13.3
Impact Factor
Theranostics 2024; 14(13):5336-5370. doi:10.7150/thno.99961 This issue Cite
Review
Nanomedicines as Guardians of the Heart: Unleashing the Power of Antioxidants to Alleviate Myocardial Ischemic Injury
1. Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, China.
2. Key Laboratory of Cardiac Injury and Repair of Henan Province, Zhengzhou, China.
Received 2024-6-22; Accepted 2024-8-16; Published 2024-8-26
Abstract
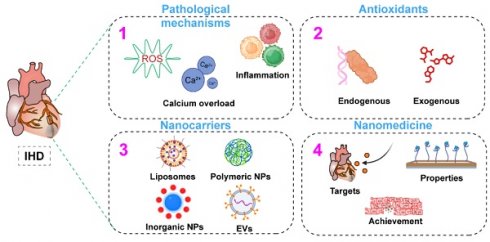
Ischemic heart disease (IHD) is increasingly recognized as a significant cardiovascular disease with a growing global incidence. Interventions targeting the oxidative microenvironment have long been pivotal in therapeutic strategies. However, many antioxidant drugs face limitations due to pharmacokinetic and delivery challenges, such as short half-life, poor stability, low bioavailability, and significant side effects. Fortunately, nanotherapies exhibit considerable potential in addressing IHD. Nanomedicines offer advantages such as passive/active targeting, prolonged circulation time, enhanced bioavailability, and diverse carrier options. This comprehensive review explores the advancements in nanomedicines for mitigating IHD through oxidative stress regulation, providing an extensive overview for researchers in the field of antioxidant nanomedicines. By inspiring further research, this study aims to accelerate the development of novel therapies for myocardial injury.
Keywords: myocardial ischemic injury, oxidative stress, antioxidant therapy, nanomedicines, drug delivery systems
1. Introduction
Ischemic heart disease (IHD), a prevalent cardiovascular disease, often leads to chronic heart failure (HF) and significantly increases global morbidity and mortality [1]. In 2017, the World Health Organization (WHO) reported approximately 8.9 million deaths associated with IHD, with an increasing trend in annual incidence [2]. Myocardial infarction (MI) is a severe cardiac event triggered by coronary artery blockage, restricting blood flow and leading to prolonged ischemic damage [3]. Timely reperfusion, achieved via thrombolysis or primary percutaneous coronary intervention (PCI), is critical for mitigating ischemic damage and reducing the infarct size. However, this reperfusion can also provoke myocardial ischemia/reperfusion injury (MI/RI), resulting in further tissue damage and increased cardiomyocyte (CM) apoptosis [4]. As prominent diseases within IHD, MI and MI/RI necessitate ongoing research into their molecular and cellular pathways. This research is crucial for the development of targeted therapies and for deepening our understanding of these conditions.
Oxidative stress, characterized by an excess of reactive oxygen species (ROS) that overwhelms the body's antioxidant defenses, plays a pivotal role in the progression of IHD by causing oxidative damage to cellular components and exacerbating tissue injury [5]. An imbalance between elevated ROS production and a diminished antioxidant response leads to significant oxidative damage and triggers pro-inflammatory pathways, ultimately causing CM apoptosis and tissue necrosis [6]. Therefore, strategies to mitigate oxidative stress are crucial for improving cardiac function and treating IHD. Various drugs, such as scutellarin [7], adenosine [8], bakuchiol [9], and melatonin (Mel) [10], have shown promise in alleviating IHD. Despite their efficacy, these agents face challenges like poor targeting and adverse effects, highlighting the need for more effective and safer therapeutic options [11].
In recent years, nanomedicine has significantly advanced, marked by an increase in research involving preclinical and clinical trials. These nanomedicines are often designed by encapsulating therapeutic agents within various carriers, enhancing therapeutic effectiveness and safety compared to free drugs [12]. The customization of nanomedicines involves careful consideration of properties such as drug load, dimensions, morphology, surface charge, and targeting moieties to achieve the desired therapeutic effects [13]. This customization aims to optimize pharmacokinetics and pharmacodynamics, control drug release, enhance targeted delivery, prolong circulation in blood, minimize side effects, improve biocompatibility, and increase tissue penetration [14]. A wide range of inorganic and organic materials, including liposomes, polymeric NPs (PNPs), and extracellular vesicles (EVs), have been explored as carriers [15]. Notably, the development of diagnostic, therapeutic, and theranostic nanomedicines facilitates early detection and precise treatment of IHD.
Recently, there has been a surge in interest in using nanomedicines for diagnosing and treating IHD (Figure 1). This review not only summarizes current advancements but also provides new insights into optimizing nanomedicine applications for IHD treatment, identifying key areas where future research can bridge existing gaps.
2. Pathological mechanisms of IHD
Coronary atherosclerosis, a common cardiovascular disease, results from the deposition of fatty substances and cholesterol in coronary arteries, leading to obstructive plaques [16]. The severity of this condition is determined by the degree of arterial occlusion and its impact on blood flow [17]. Risk factors include age, sex, genetic factors, lifestyle choices such as smoking, poor diet, lack of exercise, as well as conditions like hypertension, diabetes, and dyslipidemia [18]. As a significant global health concern, coronary atherosclerosis is a leading cause of death and illness worldwide [19].
The groundbreaking nanomaterial schematics for precise IHD therapy and diagnosis.
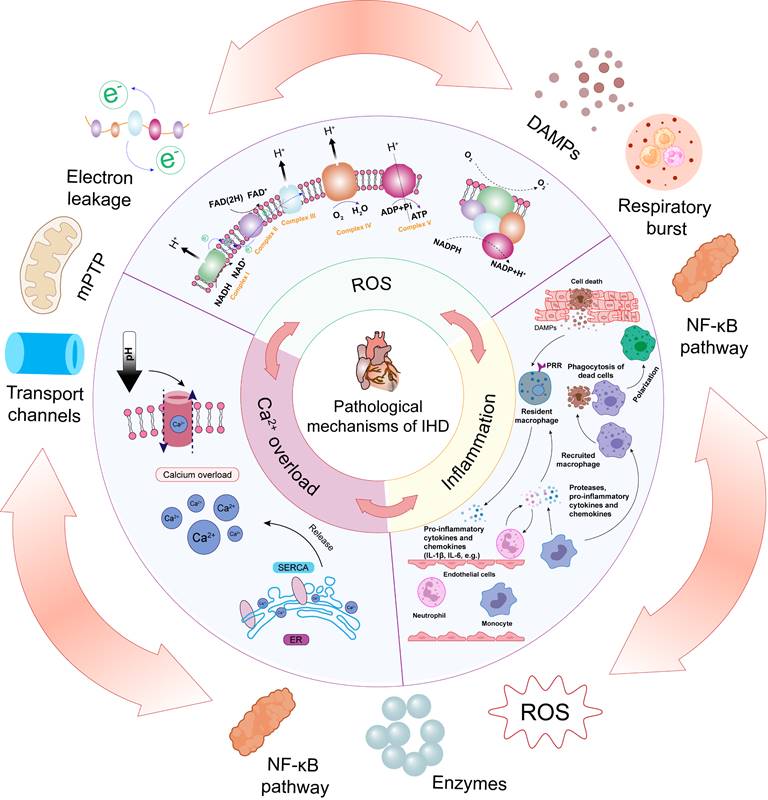
A schematic diagram of the mechanisms causing IHD, including oxidative stress, inflammation, and calcium overload.
MI, commonly known as a heart attack, is a critical medical emergency caused by the cessation of blood flow to the heart muscle, resulting in tissue damage or death [20]. The sudden arterial occlusion is typically caused by the rupture of atherosclerotic plaques, which activates the coagulation system [21]. Plaque rupture releases pro-coagulant substances, activating thrombin and platelets and leading to thrombus formation at the site of the plaque, which can obstruct or even completely block the affected vessels [22, 23]. Reperfusion therapy aims to restore blood flow to the ischemic myocardium, effectively reducing ischemic damage and minimizing the infarct size. However, reperfusion can also cause MI/RI, leading to further cardiac damage and potentially reducing the success of revascularization [24]. Understanding the pathophysiological mechanisms of IHD is crucial for targeted therapeutic interventions.
This section reviews the complex mechanisms of IHD, characterized by a complex interplay of pathophysiological processes, including inflammation, Ca2+ overload, and notably, oxidative stress (Figure 2). The intricate interaction and convergence of these factors underscore the need for a comprehensive understanding of their mechanisms, which is essential for developing effective therapeutic strategies for IHD.
2.1. Oxidative and nitrative stress
ROS are integral to the pathophysiology of IHD, functioning as both signaling molecules and mediators of cellular damage. The generation of ROS during these events is a complex, multi-step process that is tightly linked to the metabolic disturbances and bioenergetic crises that characterize ischemia and reperfusion [25]. A comprehensive understanding of the pathways involved in ROS production and their subsequent pathological roles is essential for developing targeted therapeutic strategies aimed at mitigating myocardial injury.
The mitochondrial electron transport chain (ETC) is the primary source of ROS during MI and MI/RI. Under physiological conditions, electrons from NADH and FADH2 are sequentially transferred through complexes I to IV of the ETC, culminating in the reduction of oxygen to water at complex IV. However, ischemia results in a sharp decline in oxygen availability, leading to a backlog of reduced electron carriers and excessive reduction of ETC components. Upon reperfusion, the sudden influx of oxygen reignites the ETC, causing excessive flux of electrons. This hyperactive state of the ETC results in substantial electron leakage at complexes I and III, where electrons prematurely reduce molecular oxygen to form superoxide anions (•O2-) [26].
The formation of superoxide marks the initiation of a cascading sequence of ROS generation. Superoxide dismutase (SOD) rapidly converts superoxide into hydrogen peroxide (H2O2), which, although less reactive, can freely diffuse across membranes and contribute to oxidative stress [27]. In the presence of transition metals such as iron, H2O2 is converted into hydroxyl radicals (•OH) via Fenton chemistry [28]. •OH, due to their high reactivity, inflict severe damage on lipids, proteins, and DNA, thereby compromising the structural and functional integrity of myocardial cells.
Beyond mitochondrial sources, NADPH oxidase (NOX) enzymes represent a significant non-mitochondrial pathway for ROS production in IHD. NOX enzymes are distinct in that their primary function is the deliberate generation of ROS. Upon activation by ischemic stress, inflammatory cytokines, or mechanical strain, NOX enzymes catalyze the transfer of electrons from NADPH to molecular oxygen, producing superoxide [29]. Among the NOX isoforms, NOX2 and NOX4 are particularly implicated in cardiac injury, with NOX2 contributing to the acute ROS burst during reperfusion, and NOX4 being associated with sustained ROS production and the promotion of fibrotic remodeling. The ROS generated by NOX enzymes are not merely byproducts of cellular stress; they also act as critical modulators of redox-sensitive signaling pathways. These ROS can activate a range of downstream kinases and transcription factors, such as protein kinase C (PKC) and nuclear factor-kappa B (NF-κB), which amplify inflammatory responses, induce apoptosis, and disrupt metabolic homeostasis, all of which contribute to the exacerbation of myocardial damage in IHD [30].
A less conventional but highly relevant source of ROS in the setting of IHD is the uncoupling of endothelial nitric oxide synthase (eNOS). Under normal conditions, eNOS generates nitric oxide (NO), a molecule with potent vasodilatory and cytoprotective effects. However, under oxidative stress, such as that encountered during reperfusion, eNOS can become uncoupled due to the oxidation of its cofactor tetrahydrobiopterin (BH4) or a deficiency in L-arginine. When uncoupled, eNOS shifts from producing NO to generating superoxide, which not only exacerbates oxidative stress but also diminishes NO availability, leading to endothelial dysfunction and impaired vasodilation [31]. This dual effect further perpetuates ischemic injury by impairing perfusion and increasing the oxidative burden within the myocardium.
2.2 Inflammation
Inflammation is a fundamental response to IHD, playing a crucial role in both the progression of acute myocardial damage and the subsequent remodeling of cardiac tissue. This complex inflammatory process is rapidly initiated following ischemia and further intensified upon reperfusion, driven by the activation of resident immune cells, the recruitment of circulating leukocytes, and the release of pro-inflammatory mediators [32]. Understanding the pathways and mechanisms by which inflammation is generated and perpetuated is essential for elucidating the full spectrum of myocardial injury in these conditions.
The inflammatory cascade in IHD is triggered by the release of damage-associated molecular patterns (DAMPs) from necrotic CMs and stressed cells. These DAMPs, which include high-mobility group box 1 (HMGB1), heat shock proteins, and extracellular matrix degradation products, are recognized by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs) on resident immune cells, including macrophages and dendritic cells [33]. The engagement of these receptors activates intracellular signaling cascades, notably the NF-κB and mitogen-activated protein kinase (MAPK) pathways, culminating in the transcriptional upregulation of pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6) [34].
These cytokines orchestrate a robust inflammatory response, promoting the recruitment of neutrophils and monocytes to the ischemic myocardium. Neutrophils are among the first responders, infiltrating the myocardium within hours of reperfusion. These cells release a variety of enzymes, ROS, and cytokines that exacerbate myocardial injury, not only through direct cellular damage but also by amplifying the inflammatory milieu. Monocytes, which differentiate into macrophages upon tissue entry, sustain the inflammatory response and initiate the clearance of dead cells and debris through phagocytosis [32]. Macrophages also secrete growth factors that are essential for tissue repair, yet their prolonged activation can lead to excessive inflammation and fibrosis, contributing to adverse cardiac remodeling.
A critical aspect of this inflammatory response is the polarization of macrophages within the heart. Macrophages exhibit different phenotypes, notably the pro-inflammatory M1 type and the anti-inflammatory M2 type. The transition from M1 to M2 macrophages is essential for resolving inflammation and promoting tissue repair [35]. The process of macrophage polarization is highly dynamic and influenced by various microenvironmental factors, including the presence of cytokines, growth factors, and metabolic by-products. The interplay between oxidative stress and macrophage phenotype suggests that a nuanced understanding of the redox environment within ischemic tissue could lead to more targeted and effective therapeutic strategies. Antioxidant nanomedicines, by modulating this redox balance, hold the potential not only to shift macrophages toward the M2 phenotype but also to create a microenvironment that favors long-term cardiac repair and remodeling [36].
This persistent and intense innate immune response not only leads to the necrosis and apoptosis of CMs but also exacerbates myocardial damage and diminishes the effectiveness of treatments [37]. By promoting M2 macrophage polarization, antioxidant nanomedicines help modulate the inflammatory microenvironment, mitigating myocardial damage and supporting cardiac repair. For example, antioxidant nanomedicines have been shown to enhance M2 macrophage polarization, improving cardiac function and reducing infarct size in preclinical models of MI [35]. The challenge of modulating the inflammatory microenvironment lies in the precise control of the timing and extent of M2 macrophage activation. Overactive M2 polarization may inadvertently lead to fibrosis, while insufficient M2 activity could prolong inflammation and hinder repair [38]. Therefore, future research should focus on fine-tuning antioxidant nanomedicine formulations to achieve an optimal balance, potentially through the use of stimuli-responsive systems that release therapeutic agents in response to specific inflammatory signals.
Inflammatory processes significantly boost ROS production through several mechanisms. During IHD, inflammatory cytokines such as TNF-α, IL-1β, and IL-6 activate NOX, particularly NOX2 and NOX4, in neutrophils, macrophages, and endothelial cells. These enzymes transfer electrons from NADPH to oxygen, directly generating superoxide, a primary ROS. Moreover, the activation of NF-κB and MAPK pathways by these cytokines upregulates NOX enzymes and other ROS-generating systems, further amplifying ROS production [39].
ROS, in turn, act as potent amplifiers of the inflammatory response. ROS activate redox-sensitive transcription factors like NF-κB and activator protein-1 (AP-1), leading to the transcription of pro-inflammatory genes. This results in increased production of cytokines and chemokines, which recruit more immune cells to the injured myocardium, thereby sustaining and intensifying inflammation [40]. Additionally, cause oxidative damage to cellular components, leading to the release of further DAMPs, which activate PRRs on immune cells, perpetuating the cycle of inflammation and ROS generation [39].
In summary, inflammation and ROS generation are mutually reinforcing processes in IHD. Inflammatory signaling upregulates ROS production, while ROS further intensify inflammation by activating key signaling pathways and causing oxidative damage, thus creating a self-perpetuating cycle that exacerbates myocardial injury.
2.3 Ca2+ overload
Ischemic insult precipitates a cascade of metabolic disturbances, with the initial deprivation of oxygen halting oxidative phosphorylation in mitochondria. This disruption leads to the collapse of mitochondrial membrane potential and a precipitous drop in ATP production, compelling the cell to rely on anaerobic glycolysis. The accumulation of lactic acid from glycolysis induces intracellular acidosis, which rapidly reverses the reactivation of Na+-Ca2+ exchange channels, leading to a substantial influx of Ca2+ into the cells and an increase in intracellular Ca2+ concentrations [41]. Therefore, reperfusion is considered a significant factor in exacerbating Ca2+ overload.
Elevated intramitochondrial calcium concentrations stimulate key dehydrogenases within the tricarboxylic acid (TCA) cycle, such as isocitrate dehydrogenase and α-ketoglutarate dehydrogenase. This stimulation leads to an overproduction of NADH and FADH₂, which feed into the ETC. When the ETC becomes excessively loaded with electrons beyond its oxidative capacity, electron leakage occurs, particularly at complexes I and III. These leaked electrons prematurely react with molecular oxygen, resulting in the formation of superoxide, a primary form of ROS. This calcium-induced overproduction of superoxide within mitochondria is a significant contributor to cellular oxidative stress and subsequent tissue injury [42].
In addition, calcium overload contributes to mitochondrial membrane potential depolarization, which can trigger the opening of the mitochondrial permeability transition pore (mPTP). The opening of the mPTP leads to a loss of the mitochondrial membrane potential, disrupting the proton gradient essential for ATP synthesis and causing a cessation of oxidative phosphorylation. This disruption not only diminishes the cell's energy supply but also precipitates further ROS generation, as the electron transport chain becomes dysregulated. The resultant ROS production exacerbates mitochondrial dysfunction, creating a feed-forward loop where calcium overload and ROS reinforce each other's pathological effects [43].
ROS can initiate protein denaturation, enzyme inactivation, and peroxidation of polyunsaturated fatty acids in cell membranes, thereby disrupting membrane permeability. ROS directly attack cellular components such as ion transport channels, sarcoplasm, and mitochondrial supercomplexes, leading to cellular Ca2+ overload. Moreover, ROS-induced oxidative stress exerts a profound effect on calcium signaling. Oxidative modifications of calcium-handling proteins, including the ryanodine receptors and sarcoplasmic reticulum (SR) Ca²⁺ ATPase, disrupt their normal function, exacerbating calcium mishandling and amplifying Ca²⁺ overload [44]. The pathological interplay between ROS and Ca²⁺ overload thus constitutes a vicious cycle, with each amplifying the other through a network of interrelated signaling pathways and metabolic disturbances. This cycle not only drives the acute phase of myocardial injury but also sets the stage for chronic pathological remodeling and HF.
Additionally, elevated intracellular Ca2+ levels can activate NF-κB, a key transcription factor that regulates the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. Moreover, Ca2+ overload can activate inflammasomes, particularly the NLRP3 inflammasome, which further amplifies the inflammatory response by promoting the maturation and release of IL-1β and interleukin-18 (IL-18). Conversely, inflammation exacerbates calcium overload through multiple mechanisms [45]. Pro-inflammatory cytokines can impair calcium homeostasis by downregulating the expression and function of calcium-handling proteins, such as the SR Ca²⁺-ATPase (SERCA) and plasma membrane Ca²⁺-ATPase (PMCA) [46]. Inflammatory mediators also increase oxidative stress, which disrupts calcium channels and transporters, leading to further calcium influx and overload [39].
In IHD, a self-reinforcing cycle exists between oxidative stress, inflammation, and calcium overload. ROS generated during these events amplify inflammation by activating redox-sensitive pathways, leading to the release of pro-inflammatory cytokines and further ROS production. In turn, inflammation exacerbates calcium overload by disrupting calcium-handling proteins and increasing oxidative stress. Calcium overload contributes to additional ROS generation through mitochondrial dysfunction and activation of inflammasomes, which further intensifies the inflammatory response. This vicious cycle not only exacerbates acute myocardial damage but also drives long-term pathological remodeling, highlighting the need for targeted therapies that interrupt these interconnected processes.
3. Antioxidants
In exploring the cellular redox balance, antioxidants are hypothesized to function via multiple mechanisms: (a) scavenging ROS or their precursors, (b) inhibiting ROS production, (c) binding metal ions to reduce ROS catalysis, (d) enhancing the endogenous synthesis of antioxidants, and (e) upregulating antiapoptotic genes such as Bcl-2 to mitigate cell death. Antioxidants are categorized as endogenous (synthesized within the body) or exogenous (acquired externally). These compounds display a spectrum of properties that enable them to mitigate oxidative damage and forestall the development of various diseases through a complex network of cellular defense mechanisms. Understanding their mechanisms is crucial for developing nanomedicine interventions for IHD.
3.1 Endogenous antioxidants
Endogenous antioxidants, including enzymatic antioxidants such as GPx, SOD, GSH, and catalase (CAT) and nonenzymatic antioxidants such as bilirubin, melanin, Mel, nucleic acids (NAs), and various gases, play vital roles in defending against oxidative stress in IHD. Both enzymatic and nonenzymatic antioxidant systems collaboratively maintain the body's redox balance and serve as direct or indirect targets for antioxidant interventions.
3.1.1 Enzymatic antioxidants
SOD, CAT, and GPx, along with GSH, are pivotal endogenous antioxidants. SOD, a potent enzymatic protector against oxidative stress, catalyzes the conversion of •O2- to H2O2, which is then reduced by CAT and GPx to prevent peroxynitrite formation [27]. CAT, which is prevalent in eukaryotic cells, degrades H2O2 into water and O2, thus preserving cellular redox homeostasis. Thus, the combined use of SOD and CAT is considered a superior choice as it can further reduce the harmful effects of H2O2. In conjunction with GSH, GPx can catalyze the reduction of H2O2, which is crucial for maintaining cellular redox homeostasis. A deficiency in GPx increases vulnerability to I/R injuries, whereas its overexpression can prevent left ventricular remodeling and failure post-MI [47]. The fundamental role of enzymatic antioxidants such as SOD, CAT, and GPx in maintaining redox homeostasis cannot be overstated. These enzymes not only mitigate oxidative stress by neutralizing ROS but also play critical roles in signaling pathways that govern cell survival, proliferation, and apoptosis. However, the therapeutic application of these enzymes faces significant challenges. The poor in vivo stability and bioavailability of these enzymes necessitate innovative delivery systems to enhance their therapeutic efficacy. Nanotechnology-based delivery systems, such as encapsulation within nanoparticles or conjugation with polymers, could improve the stability, bioactivity, and targeted delivery of these enzymes, potentially overcoming current limitations.
Additionally, the interplay between these enzymatic antioxidants and other cellular defense mechanisms, such as autophagy and apoptosis, warrants further exploration. Understanding how these pathways intersect could reveal new therapeutic strategies that exploit the body's natural defense systems. For instance, enhancing GPx4 activity not only prevents ferroptosis but could also be strategically targeted to modulate the overall cellular response to oxidative stress, offering a novel approach to managing conditions like MI and MI/RI [48]. Furthermore, the genetic modulation of these enzymes, either through overexpression or via CRISPR/Cas9-mediated editing, offers another potential avenue for therapeutic intervention. While current research has demonstrated the benefits of enzymatic antioxidant overexpression in preclinical models, translating these findings into clinical applications remain challenging (Table 1).
Some of the major endogenous antioxidants and their sites of action in CMs.
| Antioxidant | Site of Action | Action |
|---|---|---|
| SOD | Cytoplasm, mitochondria, extracellular space | 2•O2- + 2H+ → H2O2 + O2 |
| CAT | Peroxisomes, mitochondrial membrane | H2O2→2H2O+O2 |
| GPx | Cytoplasm, mitochondria, nucleus | H2O2+2GSH→2H2O+GSSG |
| GSH | Cytoplasm, mitochondria, nucleus | GSSG + NADPH → 2GSH + NADP+ |
| Thioredoxin | Cytoplasm, nucleus, cell membrane, mitochondria, extracellular space | 2Trx-SH ↔ Trx-S-S-Trx |
| HSPs | Cytoplasm, nucleus | Various chaperone and antioxidant functions |
| α-tocopherol | Cell membrane | Break lipid peroxidation chain and LDL reaction |
| Vitamin C (ascorbic acid) | Cytoplasm, extracellular space | Ascorbate + •ROO → Dehydroascorbate + •RO + H2O |
| CoQ10 | Mitochondria | CoQ10 + •ROO → CoQ10H + •RO |
| Metallothioneins | Cytoplasm, nucleus | MT-SH + ROS → MT-S-S-MT + reduced ROS |
| Bilirubin | Cytoplasm, extracellular space | Various antioxidant and anti-inflammatory functions |
| β-Carotene (pro-vitamin A) | Plasma | Inhibits oxidation of LDL |
3.1.2 Nonenzymatic antioxidants
Nonenzymatic antioxidants, which also originate within the body, neutralize free radicals through direct reactions. For instance, bilirubin, a byproduct of heme catabolism, acts as a natural ROS scavenger, offering antioxidative properties that surpass those of vitamins E and C [49]. Melanin, a polymer pigment, serves multiple functions, including radical scavenging and radiation protection. Synthetic analogs like polydopamine (PDA) are being developed to capture alkylperoxyl radicals [50]. NAs also exert antioxidant effects by modulating intracellular antioxidant enzymes, though their delivery is hindered by properties like charge, size, and instability [51]. Gaseous molecules, essential for cellular signaling and physiological functions, can reduce oxidative stress, and nanomedicine platforms like gas-generating nanoplatforms (GGNs) enhance their therapeutic applications [52]. Coenzyme Q10 (CoQ10), a lipid-soluble benzoquinone, is effective in energy production and antioxidant functions, highlighting its potential in treating IHD [53]. Mel, known for its potent antioxidant properties, shows promise in various oxidative stress models, especially due to its mitochondrial targeting ability [54].
3.2 Exogenous antioxidants
Exogenous antioxidants, such as vitamins, natural small molecule drugs (NSMs), and synthetic antioxidants, complement endogenous defenses by regulating oxidative balance.
3.2.1 Vitamins
Exogenous antioxidants, mainly obtained through dietary intake from fruits, vegetables, nuts, and seeds, play a crucial role in neutralizing free radicals. Vitamins A, C, and E, polyphenols, and certain minerals are particularly significant among these antioxidants [55]. They complement endogenous antioxidants, forming a vital defense system within the body. However, the effects of exogenous antioxidants vary under different experimental conditions. For instance, some studies have shown that antioxidant supplements can enhance endothelial function [56], especially when endogenous oxidative stress is high. Conversely, a study on normal domestic pigs indicated potential negative long-term cardiovascular effects from prolonged supplementation with vitamins E and C. This was attributed to increased oxidative stress in the arterial wall, possibly due to endothelial NO synthase uncoupling or the prooxidant effects of vitamin radicals [57]. These findings highlight that the benefits of antioxidants depend on the context and duration of their use. However, the stability of vitamins can be significantly affected by physical and chemical factors like light, temperature, enzymatic oxidation, metal ions, and alkaline pH. These factors often lead to the rapid degradation of vitamins into less effective forms [58]. To overcome this issue, an effective delivery system is essential.
3.2.2 NSMs
Secondary metabolites, also known as NSMs, have a wide range of biological activities, primarily through interactions with biological receptors. The antioxidant properties of NSMs are chiefly derived from four structural motifs: highly conjugated hydroxyl, amino, thiol, and isoprenoid groups [59]. Curcuminoids, a notable group within the NSMs family, exhibit significant therapeutic potential due to their antioxidant, anti-inflammatory, and anticarcinogenic properties. The antioxidant efficacy of these compounds is enhanced by the presence of a para-hydroxyl group on two phenyl rings, supported by electron-donating groups such as methoxy groups [60]. Flavonoids, another subset of NSMs, feature a C6-C3-C6 three-ring core structure and exhibit antioxidant effects primarily through hydrogen atom transfer (HAT) from their phenolic OH groups. The number of free hydroxyl groups in flavonoids correlates with their antioxidant potency, although this relationship has certain limitations [61].
Even though these compounds hold promise, over 90% of natural compounds extracted from organisms do not advance in drug development due to poor solubility, stability, or pharmacokinetic properties [59, 62, 63]. Nevertheless, the focus of pharmaceutical research is evolving toward the exploration of active phase states through non-covalent interactions, which may revolutionize drug discovery. Additionally, the continuous development of advanced delivery systems plays a crucial role in improving the pharmacodynamic characteristics of these drugs, thereby enhancing their therapeutic efficacy and bioavailability.
3.2.3 Synthetic antioxidants
Natural antioxidants derived from fruits and vegetables play a crucial role in promoting health and preventing diseases. Despite their advantages, these antioxidants often face challenges such as difficult extraction and instability, limiting their industrial application [64]. In the food industry, synthetic antioxidants are preferred due to their consistent availability and stability. These synthetic antioxidants benefit from diverse raw material sources, advanced manufacturing technologies, cost-effectiveness, fewer side effects, and ease of procurement [65]. As a result, the food industry frequently chooses synthetic options to ensure product quality and longevity. Common synthetic phenolic antioxidants in the food sector include butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), tertiary butylhydroquinone (TBHQ), and propyl gallate (PG). These antioxidants are crucial for inhibiting spoilage, stabilizing products, and extending the longevity of food items [66]. Their use provides economic benefits and enhanced food safety, although their concentration must be carefully managed to avoid adverse health implications.
The transformation of antioxidants into diverse metabolites under specific environmental or biochemical conditions is a crucial area of study. These metabolites, which vary significantly depending on the reaction conditions and the organism involved, play integral roles in antioxidative processes. Understanding these transformation mechanisms is essential for advancing our knowledge of both natural and synthetic antioxidants. Moreover, the application of nanocarriers in the synthesis and delivery of antioxidants is pivotal. Nanocarriers enhance the stability, bioavailability, and targeted delivery of antioxidants, thereby maximizing their therapeutic efficacy. This integration of nanotechnology in antioxidant research not only facilitates the precise modulation of metabolic pathways but also addresses the limitations associated with conventional antioxidant therapies. Hence, the incorporation of nanocarriers is indispensable for the effective utilization of synthetic antioxidants in therapeutic interventions.
4. Application of nanocarriers in IHD therapy
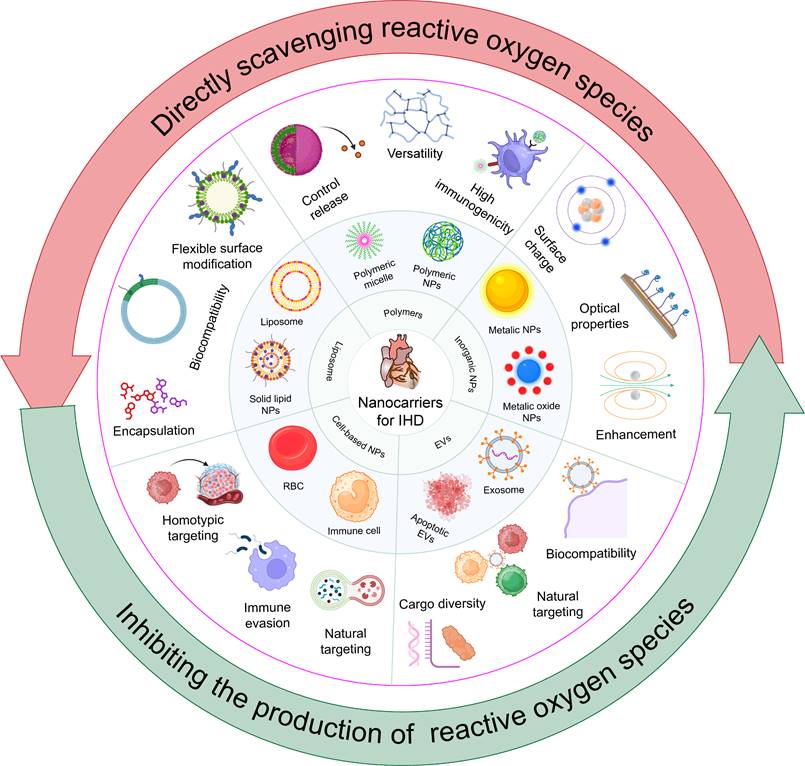
Nanomedicine, combining biomaterials and nanotechnologies, greatly improves traditional drug therapies for IHD [67, 68]. By aligning with the unique pathogeneses and pathophysiological needs of various diseases, nanomedicine offers tailored therapeutic properties and functions. The customizable size, charge, and high surface-to-volume ratio of nanomedicines enable effective drug encapsulation, enhancing pharmacokinetics and pharmacodynamics in IHD treatment [69]. Nanomedicines with multifunctional linkers, ligands, or coatings facilitate targeted delivery and controlled release, improving therapeutic outcomes in IHD [70]. Due to their diverse chemical and physical properties, a wide variety of nanomaterials—including inorganic materials (e.g., gold NPs), organic materials (e.g., liposomes), biological molecules (e.g., proteins and peptides), and synthetic polymers—are utilized for nanocarrier fabrication, each offering unique advantages in drug delivery and therapeutic efficacy. This review presents advanced nanocarriers for treating IHD, such as liposomes, synthetic PNPs, inorganic NPs, EVs, and both cell-based and biomimicry-based nanocarriers (Figure 3). This highlights the key role of nanocarriers in improving the stability, targeting, and bioavailability of antioxidant drugs, thereby enhancing IHD treatment efficacy. It offers comprehensive references for future research in carrier selection.
4.1. Liposomes
Liposomes have emerged as a vital drug delivery system for IHD treatment due to their superior biocompatibility, ability to encapsulate both hydrophilic and hydrophobic drugs, controlled release properties, and enhanced molecular engineering capabilities [53]. The inherent similarity between liposomes and cell membrane components provides them with excellent biocompatibility. This characteristic allows liposomes to be recognized and metabolized efficiently within the body [71].
Additionally, one of the key strengths of liposomes is their flexible surface modification potential. This capability enables the design of liposomes for targeted drug delivery and precise control over in vivo release. For example, Tal et al. successfully attached a ligand specific to the angiotensin II type 1 receptor (AT1) to liposomes, achieving accurate targeting of cardiac cells in vitro and after intravenous injection [72]. This surface modification flexibility allows for the customization of liposomes to meet specific therapeutic requirements.
In addition to surface modification, liposomes are exceptional in their ability to encapsulate diverse therapeutic agents, including drugs, DNA, and diagnostic substances. This encapsulation not only protects the therapeutic cargo from degradation but also allows for controlled release in response to specific stimuli, such as abnormal pH levels and temperatures at pathological sites, thereby enhancing the targeted delivery and therapeutic efficacy [73]. Polyethylene glycol (PEG)-coated liposomes further augment this benefit by offering superior stealth properties, extending drug circulation time in the bloodstream, and reducing adverse hemodynamic impacts [74].
The advantages of liposomes as versatile and efficient drug delivery vehicles. Their low toxicity and immunogenicity, coupled with their ability to be administered through multiple routes and forms, highlight their versatility in clinical applications [75]. In summary, these ingenious molecular containers have become indispensable carriers in modern drug delivery.
4.2. PNPs
PNPs have garnered significant attention in nanomedicine due to their remarkable controlled release capabilities, versatility, and high immunogenicity [76]. These attributes make PNPs highly effective for a range of therapeutic applications [77]. Controlled release is a critical feature of PNPs, facilitated by biodegradable polymers such as poly (lactic acid) (PLA) and poly (lactic-co-glycolic acid) (PLGA). These polymers allow for precise timing and location of drug release, protecting encapsulated drugs from degradation and ensuring sustained therapeutic effects [78]. This capability is particularly beneficial in treating IHD, where stable and prolonged drug activity is crucial.
The versatility of PNPs is another key advantage. They can be engineered to target specific tissues, cells, and subcellular structures, enabling precise and efficient drug delivery [79]. This adaptability enhances the stability and activity of active components, ensuring that therapeutic agents are delivered at optimal concentrations. By precisely controlling surface characteristics and particle size, PNPs can regulate permeability, adjust solubility, and manage release patterns to meet specific therapeutic needs [80]. High immunogenicity is a third significant benefit of PNPs. This property makes them excellent candidates for vaccine development and immunotherapy, as they can elicit robust immune responses [81]. Overall, these properties position PNPs as crucial components in the development of innovative medical treatments. The ideal design of PNP delivery systems involves precise control of surface characteristics and particle size to regulate permeability, adjust solubility, enhance flexibility, and manage remedial release patterns, ensuring the desired therapeutic effects at the required times and locations.
4.3. Inorganic NPs
Inorganic NPs are highly effective nanoplatforms known for their unique surface charge, optical properties, and enhancement capabilities, making them suitable for a wide range of biomedical applications. Their composition, size, shape, and structure can be precisely tailored, utilizing their large surface area and distinct surface chemical properties to meet specific needs. In recent decades, there has been a significant increase in interest in these features for biomedical use [82].
Illustration of the representative nanosystems used for IHD therapy. Different types of nanosystems offer distinct advantages, and selecting the most appropriate nanosystem depends on the specific needs of the therapy.
One primary advantage of inorganic NPs is their surface charge, which is crucial for their interactions with biological molecules. Researchers can modify the surface coatings to enhance the colloidal stability of NPs in complex biological environments, ensuring effective dispersion in aqueous solutions. This is particularly important as the surface charge effects cellular uptake, distribution, and the overall biocompatibility of the NPs [83]. Another key advantage is the optical properties of inorganic NPs. These NPs exhibit unique optical characteristics, such as plasmon resonance and fluorescence, which are invaluable for imaging and diagnostic applications. By adjusting the size and shape of the NPs, researchers can finely tune these properties, enabling high-resolution imaging and precise detection of biological targets [35].
Additionally, the enhancement potential of inorganic NPs is noteworthy. Surface engineering allows for precise control of interactions with biomolecules, leading to the development of efficient biomedical products. This capability is crucial for targeted drug delivery and improved therapeutic outcomes. However, the potential release of metal ions and the associated biological impact must be considered, as these could pose challenges for clinical applications due to potential adverse effects [84].
In summary, the surface charge, optical properties, and enhancement potential of inorganic NPs make them versatile and effective tools in biomedical research and applications. These attributes position inorganic NPs at the forefront of advancements in diagnosis, imaging, and targeted therapy, offering new possibilities for improving patient outcomes.
4.4. EVs
EVs, which include exosomes and microvesicles, are diverse membrane-bound entities originating from the endosomal system and plasma membrane. These vesicles, found in various biological fluids, play distinct roles in both physiological and pathological contexts, serving a dual role as therapeutic agents and delivery vehicles [85]. One significant advantage of EVs is their biocompatibility. Because they originate endogenously, EVs are inherently compatible with human physiology, minimizing the risk of immunogenicity and adverse immune responses. This intrinsic compatibility makes EVs safer for clinical applications compared to many synthetic delivery systems [86].
Beyond their inherent components, EVs can transport a variety of small molecules, such as proteins and NAs, which can modulate the functions of recipient cells [86, 87]. This versatile cargo-carrying capacity allows EVs to be employed in a broad range of therapeutic applications, from drug delivery to gene therapy. Another essential attribute of EVs is their natural targeting ability [85]. They can efficiently traverse biological barriers, including tissue, cellular, and intracellular barriers, to reach and modulate specific target cells. Additionally, EVs can be engineered through genetic and chemical modifications to enhance their targeting specificity, thereby improving the precision of therapeutic interventions [88]. Recent advancements have demonstrated the potential of genetically engineered hybrid nanovesicles (hNVs), which include cell-derived nanovesicles overexpressing high-affinity SIRPα variants, exosomes from human mesenchymal stem cells (MSCs), and platelet-derived nanovesicles. These hNVs significantly enhance macrophage phagocytosis of necrotic cells, mitigate inflammatory responses, and improve cardiac function in MI/RI models [89]. However, the therapeutic application of exosomes also poses potential risks, including immunogenicity, tumor-promoting effects, limited understanding of long-term effects, and high associated costs. Additionally, when used as delivery vehicles, the challenge of removing endogenous cargos from EVs to avoid unwanted side effects remains significant [90].
4.5. Cell-based and biomimicry-based nanocarriers
Cell therapy is increasingly recognized as a promising strategy for treating ischemic diseases, with various cell types demonstrating efficacy in enhancing cardiac function post-ischemia [91]. In addition to stem cell applications, the unique biological properties of various cell types are harnessed to develop sophisticated drug delivery strategies. Red blood cells (RBCs), neutrophils, monocytes, macrophages, and platelets are exemplary cell-based and biomimicry-based nanocarriers. These engineered carriers extend circulation times, target therapeutic sites, and overcome biological barriers, thereby enhancing drug delivery accuracy and intervention effectiveness [92]. Recent advancements have shown the potential of human embryonic stem cell-derived epicardial cells (hEPs) in myocardial repair. These cells enhance CM survival, angiogenesis, and lymphangiogenesis by suppressing inflammatory responses mediated by type I interferon signaling [93]. The advantages of cell-based and biomimicry-based nanocarriers stem from their capabilities in immune evasion, homotypic targeting, and functional integration.
Moreover, the cell membrane plays a crucial role in mediating interactions with the extracellular environment, such as signal transduction, recognition, adhesion, and immune modulation. Cell membrane coating is a promising technique that enhances the biointerfacial capabilities of nanocarriers, facilitating effective drug delivery to affected tissues [94]. Given the intrinsic homing abilities of each cell type, cell membrane-coated NPs can be customized for targeted drug delivery, minimizing off-target effects and improving therapeutic efficiency. This method has been successfully applied to design biomimicry-based nanocarriers for targeted cardiovascular disease treatments, including IHD, atherosclerosis, and restenosis, demonstrating significant potential in cardiovascular therapies [95].
5. Nanotechnology in the diagnosis of IHD
Beyond their delivery roles, nanomedicines can also act as molecular probes for various imaging modalities, aiding in the localization and diagnosis of various disease processes. Advanced nanocarriers can be precisely engineered to improve the detection and imaging of ischemic tissues. These diagnostic nanomedicines can be functionalized with contrast agents for magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), and fluorescence imaging, ensuring accurate localization, characterization, and monitoring of ischemic lesions (Table 2).
Diagnostic Nanomedicines for IHD.
| Imaging modality | Nanomedicine type | Advantage | Model | Ref. |
|---|---|---|---|---|
| MRI | SPIONs | High spatial, temporal resolution, high soft tissue contrast, the ability of quantitative imaging | MI | [36] |
| Hsp70-SPION | MI | [163] | ||
| Gd-CDs | MI/RI | [97] | ||
| MnO-OA | MI | [98] | ||
| CT | CNA35 | Easily available, rapid imaging, high image quality, noninvasive | MI | [100] |
| PET | Na[18F]F | MI/RI | [101] | |
| 68Ga3+ | MI | [164] | ||
| Fluorescence imaging | CD47-EVs | Noninvasive, targeting of multiple biological factors, high sensitivity | MI/RI | [103] |
| SiO2@pDA-DNA-CeO2 | MI/RI | [104] | ||
| GF/TPO | MI/RI | [105] |
5.1. MRI
MRI is a widely used noninvasive technique that provides high-resolution images of soft tissues. The incorporation of nanomedicines, especially those based on iron oxide, gadolinium (Gd), and manganese (Mn) as contrast agents, has significantly enhanced MRI capabilities. These cutting-edge in vivo imaging technologies enable real-time visualization of myocardial viability and the pathophysiology of myocardial ischemia.
Iron oxide NPs exhibit superparamagnetism, becoming magnetized only in the presence of an external magnetic field, making them ideal T2 contrast agents for MRI. The use of superparamagnetic iron oxide NPs (SPIONs) and ultrasmall superparamagnetic iron oxide NPs (USPIOs) has significantly enhanced the accuracy of MRI in diagnosing IHD. For example, Chen et al. reported that PP/PS@MIONs, superparamagnetic iron oxide NPs encapsulated with dual surfactants, improve detection of early-stage myocardial ischemia by targeting and accumulating in ischemic tissue, resolving inflammation, and enhancing MRI signals [36]. Additionally, SPIONs have been extensively validated for their exceptional diagnostic performance in identifying IHD in clinical applications [96]. These NPs significantly improve the precision of MI localization and detailed characterization, surpassing traditional imaging agents by providing clear, high-resolution images of infarcted tissue. Furthermore, SPIONs exhibit excellent biocompatibility and low toxicity, making them ideal for repeated clinical use. This innovation marks a significant breakthrough in the noninvasive diagnosis and monitoring of myocardial ischemia, promising improved patient outcomes through early and accurate detection.
Gd-based NPs, such as Gd-doped carbon dots (Gd-CDs), enhance MRI detection of IHD by providing dual magnetic resonance and fluorescence imaging. This technique overcomes the limitations of traditional Gd chelates, such as short circulation time, low relaxivity, and high dosage requirements. Gd-CDs offer precise imaging, renal clearance, and low cytotoxicity, making them promising for clinical application [97]. Moreover, Mn ions are valuable for cardiac imaging due to their excellent paramagnetic properties and ability to enter CMs via L-type voltage-dependent Ca2+ channels, where they remain for several hours. Recently, Zheng et al. developed highly crystalline MnO NPs through the thermal decomposition of Mn oleate. These MnO-based NPs demonstrated high longitudinal relaxivity (r1) and relaxation rates without significant toxicity, making them promising for advanced cardiac imaging applications [98].
5.2. CT
CT is a pivotal imaging technique that provides detailed anatomical insights, particularly valuable in diagnosing IHD. CT scans offer rapid image acquisition, high-resolution details, and the ability to visualize both bone and soft tissues, making them essential in emergency settings for quick diagnosis and treatment planning [99]. However, traditional CT imaging faces limitations such as exposure to ionizing radiation, potential allergic reactions to iodinated contrast agents, and challenges in differentiating soft tissue structures.
Nanomedicine holds the potential to significantly enhance the diagnostic capabilities of traditional CT imaging, particularly in the context of IHD. Gold NPs (AuNPs) are particularly promising due to their large scattering cross-section and low toxicity. They have been shown to improve imaging acquisition speed and reduce nephrotoxicity. For example, Kee et al. developed collagen-binding adhesion protein 35 (CNA35)-functionalized AuNPs for molecular imaging of myocardial scars. These CNA35-AuNPs demonstrated long blood circulation times and specific targeting capabilities to collagen in myocardial scars. In a rat MI/RI model, specific signal amplification in the myocardial scar was observed six hours after intravenous administration of CNA35-AuNPs, highlighting their potential for targeted imaging. Despite these promising results, further studies on the biodistribution, toxicity, and biocompatibility of AuNPs in humans are necessary before they can be widely adopted in clinical practice [100].
5.3. PET
PET is a sophisticated imaging technique that employs radioactive tracers to produce detailed heart images, essential for diagnosing IHD. PET is known for its high sensitivity and specificity in detecting coronary artery disease (CAD) and assessing myocardial viability and perfusion. Nanomedicine offers significant enhancements to PET imaging for IHD diagnosis. Recent studies have highlighted the use of sodium [18F] fluoride (Na[18F]F) as a PET contrast agent to image MI/RI in rat models. Na[18F]F uptake was notably higher in infarcted areas, correlating with CM apoptosis and positive Ca2+ staining [101]. These results demonstrate the potential of PET imaging for accurate MI/RI diagnosis.
In addition, NP-based PET tracers can quantify myocardial blood flow (MBF) and myocardial flow reserve (MFR), providing valuable insights into the severity of ischemia and assisting in patient risk stratification [102]. These advancements make PET imaging a powerful, noninvasive tool for diagnosing myocardial ischemia, essential for effective clinical decision-making. Future research should aim to optimize these NP tracers to enhance targeting, reduce toxicity, and improve imaging capabilities, ensuring their practical clinical application.
5.4. Fluorescence imaging
MRI, CT, and PET are effective for tissue-level observation but have limitations in spatiotemporal resolution, preventing clear visualization of cellular changes in the pathophysiological microenvironment. In contrast, fluorescence imaging offers high spatiotemporal resolution and sensitivity, making it superior for noninvasive and precise monitoring of IHD.
Fluorescently labeled NPs, such as fluorescence-labeled CD47-EVs (CD47-EVs), are used to track drug distribution and delivery in myocardial tissues. These CD47-EVs have shown prolonged circulation times and preferential accumulation in the myocardium, providing real-time monitoring of therapeutic effects and drug delivery efficiency [103]. Similarly, Yang et al. developed a fluorescent SiO2@PDA-DNA-CeO2 nanocomposite for detecting exogenous molecules in MI/RI models. This nanocomposite allowed simultaneous detection of intracellular miRNA and H2O2 in vivo, specifically targeting apoptotic CMs, and revealing significant miR-21 expression in response to oxidative stress [104].
Further advancements include Gd ferrate and trigadolinium pentairon (III) oxide NPs (GF/TPO NPs) grafted with fluorescent pigment indocyanine green (ICG). These NPs efficiently accumulated in infarcted areas, enhancing vascular permeability and providing clear fluorescence signals in ischemic tissues. This dual imaging capability facilitates the detailed visualization of IHD and assessment of therapeutic interventions [105].
These imaging methods collectively enhance the precision, sensitivity, and specificity of IHD diagnosis, each with unique benefits tailored to different diagnostic needs. Future research should focus on optimizing these NP-based imaging agents for better targeting, reduced toxicity, and enhanced imaging capabilities. This will ensure their effective clinical application and improve patient outcomes through precise and early diagnosis of ischemic heart conditions.
6. Antioxidant nanomedicines for IHD treatment
ROS are crucial targets in IHD, as they perpetuate a vicious cycle of inflammation and Ca2+ overload [6]. Antioxidant therapy, which utilizes antioxidants to neutralize excessive ROS, not only mitigates oxidative stress but also limits subsequent cell apoptosis and inflammatory responses, making it a preferred strategy for treating IHD. Preclinical studies have explored a range of antioxidants, including antioxidant enzymes, nonenzymatic antioxidants, NSMs, inorganic and other substances [106]. However, their poor solubility, short half-life, and limited bioavailability significantly impede their potential for clinical translation [107, 108]. To address these challenges, multifunctional nanocarriers can improve this strategy by enhancing the pharmacokinetic properties of antioxidants and facilitating their accumulation in damaged cardiac tissue. This approach offers a promising perspective for the clinical treatment of IHD. In this section, we highlight the recent progress in nanomedicines targeting oxidative regulation, categorizing and summarizing key studies based on their antioxidant mechanisms (Table 3).
The strategies, including achievements and limitations in targeted delivery of nanomedicines in reducing oxidative stress.
| Phase of cascade | Type of materials | Cargos | Achievement | Limitation | Model | Administration | Ref. |
|---|---|---|---|---|---|---|---|
| Enzymatic antioxidants | PEG-PBD/PEG-PPO | SOD | •O2- → H2O2 | Short half-life and limited stability of SOD | MI/R | Intramyocardial | [113] |
| PCADK | SOD1 | •O2- → H2O2 | Inadequate concentration | MI/R | Intramyocardial | [107] | |
| ZrMOF | SOD | •O2- → H2O2 | Potential toxicity and long-term stability | MI | Intramyocardial | [112] | |
| Nonenzymatic antioxidants | PEG-allomelanin | Allomelanin | Antioxidant and anti-inflammatory | Poor solubility and potential immunogenicity | MI | Tail vein | [120] |
| EVs | Mel | Scavenging ROS | Difficulty in controlling the release and bioavailability | MI | Intramyocardial | [121] | |
| PEG-bilirubin | Bilirubin | Antioxidant | Poor stability and potential toxicity | MI/R | Intraperitoneal | [24] | |
| PEG-PDA | PDA | Scavenging •O2- and •OH and alleviating Fe2+ accumulation | Long-term toxicity | MI/R | Tail vein | [106] | |
| Macrophage membrane-coated PDA | PDA | Scavenging •O2- and •OH | Complexity of manufacturing | MI/R | Tail vein | [117] | |
| Liposome | EGCG and CoQ10 | Eradicating ROS and mitigating apoptosis | Potential degradation of encapsulated drugs | MI | Tail vein | [71] | |
| Liposome | CoQ10 | Antioxidant | Potential for drug leakage and limited precise targeting | MI | Coronary infusion | [53] | |
| PLGA | LA | Reducing oxidative stress, senescence, DNA damage, cytokine-related processes, apoptosis, and ferroptosis | Slow and incomplete release of drugs | MI | Hydrogel delivery | [127] | |
| NSMs | MSN | Que | Inhibiting cell apoptosis and oxidative stress | Poor bioavailability and rapid metabolism | MI/R | Intravenous | [62] |
| PLGA | Que | Antioxidant | Rapid clearance and limited targeting efficiency | MI/R | / | [63] | |
| ZIF-8 cored QSF@Z-NCs | Que | Reducing apoptosis and promoting regeneration | Complex production and safety concerns | MI | Intramyocardial | [165] | |
| MOF | Que | Antioxidant and anti-inflammatory | Long-term toxicity and limited targeting efficiency | MI | Tail vein | [38] | |
| Solid lipid NPs | PUE | Antioxidant | Limited drug release control | MI | Intravenous | [129] | |
| PEG-SLNs | BN | Antioxidant | Poor stability and potential for drug leakage | MI | Intraperitoneal | [108] | |
| Hydrogel | EGCG and Rhein | Antioxidant and anti-inflammatory | Complexity of application and long-term efficacy concerns | MI/R | Intramyocardial | [166] | |
| EVs | Curcumin and miR-144-3p | Antioxidant and inhibiting of apoptosis | Limited targeting specificity and Complex production | MI | Tail vein | [167] | |
| PGMA | Curcumin | Antioxidant | Limited targeting precision and potential toxicity | MI/R | Coronary artery perfusion | [168] | |
| EVs | Curcumin | Antioxidant | Limited drug loading capacity and challenges in large-scale production | MI | Intravenous | [131] | |
| β-MEND | RSV | Maximized cell respiration | Limited mitochondrial targeting efficiency | / | / | [169] | |
| mPEG-b-O (D, L-Leu) | RSV | Inhibiting of apoptosis | Limited bioavailability and potential aggregation | MI/R | Subcutaneous | [170] | |
| IMTP-ployHis-PEG3400-hyd-PLGA/ SS-31-PEG8-PLGA | RSV | Antioxidant | Limited targeting accuracy and complex delivery mechanism | MI/R | Intravenous | [78] | |
| PLGA | RSV | Antioxidant and anti-inflammatory | Limited drug release control | MI | per oral | [77] | |
| Lipid-polymer hybrid NPs | Salvianolic acid B and PNS | Enhancing targeted drug delivery efficiency | Limited receptor targeting efficiency | MI | Tail vein | [76] | |
| MSN | Salvianolic acid B | Inhibiting of oxidative stress and apoptosis | Potential for limited drug loading capacity | MI/R | Ingastric administration | [171] | |
| PEG-b-PPS | Rg3 | Inhibiting oxidative stress, inflammation, and fibrosis promotion | Limited ROS responsiveness | MI/R | Intramyocardial | [132] | |
| Silica NPs | Notoginsenoside R1 | Inhibiting oxidative stress, inflammation, and apoptosis | Limited targeting efficiency and potential off-target | MI | Tail vein | [84] | |
| mPEG-PLGA | Panax notoginseng | Antioxidant | Limited long-term stability | MI/R | Orally | [130] | |
| PEG-SLNs | Sch B | Reducing the infarction size | Limited MMP sensitivity | MI | Tail vein | [73] | |
| mPEG-PLA-TPGS | Tanshinone IIA | Reducing inflammation, apoptosis, and fibrosis | Limited specificity | MI | Intravenous | [80] | |
| Inorganic nanoenzymes | CVNRs | / | SOD-like activities | Limited efficacy | MI/R | Intravenous | [138] |
| Pd@CeO2 | / | CAT- and SOD-like activities | Complexity manufacturing and uncertain long-term biocompatibility | MI/R | Intravenous | [35] | |
| TA-Ce | / | CAT- and SOD-like activities | Limited targeting specificity | MI/R | Intravenous | [161] | |
| Cu-TCPP-Mn | / | CAT- and SOD-like activities | Potential instability and limited ROS scavenging efficiency | MI | Intravenous | [172] | |
| RuO2@BSA | / | CAT- and SOD-like activities | Potential cytotoxicity | MI/R | Intravenous | [173] | |
| ZIF-8 | / | CAT- and SOD-like activities | Potential for incomplete ROS scavenging | MI | In situ delivery | [174] | |
| Mn3O4@PDA | MSCs | CAT- and SOD-like activities | Potential for limited MRI tracking sensitivity | MI | Intravenous | [140] | |
| CuCe | / | CAT- and SOD-like activities | Uncertain long-term biocompatibility | MI | Intramyocardial | [139] | |
| Au@Pt | / | CAT- and SOD-like activities | Limited long-term stability | MI | Intramyocardial | [175] | |
| Au@Se | L-Arg | CAT- and SOD-like activities | Limited targeting specificity | MI/R | Intravenous | [176] | |
| SSSe | / | CAT- and SOD-like activities | Limited long-term stability of the self-sustaining antioxidant system | MI | Intravenous | [141] | |
| AS-I/SNCs | SS31 | CAT-, SOD-, and GPx-like activities | Limited targeting specificity | MI/R | Intravenous | [83] | |
| ZIF-8zyme | / | CAT-, SOD-, and GPx-like activities | Limited targeting efficiency | MI | / | [177] | |
| Fe-Cur@TA | Curcumin | CAT-, SOD-, and POD-like activities | Limited targeting efficiency | MI | Intravenous | [178] | |
| MnO2 Fenozymes | / | CAT-, SOD-, and POD-like activities | Limited mitochondrial targeting efficiency | MI/R | Hydrogel delivery | [179] | |
| PtsaN-C | / | CAT-, SOD-, and POD-like activities | Limited targeting specificity | MI/R | Intramyocardial | [180] | |
| Gas-generating nanomedicine | PolyPHb | Hemoglobin | Elevating SOD activity and preserving mitochondrial ATP synthesis | Limited long-term efficacy | MI/R | / | [144] |
| PEGy-Hb | Hemoglobin | Reducing infarct size | Limited targeting specificity | MI/R | Intraperitoneal | [142] | |
| PCNP/O2 | / | Enhancing cardiac cell survival, stimulating, angiogenesis, and suppressing fibrosis | Limited Long-Term Efficacy and safety concerns | MI | Intravenous | [52] | |
| PUAO-CPO-Collagen | Ca2+ peroxide | Reducing scar formation, attenuating adverse cardiac remodelling and decreasing oxidative stress | Limited in vivo testing | MI | Intravenous | [85] | |
| SOD/PAC | G-CSF, NO/H2S | Reducing ROS, inflammation level and relieving Ca2+ overload | Limited targeted delivery efficiency | MI/R | Intravenous | [181] | |
| B-P@PLT | BNN6 | Promoting angiogenesis, reducing ROS production | Challenges in controlled release | MI/R | Intravenous | [149] | |
| Chitosan hydrogel | NO | Antioxidant | Uncertain biodegradability and biocompatibility | MI/R | Intramyocardial | [182] | |
| DATS-MSN | H2S | Inhibiting oxidative stress and inflammation | Delayed response due to slow release | MI/R | Tail vein | [150] | |
| Pluronic F-127/KAT | Keratin and H2S | Ameliorating microvascular obstruction, preventing myocardial fibrosis, and attenuating cardiac inflammation | Unclear long-term safety | MI/R | Myocardial surface | [183] | |
| C3F8-loaded microbubbles | H2S | Inhibiting oxidative stress and inflammation | Inconsistent dosage delivery | MI/R | Tail vein | [184] | |
| Microbubble | H2 | Antioxidant | Limited precision in targeted Delivery | MI/R | Tail vein | [151] | |
| Others | PEG-SLNs | ONO-1301 | Anti-inflammatory | Complex production process | MI/R | Intravenous | [156] |
| PTK | CsA | Scavenging ROS | Unclear relevance to chronic inflammation | MI/R | Tail vein | [154] | |
| PLGA | CsA | Inhibiting mPTP opening | Limited efficiency in targeted delivery | MI/R | Intravenous | [185] | |
| Platelet Membrane-Encapsulated MSN | SS31 | Scavenging ROS | Potential immune response | MI/R | Tail vein | [186] | |
| PLGA-TK-PEG/ HA-Diol-HYD | SS31/CsA | Scavenging ROS | Uncertain long-term effects | MI/R | Intramyocardial | [79] | |
| PLL-PEG-PLL | Exenatide | Attenuating the oxidative stress | Uncertain drug stability | MI/R | Subcutaneous | [187] | |
| PGMA | Alpha-interacting domain (AID) | Reducing in release of creatine kinase and lactate dehydrogenase | Insufficient long-term efficacy | MI/R | Perfusion on Langendorff's apparatus | [188] | |
| HPOX/PVAX | HBA/VA | Antioxidant | Potential toxicity | MI/R | Intramyocardial | [157] | |
| Platelet membrane-coated PLGA | microRNA | Inhibiting of ROS and apoptosis | Variable targeting efficiency | MI/R | Tail vein | [51] | |
| EVs | microRNA | Inhibiting apoptosis and inflammatory | Potential immune response | MI/R | Tail vein | [158] | |
| DNA nanostructures | / | Reducing the ROS production | Uncertain long-term biocompatibility | MI/R | / | [189] |
(A) Schematic of the construction of SOD-ZrMOF. (B-C) The ROS level in CMs. Adapted with permission from [112], copyright 2022 Elsevier. (D) Representative SEM of empty polymer (PK) and PKSOD. (E-F) Extracellular and intracellular superoxide concentration. Adapted with permission from [107], copyright 2010 Elsevier. (G) Schematic of PEG-PBD polymer. (H-I) Masson's Trichrome and Picrosirius red staining with quantitative analysis for the respective images. Adapted with permission from [113], copyright 2021 John Wiley and Sons.
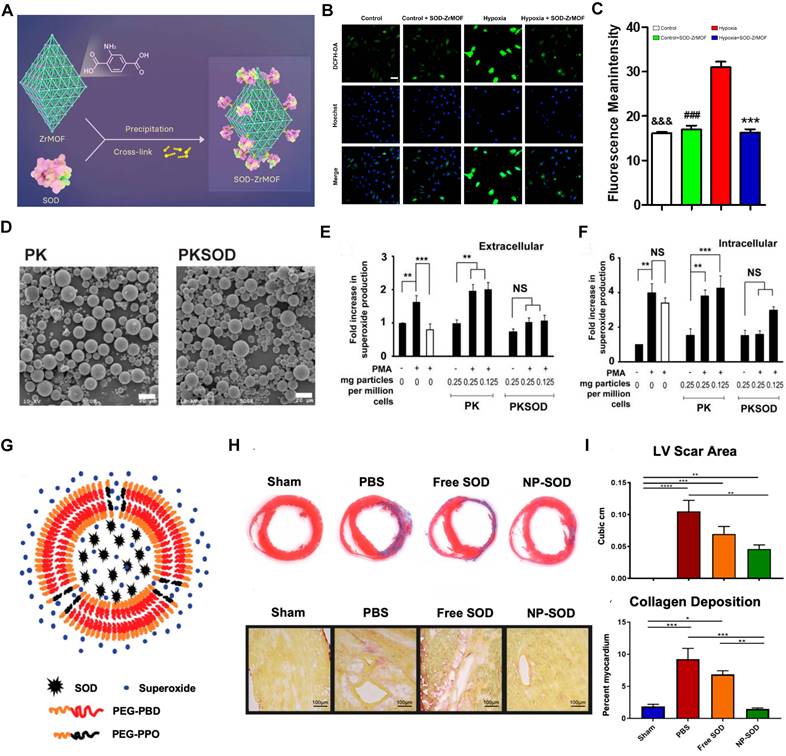
6.1. Antioxidant enzyme-based nanomedicines
Oxidative stress, while essential for cellular signaling and homeostasis at low concentrations, becomes harmful at elevated levels [25]. Myocardial ischemia impairs oxidative phosphorylation, leading to a dysregulated oxidative environment within cells [109]. Intracellular antioxidant enzymes are the primary defense against oxidative imbalance, but they are often overwhelmed by severe oxidative challenges [110]. Therapeutically enhancing these enzymes could bolster cellular resilience and mitigate oxidative injury.
Metal-organic frameworks (MOFs) are crystalline materials composed of metal ions or clusters coordinated to organic ligands, forming a porous structure. Due to their highly tunable porosity, large surface area, and versatile functionality, MOFs have garnered significant interest in various applications, including gas storage, catalysis, and drug delivery [111]. Guo et al. developed the SOD-ZrMOF nanoconstruct, integrating SOD with a zirconium-based framework, which exhibits exceptional biocompatibility and effectively neutralizes ROS (Figure 4A). These NPs present advanced approaches for MI repair, promising enhanced cardiac recovery post-ischemia after intracardiac administration [112]. Concurrently, SOD encapsulated within microparticles has shown protective effects against MI/RI in rat hearts, reducing superoxide-mediated damage (Figure 4B-C). Although Zr-MOFs exhibit superior efficacy compared to that of native SOD proteins, they face challenges such as stability issues, complex synthesis, potential toxicity, and limited functionalization. The application of MOFs in antioxidant enzyme delivery requires further investigation into their long-term biocompatibility and degradation within the body. Research should also explore the potential of integrating MOFs with other nanomaterials to form hybrid systems that can provide more controlled release profiles and target multiple oxidative stress pathways simultaneously. Additionally, the role of these nanomedicines in complex in vivo environments, including interactions with immune cells and other components of the ischemic tissue, needs to be better understood to optimize therapeutic outcomes. In contrast, polymer-based nanocarriers offer superior biocompatibility, customizable drug release profiles, enhanced stability, versatile functionalization, and ease of large-scale manufacturing [76, 77]. For instance, researchers have encapsulated SOD1 within the economically feasible and more stable poly (cyclohexane-1,4-diyl acetone dimethylene ketal) (PCADK) polymer (Figure 4D), resulting in PKSOD, which reduces the concentration of superoxide both intracellularly and extracellularly (Figure 4E-F) [107].
The dense structure of polymeric carriers makes it challenging for ROS to directly interact with the encapsulated drugs, hindering rapid onset of antioxidative effects. To address this, Atluri and colleagues incorporated the diblock copolymer PEG-PPO into the PEG-PBD polymer, resulting in a nanocarrier that facilitates the delivery of unmodified enzymes, protects against proteolysis, and allows access to ROS via a highly porous membrane (Figure 4G). This construction accommodates and retains antioxidant enzymes within the NPs while allowing small molecules, such as free •O2-, to pass through the polymer's permeable membrane into the carrier interior. Research indicates that the SOD-encapsulated PEG-PBD polymer can reduce the area of MI and diminish negative cardiac remodeling (Figure 4H-I) [113]. These results suggest that exogenous supplementation of antioxidant enzymes can facilitate myocardial repair by modulating oxidative balance. However, while SOD quenches •O2- to combat oxidative stress, the resulting H2O2 presents new challenges for clinical application, necessitating the concurrent use of CAT to detoxify H2O2.
6.2. Nonenzymatic-based nanomedicines
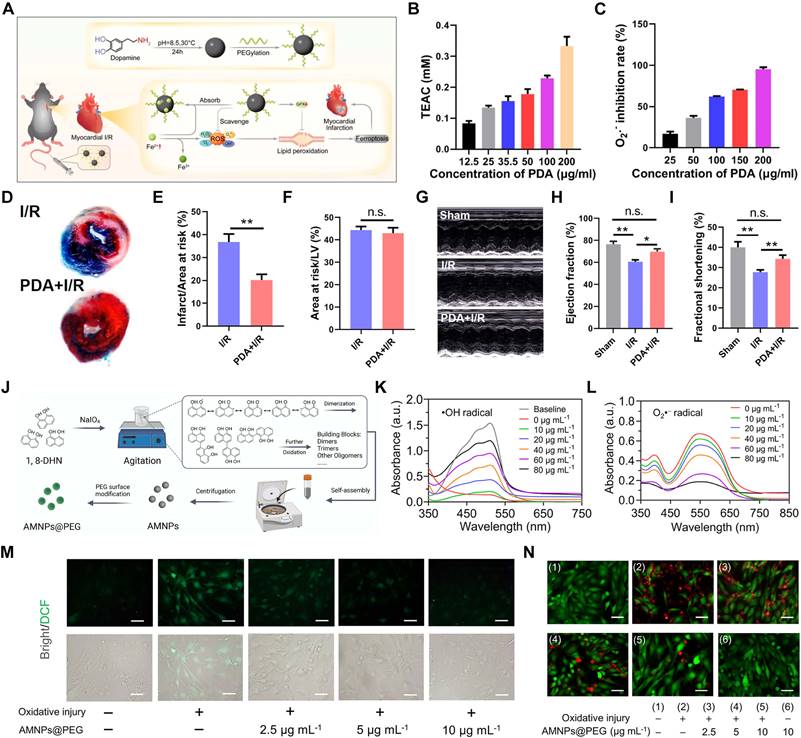
Nonenzymatic antioxidants typically react rapidly within the body to neutralize various types of free radicals, thereby mitigating the damage caused by oxidative stress. Dopamine, a crucial neurotransmitter present in the brain, plays a key role in regulating emotions, reward mechanisms, and pleasure [114]. Research has shown that dopamine can be polymerized into PDA through a process called oxidative polymerization under alkaline conditions, involving oxidation and cyclization steps that lead to the formation of melanin-like structures [115]. In addition, PDA has been identified as an antioxidant that can inhibit inflammation and oxidative stress [116].
Li's team employed PEG-modified PDA NPs as cardioprotective agents to alleviate MI/RI in mice (Figure 5A). Unlike typical nanocarriers, PDA NPs themselves exhibit inherent antioxidant properties, effectively scavenging ROS such as •OH and •O2- (Figure 5B-C). Intravenous injection of PDA NPs into mice with MI/RI reduced infarct size (Figure 5D-F) and improved cardiac function (Figure 5G-I) [106]. Although PEGylation can extend the blood circulation time of PDA NPs to some extent; however, cell membrane coatings are superior to PEGylation in prolonging blood circulation time due to their enhanced biocompatibility, natural immune evasion properties, and improved functionalization potential. The biomimetic nanoplatform (PDA@M) created by coating PDA NPs with macrophage membranes not only reduces the clearance of PDA NPs by monocytes/macrophages but also enhances their targeting ability to CMs, thereby providing more precise antioxidant therapy [117].
The design of PDA is fundamentally based on emulating the properties of natural melanin. Unlike melanin, which is challenging to extract and purify, PDA provides enhanced stability, scalability, and multifunctionality, making it ideal for various biomedical applications [118]. Additionally, researchers are actively pursuing breakthroughs in new natural melanin analogs. Allomelanins, a novel melanin analog synthesized by fungi using non-nitrogenous and non-sulfurous 1,8-dihydroxynaphthalene (1,8-DHN), has shown considerable potential. Research indicates that 1,8-DHN can form nanomedicines with robust HAT properties, providing potent antioxidant effects [119]. PEG-modified allomelanin NPs (AMNPs@PEG) have been developed for targeted therapy of MI/RI (Figure 5J). AMNPs@PEG demonstrated significant efficacy in scavenging •OH and •O2- in vitro (Figure 5K-L). Further cellular studies confirmed the protective role of AMNPs@PEG in CMs, as AMNPs@PEG effectively alleviated intracellular ROS and ROS-induced damage (Figure 5M-N) [120].
(A) Schematic diagram of the ROS-scavenging and Fe2+-chelating abilities of PDA NPs. (B) ABTS measurement of the antioxidant capacity of PDA NPs. (C) •O2--scavenging activity of PDA NPs. (D) Representative Evans Blue and TTC stained heart tissue sections. (E-F) The infarct size relative to area at risk (AAR) and the AAR relative to the area of left ventricle (LV). (G) Representative M-mode echocardiograms of myocardial I/R mice exposed to different treatments collected 24 h postoperatively. (H-I) Calculations of ejection fraction and fractional shortening percentages. Adapted with permission from [106], copyright 2021 American Chemical Society. (J) Schematic illustration of the fabrication procedure of AMNPs. (K-L) Representative UV-vis absorption results of AMNPs on scavenging •OH and •O2-. (M) Representative fluorescent images of intracellular ROS levels. (N) Fluorescence images of myocardial cell activity. Adapted with permission from [120], copyright 2022 Elsevier.
Unlike PDA, which primarily quenches ROS through electron transfer redox mechanisms, bilirubin functions by scavenging peroxyl radicals and directly neutralizing ROS. PEGylated bilirubin facilitates the self-assembly of bilirubin NPs (BRNPs), which effectively target the MI/RI site. Research indicates that BRNPs mitigate oxidative stress and inflammation, suggesting a novel therapeutic avenue for MI/RI [24].
While PDA and bilirubin can naturally self-assemble into NPs, many nonenzymatic antioxidants require appropriate carriers for effective delivery. Among various exogenous carriers, EVs have recently emerged as a popular candidate. One of the key advantages of EVs over artificial delivery systems is their inherent biocompatibility and natural origin. Researchers engineered EVs derived from adipose-derived stem cells (ADSCs) to load Mel, creating Mel@NVs. The impact of Mel@NVs on cellular oxidative stress and MI repair was studied. The results indicated that treatment with Mel@NVs under ischemic conditions reduced cell apoptosis from 42.59 ± 2.69% to 13.88 ± 1.77%. Furthermore, Mel@NVs ameliorated excessive ROS generation, promoted microvessel formation, and attenuated cardiac fibrosis [121].
CoQ10 is a vital endogenous antioxidant that plays a crucial role in oxidative phosphorylation [122]. Previous research has demonstrated the potential of CoQ10 in treating and preventing IHD, hypertension, hyperlipidemia, CAD, and HF [123, 124]. However, due to the high lipophilicity and low solubility of CoQ10, delivering it within cells presents challenges. Liposomes, as nanoscale drug delivery carriers, have been proven to enhance the therapeutic efficacy of CoQ10. A research team in the United States has employed various methods to prepare CoQ10-loaded liposomes, optimizing the formulation to achieve maximum payload and stability. These CoQ10-containing liposomes have shown significant cardioprotective effects against MI/RI, offering a new approach to protecting the ischemic myocardium [53].
Alpha-lipoic acid (LA) is a natural antioxidant compound featuring two thiol groups that can be oxidized or reduced, allowing it to participate in various redox biochemical reactions [125]. However, its therapeutic application is limited by rapid and extensive distribution, high metabolic clearance, a short half-life of approximately 25 minutes, and a high systemic pre-clearance rate [126]. Incorporating LA into a PLGA copolymer and forming a film via electrospinning technology (referred to as LA@PLGA) significantly improves these limitations. LA@PLGA enables controlled release, enhancing the stability and bioavailability of LA. Studies have demonstrated that LA@PLGA exhibits potent antioxidant and anti-apoptotic effects in primary CMs treated with H2O2, and its application to the surface of the heart in mice with MI significantly improves cardiac function and reduces cardiac fibrosis throughout the ventricular remodeling process [127].
In summary, nanocarriers play a crucial role in enhancing antioxidant therapy for IHD by improving the stability, bioavailability, and targeted delivery of nonenzymatic drugs, thus addressing the limitations of traditional therapies. These advanced carriers, including liposomes and polymeric systems, exhibit excellent biocompatibility and customizable drug release profiles, enabling precise therapeutic effects. Additionally, some nonenzymatic antioxidant drugs can self-assemble into nanostructures, and with simple modifications, their properties can be further enhanced to achieve better therapeutic outcomes. Future research should focus on overcoming the production and therapeutic challenges of nonenzymatic nanomedicines, enhancing the targeting, scalability, and consistency of nanomedicines, and conducting extensive in vivo studies to understand their long-term effects. The advancement of nonenzymatic antioxidant nanomedicines could benefit from exploring synergies with other therapeutic agents, such as anti-inflammatory molecules or growth factors, to enhance the overall therapeutic effect. The potential of these nanomedicines in long-term treatment and chronic disease management should also be investigated, focusing on their impact on tissue remodeling and regeneration post-injury. Furthermore, the development of next-generation biomimetic nanoplatforms that closely mimic the native cellular environment could offer more effective and targeted therapies.
6.3. NSM-based nanomedicines
Small molecular compounds, derived from both natural sources and synthetic modifications, are integral to the prevention and treatment of various diseases due to their potent antioxidant effects [76]. Small molecular antioxidants like curcumin, resveratrol (RSV), baicalin (BN), and quercetin (Que) are effective in combating oxidative stress but face significant delivery challenges, such as poor solubility, rapid metabolism, and lack of specific targeting. Nanotechnology-based delivery systems have revolutionized their clinical potential, ensuring more effective therapeutic outcomes.
Que, a typical flavonoid belonging to the flavonol class, has been confirmed to provide robust cardioprotection in IHD by modulating inflammation, oxidative stress, and CM apoptosis [128]. However, its clinical application is limited due to its low water solubility, short half-life, poor transference, and low bioavailability. Mesoporous silica NPs (MSNs) have emerged as a promising platform for drug delivery due to their unique structural and functional properties. One of the primary advantages of MSNs is their high surface area and large pore volume, allowing for substantial drug loading capacity. The tunable pore size and surface chemistry of MSNs enable precise control over drug release kinetics, enhancing the therapeutic efficacy of encapsulated drugs. Encapsulating que within MSNs has been shown to extend its half-life in vivo, enhancing drug absorption. Both in vitro and in vivo, que-loaded MSNs (Q-MSNs) exhibit superior efficacy in inhibiting apoptosis and oxidative stress, reducing MI size, and improving ventricular remodeling and cardiac function, compared to que alone [62].
In comparison to metal nanocarriers, MOF nanocarriers exhibit significant advantages, such as tunable porosity, biocompatibility, and stimuli-responsive release, making them highly effective for antioxidant delivery [111]. For instance, the encapsulation of Que within these multifunctional nanocarriers has demonstrated improved stability and bioavailability (Figure 6A). Que@MOF/Man facilitated real-time imaging of the therapeutic process, providing critical insights into drug distribution and efficacy, thereby paving the way for personalized medicine in treating MI/RI (Figure 6B). Que@MOF/Man exhibited a concentration-dependent scavenging effect on •NO, •O2-, and •OH radicals, indicating the effective elimination of these harmful free radicals by Que@MOF/Man (Figure 6C-E). Interestingly, co-culturing with Que@MOF/Man-treated macrophages appears to inhibit ROS production in injured cardiomyocytes (Figure 6F). Additionally, in vivo studies have shown that Que@MOF/Man significantly mitigate inflammation (Figure 6G-H), leading to a marked reduction in infarct size [38].
(A) Schematic diagram of the construction of Que@MOF/Man. (B) Representative ex vivo fluorescence images and quantification analysis. (C-D) Figure illustrating the scavenging efficacy of Que@MOF/Man against ABTS•+ (A) and DPPH• radicals. (E) UV-Vis spectra depicting the radical scavenging activities of Que@MOF/Man against •NO, •O2-, and •OH radicals. (F) Representative images of ROS production. (G) Representative images of CD68+ macrophages. (H) Representative images of CD206+ macrophages. Adapted with permission from [38], copyright 2021 John Wiley and Sons.
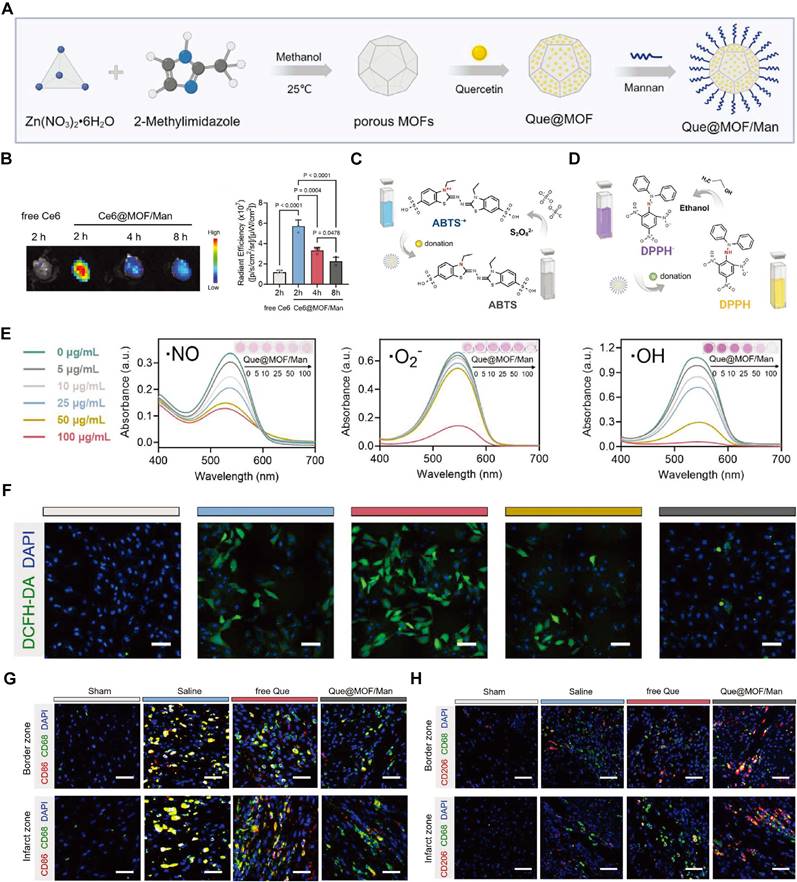
However, despite these advantages, the biodegradation and excretion of metal-based materials need to be thoroughly understood to ensure long-term safety. Therefore, using nanocarriers with good biodegradability in vivo may be a more favored choice. For instance, Lu et al. developed a novel RGD modified and PEGylated nanostructured lipid carrier (RGD/PEG-PUE-SLN) for puerarin (PUE), which enhances its bioavailability in vivo, and amplifies its protective effects. In vitro studies assessing the cytotoxicity of PUE-loaded SLN and free PUE at varying concentrations revealed that the RGD/PEG-PUE-SLN, PEG-PUE-SLN, and PUE-SLN groups showed no significant cytotoxicity relative to the saline control, underscoring the superior biocompatibility of SLN. In in vivo applications, it was evident that the RGD/PEG-PUE-SLN formulation resulted in the smallest infarct size among all the formulations tested [129]. Researchers have taken advantage of the properties of liposomes to design nanocarriers for various small-molecule drugs. For example, Han's team encapsulated poorly water-soluble Schisandrin B (Sch B) into PEG-modified solid lipid NPs (SLNs). They also modified the liposome surfaces with matrix metalloproteinase (MMP)-targeting peptides, creating MMP-Sch B SLNs nanomedicines. The profile demonstrated that the plasma concentration of Sch B solution decreased rapidly and was cleared from circulation within 4 hours. In contrast, Sch B loaded SLNs exhibited a significantly prolonged plasma circulation time, indicating that liposomes can effectively extend the circulation time of Sch B. The prolonged blood circulation time facilitates drug accumulation in the heart, thereby reducing the required dosage and improving therapeutic outcomes [73]. Furthermore, researchers developed novel panax notoginsenoside (PNS)-loaded core-shell hybrid liposomal vesicles (PNS-HLV) to overcome the limited bioavailability of PNS and enhance its protective effects. PNS-HLV demonstrated controlled drug release profiles, suggesting promising prospects for improving the bioactivity of free drugs [130].
In addition to liposomes, PLGA is a nanocarrier material known for its excellent biodegradability and high safety in applications. Zhou et al. designed a multistage targeted drug delivery system, named MCTD-NPs, by encapsulating RSV in PLGA NPs modified with ischemic myocardial-targeting peptide (IMTP) and SS-31 (Figure 7A). In vitro cell experiments indicated that MCTD-NPs were more readily absorbed by H/R-injured H9c2 cells compared to PLGA-NPs and RSV. The findings revealed that MCTD-NPs were more effective in delivering RSV to H/R injured H9c2 cells compared to PLGA-NPs, which were less effective. The SS-31 modification enabled further mitochondria accumulate of RSV, restoring membrane potential and reducing ROS production caused by mitochondrial dysfunction (Figure 7B). In vivo cardiac targeting studies revealed that MCTD-NPs accumulate significantly in the hearts of MI/RI rats compared to PLGA-NPs (Figure 7C). The increased cardiac specificity of the MCTD-NPs significantly reduced the percentage of infarct size, apoptosis, and inflammation in the ischemic myocardium, and stabilized mitochondrial function [78].
Compared to synthetic nanodelivery systems, EVs provide superior biocompatibility and safety. EVs are naturally secreted by cells, making them highly compatible with the human body and less likely to induce an immune response or toxicity [86]. Curcumin loaded into DOPE-PEG-CTP-modified MSC-derived exosomes can greatly improve its heart-targeting ability and antioxidant therapeutic outcomes [131]. This targeting capability is especially beneficial for the precise delivery of antioxidants to ischemic myocardial tissues, thereby reducing off-target effects and enhancing therapeutic outcomes.
Choosing biocompatible nanocarrier materials is essential, but addressing drug toxicity due to off-target effects is equally crucial. This can be effectively managed through the rational design of nanocarriers. ROS-responsive nanocarriers provide a sophisticated solution by significantly reducing drug toxicity. These carriers are designed with linkers or materials that degrade in the presence of ROS, ensuring that drug release is confined to pathological environments where ROS levels are elevated. This targeted release mechanism minimizes systemic drug distribution, thereby reducing off-target effects and associated toxicity. By specifically responding to the oxidative stress in damaged tissues, ROS-responsive nanocarriers enhance therapeutic efficacy while mitigating adverse effects. Thus, an amphiphilic block copolymer composed of hydrophilic PEG and hydrophobic poly (propylene sulfide) (PPS) was fabricated as an excellent carrier for Ginsenoside Rg3 (PEG-b-PPS-Rg3) (Figure 7D). Under ROS conditions, PPS transitions from hydrophobic to hydrophilic upon oxidation, causing the polymer to disassemble and release the encapsulated Rg3. ROS fluorescence staining indicated that Rg3 significantly reduced ROS levels induced by H/R in H9C2 cells (Figure 7E-F). The antioxidative effect of Rg3 was abolished after FoxO3a was knocked out, indicating that Rg3 exerts its effects by modulating FoxO3a (Figure 7G). Upon intracardiac injection, PEG-b-PPS-Rg3 significantly inhibited the generation of ROS within the injured myocardium, thereby reducing infarct size (Figure 7H) [132].
In summary, nanomedicines derived from NSMs show great potential in treating IHD by effectively reducing oxidative stress. Despite the significant therapeutic effects observed in these nanoformulations, there is a need for further exploration of their long-term safety and biocompatibility. Current challenges include optimizing drug formulations to suit different stages of IHD and diverse patient profiles, as well as refining nanocarrier designs to enhance delivery efficiency and minimize adverse reactions. Additionally, integrating real-time monitoring capabilities within these nanocarriers could provide clinicians with valuable feedback on drug efficacy and tissue response, enabling more personalized treatment regimens. Future research should also consider the scalability of these nanomedicines and their compatibility with current clinical practices to facilitate smoother transitions from bench to bedside.
(A) Schematic diagram of the construction of MCTD-NPs. (B) Representative image of mitochondrial membrane potential. (C) Ex vivo distribution of cy7.5 labeled MCTD-NPs. (E) Evans Blue and TTC staining. Adapted with permission from [78], copyright 2019 Elsevier. (D) Schematic diagram of the construction of PEG-b-PPS-Rg3. (E-F) Representative ROS staining and quantification after administration Rg3. (G) Representative ROS staining with or without knockdown of FoxO3a after administration PEG-b-PPS-Rg3. (H) Representative DHE staining. Adapted with permission from [132], copyright 2019 Elsevier.
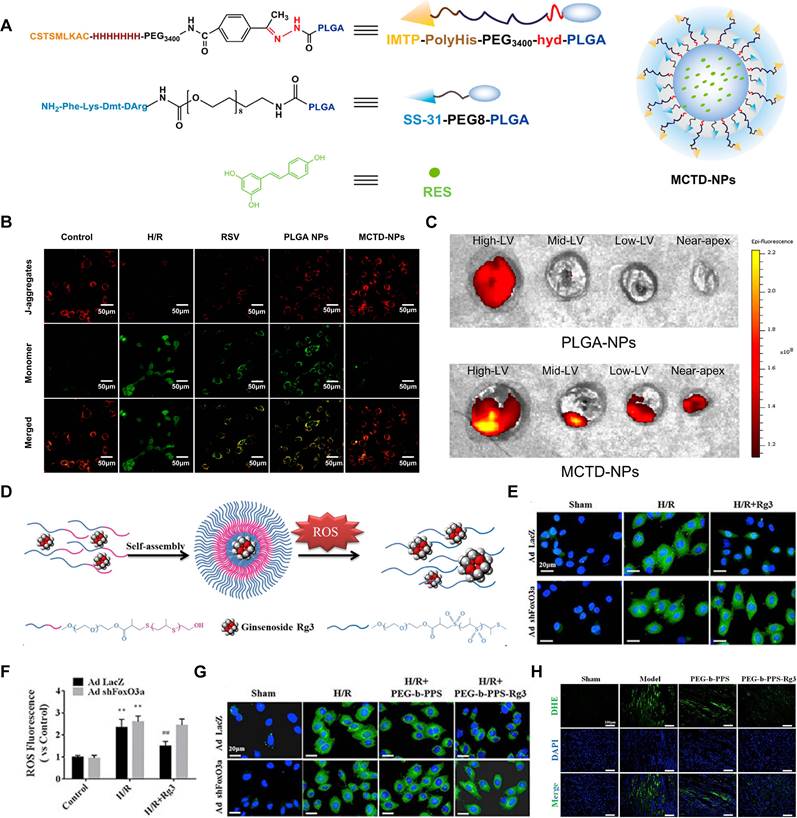
6.4. Inorganic nanomedicines
Recently, inorganic nanomedicines have garnered considerable interest due to the intriguing electronic properties of transition metals [133]. These nanomedicines exhibit redox, optical, and magnetic characteristics, making them highly effective agents for treating IHD [134]. Constructed from inorganic metals, these nanomedicines function primarily by emulating the activity of antioxidant enzymes and are termed "nanozymes". Compared to natural enzymes and other nanomaterials, nanozymes offer superior catalytic activity, selectivity, stability, and a wider substrate range, addressing many of the limitations associated with natural enzymes [135]. Nanozymes demonstrate a robust capacity to neutralize various free radicals, with some even displaying multi-enzyme activities. This enables them to efficiently eliminate ROS through cascade reactions with minimal use [136]. The inherent nanostructure and catalytic capabilities of nanozymes facilitate direct drug application. Furthermore, the activity of nanozymes can be modulated by targeting molecules, facilitating both passive and active targeting of injured myocardial sites. This targeted approach effectively protects ischemic myocardial tissue and reduces infarct size.
Cerium (Ce)-containing nanozymes have attracted widespread attention due to their ability to undergo a valence transition between the Ce3+ (reduced) and Ce4+ (oxidized) forms, exhibiting significant SOD-like activity [137]. Zhang's team reported a type of Ce vanadate nanorods (CVNRs) that possess superior SOD-like activity and act as effective ROS scavengers (Figure 8A). The SOD-like activity of CVNRs is attributed to the redox-active Ce centers and structure-stabilizing V centers (Figure 8B). This SOD-like activity effectively reduces intracellular ROS levels in human cardiac microvascular endothelial cells (HCMECs), protecting against Hcy and Cu2+-induced cell death under H/R conditions (Figure 8C). In vivo experiments showed that a 20 mg/kg CVNRs treatment significantly restored cardiac microvascular integrity and decreased apoptosis in microvascular endothelial cells (Figure 8D-E). These findings suggest that CVNRs are potent ROS scavengers for treating MI/RI [138].
While SOD converts •OH and •O2- into H2O2, the resulting H2O2 can still cause cellular damage. To address this, researchers combined SOD with CAT. They synthesized copper-deposited Ce NPs (CuCe NPs), where Ce NPs serve as antioxidant carriers, transporting and delivering copper ions through biotransformation, enabling dual enzymatic catalysis similar to SOD1 and CAT (Figure 8D). In antioxidant assays, CuCe NPs displayed slightly lower performance than Ce NPs with the same Ce content, likely due to copper deposition masking the Ce surface (Figure 8E). However, CuCe NPs demonstrated a greater ability to scavenge ROS compared to Ce NPs (Figure 8F). The removal of ROS supports the transition of macrophages to an anti-inflammatory phenotype. Dot blot analysis indicated that CuCe NPs significantly reduced the expression of most proinflammatory cytokines compared to Ce NPs. The expression levels of representative pro-inflammatory cytokines (TNF-α and iNOS) and an anti-inflammatory cytokine (TGF-β) showed that CuCe NP treatments mitigated these effects compared to the non-treated group (Figure 8G) [139]. The antioxidant and anti-inflammatory effects of CuCe NPs significantly enhance their therapeutic efficacy in treating IHD.
In addition to Ce-based nanozymes, Mn can also be utilized to construct SOD-mimicking nanozymes, such as Mn3O4 nanozyme. These nanozymes mimic the natural antioxidant functions of SOD by efficiently catalyzing the dismutation of •O2- into O2 and H2O2, thereby reducing oxidative stress in IHD. The catalytic properties of Mn3O4 nanozymes are derived from their unique crystal structure and oxidation states, which facilitate electron transfer processes essential for ROS neutralization. Liu et al. modified the surface of Mn3O4 nanozyme with PDA through a polymerization reaction, creating Mn3O4@PDA (Figure 9A). This modification increased the biocompatibility of Mn3O4, improving its interaction with stem cells and enhancing its SOD-mimicking activity. The scavenging capacities of Mn3O4 and Mn3O4@PDA for H2O2, •OH, and •O2- were evaluated. Compared to Mn3O4, Mn3O4@PDA showed a significantly higher rate of ROS elimination (Figure 9B). The antioxidant effect of Mn3O4@PDA significantly reduced the ROS concentration in MSCs (Figure 9C). In addition, in vivo studies have demonstrated that Mn3O4@PDA nanozymes possess reliable cardiac protective effects, evidenced by reduced infarct size and improved recovery of cardiac function (Figure 9D-F) [140].
In a recent study, researchers developed an innovative self-sustaining antioxidant strategy to treat MI using self-sustaining selenium-embedded NPs (SSSe NPs) (Figure 9G). These SSSe NPs exhibited potent scavenging effects on •O2-, •OH, and H2O2 (Figure 9H-J). They efficiently entered CMs under H2O2 stimulation, colocalizing with mitochondria, and subsequently scavenged excess mitochondrial ROS (Figure 9K). This mitochondrial stabilization by SSSe NPs significantly reduced oxidative stress-induced apoptosis in vivo (Figure 9L). This strategy not only offers a promising treatment option for MI but also provides inspiration for other ischemic diseases [141].
To summarize, the use of inorganic nanomedicines, while promising, must consider the potential for long-term toxicity due to metal accumulation in tissues. Developing biodegradable or bioresorbable variants of these nanomaterials could mitigate these concerns. Furthermore, exploring the combination of inorganic nanozymes with organic polymers or natural carriers might offer a balance between high catalytic activity and enhanced biocompatibility. Researchers should also investigate the use of these nanozymes in conjunction with advanced imaging techniques to monitor real-time treatment efficacy and dynamically adjust therapeutic strategies.
(A) TEM images of CVNRs. (B) The SOD-like activity of CVNRs. (C) Flow cytometric analysis of ROS levels in HCMECs. (D) Immunofluorescence of VE-cadherin. (E) TUNEL assay. Adapted with permission from [138], copyright 2024 Elsevier. (F) Schematic illustration of the function of CuCe NPs. (G) ROS scavenging effect of Ce NPs and CuCe NPs. (H) Representative fluorescence images of ROS staining. (I) Expression and quantification of immunomodulatory cytokines analyzed with mouse cytokine array. Adapted with permission from [139], copyright 2023 John Wiley and Sons.
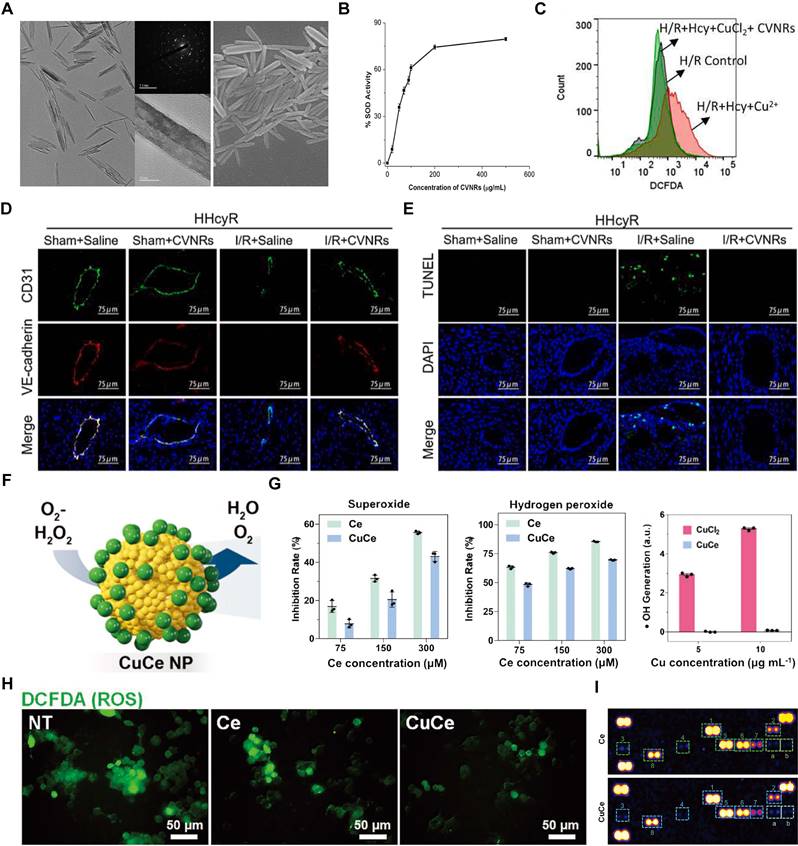
(A) Diagram showing the preparation of Mn3O4@PDA-MSCs and their use in in vivo MRI tracking and enhancement of the microenvironment for improved MI therapy. (B) ROS scavenging effect of Mn3O4 and Mn3O4@PDA-MSCs. (C) Representative fluorescent images of ROS levels in MSCs. (D) Representative images of Masson's trichrome staining. (E-F) Ejection fraction (EF%) and fractional shortening (FS%). Adapted with permission from [140], copyright 2024 John Wiley and Sons. (G) TEM image of SSSe NPs. (H-J) ROS scavenging effect of SSSe NPs. (K) MitoSOX staining. (L) Representative fluorescence images of TUNEL staining. Adapted with permission from [141], copyright 2022 John Wiley and Sons.
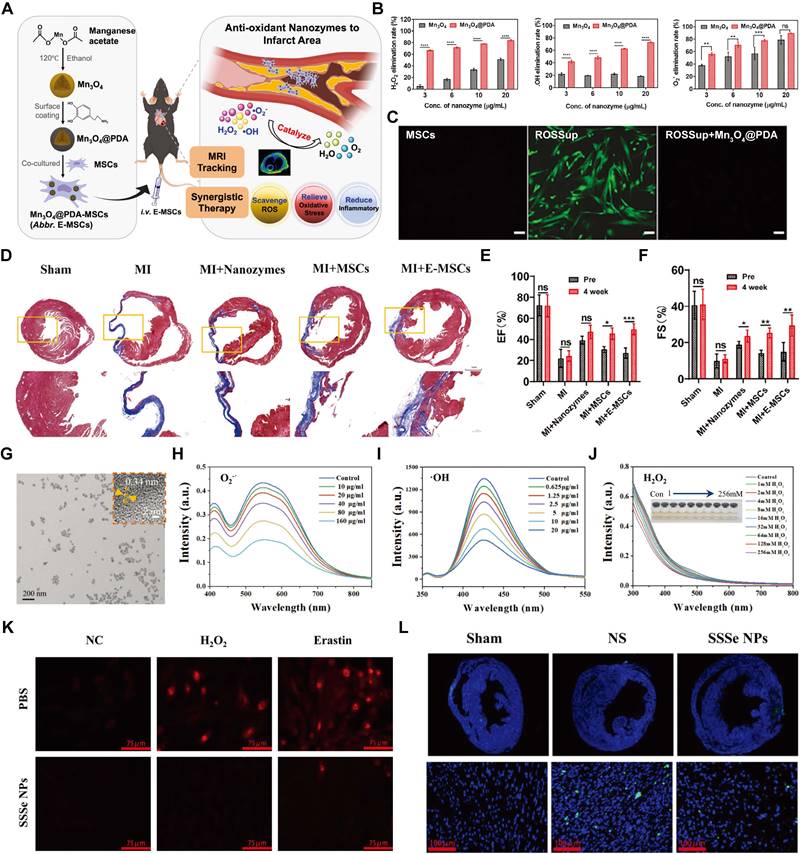
6.5. Gas-supplying nanomedicines
Gas therapy, including the use of therapeutic gases such as O2, NO, hydrogen sulfide (H2S), and H2, has emerged as a novel approach for treating various cardiovascular diseases [142]. However, the clinical application of gas therapy faces significant challenges due to the inherent difficulties in controlling the delivery, dosage, and stability of these gaseous molecules. Nanocarriers offer a transformative solution by addressing these limitations and enhancing the therapeutic efficacy of gas therapy.
O2 therapy has been extensively investigated for enhancing cell survival in the ischemic myocardium by elevating O2 levels in infarcted tissues and blood [143]. Exogenous O2 sustains the viability and metabolism of cardiac cells during acute ischemia, with the potential to mitigate infarct size and decrease the likelihood of lethal arrhythmias. Hemoglobin, a tetrameric protein comprising two α- and two β-polypeptide chains, each containing an iron-containing heme group capable of binding a single O2, has been utilized as an O2 carrier. Li et al. demonstrated that polymerized placenta hemoglobin (PolyPHb) safeguarded isolated hearts from I/RI by alleviating of NO-mediated myocardial apoptosis and the restoration of nitroso-redox balance [144]. Compared to PolyHb, PEG-conjugated hemoglobin (PEG-Hb) possesses a larger molecular radius, making it less prone to extravasation or the induction of hypertension. Additionally, surface conjugation with PEG reduces immunogenicity and increases plasma viscosity, rendering it a more optimized O2 carrier. The carbon monoxide form of PEGylated hemoglobin has been shown to reduce infarct size when administered either before left anterior descending coronary artery (LAD) occlusion or during reperfusion [142].
Although artificial O2 carriers utilizing hemoglobin have demonstrated certain preclinical advantages, these systems fail to achieve sustained O2 release, a crucial factor for cell survival before the establishment of angiogenesis. To address the limitations in O2 delivery for treating IHD, Guan et al. developed O2-generating microspheres capable of gradual O2 release. These NPs were engineered with a degradable polymer shell and a core composed of a polyvinylpyrrolidone (PVP)/H2O2 complex (PCNP/O2) (Figure 10A-B). Under conditions simulating infarcted cardiac tissue with 1% O2, the NPs exhibited sustained O2 release for 4 weeks (Figure 10C), with the O2 concentration reaching approximately 12% within 24 hours. PCNP/O2 NPs were retained in the infarcted heart for up to 28 days (Figure 10D). These results indicate that PCNP/O2 NPs have prolonged retention in ischemic heart tissue and provide sustained O2 delivery to the surrounding environment. After 4 weeks of treatment with PCNP/O2 NPs, the infarct size was reduced compared to the MI and PCNP groups (Figure 10E). Additionally, the PCNP/O2 group exhibited a significantly higher left ventricular ejection fraction (EF%) and fractional shortening (FS%) (Figure 10F-G). Moreover, the released O2 did not induce oxidative stress in the infarcted myocardium (Figure 10H-I) [52].
NO has been demonstrated in numerous experimental studies to modulate IHD [145, 146]. Administration of NOS inhibitors has been reported to exacerbate myocardial necrosis, supporting the concept that endogenous NO confers protection against MI/RI [147]. Abundant evidence suggests that supplemental exogenous NO is efficacious against MI/RI through multifaceted mechanisms, including the regulation of vasomotor tone and angiogenesis, reduction of myocardial ROS production, and inhibition of apoptosis, among others [148]. A platelet membrane-coated NP (B-P@PLT) was developed, featuring a polymeric core loaded with BNN6, an ultrasound-responsive NO donor, designed for the targeted treatment of MI/RI. B-P@PLTs can release NO during ultrasound irradiation, safeguarding CMs both in vitro and in vivo by reducing ROS and promoting vascular regeneration [149].
Additionally, Wang et al. developed a novel GSH-activated, water-dispersible, slow, and controllable H2S release system (DATS-MSN) utilizing diallyl trisulfide (DATS) and MSNs. DATS-MSNs exhibit a significantly slower process of H2S generation, thereby demonstrating superior cardioprotective effects in MI/R models by enhancing antioxidant enzyme activities [150]. Hydrogen gas (H2), a well-known molecule, has also been proposed for its potential in preventing and treating organ dysfunction induced by MI/RI. Zheng et al. developed an ultrasound-visible H2 delivery system by encapsulating H2 inside microbubbles (H2-MBs). This system enables a significant increase in the concentration of H2 in a unit volume under normal temperature and pressure conditions. H2-MBs can be visually tracked using ultrasound imaging systems and can effectively release therapeutic gas. In vivo results demonstrated that this approach markedly attenuated CM apoptosis and reduced myocardial inflammation and oxidative damage in MI/RI rats [151].
In summary, for gas-supplying nanomedicines, future research could explore the use of smart responsive materials that modulate gas release in response to specific physiological signals such as pH, temperature, or enzyme activity, thereby enhancing the precision of therapy. Additionally, combining gas therapy with other therapeutic agents, such as angiogenic factors or anti-apoptotic drugs, could create a synergistic effect, improving the overall outcome. Evaluating these systems in larger animal models and early-phase clinical trials will be critical for understanding their safety, efficacy, and potential for human application.
6.6. Other antioxidant nanomedicines
Cyclosporine A (CsA) is a cyclic polypeptide consisting of 11 amino acids [152]. It inhibits the excessive opening of the mPTP by binding to cyclophilin D on the inner mitochondrial membrane, thereby suppressing the release of ROS from mitochondria and CM apoptosis [153]. However, CsA exhibits poor solubility in water, and its natural distribution in vivo lacks myocardial targeting. To address this issue, CsA was encapsulated with Poly (5,5-dimethyl-4,6-dithio-propylene glycol azelate) (PTK) to form CsA@PTK, which was further enveloped with platelet membranes to create a Treg biomimetic NP (CsA@PPTK). Experimental results indicated that CsA@PPTK actively accumulated in the ischemic myocardium of MI/RI mice and effectively scavenged ROS. The construction of these biomimetic NPs has a significant therapeutic effect on MI/RI and holds great potential for application in MI/RI treatment [154].
(A) Schematic illustration of the design of PCNP/O2. (B) TEM images of NP/O2 and PCNP/O2. (C) Oxygen release kinetics of PCNP/O2 (D) Fluorescent images of the infarcted regions of the myocardium. (E) Representative images of Masson's trichrome staining. (F-G) Cardiac functional measurement including EF% and FS%. (H-I) Representative images and quantification of dihydroethidium staining. Adapted with permission from [52], copyright 2022 John Wiley and Sons.
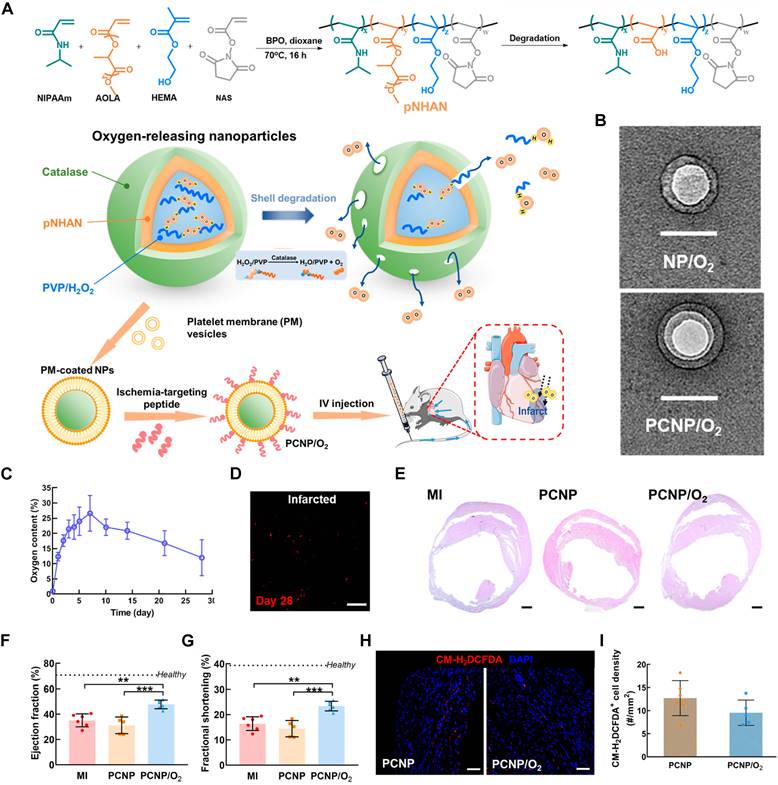
ONO-1301, a novel drug, is a synthetic prostacyclin IP receptor agonist with atypical prostanoid structures [155]. This unique feature enhances the biological and chemical stability of the compound, leading to prolonged prostacyclin activity in vivo. Studies have demonstrated that ONO-1301 binds to IP receptors on endothelial cells, vascular smooth muscle cells, or fibroblasts, triggering the release of protective cytokines. Encapsulation of ONO-1301 into PEGylated liposomes prolongs its circulation time in the bloodstream and enhances its accumulation within cardiac tissue. Results showed that intravenously injected ONO-1301-containing NPs (ONO-1301NPs) selectively accumulate in the injured myocardium of rats. Consequently, rats injected with ONO-1301NPs exhibited a smaller infarct size, better-preserved capillary networks, and improved MBF [156]. Additionally, a novel H2O2-responsive antioxidant copolyoxalate, composed of hydroxybenzyl alcohol (HBA) (HPOX) and vanillyl alcohol (VA) (PVAX), has been developed for antioxidant therapy in MI/RI. PVAX and HPOX were synthesized using naturally occurring compounds with intrinsic antioxidant and anti-inflammatory properties, VA and HBA, respectively. These antioxidant polymeric prodrug NPs can be rapidly activated by H2O2 at the site of ROS generation, mitigating I/R-induced injuries through antioxidant, anti-inflammatory, and antiapoptotic mechanisms [157].
Moreover, maintenance intracellular oxidative stress necessitates the involvement of multiple signaling molecules. Modulating cellular signaling pathways through small interfering RNA (siRNA) offers a method for intervening in oxidative stress. Researchers have proposed a novel approach using platelet membrane-camouflaged PLGA NPs (PMVs@PLGA complexes) for the systemic delivery of miRNA inhibitors. By targeting the Nrf2 regulatory pathway, these NPs can potentially enhance cardiac resilience against MI/RI [51]. Ji et al. encapsulated microRNA-146a (miR-146a) into milk exosomes (MEs) to create MEs-miR-146a, which can be administered both orally and intravenously. MEs-miR-146a exerts significant antiapoptotic and anti-inflammatory effects by inhibiting the IRAK1/TRAF6/NF-κB signaling pathway [158]. For future research, combining traditional antioxidant therapies with cutting-edge molecular technologies, such as CRISPR/Cas9 for gene editing or RNA interference, could offer novel therapeutic avenues. Exploring the potential of integrating diagnostic capabilities into these therapeutic platforms could enable real-time monitoring of disease progression and treatment efficacy, leading to more personalized and adaptive therapeutic strategies. Furthermore, considering the scalability of these advanced nanomedicines and their integration into existing clinical frameworks will be crucial for their successful translation into clinical practice.
7. Conclusion and future outlook
This article thoroughly reviews advancements in nanomedicines for addressing IHD. Understanding the pathological mechanisms of IHD is essential for the strategic selection of therapeutics. This review progresses by evaluating antioxidant mechanisms and drug molecules through both in vitro and in vivo studies. Attention is then given to the advent of nanocarriers that improve drug delivery, focusing particularly on their role in enhancing antioxidant nanomedicines for treating IHD. These nanocarriers overcome traditional drug limitations by increasing drug localization at the target site, reducing the overall dosage, and minimizing side effects, thereby markedly enhancing treatment efficacy and safety. This review concludes with a systematic summary of delivery technologies for various therapeutic agents aimed at IHD. Our review uniquely narrows down to antioxidant nanomedicines, providing an in-depth exploration of their design, targeted delivery mechanisms, and multifunctional nanomaterials. By specifically focusing on MI and MI/RI, we offer detailed discussions of their pathophysiology and targeted treatment strategies. We highlight cutting-edge nanocarrier systems, including innovative gas therapy-based nanocarriers, and provide clear categorization of antioxidant nanomedicines, enhancing logical flow and reader comprehension. Additionally, we integrate interdisciplinary insights from materials science, nanotechnology, and cardiology, presenting a holistic view and underscoring the collaborative efforts required to advance this field.
Despite significant strides in the development of cardioprotective drugs and cellular therapies for IHD [78, 85, 159], the efficacy of these interventions remains largely limited to preclinical studies. ROS have been identified as primary contributors to cellular damage under both hypoxic and reoxygenation conditions [6, 25], yet effective interventions remain elusive. Our comprehensive analysis addresses several critical factors: initially, there are considerable differences between commonly used animal models in preclinical studies and the human physiological context. Many studies utilize young, healthy animals, which can skew results due to their superior regenerative capabilities compared to the typically older, multimorbid human cardiac patients observed in clinical environments. Additionally, human research is plagued by numerous confounding variables, such as diet, sex, ethnicity, and psychological factors, which significantly complicate the interpretation of results. Moreover, the intricate homeostatic mechanisms in humans necessitate a multifaceted approach to therapy, targeting multiple pathways rather than a single molecular target. Finally, the current clinical use of these drugs is limited by their narrow antioxidant scope and inherent toxicity, which restricts their feasible dosage and thus their observable therapeutic impacts.
Can nanomedicines represent a significant advance over traditional pharmaceuticals in therapeutic efficacy? Theoretically, engineered carriers offer promising improvements in drug stability and targeted delivery. These nanocarriers, categorized by their carrier materials, exhibit unique properties that cater to the diverse requirements of therapeutic agents. PNPs, such as those made from PLGA and PEG, have shown remarkable capabilities in stabilizing and prolonging the circulation time of enzymatic antioxidants like SOD and CAT [113]. By encapsulating these enzymes, PNPs protect them from degradation, ensuring a sustained therapeutic effect in mitigating oxidative stress. Similarly, liposomes have emerged as versatile carriers for various antioxidants, such as curcumin and RSV [108], by enhancing their solubility and bioavailability. The amphiphilic nature of liposomes facilitates the incorporation of hydrophobic small molecules, providing a controlled-release profile and targeted delivery to ischemic myocardial tissues.
In the realm of inorganic nanomedicines, MOFs and Ce NPs have gained attention for their intrinsic catalytic activities that mimic natural antioxidant enzymes. MOFs, with their high surface area and tunable porosity, effectively deliver SOD-mimetic and CAT-mimetic agents, offering a robust defense against ROS [112]. Ce NPs, known for their redox cycling between Ce3+ and Ce4+, exhibit multi-enzyme activities, scavenging a wide range of ROS and thus protecting cardiac tissues from oxidative damage [139]. Moreover, GGNs, which release therapeutic gases like H2S and NO, have shown promise in modulating oxidative stress and inflammatory responses. These nanocarriers ensure the controlled and sustained release of gases, targeting the ischemic myocardium and enhancing the therapeutic outcomes [150].
The development of MSNs and NLCs has further advanced the delivery of small molecule drugs. MSNs, with their large surface area and customizable pore sizes, enable high drug loading and controlled release of antioxidants like que and BN [62]. NLCs, combining solid and liquid lipids, offer improved drug encapsulation efficiency and stability, prolonging the retention time of therapeutic agents in the bloodstream [108]. These innovative nanocarrier systems not only enhance the pharmacokinetic profiles of antioxidant therapies but also improve their targeting capabilities, thereby maximizing their efficacy in treating IHD. Therefore, these advanced nanotechnologies undoubtedly have significant effects and broad prospects for improving antioxidant drugs.
However, the field of nanomedicine faces numerous unresolved challenges in both research and practical applications. For instance, the introduction of biomaterials may induce unknown biological toxicity and risks [160]. Additionally, achieving specific functionalities often requires complex modifications, which introduce additional exogenous substances into the organism and potentially increase the physiological burden. Furthermore, the biological processes of many biomaterials within the body remain poorly understood, presenting significant challenges to their safe and effective use. Despite enhancements in drug performance, current scientific techniques fall short of achieving optimal therapeutic effects, highlighting the gap between current capabilities and ideal outcomes. Ultimately, while nanomedicines have facilitated incremental improvements in certain areas, they have not fundamentally changed the paradigm of disease treatment research. To achieve substantial progress, a deeper understanding of the complex composition and regulatory mechanisms of biological systems is essential.
Nanomedicines primarily act as carriers for antioxidant drugs, enhancing their delivery and efficacy. However, it is important to recognize that certain nanocarriers themselves possess inherent antioxidant properties and contribute directly to therapeutic effects. For instance, nanozymes like Ce oxide NPs exhibit intrinsic antioxidant activities, neutralizing ROS independently of any encapsulated drugs [161]. Additionally, nanocarriers such as PDA NPs also exhibit intrinsic antioxidant properties, offering a dual function as both carriers and active agents in mitigating oxidative stress [106]. These examples underscore the dual role of specific nanocarriers in antioxidant therapy, functioning both as carriers and active therapeutic agents. This dual functionality enhances the therapeutic potential of nanomedicines, offering a more comprehensive approach to treating IHD.
What should be the focus of our future research efforts? Initially, enhancing our understanding of the pathophysiology of diseases is crucial, as it allows for the identification of truly effective intervention targets that could lead to substantial breakthroughs. The challenges in treating IHD extend beyond oxidative stress to include autophagy and apoptosis dysregulation, endothelial dysfunction, ER stress, ischemia-induced angiogenesis, metabolic dysregulation, and fibrosis, all of which form the basis for current design strategies of nanomedicines [162]. These factors highlight the complexity of IHD and the necessity for comprehensive approaches that address multiple aspects of the disease. Although this review focuses specifically on the challenges associated with oxidative stress, it is essential to consider the broader array of issues in future research to develop more effective therapeutic strategies. By tackling these multifaceted challenges, we can advance the field of nanomedicine and improve outcomes for patients suffering from IHD.
Furthermore, the development of novel biomaterials needs to be advanced, prioritizing materials that can be effective without complex modifications. This will simplify their application in therapeutic interventions. Finally, a comprehensive understanding of the pathways, interference factors, and metabolic processes of biomaterials within the body is essential for their successful clinical translation.
Abbreviations
IHD: Ischemic heart disease
MI: Myocardial infarction
ROS: Reactive oxygen species
Mel: Melatonin
HF: Heart failure
WHO: World Health Organization
PCI: Percutaneous coronary intervention
MI/RI: Myocardial ischemia/reperfusion injury
EVs: Extracellular vesicles
ETC: Electron transport chain
hNVs: Hybrid nanovesicles
NOX: NADPH oxidases
PKC: Protein kinase C
NF-κB: Nuclear factor-kappa B
eNOS: Nitric oxide synthase
DAMPs: Damage-associated molecular patterns
NO: Nitric oxide
•O2-: Superoxide anions
•OH: Hydroxyl radicals
H2O2: Hydrogen peroxide
GPx: Glutathione peroxidase
CM: Cardiomyocyte
PRRs: Pattern recognition receptors
TLRs: Toll-like receptors
NLRs: Nucleotide-binding oligomerization domain-like receptors
MAPKs: Mitogen-activated protein kinase
AP-1: Activator protein-1
TCA: Tricarboxylic acid
HMGB-1: High mobility group box-1
HSPs: Heat shock proteins
TNF-α: Tumor necrosis factor-alpha
IL-1β: Interleukin 1-beta
IL-6: Interleukin-6
IL-18: Interleukin-18
SR: Sarcoplasmic reticulum
SERCA: Sarcoplasmic/endoplasmic reticulum Ca2+-ATPase
PMCA: Plasma membrane Ca²⁺-ATPase
SOD: Superoxide dismutase
GSH: Glutathione
CAT: Catalase
PDA: Polydopamine
NAs: Nucleic acids
GGNs: Gas-generating nanoplatforms
CoQ10: Coenzyme Q10
NSMs: Natural small molecule drugs
HAT: Hydrogen atom transfer
BHA: Butylated hydroxyanisole
BHT: Butylated hydroxytoluene
TBHQ: Tertiary butylhydroquinone
PG: Propyl gallate
PNPs: Polymeric NPs
PEG: Polyethylene glycol
PLA: Poly (lactic acid)
PLGA: Poly (lactic-co-glycolic acid)
MSCs: Mesenchymal stem cells
RBCs: Red blood cells
hEPs: Human embryonic stem cell-derived epicardial cells
MRI: Magnetic resonance imaging
CT: Computed tomography
PET: Positron emission tomography
SPIONs: Superparamagnetic iron oxide NPs
USPIOs: Ultrasmall superparamagnetic iron oxide NPs
Gd-CDs: Gadolinium-doped carbon dots
CAD: Coronary artery disease
MBF: Myocardial blood flow
MFR: Myocardial flow reserve
ICG: Indocyanine green
Mn: Manganese
MOF: Metal-organic frameworks
1,8-DHN: 1,8-dihydroxynaphthalene
ADSCs: Adipose-derived stem cells
LA: Alpha-lipoic acid
MSNs: Mesoporous silica NPs
PUE: Puerarin
RSV: Resveratrol
Que: Quercetin
BN: Baicalin
AUC: Area under the curve
Sch B: Schisandrin B
SLNs: Solid lipid NPs
MMP: Matrix metalloproteinase
PNS: Panax notoginsenoside
IMTP: Ischemic myocardial-targeted peptide
Rg3: Ginsenoside Rg3
PPS: Poly (propylene sulfide)
Ce: Cerium
CVNRs: Ce vanadate nanorods
H2S: Hydrogen sulfide
HCMECs: Human cardiac microvascular endothelial cells
PolyPHb: Polymerized placenta hemoglobin
LAD: Left anterior descending coronary artery
PVP: Polyvinylpyrrolidone
DATS: Diallyl trisulfide
mPTP: Mitochondrial permeability transition pore
EF%: Ejection fraction
FS%: Fractional shortening
H2: Hydrogen gas
CsA: Cyclosporine A
PTK: Poly (5,5-dimethyl-4,6-dithio-propylene glycol azelate)
HBA: Hydroxybenzyl alcohol
VA: Vanillyl alcohol
SiRNA: Small interfering RNA
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 82170271 to DL.S.). The Henan Key Research and Development Projects (Grant Nos. 241111311600 to DL.S.). We gratefully acknowledge the use of BioRender (https://biorender.com) for providing high-quality illustration tools, which significantly contributed to the professional and clear visualizations in this paper.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Berry C, Corcoran D, Hennigan B, Watkins S, Layland J, Oldroyd KG. Fractional flow reserve-guided management in stable coronary disease and acute myocardial infarction: recent developments. Eur Heart J. 2015;36:3155-64
2. Collaborators GBDCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736-88
3. Shi H, Huang Z, Xu T, Sun A, Ge J. New diagnostic and therapeutic strategies for myocardial infarction via nanomaterials. EBioMedicine. 2022;78:103968
4. Xiao H, Zhang M, Wu H, Wu J, Hu X, Pei X. et al. CIRKIL exacerbates cardiac ischemia/reperfusion injury by interacting with Ku70. Circ Res. 2022;130:e3-e17
5. Zhai M, Li B, Duan W, Jing L, Zhang B, Zhang M. et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res. 2017;63:e12419
6. Bugger H, Pfeil K. Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165768
7. Wang L, Ma Q. Clinical benefits and pharmacology of scutellarin: A comprehensive review. Pharmacol Ther. 2018;190:105-27
8. Mubagwa K, Flameng W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc Res. 2001;52:25-39
9. Ma W, Guo W, Shang F, Li Y, Li W, Liu J. et al. Bakuchiol alleviates hyperglycemia-induced diabetic cardiomyopathy by reducing myocardial oxidative stress via activating the SIRT1/Nrf2 signaling pathway. Oxid Med Cell Longev. 2020;2020:3732718
10. Zhang Y, Wang Y, Xu J, Tian F, Hu S, Chen Y. et al. Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J Pineal Res. 2019;66:e12542
11. Zhang Z, Dalan R, Hu Z, Wang J-W, Chew NWS, Poh K-K. et al. Reactive oxygen species scavenging nanomedicine for the treatment of ischemic heart disease. Advanced Matererials. 2022;34:e2202169
12. Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS. et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16:71
13. Jo DH, Kim JH, Lee TG, Kim JH. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine. 2015;11:1603-11
14. Zhao D, Huang X, Zhang Z, Ding J, Cui YC, Chen X. Engineered nanomedicines for tumor vasculature blockade therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13:e1691
15. Lakshmi BA, Kim S. Current and emerging applications of nanostructured metal-organic frameworks in cancer-targeted theranostics. Mater Sci Eng C. 2019;105:110091
16. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481-8
17. Bruning RS, Sturek M. Benefits of exercise training on coronary blood flow in coronary artery disease patients. Prog Cardiovasc Dis. 2015;57:443-53
18. Joshi PH, Nasir K. Discordance between risk factors and coronary artery calcium: Implications for guiding treatment strategies in primary prevention settings. Prog Cardiovasc Dis. 2015;58:10-8
19. Vogel B, Claessen BE, Arnold SV, Chan D, Cohen DJ, Giannitsis E. et al. ST-segment elevation myocardial infarction. Nat Rev Dis Primers. 2019;5:39
20. Wendelboe AM, Raskob GE. Global burden of thrombosis epidemiologic aspects. Circ Res. 2016;118:1340-7
21. PenaDuque MA, RomeroIbarra JL, GaxiolaMacias MBA, AriasSanchez EA. Coronary atherosclerosis and interventional cardiology. Arch Med Res. 2015;46:372-8
22. Charnock JS. Lipids and Cardiac-Arrhythmia. Progress in Lipid Research. 1994;33:355-85
23. Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852-66
24. Ai W, Bae S, Ke Q, Su S, Li R, Chen Y. et al. Bilirubin nanoparticles protect against cardiac ischemia/reperfusion injury in mice. J Am Heart Assoc. 2021;10:e021212
25. D'Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Bio. 2007;8:813-24
26. Jang S, Lewis TS, Powers C, Khuchua Z, Baines CP, Wipf P. et al. Elucidating mitochondrial electron transport chain supercomplexes in the heart during ischemia-reperfusion. Antioxid Redox Signal. 2017;27:57-69
27. Zhao H, Zhang R, Yan X, Fan K. Superoxide dismutase nanozymes: an emerging star for anti-oxidation. J Mater Chem B. 2021;9:6939-57
28. Bokare AD, Choi W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J Hazard Mater. 2014;275:121-35
29. Lambeth JD, Krause KH, Clark RA. NOX enzymes as novel targets for drug development. Semin Immunopathol. 2008;30:339-63
30. Brandes RP, Weissmann N, Schröder K. Redox-mediated signal transduction by cardiovascular Nox NADPH oxidases. J Mol Cell Cardiol. 2014;73:70-9
31. Das M, Devi KP, Belwal T, Devkota HP, Tewari D, Sahebnasagh A. et al. Harnessing polyphenol power by targeting eNOS for vascular diseases. Crit Rev Food Sci Nutr. 2023;63:2093-118
32. Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119:91-112
33. Silvis MJM, Kaffka Genaamd Dengler SE, Odille CA, Mishra M, van der Kaaij NP, Doevendans PA. et al. Damage-Associated Molecular Patterns in Myocardial Infarction and Heart Transplantation: The Road to Translational Success. Front Immunol. 2020;11:599511
34. Fang Y, Hu J. Toll-like receptor and its roles in myocardial ischemic/reperfusion injury. Med Sci Monit. 2011;17:RA100-9
35. Li B, Zhang Q, Du W, Wu J, Cheng J, Zhang Y. et al. Reshaping cardiac microenvironments by macrophage-derived extracellular vesicles-coated Pd@ CeO2 heterostructures for myocardial ischemia/reperfusion injury therapy. Mater Today. 2023;65:47-61
36. Chen J, Yang J, Liu R, Qiao C, Lu Z, Shi Y. et al. Dual-targeting theranostic system with mimicking apoptosis to promote myocardial infarction repair via modulation of macrophages. Theranostics. 2017;7:4149
37. Wang K, Liu CY, Zhou LY, Wang JX, Wang M, Zhao B. et al. APF lncRNA regulates autophagy and myocardial infarction by targeting miR-188-3p. Nat Commun. 2015;6:6779
38. Hu D, Li R, Li Y, Wang M, Wang L, Wang S. et al. Inflammation-Targeted Nanomedicines Alleviate Oxidative Stress and Reprogram Macrophages Polarization for Myocardial Infarction Treatment. Adv Sci (Weinh). 2024;11:2308910
39. Xiang Q, Yi X, Zhu X, Wei X, Jiang D. Regulated cell death in myocardial ischemia-reperfusion injury. Trends Endocrinol Metab. 2023;35:219-34
40. Christman JW, Blackwell TS, Juurlink BH. Redox regulation of nuclear factor kappa B: therapeutic potential for attenuating inflammatory responses. Brain Pathol. 2000;10:153-62
41. Guaricci AI, Bulzis G, Pontone G, Scicchitano P, Carbonara R, Rabbat M. et al. Current interpretation of myocardial stunning. Trends Cardiovasc Med. 2018;28:263-71
42. Zhao R, Jiang S, Zhang L, Yu Z. Mitochondrial electron transport chain, ROS generation and uncoupling. Int J Mol Med. 2019;44:3-15
43. Neginskaya MA, Pavlov EV, Sheu SS. Electrophysiological properties of the mitochondrial permeability transition pores: Channel diversity and disease implication. Biochim Biophys Acta Bioenerg. 2021;1862:148357
44. Zaidi A. Plasma membrane Ca2+-ATPases: Targets of oxidative stress in brain aging and neurodegeneration. World J Biol Chem. 2010;1:271
45. Blevins HM, Xu Y, Biby S, Zhang S. The NLRP3 inflammasome pathway: a review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front Aging Neurosci. 2022;14:879021
46. Vangheluwe P, Raeymaekers L, Dode L, Wuytack F. Modulating sarco (endo) plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium. 2005;38:291-302
47. Forgione MA, Cap A, Liao R, Moldovan NI, Eberhardt RT, Lim CC. et al. Heterozygous cellular glutathione peroxidase deficiency in the mouse: abnormalities in vascular and cardiac function and structure. Circulation. 2002;106:1154-8
48. Shen Y, Wang X, Shen X, Wang Y, Wang S, Zhang Y. et al. Geniposide possesses the protective effect on myocardial injury by inhibiting oxidative stress and ferroptosis via activation of the Grsf1/GPx4 axis. Front Pharmacol. 2022;13:879870
49. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043-6
50. Islam MN, Rauf A, Fahad FI, Bin Emran T, Mitra S, Olatunde A. et al. Superoxide dismutase: an updated review on its health benefits and industrial applications. Crit Rev Food Sci Nutr. 2022;62:7282-300
51. Wang T, Zhou T, Xu M, Wang S, Wu A, Zhang M. et al. Platelet membrane-camouflaged nanoparticles carry microRNA inhibitor against myocardial ischaemia-reperfusion injury. J Nanobiotechnology. 2022;20:434
52. Guan Y, Niu H, Wen J, Dang Y, Zayed M, Guan J. Rescuing cardiac cells and improving cardiac function by targeted delivery of oxygen-releasing nanoparticles after or even before acute myocardial infarction. ACS Nano. 2022;16:19551-66
53. Verma DD, Hartner WC, Thakkar V, Levchenko TS, Torchilin VP. Protective effect of coenzyme Q10-loaded liposomes on the myocardium in rabbits with an acute experimental myocardial infarction. Pharm Res. 2007;24:2131-7
54. Yong W, Ma H, Na M, Gao T, Zhang Y, Hao L. et al. Roles of melatonin in the field of reproductive medicine. Biomed Pharmacother. 2021;144:112001
55. Mason SA, Trewin AJ, Parker L, Wadley GD. Antioxidant supplements and endurance exercise: current evidence and mechanistic insights. Redox Biol. 2020;35:101471
56. Behrendt D, Beltrame J, Hikiti H, Wainstein M, Kinlay S, Selwyn AP. et al. Impact of coronary endothelial function on the progression of cardiac transplant-associated arteriosclerosis: effect of anti-oxidant vitamins C and E. J Heart Lung Transplant. 2006;25:426-33
57. Caritá AC, Fonseca Santos B, Shultz JD, Michniak Kohn B, Chorilli M, Leonardi GR. Vitamin C: one compound, several uses. Advances for delivery, efficiency and stability. Nanomedicine. 2020;24:102117
58. Herbig AL, Renard CM. Factors that impact the stability of vitamin C at intermediate temperatures in a food matrix. Food Chem. 2017;220:444-51
59. Charlton NC, Mastyugin M, Török B, Török M. Structural features of small molecule antioxidants and strategic modifications to improve potential bioactivity. Molecules. 2023;28:1057
60. Cunha Neto F, Marton LT, de Marqui SV, Lima TA, Barbalho SM. Curcuminoids from Curcuma Longa: New adjuvants for the treatment of crohn's disease and ulcerative colitis? Crit Rev Food Sci Nutr. 2019;59:2136-43
61. Wang Y, Liu X, Chen J, Cao J, Li X, Sun C. Citrus flavonoids and their antioxidant evaluation. Crit Rev Food Sci Nutr. 2022;62:3833-54
62. Liu C, Yao L, Hu Y, Zhao B. Effect of quercetin-loaded mesoporous silica nanoparticles on myocardial ischemia-reperfusion injury in rats and its mechanism. Int J Nanomedicine. 2021;16:741-52
63. Lozano O, LazaroAlfaro A, SilvaPlatas C, OropezaAlmazan Y, TorresQuintanilla A, BernalRamirez J. et al. Nanoencapsulated quercetin improves cardioprotection during hypoxia-reoxygenation injury through preservation of mitochondrial function. Oxid Med Cell Longev. 2019;2019:7683051
64. Tao T, Liu M, Chen M, Luo Y, Wang C, Xu T. et al. Natural medicine in neuroprotection for ischemic stroke: Challenges and prospective. Pharmacol Ther. 2020;216:107695
65. Xu X, Liu A, Hu S, Ares I, Martínez-Larrañaga M-R, Wang X. et al. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chemistry. 2021;353:129488
66. Liu R, Mabury SA. Synthetic phenolic antioxidants: A review of environmental occurrence, fate, human exposure, and toxicity. Environ Sci Technol. 2020;54:11706-19
67. Zhang Z, Dalan R, Hu Z, Wang JW, Chew NW, Poh K. et al. Reactive oxygen species scavenging nanomedicine for the treatment of ischemic heart disease. Adv Mater. 2022;34:2202169
68. Zhao T, Wu W, Sui L, Huang Q, Nan Y, Liu J. et al. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact Mater. 2022;7:47-72
69. Qiu M, Singh A, Wang D, Qu J, Swihart M, Zhang H. et al. Biocompatible and biodegradable inorganic nanostructures for nanomedicine: Silicon and black phosphorus. Nano Today. 2019;25:135-55
70. Majumder J, Taratula O, Minko T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv Drug Deliv Rev. 2019;144:57-77
71. Lei W, Yang J, Wang J, Xiao Z, Zhou P, Zheng S. et al. Synergetic EGCG and coenzyme Q10 DSPC liposome nanoparticles protect against myocardial infarction. Biomater Sci. 2023;11:6862-70
72. Dvir T, Bauer M, Schroeder A, Tsui JH, Anderson DG, Langer R. et al. Nanoparticles targeting the infarcted heart. Nano Lett. 2011;11:4411-4
73. Shao M, Yang W, Han G. Protective effects on myocardial infarction model: delivery of schisandrin B using matrix metalloproteinase-sensitive peptide-modified, PEGylated lipid nanoparticles. Int J Nanomedicine. 2017;12:7121-30
74. Takahama H, Minamino T, Asanuma H, Fujita M, Asai T, Wakeno M. et al. Prolonged targeting of ischemic/reperfused myocardium by liposomal adenosine augments cardioprotection in rats. J Am Coll Cardiol. 2009;53:709-17
75. Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145-60
76. Qiu J, Cai G, Liu X, Ma D. αvβ3 integrin receptor specific peptide modified, salvianolic acid B and panax notoginsenoside loaded nanomedicine for the combination therapy of acute myocardial ischemia. Biomed Pharmacother. 2017;96:1418-26
77. Sun L, Hu Y, Mishra A, Sreeharsha N, Moktan JB, Kumar P. et al. Protective role of poly(lactic-co-glycolic) acid nanoparticle loaded with resveratrol against isoproterenol-induced myocardial infarction. BioFactors. 2020;46:421-31
78. Cheng Y, Liu D, Zhang C, Cui H, Liu M, Zhang B. et al. Mitochondria-targeted antioxidant delivery for precise treatment of myocardial ischemia-reperfusion injury through a multistage continuous targeted strategy. Nanomedicine. 2019;16:236-49
79. Zhang X, Sun Y, Yang R, Liu B, Liu Y, Yang J. et al. An injectable mitochondria-targeted nanodrug loaded-hydrogel for restoring mitochondrial function and hierarchically attenuating oxidative stress to reduce myocardial ischemia-reperfusion injury. Biomaterials. 2022;287:121656
80. Mao S, Wang L, Chen P, Lan Y, Guo R, Zhang M. Nanoparticle-mediated delivery of Tanshinone IIA reduces adverse cardiac remodeling following myocardial infarctions in a mice model: role of NF-κB pathway. Artif Cells Nanomed Biotechnol. 2018;46:707-16
81. de Paula Peres L, da Luz FAC, dos Anjos Pultz B, Brigido PC, de Araujo RA, Goulart LR. et al. Peptide vaccines in breast cancer: The immunological basis for clinical response. Biotechnol Adv. 2015;33:1868-77
82. Chou LY, Ming K, Chan WC. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40:233-45
83. Sun Y, Zhang P, Li Y, Hou Y, Yin C, Wang Z. et al. Light-activated gold-selenium core-shell nanocomposites with NIR-II photoacoustic imaging performances for heart-targeted repair. ACS Nano. 2022;16:18667-81
84. Li H, Zhu J, Xu Y, Mou F, Shan X, Wang Q. et al. Notoginsenoside R1-loaded mesoporous silica nanoparticles targeting the site of injury through inflammatory cells improves heart repair after myocardial infarction. Redox Biol. 2022;54:102384
85. Ahmad Shiekh P, Anwar Mohammed S, Gupta S, Das A, Meghwani H, Kumar Maulik S. et al. Oxygen releasing and antioxidant breathing cardiac patch delivering exosomes promotes heart repair after myocardial infarction. Chem Eng J. 2022;428:132490
86. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183-95
87. Song H, Chen X, Hao Y, Wang J, Xie Q, Wang X. Nanoengineering facilitating the target mission: targeted extracellular vesicles delivery systems design. J Nanobiotechnology. 2022;20:431
88. Xu M, Feng T, Liu B, Qiu F, Xu Y, Zhao Y. et al. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11:8926-44
89. Lai J, Pan Q, Chen G, Liu Y, Chen C, Pan Y. et al. Triple hybrid cellular nanovesicles promote cardiac repair after ischemic reperfusion. ACS Nano. 2024;18:4443-55
90. Surman M, Drozdz A, Stepien E, Przybylo M. Extracellular vesicles as drug delivery systems - methods of production and potential therapeutic applications. Curr Pharm Des. 2019;25:132-54
91. Mu L, Dong R, Guo B. Biomaterials-based cell therapy for myocardial tissue regeneration. Adv Healthc Mater. 2023;12:2202699
92. Li T, Dong H, Zhang C, Mo R. Cell-based drug delivery systems for biomedical applications. Nano Res. 2018;11:5240-57
93. Luo X, Jiang Y, Li Q, Yu X, Ma T, Cao H. et al. hESC-Derived Epicardial Cells Promote Repair of Infarcted Hearts in Mouse and Swine. Adv Sci (Weinh). 2023;10:2300470
94. Fang RH, Kroll AV, Gao W, Zhang L. Cell membrane coating nanotechnology. Adv Mater. 2018;30:1706759
95. Liu Y, Luo J, Chen X, Liu W, Chen T. Cell membrane coating technology: a promising strategy for biomedical applications. Nanomicro Lett. 2019;11:1-46
96. Yilmaz A, Dengler MA, van der Kuip H, Yildiz H, Rösch S, Klumpp S. et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur Heart J. 2013;34:462-75
97. Li Y, Li B, Wang X, Meng Y, Bai L, Zheng Y. Safe and efficient magnetic resonance imaging of acute myocardial infarction with gadolinium-doped carbon dots. Nanomedicine. 2020;15:2385-98
98. Zheng Y, Zhang H, Hu Y, Bai L, Xue J. MnO nanoparticles with potential application in magnetic resonance imaging and drug delivery for myocardial infarction. Int J Nanomedicine. 2018;13:6177-88
99. Assen Mv, Vonder M, Pelgrim G, Von Knebel Doeberitz P, Vliegenthart R. Computed tomography for myocardial characterization in ischemic heart disease: a state-of-the-art review. Eur Radiol Exp. 2020;4:1-13
100. Kee PH, Danila D. CT imaging of myocardial scar burden with CNA35-conjugated gold nanoparticles. Nanomedicine. 2018;14:1941-7
101. Choi H, Han JH, Lim SY, Lee I, Cho Y-S, Chun EJ. et al. Imaging of Myocardial Ischemia-Reperfusion Injury Using Sodium [18F] Fluoride Positron Emission Tomography/Computed Tomography in Rats and Humans. Mol Imaging. 2017;16:1536012117704767
102. Nammas W, Maaniitty T, Knuuti J, Saraste A. Cardiac perfusion by positron emission tomography. Clin Physiol Funct Imaging. 2021;41:385-400
103. Wei Z, Chen Z, Zhao Y, Fan F, Xiong W, Song S. et al. Mononuclear phagocyte system blockade using extracellular vesicles modified with CD47 on membrane surface for myocardial infarction reperfusion injury treatment. Biomaterials. 2021;275:121000
104. Yang L, Ren Y, Pan W, Yu Z, Tong L, Li N. et al. Fluorescent nanocomposite for visualizing cross-talk between microRNA-21 and hydrogen peroxide in ischemia-reperfusion injury in live cells and in vivo. Anal Chem. 2016;88:11886-91
105. Korolev DV, Shulmeyster GA, Istomina MS, Evreinova NV, Aleksandrov IV, Krasichkov AS. et al. Fluorescently Labeled Gadolinium Ferrate/Trigadolinium Pentairon (III) Oxide Nanoparticles: Synthesis, Characterization, In Vivo Biodistribution, and Application for Visualization of Myocardial Ischemia-Reperfusion Injury. Materials. 2022;15:3832
106. Zhang Y, Ren X, Wang Y, Chen D, Jiang L, Li X. et al. Targeting ferroptosis by polydopamine nanoparticles protects heart against ischemia/reperfusion injury. ACS Appl Mater Interfaces. 2021;13:53671-82
107. Seshadri G, Sy JC, Brown M, Dikalov S, Yang SC, Murthy N. et al. The delivery of superoxide dismutase encapsulated in polyketal microparticles to rat myocardium and protection from myocardial ischemia-reperfusion injury. Biomaterials. 2010;31:1372-9
108. Zhang S, Wang J, Pan J. Baicalin-loaded PEGylated lipid nanoparticles: characterization, pharmacokinetics, and protective effects on acute myocardial ischemia in rats. Drug Deliv. 2016;23:3696-703
109. Ornatowski W, Lu Q, Yegambaram M, Garcia AE, Zemskov EA, Maltepe E. et al. Complex interplay between autophagy and oxidative stress in the development of pulmonary disease. Redox Biol. 2020;36:101679
110. Venardos KM, Kaye DM. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: a review. Curr Med Chem. 2007;14:1539-49
111. Kitagawa S. Metal-organic frameworks (MOFs). Chem Soc Rev. 2014;43:5415-8
112. Guo J, Yang Z, Lu Y, Du C, Cao C, Wang B. et al. An antioxidant system through conjugating superoxide dismutase onto metal-organic framework for cardiac repair. Bioact Mater. 2022;10:56-67
113. Altshuler PJ, Schiazza AR, Luo L, Helmers MR, Chhay B, Han JJ. et al. Superoxide dismutase-loaded nanoparticles attenuate myocardial ischemia-reperfusion injury and protect against chronic adverse ventricular remodeling. Adv Ther (Weinh). 2021;4:2100036
114. Berke JD. What does dopamine mean? Nat Neurosci. 2018;21:787-93
115. Wu H, Wei M, Xu Y, Li Y, Zhai X, Su P. et al. PDA-based drug delivery nanosystems: a potential approach for glioma treatment. Int J Nanomedicine. 2022;17:3751
116. Bao X, Zhao J, Sun J, Hu M, Yang X. Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano. 2018;12:8882-92
117. Wei Y, Zhu M, Li S, Hong T, Guo X, Li Y. et al. Engineered biomimetic nanoplatform protects the myocardium against ischemia/reperfusion injury by inhibiting pyroptosis. ACS Appl Mater Interfaces. 2021;13:33756-66
118. Cheng W, Zeng X, Chen H, Li Z, Zeng W, Mei L. et al. Versatile polydopamine platforms: synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano. 2019;13:8537-65
119. Zhou X, McCallum NC, Hu Z, Cao W, Gnanasekaran K, Feng Y. et al. Artificial allomelanin nanoparticles. ACS Nano. 2019;13:10980-90
120. Mo X, Xiang H, Wei L, Xia L, Chen X, Chen Y. et al. Nature-inspired allomelanin nanomedicine alleviates cardiac ischemia/reperfusion injury via scavenging free radicals and ameliorating myocardial microenvironment. Nano Today. 2022;46:101589
121. Zhang Y, Yang N, Huang X, Zhu Y, Gao S, Liu Z. et al. Melatonin engineered adipose-derived biomimetic nanovesicles regulate mitochondrial functions and promote myocardial repair in myocardial infarction. Front Cardiovasc Med. 2022;9:789203
122. Noh Y, Kim K, Shim M, Choi S, Choi S, Ellisman M. et al. Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death Dis. 2013;4:e820-e
123. Kumar A, Kaur H, Devi P, Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol Ther. 2009;124:259-68
124. Rosenfeldt F, Hilton D, Pepe S, Krum H. Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. Biofactors. 2003;18:91-100
125. Li Y, Zhao Y, Yu W, Jiang S. Scavenging ability on ROS of alpha-lipoic acid (ALA). Food Chem. 2004;84:563-7
126. Gorąca A, HukKolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid-biological activity and therapeutic potential. Pharmacol Rep. 2011;63:849-58
127. Xie D, Zhong Q, Xu X, Li Y, Chen S, Li M. et al. Alpha lipoic acid-loaded electrospun fibrous patch films protect heart in acute myocardial infarction mice by inhibiting oxidative stress. Int J Pharm. 2023;632:122581
128. Zhang Y, Zhang Z, Wang R. Protective mechanisms of quercetin against myocardial ischemia reperfusion injury. Front Physiol. 2020;11:506938
129. Dong Z, Guo J, Xing X, Zhang X, Du Y, Lu Q. RGD modified and PEGylated lipid nanoparticles loaded with puerarin: formulation, characterization and protective effects on acute myocardial ischemia model. Biomed pharmacother. 2017;89:297-304
130. Zhang J, Han X, Li X, Luo Y, Zhao H, Yang M. et al. Core-shell hybrid liposomal vesicles loaded with panax notoginsenoside: preparation, characterization and protective effects on global cerebral ischemia/reperfusion injury and acute myocardial ischemia in rats. Int J Nanomed. 2012;7:4299-310
131. Chen M, Wang S, Chen Y, Shen H, Chen L, Ding L. et al. Precision cardiac targeting: empowering curcumin therapy through smart exosome-mediated drug delivery in myocardial infarction. Regen Biomater. 2024;11:rbad108
132. Li L, Wang Y, Guo R, Li S, Ni J, Gao S. et al. Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J Control Release. 2020;317:259-72
133. Huang H, Feng W, Chen Y, Shi J. Inorganic nanoparticles in clinical trials and translations. Nano Today. 2020;35:100972
134. Kang T, Kim YG, Kim D, Hyeon T. Inorganic nanoparticles with enzyme-mimetic activities for biomedical applications. Coord Chem Rev. 2020;403:213092
135. Zhang Y, Yu W, Zhang L, Li P. Nanozyme-based visual diagnosis and therapeutics for myocardial infarction: The application and strategy. J Adv Res. 2024 S2090-1232: 00162
136. Liang S, Tian X, Wang C. Nanozymes in the treatment of diseases caused by excessive reactive oxygen specie. J Inflamm Res. 2022;15:6307-28
137. Pansambal S, Oza R, Borgave S, Chauhan A, Bardapurkar P, Vyas S. et al. Bioengineered cerium oxide (CeO2) nanoparticles and their diverse applications: a review. Appl Nanosci. 2023;13:6067-92
138. Ding L, Zhang S, Li Y, Wu Y, Liu X, Xu D. et al. Superoxide dismutase mimetic nanozymes attenuate cardiac microvascular ischemia-reperfusion injury associated with hyperhomocysteinemia. Chem Eng J. 2024;486:150177
139. Im GB, Kim YG, Yoo TY, Kim YH, Kim K, Hyun J. et al. Ceria nanoparticles as copper chaperones that activate SOD1 for synergistic antioxidant therapy to treat ischemic vascular diseases. Adv Mater. 2023;35:2208989
140. Le W, Sun Z, Li T, Cao H, Yang C, Mei T. et al. Antioxidant nanozyme-engineered mesenchymal stem cells for In vivo MRI tracking and synergistic therapy of myocardial infarction. Adv Funct Mater. 2024;34:2314328
141. Sun Q, Ma H, Zhang J, You B, Gong X, Zhou X. et al. A self-sustaining antioxidant strategy for effective treatment of myocardial infarction. Adv Sci (Weinh). 2023;10:2204999
142. Ananthakrishnan R, Li Q, O'Shea KM, Quadri N, Wang L, Abuchowski A. et al. Carbon monoxide form of PEGylated hemoglobin protects myocardium against ischemia/reperfusion injury in diabetic and normal mice. Artif Cells Nanomed Biotechnol. 2013;41:428-36
143. Hofmann R, James SK, Jernberg T, Lindahl B, Erlinge D, Witt N. et al. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med. 2017;377:1240-9
144. Li T, Li J, Liu J, Zhang P, Wu W, Zhou R. et al. Polymerized placenta hemoglobin attenuates ischemia/reperfusion injury and restores the nitroso-redox balance in isolated rat heart. Free Radic Biol Med. 2009;46:397-405
145. Bice JS, Jones BR, Chamberlain GR, Baxter GF. Nitric oxide treatments as adjuncts to reperfusion in acute myocardial infarction: a systematic review of experimental and clinical studies. Basic Res Cardiol. 2016;111:23
146. Cohen MV, Yang X, Downey JM. Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res. 2006;70:231-9
147. Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674-99
148. Zhu D, Hou J, Qian M, Jin D, Hao T, Pan Y. et al. Nitrate-functionalized patch confers cardioprotection and improves heart repair after myocardial infarction via local nitric oxide delivery. Nat commun. 2021;12:4501
149. Xu L, Chen Y, Jin Q, Gao T, Deng C, Wang R. et al. A novel ultrasound-responsive biomimetic nanoparticle for targeted delivery and controlled release of nitric oxide to attenuate myocardial ischemia reperfusion injury. Small Struct. 2023;4:2300004
150. Sun X, Wang W, Dai J, Jin S, Huang J, Guo C. et al. A long-term and slow-releasing hydrogen sulfide donor protects against myocardial ischemia/reperfusion injury. Sci Rep. 2017;7:3541
151. He Y, Zhang B, Chen Y, Jin Q, Wu J, Yan F. et al. Image-guided hydrogen gas delivery for protection from myocardial ischemia-reperfusion injury via microbubbles. ACS Appl Mater Interfaces. 2017;9:21190-9
152. Gui Q, Jiang Z, Zhang L. Insights into the modulatory role of cyclosporine A and its research advances in acute inflammation. Int Immunopharmacol. 2021;93:107420
153. Hausenloy DJ, Ong SB, Yellon DM. The mitochondrial permeability transition pore as a target for preconditioning and postconditioning. Basic Res Cardiol. 2009;104:189-202
154. Li F, Liu D, Liu M, Ji Q, Zhang B, Mei Q. et al. Tregs biomimetic nanoparticle to reprogram inflammatory and redox microenvironment in infarct tissue to treat myocardial ischemia reperfusion injury in mice. J Nanobiotechnology. 2022;20:251
155. Nakamura K, Sata M, Iwata H, Sakai Y, Hirata Y, Kugiyama K. et al. A synthetic small molecule, ONO-1301, enhances endogenous growth factor expression and augments angiogenesis in the ischaemic heart. Clin Sci. 2007;112:607-16
156. Yajima S, Miyagawa S, Fukushima S, Sakai Y, Iseoka H, Harada A. et al. Prostacyclin analogue-loaded nanoparticles attenuate myocardial ischemia/reperfusion injury in rats. JACC Basic Transl Sci. 2019;4:318-31
157. Bae S, Park M, Kang C, Dilmen S, Kang TH, Kang DG. et al. Hydrogen Peroxide-Responsive Nanoparticle Reduces Myocardial Ischemia/Reperfusion Injury. J Am Heart Assoc. 2016;5:e003697
158. Meng W, Zhu J, Wang Y, Shao C, Li X, Lu P. et al. Targeting delivery of miR-146a via IMTP modified milk exosomes exerted cardioprotective effects by inhibiting NF-κB signaling pathway after myocardial ischemia-reperfusion injury. J Nanobiotechnology. 2024;22:382
159. Caldas M, Santos AC, Veiga F, Rebelo R, Reis RL, Correlo VM. Melanin nanoparticles as a promising tool for biomedical applications-a review. Acta Biomater. 2020;105:26-43
160. Lin Y, Ren J, Qu X. Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc Chem Res. 2014;47:1097-105
161. Wang L, Qiu S, Li X, Zhang Y, Huo M, Shi J. Myocardial-targeting tannic cerium nanocatalyst attenuates ischemia/reperfusion injury. Angew Chem Int Ed. 2023;62:e202305576
162. Wang X, Guo Z, Ding Z, Mehta JL. Inflammation, autophagy, and apoptosis after myocardial infarction. Journal of the American Heart Association. 2018;7:e008024
163. Shevtsov MA, Nikolaev BP, Ryzhov VA, Yakovleva LY, Dobrodumov AV, Marchenko YY. et al. Detection of experimental myocardium infarction in rats by MRI using heat shock protein 70 conjugated superparamagnetic iron oxide nanoparticle. Nanomedicine. 2016;12:611-21
164. Zhu K, Jiang D, Wang K, Zheng D, Zhu Z, Shao F. et al. Conductive nanocomposite hydrogel and mesenchymal stem cells for the treatment of myocardial infarction and non-invasive monitoring via PET/CT. J nanobiotechnology. 2022;20:211
165. Hao J, Lu A, Li X, Li Y. A convergent fabrication of silk fibroin nanoparticles on quercetin loaded metal-organic frameworks for promising nanocarrier of myocardial infraction. Heliyon. 2023;9:e20746
166. Liao X, Song X, Li J, Li L, Fan X, Qin Q. et al. An injectable co-assembled hydrogel blocks reactive oxygen species and inflammation cycle resisting myocardial ischemia-reperfusion injury. Acta Biomater. 2022;149:82-95
167. Kang J, Kim H, Mun D, Yun N, Joung B. Co-delivery of curcumin and miRNA-144-3p using heart-targeted extracellular vesicles enhances the therapeutic efficacy for myocardial infarction. J Control Release. 2021;331:62-73
168. Hardy N, Viola HM, Johnstone VP, Clemons TD, Cserne Szappanos H, Singh R. et al. Nanoparticle-mediated dual delivery of an antioxidant and a peptide against the L-Type Ca2+ channel enables simultaneous reduction of cardiac ischemia-reperfusion injury. ACS Nano. 2015;9:279-89
169. Tsujioka T, Sasaki D, Takeda A, Harashima H, Yamada Y. Resveratrol-encapsulated mitochondria-targeting liposome enhances mitochondrial respiratory capacity in myocardial cells. Int J Mol Sci. 2022;23:112
170. Zhou H, Shan Y, Tong F, Zhang Y, Tang J, Shen R. et al. Resveratrol nanoparticle complex: potential therapeutic applications in myocardial ischemia reperfusion injury. J Biomed Nanotechnol. 2020;16:382-9
171. Yang M, Han X, Qiao O. Effect of salvianolic acid B-loaded mesoporous silica nanoparticles on myocardial ischemia-reperfusion injury. Tradit Med Res. 2023;8:45
172. Xiang K, Wu H, Liu Y, Wang S, Li X, Yang B. et al. MOF-derived bimetallic nanozyme to catalyze ROS scavenging for protection of myocardial injury. Theranostics. 2023;13:2721
173. Li X, Ren X, Xie M, Zhu M, Zhang Y, Li T. et al. Biominerallized noble metal-based RuO2 nanozymes against myocardial ischemic/reperfusion injury. Adv Nanobiomed Res. 2023;3:2200144
174. Zhong Y, Yang Y, Xu Y, Qian B, Huang S, Long Q. et al. Design of a Zn-based nanozyme injectable multifunctional hydrogel with ROS scavenging activity for myocardial infarction therapy. Acta Biomater. 2024;177:62-76
175. Liu W, Zhao N, Yin Q, Zhao X, Guo K, Xian Y. et al. Injectable hydrogels encapsulating dual-functional Au@Pt core-shell nanoparticles regulate infarcted microenvironments and enhance the therapeutic efficacy of stem cells through antioxidant and electrical integration. ACS Nano. 2023;17:2053-66
176. Wang Z, Yang N, Hou Y, Li Y, Yin C, Yang E. et al. L-arginine-loaded gold nanocages ameliorate myocardial ischemia/reperfusion injury by promoting nitric oxide production and maintaining mitochondrial function. Adv Sci (Weinh). 2023;10:2302123
177. Chen S, Luo X, Sun Y, Jin W, He R. A novel metabolic reprogramming strategy for the treatment of targeting to heart injury-mediated macrophages. Int Immunopharmacol. 2023;122:110377
178. Liu X, Chen B, Chen J, Wang X, Dai X, Li Y. et al. A Cardiac-targeted nanozyme interrupts the inflammation-free radical cycle in myocardial infarction. Adv Mater. 2024;36:2308477
179. Zhang Y, Khalique A, Du X, Gao Z, Wu J, Zhang X. et al. Biomimetic design of mitochondria-targeted hybrid nanozymes as superoxide scavengers. Adv Mater. 2021;33:2006570
180. Ye T, Chen C, Wang D, Huang C, Yan Z, Chen Y. et al. Protective effects of Pt-N-C single-atom nanozymes against myocardial ischemia-reperfusion injury. Nat Commun. 2024;15:1682
181. Li N, Huang C, Zhang J, Zhang J, Huang J, Li S. et al. Chemotactic NO/H2S nanomotors realizing cardiac targeting of G-CSF against myocardial ischemia-reperfusion injury. ACS Nano. 2023;17:12573-93
182. Hao T, Qian M, Zhang Y, Liu Q, Midgley AC, Liu Y. et al. An injectable dual-function hydrogel protects against myocardial ischemia/reperfusion injury by modulating ROS/NO disequilibrium. Adv Sci (Weinh). 2022;9:2105408
183. Zhang Q, Wang L, Yin Y, Shen J, Xie J, Yuan J. Hydrogen sulfide releasing hydrogel for alleviating cardiac inflammation and protecting against myocardial ischemia-reperfusion injury. J Mater Chem B. 2022;10:5344-51
184. Chen G, Yang L, Zhong L, Kutty S, Wang Y, Cui K. et al. Delivery of hydrogen sulfide by ultrasound targeted microbubble destruction attenuates myocardial ischemia-reperfusion injury. Sci Rep. 2016;6:30643
185. Ikeda G, Matoba T, Nakano Y, Nagaoka K, Ishikita A, Nakano K. et al. Nanoparticle-mediated targeting of cyclosporine A enhances cardioprotection against ischemia-reperfusion injury through inhibition of mitochondrial permeability transition pore opening. Sci Rep. 2016;6:20467
186. Zhang Z, Chen Z, Yang L, Zhang J, Li Y, Li C. et al. Platelet membrane-encapsulated MSNs loaded with SS31 peptide alleviate myocardial ischemia-reperfusion injury. J Funct Biomater. 2022;13:181
187. Zhang Y, Qian P, Zhou H, Shen R, Hu B, Shen Y. et al. Pharmacological signatures of the exenatide nanoparticles complex against myocardial ischemia reperfusion injury. Kidney Blood Press Res. 2018;43:1273-84
188. Clemons TD, Viola HM, House MJ, Iyer KS, Hool LC. Examining efficacy of “tat-less” delivery of a peptide against the L-type calcium channel in cardiac ischemia-reperfusion injury. ACS Nano. 2013;7:2212-20
189. Zhang M, Zhu J, Qin X, Zhou M, Zhang X, Gao Y. et al. Cardioprotection of tetrahedral DNA nanostructures in myocardial ischemia-reperfusion injury. ACS Appl Mater Interfaces. 2019;11:30631-9
Author contact
![]() Corresponding authors: Dongjian Han, Address: No. 1 East Construction Road, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, China. Phone: +86 18838281942; Email: hdj02012859zzu.edu.cn (DJ. Han). Deliang Shen, Address: No. 1 East Construction Road, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, China. Phone: +86 18595871805; Email: dlshenedu.cn (DL. Shen).
Corresponding authors: Dongjian Han, Address: No. 1 East Construction Road, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, China. Phone: +86 18838281942; Email: hdj02012859zzu.edu.cn (DJ. Han). Deliang Shen, Address: No. 1 East Construction Road, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, China. Phone: +86 18595871805; Email: dlshenedu.cn (DL. Shen).
 Global reach, higher impact
Global reach, higher impact