13.3
Impact Factor
Theranostics 2025; 15(3):993-1016. doi:10.7150/thno.104872 This issue Cite
Review
Metformin-based nanomedicines for reprogramming tumor immune microenvironment
1. Department of Radiology, Huaxi MR Research Center (HMRRC), Institution of Radiology and Medical Imaging, Breast Center, Institute of Breast Health Medicine, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu 610041, China.
2. Functional and Molecular Imaging Key Laboratory of Sichuan Province, NHC Key Laboratory of Transplant Engineering and Immunology, Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu 610041, China.
3. Xiamen Key Lab of Psychoradiology and Neuromodulation, Department of Radiology, West China Xiamen Hospital of Sichuan University, Xiamen 361021, China.
Received 2024-10-9; Accepted 2024-11-15; Published 2025-1-1
Abstract
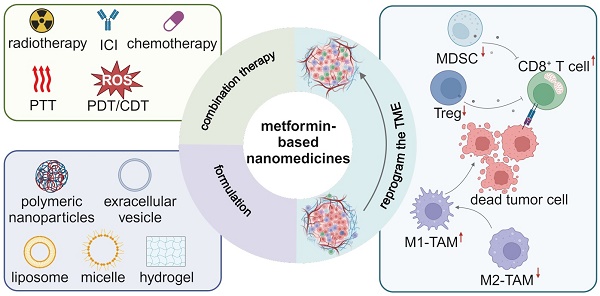
Immunotherapy has transformed current cancer management, and it has achieved significant progress over last decades. However, an immunosuppressive tumor microenvironment (TME) diminishes the effectiveness of immunotherapy by suppressing the activity of immune cells and facilitating tumor immune-evasion. Adenosine monophosphate-activated protein kinase (AMPK), a key modulator of cellular energy metabolism and homeostasis, has gained growing attention in anti-tumor immunity. Metformin is usually considered as a cornerstone in diabetes management, and its role in activating the AMPK pathway has also been extensively explored in cancer therapy although the findings on its role remain inconsistent. Metformin in a nanomedicine formulation has been found to hold potential in reprogramming the immunosuppressive TME through immunometabolic modulation of both tumor and immune cells. This review elaborates the foundation and progress of immunometabolic reprogramming of the TME via metformin-based nanomedicines, offering valuable insights for the next generation of cancer therapy.
Keywords: Metformin, AMPK, Tumor immunity, Tumor microenvironment, Nanomedicine
Introduction
Over the past decades, extensive studies have gradually unveiled the characteristics of the tumor microenvironment (TME), which is considered as an evolving entity composed of heterogeneous cell populations, abnormal vasculature, cytokines and extracellular matrix (ECM) [1]. There are various cell populations within the TME, including immune cells, tumor cells and stromal cells, and the specific proportion of each cell type varies in different cancer types [2]. The ECM is a non-cell component primarily secreted by cancer-associated fibroblasts (CAFs), and it serves as a reservoir for various immunosuppressive cytokines and growth factors [3]. Studies have indicated that the heterogeneous TME supports tumor progression and impedes immunotherapeutic action from multiple aspects. (I) A high interstitial pressure and a dense ECM within the TME are two physical barriers to block deep penetration of drugs and immune cells [4]; (II) Various suppressive cytokines, which are secreted by tumor cells and immunosuppressive cells such as tumor-associated macrophages (TAMs), regulatory T (Treg) cells and myeloid derived suppressor cells (MDSCs), facilitate tumor immunoevasion [5]; (III) Overexpressed immune checkpoint molecules suppress T cell function and proliferation [6]; (IV) Metabolic stress impairs anti-tumor immunity by depriving essential nutrients and allowing suppressive metabolite accumulation, leading to the exhaustion of cytotoxic T lymphocytes (CTLs) [7] and the proliferation of TAMs [8] and Treg cells [9]. It is noted that metabolic reprogramming of the TME has become as a novel approach to enhancing anti-tumor immunotherapeutic effects [10-15].
Metformin is commonly regarded as the primary medication for individuals with type 2 diabetes mellitus (T2DM) due to its proven ability to lower the blood sugar level as well as improve insulin sensitivity. In 1922, metformin was first synthesized, and its blood glucose-lowering function was confirmed in rabbits by 1929 [16]. Finally in 1994, metformin was approved by the Food and Drug Administration (FDA) and it is currently widely used in clinical practice to treat T2DM. The most acknowledged anti-diabetic mechanisms of metformin involve in reducing hepatic gluconeogenesis and increasing intestinal secretion of glucagon-like peptide 1 (GLP1) through activating the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway [17, 18]. The extensively studied phosphatidylinositol-3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) signaling pathways are critical regulatory hubs for cell metabolism, survival, and proliferation [19-21]. Thus, inhibitors of PI3K and rat sarcoma (RAS) are considered as highly specific medicines approved to treat patients with cancer [22, 23]. Interestingly, while the AMPK pathway is essential for maintaining cellular homeostasis, its tumor-suppressive mechanism remains unclear. Abundant clinical evidence suggests that diabetes occur frequently in patients with many kinds of cancer, indicating metformin as an AMPK pathway activator is a potential adjuvant in cancer management [24]. Emerging evidence shows that metformin-mediated AMPK activation is closely linked to its anti-tumor immune response. On the one hand, it directly suppresses tumor growth through inhibiting various anabolic processes, on the other hand, it reprograms the immunosuppressive TME by increasing the metabolic fitness of inflammatory immune cells [25, 26]. Hence, metformin holds promise as an adjuvant of cancer treatment, extending its benefits beyond diabetes management.
The poor pharmacokinetics and bioavailability of metformin hamper its clinical application in cancer therapy. Advances in nanotechnology promote the development of nanomedicines, which are a nano-formulation of therapeutic drugs including metformin. Nanomedicines have played an impactful role in the metabolic reprogramming approach [27]. Upon intravenous administration, vascular endothelial gaps and impaired lymphatic drainage allow nanomedicines to concentrate within tumor sites through the enhanced permeability and retention (EPR) effect [28]. Commonly overexpressed receptors on target cells can be utilized in preparing nanomedicines to target the cell type of interest via receptor-ligand interaction, thus achieving high-efficiency delivery of therapeutic drugs into tumor tissues and revitalizing anti-tumor immune response [29, 30]. Besides, multiple therapeutic drugs can be integrated into one nanomedicine to induce immunogenic cell death (ICD) via different treatment modalities. For instance, photothermal therapy (PTT) can damage tumor cells through local heat, while chemodynamic therapy (CDT) and photodynamic therapy (PDT) generate reactive oxygen species (ROS) to induce tumor cell apoptosis [31]. Impaired or dead tumor cells release damage-associated molecular patterns (DAMPs) including ATP, calreticulin (CRT) and high mobility group box 1 (HMGB1) to activate dendritic cells (DCs) and subsequent T cells for anti-tumor immunity initiation [32]. Chemotherapy and radiotherapy can also induce ICD to recruit lymphocytes to initiate the tumor-immunity cycle, creating an immunologically hot TME [33, 34]. Therefore, a myriad of therapeutic drugs for different treatment modalities have been incorporated into metformin-containing nanomedicines, which have shown remarkable effects on tumor regression.
In this review, we first introduce the basic structure and function of the AMPK complex, and then delve into the anti-tumor mechanisms of metformin by targeting the AMPK pathway in tumor cells, T cells and TAMs. We examine the efficacy of metformin in its current clinical use for cancer treatment, and demonstrate clinical benefits of metformin-containing nanomedicines in comparison with free metformin. Finally, we offer prospective insights into reprogramming the TME via metformin, which could help transition of metformin-containing nanomedicines to clinical use in cancer therapy. Given that metformin exerts anti-tumor effects predominately through inhibition of oxidative phosphorylation (OXPHOS) and AMPK-dependent manner, this review specifically focuses on the impact of above two mechanisms on immunometabolic modulation, excluding other AMPK-independent anti-tumor mechanisms of metformin such as increasing reactive oxygen (ROS) and inhibiting the oncogenic wingless-related integration site (Wnt) pathway [35].
Metformin reprograms the tumor microenvironment through activating the AMPK pathway
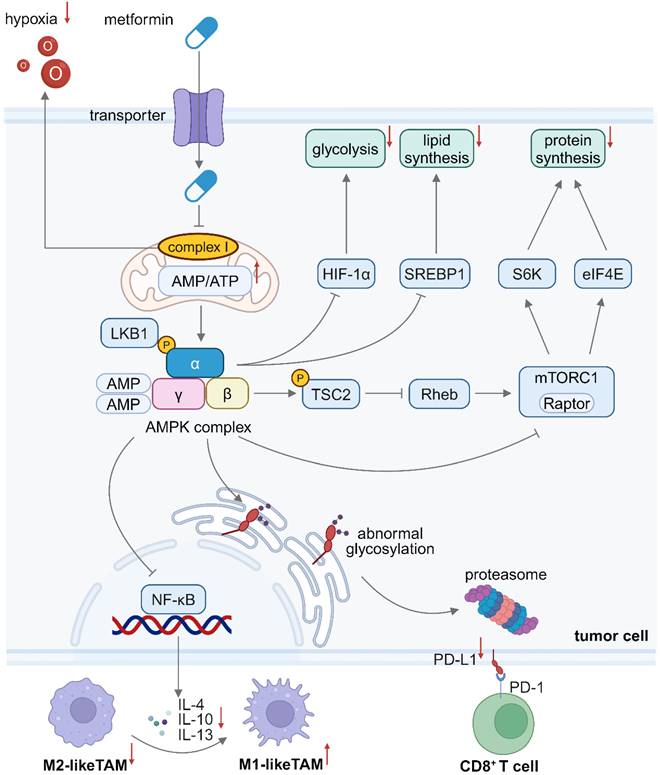
The AMPK trimer, a critical regulator of cellular energy metabolism, consists of one catalytic α subunit and two regulatory β and γ subunits, each with different isoforms. For instance, the α and β subunits have two subtypes, while the γ subunit has three [36]. The heterotrimeric structure of AMPK allows for coordinated and intricate energy-regulating function, and regulatory β and γ subunits play roles in maintaining AMPK complex stability and binding to AMP as an AMPK stimulator, respectively [37]. Metformin-mediated mitochondrial complex I inhibition reduces ATP production and induces energy stress, resulting in an elevation in the AMP level. After AMP binds to the γ subunit, upstream liver kinase B1 (LKB1) is activated to phosphorylate Thr172 on the α subunit, thereby activating the AMPK complex [38]. Additionally, an increased level of intracellular Ca2+ activates the AMPK pathway via calcium/calmodulin-dependent kinase kinase (CaMKK2), which is a non-canonical, LKB1-independent mechanism of AMPK activation [39].
The AMPK signaling pathway is a central regulator of energy metabolism in eukaryotes. AMPK activation typically inhibits the mTOR activity, thereby suppressing biosynthetic processes for proteins and lipids, while enhancing breakdown processes such as fatty acid oxidation (FAO) [40]. In addition, AMPK activation promotes autophagy and mitophagy via unc51-like kinase-1 (ULK1) phosphorylation to control the mitochondrial mass [41]. Mitochondrial biogenesis can also be promoted by AMPK activation via phosphorylating peroxisome proliferator activated receptor γ coactivator-1α (PGC-1α), a transcriptional coactivator, and an increase in the mitochondrial mass helps coping with intracellular energy stress [42].
Although metformin is well known as the first-line treatment drug for T2DM, recent clinical trial studies have demonstrated its potential in cancer therapy [43, 44]. Accumulating evidence suggests that metformin as an AMPK activator can boost anti-tumor immune response and inhibit tumor progression [45, 46]. For instance, the metformin-activated AMPK signaling pathway regulates the TME by reducing programmed cell death 1 ligand 1 (PD-L1) expression and inhibiting anabolic metabolism in tumor cells, repolarizing TAMs, and improving T cell metabolic fitness. In this section, we elaborate immunometabolic modulation in different cell types within the TME by metformin and reveal its tumor-suppressing effects as evidenced by recent clinical trial findings.
Metformin suppresses anabolic metabolism and PD-L1 expression in tumor cells
Tumor cells alter their metabolic profiles and rely on aerobic glycolysis more than OXPHOS even in the presence of sufficient oxygen, because aerobic glycolysis generates a great number of intermediate metabolites for biosynthesis of proteins, lipids and nucleotides, which is referred to the “Warburg effect” [44]. The PI3K- protein kinase B (AKT)-mammalian target of rapamycin (mTOR) axis is frequently hyperactivated during tumor progression, which can induce the overexpression of hexokinase 2 (HK2) and glucose transporter 1 (GLUT1), thereby sustaining a high glycolysis flux [47]. The PI3K and AMPK signaling pathways typically exert an opposing effect on tumor metabolism. Metformin-mediated AMPK activation can inhibit the mammalian target of rapamycin complex 1 (mTORC1) activity via phosphorylating Raptor as a scaffolding protein and tuberous sclerosis complex 2 (TSC2) as a negative regulator, thereby suppressing various anabolic processes in tumor cells [48]. Inhibition of mTORC1 downregulates the expression of its downstream targets, such as hypoxia inducible factor-1α (HIF-1α), ribosomal protein S6 kinase (S6K) and eukaryotic initiation factor 4E (eIF4E), thereby blocking glycolysis and preventing protein translation [49]. Apart from glucose and amino acid metabolism, lipid metabolism is crucial for cell growth [50]. Since fatty acids and cholesterol are vital for stabilizing cell membranes, fatty acid synthesis (FAS) and cholesterol production are often upregulated in tumor cells [51]. AMPK activation downregulates the expression of sterol regulatory element-binding protein 1 (SREBP1), a key transcriptional regulator for fatty acids and cholesterol metabolism [52]. Besides, acetyl-CoA carboxylase (ACC) is a key regulator for FAS, which facilitates the transformation of acetyl-CoA into malonyl-CoA for subsequent synthesis reactions. AMPK activation can phosphorylate ACC1 and ACC2, thus inhibiting their activity and lowering the FAS level [53]. It has been shown that AICAR as an AMPK activator could decrease the cholesterol level through downregulating the HMG-CoA reductase (HMGCR) pathway [54]. Therefore, AMPK activation can inhibit anabolic metabolism for synthesis of amino acids and lipids that are critical to tumor cell growth, suggesting metformin-mediated AMPK activation has the potential to reprogram tumor metabolism and enhance immunometabolic therapeutic effects.
In addition to disrupting tumor cell metabolism, metformin has been reported to promote proteasomal degradation of PD-L1. One of predominant immunosuppressive mechanisms in tumor cells is overexpression of immune checkpoint molecules. For instance, upregulated PD-L1 on tumor cells typically engages with programmed cell death 1 (PD-1) on effector T (Teff) cells, leading to a reduction in effector cytokine secretion and inducing apoptosis of Teff cells [55]. An array of immune checkpoint inhibitors (ICIs) has been developed to mitigate PD-L1/PD-1-mediated immunosuppression, but their therapeutic outcomes are suboptimal and their application is accompanied with severe side effects [56]. Interestingly, AMPK activation induces abnormal glycosylation of PD-L1, leading to its translocation from endoplasmic reticulum to cytoplasmic proteasomes, where mis-aligned proteins undergo degradation [57]. Therefore, metformin may hold great potential in disrupting the immunosuppressive signaling from PD-L1 and restoring tumoricidal ability of Teff cells.
Hypoxia is considered as a prevalent characteristic of solid cancers. Rapid tumor cell proliferation drives abnormal angiogenesis and the resulting disorganized vasculature prevents oxygen diffusion into distal blood vessels, resulting in a hypoxic TME that contributes to tumor progression [58]. Hypoxia facilitates tumor immune evasion by impairing both innate and adaptive anti-tumor immunity. Specifically, under hypoxia stress, tumor cells secrete interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) to drive M2-TAM polarization [59], and they upregulate the expression of CD39 and CD73 to generate adenosine as a toxic metabolite, which diminishes CD8+ T cell cytotoxicity [60]. Notably, hypoxia also decreases HIF-1α degradation and upregulates the PD-L1 expression in the TME, thus facilitating tumor immunoevasion [61]. Metformin could be applied to alleviate hypoxia in the TME via inhibiting complex I and mitochondrial respiration in tumor cells, thus mitigating hypoxia-induced immunosuppression [62]. An elevated oxygen concentration also enhances the efficacy of radiotherapy and PDT because both therapies are highly dependent on cytotoxic ROS generated from oxygen.
In conclusion, metformin contributes to creation of an immunogenic TME via several approaches. The metformin-activated AMPK signaling pathway reprograms metabolic processes of tumor cells, including protein and lipid biosynthesis via mTOR inhibition, thereby inhibiting tumor growth. Metformin also downregulates PD-L1 expression and ameliorates hypoxia within the TME, fostering a more immunogenic milieu against tumor immune evasion (Figure 1). Furthermore, an increase in the oxygen level in the TME enhances the efficacy of oxygen-dependent treatment modalities.
Metformin enhances mitochondrial biogenesis and memory differentiation of T cells
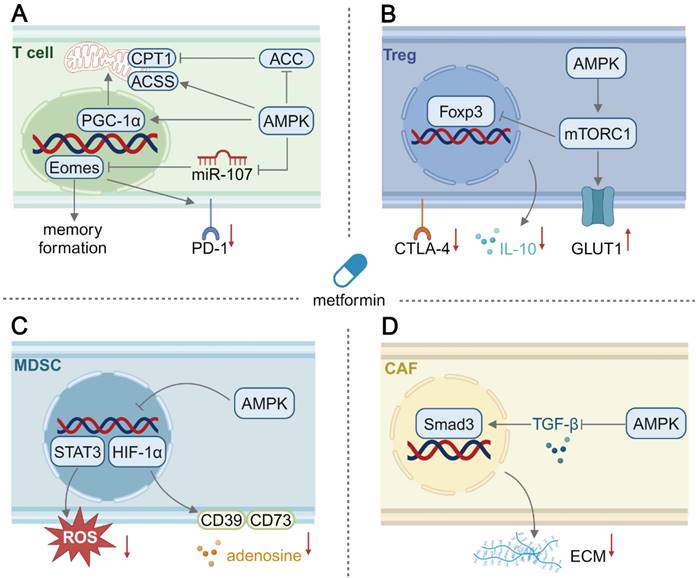
During tumor regression, T cells and their metabolic fitness are crucial for effective anti-tumor immunity. Importantly, the T cell metabolic profile changes dynamically during their development course. For example, naïve T cells exhibit a resting metabolic phenotype and they harness OXPHOS to produce ATP. Upon stimulation by tumor antigens and co-stimulatory molecules, glycolysis is significantly upregulated in naïve T cells and they become activated. Activated Teff cells are the major tumor-killing immune cells, and they exhibit an elevated level of glycolysis and OXPHOS [63]. After curbing tumor growth, the majority of Teff cells perish through apoptosis while a small subset survives and transforms into memory T (Tm) cells that respond to future encounters with tumor antigens for a long time [64]. Unfortunately, T cells can not compete with tumor cells for nutrients, resulting in an immunosuppressive TME. For example, glucose and glutamine deficiencies inhibit the activation of Teff cells and prevent interferon-γ (IFN-γ) production from them [7]. In addition, Tm cells predominately perform OXPHOS and fatty acid oxidation (FAO) to maintain self-renewal and cope with metabolic stress [56]. The AMPK signaling pathway serves as an energy sensor in glucose-starved Teff cells, and it helps restoring a quiescent metabolic profile and facilitates Tm cell generation. Therefore, promoting memory immunity of T cells through activating the AMPK pathway offers a promising strategy to enhance anti-tumor immune response.
The precise impact of AMPK activation on T cell immunity remains to be unveiled. It is known that metformin activates AMPK to downregulate glycolysis in tumor cells partially through inhibiting mTORC1 and HIF-1α, and it may impair glycolysis in Teff cells in a similar manner, weakening their anti-tumor immune response. Nevertheless, AMPK is crucial for Teff cells to mount a rapid secondary immune response, as their differentiation into Tm cells entails a metabolic transition of hyperactive glycolysis to quiescent OXPHOS [65]. Although glucose-derived acetyl-CoA is the primary fuel for the tricarboxylic acid (TCA) cycle, metformin-treated CAR-T cells upregulate acyl-coA synthetase short-chain family member 1 (ACSS1) to produce acetyl-CoA from acetate to fuel the TCA cycle [66], enhancing their proliferation and tumor-killing capability through effective OXPHOS and energy production [67].
Beyond OXPHOS, FAO is essential for Tm cell formation and tumor immunosurveillance. TNF receptor-associated factor 6 (TRAF6) promotes Tm cell differentiation by upregulating FAO. AMPK activation and memory formation are impaired in TRAF6-deficient T cells, while they can be restored after metformin interventions, suggesting that metformin promotes Tm cell generation via AMPK activation and FAO [68].
Schematic illustration of the mechanism of inhibiting tumor cell growth and reprogramming the TME by metformin. Metformin inhibits mitochondrial complex I, reducing oxygen consumption in tumor cells and alleviating hypoxia in the TME. Metformin-induced AMPK activation leads to mTORC1 inhibition in tumor cells, thus reducing glycolysis, protein synthesis and lipid synthesis. The AMPK pathway activation in tumor cells also inhibits the secretion of immunosuppressive cytokines, which facilitates M1-to-M2 polarization of TAMs. Besides, AMPK activation in tumor cells induces abnormal glycosylation and proteasomal degradation of PD-L1 proteins. Downregulated PD-L1 expression on tumor cells weakens the immunosuppressive signal from PD-L1/PD-1 engagement, thus enhancing CD8+ T cell cytotoxicity. Created in https://BioRender.com. AMPK: adenosine monophosphate-activated protein kinase; eIF4E: eukaryotic initiation factor 4E; HIF-1α: hypoxia inducible factor-1α; LKB1: liver kinase B1; mTORC1: mammalian target of rapamycin complex 1; NF-κB: nuclear factor kappa-B; PD-L1: programmed cell death 1 ligand 1; PD-1: programmed cell death 1; S6K: ribosomal protein S6 kinase; SREBP: sterol regulatory element-binding protein; TME: tumor microenvironment.
FAO and fatty acid synthesis (FAS) typically have opposite effects. Malonyl-CoA, an intermediate metabolite produced during FAS, can inhibit carnitine palmitoyl-transferase 1A (CPT1A) as a crucial enzyme involved in FAO [69]. AMPK activation induces inhibitory phosphorylation of Ser79 and Ser212 on two key FAS enzymes ACC1 and ACC2, respectively, thereby promoting FAO [70]. Interestingly, unlike Teff cells, Tm cells primarily synthesize endogenous fatty acids via glycolysis rather than engulfing exogenous fatty acids via CD36. The synthesized lipids are broken down in lysosomes to produce free fatty acids for FAO in the mitochondria. Although this is an inefficient cycle for lipid metabolism in Tm cells, primed fatty acids may stimulate Tm cells for rapid reactivation [71]. Moreover, metformin has been reported to promote lysosomal lipolysis and mitochondrial FAO, indicating it may play a role in supporting Tm cell development [72]. It is well established that Teff cell expansion primarily relies on glycolysis for ATP production, and their transition to quiescent Tm cells triggers a metabolic shift. However, the precise role of metformin in regulating Tm cell differentiation and enhancing anti-tumor immunity remains to be elucidated.
Since OXPHOS and FAO occur primarily in the mitochondria, the mitochondrial fitness of immune cells is crucial against tumor progression. Dysfunctional tumor-infiltrating cells (TILs) often exhibit a metabolic disorder due to an insufficient mitochondrial mass. The loss of mitochondrial function in TILs has been ascribed to chronic tumor antigen stimulation, which persistently activates the AKT signaling pathway and suppresses the forkhead box O (Foxo)-PGC1α axis, impairing PGC1α-mediated mitochondrial biogenesis [73]. AMPK activation can enhance the PGC1α activity to promote mitochondrial biogenesis and OXPHOS in T cells, thereby supporting long-term anti-tumor immunity [74]. Beyond mitochondrial dysfunction, PD-1 on T cells interacting with PD-L1 within the TME also induces T cell exhaustion. Downregulation of PD-1 on T cells offers an alternative strategy for restoring anti-tumor immunity. It has been found that AMPK activation downregulates miR-107, a negative regulator for a transcription factor eomesodermin (Eomes), which is crucial for Tm cell differentiation and PD-1 expression reduction [75]. Thus, metformin-mediated AMPK activation promotes Tm cell formation and downregulates PD-1 expression on their surfaces, contributing to a sustained tumor immunosurveillance.
Studies have shown that inhibiting AKT and glycolysis can enhance FAO in Tm cells and improve anti-tumor immune responses [76]. Metformin-mediated AMPK activation in T cells is considered as a promising approach to enhancing metabolic adaptation and promoting their memory immune response formation (Figure 2). Moreover, maintenance in mitochondrial fitness and mitigation in T cells exhaustion by metformin support long-term anti-tumor immunity within an immunosuppressive TME.
Schematic illustration of the mechanism of immunometabolic reprogramming of other cells in the TME by metformin. (A) In T cells, activation of the AMPK pathway promotes mitochondrial biogenesis and upregulates the FAO level. Besides, the AMPK pathway activation leads to memory differentiation and reduced PD-1 expression in T cells. (B) In Treg cells, AMPK activation surprisingly upregulates glycolysis, inhibiting inducible Treg differentiation and their CTLA-4 expression and IL-10 production. (C) In MDSCs, the production of suppressive adenosine and ROS secretion can be downregulated by metformin-mediated AMPK activation. (D) In CAFs, AMPK activation could suppress profibrotic signaling and reduce extracellular matrix deposition. Created in https://BioRender.com. ACC: acetyl-coA carboxylase; ACSS1: acyl-coA synthetase short-chain family member 1; CPT1: carnitine palmitoyltransferase 1; CTLA-4: cytotoxic T lymphocyte associate protein-4; ECM: extracellular matrix; Eomes: eomesodermin; Foxp3: forkhead box p3; GLUT1: glucose transporter 1; PGC-1α: peroxisome proliferator activated receptor γ coactivator-1α; STAT3: signal transducer and activator of transcription 3; TGF-β: transforming growth factor-β.
Metformin-induced repolarization of tumor-associated macrophages: shifting toward an anti-tumor phenotype
TAMs, innate immune cells within the TME, exhibit heterogeneous metabolic preferences and play distinct roles in tumor progression. M1-like TAMs primarily rely on glycolysis and exert anti-tumor effects by secreting inflammatory cytokines including IL-12 and nitric oxide (NO). In contrast, M2-like TAMs prefer OXPHOS and FAO, and they inhibit T cell tumoricidal activity and support tumor progression through releasing immunosuppressive cytokines including IL-10 and IL-4 [77].
In a recent clinical trial using metformin to treat esophageal squamous cell carcinoma, the infiltration of tumor-suppressing macrophages was evaluated, and the result suggested that metformin could induce the M2-to-M1 repolarization of TAMs [44]. Metformin treatment inhibited mitochondrial complex I and decreased the respiratory capacity, leading to adaptive upregulation of glycolysis to maintain energy production. Such a metabolic shift from OXPHOS to glycolysis triggered the repolarization of M2-like TAMs to M1-like, thus rewriting the immunosuppressive TME [78]. Therefore, metformin could be harnessed to repolarize TAMs and mitigate an immunosuppressive TME through triggering the metabolic shift from OXPHOS to glycolysis.
However, the influence of AMPK activation on macrophage polarization is complex and sometimes contradictory. For example, acadesine (AICAR), an AMPK activator, suppresses inflammatory response by inhibiting TNF-α secretion from M1-like macrophages [79], and the AMPK α1 subunit is critical in inducing the conversion of macrophages into an anti-inflammatory M2-like phenotype via IL-10 [80]. In diabetic cardiomyopathy, metformin is reported to induce the differentiation of M2-like macrophages by inhibition of mTOR and NLRP3 inflammasomes through AMPK activation [81], which indicates AMPK activation can alleviate excessive inflammation in autoimmune diseases. Interestingly, AMPK activation may play an anti-tumor role via repolarization of M2-like macrophages. Immunosuppressive IL-4, IL-10 and IL-13 can induce polarization of macrophages to a M2-like phenotype, while metformin-treated cancer cells exhibit a decrease in the secretion of these immunosuppressive cytokines by activating AMPK and downregulating nuclear factor kappa-B (NF-κB) p65 phosphorylation [82]. Additionally, activation of the AMPK α1 subunit in macrophages is shown to play an essential role in inhibiting M2 phenotype polarization induced by IL-13 [83]. In this context, direct effects of metformin treatment on macrophage polarization are still under debate. The role of AMPK activation in macrophage polarization may be highly dependent on interactions between cancer cells and macrophages under different pathophysiological conditions, and these interactions remain to be unveiled.
Metformin-mediated immunometabolic regulation in other cells
The effects of metformin may be exerted on other cell types within the TME (Figure 2), such as Treg cells, MDSCs [84] and CAFs [85]. Treg cell, a subset of CD4+ T cells, can prevent autoimmune conditions and promote tumor progression by suppressing CTLs. The metformin-activated AMPK signaling pathway has been reported to inhibit tumor cell growth via inhibiting mTOR [86], which could be applied to Treg cells. Indeed, AMPK activation has been reported to sustain Treg cell function via inhibiting mTOR and upregulating FAO in T1DM [87]. It was surprisingly found from one study that metformin treatment reduced the generation of tumor-infiltrating Treg cells in vitro through activating AMPK and subsequent mTOR signaling, and an elevated glycolysis/OXPHOS ratio was found to contribute to a decrease in the expression of the master transcription factor forkhead box protein P3 (Foxp3) [88]. AMPK activation to inhibit or activate mTOR in Treg cells should be investigated both in vitro and in vivo. MDSCs originate from hematopoietic stem cells, and their differentiation into mature myeloid cells is often blocked during tumor progression. Immature MDSCs contribute to an immunosuppressive TME via producing a variety of suppressive cytokines and metabolites [89]. Inhibiting the immunosuppressive activity of MDSCs represents an effective approach to reprogramming the TME. AMPK activation in MDSCs induced by metformin inhibits the signal transducer and activator of transcription 3 (STAT3) signaling pathway and its downstream ROS production, thus reducing their suppressive effect on CD4+ T cells [90]. In addition, metformin interventions result in AMPK phosphorylation to inhibit HIF-1α, thus reducing the expression of CD39 and CD73 on MDSCs and decreasing adenosine production, ultimately forming an improved immune-supportive TME [91]. Interestingly, in another study, the AMPK α1 subunit helped maintaining the immunosuppressive activity of MDSCs, while AMPK-deficient MDSCs exhibited tumoricidal activity by producing cytotoxic NO [92]. Similar to Treg cells, the role of metformin-mediated AMPK activation in modulating immune response of MDSCs is controversial, and the effects of AMPK activation may be distinguishable by investigating different subtypes of MDSCs. CAFs secrete major components of the dense ECM to form a physical barrier for infiltration of immune cells and therapeutics [93], thus a strategy could be developed to reduce ECM formation and improve an immunosuppressive TME. In stroma-rich pancreatic ductal adenocarcinoma, the dense stroma could be effectively disrupted by metformin because it activated the AMPK pathway and inhibited the secretion of profibrogenic TGF-β to reduce ECM protein secretion by stellate cells [94]. Consequently, after metformin treatment, the dense ECM became thinner to allow the penetration of a gemcitabine-loaded nanomedicine [95]. In conclusion, metformin could act as a potent cancer therapy adjuvant, but its effects on immunometabolic modulation in other cell types within the TME remain to be unveiled through thorough investigations.
Lessons learned from clinical trials
The use of metformin as an adjuvant in cancer treatment has been widely explored for various cancer types (Table 1). The most recognized anti-tumor mechanism of metformin is its activation of the AMPK signaling pathway, which in turn inhibits the typically upregulated PI3K/AKT/mTOR pathway involved in tumor progression [96]. Melanoma often displays encouraging immunogenicity, indicating its favorable response to ICI-based immunotherapy [97]. Disrupting the PD-1/PD-L1 pathway is conducive to inducing objective response in advanced melanoma, and the combination of metformin treatment and immunotherapy could be particularly promising [98, 99]. However, a randomized controlled phase III clinical trial found that metformin did not enhance the efficacy of pembrolizumab in resected melanoma, and recurrence-free survival (RFS) was not significantly extended in the pembrolizumab-treated group. Notably, although metformin is the first-line prescribed drug for T2DM, cancer patients with T2DM generally experienced the worse RFS in comparison with those without T2DM [100]. Fortunately, there was no evidence to show that metformin treatment worsened the prognosis in melanoma patients, suggesting metformin treatment of melanoma is quite safe but its clinical benefits may be limited [101]. A more recent study reported that metformin prolonged the overall survival (OS) rather than the cancer specific survival (CSS) in individuals with cutaneous melanoma. The positive effect was dose-dependent, indicating that metformin benefited melanoma patients through indirect comorbidity control rather than direct cancer prevention [102]. Colorectal cancer often begins with precancerous conditions such as hyperplastic polyps or adenomas, thus polypectomy could be used as an effective preventive measure [103]. In one trial, the use of metformin was evaluated in post-polypectomy patients without diabetes. Long-term administration of metformin (250 mg/day) inhibited neoplasia or polyp recurrence, suggesting mTOR inhibition mediated by metformin could suppress protein biosynthesis and proliferation of tumor cells [104]. Breast cancer ranks as the second most prevalent cancer affecting women globally, and its incidence continues to rise [105]. The expression level of estrogen and progesterone receptors is well correlated with breast cancer prognosis, while approximately 10-15% of breast cancer patients lack both hormone receptors and human epidermal growth factor receptor 2 (HER2), and this breast cancer is classified as triple-negative breast cancer. A significant number of breast cancer patients are resistant to immunotherapy, thus the combination of immunotherapy with other treatment modalities could be advantageous in treating breast cancer [106]. A phase III clinical trial result indicated that metformin failed to improve the disease-free survival (DFS) for invasive breast cancer patients without diabetes, suggesting metformin as an adjuvant agent may be insufficient to enhance anti-tumor immune response in breast cancer treatment [107].
Although metformin has been reported to lower cancer risks and improve patient outcomes, these results remain controversial and the anti-tumor effects of metformin may be diminished when confounding factors are accounted for [114]. One of the predominant factors is insufficient metformin accumulation in tumor sites. It is important to note that metformin has been reported to accumulate preferentially in the liver and gastrointestinal system rather than in tumor tissues, thus hepatic and intestinal uptake of orally administered metformin often results in poor systemic biodistribution and low bioavailability [115]. Interestingly, it has been recently reported that the plasma concentration of metformin in breast cancer patients was at a micromolar level, whereas an effective anti-tumor effect through inhibition of complex I by metformin requires a dose at a millimolar level [116]. It is also confirmed that high-dose metformin exhibits direct anti-tumor effect in vitro [117], but oral administration of metformin at a high dose may cause severe side effects, such as lactic acidosis [118]. These findings suggest that targeted delivery of metformin to tumor sites could resolve challenging issues associated with traditional oral administration.
Metformin as an adjuvant cancer therapy in clinical trials
| Stage | Treatment | Indication | NCT code |
|---|---|---|---|
| Early phase I | Atorvastatin + Metformin | Operable breast cancer | NCT01980823 |
| Phase I | Erlotinib + Metformin | Triple negative breast cancer | NCT01650506 |
| Phase I | Sapanisertib + Metformin | Advanced solid tumors | NCT03017833 [108] |
| Phase I | Temsirolimus + Metformin | Advanced solid tumors | NCT00659568 |
| Phase II | Levonorgestrel-releasing intrauterine device + Metformin | Endometrial cancer | NCT02035787 |
| Phase II | Capecitabine + Radiotherapy + Metformin | Locally advanced rectal cancer | NCT02437656 |
| Phase II | Abiraterone + Metformin | Metastatic prostate cancer | NCT01677897 [109] |
| Phase II | Letrozole + Metformin | ER (+) postmenopausal breast cancer | NCT01589367 [110] |
| Phase II | Docetaxel + Metformin | Castration-resistant prostate cancer | NCT01796028 [111] |
| Phase II | Letrozole + Metformin | Postmenopausal breast cancer | NCT05053841 [112] |
| Phase II | Standard chemotherapy + Metformin | Metastatic breast cancer. | NCT01310231 [113] |
| Phase II | Nivolumab + Metformin | Metastatic colon cancer | NCT03800602 [43] |
| Phase III | Leucovorin + Fluorouracil + Oxaliplatin + Metformin | Metastatic colon cancer | NCT05921942 |
The use of metformin has been explored in many preclinical and clinical cancer studies, while outcomes from these studies are controversial. Oral administration of metformin could be one of contributors for the controversial outcomes since there are challenging issues, such as unspecific biodistribution, poor tumor accumulation and non-selective targeting [119]. A prospective solution is to switch conventional oral administration of metformin to the use of metformin-containing nanomedicines. Nanomedicines could deliver metformin to targeted tissues or cells to realize spatiotemporal immunometabolic regulation at specific sites of interest, meanwhile, nanomedicines can improve bioavailability and pharmacokinetics of metformin. Its optimal doses can be reduced, in this way, its side effects can be minimized. Therefore, the challenges associated with traditional direct administration of metformin could be overcome via the nanomedicines [120]. The role of metformin in clinical cancer treatment could be demystified and its effectiveness in eradicating cancer could be enhanced via well-controlled nanomedicines.
Metformin-based nanomedicines in reprogramming the tumor microenvironment
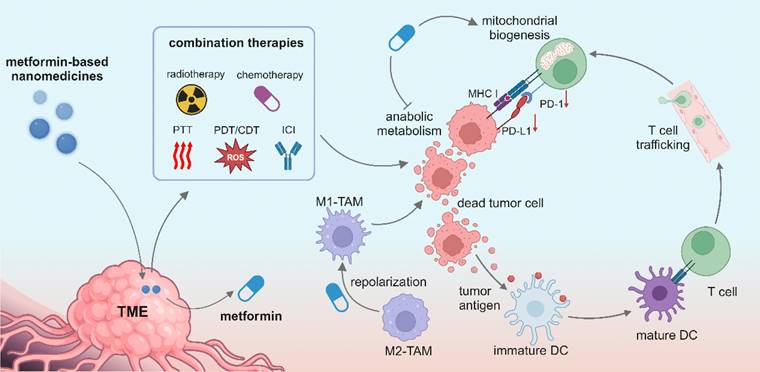
Nanomedicine is rapidly revolutionizing cancer treatment methods because of its multifunctionality and accommodation of multiple drugs within one nanocarrier, and it opens a new avenue for treating advanced cancers such as triple-negative breast cancer. Great efforts have been made to pursue smart nanomedicines which could preferably accumulate in tumor sites and specifically recognize targeted cells, thereby reducing the damage to normal cells [121]. Meanwhile, a variety of combination therapies can be realized in synergy with nanomedicine, including immunotherapy, radiotherapy, chemotherapy, CDT, PDT and PTT (Figure 3). Metformin-derived nanomedicines are predominantly developed for targeting tumor cells, and they have been recently used to target immune cells and reprogram the immunosuppressive TME. In this section, we discuss the use of metformin-derived nanomedicines to reprogram a suppressive TME and promote tumor regression (Table 2).
Improving metformin delivery efficiency via nanocarriers
Oral metformin is predominately absorbed by gastrointestinal tracts, and it exhibits unspecific biodistribution due to the broad presence of its corresponding transporters [146]. Besides, high hydrophilicity and rapid renal elimination of metformin lead to poor cellular permeation and short half-time in blood [147, 148]. All of these above factors restrain the entrance of metformin to cancer cells in which it could interact with mitochondria and activate the AMPK pathway for tumor-suppressive effects. Achieving effective therapeutic outcomes requires enhanced pharmacological properties and sufficient drug accumulation in tumor tissues [149]. As a result, there is growing interest in developing nanomedicines that improve pharmacokinetics, targeting, and bioavailability of metformin by encapsulating it in nano-scale carriers [150].
Nanomedicines could prolong half-life and improve tissue distribution of metformin [151]. The EPR effect has been the primary mechanism for passive accumulation of nanomedicines in tumor tissues via leaky blood vessels and abnormal lymphatics since 1987 [152]. A novel concept, active transport and retention (ATR) is proposed. During ATR, tumor endothelial cells can actively transport nanomedicines from the bloodstream to tumor sites through transcytosis [153].
Metformin-based nanomedicines reprogram the tumor microenvironment
| Material type | Nanoformulation | Function | Combination therapy | Indication | Refs. |
|---|---|---|---|---|---|
| Peptide-based nanoparticle | MA-pepA-Ce6 | MMP-2 responsiveness; αvβ3-mediated tumor targeting; downregulation of PD-L1 expression | PDT | Breast cancer | [122] |
| Polymeric Nanoparticle | HA-CDDP/PMet | Hyaluronic acid-mediated tumor targeting; reversal of chemotherapy resistance; inducing tumor cell apoptosis | Chemotherapy | Lung cancer | [123] |
| aPD-L1-PolyMet/BPN | Anti-PD-L1 antibody-mediated tumor targeting; inhibiting primary and abscopal tumor growth and metastasis | PTT; chemotherapy and immunotherapy | Breast cancer | [124] | |
| LPH-PolyMet-siVEGF | Inducing tumor cell autophagy and apoptosis through downregulating mTOR; decreasing VEGF production | Gene therapy | Non-small-cell lung cancer | [125] | |
| TA-Met@MS | Pulsed release of metformin and tumor antigens; promoting FAO and memory phenotype differentiation of CD8+ T cells | PTT | Breast cancer and melanoma | [126] | |
| Metformin-loaded PLGA-PEG NPs | Inhibition of mTOR and reduction in telomerase reverse transcriptase expression; G1 phase arrest; inducing apoptosis | -- | Breast cancer | [127] | |
| Met-loaded FA-PLGA-PEG NPs | Folate-mediated tumor targeting; upregulating the expression of pro-apoptotic Bax, Caspase 7, Caspase 3, p53 and anti-apoptotic Bcl-2 | -- | Breast cancer | [128] | |
| Polymet | Sensitization of anti-PD-L1 antibody; increasing IFN-γ production and infiltration of T cells; increasing anti-tumor intestinal microbes | -- | Colorectal cancer | [129] | |
| Micelle | FucOMDs | Fucoidan-mediated premetastatic site targeting; inhibition of adhesion of CTCs to endothelial cells; downregulating the expression of fibronectin and MMP-9 | Chemotherapy | Breast cancer | [130] |
| PMD | Downregulating PD-L1 expression; inhibiting Treg cell suppressive activity; promoting DC maturation | Chemotherapy; Immunotherapy | Breast cancer | [131] | |
| Liposome | IR775@Met@Lip | Reversal of hypoxia and enhancing PDT; downregulating PD-L1 expression; enhancing IFN-γ secretion and infiltration of CD8+ T cells | PDT | Colorectal cancer | [132] |
| Met-HCe6-Liposome | Sustainable release of metformin in tumor cells; improving hypoxia | PDT | Colorectal cancer | [133] | |
| Met-oxa (IV)-liposome | Relieving hypoxia; repolarizing TAMs to an M1 phenotype | Chemotherapy; Immunotherapy | Colorectal cancer | [134] | |
| Hydrogel | D@HPMNG | GSH responsiveness; hyaluronic acid-mediated tumor targeting; repolarization of TAMs to an M1 phenotype; enhancing DC maturation and CD8+ T cell infiltration | Chemotherapy | Melanoma | [135] |
| CAR-T@Met/gel | Sustain release of CAR-T cells and metformin; inhibiting glycolysis and OXPHOS in tumor cells; upregulating ACSS1 expression and promotion of OXPHOS and memory phenotype differentiation in CAR-T cells | Immunotherapy | Gastric carcinoma | [67] | |
| PMI | Reshaping an M2-like TAM phenotype; downregulating PD-L1 expression in tumor cells; enhancing the infiltration of CD8+ T cells | Immunotherapy | Breast cancer | [136] | |
| MCGPD/RGD NPs | MMP-2 responsiveness; RGD peptide-mediated tumor cell targeting; improving the hypoxia; reducing ATP production | PDT; PTT and chemotherapy | Breast cancer | [137] | |
| Extracellular vesicles | Met@HMnER | Oxygen generation and hypoxia alleviation; sensitizing radiotherapy; G2/M phase arrest; activating the cGAS-STING pathway | Radiotherapy | Breast cancer | [138] |
| SPI@hEL-RS17 NPs | RS17 peptide-mediated tumor targeting; inhibiting pyruvate kinase-M2 and damaging mitochondria; enhancing macrophage phagocytosis | PDT and chemotherapy | Breast cancer and melanoma | [139] | |
| Met@Man-MP | Mannose-mediated M2-like TAM targeting; repolarization of TAMs to an M1 phenotype; recruitment of CD8+ T cells; reducing immunosuppressive MDSCs and Treg cells | Immunotherapy | Hepatocellular carcinoma | [140] | |
| Inorganic material | CS-metformin@MnO2 | pH-responsive release; downregulating PD-L1 expression; promoting wound healing | -- | Breast cancer | [141] |
| HMMDN-Met@PM | GSH and pH responsiveness; peptide-mediated M2-like TAM targeting; repolarization of TAMs to an M1 phenotype; MR imaging | -- | Breast cancer | [142] | |
| NMC + NTC | Aptamer and magnetics-mediated tumor targeting; downregulating the expression of HK2 and PD-L1; inhibition of glycolysis; alleviating hypoxia; dual modality imaging | PTT; PDT and Immunotherapy | Hepatocellular carcinoma | [143] | |
| Met@BF | pH-responsive charge reversal; downregulation of PD-L1 expression; inducing DC maturation | CDT | Melanoma | [144] | |
| Mn-MSN@Met-M NPs | GSH and pH responsiveness; homologous tumor cell targeting; stabilizing the STING protein and activating the cGAS-STING pathway | Immunotherapy | Lung cancer | [145] |
ACSS1: Acyl-CoA synthetase short chain family member 1; CAR-T: chimeric antigen receptor T cell; cGAS-STING: cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes; PD-L1: programmed cell death 1 ligand 1; DC: dendritic cell; CDT: chemodynamic therapy; FAO: fatty acid oxidation; PTT: photothermal therapy; MMP-2: matrix metalloproteinase-2; PDT: photodynamic therapy; GSH: glutathione; TAM: tumor-associated macrophage; CTCs: circulating tumor cells; HK2: hexokinase 2; OXPHOS: oxidative phosphorylation; MDSC: myeloid-derived suppressor cell; Treg: regulatory T cell.
Illustration of metformin-based nanomedicines in combination with other therapeutic modalities for reprogramming the tumor microenvironment. Created in https://BioRender.com.
Additional active transfer mechanisms include vesicular transport and blood vessel leakage facilitated by neutrophil extravasation, which help nanomedicines enter tumor tissues [154]. However, ATR is not universally accepted for different types of solid tumors, thus personalized nanomedicines have been developed to tailor to specific biological characteristics of different cancer types [155]. Active targeting molecules for specific biological characteristics of tumors, such as antibodies, peptides, and aptamers, have been identified and incorporated into nanomedicines to increase their binding affinity to tumor cells, thus enhancing tumor accumulation [156]. Similar active targeting strategies could hold promise in targeting immune cells [157, 158]. It is important to note that evidence from the past decade supports that only 0.7% of nanoparticles successfully reach tumor tissues due to their elimination via the mononuclear phagocytic system [159]. Thus, optimization of the size, charge, and surface coating of nanocarriers is crucial to reduce their uptake by mononuclear phagocytes in the liver and spleen [160]. Therefore, nanocarriers with optimized physio-chemical properties could act as an effective delivery system for metformin.
Nanomedicines could also enhance overall biocompatibility and pharmacokinetics of metformin while achieving a high localized concentration at tumor sites, resulting in improved efficacy and reduced toxicity [29, 159]. Over the past decades, a variety of advanced nanocarriers have been developed to deliver therapeutics to tumor sites [161-163]. Polymeric nanoparticles are nanoscale biomaterials made from polymers, and poly lactic-co-glycolic acid (PLGA) nanoparticles are the most widely used due to their excellent biocompatibility [164-167]. PLGA nanoparticles have been reported to protect metformin from early release and realize sustainable release over 160 hours [168]. It is noted that polymetformin (Polymet) is a polymeric form of metformin with positive charge. Polymet has been employed to deliver siRNA and form nano-composites with therapeutic photothermal agents for tumor treatment [124, 125]. Liposomes are an effective drug delivery system with improved pharmacokinetics and bioavailability [169]. It was reported that liposomes could improve the entrapment efficiency of metformin (~65%) [170]. Micelles are self-assembled nanoscale spheres composed of amphiphilic molecules, and they have a hydrophobic tail and a hydrophilic head [171]. Metformin-containing polymeric micelles could release metformin via pH-responsiveness and exhibit more potent cytotoxicity against breast cancer [172]. Hydrogels are highly biocompatible and biodegradable networks of cross-linked polymer chains [173]. Beyond controlled and sustained release of metformin, hydrogels have been reported to realize transdermal administration of metformin, which could be a potential administration route for metformin [174]. Notably, nanomedicines have been successful in prolonging blood circulation, enhancing tissue accumulation and penetration, facilitating cellular internalization, and ultimately controlling drug release [175, 176]. In this context, the dimension, shape, and surface characteristics of nanocarriers could be meticulously fine-tuned to achieve great effectiveness of metformin-based nanomedicines.
Metformin-loaded nanomedicines inhibit tumor growth in combination with other treatments
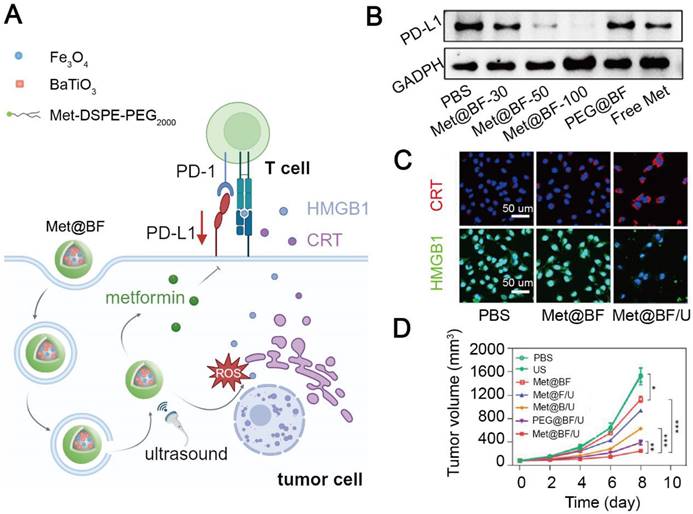
Blocking the PD-L1/PD-1 engagement can resume proliferation of T cell and recover their function. However, PD-1-based ICIs including pembrolizumab and nivolumab are costly for cancer treatment (over 100,000 dollars per year) [177]. In addition, unspecific engagement of anti-PD-1 antibodies to PD-1 expressed on normal cells can lead to systemic toxicity [178]. Therefore, cheap and safe agents have been developed to prevent the immunosuppressive signaling from PD-L1/PD-1 interaction. Wang et al. constructed a stimuli-responsive nanohybrid, Met@BF with BaTiO3 and Fe3O4 nanoparticles, to deliver metformin and induce CDT in melanoma [144]. The imine bond moiety of Met@BF realized a charge-reversal in an acidic TME: Met@BF exhibited a negative potential to reduce macrophage capture during blood circulation, but a positive potential in an acidic tumor site to facilitate tumor cell endocytosis. In addition, BaTiO3 produced H2O2 upon ultrasonic irradiation to increase Fe3O4-mediated ROS generation, inducing an ICD effect. Dead tumor cells released HMGB1 and CRT to promote dendritic cell maturation and subsequent T cell activation, triggering anti-tumor immunity. Finally, released metformin from the Met@BF nanohybrid at tumor sites significantly downregulated the PD-L1 level on tumor cells and subsequently enhanced T cell infiltration, thus curbing the development of primary and metastatic B16F10 tumor masses in mice (Figure 4). However, nanomedicines without tumor targetability may induce off-target effects and systemic toxicity. Hu et al. constructed tumor cell-targeting nanoparticles, MA-pepA-Ce6 NPs, to specifically deliver metformin and chlorin e6 (Ce6) as a photosensitizer to breast cancer cells. The nanoparticles were cleaved by abundant matrix metalloproteinase-2 within the TME to release peptide-conjugated metformin and integrin αvβ3 ligand-modified Ce6. Ce6 was bound to αvβ3-overexpressed tumor cells and induce an ICD effect under radiation. Metformin with a positive potential was easily internalized by tumor cells to promote PD-L1 proteasomal degradation and increase IFN-γ secretion from CD8+ T cells. Therefore, MA-pepA-Ce6 NPs mitigated the immunosuppressive level in the TME via downregulating PD-L1 and inducing ICD and inhibited 4T1 tumor growth without exerting significant systemic toxicity [122].
Metformin-based nanomedicine in combination with chemodynamic therapy. (A) Schematic illustration of the mechanism of action of Met@BF nanohybrids for cancer therapy. Created in https://BioRender.com. (B) Western blotting of PD-L1 after different treatments. (C) Confocal fluorescence images of CRT and HMGB1 released from dead tumor cells treated with Met@BF upon ultrasound irradiation. (D) The degree of tumor growth inhibition in B16F10 tumor-bearing mice after different treatments. Adapted with permission from [144], copyright 2024 American Chemical Society.
Apart from ameliorating an immunosuppressive TME via promoting PD-L1 degradation, metformin exhibits direct cytotoxic effects on tumor cells. Jafari et al. constructed folate-modified-PLGA-polyethylene glycol (PEG) nanoparticles to improve blood circulation and bioavailability of metformin [128]. The nano-formulated metformin induced more pronounced apoptosis of breast cancer cells than free metformin. However, metformin-dependent cytotoxicity is not sufficient to induce tumor regression. Therefore, combining metformin-based nanomedicines with other treatment modalities including radiotherapy and chemotherapy could lead to more potent ICD.
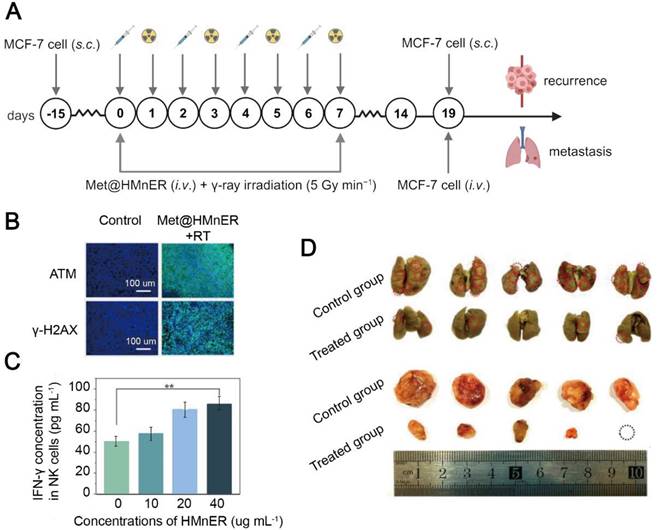
Hypoxia is one of the culprits for a low efficacy of clinical radiotherapy [179], and insufficient oxygen in the severely hypoxic solid tumor microenvironment results in a lower level of ROS generated from MnO2, a traditional radiosensitizer [180]. It was found that metformin sensitized radiotherapy by blocking mitochondrial complex I, thereby decreasing the oxygen consumption in cancer cells [62]. To improve the efficiency in the delivery of metformin to tumor tissues, Yang et al. employed natural extracellular vesicles as nanocarriers to realize prolonged circulation and immune escape to increase cellular uptake of the metformin-containing nanomedicines [181]. MnO2 nanocomposites were first constructed from hollow MnO2 nanoparticles. Metformin and the MnO2 nanocomposites were encapsulated by RGD-modified extracellular vesicles to form a radiosensitive nanomedicine, Met@HMnER [138]. Tumor cell uptake of Met@HMnER was significantly enhanced through RGD-αvβ3 binding, and oxygen and Mn2+ were released via stimuli-responsiveness from excessive H2O2 and glutathione (GSH) within the TME, respectively. Promotion of oxygen generation in conjunction with metformin-mediated inhibition of oxygen consumption alleviated hypoxia in tumor tissues and enhanced the radiotherapeutic efficacy, while Mn2+ promoted IFN-γ secretion from NK cells via activating the cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS)-stimulator of interferon genes (STING) pathway [182]. Met@HMnER simultaneously augmented innate anti-tumor immunity and improved the radiotherapy efficacy to inhibit tumor metastasis and recurrence in MCF-7 tumor-bearing mice (Figure 5). Notably, metformin has also been reported to activate the cGAS-STING pathway through AXIN1-dependent STING stabilization. Dou et al. constructed Mn-MSN@Met-M nanoparticles with a coating of cancer cell membranes to specifically deliver metformin and Mn2+ to lung cancer cells in the mice with LKB1 mutation. The loss of LKB1 in the mice usually leads to inhibition of the STING pathway and reduction in IFN-β secretion [183]. Intravenous administration of the nanomedicine resulted in an increase in localized enrichment of metformin and Mn2+ in cancer cells, both of which synergistically upregulated the cGAS-STING pathway and enhanced T cell tumor-killing ability [145].
Metformin has also been combined with chemotherapeutic agents. Cisplatin is one of the most popular agents for chemotherapy, but its efficacy is diminished due to upregulation of DNA repair in tumor cells. Nucleotide excision repair and interstrand crosslink repair are credited to the excision repair cross-complementing 1 (ERCC1) protein [184]. To reduce chemoresistance of cisplatin in lung cancer, Yang et al. constructed self-assembled nanoparticles to deliver cisplatin and metformin in a nanomedicine format, HA-CDDP/PMet. This nanomedicine specifically targeted cancer cells through hyaluronic acid-CD44 binding. Remarkably, metformin inhibited the mTOR activity and helped downregulating the expression of ERCC1, thus inhibiting DNA repair and overcoming the resistance of cisplatin-based chemotherapy in the LLC tumor-bearing mice [123]. However, DNA damage can induce an elevation in the intracellular Ca2+ level and activation of AMPK for DNA repair via inhibiting exonuclease 1 (EXO1) and p53-binding protein 1 (53BP1) [185, 186]. Arguably, metformin may assist in DNA repair rather than promote cisplatin-mediated DNA damage, and the precise mechanism of action of metformin-enhanced cisplatin chemotherapy needs to be unveiled. In another study on the combination of metformin with cisplatin by Saber et al., metabolic changes in tumor cells were detected. Self-assembled nano-cubosomes were prepared to encapsulate metformin and cisplatin to treat colorectal cancer [187]. Metformin was released from the nano-cubosomes to inhibit glycolysis and ATP production via downregulating the mTOR activity, thus eliciting oxidative stress and apoptosis and ultimately enhancing cytotoxicity of cisplatin. Metformin-mediated mTOR inhibition suppressed tumorigenesis-associated glycolysis and promoted tumor cell apoptosis, confirming a critical role of metformin in combinational cancer treatments.
Metformin as an adjuvant exhibits an anti-tumor effect, while its targetability and bioavailability are often quite poor after direct administration. Metformin-containing nanomedicines can selectively deliver metformin to tumor cells to downregulate PD-L1 and inhibit mitochondrial respiration. Notably, metformin alone could not exert potent cytotoxic effects on tumor cells, while it could be combined with ICD-inducing treatment modalities such as PDT, radiotherapy and chemotherapy. Continuous efforts should be concentrated on revealing the mechanisms of reprogramming an immunosuppressive TME via metformin in nanomedicines.
Metformin-loaded nanomedicines promote T cell oxidative metabolism and memory differentiation
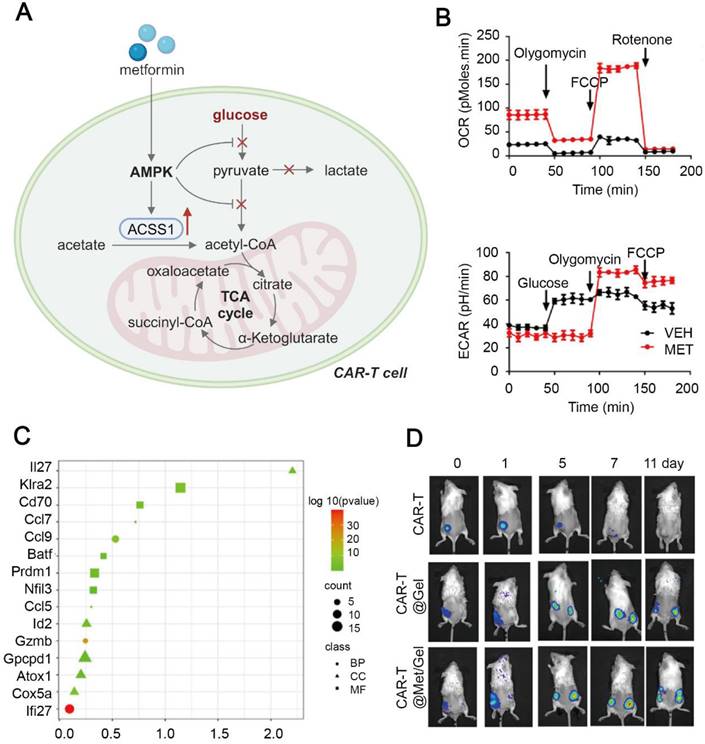
Immunometabolic modulation of T cells via metformin has been extensively explored. Polymeric metformin has been used to enhance T cell infiltration via AMPK activation and improve the TME, and it has been combined with ICI therapy in colorectal cancer treatment [129]. Tumor relapse is a predominant indicator of poor outcomes in cancer patients, while long-living Tm cells can prevent tumors from recurrence. It is well established that maintenance of Tm cells is principally dependent on mitochondrial metabolism, such as OXPHOS and FAO [188]. Therefore, Chao et al. constructed a hydrogel scaffold to store and gradually release chimeric antigen receptor T (CAR-T) cells and metformin, and implanted the scaffold in the post-resection tumor site to evaluate the anti-tumor effect [67]. Interestingly, released metformin from the scaffold inhibited glycolysis and OXPHOS in tumor cells, while it helped strengthening the TCA cycle and mitochondrial respiration in the T cells. In CAR-T cells, upregulation of ACSS1 boosted acetyl-CoA production, fueling the TCA cycle and OXPHOS. Metabolic reprogramming of CAR-T cells enhanced their proliferation and secretion of effector cytokines. The scaffold fine-tuned CAR-T cells into a more persistent and memory-like phenotype, resulting in significant inhibition of primary and metastatic tumors in the HGC-27 tumor-bearing mice (Figure 6). However, distinct metabolic influences by metformin on cancer cells and T cells remain unknown. We believe metformin improves T cell viability via altering their engagement with cancer cells, since metformin fails to exert such effects in the absence of cancer cells. Interestingly, different concentrations of metformin can exert absolutely different effects on the mitochondrial activity [189], indicating the importance of designing a proper dose of metformin to tumor cells and T cells for activating effective anti-tumor effects.
Metformin-based nanomedicine in combination with radiotherapy. (A) Schematic illustration of Met@HMnER to inhibit cancer recurrence and metastasis in the MCF7 tumor-bearing mice. Created in https://BioRender.com. (B) Fluorescence microscope images for ATM and γ-H2AX. Elevation in the expression of both of them indicated significant DNA damage and enhanced radiotherapy sensitization after treatment with Met@HMnER. (C) The expression levels of IFN-γ secreted by NK cells through activating the cGAS-STING pathway by Met@HMnER. (D) Photos of lungs (above) and recurrent tumors (below) after treatment with Met@HMnER, confirming its anti-recurrence and anti-metastasis efficacy. Adapted with permission from [138], copyright 2023 Elsevier Ltd.
Metformin-based hydrogel scaffold to promote anti-tumor immune response of CAR-T cells. (A) Schematic illustration of metabolic reprogramming in CAR-T cells treated with the metformin-containing hydrogel. Created in https://BioRender.com. (B) Metabolic flux analysis of the oxygen consumption rate and the extracellular acidification rate in CAR-T cells treated with metformin, indicating suppression of glycolysis and enhancement in oxidative phosphorylation. (C) Upregulation of memory phenotype-related transcripts in CAR-T cells treated with CAR-T@Met/gel. (D) Bioluminescence images of CAR-T cell proliferation in primary and distant lesions in the HGC-27 tumor-bearing mice. Adapted with permission from [67], copyright 2023 Elsevier Ltd.
The exposure of tumor antigens to T cells is required for T cell activation, but long-term stimulation by tumor antigens as anti-tumor vaccines can exhaust T cells and block their differentiation to a memory-like phenotype. Metformin can downregulate the expression level of immunosuppressive PD-1 and prolong Tm cell survival by strengthening their mitochondrial function and elevating their FAO level. Luo et al. developed a metformin-based tumor vaccine to induce generation of Tm cells [126]. They initially treated the tumor lesion with PTT to acquire anti-tumor immunogenicity. The tumor antigens were collected and co-encapsulated with metformin and hollow gold nanoparticles as a photothermal agent into biodegradable PLGA microspheres to obtain TA-Met@MS as a vaccine. TA-Met@MS released pulsed tumor antigens and metformin under near infrared radiation to promote T cell activation and subsequent central Tm cell formation via interfering with FAO. Thus, TA-Met@MS pronouncedly inhibited tumor growth and metastasis and a great number of CD8+ Tm cells were produced in the 4T1 and B16F10 tumor-bearing mice. Since effectiveness of cancer vaccines relies on rapid response from Tm cells, central Tm cells could be endowed with a hyper proliferative ability in response to tumor antigen reencounter [190]. Notably, the metformin-activated AMPK signaling pathway promotes catabolism and Tm cell differentiation, but it could impair Teff cell differentiation [46], therefore, it is suggested to induce Tm cell differentiation via metformin during the T cell contraction phase, which could maximize in maintaining anti-tumor immune response.
Novel T cell-targeting nanomedicines have emerged for cancer treatment. For example, engineered T cells anchored with a nanomedicine resolved the issue of vasculature extravasation of nanomedicines and they precisely acted on T cells without any physiological changes [191]. More recently, a tri-specific nano-antibody has been reported to simultaneously bind to tumor cells via targeting PD-L1, and T cells and NK cells via targeting 4-1BB and natural killer group 2 member A (NKG2A) [192]. The tri-specific nano-antibody exhibited more effective targetability and better anti-tumor immunity compared with clinical monoclonal antibodies and bispecific monoclonal antibodies. Metformin could be combined with these innovative and effective strategies in the nano-medicinal format for advanced solid tumors.
Emerging immunological evidence indicates precursor exhausted T cells in lymph nodes are emerging as novel targeting candidates, since these precursor cells can respond to ICIs and then proliferate and replenish functional T cells at the tumor site [193]. However, upon prolonged stimulation by tumor antigens, precursor exhausted T cells inevitably generate terminally exhausted progeny [194]. Mitochondrial dysfunction has been found to be linked to T cell exhaustion [195, 196], and studies have confirmed that PGC-1α-overexpressed T cells exhibit significant improvements in mitochondrial biogenesis and expansion [197]. Since metformin can activate PGC-1α via the AMPK signaling pathway, it could be employed to enhance the mitochondrial fitness of precursor exhausted T cells to mitigate the exhaustion process and maintain their tumor-killing ability. However, metformin-containing nanomedicines should be designed and constructed to break through barriers in lymph nodes [198, 199].
Given that T cells are pivotal in driving tumor regression, it is critical to promote their growth, sustain their survival, and boost their production of effector cytokines. With the help of a nanocarrier, metformin as a safe and accessible immunologic adjuvant could be harnessed to improve oxidative metabolism of T cells via activating the AMPK pathway, which can upregulate the associated enzymes and promote mitochondrial biogenesis, thus reinvigorating their anti-tumor immunity and modulating the immunosuppressive TME.
Metformin-loaded nanomedicines repolarize M2-like tumor-associated macrophages to M1 phenotype
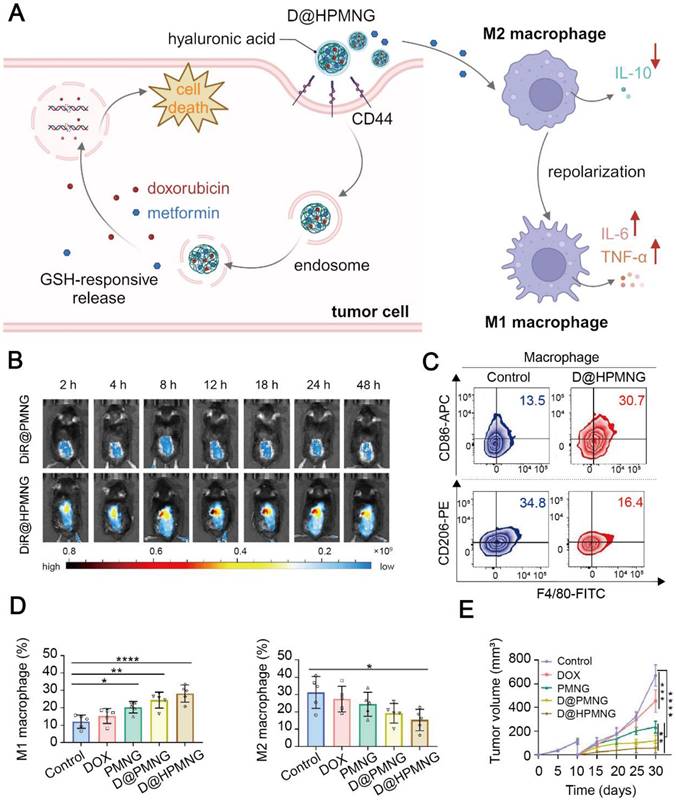
TAMs are a well-recognized cell population in the TME, and they are usually educated to an M2 phenotype to express PD-L1 and secrete suppressive cytokines, thus inhibiting T cells. The use of metformin via nanomedicines to regulate the TAM phenotype is a promising approach to revitalizing the immunosuppressive TME. Tang et al. constructed a GSH-responsive nanogel, PMNG, from carboxymethyl chitosan, metformin and cystamine [135]. The nanogel exhibited prominent deformability, facilitating its penetration into deep tumor tissues [200]. PMNG was then loaded with doxorubicin (DOX) and coated with hyaluronic acid (HA) to obtain a D@HPMNG nanomedicine. Tumor-targeting by the nanomedicine was realized via specific interaction between CD44 and HA. The HA coating also prevented D@HPMNG from immune clearance and improved biocompatibility. After the nanomedicine was effectively uptaken by tumor cells, overexpressed GSH in tumor cells immediately cleaved the disulfide bond in D@HPMNG to release DOX to induce tumor cell apoptosis without inducing cardiotoxicity. Besides, D@HPMNG reprogrammed the TME via inducing the M2-to-M1 repolarization of TAMs, increasing the portion of Teff cells, and reducing collagen deposition, ultimately inhibiting tumor growth and relapse in the B16F10 tumor-bearing mice (Figure 7). Another metformin-containing nanogel developed by Tian et al. repolarized the TAM phenotype in vitro and activation of the AMPK signaling pathway was found to contribute to the phenotype conversion [136].
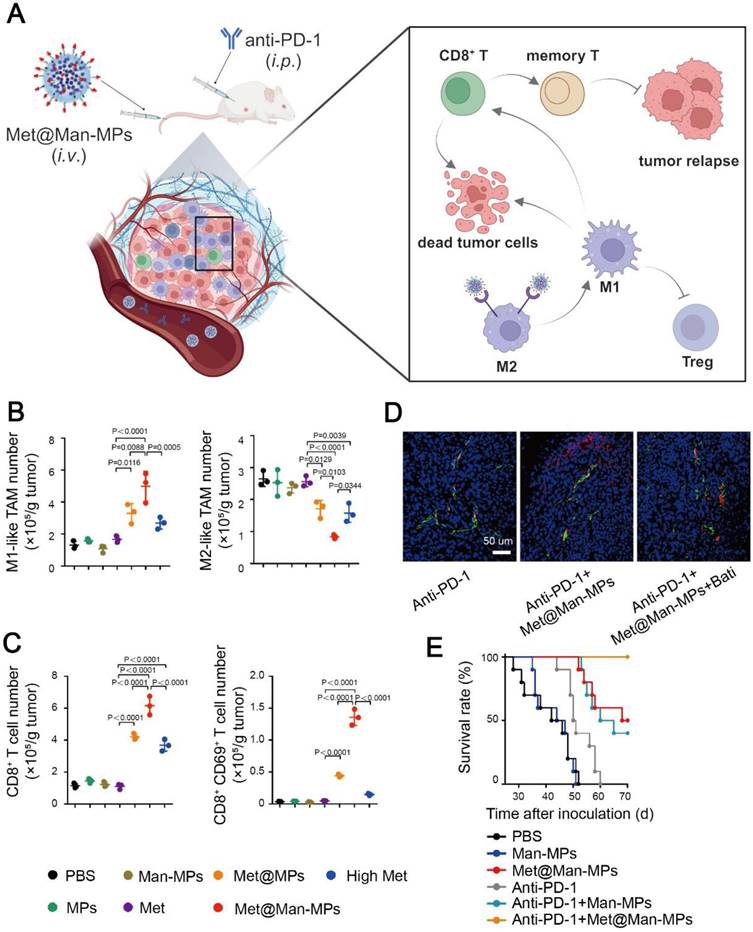
However, the above nanogels were not specifically delivered to M2-like TAMs. To exclusively study the influence of metformin on TAM repolarization, Wei et al. developed macrophage-derived microparticles to load metformin, and then modified the microparticles with mannose, resulting in a Met@Man-MP nanomedicine. The mannose moiety on the microparticle could specifically bind to overexpressed CD206 on M2-like TAMs [140]. Met@Man-MP displayed excellent stability, biocompatibility and safety during blood circulation, and it exhibited a matrix metalloproteinase-like activity to degrade the ECM in the TME. Therefore, Met@Man-MP selectively targeted M2-like TAMs and reshaped them to an M1 phenotype, and it boosted the infiltration of Teff cells as well as anti-PD-1 antibodies through promoting collagen degradation. Interestingly, the decomposition of collagen in the ECM mediated by Met@Man-MP did not result in tumor metastasis. Eventually, Met@Man-MP reprogramed the immunosuppressive TME via repolarizing M2-like TAMs to an M1 phenotype and elevating the portion of Teff cells, and enhanced the effectiveness of ICIs in the H22 tumor-bearing mice (Figure 8). Both nanogels and microparticles could repolarize TAMs from an M2 to M1 phenotype, but the specific mechanism of AMPK activation for repolarizing the TAM phenotype via metformin remained to be unveiled.
Experimental results have confirmed that metformin could reshape the TAM phenotype, but more studies should be conducted to verify its effectiveness. The mechanism of conversion of the TAM phenotype is not revealed. Metformin may be directly involved in TAM phenotype repolarization via the AMPK pathway. Alternatively, it may impair the reeducating ability of tumor cells to induce M2-like TAM differentiation by blocking the secretion of immunosuppressive cytokines. A comprehensive understanding of the immunometabolism-modulating mechanism of metformin is conducive to developing potent metformin-containing nanomedicines in reprogramming the TME.
Metformin-based nanomedicine in combination with chemotherapy. (A) Schematic illustration of the mechanism of action for D@HPMNG to combat cancer and repolarize the TAM phenotype. Created in https://BioRender.com. (B) Fluorescence images of biodistribution of DiR-labeled HPMNG nanogels in the mice, indicating excellent targeting ability of the nanogel. Representative flow cytometry plots of M1-like and M2-like TAMs (C) and their quantitative results (D) at recurrent tumor sites after treatment with D@HPMNG and its controls. (E) The recurrent tumor growth profile after different treatments. Adapted with permission from [135], copyright 2024 American Chemical Society.
Metformin-based nanomedicine in combination with immunotherapy. (A) Schematic illustration of the anti-tumor mechanism of Met@Man-MPs in synergy with anti-PD-1 antibody. Created in https://BioRender.com. The portion of M1-like TAMs, M2-like TAMs (B) and CD8+ T cells (C) in tumor sites after different treatments. (D) Colocalization of the anti-PD-1 antibody (red) and endothelial cells (green), indicating Met@Man-MPs strengthened the penetration of the anti-PD-1 antibody in tumor sites. (E) Survival curves of H22 tumor-bearing mice after different combined treatment methods. Adapted with permission from [140], copyright 2021 Springer Nature.
Conclusion
Metformin, a first-line treatment drug for T2DM to lower the blood glucose concentration through AMPK activation, has recently gained attention as a potential adjuvant in cancer therapy due to its direct and indirect anti-tumor effects. Metformin-mediated AMPK activation can induce PD-L1 degradation and interfere with anabolic processes in tumor cells via inhibiting mTOR. Additionally, inhibition of mitochondrial complex I by metformin can reduce oxygen consumption in tumor cells and remodel a hypoxic TME. Inspiringly, metformin reprograms the TME via enhancing the effector activity of anti-tumor immune cells. For example, AMPK activation in T cells favors energy metabolism and promotes memory phenotype formation, and it also contributes to anti-tumor repolarization of TAMs. However, direct administration of metformin has shown limited benefits in cancer therapy, which may be due to significant variations in the local concentration of metformin at tumor sites. To address this issue, metformin-containing nanomedicines have been developed to increase its localized concentration in tumor tissues, thereby revealing its role as an anti-tumor adjuvant and confirming its tumor-suppressing effects. The introduction of the nanomedicine formulation endows metformin with improved targetability and bioavailability through diversified strategies for targeting and responsive release in response to characteristic stimuli in the TME, in this way, the toxicity of metformin can be significantly diminished. A variety of nanomaterials have been explored to encapsulate metformin in nanomedicines. More encouragingly, metformin combined with other therapeutic drugs can be incorporated into one single nanomedicine to realize combination therapy. In summary, repurposing metformin in a nano-medicinal formulation holds great potential for effectively reprogramming an immunosuppressive TME and comprehensively activating anti-tumor immunity.
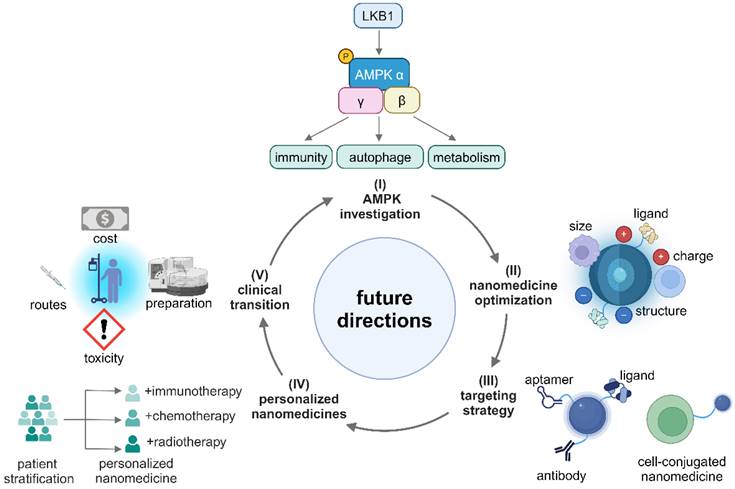
Although metformin-containing nanomedicines have shown promise in reprogramming an immunosuppressive TME, there are very few fundamental mechanistic studies in this area. To fully leverage the potential of metformin in cancer nanomedicines, the following future research directions are highly recommended (Figure 9). (I) Conducting systematical studies on the AMPK signaling network to identify specific targets that induce tumor-suppressive effects in different immune cells; (II) Enhancing efficiencies in accumulation and penetration of nanomedicines in the TME by optimizing their size, charge, coating, and ligands. In addition, exploring and designing immune-regulating nanomaterials for metformin-containing nanomedicines is conducive to enhancing anti-tumor immune response; (III) Improving active targetability and reducing off-target effects of metformin-based nanomedicines via exploring high-affinity peptides, antibodies, aptamers and developing cell-conjugated nanomedicines; (IV) Developing personalized nanomedicines through patient stratification by tissue biomarkers and biological characteristics of different cancer types; (V) Finally, reducing the cost, simplifying the preparation process, selecting the best administration routes, and mitigating toxicities from nano-formulations so that the nanomedicines can be readily translated into clinical use.
Illustration of the future directions of metformin-based nanomedicines. Created in https://BioRender.com.
In conclusion, metformin in a nanomedicine formulation has emerged as a promising adjuvant to activate the AMPK signaling pathway which is a key regulator in cellular metabolic activities, energy production and immune response, and it can reprogram the TME by remodeling an immunologically cold TME into an immunologically hot one to reactivate the suppressed anti-tumor immunity. In the nanomedicine formulation, metformin can work in synergy with other therapeutic agents or modalities to eliminate malignancies. The mechanisms of regulating immunometabolism within the TME via AMPK activation by metformin remain mysterious because of the complex interplay between the components of the AMPK signaling pathway and unveiling of these mechanisms could offer great potential in restoring suppressed anti-tumor immunity within the TME. Overall, repurposing metformin in a nanomedicine formulation to reprogram the TME will advance cancer treatment and benefit cancer patients.
Abbreviations
TME: tumor microenvironment; T2DM: type 2 diabetes mellitus; AMPK: adenosine monophosphate-activated protein kinase; GLP1: glucagon-like peptide 1; ECM: extracellular matrix; TAM: tumor-associated macrophage; CAFs: cancer-associated fibroblasts; CAR-T: chimeric antigen receptor T cell; MDSC: myeloid-derived suppressor cell; Treg: regulatory T cell; Teff: effector T cell; Tm: memory T cell; CTLs: cytotoxic T lymphocytes; DC: dendritic cell; EPR: enhanced permeability and retention; CDT: chemodynamic therapy; PTT: photothermal therapy; PDT: photodynamic therapy; ICD: immunogenic cell death; ROS: reactive oxygen species; DAMPs: damage-associated molecular patterns; CRT: calreticulin; HMGB1: high mobility group box 1; LKB1: liver kinase B1; mTOR: mechanistic target of rapamycin; eIF4E: eukaryotic initiation factor 4E; S6K: ribosomal protein S6 kinase; HIF-1α: hypoxia inducible factor-1α; SREBP1: sterol regulatory element-binding protein 1; NF-κB: nuclear factor kappa-B; Eomes: eomesodermin; CaMKK2: calcium/calmodulin-dependent kinase kinase; PGC-1α: peroxisome proliferator activated receptor γ coactivator-1α; Foxp3: forkhead box p3; STAT3: signal transducer and activator of transcription 3; RFS: recurrence-free survival; OS: overall survival; CSS: cancer specific survival; DFS: disease free survival; OXPHOS: oxidative phosphorylation; HK2: hexokinase 2; FAS: fatty acid synthesis; FAO: fatty acid oxidation; GLUT1: glucose transporter 1; HMGCR: HMG-CoA reductase; ACC: acetyl-coA carboxylase; ACSS: acyl-coA synthetase short-chain family; CPT: carnitine palmitoyltransferase; PD-L1: programmed cell death 1 ligand 1; PD-1: programmed cell death 1; MMP-2: matrix metalloproteinase-2; GSH: glutathione; cGAS-STING: cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon genes; CTCs: circulating tumor cells.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (52303198, 32271445), National Science and Technology Major Project of China, National Key Research and Development Program of China (2023YFB3810004, 2022YFC2009900, 2023ZD0502304), Department of Science and Technology of Sichuan Province (2024NSFJQ0050, 2024NSFSC1019), National Guidance Fund on Developing Local Science and Technology for Sichuan Province (2023ZYD0167), 1‧3‧5 project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYGD23026). All diagrams, including the graphical abstract image and figures, were created with BioRender.com (https://www.biorender.com/). The funders play no role in paper design, data collection, data analysis, interpretation, and writing of the paper.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jin M-Z, Jin W-L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5:166
2. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541-50
3. Caligiuri G, Tuveson DA. Activated fibroblasts in cancer: Perspectives and challenges. Cancer Cell. 2023;41:434-49
4. Zhang T, Jia Y, Yu Y, Zhang B, Xu F, Guo H. Targeting the tumor biophysical microenvironment to reduce resistance to immunotherapy. Adv Drug Del Rev. 2022;186:114319
5. Lyssiotis CA, Kimmelman AC. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 2017;27:863-75
6. Nirschl CJ, Drake CG. Molecular Pathways: Coexpression of Immune Checkpoint Molecules: Signaling Pathways and Implications for Cancer Immunotherapy. Clin Cancer Res. 2013;19:4917-24
7. Blagih J, Coulombe F, Vincent EE, Dupuy F, Galicia-Vázquez G, Yurchenko E. et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity. 2015;42:41-54
8. Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q, Saghatelian A. et al. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci U S A. 2017;114:580-5
9. Zou Z-f, Yang L, Nie H-j, Gao J, Lei S-m, Lai Y. et al. Tumor-targeted PROTAC prodrug nanoplatform enables precise protein degradation and combination cancer therapy. Acta Pharmaco Sin. 2024;45:1740-51
10. Cui Y, Liu J, Cui L, Wei C, Xu M, Wu Z. et al. Tumor-targeting and activatable biomimetic nanococktails synergistically regulate immune responses for spatiotemporal immunotherapy of low immunogenic solid tumors. Nano Today. 2024;57:102380
11. Yang J, Song L, Shen M, Gou X, Bai L, Wang L. et al. Hierarchically Responsive Tumor-Microenvironment-Activated Nano-Artificial Virus for Precise Exogenous and Endogenous Apoptosis Coactivation. Adv Funct Mater. 2021;31:2104423
12. Liu J, Bai Y, Li Y, Li X, Luo K. Reprogramming the immunosuppressive tumor microenvironment through nanomedicine: an immunometabolism perspective. EBioMedicine. 2024;107:105301
13. Li Y, Wu Y, Fang Z, Zhang Y, Ding H, Ren L. et al. Dendritic Nanomedicine with Boronate Bonds for Augmented Chemo-Immunotherapy via Synergistic Modulation of Tumor Immune Microenvironment. Adv Mater. 2024;36:e2307263
14. Wang L, He S, Liu R, Xue Y, Quan Y, Shi R. et al. A pH/ROS dual-responsive system for effective chemoimmunotherapy against melanoma via remodeling tumor immune microenvironment. Acta Pharm Sin B. 2024;14:2263-80
15. Lin Y, Wang X, He S, Duan Z, Zhang Y, Sun X. et al. Immunostimulatory gene therapy combined with checkpoint blockade reshapes tumor microenvironment and enhances ovarian cancer immunotherapy. Acta Pharm Sin B. 2024;14:854-68
16. Foretz M, Guigas B, Viollet B. Metformin: update on mechanisms of action and repurposing potential. Nat Rev Endocrinol. 2023;19:460-76
17. LaMoia TE, Shulman GI. Cellular and Molecular Mechanisms of Metformin Action. Endocr Rev. 2021;42:77-96
18. Gribble FM, Reimann F. Metabolic Messengers: glucagon-like peptide 1. Nat Metab. 2021;3:142-8
19. Bahar ME, Kim HJ, Kim DR. Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal Transduct Target Ther. 2023;8:455
20. Zheng Q, Li S, Wang A, Zhe M, Yang P, Wu Y. et al. p38 mitogen-activated protein kinase: Functions and targeted therapy in diseases. MedComm-Oncol. 2023;2:e53
21. Talukdar J, Srivastava TP, Sahoo OS, Karmakar A, Rai AK, Sarma A. et al. Cancer stem cells: Signaling pathways and therapeutic targeting. MedComm-Oncol. 2023;2:e62
22. Castel P, Toska E, Engelman JA, Scaltriti M. The present and future of PI3K inhibitors for cancer therapy. Nat Cancer. 2021;2:587-97
23. Yin H, Tang Q, Xia H, Bi F. Targeting RAF dimers in RAS mutant tumors: From biology to clinic. Acta Pharm Sin B. 2024;14:1895-923
24. Schlesinger S, Neuenschwander M, Barbaresko J, Lang A, Maalmi H, Rathmann W. et al. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: umbrella review of meta-analyses of prospective studies. Diabetologia. 2022;65:275-85
25. Arner EN, Rathmell JC. Metabolic programming and immune suppression in the tumor microenvi-ronment. Cancer Cell. 2023;41(3):421-433
26. Zhang L, Wang Z, Liu K, Liu Y, Wang S, Jiang W. et al. Targets of tumor microenvironment for potential drug development. MedComm-Oncol. 2024;3:e68
27. Li X, Duan Z, Chen X, Pan D, Luo Q, Gu L. et al. Impairing Tumor Metabolic Plasticity via a Stable Metal-Phenolic-Based Polymeric Nanomedicine to Suppress Colorectal Cancer. Adv Mater. 2023;35:e2300548
28. Wang N, Liu C, Li Y, Huang D, Wu X, Kou X. et al. A cooperative nano-CRISPR scaffold potentiates immunotherapy via activation of tumour-intrinsic pyroptosis. Nat Commun. 2023;14(1):779
29. Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019;18:175-96
30. Tietjen GT, Bracaglia LG, Saltzman WM, Pober JS. Focus on Fundamentals: Achieving Effective Nanoparticle Targeting. Trends Mol Med. 2018;24:598-606
31. He M, Wang M, Xu T, Zhang M, Dai H, Wang C. et al. Reactive oxygen species-powered cancer immunotherapy: Current status and challenges. J Control Release. 2023;356:623-48
32. Shi G, Cui Y, Zhao J, Liu J, Wang Y, Yang Y. et al. Identifying TOPK and Hypoxia Hallmarks in Esophageal Tumors for Photodynamic/Chemo/Immunotherapy and Liver Metastasis Inhibition with Nanocarriers. ACS Nano. 2023;17:6193-6207
33. Liu J, Cui Y, Cabral H, Tong A, Yue Q, Zhao L. et al. Glucosylated Nanovaccines for Dendritic Cell-Targeted Antigen Delivery and Amplified Cancer Immunotherapy. ACS Nano. 2024;18:25826-25840
34. Zhang X, Wu Y, Lin J, Lu S, Lu X, Cheng A. et al. Insights into therapeutic peptides in the cancer-immunity cycle: Update and challenges. Acta Pharm Sin B. 2024;14:3818-33
35. Misirkic Marjanovic MS, Vucicevic LM, Despotovic AR, Stamenkovic MM, Janjetovic KD. Dual anticancer role of metformin: an old drug regulating AMPK dependent/independent pathways in metabolic, oncogenic/tumorsuppresing and immunity context. Am J Cancer Res. 2021;11:5625-43
36. Ross FA, MacKintosh C, Hardie DG. AMP-activated protein kinase: a cellular energy sensor that comes in 12 flavours. FEBS J. 2016;283:2987-3001
37. Zhang YL, Guo H, Zhang CS, Lin SY, Yin Z, Peng Y. et al. AMP as a low-energy charge signal autonomously initiates assembly of AXIN-AMPK-LKB1 complex for AMPK activation. Cell Metab. 2013;18:546-55
38. Zhang CS, Jiang B, Li M, Zhu M, Peng Y, Zhang YL. et al. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metab. 2014;20:526-40
39. Marcelo KL, Means AR, York B. The Ca(2+)/Calmodulin/CaMKK2 Axis: Nature's Metabolic CaMshaft. Trends Endocrinol Metab. 2016;27:706-18
40. Song L, Wang L, Hou Y, Zhou J, Chen C, Ye X. et al. FGF4 protects the liver from nonalcoholic fatty liver disease by activating the AMP-activated protein kinase-Caspase 6 signal axis. Hepatology. 2022;76:1105-20
41. Hung C-M, Lombardo PS, Malik N, Brun SN, Hellberg K, Van Nostrand JL. et al. AMPK/ULK1-mediated phosphorylation of Parkin ACT domain mediates an early step in mitophagy. Sci Adv. 2021;7:eabg4544
42. Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017-22
43. Akce M, Farran B, Switchenko JM, Rupji M, Kang S, Khalil L. et al. Phase II trial of nivolumab and metformin in patients with treatment-refractory microsatellite stable metastatic colorectal cancer. J Immunother Cancer. 2023;11:e007235
44. Wang S, Lin Y, Xiong X, Wang L, Guo Y, Chen Y. et al. Low-Dose Metformin Reprograms the Tumor Immune Microenvironment in Human Esophageal Cancer: Results of a Phase II Clinical Trial. Clin Cancer Res. 2020;26:4921-32
45. Afzal MZ, Mercado RR, Shirai K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. J Immunother Cancer. 2018;6:64
46. Pereira FV, Melo ACL, Low JS, de Castro Í A, Braga TT, Almeida DC. et al. Metformin exerts antitumor activity via induction of multiple death pathways in tumor cells and activation of a protective immune response. Oncotarget. 2018;9:25808-25
47. Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20:74-88
48. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS. et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214-26
49. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V. et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684
50. Zhou J, Ji J, Li X, Zhang Y, Gu L, Zheng X. et al. Homomultivalent Polymeric Nanotraps Disturb Lipid Metabolism Homeostasis and Tune Pyroptosis in Cancer. Adv Mater. 2024;36:e2312528
51. Du A, Wang Z, Huang T, Xue S, Jiang C, Qiu G, Yuan K. Fatty acids in cancer: Metabolic functions and potential treatment. MedComm-Oncol. 2023;2:e25
52. Peterson Timothy R, Sengupta Shomit S, Harris Thurl E, Carmack Anne E, Kang Seong A, Balderas E. et al. mTOR Complex 1 Regulates Lipin 1 Localization to Control the SREBP Pathway. Cell. 2011;146:408-20
53. Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP. et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649-54
54. Liu S, Jing F, Yu C, Gao L, Qin Y, Zhao J. AICAR-Induced Activation of AMPK Inhibits TSH/SREBP-2/HMGCR Pathway in Liver. PLoS One. 2015;10:e0124951
55. Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B. et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:10
56. Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S. et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res. 2017;23:1920-8
57. Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO. et al. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Mol Cell. 2018;71:606-20
58. Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer. 2011;11:393-410
59. Guo X, Xue H, Shao Q, Wang J, Guo X, Chen X. et al. Hypoxia promotes glioma-associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF-beta and M-CSFR. Oncotarget. 2016;7:80521
60. Hatfield SM, Kjaergaard J, Lukashev D, Schreiber TH, Belikoff B, Abbott R. et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci Transl Med. 2015;7:277ra30
61. Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665-74
62. Vancura A, Bu P, Bhagwat M, Zeng J, Vancurova I. Metformin as an anticancer agent. Trends Pharmacol Sci. 2018;39:867-78
63. Geltink RIK, Kyle RL, Pearce EL. Unraveling the Complex Interplay Between T Cell Metabolism and Function. Annu Rev Immunol. 2018;36:461-88
64. van der Windt GJ, O'Sullivan D, Everts B, Huang SC, Buck MD, Curtis JD. et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc Natl Acad Sci U S A. 2013;110:14336-41
65. Rolf J, Zarrouk M, Finlay DK, Foretz M, Viollet B, Cantrell DA. AMPKα1: a glucose sensor that controls CD8 T-cell memory. Eur J Immunol. 2013;43:889-96
66. Comerford SA, Huang Z, Du X, Wang Y, Cai L, Witkiewicz AK. et al. Acetate dependence of tumors. Cell. 2014;159:1591-602
67. Chao Y, Wei T, Li Q, Liu B, Hao Y, Chen M. et al. Metformin-containing hydrogel scaffold to augment CAR-T therapy against post-surgical solid tumors. Biomaterials. 2023;295:122052
68. Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103-7
69. Hunt EG, Hurst KE, Riesenberg BP, Kennedy AS, Gandy EJ, Andrews AM. et al. Acetyl-CoA carboxylase obstructs CD8(+) T cell lipid utilization in the tumor microenvironment. Cell Metab. 2024;36:969-83
70. Zordoky BNM, Nagendran J, Pulinilkunnil T, Kienesberger PC, Masson G, Waller TJ. et al. AMPK-Dependent Inhibitory Phosphorylation of ACC Is Not Essential for Maintaining Myocardial Fatty Acid Oxidation. Circ Res. 2014;115:518-24
71. O'Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD. et al. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41:75-88
72. Lettieri Barbato D, Tatulli G, Aquilano K, Ciriolo MR. FoxO1 controls lysosomal acid lipase in adipocytes: implication of lipophagy during nutrient restriction and metformin treatment. Cell Death Dis. 2013;4:e861
73. Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC. et al. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity. 2016;45:374-88
74. Chamoto K, Chowdhury PS, Kumar A, Sonomura K, Matsuda F, Fagarasan S, Honjo T. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc Natl Acad Sci U S A. 2017;114:E761-E70
75. Zhang Z, Li F, Tian Y, Cao L, Gao Q, Zhang C. et al. Metformin Enhances the Antitumor Activity of CD8(+) T Lymphocytes via the AMPK-miR-107-Eomes-PD-1 Pathway. J Immunol. 2020;204:2575-88
76. Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL. et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015;75:296-305
77. Jeong H, Kim S, Hong B-J, Lee C-J, Kim Y-E, Bok S. et al. Tumor-associated macrophages enhance tumor hypoxia and aerobic glycolysis. Cancer Res. 2019;79:795-806
78. Uehara T, Eikawa S, Nishida M, Kunisada Y, Yoshida A, Fujiwara T. et al. Metformin induces CD11b+-cell-mediated growth inhibition of an osteosarcoma: implications for metabolic reprogramming of myeloid cells and anti-tumor effects. Int Immunol. 2019;31:187-98
79. Pilon G, Dallaire P, Marette A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J Biol Chem. 2004;279:20767-74
80. Zhu YP, Brown JR, Sag D, Zhang L, Suttles J. Adenosine 5'-monophosphate-activated protein kinase regulates IL-10-mediated anti-inflammatory signaling pathways in macrophages. J Immunol. 2015;194:584-94
81. Yang F, Qin Y, Wang Y, Meng S, Xian H, Che H. et al. Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int J Biol Sci. 2019;15:1010
82. Chiang CF, Chao TT, Su YF, Hsu CC, Chien CY, Chiu KC. et al. Metformin-treated cancer cells modulate macrophage polarization through AMPK-NF-κB signaling. Oncotarget. 2017;8:20706-18
83. Ding L, Liang G, Yao Z, Zhang J, Liu R, Chen H. et al. Metformin prevents cancer metastasis by inhibiting M2-like polarization of tumor associated macrophages. Oncotarget. 2015;6:36441-55
84. Li Z, Zhang Q, Li Z, Ren L, Pan D, Gong Q. et al. Branched glycopolymer prodrug-derived nanoassembly combined with a STING agonist activates an immuno-supportive status to boost anti-PD-L1 antibody therapy. Acta Pharm Sin B. 2024;14:2194-209
85. Zhang Y, Fang Z, Pan D, Li Y, Zhou J, Chen H. et al. Dendritic Polymer-Based Nanomedicines Remodel the Tumor Stroma: Improve Drug Penetration and Enhance Antitumor Immune Response. Adv Mater. 2024;36:e2401304
86. Zheng L, Yang W, Wu F, Wang C, Yu L, Tang L. et al. Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin Cancer Res. 2013;19:5372-80
87. Duan W, Ding Y, Yu X, Ma D, Yang B, Li Y. et al. Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting Treg production. Am J Transl Res. 2019;11:2393-402
88. Kunisada Y, Eikawa S, Tomonobu N, Domae S, Uehara T, Hori S. et al. Attenuation of CD4(+)CD25(+) Regulatory T Cells in the Tumor Microenvironment by Metformin, a Type 2 Diabetes Drug. EBioMedicine. 2017;25:154-64
89. Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin. Cancer Biol. 2012;22:275-81
90. Xu P, Yin K, Tang X, Tian J, Zhang Y, Ma J. et al. Metformin inhibits the function of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Biomed Pharmacother. 2019;120:109458
91. Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z. et al. Metformin-Induced Reduction of CD39 and CD73 Blocks Myeloid-Derived Suppressor Cell Activity in Patients with Ovarian Cancer. Cancer Res. 2018;78:1779-91
92. Trillo-Tinoco J, Sierra RA, Mohamed E, Cao Y, de Mingo-Pulido Á, Gilvary DL. et al. AMPK Alpha-1 Intrinsically Regulates the Function and Differentiation of Tumor Myeloid-Derived Suppressor Cells. Cancer Res. 2019;79:5034-47
93. Li Y, Shen X, Ding H, Zhang Y, Pan D, Su L. et al. Dendritic nanomedicine enhances chemo-immunotherapy by disturbing metabolism of cancer-associated fibroblasts for deep penetration and activating function of immune cells. Acta Pharm Sin B. 2024;14:3680-96
94. Meng X-m, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325-38
95. Han H, Hou Y, Chen X, Zhang P, Kang M, Jin Q. et al. Metformin-Induced Stromal Depletion to Enhance the Penetration of Gemcitabine-Loaded Magnetic Nanoparticles for Pancreatic Cancer Targeted Therapy. J Am Chem Soc. 2020;142:4944-54
96. Saini N, Yang X. Metformin as an anti-cancer agent: actions and mechanisms targeting cancer stem cells. Acta Biochim Biophys Sin. 2018;50:133-43
97. Nong C, Guan P, Li L, Zhang H, Hu H. Tumor immunotherapy: Mechanisms and clinical applications. MedComm-Oncol. 2022;1:e8
98. Haanen JB. Immunotherapy of melanoma. EJC Suppl. 2013;11:97-105
99. Chen Y, Zhou Q, Jia Z, Cheng N, Zhang S, Chen W, Wang L. Enhancing cancer immunotherapy: Nanotechnology-mediated immunotherapy overcoming immunosuppression. Acta Pharm Sin B. 2024;14:3834-54
100. Spoerl S, Gerken M, Schimnitz S, Taxis J, Fischer R, Lindner SR. et al. Prognostic Relevance of Type 2 Diabetes and Metformin Treatment in Head and Neck Melanoma: Results from a Population-Based Cohort Study. Curr Oncol. 2022;29:9660-70
101. Kennedy OJ, Kicinski M, Valpione S, Gandini S, Suciu S, Blank CU. et al. Prognostic and predictive value of metformin in the European Organisation for Research and Treatment of Cancer 1325/KEYNOTE-054 phase III trial of pembrolizumab versus placebo in resected high-risk stage III melanoma. Eur J Cancer. 2023;189:112900
102. Krakowski I, Häbel H, Nielsen K, Ingvar C, Andersson TML, Girnita A. et al. Association of metformin use and survival in patients with cutaneous melanoma and diabetes. Br J Dermatol. 2023;188:32-40
103. Imperiale TF, Glowinski EA, Lin-Cooper C, Larkin GN, Rogge JD, Ransohoff DF. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med. 2008;359:1218-24
104. Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E. et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17:475-83
105. Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A. et al. Breast Cancer Statistics, 2022. CA Cancer J Clin. 2022;72:524-41
106. Ye F, Dewanjee S, Li Y, Jha NK, Chen ZS, Kumar A. et al. Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer. Mol Cancer. 2023;22:105
107. Goodwin PJ, Chen BE, Gelmon KA, Whelan TJ, Ennis M, Lemieux J. et al. Effect of Metformin vs Placebo on Invasive Disease-Free Survival in Patients With Breast Cancer: The MA.32 Randomized Clinical Trial. JAMA. 2022;327:1963-73
108. Subbiah V, Coleman N, Piha-Paul SA, Tsimberidou AM, Janku F, Rodon J. et al. Phase I Study of mTORC1/2 Inhibitor Sapanisertib (CB-228/TAK-228) in Combination with Metformin in Patients with mTOR/AKT/PI3K Pathway Alterations and Advanced Solid Malignancies. Cancer Res Commun. 2024;4:378-87
109. Mark M, Klingbiel D, Mey U, Winterhalder R, Rothermundt C, Gillessen S. et al. Impact of Addition of Metformin to Abiraterone in Metastatic Castration-Resistant Prostate Cancer Patients With Disease Progressing While Receiving Abiraterone Treatment (MetAb-Pro): Phase 2 Pilot Study. Clin Genitourin Cancer. 2019;17:e323-e8
110. Kim J, Lim W, Kim EK, Kim MK, Paik NS, Jeong SS. et al. Phase II randomized trial of neoadjuvant metformin plus letrozole versus placebo plus letrozole for estrogen receptor positive postmenopausal breast cancer (METEOR). BMC Cancer. 2014;14:170
111. Pujalte Martin M, Borchiellini D, Thamphya B, Guillot A, Paoli JB, Besson D. et al. TAXOMET: A French Prospective Multicentric Randomized Phase II Study of Docetaxel Plus Metformin Versus Docetaxel Plus Placebo in Metastatic Castration-Resistant Prostate Cancer. Clin Genitourin Cancer. 2021;19:501-9
112. El-Attar AA, Ibrahim OM, Alhassanin SA, Essa ES, Mostafa TM. Effect of metformin as an adjuvant therapy to letrozole on estradiol and other biomarkers involved in the pathogenesis of breast cancer in overweight and obese postmenopausal women: a pilot study. Eur J Clin Pharmacol. 2023;79:299-309
113. Pimentel I, Lohmann AE, Ennis M, Dowling RJO, Cescon D, Elser C. et al. A phase II randomized clinical trial of the effect of metformin versus placebo on progression-free survival in women with metastatic breast cancer receiving standard chemotherapy. Breast. 2019;48:17-23
114. O'Connor L, Bailey-Whyte M, Bhattacharya M, Butera G, Hardell KNL, Seidenberg AB. et al. Association of metformin use and cancer incidence: a systematic review and meta-analysis. J Natl Cancer Inst. 2024;116:518-29
115. He L. Metformin and systemic metabolism. Trends Pharmacol Sci. 2020;41:868-81
116. Khan AS, Frigo DE. A spatiotemporal hypothesis for the regulation, role, and targeting of AMPK in prostate cancer. Nat Rev Urol. 2017;14:164-80
117. Lee JE, Lim JH, Hong YK, Yang SH. High-Dose Metformin Plus Temozolomide Shows Increased Anti-tumor Effects in Glioblastoma In Vitro and In vivo Compared with Monotherapy. Cancer Res Treat. 2018;50:1331-42
118. Stang M, Wysowski DK, Butler-Jones D. Incidence of lactic acidosis in metformin users. Diabetes Care. 1999;22:925-7
119. Triggle CR, Mohammed I, Bshesh K, Marei I, Ye K, Ding H. et al. Metformin: Is it a drug for all reasons and diseases? Metabolism. 2022;133:155223
120. Song Y, Du Y, Hu C, Lei L, Yang L, Wang X. et al. Metformin-Mediated Immunosuppressive Microenvironment Remodeling in Combination with Chemotherapy via a Spatial-Specific Multi-Responsive Carrier-Free Self-Assembled Nanoparticle. Adv Funct Mater. 2024;34:2316145
121. Wang B, Hu S, Teng Y, Chen J, Wang H, Xu Y. et al. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct Target Ther. 2024;9:200
122. Hu C, He X, Chen Y, Yang X, Qin L, Lei T. et al. Metformin Mediated PD-L1 Downregulation in Combination with Photodynamic-Immunotherapy for Treatment of Breast Cancer. Adv Funct Mater. 2021;31:2007149
123. Yang T, Yu S, Liu L, Sun Y, Lan Y, Ma X. et al. Dual polymeric prodrug co-assembled nanoparticles with precise ratiometric co-delivery of cisplatin and metformin for lung cancer chemoimmunotherapy. Biomater Sci. 2020;8:5698-714
124. Mei Y, Tang L, Zhang L, Hu J, Zhang Z, He S. et al. A minimally designed PD-L1-targeted nanocomposite for positive feedback-based multimodal cancer therapy. Mater Today. 2022;60:52-68
125. Zhao Y, Wang W, Guo S, Wang Y, Miao L, Xiong Y, Huang L. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat Commun. 2016;7:11822
126. Luo L, Li X, Zhang J, Zhu C, Jiang M, Luo Z. et al. Enhanced immune memory through a constant photothermal-metabolism regulation for cancer prevention and treatment. Biomaterials. 2021;270:120678
127. Javidfar S, Pilehvar-Soltanahmadi Y, Farajzadeh R, Lotfi-Attari J, Shafiei-Irannejad V, Hashemi M, Zarghami N. The inhibitory effects of nano-encapsulated metformin on growth and hTERT expression in breast cancer cells. J Drug Del Sci Tech. 2018;43:19-26
128. Jafari-Gharabaghlou D, Dadashpour M, khanghah OJ, Salmani-Javan E, Zarghami N. Potentiation of Folate-Functionalized PLGA-PEG nanoparticles loaded with metformin for the treatment of breast Cancer: possible clinical application. Mol Biol Rep. 2023;50:3023-33
129. Xiong Y, Song W, Shen L, Wang Y, Zhang J, Hu M. et al. Oral Metformin and Polymetformin Reprogram Immunosuppressive Microenvironment and Boost Immune Checkpoint Inhibitor Therapy in Colorectal Cancer. Adv Ther. 2020;3:2000168
130. Jiang T, Chen L, Huang Y, Wang J, Xu M, Zhou S. et al. Metformin and Docosahexaenoic Acid Hybrid Micelles for Premetastatic Niche Modulation and Tumor Metastasis Suppression. Nano Lett. 2019;19:3548-62
131. Zhu H, Ma K, Ruan R, Yang C, Yan A, Li J. et al. Tumor-targeted self-assembled micelles reducing PD-L1 expression combined with ICIs to enhance chemo-immunotherapy of TNBC. Chin Chem Lett. 2024;35:108536
132. Xiong W, Qi L, Jiang N, Zhao Q, Chen L, Jiang X. et al. Metformin Liposome-Mediated PD-L1 Downregulation for Amplifying the Photodynamic Immunotherapy Efficacy. ACS Appl Mater Interfaces. 2021;13:8026-41
133. Song X, Feng L, Liang C, Gao M, Song G, Liu Z. Liposomes co-loaded with metformin and chlorin e6 modulate tumor hypoxia during enhanced photodynamic therapy. Nano Res. 2017;10:1200-12
134. Song L, Hao Y, Wang C, Han Y, Zhu Y, Feng L. et al. Liposomal oxaliplatin prodrugs loaded with metformin potentiate immunotherapy for colorectal cancer. J Control Release. 2022;350:922-32
135. Tang L, Fu C, Liu H, Yin Y, Cao Y, Feng J. et al. Chemoimmunotherapeutic Nanogel for Pre- and Postsurgical Treatment of Malignant Melanoma by Reprogramming Tumor-Associated Macrophages. Nano Lett. 2024;24:1717-28
136. Tian H, Li W, Wang G, Tian Y, Yan J, Zhou S. et al. Self-Degradable Nanogels Reshape Immunosuppressive Tumor Microenvironment via Drug Repurposing Strategy to Reactivate Cytotoxic CD8(+) T Cells. Adv Sci (Weinh). 2023;10:e2301661
137. Meng X, Song J, Lei Y, Zhang X, Chen Z, Lu Z. et al. A metformin-based nanoreactor alleviates hypoxia and reduces ATP for cancer synergistic therapy. Biomater Sci. 2021;9:7456-70
138. Yang J, Zhang C, Chen X, Zhou D, Sun Z, Niu R. et al. Ultra-efficient radio-immunotherapy for reprogramming the hypoxic and immunosuppressive tumor microenvironment with durable innate immune memory. Biomaterials. 2023;302:122303
139. Tang L, Yin Y, Cao Y, Fu C, Liu H, Feng J. et al. Extracellular Vesicles-Derived Hybrid Nanoplatforms for Amplified CD47 Blockade-Based Cancer Immunotherapy. Adv Mater. 2023;35:2303835
140. Wei Z, Zhang X, Yong T, Bie N, Zhan G, Li X. et al. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat Commun. 2021;12:440
141. Wen Y, Liu Y, Chen C, Chi J, Zhong L, Zhao Y, Zhao Y. Metformin loaded porous particles with bio-microenvironment responsiveness for promoting tumor immunotherapy. Biomater Sci. 2021;9:2082-9
142. Chong L, Jiang Y-W, Wang D, Chang P, Xu K, Li J. Targeting and repolarizing M2-like tumor-associated macrophage-mediated MR imaging and tumor immunotherapy by biomimetic nanoparticles. J Nanobiotechnol. 2023;21:401
143. Bai Z, Guo L, Huang J, Li H, An G, Zheng H. et al. Biomimetic metal organic frameworks mediated by metformin and 1-MT for enhancing the synergistic treatment of photodynamic therapy, photothermal therapy and immunotherapy. Chem Eng J. 2024;479:147932
144. Wang Y, Tang Q, Wu R, Yang S, Geng Z, He P. et al. Metformin-Mediated Fast Charge-Reversal Nanohybrid for Deep Penetration Piezocatalysis-Augmented Chemodynamic Immunotherapy of Cancer. ACS Nano. 2024;18:6314-32
145. Dou Y, Zheng J, Kang J, Wang L, Huang D, Liu Y. et al. Mesoporous manganese nanocarrier target delivery metformin for the co-activation STING pathway to overcome immunotherapy resistance. iScience. 2024;27:110150
146. Gedawy A, Al-Salami H, Dass CR. Role of metformin in various pathologies: state-of-the-art microcapsules for improving its pharmacokinetics. Ther Deliv. 2020;11:733-53
147. Cetin M, Sahin S. Microparticulate and nanoparticulate drug delivery systems for metformin hydrochloride. Drug Deliv. 2016;23:2796-805
148. Mallik R, Chowdhury TA. Metformin in cancer. Diabetes Res Clin Pract. 2018;143:409-19
149. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101-24
150. Zhang Y, Xiao W, He S, Xia X, Yang W, Yang Z, Hu H, Wang Y, Wang X, Li H, Huang Y, Gao H. Lipid-mediated protein corona regulation with increased apolipoprotein A-I recruitment for glioma targeting. J Control Release. 2024;368:42-51
151. Li H, Feng Y, Luo Q, Li Z, Li X, Gan H. et al. Stimuli-activatable nanomedicine meets cancer theranostics. Theranostics. 2023;13:5386-417
152. Matsumura Y, Oda T, Maeda H. General mechanism of intratumor accumulation of macromolecules: advantage of macromolecular therapeutics. Gan To Kagaku Ryoho. 1987;14:821-9
153. Kingston BR, Lin ZP, Ouyang B, MacMillan P, Ngai J, Syed AM. et al. Specific Endothelial Cells Govern Nanoparticle Entry into Solid Tumors. ACS Nano. 2021;15:14080-94
154. Naumenko VA, Vlasova KY, Garanina AS, Melnikov PA, Potashnikova DM, Vishnevskiy DA. et al. Extravasating Neutrophils Open Vascular Barrier and Improve Liposomes Delivery to Tumors. ACS Nano. 2019;13:12599-612
155. Nguyen LNM, Ngo W, Lin ZP, Sindhwani S, MacMillan P, Mladjenovic SM, Chan WCW. The mechanisms of nanoparticle delivery to solid tumours. Nat Rev Bioeng. 2024;2:201-13
156. Sykes EA, Dai Q, Sarsons CD, Chen J, Rocheleau JV, Hwang DM. et al. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc Natl Acad Sci U S A. 2016;113:E1142-E51
157. Pan H, Zheng M, Ma A, Liu L, Cai L. Cell/bacteria-based bioactive materials for cancer immune modulation and precision therapy. Adv Mater. 2021;33:2100241
158. Agnello L, Camorani S, Fedele M, Cerchia L. Aptamers and antibodies: rivals or allies in cancer targeted therapy? Explor Target Anti-tumor Ther. 2021;2:107
159. Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014
160. Elci SG, Jiang Y, Yan B, Kim ST, Saha K, Moyano DF. et al. Surface charge controls the suborgan biodistributions of gold nanoparticles. ACS Nano. 2016;10:5536-42
161. Zhang Z, Xu X, Du J, Chen X, Xue Y, Zhang J. et al. Redox-responsive polymer micelles co-encapsulating immune checkpoint inhibitors and chemotherapeutic agents for glioblastoma therapy. Nat Commun. 2024;15:1118
162. Kong H, Zheng C, Yi K, Mintz RL, Lao Y-H, Tao Y, Li M. An antifouling membrane-fusogenic liposome for effective intracellular delivery in vivo. Nat Commun. 2024;15:4267
163. Liu X, Zhuang Y, Huang W, Wu Z, Chen Y, Shan Q. et al. Interventional hydrogel microsphere vaccine as an immune amplifier for activated antitumour immunity after ablation therapy. Nat Commun. 2023;14:4106
164. Pelaz B, Alexiou C, Alvarez-Puebla RA, Alves F, Andrews AM, Ashraf S. et al. Diverse Applications of Nanomedicine. ACS Nano. 2017;11:2313-81
165. Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS. et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16:71
166. Shakeri S, Ashrafizadeh M, Zarrabi A, Roghanian R, Afshar EG, Pardakhty A. et al. Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics. Biomedicines. 2020;8:13
167. Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14:85
168. Hassani N, Jafari-Gharabaghlou D, Dadashpour M, Zarghami N. The Effect of Dual Bioactive Compounds Artemisinin and Metformin Co-loaded in PLGA-PEG Nano-particles on Breast Cancer Cell lines: Potential Apoptotic and Anti-proliferative Action. Appl Biochem Biotech. 2022;194:4930-45
169. Pendhari J, Savla H, Bethala D, Vaidya S, Shinde U, Menon M. Mitochondria targeted liposomes of metformin for improved anticancer activity: Preparation and evaluation. J Drug Del Sci Tech. 2022;76:103795
170. Shukla SK, Kulkarni NS, Chan A, Parvathaneni V, Farrales P, Muth A, Gupta V. Metformin-encapsulated liposome delivery system: an effective treatment approach against breast cancer. Pharmaceutics. 2019;11:559
171. Ghezzi M, Pescina S, Padula C, Santi P, Del Favero E, Cantù L, Nicoli S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J Control Release. 2021;332:312-36
172. Xiao Y, Wang S, Zong Q, Yin Z. Co-delivery of metformin and paclitaxel via folate-modified pH-sensitive micelles for enhanced anti-tumor efficacy. AAPS PharmSciTech. 2018;19:2395-406
173. Jose J, Athira V, Michel H, Hafeela A, Bhat SG, Thomas S, Maria LP. Hydrogels: An overview of the history, classification, principles, applications, and kinetics. Sustainable Hydrogels. 2023:1-22
174. Migdadi EM, Courtenay AJ, Tekko IA, McCrudden MTC, Kearney M-C, McAlister E. et al. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J Control Release. 2018;285:142-51
175. Sun M, Yue T, Wang C, Fan Z, Gazit E, Du J. Ultrasound-responsive peptide nanogels to balance conflicting requirements for deep tumor penetration and prolonged blood circulation. ACS Nano. 2022;16:9183-94
176. Huang X, He T, Liang X, Xiang Z, Liu C, Zhou S. et al. Advances and applications of nanoparticles in cancer therapy. MedComm-Oncol. 2024;3:e67
177. Tartari F, Santoni M, Burattini L, Mazzanti P, Onofri A, Berardi R. Economic sustainability of anti-PD-1 agents nivolumab and pembrolizumab in cancer patients: Recent insights and future challenges. Cancer Treat Rev. 2016;48:20-4
178. Li X, Duan Z, Li Z, Gu L, Li Y, Gong Q. et al. Dendritic polymer-functionalized nanomedicine potentiates immunotherapy via lethal energy crisis-induced PD-L1 degradation. Biomaterials. 2023;302:122294
179. Li H, Gong Q, Luo K. Biomarker-driven molecular imaging probes in radiotherapy. Theranostics. 2024;14:4127-46
180. Wu M, Hou P, Dong L, Cai L, Chen Z, Zhao M, Li J. Manganese dioxide nanosheets: from preparation to biomedical applications. Int J Nanomedicine. 2019;14:4781-800
181. Cocozza F, Grisard E, Martin-Jaular L, Mathieu M, Théry C. SnapShot: Extracellular Vesicles. Cell. 2020;182:262 -
182. Fan X, Song X, Chen W, Liang H, Nakatsukasa H, Zhang D. cGAS-STING signaling in cancer: Regulation and therapeutic targeting. MedComm-Oncol. 2023;2:e49
183. Wang Z, Lu C, Zhang K, Lin C, Wu F, Tang X. et al. Metformin Combining PD-1 Inhibitor Enhanced Anti-Tumor Efficacy in STK11 Mutant Lung Cancer Through AXIN-1-Dependent Inhibition of STING Ubiquitination. Front Mol Biosci. 2022;9:780200
184. Li W, Melton DW. Cisplatin regulates the MAPK kinase pathway to induce increased expression of DNA repair gene ERCC1 and increase melanoma chemoresistance. Oncogene. 2012;31:2412-22
185. Jiang Y, Dong Y, Luo Y, Jiang S, Meng FL, Tan M. et al. AMPK-mediated phosphorylation on 53BP1 promotes c-NHEJ. Cell Rep. 2021;34:108713
186. Li S, Lavagnino Z, Lemacon D, Kong L, Ustione A, Ng X. et al. Ca(2+)-Stimulated AMPK-Dependent Phosphorylation of Exo1 Protects Stressed Replication Forks from Aberrant Resection. Mol Cell. 2019;74:1123-37
187. Saber MM, Al-mahallawi AM, Nassar NN, Stork B, Shouman SA. Targeting colorectal cancer cell metabolism through development of cisplatin and metformin nano-cubosomes. BMC Cancer. 2018;18:822
188. Corrado M, Pearce EL. Targeting memory T cell metabolism to improve immunity. J Clin Investig. 2022;132:e148546
189. Wang Y, An H, Liu T, Qin C, Sesaki H, Guo S. et al. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Reports. 2019;29:1511-23
190. Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S. et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509-24
191. Hao M, Hou S, Li W, Li K, Xue L, Hu Q. et al. Combination of metabolic intervention and T cell therapy enhances solid tumor immunotherapy. Sci Transl. 2020;12:eaaz6667
192. Ye Q-N, Zhu L, Liang J, Zhao D-K, Tian T-Y, Fan Y-N. et al. Orchestrating NK and T cells via tri-specific nano-antibodies for synergistic antitumor immunity. Nat Commun. 2024;15:6211
193. Dammeijer F, van Gulijk M, Mulder EE, Lukkes M, Klaase L, van den Bosch T. et al. The PD-1/PD-L1-Checkpoint Restrains T cell Immunity in Tumor-Draining Lymph Nodes. Cancer Cell. 2020;38:685-700
194. Gebhardt T, Park SL, Parish IA. Stem-like exhausted and memory CD8+ T cells in cancer. Nat Rev Cancer. 2023;23:780-98
195. Scharping NE, Rivadeneira DB, Menk AV, Vignali PDA, Ford BR, Rittenhouse NL. et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat Immunol. 2021;22:205-15
196. Hou X, Pan D, Zhong D, Gong Q, Luo K. Dendronized Polymer-Derived Nanomedicines for Mitochondrial Dynamics Regulation and Immune Modulation. Adv Mater. 2024;36:e2400582
197. Dumauthioz N, Tschumi B, Wenes M, Marti B, Wang H, Franco F. et al. Enforced PGC-1α expression promotes CD8 T cell fitness, memory formation and antitumor immunity. Cell Mol Immunol. 2021;18:1761-71
198. Qin L, Cao J, Shao K, Tong F, Yang Z, Lei T, Wang Y, Hu C, Umeshappa CS, Gao H, Peppas NA. A tumor-to-lymph procedure navigated versatile gel system for combinatorial therapy against tumor recurrence and metastasis. Sci Adv. 2020;6:eabb3116
199. Li Y, Tong F, Wang Y, Wang J, Wu M, Li H. et al. In situ tumor vaccine with optimized nanoadjuvants and lymph node targeting capacity to treat ovarian cancer and metastases. Acta Pharm Sin B. 2024;14:4102-17
200. Peng Y, Gao Y, Yang C, Guo R, Shi X, Cao X. Low-Molecular-Weight Poly(ethylenimine) Nanogels Loaded with Ultrasmall Iron Oxide Nanoparticles for T(1)-Weighted MR Imaging-Guided Gene Therapy of Sarcoma. ACS Appl Mater Interfaces. 2021;13:27806-13
Author contact
![]() Corresponding authors: West China Hospital, Sichuan University, China. E-mail addresses: qiyonggongorg.cn (Q. Gong), luokuiedu.cn (K. Luo).
Corresponding authors: West China Hospital, Sichuan University, China. E-mail addresses: qiyonggongorg.cn (Q. Gong), luokuiedu.cn (K. Luo).
 Global reach, higher impact
Global reach, higher impact