13.3
Impact Factor
Theranostics 2025; 15(1):300-323. doi:10.7150/thno.104594 This issue Cite
Review
Targeting cancer with precision: strategical insights into TCR-engineered T cell therapies
1. Stomatological Hospital, School of Stomatology, Southern Medical University, Guangzhou, 510280, Guangdong, China.
2. Department of Dentistry, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, 510080, China.
3. School of Dentistry, University of California, Los Angeles, Los Angeles, 90095, CA, USA.
*Equal contributors
Received 2024-10-3; Accepted 2024-11-11; Published 2025-1-1
Abstract
T cell receptor-engineered T (TCR-T) cell therapies are at the forefront of cancer immunotherapy, offering a transformative approach that significantly enhances the ability of T cells to recognize and eliminate cancer cells. This innovative method involves genetically modifying TCRs to increase their affinity for tumor-specific antigens. While these enhancements improve the ability of T cells to recognize and bind to antigens on cancer cells, rigorous assessment of specificity remains crucial to ensure safety and targeted responses. This dual focus on affinity and specificity holds significant promise for the treatment of solid tumors, enabling precise and efficient cancer cell recognition. Despite rapid advancements in TCR engineering and notable progress in TCR screening technologies, as evidenced by the growing number of specific TCRs entering clinical trials, several technical and clinical challenges remain. These challenges primarily pertain to the specificity, affinity, and safety of engineered TCRs. Moreover, the accurate identification and selection of TCRs that are both effective and safe are essential for the success of TCR-T cell therapies in cancer treatment. This review provides a comprehensive examination of the theoretical foundations of TCR therapy, explores strategies for screening specific TCRs and antigens, and highlights the ongoing challenges in this evolving therapeutic landscape.
Keywords: T cell receptor-engineered T cell therapy, screening strategy, cancer immunotherapy
Introduction
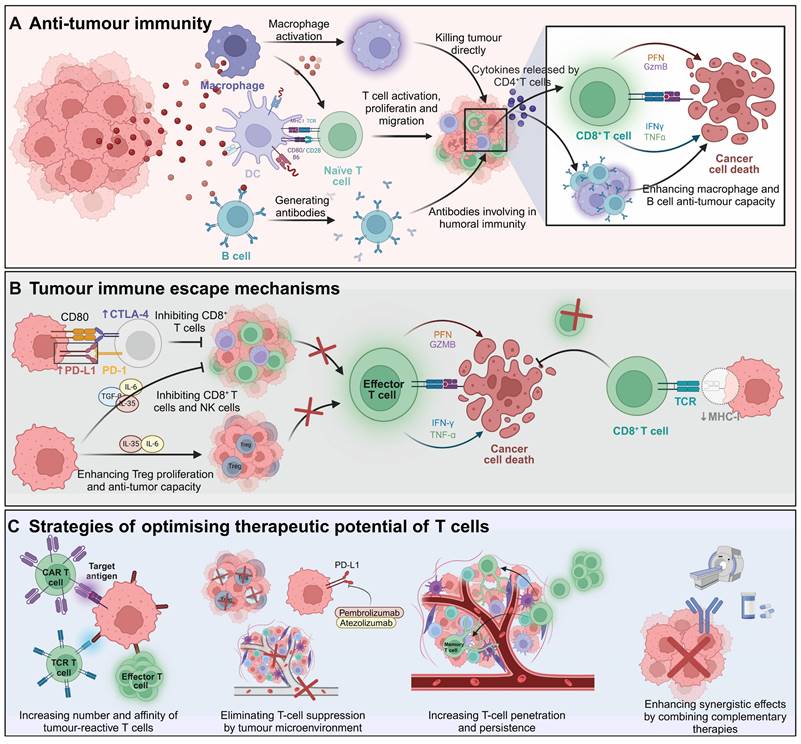
T cells, a crucial subset of lymphocytes, exhibit intrinsic anti-tumor properties and are central to the immune defense against cancer. The anti-cancer response is mediated through a synergistic action of cytotoxic T cells (CD8+) and helper T cells (CD4+). CD8+ T cells target and eradicate malignant cells and proliferate rapidly upon encountering specific tumor antigens, thereby significantly contributing to the immune counterattack against cancer. Simultaneously, CD4+ T cells play a critical role in shaping adaptive immune responses and enhance these responses by activating CD8+ T cells [1, 2] (Figure 1A). Nevertheless, the effectiveness of this immune mechanism is often undermined by tumor strategies that evade immune detection, involving limited antigen presentation, immunosuppression, and the induction of T cell exhaustion [3, 4] (Figure 1B). These challenges underscore the imperative to develop strategies that enhance the therapeutic efficacy of T cells. Such strategies may include augmenting CD8+ T cell populations, boosting their antigen recognition capabilities, reducing factors that lead to exhaustion or suppression, and optimizing the supportive role of CD4+ T cells while curbing the generation of immunosuppressive regulatory T cells (Tregs) (Figure 1C).
Overview of T-cell immunity against tumors. A) Tumor antigens are recognized by DCs, which activate naïve T cells and stimulate the generation of antigen-specific cytotoxic CD8+ T cells and helper CD4+ T cells. CD8+ T cells release PFN and GZMB to induce cancer cell apoptosis. CD4+ T cells secrete cytokines such as IFN-γ and TNF-α, enhancing CD8+ T cell cytotoxicity and promoting macrophage activation and B cell-mediated humoral immunity. B) Tumors evade immune surveillance through multiple mechanisms. They upregulate inhibitory molecules like PD-L1, which engage co-inhibitory receptors such as CTLA-4 on CD8+ T cells, thereby suppressing their effector functions. Additionally, tumors downregulate MHC class I molecules, reducing the ability of CD8+ T cells to recognize tumor antigens via TCR, leading to impaired recognition and elimination of cancer cells. Tumor-secreted cytokines, including IL-6, TGF-β, and IL-10, further inhibit the activity of CD8+ T cells and NK cells. These cytokines, along with IL-35, also promote the proliferation of Tregs, which play a crucial role in suppressing anti-tumor immune responses, further enabling tumor immune evasion. C) Strategies include CAR T cells and TCR-engineered T cells to enhance the number and affinity of tumor-reactive T cells. Immune checkpoint inhibitors such as pembrolizumab and atezolizumab restore T cell activity by counteracting the immunosuppressive TME. Additional approaches focus on improving T cell infiltration, persistence, and combining therapies for synergistic effects against cancer. The images in the figures were created using BioRender (https://www.biorender.com/).
Advancements in basic science have catalyzed the development of novel therapeutic approaches based on T cell-mediated immune responses. Checkpoint blockade therapy, for example, enhances T cell activity against tumors by blocking inhibitory pathways [2]. Similarly, cellular immunotherapy has progressed significantly through TCR engineering, which improves T cell specificity by modifying their TCRs to better recognize specific tumor antigens, thus offering a promising strategy for personalized cancer immunotherapy [1, 5]. Notably, TCR-T cell therapy offers distinct advantages over other cellular therapies, such as dendritic cell (DC) vaccines, CAR-T, and tumor-infiltrating lymphocyte (TIL) therapies, due to its unique capacity to target intracellular antigens presented by major histocompatibility complex (MHC) molecules. In contrast to CAR-T cells, which are limited to recognizing surface antigens via engineered receptors, TCR-T cells can engage a broader range of tumor-associated antigens, including those derived from intracellular proteins [6]. This enhances their applicability to a wider spectrum of cancers, particularly solid tumors, where intracellular antigens are more common. Moreover, compared to TIL therapy, which requires the expansion of tumor-reactive T cells from patient biopsies—a process that can be lengthy and inefficient—TCR-T cells can be rapidly engineered to target specific antigens, streamlining the treatment process [7]. While DC vaccines aim to prime the immune system by presenting antigens to T cells, their efficacy is often limited by the patient's existing immune landscape [8]. In contrast, TCR-T cells provide a direct and potent antitumor response by engineering T cells with predefined specificity and affinity for tumor antigens. These factors make TCR-T cell therapy a versatile and powerful approach in the context of cancer immunotherapy, offering advantages in targeting a wider array of tumors with greater precision [9].
This review highlights several critical aspects of TCR-T cell therapy. First, we critically evaluate the current strategies for TCR screening, focusing on the methods used to identify and select tumor-specific TCRs with optimal therapeutic potential. Second, we systematically summarize the key clinical trials and preclinical studies of TCR-T therapy, providing an overview of its progress and therapeutic efficacy across various cancer types. Finally, we objectively analyze the challenges currently faced by TCR-T therapy, such as antigen selection, TCR affinity, and safety concerns, and propose potential strategies to overcome these barriers, aiming to enhance the clinical impact and applicability of TCR-T cell therapies in cancer treatment.
Overview of TCR biology
TCR structure and antigen recognition
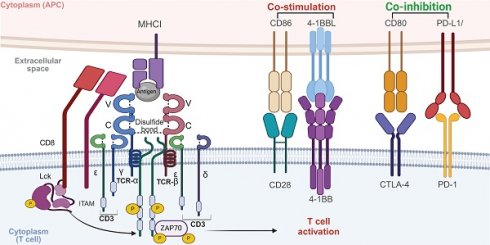
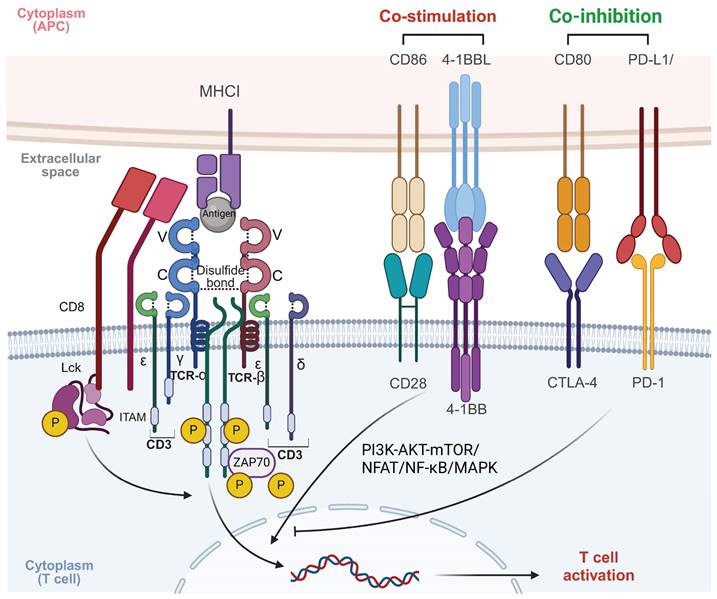
The TCR complex, chiefly composed of TCRαβ or TCRγδ heterodimers alongside the CD3 signaling subunits, plays a pivotal role in T cell immune function. These TCR chains, transmembrane glycoproteins encoded by specific loci, couple non-covalently to form heterodimers with variable and constant immunoglobulin-like domains, a transmembrane domain, and a short cytoplasmic tail [10]. Antigen recognition by the TCR involves three complementarity-determining regions (CDRs) on the α and β chains, interacting with peptide-MHC (pMHC) complexes [11]. The CDR3 region is formed by DNA recombination involving juxtaposition of Vα and Jα segments for the α chain genes, and of Vβ, D, and Jβ segments for the β chain genes [12]. The CDR1 and CDR2 regions, which exhibit limited diversity due to their germline-encoded nature, work in tandem with the highly variable CDR3 region, a key player in recognizing a wide range of antigens. The Vα CDR1 and CDR2 loops closely interact with the helices of the pMHC complex, whereas the Vβ CDR1 and CDR2 loops engage with the pMHC at the carboxy terminus of the bound peptide [13, 14]. The TCR docks diagonally over the pMHC to initiate T cell activation, differentiation, and proliferation, ensuring consistent contact. This interaction is enhanced by CD4 and CD8 co-receptors [15]. Importantly, the TCRα chain is positioned above the MHC-I α2-helix or MHC-II β1-helix, while the TCRβ chain docks above the α1-helix of both MHC-I and MHC-II, underlining the conserved docking topology crucial for effective immune responses [16, 17].
TCR signaling and activation
Signal transduction in the TCR is primarily driven by the CD3 protein complex, due to the TCR's short intracellular domain. Hydrophobic interactions enable TCR binding to the CD3εδ, CD3εγ, and CD3ζζ dimers, forming the functional TCR-CD3 complex [18]. Each CD3 subunit contains one to three immunoreceptor tyrosine-based activation motifs (ITAMs), essential for signal transduction. Upon TCR-pMHC interaction, a conformational shift and phosphorylation of ITAMs occur, initiating antigen-specific immune responses [19]. T cell activation extends beyond TCR-pMHC binding, requiring interactions between various adhesion molecules on antigen-presenting cells (APCs) and T cells. These molecules deliver co-stimulatory signals crucial for stable and efficient T cell activation [20]. After the primary TCR-pMHC signal, secondary signals are induced by co-stimulatory receptors like CD28 and ICOS, which interact with ligands such as B7-1 (CD80), B7-2 (CD86), ICOSL [21, 22]. TNFSF/TNFRSF members, such as 4-1BB/CD137, are also critical co-stimulatory molecules, especially in CAR-T cell design [23]. Notably, co-stimulatory molecules enhance TCR signals and significantly amplify T cell activation, proliferation, and differentiation by activating key signaling pathways such as PI3K-AKT-mTOR, NFAT, NF-κB, and MAPK [24]. Additionally, co-inhibitory molecules on T cell surfaces, such as CTLA-4 and PD-1, transmit inhibitory signals that down-regulate or terminate T cell activation [25]. These molecules are essential for maintaining immune homeostasis and self-tolerance, thus preventing autoimmune diseases and excessive immune responses [22] (Figure 2).
TCR repertoire diversity
TCR diversity is primarily achieved through the combinational diversity of α and β chains and junctional variations at their joining segments [26]. The α chain's variable region is encoded by V and J gene segments (TRAV and TRAJ), while the β chain includes V, D, and J segments (TRBV, TRBD, and TRBJ) [27]. During thymic maturation, these segments undergo V-J or V-D-J recombination, generating diverse α and β chains that enhance TCR's ability to specifically recognize pMHC molecules [28]. This process, coupled with random pairing of different α and β chains post-rearrangement, produces unique αβ TCR pairs with distinct antigen-binding capabilities [29]. Junctional diversity, resulting from inaccuracies like frame shifts and nucleotide deletions during VDJ recombination, alters the amino acid composition of the V region, enhancing TCR specificity. Additionally, terminal deoxynucleotidyl transferase introduces random non-template nucleotides at the V(D)J junctions during rearrangement, greatly expanding the TCR repertoire [30]. Consequently, VDJ recombination and non-template additions yield a theoretical TCR repertoire of approximately 2 × 1019 unique αβ TCR pairs [31]. This extensive diversityenables recognition of a wide range of antigen peptides presented on pMHC.
Mechanism of T cell activation through TCR signaling. The TCR complex recognizes antigenic peptides presented by MHC I molecules on APCs. CD8 co-receptor binds to MHC I, stabilizing the interaction. Upon antigen recognition, the ITAMs on CD3 are phosphorylated by the associated kinase LCK, leading to the recruitment and activation of ZAP70. This initiates downstream signaling cascades involving pathways such as PI3K-AKT-mTOR, NFAT, NF-κB, and MAPK, which ultimately result in T cell activation, proliferation, and effector functions. Co-stimulatory signals, provided by CD28 binding to CD86 and 4-1BBL binding to 4-1BB, are crucial for full T cell activation. These signals enhance the activation of downstream signaling pathways, promoting T cell survival, proliferation, and cytokine production. In contrast, co-inhibitory signals, mediated by CTLA-4 and PD-1 interacting with CD80 and PD-L1, respectively, dampen T cell activation. These inhibitory signals play a critical role in maintaining immune homeostasis and preventing overactivation, but in the context of cancer, they can contribute to immune evasion by tumors. Balancing co-stimulatory and co-inhibitory signals is essential for regulating T cell responses in both immunity and immunotherapy. The images in the figures were created using BioRender (https://www.biorender.com/).
Tumor antigens and their relevance to TCR-T cell therapy
Classification of tumor antigens
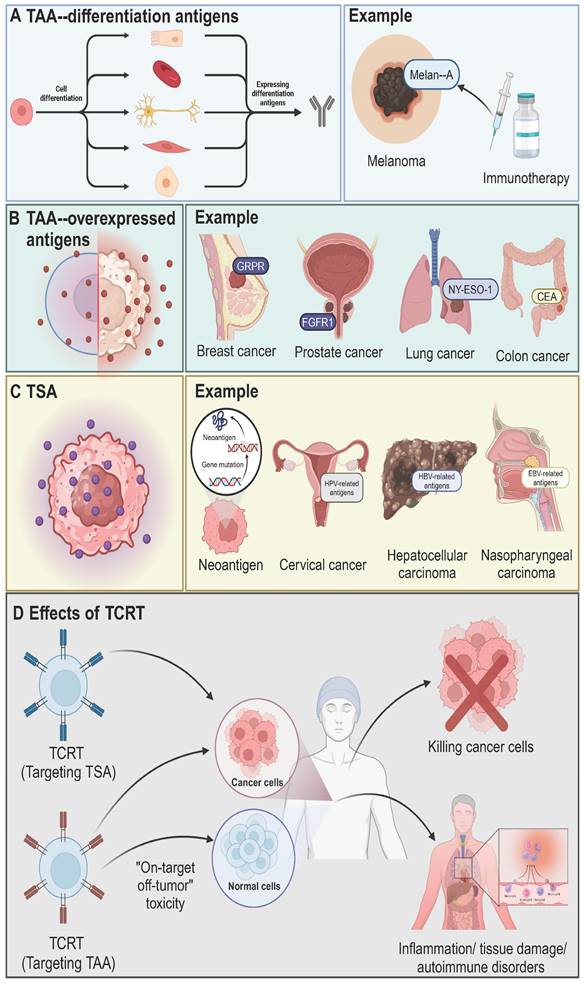
The therapeutic efficacy and safety of TCR-T cell therapy depend on the careful selection of tumor antigens. Successful tumor eradication relies on strong TCR-antigen binding, influenced by antigen processing and presentation. Tumor antigens are broadly classified into two categories: tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs) [32] (Figure 3). TAAs are expressed in both normal and tumor cells and include differentiation and overexpressed antigens. Differentiation antigens, such as Melan-A, are linked to specific cell types and are used in cancer diagnosis and therapy [33]. Overexpressed antigens, like FGFR1, NY-ESO-1, and carcinoembryonic antigen (CEA), show higher expression in tumors compared to normal tissues [34, 35]. Cancer germline antigens (CGAs), a subset of TAAs, are aberrantly expressed in cancer cells and are naturally found only in germline cells of healthy tissues, such as testes [36]. CGAs are promising targets for cancer treatments, with researched antigens including MAGE-A1, MAGE-A3, MAGE-A4, NY-ESO-1, PRAME, CT83, and SSX2, expressed in various solid tumors like melanoma and liver, lung, bladder, and neuroblastoma cancers [37]. Nevertheless, targeting TAAs poses risks such as "on-target off-tumor" toxicity, leading to side effects like inflammation and tissue damage, and is further limited by restricted expression across cancer types, narrow HLA compatibility, and intra-tumoral heterogeneity [38, 39]. TSAs, on the other hand, are exclusively expressed in tumor cells, reducing the risk of toxicity in normal tissues. High-affinity T cells that evade thymic negative selection can be isolated from patients or donors, making TSAs ideal targets [9]. TSAs are classified into viral antigens, such as those from HPV and Epstein-Barr virus, and neoantigens, which arise from tumor-specific mutations [32, 40]. Neoantigens are particularly promising due to their patient-specific nature, low toxicity, and ability to escape negative selection, making them a key focus of current immunotherapy research [41].
Processing and presentation of different types of antigens
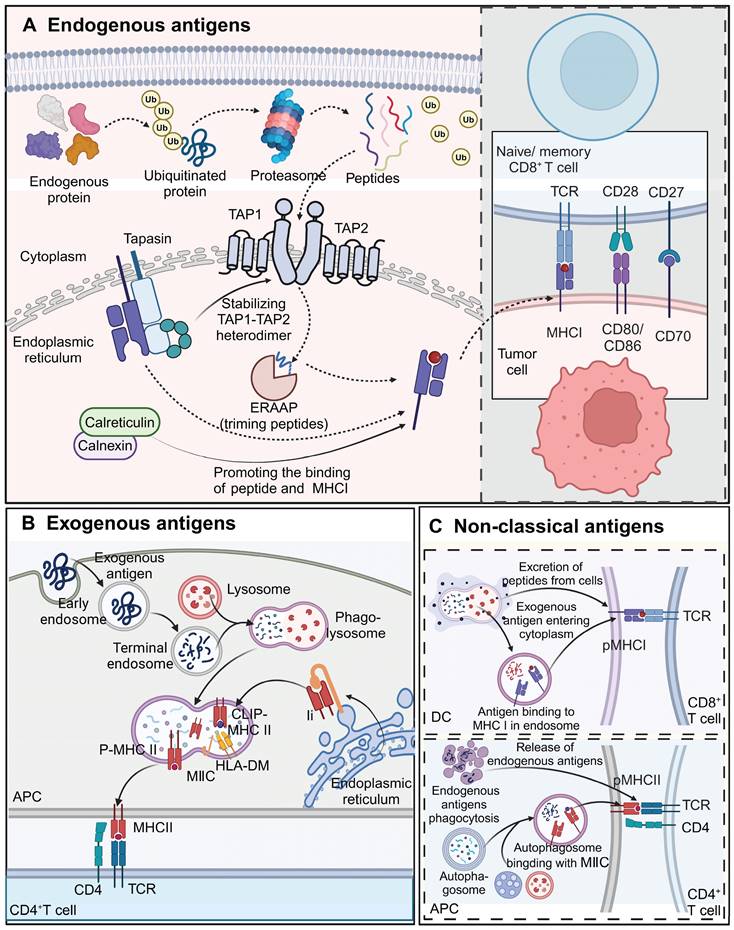
Processing and presentation of endogenous antigens
Endogenous antigens, including viral proteins, tumor antigens, and certain self-antigens, are processed via the MHC class I pathway and presented to CD8+ T cells. This process begins with the degradation of defective proteins by the ubiquitin-proteasome system [42]. The resulting peptides are translocated into the ER lumen by the ATP-dependent transporter associated with antigen processing (TAP), composed of TAP1 and TAP2 subunits. Some peptides undergo further trimming by ER-associated aminopeptidase (ERAAP) to fit the MHC class I binding groove optimally [43, 44]. The peptide-loading complex, consisting of TAP, tapasin, ERp57, and calreticulin or calnexin, facilitates peptide binding to MHC class I molecules [45]. Tapasin stabilizes the TAP heterodimer and promotes peptide loading onto MHC-I molecules [46]. The loaded MHC-I complexes are then transported to the cell surface for recognition by CD8+ T cells. Since TCR-T cell therapies typically target endogenous antigens and rely on CD8+ T cells, the MHC class I pathway is central to TCR-T cell recognition and tumor elimination [47] (Figure 4A).
Processing and presentation of exogenous antigens
Exogenous antigens, such as bacterial toxins, proteins, and pathogens, are processed via the MHC class II pathway and presented to CD4+ T cell. APCs, including DCs, B cells, macrophages, and thymic epithelial cells, internalize exogenous antigens through phagocytosis and endocytosis. Internalized proteins are degraded into peptides in phagolysosomes, which then merge with MHC class II compartments (MⅡCs) for peptide loading [48]. Most MHC-II peptides are loaded through the invariant chain (Ii)-dependent pathway, where the Ii protein blocks the binding groove until hydrolysis generates class II-associated invariant chain peptide (CLIP), which is then displaced by HLA-DM, allowing antigenic peptide binding [49]. These peptide-MHC-II complexes are transported to the APC surface for recognition by CD4+ T cells, a crucial step in immunotherapies targeting exogenous antigens (Figure 4B).
Non-classical antigen processing and presentation pathways
Cross-presentation enables the presentation of exogenous antigens via MHC class I to CD8+ T cells or endogenous antigens via MHC class II to CD4+ T cells. DCs play a primary role in cross-presentation [50]. Mechanisms include the direct translocation of exogenous antigens into the cytoplasm or their escape from phagolysosomes for binding to MHC-I molecules, or the binding of lysosome-generated peptides to surface MHC-I molecules via exocytosis [51]. This pathway is important for stimulating CD8+ T cells in response to vaccines and pathogens, enhancing antitumor immunity. Moreover, MHC-II molecules have been implicated in presenting endogenous antigens to CD4+ T cells, with autophagy potentially facilitating the cross-presentation of cytoplasmic and nuclear proteins [52] (Figure 4C).
Challenges in tumor antigen identification and targeting
The therapeutic success of TCR-T cell therapy depends on the precise selection and effective binding of engineered TCRs to tumor antigens. While theoretically capable of targeting any tumor antigen, the selection of targets that ensure both safety and efficacy remains a significant challenge [53]. Targeting antigens like viral antigens, neoantigens, and cancer-testis antigens, which are predominantly expressed in tumor cells and minimally in non-essential tissues, reduces the risk of damage to normal cells [54]. However, TAAs, even with elevated expression in tumors, may cause autoimmune toxicity due to their presence in normal tissues. The balance between TCR affinity and antigen recognition is crucial; insufficient affinity risks inadequate tumor cell recognition, while excessively high affinity can lead to overactivation, immune toxicity, and severe adverse effects [55].
Classification of tumor antigens. A) Differentiation antigens are restricted to specific cell lineages and are expressed during cell differentiation. These antigens, such as Melan-A in melanoma, are highly immunogenic and can serve as targets for immunotherapy. Melan-A is selectively expressed in melanocytes and melanoma cells, and immunotherapies targeting this antigen have been developed to elicit specific anti-tumor immune responses. B) Overexpressed antigens are present at elevated levels in tumor cells compared to normal tissues. Examples include MUC1 in breast cancer, PSA in prostate cancer, NY-ESO-1 in lung cancer, and CEA in colon cancer. These antigens are frequently utilized as biomarkers and therapeutic targets due to their high expression levels in tumors, allowing for more selective cancer treatments and monitoring of disease progression. C) TSAs are derived from tumor-specific mutations or viral infections and are exclusively expressed by cancer cells. Neoantigens, arising from somatic mutations, are key targets for personalized immunotherapies. Viral antigens, such as those associated with HPV in cervical cancer, HBV in hepatocellular carcinoma, and EBV in nasopharyngeal carcinoma, also represent critical targets for immune-based interventions. TSAs play a central role in driving specific immune responses against tumors, minimizing off-target effects on normal tissues. D) TCR-T can target either TSAs or TAAs. Targeting TSAs is associated with a higher likelihood of selectively killing cancer cells without affecting normal cells due to the tumor-specific nature of these antigens. However, TCR-T targeting TAAs, which are overexpressed in tumors but also present in normal tissues, can result in "on-target, off-tumor" toxicity, leading to adverse effects such as tissue damage, inflammation, and autoimmune disorders. The images in the figures were created using BioRender (https://www.biorender.com/).
Processing and presentation of different types of antigens. A) Endogenous proteins are ubiquitinated and degraded into peptides by the proteasome. Peptides are transported by the TAP1-TAP2 complex into the endoplasmic reticulum, where they are trimmed by ERAAP and loaded onto MHC I molecules. Calreticulin and calnexin assist in peptide loading and stabilization of MHC I. Once the peptide-MHC I complex is formed, it is transported to the cell surface to be recognized by CD8+ T cells, promoting cytotoxic T cell responses against tumor cells expressing these antigens. Co-stimulatory molecules such as CD80/CD86 and CD70 further enhance T cell activation. B) Exogenous antigens are taken up by APCs via endocytosis or phagocytosis. These antigens are processed in lysosomes and loaded onto MHC II molecules within the MIIC compartment, facilitated by HLA-DM, which removes CLIP from the MHC II complex. The peptide-MHC II complex is transported to the cell surface, where it is recognized by CD4+ T cells. This interaction triggers helper T cell activation and subsequent immune responses. C) Exogenous antigens can also enter the cytoplasm of APCs and be cross-presented on MHC I molecules, enabling CD8+ T cell activation. Additionally, endogenous antigens can be processed through autophagy, with autophagosomes delivering cytoplasmic contents, including endogenous antigens, to the MIIC for loading onto MHC II. Both cross-presentation and autophagy-mediated presentation allow for the activation of distinct subsets of T cells, broadening immune recognition of tumor antigens. The images in the figures were created using BioRender (https://www.biorender.com/).
Tumor antigen heterogeneity, characterized by variability in antigen expression across different tumors or even within the same tumor, presents additional challenges for consistent therapeutic outcome. This phenotypic diversity, driven by genetic mutations and environmental factors, allows some tumor cells to evade targeted therapy, leading to potential resistance to treatments. Moreover, inter-individual differences, particularly polymorphisms in MHC molecules, can result in varying immune responses to the same tumor antigen, affecting the efficacy of TCR-T cell therapy across patients. Strategies to address this issue, such as targeting multiple antigens or promoting epitope spreading via genetic engineering, are being explored [56].
Tumor cells employ multiple strategies to undermine the efficacy of TCR-T cell therapy, such as reducing TCR-antigen binding, altering antigen presentation, upregulating immune checkpoint molecules, and fostering an immunosuppressive tumor microenvironment that inhibits T cell function [57, 58]. Tumor cells can impair antigen presentation by reducing MHC class I expression, a phenomenon linked to mutations or epigenetic silencing of HLA genes. Additionally, defects in the antigen processing machinery pathway, crucial for MHC-antigen complex formation, can enable tumors to evade immune detection [59]. The tumor microenvironment exacerbates immune evasion by promoting immunosuppressive conditions, such as cytokine release and hypoxia-induced lactic acid production, which impair TCR-T cell function [60, 61].
In addition, engineered TCR-T cells' inability to distinguish between cancerous and normal cells expressing the same antigens remains a significant challenge. This lack of specificity, particularly concerning TAAs, can lead to severe off-target effects in healthy tissues. For example, fatal cardiotoxicity has been observed in metastatic melanoma patients treated with TCR-T cells targeting MART-1 and MAGE-A3, likely due to antigen expression in cardiac tissues [57]. Moreover, MART-1 and gp100-targeted TCR-T cells have been associated with ocular, cutaneous, and auditory toxicities due to TAA expression in melanocytes [62]. Off-target effects, characterized by unintended damage to non-target tissues, and cross-reactivity, where TCR-T cells recognize structurally similar antigens in unrelated tissues, such as MAGE-A3 TCRs binding to epitopes from MAGE-A12 or the TITIN protein, complicate the application of TCR-T cell therapies [63]. To mitigate these risks, approaches such as optimizing TCR structure and expression, as well as utilizing gene-editing technologies like CRISPR-Cas9 for antigen refinement, are under development. Close monitoring and effective management of adverse events are critical to ensuring the safety and efficacy of TCR-T cell therapies.
TCR screening approaches for optimizing TCR-T cell therapy
TCR screening is critical for the success of TCR-T cell therapy, particularly in personalized cancer immunotherapy. This process involves selecting TCRs with the appropriate specificity and affinity for tumor antigens to ensure precise targeting of cancer cells while minimizing off-target effects on normal tissues. Effective TCR screening is essential for developing tailored TCR-engineered therapies, improving therapeutic efficacy and reducing the risk of immune-related adverse events. Additionally, this approach facilitates the identification of novel tumor-specific antigens, expanding the potential applications of immunotherapy in cancer treatment [64].
Cell-based screening
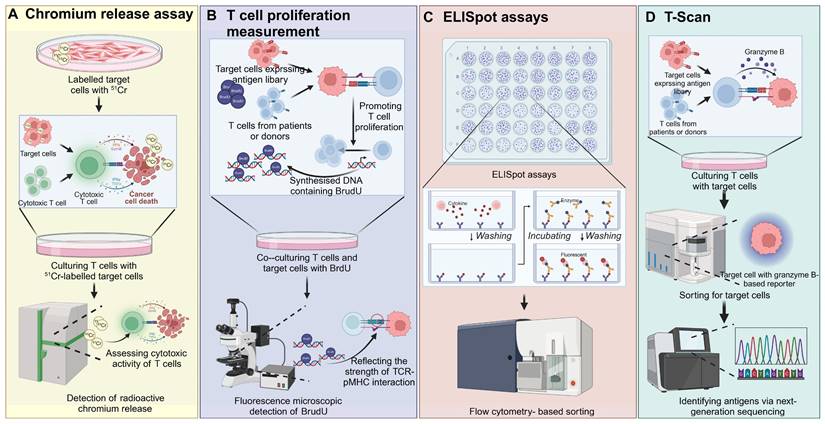
Cell-based screening strategies are indispensable in immunology for evaluating TCR-pMHC interactions and assessing the functional capacity of T cells after exposure to APCs expressing pMHC complexes. Several key techniques are utilized, each providing unique insights into T cell function [65]. T cell proliferation assays quantify the expansion of T cells in response to antigenic stimulation, often using [3H]-thymidine incorporation to measure DNA synthesis or CFSE dilution to track cell division. These assays provide a direct measure of T cell activation induced by TCR engagement with specific pMHC complexes, indicating the strength of the interaction and the capacity of T cells to proliferate in response to antigen [66]. Chromium release assays assess T cell cytotoxicity by labeling target cells with the radioactive isotope 51Cr. Upon T cell-mediated lysis, the release of 51Cr into the culture medium is measured, with higher levels indicating stronger cytolytic activity [67]. ELISpot assays are highly sensitive and allow for the detection of cytokine secretion at the single-cell level. In this assay, T cells are incubated on antibody-coated plates, where cytokines such as IFN-γ secreted in response to antigen stimulation are captured. A secondary antibody, linked to an enzyme, produces visible spots after binding to the captured cytokine, providing a quantitative measure of antigen-specific T cell responses [68]. Peptide-based ELISpot assays were used to screen for potential glioma neoepitopes in MHC-humanized mice, leading to the identification of MHCII-restricted CICR215W/Q as immunogenic. Following vaccination, TCR sequencing from neoepitope-specific T-cell lines enabled the discovery of CICR215W-specific TCRs. Adoptive intraventricular transfer of these TCR-transgenic T cells showed antitumor efficacy in a glioma model [69]. Similarly, using exome sequencing data from TCGA, shared neoantigen peptides were predicted, and ELISpot assays were employed to screen for CD8+ T cells reactive to the mutated FGFR3Y373C peptide in HLA-A*02:06 donors. FGFR3Y373C-specific TCRs were identified and expressed in engineered T cells, which selectively recognized the mutated peptide and exhibited cytotoxic activity [70]. Intracellular cytokine staining involves the fixation and permeabilization of activated T cells, allowing antibodies to access intracellular cytokines produced during TCR engagement. T cells are stained with fluorescently labeled antibodies targeting cytokines such as IFN-γ, TNF-α, or IL-2, and analyzed via flow cytometry to quantify the proportion of cytokine-producing T cells. To enhance detection, inhibitors such as Brefeldin A or Monensin are used to block cytokine secretion, ensuring their accumulation within the cells for accurate measurement [71]. T-Scan technology is a high-throughput, genome-wide approach that identifies antigens recognized by T cells. Using MHC-I molecules to display peptides from pathogen or human protein libraries, this system employs a granzyme B-based reporter to detect T cell-mediated killing. Target cells expressing specific antigens that induce T cell cytotoxicity are isolated, and the corresponding antigens are identified using next-generation sequencing (NGS). Unlike traditional methods that focus on TCR-pMHC binding affinity, T-Scan emphasizes T cells' natural cytolytic activity, effectively identifying both known and novel peptide epitopes and providing a comprehensive map of TCR specificity [62] (Figure 5).
Cell-based TCR screening strategies. A) Target cells are labeled with radioactive chromium (51Cr) and co-cultured with cytotoxic T cells. Upon recognition and lysis of target cells by T cells, chromium is released into the supernatant. The level of chromium release is measured to assess T cell cytotoxicity, providing a quantitative evaluation of the T cell-mediated killing of cancer cells. B) T cells from patients or donors are co-cultured with target cells expressing a library of antigens. The proliferation of T cells is monitored by the incorporation of BrdU into newly synthesized DNA during cell division. The level of BrdU incorporation, detected through fluorescence microscopy, reflects the strength of the TCR-pMHC interaction, providing insights into T cell responses against specific antigens. C) ELISpot is used to detect cytokine secretion from individual T cells in response to target cells. T cells are co-cultured with target cells, and cytokine release is captured on a membrane pre-coated with specific antibodies. After incubation and washing, spots representing single T cell cytokine release are visualized. Flow cytometry-based sorting can then be used to isolate specific subsets of T cells for further analysis. D) In T-Scan, T cells are cultured with target cells expressing an antigen library. Granzyme B release upon target cell recognition is measured, serving as an indicator of cytotoxic activity. Target cells are sorted based on granzyme B reporter expression, and next-generation sequencing is used to identify the antigen that triggered the T cell response, enabling the identification of novel antigens. The images in the figures were created using BioRender (https://www.biorender.com/).
Yeast / phage / mammalian cell display library
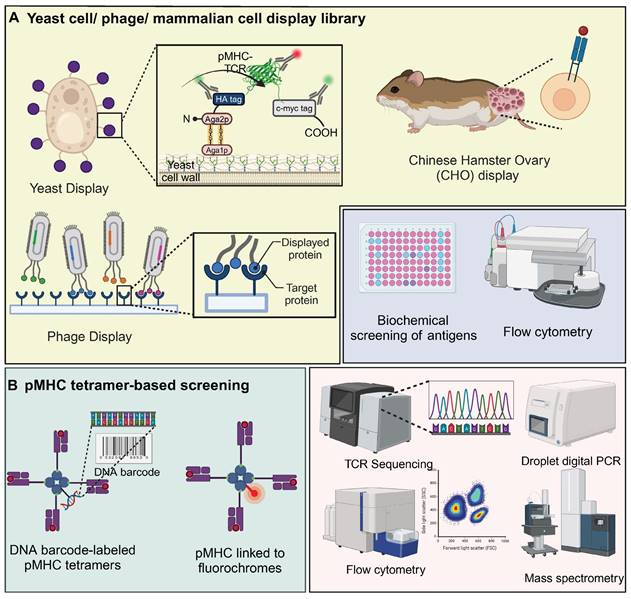
The complexity of pMHC-TCR interactions is largely driven by MHC polymorphism and the extensive diversity of peptides and TCRs. The integration of traditional biochemical methods with combinatorial biology techniques, particularly through the use of peptide or protein libraries, has proven to be an effective strategy for elucidating these complex biological interactions [72]. Yeast display of pHLA libraries is a scalable method for identifying TCR ligands, enabling the screening of millions to billions of epitopes. These libraries, comprising approximately 107 to 109 unique clones, offer benefits such as low reagent costs and rapid cycle times of 2-3 weeks for library generation and selection [73]. In this system, the pMHC complex is anchored to the yeast surface protein AGA2P, with the TCR featuring Vα and Vβ domains connected by a GlySer linker attached to the C-terminus of AGA2P [72]. This configuration ensures accurate orientation and stable presentation of TCR-pMHC interactions. The use of epitope tags and specific probes allows for monitoring of protein expression and functionality, with flow cytometry providing precise antigen screening [72]. Notably, yeast-display libraries were utilized to identify antigens for "orphan" TCRs on tumor-infiltrating lymphocytes from colorectal adenocarcinoma, revealing that four TIL-derived TCRs selectively bound peptides from a diverse pHLA-A∗02:01 library. Three TCRs targeted non-mutated self-antigens, including a shared antigen derived from U2AF2, demonstrating yeast display's effectiveness in uncovering TCR specificities and identifying tumor antigens [74]. Interestingly, the yeast agglutination mediated TCR antigen discovery system (YAMTAD) enhances the screening of TCR-pMHC interactions, achieving rapid, high-throughput identification of TCRs and their cognate antigens without the need for purification. Demonstrating high sensitivity and specificity, YAMTAD supports personalized immunotherapy by facilitating the enrichment of high-affinity TCR-pMHC interactions, offering a practical solution for library-on-library screening [75].
Phage display is employed to create diverse, functional TCR libraries by presenting human TCRs on the surface of M13 phages, utilizing a stabilized TCR heterodimer with an added disulfide bond for increased stability and compatibility with various TCR sequences and MHC molecules [76, 77]. For example, phage display facilitated the development of a TCR-like antibody, 2D2, targeting the NY-ESO-1/HLA-A2 complex, essential for recognizing intracellular cancer targets. This technology enabled the creation of CAR-T cells that effectively targeted and reduced tumor growth in melanoma and breast cancer models, showcasing its potential to expand antigen recognition in immunotherapy [78]. Additionally, a novel phage display screening strategy was developed to enable fast and easy selection of thermostabilized proteins. Random mutant scTCR phage libraries were prepared in E. coli overexpressing the ectoplasmid chaperone protein FkpA, which screened for and isolated heat-stable scTCR (mutant) variants. Then the tumour-specific reactivity of this scTCR was demonstrated by surface plasmon resonance technique [79].
Additionally, the Chinese Hamster Ovary (CHO) display has been utilized for the affinity engineering of TCRs, specifically in the development of bispecific TCR-based therapies. This system enables stable integration of TCR molecules, allowing for the efficient selection of high-affinity variants targeting cancer-specific antigens like PRAME. This approach demonstrates significant therapeutic potential, notably in eliciting strong immune responses against tumor cells [80]. Similarly, mammalian cell display was used to engineer the CMV-specific TCR RA14 for high-affinity and soluble expression. By screening CDR3 libraries, clones with enhanced pMHC affinity were identified. These high-affinity TCRs retained peptide specificity and activation in Jurkat T cells. Soluble expression was optimized via Fc fusion, disulfide bonding, and glycosylation site disruption, yielding a variant with 50 nM pMHC affinity and specific cell staining [81].
pMHC tetramer-based screening
pMHC multimers are essential tools for detecting antigen-specific T cells, as they replicate the natural presentation of peptides by MHC molecules on cell surfaces that T cells recognize. When conjugated with fluorochromes, these multimers allow for T cell detection via conventional flow cytometry [82]. pMHC tetramer-based screening with DNA barcoding improves the detection of rare antigen-specific T cells, offering higher sensitivity than conventional methods. By using photocleavable linkers, DNA-barcoded tetramers enable efficient T cell staining and barcode recovery. This approach allows for simultaneous detection of multiple antigen-specific T cell populations, facilitating precise monitoring of immune responses in cancer and vaccine studies [83]. Similarly, pMHC tetramer-based screening enhanced with magnetic nanoparticles and barcoded DNA linkers efficiently isolates neoantigen-specific CD8+ T cells. This method shows superior recovery over traditional approaches and is valuable for monitoring immune responses in cancer immunotherapy [84].
Notably, pMHC tetramer-based screening revealed that patients with metastatic urothelial carcinoma who responded to PD-L1 blockade exhibited an expansion of neoantigen-reactive CD8+ T cell (NART) populations post-treatment. NARTs displaying a PD1+ Ki67+ effector phenotype and high CD39 expression were associated with disease control, distinguishing them from bystander T cells during immune checkpoint blockade. Among 37 predicted neoantigens, tetramer staining identified potential HCC-dominant neoantigens restricted by HLA-A11:01, HLA-A24:02, or HLA-A02:01. Specifically, HLA-A24:02-restricted FYAFSCYYDL and HLA-A*02:01-restricted WVWCMSPTI demonstrated strong immunogenicity, which was confirmed using the Co-HA system. This method validated neoantigen-specific T cells and their antitumor efficacy, highlighting the role of tetramer-based screening in verifying clinically relevant neoantigens [85]. Furthermore, Tetramer-Associated T-Cell Receptor Sequencing (TetTCR-Seq) enables high-throughput linking of TCR sequences to their cognate antigens using DNA-barcoded pMHC tetramers. This method allows for the rapid identification of antigen-specific TCRs, including those recognizing cancer neoantigens without cross-reactivity to wild-type antigens [86]. Advances in using pMHC multimers attached to rare metal ions for detection via time-of-flight mass spectrometry (CyTOF) overcome the limitations of flow cytometry and enable more complex cell analysis. Using CyTOF and metal-labeled probes, CD8+ T cells were analyzed for surface markers, cytokines, and antigen specificity via peptide-MHC tetramers. This revealed extensive phenotypic and functional diversity within CD8+ T cells, including a nearly combinatorial pattern of cytokine expression despite sharing antigen specificity. Principal component analysis highlighted the continuous nature of CD8+ T cell differentiation and the complexity of the compartment, underscoring their flexibility in immune responses to pathogens [87]. Additionally, a method combining mass cytometry with combinatorial peptide-MHC tetramer staining enables rapid identification and characterization of T cells specific for numerous epitopes. Screening up to 109 peptide-MHC tetramers in a single blood sample while analyzing 23 additional markers revealed six rotavirus-specific T cell epitopes in HLA-A*0201 individuals. T cells targeting the VP3 protein showed distinct phenotypes and were abundant in intestinal epithelium, demonstrating the utility of this approach for analyzing T cell responses to infections or vaccines [88].
Trogocytosis
Trogocytosis is a process in which immune cells, particularly T cells, acquire membrane proteins such as MHC molecules from APCs during intercellular interactions. This bidirectional transfer of surface molecules occurs at the immunological synapse, where T cells recognize antigens presented by MHC molecules on APCs via their TCRs [89]. During synapse formation, T cells internalize fragments of the APC membrane, including MHC-peptide complexes, through vesicular transport mechanisms, while APCs can simultaneously acquire T cell-specific molecules, including TCRs. This exchange allows APCs to retain a molecular "fingerprint" of their interaction with antigen-specific T cells, thereby facilitating the identification of matching TCRs [90]. In TCR screening applications, trogocytosis enables the isolation of antigen-specific TCRs by tracking the acquired MHC-peptide complexes. This process is valuable for the identification of TCRs with high antigen specificity, advancing TCR-based immunotherapies and vaccine development. For example, trogocytosis was employed to identify tumor-specific TCRs from the exhausted T cell pool in post-allogeneic hematopoietic stem cell transplant patients. Although TAA-specific T cells were present in 90% of patients, they exhibited an exhaustion phenotype with limited effector function. Using trogocytosis and ligandome-on-chip technology, it was possible to isolate TCRs, including those recognizing novel acute myeloid leukemia antigens, highlighting the potential for TCR-based cancer immunotherapy [91] (Figure 6B).
Microfluidic technology in TCR screening
Microfluidic technology, with its ability to precisely control small fluid volumes, plays a critical role in TCR screening by enabling high-throughput, single-cell analysis in highly controlled microenvironments. Through microfluidic chips, individual T cells are isolated and exposed to peptide-MHC complexes, allowing for the real-time observation of antigen-specific T cell activation [92]. This approach integrates advanced detection methods, such as fluorescence or barcode-based systems, to assess TCR-antigen interactions with high sensitivity and specificity. Microfluidics significantly enhances screening efficiency by enabling rapid, parallel analysis of TCR functionality, facilitating the identification of therapeutically relevant TCRs. For instance, droplet microfluidics enables rapid, multiplexed functional screening of single TCR T cells by monitoring real-time activation upon tumor cell recognition. The platform features clone tracking and a highly specific sorting system, with 100% accuracy confirmed by single-cell RT-PCR and TCR sequencing. This microfluidic-based approach streamlines TCR screening, accelerating the development of effective T cell therapies [93]. Similarly, a high-throughput microfluidic approach was developed to identify TCRs with high functional avidity from diverse human T cell repertoires. By generating libraries of full-length TCRαβ clones from millions of primary T cells, expressed in Jurkat cells, this method enabled repeated screening. Over 2.9 million TCRαβ clonotypes were captured, including rare viral-antigen-reactive and tumor-specific TCRs from melanoma patient samples, which mediated effective tumor cell killing [94]. Additionally, microfluidic technology isolates peptide-specific CD8+ T cells using magnetic nanoparticles functionalized with peptide-MHC tetramers. This platform captures antigen-specific T cells, preserving the link between peptide and TCR gene identity, and allows simultaneous analysis of multiple peptides with just 100,000 cells. It offers 1,000-fold greater sensitivity than bulk methods, enabling the detection of rare viral antigen-specific TCRs [95]. Notably, a novel microfluidic-based approach identifies and recovers potent T cell clones by measuring cellular avidity between T cells and tumor cells. This method probes up to 10,000 T cell-tumor interactions per run and recovers highly avid T cells with 100% purity in 30 minutes. The recovered T cells retain cytotoxicity, activation, and avidity markers upon re-exposure to tumor cells, accelerating the selection process for therapeutic T cells and advancing precision cancer immunotherapy [96]. Likewise, a novel droplet microfluidic platform enables high-throughput screening of pMHC and TCR pairs with high sensitivity and minimal background noise. By integrating DNA barcoding technology, antigen-loaded cells and reporter cells can be labeled to identify pMHC-TCR specificity. Coupled with next-generation sequencing, this platform precisely maps pMHC-TCR interactions, offering potential for cross-reactivity and off-target screening in clinical TCR applications [97].
TCR screening using display libraries and pMHC tetramer-based assays. A) TCR libraries displayed on yeast, phages, and mammalian cells enable the high-throughput screening of antigen specificity. Yeast display incorporates TCRs anchored to the cell wall via Aga2p, facilitating the screening against labeled pMHC complexes. Phage display vectors present TCRs on capsid proteins, allowing for rapid affinity maturation through successive biopanning rounds. CHO cells expressing TCR-pMHC constructs provide a mammalian context for functional analyses. The resulting interactions are analyzed via biochemical screening, flow cytometry, and other downstream assays to identify TCRs with desired antigen specificities. B) pMHC tetramer-based screening utilizes DNA barcode-labeled pMHC tetramers linked to fluorochromes, facilitating simultaneous analysis of multiple TCR-pMHC interactions through flow cytometry. The barcodes allow for precise identification and quantification of TCR engagement, while TCR sequencing and droplet digital PCR provide detailed insights into the TCR repertoire. Mass spectrometry complements these analyses by identifying specific peptide-MHC interactions, enhancing the understanding of TCR specificity and affinity. The images in the figures were created using BioRender (https://www.biorender.com/).
Single-cell sequencing in TCR screening
Single-cell sequencing is a critical method for the high-throughput isolation of tumor-specific TCR encoding genes, essential for elucidating immune responses against cancer. This technique involves isolating individual T cells using flow cytometry or microfluidics, followed by cell lysis and reverse transcription to convert RNA into cDNA that includes TCR sequences [98]. Targeted sequencing, often facilitated by RNA baits that specifically hybridize to V and J segments of the TCRα and TCRβ chains, allows for selective enrichment and sequencing of these critical TCR components. This approach preserves the native TCR pairings and enables a comprehensive analysis of the TCR repertoire across thousands of T cells, providing detailed insights into the diversity and functionality of immune responses at the cellular level. Such precise capture of TCR sequences is fundamental for assessing antigen specificity and T cell diversity within the TME [12].
For instance, SEQTR, a high-throughput approach, enhances the sensitivity, reproducibility, and accuracy of analyzing human and mouse TCR repertoires, surpassing traditional assays in capturing the complexity of blood and tumor TCR landscapes. This method, combined with a novel TCR cloning strategy, streamlines the discovery, screening, and engineering of tumor-specific TCRs, significantly advancing TCR repertoire analyses and accelerating the development of cellular therapies in clinical settings [99]. Additionally, single-cell sequencing enables efficient isolation of neoantigen-specific TCRs by analyzing tumor-infiltrating T cells stimulated with neoantigen-loaded DCs. By sequencing TCRs and activation markers, this method identified 28 unique TCRs from melanoma and colorectal tumor samples, showing high reliability when identical sequences were detected in multiple cells. This high-throughput approach streamlines TCR identification for both research and clinical applications in cancer immunotherapy [98]. Notably, by analyzing TILs co-cultured with APCs, single-cell sequencing identifies TCR sequences linked to high IFN-γ and IL-2 expression. This method streamlines the isolation of neoantigen-specific TCRs, enabling the transduction of donor T cells for improved neoantigen recognition in cancer therapy [100].
Computational methods for TCR screening
Computational methods have become essential for the effective screening of TCR, enabling detailed characterization and prediction of TCR-antigen interactions at a significant scale. These approaches employ advanced algorithms to predict the binding affinity of TCRs with pMHC complexes, thereby streamlining the selection of potential therapeutic targets. Computational tools also analyze TCR repertoires from high-throughput sequencing data, interpreting complex sequence information to uncover diversity and clonality within a population. Such methodologies not only accelerate the identification of tumor-specific TCRs but also enhance our understanding of T cell mediated immunity. This supports the development of personalized T cell therapies and provides critical insights for the manipulation of immune responses in various pathologies. For instance, the TRUST algorithm was used to assemble TCR CDR3 regions from 9,700 tumor RNA-seq samples, while the iSMART methodology clustered similar TCRs to infer shared antigen specificity. Computational integration of multi-omics data facilitated the profiling of T cell behavior and the discovery of novel cancer antigens, enhancing the potential for targeted immune therapies in oncology [101]. Addtionally, TABR-BERT, a deep learning model based on BERT, enhances the prediction of TCR-pMHC binding by capturing key information from TCR sequences, antigen epitopes, and their interactions. This approach shows improved performance in benchmark tests, especially for unseen epitopes, demonstrating its potential to refine TCR-based cancer immunotherapy development [102]. Moreover, the TCR-ESM model, leveraging peptide embeddings from the evolutionary scale modeling protein language model, enhances TCR-pMHC binding predictions. Training on paired TCR data incorporating both CDR3α and CDR3β chain information yields superior performance compared to using only CDR3β, underscoring the contribution of both chains to specificity. This approach demonstrates generalizability across external datasets, spotlighting deep learning's potential to advance understanding of TCR specificity and interaction dynamics [103]. TULIP, a transformer-based unsupervised language model, addresses the limitations of existing TCR-epitope binding prediction methods by utilizing incomplete datasets and unsupervised learning. This flexible model integrates various data qualities and has demonstrated reduced bias from sampling procedures common in supervised approaches [104].
Computational methods also play a critical role in enhancing the prediction of peptide-MHC binding interactions, providing crucial insights for immunological research and therapeutic development. NetMHCpan-2.0 predicts peptide-MHC class I interactions across species, including uncharacterized human and non-human MHC molecules. Trained on extensive data, it accurately identifies binding peptides, aiding immunological research across diverse populations [105]. Similarly, NetMHCpan-4.1 and NetMHCIIpan-4.0 are web servers designed to predict peptide binding to MHC Class I and Class II molecules, respectively. These tools utilize advanced machine learning techniques to integrate binding affinity data and mass spectrometry-eluted ligands, enhancing the accuracy of predictions. This improvement in predictive capability is crucial for determining the specificity of T cell responses, significantly advancing TCR screening and immune response profiling [106]. In addition, MHCSeqNet, a deep learning model, advances MHC binding prediction by employing neural network architectures akin to those used in natural language processing. This approach models amino acid sequences of MHC alleles and epitope peptides as sentences, enhancing performance across both binding affinity and ligand peptidome datasets. Its ability to generalize to novel MHC class I alleles and accept peptides of varying lengths makes it a robust tool for neoepitope screening in cancer vaccine development [107].
Furthermore, recent computational advancements have transformed the analysis of TCR repertoires, enabling precise pMHC-TCR clustering, pairing, and deorphanization. The GLIPH algorithm clusters TCRs by conserved CDR3 motifs and sequence similarity, enabling pMHC-TCR pairing and large-scale deorphanization across donors. Validated with Mycobacterium tuberculosis-reactive TCRs, GLIPH predicts HLA restriction and antigenic ligands, aiding in T cell response analysis for immunotherapy [108]. GLIPH2, an advanced version, efficiently processes millions of TCRs, identifying specificity groups and epitopes. Applied to 19,044 TCRβ sequences from M. tuberculosis-infected individuals, GLIPH2 identified at least five PPE proteins as T-cell targets, enhancing pMHC-TCR pairing for complex pathogens [109]. Notably, GIANA (Geometric Isometry-based TCR Alignment Algorithm) clusters TCR sequences by similarity with 600-fold greater efficiency than TCRdist, while maintaining clustering specificity. Capable of rapid querying across large reference cohorts, GIANA identifies candidate disease-specific receptors and advances TCR repertoire classification. By supporting pMHC-TCR pairing and TCR deorphanization, GIANA provides a foundation for a non-invasive, TCR-based diagnostic platform across cancer, infectious, and autoimmune diseases. TCRpcDist offers a 3D-based method for clustering TCRs by calculating similarities based on the physico-chemical properties of loop residues interacting with epitopes. This approach overcomes limitations of sequence-based techniques, particularly in data-scarce scenarios, and accurately identifies TCRs likely to bind the same epitopes. Validated to determine neoantigen and tumor-associated antigen specificities of orphan TILs in cancer patients, TCRpcDist advances TCR repertoire analysis and deorphanization for personalized cancer immunotherapy [110]. Moreover, HeteroTCR, a supervised model based on Heterogeneous Graph Neural Networks, predicts peptide-TCR binding probabilities by capturing both within-type (TCR-TCR, peptide-peptide) similarities and between-type (peptide-TCR) interactions. Addressing limitations of existing models, HeteroTCR excels in predicting interactions for novel peptides and TCRs. Validation on independent and single-cell datasets confirms its accuracy and robustness, demonstrating enhanced pMHC-TCR pairing capabilities crucial for immunogenicity studies and TCR deorphanization [111].
Additionally, artificial intelligence (AI) is transforming the identification of tumor-reactive TCRs, distinguishing them from bystander TCRs and facilitating the development of personalized T cell therapies. Using high-throughput TCR cloning and single-cell RNA sequencing, the machine learning tool predicTCR distinguishes tumor-reactive TCRs from bystanders in an antigen-agnostic manner. PredicTCR significantly improves specificity and sensitivity, achieving a geometric mean accuracy of 0.74 versus 0.38 for traditional methods. This advancement enables rapid prioritization of tumor-reactive TCR clonotypes, streamlining the development of personalized T cell therapies [112]. Importantly, TRTpred, an antigen-agnostic in silico predictor, outperforms existing tools across diverse tumor datasets, enabling detailed analysis of tumor-reactive TIL repertoires. Combined with high-avidity TCR prediction and clustering (MixTRTpred), TRTpred identifies clinically relevant TCRs, validated both in vitro and in vivo, and supports the inclusion of orphan and private tumor-reactive TCRs for patients lacking common tumor antigens. This approach accelerates the selection of viable TCRs for TIL or TCR T cell therapy, advancing personalized treatments for solid tumors [113].
Multifunctional TCR screening platforms
Multifunctional TCR screening platforms are integrated systems designed for high-throughput identification and characterization of TCRs. These platforms merge advanced technologies like synthetic biology, microfluidics, and next-generation sequencing to efficiently identify TCRs specific to antigens on MHC molecules. They enable the generation of synthetic TCR libraries and the performance of functional assays to assess T cell responses. Additionally, many incorporate computational models to predict and analyze TCR-antigen interactions, improving antigen discovery essential for therapeutic developments. These platforms aim to accelerate the creation of targeted T-cell-based therapies, crucial for advancing precision medicine in oncology. Their comprehensive capabilities not only enhance the scope and accuracy of TCR screening but also establish these systems as indispensable in immunotherapy research.
For instance, DoubletSeeker, a high-throughput method, identifies ligand-receptor interactions by detecting cell doublets formed via specific membrane interactions. This approach captures paired TCR-pMHC information during complex library screenings, enabling precise identification of TCR-antigen interactions. DoubletSeeker uncovered mutant TCRs specific to the MART-1 epitope, advancing antigen discovery for immunotherapy development [114]. Similarly, a high-throughput personalized TCR discovery pipeline enables the assembly of synthetic TCR libraries in a one-pot reaction, followed by pooled expression in reporter T cells and functional screening against patient-derived tumor cells. This method screens patient-derived TCRs for tumor specificity, identifying both MHC class I- and II-restricted TCRs targeting tumor-associated antigens, including neoantigens [115]. Notably, a streamlined approach matches TCR sequences with cognate antigens through on-demand cloning, expression, and screening against candidate antigens. This system identifies viral- and neoantigen-specific TCRs, enabling rapid assessment of antigen specificity and functional avidity. Applied to melanoma and CLL, the method facilitates TCR discovery and aids in the development of T-cell-based immunotherapies [116]. Interestingly, TCR-MAP is an antigen discovery method that uses a synthetic TCR circuit to activate sortase-mediated tagging of APCs presenting peptides on MHCs. Tagged APCs are purified for sequencing, enabling pooled screening of unknown TCR specificities against barcoded peptide libraries. This method captures both MHC class I- and class II-restricted TCR reactivities, identifying self, viral, and cancer-related targets with high sensitivity [117]. TScan-II is a genome-scale CD4+ antigen discovery platform that integrates endogenous HLA-II processing in synthetic APCs with TCR signaling, enabling simultaneous screening of multiple HLAs and TCRs. Using human, virome, and mutagenesis libraries, it uncovers novel antigens and explores TCR specificity. TScan-II identified cancer-reactive and Sjögren's disease-specific antigens, highlighting its utility in both basic and translational research [118]. Moreover, nanovials coated with peptide-MHC monomers capture and activate antigen-reactive T cells, accumulating secreted effector molecules like IFN-γ and granzyme B. Microfluidic single-cell sequencing recovers paired TCR αβ-chains from sorted cells. Oligo-barcoded nanovials and detection antibodies link TCRs to specific targets, enabling functional ranking. This approach expands the repertoire of functional TCRs, including rare cancer-specific TCRs [119].
Clinical applications of TCR-T cell therapy
The clinical application of TCR T cell therapies targeting TAAs and TSAs is a critical development in precision oncology. These therapies are currently being evaluated for their ability to precisely target antigens predominantly expressed by tumor cells, optimizing therapeutic outcomes while minimizing unintended effects. The selectivity of TCRs for TAAs and TSAs enhances therapeutic efficacy, potentially transforming the management of advanced and refractory cancers. Advances in genomic profiling and TCR validation are expected to refine these therapies further, facilitating their broader integration into clinical practice.
Clinical trials of TCR-T cell therapy targeting TAAs
The MAGE family encodes TAAs, which are highly expressed in various cancers but minimally in normal tissues. These antigens are prime targets for cancer immunotherapy, enabling the development of targeted vaccines and cellular therapies. For instance, TCR-T cell therapy targeting MAGE-A1 showed a manageable safety profile in a first-in-human trial involving patients with advanced solid tumors. IMA202, consisting of engineered autologous CD8+ T cells, demonstrated sustained biological activity, with detectable persistence in peripheral blood and tumor tissues. Notably, a substantial proportion of patients achieved stable disease, with some showing tumor shrinkage, underscoring the potential efficacy of this approach in solid tumor immunotherapy [120]. Similarly, TCR-engineered T cells targeting MAGE-A10 demonstrated safety and therapeutic potential in a phase I trial for advanced non-small cell lung cancer. Patients received escalating doses of ADP-A2M10 SPEAR T cells, with observed responses ranging from stable disease to partial remission. While the trial confirmed the persistence of these cells in blood and tumor tissue, it was closed in favor of pursuing therapies targeting MAGE-A4 due to overlapping antigen expression [121]. Afamitresgene autoleucel (TECELRA®) is an HLA-restricted autologous T cell therapy that targets MAGE A4 in HLA-A02-eligible patients with solid tumors, showing early and durable responses in patients with relapsed/refractory metastatic solid tumors. Notably, TECELRA® received FDA approval in the USA in August 2024 and is prescribed for adults with unresectable or metastatic synovial sarcoma who have previously undergone chemotherapy, following positive outcomes in clinical trials [122]. In a phase 1 trial, the overall response rate was 24%, with 44% in synovial sarcoma patients. The therapy infiltrated tumors, triggered interferon-γ-driven immune responses, and showed an acceptable safety profile, with cytokine release syndrome being the most common adverse event [123]. In a phase 2 trial, TECELRA® also showed promising efficacy and manageable safety in patients with advanced synovial sarcoma or myxoid round cell liposarcoma expressing HLA-A*02. In a heavily pre-treated cohort, an overall response rate of 37% was observed, with durable responses and manageable adverse events, such as cytokine release syndrome and cytopenias [124].
NY-ESO-1 is a cancer/testis antigen predominantly expressed in various tumors but rarely in normal tissues, making it a prominent target for cancer immunotherapy. Its distinct expression pattern supports the development of vaccines and TCR-based therapies to target cancer cells. A Phase 1 trial combining NY-ESO-1-specific TCR-engineered T-cell therapy with a lymph node-targeting nanoparticulate peptide vaccine demonstrated promising efficacy in treating refractory soft tissue sarcoma. This regimen, employing pullulan nanogel-loaded long peptide antigens, enhanced TCR-T cell accumulation and function in tumors, without lymphodepletion. Notable tumor shrinkage and prolonged TCR-T cell persistence were observed, suggesting a viable strategy for treating cold tumors [125]. Importantly, autologous T lymphocytes engineered to express a TCR targeting NY-ESO-1 showed significant efficacy in a trial involving patients with advanced or recurrent synovial sarcoma. Despite a 50% objective response rate and manageable safety profile, adverse events were common, though serious complications such as cytokine release syndrome were effectively managed. This TCR T cell therapy offers a novel therapeutic option for tumors resistant to conventional treatments [126]. Similarly, autologous T cells engineered with an affinity-enhanced TCR targeting the NY-ESO-1/LAGE1a peptide demonstrated significant therapeutic efficacy in metastatic synovial sarcoma. Treated patients exhibited durable responses and persistent T cell activity, with 50% achieving tumor shrinkage. The longevity and functionality of these T cells, characterized by a predominantly central memory phenotype, highlight their potential to sustain antitumor effects without signs of exhaustion, even under continuous antigen exposure [127].
Significantly, advancements in TCR engineered T cell therapies have shown promising results in targeting specific antigens associated with hematologic cancers, demonstrating their potential in overcoming resistance mechanisms and improving patient outcomes. For instance, in a phase 1 clinical trial, T cells engineered to express TCRs targeting the minor H antigen HA-1 were administered to patients with recurrent leukemia post-allogeneic stem cell transplantation. These HA-1 TCR-T cells, derived from disparate donors, expanded in vivo and were well-tolerated, showing preliminary signs of efficacy. Four patients achieved or maintained complete remissions, with one remaining in remission after two years [128]. In addition, engineered T cells expressing a high-affinity Wilms' Tumor Antigen 1-specific TCR (TCRC4) were infused into patients with high-risk acute myeloid leukemia post- hematopoietic cell transplantation, achieving 100% relapse-free survival at 44 months, compared to 54% in a matched control group. Modified to minimize graft-versus-host disease, these TCR-T cells persisted long-term and exhibited multifunctional capabilities, suggesting a viable strategy for preventing AML recurrence [129]. Notably, engineered T cells expressing TCRs targeted an HLA-A2-restricted Wilms' tumor antigen epitope in AML, but encountered resistance due to limited immunoproteasome activity. An alternative TCR that recognizes a proteasome-independent epitope effectively killed resistant AML and solid tumor lines in vitro and in NSG mice, suggesting a strategy to overcome proteasome-related immune evasion in AML [130].
Clinical trials of TCR-T cell therapy targeting TSAs
Viral antigens serve as ideal targets for TCR T cell therapies as they are foreign proteins expressed bor cells infected with oncogenic viruses, providing highly specific and immunogenic targets. This specificity makes them excellent candidates for developing targeted immunotherapies with minimal risk of harming normal tissues. TCR-engineered T cells targeting cytomegalovirus (CMV) demonstrated safety and efficacy as a first-line pre-emptive therapy in a phase I trial for CMV reactivation post-haploidentical peripheral blood stem cell transplantation. The treatment resulted in rapid CMV clearance in most patients without the need for additional antiviral agents, with sustained T cell expansion and minimal adverse effects, highlighting its potential as a viable alternative to conventional antiviral therapies [121]. Additionally, transiently functional TCR T cells, targeting hepatitis B virus antigens, demonstrated safety in a Phase I trial involving HCC patients post-liver transplant. These mRNA-electroporated T cells, specific to HBV-derived epitopes, showed minimal adverse effects and a tolerable profile, indicating a viable approach for addressing HBV-associated HCC recurrence [131]. A phase 1/2 trial on TCR T-cell therapy for HPV-assocy tumiated epithelial cancers demonstrated preliminary efficacy and high tolerability. Patients received high doses of autologous T cells targeting HPV16 E6, leading to significant peripheral blood engraftment and objective tumor responses, including complete and partial regressions [132]. Moreover, a novel TCR (10F04), identified from a metastatic cervical cancer patient who benefited from multiple antigens stimulating cellular therapy, has shown promise in targeting HPV18-positive tumors. This HLA-DRA/DRB1*09:01 restricted TCR redirects both CD4+ and CD8+ T cells to recognize and attack tumor cells effectively, demonstrating robust antitumor activity and safety in vitro and in vivo [133].
Neoantigens, unique peptides arising from tumor-specific mutations, are highly effective targets for TCR-engineered T cell therapies due to their exclusive expression in cancer cells and absence in normal tissues. Two TCRs specific to the KRAS-G12V mutant neoantigen presented by HLA-A*11:01 were identified. TCR-T cells constructed with these TCRs showed cytokine secretion and cytotoxicity against KRAS-G12V-expressing tumor cells. In preclinical models, these TCR-T cells demonstrated potent anti-tumor activity [134]. In addition, an automated, good manufacturing practice-compliant process was developed for the scaled production of mutant NPM1 (dNPM1)-specific TCR-engineered T cells targeting AML. The optimized process shortened manufacturing time from 12 to 8 days, producing up to 5.5 billion CD8+ T cells with early memory phenotypes. These cells demonstrated specific AML killing in vitro and in vivo, supporting their use in an upcoming phase 1/2 clinical trial for NPM1-mutated AML [135]. Interestingly, NeoScreen is a method that facilitates the sensitive identification of rare tumor neoantigens and their corresponding TCRs from tumor-infiltrating lymphocytes. T cells engineered with these specific TCRs, identified through NeoScreen, effectively mediate tumor regression in patient-derived xenograft models, demonstrating its utility in enhancing cancer immunotherapy [136]. A high-throughput platform identified a panel of TCRs targeting a public neoantigen derived from a common PIK3CA mutation, presented by HLA-A*03:01. This neoantigen's immunogenicity arises from enhanced peptide/HLA complex stability. Structural analysis revealed that a lead TCR candidate binds the epitope with high specificity via an extended CDR3β loop. In vivo, TCR-engineered T cells targeting this neoantigen led to tumor regression in PIK3CA-mutant tumors, demonstrating the therapeutic potential of targeting public neoantigens in TCR T cell therapy [137].
In addition to its success in preclinical models, TCR T cell therapy has also shown promising results in clinical trials. CRISPR-Cas9 genome editing was utilized to replace endogenous TCRs with neoantigen-specific TCRs in T cells from patients with refractory solid tumors. This first-in-human trial demonstrated that engineered T cells could home to tumors and were associated with manageable side effects. Despite only stable disease or progression observed, the approach confirms the feasibility of precise TCR replacement for targeting mutational neoantigens in cancer therapy [138]. In addition, a patient with metastatic pancreatic cancer received autologous T cells genetically engineered to express TCRs targeting the KRAS G12D mutation. The patient experienced a 72% reduction in tumor burden, and the response persisted at 6 months. Engineered T cells remained detectable, constituting over 2% of circulating T cells. This case demonstrates the potential of TCR T cell therapy to mediate significant tumor regression in KRAS-driven pancreatic cancer [139]. A library of 39 TCRs targeting TP53 mutations, shared by 7.3% of solid tumor patients, demonstrated tumor-specific reactivity. In clinical trials, adoptive cell therapy with TILs yielded limited responses due to low frequencies of reactive, exhausted TILs. However, a patient treated with peripheral blood lymphocytes transduced with a TP53-specific TCR showed 55% tumor regression, indicating that targeting shared TP53 neoantigens holds promise for cancer treatment [140]. In terms of adverse reactions, although TCR-T cell therapy has been linked to isolated, transient fevers in some patients, likely reflecting an initial immune activation as TCR-T cells target tumor antigens. This mild febrile response is typically manageable and does not appear to compromise therapeutic efficacy or patient safety.
Clinical trials of TCR-T in anti-tumor therapy
| Cancer type | Target | Type | Results | HLA | Trial ID | Phase | Ref |
|---|---|---|---|---|---|---|---|
| Metastatic melanoma synovial cell sarcoma | MART-1 F5 | TAA | \ | \ | NCT00923195 | Phase 2 | [168] |
| Metastatic melanoma | MART-1 F5 | TAA | 69% (9/13) of patients showed tumor regression | HLA-A*0201 | NCT00910650 | Phase 2 | [169] |
| Vaginal cancer Cervical cancer Anal cancer Penile cancer Oropharyngeal cancer | HPV-16 E6 | TSA | \ | \ | NCT02280811 | Phase 1 Phase 2 | NA |
| Cervical cancer Renal cancer Urothelial cancer Melanoma Breast cancer | MAGE-A3-DP4 | TAA | \ | \ | NCT02111850 | Phase 1 Phase 2 | NA |
| Melanoma Meningioma Breast cancer NSCLC Hepatocellular cancer | NY-ESO-1 | TAA | \ | \ | NCT01967823 | NA | NA |
| Ovarian cancer | NY-ESO-1 | TAA | \ | \ | NCT01567891 | Phase 1 Phase 2 | NA |
| Neoplasms | NY-ESO-1 | TAA | \ | \ | NCT02992743 | Phase 2 | NA |
| NSCLC | NY-ESO-1 | TAA | \ | \ | NCT02588612 | Phase 1 | NA |
| Metastatic cancer | p53 | TAA | \ | \ | NCT00393029 | Phase 2 | NA |
| Progressive metastatic malignancies | p53 | TAA | \ | \ | NCT00496860 | Phase 1 | NA |
| Neoplasms | NY-ESO-1 | TAA | \ | \ | NCT01343043 | Phase 1 | NA |
| Solid tumor | MAGEA1 | TAA | Of the 16 patients dosed, 11 (68.8%) patients had SD as their best overall response | HLA-A*02:01 | NCT04639245 NCT05430555 | Phase 1 Phase 2 | [120] |
| Recurrent leukemia | HA-1 | TAA | Patients achieved or maintained remission, with one lasting over 2 years | HLA-A*02:01 | NCT03326921 | Phase 1 | [128] |
| HBV-related hepatocellular carcinoma | HBV | TSA | No CRS nor neurotoxicity | HLA-A02:01, HLA-A11:01 | NCT02719782 | Phase 1 | [131] |
| Soft tissue sarcoma | NY-ESO-1 | TAA | Treatment was well-tolerated; two had mild CRS. One showed tumor shrinkage lasting over two years. | HLA-A*02:01 | JMA-IIA00346 | Phase 1 | [125] |
| Refractory solid tumors | Neoantigens | TSA | Five patients had stable disease and the other eleven had disease progression | HLA-A:02*01 | NCT03970382 | Phase 1 | [138] |
| HPV16- positive epithelial cancer | HPV | TSA | All patients had high E6 TCR T cell engraftment. Two of 12 (17%) had tumor responses | HLA-A*02:01 | \ | Phase 1 Phase 2 | [132] |
| B-Cell acute lymphoblastic leukemia | leukemia-associated antigens | TAA | Patients achieved complete remission 4 weeks | \ | NCT03953599 | Phase 1 | [170] |
| Synovial sarcoma | NY-ESO-1 | TAA | Tumor burden decreased after 4 weeks, with maximal responses in 4 patients after 3 months. | HLA-A*02:01 | NCT01343043 | Phase 1 | [127] |
| Synovial Sarcoma | NY-ESO-1 | TAA | All patients had adverse events, with 87.5% experiencing drug reactions, but no deaths occurred | HLA-A*02:01 HLA-A*02:06 | NCT03250325 | Phase 1 Phase 2 | [126] |
| Sarcoma | MAGE-A4 | TSA | Overall response rate was 37% | HLA-A*02 | NCT03132922 | Phase 2 | [124] |
| Synovial sarcoma Ovarian cancer Head and neck cancer | MAGE-A4 | TSA | All 38 patients had Grade ≥3 hematologic toxicities. CRS occurred in 55%. ORR was 24%, 44% for SS, and 9% for other cancers | HLA-A*02 | NCT03132922 | Phase 1 | [123] |
Challenges and prospects in TCR-based cancer immunotherapies
Manufacturing challenges and costs
While personalized T-cell therapy holds significant promise for treating various diseases, it faces substantial manufacturing challenges that impede broader application. These challenges include high production costs, complexities in scaling up production, intricate logistics, lengthy processing and testing times, and limited patient access. Specifically, the production of TCR-T products is both costly and complex, typically requiring 1-3 weeks [6, 141]. This process entails several labor-intensive steps such as monocyte separation, T-cell activation for gene engineering susceptibility, amplification of gene-modified cells, and their subsequent harvesting and cryopreservation. These personalized therapies inherently preclude large-scale production, leading to intricate manufacturing processes and escalated costs [142]. For example, the cost of a single infusion of therapies like axicabtagene ciloleucel or tisagenlecleucel can be as high as $373,000 and $475,000, respectively [143].
To address these significant challenges and improve the accessibility of TCR-T therapies, it is crucial to integrate more advanced manufacturing technologies. The adoption of automated bioreactors and closed-system cell processing units can streamline the complex steps involved in T-cell therapy production [144]. Automation helps standardize processes such as monocyte separation, T-cell activation, and the amplification of gene-modified cells, thereby reducing human error and variability in production. Moreover, the implementation of continuous manufacturing strategies can further enhance the efficiency of these processes. By reducing batch-to-batch variability and shortening the lengthy timelines typically associated with personalized treatments, these strategies can significantly cut production costs and increase throughput. Implementing these advanced technologies in GMP-compliant facilitie is essential not only for maintaining product quality and safety but also for reducing overhead costs and enhancing scalability. By enhancing reproducibility and reducing both the time and expense associated with producing TCR-T therapies, these technological improvements have the potential to make these innovative treatments more accessible to a wider range of patients [145, 146]. This broader accessibility is critical for the future of personalized medicine, as it promises to bring potent, targeted therapies to more individuals, ultimately improving outcomes in the treatment of complex diseases.
Dysfuction of administered T cells
Maintaining the persistence and activity of administered T cells is critical for effective anti-tumor responses in T cell immunotherapy. T cell exhaustion, stemming from repeated activation during chronic infection or tumor progression, alongside T cell anergy caused by over-activation of the TCR and strong co-inhibition, presents significant challenges that must be addressed [147]. An effective strategy to enhance T cell persistence is non-myeloablative lymphodepleting chemotherapy, which administers specific chemotherapeutic agents before T cell injection. This approach reduces competition for crucial cytokines such as IL-7 and IL-15 between infused and endogenous T cells, enhancing their survival and function within the patient's body [148, 149]. Furthermore, less differentiated T cell subsets, such as central memory T cells and stem cell memory T cells, demonstrate superior engraftment and persistence compared to more differentiated effector memory T cells and terminally differentiated effector cells [150]. Techniques to enhance T cell efficacy include shortening T cell expansion time, employing less inductive cytokines, dedifferentiating induced pluripotent stem cells (iPSCs), or rejuvenating endogenous T cells to overcome dysfunction and exhaustion [151, 152]. Enhancing TCR modification to improve T cell activity within the tumor microenvironment is also beneficial. This can be achieved by integrating co-stimulatory domains, co-expressing stimulatory CARs, or blocking inhibitory receptors [153]. Genetic modifications, such as engineering T cells to self-secrete cytokines like IL-15 or IL-12, or using a constitutively activated IL-7 receptor, can stabilize cytokine signaling and enhance T cell persistence [143]. Selective apoptosis to eliminate dysfunctional T cells helps maintain the proliferation of effective effector and memory cells. Bioengineering strategies, such as using thymus-like organ substances, growth factors, and cytokines like IL-21, support the restoration and maintenance of the thymic environment, reversing thymic involution. For instance, injecting allogeneic hematopoietic cells into a reconstituted thymus may rejuvenate the production of functional T cells [154]. These strategies aim to boost T cell persistence and activity within the patient, thereby enhancing the effectiveness of immunotherapy. However, these approaches require further research and clinical trials for validation and optimization before widespread clinical application.
Antigen selection
The selection of appropriate tumor antigens as therapeutic targets is crucial in TCR-T cell therapy. The pool of safe and effective TAAs is limited, and the ability of tumor cells to downregulate or lose the expression of targeted antigens under immune pressure can significantly compromise the efficacy of TCR-T cell therapy [154]. To address this challenge, several strategies have been proposed. One approach involves the use of high-throughput sequencing and computational biology techniques, such as HTS-IR, TraCeR, and single-cell TCR sequencing, which provide a systematic method for analyzing large datasets of TCR sequences and associated antigen information [155]. These technologies enable the identification of novel TAAs and neoantigens, offering more precise and comprehensive targets for TCR-T cell therapies. Additionally, advanced techniques like flow cytometry, mass cytometry, and microfluidic technologies allow for the identification of tumor antigen-specific T cells within a patient's immune system, facilitating the development of personalized treatments tailored to the individual's specific tumor antigen profile. Moreover, genetic engineering technologies enable the introduction of multiple TCRs with distinct tumor specificities into a single population of T cells, creating polyclonal TCR-T cells [156]. This polyclonal approach allows the T cells to target multiple antigens simultaneously, enhancing both the breadth and effectiveness of the treatment. By recognizing different tumor antigens, the therapy becomes more resilient to immune escape mechanisms, thereby broadening its scope and improving its specificity. This approach addresses the inherent complexities of antigen selection and significantly enhances the therapeutic potential of TCR-T cell therapy [157].
TCR optimization and functional expression
To fully harness the therapeutic potential of TCR-T cell therapy, one major challenge is ensuring that the TCRs bind with sufficient affinity to TAAs without causing off-target effects. Low-affinity TCRs may fail to generate a robust immune response, while overly high-affinity TCRs increase the risk of targeting healthy tissues. Another challenge involves ensuring proper surface expression of the engineered TCRs, as inefficient TCR expression or rapid internalization can limit the efficacy of TCR-T cells. Addressing these challenges is critical to improve the therapeutic effectiveness of TCR-T cell therapy.
One key strategy to improve the effectiveness of TCR-T cell therapy involves optimizing TCR affinity for specific antigens. This can be achieved through selective modifications of the CDRs, particularly in the CDR3 region of the TCR α and β chains, where amino acid substitutions can be introduced to fine-tune the affinity of the TCR for its target antigen [158]. A structure-guided design process that incorporates both positive and negative design elements enables the detailed understanding of the three-dimensional structure of TCR-antigen interactions. This strategy allows for targeted mutations to either enhance or attenuate the binding interaction, optimizing the therapeutic response while minimizing potential off-target effects. In addition, optimizing the expression and function of the TCR protein is critical for enhancing TCR-T cell activity. This can be achieved by improving the pairing efficiency of the TCR α and β chains, utilizing optimized codon usage, and minimizing TCR glycosylation, which can otherwise hinder TCR surface expression and lead to internalization of transduced TCRs [154]. Minimizing glycosylation can enhance the functional affinity of the TCR and improve its stability on the cell surface, thereby increasing the overall efficacy of the immune response.
TCR mismatch
The heterodimers formed through interactions within the TCR constant region by the TCR α/β chains might experience mismatch with the endogenous TCR α/β chains. This could, in turn, reduce the surface expression of the introduced TCR. Additionally, the mismatched TCRs may compete for the limited CD3 components for binding [159]. More concerningly, these mismatches can create entirely novel TCRs, which have bypassed thymic selection and may unexpectedly target self-antigens [160]. Therefore, the resolution of these mismatches is integral to preserving the efficacy of TCR-T cell therapy. Currently, the primary methods to enhance the expression level of introduced TCRs and to improve the matching or binding of the exogenous TCR α/β chains mainly involve modifying the TCR α/β chains. This is achieved by altering the nucleotide sequence of the TCR gene to ensure effective translation within host cells [161]. For instance, replacing leucine and valine residues in the transmembrane region of the TCR alpha chain, and introducing part or all of the gene sequences of the mouse constant region into the human TCR, have been proven to enhance the stability and surface expression of the introduced TCR alpha chain [162, 163]. Additionally, the strategy of introducing extra disulfide bonds in the TCR constant region can also enhance the stability and pairing efficiency of the introduced TCR [164, 165]. For example, by introducing cysteine at Cα residue 48 and Cβ residue 57 to create a second stable disulfide bond, this improves the correct pairing of the introduced TCR. Furthermore, methods such as using a single-chain TCR, swapping two amino acids that mediate TCR α/β chain dimerization, or employing TCR/CD3 fusion products also hold promise in resolving mismatches in the TCR beta chain [166, 167].
Conclusions
In conclusion, TCR-T cell therapy represents a major advancement in cancer immunotherapy, significantly enhancing the ability of T cells to selectively target and eliminate cancer cells. Despite substantial progress in TCR engineering and screening technologies, challenges related to the specificity, affinity, and safety of TCRs remain critical hurdles. The successful application of these therapies relies on precise and safe TCR selection. As research continues to evolve, addressing these challenges will be crucial to fully realizing the therapeutic potential of TCR-T cell therapy in cancer treatment.
Abbreviations
APC: Antigen-presenting Cell
CDR: Complementarity-determining Region
CEA: Carcinoembryonic Antigen
CGA: Cancer Germline Antigen
CMV: Cytomegalovirus
DC: Dendritic Cell
ERAAP: ER-associated Aminopeptidase
ITAM: Immunoreceptor Tyrosine-based Activation Motif
MHC: Major Histocompatibility Complex
MⅡCs: MHC class Ⅱ Compartments
NART: Neoantigen-reactive CD8+ T Cell
NGS: Next-generation Sequencing
TAA: Tumor-associated Antigens
TCR-T: T Cell Receptor-engineered T
TIL: Tumor-infiltrating Lymphocyte
Treg: Regulatory T Cell
TSA: Tumor-specific Antigens
Acknowledgements
Funding
This work was supported by National Natural Science Foundation of China (81901006, 82372905), Young Top-notch Talent of Pearl River Talent Plan (0920220228), Guangdong Provincial Science and Technology Project Foundation (2022A0505050038), and Science Research Cultivation Program of Stomatological Hospital, Southern Medical University (PY2020002, PY2022019).
Author contributions
LC, and XYZ conceived of the presented idea. LC, PL, XYZ, YFL, ZZM, YCZ, JRZ and ZHZ wrote the manuscript. PL created the graphs. All authors contributed to and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Shimu AS, Wei HX, Li Q, Zheng X, Li B. The new progress in cancer immunotherapy. Clin Exp Med. 2023;23:553-67
2. Rodrigues S, Figueiredo C. Recent insights into the use of immune checkpoint inhibitors in gastric cancer. Porto Biomed J. 2022;7:e162
3. Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21:298-312
4. Laine A, Labiad O, Hernandez-Vargas H, This S, Sanlaville A, Leon S. et al. Regulatory T cells promote cancer immune-escape through integrin alphavbeta8-mediated TGF-beta activation. Nat Commun. 2021;12:6228
5. Xu Y, Jiang J, Wang Y, Wang W, Li H, Lai W. et al. Engineered T Cell Therapy for Gynecologic Malignancies: Challenges and Opportunities. Front Immunol. 2021;12:725330
6. Lee SW, Lee HM. Engineered T Cell Receptor for Cancer Immunotherapy. Biomol Ther (Seoul). 2024;32:424-31
7. Neo SY, Xu S, Chong J, Lam KP, Wu J. Harnessing novel strategies and cell types to overcome immune tolerance during adoptive cell therapy in cancer. J Immunother Cancer. 2023;11:e006434
8. Yu J, Sun H, Cao W, Song Y, Jiang Z. Research progress on dendritic cell vaccines in cancer immunotherapy. Exp Hematol Oncol. 2022;11:3
9. Baulu E, Gardet C, Chuvin N, Depil S. TCR-engineered T cell therapy in solid tumors: State of the art and perspectives. Sci Adv. 2023;9:eadf3700
10. Xu X, Li H, Xu C. Structural understanding of T cell receptor triggering. Cell Mol Immunol. 2020;17:193-202
11. Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J. T cell antigen receptor recognition of antigen-presenting molecules. Annu Rev Immunol. 2015;33:169-200
12. Sun Y, Li F, Sonnemann H, Jackson KR, Talukder AH, Katailiha AS. et al. Evolution of CD8(+) T Cell Receptor (TCR) Engineered Therapies for the Treatment of Cancer. Cells. 2021;10:2379
13. Stadinski BD, Trenh P, Smith RL, Bautista B, Huseby PG, Li G. et al. A role for differential variable gene pairing in creating T cell receptors specific for unique major histocompatibility ligands. Immunity. 2011;35:694-704
14. Alba J, D'Abramo M. The Full Model of the pMHC-TCR-CD3 Complex: A Structural and Dynamical Characterization of Bound and Unbound States. Cells. 2022;11:668
15. Li Y, Yin Y, Mariuzza RA. Structural and biophysical insights into the role of CD4 and CD8 in T cell activation. Front Immunol. 2013;4:206
16. Van Kaer L, Wu L, Joyce S. Mechanisms and Consequences of Antigen Presentation by CD1. Trends Immunol. 2016;37:738-54
17. Zareie P, Szeto C, Farenc C, Gunasinghe SD, Kolawole EM, Nguyen A. et al. Canonical T cell receptor docking on peptide-MHC is essential for T cell signaling. Science. 2021;372:eabe9124
18. Saotome K, Dudgeon D, Colotti K, Moore MJ, Jones J, Zhou Y. et al. Structural analysis of cancer-relevant TCR-CD3 and peptide-MHC complexes by cryoEM. Nat Commun. 2023;14:2401
19. Hartl FA, Beck-Garcia E, Woessner NM, Flachsmann LJ, Cardenas RMV, Brandl SM. et al. Noncanonical binding of Lck to CD3epsilon promotes TCR signaling and CAR function. Nat Immunol. 2020;21:902-13
20. Yashiro Y, Tai XG, Toyo-oka K, Park CS, Abe R, Hamaoka T. et al. A fundamental difference in the capacity to induce proliferation of naive T cells between CD28 and other co-stimulatory molecules. Eur J Immunol. 1998;28:926-35
21. Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939-51
22. Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227-42
23. Zhang K, Chen H, Li F, Huang S, Chen F, Li Y. Bright future or blind alley? CAR-T cell therapy for solid tumors. Front Immunol. 2023;14:1045024
24. Zhang Z, Liu S, Zhang B, Qiao L, Zhang Y, Zhang Y. T Cell Dysfunction and Exhaustion in Cancer. Front Cell Dev Biol. 2020;8:17
25. Mazerolles F, Rieux-Laucat F. PD-L1 is expressed on human activated naive effector CD4+ T cells. Regulation by dendritic cells and regulatory CD4+ T cells. PLoS One. 2021;16:e0260206
26. Pertseva M, Follonier O, Scarcella D, Reddy ST. TCR clustering by contrastive learning on antigen specificity. Brief Bioinform. 2024;25:bbae375
27. Song I, Gil A, Mishra R, Ghersi D, Selin LK, Stern LJ. Broad TCR repertoire and diverse structural solutions for recognition of an immunodominant CD8(+) T cell epitope. Nat Struct Mol Biol. 2017;24:395-406
28. Tuosto L. Editorial: Insights in T Cell Biology: 2021. Front Immunol. 2022;13:1039602
29. Pietropaolo M, Surhigh JM, Nelson PW, Eisenbarth GS. Primer: immunity and autoimmunity. Diabetes. 2008;57:2872-82
30. Jackson KJ, Gaeta B, Sewell W, Collins AM. Exonuclease activity and P nucleotide addition in the generation of the expressed immunoglobulin repertoire. BMC Immunol. 2004;5:19
31. Pai JA, Satpathy AT. High-throughput and single-cell T cell receptor sequencing technologies. Nat Methods. 2021;18:881-92
32. Li B. Why do tumor-infiltrating lymphocytes have variable efficacy in the treatment of solid tumors? Front Immunol. 2022;13:973881
33. Larrieu P, Renaud V, Godet Y, Jotereau F, Fonteneau JF. A HLA-Cw*0701 restricted Melan-A/MART1 epitope presented by melanoma tumor cells to CD8+ tumor infiltrating lymphocytes. Cancer Immunol Immunother. 2008;57:745-52
34. Elsayed A, Pluss L, Nideroest L, Rotta G, Thoma M, Zangger N. et al. Optimizing the Design and Geometry of T Cell-Engaging Bispecific Antibodies Targeting CEA in Colorectal Cancer. Mol Cancer Ther. 2024;23:1010-20
35. Yang F, Gao Y, Geng J, Qu D, Han Q, Qi J. et al. Elevated expression of SOX2 and FGFR1 in correlation with poor prognosis in patients with small cell lung cancer. Int J Clin Exp Pathol. 2013;6:2846-54
36. Chamorro DF, Somes LK, Hoyos V. Engineered Adoptive T-Cell Therapies for Breast Cancer: Current Progress, Challenges, and Potential. Cancers (Basel). 2023;16:124
37. Liu Q, Li J, Zheng H, Yang S, Hua Y, Huang N. et al. Adoptive cellular immunotherapy for solid neoplasms beyond CAR-T. Mol Cancer. 2023;22:28
38. Li Y, Zheng Y, Liu T, Liao C, Shen G, He Z. The potential and promise for clinical application of adoptive T cell therapy in cancer. J Transl Med. 2024;22:413
39. Schossig P, Coskun E, Arsenic R, Horst D, Sehouli J, Bergmann E. et al. Target Selection for T-Cell Therapy in Epithelial Ovarian Cancer: Systematic Prioritization of Self-Antigens. Int J Mol Sci. 2023;24:2292
40. Norberg SM, Hinrichs CS. Engineered T cell therapy for viral and non-viral epithelial cancers. Cancer Cell. 2023;41:58-69
41. Pang Z, Lu MM, Zhang Y, Gao Y, Bai JJ, Gu JY. et al. Neoantigen-targeted TCR-engineered T cell immunotherapy: current advances and challenges. Biomark Res. 2023;11:104
42. Pishesha N, Harmand TJ, Ploegh HL. A guide to antigen processing and presentation. Nat Rev Immunol. 2022;22:751-64
43. Concha-Benavente F, Srivastava R, Ferrone S, Ferris RL. Immunological and clinical significance of HLA class I antigen processing machinery component defects in malignant cells. Oral Oncol. 2016;58:52-8
44. Serwold T, Gonzalez F, Kim J, Jacob R, Shastri N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature. 2002;419:480-3
45. Barbosa CRR, Barton J, Shepherd AJ, Mishto M. Mechanistic diversity in MHC class I antigen recognition. Biochem J. 2021;478:4187-202
46. Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145-57
47. Sun Q, Zhang X, Wang L, Gao X, Xiong Y, Liu L. et al. T-cell receptor gene therapy targeting melanoma-associated antigen-A4 by silencing of endogenous TCR inhibits tumor growth in mice and human. Cell Death Dis. 2019;10:475
48. Roche PA, Cresswell P. Antigen Processing and Presentation Mechanisms in Myeloid Cells. Microbiol Spectr. 2016;4:10.1128 /microbiolspec.MCHD-0008-2015
49. Munz C. Antigen Processing for MHC Class II Presentation via Autophagy. Front Immunol. 2012;3:9
50. Cruz FM, Colbert JD, Merino E, Kriegsman BA, Rock KL. The Biology and Underlying Mechanisms of Cross-Presentation of Exogenous Antigens on MHC-I Molecules. Annu Rev Immunol. 2017;35:149-76
51. Blander JM. Regulation of the Cell Biology of Antigen Cross-Presentation. Annu Rev Immunol. 2018;36:717-53
52. Duraes FV, Niven J, Dubrot J, Hugues S, Gannage M. Macroautophagy in Endogenous Processing of Self- and Pathogen-Derived Antigens for MHC Class II Presentation. Front Immunol. 2015;6:459
53. Xie M, Viviani M, Fussenegger M. Engineering precision therapies: lessons and motivations from the clinic. Synth Biol (Oxf). 2021;6:ysaa024
54. Hutchison S, Pritchard AL. Identifying neoantigens for use in immunotherapy. Mamm Genome. 2018;29:714-30
55. Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC. et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013;5:197ra03
56. Lu SJ, Feng Q. CAR-NK cells from engineered pluripotent stem cells: Off-the-shelf therapeutics for all patients. Stem Cells Transl Med. 2021;10(Suppl 2):S10-S7
57. Oreskovic E, Wheeler EC, Mengwasser KE, Fujimura E, Martin TD, Tothova Z. et al. Genetic analysis of cancer drivers reveals cohesin and CTCF as suppressors of PD-L1. Proceedings of the National Academy of Sciences of the United States of America. 2022;119:e2120540119
58. Franzolin G, Tamagnone L. Semaphorin Signaling in Cancer-Associated Inflammation. International journal of molecular sciences. 2019;20:377
59. Gong X, Karchin R. Clustering by antigen-presenting genes reveals immune landscapes and predicts response to checkpoint immunotherapy. Scientific reports. 2023;13:950
60. Yan J, Wu X, Zhu Y, Cang S. Genome-wide DNA methylation profile analysis identifies an individualized predictive signature for melanoma immune response. J Cancer Res Clin Oncol. 2023;149:343-56
61. Zhang Q, Liu J, Lin H, Lin B, Zhu M, Li M. Glucose metabolism reprogramming promotes immune escape of hepatocellular carcinoma cells. Explor Target Antitumor Ther. 2023;4:519-36
62. Kula T, Dezfulian MH, Wang CI, Abdelfattah NS, Hartman ZC, Wucherpfennig KW. et al. T-Scan: A Genome-wide Method for the Systematic Discovery of T Cell Epitopes. Cell. 2019;178:1016-28 e13
63. Chen H, Li Z, Qiu L, Dong X, Chen G, Shi Y. et al. Personalized neoantigen vaccine combined with PD-1 blockade increases CD8(+) tissue-resident memory T-cell infiltration in preclinical hepatocellular carcinoma models. Journal for immunotherapy of cancer. 2022;10:e004389
64. He J, Xiong X, Yang H, Li D, Liu X, Li S. et al. Defined tumor antigen-specific T cells potentiate personalized TCR-T cell therapy and prediction of immunotherapy response. Cell Res. 2022;32:530-42
65. Joglekar AV, Leonard MT, Jeppson JD, Swift M, Li G, Wong S. et al. T cell antigen discovery via signaling and antigen-presenting bifunctional receptors. Nat Methods. 2019;16:191-8
66. Zhao Y, Nguyen P, Vogel P, Li B, Jones LL, Geiger TL. Autoimmune susceptibility imposed by public TCRbeta chains. Sci Rep. 2016;6:37543
67. Rochigneux P, Garcia AJ, Chanez B, Madroszyk A, Olive D, Garon EB. Medical Treatment of Lung Cancer: Can Immune Cells Predict the Response? A Systematic Review. Front Immunol. 2020;11:1036
68. Malyguine AM, Strobl S, Dunham K, Shurin MR, Sayers TJ. ELISPOT Assay for Monitoring Cytotoxic T Lymphocytes (CTL) Activity in Cancer Vaccine Clinical Trials. Cells. 2012;1:111-26
69. Kilian M, Friedrich M, Sanghvi K, Green E, Pusch S, Kawauchi D. et al. T-cell Receptor Therapy Targeting Mutant Capicua Transcriptional Repressor in Experimental Gliomas. Clin Cancer Res. 2022;28:378-89
70. Tate T, Matsumoto S, Nemoto K, Leisegang M, Nagayama S, Obama K. et al. Identification of T Cell Receptors Targeting a Neoantigen Derived from Recurrently Mutated FGFR3. Cancers (Basel). 2023;15:1031
71. Liu C, Chu D, Kalantar-Zadeh K, George J, Young HA, Liu G. Cytokines: From Clinical Significance to Quantification. Adv Sci (Weinh). 2021;8:e2004433
72. Birnbaum ME, Dong S, Garcia KC. Diversity-oriented approaches for interrogating T-cell receptor repertoire, ligand recognition, and function. Immunol Rev. 2012;250:82-101
73. Chao G, Lau WL, Hackel BJ, Sazinsky SL, Lippow SM, Wittrup KD. Isolating and engineering human antibodies using yeast surface display. Nat Protoc. 2006;1:755-68
74. Gee MH, Han A, Lofgren SM, Beausang JF, Mendoza JL, Birnbaum ME. et al. Antigen Identification for Orphan T Cell Receptors Expressed on Tumor-Infiltrating Lymphocytes. Cell. 2018;172:549-63 e16
75. Wang L, Lan X. Rapid screening of TCR-pMHC interactions by the YAMTAD system. Cell Discov. 2022;8:30
76. Li Y, Moysey R, Molloy PE, Vuidepot AL, Mahon T, Baston E. et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23:349-54
77. Loset GA, Lunde E, Bogen B, Brekke OH, Sandlie I. Functional phage display of two murine alpha/beta T-cell receptors is strongly dependent on fusion format, mode and periplasmic folding assistance. Protein Eng Des Sel. 2007;20:461-72
78. Liu X, Xu Y, Xiong W, Yin B, Huang Y, Chu J. et al. Development of a TCR-like antibody and chimeric antigen receptor against NY-ESO-1/HLA-A2 for cancer immunotherapy. J Immunother Cancer. 2022;10:e004035
79. Gunnarsen KS, Kristinsson SG, Justesen S, Frigstad T, Buus S, Bogen B. et al. Chaperone-assisted thermostability engineering of a soluble T cell receptor using phage display. Sci Rep. 2013;3:1162
80. Dilchert J, Hofmann M, Unverdorben F, Kontermann R, Bunk S. Mammalian Display Platform for the Maturation of Bispecific TCR-Based Molecules. Antibodies (Basel). 2022;11:34
81. Wagner EK, Qerqez AN, Stevens CA, Nguyen AW, Delidakis G, Maynard JA. Human cytomegalovirus-specific T-cell receptor engineered for high affinity and soluble expression using mammalian cell display. J Biol Chem. 2019;294:5790-804
82. Bentzen AK, Hadrup SR. Evolution of MHC-based technologies used for detection of antigen-responsive T cells. Cancer Immunol Immunother. 2017;66:657-66
83. Dahotre SN, Chang YM, Romanov AM, Kwong GA. DNA-Barcoded pMHC Tetramers for Detection of Single Antigen-Specific T Cells by Digital PCR. Anal Chem. 2019;91:2695-700
84. Peng S, Zaretsky JM, Ng AHC, Chour W, Bethune MT, Choi J. et al. Sensitive Detection and Analysis of Neoantigen-Specific T Cell Populations from Tumors and Blood. Cell Rep. 2019;28:2728-38 e7
85. Chen P, Chen D, Bu D, Gao J, Qin W, Deng K. et al. Dominant neoantigen verification in hepatocellular carcinoma by a single-plasmid system coexpressing patient HLA and antigen. J Immunother Cancer. 2023;11:e006334
86. Zhang SQ, Ma KY, Schonnesen AA, Zhang M, He C, Sun E. et al. High-throughput determination of the antigen specificities of T cell receptors in single cells. Nat Biotechnol. 2018
87. Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity. 2012;36:142-52
88. Newell EW, Sigal N, Nair N, Kidd BA, Greenberg HB, Davis MM. Combinatorial tetramer staining and mass cytometry analysis facilitate T-cell epitope mapping and characterization. Nat Biotechnol. 2013;31:623-9
89. Schriek P, Villadangos JA. Trogocytosis and cross-dressing in antigen presentation. Curr Opin Immunol. 2023;83:102331
90. Miyake K, Karasuyama H. The Role of Trogocytosis in the Modulation of Immune Cell Functions. Cells. 2021;10:1255
91. Manfredi F, Stasi L, Buonanno S, Marzuttini F, Noviello M, Mastaglio S. et al. Harnessing T cell exhaustion and trogocytosis to isolate patient-derived tumor-specific TCR. Sci Adv. 2023;9:eadg8014
92. Seah YFS, Hu H, Merten CA. Microfluidic single-cell technology in immunology and antibody screening. Mol Aspects Med. 2018;59:47-61
93. Segaliny AI, Li G, Kong L, Ren C, Chen X, Wang JK. et al. Functional TCR T cell screening using single-cell droplet microfluidics. Lab Chip. 2018;18:3733-49
94. Spindler MJ, Nelson AL, Wagner EK, Oppermans N, Bridgeman JS, Heather JM. et al. Massively parallel interrogation and mining of natively paired human TCRalphabeta repertoires. Nat Biotechnol. 2020;38:609-19
95. Ng AHC, Peng S, Xu AM, Noh WJ, Guo K, Bethune MT. et al. MATE-Seq: microfluidic antigen-TCR engagement sequencing. Lab Chip. 2019;19:3011-21
96. Ashby JF, Schmidt J, Kc N, Kurum A, Koch C, Harari A. et al. Microfluidic T Cell Selection by Cellular Avidity. Adv Healthc Mater. 2022;11:e2200169
97. Li Y, Qi J, Liu Y, Zheng Y, Zhu H, Zang Y. et al. High-Throughput Screening of Functional Neo-Antigens and Their Specific T-Cell Receptors via the Jurkat Reporter System Combined with Droplet Microfluidics. Anal Chem. 2023;95:9697-705
98. Lu YC, Zheng Z, Lowery FJ, Gartner JJ, Prickett TD, Robbins PF. et al. Direct identification of neoantigen-specific TCRs from tumor specimens by high-throughput single-cell sequencing. J Immunother Cancer. 2021;9:e002595
99. Genolet R, Bobisse S, Chiffelle J, Arnaud M, Petremand R, Queiroz L. et al. TCR sequencing and cloning methods for repertoire analysis and isolation of tumor-reactive TCRs. Cell Rep Methods. 2023;3:100459
100. Lu YC, Zheng Z, Robbins PF, Tran E, Prickett TD, Gartner JJ. et al. An Efficient Single-Cell RNA-Seq Approach to Identify Neoantigen-Specific T Cell Receptors. Mol Ther. 2018;26:379-89
101. Zhang H, Liu L, Zhang J, Chen J, Ye J, Shukla S. et al. Investigation of Antigen-Specific T-Cell Receptor Clusters in Human Cancers. Clin Cancer Res. 2020;26:1359-71
102. Zhang J, Ma W, Yao H. Accurate TCR-pMHC interaction prediction using a BERT-based transfer learning method. Brief Bioinform. 2023;25:bbad436
103. Yadav S, Vora DS, Sundar D, Dhanjal JK. TCR-ESM: Employing protein language embeddings to predict TCR-peptide-MHC binding. Comput Struct Biotechnol J. 2024;23:165-73
104. Meynard-Piganeau B, Feinauer C, Weigt M, Walczak AM, Mora T. TULIP: A transformer-based unsupervised language model for interacting peptides and T cell receptors that generalizes to unseen epitopes. Proc Natl Acad Sci U S A. 2024;121:e2316401121
105. Rapin N, Hoof I, Lund O, Nielsen M. MHC motif viewer. Immunogenetics. 2008;60:759-65
106. Reynisson B, Alvarez B, Paul S, Peters B, Nielsen M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020;48:W449-W54
107. Phloyphisut P, Pornputtapong N, Sriswasdi S, Chuangsuwanich E. MHCSeqNet: a deep neural network model for universal MHC binding prediction. BMC Bioinformatics. 2019;20:270
108. Glanville J, Huang H, Nau A, Hatton O, Wagar LE, Rubelt F. et al. Identifying specificity groups in the T cell receptor repertoire. Nature. 2017;547:94-8
109. Huang H, Wang C, Rubelt F, Scriba TJ, Davis MM. Analyzing the Mycobacterium tuberculosis immune response by T-cell receptor clustering with GLIPH2 and genome-wide antigen screening. Nat Biotechnol. 2020;38:1194-202
110. Zhang H, Zhan X, Li B. GIANA allows computationally-efficient TCR clustering and multi-disease repertoire classification by isometric transformation. Nat Commun. 2021;12:4699
111. Yu Z, Jiang M, Lan X. HeteroTCR: A heterogeneous graph neural network-based method for predicting peptide-TCR interaction. Commun Biol. 2024;7:684
112. Tan CL, Lindner K, Boschert T, Meng Z, Rodriguez Ehrenfried A, De Roia A. et al. Prediction of tumor-reactive T cell receptors from scRNA-seq data for personalized T cell therapy. Nat Biotechnol. 2024
113. Petremand R, Chiffelle J, Bobisse S, Perez MAS, Schmidt J, Arnaud M. et al. Identification of clinically relevant T cell receptors for personalized T cell therapy using combinatorial algorithms. Nat Biotechnol. 2024
114. Wang Y, Wang Z, Yang J, Lei X, Liu Y, Frankiw L. et al. Deciphering Membrane-Protein Interactions and High-Throughput Antigen Identification with Cell Doublets. Adv Sci (Weinh). 2024;11:e2305750
115. Moravec Z, Zhao Y, Voogd R, Cook DR, Kinrot S, Capra B. et al. Discovery of tumor-reactive T cell receptors by massively parallel library synthesis and screening. Nat Biotechnol. 2024
116. Hu Z, Anandappa AJ, Sun J, Kim J, Leet DE, Bozym DJ. et al. A cloning and expression system to probe T-cell receptor specificity and assess functional avidity to neoantigens. Blood. 2018;132:1911-21
117. Kohlgruber AC, Dezfulian MH, Sie BM, Wang CI, Kula T, Laserson U. et al. High-throughput discovery of MHC class I- and II-restricted T cell epitopes using synthetic cellular circuits. Nat Biotechnol. 2024
118. Dezfulian MH, Kula T, Pranzatelli T, Kamitaki N, Meng Q, Khatri B. et al. TScan-II: A genome-scale platform for the de novo identification of CD4(+) T cell epitopes. Cell. 2023;186:5569-86 e21
119. Koo D, Mao Z, Dimatteo R, Noguchi M, Tsubamoto N, McLaughlin J. et al. Defining T cell receptor repertoires using nanovial-based binding and functional screening. Proc Natl Acad Sci U S A. 2024;121:e2320442121
120. Wermke M, Holderried TAW, Luke JJ, Morris VK, Alsdorf WH, Wetzko K. et al. First-in-human dose escalation trial to evaluate the clinical safety and efficacy of an anti-MAGEA1 autologous TCR-transgenic T cell therapy in relapsed and refractory solid tumors. J Immunother Cancer. 2024;12:e008668
121. Blumenschein GR, Devarakonda S, Johnson M, Moreno V, Gainor J, Edelman MJ. et al. Phase I clinical trial evaluating the safety and efficacy of ADP-A2M10 SPEAR T cells in patients with MAGE-A10(+) advanced non-small cell lung cancer. J Immunother Cancer. 2022;10:e003581
122. Keam SJ. Afamitresgene Autoleucel: First Approval. Mol Diagn Ther. 2024;28:861-6
123. Hong DS, Van Tine BA, Biswas S, McAlpine C, Johnson ML, Olszanski AJ. et al. Autologous T cell therapy for MAGE-A4(+) solid cancers in HLA-A*02(+) patients: a phase 1 trial. Nat Med. 2023;29:104-14
124. D'Angelo SP, Araujo DM, Abdul Razak AR, Agulnik M, Attia S, Blay JY. et al. Afamitresgene autoleucel for advanced synovial sarcoma and myxoid round cell liposarcoma (SPEARHEAD-1): an international, open-label, phase 2 trial. Lancet. 2024;403:1460-71
125. Ishihara M, Nishida Y, Kitano S, Kawai A, Muraoka D, Momose F. et al. A phase 1 trial of NY-ESO-1-specific TCR-engineered T-cell therapy combined with a lymph node-targeting nanoparticulate peptide vaccine for the treatment of advanced soft tissue sarcoma. Int J Cancer. 2023;152:2554-66
126. Kawai A, Ishihara M, Nakamura T, Kitano S, Iwata S, Takada K. et al. Safety and Efficacy of NY-ESO-1 Antigen-Specific T-Cell Receptor Gene-Transduced T Lymphocytes in Patients with Synovial Sarcoma: A Phase I/II Clinical Trial. Clin Cancer Res. 2023;29:5069-78
127. D'Angelo SP, Melchiori L, Merchant MS, Bernstein D, Glod J, Kaplan R. et al. Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 (c259)T Cells in Synovial Sarcoma. Cancer Discov. 2018;8:944-57
128. Krakow EF, Brault M, Summers C, Cunningham TM, Biernacki MA, Black RG. et al. HA-1-targeted T-cell receptor T-cell therapy for recurrent leukemia after hematopoietic stem cell transplantation. Blood. 2024;144:1069-82
129. Chapuis AG, Egan DN, Bar M, Schmitt TM, McAfee MS, Paulson KG. et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med. 2019;25:1064-72
130. Lahman MC, Schmitt TM, Paulson KG, Vigneron N, Buenrostro D, Wagener FD. et al. Targeting an alternate Wilms' tumor antigen 1 peptide bypasses immunoproteasome dependency. Sci Transl Med. 2022;14:eabg8070
131. Yang F, Zheng X, Koh S, Lu J, Cheng J, Li P. et al. Messenger RNA electroporated hepatitis B virus (HBV) antigen-specific T cell receptor (TCR) redirected T cell therapy is well-tolerated in patients with recurrent HBV-related hepatocellular carcinoma post-liver transplantation: results from a phase I trial. Hepatol Int. 2023;17:850-9
132. Burki TK. TCR gene therapy for HPV-associated epithelial cancers. Lancet Oncol. 2019;20:e474
133. Long J, Chen X, He M, Ou S, Zhao Y, Yan Q. et al. HLA-class II restricted TCR targeting human papillomavirus type 18 E7 induces solid tumor remission in mice. Nat Commun. 2024;15:2271
134. Lu D, Chen Y, Jiang M, Wang J, Li Y, Ma K. et al. KRAS G12V neoantigen specific T cell receptor for adoptive T cell therapy against tumors. Nat Commun. 2023;14:6389
135. Yonezawa Ogusuku IE, Herbel V, Lennartz S, Brandes C, Argiro E, Fabian C. et al. Automated manufacture of DeltaNPM1 TCR-engineered T cells for AML therapy. Mol Ther Methods Clin Dev. 2024;32:101224
136. Arnaud M, Chiffelle J, Genolet R, Navarro Rodrigo B, Perez MAS, Huber F. et al. Sensitive identification of neoantigens and cognate TCRs in human solid tumors. Nat Biotechnol. 2022;40:656-60
137. Chandran SS, Ma J, Klatt MG, Dundar F, Bandlamudi C, Razavi P. et al. Immunogenicity and therapeutic targeting of a public neoantigen derived from mutated PIK3CA. Nat Med. 2022;28:946-57
138. Foy SP, Jacoby K, Bota DA, Hunter T, Pan Z, Stawiski E. et al. Non-viral precision T cell receptor replacement for personalized cell therapy. Nature. 2023;615:687-96
139. Leidner R, Sanjuan Silva N, Huang H, Sprott D, Zheng C, Shih YP. et al. Neoantigen T-Cell Receptor Gene Therapy in Pancreatic Cancer. N Engl J Med. 2022;386:2112-9
140. Kim SP, Vale NR, Zacharakis N, Krishna S, Yu Z, Gasmi B. et al. Adoptive Cellular Therapy with Autologous Tumor-Infiltrating Lymphocytes and T-cell Receptor-Engineered T Cells Targeting Common p53 Neoantigens in Human Solid Tumors. Cancer Immunol Res. 2022;10:932-46
141. Dai X, Mei Y, Nie J, Bai Z. Scaling up the Manufacturing Process of Adoptive T Cell Immunotherapy. Biotechnol J. 2019;14:e1800239
142. Rollins MR, Spartz EJ, Stromnes IM. T Cell Receptor Engineered Lymphocytes for Cancer Therapy. Curr Protoc Immunol. 2020;129:e97
143. Shafer P, Kelly LM, Hoyos V. Cancer Therapy With TCR-Engineered T Cells: Current Strategies, Challenges, and Prospects. Frontiers in immunology. 2022;13:835762
144. Song HW, Somerville RP, Stroncek DF, Highfill SL. Scaling up and scaling out: Advances and challenges in manufacturing engineered T cell therapies. International reviews of immunology. 2022;41:638-48
145. Bareke H, Ibanez-Navarro A, Guerra-Garcia P, Gonzalez Perez C, Rubio-Aparicio P, Plaza Lopez de Sabando D. et al. Prospects and Advances in Adoptive Natural Killer Cell Therapy for Unmet Therapeutic Needs in Pediatric Bone Sarcomas. International journal of molecular sciences. 2023;24:8324
146. Xu R, Du S, Zhu J, Meng F, Liu B. Neoantigen-targeted TCR-T cell therapy for solid tumors: How far from clinical application. Cancer letters. 2022;546:215840
147. Kasakovski D, Xu L, Li Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J Hematol Oncol. 2018;11:91
148. Zhao Z, Zhang J, Bian J, Lu X. The efficacy and safety of chimeric antigen receptor T cells in digestive system cancers: a systematic review and meta-analysis. Ann Transl Med. 2022;10:508
149. Chuntova P, Downey KM, Hegde B, Almeida ND, Okada H. Genetically Engineered T-Cells for Malignant Glioma: Overcoming the Barriers to Effective Immunotherapy. Front Immunol. 2018;9:3062
150. Guo J, Wang C, Luo N, Wu Y, Huang W, Zhu J. et al. IL-2-free tumor-infiltrating lymphocyte therapy with PD-1 blockade demonstrates potent efficacy in advanced gynecologic cancer. BMC Med. 2024;22:207
151. Hudson WH, Wieland A. Technology meets TILs: Deciphering T cell function in the -omics era. Cancer Cell. 2023;41:41-57
152. Luo C, Wang P, He S, Zhu J, Shi Y, Wang J. Progress and Prospect of Immunotherapy for Triple-Negative Breast Cancer. Front Oncol. 2022;12:919072
153. Netsrithong R, Wattanapanitch M. Advances in Adoptive Cell Therapy Using Induced Pluripotent Stem Cell-Derived T Cells. Front Immunol. 2021;12:759558
154. Liu Y, Yan X, Zhang F, Zhang X, Tang F, Han Z. et al. TCR-T Immunotherapy: The Challenges and Solutions. Front Oncol. 2021;11:794183
155. Roddy H, Meyer T, Roddie C. Novel Cellular Therapies for Hepatocellular Carcinoma. Cancers (Basel). 2022;14:504
156. Landoni E, Smith CC, Fuca G, Chen Y, Sun C, Vincent BG. et al. A High-Avidity T-cell Receptor Redirects Natural Killer T-cell Specificity and Outcompetes the Endogenous Invariant T-cell Receptor. Cancer Immunol Res. 2020;8:57-69
157. Zhang P, Zhang G, Wan X. Challenges and new technologies in adoptive cell therapy. J Hematol Oncol. 2023;16:97
158. Nicholson E, Ghorashian S, Stauss H. Improving TCR Gene Therapy for Treatment of Haematological Malignancies. Adv Hematol. 2012;2012:404081
159. Casey NP, Fujiwara H, Tanimoto K, Okamoto S, Mineno J, Kuzushima K. et al. A Functionally Superior Second-Generation Vector Expressing an Aurora Kinase-A-Specific T-Cell Receptor for Anti-Leukaemia Adoptive Immunotherapy. PLoS One. 2016;11:e0156896
160. Li X, Liang H, Fan J. Prospects of Cytomegalovirus-Specific T-Cell Receptors in Clinical Diagnosis and Therapy. Viruses. 2023;15:1334
161. Jahn L, Hagedoorn RS, van der Steen DM, Hombrink P, Kester MG, Schoonakker MP. et al. A CD22-reactive TCR from the T-cell allorepertoire for the treatment of acute lymphoblastic leukemia by TCR gene transfer. Oncotarget. 2016;7:71536-47
162. Lo Presti V, Buitenwerf F, van Til NP, Nierkens S. Gene Augmentation and Editing to Improve TCR Engineered T Cell Therapy against Solid Tumors. Vaccines (Basel). 2020;8:733
163. Haga-Friedman A, Horovitz-Fried M, Cohen CJ. Incorporation of transmembrane hydrophobic mutations in the TCR enhance its surface expression and T cell functional avidity. J Immunol. 2012;188:5538-46
164. Cohen CJ, Li YF, El-Gamil M, Robbins PF, Rosenberg SA, Morgan RA. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 2007;67:3898-903
165. Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C. et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109:2331-8
166. Voss RH, Willemsen RA, Kuball J, Grabowski M, Engel R, Intan RS. et al. Molecular design of the Calphabeta interface favors specific pairing of introduced TCRalphabeta in human T cells. J Immunol. 2008;180:391-401
167. Sillito F, Holler A, Stauss HJ. Engineering CD4+ T Cells to Enhance Cancer Immunity. Cells. 2020;9:1721
168. Abate-Daga D, Hanada K, Davis JL, Yang JC, Rosenberg SA, Morgan RA. Expression profiling of TCR-engineered T cells demonstrates overexpression of multiple inhibitory receptors in persisting lymphocytes. Blood. 2013;122:1399-410
169. Chodon T, Comin-Anduix B, Chmielowski B, Koya RC, Wu Z, Auerbach M. et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin Cancer Res. 2014;20:2457-65
170. Wang J, Zhang X, Zhou Z, Liu Y, Yu L, Jia L. et al. A novel adoptive synthetic TCR and antigen receptor (STAR) T-Cell therapy for B-Cell acute lymphoblastic leukemia. Am J Hematol. 2022;97:992-1004
Author contact
![]() Corresponding authors: Xinyuan Zhao, Email: zhaoxinyuan1989edu.cn; Li Cui, Email: licuiedu.cn.
Corresponding authors: Xinyuan Zhao, Email: zhaoxinyuan1989edu.cn; Li Cui, Email: licuiedu.cn.
 Global reach, higher impact
Global reach, higher impact