13.3
Impact Factor
Theranostics 2024; 14(4):1764-1780. doi:10.7150/thno.89174 This issue Cite
Research Paper
Mrc1+ macrophage-derived IGF1 mitigates crystal nephropathy by promoting renal tubule cell proliferation via the AKT/Rb signaling pathway
1. Department of Cell Biology, Naval Medical University (Second Military Medical University), Shanghai, China.
2. Department of Nephrology, Changhai Hospital, Naval Medical University (Second Military Medical University), Shanghai, China.
3. Department of Nephrology, PLA Navy No.905 Hospital, Naval Medical University (Second Military Medical University), Shanghai, China.
4. Department of Nephrology, Nantong Third People's Hospital, Affiliated Nantong Hospital 3 of Nantong University, Nantong 226006, Jiangsu, China.
5. Nanjing Medical University, Nanjing, Jiangsu, China.
6. Faculty of Pharmacy, Naval Medical University, Shanghai, China.
# These authors equally contributed to this work.
Abstract
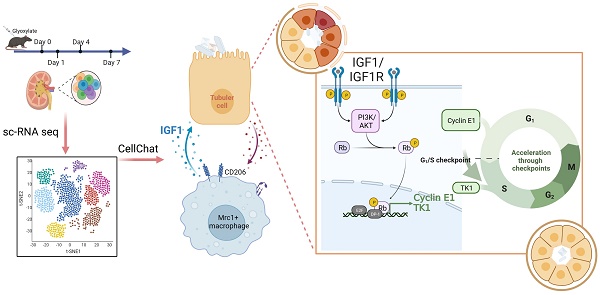
Rationale: The present understanding of the cellular characteristics and communications in crystal nephropathy is limited. Here, molecular and cellular studies combined with single-cell RNA sequencing (scRNA-seq) were performed to investigate the changes in cell components and their interactions in glyoxylate-induced crystallized kidneys to provide promising treatments for crystal nephropathy.
Methods: The transcriptomes of single cells from mouse kidneys treated with glyoxylate for 0, 1, 4, or 7 days were analyzed via 10× Genomics, and the single cells were clustered and characterized by the Seurat pipeline. The potential cellular interactions between specific cell types were explored by CellChat. Molecular and cellular findings related to macrophage-to-epithelium crosstalk were validated in sodium oxalate (NaOx)-induced renal tubular epithelial cell injury in vitro and in glyoxylate-induced crystal nephropathy in vivo.
Results: Our established scRNA atlas of glyoxylate-induced crystalline nephropathy contained 15 cell populations with more than 40000 single cells, including relatively stable tubular cells of different segments, proliferating and injured proximal tubular cells, T cells, B cells, and myeloid and mesenchymal cells. In this study, we found that Mrc1+ macrophages, as a subtype of myeloid cells, increased in both the number and percentage within the myeloid population as crystal-induced injury progresses, and distinctly express IGF1, which induces the activation of a signal pathway to dominate a significant information flow towards injured and proliferating tubule cells. IGF1 promoted the repair of damaged tubular epithelial cells induced by NaOx in vitro, as well as the repair of damaged tubular epithelial cells and the recovery of disease outcomes in glyoxylate-induced nephrolithic mice in vivo.
Conclusion: After constructing a cellular atlas of glyoxylate-induced crystal nephropathy, we found that IGF1 derived from Mrc1+ macrophages attenuated crystal nephropathy through promoting renal tubule cell proliferation via the AKT/Rb signaling pathway. These findings could lead to the identification of potential therapeutic targets for the treatment of crystal nephropathy.
Keywords: single-cell RNA sequencing, bioinformatic analysis, crystal nephropathy, intercellular communications, macrophage, IGF1
 Global reach, higher impact
Global reach, higher impact