13.3
Impact Factor
Theranostics 2024; 14(4):1430-1449. doi:10.7150/thno.90945 This issue Cite
Research Paper
Enhancing anti-tumor potential: low-intensity vibration suppresses osteosarcoma progression and augments MSCs' tumor-suppressive abilities
1. Department of Pharmacology, School of Pharmacy, Harbin Medical University, Harbin 150081, China.
2. Department of Biomedical Engineering, Indiana University Purdue University Indianapolis, Indianapolis, IN 46202, USA.
3. Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907, USA.
4. Department of Physics, Indiana University Purdue University Indianapolis, Indianapolis, IN 46202, USA.
5. Department of Mechanical Engineering, Pusan National University, Busan 46241, Korea.
6. Department of Mechanical Engineering, Ulsan College, Ulsan 44022, Korea.
7. Indiana University Simon Comprehensive Cancer Center, Indiana University School of Medicine; Indianapolis, IN 46202, USA.
8. Department of Pediatrics, Indiana University School of Medicine; Indianapolis, IN 46202, USA.
9. Department of Pediatric Hematology and Oncology, Indiana University School of Medicine; Indianapolis, IN 46202, USA.
10. Department of Orthopaedic Surgery, Indiana University School of Medicine; Indianapolis, IN 46202, USA.
11. Indiana Center for Musculoskeletal Health, Indiana University School of Medicine; Indianapolis, IN 46202, USA.
12. Department of Medical and Molecular Genetics, Indiana University School of Medicine, Indianapolis, IN 46202 USA.
13. Department of Physical Therapy, Indiana University, Indianapolis, IN 46202, USA.
Abstract
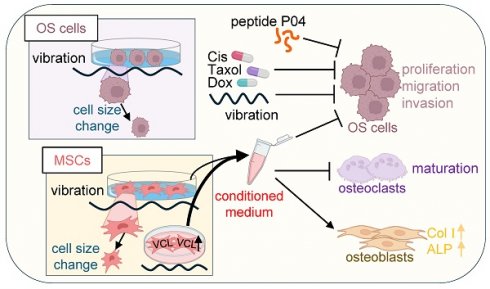
Rationale: Osteosarcoma (OS), a common malignant bone tumor, calls for the investigation of novel treatment strategies. Low-intensity vibration (LIV) presents itself as a promising option, given its potential to enhance bone health and decrease cancer susceptibility. This research delves into the effects of LIV on OS cells and mesenchymal stem cells (MSCs), with a primary focus on generating induced tumor-suppressing cells (iTSCs) and tumor-suppressive conditioned medium (CM).
Methods: To ascertain the influence of vibration frequency, we employed numerical simulations and conducted experiments to determine the most effective LIV conditions. Subsequently, we generated iTSCs and CM through LIV exposure and assessed the impact of CM on OS cells. We also explored the underlying mechanisms of the tumor-suppressive effects of LIV-treated MSC CM, with a specific focus on vinculin (VCL). We employed cytokine array, RNA sequencing, and Western blot techniques to investigate alterations in cytokine profiles, transcriptomes, and tumor suppressor proteins.
Results: Numerical simulations validated LIV frequencies within the 10-100 Hz range. LIV induced notable morphological changes in OS cells and MSCs, confirming its dual role in inhibiting OS cell progression and promoting MSC conversion into iTSCs. Upregulated VCL expression enhanced MSC responsiveness to LIV, significantly bolstering CM's efficacy. Notably, we identified tumor suppressor proteins in LIV-treated CM, including procollagen C endopeptidase enhancer (PCOLCE), histone H4 (H4), peptidylprolyl isomerase B (PPIB), and aldolase A (ALDOA). Consistently, cytokine levels decreased significantly in LIV-treated mouse femurs, and oncogenic transcript levels were downregulated in LIV-treated OS cells. Moreover, our study demonstrated that combining LIV-treated MSC CM with chemotherapy drugs yielded additive anti-tumor effects.
Conclusions: LIV effectively impeded the progression of OS cells and facilitated the transformation of MSCs into iTSCs. Notably, iTSC-derived CM demonstrated robust anti-tumor properties and the augmentation of MSC responsiveness to LIV via VCL. Furthermore, the enrichment of tumor suppressor proteins within LIV-treated MSC CM and the reduction of cytokines within LIV-treated isolated bone underscore the pivotal tumor-suppressive role of LIV within the bone tumor microenvironment.
Keywords: vibration, osteosarcoma, MSCs, iTSCs, conditioned medium, glycolytic enzymes
 Global reach, higher impact
Global reach, higher impact