13.3
Impact Factor
Theranostics 2024; 14(10):3963-3983. doi:10.7150/thno.96959 This issue Cite
Review
The emerging role of Piezo1 in the musculoskeletal system and disease
1. Musculoskeletal Research Laboratory and Centre of Musculoskeletal Aging and Regeneration, Department of Orthopaedics and Traumatology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China.
2. The Sir Yue-Kong Pao Cancer Centre, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China.
3. Joint Laboratory of Chinese Academic of Science and Hong Kong for Biomaterials, The Chinese University of Hong Kong, Hong Kong SAR, China.
Received 2024-4-4; Accepted 2024-5-15; Published 2024-6-24
Abstract
Piezo1, a mechanosensitive ion channel, has emerged as a key player in translating mechanical stimuli into biological signaling. Its involvement extends beyond physiological and pathological processes such as lymphatic vessel development, axon growth, vascular development, immunoregulation, and blood pressure regulation. The musculoskeletal system, responsible for structural support, movement, and homeostasis, has recently attracted attention regarding the significance of Piezo1. This review aims to provide a comprehensive summary of the current research on Piezo1 in the musculoskeletal system, highlighting its impact on bone formation, myogenesis, chondrogenesis, intervertebral disc homeostasis, tendon matrix cross-linking, and physical activity. Additionally, we explore the potential of targeting Piezo1 as a therapeutic approach for musculoskeletal disorders, including osteoporosis, muscle atrophy, intervertebral disc degeneration, and osteoarthritis.
Keywords: Piezo1, bone, muscle, cartilage, intervertebral disc
Introduction
Mechanotransduction enables the cells to translate mechanical forces into biochemical signals [1], thus triggering a series of biological responses. Mechanically activated ion channels, as force-sensing integral membrane proteins, can couple their structural dynamics and membrane proteins to adapt mechanical stimuli to cell plasma membrane [2]. These channels are involved in various physiological processes, such as touch, hearing, proprioception, and osmoregulation [3-5]. In 2010, Coste et al. discovered the Piezo family of mechanosensitive ion channels encoded by the Piezo1/FAM38A and Piezo2/FAM38B genes [6]. Piezo1 is predominantly expressed in non-sensory tissues and responds to mechanical loading, whereas Piezo2 is located in sensory tissues and senses touch [7].
Cell membrane deformations caused by mechanical forces can activate the Piezo1 channel [8]. This channel can directly convert mechanical forces, such as shear stress and osmotic pressure, into physiological changes through depolarization of excited cells or inducing cationic influx in non-excitable cells [9]. Piezo1 exhibits permeability to both monovalent and divalent ions, such as Ca2+, Na+, K+, and Mg2+, with a preference of Ca2+. A wealth of circumstantial evidence suggests that Piezo1 expressed in most mammals is engaged in various physiological processes, such as lymphatic vessel development [7], axon growth [10], vascular development [11], immunoregulation [12], and blood pressure regulation [13], indicating the universal significance of the Piezo1 channel.
Piezo1 channel plays a crucial role in sensing mechanical stimulation and regulating cell behaviors, such as proliferation, migration, and apoptosis [14-16]. For instance, acoustic radiation force can promote osteoblasts migration and proliferation by upregulating Piezo1 expression [17]. It is worth noting that Piezo1 also participates in stem cell fate determination. Piezo1 is robustly expressed in stem cells and its modulation can impact differentiation outcomes [18]. Piezo1 knockdown promotes astrogenesis and suppresses neurogenesis in human neural stem cells [19]. In addition, Piezo1 enhances the osteogenic differentiation of mesenchymal stem cells (MSCs) via increasing BMP2 expression [20]. Notably, Piezo1 is expressed stably in cells resident in bone [21], cartilage [22], skeletal muscle [23], tendon [24], intervertebral disc [25], and even their surrounding connective tissues [26]. Piezo1 promotes angiogenesis to accelerate bone fracture healing [27]. Besides, Piezo1 induces myotube formation by controlling Ca2+ influx [28], as well as enhances tendon stiffness through modifying collagen cross-linking [24]. Of note, Piezo1 activation however promotes the progression of osteoarthritis (OA) [29].
Recently, two reviews [30, 31] have summarized the role of Piezo1 in the skeleton and muscular tissues, including bone, cartilage, tendons, and skeletal muscles. Here, we update the current understanding of the physiological and pathophysiological roles mediated by Piezo1 in the musculoskeletal system and discuss why Piezo1 should be regarded as a therapeutic target for musculoskeletal disorders, including osteoporosis (OP), OA, muscle atrophy, and intervertebral disc degeneration (IDD). Figure 1 provides an overview of the physiological functions of Piezo1 in the musculoskeletal system.
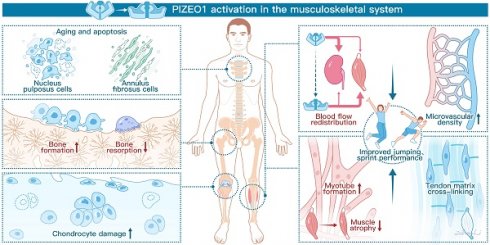
Schematic diagram showing the crucial roles of Piezo1 in the musculoskeletal system. The left panel demonstrates that Piezo1 activation enhances bone formation and reduces bone resorption. However, Piezo1 activation accelerates chondrocyte damage and promotes nucleus pulposus (NP) and annulus fibrosus (AF) cell senescence and apoptosis. The right panel highlights the positive effects of Piezo1 activation on physical performance. Specifically, Piezo1 activation leads to increased blood flow redistribution and microvascular density in muscles. Furthermore, Piezo1 activation promotes myotube formation and increases tendon stiffness.
Overview of Piezo1 channel
Structure of Piezo1
Deciphering the structure of Piezo1 is crucial for gaining a comprehensive understanding of its function. Piezo1 is a large transmembrane protein without repetitive sequence and sequence homology to other known ion channels [32]. Through cryo-electron microscopy techniques, it has been determined that Piezo1 consists of 2547 residues [33], and adopts a three-bladed, trimeric propeller-like architecture. This architecture features a central ion-conducting pore module topped with an extracellular cap domain [32]. Each subunit of the Piezo1 structure contains a central ion-conducting pore and two peripheral modules [34]. The central ion-conducting pore consists of outer helices, inner helices, intracellular C-terminal domains, and extracellular C-terminal domains (CEDs, which govern unitary conductance, ion permeability, and selectivity) [33]. The peripheral mechanotransduction module includes a long beam-like structure and an anchor domain [8]. The peripheral propeller blades house 38 transmembrane helices serve as the mechanosensing module [8]. The detail of the structure and function of Piezo1 are described elsewhere [8]. Overall, the trimeric structure of Piezo1 enables cooperative sensing of mechanical stimuli and subsequent activation of ion conductance. The precise arrangement and interactions of these domains enable Piezo1 to convert mechanical forces into electrical signals, contributing to its role in mechanotransduction.
Pharmacological Modulators of Piezo1
Although mechanical stimulation plays a primary role in Piezo1 activity, it can also be regulated by pharmacology. Yoda1 was initially identified as a Piezo1-specific allosteric activator through high-throughput chemical library screening [35]. It induces local conformational changes that result in the opening of Piezo1 pore, reducing the mechanical threshold required for channel activation [36]. Yoda1 binds to the proximal end of the blade (residue 1961-2063) of Piezo1, enhancing membrane tension-induced blade motion through a wedge-like effect [36]. The sensitivity of Yoda1 to protein mutations and structural modifications led to the development of analogues such as KC159, which contains 4-benzoic acid instead of the pyrazine moiety in Yoda1, and its potassium salt (KC289). These analogues have demonstrated equivalent or improved reliability, efficacy, and potency compared to Yoda1 in functional assays [37]. Jedi1 and Jedi2 are alternative Piezo1 agonists that activate Piezo1 from the extracellular side of the blade [38]. Yoda1 and Jedi1/2 exhibit synergistic effects, suggesting distinct activation mechanisms. Specifically, Yoda1 acts on the downstream beam, while Jedi1 and Jedi2 act on the upstream blade [38].
Several non-specific inhibitors of Piezo1 have been identified, including Grammostola spatulata mechanotoxin 4 (GsMTx4), ruthenium red (RR), and gadolinium (Gd3+). GsMTx4, a spider venom peptide, inhibits cation-sensitive mechanosensitive channels by reducing tension and lateral pressure on the membrane through insertion into the lipid bilayer [39]. RR and Gd3+ are small molecules that can non-specifically block Piezo1 [40]. In addition to these non-specific blockers, Dooku-1 is a specific inhibitor that demonstrates inhibitory effects not only on Yoda1-induced activation of Piezo1 [41], but also on the constitutively open Piezo1 channel [42]. Certain Piezo1-interacting proteins, such as sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) [36] and polycystin-2 (PC2) [43], can bind directly to Piezo1 and weaken the mechanosensitive current. Saturated and polyunsaturated fatty acids, such as arachidonic acid and eicosapentaenoic acid, can also inhibit Piezo1 activity in a non-specific manner [44].
Expression and Distribution of Piezo1 in Musculoskeletal Tissues
The musculoskeletal system is vital for providing structural support, enabling movement, and maintaining overall homeostasis in the human body. Currently, there is significant research focus on understanding the role of Piezo1 in the musculoskeletal system. It is imperative to summarize the expression and distribution of Piezo1 based on current research findings. Within bone tissues, Piezo1 has been identified in various cell types residing in bone, including bone marrow mesenchymal stromal cells (BMSCs) [45], periosteal stem cells (PSCs) [46], osteoblasts [26], osteocytes [26, 47, 48], hypertrophic chondrocytes [26], and endothelial cells (ECs) [27]. Piezo1 is predominantly expressed in differentiating osteoblasts and hypertrophic chondrocytes during limb development, suggesting its involvement in mechanotransduction during bone development [26]. The expression of Piezo1 is upregulated in young mice after birth [26], but decreases with aging in cortical bone [49]. In addition to aging, mechanical stimulation controls Piezo1 expression. Mechanical unloading has been observed to reduce Piezo1 expression [45], whereas mechanical stimulation, such as fluid shear stress (FSS) can increase its expression [50]. In cartilage, transcriptomic analyses have revealed high expression of Piezo1 in both mice and human cartilage [51, 52]. Following OA, Piezo1 is widely expressed in chondrocytes, as well as infrapatellar fat pad (IFP) and synovial membrane (SM) vessels [53]. Within muscles, Piezo1 exhibits high expression in muscle stem cells (MuSCs), myotubes, and mature myofibers [23]. Its expression increases at both mRNA and protein levels during myoblast differentiation [54]. In addition, Piezo1 prefers to express in quiescent MuSCs, suggesting the role of Piezo1 in maintaining the MuSCs pool in skeletal muscles [23, 55]. In tendon tissues, single-cell RNA sequencing revealed that Piezo1 is mainly expressed in cells with high expression of Mkx and Scx genes, but relatively low expression in Myod1+ Cluster [56]. In intervertebral discs, Piezo1 is functionally expressed in nucleus pulposus (NP) and annulus fibrosus (AF) cells [57-59], and can be increased by mechanical stimulation [60]. Understanding the expression patterns of Piezo1 in different musculoskeletal tissues provides valuable insights into its potential roles and mechanisms of action.
Piezo1 in Musculoskeletal Physiology
The musculoskeletal system contains bone, cartilage, ligament, synovium, skeletal muscle, intervertebral disc, tendon, and the surrounding connective tissues [61]. The relationship between the musculoskeletal system and mechanical stimulation is well established, particularly in relation to the Piezo1 channel [62-64]. Here, we updated the current understanding of the physiological and pathophysiological roles mediated by Piezo1 in the musculoskeletal system. To date, different types of physical stimulations, such as hydrostatic pressure (HP) [65], FSS [66], Low-intensity ultrasound stimulation (LIPUS) [17], pulsed electromagnetic fields (PEMF) [67], and longitudinal substrate stretching [68] have been applied to elucidate the mechanosensitive processes in cells resident in the musculoskeletal system. We summarized the various physical stimulations on cell fate determination via Piezo1 activation in Table 1.
Bone Formation and Remodeling
Bone is highly specialized and dynamic connective tissue, which is required for supporting muscles and providing the basis for mobility [69]. Maintaining bone homeostasis relies on the delicate balance between osteoblast-mediated bone formation and osteoclast-mediated bone resorption [70]. Mechanical loading is an essential regulatory factor in bone homeostasis [71]. Cells within bone tissues, including MSCs [72], osteoblasts, osteoclasts, and osteocytes, process mechanosensory capabilities [73]. These cells can sense mechanical stress through focal adhesion, plasma membrane receptors, and mechanosensitive ion channels [74]. Recent investigations have highlighted the involvement of Piezo1 in bone development and mechanosensing for bone formation. Conditional deletion of Piezo1 in specific populations, such as osteoblasts and osteocytes, leads to compromised bone structure and reduced strength [75], consequently resulting in developmental bone defects and an increased susceptibility to bone fractures [26]. Notably, these detrimental outcomes are proposed to be related to mechanical loading, given that the low bone mass phenotype in Piezo1Prrx1 mice only occurs in load-bearing bones [76]. Here, we summarized the role of Piezo1 in different cell populations resident in bone tissues.
Piezo1 resident in different cells regulates bone development and affects bone formation
Bone marrow mesenchymal stromal cells (BMSCs)
Deletion of Piezo1 in MSCs disrupts osteoblast differentiation and promotes bone resorption, resulting in the occurrence of spontaneous bone fractures [26, 77, 78]. Conversely, Piezo1 activation by Yoda1 enhances the proliferation and osteogenic differentiation capability of Gli1+ BMSCs in vitro and in vivo [45]. Mechanosensitive ion channels, including TRPV4, Piezo1, and Piezo2, have been implicated in mediating the osteogenic differentiation of BMSCs in response to mechanical stimulation [77]. Yoda1 has been shown to induce a TRPV4-dependent Ca2+ response by activating Piezo1 [79]. However, the specific interplay and functional relationship between Piezo channels and TRPV4 in the context of osteogenic differentiation remains to be explored. Future research is warranted to elucidate the precise mechanisms and signaling pathways involved in the crosstalk between TRPV4 and Piezo channels during osteogenic differentiation of BMSCs. Notably, a study has demonstrated that the C-terminus of Piezo1 is crucial for the mechano-transduction of osteoblastic differentiation in BMSCs via the ERK1/2 signaling pathway [80]. Under unloading conditions, Piezo1 activation by Yoda1 mitigates bone loss through the Wnt/β-Catenin signaling pathway [45]. Besides, Piezo1 expression has been identified in Oln+ BMSCs [75, 81]. In the central bone marrow and near the endosteum of diaphyseal bone, a peri-arteriolar niche that harbors leptin receptor (LEPR) and osteolectin (Oln) double-positive stromal cells was identified by Shen et al. [82]. These LEPR+Oln+ BMSCs exhibit high proliferation rates and preferentially differentiate towards osteogenesis and lymphopoiesis through Piezo1 signaling. In addition, the number of LEPR+Oln+ BMSCs decreases with aging, suggesting their involvement in age-related bone loss. Deletion of Piezo1 in LEPR+Oln+ BMSCs leads to reduced bone mineral density and cortical bone thickness. In contrast, mechanical and pharmacological stimulation of Piezo1 with Yoda1 on LEPR+Oln+ BMSCs promotes bone formation and supports bacterial clearance following bone fracture [82].
Different types of physical stimulations on cell fate determination via Piezo1 in the musculoskeletal system
| Cell type | Mechanical stimulation | Parameters | Frequency | Device | Outcomes | Mechanism |
|---|---|---|---|---|---|---|
| Aged human BMSCs [178] | Wearable pulsed triboelectric nanogenerator (WP-TENG) | 30 µA | 30 min/day, 7 days in total | Fabricated by a nylon sheet, foam paper, Cu foil, Al foil, PTFE film, petri dish, and needle | Osteogenesis↑; WP-TENG treated BMSCs for 1 week enhance tube formation | Rejuvenation of Aged BMSCs by the activation of mechanosensitive ion channel Piezo1, stimulating Ca2+ influx, and regulating HIF-1α. Thus, osteogenic and angiogenic markers elevate. |
| Mouse BMSCs [77] | Cyclic tensile strain | 0.5 Hz | 0%, 3%, 8%, 13%, and 18%, 8 h/day for 3 days | FX‐4000TM Tension System (Flexcell International Corporation) | Osteogenesis↑ | Mechanical stretch promotes osteogenic differentiation via mechanosensitive ion channels (TRPV4, Piezo1, and Piezo2). |
| Mouse BMSCs [192] | SMF | 0, 50, 100, and 200 mT | 2 weeks | Generated by a set of permanent magnets (35 mm in diameter and 10 mm in thickness) | Migration↑; Chondrogenic marker (Col2, and Sox9↑); MMP13↓ | SMF activates SDF-1/CXCR4 signaling pathway through Piezo1 |
| Human UE7T-13 cells [20] | HP | 0.01 MPa | 10 days | Custom-made pressure chamber | Piezo1, and BMP2↑; osteogenesis↑; adipogenesis↓ | Piezo1 acts as a receptor for HP and functions at the branch points of cell fate decisions of MSCs by regulating BMP2 expression. |
| Mouse MC3T3-E1 cells [47] | FSS | 100-120 rpm (about 2 Pa at the edge) | 1 h | Horizontal shaking apparatus | Piezo1 protein↑; Runx-2 gene↑ | Piezo1 induced by FSS activates AKT/GSK‐3β/β‐catenin pathway following the phosphorylation of Akt and phosphorylation of GSK‐3β. Then the β‐catenin translocates to the nucleus to modulate Runx‐2 gene expression. |
| MC3T3-E1 [78] | LFF | 10 dynes/cm2 | 1 h | FlexCell; STR-4000 | Ptgs2 and Serpine1 expression↑ | LFF induces the expression of mechanoresponsive genes via Piezo1 activation. |
| MC3T3-E1 [17] | LIPUS | 1-Hz pulse repetition frequency, 20% duty cycle, 200-mV amplitude, and 2.25-MHz burst sin wave and amplified by a radio-frequency power amplifier | 3 and 6 min | Generator: AFG3021, Tektronix Inc, Beaverton, OR; Amplifier: E&I 2100 L, Electronics & Innovation, Ltd., Rochester, NY; Transducer: Shinjuku, Tokyo, Japan | MC3T3-E1 migration and proliferation | LIPUS activates ERK1/2 phosphorylation and perinuclear F-actin polymerization via Piezo1 activation. |
| Mouse MC3T3-E1 [175] | PMVS | 120 Hz | 1 h | Microchip pulse generator (Microchip Technology Inc. Chandler, AZ, USA), a micro-amplifier (Texas Instruments, Dallas, TX, USA), a pulse wave modulator (Texas Instruments, Dallas, TX, USA), and 6 ceramic piezoelectric vibration transducers (1-cm in diameter; Steiner & Martins, Inc., Davenport, FL, USA) | Piezo1 gene and miR-29a gene↑; Osteogenic markers (Runx2, Ocn expression↑) | PMVS activates Piezo1 signaling, thus, increasing Wnt and miR-29a signaling to promote osteoblastic activity. |
| Mouse MC3T3-E1 [174] | WH NPs and LIPUS | LIPUS: 1 MHz, 0.3 W/cm2, and 20% (pulsed ratio 1:4) | WH: 100 μg/ml for 24 h; LIPUS :20 min daily at 37 oC | Custom-made WH NPs | Osteogenic markers (Runx2, Ocn, Opn, Piezo1 and TRPV4 expression↑) | WH NPs promotes osteogenic differentiation via increasing Piezo1 and TRPV4 mRNA level. |
| Mouse MC3T3-E1 [81] | FSS | 12 dynes/cm2 | 2 h | Generated by the patch-clamp recording at an angle of 80o using a fire-polished glass pipette (tip diameter 3-4 mm) | Piezo1 and osteogenic maker (alp, bglap, and Col1α1)↑ | Piezo1 senses FSS and consequently regulates its expression, osteoblast function and bone formation. |
| Murine MLO-Y4 (osteocytes) [50] | FSS | 2, 4, 8, and 16 dynes/cm2 | 10 min | Created by parallel plate flow chambers separated by a gasket of defined thickness with gravity-driven fluid flow using a peristaltic pump | Activate Piezo1, enhance the colocalization of Piezo1 and Cx43 HCs, and induce the opening of Cx43 HCs | Piezo1 activation by FSS further activates Cx43 HCs and Panx1 channels through activating the PI3K-Akt signaling pathway. |
| Mouse MLO-Y4 osteocytes [87] | FSS | 9 dynes/cm2 | 30 min | Flow loop apparatus [205] | Piezo1 protein, OPG expression↑; NOTCH3, and RANKL↓ | Piezo1 mediated FSS promotes the expression of OPG and inhibits the expression of RANKL via NOTCH3. |
| Murine IDG-SW3 cells (osteocytes) [48] | Mechanical stretch | 5 Hz, Stretch ratio: 5% | continuous cyclic stretching for 10, 30, 60, 120, 180 min | ShellPa Pro, Menicon Life Science | Rapid Ser473 phosphorylation of Akt; Sost gene↓ | Mechanical stimulation of osteocytes suppresses Sost expression via the Piezo1-Akt pathway. |
| Murine RAW264.7 [100] | Mechanical stretch | cyclic sinusoidal continuous tensile strain (10%, 0.5 Hz) | 2 h | Flexcell® FX-5000™ Tension System (Flexcell International Corporation) | M2-type macrophage transformation↑; Macrophage-derived medium enhance proliferation, migration, and osteogenic differentiation of BMSCs | Mechanical tension causes calcium influx, p53 deacetylation, macrophage polarization towards M2 and TGF-β1 release through Piezo1. |
| Human SCP-1 cells [67] | Extremely low frequency pulsed electromagnetic fields (ELF-PEMF) | 16 Hz | 10 min exposure every 8 h for 3 days | Somagen®, CE 0482, compliant with EN ISO 13485: 2016 + EN ISO 14971: 2012 | Osteogenic differentiation↑, Piezo1 expression ↑ | Intermittent exposure ELF-PEMF promotes maturation of osteoprogenitor cells mediated by increased Piezo1 expression. |
| Human chondrocytes [195] | Cyclic stress | 6 cycles per minute with 20% surface elongation | 24 h | Multi-channel stress loading system FX-4000T (Flexercell International, McKeesport, USA) | Apoptosis↑; actin polymerization↑; Piezo1 activation | NA |
| Human chondrocytes [187] | Cyclic stress | 10 cycles per minute with 20% surface elongation | 0 h, 2 h, 12 h, 24 h, 48 h | Multichannel cell stretch stress loading system FX-4000T (Flexcell, USA) | Piezo1 activation; Kif8a gene and protein↑ | Mechanical stretch activates Piezo1, resulting in the overexpression of Kif8a, which leads to microtubule depolymerization, destroys the integrity of cytoskeleton, and inhibits the mitosis of cells. |
| Rat chondrocytes [110] | Excessive mechanical strain | 20% elongation, 0.1 Hz frequency | 24 h | Flexcell Tension System (FX4000T; FlexCell International Corporation, Burlington, NC, USA) | Apoptosis↑; anabolic and catabolic imbalance | Excessive mechanical strain induces apoptosis and the anabolic and catabolic imbalance via CaN/NFAT1 Signaling Axis mediated by Piezo1. |
| Mouse chondrocytes [189] | Excessive mechanical strain | 1 MPa, at a frequency of 1 Hz | 1 h | Pneumatic component (FESTO, Germany) | GPX4 gene and protein↓; ROS production↑ | Mechanical overloading induces ferroptosis in chondrocytes through the Piezo1 channel |
| Mouse myoblasts [54] | Mechanical strain | After an initial 1 min rest period (0% stretch), stretch is applied at 3% (0.3 mm), 6% (0.6 mm), and 9% (0.9 mm) for 1 min, followed by rest for 1 min in between | Modified elastic silicone chambers (Strexcell, Ooyodonaka, Reference; STB-CH-0.02) | Piezo1 activation; myotube formation↑ | Piezo1 expression and activity is crucial for Ca2+ regulation in muscle function. | |
| Human NP cells [57] | Cyclic stress | 6 cycles per minute with 20% surface elongation | 6 h, 12 h, and 24 h | Multi-channel stretch loading system FX-4000T (Flexercell International, McKeesport, USA) | Piezo1 and NLRP3 inflammasome activation; | Mechanical stretch activates Piezo1-dependent NLRP3 inflammasome via NF-κB pathway. |
| Human NP cells [60] | Mechanical stress | 1.0 Hz with 15% or 1.5% compression intensity | 12 h, 24 h, and 48 h | Flexcell FX5000 Compression system (Flexcell International, McKeesport, USA) | Piezo1 gene and protein↑; inflammatory cytokine (TNF-α, IL-6, and IL-1β) ↑; mitochondrial damage; senescence marker (p53 and p16)↑ | Excessive mechanical stress promotes the apoptosis, senescence, and proinflammatory cytokines of NP cells via Piezo1 activation. |
| AF cells [58] | Cyclic stress | 0.5 Hz, 5-20% stretch deformation | 36 h | Flex-Cell 5000 tension system (Flexcell International) | AF cells apoptosis↑; Piezo1 activation | Excessive mechanical loading promotes AF cell senescence via Piezo1/Ca2+/Calpain2/Caspase3 pathway |
| Human NP cells [143] | Mechanical stress | Cell shape variable was 15%, deformation period 20 s | 24 h | Flexcell mechanical distraction stress loading instrument (Flexcell® International Corporation, Burlington, VT) | NP cells apoptosis↑; Piezo1 activation | Excessive mechanical stress promotes the apoptosis of NP cells via Piezo1 activation. |
NP cells, nucleus pulposus cells; AF cells, annulus fibrosus cells; FSS, fluid shear stress; SMF, static magnetic field; LIPUS, low-intensity ultrasound stimulation; HP, hydrostatic pressure; LFF, laminar fluid flow; PMVS, piezoelectric microvibration stimulation; BMSCs, bone marrow stromal cells; HIF-1α, Hypoxia-inducible factor 1-alpha; Col2, Collagen type 2; Sox9, SRY-Box Transcription Factor 9; Runx2, Runt-related transcription factor 2; Ocn, osteocalcin; OPN, osteopontin; MMP13, matrix metalloproteinase 13; SDF-1, stromal-cell derived factor-1; CXCR4, C-X-C Motif Chemokine Receptor 4; BMP2, Bone Morphogenetic Protein 2; GSK-3β, Glycogen synthase kinase-3 beta; Ptgs2, Prostaglandin-Endoperoxide Synthase 2; and Serpine1, Serpin Family E Member 1; ERK1/2, The extracellular signal-regulated kinase 1/2; Cx43 HCs, connexin 43 hemichannels; Panx1, Pannexin 1; NOTCH3, Neurogenic locus notch homolog protein 3; RANKL, Receptor activator of NF-kB ligand; PI3K-Akt, TGF-β1, Transforming growth factor beta 1; Kif8a, Kinase-like protein 18A; NFAT1, nuclear factor of activated T cells 1; OPG, osteoprotegerin; NLRP3, Nod-like receptor protein 3; NA, not applicable.
Periosteal stem cells (PSCs)
PSCs resident in the periosteum are vital for bone fracture healing [83]. Single-cell RNA sequencing data showed that PSCs are the ancestors of osteoblasts [46]. Piezo1 is upregulated after bone fracture [46]. Yoda1 treatment directly enhances migration and osteogenic differentiation of PSCs, indirectly promoting angiogenesis in vitro and in vivo. Yap/β-catenin pathway is another downstream effector of the Piezo1 channel [46].
Osteoblasts
Piezo1 is essential for osteoblast proliferation, migration, and differentiation [26, 75, 79]. Conditional deletion of Piezo1 in osteoblast, using mouse models such as Piezo1OcnCre, Piezo1Dmp1Cre, Piezo1Sp7Cre mice, results in impaired bone formation and reduced bone mass [26, 81]. Specifically, deletion of Piezo1 in Prrx1+ cells (Piezo1Prrx1Cre) results in multiple bone fracture, shortened long bones, and these effects are further exacerbated in Piezo1/2 double knockout mice. Of note, Piezo2 conditional knockout mice (Piezo2Prrx1Cre) undergo normal skeletal development, indicating that Piezo1 plays a major role in MSCs during bone development [26]. The authors also generate Piezo1Sp7Cre and Piezo2Sp7Cre mice to demonstrate that Piezo1/2 are essential for bone mass maintenance through mechanical stimulation [26]. Bulk RNA Sequencing and further experiments revealed that Piezo1/2 regulate osteoblast differentiation by modulating Wnt/Ctnnb1 and Yap1 pathways [26]. Apart from intramembranous ossification, Piezo1 also has a key function in the early stages of osteoblast differentiation, affecting bone formation through endochondral ossification [78]. Piezo1Runx2Cre mice exhibit reduced cortical thickness and increased cortical porosity, with a phenotype that is more severe and distinct from that of Piezo1Dmp1Cre mice. Osteoblasts isolated from Piezo1Runx2Cre display an unusual flattened appearance, increased chondrogenic differentiation potential, and reduced osteogenic differentiation ability [78].
The effects of mechanical loading on bone formation are partly attributed to the promotion of Piezo1 signaling in osteoblasts [75, 81]. In vitro study revealed that FSS exposure for 1 hour increases Runx-2 gene expression in MC3T3-E1 osteoblasts, and the increased Runx-2 gene expression is eliminated by Piezo1 gene deletion [47]. Piezo1 activates the Akt/GSK-3β/β-catenin pathway following the phosphorylation of Akt and phosphorylation of GSK-3β, which is recognized as a partial mechanism [47], and it induces NFAT/YAP1/ß-catenin complex formation by stimulating Calcineurin [26]. Apart from FSS, LIPUS induces Erk1/2 phosphorylation and perinuclear F-actin polymerization in a Piezo1-dependent manner to promote MC3T3-E1 cell migration and proliferation [17]. Also, Piezo1 regulates the phosphorylation of Erk1/2 and p38, and enhances BMP2 expression through HP or Yoda1 treatment [26]. Dentin matrix protein 1 (DMP1), an extracellular matrix protein belonging to the small integrin-binding ligand N-linked glycoprotein (SIBLING) family, is crucial for bone mineralization [84]. Mechanical loading, such as body weight-bearing, increases the production of kinase FAM20C in osteoblasts, promoting DMP1 secretion via activating Piezo1 channel [85]. The secreted DMP1 can convert type H vessels into type L, inhibiting bone growth and promoting bone mineralization by impeding VEGF signaling. However, Piezo1 in the gut has negative effects on osteoblast activity. Conditional deletion of Piezo1 in the gut increases bone mass accompanied by decreased serum 5-HT levels [21]. Sugisawa et al. generated LysM-Piezo1flox/flox mice and Col1a1-Piezo1flox/flox mice, and found no significant change of bone volume and serum bone markers after Piezo1 deletion in myeloid and osteoblast [21]. They hold the view that Piezo1 in myeloid and osteoblast is not that essential for bone metabolism [21]. Such discrepancy warrants further study to reach a consensus.
Osteoclasts
Conditional knock-out Piezo1 in osteoclast (Piezo1CtskCre) exhibited normal body weight, bone mass, and bone resorption process [76]. However, multiple evidence showed that Piezo1 in osteoblasts responds to mechanical stimulation to maintain bone size and mass via regulating osteoblast-osteoclast crosstalk [75, 76].
Osteocytes
Osteocytes, the most abundant cell type in bone tissue, play crucial roles in sensing and transducing mechanical stimulation and regulating bone formation and remodeling [86]. To investigate the role of Piezo1 in osteoblasts and osteocytes, Piezo1Dmp1Cre mice were generated. These mice exhibited low bone mineral density, decreased cortical bone thickness, and a diminished response to mechanical loading. The authors identified that Wnt1, Yap1, and TAZ are the downstream effectors of Piezo1 [75].
Piezo1 is co-localized with Connexin43 hemichannels (Cx43 HCs), which facilitates the exchange of small molecules in the extracellular environment on the surface of osteocytes [50]. FSS increases the expression of Piezo1 and enhances the co-localization of Piezo1 and Cx43 HCs [50]. Piezo1 activation by Yoda1 increases intracellular Ca2+, which opens Cx43 HCs and Panx1 channels through activating the PI3K-Akt signaling pathway. Additionally, these activated channels promote ATP release, which in turn activates P2X receptors and sustains intracellular Ca2+ signaling [50]. In MLOY4 osteocytes, Piezo1-mediated FSS enhances Osteoprotegerin (OPG) expression and reduces nuclear factor-Kappa-B Ligand (RANKL) expression through NOTCH3 [87]. Knockdown of Piezo1 in osteocytes reduces osteogenic makers in osteoblasts, even when exposed to LIPUS [88].
Aging is a natural and inevitable process that occurs in living organisms. It is associated with an increased risk of developing various age-related health conditions and diseases. Loss of bone mass occurs in the aging skeleton, often characterized by osteoporosis and an increased risk of fracture [89]. Piezo1Dmp1Cre mice with aging exhibited enhanced endocortical expansion, cortical porosity, and increased osteoclast formation through elevating Tnfrsf11b expression [49]. Sclerostin, highly expressed in osteocytes, is a bone formation inhibitor and one of the molecular regulators in bone homeostasis [90]. Mechanical stretch increases the phosphorylation of Akt and then reduces Sost expression through Piezo1 activation [48]. However, increased Sost (gene of Sclerostin) expression was observed in Piezo1OcnCre mice, which indicates that osteocytes may coordinate with osteoblasts in bone homeostasis [81].
Chondrocytes
The involvement of chondrocytes in endochondral ossification is closely linked to bone formation. Conditional knockout Piezo1 in chondrocytes (Piezo1Col2a1Cre) reduces trabecular bone formation, suggesting that the presence of Piezo1 in growth plate chondrocytes is responsible for trabecular bone formation [78]. Endochondral ossification is one of the most essential mechanisms involved in ankylosis progression in ankylosing spondylitis (AS). Ablation of Piezo1 in chondrocytes (Piezo1Col2a1Cre) can inhibit pathological new bone volume and alleviate the AS phenotype. CaMKII activation is the downstream pathway of Piezo1-mediated pathological new bone formation in AS [91].
Endothelial cells (ECs)
Piezo1 is essential for local vascular growth. Global knockout of Piezo1 in mice leads to fetal lethality due to obvious deformity during vascular development [92]. Piezo1 in ECs induces angiogenesis, thereby promoting bone fracture healing [27]. Piezo1Cdh5Cre impedes bone fracture healing by altering osteoblastic activity in the early stages and reducing bone remodeling in the late stages [27]. In addition, both Piezo1 and Piezo2 are expressed in gut epithelial cells [93, 94], and both are involved in the production of serotonin (5-HT) [21, 95]. 5-HT produced by the gut is a negative regulator of bone metabolism [96]. It has been reported that conditional deletion of Piezo1 in intestinal epithelium leads to increased bone mass [21]. This phenomenon can be attributed to impaired 5-HT production in the gut [21], highlighting the relationship between 5-HT production and bone metabolism.
Myeloid-lineage cells (MCs)
The periosteum is responsible for cortical bone development and strain-adaptive remodeling [97]. It consists of nerves, blood vessels, and multiple types of cells resident in the periosteum, including periosteal progenitors and myeloid-lineage cells, which collectively create a pro-osteogenic microenvironment [98]. Among them, macrophages are crucial for bone remodeling and regeneration. Deng et al. have identified a specific subtype of macrophages, CD68+F4/80+ macrophages, that regulate bone remodeling in response to mechanical stimulation. Specifically, compression increases the number of CD68+F4/80- MCs and promotes their differentiation into CD68+F4/80+ macrophages by upregulating Piezo1 expression. CD68+F4/80+ macrophages secret latent TGF-β1, and Thbs1, which activate TGF-β1, consequently mobilizing and recruiting more osteoprogenitor cells to the periosteal bone surface, thereby promoting bone regeneration under mechanical stimulation [99]. The role of macrophages in osteogenesis is further supported by a recent study conducted by Cai et al. [100]. They identified that mechanical stretch enhances M2 macrophage polarization and secretion of TGF-β1 through the Piezo1 channel to promote the proliferation, migration, and osteogenic differentiation of BMSCs [100].
Taken together, Piezo1, in response to mechanical loading, is strategically situated within a multitude of cells, such as bone cells, chondrocytes, macrophages, and endothelial cells, exerting an impact on bone homeostasis. In this regard, Piezo1 as a key mechanosensor for bone formation, may be a novel therapeutic target for OP treatment. The roles of Piezo1 in different cell types and the related signaling pathways are illustrated in Figure 2.
Cartilage Homeostasis
Articular cartilage is a thin layer of specialized connective tissue that provides a smooth surface to minimize friction and transmits mechanical loading to the subchondral bone [101]. Chondrocytes, the cells within cartilage, sense and respond to mechanical stress to maintain cartilage homeostasis [102]. Excessive mechanical loading can cause changes in chondrocyte volume and deformations [103]. Piezo channels communicate with each other and even other ion channels, like the TRPV4 channel [104-106]. Both Piezo1 and TRPV4 are active in chondrocytes. They show similar responses to nanoscale deflection-stimuli in chondrocytes, and the function deficiency of each ion channel can be compensated by the other [105]. TRPV4 mainly senses physiologic stimulation in cartilage, and Piezo channels mediate excessive mechanical loading [107]. Mechanical loading influences chondrocyte death [106, 108] and recruits more stem cells, contributing to cartilage repair via the Piezo-mediated pathway [109]. Nevertheless, excessive mechanical loading induces Ca2+ influx with increased Piezo1 level, resulting in chondrocytes apoptosis [110] and senescence [111]. GsMTx4, an effective Piezo1 inhibitor, can enhance chondrogenic markers [78], increase cartilage matrix production, inhibit chondrocytes apoptosis, and protect articular cartilage from mechanical injury through the calcineurin (CaN)/ nuclear factor of activated T cells 1 (NFAT1) signaling axis [106, 110]. However, GsMTx4 exhibits non-specificity towards Piezo1, indicating that the observed effects may not be mediated solely by this channel. In addition, Piezo1 activation induced by extreme mechanical stimulation and Yoda1 accelerates chondrocyte senescence [112, 113], and Yoda1 reduces Col10a1 gene expression in ATDC5 chondrogenic cells [78]. Urocortin (Ucn1), a 40 amino acid long peptide, has an antiresorptive effect in bone tissue [114]. Recently, a new pathway of Ucn1 that promotes chondrocyte survival has been identified. Ucn1 protects chondrocytes through maintaining Piezo1 in a closed conformation mediated by the corticotropin-releasing factor receptor 1 (CRF-R1 receptor) of Ucn1 [115, 116]. The above evidence indicates that antagonism of Piezo1 may be a promising therapeutic approach for OA patients. Figure 3. shows the role of Piezo1 in cartilage metabolism.
Skeletal Muscle
Skeletal muscle accounts for approximately 40% of total body mass [117], and plays a vital role in facilitating movement, providing stability, maintaining posture, and orchestrating various essential physiological processes in the body [118]. Skeletal muscle with high plasticity and exceptional regeneration capacity is largely affected by mechanical stimulation [119]. Mechanosensitive ion channels are key players in skeletal muscle homeostasis. They can enhance the cytoskeleton and prevent cell lysis by sensing excessive loading on the sarcolemma [120]. Among them, Piezo1 is a crucial mechanosensitive ion channel expressed stably in satellite cells and is responsible for myotube formation [54].
Piezo1 controls myotube formation through regulating muscle stem cell (MuSCs) fate
Myotubes formed by myoblast fusion are one of the essential steps of skeletal muscle development [121]. This process of myoblast fusion requires membrane remodeling and mechanical forces [54]. Various cellular events take place during myoblast fusion, such as cell-cell communication, elongation, adhesion, and alignment of myoblast membranes [121]. Within skeletal muscle tissue, ion channels play a pivotal role in muscle growth. Piezo1, as one of the mechanosensitive ion channels, regulates myotube formation and cortical actomyosin assembly through controlling the influx of Ca2+ across the cell membrane [28, 122]. In a study by Ortuste and colleagues, treatment of myotubes with Yoda1 (at concentrations of 30 and 100 µM) for 1 minute significantly induced cell fusion, whereas a 30-minute treatment with 100 µM of Yoda1 reduced cell fusion [54]. Knockdown of Piezo1 in myoblast reduces myoblast fusion and myomaker expression, thereby impeding myotube formation [54]. In Piezo1-deficient C2C12 cell lines, abnormal morphology is observed during myotube formation, characterized by excessive cell fusion and defects in cell elongation [28]. Phosphatidylserine (PS), a phospholipid with a negative charge, is typically located in the inner leaflet of the plasma membrane [123]. During myotube formation, the inner leaflet PS moves to the outer leaflet and then can be recognized by PS receptors to facilitate fusion with adjacent myoblasts [124, 125]. The tempo-spatial activation of Peizo1 is regulated by PS flippase-mediated translocation, consequently influencing myoblast fusion and elongation [28].
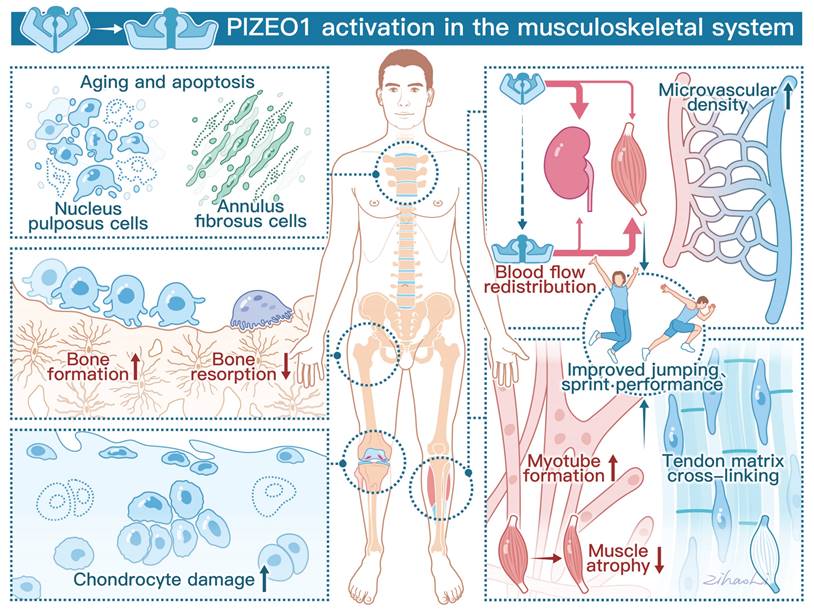
Piezo1 located in different cells regulates bone formation. (A) Mechanical stimulation by fluid shear stress (FSS), ECM rigidity, hydrostatic pressure (HP), and biomaterials (WP-TENG, D-PCL@A, and Yoda1 Bilayer membrane) activates Piezo1 channels in mesenchymal stem cells (MSCs), further activates downstream signaling pathway. (B) Activation of Piezo1 in osteoblasts (MSC-derived osteoblasts and MC3TC-E1) by FSS, LIPUS, Piezoelectric micro-vibration stimulation (PMVS), and increasing body weight, and the downstream signaling pathway. (C) FSS, mechanical stretch (MS), and Yoda1 activate Piezo1 in osteocytes to enhance bone formation. (D) Piezo1 mediates mechanical loading enhances M2 macrophage polarization and promotes proliferation, migration, and osteogenic differentiation of BMSCs via secreting TGF-β1. (E) Conditional knockout Piezo1 in endothelial cells (Piezo1Cdh5Cre) impedes bone formation, while Piezo1 activation in intestinal epithelium inhibits bone formation mediated by 5-HT production. Created with BioRender.com.
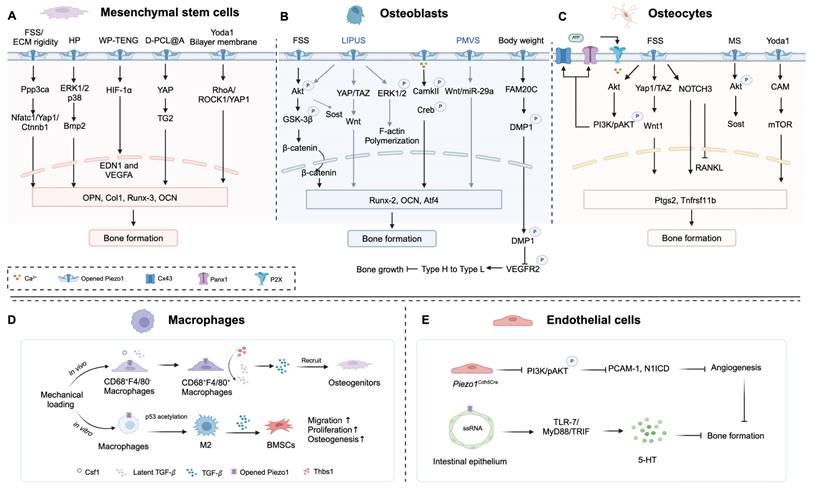
Piezo1 mediated chondrocytes mechanotransduction and potential therapeutic targets for OA. (A) Inflammation cues increase the mechanosensitivity of chondrocytes mediated by Piezo1 to mechanical loading. (B) Mechanical overloading induces chondrocyte ferroptosis in OA via Piezo1 activation (C) Static magnetic field (SMF), and appropriate mechanical loading promotes BMSCs chondrogenic differentiation via Piezo1 activation. Urocortin-1 (Ucn1), G protein-coupled estrogen receptor (GPER), GsMTx4 (a peptide of Piezo1 inhibitor), and Artemisinin (ART) protect chondrocytes from damage to alleviate OA symptoms. Created with BioRender.com.
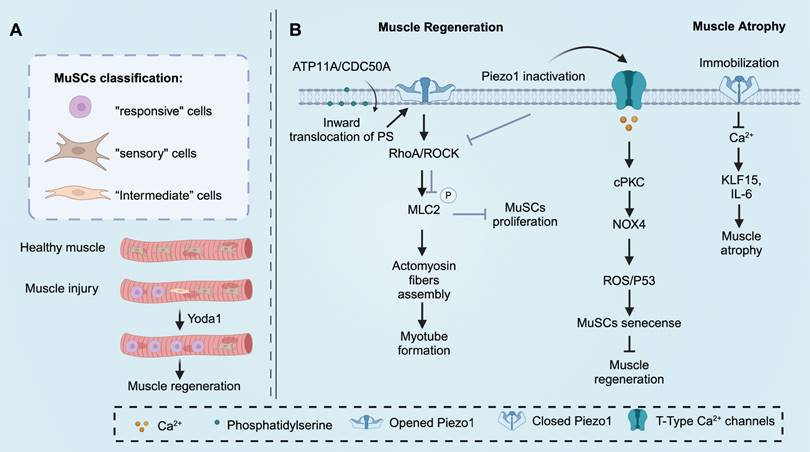
Muscle stem cells (MuSCs) play a critical role in muscle growth and regeneration, both in physiological and pathological states [126]. These cells respond promptly to exercise and injury, undergoing activation, proliferation, and differentiation into myoblasts. A small fraction of MuSCs retain their quiescent state within the MuSCs pool through self-renewal [127]. Currently, little is known about how the surrounding environment influences the transition of MuSCs between quiescence and activation. Aging populations often exhibit muscle weakness and reduced muscle regeneration [128]. Piezo1 is indispensable for MuSCs proliferation, differentiation, and even alleviation of cellular senescence [55]. To investigate the impact of Piezo1 in MuSCs on muscle injury, a conditional knockout strain of Piezo1 in MuSCs (Piezo1Pax7Cre) was generated. In Piezo1Pax7Cre mice, elevated levels of P53, increased ROS formation, and decreased muscle regeneration ability were observed [55]. It is suggested that cPKC activation, mediated by increased Ca2+ influx through T-Type Ca2+ channels, plays a key role in this process [55]. This is consistent with the notion that the concentration of Ca2+ is associated with cellular senescence in muscle fibers [129]. The administration of Pifithrin-α (PFT-α), a P53 inhibitor, reduced the rate of MuSCs senescence, ultimately improving muscle regeneration. Piezo1 is involved in the regulation of P53 expression and ROS production, thereby contributing to the maintenance of the MuSCs pool by inhibiting MuSCs senescence.
The morphology of MuSCs also plays a crucial role in determining their functions [130]. Isolated MuSCs with few protrusions are considered relatively fragile and more susceptible to the influence of the surrounding environment [131]. Recent studies have demonstrated that MuSCs with fewer protrusions exhibit an initial response after injury, followed by MuSCs with more protrusions, indicating their rapid responsiveness [130]. The authors categorized quiescent MuSCs into three subtypes, “responsive” cells (small and round cells with zero or one protrusion), “intermediate” cells (middle-size cells with two or three protrusions), and “sensory” cells (large and less rounded cells with four or more protrusions). Pharmacological activation of Piezo1 by Yoda1 has been shown to promote a shift of MuSCs towards the “responsive” cell subtype in Pax7-EGFP mice [130]. To investigate the role of Piezo1 in the functional transition between these subtypes, Piezo1Pax7Cre mice were utilized. After muscle injury, Piezo1Pax7Cre mice exhibited an increase in "intermediate" cells and a decrease in "responsive" cells [130]. These findings suggest that Piezo1 is essential for maintaining the morphology of MuSCs, and downregulation of Piezo1 may lead to reduced proliferation of MuSCs, thereby disrupting muscle homeostasis and regeneration [130]. The function of Piezo1 in skeletal muscle is shown in Figure 4.
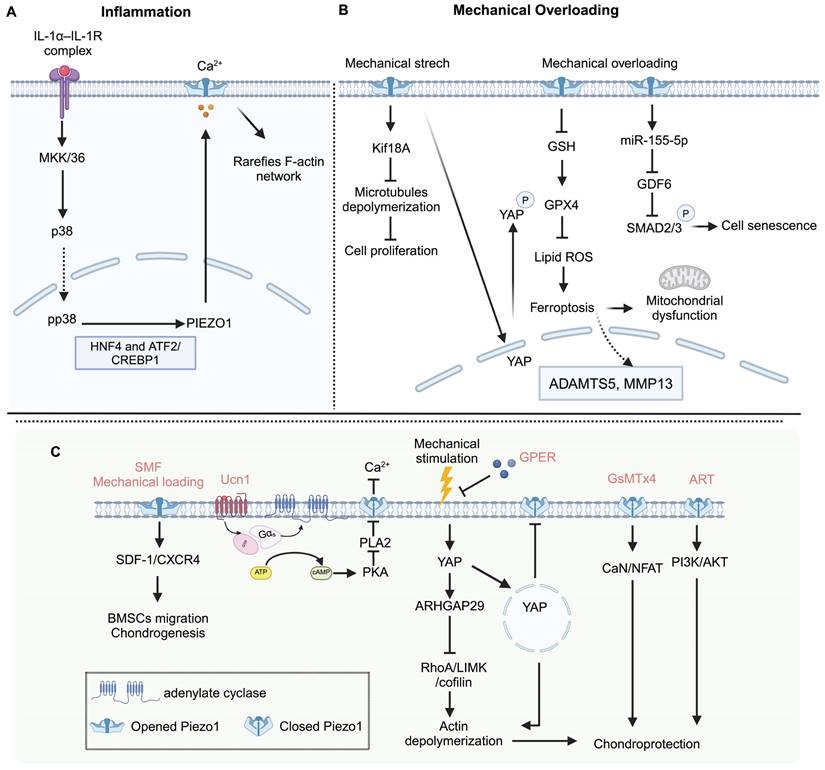
Piezo1 functions in skeletal muscle. (A) Piezo1 orchestrates muscle satellite cells (MuSCs) morphological states after muscle injury, which is essential for muscle regeneration and maintenance. Pharmacologically activate Piezo1 by Yoda1 prime MuSCs toward more “responsive” cells. (B) The inward translocation of phosphatidylserine is the precondition of Piezo1 activation. Piezo1 activation enhances myotube formation, MuSCs proliferation, and inhibits MuSCs senescence to promote muscle regeneration. In addition, Piezo1 downregulation results in muscle atrophy. Created with BioRender.com.
Tendon
Tendons are mechanosensitive soft tissues that connect muscle to bone to enable ambulation and suffer high mechanical loading transmitted by muscles [132]. Mechanical loading is essential for tendon homeostasis in humans and animal models [133], and it can have dual effects on the tendon healing process [134]. Mechanical overloading is a well-known extrinsic factor that results in tendon injury [135]. However, the prolonged unloading process also exerts detrimental effects on tendon mechanical properties [136]. Besides, under aberrant mechanical stimulation, excessive biological factors, like prostaglandins, metalloproteinases, and some growth factors can be produced [137], and the differentiation capabilities of tendon stem cells are altered [138]. However, the underlying mechanism of how resident tendon cells respond to mechanical forces and translate into biological signals remains unknown.
Piezo1 activation enhances tendon stiffness
Currently, only two research groups have published research on the role of Piezo1 in tendons. Piezo1 is essential for tendon function [24, 56]. Both loss-of-function mice and gain-of-function mice have been utilized to investigate its effects. Passini and his colleagues developed two devices, a tensile stretching device, and a microfluidics flow chamber to examine the Ca2+ influx after mechanical stimulation ex vivo. Mechanical forces were found to trigger Ca2+ influx in tenocytes [24]. Similarly, Nakamichi et al. observed an accelerated Ca2+ influx after the specific agonist Yoda1 treatment, consistent with the findings of Passini et al. [56]. Knocking out several general ion channels by CRISPR-Cas9 genome editing technique revealed that only cells with Piezo1 depletion exhibited a decreased response to shear stress [24], suggesting that the Piezo1 channel may be the primary sensor of shear force stimulation. To further identify the crucial role of Piezo1 in responding to mechanical loading, Passini et al. generated the conditional depletion of Piezo1 in tenocytes (Piezo1ScxCre) mice. The results showed reduced tendon stiffness in Piezo1ScxCre mice [24], which could be increased over two weeks of Yoda1 treatment.
Over 25 gene mutations in Piezo1 are associated with human diseases [139]. E756del, a common Piezo1 Allele, is prevalent in one out of three African populations, including African Americans and Jamaicans [140]. Emerging evidence showed that the E756del mutation regulated human physical performance. One double-blinded trial investigated the E756del gene in healthy African Americans [24]. The step motion, such as countermovement jump, is often a training process in athletics [141]. The human E756del carriers performed better in the drop countermovement jump test, which could induce high degrees of tendon loading [24]. Another research group found an increased frequency of E756del in Jamaican sprinters compared with controls from Jamaica [56]. These data indicated that the Piezo1 E756del is involved in higher physical performance. To mimic the gain-of-function variant E756del in humans, the R2482H mutation of Piezo1 was introduced in mice [140]. Passini et al. observed that R2482H Piezo1 mice had elevated stiffness and stronger plantaris tendons due to the denser collagen cross-link network [24]. Nakamichi et al. also found that the width and the cross-section of collagen fibrils of Achilles tendon R2482H Piezo1 mice were 1.2-fold wider than WT mice [56]. Piezo1GOF mice performed better jumping and high-speed running abilities [56]. However, this phenotype was not observed in muscle-specific Piezo1 gain-of-function mice [56], the contraction of muscles transmits the kinetic energy to the joints by tendons and ligaments [56]. So, it is reasonable to speculate that damaged tendons induce secondary changes in skeletal muscle tissues. In addition, RNA sequencing showed upregulation of tendon-related genes (Mkx and Scx), collagen matrix, and non-collagen matrix genes in R2482H Piezo1 mice [56]. Also, Piezo1 enhances Mkx and Scx, promoting tendon synthesis by inducing the nuclear translocation of multiple NFATCs (NFATC1, NFATC2, NFATC3, and NFATC4). Therefore, controlling Piezo1 activation may hold promise for improving tendon function in tendon-related diseases such as tendon rupture and tendinopathy.
Intervertebral Disc
Piezo1 activation increases NP and AF cell apoptosis and senescence
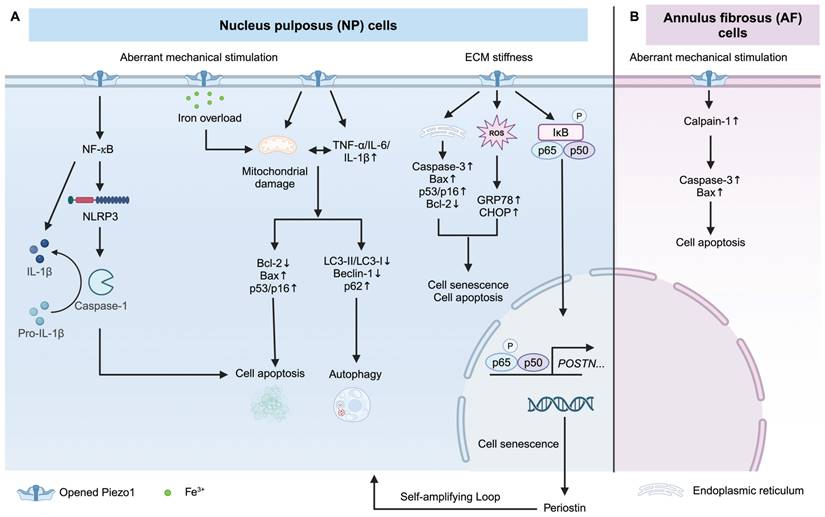
Intervertebral disc (IVD) consists of NP in the center, surrounding AF, and cartilaginous endplate (CEP), which is linked to superior and inferior vertebral bodies. Piezo1 mediates inflammation by activating Nod-like receptor protein 3 (NLRP3) inflammasome [57, 142]. Mechanical stretch on NP cells induces IL-1β production via Piezo1-mediated NLRP3 inflammasome activation [57], with the downstream effector being the NF-κB pathway [57, 59]. Excessive mechanical stress leads to senescence and apoptosis of human NP cells via Piezo1 overexpression and secretes some pro-inflammatory factors (such as TNF-α, IL-6, and IL-1β), resulting in ECM reduction and autophagy inhibition [60]. Knockdown of Piezo1 protects NP cells from apoptosis by reducing the ratio of mitochondrial membrane potential turnover induced by aberrant mechanical stimulation [143]. In addition, the stiffness of the ECM during IDD contributes to the activation of Piezo1 [144]. A stiff matrix (25 kPa) activated Piezo1, leading to endoplasmic reticulum (ER) stress and oxidative stress, thereby inducing senescence and apoptosis of human NP cells [25]. It was observed that a stiff matrix increases the secretion of periostin from human NP cells, which in turn activates the NF-κB pathway. This activation further enhances periostin expression, accelerating the senescence of NP cells [59]. This self-amplifying loop between periostin and NF-kB can be triggered by Piezo1 activation, resulting in IDD [59]. Besides, Ke et al. also identified that Piezo1 activation by matrix stiffness promotes NP cell apoptosis via activating the ERK1/2 pathway [145]. Piezo1-mediated iron overload disrupts iron metabolism and exacerbates ferroptosis in NP cells. Importantly, Piezo1-induced iron influx is independent of the transferrin receptor (TFRC), a well-recognized iron gatekeeper [146]. In AF cells, excessive mechanical loading promotes AF cell senescence via the Piezo1/Ca2+/Calpain2/Caspase3 pathway [58]. Overall, Piezo1 activation enhances inflammatory response and promotes NP and AF cells senescence, and apoptosis. Piezo1 may be a potential therapeutic target for IDD treatment. How Piezo1 regulates NP and AF cell fate is shown in Figure 5.
Piezo1 mediates apoptosis and senescence of NP and AF cells. (A) Piezo1 activation under excessive mechanical stimulation and stiff matrix triggers apoptosis and senescence of NP cells. (B) Excessive mechanical stimulation induces AF cell apoptosis via Piezo1 activation. Created with BioRender.com.
Piezo1 in the Physical Performance
One of the benefits of exercise comes from the increased blood flow [147]. Currently, the idea about how blood flow sensing during exercise is contradictory [148, 149]. Baroreflex is an essential part of regulating blood pressure. Baroreceptor nerve endings can sense increased blood pressure, thus transferring the afferent signals to the central nervous system to redistribute blood flow [150]. Piezo1 works as the baroreceptor mechanosensor for blood pressure regulation. Piezo1 is expressed in nodose and petrosal sensory ganglia, where baroreceptor cell bodies are located [151]. Conditional deletion of Piezo1 in nodose and petrosal sensory ganglia results in disturbing baroreflex and aortic depressor nerve activity [151]. However, the role of Piezo1 in regulating blood pressure is not restricted to controlling the function of the baroreceptor reflex.
Endothelial cells lie between blood flow and the vascular wall and are essential for physiology and pathology. These cells experience hemodynamic forces, especially shear stress caused by fluid flow [152]. Identifying the molecular sensor will enhance our understanding of the benefits of exercise. Piezo1 channel, highly expressed in endothelial cells [92], is crucial for vascular development. Deletion of endothelial Piezo1 leads to embryonic lethality [92]. Piezo1 is a key shear stress sensor of elevated blood pressure during physical activity [153]. Physical activity-induced increases in blood pressure were mitigated in Piezo1Cdh5Cre mice, whereas no significant blood pressure elevations were observed during periods of physical inactivity [153]. Piezo1 controls the vascular tone and blood pressure by regulating flow-induced ATP release and initiating the downstream signaling pathways [13]. Besides, the lack of Piezo1 in endothelial cells reduces running wheel performance and causes weight loss in mice, indicating that Piezo1 regulates physical performance via redistributing blood flow [153]. Recently, Wang et al. found a novel Piezo1 regulator, cartilage oligomeric matrix protein (COMP). They identified that COMP increased Ca2+ influx, eNOS activity, and nitric oxide production to regulate blood pressure [154]. In addition, microvascular density is essential in cardiovascular function and maintaining normal physical performance [155]. Piezo1Cdh5Cre mice showed lower physical activity without altering the desire for exercise [156], owing to microvascular rarefaction in muscle mediated by endothelial cell apoptosis [156].
Nitric oxide (NO), one of the vasoactive mediators triggered by FSS, is an essential endothelial vasodilator factor for controlling vascular tone and blood pressure [157]. Piezo1 deficiency in endothelium impedes NO formation and vasodilation in response to flow, leading to the development of hypertension [13]. Utilizing titanium dioxide-trapping combined with mass spectrometry, it has been revealed that the deletion of Piezo1 affects endothelial nitric oxide synthase (eNOS) under both static and shear-stress conditions [92]. Also, increased shear stress by elevated blood flow during physical exercise activates the Piezo1 channel, thereby maintaining the microvascular structure by regulating eNOS/TSP2 paracrine signaling to stabilize muscle function [156].
The excellent physical performance relies on strong tendons [158]. The tendon stiffness directly affects the muscle power outputs [159]. Notably, Piezo1 gain-of-function in tendons, resulting in increased stiffness, has been found to enhance the physical performance in both humans and mice [24, 56]. Intriguingly, Piezo1 senses physical activity to redistribute blood flow, which is closely associated with physical activity [153]. Piezo1 activation mimics the effects of physical exercise. Beech et al. defined Piezo1 as an “exercise sensor”, and the specific activator, Yoda1, was named as “exercise pill” [160]. In clinical scenarios, appropriate physical activity is beneficial for patients with musculoskeletal disorders, including OP [161], OA [162], muscle atrophy [163], IDD [164], and tendinopathy [165]. In future work, this “exercise pill” may be applied to mimic the benefits of exercise-based physical therapy to treat bone, cartilage, muscle, and tendon-related pathologies in the rehabilitation phase.
Implications of Piezo1 in Musculoskeletal Disorders
Osteoporosis and Bone Health
OP is an aging-related disease with low bone mineral density (BMD), which leads to bone fragility and prone to fractures. With the increasing age and a higher prevalence among females, it becomes an increasing burden on health care worldwide [166]. The incidence of OP is approximately 13% in China [167]. In the United States, 10 million people aged over 50 were diagnosed with OP [168]. Piezo1 expression is declined in OP patients [81]. Conditional deletion of Piezo1 in osteoblasts and chondrocytes results in severe OP [76, 78]. However, Piezo1 activation can attenuate bone loss under the conditions of unloading, OVX, and aged mice models [45]. Interestingly, genetic variants on Piezo1 are associated with human low BMD and fracture. 14 Top overlapped Piezo1 single nucleotide polymorphisms (SNPs) were found to be related to low BMD. Among them, SNPs rs62048221 from calcaneus significantly affects BMD by regulating Piezo1 expression and the activity of cis-regulatory elements [169]. A bioinformatic analysis of the DEGs affected by the Piezo1 in different bone tissues and cells showed that Piezo1 gene is associated with mineral absorption [170]. Marouli et al. have also identified the relationship between Piezo1 SNPs and human body height reduction [171]. Furthermore, the lower Piezo1 expression contributes to bone aging [26]. Apart from disuse osteoporosis, Piezo1 activation can also reduce bone loss in OVX and aging mice models [45].
Numerous biomaterials have been developed to enhance bone regeneration. Shi et al. conducted a study where they modified polycaprolactone (PCL) surfaces using 3,4-Dihydroxyphenylalanine (DOPA) and alendronate (AL). They applied a primary coating of DOPA on PCL surfaces, followed by grafting AL on the DOPA coatings using genipin (GP) crosslinking as a secondary coating. This modified PCL scaffold was referred to as D-PCL@AL. The authors demonstrated that D-PCL@AL partially promoted bone regeneration in bone defect models by activating the Piezo1-YAP-TG2 axis [172]. Bioelectricity is indispensable in cell division, intracellular communication, neuronal activities, and ion transport in living systems [173]. Piezoelectric biomaterials have raised much attention in tissue regeneration because they can generate electrical activity under deformation stimulation without requiring an external power source [173]. Kaliannagounder and colleagues developed a piezoelectric whitlockite (Ca18Mg2(HPO4)2(PO4)12) nanoparticles (WH NPs) to mimic bioelectric activity and enhance tissue regeneration [174]. They discovered that piezoelectric WH nanoparticles could promote osteogenic differentiation of MC3T3E1 cells w/o LIPUS stimulation in vitro, likely due to the activation of Piezo1 and TRPV4 channels [174]. In addition, piezoelectric micro-vibration stimulation (PMVS) inhibits bone resorption and promotes osteogenic differentiation, improving trabecular morphology in ovariectomized (OVX) mice through activating Piezo1, miR-29a and Wnt signaling [175].
Pharmacological activation of Piezo1 by Yoda1 has been reported to promote osteogenesis and angiogenesis [46]. Yoda1 mimics the beneficial effect of mechanical loading on bone cells [75]. Interestingly, Yoda1 can significantly increase bone mass without affecting the body weight of mice in vivo [75]. Yang et al. fabricated a Yoda1 loaden membrane with inner and outer layers. The inner layer is responsible for bone regeneration by controlled release of Yoda1, promoting cell proliferation and osteogenic differentiation. Simultaneously, the outer layer acts as a barrier to prevent infiltration of fibrous connective tissue into the bone defect area. The researchers found that the Yoda1 bilayer membrane promotes bone healing via the Piezo1/RhoA/ROCK1/YAP1 signaling pathway [176]. Kong et al. developed a TiO2 Nanotube and found that TiO2 nanotubes promote osteogenesis both in vitro and in vivo via the Piezo1-Yap1 signaling pathway [177]. Wang et al. fabricated a wearable pulsed triboelectric nanogenerator (WP-TENG) that generates electric flow during human body movement [178]. The WP-TENG enhances osteogenic differentiation and proangiogenic functions of aged BMSCs by Piezo1 activation in vitro, leading to increased Ca2+ influx and regulation of HIF-1α transcriptional activity. Eventually, this results in the upregulation of osteogenic markers (Col1a, Runx2, and OCN) and angiogenic markers (EDN1 and VEGFA). In vivo, the WP-TENG activates aged BMSCs to enhance bone defect repair and regeneration in a Piezo1-dependent manner. Chen et al. have identified that intermittent exposure to extremely low-frequency pulsed electromagnetic fields (ELF-PEMF) showed better effects on the maturation of osteoprogenitor cells than continuous ELF-PEMF, and this effect was mediated by increased Piezo1 expression [67]. Besides, a 3D stiff matrix activates Piezo1/AMPK/autophagy in MC3T3-E1 cells, contributing to osteogenic processes [179].
Osteoarthritis and Joint Health
OA refers to the most common joint disease worldwide, affecting approximately 9.6% of men and 18% of women aged over 60 [180]. It is the major source of pain, disability, and decreased quality of life in aging individuals [181]. Chronic overload and resultant inflammation are key factors in OA progression [181]. Currently, the treatment strategies of OA primarily focus on pain management and joint replacement for end-stage OA [182]. The mechanosensitive ion channel, Piezo1, is expressed in chondrocytes and is involved in the pathogenesis of OA [53]. Study has shown that higher expression of Piezo1 was found in osteoarthritic cartilage compared with healthy human cartilage [183]. Cytoskeleton proteins are key players in mechanical signal transduction during OA [184]. Piezo1 regulates the polymerization and depolymerization of the cytoskeleton [185], and the cytoskeleton reduces Piezo1 activation and vice versa [183, 186, 187], suggesting that Piezo1 is closely associated with the cytoskeleton [184]. Inhibition of Piezo1 can reduce the abnormal proliferation of chondrocytes caused by excessive mechanical loading. This is closely related to cytoskeleton protection [183, 187].
Chondrocyte ferroptosis, a form of cell death during degeneration and aging, has been identified as a crucial factor in the pathogenesis of OA [188]. Recent research has highlighted the role of Piezo1 in this process. Studies have shown that mechanical overloading results in human chondrocyte ferroptosis mediated by the Piezo1 channel [189]. Activation of Piezo1 by Yoda1 is associated with increased dead cells in chondrocytes under excessive loading stimulation, along with a decrease in glutathione peroxidase 4 (GPX-4), a marker of cell ferroptosis [189]. Inhibiting ferroptosis by injecting ferrostatin-1 (Fsp1) could attenuate OA progression [190]. Furthermore, Zhao et al. found that GPX-4 knockout impairs the basic response to mechanical overloading, suggesting the importance of GPX-4 in protecting against ferroptosis in chondrocytes [189].
Inflammatory factors, for example, IL-1α, secreted by chondrocytes during the development of OA, have been shown to enhance the sensitivity of Piezo1-related pathways, making chondrocytes more susceptible to mechanical overloading [183]. This increased vulnerability can be attributed to a pathogenic feed-forward signaling mechanism involving p38 MAP-kinase and transcription factors hepatocyte nuclear factor 4 (HNF4) and activating transcription factor 2 (ATF2) /CREBP1 [183]. In addition, IL-1β can upregulate the expression of Piezo1 in human chondrocytes and then inhibit chondrocyte autophagy and enhance chondrocyte apoptosis through the PI3K/AKT/mTOR pathway [191]. Static magnetic field (SMF) promotes MSCs migration and chondrogenic differentiation to enhance cartilage repair and alleviate OA symptoms [192]. Piezo1-induced CXCR4 is further identified as key mechanism behind SMF-enhanced MSC recruitment and subsequent repair [192].
Heterochromatin instability, a defining characteristic and influential factor in senescence, plays a crucial role in the regulation of the senescence-associated secretory phenotype, which triggers inflammation and leads to cartilage damage [111]. AURKB, a vital component of the chromosomal passenger complex, is associated with the destabilization of heterochromatin [193]. Ren et al. have demonstrated that mechanical overloading increases in AURKB levels via the Piezo1 channel. Interestingly, the utilization of Barasertib, an inhibitor of AURKB, has shown the potential to enhance heterochromatin stability and reduce chondrocyte senescence, thereby alleviating the progression of OA [111]. Additionally, mechanical overloading also increases miR-155-5p expression and reduces GDF6 through Piezo1 activation. However, the administration of exogenous GDF6 has been found to attenuate OA progression by activating SMDA2/3 phosphorylation [194].
The G protein-coupled estrogen receptor (GPER) inhibits Piezo1 expression by reducing actin polymerization and inhibiting the RhoA/LIMK/cofilin pathway [195]. Additionally, Yoda1 aggravates OA progression while Artemisinin (ART) protects cartilage from damage by inhibiting the Piezo1-PI3K-AKT signaling pathway [196]. Of note, the role of Piezo1 in OA alleviation remains controversial. Young et al. found that Piezo1 and Piezo2 conditional knockout (Piezo1Gdf5Cre; Piezo2Gdf5Cre) cannot protect cartilage from injury [197]. However, a recent study by Laura et al. found that inactivation of Piezo1 in chondrocytes (Piezo1Col2a1Cre) alleviates cartilage degeneration and osteophyte formation following OA [198]. The different results may be attributed to the type of mice used, gene ablation efficiency, and surgery methods. Although, Ptgs2, and Ccn2 are identified as potential downstream genes of Piezo1 in chondrocytes [198], the molecular mechanism of the role of Piezo channels in cartilage still needs to be further investigated.
Muscle Atrophy and Regeneration
Muscle mass depends on the balance of protein synthesis and degradation. A reduced muscle mass impedes the body response to stress stimulation and chronic disease [117]. In clinical situations, Duchenne muscular dystrophy (DMD) represents a life-threatening genetic neuromuscular disorder that leads to progressive muscle weakness in skeletal and cardiac muscle [199]. Improper MuSC activation exits during the progression of DMD [200]. In dystrophic mice, more “sensory” cells and less “responsive” cells are located in dystrophic muscles along with Piezo1 reduction [130], reactivation of Piezo1 by Yoda1 improves the function of dystrophic MuSCs by shortening the protrusion length and enhancing MuSCs function upon repetitive injuries [130]. Skeletal muscle adapts to reduced activity by undergoing atrophy. Immobilization is one of the most common reasons for muscle atrophy [201]. During immobilization, the diminished influx of Ca2+ acts as one of the contributing factors to the initiation of skeletal muscle atrophy [202]. Mice hind limb immobilization in a cast for three days results in a 10% to 15% decrease in muscle mass [202]. Previous evidence found that Piezo1 activation enhances the fusion index of myotubes [54]. Recent evidence showed that Piezo1 inhibition by GsMTx-4 increases muscle atrophy-related genes (Klf15 and Il6), and Piezo1 activation by Yoda1 reduces the expression of Klf15 and Il6 [202]. The atrophy-related genes were upregulated in skeletal muscle conditional knockdown Piezo1 mice [202]. Clinical samples harvested from the patients who suffered cast immobilization after bone fracture had less Piezo1 gene expression in muscles [202]. Thus, we suggest that Piezo1 is involved in regulating skeletal muscle atrophy.
Intervertebral Disc Degeneration
IDD, a type of chronic skeletal disease, is one of the common causes of low back pain. Excessive mechanical stimulation induces apoptosis and senescence of NP and AF cells, which play critical roles in the development of IDD [203]. NP-like differentiation of stem cells is essential for IDD regeneration. Huang et al. fabricated injectable upper critical temperature (UCST) microgels to measure the effects of static stretch by swelling microgels on stem cell fate determination [204]. They found that UCST microgels combined with adipose-derived mesenchymal stem cells (ADSCs) promoted NP-like differentiation of stem cells as enhanced by Piezo1 and TRPV4 activation in vitro. In vivo, ADSCs-loaded UCST microgels injection increased ECM production and water content, suggesting that mechanical stimulation produced by injectable microgel may be an effective approach for IDD repair [204].
Conclusion and Perspectives
Piezo1, as a key mechanosensitive ion channel, holds great promise in unraveling the intricate mechanisms underlying musculoskeletal physiology and pathology. In summary, the high expression of Piezo1 in different types of cells in the musculoskeletal system highlights its importance on bone formation, OA progression, myotube formation, tendon stiffness, and AF and NP cell apoptosis and senescence. However, the understanding of thePiezo1's role in the musculoskeletal system is still in its infancy. Most of the studies just focus on phenotypic changes, such as the direct effects of Piezo1 activity on stem cell function. Besides, Piezo1 may enhance other events, like CGRP release and angiogenesis, to guide stem cell behaviors indirectly. In addition, research needs to be conducted in other tissues within the musculoskeletal system, such as ligament and tendon-bone-junction. More importantly, Piezo1 plays an essential role in the regulation of physical activity, which is essential for aging people with musculoskeletal disorders. Exploring ways to enhance physical performance via regulating Piezo1 expression is an important avenue for investigation. Most recently, bioinformatics analysis showed that some key genes, such as Lcn2, Dkk3, and Tnnt1 are negatively associated with Piezo1. However, the exact relationship between Piezo1 and these genes-related pathways is still unclear. Manipulation of Piezo1 activity by drugs and mechanical stimulation will likely be developed as a new useful strategy to treat musculoskeletal disorders. Although GsMTx4 benefits OA and IDD, it is not specific to Piezo1. More investigations are needed to resolve the molecular structure of Piezo1 to fabricate additional modulators.
Abbreviations
AS: ankylosing spondylitis; AL: alendronate; ART: artemisinin; ADSCs: adipose-derived mesenchymal stem cells; AF: annulus fibrosus; BMD: bone mineral density; BMSCs: bone marrow mesenchymal stromal cells; Cx43 HCs: connexin43 hemichannels; COMP: cartilage oligomeric matrix protein; CEP: cartilaginous endplate; CaN: calcineurin; DOPA: dihydroxyphenylalanine; DMP: dentin matrix protein 1; DMD: Duchenne muscular dystrophy; ER: endoplasmic reticulum; ECs: endothelial cells; FSS: fluid shear stress; Fsp1: ferrostatin-1; GsMTx4: grammostola spatulata mechanotoxin 4; Gd3+: gadolinium; GPX-4: glutathione peroxidase 4; GPER: G protein-coupled estrogen receptor; GP: genipin; HNF4: hepatocyte nuclear factor 4; HP: hydrostatic pressure; IFP: infrapatellar fat pad; IDD: intervertebral disc degeneration; LIPUS: low-intensity ultrasound stimulation; LEPR: leptin receptor; MCs: myeloid-lineage cells; MuSCs: muscle stem cells; MSCs: mesenchymal stem cells; NFAT1: nuclear factor of activated T cells 1; NLRP3: nod-like receptor protein 3; NO: nitric oxide; NP: nucleus pulposus; OA: osteoarthritis; OP: osteoporosis; Oln: osteolectin; OVX: ovariectomized; OPG: osteoprotegerin; PC2: polycystin-2; PSCs: periosteal stem cells; PEMF: pulsed electromagnetic fields; PCL: polycaprolactone; PS: phosphatidylserine; Pifithrin-α: PFT-α; PMVS: piezoelectric micro-vibration stimulation; RR: ruthenium red; SERCA: sarco/endoplasmic reticulum Ca2+-ATPase; SM: synovial membrane; SNPs: single nucleotide polymorphisms; SIBLING: small integrin-binding ligand N-linked glycoprotein; SMF: static magnetic field; TFRC: transferrin receptor; Ucn: urocortin; WP-TENG: wearable pulsed triboelectric nanogenerator; 5-HT: serotonin.
Acknowledgements
Funding
This research was supported by Areas of Excellence Scheme (AoE/M402/20) and General Research Fund (14109421) under the Research Grant Council of Hong Kong, and Direct Grants from research Committee of the Chinese University of Hong Kong (4054759).
Author contributions
LL drafted the manuscript. All the authors revised the manuscript. All the authors have read and agreed to the final version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Douguet D, Honoré E. Mammalian Mechanoelectrical Transduction: Structure and Function of Force-Gated Ion Channels. Cell. 2019;179:340-54
2. Ridone P, Vassalli M, Martinac B. Piezo1 mechanosensitive channels: what are they and why are they important. Biophys Rev. 2019;11:795-805
3. Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. 2004;117:2449-60
4. Ranade SS, Syeda R, Patapoutian A. Mechanically Activated Ion Channels. Neuron. 2015;87:1162-79
5. Honoré E, Martins JR, Penton D, Patel A, Demolombe S. The Piezo Mechanosensitive Ion Channels: May the Force Be with You!. Rev Physiol Biochem Pharmacol. 2015;169:25-41
6. Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ. et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55-60
7. Wu J, Lewis AH, Grandl J. Touch, Tension, and Transduction - The Function and Regulation of Piezo Ion Channels. Trends Biochem Sci. 2017;42:57-71
8. Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J. et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;554:487-92
9. Beech DJ, Kalli AC. Force Sensing by Piezo Channels in Cardiovascular Health and Disease. Arterioscler Thromb Vasc Biol. 2019;39:2228-39
10. Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK. et al. Mechanosensing is critical for axon growth in the developing brain. Nat Neurosci. 2016;19:1592-8
11. Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR. et al. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Rep. 2015;13:1161-71
12. Solis AG, Bielecki P, Steach HR, Sharma L, Harman CCD, Yun S. et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019;573:69-74
13. Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest. 2016;126:4527-36
14. McHugh BJ, Buttery R, Lad Y, Banks S, Haslett C, Sethi T. Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J Cell Sci. 2010;123:51-61
15. Shinge SAU, Zhang D, Din AU, Yu F, Nie Y. Emerging Piezo1 signaling in inflammation and atherosclerosis; a potential therapeutic target. Int J Biol Sci. 2022;18:923-41
16. Xing Y, Yang B, He Y, Xie B, Zhao T, Chen J. Effects of mechanosensitive ion channel Piezo1 on proliferation and osteogenic differentiation of human dental follicle cells. Ann Anat. 2022;239:151847
17. Zhang G, Li X, Wu L, Qin Y-X. Piezo1 channel activation in response to mechanobiological acoustic radiation force in osteoblastic cells. Bone Res. 2021;9:16
18. Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J. et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell. 2014;15:471-87
19. Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DTT. et al. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci U S A. 2014;111:16148-53
20. Sugimoto A, Miyazaki A, Kawarabayashi K, Shono M, Akazawa Y, Hasegawa T. et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci Rep. 2017;7:17696
21. Sugisawa E, Takayama Y, Takemura N, Kondo T, Hatakeyama S, Kumagai Y. et al. RNA Sensing by Gut Piezo1 Is Essential for Systemic Serotonin Synthesis. Cell. 2020;182:609-624.e21
22. Zhao Z, Li Y, Wang M, Zhao S, Zhao Z, Fang J. Mechanotransduction pathways in the regulation of cartilage chondrocyte homoeostasis. J Cell Mol Med. 2020;24:5408-19
23. Bosutti A, Giniatullin A, Odnoshivkina Y, Giudice L, Malm T, Sciancalepore M. et al. "Time window" effect of Yoda1-evoked Piezo1 channel activity during mouse skeletal muscle differentiation. Acta Physiol (Oxf). 2021;233:e13702
24. Passini FS, Jaeger PK, Saab AS, Hanlon S, Chittim NA, Arlt MJ. et al. Shear-stress sensing by PIEZO1 regulates tendon stiffness in rodents and influences jumping performance in humans. Nat Biomed Eng. 2021;5:1457-71
25. Wang B, Ke W, Wang K, Li G, Ma L, Lu S. et al. Mechanosensitive Ion Channel Piezo1 Activated by Matrix Stiffness Regulates Oxidative Stress-Induced Senescence and Apoptosis in Human Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2021;2021:8884922
26. Zhou T, Gao B, Fan Y, Liu Y, Feng S, Cong Q. et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. Elife. 2020;9:e52779
27. Chen P, Zhang G, Jiang S, Ning Y, Deng B, Pan X. et al. Mechanosensitive Piezo1 in endothelial cells promotes angiogenesis to support bone fracture repair. Cell Calcium. 2021;97:102431
28. Tsuchiya M, Hara Y, Okuda M, Itoh K, Nishioka R, Shiomi A. et al. Cell surface flip-flop of phosphatidylserine is critical for PIEZO1-mediated myotube formation. Nat Commun. 2018;9:2049
29. Lee W, Guilak F, Liedtke W. Role of Piezo Channels in Joint Health and Injury. Curr Top Membr. 2017;79:263-73
30. Dienes B, Bazsó T, Szabó L, Csernoch L. The Role of the Piezo1 Mechanosensitive Channel in the Musculoskeletal System. Int J Mol Sci. 2023;24:6513
31. Savadipour A, Palmer D, Ely EV, Collins KH, Garcia-Castorena JM, Harissa Z. et al. The role of PIEZO ion channels in the musculoskeletal system. Am J Physiol Cell Physiol. 2023;324:C728-C40
32. Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. 2018;554:481-6
33. Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P. et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015;527:64-9
34. Fang X-Z, Zhou T, Xu J-Q, Wang Y-X, Sun M-M, He Y-J. et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci. 2021;11:13
35. Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T. et al. Chemical activation of the mechanotransduction channel Piezo1. Elife. 2015;4:e07369
36. Zhang T, Chi S, Jiang F, Zhao Q, Xiao B. A protein interaction mechanism for suppressing the mechanosensitive Piezo channels. Nat Commun. 2017;8:1797
37. Parsonage G, Cuthbertson K, Endesh N, Murciano N, Hyman AJ, Revill CH. et al. Improved PIEZO1 agonism through 4-benzoic acid modification of Yoda1. Br J Pharmacol. 2023;180:2039-63
38. Wang Y, Chi S, Guo H, Li G, Wang L, Zhao Q. et al. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat Commun. 2018;9:1300
39. Gnanasambandam R, Ghatak C, Yasmann A, Nishizawa K, Sachs F, Ladokhin AS. et al. GsMTx4: Mechanism of Inhibiting Mechanosensitive Ion Channels. Biophys J. 2017;112:31-45
40. Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS. et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176-81
41. Evans EL, Cuthbertson K, Endesh N, Rode B, Blythe NM, Hyman AJ. et al. Yoda1 analogue (Dooku1) which antagonizes Yoda1-evoked activation of Piezo1 and aortic relaxation. Br J Pharmacol. 2018;175:1744-59
42. Szabó L, Balogh N, Tóth A, Angyal Á, Gönczi M, Csiki DM. et al. The mechanosensitive Piezo1 channels contribute to the arterial medial calcification. Front Physiol. 2022;13:1037230
43. Peyronnet R, Martins JR, Duprat F, Demolombe S, Arhatte M, Jodar M. et al. Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO Rep. 2013;14:1143-8
44. Romero LO, Massey AE, Mata-Daboin AD, Sierra-Valdez FJ, Chauhan SC, Cordero-Morales JF. et al. Dietary fatty acids fine-tune Piezo1 mechanical response. Nat Commun. 2019;10:1200
45. Hu Y, Tian H, Chen W, Liu Y, Cao Y, Pei H. et al. The Critical Role of The Piezo1/β-catenin/ATF4 Axis on The Stemness of Gli1+ BMSCs During Simulated Microgravity-Induced Bone Loss. Adv Sci (Weinh). 2023: e2303375.
46. Liu Y, Tian H, Hu Y, Cao Y, Song H, Lan S. et al. Mechanosensitive Piezo1 is crucial for periosteal stem cell-mediated fracture healing. Int J Biol Sci. 2022;18:3961-80
47. Song J, Liu L, Lv L, Hu S, Tariq A, Wang W. et al. Fluid shear stress induces Runx-2 expression via upregulation of PIEZO1 in MC3T3-E1 cells. Cell Biol Int. 2020;44:1491-502
48. Sasaki F, Hayashi M, Mouri Y, Nakamura S, Adachi T, Nakashima T. Mechanotransduction via the Piezo1-Akt pathway underlies Sost suppression in osteocytes. Biochem Biophys Res Commun. 2020;521:806-13
49. Li X, Zhang C, Bowman HH, Stambough JB, Stronach BM, Mears SC. et al. Piezo1 opposes age-associated cortical bone loss. Aging Cell. 2023;22:e13846
50. Zeng Y, Riquelme MA, Hua R, Zhang J, Acosta FM, Gu S. et al. Mechanosensitive piezo1 calcium channel activates connexin 43 hemichannels through PI3K signaling pathway in bone. Cell Biosci. 2022;12:191
51. Nakamoto H, Katanosaka Y, Chijimatsu R, Mori D, Xuan F, Yano F. et al. Involvement of Transient Receptor Potential Vanilloid Channel 2 in the Induction of Lubricin and Suppression of Ectopic Endochondral Ossification in Mouse Articular Cartilage. Arthritis Rheumatol. 2021;73:1441-50
52. Hu Y, Li K, Swahn H, Ordoukhanian P, Head SR, Natarajan P. et al. Transcriptomic analyses of joint tissues during osteoarthritis development in a rat model reveal dysregulated mechanotransduction and extracellular matrix pathways. Osteoarthritis Cartilage. 2023;31:199-212
53. Emmi A, Stocco E, Boscolo-Berto R, Contran M, Belluzzi E, Favero M. et al. Infrapatellar Fat Pad-Synovial Membrane Anatomo-Fuctional Unit: Microscopic Basis for Piezo1/2 Mechanosensors Involvement in Osteoarthritis Pain. Front Cell Dev Biol. 2022;10:886604
54. Ortuste Quiroga HP, Ganassi M, Yokoyama S, Nakamura K, Yamashita T, Raimbach D. et al. Fine-Tuning of Piezo1 Expression and Activity Ensures Efficient Myoblast Fusion during Skeletal Myogenesis. Cells. 2022;11:393
55. Peng Y, Du J, Günther S, Guo X, Wang S, Schneider A. et al. Mechano-signaling via Piezo1 prevents activation and p53-mediated senescence of muscle stem cells. Redox Biol. 2022;52:102309
56. Nakamichi R, Ma S, Nonoyama T, Chiba T, Kurimoto R, Ohzono H. et al. The mechanosensitive ion channel PIEZO1 is expressed in tendons and regulates physical performance. Sci Transl Med. 2022;14:eabj5557
57. Sun Y, Leng P, Song M, Li D, Guo P, Xu X. et al. Piezo1 activates the NLRP3 inflammasome in nucleus pulposus cell-mediated by Ca2+/NF-κB pathway. Int Immunopharmacol. 2020;85:106681
58. Liu C, Gao X, Lou J, Li H, Chen Y, Chen M. et al. Aberrant mechanical loading induces annulus fibrosus cells apoptosis in intervertebral disc degeneration via mechanosensitive ion channel Piezo1. Arthritis Res Ther. 2023;25:117
59. Wu J, Chen Y, Liao Z, Liu H, Zhang S, Zhong D. et al. Self-amplifying loop of NF-κB and periostin initiated by PIEZO1 accelerates mechano-induced senescence of nucleus pulposus cells and intervertebral disc degeneration. Mol Ther. 2022;30:3241-56
60. Shi S, Kang X-J, Zhou Z, He Z-M, Zheng S, He S-S. Excessive mechanical stress-induced intervertebral disc degeneration is related to Piezo1 overexpression triggering the imbalance of autophagy/apoptosis in human nucleus pulpous. Arthritis Res Ther. 2022;24:119
61. Farley A, McLafferty E, Hendry C. The anatomy and physiology of the locomotor system. Nurs Stand. 2012;27:35-43
62. Qin L, He T, Chen S, Yang D, Yi W, Cao H. et al. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 2021;9:44
63. Xu X, Liu S, Liu H, Ru K, Jia Y, Wu Z. et al. Piezo Channels: Awesome Mechanosensitive Structures in Cellular Mechanotransduction and Their Role in Bone. Int J Mol Sci. 2021;22:6429
64. Bernareggi A, Bosutti A, Massaria G, Giniatullin R, Malm T, Sciancalepore M. et al. The State of the Art of Piezo1 Channels in Skeletal Muscle Regeneration. Int J Mol Sci. 2022;23:6616
65. Takai E, Mauck RL, Hung CT, Guo XE. Osteocyte viability and regulation of osteoblast function in a 3D trabecular bone explant under dynamic hydrostatic pressure. J Bone Miner Res. 2004;19:1403-10
66. Gardinier JD, Majumdar S, Duncan RL, Wang L. Cyclic Hydraulic Pressure and Fluid Flow Differentially Modulate Cytoskeleton Re-Organization in MC3T3 Osteoblasts. Cell Mol Bioeng. 2009;2:133-43
67. Chen Y, Braun BJ, Menger MM, Ronniger M, Falldorf K, Histing T. et al. Intermittent Exposure to a 16 Hz Extremely Low Frequency Pulsed Electromagnetic Field Promotes Osteogenesis In Vitro through Activating Piezo 1-Induced Ca2+ Influx in Osteoprogenitor Cells. J Funct Biomater. 2023;14:165
68. Neidlinger-Wilke C, Wilke HJ, Claes L. Cyclic stretching of human osteoblasts affects proliferation and metabolism: a new experimental method and its application. J Orthop Res. 1994;12:70-8
69. Wittkowske C, Reilly GC, Lacroix D, Perrault CM. Bone Cell Models: Impact of Fluid Shear Stress on Bone Formation. Front Bioeng Biotechnol. 2016;4:87
70. Uda Y, Azab E, Sun N, Shi C, Pajevic PD. Osteocyte Mechanobiology. Curr Osteoporos Rep. 2017;15:318-25
71. Ozcivici E, Luu YK, Adler B, Qin Y-X, Rubin J, Judex S. et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6:50-9
72. Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15:208-16
73. Rubin CT. Skeletal strain and the functional significance of bone architecture. Calcif Tissue Int. 1984;36(Suppl 1):S11-S8
74. Liedert A, Kaspar D, Blakytny R, Claes L, Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun. 2006;349:1-5
75. Li X, Han L, Nookaew I, Mannen E, Silva MJ, Almeida M. et al. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife. 2019;8:e49631
76. Wang L, You X, Lotinun S, Zhang L, Wu N, Zou W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat Commun. 2020;11:282
77. Wu T, Yin F, Wang N, Ma X, Jiang C, Zhou L. et al. Involvement of mechanosensitive ion channels in the effects of mechanical stretch induces osteogenic differentiation in mouse bone marrow mesenchymal stem cells. J Cell Physiol. 2021;236:284-93
78. Hendrickx G, Fischer V, Liedert A, von Kroge S, Haffner-Luntzer M, Brylka L. et al. Piezo1 Inactivation in Chondrocytes Impairs Trabecular Bone Formation. J Bone Miner Res. 2021;36:369-84
79. Yoneda M, Suzuki H, Hatano N, Nakano S, Muraki Y, Miyazawa K. et al. PIEZO1 and TRPV4, which Are Distinct Mechano-Sensors in the Osteoblastic MC3T3-E1 Cells, Modify Cell-Proliferation. Int J Mol Sci. 2019;20:4960
80. Sugimoto A, Iwata K, Kurogoushi R, Tanaka M, Nakashima Y, Yamakawa Y. et al. C-terminus of PIEZO1 governs Ca2+ influx and intracellular ERK1/2 signaling pathway in mechanotransduction. Biochem Biophys Res Commun. 2023;682:39-45
81. Sun W, Chi S, Li Y, Ling S, Tan Y, Xu Y. et al. The mechanosensitive Piezo1 channel is required for bone formation. Elife. 2019;8:e47454
82. Shen B, Tasdogan A, Ubellacker JM, Zhang J, Nosyreva ED, Du L. et al. A mechanosensitive peri-arteriolar niche for osteogenesis and lymphopoiesis. Nature. 2021;591:438-44
83. Roberts SJ, van Gastel N, Carmeliet G, Luyten FP. Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone. 2015;70:10-8
84. Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B. et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310-5
85. Dzamukova M, Brunner TM, Miotla-Zarebska J, Heinrich F, Brylka L, Mashreghi M-F. et al. Mechanical forces couple bone matrix mineralization with inhibition of angiogenesis to limit adolescent bone growth. Nat Commun. 2022;13:3059
86. Qin L, Liu W, Cao H, Xiao G. Molecular mechanosensors in osteocytes. Bone Res. 2020;8:23
87. Liu Z, Tang Y, He L, Geng B, Lu F, He J. et al. Piezo1-mediated fluid shear stress promotes OPG and inhibits RANKL via NOTCH3 in MLO-Y4 osteocytes. Channels (Austin). 2022;16:127-36
88. Inoue S, Li C, Hatakeyama J, Jiang H, Kuroki H, Moriyama H. Higher-intensity ultrasound accelerates fracture healing via mechanosensitive ion channel Piezo1. Bone. 2023;177:116916
89. Roberts S, Colombier P, Sowman A, Mennan C, Rölfing JHD, Guicheux J. et al. Ageing in the musculoskeletal system. Acta Orthop. 2016;87:15-25
90. Jiao Z, Chai H, Wang S, Sun C, Huang Q, Xu W. SOST gene suppression stimulates osteocyte Wnt/β-catenin signaling to prevent bone resorption and attenuates particle-induced osteolysis. J Mol Med (Berl). 2023;101:607-20
91. Chen S, Li Z, Chen D, Cui H, Wang J, Li Z. et al. Piezo1-mediated mechanotransduction promotes entheseal pathological new bone formation in ankylosing spondylitis. Ann Rheum Dis. 2023;82:533-545
92. Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ. et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279-82
93. Bai T, Li Y, Xia J, Jiang Y, Zhang L, Wang H. et al. Piezo2: A Candidate Biomarker for Visceral Hypersensitivity in Irritable Bowel Syndrome? J Neurogastroenterol Motil. 2017;23:453-63
94. Lang K, Breer H, Frick C. Mechanosensitive ion channel Piezo1 is expressed in antral G cells of murine stomach. Cell Tissue Res. 2018;371:251-60
95. Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, Linden DR. et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci U S A. 2018;115:E7632-E41
96. Yadav VK, Ryu J-H, Suda N, Tanaka KF, Gingrich JA, Schütz G. et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825-37
97. Debnath S, Yallowitz AR, McCormick J, Lalani S, Zhang T, Xu R. et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562:133-9
98. Bonnet N, Standley KN, Bianchi EN, Stadelmann V, Foti M, Conway SJ. et al. The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. J Biol Chem. 2009;284:35939-50
99. Deng R, Li C, Wang X, Chang L, Ni S, Zhang W. et al. Periosteal CD68 F4/80 Macrophages Are Mechanosensitive for Cortical Bone Formation by Secretion and Activation of TGF-β1. Adv Sci (Weinh). 2022;9:e2103343
100. Cai G, Lu Y, Zhong W, Wang T, Li Y, Ruan X. et al. Piezo1-mediated M2 macrophage mechanotransduction enhances bone formation through secretion and activation of transforming growth factor-β1. Cell Prolif. 2023;56:e13440
101. Carballo CB, Nakagawa Y, Sekiya I, Rodeo SA. Basic Science of Articular Cartilage. Clin Sports Med. 2017;36:413-25
102. Sanchez-Adams J, Leddy HA, McNulty AL, O'Conor CJ, Guilak F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr Rheumatol Rep. 2014;16:451
103. Madden R, Han S-K, Herzog W. Chondrocyte deformation under extreme tissue strain in two regions of the rabbit knee joint. J Biomech. 2013;46:554-60
104. Steinecker-Frohnwieser B, Lohberger B, Toegel S, Windhager R, Glanz V, Kratschmann C. et al. Activation of the Mechanosensitive Ion Channels Piezo1 and TRPV4 in Primary Human Healthy and Osteoarthritic Chondrocytes Exhibits Ion Channel Crosstalk and Modulates Gene Expression. Int J Mol Sci. 2023;24:7868
105. Servin-Vences MR, Moroni M, Lewin GR, Poole K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife. 2017;6:e21074
106. Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL. et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci U S A. 2014;111:E5114-E22
107. Du G, Li L, Zhang X, Liu J, Hao J, Zhu J. et al. Roles of TRPV4 and piezo channels in stretch-evoked Ca response in chondrocytes. Exp Biol Med (Maywood). 2020;245:180-9
108. Chen F, Sun M, Peng F, Lai Y, Jiang Z, Zhang W. et al. Compressive stress induces spinal vertebral growth plate chondrocytes apoptosis via Piezo1. J Orthop Res. 2023;41:1792-1802
109. Li J, Wang X, Li X, Liu D, Zhai L, Wang X. et al. Mechanical Loading Promotes the Migration of Endogenous Stem Cells and Chondrogenic Differentiation in a Mouse Model of Osteoarthritis. Calcif Tissue Int. 2023;112:363-76
110. Ren X, Zhuang H, Li B, Jiang F, Zhang Y, Zhou P. Gsmtx4 Alleviated Osteoarthritis through Piezo1/Calcineurin/NFAT1 Signaling Axis under Excessive Mechanical Strain. Int J Mol Sci. 2023;24:4022
111. Ren X, Zhuang H, Jiang F, Zhang Y, Zhou P. Barasertib impedes chondrocyte senescence and alleviates osteoarthritis by mitigating the destabilization of heterochromatin induced by AURKB. Biomed Pharmacother. 2023;166:115343
112. Liu Y, Zhang Z, Li J, Chang B, Lin Q, Wang F. et al. Piezo1 transforms mechanical stress into pro senescence signals and promotes osteoarthritis severity. Mech Ageing Dev. 2023;216:111880
113. Ren X, Li B, Xu C, Zhuang H, Lei T, Jiang F. et al. High expression of Piezo1 induces senescence in chondrocytes through calcium ions accumulation. Biochem Biophys Res Commun. 2022;607:138-45
114. Combs CE, Fuller K, Kumar H, Albert AP, Pirianov G, McCormick J. et al. Urocortin is a novel regulator of osteoclast differentiation and function through inhibition of a canonical transient receptor potential 1-like cation channel. J Endocrinol. 2012;212:187-97
115. Lawrence KM, Jones RC, Jackson TR, Baylie RL, Abbott B, Bruhn-Olszewska B. et al. Chondroprotection by urocortin involves blockade of the mechanosensitive ion channel Piezo1. Sci Rep. 2017;7:5147
116. Jones RC, Lawrence KM, Higgins SM, Richardson SM, Townsend PA. Urocortin-1 Is Chondroprotective in Response to Acute Cartilage Injury via Modulation of Piezo1. Int J Mol Sci. 2022;23:5119
117. Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96:183-95
118. Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447-531
119. Dumont NA, Bentzinger CF, Sincennes M-C, Rudnicki MA. Satellite Cells and Skeletal Muscle Regeneration. Compr Physiol. 2015;5:1027-59
120. Huang H, Bae C, Sachs F, Suchyna TM. Caveolae regulation of mechanosensitive channel function in myotubes. PLoS One. 2013;8:e72894
121. Lehka L, Rędowicz MJ. Mechanisms regulating myoblast fusion: A multilevel interplay. Semin Cell Dev Biol. 2020;104:81-92
122. Hindi SM, Tajrishi MM, Kumar A. Signaling mechanisms in mammalian myoblast fusion. Sci Signal. 2013;6:re2
123. Murate M, Abe M, Kasahara K, Iwabuchi K, Umeda M, Kobayashi T. Transbilayer distribution of lipids at nano scale. J Cell Sci. 2015;128:1627-38
124. Hochreiter-Hufford AE, Lee CS, Kinchen JM, Sokolowski JD, Arandjelovic S, Call JA. et al. Phosphatidylserine receptor BAI1 and apoptotic cells as new promoters of myoblast fusion. Nature. 2013;497:263-7
125. van den Eijnde SM, van den Hoff MJ, Reutelingsperger CP, van Heerde WL, Henfling ME, Vermeij-Keers C. et al. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J Cell Sci. 2001;114:3631-42
126. Chang NC, Rudnicki MA. Satellite cells: the architects of skeletal muscle. Curr Top Dev Biol. 2014;107:161-81
127. Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA. et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289-301
128. Blau HM, Cosgrove BD, Ho ATV. The central role of muscle stem cells in regenerative failure with aging. Nat Med. 2015;21:854-62
129. Mijares A, Allen PD, Lopez JR. Senescence Is Associated With Elevated Intracellular Resting [Ca] in Mice Skeletal Muscle Fibers. An in vivo Study. Front Physiol. 2020;11:601189
130. Ma N, Chen D, Lee J-H, Kuri P, Hernandez EB, Kocan J. et al. Piezo1 regulates the regenerative capacity of skeletal muscles via orchestration of stem cell morphological states. Sci Adv. 2022;8:eabn0485
131. Verma M, Asakura Y, Murakonda BSR, Pengo T, Latroche C, Chazaud B. et al. Muscle Satellite Cell Cross-Talk with a Vascular Niche Maintains Quiescence via VEGF and Notch Signaling. Cell Stem Cell. 2018;23:530-543
132. Nakajima T, Nakahata A, Yamada N, Yoshizawa K, Kato TM, Iwasaki M. et al. Grafting of iPS cell-derived tenocytes promotes motor function recovery after Achilles tendon rupture. Nat Commun. 2021;12:5012
133. Heinemeier KM, Kjaer M. In vivo investigation of tendon responses to mechanical loading. J Musculoskelet Neuronal Interact. 2011;11:115-23
134. Snedeker JG, Foolen J. Tendon injury and repair - A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017;63:18-36
135. Nourissat G, Berenbaum F, Duprez D. Tendon injury: from biology to tendon repair. Nature Reviews Rheumatology. 2015;11:223-33
136. Schizas N, Li J, Andersson T, Fahlgren A, Aspenberg P, Ahmed M. et al. Compression therapy promotes proliferative repair during rat Achilles tendon immobilization. J Orthop Res. 2010;28:852-8
137. Davis ME, Gumucio JP, Sugg KB, Bedi A, Mendias CL. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. J Appl Physiol (1985). 2013;115:884-91
138. Zhang J, Wang JHC. The effects of mechanical loading on tendons-an in vivo and in vitro model study. PLoS One. 2013;8:e71740
139. Zhu D, Zhang G, Guo X, Wang Y, Liu M, Kang X. A New Hope in Spinal Degenerative Diseases: Piezo1. Biomed Res Int. 2021;2021:6645193
140. Ma S, Cahalan S, LaMonte G, Grubaugh ND, Zeng W, Murthy SE. et al. Common PIEZO1 Allele in African Populations Causes RBC Dehydration and Attenuates Plasmodium Infection. Cell. 2018;173:443-455
141. Bobbert MF, Casius LJR. Is the effect of a countermovement on jump height due to active state development? Med Sci Sports Exerc. 2005;37:440-6
142. Sun Z, Zheng X, Li S, Zeng B, Yang J, Ling Z. et al. Single Impact Injury of Vertebral Endplates Without Structural Disruption, Initiates Disc Degeneration Through Piezo1 Mediated Inflammation and Metabolism Dysfunction. Spine (Phila Pa 1976). 2022;47:E203-E13
143. Yang Q, Zhou Y, Wang J, Fu W, Li X. Study on the mechanism of excessive apoptosis of nucleus pulposus cells induced by shRNA-Piezo1 under abnormal mechanical stretch stress. J Cell Biochem. 2019;120:3989-97
144. Iatridis JC, Setton LA, Weidenbaum M, Mow VC. Alterations in the mechanical behavior of the human lumbar nucleus pulposus with degeneration and aging. J Orthop Res. 1997;15:318-22
145. Ke W, Wang B, Liao Z, Song Y, Li G, Ma L. et al. Matrix stiffness induces Drp1-mediated mitochondrial fission through Piezo1 mechanotransduction in human intervertebral disc degeneration. J Transl Med. 2023;21:711
146. Xiang Z, Zhang P, Jia C, Xu R, Cao D, Xu Z. et al. Piezo1 channel exaggerates ferroptosis of nucleus pulposus cells by mediating mechanical stress-induced iron influx. Bone Res. 2024;12:20
147. Green DJ, Hopman MTE, Padilla J, Laughlin MH, Thijssen DHJ. Vascular Adaptation to Exercise in Humans: Role of Hemodynamic Stimuli. Physiol Rev. 2017;97:495-528
148. Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest. 2016;126:821-8
149. Baratchi S, Khoshmanesh K, Woodman OL, Potocnik S, Peter K, McIntyre P. Molecular Sensors of Blood Flow in Endothelial Cells. Trends Mol Med. 2017;23:850-68
150. Wehrwein EA, Joyner MJ. Regulation of blood pressure by the arterial baroreflex and autonomic nervous system. Handb Clin Neurol. 2013;117:89-102
151. Zeng W-Z, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM. et al. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science. 2018;362:464-7
152. Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327-87
153. Rode B, Shi J, Endesh N, Drinkhill MJ, Webster PJ, Lotteau SJ. et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat Commun. 2017;8:350
154. Wang H, Yuan Z, Wang B, Li B, Lv H, He J. et al. COMP (Cartilage Oligomeric Matrix Protein), a Novel PIEZO1 Regulator That Controls Blood Pressure. Hypertension. 2022;79:549-61
155. Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K. et al. The Human Microcirculation: Regulation of Flow and Beyond. Circ Res. 2016;118:157-72
156. Bartoli F, Debant M, Chuntharpursat-Bon E, Evans EL, Musialowski KE, Parsonage G. et al. Endothelial Piezo1 sustains muscle capillary density and contributes to physical activity. J Clin Invest. 2022;132:e141775
157. Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010;459:793-806
158. Roberts TJ, Marsh RL, Weyand PG, Taylor CR. Muscular force in running turkeys: the economy of minimizing work. Science. 1997;275:1113-5
159. Lichtwark GA, Barclay CJ. The influence of tendon compliance on muscle power output and efficiency during cyclic contractions. J Exp Biol. 2010;213:707-14
160. Beech DJ. Endothelial Piezo1 channels as sensors of exercise. J Physiol. 2018;596:979-84
161. Aibar-Almazán A, Voltes-Martínez A, Castellote-Caballero Y, Afanador-Restrepo DF, Carcelén-Fraile MDC, López-Ruiz E. Current Status of the Diagnosis and Management of Osteoporosis. Int J Mol Sci. 2022;23:9465
162. Goh S-L, Persson MSM, Stocks J, Hou Y, Lin J, Hall MC. et al. Efficacy and potential determinants of exercise therapy in knee and hip osteoarthritis: A systematic review and meta-analysis. Ann Phys Rehabil Med. 2019;62:356-65
163. He N, Ye H. Exercise and Muscle Atrophy. Adv Exp Med Biol. 2020;1228:255-67
164. Zhou W, Shi Y, Wang H, Chen L, Yu C, Zhang X. et al. Exercise-induced FNDC5/irisin protects nucleus pulposus cells against senescence and apoptosis by activating autophagy. Exp Mol Med. 2022;54:1038-48
165. Breda SJ, Oei EHG, Zwerver J, Visser E, Waarsing E, Krestin GP. et al. Effectiveness of progressive tendon-loading exercise therapy in patients with patellar tendinopathy: a randomised clinical trial. Br J Sports Med. 2021;55:501-9
166. Aspray TJ, Hill TR. Osteoporosis and the Ageing Skeleton. Subcell Biochem. 2019;91:453-76
167. Wang Y, Tao Y, Hyman ME, Li J, Chen Y. Osteoporosis in china. Osteoporos Int. 2009;20:1651-62
168. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-75
169. Bai W-Y, Wang L, Ying Z-M, Hu B, Xu L, Zhang G-Q. et al. Identification of PIEZO1 polymorphisms for human bone mineral density. Bone. 2020;133:115247
170. Zhou Y, Zhang C, Zhou Z, Zhang C, Wang J. Identification of Key Genes and Pathways Associated with PIEZO1 in Bone-Related Disease Based on Bioinformatics. Int J Mol Sci. 2022;23:5250
171. Marouli E, Graff M, Medina-Gomez C, Lo KS, Wood AR, Kjaer TR. et al. Rare and low-frequency coding variants alter human adult height. Nature. 2017;542:186-90
172. Shi W, Shen Z, Bian L, Gao Y, Wu Y, Zhao F. et al. A mussel-bioinspired coating strategy to licence PCL osteoinductive activity for repairing bone defects. Journal of Materials Research and Technology. 2022;18:2025-36
173. Rajabi AH, Jaffe M, Arinzeh TL. Piezoelectric materials for tissue regeneration: A review. Acta Biomater. 2015;24:12-23
174. Kaliannagounder VK, Raj NPMJ, Unnithan AR, Park J, Park SS, Kim S-J. et al. Remotely controlled self-powering electrical stimulators for osteogenic differentiation using bone inspired bioactive piezoelectric whitlockite nanoparticles. Nano Energy. 2021;85:105901
175. Wu R-W, Lian W-S, Chen Y-S, Ko J-Y, Wang S-Y, Jahr H. et al. Piezoelectric Microvibration Mitigates Estrogen Loss-Induced Osteoporosis and Promotes Piezo1, MicroRNA-29a, and Wnt3a Signaling in Osteoblasts. Int J Mol Sci. 2021;22:9476
176. Yang J, Yuan K, Zhang T, Zhou S, Li W, Chen Z. et al. Accelerated Bone Reconstruction by the Yoda1 Bilayer Membrane via Promotion of Osteointegration and Angiogenesis. Adv Healthc Mater. 2023;12:e2203105
177. Kong K, Chang Y, Hu Y, Qiao H, Zhao C, Rong K. et al. TiO2 Nanotubes Promote Osteogenic Differentiation Through Regulation of Yap and Piezo1. Front Bioeng Biotechnol. 2022;10:872088
178. Wang B, Li G, Zhu Q, Liu W, Ke W, Hua W. et al. Bone Repairment via Mechanosensation of Piezo1 Using Wearable Pulsed Triboelectric Nanogenerator. Small. 2022;18:e2201056
179. Wu Y, Xu X, Liu F, Jing Z, Shen D, He P. et al. Three-Dimensional Matrix Stiffness Activates the Piezo1-AMPK-Autophagy Axis to Regulate the Cellular Osteogenic Differentiation. ACS Biomater Sci Eng. 2023;9:4735-46
180. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646-56
181. Abramoff B, Caldera FE. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med Clin North Am. 2020;104:293-311
182. Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H. et al. Osteoarthritis. Lancet. 2015;386:376-87
183. Lee W, Nims RJ, Savadipour A, Zhang Q, Leddy HA, Liu F. et al. Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis. Proc Natl Acad Sci U S A. 2021;118:e2001611118
184. Masner M, Lujea N, Bisbal M, Acosta C, Kunda P. Linoleic and oleic acids enhance cell migration by altering the dynamics of microtubules and the remodeling of the actin cytoskeleton at the leading edge. Sci Rep. 2021;11:14984
185. Syeda R, Florendo MN, Cox CD, Kefauver JM, Santos JS, Martinac B. et al. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016;17:1739-46
186. Lewis AH, Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. Elife. 2015;4:e12088
187. Sun Y, Leng P, Li D, Gao H, Li Z, Li C. et al. Mechanism of Abnormal Chondrocyte Proliferation Induced by Piezo1-siRNA Exposed to Mechanical Stretch. Biomed Res Int. 2020;2020:8538463
188. Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280-96
189. Wang S, Li W, Zhang P, Wang Z, Ma X, Liu C. et al. Mechanical overloading induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis via Piezo1 channel facilitated calcium influx. Journal of Advanced Research. 2022;41:63-75
190. Yao X, Sun K, Yu S, Luo J, Guo J, Lin J. et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J Orthop Translat. 2021;27:33-43
191. Chen W, Zhang H. Elucidating the mechanism of IL-1β-Mediated Piezo1 expression regulation of chondrocyte autophagy and apoptosis via the PI3K/AKT/mTOR signaling Pathway. Tissue Cell. 2024;86:102291
192. Sun Y, Fang Y, Li X, Li J, Liu D, Wei M. et al. A static magnetic field enhances the repair of osteoarthritic cartilage by promoting the migration of stem cells and chondrogenesis. J Orthop Translat. 2023;39:43-54
193. Fischle W, Tseng BS, Dormann HL, Ueberheide BM, Garcia BA, Shabanowitz J. et al. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature. 2005;438:1116-22
194. Qin C, Feng Y, Yin Z, Wang C, Yin R, Li Y. et al. The PIEZO1/miR-155-5p/GDF6/SMAD2/3 signaling axis is involved in inducing the occurrence and progression of osteoarthritis under excessive mechanical stress. Cell Signal. 2024;118:111142
195. Sun Y, Leng P, Guo P, Gao H, Liu Y, Li C. et al. G protein coupled estrogen receptor attenuates mechanical stress-mediated apoptosis of chondrocyte in osteoarthritis via suppression of Piezo1. Mol Med. 2021;27:96
196. Gan D, Tao C, Jin X, Wu X, Yan Q, Zhong Y. et al. Piezo1 activation accelerates osteoarthritis progression and the targeted therapy effect of artemisinin. J Adv Res. 2023: s2090-1232(23)00289-8.
197. Young C, Kobayashi T. Limited roles of Piezo mechanosensing channels in articular cartilage development and osteoarthritis progression. Osteoarthritis Cartilage. 2023;31:775-9
198. Brylka LJ, Alimy A-R, Tschaffon-Müller MEA, Jiang S, Ballhause TM, Baranowsky A. et al. Piezo1 expression in chondrocytes controls endochondral ossification and osteoarthritis development. Bone Res. 2024;12:12
199. Falzarano MS, Scotton C, Passarelli C, Ferlini A. Duchenne Muscular Dystrophy: From Diagnosis to Therapy. Molecules. 2015;20:18168-84
200. Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P. et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143:1059-71
201. Jagoe RT, Goldberg AL. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Curr Opin Clin Nutr Metab Care. 2001;4:183-90
202. Hirata Y, Nomura K, Kato D, Tachibana Y, Niikura T, Uchiyama K. et al. A Piezo1/KLF15/IL-6 axis mediates immobilization-induced muscle atrophy. J Clin Invest. 2022;132:1-13
203. Li P, Zhang R, Wang L, Gan Y, Xu Y, Song L. et al. Long-term load duration induces N-cadherin down-regulation and loss of cell phenotype of nucleus pulposus cells in a disc bioreactor culture. Biosci Rep. 2017;37:BSR20160582
204. Huang X, Chen D, Liang C, Shi K, Zhou X, Zhang Y. et al. Swelling-Mediated Mechanical Stimulation Regulates Differentiation of Adipose-Derived Mesenchymal Stem Cells for Intervertebral Disc Repair Using Injectable UCST Microgels. Adv Healthc Mater. 2023;12:e2201925
205. Frangos JA, McIntire LV, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotechnol Bioeng. 1988;32:1053-60
Author contact
![]() Corresponding authors: Prof. Jiankun Xu (jiankunxuedu.hk), Prof. Patrick Shu-hang Yung (patrickyungedu.hk).
Corresponding authors: Prof. Jiankun Xu (jiankunxuedu.hk), Prof. Patrick Shu-hang Yung (patrickyungedu.hk).
 Global reach, higher impact
Global reach, higher impact