13.3
Impact Factor
Theranostics 2024; 14(12):4598-4621. doi:10.7150/thno.97096 This issue Cite
Review
Exosome-like nanoparticles derived from fruits, vegetables, and herbs: innovative strategies of therapeutic and drug delivery
1. Department of Pharmacy, Ningbo Municipal Hospital of Traditional Chinese Medicine (TCM), Affiliated Hospital of Zhejiang Chinese Medical University, Ningbo 315016, China.
2. State Key Laboratory of Advanced Drug Delivery and Release Systems, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China.
3. Department of Dermatology Medical Cosmetology Center, Ningbo Municipal Hospital of Traditional Chinese Medicine (TCM), Affiliated Hospital of Zhejiang Chinese Medical University, Ningbo 315016, China.
* These authors contributed equally to this work.
Received 2024-4-8; Accepted 2024-7-19; Published 2024-8-1
Abstract
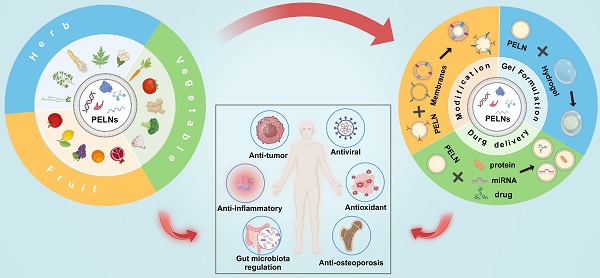
Over the past ten years, significant advancements have been made in exploring plant-derived exosome-like nanoparticles (PELNs) for disease therapeutics and drug delivery. PELNs, as inherent nanoscale particles comprised of proteins, lipids, nucleic acids, and secondary metabolites, exhibit the capacity for cellular uptake by human cells. This intercellular interaction transcends biological boundaries, effectively influencing biological functions in animals. PELNs have outstanding biocompatibility, low immunogenicity, enhanced safety, and environmentally friendly sustainability. This article summarized the preparation methods and characteristics of PELNs. It provided a systematic review of the varied roles of PELNs derived from fruits, vegetables, and herbs in disease therapeutics and drug delivery. The challenges in their production and application were discussed, and future prospects in this rapidly evolving field were explored.
Keywords: exosomes, plant-derived exosome-like nanoparticles, biotherapy, drug delivery, challenge
1. Introduction
There have been significant advancements over the past decade in therapeutic strategies of cell-derived products, particularly extracellular vesicles (EVs). Exosomes, the smallest and most extensively studied EVs, can be produced by bacteria, animals, and plants [1] to facilitate intercellular communication [2]. There is accumulating evidence that exosomes are involved in the progression of diverse disease processes and possess multifaceted biological functions, such as antigen presentation, modulation of immune responses, and cytokine transport [3-5]. Due to their favorable biocompatibility, low cytotoxicity, and minimal immunogenicity, exosomes have been applied in the diagnosis and treatment of various diseases [6]. Furthermore, exosomes exhibit specific targeting capabilities and the ability to traverse biological barriers, positioning them as promising carriers for drug delivery.
Traditionally, research has primarily focused on exosomes derived from stem cells and tumor cells but with advancements in nanoscience, increasing attention is being directed towards the presence and role of exosomes in edible plants. The exploration of plant exosomes commenced when Regente et al. first isolated exosomes in plants (sunflower seedlings) through transmission electron microscopy and proteomic analysis in 2009 [7]. However, there is a lack of consensus definitions, consistent nomenclature, and standardized practices for the extracted biological vesicles derived from plants owing to the lack of well-defined biological pathways and rigorous physicochemical characterization. These nanoparticles have been termed plant-derived exosome-like nanoparticles (PELNs) in this review. Their therapeutic potential has been examined, revealing structural, cargo, and release mechanisms similar to exosomes from animal sources [8]. Particularly, microRNAs (miRNAs) from plants discovered in the serum of healthy Chinese males and females were found to regulate the translation of mammalian LDLRAP1 similarly to mammalian miRNAs [9]. Therefore, miRNAs derived from plants may exert biological effects across different species. It is noteworthy that a multitude of miRNAs have been detected in PELNs, which are absorbed by the human body and exert therapeutic effects [10-13]. PELNs could potentially serve as a pathway for plants to transfer miRNAs to animals.
PELNs have garnered significant attention due to their abundant sources and broad potential applications in biomedical and nanotechnology fields. This article categorized plants into three groups: fruits, vegetables, and herbs, and explored the biomedical applications of PELNs derived from each group. Fruits, such as grapes, lemons, and apples, are commonly consumed as desserts or snacks. Vegetables, including ginger, broccoli, and bitter melon, are predominantly used in cooking or salads. Herbs, such as ginseng, pueraria lobata, and lonicera japonica, are typically used for medicinal purposes. PELNs derived from these sources exhibit therapeutic functions such as anti-inflammatory, anti-tumor, and gut microbiota modulation properties. Fruit-derived PELNs demonstrate antioxidant capabilities, vegetable-derived PELNs show antiviral and insulin resistance regulation abilities, and herb-derived PELNs have regenerative and anti-osteoporosis effects. Furthermore, PELNs have been applied as drug delivery carriers owing to their exceptional biocompatibility, stability, and ability to deliver both hydrophilic and hydrophobic cargo in various therapeutic applications, such as tumor-targeted delivery, colon-targeted delivery, transdermal delivery, and gene delivery [14-17]. This article provides an overview of the biogenesis, composition, and uptake mechanisms of PELNs to solidify the substantial potential of PELNs derived from fruits, vegetables, and herbs in biotherapy and drug delivery while highlighting the limitations and challenges associated with their applications.
2. Overview of PELNs
Plant cells release PELNs in response to various environmental stresses such as pathogenic infections and these PELNs share similarities with exosomes derived from animal sources in terms of surface charge, morphology, and content composition [18]. PELNs contain miRNA, lipids, and proteins, serving as extracellular messengers that mediate intercellular communication. Moreover, PELNs have an inherent capability to deliver chemical molecules. The significance of PELNs in inter-species communication arises from their diversity of biomolecules (proteins, lipids, nucleic acids, and secondary metabolites) and their ease of internalization by mammalian cells [19]. Hence, PELNs present a promising avenue for the efficient delivery of targeted drugs, offering a natural therapeutic strategy for a wide range of diseases. Furthermore, unlike exosomes derived from mammals, PELNs provide an abundant and renewable source that allows for large-scale production [20].
2.1 Biogenesis of PELNs
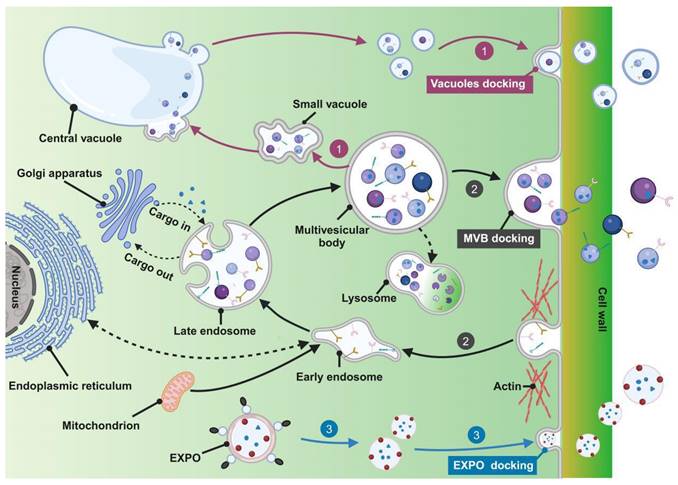
The biological genesis of PELNs encompasses three pathways: the vacuolar, the multivesicular bodies (MVBs), and the exocyst-positive organelle (EXPO) pathways (Figure 1) [18].
The MVBs pathway is a key route for exosome formation in animal cells and is considered fundamental in PELN biogenesis. Initially, the cytoplasmic membrane undergoes inward budding, leading to endocytic vesicle formation to internalize various components including lipids, proteins, small molecules, and diverse metabolites, initiating the formation of early endosomes. Fusion of early endosomes with components from the endoplasmic reticulum, trans-Golgi network, and mitochondria facilitates cargo acquisition. Subsequent maturation of early endosomes forms late endosomes, known as MVBs, encapsulating cargo within vesicles. It is important to highlight that MVBs may fuse with lysosomes, resulting in degradation due to the presence of ubiquitinated cargo. Transport of MVBs, along with their eventual fusion with the plasma membrane and release of vesicular contents, is mediated by specific components of the microtubule scaffold, actin, and the Rab family [21-23]. Alternatively, escaped MVBs may fuse with the plasma membrane to release exosomes [24].
The vacuolar pathway involves the recently discovered fusion between vacuoles containing hydrolytic enzymes and defense components with the plasma membrane. Pseudomonas syringae pv. tomato DC3000 (Pst DC3000) potentially represents a defensive signaling response to bacterial infection when expressed in plants [25]. A study in Arabidopsis demonstrates that during infection by the pathogenic bacterium Pst DC3000, fusion occurs between the vacuolar membrane and the plasma membrane, releasing antimicrobial proteins and hydrolytic enzymes into the extracellular space, serving as a defense strategy against pathogenic invasion [26]. Furthermore, PELNs were identified within the central vacuole of grapefruit epidermal cells [27] and plant cells contain small vacuoles (SVs) in early developmental cortical cells, harboring vesicles derived from the fusion of maturing MVBs. The central vacuole originates from the MVB-to-SV transition and subsequent fusion of SVs, suggesting potential connections between the vacuolar pathway and the MVB pathway [28].
The EXPO pathway represents an unconventional secretion route in plants characterized by a double-membrane EXPO that mediates exocytosis from the cytoplasm to the cell wall. In contrast to autophagosomes, EXPO is not induced by starvation and does not merge with endosomes or lytic compartments, instead, they fuse with the plasma membrane to release single-membrane vesicles, including exosomes, into the cell wall [29].
2.2 Preparations of PELNs
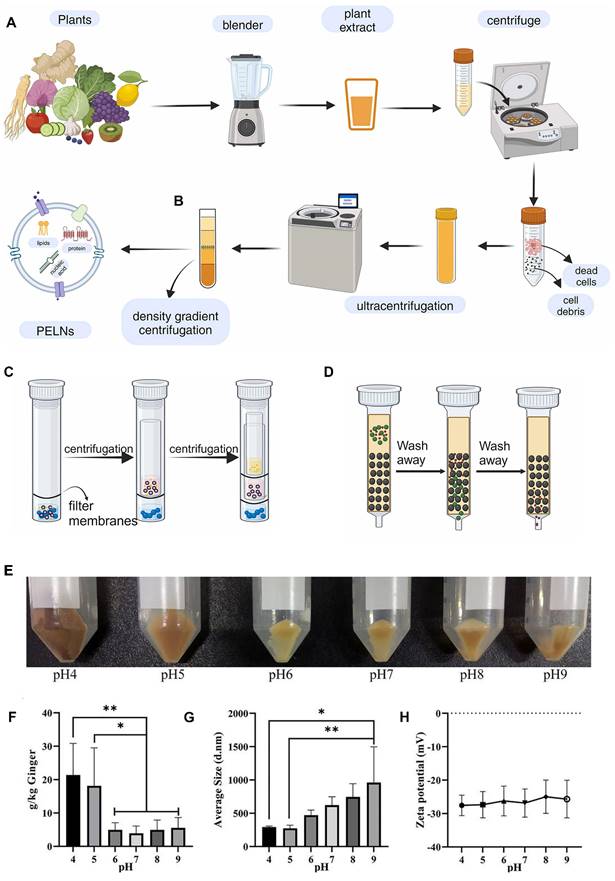
Unlike the methods used for isolating animal exosomes, the study of PELNs requires pretreatment of plant tissues prior to isolation and purification. In this paper, we briefly summarize the principles, advantages, and disadvantages of these techniques, as shown in Table 1.
2.2.1 Tissue pretreatment methods
Plant materials are initially cleaned to remove dust and soil, then washed with phosphate-buffered saline (PBS) to eliminate ions and elements from tap water. Subsequently, the materials are processed to obtain the plant's juice or apoplast fluid.
Plant tissues with a high water content can be blended to extract plant juice, such as grapes and ginger [14, 30]. However, while this method facilitates the separation of nanovesicles from plants, there is a potential risk of cellular damage, leading to the mixing of organelle or membrane structures with EVs [31].
Plant roots or leaves are immersed in buffer solutions such as 2-(N-morpholino) ethanesulfonic acid (MES), which closely mimic native apoplastic fluids, to obtain apoplastic fluid. A widely used technique for extracting apoplastic fluid is the infiltration-centrifugation method, which involves vacuum infiltration with an infiltration buffer, without the need for homogenization [26, 32]. However, the utilization of buffer solutions may dilute the native PELN fluid, resulting in a comparatively reduced concentration of PELNs. Additionally, the addition of washing liquids to dilute the PELN fluid surrounding plant cells may distort the plant's metabolic profile [33].
Biogenesis of PELNs. 1) Vacuolar pathway; 2) MVBs pathway; 3) EXPO pathway. (Created with Biorender.com).
Characteristics of main pre-treatment/isolation and purification methods of PELNs
| Pretreatment/separation and purification method | Advantages | Disadvantages | Main plants | Yield | Ref. | |
|---|---|---|---|---|---|---|
| Pretreatment method | Blender juice extraction | Convenient; fast; high concentration | Cell damage may lead to degraded plasma membranes and cellular fragments | Most fruits and vegetables | / | [31, 81] |
| Tissue infiltration homogenization | Separable nanoparticles for drying plants; high purity | Low concentration; potential cell damage; distorted plant metabolite profile | Mulberry leaf, tea leaf, panax notoginseng, lonicera japonica, sophora | / | [33] | |
| Separation and purification methods | Ultracentrifugation | High sample capacity; high production | Expensive equipment; low purity; time-consuming | Grapefruit | 9 × 1010 particles/mL juice | [136] |
| Tomato | 6 × 109 particles/mL juice | [137] | ||||
| Orange | 1 mg protein/350 mL juice | [41] | ||||
| Ginger | 240 mg protein/kg ginger | [138] | ||||
| Mushroom | 0.8-9.7 × 1011 particles/g mushroom | [139] | ||||
| Cabbage | 0.432 × 109 particles/μg protein | [122] | ||||
| Dendropanax morbifera | 1.53 × 109 particles/g leaf and 4.98 × 108 particles/g stem, respectively | [63] | ||||
| Density gradient centrifugation | High purity; Exosome structure preserved | Low production; time-consuming | Grapefruit | 2.21 ± 0.044 g/kg grapefruit | [56] | |
| Tomato | 0.44 ± 0.02 g/kg tomato | [56] | ||||
| Grape | 1.76 ± 0.15 g/kg grape | [56] | ||||
| Ginger | 1 × 1012 particles/kg ginger | [54] | ||||
| 48.5 ± 4.8 mg protein/kg ginger | [66] | |||||
| 17.5 mg protein/kg ginger | [140] | |||||
| Ginseng | 500 mg protein/kg ginseng | [44] | ||||
| 168 mg protein/kg ginseng | [45] | |||||
| 5.62 × 1011 particles/g ginseng | [141] | |||||
| Turmeric | 50-100 mg protein/kg turmeric | [142] | ||||
| Ultrafiltration | Convenient; fast | Compromised EV structure; moderate purity | Blueberry, arabidopsis | / | [143] | |
| Size exclusion chromatography | Highly automated; the structure of PELNs remains intact; high purity | Low yield; expensive equipment | Cabbage | 0.242 × 109 particles/μg protein | [122] |
EV: extracellular vesicle.
2.2.2 Separation and purification methods
After obtaining heterogeneous PELN mixtures through methods like blender juice extraction or tissue infiltration-centrifugation, additional steps for separation and purification are necessary. PELN separation and purification primarily employs techniques such as ultracentrifugation, density gradient centrifugation, ultrafiltration, and size exclusion chromatography (Figure 2A-D).
PELN extraction methods are often adapted from established exosome protocols and typically involve ultracentrifugation, effectively eliminating fibrous debris from the plant tissues [34] and facilitating PELN sedimentation [35]. To enhance the purity of PELNs, the obtained precipitate is typically resuspended in PBS and subjected to a second round of high-speed centrifugation. However, this approach often leads to a decreased yield [36]. While this technique lacks precision in isolating exosomes, the presence of high-molecular-weight components such as cellulose and starch in plant juice often leads to lower purity after centrifugation. Consequently, the purification of exosomes obtained after ultracentrifugation remains a frequently utilized method for PELN extraction.
Density gradient centrifugation can be considered a more precise method of ultracentrifugation, and research has also investigated its application for further purification following ultracentrifugation of PELNs [31]. The primary PELN purification method is density gradient centrifugation using sucrose or iodixanol (typically at concentrations of 8%, 15%, 30%, 45%, and 60% w/v). The purified exosomes usually reside in the intermediate layer of a 30%-45% sucrose solution [10]. Ginger-derived PELNs were purified from the interfaces of 8/30% and 30/45% sucrose gradients, with those at 30/45% demonstrating notable biological activity. It is noteworthy that from an initial material of 1000 g of ginger, approximately 50 mg of ginger PELNs were obtained, underscoring the necessity of purification [20]. Although PELNs separated by density gradient centrifugation typically exhibit high purity, the resulting yield is often relatively low [37].
PELNs have also been extracted by ultrafiltration, followed by size-exclusion chromatography purification and pre-concentration of elution fluids, with the separation process monitored using capillary electrophoresis [38]. A study compared the efficiency of PELN separation using ultrafiltration and ultracentrifugation, and found that the ultrafiltration method retained the optimal PELN morphology, albeit with the highest level of protein contamination [39].
Size exclusion chromatography is a separation method based on particle fluid dynamics, primarily used to separate vesicles of varying sizes from other biomolecules using gel filtration matrices or resin filtration [40]. The orange-derived products could be subdivided into either smaller (<50 nm) or larger (>150 nm) PELNs through size exclusion chromatography [41]. This method yields PELNs with high purity, characterized by minimal impurities from non-nanoparticle proteins and other large molecules, thereby preserving their structural integrity. However, it has a low yield, requires specialized equipment, and involves relatively complex procedures [42].
Extraction method of PELNs and the influence of pH on PELNs extraction. (A) Ultracentrifugation, (B) density gradient centrifugation, (C) ultrafiltration and (D) size exclusion chromatography method. Adapted with permission from [175], copyright 2024, Biomedicine & Pharmacotherapy. (E) Photomicrographs, (F) total yield, (G) average size, and (H) average zeta potential of ginger PELNs isolated under different pH. Adapted with permission from [43], copyright 2021, ACS Omega. PDNs: Panax PELNs.
Summary of the active contents in PELNs
| Plant type | Plant source | Contents | Effects | Whether or not presence in humans | Ref. |
|---|---|---|---|---|---|
| Fruit | Grapefruit | Ascorbic acid | Anti-leukemic effect | No | [136] |
| Broccoli, pomegranate, apple, orange | miR159a, miR162a, miR166b, miR396b | Toxic effect on Caco-2 cells | No | [118] | |
| Vegetable | Ginger | PA | Induced Foxa2 expression in intestinal epithelial cells, interacted with HBP35 | Yes | [54] |
| aly-miR159a | Decreased the attachment of Porphyromonas gingivalis to TIGK cells | No | [55] | ||
| rlcv-miR-rL1-28-3p, aly-miR396a-5p | Inhibited the expression of spike genes and Nsp3 | No | [58] | ||
| osa-miR-530-5p | Inhibited the synthesis of ORF1b | No | [59] | ||
| mdo-miR7267-3p | Targeted the monooxygenase ycnE of LGG | No | [79] | ||
| Garlic | han-miR3630-5p | Bound to the 3′ UTR of TLR4 | No | [144] | |
| PA | Bound to microglial cell BASP1 | Yes | [100] | ||
| miR-396e | Regulated PFKFB3 expression | No | [95] | ||
| Broccoli | miR5266 | Bound with the 3′UTR of MMP-9 | No | [145] | |
| Cucumber | Cucurbitacin B | Anti-tumor effect | No | [146] | |
| Herb | Rehmanniae radix | rgl-miR-7972 | Targeted GPR161 to inhibit inflammation | No | [105] |
| Oat | β-Glucan | Bound to microglial hippocalcin | No | [64] | |
| Houttuynia cordata | miR168b-3p, miR166a-3p, miR159a | Targeted to SARS-CoV-2 | No | [125] | |
| Lonicera japonica | miR2911 | Targeted to E6 and E7 genes of HPV16/18 | No | [147] | |
| Sophora | Rutin | Promoted nerve regeneration | No | [63] |
BMDM: bone marrow-derived macrophage; LGG: Lactobacillus rhamnosus GG; PFKFB3: 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase.
The extracted PELNs can be significantly influenced by the adjusted pH of the plant juice before extraction. For instance, compared to ginger juice with a pH of 5, adjusting ginger juice to a pH of 4 yields more PELNs with increased biological activity content and miRNA (Figure 2E-H) [43]. While numerous methodologies have been developed for the extraction and isolation of exosomes, achieving consistent levels of purity and concentration remains a challenge. Even with the same isolation method, the yields of PELNs can vary significantly. For instance, ginseng PELNs extracted using ultracentrifugation and density gradient centrifugation have shown different yields across studies (168 mg protein/kg ginseng and 500 mg protein/kg ginseng) [44, 45]. These differences may arise from factors such as the plant source, harvest season, and freshness. Additionally, studies use diverse units to quantify PELN yield (Table 1). Therefore, standardizing the units of measurement for PELN yield is recommended to facilitate easier comparison of yields obtained from different isolation methods.
2.3 Composition of PELNs
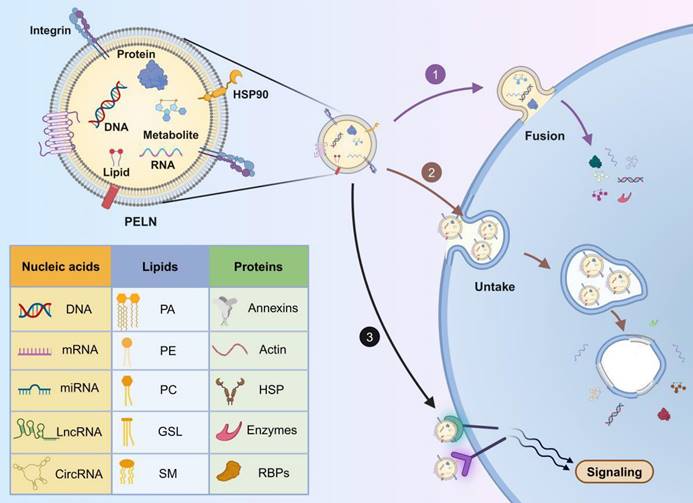
Under physiological conditions, exosomes conventionally transfer functional biomolecules like mRNA, microRNA, long non-coding RNA, circular RNA, and DNA between cells, thus facilitating intercellular communication [46]. Due to the shared evolutionary origin of plants and animals, there are similar components in PELNs and mammalian EVs [43]. However, the types and quantities of cargo in PELNs are fewer compared to those in mammalian EVs, indicating higher safety and easier functional resolution of PELNs. For instance, PELNs extracted from grapes contain 28 proteins and 96 miRNAs in contrast to the typical profile of mammalian exosomes, which commonly feature over 1000 proteins and 100-300 miRNAs [47]. Furthermore, several studies have shown that components within PELNs can target human genes and offer therapeutic effects (Table 2).
2.3.1 Proteins
Penetration 1 (PEN1), PEN3, and Tetraspanin-8 (TET-8) are potential protein markers for identification in PELNs [48]. PEN1 co-localizes with the amphiphilic styryl dye FM4-64 outside the plasma membrane, accumulating extracellularly in papillae, suggesting its secretion via exosomes [49]. TET-8 shares homology with mammalian exosomal markers (CD63, CD81, and CD9), and co-localizes with intracellular MVB markers in plant cells, suggesting its potential utility as a marker for plant exosome-like vesicles [50].
Moreover, PELNs generally exhibit a lower protein concentration, predominantly consisting of cytoplasmic and transmembrane proteins [18]. Analysis of ginger-derived PELNs revealed the presence of proteins like actin, proteases, and various membrane proteins, including transport proteins such as aquaporins and chloride ion channels, which may be more relevant to the endocytosis function of PELNs [20]. However, substantive studies on the proteins in PELNs are limited, with most relying on proteomics and bioinformatics to predict the functions of the identified proteins. For instance, Wang et al. conducted protein structure analysis and GO/KEGG enrichment analysis on proteins extracted from bitter melon PELNs, suggesting that the proteins may possess antioxidative capabilities, potentially alleviating disease symptoms through their antioxidative effects [51].
2.3.2 Lipids
Lipids, a crucial yet relatively underexplored component of exosomes, encompass sphingolipids, phosphatidic acid (PA), sphingomyelin (SM), phosphatidylserine (PS), and cholesterol [22]. Lipids not only contribute to the structural integrity of PELN membranes but also actively participate in PELN formation and release [52]. Lipidomic analysis of PELNs has revealed two distinct classes of lipids, named phospholipids and glycerol lipids, which are involved in the uptake and various biological functions of PELNs. However, the presence of cholesterol in PELNs has not been confirmed [10]. Interestingly, the lipid content of PELNs may be different from that of their parent plants. For example, PELNs from grapefruits exhibited a lipid composition similar to grapefruit tissues, with a higher proportion of sphingolipids, particularly hexosylceramides and ceramides [53].
PELN-derived lipids are believed to possess biotherapeutic effects. For example, ginger PELNs contain abundant PA, which induces Foxa2 expression in intestinal epithelial cells, thereby preventing high-fat diet-induced obesity and insulin resistance [54]. Additionally, PA directly interacts with the HBP35 protein in Porphyromonas gingivalis conferring antibacterial properties [55].
Differential centrifugation and ultrasonication were used to extract lipids from PELNs, which can be reassembled into lipid-based PELNs as a potential drug delivery platform. These lipid PELNs demonstrate minimal toxicity, possess the potential for targeted delivery through modifications, and can deliver various agents. For instance, grapefruit lipid PELNs loaded with a Stat3 inhibitor demonstrate remarkable in vitro uptake capacity and targeted delivery to brain tumors, effectively inhibiting tumor growth [56].
2.3.3 Nucleic acids
Nucleic acids encapsulated in EVs act as mediators for intracellular communication and constituents of diverse signaling pathways. Among these, miRNAs have been shown to have a central role in the therapeutic effects of most EV treatments [57]. Notably, discrepancies in the types of nucleic acids present may exist between PELNs and their source plant tissues. For instance, ginger-derived PELNs have a more abundant miRNA cargo compared to ginger tissues, coupled with a reduced tRNA content [58]. Bioinformatics enables the prediction of miRNA targets within the human genome, facilitating the investigation of plant-derived miRNAs in human diseases. For example, Osa-miR-530-5p in ginger PELNs indirectly hinders ORF1b synthesis by targeting the ribosomal slippage site in the ORF1ab gene, thereby impeding the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [59].
The analysis of 11 different PELNs, including coconut, ginger, and hamimelon, identified 418 distinct miRNAs. The abundant miRNAs present in multiple plants are referred to as frequent miRNAs, whereas those existing in individual plant species are known as rare miRNAs. Notably, "frequent" miRNA categories are less numerous than "rare" miRNA categories, despite the former exhibiting higher cumulative expression levels [60]. Certain miRNAs present in substantial quantities across multiple plant species potentially serve as plant-specific miRNAs.
2.3.4 Secondary metabolites
PELNs transport small secondary metabolites produced by plants, such as flavonoids, saponins, polysaccharides, alkaloids, etc. [61]. Active metabolites, specifically 6-gingerol, and 6-shogaol, have been identified in ginger PELNs [20] and PELNs isolated from sucrose gradients of 8%/30% exhibited higher concentrations of shogaol compared to those between 30%/45%, suggesting potential specific variations based on PELN density [62].
Secondary metabolites in PELNs may also be used in the treatment of human diseases. Our previous research has extracted PELNs from the embryonic layer of Flos Sophorae Immaturus and identified rutin, a crucial therapeutic component for the treatment of spinal cord injuries [63]. The metabolites encapsulated in PELNs demonstrate a propensity to enhance cellular absorption and accumulation within recipient cells. For instance, the β-glucans in oat-derived PELNs facilitate a dose-dependent uptake of PELNs by microglia [64].
2.4 Uptake mechanism of PELNs
PELNs can be internalized by diverse mammalian cells including cancer cells (breast, lung, colon, and glioma cells), lymphocytes (T cells and B cells), neural cells (neurons, microglia, astrocytes, and brain microvascular endothelial cells), as well as normal human skin keratinocytes (HaCat cells) [56, 60, 65]. Based on current reports, PELNs demonstrate uptake rates exceeding 40% in most cell lines [66-69]. Even in difficult-to-transfect T cells and B cells, they achieve uptake rates of 14.1% and 19.8%, respectively [56].
It is widely accepted that PELNs can enter target cells through three distinct mechanisms: ① direct fusion with the target cell membrane, releasing their cargo into the cytoplasm. This fusion involves the formation of a hemi-fusion stalk between the hydrophobic lipid bilayers of the PELN and the plasma membrane, followed by expansion into a cohesive structure and is likely facilitated by the SNARE and Rab protein families [70]; ② internalization through endocytosis into target cells, followed by the release of cargo into the cytoplasm. This uptake process is rapid and temperature-sensitive, with lower temperatures attenuating internalization [71]. Common endocytic pathways, such as clathrin-mediated endocytosis, lipid raft-associated membrane invagination, and caveolin-dependent endocytosis, mediate exosome internalization [72]; ③ binding to receptors on the target cell membrane, initiating receptor-ligand interactions and downstream signaling cascades to activate the target cell [73] (Figure 3).
Transport proteins located on the surface of PELNs may influence their uptake mechanisms. Studies have indicated that the endocytosis of garlic-derived PELNs by HepG2 cells depends on the interaction between the surface protein II agglutinin on PELNs and CD98 on HepG2 cells [74]. Therefore, the role of transport proteins on the surface of PELNs in cellular uptake mechanisms warrants further exploration. Additionally, PELN uptake may depend on time and dosage. Ginseng-derived PELNs are internalized by bone marrow mesenchymal stem cells (BMSCs) in a time-dependent manner, reaching a peak after 12 hours [75], whereas PELNs from blueberry demonstrate dosage-dependent internalization into the human stable endothelial cell line EA.hy926 [76].
Understanding the intracellular transport pathway and fate of PELNs following cellular uptake is crucial for ongoing research. Currently, only one study has observed that grapefruit-derived PELNs co-localize with endosomes and lysosomes within HaCaT cells six hours post-uptake [77]. This suggests a potential pathway where PELNs might be degraded by lysosomes within the cell. Overall, research on the mechanisms underlying the intracellular fate of PELNs remains incomplete. Elucidating the molecular mechanisms involved in their internalization process will pave the way for more effective treatments and drug delivery strategies.
The composition and uptake of PELNs. 1) Fusion with the target cell membrane; 2) internalization through endocytosis into target cells; 3) binding to receptors on the target cell membrane. PA: phosphatidic acid; PC: phosphatidylcholine; PE: phosphatidylethanolamine; GSL: glycosphingolipids; SM: sphingomyelin; HSP: heat shock proteins; RBPs: RNA binding proteins. Created with Biorender.com.
2.5 Distribution of PELNs
The distribution of PELNs within living organisms is influenced by the method of administration including oral ingestion, intravenous injection, transdermal delivery, nasal administration, intraperitoneal injection, and intramuscular injection. Among these, upon oral administration, PELNs are primarily distributed in the gastrointestinal tract. For example, after mice were given orange PELNs by gastric gavage, fluorescence was detected in the gastrointestinal tract after 6 hours, and significant metabolism was observed within 24 hours [41, 78]. Interestingly, ginger-derived PELNs are also distributed within the gastrointestinal tract and are taken up by intestinal bacteria, affecting both the microbiota composition and host physiology [79]. Therefore, oral administration of PELNs is well-suited for gastrointestinal conditions such as gastrointestinal cancer and colitis. PELNs exhibit good stability in gastric and intestinal environments. In simulated gastric acid (pH=2.0, supplemented with pepsin), yam-derived PELN vesicles maintained their quantity despite an increase in particle size, retaining their functional targeting and communication roles [80]. Moreover, Zhang et al. investigated the variations in size and surface charge of grapefruit, carrot, and ginger PELNs in simulated gastric acid and intestinal fluid (pH=6.5, supplemented with bile and pancreatin). They observed some subsets separated or merged for PELNs, with a decrease in negative charge in simulated intestinal fluid, thereby enhancing their resistance to gastrointestinal digestion [81]. In summary, PELNs demonstrate robust resistance to degradation in gastric and intestinal fluids, underscoring oral administration as a crucial delivery method for PELNs.
Intravenous injection avoids the first-pass effect and allows for the distribution and targeting of PELNs in tissues outside the gastrointestinal tract. PELNs derived from Panax notoginseng targeted the brain in rats with cerebral ischemia 8 to 12 hours post-intravenous injection [31]. Similarly, bitter melon-derived PELNs exhibited targeted accumulation in tumors after intravenous administration in tumor-bearing mice, with sustained high levels even after 72 hours, suggesting a potentially extended in vivo half-life [82]. Most bitter melon PELNs accumulate in the liver, indicating predominant metabolism through the liver [82]. However, not all PELNs can effectively target organ lesions, necessitating engineering modifications to enhance their targeting ability after intravenous injection. For instance, modifying the outer membrane of ginseng-derived PELNs improved their targeting of inflammatory areas [83]. The blood circulation characteristics of PELNs after intravenous injection vary depending on their source. For instance, ginger lipid-derived PELNs remain stable and detectable in the bloodstream for up to 48 hours post-injection [66]. Conversely, corn-derived PELNs are rapidly cleared from the systemic circulation following intravenous administration. However, when corn PELNs are modified with polyethylene glycol (PEG), they exhibit significantly enhanced distribution in tumor tissues and prolonged circulation time [84]. Therefore, it is important to investigate the blood circulation characteristics of PELNs, and explore modifications to extend their systemic circulation time in PELN research.
Various administration routes have been investigated for PELNs, including transdermal, nasal, intraperitoneal, and intramuscular routes. For instance, PELNs derived from broccoli have demonstrated superior penetration through both the stratum corneum and dermis of pig skin when administered transdermally [15]. Grapefruit-derived PELNs, upon nasal administration, were found to distribute exclusively in the brain [56, 85] and lungs [56]. Furthermore, following intraperitoneal injection, grapefruit PELNs predominantly distributed in liver, lung, kidney, and spleen tissues, similar to intravenous injection [56]. Conversely, intramuscular injection of grapefruit PELNs resulted in their distribution exclusively within muscle tissue. Therefore, specific administration methods can be selected based on the site of disease manifestation [56].
The in vivo biodistribution mechanisms of PELNs sourced derived from different plant sources are complex, necessitating further foundational research exploration.
3. Biotherapeutic applications of PELNs
PELNs present substantial potential in treating human diseases owing to their anti-inflammatory [86], antiviral [87], antioxidant [88], anti-tumor [89], gut microbiota regulation [90], insulin resistance regulation [91], anti-osteoporotic [92] and regenerative effects [93]. Compared to traditional cell therapy or drug therapy, PELNs offer significant advantages including lower immunogenicity, improved targeting, and scalability. Furthermore, the modification or engineering of PELNs may enhance their therapeutic capabilities for specific diseases.
PELNs derived from fruits, vegetables, and herbs collectively exhibit common biological therapeutic effects, such as anti-inflammatory properties, anti-tumor activity, and regulation of gut microbiota (Table 3). Additionally, PELNs from these sources each display distinct biological activities. Fruit-derived PELNs exhibit antioxidant effects, vegetable-derived PELNs show antiviral properties and regulation of insulin resistance, whereas herb-derived PELNs possess anti-osteoporosis and regenerative properties (Table 4).
Summary of common applications of PELNs derived from fruit, vegetable and herb in biotherapeutics
| Biological activity | Plant type | Disease | Plant source | Main findings | Ref. |
|---|---|---|---|---|---|
| Anti-inflammatory | Fruit | Dermatitis | Apple | Downregulated NF-κB signaling pathway leads to changes in ECM production by dermal fibroblasts | [148] |
| Grapefruit | Reduced expression of inflammatory factors and reshaping of the imbalanced immune microenvironment | [53] | |||
| Colitis | Regulated inflammatory cytokine expression leading to improvement in colitis in mice | [86] | |||
| Grape | Mucosal epithelial regeneration restored the entire intestinal structure in colitis mice | [14] | |||
| Obesity | Orange | Ameliorated intestinal inflammation accelerated the restoration of intestinal function | [41] | ||
| Vegetable | Colitis | Ginger | Regulated inflammatory cytokine expression leading to enhanced intestinal repair | [20, 149] | |
| Bitter melon | Regulated oxidative stress and inflammatory markers in the blood of mice, safeguarding the colonic mucosa | [51] | |||
| Obesity | Garlic | Reduced inflammatory cytokine expression and inhibited c-Myc-mediated STING expression | [100] | ||
| PFKFB3 expression in PELNs was regulated by miR-396e influencing metabolic reprogramming in macrophages | [95] | ||||
| Acute liver injury | Reduced macrophage infiltration via CCR2/CCR5 signaling inhibition | [150] | |||
| Shiitake Mushroom | NLRP3 inflammasome activation was inhibited in macrophages | [139] | |||
| Photodamage | Potato | Inhibited MMP and inflammatory cytokine expression preventing collagen degradation and promoting cell proliferation | [99] | ||
| Herb | Colitis | Mulberry bark | Activated the AhR signaling pathway, mediated by HSPA8 to induce COPS8 expression | [151] | |
| Cerebral ischemia-reperfusion injury | Panax notoginseng | Induced a shift in microglial phenotypes from M1 to M2 and activated the PI3K/Akt signaling pathway | [31] | ||
| Hypertension | Dandelion | Increased butyric acid production and suppressed systemic inflammation and vascular remodeling | [152] | ||
| Brain inflammation | Oat | Modulated the assembly of the HPCA/Rab11a/dectin-1 complex | [64] | ||
| Anti-tumor | Fruit | Leukemia | Grapefruit | Inhibited leukemia cell proliferation and elevated ROS levels in leukemia progenitor cells | [136] |
| Inflammatory tumor | Targeted delivery to tissues with inflammatory tumors | [16] | |||
| Gastric cancer | Lemon | Induced ROS generation causing S-phase arrest and apoptosis in gastric cancer cells | [78] | ||
| P53-inactivated colorectal cancer | Activated the macropinocytosis pathway and inhibited tumor cell proliferation | [153] | |||
| Colorectal cancer | Inhibited cellular lipid metabolism and suppressed tumor cell growth | [154] | |||
| Vegetable | Non-small cell lung cancer | Cucumber | Cucurbitacin B suppressed ROS generation induced by the STAT3 signaling pathway resulting in cell cycle arrest | [146] | |
| Triple-negative breast cancer | Ginger | Induced apoptosis, cell cycle arrest, and cell migration | [30] | ||
| Colorectal cancer | Corn | Stimulated the release of inflammatory factors from Raw264.7 cells, and inhibited the colon26 cell proliferation | [101] | ||
| Herb | Breast cancer | Tea flower/leaf | Increased ROS production leading to mitochondrial damage and cell cycle arrest | [68, 69] | |
| Hepatocellular carcinoma | Morus nigra L. | Enhanced oxidative stress caused mitochondrial damage within tumor cells and suppressed their proliferation | [155] | ||
| Cervical cancer | Lonicera japonica | MiR2911 bound to HPV16/18 E6 and E7 oncogenes in PELNs inducing apoptosis | [147] | ||
| Leukemia, cervical cancer | Moringa oleifera Lam | Reduced BCL2 protein expression and mitochondrial membrane potential to promote apoptosis in tumor cells | [156] | ||
| Colorectal cancer | Rice bran | Induced cell cycle arrest and apoptosis, and reduced the expression of proliferative proteins | [157] | ||
| Triple-negative breast cancer | Brucea javanica | Inhibited tumor growth, metastasis, and angiogenesis and activated the PI3K/Akt signaling pathway | [158] | ||
| Lung cancer | Ginseng | Induced thymidine phosphorylase expression and downregulated the pentose phosphate pathway | [159] | ||
| Melanoma | Induced TAM polarization from M2 to M1 phenotype through the TLR4/MyD88 pathway and increased ROS production and cell apoptosis | [44] | |||
| Increased tumor-infiltrating lymphocyte infiltration, inhibited hot tumor growth and converted cold tumors into hot tumors | [103] | ||||
| Colorectal cancer | Reprogrammed TAMs and enhanced CD8 Teff function via the mTOR-T-bet axis and downregulated immune checkpoint expression on T cells | [102] | |||
| Glioma | Promoted TAM proliferation, modulated the immune system and silenced the c-MYC gene mediated by ptc-miR396f in PELNs | [45] | |||
| Gut microbiota regulation | Fruit | Intestinal bacterial infection | Lemon | Inhibited the production of Msp1 and Msp3 through RNase P-mediated specific tRNA decay, and increased bile tolerance for LGG | [160] |
| Manipulated probiotics to mediate bile resistance and intestinal survival | [161] | ||||
| Vegetable | Colitis | Garlic | Inhibited the TLR4/MyD88/NF-κB signaling pathway, modulated the gut microbiota, and improved tight junction protein dysfunction | [144] | |
| Bacteroides thetaiotaomicron growth was promoted by p-miR2916-p3 in PELNs | [90] | ||||
| Diabetes and brain inflammation | Ginger | Induced the generation of indole-3-aldehyde and IL-22, activated antimicrobial immunity and promoted tissue repair at the mucosal surface | [79] | ||
| Garlic | The OMVs released by A. muciniphila were modulated, inhibiting cGas-STING-mediated inflammatory responses and crosstalk between GLP-1R and insulin pathways. | [107] | |||
| Constipation | Broccoli | Restored tryptophan metabolism, ameliorated gut microbiota dysbiosis and accelerated intestinal transit | [162] | ||
| Herb | Colitis | Ginseng | Balanced the gut microbiota at the intestinal barrier | [67] | |
| Portulaca oleracea L. | Promoted Lactobacillus reuteri growth and reprogrammed conventional CD4+ T cells into double-positive CD4+CD8+ T cells | [163] | |||
| Modulated colonic barrier dysfunction and gut microbiota abundance | [106] | ||||
| Tea leaf | Enhanced the diversity and overall abundance of the gut microbiota | [104] | |||
| Osteoporosis | Pueraria lobata | Promoted osteogenic differentiation and functionality of hBMSCs and increased cellular autophagy | [92] | ||
| Lung inflammation | Rehmanniae radix | Activated the Hedgehog pathway and inhibited E. coli biofilm formation by targeting the virulence gene sxt2 | [105] |
ECM: extracellular matrix; LGG: Lactobacillus rhamnosus GG; MMP: matrix metalloproteinases; PFKFB3: 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; OMVs: outer membrane vesicles; HSPA8: heat shock protein family A member 8; COPS8: COP9 Constitutive Photomorphogenic Homolog Subunit 8; TAMs: tumor-associated macrophages; AhR: aryl hydrocarbon receptor.
Summary of unique applications of PELNs derived from fruit, vegetable and herb in biotherapeutics.
| Plant type | Biological activity | Disease | Plant source | Main findings | Ref. |
|---|---|---|---|---|---|
| Fruit | Antioxidant | Colitis | Lemon | Probiotics were manipulated to mediate bile resistance and enhance intestinal survival | [160, 161] |
| Leaky gut and liver injury | Pomegranate | Reduced hepatic oxidative stress and apoptosis markers preventing intestinal leakage | [111] | ||
| Non-alcoholic fatty liver disease | Blueberry | Oxidative stress and cell apoptosis were mitigated thereby ameliorating insulin resistance and hepatic dysfunction | [164] | ||
| Photoaging | Golden cherry | Scavenged free radicals | [133] | ||
| Vegetable | Antiviral | SARS-CoV-2 | Ginger | ORF1b synthesis was inhibited by targeting the ribosomal slippage site in the ORF1ab gene | [59] |
| Spike genes and Nsp3 expression were inhibited by rlcv-miR-rL1-28-3p and aly-miR396a-5p in PELNs | [58] | ||||
| Regulation of insulin resistance | Insulin resistance and obesity | Ginger | Inhibited the expression of the aromatic hydrocarbon receptor improving host glucose tolerance and insulin response | [91] | |
| Foxa2 expression in intestinal epithelial cells was induced by PA in PELNs | [54] | ||||
| Herb | Regenerative effect | Neural differentiation in vitro | Ginseng | Upregulated the PI3K signaling pathway, stimulating the differentiation and development of BMSCs | [75] |
| Skin wound | Angiogenesis in endothelial cells was facilitated through the ERK and AKT/mTOR pathways | [113] | |||
| Enhanced the angiogenesis and nascent vessel network reconstruction in full-thickness diabetic complicated skin ulcer wounds | [138] | ||||
| Wheat | Induced proliferation, migration, and angiogenesis | [93] | |||
| Bacterial infection | Dandelion | Neutralized Staphylococcus aureus exotoxins, and promote the healing of wounds | [114] | ||
| Muscle atrophy | Lycium barbarum L. | Activated the muscle regeneration and AMPK/SIRT1/PGC1α signaling pathway | [165] | ||
| Anti-osteoporotic effect | Osteoporosis | Ginseng | Suppressed the IκBα, c-JUN N-terminal kinase and ERK signaling pathways and regulated genes associated with osteoclast maturation | [115] | |
| Pueraria lobata | Promoted osteogenic differentiation of hBMSCs and enhanced intracellular autophagy | [92] | |||
| Yam | Activated the BMP-2/p-p38-dependent Runx2 pathway, and promoted differentiation and mineralization of osteoblasts | [80] |
PA: phosphatidic acid; BMSCs: bone marrow mesenchymal stem cells.
3.1 Anti-inflammatory activity of PELNs
PELNs can modulate macrophages, key participants in the pathogenesis of numerous chronic inflammatory and autoimmune diseases. For example, pueraria lobata PELNs promote the polarization of M1 macrophages towards the M2 phenotype by downregulating the expression of pro-inflammatory genes [94]. Additionally, miR-396e in garlic-derived PELNs regulated the expression of PFKFB3, influencing metabolic reprogramming in macrophages and alleviating inflammation in adipocytes of obese mice through crosstalk between macrophages and adipocytes [95].
Intriguingly, some studies suggest that oral administration of PELNs exhibits notable therapeutic efficacy against intestinal inflammation. Oral administration of grape and orange PELNs promotes mucosal epithelial regeneration and rapid recovery of the entire intestinal structure in dextran sulfate sodium (DSS)-induced colitis mice [14, 41, 96]. Similar effects have been observed in PELNs derived from celery [97], allium tuberosum [98], potato [99], and bitter melon [51]. The findings underscore the pivotal role of PELNs in promoting intestinal function, thereby supporting the importance of increased fruit and vegetable consumption and adherence to a well-balanced diet, such as the Mediterranean diet.
Impact of ginseng-derived PELNs on TAMs and modulation of immune cells in TME. (A) Flow cytometry analysis of the effect of ginseng-derived PELN treatment on the surface marker expression of M2 macrophages. (B) Quantitative mRNA expression of M1 marker genes and M2 marker genes. Adapted with permission from [44], copyright 2019, Journal for ImmunoTherapy of Cancer. (C) Heat map analysis of the effect of ginseng-derived PELNs on M1-M2 related gene expression in M2-like macrophages. (D) Single-cell sequencing analysis of the effect of G (ginseng-derived PELNs) and V (Vehicle) on the proportion of immune cells in MC38 mouse cancer. (E) Heat map showing the expression of cytotoxicity, chemokine, naive, and inhibitory receptor in CD8+ T cell subsets (naive T, CD8 Teff, and CD8 Tex) in G and V groups. Adapted with permission from [103], copyright 2023, Journal of Experimental & Clinical Cancer Research.
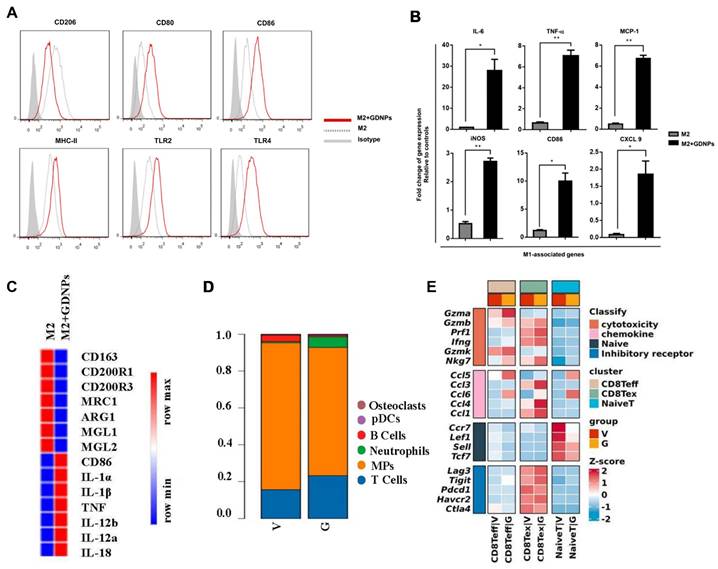
Furthermore, garlic-derived PELNs containing PA (36:4) interact with BASP1 in microglial cells, inhibiting c-Myc-mediated STING expression and reducing the expression of inflammatory cytokines, thereby reducing inflammation induced by a high-fat diet [100]. Oat bran-derived PELNs ameliorate alcohol-induced brain inflammation by modulating the assembly of the HPCA/Rab11a/dectin-1 complex [64]. These findings confirm that dietary-derived factors have the potential to impact brain health through their anti-inflammatory effects.
3.2 Anti-tumor activity of PELNs
Various PELNs have demonstrated potent anti-tumor effects including promoting apoptosis, cell cycle arrest, inhibition of cell proliferation, inducing mitochondrial damage, and inhibiting epithelial-mesenchymal transition. For example, lemon PELNs induce ROS generation, upregulate GADD45α expression leading to cell cycle arrest in the S phase, and induce apoptosis in gastric cancer cells [78]. Furthermore, PELNs derived from corn have been shown to stimulate the release of inflammatory factors from Raw264.7 cells, leading to the inhibition of colon26 cell proliferation and suppression of subcutaneous colon26 tumor growth in mice [101].
Several notable studies highlight the therapeutic impact of ginseng-derived PELNs on both "cold" and "hot" tumors by modulating tumor-associated macrophages (TAMs) [44, 45, 102, 103]. Ginseng-derived PELNs induce the polarization of TAMs from M2 to M1 phenotype through a TLR4/MyD88-dependent mechanism, resulting in elevated total ROS production and increased apoptosis in B16F10 melanoma cells in mice, thereby decelerating tumor progression (Figure 4A-C) [44]. Additionally, ginseng-derived PELNs reprogram TAMs and enhance the function of CD8 Teff via the mTOR-T-bet axis to regulate arginase-1 (ARG1) release thereby downregulating immune checkpoint expression on T cells in the tumor microenvironment (TME) and ameliorating T cell exhaustion (Figure 4D-E). This recruitment of CD8+ T cells inhibits the growth of hot tumors, with a marked synergistic effect when combined with PD-1 antibody therapy. Notably, combining ginseng-derived PELNs with PD-1mAb optimizes the TME by increasing tumor-infiltrating lymphocytes, thus converting cold tumors into hot tumors [102, 103]. Therefore, PELNs, epitomized by ginseng, offer a strategy for targeting TAMs in cancer therapy.
3.3 Gut microbiota regulation of PELNs
Certain PELNs can regulate gut microbiota abundance, thereby maintaining gut homeostasis [67, 69, 90, 104-106]. For example, the p-miR2916-p3 within garlic-derived PELNs specifically promotes the growth of Bacteroides thetaiotaomicron, consequently suppressing DSS-induced colitis [90]. Rgl-miR-7972 within Rehmanniae radix PELNs have been reported toinhibits Escherichia coli biofilm formation by targeting the virulence gene sxt2, thereby alleviating LPS-induced lung inflammation and recovering gut microbiota dysbiosis [105]. Therefore, the miRNAs present in PELNs could potentially play a crucial role in regulating the composition of gut microbiota.
An intriguing study revealed the capacity of garlic-derived PELNs to train human gut Akkermansia muciniphila (A. muciniphila) to release outer membrane vesicles (OMVs), leading to elevated expression of insulin receptor substrates (IRS1 and IRS2) in microglial cells. This offers a promising avenue for addressing high-fat diet-induced type 2 diabetes and alleviating brain inflammation [107]. In addition, portulaca oleracea L. PELNs demonstrate a dose-dependent modulation of gut microbiota abundance, thus ameliorating intestinal and pulmonary inflammation at the gut-lung axis level [106]. The intestinal microbiota plays a critical role in establishing connections between the intestine and other organs, including the brain-gut axis, liver-gut axis, and lung-gut axis, thus the regulatory influence of PELNs on the intestinal microbiota may also extend to other organs associated with the intestine.
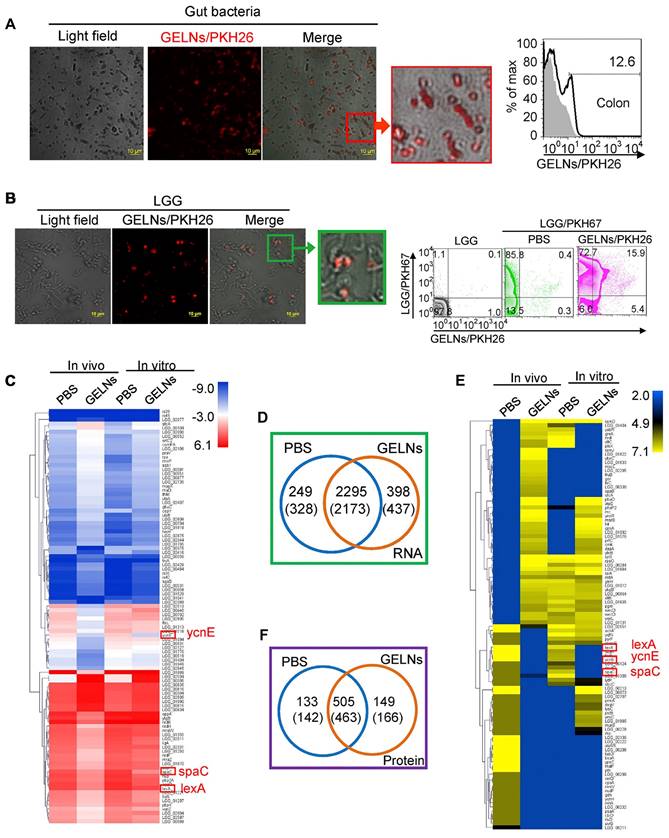
Notably, ginger PELNs demonstrate promising capabilities in mitigating intestinal inflammation by selectively regulating the mRNA and proteins within the gut microbiota (Figure 5). It has been reported that mdo-miR7267-3p in ginger PELNs efficiently target the monooxygenase ycnE of LGG in a lipid-dependent manner. This targeted interaction subsequently activates antimicrobial immunity and promotes tissue repair at the mucosal surface, thereby improving intestinal barrier function in a DSS-induced colitis mouse model [79].
Additionally, PELNs indirectly impact disease progression by modulating the metabolites of gut microbiota. For example, pueraria lobata PELNs accelerate the degradation of trimethylamine-N-oxide (TMAO), a gut microbiota metabolite, leading to increased cellular autophagy, ultimately promoting the osteogenic differentiation and functionality of human bone marrow mesenchymal stem cells (hBMSCs) [92]. The metabolites produced by the gut microbiota have garnered considerable attention in recent research and the convergence of these metabolites with PELNs presents an exciting prospect for further exploration.
3.4 Antioxidant activity of fruit-derived PELNs
A variety of antioxidant compounds are found in fruit-derived PELNs, such as ascorbic acid, catalase, anthocyanin, and glutathione, which may account for their unique antioxidant activity [53, 108, 109]. Studies have reported that PELNs extracted from a juice mixture of asparagus, grape, kiwi, cherry, blood orange, orange, tomato, papaya, grapefruit, lemon, mango, and bergamot significantly reduced the levels of ROS and malondialdehyde (MDA) in mice subjected to H2O2-induced oxidative stress [110]. Oral administration of pomegranate PELNs reduced hepatic oxidative stress, preventing intestinal leakage, thus suggesting their potential as antioxidants for liver or intestinal protection [111]. Notably, the miRNAs present in blueberry PELNs also possess anti-inflammatory and antioxidant properties [76], implying that the antioxidative effects might not solely derive from secondary metabolites. This underscores the need for further investigation into the constituents of PELNs.
3.5 Antiviral activity of vegetable-derived PELNs
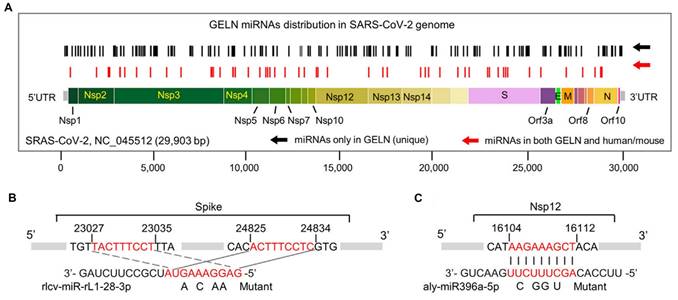
The treatment of COVID-19 induced by SARS-CoV-2 remains particularly challenging, especially in patients with comorbidities [112]. Ginger, recognized for its dual role in medicine and culinary applications, has demonstrated significant potential through the miRNAs contained within ginger-derived PELNs to target RNA from the SARS-CoV-2 virus (Figure 6). For instance, osa-miR-530-5p in ginger PELNs indirectly inhibits the synthesis of ORF1b by targeting the ribosomal slippage site in the ORF1ab gene, thereby preventing SARS-CoV-2 replication [59]. Moreover, rlcv-miR-rL1-28-3p and aly-miR396a-5p in ginger PELNs mediate the inhibition of spike genes and Nsp3 expression, thereby suppressing the cytopathic effects induced by exosomes secreted from lung epithelial cells infected with COVID-19 [58]. Ginger, recognized for its dual role in medicine and culinary applications, has demonstrated significant potential through the miRNAs contained within ginger-derived PELNs to target RNA from the SARS-CoV-2 virus.
3.6 Regulation of insulin resistance by vegetable-derived PELNs
Ginger PELNs demonstrate regulatory effects on insulin resistance and obesity. They can increase the expression of miR-375 and VAMP7 in high-fat diet mice small intestinal tissue, and inhibit the expression of the aromatic hydrocarbon receptor, thereby improving host glucose tolerance and insulin response [91]. Furthermore, PA in ginger-derived PELNs induces Foxa2 expression in intestinal epithelial cells, thereby preventing high-fat diet-induced obesity and insulin resistance [54]. Thus, improved insulin resistance by vegetable-derived PELNs is mainly achieved through the regulation of intestinal function, thereby indirectly impacting metabolic processes.
Absorption of ginger PELNs by LGG and regulation of LGG mRNA and proteins. (A) Representative confocal microscopy images (scale bar: 10 μm) and flow cytometry quantification of fecal samples from mice fed with ginger PELNs; (B) Confocal microscopy images (scale bar: 10 μm) and flow cytometry quantification of the colocalization between ginger PELNs and LGG colonies; (C) Heatmap illustrating the influence of GELNs on LGG mRNA expression as determined by next-generation sequencing; (D) Venn diagram of all mRNA detected in LGG. Numbers in parentheses represent in vitro results. (E) Heatmap of the impact of GELNs on LGG protein expression based on LC-MS data; (F) Venn diagram of all proteins detected in LGG. Adapted with permission from [79], copyright 2018, cell host & microbe.
The hypothetical targeting of SARS-CoV-2 RNA by ginger PELN miRNA. (A) Schematic representation and positioning of putative binding sites for ginger PELN miRNAs across the SARS-CoV-2 genome. (B-C) Predicted complementary pairing between target regions within the spike gene (B) and Nsp12 gene (C), and ginger PELN rlcv-miR-rL1-28-3p (B) and aly-miR396a-5p (C), respectively. The miRNA seed matches within the target RNAs are mutated as indicated. GELN: ginger PELN; UTR: untranslated region. Adapted with permission from [58], copyright 2021, Molecular Therapy.
3.7 Regenerative effect of herb-derived PELNs
Herbs have long been used for disease treatment since ancient times but herb-derived PELNs, as novel nano derivatives, may possess different compositions and mechanisms compared to the herbs, necessitating further research. Herb-derived PELNs demonstrate regenerative properties by promoting skin healing, stimulating nerve regeneration, and enhancing angiogenesis. Ginseng and wheat PELNs can promote wound healing by enhancing the migration of endothelial cells and facilitating angiogenesis [93, 113]. Dandelion-derived PELNs can neutralize exotoxins produced Staphylococcus aureus. When incorporated into gelatin methacryloyl hydrogels, these PELNs are continuously released, facilitating the healing process in wounds infected with Staphylococcus aureus exotoxins [114]. These findings suggest potential applications in the treatment of skin wounds. In addition, ginseng-derived PELNs promote neurodifferentiation while enhancing neural regeneration through the promotion of neurotrophic factors and influencing the Ras/Erk pathway [75].
3.8 Anti-osteoporotic effect of herb-derived PELNs
Herb-derived PELNs have anti-osteoporotic properties, primarily by regulating the differentiation of osteoblasts and osteoclasts. Reportedly, Pueraria lobata PELNs can promote osteogenic differentiation of hBMSCs by enhancing intracellular autophagy, significantly alleviating osteoporosis in rats [92]. Ginseng-derived PELNs significantly suppress IκBα, c-JUN N-terminal kinase, and ERK signaling pathways in receptor activator of nuclear factor-kappa B ligand (RANKL)-induced osteoclasts, thereby regulating genes associated with osteoclast maturation. Consequently, ginseng-derived PELNs exhibit inhibitory effects on osteoclast differentiation in an LPS-induced bone resorption mouse model [115].
In summary, herb-derived PELNs possess unique therapeutic functions that distinguish them from PELNs obtained from other sources, potentially attributed to the inherent therapeutic properties of the herbs.
4. Drug delivery applications of PELNs
Synthetic nanoparticles, such as liposomes, are currently the preferred carriers for drug delivery. Recent research indicates that PELNs, similar to synthetic nanoparticles, possess innate capabilities for drug delivery [19]. PELNs and liposomes share fundamental characteristics, including a lipid bilayer structure enabling delivery of both hydrophilic and hydrophobic cargoes [37]. However, PELNs offer several key advantages over liposomes: ① Synthetic liposomes may induce adverse effects like cellular stress [116, 117], whereas PELNs demonstrate greater biocompatibility and reduced toxicity. For example, Wang et al. showed that intravenous administration of grapefruit PELNs and DOPE liposomes at equivalent doses resulted in liver function damage only with liposomes [56]. ② Unlike synthetic nanoparticles used solely as drug carriers, PELNs possess inherent therapeutic properties due to their natural cargo. ③ PELNs exhibit enhanced cellular uptake; a recent study reported internalization rates exceeding 80% for PELNs compared to 40% for liposomes [56]. In summary, PELNs offer significant advantages over traditional liposomes across multiple critical domains, thereby presenting new avenues for future disease treatments.
Summary of the applications of PELNs in drug delivery
| Plant type | Plant source | Medications carried | Target cell/organ | Ref. |
|---|---|---|---|---|
| Fruit | Grape | Fisetin | MOLT-4 cell | [166] |
| Curcumin | / | [38] | ||
| Grapefruit | Methotrexate | Intestinal macrophage | [86] | |
| HSP70, variants of BSA | Human peripheral blood mononuclear cells, colon cancer cells | [167] | ||
| HSP70 | Glioma cells | [137] | ||
| Doxorubicin, curcumin | Colonic tissue | [16] | ||
| CX5461 | Inflammatory skin tissue | [53] | ||
| Doxorubicin, heparin nanoparticles | Glioma | [121] | ||
| siRNA | HaCaT cell | [77] | ||
| JSI-124, siRNA, paclitaxel, folic acid | Tumor | [56] | ||
| Apple, orange, pomegranate | ath-miR159a, ath-miR162a-3p, ath-miR166b-3p, ath-miR396b-5p | Caco-2 cells | [118] | |
| Orange | mRNA | T cell | [120] | |
| Vegetable | Celery | Doxorubicin | Tumor | [168] |
| Sesame leaf | Luteolin | Gastrointestinal tract | [169] | |
| Allium tuberosum | Dexamethasone | Microglial cell | [98] | |
| Broccoli | ath-miR159a, ath-miR159b-3p, ath-miR166b-3p, ath-miR319a, ath-miR403-3p | Caco-2 cell | [119] | |
| Ginger | Infliximab | Gastrointestinal tract | [140] | |
| Doxorubicin, folic acid | Tumor | [66] | ||
| Dmt1 siRNA | Duodenum | [170] | ||
| CD98 siRNA | Colonic tissue | [171] | ||
| Herb | Ginseng | miR-182-5p | Colonic tissue | [83] |
MOLT-4: human acute lymphoblastic leukemia cells; HSP70: heat shock protein 70.
PELNs, as potential drug delivery systems, can effectively transport compounds, proteins, and nucleic acids to target organs (Table 5).
Curcumin interacts with grape PELNs through hydrogen bonding, existing in an amorphous form within the PELNs, significantly enhancing its solubility, stability, and bioavailability [38]. Hence, the interaction between active compounds and vesicles can significantly influence the overall physical and chemical properties, as well as the functionality of the entire system. Moreover, it is worthwhile to investigate whether the mode of action differs between endogenous secondary metabolites and exogenous active compounds when loaded into PELNs and their respective effects on PELNs. Apart from serving as effective delivery carriers for compounds, PELNs can also deliver nucleic acids and protect them from nuclease degradation [118, 119]. Notably, orange PELNs represent a promising RNA-based vaccine delivery platform, leveraging their natural membrane envelopment to protect and deliver nucleic acids. Administering mice with orange PELNs loaded with specific mRNA via various routes generates specific IgM and IgG blocking antibodies along with T-cell immune responses, emphasizing the dual role of orange PELNs as carriers for RNA vaccines and inducers of immune responses through oral and intranasal administration [120].
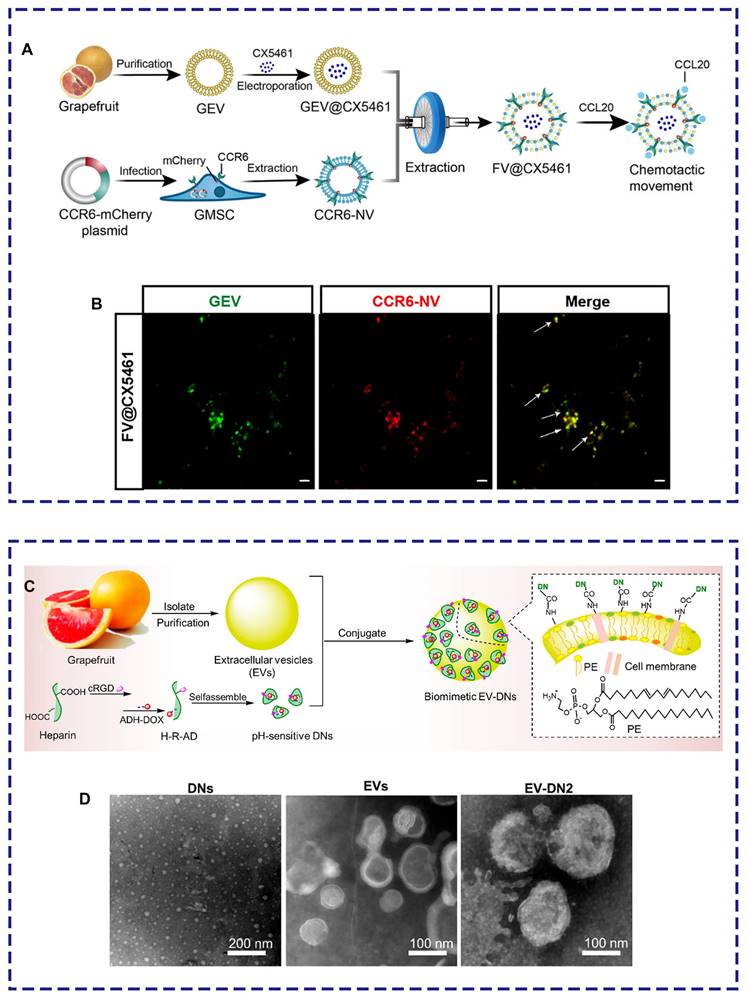
Several compelling studies have focused on the modification of PELNs to improve their ability to target specific lesions. Thus, it is essential to explore various engineering approaches, including nanobiotechnology. For example, grapefruit lipid-derived PELNs loaded with the immune suppressor CX5461 fused with engineered gingiva-derived mesenchymal stem cells (GMSCs) overexpressing CCR6 selectively targeted the inflammatory area of autoimmune skin diseases upon intravenous injection, reshaping the imbalanced immune microenvironment (Figure 7A-B) [53]. Additionally, modifying PELNs can enhance their drug-loading capacity. Niu et al. demonstrated an unprecedented four-fold increase in drug loading capacity by incorporating doxorubicin-loaded heparin nanoparticles (DNs) onto the surface of grapefruit lipid-derived PELNs using catalytic infiltration. This method effectively improved the therapeutic effectiveness against glioblastoma (Figure 7C-D) [121].
5. Toxicity of PELNs
The safety of PELNs is a crucial prerequisite for their extensive research and clinical translation. PELNs, devoid of zoonotic or human pathogens, have demonstrated favorable biocompatibility both in vitro and across various in vivo administration routes. For instance, cabbage-derived PELNs at concentrations of 1×1011 and 2×1011 particles/mL showed no significant reduction in cell viability after 72 hours of incubation in HaCaT, HDF, and Raw264.7 cells [122]. To date, no toxicity reports have emerged from oral administration studies of PELNs. Daily oral administration of grapefruit PELNs (10 mg/kg) to mice for 7 days did not alter serum IFN-γ levels, liver enzymes, or AST/ALT levels [86]. In addition, no significant toxicity has been observed with other administration routes, including intraperitoneal injection [110] and intranasal delivery [85].
PELNs-based biomimetic drug delivery system. (A) Schematic illustration. (B) Laser confocal microscopy image demonstrating the colocalization of PELNs and CCR6-GMSCs exosomes (scale bar: 2 μm). Adapted with permission from [53], copyright 2023, Journal of Extracellular Vesicles. (C) Schematic illustration. (D) Transmission electron microscope images of DNs, grapefruit lipid-derived PELNs, and PELNs-DNs. Adapted with permission from [121], copyright 2021, Nano Letters.
Most PELNs intended for intravenous injection, such as those derived from grapefruit [56], ginger [66], lemon [123], and ginseng [44], have shown no evidence of potential toxicity. However, PELNs sourced from certain herbs may exhibit significant toxicity when administered intravenously. For instance, PELNs derived from tea leaves and camellia flowers have been found to induce notable hepatic and renal toxicity following intravenous injection, whereas oral administration poses no such risks [68, 69]. Given the varied compositions and characteristics of PELNs derived from different sources, thorough safety assessments are indispensable prior to their potential application via intravenous administration.
6. Challenges in PELNs Application
PELNs have garnered significant attention for their potential therapeutic applications across different diseases. However, challenges in their application have led to a limited number of clinical trials. At present, only four clinical trials focusing on PELNs (NCT01668849, NCT04879810, NCT01294072, NCT03493984) have been undertaken, yet substantive data remain elusive despite the culmination of their primary timelines [124]. This circumstance may be attributed to the ongoing status of these investigations, the deadline for results submission, or exemption of certain studies from results submission requirements. Given the current scarcity of clinical trials, it is evident that addressing these challenges and furthering clinical experiments is necessary to propel the application of PELNs within future clinical therapies.
The production of PELNs faces significant challenges, especially in isolation and content standardization. In comparison to EVs derived from cells, the isolation of PELNs is limited due to the additional preprocessing steps required for plant-derived materials. Notably, among these challenges, heterogeneity poses a critical concern in PELNs manufacturing, since EV mixtures obtained through diverse extraction methods fail to qualify unequivocally as pure exosomes. In murine models, the administration of PELNs typically involves dosages spanning from 3-10 mg/kg (protein content), equating to 77.58 mg upon extrapolation to a 60 kg adult using the body surface area methodology. Despite the abundance of sources for PELNs facilitating their mass production compared to exosomes from animal origins, refining extraction methods is essential to achieve large-scale, high-purity production of PELNs. Therefore, achieving precise PELNs extraction with identical defined contents necessitates the deployment of highly accurate tools and innovative extraction methods.
The second pivotal challenge associated with PELNs is related with the uncertainty regarding their content. Plants have diverse components and functionalities in various parts, including roots, stems, leaves, flowers, bark, fruits, seeds, and dried aerial portions. Evident differentiations in miRNA content and disease-targeting profiles exist between the underground roots and above-ground stems/leaves of houttuynia cordata-derived PELNs. Additionally, discernible dissimilarities manifest between PELNs extracted from dried and fresh plant samples. Dry plants may present greater difficulties in obtaining PELNs compared to fresh counterparts, and the cargo of PELNs derived may also exhibit variability [125]. Furthermore, the cargo content of PELNs sourced from mature and immature plant samples also varies noticeably. For instance, the miRNA abundance in mature coconut-derived PELNs surpasses that in immature coconut-derived PELNs [126].
Preserving PELNs presents great challenges, particularly regarding storage conditions. Conventionally, freezing PELN samples below their reactivity threshold is a standard practice in research sample preservation. Storing ginger-derived PELNs at -80°C has proven effective in inhibiting IL-1β secretion and Casp1 self-cleavage, thereby facilitating prolonged preservation [127]. However, the recurrence of freeze-thaw cycles may result in PELN aggregation. Thus, the incorporation of cryoprotectants such as glucose, sucrose, and trehalose forms a hydrating shield around PELNs during freeze-drying. While this approach holds promise for improving PELN quality during storage and transportation, its significance and effectiveness warrant further investigation [128].
The primary challenges associated with using PELNs in therapeutic applications relate to their targeting capabilities and safety. Targeting lesion areas effectively is crucial, yet it remains a significant challenge for PELNs in disease treatment. Refining their targeting capabilities through methods such as incorporating targeting ligands or cell membrane-derived vesicles can enhance their efficiency in intracellular delivery, especially in intravenous administration [129]. Furthermore, it is necessary to rigorously evaluate the safety of PELNs. Although most administration routes of PELNs have shown no toxicity in current studies, concerns about safety have arisen specifically with intravenous injection in some investigations [31, 68, 69], presenting a challenge for their therapeutic application. Additionally, the safe dosages and minimum effective doses may vary across different types of PELNs. Therefore, prior to using PELNs as therapeutic agents and drug carriers, comprehensive quality control measures are imperative to address concerns regarding biological safety and potential toxicity from unidentified bioactive components. A thorough evaluation encompassing morphological characteristics, quantitative parameters, safe dosage levels and active constituents is indispensable.
Comparison between PELN and mammalian cell-derived exosomes
| Source of exosomes | Size | Contents | Distribution | Functionality | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| PELN | 30-800nm | Protein, nucleic acid, lipid, secondary metabolite | Liver, kidney, spleen, lungs, brain, stomach, intestine | Anti-inflammatory, anti-tumor, gut microbiota regulation, regenerative, drug carrier, etc. | Promising for mass production, enriched biological activities, low toxicity, high biocompatibility, and environmental-friendliness | High heterogeneity, seasonal harvest, and lack of standardized isolation methods | [10, 37, 47, 129, 172, 173] |
| Mammalian cell-derived exosomes | 30-150nm | Protein, nucleic acid, lipid | Liver, kidney, spleen, lungs, brain, stomach, intestine | Anti-inflammatory, anti-tumor, antioxidant, immunomodulatory, drug carrier, etc. | High biocompatibility, noninvasive biomarker, and potential therapeutic efficacy | Complex preparation steps, low productivity, and high heterogeneity | [72, 174-176] |
7. Summary and outlook
In recent years, research on cell-free therapeutic approaches based on PELNs have attracted considerable attention. Plant-derived PELNs offer several advantages over exosomes derived from mammalian cells, including high safety, environmental sustainability, cost-effectiveness, and enriched biological activities (Table 6). As drug carriers, PELNs can exert synergistic therapeutic effects through efficient drug delivery based on their inherent biological properties combined with site-specific modifications.
At present, molecular mechanisms underlying cargo uptake and release into recipient cells remain incompletely understood. Thus, it is crucial to explore how to regulate the biogenesis of PELNs and their functions. Currently, the nomenclature of PELNs has been inconsistent in studies, which may be attributed to the differences in isolation methods as well as the particular terms such as "EVs" and "exosomes". The generally accepted size range of exosomes span from 30 nm to 200 nm. Notably, several studies have reported the size of PELNs to be more than 200 nm. For plant-derived PELNs, the plant can be named in Latin and English. What's important is to keep consistent in their nomenclature. Therefore, we should modify the protocols for the isolation and definition of PELNs and improve the clarity in terminology. Recently, a consensus statement has been issued by the Chinese Expert Committee on Research and Application of Chinese Herbal Vesicles. It involves the nomenclature, isolation methods, quality control, and applications of PELNs, providing unified recommendations for plant vesicles primarily derived from traditional Chinese herbs. With periodic updates and revisions, it was aimed to guide researchers in the field of PELNs [130].
PELNs serve as carriers for various cargo, including proteins, nucleic acids, lipids, and metabolites. As evidenced by recent studies, they are potential for therapeutic interventions. Although numerous studies have identified the components of PELNs through sequencing, elucidating the specific functions of these components should be a primary focus for future research. Secondary metabolites of PELNs have garnered significant attentions for years. In developing countries, herbal remedies usually play a predominant role in disease management. The metabolites in these herbs can regulate the functions of blood or cell-derived exosomes, thereby exerting therapeutic effects on diseases [131, 132]. Thus, future research should explore the synergistic effects of PELNs and cell-derived exosomes in the treatments of diseases.
Owing to their targeting tendency for gastrointestinal tract and tumors, PELNs are primarily applied in gastrointestinal disorders, particularly colitis. Meanwhile, the significant regulatory role of PELNs in gut microbiota should not be underestimated. PELNs are primarily delivered orally, but their efficacy is limited by restricted distribution in tissues beyond the gastrointestinal tract. Engineering modifications to PELNs can effectively make them suitable for specific tissues and organs, thus addressing the challenges encountered by conventional drug delivery systems. For instance, incorporating cell membranes containing targeting ligands onto the surface of PELNs facilitates targeted delivery to specific regions, thereby achieving precise delivery of PELNs [83]. PEG-modified PELNs can increase retention of PELNs in both the systemic circulation and tumor tissues, thereby further enhancing their therapeutic efficacy [16, 84]. Hydrogel-loaded PELNs exhibit pseudoplastic flow behavior and viscosity suitable for local application, making them more appropriate for transdermal drug delivery [75, 133]. Furthermore, incorporating drug-loaded nanocarrier structures either embedded within PELNs or patched onto their surface enhances the drug-carrying efficacy and stability of PELNs [121, 134]. Although research on PELNs is still in the emerging stage compared to other engineered nanoparticles, it warrants more in-depth investigation.
Fruits and vegetables, owing to their high lipid content, serve not only biological functions but also as potential carriers for drug delivery. PELNs derived from herbs, enriched with active herbal components, exhibit diverse biological effects such as regenerative and anti-osteoporotic properties. However, research exploring their potential as drug delivery carriers is limited, leaving their role in this capacity uncertain. In addition to medicinal field, PELNs is also promising in the domain of skincare. They can facilitate the penetration of biologically active substances derived from plant cells into the skin. Besides, PELNs also have a potential in releasing encapsulated nutrients to achieve sustained nourishment. For instance, a skin-rejuvenating formulation utilizing Aloe vera-derived PELNs, which are rich in heat shock proteins, has been developed to enhance skin texture and complexion, as well as improve the appearance of scars and skin abrasions [135].
Despite the significant challenges in their production and application, the potential of PELNs in the biomedicine is immense. Over the past decade, numerous researches have been carried out for understanding the biomedical efficacy of PELNs. However, further in-depth exploration is still necessary in future. Furthermore, several promising PELNs, such as those derived from grapefruit, ginger, and ginseng, warrant further development. Grapefruit PELNs, capable of delivering nucleic acids, proteins, and small molecules, show considerable promise as drug delivery carriers. Ginger PELNs, owing to their broad therapeutic properties, represent valuable candidates for development as therapeutic agents. Ginseng PELNs have demonstrated remarkable efficacy in treating various tumors, underscoring their potential as future anti-cancer drugs. Overall, the transition of PELNs from lab to commercial production necessitates substantial endeavors, including the establishment of standardized production protocols, exploration of stable long-term storage methodologies, and the initiation of clinical trials.
Abbreviations
AhR: aryl hydrocarbon receptor; ARG1: arginase-1; DSS: dextran sulfate sodium; EV: extracellular vesicle; EXPO: exocyst-positive organelle; HFD: high-fat diet; IRS: insulin receptor substrates; MSC: mesenchymal stem cells; MVB: multivesicular body; OMV: outer membrane vesicle; PA: phosphatidic acid; PELN: plant-derived exosome-like nanoparticle; PEN1: penetration 1; PS: phosphatidylserine; RANKL: receptor activator of nuclear factor-kappa B ligand; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SM: sphingomyelin; SV: small vacuole; TAM: tumor-associated macrophage; TET-8: tetraspanin-8; TME: tumor microenvironment.
Acknowledgements
This work was supported by the Development Project of Zhejiang Province's "Lingyan" (2024C03285 (SD2)), the NINGBO Medical & Health Leading Academic Discipline Project (2022-ZF02) and NINGBO Medical & Health Brand Discipline Project (No. PPXK2024-08).
Competing Interests
The authors have declared that no competing interest exists.
References
1. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-28
2. Jansen F, Li Q. Exosomes as diagnostic biomarkers in cardiovascular diseases. Adv Exp Med Biol. 2017;998:61-70
3. Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM. et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756-66
4. Baulch JE, Acharya MM, Allen BD, Ru N, Chmielewski NN, Martirosian V. et al. Cranial grafting of stem cell-derived microvesicles improves cognition and reduces neuropathology in the irradiated brain. Proc Natl Acad Sci U S A. 2016;113:4836-41
5. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177-82
6. Jiang XC, Gao JQ. Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm. 2017;521:167-75
7. Regente M, Corti-Monzón G, Maldonado AM, Pinedo M, Jorrín J, de la Canal L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009;583:3363-6
8. Cao M, Diao N, Cai X, Chen X. Plant exosome nanovesicles (PENs): green delivery platforms. Mater Horiz. 2023;10:3879-94
9. Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang Y. et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22:107-26
10. Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29:13-31
11. Li M, Chen T, Wang R, Luo JY, He JJ, Ye RS. et al. Plant MIR156 regulates intestinal growth in mammals by targeting the Wnt/β-catenin pathway. Am J Physiol Cell Physiol. 2019;317:C434-C48
12. Jeong DH, Thatcher SR, Brown RS, Zhai J, Park S, Rymarquis LA. et al. Comprehensive investigation of microRNAs enhanced by analysis of sequence variants, expression patterns, ARGONAUTE loading, and target cleavage. Plant Physiol. 2013;162:1225-45
13. Cavalieri D, Rizzetto L, Tocci N, Rivero D, Asquini E, Si-Ammour A. et al. Plant microRNAs as novel immunomodulatory agents. Sci Rep. 2016;6:25761
14. Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H. et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther. 2013;21:1345-57
15. Yepes-Molina L, Martínez-Ballesta MC, Carvajal M. Plant plasma membrane vesicles interaction with keratinocytes reveals their potential as carriers. J Adv Res. 2020;23:101-11
16. Wang Q, Ren Y, Mu J, Egilmez NK, Zhuang X, Deng Z. et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015;75:2520-9
17. Li Z, Wang H, Yin H, Bennett C, Zhang HG, Guo P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci Rep. 2018;8:14644
18. Mu N, Li J, Zeng L. Plant-derived exosome-like nanovesicles: current progress and prospects. Int J Nanomedicine. 2023;18:4987-5009
19. Orefice NS, Di Raimo R, Mizzoni D, Logozzi M, Fais S. Purposing plant-derived exosomes-like nanovesicles for drug delivery: patents and literature review. Expert Opin Ther Pat. 2023;33:89-100
20. Zhang M, Viennois E, Prasad M, Zhang Y, Wang L, Zhang Z. et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321-40
21. Lee EC, Ha TW. Utility of exosomes in ischemic and hemorrhagic stroke diagnosis and treatment. Int J Mol Sci. 2022;23:8367
22. Hade MD, Suire CN. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021;10:1959
23. An Q, van Bel AJ, Hückelhoven R. Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal Behav. 2007;2:4-7
24. Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A. et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677-85
25. Qiu D, Lange E, Haas TM, Prucker I. Bacterial pathogen Infection triggers magic spot nucleotide signaling in arabidopsis thaliana chloroplasts through specific RelA/SpoT homologues. J Am Chem Soc. 2023;145:16081-9
26. Rutter BD, Innes RW. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017;173:728-41
27. Ito Y, Taniguchi K. Uptake of microRNAs from exosome-like nanovesicles of edible plant juice by rat enterocytes. Int J Mol Sci. 2021;22:3749
28. Cui Y, Cao W, He Y, Zhao Q. A whole-cell electron tomography model of vacuole biogenesis in Arabidopsis root cells. Nat Plants. 2019;5:95-105
29. Wang J, Ding Y, Wang J, Hillmer S, Miao Y, Lo SW. et al. EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. Plant Cell. 2010;22:4009-30
30. Anusha R, Ashin M, Priya S. Ginger exosome-like nanoparticles (GELNs) induced apoptosis, cell cycle arrest, and anti-metastatic effects in triple-negative breast cancer MDA-MB-231 cells. Food Chem Toxicol. 2023;182:114102
31. Li S, Zhang R, Wang A, Li Y, Zhang M, Kim J. et al. Panax notoginseng: derived exosome-like nanoparticles attenuate ischemia reperfusion injury via altering microglia polarization. J Nanobiotechnology. 2023;21:416
32. Nouchi I, Hayashi K, Hiradate S, Ishikawa S, Fukuoka M, Chen CP. et al. Overcoming the difficulties in collecting apoplastic fluid from rice leaves by the infiltration-centrifugation method. Plant Cell Physiol. 2012;53:1659-68
33. Feng J, Xiu Q, Huang Y, Troyer Z, Li B, Zheng L. Plant-derived vesicle-like nanoparticles as promising biotherapeutic tools: present and future. Adv Mater. 2023;35:e2207826
34. Rahmati S, Karimi H, Alizadeh M, Khazaei AH, Paiva-Santos AC, Rezakhani L. et al. Prospects of plant-derived exosome-like nanocarriers in oncology and tissue engineering. Hum Cell. 2023;37:121-38
35. Sidhom K, Obi PO, Saleem A. A review of exosomal isolation methods: Is size exclusion chromatography the best option? Int J Mol Sci. 2020;21:6466
36. Driedonks TAP, Nijen Twilhaar MK, Nolte-'t Hoen ENM. Technical approaches to reduce interference of Fetal calf serum derived RNA in the analysis of extracellular vesicle RNA from cultured cells. J Extracell Vesicles. 2019;8:1552059
37. Lian MQ, Chng WH, Liang J, Yeo HQ, Lee CK, Belaid M. et al. Plant-derived extracellular vesicles: recent advancements and current challenges on their use for biomedical applications. J Extracell Vesicles. 2022;11:12283
38. Liu H, Song J, Zhou L, Peng S. Construction of curcumin-fortified juices using their self-derived extracellular vesicles as natural delivery systems: grape, tomato, and orange juices. Food Funct. 2023;14:9364-76
39. Liu NJ, Wang N, Bao JJ, Zhu HX, Wang LJ, Chen XY. Lipidomic analysis reveals the importance of GIPCs in arabidopsis leaf extracellular vesicles. Mol Plant. 2020;13:1523-32
40. Kreimer S, Ivanov AR. Rapid isolation of extracellular vesicles from blood plasma with size-exclusion chromatography followed by mass spectrometry-based proteomic profiling. Methods Mol Biol. 2017;1660:295-302
41. Berger E, Colosetti P, Jalabert A, Meugnier E, Wiklander OPB, Jouhet J. et al. Use of nanovesicles from orange juice to reverse diet-induced gut modifications in diet-induced obese mice. Mol Ther Methods Clin Dev. 2020;18:880-92
42. Liu Y, Xiao S, Wang D, Qin C, Wei H, Li D. A review on separation and application of plant-derived exosome-like nanoparticles. J Sep Sci. 2024;47:2300669
43. Suresh AP, Kalarikkal SP. Low pH-based method to increase the yield of plant-derived nanoparticles from fresh ginger rhizomes. ACS Omega. 2021;6:17635-41
44. Cao M, Yan H, Han X, Weng L, Wei Q, Sun X. et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019;7:326
45. Kim J, Zhu Y, Chen S, Wang D, Zhang S, Xia J. et al. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood-brain-barrier penetration and tumor microenvironment modulation. J Nanobiotechnology. 2023;21:253
46. Mateescu B, Kowal EJ. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA - an ISEV position paper. J Extracell Vesicles. 2017;6:1286095
47. Zhang M, Viennois E, Xu C, Merlin D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers. 2016;4:e1134415
48. Pinedo M, de la Canal L. A call for Rigor and standardization in plant extracellular vesicle research. J Extracell Vesicles. 2021;10:e12048
49. Meyer D, Pajonk S, Micali C, O'Connell R, Schulze-Lefert P. Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. Plant J. 2009;57:986-99
50. He B, Cai Q. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat Plants. 2021;7:342-52
51. Wang F, Yuan M, Shao C, Ji N, Zhang H, Li C. Momordica charantia-derived extracellular vesicles provide antioxidant protection in ulcerative colitis. Molecules. 2023;28:6182
52. Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41-51
53. Huang R, Jia B. Plant exosomes fused with engineered mesenchymal stem cell-derived nanovesicles for synergistic therapy of autoimmune skin disorders. J Extracell Vesicles. 2023;12:e12361
54. Kumar A, Sundaram K, Teng Y, Mu J, Sriwastva MK, Zhang L. et al. Ginger nanoparticles mediated induction of Foxa2 prevents high-fat diet-induced insulin resistance. Theranostics. 2022;12:1388-403
55. Sundaram K, Miller DP, Kumar A, Teng Y, Sayed M, Mu J. et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of porphyromonas gingivalis. iScience. 2019;21:308-27
56. Wang Q, Zhuang X, Mu J, Deng ZB, Jiang H, Zhang L. et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867
57. Zhang ZG, Buller B, Chopp M. Exosomes-beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15:193-203
58. Teng Y, Xu F, Zhang X, Mu J, Sayed M, Hu X. et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol Ther. 2021;29:2424-40
59. Kalarikkal SP, Sundaram GM. Edible plant-derived exosomal microRNAs: Exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicol Appl Pharmacol. 2021;414:115425
60. Xiao J, Feng S, Wang X, Long K, Luo Y, Wang Y. et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6:e5186
61. Wang K, Chen Q, Shao Y, Yin S, Liu C, Liu Y. et al. Anticancer activities of TCM and their active components against tumor metastasis. Biomed Pharmacother. 2021;133:111044
62. Zhuang X, Deng ZB, Mu J, Zhang L, Yan J, Miller D. et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713
63. Chen J, Wu J, Mu J, Li L, Hu J, Lin H. et al. An antioxidative sophora exosome-encapsulated hydrogel promotes spinal cord repair by regulating oxidative stress microenvironment. Nanomedicine. 2023;47:102625
64. Xu F, Mu J, Teng Y, Zhang X, Sundaram K, Sriwastva MK. et al. Restoring oat nanoparticles mediated brain memory function of mice fed alcohol by sorting inflammatory dectin-1 complex into microglial exosomes. Small. 2022;18:e2105385
65. Stanly C, Alfieri M, Ambrosone A. Grapefruit-derived micro and nanovesicles show distinct metabolome profiles and anticancer activities in the A375 human melanoma cell line. Cells. 2020;9:2722
66. Zhang M, Xiao B, Wang H, Han MK, Zhang Z, Viennois E. et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther. 2016;24:1783-96
67. Kim J, Zhang S, Zhu Y, Wang R, Wang J. Amelioration of colitis progression by ginseng-derived exosome-like nanoparticles through suppression of inflammatory cytokines. J Ginseng Res. 2023;47:627-37
68. Chen Q, Zu M, Gong H, Ma Y, Sun J, Ran S. et al. Tea leaf-derived exosome-like nanotherapeutics retard breast tumor growth by pro-apoptosis and microbiota modulation. J Nanobiotechnology. 2023;21:6
69. Chen Q, Li Q, Liang Y, Zu M, Chen N, Canup BSB. et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm Sin B. 2022;12:907-23
70. Chernomordik LV, Melikyan GB, Chizmadzhev YA. Biomembrane fusion: A new concept derived from model studies using two interacting planar lipid bilayers. Biochim Biophys Acta. 1987;906:309-52
71. Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108
72. Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19:47
73. Tkach M, Kowal J. Qualitative differences in T-cell activation by dendritic cell-derived extracellular vesicle subtypes. EMBO J. 2017;36:3012-28
74. Song H, Canup BSB, Ngo VL, Denning TL, Garg P, Laroui H. Internalization of garlic-derived nanovesicles on liver cells is triggered by interaction with CD98. ACS Omega. 2020;5:23118-28
75. Xu XH, Yuan TJ, Dad HA, Shi MY, Huang YY, Jiang ZH. Plant exosomes as novel nanoplatforms for microRNA transfer stimulate neural differentiation of stem cells in vitro and in vivo. Nano Lett. 2021;21:8151-9
76. De Robertis M, Sarra A, D'Oria V, Mura F, Bordi F, Postorino P. Blueberry-derived exosome-like nanoparticles counter the response to TNF-α-induced change on gene expression in EA.hy926 cells. Biomolecules. 2020;10:742
77. Itakura S, Shohji A, Amagai S, Kitamura M, Takayama K, Sugibayashi K. et al. Gene knockdown in HaCaT cells by small interfering RNAs entrapped in grapefruit-derived extracellular vesicles using a microfluidic device. Sci Rep. 2023;13:3102
78. Yang M, Liu X, Luo Q, Xu L, Chen F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J Nanobiotechnology. 2020;18:100
79. Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar A. et al. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe. 2018;24:637-52
80. Hwang J-H, Park Y-S, Kim H-S, Kim D-h, Lee S-H, Lee C-H. et al. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. J Control Release. 2023;355:184-98
81. Mu J, Zhuang X, Wang Q, Jiang H, Deng ZB, Wang B. et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58:1561-73
82. Feng T, Wan Y, Dai B, Liu Y. Anticancer activity of bitter melon-derived vesicles extract against breast cancer. Cells. 2023;12:824
83. Ma C, Liu K, Wang F, Fei X, Niu C, Li T. et al. Neutrophil membrane engineered Panax ginseng root derived exosomes loaded miRNA 182-5p targets NOX4/Drp-1/NLRP3 signal pathway to alleviate acute lung injury in sepsis: experimental studies. Int J Surg. 2023;110:72-86
84. Sasaki D, Kusamori K, Nishikawa M. Delivery of corn-derived nanoparticles with anticancer activity to tumor tissues by modification with polyethylene glycol for cancer therapy. Pharm Res. 2023;40:917-26
85. Zhuang X, Teng Y, Samykutty A, Mu J, Deng Z, Zhang L. et al. Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression. Mol Ther. 2016;24:96-105
86. Wang B, Zhuang X, Deng ZB, Jiang H, Mu J, Wang Q. et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22:522-34
87. Huang Y, Liu H, Sun X, Ding M, Tao G, Li X. Honeysuckle-derived microRNA2911 directly inhibits varicella-zoster virus replication by targeting IE62 gene. J Neurovirol. 2019;25:457-63
88. Perut F, Roncuzzi L, Avnet S. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules. 2021;11:87
89. Raimondo S, Naselli F, Fontana S, Monteleone F, Lo Dico A, Saieva L. et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6:19514-27
90. Wang X, Liu Y, Dong X, Duan T, Wang C, Wang L. et al. peu-MIR2916-p3-enriched garlic exosomes ameliorate murine colitis by reshaping gut microbiota, especially by boosting the anti-colitic Bacteroides thetaiotaomicron. Pharmacol Res. 2024;200:107071
91. Kumar A, Ren Y, Sundaram K, Mu J, Sriwastva MK, Dryden GW. et al. miR-375 prevents high-fat diet-induced insulin resistance and obesity by targeting the aryl hydrocarbon receptor and bacterial tryptophanase (tnaA) gene. Theranostics. 2021;11:4061-77
92. Zhan W, Deng M, Huang X, Xie D, Gao X, Chen J. et al. Pueraria lobata-derived exosome-like nanovesicles alleviate osteoporosis by enhacning autophagy. J Control Release. 2023;364:644-53
93. Şahin F, Koçak P, Güneş MY, Özkan İ, Yıldırım E, Kala EY. In vitro wound healing activity of wheat-derived nanovesicles. Appl Biochem Biotechnol. 2019;188:381-94
94. Wu J, Ma X, Lu Y, Zhang T, Du Z, Xu J. et al. Edible pueraria lobata-derived exosomes promote M2 macrophage polarization. Molecules. 2022;27:8184
95. Bian Y, Li W, Jiang X, Yin F, Yin L, Zhang Y. et al. Garlic-derived exosomes carrying miR-396e shapes macrophage metabolic reprograming to mitigate the inflammatory response in obese adipose tissue. J Nutr Biochem. 2023;113:109249
96. Bruno SP, Paolini A, D'Oria V, Sarra A, Sennato S, Bordi F. et al. Extracellular vesicles derived from citrus sinensis modulate inflammatory genes and tight junctions in a human model of intestinal epithelium. Front Nutr. 2021;8:778998
97. Taşlı PN. Usage of celery root exosome as an immune suppressant; Lipidomic characterization of apium graveolens originated exosomes and its suppressive effect on PMA/ionomycin mediated CD4(+) T lymphocyte activation. J Food Biochem. 2022;46:e14393
98. Ishida T, Kawada K. Exosome-like nanoparticles derived from Allium tuberosum prevent neuroinflammation in microglia-like cells. J Pharm Pharmacol. 2023;75:1322-31
99. Lee Y, Jeong DY, Jeun YC, Choe H, Yang S. Preventive and ameliorative effects of potato exosomes on UVB-induced photodamage in keratinocyte HaCaT cells. Mol Med Rep. 2023;28:167
100. Sundaram K, Mu J, Kumar A, Behera J, Lei C, Sriwastva MK. et al. Garlic exosome-like nanoparticles reverse high-fat diet induced obesity via the gut/brain axis. Theranostics. 2022;12:1220-46
101. Sasaki D, Kusamori K, Takayama Y, Itakura S, Todo H, Nishikawa M. Development of nanoparticles derived from corn as mass producible bionanoparticles with anticancer activity. Sci Rep. 2021;11:22818
102. Han X, Wei Q, Lv Y, Weng L, Huang H, Wei Q. et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol Ther. 2022;30:327-40
103. Lv Y, Li M, Weng L, Huang H, Mao Y, Yang DA. et al. Ginseng-derived nanoparticles reprogram macrophages to regulate arginase-1 release for ameliorating T cell exhaustion in tumor microenvironment. J Exp Clin Cancer Res. 2023;42:322
104. Zu M, Xie D, Canup BSB, Chen N, Wang Y, Sun R. et al. 'Green' nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials. 2021;279:121178
105. Qiu FS, Wang JF, Guo MY, Li XJ, Shi CY, Wu F. et al. Rgl-exomiR-7972, a novel plant exosomal microRNA derived from fresh Rehmanniae Radix, ameliorated lipopolysaccharide-induced acute lung injury and gut dysbiosis. Biomed Pharmacother. 2023;165:115007
106. Lu Y, Xu J, Tang R, Zeng P, Li Z, You J. et al. Edible pueraria lobata-derived exosome-like nanovesicles ameliorate dextran sulfate sodium-induced colitis associated lung inflammation through modulating macrophage polarization. Biomed Pharmacother. 2024;170:116098
107. Sundaram K, Teng Y, Mu J, Xu Q, Xu F, Sriwastva MK. et al. Outer membrane vesicles released from garlic exosome-like nanoparticles (GaELNs) train gut bacteria that reverses type 2 diabetes via the gut-brain axis. Small. 2024;20:e2308680
108. Yıldırım M, Ünsal N, Kabataş B, Eren O, Şahin F. Effect of solanum iycopersicum and citrus limon-derived exosome-like vesicles on chondrogenic differentiation of adipose-derived stem cells. Appl Biochem Biotechnol. 2023;196:203-19
109. Teng Y, He J, Zhong Q, Zhang Y, Lu Z, Guan T. et al. Grape exosome-like nanoparticles: A potential therapeutic strategy for vascular calcification. Front Pharmacol. 2022;13:1025768
110. Di Raimo R, Mizzoni D, Spada M, Dolo V, Fais S, Logozzi M. Oral treatment with plant-derived exosomes restores redox balance in H2O2-treated mice. Antioxidants. 2023;12:1169
111. Kim JS, Kim DH, Gil MC. Pomegranate-derived exosome-like nanovesicles alleviate binge alcohol-induced leaky gut and liver injury. J Med Food. 2023;26:739-48
112. Fiorucci S, Urbani G, Biagioli M, Sepe V, Distrutti E, Zampella A. Bile acids and bile acid activated receptors in the treatment of Covid-19. Biochem Pharmacol. 2023: 115983.
113. Yang S, Lu S, Ren L, Bian S, Zhao D, Liu M. et al. Ginseng-derived nanoparticles induce skin cell proliferation and promote wound healing. J Ginseng Res. 2023;47:133-43
114. Tan S, Liu Z, Cong M, Zhong X, Mao Y, Fan M. et al. Dandelion-derived vesicles-laden hydrogel dressings capable of neutralizing Staphylococcus aureus exotoxins for the care of invasive wounds. J Control Release. 2024;368:355-71
115. Seo K, Yoo JH. Ginseng-derived exosome-like nanovesicles extracted by sucrose gradient ultracentrifugation to inhibit osteoclast differentiation. Nanoscale. 2023;15:5798-808
116. Hu M, Palić D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020;37:101620
117. Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36-48
118. López de Las Hazas MC, Tomé-Carneiro J, Del Pozo-Acebo L, Del Saz-Lara A, Chapado LA, Balaguer L. et al. Therapeutic potential of plant-derived extracellular vesicles as nanocarriers for exogenous miRNAs. Pharmacol Res. 2023;198:106999
119. Del Pozo-Acebo L, López de Las Hazas MC, Tomé-Carneiro J, Del Saz-Lara A, Gil-Zamorano J, Balaguer L. et al. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol Res. 2022;185:106472
120. Pomatto MAC, Gai C, Negro F, Massari L, Deregibus MC, Grange C. Plant-derived extracellular vesicles as a delivery platform for RNA-based vaccine: feasibility study of an oral and intranasal SARS-CoV-2 vaccine. Pharmaceutics. 2023;15:974
121. Niu W, Xiao Q, Wang X, Zhu J, Li J, Liang X. et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21:1484-92
122. You JY, Kang SJ, Rhee WJ. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater. 2021;6:4321-32
123. Xiao Q, Zhao W, Wu C, Wang X, Chen J, Shi X. et al. Lemon-derived extracellular vesicles nanodrugs enable to efficiently overcome cancer multidrug resistance by endocytosis-triggered energy dissipation and energy production reduction. Adv Sci. 2022;9:2105274
124. Wu K, Xing F, Wu SY, Watabe K. Extracellular vesicles as emerging targets in cancer: Recent development from bench to bedside. Biochim Biophys Acta Rev Cancer. 2017;1868:538-63
125. Zhu H, Chang M, Wang Q, Chen J, Liu D, He W. Identifying the potential of miRNAs in houttuynia cordata-derived exosome-like nanoparticles against respiratory RNA viruses. Int J Nanomedicine. 2023;18:5983-6000
126. Zhao Z, Yu S. Isolation of exosome-like nanoparticles and analysis of microRNAs derived from coconut water based on small RNA high-throughput sequencing. J Agric Food Chem. 2018;66:2749-57
127. Chen X, Zhou Y, Yu J. Exosome-like nanoparticles from Ginger Rhizomes inhibited NLRP3 inflammasome activation. Mol Pharm. 2019;16:2690-9
128. Karn V, Ahmed S, Tsai LW, Dubey R, Ojha S. Extracellular vesicle-based therapy for COVID-19: promises, challenges and future prospects. Biomedicines. 2021;9:1373
129. Kim J, Li S, Zhang S, Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J Pharm Sci. 2022;17:53-69
130. Zhao Q, Wang T, Wang H, Cao P, Jiang C, Qiao H. et al. Consensus statement on research and application of Chinese herbal medicine derived extracellular vesicles-like particles (2023 edition). Chin Herb Med. 2023;16:3-12
131. Peng Y, Fan JY, Xiong J, Lou Y, Zhu Y. miR-34a enhances the susceptibility of gastric cancer to Platycodin D by targeting survivin. Pathobiology. 2019;86:296-305
132. Yang Q, Luo Y. Emodin ameliorates severe acute pancreatitis-associated acute lung injury in rats by modulating exosome-specific miRNA expression profiles. Int J Nanomedicine. 2023;18:6743-61
133. Setiadi VE, Adlia A, Barlian A, Ayuningtyas FD, Rachmawati H. Development and characterization of a gel formulation containing golden cherry exosomes (physalis minima) as a potential anti-photoaging. Pharm Nanotechnol. 2023;12:56-67
134. Gu W, Guo W, Ren Z, Zhang Y, Han M, Zhao Q. et al. A bioactive nanocomposite integrated specific TAMs target and synergistic TAMs repolarization for effective cancer immunotherapy. Bioact Mater. 2024;38:472-85
135. Buehrer Benjamin LJ, Pieraccini Peter. Plant-based exosome compositions and use thereof for rejuvenating skin. US. 2020 https://worldwide.espacenet.com/. Patent No: WO2020180311A1
136. Castelli G, Logozzi M, Mizzoni D. Ex vivo anti-leukemic effect of exosome-like grapefruit-derived nanovesicles from organic farming-the potential role of ascorbic acid. Int J Mol Sci. 2023;24:15663
137. Kilasoniya A, Garaeva L, Shtam T. Potential of plant exosome vesicles from grapefruit (Citrus × paradisi) and tomato (Solanum lycopersicum) Juices as functional ingredients and targeted drug delivery vehicles. Antioxidants (Basel). 2023;12:943
138. Tan M, Liu Y, Xu Y, Yan G, Zhou N, Chen H. et al. Plant-derived exosomes as novel nanotherapeutics contrive glycolysis reprogramming-mediated angiogenesis for diabetic ulcer healing. Biomater Res. 2024;28:0035
139. Liu B, Lu Y, Chen X. Protective role of shiitake mushroom-derived exosome-like nanoparticles in D-Galactosamine and Lipopolysaccharide-induced acute liver injury in mice. Nutrients. 2020;12:477
140. Mao Y, Han M, Chen C, Wang X, Han J, Gao Y. et al. A biomimetic nanocomposite made of a ginger-derived exosome and an inorganic framework for high-performance delivery of oral antibodies. Nanoscale. 2021;13:20157-69
141. Jang J, Jeong H, Jang E, Kim E, Yoon Y, Jang S. et al. Isolation of high-purity and high-stability exosomes from ginseng. Front Plant Sci. 2022;13:1064412
142. Gao C, Zhou Y, Chen Z, Li H, Xiao Y, Hao W. et al. Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis. Theranostics. 2022;12:5596-614
143. Konoshenko MY, Lekchnov EA. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;2018:8545347
144. Zhu Z, Liao L, Gao M, Liu Q. Garlic-derived exosome-like nanovesicles alleviate dextran sulphate sodium-induced mouse colitis via the TLR4/MyD88/NF-κB pathway and gut microbiota modulation. Food Funct. 2023;14:7520-34
145. Cai H, Huang LY, Hong R, Song JX, Guo XJ, Zhou W. et al. Momordica charantia exosome-like nanoparticles exert neuroprotective effects against ischemic brain injury via inhibiting matrix metalloproteinase 9 and activating the AKT/GSK3β signaling pathway. Front Pharmacol. 2022;13:908830
146. Chen T, Ma B, Lu S, Zeng L, Wang H, Shi W. et al. Cucumber-derived nanovesicles containing Cucurbitacin B for non-small cell lung cancer therapy. Int J Nanomedicine. 2022;17:3583-99
147. Chi Y, Shi L, Lu S, Cui H, Zha W, Shan L. et al. Inhibitory effect of Lonicera japonica-derived exosomal miR2911 on human papilloma virus. J Ethnopharmacol. 2024;318:116969
148. Trentini M, Zanolla I, Zanotti F, Tiengo E, Licastro D. Apple derived exosomes improve collagen type I production and decrease MMPs during aging of the skin through downregulation of the NF-κB pathway as mode of action. Cells. 2022;11:3950
149. Yin L, Yan L, Yu Q, Wang J, Liu C. Characterization of the microRNA profile of ginger exosome-like nanoparticles and their anti-inflammatory effects in intestinal Caco-2 cells. J Agric Food Chem. 2022;70:4725-34
150. Zhao X, Yin F, Fu L, Ma Y, Ye L, Huang Y. et al. Garlic-derived exosome-like nanovesicles as a hepatoprotective agent alleviating acute liver failure by inhibiting CCR2/CCR5 signaling and inflammation. Biomater Adv. 2023;154:213592
151. Sriwastva MK, Deng ZB, Wang B, Teng Y, Kumar A, Sundaram K. et al. Exosome-like nanoparticles from Mulberry bark prevent DSS-induced colitis via the AhR/COPS8 pathway. EMBO Rep. 2022;23:e53365
152. Zhang X, Pan Z, Wang Y, Liu P, Hu K. Taraxacum officinale-derived exosome-like nanovesicles modulate gut metabolites to prevent intermittent hypoxia-induced hypertension. Biomed Pharmacother. 2023;161:114572
153. Takakura H, Nakao T, Narita T. Citrus limonL.-derived nanovesicles show an inhibitory effect on cell growth in p53-inactivated colorectal cancer cells via the macropinocytosis pathway. Biomedicines. 2022;10:1352
154. Raimondo S, Saieva L, Cristaldi M, Monteleone F, Fontana S, Alessandro R. Label-free quantitative proteomic profiling of colon cancer cells identifies acetyl-CoA carboxylase alpha as antitumor target of Citrus limon-derived nanovesicles. J Proteomics. 2018;173:1-11
155. Gao Q, Chen N, Li B, Zu M, Ma Y, Xu H. et al. Natural lipid nanoparticles extracted from Morus nigra L. leaves for targeted treatment of hepatocellular carcinoma via the oral route. J Nanobiotechnology. 2024;22:4
156. Potestà M, Roglia V, Fanelli M, Pietrobono E, Gismondi A, Vumbaca S. Effect of microvesicles from Moringa oleifera containing miRNA on proliferation and apoptosis in tumor cell lines. Cell Death Discov. 2020;6:43
157. Sasaki D, Suzuki H, Kusamori K, Itakura S, Todo H, Nishikawa M. Development of rice bran-derived nanoparticles with excellent anti-cancer activity and their application for peritoneal dissemination. J Nanobiotechnology. 2024;22:114
158. Yan G, Xiao Q, Zhao J, Chen H, Xu Y, Tan M. et al. Brucea javanica derived exosome-like nanovesicles deliver miRNAs for cancer therapy. J Control Release. 2024;367:425-40
159. Yang L, Jin WQ, Tang XL, Zhang S, Ma R, Zhao DQ. et al. Ginseng-derived nanoparticles inhibit lung cancer cell epithelial mesenchymal transition by repressing pentose phosphate pathway activity. Front Oncol. 2022;12:942020
160. Lei C, Teng Y, He L, Sayed M, Mu J, Xu F. et al. Lemon exosome-like nanoparticles enhance stress survival of gut bacteria by RNase P-mediated specific tRNA decay. iScience. 2021;24:102511
161. Lei C, Mu J, Teng Y, He L, Xu F, Zhang X. et al. Lemon exosome-like nanoparticles-manipulated probiotics protect mice from C. d iff Infection. iScience. 2020;23:101571
162. Duan T, Wang X, Dong X, Wang C, Wang L, Yang X. Broccoli-derived exosome-like nanoparticles alleviate loperamide-induced constipation, in correlation with regulation on gut microbiota and tryptophan metabolism. J Agric Food Chem. 2023;71:16568-80
163. Zhu MZ, Xu HM, Liang YJ, Xu J, Yue NN, Zhang Y. et al. Edible exosome-like nanoparticles from portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4(+)CD8(+)T cells expansion. J Nanobiotechnology. 2023;21:309
164. Zhao WJ, Bian YP, Wang QH, Yin F, Yin L, Zhang YL. et al. Blueberry-derived exosomes-like nanoparticles ameliorate nonalcoholic fatty liver disease by attenuating mitochondrial oxidative stress. Acta Pharmacol Sin. 2022;43:645-58
165. Zhou X, Xu S, Zhang Z, Tang M, Meng Z, Peng Z. et al. Gouqi-derived nanovesicles (GqDNVs) inhibited dexamethasone-induced muscle atrophy associating with AMPK/SIRT1/PGC1α signaling pathway. J Nanobiotechnology. 2024;22:276
166. Sarvarian P, Samadi P, Gholipour E, Khodadadi M, Pourakbari R, Akbarzadelale P. et al. Fisetin-loaded grape-derived nanoparticles improve anticancer efficacy in MOLT-4 cells. Biochem Biophys Res Commun. 2023;658:69-79
167. Garaeva L, Kamyshinsky R, Kil Y, Varfolomeeva E, Verlov N, Komarova E. et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci Rep. 2021;11:6489
168. Lu X, Han Q, Chen J, Wu T, Cheng Y, Li F. et al. Celery (Apium graveolens L.) exosome-like nanovesicles as a new-generation chemotherapy drug delivery platform against tumor proliferation. J Agric Food Chem. 2023;71:8413-24
169. Jiang D, Li Z, Liu H, Liu H, Xia X, Xiang X. Plant exosome-like nanovesicles derived from sesame leaves as carriers for luteolin delivery: Molecular docking, stability and bioactivity. Food Chem. 2024;438:137963
170. Wang X, Zhang M. Oral administration of ginger-derived lipid nanoparticles and Dmt1 siRNA potentiates the effect of dietary iron restriction and mitigates pre-existing iron overload in hamp KO mice. Nutrients. 2021;13:1686
171. Zhang M, Wang X, Han MK, Collins JF, Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine (Lond). 2017;12:1927-43
172. Cong M, Tan S, Li S, Gao L, Huang L, Zhang H-G. et al. Technology insight: Plant-derived vesicles—How far from the clinical biotherapeutics and therapeutic drug carriers? Adv Drug Deliv Rev. 2022;182:114108
173. Fang Z, Liu K. Plant-derived extracellular vesicles as oral drug delivery carriers. J Control Release. 2022;350:389-400
174. Zhao Y, H T, Zhang J, Pan B, Wang N, Chen T. et al. Plant-derived vesicles: a new era for anti-cancer drug delivery and cancer treatment. Int J Nanomedicine. 2023;18:6847-68
175. Bai C, liu J, Zhang X, Li Y, Qin Q, Song H. et al. Research status and challenges of plant-derived exosome-like nanoparticles. Biomed Pharmacother. 2024;174:116543
176. Feng J, Xiu Q, Huang Y, Troyer Z, Li B, Zheng L. Plant-derived vesicle-like nanoparticles as promising biotherapeutic tools: present and future. Adv Mater. 2023;35:2207826
Author contact
![]() Corresponding authors: Ninggang Chen, 13486656300com; Jianqing Gao, gaojianqingedu.cn.
Corresponding authors: Ninggang Chen, 13486656300com; Jianqing Gao, gaojianqingedu.cn.
 Global reach, higher impact
Global reach, higher impact