13.3
Impact Factor
Theranostics 2024; 14(3):940-953. doi:10.7150/thno.91268 This issue Cite
Review
Peptide receptor radionuclide therapy combinations for neuroendocrine tumours in ongoing clinical trials: status 2023
1. Department of Nuclear Medicine, Medical University of Innsbruck, Innsbruck, Austria.
2. Department of Experimental and Clinical Medicine, “Magna Graecia” University of Catanzaro, Catanzaro, Italy.
Received 2023-10-18; Accepted 2023-12-4; Published 2024-1-1
Abstract
A growing body of literature reports on the combined use of peptide receptor radionuclide therapy (PRRT) with other anti-tumuor therapies in order to anticipate synergistic effects with perhaps increased safety issues. Combination treatments to enhance PRRT outcome are based on improved tumour perfusion, upregulation of somatostatin receptors (SSTR), radiosensitization with DNA damaging agents or targeted therapies. Several Phase 1 or 2 trials are currently recruiting patients in combined regimens. The combination of PRRT with cytotoxic chemotherapy, capecitabine and temozolomide (CAPTEM), seems to become clinically useful especially in pancreatic neuroendocrine tumours (pNETs) with acceptable safety profile. Neoadjuvant PRRT prior to surgery, PRRT combinations of intravenous and intraarterial routes of application, combinations of PRRT with differently radiolabelled (alpha, beta, Auger) SSTR-targeting agonists and antagonists, inhibitors of immune checkpoints (ICIs), poly (ADP-ribose) polymerase-1 (PARP1i), tyrosine kinase (TKI), DNA-dependent protein kinase, ribonucleotide reductase or DNA methyltransferase (DMNT) are tested in currently ongoing clinical trials. The combination with [131I]I-MIBG in rare NETs (such as paraganglioma, pheochromocytoma) and new non-SSTR-targeting radioligands are used in the personalization process of treatment. The present review will provide an overview of the current status of ongoing PRRT combination treatments.
Keywords: PRRT, combination treatments, chemotherapy, neuroendocrine tumours, SSTR
Introduction
The current status of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumour (NET) patients involves a standardized treatment protocol with [177Lu]Lu-DOTATATE given in four cycles (7.4 GBq, eight weeks apart), under amino acid infusion for reduction of the absorbed kidney radiation dose [1,2]. Increased availability through the industry based on FDA and EMA appropriation has led to an increased experience world-wide and to the incorporation of PRRT into various oncological guidelines [3-11]. The clinical impact of somatostatin receptor (SSTR) positron emission tomography/computed tomography (PET/CT) [12] for the follow-up and clinical management was demonstrated for more than one third of NET patients [13]. Furthermore, the role of [18F]F-FDG PET/CT in NET patients has been under debate in recent years, and several groups have evaluated the potential use of dual tracer PET/CT in NET patients [14-16] resulting also in prognostic grading proposals [17].
The first disease control data with [177Lu]Lu-DOTATATE in gastroenteropancreatic (GEP)-NET patients were already published in 2008 by the Rotterdam group [18] who also reported similar effective long-term data for bronchial carcinoids in 2017 [19] with a median overall survival (OS) ranging from 52 to 71 months. In the Phase 3 NETTER-1 study [2], with a median follow-up of >6.3 years, the pre-specified final analysis of OS in the intention-to-treat population did not reach statistical significance (HR, 0.84 [95% CI: 0.60, 1.17]; p = 0.30, two-sided) which was potentially impacted by a high rate of cross-over (36%) of patients in the control arm to PRRT. The median OS was 48.0 months in the [177Lu]Lu-DOTATATE arm and 36.3 months in the control arm (i.e. 11.3 months survival benefit). The NETTER-1 safety data showed a low incidence of long-term side-effects regarding haematotoxicity and nephrotoxicity (6/111 (5.4%) patients had ≥Grade 3 nephrotoxicity, no new cases of myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML)). In our own retrospective long-term study report [20] the median OS was 9 years for responders and 2 years for non-responders, and one third of patients was still alive after the ≥12-year follow-up.
The European Association of Nuclear Medicine (EANM) focus guideline [11] suggests PRRT as a) first line treatment in non-resectable or disseminated NET in a minority of highly selected patients with high SSTR-expression (based on risk and symptoms, primary tumour location), b) PRRT as second line treatment for GEP-NET, if there is sufficient SSTR-expression in all lesions, c) consideration of PRRT in GEP-NET G1 and G2 (Ki-67 <20%) patients at first disease progression when all lesions are matched in [68Ga]Ga-DOTA-SSTR / [18F]F-FDG PET/CT and d) PRRT in a minority of patients with G3 NET (Ki-67 >20%), preferably within clinical study protocols, such as in combination with capecitabine (CAP) and temozolomide (TEM). Re-challenge PRRT should be considered in patients with disease stabilization or remission for at least one year after end of first PRRT. Our own [14, 21] and other [22, 23] data strongly suggest that re-challenge PRRT can be as effective as the first course of PRRT with similar safety profile.
The combined use of PRRT with other anti-tumour therapies anticipates synergistic effects with perhaps increased safety issues. Only a few studies have so far evaluated the combination of PRRT with other treatments such as cytotoxic chemotherapy or molecular targeted treatments. Generally, combination treatments to enhance PRRT outcome are based on either improved tumour perfusion, upregulation of SSTR or radiosensitization with DNA damaging agents or targeted therapies [24].
Several Phase 1 or 2 trials are currently recruiting patients in combined regimens. Here we overview the current status of ongoing PRRT combination therapies.
Role of diagnostic imaging in combination treatments
NETs are heterogeneous neoplasms exhibiting intra- and inter-lesion variability that could impact treatment success and prognosis. The growing knowledge about different imaging pathways as well as the progressive introduction of new radiopharmaceuticals for diagnosis and treatment could provide a real whole-body “in vivo” study to characterize each lesion and its receptor expression [25]. In other words, molecular images could overcome the limitation of a single-site biopsy, giving a better understanding of disease variability, and guiding to the best treatment option for each patient [26]. In clinical practice, advances in molecular imaging using dual tracer PET with [68Ga]Ga-DOTA-SSTR and [18F]F-FDG are emerging as a potential tool to investigate lesion differentiation, affecting patient management [27]. Namely, highly SSTR-avid (WHO G1/G2) NETs are usually treated with octreotide long-acting repeatable (LAR) followed by PRRT, whereas highly FDG-avid (WHO G3) NETs are commonly treated with chemotherapy. However, an intermediate “gray zone” exists, represented by those patients with high uptake on both [68Ga]Ga-DOTA-SSTR and [18F]F-FDG dual tracer PET/CTs [28]. For these patients, a therapeutic approach combining PRRT with chemotherapy may represent an effective strategy. As shown in Table 1A and B, most of the published data about the combination of PRRT plus chemotherapy include in the baseline assessment [18F]F-FDG PET/CT beyond SSTR expression. For instance, Nicolini et al. in their prospective study included [18F]F-FDG positive patients with a SUV >2.5 in at least one documented lesion [29]. Also, the Australian group used dual tracer imaging as part of the baseline assessment [30,31] showing a 27% of complete metabolic responders on [18F]F-FDG PET/CT after combined treatment. Similar results were also shown by Yordanova et al. [32]. This deserves a mention in order to underline the possible impact of combination therapies in [18F]F-FDG positive patients, who did not respond to PRRT alone, and the need for comparative studies for this setting of patients.
Moreover, the thera(g)nostics concept is not limited to the use of [18F]F-FDG, but could also be extended to the other combined treatments, running from the “old” use of [123/131I]I-MIBG to other potential targets including the gastrin releasing peptide receptor (GRP-R) [33], or glucagon-like peptide 1 (GLP-1) [34], as well as the fibroblast activating protein (FAP) [35]. In addition, the immuno-PET could pave the way for the study of PD-1/PD-L1 expression [36] and the poly (ADP-ribose) polymerase-1 (PARP1) imaging-based analysis was also proposed [37]. These possibilities could change the perspective of NET patients, allowing better patient selection, response prediction and follow-up, adapting treatment to the characteristics of each patient during the course of the disease.
Combination of PRRT with Chemotherapy (Figure 1; Table 1A, B)
The role of chemotherapy in NETs has evolved in recent years. Radiosensitizing low-dose chemotherapy may exert its effect via inhibition of DNA repair, cell proliferation arrest, increased DNA damage, or apoptosis. The CAPTEM regimen demonstrated significant anti-tumour activity in both pancreatic (p) NETs [38] and non-pancreatic NETs following several retrospective studies [39-42]. Owen et al. [41] demonstrated for pNET as well as non pNET a progression free survival (PFS) of 13 months and an OS of 29.3 months. In this study, partial remission (PR) was seen in 11 (38%), stable disease (SD) in 15 (52%) of patients giving a disease control rate (DCR) of 90%. A trend of increased response rate in patients with low O6-methylguanine DNA methyltransferase (MGMT) activity was also seen. On the other hand, in the study of Cives et al. [43] response to CAPTEM was not influenced by MGMT, proliferative activity or alternative lengthening telomeres (ALT) pathway activation.
Combination of PRRT with CHEMOTHERAPY
| Treatment Combination | n | Patients | Safety | Results | Reference |
|---|---|---|---|---|---|
| PRRT (5x5.5GBq) + CAP (1000 or 1500 mg/d/14d) [111In]In-Pentetreotide [68Ga]Ga-DOTATOC PET/CT [18F]F-FDG PET/CT | 37 | GEP-NET (1-3, Ki<55%) | 4xG3/4 neutropenia 1xG4 thrombocytopenia 1xG3 lymphopenia fatigue 5.4% diarrhea 5.4% | PR 30%, SD 55% DCR 85% PFS 31.4mo, OS not reached at 38mo | Nicolini 2021 (29) |
| PRRT+5-FU (200 mg/m2/24h) [111In]In-Pentetreotide [68Ga]Ga-DOTATOC PET/CT [18F]F-FDG PET/CT | 68 | mNET | not reported | OS 72.1% and 52.1% at 2 and 5 years, respectively | Kong 2014 (30) |
| PRRT + 5-FU (200mg/m2/24h) [111In]In-Pentetreotide [68Ga]Ga-DOTATOC PET/CT [18F]F-FDG PET/CT | 52 | mNET | 1xG4 thrombocytopenia 2xG3 thrombocytopenia 1xG3 liver failure | CR 2%, PR 28%, SD 68%, DCR 98% Metabolic response 27% Biochemical response 45% PFS 48mo, OS not reached at 36mo | Kashyap 2015 (31) |
| PRRT+ CAP (1650 mg/m2/d/14d) [111In]In-Pentetreotide | 7 | GEP-NET | 1xG3 thrombocytopenia | Not Reported | van Essen 2008 (45) |
| PRRT +TEM (150-250mg/m2) + CAPTEM (500-1000mg/m2) [68Ga]Ga-DOTATOC PET/CT [18F]F-FDG PET/CT | 2 12 | mNET mNET | 1xG4 liver failure 4xG3 liver failure | DCR (CT) 55% DCR (FDG) 38% DCR (Ga-DOTATOC) 44% PFS 7.1mo, OS 25.3mo | Yordanova 2019 (32) |
| PRRT + 5-FU (200 mg/m2/24h) PRRT + CAP (1500mg/b.i.d.) [111In]In-Pentetreotide | 27 2 | mNET | 1xG4 lymphopenia 1xG4 late anaemia and thrombocytopenia | OS 34mo | Hubble 2010 (46) |
| PRRT + CAP (1650 mg/m2/d/14d) [111In]In-Pentetreotide | 33 | mNET | 1xG3 thrombocytopenia 3xG3 angina | PR 24%, SD 70%, PD 6% DCR 94% | Claringbold 2011 (50) |
| PRRT + CAPTEM CAP (1500 mg/m2/d/14d) TEM (100-200 mg/m2/d/5d) | 35 | mNET | 1xG3 nausea/vomiting 2xG3 neutropenia 2xG3 angina | CR 15%, PR 38%, SD 38% DCR 91% PFS 31mo, OS not reached at 24mo | Claringbold 2012 (51) |
| PRRT+CAPTEM CAP (1500 mg/m2/d/14d) TEM (200 mg/m2/d/5d) | 30 | pNET | 3xG3 thrombocytopenia | CR 13%, PR 67%, SD 20%, Response rate 80% PFS 48mo, OS not reached at 33mo | Clairingbold 2016 (52) |
| PRRT + CAPTEM CAP (1500 mg/m2/d) TEM (200 mg/m2/24h) [111In]In-Pentetreotide | 12/56 | mNET (unknown primary) | 1xG3 HFS | PFS 10.8mo in Grade 2 PFS 7.0mo in Grade 3 | Chauhan 2018 (54) |
Abbreviations: PRRT, peptide receptor radionuclide therapy; CAP, capecitabine; TEM, temozolomide; mNET, metastatic neuroendocrine tumour; MTC; medullary thyroid cancer; Pheo/Para, pheochromocytoma/paraganglioma; pNET, pancreatic neuroendocrine tumour; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; DCR, disease control rate; PFS, progression-free survival; OS, overall survival; GEP, gastroenteropancreatic; HFS, hand-foot syndrome; PET, positron emission tomography; CT, computed tomography; FDG, fluorodeoxyglucose.
Combination of PRRT with CHEMOTHERAPY - Ongoing Prospective Studies (Status 1.9.2023)
| Treatment Combination | Centre/Sponsor | n | Patients | Study Phase | No trial (reference) | Status |
|---|---|---|---|---|---|---|
| PRRT+ CAPTEM vs CAPTEM alone PRRT+ CAPTEM vs PRRT alone | Australia | 72 | pNET mid gut mNET | 2 | NCT02358356 (56) | Completed |
| PRRT + Capecitabine vs PRRT alone (FDG-positive GEP-NET) | Italy | 35 | GEP-NET | 2 | NCT02736448 (57) | Unknown |
| PRRT+CAPTEM | Poland | 25 | mGEP-NET | 2 | NCT04194125 (58) | Unknown |
| PRRT + Capecitabine | Italy | 37 | mGEP-NET | 1/2 | NCT02736500 (59) | Unknown |
| PRRT+Capecitabine | Sweden | 300 | mNET | 3 | NCT05387603 (60) | Not yet recruiting |
| PRRT+Carboplatin, Etoposide, Tislelizumab | Novartis | 39 | ES-SCLC | 1 | NCT05142696 (61) | Recruiting |
Abbreviations: PRRT, peptide receptor radionuclide therapy; CAP, capecitabine; TEM, temozolomide; pNET, pancreatic neuroendocrine tumour; mNET, metastatic neuroendocrine tumour; mGEP-NET, metastatic gastroenteropancreatic neuroendocrine tumour; FDG, fluorodeoxyglucose; ES-SCLC, extensive stage small cell lung cancer
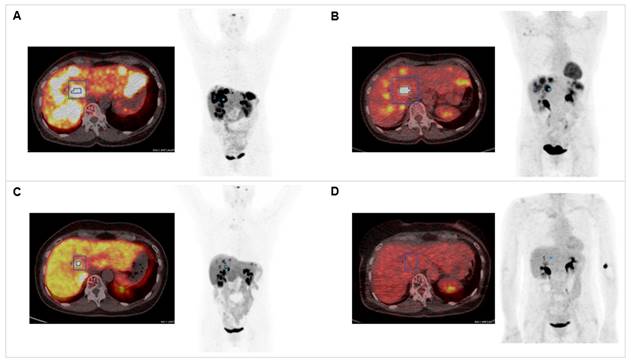
Combination of PRRT with CAPTEM. The patient was diagnosed with pancreatic NET (Ki-67 15%, pT3N0M0R0) following partial pancreatectomy in 2012. Dual tracer PET/CT with (A) [68Ga]Ga-DOTATOC and (B) [18F]F-FDG indicated multiple liver metastases in 2020. The combination treatment with 1500 mg/m2 capecitabine (CAP) and 200 mg/m2 temozolomide (TEM) with 177Lu[Lu]Lu-DOTATATE (accumulated activity 29.54 GBq) resulted in partial response in (C) [68Ga]Ga-DOTATOC PET/CT (SUVmax decreased from 57.43 to 24.92) and (D) complete response in 18[F]F-FDG PET/CT (SUVmax decreased from 14.51 to 4.76).
A retrospective critical analysis of the cytoreductive impact of systemic therapies in advanced pNETs identified a multi-agent chemotherapy in combination with PRRT as probably the best treatment strategy [44]. In an initial study on the combination of [177Lu]Lu-DOTATATE-PRRT with CAP in 7 patients, no Grade 4 haematotoxicity was reported and Grade 3 thrombocytopenia in one single patient only [45]. One of the first retrospective reports by the Melbourne group after high activity [111In]In-pentetreotide PRRT concluded that re-challenge PRRT with [177Lu]Lu-DOTATATE with either radiosensitizing infusional 5-Fluorouracil (5-FU, n=27) or CAP (n=2) is safe and well-tolerated with an OS of 34 months [46]. However, caution was recommended in patients with bone metastases with one single case of Grade 4 lymphocytopenia only. Hence, the authors concluded that in terms of safety, also the sequence of [177Lu]Lu-DOTATATE plus radiosensitizing chemotherapy after [90Y]Y-DOTATOC-PRRT should be safe [47] as Grade 3 or 4 haematotoxicity was found only in 3.6% in a large number of patients after re-challenge PRRT with [177Lu]Lu-DOTATATE [48]. Renal failure as the dose-limiting factor did not appear, and this probably is somewhat higher in a combination with [90Y]Y-based-PRRT [49].
A Phase 2 study [50] in 33 metastatic NET (mNET) patients treated with PRRT plus CAP (1650 mg/m2/d/14d) revealed 24% PR, 70% SD (i.e. DCR 94%) despite one single case of Grade 3 thrombocytopenia and 3 cases of Grade 3 angina. The same group demonstrated for on top-treatment with escalating doses of TEM (100-200 mg/m2/5d) no dose-limiting toxicities [51]. The commonest toxicities were Grade 3 nausea in 1 (3%) patient, Grade 3 neutropenia in 2 (6%) patients and Grade 3 angina in other 2 (6%) cases. Complete remission (CR) was achieved in 15%, PR in 38%, SD in 38% (DCR 91%). PFS was 31 months, OS was not reached at the 24-month follow-up. In 30 patients with pNET the same authors demonstrated an overall response rate of 80% (CR in 13%, PR in 67%), PFS 48 months, OS was not reached at the 33-month follow-up. In this study Grade 3 thrombocytopenia was observed in 10% of patients [52].
Nicolini et al. [29] reported for the combined use of PRRT (5x5.5 GBq) and CAP (1000 or 1500 mg/d) in 37 patients, assessed by dual-tracer PET/CT (Ki-67≤ 55%), haematotoxicity Grade 3 or 4 in 16.2% of patients, diarrhea in 5.4% and asthenia/fatigue in 5.4%. PR was seen in 10 patients (30%), SD in 18 patients (55%) and DCR in 85%, PFS was 31.4 months and OS was not reached at the 38th month of follow-up.
Kong et al. [30] assessed predictors of response and long-term survival following radiosensitizing infusional chemotherapy with 5-FU (200 mg/m2/24h) in combination with PRRT. A high proportion (70%) of patients received benefit from the treatment with OS rates at 2 and 5 years of 72.1% and 52.1%, respectively. Patients with pNET and lesions >5 cm appeared to have a lower objective response rate, thus needing a more aggressive approach. The same group previously reported under the same treatment regimen a complete metabolic response in 27% of FDG-avid patients and a biochemical response (i.e.>25% chromogranin-A decrease) in 45% of patients [31] and for patients with Ki >55% a PFS of 4 months and OS of 7 months [53].
Chauhan et al. [54] reported in 12 patients with unknown origin a PFS of 10.8 months for Grade 2 and 7 months for Grade 3 mNET patients. In this study, one patient developed the foot-hand syndrome Grade 3.
Yordanova et al. [32] reported for 15 patients with mNET a PFS of 7.1 months and OS of 25.3 months. The DCR was 55% when assessed by CT, 28% by [18F]F-FDG PET/CT and 44% by [68Ga]Ga-DOTATOC PET/CT.
The Australian study [55, 56] reported for pNETs a PFS of 61.1% when treated with PRRT+CAPTEM versus 33.3% for patients treated by CAPTEM only at the 27-month follow-up. In their evaluation, no difference was found for mNETs at the 36-month follow-up between both groups. However, the long-term results are not yet published.
Several other studies are currently recruiting NET patients combining PRRT with chemotherapy, particularly with CAPTEM [57-61]
New radioisotopes such as [225Ac]Ac-DOTATATE in combination with CAP (2 g/d, days 1-14) may impact future PRRT outcomes [62]. In this study the authors reported an improved OS also for patients who received prior [117Lu]Lu-DOTATATE. A poorer OS was estimated for patients with bone metastases by multivariate analysis while the OS probability at the 24-month follow-up was 70.8%.
Conclusion on PRRT plus Chemotherapy
Although prospective data are needed, PRRT in combination with chemotherapy seems to become an effective treatment option in patients with a wide variety of advanced metastatic NETs. The indication for combined treatment may be based on FDG-positivity and/or Ki-67 values greater than 20%, basically Grade 3 disease. No clinically significant toxicity has been reported so far for this combination, even in patients who have failed prior conventional therapies, except of one case of neutropenic sepsis [63]. The DCR seems to come up >90% depending on the response criteria used.
Combination of PRRT with PARP Inhibitors (Table 2)
Rendering the tumour cell more sensitive to radiation is one treatment strategy. Thus, inhibition of the DNA repair mechanism that repairs the DNA damage induced by radiation of PRRT could be a new treatment basis. The DNA damage consists of double- and single-strand DNA damages that are repaired by poly (ADP-ribose) polymerase-1 (PARP1) [64]. Hence, inhibitors of PARP1 (PARP1i) have become an important tool for inhibition therapy especially in BRCA1/2mut patients, leading to cytotoxicity which is termed “synthetic lethality”. In this context, the targeting of PARP1 has emerged as a nuclear imaging and thera(g)nostic modality. PARP-imaging agents may be potentially useful as a guidance to predict therapy response to PARP1i, or to monitor therapy response. In the past, several radiolabelled compounds were synthesized and also used in preclinical and clinical studies [65]. Among the tracers reported 18F-olaparib [66], a direct analog of olaparib, has recently gained significant attention and the alpha emitting compound [211At]At-MM4 [67] has shown encouraging anti-tumour activity despite of methodological challenges regarding radiochemistry on the one, and biodistribution, on the other hand.
Recently, PARP1i in combination with [117Lu]Lu-DOTATATE increased the anti-tumour activity in experimental animals [68, 68] as well as in human NET cells [70, 71].
Three Phase 1/2 studies are currently recruiting using escalating doses of either olaparib [72-74] or talazoparib [75] in combination with PRRT.
Combination of PRRT with PARP Inhibitors - Ongoing Prospective Studies (Status 1.9.2023)
| Treatment Combination | Centre/Sponsor | n | Patients | Study Phase | No trial (reference) | Status |
|---|---|---|---|---|---|---|
| 177Lu-DOTATATE + Olaparib p.o. 2 days before to 4 weeks after PRRT | NIH, USA Bethesda, Maryland | 37 | GEP-NET | 1/2 | NCT04086485 (72) | Recruiting |
| 177Lu-DOTATATE + Olaparib dose escalation study (3 doses) 100 + 200 + 300 mg/d, 18 days | Netherlands Erasmus Medical Center | 24 | locally advanced or mNET (G1-3) | 1 | NCT05870423 (73) | Recruiting |
| 177Lu-DOTA-TATE + Olaparib | Gothenburg, Sweden | 18 | SSRT-positive tumours | 1 | NCT04375267 (74) | Unknown |
| 177Lu-DOTATATE+Talazoparib dose escalation study (4 doses) 0.1, 0.25, 0.5 and 1 mg/d/days 2-6 | Australia Peter MacCallum Centre | 24 | mNET | 1 | NCT05053854 (75) | Recruiting |
Abbreviations: GEP-NET, gastroenteropancreatic neuroendocrine tumour; mNET, metastatic neuroendocrine tumour; SSRT, somatostatin receptor
Combination of PRRT with Checkpoint Inhibitors - Ongoing Prospective Studies (Status 1.9.2023)
| Treatment Combination | Centre/Sponsor | n | Patients | Study Phase | No trial (reference) | Status |
|---|---|---|---|---|---|---|
| PRRT+ Nivolumab (240 mg iv, d1+d15/28d cycle) | Spain (Multicentre) | 30 | NET G3 or NEC | 2 | NCT04525638 (81) | Active*) |
| PRRT+ Avelumab (10 mg/kg/ 2 we/24 mo) | Australia (Multicentre) | 38 | Merkel Cell Cancer | 1/2 | NCT04261855 (82) | Recruiting |
| PRRT+ Pembrolizumab vs Pembrolizumab+TAE vs Pembrolizumab+RE | University California, USA | 32 | mNET (Ki>20%, liver burden<75%) | 2 | NCT03457948 (83) | Active, not recruiting |
| PRRT+ Pembrolizumab (400 mg/6 we/24 mo) | Weill Medical College, Cornell, USA | 18 | Merkel Cell Cancer | 2 | NCT05583708 (84) | Recruiting |
*) some data published. Abbreviations: NET, neuroendocrine tumour; NEC, neuroendocrine carcinoma; mNET, metastatic neuroendocrine tumour; TAE, transarterial embolization; RE, radio embolization.
Combination of PRRT with Ribonucleotide Reductase, Tyrosin Kinase, DNA-dependent Protein Kinase or DNMT Inhibitors - Prospective Ongoing Studies (Status 1.9.2023)
| Treatment Combination | Centre/Sponsor | n | Patients | Study Phase | No trial (reference) | Status |
|---|---|---|---|---|---|---|
| 177Lu-DOTATATE+ Triapine p.o. days 1-14 | NIH, USA | 29 | mNET | 1 | NCT04234568 (86) | Active, not recruiting |
| 177Lu-DOTATATE+ Triapine | NCI, USA | 94 | NET | 2 | NCT05724108 (87) | Recruiting |
| 177Lu-DOTATATE + Peposertib p.o. days 1-21 | NIH, USA | 29 | pNET | 1 | NCT04750954 (89) | Recruiting |
| 177Lu-DOTATATE + Sunitinib malate p.o. days 1 - 28 | NIH, USA | 24 | pNET | 1 | NCT05687123 (90) | Recruiting |
| 177Lu-DOTATATE + Cabozantinib malate p.o. escalating 20, 40, 60 mg | Oregon, USA | 90 | mNET | 1 | NCT05249114 (91) | Recruiting |
| 177Lu-DOTATATE+ ASTX727 Cedazuridine 100 mg + Decitabine 35 mg days 0-5 | London, UK | 27 | NET | 1 | NCT05178693 (92) | Recruiting |
Abbreviations: mNET, metastatic neuroendocrine tumour; pNET, pancreatic neuroendocrine tumour; NET, neuroendocrine tumour
Conclusion on PRRT plus PARPi
Based on the preclinical studies the combination with PARPis has immense clinical potential and results of the ongoing clinical trials remain to be awaited.
Combination of PRRT with Immune-Checkpoint-Inhibitors (ICIs) (Table 3)
ICIs are considered to be a revolutionary treatment option in the field of primarily solid tumours and are increasingly used in multiple other tumour types [76, 77]. As for NET, only limited data are available. In an animal model Esfahani et al. [78] recently demonstrated that the combination of PRRT and anti-PD1 treatment with pembrolizumab showed the most robust inflammatory response to NETs and a better overall outcome than ICIs or PRRT alone. The most effective regimen is PRRT preceding anti-PD1 administration by several days. The success of a combination treatment of PRRT with ICIs may be ruled out by prior immuno-PET, a new methodology emerged as a promising imaging tool for the prediction of response as well as the monitoring of response to treatment [79]. As both, ICIs and PRRT are registered products today, this approach is now translating into the clinic.
No dose limiting toxicity was observed in an initial small cohort of 9 NET patients reported by Kim at al. [80] with predominantly small cell lung cancer. The patients were treated by PRRT at half dose (3.7 GBq) in combination with nivolumab (days 1 and 15; 240 mg i.v.). In this heavily pretreated cohort one patient showed PR and two patients SD. This study is still recruiting [81].
Other studies are on the way including PRRT in combination with avelumab [82] or pembrolizumab [83] in Merkel Cell Cancer patients. A study combining PRRT and pembrolizumab versus pembrolizumab plus transarterial embolization (TAE) or pembrolizumab plus radioembolization (RE) for liver metastatic NET with a Ki-67 >20% is also active [84].
Conclusion on PRRT plus ICIs
Based on the preclinical studies the combination with ICIs has immense clinical potential and results of the ongoing clinical trials remain to be awaited.
Combination of PRRT with Inhibitors of Ribonucleotide Reductase, DNA-dependent Protein Kinase, Tyrosin Kinase (TKI) or DNA Methyltransferase (DNMT) (Table 4)
The ribonucleotide reductase inhibitor triapine [85] is currently tested in a PRRT combination study in mNET [86, 87]. The DNA-dependent protein kinase inhibitor peposertib [88] is tested in combination with PRRT in pNET [89]. The tyrosin kinase inhibitors sunitinib [90] and cabozantinib [91] are also tested in combination with PRRT. On the basis of SSTR2 upregulation also DNMT inhibitors such ASTX727 (i.e. cedazuridine and decitabine) is currently tested in combination with PRRT [92].
Conclusion on PRRT plus Anticancer Drugs
Based on the preclinical studies the combination with anticancer drugs has immense clinical potential and results of the ongoing clinical trials remain to be awaited.
Improvement of PRRT by Different Concepts (Table 5)
Combination of PRRT with [131I]I-MIBG
[131I] I-MIBG, a guanethidine analog of norepinephrine, has been used for the treatment of paraganglioma, pheochromocytoma and neuroblastoma as well as other NETs over decades. The “old” strategy of [123/131I]I-MIBG [93-95] is currently challenged by the new treatment paradigm of [68Ga]Ga / [177Lu]Lu- or [90Y]Y-SSTR-based thera(g)nostics [96,97], or supposedly also a combination of both. However, results are difficult to interpret as reports are mostly retrospective with low patient numbers only [11]. A recent Phase 1 study in a limited number of patients investigated the combined use of [131I]I-MIBG with [90Y]Y-DOTATOC in a dose escalating manner [98]. The calculated absorbed tumour dose estimates suggested an increase of 34 to 83% for the combination as opposed to PRRT alone with dose limits of 19 Gy to the kidneys and 0.15 Gy to the bone marrow. No dose-limiting toxicities were observed despite of one case Grade 3 thrombocytopenia.
A clinical trial evaluating the safety of PRRT in combination in with Azedra(R) to treat mNET (SPORE-3) is currently active [99]. No other clinical trials are currently on the way and such a combination remains for individual cases based on positive imaging results as presented in Figure 2.
Combination of PRRT with Neoadjuvant Surgery
Early PRRT can be applied for downstaging of the disease or as neoadjuvant treatment in order to make resection possible or improve the cure rate. Mainly reported for pNETs, neoadjuvant PRRT may also result in fewer surgical complications [100, 101].
Improvement of PRRT by Different Concepts
| Concept | Centre/Sponsor | n | Patients | Study Phase | No trial (reference) | Status |
|---|---|---|---|---|---|---|
| PRRT + 131I-MIBG | Iowa, USA | 50 | GEP-NET | 1/2 | NCT04614766 (99) | Recruiting*) |
| Neoadjuvant PRRT (2cycles) + surgery + PRRT (2cycles) | Standford, USA | 10 | mGEP-NET | 1 | NCT04609592 (103) | Recruiting |
| Neoadjuvant PRRT + surgery | Milano, Italy | 31 | pNET | 2 | NCT04385992 (104) | Completed |
| New SSTR-based radioligand 177Lu-OPS201 | IPSEN | 40 | mNET | 1/2 | NCT02592707 (116) | Terminated |
| combination with other radioligands 177Lu-DOTATOC+161Tb-DOTA-LM3 0.5-1 GBq + 0.5-1 GBq „cross-over“design | Basel, Switzerland | 16 | mGEP-NET | 1 | NCT05359146 (122) | Recruiting |
| New SSTR-based radioligand 212Pb-DOTAMTATE | Radiomedix | 33 | mNET | 1 | NCT03466216 (125) | Unknown |
| Combination of i.v. and i.a. routes (2 cycles i.a. then 2 cycles i.v.) | Memorial SKCC, USA | 10 | mGEP-NET, bronchial or unknown NET | 1 | NCT04544098 (131) | Recruiting |
| Combination of i.v. and i.a. routes (i.a. PRRT after 4 cycles i.v. PRRT) | Bordeaux, France | 20 | mGEPNET | 2 | NCT04837885 (132) | Recruiting |
*) some data published. Abbreviations: PRRT, peptide receptor radionuclide therapy; mGEP-NET, metastatic gastroenteropancreatic neuroendocrine tumour; pNET, pancreatic neuroendocrine tumour; mNET, metastatic neuroendocrine tumour
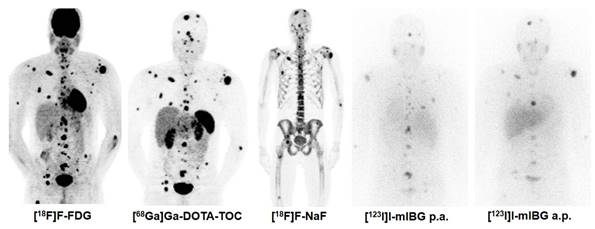
Combination Treatment of PRRT with [131I]I-mIBG. [68Ga]Ga-DOTA-TOC, [18F]F-FDG, [131I]I-mIBG, and [18F]F-NaF imaging in a 30 year old male patient with metastatic pheochromocytoma (functional tumour). In such rare cases, the combination treatment of PRRT with [131I]I-mIBG can be considered based on tumour accumulation of both thera(g)nostics.
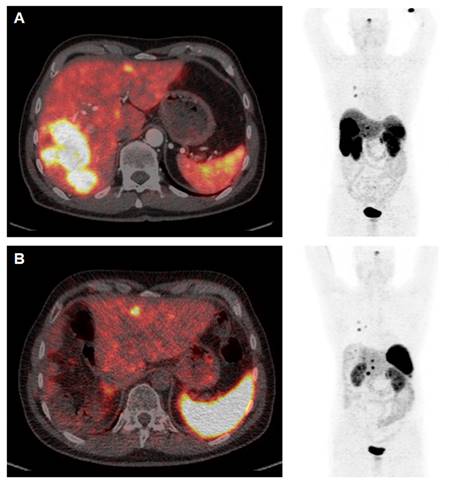
[68Ga]Ga-DOTATOC PET/CT Study Prior to (A) and 12 weeks after (B) Cytoreductive Surgery. The patient (64 y) was diagnosed with medullary thyroid cancer (MTC) at stage pT1a N1a R0 Mx in 2005. After complete thyroidectomy in July 2005 the disease was stable until 2014 when SSTR-positive liver metastases were diagnosed. PRRT with [177Lu]Lu-DOTATATE (accumulated activity 29.47 GBq) then resulted in disease stabilisation until January 2022. Appearance of increased size of liver metastases (A) were associated with increased episodes of watery diarrhea, not responsive to symptomatic therapy. In April 2023 the patient underwent cytoreductive surgery (B) and currently receives a postsurgery second period of [177Lu]Lu-DOTATATE PRRT. Symptoms are completely relieved and the patient has gained >10 kg body weight (September 2023).
Recently, Minczeles et al. [102] reported that early administration of PRRT followed by surgery is associated with favourable long-term outcomes in patients with locally advanced or oligometastatic pNET and can be considered for selected patients with vascular involvement and/or increased risk of recurrence. Two clinical studies are currently recruiting patients with either GEP-NET [103] or pNET [104] receiving neoadjuvant PRRT followed by surgery.
We include a clinical case of a patient with medullary thyroid cancer with mainly liver metastases who improved dramatically with the combination of cytoreductive surgery and PRRT (Figure 3).
Increasing PRRT Results by Addition of Long-Acting SST Analogs
The anti-proliferative effect of somatostatin (SST) analogs was established in the PROMID [105] and CLARINET [106] trials, and today PRRT is usually applied in combination with long-acting SST analogs. However, this basic combination therapy is not well established. Yordanova et al. [107] reported higher tumour response rates, especially in patients with higher tumour burden or higher Ki-67 values for the combination group. The results are controversial and no final statement was ever discussed [108-110].
Increasing PRRT Results by (Individualized) Dosing - Dosimetry
Basically, today dosimetry is not (anymore) a prerequisite for PRRT as we have come from single photon emission tomography (SPECT)/CT to PET/CT. However, for small-sized lesions (especially <2 cm) SSTR PET/CT increases the so-called “Krenning Scores” which were historically introduced to define patients for treatment with PRRT using [111In]In-DTPA-D-Phe1-octreotide [111]. Furthermore, Roth et al. [112] reported that the tumour absorbed dose decreases from cycle 1 to cycle 2 of PRRT by 6% for G1 tumours and 14% for G2 tumours. This may be caused by lower uptake in the tumour lesions and decreasing tumour volume as response to treatment. There are also estimations that a cumulative dose <29.6 GBq of [177Lu]Lu-DOTA-TATE is less efficacious in terms of tumour response and survival compared to patients receiving 29.6 GBq [113]. Dosimetry-based personalization of PRRT by increasing the injected activity until an absorbed kidney dose of 23 Gy has shown that a high proportion of patients is probably undertreated [114] using the one-size-fits-all regime [1, 2].
The debate on dosimetry cannot easily be solved [115]. While for the clinical setting, the “real world” use of PRRT, dosimetry may play a minor role for most patients, accurate dosimetry seems important in patients receiving PPRT combinations.
Increasing PRRT Results with New SSTR-Based Peptides/Radioligands
New SSTR-based radioligands include the group of antagonists. Among these, [177Lu]Lu-OPS201 [116, 117] and LM3 [118] have shown increased in vitro and in vivo binding to NET tumours despite of decreased kidney absorbed dose. By introducing an albumin binding moiety, the bioconjugate-modified [177Lu]Lu-EB-TATE has recently shown clinical potential [119, 120] in terms of increased SSTR-based tumour uptake. The concept of [64/67Cu]Cu-thera(g)nostics has so far had no real breakthrough due to technical production problems [121].
Increasing PRRT Results with New Isotopes, i.e. Alpha
Alpha-labelled SSTR-based analogs have recently gained increased interest in the clinical setting. The [161Tb]Tb-labelled SSTR antagonist LM3 currently is investigated in a combination setting with [177Lu]Lu-DOTATOC [122]. The new SSTR ligand [211Pb]Pb-DOTAMATE has also gained attraction in an ongoing clinical study [123]. Other possibilities in NET include [225Ac]Ac-labelled compounds such as [225Ac]Ac-DOTATATE [124] which has already shown good tolerability in PRRT-naïve patients [125]. “Dual PRRT” in general is based on combining SSTA analogs radiolabelled with different isotopes. Such combinations were [90Y]Y-labelled with [177Lu]Lu-labelled analogs in the past [126, 127] and nowadays a move towards a combination with alpha-emitting isotopes is registered based on higher energy transfer and lesser penetration range. In these combinations the lower energy and shorter tissue penetration range of maximal 2-4 mm for [177Lu]Lu products and of maximal 11 mm for [90Y]Y products are combined for large and bulky NET metastases. While several results for this combination are promising from the past [126, 127] the study NCT04029428 [128] is currently recruiting 150 NET patients of various origin using [90Y]Y (4x 3.7 GBq, [177Lu]Lu (4x5.55 GBq) or the mixed PRRT combination.
Increasing PRRT Results by Combination of Intravenous and Intraarterial Routes of Administration
Several attempts have been made in liver predominant disease to use the liver-directed intraarterial route for PRRT application in combination with the intravenous route of PRRT application [129]. As about two thirds of NET patients have metastatic liver disease and as the tissue penetration of [177Lu]Lu-DOTATATE is only 2-4 mm radioembolization of especially larger liver metastases with [166Ho]Ho or [90Y]Y seems to be meaningful. As the technique may lead to severe hepatotoxicity along with radioembolization, a multidisciplinary team is essential in the decision making process. Kratochwil et al. [130] reported successful results after intraarterial administration of [213Bi]Bi-DOTATOC in patients with liver metastases resistant to [90Y]Y/[177Lu]Lu-DOTATOC. Two studies are currently evaluating the combination of i.v. and i.a. routes of PRRT [131,132].
Increasing PRRT Results by Use of New Non-SSTR-based Radioligands
Several non-SSTR-receptors have been addressed to be potential targets for PRRT [133]. Such potential targets include the gastrin releasing peptide receptor (GRP-R), cholecystokinin receptors (CCK2) or glucagon-like peptide 1 (GLP-1). The NEORAY Phase 1 study is currently recruiting patients with solid tumours [134]. The CCK2R antagonist [177Lu]Lu-PP-F11N has recently shown clinical safety in patients with medullary thyroid cancer (MTC) [135], and further studies are under way including our own derivative [136; Figure 4]. Other new peptide tracers are promising such as exendin in insulinoma [137], and novel therapy concepts are to be expected.
Furthermore, the present “hype” of thera(g)nostics for fibroblast activating protein (FAP) [138] may be adopted also for NET patients not responsive to PRRT with SSTR compounds.
Conclusions on the Improvement of PRRT by Different Concepts
The published data are mostly retrospective, limited patient numbers, and uncontrolled. Data on the use of long-acting octreotide or lanreotide from Phase 3 studies have led to their incorporation in the standard setting of PRRT combinations. Dosimetry and/or individualized dosing does not hold in the so-called “real life experience”. New antagonists, especially when labelled with alpha-emitting radioisotopes, seem most promising in the future. For further personalization of PRRT, the combination of intravenous and intraarterial routes of application seem reasonable in individual cases as does the use of new radioligands.
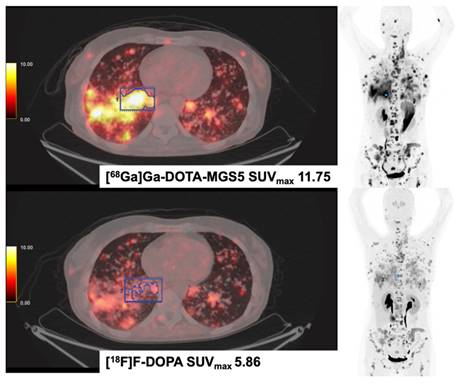
[68Ga]Ga-DOTA-MGS5 (DOTA-DGlu-Ala-Tyr-Gly-Trp-(N-Me)Nle-Asp-1-NaI-NH2) and [18F]F-DOPA PET/CT in a Patient with Medullary Thyroid Cancer (MTC). The patient was diagnosed with medullary thyroid cancer in 2014 (Ki-67 15%). Disease progression was evidenced after treatment with sorafenib, vandetanib, cabozantinib and long-acting octreotide. In 2023, disseminated metastases were seen in both examinations, [18F]F-DOPA and [68Ga]Ga-MGS5 PET/CT. The patient currently receives treatment with the CCK2-targeting [177Lu]Lu-labelled antagonist [177Lu]-Lu-PP-F11N (DOTA-(DGlu)6-Ala-Tyr-Gly-Trp-Nle-Asp-Phe-NH2) in Basel, Switzerland.
Perspectives
Three multicenter Phase 3 trials are currently active or still recruiting [139-141]. The COMPETE study [141] evaluates the efficacy and safety of [177Lu]Lu-edotreotide PRRT (4 cycles, 12 weeks apart) in Grade 1 and 2 GEP-NET patients versus everolimus (10 mg/d) whereas the COMPOSE study [139] evaluates against best standard of care in Grade 2 and 3 GEP-NET patients. Efficacy and safety results in Grade 2 and Grade 3 GEP-NET patients under PRRT with [177Lu]Lu-PRRT (4 cycles, 8 weeks apart) in combination with long-acting octreotide versus high dose octreotide are evaluated in the NETTER-2 trial [140]. The results of these three larger studies may lead to a broader application of PRRT in the near future, especially in G3 NET patients. As experimental preclinical studies with PARPis and ICIs are very promising, the results of the ongoing clinical studies in patients are to be anticipated. Furthermore, the combination results of sunitinib as a potential radiosensitizer for PRRT patients are to be awaited soon. The future is bright with all other combinations in prospective studies using various other modern anti-tumour substances. Certainly, further personalization of PRRT combinations on the one hand, and clinical safety of these combinations at the same time on the other hand, remain the major challenge. The treatment of NET patients is complex due to the heterogeneity of the disease and the current different combination possibilities tested in a variety of clinical studies are highly valuable. In this scenario, the possibility of the translating thera(g)nostic concept to clinical reality also using non-SSTR-receptors as well as immuno-PET can pave the way for the application of new combined treatments strategies. This gains increased importance considering the chance to choose a “tailored” treatment option for every NET patient in the era of personalized medicine. Last but not least, the ongoing development of combined treatments underlines the need for a multidisciplinary approach, not only in terms of treatment strategies, but also in terms of skills and knowledge. Therefore, it is crucial to train specialists who take care of the patient in every medical aspect, especially when using combined treatments.
Consent to publish
Informed consent for the publication of images was received from all participants who appear in the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B. et al. NETTER-1 Trial Investigators. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125-135
2. Strosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar A. et al. NETTER-1 Investigators. 177Lu-Dotatate plus long-acting octreotide versus highdose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021; 22(12):1752-1763. Epub 2021 Nov 15. Erratum in: Lancet Oncol. 2022;23(2):e59
3. Zaknun J, Bodei L, Mueller-Brand J, Pavel ME, Baum RP, Hörsch D. et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40(5):800-816
4. Kwekkeboom DJ, Krenning EP, Lebtahi R, Komminoth P, Kos-Kudła B, de Herder WW, Plöckinger U; Mallorca Consensus Conference Participants; European Neuroendocrine Tumor Society. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: peptide receptor radionuclide therapy with radiolabeled somatostatin analogs. Neuroendocrinology. 2009;90(2):220-6
5. Pavel M, O'Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R. et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103(2):172-85
6. Hicks RJ, Kwekkeboom DJ, Krenning E, Bodei L, Grozinsky-Glasberg S, Arnold R. et al. ENETS Consensus Guidelines for the standards of care in neuroendocrine neoplasia: peptide receptor radionuclide therapy with radiolabeled somatostatin analogues. Neuroendocrinology. 2017;105(3):295-309
7. Shah MH, Goldner WS, Benson AB, Bergsland E, Blaszkowsky LS, Brock P. et al. Neuroendocrine and adrenal tumors, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(7):839-868
8. Halfdanarson TR, Strosberg JR, Tang L, Bellizzi AM, Bergsland EK, O'Dorisio TM. et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for surveillance and medical management of pancreatic neuroendocrine tumors. Pancreas. 2020;49(7):863-881
9. Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(7):844-860
10. Hope TA, Bodei L, Chan JA, El-Haddad G, Fidelman N, Kunz PL. et al. NANETS/SNMMI consensus statement on patient selection and appropriate use of 177Lu-DOTATATE peptide receptor radionuclide therapy. J Nucl Med. 2020;61(2):222-227
11. Ambrosini V, Kunikowska J, Baudin E, Bodei L, Bouvier C, Capdevila J. et al. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur J Cancer. 2021;146:56-73
12. Bozkurt MF, Virgolini I, Balogova S, Beheshti M, Rubello D, Decristoforo C. et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur J Nucl Med Mol Imaging. 2017;44(9):1588-1601 Erratum in: Eur J Nucl Med Mol Imaging. 2017
13. Barrio M, Czernin J, Fanti S, Ambrosini V, Binse I, Du L. et al. The impact of somatostatin receptor-directed PET/CT on the management of patients with neuroendocrine tumor: a systematic review and meta-analysis. J Nucl Med. 2017;58(5):756-761
14. Rodrigues M, Winkler KK, Svirydenka H, Nilica B, Uprimny C, Virgolini I. Long-Term survival and value of 18F-FDG PET/CT in patients with gastroenteropancreatic neuroendocrine tumors treated with second peptide receptor radionuclide therapy course with 177Lu-DOTATATE. Life (Basel). 2021;11(3):198
15. Nicolini S, Severi S, Ianniello A, Sansovini M, Ambrosetti A, Bongiovanni A. et al. Investigation of receptor radionuclide therapy with 177Lu-DOTATATE in patients with GEP-NEN and a high Ki-67 proliferation index. Eur J Nucl Med Mol Imaging. 2018;45(6):923-930
16. Thang SP, Lung MS, Kong G, Hofman MS, Callahan J, Michael M, Hicks RJ. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) Grade 3 (G3) neuroendocrine neoplasia (NEN) - a single-institution retrospective analysis. Eur J Nucl Med Mol Imaging. 2018;45(2):262-277
17. Chan DL, Pavlakis N, Schembri GP, Bernard EJ, Hsiao E, Hayes A. et al. Dual somatostatin receptor / FDG PET/CT imaging in metastatic neuroendocrine tumours: proposal for a novel grading scheme with prognostic significance. Theranostics. 2017;7(5):1149-1158
18. Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP. et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26(13):2124-30
19. Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW. et al. Long-Term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res. 2017;23(16):4617-4624
20. Gabriel M, Nilica B, Kaiser B, Virgolini IJ. Twelve-Year Follow-up After Peptide Receptor Radionuclide Therapy. J Nucl Med. 2019;60(4):524-529
21. Virgolini I & Innsbruck Team. Peptide receptor radionuclide therapy (PRRT): clinical significance of re-treatment? Eur J Nucl Med Mol Imaging. 2015;42:1949-54
22. Yordanova A, Mayer K, Brossart P, Gonzalez-Carmona MA, Strassburg CP, Essler M, Ahmadzadehfar H. Safety of multiple repeated cycles of 177Lu-octreotate in patients with recurrent neuroendocrine tumour. Eur J Nucl Med Mol Imaging. 2017;44(7):1207-1214
23. van der Zwan WA, Brabander T, Kam BLR, Teunissen JJM, Feelders RA, Hofland J. et al. Salvage peptide receptor radionuclide therapy with [177Lu-DOTA,Tyr3]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2019;46(3):704-717
24. Adant S, Shah GM, Beauregard JM. Combination treatments to enhance peptide receptor radionuclide therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2020;47(4):907-921
25. Reccia I, Pai M, Kumar J, Spalding D, Frilling A. Tumour Heterogeneity and the Consequent Practical Challenges in the Management of Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers. 2023;15:1861
26. Gerlinger M, Rowan AJ, Horswell S. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing [published correction appears in N Engl J Med. 2012 Sep 6;367(10):976]. N Engl J Med. 2012;366(10):883-892
27. Nilica B, Waitz D, Stevanovic V. et al. Direct comparison of (68)Ga-DOTA-TOC and (18)F-FDG PET/CT in the follow-up of patients with neuroendocrine tumour treated with the first full peptide receptor radionuclide therapy cycle. Eur J Nucl Med Mol Imaging. 2016;43(9):1585-1592
28. Basu S, Parghane RV, Kamaldeep, Chakrabarty S. Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors. Semin Nucl Med. 2020;50(5):447-464 doi:10.1053/j.semnuclmed.2020.05.004
29. Nicolini S, Bodei L, Bongiovanni A, Sansovini M, Grassi I, Ibrahim T. et al. Combined use of 177Lu-DOTATATE and metronomic capecitabine (Lu-X) in FDG-positive gastro-entero-pancreatic neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2021;48(10):3260-3267
30. Kong G, Thompson M, Collins M, Herschtal A, Hofman MS, Johnston V. et al. Assessment of predictors of response and long-term survival of patients with neuroendocrine tumour treated with peptide receptor chemoradionuclide therapy (PRCRT). Eur J Nucl Med Mol Imaging. 2014;41(10):1831-44
31. Kashyap R, Hofman MS, Michael M, Kong G, Akhurst T, Eu P. et al. Favourable outcomes of (177)Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2015;42(2):176-85
32. Yordanova A, Ahrens H, Feldmann G, Brossart P, Gaertner FC, Fottner C. et al. Peptide receptor radionuclide therapy combined with chemotherapy in patients with neuroendocrine tumors. Clin Nucl Med. 2019;44(5):e329-e335
33. Moreno P, Ramos-Álvarez I, Moody TW, Jensen RT. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin Ther Targets. 2016;20(9):1055-1073 doi:10.1517/14728222.2016.1164694
34. Brom M, Joosten L, Laverman P. et al. Preclinical evaluation of 68Ga-DOTA-minigastrin for the detection of cholecystokinin-2/gastrin receptor-positive tumors. Mol Imaging. 2011;10(2):144-152
35. Kreppel B, Gonzalez-Carmona MA, Feldmann G. et al. Fibroblast activation protein inhibitor (FAPi) positive tumour fraction on PET/CT correlates with Ki-67 in liver metastases of neuroendocrine tumours. Nuklearmedizin. 2021;60(5):344-354
36. González Trotter DE, Meng X, McQuade P. et al. In Vivo Imaging of the Programmed Death Ligand 1 by 18F PET. J Nucl Med. 2017;58(11):1852-1857 doi:10.2967/jnumed.117.191718
37. Lee HS, Schwarz SW, Schubert EK. et al. The Development of 18F Fluorthanatrace: A PET Radiotracer for Imaging Poly (ADP-Ribose) Polymerase-1. Radiol Imaging Cancer. 2022;4(1):e210070
38. Kunz PL, Graham NT, Catalano PJ, Nimeiri HS, Fisher GA, Longacre TA. et al. Randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors (ECOG-ACRIN E2211). J Clin Oncol. 2023;41(7):1359-1369
39. Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, Chen DT. et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117(2):268-75
40. Peixoto RD, Noonan KL, Pavlovich P, Kennecke HF, Lim HJ. Outcomes of patients treated with capecitabine and temozolamide for advanced pancreatic neuroendocrine tumors (PNETs) and non-PNETs. J Gastrointest Oncol. 2014;5(4):247-52
41. Owen DH, Alexander AJ, Konda B, Wei L, Hemminger JA, Schmidt CR. et al. Combination therapy with capecitabine and temozolomide in patients with low and high grade neuroendocrine tumors, with an exploratory analysis of O6-methylguanine DNA methyltransferase as a biomarker for response. Oncotarget. 2017;8(61):104046-104056
42. Ramirez RA, Beyer DT, Chauhan A, Boudreaux JP, Wang YZ, Woltering EA. The role of capecitabine/temozolomide in metastatic neuroendocrine tumors. Oncologist. 2016;21(6):671-5
43. Cives M, Ghayouri M, Morse B, Brelsford M, Black M, Rizzo A. et al. Analysis of potential response predictors to capecitabine / temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2016;23(9):759-67
44. Pozzari M, Maisonneuve P, Spada F, Berruti A, Amoroso V, Cella CA. et al. Systemic therapies in patients with advanced well-differentiated pancreatic neuroendocrine tumors (PanNETs): When cytoreduction is the aim. A critical review with meta-analysis. Cancer Treat Rev. 2018;71:39-46
45. van Essen M, Krenning EP, Kam BL, de Herder WW, van Aken MO, Kwekkeboom DJ. Report on short-term side effects of treatments with 177Lu-octreotate in combination with capecitabine in seven patients with gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2008;35(4):743-748
46. Hubble D, Kong G, Michael M, Johnson V, Ramdave S, Hicks RJ. 177Lu-octreotate, alone or with radiosensitising chemotherapy, is safe in neuroendocrine tumour patients previously treated with high-activity 111In-octreotide. Eur J Nucl Med Mol Imaging. 2010;37(10):1869-1875
47. Forrer F, Uusijärvi H, Storch D, Maecke HR, Mueller-Brand J. Treatment with 177Lu-DOTATOC of patients with relapse of neuroendocrine tumors after treatment with 90Y-DOTATOC. J Nucl Med. 2005;46(8):1310-1316
48. Kwekkeboom DJ, de Herder WW, Krenning EP. Somatostatin receptor-targeted radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40(1):173-9
49. Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M. et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42(1):5-19
50. Claringbold PG, Brayshaw PA, Price RA, Turner JH. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38(2):302-311
51. Claringbold PG, Price RA, Turner JH. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother Radiopharm. 2012;27(9):561-569
52. Claringbold PG, Turner JH. Pancreatic neuroendocrine tumor control: durable objective response to combination 177Lu-Octreotate-Capecitabine-Temozolomide radiopeptide chemotherapy. Neuroendocrinology. 2016;103(5):432-9
53. Thang SP, Lung MS, Kong G, Hofman MS, Callahan J, Michael M, Hicks RJ. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN) - a single-institution retrospective analysis. Eur J Nucl Med Mol Imaging. 2018;45(2):262-277 Erratum in: Eur J Nucl Med Mol Imaging. 2017
54. Chauhan A, Farooqui Z, Murray LA, Weiss HL, War Myint Z, Raajasekar AKA. et al. Capecitabine and temozolomide in neuroendocrine tumor of unknown primary. J Oncol. 2018;2018:3519247
55. Pavlakis N, Randsom D, Wyld D, Sjoquist K, Wilson K, Gebski V. et al. Australasian Gastrointestinal Trials Group (AGITG) CONTROL NET Study: 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT) and capecitabine plus temozolomide (CAPTEM) for pancreas and midgut neuroendocrine tumours (pNETS, mNETS)—Final results. Journal of Clinical Oncology. 2022;40:4122-4122
56. Capecitabine on Temozolomide radionuclide therapy octreotate Lutetium-177 neuroendocrine tumours study (CONTROL NETS). NCT02358356. https://classic.clinicaltrials.gov/ct2/show/NCT02358356.
57. 177Lutethium - peptide receptor radionuclide therapy (Lu-PRRT) plus capecitabine versus Lu-PRRT in FDG positive, gastro-entero-pancreatic neuroendocrine tumors (Lu-Ca-S). NCT02736448. https://classic.clinicaltrials.gov/ct2/show/NCT02736448.
58. Personalized CAPTEM radiopeptide therapy of advanced, non-resectable neuroendocrine cancer. NCT04194125. https://classic.clinicaltrials.gov/ct2/show/NCT04194125.
59. Peptide receptor radionuclide therapy with 177Lu-Dotatate associated with metronomic Capecitabine in patients affected by aggressive gastro-etero-pancreatic neuroendocrine tumors (LuX). NCT02736500. https://classic.clinicaltrials.gov/ct2/show/NCT02736500.
60. Systemic targeted adaptive radio therapy of neuroendocrine tumors. (START-NET). NCT05387603. https://classic.clinicaltrials.gov/ct2/show/NCT05387603.
61. A safety study of [177Lu]Lu-DOTA-TATE in newly diagnosed extensive stage small cell lung cancer (ES-SCLC) patients in combination with carboplatin, etoposide, tislelizumab. NCT05142696. https://classic.clinicaltrials.gov/ct2/show/NCT05142696.
62. Ballal S, Yadav MP, Tripathi M, Sahoo RK, Bal C. Survival outcomes in metastatic gastroenteropancreatic neuroendocrine tumor patients receiving concomitant 225Ac-DOTATATE targeted alpha therapy and capecitabine: a real-world scenario management based long-term outcome study. J Nucl Med. 2022;122:264043
63. Özdirik B, Amthauer H, Schatka I, Goretzki PE, Mogl MT, Fehrenbach U. et al. A rare case of a patient with a high grade neuroendocrine tumor developing neutropenic sepsis after receiving PRRT combined with Capecitabine or Temozolomide: A case report. Mol Clin Oncol. 2021;14(1):20
64. Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18(10):610-621
65. Ambur Sankaranarayanan R, Kossatz S, Weber W, Beheshti M, Morgenroth A, Mottaghy FM. Advancements in PARP1 targeted nuclear imaging and theranostic probes. J Clin Med. 2020;9(7):2130
66. Chen DL. PET Imaging of PARP expression using 18F-olaparib. J Nucl Med. 2019;60(4):502-503
67. Makvandi M, Lee H, Puentes LN, Reilly SW, Rathi KS, Weng CC. et al. Targeting PARP-1 with alpha-particles is potently cytotoxic to human neuroblastoma in preclinical models. Mol Cancer Ther. 2019;18(7):1195-1204
68. Cullinane C, Waldeck K, Kirby L, Rogers BE, Eu P, Tothill RW, Hicks RJ. Enhancing the anti-tumour activity of 177Lu-DOTA-octreotate radionuclide therapy in somatostatin receptor-2 expressing tumour models by targeting PARP. Sci Rep. 2020;10(1):10196
69. Nonnekens J, van Kranenburg M, Beerens CE, Suker M, Doukas M, van Eijck CH de Jong M, van Gent DC. Potentiation of peptide receptor radionuclide therapy by the PARP Inhibitor Olaparib. Theranostics. 2016;6(11):1821-32
70. Purohit NK, Shah RG, Adant S, Hoepfner M, Shah GM, Beauregard JM. Potentiation of 177Lu-octreotate peptide receptor radionuclide therapy of human neuroendocrine tumor cells by PARP inhibitor. Oncotarget. 2018;9(37):24693-24706
71. Soni A, Li F, Wang Y, Grabos M, Krieger LM, Chaudhary S. et al. Inhibition of Parp1 by BMN673 effectively sensitizes cells to radiotherapy by upsetting the balance of repair pathways processing DNA double-trand breaks. Mol Cancer Ther. 2018;17(10):2206-2216
72. Lu-177-DOTATATE (Lutathera) in combination with olaparib in inoperable gastroenteropancreatico neuroendocrine tumors (GEP-NET). NCT04086485. https://clinicaltrials.gov/study/NCT04086485?tab=results.
73. Improving peptide receptor radionuclide therapy with PARP inhibitors (PRRT-PARPi).NCT05870423. https://classic.clinicaltrials.gov/ct2/show/NCT05870423
74. 177Lu-DOTA-TATE and olaparib in somatostatin receptor positive zumours (LuPARP). NCT04375267. https://www.clinicaltrials.gov/study/NCT04375267.
75. PARP inhibitor with 177Lu-DOTA-octreotate PRRT in patients with neuroendocrine tumours (PARLuNET). NCT05053854. https://classic.clinicaltrials.gov/ct2/show/NCT05053854.
76. Strosberg J, Mizuno N, Doi T, Grande E, Delord JP, Shapira-Frommer R. et al. Efficacy and safety of pembrolizumab in previously treated advanced neuroendocrine tumors: results from the Phase II KEYNOTE-158 study. Clin Cancer Res. 2020;26(9):2124-2130
77. Vijayvergia N, Dasari A, Deng M, Litwin S, Al-Toubah T, Alpaugh RK. et al. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: joint analysis of two prospective, non-randomised trials. Br J Cancer. 2020;122(9):1309-1314
78. Esfahani SA, De Aguiar Ferreira C, Summer P, Mahmood U, Heidari P. Addition of peptide receptor radiotherapy to immune checkpoint inhibition therapy improves outcomes in neuroendocrine tumors. J Nucl Med. 2023;64(7):1056-1061
79. Bensch F, van der Veen EL, Lub-de Hooge MN, Jorritsma-Smit A, Boellaard R, Kok IC. et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24(12):1852-1858
80. Kim C, Liu SV, Subramaniam DS, Torres T, Loda M, Esposito G, Giaccone G. Phase I study of the 177Lu-DOTA0-Tyr3-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung. J Immunother Cancer. 2020;8(2):e000980
81. A clinical study to assess the combination of two drugs (177Lu-DOTATATE and Nivolumab) in neuroendocrine tumours. NCT04525638. https://classic.clinicaltrials.gov/ct2/show/NCT04525638.
82. Targeted therapy and avelumab in merkel cell carcinoma (GoTHAM). NCT04261855. https://classic.clinicaltrials.gov/ct2/show/NCT04261855.
83. Pembrolizumab with liver-directed or peptide receptor radionuclide therapy for neuroendocrine tumors and liver metastases. NCT03457948. https://classic.clinicaltrials.gov/ct2/show/NCT03457948.
84. Phase II study of peptide receptor radionuclide therapy in combination with immunotherapy for patients with merkel cell cancer (iPRRT). NCT05583708. https://clinicaltrials.gov/study/NCT05583708?tab=table.
85. Finch RA, Liu MC, Cory AH, Cory JG, Sartorelli AC. Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone; 3-AP): an inhibitor of ribonucleotide reductase with antineoplastic activity. Adv Enzyme Regul. 1999;39:3-12
86. Testing the addition of an anti-cancer drug, triapine, to the usual radiation-based treatment (Lutetium Lu 177 Dotatate) for neuroendocrine tumors. NCT04234568. https://classic.clinicaltrials.gov/ct2/show/NCT04234568.
87. Testing the Effectiveness of an anti-cancer drug, triapine, when used with targeted radiation-based treatment (Lutetium Lu 177 Dotatate), compared to lutetium Lu 177 Dotatate alone for metastatic neuroendocrine tumors. NCT05724108. https://www.clinicaltrials.gov/study/NCT05724108.
88. Zenke FT, Zimmermann A, Sirrenberg C, Dahmen H, Kirkin V, Pehl U. et al. Pharmacologic inhibitor of DNA-PK, M3814, potentiates radiotherapy and regresses human tumors in mouse models. Mol Cancer Ther. 2020;19(5):1091-1101
89. Testing the addition of an anti-cancer drug, M3814 (peposertib), to the usual radiation-based treatment (Lutetium Lu 177 Dotatate) for pancreatic neuroendocrine tumors. NCT04750954. https://classic.clinicaltrials.gov/ct2/show/NCT04750954.
90. Testing the addition of sunitinib malate to lutetium Lu 177-Dotatate (Lutathera) in pancreatic neuroendocrine tumors. NCT05687123. https://classic.clinicaltrials.gov/ct2/show/NCT05687123.
91. Study of cabozantinib with Lu-177 in patients with somatostatin receptor 2 positive neuroendocrine tumors. NCT05249114. https://clinicaltrials.gov/study/NCT05249114.
92. Lutathera and ASTX727 in neuroendocrine tumours (LANTana). NCT05178693. https://classic.clinicaltrials.gov/ct2/show/NCT05178693.
93. Kane A, Thorpe MP, Morse MA, Howard BA, Oldan JD, Zhu J. et al. Predictors of survival in 211 patients with stage IV pulmonary and gastroenteropancreatic MIBG-positive neuroendocrine tumors treated with 131I-MIBG. J Nucl Med. 2018;59(11):1708-1713
94. Taïeb D, Hicks RJ, Hindié E, Guillet BA, Avram A, Ghedini P. et al. European Association of Nuclear Medicine Practice Guideline/Society of nuclear medicine and molecular imaging procedure standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2019;46(10):2112-2137
95. Pryma DA, Chin BB, Noto RB, Dillon JS, Perkins S, Solnes L. et al. Efficacy and safety of high-specific-activity 131I-MIBG therapy in patients with advanced pheochromocytoma or paraganglioma. J Nucl Med. 2019;60(5):623-630
96. Kroiss A, Shulkin BL, Uprimny C, Frech A, Gasser RW, Url C. et al. (68)Ga-DOTATOC PET/CT provides accurate tumour extent in patients with extraadrenal paraganglioma compared to (123)I-MIBG SPECT/CT. Eur J Nucl Med Mol Imaging. 2015;42(1):33-41
97. Jha A, Taïeb D, Carrasquillo JA, Pryma DA, Patel M, Millo C. et al. High-specific-activity-131I-MIBG versus 177Lu-DOTATATE targeted radionuclide therapy for metastatic pheochromocytoma and paraganglioma. Clin Cancer Res. 2021;27(11):2989-2995
98. Bushnell DL, Bodeker KL, O'Dorisio TM, Madsen MT, Menda Y, Graves S. et al. Addition of 131I-MIBG to PRRT (90Y-DOTATOC) for personalized treatment of selected patients with neuroendocrine tumors. J Nucl Med. 2021;62(9):1274-1277
99. A Clinical Trial Evaluating the Safety of Combining Lutathera(R), Azedra(R) to Treat Mid-gut Neuroendocrine Tumors (SPORE-3). NCT04614766. https://clinicaltrials.gov/study/NCT04614766.
100. van Vliet EI, van Eijck CH, de Krijger RR, Nieveen van Dijkum EJ, Teunissen JJ, Kam BL. et al. Neoadjuvant treatment of nonfunctioning pancreatic neuroendocrine tumors with [177Lu-DOTA0,Tyr3]octreotate. J Nucl Med. 2015;56(11):1647-53
101. Partelli S, Bertani E, Bartolomei M, Perali C, Muffatti F, Grana CM. et al. Peptide receptor radionuclide therapy as neoadjuvant therapy for resectable or potentially resectable pancreatic neuroendocrine neoplasms. Surgery. 2018;163(4):761-767
102. Minczeles NS, van Eijck CHJ, van Gils MJ, van Velthuysen MF, Nieveen van Dijkum EJM, Feelders RA. et al. Induction therapy with 177Lu-DOTATATE procures long-term survival in locally advanced or oligometastatic pancreatic neuroendocrine neoplasm patients. Eur J Nucl Med Mol Imaging. 2022;49(9):3203-3214
103. Study of PRRT in metastatic, world health organization (WHO) grade 1 or 2, SSTR positive, GEP-NET who are candidates for cytoreductive surgery. NCT04609592. https://clinicaltrials.gov/study/NCT04609592.
104. Neoadjuvant PRRT With 177Lu-DOTATATE followed by surgery for resectable panNET (NeoLuPaNET). NCT04385992. https://www.clinicaltrials.gov/study/NCT04385992.
105. Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M. et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656-63
106. Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E. et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224-33
107. Yordanova A, Wicharz MM, Mayer K, Brossart P, Gonzalez-Carmona MA, Strassburg CP. et al. The role of adding somatostatin analogues to peptide receptor radionuclide therapy as a combination and maintenance therapy. Clin Cancer Res. 2018;24(19):4672-4679
108. Ayati N, Lee ST, Zakavi R, Pathmaraj K, Al-Qatawna L, Poon A, Scott AM. Long-acting somatostatin analog therapy differentially alters 68Ga-DOTATATE uptake in normal tissues compared with primary tumors and metastatic lesions. J Nucl Med. 2018;59(2):223-227
109. Gålne A, Almquist H, Almquist M, Hindorf C, Ohlsson T, Nordenström E. et al. A Prospective observational study to evaluate the effects of long-acting somatostatin analogs on 68Ga-DOTATATE uptake in patients with neuroendocrine tumors. J Nucl Med. 2019;60(12):1717-1723
110. Aalbersberg EA, de Wit-van der Veen BJ, Versleijen MWJ, Saveur LJ, Valk GD, Tesselaar MET, Stokkel MPM. Influence of lanreotide on uptake of 68Ga-DOTATATE in patients with neuroendocrine tumours: a prospective intra-patient evaluation. Eur J Nucl Med Mol Imaging. 2019;46(3):696-703
111. Hope TA, Calais J, Zhang L, Dieckmann W, Millo C. 111In-Pentetreotide Scintigraphy Versus 68Ga-DOTATATE PET: Impact on Krenning Scores and effect of tumor burden. J Nucl Med. 2019;60(9):1266-1269
112. Roth D, Gustafsson J, Warfvinge CF, Sundlöv A, Åkesson A, Tennvall J, Gleisner KS. Dosimetric quantities in neuroendocrine tumors over treatment cycles with 177Lu-DOTATATE. J Nucl Med. 2022;63(3):399-405
113. Minczeles NS, de Herder WW, Feelders RA, Verburg FA, Hofland J, Brabander T. Long-term outcomes of submaximal activities of peptide receptor radionuclide therapy with 177Lu-DOTATATE in neuroendocrine tumor patients. J Nucl Med. 2023;64(1):40-46
114. Del Prete M, Buteau FA, Beaulieu A, Beauregard JM. Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumors: initial dosimetry and safety results of the P-PRRT trial. J Nucl Med. 2019;46:728-742
115. Gleisner K, Spezi E, Solny P, Gabina M, Cicone F, Stokke C. et al. Variations in the practice of molecular radiotherapy and implementation of dosimetry: results from a European survey. EJNMMI Phys. 2017;4(1):28
116. Ginj M, Zhang H, Waser B, Cescato R, Wild D, Wang X. et al. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci U S A. 2006;103:16436-41
117. Wild D, Grønbæk H, Navalkissoor S, Haug A, Nicolas GP, Pais B. et al. A phase I/II study of the safety and efficacy of [177Lu]Lu-satoreotide tetraxetan in advanced somatostatin receptor-positive neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2023;51:183-195
118. Baum RP, Zhang J, Schuchardt C, Müller D, Mäcke H. First-in-Humans Study of the SSTR Antagonist 177Lu-DOTA-LM3 for peptide receptor radionuclide therapy in patients with metastatic neuroendocrine neoplasms: dosimetry, safety, and efficacy. J Nucl Med. 2021;62(11):1571-1581
119. Tian R, Jacobson O, Niu G, Kiesewetter DO, Wang Z, Zhu G, Ma Y, Liu G, Chen X. Evans blue attachment enhances somatostatin receptor subtype-2 imaging and radiotherapy. Theranostics. 2018;8(3):735-745
120. Liu Q, Zang J, Sui H, Ren J, Guo H, Wang H. et al. Peptide receptor radionuclide therapy of late-stage neuroendocrine tumor patients with multiple cycles of 177Lu-DOTA-EB-TATE. J Nucl Med. 2021;62(3):386-392. Erratum in: J Nucl Med. 2021;62(7):1015
121. Cullinane C, Jeffery CM, Roselt PD, van Dam EM, Jackson S, Kuan K. et al. Peptide receptor radionuclide therapy with 67Cu-CuSarTATE is highly efficacious against a somatostatin-positive neuroendocrine tumor model. J Nucl Med. 2020;61(12):1800-1805
122. Combined beta-plus auger electron therapy using a novel somatostatin receptor subtype 2 antagonist labelled with terbium-161 (161Tb-DOTA-LM3) (Beta plus). NCT05359146. https://classic.clinicaltrials.gov/ct2/show/NCT05359146.
123. Delpassand ES, Tworowska I, Esfandiari R, Torgue J, Hurt J, Shafie A, Núñez R. Targeted α-emitter therapy with 212Pb-DOTAMTATE for the treatment of metastatic SSTR-expressing neuroendocrine tumors: first-in-humans dose-escalation clinical trial. J Nucl Med. 2022;63(9):1326-1333
124. Yadav MP, Ballal S, Sahoo RK, Bal C. Efficacy and safety of 225Ac-DOTATATE targeted alpha therapy in metastatic paragangliomas: a pilot study. Eur J Nucl Med Mol Imaging. 2022;49(5):1595-1606
125. NCT03466216. Phase 1 Study of AlphaMedix™ in Adult Subjects With SSTR (+) NET https://clinicaltrials.gov/study/NCT03466216
126. Kunikowska J, Zemczak A, Kołodziej M, Gut P, Łoń I, Pawlak D. et al. Tandem peptide receptor radionuclide therapy using 90Y / 177Lu-DOTATATE for neuroendocrine tumors efficacy and side-effects - Polish multicenter experience. Eur J Nucl Med Mol Imaging. 2020;47(4):922-933
127. Pfeifer AK, Gregersen T, Grønbæk H, Hansen CP, Müller-Brand J, Herskind Bruun K. et al. Peptide receptor radionuclide therapy with Y-DOTATOC and (177)Lu-DOTATOC in advanced neuroendocrine tumors: results from a Danish cohort treated in Switzerland. Neuroendocrinology. 2011;93(3):189-96
128. Peptide receptor radionuclide therapy in the treatment of advanced, non-resectable and/or symptomatic tumors with SSTR overexpression (POLNETS_PRRT). NCT04029428. https://clinicaltrials.gov/study/NCT04029428.
129. Kratochwil C, López-Benítez R, Mier W, Haufe S, Isermann B, Kauczor HU. et al. Hepatic arterial infusion enhances DOTATOC radiopeptide therapy in patients with neuroendocrine liver metastases. Endocr Relat Cancer. 2011;18(5):595-602
130. Kratochwil C, Giesel FL, Bruchertseifer F, Mier W, Apostolidis C, Boll R. et al. ²¹³Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging. 2014;41(11):2106-19
131. Lutathera in people with gastroenteropancreatic (GEP), bronchial or unknown primary neuroendocrine tumors that have spread to the liver. NCT04544098. https://classic.clinicaltrials.gov/ct2/show/NCT04544098.
132. Intra-arterial hepatic (IAH) infusion of radiolabelled somatostatin analogs in GEP-NET patients with dominant liver metastases (LUTARTERIAL). NCT04837885. https://clinicaltrials.gov/study/NCT04837885.
133. Fani M, Maecke HR, Okarvi SM. Radiolabeled peptides: valuable tools for the detection and treatment of cancer. Theranostics. 2012;2(5):481-501
134. [177Lu]-NeoB in patients with advanced solid tumors and with [68Ga]-NeoB lesion uptake (NeoRay). NCT03872778. https://clinicaltrials.gov/study/NCT03872778.
135. Rottenburger C, Nicolas GP, McDougall L, Kaul F, Cachovan M, Vija AH. et al. Cholecystokinin 2 receptor agonist 177Lu-PP-F11N for radionuclide therapy of medullary thyroid carcinoma: results of the Lumed Phase 0a Study. J Nucl Med. 2020;61(4):520-526
136. von Guggenberg E, Uprimny C, Klinger M, Warwitz B, Sviridenko A, Bayerschmidt S. et al. Preliminary clinical experience with cholecystokinin-2 receptor PET/CT using the 68Ga-labeled minigastrin analog DOTA-MGS5 in patients with medullary thyroid cancer. J Nucl Med. 2023;64(6):859-862
137. Zhang M, Jacobson O, Kiesewetter DO, Ma Y, Wang Z, Lang L. et al. Improving the theranostic potential of exendin 4 by reducing the renal radioactivity through brush border membrane enzyme-mediated degradation. Bioconjug Chem. 2019;30(6):1745-1753
138. Zhao L, Chen J, Pang Y, Fu K, Shang Q, Wu H. et al. Fibroblast activation protein-based theranostics in cancer research: A state-of-the-art review. Theranostics. 2022;12(4):1557-1569
139. Lutetium 177Lu-Edotreotide Versus Best Standard of care in well-differentiated aggressive Grade-2, Grade-3 GastroEnteroPancreatic NeuroEndocrine Tumors (GEP-NETs) - COMPOSE (COMPOSE). NCT04919226. https://www.clinicaltrials.gov/study/NCT04919226.
140. Study to evaluate the efficacy, safety of lutathera in patients with grade 2, grade 3 advanced GEP-NET (NETTER-2). NCT03972488. https://clinicaltrials.gov/study/NCT03972488.
141. Efficacy safety of 177Lu-edotreotide PRRT in GEP-NET patients (COMPETE). NCT03049189. https://clinicaltrials.gov/study/NCT03049189.
Author contact
![]() Corresponding author: irene.virgoliniat.
Corresponding author: irene.virgoliniat.
 Global reach, higher impact
Global reach, higher impact