13.3
Impact Factor
Theranostics 2024; 14(2):830-842. doi:10.7150/thno.90071 This issue Cite
Research Paper
Quaternization drives spleen-to-lung tropism conversion for mRNA-loaded lipid-like nanoassemblies
1. Department of Infectious Disease, Shenzhen People's Hospital, The First Affiliated Hospital of Southern University of Science and Technology & The Second Clinical Medical College of Jinan University, Shenzhen 518020, China.
2. School of Medicine, Southern University of Science and Technology, Shenzhen, 518055, China.
Abstract
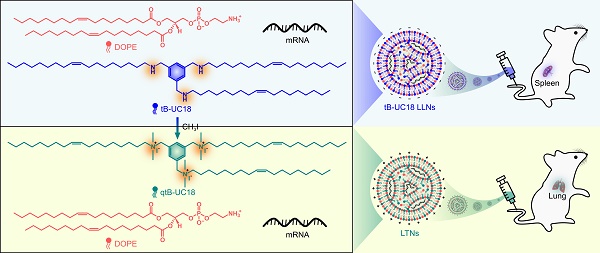
Background: As the overwhelming majority of advanced mRNA delivery systems are preferentially accumulated in the liver, there is an accelerating growth in the demand for the development of non-liver mRNA delivery platforms.
Methods: In this study, we prepared cationic lipid-like nanoassemblies through a N-quaternizing strategy. Their physicochemical properties, in vitro mRNA delivery efficiency, and organ tropism in mice were investigated.
Results: Introduction of quaternary ammonium groups onto lipid-like nanoassemblies not only enhances their mRNA delivery performance in vitro, but also completely alters their tropism from the spleen to the lung after intravenous administration in mice. Quaternized lipid-like nanoassemblies exhibit ultra-high specificity to the lung and are predominantly taken up by pulmonary immune cells, leading to over 95% of exogenous mRNA translation in the lungs. Such mRNA delivery carriers are stable even after more than one-year storage at ambient temperature.
Conclusions: Quaternization provides an alternative method for design of new lung-targeted mRNA delivery systems without incorporation of targeting ligands, which should extend the therapeutic applicability of mRNA to lung diseases.
Keywords: quaternization, lipid-like nanoassembly, systemic mRNA delivery, lung targeting, ultra-high selectivity
 Global reach, higher impact
Global reach, higher impact