13.3
Impact Factor
Theranostics 2025; 15(2):384-407. doi:10.7150/thno.101697 This issue Cite
Review
Recent advances in ferrocene-based nanomedicines for enhanced chemodynamic therapy
1. Department of Hepatopancreatobiliary Surgery, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan 421001, China.
2. Center for Molecular Imaging Probe of Cancer Research Institute, Hengyang Medical School, University of South China, Hengyang, Hunan 421001, China.
3. Department of general Surgery, Turpan City People's Hospital, Tulufan 838000, China.
Received 2024-7-31; Accepted 2024-9-25; Published 2025-1-1
Abstract
Malignant tumors have been a serious threat to human health with their increasing incidence. Difficulties with conventional treatments are toxicity, drug resistance, and recurrence. For this reason, non-invasive treatment modalities such as photothermal therapy (PTT), photodynamic therapy (PDT), chemodynamic therapy (CDT), and others have received much attention. Among them, Ferrocene (Fc)-based nanomedicines for enhanced Chemodynamic Therapy (ECDT) is a new therapeutic strategy based on the Fenton reaction. Based on ferrocene's good biocompatibility, potentiation in medicinal chemistry, and good stability of divalent iron ions, scientists are increasingly using it as a Fenton's iron donor for tumor therapy. Such ferrocene-based ECDT nanoplatforms have shown remarkable promise for clinical applications and have significantly increased the efficacy of CDT treatment. Ferrocene-based nanomedicines exhibit exceptional consistency owing to their low toxicity, high stability, enhanced bioavailability, and a multitude of advantages over conventional approaches to cancer treatment. As a consequence, a number of tactics have been investigated in recent years to raise the effectiveness of ferrocene-based ECDT. In this review, we detail the different forms and strategies used to enhance Ferrocene-based ECDT efficiency.
Keywords: Ferrocene, Enhanced Chemodynamic therapy (ECDT), Synergistic therapy, Nanomedicine
Introduction
The incidence of malignant tumors is surging, with the number of malignant tumor patients increasing to 28.4 million by 2040, posing a great danger to human health [1]. The first-line cancer clinical treatments, including surgery, chemotherapy (CT), and radiotherapy, could partly cure the primary tumor while causing irreversible harm to the patient's body organs and tissues, leading to decreased immunity, severe pain, drug resistance, uncontrolled metastasis, and other adverse effects [2-9]. Therefore, the rational design of non-invasive, efficient, precise, and biologically safer cancer treatment methods is of great significance for the future development of cancer therapy.
Recently, anticancer research has been gradually focusing on some emerging technologies, such as photothermal therapy (PTT) [10-15], photodynamic therapy (PDT) [16-20], gas therapy (GT) [21-27], and chemodynamic therapy (CDT) [28-34]. For instance, Ding et al. have created novel compounds, such as PFW-DOX/glucose oxidase (GOD) [35] and SPN-TAPP-PCB4 [36], which have expanded the possibilities for cancer detection and therapy. Among them, CDT is an innovative treatment strategy for sufficiently inhibiting tumor growth. CDT utilizes the Fenton/Fenton-like reaction that could in situ convert intratumoral hydrogen peroxide (H2O2) into highly toxic hydroxyl radicals (•OH) by transition metal ions in a weakly acidic environment (typical reaction scheme: Fe2+ + H2O2 → Fe3+ + •OH + OH-) [37-43]. The •OH is the most reactive among the reactive oxygen species, which results in DNA damage and protein denaturation to cause senescence, death, and carcinogenesis of tumor cells [44-46]. Notably, the Fenton/Fenton-like reaction would not work in normal tissues due to their neutral pH and insufficient H2O2 concentration, thus allowing for highly selective tumor therapy [47-49]. Therefore, CDT has several manifest superiorities: low invasiveness, superior selectivity, and fewer adverse effects. Nevertheless, there are still some problems that hinder the further development of CDT. First, the Fenton/Fenton-like reaction should have exerted its highest stage in an acidic environment (pH at 2-4), while the pH of solid tumors is at approximately 6.5, constraining an efficient intratumoral Fenton/Fenton reaction [37, 50, 51]. Second, the limited H2O2 level inside the cancerous tissue cannot afford to produce •OH continuously, thereby rendering individual CDT treatments ineffectual. Moreover, antioxidants within cells possess a powerful scavenging effect on short-lived, highly reactive •OH and diffusion distance, further limiting the antitumor efficiency. In addition, persistent harm to tumor cells in a short amount of time by •OH depends on its generation rate, which is mainly limited by the performance of the catalyst [52-54]. Therefore, optimizing the effectiveness of CDT by designing innovative catalysts and promoting their composition and organization would be beneficial to potential clinical cancer therapy [55].
Metallocene is an organometallic complex by doping metal ions into organic scaffolds. The ferrocene as the typical of metallocene was discovered in 1951 and determined with a sandwich structure [Fe(η5-C5H5)2] by Wilkinson and Fischer owing to the formation of a symmetrical covalent bond with the d-orbitals of Fe2+ cation, which were awarded the Nobel Prize in chemistry in 1973 [56, 57]. The non-toxic and lipophilic nature of ferrocene makes it an attractive core for grafting with bioactive pharmacophores to influence their biological activity and confer drug-like properties. With the development of nanotechnology in medicinal chemistry, cancer nanomedicines based on nanomaterials and devices to realize drug delivery, diagnosis and imaging, and synthetic vaccine development open a new chapter for the human fight against cancer. Ferrocene-based nanomedicine has been extensively reported in the biomedical field owing to the nature of low toxicity, high stability, and reversible redox in non-oxidizing environments [58, 59]. Ferrocene-based nanomedicine mainly triggers the generation of •OH to exert anticancer effects by the Fenton reaction between the ferrocene group and H2O2 in the tumor microenvironment. In turn, the process of reaction would result in the creation and build-up of cytotoxic quinone-methide, which causes DNA damage or apoptosis via an intracellular redox event [60]. Furthermore, the moiety of ferrocene increases the lipid solubility of the overall nanomedicine and facilitates their delivery to the desired targets [55]. However, single CDT therapy based on ferrocene-based nanomedicine has not been very effective for complete tumor elimination. Therefore, scientists are currently focusing on enhanced chemodynamic therapy (ECDT) based on ferrocene-based nanomedicine. The use of ferrocene in ECDT combination therapy presents both challenges and opportunities. The main challenges are that ferrocene as an organic small molecule is difficult to apply to biological systems, and the selective delivery of Fe2+ to tumor sites is also challenging, and this has become a challenge for the ECDT application of ferrocene molecules [61, 62]. However, at the same time, the iron cyclopentadienyl (Fe-Cp) bond has a large dissociation energy (≈ 90 kcal mol-1), which is less prone to be oxidized to the trivalent state (Fe3+) than the ionic state of Fe2+ [63]. This unique advantage makes ferrocene-based nanomedicines uniquely advantageous for ECDT biologic applications.
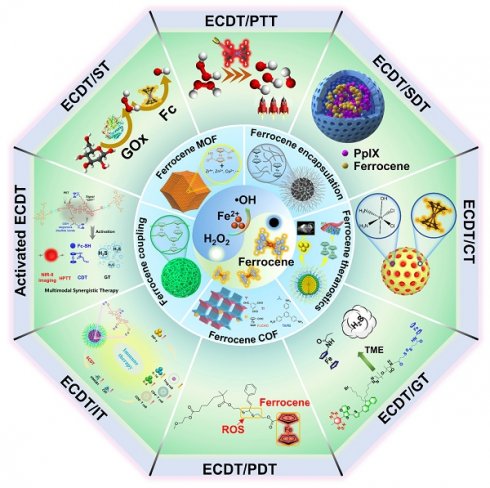
Considering the significance of ECDT anticancer tactics and the existing gaps in reviews in the field, there is a pressing requirement for a thorough analysis of the most recent developments in ferrocene-based nanomedicines for ECDT. In this work, we first summarize the recent significant progress in different ferrocene-based nanomedicines with different forms of ferrocene molecules, such as ferrocene-encapsulated nanoparticles, ferrocene-coupled nanoparticles, ferrocene-based nanotheranostics, ferrocene-based metal-organic frameworks (MOF), and ferrocene-based covalent organic frameworks (COF) (Scheme 1). Then, we comprehensively review the recent remarkable advances in ferrocene-based ECDT synergistically with other therapeutic models and explicitly describe the design advantages and possibilities for the clinical application of the state-of-the-art ferrocene-based nanoplatforms. Finally, we exhaustively analyze the existing challenges, constraints, and potential advancements of ferrocene-based nanomedicines for ECDT.
2. Different kinds of ferrocene-based nanomedicines for CDT
In recent years, inspired by the development in nanotechnology and in-depth comprehension of the ECDT mechanism [31], researchers have attempted to construct efficient and precise ferrocene-based nanomedicines in chemodynamic cancer therapy. However, to the best of our knowledge, there is no comprehensive review for exploring the structural forms of different ferrocene-based nanomedicines and their effects. Therefore, this section will specifically discuss the structure-activity relationship between the newly developed ferrocene-based nanomedicines and tumor therapy applications based on them. These types of ferrocene-based nanomedicines are summarized as follows: ferrocene-encapsulated nanomedicines, ferrocene-coupled nanomedicines, ferrocene-based nanotheranostics, ferrocene-based metal-organic frameworks (MOF), and ferrocene-based covalent organic frameworks (COF).
2.1 Ferrocene-encapsulated nanomedicines
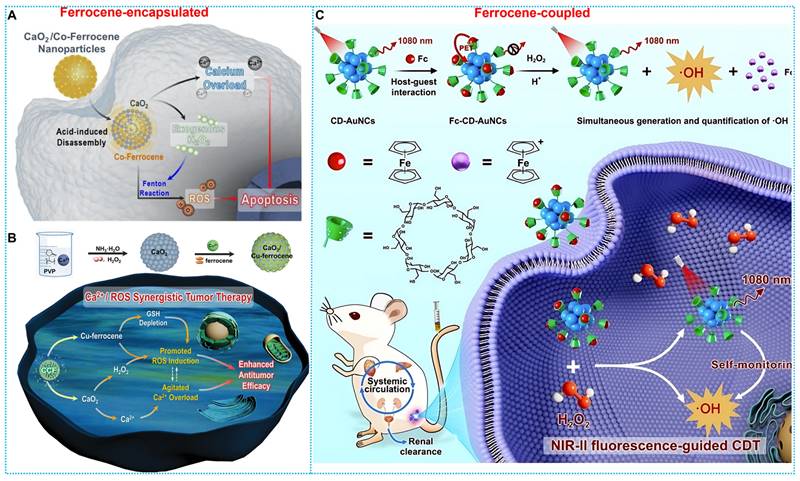
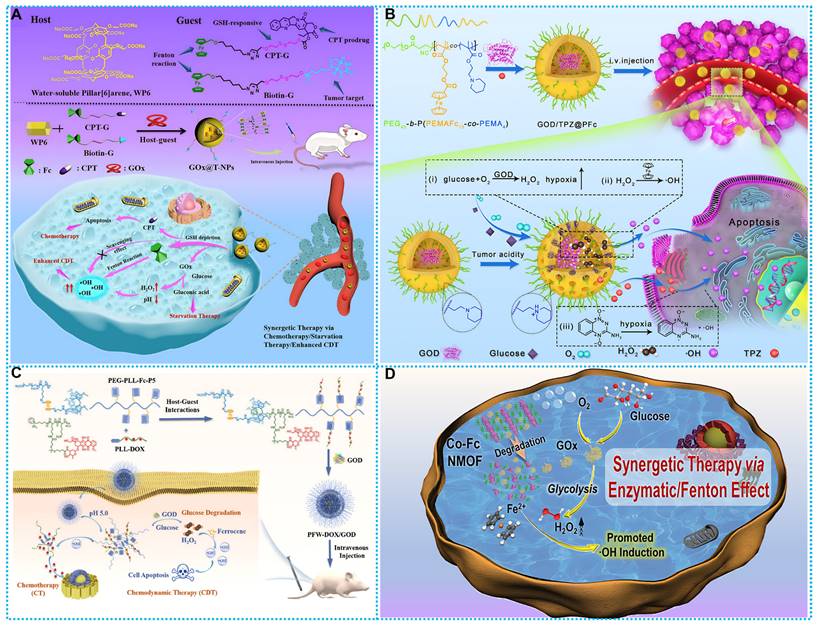
Based on the good biosafety and stability of ferrocene, researchers have developed ferrocene-based nanomedicines by encapsulating ferrocene in the form of loading or self-assembly. After these ferrocene-based nanomedicines enter the tumor cells, the ferrocene molecules would be released and react with H2O2 within the tumor to generate •OH to induce cell apoptosis. For instance, Zhou and colleagues developed a ferrocene-based nanoplatform CaO2@Co-Fc, which could decompose CaO2 and produce large quantities of H2O2. This strategy resulted in calcium overload and ferrocene-based CDT at the tumor site, and the combined effect resulted in a favorable tumor-suppressive effect (Figure 1A) [64]. Another typical paradigm is reported by Kong and coworkers, who developed CaO2 nanoparticles that are loaded with Cu-ferrocene molecules at the surface (CaO2/Cu-ferrocene, CCF). The synergistic effect of the three metal ions, copper, iron, and calcium, produced a good tumor suppression effect (Figure 1B) [65]. Furthermore, Lin and Chen's team [66] designed host-guest supramolecular Fc-CD-AuNCs for NIR-II fluorescence-guided cancer CDT by integrating the Fenton, redox, and PET activities of Fc (Figure 1C).
The schematic illustration for the type of Ferrocene-based nanomedicines and applications of enhanced CDT.
(A) Synthesis and therapeutic mechanisms of CaO2/Co-Ferrocene NPs. Reproduced with permission from ref [64]. Copyright 2021, Springer. (B) Working principle and preparation of CaO2/Cu-ferrocene NPs. Reproduced with permission from ref [65]. Copyright 2021, Wily. (C) Synthesis route and workflow of Fc-CD-AuNCs. Reproduced with permission from ref [66]. Copyright 2024, Wily.
2.2 Ferrocene-coupled nanomedicines
The encapsulated-typed ferrocene usually confronts the leakage problem during the preparation, storage, and subsequent internal circulation process of nanomedicines, which aggravates the concern about biological safety and reduces the eventual treatment effect. Moreover, the Fenton or Fenton-like reaction could generate •OH by catalyzing H2O2 with highly dynamic low-valence Fenton metal ions while easily converting to high-valence (e.g., Fe2+ to Fe3+ and Cu+ to Cu2+) state with low activity after reaction [67]. In this process, the leakage of metal ions becomes a key factor in affecting the efficiency of the Fenton reaction and clinical conversion [68]. Chemically coupled ferrocene-based nanomedicines provide significant biosafety optimization as well as the potential to address molecular stability and leakage. Therefore, the chemical coupling strategy is employed to improve the efficiency of the Fenton reaction by increasing the single-molecule ferrocene loading and avoiding molecular leakage. In addition, although exhibiting excellent ferrous ion stability and low toxicity, ferrocene's poor biocompatibility and hydrophobicity could not afford its clinical conversion application. Consequently, Strategies for coupling ferrocene to amphiphilic bioactive can effectively enhance the ability to change the hydrophobic properties of ferrocene organic molecules and thus improve their applicability in biological environments.
For instance, Ding and colleagues synthesized two polymer-modified ferrocene-based nanomedicines named PFW-DOX/GOD (DOX: doxorubicin, GOD: glucose oxidase), which exhibited minimal adverse effects and high accumulation at the tumor site. The disruption of the pH-sensitive benzoicimine bond will cause the dismantling of PFW-DOX/GOD. The declared GOD catalyzes the breakdown of intratumoral glucose to produce a significant amount of gluconic acid and H2O2, which both deplete the tumor's energy source and generate hydroxyl radicals via the Fenton reaction [35]. Another typical example is reported by Xu and coworkers, who developed a self-immolated amphiphilic poly(ferrocenes) named BPnbs-Fc, which is made up of self-immolated framework and aminoferrocene (AFc) side chains as well as end-capping poly (ethylene glycol) monomethyl ether. Following the tumor's acidic environment, the BPnbs-Fc nanoparticles break down easily, releasing components of azaquinone methide (AQM), which may effectively exhaust glutathione (GSH) to cascade discharge AFc. Thereby raising the risk of oxidative damage to the cancerous cells [69]. In addition, the team led by Sun has created a DNA-adjuvant hydrogel-optimized enzymatic cascade that accurately regulates the distance between glucose oxidase and ferrocene, which has resulted in a substantial improvement in the production of reactive oxygen species (ROS), leading to more efficient destruction of tumor cells. This DNA-adjuvant hydrogel-optimized enzymatic cascade has the potential to effectively treat solid tumors by enhancing the combined effects of CDT and immunotherapy [70].
2.3 Ferrocene-based phototheranostic platform
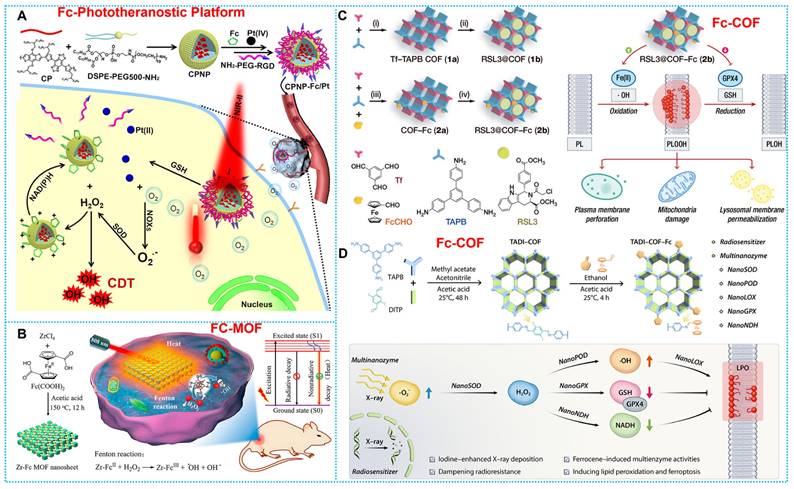
The ferrocene-based nanomedicines play an indispensable role in cancer therapy. Before initiating treatment of various cancers, it is essential to carry out diagnostic imaging to understand the cellular phenotype(s) and heterogeneity of the tumor. On this concept, the construction of ferrocene-based nanotheranostics is emerging as a revolutionary and promising method for cancer therapy. The clinical interventions used today are out of step with the clinical diagnosis, restricting tumor treatment's effectiveness and precision to some degree [71]. The ultimate goal of ferrocene-based nanotheranostics is to gain the ability to image and monitor the cancer tissue, delivery kinetics, and treatment efficacy with the long-term hope of gaining the ability to tune the therapy. In light of its excellent specificity and sensitivity, fluorescence imaging (FLI) has generated a great deal of interest in the diagnostic field [72-74]. The biological imaging window has steadily increased from the visible light area (400-700 nm) to the first area of near-infrared (NIR-I, 700-900 nm) and now to the second near-infrared region (NIR-II, 1000-1700 nm). Consequently, making use of integrated cancer theranostics is essential for combating cancer and raising the chances of recovery and survival for patients. Combined ferrocene-based therapeutic agents with NIR-II imaging connect therapeutic approaches with deep-tissue diagnostic imaging in a synergistic way that promises simultaneous precision therapy and early cancer detection [75, 76]. Hence, Wei and coworkers established a rare-earth-nanocrystal (RENC)-based Fe2+ transfer device using light-control strategies and DNA nanotechnology to achieve controllable Fe2+ distribution. RENC's unique design provides the transport framework with the capacity to both regulate and diagnose deliveries [77]. In addition, organic conjugated fluorescent molecules, with their excellent luminescence performance and carbon structure of good biocompatibility, are also excellent diagnoses and treatments. For example, Wu and coworkers developed IR-FEP-RGD-S-S-S-S-Fc, a NIR-II organic phototheranostic platform activated by the high GSH concentration Tumor microenvironment. The intrinsic fluorescence of IR-FEP-RGD-S-S-S-S-Fc is removed via photoinduced electron transfer (PET) between NIR-II fluorescence cores with ferrocene, and the fluorescence signal is triggered when the cleavable linker unit breaks in the presence of the high GSH concentration Tumor microenvironment. This "activatable" biotargeting NIR-II organic photodiagnostic platform reduces the unanticipated toxicity of the substance and provides a theoretical framework for the creation of highly accurate tumor-targeting combination therapy approaches [78]. Furthermore, He et al. constructed a nano-amplifier CPNP-Fc/Pt by self-assembly of conjugated polymer nanoparticles (CPNPs) modified with ferrocene (Fc) and cisplatin prodrugs (Pt (IV)). The CPNPs showed a strong photothermal response when exposed to light at 1064 nm. Meanwhile, Fe (II) in Fc exhibits more readily reversible redox properties compared to free Fe2+; thus, intracellular nicotinamide adenine nucleotide phosphate (NADPH) can regenerate Fc by reducing the Fenton reaction product, Fe (III) to Fe (II). The effective restoration of Fc further enhanced the CDT (Figure 2A) [79].
2.4 Ferrocene-based metal-organic frameworks (MOFs)
The first MOFs were created with a kind of organic-inorganic porous material by the coordination between metal ions and organic ligands [80]. The movable framework, high porosity, facile functionalization, and stimuli-responsiveness of metal-organic frameworks (MOFs) have expanded their potential biological applications [81], encompassing bioimaging [82], antitumor [83], anti-bacterial [84], and biocatalysis [85]. Concretely, MOFs' variable selection towards coordinated metal ions and induced sensitivity to the TME make them ideal options for CDT agents. Meanwhile, MOFs' excess porosity and transparency allow them to be employed as transporters for a variety of species, from medicines to diagnostics, where the release of the functional molecules enhances the start and enhancement of CDT under the influence of the TME. Moreover, MOFs may be used as direct chemodynamic agents when their building blocks are active compounds with Fenton/Fenton-like reaction capabilities [86-88]. MOFs were usually employed as carriers by employing their porosity for delivering and releasing a variety of functional molecules [89, 90]. Ferrocene derivatives (such as 1,1'-Ferrocenedicarboxylic acid (Fc(COOH)2) as ligands can form multifunctional MOF nanomaterials by binding multiple metal ions, showing good potential for tumor therapy. For instance, Fang et al. constructed a Co-Fc NMOF incorporated with GOx molecules named Co-Fc@GOx. In this platform, along with the plentiful H2O2 production and the reduction in pH by GOx catalysis, the limiting parameters of the Fenton reaction were thoroughly optimized [91]. Another typical paradigm is reported by Deng and coworkers, who developed a therapeutic platform that combines PTT and CDT in harmony via the Zr-Fc MOF nanosheet application. The Fc(COOH)2 ligand endows the Zr-Fc MOF nanosheet with catalytic activity to facilitate the production of •OH for CDT (Figure 2B) [92]. The studies mentioned above indicated that the loose and porous structural units of MOFs possess beneficial compounds with Fenton/Fenton-like reactivity. This fundamentally enhances the efficiency of the Fenton reaction of ferrocene. Additionally, this unique property also allows for the possibility of modifying and loading multiple substances, thereby improving the therapeutic effectiveness of Ferrocene-based nanomedicines through multiple synergistic effects.
(A) Working principle and preparation of CPNPs. Reproduced with permission from ref [79]. Copyright 2021, ACS. (B) Synthesis and therapeutic mechanisms of Zr-Fc MOF. Reproduced with permission from ref [92]. Copyright 2020, ACS. (C) Synthesis route and workflow of RSL3@COF-Fc. Reproduced with permission from ref [93]. Copyright 2021, Wily. (D) Schematic diagram of therapeutic mechanisms of TADI-COF-Fc. Reproduced with permission from ref [94]. Copyright 2023, Wiley-VCH.
2.5 Ferrocene-based covalent organic frameworks (COFs)
Being different from metal-organic frameworks (MOFs), COFs belong to a new type of crystalline porous materials, which are composed of light elements and linked by robust covalent bonds. COFs have garnered a lot of interest in biological research because of their high surface area, changeable pore size, and low density [60]. Based on this concept, COFs have been employed by Fenton/Fenton-like catalyst doping in Ferrocene-based nanomedicines. For instance, Zhou et al. developed a COF-based therapeutic system called RSL3@COF-Fc. Once RSL3@COF-Fc is endocytosed by tumor cells, the progressively released methyl (1S,3R)-2-(2-chloroacetyl)-1-(4-(methoxycarbonyl)phenyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylate (RSL3) inhibits GPX4 (a central checkpoint of the antioxidant system of the tumor cells) to perturb the redox homeostasis, whereas the Fc induces the production of •OH via Fenton-like reactions, leading to lipid peroxidation (Figure 2C) [93]. Another typical example is developed by Zhou et al., who designed a multifunctional COF nanozyme (TADI-COF-Fc), which features iodine atoms and ferrocene (Fc)-based for use in treating radioresistant esophageal carcinoma in conjunction with radiation therapy. TADI-COF-Fc not only generates multiple reactive oxygen species (ROS) in situ but furthermore produces H2O2 from superoxide, which results in decreased nicotinamide adenine dinucleotide (NADH) dehydrogenation, lipid peroxidation, glutathione (GSH) oxidation, and the generation of •OH. Eventually, increment in cancer cell death (Figure 2D) [94]. The above investigations have verified that crystalline porous structural units in COFs, similar to MOFs, exhibit enormous activity as compounds with Fenton/Fenton-like reactivity. COFs composed of light elements possess desirable characteristics such as high specific surface area, variable pore size, and low density. These properties enable multiple modification options and excellent biocompatibility for the Ferrocenyl Fenton reaction.
3. Ferrocene-based synergetic enhanced chemodynamic therapy
Based on the above design mechanisms and synthesis methods, different types of ferrocene-based ECDT nanoplatforms have been established and have become a hot area for tumor therapy combined with other efficient modalities and strategies, such as ECDT in combination with starvation therapy (ST), ECDT in combination with chemotherapy, ECDT in combination with PTT, ECDT in combination with PDT, ECDT in combination with tumor TEM-activated therapy, ECDT in combination with Sonodynamic Therapy (SDT) and gene therapy, ECDT in combination with immunotherapy (IT), and ECDT in combination with gas therapy (GT). In addition, Chen and Lin's team found that ECDT can be achieved through various strategies such as modulation of cell membrane unsaturation and down-regulation [95] of ferritin heavy chain [96]. We present the latest Ferrocene-based nanomedicine types, ECDT mechanisms, and related enhancement strategies categorized in Table 1.
3.1 ECDT in combination with ST
Starvation therapy is mainly used to “starve” tumor cells to death by blocking the nutritional supply to the tumor. Most works have focused on harnessing the potential of GOx-based catalytic reactions for CDT co-enhancement strategies [112-116], which provides a non-invasive strategy for cancer therapy to cut off the energy source of the tumor [117, 118]. Moreover, the generation of H2O2 via the glucose oxidase (GOx)-mediated catalytic pathway significantly amplifies tumor oxidative stress, thereby inducing the destruction of cancer cells [112, 119]. In addition, the generation of gluconic acid would decrease the intratumor pH to amplify the CDT effects.
Summary of Nanomedicine Platform centered on Ferrocene-based ECDT.
| Ferrocene-based Nanomedicines | Type of Nanomedicines | Mechanism | Advantages and Shortcomings | In vivo | Administration Method | Enhance Strategies | Reference |
|---|---|---|---|---|---|---|---|
| GOx@T-NPs | Ferrocene-encapsulated nanomedicines | Amplifying H2O2, decreasing pH | Three-modality therapy is novel but lacks validation of tumor recurrence or not. | HT-29 cells | Intravenous injection | ST/CDT | [97] |
| GOD/TPZ@PFc | Ferrocene-coupled nanomedicines | Amplifying H2O2, decreasing pH | Tumor acid response nanoreactors exhibit better tumor targeting ability but lack studies on related mechanisms. | H22 cells | Intravenous injection | ST/CDT | [98] |
| PFW-DOX/GOD | Ferrocene-coupled nanomedicines | Photothermal energy conversion, amplifying H2O2, decreasing pH | Self-Immolative amphiphilic poly(ferrocenes) for synergistic amplification of oxidative stress in tumor therapy. | HeLa cells | Intravenous injection | ST/CT/CDT | [35] |
| Co-Fc NMOF | Ferrocene-based Metal-organic Frameworks (MOFs) | Amplifying H2O2, decreasing pH | Unique cascade enzyme/Fenton effect that promotes kinetic chemotherapy treatment, but long-term toxicity needs to be considered. | 4T1 cells | Intravenous injection | ST/CDT | [91] |
| G-b-PPLGFc@Dox | Ferrocene-coupled nanomedicines | Amplifying H2O2 and depletion of GSH | Ferrocene-based polymeric nanoparticles carrying doxorubicin for combination of chemotherapy and ferroptosis, but Lack of studies on related mechanisms. | DU145 cells | Intravenous injection | CDT/CT | [99] |
| cis-CD-Fc | Ferrocene-coupled nanomedicines | Amplifying H2O2 | PCSNs exhibited excellent biocompatibility and minimized side effects, which would be more convincing if validated with organoid models. | 4T1 cells | Intravenous injection | CDT/CT | [100] |
| Nut@FSSG | Ferrocene-coupled nanomedicines | Depletion of GSH | Nut@FSSG case apoptosis by the p53 activation, leading to good combination therapy with GEM in PDAC treatment | Panc02 cells | Intravenous injection | CDT/CT | [101] |
| FcNEt-F4 NPs | Ferrocene-coupled nanomedicines | Thermal acceleration and GSH depletion | Nut@FSSG leads to apoptosis by activating p53, thus forming a good combination therapy with GEM in PDAC treatment, but lacks validation of tumor recurrence or not | 4T1 cells | Intravenous injection | PTT/CDT | [102] |
| Fc-HP/HD/GOx | Ferrocene-coupled nanomedicines | Photothermal energy conversion, amplifying H2O2, and decreasing pH | Fc-HP cross-linked injectable hydrogels demonstrate shear-thinning behavior, self-healing properties, and high feasibility in clinical applications but need to advance preclinical studies | 4T1 cells | Peritumoral injection | PTT/CDT/ ST | [103] |
| Lac-FcMOF | Ferrocene-encapsulated nanomedicines | Thermal acceleration and GSH depletion | The Lac-FcMOF complex demonstrates efficient targeting of HepG2 cells, and its porous structure provides excellent capacity for delivering drugs. However, it requires more extensive validation to ensure its biosafety. | HepG2 cells | Peritumoral injection | MMHT/CDT | [104] |
| Ce6-CD / Fc-pep-PEG | Ferrocene-based phototheranostic platform | Thermal acceleration | Fc-pep-PEG recombined to nanofibers, and Ce6-CD maintained the form of a spherical micelle with a smaller size, enhancing the penetration and retention, but Lack of Studies on Related Mechanisms. | 4T1 cells | Intravenous injection | PTT/CDT/IT | [105] |
| PCF-PDP NPs | Ferrocene-coupled nanomedicines | GSH depletion | PCF-PDP NPs could achieve ROS cascade-amplifying through the positive feedback loop for dual-dynamic cascade cancer therapy but need to advance preclinical studies | 4T1 cells | Intravenous injection | PDT/CDT | [106] |
| BPnbs-Fc | Ferrocene-coupled nanomedicines | GSH depletion | BPnbs-Fc exhibits exceptional photothermal conversion efficiency by the integration of a stiff planar donor-acceptor structure with a molecular rotor, but Lack of Studies on Related Mechanisms | 4T1 cells | Intravenous injection | CDT and TEM-activated therapy | [69] |
| IR-FEP-RGD-S-S-S-Fc | Ferrocene-based phototheranostic platform | Thermal acceleration, GSH depletion, and accelerated Fenton cycling. | The therapeutic benefits of IR-FEP-RGD-S-S-S-Fc are achieved by a four-mode activation design, although molecular-level mechanistic research is absent. | 4T1 cells | Intravenous injection | HPTT/CDT/GT and TEM-activated therapy | [78] |
| PpIX@MFc | Ferrocene-coupled nanomedicines | GSH depletion | PpIX@MFc were constructed to realize the controlled production of Fe2+, •OH, and 1O2 in the tumor site, and non-invasive deep-penetration ultrasound. , but need to advance preclinical studies. | 4T1 cells | Intravenous injection | SDT/CDT | [63] |
| RENC | Ferrocene-based phototheranostic platform | GSH depletion and accelerated Fenton cycling. | DNA-modified RENCs work as a programmable remoter, providing a practical strategy for the safe and efficient delivery of Fe2+, showing great significance for the clinical applications of CDT, but need to advance preclinical studies | 4T1 cells | Intravenous injection | CDT and gene therapy | [77] |
| N@Fc/IM | Ferrocene-coupled nanomedicines | GSH depletion. | N@Fc/IM stimulates anti-tumor immunity by triggering a potent Fenton reaction, yet a single treatment approach still has limitations. | 4T1 cells | Intravenous injection | CDT/IT | [107] |
| IR-FE-Fc@DSPE-S-S-PEG | Ferrocene-based phototheranostic platform | GSH depletion | The IR-FE-Fc@DSPE-S-S-PEG compound demonstrates innovative approaches to iron-induced cell death, but the molecular mechanisms need to be investigated. | 4T1 cells | Intravenous injection | PTT/CDT/IT | [108] |
| Nanobombig | Ferrocene-coupled nanomedicines | GSH depletion | The Nanobombig system exhibits enhancing cell toxicity in tumors, but more research is required to assess its biosafety. | CT26 cells | Intravenous injection | CDT/IT | [109] |
| FTEB-TBFc | Ferrocene-based phototheranostic platform | Thermal acceleration, GSH depletion, and accelerated Fenton cycling. | FTEB-TBFc has developed diagnostic approaches that are triggered by several modes. However, a thorough safety evaluation is still lacking. | 4T1 cells | Intravenous injection | HPTT/PDT/CDT/GT and TEM-activated therapy | [110] |
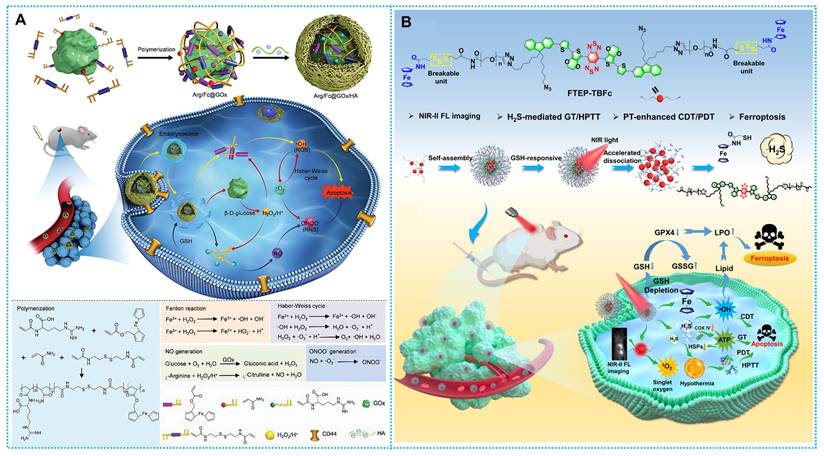
| Arg/Fc@GOx/HA | Ferrocene-coupled nanomedicines | Thermal acceleration, GSH depletion, and accelerated Fenton cycling. | The Arg/Fc@GOx/HA stimulates the generation of reactive oxygen and nitrogen species (RONS) free radicals inside cancer cells, effectively suppressing the growth of tumors while little affecting healthy tissues. | 4T1 cells | Intravenous injection | ST/CDT/GT | [111] |
(A) Synthesis and therapeutic mechanisms of GOx@T-NPs. Reproduced with permission from ref [97]. Copyright 2021, ACS. (B) Working principle and preparation of GOD/TPZ@PFc. Reproduced with permission from ref [98]. Copyright 2021, Elsevier. (C) Synthesis route and workflow of PFW-DOX/GOD. Reproduced with permission from ref [35]. Copyright 2022, Elsevier. (D) Schematic diagram of therapeutic mechanisms of Co-Fc NMOF. Reproduced with permission from ref [91]. Copyright 2020, Wiley-VCH.
Drawing from these exhilarating advantages, Liu and coworkers successfully prepared a columnar [6]-fluorene-based supramolecular GOx@T-NPs for rapidly facilitating the transformation of glucose to gluconic acid and H2O2. The multimodal synergistic effect of ferrocene-initiated CDT, starvation therapy ST (GOx), and chemotherapy (CPT) was achieved (Figure 3A) [97]. However, this work could not avoid the problem that the hypoxic tumor microenvironment constrains the efficiency of H2O2 generation. Further, Wang et al. developed a synergistic pH-responsive polymerset nanoreactor of glucose oxidase (GOD) and tirapazamine (TPZ), GOD/TPZ@PFc, which not only generates the highly toxic •OH through ferrocene-based Fenton reaction but also produces •OH from the anticancer prodrug TPZ, under hypoxic conditions. The antitumor medication TPZ may be distributed and enabled to create more •OH when the hypoxic situation develops after O2 is depleted (Figure 3B) [98].
Similarly, Ding et al. developed a supramolecular peptide nano-drug (PFW-DOX/GOD). The coating of PEG sections and mean hydrodynamic diameter of 200 nm of PFW-DOX/GOD resulted in significant tumor retention with little adverse effect. In an internal acidic environment, PFW-DOX/GOD exhibits pH-triggered disintegration, distributing GOD, which then catalyzes intratumoral glucose into H2O2. After that, the Fenton reaction transforms sufficiently H2O2 into •OH, which is highly toxic to CDT. This strategy activated free doxorubicin (DOX) and glucose degradation to achieve synergistic antitumor effects of CDT and CT (Figure 3C) [35]. Further, nanoscale ferrocene copolymer MOF was first discovered by Fang and coworkers. Co-Fc NMOF showed unique Fenton activity, which was mainly derived from Fe2+ containing ferrocene. The unique cascade enzyme/Fenton effect facilitated CDT and ST. Demonstrated superior ability in cancer cell inhibition and tumor regression (Figure 3D) [91].
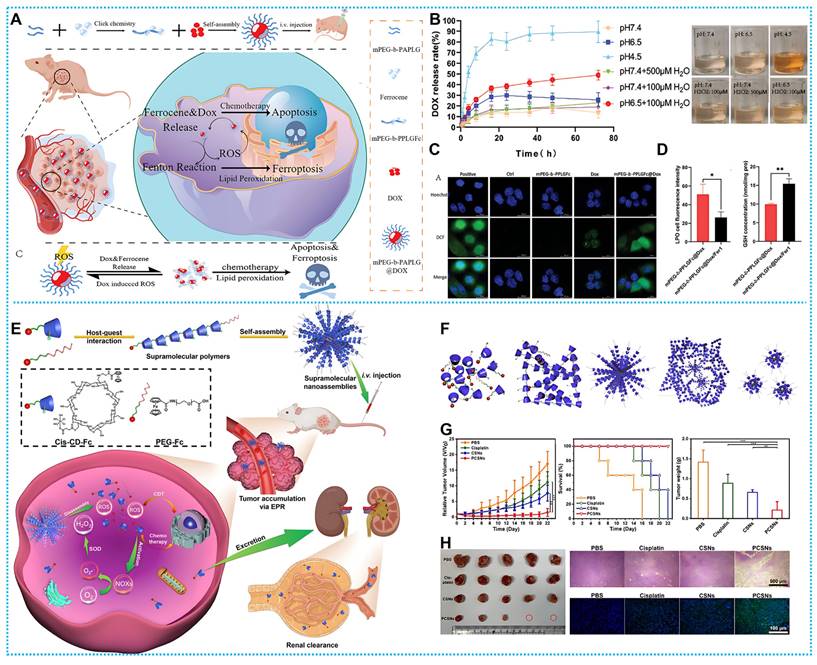
(A) Synthesis process and therapeutic mechanisms of mPEG-b-PPLGFc@Dox. (B) The dox release rate of mPEG-b-PPLGFc@Dox and variations in coloring of the liquids after 72 hours. (C) The degree of ROS in various treatments (D) LPO and GSH quantities in various treatment conditions. Reproduced with permission from ref [99]. Copyright 2023, Wiley-VCH. (E) Working principle and preparation of cis-CD-Fc. (F) Representative TEM images of cis-CD-Fc. (G) The tumor volume, survival, and final tumor weight curves. (H) photographs of the dissected tumors and H&E, TUNEL staining image. Reproduced with permission from ref [100]. Copyright 2021, Wiley-VCH.
3.2 ECDT in combination with CT
Chemotherapeutic agents with in situ self-supply of H2O2 are ideal supplements to replenish CDT. Some anticancer chemotherapeutic drugs, such as doxorubicin (DOX) and cisplatin, could produce •O22- and subsequently converted by superoxide dismutase (SOD) to form H2O2, thus expecting to serve as an effective support for CDT [120-122]. Packaging strategies for the co-delivery of CDT agents with chemotherapeutic drugs inevitably suffer from insufficient drug loading, premature leakage, and limited reproducibility, leading to catalytic effects of H2O2 in cells and inefficiency [40, 123]. In addition, chemotherapy agents used in clinical settings often lack selectivity and sensitivity towards tumor cells, leading to toxicity in normal cells, multidrug resistance (MDR), suboptimal pharmacokinetic profiles, and other limitations that hinder treatment efficacy, consequently resulting in severe adverse effects. Therefore, it is expected to construct ferrocene-based nanoplatforms incorporating chemotherapeutic drugs to decrease the adverse effects of chemotherapy on healthy tissues and enhance the CDT efficiency on tumor tissues.
Based on the above design concepts, Lin and colleagues designed mPEG-b-PPLGFc@Dox, an amphiphilic polymeric nanoparticle (Figures 4A). After the mPEG-b-PPLGFc@Dox reached tumor cells, endogenous H2O2 degraded it, releasing Dox and upsetting the redox equilibrium (Figures 4B). Fe (II) produced from ferrocene initiated the Fenton reaction, which resulted in ·OH and ferroptosis (Figures 4C-D). The absence of stimulus reactivity in normal tissue decreased Dox's organ toxicity [99]. In addition to DOX, platinum drugs in combination with ferrocene have also reported very successful paradigms. For instance, Wang's team developed an H2O2-responsive nanomedicine cis-CD-Fc. Self-assembled cis-CD-Fc monomers with Fenton catalyst Fc as the guest and CD as the host (Figures 4E-F). The cis-CD-Fc obtained exhibits a substantial drug loading capacity and allows for the adjustable ratio of platinum (IV) complexes to Fc. Therefore, the medication is released by means of a sequential process of generating and dissolving reactive oxygen species (ROS) (Figures 4G-H) [100].
There are also cases of synergistic application of classical chemotherapeutic agents, such as Liang's team developed a small molecule prodrug (Nut@FSSG) consisting of Fc and Gemcitabine (GEM). In TME, FSSG can trigger iron death to generate LPO, leading to apoptosis. Overexpression of GSH triggers the cleavage of disulfide bonds in FSSG, which induces the decomposition of nano prodrugs, activates GEM, and releases nutlin-3a. The released nutlin-3a not only activates the p53 signaling pathway to enhance apoptosis of GEM but also inhibits cystine uptake and promotes iron death of Fc. FSSG achieves co-delivery of the cystine uptake inhibitor, Nutlin-3a, with the chemotherapeutic drug, GEM, and the iron-death drug, Fc, in the nano-remedy, which decreases chemoresistance in GEM and the pancreatic ductal adenocarcinoma (PDAC) treatment yielded good anti-tumor efficiency [101].
3.3. ECDT in combination with thermotherapeutic
Thermotherapy is a non-invasive cancer treatment that is primarily based on external energy sources such as photos or magnets that cause an elevation of the internal temperature of the cancerous tissue. When the local temperature of the tumor increased to over 42 °C, malignant cells could be ablated sufficiently [124]. Thermotherapeutic also boosts the catalytic efficiency of ferrocene-based Fenton reaction. The therapeutic concept of combining ferrocene-based ECDT with Thermotherapeutic is proposed by constructing ferrocene-based nanomaterials. Notably, PTT causes an area-specific temperature rise that significantly speeds up the circulation inside the tumor., thereby increasing the oxygen level in the tumor region and alleviating hypoxic conditions to facilitate CDT owing to the Fenton reaction strongly depends on the involvement of oxygen for the generation of H2O2 [125].
(A) Enhanced the Fc-based complex and its anti-tumor mechanism through schematic representation. Reproduced with permission from ref [102]. Copyright 2022, RSC. (B) Schematic diagram of therapeutic mechanisms of Fc-HP/HD/GOx. Reproduced with permission from ref [103]. Copyright 2022, Elsevier. (C) Schematic description of the fabrication and anticancer mechanism of Lac-FcMOF. Reproduced with permission from ref [104]. Copyright 2024, Wily.
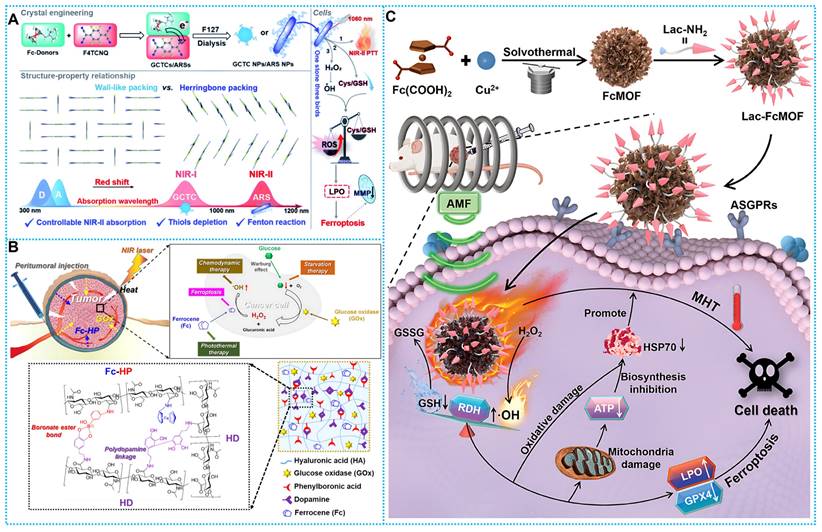
Based on this concept, Dong's team fabricated a variety of complexes utilizing F4TCNQ, employing ferrocene and its derivatives as electron donors. These ferrocenyl charge-transfer complexes (CTCs) exhibit photophysical properties that are contingent upon their stacking behavior. Notably, anion-radical salts (ARS) NPs like FcN-F4, FcNEt-F4, and FcNOH-F4 NPs exhibit a linear dimer configuration with close π-π stacking distances. This arrangement confers upon the nanoparticles a remarkable NIR-II absorptivity and exceptional photothermal effects upon exposure to 1060 nm laser irradiation. Furthermore, ARS NPs showcase characteristics conducive to the biothiol/H2O2 cascade reaction. This prompts GSH depletion, accumulation of ROS/LPO, and facilitates ROS-mediated cellular iron death. These discoveries stem from the crystal engineering of CTCs and offer valuable insights into the mechanisms governing optimal NIR-II uptake (Figure 5A) [102].
However, conventional ECDT/PTT needs to be performed multiple times. Thus, reducing the frequency of administration can improve patient compliance. Based on this concept, Lee et al. developed crosslinked injectable hydrogels based on opioid amine polymerization Fc-HP/HD/Gox. In anticancer applications, Fc and GOx can be sustainably released from the crosslinked hydrogel reactor system, and it contributes to effective tumor growth inhibition. Hydrogels containing Fc-HP/HD/GOx, when absorbed by a tumor following exposure to NIR laser, might potentially enhance the efficacy of breast cancer therapy by enabling a more precise control method, including ST/CDT/PTT/ferroptosis. (Figure 5B) [103] Further, Magnetic thermotherapeutic methods have proven to be highly effective in delivering heat to targeted areas. Pei's research team has pioneered a groundbreaking advancement by developing the first-of-its-kind lactose derivative-modified paramagnetic FcMOF, termed Lac-FcMOF. Lac-FcMOF exhibits dual functionality, possessing both magneto-thermal properties and exceptional thermal stability. Lac-FcMOF demonstrates remarkable efficacy in targeting HepG2 cells and modulating bi-directionally regulated RDH, a crucial pathway in cellular function. In addition, the porous nature of FcMOF provides enhanced drug-carrying properties in comparison to other ferrite materials. This presents new opportunities for integrating magnetothermal therapy with other therapeutic modalities, therefore providing a viable strategy for synergistic tumor treatment (Figure 5C) [104].
3.4. ECDT in combination with PDT
Photodynamic therapy (PDT) depends on laser irradiation at a specific wavelength to excite the photosensitizer delivered into the tissue. The photosensitizer in the excited state transfers the energy to the surrounding oxygen, then generates highly reactive ROS to induce oxidative reactions with neighboring biomolecules to cause cellular damage and even death. Unlike oxygen-dependent PDT, ferrocene-based CDT operates irrespective of local oxygen levels. Moreover, the initiation of ferrocene-based CDT relies on endogenous chemical stimulation instead of external energy input, thereby circumventing rapid energy dissipation [126]. Furthermore, the key challenge of PDT combined with ferrocene-based CDT therapy is the TME's low oxygen level and restricted supply of hydrogen peroxide. Consequently, this field has emerged as a focal point for scientists to investigate.
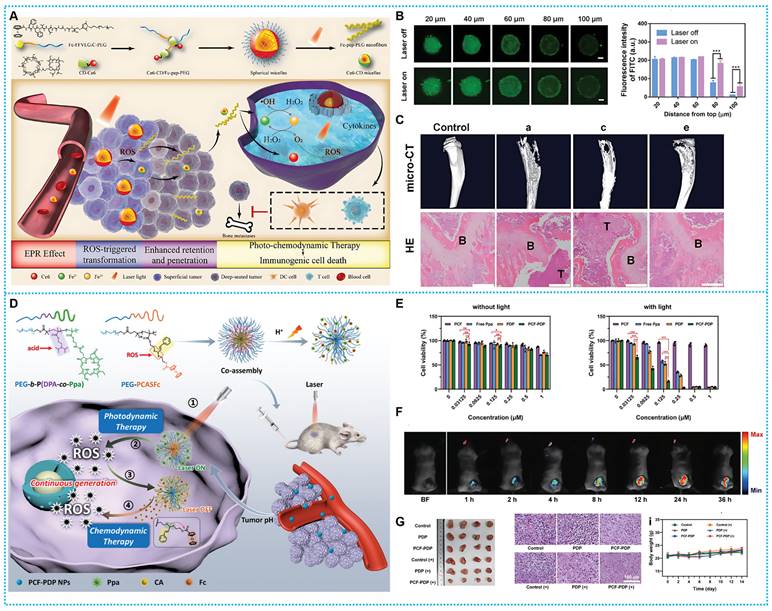
Based on this concept, Qin et al. constructed a self-delivery supramolecular nanoplatform with shape-shifting ability (Ce6-CD/Fc-pep-PEG). Ce6 is a good photosensitizer for mediating PDT, converting oxygen to H2O2 in response to light. The material undergoes a morphological transformation stimulated by ROS, and after passive accumulation mediated by the enhanced permeability and retention (EPR) effect, Fc is oxidized by a large amount of ROS within the tumor tissue to Fc+. Fc+-pep-PEG dissociates from the hydrophobic cavities of Ce6-CD and reassembles into nanofibers, which increase the accumulation in the tumor. At the same time, Ce6-CD fragments maintain the morphology of spherical micelles with smaller sizes and penetrate deeper into the tumor (Figure 6A-B). Through this strategy, Ce6 goes deep into the tumor for PDT, and more H2O2 is generated. In response, the conserved Fc may stimulate the Fenton reaction chain reaction, which produces •OH and O2, boosting PDT's effectiveness and establishing an adverse reinforcement cycle. Meanwhile, combining CDT with PDT also induces immunogenic cell death (ICD), induces the maturation of DC cells and the activation of T cells, and thus inhibits the expansion of initial tumors and bone metastases (Figure 6C) [105].
The poor selection might be the cause of CDT's systemic adverse effects. In view of this, developing a therapeutic strategy that can consistently and successfully create ROS while retaining an excellent degree of specificity remains challenging. Based on this line of thought, Zhang and coworkers reported a ROS nano-amplifier (PCF-PDP NPs) that can be designed to induce photoactivated persistent ROS generation via the PDT-CDT chain reaction to enhance cancer therapy. Under light irradiation, PCF-PDP NPs generate a significant quantity of ROS, which may be utilized to damage malignant cells and disrupt the particles' thioacetal junction, releasing the functional molecule cinnamaldehyde (CA) and the organocatalyst Fc simultaneously (Figures 6D). Hence increasing the rate of •OH production in the -Fc-catalyzed Fenton reaction and the therapeutic efficacy of CDT without the presence of light. It was possible to get around the limitations of the ROS-based treatment strategy and avert harm to tissue from overboard laser irradiation by utilizing a positive feedback loop of light-triggered production of ROS, ROS-responsive prodrug stimulation, and Fenton-mediated ROS cyclic regeneration amplifiers (Figures 6E-G) [106].
3.5. ECDT based on the TME-activated strategies
Developing tumor microenvironment (TME)-activated therapeutic agents continues to pose a formidable challenge. The agents in question possess both diagnostic and therapeutic properties, with the ability to "trigger" inside the tumor and "inhibit" within normal tissue. At present, the prevailing approach typically depends on a multi-component encapsulation nanoplatform. Nonetheless, this strategy could encounter potential instability within the intricate in vivo environment. Therefore, investigating single-component TME-activated phototheranostic compounds could provide a way around this problem. Concurrently, biosafety stands as a significant hurdle in the progression of cancer phototherapy [127, 128]. At present, the majority of TME-activated agents comprise inorganic metals and organic polymers [129]. However, the potential toxicity arising from the release of metal ions poses a significant barrier to their clinical translation. Therefore, the development of a molecular phototheranostic platform with robust stability and biocompatibility emerges as a logical strategy for advancing TME-activated phototheranostic agents in multimodal cascade therapy. Recent years have also yielded very prospective research discoveries on ferrocene-based activated nanopreparations.
(A) Synthesis process and therapeutic mechanisms of Ce6-CD / Fc-pep-PEG. (B) CLSM of 4T1 MSCs incubated with Ce6-CD/Fc-pep-PEG-FITC (4 h) and fluorescence semi-quantification. (C) Micro-CT images and H&E staining images of the metastatic tumor-bearing tibias. Reproduced with permission from ref [105]. Copyright 2021, Wiley-VCH. (D) Synthesis route and workflow of PCF-PDP NPs. (E) Cell viability at various treatments. (F) FL of mice injected with PCF-PDP NPs. (G) Evaluation of treatment effects of PCF-PDP NPs Reproduced with permission from ref [106]. Copyright 2023, Wiley-VCH.
(A) Synthesis process and therapeutic mechanisms of BPnbs-Fc. (B) GPC date for the micellar dispersion, TEM images of the dispersion of BPnbs-Fc, and Time-dependent changes of BPnbs-Fc with different treatments. (C) Reaction scheme of iron ions produced from BPnbs-Fc, time-dependent development of absorbance intensity of BPnbs-Fc at different pH values, and BPnbs-Fc iron ions in vitro under various treatments. (D) NIR FL image after intravenous injection of BPnbs-Fc and BPBn-Fc. Reproduced with permission [69], copyright 2023, Wiley-VCH. (E) Activation mechanism of IR-FEP-RGD-S-S-S-Fc. (F) CLSM images of Intracellular H2S and COX IV. (G) NIR-II fluorescence images of IR-FEP-RGD-S-S-S-Fc. Reproduced with permission [78], copyright 2023, Wiley-VCH.
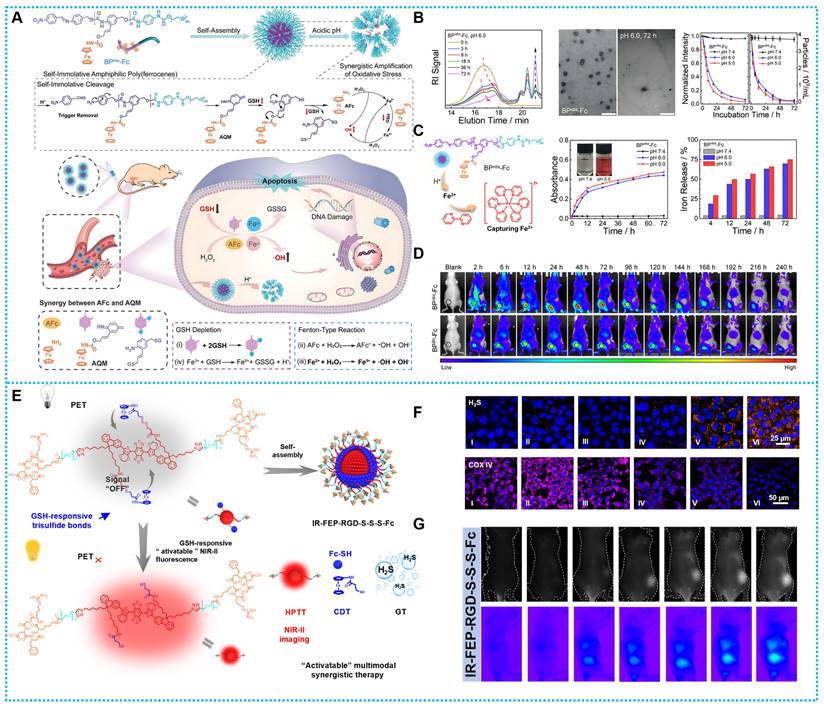
Based on these challenges, Xu and colleagues recently published research on self-immolating amphiphilic poly(ferrocene) that, in acidic settings, may self-promote ROS bursts by depolymerizing and releasing highly payloaded derivatives of azaquinone methide (AQM) and aminoferrocene (AFc) fractions. Specifically, AQM-derived products can quickly and effectively react with tumor cells' cytoplasmic GSH to lower internal GSH stages, greatly increasing the susceptibility of tumor cells to oxidative stress (Figures 7A-B). Additionally, when AFc used its catalytic abilities, it interacted to create free iron ions, enabling the intracellular recycling of iron ions, which partially resolved the difficult issue of Fc-based nanoparticle inefficiency. Concurrently, endogenous hydrogen peroxide (H2O2) in tumor cells was transformed into highly reactive hydroxyl radicals (•OH) by AFc and Fe2+, which functioned as efficient Fenton reagents, damaging intracellular materials in situ. A logical combination between GSH depletion and •OH explosion in tumor CDT is made possible by the powerful self-incineration degradation of amphiphilic poly(ferrocene) (Figures 7C-D) [69].
Therefore, creating a molecular phototheranostic platform that is both stable and biocompatible is a sensible idea for creating a multimodal cascade treatment led by a TEM-activated strategy. Our research team has created IR-FEP-RGD-S-S-S-Fc based on this idea for improved gas-chemo-photothermal synergistic treatment that is GSH-activatable and guided by NIR-II imaging. Through a variety of chemical synthesis designs and spectroscopic studies, this study performed the first investigation of the link between PET efficiency and molecular spacing. The IR-FEP-RGD-S-S-S-Fc produced tumor site cascade-specific lighting by blocking the PET process by GSH shearing the trisulfide bond and accomplishing active tumor targeting via cRGDfk peptide (Figures 7E). Adenosine triphosphate (ATP)-dependent heat shock proteins (HSPs) were shown to be less expressed when H2S was released (Figures 7F-G) [78].
3.6 ECDT in combination with gene therapy
Gene therapy, such as antisense therapy, is a promising treatment for cancer. Tumor-targeted gene therapy is an emerging and promising approach for the efficient treatment of cancer. Utilizing vectors in gene therapy to introduce nucleic acids into cells and modify gene expression has the potential to prevent or reverse the aggressive growth of tumors. Global clinical studies for gene therapy are on the rise. Despite tremendous advancements in tumor-selective delivery systems over the last two decades, the generation of therapeutic vectors based on promoters that are exclusively expressed in cancer cells remains a challenging task. Consequently, several approaches have been developed that use certain gene enhancers, promoters, and 5′-untranslated regions, which are responsive to transcription factors that specifically target tumors. These tactics aim to increase the expression of tumor suppressor genes or decrease the expression of cancer-related genes [130]. Refinements in genetics and molecular biology have illuminated the intricate relationship between cancer development and a myriad of genetic anomalies. Cancer gene therapy involves the delivery of targeted nucleic acids to tumor cells to correct or alter genetic conditions [131]. In the past few decades, there has been a proliferation of gene therapies for various types of cancers [132, 133]. It is noteworthy that gene therapy is a promising cancer treatment with excellent effectiveness with few adverse effects [134]. Therefore, the combination of gene therapy and Ferrocene-based ECDT can achieve highly effective tumor treatment with few side effects.
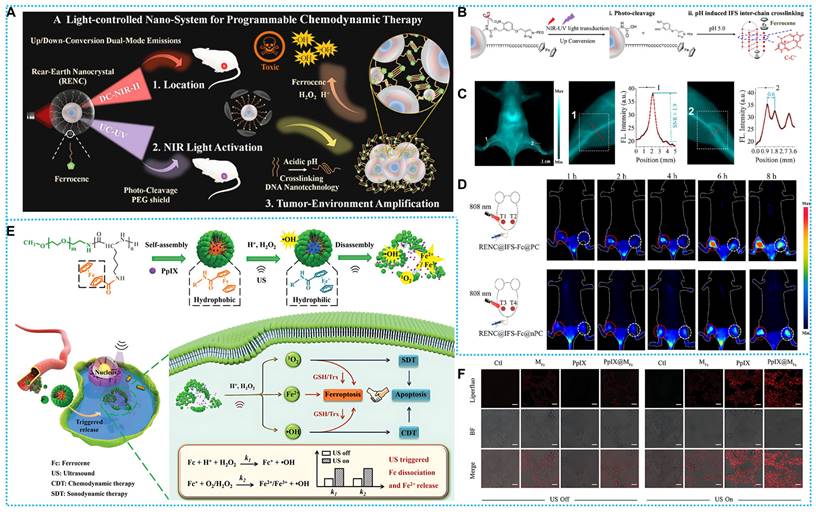
(A) Synthesis process and therapeutic mechanisms of RENC@IFS-Fc@PC. (B) Flow chart of PEG cleavage and pH response of IFS-Fc. (C) NIR-II FL imaging and peripheral tissues. (D) NIR-II FL imaging of RENC@IFS-Fc@PC and RENC@IFS-Fc@nPC. Reproduced with permission [77], copyright 2023, ACS. (E) Synthesis route and workflow of PpIX@MFc (F) LPO under different experimental conditions. Reproduced with permission [63], copyright 2022, Wiley-VCH.
For example, Wei et al. modified Fc on a DNA strand to act as a linker arm, effectively coupling Fc to the surface of rare earth nanocrystals (RENC@IFS-Fc@PC). The surface-formed photocleavable PEG network protects the IFS from degradation and “locks in” the toxicity of iron ions in the circulation (Figure 8A). Upconverted UV light stimulates the catalytic activity of Fe2+ by stripping off the PEG network. Down-converted NIR-II fluorescence can localize tumors, allowing for precise treatment of tumors (Figure 8B-C). This method enhances the accumulation of Fe2+ in tumors by altering the Fe delivery mode, which has been shown to result in a 4.5-fold increase in tumor accumulation by the nanosystem (Figure 8D) [77]. Although this design offers a unique and effective treatment concept, the discussion on the biosafety of the gene therapy issue has been limited. In general, it is crucial to do future safety studies.
3.7 ECDT in combination with SDT
Chemodynamic therapy (CDT) is a novel treatment approach that is driven by the natural characteristics of tumor microenvironments, such as acidity and hydrogen peroxide (H2O2), to produce a harmful hydroxyl radical (•OH) through a Fenton or Fenton-like reaction [20-23]. Extensive study has shown that, apart from Fe2+, other metal ions such as Mn2+, Cu2+, and Ti3+ may also function as Fenton-type agents and exhibit exceptional catalytic capabilities [24]. SDT is a novel treatment similar to PDT that eliminates cancer cells by generating large amounts of ROS under US irradiation of a sonic sensitizer. Since ultrasound has a greater ability to penetrate biological tissues, SDT is effective in treating deep-seated tumors. However, O2-dependent SDT may be completely ineffective due to hypoxia and increased drug resistance in tumor tissues. Both CDT and SDT are tumor therapeutic paradigms that rely on ROS. External sonication can enhance the production of •OH by directly affecting unstable molecules or through indirect thermogenesis, thereby enhancing CDT. Therefore, combining CDT with SDT is a highly recommended cancer synergistic strategy. The rapid advancement of nanotechnology may effectively establish a connection between CDT (Cancer Diagnosis and Treatment) and SDT (Sonodynamic Therapy) [25].
Based on the above concept, Li and coworkers designed a novel “jumping frog” polymer micelle (PpIX@MFc). After sonication, both 1O2 and •OH will be produced via a combination of SDT and CDT is used to activate apoptosis. Meanwhile, ROS-induced GSH depletion can trigger iron death, which can be augmented by the conversion of Fc to Fe2+ after sonication in the presence of H2O2 (Figures 8E-F). In addition, the triggered dissociation of Fc enables the triggered release of the acoustic sensitizer (PpIX), which also facilitates the short half-life and the limited diffusion distance of therapeutic ROS [63].
3.8 ECDT in combination with IT
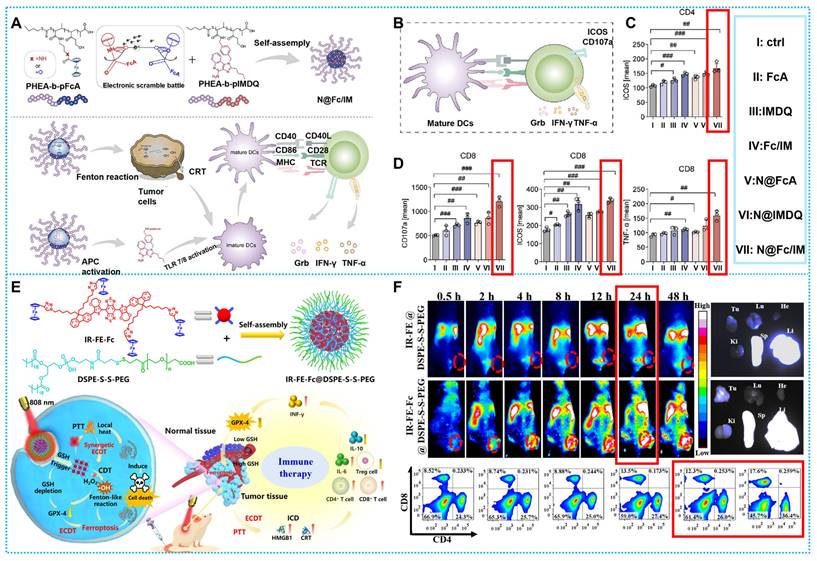
Cancer immunotherapy (IT) has shown considerable promise in the treatment of solid tumors by stimulating the innate immune system to limit tumor growth [135, 136]. In this case, increased pro-tumoral M2 macrophage polarization and anti-tumoral M1 phenotype, together with increased hydrogen peroxide from polarized M1 macrophages, produce immunogenic TME, whereas Fenton/Fenton-like reactions are enhanced [137, 138]. Tumor cells undergo ferroptosis when exposed to high concentrations of •OH produced by NIR-II irradiation. Simultaneously, exposure to tumor antigens causes cell death, which raises TME's immunogenicity. This results in a two-way loop of immunological and CDT, improving the persistence that is lacking in traditional CDT therapy.
According to current studies, CDT may elicit an immunological response by means of immunogenic cell death (ICD), a particular cancer-killing mechanism [139]. Exposure to calreticulin (CRT), referred to as the “eat-me” signal, induces dendritic cell (DC) maturation, which stimulates T lymphocyte activation [32]. Nevertheless, ICD alone is unable to sufficiently stimulate immunity that kills tumors, and new research indicates that immunological adjuvant combinations may be helpful [140]. Based on this concept, Zhang's Team developed a polymer platform (N@Fc/IM). By breaking down H2O2 to create •OH, Fc in N@Fc/IM promoted the Fenton reaction, which upset the critical redox equilibrium and caused the ICD of tumor cells exposed to CRT. Subsequent to IMDQ-induced activation of TLR7/8, the matured dendritic cells (DCs) enhanced T-lymphocyte infiltration, thereby enhancing a robust antitumor immune response (Figures 9A). Using four female BALB/c mouse models carrying T1, N@Fc / IM exhibited potent therapeutic effects and triggered a strong immunological response within the tumor microenvironment (TME) in vivo (Figures 9B-D) [107]. Nevertheless, the correlation between the immune response and Fenton's therapy, as well as other diagnostic and therapeutic processes, has not been extensively researched. Therefore, there is a need to delve further into the specific mechanisms that are directly linked to it.
Impressively, Wu and colleagues used a straightforward and practical rational design method to build IR-FE-Fc@DSPE-S-S-PEG [108]. By producing photothermal effects and converting less endogenous reactive hydrogen peroxide (H2O2) into •OH, the nanoplatform may kill tumor cells and cause tumor iron death by depleting GSH. Notably, it was shown that exposure to laser radiation had markedly higher levels of CD4+ and CD8+, indicating that the mice's autoimmunity was, in fact, enhanced after the combined therapy (Figures 9E-F) [108].
Liu's team has introduced a groundbreaking cancer nanobomb, termed ig, which comprises a (Pt (IV)) prodrug (Artoxplatin) and an oxidatively cleavable poly igniter embedded with Fc units. The nanobombig liberates Oxaliplatin (Oxa), artesunate. (ART), and Fc, thereby initiating a cascade leading to the production of highly cytotoxic ROS through the catalytic reaction of ART and Fc. This amplifies cytotoxicity and augments ICD effects, culminating in the induction of robust anti-tumor immunity. Furthermore, metabolomics analysis reveals that the nanobombig dramatically alters glutamine-related metabolic pathways, consequently reducing the levels of glutathione (GSH) within cancer cells. These findings underscore a promising strategy to bolster cancer chemoimmunotherapy when combined with immune checkpoint blockade, thus offering significant potential for clinical implementation and translational advancement [109].
3.9 ECDT in combination with GT
Gas therapy (GT) is an innovative treatment approach such as treatment with Nitric oxide (NO), hydrogen (H2), [141] hydrogen sulfide (H2S),[21] and carbon monoxide (CO) [23]. Due to its inherent connection with different metabolic pathways, the accessible gas, when present at optimal concentrations, exerts an anticancer impact by suppressing the production of HSPs and reversing the Warburg effect in cancer cells without negatively affecting normal cells. Thus, the integration of ferrocene-based CDT with GT holds promise in realizing a synergistic HPTT approach with heightened efficacy against malignant tumors. In addition, certain reducing agents, such as H2S, can expedite the conversion of Fe3+/Fe2+, thereby augmenting •OH generation and enhancing the therapeutic potential of CDT.
(A) Synthesis of pHEA-b-pFcAam and pHEA-b-pIMDQ and CDT destroys tumor cells and further induces ICD and enhances immune response. (B-D) Schematic representation and timing of important proteins or substances expressed or secreted by stimulated T cells. Reproduced with permission [107], copyright 2023, Elsevier. (E) Synthesis and therapeutic modalities of IR-FE-Fc@DSPE-S-S-PEG. (F) Tumor in vivo imaging and immune changes. Reproduced with permission [108], copyright 2023, Elsevier.
(A) Synthesis and therapeutic mechanisms of Arg/Fc@GOx/HA. Reproduced with permission [111]. Copyright 2023, Wiley-VCH. (B) Synthesis and therapeutic mechanisms of FTEB-TBFc. Reproduced with permission [110], copyright 2023, ACS.
Nitric oxide (NO) radicals are the main kind of reactive nitrogen species (RNS) that damage mitochondria and DNA and may immediately trigger apoptosis [142, 143] and subsequently increase the effectiveness of PDT and SDT via quickening the metabolism of intracellular glutathione (GSH) and widening blood arteries to provide oxygen [144, 145]. More importantly, NO has further reactions with ROS (e.g., •O2-) to produce peroxynitrite anions (ONOO-), [146] another RNS that is more hazardous than the majority of ROS since it may cause direct oxidation and nitration processes mediated by free radicals [147, 148]. Inspired by the natural mechanism by which inflammatory cells generate a storm of free radicals through activated and tunable biocatalysis, Liu et al. developed a biomimetic nano-enzyme capable of generating reactive oxygen and nitrogen species (RONS) in situ for cancer therapy. The nano-enzyme gets separated by internal glutathione, releasing GOx, which breaks down glucose to generate gluconic acid and H2O2. Under this acidifying condition, H2O2 efficiently oxidizes side-chain arginine residues to generate nitric oxide, which is converted to Highly hazardous hydroxyl radicals and superoxide anion. This process mainly produces peroxynitrite anion (ONOO-). This reactive nitrogen (RNS) is more toxic than most ROS. Enhanced tumor-killing effect (Figure 10A) [111].
Furthermore, the activatable nanomaterials would be a dependable and effective tumor treatment with excellent effectiveness, powerful targeting, flexible controllability, and few side effects. The logical design of FTEP-TBFc NPs was shown by Wu et al. based on this concept. This molecularly engineered photothermal nanoplatform produces hydrogen sulfide (H2S) gas in response to glutathione (GSH) and transports ferrocene molecules into the TME. This strategy improves photodynamic treatment (PDT) and chemodynamic therapy (CDT) at a biosafe laser power of 0.33 W/cm2 (Figure 10B) [110].
4. Summary, Prospects, and Challenges
This article summarizes the latest research progress of ferrocene-based nanomedicines for synergistically enhanced chemodynamic therapy. Ferrocene-based chemodynamic therapy has several distinct advantages of excellent stability, high efficiency, flexible functionalization, and low toxicity, exhibiting brilliant prospects for oncological applications. For the first time, this paper focuses on the different types of ferrocene-containing nanomedicines and describes the relationship between the functionality and various states of ferrocene that exist in nanomedicine, followed by recent progress in cancer-enhanced chemodynamic therapy applications with other treatment patterns. Additionally, the recently reported Ferrocene-based ECDT nanoplatform is highlighted, shedding light on the molecular mechanisms behind reinforcement strategies to promote the development of ferrocene-based ECDT anticancer methods. But before they may be used in clinical settings, some unresolved concerns need to be addressed, which are listed as follows.
(1) The therapeutic mechanisms of ferrocene-based CDT in complicated TME should be thoroughly elucidated. Currently, most of the research on the mechanism and properties of the Fenton reaction has been performed in simulated humoral environments, which may differ from intricate physiological circumstances. It is foreseeable that abundant enzymes, proteins, and bioactive molecules are likely to inhibit the Fenton reaction under pathological conditions. In addition, due to tumor heterogeneity, the therapeutic approach should be individualized for different tumors. Therefore, there is an urgent need for an in-depth study of the response mechanism of ferrocene-based CDT under specific pathological conditions, which requires targeted cellular or in vivo models for effective validation. Meanwhile, the creation of an online immediate form ROS auditing system to direct the logical design of upcoming effective Fenton reagents.
(2) The controllable manufacture of ferrocene-based nanomedicines with active ingredient encysted and the species of ferrocene-based CDT drugs and the regimen for dosage are tailored to different patients for meeting the next phase of clinical medicine [149]. So far, the efficiency of ferrocene-based CDT is closely related to the TME and biochemical reaction, which changes across tumor varieties and phases, as well as individuals [150, 151]. The good news is developments in big data, artificial intelligence (AI), and tumor whole-genome sequencing technologies may enable us to build a multivariate database that crosses TME, spatiotemporal distribution of nanomedicines, and ferrocene CDT results. Moving forward, data-driven nano bioinformatics has great prospects for guiding the design of customized ferrocene CDT nanopharmaceuticals and achieving precise ferrocene CDT.
(3) Leakage of ferrocene-based nanomedicines with releasing ferrocene into the bloodstream would cause toxicity and inefficient treatment. In general, Transition ferrocene-encapsulated nanoplatforms have been suffering from ferrocene leakage problems. Consequently, Monitoring blood Fe concentrations and long-term in vivo toxicity are essential. The majority of the existing ferrocene nanoplatforms are molecularly encapsulated. Recent investigations suggest an evolution towards completely manufactured, chemically coupled, single-molecule medicines, which provide the benefit of guaranteeing stability inside a living organism. Simultaneously, it is necessary to conduct realistic and thorough toxicological assessments, as well as clinical and preclinical investigations, in order to provide a foundation for the ongoing development of future medications.
(4) The biodegradability and biosafety of ferrocene-based agents remain to be major concerns. Despite a number of studies that have examined the safety and biocompatibility of nanocarriers in pathology and blood, however, there are currently insufficient long-term safety evaluations across a variety of experimental models. In order to effectively translate intriguing hypotheses into pre-clinical and clinical trials, it is crucial to take into account the physical compatibility, pharmacological synergy, and pharmaceutical features such as stability, scalability, and pharmacokinetics from an early stage. By using this method and implementing strong production procedures, several drug-combination nanoparticles have advanced to trials, including non-human primates and humans. Meanwhile, several labeling methods, such as isotope labeling and Positron Emission Tomography (PET), may be used to track the destiny of nanomaterials in vivo. For clinical translation, a thorough safety evaluation by conducting nanotoxicity investigations on delivery platforms involving larger animals and primates is required.
(5) The development of ferrocene-based nanomedicines with low toxicity, strong tumor targeting, and immune escape remains a major challenge for CDT therapy. Despite the advancement of targeted strategies, some challenges still exist. Examples include the creation of “protein crowns” during blood transport, the immune system's rejection of nanoparticles, and the stability and targeting capacity of nanomaterials in vivo. In the last several years, nanobionic therapeutic platforms have received widespread attention, allowing nanomedicines to self-recognize substances and avoid recognition by the immune system. Specifically, Cancer cells' membrane surface antigen homology offers opportunities for immune evasion and homologous adhesion. Replicating these functions in full using synthetic materials is difficult. To facilitate further progress, it is recommended to develop customized nanoplatforms by the loading or chemical coupling of cancer cells obtained from patient tissues utilizing biomimetic techniques. This novel design is anticipated to enhance the targeting efficacy of the treatments and circumvent detection by the immune system.
(6) Ferrocene-based activatable theranostics with the integration of diagnosis and treatment function would facilitate precise tumor detection and treatment with minimal non-specific damage. Thus obtaining a high tumor-to-normal tissue (T/N) signal ratio, sound sensitivity, and avoiding “false positives.” Furthermore, they are able to overcome undesirable toxic side effects during diagnosis and therapy due to their higher selectivity. It is thus very valuable to research the activatable Ferrocene-based ECDT diagnostic and therapeutic platforms. For instance, the work put forward by our team and Chen et al. on activation-based research serves as a valuable reference for investigators. Activation probes having a high signal ratio between target and non-target regions, as well as excellent sensitivity, may be developed by molecular design using PET, ICT, or FRET mechanisms.
(7) To manage the tumor as a chronic condition, the ferrocene-based ECDT should focus more on creating innovative nanomedicine that targets tumor evolution. Because tumor cells' genomes are unstable, these cancerous cells may undergo natural selection in response to pressures from the tumor's survival or outside treatments [152-154]. The three main benefits of nanomedicines—exact controllability, durability, and inclusivity—should be thoroughly understood in the coping strategy. In response strategies, interventions should be developed to target tumor cells or the tumor microenvironment. Regardless of how the tumor evolves, it will build up a tumor microenvironment. Investigating the evaluation and management of the tumor microenvironment has the potential to provide precise and timely diagnosis and therapy. Moreover, with the advanced technique of whole tumor genome sequencing, a thorough examination of the patient's tumor cells may be conducted to acquire a tailored diagnosis and treatment strategy, thereby addressing the challenge at present.
In summary, although much effort has been made to enhance ferrocene CDT's activity, more work has to be done prior to it entering clinical applications. Urgent issues such as safety, efficiency, stability, and reproducibility of ferrocene CDT reagent design should be fully considered. Meanwhile, unresolved scientific issues need to be resolved through interdisciplinary collaboration among clinical medicine, chemistry, pharmacy, and biology in order to further accelerate the discovery of ferrocene CDT. Since ferrocene CDT has been demonstrated to have promise for usage in medical settings in earlier studies, we anticipate that its real clinical uses will benefit a greater number of individuals.
Acknowledgements
Q.Y. thanks to the National Science Foundation of China (22374065) and the Outstanding Youth Foundation of Hunan Province (2024JJ2047). G-L W. thanks the Natural Science Foundation of Hunan Province of China (2024JJ6374) for funding support. G.C. thanks the Scientific research key project of Hunan Education Department (No.21A0258), Hunan Provincial Clinical Medical Technology Innovation Guide Project (No.2020SK51817) and University of South China Clinical Research 4310 Program (20224310NHYCG01, 20214310NHYPY05). Xinjiang Uygur Autonomous Region Department of Science and Technology-Centralized Guided Local Science and Technology Development Fund Project (2060404).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel R L, Laversanne M, Soerjomataram I, Jemal A. et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209
2. Pan W, Cui B, Wang K, Shi M, Lu F, Li N. et al. Atp-triggered mitochondrial cascade reactions for cancer therapy with nanoscale zeolitic imidazole framework-90. Theranostics. 2021;11:7869
3. Ding Y, Xiao X, Zeng L, Shang Q, Jiang W, Xiong S. et al. Platinum-crosslinking polymeric nanoparticle for synergetic chemoradiotherapy of nasopharyngeal carcinoma. Bioact Mater. 2021;6:4707
4. Cheng X, Li D, Xu J, Wei B, Fang Q, Yang L. et al. Self-assembled ternary hybrid nanodrugs for overcoming tumor resistance and metastasis. Acta Pharm Sin B. 2021;11:3595
5. Zheng T, Wang W, Wu F, Zhang M, Shen J, Sun Y. Zwitterionic polymer-gated au@tio(2) core-shell nanoparticles for imaging-guided combined cancer therapy. Theranostics. 2019;9:5035
6. Jyotsana N, Zhang Z, Himmel L E, Yu F, King M R. Minimal dosing of leukocyte targeting trail decreases triple-negative breast cancer metastasis following tumor resection. Sci Adv. 2019;5:eaaw4197
7. Chen Y, Huang Y, Zhou S, Sun M, Chen L, Wang J. et al. Tailored chemodynamic nanomedicine improves pancreatic cancer treatment via controllable damaging neoplastic cells and reprogramming tumor microenvironment. Nano Lett. 2020;20:6780
8. An S, Tiruthani K, Wang Y, Xu L, Hu M, Li J. et al. Locally trapping the c-c chemokine receptor type 7 by gene delivery nanoparticle inhibits lymphatic metastasis prior to tumor resection. Small. 2019;15:e1805182
9. Sorski L, Melamed R, Matzner P, Lavon H, Shaashua L, Rosenne E. et al. Reducing liver metastases of colon cancer in the context of extensive and minor surgeries through beta-adrenoceptors blockade and cox2 inhibition. Brain Behav Immun. 2016;58:91
10. Ma Z, Foda M F, Liang H, Zhao Y, Han H. In situ nanozyme-amplified nir-ii phototheranostics for tumor-specific imaging and therapy. Adv Funct Mater. 2021;31:2103765
11. Zou B, Xiong Z, He L, Chen T. Reversing breast cancer bone metastasis by metal organic framework-capped nanotherapeutics via suppressing osteoclastogenesis. Biomaterials. 2022;285:121549
12. Chen S, Pan Y, Chen K, Chen P, Shen Q, Sun P. et al. Increasing molecular planarity through donor/side-chain engineering for improved nir-iia fluorescence imaging and nir-ii photothermal therapy under 1064 nm. Angew Chem Int Ed. 2022;62:e202215372
13. Hu H, Li D, Dai W, Jin Q, Wang D, Ji J. et al. A nir-ii aiegen-based supramolecular nanodot for peroxynitrite-potentiated mild-temperature photothermal therapy of hepatocellular carcinoma. Adv Funct Mater. 2023;33:2213134
14. Liu D, Dai X, Zhang W, Zhu X, Zha Z, Qian H. et al. Liquid exfoliation of ultrasmall zirconium carbide nanodots as a noninflammatory photothermal agent in the treatment of glioma. Biomaterials. 2023;292:121917
15. Zheng Z, Duan A, Dai R, Li Y, Chen X, Qin Y. et al. A “dual-source, dual-activation” strategy for an nir-ii window theranostic nanosystem enabling optimal photothermal-ion combination therapy. Small. 2022;18:2201179
16. Guo X, Yang N, Ji W, Zhang H, Dong X, Zhou Z. et al. Mito-bomb: Targeting mitochondria for cancer therapy. Adv Mater. 2021;33:e2007778
17. Zheng Z, Gao J, Wang R, Dong C, Dong X, Sun J. et al. Molecular engineering of luminogens for high-integrity imaging of hydrogen polysulfides via activatable aggregation-induced dual-color fluorescence. ACS Nano. 2023;17:22060
18. Chen D, Dai H, Wang W, Cai Y, Mou X, Zou J. et al. Proton-driven transformable 1o2-nanotrap for dark and hypoxia tolerant photodynamic therapy. Adv Sci. 2022;9:2200128
19. Chen Y, Liu Y, Guo C, Yin C, Xie C, Fan Q. Self-amplified competitive coordination of mno2-doped ceo2 nanozyme for synchronously activated combination therapy. Adv Funct Mater. 2022;33:2209927
20. Wan J, Zhang X, Tang D, Liu T, Xiao H. Biodegradable nir-ii pseudo conjugate polymeric nanoparticles amplify photodynamic immunotherapy via alleviation of tumor hypoxia and tumor-associated macrophage reprogramming. Adv Mater. 2023;35:2209799
21. Tian Q, An L, Tian Q, Lin J, Yang S. Ellagic acid-fe@bsa nanoparticles for endogenous h2s accelerated fe(iii)/fe(ii) conversion and photothermal synergistically enhanced chemodynamic therapy. Theranostics. 2020;10:4101
22. Xie C, Cen D, Ren Z, Wang Y, Wu Y, Li X. et al. Fes@bsa nanoclusters to enable h2s-amplified ros-based therapy with mri guidance. Adv Sci. 2020;7:1903512
23. Yingshuai Wang T Y, Qianjun He. Strategies for engineering advanced nanomedicines for gas therapy of cancer. Natl Sci Rev. 2020;7:1485
24. Ma G, Liu Z, Zhu C, Chen H, Kwok R T K, Zhang P. et al. H2o2-responsive nir-ii aie nanobomb for carbon monoxide boosting low-temperature photothermal therapy. Angew Chem Int Ed. 2022;61:e202207213
25. Wang K, Li Y, Wang X, Zhang Z, Cao L, Fan X. et al. Gas therapy potentiates aggregation-induced emission luminogen-based photoimmunotherapy of poorly immunogenic tumors through cgas-sting pathway activation. Nat Commun. 2023;14:2950
26. Yang Y, Ge J, Li G, Lei H, Chen L, Gong Y. et al. Manganese-doping titanium disulfide cascade nanobioreactors for sequential gas-sonodynamic strategy with immune checkpoint blockade therapy of cancer. Nano Today. 2022;46:101585
27. Zhang T, Pan Y, Suo M, Lyu M, Lam J W Y, Jin Z. et al. Photothermal-triggered sulfur oxide gas therapy augments type i photodynamic therapy for potentiating cancer stem cell ablation and inhibiting radioresistant tumor recurrence. Adv Sci. 2023;10:2304042
28. Pignatello J J, Oliveros E, MacKay A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit Rev Env Sci Tec. 2006;36:1
29. Tang Z, Zhao P, Wang H, Liu Y, Bu W. Biomedicine meets fenton chemistry. Chem Rev. 2021;121:1981
30. Liu G, Zhu J, Guo H, Sun A, Chen P, Xi L. et al. Mo(2) c-derived polyoxometalate for nir-ii photoacoustic imaging-guided chemodynamic/photothermal synergistic therapy. Angew Chem Int Ed. 2019;58:18641
31. Sun P, Qu F, Zhang C, Cheng P, Li X, Shen Q. et al. Nir-ii excitation phototheranostic platform for synergistic photothermal therapy/chemotherapy/chemodynamic therapy of breast cancer bone metastases. Adv Sci. 2022;9:e2204718
32. Wang W J, Ling Y Y, Zhong Y M, Li Z Y, Tan C P, Mao Z W. Ferroptosis-enhanced cancer immunity by a ferrocene-appended iridium(iii) diphosphine complex. Angew Chem Int Ed. 2022;61:e202115247
33. Wang X, Zhong X, Bai L, Xu J, Gong F, Dong Z. et al. Ultrafine titanium monoxide (tio1+x) nanorods for enhanced sonodynamic therapy. J Am Chem Soc. 2020;142:6527
34. Zheng Z, Chen X, Ma Y, Dai R, Wu S, Wang T. et al. Dual h2o2 -amplified nanofactory for simultaneous self-enhanced nir-ii fluorescence activation imaging and synergistic tumor therapy. Small. 2022;18:2203531
35. Ding Y, Wang C, Ma Y, Zhu L, Lu B, Wang Y. et al. Tumor microenvironment responsive polypeptide-based supramolecular nanoprodrugs for combination therapy. Acta Biomaterialia. 2022;146:396
36. Ding Y, Yu W, Shen R, Zheng X, Zheng H, Yao Y. et al. Hypoxia-responsive tetrameric supramolecular polypeptide nanoprodrugs for combination therapy. Adv Healthc Mater. 2023;13:2303308
37. Wang X, Zhong X, Liu Z, Cheng L. Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today. 2020;35:100946
38. Lin L S, Huang T, Song J, Ou X Y, Wang Z, Deng H. et al. Synthesis of copper peroxide nanodots for h2o2 self-supplying chemodynamic therapy. J Am Chem Soc. 2019;141:9937
39. Zhang C, Bu W, Ni D, Zhang S, Li Q, Yao Z. et al. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction. Angew Chem Int Ed. 2016;55:2101
40. Tang Z, Liu Y, He M, Bu W. Chemodynamic therapy: Tumour microenvironment-mediated fenton and fenton-like reactions. Angew Chem Int Ed. 2019;58:946
41. Zhao Z, Wang W, Li C, Zhang Y, Yu T, Wu R. et al. Reactive oxygen species-activatable liposomes regulating hypoxic tumor microenvironment for synergistic photo/chemodynamic therapies. Adv Funct Mater. 2019;29:1905013
42. Tang Z, Zhang H, Liu Y, Ni D, Zhang H, Zhang J. et al. Antiferromagnetic pyrite as the tumor microenvironment-mediated nanoplatform for self-enhanced tumor imaging and therapy. Adv Mater. 2017;29:1701683
43. Fan X, Li Y, Feng Z, Chen G, Zhou J, He M. et al. Nanoprobes-assisted multichannel nir-ii fluorescence imaging-guided resection and photothermal ablation of lymph nodes. Adv Sci. 2021;8:2003972
44. Li Z, Chu Z, Yang J, Qian H, Xu J, Chen B. et al. Immunogenic cell death augmented by manganese zinc sulfide nanoparticles for metastatic melanoma immunotherapy. ACS Nano. 2022;16:15471
45. Yang T, Zhou M, Gao M, Qin W, Wang Q, Peng H. et al. Carrier-free h2o2 self-supplier for amplified synergistic tumor therapy. Small. 2022;19:2205692
46. Bhattacharjee S, Chatterjee S, Jiang J, Sinha B K, Mason R P. Detection and imaging of the free radical DNA in cells-site-specific radical formation induced by fenton chemistry and its repair in cellular DNA as seen by electron spin resonance, immuno-spin trapping and confocal microscopy. Nucleic Acids Res. 2012;40:5477
47. Zhao P, Tang Z, Chen X, He Z, He X, Zhang M. et al. Ferrous-cysteine-phosphotungstate nanoagent with neutral ph fenton reaction activity for enhanced cancer chemodynamic therapy. Mater Horiz. 2019;6:369
48. Meng X, Zhang X, Liu M, Cai B, He N, Wang Z. Fenton reaction-based nanomedicine in cancer chemodynamic and synergistic therapy. Appl Mater Today. 2020;21:100864
49. Fan J X, Peng M Y, Wang H, Zheng H R, Liu Z L, Li C X. et al. Engineered bacterial bioreactor for tumor therapy via fenton-like reaction with localized h2 o2 generation. Adv Mater. 2019;31:e1808278
50. Ma B, Wang S, Liu F, Zhang S, Duan J, Li Z. et al. Self-assembled copper-amino acid nanoparticles for in situ glutathione “and” h2o2 sequentially triggered chemodynamic therapy. J Am Chem Soc. 2018;141:849
51. Bao W, Liu M, Meng J, Liu S, Wang S, Jia R. et al. Mofs-based nanoagent enables dual mitochondrial damage in synergistic antitumor therapy via oxidative stress and calcium overload. Nat Commun. 2021;12:6399
52. Mao H, Wen Y, Yu Y, Li H, Wang J, Sun B. Ignored role of polyphenol in boosting reactive oxygen species generation for polyphenol/chemodynamic combination therapy. Mater Today Bio. 2022;16:100436
53. Wu A, Han M, Ding H, Rao H, Lu Z, Sun M. et al. Fe3s4 nanozyme inhibits tumor growth by synergistic effects of ferroptosis and apoptosis. Chem Eng J. 2023;474:145920
54. Yang S, Wu Y, Zhong W, Chen R, Wang M, Chen M. Gsh/ph dual activatable crosslinked and fluorinated pei for cancer gene therapy through endogenous iron de-hijacking and in situ ros amplification. Adv Mater. 2023;36:2304098
55. Amjad S, Naeem A, Sabahat S. In vitro investigation of anti-cancer activity of ferrocene-functionalized gold nanoparticles. Chem Pap. 2019;74:125
56. Miller S A, Tebboth J A, Tremaine J F. 114. Dicyclopentadienyliron. J Chem Soc. 1952: 632.
57. Metzler-Nolte* D R v S a N. Bioorganometallic chemistry of ferrocene. Chem Rev. 2004;104:5931-5985
58. Liu C-X, Gu Q, You S-L. Asymmetric c-h bond functionalization of ferrocenes: New opportunities and challenges. Trends Chem. 2020;2:737
59. Metzler-Nolte D R v S a N. Bioorganometallic chemistry of ferrocene. Chem Rev. 2004;104:5931-5985
60. Cázares-Marinero J d J, Top S, Vessières A, Jaouen G. Synthesis and antiproliferative activity of hydroxyferrocifen hybrids against triple-negative breast cancer cells. Dalton T. 2014;43:817
61. Zhao P, Jiang Y, Tang Z, Li Y, Sun B, Wu Y. et al. Constructing electron levers in perovskite nanocrystals to regulate the local electron density for intensive chemodynamic therapy. Angew Chem Int Ed. 2021;60:8905
62. Koo S, Park O K, Kim J, Han S I, Yoo T Y, Lee N. et al. Enhanced chemodynamic therapy by cu-fe peroxide nanoparticles: Tumor microenvironment-mediated synergistic fenton reaction. ACS Nano. 2022;16:2535
63. Xiong Y, Li J, He S. Zinc protects against heat stress-induced apoptosis via the inhibition of endoplasmic reticulum stress in tm3 leydig cells. Biol Trace Elem Res. 2022;200:728
64. Kong H, Fang C, Chu Q, Hu Z, Fu Y, Han G. et al. Catalytic core-shell nanoparticles with self-supplied calcium and h2o2 to enable combinational tumor inhibition. J Nanobiotechnol. 2021;19:313
65. Kong H, Chu Q, Fang C, Cao G, Han G, Li X. Cu-ferrocene-functionalized cao2 nanoparticles to enable tumor-specific synergistic therapy with gsh depletion and calcium overload. Adv Sci. 2021;8:e2100241
66. Yu M, Ye Z, Liu S, Zhu Y, Niu X, Wang J. et al. Redox-active ferrocene quencher-based supramolecular nanomedicine for nir-ii fluorescence-monitored chemodynamic therapy. Angew Chem Int Ed. 2024;63:e202318155
67. Wang N, Zeng Q, Zhang R, Xing D, Zhang T. Eradication of solid tumors by chemodynamic theranostics with h2o2-catalyzed hydroxyl radical burst. Theranostics. 2021;11:2334
68. Zhou Y, Fan S, Feng L, Huang X, Chen X. Manipulating intratumoral fenton chemistry for enhanced chemodynamic and chemodynamic-synergized multimodal therapy. Adv Mater. 2021;33:2104223
69. Xu J, Tan J, Song C, Zhang G, Hu X, Liu S. Self-immolative amphiphilic poly(ferrocenes) for synergistic amplification of oxidative stress in tumor therapy. Angew Chem Int Ed. 2023;62:e202303829
70. Zhao Y, Du J, Xu Z, Wang L, Ma L, Sun L. DNA adjuvant hydrogel-optimized enzymatic cascade reaction for tumor chemodynamic-immunotherapy. Adv Sci. 2024;11:2308229
71. Guo B, Huang Z, Shi Q, Middha E, Xu S, Li L. et al. Organic small molecule based photothermal agents with molecular rotors for malignant breast cancer therapy. Adv Funct Mater. 2019;30:1907093
72. Li J, Liu Y, Xu Y, Li L, Sun Y, Huang W. Recent advances in the development of nir-ii organic emitters for biomedicine. Coordin Chem Rev. 2020;415:213318
73. Wan H, Du H, Wang F, Dai H. Molecular imaging in the second near-infrared window. Adv Funct Mater. 2019;29:1900566
74. Xu W, Wang D, Tang B Z. Nir-ii aiegens: A win-win integration towards bioapplications. Angew Chem Int Ed. 2020;60:7476
75. Feng G, Zhang G-Q, Ding D. Design of superior phototheranostic agents guided by jablonski diagrams. Chem Soc Rev. 2020;49:8179
76. Dai H, Wang X, Shao J, Wang W, Mou X, Dong X. Nir-ii organic nanotheranostics for precision oncotherapy. Small. 2021;17:2102646
77. Wei Z, Chao Z, Zhang X, Yu J, Xiao F, Zhang X. et al. Nir-ii luminescent and multi-responsive rare earth nanocrystals for improved chemodynamic therapy. Acs Appl Mater Inter. 2023;15:11575
78. Wu G-L, Liu F, Li N. et al. Trisulfide bond-mediated molecular phototheranostic platform for “activatable” nir-ii imaging-guided enhanced gas/chemo- hypothermal photothermal therapy. Adv Sci. 2023;10:2304104
79. He Y, Jin X, Guo S, Zhao H, Liu Y, Ju H. Conjugated polymer-ferrocence nanoparticle as an nir-ii light powered nanoamplifier to enhance chemodynamic therapy. Acs Appl Mater Inter. 2021;13:31452
80. Yang J, Yang Y W. Metal-organic frameworks for biomedical applications. Small. 2020;16:1906846
81. Tan L-L, Li H, Qiu Y-C, Chen D-X, Wang X, Pan R-Y. et al. Stimuli-responsive metal-organic frameworks gated by pillar[5]arene supramolecular switches. Chem Sci. 2015;6:1640
82. Hang L, Zhou F, Men D, Li H, Li X, Zhang H. et al. Functionalized periodic au@mofs nanoparticle arrays as biosensors for dual-channel detection through the complementary effect of spr and diffraction peaks. Nano Res. 2017;10:2257
83. Wu M X, Yang Y W. Metal-organic framework (mof)-based drug/cargo delivery and cancer therapy. Adv Mater. 2017;29:1606134
84. Brownlee C. In nano, volume 15, issue 1. ACS Nano. 2021;15:3
85. Liu J, Liang J, Xue J, Liang K. Metal-organic frameworks as a versatile materials platform for unlocking new potentials in biocatalysis. Small. 2021;17:2100300
86. Gao S, Han Y, Fan M, Li Z, Ge K, Liang X-J. et al. Metal-organic framework-based nanocatalytic medicine for chemodynamic therapy. Sci China Mater. 2020;63:2429
87. Cai W, Wang J, Chu C, Chen W, Wu C, Liu G. Metal-organic framework-based stimuli-responsive systems for drug delivery. Adv Sci. 2018;6:1801526
88. Di X, Pei Z, Pei Y, James T D. Tumor microenvironment-oriented mofs for chemodynamic therapy. Coordin Chem Rev. 2023;484:215098
89. Zhang X, Liu Y, Guo Q. et al. Light-responsive Au@Zn-TCPP nanozyme functionalized with cell-penetrating peptide and antisense oligonucleotide for sensing living bacteria and synergistic therapy of diabetic wounds. Chem Eng J. 2024;488:150945
90. Gao S, Han Y, Fan M, Li Z, Ge K, Liang X-J. et al. Metal-organic framework-based nanocatalytic medicine for chemodynamic therapy. Science China Materials. 2020;63:2429
91. Fang C, Deng Z, Cao G, Chu Q, Wu Y, Li X. et al. Co-ferrocene mof/glucose oxidase as cascade nanozyme for effective tumor therapy. Adv Funct Mater. 2020;30:396
92. Deng Z, Fang C, Ma X, Li X, Zeng Y-J, Peng X. One stone two birds: Zr-fc metal-organic framework nanosheet for synergistic photothermal and chemodynamic cancer therapy. Acs Appl Mater Inter. 2020;12:20321
93. Zhou L L, Guan Q, Li W Y, Zhang Z, Li Y A, Dong Y B. A ferrocene-functionalized covalent organic framework for enhancing chemodynamic therapy via redox dyshomeostasis. Small. 2021;17:e2101368
94. Zhou L-L, Guan Q, Zhou W, Kan J-L, Teng K, Hu M. et al. A multifunctional covalent organic framework nanozyme for promoting ferroptotic radiotherapy against esophageal cancer. ACS Nano. 2023;17:20445
95. Zhu Y, Gong P, Wang J, Cheng J, Wang W, Cai H. et al. Amplification of lipid peroxidation by regulating cell membrane unsaturation to enhance chemodynamic therapy. Angew Chem Int Ed. 2023;62:e202218407
96. Wang J, Ding H, Zhu Y, Liu Y, Yu M, Cai H. et al. Iron-sirna nanohybrids for enhanced chemodynamic therapy via ferritin heavy chain downregulation. Angew Chem Int Ed. 2023;62:e202302255
97. Liu X, Liu J, Meng C, Zhu P, Liu X, Qian J. et al. Pillar[6]arene-based supramolecular nanocatalysts for synergistically enhanced chemodynamic therapy by the intracellular cascade reaction. Acs Appl Mater Inter. 2021;13:53574
98. Wang Y, Zhang S, Wang J, Zhou Q, Mukerabigwi J F, Ke W. et al. Ferrocene-containing polymersome nanoreactors for synergistically amplified tumor-specific chemodynamic therapy. J Control Release. 2021;333:500
99. Lin J, Yang H, Zhang Y, Zou F, He H, Xie W. et al. Ferrocene-based polymeric nanoparticles carrying doxorubicin for oncotherapeutic combination of chemotherapy and ferroptosis. Small. 2023;19:e2205024
100. Yang K, Yu G, Yang Z, Yue L, Zhang X, Sun C. et al. Supramolecular polymerization-induced nanoassemblies for self-augmented cascade chemotherapy and chemodynamic therapy of tumor. Angew Chem Int Ed. 2021;60:17570
101. Yao Z, Hu Q, Jin P, Li B, Huang Y, Zhang F. et al. A self-assembly combined nano-prodrug to overcome gemcitabine chemo-resistance of pancreatic tumors. Adv Funct Mater. 2023;33:2214598
102. Ge W, Liu C, Xu Y, Zhang J, Si W, Wang W. et al. Crystal engineering of ferrocene-based charge-transfer complexes for nir-ii photothermal therapy and ferroptosis. Chem Sci. 2022;13:9401
103. Lee S Y, Park J, Jeong D I, Hwang C, Lee J, Lee K. et al. Ferrocene and glucose oxidase-installed multifunctional hydrogel reactors for local cancer therapy. J Control Release. 2022;349:617
104. Wang Y, Chen Z, Li J, Wen Y, Li J, Lv Y. et al. A paramagnetic metal-organic framework enhances mild magnetic hyperthermia therapy by downregulating heat shock proteins and promoting ferroptosis via aggravation of two-way regulated redox dyshomeostasis. Adv Sci. 2024;11:e2306178
105. Qin Y, Tong F, Zhang W, Zhou Y, He S, Xie R. et al. Self-delivered supramolecular nanomedicine with transformable shape for ferrocene-amplified photodynamic therapy of breast cancer and bone metastases. Adv Funct Mater. 2021;31:2104645
106. Zhang X, Zhang X, Guo H, Jia S, Li Y, Xing S. et al. A photo-activated continuous reactive oxygen species nanoamplifier for dual-dynamic cascade cancer therapy. Adv Healthc Mater. 2023;12:2301469
107. Hao Y, Li H, Ren F, Feng R, Liu Y, Li X. et al. Ferrocene-conjugated polymeric platform via amide bond formation facilitates enhanced in situ fenton reaction and robust immune responses in combination with toll-like receptor 7/8 agonist. Chem Eng J. 2023;472:144909
108. Wu G-l, Sun B, He Y, Tan X, Pan Q, Yang S. et al. Rational design of molecular phototheranostic platform for nir-ii fluorescence imaging guided chemodynamic-photothermal combined therapy. Chem Eng J. 2023;463:142372
109. Gao Y, Zhang H, Tang L, Li F, Yang L, Xiao H. et al. Cancer nanobombs delivering artoxplatin with a polyigniter bearing hydrophobic ferrocene units upregulate pd-l1 expression and stimulate stronger anticancer immunity. Adv Sci. 2024;11:e2300806
110. Wu G-l, Liu F, Li N, Wang F, Yang S, Wu F. et al. Tumor microenvironment-responsive one-for-all molecular-engineered nanoplatform enables nir-ii fluorescence imaging-guided combinational cancer therapy. Anal Chem. 2023;95:17372-17383
111. Liu X, Li W, Wang M, Liu N, Yang Q, He Y. et al. Inflammatory cell-inspired cascade nanozyme induces intracellular radical storm for enhanced anticancer therapy. Small Methods. 2023;7:2201641
112. Fan W, Lu N, Huang P. et al. Glucose-responsive sequential generation of hydrogen peroxide and nitric oxide for synergistic cancer starving-like/gas therapy. Angew Chem Int Ed. 2016;55:1
113. Huo M, Wang L, Chen Y, Shi J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat Commun. 2017;8:357
114. Li S Y, Cheng H, Xie B R, Qiu W X, Zeng J Y, Li C X. et al. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy. ACS Nano. 2017;11:7006
115. Zhang R, Feng L, Dong Z, Wang L, Liang C, Chen J. et al. Glucose & oxygen exhausting liposomes for combined cancer starvation and hypoxia-activated therapy. Biomaterials. 2018;162:123
116. Zhao W, Hu J, Gao W. Glucose oxidase-polymer nanogels for synergistic cancer-starving and oxidation therapy. Acs Appl Mater Inter. 2017;9:23528
117. Fu L H, Qi C, Lin J, Huang P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem Soc Rev. 2018;47:6454
118. Wang C, Ye Y, Hochu G M, Sadeghifar H, Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-pd1 antibody. Nano Lett. 2016;16:2334
119. Zhang L, Hu X, Chen Y, Zhu J, Liu Q, Wan Z. et al. Multiple in-situ reactions induced by biodegradable iodides: A synergistically chemodynamic-photothermal therapy platform. Chem Eng J. 2023;465:142699
120. Dai Y, Yang Z, Cheng S, Wang Z, Zhang R, Zhu G. et al. Toxic reactive oxygen species enhanced synergistic combination therapy by self-assembled metal-phenolic network nanoparticles. Adv Mater. 2018;30:1704877
121. Ma P a, Xiao H, Yu C, Liu J, Cheng Z, Song H. et al. Enhanced cisplatin chemotherapy by iron oxide nanocarrier-mediated generation of highly toxic reactive oxygen species. Nano Lett. 2017;17:928
122. Ren Z, Sun S, Sun R, Cui G, Hong L, Rao B. et al. A metal-polyphenol-coordinated nanomedicine for synergistic cascade cancer chemotherapy and chemodynamic therapy. Adv Mater. 2019;32:1906024
123. Yang K, Qi S, Yu X, Bai B, Zhang X, Mao Z. et al. A hybrid supramolecular polymeric nanomedicine for cascade-amplified synergetic cancer therapy. Angew Chem Int Ed. 2022;61:e202203786
124. Abedi M H, Yao M S, Mittelstein D R, Bar-Zion A, Swift M B, Lee-Gosselin A. et al. Ultrasound-controllable engineered bacteria for cancer immunotherapy. Nat Commun. 2022;13:1585
125. Zhang X, Lyu Y, Li J. et al. Bimetallic Nanozymes-Integrated Parachute-Like Au2Pt@PMO@ICG Janus Nanomotor with Dual Propulsion for Enhanced Tumor Penetration and Synergistic PTT/PDT/CDT Cancer Therapy. Adv Func Mater. 2024;34:2406059
126. Li L, Yang Z, Fan W, He L, Cui C, Zou J. et al. In situ polymerized hollow mesoporous organosilica biocatalysis nanoreactor for enhancing ros-mediated anticancer therapy. Adv Funct Mater. 2020;30:1907716
127. Zhang N N, Lu C Y, Chen M J, Xu X L, Shu G F, Du Y Z. et al. Recent advances in near-infrared ii imaging technology for biological detection. J Nanobiotechnology. 2021;19:132
128. Cheng P H, Pu K Y. Molecular imaging and disease theranostics with renal-clearable optical agents. Nat Rev Mater. 2021;6:1095
129. Ling S, Yang X, Li C, Zhang Y, Yang H, Chen G. et al. Tumor microenvironment-activated nir-ii nanotheranostic system for precise diagnosis and treatment of peritoneal metastasis. Angew Chem Int Ed. 2020;59:7219
130. Shen J, Chen J, Ma J, Fan L, Zhang X, Yue T. et al. Enhanced lysosome escape mediated by 1,2-dicarboxylic-cyclohexene anhydride-modified poly-l-lysine dendrimer as a gene delivery system. Asian J Pharm Sci. 2020;15:759
131. Deverman B E, Ravina B M, Bankiewicz K S, Paul S M, Sah D W Y. Gene therapy for neurological disorders: Progress and prospects. Nat Rev Drug Discov. 2018;17:641
132. Huang R-Y, Lin Y-H, Lin S-Y, Li Y-N, Chiang C-S, Chang C-W. Magnetic ternary nanohybrids for nonviral gene delivery of stem cells and applications on cancer therapy. Theranostics. 2019;9:2411
133. Liu J, Song L, Liu S, Jiang Q, Liu Q, Li N. et al. A DNA-based nanocarrier for efficient gene delivery and combined cancer therapy. Nano Lett. 2018;18:3328
134. Dong Y, Yu T, Ding L, Laurini E, Huang Y, Zhang M. et al. A dual targeting dendrimer-mediated sirna delivery system for effective gene silencing in cancer therapy. J Am Chem Soc. 2018;140:16264
135. Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432
136. Ohaegbulam K C, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the pd-1 and pd-l1 pathway. Trends Mol Med. 2015;21:24
137. Song X, Xu J, Liang C, Chao Y, Jin Q, Wang C. et al. Self-supplied tumor oxygenation through separated liposomal delivery of h2o2 and catalase for enhanced radio-immunotherapy of cancer. Nano Lett. 2018;18:6360
138. Zou W. Regulatory t cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295
139. Li B, Hao G, Sun B, Gu Z, Xu Z P. Engineering a therapy-induced “immunogenic cancer cell death” amplifier to boost systemic tumor elimination. Adv Funct Mater. 2020;30:1909745
140. Stickdorn J, Stein L, Arnold-Schild D, Hahlbrock J, Medina-Montano C, Bartneck J. et al. Systemically administered tlr7/8 agonist and antigen-conjugated nanogels govern immune responses against tumors. ACS Nano. 2022;16:4426
141. Zhao P, Jin Z, Chen Q, Yang T, Chen D, Meng J. et al. Local generation of hydrogen for enhanced photothermal therapy. Nat Commun. 2018;9:4241
142. Deng Y, Jia F, Chen S, Shen Z, Jin Q, Fu G. et al. Nitric oxide as an all-rounder for enhanced photodynamic therapy: Hypoxia relief, glutathione depletion and reactive nitrogen species generation. Biomaterials. 2018;187:55
143. Zhang X, Tian G, Yin W, Wang L, Zheng X, Yan L. et al. Controllable generation of nitric oxide by near-infrared-sensitized upconversion nanoparticles for tumor therapy. Adv Funct Mater. 2015;25:3049
144. Zhang K, Xu H, Jia X, Chen Y, Ma M, Sun L. et al. Ultrasound-triggered nitric oxide release platform based on energy transformation for targeted inhibition of pancreatic tumor. ACS Nano. 2016;10:10816
145. Zhang X, Du J, Guo Z, Yu J, Gao Q, Yin W. et al. Efficient near infrared light triggered nitric oxide release nanocomposites for sensitizing mild photothermal therapy. Adv Sci. 2018;6:1801122
146. Gehring J, Trepka B, Klinkenberg N, Bronner H, Schleheck D, Polarz S. Sunlight-triggered nanoparticle synergy: Teamwork of reactive oxygen species and nitric oxide released from mesoporous organosilica with advanced antibacterial activity. J Am Chem Soc. 2016;138:3076
147. Lundberg J O, Gladwin M T, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Gastro Hepat. 2015;14:623
148. Wang Z, Zhan M, Li W, Chu C, Xing D, Lu S. et al. Photoacoustic cavitation-ignited reactive oxygen species to amplify peroxynitrite burst by photosensitization-free polymeric nanocapsules. Angew Chem Int Ed. 2021;60:4720
149. Hodson R. Precision medicine. Nature. 2016;537:S49
150. Yang M, Li J, Gu P, Fan X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact Mater. 2021;6:1973
151. Yeldag G, Rice A, Del Río Hernández A. Chemoresistance and the self-maintaining tumor microenvironment. Cancers. 2018;10:471
152. Kakiuchi N, Ogawa S. Clonal expansion in non-cancer tissues. Nat. Rev. Cancer. 2021;21:239
153. Choi C-H R, Bakir I A, Hart A L, Graham T A. Clonal evolution of colorectal cancer in ibd. Nat Rev Gastro Hepat. 2017;14:218
154. Craig A J, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastro Hepat. 2019;17:139
Author biography
 Gui-long Wu received his Ph.D. at the University of South China in 2024 under the supervision of Prof. Qinglai Yang. In 2024, Dr. Wu joined Qinglai Yang group at the Center for Molecular Imaging Probe, Hunan Province Key Laboratory of Tumor Cellular and Molecular Pathology, Cancer Research Institute, Hengyang Medical School, University of South China. The University of South China. Her research interests focus on molecular fluorescence imaging for cancer therapy.
Gui-long Wu received his Ph.D. at the University of South China in 2024 under the supervision of Prof. Qinglai Yang. In 2024, Dr. Wu joined Qinglai Yang group at the Center for Molecular Imaging Probe, Hunan Province Key Laboratory of Tumor Cellular and Molecular Pathology, Cancer Research Institute, Hengyang Medical School, University of South China. The University of South China. Her research interests focus on molecular fluorescence imaging for cancer therapy.
 Senyou Tan is currently studying clinical medicine in Hengyang Medical College, University of South China. At the same time, he is participating in research projects under Professor Yang Qinglai at the Molecular Imaging Probe Center of Hunan Key Laboratory of Tumor Cell and Molecular Pathology, Cancer Research Institute, Hengyang Medical College, University of South China. His research interest covers the design and application of NIR-II fluorescent molecular probes.
Senyou Tan is currently studying clinical medicine in Hengyang Medical College, University of South China. At the same time, he is participating in research projects under Professor Yang Qinglai at the Molecular Imaging Probe Center of Hunan Key Laboratory of Tumor Cell and Molecular Pathology, Cancer Research Institute, Hengyang Medical College, University of South China. His research interest covers the design and application of NIR-II fluorescent molecular probes.
 Xiaofeng Tan received his Ph.D. at the University of Jinan in 2021 under the supervision of Prof. Gengxiu Zheng and He Li. In 2021, Dr. Tan joined Qinglai Yang group at the University of South China. His scientific interests are primarily in developing various functional nanomaterials for cancer diagnosis and antibacterial therapy.
Xiaofeng Tan received his Ph.D. at the University of Jinan in 2021 under the supervision of Prof. Gengxiu Zheng and He Li. In 2021, Dr. Tan joined Qinglai Yang group at the University of South China. His scientific interests are primarily in developing various functional nanomaterials for cancer diagnosis and antibacterial therapy.
 Guodong Chen received his PhD from Southern Medical University under the guidance of Professor of Prof. Zonghai Huang. Presently, he holds the position of vice president at the First Affiliated Hospital of Nanhua University and serves as the deputy director of the Hunan Engineering Research Centre for Early Diagnosis and Treatment of Liver Cancer. His primary scientific research interests are in the field of hepatobiliary and pancreatic malignant tumours, with a specific emphasis on studying near-infrared two-region organic probes for the purpose of diagnosing and treating liver cancer.
Guodong Chen received his PhD from Southern Medical University under the guidance of Professor of Prof. Zonghai Huang. Presently, he holds the position of vice president at the First Affiliated Hospital of Nanhua University and serves as the deputy director of the Hunan Engineering Research Centre for Early Diagnosis and Treatment of Liver Cancer. His primary scientific research interests are in the field of hepatobiliary and pancreatic malignant tumours, with a specific emphasis on studying near-infrared two-region organic probes for the purpose of diagnosing and treating liver cancer.
 Qinglai Yang received his Ph.D. at Shanghai Jiao Tong University in 2015 under the supervision of Prof. Yumei Shen and Bing Gong. After two years of postdoctoral research at the Research Institute of Tsinghua University in Shenzhen under the supervision of Prof. Hongjie Dai. He joined Shanghai Jiao Tong University School of Medicine, Prof. Weihong Tan's group, as an associate research fellow in 2018 and then joined the University of South China Cancer Research Institute of Medical College in 2020 as a professor. His research interests focus on materials design and synthesis for molecular imaging and therapy.
Qinglai Yang received his Ph.D. at Shanghai Jiao Tong University in 2015 under the supervision of Prof. Yumei Shen and Bing Gong. After two years of postdoctoral research at the Research Institute of Tsinghua University in Shenzhen under the supervision of Prof. Hongjie Dai. He joined Shanghai Jiao Tong University School of Medicine, Prof. Weihong Tan's group, as an associate research fellow in 2018 and then joined the University of South China Cancer Research Institute of Medical College in 2020 as a professor. His research interests focus on materials design and synthesis for molecular imaging and therapy.
![]() Corresponding authors: Qinglai Yang, The First Affiliated Hospital & Center for Molecular Imaging Probe of Cancer Research Institute, Hengyang Medical School, University of South China, Hengyang, Hunan 421001, China. Email: qingyu513edu.cn. Guodong Chen, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan 421001, China. Email: chenguodongedu.cn. Xiaofeng Tan, Center for Molecular Imaging Probe of Cancer Research Institute, Hengyang Medical School, University of South China, Hengyang, Hunan 421001, China. Email: tanxiaofengedu.cn.
Corresponding authors: Qinglai Yang, The First Affiliated Hospital & Center for Molecular Imaging Probe of Cancer Research Institute, Hengyang Medical School, University of South China, Hengyang, Hunan 421001, China. Email: qingyu513edu.cn. Guodong Chen, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan 421001, China. Email: chenguodongedu.cn. Xiaofeng Tan, Center for Molecular Imaging Probe of Cancer Research Institute, Hengyang Medical School, University of South China, Hengyang, Hunan 421001, China. Email: tanxiaofengedu.cn.
 Global reach, higher impact
Global reach, higher impact