13.3
Impact Factor
Theranostics 2024; 14(3):954-972. doi:10.7150/thno.90538 This issue Cite
Research Paper
Endometrial organoids: a reservoir of functional mitochondria for uterine repair
1. Department of Biomedical Science, School of Life Science, CHA University; 335 Pangyo-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, South Korea.
2. Department of Biochemistry, Research Institute for Basic Medical Science, School of Medicine, CHA University; 335 Pangyo-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, South Korea.
3. CHA Fertility Center Bundang; 59, Yatap-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, South Korea.
#These authors contributed equally to this work.
Abstract
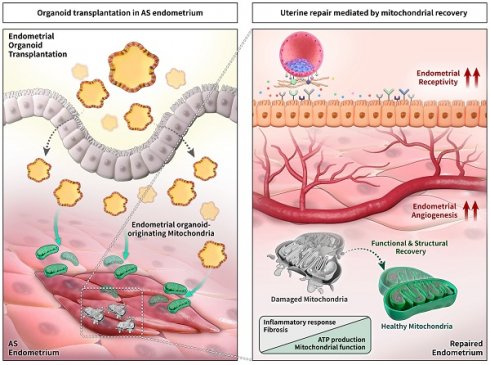
Background: Asherman's syndrome (AS) is a dreadful gynecological disorder of the uterus characterized by intrauterine adhesion with severe fibrotic lesions, resulting in a damaged basalis layer with infertility. Despite extensive research on overcoming AS, evidence-based effective and reproducible treatments to improve the structural and functional morphology of the AS endometrium have not been established.
Methods: Endometrial organoids generated from human or mouse endometrial tissues were transplanted into the uterine cavity of a murine model of AS to evaluate their transplantable feasibility to improve the AS uterine environment. The successful engraftment of organoid was confirmed by detection of human mitochondria and cytosol (for human endometrial organoid) or enhanced green fluorescent protein signals (for mouse endometrial organoid) in the recipient endometrium. The therapeutic effects mediated by organoid transplantation were examined by the measurements of fibrotic lesions, endometrial receptivity and angiogenesis, and fertility assessment by recording the number of implantation sites and weighing the fetuses and placenta. To explore the cellular and molecular mechanisms underlying the recovery of AS endometrium, we evaluated the status of mitochondrial movement and biogenetics in organoid transplanted endometrium.
Results: Successfully engrafted endometrial organoids with similar morphological and molecular features to the parental tissues dramatically repaired the AS-induced damaged endometrium, significantly reducing fibrotic lesions and increasing fertility outcomes in mice. Moreover, dysfunctional mitochondria in damaged tissues, which we propose might be a key cellular feature of the AS endometrium, was fully recovered by functional mitochondria transferred from engrafted endometrial organoids. Endometrial organoid-originating mitochondria restored excessive collagen accumulation in fibrotic lesions and shifted uterine metabolic environment to levels observed in the normal endometrium.
Conclusions: Our findings suggest that endometrial organoid-originating mitochondria might be key players to mediate uterine repair resulting in fertility enhancement by recovering abrogated metabolic circumstance of the endometrium with AS. Further studies addressing the clinical applicability of endometrial organoids may aid in identifying new therapeutic strategies for infertility in patients with AS.
Keywords: Asherman's syndrome, Endometrial organoid, Mitochondria, Infertility, Uterine repair
 Global reach, higher impact
Global reach, higher impact