13.3
Impact Factor
Theranostics 2025; 15(2):521-545. doi:10.7150/thno.103384 This issue Cite
Review
Targeting ion channels: innovative approaches to combat cancer drug resistance
1. School of Pharmacy, Hangzhou Normal University, Hangzhou, Zhejiang, China.
2. Department of Breast Surgery, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China.
3. General Surgery, Cancer Center, Department of Gastrointestinal-Pancreatic Surgery, Zhejiang Provincial People's Hospital, Hangzhou Medical University, Hangzhou, Zhejiang, China.
Received 2024-9-7; Accepted 2024-10-21; Published 2025-1-1
Abstract
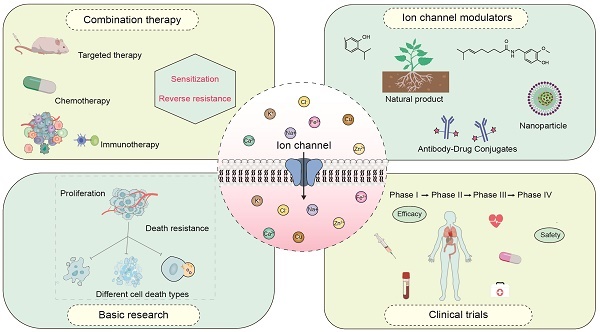
Ion channels, as functional molecules that regulate the flow of ions across cell membranes, have emerged as a promising target in cancer therapy due to their pivotal roles in cell proliferation, metastasis, apoptosis, drug resistance, and so on. Recently, increasing evidence suggests that dysregulation of ion channels is a common characteristic of cancer cells, contributing to their survival and the resistance to conventional therapies. For example, the aberrant expression of sodium (Na+) and potassium ion (K+) channels is significantly correlated with the sensitivity of chemotherapy drugs. The endogenous calcium (Ca2+) channels contribute to the acquired resistance of osimertinib in epidermal growth factor receptor (EGFR) mutant non-small cell lung cancer cell lines. Ferrous ions (Fe2+) enhance the sensitivity of breast cancer cells to doxorubicin treatment. Preclinical models have also demonstrated the effect of specific ion channel blockers or modulators on anticancer drug resistance. This review describes the current understanding about the interaction between ion channels and the therapeutic efficacy of anticancer drugs. Then, the therapeutic potential of ion channel blockers or modulators in enhancing the sensitivity or overcoming the resistance of cancer cells to anticancer therapies is discussed. Targeting ion channels will hopefully offer a novel and promising strategy for overcoming cancer drug resistance.
Keywords: Ion channels, Cancer, Sensitization, Drug resistance.
1. Introduction
The asymmetric ion distribution across the cell membrane establishes a gradient fundamental to cellular function. Ion channels, a class of membrane-spanning proteins, provide selective conduits for specific ions, which orchestrate the delicate balance of ionic concentrations on either side of the membrane [1]. These channels are pivotal in regulating transmembrane ion flux, maintaining cellular homeostasis, and propagating of signaling pathways essential for numerous biological processes including nerve impulse transmission, muscle contraction, and cancer cell signaling transduction [2-4]. Ion channels play a crucial role in both the physiological and pathological processes of organisms, such as cell proliferation, migration, and apoptosis. Increasing researches have demonstrated that the expression of ion channels in cancer cells is frequently dysregulated. This abnormal expression and/or function can disrupt normal cellular processes, leading to the malignant transformation of normal cells. Consequently, this dysregulation is manifested as uncontrolled proliferation and spread, which are hallmark characteristics of cancer cells. The strong association between cancer hallmarkers and ion channel dysfunction leads us to classify cancer as a specific type of channel lesions, termed oncochannelopathies. Classic channelopathies often result from inherited mutations in ion channel genes, which alter the biophysical properties of the channel and then cause the disease. In contrast, oncochannelopathies often involve various malignancies in multiple ion channels, while channelopathies have traditionally been viewed as one channel diseases [1, 5].
Cancer remains the principal cause of mortality globally, significantly impeding the extension of life expectancy [6]. The current therapeutic landscape for oncology is fraught with challenges, predominantly characterized by the emergence of drug resistance. This resistance confers a resilient phenotype upon many cancer cells, rendering them impervious to existing treatments [7]. The ion channel-targeted therapy introduces a novel and potentially transformative strategy within the oncological domain. This paradigm has the capacity to surmount substantial challenges including the evolution of drug resistance, inequities in therapeutic delivery, and so on. Therefore, the strategic engagement of ion channels may enhance the therapeutic efficacy of cancer treatments and optimize the prognosis of cancer patients [8, 9].
Several ion channels exhibit differential expression in various malignancies and play pivotal roles in promoting malignant transformation through specific mechanisms. The combination of ion channel modulators with other anticancer drugs can generate synergistic effects, resulting in enhancing therapeutic efficacy or combating the drug resistance. This review systematically analyzes the interaction between the common ion channels (sodium, potassium, calcium, and chloride ion channels), trace element ion channels (iron, copper, zinc, and so on), and the therapeutic efficacy of anticancer drugs. The progress of ion channel blockers or modulators in augmenting the sensitivity of anticancer drugs or overcoming their resistance is also described. Our increasing understanding of ion channels in regulating therapeutic efficacy of anticancer drugs will hopefully offer a novel and promising strategy for combating cancer drug resistance.
2. The role of ion channels in cancer
Ion channels, govern the transmembrane movement of ions, are integral to maintaining cellular homeostasis. The role of ion channels in cancer is multifaceted and pivotal, influencing fundamental cellular processes in oncogenesis. The aberrant regulation of ion channels has been extensively documented across various cancer types (Table 1).
2.1 Sodium channel
In tumor regions, the concentration of sodium ions (Na+) is significantly higher than in normal tissues. This disparity is closely related to the formation and maintenance of the tumor immune microenvironment (TME), and interacts with the complex biological processes of tumorigenesis. Voltage-Gated Sodium Channels (VGSCs) contribute to an increase in Na+ influx, triggering a cascade of intracellular physiological reactions. These reactions include disruptions in Ca2+ concentration, pH balance, and overall cellular homeostasis [10]. The SCNN1B gene encodes the essential β-subunit of the epithelial sodium channel (ENaC) complex. Elevated SCNN1B expression serves as an independent prognostic marker for prolonged survival in patients with advanced gastric cancer (GC). SCNN1B initiates the unfolded protein response (UPR) by degrading GRP78, which activates PERK, ATF4, XBP1s, and C/EBP homologous protein, ultimately leading to cancer cell apoptosis [11]. In colorectal cancer (CRC), SCNN1B expression is significantly downregulated, indicating its role as a tumor suppressor. Experimental evidence shows that SCNN1B inhibits colon cancer cell proliferation and enhances apoptosis by regulating the c-Raf and MAPK signaling pathways [12]. Increased expression of Nav1.5 has been demonstrated in oral squamous cell carcinoma (OSCC). Nav1.5 influences the proliferation, migration, and invasive capabilities of OSCC cells [13]. So, sodium ion channels are closely related with carcinogenesis and cancer development.
2.2 Potassium channel
Potassium channels, the most diverse and well-studied ion channel family, play crucial roles in cell proliferation, apoptosis, cell volume regulation, and maintaining membrane potential. These functions make them a primary focus of cancer treatment [14, 15]. Potassium channels are classified into four main groups: voltage-gated potassium channels, calcium-activated potassium channels, inwardly rectifying potassium channels, and two-pore domain potassium channels [16]. KCNMA1 encodes the large-conductance calcium-activated potassium channel (BKCa), which acts as a pivotal tumor suppressor gene in carcinogenesis. Abnormal methylation of its promoter region modulates the expression of the key apoptotic gene PTK2 influencing the progression of GC [17]. Activation of BKCa channels regulates the MEK/ERK pathway, impacting the development and progression of endometrial adenocarcinoma [18]. Early studies show that inhibition of the Intermediate Conductance Calcium-Activated Potassium Channel 1 (IKCa1) potassium channel suppresses the proliferation of prostate cancer cells. This suppression might be related to IKCa1 activation-induced membrane potential hyperpolarization, which drives Ca2+ influx [19]. Rapidly proliferating embryonic cells, stem cells, or cancer cells generally show a more depolarized state, cancer cells may exhibit this depolarized property to support their uncontrolled proliferation [20, 21]. Elevated levels of KCa3.1 (KCNN4) have been identified across a spectrum of malignant neoplasms, including pancreatic cancer [22], breast cancer [23], non-small cell lung cancer(NSCLC) [24], and melanoma [25]. KCa3.1 expressed on the mitochondrial inner membrane affects the survival of pancreatic ductal adenocarcinoma (PDAC) and melanoma cells, which is associated with mitochondrial function and intracellular calcium homeostasis [26].
The expression of Kv10.1 channels is upregulated in over 70% of cancers [27]. In human breast adenocarcinoma exposure to the hERG1 channel activator NS1643 results in G0/G1 cell cycle arrest by activating of the senescence program, which is evidenced by increased protein levels of p21 and p16INK4a, and β-galactosidase activity [28]. The potassium channel Kv1.3 is highly expressed in the mitochondria of various cancer cells and exists on the plasma membrane of different cell types. On the plasma membrane, Kv1.3 is involved in cell proliferation, while in the mitochondria, it plays a role in apoptosis in multiple types of tumor cells (mitochondrial channel) [29, 30]. Caveolins regulate cell survival and participate in the plasma membrane targeting of Kv1.3. Specifically, the interaction between Kv1.3 and Cav1 in mitochondria, counteracts apoptosis, resulting in preventing Kv1.3-mediated cell death [29, 30]. In osteosarcoma, silencing Kv1.5 inhibits cancer cell proliferation, induces G0/G1 cell cycle arrest, and promotes cell apoptosis [31]. The ability to resist cell death is a hallmark of cancer, even under stressful conditions. Polycomb proteins (PcG) can regulate voltage-gated potassium channel genes in stem cells [32]. PcG-dependent inhibition of the Kv1.5 channel gene KCNA5 contributes to cancer cell survival under stressful conditions [33]. The activation of Kv11.1 channel increases the oxidative stress level, inhibits the NRF2-mediated antioxidant response mechanism, and enhances the lethal effect of the Kv11.1 channel activator on breast cancer cells [34]. In GC, the gene encoding the voltage-gated potassium channel, KCNE2, is downregulated to contribute to the suppression of tumor proliferation, which may be associated with the downregulation of Cyclin D1 [35].
Inwardly rectifying potassium channels, such as Kir2.2, enhance RelA phosphorylation and facilitate its cytoplasmic-to-nuclear translocation. This process activates the transcription factor NF-κB and upregulating its target genes including Cyclin D1, MMP9, and VEGF [36]. Recent studies have demonstrated that the acid-sensing potassium channel KCNK3 impact various cancer types, such as prostate cancer [37], pancreatic cancer [38], hepatocellular carcinoma (HCC) [39], and NSCLC [40]. Aberrant glucose metabolism stands as a defining characteristic of cancer [41]. Overexpression of KCNK3 significantly inhibits the proliferative capacity and glycolytic processes of lung adenocarcinoma cells, which is associated with the activation of the AMPK-TXNIP pathway [42].
K+ transport is central to antitumor function and can be targeted to change T-cell exhaustion and augment cancer immunotherapy [43]. The high K+ environment impacts the phosphorylation of the T-cell receptor (TCR)-mediated Akt-mTOR signaling pathway, independently of membrane potential changes. Overexpression of the Kv1.3 channel facilitates K+ efflux in T cells, thereby restoring T-cell function [44]. Additionally, KCNAB2 is associated with immune infiltration defects. Its overexpression increases the expression of chemokines, among which CCL2 is crucial for immune cell recruitment [45]. So, potassium channels may regulate immune cell functions within the TME, thereby enhancing their anti-tumor activity.
2.3 Calcium channel
Calcium, a ubiquitous and diffusible second messenger, plays a pivotal role in cell signaling mechanisms, orchestrating numerous fundamental physiological processes [46]. The CACNG4 gene, encoding an L-type voltage-gated calcium channel γ subunit, influences tumor cell survival in breast cancer by closing channel pores, inhibiting Ca2+ influx, and altering crucial genes [47]. Similarly, the CACNA1D gene, which encodes the CaV1.3 α1D subunit, is overexpressed in various cancers, including prostate, uterine, and colon cancer [48]. The α1D subunit promotes the proliferation of endometrial cancer cells mediated by 17β-estradiol through the G protein-coupled estrogen receptor (GPER), leading to the phosphorylation of downstream molecules ERK1/2 and CREB [49]. Additionally, CACNA1E enhances the proliferation of NSCLC cells by increasing current density and Ca2+ influx, activating the epidermal growth factor receptor (EGFR) signaling pathway [50]. This gene is also associated with favorable histological outcomes in nephroblastoma recurrence [51].
Mitochondria are crucial in intracellular Ca2+ regulation and reactive oxygen species (ROS) generation [52]. In colon cancer cells, RIPK1 interacts with the mitochondrial calcium uniporter (MCU), promoting proliferation by enhancing mitochondrial Ca2+ uptake and energy metabolism [53]. MCU is regulated by MICU1, and its absence can lead to persistent mitochondrial calcium loading, excessive ROS production, and increased sensitivity to apoptotic stress [54]. Metabolic dysregulations, particularly aerobic glycolysis, are implicated in tumor growth and chemoresistance [55, 56]. In ovarian cancer, silencing MICU1 activates PDH by stimulating the PDPhosphatase-phosphoPDH-PDH axis, resulting in increased oxygen consumption, reduced lactate production, and inhibition of clonal growth of ovarian cancer cells [57].
In non-excitable cells, including most cancer cells, store-operated calcium entry (SOCE) serves as a crucial pathway for Ca2+ influx. SOCE activation are primarily involved in the interaction between stromal interaction molecule 1 (STIM1) and Orai1 [58]. STIM1 acts as a calcium sensor that triggers Ca2+ influx upon depletion of endoplasmic reticulum Ca2+ levels [59]. Silencing STIM1 arrests the cervical cancer cell cycle at the S phase and G2/M phase [60]. The molecular complexes in SOCE promote the proliferation, metabolism, migration, and invasion of GC cell by targeting MACC1 [61]. Knockdown of Orai3, a highly conserved paralog of Orai1 [62], enhances SOCE in PDAC, leading to mitotic catastrophe and apoptosis in cancer cells [63].
Transient receptor potential canonical (TRPC) channels may be associated with both SOCE and non-capacitative calcium entry (NCCE) [64]. Experimental evidence indicates that TRPC6 correlates with SOCE amplitude and the proliferation of HCC cells [65]. In human follicular thyroid ML-1 cancer cells, TRPC1 plays a crucial role in the proliferation of thyroid cancer cells by regulating the expression of S1P3 and VEGFR2 in calcium-dependent mechanisms [66].
The ion channels involved in cancer and their molecular mechanisms.
| Types of ion channels | Cancer Type | Cancer cell lines | Biological effect | Molecular mechanisms | Ref |
|---|---|---|---|---|---|
| Na+ (SCNN1B) | Gastric | AGS, BGC823, MKN45 | Apoptosis↑, Proliferation↓ | GRP78↓, PERK↑, ATF4↑, XBP1s↑, CHOP↑ | [11] |
| Na+ (SCNN1B) | Colorectal | DLD1, SW1116 | Apoptosis↑, Proliferation↓ | c-Raf↓, p-ERK↓, p-AKT↓, p-MEK↓ | [12] |
| Na+ (Nav1.5) | Oral | HSC-3 | Proliferation↑ | β-catenin↑, c-Myc↑, Cyclin D1↑ | [13] |
| K+ (KCNMA1) | Gastric | MGC803, BGC823 | Apoptosis↑ | PTK2↓ | [17] |
| K+ (KCNMA1) | Endometrial | Ishikawa | Proliferation↑ | MEK/ERK↑ | [18] |
| K+ (IKCa1) | Prostate | LNCaP, PC-3, DU-145 | Proliferation↑ | Ca2+↑ | [19] |
| K+ (HERG1) | Breast | SKBR3, MDA-MB-231 | Proliferation↓ | p21↑, p16INK4a↑ | [28] |
| K+ (Kv1.3) | Melanoma | B16F10 | Apoptosis↓ | Caveolin-Kv1.3 axis↑ | [29] |
| K+ (KCNA5) | Ewing sarcoma, Neuroblastoma | HuVEC, HL-1, TC-71 | Apoptosis↑ | Caspase-3↑ | [33] |
| K+ (Kv11.1) | Breast | MCF7, MDA-MB-231 | Apoptosis↑ | NRF2↑, ROS↑ | [34] |
| K+ (KCNE2) | Gastric | SGC7901 | Proliferation↓ | Cyclin D1↓ | [35] |
| K+ (Kir2.2) | Prostate | PC-3 | Proliferation↑ | p-RelA↑, NF-κB↑, Cyclin D1↑ | [36] |
| K+ (KCNK3) | Lung adenocarcinoma | H1975, H1299 | Proliferation↓ | AMPK-TXNIP↑ | [42] |
| K+ (Kv1.3) | Melanoma | Pmel-1, B16, Mel624 | Immune↑ | [K+]i↑, Akt-mTOR↑ | [44] |
| K+ (KCNAB2) | Lung adenocarcinoma | A549, H23 | Immune↑ | CCL2↑, CCL3↑, CCL4↑, CCL18↑, CXCL9↑, CXCL10↑, CXCL12↑ | [45] |
| Ca2+ (Cav1.3) | Endometrial | Ishikawa | Proliferation↑ | GPER↑, Ca2+↑, p-ERK1/2↑, p-CREB↑ | [49] |
| Ca2+ (CACNA1E) | NSCLC | H1299, H1975 | Proliferation↑ | Ca2+↑, EGFR↑, p-Akt↑, p-Erk↑ | [50] |
| Ca2+ (MCU) | Colorectal | HT29 | Proliferation↑ | RIPK1-MCU↑, Ca2+↑ | [53] |
| Ca2+ (MICU1) | Ovarian | CP20, OV90 | Aerobic glycolysis↑ | PDH↑ | [57] |
| Ca2+ (STIM1) | Cervical | SiHa, CaSki | Proliferation↑ | p21↓, Cdc25c↑ | [60] |
| Ca2+ (SOCE) | Gastric | BGC-803, MKN-45 | Proliferation↑ | MACC1↑, p21↓, Cycline D1↑ | [61] |
| Ca2+ (Orai3) | Pancreatic | MiaPaCa2 | Proliferation↑, Apoptosis↓ | SOCE↓, c-PARP↓ | [63] |
| Ca2+ (TRPC6) | Liver | Huh-7 | Proliferation↑ | SOCE↑, Cycline D1↑ | [65] |
| Ca2+ (TRPC1) | Thyroid | ML-1, FTC-133 | Proliferation↑ | p21↓, p27↓, Cycline D2↑, Cycline D3↑, CDK6↑ | [66] |
| Ca2+ (ORAI1) | Lung | H1299 | Immune↑ | Ca2+↑, Calpain↑, MLPH↑, SLP2-a↑, sEV PD-L1↑ | [67] |
| Cl- (ClC-3) | Nasopharyngeal carcinoma | CNE-2Z | Proliferation↑ | p21↓, p27↓, CDK4/6↑ | [69] |
| Cl- (ANO1) | Breast | HCC1954, ZR75-1 | Proliferation↑ | EGFR↑, CAMK↑ | [70] |
| H+ (ASIC1a) | Glioblastoma | R54, R8 | Necroptosis↑ | p-RIPK1↑ | [73] |
| Fe2+ (TFRC) | Colorectal | NCM460, CT26 | Ferroptosis↑, Immune↑ | Fe2+↑, ROS↑ | [79] |
| Fe2+ (TRPML1) | Melanoma | A375, M214, M481, M491 | Proliferation↑ | p-ERK↓, p-TSC2↓, p-S6K↓, mTORC1↓ | [83] |
| Fe2+ (FPN1) | Myeloma | ARP1, OCI-MY5 | Apoptosis↑, Proliferation↓ | p-STAT3↓, MCL-1↓ | [84] |
| Zn2+ (TRPML1) | Melanoma | MeWo, M12 | Necrosis↑ | Zn2+↑ | [88] |
| Cu2+ (CTR1) | Breast | MDA-MB-231 | Tumorigenesis↑ | Cu2+↑, PDK1↑, p-AKT↑ | [89] |
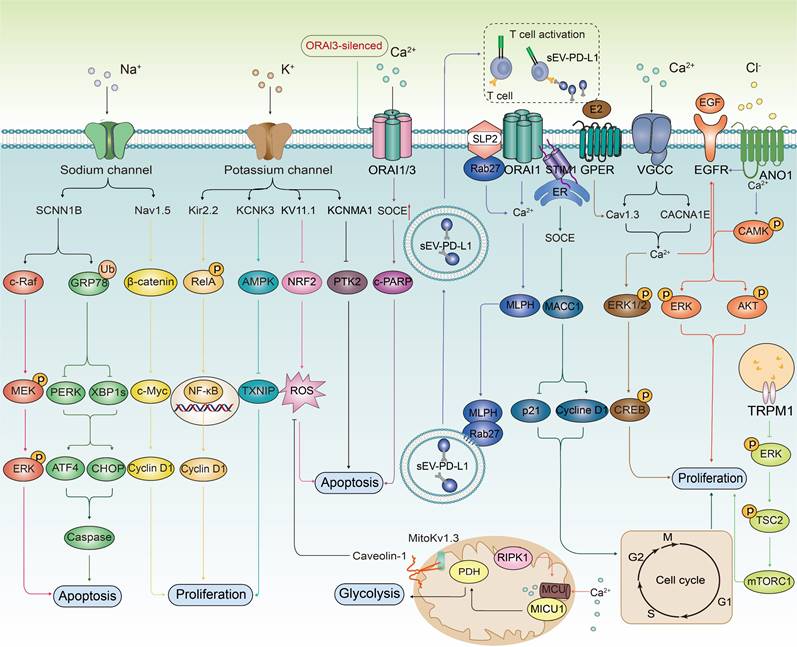
An imbalance between immune surveillance and tumor inflammation can lead to immunosuppression, ultimately resulting in tumor immune evasion [32]. PD-L1 is a crucial checkpoint molecule in this context. By inhibiting the ORAI1 channel, intracellular calcium signaling is disrupted, hindering the release of PD-L1 from small extracellular vesicles (sEVs). This inhibition of PD-L1 release can suppress tumor growth and enhance systemic anti-tumor immunity. Ca2+-dependent proteins such as melanophilin and Synaptotagmin-like protein 2 are involved in the release of PD-L1 carried by sEVs [67]. Therefore, calcium channel plays an important role in manipulating immune checkpoint blockade by PD-L1.
2.4 Other ion channels
Chloride ion channels play a crucial role in regulating the cell cycle and cell proliferation. These channels are often overexpressed in various tumors [68]. ClC-3 upregulates Cyclin D1-CDK4/6 in nasopharyngeal carcinoma cells by inhibiting the expression of p21/p27, thereby affecting the cell cycle [69]. ANO1, a calcium-activated chloride channel, is a significant oncogenic factor in the 11q13 amplification of breast cancer and other malignancies. It promotes breast cancer progression by activating the EGFR and CAMK signaling pathways [70]. Conversely, overexpression of CLCA2 in nasopharyngeal carcinoma cells significantly reduces cell proliferation by inhibiting the FAK/ERK signaling pathway [71]. Persistent acidosis is a prevalent characteristic of the TME across various cancer types, including glioblastoma multiforme (GBM) [72]. ASIC1a, an acid-sensing ion channel, is highly sensitive to extracellular protons. Acidosis triggers RIPK1-dependent death in glioblastoma stem cells via the activation of ASIC1a [73].
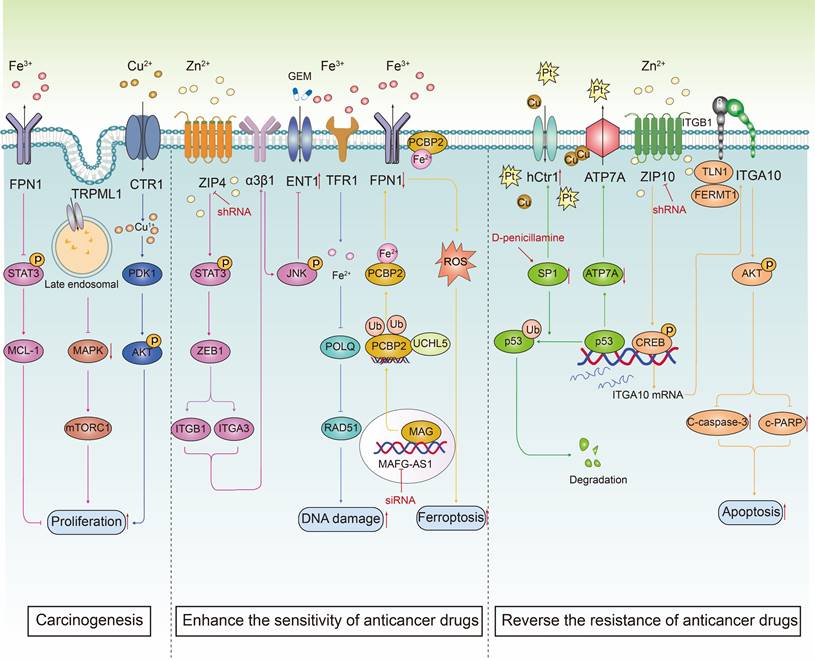
Ion channels that transport trace elements, alongside common ion channels like sodium, potassium, and calcium, are crucial for maintaining cellular stability and contributing to cancer development. Iron is essential for DNA synthesis, cellular metabolism, and proliferation [74]. Transferrin receptor (TFR) is overexpressed in many types of cancers, such as liver cancer [75], kidney cance [76], glioma [77], and pancreatic cancer [78], compared to non-tumor tissues, which makes it a promising target for cancer therapy. Excess cellular iron is toxic, recent studies have found that OTUD1 acts as a deubiquitinating enzyme of iron-responsive element-binding protein 2 (IREB2), which can block its degradation, thereby promote the expression of transferrin receptor protein 1 (TFRC) and enhance cellular iron uptake, leading to increased intracellular ROS production and ferroptosis in CRC [79]. In addition to increasing iron absorption and reducing iron storage, cancer cells also reduce iron export [80]. MCOLN1/ mucolipin TRP channel 1 (TRPML1), a non-selective cation channel localized in lysosomes, functions as a Fe2+ permeable channel in late endosomes and lysosomes [81], and is involved in vesicle fusion and fission processes [82]. TRPML1 can attenuate MAPK and mTORC1 signaling, maintain protein homeostasis and facilitate macropinocytosis, thereby promoting the survival and proliferation of melanoma cells [83]. The iron exporter ferroportin 1 (FPN1), the only known iron exporter in vertebrates, plays a crucial role in myeloma. Restoration of FPN1 expression is shown to decrease intracellular liable iron pool, inhibit STAT3-MCL-1 signaling, which in turn suppresses myeloma cell proliferation [84]. Additionally, cancer cells upregulate autophagy processes to meet their nutritional needs. MCOLN1/TRPML1, a key player in autophagy, is implicated in various cancers by promoting carcinogenic autophagy. TRPML1 serves as a crucial ion channel that mediates the release of metal ions from lysosomes [85]. Besides mediating Ca2+ influx, MCOLN1 also facilitates lysosomal zinc (Zn2+) inflow. This action blocks the interaction between STX17 and VAMP8, thereby regulating carcinogenic autophagy [86]. Zn2+ is an essential trace element necessary for cell function. However, excessive Zn2+ release can impair mitochondrial functions, particularly the electron transport chain, leading to energy depletion and cell death. In metastatic melanoma, the protein TRPML1 is upregulated [87]. Activation of TRPML1, rather than its inhibition, induces cell death. Specifically, activation of ML-SAs (presumably TRPML1 activators) results in lysosomal Zn2+-dependent necrotic cell death [88]. Copper also plays a crucial role in metabolic homeostasis. The E3 ubiquitin ligase Nedd4l inhibits the expression of Copper Transporter 1 (CTR1) through ubiquitination. This Nedd4l-CTR1 signaling pathway modulates AKT kinase activity in a copper-PDK1 binding-dependent manner. Therefore, targeting the CTR1-copper pathway to counteract hyperactive AKT-driven cancers represents a potential therapeutic strategy [89]. So, Ion channels that transport trace elements influence cellular physiological functions by regulating the balance and transport of trace ions, thereby promoting or inhibiting cancer development.
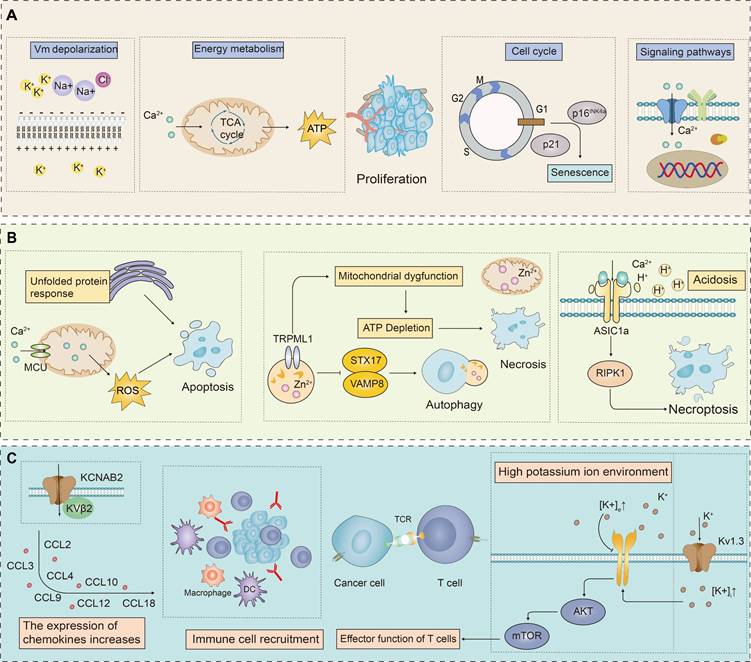
In summary, the biological role of ion channels in tumor cells is both multi-dimensional and complex, with their abnormal expression being a critical phenomenon in tumorigenesis. This abnormal expression is intricately involved in the proliferation and death resistance of tumor cells as well as the reconstruction of the TME. Specifically, in tumor cells, the expression levels of certain ion channels are abnormally elevated, which may promote the proliferation and survival of these cells. These ion channels regulate the membrane potential by controlling the concentrations of K+ and Na+ ions inside and outside the cell, thereby influencing the proliferative state of the tumor cells. In mitochondria, the flow of Ca2+ can induce membrane depolarization, promote ATP synthesis, and thus enhance cell proliferation (Figure 1A). Conversely, some studies have found that the expression of certain ion channels in tumor cells is significantly down-regulated to resist programmed cell death, potentially acting as tumor suppressors. Activation or ectopic expression of these ion channels can induce programmed cell death in cancer cells (Figure 1B). The activation of potassium ion channels can activate AKT/mTOR signaling pathway, enhance T cell function, and promote the infiltration of immune cells by increasing the expression of chemokines, thereby enhancing the immune response to tumors (Figure 1C). Ion channels, as key membrane proteins, exhibit highly selective permeability and regulate the transmembrane transport of specific ions. Through this interaction, they significantly influence intracellular signaling pathways. These channels are deeply involved in the pathophysiological characteristics of various cancer markers, affecting them to varying degrees through a multitude of distinct signaling mechanisms, as shown in Figure 2. These findings provide a theoretical basis for developing novel anti-cancer therapeutic strategies targeting ion channels.
The role of ion channels in the cell proliferation, death and tumor immune microenvironment (TME). Compared to normal cells, ion channels in cancer cells often show abnormal expression patterns, which impact cell proliferation, death resistance, and the remodeling of the TME. (A) Ion channels significantly influence tumor cell proliferation by regulating cell membrane potential, energy metabolism, cell cycle, and intracellular Ca2+ concentration These processes modulate signaling pathways that are critical for cancer cell proliferation. (B) By activating certain ion channels, it interferes with the physiological processes of cancer cells, leading to cell death. Ion channels induce apoptosis in cancer cells by generating excessive ROS through the endoplasmic reticulum's UPR and by modulating mitochondrial Ca2+ concentrations. Additionally, ion channels affect mitochondrial function by altering Zn2+ concentrations, leading to ATP depletion and necrotic cell death. They also regulate autophagy by mediating the release of Zn2+ from lysosomes into the cytosol. Furthermore, acidosis in the TME induces RIPK1-dependent necroptosis via acid-sensing ion channels. (C) Ion channels, particularly potassium ion channels, play a crucial role in tumor immunity. They influence the TME and the function of immune cells through various mechanisms.
3. Targeting ion channels to overcome cancer resistance
During cancer treatment, the issue of reduced sensitivity and drug resistance due to cellular evolution has emerged as a significant barrier to therapeutic efficacy. Ion channel modulators will hopefully offer a promising strategy to enhance the sensitivity or reverse the resistance of anticancer drugs (Table 2).
3.1 Ion channels and chemotherapy
3.1.1 Enhancing anticancer drug sensitivity
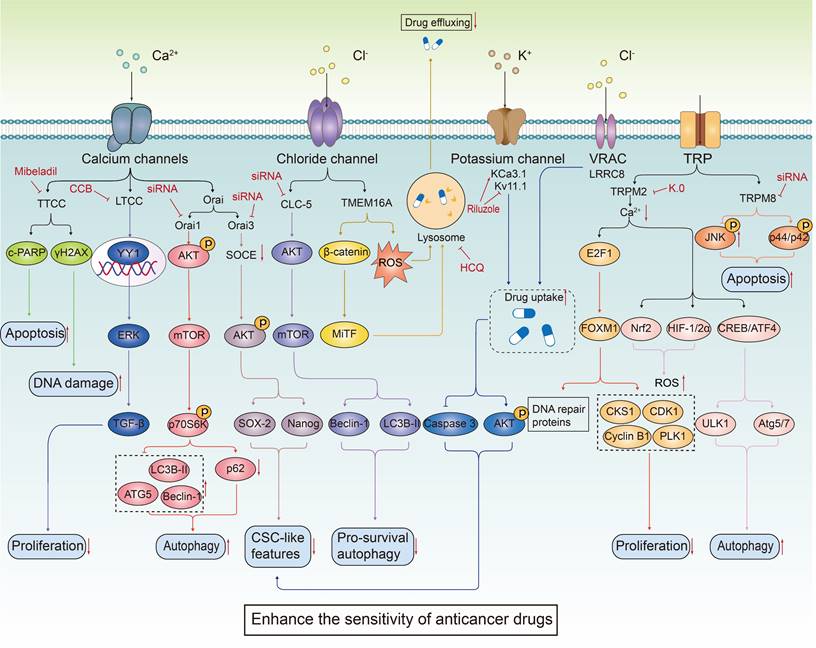
In the field of cancer therapy, enhancing the sensitivity of tumor cells to therapeutic interventions is a pivotal strategy for improving overall treatment efficacy. The KCNG1 gene expression is significantly higher in triple-negative breast cancer (TNBC) compared to other breast cancer subtypes. This elevated expression is positively correlated with increased sensitivity to chemotherapy drugs such as cisplatin and oxaliplatin, suggesting that targeting KCNG1 with its inhibitor guanidine hydrochloride (GuHCl) may be an effective treatment strategy for TNBC [90]. The dual-action drug liluzole, combined with a KCa3.1 activator and a Kv11.1 inhibitor, significantly enhanced cisplatin uptake. This combination synergistically increased cisplatin-induced apoptosis and anti-proliferative effects in CRC cells [91, 92]. The combination of the IK1 channel activator 1-EBIO with cisplatin has been demonstrated to enhance caspase-3/7 activity, thereby augmenting the apoptotic cell death induced by cisplatin. This finding underscores the potential of IK1 channel activation in improving the efficacy of cisplatin treatment [93]. Additionally, other research has identified a significant relationship between cell volume regulation and cisplatin sensitivity, highlighting the role of the VSOR Cl- channel, which exhibits a similar mode of action [94]. In parallel, the combination of sparfloxacin (SPFX), a HERG K+ channel blocker, with 5-fluorouracil has been shown to synergistically inhibit the proliferation and induce apoptosis of colon cells [95]. The Nav1.5 activator veratridine has been found to increase 5-FU-induced apoptosis in CRC cells, thereby enhancing the sensitivity of chemotherapy [96]. Calcium channel blockers (CCBs) such as lercanidipine and amlodipine have been shown to inhibit YY1/ERK/TGF-β-mediated transcription, thereby increasing the sensitivity of GC cells to doxorubicin. The potential use of these blockers in targeted and combined therapies for GC is suggested by recent finding [97]. Similarly, the combined use of mibefradil, an inhibitor of T-type calcium channels, with carboplatin has been found to synergistically enhance apoptosis in ovarian cancer cells in vitro. Mibefradil achieves this by reducing AKT phosphorylation, increasing the levels and nuclear retention of FOXO transcription factors, and suppressing the expression of the anti-apoptotic gene BIRC5 [98]. In liver cancer, increased expression of Orai1 is observed. Inhibition of Orai1-mediated Ca2+ influx, known as store-operated Ca2+ entry (SOCE), significantly enhances the sensitivity of the human liver cancer cell line HepG2 to 5-FU. This is accomplished by potentiating the inhibition of the PI3K/AKT/mTOR pathway and promoting autophagic cell death induced by 5-FU [99]. Cancer stem cells (CSCs), a small subset of tumor cells with stem cell characteristics, have been implicated in the resistance to chemotherapy, potentially leading to tumor rebound and recurrence [100, 101]. In epithelial ovarian cancer, the resistance is linked to the presence of CSCs. From a library of FDA-approved compounds, four CCBs were identified for their ability to disrupt the characteristics of ovarian CSCs by inhibiting the AKT and ERK signaling pathways and inducing apoptosis. When combined with cisplatin, these CCBs synergistically suppress the activity and proliferation of ovarian CSCs [102]. In NSCLC cells, cisplatin treatment leads to the upregulation of Orai3 and CSC markers. This process involves the modulation of Ca2+ influx, which increases the expression of CSC markers Nanog and SOX-2 via the PI3K/AKT pathway. Silencing Orai3 or altering extracellular Ca2+ levels has been shown to enhance sensitivity to cisplatin [103]. Furthermore, the voltage-dependent calcium channel α2δ1 subunit has been proposed as a potential marker for GC stem cells. The knockdown of α2δ1 significantly diminishes the spherogenesis and tumorigenic capacity of these stem cells, while concurrently increasing the sensitivity of cisplatin in vitro [104]. The small-cell lung cancer (SCLC) cells expressing the α2δ1 subunit exhibit cancer stem cell-like characteristics, which may contribute to chemotherapy resistance. The 1B50-1 antibody, a monoclonal antibody specifically targeting the α2δ1 subunit, is observed to improve the efficacy of chemotherapy and delay relapse, particularly in cases with a relatively low proportion of α2δ1+ SCLC cells. [105]. The inhibition of transient receptor potential melastatin-2 (TRPM2), an important regulator of Ca2+ influx, has been shown to induce cell death in several malignancies, including T-cell leukemia [106]. In neuroblastoma, TRPM2 knockout results in reduced tumor proliferation and increased sensitivity to doxorubicin, which are associated with Ca2+ activity [107]. Similarly, TRPM2 is highly expressed in acute myeloid leukemia (AML), and its knockout leads to inhibit cell proliferation and heighten the sensitivity to adriamycin. This effect is attributed to impaired mitochondrial function, disrupted autophagy, and elevated ROS levels [108]. TRPM8, another calcium-permeable cation channel, enhances epirubicin-induced apoptosis when its expression is knocked down, primarily by reducing phosphorylated p44/p42 levels and promoting JNK activation triggered by epirubicin [109]. Autophagy plays an important role in cell survival and chemotherapy sensitivity [110-112]. TMEM16A ion channel is responsible for calcium-activated chloride transport in epithelial tissues and its overexpression is considered to be associated with cisplatin resistance by promoting lysosomal flux in squamous cell carcinoma of the head and neck (SCCHN). Notably, the lysosomal inhibitor hydroxychloroquine (HCQ) has been demonstrated to synergistically enhance the cytotoxic effects of cisplatin on SCCHN cells in vitro [113]. ClC5, a member of the chloride channel family, contributes to the chemoresistance of multiple myeloma cells to bortezomib (BZ) treatment by promoting pro-survival autophagy. Interestingly, the knockout of ClC5 increases the sensitivity of these cells to BZ, highlighting its potential as a therapeutic target [114]. The transport mechanisms of platinum-based drugs involve various drug transporters, such as volume-regulated anion channels (VRACs). In head and neck cancer cells, the expression level of VRAC is crucial for determining the responsiveness of platinum drugs [115]. The substrate selectivity of the VRAC channel is determined by the composition of LRRC8 subunits, which regulate the intracellular uptake of cisplatin and carboplatin and promote apoptosis. Specifically, the LRRC8D subunit is essential for maintaining cell volume homeostasis and may significantly influence tumor responsiveness to cisplatin/carboplatin [116].
Ion channels trigger key biological effect on cancer cells by regulating multiple signaling pathways. Ion channels play a crucial role in regulating the biological behavior of cancer cells. They influence the proliferative ability of cancer cells by modulating multiple signal transduction pathways, including NF-κB, β-catenin, AMPK, MACC1, MAPK/ERK, Ca2+/CaMK, and PI3K/Akt. Furthermore, ion channels are involved in the regulation of apoptosis through pathways such as MAPK, endoplasmic reticulum stress, oxidative stress signaling (NRF2), PTK2, PARP, and apoptosis-related proteins. In the context of tumor immune escape mechanisms, ion channels enhance the immune evasion capabilities of tumor cells by regulating the release of programmed death PD-L1 in sEV. Mitochondrial ion channels influence the activity of pyruvate dehydrogenase (PDH), thereby affecting the glycolysis process, which provides energy for cancer cells and promotes their survival.
Targeting ion channels enhances the sensitivity or reverses the resistance of anticancer drugs.
| Synergistic | Treatment | Types of ion channels | Cancer Types | Cancer cell lines | Anticancer Drugs | Biological effect | Molecular mechanisms | Ref |
|---|---|---|---|---|---|---|---|---|
| Enhancing the sensitivity | Riluzole | K+ (KCa3.1↑, Kv11.1↓) | Colorectal | HCT116 | Cisplatin | Apoptosis↑, Proliferation↓ | Caspase 3↑, p-AKT↓, Cisplatin uptake↑ | [91] |
| 1-EBIO | K+ (IK1↑) | Epidermoid | KB | Cisplatin | Apoptosis↑ | Caspase-3/7↑ | [93] | |
| Sparfloxacin | K+ (HERG K+↓) | Colon | HCT 116, HT-29 | 5-FU | Proliferation↓, Apoptosis↑ | - | [95] | |
| Veratridine | Na+ (Nav1.5↑) | Colorectal | SW480, DLD1 | 5-FU | Apoptosis↑ | Ca2+↑, p53↑ | [96] | |
| CCBs | Ca2+ | Gastric | MKN45, AGS | Doxorubicin | Proliferation↓ | YY1/ERK/TGF-β↓ | [97] | |
| Mibeladil | Ca2+ (T-Type↓) | Ovarian | A2780Cis, IGROV-1 | Carboplatin | Apoptosis↑, DNA damage↑ | c-PARP↑, γH2AX↑ | [98] | |
| siRNA/SKF96365 | Ca2+ (Orai1↓) | Liver | HepG2 | 5-FU | Autophagy↑ | p-AKT↓, mTOR↓, p-p70S6K↓, LC3B-II↑, Beclin-1↑, ATG5↑, p62↓ | [99] | |
| CCBs | Ca2+ (T-Type, L-Type↓) | Ovarian | A2780/A2780-SP | Cisplatin, Paclitaxel | Proliferation↓, CSC↓, Apoptosis↑ | C-caspase3↑, ABCG2↓, ALDH↓ | [102] | |
| siRNA | Ca2+ (Orai3↓) | NSCLC | H23, A549 | Cisplatin | Apoptosis↑, CSC↓ | SOCE↓, p-AKT/AKT↓, Nanog↓, SOX-2↓ | [103] | |
| shRNA | Ca2+ (α2δ1↓) | Gastric | HGC-27 | Cisplatin | Apoptosis↑, CSC↓ | - | [104] | |
| 1B50-1 antibody | Ca2+ (α2δ1↓) | SCLC | H1048 | Etoposide, Cisplatin | CSC↓, Tumor growth↓ | ERK↓ | [105] | |
| Knockout (CRISPR-Cas9) | Ca2+ (TRPM2↓) | Neuroblastoma | SH-SY5Y | Doxorubicin | Proliferation↓, DNA damage↑ | Ca2+↓, FOXM1↓, E2F1↓, Cyclin B1↓, CDK1↓, PLK1↓, CKS1↓ | [107] | |
| Knockout (CRISPR-Cas9) | Ca2+ (TRPM2↓) | AML | U937 | Doxorubicin | Proliferation↓, Autophagy↓ | Ca2+↓, ROS↑, ATP↓, HIF-1/2α↓、Nrf2↓, ATF4↓, CREB↓, ULK1↓, Atg5/7↓ | [108] | |
| siRNA | Ca2+ (TRPM8↓) | Osteosarcoma | MG-63, U2OS | Epirubicin | Apoptosis↑ | p-JNK↑, p-p44/p42↓ | [109] | |
| HCQ | Cl- (TMEM16A) | SCCHN | UM-SCC-1, OSC19, HN30, HN31 | Cisplatin | Cell viability↓ | ROS↓, β-catenin↓, MiTF↓ | [113] | |
| siRNA | Cl- (ClC-5↓) | Myeloma | ARH77, U266, SKO-007 | Bortezomib | Autophagy↓ | AKT-mTOR↓, Beclin-1↓, LC3B-II↓ | [114] | |
| Knockout (CRISPR-Cas9) | Cl- (VRAC↓) | Head and Neck | Pica | Cisplatin | Cell viability↓, DNA damage↑ | - | [115] | |
| Knockout (CRISPR-Cas9) | Cl- (LRRC8↓) | Colorectal | HCT116 | Cisplatin/carboplatin | Drug uptake↑, Apoptosis↑ | Caspase-3↑ | [116] | |
| shRNA | Fe2+ (TFRC↓) | Ovarian | A2780, Kuramochi | Carboplatin | DNA damage↑ | Fe2+↓, FTH1↓, POLQ-RAD51↑ | [117] | |
| ML-SI1 | Fe2+ (TRPML1↓) | Breast | SUM159, MCF7 | Doxorubicin | Ferroptosis↑ | - | [121]. | |
| siRNA | MAFG-AS1↓ | Bladder urothelial carcinoma | T24, RT4 | Cisplatin | Ferroptosis↑ | UCHL5-PCBP2↓, FPN1↓, Fe2+↑, ROS↑, MDA↑ | [122] | |
| shRNA | Zn2+ (ZIP4↓) | Pancreatic | MIA PaCa-2; AsPC-1 | Gemcitabine | Cell viability↓ | ZEB1↓, ITGA3↓, ITGB1↓, α3β1↓, JNK↓, ENT1↑ | [124] | |
| Tetrathiomolybdate | Cu2+ (CTR1↓) | Ovarian | SiHa | Cisplatin | Proliferation↓ | - | [127] | |
| TRAM-34 | K+ (KCa3.1↓) | Melanoma | A-375 | Vemurafenib (BRAF-TKI) | Apoptosis↑ | ROS↑, Caspase-3↑ | [173] | |
| NIFE | Ca2+ (L-type↓) | Colorectal | HCT116, SW620 | PD-1 | Proliferation↓, Immune↑ | Ca2+↓, NFAT2-STAT3↓, LASP1↑, PD-L1↓, PD-1↓ | [184] | |
| HEI3090 | Ca2+ (P2RX7↑) | NSCLC | LLC | αPD-1 | Tumor growth↓, Immune↑ | IL-18↑, IFN-γ↑ | [188] | |
| WT-iRGD | Fe2+ (TRPML1↓) | Prostate, breast | PC3, T47D | PD-1 | Ferroptosis↑, Tumor growth↓ | TRPML1-ARL8B↓, CD4 T↑, CD8 T↑ | [185] | |
| Reversing the resistance | shRNA | K+ (Kir2.1↓) | SCLC | H69AR, H446AR | Adriamycin | Apoptosis↑, Proliferation↓ | MRP1/ABCC1↓ | [129] |
| siRNA | K+ (KCNQ1OT1↓) | AML | HL60/ADR, K562/ADR | Adriamycin | Apoptosis↑, Proliferation↓, Migration↓, Invasion↓ | miR-193a-3p↑, Tspan3↓, MRP1↓, P-gp↓, LRP↓ | [130] | |
| CCBs | Ca2+ (L-type↓) | Pancreatic | PANC-1-GR | Gemcitabine | Apoptosis↑ | ERK↓, C-caspase-3↑ | [131] | |
| shRNA | Ca2+ (Cav3.1↓) | Glioblastoma | A172-RC1/RC2 | Temozolomide | Apoptosis↑ | p62/SQSTM1↓ | [138] | |
| Plasmid | Ca2+ (Orai3↑) | Breast | T47D | Cisplatin, 5-FU, Paclitaxel | Chemoresistance↑ | PI3K/Sgk-1/Sek-1↑, p53↓ | [139] | |
| AMG9810 | Ca2+ (TRPV1↓) | Cervical | CaSki CR, SiHa CR, HeLa CR | Cisplatin | Apoptosis↑, Autophagy↓, | LC3B↓, EGF↓, p-EGFR↓, p-AKT↓, MCL1↓ | [143] | |
| Tranilast | Ca2+ (TRPV2↓) | Gastric | KATO-III | Cisplatin | Apoptosis↑ | Ca2+↓, c-PARP↑, CASP3↓ | [146] | |
| siRNA | Ca2+ (TRPC5↓) | Breast | MCF-7/ADM | Adriamycin | Proliferation↓, autophagy↓ | Ca2+↓, p-CaMKKβ↓, p-AMPKα↓, p-mTOR↑, LC3-II↓ | [147] | |
| ML-SI1 | Ca2+ (TRPML1↓) | Ovarian | OVCAR8 | Cisplatin | Cell viability↓ | Arginine↓, Glutamic acid↓, Cysteine↓, Creatine↓ | [148] | |
| siRNA | Cl- (ClC-3↓) | Lung, breast | MCF-7/DOX, A549/Taxol | Taxol, DOX | Chemoresistance↓ | NF-κB↓, P-gp↓ | [150] | |
| siRNA | Cl- (ClC-3↓) | Ovarian | A2780/PTX | Paclitaxel | Proliferation↓ | MDR↓, β-tubulin↓ | [154] | |
| siRNA | Cl- (CLIC1↓) | Gastric | SGC-7901/VCR | Vincristine | Cell viability↓ | P-gp↓, Bcl-2↓ | [155] | |
| PcTx1/shRNA | Ca2+ (ASIC1a↓) | Liver | HepG2/R, Bel7402/R | Oxaliplatin, 5-FU | Proliferation↓, Migration↓, Invasion↓ | p-AKT↓, p-GSK3β↓, Snail↓ | [160] | |
| Amiloride | Ca2+ (ASIC1a↓) | Liver | Bel7402/FU, HepG2/ADM | 5-FU, Doxorubicin | Proliferation↓ | Ca2+↓, PI3K/AKT↓ | [159] | |
| Plasmid | Fe2+ (TFRC↑) | Breast | MCF-7, MCF-7/ADR | Doxorubicin | Ferroptosis↑, Proliferation↓ | Fe2+↑, MDR↑ | [161] | |
| shRNA | Zn2+ (ZIP10↓) | Osteosarcoma | 143BR | Cisplatin | Proliferation↓, Apoptosis↑ | p-CREB↓, ITGA10↓, p-AKT↓, c-PARP↑, C-caspase-3↑ | [163] | |
| D-penicillamine | Cu2+ (CTR1↓) | Ovarian | S3 | Oxaliplatin | Cell viability↓ | Sp1↑, hCtr1↑, p53↓, ATP7A↓, Pt/DNA↑ | [167] | |
| PAPTP | K+ (MitoKv1.3↓) | Leukemia | B lymphocytes | Ibrutinib (BTK-TKI) | Apoptosis↑ | ROS↑, Cytochrome c↑ | [169] | |
| Memantine | K+ (Kv1.3↓) | Melanoma | MeWo, MeWoEto | BSc2189 (proteasome inhibitor) | Apoptosis↑ | Bak↑, Noxa↑ | [171] | |
| Senicapoc | K+ (KCa3.1↓) | Lung | A549, A549-3R | Erlotinib (EGFR-TKI) | Proliferation↓, Migration↓ | - | [175] | |
| NNC 55-0396 | Ca2+ (TTCC↓) | Melanoma | A375, SK-MEL-28, HT144 | Mibefradil (MAPK inhibitor) | Apoptosis↑, Differentiation↑ | Sox2↓ | [178] | |
| Mibefradil | Ca2+ (Cav3.1↓) | Melanoma | A375-R, M3-R | Verofenil (BRAF inhibitor) | Autophagy↓, Apoptosis↑, Migration↓ | p62↑, LC3II↑ | [179] | |
| D9/shRNA | Ca2+ (TRPM2↓) | Lung | PC-9/AR, HCC827/AR | Osimertinib (EGFR-TKI) | Apoptosis↑, DNA damage↑ | Ca2+↓, c-PARP↑, C-caspase-3↑, ROS↑, γ-H2AX↑ | [180] | |
| siRNA | Cl- (ClC-3↓) | Breast | YMB-1, MDA-MB-453 | anti-HER2 | Drug resistance↓ | HER2↓, PI3K/AKT/mTOR↑, p-STAT3↓ | [182] |
Ion channels also play a crucial role in regulating trace element transport and influencing cancer cell sensitivity to chemotherapy. Iron, for instance, is implicated in promoting ovarian cancer through its absorption via transferrin receptor TFRC. Iron facilitates DNA damage repair through the FTH1/FTL/POLQ/RAD51 pathway, and iron chelators enhance the sensitivity of ovarian cancer to carboplatin [117]. In mammals, TFR1 mediates the endocytosis of transferrin-bound iron from the extracellular environment, while divalent metal transporter 1 (DMT1) translocates iron ions from endocytosomes into the cytoplasm [118]. DMT1 is antagonized by GSK-3β, which manipulates iron-induced cell death, offering insights into potential chemotherapy targets [119]. Iron metabolism exhibits a dual role in tumor development, both promoting and inhibiting it. Iron catalyzes the conversion of hydrogen peroxide into ROS, and excess ROS can trigger lipid peroxidation, leading to ferroptosis when the antioxidant system is overwhelmed [120]. TRPML1 regulates lysosomal iron release into the cytoplasm, and its inhibition promotes ferroptosis in breast CSCs to reduce their stemness and enhance the sensitivity of breast cancer cells to doxorubicin [121]. Intracellular Fe2+ is exported by the membrane protein FPN1 and lncRNA MAF transcription factor G antisense RNA 1 (MAFG-AS1) in bladder urothelial carcinoma (BUC) cells. Inhibition of MAFG-AS1 expression increases cisplatin sensitivity in BUC cells by promoting ferroptosis [122]. Zinc, another essential trace element, is involved in DNA synthesis, enzyme activity, and nucleic acid metabolism [123]. ZIP4, a regulator of intracellular zinc, inhibits the gemcitabine transporter ENT1, thereby reducing the sensitivity of pancreatic cancer cells to gemcitabine [124]. Copper ions, when in excess, lead to the endocytosis and degradation of CTR1 [125, 126], resulting in the decrease of cisplatin uptake. In a mouse model of human cervical cancer, combining copper chelators with cisplatin enhances the therapeutic efficacy of cisplatin [127]. These results are summarized in Table 2.
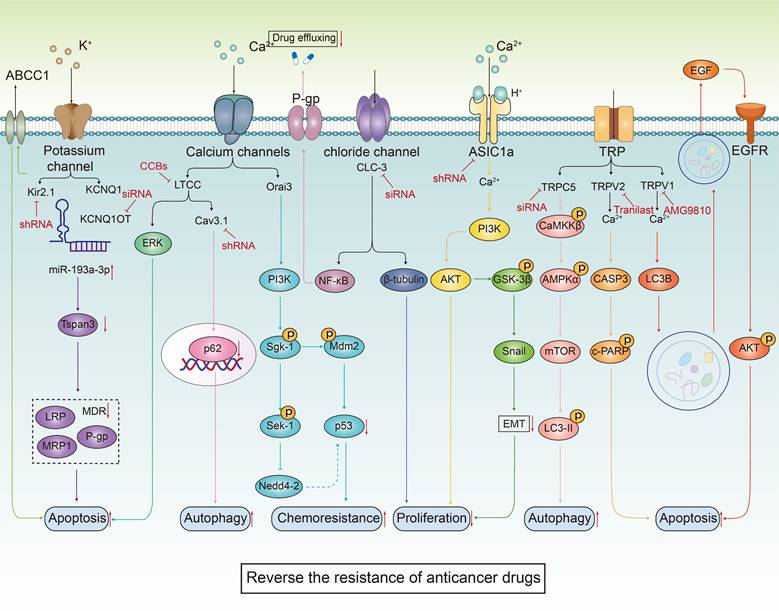
3.1.2 Reversing anticancer drug resistance
Ion channels are also pivotal in the development of drug resistance in tumor cells. Multidrug resistance (MDR) is a primary cause of chemotherapy failure, responsible for up to 90% of cases, making it crucial to combat tumor resistance to enhance the efficacy of anticancer therapy [128]. A promising approach to counteract this resistance is targeting ion channels to reverse the resistance of tumor cells to anticancer drugs. In SCLC, for instance, the KCNJ2/Kir2.1 channel is expressed in 44.23% of tissues and influences cell growth and drug resistance by regulating the expression of MDR protein 1 (MRP1/ABCC1). This channel is modulated by the Ras/MAPK pathway and miR-7, exhibiting its potential as both a prognostic biomarker and a therapeutic target for overcoming chemotherapy resistance in SCLC [129]. Additionally, in AML, the long non-coding RNA potassium voltage-gated channel subfamily Q member 1 overlapping transcript 1 (KCNQ1OT1) shows elevated expression levels in the cells resistant to the chemotherapy drug adriamycin (ADR). Targeting the KCNQ1OT1/miR-193a-3p/Tspan3 axis presents a potential therapeutic strategy for overcoming chemoresistance in AML [130]. The calcium/calmodulin signaling pathway is particularly active in gemcitabine-resistant tumor cell subsets in PDAC. Calcium channel blockers (CCBs) have demonstrated their ability to inhibit survival-promoting ERK signaling in vitro, significantly enhancing the therapeutic efficacy of gemcitabine in both orthotopic xenograft and transgenic PDAC models [131]. Furthermore, CCBs have been shown to reverse MDR induced by docetaxel and vincristine in NSCLC cells [132, 133]. The combination therapy of nifedipine (a dihydropyridine class CCB) and cisplatin has been found to synergistically inhibit tumor cell proliferation and primary tumor growth both in vitro and in vivo, inducing apoptosis in cisplatin-resistant human glioblastoma cells [134]. Autophagy, known as a pro-survival signal, can contribute to the chemoresistance [135-137]. In temozolomide-resistant GBM models, the knockdown of Cav3.1 calcium channel reduced GBM cell viability and slowed tumor progression, which was associated with the transcriptional downregulation of p62/SQSTM1 and defects in autophagy [138]. In breast cancer, the overexpression of Orai3 calcium channel plays a critical role in promoting cell growth and survival, thereby conferring resistance to chemotherapeutic drugs. Mechanistically, this process involves the downregulation of the p53 tumor suppressor protein, which is mediated through the pro-survival PI3K/Sgk-1/Akt-1 signaling pathway. The degradation of p53 is further associated with the actions of Mdm2 and Nedd4-2, which are key regulators in this pathway [139]. Additionally, inhibitor of apoptosis-stimulating protein of p53 (iASPP) maintains intracellular Ca2+ balance by preventing Gp78-mediated degradation of Ca2+-channel protein transmembrane and coiled-coil domains 1 (TMCO1), thereby inhibiting tumor cell growth and overcoming the resistance to therapeutic drugs in colon cancer [140]. EGFR overactivation is associated with resistance to various therapies, including chemotherapy, radiotherapy, and immunotherapy [141, 142]. In cervical cancer, TRPV1 promotes autophagy-mediated EGF secretion via Ca2+ influx, which induces the acquisition of cisplatin resistance. TRPV1 inhibition using a small-molecule agent AMG9810 can effectively overcome cisplatin resistance in cervical cancer cells [143]. Disruption of intracellular Ca2+ balance is linked to cellular escape from death [144, 145], and abnormal expression of calcium regulatory genes may confer cisplatin resistance. Tranilast in combination with cisplatin significantly enhances apoptosis and reverses drug resistance by inhibiting TRPV2 channels and preventing the efflux of Ca2+ ions [146]. In breast cancer, doxorubicin up-regulates TRPC5 expression and promotes autophagy through the CaMKKβ/AMPKα/mTOR signaling pathway. Silencing TRPC5 and inhibiting autophagy can reverse the resistance of breast cancer cell to adriamycin [147].
Lysosomes play a critical role in promoting the sequestration of drugs, making them a promising target for overcoming chemical resistance. Modulating TRPML1-mediated lysosomal exocytosis can regulate cisplatin resistance. This regulation is associated with altered metabolomic signatures due to TRPML1 inhibition [148]. Changes in cellular metabolism can contribute to the development of resistance to chemotherapeutic drugs [149]. In recent years, the role of chloride channel-3 (ClC-3) in tumor drug resistance mechanisms has gained significant attention. In human lung adenocarcinoma and breast cancer cell lines, ClC-3 is highly expressed, inducing MDR through the activation of the NF-κB signaling pathway and the up-regulation of P-gp expression [150]. Furthermore, ClC-3 is crucial in mediating cisplatin resistance in human erythroleukemia cells, glioma cells, and cholangiocarcinoma cells [151-153]. This highlights ClC-3 as a potential target for overcoming chemotherapy resistance in cancer treatment. In paclitaxel-resistant ovarian cancer cells, ClC-3 overexpression enhances the interaction with β-tubulin, thereby increasing drug resistance. Notably, silencing ClC-3 partially restores the sensitivity of these cancer cells to paclitaxel, underscoring its potential as a therapeutic target [154]. Numerous studies have demonstrated a close relationship between exosomes and chemotherapy resistance across various cancers. In the context of GC cells, vincristine-resistant cell lines exhibit elevated levels of chloride intracellular channel 1 (CLIC1) expression. This resistance is potentially linked to the up-regulation of P-gp and Bcl-2, facilitated by exosome-mediated CLIC1 transfer, which induces vincristine resistance in vitro. Notably, silencing CLIC1 expression significantly reduces the semi-inhibitory concentration (IC50) of vincristine [155]. Furthermore, CLIC1 is associated with drug resistance in human choriocarcinoma [156]. Changes in extracellular pH homeostasis are prevalent in most solid tumors, influencing cancer proliferation and migration. Acid sensing ion channel 1a (ASIC1a), a H+-gated cation channel, plays a significant role in these processes [157, 158]. Notably, ASIC1a is overexpressed in drug-resistant HCC cells and is implicated in drug resistance through the Ca2+/PI3K/AKT pathway. ASIC1a knockout can overcome drug resistance in HCC cells [159]. EMT induced by the tumor microenvironment is closely related to tumor invasion and drug resistance. The inactivation of ASIC1a has been shown to suppress cell migration and invasion. This mechanism is specifically regulated by the AKT/GSK-3β/Snail pathway, driven by the TGFβ/Smad signal, which modulates the expression of α-catenin, β-catenin, vimentin, and fibronectin [160].
Ferroptosis, a process involving TFRC, is closely linked to tumor cells and plays a significant role in breast cancer drug resistance. The upregulation of TFRC in ADR-resistant breast cancer cells can activate ferroptosis, thereby reversing ADR resistance [161]. Zinc transporters in the cell membrane, including the Znt/SLC30 family (responsible for Zn efflux) and the ZIP/SLC39 family (absorbing Zn from the extracellular environment), are crucial in this context [162]. Specifically, the expression of ZIP10 influences the sensitivity of cancer cells to chemotherapeutic drugs and affects the clinical outcomes of osteosarcoma. Knocking out ZIP10 inhibits osteosarcoma cell proliferation and impairs the chemoresistance through the ZIP10-ITGA10-PI3K/AKT axis [163]. The copper transporter CTR1 affects the influx of platinum drugs into cells. Lower CTR1 levels are generally associated with increased cisplatin resistance in tumors [164], while higher CTR1 expression in NSCLC patients is associated with higher survival rates [165]. Consequently, manipulating CTR1 with copper-chelating drugs can selectively reverse resistance to platinum treatment [166]. In human cervical cancer oxaliplatin-resistant S3 cells, the copper chelator D-penicillamine increases the therapeutic efficacy of platinum drugs in oxaliplatin resistant tumors [167]. Therefore, targeted ion channel therapies have demonstrated significant potential in overcoming chemotherapeutic drug resistance (Table 2).
3.2 Ion channels and targeted therapy
The development of resistance to targeted cancer therapies presents an increasingly formidable clinical challenge. Tumor cells exhibit resistance to these drug treatments through intricate molecular adaptive changes, which limits the long-term efficacy of precision medicine. Ion channels are emerging as important tumor targets and potential cancer biomarkers [168]. Primary chronic lymphocytic leukemia (CLL) cells from ibrutinib-resistant patients can be killed with Kv1.3 potassium channel inhibitor PAPTP. Thus, PAPTP emerges as a potential alternative therapeutic option for CLL [169]. For patients with BRAF-mutant melanoma, the primary and acquired resistance of BRAF and MEK inhibitors remain major treatment challenges [170]. The combination of proteasome and Kv1.3 channel inhibitors results in synergistic effects and prevents the outgrowth of both drug-resistant and -sensitive BRAF-mutant melanoma cells [171]. TRAM-34, a specific inhibitor of the KCa3.1 channel [172], is found to significantly enhance the induction of apoptosis by vemurafenib in melanoma cells [173]. NSCLC treatment also faces challenges, particularly with the development of resistance to EGFR tyrosine kinase inhibitors (TKIs) after initial efficacy [174]. Recent studies indicate that combining the blockade of the KCa3.1 channel with the EGFR TKI erlotinib can effectively enhance tumor cell responsiveness to erlotinib [175]. Clinically, the use of BRAF inhibitors, MEK inhibitors, or a combination of both has been proven to significantly extend the progression-free survival and overall survival of patients with malignant melanoma [176, 177]. However, resistance to these treatments remains a significant issue. Inhibiting T-type calcium channels has been found to induce differentiation and death of drug-resistant melanoma cells in vitro and reverse the resistance to MAPK inhibitors in vivo [178]. The melanoma patients with BRAFV600E mutation can be treated with kinase inhibitors, such as vemurafenib, but these patients frequently develop the acquire resistance to these drugs. T-type calcium channel (TTCC) blocker mibefradil can induce apoptosis via autophagy inhibition in drug-resistant BRAF melanoma cells, which provides a therapeutic strategy toward BRAF inhibitor resistance [179]. The elevated TRPM2 expression was observed in EGFR mutant non-small cell lung cancer cell lines treated with osimertinib. The knockdown of TRPM2 inhibits the endogenous flow of Ca²+, thereby enhancing the apoptotic effect of osimertinib in vitro and in vivo. Therefore, targeting TRPM2 is expected to be a promising strategy for overcoming and preventing osimertinib acquired resistance [180]. The intracellular Cl- channel protein ClC-3 contributes to the resistance of cancer cells to chemotherapy drugs and HER2-targeted therapies [181]. The intracellular Cl- regulation by ANO1/ClC-3 is closely related to the transcription of the HER2 gene in HER2-positive breast cancer cells. Consequently, inhibitors of ANO1/ClC-3 may represent an effective therapeutic strategy for patients with resistance to anti-HER2 therapies [182]. Additionally, the inhibition of TMEM16A/ANO1, a Ca2+-activated Cl- channel, increases the sensitivity to EGFR and HER2/ERBB2 targeted therapies [183]. In summary, targeting ion channels presents a promising strategy to overcome resistance in various cancers, including CLL, melanoma, and NSCLC. Further researches into these mechanisms may lead to more effective and durable anticancer therapies.
3.3 Ion channels and immunotherapy
Ion channel modulators can enhance the immune system's attack on tumors through various mechanisms, thereby improving therapeutic efficacy of immunotherapy. Nifedipine (NIFE), a calcium channel blocker, inhibits the expression of programmed death-ligand 1 (PD-L1) on CRC cells and programmed death-1 (PD-1) on CD8+ T cells. This finding reveals the significant role of calcium channel-related signaling pathways in PD-1/PD-L1-mediated tumor immune escape. In the further study, the researchers show that NIFE is more effective against tumors when used in combination with anti-PD-1 antibodies. This combination therapy suggests a potential synergistic effect, offering a more effective strategy for enhancing PD-1-based antitumor immunotherapy [184]. TRPML1-mediated ferroptosis plays a critical role in AKT-driven tumorigenesis and anticancer drug resistance. The inhibition of TRPML1 can suppress AKT-driven tumorigenesis and enhance the sensitivity of tumor cells to ferroptosis induction therapy, radiation therapy, and immunotherapy. Specifically, WT-iRGD, a peptide targeting TRPML1, inhibits tumorigenesis and promotes cancer therapy by blocking the interaction between TRPML1 and ARL8B. This blockade leads to increased lipid peroxidation levels and the activation of cytotoxic CD4+ and CD8+ T cells in mice, particularly when combined with PD1 antibodies [185]. The P2RX7 receptor (also known as P2X7R) is an ATP-gated ion channel, primarily presents in immune cells and some tumor cells [186]. P2RX7 has been demonstrated to play a crucial role in the maturation of macrophages and dendritic cells (DCs), as well as in the secretion of the proinflammatory cytokines IL-1β and IL-18. Moreover, P2RX7 coordinates immunogenic cell death (ICD) and enhances the ability of DCs to activate and present tumor antigens to T cells, positioning it as a positive regulator of the anti-tumor immune response [187]. In immune cells, P2RX7 negatively regulates the number and function of regulatory T cells (TREGs), thereby inhibiting their immunosuppressive activity. This regulatory function is particularly relevant in the treatment of NSCLC, where only a subset of patients responds to immunotherapy. The P2RX7 activator, HEI3090, has been shown to augment an anti-tumor immune response, when used in combination with PD-1 immune checkpoint inhibitor. Mechanistically, the activation of P2RX7 leads to an increase in IL-18 production in an NLRP3-dependent manner, which subsequently activates NK cells and CD4+ T cells to produce IFN-γ, thereby enhancing tumor immunogenicity [188].
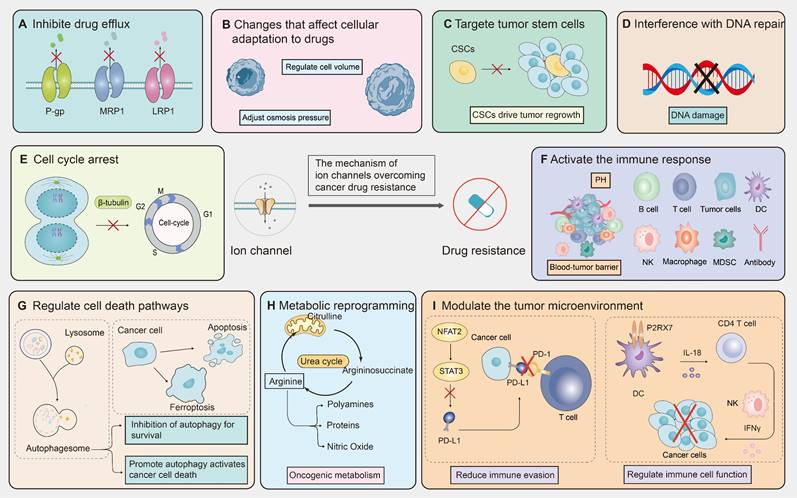
The ion channel regulators, when combined with conventional cancer therapies, can combat anticancer drug resistance through multiple mechanisms. These mechanisms include inhibiting drug efflux (Figure 3A), regulating cell volume changes (Figure 3B), targeting tumor stem cells (Figure 3C), interfering with DNA repair mechanisms (Figure 3D), inhibiting cell proliferation processes (Figure 3E), activating immune responses (Figure 3F), promoting cell death (Figure 3G), inhibiting metabolic reprogramming (Figure 3H), and regulating tumor microenvironment (Figure 3l). The combination of ion channel-targeting modulators with anticancer drugs presents a promising strategy to overcome drug resistance. This approach can be specifically categorized into two mechanisms: enhancing drug sensitivity (Figure 4) and reversing drug resistance (Figure 5). Recent studies have revealed that ion channels are also involved in the transport of essential trace elements such as iron, zinc, and copper, which play a crucial role in tumor development and drug resistance. By participating in the regulation of enzyme activity and signal transduction pathways associated with cell proliferation and apoptosis, those modulators of trace elements enhance the sensitivity of anticancer drugs or reverse their resistance (Figure 6).
4. The current drugs that target ion channels
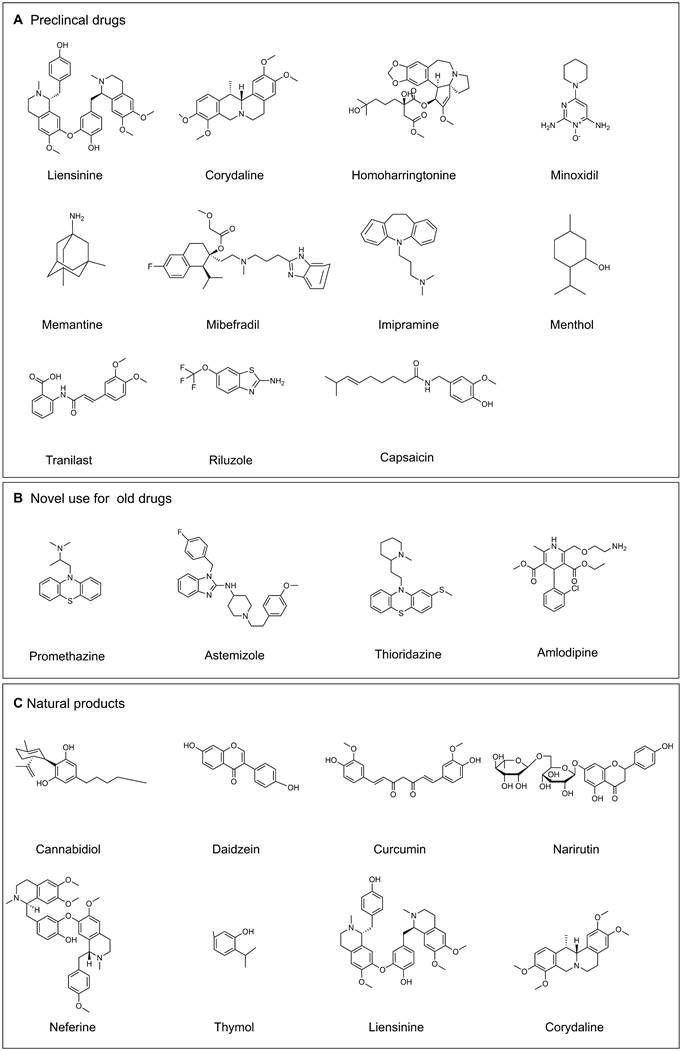
The role of ion channels in cancer drug resistance has been increasingly highlighted in recent literature, which promotes researchers to explore the novel agents for targeting these ion channels. The ion channel modulators include preclinical drugs (Figure 7A), novel use for old drugs that have been approved by U.S. Food and Drug Administration (FDA) (Table 3, Figure 7B) and some natural products (Table 3, Figure 7C).
Therapeutic mechanisms targeting ion channels to overcome cancer drug resistance. Targeting ion channels is a therapeutic strategy to overcome anti-cancer resistance and involves multiple pathways. (A) Firstly, they influence efflux pumps, inhibiting drug efflux and thereby increasing intracellular drug concentration. (B) Modulating cellular adaptive changes to chemotherapy drugs, such as osmoregulation, which affects cell volume. (C) Targeting specific markers or signaling pathways of tumor stem cells to reduce tumor resistance. (D) Interfering with DNA repair pathways to reduce cancer cells' ability to repair DNA damage induced by chemotherapy. (E) modulating cyclins or arresting the cell cycle by inhibiting mitosis. (F) Promoting drug efficacy by altering the composition of immune cells, PH values, and the blood-brain barrier within the TME. (G) Enhancing the killing effect of chemotherapeutic drugs by promoting apoptosis, autophagy, ferroptosis, and other cell death pathways. (H) Modulating tumor metabolic pathways to reduce resistance to chemotherapy drugs. (I) Regulating the immune system by reducing immune evasion and influencing the function of immune cells.
Two different types of drugs target ion channels to treat cancer.
| Drug name | Drug | Cancer type | Target | Mechanism | Ref |
|---|---|---|---|---|---|
| Novel use for old drugs | Imipramine, Promethazine | Lung, Pancreatic neuroendocrine tumors, Merkel cell carcinoma | hEag1 inhibitor | Induce apoptosis and reverse chemotherapy drug resistance | [189, 191] |
| Amitriptyline | Glioblastoma multiforme | Kv10.1 inhibitor | Prolong the overall survival of patients | [192] | |
| Astemizole, Imipramine | Acute myeloid leukemia | hEag1 inhibitor | Induce apoptosis, increase chemotherapy sensitivity in PLB-985 cells | [193] | |
| Thioridazine | Medulloblastoma | EAG2 inhibitor | Reduced MB growth and metastasis | [194] | |
| Amlodipine, Felodipine, Mannipine, Cilnidipine | Breast, Pancreatic | Calcium channel inhibitor | Reduced invasion | [195] | |
| Natural products | Cannabidiol | Glioblastoma, Multiple myeloma, Breast, Endometrial cancer | TRPV2 agonists | Induce apoptosis, increase chemotherapy sensitivity | [200-202] |
| Cannabidiol | NSCLC | TRPV2 agonists | Induce apoptosis, reverse chemotherapy drug resistance | [203] | |
| Curcumin | Colorectal cancer | TRPA1 agonists | Inhibits cell proliferation and reduces cholesterol absorption | [207] | |
| Maclura pomifera | Breast cancer | TRPV1 agonists | Induce apoptosis | [208] | |
| Natural Borneol | Lung adenocarcinoma | TRPM8 agonists | Induce apoptosis, increase chemotherapy sensitivity | [209] | |
| Neferine | Colorectal cancer | RyRs agonists | Induce apoptosis, autophagy | [210] | |
| Narirutin | Lung cancer | TMEM16A inhibitor | Induce apoptosis, inhibit cell proliferation, reverse chemotherapy drug resistance | [211] | |
| Daidzein, Homoharringtonine | Lung cancer | TMEM16A inhibitor | Inhibit cell proliferation and migration | [212] | |
| Liensinine, Corydaline | Liver cancer | Kv10.1 inhibitor | Inhibit cell proliferation and migration | [214, 215] | |
| Mallotus apelta | Prostate cancer, oral squamous cell carcinoma | ANO1 inhibitor | Induce apoptosis | [216] | |
| Capsaicin | Thyroid cancer | TRPV1 agonists | Induce apoptosis, autophagy | [204, 205] | |
| Thymol | Prostate cancer | TRPV3 agonists | Decrease cell viability | [206] |
The combined use of ion channel-targeting agents with anticancer drugs enhances their sensitivity through related signaling pathways. Given the close relationship between ion channels and cancer, drugs targeting ion channels, when used in combination with anticancer drugs, exhibit synergistical effect on tumor cells. By precisely regulating the ion balance within these cells, they effectively inhibit abnormal proliferation. This regulation significantly enhances apoptosis and autophagy processes while exacerbating DNA damage. These combined effects not only undermine the fundamental survival mechanisms of tumor cells but also markedly increase their sensitivity to traditional anticancer drugs.
4.1 Novel use for old drugs that have been approved by the FDA
Ion channels are currently essential drug targets for the treatment of a variety of diseases, including type 2 diabetes, hypertension, epilepsy, arrhythmia, anxiety disorders, and cancer. The novel use of FDA-approved old drugs as Ion channel modulators is a promising approach for anticancer treatment. Ion channels are not the primary targets of imipramine or astemizole, but these drugs can block various channels due to their high affinity. Specifically, imipramine blocks sodium, potassium, and calcium ion channels, while astemizole affects several potassium channels associated with Kv10.1 [189]. Promethazine, known as a nonspecific Eag1 (KV 10.1) blocker, can inhibit the growth of SCLC, pancreatic neuroendocrine tumors, and Merkel cell carcinoma [190]. Additionally, cisplatin-resistant tumors remain sensitive to imipramine treatment. Therefore, imipramine and other related TCAs might serve as second-line treatment for patients with SCLC refractory to cisplatin/etoposide [191]. The efficacy of TCAs is attributed to their non-specific and "non-targeted" mode of action, which impacts multiple molecules on the surface of cancer cells. For instance, the use of the TCA amitriptyline has been linked to significantly prolonged overall survival of patients with brain metastases from various carcinomas or GBM [192]. hEag1 blockers such as astemizole and mAb56, when combined with commonly used chemotherapeutic drugs, can increase the apoptotic response of PLB-985 cells in AML [193]. The antihistamine drug astemizole shows its ability to reduce breast cancer cell proliferation through selective blockade of Eag1 channels [189]. Additionally, astemizole and the antipsychotic drug thioridazine, as Eag2 channel blockers, potentially reduces the growth and metastasis of intracranial xenograft medulloblastoma [194]. Calcium channel blocker analogs of antihypertensive drugs including amlodipine, felodipine, manidipine, and cilnidipine, have shown significant efficacy in inhibiting filopodial formation, directional migration, and cell invasion in breast and pancreatic cancer [195]. These findings underscore the potential of repurposing existing drugs to target ion channels for cancer therapy.
The combined use of ion channel-targeting agents with anticancer drugs reverses the resistance via multiple signaling pathways. The combination of ion channel targeting drugs and anticancer drugs can effectively inhibit the abnormal proliferation of tumor cells and significantly enhance the process of apoptosis and autophagy by regulating ion homeostasis and related signaling pathways. This combination therapy especially targets tumor cells that have shown resistance to conventional anticancer drugs, showing a significant resistance reversal effect. In addition, abnormal function of specific ion channels such as Orai3 is directly related to the development of chemotherapy resistance in tumor cells. This resistance occurs because the degradation of p53 reduces the cell's response to chemotherapeutic-induced apoptosis.
4.2 Natural products
Long-term use of chemotherapeutic drugs can produce a series of side effects, including toxicity and drug resistance [196]. Natural products can serve as invaluable sources for studying biological systems and drug discovery, particularly in anticancer research [197, 198]. These products have very favorable advantages due to their chemical diversity, low toxicity, safety, and availability, and can also enhance the efficacy of anticancer drugs [199]. Cannabidiol (CBD), a natural product that activates the TRPV2 channel, increases the sensitivity of glioblastoma cells to cytotoxic chemotherapeutics by enhancing drug uptake. In multiple myeloma cells, CBD exhibits a synergistic effect when combined with bortezomib. Furthermore, CBD enhances the chemosensitivity in the treatment of TNBC and endometrial cancer cells [200-202]. Additionally, CBD induces apoptosis in cisplatin-resistant NSCLC cells by modulating oxidative stress pathways [203]. Capsaicin, another natural product and a TRPV1 agonist, triggers excessive Ca2+ influx leading to mitochondrial dysfunction. This process activates the mitochondrial permeability transition pore (mPTP), and initiates apoptosis in thyroid cancer cells. Capsaicin also induces autophagy in anaplastic thyroid carcinoma (ATC) cells via Ca2+ influx [204, 205]. Thymol, a TRPV3 activator, affects Ca2+ homeostasis and reduces the viability of prostate cancer cells [206]. Curcumin inhibits the proliferation of colon cancer cells and reduces cholesterol absorption in Caco-2 cells by activating TRPA1 channels [207]. Both male and female M. pomifera plant extracts trigger intracellular Ca2+ overload via TRPV1, subsequently inducing apoptosis through multiple pathways in ER-positive MCF-7 and T47D breast cancer cells [208]. Natural Borneol pretreatment enhances the sensitivity of A549 cells to low dose doxicin (DOX) and increases apoptosis. Through surface plasmonic resonance (SPR) and liquid chroorubmatography-mass spectrometry (MS-SPRI) analysis, TRPM8 is found to be a potential target for natural borneol as a sensitizer in chemotherapy [209]. Neferine, a natural alkaloid, induces autophagy and reverses drug resistance by activating ryanodine receptors and promoting Ca2+ release [210]. Narirutin, a functional food, significantly improves the therapeutic effect and eliminates the side effects of cisplatin [211]. Daidzein is confirmed to inhibit the growth of lung adenocarcinoma by suppressing TMEM16A channel in a dose-dependent manner [212, 213]. Homoharringtonine (HHT) is proven to be a novel TMEM16A inhibitor to suppress the growth of lung cancer [212, 213]. The novel food-derived compound Liensinine can serve as a lead compound for anti-HCC drugs by targeting Kv10.1. Corydaline inhibits HCC by binding to the druggable pocket of the hEAG1 channel [214, 215]. Extracts from Mallotus apelta act as novel ANO1 inhibitors to exhibit anticancer activity [216]. Natural products targeting ion channels hold significant promise for cancer therapy. They are particularly effective in enhancing therapeutic outcomes and reversing drug resistance. However, their clinical application necessitates further research and validation. Ensuring safety and efficacy, as well as optimizing dosing strategies, are critical steps that must be addressed.
The transport trace elements are involved in carcinogenesis and anticancer drug resistance. Metal ions, including Fe, Zn and Cu, interact with ion channels on the cell membrane, thereby regulating intracellular ion concentrations. This regulation plays a crucial role in fine-tuning cell signal transduction pathways, which in turn influences key biological processes such as cell proliferation and apoptosis. It is worth noting that the combination of ion channel modulators transporting metal elements and anticancer drugs can inhibit drug efflux, promote cell apoptosis, increase DNA damage, cause iron sag, effectively improve the sensitivity of anticancer treatment and reverse drug resistance of cancer cells.
5. Conclusions and Perspectives
Although the advancements in healthcare have extended human lifespan, cancer remains a significant global health burden. Recent research highlights the intricate relationship between ion channels and cancer. Ion channels play crucial roles in cancer initiation, progression, and therapeutic response, thus termed oncogenic channels. Aberrant expression and dysregulation of ion channels have been associated with the drug resistance to conventional cancer treatment through various mechanisms. Therefore, targeting ion channels will hopefully offer a novel and promising strategy for overcoming cancer drug resistance.
Despite the identification of ion channels whose expression and/or functional alterations promote our understanding for cancer drug resistance, only a few therapeutic strategies targeting these channels have progressed to early-stage clinical trials (Table 4, Figure 7A). A viable ion channel therapeutic target for cancer must have low expression in normal tissue, high expression in tumor tissue, high selectivity with ligands, and minimal side effects [1]. However, many drugs fail to meet these stringent criteria, underscoring the urgent need for clinical trials. Consequently, developing drugs that effectively target ion channels presents numerous challenges. Firstly, the crosstalk between ion channels and their biological function is complicated, which depends on cancer cell types, intracellular ion concentration, and other circumstances. For instance, both BKCa and calcium-activated chloride channel (CLCA) can be regulated by Ca2+. Sodium-calcium exchangers (NCX) function to pump Ca2+ out of the cell but Na+ in cellular uptake [217-219]. Therefore, the ion channel modulators may exert different possibilities, which should be considered in the personalized medicine design for cancer patients. Secondly, ion channels are highly druggable targets, primarily due to their membrane localization. Ion channels are extensively distributed across various cells and tissues, and their activation or inhibition can significantly impact the function of multiple organ systems, potentially leading to unexpected anti-tumor side effects. For instance, TMEM16A plays a crucial role not only in tumor progression but also in regulating chloride ion transport through epithelial cells, and modulating electrical signals in smooth muscle and specific neurons [220-222]. Achieving selectivity among ion channel subtypes is challenging due to their high homology. This highlights the need for cancer-specific targets and novel targeted drug delivery systems via ion channels [223]. Biological agents, notably antibodies and peptides, can exhibit high selectivity for subtypes and off-targets compared to small molecules. These biological agents are metabolized normally without metabolism-mediated toxicity or drug interactions [224]. The antibodies often possess strong specificity, potency, and long circulatory half-life [224-231]. They can also be used for targeted cancer therapy by attaching to toxins or radioactive molecules. An antibody targeting a P2X7 variant is currently in clinical trials for basal cell carcinoma [232]. The evolution of drug delivery systems highlights the need for selective cytotoxic delivery to cancer cells, which makes ion channels potential targets for anticancer drugs. Natural product-drug conjugates may provide new research avenues for prostate cancer patients over-expressing TRPV1 channel [233]. Based on 3D hydrogels, limonin, a novel TMEM16A inhibitor from herbal medicine, is found to have the anticancer potential in lung cancer by targeting specific high expressed TMEM16A ion channel [234]. Another natural product, Silibinin, is also found to serve as an inhibitor of TMEM16A [235]. Thirdly, given the complexity of ion channel function and tumor heterogeneity, clinical decisions require precise modulation (activation or inhibition) of ion channels and personalized treatment for cancer patients. Iron metabolism has dual roles in tumorigenesis and development. Upregulation of TFRC in ADR-resistant breast cancer reverses drug resistance via ferroptosis activation, whereas increased iron absorption in ovarian cancer enhances DNA repair and drug resistance [117, 161]. To address this, deepening understanding of ion channel biology, signal transduction, and their tumor microenvironment interactions is essential. Advances in electrophysiology, particularly patch-clamp technology, and mature high-throughput screening (HTS) for ion channels have accelerated ion channel research and drug discovery. At last, effective biomarkers predicting the sensitivity or resistance of cancer cells to ion channel modulators are lacking. Thus, it is essential to discover and validate these biomarkers through genomics, proteomics, and metabolomics.
In summary, addressing the challenge of cancer resistance through targeting ion channels involves several key issues. Moreover, the current lack of sufficient clinical data, which limits the practical application of these strategies. Future advancements in technologies such as high-throughput sequencing, single-cell analysis, and structural biology are expected to enhance our comprehension for the molecular mechanisms by which ion channel modulators overcome drug resistance. Additionally, progress in drug design, delivery systems, biomarker development, and personalized treatment will be essential. Although these issues, we think targeting ion channels will hopefully provide us new treatment methods to prevent or combat the emergence of drug resistance.
Selected drugs that target ion channels. (A) Preclinical drugs. (B) Novel use for old drugs. (C) Natural products. (Drawn by chemdraw)
Advances in clinical trials of anticancer drugs targeting ion channels over the past few decades.
| Posted | Identifiers | Interventions | Target | Cancer Types | Phase |
|---|---|---|---|---|---|
| 2004 | NCT00130962 | ALGRX 4975 | TRPV1 | Neuroma | Ⅱ |
| 2011 | NCT01303341 | Riluzole+Sorafenib Tosylate | VGSC | Melanoma, Advanced solid tumors | Ⅰ |
| 2011 | NCT01916317 | Lidocaine+Surgery | VGSC | Breast cancer | Ⅲ |
| 2011 | NCT01298310 | Lidocaine | VGSC | Morton's Neuroma | Ⅰ |
| 2012 | NCT01480050 | Mibefradil Dihydrochloride+Temozolomide | TTCC | Brain and central nervous system tumors | Ⅰ |
| 2012 | NCT01578564 | SOR-C13 | TRPV6 | Ovarian cancer | Ⅰ |
| 2013 | NCT02587819 | BSCT | P2X7 | Basal cell carcinoma | Ⅰ |
| 2013 | NCT01855607 | Menthol | TRPM8 | Breast, Gastrointestinal, Gynecological cancer | Ⅱ |
| 2014 | NCT02037464 | Capsaicin | TRPV1 | Prostate cancer | Ⅱ |
| 2021 | NCT05272462 | Minoxidil+Platinum | Kir6/SUR | Epithelial ovarian cancer | Ⅱ |
| 2021 | NCT04801342 | WBRT+Memantine | TRPM2 | Brain cancer | Ⅱ |
| 2022 | NCT04863950 | Imipramine Hydrochloride+Lomustine | EAG1 | Glioblastoma | Ⅱ |
| 2022 | NCT05626829 | Tranilast+Radiotherapy | TRPV2 | Nasopharyngeal carcinoma | Ⅱ |
| 2023 | NCT06007846 | Memantine | Kv1.3 | Liver cancer | Ⅱ/Ⅲ |
| 2023 | NCT04761614 | Riluzole+mFOLFOX6+Bevacizumab | VGSC | Colorectal cancer | Ⅰ |
| 2024 | NCT06515678 | CCB+ACEi+Bevacizumab | LTCC | Ovarian cancer | - |
| 2014 | NCT02037464 | Capsaicin | TRPV1 | Prostate cancer | Ⅱ |
Abbreviations
AML: Acute myeloid leukemia; ADR: Adriamycin; ASIC1a: Acid sensing ion channel 1a; BKCa: The large-conductance calcium-activated potassium channel; BTB: Blood-tumor barrier; BZ: Bortezomib; BUC: Bladder urothelial carcinoma; CRC: Colorectal cancer; CTR1: Copper Transporter 1; CSC: Cancer stem cell; CCBs: Calcium channel blockers; ClC-3: Chloride channel-3; CLIC1: Chloride intracellular channel 1; CLL: Chronic lymphocytic leukemia; CBD: Cannabidiol; CLCA: Calcium-activated chloride channel; DMT1: Divalent metal transporter 1; DCs: Dendritic cells; DOX: Doxicin; ENaC: Epithelial sodium channel; EGFR: Epidermal growth factor receptor; FPN1: Ferroportin 1; FDA: Food and Drug Administration; GC: Gastric cancer; GPER: G protein-coupled estrogen receptor; GBM: Glioblastoma multiforme; GuHCl: Guanidine hydrochloride; HCC: Hepatocellular carcinoma; HCQ: Hydroxychloroquine; HHT: Homoharring-tonine; IKCa1: Intermediate Conductance Calcium-Activated Potassium Channel 1; IREB2: Iron-responsive element-binding protein 2; iASPP: Inhibitor of apoptosis-stimulating protein of p53; IC50: Semi-inhibitory concentration; ICD: Immunogenic cell death; KCNQ1OT1: Potassium voltage-gated channel subfamily Q member 1 overlapping transcript 1; MCU: Mitochondrial calcium uniporter; MDR: Multidrug resistance; MAFG-AS1: MAF transcription factor G antisense RNA 1; MDR: Multidrug resistance; mPTP: Mitochondrial permeability transition pore; NSCLC: non-small cell lung cancer; NCCE: Non-capacitative calcium entry; NIFE: Nifedipine; NCX: Sodium-calcium exchangers; OSCC: Oral squamous cell carcinoma; PDAC: Pancreatic ductal adenocarcinoma; PcG: Polycomb proteins; PD-L1: Programmed death-ligand 1; PD-1: Programmed death-1; ROS: Reactive oxygen species; SOCE: Store-operated calcium entry; sEVs: Small extracellular vesicles; STIM1: Stromal interaction molecule 1; SPFX: Sparfloxacin; SCLC: Small-cell lung cancer; SCCHN: Squamous cell carcinoma of the head and neck; SPR: Surface plasmonic resonance; TME: Tumor immune microenvironment; TRPC: Ttransient receptor potential canonical; TFR: Transferrin receptor; TCAs: Tricyclic antidepressants; TCR: T-cell receptor; TRPML1: MCOLN1/ mucolipin TRP channel 1; TNBC: Triple-negative breast cancer; TRPM2: Transient receptor potential melastatin-2; TMCO1: Transmembrane and coiled-coil domains 1; TKIs: Tyrosine kinase inhibitors; TTCC: T-type calcium channel; TREGs: Regulatory T cells; UPR: Unfolded protein response; VGSCs: Voltage-Gated Sodium Channels; VRACs: Volume-regulated anion channels.
Acknowledgements
This work was supported in part by the grants from the National Natural Science Foundation of China (grant No. U23A20518), Zhejiang Provincial Natural Science Foundation of China (grant No. LY22H290004), and Zhejiang Provincial Science and Technology Program of Traditional Chinese Medicine (No. GZY-ZJ-KJ-24056).
Author contributions
Xinbing Sui, Tian Xie, Xidong Gu, and Yiping Mou guided and designed this review. Qian Shi wrote the original manuscript and drew the figures and tables. Zijing Yang, Huan Yang, Lihui Xu, Jing Xia, Jie Gu, Mengting Chen, Yan Wang, Xiaohong Zhao, and Zehua Liao performed the data collection. All authors read and approved the article.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Prevarskaya N, Skryma R, Shuba Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol Rev. 2018;98:559-621
2. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11-21
3. Jentsch TJ, Pusch M. CLC Chloride Channels and Transporters: Structure, Function, Physiology, and Disease. Physiol Rev. 2018;98:1493-590
4. Catterall WA. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron. 2010;67:915-28
5. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70
6. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49
7. Saez-Ibañez AR, Upadhaya S, Partridge T, Winkelman D, Correa D, Campbell J. The changing landscape of cancer cell therapies: clinical trials and real-world data. Nat Rev Drug Discov. 2024;23:736-7
8. Capatina AL, Lagos D, Brackenbury WJ. Targeting Ion Channels for Cancer Treatment: Current Progress and Future Challenges. Rev Physiol Biochem Pharmacol. 2022;183:1-43
9. Leanza L, Managò A, Zoratti M, Gulbins E, Szabo I. Pharmacological targeting of ion channels for cancer therapy: In vivo evidences. Biochim Biophys Acta. 2016;1863:1385-97
10. Cameron IL, Smith NK, Pool TB, Sparks RL. Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res. 1980;40:1493-500
11. Qian Y, Wong CC, Xu J, Chen H, Zhang Y, Kang W. et al. Sodium Channel Subunit SCNN1B Suppresses Gastric Cancer Growth and Metastasis via GRP78 Degradation. Cancer Res. 2017;77:1968-82
12. Qian Y, Zhou L, Luk STY, Xu J, Li W, Gou H. et al. The sodium channel subunit SCNN1B suppresses colorectal cancer via suppression of active c-Raf and MAPK signaling cascade. Oncogene. 2023;42:601-12
13. Xu X, Dai Y, Feng L, Zhang H, Hu Y, Xu L. et al. Knockdown of Nav1.5 inhibits cell proliferation, migration and invasion via Wnt/β-catenin signaling pathway in oral squamous cell carcinoma. Acta Biochim Biophys Sin (Shanghai). 2020;52:527-35
14. Bortner CD, Cidlowski JA. Ion channels and apoptosis in cancer. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130104
15. Li M, Tian P, Zhao Q, Ma X, Zhang Y. Potassium channels: Novel targets for tumor diagnosis and chemoresistance. Frontiers in oncology. 2022;12:1074469
16. Pardo LA, Stühmer W. The roles of K(+) channels in cancer. Nat Rev Cancer. 2014;14:39-48
17. Ma G, Liu H, Hua Q, Wang M, Du M, Lin Y. et al. KCNMA1 cooperating with PTK2 is a novel tumor suppressor in gastric cancer and is associated with disease outcome. Mol Cancer. 2017;16:46
18. Wang F, Chen Q, Huang G, Guo X, Li N, Li Y. et al. BKCa participates in E2 inducing endometrial adenocarcinoma by activating MEK/ERK pathway. BMC Cancer. 2018;18:1128
19. Lallet-Daher H, Roudbaraki M, Bavencoffe A, Mariot P, Gackière F, Bidaux G. et al. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene. 2009;28:1792-806
20. Yu H. Depolarization or hyperpolarization: Emerging role of altered bioelectricity in breast cancer metastasis. EBioMedicine. 2022;76:103853
21. Sundelacruz S, Levin M, Kaplan DL. Depolarization alters phenotype, maintains plasticity of predifferentiated mesenchymal stem cells. Tissue Eng Part A. 2013;19:1889-908
22. Kovalenko I, Glasauer A, Schöckel L, Sauter DR, Ehrmann A, Sohler F. et al. Identification of KCa3.1 Channel as a Novel Regulator of Oxidative Phosphorylation in a Subset of Pancreatic Carcinoma Cell Lines. PLoS ONE. 2016;11:e0160658
23. Mohr CJ, Gross D, Sezgin EC, Steudel FA, Ruth P, Huber SM. et al. K(Ca)3.1 Channels Confer Radioresistance to Breast Cancer Cells. Cancers (Basel). 2019;11:1285
24. Bulk E, Todesca LM, Bachmann M, Szabo I, Rieke M, Schwab A. Functional expression of mitochondrial K(Ca)3.1 channels in non-small cell lung cancer cells. Pflugers Arch. 2022;474:1147-57
25. Quast SA, Berger A, Buttstädt N, Friebel K, Schönherr R, Eberle J. General Sensitization of melanoma cells for TRAIL-induced apoptosis by the potassium channel inhibitor TRAM-34 depends on release of SMAC. PLoS ONE. 2012;7:e39290
26. Bachmann M, Rossa A, Varanita T, Fioretti B, Biasutto L, Milenkovic S. et al. Pharmacological targeting of the mitochondrial calcium-dependent potassium channel KCa3.1 triggers cell death and reduces tumor growth and metastasis in vivo. Cell Death Dis. 2022;13:1055
27. Hemmerlein B, Weseloh RM, Mello de Queiroz F, Knötgen H, Sánchez A, Rubio ME. et al. Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer. 2006;5:41
28. Lansu K, Gentile S. Potassium channel activation inhibits proliferation of breast cancer cells by activating a senescence program. Cell Death Dis. 2013;4:e652
29. Capera J, Pérez-Verdaguer M, Peruzzo R, Navarro-Pérez M, Martínez-Pinna J, Alberola-Die A. et al. A novel mitochondrial Kv1.3-caveolin axis controls cell survival and apoptosis. Elife. 2021;10:e69099
30. Leanza L, Romio M, Becker KA, Azzolini M, Trentin L, Managò A. et al. Direct Pharmacological Targeting of a Mitochondrial Ion Channel Selectively Kills Tumor Cells In Vivo. Cancer Cell. 2017;31:516-31.e10
31. Wu J, Chen Z, Liu Q, Zeng W, Wu X, Lin B. Silencing of Kv1.5 Gene Inhibits Proliferation and Induces Apoptosis of Osteosarcoma Cells. Int J Mol Sci. 2015;16:26914-26
32. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74
33. Ryland KE, Svoboda LK, Vesely ED, McIntyre JC, Zhang L, Martens JR. et al. Polycomb-dependent repression of the potassium channel-encoding gene KCNA5 promotes cancer cell survival under conditions of stress. Oncogene. 2015;34:4591-600
34. Senyuk V, Eskandari N, Jiang Y, Garcia-Varela R, Sundstrom R, Leanza L. et al. Compensatory expression of NRF2-dependent antioxidant genes is required to overcome the lethal effects of Kv11.1 activation in breast cancer cells and PDOs. Redox Biol. 2021;45:102030
35. Yanglin P, Lina Z, Zhiguo L, Na L, Haifeng J, Guoyun Z. et al. KCNE2, a down-regulated gene identified by in silico analysis, suppressed proliferation of gastric cancer cells. Cancer Lett. 2007;246:129-38
36. Lee I, Lee SJ, Kang TM, Kang WK, Park C. Unconventional role of the inwardly rectifying potassium channel Kir2.2 as a constitutive activator of RelA in cancer. Cancer Res. 2013;73:1056-62
37. Voloshyna I, Besana A, Castillo M, Matos T, Weinstein IB, Mansukhani M. et al. TREK-1 is a novel molecular target in prostate cancer. Cancer Res. 2008;68:1197-203
38. Mori Y, Yokota H, Hoshino I, Iwatate Y, Wakamatsu K, Uno T. et al. Deep learning-based gene selection in comprehensive gene analysis in pancreatic cancer. Scientific reports. 2021;11:16521
39. Li WC, Xiong ZY, Huang PZ, Liao YJ, Li QX, Yao ZC. et al. KCNK levels are prognostic and diagnostic markers for hepatocellular carcinoma. Aging. 2019;11:8169-82
40. Leithner K, Hirschmugl B, Li Y, Tang B, Papp R, Nagaraj C. et al. TASK-1 Regulates Apoptosis and Proliferation in a Subset of Non-Small Cell Lung Cancers. PLoS ONE. 2016;11:e0157453
41. Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297-308
42. Lin G, Lin L, Lin H, Chen W, Chen L, Chen X. et al. KCNK3 inhibits proliferation and glucose metabolism of lung adenocarcinoma via activation of AMPK-TXNIP pathway. Cell Death Discov. 2022;8:360
43. Collier C, Wucherer K, McWhorter M, Jenkins C, Bartlett A, Roychoudhuri R. et al. Intracellular K+ Limits T-cell Exhaustion and Preserves Antitumor Function. Cancer Immunol Res. 2024;12:36-47
44. Eil R, Vodnala SK, Clever D, Klebanoff CA, Sukumar M, Pan JH. et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature. 2016;537:539-43
45. Lyu Y, Wang Q, Liang J, Zhang L, Zhang H. The Ion Channel Gene KCNAB2 Is Associated with Poor Prognosis and Loss of Immune Infiltration in Lung Adenocarcinoma. Cells. 2022;11:3438
46. Brini M, Calì T, Ottolini D, Carafoli E. Intracellular calcium homeostasis and signaling. Met Ions Life Sci. 2013;12:119-68
47. Kanwar N, Carmine-Simmen K, Nair R, Wang C, Moghadas-Jafari S, Blaser H. et al. Amplification of a calcium channel subunit CACNG4 increases breast cancer metastasis. EBioMedicine. 2020;52:102646
48. Wang CY, Lai MD, Phan NN, Sun Z, Lin YC. Meta-Analysis of Public Microarray Datasets Reveals Voltage-Gated Calcium Gene Signatures in Clinical Cancer Patients. PLoS ONE. 2015;10:e0125766
49. Hao J, Bao X, Jin B, Wang X, Mao Z, Li X. et al. Ca2+ channel subunit α 1D promotes proliferation and migration of endometrial cancer cells mediated by 17β-estradiol via the G protein-coupled estrogen receptor. FASEB J. 2015;29:2883-93
50. Gao SH, Wang GZ, Wang LP, Feng L, Zhou YC, Yu XJ. et al. Mutations and clinical significance of calcium voltage-gated channel subunit alpha 1E (CACNA1E) in non-small cell lung cancer. Cell Calcium. 2022;102:102527
51. Natrajan R, Little SE, Reis-Filho JS, Hing L, Messahel B, Grundy PE. et al. Amplification and overexpression of CACNA1E correlates with relapse in favorable histology Wilms' tumors. Clin Cancer Res. 2006;12:7284-93
52. Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12:685-98
53. Zeng F, Chen X, Cui W, Wen W, Lu F, Sun X. et al. RIPK1 Binds MCU to Mediate Induction of Mitochondrial Ca(2+) Uptake and Promotes Colorectal Oncogenesis. Cancer Res. 2018;78:2876-85
54. Mallilankaraman K, Doonan P, Cárdenas C, Chandramoorthy HC, Müller M, Miller R. et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell. 2012;151:630-44
55. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152
56. Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:e532
57. Chakraborty PK, Mustafi SB, Xiong X, Dwivedi SKD, Nesin V, Saha S. et al. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat Commun. 2017;8:14634
58. Prakriya M, Lewis RS. Store-Operated Calcium Channels. Physiol Rev. 2015;95:1383-436
59. Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr. et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235-41
60. Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY. et al. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci U S A. 2011;108:15225-30
61. Xia J, Wang H, Huang H, Sun L, Dong S, Huang N. et al. Elevated Orai1 and STIM1 expressions upregulate MACC1 expression to promote tumor cell proliferation, metabolism, migration, and invasion in human gastric cancer. Cancer Lett. 2016;381:31-40
62. Shuttleworth TJ. Orai3-the 'exceptional' Orai? J Physiol. 2012;590:241-57
63. Dubois C, Kondratska K, Kondratskyi A, Morabito A, Mesilmany L, Farfariello V. et al. ORAI3 silencing alters cell proliferation and promotes mitotic catastrophe and apoptosis in pancreatic adenocarcinoma. Biochim Biophys Acta, Mol Cell Res. 2021;1868:119023
64. Lopez JJ, Jardin I, Sanchez-Collado J, Salido GM, Smani T, Rosado JA. TRPC Channels in the SOCE Scenario. Cells. 2020;9:126
65. El Boustany C, Bidaux G, Enfissi A, Delcourt P, Prevarskaya N, Capiod T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology. 2008;47:2068-77
66. Asghar MY, Magnusson M, Kemppainen K, Sukumaran P, Löf C, Pulli I. et al. Transient Receptor Potential Canonical 1 (TRPC1) Channels as Regulators of Sphingolipid and VEGF Receptor Expression: IMPLICATIONS FOR THYROID CANCER CELL MIGRATION AND PROLIFERATION. J Biol Chem. 2015;290:16116-31
67. Chen X, Li J, Zhang R, Zhang Y, Wang X, Leung EL. et al. Suppression of PD-L1 release from small extracellular vesicles promotes systemic anti-tumor immunity by targeting ORAI1 calcium channels. J Extracell Vesicles. 2022;11:e12279
68. Zhu L, Yang H, Zuo W, Yang L, Zhang H, Ye W. et al. Differential expression and roles of volume-activated chloride channels in control of growth of normal and cancerous nasopharyngeal epithelial cells. Biochem Pharmacol. 2012;83:324-34
69. Ye D, Luo H, Lai Z, Zou L, Zhu L, Mao J. et al. ClC-3 Chloride Channel Proteins Regulate the Cell Cycle by Up-regulating cyclin D1-CDK4/6 through Suppressing p21/p27 Expression in Nasopharyngeal Carcinoma Cells. Scientific reports. 2016;6:30276
70. Britschgi A, Bill A, Brinkhaus H, Rothwell C, Clay I, Duss S. et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc Natl Acad Sci U S A. 2013;110:E1026-34
71. Qiang YY, Li CZ, Sun R, Zheng LS, Peng LX, Yang JP. et al. Along with its favorable prognostic role, CLCA2 inhibits growth and metastasis of nasopharyngeal carcinoma cells via inhibition of FAK/ERK signaling. J Exp Clin Cancer Res. 2018;37:34
72. Corbet C, Feron O. Tumour acidosis: from the passenger to the driver's seat. Nat Rev Cancer. 2017;17:577-93
73. Clusmann J, Franco KC, Suárez DAC, Katona I, Minguez MG, Boersch N. et al. Acidosis induces RIPK1-dependent death of glioblastoma stem cells via acid-sensing ion channel 1a. Cell Death Dis. 2022;13:702
74. Raza M, Chakraborty S, Choudhury M, Ghosh PC, Nag A. Cellular iron homeostasis and therapeutic implications of iron chelators in cancer. Curr Pharm Biotechnol. 2014;15:1125-40
75. Adachi M, Kai K, Yamaji K, Ide T, Noshiro H, Kawaguchi A. et al. Transferrin receptor 1 overexpression is associated with tumour de-differentiation and acts as a potential prognostic indicator of hepatocellular carcinoma. Histopathology. 2019;75:63-73
76. Greene CJ, Attwood K, Sharma NJ, Gross KW, Smith GJ, Xu B. et al. Transferrin receptor 1 upregulation in primary tumor and downregulation in benign kidney is associated with progression and mortality in renal cell carcinoma patients. Oncotarget. 2017;8:107052-75
77. Rosager AM, Sørensen MD, Dahlrot RH, Hansen S, Schonberg DL, Rich JN. et al. Transferrin receptor-1 and ferritin heavy and light chains in astrocytic brain tumors: Expression and prognostic value. PLoS ONE. 2017;12:e0182954
78. Jeong SM, Hwang S, Seong RH. Transferrin receptor regulates pancreatic cancer growth by modulating mitochondrial respiration and ROS generation. Biochem Biophys Res Commun. 2016;471:373-9
79. Song J, Liu T, Yin Y, Zhao W, Lin Z, Yin Y. et al. The deubiquitinase OTUD1 enhances iron transport and potentiates host antitumor immunity. EMBO Rep. 2021;22:e51162
80. Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S. et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191-200
81. Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T. et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992-6
82. LaPlante JM, Ye CP, Quinn SJ, Goldin E, Brown EM, Slaugenhaupt SA. et al. Functional links between mucolipin-1 and Ca2+-dependent membrane trafficking in mucolipidosis IV. Biochem Biophys Res Commun. 2004;322:1384-91
83. Kasitinon SY, Eskiocak U, Martin M, Bezwada D, Khivansara V, Tasdogan A. et al. TRPML1 Promotes Protein Homeostasis in Melanoma Cells by Negatively Regulating MAPK and mTORC1 Signaling. Cell Rep. 2019;28:2293-305.e9
84. Gu Z, Wang H, Xia J, Yang Y, Jin Z, Xu H. et al. Decreased ferroportin promotes myeloma cell growth and osteoclast differentiation. Cancer Res. 2015;75:2211-21
85. Venkatachalam K, Wong CO, Zhu MX. The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium. 2015;58:48-56
86. Qi J, Xing Y, Liu Y, Wang MM, Wei X, Sui Z. et al. MCOLN1/TRPML1 finely controls oncogenic autophagy in cancer by mediating zinc influx. Autophagy. 2021;17:4401-22
87. Sheline CT, Behrens MM, Choi DW. Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis. J Neurosci. 2000;20:3139-46
88. Du W, Gu M, Hu M, Pinchi P, Chen W, Ryan M. et al. Lysosomal Zn(2+) release triggers rapid, mitochondria-mediated, non-apoptotic cell death in metastatic melanoma. Cell Rep. 2021;37:109848
89. Guo J, Cheng J, Zheng N, Zhang X, Dai X, Zhang L. et al. Copper Promotes Tumorigenesis by Activating the PDK1-AKT Oncogenic Pathway in a Copper Transporter 1 Dependent Manner. Advanced science (Weinheim, Baden-Wurttemberg, Germany). 2021;8:e2004303
90. Bakhsh MR, Rouhi L, Ghaedi K, Hashemi M, Peymani M, Samarghandian S. Therapeutic effects of guanidine hydrochloride on breast cancer through targeting KCNG1 gene. Biomed Pharmacother. 2023;164:114982
91. Pillozzi S, D'Amico M, Bartoli G, Gasparoli L, Petroni G, Crociani O. et al. The combined activation of K(Ca)3.1 and inhibition of K(v)11.1/hERG1 currents contribute to overcome Cisplatin resistance in colorectal cancer cells. Br J Cancer. 2018;118:200-12
92. Fortunato A. The role of hERG1 ion channels in epithelial-mesenchymal transition and the capacity of riluzole to reduce cisplatin resistance in colorectal cancer cells. Cell Oncol (Dordr). 2017;40:367-78
93. Lee EL, Hasegawa Y, Shimizu T, Okada Y. IK1 channel activity contributes to cisplatin sensitivity of human epidermoid cancer cells. Am J Physiol Cell Physiol. 2008;294:C1398-406
94. Ise T, Shimizu T, Lee EL, Inoue H, Kohno K, Okada Y. Roles of volume-sensitive Cl- channel in cisplatin-induced apoptosis in human epidermoid cancer cells. J Membr Biol. 2005;205:139-45
95. Gong JH, Liu XJ, Shang BY, Chen SZ, Zhen YS. HERG K+ channel related chemosensitivity to sparfloxacin in colon cancer cells. Oncol Rep. 2010;23:1747-56
96. Sui Q, Peng J, Han K, Lin J, Zhang R, Ou Q. et al. Voltage-gated sodium channel Na(v)1.5 promotes tumor progression and enhances chemosensitivity to 5-fluorouracil in colorectal cancer. Cancer Lett. 2021;500:119-31
97. Panneerpandian P, Rao DB, Ganesan K. Calcium channel blockers lercanidipine and amlodipine inhibit YY1/ERK/TGF-β mediated transcription and sensitize the gastric cancer cells to doxorubicin. Toxicol In Vitro. 2021;74:105152
98. Dziegielewska B, Casarez EV, Yang WZ, Gray LS, Dziegielewski J, Slack-Davis JK. T-Type Ca2+ Channel Inhibition Sensitizes Ovarian Cancer to Carboplatin. Mol Cancer Ther. 2016;15:460-70
99. Tang BD, Xia X, Lv XF, Yu BX, Yuan JN, Mai XY. et al. Inhibition of Orai1-mediated Ca(2+) entry enhances chemosensitivity of HepG2 hepatocarcinoma cells to 5-fluorouracil. J Cell Mol Med. 2017;21:904-15
100. Zhang W, Sui Y, Ni J, Yang T. Insights into the Nanog gene: A propeller for stemness in primitive stem cells. Int J Biol Sci. 2016;12:1372-81
101. Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275-84
102. Lee H, Kim JW, Kim DK, Choi DK, Lee S, Yu JH. et al. Calcium Channels as Novel Therapeutic Targets for Ovarian Cancer Stem Cells. Int J Mol Sci. 2020;21:2327
103. Daya HA, Kouba S, Ouled-Haddou H, Benzerdjeb N, Telliez MS, Dayen C. et al. Orai3-Mediates Cisplatin-Resistance in Non-Small Cell Lung Cancer Cells by Enriching Cancer Stem Cell Population through PI3K/AKT Pathway. Cancers (Basel). 2021;13:2314
104. Zhang Z, Zhao W, Lin X, Gao J, Zhang Z, Shen L. Voltage-dependent calcium channel α2δ1 subunit is a specific candidate marker for identifying gastric cancer stem cells. Cancer Manag Res. 2019;11:4707-18
105. Yu J, Wang S, Zhao W, Duan J, Wang Z, Chen H. et al. Mechanistic Exploration of Cancer Stem Cell Marker Voltage-Dependent Calcium Channel α2δ1 Subunit-mediated Chemotherapy Resistance in Small-Cell Lung Cancer. Clin Cancer Res. 2018;24:2148-58
106. Klumpp D, Misovic M, Szteyn K, Shumilina E, Rudner J, Huber SM. Targeting TRPM2 Channels Impairs Radiation-Induced Cell Cycle Arrest and Fosters Cell Death of T Cell Leukemia Cells in a Bcl-2-Dependent Manner. Oxid Med Cell Longev. 2016;2016:8026702
107. Hirschler-Laszkiewicz I, Festa F, Huang S, Moldovan GL, Nicolae C, Dhoonmoon A. et al. The human ion channel TRPM2 modulates cell survival in neuroblastoma through E2F1 and FOXM1. Sci Rep. 2022;12:6311
108. Chen SJ, Bao L, Keefer K, Shanmughapriya S, Chen L, Lee J. et al. Transient receptor potential ion channel TRPM2 promotes AML proliferation and survival through modulation of mitochondrial function, ROS, and autophagy. Cell Death Dis. 2020;11:247
109. Wang Y, Yang Z, Meng Z, Cao H, Zhu G, Liu T. et al. Knockdown of TRPM8 suppresses cancer malignancy and enhances epirubicin-induced apoptosis in human osteosarcoma cells. Int J Biol Sci. 2013;10:90-102
110. Song YJ, Zhang SS, Guo XL, Sun K, Han ZP, Li R. et al. Autophagy contributes to the survival of CD133+ liver cancer stem cells in the hypoxic and nutrient-deprived tumor microenvironment. Cancer Lett. 2013;339:70-81
111. Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10:1533-41
112. White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401-10
113. Vyas A, Gomez-Casal R, Cruz-Rangel S, Villanueva H, Sikora AG, Rajagopalan P. et al. Lysosomal inhibition sensitizes TMEM16A-expressing cancer cells to chemotherapy. Proc Natl Acad Sci U S A. 2022;119:e2100670119
114. Zhang H, Pang Y, Ma C, Li J, Wang H, Shao Z. ClC5 Decreases the Sensitivity of Multiple Myeloma Cells to Bortezomib via Promoting Prosurvival Autophagy. Oncol Res. 2018;26:421-9
115. Siemer S, Fauth T, Scholz P, Al-Zamel Y, Khamis A, Gül D. et al. Profiling Cisplatin Resistance in Head and Neck Cancer: A Critical Role of the VRAC Ion Channel for Chemoresistance. Cancers (Basel). 2021;13:4831
116. Planells-Cases R, Lutter D, Guyader C, Gerhards NM, Ullrich F, Elger DA. et al. Subunit composition of VRAC channels determines substrate specificity and cellular resistance to Pt-based anti-cancer drugs. EMBO J. 2015;34:2993-3008
117. Zhang Q, Chen C, Zou X, Wu W, Di Y, Li N. et al. Iron promotes ovarian cancer malignancy and advances platinum resistance by enhancing DNA repair via FTH1/FTL/POLQ/RAD51 axis. Cell Death Dis. 2024;15:329
118. Muckenthaler MU, Rivella S, Hentze MW, Galy B. A Red Carpet for Iron Metabolism. Cell. 2017;168:344-61
119. Wang L, Ouyang S, Li B, Wu H, Wang F. GSK-3β manipulates ferroptosis sensitivity by dominating iron homeostasis. Cell Death Discov. 2021;7:334
120. Torti SV, Manz DH, Paul BT, Blanchette-Farra N, Torti FM. Iron and Cancer. Annu Rev Nutr. 2018;38:97-125
121. Fan C, Wu H, Du X, Li C, Zeng W, Qu L. et al. Inhibition of lysosomal TRPML1 channel eliminates breast cancer stem cells by triggering ferroptosis. Cell Death Discov. 2024;10:256
122. Xiang L, Zeng Q, Liu J, Xiao M, He D, Zhang Q. et al. MAFG-AS1/MAFG positive feedback loop contributes to cisplatin resistance in bladder urothelial carcinoma through antagonistic ferroptosis. Sci Bull (Beijing). 2021;66:1773-88
123. Wang J, Zhao H, Xu Z, Cheng X. Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer Biol Med. 2020;17:612-25
124. Liu M, Zhang Y, Yang J, Cui X, Zhou Z, Zhan H. et al. ZIP4 Increases Expression of Transcription Factor ZEB1 to Promote Integrin α3β1 Signaling and Inhibit Expression of the Gemcitabine Transporter ENT1 in Pancreatic Cancer Cells. Gastroenterology. 2020;158:679-92.e1
125. Ooi CE, Rabinovich E, Dancis A, Bonifacino JS, Klausner RD. Copper-dependent degradation of the Saccharomyces cerevisiae plasma membrane copper transporter Ctr1p in the apparent absence of endocytosis. EMBO J. 1996;15:3515-23
126. Petris MJ, Smith K, Lee J, Thiele DJ. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem. 2003;278:9639-46
127. Ishida S, McCormick F, Smith-McCune K, Hanahan D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell. 2010;17:574-83
128. Klopfleisch R, Kohn B, Gruber AD. Mechanisms of tumour resistance against chemotherapeutic agents in veterinary oncology. Vet J. 2016;207:63-72
129. Liu H, Huang J, Peng J, Wu X, Zhang Y, Zhu W. et al. Upregulation of the inwardly rectifying potassium channel Kir2.1 (KCNJ2) modulates multidrug resistance of small-cell lung cancer under the regulation of miR-7 and the Ras/MAPK pathway. Mol Cancer. 2015;14:59
130. Sun H, Sun Y, Chen Q, Xu Z. LncRNA KCNQ1OT1 contributes to the progression and chemoresistance in acute myeloid leukemia by modulating Tspan3 through suppressing miR-193a-3p. Life Sci. 2020;241:117161
131. Principe DR, Aissa AF, Kumar S, Pham TND, Underwood PW, Nair R. et al. Calcium channel blockers potentiate gemcitabine chemotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2022;119:e2200143119
132. Chiu LY, Ko JL, Lee YJ, Yang TY, Tee YT, Sheu GT. L-type calcium channel blockers reverse docetaxel and vincristine-induced multidrug resistance independent of ABCB1 expression in human lung cancer cell lines. Toxicol Lett. 2010;192:408-18
133. Wong BS, Chiu LY, Tu DG, Sheu GT, Chan TT. Anticancer Effects of Antihypertensive L-Type Calcium Channel Blockers on Chemoresistant Lung Cancer Cells via Autophagy and Apoptosis. Cancer Manag Res. 2020;12:1913-27
134. Kondo S, Yin D, Morimura T, Kubo H, Nakatsu S, Takeuchi J. Combination therapy with cisplatin and nifedipine induces apoptosis in cisplatin-sensitive and cisplatin-resistant human glioblastoma cells. Br J Cancer. 1995;71:282-9
135. Zhang L, Zhang J, Chen L, Wang J. Autophagy in human skin squamous cell carcinoma: Inhibition by 3-MA enhances the effect of 5-FU-induced chemotherapy sensitivity. Oncol Rep. 2015;34:3147-55
136. Sui X, Kong N, Wang X, Fang Y, Hu X, Xu Y. et al. JNK confers 5-fluorouracil resistance in p53-deficient and mutant p53-expressing colon cancer cells by inducing survival autophagy. Scientific reports. 2014;4:4694
137. Lv L, Liu HG, Dong SY, Yang F, Wang QX, Guo GL. et al. Upregulation of CD44v6 contributes to acquired chemoresistance via the modulation of autophagy in colon cancer SW480 cells. Tumour Biol. 2016;37:8811-24
138. Visa A, Sallán MC, Maiques O, Alza L, Talavera E, López-Ortega R. et al. T-Type Ca(v)3.1 Channels Mediate Progression and Chemotherapeutic Resistance in Glioblastoma. Cancer Res. 2019;79:1857-68
139. Hasna J, Hague F, Rodat-Despoix L, Geerts D, Leroy C, Tulasne D. et al. Orai3 calcium channel and resistance to chemotherapy in breast cancer cells: the p53 connection. Cell Death Differ. 2018;25:693-707
140. Zheng S, Zhao D, Hou G, Zhao S, Zhang W, Wang X. et al. iASPP suppresses Gp78-mediated TMCO1 degradation to maintain Ca(2+) homeostasis and control tumor growth and drug resistance. Proc Natl Acad Sci U S A. 2022;119:e2111380119
141. Uribe ML, Marrocco I, Yarden Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers (Basel). 2021;13:2748
142. Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160-74
143. Oh SJ, Lim JY, Son MK, Ahn JH, Song KH, Lee HJ. et al. TRPV1 inhibition overcomes cisplatin resistance by blocking autophagy-mediated hyperactivation of EGFR signaling pathway. Nat Commun. 2023;14:2691
144. Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer. 2007;7:519-30
145. Danese A, Patergnani S, Bonora M, Wieckowski MR, Previati M, Giorgi C. et al. Calcium regulates cell death in cancer: Roles of the mitochondria and mitochondria-associated membranes (MAMs). Biochim Biophys Acta, Bioenerg. 2017;1858:615-27
146. Laurino S, Mazzone P, Ruggieri V, Zoppoli P, Calice G, Lapenta A. et al. Cationic Channel TRPV2 Overexpression Promotes Resistance to Cisplatin-Induced Apoptosis in Gastric Cancer Cells. Front Pharmacol. 2021;12:746628
147. Zhang P, Liu X, Li H, Chen Z, Yao X, Jin J. et al. TRPC5-induced autophagy promotes drug resistance in breast carcinoma via CaMKKβ/AMPKα/mTOR pathway. Sci Rep. 2017;7:3158
148. Kim B, Kim G, Kim H, Song YS, Jung J. Modulation of Cisplatin Sensitivity through TRPML1-Mediated Lysosomal Exocytosis in Ovarian Cancer Cells: A Comprehensive Metabolomic Approach. Cells. 2024;13:115
149. Chen X, Chen S, Yu D. Metabolic Reprogramming of Chemoresistant Cancer Cells and the Potential Significance of Metabolic Regulation in the Reversal of Cancer Chemoresistance. Metabolites. 2020;10:289
150. Chen Q, Liu X, Luo Z, Wang S, Lin J, Xie Z. et al. Chloride channel-3 mediates multidrug resistance of cancer by upregulating P-glycoprotein expression. J Cell Physiol. 2019;234:6611-23
151. Xu Y, Zheng H, Kang JS, Zhang L, Su J, Li HY. et al. 5-Nitro-2-(3-phenylpropylamino) benzoic acid induced drug resistance to cisplatin in human erythroleukemia cell lines. Anat Rec (Hoboken). 2011;294:945-52
152. Su J, Xu Y, Zhou L, Yu HM, Kang JS, Liu N. et al. Suppression of chloride channel 3 expression facilitates sensitivity of human glioma U251 cells to cisplatin through concomitant inhibition of Akt and autophagy. Anat Rec (Hoboken). 2013;296:595-603
153. Han Y, Zhou Y, Zhou L, Jia X, Yu X, An X. et al. Blockade of chloride channel-3 enhances cisplatin sensitivity of cholangiocarcinoma cells though inhibiting autophagy. Can J Physiol Pharmacol. 2022;100:584-93
154. Feng J, Peng Z, Gao L, Yang X, Sun Z, Hou X. et al. ClC-3 promotes paclitaxel resistance via modulating tubulins polymerization in ovarian cancer cells. Biomed Pharmacother. 2021;138:111407
155. Zhao K, Wang Z, Li X, Liu JL, Tian L, Chen JQ. Exosome-mediated transfer of CLIC1 contributes to the vincristine-resistance in gastric cancer. Mol Cell Biochem. 2019;462:97-105
156. Wu J, Wang D. CLIC1 Induces Drug Resistance in Human Choriocarcinoma Through Positive Regulation of MRP1. Oncol Res. 2017;25:863-71
157. Jin C, Ye QH, Yuan FL, Gu YL, Li JP, Shi YH. et al. Involvement of acid-sensing ion channel 1α in hepatic carcinoma cell migration and invasion. Tumour Biol. 2015;36:4309-17
158. Chen X, Sun X, Wang Z, Zhou X, Xu L, Li F. et al. Involvement of acid-sensing ion channel 1a in gastric carcinoma cell migration and invasion. Acta Biochim Biophys Sin (Shanghai). 2018;50:440-6
159. Zhang Y, Zhang T, Wu C, Xia Q, Xu D. ASIC1a mediates the drug resistance of human hepatocellular carcinoma via the Ca(2+)/PI3-kinase/AKT signaling pathway. Lab Invest. 2017;97:53-69
160. Zhang Y, Cao N, Gao J, Liang J, Liang Y, Xie Y. et al. ASIC1a stimulates the resistance of human hepatocellular carcinoma by promoting EMT via the AKT/GSK3β/Snail pathway driven by TGFβ/Smad signals. J Cell Mol Med. 2022;26:2777-92
161. Yu X, Cheng L, Liu S, Wang M, Zhang H, Wang X. et al. Correlation between ferroptosis and adriamycin resistance in breast cancer regulated by transferrin receptor and its molecular mechanism. FASEB J. 2024;38:e23550
162. Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153-76
163. Li H, Shen X, Ma M, Liu W, Yang W, Wang P. et al. ZIP10 drives osteosarcoma proliferation and chemoresistance through ITGA10-mediated activation of the PI3K/AKT pathway. J Exp Clin Cancer Res. 2021;40:340
164. Lee YY, Choi CH, Do IG, Song SY, Lee W, Park HS. et al. Prognostic value of the copper transporters, CTR1 and CTR2, in patients with ovarian carcinoma receiving platinum-based chemotherapy. Gynecol Oncol. 2011;122:361-5
165. Xu X, Ren H, Zhou B, Zhao Y, Yuan R, Ma R. et al. Prediction of copper transport protein 1 (CTR1) genotype on severe cisplatin induced toxicity in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2012;77:438-42
166. Arnesano F, Nardella MI, Natile G. Platinum drugs, copper transporters and copper chelators. Coord Chem Rev. 2018;374:254-60
167. Chen SJ, Kuo CC, Pan HY, Tsou TC, Yeh SC, Chang JY. Mechanistic basis of a combination D-penicillamine and platinum drugs synergistically inhibits tumor growth in oxaliplatin-resistant human cervical cancer cells in vitro and in vivo. Biochem Pharmacol. 2015;95:28-37
168. Lastraioli E, Iorio J, Arcangeli A. Ion channel expression as promising cancer biomarker. Biochim Biophys Acta. 2015;1848:2685-702
169. Severin F, Urbani A, Varanita T, Bachmann M, Azzolini M, Martini V. et al. Pharmacological modulation of Kv1.3 potassium channel selectively triggers pathological B lymphocyte apoptosis in vivo in a genetic CLL model. J Exp Clin Cancer Res. 2022;41:64
170. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L. et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521-32
171. Cammann C, Kulla J, Wiebusch L, Walz C, Zhao F, Lowinus T. et al. Proteasome inhibition potentiates Kv1.3 potassium channel expression as therapeutic target in drug-sensitive and -resistant human melanoma cells. Biomed Pharmacother. 2023;168:115635
172. Glaser N, Little C, Lo W, Cohen M, Tancredi D, Wulff H. et al. Treatment with the KCa3.1 inhibitor TRAM-34 during diabetic ketoacidosis reduces inflammatory changes in the brain. Pediatr Diabetes. 2017;18:356-66
173. Bauer D, Werth F, Nguyen HA, Kiecker F, Eberle J. Critical role of reactive oxygen species (ROS) for synergistic enhancement of apoptosis by vemurafenib and the potassium channel inhibitor TRAM-34 in melanoma cells. Cell Death Dis. 2017;8:e2594
174. Díaz-Serrano A, Gella P, Jiménez E, Zugazagoitia J, Paz-Ares Rodríguez L. Targeting EGFR in Lung Cancer: Current Standards and Developments. Drugs. 2018;78:893-911
175. Glaser F, Hundehege P, Bulk E, Todesca LM, Schimmelpfennig S, Nass E. et al. K(Ca) channel blockers increase effectiveness of the EGF receptor TK inhibitor erlotinib in non-small cell lung cancer cells (A549). Scientific reports. 2021;11:18330
176. Stadler S, Weina K, Gebhardt C, Utikal J. New therapeutic options for advanced non-resectable malignant melanoma. Adv Med Sci. 2015;60:83-8
177. Long GV, Flaherty KT, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F. et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-9
178. Granados K, Hüser L, Federico A, Sachindra S, Wolff G, Hielscher T. et al. T-type calcium channel inhibition restores sensitivity to MAPK inhibitors in de-differentiated and adaptive melanoma cells. Br J Cancer. 2020;122:1023-36
179. Barceló C, Sisó P, Maiques O, García-Mulero S, Sanz-Pamplona R, Navaridas R. et al. T-Type Calcium Channels as Potential Therapeutic Targets in Vemurafenib-Resistant BRAF(V600E) Melanoma. J Invest Dermatol. 2020;140:1253-65
180. Chen Z, Vallega KA, Boda VK, Quan Z, Wang D, Fan S. et al. Targeting Transient Receptor Potential Melastatin-2 (TRPM2) Enhances Therapeutic Efficacy of Third Generation EGFR Inhibitors against EGFR Mutant Lung Cancer. Adv Sci (Weinh). 2024;11:e2310126
181. Hong S, Bi M, Wang L, Kang Z, Ling L, Zhao C. CLC-3 channels in cancer (review). Oncol Rep. 2015;33:507-14
182. Fujimoto M, Kito H, Kajikuri J, Ohya S. Transcriptional repression of human epidermal growth factor receptor 2 by ClC-3 Cl(-) /H(+) transporter inhibition in human breast cancer cells. Cancer Sci. 2018;109:2781-91
183. Kulkarni S, Bill A, Godse NR, Khan NI, Kass JI, Steehler K. et al. TMEM16A/ANO1 suppression improves response to antibody-mediated targeted therapy of EGFR and HER2/ERBB2. Genes Chromosomes Cancer. 2017;56:460-71
184. Wu L, Lin W, Liao Q, Wang H, Lin C, Tang L. et al. Calcium Channel Blocker Nifedipine Suppresses Colorectal Cancer Progression and Immune Escape by Preventing NFAT2 Nuclear Translocation. Cell Rep. 2020;33:108327
185. Zhang HL, Hu BX, Ye ZP, Li ZL, Liu S, Zhong WQ. et al. TRPML1 triggers ferroptosis defense and is a potential therapeutic target in AKT-hyperactivated cancer. Sci Transl Med. 2024;16:eadk0330
186. Benzaquen J, Heeke S, Janho Dit Hreich S, Douguet L, Marquette CH, Hofman P. et al. Alternative splicing of P2RX7 pre-messenger RNA in health and diseases: Myth or reality? Biomed J. 2019;42:141-54
187. Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170-8
188. Douguet L, Janho Dit Hreich S, Benzaquen J, Seguin L, Juhel T, Dezitter X. et al. A small-molecule P2RX7 activator promotes anti-tumor immune responses and sensitizes lung tumor to immunotherapy. Nat Commun. 2021;12:653
189. García-Ferreiro RE, Kerschensteiner D, Major F, Monje F, Stühmer W, Pardo LA. Mechanism of block of hEag1 K+ channels by imipramine and astemizole. J Gen Physiol. 2004;124:301-17
190. Toplak Ž, Hendrickx LA, Abdelaziz R, Shi X, Peigneur S, Tomašič T. et al. Overcoming challenges of HERG potassium channel liability through rational design: Eag1 inhibitors for cancer treatment. Med Res Rev. 2022;42:183-226
191. Jahchan NS, Dudley JT, Mazur PK, Flores N, Yang D, Palmerton A. et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer discovery. 2013;3:1364-77
192. Martínez R, Stühmer W, Martin S, Schell J, Reichmann A, Rohde V. et al. Analysis of the expression of Kv10.1 potassium channel in patients with brain metastases and glioblastoma multiforme: impact on survival. BMC cancer. 2015;15:839
193. Agarwal JR, Griesinger F, Stühmer W, Pardo LA. The potassium channel Ether à go-go is a novel prognostic factor with functional relevance in acute myeloid leukemia. Mol Cancer. 2010;9:18
194. Huang X, He Y, Dubuc AM, Hashizume R, Zhang W, Reimand J. et al. EAG2 potassium channel with evolutionarily conserved function as a brain tumor target. Nature neuroscience. 2015;18:1236-46
195. Jacquemet G, Baghirov H, Georgiadou M, Sihto H, Peuhu E, Cettour-Janet P. et al. L-type calcium channels regulate filopodia stability and cancer cell invasion downstream of integrin signalling. Nat Commun. 2016;7:13297
196. Liu S, Khan AR, Yang X, Dong B, Ji J, Zhai G. The reversal of chemotherapy-induced multidrug resistance by nanomedicine for cancer therapy. J Control Release. 2021;335:1-20
197. Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311-35
198. Rodrigues T, Sieglitz F, Bernardes GJ. Natural product modulators of transient receptor potential (TRP) channels as potential anti-cancer agents. Chem Soc Rev. 2016;45:6130-7
199. Naeem A, Hu P, Yang M, Zhang J, Liu Y, Zhu W. et al. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules. 2022;27:8367
200. Nabissi M, Morelli MB, Santoni M, Santoni G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis. 2013;34:48-57
201. Morelli MB, Offidani M, Alesiani F, Discepoli G, Liberati S, Olivieri A. et al. The effects of cannabidiol and its synergism with bortezomib in multiple myeloma cell lines. A role for transient receptor potential vanilloid type-2. Int J Cancer. 2014;134:2534-46
202. Marinelli O, Morelli MB, Annibali D, Aguzzi C, Zeppa L, Tuyaerts S. et al. The Effects of Cannabidiol and Prognostic Role of TRPV2 in Human Endometrial Cancer. Int J Mol Sci. 2020;21:5409
203. Misri S, Kaul K, Mishra S, Charan M, Verma AK, Barr MP. et al. Cannabidiol Inhibits Tumorigenesis in Cisplatin-Resistant Non-Small Cell Lung Cancer via TRPV2. Cancers (Basel). 2022;14:1181
204. Xu S, Cheng X, Wu L, Zheng J, Wang X, Wu J. et al. Capsaicin induces mitochondrial dysfunction and apoptosis in anaplastic thyroid carcinoma cells via TRPV1-mediated mitochondrial calcium overload. Cell Signal. 2020;75:109733
205. Wu L, Xu S, Cheng X, Zhang L, Wang Y, Wu J. et al. Capsaicin inhibits the stemness of anaplastic thyroid carcinoma cells by triggering autophagy-lysosome mediated OCT4A degradation. Phytother Res. 2022;36:938-50
206. Yeh JH, Chou CT, Chen IS, Lu T, Lin KL, Yu CC. et al. Effect of thymol on Ca²⁺ homeostasis and viability in PC3 human prostate cancer cells. Chin J Physiol. 2017;60:32-40
207. Qin S, Su Q, Li X, Shao M, Zhang Y, Yu F. et al. Curcumin suppresses cell proliferation and reduces cholesterol absorption in Caco-2 cells by activating the TRPA1 channel. Lipids Health Dis. 2023;22:6
208. Rumpa MM, Maier C. TRPV1-Dependent Antiproliferative Activity of Dioecious Maclura pomifera Extracts in Estrogen Receptor-Positive Breast Cancer Cell Lines Involves Multiple Apoptotic Pathways. Int J Mol Sci. 2024;25:5258
209. Lai H, Liu C, Hou L, Lin W, Chen T, Hong A. TRPM8-regulated calcium mobilization plays a critical role in synergistic chemosensitization of Borneol on Doxorubicin. Theranostics. 2020;10:10154-70
210. Law BYK, Michelangeli F, Qu YQ, Xu SW, Han Y, Mok SWF. et al. Neferine induces autophagy-dependent cell death in apoptosis-resistant cancers via ryanodine receptor and Ca(2+)-dependent mechanism. Scientific reports. 2019;9:20034
211. Shi S, Bai X, Ji Q, Wan H, An H, Kang X. et al. Molecular mechanism of ion channel protein TMEM16A regulated by natural product of narirutin for lung cancer adjuvant treatment. Int J Biol Macromol. 2022;223:1145-57
212. Wang X, Hao A, Song G, Elena V, Sun Y, Zhang H. et al. Inhibitory effect of daidzein on the calcium-activated chloride channel TMEM16A and its anti-lung adenocarcinoma activity. Int J Biol Macromol. 2023;253:127261
213. Guo S, Bai X, Shi S, Deng Y, Kang X, An H. TMEM16A, a Homoharringtonine Receptor, as a Potential Endogenic Target for Lung Cancer Treatment. Int J Mol Sci. 2021;22:10930
214. Ma B, Shi S, Guo W, Zhang H, Zhao Z, An H. Liensinine, a Novel and Food-Derived Compound, Exerts Potent Antihepatoma Efficacy via Inhibiting the Kv10.1 Channel. J Agric Food Chem. 2024;72:4689-702
215. Ma B, Shi S, Ren S, Qu C, Zhao Z, An H. Corydaline binds to a druggable pocket of hEAG1 channel and inhibits hepatic carcinoma cell viability. Eur J Pharmacol. 2024;962:176240
216. Seo Y, Anh NH, Heo Y, Park SH, Kiem PV, Lee Y. et al. Novel ANO1 Inhibitor from Mallotus apelta Extract Exerts Anticancer Activity through Downregulation of ANO1. Int J Mol Sci. 2020;21:6470
217. Bloch M, Ousingsawat J, Simon R, Schraml P, Gasser TC, Mihatsch MJ. et al. KCNMA1 gene amplification promotes tumor cell proliferation in human prostate cancer. Oncogene. 2007;26:2525-34
218. Elble RC, Walia V, Cheng HC, Connon CJ, Mundhenk L, Gruber AD. et al. The putative chloride channel hCLCA2 has a single C-terminal transmembrane segment. J Biol Chem. 2006;281:29448-54
219. Fourbon Y, Guéguinou M, Félix R, Constantin B, Uguen A, Fromont G. et al. Ca(2+) protein alpha 1D of CaV1.3 regulates intracellular calcium concentration and migration of colon cancer cells through a non-canonical activity. Scientific reports. 2017;7:14199
220. Li S, Wang Z, Geng R, Zhang W, Wan H, Kang X. et al. TMEM16A ion channel: A novel target for cancer treatment. Life Sci. 2023;331:122034
221. Lam AK, Dutzler R. Mechanistic basis of ligand efficacy in the calcium-activated chloride channel TMEM16A. EMBO J. 2023;42:e115030
222. Paulino C, Kalienkova V, Lam AKM, Neldner Y, Dutzler R. Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM. Nature. 2017;552:421-5
223. Liu Y, Wang K. Exploiting the Diversity of Ion Channels: Modulation of Ion Channels for Therapeutic Indications. Handb Exp Pharmacol. 2019;260:187-205
224. Wulff H, Christophersen P, Colussi P, Chandy KG, Yarov-Yarovoy V. Antibodies and venom peptides: new modalities for ion channels. Nat Rev Drug Discov. 2019;18:339-57
225. Stortelers C, Pinto-Espinoza C, Van Hoorick D, Koch-Nolte F. Modulating ion channel function with antibodies and nanobodies. Curr Opin Immunol. 2018;52:18-26
226. Bednenko J, Harriman R, Mariën L, Nguyen HM, Agrawal A, Papoyan A. et al. A multiplatform strategy for the discovery of conventional monoclonal antibodies that inhibit the voltage-gated potassium channel Kv1.3. mAbs. 2018;10:636-50
227. Gómez-Varela D, Zwick-Wallasch E, Knötgen H, Sánchez A, Hettmann T, Ossipov D. et al. Monoclonal antibody blockade of the human Eag1 potassium channel function exerts antitumor activity. Cancer Res. 2007;67:7343-9
228. Lin FF, Elliott R, Colombero A, Gaida K, Kelley L, Moksa A. et al. Generation and characterization of fully human monoclonal antibodies against human Orai1 for autoimmune disease. J Pharmacol Exp Ther. 2013;345:225-38
229. Cox JH, Hussell S, Søndergaard H, Roepstorff K, Bui JV, Deer JR. et al. Antibody-mediated targeting of the Orai1 calcium channel inhibits T cell function. PLoS ONE. 2013;8:e82944
230. Qiang M, Dong X, Zha Z, Zuo XK, Song XL, Zhao L. et al. Selection of an ASIC1a-blocking combinatorial antibody that protects cells from ischemic death. Proc Natl Acad Sci U S A. 2018;115:E7469-E77
231. Sun H, Luo L, Lal B, Ma X, Chen L, Hann CL. et al. A monoclonal antibody against KCNK9 K(+) channel extracellular domain inhibits tumour growth and metastasis. Nat Commun. 2016;7:10339
232. Gilbert SM, Gidley Baird A, Glazer S, Barden JA, Glazer A, Teh LC. et al. A phase I clinical trial demonstrates that nfP2X(7) -targeted antibodies provide a novel, safe and tolerable topical therapy for basal cell carcinoma. Br J Dermatol. 2017;177:117-24
233. Baker C, Rodrigues T, de Almeida BP, Barbosa-Morais NL, Bernardes GJL. Natural product-drug conjugates for modulation of TRPV1-expressing tumors. Bioorg Med Chem. 2019;27:2531-6
234. Chang L, Chang R, Shen J, Wang Y, Song H, Kang X. et al. Self-healing pectin/cellulose hydrogel loaded with limonin as TMEM16A inhibitor for lung adenocarcinoma treatment. Int J Biol Macromol. 2022;219:754-66
235. Yin L, Duan W, Chen Y, Chen D, Wang Y, Guo S. et al. Biodegradable hydrogel from pectin and carboxymethyl cellulose with Silibinin loading for lung tumor therapy. Int J Biol Macromol. 2023;243:125128
Author contact
![]() Corresponding author: hzzjuedu.cn (X.S.), xbsedu.cn (T.X.), guxidongcom (X.G.), yipingmoucom (Y.M.)
Corresponding author: hzzjuedu.cn (X.S.), xbsedu.cn (T.X.), guxidongcom (X.G.), yipingmoucom (Y.M.)
 Global reach, higher impact
Global reach, higher impact