13.3
Impact Factor
Theranostics 2024; 14(4):1583-1601. doi:10.7150/thno.92848 This issue Cite
Research Paper
MAGL protects against renal fibrosis through inhibiting tubular cell lipotoxicity
1. State Key Laboratory of Organ Failure Research, National Clinical Research Center of Kidney Disease, Guangdong Provincial Clinical Research Center for Kidney Disease, Guangdong Provincial Key Laboratory of Nephrology, Division of Nephrology, Nanfang Hospital, Southern Medical University, Guangzhou, China.
2. Department of Health Care, Zhongshan Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, China.
3. Nephrology Department, The First People's Hospital of Foshan, Foshan, China.
4. Division of Nephrology, Department of medicine, The Fifth Affiliated Hospital Sun Yat-Sen University, Zhuhai, China.
5. School of Pharmaceutical Sciences and School of Biomedical Engineering, Southern Medical University, Guangzhou, China.
*These authors contributed equally: Shan Zhou, Xian Ling, Jielin Zhu, Ye Liang, Qijian Feng, Chao Xie.
Abstract
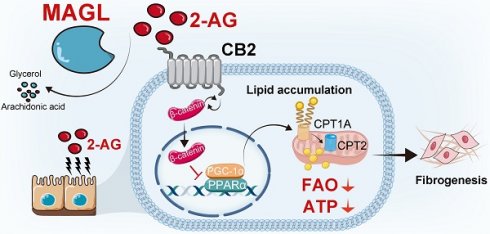
Rationale: Renal fibrosis, with no therapeutic approaches, is a common pathological feature in various chronic kidney diseases (CKD). Tubular cell injury plays a pivotal role in renal fibrosis. Commonly, injured tubular cells exhibit significant lipid accumulation. However, the underlying mechanisms remain poorly understood.
Methods: 2-arachidonoylglycerol (2-AG) levels in CKD patients and CKD model specimens were measured using mass spectrometry. 2-AG-loaded nanoparticles were infused into unilateral ureteral obstruction (UUO) mice. Lipid accumulation and renal fibrosis were tested. Furthermore, monoacylglycerol lipase (MAGL), the hydrolyzing enzyme of 2-AG, was assessed in CKD patients and models. Tubular cell-specific MAGL knock-in mice were generated. Moreover, MAGL recombination protein was also administered to unilateral ischemia reperfusion injury (UIRI) mice. Besides, a series of methods including RNA sequencing, metabolomics, primary cell culture, lipid staining, etc. were used.
Results: 2-AG was increased in the serum or kidneys from CKD patients and models. Supplement of 2-AG further induced lipid accumulation and fibrogenesis through cannabinoid receptor type 2 (CB2)/β-catenin signaling. β-catenin knockout blocked 2-AG/CB2-induced fatty acid β-oxidation (FAO) deficiency and lipid accumulation. Remarkably, MAGL significantly decreased in CKD, aligning with lipid accumulation and fibrosis. Specific transgene of MAGL in tubular cells significantly preserved FAO, inhibited lipid-mediated toxicity in tubular cells, and finally retarded fibrogenesis. Additionally, supplementation of MAGL in UIRI mice also preserved FAO function, inhibited lipid accumulation, and protected against renal fibrosis.
Conclusion: MAGL is a potential diagnostic marker for kidney function decline, and also serves as a new therapeutic target for renal fibrosis through ameliorating lipotoxicity.
Keywords: MAGL, lipotoxicity, renal fibrosis, 2-AG, FAO
 Global reach, higher impact
Global reach, higher impact