13.3
Impact Factor
Theranostics 2024; 14(3):1224-1240. doi:10.7150/thno.91119 This issue Cite
Research Paper
Tumor cell senescence-induced macrophage CD73 expression is a critical metabolic immune checkpoint in the aging tumor microenvironment
1. Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China
2. Institute of Radiation Oncology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China
3. Hubei Key Laboratory of Precision Radiation Oncology, Wuhan 430022, China
* These authors have contributed equally to this article.
Abstract
Background: The role of senescent cells in the tumor microenvironment (TME) is usually bilateral, and diverse therapeutic approaches, such as radiotherapy and chemotherapy, can induce cellular senescence. Cellular interactions are widespread in the TME, and tumor cells reprogram immune cells metabolically by producing metabolites. However, how senescent cells remodel the metabolism of TME remains unclear. This study aimed to explore precise targets to enhance senescent cells-induced anti-tumor immunity from a metabolic perspective.
Methods: The in vivo senescence model was induced by 8 Gy×3 radiotherapy or cisplatin chemotherapy, and the in vitro model was induced by 10 Gy-irradiation or cisplatin treatment. Metabonomic analysis and ELISA assay on tumor interstitial fluid were performed for metabolites screening. Marker expression and immune cell infiltration in the TME were analyzed by flow cytometry. Cell co-culture system and senescence-conditioned medium were used for crosstalk validation in vitro. RNA sequencing and rescue experiments were conducted for mechanism excavation. Immunofluorescence staining and single-cell transcriptome profiling analysis were performed for clinical validation.
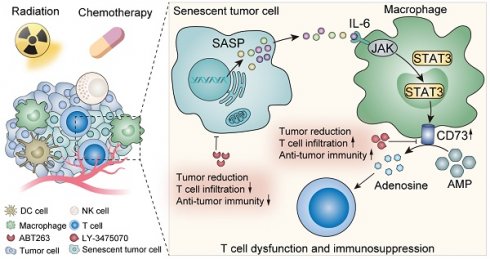
Results: We innovatively reveal the metabolic landscape of the senescent TME, characterized with the elevation of adenosine. It is attributed to the senescent tumor cell-induced CD73 upregulation of tumor-associated macrophages (TAMs). CD73 expression in TAMs is evoked by SASP-related pro-inflammatory cytokines, especially IL-6, and regulated by JAK/STAT3 pathway. Consistently, a positive correlation between tumor cells senescence and TAMs CD73 expression is identified in lung cancer clinical specimens and databases. Lastly, blocking CD73 in a senescent background suppresses tumors and activates CD8+ T cell-mediated antitumor immunity.
Conclusions: TAMs expressed CD73 contributes significantly to the adenosine accumulation in the senescent TME, suggesting targeting CD73 is a novel synergistic anti-tumor strategy in the aging microenvironment.
Keywords: senescence, CD73, tumor-associated macrophages, cancer immunotherapy, tumor microenvironment
 Global reach, higher impact
Global reach, higher impact