13.3
Impact Factor
Theranostics 2024; 14(15):5999-6015. doi:10.7150/thno.98149 This issue Cite
Research Paper
Nicotinamide mononucleotide enhances fracture healing by promoting skeletal stem cell proliferation
1. School of Life Science and Technology, ShanghaiTech University, Shanghai 201210, PR China.
2. Shanghai Clinical Research and Trial Center, Shanghai 200000, PR China.
3. Key Laboratory of Multi-Cell Systems, Shanghai Institute of Biochemistry and Cell Biology, Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences; University of Chinese Academy of Sciences, Shanghai 200031, PR China.
4. Division of Sports Medicine and Adult Reconstructive Surgery, Department of Orthopedic Surgery, Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, 321 Zhongshan Road, Nanjing, Jiangsu 210008, PR China.
5. Department of Orthopedic Surgery, the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310000, PR China.
6. Musculoskeletal Research Laboratory, Department of Orthopedics & Traumatology, The Chinese University of Hong Kong, Hong Kong 999077, PR China.
†These authors contributed equally to this work.
Abstract
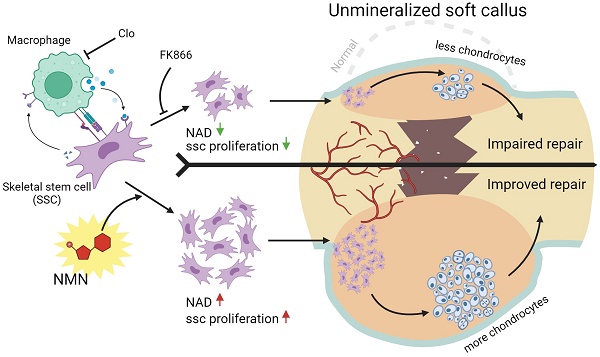
The process of skeletal regeneration initiated by stem cells following injury, especially in fractures, is significantly impaired by aging and adverse factors. Nicotinamide mononucleotide (NMN), a critical endogenous precursor of nicotinamide adenine dinucleotide (NAD), has garnered extensive attention for its multifaceted regulatory functions in living organisms and its wide-ranging therapeutic potential. However, whether NMN contributes to trauma-induced skeletal regeneration remains unclear.
Methods: The transverse femoral shaft fracture model was employed to evaluate the potential advantages of NMN administration for overall repair during the initial fracture stages in male mice through micro-CT analysis, histochemistry, and biomechanical testing. The pro-proliferative function of NMN on skeletal stem cells (SSCs) was investigated through flow cytometry, qRT-PCR, NAD content measurement, and cell proliferation assay.
Results: In this study, we observed that the administration of NMN during the initial phase of fracture in mice led to a larger callus and corresponding improvement in micro-CT parameters. NMN enhances the cartilaginous component of the callus by elevating the NAD content, consequently accelerating subsequent endochondral ossification and the fracture healing process. Subsequent analyses elucidated that NMN was beneficial in promoting the expansion of diverse stem cells in vivo and in vitro potentially via modulation of the Notch signaling pathway. Moreover, the depletion of macrophages profoundly obstructs the proliferation of SSCs.
Conclusion: Our discoveries provide a potential strategy for enhancing fracture healing through stimulation of callus SSC proliferation at an early stage, shedding light on the translational value of NMN as an enhancer for skeletal regeneration and highlighting the pivotal role of macrophage-stem cell interactions in governing the regenerative influence of NMN on stem cells.
Keywords: fracture healing, callus formation, skeletal stem cells, NAD
 Global reach, higher impact
Global reach, higher impact