13.3
Impact Factor
Theranostics 2024; 14(7):2915-2933. doi:10.7150/thno.93124 This issue Cite
Research Paper
Comprehensive multi-omics analysis of pyroptosis for optimizing neoadjuvant immunotherapy in patients with gastric cancer
1. Department of Gastric Surgery, Fujian Medical University Union Hospital, Fuzhou, China.
2. Key Laboratory of Ministry of Education of Gastrointestinal Cancer, Fujian Medical University, Fuzhou, China.
3. Fujian Key Laboratory of Tumour Microbiology, Fujian Medical University, Fuzhou, China.
4. Department of Pathology, Fujian Medical University Union Hospital, Fuzhou, China.
5. Department of Gastrointestinal and Hernia Surgery, The First Affiliated Hospital of Kunming Medical University, Kunming, China.
6. Department of Gastrointestinal Surgery, The First Affiliated Hospital of Bengbu Medical College, Bengbu, China.
7. Department of Gastrosurgery, Liaoning Cancer Hospital & Institute, Cancer Hospital of China Medical University, Shenyang, China.
8. Department of Gastrointestinal Surgery, The Affiliated Tumor Hospital of Guangxi Medical University, Nanning, China.
9. Gastrointestinal Cancer Institute, Fujian Medical University, Fuzhou, 350001, China.
# These authors contributed equally to this study
Abstract
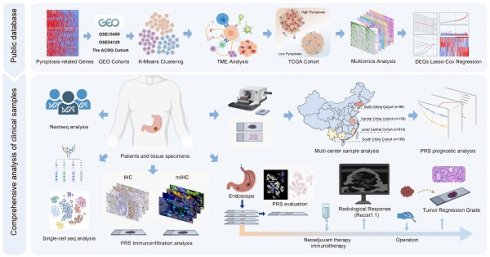
Background: Pyroptosis plays a crucial role in immune responses. However, the effects of pyroptosis on tumor microenvironment remodeling and immunotherapy in gastric cancer (GC) remain unclear.
Patients and Methods: Large-sample GEO data (GSE15459, GSE54129, and GSE62254) were used to explore the immunoregulatory roles of pyroptosis. TCGA cohort was used to elucidate multiple molecular events associated with pyroptosis, and a pyroptosis risk score (PRS) was constructed. The prognostic performance of the PRS was validated using postoperative GC samples from three public databases (n=925) and four independent Chinese medical cohorts (n=978). Single-cell sequencing and multiplex immunofluorescence were used to elucidate the immune cell infiltration landscape associated with PRS. Patients with GC who received neoadjuvant immunotherapy (n=48) and those with GC who received neoadjuvant chemotherapy (n=49) were enrolled to explore the value of PRS in neoadjuvant immunotherapy.
Results: GC pyroptosis participates in immune activation in the tumor microenvironment and plays a powerful role in immune regulation. PRS, composed of four pyroptosis-related differentially expressed genes (BATF2, PTPRJ, RGS1, and VCAN), is a reliable and independent biomarker for GC. PRSlow is associated with an activated pyroptosis pathway and greater infiltration of anti-tumor immune cells, including more effector and CD4+ T cells, and with the polarization of tumor-associated macrophages in the tumor center. Importantly, PRSlow marks the effectiveness of neoadjuvant immunotherapy and enables screening of GC patients with combined positive score ≥1 who benefit from neoadjuvant immunotherapy.
Conclusion: Our study demonstrated that pyroptosis activates immune processes in the tumor microenvironment. A low PRS correlates with enhanced infiltration of anti-tumor immune cells at the tumor site, increased pyroptotic activity, and improved patient outcomes. The constructed PRS can be used as an effective quantitative tool for pyroptosis analysis to guide more effective immunotherapeutic strategies for patients with GC.
 Global reach, higher impact
Global reach, higher impact