13.3
Impact Factor
Theranostics 2025; 15(3):915-942. doi:10.7150/thno.103266 This issue Cite
Review
Advances in adhesive hydrogels applied for ophthalmology: An overview focused on the treatment
1. Department of Ophthalmology, The First Affiliated Hospital of University of South China, Hengyang Medical School, University of South China, Hengyang Hunan 421001, China.
2. Xiamen University affiliated Xiamen Eye Center, Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, Fujian Engineering and Research Center of Eye Regenerative Medicine, Eye Institute of Xiamen University, School of Medicine, Xiamen University, Xiamen Fujian 361005, China.
Received 2024-9-4; Accepted 2024-11-18; Published 2025-1-1
Abstract
Adhesive hydrogels, composed of hydrophilic polymers arranged in a three-dimensional network, have emerged as a pivotal innovation in ophthalmology due to their ability to securely adhere to ocular tissues while providing sustained therapeutic effects. The eye, with its delicate structure and specific needs, presents unique challenges for drug delivery and tissue regeneration. This review explores the transformative potential of adhesive hydrogels in addressing these challenges across a range of ocular conditions, including corneal injuries, cataracts, glaucoma, vitreoretinal disorders, and ocular trauma. By detailing the mechanisms of polymerization and adhesion, this paper highlights how these materials can be customized for specific ophthalmic applications, offering insights into their current use and future possibilities. The emphasis is placed on the clinical significance and future directions of adhesive hydrogels in advancing ophthalmic therapy, potentially revolutionizing the treatment of complex eye diseases.
Keywords: adhesive hydrogels, ophthalmology, polymerization and adhesion mechanisms, treatment of ocular diseases
1. Introduction
The eye is a vital organ and the sole access to vision. Numerous ocular diseases, including corneal and conjunctival trauma, chemical damage, ocular surface diseases such as dry eye [1, 2], refractive errors [3, 4], cataracts [5, 6], glaucoma [7, 8], diabetic retinopathy [9, 10], and retinal detachment [11, 12], affect vision quality to varying degrees. The quality of vision is closely related to a person's quality of life. The World Health Organization (WHO) reported that globally, at least 2.2 billion people suffer from impaired vision at near or far distances [13]. At least 1 billion of these cases could have been prevented or are yet to be addressed. Visual impairment also imposes an enormous economic burden, with an estimated annual global productivity loss of $411 billion [13].
Currently, ophthalmic treatments are primarily categorized into medications and surgery. For the anterior segment of the eye, medications are mainly administered as eye drops. However, the efficacy of eye drops is limited by their short duration of action, due to the physical flushing action of tears and blinking, which necessitates frequent applications or higher drug concentrations to achieve therapeutic levels. This often results in poor patient compliance and inconsistent medication adherence. For the posterior segment of the eye, treatments typically involve invasive vitreous injections, requiring multiple doses to compensate for rapid drug metabolism. This approach is associated with a high risk of ocular complications and potential retinal toxicity [14, 15]. On the other hand, the surgical treatment of refractive errors, cataracts, glaucoma, and retinal detachment essentially involves ocular wound repair, including the healing of corneal, conjunctival, scleral and retinal wounds. Tissue wound repair requires a certain amount of time, and when patient compliance is poor, it is prone to wound dehiscence, secondary scarring, inflammation, and rejection due to the presence of suture nodules [16-18].
Hydrogels are hydrophilic molecules with three-dimensional (3D) networks that absorb and retain large amounts of water [19]. In the last decade, hydrogels have been widely used for ocular drug delivery and wound repair, with several products already on the market, such as ReSure® [20], DEXTENZA® [21]. Notably, hydrogels with strong adhesive properties have been developed for drug delivery and ocular tissue repair, offering new methods and ideas for clinical ocular therapy. Since ophthalmic tailored materials must ensure both the structural integrity of the eye and its refractive function, adhesive hydrogels for ocular applications need to possess the following characteristics: 1) excellent biocompatibility and non-toxicity; 2) a suitable surface microstructure to promote cell proliferation and migration; 3) strong adhesion in humid environments; 4) good light transmission; 5) adequate mechanical strength; 6) high stability against significant changes in the ocular environment; 7) simultaneous degradation during the healing process or easy removal after repair is complete; and 8) easy handling.
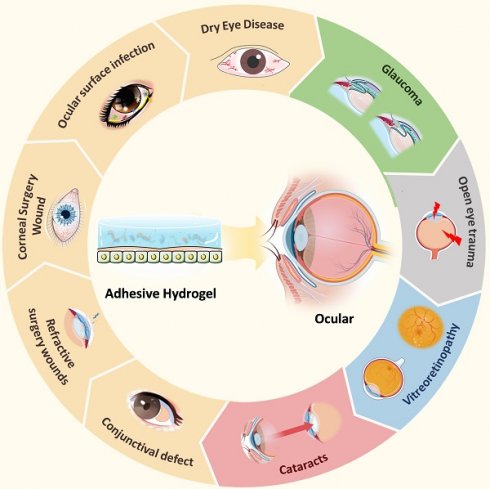
With advances in materials science, researchers have developed various multifunctional ocular adhesive hydrogels that address the challenges mentioned above. However, a comprehensive overview of their design and applications has been lacking. In this review, we present, for the first time, an in-depth analysis of the latest developments in adhesive hydrogels for various ocular tissues. We explore the polymerization and adhesion mechanisms of the currently studied adhesive hydrogels. The main section of the review introduces bioadhesive hydrogel materials specifically designed for different ocular diseases (Figure 1) and critically examines their advantages and limitations. Additionally, we summarize current research trends and offer insights into the future design and application of ocular adhesive hydrogels. The aim of this paper is to enhance the understanding of adhesive hydrogels for various ocular tissues, guide the improved design of these materials for diverse ocular applications, and stimulate progress in the development of ocular hydrogels.
Classification of adhesive hydrogels for ocular applications.
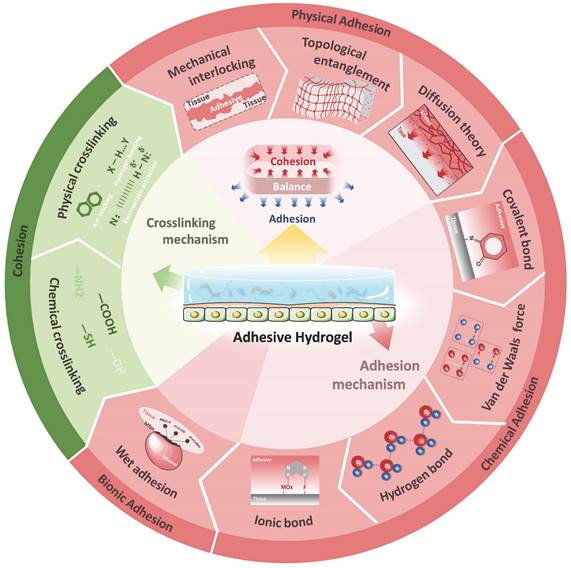
Crosslinking and adhesion mechanisms of adhesive hydrogels.
2. Overview of adhesive hydrogel
With the vigorous development of research, hydrogels have gradually evolved from a single network structure to a network structure with multiple interactions. Adhesive hydrogels have also evolved from basic adhesion to multifunctional adhesion with intelligent response. As a special class of hydrogels, this part will briefly describe the crosslinking mechanism of ocular adhesive hydrogels, focusing on the adhesion mechanism (Figure 2).
2.1 Crosslinking mechanisms for hydrogel
2.1.1 Physical crosslinking
The physical cross-linking method primarily involves physical effects (such as temperature, pressure, electric field, etc.) to induce polymer chain cross-linking, forming a network structure. This method typically does not require the addition of chemical crosslinking agents, making the preparation process relatively simple and environmentally friendly. However, most physical cross-linking is reversible and mechanically unstable.
Some polymer hydrogels undergo sol-gel transition due to changes in temperature affecting the entanglement of polymer chains, which is known as temperature-sensitive hydrogel [22]. The cornea, conjunctiva, and eyelid tissues, due to their exposure to the external environment, generally maintain a temperature of 32-34°C under normal conditions [23]. Commonly used temperature-sensitive polymers include poly N-isopropyl acrylamide (pNIPAAm) [24], poloxamer [25], chitosan [26] and Pluronic F127 [27], all of which can form gels at 37°C. Therefore, these materials can remain in a liquid state at room temperature and undergo a sol-gel transition upon contact with the eye surface, enhancing their adhesive properties.
2.1.2 Chemical crosslinking
Chemical crosslinking involves the formation of polymers through chemical reactions between various functional groups. Compared to hydrogels synthesized by physical crosslinking, those synthesized by chemical crosslinking methods exhibit better mechanical strength, tissue adhesion, and stability [28]. The commonly used cross-linkable groups include amino (-NH2), carboxyl (-COOH), sulfhydryl (-SH), aldehyde (-CHO) and hydroxyl (-OH). Common chemical crosslinking methods include aldimine condensation, the glutaraldehyde method, and the carbodiimide method. The gel structure formed by chemical crosslinking is generally irreversible unless the chemical bonds are broken. Therefore, this crosslinking method is more advantageous for designing robust adhesive hydrogels and hydrogel patches for ophthalmic applications.
2.2 Adhesion mechanism of hydrogel
The adhesive strength of hydrogels is influenced by two crucial factors: cohesion and adhesion. Cohesion refers to the internal force that binds the hydrogel network together, while adhesion measures the strength of the bond between the hydrogel adhesive and the surface of the target tissue [29]. Failures in adhesive hydrogels can often be traced back to cohesive failure caused by the rupture of interfacial adhesion or insufficient mechanical stability. Different adhesion mechanisms are suited to different application scenarios, and achieving a balance between cohesion and adhesion is critical. For the complex and tightly structured organ like the eye, hydrogels should be designed by combining various adhesion mechanisms according to the specific characteristics of the adhesion interface. The adhesion mechanisms of hydrogels are diverse and can generally be categorized into physical adhesion (mechanical interlocking, diffusion theory, topological entanglement), chemical adhesion (van der Waals forces, hydrogen bonds, ionic bonds and covalent bonds) and bionic adhesion (catechols, dopamine, and other wet adhesion mechanisms).
2.2.1 Physical adhesion
Mechanical interlocking is a traditional hydrogel adhesion strategy, first discovered on wood by McBain and Hopkins in 1925 [30]. Mechanical interlocking refers to the interlocking between the adhesive and the microrough structure on the surface of the bonded object [31]. According to diffusion theory, adhesion is established through the intermixing (or mutual diffusion) of molecules between the substrate and the adhesive to achieve mutual adhesion. Hydrogels can achieve interdiffusion between polymers through an interpenetrating network structure, enhancing their adhesion properties. Topological entanglement is an emerging approach for hydrogels with prefabricated networks, where the bonding polymer chains act as sutures to bind the adherends through topological connections.
Given the complex structure of ocular tissues, the application of physical adhesion requires consideration of the physical properties and structure of both the polymer and the adhesion interface. Healthy ocular tissues are smooth and tightly bound with a tear film, whereas in pathological states, the cornea and conjunctiva may become rough and edematous, making the tissue structure looser and the tear film uneven. Thus, polymer hydrogels can adaptively fill rough adhesion interfaces through mechanical interlocking and topological entanglement, promoting diffusion within loose tissue structures to increase adhesion, thereby achieving seamless repair or extending drug delivery time.
2.2.2 Chemical adhesion
At the molecular level, chemical adhesion arises from interactions between molecules, such as hydrogen bonds, van der Waals forces, ionic bonds and covalent bonds. Van der Waals forces, also known as intermolecular forces, exist in all polar or nonpolar molecules. These forces are generated by the temporary dipole moments produced by the instantaneous positions of electrons in the molecule. Van der Waals forces are particularly diminished in water, making them less significant for the adhesive properties of hydrogels. However, in ocular applications where external tissues are damaged, van der Waals forces can facilitate reversible adhesion to prevent fluid loss and provide a foundation for subsequent wound treatment.
Hydrogen bonds, stronger than van der Waals forces, involve interactions between a hydrogen atom and an electronegative atom like oxygen or nitrogen [32]. Since the eye is a highly hydrated organ, hydrogen bonding plays a crucial role in enhancing adhesion between hydrogels and moist or biological tissue surfaces, making them ideal for eye applications. Additionally, the reversibility of hydrogen bonds balances adhesion with flexibility, adapting hydrogels to ocular movement (blinking and rotation).
Ionic bonds, formed when atoms or chemical groups gain or lose electrons, typically form stronger bonds in moist environments and can break and reform under certain conditions, providing self-healing properties. This reversibility is especially useful for medical hydrogels in prolonged tissue contact, such as those used in the eye.
Covalent bonds, which form when atoms share electrons, create strong, stable connections between molecules [33]. Static covalent bonds, such as carbon, amide, siloxane, and carbon-nitrogen bonds, are suitable for applications requiring long-term adhesion (retinal tear repair and biosensors in the fundus). In contrast, dynamic covalent bonds, which can reversibly dissociate and reconfigure in response to environmental changes (pH, heat, light or redox), provide self-healing properties, making them valuable for smart hydrogels and drug delivery systems within the eye [34].
2.2.3 Bionic adhesion
Bionic adhesion mechanisms, often referred to as bio-inspired adhesion, are exemplified by various organisms that exhibit wet adhesion, including marine organisms (mussels, abalone) and amphibians (salamanders, tree frogs). These organisms achieve strong adhesion through chemical reactions or unique microstructures. Inspired by the remarkable adhesion properties of mussel foot proteins, the catechol fraction (dopamine) derived from mussels has gained significant attention [35, 36]. Most biological tissues have amino groups on their surfaces, and the eye is no exception. The hydroxyl structure of dopamine can form hydrogen bonds with amino groups at the tissue interface to enhance adhesion. Under certain conditions, DOPA's catechol structure can also form π-cation interactions and covalent bonds, enabling long-lasting adhesion. This capability has been applied in the development of hydrogels for ocular applications, such as retinal hole repair and bio-sensors.
Barnacles and mollusks, which can adhere in moist and variable marine environments, secrete adhesive proteins and polysaccharides, which can be integrated into hydrogel systems to create strong adhesives for treating ocular injuries, modifying the surfaces of ocular implants, and more.
In summary, the design and development of adhesive hydrogels necessitate a thorough consideration of the polymer network type, cross-linking mode, and adhesion mechanism. By rationally combining these factors, hydrogels can be tailored to meet the specific needs of their intended applications, particularly in the complex and dynamic environment of the eye.
3. Application of adhesive hydrogel in treatment of ocular disease
The eye is a remarkably intricate and delicate closed cavity structure. Anatomically, the eyeball is segmented into two distinct parts: the anterior segment and the posterior segment. The anterior segment encompasses the cornea, conjunctiva, aqueous humor, iris, and lens, whereas the posterior segment comprises the retina, choroid, sclera, optic nerve, and vitreous humor [37]. While each segment exhibits a unique organizational structure, both play a pivotal role in the development and maintenance of visual quality within the eye [38].
Adhesive hydrogel systems tailored to meet the demands of various diseases exhibit diverse functionalities and properties. For instance, in cases of dry eye, glaucoma, and ocular infectious diseases, hydrogels are designed to provide localized adhesion, extending the drug's efficacy and reducing the need for frequent administrations, thus enhancing patient compliance [39-41]. For corneal transplantation surgeries, pterygium excision, conjunctival mass removal, and cataract lens replacement, hydrogels focus on sealing wounds and promoting tissue repair [42, 43]. In the context of vitreoretinal diseases, hydrogels must not only facilitate the delivery of biologically active ocular substances but also ensure stability and adhesion within the retina [44-46]. Bearing these considerations in mind, we will delve into the adhesive hydrogel systems that have been explored based on the classification of ocular diseases.
3.1 Ocular surface diseases
Ocular surface diseases refer to diseases that impair the normal structure and function of the corneal conjunctival ocular surface. Here we will detail the research on adhesive hydrogels for the treatment of the corresponding diseases in five areas: dry eye, ocular surface infections, corneal wound surgery, refractive surgery wounds, and conjunctival defects (Table 1).
3.1.1 Dry eye
Dry eye disease (DED), a prevalent ocular condition, manifests as an alteration in the quality and quantity of tears stemming from diverse causes [47]. Traditional clinical medications for DED primarily consist of eye drops, which are prone to the mechanical movements of tears and eyelids, necessitating frequent administration [48]. Consequently, current research endeavors exploring adhesive hydrogels for DED have centered on extending the duration of drug efficacy on the ocular surface, aiming to enhance the treatment of this condition [49, 50].
Han et al. successfully formulated a thermoresponsive hydrogel loaded with tacrolimus (FK506), utilizing monofunctional polyhedral oligomeric sesquicarbophilic siloxanes (POSS), polyethylene glycol (PEG), polypropylene glycol (PPG) and polyurethane (MPEP) as its constituents (Figure 3A) [51]. Their results indicated that the MPOSS-PEG-PPG-FK506 (MPEP-FK506) hydrogel exhibited a significantly superior therapeutic effect on dry eye disease (DED) compared to commercial FK506 and PEG-PPG-FK506 (F127-FK506) hydrogels. Notably, the MPEP-FK506 hydrogel adhered uniformly to the cornea even after mice blinked. Furthermore, surface plasmon resonance (SPR) analysis revealed that the hydrophobic POSS groups within the copolymer interact strongly with the exposed hydrophobic domains surrounding the mucin in the cornea, contributing to the hydrogel's adhesion and extending the duration of drug action. This study not only improved the solubility of FK506 but also prolonged its therapeutic effect and enhanced drug utilization, thanks to the strong affinity between the POSS moiety and mucin. Future research could explore additional adhesion mechanisms of this polymer and determine if the POSS moiety possesses a similarly robust affinity for other proteins or tissues.
Drawing inspiration from barnacles' use of the amyloid system to create stable aqueous adhesive surfaces on solids, Qin et al. crafted a functional therapeutic contact lens coated with human lactoferrin (HLF) nanomembranes that encapsulate cyclosporine A (CsA) (Figure 3B) [52]. This innovative design allows for controlled release of CsA only upon application to the eye, resulting in a remarkable 82% increase in bioavailability compared to commercial CsA Restasis®. This study not only introduces a novel therapeutic method for ophthalmic drug delivery, but also holds the potential to accelerate research into biocompatible, wearable contact lens devices. Should the retention of the nanomembrane be detectable post-removal, such devices would offer a more economical solution for clinical applications.
Adhesive hydrogels for ocular anterior section applications.
| Application | Adherent tissue | Materials | Adhesion mechanism | Clinical research process | Ref. |
|---|---|---|---|---|---|
| Dry Eye | Cornea | MPEP(POSS-PEG-PPG) | Hydrogen bonds, hydrophobicity, mechanical interlocking | Pre-clinic | [51] |
| Contact lens | PEG | Wet adhesive | Approved | [52] | |
| Cornea | AF127, Cu2-x Se NPs | Ionic bonds, covalent bonds (Schiff base) | Pre-clinic | [53] | |
| Lacrimal duct | CMC | Mechanical interlocking, cohesion | Pre-clinic | [55] | |
| Cornea | PBA, CMC, GSH | Hydrogen bonds, covalent bonds | Pre-clinic | [60] | |
| Eye surface infection | Cornea | PAM, QCS, TA | Hydrogen bonds, electrostatic force, π-π stacking | Pre-clinic | [68] |
| Cornea | F127, PEG-PPG-PEG, PLGA-PEI-PEG | Cohesion, mechanical interlocking, hydrogen bonds | Pre-clinic | [69] | |
| Cornea | FNPs, ISG | Cohesion, ionic bonds, hydrogen bonds | Pre-clinic | [70] | |
| Cornea | Gel | Mechanical interlocking | Pre-clinic | [71] | |
| Cornea | GAMA, contact lenses (with carboxylic acid) | Covalent bonds | Pre-clinic | [72] | |
| Corneal wound surgery | Cornea | DPC-ECM | / | Approved | [78] |
| Cornea | DPC- ECM, alginate, DOPA | Covalent bonds, ionic bonds, crosslinked structure | Pre-clinic | [79] | |
| Cornea | HA-cyclodextrin, HA-adamantane | Covalent bonds, cross-linked structures | Pre-clinic | [80] | |
| Cornea | Fibrin | Fibrinogen polymerization | Approved | [85] | |
| Cornea | PEG | Cohesion, hydrogen bonds, mechanical interlocking | Approved | [20,86-88] | |
| Cornea | 4-arm-PEG-NHS, lysozyme | Covalent bonds, mechanical interlocking | Pre-clinic | [89] | |
| Cornea | Gel, TEA, N-VC | Hydrogen bonds, mechanical interlocking | Pre-clinic | [93] | |
| Cornea | GelMA, HAGM, PEGDA | Hydrogen bonds, covalent bonds, cross-linked structures, mechanical interlocking | Pre-clinic | [94] | |
| Cornea | Collagen I, F127DA, AF127,GelMA | Cross-linked structures, covalent bonds (Schiff base), hydrogen bonds, π-π stacking | Pre-clinic | [95] | |
| Cornea | GelMA, Odex | Dual network structure, covalent bonds (Schiff base), hydrogen bonds | Pre-clinic | [96] | |
| Cornea | GelMA, HA-NB | Wet adhesive, hydrogen bonds | Pre-clinic | [97] | |
| Cornea | Gel, DMA | Hydrogen bonds, covalent bonds | Pre-clinic | [98] | |
| Cornea | Gel, PEG, bovine stromal corneal extracellular matrix | Covalent bonding, double network structure | Pre-clinic | [99] | |
| Cornea | F127DA, HADA, DOPA | Wet adhesive, hydrogen bonds, crosslinked structure | Pre-clinic | [100] | |
| Cornea | AlgMA, ODex, dendritic polymers | Covalent bonding, inion bonds, triple-crosslinked double-network | Pre-clinic | [101] | |
| Refractive surgery wounds | Cornea | PEG | Cohesion, hydrogen bonds, mechanical interlocking effects | Approved | [105-107] |
| Conjunctival defect | Conjunctiva | Acrylic resins | Hydrogen bonds | Pre-clinic | [110] |
| Conjunctiva | PEG | Cohesion, hydrogen bonds, mechanical interlocking | Pre-clinic | [113] | |
| Conjunctiva | GelMA, OHA | Semi-interpenetrating polymer network, crosslinked structure, covalent bonds | Pre-clinic | [114] | |
| Conjunctiva, cornea | PEGDA, Alg-NHS, TA, Fe3+ | Hydrogen bonds, covalent bonds, inion bonds | Pre-clinic | [116] |
Representative diagram of adhesive hydrogels applied for dry eye disease. A) Effectiveness of an ocular adhesive polyhedral oligomeric silsesquioxane hybrid thermo-responsive FK506 hydrogel in a murine model of dry eye. Adapted with permission from [51], Copyright 2022 Elsevier. B) Instant Adhesion of Amyloid-like Nanofilms with Wet Surfaces. Adapted with permission from [52], Copyright 2022 American Chemical Society. C) A tissue-adhesive F127 hydrogel delivers antioxidative copper-selenide nanoparticles for the treatment of dry eye disease. Adapted with permission from [53], Copyright 2024 Elsevier. D) Mucoadhesive phenylboronic acid-grafted carboxymethyl cellulose hydrogels containing glutathione for treatment of corneal epithelial cells exposed to benzalkonium chloride. Adapted with permission from [61], Copyright 2024 Elsevier.
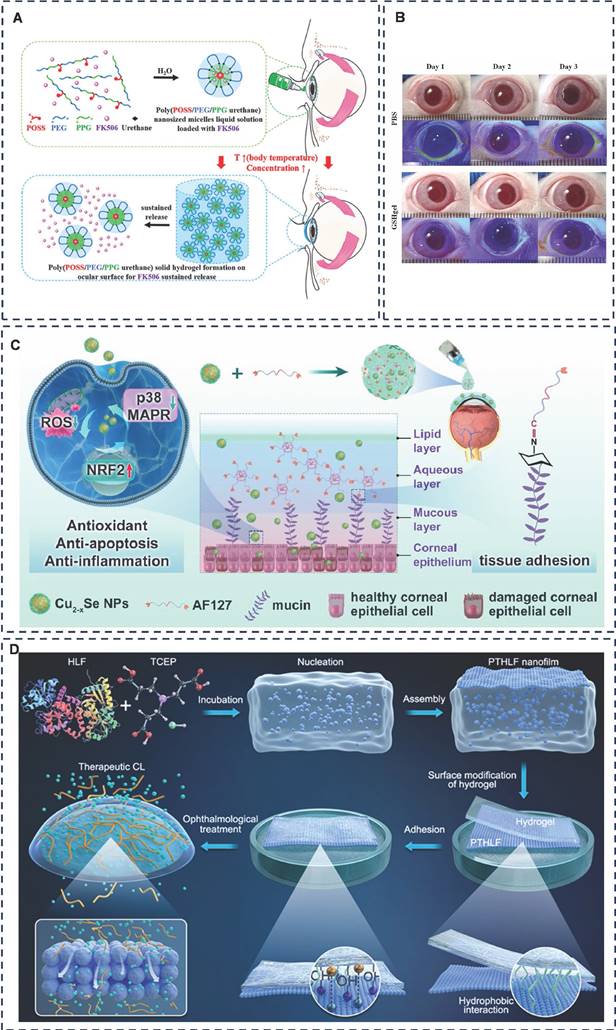
Additionally, Ou et al. developed aldehyde-functionalized F127 (AF127) hydrogel eye drops containing multifunctional antioxidant Cu2-x selenium nanoparticles (Cu2-x Se NPs) to treat dry eye disease (DED) (Figure 3C) [53]. The investigation revealed that the Cu2-x Se NPs @AF127 aqueous gel eye drops adhered well to the ocular surface due to the formation of Schiff base bonds. Compared to F127, the AF127 group exhibited minimal loss of adhesion even after 60 minutes of dispensing, indicating superior adhesion and extended treatment time of the aqueous gel. However, since the study was conducted on anesthetized mice with resting eye muscles, a dynamic assessment of the adhesion effect is necessary. Using larger animals or isolated porcine eyes to mimic human ocular movement would provide a more accurate simulation.
Carboxymethylcellulose (CMC) is one of U.S. Food and Drug Administration (FDA)-approved gel formulations for ophthalmic drug delivery [54]. CMC exhibits thermosensitive properties. Lin et al. utilized CMC hydrogel by injecting it through the punctum into the lacrimal duct. The CMC undergoes a sol-gel transition in response to temperature changes, leading to increased viscosity and enhanced cohesion, allowing it to settle within the lacrimal duct [55]. This adaptation reduces tear drainage, making it a promising approach for managing dry eye disease. Moreover, CMC is inherently viscous and binds to fibronectin and collagen to promote adhesion of corneal epithelial cells [56, 57]. Phenylboronic acid (PBA) has two hydroxyl groups on the boron atom and can form high-affinity complexes with the 1.2-cis-diol moiety of sialic acid in mucins, and PBA nanoparticles bind to mucins 4.7-9.1 times more than other mucosal adhesion nanoparticles, such as chitosan-based and sulfate nanoparticles [58, 59]. Combining these two points, Cheng et al. designed a glutathione (GSH)-rich PBA-grafted CMC hydrogel (PBA-CMC-GSH) (Figure 3D) [60]. The team demonstrated that the PBA-CMC-GSH hydrogel was effective in alleviating benzalkonium chloride (BAC)-induced apoptosis of corneal epithelial cells and exhibited good biocompatibility without significant eye irritation [61]. BAK can induced dry eye-like changes. However, the therapeutic effect of this study was limited to cellular experiments, and further in vivo animal experiments must be carried out to validate its therapeutic potential for dry eye disease (DED) by adding a more classical dry eye animal model for further in vivo animal experiments. Additionally, studies have explored the use of nano-hydrogels for drug delivery in the treatment of dry eye disease, often utilizing temperature-induced sol-gel transitions to enhance adhesion effects [62, 63].
DED is a complex, multifaceted disease. With advancing knowledge about its underlying mechanisms, the pathogenesis of DED has become increasingly clear. Consequently, the prospect of utilizing ocular surface adhesive hydrogels for DED treatment is highly promising, yet this area of research remains relatively underexplored, particularly in terms of drug delivery. Given that hydrogels are inherently water-retaining materials, they offer inherent advantages in treating dry eye conditions. Thus, we should focus on developing hydrogels loaded with suitable drugs or designing polymer systems that can inhibit inflammation and oxidative stress, tailored specifically to the pathogenesis of dry eye. This approach, which is grounded in maintaining the aqueous balance of the ocular surface, will significantly contribute to more precise and effective treatments for dry eye.
3.1.2 Ocular surface infection
Ocular surface infections are broadly classified into bacterial, viral, fungal, and other microbial types [64]. Currently, the treatment of these infections almost exclusively relies on topical medications [65]. However, the application of these topical drugs is hampered by challenges such as poor solubility, limited bioavailability, and significant drug loss, necessitating frequent dosing and often leading to suboptimal patient compliance [66, 67]. Consequently, there is a pressing need for effective drug delivery methods that can enhance local drug penetration and extend the duration of therapeutic action.
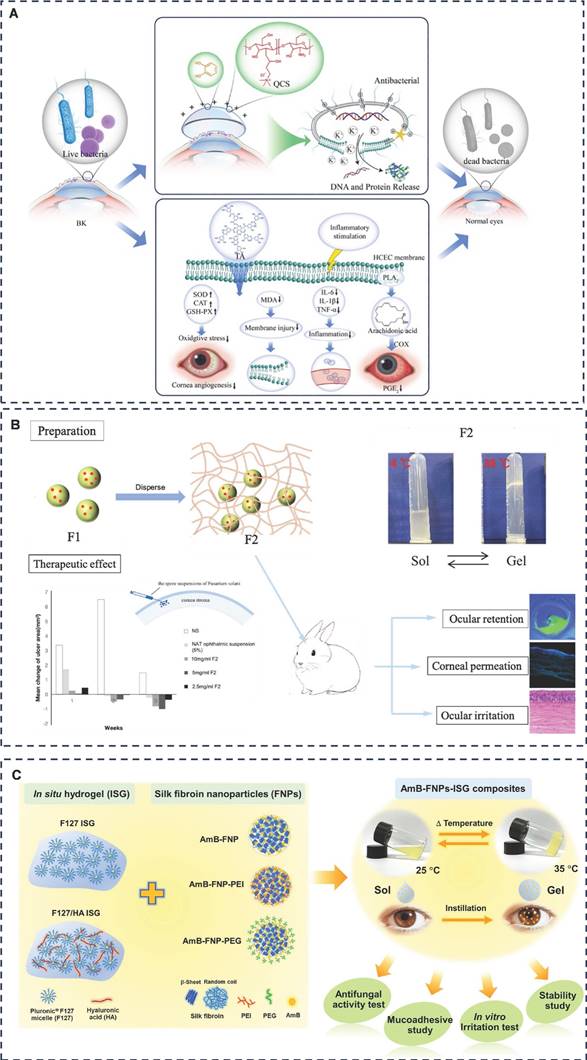
Bacterial keratitis, a prevalent ocular infection, was addressed by Jiao et al. through the development of a unique polyacrylamide (PAM) semi-interpenetrating network hydrogel contact lens dubbed PAM-QCS-TA (Figure 4A) [68]. This innovative material is synthesized from quaternate chitosan (QCS) and tannic acid (TA), imparting it with antibacterial and antioxidant qualities. The intricate interpenetration of PAM, QCS, and TA, mediated by hydrogen bonding, electrostatic interactions and π-π stacking, results in a hydrogel that exhibits remarkable resistance to swelling and boasts excellent mechanical properties. Moreover, in a rabbit model of bacterial keratitis, this hydrogel displayed pronounced antibacterial, anti-inflammatory, and cornea-repair-promoting effects.
Fungal keratitis (FK) is a symptomatic, rapidly progressive condition that can severely damage the cornea, including ulceration, perforation, and potentially blindness. The primary therapeutic agents currently employed to treat FK include polyene macrolide antibiotics (5% natamycin [NAT] and 0.25% amphotericin B [AmB]), as well as imidazole and pyrimidine antifungal agents. However, these drugs face significant challenges due to their poor solubility and low bioavailability, often resulting in inadequate disease control and severe consequences. To address these limitations, nano drug delivery systems have emerged as a promising approach to enhance drug solubility and permeability. Sha et al. have pioneered the development of NAT-loaded triblock polymer nanoparticles embedded in Pluronic® gel (F127 and PEG-PPG-PEG), creating a thermosensitive hydrogel polymer system with adhesive properties (Gel@PLGA-PEI-PEG@NAT) specifically designed for FK treatment (Figure 4B) [69]. The system increased cohesion through temperature responsive phase transition, and the enhancement of cohesion in this system, together with mechanical chain effect and hydrogen bonding, played a role in ocular surface adhesion, which ultimately achieved the effect of prolonging drug residence time and increasing drug permeability. Notably, in a rabbit model of FK, a 2.5 mg/mL hydrogel system achieved therapeutic efficacy comparable to a 50 mg/mL NAT ophthalmic suspension, significantly enhancing drug bioavailability and reducing the need for frequent dosing. Furthermore, Chomchalao et al. have introduced a combination system utilizing filipin protein nanoparticles encapsulating AmB and incorporated into a thermosensitive in situ hydrogel (AmB-FNPs ISG) (Figure 4C) [70]. This system similarly improved cohesion through a temperature-responsive phase transition. Ionic and hydrogen bonds were also formed between the polymer and the mucin of the mucosa, which greatly improved the adhesion of the system. The polymer system was formed at a gel-forming temperature of 35 ± 1 °C, and ocular surface adhesion was maintained for more than 6 hours, which not only prolonged the ocular retention time of AmB, but also reduced the frequency of administration during treatment.
Amit et al. designed a cornea-specific cell-penetrating the peptide for the treatment of FK, but the duration of action was only 4 hours. In order to improve the bioavailability and delivery of this peptide, they introduced the cell-penetrating peptide into a gelatin hydrogel system and succeeded in extending the antifungal activity of the biopeptide to 24 hours, which reduces the number of applications and is more favorable for the treatment of FK [71]. This study did not elaborate on the adhesion mechanism, but analyzed actin expression in corneal epithelial cells inoculated with soft and hard hydrogels, respectively. The results showed that corneal epithelial cells adhering to the soft hydrogel exhibited prominent stress fibers compared to the hard hydrogel. Here, we hypothesized that it may be mechanical effects such as the pores and flexible surface of gelatin that promote cell adhesion, thus enabling the polymer system to prolong drug action. Adhesive hydrogels have diverse applications, ranging from drug delivery for infectious ocular diseases to assisting in the diagnosis of various ocular surface infections. Swift et al. have pioneered two methods for specifically and swiftly detecting and differentiating between Gram-positive, Gram-negative bacteria, and fungi in infected corneas [72]. The first method involves a glycerol mono-methacrylate (GAMA) hydrogel swab coupled with a highly branched polymer additive functionalized with vancomycin (VAN), polymyxin (PMX), and amphotericin (AMP) ligands, exhibiting high selectivity for bacterial and fungal strains. The second method utilizes modified commercial contact lenses (carboxylate contact lenses) functionalized with similar ligands, though it is less specific than the first approach. Both methods are based on functionalisation of GAMA swabs or contact lenses with highly branched polymer additives that increase affinity for bacterial or fungal isolates by forming covalent bonds such as amide bonds. These methods rapidly determine the type of infection (Gram-positive, Gram-negative, or fungal) in less than 30 minutes, greatly improving clinical diagnosis and treatment.
Ocular surface infections, though common, are complex eye diseases that require prompt and targeted diagnosis and treatment. However, misuse of antibiotics often leads to atypical presentations, potentially misleading doctors and delaying treatment. While numerous studies have focused on improving drug delivery efficiency, fewer have addressed diagnostic efficiency. Therefore, enhancing diagnostic methods for ocular surface infections represents a promising direction for future research.
3.1.3 Corneal wound surgery
Corneal disease stands as a leading cause of visual impairment and blindness globally. Although corneal transplantation is the most effective treatment for advanced cases, only 5% of patients have access to this procedure due to the scarcity of donors and the associated high costs [73]. Furthermore, allogeneic grafts pose inherent risks of immune rejection and infection [74, 75]. Beyond corneal transplantation, any intraocular surgery involving corneal incision, such as cataract IOL replacement or vitrectomy, typically requires sutures for closure. However, these sutures inflict additional trauma on corneal tissues, potentially leading to localized inflammation or infection. Moreover, corneal sutures often result in uneven healing, causing astigmatism that can significantly impact visual formation [76].
Representative diagram of adhesive hydrogels applied for eye surface infection. A) Drug-free contact lens based on quaternized chitosan and tannic acid for bacterial keratitis therapy and corneal repair. Adapted with permission from [68] Copyright 2022 Elsevier. B) Thermosensitive tri-block polymer nanoparticle-hydrogel composites as payloads of natamycin for antifungal therapy against fusarium solani. Adapted with permission from [69], Copyright 2022 Dove Medical Press. C) Mucoadhesive hybrid system of silk fibroin nanoparticles and thermosensitive in situ hydrogel for amphotericin b delivery: a potential option for fungal keratitis treatment. Adapted with permission from [70], Copyright 2024 MDPI.
Grinstaff et al. proposed a set of ideal properties for corneal adhesives to address these challenges [76]. These include: strong wet adhesion capable of withstanding intraocular pressure (IOP) exceeding 80 mmHg; controlled rheological properties with a viscosity below 100 cP; rapid curing within 30 seconds; swift IOP recovery within 24 hours; refractive index matching the natural cornea (1.42); solute diffusion properties conducive to normal corneal healing (exceeding 2×10-7 cm2/s for small molecules/nutrients); biocompatibility; elasticity exceeding corneal tissue to prevent astigmatism during healing; provision of a microbial barrier for 2-3 days; and timed removal from the wound, aligning with tissue regeneration through bio-absorption or exudation (ranging from days to months depending on the application).
Biomaterials used as tissue adhesives for corneal sealing and repair can be broadly categorized into two types based on their source: (i) naturally derived materials, such as extracellular matrix components (gelatin, collagen, fibrin) and polysaccharides (hyaluronic acid, chitosan), and (ii) synthetic materials, such as cyanoacrylate and polyethylene glycol (PEG). Each type has its own advantages and disadvantages. Naturally derived polymers generally offer better biocompatibility, but they often exhibit lower mechanical stability and adhesive strength. In contrast, synthetic polymers allow for tailored formulations to meet specific requirements but tend to have poorer tissue regeneration and cell adhesion properties.
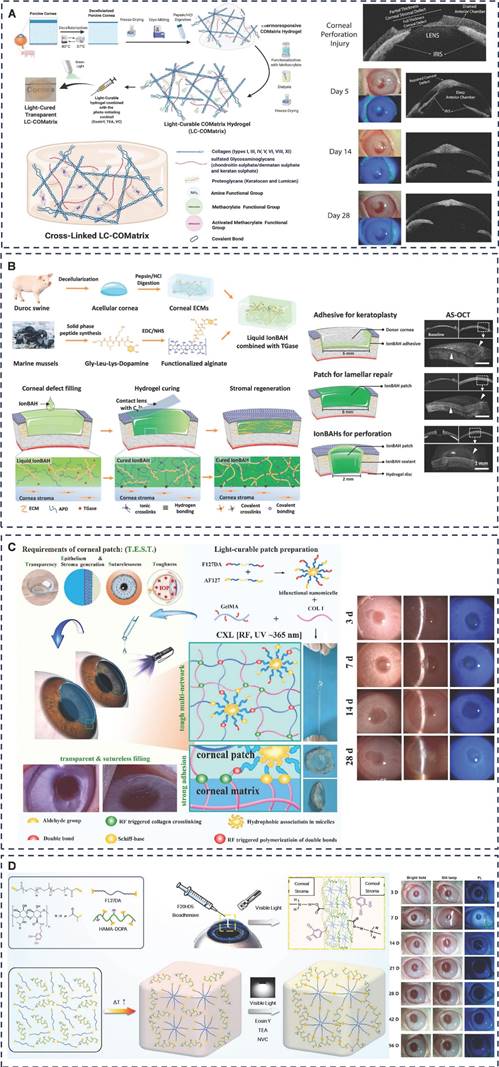
The extracellular matrix (ECM) of the corneal stroma is a complex network comprising primarily water, collagen, and glycosaminoglycans (GAGs) [77]. Researchers have explored various materials and strategies to mimic this ECM for corneal tissue engineering. Yazdanpanah et al. developing a light-cured corneal stroma (LC-COMatrix) based on decellularized porcine corneal ECM (DPC-ECM). This material exhibits suitable swelling behavior, biodegradability, and viscosity, and can be crosslinked in situ under visible light to significantly enhance biomechanical strength, stability, and adhesion (Figure 5A) [78]. Crosslinked LC-COMatrix showed strong adhesion to isolated human corneas and effectively closed full-layer corneal perforations and tissue defects. The naturally derived and biocompatible nature of these materials facilitates clinical translation. Zhao et al. pioneered the development of an ion-activated bio-adhesive hydrogel (IonBAH) specifically for corneal repair (Figure 5B) [79]. This innovative hydrogel comprises DPC-ECM, peptide-modified alginate, and transglutaminases (TGases) utilizing the synergistic effect of covalent and ionic bonding of DOPA and TGase-mediated cross-linking to achieve robust adhesion to the recipient cornea. After six months of observation, IonBAH exhibited remarkable results, facilitating rapid regeneration of corneal epithelium, stroma, and nerves, restoring transparency and thickness, thus achieving therapeutic effects comparable to donor corneal transplantation.
Another interesting approach was demonstrated by Fernandes-Cunha et al., who utilized supramolecular noncovalent host-guest interactions between HA-cyclodextrin and HA-adamantane to create shear-thinning HA hydrogels. These hydrogels promoted adhesion and spreading of encapsulated human corneal epithelial cells in ex vivo models and improved corneal wound healing in vivo [80]. In summary, researchers have explored a variety of strategies to mimic the corneal ECM using synthetic and naturally derived materials. These advances hold promise for the development of novel corneal substitutes and adhesives that can effectively repair corneal defects and improve patient outcomes.
Achieving a watertight closure of clear corneal incisions during cataract surgery is crucial for minimizing the risk of infection and other leakage-related complications, as it prevents fluid from infiltrating the eye and thus reduces the likelihood of intraocular infection [81]. As early as 1996, a team began investigating the use of fibrin glue to seal corneal incisions after cataract surgery [82]. However, due to the toxicity of cyanoacrylates and the heat generated during polymerization, they were not widely used clinically, and further researchers looked to the much less toxic fibrin glue [83, 84]. Banitt et al. compared the healing effects of fibrin glue (Tisseel®), cyanoacrylate glue (Histoacryl), and suture closure on post-cataract corneal incisions of different widths [85]. The researchers found that all three did a good job of sealing corneal incisions created by cataract surgery. But there were differences in maintaining a certain amount of intraocular pressure after surgery. Tisseel® maintained better IOP than sutures when sealing 3-mm incisions, but did not achieve ideal IOP when sealing 4.5-mm or 6-mm incisions. This suggests that it may be caused by the fact that Tisseel® is of human origin and degrades easily.
In recent years, the FDA-approved polyethylene glycol hydrogel Resure® has been proven effective in corneal wound closure in cataract surgery. Studies conducted by Tong et al. and Masket et al. revealed that Resure® performs comparably to suture closure in sealing cataract surgical wounds, without causing adverse effects such as a foreign body sensation or an increase in surgically-induced astigmatism [20, 86].
Representative diagram of adhesive hydrogels applied for corneal wound surgery. A) A Light-Curable and Tunable Extracellular Matrix Hydrogel for In Situ Suture-Free Corneal Repair. Adapted with permission from [78], Copyright 2022 Wiley. B) Natural Dual-Crosslinking Bioadhesive Hydrogel for Corneal Regeneration in Large-Size Defects. Adapted with permission from [79], Copyright 2022 Wiley. C) A "T.E.S.T." Hydrogel Bioadhesive Assisted by Corneal Cross-linking for In Situ Sutureless Corneal Repair. Adapted with permission from [95], Copyright 2023 Wiley. D) Photocurable and Temperature-Sensitive Bioadhesive Hydrogels for Sutureless Sealing of Full-Thickness Corneal Wounds. Adapted with permission from [100], Copyright 2024 Wiley.
Nallasamy et al. further investigated the efficacy of Resure® in both routine and complex cataract surgeries, finding that a hydrogel ocular sealant was more suitable due to its ability to provide a more stable positioning for complex multi-intersection adjustable IOLs [87]. Spierer et al. examined the effectiveness of Resure® in sealing corneal incisions during Descemet's stripping endothelial keratoplasty (DSEK) surgery, both alone and combined with cataract surgery. They concluded that Resure® effectively and rapidly closed corneal incisions in DSEK surgery, with or without cataract surgery, significantly reducing foreign body sensation and inflammation associated with sutures, while also saving surgical time [88]. In summary, Resure® sealants with polyethylene glycol (PEG) as the main ingredient, this polymer has excellent biocompatibility and water solubility, and its strong cohesion is one of the reasons for its adhesion. When used on tissue surfaces, they can generate hydrogen bonds and mechanical interlocking effects, resulting in better adhesion. Building on its strong mechanical and adhesive properties, Zhou et al. developed an injectable hydrogel based on PEG-lysozyme for repairing corneal stromal defects [89]. The NHS group of 4-arm-PEG-NHS reacts with amine groups on corneal tissue, forming amide bonds that serve as the primary adhesive mechanism between the hydrogel and the corneal surface. Additionally, the 4-arm-PEG-NHS and lysozyme mixture maintains suitable fluidity before curing, allowing it to fill irregular defects. This promotes mechanical interlocking adhesion and enables the formation of a highly shape-adaptive hydrogel implant.
Gelatin (Gel), a natural protein polymer approved by the U.S. Food and Drug Administration (FDA), is derived from animal sources through the hydrolytic degradation of type I collagen [90, 91]. It boasts high biodegradability, biocompatibility, and non-toxicity. GelMA, a modified version of Gelatin, incorporates meth-acrylamide groups that enable its photoinitiated, free radical polymerization to create covalently crosslinked hydrogels [92]. This controlled photoinitiated polymerization renders GelMA a favorable choice for ophthalmic clinical applications, leading to the development of numerous GelMA-based polymer systems.
Sani et al. developed GelCORE, a gelatin-based adhesive hydrogel for the swift and enduring repair of corneal interstitial defects, by crosslinking with Type II initiators, Eosin Y, triethanolamine (TEA), and N-vinylcaprolactam (VC) through a free radical polymerization process [93]. After just four minutes of photo-crosslinking, this hydrogel forms a robust, transparent, and adherent gel on corneal tissue. Furthermore, its burst pressure test results on isolated rabbit eyes surpassed those of the commercially available Resure® hydrogel. However, the team noted a limitation of GelCORE: its high liquid content, which led to easy diffusion and loss after application, rendering it unsuitable for bonding full-thickness corneal injuries. To address this challenge, the team developed a light-polymerized hydrogel patch based on GelMA (gelatin methacrylate), incorporating hyaluronic acid glycidyl methacrylate (HAGM) and PEG diacrylate (PEGDA) [94]. HAGM increased the viscosity of the polymer system, while PEGDA enhanced its flexibility and extensibility, resulting in improved adhesion. The average burst pressure of this new hydrogel was 3-4 times greater than that of Resure® hydrogel. This multi-source polymer system effectively balanced the properties of its components, leading to a more versatile adhesive hydrogel for corneal injury repair.
In addressing the concern for corneal repair following cross-linking surgery in patients with conical corneas, Zhou et al. emphasized the need for corneal substitutes to fulfill four critical properties, namely "transparency," "epithelial and stromal production," "suture-free," and "toughness," collectively referred to as "T.E.S.T." Type I collagen plays a pivotal role in the properties of corneal substitutes (Figure 5C) [95]. To this end, the research team combined collagen type I (COL-I), Pluronic F127 diacrylate (F127DA), aldolized Pluronic F127 (AF127), and gelatin methacrylate (GelMA) to create an advanced bio-adhesive hydrogel substitute rooted in GelMA that fulfills the "T.E.S.T." criteria. The hydrogel's superior tissue adhesion ability stems from the aldehyde groups in the micelles, the corneal cross-linking-induced COL-I connection to the corneal ECM, and the in-situ network formation through light curing. To validate its efficacy, the corneal tissue adhesion of these hydrogels was rigorously evaluated both in vitro using porcine corneas and in vivo in rabbit models, encompassing lamellar corneal transplantation and the repair and replacement of deep corneal defects. This comprehensive study, leveraging materials approved by the U.S. Food and Drug Administration, holds promising prospects for clinical translation.
Zhao et al. also integrated oxidized dextran (ODex) into the rigid photopolymerized GelMA hydrogel, facilitating the formation of a dual network under visible light to bolster its adhesive properties. Further assessment of the adhesion effect, biocompatibility, and potential clinical applications was conducted in a New Zealand rabbit corneal lamellar graft model [96]. Wang et al. crafted GelMA/HA-NB hydrogels by fine-tuning the ratio of GelMA to butyramide (NB)-modified hyaluronic acid (HA-NB), mirroring the human corneal collagen-to-glycosaminoglycan ratio [97]. This hydrogel boasts robust wet tissue adhesion, swift gelation, minimal swelling, and exceptional biocompatibility. A long-term study using the New Zealand rabbit large-diameter corneal defect regeneration model explored the biocompatibility and clinical potential of this ECM-mimicking bonding hydrogel. Qian et al. developed a dopamine methacrylamide (DMA)-based adhesive hydrogel, building upon the transparent Gel/DMA base, through oxidative radical polymerization. This hydrogel excels in transparency, adhesion, oxidation resistance, and biocompatibility [98]. Notably, the adhesion of Gel/DMA, primarily attributed to the catecholamine groups of DMA, surpasses even clinically used PEG-based adhesives (Evicel). Furthermore, a rabbit corneal stromal defect model demonstrated that this bioadhesive significantly accelerated epithelialization in damaged corneas in vivo. Additionally, an adhesive hydrogel (dCor/Gel-PEG) has been developed based on GelMA, combined with decellularized bovine corneal stroma and PEG, to treat corneal defects, offering a new design approach for corneal adhesive hydrogels [99]. Shear testing indicates that dCor/Gel-PEG hydrogel achieves a shear strength of approximately 945 ± 23 kPa on dry substrates and 732 ± 29 kPa on wet substrates, surpassing or equaling the adhesive strength of commercial surgical sealants (Evicel at 207.7 ± 67.3 kPa, CoSEAL at 69.7 ± 20.6 kPa, and cyanoacrylate tissue adhesives at 115 ± 22 kPa). This impressive adhesive strength is primarily attributed to the formation of a double network structure that enhances the topology, and covalent bonding between the -CH2 groups of PEGDA and the -NH2 groups of lysine in dCor.
On a different note, Wang et al. successfully synthesized an injectable, light-curable bioadhesive hydrogel (F20HD5) utilizing polyether F127 diacrylate (F127DA) and dopamine-modified hyaluronic acid methacrylate (Figure 5D) [100]. This hydrogel is specifically designed for the seamless closure of total corneal incision wounds. The study revealed that F20HD5 exhibits remarkable transparency, optimal viscosity, biodegradability, and exceptional biocompatibility. It effectively seals various types of rabbit corneal wounds, maintaining corneal curvature and clarity even after 56 days of follow-up.
The cornea is a resilient tissue with tightly arranged structures, so materials suitable for corneal adhesion must also exhibit good mechanical compliance with the underlying tissue. Zhang et al. adjusted the crosslinking degree of alginate by MA (AlgMA) crosslinking and calcium ion crosslinking, then combined it with ODex and dendritic polymers to form a triple-crosslinked double-network hydrogel for corneal tissue bonding [101]. Shear tests on regenerated cellulose membranes and biological amniotic membranes revealed that calcium ion chelation improved adhesion by twofold, and the triple-crosslinked double-network hydrogel further enhanced this by an additional factor of one. This study suggests that increasing the crosslinking density of polymers and designing multiple crosslinking, interpenetrating network polymers are effective strategies to improve corneal adhesives. However, a limitation is that multiple crosslinking often affects the transparency of the polymer. Transparency is critical for corneal adhesives, so balancing crosslinking density and adhesive strength, while considering tissue characteristics, is an important area for further research.
Current research predominantly centers on enhancing adhesion, biocompatibility, and promoting corneal healing. However, interstitial corneal fibrosis, a prevalent cause of corneal injury, inflammation, and vision loss post-surgery [102], remains a challenge. While researchers have formulated polymer hydrogels to prevent corneal scarring and stromal fibrosis in lamellar corneal transplants [103], these hydrogels often lack robust adhesive properties. Therefore, future research on corneal adhesives should aim to not only ensure strong adhesion but also inhibit corneal stromal fibrosis and scar formation, ultimately leading to improved postoperative vision outcomes.
3.1.4 Refractive surgery wounds
Excimer laser in situ keratomileusis (LASIK) is a widely practiced surgical procedure for correcting refractive errors [3]. Since 1995, over 8.5 million individuals in the United States have undergone refractive surgery, with a total of 13 million eyes treated via excimer laser keratomileusis [104][64]. However, corneal wound healing following LASIK surgery is a slow and incomplete process, often taking 3 to 4 years to complete. Microscopic examinations reveal that the LASIK flap heals through the development of oligocellular primary mesenchymal scarring at the central and paracentral interfaces, and multicellular fibrous mesenchymal scarring along the flap's edges. In some cases, inward growth of the corneal epithelium has been reported following LASIK, typically when surgical intervention is inadequate. Conversely, when the procedure is coupled with the use of ocular sealing hydrogels, such as ReSure®, a significant reduction in this inward growth has been observed [105-107].
Despite the limited research in this field, the escalating number of patients seeking refractive error correction through excimer laser corneal surgery underscores the need for ocular adhesives. Swift and precise adhesive bonding is more effective than natural corneal adhesion in enhancing corneal alignment restoration and minimizing postoperative complications, including astigmatism and dry eye. Given that laser surgery can damage subepithelial corneal nerve fibers, the development of adhesive hydrogels that promote corneal nerve growth remains a promising area of investigation.
3.1.5 Conjunctival defect
The conjunctiva, a vital structure of the ocular surface, plays a crucial role in maintaining ocular health. Its cup cells secrete mucin, a primary component of the tear film's mucin layer, essential for lubricating and protecting the eye. Additionally, the conjunctiva functions as a vital immune tissue, safeguarding the eye from external threats while housing numerous immune cells, including macrophages and CD4/CD8-positive T cells. However, in cases of significant conjunctival defects resulting from ocular chemical burns, Steven-Johnson syndrome, pterygium, or conjunctival swelling, conjunctival scarring and contracture often arise due to the tissue's loose structure and abundant fibroblasts. Here, hydrogels emerge as promising therapeutic aids, mimicking the extracellular matrix (ECM) to facilitate conjunctival epithelial repair and providing scaffolds to guide the healing process.
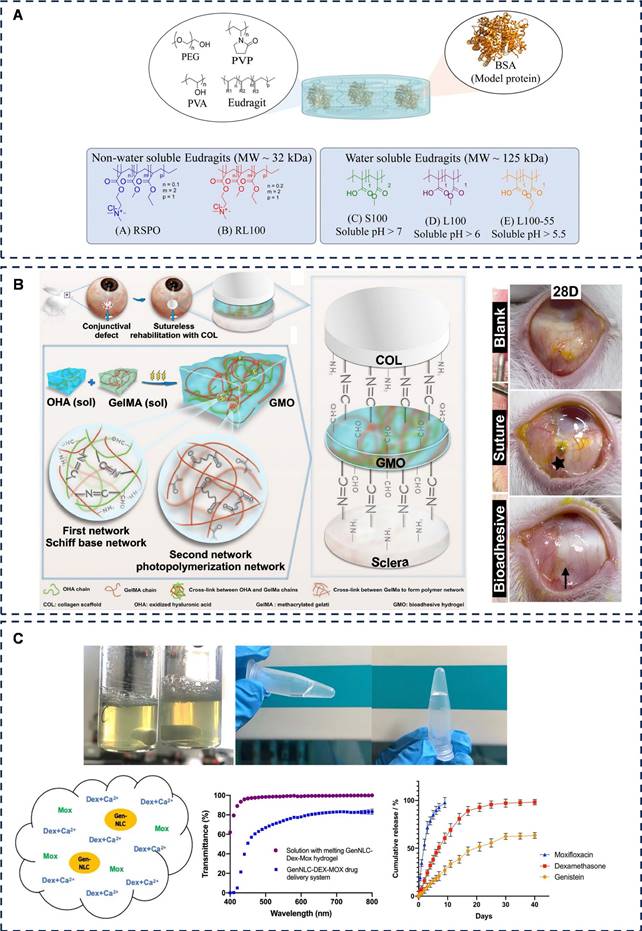
Eudragits® polymers, renowned for their gastrointestinal mucosal adhesion [108] and ocular drug delivery capabilities [109], have shown potential in conjunctival applications. Esporrín-Ubieto et al. have investigated the adhesion of Eudragits®-based hydrogels to the conjunctiva, demonstrating the feasibility of creating hydrogels with enhanced conjunctival adhesion for drug delivery and tissue repair purposes by optimizing the ingredient ratios (Figure 6A) [110].
Autologous conjunctival grafts are presently the gold standard for preventing pterygium recurrence, often affixed to the sclera via sutures. However, recent advancements have introduced fibrin glue as a suture-free alternative, offering reduced inflammation, lessened pain, expedited surgery time, and potentially lower recurrence rates [111, 112]. Bondalapati et al. experimented with ReSure®, a corneal sealant commonly used in cataract surgery, during amniotic membrane grafting in pterygium surgeries. Notably, after excision in nine eyes, they observed no graft dislocation or failure, and there were no recurrences during follow-up [113]. This promising finding suggests that ReSure® could serve as a potential amniotic adhesion sealant in suture-free pterygium surgeries.
Furthermore, Liu et al. have devised a semi-interpenetrating polymer network (sIPN) tissue-adhesive hydrogel, GMO, comprising GelMA and an interfacial initiator (OHA), for ocular surface reconstruction (Figure 6B) [114]. Their study on conjunctival defects in New Zealand rabbits demonstrated that GMO, paired with collagen scaffolds, promoted conjunctival epithelial hyperplasia and repair, without postoperative scarring or conjunctival contracture, outperforming suturing methods. This sIPN-based bioadhesive, characterized by the molecular-level penetration of macromolecules into polymer networks, offers both robust mechanical strength and adjustable composition ratios for tailored adhesion [115]. Compared to interpenetrating polymer networks (IPNs), sIPNs provide an enhanced combination of strength and flexibility, making them a promising material for ophthalmic applications. Zheng et al. developed an elastic and resilient hydrogel patch named APTF, designed with strong cohesion to ensure robust adhesion [116]. APTF is primarily structured with poly (ethylene glycol) diacrylate (PEGDA) as its main backbone. Its elasticity and toughness are achieved through ionic interactions between N-hydroxysuccinimide (NHS)-conjugated alginate (Alg-NHS) and Fe3+, as well as reversible hydrogen bonding between PEGDA and tannic acid (TA). The adhesive strength of APTF is further enhanced by the wet adhesion properties of TA (via hydrogen and covalent bonds) and the covalent bonding between NHS groups and amine groups on tissue surfaces. In lap shear tests, APTF demonstrated robust adhesion (77.28 ± 3.39 kPa), securely attaching to corneal (19.61 ± 3.26 kPa) and conjunctival tissues (11.87 ± 2.57 kPa). Moreover, APTF can be easily removed from these tissues using a urea solution, offering a promising approach for ocular surface and conjunctival repair that accounts for removal considerations. This design shows significant potential for clinical application.
The conjunctiva, though not directly involved in the formation of the optical pathway, plays a crucial role in ocular surface immunity. However, significant conjunctival defects often result in scarring due to the tissue's laxity, ultimately leading to eyelid contracture that impacts vision and aesthetics. Consequently, the development of soft and flexible hydrogel polymers tailored for conjunctival tissue is imperative. These polymers must support conjunctival epithelial repair while inhibiting scar formation. Furthermore, given the direct contact between the eye and the external environment, as well as the potential for microbial colonization on the ocular surface, hydrogels with improved anti-inflammatory and antimicrobial properties are essential. Currently, hydrogels used for conjunctival defect repair primarily focus on adhesion and biocompatibility. In the future, multifunctional adhesive hydrogels loaded with antifibrotic, anti-inflammatory, and antimicrobial bioactive molecules or drugs could be designed to effectively inhibit conjunctival scar formation and enhance anti-inflammatory and antimicrobial effects.
Representative diagram of adhesive hydrogels applied for conjunctival defect and cataract. A) The role of Eudragit® as a component of hydrogel formulations for medical devices. Adapted with permission from [110], Copyright 2023 Royal Society of Chemistry. B) Sutureless transplantation using a semi-interpenetrating polymer network bioadhesive for ocular surface reconstruction. Adapted with permission from [114], Copyright 2022 Elsevier. C) Thermoresponsive genistein NLC-dexamethasone-moxifloxacin multi-drug delivery system in lens capsule bag to prevent complications after cataract surgery. Adapted with permission from [128], Copyright 2021 Nature Portfolio.
Adhesive hydrogels for ocular posterior segment applications.
| Application | Adherent tissue | Materials | Adhesion mechanism | Clinical research process | Ref. |
|---|---|---|---|---|---|
| Cataract | Cornea | Pluronic® F127 | Hydrogen bonds, cohesion | Approved | [125] |
| Anterior chamber | GenNLC, F127, F68 | Hydrogen bonds, cohesion | Pre-clinic | [128] | |
| Glaucoma | Cornea | PPI | Hydrogen bonds, covalent bonds | Pre-clinic | [138] |
| Cornea | Thermosensitive elastin, silk elastin-like recombinants | Cohesion | Pre-clinic | [139] | |
| Cornea, conjunctiva | PAMAM, PLGA | Covalent bonds | Pre-clinic | [140] | |
| sclera | AOAQ, DMAA | Crosslinked structure, covalent bonds, hydrogen bonds | Pre-clinic | [142] | |
| Suprachoroidal space | HA-SH, PEGDA | Covalent bonds, hydrogen bonds, crosslinked structure | Pre-clinic | [143] | |
| Vitreoretinopathy | Retina | PDA, GelCA, Cur | π-π stacking, hydrogen bonds | Pre-clinic | [147] |
| Vitreous humor | PNaAMPS, PDMAAm | Hydrogen bonds, mechanical interlocking | Pre-clinic | [148] | |
| Retina | CS, Odex | Covalent bonds (Schiff base) | Pre-clinic | [153] | |
| Retina | Gel, HA, PDA | Wet adhesive | Pre-clinic | [157] | |
| Retina | Fibrin glue | Mechanical interlocking, ionic bonds | Pre-clinic | [163] | |
| Retina | PEG | / | Approved | [164.165] | |
| Retina | HA | Hydrogen bonds, ionic bonds, mechanical interlocking | Approved | [166] | |
| Open eye trauma | Cornea, conjunctiva, sclera | N-isopropylacrylamide, butyl acrylate | Mechanical interlocking, diffusion, hydrogen bonds, covalent bonds | Pre-clinic | [171] |
| Cornea | PEG, CV | Hydrogen bonds, mechanical interlocking | Pre-clinic | [172] | |
| Cornea | PDA | Cohesion, covalent bonds | Pre-clinic | [173] |
In summary, the focus of research on adhesive hydrogels for ocular surface diseases has shifted from pure adhesion, which binds tissues together, to the design of biocompatible adhesive hydrogels with a variety of functions, including, but not limited to, facilitating repair, loading of drugs, inhibition of oxidative stress, and inhibition of inflammatory responses. Next, we will present research on adhesive hydrogels for the treatment of the corresponding diseases in four areas of ocular diseases, including cataracts, glaucoma, vitreoretinopathy, and open ocular trauma (Table 2).
3.2 Cataracts
Cataract stands as the foremost cause of blindness globally. Presently, the primary treatment modality for cataracts is surgical, encompassing extracapsular extraction, lens ultrasonic emulsification techniques, and intraocular lens (IOL) replacement. These procedures are highly effective in significantly enhancing vision. Nonetheless, as cataract surgery involves intraocular manipulation, it can occasionally lead to severe complications such as intraocular inflammation, infection, or posterior capsule opacification (PCO) [5]. Given that cataract surgery necessitates a corneal incision, and we have previously delved into hydrogels for corneal adhesion, this section will primarily delve into the research surrounding adhesive hydrogels for drug delivery post-cataract surgery.
After cataract surgery, antibiotic and glucocorticoid drops are routinely administered to safeguard against intraocular infections by providing antibacterial and anti-inflammatory treatments. In order to enhance patient compliance during the postoperative period, numerous efforts have been made to devise ocular drug delivery systems as alternatives to traditional eye drops. These include the anterior chamber implants such as Surodex® [117-120], DEXYCU® [121] and IBI-10090® [122]. The positive outcomes of clinical trials have bolstered confidence in the field of ocular anti-inflammatory drug delivery, leading researchers to explore the delivery of diverse drug types.
Polyether F127 is a synthetic A-B-A triblock copolymer with the molecular structure of poly(ethylene glycol)-poly(propylene glycol)-poly(ethylene glycol) [123]. The poly (propylene glycol) chain segments in the middle of the molecule are relatively hydrophobic while the poly (ethylene glycol) chain segments at the ends are relatively hydrophilic. This hydrophilicity and hydrophobicity make it self-assembling into nanomembranes in water, which is often used for solubilisation and loading of hydrophobic drugs. In addition, F127 has reversible temperature-raising thermogenic gelation properties [27, 124]. Sapino et al. developed an ophthalmic nanocomposite hydrogel drug delivery system consisting of solid lipid nanoparticles loaded with dodecyl cefuroxime (dCEF) and an oil-in-water microemulsion with Pluronic® F127 (20% w/v) [125]. This system, with increased cohesion due to hydrogen bonding at elevated temperatures, formed an in-situ hydrogel with some viscosity at the ocular surface to achieve a slow-release effect, thus prolonging the efficacy of CEF and enabling its sustained release for up to one week.
Posterior cataract is a common complication after cataract IOL implantation [126]. Liu et al. introduced a nanostructured lipid carrier (NLC) that prevents intracapsular collagen deposition and lens fibrosis [127]. Yan et al. further advanced this area of research by combining a nanostructured lipid carrier loaded with genistein flavonoid (GenNLC) with a F127/F68 hydrogel containing dexamethasone (Dex) and moxifloxacin (Mox) (Figure 6C) [128]. The result produced a temperature-sensitive in situ hydrogel suitable for injection into the anterior chamber after cataract surgery. The system reduces inflammation, prevents infection and minimises posterior capsule opacity. The system forms a moderately viscous gel at approximately 32°C, providing the operator with sufficient time to perform anterior chamber injections, while ensuring that the gel forms rapidly upon entry into the lens capsule and is less prone to dislodgement, helping to reduce the incidence of posterior cataract and endophthalmitis.
For post-cataract surgery anti-infective treatment, patient compliance holds paramount importance, and the utilization of adherent hydrogels has the potential to minimize the need for postoperative medications and enhance patient adherence. Nevertheless, the precision of lens selection and measurement before the procedure is also essential for successful visual recovery [129, 130]. Consequently, the application of adhesive hydrogels in cataract surgery should not be confined to intra- and post-operative phases alone. Looking ahead, researchers can explore integrating these hydrogels with biosensors to develop ocular biosensors capable of measuring axial and corneal curvature, facilitating lens calculations. This approach could pave the way for personalized and precise designs that contribute significantly to enhancing visual function.
3.3 Glaucoma
Glaucoma, a complex, progressive neurodegenerative disorder, is marked by the deterioration of the optic nerve and the loss of retinal ganglion cells, ultimately resulting in vision impairment and the narrowing of the visual field. Statistically, glaucoma stands as one of the four primary culprits behind blindness globally, yet it is a condition that can be managed and intervened with medication and surgical procedures [131]. Among the prevalent treatment options for glaucoma patients, the administration of medications to regulate intraocular pressure (IOP) within healthy limits is commonplace. This approach necessitates the regular application of eyedrops to lower IOP and hinder disease progression. However, the frequent use of eyedrops can be cumbersome for patients, and the preservatives present in these drops have the potential to damage the ocular surface. To mitigate this issue, numerous studies have been undertaken, aiming to devise methods for sustained release and dose reduction through the formation of in situ hydrogels with adhesive properties on the ocular surface.
Dendritic polymers were first invented and successfully synthesized by Dr. Tomalia DA, an American chemist, in the early 1980s [132]. Nowadays, dendritic polymers are not only used as delivery carriers for bioactives and drugs, but they are also viscous and have been subjected to photocrosslinking and nucleophilic reagent-electrophilic reagent crosslinking by a number of researchers to create adhesive, transparent, elastic, hydrophilic and soft hydrogels [76, 133-137]. For the treatment of glaucoma, Mishra et al. developed a polypropyleneimine (PPI) dendritic polymer nanostructure loaded with acetazolamide (ACZ) for the treatment of glaucoma [138]. The polymer system produced adhesion through hydrogen bonding and peripheral functional groups (amine groups) of the dendritic molecule, which prolonged the ocular retention time of ACZ and enhanced the IOP-lowering effect.
Fernández-Colino et al. evaluated thermosensitive elastin and silk elastin-like recombinants as innovative pharmaceutical dosage forms for topical administration of timolol, a system that can turn into a gel at physiological temperatures and adhere to the ocular surface (Figure 7A) [139]. The specific mechanism of adhesion was not mentioned in this study, and we hypothesized that it is the balance between the cohesive and adhesive forces of the liquid in the presence of temperature that produces the adhesion effect.
Yang et al. designed a novel hybridized polyamidoamine (PAMAM) dendritic polymer hydrogel/poly (lactic acid-glycolic acid co-polyester) (PLGA) nanoparticle platform (HDNP) for the co-administration of two anti-glaucoma drugs, brimonidine and timolol maleate. These two IOP-lowering drugs can be maintained in vitro for 28-35 days, and a single application in a rabbit model can maintain the IOP-lowering effect for 4 days [140]. In this system, the partially crosslinked PAMAM dendritic polymer G3.0-PEG-acrylate carries a large number of amine groups, which create mucosal adhesion through covalent bonding and enhance interaction with the ocular surface cornea and conjunctiva. This study significantly reduced the dosing frequency of topical formulations and helped improve long-term patient compliance.
Representative diagram of adhesive hydrogels applied for glaucoma. A) Self-assembling elastin-like hydrogels for timolol delivery: development of an ophthalmic formulation against glaucoma. Adapted with permission from [139], Copyright 2017 American Chemical Society. B) Prevention of ocular tenon adhesion to sclera by a PDMAA polymer to improve results after glaucoma surgery. Adapted with permission from [142], Copyright 2020 Wiley. C) Drug-free, nonsurgical reduction of intraocular pressure for four months after suprachoroidal injection of hyaluronic acid hydrogel. Adapted with permission from [143], Copyright 2021 Wiley.
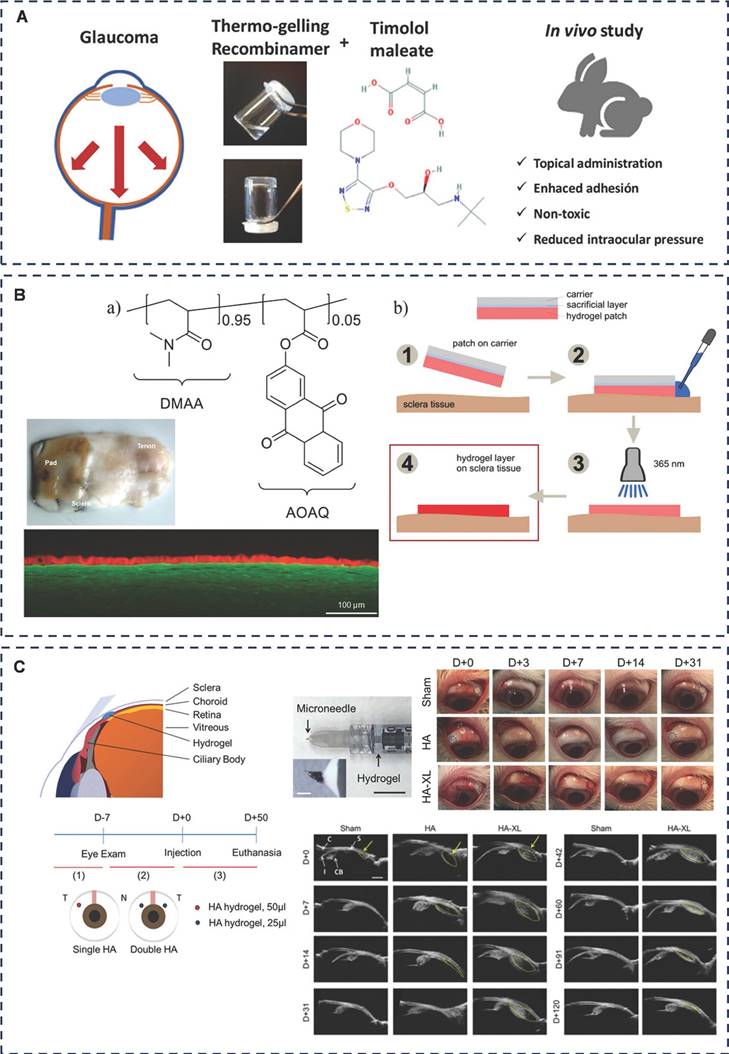
Another viable treatment option for glaucoma is surgical intervention, specifically filtering glaucoma surgery, which is often recommended for various glaucoma types. However, a common post-surgical complication is scarring, often triggered by the disruption of the blood-atrial fluid barrier, and excessive scarring can ultimately result in fistula occlusion [141]. To address this challenge, Martin et al. have formulated a hydrogel polymer comprising 2-acryloyloxyanthraquinone (AOAQ) and dimethylacrylamide (DMAA) (Figure 7B) [142]. This polymer serves as an effective barrier against fibroblasts, thereby preventing scar tissue formation at the surgical site. Their team has discovered that this polymer exhibits superior adhesion to isolated porcine sclera and is now poised to test its application on rabbit sclera. This study aims to validate the feasibility of the surgical procedures and demonstrate the long-term adhesion effectiveness of this innovative polymer pad.
Chae et al. designed a microneedle injection of an in situ molded hydrogel for the treatment of glaucoma by lowering intraocular pressure (IOP) in a drug-free, non-surgical manner (Figure 7C) [143]. The system (HA-XL) consists of thiol-based modified hyaluronic acid (HA-SH) and polyethylene glycol diacrylate (PEGDA.) HA-XL is viscous, and when injected into the suprachoroidal space (SCS), the hydrogel adheres and stays there to keep the SCS open by solubilizing, thus lowering IOP in patients with glaucoma for up to 4 months. The research team is now working to extend the duration of efficacy by modifying the polymer material (hyaluronic acid) with a view to achieving at least 6 months of efficacy. This would coincide with many patients' visits to the clinic.
The critical issue with glaucoma lies in the prolonged elevation of intraocular pressure (IOP), which can trigger optic nerve atrophy in the fundus. Consequently, adhesive hydrogels play a therapeutic role in glaucoma by effectively reducing IOP. This is achieved through their adhesive properties, improved drug retention for extended drug action, and assistance in establishing ocular aqueous drainage channels. Glaucoma patients necessitate ongoing IOP monitoring, yet these measurements are often conducted in hospitals. Although implantable IOP monitoring sensors exist, they are associated with adverse reactions such as anterior chamber inflammation and implantation trauma [144]. To address this, future research could focus on developing a miniature, real-time IOP monitoring biosensor that integrates the adhesive, biocompatible, and multifunctional properties of viscous hydrogels. This innovative device would significantly contribute to the management of patients with chronic glaucomatous eye disease.
3.4 Vitreoretinopathy
The retina is an important tissue structure in the eye that converts light signals into neuroelectric signals. With its fine and fragile tissue structure and high oxygen demand, the retina is particularly susceptible to oxidative damage, and oxidative stress damage to retinal tissue, especially that caused by reactive oxygen species (ROS), is present in many diseases [145, 146].
To combat ROS-related challenges, Liu et al. constructed a photocrosslinked, injectable, multifunctional nanocomposite hydrogel, Cur@PDA@GelCA, consisting of cinnamic acid crosslinked gelatin GelCA and curcumin-rich dopamine nanoparticles (Figure 8A) [147]. This innovative hydrogel showed better biocompatibility, stronger tissue adhesion and oxidative stress inhibition in a mouse retinal injury model. In this system, adhesion was mainly generated by PDA and Cur@PDA NP with amines and thiols on the tissue surface through π-π stacking and hydrogen bonding. This study highlights the promising biomedical applications of adhesive hydrogels in ophthalmology and regenerative medicine, showcasing their versatility and potential impact in these crucial medical fields.
In retinal degenerative diseases such as age-related macular degeneration and retinitis pigmentosa, retinal neurons fail to regenerate. Chen et al. tackled this challenge by introducing a novel adhesive-based, protein-free synthetic hydrogel formulated from poly(2-acrylamido-2-methylpropanesulfonic acid sodium salt) (PNaAMPS) and poly(N,N-dimethylacrylamide) (PDMAAm) [148]. This hydrogel serves as an RPE cell culture matrix, enabling in vitro cultivation of human RPE cell monolayers with low ROS levels, aiming for potential intravitreal placement to foster retinal repair in the future.
While retinal progenitor cell (RPC) transplantation has been proposed to decelerate disease progression [149, 150], its efficacy faces limitations due to the restricted proliferation and differentiation of RPCs post-transplantation. Solid polymer scaffolds, although enhancing RPC proliferation in mice, suffer from issues such as disorganized cell delivery, reduced cell survival, and lack of adaptability and tight integration with the retina [151, 152]. Addressing these challenges, Jiang et al. developed a self-healing injectable hydrogel, CS-Odex, utilizing chitosan hydrochloride (CS) and oxidized dextran (Odex) as its base (Figure 8B) [153]. This hydrogel, adhering through dynamic Schiff bases, exhibits high biocompatibility and stimulates AKT and ERK pathways, thereby promoting RPC proliferation and differentiation.
Representative diagram of adhesive hydrogels applied for retinal regeneration. A) Injectable, antioxidative, and tissue-adhesive nanocomposite hydrogel as a potential treatment for inner retina injuries. Adapted with permission from [147], Copyright 2024 Wiley. B) Enhanced proliferation and differentiation of retinal progenitor cells through a self-healing injectable hydrogel. Adapted with permission from [153], Copyright 2019 Royal Society of Chemistry. C) Mussel-inspired injectable hydrogel and its counterpart for actuating proliferation and neuronal differentiation of retinal progenitor cells. Adapted with permission from [157], Copyright 2019 Elsevier.
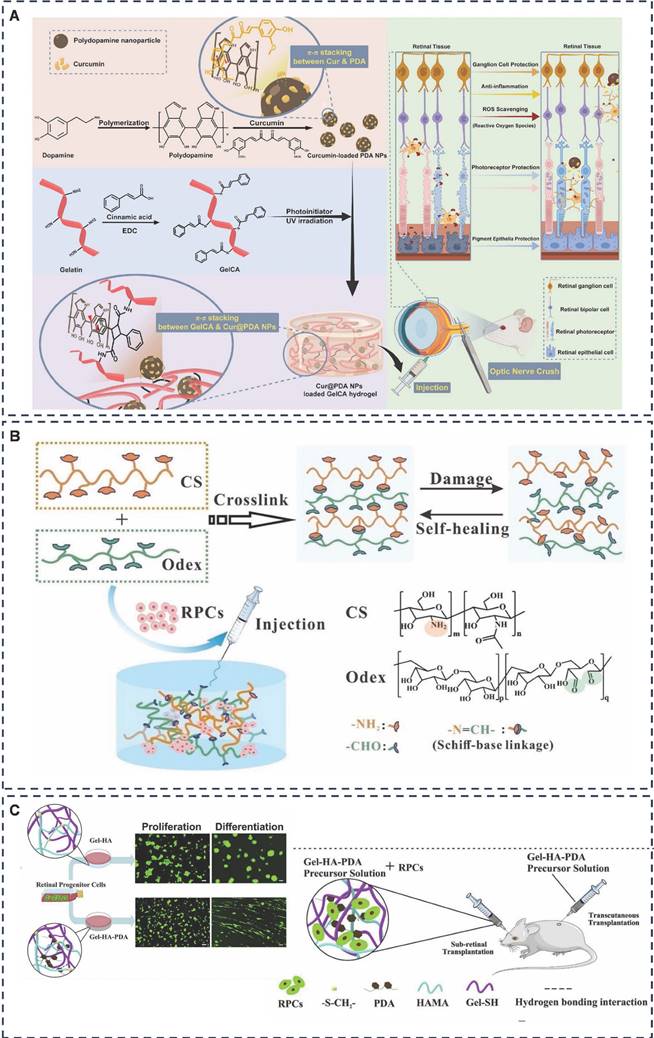
Inspired by the adhesive and proliferative properties of polydopamine (PDA), which promotes synaptic growth, gel-based scaffolds that enhance stem cell proliferation [154, 155], and hyaluronic acid (HA), a key component of the subretina [156]. Tang et al. devised Gel-HA-PDA, a highly biocompatible and adhesive injectable hydrogel (Figure 8C) [157]. This hydrogel significantly enhanced the proliferation, migration, and neuronal differentiation (e.g., photoreceptors) of RPCs, presenting a novel biomaterial platform for RPC transplantation-based therapies.
As we age, the vitreous body liquefies, causing it to detach from the retinal surface, resulting in retinal breakage. Liquefied vitreous can enter and accumulate in the subretinal space between the retinal neuroepithelium and retinal pigment epithelium (RPE), leading to fornix retinal detachment (RRD) [158]. RRD can be reattached by surgical removal of the vitreous and filling with silicone oil, but the rate of first retinal attachment is about 85% ± 11% [159-161]. On the other hand, postoperative proliferative vitreoretinopathy (PVR) may occur due to the presence of a retinal tear [162].
Fibrin glue has been mentioned in corneal wound repair and can exert better tissue adhesion through mechanisms such as mechanical interlocking and ionic bonding. In retinal laceration repair, Tyagi et al. conducted a clinical trial of fibrin glue as a repair agent for idiopathic macular laceration, and the results suggest that fibrin glue can be used in patients who have difficulty with postoperative fixation after retinal laceration repair surgery [163]. Hoshi et al. developed two absorbable PEG hydrogels for the treatment of RD and verified in a rabbit RD model that both PEG hydrogels adhered firmly to the rabbit retina and that FocalSeal treatment restored retinal function after 28 days of follow-up (Figure 9A) [164, 165]. Zheng et al. focused on another FDA-approved hydrogel, hyaluronic acid. They designed an injectable HA-engineered hydrogel based on Healaflow®, a commercially available retinal patch, with physical properties such as pH, osmolality, specific gravity, and refractive index consistent with those of Healaflow®, and strong adhesion through hydrogen bonding, ionic bonding, and mechanical interlocking (Figure 9B) [166]. In vitro, it maintains adhesion to RRD patients for 14 days and adheres to the retina. In a rabbit model of RRD, HA-engineered hydrogel was shown to adhere well to the retina, and at 3-month follow-up, it helped the retina to completely reattach and regain function without the need for expanding gas or silicone oil endothelial tamponade. The results of the current study are a boon to clinical RRD patients, but further long-term follow-up and clinical studies are needed.
Given the crucial role of the retina in converting visual signals into electrical impulses, its treatment demands materials of exceptional biocompatibility. In designing such materials, natural substances like hyaluronic acid, gelatin, sodium alginate, and chitosan are prime candidates due to their high biocompatibility. Adjusting the ratios of these substances can yield polymer systems with dual or multiple networks, balancing flexibility and rigidity. To further enhance retinal nerve regeneration, bioactive proteins like elastin and filipin can be incorporated. These proteins form robust bonds with amino acids on tissue surfaces through hydrogen bonds, π-π interactions, and electrostatic forces, fostering stronger adhesion between tissues and cells [167]. Moreover, in evaluating retinal function restoration, while the retinal electrophysiological test remains a cornerstone, advancements can be made by introducing additional tests. Designing full-field photosensitivity threshold tests (FST), multi-luminance mobility tests (MLMT), and other animal-specific evaluations would greatly enrich our means of assessing retinal function.
3.5 Open eye trauma
The eye, a closed cavity under high internal pressure, is susceptible to open trauma caused by external forces, sharp objects, or explosions. Although infrequent in urban settings, such trauma can significantly impair vision and predispose to intraocular infections, drastically reducing patients' quality of life. Immediate treatment involves thorough debridement and wound closure using sutures and adhesives. However, currently approved adhesives like fibrin glue [168, 169] and cyanoacrylates [84, 170] fall short due to their cumbersome handling, inadequate adhesive strength, and high toxicity, rendering them unsuitable for open ocular trauma.
Bayat et al. introduced an injectable, non-biodegradable liquid sealant formulated from a physically crosslinked copolymer of N-isopropylacrylamide and butyl acrylate (Figure 9C) [171]. The sealant can be modulated to form a gel at body temperature that adheres to the surface of the eye by mechanical interlocking, diffusion, hydrogen and covalent bonding to effectively seal ocular wounds. Once gelled, it restores intraocular pressure (IOP) and can be removed non-invasively for further treatment within a few days by changing the temperature without damaging the surrounding tissues.
Representative diagram of adhesive hydrogels applied for retinal detachment and open eye trauma. A) Patching retinal breaks with polyethylene glycol-based synthetic hydrogel sealant for retinal detachment in rabbits. Adapted with permission from [164], Copyright 2018 Elsevier. B) Biocompatibility and efficacy of a linearly cross-linked sodium hyaluronic acid hydrogel as a retinal patch in rhegmatogenous retinal detachment repairment. Adapted with permission from [166], Copyright 2022 Frontiers. C) A reversible thermoresponsive sealant for temporary closure of ocular trauma. Adapted with permission from [171], Copyright 2017 American Association for the Advancement of Science. D) Application of a collagen-based membrane and chondroitin sulfate-based hydrogel adhesive for the potential repair of severe ocular surface injuries. Adapted with permission from [172] , Copyright 2014 Oxford Academic.
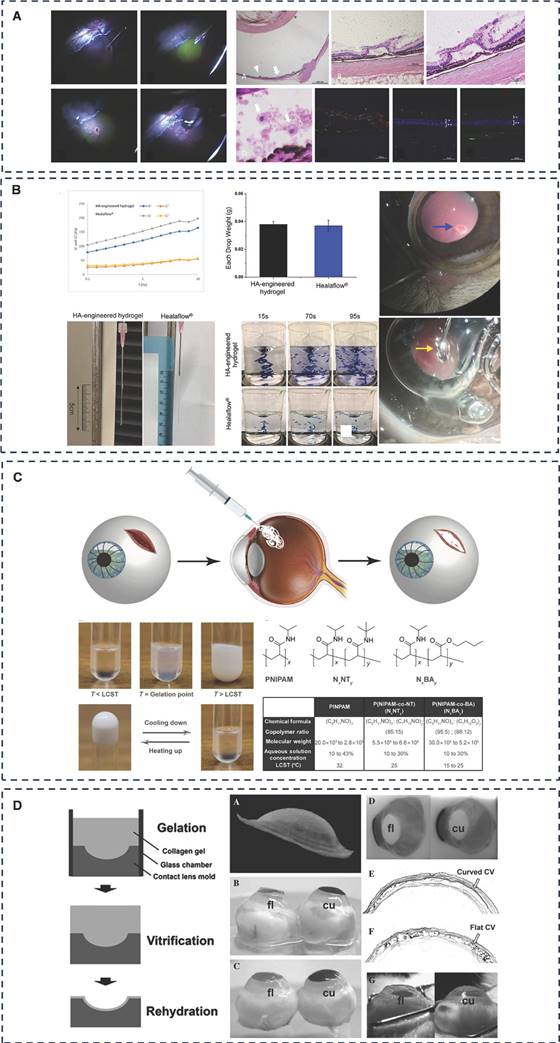
Chae et al. developed a viscous hydrogel comprising chondroitin sulfate-polyethylene glycol (CS-PEG) and a collagen vitreous gel (CV) membrane, infused with antibiotics (Figure 9D) [172]. This CS-PEG-CV biobandage has antimicrobial properties and has some adhesion properties due to hydrogen bonding and mechanical interlocking, and can be used as a biological dressing after ocular trauma. In addition, this dressing is transparent and can close larger corneal wounds without the need for surgical microscopy, making it suitable for emergency situations or battlefields. Future research aims to design bandages of varying sizes and investigate their long-term preservation efficacy.
Bhattacharjee et al. addressed both corneal injury closure and infection control by designing an antimicrobial adhesive hydrogel sealant [173]. The aldehyde groups in polyglucose aldehyde (PDA) interact with amino groups on tissue proteins, promoting hydrogel adhesion to the tissue surface. Corneal burst pressure tests showed that the BacSeal-3 hydrogel, when used to seal corneal incisions, can withstand pressures comparable to those of cyanoacrylate adhesives (~68 kPa) and demonstrates excellent biocompatibility and antimicrobial effectiveness.
Open ocular trauma, predominantly encountered in war or emergency medical scenarios, demands swift and robust sealing of the eye using adhesive materials with exceptional adhesion properties. This not only involves achieving a dense closure within a short time frame but also necessitates the evaluation of adhesion under diverse environmental conditions, spanning high temperatures, dryness, humidity, underwater settings, and low temperatures. Therefore, enhancing the comprehensive adhesion capability stands at the forefront of designing adhesive hydrogels for this condition. To achieve this, bionic polymeric materials can be crafted, drawing inspiration from mussel-like marine organisms like barnacles and abalone, or by incorporating adhesive agents like DOPA, tannic acid, and tea polyphenols. Alternatively, hydrogel networks can be formulated by modifying reactive groups, such as double bonds, and initiating free radical polymerization on the surface. This approach enables the design of network structures that yield exceptionally strong interfacial adhesion, crucial for effective wound sealing in open ocular trauma.
4. Summary and outlook
The ocular surface environment is inherently dynamic and humid, constantly subjected to external factors like wind, tears, dust and varying temperatures. Additionally, mechanical interactions between eyelids and the eyeball during regular activities like blinking, eye rubbing, and sleeping can leave the ocular surface vulnerable to wounds without adequate rest and protection. These complexities pose significant challenges in the design and application of adhesive hydrogels for the ocular surface. To address these challenges, researchers have developed bi- or multi-polymer network hydrogels (interpenetrating or semi-interpenetrating) by incorporating rigid and flexible polymer hydrogels. By adjusting the ratio of these two components, with or without the introduction of adhesive groups like tannic acid and DOPA, they have found formulations that are suitable for ocular surface tissues, further enhancing the overall adhesion of the system [100, 114, 147, 153]. Inspired by the asymmetric structure of the peritoneum, Liang et al. have successfully designed and prepared PVA hydrogels (JPVA) with a "Janus" porous structure using a top-down solvent exchange-solvite-hydration strategy [174]. This unique property of Janus hydrogels could potentially be applied to ocular adhesive hydrogels, particularly for intraocular retinopathy, paving the way for future advancements in this field.
Injectable hydrogels are polymer systems with good fluidity that, upon reaching the target site, undergo gelation triggered by factors such as self-crosslinking, temperature, pH, or light, enabling stable adhesion at the intended site. This distinct property makes injectable hydrogels especially well-suited for treating nasolacrimal duct disorders, minimally invasive glaucoma therapies, and vitreoretinal diseases. Although there has been significant research on injectable adhesive hydrogels for ocular applications, their use in treating diseases of ocular adnexa remains relatively limited [100, 147, 171]. For example, future designs could involve adhesive hydrogels that can be injected through the punctum to seal the lacrimal duct opening, potentially serving as punctal plugs. Furthermore, these hydrogels could be engineered to carry drugs, exosomes, or other therapeutic agents for controlled release, offering both structural and therapeutic benefits.
The ocular surface microenvironment is intricately intertwined with the external milieu, and its microbiota plays a crucial role in maintaining microenvironmental homeostasis. When this microbiota is thrown into imbalance, the microenvironment is disrupted, potentially resulting in delayed wound healing and even exacerbating ocular infections. To address this, incorporating antimicrobial substances into material design is a viable strategy. Regarding ocular adhesive polymer hydrogel formulations, the majority are currently in the form of liquid drops or flexible solid tablets. However, the future may witness the emergence of ocular spray hydrogels. Compared to in situ hydrogels forming liquid drops, sprayed hydrogels offer a broader coverage area, swiftly forming a lightweight coating to minimize intraocular foreign body sensation. Moreover, the spray method significantly reduces bacterial contamination during application [175-177]. Three-dimensionally printed hydrogels represent a rapid and efficient physical production method, allowing for continuous trial-and-error adjustments, and are currently a research hotspot [178, 179]. Given that the shape of the eyeball and the curvature of the cornea vary among individuals, artificial intelligence could potentially recognize the unique shape of the ocular surface and cornea. By combining this technology with 3D printing, hydrogels that are more compatible with tissue trauma could be tailored specifically for each patient.
Regarding the biological properties of ocular adhesive hydrogels, there is a dearth of drug-device combination studies that seamlessly integrate the dual aspects of drug delivery and adhesive repair [180, 181]. Such studies have the potential to augment repair mechanisms by adhering to wounds, encapsulating, and delivering drugs or bioactive substances for slow-release and controlled-release effects.
In summary, the eye is indispensable for vision, and while ocular adhesive hydrogels have garnered significant attention in recent research, numerous challenges persist due to the unique features of the eye, as well as the intricacies and variability of adhesive systems. Nonetheless, the application of adhesive polymer hydrogels in ophthalmology remains highly promising. To this end, we ought to devise multifunctional adhesive hydrogels tailored to specific ocular tissues and diseases, aiming to facilitate the diagnosis and treatment of ocular disorders, ultimately contributing to the advancement of human physical and mental well-being.
Acknowledgements
Authors would like to acknowledge the support provided by grants from the National Natural Science Foundation of China (No. 82271054, ZL; No. 82401321, YH), the China Postdoctoral Science Foundation (No. 2023M741608, YH), the Natural Science Foundation of Hunan Province (No. 2024JJ6402, YH).
Author contributions
Conceptualization: KY, YH. Visualization: KY, QZ. Supervision: QL, ZL, YH. Writing—original draft: KY. Writing—review & editing: QL, ZL, YH.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F. et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334-65
2. Clayton JA. Dry Eye. N Engl J Med. 2018;378:2212-23
3. Chuck RS, Jacobs DS, Lee JK, Afshari NA, Vitale S, Shen TT. et al. Refractive errors & refractive surgery preferred practice Pattern®. Ophthalmology. 2018;125:P1-p104
4. Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379:1739-48
5. Liu YC, Wilkins M, Kim T, Malyugin B, Mehta JS. Cataracts. Lancet. 2017;390:600-12
6. Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S. Age-related cataract. Lancet. 2005;365:599-609
7. Jayaram H, Kolko M, Friedman DS, Gazzard G. Glaucoma: now and beyond. Lancet. 2023;402:1788-801
8. Kang JM, Tanna AP. Glaucoma. Med Clin North Am. 2021;105:493-510
9. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124-36
10. Pandit S, Ho AC, Yonekawa Y. Recent advances in the management of proliferative diabetic retinopathy. Curr Opin Ophthalmol. 2023;34:232-6
11. Lin JB, Narayanan R, Philippakis E, Yonekawa Y, Apte RS. Retinal detachment. Nat Rev Dis Primers. 2024;10:18
12. Kwok JM, Yu CW, Christakis PG. Retinal detachment. Cmaj. 2020;192:E312
13. World Health Organization. Blindness and vision impairment. Revised 10 August 2023. https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment
14. De Andrade LM, Isenberg SJ. Does general anesthesia or intravitreal injection affect neurodevelopment in children undergoing ophthalmic procedures? Curr Opin Ophthalmol. 2019;30:326-30
15. Moisseiev E, Loewenstein A. Intravitreal injection-a small procedure for the eye, agiant leap for ophthalmology. Harefuah. 2019;158:121-5
16. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265-6
17. Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10:200223
18. Peña OA, Martin P. Cellular and molecular mechanisms of skin wound healing. Nat Rev Mol Cell Biol. 2024;25:599-616
19. Cao H, Duan L, Zhang Y, Cao J, Zhang K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct Target Ther. 2021;6:426
20. Masket S, Hovanesian JA, Levenson J, Tyson F, Flynn W, Endl M. et al. Hydrogel sealant versus sutures to prevent fluid egress after cataract surgery. J Cataract Refract Surg. 2014;40:2057-66
21. Ibach MJ, Shafer BM, Wallin DD, Puls-Boever KR, Thompson VM, Berdahl JP. The effectiveness and safety of Dextenza 0.4 mg for the treatment of postoperative inflammation and pain in patients after photorefractive keratectomy: the RESTORE trial. J Refract Surg. 2021;37:590-4
22. Jiang Y, Wang Y, Li Q, Yu C, Chu W. Natural polymer-based stimuli-responsive hydrogels. Current Medicinal Chemistry. 2020;27:2631-57
23. Gu Z, Cao G, Wu C, Huang Y, Xu B, Zhuang S. et al. Comparing the ocular surface temperature and dry eye condition of keratoconus with normal eyes using infrared thermal imaging. Int Ophthalmol. 2023;43:4781-9
24. Nagase K. Thermoresponsive interfaces obtained using poly (N-isopropylacrylamide)-based copolymer for bioseparation and tissue engineering applications. Adv Colloid Interface Sci. 2021;295:102487
25. Soliman KA, Ullah K, Shah A, Jones DS, Singh TRR. Poloxamer-based in situ gelling thermoresponsive systems for ocular drug delivery applications. Drug Discov Today. 2019;24:1575-86
26. Rashki S, Asgarpour K, Tarrahimofrad H, Hashemipour M, Ebrahimi MS, Fathizadeh H. et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr Polym. 2021;251:117108
27. Li S, Yang C, Li J, Zhang C, Zhu L, Song Y. et al. Progress in pluronic F127 derivatives for application in wound healing and repair. Int J Nanomedicine. 2023;18:4485-505
28. Konieczynska MD, Grinstaff MW. On-demand dissolution of chemically cross-linked hydrogels. Acc Chem Res. 2017;50:151-60
29. Kim BJ, Oh DX, Kim S, Seo JH, Hwang DS, Masic A. et al. Mussel-mimetic protein-based adhesive hydrogel. Biomacromolecules. 2014;15:1579-85
30. McBain JW, Hopkins DG. On Adhesives and Adhesive Action. The Journal of Physical Chemistry. 1925;29:188-204
31. Liu X, Wang L, Qiao Y, Sun X, Ma S, Cheng X. et al. Adhesion of liquid food to packaging surfaces: Mechanisms, test methods, influencing factors and anti-adhesion methods. J Food Eng. 2018;228:102-17
32. Jabłoński M. Hydrogen Bonds. Molecules. 2023;28:1616
33. Zheng P, Li H. Highly covalent ferric-thiolate bonds exhibit surprisingly low mechanical stability. J Am Chem Soc. 2011;133:6791-8
34. Yang J, Bai R, Chen B, Suo Z. Hydrogel adhesion: a supramolecular synergy of chemistry, topology, and mechanics. Adv Funct Mater. 2020;30:1901693
35. Maier GP, Rapp MV, Waite JH, Israelachvili JN, Butler A. Biological adhesives: Adaptive synergy between catechol and lysine promotes wet adhesion by surface salt displacement. Science. 2015;349:628-32
36. Mu Y, Mu P, Wu X, Wan X. The two facets of the synergic effect of amine cation and catechol on the adhesion of catechol in underwater conditions. Appl Surf Sci. 2020;530:146973
37. Nema HV, Nema N. Ophthalmology. Beijing: Tsinghua University Press Publisher. 2016
38. Stern JH, Tian Y, Funderburgh J, Pellegrini G, Zhang K, Goldberg JL. et al. Regenerating eye tissues to preserve and restore vision. Cell Stem Cell. 2018;22:834-49
39. Thacker M, Singh V, Basu S, Singh S. Biomaterials for dry eye disease treatment: Current overview and future perspectives. Exp Eye Res. 2023;226:109339
40. Fea AM, Novarese C, Caselgrandi P, Boscia G. Glaucoma treatment and hydrogel: current insights and state of the art. Gels. 2022;8:510
41. Zielińska A, Eder P, Rannier L, Cardoso JC, Severino P, Silva AM. et al. Hydrogels for modified-release drug delivery systems. Curr Pharm Des. 2022;28:609-18
42. Al-Kinani AA, Zidan G, Elsaid N, Seyfoddin A, Alani AWG, Alany RG. Ophthalmic gels: Past, present and future. Adv Drug Deliv Rev. 2018;126:113-26
43. Caffin F, Boccara D, Piérard C. The use of hydrogel dressings in sulfur mustard-induced skin and ocular wound management. Biomedicines. 2023Jun2;11(6):1626
44. Hoon M, Okawa H, Della Santina L, Wong RO. Functional architecture of the retina: development and disease. Prog Retin Eye Res. 2014;42:44-84
45. Cooper RC, Yang H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J Control Release. 2019;306:29-39
46. Tan CS, Ngo WK, Chay IW, Ting DS, Sadda SR. Neovascular age-related macular degeneration (nAMD): A Review of emerging treatment options. Clin Ophthalmol. 2022;16:917-33
47. Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK. et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276-83
48. Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX. et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575-628
49. Li Q, Cao Y, Wang P. Recent advances in hydrogels for the diagnosis and treatment of dry eye disease. Gels. 2022Dec11;8(12):816
50. Joshi VP, Singh S, Thacker M, Pati F, Vemuganti GK, Basu S. et al. Newer approaches to dry eye therapy: Nanotechnology, regenerative medicine, and tissue engineering. Indian J Ophthalmol. 2023;71:1292-303
51. Han Y, Jiang L, Shi H, Xu C, Liu M, Li Q. et al. Effectiveness of an ocular adhesive polyhedral oligomeric silsesquioxane hybrid thermo-responsive FK506 hydrogel in a murine model of dry eye. Bioact Mater. 2022;9:77-91
52. Qin R, Guo Y, Ren H, Liu Y, Su H, Chu X. et al. Instant adhesion of amyloid-like nanofilms with wet surfaces. ACS Cent Sci. 2022;8:705-17
53. Ou L, Wu Z, Hu X, Huang J, Yi Z, Gong Z. et al. A tissue-adhesive F127 hydrogel delivers antioxidative copper-selenide nanoparticles for the treatment of dry eye disease. Acta Biomater. 2024;175:353-68
54. Administration USFaD. U.S. food and drug administration (FDA)-approved carboxymethylcellulose. Revised 23 June 2024. https://nctr-crs.fda.gov/fdalabel/ui/spl-summaries/criteria/639487
55. Lin T, Lu Y, Zhang X, Gong L, Wei C. Treatment of dry eye by intracanalicular injection of a thermosensitive chitosan-based hydrogel: evaluation of biosafety and availability. Biomater Sci. 2018;6:3160-9
56. Garrett Q, Simmons PA, Xu S, Vehige J, Zhao Z, Ehrmann K. et al. Carboxymethylcellulose Binds to Human Corneal Epithelial Cells and Is a Modulator of Corneal Epithelial Wound Healing. Invest Ophthalmol Vis Sci. 2007;48:1559-67
57. Lee JS, Lee SU, Che CY, Lee JE. Comparison of cytotoxicity and wound healing effect of carboxymethylcellulose and hyaluronic acid on human corneal epithelial cells. Int J Ophthalmol. 2015;8:215-21
58. Janagam DR, Wu L, Lowe TL. Nanoparticles for drug delivery to the anterior segment of the eye. Adv Drug Deliv Rev. 2017;122:31-64
59. Liu S, Chang CN, Verma MS, Hileeto D, Muntz A, Stahl U. et al. Phenylboronic acid modified mucoadhesive nanoparticle drug carriers facilitate weekly treatment of experimentallyinduced dry eye syndrome. Nano Res. 2015;8:621-35
60. Huang C, Chen W, Chen Y, Liu Z. Toxicity research status of benzalkonium chloride on ocular surface. Zhonghua Yan Ke Za Zhi. 2014;50:303-6
61. Cheng YH, Huang HP, Chen HH. Mucoadhesive phenylboronic acid-grafted carboxymethyl cellulose hydrogels containing glutathione for treatment of corneal epithelial cells exposed to benzalkonium chloride. Colloids Surf B Biointerfaces. 2024;238:113884
62. Wei W, Cao H, Shen D, Sun X, Jia Z, Zhang M. Antioxidant carbon dots nanozyme loaded in thermosensitive in situ hydrogel system for efficient dry eye disease treatment. Int J Nanomedicine. 2024;19:4045-60
63. Wang JJ, Liu XX, Zhu CC, Wang TZ, Wang SY, Liu Y. et al. Improving ocular bioavailability of hydrophilic drugs through dynamic covalent complexation. J Control Release. 2023;355:395-405
64. Cabrera-Aguas M, Khoo P, Watson SL. Infectious keratitis: A review. Clin Exp Ophthalmol. 2022;50:543-62
65. Chiang MC, Chern E. More than antibiotics: Latest therapeutics in the treatment and prevention of ocular surface Iinfections. J Clin Med. 2022Jul19;11(14):4195
66. Sharma A, Taniguchi J. Review: Emerging strategies for antimicrobial drug delivery to the ocular surface: Implications for infectious keratitis. Ocul Surf. 2017;15:670-9
67. Ruan M, Wang R, He Y. Novel drug delivery systems for the management of Fungal keratitis. J Ocul Pharmacol Ther. 2024;40:160-72
68. Jiao Z, Huo Q, Lin X, Chu X, Deng Z, Guo H. et al. Drug-free contact lens based on quaternized chitosan and tannic acid for bacterial keratitis therapy and corneal repair. Carbohydr Polym. 2022;286:119314
69. Sha X, Chan L, Fan X, Guo P, Chen T, Liu L. et al. Thermosensitive tri-block polymer nanoparticle-hydrogel composites as payloads of natamycin for antifungal therapy against fusarium solani. Int J Nanomedicine. 2022;17:1463-78
70. Chomchalao P, Saelim N, Lamlertthon S, Sisopa P, Tiyaboonchai W. Mucoadhesive hybrid system of silk fibroin nanoparticles and thermosensitive in situ hydrogel for amphotericin B delivery: A potential option for fungal keratitis treatment. Polymers (Basel). 2024Jan3;16(1):148
71. Amit C, Muralikumar S, Janaki S, Lakshmipathy M, Therese KL, Umashankar V. et al. Designing and enhancing the antifungal activity of corneal specific cell penetrating peptide using gelatin hydrogel delivery system. Int J Nanomedicine. 2019;14:605-22
72. Swift T, Pinnock A, Shivshetty N, Pownall D, MacNeil S, Douglas I. et al. Generation and use of functionalised hydrogels that can rapidly sample infected surfaces. MethodsX. 2022;9:101684
73. Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F. et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134:167-73
74. Gauthier AS, Castelbou M, Garnier MB, Pizzuto J, Roux S, Gain P. et al. Corneal transplantation: study of the data of a regional eye bank for the year 2013 and analysis of the evolution of the adverse events reported in France since 2010. Cell Tissue Bank. 2017;18:83-9
75. Lau N, Hajjar Sesé A, Augustin VA, Kuit G, Wilkins MR, Tourtas T. et al. Fungal infection after endothelial keratoplasty: association with hypothermic corneal storage. Br J Ophthalmol. 2019;103:1487-90
76. Grinstaff MW. Designing hydrogel adhesives for corneal wound repair. Biomaterials. 2007;28:5205-14
77. Gundersen T. Cornea and sclera. AMA Arch Ophthalmol. 1956;55:274-92
78. Yazdanpanah G, Shen X, Nguyen T, Anwar KN, Jeon O, Jiang Y. et al. A light-curable and tunable extracellular matrix hydrogel for in situ suture-free corneal repair. Adv Funct Mater. 2022Jun10;32(24):2113383
79. Zhao L, Shi Z, Sun X, Yu Y, Wang X, Wang H. et al. Natural dual-crosslinking bioadhesive hydrogel for corneal regeneration in large-size defects. Adv Healthc Mater. 2022;11:e2201576
80. Fernandes-Cunha GM, Jeong SH, Logan CM, Le P, Mundy D, Chen F. et al. Supramolecular host-guest hyaluronic acid hydrogels enhance corneal wound healing through dynamic spatiotemporal effects. Ocul Surf. 2022;23:148-61
81. Matossian C, Makari S, Potvin R. Cataract surgery and methods of wound closure: a review. Clin Ophthalmol. 2015;9:921-8
82. Alió JL, Mulet ME, Garcia JC. Use of cyanoacrylate tissue adhesive in small-incision cataract surgery. Ophthalmic Surg Lasers. 1996;27:270-4
83. Chen WL, Lin CT, Hsieh CY, Tu IH, Chen WY, Hu FR. Comparison of the bacteriostatic effects, corneal cytotoxicity, and the ability to seal corneal incisions among three different tissue adhesives. Cornea. 2007;26:1228-34
84. Vote BJ, Elder MJ. Cyanoacrylate glue for corneal perforations: a description of a surgical technique and a review of the literature. Clin Exp Ophthalmol. 2000;28:437-42
85. Banitt M, Malta JB, Soong HK, Musch DC, Mian SI. Wound integrity of clear corneal incisions closed with fibrin and N-butyl-2-cyanoacrylate adhesives. Curr Eye Res. 2009;34:706-10
86. Tong AY, Gupta PK, Kim T. Wound closure and tissue adhesives in clear corneal incision cataract surgery. Curr Opin Ophthalmol. 2018;29:14-8
87. Nallasamy N, Grove KE, Legault GL, Daluvoy MB, Kim T. Hydrogel ocular sealant for clear corneal incisions in cataract surgery. J Cataract Refract Surg. 2017;43:1010-4
88. Spierer O, O'Brien TP. Endothelial keratoplasty combined with cataract surgery or alone using polyethylene glycol hydrogel sealant for closure of corneal incisions. J Cataract Refract Surg. 2015;41:492-6
89. Zhou H, Zhang S, Lei M, Cai Y, Wang H, Sun J. et al. A suture-free, shape self-adaptive and bioactive PEG-Lysozyme implant for Corneal stroma defect repair and rapid vision restoration. Bioact Mater. 2023;29:1-15
90. Ladewig K. Drug delivery in soft tissue engineering. Expert Opin Drug Deliv. 2011;8:1175-88
91. Patel CG, Dalwadi AC. Recent patents on stimuli responsive hydrogel drug delivery system. Recent Pat Drug Deliv Formul. 2013;7:206-15
92. Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254-71
93. Shirzaei Sani E, Kheirkhah A, Rana D, Sun Z, Foulsham W, Sheikhi A. et al. Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Sci Adv. 2019;5:eaav1281
94. Jumelle C, Yung A, Sani ES, Taketani Y, Gantin F, Bourel L. et al. Development and characterization of a hydrogel-based adhesive patch for sealing open-globe injuries. Acta Biomater. 2022;137:53-63
95. Li M, Wei R, Liu C, Fang H, Yang W, Wang Y. et al. A "T.E.S.T." hydrogel bioadhesive assisted by corneal cross-linking for in situ sutureless corneal repair. Bioact Mater. 2023;25:333-46
96. Zhao X, Li S, Du X, Li W, Wang Q, He D. et al. Natural polymer-derived photocurable bioadhesive hydrogels for sutureless keratoplasty. Bioact Mater. 2022;8:196-209
97. Wang F, Zhang W, Qiao Y, Shi D, Hu L, Cheng J. et al. ECM-Like adhesive hydrogel for the regeneration of large corneal stromal defects. Adv Healthc Mater. 2023;12:e2300192
98. Qian Y, Xu K, Shen L, Dai M, Zhao Z, Zheng Y. et al. Dopamine-based high-transparent hydrogel as bioadhesive for sutureless ocular tissue repair. Adv Funct Mater. 2023;33:2300707
99. Borouman S, Sigaroodi F, Ahmadi Tafti SM, Khoshmaram K, Soleimani M, Khani MM. ECM-based bioadhesive hydrogel for sutureless repair of deep anterior corneal defects. Biomater Sci. 2024;12:2356-68
100. Wang Q, Zhao X, Yu F, Fang PH, Liu L, Du X. et al. Photocurable and temperature-sensitive bioadhesive hydrogels for sutureless sealing of full-thickness corneal wounds. Small Methods. 2024;8:e2300996
101. Zhang W, Liu S, Wang L, Li B, Xie M, Deng Y. et al. Triple-crosslinked double-network alginate/dextran/dendrimer hydrogel with tunable mechanical and adhesive properties: A potential candidate for sutureless keratoplasty. Carbohydr Polym. 2024;344:122538
102. Barrientez B, Nicholas SE, Whelchel A, Sharif R, Hjortdal J, Karamichos D. Corneal injury: Clinical and molecular aspects. Exp Eye Res. 2019;186:107709
103. Li Z, Liu R, Zhang X, Guo Z, Geng X, Chu D. et al. An injectable thermoresponsive-hydrogel for lamellar keratoplasty: In-situ releases celastrol and hampers corneal scars. J Control Release. 2024;369:604-16
104. Jacobs DS, Afshari NA, Bishop RJ, Keenan JD, Lee J, Shen TT. et al. Refractive errors preferred practice Pattern®. Ophthalmology. 2023;130:P1-p60
105. Ramsook SS, Hersh PS. Use of a hydrogel sealant in epithelial ingrowth removal after laser in situ keratomileusis. J Cataract Refract Surg. 2015;41:2768-71
106. Yesilirmak N, Diakonis VF, Battle JF, Yoo SH. Application of a hydrogel ocular sealant to avoid recurrence of epithelial ingrowth after LASIK enhancement. J Refract Surg. 2015;31:275-7
107. Thulasi P, Kim SW, Shetty R, Randleman JB. Recalcitrant epithelial ingrowth after SMILE treated with a hydrogel ocular sealant. J Refract Surg. 2015;31:847-50
108. Mahmood S, Buabeid MA, Ullah K, Murtaza G, Mannan A, Khan SA. Synthesis, characterization and safety profiling of eudragit-based pH responsive hydrogels: A promising platform for colonic delivery of losartan potassium. Curr Drug Deliv. 2019;16:548-64
109. Zhu Q, Liu C, Sun Z, Zhang X, Liang N, Mao S. Inner layer-embedded contact lenses for pH-triggered controlled ocular drug delivery. Eur J Pharm Biopharm. 2018;128:220-9
110. Esporrín-Ubieto D, Sonzogni AS, Fernández M, Acera A, Matxinandiarena E, Cadavid-Vargas JF. et al. The role of Eudragit® as a component of hydrogel formulations for medical devices. J Mater Chem B. 2023;11:9276-89
111. Romano V, Cruciani M, Conti L, Fontana L. Fibrin glue versus sutures for conjunctival autografting in primary pterygium surgery. Cochrane Database Syst Rev. 2016;12:Cd011308
112. Karalezli A, Kucukerdonmez C, Akova YA, Altan-Yaycioglu R, Borazan M. Fibrin glue versus sutures for conjunctival autografting in pterygium surgery: a prospective comparative study. Br J Ophthalmol. 2008;92:1206-10
113. Bondalapati S, Ambati B. Minimally invasive pterygium surgery: sutureless excision with amniotic membrane and hydrogel sealant. Case Rep Ophthalmol. 2016;7:79-84
114. Liu J, Huang Y, Yang W, Sun X, Xu Y, Peng Y. et al. Sutureless transplantation using a semi-interpenetrating polymer network bioadhesive for ocular surface reconstruction. Acta Biomater. 2022;153:273-86
115. Sougata Jana, Subrata Jana. Semi-interpenetrating polymer network. 3.0.1 ed: International Union of Pure and Applied Chemistry (IUPAC). 2019
116. Zheng Y, Baidya A, Annabi N. Molecular design of an ultra-strong tissue adhesive hydrogel with tunable multifunctionality. Bioact Mater. 2023;29:214-29
117. Lee SY, Chee SP, Balakrishnan V, Farzavandi S, Tan DT. Surodex in paediatric cataract surgery. Br J Ophthalmol. 2003;87:1424-6
118. Tan DT, Chee SP, Lim L, Lim AS. Randomized clinical trial of a new dexamethasone delivery system (Surodex) for treatment of post-cataract surgery inflammation. Ophthalmology. 1999;106:223-31
119. Tan DT, Chee SP, Lim L, Theng J, Van Ede M. Randomized clinical trial of Surodex steroid drug delivery system for cataract surgery: anterior versus posterior placement of two Surodex in the eye. Ophthalmology. 2001;108:2172-81
120. Wadood AC, Armbrecht AM, Aspinall PA, Dhillon B. Safety and efficacy of a dexamethasone anterior segment drug delivery system in patients after phacoemulsification. J Cataract Refract Surg. 2004;30:761-8
121. Donnenfeld E, Holland E. Dexamethasone intracameral drug-delivery suspension for inflammation associated with cataract surgery: A randomized, placebo-controlled, phase III trial. Ophthalmology. 2018;125:799-806
122. Donnenfeld ED, Solomon KD, Matossian C. Safety of IBI-10090 for inflammation associated with cataract surgery: Phase 3 multicenter study. J Cataract Refract Surg. 2018;44:1236-46
123. Shamma RN, Sayed RH, Madry H, El Sayed NS, Cucchiarini M. Triblock copolymer bioinks in hydrogel three-dimensional printing for regenerative medicine: A focus on pluronic F127. Tissue Eng Part B Rev. 2022;28:451-63
124. Giuliano E, Paolino D, Fresta M, Cosco D. Mucosal applications of poloxamer 407-based hydrogels: An overview. Pharmaceutics. 2018;10:159
125. Sapino S, Peira E, Chirio D, Chindamo G, Guglielmo S, Oliaro-Bosso S. et al. Thermosensitive nanocomposite hydrogels for intravitreal delivery of cefuroxime. Nanomaterials (Basel). 2019Oct15;9(10):1461
126. Wormstone IM, Wormstone YM, Smith AJO, Eldred JA. Posterior capsule opacification: What's in the bag? Prog Retin Eye Res. 2021;82:100905
127. Liu JL, Zhang WJ, Li XD, Yang N, Pan WS, Kong J. et al. Sustained-release genistein from nanostructured lipid carrier suppresses human lens epithelial cell growth. Int J Ophthalmol. 2016;9:643-9
128. Yan T, Ma Z, Liu J, Yin N, Lei S, Zhang X. et al. Thermoresponsive genisteinNLC-dexamethasone-moxifloxacin multi drug delivery system in lens capsule bag to prevent complications after cataract surgery. Sci Rep. 2021;11:181
129. Kane JX, Chang DF. Intraocular lens power formulas, biometry, and intraoperative aberrometry: a review. Ophthalmology. 2021;128:e94-e114
130. Wang L, Koch DD. Intraocular lens power calculations in eyes with previous corneal refractive surgery: Review and expert opinion. Ophthalmology. 2021;128:e121-e31
131. Causes of blindness, vision impairment in 2020, trends over 30 years, prevalence of avoidable blindness in relation to VISION 2020. the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144-e60
132. Aulenta F, Hayes W, Rannard SP. Dendrimers: a new class of nanoscopic containers and delivery devices. Eur Polym J. 2003;39:1741-71
133. Kim ID, Shin JH, Kim SW, Choi S, Ahn J, Han PL. et al. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol Ther. 2012;20:829-39
134. Zhang L, Zhu S, Qian L, Pei Y, Qiu Y, Jiang Y. RGD-modified PEG-PAMAM-DOX conjugates: in vitro and in vivo studies for glioma. Eur J Pharm Biopharm. 2011;79:232-40
135. Sun X, Pang Z, Ye H, Qiu B, Guo L, Li J. et al. Co-delivery of pEGFP-hTRAIL and paclitaxel to brain glioma mediated by an angiopep-conjugated liposome. Biomaterials. 2012;33:916-24
136. Guo L, Fan L, Ren J, Pang Z, Ren Y, Li J. et al. Combination of trail and actinomycin D liposomes enhances antitumor effect in non-small cell lung cancer. Int J Nanomedicine. 2012;7:1449-60
137. Arkas M, Vardavoulias M, Kythreoti G, Giannakoudakis DA. Dendritic polymers in tissue engineering: contributions of PAMAM, PPI PEG and PEI to injury restoration and bioactive scaffold evolution. Pharmaceutics. 2023Feb4;15(2):524
138. Mishra V, Jain NK. Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int J Pharm. 2014;461:380-90
139. Fernández-Colino A, Quinteros DA, Allemandi DA, Girotti A, Palma SD, Arias FJ. Self-Assembling Elastin-Like Hydrogels for Timolol Delivery: Development of an Ophthalmic Formulation Against Glaucoma. Mol Pharm. 2017;14:4498-508
140. Yang H, Tyagi P, Kadam RS, Holden CA, Kompella UB. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one Week and antiglaucoma effects for four days following one-time topical administration. ACS Nano. 2012;6:7595-606
141. Herschler J, Claflin AJ, Fiorentino G. The effect of aqueous humor on the growth of subconjunctival fibroblasts in tissue culture and its implications for glaucoma surgery. Am J Ophthalmol. 1980;89:245-9
142. Martin G, Lübke J, Schefold S, Jordan JF, Schlunck G, Reinhard T. et al. Prevention of ocular tenon adhesion to sclera by a PDMAA polymer to improve results after glaucoma surgery. Macromol Rapid Commun. 2020;41:e1900352
143. Chae JJ, Jung JH, Zhu W, Gerberich BG, Bahrani Fard MR, Grossniklaus HE. et al. Drug-free, nonsurgical reduction of intraocular pressure for four months after suprachoroidal injection of hyaluronic acid hydrogel. Adv Sci (Weinh). 2021;8:2001908
144. Enders P, Cursiefen C. Device profile of the EYEMATE-IO™ system for intraocular pressure monitoring: overview of its safety and efficacy. Expert Rev Med Devices. 2020;17:491-7
145. Wang J, Li M, Geng Z, Khattak S, Ji X, Wu D. et al. Role of Oxidative Stress in Retinal Disease and the Early Intervention Strategies: A Review. Oxid Med Cell Longev. 2022Oct14;2022:7836828
146. Rohowetz LJ, Kraus JG, Koulen P. Reactive oxygen species-mediated damage of retinal neurons: drug development targets for therapies of chronic neurodegeneration of the retina. Int J Mol Sci. 2018Oct27;19(11):3362
147. Liu YC, Lin YK, Lin YT, Lin CW, Lan GY, Su YC. et al. Injectable, antioxidative, and tissue-adhesive nanocomposite hydrogel as a potential treatment for inner retina injuries. Adv Sci (Weinh). 2024;11:e2308635
148. Chen YM, Liu ZQ, Feng ZH, Xu F, Liu JK. Adhesive protein-free synthetic hydrogels for retinal pigment epithelium cell culture with low ROS level. J Biomed Mater Res A. 2014;102:2258-67
149. Li SY, Yin ZQ, Chen SJ, Chen LF, Liu Y. Rescue from light-induced retinal degeneration by human fetal retinal transplantation in minipigs. Curr Eye Res. 2009;34:523-35
150. Klassen HJ, Ng TF, Kurimoto Y, Kirov I, Shatos M, Coffey P. et al. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest Ophthalmol Vis Sci. 2004;45:4167-73
151. Ni N, Ji J, Chen S, Zhang D, Wang Z, Shen B. et al. Poly(1,3-propylene sebacate) and Poly(sebacoyl diglyceride): A Pair of Potential Polymers for the Proliferation and Differentiation of Retinal Progenitor Cells. Macromol Biosci. 2016;16:1334-47
152. Redenti S, Neeley WL, Rompani S, Saigal S, Yang J, Klassen H. et al. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials. 2009;30:3405-14
153. Jiang F, Tang Z, Zhang Y, Ju Y, Gao H, Sun N. et al. Enhanced proliferation and differentiation of retinal progenitor cells through a self-healing injectable hydrogel. Biomater Sci. 2019;7:2335-47
154. Tseng T-C, Tao L, Hsieh F-Y, Wei Y, Chiu I-M, Hsu S-h. An injectable, self-healing hydrogel to repair the central nervous system. Adv Mater. 2015;27:3518-24
155. Liu L, Yoshioka M, Nakajima M, Ogasawara A, Liu J, Hasegawa K. et al. Nanofibrous gelatin substrates for long-term expansion of human pluripotent stem cells. Biomaterials. 2014;35:6259-67
156. Clark SJ, Keenan TDL, Fielder HL, Collinson LJ, Holley RJ, Merry CLR. et al. Mapping the differential distribution of glycosaminoglycans in the adult human retina, choroid, and sclera. Invest Ophthalmol Vis Sci. 2011;52:6511-21
157. Tang Z, Jiang F, Zhang Y, Zhang Y, YuanYang, Huang X. et al. Mussel-inspired injectable hydrogel and its counterpart for actuating proliferation and neuronal differentiation of retinal progenitor cells. Biomaterials. 2019;194:57-72
158. Feltgen N, Walter P. Rhegmatogenous retinal detachment-an ophthalmologic emergency. Dtsch Arztebl Int. 2014;111:12-21 quiz 2
159. Oshima Y, Wakabayashi T, Sato T, Ohji M, Tano Y. A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology. 2010;117:93-102.e2
160. Bourla DH, Bor E, Axer-Siegel R, Mimouni K, Weinberger D. Outcomes and complications of rhegmatogenous retinal detachment repair with selective sutureless 25-gauge pars plana vitrectomy. Am J Ophthalmol. 2010;149:630-4.e1
161. Hillier RJ, Felfeli T, Berger AR, Wong DT, Altomare F, Dai D. et al. The pneumatic retinopexy versus vitrectomy for the management of primary rhegmatogenous retinal detachment outcomes randomized trial (PIVOT). Ophthalmology. 2019;126:531-9
162. Pastor JC, Rojas J, Pastor-Idoate S, Di Lauro S, Gonzalez-Buendia L, Delgado-Tirado S. Proliferative vitreoretinopathy: A new concept of disease pathogenesis and practical consequences. Prog Retin Eye Res. 2016;51:125-55
163. Tyagi M, Reddy NG, Sahoo NK. Fibrin-glue-assisted surgery for idiopathic macular holes. Semin Ophthalmol. 2024;39:172-5
164. Hoshi S, Okamoto F, Arai M, Hirose T, Sugiura Y, Murakami T. et al. Patching retinal breaks with polyethylene glycol-based synthetic hydrogel sealant for retinal detachment in rabbits. Exp Eye Res. 2018;177:117-21
165. Hoshi S, Okamoto F, Arai M, Hirose T, Sugiura Y, Kaji Y. et al. In vivo and in vitro feasibility studies of intraocular use of polyethylene glycol-based synthetic sealant to close retinal breaks in porcine and rabbit eyes. Invest Ophthalmol Vis Sci. 2015;56:4705-11
166. Zheng C, Xi H, Wen D, Ke Y, Zhang X, Ren X. et al. Biocompatibility and efficacy of a linearly cross-linked sodium hyaluronic acid hydrogel as a retinal patch in rhegmatogenous retinal detachment repairment. Front Bioeng Biotechnol. 2022;10:914675
167. Soucy JR, Shirzaei Sani E, Portillo Lara R, Diaz D, Dias F, Weiss AS. et al. Photocrosslinkable gelatin/tropoelastin hydrogel adhesives for peripheral nerve repair. Tissue Eng Part A. 2018;24:1393-405
168. Koranyi G, Seregard S, Kopp ED. Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol. 2004Jul;88(7):911-4
169. Hall RC, Logan AJ, Wells AP. Comparison of fibrin glue with sutures for pterygium excision surgery with conjunctival autografts. Clin Exp Ophthalmol. 2009Aug;37(6):584-9
170. Forseth M, O'Grady K, Toriumi DM. The current status of cyanoacrylate and fibrin tissue adhesives. J Long Term Eff Med Implants. 1992;2(4):221-33
171. Bayat N, Zhang Y, Falabella P, Menefee R, Whalen JJ, Humayun MS. et al. A reversible thermoresponsive sealant for temporary closure of ocular trauma. Sci Transl Med. 2017Dec6;9(419):eaan3879
172. Chae JJ, Mulreany DG, Guo Q, Lu Q, Choi JS, Strehin I. et al. Application of a collagen-based membrane and chondroitin sulfate-based hydrogel adhesive for the potential repair of severe ocular surface injuries. Mil Med. 2014Jun;179(6):686-94
173. Bhattacharjee B, Tabbasum K, Mukherjee R, Garg P, Haldar J. Functionalized chitosan based antibacterial hydrogel sealant for simultaneous infection eradication and tissue closure in ocular injuries. Int J Biol Macromol. 2024Jul;273(Pt 1):132838
174. Liang W, He W, Huang R, Tang Y, Li S, Zheng B. et al. Peritoneum-Inspired Janus Porous Hydrogel with Anti-Deformation, Anti-Adhesion, and Pro-Healing Characteristics for Abdominal Wall Defect Treatment. Adv Mater. 2022Apr;34(15):e2108992
175. Chen S, Luo Y, He Y, Li M, Liu Y, Zhou X. et al. In-situ-sprayed therapeutic hydrogel for oxygen-actuated Janus regulation of postsurgical tumor recurrence/metastasis and wound healing. Nat Commun. 2024Jan27;15(1):814
176. Zhong G, Lei P, Guo P, Yang Q, Duan Y, Zhang J. et al. A photo-induced cross-linking enhanced A and B combined multi-functional spray hydrogel instantly protects and promotes of irregular dynamic wound healing. Small. 2024Jun;20(23):e2309568
177. Li X, Lin H, Yu Y, Lu Y, He B, Liu M. et al. In situ rapid-formation sprayable hydrogels for challenging tissue injury management. Adv Mater. 2024May;36(19):e2400310
178. Liu Q, Dong X, Qi H, Zhang H, Li T, Zhao Y. et al. 3D printable strong and tough composite organo-hydrogels inspired by natural hierarchical composite design principles. Nat Commun. 2024Apr15;15(1):3237
179. Liu P, Mao Z, Zhao Y, Yin J, Chu C, Chen X. et al. Hydrogel-reactive-microenvironment powering reconfiguration of polymer architectures. Adv Sci (Weinh). 2024Jun;11(24):e2307830
180. Zhao X, Chen X, Deng Y, Wu C, Ruan Z, Li C. et al. A novel adhesive dual-sensitive hydrogel for sustained release of exosomes derived from M2 macrophages promotes repair of bone defects. Mater Today Bio. 2023Nov2;23:100840
181. Gu C, Li Y, Liu J, Liu S, Long J, Zhang Q. et al. Neural stem cell-derived exosomes-loaded adhesive hydrogel controlled-release promotes cerebral angiogenesis and neurological function in ischemic stroke. Exp Neurol. 2023Dec;370:114547
Author contact
![]() Corresponding authors: Zuguo Liu, PhD, MD, Department of Ophthalmology, The First Affiliated Hospital of University of South China, Hengyang Medical School, University of South China, Hengyang Hunan 421001, China; Email: zuguoliuedu.cn. Yi Han, PhD, MD, Department of Ophthalmology, The First Affiliated Hospital of University of South China, Hengyang Medical School, University of South China, Hengyang Hunan 421001, China; Email: hanyixmucom; hanyiedu.cn. Qiuping Liu, PhD, MD, Department of Ophthalmology, The First Affiliated Hospital of University of South China, Hengyang Medical School, University of South China, Hengyang Hunan 421001, China; Email: 76655086com.
Corresponding authors: Zuguo Liu, PhD, MD, Department of Ophthalmology, The First Affiliated Hospital of University of South China, Hengyang Medical School, University of South China, Hengyang Hunan 421001, China; Email: zuguoliuedu.cn. Yi Han, PhD, MD, Department of Ophthalmology, The First Affiliated Hospital of University of South China, Hengyang Medical School, University of South China, Hengyang Hunan 421001, China; Email: hanyixmucom; hanyiedu.cn. Qiuping Liu, PhD, MD, Department of Ophthalmology, The First Affiliated Hospital of University of South China, Hengyang Medical School, University of South China, Hengyang Hunan 421001, China; Email: 76655086com.
 Global reach, higher impact
Global reach, higher impact