13.3
Impact Factor
Theranostics 2025; 15(1):189-201. doi:10.7150/thno.103999 This issue Cite
Research Paper
A H2S-activated NIR-II imaging probe for precise diagnosis and pathological evaluation of colorectal tumor
1. Lab of Molecular Imaging and Translational Medicine (MITM), Engineering Research Center of Molecular and Neuro Imaging, Ministry of Education, School of Life Science and Technology, Xidian University & International Joint Research Center for Advanced Medical Imaging and Intelligent Diagnosis and Treatment, Xi'an, Shaanxi, 710126, P. R. China.
2. Guangzhou Institute of Technology, Xidian University, Guangzhou, GuangDong, 510000, P. R. China.
3. Department of Neurogastroenterology and Pelvic Floor Surgery, Hospital of Gastroenterology, Xi'an International Medical Center Hospital, Xi'an, Shaanxi, 710100, P. R. China.
# These authors contributed to this work equally.
Received 2024-9-21; Accepted 2024-10-16; Published 2025-1-1
Abstract
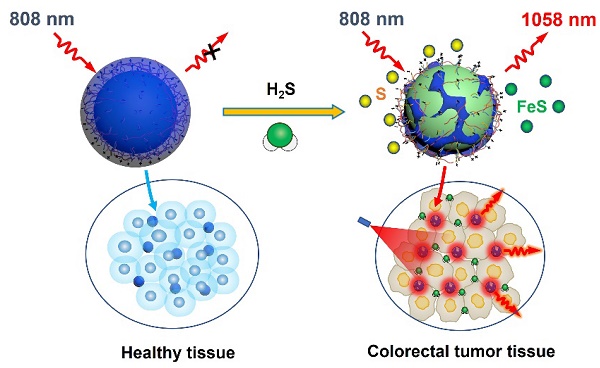
Rationale: The quick and accurate detection of colorectal cancer (CRC) is essential for improving the treatment efficacy and patient survival, which nevertheless remains challenging due to low specificity and sensitivity of current CRC diagnostic approaches. Therefore, providing a robust solution for real-time and accurate tumor delineation is highly desirable.
Methods: We report a novel polyacrylic acid-mediated strategy to develop the endogenous hydrogen sulfide (H2S)-activated NIR-II probe DCNP@PB for specific visualization of CRC and image-guided tumor surgery. The stability, biocompatibility, H2S-responsiveness, and NIR-II imaging capability were evaluated in vitro and in vivo. Human CRC tissues were used to evaluated the performance of the DCNP@PB.
Results: By exploiting the effective inner filter effect (IFE) and fluorescence resonance energy transfer (FRET) between DCNPs and PB, luminescence of DCNP@PB can be rapidly switched ON in response to H2S in colorectal tumor, affording high tumor-to-background ratio (TBR). Notably, H2S-responsive range of DCNP@PB well matches the H2S concentration at tumor site, thereby minimizing nonspecific activation by other sulfur-containing substances in a complicated biological environment. Such accurate H2S responsiveness not only benefits the differentiation between tumor and normal tissues in the mouse model, but also clearly delineates the cancerous boundaries in human tissues specimens.
Conclusion: This work presents not only a promising example of H2S-activated NIR-II optical probe that could be intravenously injected for in vivo applications to afford reliable information and quick feedback, but also an effective strategy to design the activatable imaging probes for precise tumor diagnosis and intraoperative decision support.
Keywords: hydrogen sulfide, NIR-II imaging, molecular probe, tumor imaging, colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most common cancer diagnosis and the second leading cause of cancer death in the world [1]. Over 1,918,030 new colorectal cancer cases and 609,360 deaths were estimated to occur in 2022, accounting for 10% of all cancer cases and deaths worldwide [2]. Colorectal cancer survival largely depends on the stage at diagnosis, with later-stage diagnosis having poorer survival, which highlights importance of early detection and accurate diagnosis [3-6]. Although several screening methods can help detect colorectal cancer, including colonoscopy, biopsy, stool sample testing, biomarker testing of the tumor, and computed tomography (CT) scan of the colon, they still suffer from certain drawbacks such as invasive testing way, low specificity and sensitivity and potential radiation risks [7-9].
Optical imaging, as a fast and real-time feedback technique, has attracted increasing attention in biomedical fields owing to its high sensitivity and selectivity, noninvasiveness, and radiation-free feature [10-13]. Especially, the advent of imaging in the second near-infrared window (NIR-II, 1000-1700 nm) has further accelerated in vivo applications of optical imaging [14-20]. Compared to conventional optical imaging approaches at shorter emission wavelengths, NIR-II imaging can significantly reduce the tissue autofluorescence, photon absorption and scattering, offering superior signal-to-background ratio and improved spatial resolution [21-23]. Indeed, the past few years have witnessed the rapid development of optical probes with emission in the NIR-II region, such as single-walled carbon nanotubes (SWCNTs) [24-25], quantum dots (QDs) [26-27], rare-earth (RE)-doped nanoparticles (RENPs) [28-30], and organic molecules-based probes [31-32]. Despite great progress of applying NIR-II probes for tumor imaging and imaging-guided surgery, there is still a challenge to customize a highly specific and sensitive NIR-II probe to improve the diagnostic accuracy of CRC. The utilization of RENPs-based NIR-II probes to achieve this goal has been even less explored.
Hydrogen sulfide (H2S), as the third gaseous transmitter, has been proven to play important roles in various physiological and pathophysiological processes [33-34]. The increased production of H2S, generated by overexpressed cystathionine-β-synthase (CBS) in colorectal cancer cells contributes to cancer cell proliferation, migration, and invasion, which makes H2S a promising chemical target for identification of CRC [35-37]. Therefore, the H2S-responsive probes have been recently fabricated and used to detect CRC. However, most of the H2S-responsive probes are administrated intratumorally, which may not reflect the complexity of in vivo situation [38]. Additionally, although emphasis is often placed on the detection limit of probes toward H2S, the detection window is more crucial to guarantee detection specificity. The probe whose H2S-responsive range matches the H2S concentration at tumor site helps to avoid the interference from other diseases or organs that also contain H2S or other sulfur species, holding great potential for in vivo imaging of CRC [39]. Therefore, to develop a H2S-activatable NIR-II optical probe with a good stability and highly matched responsive-range is extremely desirable for sensitive and accurate CRC detection.
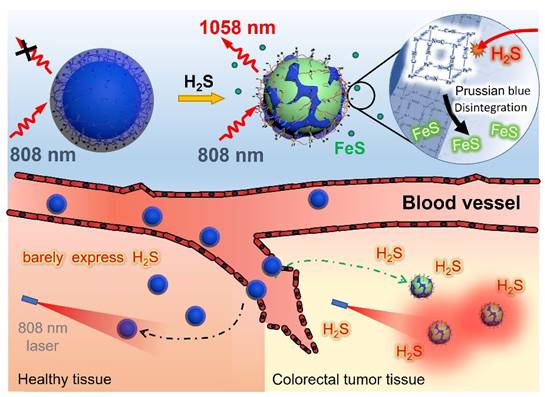
Herein, we proposed a novel polyacrylic acid (PAA)-mediated strategy to fabricate an endogenous H2S-activated NIR-II probe based on Nd3+-doped downconversion nanoparticles (DCNPs) coated with Prussian Blue (PB) layer (termed DCNP@PB) for sensitive and highly specific detection of CRC (Scheme 1). Besides serving as a H2S-responsive motif, PB plays a crucial role in regulating the emission properties of DCNP@PB. After encapsulation by PB, the luminescence of DCNPs is greatly quenched via both inner filter effect (IFE) and fluorescence resonance energy transfer (FRET) due to spectral overlap between absorption of PB and excitation/emission spectra of DCNPs. However, once at tumor site, the Fe(III) in PB reacts with highly expressed H2S and causes the decomposition of PB layer, leading to a significant decrease in PB absorbance. As a result, the luminescence of DCNPs can be recovered to illuminate the colorectal tumor with high sensitivity and specificity. Most importantly, H2S-responsive range of DCNP@PB well matches the H2S concentration at tumor site (0.3-3.4 mmol L-1) [40], allowing the specific detection of colorectal tumor in complicated physiological environment. With these favorable features, intravenously administrated DCNP@PB not only exhibits superior capability for in vivo CRC imaging in mouse models, but also well defines margins of the tumor lesions with high accuracy in human colorectal tissues.
Materials and methods
Reagents and instrumentation
All chemical reagents, if not specified, were purchased from Alfa Aesar or Sigma-Aldrich and used without purification. Deionized water (DI water) was used in all experiments. Murine mammary carcinoma cell line 4T1, HCT116, Luciferase-expressing HCT116 were obtained from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. (Shanghai, China). Penicillin streptomycin, RPMI 1640 Medium, bovine serum (FBS) and 0.25% (w/v) trypsin solution were purchased from Gibco Life Technologies (AG, Switzerland). Phosphate-Buffered Saline (PBS) was purchased from OpticsPlanet, Inc. (USA). BALB/c nude female mice (6-7 weeks old and weighing 18-22 g) were supplied by the Animal Center of the Fourth Military Medical University (Xi'an, China). Animal protocols related to this study were reviewed and approved by the Institutional Animal Care and Use Committee of the Fourth Military Medical University (approval number: 20220312). The tissues taken from the patients related to this study were reviewed and approved by the Medical Research Ethics Committee of Xi 'an International Medical Center Hospital (approval number: GJYX-KY-2024-023). All participants provided written informed consent before study enrollment. Transmission electron microscopy (TEM) images were obtained using a JEM-2100F electron microscope. NIR-II live animal imaging system (Series II 900/1700-H, China) and home-built small animal NIR-II imaging system by InGaAs-NIRvana640LN camera were applied for in vivo imaging. NIR-II microscopy platform (InGaAs CCD camera equipped with a microscope objective lens) was self-built [41].
Synthesis of NaGdF4: Nd nanoparticles
In a typical synthetic procedure of NaGdF4:5%Nd, 1 mmol of CF3COONa, 0.95 mmol of Gd(CF3COO)3, 0.05 mmol of Nd(CF3COO)3 were mixed with oleic acid (10 mmol), oleylamine (10 mmol) and 1-octadecene (20 mmol) in a two-neck reaction flask. The slurry mixture was heated to 120 °C under vacuum for 30 min to remove water and oxygen. Then the solution was heated to 300 °C with a rate of 10 °C/min under dry argon flow, and then maintained at 300 °C for 60 min with stirring. After cooling to room temperature, an excess amount of ethanol was poured into the solution. The resultant nanoparticles were centrifuged at 5,000 r.p.m. for 20 min. After centrifugal washing with hexane/ethanol, NaGdF4:5%Nd nanoparticles were re-dispersed in 5 mL of hexane for further coating.
Synthesis of NaGdF4: Nd@NaGdF4 nanoparticles
Typically, 5.0 mL of as-prepared NaGdF4:5%Nd solution, 1 mmol of CF3COONa, 1 mmol of Gd(CF3COO)3 were mixed with oleic acid (20 mmol) and 1-octadecene (20 mmol) in a two-neck reaction flask. Then the mixture was heated to 120 °C under vacuum for 30 min to remove hexane and water. The flask was then heated to 305 °C for 75 min with stirring. After cooling to room temperature, an excess amount of ethanol was poured into the solution. The resultant nanoparticles were centrifuged at 5,000 r.p.m. for 20 min. After centrifugal washing with hexane/ethanol, NaGdF4:5%Nd@NaGdF4 nanoparticles were re-dispersed in 5 mL of hexane for further coating.
Synthesis of NaGdF4: Nd@NaGdF4@NaGdF4 nanoparticles
The synthetic procedure of NaGdF4:5%Nd@NaGdF4@NaGdF4 was the same as that used to synthesize NaGdF4:5%Nd@NaGdF4 nanoparticles, except that 5 mL of as-prepared NaGdF4:5%Nd@NaGdF4 solution, 1 mmol of CF3COONa, 1 mmol of Nd(CF3COO)3 were mixed with oleic acid (20 mmol) and 1-octadecene (20 mmol) in a two-neck reaction flask. The as-synthesized NaGdF4:5%Nd@NaGdF4@NaGdF4 nanoparticles were re-dispersed in 10 mL of chloroform for further modification.
Schematic illustrations of an endogenous H2S-activatable NIR-II nanoprobe DCNP@PB for highly specific imaging of colon tumor.
Synthesis of NaGdF4: Nd@NaGdF4@NaGdF4@PVP nanoparticles
2 g of polyvinyl pyrrolidone (PVP) was dissolved in 20 mL of DI water in 5 mL flask, and then 1 mL NaGdF4:5%Nd@NaGdF4@NaGdF4 in chloroform was added dropwise, followed by 6 h of stirring at room temperature. Subsequently, after centrifugation at 14,000 r.p.m for 1 h, the excess surfactant was removed to obtain hydrophilic nanoparticles NaGdF4:5%Nd@NaGdF4@NaGdF4@PVP, which were dissolved in 10 mL of DI water for later use coating.
Preparation of DCNP@PB probe (NaGdF4: Nd@NaGdF4@NaGdF4@PVP@PB)
4 mg Polyacrylic acid (PAA, MW: 1800) added into 10 mL of NaGdF4:5%Nd@NaGdF4@NaGdF4@PVP, followed by 1 h of stirring at room temperature. Then 2 mg of FeCl3 dissolved in 1 mL of DI water was added to solution under magnetic stirring, followed by 1h of stirring at room temperature. Afterwards, 30 mL isopropanol was added to the above mixed suspension under stirring for 30 min. Then 5 mg K₄[Fe (CN)6] aqueous solution was added to the above mixed suspension, followed by 12 h of stirring at room temperature. Subsequently, after centrifugation at 10000 r.p.m. for 10 min, the excess surfactant was removed to obtain hydrophilic nanoparticles NaGdF4:5%Nd@NaGdF4@NaGdF4@PVP@PB probe, which were dissolved in 1 mL of DI water.
Preparation of NdNP@PAA-mPEG probe
4 mg Polyacrylic acid (PAA, MW: 1800) added into 10 mL of NaGdF4:5%Nd@NaGdF4@NaGdF4@PVP, followed by 1 h of stirring at room temperature to obtain NdNP@PAA solution. Then 4 mg mPEG-NH2 (5k) mixed in 3 mL MES solution is added to above NdNP@PAA solution. 8 mg EDC is added then. The solution is shakily reacted for 3 hours. Then 20 mg Tris-base and 10 mg EDC is added into above solution. The solution is shakily reacted for another 3 hours. The solution is centrifuged at 4500 r.p.m. for 30 minutes to get rid of potential large floccules. Then the supernate is washed against DI water by 100 K centrifugal filter for 3 times. The final ErNPs-mixPEG are dispersed in 800 μL MES solution.
Synthesis of ErNP, ErNP@PVP and ErNP@PB
The ErNP nanoparticle was synthesized by the literature method [42]. The synthetic procedure of ErNP@PVP and ErNP@PB was the same as that used to synthesize NaGdF4@PVP and DCNP@PB nanoparticles.
Detection of H2S in vitro
The H2S standard solution was prepared by adjusting the pH of freshly formulated NaHS solution to neutral (pH = 7.4) with diluted hydrochloric acid solution. All H2S standard solutions and DCNP@PB nanoprobes suspension were used immediately after formulation. To set up the standard curve for H2S detection, 1 mL DCNP@PB nanoprobes solution (1 mg mL-1) was added into 1 mL H2S standard solutions with concentrations from 0 μM to 800 μM, respectively. After incubated for 10 min, the UV-vis-NIR absorbance spectra and NIR spectra of all samples were determined, respectively.
The Signal-to-Noise Ratio Method is used in the calculation of LOD (Limit of Detection):
LOD=3σ/S
where σ is the standard deviation of the background noise and S is the slope of the calibration curve (response versus concentration).
The same method to demonstrate the selectivity of DCNP@PB nanoprobes, including common biosulfurs (reduced glutathione (GSH), cysteine (Cys), glutamic acid (Glu), SO32-), essential/non-essential amino acids, reactive oxygen species (Vitamin C (VC), HClO, H2O2, and ONOO-), cations (K+, Na+, Ca2+, Mg2+, Mn2+, Zn2+, and NH4+), anions (Cl-, PO43-), dopamine, bovine serum albumin (BSA). The concentration of studied biomolecules and ions was 10 mM.
To demonstrate the speed of responsive of DCNP@PB nanoprobes, 1 mL DCNP@PB nanoprobes suspension (10 mg mL-1) were monitored dynamically per 6 s by UV-vis-NIR absorbance spectra. Time to 2 min, 1 mL H2S standard solution (600, 300, 0 μM) was added rapidly. Continuous monitoring to 5 min, UV-vis-NIR absorbance spectra of all samples were determined.
Stability of DCNP@PB
The intensity at 1058 nm of DCNP@PB nanoprobes in various biological solutions (DI water, PBS (pH 7.4, pH 6.5, saline, 0.35 mg mL-1) were recorded by and NIR spectra within 120 min. The DLS of DCNP@PB after various storage time points were determined after voticity for 2 h.
Cell lines culture
Murine mammary carcinoma cell line 4T1, human colon cancer cell line HCT116 and luciferase HCT116 (HCT116-luc), were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) at 37 °C under 5% CO2.
Cytotoxicity test in vitro
The cell viability was measured using conventional MTT assay. The used cell lines were human colon cancer cell line HCT116. Typically, cells were respectively seeded at a density of 5000 cells per well in a 96-well plate. After 12 h of incubation, fresh cell culture medium containing various concentrations (0, 0.5, 1, 2, 4, 6, 8 and 10 mg mL-1) of DCNP@PB was added for further incubation of 24 h. To determine the cell viability, cells were washed with PBS for three times and incubated with MTT (5 mg mL-1) solution for another 4 h, followed by 150 μL of DMSO was added to each and optical absorption measurement at 492 nm to calculate cell viability.
Cell imaging
2×104 HCT116 cells were pretreated with PBS, aminooxyacetic acid AOAA (2 mM) for 24 h, 2×104 4T1 cells were pretreated with PBS for 24 h. Group1 cells with various pretreatments were then incubated with DCNP@PB nanoprobes suspension (1 mg mL-1) for 4 h, Group2 cells with various pretreatments were then incubated with DCNP@PB nanoprobes suspension (1 mg mL-1) and NaHS (200 μM) for 4 h, washed with PBS several time. The cell imaging was taken by NIR-II live animal imaging systemexicted by 808 nm laser (100 mW cm-2). For calibration experiments, the cultured cells were counted using an automated cell counter. Cell suspensions with different cell concentrations were added to a row of wells. Following this, 10 μL of 1 mg mL-1 D-luciferin-K+ salt bioluminescent substrate in PBS is added to each well. After a 3 min reaction time, NIR-II imaging ex vivo bioluminescence imaging is performed.
Animal models construction
To establish colon cancer and breast cancer mice model, approximately 5×105 HCT116 and 4T1 cells in 5 μL phosphate buffered saline were intracranially injected into the right hindlimb of the BALB/c mice subcutaneously.
In vivo imaging
Before imaging, the hair of all the mice was removed by using hair clipper and depilatory cream. The mice were intravenously injected with 100 µL (10 mg mL-1) probes. 50 µL 10 mg mL-1 NaHS solution was injected subcutaneously around the tumor during the 4T1 tumor imaging. Human ex vivo tissues were immersed in a 10 mg mL-1 probe solution for 6 hours. Fluorescence images were acquired by Series II 900/1700-H. An 808 nm laser diode was decided to be the excitation laser for both contrast agents at a power density of 400 mW cm-2 (lower than the safe exposure limit of 1.0 W cm-2 determined by the International Commission on Nonionizing Radiation Protection). A 1000 nm long-pass filter were applied to collect the emission of the probes. Exposure time was 50 ms for all images.
Statistical analysis
Data analysis was conducted using Origin 9, GraphPad Prism 8, LivingImage and ImageJ v.2.9.0. All data, presented as mean ± s.d., were calculated using Origin 9 or GraphPad Prism 8. Statistics were performed on SPSS17.0 statistical analysis software. All the error bars indicated mean ± standard deviation. Statistical analysis was performed using Student's t test (two-tailed). Statistical significance was established as indicated * P < 0.05; ** P < 0.01 and *** P < 0.001.
Results and Discussion
Synthesis and characterization of DCNP@PB nanoprobe
DCNP@PB probe composed of Nd3+-doped downconversion nanoparticles (DCNPs) NaGdF4: Nd@NaGdF4 interior and a PB coating were synthesized using a novel PAA-mediated strategy (Figure 1A and Figure S1A-1C). Briefly, oleic acid-capped DCNPs were first modified with a water-soluble polymer, PVP through a ligand-exchange approach. PAA was then introduced onto the surface of DCNPs via an electrostatic interaction, endowing the DCNPs with negatively charged surface. Fe3+ was added to PAA-capped DCNPs to yield a PAA/Fe3+ solution, followed by adjusting pH of reaction system. Afterward, isopropanol and K4[Fe(CN)6] were added to this mixture for PB growth and mineralization to finally generate DCNP@PB. The representative TEM images in Figure 1B and C showed that as-prepared DCNPs (88.59 nm ± 2.1 nm) were homogenously encapsulated by a PB layer, resulting in particle with an average diameter of approximately 140.16 ± 1.7 nm. Further elemental mapping analysis demonstrated that the obtained DCNP@PB was uniformly composed of Gd (green), Nd (purple), and Fe (red) (Figure S1D). The successful formation of DCNP@PB was also confirmed by X-ray diffraction (XRD) patterns results, which clearly showed combined characteristic peaks of hexagonal phase of DCNPs and PB (Figure 1D). In addition, shift in zeta potential of DCNPs surface indicated the fabrication of DCNP@PB, which has a near-neutral net surface charge at physiological pH (Figure 1E).
Characterization of the DCNP@PB nanoprobes. (A) Schematic illustrations of synthesis of DCNP@PB. (B) TEM image of DCNPs. (C) TEM image of DCNP@PB. (D) X-ray diffraction spectra of DCNPs, PB and DCNP@PB. (E) Zeta potential of DCNP@PVP, DCNP@PAA and DCNP@PB. (F) Absorption spectrum of PB, excitation and emission spectra of DCNPs. (G) Absorption spectra of DCNPs and DCNP@PB. (H) NIR-II emission spectra of DCNPs, DCNPs in PB solution and DCNP@PB.
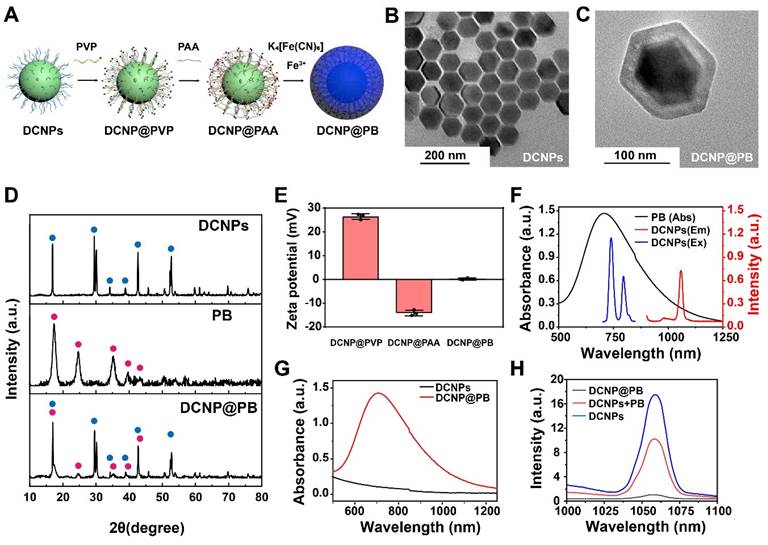
To evaluate the luminescence behaviors of DCNP@PB, we first measured the optical spectra of DCNPs and PB, respectively. As shown in Figure 1F, excitation and emission spectra of DCNPs effectively overlapped with absorption spectra of PB, indicating the possibility of IFE or FRET. To further investigate the effects of the PB on emission of DCNP, we tracked the absorption and emission spectra of DCNPs before and after PB coating. The mixture of DCNPs with PB was used for comparison. As shown in Figure 1G, DCNP@PB displayed a typical broad absorbance band of PB extending to beyond 1200 nm with peak absorbance at 720 nm, suggesting the presence of PB shell. Meanwhile, the luminescence of DCNP@PB was almost completely quenched compared with that of pure DCNPs, which exhibited characteristic emission bands of Nd3+ at 1058 nm under an 808 nm laser irradiation (Figure 1H). However, when DCNPs directly mixed with PB, in which the DCNPs and PB was not too close to induce significant FRET, the emission intensity of DCNPs only decreased by half (Figure 1H). These results indicated that single IFE between DCNPs and PB is not enough to strongly quench luminescence of DCNPs and both IFE and FRET mechanisms may be responsible for substantial reduction in DCNP@PB luminescence.
To verify our assumption, we fabricated a PB coated Er3+-doped nanoparticles with 1525 nm emission (denoted as ErNP@PB) to diminish the spectra overlap between absorbance of PB and emission of ErNPs. In this way, the FRET could be excluded. By keeping the absorbance at 720 nm the same (Figure S2A, 2.5 mg of PB), we further compared the emission of ErNP@PB with that of mixture of ErNPs and PB. As shown in Figure S2B, luminescence of both groups declined slightly compared with that of pure ErNPs. In addition, there is almost no difference in their emission intensity (2.5 mg of PB), which indicated that the luminescence quenching of ErNP@PB can be mainly attributed to the IFE. Moreover, the emission intensity of the mixture was gradually dropped with the increasing amount of PB, further indicating the existence of IFE (Figure S2B). We also investigated the emission spectra of ErNPs with or without PB coating under 980 nm or 808 nm excitation, which would result in the different degree of spectral overlap between ErNPs and PB. As shown in Figure S2C and D, when excited by 808 nm laser, luminescence of ErNP@PB decreased more sharply than that under 980 nm excitation, indicating the existence of IFE. Taken together, it can be reasoned that the significant luminescence quenching of DCNP@PB could be attributed to both FRET and IFE between DCNPs and PB. In addition to the matched optical properties, the distance between DCNPs and PB is crucial. Benefiting from our innovative surface functionalization strategy, PB could be evenly and densely coated onto DCNPs surface to permit optimal FRET, considering that the energy donor-acceptor pair must be close enough (typically 1 - 10 nm) for effective FRET.
In vitro evaluation the H2S response characteristics of DCNP@PB nanoprobe
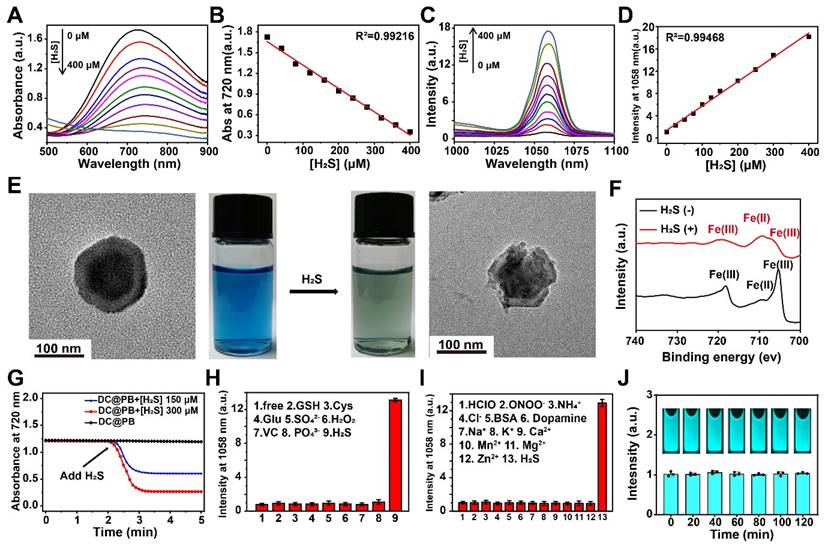
To explore the H2S-responsive performance of DCNP@PB, the changes in absorption spectra of PB and emission spectra of DCNP@PB were monitored at different H2S concentration. As shown in Figure 2A and B, as the concentration of H2S increased from 0 to 400 µM, the absorbance of PB gradually decreased, demonstrating the decomposition of the PB shells in the presence of H2S. As a result, the luminescence of DCNP@PB at 1058 nm enhanced dramatically (more than 17-fold), indicating the fast activation of DCNP@PB in response to H2S (Figure 2C and D). This H2S-responsive decomposition process of the DCNP@PB could also be confirmed by the TEM results and photographs observed in Figure 2E, which clearly showed that the intact PB shell was disintegrated after addition of H2S along with a color change from blue to dark green. Consistently, Fe 2p X-ray photoelectron spectroscopy (XPS) spectra supported the reduction of Fe(III) and the formation of FeS (Figure 2F) in the presence of H2S. DCNP@PB responded to the addition of the H2S in less than 20 s (Figure 2G). This fast response makes DCNP@PB suitable for real-time tumor imaging in vivo.
Furthermore, the H2S selectivity of DCNP@PB was evaluated with various analytes, including other reducing biological species, such as cysteine (Cys) and glutathione (GSH) at 100 μM. The results presented in Figure 2H-I and Figure S3 clearly showed that only H2S could cause a dramatic change in the emission and absorption of DCNP@PB under the identical conditions, demonstrating that DCNP@PB was promising for specific detection of H2S in a complicated biological environment. Notably, DCNP@PB could detect H2S with a detection limit of 10 µM according to the linear correlation between the NIR-II luminescence intensities and NaHS concentrations of 0-400 µM. This detection window just fell in the concentration range of H2S in CRC, implying that DCNP@PB may be able to specifically distinguish the colorectal cancer from other organs or disease based on the difference in concentration of H2S.
Furthermore, DCNP@PB showed great photostability under 808 nm laser irradiation (Figure 2J), which is favorable for intraoperative imaging. The stability of DCNP@PB in water, PBS buffer, DMEM, RPMI 1640 Medium, and 10% fetal bovine serum complete medium were tested by measuring the hydrodynamic size of DCNP@PB. As shown in Figure S4A, the size of DCNP@PB remained nearly unchanged over 12 h and exhibited excellent colloidal stability in physiological media. Additionally, the emission intensity of DCNP@PB at 1058 nm has barely changed for 2 h even at acidic tumor extracellular pH (6.5), indicating that DCNP@PB is suitable for imaging of CRC with stable NIR-II luminescence (Figure S4B). In addition, the cytotoxicity of DCNP@PB towards colorectal cancer cells HCT116 as assessed. As shown in Figure S5, no significant cytotoxicity was observed at various concentrations (up to 10 mg mL-1), suggesting excellent biocompatibility of DCNP@PB.
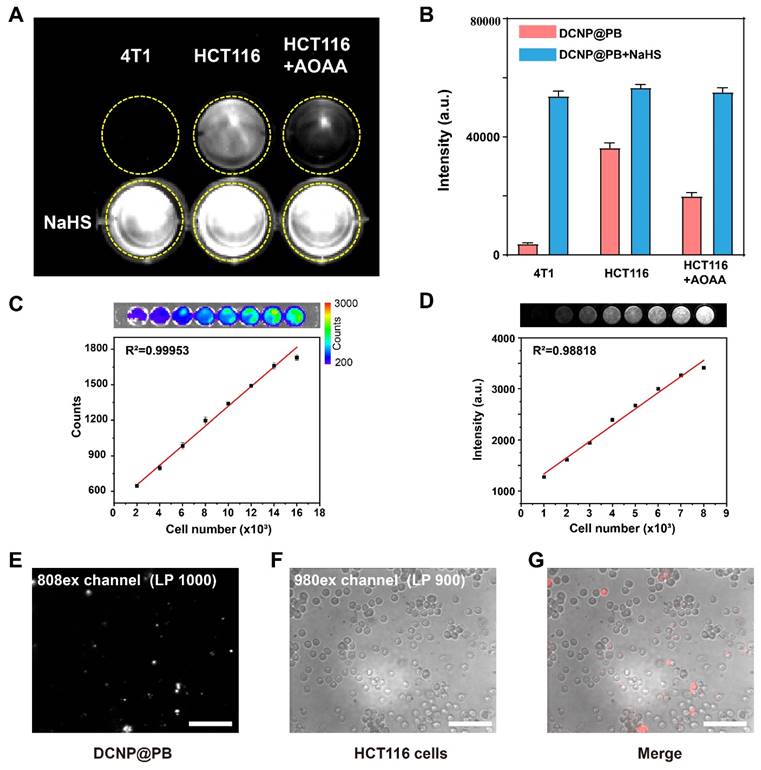
To further verify whether the DCNP@PB could target H2S-expressed colorectal cancer and initiate DCNP@PB emission effectively, H2S-rich HCT116 human colorectal cancer cells and H2S-deficient 4T1 breast cancer cells were used to evaluate the cell imaging performance of DCNP@PB. As shown in Figure 3A, HCT116 cells incubated with DCNP@PB afforded bright NIR-II luminescence under excitation of 808 nm laser, while same treatment with 4T1 cells did not elicit detectable signals. To confirm that the luminescence of DCNP@PB was indeed activated by endogenous H2S in HCT116 cells, AOAA, a frequently used CBS inhibitor, was utilized to suppress the synthesis of H2S in cells. As depicted in Figure 3A and B, pretreatment of HCT116 cells with AOAA did result in a substantial decrease in DCNP@PB luminescence, indicative of the involvement of H2S in DCNP@PB activation. In contrast, when NaHS was introduced to up-regulate H2S content in cells before incubated with DCNP@PB, enhanced luminescence signals can be observed for both 4T1 and HCT116 cells. Moreover, the luminescence intensity of DCNP@PB in HCT116 cells increased linearly with the number of cells (Figure 3C and D). All these results demonstrated that the NIR-II luminescence recovery of DCNP@PB probe is closely related with H2S contents in cells, which enables imaging of H2S-rich HCT116 cells with high sensitivity and specificity. Furthermore, HCT116 cells incubated with DCNP@PB showed clear and independent luminescence (Figure 3E-G), indicating that DCNP@PB can be internalized by HCT116 cells and activated by H2S effectively.
Evaluation of H2S response characteristics of DCNP@PB nanoprobe. (A) Absorption spectra of the DCNP@PB in H2S solutions with different concentration (0-400 μM). (B) The absorbance at 720 nm of DCNP@PB was plotted against the H2S concentration generate an empirical calibration formula with R2 = 0.992. (C) NIR-II emission spectra of DCNP@PB in H2S solutions with different concentration (0-400 μM) under excitation wavelengths of 808 nm. (D) The intensity at 1058 nm of DCNP@PB was plotted against the H2S concentration generate an empirical calibration formula with R2 = 0.995. (E) TEM images and photo of DCNP@PB solution (left) and H2S-responsed DCNP@PB solution (right). (F) Fe2p XPS spectra of DCNP@PB with/without H2S addition. (G) The response time reflected by changes in the absorbance at 720 nm of DCNP@PB. (H) and (I) The normalized luminescence intensity of DCNP@PB in the presence of H2S or other active substances. (J) Photostability of DCNP@PB solution under continuous 808 nm laser irradiation.
Sensitivity and specificity of DCNP@PB for in vivo tumor imaging
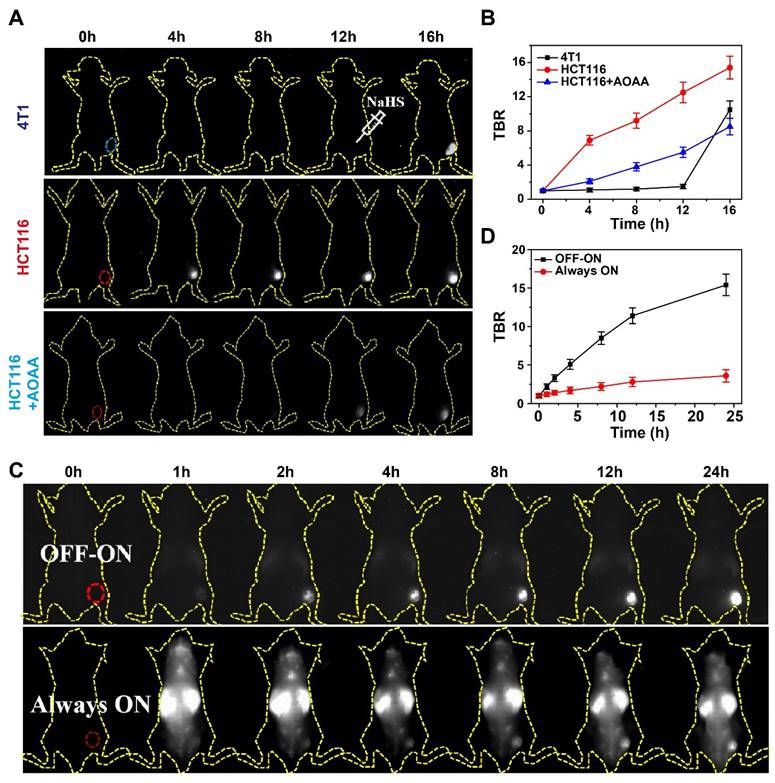
Encouraged by the impressive H2S-responsiveness of DCNP@PB in vitro, we further evaluated the capability of DCNP@PB to identify the colorectal cancer in vivo. Toward this goal, DCNP@PB was first intratumorally injected into HCT116 or 4T1 tumor-bearing mice models (n = 5 for each group). As shown in Figure 4A, DCNP@PB failed to detect 4T1 tumor even at 12 h after injection until the tumor received an intratumoral injection of NaHS. In contrast, the HCT116 tumor region was clearly illuminated with a strong NIR-II luminescence at 4 h post-injection with DCNP@PB. However, the pretreatment of HCT116 tumor with AOAA significantly retarded the imaging process of DCNP@PB, reinforcing the key role of endogenous H2S in DCNP@PB activation. These results were supported by the quantitative analysis in Figure 4B, which showed that the DCNP@PB yielded a higher luminescence contrast, defined as a tumor-to-background ratio (TBR), in the HCT116 tumor than in the 4T1 tumor, consistent with in vitro results above.
To better investigate the performance of DCNP@PB for imaging of colorectal tumor, H2S-activatable DCNP@PB probe (OFF-ON) and non-activatable probe without PB shell (NdNP@PAA-mPEG), which was used as an Always-ON control probe were separately injected into HCT116 tumor-bearing mice via tail vein (n = 5 for each group). Compared with the whole-body distribution of NdNP@PAA-mPEG, clearly delineated tumor with a bright luminescence could be observed at 2 h post-injection with DCNP@PB (Figure 4C). Meanwhile, the signal was barely visible over non-tumor areas, indicating an exceptional ability of DCNP@PB to distinguish the tumor from normal tissue with high sensitivity and specificity. Further quantitative analysis clearly demonstrated a much higher contrast of DCNP@PB in the tumor than NdNP@PAA-mPEG (Figure 4D). The maximum contrast was reached in the DCNP@PB group at 24 h post-injection (TBR ~15.4) whereas the Always-ON probe had a minimal luminescence increase in the tumor over a 24 h period (TBR ~3.4). Such a marked colorectal tumor imaging ability could be attributed to the suitable H2S-response range of DCNP@PB, which helped to reduce the interference from other biomolecules in vivo, thereby ensuring desirable specificity toward H2S in colorectal tumor.
H2S-responsive performance of DCNP@PB on cells. (A) NIR-II imaging of 4T1 cells and HCT116 cells incubated with DCNP@PB after treatment with AOAA or NaHS. (B) Quantitative luminescence intensity of (A). (C) Ex vivo bioluminescence imaging of luciferase-expressing HCT116 cells in PBS solutions with different cell numbers (upper); and the corresponding luminescence intensity counts were plotted against the cell numbers (lower). (D) NIR-II imaging of luciferase-expressing HCT116 cells incubated with DCNP@PB in PBS solutions with different cell numbers (upper); and the corresponding intensity were plotted against the cell numbers (lower). (E) 20x NIR-II micro-imaging of HCT116 cells incubated with DCNP@PB in 808-EX channel (long pass 1000 nm), (F) 980-EX channel (long pass 900 nm) and (G) merging image of two channels. Scale bar: 100 μm.
NIR-II luminescence-guided surgery by DCNP@PB
The great tumor recognition capability of DCNP@PB inspired us to further examine the feasibility of DCNP@PB for NIR-II luminescence-guided surgery (Supplementary Video). DCNP@PB was injected intravenously into HCT116 tumor-bearing mice 12-24 h before surgery. After resection of the tumor under the guidance of DCNP@PB illumination, there was no detectable signals can be observed in the tumor bed, indicating that the tumor was excised completely (Figure S6A). Consistent with in vivo imaging result, DCNP@PB generated remarkable TBR (more than 10, Figure S6B). Meanwhile, ex vivo imaging of the tumor and major organs collected from mice that were sacrificed post-injection was performed to examine the biodistribution of the DCNP@PB. As shown in Figure S6C and D, DCNP@PB exhibited higher NIR-II signals in the tumor over normal organs, which was probably due to specific activation of DCNP@PB by H2S in the tumor region. As a result, the background signals can be effectively suppressed, giving rise to enhanced contrast for specific tumor visualization. All these results strongly supported the ability of DCNP@PB to specifically image the tumor rather than the normal tissue. Additionally, multiple physiological indicators in the blood biochemistry suggested good biocompatibility of DCNP@PB (Figure S7).
The specificity of DCNP@PB for tumor extracellular H2S-responsive imaging. (A) Time-dependent NIR-II luminescence imaging of mice bearing 4T1 and HCT116 cells tumor after intravenous injection of DCNP@PB under different conditions. (B) TBR as a function of time from A. (C) Time-dependent NIR-II luminescence imaging of mice bearing HCT116 cells tumor after intravenous injection of DCNP@PB (upper) and NdNP@PAA-mPEG (lower), respectively. (D) TBR as a function of time from (C).
Specific imaging of tumor tissues in patients with CRC
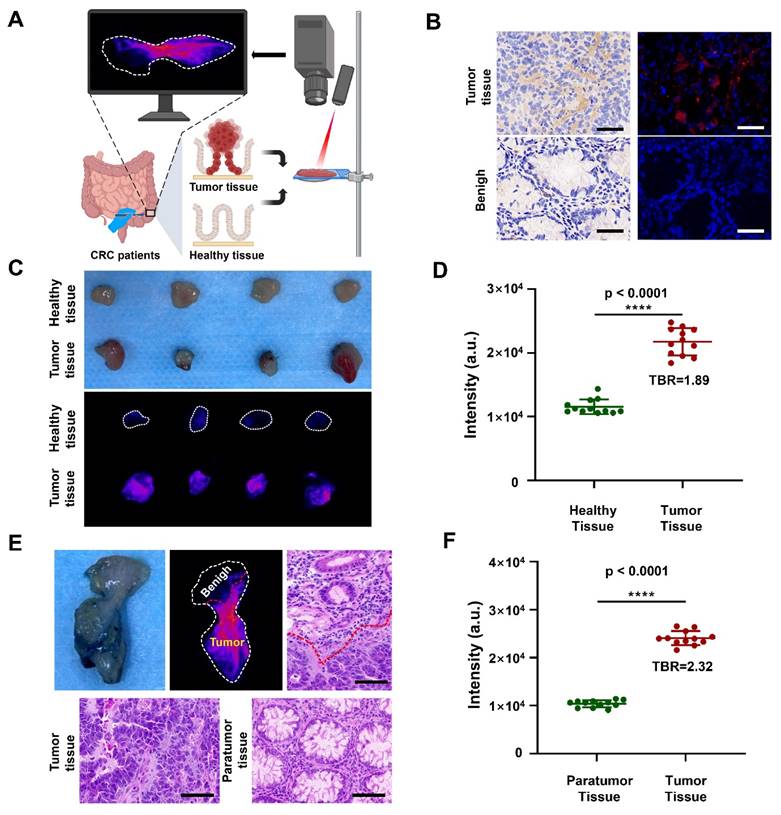
Having demonstrated the remarkable capability of DCNP@PB to detect the tumor in mouse models, we further explored its feasibility for precise discrimination between cancerous and normal human colorectal tissues (Figure 5A). Before that, we first measured the expression of CBS in tumor tissue of CRC patients. As shown in Figure 5B, immunohistochemical staining and immunofluorescence staining validated that CBS were highly expressed in tumor tissues of CRC patients, but hardly observed in benign tissues, indicating that the H2S level was indeed higher in human tumor than in normal tissues. These results encouraged us to employ DCNP@PB to image the resected human specimens during surgery, which were assessed by pathologic evaluation. As shown in Figure 5C and D, the tumor tissues were illuminated by DCNP@PB with high TBR (1.89), while adjacent healthy tissues only showed very weak signals. H&E staining confirmed that the NIR-II signals were highly tumor specific, indicating the effectiveness of DCNP@PB as a promising option for fast and precise tumor identification and histologic evaluation (Figure S8). It is noteworthy that our DCNP@PB excelled in delineating the tumor margin, validating the reliability of DCNP@PB to differentiating between malignant and benign lesions (Figure 5E and Figure S9). Such accurate tumor recognition of DCNP@PB could probably be attributed to its tumor-specific H2S responsiveness, which effectively reduced nonspecific activation by other sulfur-containing substances in complicated physiological environment.
NIR-II imaging of tissues taken from the patients. (A) Schematic illustrations of NIR-II imaging of tumor tissues and healthy tissues taken from the patients. (B) Immunohistochemical staining (left) and immumofluorescence staining (right) of CBS in tumor tissues (upper) and benign (lower) taken from the patients. (CBS is red, scale bar: 50 μm) (C) Photo (upper) and NIR-II imaging (lower) of healthy tissues and tumor tissues taken from the patients. (D) Quantification of luminescence signals of healthy tissues and tumor tissues taken from the patients in (C). (E) Photo, NIR-II imaging and d H&E staining of tissue where both tumor and paratumor tissue of colon tissues taken from the patients. (scale bar: 100 μm) (F) Quantification of luminescence signals of paratumor tissue and tumor tissue taken from the patients in (E).
Conclusions
In summary, we successfully developed a H2S-activatable NIR-II probe DCNP@PB for highly specific imaging of CRC in vivo. By exploiting a novel PAA-mediated strategy, a homogeneous PB outer shell could be uniformly coated on the DCNP surface to ensure effective IFE and FRET between DCNPs and PB. As a result, luminescence of DCNP@PB can be efficiently switched from OFF to ON state in response to H2S. In the absence of H2S, DCNP@PB exhibited good stability and very weak emission. Upon arriving in the H2S-rich colorectal tumor, however, the luminescence of DCNP@PB at 1058 nm was recovered rapidly to illuminate the tumor. Notably, H2S-responsive range of DCNP@PB fell right into the H2S concentration range at the tumor site, which greatly reduced interference from other sulfur-containing substances in complicated physiological environment. Benefiting from such accurate responsiveness, DCNP@PB can be specifically activated by H2S in colorectal tumor rather than in normal organs/tissues even when DCNP@PB was intravenously administrated in vivo. Compared to the non-activatable probe, the H2S-activatable DCNP@PB probe can afford much higher TBR in tumor regions (16 vs 3), making DCNP@PB ideal candidate for real-time CRC imaging and image-guided surgery. Notably, DCNP@PB could not only possess the superior capability of DCNP@PB to detect the tumor in mouse models, but also ensure the precise discrimination between cancerous and normal human colorectal tissues. This work presents not only a promising example of H2S-activated NIR-II optical probe that could be intravenously injected for in vivo applications, but also an effective strategy to design the activatable probes for precise tumor imaging and fast histologic evaluation.
Supplementary Material
Supplementary figures.
Supplementary video.
Acknowledgements
This work was funded by the National Key Research and Development Program of China (No. 2022YFB3203800), National Natural Science Foundation of China (Nos. 82272159, 22404131, W2411083), China Postdoctoral Science Foundation (No. 2022TQ0249), the Natural Science Basic Research Program of Shaanxi (No. 2024JC-YBQN-0111), the Postdoctoral Research Project of Shaanxi Province (No. 2023BSHEDZZ155), Innovation Capability Support Program of Shaanxi (No. 2022TD-52), Shaanxi Fundamental Science Research Project for Chemistry and Biology (No. 23JHZ005), Guangdong Basic and Applied Basic Research Foundation (No. 2023A1515110803), Key Research and Development Program of Shaanxi (No. 2024SF-YBXM-126), the Fundamental Research Funds for the Central Universities (Nos. QTZX22068, XJSJ23189), and the Open Project Program of the State Key Laboratory of Holistic Integrative Management of Gastrointestinal Cancers (Xijing Hospital of Digestive Diseases, Fourth Military Medical University) (No. CBSKL2022ZDKF14).
Author contributions
# Yu Ji and Qinxian Huang contributed equally to this work.
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. Yu Ji: Data curation, Investigation, Methodology, Validation, Writing - original daft. Qinxian Huang: Investigation, Data curation, Methodology. Qian Jia: Writing - original draft, Project administration, Supervision, Funding acquisition. Haohao Yan: Investigation, Formal analysis. Yajing Chi: Formal analysis. Yuanyuan Jia: Investigation, Formal analysis. Chaoqiang Qiao: Formal analysis, Supporting. Yanbin Feng: Validation, Supporting. Zuo Yang: Investigation, Methodology. Ruili Zhang: Writing - original draft, Conceptualization, Supervision, Funding acquisition. Zhongliang Wang: Writing - review & editing, Conceptualization, Supervision, Funding acquisition.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel R, Leversanne M, Soerjomataram I, Jemal A. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;71:209-249
2. Siegel R, Miller K, Fuchs H, Jemal A. Cancer statistics. CA Cancer J Clin. 2022;72:7-33
3. Veettil S, Wong T, Loo Y, Playdon M, Chaiyakunapruk N. Role of diet in colorectal cancer incidence. JAMA Netw Open. 2021;4:e2037341
4. Clay S, Fonseca-Pereira D, Garrett W. Colorectal cancer: the facts in the case of the microbiota. J Clin Invest. 2022;132:e155101
5. Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D. et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607-615
6. Xu J, Lv Z, Wang L, Wu X, Tan B, She X. et al. Tuning tumor targeting and ratiometric photoacoustic imaging by fine-tuning torsion angle for colorectal liver metastasis diagnosis. Chem Eur J. 2024;30:e202402019
7. Shaukat A, Levin T. Current and future colorectal cancer screening strategies. Nat Rev Gastroenterol Hepatol. 2022;19:521-531
8. Biller L, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325:669-685
9. Sawicki T, Ruszkowska M, Danielewicz A, Niedźwiedzka E, Arłukowicz T, Przybyłowicz K. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers. 2021;13:2025
10. Jia Q, Zhang R, Wang Y, Yan H, Li Z, Feng Y. et al. A metabolic acidity-activatable calcium phosphate probe with fluorescence signal amplification capabilities for non-invasive imaging of tumor malignancy. Sci Bull. 2022;67:288-298
11. Li J, Pu K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem Soc Rev. 2019;48:38-71
12. Cheng P, Pu K. Molecular imaging and disease theranostics with renal-clearable optical agents. Nat Rev Mater. 2021;6:1095-1113
13. Zhang S, Chen H, Wang L, Qin X, Jiang B, Jie S. et al. A general approach to design dual ratiometric fluorescent and photoacoustic probe for quantitatively visualizing tumor hypoxia levels in vivo. Angew Chem Int Ed Engl. 2022;134:e202107076
14. Zhang F, Tang B. Near-infrared luminescent probes for bioimaging and biosensing. Chem Sci. 2021;12:3377-3378
15. Lei Z, Zhang F. Molecular engineering of NIR-II fluorophores for improved biomedical detection. Angew Chem Int Ed Engl. 2021;60:16294-16308
16. Zhu S, Tian R, Antaris A, Chen X, Dai H. Near-infrared-II molecular dyes for cancer imaging and surgery. Adv Mater. 2019;31:1900321
17. Jia Q, Zhang R, Yan H, Feng Y, Sun F, Yang Z. et al. An activatable near-infrared fluorescent probe for precise detection of the pulmonary metastatic tumors: a traditional molecule having a stunning turn. Angew Chem Int Ed Engl. 2023;62:e202313420
18. Elizabeth L, Ou Z, Erving X, Cui H, Carl H, Daniel J. et al. Near-infrared II fluorescence imaging. Nat Rev Methods Primers. 2024;4:23
19. Lei S, Jiang K, Zhang C, Sun W, Pan Y, Wang D. et al. A FRET-based ratiometric H2S sensor for sensitive optical molecular imaging in second near-infrared window. Research. 2023;6:0286
20. Guo Y, Li Z, Guo B, Wang B, Tu Y. Targeting-specific nanoprobes in the second near-infrared window for biomedical applications. Nano Biomed Eng. 2024;16:135-151
21. Smith A, Mancini M, Nie S. Bioimaging: second window for in vivo imaging. Nat Nanotechnol. 2009;4:710-711
22. Hu Z, Fang B, Li B, Zhang Z, Tian J. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat Biomed Eng. 2020;4:259-271
23. Jia Q, Li Z, Bai M, Yan H, Zhang R, Ji Y. et al. Estimating dynamic vascular perfusion based on Er-based lanthanide nanoprobes with enhanced down-conversion emission beyond 1500 nm. Theranostics. 2021;11:9859-9872
24. Brozena A, Kim M, Powell R, Wan Y. Controlling the optical properties of carbon nanotubes with organic colour-centre quantum defects. Nat Rev Chem. 2019;3:375-392
25. Hong G, Diao S, Antaris A, Dai H. Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem Rev. 2015;115:10816-10906
26. Li C, Li F, Zhang Y, Zhang W, Zhang X, Wang Q. Real-time monitoring surface chemistry-dependent in vivo behaviors of protein nanocages via encapsulating an NIR-II Ag2S quantum dot. ACS Nano. 2015;9:12255
27. Vasilopoulou M, Kim H, Kim B, Papadakis M, Yusoff A. Efficient colloidal quantum dot light-emitting diodes operating in the second near-infrared biological window. Nat Photonics. 2019;14:50-56
28. Zhong Y, Dai H. A mini-review on rare-earth down-conversion nanoparticles for NIR-II imaging of biological systems. Nano Res. 2020;13:1281-1294
29. Yang Z, Ji Y, Jia Q, Feng Y, Ji R, Bai M. et al. Real-time detection and resection of sentinel lymph node metastasis in breast cancer through a rare earth nanoprobe based NIR-IIb fluorescence imaging. Mater Today Bio. 2024;28:101166
30. Zhong Y, Ma Z. Wang F, Wang X, Dai H. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat Biotechnol. 2019;37:1322-1331
31. He S, Song J, Qu J, Chen Z. Crucial breakthrough of second near-infrared biological window fluorophores: design and synthesis toward multimodal imaging and theranostics. Chem Soc Rev. 2018;47:4258-4278
32. Antaris A, Hao C, Kai C, Yao S, Hong G, Qu C. et al. A small-molecule dye for NIR-II imaging. Nat Mater. 2019;15:235-242
33. Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41:113-121
34. Jha S, Calvert J, Duranski M, Ramachandran A, Lefer D. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295:H801
35. Khattak S, Rauf M, Khan N, Zhang Q, Chen H, Muhammad P. et al. Hydrogen sulfide biology and its role in cancer. Molecules. 2022;27:3389
36. Corvino A, Severino B, Fiorino F, Frecentese F, Magli E, Perissutti E. et al. Fragment-based de novo design of a cystathionine γ-lyase selective inhibitor blocking hydrogen sulfide production. Sci Rep. 2016;6:34398
37. Csaba S, Ciro C, Celia C, Ketalin M, Bartosz S, Andreas P. et al. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc Natl Acad Sci U S A. 2013;110:12474-12479
38. Feng L, Zhao Y. Research progress in endogenous H2S activatable nanoplatforms for cancer theranostics. View. 2020;1:e15
39. Filipovic M R, Jasmina Z, Beatriz A, Ruma B. Chemical biology of H2S signaling through persulfidation. Chem Rev. 2018;118:1253-1337
40. An L, Wang X, Rui X, Lin J, Yang H, Tian Q. et al. The In situ sulfidation of Cu2O by endogenous H2S for colon cancer theranostics. Angew Chem Int Ed Engl. 2018;57:15782-15786
41. Fang Z, Wang C, Yang J, Song Z, Xie C, Ji Y. et al. Oxyhaemoglobin saturation NIR-IIb imaging for assessing cancer metabolism and predicting the response to immunotherapy. Nat Nanotechnol. 2024;19:124-130
42. Zhong Y, Ma Z, Wang F, Wang X, Yang Y, Liu Y. et al. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat Biotechnol. 2019;37:1322-1331
Author contact
![]() Corresponding authors: Qian Jia, Email: jiaqianedu.cn; Ruili Zhang, Email: rlzhangedu.cn; Zhongliang Wang, Email: wangzledu.cn.
Corresponding authors: Qian Jia, Email: jiaqianedu.cn; Ruili Zhang, Email: rlzhangedu.cn; Zhongliang Wang, Email: wangzledu.cn.
 Global reach, higher impact
Global reach, higher impact