13.3
Impact Factor
Theranostics 2024; 14(13):5235-5261. doi:10.7150/thno.99181 This issue Cite
Review
Processed microalgae: green gold for tissue regeneration and repair
1. College of Life Science, Mudanjiang Medical University, Mudanjiang, China.
2. Department of Neurology, the Affiliated Hongqi Hospital, Mudanjiang Medical University, Aimin District, Mudanjiang 157011, China.
3. Pathology Department of the Second Affiliated Hospital of Mudanjiang Medical College, Mudanjiang, China.
4. Department of Dermatology, Shanghai Ninth People's Hospital, Shanghai Jiaotong University, Shanghai, China.
5. Changhai Clinical Research Unit, Shanghai Changhai Hospital, Naval Medical University, Shanghai, China.
6. Shanghai Key Laboratory of Nautical Medicine and Translation of Drugs and Medical Devices, Shanghai 200433, China.
Received 2024-6-2; Accepted 2024-8-15; Published 2024-8-19
Abstract
As novel biomedical materials, microalgae have garnered significant interest because of their ability to generate photosynthetic oxygen, their antioxidant activity, and their favorable biocompatibility. Many studies have concentrated on the hypoxia-alleviating effects of microalgae within tumor microenvironments. However, recent findings indicate that microalgae can significantly increase the regeneration of various tissues and organs. To augment microalgae's therapeutic efficacy and mitigate the limitations imposed by immune clearance, it is essential to process microalgae through various processing strategies. This review examines common microalgal species in biomedical applications, such as Chlorella, Chlamydomonas reinhardtii, diatoms, and Spirulina. This review outlines diverse processing methods, including microalgae extracts, microalgae‒nanodrug composite delivery systems, surface modifications, and living microalgae‒loaded hydrogels. It also discusses the latest developments in tissue repair using processed microalgae for skin, gastrointestinal, bone, cardiovascular, lung, nerve, and oral tissues. Furthermore, future directions are presented, and research gaps for processed microalgae are identified. Collectively, these insights may inform the innovation of processed microalgae for various uses and offer guidance for ongoing research in tissue repair.
Keywords: microalgae, processing, tissue damage repair, drug delivery, wound healing
Introduction
In the realm of medical science, a pivotal area of investigation is tissue regeneration, which refers to the intricate process by which the body repairs and restores damaged local cells and tissues [1]. This process is essential for all living organisms because it maintains tissue integrity and biological function and prevents infections and diseases. The efficacy of tissue regeneration is highly dependent on microenvironmental conditions. As the “soil”, the tissue microenvironment regulates the function of parenchymal cells, which are the “seeds”. Therefore, maintaining the stability of the tissue microenvironment is vital for normal cell proliferation, differentiation, and metabolism. Abnormalities in the extracellular matrix, growth factors, chemokines, or other components in the tissue microenvironment may lead to cell damage [2]. Microdamage to local tissues can be alleviated through regulatory mechanisms in the human body. However, impaired tissue repair can lead to delayed or dysregulated wound healing. In such cases, traditional “3Rs” treatment (i.e., resection, repair, and replacement) fails to mimic the regenerative microenvironment. It may result in fibrosis or scarring, which can impair normal tissue function and lead to organ failure and death [2-4]. Owing to the drawbacks of traditional therapies, researchers have attempted to develop novel strategies that specifically target the tissue microenvironment to improve tissue regeneration and repair.
The use of microalgae as natural biomedical materials is gradually increasing in the field of tissue regeneration and repair. Microalgae are unicellular or multicellular photosynthetic autotrophic microorganisms widely distributed in seawater and freshwater [5]. They consist of lipids, proteins, carbohydrates, and various other components, and their size ranges from a few micrometres to a few hundred micrometres. Therefore, microalgae are small, simple microorganisms with a wide range of sources and low culture costs [6,7]. Recent studies have shown that microalgae can regulate microenvironmental conditions at the trauma site during tissue repair and promote wound healing through anti-inflammatory, antioxidative, and antibacterial effects [8-10]. In addition, microalgae are rich in various photosynthetic pigments, such as chlorophyll a and b and carotenoids. They can not only efficiently utilize light, carbon dioxide, and water to synthesize oxygen and carbohydrates but also emit fluorescence for imaging purposes. Chlorophyll, a natural fluorescent pigment, has an absorption peak at a wavelength of 640-660 nm and can emit red fluorescence when stimulated with a laser. Therefore, microalgae can be used in imaging-guided integrated diagnosis and treatment to monitor the development of lesions continuously and enhance therapeutic efficacy [6,11]. Owing to these advantages, microalgae hold great promise as natural biomedical materials in the field of tissue repair. In addition to their applications in the food, nutraceutical, and fuel industries, microalgae are widely used to promote the repair of various tissues, including skin, gastrointestinal, bone, cardiovascular, lung, nerve, and oral tissues. On the basis of in-depth microalgae research, the bioactivity, targeting ability, and functionality of microalgae can be enhanced via the use of microalgae extracts, microalgae-nanodrug composite drug delivery systems, surface modifications, and microalgae-loaded hydrogels. Improving these properties may enhance therapeutic efficacy and promote tissue repair and regeneration. In addition, it can address the limitations imposed on the therapeutic effects of microalgae by these factors, such as microalgae vitality and phagocytosis and clearance by immune cells.
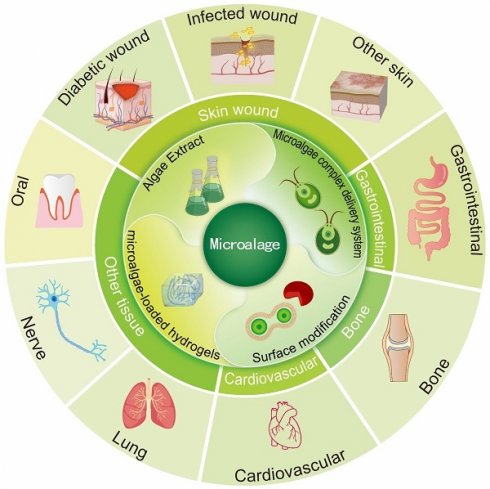
At present, a comprehensive review summarizing the applications of processed microalgae in the field of tissue repair is lacking. To address this knowledge gap, this review summarizes common processing methods used to process microalgae and discusses the advantages and disadvantages of different types of processed microalgae in tissue repair. In addition, recent research progress on microalgae and future research avenues are described, and the potential applications of processed microalgae in tissue repair are highlighted (Fig. 1).
Microalgae in the biomedical field are commonly processed through methods such as bioactive substance extraction, the creation of microalgae-based composite drug delivery systems, surface modification, and living microalgae-loaded hydrogels. These processed forms are utilized to promote regeneration and repair across a range of tissues, including skin, gastrointestinal, bone, cardiovascular, lung, nerve, and oral tissues. (Created with Adobe lilustrator.com).
Microalgae classification and major medical applications
Microalgae vary in terms of species and morphological characteristics. Approximately 800,000 species of microalgae are found worldwide [12]. However, only a few microalgae are used in biomedical applications. These microalgae can be classified as eukaryotic (mainly including Chlorella, C. reinhardtii, and diatoms) or prokaryotic (cyanobacteria, mainly Spirulina) on the basis of the presence or absence of chloroplasts, respectively [12].
Chlorella
Chlorella, a spherical unicellular green alga, has a strong cell wall and is rich in intracellular components such as lipids, proteins, polysaccharides, and chlorophylls. It possesses antimicrobial, antioxidant, hydrogen-producing, and oxygen-producing properties [13,14]. Chlorellin, derived from Chlorella extracts, has antimicrobial activity and can be used as an alternative to antibiotics in certain cases, preventing the development of drug resistance [15]. Chlorella has high antioxidant levels and can promote tissue regeneration by scavenging reactive oxygen species (ROS) [16]. In addition, it can convert solar energy to biohydrogen under anaerobic conditions, and this biohydrogen can be used as an antioxidant to reduce ROS levels, thereby alleviating oxidative stress and inflammation [17]. Moreover, Chlorella has the ability to produce photosynthetic oxygen. Chlorella can act as an efficient oxygen producer for treating hypoxia-related diseases. In one study, the amount of oxygen produced by an autotrophic light-activated green oxygenation system composed of calcium alginate-coated Chlorella pyrenoidosa was three times greater than that produced by inorganic oxygen production materials. Consequently, Chlorella can promote tissue repair by improving the hypoxic tissue microenvironment and serve as a promising therapeutic agent for hypoxia-related diseases [18]. Despite its benefits, the application of Chlorella in tissue repair faces certain challenges. The cell wall of Chlorella is notably robust, impeding efficient enzymatic breakdown in the human digestive tract and thus restricting nutrient absorption [19]. Additionally, the extraction methods for active substances from Chlorella vulgaris are diverse, resulting in substantial variability in the potency of the resulting products [20]. To fully leverage the therapeutic potential of Chlorella in tissue repair, additional research and technological progress are crucial.
Chlamydomonas reinhardtii
Chlamydomonas reinhardtii (C. reinhardtii), a eukaryotic unicellular green microalga, is mostly spherical or ovate. Because its cell wall is negatively charged, it can be loaded with positively charged drugs or materials through electrostatic adsorption [21]. The anterior end of C. reinhardtii has two equal-length flagella that can oscillate to swim directionally at a speed of 100 μm/s [22,23]. This active movement helps promote drug diffusion and penetration in the human body, thus improving drug delivery efficiency. These attributes make C. reinhardtii a promising candidate for drug delivery systems. Additionally, the complete sequencing of the C. reinhardtii triad of genomes—nuclear, chloroplast, and mitochondrial—has been achieved. Through genetic engineering, a microalga gene recombinant expression platform can be constructed, facilitating the tailored design of its functional capabilities [24-26]. For example, Jarquín-Cordero et al. developed a novel C. reinhardtii strain via a transgenic approach to produce the growth factor hVEGF-165, which promotes angiogenesis during wound healing [27]. Although the gene editing technology of C. reinhardtii is relatively mature, its application still faces challenges. First, poor and inconsistent expression of nuclear transgenes remains an obstacle for both fundamental and applied research endeavors [28]. Second, genetic manipulation has many disadvantages, such as strict codon usage, low transgene expression and gene silencing, and variable gene expression due to position effects [29].
Diatoms
Diatoms, tiny unicellular microalgae with lengths ranging from a few micrometres to tens of micrometres, have a porous cell wall composed of silica. This porous structure allows the encapsulation of drugs, making diatoms promising vehicles for drug delivery [30]. Coating the surface of diatoms with polydopamine (PDA) can prevent their degradation and removal in vivo and improve their viability [31]. In addition, a novel nanomaterial formed by combining diatoms and graphene oxide has been shown to release chemotherapeutic drugs under acidic conditions for a localized effect, which can regulate the rate of drug release on the basis of the pH of the intestine, suggesting a novel strategy for the treatment of intestinal diseases [32]. When diatoms die, their porous shells (i.e., silica) sink to the bottom of the water body. The accumulation of these shells eventually leads to the formation of diatomaceous earth, also referred to as biosilica [33,34]. Owing to its three-dimensional porous and hollow structure and silanol groups on its surface, diatomaceous earth can concentrate coagulation factors and promote coagulation through endogenous pathways, thereby accelerating hemostasis [34,35]. Compared with synthetic silica, diatomaceous earth has a larger microscopic size and is superhydrophilic and superhematotropic. Therefore, it can be used as a novel hemostatic material [36]. While natural diatoms offer numerous advantages, the microscopic size of biosilica somewhat limits its route of administration. In areas with thin blood vessels and restricted blood flow, such as the vitreous cavity of the eye, biosilica tends to form a buildup. In addition, although intravenous biosilica is excreted by the kidneys, silica particles can still be detected in the liver, lungs, and glomeruli [37].
Spirulina platensis
Spirulina platensis (SP), a typical spiral-shaped blue-green alga (200-500 μm in size), possesses anti-inflammatory and antioxidative properties [38]. Excessive accumulation of ROS disrupts DNA structure, oxidizes proteins and lipids, delays tissue regeneration, and causes cell and tissue death [39-41]. Therefore, the inhibition of ROS production or removal of excess ROS can attenuate inflammatory responses, reduce oxidative damage, and promote wound healing and tissue regeneration. C-Phycocyanin, which is extracted from SP, has demonstrated potent antioxidative, anti-inflammatory, and neuroprotective properties. It is a natural antioxidant that neutralizes ROS and reduces ROS-induced cell damage [42]. Additionally, C-phycocyanin inhibits inflammatory responses and alleviates tissue damage, making it a promising drug or functional food for treating inflammatory diseases, oxidative stress, and neurodegenerative diseases [43-46]. In addition, the surface and morphological features of SPs are very favorable for drug delivery. First, the negatively charged surface of SPs can be loaded with positively charged small-molecule drugs through electrostatic adsorption [47]; second, the presence of water channels and connecting pore structures in the cell membrane facilitate the smooth entry of small-molecule drugs into the membrane [47,48]; third, the spiral structure is conducive to embedding into the villi of the small intestine, thereby becoming a drug carrier with a relatively high loading rate, which enhances the intestinal drug delivery efficiency and bioavailability [47,48]; fourth, the intrinsic fluorescence of chlorophyll within Spirulina enables noninvasive in vivo tracking without the need for additional fluorescent markers[48]. To fully harness the potential of Spirulina as a pharmaceutical carrier, several pivotal technical challenges must be addressed, including the enhancement of targeting precision, ensuring the stability of the carrier, guaranteeing its safety, and achieving controlled degradation under physiological conditions.
Methods for processing microalgae
The biological functions of natural microalgae may be limited by immune clearance and the ability of light to penetrate human tissues. Processing approaches can improve the delivery efficiency and targeting ability of microalgae, thus enhancing their efficacy in tissue repair (Table 1). Currently, microalgae extracts, microalgae-nanodrug composite delivery systems, surface modifications, and living microalgae-loaded hydrogels are commonly used in biomedical applications. The combined use of different processing methods can further promote the multifunctionality of microalgae, providing a valuable reference for the future design and development of processed microalgae.
Processing methods for microalgae
| Engineering strategy | Classification | Processing options | Advantage | Disadvantage | Operational difficulties | Applicable scenarios | Refs. |
|---|---|---|---|---|---|---|---|
| Microalgae extract | Lipids extraction | Conventional organic solvents extraction | Simple operation, low cost | Long extraction time; high toxicity of some organic solvents (e.g. chloroform and methanol) | Extraction efficiency is often influenced by operating conditions such as temperature, pressure, microalgal state (concentration, dry/wet state, growth stage), and scale | Drug carriers; nutraceuticals and biomedical applications | [161] |
| Ionic liquids extraction | Low toxicity, flexible synthesis, nonvolatile, thermally and chemically stable | High cost, only small-scale preparation | |||||
| Supercritical fluid extraction (e.g. supercritical C02 extraction) | Nontoxic, nonhazardous, low cost, mild critical pressure, and low critical temperature | The low polarity of carbon dioxide makes it harder to penetrate the polar cell membranes and hard cell walls of microalgae. | |||||
| Accelerated solvent extraction | Low solvent consumption, fast and efficient | requirements, high energy consumption | |||||
| Pulsed electric fields | Simple operation | High energy consumption | |||||
| Ultrasound-assisted extraction | Simple operation, energy-saving, and high efficiency | The intensity and time of ultrasound need to be controlled to avoid negative effects | |||||
| Microwave-assisted extraction | Fast and efficient | The polarity of the solvent significantly affects the extraction efficiency and selectivity | |||||
| Enzyme-assisted extraction | mild operating conditions, energy-saving. | High cost; it is necessary to optimize the conditions to get the highest extraction rate | |||||
| Protein extraction | 1. Ball milling method | Simple equipment, gentle and efficient | High energy consumption | Effective cleavage of cell walls, protein recovery, issues such as product purity, energy consumption, and nondestruction of active substances during the extraction process | Nutraceuticals and biomedical applications | [162-168] | |
| 2. Ultrasound-assisted extraction techniques | Simple operation, energy-saving, high efficiency | Small-scale preparation, thermal effects affect protein quality | |||||
| 3. High-pressure homogenization | High efficiency | High energy consumption | |||||
| 4. Pulsed electric field treatment | Gentle, efficient, high protein purity | Low extraction rate, high energy consumption | |||||
| 5. Aqueous enzymatic method | Mild operating conditions, specialization | Long extraction time, high cost, and difficulty in selecting specific enzymes for each microalgae; | |||||
| 6. Ionic liquids extraction | Low volatility, good stability | High cost | |||||
| 7. Repeated freezing method | Simple operation, high stability of proteins | Long extraction times, small-scale preparation | |||||
| 8. Supercritical fluid extraction | Short extraction time, high efficiency | Low extraction rate, high cost, low extraction rate | |||||
| 9. Microfluidization | High efficiency | High cost, low extraction rate, high equipment requirements | |||||
| Polysaccharides extraction | Hot-water extraction methods | Simple operation; low cost | Long extraction time, low extraction rate and purity | Extraction efficiency is often influenced by operating conditions such as extracting times, time, PH, etc. | Nutraceuticals and biomedical applications | [169,170] | |
| Alkali extraction methods | High extraction efficiency | High cost, | |||||
| Ultrasound-assisted extraction techniques | Simple operation, energy-saving, and high efficiency | Easy to damage polysaccharide structure | |||||
| Microwave-assisted extraction | Simple operation, energy-saving | Easy to damage polysaccharide structure | |||||
| Repeated freezing method | Simple operation | Low-efficiency, small-scale preparation | |||||
| Enzyme-assisted solvent extraction | Simple operation, high efficiency, mild operating conditions, protecting polysaccharide bioactivity | Unstable enzyme activity | |||||
| Pigments extraction | The traditional methods: solid‒liquid extraction, liquid‒liquid extraction, and Soxhlet extraction; The innovative methods: Supercritical fluid extraction, Pressurized liquid extraction, Microwave-assisted extraction, Ultrasound-assisted extraction, and Enzyme assisted extraction, etc. | Innovative approach: less time-consuming, less solvent consumption, large amounts of purified extraction fractions | Traditional methods: lower efficiency, longer time, toxicity, higher solvent consumption | Organic solvents pose environmental and safety risks; enzyme-assisted extraction requires control of specificity and stability | Nutraceuticals and biomedical applications | [171] | |
| Microalgae-based composite drug delivery systems | Covalent bonds | 1. Amidation reactions 2. Click Chemistry | Improved stability of microalgae drug delivery systems | Irreversible; affects microalgal activity | Requiring specific reaction conditions, complex processing | Drug delivery | [77,172] |
| Noncovalent Bonds | Electrostatic adsorption | Reversible, allowing dynamic and controlled assembly and disassembly of biological systems | Less stable than covalent bonds | Vulnerable to factors such as pH, temperature, and ionic strength, etc. | Drug delivery | [77,173] | |
| Surface modification | Cell membrane encapsulation | 1. Stirring method 2. Ultrasonication 3. Mechanical extrusion | Prolonged circulation of microalgae in vivo; targeting; preservation of cell membrane surface antigens | Immune cell membranes encapsulating microalgae may induce or exacerbate inflammation through interactions with the body's immune system. | Stirring method has low fusion efficiency; the fusion efficiency frequency of the ultrasonication method is limited by time and vibration amplitude; the Mechanical extrusion method is unsuitable for large-scale production. | Targeted therapy, drug delivery, in vivo fluorescence imaging | [6,84,174] |
| Magnetic nanoparticles modification | 1. Electrostatic adsorption 2. Internalization and uptake of metal cations | Easy to assemble; highly precise motion capability under remote external magnetic field control; noninvasive dual-modality imaging using autofluorescence and MRI signals | A higher concentration of magnetic nanoparticles inhibits microalgae growth; Loaded with magnetic nanoparticles; Inhibits the deposition of therapeutic agents on the microalgae's surface. | The efficiency of random interactions between microalgae and magnetic nanoparticles is suboptimal; The internalization of metal cations is constrained by factors such as their concentration, the duration of incubation, and the density of microalgal cells. | Targeted therapy, drug delivery, in vivo fluorescence imaging, Photothermal inhibition | [22,90,175,176] | |
| Living microalgae-loaded hydrogels | Hydrogel-encapsulated | A multifunctional bioactive hydrogel system is formed by loading active microalgae into a hydrogel matrix. | Good biocompatible; providing an aqueous environment for embedded microalgae, maintaining microalgae activity | Restriction of cell growth | Precise control of reaction conditions such as pH, concentration, temperature, etc. | Drug delivery, wound dressings, applying to gastrointestinal injuries due to heavy metal poisoning | [172,177,178] |
| 3D bioprinting technology | Utilizing microalgae in conjunction with hydrogel substances (e.g., sodium alginate, carrageenan, etc.) to develop bioinks, allows for the 3D printing of biological materials with sophisticated three-dimensional configurations | Good biocompatible, providing a three-dimensional culture environment, maintaining microalgae activity, customizable shape | The printing process reduces the survival of microalgae; complex processing | Stabilization of bioinks; control of printing parameters; maintenance of microalgal activity | Drug delivery; tissue-engineered scaffolds | [179,180] |
Microalgae extracts
Microalgae extracts contain many bioactive substances, such as lipids, carbohydrates, and proteins [49]. First, microalgae are rich in polyunsaturated fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are necessary for nerve cells, and essential fatty acids, such as linolenic acid and linoleic acid [50]. In 1944, microalgae fatty acids (chlorellin) were first extracted and shown to inhibit the growth of gram-positive and gram-negative bacteria [15]. Since then, the antimicrobial effects of microalgal fatty acids have gradually attracted widespread attention. Researchers have attempted to assess whether microalgal fatty acids can be used as alternatives to classical antibiotics or whether their synergism with antibiotics can enhance their antibacterial effects. Among the major compounds extracted and purified from three microalgae (Isochrysis galbana, Scenedesmus sp. NT8c, and Chlorella sp. FN1) by Alsenani et al., linoleic acid, oleic acid, DHA, and EPA have been shown to inhibit the growth of gram-positive bacteria [51]. Furthermore, microalgal lipids can be used for drug delivery. On the basis of their class and molecular stacking parameters, conventional lipids can be classified as lamellar or nonlamellar [52]. Lamellar lipids, which have a self-assembled bilayer structure with one or more vesicular morphologies, are characterized by their ease of design, modeling, and similarity to biological membrane structures [52]. Compared with lamellar liposomes, nonlamellar structures, such as cuboidal structures, have more complex configurations, higher surface area-to-volume ratios, and enhanced cargo encapsulation and sustained delivery [53-55]. Clemente et al. extracted F&M-M24 lipids from Nannochloropsis oceanica and self-assembled them into cubes and liposomes for loading and delivery of natural antioxidants such as curcumin, α-tocopherol, and piperine [55]. Second, in microalgae, carbohydrates are usually present in the cytoplasm and chloroplasts as cellulose, monosaccharides, polysaccharides, and starch, such as glucose, galactose, mannose, and arabinose [56]. Microalgal polysaccharides exhibit antibacterial, antiviral, and antioxidative activities [57]. The antimicrobial mechanism of microalgal polysaccharides is very complex. In addition to inhibiting bacterial adhesion, disrupting biofilms, and increasing cell membrane permeability, polysaccharides can act on bacterial ribosomes, affecting protein biosynthesis, metabolism, and nutrient uptake [58,59]. Compared with unmodified polysaccharides, polysaccharide derivatives obtained through chemical modification (such as sulfation, carboxymethylation, and acetylation) of natural polysaccharides possess superior antimicrobial properties [60]. In antiviral terms, the sulfated polysaccharides p-KG03 [61], Naviculan [62], and Ca-SP [63] extracted from microalgae have been shown to possess antiviral properties. These polysaccharides exert antiviral effects by interfering with the viral life cycle [64]. However, these effects strongly rely on the specific characteristics of the polysaccharide, such as the degree and type of sulfation, molecular weight, monosaccharide composition, three-dimensional structure, and hydrogen bond formation [64,65]. In terms of antioxidative effects, the Spirulina polysaccharide complex (SPC) scavenges superoxide by upregulating superoxide dismutase 2 (SOD2) in aging fibroblasts, thereby restoring mitochondrial function and promoting collagen production to rejuvenate fibroblasts [66]. In addition, microalgae are rich in proteins and other nutrients, including pigments, vitamins, and minerals such as carotenoids, chlorophyll, phycocyanin, vitamin A, vitamin E, folic acid, iron, and calcium. These components are used to treat various diseases, including cancer, cardiac disease, and immunodeficiency syndromes [67]. For example, microalgae-derived β-carotene, a precursor of vitamin A, acts as an antioxidant [68]. Phycocyanin, a microalgal-derived pigment protein, is a potential antioxidant, fluorescent molecular probe, and photosensitizer [69,70].
Microalgae‒nanodrug composite delivery systems
In physiological media, natural drugs exhibit poor solubility, low bioavailability, and short effective action times, significantly weakening their therapeutic effects [71]. Microalgae‒nanodrug composite delivery systems constructed from living microalgae provide an innovative and effective platform for drug delivery. Active microalgae, one of the most promising carriers for drug delivery, have negatively charged cell walls. Therefore, they can noncovalently bind to positively charged small-molecule drugs through electrostatic adsorption [72]. In particular, the diatom cell wall, which is composed of silica and other components, can carry drugs and exhibit some resistance to strongly acidic reagents. Therefore, diatoms are not easily disrupted or digested [73]. The surface and morphological characteristics of microalgae are conducive to improving the bioavailability of drugs. For example, small-molecule drugs can penetrate the membrane of Spirulina through water channels and connect pores (14-16 nm) on its surface, increasing the drug loading capacity [74]. The unique flagellar structure of C. reinhardtii can generate strong propulsive forces in simulated intestinal fluids with sustained motility, facilitating the widespread distribution and retention of drugs in the small intestine [75]. In one study, C. reinhardtii (flagellated) and static microalgae (nonflagellated) were encapsulated in protective capsules with internal hydrophobic and external enteric coatings, respectively, and their distributions in the gastrointestinal tract were assessed via fluorescence labeling after 5 h of oral administration. Compared with static microalgae, C. reinhardtii is more widely distributed in intestinal tissues. These findings indicate that the flagellar motility of C. reinhardtii plays an important role in enhancing its interaction with the intestinal wall and its ability to stay in the intestine [75]. In contrast to spherical Chlorella vulgaris, helical Spirulina can be captured between the villi of the small intestine, increasing the retention time and bioavailability of drugs in the small intestine [47]. Owing to their abovementioned properties and natural phototropism, microalgae have emerged as an important platform for constructing microalga‒nanodrug composite delivery systems. For example, the cell wall of C. reinhardtii is composed of glycoproteins enriched with 4-hydroxyproline (4-HP) residues. Weibel et al. achieved the binding of 4-HP glycopeptide-modified polystyrene particles to the surface of microalgae through noncovalent interactions. The particles are guided to reach the target site by the intrinsic phototropism of the microalgae and release polystyrene beads through photochemical reactions [76]. The surface of microalgae is rich in chemical groups that serve as binding sites. These surfaces are commonly adorn with natural functional groups, such as carboxyl (-COOH) and amine (-NH2) groups, which originate from proteins or sugar components. By harnessing these inherent characteristics, the construction of stable covalent bonds can be efficiently achieved through methods such as amidation and click chemistry [77]. For example, Zhang et al. utilized the amino group on the surface of microalgae to form an ester bond with N-hydroxysuccinimide (NHS) and reacted the drug with dibenzocyclooctyne (DBCO) via click chemistry to achieve covalent bonding between microalgae and the drug carrier [78]. Overall, live algae are promising drug carriers that have attracted substantial attention in recent years owing to their unique structural properties, biocompatibility, self-propulsion, and phototropism.
Surface modification
The widespread application of surface modification has provided novel opportunities in the biomedical field. Surface modification is crucial for the use of microalgae in the field of tissue regeneration and repair. Materials currently used to modify microalgae can be classified into cell membranes and magnetic nanomaterials. Cell membrane encapsulation is frequently used in studies. NPs are “camouflaged” into a cellular form by coating them with natural cell membranes through stirring, sonication, or mechanical extrusion. In this form, the proteins and polysaccharides present on the surface of the cell membrane can be used to protect the nanoparticles from immune clearance without affecting their inherent properties [79]. Therefore, coating the surface of microalgae with a cell membrane can improve their circulation, targeting, and accumulation in vivo and effectively enhance their biocompatibility, thus improving their efficacy in tissue repair. On the basis of different requirements, cell membranes from different sources, such as erythrocytes, platelets, macrophages, and tumor cell membranes, perform different physiological functions. In particular, erythrocyte membranes can increase the blood circulation time and stability of microalgae [80]. Platelet membranes can inhibit immune clearance [81] and actively target tissues or cells, such as tumor cells and damaged vascular tissues [82,83]. Macrophage membranes can help evade immune clearance and actively target inflammatory and tumor tissues [84,85]. Tumor cell membranes can specifically recognize and target corresponding tumors [86-88]. Qiao et al. modified the surface of Chlorella vulgaris with a red blood cell membrane (RBCM) to design a novel biomaterial named RBCM-Algae via the stirring method. In vivo imaging revealed that compared with unmodified microalgae, RBCM-Algae effectively evades immune clearance and is efficiently delivered to tumor tissues owing to the presence of natural markers on the surface of RBCMs (e.g., CD47, sialic acid, and polysaccharides) [6]. Additionally, ultrasonic treatment and mechanical extrusion are frequently employed techniques. Cheng et al. developed chlorella encapsulated within macrophage membranes for drug delivery applications. This was achieved by blending the cell membranes with chlorella, subjecting the mixture to ultrasonication for 2 minutes, and subsequently extruding it 40 times via a liposome extruder [84].
In addition, magnetic nanomaterials can also be used to modify microalgae. In recent years, many researchers have modified microalgae with magnetic nanoparticles by electrostatic adsorption or internalization and uptake of metal cations to achieve directional movement of microalgae under the action of an external magnetic field. For example, Spirulina has been modified with Fe3O4 via the dip-coating technique to achieve directional movement to the target site under the influence of an external magnetic field [89]. Yasa et al. developed a biohybrid microswimmer by modifying C. reinhardtii with magnetic polystyrene particles (1 μm in diameter) through electrostatic adsorption for the targeted delivery of the particles under the influence of a magnetic field [22]. Although magnetic nanoparticles can be attached to the surface of microalgae, this approach hinders cell movement and impedes drug loading on the surface of microalgae. To address this shortcoming, Santomauro et al. incubated microalgae with a medium containing terbium ions (Tb3+) by the internalization and uptake of metal cations, using functional groups on the cell wall to adsorb the ions. Small amounts of Tb3+ were translocated to the cytoplasm through internalization. Subsequently, magnetized microalgae containing Tb3+ were obtained by washing the cells with a dilute HCl solution and dissolving ions adsorbed on the cell surface [90]. The inherent fluorescence properties of microalgae and the luminescent properties of Tb3+ can facilitate in vivo imaging. These findings suggest that surface modification of microalgae with cell membranes (e.g., erythrocyte, macrophage, and platelet membranes) or magnetic nanomaterials (e.g., Fe3O4, magnetic polystyrene particles, and terbium ions) can endow them with novel properties. Therefore, surface modification can help overcome the shortcomings of individual microalgae (e.g., immune clearance in vivo) and improve the targeting ability of processed microalgae in vivo for enhanced therapeutic effects.
Living microalgae-loaded hydrogels
Hydrogel is a type of general-purpose soft material. Hydrogels can be divided into natural polymer hydrogels and synthetic polymer hydrogels according to the type of synthetic material. The gelators in natural polymer hydrogels are mainly natural polymers, such as starch, cellulose, alginate, and collagen. In contrast, gelators in synthetic polymer gels are mainly synthetic polymers, such as polyacrylic acid and polyethylene glycol [91]. Currently, the majority of research endeavors have focused on the fabrication of living microalgae-loaded hydrogels through encapsulation within hydrogels and the utilization of 3D bioprinting techniques. These innovative approaches are predominantly leveraged in the realms of pharmaceutical delivery systems and tissue engineering. Gastrointestinal drug delivery systems constructed using living microalgae-loaded hydrogels can penetrate the gastrointestinal barrier. Ren et al. incubated insulin-loaded microalgae with sodium alginate and crosslinked them with calcium chloride to develop microalgae encapsulated with sodium alginate. This method preserves the inherent properties of microalgae and prolongs insulin retention in the intestine by preventing the rapid degradation of insulin in gastric acid [72]. Hydrogels can mimic the extracellular matrix (ECM) and keep microalgae alive. Therefore, they can be used to load living microalgae or microalgae extracts for subsequent application in tissue repair. In addition, hydrogels can prevent infection in the early stage of skin injury, inhibit bacterial growth at the wound site, improve the local microenvironment, and support cell migration and differentiation, thereby promoting whole-layer skin repair. Chen et al. designed a microalgal gel patch capable of generating dissolved oxygen, which can be delivered to wounds to alleviate both acute and chronic tissue hypoxia, significantly facilitating wound healing [92]. The rapid development of 3D bioprinting technology has revolutionized methods for processing microalgae, with 3D-printed hydrogels using activated microalgae as bioinks. Zhao et al. used 3D-printed filamentous protein hydrogels and Platymonas sp., a marine microalga, as bioinks. Experimental data have shown that hydrogels support microalgal proliferation for at least 4 weeks and maintain photosynthetic activity for more than 90 days [93]. In situ 3D printing enables the direct application of living microalgae-loaded hydrogel scaffolds to damaged tissue sites. Wang et al. achieved in situ printing of microalgae-loaded hydrogel scaffolds on skin wounds via microfluidics in combination with 3D printing. This approach improved the bridging of hydrogels with the surrounding tissues and effectively accelerated the healing of chronic wounds [94]. Living microalgae-loaded hydrogels can be developed into various structures according to specific requirements, such as microneedles, wound patches, and 3D scaffolds, offering the dual advantages of sustaining microalgae vitality and enabling functional modifications. The synergy between hydrogels and microalgae has significant therapeutic effects, including anti-inflammatory, antioxidative, and anti-infective effects and homeostasis regulation.
Applications of processed microalgae in tissue repair
Processed microalgae play pivotal roles in enhancing drug targeting and therapeutic effectiveness and preserving the viability of microalgae. Many studies have demonstrated that microalgae can substantially improve therapeutic outcomes across various applications, including skin, gastrointestinal, bone, cardiovascular, lung, oral, and neural tissue treatments. In this section, we delve into the applications, design, therapeutic efficacy, and limitations of microalgae, aiming to provide insights into the current state and future directions of processed microalgae research in tissue repair (Table 2).
Skin tissue
As the body's protective barrier, the skin protects against environmental damage. However, cutaneous wounds may lead to various local or systemic physiological and pathological changes, imposing a heavy burden on patients and society. Wound healing is a dynamic and continuous process involving multiple overlapping spatial and temporal phases, including hemostasis, inflammation, cell proliferation, and tissue reconstruction [95,96]. During this process, different types of cells and biomolecules interact with each other to promote wound healing and restore the barrier function of the skin. Successful completion of both the spatial and temporal phases is necessary for achieving complete wound healing and restoring skin function [97,98].
Diabetic wound healing
The complex microenvironment of diabetic wounds, which are typically chronic, poses a serious challenge to healing. High levels of blood glucose lead to the accumulation of advanced glycosylation end products (AGEs), which exacerbate oxidative stress, suppress the phagocytic function of macrophages, and increase the secretion of inflammatory factors.
Role of processed microalgae in the field of tissue regeneration and repair.
| Processing strategy | Microalgal species | Processed microalgae | Cellular or animal mode | Role and mechanism | Refs. |
|---|---|---|---|---|---|
| Microalgae extract | Red alga | κ-carrageenan | NIH 3T3 cells | Bionic skin ECM; promoting the adhesion, growth, survival, and proliferation of fibroblasts and upregulating genes related to cell adhesion and cytoskeletal matrix formation | [125] |
| Hematococcus pluvialis | Astaxanthin | Osteoporotic rats | Inhibition of osteoclast-mediated bone resorption | [136] | |
| Isochrysis zhanjiangensis Microalgae | Polypeptide | Human umbilical vein endothelial cell vascular injury model | PIZ antagonizes ACE in a noncompetitive binding manner and inhibits the Ang II-induced secretion and expression of vascular factors by blocking the NF-κB, Nrf2, MAPK, and Akt signaling pathways | [150] | |
| Porphyridium sp. | Polysaccharides | Human coronary artery endothelial cell inflammation model | Inhibition of NF-κB activation and TNF-α-induced oxidative stress in HCAECs | [181] | |
| C. reinhardtii | Channel rhodopsin-2 | Retinal degeneration mouse model; cochlear cells | Modulation of cation channel activity in nerve cells, modulation of neuronal and muscle cell activity, and improvement of visual or auditory perception | .[157,182] | |
| Spirulina | Spirulina protein | Full-thickness skin excision wound mouse | Modulation of the Akt, ERK, and TGF-β1 signaling pathways promotes skin wound repair in mice | [123] | |
| Spirulina | Spirulina extract | Dextran-sulfate-sodium induced ulcerative colitis in rats | Hydroalcoholic extracts of Spirulina attenuate dextran sulfate sodium-induced inflammation | [183] | |
| Microalgae-based composite drug delivery systems | C. reinhardtii | Microalgae--nanoparticle microbots | Acute pseudomonas aeruginosa pneumonia mouse | Improved efficiency of drug delivery to the lungs and uniform distribution throughout lung tissues | [78] |
| C. reinhardtii | C. reinhardtii loaded with Adriamycin | Oral administration to mice | Promoting drug distribution and retention in the gastrointestinal tract by using the efficient and long-lasting motility of C. reinhardtii and the protective ability of oral capsules | [75] | |
| C. reinhardtii | Chitosan-heparin nano complex coated microalgae | Chronic diabetic mice wound | Oxygen production via photosynthesis and anti-inflammatory effects; capable of penetrating moderately dense blood clots to reach deep wounds | [184] | |
| Diatoms | Diatom silica shells loaded with sodium alendronate | J774 cells; Bone marrow stem cells; Human SaOS-2 osteoblastic cell line | Increased drug delivery rates | [140] | |
| Spirulina | Magnetic spirulina composites constructed from ferrite nanoparticle-modified Spirulina | Infection mouse model | Magnetic properties, photothermal properties, and antimicrobial effects | [121] | |
| Spirulina | Spirulina platensis loaded with astaxanthin nanoparticles | Rat small intestinal epithelial cells; acute radiation enteritis mouse | Extension of median survival of mice with acute radiation enteritis to 29 d and amelioration of radiation-induced intestinal damage | [132] | |
| Spirulina | SP-mediated delivery of amifostine | Rat small intestinal epithelial cells; early intestinal radiation injury mouse; delayed intestinal radiation injury mouse; orthotopic colorectal cancer irradiated mouse | The SP@AMF drug delivery system can pass through the acidic gastric environment and distribute more evenly and widely in the intestinal tract, exerting comprehensive protective effects on the entire small intestine | [130] | |
| Surface modification | C. reinhardtii | Noncovalent bonding of magnetic polystyrene particles with C. reinhardtii | NIH 3T3 cells; HeLa cells; OVCAR-3 cells. | Realization of targeted therapy in the presence of an external magnetic field | [22] |
| C. reinhardtii | C. reinhardtii loaded with terbium ions (Tb3+) | Human breast cancer cells; mouse fibroblasts | Realization of directional movement under the influence of a magnetic field and in vivo fluorescence imaging | [90] | |
| Living microalgae-loaded hydrogels | Chlorella vulgaris | Algal gel patches prepared using Chlorella vulgaris and Bacillus licheniformis | Diabetic mouse wound | Sustained hydrogen production under anaerobic conditions; promotion of diabetic chronic wound healing in vivo by alleviating oxidative stress and inflammation | [17] |
| Chlorella vulgaris | Coencapsulation of Chlorella vulgaris and Weissella in calcium alginate hydrogels | Diabetic mouse wound | Alleviation of chronic inflammation and hypoxia by alternating NO and O2 production, reduction in the expression of pro-inflammatory cytokines, and improvement in neovascularization and tissue regeneration | [108] | |
| Chlorella vulgaris | Polyacrylamide-sodium alginate hydrogel loaded with Chlorella | Diabetic mouse wound | The sustained release of dissolved oxygen, augmenting cell proliferation, migration, and angiogenesis | [109] | |
| Chlorella vulgaris | Microneedles constructed using Chlorella and antimicrobial polyionic liquid | Infected diabetic mouse wound | The continuous production of oxygen by Chlorella via photosynthesis and the bactericidal activity of PIL promote wound healing | [185] | |
| Spirulina | Natural polymer carboxymethyl chitosan-coated spirulina | Infected diabetic mouse wound | Oxygen production via photosynthesis and continuous production of dissolved oxygen; Chlorophyll in Spirulina produces ROS under light to exert an antimicrobial effect | [120] | |
| Spirulina | SP@Rh-gel hydrogels prepared using Spirulina and rhein | Chronic colitis mouse model | Improved drug bioavailability; inhibition of the NF-κB-caspase-1 signaling pathway reduces intestinal inflammation and maintains intestinal homeostasis; reduction in the release of pro-inflammatory cytokines and lipopolysaccharides and preventing their penetration through the blood‒brain barrier, thereby inhibiting neuroinflammation | [186] | |
| Spirulina | Spirulina hydrogel microspheres | Chronic periodontitis patients | Antioxidant | [160] | |
| Spirulina | Hydrogel containing 12% Spirulina and 20% chitosan | Diabetic rat tooth extraction wound | Downregulation of the pro-inflammatory factors IF-1β and TGF-α and upregulation of the anti-inflammatory factor IF-10 promote wound healing after tooth extraction | [187] | |
| Chlorella pyrenoidosa | Photosynthetic microalgae scaffolding material | Chronic diabetic murine wound | Oxygen production via photosynthesis, accelerated neovascularization, and collagen deposition; real-time matching and rapid repair of tissue defects of any shape and depth | [94] | |
| S. elongatus | Alginate hydrogel particles loaded with S. elongatus | A murine model of myocardial ischemia | Relief of cardiomyocyte hypoxia and reduction of cardiomyocyte apoptosis | [147] | |
| S. elongatus PCC7942 | Microalgae hydrogel patch | Chronic diabetic murine wound | Oxygen production and delivery of dissolved oxygen to the wounds to promote angiogenesis | [92] | |
| HEA | GelMA hydrogel loaded with microalgae HEA | Infected diabetic mouse wound | Oxygen production via photosynthesis, ROS scavenging, antimicrobial effects, anti-inflammatory effects, and modulation of macrophage polarization for the overall rapid healing of diabetic wounds | [114] | |
| Microalgae | A carbomer gel containing microalgae, basic fibroblast growth factor, and covalent organic framework | Diabetic mouse wound | Oxygen production via photosynthesis, ROS scavenging, anti-inflammatory effects, and promoting angiogenesis | [188] | |
| Microalgae extract; Living microalgae-loaded hydrogels | Diatoms | Chitosan-coated diatomaceous earth | Rat-tail amputation model | Improved biocompatibility, shortened clotting time, and reduced bleeding | [189] |
| Microalgae-nanodrug composite delivery system; Living microalgae-loaded hydrogels | Chlorella vulgaris | Preparation of composite hydrogels using Chlorella vulgaris, Berberine, and carboxymethyl chitosan-sodium alginate | Chronic lead poisoning mouse | Adsorption and removal of metal ions from the body; extended retention time of microalgae in the gastrointestinal tract by hydrogels | [127] |
In addition, AGEs accumulate more neutrophils and macrophages in wounds [99]. Excess macrophages and neutrophils increase the production of ROS and inflammatory factors, leading to chronic inflammation. Furthermore, inflammatory cells consume oxygen and nutrients, aggravating hypoxia and malnutrition [100,101]. Oxygen plays indispensable roles in cell proliferation, neovascularization, collagen synthesis, and other processes involved in wound healing. Therefore, increasing the concentration of oxygen at the wound site can effectively accelerate wound healing [102]. Localized rupture or constriction of blood vessels in the wound may impede the supply of oxygen, resulting in hypoxia, which is detrimental to wound healing. When the oxygen partial pressure decreases below the hypoxic threshold, the healing of chronic wounds is delayed [103]. To address this challenge, localized or hyperbaric oxygen therapy is commonly used to treat chronic wounds in clinical settings. However, both of these traditional treatments have certain shortcomings. For example, the therapeutic effect of localized oxygen therapy is limited by the penetration of external gases into the skin, and only a trace amount of oxygen can enter the body through the skin and body fluids [104]. In contrast to topical oxygen therapy, hyperbaric oxygen therapy involves the placement of patients in a high-pressure environment (for example, in a hyperbaric chamber), wherein they can inhale pure or highly concentrated oxygen to increase the partial pressure of oxygen and the content of blood and tissue oxygen, which promotes capillarization and wound healing. However, hyperbaric oxygen therapy needs to be performed in hospitals under the supervision of medical professionals, which greatly limits its application in the field of skin tissue repair [105,106]. Therefore, ongoing research is focused on realizing prolonged oxygen delivery and improving the efficiency of oxygen delivery.
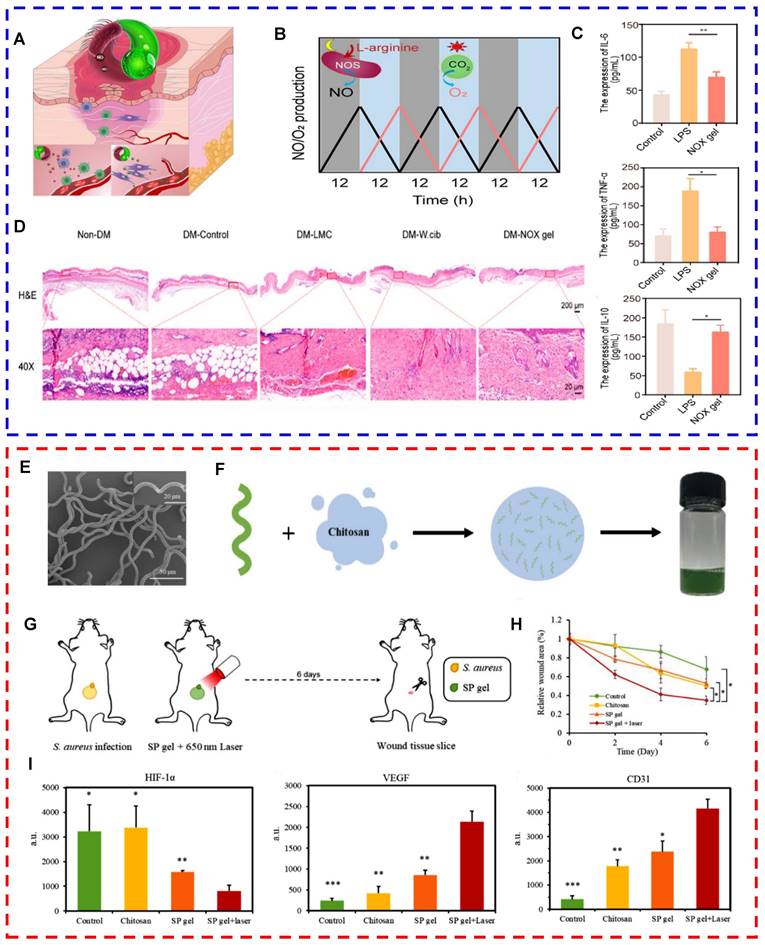
Microalgae have gradually attracted attention as natural oxygenic organisms [107]. Chen et al. developed a wound dressing containing living Synechococcus elongatus PCC7942 and used it to heal chronic wounds in patients with diabetes. By leveraging the oxygen-producing photosynthetic capabilities of the microalgae, they created a localized moist environment with elevated oxygen levels, facilitating the delivery of dissolved oxygen to the wound. The penetration efficiency of this method was nearly 100 times greater than that of traditional localized oxygen therapy. The wound dressing effectively promoted oxygenation at the wound site and stimulated aerobic metabolism and angiogenesis in hypoxic tissues [92]. Furthermore, Chen et al. applied a microalgal gel patch to skin graft wounds with poor microvessel formation and evaluated the effects of the patch on a mouse model of autologous skin grafts. The results revealed that mice treated with microalgal gel patches presented significantly increased microvessel density, intact epithelial structures, abundant granulation tissue, a large amount of well-aligned collagen, and a strong resemblance to the healthy epidermis of intact skin [92]. Subsequently, Chen et al. developed a NOX hydrogel by integrating Weissella and Chlorella. They innovatively used nitric oxide synthase present in Weissella to catalyze the reaction of L-arginine with molecular oxygen to produce NO. Moreover, microalgae can act as an oxygen donor. The hydrogel unidirectionally delivered NO and O2 on the basis of circadian rhythms to alleviate chronic inflammation and hypoxia. The findings demonstrated that the hydrogel significantly reduced the expression of proinflammatory cytokines and improved neovascularization and tissue regeneration in a diabetic wound healing model and a diabetic flap transplantation model (Figs. 2A-D) [108]. Expanding upon the foundational work of prior studies, our research team has also further explored the therapeutic potential of microalgae in the context of diabetic wound healing. We developed a novel hydrogel, denoted CHPS, which is a polyacrylamide‒sodium alginate hydrogel loaded with Chlorella. Upon exposure to 680 nm near-infrared irradiation, the sustained release of dissolved oxygen has been shown to markedly increase cell proliferation, migration, and angiogenesis [109].
As facultative anaerobic organisms, microalgae can convert solar energy to biohydrogen under anoxic conditions [110]. Hydrogen acts as an antioxidant, selectively reducing the levels of hydroxyl radicals and peroxynitrite, promoting antioxidant enzyme expression, and decreasing inflammatory factor levels[111,112]. Traditional treatments such as hydrogen gas, hydrogen-rich water (HRW), and hydrogen-rich saline (HRS) have limited therapeutic efficacy owing to the short effective reaction time, explosive nature of hydrogen, and permeability of hydrogen to damaged tissues [17,113]. However, as novel biomedical materials, microalgal hydrogel patches enable continuous generation and transdermal delivery of hydrogen to improve therapeutic efficacy. This approach can scavenge ROS, alleviate chronic inflammation at the wound site, and promote wound healing in diabetic wounds [17]. Chen et al. proposed a symbiotic algal-bacterial wound dressing containing living Chlorella vulgaris and Bacillus licheniformis. The dressing functions through continuous consumption of oxygen in calcium alginate hydrogel beads by Bacillus licheniformis via the respiratory function of Bacillus licheniformis. Under hypoxic conditions, Chlorella vulgaris performs photosynthesis for 60 hours to produce hydrogen, thereby scavenging ROS and reducing -OH and ONOO- levels. The reduction in ROS levels promoted the polarization of macrophages to the M2 reparative phenotype and attenuated the inflammatory response. In vivo experiments revealed that on the third day after treatment initiation, the wound dressing promoted cell proliferation and led to approximately 50% healing of diabetic wounds [17]. Processing microalgae for programmed treatment can improve the microenvironment of diabetic wounds. Kang et al. encapsulated live Hematococcus (HEA) in a conventional GelMA gel [114]. During the experiment, high-intensity laser irradiation (658 nm, 0.5 W/cm2) was initially used to eliminate bacteria rapidly via the photothermal conversion effect. The light intensity (658 nm, 0.1 W/cm2) was subsequently reduced to allow the microalgae to continuously generate oxygen to ameliorate tissue hypoxia and promote vascular regeneration. Continuous light exposure promoted the accumulation of astaxanthin (AST) in HEA cells, effectively removing excess ROS. In addition, HEA cells further regulate macrophage polarization by secreting AST-rich vesicles [114]. This programmed therapeutic strategy has a strong ability to regulate the wound microenvironment.
Overall, microalgae can promote the healing of chronic or diabetic wounds by producing oxygen or hydrogen through photosynthesis, exerting anti-inflammatory and antioxidative effects, and alleviating hypoxia. Wound dressings prepared using living microalgae-loaded hydrogels offer the following advantages: (1) the use of hydrogels to cover wounds creates a moist environment and provides a temporary barrier against external infections, and (2) this approach maintains the survival of microalgae and allows for the sustained delivery of dissolved oxygen to wounds in a moist environment to improve oxygenation. Patients with chronic diabetic wounds should be treated with a combination of hydrogels and microalgae‒nanodrug composite delivery systems to alleviate infection and inflammation. In addition, the photosynthetic activity of microalgae can increase oxygenation at the wound site, synergistically promoting wound healing.
Infected wounds
Wound infections pose a significant challenge in modern medicine, with millions of deaths attributed to untreated infections each year [115]. The formation of infectious wounds is a complex process involving many factors, including the invasion of microorganisms, the host immune response, the activation of the inflammatory response, and possible complications [116]. Antibiotics remain the mainstay of treatment for infections in clinical practice. However, poor targeting ability and nonselectivity to the focal area limit the efficiency of drug delivery. Achieving the required therapeutic dose often requires multiple administrations. Moreover, misuse of antibiotics tends to induce the formation of drug-resistant bacteria, resulting in less effective or ineffective treatment [117].
Processed microalgae offer various strategies for treating infected wounds, including the following: (1) Microalgae extracts exert antimicrobial effects. For example, chlorellin, which is extracted from Chlorella, can inhibit the growth of both gram-positive and gram-negative bacteria [15]. Therefore, chlorellin can be used as an alternative to antibiotics to a certain extent, preventing the development of drug resistance. (2) Microalgae-nanomedicine composite drug delivery systems can be used to treat infected wounds. Shchelik et al. functionalized C. reinhardtii through azide cycloaddition using N-hydroxysuccinimide. In particular, they covalently attached vancomycin on the surface of the microalga via a cleavable o-nitrobenzyl [118]. However, this antibiotic system does not function in the conjugated state. However, irradiation with 365 nm light disrupted the covalent bonds, resulting in the targeted release of the drug for the treatment of skin or soft tissue infections [118]. (3) Microalgae rich in chlorophyll can generate ROS and function as photosensitizers for photodynamic therapy (PDT) under laser irradiation [119]. Li et al. successfully prepared a hydrogel with photosynthetic oxygen production and antimicrobial activity via a one-step synthesis method. They utilized living Spirulina as the core material, encapsulating it within biocompatible carboxymethyl chitosan. The photodynamic bactericidal effects of Spirulina and the continuous release of dissolved oxygen improved the hypoxic microenvironment of the wound, alleviating infection and promoting healing (Figs. 2E-I) [120]. (4) Magnetic nanocarrier-modified microalgae can be used for photothermal therapy (PTT) under laser irradiation. To date, no studies have used magnetic nanocarrier-modified microalgae to treat infected wounds. However, in terms of antimicrobial properties, researchers have used magnetite nanoparticles to modify the surface of Spirulina. Researchers have subsequently coated the surface of Spirulina with polydopamine to develop magnetic microalgal composites with improved performance. Under photoacoustic image guidance, these modified Spirulina materials possess magnetic properties and exhibit excellent photothermal activity. After 6 minutes of 808 nm laser irradiation, the microalgal composites can warm to >50 °C and exert antimicrobial effects [121].
Taken together, these findings indicate that microalgae possess excellent potential in the treatment of infected wounds. Microalgae extracts have antimicrobial properties. Microalgae rich in chlorophyll can generate ROS upon laser irradiation, enabling PDT for antimicrobial treatment. Additionally, microalgae can be loaded with drugs and magnetic nanoparticles, leveraging their magnetic properties for targeted movement and activation and achieving drug release or PTT.
Repair of other types of skin injuries
Microalgae have shown promising therapeutic effects against acute wounds caused by surgery and various inflammatory skin diseases (e.g., atopic dermatitis, psoriasis, and vitiligo). Lim et al. reported that the microalgae extract KSF0041 had oxygen radical-scavenging activity. KSF0041 effectively protected HaCaT cells against oxidative stress-induced damage [122]. Furthermore, KSF0041 significantly alleviated clinical symptoms of psoriasis, including desquamation, redness, skin moisture loss, and weight loss, and inhibited inflammatory cytokine upregulation in imiquimod-induced psoriasis in mice [122]. Liu et al. reported that spirochete protein (SPCP) promoted the repair of wounds caused by total dermal excision in C57BL/6 mice by upregulating the Akt, ERK, and TGF-β1 signaling pathways [123]. With respect to surgical wounds, Centeno-Cerdas et al. pioneered the integration of genetically processed C. reinhardtii into surgical sutures, creating photosynthetic sutures. These innovative sutures enable the continuous and stable release of oxygen and recombinant human growth factors locally at the wound site, effectively promoting wound healing [124]. This attempt not only led to the development of a new generation of bioactive sutures with increased regenerative capacity but also provided valuable insights into the development of microalgae-based biomaterials.
With respect to skin tissue engineering scaffolds, κ-carrageenan, a naturally sulfated algal polysaccharide derived from microalgae, has been found to be highly similar to the natural glucosaminoglycans (GAGs) present in the ECM of the skin [125]. Singh et al. coated electrospun nanofibers with ECM-mimicking κ-carrageenan to construct skin tissue engineering scaffolds. The experimental data revealed that the scaffolds significantly promoted fibroblast adhesion, growth, survival, and proliferation and upregulated the expression of genes related to cell adhesion and cytoskeletal matrix formation. These findings indicated the formation of a favorable environment for fibroblast growth on the scaffolds [125].
As mentioned earlier, the use of microalgae to accelerate skin tissue repair is innovative, safe, and efficient. However, processed microalgae have many limitations in the field of skin tissue repair. At present, relevant research is at an early stage. In addition, the application of microalgae is limited mostly to the backs of experimental animals, which have relatively little movement. Wounds at these sites are significantly different from tension wounds at the neck, knee, elbow, or other joints. Resolution of these limitations is crucial for the clinical translation of microalgae.
Gastrointestinal tissue
Oral administration is often the preferred route for the clinical treatment of gastrointestinal disorders. Microalgae offer the advantages of convenience and safety as oral medications. Fields et al. investigated the effects of consuming freeze-dried C. reinhardtii on gastrointestinal health in mice and humans. Experimental data revealed that mice with acute colitis fed PBS lost an average of 9% of their body weight, whereas those fed freeze-dried C. reinhardtii lost only 7% of their body weight after 12 days of treatment [126]. These findings suggest that dietary supplementation with C. reinhardtii can mitigate colitis-associated weight loss. Furthermore, the addition of C. reinhardtii to the diet of patients with frequent gastrointestinal distress significantly reduced symptoms, facilitated regular bowel movements, and improved stool quality. In addition, when the gut microflora of these patients was examined, the consumption of C. reinhardtii was not found to have significant effects on the composition of gut microbes [126]. Microalgae can also be used to treat heavy metal-induced tissue damage in the gastrointestinal tract. They can remove heavy metal ions from the body through electrostatic adsorption and chelation. Liu et al. engineered an innovative hydrogel, designated BBR-CV@ALG, for the remediation of lead intoxication. This composite material integrated Chlorella vulgaris (CV), berberine (BBR), and a composite of carboxymethyl chitosan and sodium alginate. The surface groups of Chlorella vulgaris can bind to lead ions, thereby exhibiting superior detoxification capabilities. Moreover, BBR mitigated the inflammatory responses induced by lead exposure through the neutralization of ROS. Moreover, the hydrogel formulation extended the gastrointestinal residence time of the microalgae, offering a novel in vivo detoxification approach for managing acute, subacute, and chronic lead toxicity [127].
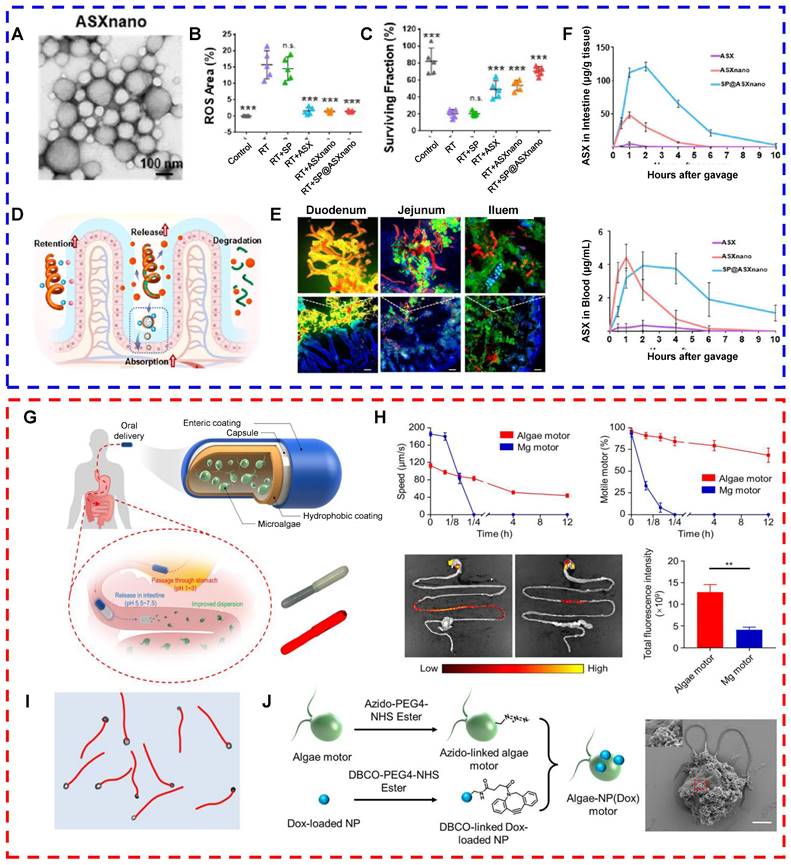
Moreover, a drug-carrying system based on living microalgae has been developed and used before and after radiotherapy to protect normal intestinal tissues. Radiotherapy, a conventional treatment for tumors, is administered to more than 70% of cancer patients [128]. In addition to killing tumor cells, radiotherapy exerts toxic effects on normal tissues either systemically or locally, causing acute or chronic radiation syndrome and organ damage [129]. For example, radiation therapy for tumors in the abdominal or pelvic cavity, such as pancreatic, prostate, and colorectal cancers, often damages the small intestine because of its high sensitivity to radiation and large size. Damage to the small intestine may lead to gastrointestinal dysfunction or death [130]. Thus, minimizing radiotherapy-induced damage to the small intestine is essential. Drugs such as amphotericin have been approved to protect normal tissues from radiation. However, when these drugs are administered orally, they are readily affected by gastric acid and are rapidly metabolized, resulting in limited protective effects, a short half-life, and poor stability. Moreover, the potential side effects of these drugs limit their widespread application in clinical settings [131]. Therefore, compared with traditional oral drug delivery systems, drug delivery systems constructed from living microalgae can overcome the limitations of traditional oral drug delivery, such as poor solubility, low bioavailability, and short half-lives of drugs [132]. Zhang et al. constructed an oral microalga-nanointegrated system (SP@ASXNano) using Spirulina platensis (SP) as a drug delivery carrier and loaded astaxanthin nanoparticles (ASXNano). This system extended the median survival of mice with acute radiation enteritis to 29 days and ameliorated radiation-induced intestinal damage (Figs. 3A-F) [132]. Moreover, compared with that of spherical algae, the spiral structure of Spirulina makes it better suited as a drug carrier. In one study, SPs loaded with curcumin were used to treat two intestinal diseases, namely, colitis and colon cancer. Researchers compared the retention times of Chlorella vulgaris- and Spirulina-based drug carriers in the intestine. A study revealed that Spirulina-based carriers were more likely to be captured by intestinal villi, prolonging the drug retention time and enhancing the absorption efficiency [47]. Furthermore, microalgae can be loaded with drugs that are used to protect normal tissues from radiation, such as amifostine (AMF). Zhang et al. used a dehydration‒rehydration synthesis strategy to develop SP-based delivery vehicles for amifostine (SP@AMF). In vivo fluorescence imaging and SEM images of the gastrointestinal tract revealed that the SP@AMF carrier penetrated gastric tissues in an acidic environment and was more uniformly and widely distributed in the intestine. This system is capable of exerting a long-lasting effect, resulting in a comprehensive protective effect on the entire small intestine [130].
Oxygen-producing microalgal gel patches for repairing chronic diabetic wounds (reproduced with permission [108]. Copyright 2023, American Chemical Society). (A) Mechanism of action of the NOX gel patch for the treatment of chronic diabetic wounds. (B) Functional regulation of NO (gray area) and O2 (blue area) production in lipid membrane-coated Chlorella vulgaris and Weissella. (C) Expression of IL-6, TNF-α, and IL-10 in different groups of LPS-induced RAW264.7 cells. (D) Representative images of diabetic wounds treated with or without NOX gel. (E-I) Microalgae-loaded hydrogels promoted the healing of infected wounds by improving the hypoxic microenvironment and exerting antimicrobial effects (reproduced with permission [120]. Copyright 2020, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim). (E) SEM image of the SP gel. (F) Schematic illustration of the synthesis of the SP gel. (G) Schematic illustration of the in vivo experiments. (H) Measurement of the wound area at each time point and calculation of the relative wound area using the initial wound area on day 0. (I) Quantification of the results of immunohistochemistry (HIF-1α, VEGF, and CD31) using Image-Pro Plus to characterize the mechanisms through which the SP gel + laser group promoted wound healing.
In contrast to Spirulina-based drug delivery systems, C. reinhardtii-based drug delivery systems offer certain advantages because of the unique flagellar structure of microalgae. This structure endows C. reinhardtii with the ability to swim rapidly and persistently in the intestinal fluid. It enhances the retention and absorption of drugs in the small intestine and improves the bioavailability of orally administered drugs. Therefore, C. reinhardtii-based drug delivery systems can be used for the diagnosis or treatment of gastrointestinal diseases. Zhang et al. developed nanorobots based on live C. reinhardtii with long-lasting self-propulsion capability. These nanorobots are capable of stable and continuous movement in the intestinal fluid at normal body temperature for more than 12 hours. The longer lifetime and more efficient motility of the algal motors than the metal micromotor facilitated the distribution and retention of drugs in the gastrointestinal tract (Figs. 3G-J) [75]. Future studies should explore additional functionalities of algal motors to broaden their applications in gastrointestinal drug delivery. For example, photosensitizers such as chlorophyll in microalgae can enable visualization of the movement of microalgae as they function in the gut. In addition, the conjugation of magnetic particles to the surface of microalgae can more precisely direct the microalgal motors to the target site under the influence of an external magnetic field, enabling targeted delivery to specific regions in the gastrointestinal tract. These approaches may improve the precision and targeting ability of algal motor systems and enhance their efficacy in gastrointestinal drug delivery.
Bone tissue
To maintain the structural integrity of the skeleton, bones should be constantly remodeled. Osteoclasts remove old bone and are replaced by osteoblasts that synthesize new bone. Disruption of the balance between bone formation and bone resorption leads to disturbances in bone metabolism, which in turn triggers the development of several bone diseases, such as osteolysis, osteoporosis, osteoarthritis, and rheumatoid arthritis [133,134]. Compared with individual microalgae, microalgae extracts exert more significant therapeutic effects against bone diseases. Astaxanthin, a component derived from microalgae, has been shown to have significant efficacy in improving the microstructure and thickness of bones [135]. Hematococcus pluvialis, a natural antioxidant, is a rich source of astaxanthin. El-Baz et al. administered Hematococcus pluvialis (BHP, 450 mg/kg), the polar fraction (PHP, 30 mg/kg, nonastaxanthin component), and Hematococcus pluvialis extract (CHP, 30 mg/kg, enriched with astaxanthin) to osteoporotic rats orally for 14 days. Microcomputed tomography, serum biochemical tests, and other methods were subsequently used to compare the effects of the three treatments. The results showed that oral administration of BHP and PHP partially increased tibial bone mineral density and serum phosphorus levels while partially decreasing serum calcium, bone alkaline phosphatase, interleukin 6, osteoprotegerin (OPG), and nuclear factor-κβ ligand (RANKL) levels. Oral administration of CHP almost completely restored the abovementioned parameters to normal values, resulting in more significant therapeutic effects and confirming that astaxanthin inhibits osteoclast-mediated bone resorption [136]. The ability of astaxanthin to increase the activity of osteoblasts has been demonstrated in an experimental model of periodontitis [137]. For bone tissue repair, an ideal biomaterial should have excellent porosity, mechanical properties, drug-carrying efficiency, biocompatibility, biodegradability, antimicrobial activity, antioxidant activity, anti-inflammatory activity, and bone repair-promoting properties [138]. Natural green microalgae can not only be extracted as bioactive substances but also be used as binders and pore-forming agents for the development of scaffolds for bone repair (Fig. 4) [12]. Barua et al. constructed three new types of interconnected porous bone scaffolds via solvent casting. These scaffolds were composed of green microalgae and hydroxyapatite (HA) at ratios of 1:2, 2:1, and 1:1 (w/w). Comparative analysis revealed that scaffolds with equal microalgae-to-HA weight ratios (i.e., 1:1) had higher compressive and mechanical strengths, which met the requirements for cell growth and support [139]. The silica shell of diatoms is a new type of biomaterial that holds substantial promise in bone repair. Owing to their complex structure, silica shells can be used as drug carriers with sustained drug release properties. Cicco et al. reported that sodium alendronate (NaALE)-loaded diatom silica shells not only improved the drug delivery rate but also prevented the side effects caused by prolonged systemic administration of drugs [140]. Furthermore, the porous structure of diatom silica shells facilitates the adhesion and differentiation of osteoblasts. Amoda et al. sintered diatomaceous earth to construct a 3D cell growth platform for MC3T3-E1 preosteoblasts. The material exhibited excellent biocompatibility, with the cells beginning to attach to it by the second day. Von-Kossa staining revealed the presence of mineral deposits in osteoblasts after 21 days. The material was autoclaved and reused without leading to any adverse effects on subsequent cell culture [141]. López-Álvarez et al. compared the effects of Si-HA coatings prepared from two silica sources (diatomaceous earth and quartz) and HA on a human osteoblast-like cell line (SaOS-2). An assessment of dsDNA and alkaline phosphatase (ALP) activity confirmed that Si-HA coatings prepared with diatoms were superior to those prepared with quartz in promoting the activity and proliferation rate of osteoblasts [142]. The medicinal properties of microalgae, drug delivery efficiency, ability to stimulate bone tissue regeneration, and surface modification of scaffolding materials are considered key indicators of the efficacy of a biomaterial in bone repair [12]. The development and design of different types of processed microalgae provide novel strategies for bone repair.
(A-F) Oral microalgae-nanointegrated system against radiation damage (reproduced with permission [132]. Copyright 2023, American Chemical Society). (A) TEM images of ASXnano. (B) Fluorescence area measured on the basis of fluorescence (green) images of ROS in IEC-6 cells after 6-Gy X-ray irradiation (RT) of different materials for 6 hours. (C) Quantification of surviving colonies of IEC-6 cells after different treatments. (D) Schematic diagram of SP@ASXnano administration. (E) Fluorescence images of small intestinal sites 4 hours after the administration of SP@ASXnano via gavage. (F) Pharmacokinetic distribution of ASX in mouse intestinal tissues and blood after the administration of ASX, ASXnano, or SP@ASXnano via gavage. (G-J) Live algal nanorobots based on C. reinhardtii for gastrointestinal drug delivery (reproduced with permission [75]. Copyright 2022, Author). (G) Schematic representation of algae motors loaded inside a protective capsule containing an internal hydrophobic coating and an external enteric coating for oral delivery; bright field and fluorescence images of the capsule containing the algal motor are shown. (H) Comparison of the distributions of algal and magnesium motors in the gastrointestinal tract. (I) Representative trajectories of the autonomous movement of algal motors in simulated intestinal fluid. (J) Schematic illustration and SEM images of the fabrication process of algal motors loaded with doxorubicin.
Schematic diagram of the combination of microalgae extracts (lipids, proteins, nucleic acids, and carbohydrates) and biomaterials for bone tissue repair [12] (created with BioRender.com).
Cardiovascular system
Cardiovascular diseases (CVDs) involve lesions of the myocardium and vascular tissue, including myocardial infarction (MI), atherosclerosis, and heart failure. A deficiency in oxygen supply is a critical etiological factor for numerous cardiovascular conditions, with particular emphasis on the scenario of profound myocardial hypoxia. In such cases, the ischemic regions are at risk of sustaining irreversible damage, potentially leading to necrosis [143-145]. Owing to their photosynthetic oxygen production activity, microalgae represent revolutionary and promising therapeutic agents for hypoxia-related CVDs. Microalgae can convert carbon dioxide to oxygen during myocardial ischemia, thereby improving the oxygen deficit in the microenvironment, rescuing ischemic cardiomyocytes, and maintaining their survival [146]. To increase the survival of microalgae in the human body, Stapleton et al. designed alginate-based hydrogel particles (S. elongatus-HMPs) containing Synechococcus elongatus (S. elongatus, a type of cyanobacterium) [147]. In vitro experiments revealed that the cyanobacteria in HMPs survived longer than those suspended in a phosphate-buffered solution. In addition, S. elongatus-HMPs significantly alleviated cardiomyocyte hypoxia and reduced cardiomyocyte apoptosis. In vivo experiments revealed that S. elongatus-HMPs suppressed apoptosis and improved left ventricular function in mice with ischemia‒reperfusion injury (Figs. 5A-E) [147]. EPA and DHA found in microalgae can reduce the morbidity and mortality rates of CVD. Microalgae-derived peptides with antioxidative and anti-inflammatory properties may alleviate hypertension and regulate dyslipidemia by inhibiting angiotensin-converting enzyme (ACE) and endothelial nitric oxide synthase [148,149]. Chen et al. assessed the protective effects of a peptide, I. zhanjiangensis (PIZ, FEIHCC), derived from the marine microalga Isochrysis zhanjiangensis, which is a golden-brown flagellate species isolated from Nansan Island, Zhanjiang, Guangdong Province, China. This evaluation focused on its potential to mitigate endothelial damage in human umbilical vein endothelial cells (HUVECs) [150]. The results showed that PIZ (IC50 = 61.38 μM) antagonized ACE in a noncompetitive binding manner and inhibited angiotensin II (Ang II)-induced secretion and expression of vascular factors by blocking the NF-κB, Nrf2, MAPK, and Akt signaling pathways [150]. When surgery or trauma leads to severe damage and rupture of arterial or venous vessels or when specific bleeding wounds develop (e.g., irregular wounds, deep luminal wounds, and multiple tiny oozing wounds), traditional hemostatic strategies, such as tourniquets and hemostatic agents, may not be able to control the bleeding effectively. Diatomaceous earth has silanol groups on its surface and a multistage porous structure that can rapidly absorb plasma and bind to blood components (i.e., red blood cells and platelets), thereby concentrating coagulation factors and facilitating coagulation. Therefore, diatomaceous earth is a novel hemostatic biomaterial [35,151]. Using dopamine as a cross-linking agent, Li et al. developed chitosan/diatom-bBiosilica aerogels (CDDs-TBA) via the "alkaline precipitation-tertiary butanol replacement-freeze-drying method". The results revealed that CDD-TBA had a large specific surface area (74.441 m2 g-1) and a high water absorption rate (316.83 ± 2.04%), with a clotting time of <80 s in vitro. The therapeutic effects of CDDs-TBA were subsequently examined in a rat tail-breaking model and a femoral arteriovenous dissection model. Compared with commercial products, CDD-TBA led to significant improvements in the clotting time and bleeding volume metrics (Figs. 5F-G) [35]. As mentioned earlier, processed microalgae-based strategies for cardiovascular repair can alleviate hypoxia, control coagulation, and mitigate the impact of CVD risk factors. In addition, these strategies can improve the quality of life of patients with CVD.
Other tissues
The applications of processed microalgae extend to several tissues other than those mentioned above, such as lung, nerve, and oral tissues. For the treatment of lung injury, microalgae‒nanodrug composite delivery systems can efficiently encapsulate drugs and deliver them to lung tissues. These systems can improve drug bioavailability and overcome the limitations imposed by the unique physiological barriers and defense mechanisms of the lungs, such as mucociliary clearance in the upper respiratory tract and phagocytosis by macrophages in the alveoli [78]. Zhang et al. functionalized the surface of C. reinhardtii via azido N-hydroxysuccinimide (NHS) ester, followed by conjugation with dibenzocyclooctyne (DBCO)-modified neutrophil membrane/polymer NPs via click chemistry. They successfully constructed a microalgae-nanoparticle microrobot (algae-NP-robot) for drug delivery to the lungs. Owing to the motility of the flagella of C. reinhardtii, algae-NP-robots presented high drug delivery efficiency and improved the therapeutic efficacy of the drug (Fig. 6A) [78]. On the basis of the autofluorescence characteristics of algal chloroplasts, fluorescence imaging of isolated lungs was performed at different time points to observe the distribution of algae-NP-robots. The results revealed that the algae-NP-robots penetrated the whole lung tissue within 1 h and were retained for at least 24 h, with an even distribution throughout the lung tissue [78]. Microalgae extracts are the predominant processed microalgae used in the neurological field. Most microalgal extracts (e.g., omega-3, C-phycocyanin, and fucoxanthin) intervene with or repair neuronal cell damage by alleviating oxidative stress [152], inhibiting neuroinflammation [153,154] and promoting neuronal cell growth [155]. Among microalgae extracts, the photosensitive ion channel protein ChR2 extracted from C. reinhardtii has attracted substantial attention owing to its ability to manipulate the activity of cation channels in neuronal cells. ChR2, analogous to a circuit switch, manipulates the initiation and inhibition of various physiological activities in the neurons and muscle cells of living organisms [156]. Boyden et al. used lentiviral vectors to transfect laboratory-cultured neuronal cells with ChR2 and irradiated the transfected cells with blue light. The ChR2 protein captures light, allowing extracellular Ca2+, Na+, and other cations to enter nerve cells and cause excitation, thus enabling the manipulation of nerve cell activity with millisecond-level temporal precision [156]. On the basis of these findings, the effects of ChR2 on different mouse tissues were examined. The results showed that ChR2 controlled cardiac muscle contraction and promoted the recovery of respiration after SCI by activating neural circuits. Furthermore, ChR2 was introduced into retinal ganglion cells or cochlear cells to improve visual or auditory perception, respectively (Figs. 6B-C) [157]. Microalgae have shown strong potential in the repair of oral tissues [158]. Yuce et al. evaluated the effects of astaxanthin, a component derived from microalgae, on alveolar bone in a rat model of ligation-induced periodontitis. They measured changes in alveolar bone levels and performed immunohistochemical staining. The results revealed that astaxanthin promoted osteoblast-mediated bone formation, suppressed osteoclast-mediated bone resorption, and significantly reduced alveolar bone loss in rats [137]. Owing to the moist and dynamic environment of the oral cavity (e.g., temperature changes, salivation, swallowing, and chewing) and the presence of various bacteria [159], orally administered microalgae extracts have a short local retention time and inefficient drug delivery capacity. To address these issues, Kaipa et al. engineered a spirulina-enriched hydrogel microsphere specifically designed for targeted treatment of chronic periodontitis. These microspheres facilitate the direct delivery of spirulina to affected oral regions, leveraging its antioxidant properties to significantly ameliorate this condition [160] (Fig. 6D). Taken together, the processing of microalgae may endow them with desirable properties for the repair of lung, nerve, and oral tissues. However, further research is warranted to assess the application potential of different processing techniques.
(A-E) S. elongatus-containing hydrogel particles (S. elongatus-HMPs) for the repair of ischemic myocardium (reproduced with permission [147]. Copyright 2023, The Society for Biotechnology, Japan. All rights reserved). (A) Schematic diagram of the microfluidic device used to fabricate S. elongatus-HMPs. (B) Microscopy image of S. elongatus encapsulated in HMPs. (C) S. elongatus-HMPs delivered to the ischemic myocardial tissue stopped bleeding by temporarily blocking the left anterior descending (LAD) artery. (D) ESPVR values obtained by comparing the systolic pressure‒volume relationship (ESPVR) values of each animal with the ESPVR values at baseline after 45 minutes of treatment. (E) Effects of S. elongatus-HMPs on the ejection fraction 4 weeks after ischemia-reperfusion. (F-J) Chitosan/Diatom-Biosilica aerogels (CDDs-TBA) for rapid hemostasis (reproduced with permission [35]. Copyright 2020, Wiley-VCH GmbH). (F) Representative images of 2% CDDs, 2% CDDs-5% TBA, 2% CDDs-10% TBA, 2% CDDs-20% TBA, 2% CDDs-30% TBA, 2% CDDs- 60% TBA, and 2% CDDs-100% TBA after freeze-drying. (G) Formation of clots at vascular wounds after the application of CDDs-TBA. (H) Representative images of the hemostatic effects of CDDs-TBA. (I) Clotting time. (J) Bleeding volume in a rat model of femoral arteriovenous dissection.
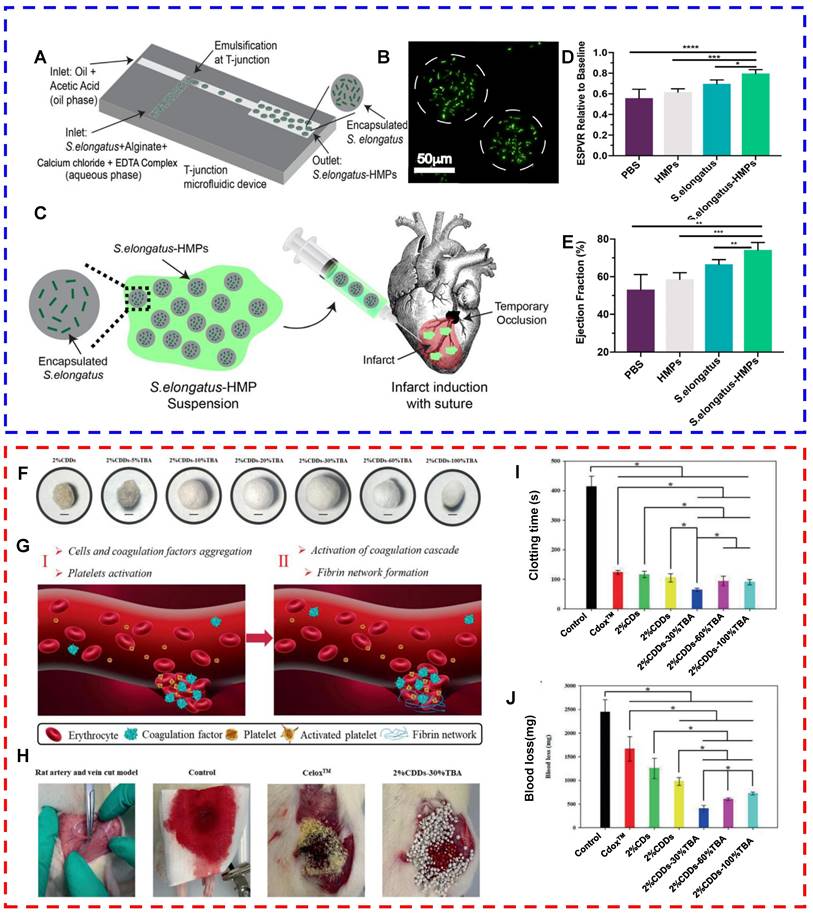
Microalgae are processed for the repair of lung, nerve, and oral tissues. (A) Schematic diagram of a microalga‒nanodrug composite delivery system that can actively deliver drugs to the lungs [78] (created with BioRender.com). (B) Schematic diagram of the photosensitive channel protein rhodopsin. (C) Light transmission to the rodent brain via optical fibers, neuronal activation after light stimulation, and expression of ChR2 on the membranes of neurons (reproduced with permission [157]. Copyright 2013, Elsevier B.V.). (D) Schematic illustration of the synthesis process for microalgae-based hydrogel microspheres and their application in chronic periodontitis therapy [160] (created with BioRender.com).
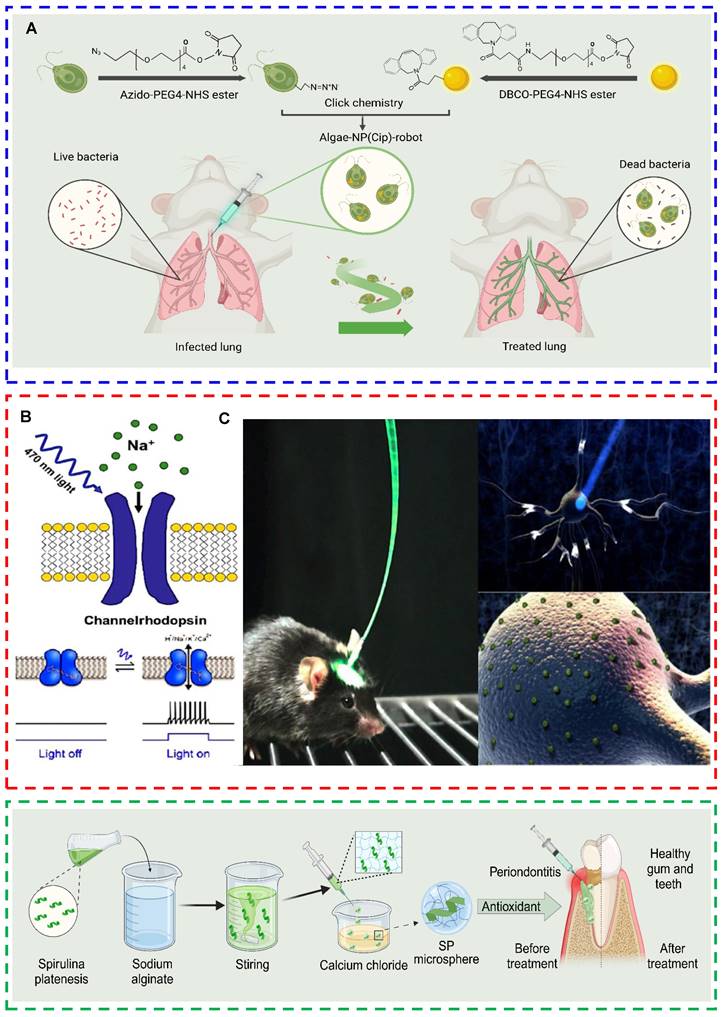
Conclusion
In this study, the applications of processed microalgae in tissue repair were systematically summarized. The extraction of organic matter from microalgae can improve the bioavailability of their active components and enhance therapeutic efficacy. Microalgae-based composite drug delivery systems can improve the delivery rate and bioavailability of drugs. Surface modification of microalgae with cell membranes or nanomaterials can help them evade immune clearance and enhance their targeting ability in vivo. Living microalgae-loaded hydrogels can maintain the activity of microalgae for improved therapeutic effects. In addition, microalgae have good metal adsorption capacity and can be used as biocarriers to adsorb and remove heavy metals such as lead and mercury from the body, thus alleviating heavy metal poisoning. Owing to the presence of fluorescent chlorophyll pigments, microalgae can be used for fluorescence imaging in vivo and can be combined with PDT. Moreover, the distinct characteristics of various tissues and organs necessitate a tailored approach. Consequently, microalgae offer a diverse array of future research avenues, specifically for the treatment of particular diseases or tissues. For example, in the treatment of skin wounds, the focus should be on increasing local oxygen concentrations to increase the efficiency of oxygen production. Given the limited light conditions within internal organs, the integration of upconversion luminescent materials or wireless luminescent devices is essential for addressing hypoxic diseases in vivo. In the context of gastrointestinal and pulmonary diseases, attention must be given to the targeting and drug-loading capabilities of microalgae as drug carriers, as well as their self-luminescent properties, to increase the precision of disease diagnosis and treatment. Concurrently, in the realm of bone tissue engineering, the development of microalgae-based scaffolds represents a promising research direction.
In addition, the synergistic application of microalgae with biomaterials could encompass future advancements such as functionalization, customization, and the application of genetic engineering techniques. First, functionalization is an important direction for the development of processed microalgae, especially for drug delivery. Endowing microalgae with specific functions by processing methods can improve drug delivery efficiency and treatment efficacy, thus promoting more comprehensive and effective tissue repair. Second, in the current era of precision medicine and digitalization, the demand for processed microalgae is increasing. Processed microalgae that are "tailor-made" according to the specific conditions of each patient and the diverse requirements of tissue repair can facilitate individualized treatment. For example, introducing photosynthetically living microalgae into hydrogel scaffolds or dressings via 3D printing can enable individualized treatment. 3D printing can be used to combine drug-loaded microalgae and hydrogels and customize them with various drug release properties, such as the ability to release drugs in response to external stimuli (e.g., temperature, pH, and light). This design allows processed microalgae to flexibly adjust the release of drugs in response to changes in the external environment for different therapeutic requirements. Third, genetic engineering of microalgae is another important direction for future research. Precise modification of microalgae through genetic engineering can enable them to produce more bioactive substances (e.g., proteins, lipids, carotenoids, and biohydrogen) or have specific functional expression, improving their efficacy in tissue repair.
Although the prospect of utilizing processed microalgae in tissue injury repair is encouraging, research into their efficacy in promoting healing also faces several challenges. First, studies investigating the applications of processed microalgae in tissue repair are insufficient. Efforts to improve the survival of microalgae in organisms and increase their ability to accumulate at target sites effectively are ongoing. Second, the majority of microalgae processed for tissue repair applications remain confined to the preclinical experimental stage. In addition, existing clinical studies on processed microalgae have focused mostly on microalgae extracts (Table 3), whereas clinical studies on other processed microalgae are relatively limited. To verify the application value of processed microalgae in clinical treatment, it is necessary to conduct clinical studies on more types of processed microalgae. In addition, the optimal therapeutic dose and expected therapeutic effects of processed microalgae and the duration of treatment warrant further investigation. Future research should focus on processed microalgae to maximize their potential as biomaterials. Processed microalgae may introduce novel avenues for tissue repair and regeneration and promote the continuous development of microalgae in the biomedical field.
Clinical trials related to the use of microalgae extracts in tissue repair. (Source: https://clinicaltrials.gov/)
| Clinical trial code | Experimental phase | Condition or disease | Interventions/treatments | Trial purpose |
|---|---|---|---|---|
| NCT04851899 | Not Applicable | Cognitive function | Participants were administered oral placebo or microalgae extract PhaeoSOL for 1 month | To assess whether PhaeoSOL composition affects the cognitive function of participants |
| NCT04832412 | Not Applicable | Cognitive impairment and neuroprotection | Participants were orally administered maltodextrin as a placebo or the microalgae extract BrainPhyt for 24 weeks | To assess the effects of BrainPhyt on cognitive function (spatial working memory scores, attention and alertness, situational memory, and executive function), stress, mood, sleep quality, and biomarkers in healthy older adults |
| NCT05759910 | Not Applicable | Age-associated memory impairment | Participants were orally administered maltodextrin capsules as a placebo or the microalgae extract Brainphyt capsules for 12 weeks | To assess the effects of the microalgae extract BrainPhyt on cognitive function in healthy older adults |
| NCT05267301 | Phase 4 | Heart health | Participants received the oral dietary supplement AlmegaPL, an EPA-rich microalgae extract, for 6 months | To assess the effectiveness of Almega PL in improving blood markers associated with heart health over the period of evaluation |
| NCT01437930 | Not Applicable | Cardiovascular disease | Participants were orally administered a placebo or microalgae-derived n-3 PUFA | To assess the effects of dietary supplementation of n-3 PUFA on cardiovascular risk factors in patients with hypertriglyceridemia |
| NCT00728338 | Not Applicable | Cardiovascular disease | Patients were orally administered olive oil as a placebo or microalgae-derived DHA | To evaluate the effects of dietary supplementation of DHA on cardiovascular risk factors in male patients with hyperlipidemia |
| NCT03625284 | Not Applicable | Nonalcoholic fatty liver disease | Participants were orally administered capsules of edible oil as a placebo or microalgae-derived fucoxanthin capsules | To investigate the effects of microalgae-derived fucoidan on biochemical clinical markers related to liver health |
| NCT01742468 | Not Applicable | Rheumatoid arthritis | Participants were orally administered sunflower oil as a placebo or microalgae-derived long-chain n-3 PUFA for 10 weeks | To assess the effects of microalgae-derived long-chain n-3 PUFA on disease activity, inflammatory markers, and cardiovascular risk factors in patients with rheumatoid arthritis |
| NCT03960164 | Phase 1 | Acute Wounds | The integration of Chlamydomonas reinhardtii microalgae within Evicel human fibrin sealant constructs a dermal regeneration photosynthetic matrix (DRPM) | To assess the effects of DRPM on the treatment of acute wounds |
Abbreviations
ACE: angiotensin-converting enzyme; AGEs: advanced glycosylation end products; ALP: alkaline phosphatase; algae-NP-robot: a microalgae-nanoparticle microrobot; AMF: amifostine; Ang II: angiotensin II; AST: astaxanthin; ASXNano: astaxanthin nanoparticles; BBR: berberine; BHP: Hematococcus pluvialis biomass; CDDs-TBA: Chitosan/Diatom-Biosilica aerogels; ChR2: Channel rhodopsin-2; CHP: Hematococcus pluvialis extract rich in Astaxanthin; CHPS: a polyacrylamide-sodium alginate hydrogel loaded with Chlorella; C.reinhardtii: Chlamydomonas reinhardtii; CV: Chlorella vulgaris; CVDs: Cardiovascular diseases; DBCO: dibenzocyclooctyne; DHA: docosahexaenoic acid; DRPM: dermal regeneration photosynthetic matrix; ECM: extracellular matrix; EPA: eicosapentaenoic acid; GAGs: glucosaminoglycans; HA: hydroxyapatite; HEA: Hematococcus; HRS: hydrogen-rich saline; HRW: hydrogen-rich water; HUVECs: human umbilical vein endothelial cells; MI: myocardial infarction; Na ALE: sodium alendronate; NHS: N-hydroxysuccinimide; OPG: osteoprotegerin; PDA: polydopamine; PDT: photodynamic therapy; PHP: The polar components of Heamatococcus pluvialis; PIZ: I. zhanjiangensis; PTT: photothermal therapy; RANKL: nuclear factor-κβ ligand; RBCM: red blood cell membrane; ROS: reactive oxygen species; S. elongatus: Synechococcus elongatus; S. elongatus-HMPs: Synechococcus elongatus-containing hydrogel particles; SOD2: superoxide dismutase 2; SP: Spirulina platensis; SPC: Spirulina polysaccharide complex; SPCP: spirochete protein; SP@AMF: Amifostine-loaded Spirulina platensis; SP@ASXNano: Astaxanthin nanoparticles-loaded Spirulina platensis; 4-HP: 4-hydroxyproline.
Acknowledgements
This research was funded by the National Natural Science Foundation of China (No. 82072051) and the Guiding Science and Technology Plan Project of Mudanjiang City (HT2022JG125).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Fu X-B. Repair cell first, then regenerate the tissues and organs. Military Med Res. 2021;8:2
2. Gao J, Yu X, Wang X, He Y, Ding J. Biomaterial-Related Cell Microenvironment in Tissue Engineering and Regenerative Medicine. Engineering-prc. 2022;13:31-45
3. Sachs PC, Mollica PA, Bruno RD. Tissue specific microenvironments: a key tool for tissue engineering and regenerative medicine. J Biol Eng. 2017;11:34
4. Nakkala JR, Li Z, Ahmad W, Wang K, Gao C. Immunomodulatory biomaterials and their application in therapies for chronic inflammation-related diseases. Acta Biomaterialia. 2021;123:1-30
5. Yu Z, Hong Y, Xie K, Fan Q. Research Progresses on the Physiological and Pharmacological Benefits of Microalgae-Derived Biomolecules. Foods. 2022;11:2806
6. Qiao Y, Yang F, Xie T, Du Z, Zhong D, Qi Y. et al. Engineered algae: A novel oxygen-generating system for effective treatment of hypoxic cancer. Sci Adv. 2020;6:eaba5996
7. Singh S, Kate BN, Banerjee UC. Bioactive Compounds from Cyanobacteria and Microalgae: An Overview. Critical Reviews in Biotechnology. 2005;25:73-95
8. Vornoli A, Grande T, Lubrano V, Vizzarri F, Gorelli C, Raffaelli A. et al. In Vitro Characterization of Antioxidant, Antibacterial and Antimutagenic Activities of the Green Microalga Ettlia pseudoalveolaris. Antioxidants-basel. 2023;12:1308
9. Coulombier N, Jauffrais T, Lebouvier N. Antioxidant Compounds from Microalgae: A Review. Mar Drugs. 2021;19:549
10. Rojas V, Rivas L, Cárdenas C, Guzmán F. Cyanobacteria and Eukaryotic Microalgae as Emerging Sources of Antibacterial Peptides. Molecules. 2020;25:5804
11. Nürnberg DJ, Morton J, Santabarbara S, Telfer A, Joliot P, Antonaru LA. et al. Photochemistry beyond the red limit in chlorophyll f-containing photosystems. Science. 2018;360:1210-3
12. Arrieta Payares LM, Gutiérrez Púa LDC, Di Mare Pareja LA, Paredes Méndez SC, Paredes Méndez VN. Microalgae Applications to Bone Repairing Processes: A Review. Acs Biomater Sci Eng. 2023;9:2991-3009
13. Zhao W, Duan M, Zhang X, Tan T. A mild extraction and separation procedure of polysaccharide, lipid, chlorophyll and protein from Chlorella spp. Renew Energ. 2018;118:701-8
14. De Melo RG, De Andrade AF, Bezerra RP, Viana Marques DDA, Da Silva VA, Paz ST. et al. Hydrogel-based Chlorella vulgaris extracts: a new topical formulation for wound healing treatment. J Appl Phycol. 2019;31:3653-63
15. Pratt R, Daniels TC, Eiler JJ, Gunnison JB, Kumler WD, Oneto JF. et al. Chlorellin, an Antibacterial Substance from Chlorella. Science. 1944;99:351-2
16. Napolitano G, Fasciolo G, Salbitani G, Venditti P. Chlorella sorokiniana Dietary Supplementation Increases Antioxidant Capacities and Reduces ROS Release in Mitochondria of Hyperthyroid Rat Liver. Antioxidants. 2020;9:883
17. Chen H, Guo Y, Zhang Z, Mao W, Shen C, Xiong W. et al. Symbiotic Algae-Bacteria Dressing for Producing Hydrogen to Accelerate Diabetic Wound Healing. Nano Lett. 2022;22:229-37
18. Zhou T-J, Xing L, Fan Y-T, Cui P-F, Jiang H-L. Light triggered oxygen-affording engines for repeated hypoxia-resistant photodynamic therapy. J Control Release. 2019;307:44-54
19. Lopes PA, Coelho D, Prates JAM. Testimony on a successful lab protocol to disrupt Chlorella vulgaris microalga cell wall. Koller M, Ed. PLoS ONE. 2022;17:e0268565
20. Cunha SA, Coscueta ER, Nova P, Silva JL, Pintado MM. Bioactive Hydrolysates from Chlorella vulgaris: Optimal Process and Bioactive Properties. Molecules. 2022;27:2505
21. Miller DH, Lamport DTA, Miller M. Hydroxyproline Heterooligosaccharides in Chlamydomonas. Science. 1972;176:918-20
22. Yasa O, Erkoc P, Alapan Y, Sitti M. Microalga-Powered Microswimmers toward Active Cargo Delivery. Adv Mater. 2018;30:e1804130
23. Ringo DL. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. The Journal of Cell Biology. 1967;33:543-71
24. Shamriz S, Ofoghi H. Outlook in the application of Chlamydomonas reinhardtii chloroplast as a platform for recombinant protein production. Biotechnology and Genetic Engineering Reviews. 2016;32:92-106
25. Chávez MN, Schenck TL, Hopfner U, Centeno-Cerdas C, Somlai-Schweiger I, Schwarz C. et al. Toward autotrophic tissue engineering: Photosynthesis-related gene therapy for regeneration. Biomaterials. 2016;75:25-36
26. Li X, Patena W, Fauser F, Jinkerson RE, Saroussi S, Meyer MT. et al. A genome-wide algal mutant library and functional screen identifies genes required for eukaryotic photosynthesis. Nat Genet. 2019;51:627-35
27. Jarquín-Cordero M, Chávez MN, Centeno-Cerdas C, Bohne A-V, Hopfner U, Machens H-G. et al. Toward a biotechnological platform for the production of human pro-angiogenic growth factors in the green alga Chlamydomonas reinhardtii. Appl Microbiol Biot. 2020;104:725-39
28. López-Paz C, Liu D, Geng S, Umen JG. Identification of Chlamydomonas reinhardtii endogenous genic flanking sequences for improved transgene expression. The Plant Journal. 2017;92:1232-44
29. Chen H, Yang Q, Xu J, Deng X, Zhang Y, Liu T. et al. Efficient methods for multiple types of precise gene-editing in Chlamydomonas. The Plant Journal. 2023;115:846-65
30. Saxena A, Dutta A, Kapoor N, Kumar A, Tiwari A. Envisaging marine diatom Thalassiosira weissflogii as a 'SMART' drug delivery system for insoluble drugs. J Drug Deliv Sci Tec. 2022;68:102983
31. Vona D, Cicco SR, Ragni R, Vicente-Garcia C, Leone G, Giangregorio MM. et al. Polydopamine coating of living diatom microalgae. Photochem Photobiol Sci. 2022;21:949-58
32. Kumeria T, Bariana M, Altalhi T, Kurkuri M, Gibson CT, Yang W. et al. Graphene oxide decorated diatom silica particles as new nanohybrids: toward smart natural drug microcarriers. J Mater Chem B. 2013;1:6302
33. Kaufhold S, Dohrmann R, Ulrichs Ch. Shelf life stability of diatomites. Appl Clay Sci. 2008;41:158-64
34. Afzal S, Jin L, Pan K, Duan D, Wei Y, Ahmad M. et al. CoM/diatomite (M = Mg and Al) for the activation of peroxymonosulfate to degrade atrazine in water: Effect of preparation method, role of acidic/basic sites, and catalytic mechanism. Appl Surf Sci. 2022;600:154026
35. Li J, Sun X, Zhang K, Yang G, Mu Y, Su C. et al. Chitosan/Diatom-Biosilica Aerogel with Controlled Porous Structure for Rapid Hemostasis. Adv Healthc Mater. 2020;9:2000951
36. Lee J, Lee HA, Shin M, Juang LJ, Kastrup CJ, Go GM. et al. Diatom Frustule Silica Exhibits Superhydrophilicity and Superhemophilicity. Acs Nano. 2020;14:4755-66
37. Lim H, Seo Y, Kwon D, Kang S, Yu J, Park H. et al. Recent Progress in Diatom Biosilica: A Natural Nanoporous Silica Material as Sustained Release Carrier. Pharmaceutics. 2023;15:2434
38. Wu Q, Liu L, Miron A, Klímová B, Wan D, Kuča K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol. 2016;90:1817-40
39. Cobbaut M, Van Lint J. Function and Regulation of Protein Kinase D in Oxidative Stress: A Tale of Isoforms. Oxidative Medicine and Cellular Longevity. 2018;2018:1-10
40. Zheng Y, Chen X, Zhang Q, Yang L, Chen Q, Chen Z. et al. Evaluation of Reactive Oxygen Species Scavenging of Polydopamine with Different Nanostructures. Adv Healthcare Materials. 2024;13:2302640
41. Lennicke C, Cochemé HM. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Molecular Cell. 2021;81:3691-707
42. Fratelli C, Burck M, Amarante MCA, Braga ARC. Antioxidant potential of nature's “something blue”: Something new in the marriage of biological activity and extraction methods applied to C-phycocyanin. Trends in Food Science & Technology. 2021;107:309-23
43. Alzokaky AA, Abdelkader EM, El-Dessouki AM, Khaleel SA, Raslan NA. C-phycocyanin protects against ethanol-induced gastric ulcers in rats: Role of HMGB1/NLRP3/NF-κB pathway. Basic Clin Pharma Tox. 2020;127:265-77
44. Paramanya A, Oyesiji Abiodun A, Shamsul Ola M, Ali A. Enhancing the quality and antioxidant capacity of phycocyanin extracted from Spirulina platensis PCC 7345: A quality-by-design approach. Arabian Journal of Chemistry. 2024;17:105653
45. Pentón-Rol G, Marín-Prida J, McCarty MF. C-Phycocyanin-derived Phycocyanobilin as a Potential Nutraceutical Approach for Major Neurodegenerative Disorders and COVID-19- induced Damage to the Nervous System. CN. 2021;19:2250-75
46. Silva-Neto AF, Fratelli C, Pucci VG, Boldarine VT, Ferreira YAM, Telles MM. et al. C-phycocyanin extracted from Spirulina using a green solvent approach presents an anti-obesity characteristic in mice fed a hyperlipidic diet. Journal of Functional Foods. 2023;108:105747
47. Zhong D, Zhang D, Chen W, He J, Ren C, Zhang X. et al. Orally deliverable strategy based on microalgal biomass for intestinal disease treatment. Sci Adv. 2021;7:eabi9265
48. Zhong D, Zhang D, Xie T, Zhou M. Biodegradable Microalgae-Based Carriers for Targeted Delivery and Imaging-Guided Therapy toward Lung Metastasis of Breast Cancer. Small. 2020;16:e2000819
49. Barsanti L, Birindelli L, Gualtieri P. Paramylon and Other Bioactive Molecules in Micro and Macroalgae. Int J Mol Sci. 2022;23:8301
50. Chen W, Li T, Du S, Chen H, Wang Q. Microalgal polyunsaturated fatty acids: Hotspots and production techniques. Front Bioeng Biotech. 2023;11:1146881
51. Alsenani F, Tupally KR, Chua ET, Eltanahy E, Alsufyani H, Parekh HS. et al. Evaluation of microalgae and cyanobacteria as potential sources of antimicrobial compounds. Saudi Pharm J. 2020;28:1834-41
52. Clemente I, Lamponi S, Tamasi G, Rodolfi L, Rossi C, Ristori S. Structuring and De-Structuring of Nanovectors from Algal Lipids: Simulated Digestion, Preliminary Antioxidant Capacity and In Vitro Tests. Pharmaceutics. 2022;14:1847
53. Abourehab MAS, Ansari MJ, Singh A, Hassan A, Abdelgawad MA, Shrivastav P. et al. Cubosomes as an emerging platform for drug delivery: a review of the state of the art. J Mater Chem B. 2022;10:2781-819
54. Barriga HMG, Holme MN, Stevens MM. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew Chem Int Ed. 2019;58:2958-78
55. Clemente I, Bonechi C, Rodolfi L, Bacia-Verloop M, Rossi C, Ristori S. Lipids from algal biomass provide new (nonlamellar) nanovectors with high carrier potentiality for natural antioxidants. Eur J Pharm Biopharm. 2021;158:410-6
56. Schulze C, Strehle A, Merdivan S, Mundt S. Carbohydrates in microalgae: Comparative determination by TLC, LC-MS without derivatization, and the photometric thymol-sulfuric acid method. Algal Research. 2017;25:372-80
57. Kalita P, Ahmed AB, Sen S, Chakraborty R. A comprehensive review on polysaccharides with hypolipidemic activity: Occurrence, chemistry and molecular mechanism. Int J Biol Macromol. 2022;206:681-98
58. Shannon E, Abu-Ghannam N. Antibacterial Derivatives of Marine Algae: An Overview of Pharmacological Mechanisms and Applications. Marine Drugs. 2016;14:81
59. Pierre G, Sopena V, Juin C, Mastouri A, Graber M, Maugard T. Antibacterial activity of a sulfated galactan extracted from the marine alga Chaetomorpha aerea against Staphylococcus aureus. Biotechnol Bioproc E. 2011;16:937-45
60. Zhang J, Cao Y, Wang J, Guo X, Zheng Y, Zhao W. et al. Physicochemical characteristics and bioactivities of the exopolysaccharide and its sulphated polymer from Streptococcus thermophilus GST-6. Carbohydrate Polymers. 2016;146:368-75
61. Kim M, Yim JH, Kim S-Y, Kim HS, Lee WG, Kim SJ. et al. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir Res. 2012;93:253-9
62. Lee J-B, Hayashi K, Hirata M, Kuroda E, Suzuki E, Kubo Y. et al. Antiviral Sulfated Polysaccharide from Navicula directa, a Diatom Collected from Deep-Sea Water in Toyama Bay. Biological & Pharmaceutical Bulletin. 2006;29:2135-9
63. Hayashi T, Hayashi K, Maeda M, Kojima I. Calcium Spirulan, an Inhibitor of Enveloped Virus Replication, from a Blue-Green Alga Spirulina platensis. J Nat Prod. 1996;59:83-7
64. Wei Q, Fu G, Wang K, Yang Q, Zhao J, Wang Y. et al. Advances in Research on Antiviral Activities of Sulfated Polysaccharides from Seaweeds. Pharmaceuticals-base. 2022;15:581
65. Lu W, Yang Z, Chen J, Wang D, Zhang Y. Recent advances in antiviral activities and potential mechanisms of sulfated polysaccharides. Carbohydrate Polymers. 2021;272:118526
66. Machihara K, Oki S, Maejima Y, Kageyama S, Onda A, Koseki Y. et al. Restoration of mitochondrial function by Spirulina polysaccharide via upregulated SOD2 in aging fibroblasts. iScience. 2023;26:107113
67. Sirohi P, Verma H, Singh SK, Singh VK, Pandey J, Khusharia S. et al. Microalgal Carotenoids: Therapeutic Application and Latest Approaches to Enhance the Production. CIMB. 2022;44:6257-79
68. Arias D, Arenas-M A, Flores-Ortiz C, Peirano C, Handford M, Stange C. Daucus carota DcPSY2 and DcLCYB1 as Tools for Carotenoid Metabolic Engineering to Improve the Nutritional Value of Fruits. Front Plant Sci. 2021;12:677553
69. Shang M, Sun J, Bi Y, Xu X, Zang X. Fluorescence and antioxidant activity of heterologous expression of phycocyanin and allophycocyanin from Arthrospira platensis. Front Nutr. 2023;10:1127422
70. Du S-W, Zhang L-K, Han K, Chen S, Hu Z, Chen W. et al. Combined Phycocyanin and Hematoporphyrin Monomethyl Ether for Breast Cancer Treatment via Photosensitizers Modified Fe 3 O 4 Nanoparticles Inhibiting the Proliferation and Migration of MCF-7 Cells. Biomacromolecules. 2018;19:31-41
71. Huang L, Huang X-H, Yang X, Hu J-Q, Zhu Y-Z, Yan P-Y. et al. Novel nanodrug delivery system for natural products and their application. Pharmacological Research. 2024;201:107100
72. Ren C, Zhong D, Qi Y, Liu C, Liu X, Chen S. et al. Bioinspired pH-Responsive Microalgal Hydrogels for Oral Insulin Delivery with Both Hypoglycemic and Insulin Sensitizing Effects. Acs Nano. 2023;17:14161-75
73. Ruggiero I, Terracciano M, Martucci NM, De Stefano L, Migliaccio N, Tatè R. et al. Diatomite silica nanoparticles for drug delivery. Nanoscale Res Lett. 2014;9:329
74. An X, Zhong D, Wu W, Wang R, Yang L, Jiang Q. et al. Doxorubicin-Loaded Microalgal Delivery System for Combined Chemotherapy and Enhanced Photodynamic Therapy of Osteosarcoma. Acs Appl Mater Inter. 2024;16:6868-78
75. Zhang F, Li Z, Duan Y, Abbas A, Mundaca-Uribe R, Yin L. et al. Gastrointestinal tract drug delivery using algae motors embedded in a degradable capsule. Sci Robot. 2022;7:eabo4160
76. Weibel DB, Garstecki P, Ryan D, DiLuzio WR, Mayer M, Seto JE. et al. Microoxen: microorganisms to move microscale loads. Proc Natl Acad Sci U S A. 2005;102:11963-7
77. Zhang F, Li Z, Chen C, Luan H, Fang RH, Zhang L. et al. Biohybrid Microalgae Robots: Design, Fabrication, Materials, and Applications. Adv Mater. 2023 2303714
78. Zhang F, Zhuang J, Li Z, Gong H, de Ávila BE-F, Duan Y. et al. Nanoparticle-modified microrobots for in vivo antibiotic delivery to treat acute bacterial pneumonia. Nat Mater. 2022;21:1324-32
79. Fang RH, Kroll AV, Gao W, Zhang L. Cell Membrane Coating Nanotechnology. Adv Mater. 2018;30:e1706759
80. Hayashi K, Yamada S, Sakamoto W, Usugi E, Watanabe M, Yogo T. Red Blood Cell-Shaped Microparticles with a Red Blood Cell Membrane Demonstrate Prolonged Circulation Time in Blood. ACS Biomater Sci Eng. 2018;4:2729-32
81. Gao W, Xiao L, Mu Y, Xiao Y. Targeting macrophage endocytosis via platelet membrane coating for advanced osteoimmunomodulation. Iscience. 2022;25:105196
82. Bahmani B, Gong H, Luk BT, Haushalter KJ, DeTeresa E, Previti M. et al. Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat Commun. 2021;12:1999
83. Chen L, Zhou Z, Hu C, Maitz MF, Yang L, Luo R. et al. Platelet Membrane-Coated Nanocarriers Targeting Plaques to Deliver Anti-CD47 Antibody for Atherosclerotic Therapy. Research. 2022;2022:2022 /9845459
84. Cheng Gao, Kwong CHT, Wang Q, Kam H, Xie B, Lee SM-Y. et al. Conjugation of Macrophage-Mimetic Microalgae and Liposome for Antitumor Sonodynamic Immunotherapy via Hypoxia Alleviation and Autophagy Inhibition. ACS Nano. 2023;17:4034-49
85. Lopes J, Lopes D, Pereira-Silva M, Peixoto D, Veiga F, Hamblin MR. et al. Macrophage Cell Membrane-Cloaked Nanoplatforms for Biomedical Applications. Small Methods. 2022;6:2200289
86. Rao L, Bu L, Cai B, Xu J, Li A, Zhang W. et al. Cancer Cell Membrane-Coated Upconversion Nanoprobes for Highly Specific Tumor Imaging. Adv Mater. 2016;28:3460-6
87. Zhu J-Y, Zheng D-W, Zhang M-K, Yu W-Y, Qiu W-X, Hu J-J. et al. Preferential Cancer Cell Self-Recognition and Tumor Self-Targeting by Coating Nanoparticles with Homotypic Cancer Cell Membranes. Nano Lett. 2016;16:5895-901
88. Krishnan N, Fang RH, Zhang L. Cell membrane-coated nanoparticles for the treatment of cancer. Clinical & Translational Med. 2023;13:e1285
89. Zhong D, Li W, Qi Y, He J, Zhou M. Photosynthetic Biohybrid Nanoswimmers System to Alleviate Tumor Hypoxia for FL/PA/MR Imaging-Guided Enhanced Radio-Photodynamic Synergetic Therapy. Adv Funct Materials. 2020;30:1910395
90. Santomauro G, Singh AV, Park B, Mohammadrahimi M, Erkoc P, Goering E. et al. Incorporation of Terbium into a Microalga Leads to Magnetotactic Swimmers. Adv Biosyst. 2018;2:1800039
91. Song Y, Zhang Y, Qu Q, Zhang X, Lu T, Xu J. et al. Biomaterials based on hyaluronic acid, collagen and peptides for three-dimensional cell culture and their application in stem cell differentiation. Int J Biol Macromol. 2023;226:14-36
92. Chen H, Cheng Y, Tian J, Yang P, Zhang X, Chen Y. et al. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Sci Adv. 2020;6:eaba4311
93. Zhao S, Guo C, Kumarasena A, Omenetto FG, Kaplan DL. 3D Printing of Functional Microalgal Silk Structures for Environmental Applications. ACS Biomater Sci Eng. 2019;5:4808-16
94. Wang X, Yang C, Yu Y, Zhao Y. In Situ 3D Bioprinting Living Photosynthetic Scaffolds for Autotrophic Wound Healing. Research (Wash D C). 2022;2022:9794745
95. Liu W, Zu L, Wang S, Li J, Fei X, Geng M. et al. Tailored biomedical materials for wound healing. Burns Trauma. 2023;11:tkad040
96. Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10:200223
97. Wang M, Sun Y, Li L, Wu P, Dkw O, Shi H. Calcium Channels: Noteworthy Regulators and Therapeutic Targets in Dermatological Diseases. Front Pharmacol. 2021;12:702264
98. Hazrati R, Davaran S, Omidi Y. Bioactive functional scaffolds for stem cells delivery in wound healing and skin regeneration. Reactive and Functional Polymers. 2022;174:105233
99. Bezold V, Rosenstock P, Scheffler J, Geyer H, Horstkorte R, Bork K. Glycation of macrophages induces expression of pro-inflammatory cytokines and reduces phagocytic efficiency. Aging (Albany NY). 2019;11:5258-75
100. Zhou Z, Deng T, Tao M, Lin L, Sun L, Song X. et al. Snail-inspired AFG/GelMA hydrogel accelerates diabetic wound healing via inflammatory cytokines suppression and macrophage polarization. Biomaterials. 2023;299:122141
101. Liu J, Chen Z, Liu H, Qin S, Li M, Shi L. et al. Nickel-Based Metal-Organic Frameworks Promote Diabetic Wound Healing via Scavenging Reactive Oxygen Species and Enhancing Angiogenesis. Small. 2023;20:2305076
102. Cui H, Su Y, Wei W, Xu F, Gao J, Zhang W. How Microalgae is Effective in Oxygen Deficiency Aggravated Diseases?. A Comprehensive Review of Literature. Int J Nanomed. 2022;17:3101-22
103. Darby IA, Hewitson TD. Hypoxia in tissue repair and fibrosis. Cell Tissue Res. 2016;365:553-62
104. Liakos A, Liakopoulou P, Tsapas A. Cyclical pressurized topical wound oxygen therapy increased healing of refractory diabetic foot ulcers. Ann Intern Med. 2020;172:JC27
105. Huang X, Liang P, Jiang B, Zhang P, Yu W, Duan M. et al. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast proliferation and endothelial cell angiogenesis. Life Sciences. 2020;259:118246
106. Oropallo AR, Serena TE, Armstrong DG, Niederauer MQ. Molecular Biomarkers of Oxygen Therapy in Patients with Diabetic Foot Ulcers. Biomolecules. 2021;11:925
107. Chávez MN, Moellhoff N, Schenck TL, Egaña JT, Nickelsen J. Photosymbiosis for Biomedical Applications. Front Bioeng Biotechnol. 2020;8:577204
108. Chen H-H, Fu F-S, Chen Q-W, Zhang Y, Zhang X-Z. Two-Pronged Microbe Delivery of Nitric Oxide and Oxygen for Diabetic Wound Healing. Nano Lett. 2023;23:5595-602
109. Wu Y, Li M, He R, Xiao L, Liu S, Chen K. et al. Photosynthetic live microorganism-incorporated hydrogels promote diabetic wound healing via self-powering and oxygen production. Chemical Engineering Journal. 2024;485:149545
110. Wang Y, Yang H, Zhang X, Han F, Tu W, Yang W. Microalgal Hydrogen Production. Small Methods. 2020;4:1900514
111. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K. et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688-94
112. Wang S, Liu K, Zhou Q, Xu C, Gao J, Wang Z. et al. Hydrogen-Powered Microswimmers for Precise and Active Hydrogen Therapy Toward Acute Ischemic Stroke. Adv Funct Mater. 2021;31:2009475
113. Ding J, Xu K, Xu H, Ji J, Qian Y, Shen J. Advances in Gas Therapeutics for Wound Healing: Mechanisms, Delivery Materials, and Prospects. Small Struct. 2024;5:2300151
114. Kang Y, Xu L, Dong J, Yuan X, Ye J, Fan Y. et al. Programmed microalgae-gel promotes chronic wound healing in diabetes. Nat Commun. 2024;15:1042
115. Aliakbar Ahovan Z, Esmaeili Z, Eftekhari BS, Khosravimelal S, Alehosseini M, Orive G. et al. Antibacterial smart hydrogels: New hope for infectious wound management. Mater Today, Bio. 2022;17:100499
116. Malone M, Schultz G. Challenges in the diagnosis and management of wound infection. Brit J Dermatol. 2022;187:159-66
117. Nazli A, He DL, Liao D, Khan MZI, Huang C, He Y. Strategies and progresses for enhancing targeted antibiotic delivery. Adv Drug Deliver Rev. 2022;189:114502
118. Shchelik IS, Sieber S, Gademann K. Green Algae as a Drug Delivery System for the Controlled Release of Antibiotics. Chemistry A European J. 2020;26:16644-8
119. Ma J, Fang Y, Yu H, Yi J, Ma Y, Lei P. et al. Recent Advances in Living Algae Seeding Wound Dressing: Focusing on Diabetic Chronic Wound Healing. Adv Funct Materials. 2024;34:2308387
120. Li W, Wang S, Zhong D, Du Z, Zhou M. A Bioactive Living Hydrogel: Photosynthetic Bacteria Mediated Hypoxia Elimination and Bacteria-Killing to Promote Infected Wound Healing. Adv Ther-germany. 2021;4:2000107
121. Xie L, Pang X, Yan X, Dai Q, Lin H, Ye J. et al. Photoacoustic Imaging-Trackable Magnetic Microswimmers for Pathogenic Bacterial Infection Treatment. ACS Nano. 2020;14:2880-93
122. Lim Y, Park S-H, Kim EJ, Lim H, Jang J, Hong I-S. et al. Polar microalgae extracts protect human HaCaT keratinocytes from damaging stimuli and ameliorate psoriatic skin inflammation in mice. Biol Res. 2023;56:40
123. Liu P, Choi J-W, Lee M-K, Choi YH, Nam T-J. Spirulina protein promotes skin wound repair in a mouse model of full-thickness dermal excisional wound. Int J Mol Med. 2020;46:351-9
124. Centeno-Cerdas C, Jarquín-Cordero M, Chávez MN, Hopfner U, Holmes C, Schmauss D. et al. Development of photosynthetic sutures for the local delivery of oxygen and recombinant growth factors in wounds. Acta Biomater. 2018;81:184-94
125. Singh P, Maparu AK, Shah S, Rai B, Sivakumar S. Biomimetic algal polysaccharide coated 3D nanofibrous scaffolds promote skin extracellular matrix formation. Mater Sci Eng C Mater Biol Appl. 2021;119:111580
126. Fields FJ, Lejzerowicz F, Schroeder D, Ngoi SM, Tran M, McDonald D. et al. Effects of the microalgae Chlamydomonas on gastrointestinal health. J Funct Foods. 2020;65:103738
127. Liu C, Ye Q, Hua S, Huang H, Zhong D, Liang F. et al. Microalgae-based natural oral hydrogel system for synergistic treatment of lead poisoning-related diseases. Nano Today. 2023;53:102034
128. Zhou Z, Guan B, Xia H, Zheng R, Xu B. Particle radiotherapy in the era of radioimmunotherapy. Cancer Letters. 2023;567:216268
129. Cytlak UM, Dyer DP, Honeychurch J, Williams KJ, Travis MA, Illidge TM. Immunomodulation by radiotherapy in tumor control and normal tissue toxicity. Nat Rev Immunol. 2022;22:124-38
130. Zhang D, Zhong D, Ouyang J, He J, Qi Y, Chen W. et al. Microalgae-based oral microcarriers for gut microbiota homeostasis and intestinal protection in cancer radiotherapy. Nat Commun. 2022;13:1413
131. Liu Y, Miao L, Guo Y, Tian H. Preclinical Evaluation of Safety, Pharmacokinetics, Efficacy, and Mechanism of Radioprotective Agent HL-003. Dos Santos JM, Ed. Oxidative Medicine and Cellular Longevity. 2021;2021:1-11
132. Zhang D, He J, Cui J, Wang R, Tang Z, Yu H. et al. Oral Microalgae-Nano Integrated System against Radiation-Induced Injury. ACS Nano. 2023;17:10560-76
133. Wang S, Deng Z, Ma Y, Jin J, Qi F, Li S. et al. The Role of Autophagy and Mitophagy in Bone Metabolic Disorders. Int J Biol Sci. 2020;16:2675-91
134. Borciani G, Montalbano G, Baldini N, Cerqueni G, Vitale-Brovarone C, Ciapetti G. Co-culture systems of osteoblasts and osteoclasts: Simulating in vitro bone remodeling in regenerative approaches. Acta Biomater. 2020;108:22-45
135. Hwang Y-H, Kim K-J, Kim S-J, Mun S-K, Hong S-G, Son Y-J. et al. Suppression Effect of Astaxanthin on Osteoclast Formation In Vitro and Bone Loss In Vivo. Int J Mol Sci. 2018;19:912
136. El-Baz FK, Saleh DO, Abdel Jaleel GA, Hussein RA, Hassan A. Heamatococcus pluvialis ameliorates bone loss in experimentally induced osteoporosis in rats via the regulation of OPG/RANKL pathway. Biomed Pharmacother. 2019;116:109017
137. Yuce H, Lektemur Alpan A, Gevrek F, Toker H. Investigation of the effect of astaxanthin on alveolar bone loss in experimental periodontitis. J Periodontal Res. 2018;53:131-8
138. Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioactive Materials. 2017;2:224-47
139. Barua E, Deoghare AB, Chatterjee S, Mate VR. Characterization of Mechanical and Micro-Architectural Properties of Porous Hydroxyapatite Bone Scaffold Using Green MicroAlgae as Binder. Arab J Sci Eng. 2019;44:7707-22
140. Cicco SR, Vona D, Leone G, De Giglio E, Bonifacio MA, Cometa S. et al. In vivo functionalization of diatom biosilica with sodium alendronate as osteoactive material. Materials Science and Engineering: C. 2019;104:109897
141. Amoda A, Borkiewicz L, Rivero-Müller A, Alam P. Sintered nanoporous biosilica diatom frustules as high efficiency cell-growth and bone-mineralization platforms. Mater Today, Commun. 2020;24:100923
142. López-Álvarez M, Solla EL, González P, Serra J, León B, Marques AP. et al. Silicon-hydroxyapatite bioactive coatings (Si-HA) from diatomaceous earth and silica. Study of adhesion and proliferation of osteoblast-like cells. J Mater Sci-mater M. 2009;20:1131-6
143. Liu M, Galli G, Wang Y, Fan Q, Wang Z, Wang X. et al. Novel Therapeutic Targets for Hypoxia-Related Cardiovascular Diseases: The Role of HIF-1. Front Physiol. 2020;11:774
144. Yu B, Wang X, Song Y, Xie G, Jiao S, Shi L. et al. The role of hypoxia-inducible factors in cardiovascular diseases. Pharmacology & Therapeutics. 2022;238:108186
145. Hofmann R, James SK. Routine Oxygen Supplementation in Acute Cardiovascular Disease: The End of a Paradigm? Circulation. 2018;137:320-2
146. Cohen JE, Goldstone AB, Paulsen MJ, Shudo Y, Steele AN, Edwards BB. et al. An innovative biologic system for photon-powered myocardium in the ischemic heart. Sci Adv. 2017;3:e1603078
147. Stapleton LM, Farry JM, Zhu Y, Lucian HJ, Wang H, Paulsen MJ. et al. Microfluidic encapsulation of photosynthetic cyanobacteria in hydrogel microparticles augments oxygen delivery to rescue ischemic myocardium. Journal of Bioscience and Bioengineering. 2023;135:493-9
148. Myhre PL, Kalstad AA, Tveit SH, Laake K, Schmidt EB, Smith P. et al. Changes in eicosapentaenoic acid and docosahexaenoic acid and risk of cardiovascular events and atrial fibrillation: A secondary analysis of the OMEMI trial. J Intern Med. 2022;291:637-47
149. Ejike CECC, Ezeorba TPC, Ajah O, Udenigwe CC. Big Things, Small Packages: An Update on Microalgae as Sustainable Sources of Nutraceutical Peptides for Promoting Cardiovascular Health. Glob Chall. 2023;7:2200162
150. Chen J, Tan L, Li C, Zhou C, Hong P, Sun S. et al. Mechanism Analysis of a Novel Angiotensin-I-Converting Enzyme Inhibitory Peptide from Isochrysis zhanjiangensis Microalgae for Suppressing Vascular Injury in Human Umbilical Vein Endothelial Cells. J Agr Food Chem. 2020;68:4411-23
151. Sun X, Li J, Shao K, Su C, Bi S, Mu Y. et al. A composite sponge based on alkylated chitosan and diatom-biosilica for rapid hemostasis. Int J Biol Macromol. 2021;182:2097-107
152. Wang C, Zhao Y, Wang L, Pan S, Liu Y, Li S. et al. C-phycocyanin Mitigates Cognitive Impairment in Doxorubicin-Induced Chemobrain: Impact on Neuroinflammation, Oxidative Stress, and Brain Mitochondrial and Synaptic Alterations. Neurochem Res. 2021;46:149-58
153. Sasaki K, Linh TN, Hirano A, Tominaga K, Nukaga S, Nozaki H. et al. Microalgae extract induces antidepressant-like activity via neuroinflammation regulation and enhances the neurotransmitter system. Food and Chemical Toxicology. 2022;170:113508
154. Gallego R, Valdés A, Suárez-Montenegro ZJ, Sánchez-Martínez JD, Cifuentes A, Ibáñez E. et al. Anti-inflammatory and neuroprotective evaluation of diverse microalgae extracts enriched in carotenoids. Algal Research. 2022;67:102830
155. Sasaki K, Geribaldi-Doldán N, Wu Q, Davies J, Szele FG, Isoda H. Microalgae Aurantiochytrium Sp. Increases Neurogenesis and Improves Spatial Learning and Memory in Senescence-Accelerated Mouse-Prone 8 Mice. Front Cell Dev Biol. 2021;8:600575
156. Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263-8
157. G N, Tan A, Farhatnia Y, Rajadas J, Hamblin MR, Khaw PT. et al. Channelrhodopsins: visual regeneration and neural activation by a light switch. New Biotechnol. 2013;30:461-74
158. Ferrazzano GF, Papa C, Pollio A, Ingenito A, Sangianantoni G, Cantile T. Cyanobacteria and Microalgae as Sources of Functional Foods to Improve Human General and Oral Health. Molecules. 2020;25:5164
159. Fischer NG, Münchow EA, Tamerler C, Bottino MC, Aparicio C. Harnessing biomolecules for bioinspired dental biomaterials. J Mater Chem B. 2020;8:8713-47
160. Kaipa VRK, Asif SM, Assiri KI, Saquib SA, Arem SA, Sree S. et al. Antioxidant effect of spirulina in chronic periodontitis. Medicine. 2022;101:e31521
161. Zhou J, Wang M, Saraiva JA, Martins AP, Pinto CA, Prieto MA. et al. Extraction of lipids from microalgae using classical and innovative approaches. Food Chemistry. 2022;384:132236
162. Timira V, Meki K, Li Z, Lin H, Xu M, Pramod SN. A comprehensive review on the application of novel disruption techniques for proteins release from microalgae. Critical Reviews in Food Science and Nutrition. 2022;62:4309-25
163. Günerken E, D'Hondt E, Eppink MHM, Garcia-Gonzalez L, Elst K, Wijffels RH. Cell disruption for microalgae biorefineries. Biotechnology Advances. 2015;33:243-60
164. Alavijeh RS, Karimi K, Wijffels RH, Van Den Berg C, Eppink M. Combined bead milling and enzymatic hydrolysis for efficient fractionation of lipids, proteins, and carbohydrates of Chlorella vulgaris microalgae. Bioresource Technology. 2020;309:123321
165. Käferböck A, Smetana S, De Vos R, Schwarz C, Toepfl S, Parniakov O. Sustainable extraction of valuable components from Spirulina assisted by pulsed electric fields technology. Algal Research. 2020;48:101914
166. Soto-Sierra L, Wilken LR, Dixon CK. Aqueous enzymatic protein and lipid release from the microalgae Chlamydomonas reinhardtii. Bioresour Bioprocess. 2020;7:46
167. Hildebrand G, Poojary MM, O'Donnell C, Lund MN, Garcia-Vaquero M, Tiwari BK. Ultrasound-assisted processing of Chlorella vulgaris for enhanced protein extraction. J Appl Phycol. 2020;32:1709-18
168. Ruiz-Domínguez MC, Jáuregui M, Medina E, Jaime C, Cerezal P. Rapid Green Extractions of C-Phycocyanin from Arthrospira maxima for Functional Applications. Applied Sciences. 2019;9:1987
169. Pagels F, Pereira RN, Vicente AA, Guedes AC. Extraction of Pigments from Microalgae and Cyanobacteria—A Review on Current Methodologies. Applied Sciences. 2021;11:5187
170. Pasquet V, Chérouvrier J-R, Farhat F, Thiéry V, Piot J-M, Bérard J-B. et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochemistry. 2011;46:59-67
171. Fatima I, Munir M, Qureshi R, Hanif U, Gulzar N, Sheikh AA. Advanced methods of algal pigments extraction: A review. Critical Reviews in Food Science and Nutrition. 2023:1-18
172. Xiong W, Peng Y, Ma W, Xu X, Zhao Y, Wu J. et al. Microalgae-material hybrid for enhanced photosynthetic energy conversion: a promising path toward carbon neutrality. National Science Review. 2023;10:nwad200
173. Li N, Wang P, Wang S, Wang C, Zhou H, Kapur S. et al. Electrostatic charges on microalgae surface: Mechanism and applications. Journal of Environmental Chemical Engineering. 2022;10:107516
174. Liu Y, Luo J, Chen X, Liu W, Chen T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications. Nano-Micro Lett. 2019;11:100
175. Zhang Z, Chen Y, Klausen LH, Skaanvik SA, Wang D, Chen J. et al. The Rational Design and Development of Microalgae-Based Biohybrid Materials for Biomedical Applications. Engineering. 2023;24:102-13
176. Hurtado-Gallego J, Pulido-Reyes G, González-Pleiter M, Salas G, Leganés F, Rosal R. et al. Toxicity of superparamagnetic iron oxide nanoparticles to the microalga Chlamydomonas reinhardtii. Chemosphere. 2020;238:124562
177. Martin N, Bernat T, Dinasquet J, Stofko A, Damon A, Deheyn DD. et al. Synthetic algal-bacteria consortia for space-efficient microalgal growth in a simple hydrogel system. J Appl Phycol. 2021;33:2805-15
178. Homburg SV, Patel AV. Silica Hydrogels as Entrapment Material for Microalgae. Polymers. 2022;14:1391
179. Liu H, Yu S, Liu B, Xiang S, Jiang M, Yang F. et al. Space-Efficient 3D Microalgae Farming with Optimized Resource Utilization for Regenerative Food. Advanced Materials. 2024;36:2401172
180. Kumar V, Vlaskin MS, Grigorenko AV. 3D Bioprinting to Fabricate Living Microalgal Materials. Trends Biotechnol. 2021;39:1243-4
181. Levy-Ontman O, Huleihel M, Hamias R, Wolak T, Paran E. An anti-inflammatory effect of red microalga polysaccharides in coronary artery endothelial cells. Atherosclerosis. 2017;264:11-8
182. Singh NK, Ramamourthy B, Hage N, Kappagantu KM. Optogenetics: Illuminating the Future of Hearing Restoration and UnderstandingAuditory Perception. CGT [Internet]. 2024 [cited 9 February 2024]; 24. Available at: https://www.eurekaselect.com/225203/article
183. Morsy MA, Gupta S, Nair AB, Venugopala KN, Greish K, El-Daly M. Protective Effect of Spirulina platensis Extract against Dextran-Sulfate-Sodium-Induced Ulcerative Colitis in Rats. Nutrients. 2019;11:2309
184. Choi H, Kim B, Jeong SH, Kim TY, Kim D, Oh Y. et al. Microalgae-Based Biohybrid Microrobot for Accelerated Diabetic Wound Healing. Small. 2023;19:2204617
185. Gao S, Rao Y, Wang X, Zhang Q, Zhang Z, Wang Y. et al. Chlorella-Loaded Antibacterial Microneedles for Microacupuncture Oxygen Therapy of Diabetic Bacterial Infected Wounds. Advanced Materials. 2024;36:2307585
186. Zhong D, Jin K, Wang R, Chen B, Zhang J, Ren C. et al. Microalgae-Based Hydrogel for Inflammatory Bowel Disease and Its Associated Anxiety and Depression. Advanced Materials. 2024;36:2312275
187. Hendrijantini N, Sitalaksmi RM, Ari MDA, Hidayat TJ, Putri PAN, Sukandar D. The expression of TNF-α, IL-1Î2, and IL-10 in the diabetes mellitus condition induced by the combination of spirulina and chitosan. Bali Med J. 2020;9:22-6
188. Jin N, Wu J, Ye S, Xue J, Meng T, Hu L. et al. Injectable Dynamic ROS-Responsive COF-Modified Microalgae Gels for In Vivo bFGF Delivery to Treat Diabetic Wounds. ACS Appl Mater Interfaces. 2024;16:18608-26
189. Feng C, Li J, Wu GS, Mu YZ, Kong M, Jiang CQ. et al. Chitosan-Coated Diatom Silica as Hemostatic Agent for Hemorrhage Control. Acs Appl Mater Inter. 2016;8:34234-43
Author contact
![]() Corresponding authors: E-mail addresses: wuyanedu.cn (Y. Wu), gaojiehighcleaedu.cn (J. Gao), lemonlives_drcom (M. Li).
Corresponding authors: E-mail addresses: wuyanedu.cn (Y. Wu), gaojiehighcleaedu.cn (J. Gao), lemonlives_drcom (M. Li).
 Global reach, higher impact
Global reach, higher impact