13.3
Impact Factor
Theranostics 2024; 14(4):1631-1646. doi:10.7150/thno.92463 This issue Cite
Review
Emerging role of extracellular vesicles in diabetic retinopathy
1. Department of Gynecology and obstetrics, The Affiliated Yixing Hospital of Jiangsu University, 214200, China.
2. Jiangsu Province Key Laboratory of Medical Science and Laboratory Medicine, Department of Laboratory Medicine, School of Medicine, Jiangsu University, Zhenjiang, Jiangsu 212013, China.
3. Department of clinical laboratory, The Affiliated Yixing Hospital of Jiangsu University, Yixing, 214200, China.
# Equal Contributors.
Received 2023-11-21; Accepted 2024-1-26; Published 2024-2-4
Abstract
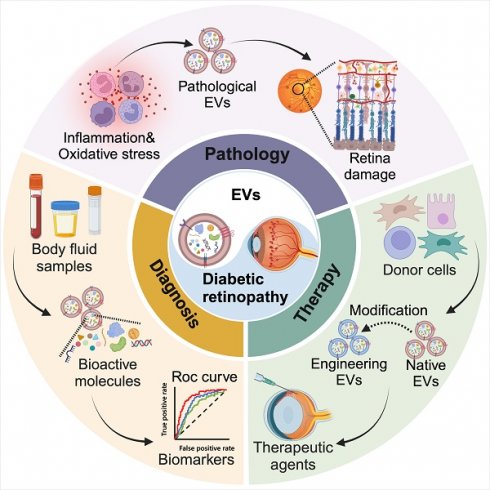
Diabetic retinopathy (DR), a complex complication of diabetes mellitus (DM), is a leading cause of adult blindness. Hyperglycemia triggers DR, resulting in microvascular damage, glial apoptosis, and neuronal degeneration. Inflammation and oxidative stress play crucial roles during this process. Current clinical treatments for DR primarily target the advanced retinal disorder but offer limited benefits with inevitable side effects. Extracellular vesicles (EVs) exhibit unique morphological features, contents, and biological properties and can be found in cell culture supernatants, various body fluids, and tissues. In DR, EVs with specific cargo composition would induce the reaction of receptor cell once internalized, mediating cellular communication and disease progression. Increasing evidence indicates that monitoring changes in EV quantity and content in DR can aid in disease diagnosis and prognosis. Furthermore, extensive research is investigating the potential of these nanoparticles as effective therapeutic agents in preclinical models of DR. This review explores the current understanding of the pathological effects of EVs in DR development, discusses their potential as biomarkers and therapeutic strategies, and paves the way for further research and therapeutic advancements.
Keywords: Extracellular vesicles, Diabetic retinopathy, Pathogenesis, Diagnosis, Therapy
1. Introduction
Diabetes mellitus (DM) is a global epidemic with prominent morbidity. According to the 2021 report from the International Diabetes Federation (IDF), approximately 537 million adults worldwide are affected by DM, and this number is projected to reach 783 million by 2045 [1]. Among DM patients, about 20% present with diabetic retinopathy (DR) at the time of diagnosis, and an additional 40-50% will develop DR as the disease progresses. DR, a microvascular complication of both type 1 and type 2 diabetes, has emerged as the major cause of vision loss [2]. Factors such as prolonged duration of diabetes, hyperglycemia, and hypertension contribute to the development of DR. Additionally, pregnancy and puberty can expedite the progression of retinal pathological changes [3]. Persistent high blood glucose levels and hypertension lead to vascular abnormality and dysfunction of retinal cells, resulting in cloudy or blurred vision in diabetic patients. At the late stage of DR, sight loss diminishes patients' quality of life and places a burden on healthcare resources. However, the pathophysiological process of DR is highly complex, involving multiple interrelated mechanisms that trigger adaptive changes and dysregulation of retina cells. Regular monitoring and stringent control of blood glucose levels have been shown to prevent the progression of the disease during its early stage. In advanced phase of DR, treatment options include intraocular injections of anti-vascular endothelial growth factor (VEGF) antibodies, laser therapy, and vitrectomy. Although these clinical treatments have shown encouraging results, most DR patients do not achieve significant clinical improvement in vision [4]. There is an urgent need to deepen our understanding of the etiology of the disease and develop new approaches for diagnosis and treatment for DR.
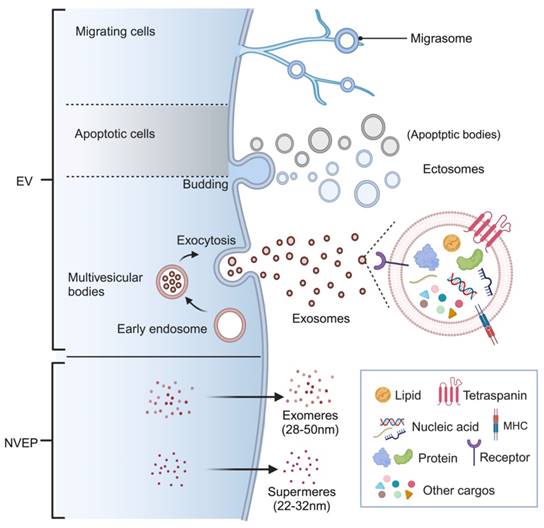
Extracellular vesicles are naturally occurring lipid bilayer-encapsulated nanoparticles with varied size, cargos, and origins and can be categorized into several subtypes [5]. According to the biogenesis, membranous EVs are mainly classified as exosomes and ectosomes (also termed as microvesicles, MVs) [6]. Exosomes are a subset of small particles (30-150nm) formed in internal multivesicular bodies (MVB) and released during exocytosis [7]. On the other hand, ectosomes are vesicles ranging from 50 nm to 10000 nm that are directly shed from the plasma membrane of both healthy and damaged cells [8]. While apoptotic bodies, one of the most well-studied subtypes of ectosomes, are submicron vesicles only derived from cells undergoing programmed cell death [9]. Recently, membranous vesicles produced by migrating cells at the near ends are identified as a novel subtype of EVs and referred to as “migrasome”, whose roles in cellular communication are under extensive exploration [10, 11]. It is worth mentioning that there is a growing interest in distinct non-vesicular extracellular nanoparticles (NVEPs) with diameter less than 50 nm, which are known as exomere and supermere [12, 13]. Different names have been used in varieties of studies to describe EVs based on the specific biogenesis, diameter, or contents, such as microparticles [14], shedding vesicles [15], and apoptotic blebs [16]. However, the International Society for Extracellular Vesicles (ISEV) has proposed the umbrella term “extracellular vesicles” to encompass all types of membrane-derived vesicles. According to the ISEV, these vesicles are further divided into small EVs (sEVs, <200 nm) and medium/large EVs (m/lEVs) based on their particle size (MISEV2018) [17]. Therefore, in this review, the term EV will be primarily exploited to refer to all nanoparticles, unless specific name is applicable based on the research context.
EVs can be released by almost all cells and isolated from the supernatant of cell cultures, a broad range of tissues and body fluids. Enclosed by a membrane, EVs contain a rich assortment of components, primarily including proteins, lipids, nucleic acids, and metabolites. Certain components found commonly in EVs serve as markers for EV identification, such as certain transmembrane proteins (CD9, CD63, and CD81), membrane transport/fusion proteins (Annexins and Rabs), MVB synthetic proteins (TSG101 and Alix), and chaperone proteins (Hsp60, Hsp70, and Hsp90). Additionally, EVs also possess unique compositions specific to their origins, reflecting the physiological or pathological states of the parent cells or tissues (Figure 1). The distinct changes in EV quantity or components make them valuable as disease biomarkers. When taken up by neighbor or distant receptor cells, the cargos carried by EVs can modify their biological functions, enabling cellular information transmitting without direct contact [18]. Presently, an increasing number of studies are dedicated to exploring the use of EVs as therapeutic agents and ideal drug delivery carriers due to their specifical biological characteristics [19]. The therapeutic benefits of EVs, especially those derived from mesenchymal stem cells (MSCs), have been proposed for a broad range of common or refractory diseases, including diverse retinal disorders [20]. In this review, we provide an overview of DR and elucidate how EVs contribute to the progression of DR pathologies. Furthermore, we summarize the potential of EVs as biomarkers and therapeutic agents for DR.
2. Development and Pathological changes of DR
As a progressive disease, DR undergoes several clinical stages with increasing diseases severity. Based on the modified Airlie House classification scale as applied in the Early Treatment Diabetic Retinopathy Study (ETDRS) [21] and 2017 position statement from the American Diabetes Association [22], the chronopathology of DR can be clarified into four overlapping phases: mild non-proliferative DR (NPDR); moderate NPDR; severe NPDR; proliferative DR (PDR). Mild NPDR is the earliest stage of the disease, characterized by the presence of microaneurysms, dot intraretinal hemorrhages, hard exudates, or cotton wool spots in the retina. As the disease progresses, there is an increasing distortion and leakage of retinal blood vessels. These detectable abnormalities result from vascular dysregulation and the detachment of endothelial cells from pericytes on histopathology. The leakage of retinal capillaries allows the passage of inflammatory mediators, proteins, and other blood contents into interstitial space, leading to the development of diabetic macular edema (DME) [23]. In the severe NPDR stage, dysfunctional microvessels fail to supply adequate blood to retina, triggering oxidative stress reaction and compensatory angiogenesis. New blood vessels, accompanied by fibrotic tissue, grow along the inner surface of the retina and even extend into the vitreous. These fibrotic vessels may contract and cause tractional retinal detachment, eventually resulting in permanent vision loss [24].
EV classification and biogenesis. Ectosomes are released by shedding from the plasma membrane, while exosomes are generated through the endosomal pathway. Apoptotic bodies, which are released by cells undergoing programmed death, fall into the category of ectosomes. Migrasomes refer to vesicles that formed on retraction fibers of migrating cells. These EVs are enclosed by a phospholipid bilayer and serve as carriers for transporting various cargos, including lipids, proteins, nucleic acids, and metabolites. Interestingly, there are also non-vesicular extracellular nanoparticles known as exomeres and supermeres, which lack a membrane structure but still play important roles in intercellular communication. (Figure created with BioRender.com).
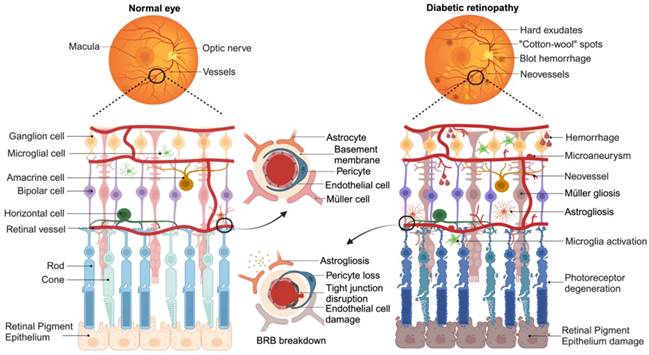
Three layers of capillary plexuses embed in the retina offer needed nutrition and maintain the retinal homeostasis. Inner blood-retina barrier (BRB) composed by the tight junction of endothelial cells, podocytes, and glial endfeets. While the single layer of retinal pigment epithelium cells (RPEs) and Bruch's membrane (BM) constitute outer BRB that regulates the exchange of metabolites from the photoreceptor cells (cones and rods) with the underlying choroidal vessels. Both the inner and outer BRB act as barriers between the systemic circulation and the retina [25]. More than a network of blood vessels, retina with multilayer structure is composed of neurons (photoreceptors, horizontal cells, bipolar cells, amacrine and ganglion cells) and glial cells (astrocytes, microglia, and Müller cells) [26], accounting for approximately 95% of the tissue [27]. Essential for the BRB formation, glial cells dysregulate after microvascular damage, including Müller cells or astrocyte gliosis and microglia activation. Activated microglia, as the primary resident innate immune cells, can present a pro-inflammatory (M1) state and release various inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α) [28], further exacerbating microangiopathy. Glial dysfunction also disrupts homeostatic and metabolic support to neuronal cells and photoreceptors, contributing to neurons death and axon degeneration [29]. These irreversible neuronal abnormalities directly contribute to vision loss in DR patients. Emerging basic and clinical studies have shown that neuronal disorder may develop before the clinical observation of microvascular lesions, even though DR is primarily diagnosed based on vascular abnormalities [30]. Moreover, the American Diabetes Association defines DR as a specific neurovascular complication of diabetes [22], highlighting the importance of both vascular and neuronal pathology in the development of DR (Figure 2).
3. EVs in DR pathology and diagnosis
3.1 The impact of EVs on pathological progression of DR
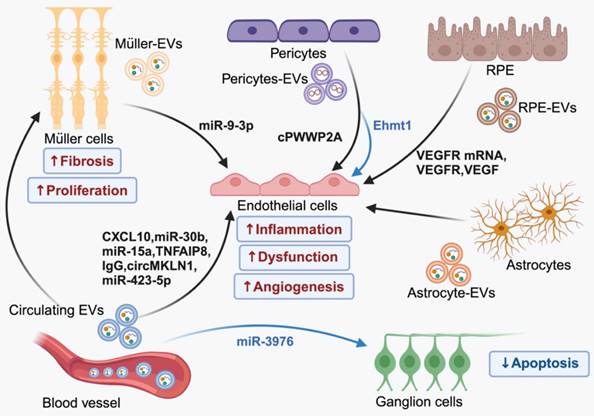
Recent studies have highlighted the significance of EV-mediated endogenous intercellular communication in maintaining retinal cell function and homeostasis. In healthy retina, EVs released by photoreceptor cells are selectively internalized by Müller cells [31]. Dysregulation of this EV communication process can exacerbate retinal degeneration [32]. Additionally, disrupting EV biogenesis using GW4869, an exosome inhibitor, leads to altered EVs profiles (increased lEVs and reduced sEVs) in retinal ganglion cells, promoting neurodegeneration in a mouse model [33]. In DR, persistent high blood glucose levels induce a cascade of pathological effects, including inflammation, oxidative stress, angiogenesis, and apoptosis [34]. These pathological stimuli are likely responsible for the changes in cargo specificity and release of EVs. Recent studies in both experimental models and humans have observed elevated levels of EVs that correlated with inflammatory conditions [35] and neurodegenerative lesions [36]. Wozniak et al. [37] disclosed that inflammasome activation contributes to caspase-1-mediated RILP cleavage, which can promote selective loading of miRNA cargos and secretion of EVs. In the retina, the activation of inflammasome triggers the release of EVs that encapsulate pro-inflammatory cytokines, such as interleukin-10. This is mediated by Gasdermin D, a terminal inflammasome component pyroptotic pore-forming protein, resulting in the propagation of inflammatory cascades [38]. Additionally, study has shown that oxidative stress can lead to the secretion of enhanced numbers of basolateral EVs by RPEs containing changed protein contents related to epithelial barrier integrity, even though tight junction integrity remains unaffected [39]. Although the exact mechanism of pathological EVs formation is still being investigated, substantial evidence suggests the involvement of disordered EVs in the development of DR, which contribute to ocular dysfunction by delivering special signals among retinal cells (Figure 3).
Schematic representation of key pathogenetic events in retina during the development of DR. The diagram illustrates the major changes that occur from a normal state (left) to an advanced stage of DR (right). Retina is composed of a complex network of capillary plexuses and multiple layers of cells. In DR, there are various vascular abnormalities, including thickening of basement membrane, endothelial injury leading to disruption of the tight junctions, and loss of pericyte. These changes result in the formation of microaneurysms, dot hemorrhages, hard exudates, and “cotton wool” spots. Compensatory neovascularization occurs as a response to the damaged vasculature. Additionally, dysfunction of glial cells (Müller cells or astrocytes) and activation of microglia contribute to the progression of the disease. Furthermore, degeneration of ganglion cells and photoreceptor cells (cones and rods) exacerbates the loss of vision associated with DR. (Figure created with BioRender.com).
3.1.1 The role of EVs in vascular lesions in DR
At the early stage of DR, vascular abnormities such as hemorrhage and neovascularization occur, characterized by thickening of the basement membrane, endothelial dysfunction, disruption of the tight junctions, pericyte loss, and the formation of acellular capillaries. Several studies have reported the involvement of aberrant circulating EVs in vascular damage during the development of DR. Jung et al. [40] suggested that elevated levels of endothelial cells (ECs)- derived EVs in peripheral blood (PB) are associated with vasculopathy of DR patients. Research indicates that circulating EVs originating from platelets, leukocytes, and endothelial cells in individuals with diabetes transport pro-angiogenic factors and proinflammatory cytokines, contributing to vascular complications such as retinopathy [41, 42]. For example, in condition of hyperglycemia, EVs derived from platelet-rich plasma (PRP) can induce retinal endothelial injury by transferring CXCL10 and upregulating the TLR4 signaling pathway [43]. Increased IgG-laden EVs in plasma can activate the classical complement pathway and promote membrane attack complex (MAC) deposition, accelerating vascular damage associated with DR [44, 45]. Additionally, miR-15a enriched EVs in the plasma of DM patients are believed to be mainly produced in pancreatic β-cells, which can travel through the bloodstream, leading to retina damage by inducing oxidative stress [46]. The serum EVs from individuals with DR have been found to exhibit elevated levels of circMKLN1. This circRNA specifically targets the miR-26a-5p/Rab11a axis, leading to the induction of retinal microvascular dysfunction [47]. Moreover, these pathogenic EVs in PB also promote thrombosis in DR patients [48], thereby increasing the risk of thrombo-embolic DR.
Retinal vessel dysfunction in DR leads to increased leukostasis and thrombosis, resulting in local blockage of capillaries and hypoxia. This process triggers a series of adaptive responses, with one of the direct consequences being the activation of hypoxia-related factors. These factors, including hypoxia-inducible factor-1alpha (HIF-1α), VEGF, erythropoietin (EPO) and EPO receptor (EPOR), progressively increase in a time dependent manner in DR process [49], whose upregulation plays a crucial role in the permeability of microvessels and BRB break down [50]. Meanwhile, these diabetes-related stress disrupts vascular stability via the EV mediated interaction between vascular endothelial cells and pericytes [51]. Under high glucose (HG) condition, there is increased level of circRNA cPWWP2A in pericytes, which can be transferred from pericytes to ECs through EVs. The aberrant expressed cPWWP2A acts as a sponge for miR-579 and promotes the expression of angiopoietin 1, occludin, and SIRT1, leading to vascular cell damage and microvascular dysfunction [52]. However, overexpressed vesicular-circEhmt1 from hypoxia-induced pericytes exhibits protective effect in ECs against HG-induced injury by suppressing NLRP3-mediated inflammasome formation [53]. In the absence of therapeutic intervention, vascular damage becomes inevitable during the progression of DR, despite the therapeutic potential of pericyte-derived EVs in communication with ECs.
Neovascularization occurs as a compensatory response to severe vessel damage following retinal ischemia or infarction. These fragile new vessels, with abnormal architecture, are susceptible to bleeding and protrude into the vitreous body where they eventually anchored by fibrovascular tissue. Recent investigations into the mechanisms involved in abnormal retinal angiogenesis have identified several novel molecular players, including ADAM10 [54] and the Netrin-1-CD146-Gαi1/3 axis [55]. However, angiogenic factors, especially VEGF, are considered to be the primary drivers of disease progression in DR. Hyperglycemia and oxidative stress stimulate the secretion of EVs loaded with VEGF, VEGF receptors (VEGFR), and VEGFR mRNA by RPEs, promoting neovascularization in PDR [56-58]. These pathological EVs not only deliver VEGF-related molecules directly but also induce the activation of VEGF signaling in ECs. In case of PDR, elevated levels of miR-9-3p are transferred to retinal endothelial cells through EVs derived from Müller glia. This transfer activates S1P1/AKT/VEGFR2 pathway, exacerbating abnormal angiogenesis [59]. Additionally, the increased tumor necrosis factor-α-induced protein 8 (TNFAIP8) and miR-30b in plasma EVs have been revealed to promote proliferation in human retinal microvascular endothelial cells [60, 61], making them crucial mediators of angiogenesis. Recent research has also discovered low levels of lncRNA PPT2-EGFL8 and elevated miR-423-5p in plasma EVs derived from PDR patients. Reduced PPT2-EGFL8/miR-423-5p binding activates hypoxia-induced peroxisome proliferator-activated receptor-β/δ (PPARD)/angiopoietin-like 4 (ANGPTL4) signaling pathway, leading to diabetes-related neovascularization and retinal vessel breakage [62]. Luo et al. [63] made an interesting discovery regarding a cluster of Tsp-1+ microglia cells that mitigate retinal neovascularization in mice with oxygen-induced retinopathy (OIR). Mechanically, these microglial cells release EVs containing Tsp-1, which are then transported to ECs and inhibit their migration and proliferation by regulating the miR-27a-5p/Smad3 axis. This finding offers a potential target for inhibiting pathologic neovascularization in DR.
3.1.2 Role of EVs in neuronal and glial cell damage
Vessels, neurons, glia, and retina-resident immune cells constitute the retinal neurovascular unit. Retinal vasculature supplies essential nutrients to neural tissue. While neural and glial cells can regulate vascular cells as feedback, ensuring the normal function of the retina [64]. Disrupted BRB in DR can jeopardize neurovascular homeostasis, leading to dysfunction of neural and glial cells and eventual vision loss. Loss of tight junction allows circulating EVs to cross the damaged endothelial barrier and enter interstitial space [65], which may influence retinal cell viability. In vitro, studies have demonstrated that EVs isolated from the PRP of diabetic rats upregulate both the proliferation and fibrogenic activity of retinal Müller glia cells though the Yes-associated protein (YAP)/PI3K/Akt pathway [66]. Notably, although overexpression of miRNA-3976 promotes the apoptosis of retinal ganglion cells 5 (RGC-5), EVs carrying upregulated miRNA-3976 in plasma of DR patients have been found to increase proliferation and reduce apoptosis of RGC-5 cells, thus exerting a protective effect in vitro [67]. Further in vivo studies are required to elucidate the impact of circulating EVs on retinal resident cells.
EVs from astrocytes (AD-EVs) has long been considered as the important participants in glia-neuron crosstalk, whose cargos are reported to be stimulus-dependent and exert pro-inflammatory or neuroprotective effects based on different context [68, 69]. Under physiological conditions, AD-EVs play a role in transferring bioactive molecules that contribute to neuronal survival and neurite outgrowth [70]. However, under pathological conditions, AD-EVs can exacerbate inflammatory in neural cells [71] and even trigger peripheral immunological response when released into circulation [72]. These findings indicate a potential relationship between neurodegeneration and EVs derived from activated astrocytes in DR, given the oxidative stress and inflammation induced by chronic hyperglycemia and hypoxia [34, 73]. Moreover, retinal astrocytes under oxidative stress exhibit increased levels of autophagy and release EVs that promote the proliferation and migration of ECs [74], showing the potential to promote neovascularization.
Overall, numerous studies have demonstrated the involvement of EVs in various pathologies associated with DR, including blood vessel damage, apoptosis of retina neurons and dysfunction of glial cells. EVs derived from retinal cells, vitreous and blood transport physiological or pathological signals through the change of amounts or special cargo contents, thus mediating biological functions of neighbor cells and even others located further away. The broad participation of EVs in DM and other connected complications have been reported besides DR [75]. Given the systematic dysregulation in diabetes, it is important not to overlook the EV-mediated communication between the retina and other remote organs. As mentioned above, numerous studies have identified abnormal EVs circulating in the bloodstream that can damage retinal vascular endothelial cells. However, the organ-specific origins of these circulating EVs remain unclear. Furthermore, the existence of BRB increases the difficulty for the delivery of circulating EV into retina. Abundant evidence indicates that EVs have the ability to traverse biological barriers, including blood-brain-barrier (BBB) in the central nervous system, which is similar to BRB [76]. Multiple potential pathways for EVs to cross the endothelial barrier are currently being extensively explored [77], shedding light on the possibility that EVs shuttling between the bloodstream and retina may precede BRB leakage. In addition to inner BRB composed of ECs, the outer BRB formed by RPEs further complicates EVs transport within the retina. A comprehensive understanding of ocular structures will expand research and application possibilities for EVs in retinal disorders.
EV-mediated intercellular communication in the pathology of DR. In the context of DR, EVs derived from blood and a group of retinal cells contribute to the advancement of the diseases by facilitating the transfer of bioactive molecules between cells. Importantly, EVs originating from the same source can have both harmful and beneficial effects, which can be attributed to the different cargo they contain. (Figure created with BioRender.com).
EVs as biomarkers for DR
| Source of EVs | EV cargo sequencing (type) | EV cargos/markers | Levels | Comparison | Ref. |
|---|---|---|---|---|---|
| serum | yes (circRNA) | circMKLN1 | ↑ | DR VS DM and HC | [47] |
| plasma, vitreous humor | yes (protein) | TNFAIP8 | ↑ | DR VS DM and HC | [60] |
| serum | yes (total RNA) | lncRNA PPT2-EGFL8/ miR-423-5p | ↓/↑ | PDR VS NPDR, DM, and HC | [62] |
| serum | yes (miRNA) | miR-3976 | ↑ | DR VS DM | [67] |
| plasma | no | GPIX+ EV counts | ↑ | DR VS DM | [78] |
| plasma | no | Annexin V+ and CD14+ EV counts | ↑ | DR VS DM | [79] |
| plasma | yes (metabolite) | N, N′-dicyclohexylcarbodiimide, 4-acetamidobutyric acid, Dl-2-aminooctanoic acid/ triethanolamine | ↑ | NPDR VS DM/ PDR VS NPDR | [80] |
| plasma | no | CCR5+ EVs counts | ↑ | moderate and severe NPDR VS mild NPDR | [81] |
| vitreous humor | yes (protein) | IGLL1, TPI1, LDHA, ApoB/ ApoM, FCN3, ALDOC, SAA2-4, IGFALS, HABP2 | ↑/↓ | PDR VS HC | [82] |
| urine | yes (protein) | JUP | ↑ | DR VS DM and HC | [83] |
| plasma, serum | yes (miRNA) | miR-150-5p/miR-21-3p, miR-30b-5p | ↓/↑ | DR VS DM and HC | [85, 86] |
| plasma | no | miR-15a | ↑ | DM with retinal damage VS HC | [87] |
| serum | yes (miRNA) | miR-431-5p, | ↑ | PDR VS DM and HC | [88] |
| serum | no | miR-26b-5p | ↑ | DR VS DM and HC | [89] |
| tear | yes (miRNA) | miR-145-5p, miR-214-3p, miR-9-5p/ miR-146a-5p, miR-31-5p, miR-96-5p | ↑/↓ | DR VS HC | [92] |
| plasma | yes (lncRNA) | lncRNA DLX6-AS1/PRINS, FAM190A-3 | ↑/↓ | DR VS DM | [93] |
HC: healthy control; DM: DM without DR; VS: versus
3.2 EVs as diagnostic and prognostic biomarkers in DR
Growing evidence suggests that the regular fluctuation of EV quantities and cargos holds significant potential for providing predictors toward clinical diagnosis and prognosis in patients with DR. For example, a higher level of plasma EV numbers has been observed in PDR patients compared to those with NPDR or NDR [48]. The number of platelet-derived EVs and monocyte-derived EVs in plasma elevate with the progression of the DR [78, 79]. Moreover, an increased number of specific metabolites has been identified in the plasma EVs with progressive DR, suggesting the value of EVs contents in reflecting the progression of DR [80] (Table 1). The exploration of EVs as telltale indicators of DR is gaining more attention.
3.2.1 Protein cargos of EVs for the diagnosis of DR
In plasma of patients with DR, an observed correlation has been found between the increased number of CCR5-positive (CCR5+) EVs and the progression of DR [81]. Systematic analysis of proteomic profiles conducted by Xiao et al. [60] revealed the elevated expression of TNFAIP8 in the EVs derived from both plasma and vitreous humor in DR patients, suggesting that TNFAIP8 in plasma EVs could serve as a suitable biomarker of DR. Similarly, proteomic analysis of EVs derived from vitreous humor in the PDR patients has identified a series of differentially expressed proteins, including 4 upregulated proteins (immunoglobulin lambda-like polypeptide 1(IGLL1), triosephosphate isomerase 1 (TPI1), lactate dehydrogenase A (LDHA), apolipoprotein B(ApoB)) and 6 downregulated proteins (apolipoprotein M (ApoM), ficolin 3 (FCN3), aldolase C (ALDOC), serum amyloid protein (SAA) 2-4, insulin-like growth factor binding protein, acid-labile subunit (IGFALS), hyaluronan binding protein 2 (HABP2)), which show promise as candidates for PDR diagnosis. LDHA, FCN3, ApoB and ApoM have been further proved to be able to distinguish PDR patients from the normal controls in a cohort study with area under the curve (AUC) > 0.75 (0.777, 0.820, 0.910, 0.840) [82]. Interestingly, EVs present in urine also hold potential for identifying DR. In a study conducted by Mighty et al. [83], specific expression of junction plakoglobin (JUP) was revealed in EVs from the urine of DR patients, but not in those from healthy donors or diabetic patient without DR. Due to its non-invasiveness and easy availability, urine is considered as attractive source for biomarkers compared to vitreous humor, plasma, or serum. Therefore, further exploration of the application of urine EVs for DR diagnosis is warranted.
3.2.2 RNA cargos of EVs for the diagnosis of DR
The dysregulated miRNAs shuttled by EVs have been extensively recognized to offer diagnostic and/or prognostic information for type 2 diabetes mellitus (T2DM) and related cardiovascular complications [84], whose levels can also reflect the status of DR. Several studies focus on the alterations of circulating EV-miRNAs in biofluids from diabetic patients with/without retinopathy, as well as normal controls. Mazzeo et al. [85] discovered a specific miRNA signature in circulating EVs isolated from diabetic patients with retinopathy, which differed from both patients without complications and healthy controls, with 11 differentially expressed miRNAs identified. Upon further validation, they found that miR-150-5p, miR-21-3p, and miR-30b-5p not only involved in the pathological process of DR, but also play hold potential as biomarkers [86]. Another study showed that elevated expression of EV-miR-15a in plasma is correlated with initial retinal damage, suggesting its potential application in the early prevention of DR in patients with T2DM [87]. Similarly, miR-3976, miR-431-5p, and miR-26b-5p derived from serum EVs have been reported to be up-regulated in patients with DR [67, 88, 89], indicating their potential utility in monitoring the progression of DR. Tears, a valid source of biomarkers for ocular diseases, show aberrant proteomics or metabolomics alterations in DR [90, 91]. Moreover, EVs from tear samples have been isolated and performed transcriptomic analysis, which disclosed the upregulated miR-145-5p, miR-214-3p, and miR-9-5p and the downregulated miR-146a-5p, miR-31-5p, and miR-96-5p in diabetic patients with retinopathy compared with the control [92]. These dysregulated EV miRNAs offer solid indication for the disease-specific biomarker screen in DR diagnosis.
As the important regulator of miRNA, circRNA and lncRNA in DR patients also change specifically in DR pathogenesis. For instance, in DR patients there are increased levels of circMLKN1 in EVs from serum [47] and cPWWP2A in EVs secreted by pericytes [52], both of which mediate microvascular damage under diabetes-related stress. Xu et al. [62] separated serum EVs of healthy controls, DM individuals with NPDR, PDR, or without DR, and performed total RNA deep sequencing. They found aberrantly decreased levels of lncRNA PPT2-EGFL8 and significantly high expression of miR-423-5p due to competing endogenous RNA (ceRNA) mechanism. Another study analyzed lncRNAs in plasma EVs from individuals with T2DM and comorbid DR and identified lncRNA DLX6-AS1, PRINS, and FAM190A-3 as potential predictor of DR, with separate AUC values of 0.658, 0.798, and 0.603. Notably, the combination of DLX6-AS1 and PRINS obtained an AUC of 0.813, which even reached 0.860 in males [93].The construction of an EV-related ceRNA network in DR has revealed a series of aberrant expressed lncRNAs, miRNAs, and mRNAs [94], highlighting the broad prospects of vesicular RNAs in diagnosis applications.
The potential of EVs as non-invasive liquid biopsy has been further supported by clinical phase 1 trails conducted in various diseases, including in the diagnosis of DM [95]. As for DR, there is also promising evidence suggesting that EVs found in tear, vitreous humor, serum, plasma, and urine hold great potential as diagnostic and prognostic markers. However, in addition to comparing EVs between healthy individuals and patients with varying degrees of DR, demographic factors such as age, race, and sex should also be considered in clinical cohort studies, as they can significantly influence EV characteristics [96]. Furthermore, the availability of commercial kits and machines for EV capture and analysis plays a crucial role in facilitating the clinical translation of EV biomarkers. For instance, Pan et al. [80] reported that Fe3O4@TiO2 microbeads can efficiently and reversibly capture EVs from plasma (100 μL) within 20 min, compared to the hours required by traditional ultracentrifugation method. Another example is the iTEARS system developed by Hu et al. [92], which enables rapid isolation of EVs from small teardrop samples (less than 10 μL) with high yield and purity in only 5 min. These innovative EVs isolation methods have been successfully validated in DR patients, providing valuable insights into the pathological changes of EVs and highlighting the significant potential of EV biomarkers for clinical applications.
4. EV-based therapy in DR
Laser photocoagulation, intraocular administration of steroids and anti-VEGF agents, and vitrectomy are the most common used clinical treatment for DR, aimed at minimizing vision loss [97]. However, the limited efficacy and unavoidable side effects of these therapies remain unresolved [98]. Therefore, there is an active demand for new therapeutics. Emerging evidence suggests that EVs derived from various cell types, including MSCs, retinal cells, and immune cells, hold great promise in DR therapy (Figure 4).
4.1 MSCs-derived EVs
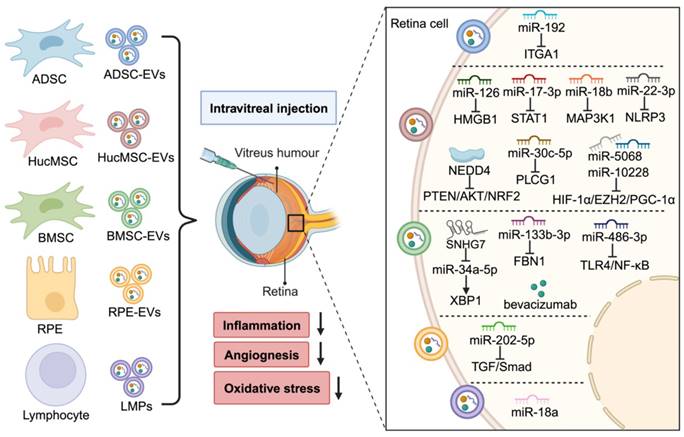
Due to their self-renewal and differentiation properties, MSCs are considered as promising candidates for tissue repair and regenerative medicine. EVs secreted from MSCs (MSC-EVs) have been widely studied as therapeutic molecules in both in vitro cell models and preclinical animal models of diabetes and its complications, including DR [99] (Table 2). Our previous research has shown that EVs from human umbilical cord MSCs (hucMSC-EVs) can reduce blood glucose levels, partially reverse peripheral insulin resistance, and alleviate β-cell destruction in a streptozotocin (STZ) induced T2DM rat model [100]. More recently, we have also found that intravitreal injection of hucMSC-EVs effectively improves retinal function, reduce retinal oxidative stress, apoptosis, inflammation, and angiogenesis in both leptin gene deficient db/db mice and STZ-induced diabetic rats. Mechanistically, miR-5068 and miR-10228, and neuronal precursor cell-expressed developmentally downregulated 4 (NEDD4) carried by hucMSC-EVs can modulate HIF-1α/EZH2/PGC-1α and PTEN/AKT/NRF2 signaling pathway separately [101, 102]. In the study by Zhang et al. [103], hucMSC-EVs treatment was shown to inhibit hyperglycemia induced retinal inflammation in both in vivo and in vitro DR models by transporting miR-126, a suppressor of the HMGB1 signaling pathway. Similarly, several studies have reported that hucMSC-EVs carrying specific miRNAs, such as miR-17-3p [104], miR-18b [105], miR-30c-5p [106], and miR-22-3p [107], have the ability to ameliorate inflammatory response and oxidative injury in DR mouse models via targeting STAT1, MAP3K1, PLCG1, and NLRP3 respectively. Moreover, intravitreal administration of hucMSC-EVs has also been shown to protect retinal ganglion cells from apoptosis, thereby reducing neurodegeneration (DRN) and improving the retinal structure in a DR rat model [108].
Bone marrow mesenchymal stem cells (BMSCs) are considered as a safe and effective treatment option for DR [109], and their EVs also show great potential for therapy. Li et al. [110] found that EVs from BMSCs (BMSC-EVs) can reduce oxidative stress and inflammation and protect the viability of HG-treated Müller cells via delivering miR-486-3p that repress TLR4/NF-κB signaling pathway. In HG-stimulated mouse retinal microvascular endothelial cells (mRMECs), BMSC-EVs loaded with miR-133b-3p have been demonstrated to reduce pathological angiogenesis and oxidative stress by targeting FBN1 [111].
Therapeutic function of MSC-EVs in DR
| Source of EVs | Therapeutic molecules | Animal models | EV dosage/per eye | Method of administration | Effect of EVs in vivo | Clinical / pre-clinical object | Cell models | Effect of EVs in vitro | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| hucMSCs | NEDD4 | STZ-induced diabetic rats | 1×106 particles | IVT | attenuate retinal apoptosis and oxidative stress | pre-clinical object | HG-treated RPEs | promote cell proliferation, reduce oxidative damage | [101] |
| hucMSCs | miR-5068, miR-10228 | STZ-induced diabetic rats and db/db mice | 1×107 particles | IVT | alleviate retinal apoptosis, inflammation, and angiogenesis | pre-clinical object | HG-treated hRMECs | mitigate cell proliferation, migration, and tube formation | [102] |
| hucMSCs | miR-126 | STZ-induced diabetic rats | 50 μg (RNA concentration) | IVT | alleviate retina inflammatory response | pre-clinical object | HG-treated hRMECs | reduce NLRP3 inflammasome | [103] |
| hucMSCs | miR-17-3p | STZ-induced diabetic mice | 50 μg | IVT | ameliorate retina inflammatory reaction and antioxidant injury | pre-clinical object | - | - | [104] |
| hucMSCs | miR-18b | STZ-induced diabetic rats | 10, 20, and 40 μg/mL | IVT | reduce inflammatory response and vascular leakage | pre-clinical object | HG-treated hRMECs | attenuate cell inflammation and apoptosis | [105] |
| hucMSCs | miR-30c-5p | STZ-induced diabetic rats | 50 μg | IVT | suppress retinal inflammatory response | pre-clinical object | HG-treated hRMECs | inhibit cell inflammation | [106] |
| hucMSCs | miR-22-3p | STZ-induced diabetic rats | 1.5 × 109 particles | IVT | alleviate retinal inflammation, improve histological morphology and BRB function | pre-clinical object | advanced glycation end-products-induced microglial cells | alleviate cell activation | [107] |
| hucMSCs | - | STZ-induced diabetic rats | 4 μL | IVT | improve retinal structure, reduce neurodegeneration | pre-clinical object | - | - | [108] |
| BMSC | miR-486-3p | STZ-induced diabetic mice | - | - | - | - | HG-treated Müller cells | inhibit cell oxidative stress, inflammation, and apoptosis, promote proliferation | [110] |
| BMSC | miR-133b-3p | KK/Upj-Ay mice | - | - | - | pre-clinical object | HG-treated mRMECs | reduce cell oxidative stress, angiogenesis, proliferation, migration, and promote apoptosis | [111] |
| BMSC | - | STZ-induced diabetic rats | 50 μg (Protein concentration) | IVT | inhibit retina oxidative stress, inflammation, and angiogenesis | pre-clinical object | - | - | [112] |
| BMSC | lncRNA SNHG7 | - | - | - | - | - | HG-treated hRMECs | suppress cell EndoMT and tube formation | [113] |
| ADSC | miR‐192 | STZ-induced diabetic rats | 5, 10, 20, and 40 μg/ml | IVT | alleviate inflammatory response and angiogenesis | pre-clinical object | HG-treated hRMECs, Müller cells, and RPEs | inhibit RPEs apoptosis, Müller cells activation, and hRMEC proliferation | [114] |
IVT: intravitreal injection; hucMSC: human umbilical cord MSCs; STZ: streptozotocin; BMSCs: bone marrow MSCs; mRMECs: mouse retinal microvascular endothelial cells; EndoMT: endothelial-to-mesenchymal transition; hRMECs: human retinal microvascular endothelial cells; ADSC: adipose MSC.
Similar results have been observed in a STZ induced DR rat model, whose retinal injury was alleviated by BMSC-EVs through the suppression of the Wnt/β-catenin signaling pathway, resulting in reduced oxidative stress, inflammatory response, and angiogenesis [112]. The BMSC-derived vesicular lncRNA SNHG7 could suppresses HG-triggered endothelial-to-mesenchymal transition (EndoMT) and tube formation in human retinal microvascular endothelial cells (hRMECs) by targeting miR-34a-5p/XBP1 axis [113]. Furthermore, EVs extracted from adipose mesenchymal stem cells (ADSC-EVs) exhibits similar benefits as BMSC-EVs in DR therapy. A study by Gu et al. [114] revealed that intravitreal administration of ADSC-EVs delivered miR‐192, which negatively regulates ITGA1, thus delaying the events of inflammation and angiogenesis. In another study of Reddy et al. [115], BMSC-EVs loaded with bevacizumab, an anti-VEGF antibody commonly used to treat DR, reduced retinal leakages, leukocytosis, and cell apoptosis in a rat model of DR. Moreover, the combination of EVs and bevacizumab prolongs the therapeutic effect from one month to two months, indicating the diverse application possibilities of MSC-EVs for DR treatment.
4.2 Retinal cells and immune cells derived EVs
RPEs are crucial for maintaining the integrity of the outer BRB and normal vision [25]. Despite that RPEs undergo pathological changes during the progression of DR, it has been suggested that RPEs derived EVs (RPE-EVs) may have a therapeutic role in DR. For example, EVs isolated from HG-stimulated RPEs are rich in miR-202-5p and can inhibit EndoMT of ECs under HG condition by targeting TGF/Smad pathway [116]. In addition, another research indicates that bevacizumab, when administered intravitreally, can be taken up by RPEs and then released though EVs, offering a novel mechanism by which therapeutic antibodies exert beneficial effects on distant non-target cells [117]. Photoreceptor cells are located in the outer retina and adjacent to RPEs, the later also have a significant impact on maintaining the normal structure and function of photoreceptor cells. Both RPEs and photoreceptor cells contribute to the progression of DR [118]. A recent study suggests that overexpression of Thioredoxin alleviates diabetics-induced degeneration of photoreceptor cells by mediating autophagy and secreting EVs, which can then be phagocytosed by RPEs and enhance their biological function during DR in vitro [119]. The EV-mediated communication between RPEs and photoreceptor cells may establish a beneficial feed-forward cycle in DR therapy.
Human T lymphocytes derived EVs (LMPs) have been reported to possess strong anti-angiogenic abilities in several ocular diseases [120-122]. LMPs-mediated inhibition of choroidal neovascularization has been proved to depend on the integrity of RPEs, which express increased levels of antiangiogenic factors (pigment epithelium-derived factor) and trigger the apoptosis of ECs by activating neurotrophin receptor p75 [120]. In the mouse model of laser-induced choroidal neovascularization, LMPs have been shown to target macrophages and prevent their proangiogenic polarization, leading to a downregulation of proangiogenic factors (e.g., VEGFa, interleukin-10) and an upregulation of antiangiogenic factors (e.g., interleukin-12, thrombospondin-1) [121]. The reduced pathological retinal neovascularization has also been observed in a mouse model of OIR after intravitreal injection of LMPs. Specifically, LMPs inhibit the proliferation of Müller cells and decrease VEGF expression, thereby attenuating the infiltration of proangiogenic macrophages [122]. A study by Yang et al. [123] analyzed the expression profiling of RNAs in LMPs and found selective enrichment of miR-18a, which inhibited retinal neovascularization in both in vitro and in vivo models of OIR. This discovery aids in identifying optimal targets and effective treatments to prevent abnormal neovascularization in DR.
Improving the circulation half-lives of anti-DR drugs is crucial to minimize side effects caused by frequent intraocular administration [124]. Utilizing EVs as a drug delivery platform is advantageous due to their specifical membrane-bound structure. The combination of drugs and EVs exerts a prolonged and better treatment benefit than either drug or native EVs administration alone [115]. However, when compared to conventional drugs, very few studies have reported the cellular uptake and vitreous pharmacokinetics of EVs after injection into retina. In a rat model of retinal ischemia, functional recovery and reduced neuro-damage can be observed after injection of BMSC-EVs into the vitreous humor 24h. These EVs have been found to be taken up by retinal neurons, ganglion cells, and microglia, and remain in the vitreous humor for four weeks [125]. However, another study found that the lifetime of EVs in rat vitreous humor is only 2.5 days. Moreover, EVs are mainly internalized by cells in the outer nuclear layer, with varying retention times in different cell types [126]. Thus, enhancing vitreous retention and targeting ability may be the key point for improving the therapeutic efficiency of EVs in DR. In a current paper by Bao et.al [127], BMSC-EVs were encapsulated within polymeric microcapsules, allowing for controlled release as the capsules gradually degrade. These microencapsulated EVs not only show enhanced biological stability but also demonstrate sustained release for more than one month following intravitreal injection in a mouse model of retinal ischemia-reperfusion injury. In EV therapy, in addition to being loaded with therapeutic cargos and combined with biomaterials, EVs membranes can also be modified to improve targeting properties and tracing abilities [128]. These engineering strategies address the limitations of native EVs, thereby expanding their potential as the next generation of nanomedicine for DR.
Therapeutic effects of EVs in DR. After intravitreal injection, MSC-EVs, RPE-EVs, and LMPs transfer various cargos and could alleviate DR progress by reducing inflammation, oxidative stress, and angiogenesis. Some research also loads the drug (e.g., bevacizumab) into EVs to improve the therapeutic effect. (Figure created with BioRender.com).
5. Perspectives and challenges
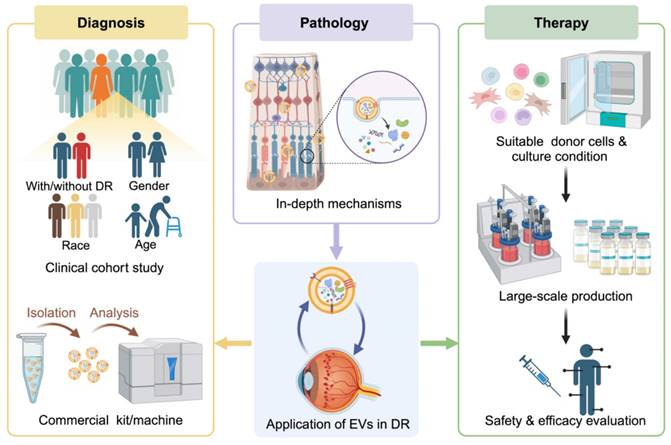
Although significant progress has been made in understanding the biological features of EVs and their specific roles in diabetic retina damage, many challenges impede the development of EV research, one of which is the heterogeneity of vesicles. Most of the EVs in published studies consist of mixed subpopulations and may even be accompanied by non-vesicular contaminants due to the technical barriers associated with standard EV isolation protocol [129, 130]. Conventional methods for isolating EVs, such as ultracentrifugation, ultrafiltration, and precipitation, often result in co-separation of lipoproteins or other contaminants that have similar physicochemical properties to EVs. These methods may even disrupt the structural and biological integrity of EVs due to external force [131]. While immunoaffinity capture technology offers the potential to isolate a particular subpopulation of EVs based on specific protein markers, its limited small-scale production cannot be ignored [132]. The “cocktail strategy”, which involves combination of multiple EV extraction protocols, is being widely researched to achieve high-purity and high-yield EV isolation in a shorter time frame [95]. Notably, advancements in technology have enabled the detection and analysis of EVs at the single-vesicle level, offering valuable insights into the study of specific EV subtypes [133]. Furthermore, thorough understanding of the complex origins, destination, and temporal dynamics of EVs in DR is limited by deficient in endogenous EV tracking system and imaging techniques that can operate across different scales. Recently, researchers have successfully constructed and utilized three-dimensional models of the retinal vasculature to investigate hyperoxia-induced vascular obliteration in a mouse model of OIR [134]. This achievement highlights the potential for visualizing EVs and elucidating their contributions within the three-dimensional structure of the retina, thereby advancing our understanding of DR mechanisms, and identifying potential biomarkers.
Given the inherent therapeutic advantages of native EVs, which can be further enhanced through engineered modifications [135], there is considerable anticipation for the clinical application of EVs as therapeutic agents for DR. However, there are still some potential issues that need to be addressed. Firstly, EVs derived from donor cells under different condition may possess distinct biological features, making it difficult to maintain the stability of treatment effects. As mentioned earlier, BMSC-EVs are generally considered to alleviate retinal damage. While EVs from BMSC cultured in diabetic-like condition (HG and/or hypoxia) have been shown to promote BRB permeability and angiogenesis in vitro [136, 137]. Therefore, more efforts should be made to explore the most suitable origin and culture condition for parental cells of therapeutic EVs. To meet the requirements for clinical administration, technologies for subsequent large-scale production of EVs, such as bioreactors that contain a dynamic monitoring system and maximized cell culture surface, are also popular directions worth investigating [138]. Secondly, the complex nature of bioactive molecules in EVs poses challenges in fully understanding their functions. The presence of unknown contents increases the risk of potential unintended side effects during clinical administration. A comprehensive safety evaluation is imperative prior to clinical administration. (Figure 5).
Taken together, this review summarizes the key characteristics of EVs and DR, and highlight the research advances of the emerging roles played by EVs in the context of DR. Accumulating evidence strongly supports the notion that EVs, acting as important mediators of intercellular and inter-tissue communication, are crucial participants in the onset and progression of DR. Moreover, their potential as biomarkers and therapeutic interventions for DR is increasingly recognized. With advancements in basic research, EVs will find broad applications in the diagnosis and treatment of DR and other diabetic complications in the future.
The potential future development of EVs as biomarkers and therapy agents in DR. Continued advancements in basic research will enhance our understanding of the involvement of EVs in the progression of DR, opening avenues for the development of new targets for disease diagnosis and treatment. However, there are still several key issues that require further exploration before the biomedical application and clinical translation of EVs can be realized.
Abbreviations
DR: diabetic retinopathy; DM: diabetes mellitus; EVs: extracellular vesicles; VEGF: vascular endothelial growth factor; MVs: microvesicles; MVB: multivesicular bodies; NVEPs: non-vesicular extracellular nanoparticles; sEVs: small EVs; lEVs: large EVs; MSCs: mesenchymal stem cells; NPDR: non-proliferative DR; PDR: proliferative DR; DME: diabetic macular edema; BRB: blood-retina barrier; RPEs: retinal pigment epithelium cells; BM: Bruch's membrane; ECs: endothelial cells; PB: peripheral blood; PRP: platelet-rich plasma; MAC: membrane attack complex; HIF-1α: hypoxia-inducible factor-1alpha; EPO: erythropoietin; EPOR: EPO receptor; HG: high glucose; VEGFR: VEGF receptors; OIR: oxygen-induced retinopathy; RGC-5: retinal ganglion cells 5; AD-EVs: EVs from astrocytes; BBB: blood-brain-barrier; CCR5+: CCR5-positive; AUC: area under the curve; JUP: junction plakoglobin; T2DM: type 2 diabetes mellitus; ceRNA: competing endogenous RNA; MSC-EVs: EVs secreted from MSCs; hucMSC: human umbilical cord MSCs; STZ: streptozotocin; BMSCs: bone marrow MSCs; mRMECs: mouse retinal microvascular endothelial cells; EndoMT: endothelial-to-mesenchymal transition; hRMECs: human retinal microvascular endothelial cells; ADSC: adipose MSC; LMPs: human T lymphocytes derived EVs.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 82272179, 81971757); Technology Development Project of Jiangsu University (Grant no. 20230593); Zhenjiang Key Laboratory of High Technology Research on Exosomes Foundation and Transformation Application (Grant no. SS2018003) and Priority Academic Program Development of Jiangsu Higher Education Institutions (Phase IV).
Author contributions
HQ and WRX conceptualized and reviewed the paper. JYZ and JH drafted the manuscript and prepared the figures and tables. YXS assisted the manuscript preparation. All authors reviewed and approved the final manuscript.
Consent for publication
All authors gave their consent for publication.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB. et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119
2. Yang Z, Tan TE, Shao Y, Wong TY, Li X. Classification of diabetic retinopathy: Past, present and future. Front Endocrinol (Lausanne). 2022;13:1079217
3. Lin KY, Hsih WH, Lin YB, Wen CY, Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig. 2021;12:1322-5
4. Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018 19
5. Jeppesen DK, Zhang Q, Franklin JL, Coffey RJ. Extracellular vesicles and nanoparticles: emerging complexities. Trends Cell Biol. 2023;33:667-81
6. van de Wakker SI, Meijers FM, Sluijter JPG, Vader P. Extracellular vesicle heterogeneity and its impact for regenerative medicine applications. Pharmacol Rev. 2023;75:1043-61
7. Dyball LE, Smales CM. Exosomes: Biogenesis, targeting, characterization and their potential as "Plug & Play" vaccine platforms. Biotechnol J. 2022;17:e2100646
8. Ashoub MH, Salavatipour MS, Kasgari FH, Valandani HM, Khalilabadi RM. Extracellular microvesicles: biologic properties, biogenesis, and applications in leukemia. Mol Cell Biochem. 2023 [Epub ahead of print]
9. Yu L, Zhu G, Zhang Z, Yu Y, Zeng L, Xu Z. et al. Apoptotic bodies: bioactive treasure left behind by the dying cells with robust diagnostic and therapeutic application potentials. J Nanobiotechnology. 2023;21:218
10. Tan X, He S, Wang F, Li L, Wang W. Migrasome, a novel organelle, differs from exosomes. Biochem Biophys Rep. 2023;35:101500
11. Jiang Y, Liu X, Ye J, Ma Y, Mao J, Feng D. et al. Migrasomes, a new mode of intercellular communication. Cell Commun Signal. 2023;21:105
12. Zhang Q, Jeppesen DK, Higginbotham JN, Graves-Deal R, Trinh VQ, Ramirez MA. et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat Cell Biol. 2021;23:1240-54
13. Tosar JP, Cayota A, Witwer K. Exomeres and Supermeres: monolithic or diverse? J Extracell Biol. 2022;1:e45
14. Rother N, Yanginlar C, Pieterse E, Hilbrands L, van der Vlag J. Microparticles in autoimmunity: cause or consequence of disease? Front Immunol. 2022;13:822995
15. Bjørge IM, Kim SY, Mano JF, Kalionis B, Chrzanowski W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine - a new paradigm for tissue repair. Biomater Sci. 2017;6:60-78
16. Faridnia R, Kalani H, Hezarjaribi HZ, Denny PW, Rafie A, Fakhar M. et al. Apoptotic blebs from Leishmania major-infected macrophages as a new approach for cutaneous leishmaniasis vaccination. Microb Pathog. 2020;147:104406
17. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750
18. Ni P, Yang L, Li F. Exercise-derived skeletal myogenic exosomes as mediators of intercellular crosstalk: a major player in health, disease, and exercise. J Physiol Biochem. 2023;79:501-10
19. Wu P, Zhang B, Ocansey DKW, Xu W, Qian H. Extracellular vesicles: A bright star of nanomedicine. Biomaterials. 2021;269:120467
20. Mead B, Tomarev S. Extracellular vesicle therapy for retinal diseases. Prog Retin Eye Res. 2020;79:100849
21. Grading diabetic retinopathy from stereoscopic color fundus photographs-an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786-806
22. Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL. et al. Diabetic retinopathy: a position statement by the american diabetes association. Diabetes Care. 2017;40:412-8
23. Robles-Osorio ML, Sabath E. Tight junction disruption and the pathogenesis of the chronic complications of diabetes mellitus: A narrative review. World J Diabetes. 2023;14:1013-26
24. Dervenis P, Dervenis N, Smith JM, Steel DH. Anti-vascular endothelial growth factors in combination with vitrectomy for complications of proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2023;5:Cd008214
25. O'Leary F, Campbell M. The blood-retina barrier in health and disease. Febs j. 2023;290:878-91
26. Zhu S, Xie T, Lv Z, Leng YB, Zhang YQ, Xu R. et al. Hierarchies in visual pathway: functions and inspired artificial vision. Adv Mater. 2023: e2301986.
27. Gardner TW, Abcouwer SF, Barber AJ, Jackson GR. An integrated approach to diabetic retinopathy research. Arch Ophthalmol. 2011;129:230-5
28. Park YG, Lee JY, Kim C, Park YH. Early microglial changes associated with diabetic retinopathy in rats with streptozotocin-induced diabetes. J Diabetes Res. 2021;2021:4920937
29. Reichenbach A, Bringmann A. Glia of the human retina. Glia. 2020;68:768-96
30. Oshitari T. The pathogenesis and therapeutic approaches of diabetic neuropathy in the retina. Int J Mol Sci. 2021;22:9050
31. Kalargyrou AA, Basche M, Hare A, West EL, Smith AJ, Ali RR. et al. Nanotube-like processes facilitate material transfer between photoreceptors. EMBO Rep. 2021;22:e53732
32. Wooff Y, Cioanca AV, Chu-Tan JA, Aggio-Bruce R, Schumann U, Natoli R. Small-medium extracellular vesicles and their miRNA cargo in retinal health and degeneration: mediators of homeostasis, and vehicles for targeted gene therapy. Front Cell Neurosci. 2020;14:160
33. Risner ML, Ribeiro M, McGrady NR, Kagitapalli BS, Chamling X, Zack DJ. et al. Neutral sphingomyelinase inhibition promotes local and network degeneration in vitro and in vivo. Cell Commun Signal. 2023;21:305
34. Andrés-Blasco I, Gallego-Martínez A, Machado X, Cruz-Espinosa J, Di Lauro S, Casaroli-Marano R. et al. Oxidative stress, inflammatory, angiogenic, and apoptotic molecules in proliferative diabetic retinopathy and diabetic macular edema patients. Int J Mol Sci. 2023;24:8227
35. Cypryk W, Nyman TA, Matikainen S. From inflammasome to exosome-does extracellular vesicle secretion constitute an inflammasome-dependent immune response? Front Immunol. 2018;9:2188
36. Xia X, Wang Y, Zheng JC. Extracellular vesicles, from the pathogenesis to the therapy of neurodegenerative diseases. Transl Neurodegener. 2022;11:53
37. Wozniak AL, Adams A, King KE, Dunn W, Christenson LK, Hung WT. et al. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J Cell Biol. 2020;219:e201912074
38. Sekar R, Wooff Y, Cioanca AV, Kurera M, Ngo C, Man SM. et al. Impairing Gasdermin D-mediated pyroptosis is protective against retinal degeneration. J Neuroinflammation. 2023;20:239
39. Hernandez BJ, Skiba NP, Plössl K, Strain M, Liu Y, Grigsby D. et al. Polarized desmosome and hemidesmosome shedding via small extracellular vesicles is an early indicator of outer blood-retina barrier dysfunction. J Extracell Biol. 2023;2:e116
40. Jung KH, Chu K, Lee ST, Bahn JJ, Kim JH, Kim M. et al. Risk of macrovascular complications in type 2 diabetes mellitus: endothelial microparticle profiles. Cerebrovasc Dis. 2011;31:485-93
41. Tokarz A, Szuścik I, Kuśnierz-Cabala B, Kapusta M, Konkolewska M, Żurakowski A. et al. Extracellular vesicles participate in the transport of cytokines and angiogenic factors in diabetic patients with ocular complications. Folia Med Cracov. 2015;55:35-48
42. Feng Q, Stork CJ, Xu S, Yuan D, Xia X, LaPenna KB. et al. Increased circulating microparticles in streptozotocin-induced diabetes propagate inflammation contributing to microvascular dysfunction. J Physiol. 2019;597:781-98
43. Zhang W, Dong X, Wang T, Kong Y. Exosomes derived from platelet-rich plasma mediate hyperglycemia-induced retinal endothelial injury via targeting the TLR4 signaling pathway. Exp Eye Res. 2019;189:107813
44. Huang C, Fisher KP, Hammer SS, Navitskaya S, Blanchard GJ, Busik JV. Plasma exosomes contribute to microvascular damage in diabetic retinopathy by activating the classical complement pathway. Diabetes. 2018;67:1639-49
45. Huang C, Fisher KP, Hammer SS, Busik JV. Extracellular vesicle-induced classical complement activation leads to retinal endothelial cell damage via MAC deposition. Int J Mol Sci. 2020;21:1693
46. Kamalden TA, Macgregor-Das AM, Kannan SM, Dunkerly-Eyring B, Khaliddin N, Xu Z. et al. Exosomal microRNA-15a transfer from the pancreas augments diabetic complications by inducing oxidative stress. Antioxid Redox Signal. 2017;27:913-30
47. Yang J, Tan C, Wang Y, Zong T, Xie T, Yang Q. et al. The circRNA MKLN1 regulates autophagy in the development of diabetic retinopathy. Biochim Biophys Acta Mol Basis Dis. 2023;1869:166839
48. Su Y, Chen J, Dong Z, Zhang Y, Ma R, Kou J. et al. Procoagulant activity of blood and endothelial cells via phosphatidylserine exposure and microparticle delivery in patients with diabetic retinopathy. Cell Physiol Biochem. 2018;45:2411-20
49. Gu L, Xu H, Zhang C, Yang Q, Zhang L, Zhang J. Time-dependent changes in hypoxia- and gliosis-related factors in experimental diabetic retinopathy. Eye (Lond). 2019;33:600-9
50. Bahrami B, Hong T, Gilles MC, Chang A. Anti-VEGF therapy for diabetic eye diseases. Asia Pac J Ophthalmol (Phila). 2017;6:535-45
51. Huang H. Pericyte-endothelial interactions in the retinal microvasculature. Int J Mol Sci. 2020;21:7413
52. Liu C, Ge HM, Liu BH, Dong R, Shan K, Chen X. et al. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc Natl Acad Sci U S A. 2019;116:7455-64
53. Ye L, Guo H, Wang Y, Peng Y, Zhang Y, Li S. et al. Exosomal circEhmt1 released from hypoxia-pretreated pericytes regulates high glucose-induced microvascular dysfunction via the NFIA/NLRP3 pathway. Oxid Med Cell Longev. 2021;2021:8833098
54. Alli-Shaik A, Qiu B, Lai SL, Cheung N, Tan G, Neo SP. et al. System-wide vitreous proteome dissection reveals impaired sheddase activity in diabetic retinopathy. Theranostics. 2022;12:6682-704
55. Li Y, Chai JL, Shi X, Feng Y, Li JJ, Zhou LN. et al. Gαi1/3 mediate Netrin-1-CD146-activated signaling and angiogenesis. Theranostics. 2023;13:2319-36
56. Atienzar-Aroca S, Flores-Bellver M, Serrano-Heras G, Martinez-Gil N, Barcia JM, Aparicio S. et al. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J Cell Mol Med. 2016;20:1457-66
57. Atienzar-Aroca S, Serrano-Heras G, Freire Valls A, Ruiz de Almodovar C, Muriach M, Barcia JM. et al. Role of retinal pigment epithelium-derived exosomes and autophagy in new blood vessel formation. J Cell Mol Med. 2018;22:5244-56
58. Maisto R, Oltra M, Vidal-Gil L, Martínez-Gil N, Sancho-Pellúz J, Filippo CD. et al. ARPE-19-derived VEGF-containing exosomes promote neovascularization in HUVEC: the role of the melanocortin receptor 5. Cell Cycle. 2019;18:413-24
59. Liu Y, Yang Q, Fu H, Wang J, Yuan S, Li X. et al. Müller glia-derived exosomal miR-9-3p promotes angiogenesis by restricting sphingosine-1-phosphate receptor S1P(1) in diabetic retinopathy. Mol Ther Nucleic Acids. 2022;27:491-504
60. Xiao J, Zhang H, Yang F, Xiao M, Zhou L, Yu R. et al. Proteomic analysis of plasma sEVs reveals that TNFAIP8 is a new biomarker of cell proliferation in diabetic retinopathy. J Proteome Res. 2021;20:1770-82
61. Wang P, Li C, Deng Y, Yu Q, Meng X, Jiang T. et al. Effect of plasma-derived extracellular vesicles on angiogenesis and the ensuing proliferative diabetic retinopathy through a miR-30b-dependent mechanism. Diabetol Metab Syndr. 2022;14:188
62. Xu Z, Yang J, Zheng H, Xie T, Yang Q, Cai J. et al. Long noncoding RNA PPT2-EGFL8 regulates pathological retinal neovascularization in PDR by functioning as a competing endogenous RNA. Diabetes. 2023;72:1012-27
63. Luo Q, Jiang Z, Jiang J, Wan L, Li Y, Huang Y. et al. Tsp-1(+) microglia attenuate retinal neovascularization by maintaining the expression of Smad3 in endothelial cells through exosomes with decreased miR-27a-5p. Theranostics. 2023;13:3689-706
64. Antonetti DA, Silva PS, Stitt AW. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat Rev Endocrinol. 2021;17:195-206
65. Wolfram J, Ferrari M. Clinical cancer nanomedicine. Nano Today. 2019;25:85-98
66. Zhang W, Jiang H, Kong Y. Exosomes derived from platelet-rich plasma activate YAP and promote the fibrogenic activity of Müller cells via the PI3K/Akt pathway. Exp Eye Res. 2020;193:107973
67. Yang S, Zhang J, Zeng T, Zheng J, Min J, Chen L. Role of circulating Exosomal miRNA-3976 in early diabetic retinopathy. Int J Nanomedicine. 2023;18:3695-709
68. Chaudhuri AD, Dastgheyb RM, Yoo SW, Trout A, Talbot CC Jr, Hao H. et al. TNFα and IL-1β modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis. 2018;9:363
69. Datta Chaudhuri A, Dasgheyb RM, DeVine LR, Bi H, Cole RN, Haughey NJ. Stimulus-dependent modifications in astrocyte-derived extracellular vesicle cargo regulate neuronal excitability. Glia. 2020;68:128-44
70. Oyarce K, Cepeda MY, Lagos R, Garrido C, Vega-Letter AM, Garcia-Robles M. et al. Neuroprotective and neurotoxic effects of glial-derived exosomes. Front Cell Neurosci. 2022;16:920686
71. Ibáñez F, Montesinos J, Ureña-Peralta JR, Guerri C, Pascual M. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte-derived extracellular vesicles. J Neuroinflammation. 2019;16:136
72. Dickens AM, Tovar YRLB, Yoo SW, Trout AL, Bae M, Kanmogne M. et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10:eaai7696
73. Haydinger CD, Oliver GF, Ashander LM, Smith JR. Oxidative stress and its regulation in diabetic retinopathy. Antioxidants. 2023;12:1649
74. Zhu L, Zang J, Liu B, Yu G, Hao L, Liu L. et al. Oxidative stress-induced RAC autophagy can improve the HUVEC functions by releasing exosomes. J Cell Physiol. 2020;235:7392-409
75. Liu J, Zhang Y, Tian Y, Huang W, Tong N, Fu X. Integrative biology of extracellular vesicles in diabetes mellitus and diabetic complications. Theranostics. 2022;12:1342-72
76. Elliott RO, He M. Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics. 2021;13:122
77. Iannotta D, A A, Kijas AW, Rowan AE, Wolfram J. Entry and exit of extracellular vesicles to and from the blood circulation. Nat Nanotechnol. 2024;19:13-20
78. Ogata N, Imaizumi M, Nomura S, Shozu A, Arichi M, Matsuoka M. et al. Increased levels of platelet-derived microparticles in patients with diabetic retinopathy. Diabetes Res Clin Pract. 2005;68:193-201
79. Ogata N, Nomura S, Shouzu A, Imaizumi M, Arichi M, Matsumura M. Elevation of monocyte-derived microparticles in patients with diabetic retinopathy. Diabetes Res Clin Pract. 2006;73:241-8
80. Pan Y, Chen T, Zhang Q, Cao L, Wang S, Cai J. et al. Highly selective purification of plasma extracellular vesicles using titanium dioxide microparticles for depicting the metabolic signatures of diabetic retinopathy. Anal Chem. 2022;94:14099-108
81. Tokarz A, Konkolewska M, Kuśnierz-Cabala B, Maziarz B, Hanarz P, Żurakowski A. et al. Retinopathy severity correlates with RANTES concentrations and CCR 5-positive microvesicles in diabetes. Folia Med Cracov. 2019;59:95-112
82. Wang J, Wang Z, Zhang Y, Li J. Proteomic analysis of vitreal exosomes in patients with proliferative diabetic retinopathy. Eye (Lond). 2023;37:2061-8
83. Mighty J, Rubio-Navarro A, Shi C, Zhou J, Flores-Bellver M, Heissel S. et al. Extracellular vesicles of human diabetic retinopathy retinal tissue and urine of diabetic retinopathy patients are enriched for the junction plakoglo bin protein. Front Endocrinol (Lausanne). 2022;13:1077644
84. Prattichizzo F, Matacchione G, Giuliani A, Sabbatinelli J, Olivieri F, de Candia P. et al. Extracellular vesicle-shuttled miRNAs: a critical appraisal of their potential as nano-diagnostics and nano-therapeutics in type 2 diabetes mellitus and its cardiovascular complications. Theranostics. 2021;11:1031-45
85. Mazzeo A, Beltramo E, Lopatina T, Gai C, Trento M, Porta M. Molecular and functional characterization of circulating extracellular vesicles from diabetic patients with and without retinopathy and healthy subjects. Exp Eye Res. 2018;176:69-77
86. Mazzeo A, Lopatina T, Gai C, Trento M, Porta M, Beltramo E. Functional analysis of miR-21-3p, miR-30b-5p and miR-150-5p shuttled by extracellular vesicles from diabetic subjects reveals their association with diabetic retinopathy. Exp Eye Res. 2019;184:56-63
87. Sangalli E, Tagliabue E, Sala L, Prattichizzo F, Uccellatore A, Spada D. et al. Circulating microRNA-15a associates with retinal damage in patients with early stage type 2 diabetes. Front Endocrinol (Lausanne). 2020;11:254
88. Yu B, Xiao M, Yang F, Xiao J, Zhang H, Su L. et al. MicroRNA-431-5p encapsulated in serum extracellular vesicles as a biomarker for proliferative diabetic retinopathy. Int J Biochem Cell Biol. 2021;135:105975
89. Zhang Y, Wei J, Zhang L, Jiang G, Wang B, Jiang L. Extracellular vesicle-derived miR-26b-5p is up-regulated in the serum of patients with diabetic retinopathy. Comb Chem High Throughput Screen. 2022;25:877-82
90. Amorim M, Martins B, Caramelo F, Gonçalves C, Trindade G, Simão J. et al. Putative biomarkers in tears for diabetic retinopathy diagnosis. Front Med (Lausanne). 2022;9:873483
91. Wen X, Ng TK, Liu Q, Wu Z, Zhang G, Zhang M. Azelaic acid and guanosine in tears improve discrimination of proliferative from non-proliferative diabetic retinopathy in type-2 diabetes patients: A tear metabolomics study. Heliyon. 2023;9:e16109
92. Hu L, Zhang T, Ma H, Pan Y, Wang S, Liu X. et al. Discovering the secret of diseases by incorporated tear exosomes analysis via rapid-isolation system: iTEARS. ACS Nano. 2022;16:11720-32
93. Ye Q, Li L, Shao Z, Xu M, Li L, Yan Q. et al. Association between lncRNAs in plasma exosomes and diabetic retinopathy. Front Endocrinol (Lausanne). 2022;13:987488
94. Wang T, Cheng MY, Shan MY, Tang CY, Pan NJ, Xu ZH. et al. Construction of a competitive endogenous RNA network related to exosomes in diabetic retinopathy. Comb Chem High Throughput Screen. 2023;26:576-88
95. Jia Y, Yu L, Ma T, Xu W, Qian H, Sun Y. et al. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics. 2022;12:6548-75
96. Noren Hooten N, Byappanahalli AM, Vannoy M, Omoniyi V, Evans MK. Influences of age, race, and sex on extracellular vesicle characteristics. Theranostics. 2022;12:4459-76
97. Wong TY, Cheung CMG, Larsen M, Sharma S, Simó R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012
98. Levine SR, Sapieha P, Dutta S, Sun JK, Gardner TW. It is time for a moonshot to find "Cures" for diabetic retinal disease. Prog Retin Eye Res. 2022;90:101051
99. Zhang J, Zheng Y, Huang L, He J. Research progress on mesenchymal stem cells for the treatment of diabetes and its complications. Int J Endocrinol. 2023;2023:9324270
100. Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B. et al. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018;12:7613-28
101. Sun F, Sun Y, Zhu J, Wang X, Ji C, Zhang J. et al. Mesenchymal stem cells-derived small extracellular vesicles alleviate diabetic retinopathy by delivering NEDD4. Stem Cell Res Ther. 2022;13:293
102. Sun F, Sun Y, Wang X, Zhu J, Chen S, Yu Y. et al. Engineered mesenchymal stem cell-derived small extracellular vesicles for diabetic retinopathy therapy through HIF-1α/EZH2/PGC-1α pathway. Bioact Mater. 2024;33:444-59
103. Zhang W, Wang Y, Kong Y. Exosomes derived from mesenchymal stem cells modulate miR-126 to ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1. Invest Ophthalmol Vis Sci. 2019;60:294-303
104. Li W, Jin LY, Cui YB, Xie N. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-17-3p ameliorates inflammatory reaction and antioxidant injury of mice with diabetic retinopathy via targeting STAT1. Int Immunopharmacol. 2021;90:107010
105. Xu Z, Tian N, Li S, Li K, Guo H, Zhang H. et al. Extracellular vesicles secreted from mesenchymal stem cells exert anti-apoptotic and anti-inflammatory effects via transmitting microRNA-18b in rats with diabetic retinopathy. Int Immunopharmacol. 2021;101:108234
106. He Y, Zhang Z, Yao T, Huang L, Gan J, Lv H. et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells relieves diabetic retinopathy through a microRNA-30c-5p-dependent mechanism. Diabetes Res Clin Pract. 2022;190:109861
107. Chen Y, Yao G, Tong J, Xie H, Zheng X, Zhang H. et al. MSC-derived small extracellular vesicles alleviate diabetic retinopathy by delivering miR-22-3p to inhibit NLRP3 inflammasome activation. Stem Cells. 2024;42:64-75
108. Fu Y, Xie TH, Zhang YL, Gu ZH. The effect of human umbilical cord mesenchymal stem cell-derived exosomes on diabetic retinal neurodegeneration in a rat model. J Chem Neuroanat. 2022;126:102181
109. Gu X, Yu X, Zhao C, Duan P, Zhao T, Liu Y. et al. Efficacy and safety of autologous bone marrow mesenchymal stem cell transplantation in patients with diabetic retinopathy. Cell Physiol Biochem. 2018;49:40-52
110. Li W, Jin L, Cui Y, Nie A, Xie N, Liang G. Bone marrow mesenchymal stem cells-induced exosomal microRNA-486-3p protects against diabetic retinopathy through TLR4/NF-κB axis repression. J Endocrinol Invest. 2021;44:1193-207
111. Liang G, Qin Z, Luo Y, Yin J, Shi Z, Wei R. et al. Exosomal microRNA-133b-3p from bone marrow mesenchymal stem cells inhibits angiogenesis and oxidative stress via FBN1 repression in diabetic retinopathy. Gene Ther. 2022;29:710-9
112. Ebrahim N, El-Halim HEA, Helal OK, El-Azab NE, Badr OAM, Hassouna A. et al. Effect of bone marrow mesenchymal stem cells-derived exosomes on diabetes-induced retinal injury: Implication of Wnt/ b-catenin signaling pathway. Biomed Pharmacother. 2022;154:113554
113. Cao X, Xue LD, Di Y, Li T, Tian YJ, Song Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021;272:119232
114. Gu C, Zhang H, Gao Y. Adipose mesenchymal stem cells-secreted extracellular vesicles containing microRNA-192 delays diabetic retinopathy by targeting ITGA1. J Cell Physiol. 2021;236:5036-51
115. Reddy SK, Ballal AR, Shailaja S, Seetharam RN, Raghu CH, Sankhe R. et al. Small extracellular vesicle-loaded bevacizumab reduces the frequency of intravitreal injection required for diabetic retinopathy. Theranostics. 2023;13:2241-55
116. Gu S, Liu Y, Zou J, Wang W, Wei T, Wang X. et al. Retinal pigment epithelial cells secrete miR-202-5p-containing exosomes to protect against proliferative diabetic retinopathy. Exp Eye Res. 2020;201:108271
117. Aboul Naga SH, Dithmer M, Chitadze G, Kabelitz D, Lucius R, Roider J. et al. Intracellular pathways following uptake of bevacizumab in RPE cells. Exp Eye Res. 2015;131:29-41
118. Tonade D, Kern TS. Photoreceptor cells and RPE contribute to the development of diabetic retinopathy. Prog Retin Eye Res. 2021;83:100919
119. Ren X, Lv J, Wang N, Liu J, Gao C, Wu X. et al. Thioredoxin upregulation delays diabetes-induced photoreceptor cell degeneration via AMPK-mediated autophagy and exosome secretion. Diabetes Res Clin Pract. 2022;185:109788
120. Tahiri H, Yang C, Duhamel F, Omri S, Picard E, Chemtob S. et al. p75 neurotrophin receptor participates in the choroidal antiangiogenic and apoptotic effects of T-lymphocyte-derived microparticles. Invest Ophthalmol Vis Sci. 2013;54:6084-92
121. Tahiri H, Omri S, Yang C, Duhamel F, Samarani S, Ahmad A. et al. Lymphocytic microparticles modulate angiogenic properties of macrophages in laser-induced choroidal neovascularization. Sci Rep. 2016;6:37391
122. Cai C, Tahiri H, Fortin C, Ortiz C, Sintjago H, Yang C. et al. Lymphocytic microparticles suppress retinal angiogenesis via targeting Müller cells in the ischemic retinopathy mouse model. Exp Cell Res. 2021;399:112470
123. Yang C, Tahiri H, Cai C, Gu M, Gagnon C, Hardy P. MicroRNA-181a inhibits ocular neovascularization by interfering with vascular endothelial growth factor expression. Cardiovasc Ther. 2018;36:e12329
124. Moon CH, Lee AJ, Jeon HY, Kim EB, Ha KS. Therapeutic effect of ultra-long-lasting human C-peptide delivery against hyperglycemia-induced neovascularization in diabetic retinopathy. Theranostics. 2023;13:2424-38
125. Mathew B, Ravindran S, Liu X, Torres L, Chennakesavalu M, Huang CC. et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. 2019;197:146-60
126. Mathew B, Torres LA, Gamboa Acha L, Tran S, Liu A, Patel R. et al. Uptake and distribution of administered bone marrow mesenchymal stem cell extracellular vesicles in retina. Cells. 2021;10:730
127. Bao H, Tian Y, Wang H, Ye T, Wang S, Zhao J. et al. Exosome-loaded degradable polymeric microcapsules for the treatment of vitreoretinal diseases. Nat Biomed Eng. 2023 [Epub ahead of print]
128. Sun Y, Sun F, Xu W, Qian H. Engineered extracellular vesicles as a targeted delivery platform for precision therapy. Tissue Eng Regen Med. 2023;20:157-75
129. Veerman RE, Teeuwen L, Czarnewski P, Güclüler Akpinar G, Sandberg A, Cao X. et al. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J Extracell Vesicles. 2021;10:e12128
130. van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23:369-82
131. Taylor DD, Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3-10
132. Zhang Q, Jeppesen DK, Higginbotham JN, Franklin JL, Coffey RJ. Comprehensive isolation of extracellular vesicles and nanoparticles. Nat Protoc. 2023;18:1462-87
133. He D, Wang H, Ho SL, Chan HN, Hai L, He X. et al. Total internal reflection-based single-vesicle in situ quantitative and stoichiometric analysis of tumor-derived exosomal microRNAs for diagnosis and treatment monitoring. Theranostics. 2019;9:4494-507
134. Chang CC, Chu A, Meyer S, Ding Y, Sun MM, Abiri P. et al. Three-dimensional imaging coupled with topological quantification uncovers retinal vascular plexuses undergoing obliteration. Theranostics. 2021;11:1162-75
135. Li M, Fang F, Sun M, Zhang Y, Hu M, Zhang J. Extracellular vesicles as bioactive nanotherapeutics: An emerging paradigm for regenerative medicine. Theranostics. 2022;12:4879-903
136. Beltramo E, Lopatina T, Berrone E, Mazzeo A, Iavello A, Camussi G. et al. Extracellular vesicles derived from mesenchymal stem cells induce features of diabetic retinopathy in vitro. Acta Diabetol. 2014;51:1055-64
137. Mazzeo A, Beltramo E, Iavello A, Carpanetto A, Porta M. Molecular mechanisms of extracellular vesicle-induced vessel destabilization in diabetic retinopathy. Acta Diabetol. 2015;52:1113-9
138. Kimiz-Gebologlu I, Oncel SS. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533-43
Author contact
![]() Corresponding authors: Wenrong Xu, iclsedu.cn (ORCID: 0000-0003-0903-1973); Hui Qian, qianhuiedu.cn (ORCID: 0000-0002-0098-3196).
Corresponding authors: Wenrong Xu, iclsedu.cn (ORCID: 0000-0003-0903-1973); Hui Qian, qianhuiedu.cn (ORCID: 0000-0002-0098-3196).
 Global reach, higher impact
Global reach, higher impact