13.3
Impact Factor
Theranostics 2024; 14(3):1260-1288. doi:10.7150/thno.89380 This issue Cite
Review
Understanding AAV vector immunogenicity: from particle to patient
1. Gene and Stem Cell Therapy Program Centenary Institute, The University of Sydney, NSW, Australia.
2. Faculty of Medicine and Health, The University of Sydney, NSW, Australia.
3. Pfizer Inc, Walton on the Hill, Surrey, UK.
4. Pfizer Australia, Sydney, NSW, Australia.
5. Pfizer Inc, New York, NY, USA.
6. Cell and Molecular Therapies, Royal Prince Alfred Hospital, Sydney, NSW, Australia.
Received 2023-8-22; Accepted 2023-12-4; Published 2024-1-20
Abstract
Gene therapy holds promise for patients with inherited monogenic disorders, cancer, and rare genetic diseases. Naturally occurring adeno-associated virus (AAV) offers a well-suited vehicle for clinical gene transfer due to its lack of significant clinical pathogenicity and amenability to be engineered to deliver therapeutic transgenes in a variety of cell types for long-term sustained expression. AAV has been bioengineered to produce recombinant AAV (rAAV) vectors for many gene therapies that are approved or in late-stage development. However, ongoing challenges hamper wider use of rAAV vector-mediated therapies. These include immunity against rAAV vectors, limited transgene packaging capacity, sub-optimal tissue transduction, potential risks of insertional mutagenesis and vector shedding. This review focuses on aspects of immunity against rAAV, mediated by anti-AAV neutralizing antibodies (NAbs) arising after natural exposure to AAVs or after rAAV vector administration. We provide an in-depth analysis of factors determining AAV seroprevalence and examine clinical approaches to managing anti-AAV NAbs pre- and post-vector administration. Methodologies used to quantify anti-AAV NAb levels and strategies to overcome pre-existing AAV immunity are also discussed. The broad adoption of rAAV vector-mediated gene therapies will require wider clinical appreciation of their current limitations and further research to mitigate their impact.
Keywords: neutralizing antibodies, humoral immunity, seroprevalence, patient exclusion, redosing
Introduction
Momentum has been increasing recently for gene therapy approvals worldwide following many years of setbacks [1, 2]. A range of vectors (delivery vehicles, or carriers of therapeutic genes) have been explored for in vivo gene therapy designed to deliver genes of interest (transgenes) to target tissues. As adeno-associated virus (AAV) lacks significant clinical pathogenicity [3-5], it provides an ideal foundation for clinical gene transfer based on a recombinant AAV (rAAV) vector [6]. rAAV can package and deliver transgenes to a wide variety of dividing and non-dividing cells and maintain stable, and potentially long-term, transgene expression (particularly in less rapidly- or non-dividing cells including the liver, retina and central nervous system) [3, 7]. Further, AAV has a relatively low immunogenicity [3, 5] and optimized production protocols allowing for high-titer and high-purity manufacturing are available for clinical use [8, 9] (Table 1). Importantly, as the rAAV genome is predominantly maintained episomally in cells, complications related to insertional mutagenesis inherent to some other viral vectors used in clinical gene therapy are minimized [3, 4].
Characteristics, benefits, and challenges of adeno-associated virus-based vectors for gene therapy.
| Characteristics | Benefits | Challenges |
|---|---|---|
| • Small, non-enveloped, ~4.7-kb single-stranded DNA genome [11, 31] • Genus Dependoparvovirus [11] • Belongs to the Parvoviridae family [11] | • Lack of significant clinical pathogenicity [3] • Predominantly non-integrating (rAAV vector integration into the target cell genome can occur at a rate of 0.1-1% of total transduction) [28, 122] • Ability to package different transgenes [4] • Ability to transduce a wide variety of dividing and non-dividing, terminally differentiated tissues, driving long-term transgene expression [7] • Inefficient at transducing antigen-presenting cells [14] • Exhibits a low immunogenicity profile [3, 5] • Optimized production protocols enable high-titer and high-purity manufacturing [8, 9] | • Capsid triggers a dose-dependent immune response [89] • AAV genomes can persist for years in host cells, either episomally or integrated within the host DNA, and be reactivated by a helper virus, such as adenovirus, herpesvirus, human papillomavirus, and vaccinia virus [14, 31] • Due to the broad cross-reactivity between AAV serotypes, NAbs recognizing most serotypes can be found in the majority of subjects [14, 31] |
AAV, adeno-associated virus; NAb, neutralizing antibody; rAAV, recombinant adeno-associated virus
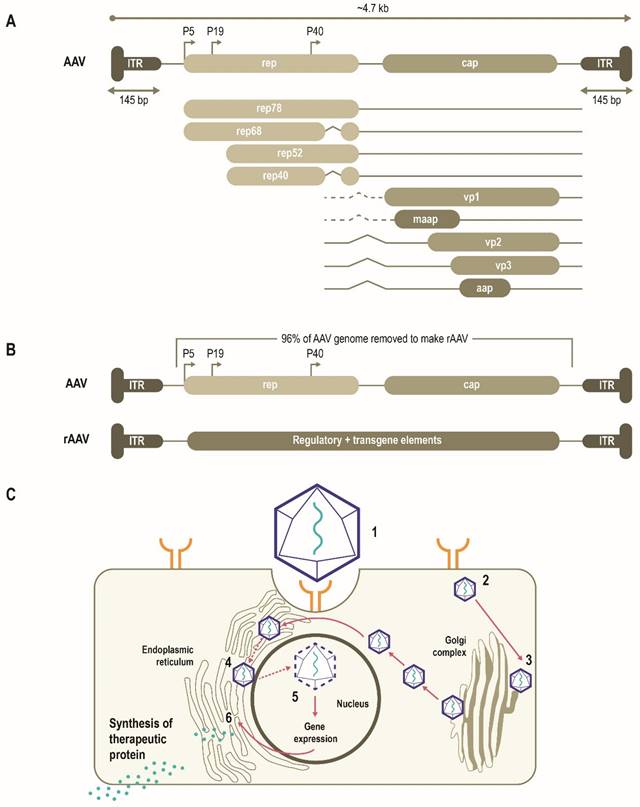
AAVs consist of a single-stranded DNA (ssDNA) genome of ~4.7 Kb enclosed by a capsid ~26 nm in diameter (Figure 1A) [10, 11]. The AAV genome is flanked by two T-shaped hairpin structures - inverted terminal repeats (ITRs) [10, 11]. Overlapping genes with alternative splicing and multiple translation initiation sites allow for efficient use of the AAV genome and result in three capsid proteins (Cap), four replication proteins (Rep) as well as an assembly-activating protein that facilitates virion assembly in some serotypes [11]. Together, these three genes mediate genome replication and packaging, capsid production, and integration [10, 11]. More recently, an additional gene product coding for a membrane-associated accessory protein (MAAP) has been identified that facilitates AAV egress and encapsulation [12, 13].
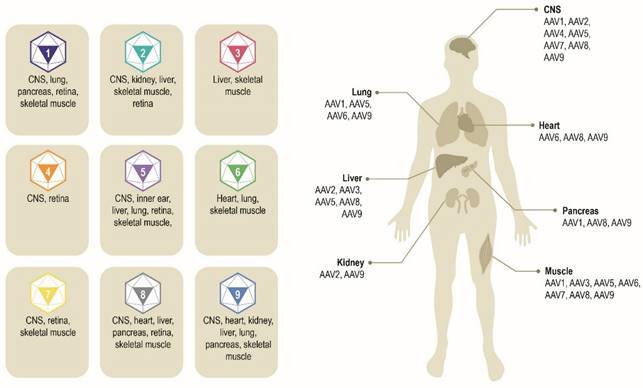
Multiple AAV serotypes exist with the same overall genome structure [4, 10, 14, 15] and over 80% homology in nucleotide sequence [16]. The difference between serotypes is primarily within the amino acid sequence of their capsid proteins [14, 17]. It is the differences in the capsid sequence and the presence of receptors or co-receptors on the host cell that determine cell/tissue tropism of a particular AAV serotype (Figure 2) [10, 14, 18, 19]. AAV2 is the most common serotype and is close in amino acid sequence similarity to all AAV serotypes except AAV4 and AAV5 [17, 20]. A comparison of phylogenies from human and non-human primate AAV serotypes revealed that human AAV4 and AAV5 serotypes are the most divergent, while the other human serotypes (AAV1, AAV6, AAV2, AAV3, and AAV9), unique non-human primate serotypes (AAV7), or serotypes found in both human and non-human primates (AAV8) clustered in groups [17, 21]. AAV1, AAV2, and AAV3 are closely related to each other, with 83% to 93% homology at the amino acid level [20]. AAV4 and AAV5 are more distantly related to AAV1, AAV2, and AAV3 based on the amino acid sequence of viral proteins VP1/VP3 ranging from 51% to 63% sequence identity for AAV4 [20] and 51% to 59% for AAV5 [20, 22, 23]. AAV6 is a hybrid of AAV1 and AAV2, with 99% homology to AAV1 [16, 24].
AAV has been bioengineered to produce rAAV vectors to target diverse monogenic disorders, and many therapies are either approved or in late-stage development (Table 2). In a rAAV vector, the AAV genome is replaced by a transgene expression cassette that includes a specialized promoter, transgene of interest, and transcriptional terminator, flanked with ITRs (Figure 1B) [11, 25]. In addition to accommodating a transgene expression cassette, this replacement of viral coding sequences contributes to lower immunogenicity and cytotoxicity (Table 1) [11]. The tissue tropism of the resultant rAAV is largely determined by the capsid used with a range of natural or engineered capsids available to target different tissues [11, 14, 25]. The selectivity and efficiency of transduction can be further enhanced by optimizing the transgene expression cassette through improved codon usage, CpG-depletion or with the inclusion of tissue- or cell-selective regulatory elements [11, 26, 27]. AAV enters cells through receptor-mediated endocytosis (Figure 1B) [10, 11, 28]. AAV then traffics through the endosomal and Golgi compartments and, after endosomal escape, undergoes nuclear transport and uncoating [10, 11]. The single-stranded AAV genome converts into a double-stranded genome through the activity of cellular DNA polymerase [10, 11]. AAV requires a helper virus (e.g., adenovirus, herpes simplex) to facilitate gene expression and viral replication [10, 11]. In the presence of a helper virus, AAV undergoes productive infection characterized by genome replication, viral gene expression, and virion production [25]. Without helper virus, the AAV can establish latency by undergoing integration into the adeno-associated virus integration site 1 (AAVS1) on chromosome 19, and also at other chromosome locations mediated by the Rep protein [25, 29]. Currently, no confirmed cases of rAAV-mediated genotoxic events have been reported in humans to date. However, due to some reports of insertional mutagenesis in murine studies, research on genomic integration of rAAV is of active interest [29, 30].
Schematic representation of AAV genome, the AAV life cycle and the engineering of a generic rAAV expression cassette [10]. (A) AAVs consist of a single-stranded DNA genome of ~4.7 Kb enclosed by a capsid ~26 nm in diameter. The AAV genome contains three open reading frames flanked by two T-shaped inverted terminal repeats (ITRs). The cap, rep, MAAP and AAP of the AAV genome encode three capsid proteins, four replication proteins, the membrane-associated accessory protein (MAAP) that facilitates AAV egress and encapsulation and the assembly-activating protein (AAP) that facilitates capsid assembly in some serotypes. (B) In rAAV, the AAV genome is replaced by a transgene expression cassette, including a specialized promoter, enhancer, transgene of interest, and terminator, flanked with ITRs. (C) rAAV vectors transduce cells through (1) binding to cell surface receptors and/or co-receptors), followed by (2) internalization by endocytosis. AAV then (3) traffics through the endosomal and Golgi compartment and after endosomal escape, undergoes (4) nuclear transport and (5) uncoats releasing the genome, which is then (6) expressed. Relative size representations are not to scale.
Exemplar AAV serotypes and their preferential tissue tropism [6, 14, 195]. Differences in the capsid sequence and the presence of specific host cell receptor(s) determine cell/tissue tropism of exemplar AAV serotypes. AAV, adeno-associated virus. CNS, central nervous system.
AAV therapies approved or in late-stage development
| Name | Indication | AAV vector | Company | Status |
|---|---|---|---|---|
| Voretigene neparvovec | Retinal dystrophy | AAV2 | Spark Therapeutics | Approved in Europe [123]/Approved in USA [124] |
| Onasemnogene abeparvovec | Spinal muscular atrophy | AAV9 | Novartis | Approved in Europe [125]/Approved in USA [126] |
| Valoctocogene roxaparvovec | Hemophilia A | AAV5 | BioMarin | Approved in Europe [127]/ Biologics License Application resubmission in progress in USA [128] |
| Etranacogene dezaparvovec | Hemophilia B | AAV5 | UniQure | Conditional approval in Europe [129]/Approved in USA [130] |
| Giroctocogene fitelparvovec | Hemophilia A | AAV2/6 | Pfizer | Phase 3 clinical: NCT03861273 [131] |
| Fidanacogene elaparvovec | Hemophilia B | AAVRh74var (also known as AAV-Spark100) | Pfizer | Phase 3 clinical: NCT03861273 [132] |
| Lenadogene nolparvovec | Leber's congenital amaurosis type 2 | rAAV2/2-ND4 | GenSight Biologics | Phase 3 clinical: NCT02652767, [133] NCT02652780, [134] NCT03406104, [135] NCT03293524 [136] |
| Delandistrogene moxeparvovec | Duchenne muscular dystrophy | AAVRh74 | Serepta Therapeutics | Approved in USA [137] |
| Eladocagene exuparvovec | Aromatic L-amino acid decarboxylase (AADC) deficiency | AAV2 | PTC Therapeutics | Approved in Europe [138] |
AAV, adeno-associated virus; rAAV2, recombinant adeno-associated virus 2.
Challenges for rAAV gene delivery
The Humoral Immune Response
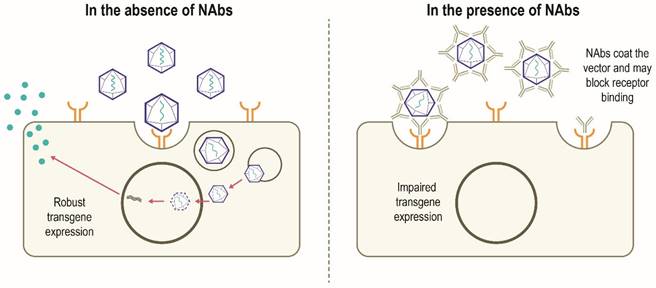
Although AAV possesses several features to make it an attractive gene therapy vector, there are challenges to be overcome in the development of widespread successful AAV-mediated therapies (Table 1). Despite having a low immunogenicity profile [3, 5], rAAV vectors can stimulate host antiviral immune responses directed against the capsid and/or the encoded transgene product, particularly when delivered systemically or at higher vector doses [14, 31]. Anti-AAV neutralizing antibodies (NAbs) can develop following exposure to naturally occurring AAVs, with increased population seropositivity correlating with age [32-34]. Passive transfer of maternal anti-AAV antibodies also occurs [33, 35]. A high degree of homology between AAV capsids allows these NAbs to cross-react with different capsids, including those used for gene therapy [14, 36]. A humoral immune response against the capsid is triggered in patients who have received gene therapy, which subsequently results in the neutralization of cross-reactive rAAVs administered [6, 14]. In this scenario, NAbs may coat the rAAV vector and 'block' its binding to the receptor(s) on the target cell and thus prevents attachment and cell entry. Alternatively, NAbs may inhibit the interaction of viral envelope protein and cell-surface receptors after the vector has bound to the cell or otherwise inhibit transduction, thereby limiting the efficacy of gene therapy. It is also possible that the NAb-coated vector binds to the cellular receptor and is internalized but productive transduction is inhibited. Alternatively, the NAb itself may bind to the cell receptor and block the rAAV binding and internalization (Figure 3) [37]. However, it has been suggested that some AAV serotypes may be less sensitive to antibody neutralization, with partial resistance to neutralization observed in recent trials of hemophilia B gene therapy using AAV5 [38-40]. NAbs may also impact vector biodistribution, directing the AAV vector away from target cells towards secondary lymphoid organs [41]. The level of anti-AAV NAbs depends on diverse patient- and therapy-related factors, including patient demographics, disease state, capsid type, transgene, and dose [14]. Approaches to minimize the effect of humoral immunity against AAV are being explored, but significant hurdles remain.
In addition to pre-existing anti-AAV NAbs that impact the initial transduction of rAAV, host cellular immune responses may reduce the long-term durability of the transgene expression [42, 43]. T-cell-mediated immunity post-cellular entry may include recognition of the capsid by toll-like receptor (TLR) 2 at the cell surface and stimulation of TLR9-mediated innate immunity. The degradation and presentation of Cap on major histocompatibility complex (MHC) class I molecules may result in the clearance of transduced cells by capsid-specific cytotoxic CD8+ T cells [44] and presentation of capsid proteins on MHC class II molecules [11, 42]. In turn, the MHC class II complex may be recognized by CD4+ T lymphocytes, resulting in the secretion of interleukins and stimulation of B lymphocytes [42]. This can lead to an anti-capsid humoral response [28, 42], thereby reducing long-term persistence of the therapy or preventing successful vector re-administration.
Seroprevalence of NAbs
Considering the prevalence of AAV infection, cross-reactivity of NAbs, and potential for NAb-mediated inhibition of rAAV-mediated gene therapy, an understanding of the seroprevalence of AAV NAbs is important. Seroprevalence has been studied in healthy individuals as well as in patients potentially amenable to gene therapy. Seroprevalence profiles for AAV serotypes 1 to 9 and bioengineered capsids in humans vary by geography, race, and age as well as assay methodologies and their sensitivities (Table 3). It is important to note that the seroprevalence studies discussed here focus largely on AAV. Therefore, caution with respect to the direct applicability of these data to specific rAAVs used in gene therapy trials is warranted.
Canonical mechanism of action of NAbs [37, 83]. In the absence of pre-existing NAbs, robust transgene expression is possible. In the presence of NAbs, developed following previous exposure to AAV, NAbs bind to the AAV vector and may prevent binding to the target cell receptor which can block vector transduction and transgene expression. The NAb may also inhibit the interaction of viral envelope protein and cell-surface receptors after the vector has bound to the cell. Other mechanisms by which NAbs impair transgene expression not shown include NAb-coated vector binds to the cellular receptor and is internalized and subsequently destroyed intracellularly; the NAbs bind to the cell receptor and block the rAAV binding and subsequent internalization. NAb, neutralizing antibody; rAAV, recombinant adeno-associated virus.
Seroprevalence of NAbs against AAVs in healthy subjects
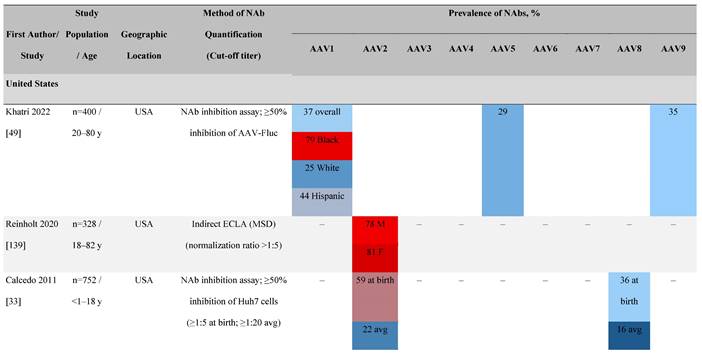
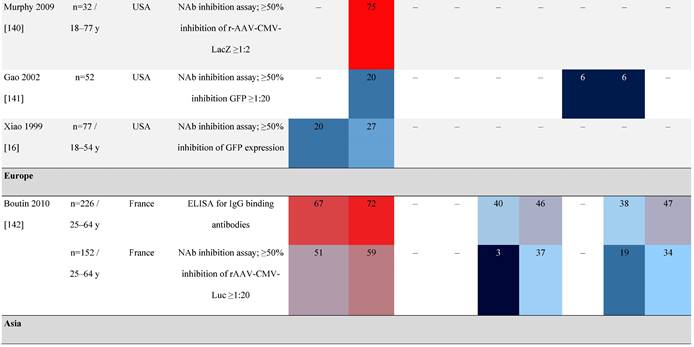
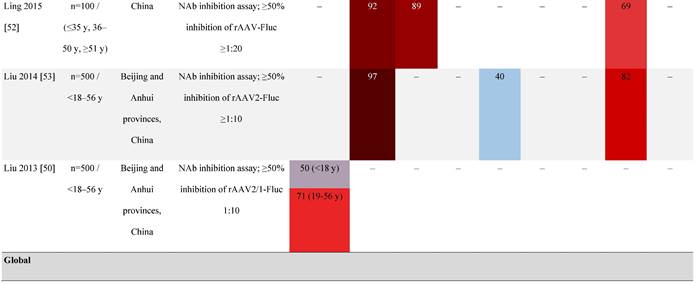
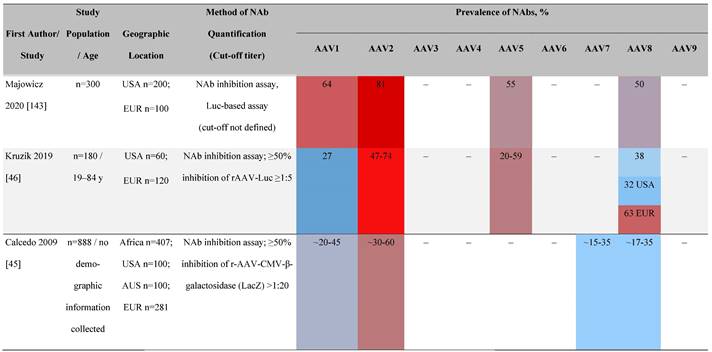
Prevalence color coding: 
AAV, adeno-associated virus; AUS, Australia; avg, average across all ages; CMV, cytomegalovirus; ECLA, electrochemiluminescence assay; ELISA, enzyme-linked immunosorbent assay; EUR, Europe; F, female; Fluc, firefly luciferase; GFP, green fluorescent protein; IgG, immunoglobulin G; LacZ, β-galactosidase; Luc, luciferase; M, male; MSD, Meso Scale Discovery; NAb, neutralizing antibody; rAAV, recombinant adeno-associated virus; S Africa, South Africa.
Geographical differences in seroprevalence
One large population-based study of healthy volunteers in 10 countries across four regions [45] showed anti-AAV2 NAbs were the most prevalent antibodies in all regions (30%-60%), followed by anti-AAV1, anti-AAV7, and anti-AAV8 NAbs [45]. The prevalence of anti-AAV1, anti-AAV7, and anti-AAV8 NAbs was lower in the United States compared with other countries [45]. In another study of healthy volunteers in the United States and Europe, the prevalence of anti-AAV2 and anti-AAV8 NAbs was high although some regional variations were noted [46]. Another observational, retrospective study of participants from 10 countries (Australia, Canada, France, Germany, Italy, Japan, South Korea, Spain, United Kingdom, and United States) who were enrolled in non-gene therapy trials showed that anti-AAV1 NAbs were the most prevalent (74.9%), followed by AAV6 (70.1%) and AAV5 (63.9%) [47]. However, prevalence varied by dilution used in the assay, with AAV5 having the lowest seroprevalence [47]. Overall, the prevalence of all AAV serotypes studied was highest in South Korea and lowest in Japan, Australia, and the United States [47].
Considerable geographic variability across serotypes has also been observed in individuals with hemophilia A, particularly for anti-AAV5 NAbs. In one study (N=546) across nine countries, AAV5 consistently exhibited the lowest seroprevalence of all serotypes [32]. The global weighted average (factoring in the country specific prevalence of hemophilia A) of AAV5 seroprevalence was 29.7%, with seropositivity rates ranging from 5.9% in the United Kingdom to 51.8% in South Africa. Another study showed the prevalence of anti-AAV5 NAbs ranged from 21% in the United States to 47% in Russia [48].
Racial differences in seroprevalence
In a study in the United States of healthy donors who self-identified as belonging to a race, prevalence of NAbs to most AAV serotypes was higher among Black and Hispanic donors compared with those who self-reported as White [49]. The study of non-gene therapy trial participants from 10 countries showed a higher seroprevalence in Asians compared with non-Asian participants [47].
Age differences in seroprevalence
Increases in the seroprevalence of anti-AAV NAbs have been observed with age both in healthy individuals [50, 51] and in a number of disease states [32, 34, 47, 51]. One study examining anti-AAV NAb seroprevalence from birth to adolescence in the United States (N=752) reported that anti-AAV2 and anti-AAV8 NAbs were higher in neonates (59% and 36%, respectively) but declined substantially in infants aged 7 to 11 months and then increased again in children and adolescents aged 3 to 18 years [33]. Interestingly, in a smaller study of patients with mucopolysaccharidosis (MPS) III and healthy individuals, the seroprevalence differed between cohorts. NAbs against all AAV serotypes were higher in healthy children aged 8 to 15 years than in those aged 2 to 7 years, whereas in children with MPS III the opposite was observed, with seroprevalence peaking before 8 years of age [34].
It is important to note that not all studies have reported an association between age and seroprevalence. For example, no age-related differences in AAV2, AAV3, AAV8, or AAVLK03 were noted in a smaller study of healthy Chinese participants (N=100) ranging in age from ≤35, 36 to 50, and ≥51 years [52].
Gender differences in seroprevalence
Some studies have proposed that the prevalence of anti-AAV NAbs can be influenced by gender. The prevalence of anti-AAV1, anti-AAV5, and anti-AAV8 NAbs was significantly higher in healthy Chinese women than male counterparts [53]. Similarly, another study found higher level of anti-AAV9 NAbs in adult females [47]. However, not all studies have reported significant gender differences in anti-AAV1 NAbs [54]. Intraracial differences have also been observed between genders, with White women and Hispanic men more seropositive compared with their gender counterparts [49].
Differences in seroprevalence according to disease state
The seroprevalence of NAbs against AAV also varies across disease states relative to healthy populations (Table 4). Evidence suggests that seropositivity in patients may be affected by the nature of the disease and/or treatment received. There are inconsistencies in exact rates across studies that could be explained by differences in study design and implementation (i.e., assay type: see section 'Preparing for rAAV gene therapy: screening for anti-AAV NAbs' [55]), geographic location, or other factors such as age, but seroprevalence trends are similar [32, 45, 47]. For example, anti-AAV2 NAbs were the most prevalent antibodies across disease states, including hemophilia [56], cystic fibrosis [57], rheumatoid arthritis [58], and primary Sjögren syndrome [59]. Furthermore, the prevalence of anti-AAV NAbs increased with age in patients with hemophilia A and cystic fibrosis [56, 57, 60].
Seroprevalence of NAbs against AAVs in patients with a monogenic disorder
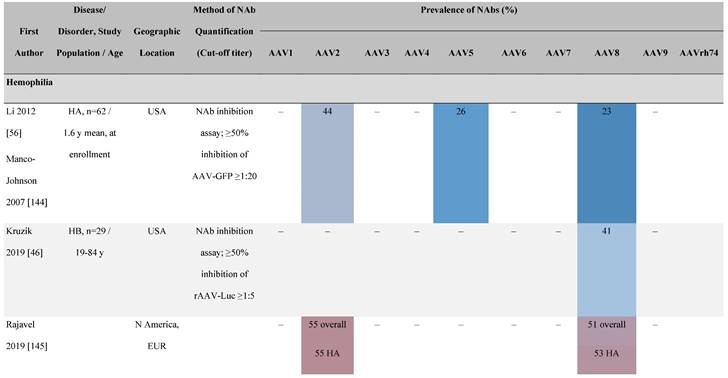
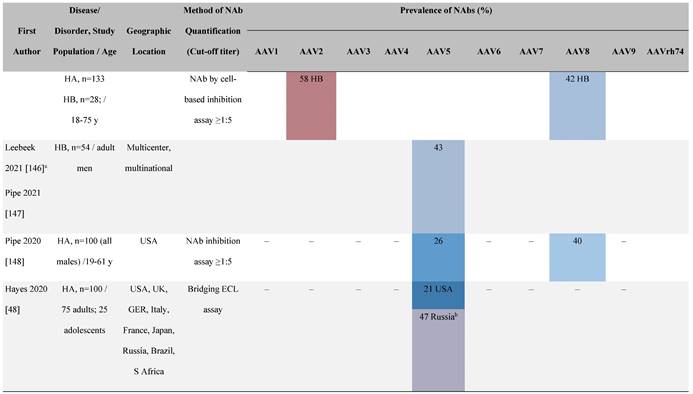
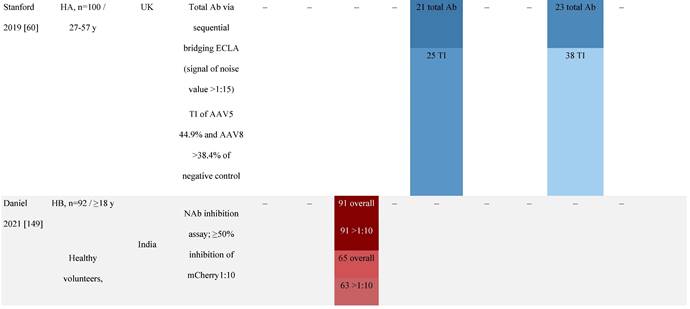
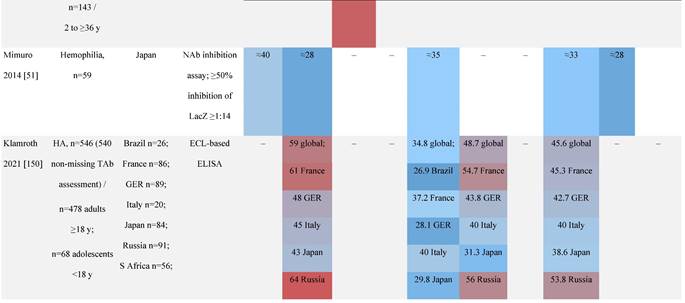
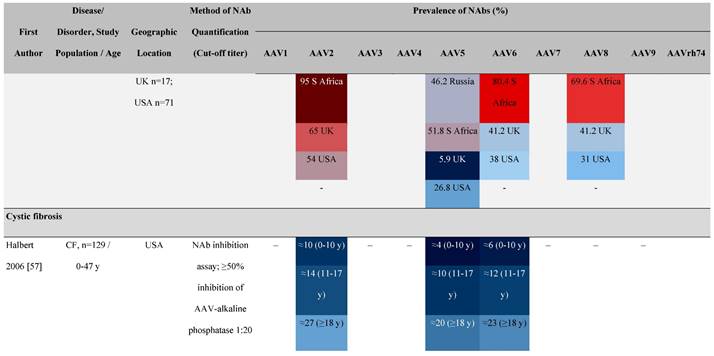
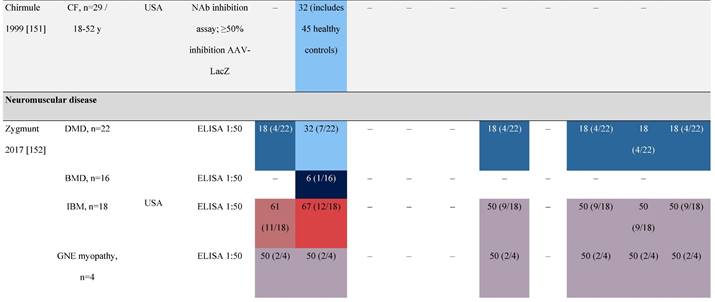
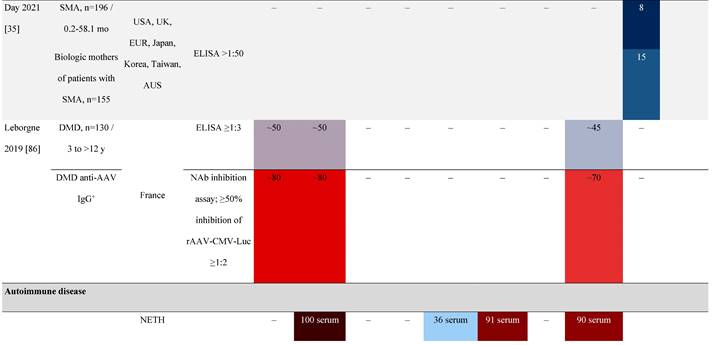
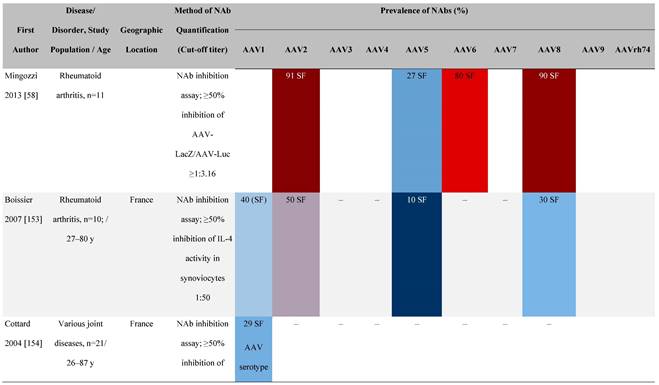
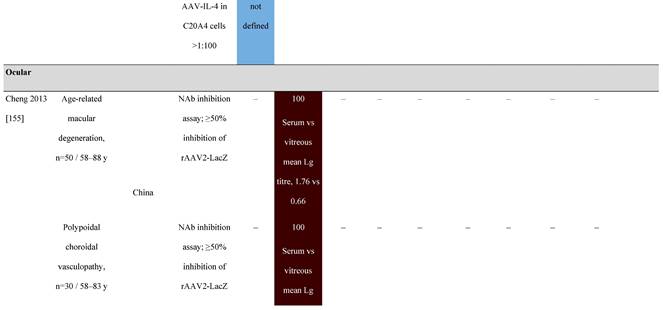
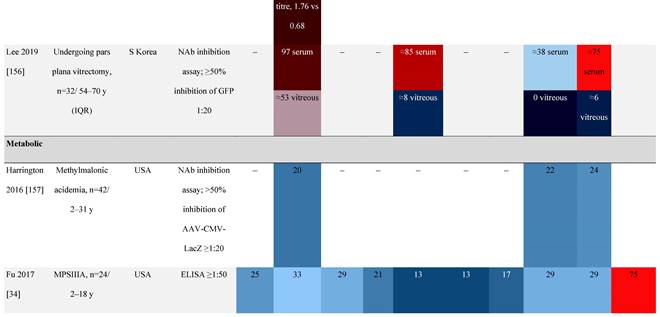
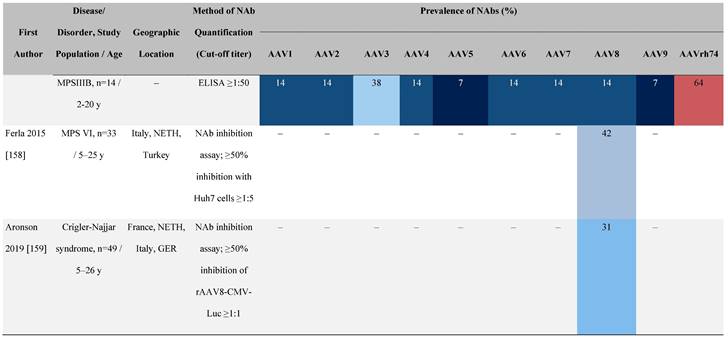
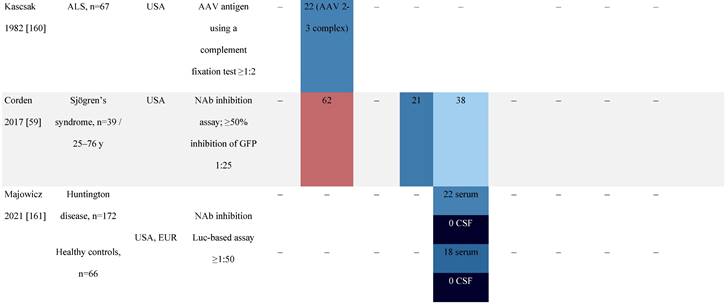
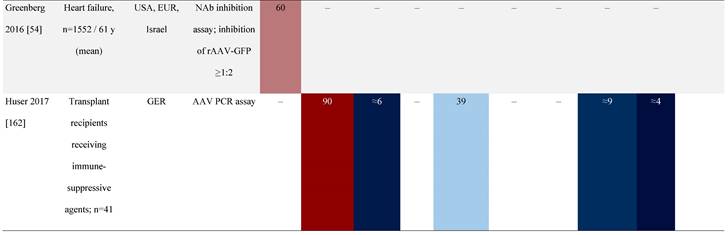
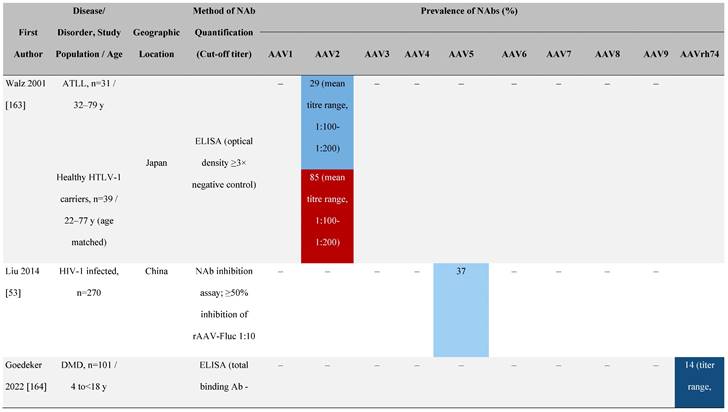

Prevalence color coding: 
a Method of NAb quantification not reported.
b Data reported only for United States (lowest) and Russian Federation (highest).
AAV, adeno-associated virus; Ab, antibody; ALS, amyotrophic lateral sclerosis; ATLL, adult T-cell leukemia lymphoma; AUS, Australia; BMD, Becker muscular dystrophy; CF, cystic fibrosis; CMV, cytomegalovirus; CSF, cerebrospinal fluid; DMD, Duchenne muscular dystrophy; ECL, electrochemiluminescent; ECLA, electrochemiluminescence assay; ELISA, enzyme-linked immunosorbent assay; EUR, Europe; F, female; Fluc, firefly luciferase; GER, Germany; GFP, green fluorescent protein; GNE, UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase; HA, hemophilia A; HB, hemophilia B; HIV, human immunodeficiency virus; HTLV, human T cell lymphotropic virus; IBM, inclusion body myositis; IgG, immunoglobulin G; IL, interleukin; IQR, interquartile range; LacZ, β-galactosidase; Lg, log10; Luc, luciferase; M, male; MPS, mucopolysaccharidosis; NAb, neutralizing antibody; NETH, Netherlands; rAAV, recombinant adeno-associated virus; S Africa, South Africa; SF, synovial fluid; SMA, spinal muscular atrophy; TAb, total antibody; TI, transduction inhibition.
There are interesting reports regarding anti-AAV NAbs among individuals who receive plasma-derived blood products. One such study noted an increase of anti-AAV8 NAbs in patients with hemophilia A exposed to plasma products, as well as an increase in anti-AAV5 and anti-AAV8 NAbs in patients exposed to hepatitis C [60]. Although the authors noted a possibility of transfusion-transmitted infection, they acknowledged further investigation was required to establish the cause [60]. This was explored further using a highly sensitive assay to detect AAV gene sequences in a range of commercially available plasma and recombinant factor VIII (FVIII) and factor IX (FIX) products [61]. Results indicated a presence of AAV and other viral serotypes in some of the plasma-derived blood products. In contrast, another study did not find an association between anti-AAV6 NAbs in patients with hemophilia B and exposure to contaminated plasma derivatives [62]. This apparent discrepancy could be explained by the AAV serotype under study. Neutralizing activity against multiple AAV serotypes is also possible, either through cross-reactivity of NAbs or the co-occurrence of NAbs as a result of multiple or co-infections within the same individual. This creates profound implications when attempting to implement alternate serotypes or engineered capsids in clinical practice [34, 46, 57].
An issue raised more recently relates to the development of anti-AAV NAbs in recipients of rAAV-based vaccines, including those against SARS COV-2 [63]. With the high frequency of cross-reactivity among different AAV serotypes, such vaccines could render recipients with genetic disorders ineligible for future rAAV-mediated gene therapy. This is a topic requiring further discussion and research.
Pre-existing NAb titer and post administration increases in NAbs
There are conflicting data regarding the relationship between titers of pre-existing anti-AAV NAbs and the efficacy of vector transduction. The presence of anti-AAV NAbs increases dramatically after administration of rAAV vectors (Table 5), which has implications for redosing [64]. In the first hemophilia B gene therapy clinical trial that used rAAV2 expressing human FIX (AAV-hFIX), there was a greater than 10,000-fold increase in anti-AAV NAb titers after vector administration. This was true for participants with or without detectable anti-AAV NAbs pre-treatment [65-67]. Therapeutic levels of FIX were achieved but reduced approximately 8 weeks post-infusion, correlating with the development of capsid-specific T cells [65, 66].
Seroprevalence of NAbs against AAVs following administration of recombinant therapeutic AAV vector serotypes
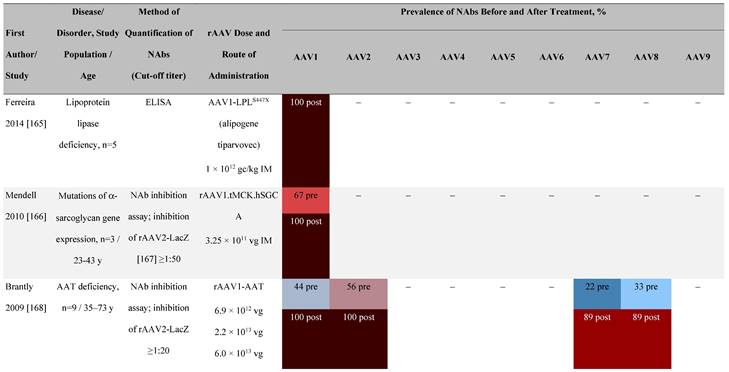
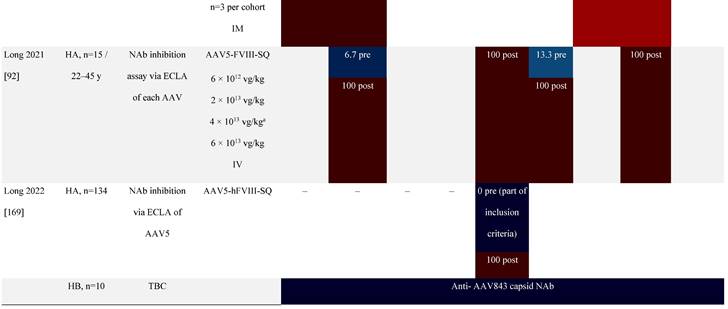
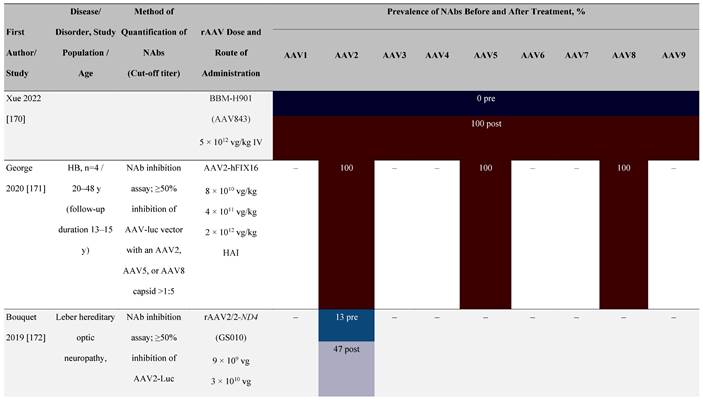
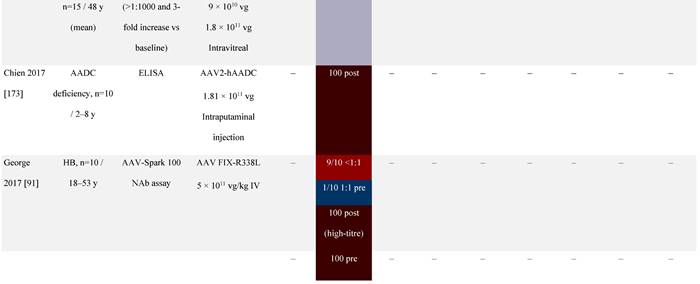
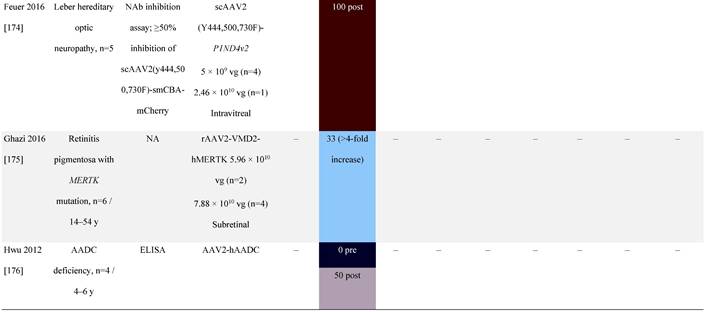
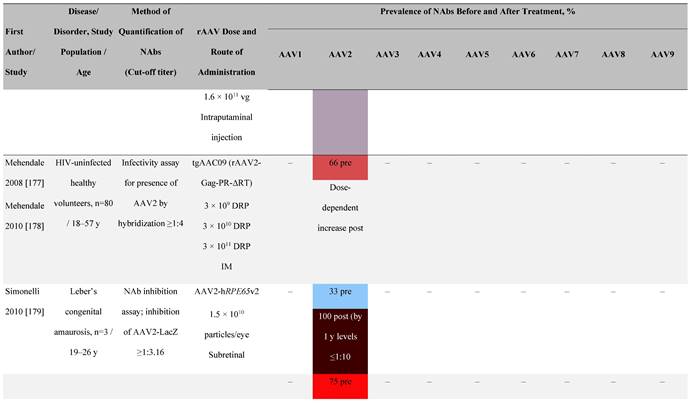
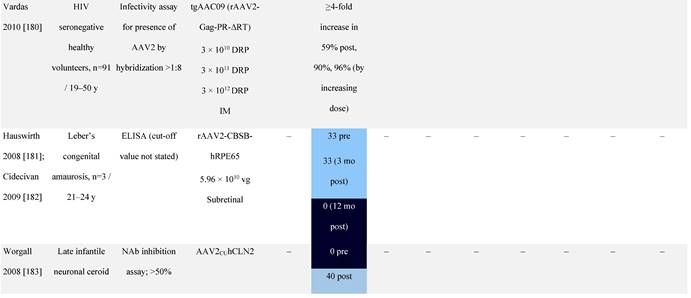
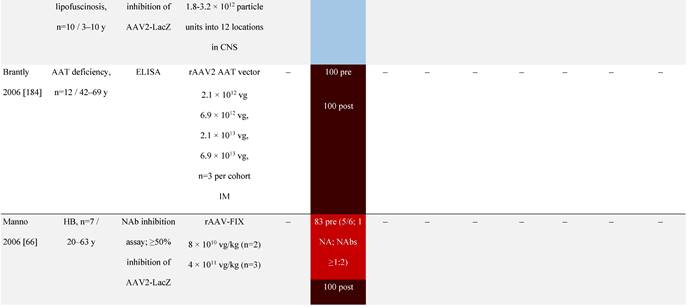
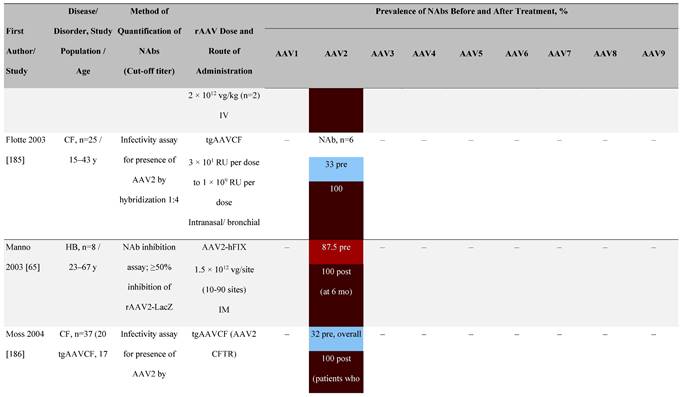
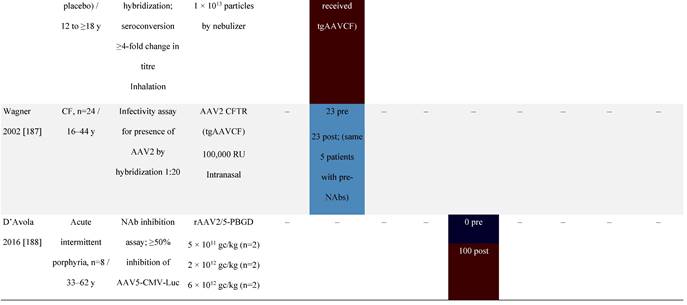
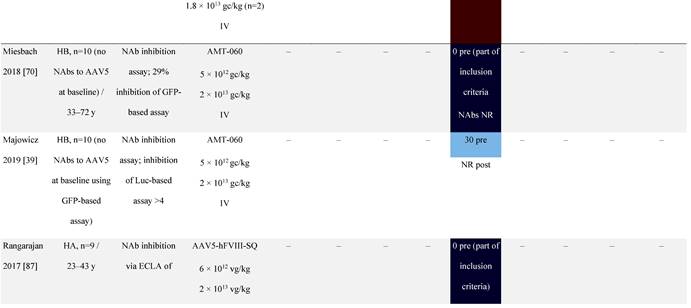
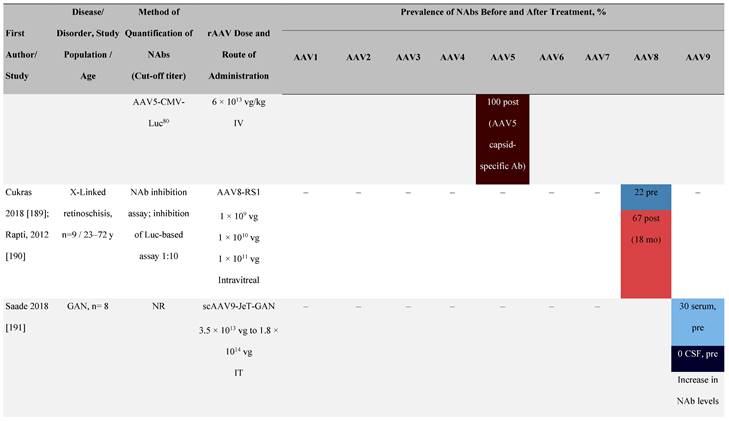


Prevalence color coding: 
a ECLA data were not available for 2 patients in this cohort.
AADC, aromatic L-amino acid decarboxylase; AAT, α-1 antitrypsin; AAV, adeno-associated virus; Ab, antibody; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; CMV, cytomegalovirus; CNS, central nervous system; CSF, cerebrospinal fluid; DRP, DNase-resistant particles; ECLA, electrochemiluminescence assay; ELISA, enzyme-linked immunosorbent assay; GAN, giant axonal neuropathy; HA, hemophilia A; HB, hemophilia B; HIV, human immunodeficiency virus; gc, genome copies; GFP, green fluorescent protein; HAI, hepatic artery infusion; IM, intramuscular; IT, intrathecal; IV, intravenous; LacZ, β-galactosidase; Luc, luciferase; NA, not available; NAb, neutralizing antibody; NR, not reported; PBGD, porphobilinogen deaminase; rAAV, recombinant adeno-associated virus; RU, replication units; SQ, subcutaneous; vg, vector genomes.
In most gene therapy trials utilizing rAAV, pre-existing anti-AAV NAbs are an exclusion criterion for patient enrollment [34]. For example, patients with pre-existing anti-AAV9 NAbs above a threshold were excluded from a phase 1 spinal muscular atrophy clinical trial [68] as were patients with anti-AAV5 NAbs from a phase 3 clinical trial for hemophilia A [69]. However, some findings have challenged exclusion based on this criterion [38, 39]. For instance, pre-existing anti-AAV5 NAbs had no effect on the efficacy of AAV5-based gene therapy expressing hFIX in a phase 1/2 hemophilia B gene therapy trial (NCT02396342) [70], even at high titers [39]. Similarly, there was no correlation between pre-existing anti-AAV5 NAbs and AAV5-mediated delivery of hFIX up to 18 months in a phase 3 hemophilia B trial (NCT03569891) [38]. Given that the observed increase in mean FIX activity was restored to near normal range at 18 months [38], these trials imply broad eligibility for AAV5-based therapies [38, 39, 71]. The NCT03569891 trial excluded patients with pre-existing anti-AAV5 NAbs based on a green fluorescent protein (GFP)-based reporter assay [39, 70]. In a follow-up study using a more sensitive luciferase-based assay, immunoglobulin (Ig)G and IgM antibodies against AAV5 were detected [39, 70]. These observations highlight the importance of the assay being used to examine NAb levels.
Apart from NAbs [6, 14], memory B cells and T cells are also generated due to natural AAV infection that can be re-activated on subsequent exposure to rAAV vectors [42, 73]. An investigation of immune responses to natural AAV1 infection by screening human peripheral blood mononuclear cells and sera from healthy donors showed no correlation between AAV1-specific T cells and anti-AAV1 NAb responses [74]. T-cell response composed mostly of effector memory CD8+ cell subsets. This suggests that patient screening should include testing for pre-existing AAV-specific cellular responses in addition to anti-AAV NAbs [74].
There is a need to standardize the quantitative relationship between neutralizing or total antibody titers and their impact on transduction for individual AAV serotypes [71]. It is important that validated assays to determine NAb titers are made available as gene therapy products are commercialized. The absence of validated assays to accompany regulatory approvals of some gene therapy products leaves clinicians with a challenging task of evaluating results from non-validated NAb assay kits.
Systemic Inflammatory Responses
Administration of gene therapy to patients with pre-existing NAbs can induce systemic inflammatory responses due to immune complex formation, enhanced vector uptake into antigen presenting cells (APCs), and complement activation, especially at higher titers. One study demonstrated that titers of NAb ≥1:100 significantly increased the innate immune response to AAV vectors with increased pro-inflammatory cytokine/chemokine secretion, vector uptake by APCs, and complement activation [75]. As complement is an important modulator of the anti-AAV immune response, inhibiting the complement pathway could help mitigate anti-AAV immune responses [76].
Preparing for rAAV gene therapy
Screening for Anti-AAV NAbs
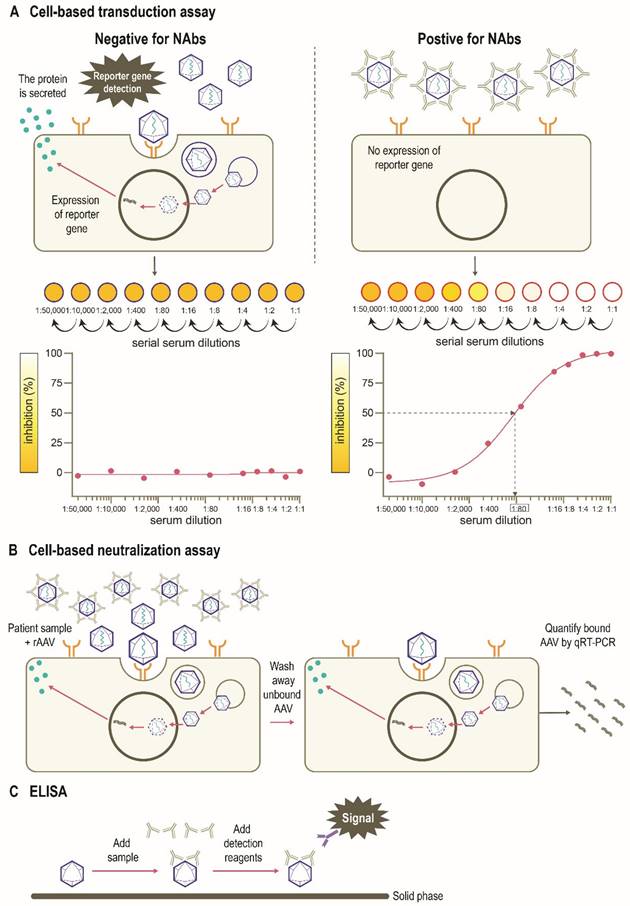
There are several assays to quantify neutralizing and total (non-neutralizing plus neutralizing) anti-AAV antibodies in humans, including in vitro cell-based assays and variants of the enzyme-linked immunosorbent assay (ELISA) (Figure 4) [77]. The protocols and reagents are not standardized and are usually adapted to accommodate the AAV serotype and/or transgene under investigation [78]. The most widely used in vitro cell-based assay is the transduction inhibition assay, which involves the measurement of transduction levels using a “reporter” gene (Figure 4A). Instead of the therapeutic transgene, a rAAV vector contains a reporter gene such as GFP, β-galactosidase, or luciferase, which provides a convenient and sensitive measure of transduction at wide dynamic ranges. The ability of NAbs to inhibit transduction of the specific AAV vector is then assessed by measuring the activity of the reporter protein (Figure 4B). The anti-AAV NAb titer is defined as the highest dilution that inhibits transgene expression by a specified amount (e.g., ≥50% [ID50]) [71, 77-79]. An advantage of this method is that it uses the same AAV capsid engineered into the gene therapy vector, recapitulating the clinical setting [71]. However, the output (e.g., 50% inhibition of transduction) may not correlate to a biologically relevant patient response [71]. As the assay measures reduction in transduction, factors other than NAbs may be involved. For example, not all AAV serotypes transduce efficiently in vitro, and the sensitivity of the assay may depend on the number of AAV vector genomes or multiplicity of infection (MOI), as well as the cell line and reporter system used [71, 80]. Indeed, the luciferase reporter system is likely to be more sensitive than a GFP-based reporter [39, 72, 81]. Additionally, the purity of the vector preparation influences NAb titer with the presence of monomeric or oligomeric capsid proteins, “empty” vectors, or vectors containing truncated genomes or genomic DNA potentially affecting the outcome of this assay [79]. Inhibition by other factors in the serum that reduce transduction such as human serum galectin 3 binding protein (G3BP) [82] can also impact the assay sensitivity. Furthermore, these assays are often proprietary, and therefore standardization and broad application remains challenging. Thus, there are pressing opportunities to harmonize such assays within and between jurisdictions as clinical gene therapies become widespread.
An alternative in vitro cell-based assay is the neutralization assay, which measures the binding of AAV vectors to target cells (Figure 4C) [71, 83]. Since anti-AAV NAbs can interfere with the binding of AAV to target cells, increased levels of NAbs in culture are associated with a measurable decrease in rAAV cell binding. The ability of the NAb to inhibit AAV binding is often assessed by measuring the activity of a reporter protein [71, 83]. As many cell-based assays are time-consuming [71], newer methods have been developed to determine NAb titers that use real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) methodology as opposed to reporter genes and can be applied to all serotypes in a fast, efficient, and cost-effective manner (Figure 4C) [80]. Other rapid cell-based assays have also been developed to provide alternative methods for in vivo determination of NAb titers [84]. However, the neutralization assay does not directly inform overall gene transfer efficiency or transduction as steps subsequent to cell binding are not evaluated [83].
Total antibody assays, such as ELISAs, can be used to measure antibody binding to the whole AAV capsid or capsid proteins [77, 78, 83]. These typically involve coating of the assay plate with AAV capsids (full or empty) or peptides, the addition of patient sample, and finally, detection of signal (Figure 4D). Peptide-based ELISAs can be more sensitive, specific and consistent than capsid ELISAs [85]. The peptide-based ELISA method has the advantage of simplicity compared with cell culture protocols, although neutralizing activity is not measured directly and levels of total anti-AAV antibodies are captured [77, 80]. The total antibody assay measures both neutralizing as well as non-neutralizing antibodies against AAV. Evidence suggests a correlation between levels of total anti-AAV antibody and NAbs [46, 86]. There have been studies suggesting that non-neutralizing antibodies may enhance the transduction rates of some serotypes including AAV8 [41] although mechanisms behind this phenomenon are yet to determined. Thus, the use of total antibody assay to determine a patient's eligibility for AAV gene therapy needs to be studied further [79].
Currently, beyond the lack of standardized assays, variations exist in cut-off values for NAb positivity between studies [31, 77, 83]. This makes comparison of NAb titers across studies difficult, particularly when the details of the assays used are lacking. Clinical trials conducted in patients with hemophilia exemplify this issue with differences in the cut-offs and types of assays used. For instance, in a rAAV5-based vector expressing FVIII in men with severe hemophilia A (NCT02576795) [87], exclusion was based on an in vitro transduction inhibition assay with a cut-off of 44.9% transduction and a total anti-AAV5 antibody assay cut-off of signal dilution ≥39.7%. The transduction inhibition assay employed the HEK293T/17 cell line, a rAAV5 vector expressing a luciferase reporter and a MOI of 25,000 vector genomes (vg)/cell [87]. A separate trial using a rAAV5-based vector expressing wild-type human FIX in adults with hemophilia B (NCT02396342) [70], exclusion was based on a 29% inhibition of transduction. The assay employed a rAAV5 vector expressing a GFP reporter, but no details of the MOI were provided. In another phase 2b trial using a rAAV5-based vector (NCT03489291) [88], patients with detectable anti-AAV5 NAbs titers were included. The NAb level was determined using the HEK293T cell line, a rAAV5 vector expressing a luciferase reporter and a MOI of 378.4 vg/cell [39, 88].
A standardized approach to reporting the number of AAV vector particles neutralized per unit volume (e.g., μL) of serum or plasma will be essential as stakeholders, including clinicians, scientists, industry, consumers and regulators, seek to compare trial results [79].
NAb screening to determine eligibility for AAV-based gene therapy
The titer of NAbs is routinely assessed prior to the administration of AAV gene therapies and is important given that even low titers can potentially to inhibit vector transduction especially after systemic administration [89]. Guidelines have proposed the development of appropriate assays to measure immune responses to gene therapy products early in the development process to monitor outcomes and inform treatment decisions [90]. However, there is no clear, consistent relationship between pre-existing anti-AAV NAb titers and clinical vector transduction levels [38, 39, 65, 66, 91]. In a recent meta-analysis of clinical AAV usage, it was determined that 45% of trials excluded subjects with pre-existing anti-AAV Nabs. This varies between therapeutic areas, with the proportions ranging from <10% for eye disorders to ~90% for blood disorders [6].
Assays used to detect immunity to AAVs. (A) An in vitro cell-based transduction inhibition assay, using a “reporter” gene such as GFP, β-galactosidase, or luciferase, which provides convenient and sensitive detection of transduction [71, 77, 78]. The degree of inhibition of reporter gene expression is plotted against the dilutions of the serum sample. The anti-AAV NAb titer is defined as the highest serum dilution that inhibits vector transduction by a specified amount (eg, ≥50%) in comparison with the negative control sample. (B) An in vitro cell-based neutralization assay measures the binding of rAAV to target cells. Increased levels of NAbs are associated with a proportionate decrease in rAAV cell binding [71, 80, 84]. (C) ELISAs can be used to measure antibody binding to the AAV capsid or other serotype-specific proteins or peptides. This method includes coating of the assay plate with AAV capsids (full or empty) or peptides, the addition of patient sample, and finally detection of signal [77, 78, 83]. AAV, adeno-associated virus; ELISA, enzyme-linked immunosorbent assay; GFP, green fluorescent protein; NAb, neutralizing antibody; qRT-PCR, quantitative reverse transcription polymerase chain reaction; rAAV, recombinant adeno-associated virus; IC50, half maximal inhibitory concentration.
A recent phase 1/2 clinical study of an AAV5-mediated gene therapy for severe hemophilia A excluded patients with pre-existing anti-AAV5 NAbs [92]. Immunogenicity data up to 3 years suggested the predominant immune response elicited by vector infusion was largely limited to the development of an anti-AAV5 antibody response with cross-reactivity to other serotypes, including AAV2, AAV6, AAV8, and AAVrh10 [92]. Patients with pre-existing anti-AAV5 NAbs were also excluded from another phase 3 study (NCT03370913) of hemophilia A that successfully reduced bleeding and the need for FVIII concentrates in participants [69]. In contrast, another study is currently recruiting patients with pre-existing anti-AAV5 NAbs [93], which will help inform whether NAbs should be an exclusion criterion especially for AAV5-based gene therapy clinical trials.
Other recent studies demonstrate that NAbs may not always impair the efficacy of gene delivery, as in the phase 1/2 trial (NCT02396342) discussed earlier [39, 70]. Indeed, the highest post-infusion FIX activity in this trial was observed in a participant with the highest pre-existing anti-AAV5 NAb titer of 1:340 [39], suggesting efficacy is not always related to anti-AAV NAb titer. In a phase 2b trial (NCT03489291) of a different AAV5-based gene therapy for hemophilia B and with pre-existing anti-AAV5 NAbs; all participants met the primary efficacy endpoint and had sustained FIX production and protection from spontaneous bleeding at the initial 26-week follow-up and after the 2-year follow-up period [88, 94]. Accordingly, pre-existing anti-AAV5 NAbs were not used as an exclusion criterion in the subsequent phase 3 trial (NCT03569891). The 18- and 24-month follow-up data demonstrated no correlation between therapeutic benefit and pre-existing anti-AAV5 NAbs [38]. Of note, one of the 54 participants enrolled had a high anti-AAV5 NAb titer of 3212 prior to vector dosing and did not respond to treatment [38].
These findings suggest clinically meaningful outcomes in patients with NAb titers at levels found in the general population may be achievable and may therefore enable a larger proportion of patients to potentially benefit from AAV-based gene therapies.
The full extent of the influence of vector dose on outcomes and clinical impact in the long-term is unclear and requires further investigation. It is also possible that AAV5 serotype is unique and less sensitive to NAbs [40]. Given the impact of pre-existing NAbs on outcomes, reliable in vitro assays to detect anti-AAV NAbs are required, not only for patient selection, but also to inform the clinical management of patients before, during and after treatment.
Strategies to Mitigate Anti-AAV NAbs
Strategies to mitigate pre-existing anti-AAV NAbs
With the majority of the global population exposed to AAV [6] and who potentially may have high levels of pre-existing NAbs against specific AAV serotypes (Table 3), it is important to develop strategies to mitigate the risks and to potentially expand the gene therapy-eligible patient population (Table 6) [5, 79]. Administering a high vector dose, particularly in the presence of low titer NAbs, may be an option to overcome the effects of NAbs [66 ]. However, both clinical and pre-clinical studies have demonstrated dorsal root ganglia toxicity after administration of high doses of AAV vectors through the cerebrospinal fluid (although no clinical signs were observed) [95, 96].
Modifying the mode or route of administration may be another option to reduce the humoral immune response to AAV-mediated gene therapy. A study conducted in macaque monkeys suggested portal vein-directed delivery of AAV8-based vectors with saline flushing is efficacious to minimize the inhibitory effect of pre-existing anti-AAV8 NAbs [97]. Similarly, intramuscular injection of a rAAV2-based gene therapy for hemophilia B was shown to result in successful gene transfer in patients with pre-existing anti-AAV NAbs, suggesting this method is less susceptible to the neutralizing activity of the NAbs [65]. It should be noted that despite successful gene transfer, circulating FIX were below therapeutic levels in this trial and capsid-specific T-cell responses can be triggered after intramuscular injection of rAAV vectors [98].
Identifying AAV capsids with altered epitopes that might be able to avoid the neutralizing activity of pre-existing NAbs offers another strategy. Alternatively, an AAV serotype with a lower seroprevalence may be used, as more than 100 naturally occurring AAVs have been identified. For instance, a comparison of NAb levels between AAV2, AAV4, AAV5, AAV12, and BAAV in serum of 39 patients with Sjögren disease and 38 healthy donors discovered lowest seroprevalence for AAV12 in both groups [59]. AAV vectors can also be engineered by directed evolution or rational design by mutagenesis of AAV capsids - this may lead to capsids with distinct tissue tropism, immunogenicity, and/or susceptibility to NAbs [5, 99-103]. However, artificial engineering of capsids for human gene therapy is associated with several challenges. Firstly, designing a capsid with all desired properties (tropism, immune evasion, packaging ability, and safety) is inefficient. This process may alter natural biology, decrease packaging ability, effect large-scale production, or even increase the antigenic load [104]. An assessment of all these aspects for each new capsid is required in pre-clinical as well as clinical settings. Nonetheless, several reports suggest that AAV capsid modification can lead to NAb evading vectors.
Additional non-genetic, chemical modifications of the AAV capsid may avoid the humoral immune response and neutralization by NAbs. For example, conjugating the AAV surface with polyethylene glycol chains (PEGylation) may protect AAV vectors from NAbs [105]. The inclusion of antibody decoys such as empty capsids in the final vector formulation may overcome the inhibitory effect of NAbs [106]. However, empty capsids may also contribute to the overall number of capsid antigens presented onto MHC class I and may also have an impact on the immunogenicity of AAV vectors [28, 107].
Immunosuppressants, given during or shortly after gene transfer, are often used to prevent the rejection of rAAV-transduced cells [5, 108 ]. However, despite their demonstrated effectiveness at dampening anti-AAV T-cell response, circumventing pre-existing NAbs using this method is challenging [5]. Plasmapheresis, with selective ex vivo removal of AAV-specific NAbs from an individual's blood using filtration- or centrifugation-based techniques may help manage seropositive patients and enable successful AAV transduction [5, 109]. Similar results might be possible using pre-treatment with immunoglobulin degrading enzymes, such as imlifidase, that can cleave IgG antibodies in seropositive patients to reduce pre-existing NAb levels [110-112]. Although these strategies have modestly decreased titers, they have not eliminated the presence of NAbs. It remains to be determined whether one or combination of some or all of the above-mentioned strategies will be most beneficial to mitigate the impacts of pre-existing NAbs.
Strategies to mitigate post-administration humoral immunity against rAAV vectors
As levels of anti-AAV NAbs can increase dramatically after administration of rAAV vectors, preventing re-administration of the same vector [64], it is important to develop strategies to mitigate humoral immunity to rAAV vectors post-administration (Table 6). Minimizing vector dosage is one such strategy, as rAAV vector immunogenicity appears to be dose dependent [113, 114]. It may be possible to manage expression of the transgene with low doses of vector together with broad-acting immunosuppressants such as corticosteroids [5, 114, 115]. These have been employed in therapies approved for inherited retinal dystrophy (1·5 × 1011 vg) [116] and in development for Crigler-Najjar syndrome (2 × 1012 vg/kg to 5 × 1012 vg/kg doses) [117]. Downregulating CD4+ helper T cells can be used to reduce anti-AAV NAb levels indirectly due to their ability to regulate the function of B cells and the antibody producing progeny plasma cell [5]. For instance, inhibiting B-cell activation has shown promise in several pre-clinical ocular models [5]. Reducing off-target expression of the transgene with tissue-specific promotors can also limit immune responses directed towards the transgene [118]. Effective strategies to overcome pre-existing NAbs and prevent the induction of humoral immunity against AAV gene therapies are likely to include a combination of approaches, particularly if the patient has a high pre-existing NAb titer.
Considerations for Pediatric Patients
There are specific considerations for pediatric patients receiving AAV-based gene therapy. When considering the challenge of pre-existing anti-AAV NAbs, seroprevalence is the lowest in patients under the age of 3 years and progressively increases into adulthood [33, 56 ] This may be an optimal therapeutic window, when the risk of anti-AAV NAbs is at its lowest and the proportion of eligible patients for gene therapy is at its highest [28]. However, when AAV vectors are delivered to pediatric patients, transgene expression may dilute with liver growth and increased blood volume [119, 120]. A single administration of an AAV-based vector may be sufficient to achieve a lifelong benefit for diseases such as hemophilia, with a relatively low therapeutic threshold [120], where even a low expression of the transgene may convert the disease phenotype from severe to mild [113]. As liver cells proliferate from birth to teenage years, episomal rAAV is expected to dilute, possibly leading to a loss of therapeutic benefit [121]. For diseases requiring robust transgene expression or treatment in childhood, vector re-administration or a methodology that uses vector genome integration or genome editing may be required [28].
Conclusions
There is compelling evidence that rAAV has become an established vector for human gene therapies. Although a number of clinical trials have shown the efficacy and safety of rAAV-mediated gene transfer, NAbs (pre-existing or induced post-therapy) remain a significant challenge. Seroprevalence profiles for AAV serotypes in humans vary by region, geography, race, and age. As NAb levels are an important patient exclusion criteria in clinical trials [53], it is important for healthcare professionals preparing for gene therapy to understand the challenges associated with NAbs and their impact on eligibility for clinical trials in AAV-based gene therapy, as well as strategies to mitigate their impact. Clarifying the relationship between NAb titer and its impact on transduction for individual AAV serotypes is required. This is especially the case for capsids like AAV5 that seem to behave differently in the presence of NAbs compared with other AAV serotypes. Clinical gene therapies require a consistent approach when reporting the number of AAV vector particles neutralized per unit volume of serum or plasma. Comprehensive and combinatorial strategies will likely be required to mitigate AAV NAbs to overcome pre-existing and post-therapy humoral immune responses. Gene therapy trials are often small, single-arm, short-term trials because eligible patients are selected from limited potential populations who have rare, severe, or advanced disease. Use of real-world evidence such as from registries and electronic health records will be an important aspect of gene therapy to understand long-term patient outcomes and real-world effectiveness.
Strategies under investigation to mitigate pre-existing anti-AAV NAbs and humoral immunity to rAAV vectors post-gene therapy [5, 77, 79, 113, 114, 121]
| Strategy | Considerations | |
|---|---|---|
| Strategies to mitigate pre-existing anti-AAV NAbs | Route of administration | • Easy to implement in clinical practice • May alter the pattern of transduction, limiting gene delivery to target cell type or tissue • Limited number of routes of administration depending on the target tissue • Target 'immune privileged' tissues |
| Alternative AAV vectors | • Increasing number of novel AAV vectors identified which may be more effective at evading NAbs. • Increasing understanding of AAV epitopes is enabling rational mutation of antigenic regions - could be applied to any vector. • In vitro and in vivo screens available to identify NAb-resistant vectors • Engineering novel AAVs can be expensive and technically challenging; novel AAVs may have unwanted/unintended properties e.g., toxicity issues | |
| AAV modification | • Non-genetic/chemical modifications are amenable to scale-up in manufacturing • Limited resistance of PEGylated AAV to NAbs; examples of other biological polymers are limited to date | |
| Decoy capsids | • Clinically translatable if 'known' serotype decoy capsids are used • Possible to adjust ratio of decoy to full capsids depending on pre-existing NAb titer • Potential to increase immune responses, including CD8+ T cell activation with destruction of transduced cells and enhanced NAb response • Would require production of more AAV capsids, which could create a manufacturing bottleneck | |
| Plasmapheresis | • Multiple rounds can reduce pre-existing NAb levels • Relatively non-invasive and safe; routinely used in other applications • Less effective with high pre-existing NAb titers • 'Rebound' phenomenon may limit effectiveness and compromise the option of repeated use | |
| Broad-acting immunosuppressants | • Approved immunosuppressants can be used • Well-characterized safety and efficacy profiles, with known toxicities • May be ineffective at completely depleting the bone marrow memory B-cells to overcome humoral immunity • Little evidence to support the approach despite efficacy of in pre-immunized models | |
| Antibody-degrading enzymes | • Effective in preclinical animal studies with low and moderate NAb titer • Clinical evidence of safety in patients undergoing graft rejection • Patients may have pre-existing humoral immunity to, or develop humoral immunity against, the enzyme • May only be partially effective against high titers of NAbs | |
| Anti-FcRn antibodies | • FcRn1 helps maintain circulating IgG levels; use of anti-FcRn antibodies can potentially reduce NAb titers by ~80% for 60 days • Specific for IgG; does not impact IgA, IgM, IgD, and IgE antibodies | |
| Strategies to mitigate humoral immunity to rAAV vectors post-gene therapy | Minimizing vector dosage | • Transgene expression may be achieved with low vector doses + corticosteroids or other mild immunosuppressive regimens • Additional strategies might be required to achieve therapeutic level of transgene expression |
| Inhibit B-cell activation | • Inhibiting B-cell function may prevent NAb formation whilst retaining cytotoxic T-cell function • Patients may be vulnerable to opportunistic pathogens and tumorigenesis while B-cell levels recover | |
| Broad-acting immunosuppressants | • Possible to utilize approved drugs to suppress immune responses; highly translatable. • Well-characterized safety and efficacy profiles and mechanisms of action • Preclinical evidence suggests this approach may not be sufficient to prevent NAb formation or enable vector re-administration. • Outcomes of clinical trials investigating gene delivery to the retina also suggest this approach may not be effective in all patients | |
| Targeted transgene expression | • Reduce off-target expression of the transgene with gene regulatory elements like tissue- and cell-specific promoters or microRNAs • Targeted gene delivery platforms improve the safety profile • Tissue-specific promoters can be weaker compared to ubiquitous promoters; identification of strong but tissue-specific promoters can be challenging |
Adapted from "Humoral immune responses to AAV gene therapy in the ocular compartment" by Whitehead M et al. which is licensed under CC BY 4.0. [5]
AAV, adeno-associated virus; FDA, US Food and Drug Administration; MicroRNA, micro ribonucleic acid; NAb, neutralizing antibody; PEG, polyethylene glycol.
Abbreviations
AAP: assembly-activating protein
AAV: adeno-associated virus
APC: antigen-presenting cells
ELISA: enzyme-linked immunosorbent assay
G3BP: galectin 3 binding protein
GFP: green fluorescent protein
IC50: half maximal inhibitory concentration
ITR: inverted terminal repeats
MAAP: membrane-associated accessory protein
MHC: Major histocompatibility complex
MOI: multiplicity of infection
Nab: neutralizing antibody
rAAV: recombinant adeno-associated virus
Rep: replica proteins
ssDNA: single-stranded DNA
TLR: toll-like receptor
Acknowledgements
Editorial support was provided by Marion James, PhD, of Engage Scientific Solutions and Michael D. Morren, RPh, MBA, of Peloton Advantage, an OPEN Health company, and funded by Pfizer. Anthony Pirrello assisted in designing the graphical abstract. No author received an honorarium or other form of financial support related to the development of this manuscript.
Author Contributions
All authors substantially contributed to the conception and/or planning of the article. All authors substantially contributed to the drafting of the manuscript. All authors contributed to the critical review and revision of the manuscript. All authors approved the final version of the manuscript for publication.
Competing Interests
Bijay P. Dhungel and John E. J. Rasko acknowledge support from Brandon Capital and Therapeutic Innovation Australia. John E. J. Rasko is supported by NHMRC (Investigator Grant 1177305 to him for “Driving clinical cell and gene therapy in Australia”), Cure the Future, and an anonymous foundation. He declares Supply of Material (MTA), consultancy, or honoraria with Bluebird Bio, Cynata, Gilead, Novartis, Pfizer, RareCyte, Roche, and SPARK Therapeutics; and shareholdings with RareCyte, Genea, and Woke. Ian Winburn, Candida da. da Fonseca Pereira, Kui Huang, and Amit Chhabra are employees of Pfizer and may own stock/options in the company.
References
1. Anguela XM, High KA. Entering the modern era of gene therapy. Annu Rev Med. 2019;70:273-88
2. High KA, Roncarolo MG. Gene therapy. N Engl J Med. 2019;381(5):455-64
3. Salganik M, Hirsch ML, Samulski RJ. Adeno-associated vrus as a mammalian DNA vector. Microbiol Spectr. 2015;3(4):10.1128 /microbiolspec.MDNA3-0052-2014
4. Tse LV, Moller-Tank S, Asokan A. Strategies to circumvent humoral immunity to adeno-associated viral vectors. Expert Opin Biol Ther. 2015;15(6):845-55
5. Whitehead M, Osborne A, Yu-Wai-Man P, Martin K. Humoral immune responses to AAV gene therapy in the ocular compartment. Biol Rev Camb Philos Soc. 2021;96(4):1616-44
6. Au HKE, Isalan M, Mielcarek M. Gene Therapy advances: a meta-analysis of AAV usage in clinical settings. Front Med (Lausanne). 2021;8:809118
7. Dudek AM, Porteus MH. Answered and unanswered questions in early-stage viral vector transduction biology and innate primary cell toxicity for ex-vivo gene editing. Front Immunol. 2021;12(e-collection 2021):660302
8. Clement N, Grieger JC. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol Ther Methods Clin Dev. 2016;3:16002
9. Grieger JC, Soltys SM, Samulski RJ. Production of recombinant adeno-associated virus vectors using suspension HEK293 cells and continuous harvest of vector from the culture media for GMP FIX and FLT1 clinical vector. Mol Ther. 2016;24(2):287-97
10. Dhungel BP, Bailey CG, Rasko JEJ. Journey to the center of the cell: tracing the path of AAV transduction. Trends Mol Med. 2021;27:172-84
11. Wang D, Tai PWL, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18(5):358-78
12. Galibert L, Hyvonen A, Eriksson RAE, Mattola S, Aho V, Salminen S. et al. Functional roles of the membrane-associated AAV protein MAAP. Sci Rep. 2021;11(1):21698
13. Elmore ZC, Patrick Havlik L, Oh DK, Anderson L, Daaboul G, Devlin GW. et al. The membrane associated accessory protein is an adeno-associated viral egress factor. Nat Commun. 2021;12(1):6239
14. Verdera HC, Kuranda K, Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol Ther. 2020;28(3):723-46
15. Ertl HCJ, High KA. Impact of AAV capsid-specific T-cell responses on design and outcome of clinical gene transfer trials with recombinant adeno-associated viral vectors: an evolving controversy. Hum Gene Ther. 2017;28(4):328-37
16. Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73(5):3994-4003
17. Vance MA, Mitchell A, Samulsk iRJ. AAV biology, infectivity and therapeutic use from bench to clinic. In: Hashad D, editor. Gene Therapy. Rijeka: IntechOpen. 2015 p. 119-43
18. Walters RW, Yi SM, Keshavjee S, Brown KE, Welsh MJ, Chiorini JA. et al. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem. 2001;276(23):20610-6
19. Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X. et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76(2):791-801
20. Mietzsch M, Jose A, Chipman P, Bhattacharya N, Daneshparvar N, McKenna R. et al. Completion of the AAV structural atlas: serotype capsid structures reveals clade-specific features. Viruses. 2021;13(1):101
21. Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X. et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78(12):6381-8
22. Bantel-Schaal U, Delius H, Schmidt R, zur Hausen H. Human adeno-associated virus type 5 is only distantly related to other known primate helper-dependent parvoviruses. J Virol. 1999;73(2):939-47
23. Georg-Fries B, Biederlack S, Wolf J, zur Hausen H. Analysis of proteins, helper dependence, and seroepidemiology of a new human parvovirus. Virology. 1984;134(1):64-71
24. Lisowski L, Tay SS, Alexander IE. Adeno-associated virus serotypes for gene therapeutics. Curr Opin Pharmacol. 2015;24:59-67
25. Adachi K, Nakai H. The role of DNA Repair Pathways in Adeno-associated virus infection and viral genome replication / recombination / integration. In: Vengrova S, editor. DNA Repair and Human Health. London: IntechOpen. 2011 p. 685-714
26. Bertolini TB, Shirley JL, Zolotukhin I, Li X, Kaisho T, Xiao W. et al. Effect of CpG Depletion of vector genome on CD8(+) T cell responses in AAV gene therapy. Front Immunol. 2021;12:672449
27. Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther. 2021;6(1):53
28. Colella P, Ronzitti G, Mingozzi F. Emerging Issues in AAV-mediated in vivo gene therapy. Mol Ther Methods Clin Dev. 2018;8:87-104
29. Sabatino DE, Bushman FD, Chandler RJ, Crystal RG, Davidson BL, Dolmetsch R. et al. Evaluating the state of the science for adeno-associated virus integration: An integrated perspective. Mol Ther. 2022;30(8):2646-63
30. Chandler RJ, LaFave MC, Varshney GK, Burgess SM, Venditti CP. Genotoxicity in mice following AAV gene delivery: a safety concern for human gene therapy? Mol Ther. 2016;24(2):198-201
31. Vandamme C, Adjali O, Mingozzi F. Unraveling the complex story of immune responses to AAV vectors trial after trial. Hum Gene Ther. 2017;28(11):1061-74
32. Klamroth R, Hayes G, Andreeva T, Gregg K, Suzuki T, Mitha IH. et al. Global seroprevalence of pre-existing immunity against aAV5 and other AAV serotypes in people with hemophilia A. Hum Gene Ther. 2022;33(7-8):432-41
33. Calcedo R, Morizono H, Wang L, McCarter R, He J, Jones D. et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol. 2011;18(9):1586-8
34. Fu H, Meadows AS, Pineda RJ, Kunkler KL, Truxal KV, McBride KL. et al. Differential prevalence of antibodies against adeno-associated virus in healthy children and patients with mucopolysaccharidosis III: perspective for AAV-mediated gene therapy. Hum Gene Ther Clin Dev. 2017;28(4):187-96
35. Day JW, Finkel RS, Mercuri E, Swoboda KJ, Menier M, van Olden R. et al. Adeno-associated virus serotype 9 antibodies in patients screened for treatment with onasemnogene abeparvovec. Mol Ther Methods Clin Dev. 2021;21:76-82
36. Meyer NL, Chapman MS. Adeno-associated virus (AAV) cell entry: structural insights. Trends Microbiol. 2022;30(5):432-51
37. Klasse PJ, Sattentau QJ. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol. 2002;83(Pt 9):2091-108
38. Pipe SW, Leebeek FWG, Recht M, Key NS, Castaman G, Miesbach W. et al. Gene therapy with etranacogene dezaparvovec for hemophilia B. N Engl J Med. 2023;388(8):706-18
39. Majowicz A, Nijmeijer B, Lampen MH, Spronck L, de Haan M, Petry H. et al. Therapeutic hFIX activity achieved after single AAV5-hFIX treatment in hemophilia B patients and NHPs with pre-existing anti-AAV5 NABs. Mol Ther Methods Clin Dev. 2019;14:27-36
40. Ronzitti G. Neutralizing antibodies for AAV vectors: the strange case of AAV5. Sci Transl Med. 2019;11(497):1
41. Fitzpatrick Z, Leborgne C, Barbon E, Masat E, Ronzitti G, van Wittenberghe L. et al. Influence of pre-existing anti-capsid neutralizing and binding antibodies on AAV vector transduction. Mol Ther Methods Clin Dev. 2018;9:119-29
42. Yang TY, Braun M, Lembke W, McBlane F, Kamerud J, DeWall S. et al. Immunogenicity assessment of AAV-based gene therapies: An IQ consortium industry white paper. Mol Ther Methods Clin Dev. 2022;26:471-94
43. Hamilton BA, Wright JF. Challenges posed by immune responses to AAV vectors: addressing root causes. Front Immunol. 2021;12:675897
44. Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE. et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13(4):419-22
45. Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199(3):381-90
46. Kruzik A, Fetahagic D, Hartlieb B, Dorn S, Koppensteiner H, Horling FM. et al. Prevalence of anti-adeno-associated virus immune responses in international cohorts of healthy donors. Mol Ther Methods Clin Dev. 2019;14:126-33
47. Rasko J BG, Petropoulos C, Wrin T, Paliwal Y, Somanathan S, Casey M, da Fonseca Pereira C, WinburnI, Chhabra A. Global seroprevalence of neutralizing antibodies against adeno-associated virus (AAV) serotypes of relevance to gene therapy. Blood. 2022;140:10668-70
48. Hayes GM, Andrea T, Klamroth R, Hardesty B, Suzuki T, Stieltjes N. et al. Global seroprevalence of pre-existing antibodies against various AAV serotypes in hemophilia A adults and adolescents [abstract MED-FP-007 (383)]. Haemophilia. 2020;26(Suppl 4):20-1
49. Khatri A, Shelke R, Guan S, Somanathan S. Higher seroprevalence of anti-adeno-associated viral vector neutralizing antibodies among racial minorities in the United States. Hum Gene Ther. 2022;33(7-8):442-50
50. Liu Q, Huang W, Zhao C, Zhang L, Meng S, Gao D. et al. The prevalence of neutralizing antibodies against AAV serotype 1 in healthy subjects in China: implications for gene therapy and vaccines using AAV1 vector. J Med Virol. 2013;85(9):1550-6
51. Mimuro J, Mizukami H, Shima M, Matsushita T, Taki M, Muto S. et al. The prevalence of neutralizing antibodies against adeno-associated virus capsids is reduced in young Japanese individuals. J Med Virol. 2014;86(11):1990-7
52. Ling C, Wang Y, Feng YL, Zhang YN, Li J, Hu XR. et al. Prevalence of neutralizing antibodies against liver-tropic adeno-associated virus serotype vectors in 100 healthy Chinese and its potential relation to body constitutions. J Integr Med. 2015;13(5):341-6
53. Liu Q, Huang W, Zhang H, Wang Y, Zhao J, Song A. et al. Neutralizing antibodies against AAV2, AAV5 and AAV8 in healthy and HIV-1-infected subjects in China: implications for gene therapy using AAV vectors. Gene Ther. 2014;21(8):732-8
54. Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Pogoda JM. et al. Prevalence of AAV1 neutralizing antibodies and consequences for a clinical trial of gene transfer for advanced heart failure. Gene Ther. 2016;23(3):313-9
55. Tenenbaum L, Lehtonen E, Monahan PE. Evaluation of risks related to the use of adeno-associated virus-based vectors. Curr Gene Ther. 2003;3(6):545-65
56. Li C, Narkbunnam N, Samulski RJ, Asokan A, Hu G, Jacobson LJ. et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 2012;19(3):288-94
57. Halbert CL, Miller AD, McNamara S, Emerson J, Gibson RL, Ramsey B. et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum Gene Ther. 2006;17(4):440-7
58. Mingozzi F, Chen Y, Edmonson SC, Zhou S, Thurlings RM, Tak PP. et al. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther. 2013;20(4):417-24
59. Corden A, Handelman B, Yin H, Cotrim A, Alevizos I, Chiorini JA. Neutralizing antibodies against adeno-associated viruses in Sjögren's patients: implications for gene therapy. Gene Ther. 2017;24(4):241-4
60. Stanford S, Pink R, Creagh D, Clark A, Lowe G, Curry N. et al. Adenovirus-associated antibodies in UK cohort of hemophilia patients: a seroprevalence study of the presence of adenovirus-associated virus vector-serotypes AAV5 and AAV8 neutralizing activity and antibodies in patients with hemophilia A. Res Pract Thromb Haemost. 2019;3(2):261-7
61. Young L, Marshall J-C, Pittman D, Smith J, Uzgiris J, Smith L. et al. AAV amplification and DNA sequencing of factor VIII and factor IX concentrates. Mol Ther. 2022;30(4 Suppl 1):721
62. Boyce S, James I, Rangarajan S, Curry N, Bagot C, Austin S. et al. Seroprevalence to adeno-associated virus type 6 in people with hemophilia B from a UK adult cohort. Res Pract Thromb Haemost. 2022;6(4):e12705
63. Aledo-Serrano A, Gil-Nagel A, Isla J, Mingorance A, Mendez-Hermida F, Hernandez-Alcoceba R. Gene therapies and COVID-19 vaccines: a necessary discussion in relation with viral vector-based approaches. Orphanet J Rare Dis. 2021;16(1):316
64. Pipe S, Leebeek FWG, Ferreira V, Sawyer EK, Pasi J. Clinical considerations for capsid choice in the development of liver-targeted AAV-based gene transfer. Mol Ther Methods Clin Dev. 2019;15:170-8
65. Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR. et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101(8):2963-72
66. Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ. et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342-7
67. Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A. et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24(3):257-61
68. Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713-22
69. Ozelo MC, Mahlangu J, Pasi KJ, Giermasz A, Leavitt AD, Laffan M. et al. Valoctocogene roxaparvovec gene therapy for hemophilia A. N Engl J Med. 2022;386(11):1013-25
70. Miesbach W, Meijer K, Coppens M, Kampmann P, Klamroth R, Schutgens R. et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2018;131(9):1022-31
71. Gorovits B, Fiscella M, Havert M, Koren E, Long B, Milton M. et al. Recommendations for the development of cell-based anti-viral vector neutralizing antibody assays. AAPS J. 2020;22(2):24
72. Wang M, Crosby A, Hastie E, Samulski JJ, McPhee S, Joshua G. et al. Prediction of adeno-associated virus neutralizing antibody activity for clinical application. Gene Ther. 2015;22(12):984-92
73. Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12(5):341-55
74. Veron P, Leborgne C, Monteilhet V, Boutin S, Martin S, Moullier P. et al. Humoral and cellular capsid-specific immune responses to adeno-associated virus type 1 in randomized healthy donors. J Immunol. 2012;188(12):6418-24
75. Smith CJ, Ross N, Kamal A, Kim KY, Kropf E, Deschatelets P. et al. Pre-existing humoral immunity and complement pathway contribute to immunogenicity of adeno-associated virus (AAV) vector in human blood. Front Immunol. 2022;13:999021
76. Muhuri M, Maeda Y, Ma H, Ram S, Fitzgerald KA, Tai PW. et al. Overcoming innate immune barriers that impede AAV gene therapy vectors. J Clin Invest. 2021;131(1):e143780
77. Schulz M, Levy DI, Petropoulos CJ, Bashirians G, Winburn I, Mahn M. et al. Binding and neutralizing anti-AAV antibodies: detection and implications for rAAV-mediated gene therapy. Mol Ther. 2023; Jan 11: doi: 10.1016/j.ymthe. 2023 01.010
78. Calcedo R, Chichester JA, Wilson JM. Assessment of humoral, innate, and T-cell immune responses to adeno-associated virus vectors. Hum Gene Ther Methods. 2018
79. Weber T. Anti-AAV antibodies in AAV gene therapy: current challenges and possible solutions. Front Immunol. 2021;12:658399
80. Guo P, Zhang J, Chrzanowski M, Huang J, Chew H, Firrman JA. et al. Rapid AAV-neutralizing antibody determination with a cell-binding assay. Mol Ther Methods Clin Dev. 2019;13:40-6
81. Falese L, Sandza K, Yates B, Triffault S, Gangar S, Long B. et al. Strategy to detect pre-existing immunity to AAV gene therapy. Gene Ther. 2017;24(12):768-78
82. Denard J, Beley C, Kotin R, Lai-Kuen R, Blot S, Leh H. et al. Human galectin 3 binding protein interacts with recombinant adeno-associated virus type 6. J Virol. 2012;86(12):6620-31
83. Mendell JR, Connolly AM, Lehman KJ, Griffin DA, Khan SZ, Dharia SD. et al. Testing preexisting antibodies prior to AAV gene transfer therapy: rationale, lessons and future considerations. Mol Ther Methods Clin Dev. 2022;25:74-83
84. Zhang J, Guo P, Huang J, Chew H, Firrman JA, Liu L. et al. Rapid AAV neutralizing antibody determination with a cell-binding assay [abstract]. American Society of Gene and Cell Therapy, Chicago, IL, May 16-19. 2018
85. Daniel H, Agbandje-McKenna M, Gabriel N, Kumar S, Coleman K, Srivastava A. et al. Development of a peptide ELISA for the screening of pre-existing anti-AAV antibodies [abstract 460]. Mol Ther. 2019;27(4 Suppl 1):218
86. Leborgne C, Latournerie V, Boutin S, Desgue D, Quéré A, Pignot E. et al. Prevalence and long-term monitoring of humoral immunity against adeno-associated virus in Duchenne muscular dystrophy patients. Cell Immunol. 2019;342:103780
87. Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M. et al. AAV5-factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377(26):2519-30
88. von Drygalski A GA, Castaman G. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 2021;3(21):3241-7
89. Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122(1):23-36
90. US Food, Drug Administration, Center for Biologics Evaluation, Research. Human gene therapy for rare diseases. Guidance for industry. 2020 (last update January 2020). https://www.fda.gov/media/113807/download (accessed 9 October 2023)
91. George LA, Sullivan SK, Giermasz A, Rasko JEJ, Samelson-Jones BJ, Ducore J. et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med. 2017;377(23):2215-27
92. Long BR, Veron P, Kuranda K, Hardet R, Mitchell N, Hayes GM. et al. Early phase clinical immunogenicity of valoctocogene roxaparvovec, an AAV5-mediated gene therapy for hemophilia A. Mol Ther. 2021;29(2):597-610
93. ClinicalTrials.gov. Gene therapy study in severe haemophilia A patients with antibodies against AAV5 (GENEr8-AAV5+) [NCT03520712]. 2022 (last update 26 September 2023). www.clinicaltrials.gov/ct2/show/NCT03520712 (accessed 10 October 2023).
94. Gomez E GA, Castaman G, Key NS, LAttimore SU, Leebeek FW, Miesbacj W, Recht M, von Drygalski A, Sawyer EK, Pipe SW. Etranacogene dezaparvovec (AAV5-Padua hFIX variant, AMT-061), an enhanced vector for gene transfer in adults with severe or moderate-severe hemophilia B: 2.5 year data from a phase 2b trial. Res Pract Thromb Haemost. 2021;5(Suppl 2):e12589
95. Hordeaux J, Buza EL, Dyer C, Goode T, Mitchell TW, Richman L. et al. Adeno-associated virus-induced dorsal root ganglion pathology. Hum Gene Ther. 2020;31(15-16):808-18
96. Mueller C, Berry JD, McKenna-Yasek DM, Gernoux G, Owegi MA, Pothier LM. et al. SOD1 suppression with adeno-associated virus and microrna in familial ALS. N Engl J Med. 2020;383(2):151-8
97. Mimuro J, Mizukami H, Hishikawa S, Ikemoto T, Ishiwata A, Sakata A. et al. Minimizing the inhibitory effect of neutralizing antibody for efficient gene expression in the liver with adeno-associated virus 8 vectors. Mol Ther. 2013;21(2):318-23
98. Kumar SRP, Duan D, Herzog RW. Immune responses to muscle-directed adeno-associated viral gene transfer in clinical studies. Hum Gene Ther. 2023;34(9-10):365-71
99. Zolotukhin S, Vandenberghe LH. AAV capsid design: a Goldilocks challenge. Trends Mol Med. 2022;28(3):183-93
100. Paulk NK, Pekrun K, Zhu E, Nygaard S, Li B, Xu J. et al. Bioengineered AAV capsids with combined high human liver transduction in vivo and unique humoral seroreactivity. Mol Ther. 2018;26(1):289-303
101. Jose A, Mietzsch M, Smith JK, Kurian J, Chipman P, McKenna R. et al. High-resolution structural characterization of a new adeno-associated virus serotype 5 antibody epitope toward engineering antibody-resistant recombinant gene delivery vectors. J Virol. 2019;93(1):e01394-18
102. Tse LV, Klinc KA, Madigan VJ, Castellanos Rivera RM, Wells LF, Havlik LP. et al. Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc Natl Acad Sci U S A. 2017;114(24):E4812-E21
103. Li C, Wu S, Albright B, Hirsch M, Li W, Tseng YS. et al. Development of patient-specific AAV vectors after neutralizing antibody selection for enhanced muscle gene transfer. Mol Ther. 2016;24(1):53-65
104. Li C, Samulski RJ. Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet. 2020;21(4):255-72
105. Lee GK, Maheshri N, Kaspar B, Schaffer DV. PEG conjugation moderately protects adeno-associated viral vectors against antibody neutralization. Biotechnol Bioeng. 2005;92(1):24-34
106. Mingozzi F, Anguela XM, Pavani G, Chen Y, Davidson RJ, Hui DJ. et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med. 2013;5(194):194ra92
107. Pien GC, Basner-Tschakarjan E, Hui DJ, Mentlik AN, Finn JD, Hasbrouck NC. et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest. 2009;119(6):1688-95
108. Corti M, Elder M, Falk D, Lawson L, Smith B, Nayak S. et al. B-cell depletion is protective against anti-AAV capsid immune response: a human subject case study. Mol Ther Methods Clin Dev. 2014;1:14033
109. Monteilhet V, Saheb S, Boutin S, Leborgne C, Veron P, Montus MF. et al. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther. 2011;19(11):2084-91
110. Leborgne C, Barbon E, Alexander JM, Hanby H, Delignat S, Cohen DM. et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat Med. 2020;26(7):1096-101
111. Ros-Ganan I, Hommel M, Trigueros-Motos L, Tamarit B, Rodriguez-Garcia E, Salas D. et al. Optimising the IgG-degrading enzyme treatment regimen for enhanced adeno-associated virus transduction in the presence of neutralising antibodies. Clin Transl Immunology. 2022;11(2):e1375
112. Elmore ZC, Oh DK, Simon KE, Fanous MM, Asokan A. Rescuing AAV gene transfer from neutralizing antibodies with an IgG-degrading enzyme. JCI Insight. 2020;5(19):e139881
113. Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC. et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357-65
114. Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA. et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114(10):2077-86
115. Xiang Z, Kuranda K, Quinn W, Chekaoui A, Ambrose R, Hasanpourghai M. et al. The effect of rapamycin and ibrutinib on antibody rresponses to adeno-associated virus vector-mediated gene transfer. Hum Gene Ther. 2022;33(11-12):614-24
116. Russell S, Bennett J, Wellman JA, Chung DC, Yu ZF, Tillman A. et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849-60
117. D'Antiga L, Beuers U, Ronzitti G, Brunetti-Pierri N, Baumann U, Di Giorgio A. et al. Gene therapy in patients with the Crigler-Najjar Syndrome. N Engl J Med. 2023;389(7):620-31
118. Sack BK, Herzog RW. Evading the immune response upon in vivo gene therapy with viral vectors. Curr Opin Mol Ther. 2009;11(5):493-503
119. Arruda VR, Samelson-Jones BJ. Obstacles and future of gene therapy for hemophilia. Expert Opin Orphan Drugs. 2015;3(9):997-1010
120. Perrin GQ, Herzog RW, Markusic DM. Update on clinical gene therapy for hemophilia. Blood. 2019;133(5):407-14
121. Muhuri M, Levy DI, Schulz M, McCarty D, Gao G. Durability of transgene expression after rAAV gene therapy. Mol Ther. 2022;30(4):1364-80
122. Deyle DR, Russell DW. Adeno-associated virus vector integration. Curr Opin Mol Ther. 2009;11(4):442-7
123. European Medicines Agency. Luxturna (voretigene neparvovec) summary of product characteristics. 2018 https://www.ema.europa.eu/en/documents/product-information/luxturna-epar-product-information_en.pdf (accessed 10 October 2023).
124. US Food and Drug Administration. Luxturna (voretigene neparvovec-rzyl) prescribing information. 2022 (last update May 2022). https://www.fda.gov/media/109906/download (accessed 10 October 2023).
125. European Medicines Agency. Zolgensma (onasemnogene abeparvovec) summary of product characteristics. 2023 https://www.ema.europa.eu/en/documents/product-information/zolgensma-epar-product-information_en.pdf (accessed 10 October 2023).
126. US Food and Drug Administration. Zolgensma (onasemnogene abeparvovec-xioi) prescribing information. 2021 (last update February 2023). https://www.fda.gov/media/126109/download (accessed 10 October 2023).
127. European Medicines Agency. Roctavian (valoctocogene roxaparvovec) summary of product characteristics. 2022 https://www.ema.europa.eu/en/documents/product-information/roctavian-epar-product-information_en.pdf (accessed 10 October 2023).
128. BioMarin. BioMarin resubmits biologics license application (BLA) for valoctocogene roxaparvovec AAV gene therapy for severe hemophilia A to the FDA [media release]. 2022 https://investors.biomarin.com/2022-09-29-BioMarin-Resubmits-Biologics-License-Application-BLA-for-Valoctocogene-Roxaparvovec-AAV-Gene-Therapy-for-Severe-Hemophilia-A-to-the-FDA (accessed 10 October 2023).
129. European Medicines Agency. Hemgenix (etranacogene dezaparvovec) summary of opinion (initial authorisation). 2022 (last update 15 December 2022). https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-hemgenix_en.pdf (accessed 9 October 2023).
130. US Food and Drug Administration. HEMGENIX (etranacogene dezaparvovec-drlb) prescribing information. 2022 (last update November 2022). https://www.fda.gov/media/163467/download (accessed 10 October 2023).
131. ClinicalTrials.gov. Study to evaluate the efficacy and safety of PF-07055480 / Giroctocogene fitelparvovec gene therapy in moderately severe to severe hemophilia A adults (AFFINE) [NCT04370054]. 2022 (last update 28 September 2023). https://clinicaltrials.gov/ct2/show/NCT04370054 (accessed 10 October 2023).
132. ClinicalTrials.gov. A study to evaluate the efficacy and safety of factor IX gene therapy with pf-06838435 in adult males with moderately severe to severe hemophilia B (BENEGENE-2) (NCT03861273). 2022 (last update 14 September 2023). https://clinicaltrials.gov/ct2/show/NCT03861273 (accessed 9 October 2023).
133. ClinicalTrials.gov. Efficacy study of GS010 for the treatment of vision loss up to 6 months from onset in LHON due to the ND4 mutation (RESCUE) [NCT02652767]. 2022 (last update 29 July 2022). https://clinicaltrials.gov/ct2/show/NCT02652767?term=Lenadogene+nolparvovec&draw=2&rank=4 (accessed 10 October 2023).
134. ClinicalTrials.gov. Efficacy study of GS010 for treatment of vision loss from 7 months to 1 year from onset in LHON due to the ND4 mutation (REVERSE) [NCT02652780]. 2022 (last update 23 January 2020). https://clinicaltrials.gov/ct2/show/NCT02652780?term=Lenadogene+nolparvovec&draw=2&rank=3 (accessed 10 October 2023).
135. ClinicalTrials.gov. RESCUE and REVERSE long-term follow-up (RESTORE) [NCT03406104]. 2022 (last update 27 July 2022). https://clinicaltrials.gov/ct2/show/NCT03406104?term=Lenadogene+nolparvovec&draw=2&rank=2 (accessed 10 October 2023).
136. ClinicalTrials.gov. Efficacy & safety study of bilateral IVT injection of GS010 in LHON subjects due to the ND4 mutation for up to 1 Year (REFLECT) [NCT03293524]. 2022 (last update 3 August 2022). https://clinicaltrials.gov/ct2/show/NCT03293524?term=Lenadogene+nolparvovec&draw=2&rank=1 (accessed 10 October 2023).
137. US Food and Drug Administration. Elevidys (delandistrogene moxeparvovec-rokl) presxribing information. 2023 https://www.fda.gov/media/169679/download (accessed 9 October 2023).
138. European Medicines Agency. Upstaza (eladocagene exuparvovec) summary of product characteristics. 2022 (last update 9 June 2023). https://www.ema.europa.eu/en/medicines/human/EPAR/upstaza (accessed 9 October 2023).
139. Reinholt B, Sterling R, Ofori J, Benson M, St. Charles J, Smith A. Seroprevalence of antibodies against two AAV serotype 2 vectors in healthy subjects and implications for clinical gene therapy programs [abstract 1210]. Mol Ther. 2020;28(4 suppl 1):521
140. Murphy SL, Li H, Mingozzi F, Sabatino DE, Hui DJ, Edmonson SA. et al. Diverse IgG subclass responses to adeno-associated virus infection and vector administration. J Med Virol. 2009;81(1):65-74
141. Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(18):11854-9
142. Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF. et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704-12
143. Majowicz A, van Waes F, Timmer N, van Deventer S, Ferreira V. Prevalence and affinity/avidity assessment of pre-existing NABs against AAV2, 5 and 8 analyzed in the serum of 300 healthy donors [poster P054]. European Association for Haemophilia and Allied Disorders, The Hague, Netherlands, February 5-7. 2020
144. Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R. et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535-44
145. Rajavel KS, Ayash-Rashkovsky M, Tang Y. Prevalence of preexisting immunity to adeno-associated virus (AAV) in adults with hemophilia: interim results from an international epidemiology study [abstract PB0304]. Res Pract Thromb Haemost. 2019;3(Suppl 1):304
146. Leebeek FW, Miesbach W, Recht M, Key NS, Lattimore S, Castaman G. et al. Clinical outcomes in adults with hemophilia B with and without pre-existing neutralizing antibodies to AAV5: 6 month data from the phase 3 etranacogene dezaparvovec HOPE-B gene therapy trial [abstract]. Res Pract Thromb Haemost. 2021;5(Suppl 1):OC 67.3
147. Pipe SW, Leebeek FW, Recht M, Key NS, Lattimore S, Castaman G. et al. 52 week efficacy and safety of etranacogene dezaparvovec in adults with severe or moderate-severe hemophilia B: data from the phase 3 HOPE-B gene therapy trial [abstract]. Res Pract Thromb Haemost. 2021;5(Suppl 2):PB0653
148. Pipe SW, Ferrante F, Reis M, Wiegmann S, Lange C, Braun M. et al. First-in-human gene therapy study of AAVhu37 capsid vector technology in severe hemophilia A - BAY 2599023 has broad patient eligibility and stable and sustained long-term expression of FVIII [abstract]. Blood. 2020;136(Suppl 1):44-5
149. Daniel HD, Kumar S, Kannangai R, Lakshmi KM, Agbandje-Mckenna M, Coleman K. et al. Prevalence of adeno-associated virus 3 capsid binding and neutralizing antibodies in healthy and hemophilia B individuals from India. Hum Gene Ther. 2021;32(9-10):451-7
150. Klamroth R, Hayes G, Andreeva T, Suzuki T, Hardesty B, Shima M. et al. Global seroprevalence of pre-existing immunity against AAV serotypes in people with hemophilia A [abstract]. Res Pract Thromb Haemost. 2021;5(Suppl 2):LPB0022
151. Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6(9):1574-83
152. Zygmunt DA, Crowe KE, Flanigan KM, Martin PT. Comparison of serum rAAV serotype-specific antibodies in patients with Duchenne muscular dystrophy, Becker muscular dystrophy, inclusion body myositis, or GNE myopathy. Hum Gene Ther. 2017;28(9):737-46
153. Boissier MC, Lemeiter D, Clavel C, Valvason C, Laroche L, Begue T. et al. Synoviocyte infection with adeno-associated virus (AAV) is neutralized by human synovial fluid from arthritis patients and depends on AAV serotype. Hum Gene Ther. 2007;18(6):525-35
154. Cottard V, Valvason C, Falgarone G, Lutomski D, Boissier MC, Bessis N. Immune response against gene therapy vectors: influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J Clin Immunol. 2004;24(2):162-9
155. Cheng Y, Huang L, Li X, Qi H, Zhou P, Yu W. et al. Prevalence of neutralizing factors against adeno-associated virus types 2 in age-related macular degeneration and polypoidal choroidal vasculopathy. Curr Gene Ther. 2013;13(3):182-8
156. Lee S, Kang IK, Kim JH, Jung BK, Park K, Chang H. et al. Relationship between neutralizing antibodies against adeno-associated virus in the vitreous and serum: effects on retinal gene therapy. Transl Vis Sci Technol. 2019;8(2):14
157. Harrington EA, Sloan JL, Manoli I, Chandler RJ, Schneider M, McGuire PJ. et al. Neutralizing antibodies against adeno-associated viral capsids in patients with mut methylmalonic acidemia. Hum Gene Ther. 2016;27(5):345-53
158. Ferla R, Claudiani P, Savarese M, Kozarsky K, Parini R, Scarpa M. et al. Prevalence of anti-adeno-associated virus serotype 8 neutralizing antibodies and arylsulfatase B cross-reactive immunologic material in mucopolysaccharidosis VI patient candidates for a gene therapy trial. Hum Gene Ther. 2015;26(3):145-52
159. Aronson SJ, Veron P, Collaud F, Hubert A, Delahais V, Honnet G. et al. Prevalence and relevance of pre-existing anti-adeno-associated virus immunity in the context of gene therapy for Crigler-Najjar Syndrome. Hum Gene Ther. 2019;30(10):1297-305
160. Kascsak RJ, Carp RI, Vilcek JT, Donnenfeld H, Bartfeld H. Virological studies in amyotrophic lateral sclerosis. Muscle Nerve. 1982;5(2):93-101
161. Majowicz A, van Waes F, Valles A, van Deventer S, Gilling M, Ehrhardt A. et al. Presence of pre-existing anti-adeno-associated virus (AAV) serotype 5 neutralizing antibodies (NABs) in serum of Huntington disease (HD) patients was not associated with detectable anti-AAV5 NABs in cerebrospinal fluid (CSF) [abstract 736]. Mol Ther. 2021;29(4 Suppl 1):357
162. Huser D, Khalid D, Lutter T, Hammer EM, Weger S, Hessler M. et al. High prevalence of infectious adeno-associated virus (AAV) in Human peripheral blood mononuclear cells indicative of T lymphocytes as sites of AAV persistence. J Virol. 2017;91(4):e02137-16
163. Walz CM, Nakamura M, Fukunaga T, Jasiewicz Y, Edler L, Schlehofer JR. et al. Reduced prevalence of serum antibodies against adeno-associated virus type 2 in patients with adult T-cell leukaemia lymphoma. J Med Virol. 2001;65(1):185-9
164. Goedeker NL, Griffin D, Dharia S, Santra S, Coy J, Yocum N. et al. Evaluation of total binding antibodies against rAAVrh74 in patients with Duchenne muscular dystrophy [abstract]. Mol Ther. 2022;30(4 Suppl 1):563
165. Ferreira V, Twisk J, Kwikkers K, Aronica E, Brisson D, Methot J. et al. Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPL(S447X)) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum Gene Ther. 2014;25(3):180-8
166. Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S. et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol. 2010;68(5):629-38
167. Johnson PR, Schnepp BC, Connell MJ, Rohne D, Robinson S, Krivulka GR. et al. Novel adeno-associated virus vector vaccine restricts replication of simian immunodeficiency virus in macaques. J Virol. 2005;79(2):955-65
168. Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT. et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A. 2009;106(38):16363-8
169. Long B, Robinson T, Day J, Yu H, Lau K, Patton K. et al. Interim 52-week analysis of immunogenicity to the vector capsid and transgene-expressed human FVIII in GENEr8-1, a phase 3 clinical study of valoctocogene roxaparvovec, an AAV5-mediated gene therapy for haemophilia A [abstract]. Haemophilia. 2022;28(S3):23-4
170. Xue F, Li H, Wu X, Liu W, Zhang F, Tang D. et al. Safety and activity of an engineered, liver-tropic adeno-associated virus vector expressing a hyperactive Padua factor IX administered with prophylactic glucocorticoids in patients with haemophilia B: a single-centre, single-arm, phase 1, pilot trial. Lancet Haematol. 2022;9(7):e504-e13
171. George LA, Ragni MV, Rasko JEJ, Raffini LJ, Samelson-Jones BJ, Ozelo M. et al. Long-term follow-up of the first in human intravascular delivery of AAV for gene transfer: AAV2-hFIX16 for severe hemophilia B. Mol Ther. 2020;28(9):2073-82
172. Bouquet C, Vignal Clermont C, Galy A, Fitoussi S, Blouin L, Munk MR. et al. Immune response and intraocular inflammation in patients with leber hereditary optic neuropathy treated with intravitreal injection of recombinant adeno-associated virus 2 carrying the ND4 gene: a secondary analysis of a phase 1/2 clinical trial. JAMA Ophthalmol. 2019;137(4):399-406
173. Chien YH, Lee NC, Tseng SH, Tai CH, Muramatsu SI, Byrne BJ. et al. Efficacy and safety of AAV2 gene therapy in children with aromatic L-amino acid decarboxylase deficiency: an open-label, phase 1/2 trial. Lancet Child Adolesc Health. 2017;1(4):265-73
174. Feuer WJ, Schiffman JC, Davis JL, Porciatti V, Gonzalez P, Koilkonda RD. et al. Gene therapy for leber hereditary optic neuropathy: initial results. Ophthalmology. 2016;123(3):558-70
175. Ghazi NG, Abboud EB, Nowilaty SR, Alkuraya H, Alhommadi A, Cai H. et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum Genet. 2016;135(3):327-43
176. Hwu WL, Muramatsu S, Tseng SH, Tzen KY, Lee NC, Chien YH. et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency. Sci Transl Med. 2012;4(134):134ra61
177. Mehendale S, van Lunzen J, Clumeck N, Rockstroh J, Vets E, Johnson PR. et al. A phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C adeno-associated virus vaccine. AIDS Res Hum Retroviruses. 2008;24(6):873-80
178. Mehendale S, Sahay S, Thakar M, Sahasrabuddhe S, Kakade M, Shete A. et al. Safety & iimmunogenicity of tgAAC09, a recombinant adeno-associated virus type 2 HIV-1 subtype C vaccine in India. Indian J Med Res. 2010;132:168-75
179. Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL. et al. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18(3):643-50
180. Vardas E, Kaleebu P, Bekker LG, Hoosen A, Chomba E, Johnson PR. et al. A phase 2 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 vaccine based on adeno-associated virus. AIDS Res Hum Retroviruses. 2010;26(8):933-42
181. Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L. et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979-90
182. Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL. et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009;20(9):999-1004
183. Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N. et al. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther. 2008;19(5):463-74
184. Brantly ML, Spencer LT, Humphries M, Conlon TJ, Spencer CT, Poirier A. et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum Gene Ther. 2006;17(12):1177-86
185. Flotte TR, Zeitlin PL, Reynolds TC, Heald AE, Pedersen P, Beck S. et al. Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: a two-part clinical study. Hum Gene Ther. 2003;14(11):1079-88
186. Moss RB, Rodman D, Spencer LT, Aitken ML, Zeitlin PL, Waltz D. et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest. 2004;125(2):509-21
187. Wagner JA, Nepomuceno IB, Messner AH, Moran ML, Batson EP, Dimiceli S. et al. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther. 2002;13(11):1349-59
188. D'Avola D, López-Franco E, Sangro B, Pañeda A, Grossios N, Gil-Farina I. et al. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J Hepatol. 2016;65(4):776-83
189. Cukras C, Wiley HE, Jeffrey BG, Sen HN, Turriff A, Zeng Y. et al. Retinal AAV8-RS1 gene therapy for X-linked retinoschisis: initial findings from a phase i/iia trial by intravitreal delivery. Mol Ther. 2018;26(9):2282-94
190. Rapti K, Louis-Jeune V, Kohlbrenner E, Ishikawa K, Ladage D, Zolotukhin S. et al. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol Ther. 2012;20(1):73-83
191. Saade D, Bharucha-Goebel D, Foley AR, Neuhaus SB, Soldatos A, Hu Y. et al. Review of CSF and peripheral immune responses following intrathecal gene transfer for giant axonal neuropathy [abstract]. American Society of Gene and Cell Therapy, Chicago, IL, May 16-19. 2018
192. Lyon AR, Babalis D, Morley-Smith AC, Hedger M, Suarez Barrientos A, Foldes G. et al. Investigation of the safety and feasibility of AAV1/SERCA2a gene transfer in patients with chronic heart failure supported with a left ventricular assist device - the SERCA-LVAD trial. Gene Ther. 2020;27(12):579-90
193. Casazza JP, Cale EM, Narpala S, Yamshchikov GV, Coates EE, Hendel CS. et al. Safety and tolerability of AAV8 delivery of a broadly neutralizing antibody in adults living with HIV: a phase 1, dose-escalation trial. Nat Med. 2022;28(5):1022-30
194. Vrouwe JPM, Meulenberg JJM, Klarenbeek NB, Navas-Cañete A, Reijnierse M, Ruiterkamp G. et al. Administration of an adeno-associated viral vector expressing interferon-β in patients with inflammatory hand arthritis, results of a phase I/II study. Osteoarthritis Cartilage. 2022;30(1):52-60
195. Pupo A, Fernandez A, Low SH, Francois A, Suarez-Amaran L, Samulski RJ. AAV vectors: the Rubik's cube of human gene therapy. Mol Ther. 2022;30(12):3515-41
Author contact
![]() Corresponding author: John E.J. Rasko, MBBS, PhD, Head of Department, Cell & Molecular Therapies, Royal Prince Alfred Hospital, Camperdown, Sydney, NSW 2050, Australia. Tel: +612 95656160. E-mail: j.raskoorg.au; ORCID: 0000-0003-2975-807X.
Corresponding author: John E.J. Rasko, MBBS, PhD, Head of Department, Cell & Molecular Therapies, Royal Prince Alfred Hospital, Camperdown, Sydney, NSW 2050, Australia. Tel: +612 95656160. E-mail: j.raskoorg.au; ORCID: 0000-0003-2975-807X.
 Global reach, higher impact
Global reach, higher impact