13.3
Impact Factor
Theranostics 2025; 15(2):460-493. doi:10.7150/thno.103725 This issue Cite
Review
Advancements in GelMA bioactive hydrogels: Strategies for infection control and bone tissue regeneration
1. Department of Endodontics, Stomatological Hospital, School of Stomatology, Southern Medical University, Guangzhou, China.
2. SCP 11A of the International Department, Guangzhou Experimental Foreign Language School, Guangzhou, China.
3. Department of Periodontology, Shaoxing Stomatological Hospital, Shaoxing, Zhejiang, China.
Received 2024-9-15; Accepted 2024-11-4; Published 2025-1-1
Abstract
Infectious bone defects present a significant clinical challenge, characterized by infection, inflammation, and subsequent bone tissue destruction. Traditional treatments, including antibiotic therapy, surgical debridement, and bone grafting, often fail to address these defects effectively. However, recent advancements in biomaterials research have introduced innovative solutions for managing infectious bone defects. GelMA, a three-dimensional network of hydrophilic polymers that can absorb and retain substantial amounts of water, has attracted considerable attention in the fields of materials science and biomedical engineering. Its distinctive properties, such as biocompatibility, responsiveness to stimuli, and customisable mechanical characteristics make GelMA an exemplary scaffold material for bone tissue engineering. This review aims to thoroughly explore the current literature on antibacterial and osteogenic strategies using GelMA hydrogels for the restoration of infected bones. It discusses their fabrication methods, biocompatibility, antibacterial effectiveness, and bioactivity. We conclude by discussing the existing challenges and future research directions in this field, with the hope of inspiring further innovations in the synthesis, modification, and application of GelMA-based hydrogels for infection control and bone tissue regeneration.
Keywords: GelMA hydrogels, infection control, bone tissue regeneration, infected bone tissue regeneration, antimicrobial materials
Introduction
Infectious bone defects present a formidable clinical challenge due to the immunological response to microbial invasion and the release of acidic metabolites. Collectively, these factors hinder osteoblastic activity, thereby obstructing the natural healing of bone defects. Such conditions are prevalent among individuals with trauma, surgical history, or diabetes, significantly affecting their quality of life [1]. Traditional treatments, including bone grafting and antibiotic therapy, are effective but limited by issues such as restricted bone supply and the emergence of secondary diseases [2,3]. Furthermore, the escalating problem of antibiotic resistance poses a considerable public health threat [4,5]. Consequently, therapeutic strategies for infectious bone defects must balance the control of antimicrobial inflammation with the promotion of bone healing.
The advent of tissue-engineering technologies has introduced novel treatment approaches in this field. By integrating biodegradable scaffolds, seed cells, and cytokines, tissue engineering offers a biological alternative for repairing infectious bone defects and provides new perspectives and methods for clinical treatment. The repair process of bone defects is both orderly and dynamic, involving multiple dimensions. The selection of an appropriate scaffold substitute is crucial for this process. With the advancements in biomedical materials, hydrogels have garnered significant attention. Hydrogels are highly hydrophilic three-dimensional networks that serve as biological scaffolds for cell growth [6,7]. They rapidly expand in water, retain a significant amount of moisture without dissolving, and resemble the extracellular matrix (ECM), providing structural support for bone defect sites and promoting healing through intrinsic mechanisms [6]. Hydrogels are simple to prepare, cost-effective, and exhibit low toxicity, making them widely used in bone tissue engineering. By mimicking the ECM environment, hydrogels provide an ideal biological scaffold for the repair of bone defects and facilitate the regeneration and healing of bone tissue [8,9].
GelMA hydrogels, which are biomaterials based on gelatine, can form crosslinked three-dimensional networks under specific conditions through methacrylation modification [10]. Gelatine, a natural protein derived from collagen, is a macromolecule obtained from animal skin, bone, and connective tissue, and possesses excellent biocompatibility and degradability [11]. The methacrylation modification endows GelMA hydrogels with improved mechanical properties and processability while retaining the biological features of gelatine [12]. GelMA hydrogels possess the versatility to amalgamate with cells, growth factors, pharmaceuticals, or exosomes, fostering microenvironments conducive to osteogenesis, angiogenesis, and antimicrobial efficacy [10]. Therefore, GelMA hydrogels are widely employed in drug delivery, tissue engineering, biological research, and hydrogel devices [12-14]. An ideal bone regeneration scaffold should possess excellent cellular/biocompatibility, outstanding bioactivity, biodegradability, suitable biomechanical properties, and necessary porous structures to facilitate the adhesion, proliferation, diffusion, nutrient transport, and gas exchange of osteoblasts [11,15]. However, pure GelMA hydrogels exhibit inadequate osteogenic activity. Therefore, to enhance the osteogenic capacity of the composite materials, it is essential to combine GelMA hydrogels with materials that possess osteogenic properties. Additionally, the mechanical strength of GelMA hydrogels is relatively weak, and their electrical conductivity is insufficient, making the development of hybrid hydrogels composed of two or more components a crucial strategy [16]. This approach leverages the unique advantages of each component and provides promising possibilities for developing tissue-engineered biomaterials. Notably, the concentration and degree of substitution of GelMA significantly influence its physicochemical properties and cellular responses, leading to a degree of uncertainty and limitation in bioactivity modulation. In bone defect repair applications, the poor mechanical stiffness and uncontrollable degradation rate of pure GelMA hydrogels severely restrict their widespread use in load-bearing bone defect treatments. Thus, multifunctional bone tissue engineering scaffolds with osteogenic and antibacterial properties can be produced by employing various strategies to modify pure GelMA hydrogels and incorporate bioactive components [15].
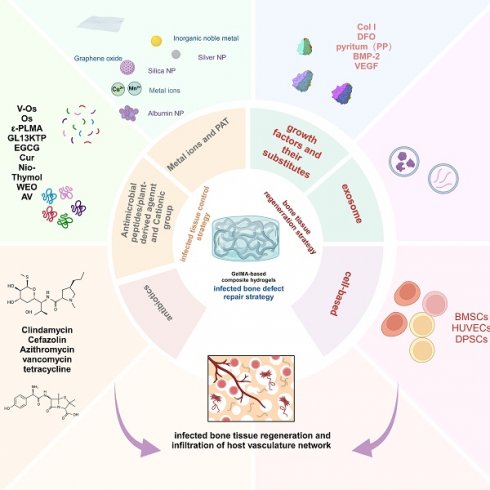
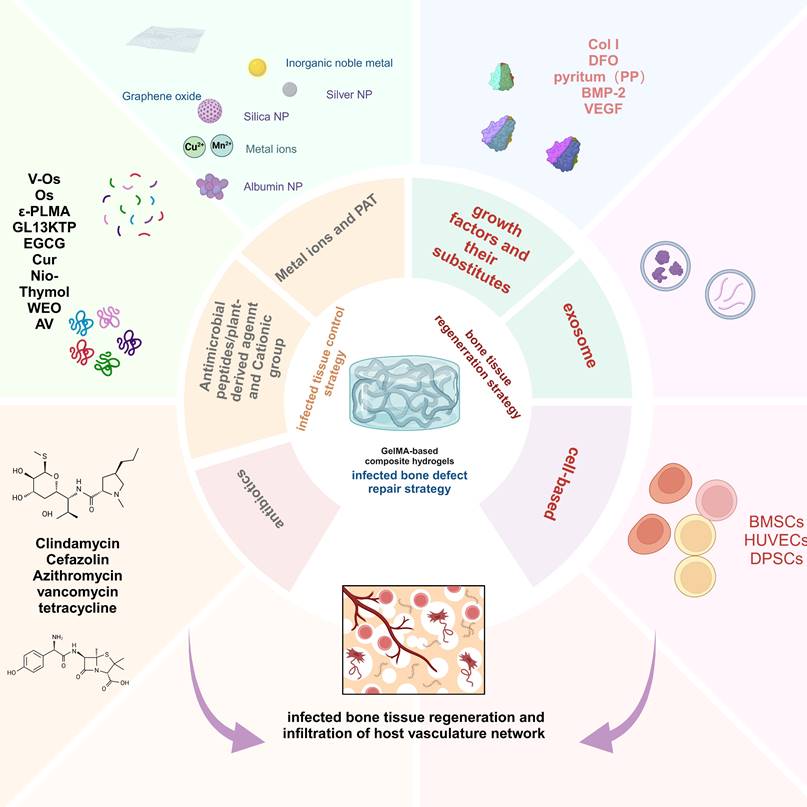
This review provides a comprehensive synopsis of the use of GelMA hydrogels for infection control and bone tissue regeneration. This section discusses the composition and structure of the GelMA hydrogels and explores their multifunctional properties. Special focus is given to the advancement of GelMA hydrogels tailored for the resolution of infectious bone defects, particularly those incorporating infection-mitigation agents such as antibiotics, metallic ions, antimicrobial peptides (AMPs), botanical active compounds, photothermal therapy (PTT), and intelligent responsive mechanisms (Scheme 1). This review provides a comprehensive overview of the incorporation of various antimicrobial agents into GelMA hydrogels for bone repair and their antimicrobial attributes, thereby providing a theoretical basis for the advancement of GelMA hydrogels in the treatment of infectious bone defects. Furthermore, it addresses the inherent challenges in the application of GelMA hydrogels. Finally, recent progress and technical directions for GelMA hydrogels are discussed, with the aim of promoting the development of novel composites based on GelMA hydrogels and expanding their future application prospects.
Hydrogel overview
Properties of hydrogels
Hydrogels are three-dimensional crosslinked porous materials characterised by excellent permeability, remarkable drug-carrying capacity, high water content, optimal mechanical strength, favourable biocompatibility, and sensitivity to environmental stimuli such as temperature, pH, and solvent type. The polymer network of hydrogels is composed of chains linked at crosslinking points, forming a three-dimensional structure whose stability and functionality are influenced by the polymer type, degree of crosslinking, and external physicochemical conditions. The polymer type primarily affects the chemical properties and biocompatibility of the hydrogel, whereas the degree of crosslinking dictates the structural density, influencing its water absorption capacity and mechanical stability. Hydrogels possess a 3D structure that closely mimics the ECM, supports the activity of biomolecules and cells, and offers an ideal platform for cell adhesion, proliferation, and migration at wound sites [17]. Furthermore, their exceptional water absorption and swelling properties allow them to absorb excess exudate without dissolving, while the pore size can be tailored to encapsulate various cells, drugs, growth factors, nucleic acids, nanoparticles, metal ions (e.g., Mg2+, Fe3+), gas molecules (e.g., NO, O₂), and therapeutic agents like platelet-rich plasma (PRP) [18,19]. Therefore, hydrogels are widely regarded as promising materials for bone tissue engineering applications.
Schematic description of GelMA-based composite hydrogels with multiple functions for infection control and bone tissue regeneration (created with BioRender.com/y41f851).
Classification of hydrogels
Natural polymer hydrogels are composed primarily of proteins, peptides, polysaccharides, and nucleic acids. Hydrogels based on proteins or peptides mainly include gelatine, collagen, fibrin, silk fibroin, and ε-polylysine (EPL), which retain a higher concentration of cell-adhesive proteins and exhibit excellent biocompatibility [20]. Polysaccharide-based hydrogels comprise chitosan, alginates, agarose, hyaluronic acid, glucans, cellulose, guar gum, and cyclodextrin polymers. Natural polymer hydrogels offer numerous advantages, including abundant sources, porous structures, multifunctional groups, favourable swelling properties, biodegradability, and low immunogenicity. However, the mechanical properties of these materials tend to be inferior. To enhance their physicochemical characteristics, chemical modifications can be used to introduce additional biologically functional sites [21]. A notable example is the methacrylation of hydrogels, which imparts remarkable photosensitivity [22]. Furthermore, the quaternization of chitosan hydrogels enhances their antibacterial properties, whereas catechol modification imparts exceptional tissue adhesion capabilities [23]. Synthetic hydrogels are derived primarily from organic polymers. The commonly synthesised hydrogels include polyacrylamide (PAM), polyethylene glycol (PEG), polyvinyl alcohol (PVA), polyurethane (PU), polyvinylpyrrolidone (PVP), polyethylene imine (PEI), poly(N-isopropylacrylamide) (PNIPAM), Pluronic F127 (PF127), polylactide-co-glycolide (PLGA), polyhydroxyalkanoates (PHA), polylactic acid (PLA), polycaprolactone (PCL), polymethacrylic acid (PMAA), and their derivatives. These synthetic hydrogels are valuable owing to their stability, structural strength, resistance to degradation, and potential for mass production. However, they may raise certain biological safety concerns due to the possible release of monomers and toxic substances during synthesis [18].
Both hydrogel categories can be combined to create hybrid hydrogels. For example, polyvinyl alcohol crosslinked with sodium alginate (PVA-SA) hybrid hydrogels exhibit good biocompatibility and mechanical properties, making them suitable for the localised delivery of phages and antibiotics for the treatment of methicillin-resistant Staphylococcus aureus infections in burn wounds [24]. Additionally, polyvinylpyrrolidone crosslinked with hyaluronic acid (PVP-HA) enhanced the mechanical strength of the microneedles, ensuring effective drug permeation. Moreover, the incorporation of nanomaterials into hydrogels endows them with stimulus-responsive characteristics [25] (Table 1).
Characterization and preparation of GelMA hydrogel
GelMA, short for gelatine methacrylate, was initially synthesized by Van Den Bulcke and colleagues in 2000 [35]. Derived from collagen hydrolysis, this material inherits the desirable solubility and low antigenicity of gelatine. Notably, the retention of the arginine-glycine-aspartate (RGD) sequence is a critical structure that facilitates cell adhesion, proliferation, and differentiation [8]. Moreover, the presence of matrix metalloproteinases (MMPs) within gelatine aids cell remodelling, thereby enhancing the physicochemical properties of the material [36].
Advantages and disadvantages of different source materials.
| Hydrogel materials | Advantages | Limitations | Application |
|---|---|---|---|
| natural hydrogel | |||
| collagen | good biocompatibility and biodegradability; good tensile strength; mimics natural extracellular matrix (ECM) of dentin [26] | poor mechanical strength [27] | soft tissue engineering, bone tissue regeneration, and wound healing [28] |
| chitosan | nontoxic and easily bioabsorbable; antibacterial activity [29] | poor mechanical performance; poor control over hydrogel pore size [29] | wound dressings, drug delivery systems, and antibacterial materials [29]. |
| silk | good biocompatibility; biodegradability; oxygen and water permeability; morphologic flexibility [30] | poor degradation performance [30] | bone tissue engineering, vascular repair, nerve regeneration, and wound healing [30] |
| alginate | nontoxic; good biocompatibility [31] | mechanically weak [31] | tissue engineering scaffolds, cell encapsulation and transplantation, and drug delivery [31] |
| chondroitin sulphate | biocompatible; biodegradable; bioactive; nonimmunogenic [32] | water soluble; low mechanical integrity [32] | soft tissue repair, plastic and cosmetic surgery, drug delivery systems [28] |
| synthetic hydrogel | |||
| polylactic acid (PLA), poly‑l‑lactic acid (PLLA), polyglycolic acid (PGA), PLGA, and polyεcaprolactone (PCL) | nontoxic and biodegradable [33] | chronic or acute inflammatory [33] | load-bearing bone scaffolds, soft tissue repair, controlled drug release systems [33] |
| hybrid hydrogel | |||
| PVA-SA | good mechanical strength; multifunctionality [34] | poor biodegradability; Complex fabrication; Potential toxicity [34] | drug delivery systems; Tissue engineering scaffolds [24] |
| PVP-HA | good mechanical strength; multifunctionality [34] | poor biodegradability; Complex fabrication; Potential toxicity [34] | drug delivery systems; Tissue engineering scaffolds [25] |
GelMA is prominent in the biomedical field because of its remarkable versatility. The extensive applications of GelMA hydrogels can be largely attributed to their unique biological properties, which facilitate superior cell attachment and proliferation across a wide array of cell types. In summary, there are four primary reasons that researchers opt for gelatine methacrylate:
I. Biocompatibility: GelMA gelatine molecules feature RGD sequences that promote the adhesion and proliferation of virtually any cell type [37]. These sequences, derived from collagen, are retained in the material to support cell adhesion, proliferation, and maturation within the construct.
II. Biodegradability: GelMA contains MMP sites that enable its enzymatic degradation by cells. As natural cells populate a construct, they progressively degrade and remodel the material, ultimately replacing the original structure with their own cells and tissues [22].
III. Tunable properties: GelMA possesses excellent tunable properties, allowing easy customisation of the hydrogels. This flexibility arises from the degree of substitution in GelMA, which directly influences the stiffness of the polymer and its mechanical properties, including compression and tensile strength. By adjusting these parameters, the soft and hard components of natural tissues can be mimicked, thereby activating biomechanical signals in specific microenvironments and guiding the maturation of embedded cells in designated orientations [10].
IV. Bioprintability: GelMA exhibits outstanding bioprintability. It is extensively utilised in both research and commercial applications because of its capacity to create complex structures with diverse characteristics. Scientists have employed GelMA to fabricate intricate 3D structures using bioprinting technology, providing cells with growth conditions similar to those in the body [10,38].
Recently, significant advances have been made in bone tissue engineering for the design and fabrication of artificial bone scaffolds. Scaffolds can be classified into two main categories: natural and synthetic. Common synthetic materials include polylactic acid, polyglycolic acid, and inorganic compounds such as titanium alloys, calcium phosphate, bioceramics, and bioactive glass [39]. The incorporation of inorganic minerals, particularly calcium phosphate, has demonstrated beneficial effects on bone regeneration, owing to their biocompatibility and osteoconductivity [16]. However, the practical application of these materials is challenging because of their fragility and limited adaptability [16]. Conversely, synthetic organic materials, such as polylactic acid, can be easily fabricated with desirable mechanical properties and acceptable biocompatibility; however, they often lack the necessary bioactivity to initiate bone repair.
Consequently, researchers have shifted their focus toward natural materials as potential sources for developing bone regeneration scaffolds with high biocompatibility and low cytotoxicity. Many researchers have integrated GelMA into bone repair material systems owing to its favourable temperature-sensitive gel properties, degradability, adjustable mechanical characteristics, and ability to promote bone differentiation and vascularisation [16,40].
Synthesis of GelMA hydrogel
Since its advent in the 21st century, GelMA has garnered widespread application in numerous subfields of tissue engineering, including antibacterial, osteogenic, drug delivery, and gene transfer applications. Over the past two decades, researchers have reported various methods for synthesising GelMA, each with its own merits but fundamentally rooted in the general synthetic route initially proposed by Bulcke and colleagues [35], albeit with varying degrees of optimisation and adjustment within this framework. A concise overview of the GelMA synthesis process is provided below.
Typically, GelMA is synthesised in phosphate buffer solution (pH 7.4) through the direct reaction of gelatine with methacrylic anhydride (MA). In this process, methacrylate substituents are introduced into the amino and hydroxyl groups of amino acid residues [41]. By controlling the amount of MA added to the reaction mixture, GelMAs with varying degrees of methacrylate substitution can be synthesised, resulting in materials with diverse physical properties. Maintaining the alkalinity of the reaction solution is crucial for augmenting the reactivity of the amino and hydroxyl groups and ensuring a higher level of substitution. In the final stage, the substitution reaction is halted by adding a five-fold diluted phosphate buffer solution to the reaction mixture [42]. Subsequently, the mixture is dialysed against deionised water using a dialysis membrane with a molecular weight cutoff of 12-14 kDa for 5-7 days to eliminate low-molecular-weight impurities that may affect cell function (e.g., unreacted MA and methacrylic acid byproducts). If required, GelMA can be preserved by freeze-drying and reconstituted for use at room temperature.
Tunability of GelMA mechanical properties
The tunability of the mechanical properties of GelMA is a defining feature of the material, which significantly contributes to its sustained interest in tissue engineering applications. By adjusting the degree of methacrylate substitution, the pore size of the GelMA hydrogels can be precisely controlled, which is a critical factor in facilitating cellular nutrient exchange. Studies have demonstrated that different concentrations of MA (1M, 5M, and 10M) can yield GelMA hydrogels with varying degrees of substitution (49.8%, 63.8%, and 73.2%, respectively). Following freeze-drying, the average pore size of these hydrogels is reported to have decreased from 49.7±11.8 μm for 1M to 23.6±5.85 μm for 10M, as confirmed by FE-SEM, indicating a decrease in pore size with increasing substitution degree [43]. This observation has been corroborated by other experiments, which revealed an inverse relationship between the degree of methacrylate substitution and the mass fraction of GelMA [43]. Although GelMA with a high degree of substitution exhibits greater mechanical strength, it hinders cell proliferation and is therefore unsuitable for cell loading and co-culture. Some researchers have conducted two-dimensional nuclear magnetic resonance (NMR) experiments to obtain detailed information on the signals present in the 1H NMR spectrum. Using this method, both methacrylamide from the methacrylate groups and unreacted impurities, such as methacrylic acid, can be identified. This quantitative cationic approach enables precise control over the substitution of GelMA, allowing for further adjustments to the hydrogel properties to meet the requirements of bone regeneration scaffolds [44]. Additionally, studies have shown that a higher degree of methacrylation in GelMA is correlated with a lower viscosity of the GelMA precursor aqueous solution, making it suitable for 3D bioprinting [18].
Despite possessing osteogenic properties, such as cell adhesion, proliferation, and differentiation, the mechanical properties of GelMA hydrogels are insufficient for use as load-bearing materials. Wang et al. [45] devised a novel two-component polymer hydrogel, GelMA-DexMA, via photopolymerization using GelMA and dextran glycidyl methacrylate (DexMA). They reported that improved mechanical properties can be attained by regulating the degree of substitution (DS) of glycidyl methacrylate in DexMA. GelMA-DexMA exhibits a honeycomb-like structure with decreasing pore size as the DS increases, displaying a lower tensile strength and higher compressive modulus. The UV dose used during polymerisation also plays a pivotal role in determining the mechanical properties of GelMA. Substituted gelatine derivatives are highly prone to photoinduced reactions because of the abundance of unsaturated photocrosslinking groups. The primary amine (-NH₂) and hydroxyl (-OH) groups predominantly participate in this substitution reaction, facilitating the introduction of methacryloyl groups into gelatine. In the presence of a photoinitiator, GelMA polymerisation occurs in an aqueous solution via a free-radical mechanism. UV irradiation of the photoinitiator generates free radicals through homolytic cleavage, which initiates chain polymerisation [46]. Photopolymerization offers numerous advantages over other methods, including injectability, rapid gelation, enhanced mechanical properties, compatibility with custom bioprinting, and ease of integration into various cell types [22,47]. However, free radicals produced during crosslinking can attack cell membranes, resulting in cell death [48,49]. This detrimental effect is contingent upon the radiation source and intensity of the UV light. Research has also demonstrated that photocrosslinking can be biocompatible under mild conditions and can be easily adjusted by reducing the intensity of the UV source and the amount of the photoinitiator used. Commonly employed photoinitiators are free radical photoinitiators, such as LAP, IC-2959, and VA086, which exhibit good biocompatibility and low immunogenicity [46]. Wang et al. [50] synthesised GelMA/polyethylene glycol diacrylate (PEGDA) gels using the UV photocrosslinking method by modifying photoinitiators I and II. They reported that the gelling time could be readily controlled by altering the amounts of I2959 and PEGDA photoinducers. It was also observed that the mechanical strength, spreading, and swelling rates of this type of gel were enhanced, with mechanical properties significantly superior to those of GelMA. Although LAP initiation proved to be more efficient, yielding hydrogels with higher moduli than those initiated by IC-2959 and VA086 under identical curing conditions, the GelMA hydrogel could also be chemically crosslinked using agents such as glutaraldehyde. However, because of its high toxicity to living organisms, glutaraldehyde is typically unsuitable for cell culture applications [51,52]. Furthermore, different synthesis systems can influence the structure and properties of GelMA. Currently, the mainstream method for GelMA synthesis involves a phosphate buffer solution (PBS) reaction system. Several research groups have reported alternative methods for synthesising GelMA. For instance, recent studies have indicated that GelMA synthesised in a carbonate-bicarbonate buffer solution (CBS) exhibits superior deprotonation of free amino groups and buffering capacity compared with GelMA synthesised in PBS [11,53]. Additionally, the synthesis of GelMA using the CBS reaction system requires a reduced amount of MA, rendering the synthesis both environmentally friendly and cost-effective [11,53]. In addition to optimising the degree of substitution and photopolymerization time, GelMA can be combined with other materials to achieve enhanced performance. Wang et al. [54] enhanced the mechanical properties of GelMA hydrogels by simple physical mixing with nanoscale hydroxyapatite nanoparticles. Pressure testing revealed that addition of 5% nanoscale hydroxyapatite results in the hydrogel withstanding a maximum compressive stress of (278.62±7.49) kPa, over three times higher than GelMA hydrogels without nanoparticles, with no significant change in pore size. It is also observed that co-modification of GelMA with type I collagen, a natural polymer, notably improves the rheological properties of the hydrogel, such as viscosity and stiffness, and enhances the viability of human umbilical vein endothelial cells (HUVECs) [55]. Zuo et al. [56] developed a novel system comprising GelMA and HA scaffolds loaded with HUVEC and human osteosarcoma-like cells (MG63). Compared with pure GelMA hydrogels, these composite hydrogels exhibit lower swelling, higher mechanical strength, and superior biocompatibility. In this age of innovative materials, the exceptional tunability of GelMA sets it apart and injects renewed vitality into the field.
Bone defect repair strategy
In the field of bone tissue engineering, strategies for the reconstruction of bone defects using GelMA hydrogels have been extensively explored [15], and a succinct overview of these strategies is presented in Table 2, focusing on the dual functionality of GelMA hydrogels in infection control and antibacterial osteogenesis for infectious bone defects. Infectious bone tissue defects often pose challenges to healing via intrinsic bone repair mechanisms, necessitating biomimetic reconstruction techniques to restore bone shape and function. This typically involves the use of materials from the field of bone tissue engineering [87]. Ideal candidates for bone regeneration should exhibit biomechanical strength, physicochemical properties, optimal porosity, biodegradability, biocompatibility, and osteoconductivity, while serving as conduits for the unhindered transport of nutrients, waste, and gases for cells embedded within the scaffold [1,4]. Integrated bone-graft materials should possess at least one of the following biological attributes: 1. osteoconductivity, providing a framework for tissue ingrowth and new bone deposition; 2. osteoinductivity, secreting factors that stimulate the differentiation of osteoprogenitor cells into bone; and 3. osteogenicity, supplying bone progenitor cells with osteogenic potential [15]. These criteria can be met by employing GelMA scaffolds as primary vehicles for the delivery of drugs, cells, growth factors, and exosomes, thereby enhancing the repair process. Consequently, the development and design of GelMA-based drug delivery systems can achieve local delivery, ensuring that the effective concentration is maintained for a sustained duration, thereby maximising the therapeutic efficacy.
Stem cell
The repair of bone defects is a multifaceted process involving two fundamental multicellular units: osteoclasts and osteoblasts [88]. Cell-based therapies have emerged as effective strategies for bone repair. Stem cell therapy typically involves injecting a suspension of stem cells into the site of the bone defect. However, the microenvironment within the defect presents challenges in maintaining the quantity and vitality of stem cells [89]. Therefore, hydrogels containing stem cells should be implanted into bone defects to create a cellular microenvironment conducive to adhesion, proliferation, and differentiation [89].
Bone marrow mesenchymal stem cells (BMSCs) can self-renew and differentiate into multiple lineages, making them the ideal seed cells for bone tissue engineering [90]. These cells secrete paracrine mediators and nutrients, regulate immunity, promote angiogenesis, and exhibit excellent osteogenic properties [91]. The hydrogel scaffolds prepared with bio-GelMA and encapsulated BMSCs demonstrate good biocompatibility and are chemically and physically similar to the natural extracellular matrix of bone cells. As a result of loading the hydrogel scaffold with BMSCs, new bone has been reported to form most effectively in rat segmental bone defect models [57].
Summary of typical GelMA-based scaffolds for accelerating bone regeneration.
| GelMA-based bone scaffolds with cells | ||||||
| GelMA hydrogel scaffolds | Cells lines | Physical properties | Bioactivities | Mechanism | ||
| bio-GelMA [57] | BMSC | injectability; porous structure | good cytocompatibility and proliferative properties, promoted new bone formation and angiogenesis | |||
| GelMA-HAP-SN [58] | BMSC | injectability | excellent injectability, cell activity, and osteogenesis | |||
| GelMA)/dextran emulsion [59] | BMSC | porous structure, good mechanical and degradation properties | good proliferation, migration, spreading, and osteogenesis | YAP signal pathway | ||
| GelMA/β-TCP/Alginate/MXene [60] | rBMSCs, RAW264.7 | excellent shear-thinning properties, and suitable viscosity, good mechanical strength | good biocompatibility, excellent antibacterial properties, promoted healing of infected bone defects | |||
| GelMA-RF [61] | KUSA-A1 | delayed photocrosslink curing and suitable mechanical function | higher cell viability, promoted osteoblast differentiation | |||
| GelMA-BMSCs/PLA-PGA-PLA-ECs [62] | BMSCs, RAOECs | sufficient mechanical properties and good permeability | increased cell viability, promoted differentiation and maturation of osteoblast | |||
| GelMA/HAMA/Alginate/GO [63] | BMSCs, BMMs | stable porous structure, suitable mechanical, swelling and degradation properties | promoted polarization of BMMS to M2 type, promoted osteogenic differentiation of BMSCs, improved osteogenic repair | |||
| GelMA/HAMA/DBM/VEGF [64] | BMSCs | high mechanical strength, appropriate biodegradation rate and controllable VEGF release | biocompatibility, excellent ectopic bone regeneration ability | |||
| Eth-DFO@GelMA/GGMA [65] | BMSCs, HUVECs | improved printability and mechanical property, slow release of DFO | promoted migration and tube formation of ECs, improved osteogenesis and angio- genesis | |||
| GelMA/OMP [66] | hMSCs | enhanced mechanical properties, prolonged oxygen release | enhanced mechanical properties, prolonged oxygen release | |||
| GelMA/SiGO [67] | hMSCs | enhanced production, retention and bioactivity of BMPs | improved mineralization and accelerated bone repair | BMP-SMAD1/5 signalling pathway | ||
| GelMA-Alg-WH/HAP-hMSCs [68] | HUVECs, hMSCs | good mechanical, swelling, degradability properties | good cytocompatibility and excellent osteogenic properties | |||
| EphrinB2-DPSC-GelMA [69] | DPSCs | good mechanical properties | improved mineralization and accelerated bone repair | |||
| GelMA-based bone scaffolds with growth factors and their substitutes | ||||||
| GelMAhydrogel scaffolds | growth factors and their substitutes | physical properties | bioactivities | mechanism | ||
| GelMA-HAp-HAD/Col I [70] | col I | excellent swelling properties, mechanical stability and delayed degradation | promoted the migration and differentiation of BMSCs, improved angiogenesis and bone regeneration | |||
| Ti2448-GelMA/DFO [71] | DFO | good osteoconductivity and osteointegration | improved the osteogenesis, angiogenesis for large bone defects | |||
| MSNs-NH2@GelMA [72] | pyritum (PP) | good mechanical properties | promoted the differentiation of BMSCs and improved the osteogenesis | |||
| C/BCM-GelMA [73] | BMP-2 | rapid molecular release | inhibited the inflammatory response and improved the osteogenesis | |||
| GelMA-PPy-Fe [74] | BMP-2 | excellent shape fidelity, enhanced conductivity | good cytocompatibility and improved osteo- genic differentiation | NOTCH/MAPK/SMAD signalling | ||
| BMSCs-BMP2-GelMA [75] | BMP-2 | appropriate mechanical properties; injectability | promoted the differentiation of BMSCs and improved the osteogenesis | |||
| BMSC/RAW/BMP-4-GelMA [76] | BMP-4 | good mechanical properties | improved anti-inflammatory and osteogenesis | |||
| GelMA/HAMA-OGP [77] | OGP | good mechanical properties and photocrosslink ability | improved the osteogenesis | |||
| GelMa-PP&VEGF [78] | VEGF | high mechanical strength, appropriate biodegradation rate and controllable VEGF release | biocompatibility, excellent ectopic bone regeneration ability | |||
| GelMA/HAMA/DBM/VEGF [15] | VEGF | high mechanical strength, appropriate biodegradation rate and controllable VEGF release | biocompatibility, excellent ectopic bone regeneration ability | |||
| GelMA-Lip@VEGF [79] | VEGF | controllable VEGF release | improved the osteogenesis | |||
| F/G-B/M [80] | BFGF and BMP-2 | controllable VEGF and bFGF release | improved the osteogenesis and angiogenesis | |||
| GelMA-PVA (GP) [81] | PTH | controllable PTH release | improved the osteogenesis | |||
| GelMA-based bone scaffolds with exosome | ||||||
| GelMAhydrogel scaffolds | exosome | physical properties | bioactivities | mechanism | ||
| hypo-ADSC-Exos-gel [82] | ADSC-exos | controllable | improved of HUVEC proliferation, migration, and angiogenesis | PI3K/AKT signalling pathway | ||
| ExoBMP2+NoBody [83] | ExoBMP2 | Delayed degradation | improved the osteogenesis | |||
| ExoLnc NEAT1-alginate/GelMA [84] | HUVEC-exos | mechanical stability and delayed degradation | improved bone regeneration, facilitate the angiogenesis, increase the infiltration of M2 polarized macrophages | DDX3X/NLRP3 axis | ||
| USCEXOs/GelMA-HAMA/nHAP [85] | USCEXOs | controllable and biocompatible | improved osteogenesis and angiogenesis | |||
| BG-gel-sEVs [86] | hUC-MSCs-sEVs | controllable and biocompatible | improved vascularized bone regeneration | PTEN/AKT signalling pathway | ||
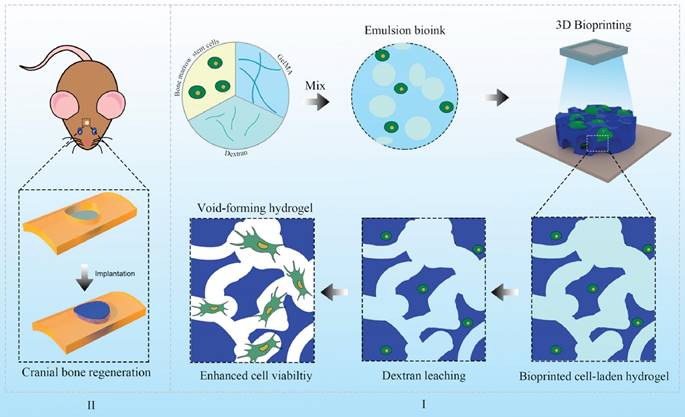
Schematic illustration of the 3D-bioprinted void-forming hydrogel constructs for implantation. (A) An aqueous emulsion bioink, where dextran solution is distributed in droplet form within a GelMA solution, was prepared by mixing GelMA, dextran, and stem cells for 3D bioprinting of a void-forming hydrogel to (B) repair the cranial defect. Reproduced from reference [61]. Copyright 2022, with permission from Elsevier.
Researchers have devised injectable hydrogels to address the clinical need for irregular bone defects. Shi et al. [58] used a bone-mimicking and injectable GelMA hydrogel (GelMA-HAP-SN) system containing mesenchymal stem cells (MSC), nano-hydroxyapatite (HAP), and nano-silicate (SN) for bone tissue engineering and systematically examined the osteogenic capacity of GelMA-HAP-SN in vitro and in vivo. The incorporation of HAP enhanced the resemblance to the components of the natural extracellular matrix of bone cells, whereas SN loading conferred injectability and osteogenic properties to the hydrogel. Consequently, GelMA-HAP-SN hydrogels exhibited increased cell viability, proliferation, and diffusion. Additionally, the GelMA-HAP-SN hydrogels augmented the expression of osteogenic biomarkers and matrix mineralisation in the encapsulated MSCs. Moreover, injection of MSC-encapsulated GelMA-HAP-SN hydrogels into critical-sized cranial bone defects in rats further confirmed their outstanding bone regeneration capacity.
Technological advances have facilitated the application of GelMA to irregular bone defects. Tao et al. [59] have integrated 3D bioprinting with stem cells to engineer cell-laden hydrogels for bone regeneration. This hydrogel was formulated by bioprinting BMSCs with a GelMA/dextran emulsion via digital light processing (DLP). 3D bioprinted hydrogels not only foster the migration, proliferation, and diffusion of encapsulated BMSCs but also stimulate the YAP signalling pathway, thereby enhancing osteogenic differentiation of BMSCs. Furthermore, in vivo therapeutic evaluations have indicated that hydrogels with pores exhibit significant potential for BMSC delivery, effectively promoting bone regeneration (Figure 1).
Growth factors and their substitutes
Growth factors are a class of peptides that facilitate communication between cells and regulate cell growth, forming the fundamental triad of biotechnological tissue engineering alongside seed cells and scaffold materials [92]. These factors play crucial roles in the promotion of cell proliferation, tissue repair, and organ regeneration [93]. In the realm of tissue engineering research, a myriad of growth factors has been recognised and new discoveries are continually emerging. Given the significant reliance of the bone healing process on biologically active factors, exogenous biologically active agents can be introduced via injection into traditional methods to expedite bone repair. However, it is imperative to acknowledge that most biologically active agents are proteins, which are susceptible to enzymatic degradation [94]. Therefore, appropriate carriers must be employed to ensure safe delivery of active agents and prevent their inactivation. Precise delivery of growth factors and minimisation of rapid diffusion are also important to avoid potential inflammatory reactions and other adverse effects [95]. GelMA hydrogels possess a favourable three-dimensional structure, adjustable swelling, and porosity, enabling the controlled release of biologically active factors in both spatial and temporal dimensions, thereby maintaining effective concentrations over extended periods [96]. In the context of bone regeneration, bone morphogenetic proteins (BMPs) [97], vascular endothelial growth factor (VEGF) [98], insulin-like growth factor (IGF) [99], and fibroblast growth factor (FGF) [100] stand as the most extensively studied and utilized growth factors. Additionally, certain pharmaceuticals and hormones, such as dexamethasone, exhibit osteogenic effects and can serve as alternatives to growth factors in bone tissue engineering.
BMP: The BMP family, a subset of the TGF-β superfamily, plays a pivotal role in cell growth, mesenchymal stem cell differentiation, and tissue regeneration and remodelling [101]. These factors can induce MSCs to differentiate into various tissues, including bone, cartilage, ligaments, tendons, and nerves. BMP-2 and BMP-7 are commonly used for bone and cartilage regeneration. Calcium carbonate (CaCO3), which has exceptional biocompatibility and bioactivity, is a well-established bone defect-filling material. Lu et al. [73] synthesised CaCO3 microspheres (CM) as intelligent carriers to load BMP-2. Subsequently, CM loaded with BMP-2 and catalase (CAT) was incorporated into GelMA hydrogels to fabricate a composite hydrogel for differential drug release. They reported that CAT within the hydrogel was rapidly released to eliminate H2O2 and generate oxygen, whereas the CM continuously released BMP-2 to facilitate rapid osteogenesis. In vitro experiments demonstrated that this composite hydrogel effectively reduced intracellular reactive oxygen species levels, thereby preventing cell damage from oxidative stress, enhancing cell survival and proliferation, and strengthening osteogenic properties. Animal experiments further revealed that this composite hydrogel could mitigate inflammation, modulate macrophage polarisation, and promote bone-defect healing.
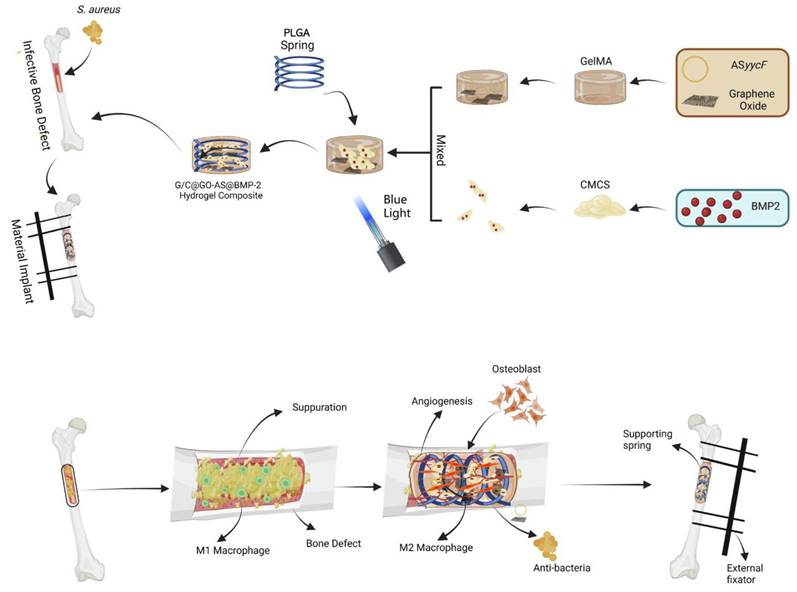
Qin et al. [102] developed a GelMA and carboxymethyl chitosan (CMCS) composite hydrogel that incorporates BMP-2 growth factor and a post-transcriptional regulation antisense technique based on graphene oxide (GO). The photocrosslinked GelMA composite hydrogel exhibited excellent biocompatibility and in vitro degradation. As the GelMA and CMCS composite hydrogels degraded, antisense yycF and BMP-2 were released. In terms of antibacterial properties, the synergistic action of CMCS, GO, and post-transcriptional regulation antisense yycF in the composite hydrogel effectively eradicated S. aureus. In in vivo experiments, in which the composite hydrogel was implanted into a rat model with an S. aureus-infected femoral defect, bone healing was significantly accelerated in an infectious microenvironment (Figure 2). To address the repair of irregular bone defects, Chai et al. [75] developed a photocrosslinked composite biologically active scaffold based on GelMA, BMSCs, and BMP-2. This composite scaffold exhibited suitable mechanical properties for stem cell adhesion and proliferation, good biocompatibility, and the ability to stimulate the osteogenic differentiation of BMSCs in vitro. Imaging and histological analyses demonstrated that the synergy between BMSCs and BMP-2 in this composite biologically active scaffold exhibited higher osteogenic potential in vivo than scaffolds loaded with BMSCs or BMP-2 alone.
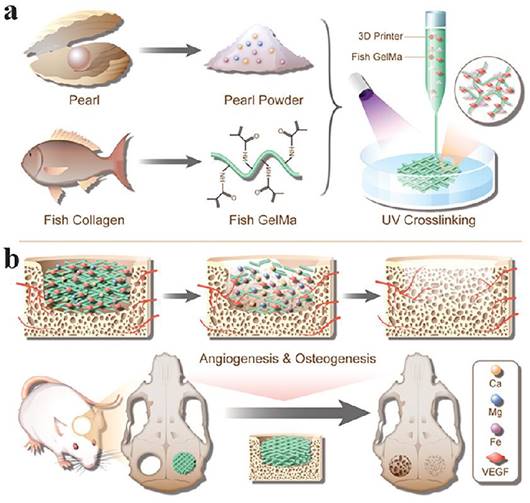
VEGF stands as a potent cytokine that fosters cell growth and angiogenesis by stimulating the proliferation and survival of endothelial cells and enhancing vascular permeability. VEGF is expressed in vascularised tissues and plays a crucial role in both normal and pathological angiogenesis [98]. The development of biologically active scaffolds that mimic the ECM of bone tissue is important for bone regeneration. A novel pearl powder (PP) mixed fish GelMA hydrogel scaffold, inspired by bone tissue composites and loaded with VEGF for bone regeneration, has been proposed by researchers [78]. By combining microfluidics with 3D printing, the composition and structure of the hybrid scaffold can be precisely adapted for clinical use. The fusion of fish skin GelMA and PP results in a hybrid scaffold with excellent biocompatibility, cell adhesion, and osteogenic differentiation capacity. Controlled release of VEGF facilitates angiogenesis. In a rat cranial defect model, this scaffold expedited bone regeneration through the synergistic effects of osteogenesis and angiogenesis (Figure 3). In another study, a heterogeneous biomimetic structure scaffold was constructed employing a 3D printed mould, simulating the outer/inner periosteum and intermediate bone matrix of natural long bones. Through the modification of mesoporous bioglass nanoparticles (MBGNs), the structural stability and osteogenic capacity of the middle layer of the scaffold were shown to be bolstered. Conversely, the incorporation of GelMA into the VEGF-loaded liposomes facilitated the controlled release of angiogenic factors from the inner and outer layers of the scaffold. This heterogeneous scaffold structure is reported to effectively guide bone regeneration and restoration of the natural bone anatomical structure [103].
The synthesis of PLAG spring supporting G/CB@GOAS hydrogel composite and application in infected femur defect models. The GelMA precursor solution loaded with the GO-ASyycF system was mixed with the CMCS solution loaded with BMP-2. After application of PLGA spring, the P-G/CB@GOAS hydrogel composite was synthesized by 405 nm blue light photo-crosslinking. The hydrogel composite was implanted into the femur infected bone defects of rats and the external fixator was applied for fixation. BMP-2 mainly induced bone regeneration. GO-ASyycF system mainly played the role of antibacterial. Meanwhile, the hydrogel composite also induced angiogenesis and promoted M2 macrophage polarization. They played synergistic roles in the treatment of infected bone defects. Reproduced from reference [103] Copyright 2023, with permission from Elsevier.
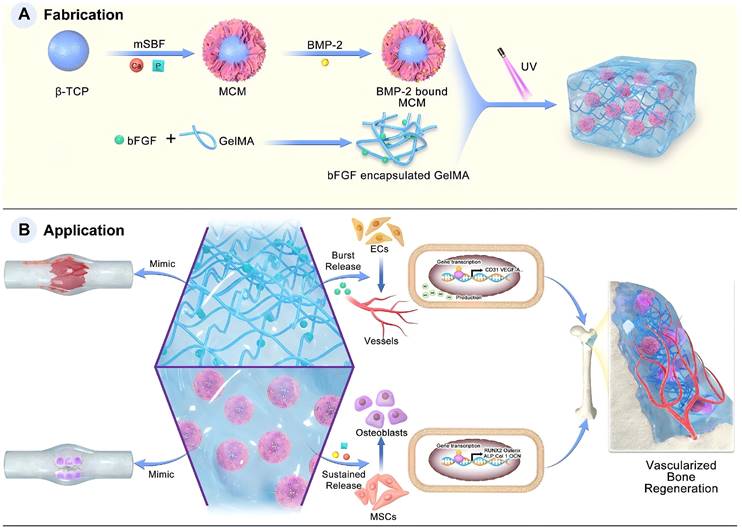
FGF plays a pivotal role in bone formation and repair, and it is involved not only in embryonic bone development but also in fracture healing and joint cartilage formation [100]. To emulate the cascade process of natural bone healing, Zhou et al. [104] have concentrated on integrating angiogenesis and osteogenesis using a hybrid dual-factor delivery system to achieve vascularised bone formation. In their study, basic fibroblast growth factor (bFGF) was encapsulated into GelMA to mimic the angiogenic signalling during the inflammatory and soft callus stages of bone healing. BMP-2 binds to mineral-coated microparticles (MCM) to simulate osteogenic signalling during the hard callus and bone remodelling stages. This design achieved the coordinated release of high initial concentrations of bFGF, along with the sustained release of BMP-2 and inorganic ions, fostering a well-coordinated osteogenic and angiogenic effect for bone regeneration. In vitro experiments demonstrated that this hybrid hydrogel significantly enhanced the formation of vascular systems by HUVECs and promoted the osteogenic differentiation of BMSCs. This underscores the potential of this system to promote bone repair and regeneration (Figure 4).
Exosomes
Exosomes, minute vesicles measuring approximately 40-100 nm in diameter, are generated within cells and released into the extracellular milieu through the fusion of multivesicular bodies with the cell membrane [105]. These microvesicles are replete with diverse bioactive substances and serve as pivotal carriers for intercellular signalling and interactions, thus playing a central role in tissue repair and regeneration. Exosomes facilitate the transfer of specific proteins, miRNAs, and bioactive factors, thereby promoting the differentiation of mesenchymal stem cells into osteoblasts by activating distinct signalling pathways that upregulate genes associated with osteogenesis, thereby fostering bone defect repair and promoting bone regeneration [105].
Schematic diagram of PP hybrid bioactive scaffold from microfluidic 3D printing for bone regeneration. (A)The composition and microfluidic 3D printing of PP hybrid bioactive scaffold. (B) The application of PP hybrid bioactive scaffold in bone regeneration. Under Creative Commons Attribution https://creativecommons.org/licenses/by/4.0/ Copyright 2023.
Schematic illustration of the fabrication and application of the F-G/B-M hybrid hydrogel. Under Creative Commons Attribution https://creativecommons.org/licenses/by/4.0/ Copyright 2023.
Schematic of the HUVECs derived exosomal NEAT1 mediated bone regeneration mediated by macrophage polarization via DDX3X/NLRP3 axis. Under Creative Commons Attribution https://creativecommons.org/licenses/by/4.0/ Copyright 2023.
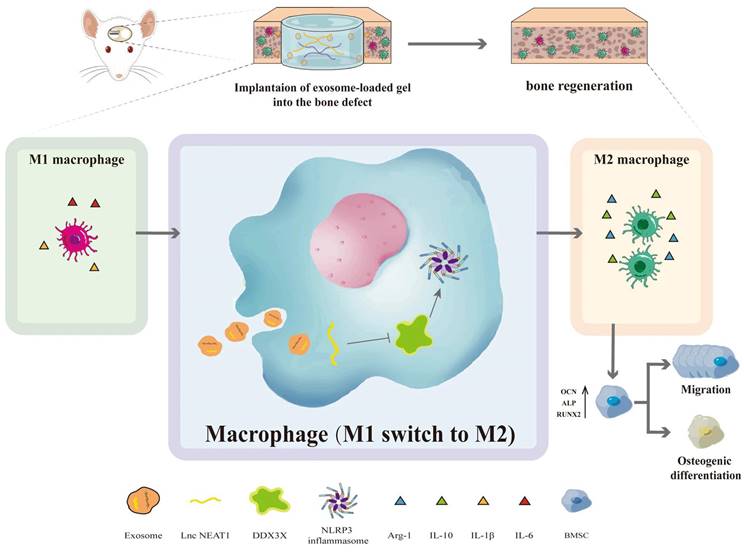
Particularly noteworthy is the pretreatment of adipose-derived stem cell (ADSC) exosomes under hypoxic conditions, which has garnered attention because of the heightened secretion and functionality of exosomes. Li et al. [82] indicated that hypo-ADSC-Exos harbour a crucial miRNA, miR-21-5p, which is a major regulator of angiogenesis. Hypo-ADSC-Exos have been demonstrated to promote proliferation, migration, and angiogenesis in HUVECs in vitro. They reported that inhibition of miR-21-5p effectively attenuates proangiogenic effects mediated by hypo-ADSC-Exos. Mechanistically, their study demonstrated that exosomes from hypo-ADSCs target SPRY1 within HUVECs to exert their regulatory effects, thereby promoting activation of the PI3K/AKT signalling pathway. Notably, silencing SPRY1 enhances PI3K/AKT activation in HUVECs and promotes proliferation, migration, and angiogenesis. The culmination of the above study involved an in vivo demonstration, validating that loading hypo-ADSC-Exos into GelMA significantly augments local H-type angiogenesis and concomitant bone regeneration, attributed to the modulation of SPRY1 by exosomes. Additionally, HUVEC-derived exosomes carrying NEAT1 substantially enhanced M2 polarisation and alleviated LPS-induced inflammation in vitro. Exosome-induced macrophage-conditioned medium indirectly promotes the migration and osteogenic differentiation of BMSCs. Mechanistically, exosomes carrying NEAT1 significantly downregulate the expression of DDX3X and NLRP3 in HUVECs. In vivo, HUVEC-derived exosomes markedly increase pro-inflammatory cytokines (IL-6 and IL-1β) and anti-inflammatory cytokines (IL-10), thus ameliorating LPS-induced inflammation. Subsequently, encapsulating HUVEC-derived exosomes in alginate/GelMA interpenetrating polymer network (IPN) hydrogels promotes bone regeneration and angiogenesis, augments M2 polarized macrophage infiltration, and reduces NLRP3 expression, as reported in a rat cranial defect model [84] (Figure 5).
Antibacterial strategy
In clinical practice, addressing infectious bone defects and fostering bone regeneration poses a formidable challenge. Upon bacterial infiltration into the bone tissue, the ensuing production of acidic metabolites and the provocation of a robust immune response dampens osteoblastic activity and impedes the restitution of bone defects [106]. Furthermore, bacterial pathogens release toxins, virulent factors, and cytotoxic substances that deleteriously impact the bone matrix, precipitating nerve and blood vessel necrosis and severely hindering bone regeneration. Consequently, interest in antimicrobial hydrogels for bone tissue engineering has rapidly increased. By employing various antimicrobial agents such as antibiotics, metal nanoparticles, and botanically active compounds, these hydrogels, specifically designed for bone restoration, can meticulously and efficiently eradicate bacteria, thereby effectively preventing infections and reducing postoperative complications [107,108]. This review systematically delineates the antimicrobial strategies anticipated for GelMA hydrogels, as outlined in Table 3.
Antibiotics
The conveyance of antibiotic molecules through carriers represents a straightforward and efficacious traditional dosing approach, affording localised administration to circumvent the systemic drug tolerance hazards inherent to intravenous administration [140]. Currently, antibiotics are broadly classified into seven categories: β-lactam antibiotics (encompassing penicillins and cephalosporins), macrolide antibiotics (such as erythromycin and azithromycin), aminoglycoside antibiotics (including gentamicin and etimicin), tetracycline antibiotics (such as tetracycline), lincosamide antibiotics (such as clindamycin), chloramphenicol antibiotics (such as chloramphenicol), and peptide antibiotics (such as vancomycin).
β-Lactam Antibiotics: The mode of action of β-lactam antibiotics primarily involves targeting bacterial penicillin-binding proteins (PBPs), thereby inhibiting the enzyme responsible for peptidoglycan synthesis in the bacterial cell wall. This inhibition impairs peptidoglycan synthesis, resulting in the formation of defective bacterial cell walls. Consequently, external water penetrates the cells, inducing swelling and rupture, which triggers bacterial autolysin activity and ultimately results in bacterial death. Vigata et al. [109] devised a hydrogel drug delivery system (termed GelMA-DDS) based on GelMA for the localised delivery of the broad-spectrum antibiotic cefazolin, with the aim of preventing and treating postoperative infections in surgical wounds. The researchers fabricated GelMA hydrogels with polymer concentrations ranging from 5% to 15% w/v and loaded them with doses of cefazolin ranging from 3μg to 90μg, followed by facile photocrosslinking for solidification. The findings demonstrated that all the GelMA groups exhibited a remarkable drug encapsulation efficiency of 99 %. Additionally, cefazolin dispensed by GelMA showed dose-dependent antimicrobial efficacy against S. aureus in broth and diffusion assays. The loaded cefazolin GelMA-DDS provides a highly adaptable and user-friendly localised delivery system and a novel modality for preventing and treating postoperative infections in surgical wounds.
Summary of typical GelMA-based hydrogel scaffolds for controlling infected tissue.
| GelMA-bases hydrogel | Metal ions | Bacteria | Application |
|---|---|---|---|
| GelMA/HA-E/Ag@MOF [120] | EGCG | E. coli + S. aureus | Antibacterial effect, wound healing |
| GelMA-NP50-Cur3 [121] | cur | E. coli + S. aureus | Antibacterial effect, wound healing |
| Nio-Thymol@GelMa [122] | Nio-Thymol | E. coli + S. aureus | Antibacterial effect, wound healing |
| GelMA-WEO [123] | WEO | E. coli + S. aureus + MRSA | Antibacterial effect, wound healing |
| GelMA/AV [124] | AV | E. faecalis | Endodontic disinfection and immunomodulation |
| GelMA-bases hydrogel | Metal ions | Bacteria | Application |
| PSBDA [125] | Ag | E. coli + S. aureus | Antibacterial effect, wound healing |
| GelMA [126] | ZF | S. aureus | Antibacterial effect, wound healing |
| GelMA [127] | SPION | S. aureus | Antibacterial effect, wound healing |
| Ce-BG/GelMA [128] | Ce | E. coli and S. aureus | Antibacterial effect, wound healing |
| GelMA-bases hydrogel | Cationic group | Bacteria | Application |
| GelMAQ [129] | DSMA | Porphyromonas gingivalis | Periodontal tissue regeneration |
| GelMA-bases hydrogel | PAT | Bacteria | Application |
| SC/gel [130] | SC | Streptococcus mutans + Lactobacillus casei | Antibacterial effect and enhanced pulp, regeneration activity |
| PAG-CuS [131] | CuS | E. coli + S. aureus | Antibacterial effect, wound healing |
| p-CQD/WS2/n-CQD/GelMA [132] | p-CQD/WS2 | E. coli + S. aureus | Osteogenic and antibacterial effects |
| GelMA-PAM [133] | MOF | E. coli + S. aureus | Antibacterial effect, wound healing |
| GelMA-EGF/ -MPDA-LZM [134] | MPDA-LZM | E. coli | Osteogenic and antibacterial effects |
| GelMA-Au NBPs@SiO2 [135] | Au NBPs | P. gingivalis | Osteogenic and antibacterial effects |
| GelMA/HA-DA/GO-βCD-BNN6 [136] | GO | E. coli + S. aureus | Antibacterial effect, wound healing |
| ZPTA-G/HMA [137] | ZnO@PDA | E. coli + S. aureus + Candida albicans | Antibacterial and anti-inflammatory effects |
| GelMA [138] | BP@Mg | E. coli + S. aureus | Antibacterial effect and innerved bone regeneration |
| CuSNP@CS-MA [139] | CuS | E. coli + S. aureus | Periodontal tissue regeneration |
Macrolide Antibiotics: The macrolide antibiotics bind to the 50S subunit of bacterial ribosomes, which are responsible for protein synthesis. Upon binding, they impede the translocation of bacterial proteins, thereby disrupting the bacterial protein synthesis process. Ayoub et al. [110] devised a novel photocrosslinkable azithromycin (AZ)-loaded GelMA fibre using electrospinning technology, which served as a localised and biodegradable drug delivery system for managing dental pulp infections. Fibres were fabricated at three different concentrations: GelMA+5%AZ, GelMA+10%AZ, and GelMA+15%AZ. Fibres comprising GelMA and GelMA+10%AZ exhibited the highest average diameters. The incorporation of AZ reduced the tensile strength of the GelMA-based fibres. Furthermore, the GelMA+15%AZ fibres demonstrated the most pronounced inhibition of bacterial growth. Importantly, the presence of AZ at all tested concentrations did not elicit a significant toxic response in their study. Additionally, findings from the subcutaneous rat model revealed abundant vascular formation in the experimental group, along with attenuated inflammation and mature collagen fibres intertwined with the engineered fibres, signifying promising prospects for tissue engineering.
Peptide Antibiotics: Chemically synthesised antibiotic peptides typically employ a mechanism of action similar to that of natural AMPs, primarily targeting the bacterial cell membrane to exert bactericidal effects. This membrane-damaging mechanism disrupts the integrity of the bacterial cell membrane, leading to pore formation and the subsequent leakage of cellular contents. Qian et al. [111] encapsulated vancomycin (Van) in poly(lactic-co-glycolic acid) (PLGA) microspheres using liquid encapsulation technology, which were then loaded into GelMA hydrogels. These microsphere-loaded hydrogels were embedded into additively manufactured porous tantalum (AM-Ta) structures to create a composite scaffold—Ta/GelMA hydrogel/PLGA/Van—for treating infectious bone defects. The physicochemical characterisation of this scaffold revealed a vancomycin-release cycle lasting for over two weeks. Subsequent biological experiments confirmed the excellent biocompatibility and antibacterial and osteoconductive properties of the scaffold, highlighting its considerable potential for clinical applications.
Tetracycline Antibiotics: Tetracycline antibiotics exert their antimicrobial effects by forming reversible complexes with the 30S subunit of bacterial ribosomes, thereby inhibiting protein synthesis. Zhang et al. [112] developed a modular microneedle (MN) patch for the effective delivery of antibiotics and cytokines to the local gingival tissue to achieve immune regulation and tissue regeneration. The MN patch comprises two components: a rapidly dissolving gelatine membrane releasing tetracycline and a biodegradable GelMA MN containing tetracycline-loaded poly(lactic-co-glycolic acid) nanoparticles and cytokine-loaded silica microparticles for sustained release. Experimental results demonstrated that antibiotic release could completely inhibit bacterial growth, while the release of TGF-β and IL-4 induced anti-inflammatory macrophage reprogramming and promoted regulatory T cell formation in vitro. When applied to the periodontal tissue in vivo, the MN patch suppressed proinflammatory factor production, promoted proregenerative signals, and facilitated tissue healing, highlighting its potential for local immune regulation in tissue regenerative therapy.
Lincosamide Antibiotics: Lincomycin, a lincosamide antibiotic produced by Lincomyces, acts on sensitive bacterial ribosomes by binding to the central loop of the 50S subunit 23SrRNA gene, preventing elongation of the peptide chain and inhibiting bacterial cell protein synthesis. Ribeiro et al. [113] used electrospinning to incorporate clindamycin (CLIN) and metronidazole (MET) into polymer solutions to prepare fibre mats. The mats were then processed by low-temperature grinding to obtain fibre particles containing CLIN or MET. These particles were used to modify the GelMA hydrogel. Morphological characterisation of the electrospun fibres and particles obtained by low-temperature grinding was performed using scanning electron microscopy (SEM). Furthermore, the swelling, degradation, and toxicity of the experimental hydrogels toward dental pulp stem cells were tested, and their antibacterial efficacy was assessed using agar diffusion and biofilm inhibition tests. The results showed that GelMA hydrogels modified with fibre particles containing antibiotics showed increased swelling and degradation rates. Dental pulp stem cell viability slightly decreased but did not show significant toxicity (cell viability >50%). All hydrogels containing antibiotic-loaded fibre particles exhibited antibiofilm activity with almost complete elimination of viable bacteria in the dentin matrix.
Antimicrobial peptides
AMPs serve as critical immune defence molecules in multicellular organisms, showcase broad-spectrum bactericidal effects, and are emerging as promising candidates for novel antimicrobial therapies. Currently, over 30 AMP drugs are undergoing clinical studies worldwide, highlighting their potential clinical applications. Recent studies have revealed synergistic effects between certain AMPs and conventional antibiotics. By combining AMPs with antibiotics, it is possible to reduce antibiotic dosage, minimise side effects, mitigate the emergence of antibiotic-resistant strains, and significantly enhance the overall therapeutic efficacy of antibiotics [141,142]. This synergy represents a promising avenue for combating bacterial infections and addressing the challenges posed by antibiotic resistance.
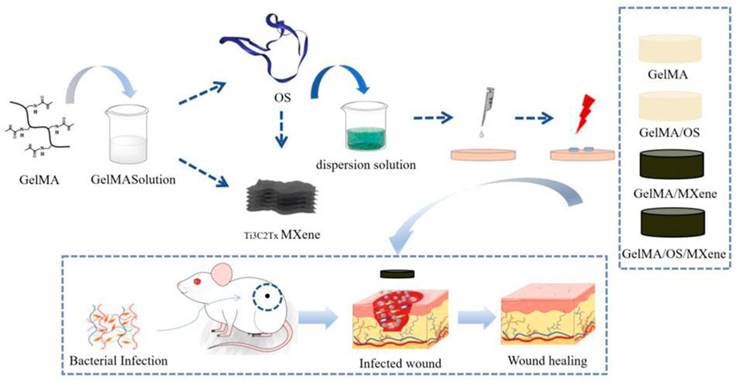
Ren et al. [115] developed a multifunctional conductive hydrogel dressing using GelMA, Ti3C2, and collagen-bound AMPs (V-Os). Electrical stimulation (ES) promotes skin wound healing. However, traditional ES strategies based on single electrodes may struggle to achieve uniform coverage over the entire wound area, affecting treatment outcomes because of mechanical property mismatches between the electrodes and the wound tissue. This dressing aimed to improve wound management by incorporating GelMA, Ti3C2 for conductivity, and modified AMPs (V-Os) to replace traditional antibiotics. Their study demonstrated that the GelMA@Ti3C2/V-Os hydrogel dressing exhibited excellent conductivity and biocompatibility with sustained bactericidal effects. V-Os were tightly bound to GelMA, thereby prolonging its antimicrobial activity. Cell experiments revealed enhanced fibroblast migration, proliferation, and tissue repair gene expression with dressings, particularly under electrical stimulation. In vitro, it promotes wound re-epithelialisation, angiogenesis, immune response mediation, and infection prevention. This dressing shows promise for wound healing because it provides moisture retention, biocompatibility, mechanical strength, and antimicrobial properties. In another study by Liang et al. [116], tick-derived AMPs (Os) were encapsulated in a GelMA hydrogel containing MXene nanoparticles. The composite hydrogel exhibited excellent mechanical strength, swelling, degradation, and antimicrobial activity and promoted cell growth, adhesion, and antimicrobial peptide activity expression of AMPs. In full-thickness rat wound-healing experiments, the composite hydrogel accelerated wound closure, reduced inflammation, and promoted epithelial cell formation and maturation. Incorporating AMPs into GelMA hydrogels is a feasible strategy for developing high-quality antimicrobial wound dressings with strong tissue regenerative capacity (Figure 6).
Schematic diagram of the experimental procedure. This experiment involves preparing GelMA solution and TBCZ@MXene dispersion solution, as well as GelMA/OS and GelMA/MXene composite materials. Subsequently, a bacterial infection wound model on mice is established, followed by the application of these materials to the infected wounds. Finally, the antibacterial effects of these materials and their impact on wound healing are assessed aiming to explore the effectiveness of GelMA-based composite materials in treating bacterial infection wounds. Under Creative Commons Attribution https://creativecommons.org/licenses/by/4.0/ Copyright 2023.
More recently, poly-L-lysine, an AMP, has gained attention owing to its high safety profile and broad-spectrum inhibitory effects against Gram-positive and Gram-negative bacteria, fungi, and other microbes. Its thermal stability and good water solubility make it suitable for a wide range of applications in the food preservation and medical fields, particularly as a coating material. Chen et al. [117] explored the advantages of polyether ether ketone (PEEK) in self-initiated graft polymerisation and the application of hydrogels in bone tissue engineering. They constructed a hydrogel coating (GPL) on the surface of PEEK using UV-induced crosslinking, composed of GelMA, methacryloyl-modified ε-poly-l-lysine (ε-PLMA), and laponite. This coating not only enhanced the hydrophilicity of PEEK but also exhibited slow-degrading characteristics, retaining approximately 80% of the coating after eight weeks of incubation in PBS. In vitro studies showed that PEEK-GPLs enhanced the viability and adhesion of human umbilical cord Wharton jelly mesenchymal stem cells (hWJ-MSCs) compared to plain PEEK. The micrometre-scale three-dimensional surface structure of the GPL coating and the synergistic effect of laponite significantly improved the ability of PEEK-GPL to induce hWJ-MSC osteogenic differentiation with increased alkaline phosphatase activity, matrix mineralisation, and osteogenic gene expression. Furthermore, PEEK-GPL demonstrated antimicrobial activity against S. aureus and E. coli, which lasted until the hydrogel fully degraded due to the covalent crosslinking of ε-PLMA in the coating. Infection resistance and bone integration are critical considerations for antimicrobial titanium implants. To address these issues, Zhang et al. [118] prepared a titanium surface with a micro-nano structure using dual-acid treatment and anodic oxidation and then coated it with a GelMA hydrogel loaded with GL13K. In vitro cell experiments, including observations of the cell cytoskeleton, cell viability, alkaline phosphatase activity, mineralisation, and osteogenic gene expression, demonstrated the biocompatibility of the released hydrogel-modified micro/nano-titanium peptide, promoting osteoblast differentiation. In vitro antimicrobial experiments showed that it could also inhibit the growth of gram-positive bacteria (S. aureus) and gram-negative bacteria (E. coli) by releasing GL13K. These studies validated the modification of titanium surfaces to develop antimicrobial titanium implants, providing a new solution for bone tissue engineering.
Plant-derived antimicrobial agents
Plant-derived antimicrobials are bioactive compounds procured from botanical sources via diverse extraction techniques, including physical, chemical, and biological methods. These compounds are broadly classified as polyphenols, anthraquinones, terpenoids, and alkaloids, each harbouring chemical constituents such as phenolic, ether, terpenoid, and ketone groups. Recently, the efficacy of plant extracts against external pathogens has emerged as a focal point of research. Owing to their eco-friendliness, minimal environmental impact, and limited adverse effects, plant-derived antimicrobials have been widely utilised in various sectors such as cosmetics, natural preservatives, and animal feed production [143,144]. The antimicrobial modalities exhibited by these compounds are as follows: 1. Direct disruption of bacterial cell integrity, leading to enhanced membrane permeability and subsequent leakage of intracellular components; 2. Genetic impairment of bacterial DNA, 3. Perturbation of bacterial intracellular enzymatic cascades, culminating in enzyme denaturation and functional incapacitation; and 4. Alterations in bacterial oxidative respiratory pathways [143].
Polyphenols
Polyphenols exhibit notable efficacy in impeding the proliferation of both bacteria and fungi, and their antimicrobial potency is positively correlated with their concentration. Tea polyphenols, primarily catechins, are widely used as botanical polyphenols [143]. These lipophilic compounds can permeate the bacterial cells and exert antimicrobial effects. Dong et al. [119] engineered a dual-network hydrogel through the synergy of hydrogen bonding interactions between tea polyphenols (TP) and glycerine and photocrosslinked N-acryloylaminoacetamide (NAGA), GelMA, and nanoclay laponite (NGL) hydrogels. The introduction of glycerine hindered the diffusion of TP into the NAGA/GelMA/laponite (NGL) hydrogel, thus preventing excessive crosslinking and facilitating the formation of a homogeneous network. This hydrogel demonstrated exceptional moisture retention, sustaining 84% moisture over 28 days. Moreover, owing to the hygroscopic nature of glycerine, the mechanical robustness (0.73-1.14 MPa) and tensile strain (207%-353%) of the hydrogel were further augmented after 14 days in an open environment. The hydrogel exhibited remarkable UV protection and antioxidant capabilities, effectively mitigating oxidative stress at the wound sites and expediting wound healing. Additionally, antimicrobial efficacy against E. coli and S. aureus was evident in the hydrogel wound dressings. Due to its dual-network design, the NGLG20/TG hydrogel dressing accelerated wound healing by promoting wound closure, angiogenesis, and collagen deposition, offering a unique wound management approach.
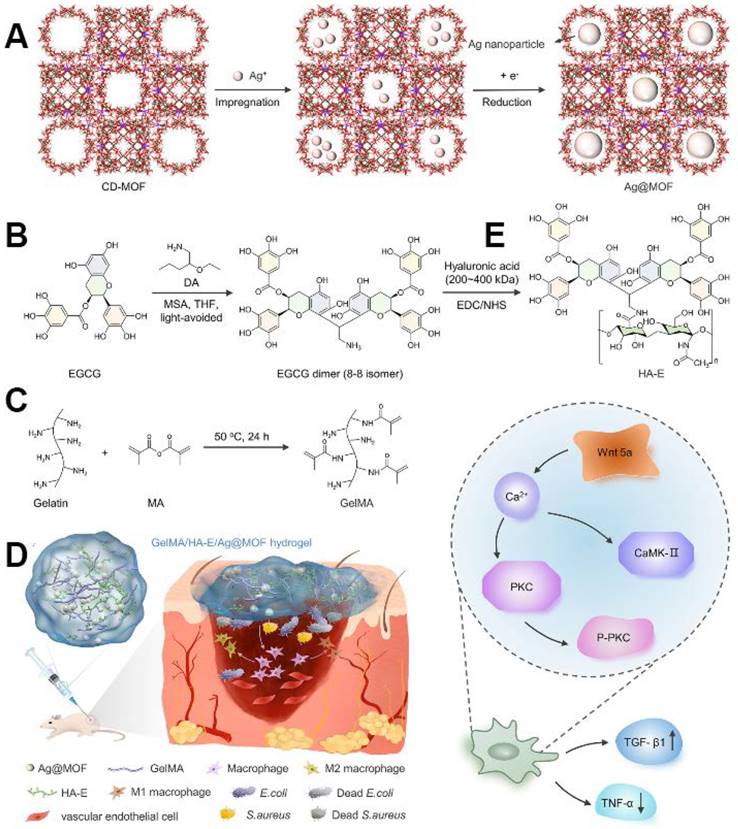
Epigallocatechin gallate (EGCG), one of the most potent constituents of tea polyphenols, has garnered significant attention for its anticancer, antimutagenic, and cardiovascular protective properties, as well as its capacity to modulate the endocrine and immune systems. Xiong et al. [120] innovated a novel GelMA hydrogel system incorporating silver nanoparticles encapsulated in γ-cyclodextrin metal-organic frameworks (Ag@MOF) and hyaluronic acid-epigallocatechin gallate (HA-EGCG). The GelMA/HA-EGCG/Ag@MOF hydrogel exhibited favourable physical attributes and sustained Ag + release. Furthermore, the hydrogel exhibited excellent biocompatibility and facilitated the polarisation of macrophages from M1 to M2 phenotype. In vivo evaluations of wound healing showed the hydrogel's ability to curtail bacterial proliferation, expedite wound closure, stimulate early angiogenesis, and modulate immune responses. Subsequent findings highlighted the significant activation of the non-canonical Wnt signalling pathway in the GelMA/HA-EGCG/Ag@MOF hydrogel-treated group (Figure 7).
(A) Schematic of CD-MOF template guided synthesis of Ag@MOF. (B) Schematic diagram of synthesis of HA-E. (C) Schematic ofsynthesis of GelMA. (D) Schematic of infectious burn wound healing process including bacterium invasion, macrophages polarization and pro-inflammation cytokines release. (E) Schematic diagram of activated noncanonical Wnt pathway in GelMA/HA-E/Ag@MOF hydrogel group. Copyright 2022, with permission from Wiley.
Terpenoids
Terpenoids, which are prevalent constituents of plant essential oils, resins, and pigments, exhibit diverse biological functions, including anti-inflammatory, antitumour, anti-HIV, and lipid-lowering effects [143]. The principal sources of terpenoids are Artemisia annua and Prunus persicae. Moghtaderi et al. [122] have harnessed thymol, a terpenoid-derived antimicrobial agent, to treat antibiotic-resistant microbes. To facilitate efficient thymol delivery, a hydrophilic polymer hydrogel with exceptional biocompatibility was synergistically amalgamated using niobium-based technology for thymol encapsulation, culminating in the formation of a GelMa composite system. Thereafter, optimisation efforts focused on enhancing the thymol capture efficiency, minimising the particle size, and reducing the polydispersity index, resulting in Nio-Thymol@GelMa achieving a peak thymol release of 60% and 42% in media with pH values of 6.5 and 7.4, respectively. Moreover, Nio-Thymol@GelMa demonstrated superior antimicrobial and anti-biofilm efficacy against both Gram-negative and Gram-positive bacteria compared with Nio-thymol and free thymol. Notably, Nio-Thymol@GelMa significantly augmented the migration of human dermal fibroblasts in vitro and upregulated the expression of pivotal growth factors (e.g., FGF-1) and MMPs (e.g., MMP-2 and MMP-13). Consequently, Nio-Thymol@GelMa has emerged as a promising novel thymol drug formulation, offering considerable potential for enhancing wound-healing processes and augmenting antimicrobial efficacy.
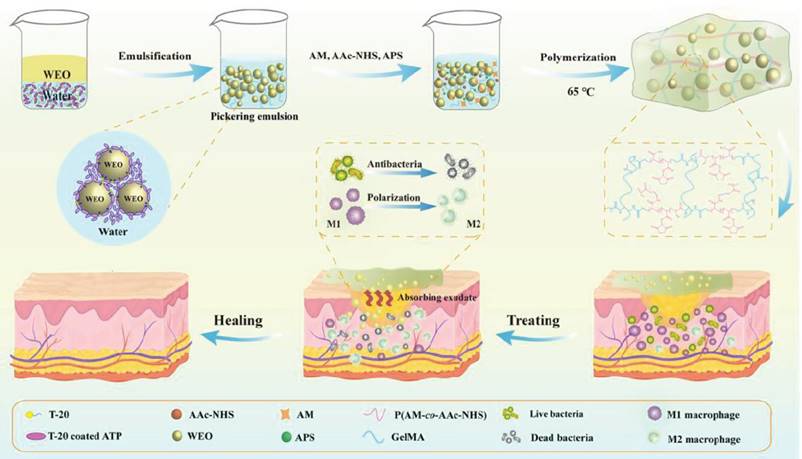
In response to the escalating conundrum of multidrug-resistant bacteria, Wang et al. [123] harnessed Artemisia annua essential oil (WEO) encapsulated within a GelMA, acrylamide (AM), and acryloyl N-hydroxysuccinimide (AAc-NHS) polymerisation process using the O/W-Pickering emulsion technique to form a multifunctional hydrogel dressing (HD-WEO). Compared to conventional emulsions, Pickering emulsions not only bolstered the encapsulation stability of the WEO but also fortified the tensile and swelling properties of the hydrogel. The diverse bioactive constituents of the WEO exhibit broad-spectrum antimicrobial activity against S. aureus, E. coli, and methicillin-resistant S. aureus (MRSA). Furthermore, HD-WEO facilitated the polarisation of macrophages from the M1 to the M2 phenotype. HD-WEO effectively promoted collagen deposition and neoangiogenesis, thereby expediting the healing of MRSA-infected diabetic wounds (Figure 8).
The preparation process diagram of HD-WEO and the mechanism to promote healing of infected diabetic wound. Reproduced from reference [124] Copyright 2023, with permission from Wiley.
Anthraquinone
Anthraquinone, a naturally occurring quinone compound, comprises various reduced products and dimers, including anthrahydroquinone, oxidised anthrahydroquinone, and anthraquinone, along with their glycosides. Within the natural realm, anthraquinones are prevalent in the metabolic byproducts of higher plants, lower plants, lichens, and fungi, showing diverse functionalities such as haemostasis, antimicrobial activity, purgative effects, and diuretic properties. Namazi et al. [124] fabricated a highly biocompatible biomaterial capable of eradicating dental pulp infections and modulating inflammation by integrating aloe vera, which is primarily enriched with aloe emodin, an antimicrobial constituent, into photo-crosslinkable GelMA nanofibers. These efforts yielded stable GelMA/AV nanofibers with an optimal (70:30) ratio achieved via electrospinning. These GelMA/AV (70:30) nanofibers showed remarkable antimicrobial efficacy against Enterococcus faecalis, maintaining sustained activity for over 14 days, along with notable biofilm reduction and minimal cytotoxicity. Furthermore, these nanofibers exhibited pro-healing, anti-inflammatory, and immunomodulatory properties.
Metal nanoparticles
Antimicrobial agents can be categorised into three main types: inorganic, organic, and natural. Organic antimicrobials are often disregarded because of their limited heat resistance, elevated toxicity, and propensity to foster antibiotic resistance. Similarly, natural antimicrobials, which are limited by their source, lack widespread applicability. Inorganic antimicrobials, predominantly metal-based, are favoured because of their expansive antimicrobial spectrum, exceptional antibacterial efficacy, and the absence of resistance development. Metallic ions have potent inhibitory and bactericidal effects, with certain metal ions posing no harm to human health [145]. Typically, the antimicrobial activity of metal ions follows this order: Ag+ > Co2+ ≥ Ni2+ ≥ Al3+ ≥ Zn2+ ≥ Cu3+ = Fe3+ > Mn2+ ≥ Sn2+ ≥ Ba2+ ≥ Mg2+ ≥ Ca2+ [146]. The mechanisms of antimicrobial action can be broadly classified as ionic, degradative, oxidative, or substitutional.
Ionic antimicrobials
Ionic antimicrobials represent a widely explored and utilised category of antimicrobial agents distinguished by their capacity to interact with bacteria through diverse mechanisms such as electrostatic attraction and ionic dissolution [147]. Metals such as copper and silver exemplify prominent instances of ionic antimicrobials, which are renowned for their broad-spectrum antimicrobial activity and efficacy. Xiang et al. [125] conducted a study on the augmentation of the antimicrobial properties of injectable wound dressings using multifunctional ionic silver nanoparticles (AgNPs). This investigation entailed the development of a biomimetic polymer copolymerised with sulfobetaine and catechol groups (PSBDA), which served as a stabilising strategy to streamline the synthesis of AgNPs. The phenolic constituent of PSBDA demonstrated the ability to reduce AgNO3 in alkaline solutions and immobilise PSBDA on the surface of AgNPs. This yielded AgNPs characterised by a uniform size distribution and markedly enhanced stability, which is pivotal for sustaining antimicrobial efficacy in physiological milieus. The precursor hydrogel exhibited excellent injectability and promptly responded to UV-induced in situ gelation. Compared to hydrogels without amphoteric ionic AgNP modification, the AgNP-incorporated hydrogel exhibited heightened antimicrobial effectiveness in both in vitro and in vivo assays. Furthermore, amphoteric ionic modifications improved hemocompatibility and biocompatibility. This hydrogel dressing facilitated the resolution of inflammation and rapid re-epithelialisation, thereby expediting healing in a full-thickness rat wound model.
Degradative antimicrobials
As research on degradative antimicrobial mechanisms has progressed, pure Mg and Mg alloys have increasingly come under the scrutiny of researchers. Robinson et al. [148] have confirmed the bactericidal effect of Mg, suggesting that its antimicrobial environment is alkaline. Additionally, the bactericidal properties of Mg-based metals have been investigated in animal experiments. These studies revealed that the bactericidal action of Mg-based metals differs from that of copper, silver, and zinc, as Mg-based metals do not rely on ionic effects. Instead, Mg-based metals exert their antibacterial effects through self-degradation in the body or environment, significantly increasing the environmental pH, thereby altering the conditions for bacterial growth and ultimately leading to bacterial eradication [149]. Researchers have also utilised polydopamine (PDA), which interacts with the amino groups in polyacrylamide (PAM) through its amino and catechol groups, to form a network with PAM. Magnesium ions (Mg2+) were incorporated into hydrogels to promote cell proliferation, differentiation, and tissue regeneration. The mixed crosslinking of covalent bonds and reversible non-covalent bonds in the PDA-PAM/Mg2+ composite endows it with excellent self-healing and adhesive properties. Under near-infrared radiation, PDA in the hydrogel demonstrated remarkable photothermal efficiency and antibacterial activity. The introduction of Mg2+ enhances the photostability and recyclability of the photothermal effect [150].
However, research on the degradation mechanisms of antimicrobial metals remains limited. This scarcity may be attributed to the rapid degradation rate of Mg2+, which could lead to bacterial infections and adversely affect biocompatibility, thus hindering its clinical development.
Oxidative antimicrobials
Oxidative antimicrobials include metals that may not inherently possess robust antibacterial properties or exhibit limited effectiveness. However, metal oxides can also exhibit potent antimicrobial activity under specific circumstances. Illustrative examples include zinc oxide and iron oxide, which undergo chemical reactions upon exposure to light under specific environmental conditions, culminating in bactericidal effects. Haghniaz et al. [126] devised an injectable, photocrosslinkable, and stretchable hydrogel sealant on GelMA fortified with antibacterial ferrimagnetic zinc oxide (ZF) nanoparticles and haemostatic silicate nanoplatelets (SN) for prompt clotting. The hydrogel demonstrated the capacity to diminish the viability of S. aureus by over 90% in vitro. Incorporating SN (2% w/v) and ZF nanoparticles (1.5 mg/mL) into GelMA (20% w/v) notably increased the bursting pressure of perforated porcine lungs by > 40%. Relative to the commercial haemostatic sealant Evicel, this fortified formulation enhanced tissue-sealing efficacy by approximately 250%. Additionally, in a rat bleeding model, the hydrogel curtailed bleeding by approximately 50%, serving as a biocompatible wound-closure adhesive with dual haemostatic and antibacterial attributes. Another study introduced a novel generation of antimicrobial gelatine bioinks based on GelMA containing varying doses of antimicrobial superparamagnetic iron oxide nanoparticles (SPION). The SPION-loaded GelMA scaffolds showed notable resistance to the proliferation of S. aureus. In vitro simulations of bacterial contamination on 3D GelMA scaffolds underscored the pivotal role of functionalized scaffolds in impeding bacterial proliferation while preserving cell viability and proliferation [127] (Figure 9).
Schematic overview of the experimental design for the fabrication and in vitro analysis of bacteriostatic.3D scaffold systems (A-E) A CAD model of the 3D geometry of interest was designed (A) and bioprinted (B) using various GelMA bioinks containing 0 (control), 100, 200, and 500 mg/mL of superparamagnetic iron oxide nanoparticles (SPIONs) to create the 3D Scaffold (C). Bioprinted scaffolds were assessed for printing fidelity and mechanical and MR properties (D), and they were seeded with human cells (fluorescently tagged endothelial cells) and/or bacteria (luminescent S. aureus) (E) for in vitro 3D culture assays. (F) Timeline for in vitro culture assays. Constructs were cultured for 5 days (bacterial and human cell coculture) or 14 days (endothelial cell culture) and examined using AlamarBlue and imaging techniques. In panel (F), grey represents the experimental steps for both bacterial and cell culture assays, brown represents the steps for the bacterial assays; and green represents steps for endothelial cell culture reproduced from reference [128] Copyright 2023, with permission from Elsevier.
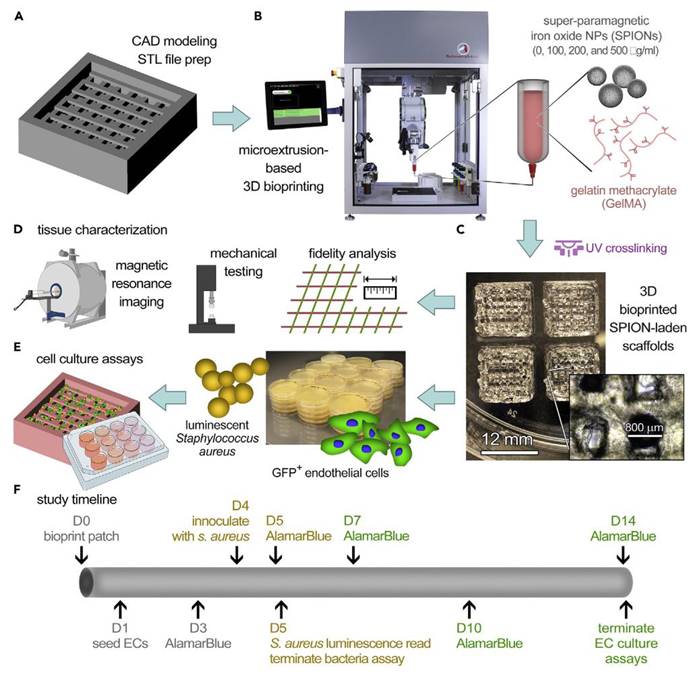
Substitutional antimicrobials
Substitutional antimicrobials operate on the premise of the structural resemblance between metal ions and essential bacterial nutrients. This structural similarity causes confusion, with antibacterial elements substituting for vital nutrients and infiltrating bacterial cells. Consequently, these elements disrupt proteins and enzymes, thereby altering the bacterial microenvironment and exerting bactericidal effects. The prominent examples include gallium and cerium [151]. Chen et al. [128] devised a multifaceted injectable composite hydrogel by integrating cerium-containing bioglass (Ce-BG) into a GelMA hydrogel. Ce-BG was synthesised via a fusion of sol-gel and template methods, effectively preserving the spherical morphology, chemical structure, and phase composition of the bioglass. The Ce-BG/GelMA hydrogel showed commendable cellular compatibility and facilitated the migration and tube formation of endothelial cells via Si ion release. In vitro antimicrobial assays revealed exceptional antibacterial attributes of the CeO2-incorporated bioglass/GelMA (5/G) composite hydrogel, particularly with 5 mol % CeO2 content. In vivo investigations revealed that the 5/G hydrogel significantly expedited wound healing in diabetic rats by accelerating granulation tissue formation, collagen deposition, and angiogenesis. Collectively, these findings underscore the therapeutic potential of the 5/G hydrogel in ameliorating diabetic wound healing.
Cationic group
Cationic polymers with positive charges play a pivotal role in the treatment of bacterial infections and various other biomedical applications [152]. They have a remarkable potential to combat antibiotic resistance and impede the formation of bacterial biofilms. Cationic polymers can proficiently dismantle preformed biofilms, and amphiphilic cationic polymers possess the capacity to self-assemble into nanoparticles capable of infiltrating biofilms, thereby enabling the polymers to eradicate both the biofilm and the bacteria residing within them. Vargas-Alfredo et al. [129] delved into the enhancement of GelMA through the incorporation of functional groups with innate antimicrobial properties. They synthesised GelMAQ by introducing modest degrees of substitution of methylacrylamide groups (DSMA) into GelMA and grafting glycidyltrimethylammonium chloride onto the free amino groups of the lysine residues in the original gelatine. Subsequently, they amalgamated GelMA with modified polymers containing elevated DSMA and GelMAQ at ratios of 50/50% or 25/75% (w/w) and juxtaposed them with the control groups of GelMA and GelMA. Chlorhexidine (CHX) was incorporated at a ratio of 0.2%. Various hydrogels were characterized using 1H-NMR spectroscopy and swelling behaviour assessments. These hydrogels were then scrutinised against P. gingivalis to gauge their antimicrobial properties, and their biocompatibility and regenerative attributes in human gingival fibroblasts were evaluated. In wound healing assays, GelMA/GelMAQ 25/75% exhibited commendable antimicrobial efficacy while demonstrating exceptional biocompatibility and regenerative potential in human fibroblasts. These findings offer novel insights into the development of hydrogels with antimicrobial and regenerative properties (Figure 10).
GelMA/GelMAQ hydrogel synthesis. (A) Synthetic chemical route for the GelMA/GelMAQ hydrogel synthesis, using I2959 as a crosslinker under UV irradiation. (B) General experimental procedure to GelMA/GelMAQ hydrogel synthesis. Under Creative Commons Attribution https://creativecommons.org/licenses/by/4.0/ Copyright 2023.
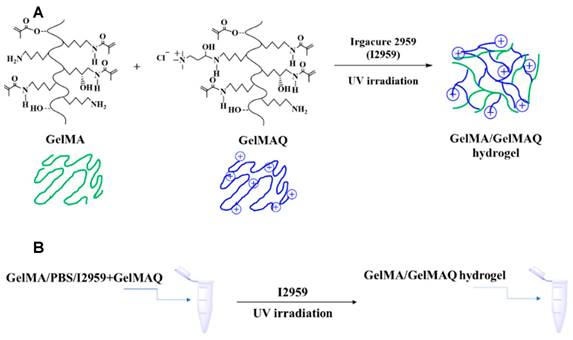
Antibacterial methods based on photothermal therapy
Recently, PTT has garnered widespread attention for its efficacy in addressing bacterial infections owing to its minimal side effects, precise controllability, minimal invasiveness, high selectivity, and resistance to fostering multidrug resistance. Furthermore, research has indicated that photothermal stimulation within the temperature range of 40-42 °C can stimulate bone regeneration, thereby conferring upon PTT a distinctive advantage in treating infectious bone defects [153]. Photothermal agents (PTAs) serve as the cornerstone of PTT, and the judicious selection of an appropriate PTA is pivotal for optimising treatment outcomes. Amid the escalating demand for antimicrobial interventions and the continual advancement of photothermal technology, PTAs have garnered considerable attention. In such systems, the heat generated by the photothermal effect not only inhibits bacterial proliferation, but also fosters tissue regeneration, thus offering dual therapeutic benefits. In the medical field, primary PTAs utilise both inorganic (IPTA) and organic (OPTA) types. IPTAs include metals such as silver (Ag), gold (Au), and copper (Cu), as well as carbon nanoparticles such as graphene oxide (GO) and carbon nanotubes (CNTs). OPTAs chiefly encompass organic dyes (such as Prussian blue [PB] and indocyanine green [ICG]) and polymer nanoparticles (e.g., polypyrrole [Ppy] and polydopamine [PDA]), the majority of which demonstrate commendable degradability and biocompatibility [153].
Inorganic photothermal agents
Metal nanoparticles are favoured for PTT owing to their cost-effectiveness, remarkable photothermal absorption properties, and broad-spectrum antimicrobial efficacy. Recently, considerable attention has been drawn towards composites that integrate metal nanoparticles into hydrogels. These composites exhibited robust stability and maintained an exceptional photothermal performance under specific light conditions. Qiu et al. [130] developed a composite hydrogel incorporating SrCuSi4O10 (SC), a microscale biomimetic ceramic composed of multilayer assembled nanosheets, in conjunction with GelMA. The SC/Gel composite hydrogel displayed proficient near-infrared photothermal conversion capabilities and diverse biological activities attributable to the sustained release of Sr2+, Cu2+, and SiO32- ions. Engineered for the treatment of infected dental pulp, this hydrogel effectively eradicated S. mutans and L. casei while impeding biofilm formation under photothermal heating. Furthermore, SC ion extracts facilitated dentinogenesis in rat dental pulp stem cells and angiogenesis in human umbilical vein endothelial cells (HUVECs). The therapeutic efficacy of the SC/Gel composite hydrogel-mediated vital pulp therapy was corroborated in a rat dental pulp infection model, demonstrating superior dentin-pulp complex repair compared to the commercially available iRoot® BP Plus. He et al. [154] introduced a PAG-CuS hydrogel, a polymer material derived from the copolymerisation of acrylic acid (AA) and GelMA modified with methacryloyl chloride, with a sodium lauroyl sulphate (LAS) coating on copper sulphide nanoparticles (CuS@LAS). This hydrogel has a porous three-dimensional structure conducive to cell adhesion and possesses remarkable water retention capabilities. The presence of CuS@LAS within the hydrogel not only confers photothermal antibacterial properties, but also serves as a physical crosslinker, augmenting its mechanical robustness. Upon near-infrared light-induced photothermal effects, the porous hydrogel released CuS@LAS, which subsequently decomposed via micelle disintegration to neutralise intracellular reactive oxygen species (ROS). This process culminates in the downregulation of MMP-9, which facilitates ECM synthesis and accelerates wound healing. Additionally, released Cu2+ from the PAG-CuS hydrogel augmented CD31 expression in endothelial cells, fostering microvascular formation, which is crucial for wound healing. In a diabetic wound model in GK rats, the application of the PAG-CuS hydrogel significantly mitigated ROS levels, augmented microvascular density, promoted epithelialisation, and expedited wound healing. Hence, this multifaceted photothermal hydrogel has the potential for sterilisation, free radical scavenging, and promotion of angiogenesis, rendering it a potent and comprehensive solution for managing the challenges posed by diabetic wounds.
Organic photothermal agents
OPTAs predominantly encompass organic dyes such as ICG and PB, as well as polymer nanoparticles such as PDA and Ppy [153]. Most OPTAs exhibit excellent biocompatibility and degradability. Despite the therapeutic promise of PTT under certain conditions, its efficacy is constrained by potential tissue damage from excessive heat and logistical hurdles in the storage and delivery of PTAs, which hinder the complete eradication of biofilms via PTT. To mitigate these challenges, Wang et al. [134] introduced a pioneering GelMA-EGF/gelatine-MPDA-LZM dual-layer hydrogel dressing engineered to combat biofilms through PTT bolstered by lysozyme (LZM) while concurrently expediting the healing of chronic wounds. Within this dual-layer hydrogel system, gelatine forms the inner layer, tasked with housing mesoporous polydopamine (MPDA) nanoparticles (MPDA-LZM) laden with lysozyme. Upon temperature elevation, these nanoparticles swiftly liquefy, facilitating substantial release. MPDA-LZM nanoparticles not only function as PTAs with antimicrobial properties but also penetrate and disrupt biofilms. The outer hydrogel layer is comprised of GelMA and EGF, which foster wound healing and tissue rejuvenation. This dual-layer hydrogel dressing exhibits notable anti-infective properties and accelerates wound healing in vivo. Furthermore, nerve innervation plays a pivotal role in bone regeneration, serving as both an instigating factor and a crucial regulator of subsequent processes such as angiogenesis, ossification, and mineralisation. Hence, this innovative dual-layer hydrogel dressing shows promise for advancing bone regeneration and repair (Figure 11).
The preparation process of GelMA-EGF/Gelatin-MPDA-LZM bilayer hydrogel dressing and its application on chronic wounds. Reproduced from reference [135] Copyright 2022, with permission from Elsevier.
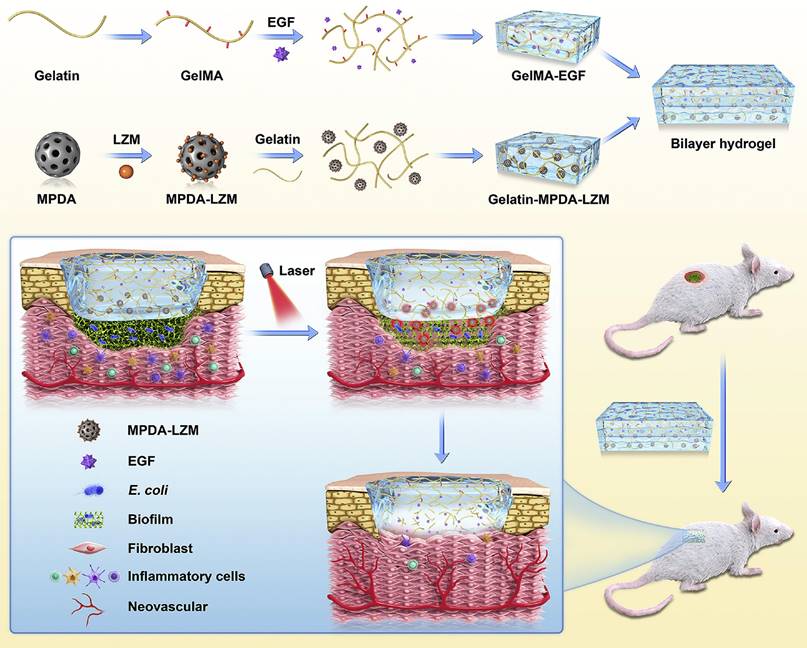
Infectious bone defects treatment strategies
Smart stimuli-responsive GelMA-based strategies
Polymer hydrogels are composed of water-soluble polymers that form a flexible three-dimensional crosslinked network through appropriate crosslinking, resulting in a multiphasic system with water. This system responds accordingly when subjected to environmental stimuli, classifying it as an intelligent polymer material or smart hydrogel [155]. These hydrogels exhibit sensitive responses to external stimuli. In contrast to traditional hydrogels, stimulus-responsive hydrogels demonstrate sensitivity in both spatial and temporal dimensions, enabling products made from these materials to possess multiple, adjustable, and controllable characteristics. The introduction of specific responsive functional groups combined with natural polymers is a key approach to imparting stimulus responsiveness to hydrogels [156]. This section provides an overview of the response strategies of GelMA hydrogels to various stimuli, including those that address internal pathological microenvironmental stimuli (such as pH, ROS, enzymes, and glucose levels) and external physical stimuli (such as thermal, electrical, optical, magnetic, and mechanical forces).
The pH- response strategies
The normal microenvironment of the human body is weakly alkaline with a pH of 7.4 [157]. In the presence of inflammation, infection, or tumours, the pH of affected tissues can be 0.5 to 1 unit lower than that of surrounding healthy tissues [158]. This mildly acidic microenvironment can be exploited to develop GelMA-based reactive biomaterials that promote bone healing and regeneration. Yao et al. [159] introduced oxidised sodium alginate (OSA) into GelMA to create pH-responsive double-network GelMA-OSA hydrogel scaffolds. In these scaffolds, light energy is converted into gentle heating (40°C - 43°C), leading to a reaction between the aldehyde groups in OSA and the amine groups in GelMA, resulting in the formation of Schiff base linkages (-C=N-). Under acidic conditions associated with bacterial infection, the Schiff base linkages in the GelMA-OSA hydrogel break, releasing gentamicin sulphate (GS, an antibacterial agent) and phenylalanine (Phe, a small-molecule activator of BMP-2) encapsulated within the GelMA, thereby exerting antibacterial and osteogenic effects. Additionally, ferritin possesses pH-dependent assembly characteristics, allowing it to be loaded with various substances (such as drugs and DNA) and release them in response to specific pH changes, thereby achieving a pH-responsive targeted drug delivery system (Figure 12).
Schematic diagram of GelMA-OSA hydrogels preparation and fast large bone defect repair. Reproduced from reference [160] Copyright 2022, with permission from Elsevier [144].
Reactive oxygen species-response strategies
ROS are a group of substances generated through the one-electron reduction of oxygen, including superoxide anions, hydrogen peroxide, hydroxyl radicals, and nitric oxide. They play vital roles in various biological processes including cellular signal transduction and metabolism [156]. However, excessive ROS production can lead to oxidative stress, resulting in the degradation of ECM mineralisation and abnormal bone metabolism [156]. Consequently, the development of reactive biomaterials with the capacity to scavenge ROS is crucial for the treatment of bone diseases and regenerative medicine. Bacterial infections can trigger inflammatory responses, leading to the accumulation of ROS and a reduction in local pH levels. In an acidic environment, osteoblast activity is inhibited, posing significant challenges to the repair and regeneration of bone defects. To address this complex microenvironment, researchers such as Qi et al. [160] utilised the unique pH and ROS responsiveness of borate complexes and combined them with GelMA to successfully synthesise a novel smart GelMA hydrogel. This hydrogel was capable of simultaneously delivering two drugs, procainamide and amikacin. The incorporation of the borate complexes improved the mechanical properties, swelling rate, degradation kinetics, and antioxidant capacity of the hydrogel system. Most notably, this hydrogel exhibited dual sensitivity to pH and ROS, enabling the coordinated regulation of drug release. They reported that during the release of proanthocyanidins, the hydrogel responds to changes in ROS levels, thereby protecting mouse osteoblast precursor cells from oxidative stress damage and promoting their differentiation into osteoblasts. Additionally, this hydrogel sensed fluctuations in pH, releasing an appropriate amount of amikacin in a timely manner to exert an effective antibacterial action (Figure 13).
Schematic diagram of GelMA-OSA hydrogels preparation and fast large bone defect repair. (A) smat hydrogel delivery system for dual delivery of proanthocyanidin and amikacin at bone defect interfaces based on the unigue pH and Ros responsiveness of boronate complexes; (B) The hydrogel can timely respond to changes in pH and Ros levels of the bone defect microenvironment, release proanthocyanidin and amikacin on demand to respectively cope with the inflammation and infection problems in the early stage, thus promoting the early healing of bone tissue. Reproduced from reference [160] Copyright 2022, with permission from Elsevier.
Enzyme-response strategies
Enzymes are a class of highly specific and selective biomolecules that play crucial roles in biological processes such as bone growth, resorption, and onset and progression of bone diseases [161]. By exploiting these properties, researchers have developed GelMA hydrogels with intelligent response characteristics that can act as biological triggers. MMPs are a family of enzymes primarily involved in ECM remodelling of the ECM [162]. Studies have shown that the expression levels of MMPs are abnormally elevated in diseases such as osteoarthritis and osteoporosis. The presence of MMP recognition sequences within the GelMA molecules allows for their degradation in environments with high MMP levels. These characteristics render GelMA an ideal material for the development of MMP-responsive drug delivery systems. Ribeiro et al. [163] synthesised a GelMA-based hydrogel that released chlorhexidine (CHX) on demand. When exposed to elevated levels of MMPs, the GelMA hydrogel released CHX, thereby exhibiting antibacterial properties. However, to date, no studies have combined MMP-responsive GelMA hydrogels with osteogenic drugs or growth factors. Therefore, the potential applications of MMP-responsive GelMA hydrogels in the treatment of osteoarthritis and bone tumours and promotion of bone formation require further investigation. The development of such intelligent hydrogel systems not only aids in disease treatment but may also provide new strategies for personalised medicine.
Photo-responsive strategies
PTT utilises PTAs to convert light energy into mild heat (40°C - 43°C), demonstrating significant application potential in the field of bone regeneration [164]. Based on this principle, researchers led by Wu et al. [165] successfully developed a GelMA/poly(methyl methacrylate) (PMMA)/polysaccharide amine nanoparticle (PDA) light-responsive hydrogel. PDA serves as a photothermal conversion agent, effectively transforming light energy into thermal energy under 808 nm laser irradiation. The results indicated that this composite light-responsive hydrogel exhibited excellent osteogenic effects in both the in vitro and in vivo calvarial defect repair experiments (Figure 14). In another study, Nie et al. [60] developed a composite biolink consisting of GelMA/β-tricalcium phosphate (β-TCP)/sodium alginate (Sr2+)/MXene (Ti3C2) (GTAM). The incorporation of MXene endowed this biolink with remarkable photothermal properties, enabling it to generate heat under near-infrared light irradiation, thereby effectively eradicating bacteria. In addition, GTAM biolink promotes osteogenic differentiation. Ultimately, this dual-functional bio-link successfully facilitated the regeneration of infected mandibular defects within bioprinted scaffolds loaded with bone marrow mesenchymal stem cells. These studies highlight the broad application prospects of integrating PTT with biomaterials in bone regenerative medicine.
(A) Preparation of GelMA/PMMA/PDA hydrogel. (B) Illustration of GelMA/PMMA/PDA hydrogel with mild PTT for skull regeneration. Reproduced from reference [165] Copyright 2022, with permission from Elsevier.
Temperature-responsive strategies
Among the various external stimuli, temperature changes are considered an easy-to-use and relatively safe source of stimulation. Temperature-sensitive hydrogels have been widely studied in the field of smart hydrogels. For thermosensitive natural polymer gels, the rapid phase transition near the lower critical solution temperature (LCST) significantly influences the gel state, which has close relevance and broad application potential in medicine and pharmaceuticals. Researchers led by Luo [166] developed a thermo/optically double-crosslinked hydrogel using GelMA and methylmethacrylate-based chitosan (MHBC). As a thermosensitive material, MHBC enables hydrogels to shrink uniformly and reversibly with increasing temperature. GelMA provides excellent biocompatibility to composite hydrogels and enhances cell adhesion. Future research directions for GelMA temperature-responsive hydrogels will focus on achieving precise temperature regulation and flexible temperature control technologies. Although there are limited studies on the application of GelMA hydrogels with temperature-responsive strategies for infectious bone regeneration, these investigations lay the groundwork for future research. It is anticipated that the GelMA composite hydrogels with temperature-responsive properties will selectively release therapeutic drugs or promote active osteogenic molecules in a time- and space-controlled manner, leading to improved treatment outcomes for bone diseases and enhanced bone regeneration.
Electrical response strategies
Polymer electrolyte gels and some composite gels doped with electrical materials exhibit specific responsiveness to electric fields. When hydrogels are placed in an external electric field, they undergo contraction or expansion, accompanied by the conversion of electrical energy into mechanical energy. This characteristic endows hydrogels with multiple functionalities, showing promising application prospects in sensors, controlled drug release, and artificial muscles within the fields of medicine and smart materials. By combining electroactive materials with biomaterials, it is possible to produce "smart" biomaterials that respond to electrical stimulation, enabling more precise regulation of cellular activities and drug release, thereby achieving better bone regeneration and therapeutic effects [167]. Although GelMA hydrogels lack electrical conductivity, they can be combined with conductive polymers such as polypyrrole (PPY) [74] and polyaniline (PANI) [168] to impart responsiveness to electrical stimulation. Dutta et al. [74] introduced Fe ions into GelMA grafted with PPY, adjusting the conductivity of the bio-link to levels comparable to that of cortical bone (approximately 0.2 mS cm-1) and trabecular bone (approximately 0.79 mS cm-1). Piezoelectric hydrogels constitute another category of electrically responsive biomaterials. The piezoelectric effect allows them to convert mechanical forces into electrical stimuli, making them particularly suitable for bone defects in pressurised areas, such as the alveolar and limb bones. Liu et al. [169] fabricated a nanocomposite hydrogel composed of piezoelectric quadrilateral BaTiO₃ nanoparticles and GelMA. Under mechanical stimulation, this hydrogel can generate electrical stimulation, improving the mitochondrial bioenergetic function in periodontal ligament stem cells (PDLSCs) and promoting their osteogenic differentiation.
Although the application of electrically responsive GelMA biomaterials in infectious bone regeneration is currently limited, the aforementioned studies indicate significant application potential for utilizing the electrical stimulation-responsive GelMA in the field of bone regeneration, with expectations for more breakthroughs in the near future.
3D‑Bioprinted GelMA‑Based Strategies
Traditional methods for fabricating tissue engineering scaffolds include porogen (or template) methods, phase separation, gas foaming, and freeze-drying [170]. With the continuous development of clinical requirements and scientific research, these methods face three main challenges: 1. difficulty in flexibly designing and precisely controlling the microscopic structure of scaffolds; 2. difficulty in manufacturing scaffolds with shapes that closely match the injured site; and 3. difficulty in constructing scaffolds with heterogeneous features. Additive manufacturing (3D printing), a computer-assisted forming technique, has the advantage of accurately and rapidly constructing the macromorphology and microstructure of scaffolds, enabling personalised tissue repair and regeneration [38]. The focus of 3D printing research has been on the development of bioinks composed of biomaterials, cells, and functional factors. In addition to traditional requirements such as biocompatibility, mechanical strength, and biodegradability, 3D printing inks must also possess printing adaptability (including structural fidelity, structural resolution, and cell viability) [38]. The rapid gelation, photopolymerization characteristics, and biocompatibility of GelMA meet the requirements of the widely used 3D printing technologies. After the bioprinting solution is extruded from the machine, it undergoes focused UV radiation for an appropriate time, leading to rapid curing under UV light at the desired location [171]. Therefore, owing to its excellent plasticity and ability to conform to defects, GelMA is often combined with 3D printing technology for bone tissue engineering applications to construct scaffolds for more complex bone defect repairs.
Currently, many 3D printing materials for bone repair are prepared using extrusion-based bioprinting methods, which have significant limitations in terms of structural integrity and manufacturing speed for bone substitutes. Tao et al. reported the encapsulation of rBMSCs in GelMA/dextran emulsions using a DLP-based 3D bioprinting platform [59]. DLP is a surface projection-based bioprinting method that offers higher printing resolution and reproducibility than the widely used extrusion printing method. Unlike the "boundaries" formed between droplets in inkjet bioprinting or between adjacent fibres in extrusion printing, DLP technology allows for smoother stacking of three-dimensional structures, significantly enhancing structural integrity and mechanical performance. This 3D hydrogel scaffold has been shown to promote the proliferation, migration, diffusion, and osteogenic differentiation of rBMSCs by modulating the YAP signalling pathway. Furthermore, in-vivo experiments on rat cranial defects have confirmed the therapeutic effects of these scaffolds.
In addition to customising scaffolds for specific bone defect shapes using bioprinting technology, it is essential to focus on the biological functions of printed structures. There have also been attempts to use decellularised matrix hydrogel bioinks. For instance, Yang et al. [172] combined a decellularised ECM (dECM) from pig hair follicles with GelMA to create bioinks. The addition of the dECM provided a supportive microenvironment for the loaded periodontal stem cells, promoting periodontal tissue regeneration (Figure 15). In a recent study, Gao et al. [20] incorporated decellularised pig bone powder into GelMA for use as a bone tissue engineering scaffold. They demonstrated that the scaffolds could induce the osteogenic differentiation of human bone marrow mesenchymal stem cells, even without induced media.
Compared to allogeneic growth factors, autologous growth factors have significant advantages such as high biosafety, low rejection rates, and excellent efficacy, although their sources are relatively limited. Researchers have attempted to use patient-specific biological cues to promote bone regeneration in printed structures. Patient-specific biological cues offer clear advantages, including easy accessibility, avoidance of immune rejection, and reduced ethical regulatory burden in clinical translation. Platelet-rich plasma (PRP) and patient-derived bone particles are currently used to prepare patient-specific bioinks based on GelMA hydrogels. Patient-derived PRP provides abundant autologous growth factors for printed structures, thereby promoting stem cell recruitment, osteogenesis, and vascular formation [173]. The presence of bone particles not only enhances the mechanical properties of printed structures but also provides viable cells and forms a complete mineralised matrix, all of which are beneficial for osteogenesis [174].
Conclusion and prospective
This review describes the latest research progress on GelMA hydrogel-based biomaterials for the treatment of infectious bone defects. First, we briefly introduced the main types of biological materials, along with their advantages and disadvantages. We focused on the synthesis and properties of GelMA hydrogels, followed by a summary of their applications in promoting osteogenesis and exhibiting antibacterial activity. Finally, we discussed the application of GelMA hydrogels for the treatment of infectious bone defects.
Although significant strides have been made in the development of GelMA-based hydrogels, challenges and unresolved issues remain. Future research directions include:
1. Source, Synthesis, and Safety of GelMA: Currently, there is no standardised framework for the origin, synthesis, or safety of GelMA, making it a valuable area of study.
Schematic overview of the 3D bioprinting of periodontal modules with GelMA/dECM bioink encapsulating human DFCs for periodontal regeneration. Under Creative Commons Attribution https://creativecommons.org/licenses/by/4.0/ Copyright 2022.
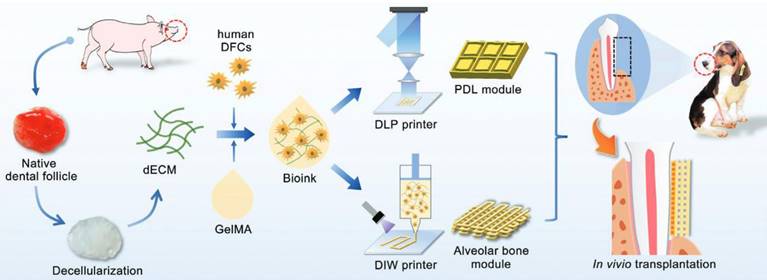
Summary of GelMA-based hydrogel scaffolds for infectious bone regeneration
| GelMA hydrogel scaffolds | Strategies | Bioactivities | Application |
|---|---|---|---|
| GPA hydrogel | ROS- response | promoting early-stage bone healing in infectious bone defect | targeted drug delivery systems [160] |
| GelMA-OSA | PH-response | antibacterial and osteogenic agents | targeted drug delivery systems [159] |
| GelMA-CHX | MMP-response | antibacterial | targeted drug delivery systems [163] |
| GelMA-PMMA-PDA | photo-responsive | osteogenic | bone tissue engineering scaffolds [165] |
| GTAM | photo-responsive | antibacterial and osteogenic agents | bone tissue engineering scaffolds [60] |
| GelMA-MHBC | temperature response | promoting cell adhesion ability | cell culture [166] |
| GelMA-BaTiO3 | electrical response | promoting cell osteogenic differentiation | bone tissue engineering scaffolds [74] |
| GelMA-dextran | DLP | promoting osteogenics | bone tissue engineering scaffolds [59] |
| GelMA-dECM | 3D-bioprinted | promoting periodontal tissue regeneration | bone tissue engineering scaffolds [172] |
| GelMA-bp-PRP | 3D-bioprinted | promoting osteogenics | bone tissue engineering scaffolds [173] |
2. Biodegradation and Stability: GelMA hydrogels suffer from rapid biodegradation and lack long-term stability, resulting in performance loss. Addressing this issue will require adjusting material formulations and improving processing techniques. For example, adding more biodegradable materials, optimising crosslinking, and introducing stabilisers can enhance the biocompatibility, stability, and photothermal effects [11]. Therefore, selecting suitable materials for specific applications is crucial, as addressing these issues is a prerequisite for advancing their clinical application. For instance, a company modified GelMA with silk fibroin to create a new product, Silk Fibroin Methacryloyl (SilMA), which combines the excellent biocompatibility, stable degradation performance, and osteogenic properties of silk fibroin. This product has garnered significant attention owing to its ability to dissolve quickly in water and to photocrosslink into hydrogels [175,176].
3. Cost and Technical Sensitivity: While materials may possess ideal properties, they can be technically sensitive and costly, limiting their feasibility for large-scale production and applications. For example, AgNPs, although highly effective with broad-spectrum antibacterial activity, are relatively expensive and prone to oxidation at room temperature. Many researchers are drawing inspiration from plant-based materials such as A. vera and curcumin, which offer broad availability and low costs. Therefore, optimising production, reducing costs, and ensuring scalability of materials are essential for their widespread use [16,22]
Several promising approaches are worth considering to further optimise construction strategies for GelMA hydrogels:
1. Smart Responsive Antibacterial and Osteogenic Hydrogels: Traditional assembly methods have limitations. Developing microenvironment-responsive materials that possess biodegradability, drug-loading capacity, and anti-infective and anti-inflammatory properties aligns better with clinical needs. To date, no studies have combined GelMA hydrogels with stimuli-responsive mechanisms such as glucose, enzymes, or magnetic fields for infectious bone defects, making this a novel and valuable avenue of research.
2. Piezoelectric Antibacterial Osteogenic Hydrogels: Recently, there has been a growing awareness of the importance of bioelectric communication between cells. Piezoelectric materials are gaining attention because of their ability to generate electrical stimuli under stress, mimic the bioelectric microenvironment of bone tissue, and enhance osteogenic bioactivity [177]. GelMA hydrogels combined with conductive materials offer a promising route for creating multifunctional tissue structures with biocompatibility and piezoelectric properties. Hybrid hydrogels with piezoelectricity and low toxicity represent the next-generation bone tissue engineering scaffolds for treating bone defects.
3. Carbon Quantum Dot (CQD) Antibacterial Osteogenic Hydrogels: CQDs are carbon particles with diameters between 1-10 nm that exhibit fluorescent properties. They have two main advantages: their unique fluorescence makes them ideal for bioimaging, and their simple, environmentally friendly synthesis makes them highly accessible [178]. Researchers have developed p-CQD/WS2 composites and p-CQD/WS2/n-CQD-coated GelMA hydrogels that effectively kill multi-drug resistant bacteria and promote bone regeneration, making them promising solutions for repairing infectious bone defects [132].
In summary, as bone tissue engineering continues to advance rapidly, it is crucial to address the challenges related to its mechanical performance, degradation behaviour, microstructure, bioactivity, and long-term biocompatibility. Despite these challenges, continuous progress in GelMA-based bone tissue engineering technologies suggests that GelMA hydrogel substitutes have great potential for the clinical treatment of infectious bone defects in the near future.
Acknowledgements
We are grateful to the Clinical Research Initiation Plan Project of the Stomatological Hospital, Southern Medical University (grant number: KQIIT2021005); New Technology New Projects of the Stomatological Hospital, Southern Medical University (grant number: NTP20220110703); and the Research and Cultivation Project of the Stomatological Hospital, Southern Medical University (grant number: PY2020029).
Author contributions
LH: conceptualisation, data curation, formal analysis, validation, visualisation, and writing of the original draft. ZYG: formal Analysis, Methodology, Supervision, Validation, Writing, Review, and Editing. XXY: Supervision, Writing, Review, and Editing. YCZ: Writing, Review, and Editing. YYL and XXC Writing, Review, and Editing. XLQ: Funding acquisition, Resources, Writing, Review, and Editing. XC. Funding Acquisition, Resources, Writing, Review, and Editing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zhou J, Zhang Z, Joseph J, Zhang X, Ferdows BE, Patel DN. et al. Biomaterials and nanomedicine for bone regeneration: progress and future prospects. Exploration. 2021;1(2):20210011
2. Liu T, Xu J, Pan X, Ding Z, Xie H, Wang X. et al. Advances of adipose-derived mesenchymal stem cells-based biomaterial scaffolds for oral and maxillofacial tissue engineering. Bioact Mater. 2021;6(8):2467-2478
3. Lopes D, Martins-Cruz C, Oliveira MB, Mano JF. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials. 2018;185:240-275
4. Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66
5. Zhao H, Wei L, Li H, Zhang M, Cao B, Bian J, Zhan S. Appropriateness of antibiotic prescriptions in ambulatory care in China: a nationwide descriptive database study. Lancet Infect Dis. 2021;21(6):847-857
6. Cai C, Wang T, Han X, Yang S, Lai C, Yuan T. et al. In situ wound sprayable double-network hydrogel: preparation and characterization. Chin Chem Lett. 2022;33(4):1963-1969
7. Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6(2):105-121
8. Liang Y, He J, Guo B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. 2021;15(8):12687-12722
9. Malik US, Duan Q, Niazi MBK, Jahan Z, Liaqat U, Sher F. et al. Vanillin cross-linked hydrogel membranes interfacial reinforced by carbon nitride nanosheets for enhanced antibacterial activity and mechanical properties. Chin Chem Lett. 2023;34(4):108071
10. Kurian AG, Singh RK, Patel KD, Lee JH, Kim HW. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact Mater. 2022;8:267-295
11. Gaglio CG, Baruffaldi D, Pirri CF, Napione L, Frascella F. GelMA synthesis and sources comparison for 3D multimaterial bioprinting. Front Bioeng Biotechnol. 2024;12:1383010
12. Su M, Ruan L, Dong X, Tian S, Lang W, Wu M. et al. Current state of knowledge on intelligent-response biological and other macromolecular hydrogels in biomedical engineering: a review. Int J Biol. Macromol. 2023;227:472-492
13. Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254-271
14. Thiele J, Ma Y, Bruekers SMC, Ma S, Huck WTS. 25th anniversary article: designer hydrogels for cell cultures: a materials selection guide. Adv Mater. 2014;26(1):125-147
15. Zhou B, Jiang X, Zhou X, Tan W, Luo H, Lei S. et al. GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: therapeutic strategies and recent advances. Biomater Res. 2023;27(1):86
16. Dong Z, Yuan Q, Huang K, Xu W, Liu G, Gu Z. Gelatin methacryloyl (GelMA)-based biomaterials for bone regeneration. RSC Adv. 2019;9(31):17737-17744
17. Guan S, Zhang K, Cui L, Liang J, Li J, Guan F. Injectable gelatin/oxidized dextran hydrogel loaded with apocynin for skin tissue regeneration. Biomater Adv. 2022;133:112604
18. Kaur H, Gogoi B, Sharma I, Das DK, Azad MA, Pramanik DD. et al. Hydrogels as a potential biomaterial for multimodal therapeutic applications. Mol Pharm. 2024;21(10):4827-4848
19. Feng W, Wang Z. Tailoring the swelling-shrinkable behavior of hydrogels for biomedical applications. Adv Sci. 2023;10(28):e2303326
20. Gao C, Sow WT, Wang Y, Wang Y, Yang D, Lee BH. et al. Hydrogel composite scaffolds with an attenuated immunogenicity component for bone tissue engineering applications. J Mater Chem B. 2021;9(8):2033-2041
21. Yuan N, Shao K, Huang S, Chen C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: a review. Int J Biol Macromol. 2023;240:124321
22. Klotz BJ, Gawlitta D, Rosenberg AJWP, Malda J, Melchels FPW. Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol. 2016;34(5):394-407
23. An S, Jeon EJ, Han SY, Jeon J, Lee MJ, Kim S. et al. pH-universal catechol-amine chemistry for versatile hyaluronic acid bioadhesives. Small. 2022;18(41):e2202729
24. Candry P, Godfrey BJ, Wang Z, Sabba F, Dieppa E, Fudge J. et al. Tailoring polyvinyl alcohol-sodium alginate (PVA-SA) hydrogel beads by controlling crosslinking pH and time. Sci Rep. 2022;12(1):20822
25. Ma Y, Bai T, Wang F. The physical and chemical properties of the polyvinylalcohol/polyvinylpyrrolidone/hydroxyapatite composite hydrogel. Mater. Sci Eng C Mater Biol Appl. 2016;59:948-957
26. Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J. et al. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34(4):421-426
27. Vaissiere G, Chevallay B, Herbage D, Damour O. Comparative analysis of different collagen-based biomaterials as scaffolds for long-term culture of human fibroblasts. Med Biol Eng Comput. 2000;38(2):205-210
28. Xu Q, Torres JE, Hakim M, Babiak PM, Pal P, Battistoni CM. et al. Collagen- and hyaluronic acid-based hydrogels and their biomedical applications. Mater Sci Eng R Rep. 2021;146:100641
29. Lv S, Zhang S, Zuo J, Liang S, Yang J, Wang J, Wei D. Progress in preparation and properties of chitosan-based hydrogels. Int J Biol Macromol. 2023;242(2):124915
30. Zheng H, Zuo B. Functional silk fibroin hydrogels: preparation, properties and applications. J Mater Chem B. 2021;9(5):1238-1258
31. Manjula B, Varaprasad K, Sadiku R, Raju KM. Preparation and characterization of sodium alginate-based hydrogels and their in vitro release studies. Adv Polym Technol. 2013 32(2)
32. Gholamali I, Vu TT, Jo SH, Park SH, Lim KT. Exploring the progress of hyaluronic acid hydrogels: synthesis, characteristics, and wide-ranging applications. Materials. 2024;17(10):2439
33. Yang D. Recent advances in hydrogels. Chem Mater. 2022;34(5):1987-1989
34. Zhu H, Zheng J, Oh XY, Chan CY, Low BQL, Tor JQ. et al. Nanoarchitecture-Integr. Hydrogel Syst. Toward Ther Appl. 2023;17:7953-7978
35. Van Den Bulcke AI, Bogdanov B, De Rooze N, Schacht EH, Cornelissen M, Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1(1):31-38
36. Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol. 2002;37(6):375-536
37. Herrera-Ruiz A, Tovar BB, García RG, Tamez MFL, Mamidi N. Nanomaterials-incorporated chemically modified gelatin methacryloyl-based biomedical composites: A novel approach for bone tissue engineering. Pharmaceutics. 2022;14(12):2645
38. Zhu Y, Yu X, Liu H, Li J, Gholipourmalekabadi M, Lin K. et al. Strategies of functionalized GelMA-based bioinks for bone regeneration: recent advances and future perspectives. Bioact Mater. 2024;38:346-373
39. Huang A, Peng X, Geng L, Zhang L, Huang K, Chen B. et al. Electrospun poly (butylene succinate)/cellulose nanocrystals bio-nanocomposite scaffolds for tissue engineering: preparation, characterization and in vitro evaluation. Polym Test. 2018;71:101-109
40. Xia Y, Chen Z, Zheng Z, Chen H, Chen Y. Nanomaterial-integrated injectable hydrogels for craniofacial bone reconstruction. J Nanobiotechnology. 2024;22(1):525
41. Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI. et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized Phase I/II trial. Stem Cells Transl Med. 2019;8(3):215-224
42. Hoch E, Schuh C, Hirth T, Tovar GEM, Borchers K. Stiff gelatin hydrogels can be photo-chemically synthesized from low viscous gelatin solutions using molecularly functionalized gelatin with a high degree of methacrylation. J Mater Sci Mater Med. 2012;23(11):2607-2617
43. Chen YC, Lin RZ, Qi H, Yang Y, Bae H, Melero-Martin JM. et al. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv Funct Mater. 2012;22(10):2027-2039
44. Li Y, Li X, Chen C, Zhao D, Su Z, Ma G. et al. Sol-gel transition characterization of thermosensitive hydrogels based on water mobility variation provided by low field NMR. J Polym Res. 2017;24(2):25
45. Wang H, Zhou L, Liao J, Tan Y, Ouyang K, Ning C. et al. Cell-laden photocrosslinked GelMA-DexMA copolymer hydrogels with tunable mechanical properties for tissue engineering. J Mater Sci Mater Med. 2014;25(9):2173-2183
46. Sharifi S, Sharifi H, Akbari A, Chodosh J. Systematic optimization of visible light-induced crosslinking conditions of gelatin methacryloyl (GelMA). Sci Rep. 2021;11(1):23276
47. Wang Z, Kumar H, Tian Z, Jin X, Holzman JF, Menard F. et al. Visible light photoinitiation of cell-adhesive gelatin methacryloyl hydrogels for stereolithography 3D bioprinting. ACS Appl Mater Interfaces. 2018;10(32):26859-26869
48. Sakr MA, Sakthivel K, Hossain T, Shin SR, Siddiqua S, Kim J. et al. Recent trends in gelatin methacryloyl nanocomposite hydrogels for tissue engineering. J Biomed Mater Res A. 2022;110(3):708-724
49. Tanadchangsaeng N, Pasanaphong K, Tawonsawatruk T, Rattanapinyopituk K, Tangketsarawan B, Rawiwet V. et al. 3D bioprinting of fish skin-based gelatin methacryloyl (GelMA) bio-ink for use as a potential skin substitute. Sci Rep. 2024;14(1):23240
50. Wang Y, Ma M, Wang J, Zhang W, Lu W, Gao Y. et al. Development of a photo-crosslinking, biodegradable GelMA/PEGDA hydrogel for guided bone regeneration materials. Materials. 2018;11(8):1345
51. Nguyen TU, Watkins KE, Kishore V. Photochemically crosslinked cell-laden methacrylated collagen hydrogels with high cell viability and functionality. J Biomed Mater Res A. 2019;107(7):1541-1550
52. Mironi-Harpaz I, Wang DY, Venkatraman S, Seliktar D. Photopolymerization of cell-encapsulating hydrogels: crosslinking efficiency versus cytotoxicity. Acta Biomater. 2012;8(5):1838-1848
53. Chen S, Wang Y, Lai J, Tan S, Wang M. Structure and properties of gelatin methacryloyl (GelMA) synthesized in different reaction systems. Biomacromolecules. 2023;24(6):2928-2941
54. Wang Y, Cao X, Ma M, Lu W, Zhang B, Guo Y. A GelMA-PEGDA-nHA composite hydrogel for bone tissue engineering. Materials. 2020;13(17):3735
55. Xu F, Wu CAM, Rengarajan V, Finley TD, Keles HO, Sung Y. et al. Three-dimensional magnetic assembly of microscale hydrogels. Adv Mater. 2011;23(37):4254-4260
56. Piraino F, Camci-Unal G, Hancock MJ, Rasponi M, Khademhosseini A. Multi-gradient hydrogels produced layer by layer with capillary flow and crosslinking in open microchannels. Lab Chip. 2012;12(3):659-661
57. Li J, Wang W, Li M, Song P, Lei H, Gui X. et al. Biomimetic methacrylated gelatin hydrogel loaded with bone marrow mesenchymal stem cells for bone tissue regeneration. Front Bioeng Biotechnol. 2021;9:770049
58. Shi Z, Zhong Q, Chen Y, Gao J, Pan X, Lian Q. et al. Nanohydroxyapatite, nanosilicate-reinforced injectable, and biomimetic gelatin-methacryloyl hydrogel for bone tissue engineering. Int J Nanomedicine. 2021;16:5603-5619
59. Tao J, Zhu S, Liao X, Wang Y, Zhou N, Li Z. et al. DLP-based bioprinting of void-forming hydrogels for enhanced stem-cell-mediated bone regeneration. Mater Today Bio. 2022;17:100487
60. Nie R, Sun Y, Lv H, Lu M, Huangfu H, Li Y. et al. 3D printing of MXene composite hydrogel scaffolds for photothermal antibacterial activity and bone regeneration in infected bone defect models. Nanoscale. 2022;14(22):8112-8129
61. Goto R, Nishida E, Kobayashi S, Aino M, Ohno T, Iwamura Y. et al. Gelatin methacryloyl-riboflavin (GelMA-RF) hydrogels for bone regeneration. Int J Mol Sci. 2021;22(4):1635
62. Shen M, Wang L, Gao Y, Feng L, Xu C, Li S. et al. 3D bioprinting of in situ vascularized tissue engineered bone for repairing large segmental bone defects. Mater Today Bio. 2022;16:100382
63. Yu K, Huangfu H, Qin Q, Zhang Y, Gu X, Liu X. et al. Application of bone marrow-derived macrophages combined with bone mesenchymal stem cells in dual-channel three-dimensional bioprinting scaffolds for early immune regulation and osteogenic induction in rat calvarial defects. ACS Appl Mater Interfaces. 2022;14(41):47052-47065
64. Hao J, Bai B, Ci Z, Tang J, Hu G, Dai C. et al. Large-sized bone defect repair by combining a decalcified bone matrix framework and bone regeneration units based on photo-crosslinkable osteogenic microgels. Bioact Mater. 2022;14:97-109
65. Li Z, Li S, Yang J, Ha Y, Zhang Q, Zhou X, He C. 3D bioprinted gelatin/gellan gum-based scaffold with double-crosslinking network for vascularized bone regeneration. Carbohydr Polym. 2022;290:119469
66. Hassan S, Wang T, Shi K, Huang Y, Urbina Lopez ME, Gan K. et al. Self-oxygenation of engineered living tissues orchestrates osteogenic commitment of mesenchymal stem cells. Biomaterials. 2023;300:122179
67. Li Z, Xiang S, Lin Z, Li EN, Yagi H, Cao G. et al. Graphene oxide-functionalized nanocomposites promote osteogenesis of human mesenchymal stem cells via enhancement of BMP-SMAD1/5 signaling pathway. Biomaterials. 2021;277:121082
68. Ghahri T, Salehi Z, Aghajanpour S, Eslaminejad MB, Kalantari N, Akrami M. et al. Development of osteon-like scaffold-cell construct by quadruple coaxial extrusion-based 3D bioprinting of nanocomposite hydrogel. Biomater Adv. 2023;145:213254
69. Wang W, Zhu Y, Li J, Geng T, Jia J, Wang X. et al. Bioprinting EphrinB2-modified dental pulp stem cells with enhanced osteogenic capacity for alveolar bone engineering. Tissue Eng Part A. 2023;29(7-8):244-255
70. Pu X, Tong L, Wang X, Liu Q, Chen M, Li X. et al. Bioinspired hydrogel anchoring 3DP GelMA/HAp scaffolds accelerates bone reconstruction. ACS Appl Mater Interfaces. 2022;14(18):20591-20602
71. Xu Q, Bai Y, Li S, Hou W, Hao Y, Yang R. et al. Enhancing osteogenesis and angiogenesis functions for Ti-24Nb-4Zr-8Sn scaffolds with methacrylated gelatin and deferoxamine. Front Bioeng Biotechnol. 2024;12:1372636
72. Zhu X, Liu H, Mei C, Chen F, Guo M, Wei C. et al. A composite hydrogel loaded with the processed pyritum promotes bone repair via stimulate the osteogenic differentiation of BMSCs. Biomater Adv. 2024;160:213848
73. Lu K, Wang D, Zou G, Wu Y, Li F, Song Q, Sun Y. A multifunctional composite hydrogel that sequentially modulates the process of bone healing and guides the repair of bone defects. Biomed Mater. 2024 19(3)
74. Dutta SD, Ganguly K, Randhawa A, Patil TV, Patel DK, Lim KT. Electrically stimulated 3D bioprinting of gelatin-polypyrrole hydrogel with dynamic semi-IPN network induces osteogenesis via collective signaling and immunopolarization. Biomaterials. 2023;294:121999
75. Chai S, Huang J, Mahmut A, Wang B, Yao Y, Zhang X. et al. Injectable photo-crosslinked bioactive BMSCs-BMP2-GelMA scaffolds for bone defect repair. Front Bioeng Biotechnol. 2022;10:875363
76. Sun X, Ma Z, Zhao X, Jin W, Zhang C, Ma J. et al. Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus. Bioact Mater. 2021;6(3):757-769
77. Liu B, Wu J, Sun X, Meng Q, Zhang J. Sustained delivery of osteogenic growth peptide through injectable photoinitiated composite hydrogel for osteogenesis. Front Bioeng Biotechnol. 2023;11:1228250
78. Yang L, Fan L, Lin X, Yu Y, Zhao Y. Pearl powder hybrid bioactive scaffolds from microfluidic 3D printing for bone regeneration. Adv Sci (Weinh). 2023;10(34):e2304190
79. Zhou X, Xi K, Bian J, Li Z, Wu L, Tang J. et al. Injectable engineered micro/nano-complexes trigger the reprogramming of bone immune epigenetics. Chem Eng J. 2023;462:142158
80. Samorezov JE, Alsberg E. Spatial regulation of controlled bioactive factor delivery for bone tissue engineering. Adv Drug Deliv Rev. 2015;84:45-67
81. Long J, Wang Y, Lu M, Etxeberria AE, Zhou Y, Gu P. et al. Dual-cross-linked magnetic hydrogel with programmed release of parathyroid hormone promotes bone healing. ACS Appl Mater Interfaces. 2023;15(30):35815-35831
82. Li X, Fang S, Wang S, Xie Y, Xia Y, Wang P. et al. Hypoxia preconditioning of adipose stem cell-derived exosomes loaded in gelatin methacryloyl (GelMA) promote type H angiogenesis and osteoporotic fracture repair. J. Nanobiotechnology. 2024;22(1):112
83. Yang Z, Li X, Gan X, Wei M, Wang C, Yang G. et al. Hydrogel armed with Bmp2 mRNA-enriched exosomes enhances bone regeneration. J Nanobiotechnology. 2023;21(1):119
84. Chen Y, Wu Y, Guo L, Yuan S, Sun J, Zhao K. et al. Exosomal Lnc NEAT1 from endothelial cells promote bone regeneration by regulating macrophage polarization via DDX3X/NLRP3 axis. J. Nanobiotechnology. 2023;21(1):98
85. Lu W, Zeng M, Liu W, Ma T, Fan X, Li H. et al. Human urine-derived stem cell exosomes delivered via injectable GelMA templated hydrogel accelerate bone regeneration. Mater Today Bio. 2023;19:100569
86. Hu H, Zhang H, Bu Z, Liu Z, Lv F, Pan M. et al. Small extracellular vesicles released from bioglass/hydrogel scaffold promote vascularized bone regeneration by transferring miR-23a-3p. Int J Nanomedicine. 2022;17:6201-6220
87. Agarwal R, García AJ. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv Drug Deliv Rev. 2015;94:53-62
88. Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. BoneKEy Rep. 2014;3:481
89. McNeely T, Leone M, Yanai H, Beerman I. DNA damage in aging, the stem cell perspective. Hum Genet. 2020;139(3):309-331
90. Shang F, Yu Y, Liu S, Ming L, Zhang Y, Zhou Z. et al. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact Mater. 2021;6(3):666-683
91. Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77(14):2771-2794
92. Toosi S, Behravan J. Osteogenesis and bone remodeling: A focus on growth factors and bioactive peptides. BioFactors. 2020;46(3):326-340
93. Devescovi V, Leonardi E, Ciapetti G, Cenni E. Growth factors in bone repair. Chir Organi Mov. 2008;92(3):161-168
94. Tessmar JK, Göpferich AM. Matrices and scaffolds for protein delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59(4-5):274-291
95. Luginbuehl V, Meinel L, Merkle HP, Gander B. Localized delivery of growth factors for bone repair. Eur J Pharm Biopharm. 2004;58(2):197-208
96. Chen FM, Zhang M, Wu ZF. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31(24):6279-6308
97. Short AR, Koralla D, Deshmukh A, Wissel B, Stocker B, Calhoun M. et al. Hydrogels that allow and facilitate bone repair, remodeling, and regeneration. J Mater Chem B. 2015;3(40):7818-7830
98. Patterson J, Siew R, Herring SW, Lin ASP, Guldberg R, Stayton PS. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials. 2010;31(26):6772-6781
99. Maihöfer J, Madry H, Rey-Rico A, Venkatesan JK, Goebel L, Schmitt G. et al. Hydrogel-guided, rAAV-mediated IGF-I overexpression enables long-term cartilage repair and protection against perifocal osteoarthritis in a large-animal full-thickness chondral defect model at one year in vivo. Adv Mater. 2021;33(16):e2008451
100. Murahashi Y, Yano F, Nakamoto H, Maenohara Y, Iba K, Yamashita T. et al. Multi-layered PLLA-nanosheets loaded with FGF-2 induce robust bone regeneration with controlled release in critical-sized mouse femoral defects. Acta Biomater. 2019;85:172-179
101. Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8(3):147-159
102. Qin B, Dong H, Tang X, Liu Y, Feng G, Wu S. et al. Antisense yycF and BMP-2 co-delivery gelatin methacryloyl and carboxymethyl chitosan hydrogel composite for infective bone defects regeneration. Int J Biol Macromol. 2023;253(5):127233
103. Wang L, Mao J, Cai F, Tang J, Xi K, Feng Y. et al. A structured scaffold featuring biomimetic heterogeneous architecture for the regeneration of critical-size bone defects. Front Bioeng Biotechnol. 2022;10:927050
104. Zhou X, Chen J, Sun H, Wang F, Wang Y, Zhang Z. et al. Spatiotemporal regulation of angiogenesis/osteogenesis emulating natural bone healing cascade for vascularized bone formation. J Nanobiotechnology. 2021;19(1):420
105. Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J. et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182(4):1044-1061.e18
106. Kwon Y, Park C, Lee J, Park DH, Jeong S, Yun CH. et al. Regulation of bone cell differentiation and activation by microbe-associated molecular patterns. Int J Mol Sci. 2021;22(11):5805
107. Hao Z, Chen R, Chai C, Wang Y, Chen T, Li H. et al. Antimicrobial peptides for bone tissue engineering: diversity, effects and applications. Front Bioeng Biotechnol. 2022;10:1030162
108. Clasky AJ, Watchorn JD, Chen PZ, Gu FX. From prevention to diagnosis and treatment: biomedical applications of metal nanoparticle-hydrogel composites. Acta Biomater. 2021;122:1-25
109. Vigata M, O'Connell CD, Cometta S, Hutmacher DW, Meinert C, Bock N. Gelatin methacryloyl hydrogels for the localized delivery of cefazolin. Polymers. 2021;13(22):3960
110. Ayoub AA, Mahmoud AH, Ribeiro JS, Daghrery A, Xu J, Fenno JC. et al. Electrospun azithromycin-laden gelatin methacryloyl fibers for endodontic infection control. Int J Mol Sci. 2022;23(22):13761
111. Qian H, Lei T, Hua L, Zhang Y, Wang D, Nan J. et al. Fabrication, bacteriostasis and osteointegration properties researches of the additively-manufactured porous tantalum scaffolds loading vancomycin. Bioact Mater. 2023;24:450-462
112. Zhang X, Hasani-Sadrabadi MM, Zarubova J, Dashtimighadam E, Haghniaz R, Khademhosseini A. et al. Immunomodulatory microneedle patch for periodontal tissue regeneration. Matter. 2022;5(2):666-682
113. Ribeiro JS, Münchow EA, Bordini EAF, Rodrigues NS, Dubey N, Sasaki H. et al. Engineering of injectable antibiotic-laden fibrous microparticles gelatin methacryloyl hydrogel for endodontic infection ablation. Int J Mol Sci. 2022;23(2):971
114. Ribeiro JS, Daghrery A, Dubey N, Li C, Mei L, Fenno JC. et al. Hybrid antimicrobial hydrogel as injectable therapeutics for oral infection ablation. Biomacromolecules. 2020;21(9):3945-3956
115. Ren D, Zhang Y, Du B, Wang L, Gong M, Zhu W. An antibacterial, conductive nanocomposite hydrogel coupled with electrical stimulation for accelerated wound healing. Int J Nanomedicine. 2024;19:4495-4513
116. Liang C, Wang H, Lin Z, Zhang C, Liu G, Hu Y. Augmented wound healing potential of photosensitive GelMA hydrogel incorporating antimicrobial peptides and MXene nanoparticles. Front Bioeng Biotechnol. 2023;11:1310349
117. Chen Y, Chen Y, Han T, Xie Z, Yang Y, Chen S. et al. Enhanced osteogenic and antibacterial properties of polyetheretherketone by ultraviolet-initiated grafting polymerization of a gelatin methacryloyl/epsilon-poly-L-lysine/laponite hydrogel coating. J Biomed Mater Res A. 2023;111(11):1808-1821
118. Zhang X, Wang W, Chen J, Lai M. Peptide GL13K releasing hydrogel functionalized micro/nanostructured titanium enhances its osteogenic and antibacterial activity. J Biomater Sci Polym Ed. 2023;34(8):1036-1052
119. Dong L, Han Z, Zhang H, Yang R, Fang J, Wang L. et al. Tea polyphenol/glycerol-treated double-network hydrogel with enhanced mechanical stability and anti-drying, antioxidant and antibacterial properties for accelerating wound healing. Int J Biol Macromol. 2022;208:530-543
120. Xiong Y, Xu Y, Zhou F, Hu Y, Zhao J, Liu Z. et al. Bio-functional hydrogel with antibacterial and anti-inflammatory dual properties to combat with burn wound infection. Bioeng Transl Med. 2023;8(1):e10373
121. Khoshmaram K, Yazdian F, Pazhouhnia Z, Lotfibakhshaiesh N. Preparation and characterization of 3D bioprinted gelatin methacrylate hydrogel incorporated with curcumin loaded chitosan nanoparticles for in vivo wound healing application. Biomater Adv. 2024;156:213677
122. Moghtaderi M, Bazzazan S, Sorourian G, Sorourian M, Akhavanzanjani Y, Noorbazargan H. et al. Encapsulation of thymol in gelatin methacryloyl (GelMa)-based nanoniosome enables enhanced antibiofilm activity and wound healing. Pharmaceutics. 2023;15(6):1699
123. Wang F, Sun Q, Li Y, Xu R, Li R, Wu D. et al. Hydrogel encapsulating wormwood essential oil with broad-spectrum antibacterial and immunomodulatory properties for infected diabetic wound healing. Adv Sci (Weinh). 2024;11(3):e2305078
124. Namazi SS, Mahmoud AH, Dal-Fabbro R, Han Y, Xu J, Sasaki H. et al. Multifunctional and biodegradable methacrylated gelatin/Aloe vera nanofibers for endodontic disinfection and immunomodulation. Biomater Adv. 2023;150:213427
125. Xiang J, Bai Y, Huang Y, Lang S, Li J, Ji Y. et al. A zwitterionic silver nanoparticle-incorporating injectable hydrogel with a durable and efficient antibacterial effect for accelerated wound healing. J Mater Chem B. 2022;10(39):7979-7994
126. Haghniaz R, Montazerian H, Rabbani A, Baidya A, Usui B, Zhu Y. et al. Injectable, antibacterial, and hemostatic tissue sealant hydrogels. Adv Healthc Mater. 2023;12(31):e2301551
127. Theus AS, Ning L, Kabboul G, Hwang B, Tomov ML, LaRock CN. et al. 3D bioprinting of nanoparticle-laden hydrogel scaffolds with enhanced antibacterial and imaging properties. iScience. 2022;25(9):104947
128. Chen YH, Rao ZF, Liu YJ, Liu XS, Liu YF, Xu LJ. et al. Multifunctional injectable hydrogel loaded with cerium-containing bioactive glass nanoparticles for diabetic wound healing. Biomolecules. 2021;11(5):702
129. Vargas-Alfredo N, Munar-Bestard M, Ramis JM, Monjo M. Synthesis and modification of gelatin methacryloyl (GelMA) with antibacterial quaternary groups and its potential for periodontal applications. Gels. 2022;8(10):630
130. Qiu Y, Tian J, Kong S, Feng Y, Lu Y, Su L. et al. SrCuSi4 O10 /GelMA Composite Hydrogel-Mediated Vital Pulp Therapy: Integrating Antibacterial Property and Enhanced Pulp Regeneration Activity. Adv. Healthc Mater. 2023;12(24):e2300546
131. Yang Y, Xu C, Xu S, Li Y, Chen K, Yang T. et al. Injectable hydrogels activated with copper sulfide nanoparticles for enhancing spatiotemporal sterilization and osteogenesis in periodontal therapy. Biomater Sci. 2024
132. Geng B, Li P, Fang F, Shi W, Glowacki J, Pan D. et al. Antibacterial and osteogenic carbon quantum dots for regeneration of bone defects infected with multidrug-resistant bacteria. Carbon. 2021;184:375-385
133. Wang Y, Nie X, Lv Z, Hao Y, Wang Q, Wei Q. A fast hemostatic and enhanced photodynamic 2-dimensional metal-organic framework loaded aerogel patch for wound management. J Colloid Interface Sci. 2024;656:376-388
134. Wang Y, Lv Q, Chen Y, Xu L, Feng M, Xiong Z. et al. Bilayer hydrogel dressing with lysozyme-enhanced photothermal therapy for biofilm eradication and accelerated chronic wound repair. Acta Pharm Sin B. 2023;13(1):284-297
135. Lin J, He Z, Liu F, Feng J, Huang C, Sun X, Deng H. Hybrid hydrogels for synergistic periodontal antibacterial treatment with sustained drug release and NIR-responsive photothermal effect. Int. J. Nanomedicine. 2020;15:5377-5387
136. Huang S, Liu H, Liao K, Hu Q, Guo R, Deng K. Correction to "functionalized GO nanovehicles with nitric oxide release and photothermal activity-based hydrogels for bacteria-infected wound healing". ACS Appl.Mater Interfaces. 2023;15(38):45536-45537
137. Liu H, Li Q, Xu Y, Sun Y, Fan X, Fang H. et al. Dual-light defined in situ oral mucosal lesion therapy through a mode switchable anti-bacterial and anti-inflammatory mucoadhesive hydrogel. Biomater Sci. 2023;11(9):3180-3196
138. Jing X, Xu C, Su W, Ding Q, Ye B, Su Y. et al. Photosensitive and conductive hydrogel induced innerved bone regeneration for infected bone defect repair. Adv. Healthc. Mater. 2023;12(3):e2201349
139. Roy S, Rhim J-W. Gelatin-based film integrated with copper sulfide nanoparticles for active packaging applications. Applied Sciences. 2021;11(14):6307
140. Boot W, Schmid T, D'Este M, Guillaume O, Foster A, Decosterd L. et al. A hyaluronic acid hydrogel loaded with gentamicin and vancomycin successfully eradicates chronic methicillin-resistant Staphylococcus aureus orthopedic infection in a sheep model. Antimicrob. Agents Chemother. 2021;65(4):e01840-20
141. Zhang QY, Yan ZB, Meng YM, Hong XY, Shao G, Ma JJ. et al. Antimicrobial peptides: mechanism of action, activity and clinical potential. Mil Med Res. 2021;8(1):48
142. Li S, Wang Y, Xue Z, Jia Y, Li R, He C, Chen H. The structure-mechanism relationship and mode of actions of antimicrobial peptides: a review. Trends Food Sci Technol. 2021;109:103-115
143. Li S, Jiang S, Jia W, Guo T, Wang F, Li J. et al. Natural antimicrobials from plants: recent advances and future prospects. Food Chem. 2024;432:137231
144. Teshome E, Forsido SF, Rupasinghe HPV, Olika Keyata E. Potentials of natural preservatives to enhance food safety and shelf life: a review. The Sci World J. 2022;2022:9901018
145. Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM. et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano Micro Lett. 2015;7(3):219-242
146. Guo L, Kong W, Che Y, Liu C, Zhang S, Liu H. et al. Research progress on antibacterial applications of metal-organic frameworks and their biomacromolecule composites. Int J Biol Macromol. 2024;261(2):129799
147. Bisht N, Dwivedi N, Kumar P, Venkatesh M, Yadav AK, Mishra D. et al. Recent advances in copper and copper-derived materials for antimicrobial resistance and infection control. Curr. Opin. Biomed Eng. 2022;24:100408
148. Robinson DA, Griffith RW, Shechtman D, Evans RB, Conzemius MG. In vitro antibacterial properties of magnesium metal against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Acta Biomater. 2010;6(5):1869-1877
149. Dong J, Lin T, Shao H, Wang H, Wang X, Song K. et al. Advances in degradation behavior of biomedical magnesium alloys: a review. J Alloys Compd. 2022;908:164600
150. Guo Z, Zhang Z, Zhang N, Gao W, Li J, Pu Y. et al. A Mg2+/polydopamine composite hydrogel for the acceleration of infected wound healing. Bioact Mater. 2022;15:203-213
151. Shi F, Ma S, Liu S, Xin R, Chen B, Ye W. et al. A novel antimicrobial strategy for bacterial infections: gallium-based materials. Colloids Interface Sci Commun. 2023;56:100735
152. Yang N, Wei L, Teng Y, Yu P, Xiang C, Liu J. Cyclodextrin-based metal-organic frameworks transforming drug delivery. Eur J Med Chem. 2024;274:116546
153. Egloff-Juras C, Bezdetnaya L, Dolivet G, Lassalle HP. NIR fluorescence-guided tumor surgery: new strategies for the use of indocyanine green. Int J Nanomedicine. 2019;14:7823-7838
154. He D, Liao C, Li P, Liao X, Zhang S. Multifunctional photothermally responsive hydrogel as an effective whole-process management platform to accelerate chronic diabetic wound healing. Acta Biomater. 2024;174:153-162
155. Montoya C, Du Y, Gianforcaro AL, Orrego S, Yang M, Lelkes PI. On the road to smart biomaterials for bone research: definitions, concepts, advances, and outlook. Bone Res. 2021;9(1):12
156. Wei H, Cui J, Lin K, Xie J, Wang X. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res. 2022;10(1):17
157. Hubbell JA. Enhancing drug function. Science. 2003;300(5619):595-596
158. Karimi M, Eslami M, Sahandi-Zangabad P, Mirab F, Farajisafiloo N, Shafaei Z. et al. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdiscip. Rev Nanomed Nanobiotechnology. 2016;8(5):696-716
159. Yao Q, Liu Y, Pan Y, Li Y, Xu L, Zhong Y. et al. Long-term induction of endogenous BMPs growth factor from antibacterial dual network hydrogels for fast large bone defect repair. J Colloid Interface Sci. 2022;607(2):1500-1515
160. Qi H, Wang B, Wang M, Xie H, Chen C. A pH/ROS-responsive antioxidative and antimicrobial GelMA hydrogel for on-demand drug delivery and enhanced osteogenic differentiation in vitro. Int J Pharm. 2024;657:124134
161. Zelzer M, Todd SJ, Hirst AR, McDonald TO, Ulijn RV. Enzyme responsive materials: design strategies and future developments. Biomater Sci. 2013;1(1):11-39
162. Paiva KBS, Granjeiro JM. Bone tissue remodeling and development: focus on matrix metalloproteinase functions. Arch Biochem Biophys. 2014;561:74-87
163. Ribeiro JS, Bordini EAF, Ferreira JA, Mei L, Dubey N, Fenno JC. et al. Injectable MMP-responsive nanotube-modified gelatin hydrogel for dental infection ablation. ACS Appl Mater Interfaces. 2020;12(14):16006-16017
164. Wan Z, Zhang P, Lv L, Zhou Y. NIR light-assisted phototherapies for bone-related diseases and bone tissue regeneration: A systematic review. Theranostics. 2020;10(25):11837-11861
165. Wu Y, Zhang X, Tan B, Shan Y, Zhao X, Liao J. Near-infrared light control of GelMA/PMMA/PDA hydrogel with mild photothermal therapy for skull regeneration. Biomater Adv. 2022;133:112641
166. Luo X, Liu Y, Pang J, Bi S, Zhou Z, Lu Z. et al. Thermo/photo dual-crosslinking chitosan-gelatin methacrylate hydrogel with controlled shrinking property for contraction fabrication. Carbohydr Polym. 2020;236:116067
167. Leppik L, Oliveira KMC, Bhavsar MB, Barker JH. Electrical stimulation in bone tissue engineering treatments. Eur J Trauma Emerg Surg. 2020;46(2):231-244
168. Hsu HH, Liu Y, Wang Y, Li B, Luo G, Xing M. et al. Mussel-inspired autonomously self-healable all-in-one supercapacitor with biocompatible hydrogel. ACS Sustainable Chem Eng. 2020;8(18):6935-6948
169. Liu X, Wan X, Sui B, Hu Q, Liu Z, Ding T. et al. Piezoelectric hydrogel for treatment of periodontitis through bioenergetic activation. Bioact Mater. 2024;35:346-361
170. Jang J-W, Min K-E, Kim C, Shin J, Lee J, Yi S. Correction: review: scaffold characteristics, fabrication methods, and biomaterials for the bone tissue engineering. Int. J. Precis. Eng. Manuf. 2023;24(5):887-887
171. Wang X, Jiang M, Zhou Z, Gou J, Hui D. 3D printing of polymer matrix composites: a review and prospective. Compos B Eng. 2017;110:442-458
172. Yang X, Ma Y, Wang X, Yuan S, Huo F, Yi G. et al. A 3D-bioprinted functional module based on decellularized extracellular matrix bioink for periodontal regeneration. Adv Sci (Weinh). 2023;10(5):e2205041
173. Jiang G, Li S, Yu K, He B, Hong J, Xu T. et al. A 3D-printed PRP-GelMA hydrogel promotes osteochondral regeneration through M2 macrophage polarization in a rabbit model. Acta Biomater. 2021;128:150-162
174. Ratheesh G, Vaquette C, Xiao Y. Patient-specific bone particles bioprinting for bone tissue engineering. Adv Healthc Mater. 2020;9(23):e2001323
175. Choi JR, Yong KW, Choi JY, Cowie AC. Recent advances in photo-crosslinkable hydrogels for biomedical applications. BioTechniques. 2019;66(1):40-53
176. Van Hoorick J, Tytgat L, Dobos A, Ottevaere H, Van Erps J, Thienpont H. et al. (Photo-)crosslinkable gelatin derivatives for biofabrication applications. Acta Biomater. 2019;97:46-73
177. Vijayakanth T, Shankar S, Finkelstein-Zuta G, Rencus-Lazar S, Gilead S, Gazit E. Perspectives on recent advancements in energy harvesting, sensing and bio-medical applications of piezoelectric gels. Chem Soc Rev. 2023;52(17):6191-6220
178. Alafeef M, Srivastava I, Aditya T, Pan D. Carbon dots: from synthesis to unraveling the fluorescence mechanism. Small. 2024;20(4):e2303937
Author contact
![]() Corresponding authors: Xiaoling Qiu, Email: linglingjojocom; Xuan Chen, Email: 406625227com.
Corresponding authors: Xiaoling Qiu, Email: linglingjojocom; Xuan Chen, Email: 406625227com.
 Global reach, higher impact
Global reach, higher impact