulty in breathing [180]. Dexamethasone has been shown to exert anti-asthmatic effects by modulating the HIF-1α-glycolysis-lactate axis and protein Kla, offering a new therapeutic approach for eosinophilic asthma [180]. In summary, lactate and histone Kla play crucial roles in the pathogenesis of various respiratory diseases, providing new strategies for their treatment.
Circulatory system diseases
Cardiovascular diseases (CVDs), including myocardial infarction (MI), heart failure, atrial fibrillation, and atherosclerosis, pose a major global health threat [181]. MI remains one of the leading causes of death and disability worldwide [181]. Post-MI, lactate exacerbates heart dysfunction and fibrosis by inducing Snail1 Kla and activating the TGF-β/Smad 2 pathway, which drives endothelial-to-mesenchymal transition [181]. Moreover, timely activation of reparative signals in monocytes and macrophages is essential to mitigate early inflammatory damage and facilitate repair [181]. Wang et al. found that treating MI-affected mice with sodium lactate increased H3K18la in circulating monocytes and infiltrating macrophages, promoting angiogenesis and improving cardiac dysfunction [35]. The study further highlighted that metabolic reprogramming and MCT1-mediated lactate transport enhance histone Kla, with GCN5 serving as a lactylation writer to regulate gene transcription. These findings provide new insights into the metabolic-epigenomic-immune mechanisms after MI, offering theoretical foundations and potential applications for improving cardiac repair with significant clinical value [35].
Myocardial ischemia/reperfusion (I/R) injury significantly contributes to adverse outcomes post-MI [182]. Xu et al. reported that lactate affects cardiomyocyte size and apoptosis under OGD/R by elevating protein Kla levels and enhancing the intracellular YTHDF2, which subsequently upregulates Ras GTPase-activating protein-binding protein 1 (G3BP1) [182]. Targeting Kla or inhibiting YTHDF2/G3BP1 may help alleviate acute injury and pathological remodeling caused by myocardial I/R, offering a potential therapeutic approach for heart diseases [182]. Yao et al. found that increased LDHA activity raises lactate levels, promotes H3K18la, upregulates HMGB1 expression, induces apoptosis, and worsens cerebral I/R injury [183]. Du et al. demonstrated that HSPA12A protects the liver from I/R injury by reducing HMGB1 Kla and secretion through glycolysis inhibition, thereby suppressing macrophage chemotaxis and inflammation [184]. Interestingly, some studies suggest maintaining lactylation could mitigate MI/R injury. Yu et al. observed that HSPA12A stabilizes HIF1α protein via SMURF1, enhancing glycolytic gene expression, sustaining aerobic glycolysis, preserving H3 lactylation, and improving cardiomyocyte survival, thus reducing MI/R injury [185]. Further research is needed to clarify the role and mechanisms of lactylation in I/R-induced diseases.
Atherosclerosis (AS) is a leading cause of CVD. Research indicates that elevated Kla levels exacerbate AS progression. For example, Dong et al. found increased aerobic glycolysis and lactate levels in endothelial cells within atherosclerotic regions [186]. ASF1A-dependent p300-mediated H3K18la promotes AS by regulating endothelial-to-mesenchymal transition (EndMT). Pharmacological inhibition and advanced PROTAC suppression of glycolysis can reduce H3K18la, SNAI1 transcription, and EndMT-induced AS [186]. Vascular smooth muscle cell (VSMC) senescence, marked by metabolic abnormalities, is another critical factor in AS development [187]. Li et al. discovered that TRAP1-mediated metabolic reprogramming promotes H4K12la by downregulating HDAC3. H4K12la enriches senescence-associated secretory phenotype (SASP) gene promoters, activating their transcription, accelerating VSMC senescence, and driving AS [187]. Xu et al. showed that sustained inflammatory damage triggers TNF-α-induced Sox10 Kla via the PI3K/AKT pathway, leading to VSMC transdifferentiation. This results in macrophage-like VSMC accumulation, vascular inflammation, pyroptosis-driven hyperplasia, and AS-related complications [185]. Interestingly, some studies suggest enhancing lactylation may reduce AS risk. Zhang et al. found that H3K18la mediated by MCT4, associated with lactate efflux, activates repair genes and prevents AS. Inhibiting macrophage MCT4 promotes repair mechanisms [188]. Chen et al. demonstrated that exercise induces MeCP2 K271 lactylation in macrophages within aortic root plaques. MeCP2 K271la-H3K36me3/RUNX1 promotes pro-repair M2 macrophage polarization, reducing plaque size, necrotic core area, and lipid deposition while increasing collagen content and suppressing AS [189]. This highlights that interventions enhancing MeCP2 K271 lactylation could promote plaque stability by increasing pro-repair M2 macrophage infiltration, thereby lowering the risk of atherosclerotic CVD [189]. The study also identified RUNX1 as a potential therapeutic target in exercise therapy, offering insights for new target discovery. In summary, the dual role of lactate in AS progression warrants further investigation.
Medial arterial calcification, common in chronic kidney disease and diabetes. Ma et al. discovered NR4A3 is a key regulator in the progression of apolipoprotein A-IV-induced atherosclerosis. NR4A3-mediated histone Kla represents a novel metabolome-epigenome signaling cascade involved in the development of medial arterial calcification [190]. Huang et al. demonstrated that lumican mediates H3K14la and H3K9la, thereby promoting aortic valve calcification and suggesting that lumican could be a potential therapeutic target for calcific aortic valve disease (CAVD) [191]. Wang et al. found that andrographolide (AGP) reduces CAVD by regulating LDHA to disrupt lactate production and inhibit VIC calcification. Additionally, the study identified p300 acetyltransferase as the molecular target of AGP in suppressing H3Kla. The suppression of H3Kla and H3K9la by AGP was associated with decreased Runx2 expression [192].
Moreover, Zhang et al. found that α-myosin heavy chain (α-MHC) undergoes lactylation at lysine 1897 (K1897). When K1897 lactylation is absent, the interaction between α-MHC and titin is reduced, impairing cardiac structure and function [28]. The study identified p300 and Sirtuin 1 as the lactyltransferase and delactylase for α-MHC, respectively. It also showed that reducing lactate production, either chemically or genetically, lowers α-MHC Kla, weakens the α-MHC-titin interaction, and worsens heart failure. On the other hand, increasing lactate levels by administering sodium lactate or inhibiting key lactate transporters in cardiomyocytes enhances K1897 Kla and strengthens the α-MHC-titin interaction, improving heart failure outcomes [28]. In conclusion, lactate and its role in Kla are increasingly recognized in cardiovascular diseases. Targeting lactate production, modulating its transport, and regulating circulating lactate levels could offer promising therapeutic strategies for heart disease.
Neurological system diseases
Lactylation in the nervous system may hold broad biological significance, varying with specific cell types, brain regions, and physiological or pathological states [193]. For example, Hagihara demonstrated that stress-induced neuronal excitation and social defeat promote histone lactylation (especially H1Kla) in brain cells, enhancing c-Fos expression, which leads to reduced social behavior and increased anxiety [193]. Similarly, Fei et al. found that hypoxia amplifies p53 Kla in microglia, driving LPS-induced pro-inflammatory activation of BV2 cells via the NF-κB pathway, thereby exacerbating neurodegeneration [194]. He et al. reported that NCOA4 K450la enhances iron autophagy and glycolysis in hippocampal neurons, accelerating ischemic brain injury [195]. However, some studies offer contrasting perspectives. For instance, Wang et al. revealed that microglial Bach1 deficiency during brain development lowers lactate levels, reducing H4K12la enrichment at the Lrrc15 promoter. This activates the JAK/STAT3 pathway, regulates astrogliogenesis, and leads to anxiety-like behaviors, including impaired exploration and social deficit [196]. Wu et al. showed that hippocampal lactate injections upregulated PSD95, SYP, and GAP43 expression in hippocampal tissues and HT22 cells, enhancing spatial memory in mice through protein Kla [197]. Additionally, Han et al. demonstrated that exercise-induced histone H3 lactylation in microglia transforms pro-inflammatory microglia into anti-inflammatory/repair phenotypes, mitigating neuroinflammation and improving cognitive function—similar to the “lactate clock” in macrophages [198]. This indicates that exercise-induced lactate and lactylation facilitate microglial phenotype transformation, playing a key role in reducing neuroinflammation and enhancing cognitive performance [198]. Yan et al. further revealed that physical exercise enhances synaptic structure and neuronal activity in the medial prefrontal cortex by mediating Kla of the synaptic protein SNAP91, increasing resilience to chronic restraint stress. Notably, SNAP91 Kla was essential for preventing anxiety-like behaviors in CRS mice. These findings underscore a previously unrecognized non-histone Kla mechanism in regulating neural function, highlighting the brain's metabolic adaptations during exercise [199]. Overall, these studies illustrate the complex, multifaceted role of Kla in the nervous system, offering potential therapeutic targets for neurological disorders.
Alzheimer's disease (AD), the most common neurodegenerative disorder, is characterized by extracellular amyloid-β (Aβ) deposits, intracellular tau hyperphosphorylation leading to neurofibrillary tangles, and microglia-mediated neuroinflammation [200]. Pan et al. identified elevated histone Kla levels in both clinical brain samples and mouse models of AD, with H4K12la being the most prominent, indicating its potential as a therapeutic target [200]. This lactate-dependent histone modification was found at glycolytic gene promoters, activating transcription and worsening microglial dysfunction through a “glycolysis-H4K12la-PKM2” positive feedback loop [200]. Deleting PKM2 in microglia reduced Aβ levels in AD mouse models and improved learning and memory by lowering glycolytic rates and suppressing H4K12la expression, thus restoring microglial function. This suggests that disrupting this feedback loop could be a viable therapeutic approach [200]. Wei et al. observed elevated lactate levels in microglia and hippocampal tissues in aged mice and AD models (FAD4T and APP/PS1), which increased global histone Kla levels, especially H3K18la. Enhanced H3K18la activated the NFκB signaling pathway by binding to Rela and NFκB1 promoters, leading to upregulation of SASP components such as IL-6 and IL-8, further exacerbating brain aging and AD [201]. Wang et al. reported reduced levels of isocitrate dehydrogenase 3β (IDH3β), a key tricarboxylic acid cycle enzyme, in AD patient brains and transgenic mice. Knockdown of IDH3β caused oxidative phosphorylation uncoupling, reduced energy metabolism, and lactate accumulation. Overexpressing IDH3β raised lactate levels, promoting histone Kla and increasing paired box gene 6 (PAX6) expression [202]. However, PAX6, a repressor of IDH3β, further suppressed its expression, causing tau hyperphosphorylation, synaptic damage, and cognitive deficits. Breaking this cycle by upregulating IDH3β or downregulating PAX6 could potentially mitigate neurodegeneration and cognitive decline in AD.
Acute ischemic stroke (AIS) occurs when cerebral vessels are blocked, resulting in ischemia and brain tissue necrosis, which can lead to severe and permanent damage to the central nervous system [203]. Zhou et al. showed that LRP1 helps transfer healthy mitochondria from astrocytes to neurons by reducing lactate production in astrocytes and lowering the Kla of ARF1 [203]. Xiong et al. found that increased lactate from astrocytes worsens ischemic brain injury by promoting the formation of protein Kla. Inhibiting lactate production or blocking its transfer to neurons significantly reduces protein Kla levels in ischemic brains. Likewise, lowering protein Kla levels—either by using the antagonist a-485 to inhibit p300 and block protein Kla formation or by knocking out LDHA—can greatly reduce neuronal death and astrocyte activation in cerebral ischemia, extend the reperfusion window, and improve functional recovery from ischemic stroke [204]. However, it is important to note that while increased lactate during the ischemic phase may promote protein Kla formation, lactate treatment during the reperfusion phase does not affect brain protein Kla levels and has a neuroprotective effect [204]. Yao et al. reported that after cerebral ischemia-reperfusion injury, Kla levels of key Ca2+ signaling proteins, SLC25A4 and SLC25A5, increase in rat brain endothelial cells, while Kla levels of VDAC1 decrease, suggesting that Kla may mediate neuronal death through Ca2+ signaling pathways [205]. Moreover, Song et al. found that the traditional Chinese medicine Buyang Huanwu Decoction reduces pan-Kla and H3K18la protein levels and Apaf-1 transcriptional activity, decreasing glycolysis and apoptosis in rat brain microvascular endothelial cells, thus slowing AIS progression [206]. In conclusion, recent studies are increasingly focusing on the relationship between Kla and neurological diseases, seeking ways to improve outcomes for patients with central nervous system disorders through Kla modulation.
Skeletal system diseases
Lactylation plays a vital role in skeletal and muscular diseases. For example, Xia et al. observed increased H3K18la levels in osteoarthritis (OA) and identified LDHA-mediated H3K18la as regulating TPI1 gene transcription, contributing to OA progression [207]. Wu et al. reported lower lactate levels in osteoporosis patients, noting that elevated lactate levels from exercise can induce histone Kla in mesenchymal stem cells, alleviating osteoporosis [208]. Zhang et al. discovered decreased glutamine levels alongside increased lactate accumulation and Kla in severely degenerated nucleus pulposus tissue; glutamine supplementation was found to prevent disc degeneration by inhibiting glycolysis and reducing AMPKα Kla [209]. Lin et al. identified lactylation sites linked to cholesterol metabolism and fascia matrix synthesis in tendon samples, concluding that increased Kla levels may signal impaired cholesterol metabolism and tendon matrix degeneration [210]. Overall, modulating lactate levels and Kla presents promising therapeutic potential for skeletal diseases, warranting further investigation to identify effective treatment targets.
Digestive system diseases
Lactylation plays a critical role in digestive diseases such as ulcerative colitis (UC) and non-alcoholic fatty liver disease (NAFLD). Research indicates that inhibiting H3K18la can mitigate UC by reducing macrophage pyroptosis, restoring intestinal immunity, and enhancing the mucosal barrier [211]. The traditional Chinese medicine Ge Gen Qin Lian Tang modulates histone Kla, preventing M1 macrophage polarization and slowing UC progression [212]. These findings suggest that regulating lactylation could be a safe and effective therapeutic approach for UC. In NAFLD, mitochondrial pyruvate carrier 1 (MPC1) expression is associated with increased liver lipid deposition. MPC1 regulates lactate levels by influencing pyruvate metabolism, impacting the Kla of various proteins, such as fatty acid synthase, particularly at the K673 site, thereby exacerbating hepatic lipid metabolism in NAFLD [213]. Despite increased liver lactate levels following MPC1 gene knockout, liver inflammation does not occur [213]. Thus, Kla modulation presents promising therapeutic strategies for these conditions. However, broader clinical studies are necessary to confirm the efficacy and safety of Kla regulation.
Endocrine system diseases
Lactylation is closely linked to endocrine disorders. Diabetic nephropathy (DN), the most common complication of diabetes, is a leading cause of death among diabetic patients [214]. Studies highlight lactate accumulation as a biomarker for DN progression [214]. ACSF2 K182la disrupts mitochondrial function, leading to tubular damage in diabetes [215]. Additionally, aging also shifts renal tubular epithelial cells from oxidative phosphorylation to glycolysis, increasing renal lactate levels [216]. Elevated lactate enhances H3K14la in diabetic kidney disease (DKD), upregulating KLF5 expression, which suppresses CDH1 transcription and accelerates EMT [216]. Reducing lactate and Kla levels could significantly mitigate tubular fibrosis in DKD patients.
Chronic kidney disease (CKD) now affects over 10% of the global population, posing a serious public health concern [217]. Research shows that the glycolytic enzyme PFKFB3 drives abnormal histone Kla levels in renal tubular epithelial cells. Targeted PFKFB3 knockout reduces H4K12la, suppresses NF-κB pathway activation, and alleviates renal inflammation and fibrosis, providing promising strategies for CKD prevention and treatment [218].
Others
Lactylation is closely associated with retinal diseases. For instance, Huang et al. found that YY1 Kla regulates the transcription of inflammatory genes such as STAT3, CCL5, IRF1, IDO1, and SEMA4D, promoting microglial activation, migration, and proliferation, which contributes to microglial dysfunction in autoimmune uveitis [17]. This study identifies p300 as the “writer” of YY1 Kla, and suggesting that targeting the lactate/p300/YY1 Kla/inflammatory gene axis could have therapeutic potential [17]. Wang et al. further revealed that under hypoxic conditions, non-histone YY1 undergoes K183la in microglia, upregulating FGF2 expression and promoting retinal neovascularization. This highlights the lactate/p300/ YY1 Kla/FGF2 axis as a potential therapeutic target for proliferative retinal diseases [219].
In pregnancy-related diseases, Kla also plays a significant role. For example, Li et al. found that reduced uteroplacental blood flow in preeclampsia leads to placental hypoxia, which induces excessive lactate production in trophoblasts, triggering histone lactylation. This process regulates the expression of genes associated with placental fibrosis in preeclampsia, such as FN1 and SERPINE1, providing new insights into placental dysfunction mechanisms [220]. Similarly, Huang et al. observed significantly elevated lactate and histone lactylation levels in patients with gestational diabetes mellitus (GDM). Histone Kla modifications in the GDM group prominently affected promoter regions, with CACNA2D1 identified as a key gene. This gene influences GDM progression by promoting cell viability and proliferation [221].
Lactylation is also important in skin injury and disease progression. For instance, Liu et al. reported elevated histone Kla levels in hypertrophic scar tissues and fibroblasts. Histone Kla enhances the transcriptional activity of SLUG while inhibiting PTEN, suppressing autophagy, and promoting collagen deposition and cell viability, thus regulating hypertrophic scarring [222]. Zhao et al. found reduced levels of ADIPOQ and H3K18la in psoriasis skin tissues. Lactylation promotes the binding of ADIPOQ to the H3K18la promoter region, and the downregulation of H3K18la inhibits psoriasis progression by suppressing ADIPOQ [223].
Lactylation detection technology and its applications in diagnosis
Recent advancements in technologies like affinity enrichment, multidimensional separation, and biospectrometry have opened new avenues for Kla proteomics development. High-performance liquid chromatography has become a standardized separation technique in proteomics. The detection of cyclic immonium ions from lactyl lysine via MS is a reliable marker for identifying lactylated peptides, as verified through affinity enrichment lactyl proteome analysis and evaluation against nonlactylated spectral libraries [11]. This approach encompasses affinity enrichment, targeted lactylation proteomics, and large-scale bioinformatics assessments of nonlactylated protein databases. Using these methods, researchers like Zhang et al. and Wan et al. identified novel lactylations, revealing widespread lactylation in the human proteome [3, 11]. Besides MS, immunodetection methods are frequently employed. For instance, a ynoic functionalized chemical reporter molecule called YnLac has been developed for detecting protein lactylation in mammalian cells, specifically targeting nonhistone proteins [42]. However, the specificity and sensitivity of such fluorescent probes for large-scale detection require further refinement.
Artificial intelligence-based analysis is increasingly employed for lactylation prediction and study. Tools like FSL-Kla (http://kla.zbiolab.cn/) predict Kla sites computationally, aiding in systematic analysis and predicting various PTM sites [224]. Additionally, Lai et al. developed an automated machine learning model, Auto-Kla, which demonstrates stable and accurate performance in predicting PTM sites. This model is accessible via http://tubic.org/Kla and https://github.com/Tubic/Auto-Kla [225]. In summary, with ongoing technological improvements, more sensitive and high-throughput methods are expected to enhance lactylation detection and analysis accuracy.
Additionally, research also highlights lactate's multiple metabolic pathways, making it ideal for clinical metabolic imaging [226]. Hyperpolarized [1-13C] pyruvate magnetic resonance spectroscopy can noninvasively detect pyruvate metabolism in real-time, with the lactate signal or pyruvate/lactate ratio correlating to lactate production [226]. This method enables clinical mapping of lactate production and enzymatic transformation on subminute timescales, particularly under the Warburg effect [226]. Currently, hyperpolarized lactate signals, combined with metabolic indices like 18F-fluorodeoxyglucose-positron emission tomography, are utilized in clinical diagnoses and prognoses, providing insights into tumor burden, staging, and invasiveness [227]. Since 2013, hyperpolarized [1-13C] pyruvate probes have been FDA-approved for prostate cancer treatment [228]. Beyond oncology, these probes are used in preclinical studies of metabolically active noncancerous tissues such as the heart, liver, and central nervous system [226]. Preclinical evidence suggests their potential for organ transplant preservation research when coupled with magnetic resonance and normothermic perfusion [226].
In summary, lactylation offers promising therapeutic opportunities. Before disease onset, interventions to modulate lactylation through diet, exercise, or lifestyle changes could enable early prevention [1]. During disease progression, particularly in tumors, metabolic disorders, and inflammatory diseases, altered lactylation levels may serve as biomarkers for early diagnosis and monitoring. Personalized treatment plans, informed by lactylation variability, could improve therapeutic outcomes and minimize side effects [226]. Finally, identifying key lactylated proteins and enzymes may lead to targeted interventions through small molecules or biologics, paving the way for innovative disease treatments.
Challenges and Perspectives
Since its discovery in 2019, Kla has attracted significant attention for its potential role in regulating various physiological and pathological processes. The identification of Kla has expanded our understanding of glucose metabolism, providing new perspectives for exploring biological functions and developing therapeutic strategies [29]. Despite existing research, the study of Kla remains in its early stages, with several key areas still unexplored.
First, while Kla primarily originates from the intracellular metabolite lactate, it is unclear whether it is an inevitable consequence of lactate accumulation. The full range of lactylation substrates also remains challenging to identify. Second, although Kla refers to the covalent linkage of a lactyl group to lysine residues, it is still unknown whether Kla can occur on other amino acids, such as glycine, cysteine, or serine. Third, KL-La is a reversible process, influenced by specific enzymes. However, the key enzymes responsible for these processes are still debated, particularly due to the lack of data on substrate specificity and kinetic parameters. Fourth, the interactions between Kla and other PTMs, including competition, coordination, or selection mechanisms, remain unclear. Finally, while Kla is recognized as a critical regulatory factor in tumor development and immunotherapy, its epigenetic regulatory networks and molecular mechanisms in physiological and pathological processes require further investigation.
Research has also highlighted the challenge of maintaining lactylation levels within physiologically and pathologically relevant ranges [29]. An inducible orthogonal translation system using pyrrolysyl-tRNA synthetase offers a promising method for selectively increasing lactylation levels in a dose-dependent manner, with fewer off-target effects. However, this approach requires complex genetic manipulations and lacks precise control over lactylation levels, often resulting in artificial increases that may not reflect true pathophysiological conditions. Current assays used to study lactylation frequently involve experimental conditions that may not accurately represent in vivo environments. For instance, the concentrations of lactoyl-CoA used in some studies are much higher than those found in cells, potentially distorting results and overestimating lactylation's role in cellular processes. Lactate supplementation or LDHA inhibition affects other cellular processes, such as redox balance and immune regulation. Inhibiting upstream regulators of glycolysis could have even broader side effects, raising questions about the specificity and efficiency of lactylation regulation. Additionally, the lack of targeted tools for manipulating lactylation, such as enzymes that selectively affect lactylation without impacting other biological processes, limits the ability to study its functions independently. Thus, developing methods to control lactylation levels under pathophysiological conditions is crucial for gaining clearer insights into its role in cancer.
Currently, targeting lactate and lactylation metabolism is a promising therapeutic strategy (Table 4). However, clinical approaches targeting Kla, including potential combination therapies, still require validation through clinical trials. Future research should focus on elucidating the mechanisms of lactylation, systematically analyzing its regulatory networks, developing precise detection tools, and evaluating its safety and efficacy as a therapeutic target.
Lactate-Lactylation in Malignancy and treatment.
| Therapeutic targets and methods | Effects | Cancer | Object | Representative drugs or antibody | Ref/Trial No. |
|---|---|---|---|---|---|
| MCT1 | Lactate uptake | Raji B cell lymphoma | Human | AZD3965 | NCT01791595 |
| Triple negative breast cancer | Human | BAY8002 | PMID: 33333023 | ||
| Neuroblastoma, lymphoma, breast cancer | Human | SR13800 | PMID: 32123312 | ||
| Breast cancer, colorectal cancer | Cell lines, mouse | AR-C155858 | PMID: 24672058 | ||
| Pancreatic cancer | Cell lines, human tissue, mouse | 7ACC2 | PMID: 32138176 | ||
| Regulation of Treg cells | HCC | Cell lines, human tissue, mouse | anti-PD 1 | PMID: 35965549 | |
| PD-1 | Regulates the cytotoxicity of CD8 + T cells | NSCLC | Cell lines, mouse | anti-PD 1 | PMID: 39137401 |
| PD-1 & MCT1/4 | Through MCT1, Treg cells actively absorbed LA, which enhanced the expression of PD-1 and dampened the expression of PD-1 by effector T cells. | MYC-amplified tumors and liver tumors | Cell lines, mouse, human and human tissues | pembrolizumab or nivolumab | PMID: 35090594 |
| Regulates the expression of Mct4/Slc16A | Melanoma | Cell lines, mouse, human tissues | pembrolizumab and nivolumab | PMID: 32123312 | |
| Inhibiting MCT4 can enhance the activity of CD8+ T cells | Hepatocellular carcinoma | Cell lines, mouse, human and human tissues | Toripalimab, MCT4 inhibition | PMID: 38522774 | |
| Combination of MCT4 and ICB increased intratumoral pH, delayed tumor growth, and prolonged survival | Colorectal carcinoma | Cell lines, mouse; human blood | anti-PD L1 antibody, MCT4 inhibition | PMID: 37880183 | |
| Lactate excretion | Myeloma | Cell lines, human tissue, mouse | Syrosingopine | PMID: 36807305 | |
| PD-1 & LDHA | Oxamate enhances the therapeutic effect of pembrolizumab. | Non-small cell lung cancer | Mouse | Oxamate, anti-PD-1 pembrolizumab | PMID: 33859941 |
| PD-1 & LDHA | Deficiency of LDH-A increased infiltration of NK cells and CD8+ cytotoxic T cells, improving the efficacy of anti-PD-1 therapy. | Melanoma | Cell Lines; mouse | Deletion of LDHA; Anti-PD-1 antibody | PMID: 30934955 |
| CAR-T therapy | HDAC inhibition induced by lactate enhanced CD8+ T cell exhaustion efficiently inhibit tumor growth. | Melanoma and colon adenocarcinoma | Cell lines, mouse, Human blood | Glucose or sodium lactate; | PMID: 36068198 |
| CAR-T therapy | Lactate increase the immunogenicity of whole tumor cell vaccines that have been irradiated with UV light. | Lymphoma | Cell lines, mouse | Lactic acid; irradiation | PMID: 31734353 |
| Promotes immune activation of tumor-infiltrating CAR T cells. | Glioblastoma | Cell lines, mouse | Oxamate,LDH-A inhibitor | PMID: 34978120 | |
| LDHA | Lactate production | Prostate and lung adenocarcinomas | Cell lines, mouse, human tissue | Oxamate | PMID: 35075123 |
| Lactate uptake, restrain LDHA | liver cancer | Cell lines, mouse | Royal jelly acid | PMID: 37453194 | |
| PDHK | Lactate production | Metastatic breast cancer, lung cancer | Human | Dichloroacetate | NCT01029925, NCT01386632 |
| Hexokinase | Lactate production | Prostate cancer, lung cancer, breast cancer, etc. | Human | 2DG | NCT00096707, NCT00588185 |
| Breast cancer, liver cancer, bladder cancer | Cell line, mouse | 3-BrPA | PMID: 32330495 | ||
| Breast cancer | Cell line | Tristetraprolin | PMID: 30650008 | ||
| Lung cancer, breast cancer, melanoma, etc. | Cell line | Lonidamine | PMID: 30472500 | ||
| GPR81 | Lactate uptake | Acute lymphoblastic leukemia, prostate cancer, etc. | Human | Curcumin | NCT05045443, NCT04731844 |
| HDAC | Lactylation | Nasopharynx cancer | Cell lines | ITSA-1 | PMID: 23946197 |
| HAT | Breast cancer, colon cancer, lung cancer, etc. | Cell lines | Garcinol | PMID: 34785033 | |
| p300/CBP | Neovascularization, pituitary adenoma, melanoma, etc. | Cell lines | A-485 | PMID: 30387898 | |
| GCN5 | lung cancer | Cell lines, human tissue | CPTH6 | PMID: 36621008 | |
| Mitochondria | Regulation of histone Kla and therapeutic cancer cell plasticity | Neuroendocrine prostate cancer | Cell lines, mouse, human tissue | Unmentioned | PMID: 36869843 |
| Induces mitochondrial apoptosis and regulates the levels of Kla and 2-hydroxyisobutyllation | Breast cancer | Cell lines, mouse | Catalpol | PMID: 36067808 | |
| Combined treatment | Reduces Kla levels | Endometrial carcinoma | Cell lines, mouse, human tissue | 2-DG and sodium acetate | PMID: 39320546 |
| Reduces lactate production | Prostatic carcinoma | Cell lines, mouse | Copanlisib and ADT | PMID: 36862086 | |
| Enhances the efficacy of bevacizumab in colorectal cancer | Colorectal cancer | Cell lines, mouse, human tissue | Kla and autophagy inhibitors | PMID: 37615625 | |
| Immunization therapy | Affects the immunosuppressive activity of regulatory T cells and the antitumor activity of CD8 + T cells | Colorectal cancer | Cell lines, mouse | Vitamin A acid | PMID: 27590114 |
| Reduces resistance to immunotherapy | Non-small cell lung cancer | Cell lines, mouse | anti-apoc2-k70-lac | PMID: 38981044 |
Conclusion
Lactylation, an important form of PTMs, has emerged in recent years as a key player in health and disease. Although research on its physiological and pathological roles is still in its early stages, evidence highlights its significance in pathology. The regulation of Kla is expected to become a pivotal focus in developing new therapeutic strategies for major diseases, such as cancer. Identifying specific lactylation targets and integrating them with high-throughput detection technologies could facilitate early diagnosis and disease classification. Targeting lactylation-related enzymes, such as lactoyltransferases and delactylases, or modulating lactate metabolism, could enable more precise drug interventions. Clinically, therapies targeting lactylation could offer new options for cancer treatment, improving both efficacy and quality of life. Drug delivery systems with lactose glycosylation-specific structures could boost drug accumulation at tumor sites, increasing treatment efficiency. Combining immunotherapy with metabolic regulation strategies may further establish lactylation as a key component of combination therapies. In summary, lactylation research not only provides new insights into disease mechanisms but also holds significant potential for innovative therapeutic development. As the field evolves, lactylation could transition from research to clinical applications, paving the way for breakthroughs in health and disease management.
Abbreviations
PTM: Protein post-translational modification; Kla: Lactylation; Kac: Acetylation; Glis1: Phosphoglycerate kinase 1; Arg1: Arginase1; Brg1: SBP (S-ribonuclease binding protein) family protein; P300: E1A binding protein p300; CBP: CREB binding protein; MCT1: Monocarboxylate transporter-1; ROS: Reactive oxygen species; NSCLC: Non-small cell lung cancer; 2-DG: 2-Deoxy-D-glucose; TME: Tumor microenvironment; CRC: Colorectal cancer; LDHA: Lactate dehydrogenase A; PKM2: Pyruvate kinase M2; ALDOA: Aldolase, fructose-bisphosphate A; AARS1/AARS2: Alanyl-tRNA synthetase 1/ Alanyl-tRNA synthetase 2; ccRCC: Clear cell renal cell carcinoma; CBX3: Chromobox protein homolog 3; CAFs: Cancer-associated fibroblasts; SAKI: sepsis-associated acute kidney injury; SLE: Systemic lupus erythematosus; CVDs: Cardiovascular diseases; MI/R: Myocardial ischemia/reperfusion; AS: Atherosclerosis; EndMT: Endothelial-to-mesenchymal transition; AD: Alzheimer's disease; AIS: Acute ischemic stroke; DN: Diabetic nephropathy; GDM: Gestational diabetes mellitus.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82472702,82173046), Shaanxi Province Funds for Distinguished Young Youths (2023-JC-JQ-66), Shaanxi Province key industrial innovation chain project (2024SF ZDCYL-04-04). Some of the materials in Figure 5 and graphical abstract were created with BioRender (https://BioRender.com) and publish with permission.
Author contributions
Conceptualization: A.Y. and R.Z.; Writing and editing: L.Z., H.Q., H.L. and W.L.; Supervision: A.Y. and R.Z.; Figure drawing: L.Z., H.Q., H.L., W.L., A.Y. and R.Z. All authors have read and approved the article.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lv X, Lv Y, Dai X. Lactate, histone lactylation and cancer hallmarks. Expert Rev Mol Med. 2023;25:e7
2. Zhang Y, Song H, Li M, Lu P. Histone lactylation bridges metabolic reprogramming and epigenetic rewiring in driving carcinogenesis: Oncometabolite fuels oncogenic transcription. Clin Transl Med. 2024;14:e1614
3. Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y. et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575-80
4. Yang Z, Zheng Y, Gao Q. Lysine lactylation in the regulation of tumor biology. Trends Endocrinol Metab. 2024;35:720-31
5. Galle E, Wong CW, Ghosh A, Desgeorges T, Melrose K, Hinte LC. et al. H3K18 lactylation marks tissue-specific active enhancers. Genome Biol. 2022;23:207
6. Gong H, Zhong H, Cheng L, Li LP, Zhang DK. Post-translational protein lactylation modification in health and diseases: a double-edged sword. J Transl Med. 2024;22:41
7. Miao Z, Zhao X, Liu X. Hypoxia induced beta-catenin lactylation promotes the cell proliferation and stemness of colorectal cancer through the wnt signaling pathway. Exp Cell Res. 2023;422:113439
8. Sun L, Zhang Y, Yang B, Sun S, Zhang P, Luo Z. et al. Lactylation of METTL16 promotes cuproptosis via m6A-modification on FDX1 mRNA in gastric cancer. Nat Commun. 2023;14(1):6523
9. Gu J, Zhou J, Chen Q, Xu X, Gao J, Li X. et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-beta signaling in regulatory T cells. Cell Rep. 2022;39:110986
10. Wang J, Yang P, Yu T, Gao M, Liu D, Zhang J. et al. Lactylation of PKM2 Suppresses Inflammatory Metabolic Adaptation in Pro-inflammatory Macrophages. Int J Biol Sci. 2022;18:6210-25
11. Wan N, Wang N, Yu S, Zhang H, Tang S, Wang D. et al. Cyclic immonium ion of lactyllysine reveals widespread lactylation in the human proteome. Nat Methods. 2022;19:854-64
12. Zhang D, Gao J, Zhu Z, Mao Q, Xu Z, Singh PK. et al. Lysine L-lactylation is the dominant lactylation isomer induced by glycolysis. Nat Chem Biol. 2024 Online ahead of print
13. Gaffney DO, Jennings EQ, Anderson CC, Marentette JO, Shi T, Schou Oxvig AM. et al. Non-enzymatic Lysine Lactoylation of Glycolytic Enzymes. Cell Chem Biol. 2020;27:206-13 e6
14. Jing F, Zhang J, Zhang H, Li T. Unlocking the multifaceted molecular functions and diverse disease implications of lactylation. Biol Rev Camb Philos Soc. 2024 Online ahead of print
15. Hu X, Huang X, Yang Y, Sun Y, Zhao Y, Zhang Z. et al. Dux activates metabolism-lactylation-MET network during early iPSC reprogramming with Brg1 as the histone lactylation reader. Nucleic Acids Res. 2024;52(10):5529-5548
16. Nunez R, Sidlowski PFW, Steen EA, Wynia-Smith SL, Sprague DJ, Keyes RF. et al. The TRIM33 Bromodomain Recognizes Histone Lysine Lactylation. ACS Chem Biol. 2024 Online ahead of print
17. Huang J, Wang X, Li N, Fan W, Li X, Zhou Q. et al. YY1 Lactylation Aggravates Autoimmune Uveitis by Enhancing Microglial Functions via Inflammatory Genes. Adv Sci (Weinh). 2024;11(19):e2308031
18. Yang Y, Yan Y, Yin J, Tang N, Wang K, Huang L. et al. O-GlcNAcylation of YTHDF2 promotes HBV-related hepatocellular carcinoma progression in an N6-methyladenosine-dependent manner. Signal Transduct Target Ther. 2023;8(1):63
19. Chen J, Zhao D, Wang Y, Liu M, Zhang Y, Feng T. et al. Lactylated Apolipoprotein C-II Induces Immunotherapy Resistance by Promoting Extracellular Lipolysis. Adv Sci (Weinh). 2024;11(38):e2406333
20. Chen H, Li Y, Li H, Chen X, Fu H, Mao D. et al. NBS1 lactylation is required for efficient DNA repair and chemotherapy resistance. Nature. 2024;631:663-9
21. Mengshu Jia XY, Weixia Sun, Qianjun Zhou, Cheng Chang, Weihua Gong, Jian Feng. et al. ULK1-mediated metabolic reprogramming regulates Vps34 lipid kinase activity by its lactylation. Sci Adv. 2023;9(22):eadg4993
22. Xie B, Zhang M, Li J, Cui J, Zhang P, Liu F. et al. KAT8-catalyzed lactylation promotes eEF1A2-mediated protein synthesis and colorectal carcinogenesis. Proc Natl Acad Sci USA. 2024;121(8):e2314128121
23. Zhu R, Ye X, Lu X, Xiao L, Yuan M, Zhao H. et al. ACSS2 acts as a lactyl-CoA synthetase and couples KAT2A to function as a lactyltransferase for histone lactylation and tumor immune evasion. Cell Metab. 2024 S1550-4131(24)00411-X
24. Sun S, Xu Z, He L, Shen Y, Yan Y, Lv X. et al. Metabolic regulation of cytoskeleton functions by HDAC6-catalyzed alpha-tubulin lactylation. Nat Commun. 2024;15:8377
25. Moreno-Yruela C, Zhang D, Wei W, Baek M, Liu W, Gao J. et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci Adv. 2022;8(3):eabi6696
26. Zhang X-W, Li L, Liao M, Liu D, Rehman A, Liu Y. et al. Thermal Proteome Profiling Strategy Identifies CNPY3 as a Cellular Target of Gambogic Acid for Inducing Prostate Cancer Pyroptosis. J Med Chem. 2024;67(12):10005-10011
27. Jin J, Bai L, Wang D, Ding W, Cao Z, Yan P. et al. SIRT3-dependent delactylation of cyclin E2 prevents hepatocellular carcinoma growth. EMBO Rep. 2023;24(5):e56052
28. Naijin Zhang YZ, Jiaqi Xu, Pengbo Wang, Boquan Wu, Saien Lu, Xinxin Lu. et al. α-myosin heavy chain lactylation maintains sarcomeric structure and function and alleviates the development of heart failure. Cell Res. 2023;33(9):679-698
29. Li H, Sun L, Gao P, Hu H. Lactylation in cancer: Current understanding and challenges. Cancer Cell. 2024;42:1803-7
30. Varner EL, Trefely S, Bartee D, von Krusenstiern E, Izzo L, Bekeova C. et al. Quantification of lactoyl-CoA (lactyl-CoA) by liquid chromatography mass spectrometry in mammalian cells and tissues. Open Biol. 2020;10(9):200187
31. Li H, Liu C, Li R, Zhou L, Ran Y, Yang Q. et al. AARS1 and AARS2 sense L-lactate to regulate cGAS as global lysine lactyltransferases. Nature. 2024;634:1229-37
32. Ju J, Zhang H, Lin M, Yan Z, An L, Cao Z. et al. The alanyl-tRNA synthetase AARS1 moonlights as a lactyltransferase to promote YAP signaling in gastric cancer. J Clin Invest. 2024;134(10):e174587
33. Mao Y, Zhang J, Zhou Q, He X, Zheng Z, Wei Y. et al. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res. 2024;34:13-30
34. Zong Z, Xie F, Wang S, Wu X, Zhang Z, Yang B. et al. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell. 2024;187:2375-92 e33
35. Wang N, Wang W, Wang X, Mang G, Chen J, Yan X. et al. Histone Lactylation Boosts Reparative Gene Activation Post-Myocardial Infarction. Circ Res. 2022;131:893-908
36. Dong H, Zhang J, Zhang H, Han Y, Lu C, Chen C. et al. YiaC and CobB regulate lysine lactylation in Escherichia coli. Nat Commun. 2022;13(1):6628
37. Niu Z, Chen C, Wang S, Lu C, Wu Z, Wang A. et al. HBO1 catalyzes lysine lactylation and mediates histone H3K9la to regulate gene transcription. Nat Commun. 2024;15(1):3561
38. Li L, Chen K, Wang T, Wu Y, Xing G, Chen M. et al. Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat Metab. 2020;2:882-92
39. Yang K, Fan M, Wang X, Xu J, Wang Y, Tu F. et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. 2022;29:133-46
40. Lu J, Fu S, Dai J, Hu J, Li S, Ji H. et al. Integrated metabolism and epigenetic modifications in the macrophages of mice in responses to cold stress. J Zhejiang Univ Sci B. 2022;23:461-80
41. Li X, Yang Y, Zhang B, Lin X, Fu X, An Y. et al. Lactate metabolism in human health and disease. Signal Transduct Target Ther. 2022;7(1):305
42. Sun Y, Chen Y, Peng T. A bioorthogonal chemical reporter for the detection and identification of protein lactylation. Chem Sci. 2022;13:6019-27
43. Maschari D, Saxena G, Law TD, Walsh E, Campbell MC, Consitt LA. Lactate-induced lactylation in skeletal muscle is associated with insulin resistance in humans. Front Physiol. 2022;13:951390
44. Xu X, Zhang DD, Kong P, Gao YK, Huang XF, Song Y. et al. Sox10 escalates vascular inflammation by mediating vascular smooth muscle cell transdifferentiation and pyroptosis in neointimal hyperplasia. Cell Rep. 2023;42:112869
45. Wang Y, Chen L, Zhang M, Li X, Yang X, Huang T. et al. Exercise-induced endothelial Mecp2 lactylation suppresses atherosclerosis via the Ereg/MAPK signalling pathway. Atherosclerosis. 2023;375:45-58
46. Ma W, Ao S, Zhou J, Li J, Liang X, Yang X. et al. Methylsulfonylmethane protects against lethal dose MRSA-induced sepsis through promoting M2 macrophage polarization. Mol Immunol. 2022;146:69-77
47. Xiong J, He J, Zhu J, Pan J, Liao W, Ye H. et al. Lactylation-driven METTL3-mediated RNA m6A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol Cell. 2022;82:1660-77.e10
48. Xie Y, Hu H, Liu M, Zhou T, Cheng X, Huang W. et al. The role and mechanism of histone lactylation in health and diseases. Front Genet. 2022;13:949252
49. Liu J, Du J, Li Y, Wang F, Song D, Lin J. et al. Catalpol induces apoptosis in breast cancer in vitro and in vivo: Involvement of mitochondria apoptosis pathway and post-translational modifications. Toxicol Appl Pharmacol. 2022;454:116215
50. Irizarry-Caro RA, McDaniel MM, Overcast GR, Jain VG, Troutman TD, Pasare C. TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc Natl Acad Sci U S A. 2020;117:30628-38
51. Ma W, Ao S, Zhou J, Li J, Liang X, Yang X. et al. Methylsulfonylmethane protects against lethal dose MRSA-induced sepsis through promoting M2 macrophage polarization. Mol Immunol. 2022;146:69-77
52. Dong Q, Zhang Q, Yang X, Nai S, Du X, Chen L. Glycolysis-Stimulated Esrrb Lactylation Promotes the Self-Renewal and Extraembryonic Endoderm Stem Cell Differentiation of Embryonic Stem Cells. Int J Mol Sci. 2024;25(5):2692
53. Li L, Chen K, Wang T, Wu Y, Xing G, Chen M. et al. Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat Metab. 2020;2:882-92
54. Zhou Y, Yan J, Huang H, Liu L, Ren L, Hu J. et al. The m(6)A reader IGF2BP2 regulates glycolytic metabolism and mediates histone lactylation to enhance hepatic stellate cell activation and liver fibrosis. Cell Death Dis. 2024;15(3):189
55. Rho H, Terry AR, Chronis C, Hay N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell Metab. 2023;35:1406-23.e8
56. Yang Q, Liu J, Wang Y, Zhao W, Wang W, Cui J. et al. A proteomic atlas of ligand-receptor interactions at the ovine maternal-fetal interface reveals the role of histone lactylation in uterine remodeling. J Biol Chem. 2022;298(1):101456
57. Yang W, Wang P, Cao P, Wang S, Yang Y, Su H. et al. Hypoxic in vitro culture reduces histone lactylation and impairs pre-implantation embryonic development in mice. Epigenetics & Chromatin. 2021;14(1):57
58. Tian Q, Zhou L-q. Lactate Activates Germline and Cleavage Embryo Genes in Mouse Embryonic Stem Cells. Cells. 2022;11(3):548
59. Zhao Y, Zhang M, Huang X, Liu J, Sun Y, Zhang F. et al. Lactate modulates zygotic genome activation through H3K18 lactylation rather than H3K27 acetylation. Cell Mol Life Sci. 2024;81:298
60. Yang W, Wang P, Cao P, Wang S, Yang Y, Su H. et al. Hypoxic in vitro culture reduces histone lactylation and impairs pre-implantation embryonic development in mice. Epigenetics Chromatin. 2021;14:57
61. Merkuri F, Rothstein M, Simoes-Costa M. Histone lactylation couples cellular metabolism with developmental gene regulatory networks. Nat Commun. 2024;15:90
62. Nian F, Qian Y, Xu F, Yang M, Wang H, Zhang Z. LDHA promotes osteoblast differentiation through histone lactylation. Biochem Biophys Res Commun. 2022;615:31-5
63. Wu J, Hu M, Jiang H, Ma J, Xie C, Zhang Z. et al. Endothelial Cell-Derived Lactate Triggers Bone Mesenchymal Stem Cell Histone Lactylation to Attenuate Osteoporosis. Adv Sci (Weinh). 2023;10:e2301300
64. Hao B, Dong H, Xiong R, Song C, Xu C, Li N. et al. Identification of SLC2A1 as a predictive biomarker for survival and response to immunotherapy in lung squamous cell carcinoma. Comput Biol Med. 2024;171:108183
65. Huang W, Su J, Chen X, Li Y, Xing Z, Guo L. et al. High-Intensity Interval Training Induces Protein Lactylation in Different Tissues of Mice with Specificity and Time Dependence. Metabolites. 2023;13(5):647
66. Dai W, Wu G, Liu K, Chen Q, Tao J, Liu H. et al. Lactate promotes myogenesis via activating H3K9 lactylation-dependent up-regulation of Neu2 expression. J Cachexia Sarcopenia Muscle. 2023;14:2851-65
67. Zhou Y, Liu X, Huang C, Lin D. Lactate Activates AMPK Remodeling of the Cellular Metabolic Profile and Promotes the Proliferation and Differentiation of C2C12 Myoblasts. Int J Mol Sci. 2022;23(22):13996
68. Desgeorges T, Galle E, Zhang J, von Meyenn F, De Bock K. Histone lactylation in macrophages is predictive for gene expression changes during ischemia induced-muscle regeneration. Molecular Metabolism. 2024;83:101923
69. Sun Y, Chen Y, Peng T. A bioorthogonal chemical reporter for the detection and identification of protein lactylation. Chem Sci. 2022;13:6019-27
70. Chen Y, Wu J, Zhai L, Zhang T, Yin H, Gao H. et al. Metabolic regulation of homologous recombination repair by MRE11 lactylation. Cell. 2024;187:294-311 e21
71. Zou Y, Cao M, Tao L, Wu S, Zhou H, Zhang Y. et al. Lactate triggers KAT8-mediated LTBP1 lactylation at lysine 752 to promote skin rejuvenation by inducing collagen synthesis in fibroblasts. Int J Biol Macromol. 2024;277:134482
72. Hu X, Huang J, Li Z, Li J, Ouyang F, Chen Z. et al. Lactate promotes microglial scar formation and facilitates locomotor function recovery by enhancing histone H4 lysine 12 lactylation after spinal cord injury. J Neuroinflammation. 2024;21:193
73. Qiu CZ, Zhou R, Zhang HY, Zhang L, Yin ZJ, Ren DL. Histone lactylation-ROS loop contributes to light exposure-exacerbated neutrophil recruitment in zebrafish. Commun Biol. 2024;7:887
74. Gao X, Pang C, Fan Z, Wang Y, Duan Y, Zhan H. Regulation of newly identified lysine lactylation in cancer. Cancer Lett. 2024;587:216680
75. Zhang R, Li L, Yu J. Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells. Open Life Sci. 2024;19:20220874
76. Jiang J, Huang D, Jiang Y, Hou J, Tian M, Li J. et al. Lactate Modulates Cellular Metabolism Through Histone Lactylation-Mediated Gene Expression in Non-Small Cell Lung Cancer. Front Oncol. 2021;11:647559
77. Zhang C, Zhou L, Zhang M, Du Y, Li C, Ren H. et al. H3K18 Lactylation Potentiates Immune Escape of Non-Small Cell Lung Cancer. Cancer Res. 2024;84(21):3589-3601
78. Yan F, Teng Y, Li X, Zhong Y, Li C, Yan F. et al. Hypoxia promotes non-small cell lung cancer cell stemness, migration, and invasion via promoting glycolysis by lactylation of SOX9. Cancer Biol Ther. 2024;25:2304161
79. Zhou C, Li W, Liang Z, Wu X, Cheng S, Peng J. et al. Mutant KRAS-activated circATXN7 fosters tumor immunoescape by sensitizing tumor-specific T cells to activation-induced cell death. Nat Commun. 2024;15(1):499
80. Zheng P, Mao Z, Luo M, Zhou L, Wang L, Liu H. et al. Comprehensive bioinformatics analysis of the solute carrier family and preliminary exploration of SLC25A29 in lung adenocarcinoma. Cancer Cell Int. 2023;23(1):222
81. Wang M, He T, Meng D, Lv W, Ye J, Cheng L. et al. BZW2 Modulates Lung Adenocarcinoma Progression through Glycolysis-Mediated IDH3G Lactylation Modification. J Proteome Res. 2023;22:3854-65
82. Liu M, Gu L, Zhang Y, Li Y, Zhang L, Xin Y. et al. LKB1 inhibits telomerase activity resulting in cellular senescence through histone lactylation in lung adenocarcinoma. Cancer Lett. 2024;595:217025
83. Wenzhe Duan WL, Shengkai Xia, Yang Zhou, Mengyi Tang, Mingxin Xu, Manqing Lin. et al. Warburg effect enhanced by AKR1B10 promotes acquired resistance to pemetrexed in lung cancer-derived brain metastasis. J Transl Med. 2023;21(1):547
84. Huang H, Chen K, Zhu Y, Hu Z, Wang Y, Chen J. et al. A multi-dimensional approach to unravel the intricacies of lactylation related signature for prognostic and therapeutic insight in colorectal cancer. J Transl Med. 2024;22(1):211
85. Chen B, Deng Y, Hong Y, Fan L, Zhai X, Hu H. et al. Metabolic Recoding of NSUN2-Mediated m(5)C Modification Promotes the Progression of Colorectal Cancer via the NSUN2/YBX1/m(5)C-ENO1 Positive Feedback Loop. Adv Sci (Weinh). 2024;11(28):e2309840
86. Li X-M, Yang Y, Jiang F-Q, Hu G, Wan S, Yan W-Y. et al. Histone lactylation inhibits RARγ expression in macrophages to promote colorectal tumorigenesis through activation of TRAF6-IL-6-STAT3 signaling. Cell Rep. 2024;43(2):113688
87. Li W, Zhou C, Yu L, Hou Z, Liu H, Kong L. et al. Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy. 2024;20:114-30
88. Sun X, He L, Liu H, Thorne RF, Zeng T, Liu L. et al. The diapause-like colorectal cancer cells induced by SMC4 attenuation are characterized by low proliferation and chemotherapy insensitivity. Cell Metab. 2023;35:1563-79.e8
89. Miao Z, Zhao X, Liu X. Hypoxia induced β-catenin lactylation promotes the cell proliferation and stemness of colorectal cancer through the wnt signaling pathway. Exp Cell Res. 2023;422(1):113439
90. Zhou J, Xu W, Wu Y, Wang M, Zhang N, Wang L. et al. GPR37 promotes colorectal cancer liver metastases by enhancing the glycolysis and histone lactylation via Hippo pathway. Oncogene. 2023;42:3319-30
91. Wang L, Li S, Luo H, Lu Q, Yu S. PCSK9 promotes the progression and metastasis of colon cancer cells through regulation of EMT and PI3K/AKT signaling in tumor cells and phenotypic polarization of macrophages. J Exp Clin Cancer Res. 2022;41(1):303
92. Gu J, Xu X, Li X, Yue L, Zhu X, Chen Q. et al. Tumor-resident microbiota contributes to colorectal cancer liver metastasis by lactylation and immune modulation. Oncogene. 2024;43(31):2389-2404
93. Yang Z, Yan C, Ma J, Peng P, Ren X, Cai S. et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat Metab. 2023;5:61-79
94. Cheng Z, Huang H, Li M, Liang X, Tan Y, Chen Y. Lactylation-Related Gene Signature Effectively Predicts Prognosis and Treatment Responsiveness in Hepatocellular Carcinoma. Pharmaceuticals (Basel). 2023;16(5):644
95. Zhao P, Qiao C, Wang J, Zhou Y, Zhang C. Histone lactylation facilitates hepatocellular carcinoma progression by upregulating endothelial cell-specific molecule 1 expression. Mol Carcinog. 2024;63(11):2078-2089
96. Liao J, Chen Z, Chang R, Yuan T, Li G, Zhu C. et al. CENPA functions as a transcriptional regulator to promote hepatocellular carcinoma progression via cooperating with YY1. Int J Biol Sci. 2023;19:5218-32
97. Yang Z, Yan C, Ma J, Peng P, Ren X, Cai S. et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat Metab. 2023;5:61-79
98. Qian J, Huang C, Wang M, Liu Y, Zhao Y, Li M. et al. Nuclear translocation of metabolic enzyme PKM2 participates in high glucose-promoted HCC metastasis by strengthening immunosuppressive environment. Redox Biol. 2024;71:103103
99. Gu J, Zhou J, Chen Q, Xu X, Gao J, Li X. et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-β signaling in regulatory T cells. Cell Rep. 2022;39(12):110986
100. Cai J, Song L, Zhang F, Wu S, Zhu G, Zhang P. et al. Targeting SRSF10 might inhibit M2 macrophage polarization and potentiate anti-PD-1 therapy in hepatocellular carcinoma. Cancer Commun (Lond). 2024;44:1231-60
101. Yao G, Yang Z. Glypican-3 knockdown inhibits the cell growth, stemness, and glycolysis development of hepatocellular carcinoma cells under hypoxic microenvironment through lactylation. Arch Physiol Biochem. 2024;130:546-554
102. Pan L, Feng F, Wu J, Fan S, Han J, Wang S. et al. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol Res. 2022;181:106270
103. Feng F, Wu J, Chi Q, Wang S, Liu W, Yang L. et al. Lactylome Analysis Unveils Lactylation-Dependent Mechanisms of Stemness Remodeling in the Liver Cancer Stem Cells. Adv Sci (Weinh). 2024;11:e2405975
104. Yang L, Niu K, Wang J, Shen W, Jiang R, Liu L. et al. Nucleolin lactylation contributes to intrahepatic cholangiocarcinoma pathogenesis via RNA splicing regulation of MADD. J Hepatol. 2024;81(4):651-666
105. Xu H, Li L, Wang S, Wang Z, Qu L, Wang C. et al. Royal jelly acid suppresses hepatocellular carcinoma tumorigenicity by inhibiting H3 histone lactylation at H3K9la and H3K14la sites. Phytomedicine. 2023;118:154940
106. Cui Z, Li Y, Lin Y, Zheng C, Luo L, Hu D. et al. Lactylproteome analysis indicates histone H4K12 lactylation as a novel biomarker in triple-negative breast cancer. Front Endocrinol (Lausanne). 2024;15:1328679
107. Li J, Chen Z, Jin M, Gu X, Wang Y, Huang G. et al. Histone H4K12 lactylation promotes malignancy progression in triple-negative breast cancer through SLFN5 downregulation. Cell Signal. 2024;124:111468
108. Hou X, Ouyang J, Tang L, Wu P, Deng X, Yan Q. et al. KCNK1 promotes proliferation and metastasis of breast cancer cells by activating lactate dehydrogenase A (LDHA) and up-regulating H3K18 lactylation. PLoS Biol. 2024;22(6):e3002666
109. Zong Z, Xie F, Wang S, Wu X, Zhang Z, Yang B. et al. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell. 2024;187(10):2375-2392.e33
110. Pandkar MR, Sinha S, Samaiya A, Shukla S. Oncometabolite lactate enhances breast cancer progression by orchestrating histone lactylation-dependent c-Myc expression. Transl Oncol. 2023;37:101758
111. Liu J, Du J, Li Y, Wang F, Song D, Lin J. et al. Catalpol induces apoptosis in breast cancer in vitro and in vivo: Involvement of mitochondria apoptosis pathway and post-translational modifications. Toxicol Appl Pharmacol. 2022;454:116215
112. Yang D, Yin J, Shan L, Yi X, Zhang W, Ding Y. Identification of lysine-lactylated substrates in gastric cancer cells. iScience. 2022;25:104630
113. Yang H, Zou X, Yang S, Zhang A, Li N, Ma Z. Identification of lactylation related model to predict prognostic, tumor infiltrating immunocytes and response of immunotherapy in gastric cancer. Front Immunol. 2023;14:1149989
114. Yang H, Yang S, He J, Li W, Zhang A, Li N. et al. Glucose transporter 3 (GLUT3) promotes lactylation modifications by regulating lactate dehydrogenase A (LDHA) in gastric cancer. Cancer Cell Int. 2023;23(1):303
115. Zhao Y, Jiang J, Zhou P, Deng K, Liu Z, Yang M. et al. H3K18 lactylation-mediated VCAM1 expression promotes gastric cancer progression and metastasis via AKT-mTOR-CXCL1 axis. Biochem Pharmacol. 2024;222:116120
116. Duan Y, Zhan H, Wang Q, Li B, Gao H, Liu D. et al. Integrated Lactylome Characterization Reveals the Molecular Dynamics of Protein Regulation in Gastrointestinal Cancers. Adv Sci (Weinh). 2024;11(35):e2400227
117. Sun L, Zhang Y, Yang B, Sun S, Zhang P, Luo Z. et al. Lactylation of METTL16 promotes cuproptosis via m(6)A-modification on FDX1 mRNA in gastric cancer. Nat Commun. 2023;14:6523
118. Li Z, Liang P, Chen Z, Chen Z, Jin T, He F. et al. CAF-secreted LOX promotes PD-L1 expression via histone Lactylation and regulates tumor EMT through TGFbeta/IGF1 signaling in gastric Cancer. Cell Signal. 2024;124:111462
119. Peng T, Sun F, Yang JC, Cai MH, Huai MX, Pan JX. et al. Novel lactylation-related signature to predict prognosis for pancreatic adenocarcinoma. World J Gastroenterol. 2024;30:2575-602
120. Huang H, Wang S, Xia H, Zhao X, Chen K, Jin G. et al. Lactate enhances NMNAT1 lactylation to sustain nuclear NAD+ salvage pathway and promote survival of pancreatic adenocarcinoma cells under glucose-deprived conditions. Cancer Lett. 2024;588:216806
121. Zhao R, Yi Y, Liu H, Xu J, Chen S, Wu D. et al. RHOF promotes Snail1 lactylation by enhancing PKM2-mediated glycolysis to induce pancreatic cancer cell endothelial-mesenchymal transition. Cancer Metab. 2024;12(1):32
122. Li F, Si W, Xia L, Yin D, Wei T, Tao M. et al. Positive feedback regulation between glycolysis and histone lactylation drives oncogenesis in pancreatic ductal adenocarcinoma. Mol Cancer. 2024;23(1):90
123. Chen M, Cen K, Song Y, Zhang X, Liou Y-C, Liu P. et al. NUSAP1-LDHA-Glycolysis-Lactate feedforward loop promotes Warburg effect and metastasis in pancreatic ductal adenocarcinoma. Cancer Lett. 2023;567:216285
124. Takata T, Nakamura A, Yasuda H, Miyake H, Sogame Y, Sawai Y. et al. Pathophysiological Implications of Protein Lactylation in Pancreatic Epithelial Tumors. Acta Histochem Cytochem. 2024;57:57-66
125. Qiao Z, Li Y, Li S, Liu S, Cheng Y. Hypoxia-induced SHMT2 protein lactylation facilitates glycolysis and stemness of esophageal cancer cells. Mol Cell Biochem. 2024;479(11):3063-3076
126. Li Q, Lin G, Zhang K, Liu X, Li Z, Bing X. et al. Hypoxia exposure induces lactylation of Axin1 protein to promote glycolysis of esophageal carcinoma cells. Biochem Pharmacol. 2024;226:116415
127. Zang Y, Wang A, Zhang J, Xia M, Jiang Z, Jia B. et al. Hypoxia promotes histone H3K9 lactylation to enhance LAMC2 transcription in esophageal squamous cell carcinoma. iScience. 2024;27:110188
128. Fu C, Jiang W, Wang C, Song SJ, Tao H, Zhang XG. et al. AP001885.4 promotes the proliferation of esophageal squamous cell carcinoma cells by histone lactylation- and NF-kappaB (p65)-dependent transcription activation and METTL3-mediated mRNA stability of c-myc. Anim Cells Syst (Seoul). 2024;28:536-50
129. Pan J, Zhang J, Lin J, Cai Y, Zhao Z. Constructing lactylation-related genes prognostic model to effectively predict the disease-free survival and treatment responsiveness in prostate cancer based on machine learning. Front Genet. 2024;15:1343140
130. Luo Y, Yang Z, Yu Y, Zhang P. HIF1α lactylation enhances KIAA1199 transcription to promote angiogenesis and vasculogenic mimicry in prostate cancer. Int J Biol Macromol. 2022;222:2225-43
131. Liu C, Armstrong CM, Ning S, Yang JC, Lou W, Lombard AP. et al. ARVib suppresses growth of advanced prostate cancer via inhibition of androgen receptor signaling. Oncogene. 2021;40:5379-92
132. Zhao J, Ning S, Lou W, Yang JC, Armstrong CM, Lombard AP. et al. Cross-Resistance Among Next-Generation Antiandrogen Drugs Through the AKR1C3/AR-V7 Axis in Advanced Prostate Cancer. Mol Cancer Ther. 2020;19:1708-18
133. Chen HJ, Yu MM, Huang JC, Lan FY, Liao HH, Xu ZH. et al. SLC4A4 is a novel driver of enzalutamide resistance in prostate cancer. Cancer Lett. 2024;597:217070
134. Kiranj Chaudagar HMH, Rimpi Khurana, Brian Labadie, Taghreed Hirz, Shenglin Mei. et al. Reversal of Lactate and PD-1-mediated Macrophage Immunosuppression Controls Growth of PTEN/p53-deficient Prostate Cancer. Clin Cancer Res. 2023;29(10):1952-1968
135. Kiranj Chaudagar HMH, Anne Kelley, Brian Labadie, Jordan Shafran, Srikrishnan Rameshbabu, Catherine Drovetsky. et al. Suppression of Tumor Cell Lactate-generating Signaling Pathways Eradicates Murine PTEN/p53-deficient Aggressive-variant Prostate Cancer via Macrophage Phagocytosis. Clin Cancer Res. 2023;29(23):4930-4940
136. Wang D, Du G, Chen X, Wang J, Liu K, Zhao H. et al. Zeb1-controlled metabolic plasticity enables remodeling of chromatin accessibility in the development of neuroendocrine prostate cancer. Cell Death Differ. 2024;31(6):779-791
137. He Y, Ji Z, Gong Y, Fan L, Xu P, Chen X. et al. Numb/Parkin-directed mitochondrial fitness governs cancer cell fate via metabolic regulation of histone lactylation. Cell Rep. 2023;42:112033
138. He C, Zhang J, Bai X, Lu C, Zhang K. Lysine lactylation-based insight to understanding the characterization of cervical cancer. Biochim Biophys Acta Mol Basis Dis. 2024;1870:167356
139. Huang C, Xue L, Lin X, Shen Y, Wang X. Histone Lactylation-Driven GPD2 Mediates M2 Macrophage Polarization to Promote Malignant Transformation of Cervical Cancer Progression. DNA Cell Biol. 2024;43:605-618
140. Meng Q, Zhang Y, Sun H, Yang X, Hao S, Liu B. et al. Human papillomavirus-16 E6 activates the pentose phosphate pathway to promote cervical cancer cell proliferation by inhibiting G6PD lactylation. Redox Biol. 2024;71:103108
141. Meng Q, Sun H, Zhang Y, Yang X, Hao S, Liu B. et al. Lactylation stabilizes DCBLD1 activating the pentose phosphate pathway to promote cervical cancer progression. J Exp Clin Cancer Res. 2024;43(1):36
142. Xie B, Lin J, Chen X, Zhou X, Zhang Y, Fan M. et al. CircXRN2 suppresses tumor progression driven by histone lactylation through activating the Hippo pathway in human bladder cancer. Mol Cancer. 2023;22:151
143. Li F, Zhang H, Huang Y, Li D, Zheng Z, Xie K. et al. Single-cell transcriptome analysis reveals the association between histone lactylation and cisplatin resistance in bladder cancer. Drug Resist Updat. 2024;73:101059
144. Kong W, He J, Zhou Q, Zhou X, Wei X, Yang Y. et al. Histone lactylation-related genes correlate with the molecular patterns and functions of cancer-associated fibroblasts and have significant clinical implications in clear cell renal cell carcinoma. Heliyon. 2024;10:e33554
145. Liu J, Chen P, Zhou J, Li H, Pan Z. Prognostic impact of lactylation-associated gene modifications in clear cell renal cell carcinoma: Insights into molecular landscape and therapeutic opportunities. Environ Toxicol. 2023;39:1360-73
146. Liu R, Zou Z, Chen L, Feng Y, Ye J, Deng Y. et al. FKBP10 promotes clear cell renal cell carcinoma progression and regulates sensitivity to the HIF2α blockade by facilitating LDHA phosphorylation. Cell Death Dis. 2024;15(1):64
147. Yang J, Luo L, Zhao C, Li X, Wang Z, Zeng Z. et al. A Positive Feedback Loop between Inactive VHL-Triggered Histone Lactylation and PDGFRβ Signaling Drives Clear Cell Renal Cell Carcinoma Progression. Int J Biol Sci. 2022;18:3470-83
148. Zhang M, Zhao Y, Liu X, Ruan X, Wang P, Liu L. et al. Pseudogene MAPK6P4-encoded functional peptide promotes glioblastoma vasculogenic mimicry development. Commun Biol. 2023;6(1):1059
149. Li L, Li Z, Meng X, Wang X, Song D, Liu Y. et al. Histone lactylation-derived LINC01127 promotes the self-renewal of glioblastoma stem cells via the cis-regulating the MAP4K4 to activate JNK pathway. Cancer Lett. 2023;579:216467
150. Li M, Sun P, Tu B, Deng G, Li D, He W. Hypoxia conduces the glioma progression by inducing M2 macrophage polarization via elevating TNFSF9 level in a histone-lactylation-dependent manner. Am J Physiol Cell Physiol. 2024;327(2):C487-C504
151. Li G, Wang D, Zhai Y, Pan C, Zhang J, Wang C. et al. Glycometabolic reprogramming-induced XRCC1 lactylation confers therapeutic resistance in ALDH1A3-overexpressing glioblastoma. Cell Metab. 2024;36:1696-710 e10
152. Wang S, Huang T, Wu Q, Yuan H, Wu X, Yuan F. et al. Lactate reprograms glioblastoma immunity through CBX3-regulated histone lactylation. J Clin Invest. 2024;134(22):e176851
153. Yue Q, Wang Z, Shen Y, Lan Y, Zhong X, Luo X. et al. Histone H3K9 Lactylation Confers Temozolomide Resistance in Glioblastoma via LUC7L2-Mediated MLH1 Intron Retention. Adv Sci (Weinh). 2024;11(19):e2309290
154. De Leo A, Ugolini A, Yu X, Scirocchi F, Scocozza D, Peixoto B. et al. Glucose-driven histone lactylation promotes the immunosuppressive activity of monocyte-derived macrophages in glioblastoma. Immunity. 2024;57:1105-23.e8
155. Yu J, Chai P, Xie M, Ge S, Ruan J, Fan X. et al. Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 2021;22:85
156. Gu X, Zhuang A, Yu J, Yang L, Ge S, Ruan J. et al. Histone lactylation-boosted ALKBH3 potentiates tumor progression and diminished promyelocytic leukemia protein nuclear condensates by m1A demethylation of SP100A. Nucleic Acids Res. 2024;52:2273-89
157. Song F, Hou C, Huang Y, Liang J, Cai H, Tian G. et al. Lactylome analyses suggest systematic lysine-lactylated substrates in oral squamous cell carcinoma under normoxia and hypoxia. Cell Signal. 2024;120:111228
158. Sun C, Zhang W, Liu H, Ding Y, Guo J, Xiong S. et al. Identification of a novel lactylation-related gene signature predicts the prognosis of multiple myeloma and experiment verification. Sci Rep. 2024;14:15142
159. Zhu M, Xiao Q, Cai X, Chen Z, Shi Q, Sun X. et al. Predicting lymphoma prognosis using machine learning-based genes associated with lactylation. Transl Oncol. 2024;49:102102
160. Yu L, Jing C, Zhuang S, Ji L, Jiang L. A novel lactylation-related gene signature for effectively distinguishing and predicting the prognosis of ovarian cancer. Transl Cancer Res. 2024;13:2497-508
161. Sun J, Feng Q, He Y, Wang M, Wu Y. Lactate activates CCL18 expression via H3K18 lactylation in macrophages to promote tumorigenesis of ovarian cancer. Acta Biochim Biophys Sin (Shanghai). 2024;56(9):1373-1386
162. Wei S, Zhang J, Zhao R, Shi R, An L, Yu Z. et al. Histone lactylation promotes malignant progression by facilitating USP39 expression to target PI3K/AKT/HIF-1α signal pathway in endometrial carcinoma. Cell Death Discov. 2024;10(1):121
163. Xiaoyang Wen JZ, Zihan Xu, Muzi Li, Xiaotong Dong, Yanbo Du. et al. Highly expressed lncRNA H19 in endometriosis promotes aerobic glycolysis and Histone lactylation. Reproduction. 2024;168(2):e240018
164. Huang ZW, Zhang XN, Zhang L, Liu LL, Zhang JW, Sun YX. et al. STAT5 promotes PD-L1 expression by facilitating histone lactylation to drive immunosuppression in acute myeloid leukemia. Signal Transduct Target Ther. 2023;8(1):391
165. Wang Z-H, Zhang P, Peng W-B, Ye L-L, Xiang X, Wei X-S. et al. Altered phenotypic and metabolic characteristics of FOXP3+CD3+CD56+ natural killer T (NKT)-like cells in human malignant pleural effusion. OncoImmunology. 2022;12(1):2160558
166. Wang X, Ying T, Yuan J. et al. BRAFV600E restructures cellular lactylation to promote anaplastic thyroid cancer proliferation. Endocr Relat Cancer. 2023;30:e220344
167. Chu X, Di C, Chang P, Li L, Feng Z, Xiao S. et al. Lactylated Histone H3K18 as a Potential Biomarker for the Diagnosis and Predicting the Severity of Septic Shock. Front Immunol. 2022;12:786666
168. An S, Yao Y, Hu H, Wu J, Li J, Li L. et al. PDHA1 hyperacetylation-mediated lactate overproduction promotes sepsis-induced acute kidney injury via Fis1 lactylation. Cell Death Dis. 2023;14(7):457
169. Qiao J, Tan Y, Liu H, Yang B, Zhang Q, Liu Q. et al. Histone H3K18 and Ezrin Lactylation Promote Renal Dysfunction in Sepsis-Associated Acute Kidney Injury. Adv Sci (Weinh). 2024;11(28):e2307216
170. Wu D, Spencer CB, Ortoga L, Zhang H, Miao C. Histone lactylation-regulated METTL3 promotes ferroptosis via m6A-modification on ACSL4 in sepsis-associated lung injury. Redox Biol. 2024;74:103194
171. Zhang J, Ji H, Liu M, Zheng M, Wen Z, Shen H. Mitochondrial DNA Programs Lactylation of cGAS to Induce IFN Responses in Patients with Systemic Lupus Erythematosus. J Immunol. 2024;213(6):795-807
172. Hair P, Goldman DW, Li J, Petri M, Krishna N, Cunnion K. Classical complement activation on human erythrocytes in subjects with systemic lupus erythematosus and a history of autoimmune hemolytic anemia. Lupus. 2020;29:1179-88
173. Caielli S, Cardenas J, de Jesus AA, Baisch J, Walters L, Blanck JP. et al. Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE. Cell. 2021;184:4464-79.e19
174. Sun Z, Gao Z, Xiang M, Feng Y, Wang J, Xu J. et al. Comprehensive analysis of lactate-related gene profiles and immune characteristics in lupus nephritis. Front Immunol. 2024;15:1329009
175. Fan W, Wang X, Zeng S, Li N, Wang G, Li R. et al. Global lactylome reveals lactylation-dependent mechanisms underlying T(H)17 differentiation in experimental autoimmune uveitis. Sci Adv. 2023;9(42):eadh4655
176. Wu J, Lv Y, Hao P, Zhang Z, Zheng Y, Chen E. et al. Immunological profile of lactylation-related genes in Crohn's disease: a comprehensive analysis based on bulk and single-cell RNA sequencing data. J Transl Med. 2024;22(1):300
177. Huachun Cui NX, Sami Banerjee, Jing Ge, Dingyuan Jiang, Tapan Dey. et al. Lung Myofibroblasts Promote Macrophage Profibrotic Activity through Lactate-induced Histone Lactylation. Am J Respir Cell Mol Biol. 2021;64(1):115-125
178. Wang P, Xie D, Xiao T, Cheng C, Wang D, Sun J. et al. H3K18 lactylation promotes the progression of arsenite-related idiopathic pulmonary fibrosis via YTHDF1/m6A/NREP. J Hazard Mater. 2024;461:132582
179. Jian Chen MZ, Yanjie Liu, Shihong Zhao, Yanxia Wang, Meng Wang, Wen Niu. et al. Histone lactylation driven by mROS-mediated glycolytic shift promotes hypoxic pulmonary hypertension. J Mol Cell Biol. 2023;14(12):mjac073
180. Chen N, Xie Q-M, Song S-M, Guo S-N, Fang Y, Fei G-H. et al. Dexamethasone protects against asthma via regulating Hif-1α-glycolysis-lactate axis and protein lactylation. Int Immunopharmacol. 2024;131:111791
181. Min Fan KY, Xiaohui Wang, Linjian Chen, P Spencer Gill, Tuanzhu Ha, Li Liu. et al. Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction. Sci Adv. 2023;9(5):eadc9465
182. Xu G-e, Yu P, Hu Y, Wan W, Shen K, Cui X. et al. Exercise training decreases lactylation and prevents myocardial ischemia-reperfusion injury by inhibiting YTHDF2. Basic Res Cardiol. 2024;119(4):651-671
183. Yao X, Li C. Lactate dehydrogenase A mediated histone lactylation induced the pyroptosis through targeting HMGB1. Metab Brain Dis. 2023;38:1543-53
184. Du S, Zhang X, Jia Y, Peng P, Kong Q, Jiang S. et al. Hepatocyte HSPA12A inhibits macrophage chemotaxis and activation to attenuate liver ischemia/reperfusion injury via suppressing glycolysis-mediated HMGB1 lactylation and secretion of hepatocytes. Theranostics. 2023;13:3856-71
185. Yu W, Kong Q, Jiang S, Li Y, Wang Z, Mao Q. et al. HSPA12A maintains aerobic glycolytic homeostasis and Histone3 lactylation in cardiomyocytes to attenuate myocardial ischemia/reperfusion injury. JCI Insight. 2024;9(7):e169125
186. Dong M, Zhang Y, Chen M, Tan Y, Min J, He X. et al. ASF1A-dependent P300-mediated histone H3 lysine 18 lactylation promotes atherosclerosis by regulating EndMT. Acta Pharm Sin B. 2024;14:3027-48
187. Li X, Chen M, Chen X, He X, Li X, Wei H. et al. TRAP1 drives smooth muscle cell senescence and promotes atherosclerosis via HDAC3-primed histone H4 lysine 12 lactylation. Eur Heart J. 2024;45(39):4219-4235
188. Yunjia Zhang HJ, Mengdie Dong, Jiao Min, Xian He, Yongkang Tan, Fuhao Liu. et al. Macrophage MCT4 inhibition activates reparative genes and protects from atherosclerosis by histone H3 lysine 18 lactylation. Cell Rep. 2024;43(5):114180
189. Chen L, Zhang M, Yang X, Wang Y, Huang T, Li X. et al. Methyl-CpG-binding 2 K271 lactylation-mediated M2 macrophage polarization inhibits atherosclerosis. Theranostics. 2024;14:4256-77
190. Ma W, Jia K, Cheng H, Xu H, Li Z, Zhang H. et al. Orphan Nuclear Receptor NR4A3 Promotes Vascular Calcification via Histone Lactylation. Circ Res. 2024;134:1427-47
191. Huang Y, Wang C, Zhou T, Xie F, Liu Z, Xu H. et al. Lumican promotes calcific aortic valve disease through H3 histone lactylation. Eur Heart J. 2024;45(37):3871-3885
192. Wang C, Wang S, Wang Z, Han J, Jiang N, Qu L. et al. Andrographolide regulates H3 histone lactylation by interfering with p300 to alleviate aortic valve calcification. Br J Pharmacol. 2024;181:1843-56
193. Hagihara H, Shoji H, Otabi H, Toyoda A, Katoh K, Namihira M. et al. Protein lactylation induced by neural excitation. Cell Rep. 2021;37:109820
194. Fei X, Chen L, Gao J, Jiang X, Sun W, Cheng X. et al. p53 lysine-lactylated modification contributes to lipopolysaccharide-induced proinflammatory activation in BV2 cell under hypoxic conditions. Neurochem Int. 2024;178:105794
195. He X, Wang Z, Ge Q, Sun S, Li R, Wang B. Lactylation of nuclear receptor coactivator 4 promotes ferritinophagy and glycolysis of neuronal cells after cerebral ischemic injury. Neuroreport. 2024;35(14):895-903
196. Wang Y, Wang W, Su L, Ji F, Zhang M, Xie Y. et al. BACH1 changes microglial metabolism and affects astrogenesis during mouse brain development. Dev Cell. 2024;59:108-24.e7
197. Wu Y, Hu H, Liu W, Zhao Y, Xie F, Sun Z. et al. Hippocampal Lactate-Infusion Enhances Spatial Memory Correlated with Monocarboxylate Transporter 2 and Lactylation. Brain Sci. 2024;14(4):327
198. Han H, Zhao Y, Du J, Wang S, Yang X, Li W. et al. Exercise improves cognitive dysfunction and neuroinflammation in mice through Histone H3 lactylation in microglia. Immun Ageing. 2023;20(1):63
199. Yan L, Wang Y, Hu H, Yang D, Wang W, Luo Z. et al. Physical exercise mediates cortical synaptic protein lactylation to improve stress resilience. Cell Metab. 2024;36(9):2104-2117.e4
200. Pan R-Y, He L, Zhang J, Liu X, Liao Y, Gao J. et al. Positive feedback regulation of microglial glucose metabolism by histone H4 lysine 12 lactylation in Alzheimer's disease. Cell Metab. 2022;34:634-48.e6
201. Wei L, Yang X, Wang J, Wang Z, Wang Q, Ding Y. et al. H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer's disease through the NFκB signaling pathway. J Neuroinflammation. 2023;20(1):208
202. Wang X, Liu Q, Yu H-t, Xie J-z, Zhao J-n, Fang Z-t. et al. A positive feedback inhibition of isocitrate dehydrogenase 3β on paired-box gene 6 promotes Alzheimer-like pathology. Signal Transduct Target Ther. 2024;9(1):105
203. Zhou J, Zhang L, Peng J, Zhang X, Zhang F, Wu Y. et al. Astrocytic LRP1 enables mitochondria transfer to neurons and mitigates brain ischemic stroke by suppressing ARF1 lactylation. Cell Metab. 2024;36(9):2054-2068.e14
204. Xiong XY, Pan XR, Luo XX, Wang YF, Zhang XX, Yang SH. et al. Astrocyte-derived lactate aggravates brain injury of ischemic stroke in mice by promoting the formation of protein lactylation. Theranostics. 2024;14:4297-317
205. Yao Y, Bade R, Li G, Zhang A, Zhao H, Fan L. et al. Global-Scale Profiling of Differential Expressed Lysine-Lactylated Proteins in the Cerebral Endothelium of Cerebral Ischemia-Reperfusion Injury Rats. Cell Mol Neurobiol. 2023;43:1989-2004
206. Song C, Fang X, Fang N, Hu F. Buyang Huanwu Decoction suppresses ischemic stroke by suppressing glycolysis and cell apoptosis in rat brain microvascular endothelial cells. Brain Res Bull. 2024;215:111032
207. Xia J, Qiao Z, Hao X, Zhang Y. LDHA-induced histone lactylation mediates the development of osteoarthritis through regulating the transcription activity of TPI1 gene. Autoimmunity. 2024;57:2384889
208. Wu J, Hu M, Jiang H, Ma J, Xie C, Zhang Z. et al. Endothelial Cell-Derived Lactate Triggers Bone Mesenchymal Stem Cell Histone Lactylation to Attenuate Osteoporosis. Adv Sci (Weinh). 2023;10(31):e2301300
209. Zhang Y, Huang Z, Han W, Wu J, Li S, Qin T. et al. Glutamine suppresses senescence and promotes autophagy through glycolysis inhibition-mediated AMPKα lactylation in intervertebral disc degeneration. Commun Biol. 2024;7(1):325
210. Lin Y, Chen M, Wang D, Yu Y, Chen R, Zhang M. et al. Multi-Proteomic Analysis Reveals the Effect of Protein Lactylation on Matrix and Cholesterol Metabolism in Tendinopathy. J Proteome Res. 2023;22:1712-22
211. Sun S, Xu X, Liang L, Wang X, Bai X, Zhu L. et al. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front Immunol. 2021;12:777665
212. Xu ZP, Shan SY, Cai EW, Wu YY. Gegen Qinlian decoction inhibited M1 macrophage polarization and ulcerative colitis progression through regulating histone lactylation. Tissue Cell. 2024;89:102468
213. Gao R, Li Y, Xu Z, Zhang F, Xu J, Hu Y. et al. Mitochondrial pyruvate carrier 1 regulates fatty acid synthase lactylation and mediates treatment of nonalcoholic fatty liver disease. Hepatology. 2023;78:1800-15
214. Darshi M, Kugathasan L, Maity S, Sridhar VS, Fernandez R, Limonte CP. et al. Glycolytic lactate in diabetic kidney disease. JCI Insight. 2024;9(11):e168825
215. Chen J, Feng Q, Qiao Y, Pan S, Liang L, Liu Y. et al. ACSF2 and lysine lactylation contribute to renal tubule injury in diabetes. Diabetologia. 2024;67(7):1429-1443
216. Zhang X, Chen J, Lin R, Huang Y, Wang Z, Xu S. et al. Lactate drives epithelial-mesenchymal transition in diabetic kidney disease via the H3K14la/KLF5 pathway. Redox Biol. 2024;75:103246
217. Chen TK, Hoenig MP, Nitsch D, Grams ME. Advances in the management of chronic kidney disease. BMJ. 2023;383:e074216
218. Wang Y, Li H, Jiang S, Fu D, Lu X, Lu M. et al. The glycolytic enzyme PFKFB3 drives kidney fibrosis through promoting histone lactylation-mediated NF-kappaB family activation. Kidney Int. 2024;106:226-40
219. Wang X, Fan W, Li N, Ma Y, Yao M, Wang G. et al. YY1 lactylation in microglia promotes angiogenesis through transcription activation-mediated upregulation of FGF2. Genome Biol. 2023;24(1):87
220. Li X, Yang N, Wu Y, Wang X, Sun J, Liu L. et al. Hypoxia regulates fibrosis-related genes via histone lactylation in the placentas of patients with preeclampsia. J Hypertens. 2022;40:1189-98
221. Xiaman Huang KY, Hanhui Nie, Ruiping Chen, Xiufang Wang, Yun Wang. et al. ChIP-seq and RNA-seq Reveal the Involvement of Histone Lactylation Modification in Gestational Diabetes Mellitus. J Proteome Res. 2024;23(6):1937-1947
222. Xiaosong Liu BW. Histone lactylation regulates autophagy of hyperplastic scar fibroblasts by inhibiting the transcriptional activity of phosphatase and tensin homologue. Wound Repair Regen. 2024;32(5):725-734
223. Zhao S, Wu T, Fu M, Zhang Z. Histone Lactylation Participates in Psoriasis Progression by Regulating the Adiponectin Expression. Clin Cosmet Investig Dermatol. 2024;17:219-227
224. Jiang P, Ning W, Shi Y, Liu C, Mo S, Zhou H. et al. FSL-Kla: A few-shot learning-based multi-feature hybrid system for lactylation site prediction. Comput Struct Biotechnol J. 2021;19:4497-509
225. Lai FL, Gao F. Auto-Kla: a novel web server to discriminate lysine lactylation sites using automated machine learning. Brief Bioinform. 2023;24(2):bbad070
226. Wang Z, Hao D, Zhao S, Zhang Z, Zeng Z, Wang X. Lactate and Lactylation: Clinical Applications of Routine Carbon Source and Novel Modification in Human Diseases. Mol Cell Proteomics. 2023;22:100641
227. Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH, Bankson JA, Brindle K, Cunningham CH. et al. Hyperpolarized (13)C MRI: Path to Clinical Translation in Oncology. Neoplasia. 2019;21:1-16
228. Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M. et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(1)(3)C]pyruvate. Sci Transl Med. 2013;5:198ra08
Author contact
![]() Corresponding authors: Angang Yang (agyangedu.cn) or Rui Zhang (ruizhangedu.cn).
Corresponding authors: Angang Yang (agyangedu.cn) or Rui Zhang (ruizhangedu.cn).
 Global reach, higher impact
Global reach, higher impact