13.3
Impact Factor
Theranostics 2024; 14(2):640-661. doi:10.7150/thno.91814 This issue Cite
Review
Current evidence and therapeutic implication of PANoptosis in cancer
1. Department of Laboratory Medicine, Lianyungang Clinical College, Jiangsu University, Lianyungang 222006, Jiangsu, P.R. China.
2. Directorate of University Health Services, University of Cape Coast, Cape Coast CC0959347, Central Region, Ghana.
3. The People's Hospital of Danyang, Affiliated Danyang Hospital of Nantong University, Zhenjiang 212300, Jiangsu, P.R. China.
4. Department of Optometry and Vision Science, School of Allied Health Sciences, College of Health and Allied Sciences, University of Cape Coast, Cape Coast CC0959347, Central Region, Ghana.
5. Department of Pathology, Nanjing Jiangning Hospital, Nanjing 211100, Jiangsu, P.R. China.
†The first two authors contributed equally to this work.
Received 2023-11-2; Accepted 2023-11-29; Published 2024-1-1
Abstract
Regulated cell death (RCD) is considered a critical pathway in cancer therapy, contributing to eliminating cancer cells and influencing treatment outcomes. The application of RCD in cancer treatment is marked by its potential in targeted therapy and immunotherapy. As a type of RCD, PANoptosis has emerged as a unique form of programmed cell death (PCD) characterized by features of pyroptosis, apoptosis, and necroptosis but cannot be fully explained by any of these pathways alone. It is regulated by a multi-protein complex called the PANoptosome. As a relatively new concept first described in 2019, PANoptosis has been shown to play a role in many diseases, including cancer, infection, and inflammation. This study reviews the application of PCD in cancer, particularly the emergence and implication of PANoptosis in developing therapeutic strategies for cancer. Studies have shown that the characterization of PANoptosis patterns in cancer can predict survival and response to immunotherapy and chemotherapy, highlighting the potential for PANoptosis to be used as a therapeutic target in cancer treatment. It also plays a role in limiting the spread of cancer cells. PANoptosis allows for the elimination of cancer cells by multiple cell death pathways and has the potential to address various challenges in cancer treatment, including drug resistance and immune evasion. Moreover, active investigation of the mechanisms and potential therapeutic agents that can induce PANoptosis in cancer cells is likely to yield effective cancer treatments and improve patient outcomes. Research on PANoptosis is still ongoing, but it is a rapidly evolving field with the potential to lead to new treatments for various diseases, including cancer.
Keywords: PANoptosis, cancer, regulated cell death, therapy, immunity
1. Introduction
PANoptosis is a complex, multi-faceted agent of the immune response that has important implications for several diseases, including cancer. It is regulated by PANoptosomes, macromolecular complexes implicated in driving innate immune responses and inflammation [1,2]. The assembly of PANoptosomes results in cell death that occurs through the collective activation of pyroptosis, apoptosis, and necroptosis [1]. The precise molecular details of this process are unclear. Still, the presence of apoptosis and necroptosis markers in various body parts have been identified in several studies, leading to the hypothesis of possible PANoptosis in multiple diseases, including cancer [3,4]. Studies that have analyzed the molecular changes of PANoptosis-related genes in cancer have reported distinct clusters that exhibit unique immune microenvironments, biological function, survival rate, and chemotherapy drug sensitivity [5-7]. These findings highlight the therapeutic and prognostic value of PANoptosis, providing a new area for exploration. The PANoptosis pathway has also been studied in other diseases, such as inflammatory diseases, infections, and periodontitis [8]. The study of PANoptosis also opens up a new frontier in RCD, not only in cancer but also in the context of stroke [4], neuroinflammation, and Alzheimer's Disease [9].
Despite some uncertainty about the specifics of the PANoptosis pathway, evidence suggests that it is deeply involved in cancer immunity and plays an important role in innate immune cells that detect and eliminate intracellular pathogens. Furthermore, several studies have shown that the expression profiles of key inflammatory cytokines are modulated in patients with cancer and that multiple cytokines are associated with disease severity, indicating that PANoptosis may be involved in regulating immune responses to cancer [10]. While the exact mechanism by which PANoptosis drives immune responses and inflammation in cancer is poorly understood, it is believed to provide an "immune system workaround" to protect the host from cancer. According to a recent study, the sensors involved in PANoptosis may vary depending on the disease, but most infections will induce the formation of PANoptosomes, which unleash inflammatory cell death to protect the host [11]. The therapeutic implications of PANoptosis in cancer are still emerging, but there is potential for the development of new treatments that target this pathway and improve existing therapies. For example, phosphorylated NFS1 was found to weaken oxaliplatin-based chemosensitivity of colorectal cancer (CRC) by preventing PANoptosis [12], implicating that the inhibition of NFS1 serves as a promising strategy for improving the outcome of platinum-based chemotherapy-induced PANoptosis in the treatment of CRC. Moreover, interferon regulatory factor 1 (IRF1) regulates PANoptosis to prevent CRC [13], and PANoptosis-based molecular clustering and prognostic signature predict patient survival and immune landscape in cancer [7]. Thus, studies of the PANoptosis pathway and related molecules may inform the development of new therapeutic, diagnostic, and prognostic markers for cancer and provide a better understanding of the role of cell death in the regulation of immune responses to cancer.
The study reviews the role of regulated cell death (RCD) in cancer therapy, the interaction between these cell deaths, and the emergence and application of PANoptosis in treatments, particularly implications in developing therapeutic strategies for cancer. This will provide a timely overview of this new and promising field of research, highlighting a great deal of potential to develop new and effective PANoptosis-based cancer therapies.
2. Types of regulated cell death and application in cancer
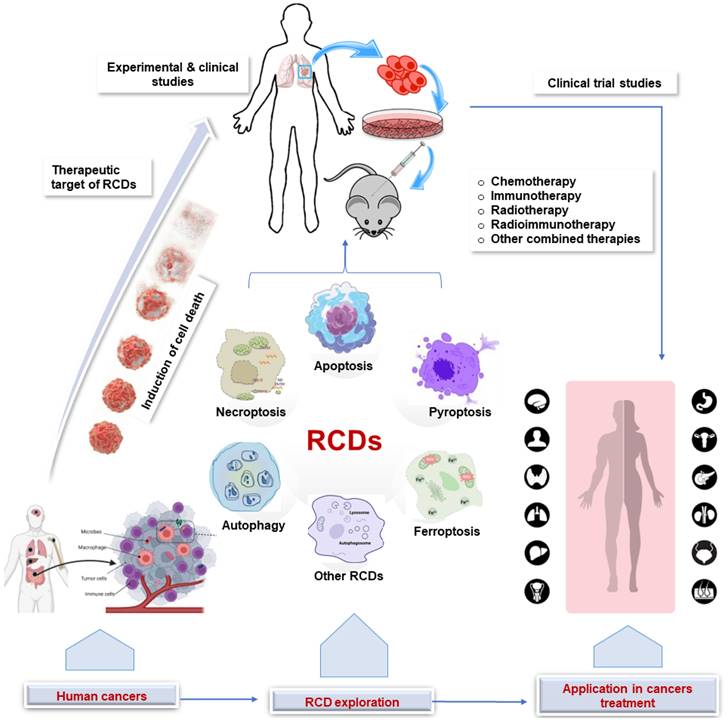
Regulated cell death (RCD) is a broad term that encompasses all forms of cell death that are controlled by genes and occur through a series of biochemical steps, whereas programmed cell death (PCD) refers to the series of events that occur within a cell leading to its death [14,15]. Thus, PCD is a subset of RCD and occurs during normal development and tissue homeostasis. Different types of RCD can be activated depending on the cell type and stimulus, each with distinct morphological and molecular characteristics, including apoptosis, autophagy, necroptosis, pyroptosis, ferroptosis, necrosis, and NETosis [16-18]. In cancer therapy, RCD can be induced by a variety of treatments, including chemotherapy, radiation therapy, and immunotherapy. While chemotherapy drugs and radiotherapy work by interfering with the cell's ability to divide, leading to the death of cancer cells, immunotherapy is a relatively newer approach to cancer treatment in which the immune system is stimulated to attack cancer cells. The implications of the various RCDs in cancer therapy are summarized in Figure 1 and elaborated below.
2.1 Apoptosis
Apoptosis, the most well-known type of PCD, is a highly regulated process that is essential for normal development, tissue homeostasis, and eliminating unwanted or damaged cells [19]. During apoptosis, the cell undergoes characteristic morphological changes, including cell shrinkage, nuclear fragmentation, chromatin condensation, and membrane blebbing. The cell's contents are then packaged into small membrane-bound vesicles called apoptotic bodies, which are engulfed and degraded by phagocytic cells without eliciting an inflammatory response [20,21]. For over 30 years, a primary focus and goal of cancer treatment has been to develop therapies that help cancer cells die by apoptosis. In other words, researchers have intensely explored the clinical translation of apoptosis modulators into therapeutic approaches that target both the intrinsic and extrinsic apoptotic pathways. Among the strategies that target the intrinsic apoptotic pathway are selective BCL-2 inhibitors (Venetoclax, BCL201/S55746, APG-2575) [22-24], BCL-XL inhibitors (ABBV-155, A-1155463, WEHI-539, -1331852) [25,26], dual BCL-2 and BCL-XL inhibitors (APG-1252, Navitoclax, AZD4320, S44563 BM-1197) [27-29], MCL1 inhibitors (AMG 176, MIK665/S64315, AZD5991, A-1210477) [30-32], and inhibitor of apoptosis (IAP) proteins and SMAC mimetics (Birinapant/TL32711, LCL161) [26,33,34]. The extrinsic apoptosis pathway is activated by signals from outside the cell that activate pro-apoptotic death receptors (DRs). DRs (including TNFR1, Fas (CD95, APO-1), DR3, DR4 (TRAILR1), DR5 (TRAILR2) and DR6) are transmembrane proteins that belong to the tumor necrosis factor receptor superfamily (TNFR) [35]. Approaches that target the extrinsic apoptotic pathway to induce cancer death include the use of DR agonists such as Fas ligand, tumor necrosis factor-alpha (TNFα), TNF-related apoptosis-inducing ligand (TRAIL), Apo2L/TRAIL, and CD95L/FasL [26,36].
Application of RCD in cancer. Clinical and experimental studies continue to explore the target of RCDs in cancer. This sets the stage for clinical trials that ultimately lead to the application in human cancer treatment. RCD, regulated cell death.
2.2 Necroptosis
Necroptosis is a form of programmed necrosis that occurs when the apoptotic pathway is inhibited. Necroptosis is characterized by programmed mitochondrial dysfunction and plasma membrane rupture induced by various stimuli, including TNF-alpha, Toll-like receptor (TLR) ligands, and chemotherapeutic drugs [37]. Unlike apoptosis, necroptosis involves the release of cellular contents into the extracellular space, which can trigger an inflammatory response. Necroptosis is regulated by several essential proteins, including receptor-interacting protein kinases (RIPKs) and mixed lineage kinase domain-like pseudokinase (MLKL) [38,39]. Necroptosis can have both tumor-promoting and tumor-suppressing effects in cancer [40]. On the one hand, necroptosis can trigger an inflammatory response that can recruit immune cells to the tumor and promote its clearance. On the other hand, necroptosis can also promote tumor metastasis by helping cancer cells detach from the primary tumor and invade other tissues [40,41]. Some of the specific applications of necroptosis in cancer therapy include necroptosis-inducing drugs, necroptosis inhibitors (receptor-interacting serine/threonine-protein kinase (RIPK)1 inhibitors, RIPK3 inhibitors, MLKL inhibitors), and necrotic cell debris removal. Drugs that induce necroptosis in cancer cells are still in the early stages of development, but they have shown promise in preclinical studies. For example, drugs such as Neoalbaconol [42], Matrine [43], Erigeron breviscapus [44], and inorganic salts and metal complexes (CoCl2, LiCl, Cisplatin, Rhenium(V) oxo complexes) [45-47] have been shown to induce necroptosis in a variety of cancer cell lines and to suppress tumor growth in animal models.
2.3 Pyroptosis
Pyroptosis is characterized by the release of proinflammatory cytokines, including IL-1β and IL-18, and the formation of large membrane pores [48]. It is a caspase-independent form of cell death triggered by stimuli such as microbial pathogens, damage-associated molecular patterns (DAMPs), and certain chemotherapeutic drugs. It can also occur spontaneously in cancer cells that have mutations in certain genes, such as caspase (CASP)1, CASP3, and NOD-like receptor protein 3 (NLRP3) [49,50]. Pyroptosis can have both tumor-promoting and tumor-suppressing effects in cancer. On the one hand, pyroptosis can trigger an inflammatory response that can recruit immune cells to the tumor and promote its clearance. On the other hand, pyroptosis can also promote tumor metastasis by helping cancer cells detach from the primary tumor and invade other tissues [51]. Studies show that Gasdermins (GSDMs)-driver pyroptosis also participates in the inflammatory cell death, PANoptosis, which makes a significant sense to the initiation and progression of diseases [52], including tumors, where GSDM genes are highly upregulated and associated with tumor microenvironment (TME) features, prognosis, clinical characteristics, and stemness scores in several cancer types [53]. Thus, pyroptosis is a promising target for cancer therapy. The association of GSDM genes (and their regulation of PANoptosis) with tumor behaviors and probable participation in carcinogenesis presents it as a potential therapeutic target in pan-cancer. Several studies have explored pyroptosis as a new bridge in tumor immunity, targeting GSDM and related pathways [54,55]. In summary, targets for pyroptosis in cancer include pyroptosis-inducing drugs (shikonin, ferrostatin-1, cisplatin), pyroptosis inhibitors (necrostatin-1, MCC950), and pyroptotic cell debris removal (A11) [56,57].
2.4 Autophagy
Autophagy is a cellular recycling process that is critical in maintaining cellular homeostasis by degrading and recycling cytoplasmic contents, including organelles and proteins. During autophagy, the cell forms a double-membraned autophagosome that sequesters the cellular components to be degraded and fuses with a lysosome to form an autolysosome. Lysosomal enzymes then degrade the contents, and the resulting molecules are released back into the cytosol for reuse [58,59]. Hyper-activation of autophagy is also believed to cause autophagy-dependent cell death (ADCD), a caspase-independent form of programmed cell death. ADCD serves as a type of cellular demise that may act as a backup cell death program in apoptosis-deficient tumors, presenting a potential cancer cell death target [60]. Researchers are developing therapies that target the autophagy pathway, specifically in cancer cells, including the inhibition of autophagy-related proteins that are essential for tumor growth and metastasis [61]. In cancer research, studies have explored autophagy-inducing drugs such as Vorinostat and Hydroxychloroquine [62], autophagy inhibitors such as 3-Methyladenine and Chloroquine [63,64], and autophagy pathway-specific drugs like ATG4B [65].
2.5 Ferroptosis
Ferroptosis is a regulated form of cell death that depends on iron and lipid peroxidation. It is characterized by the accumulation of lipid peroxides and the absence of caspase activation, distinguishing it from apoptosis and necrosis [66]. Ferroptosis is initiated by the depletion of glutathione or the inhibition of glutathione peroxidase 4, leading to lipid peroxidation and membrane damage [67,68]. Studies have implicated ferroptosis in a variety of physiological and pathological processes, including cancer, where it plays a role in tumor suppression and drug resistance and presents promising therapeutic potential [69]. In other words, ferroptosis plays an essential role in preventing cancer development but is often suppressed in many types of cancer, suggesting that regulating ferroptosis could be a promising way to treat cancer [70]. Several substances are under investigation for their ability to regulate ferroptosis to reduce tumor progression, including Simvastatin [71], P53 [72], and BAP1 [70]. A study found that KRAS mutant in tumors is associated with suppressed ferroptosis and associated gene expression changes that reduce adverse outcomes [73], presenting a novel potential avenue for therapeutic approaches in cancer. In other studies, Glutathione S-transferase zeta 1 (GSTZ1), an enzyme in the catabolism of phenylalanine, enhances sorafenib-induced ferroptosis by inhibiting the NRF2/GPX4 axis [74]. Interestingly, although many ferroptosis regulators such as GPX4, SLC7A11, and FTH1 are obviously increased in certain cancers compared with normal tissues, the treatments targeting these regulators might vary in effectiveness across different cancers [70].
2.6 Other types of regulated cell death
Other forms of regulated cell death include NETosis, entosis, parthanatos, and mitotic catastrophe. Moreover, immunogenic cell death (ICD), lysosome-dependent cell death (LDCD), alkaliptosis, and oxeiptosis have also been documented as emerging RCD pathways [75,76]. NETosis, or neutrophil extracellular trap formation, occurs in neutrophils in response to infection or inflammation. In the tumor microenvironment, NETosis can play both a tumor-suppressive role by trapping and killing cancer cells and a tumor-promoting role by providing a scaffold for tumor cell attachment and migration and releasing pro-angiogenic and pro-inflammatory factors that promote tumor growth [77,78]. NETosis is distinct from apoptosis and necrosis and is characterized by the release of DNA, histones, and antimicrobial peptides from neutrophils and the formation of extracellular traps [79]. On the other hand, entosis occurs when one living cell invades and engulfs another living cell's cytoplasm. This process involves the formation of cell-in-cell structures, where the engulfed cell is degraded and eventually dies [80]. Entotic cell death occurs in a non-apoptotic manner and usually involves the lipidation of microtubule-associated protein light chain 3 (LC3) onto entotic vacuoles. The process of entosis is triggered by the loss of attachment to the extracellular matrix and occurs in response to several stimuli, such as hypoxia, nutrient starvation, and chemotherapy [81]. The role of entosis in cancer is complex and multifaceted. Entosis can lead to the death of cancer cells, but it can also promote tumor growth and metastasis [82]. This suggests that entosis could be a potential target for therapeutic intervention, and several entosis-targeting therapies are currently in development [83].
Parthanatos is triggered by excessive activation of poly(ADP-ribose) polymerase-1 (PARP-1) and the subsequent release of apoptosis-inducing factor (AIF). Parthanatos can occur independently of caspases and is involved in the pathogenesis of various cancers and neurodegenerative diseases [39,84]. A number of parthanatos-targeting therapies are currently in preclinical or clinical development. For example, some studies have shown that inhibiting PARP1, a key enzyme involved in parthanatos, can suppress tumor growth in animal models [85,86]. Other studies have shown that stimulating parthanatos with certain drugs can kill cancer cells in vitro and in vivo [86,87]. Mitotic catastrophe is another type of PCD that occurs in response to errors in cell division, leading to abnormal mitotic events and genomic instability. During mitotic catastrophe, cells undergo premature or delayed mitosis, leading to chromosomal instability and the activation of the DNA damage response. If the DNA damage response is not resolved, cells will ultimately undergo PCD [88]. Mitotic catastrophe can be triggered by a variety of factors, such as chemotherapeutic drugs, radiation, and viral infection [88]. Mitotic catastrophe plays a vital role in cancer prevention and suppression. Mitotic failure can lead to senescent cells, which could suppress tumor growth by blocking the proliferation of neighboring cancer cells and recruiting immune cells to the tumor microenvironment [89]. Notwithstanding, cancer cells can often evade mitotic catastrophe and senescence, which allows them to continue to proliferate and form tumors [90]. Chemotherapeutic agents such as vinca alkaloids and taxanes can trigger mitotic catastrophe. Such agents can also cause cancer cells to undergo immunogenic cell death (ICD), which allows therapeutic responses to last beyond treatment discontinuation due to the induction of an antitumor immune response [91]. Researchers are also investigating the potential of combining mitotic catastrophe-inducing therapies with other cancer therapies, such as chemotherapy and immunotherapy. The goal of these combination therapies is to make cancer cells more sensitive to treatment and to prevent the development of drug resistance [92,93]. In one such study, 212Pb-radioimmunotherapy was found to potentiate paclitaxel-induced cell-killing efficacy by perturbing the mitotic spindle checkpoint, causing increased mitotic catastrophe and cell death [92].
ICD, defined by chronic exposure to DAMPs in the TME, stimulates the dysfunctional antitumor immune system and contributes to long‐lasting protective antitumor immunity. ICD induction has emerged as a novel cancer therapeutic approach. Some of the reported ICD inducers include bleomycin (an antitumor antibiotic glycopeptide), cyclophosphamide, cardiac glycosides (digoxin, digitoxin, ouabain, and lanatoside C), shikonin, anthracyclines (doxorubicin, idarubicin, and mitoxantrone), and hypericin photodynamic therapy (Hyp-PDT) [94]. For example, anthracyclines induce ICD in human prostate cancer, ovarian cancer, and acute lymphoblastic leukemia cells by increasing the maturation of DCs and tumor cell uptake by DCs, stimulating tumor-specific IFN-γ-producing T cells, reducing Tregs [95]. In LDCD, the lysosome serves as an anti-cancer target, where lysosome-disrupting agents trigger lysosomal membrane permeabilization (LMP), cathepsin protease release, and subsequent LDCD. Depletion of cancer cells through LMP is an attractive therapeutic strategy, holding particular promise for combating apoptosis-resistant cancer cell populations. Several lysosome-disrupting agents (lysosomotropic agents, sphingolipids, nanoparticles) are currently under investigation or in clinical development for cancer and other indications [96]. Alkaliptosis, a pH-dependent form of RCD, has also been recently identified as a new strategy for cancer therapy across multiple tumor types. For example, a G protein-coupled receptors (GPCRs)-targeted small molecule called JTC801 has been demonstrated to suppressed tumor growth in xenograft, orthotropic, lung metastases, and genetically engineered mouse models of pancreatic cancer in vivo by inducing alkaliptosis, but not apoptosis, necroptosis, ferroptosis, and autophagy-dependent cell death [97]. Moreover, oxeiptosis is recently identified as a reactive oxygen species (ROS)-sensitive, caspase-independent, non-inflammatory RCD pathway. Data indicates that targeting oxeiptosis-mediated tumor suppression presents a novel approach to treating cancers [98]. Regardless, data on these cell death pathways are largely lacking and calls for further exploration in future studies.
3. Key molecular interaction between the types of programmed cell death
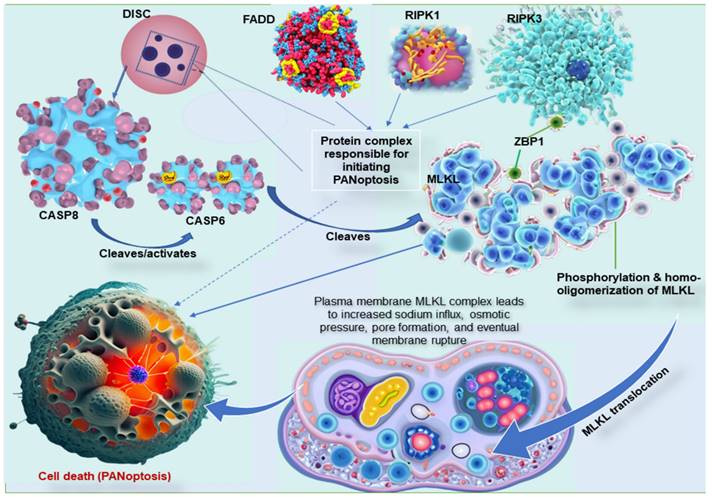
The three common pathways, necroptosis, pyroptosis, and apoptosis, have unique mechanisms, proteins, and byproducts. The key molecular interactions between these cell death pathways are complex and involve a number of different proteins [99]. The most important interactions include CASP8, RIPK1, RIPK3, MLKL, and Z-DNA binding protein 1 (ZBP1). CASP8 is a central player in all three PCD pathways [100]. In apoptosis, CASP8 is activated by a protein complex called the death-inducing signaling complex (DISC). The DISC also contains the Fas-associated death domain (FADD) proteins and RIPK1. Once CASP8 is activated, it cleaves and inactivates RIPK1, preventing the formation of the necrosome and necroptosis [99,101]. RIPK1 is another crucial player in all three PCD pathways. In apoptosis, RIPK1 is inactivated by CASP8. However, if CASP8 is inhibited, RIPK1 can form a complex with RIPK3 and FADD called the necrosome, a complex responsible for initiating necroptosis [102]. In the interaction between these cell death pathways, RIPK3 is recruited to the necrosome by RIPK1 and phosphorylates MLKL, which is the final effector of necroptosis. Once RIPK3 phosphorylates MLKL protein, it is translocated to the plasma membrane and forms pores that lead to cell death [84,99]. Moreover, ZBP1, an essential protein in these mechanisms, interacts with both CASP8 and RIPK3 and is required to assemble the PANoptosome (Figure 2) [103]. In addition to these key molecular interactions, there are a number of other proteins that are involved in regulating pyroptosis, apoptosis, and necroptosis. These proteins include inflammasomes, caspases, kinases, and phosphatases [15,17,35,104].
The complex interplay between these proteins allows the cell to fine-tune its response to various stimuli and choose the appropriate PCD pathway. For example, if the cell is infected with a virus, it may undergo pyroptosis to release inflammatory cytokines and alert the immune system. However, if the cell is damaged beyond repair, it may undergo apoptosis or necroptosis to prevent the spread of damage to other cells [15,17,35,104]. Studies have also shown that dysregulated PCD can lead to abnormal growth of cells, a hallmark feature of many cancer pathologies, causing tumor growth. An integral protein, ZBP1 participates in initiating the NLRP3 inflammasome, a known facilitator of pyroptosis. It is also demonstrated to bind to RIPK3, a regulator of necroptosis. Moreover, a unique binding partner to ZBP1, adenosine deaminase acting on RNA 1 (ADAR1), is involved in RNA editing, stress mechanisms, and diseases, including cancers [105]. Research on the molecular interactions between PCDs is still ongoing and rapidly evolving and harbors the potential to lead to new treatments for diseases like cancer.
Key molecular interaction between the types of programmed cell death. ZBP1 is essential for forming the PANoptosome, a protein complex that causes cell death. ZBP1 does this by recruiting RIPK3, another protein in the PANoptosome. RIPK3 then phosphorylates MLKL, a third protein in the PANoptosome. This phosphorylation causes MLKL to move to the plasma membrane, forming pores that kill the cell. In other words, ZBP1 is the glue that holds the PANoptosome together. It recruits the other complex proteins and helps them function properly. MLKL must form clumps (oligomerize) to move to the plasma membrane, forming pores that kill the cell. This movement to the plasma membrane happens before cell death. MLKL, either by itself or with the help of other proteins, causes sodium to flow into the cell. This influx of sodium increases the pressure inside the cell, which eventually causes the cell membrane to burst. DISC, comprising of Fas, FADD, and caspase-8, is a multi-protein complex that initiates PANoptosis, whereas RIPK1 also complexes with RIPK3 and FADD to initiate PANoptosis. ZBP1, Z-DNA binding protein 1; RIPK, receptor interacting serine/threonine kinase; MLKL, mixed lineage kinase domain like pseudokinase; DISC, death-inducing signaling complex; FADD, Fas-associated death domain.
4. The emergence of PANoptosis
The term "PANoptosis" was first coined in 2019 by a team of researchers led by Subbarao Malireddi [106]. In their study, the researchers showed that a protein complex, later dubbed PANoptosome, could induce a unique type of cell death characterized by features of pyroptosis, apoptosis, and necroptosis [106,107]. Prior to the discovery of PANoptosis, researchers had observed that certain types of cell death shared components of multiple PCD pathways. However, the molecular mechanisms underlying these hybrid cell death processes were poorly understood. The discovery of the PANoptosome and its role in PANoptosis provided a new framework for understanding these hybrid cell death processes [2,103,108]. After the discovery and description of "PANoptosis" in 2019, other key milestones in the historical evolution of PANoptosis include the demonstration of the role of PANoptosis in the development of sepsis [107,109] and tumors [13,110]. Moreover, in 2022, researchers show that PANoptosis plays a role in the neurodegeneration that occurs in Alzheimer's disease [9].
Therefore, since 2019, there has been a rapid increase in the number of studies published on PANoptosis. These studies have shed light on the molecular mechanisms of PANoptosis and its role in various diseases [6,10,52,111,112]. PANoptosis is now thought to be a critical defense mechanism against infection, but it is also believed to play a role in the development of a variety of diseases, including cancer, sepsis, inflammatory bowel disease, and Alzheimer's disease [99]. In summary, the historical evolution of the term PANoptosis is, therefore, relatively short, but it is a rapidly evolving field of research with the potential to lead to new treatments for various diseases.
5. Evidence of PANoptosis in cancer
PANoptosis serves as a multifaceted agent of the immune response with implications in driving innate immune responses and inflammation and playing a crucial role in cancer. Recent studies have demonstrated the participation of PANoptosis in several cancers. The evidence from comprehensive analyses, molecular clustering studies, and experimental validations in specific cancer cell lines collectively support the involvement of PANoptosis in cancer, highlighting its potential as a target for therapeutic interventions. For example, PANoptosis genes were found to contribute to the tumor microenvironment in lower-grade glioma patients [113], while PANoptosis-based molecular clustering demonstrated predictive capabilities for the survival and immune status of colon cancer patients [7], providing evidence of its involvement in cancer biology. By analyzing 1203 glioma samples from three public databases, the researchers found that PANoptosis is involved in tumor microenvironment interaction and patients' prognosis, and distinct PANclusters have their own molecular, immunological, and clinical profiles [113]. In a similar report, a study identified three distinct PANoptosis patterns in 1316 gastric cancer patients, each PANoptosis pattern has its own molecular, clinical, and pathological characteristics [3]. Characterizing the PANoptosis patterns predicts immunotherapy response and survival, providing a roadmap for patient stratification, not only in gastric cancer but also across pan-cancer [3]. In a proof-of-concept study, PANoptosis was shown to cause cell death in melanoma cancer cell lines. This experimental evidence reinforces the role of PANoptosis in cancer, specifically in inducing cell death [114]. Other authors indicate that PANoptosis genes are aberrantly expressed in most cancer types and are consistent with the validation of PYCARD expression, a PANoptosis gene. PANoptosis gene expression and score correlate significantly with patient survival in 21 and 14 different cancers, respectively [6], and contribute to predicting immunotherapy response in patients with tumors. A recent study found that genes and gene clusters involved in PANoptosis are associated with patient survival, immune system function, and cancer-related biological processes and pathways. The study also found that a calculated risk score can be used to predict which patients have a worse prognosis. The risk score is associated with the abundance of immune cells, the cancer stem cell index, checkpoint expression, and the response to immunotherapy and chemotherapy drugs [7]. Thus, PANoptosis-based molecular clustering and prognostic signatures can be used to predict patient survival and tumor microenvironment characteristics.
There is evidence of mutations in PANoptosis-related genes, among which NLRP3 had the highest mutation frequency, and ZBP1, AIM2(absent in melanoma 2), NLRP3, and GSDMD (Gasdermin D) had the highest copy number variation. In tumor samples, NLRP3, CASP7, RIP1 (receptor-interacting protein 1), and RIP3 are downregulated [7]. In another study, researchers identified a prognostic signature based on the expression of genes involved in PANoptosis. The signature was found to predict overall survival in patients with cutaneous melanoma, and it also provided insights into the immune infiltration landscape of the tumor [115]. The prognosis-associated PANoptosis-related genes (ZBP1, MAP3K7, and RBCK1) were highly elevated in the low-risk group and less expressed in the high-risk group, suggesting the participation of PANoptosis in cancer progression and as a potential target for therapeutic intervention in cancers [115]. Long non-coding RNAs (lncRNAs) are crucial modulators of cancer occurrence and progress. Researchers have identified several lncRNAs associated with PANoptosis and metastasis in colon adenocarcinoma. While the expression of MIR22HG and SFTA1P are decreased in colon adenocarcinoma compared with healthy individuals, SNHG6, FAM222A-AS1, SNHG11, RHPN1-AS1, SNHG16, SNHG1, and SNHG7 are highly expressed in tumors versus normal tissues. Further analysis indicated that the expression level of lncRNA SNHG7 correlates with tumor stage and lymph node metastasis. It also contributes to drug resistance and significantly relates to prognosis [111]. In addition, the PANoptosome has been identified as a cytoplasmic multimeric protein complex that controls PANoptosis [116]. This complex can engage three key modes of PCD, pyroptosis, apoptosis, and necroptosis [117], all of which are implicated in cancer. Cytosolic innate immune regulators and sensors, such as ZBP1, RIPK1, and AIM2promote the assembly of PANoptosomes to drive PANoptosis [2], and studies highlight recent progress in cancer treatment based on PANoptosis and ferroptosis induced by other small-molecule compounds, immune checkpoint inhibitors, and nanoparticles [104]. Some of the studies that report the involvement of PANoptosis in cancer are summarized in Table 1.
6. PANoptosis in immunity and its associated molecular mechanisms in cancer
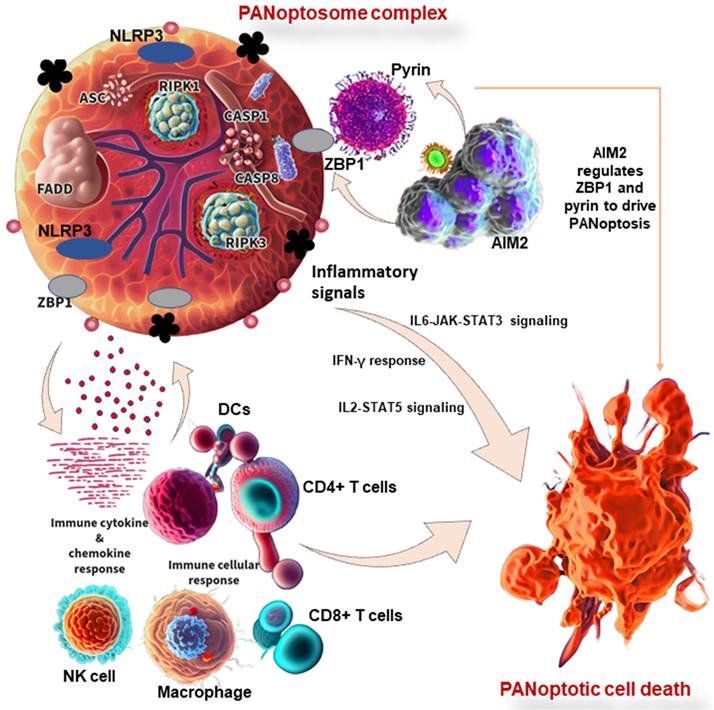
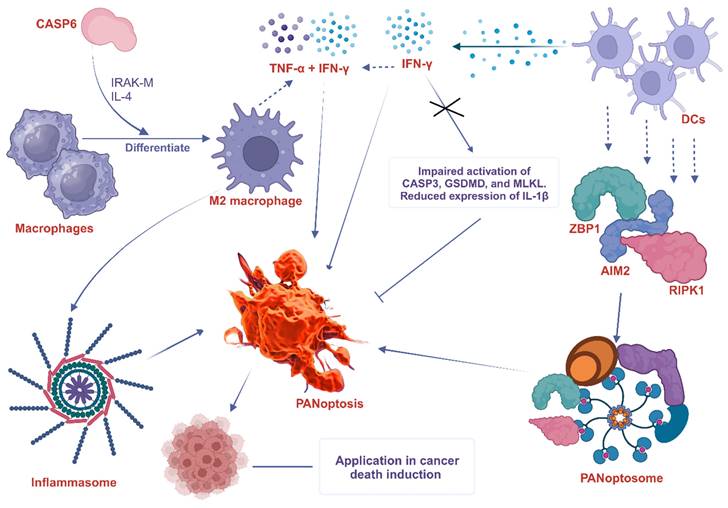
The innate immune system is made up of barriers that aim to keep pathogens and cancer cells out of the body or limit their ability to spread. During invasion, the innate immune system recognizes the tumor cell or conserved microbial molecules and engages a rapid response by producing inflammatory mediators and activating PCD pathways, including PANoptosis, pyroptosis, apoptosis, and necroptosis [112]. Studies have shown that PANoptosis is implicated in driving innate immune responses and inflammation, and it cannot be individually accounted for by pyroptosis, apoptosis, or necroptosis alone [8,112]. ZBP1 has been identified as the first innate immune sensor to form a PANoptosome and induce PANoptosis. It is reported that AIM2 regulates the innate immune sensors ZBP1 and pyrin to drive inflammatory signaling and PANoptosis, providing host protection. AIM2, ZBP1, and pyrin are members of a large multi-protein complex along with CASP1, CASP8, ASC, RIPK3, RIPK1, and FADD, that promotes inflammatory cell death (PANoptosis) (Figure 3) [11]. Moreover, pathogens induce inflammatory PCD in the form of pyroptosis, apoptosis, and necroptosis (PANoptosis), where the Zα2 domain of ZBP1 is required to promote the activation of inflammasome/PANoptosis [109]. CASP6 facilitates the RIP homotypic interaction motif (RHIM)-dependent binding of RIPK3 to ZBP1, contributing to ZBP1-mediated inflammasome activation, PANoptotic cell death, and host defense [119]. Therefore, studies have reported the functional significance of CASP6 in PANoptosis and its impact on cancer development, indicating a potential therapeutic target and strategy for tumor treatment [108]. In addition to cancer, the implications of PANoptosis in infectious diseases and autoimmunity and its potential as a therapeutic target have been the focus of ongoing research [1,8].
In cancer studies, comprehensive pathway analysis shows that PANoptosis score positively correlates with several pathways associated with immune and inflammatory responses in pan-cancer, including IL6-JAK-STAT3 signaling, the interferon-gamma (IFN-γ) response, and IL2-STAT5 signaling. Moreover, PANoptosis score is significantly associated with the tumor microenvironment, immune-related genes, and the infiltration levels of most immune cells, including NK cells, CD4+ T cells, CD8+ T cells, and DCs [6]. These observations provide PANoptosis components in cancer as potential prognostic and therapy biomarkers. Some of the innate immune cells that have been shown to be involved in PANoptosis are discussed below.
6.1 Macrophages
Macrophages are large phagocytic cells that play a crucial role in innate immunity by recognizing and engulfing pathogens, foreign substances, and cell debris. They are involved in the induction of PANoptosis in some studies. For example, a study found that PANscore, a risk-scoring system based on the PANoptosis patterns, is significantly associated with dense infiltration of M2 macrophage and cancer-associated fibroblasts, high expression of TGF-β, and low expression of immune checkpoints [3]. Infection of macrophages with pathogens results in robust cell death and the hallmarks of PANoptosis activation. Combined deletion of the PANoptotic components CASP1 (CASP1), CASP8, CASP11, and RIPK3 essentially protects macrophages from cell death induced by pathogens, while deletion of individual components provides reduced or no protection. Further analysis indicates that molecules from the apoptotic, pyroptotic, and necroptotic cell death pathways interact to form a single molecular complex termed the PANoptosome [107].
Evidence of PANoptosis in cancer
| Type of cancer | Study type | Analytical technique | Key finding | Reference |
|---|---|---|---|---|
| Lower-grade glioma | Clinical/patient samples | Database analysis of 1203 lower-grade glioma samples | Distinct PANclusters have their own molecular, clinical, and immunological profiles, and PANoptosis pattern predicts clinical survival | [113] |
| Colon adenocarcinoma | Clinical/patient samples, cell culture | Bioinformatics analysis of TCGA and GEO databases | lncRNAs are associated with metastasis and PANoptosis. lncRNA SNHG7 is linked with tumor stage and lymph node metastasis | [111] |
| Gastric and pan-cancer | Clinical/patient samples | Data acquisition and processing of RNA-seq data and corresponding clinicopathological information of samples from different databases | PANoptosis pattern score is linked with the response rate to immunotherapy and prognosis | [3] |
| Pan-cancer | Clinical/patient samples | A bioinformatic approach using the Human Protein Atlas database and RT-PCR | PANoptosis genes and scores are significantly linked with patient survival, immune and inflammatory responses, and tumor microenvironment | [6] |
| Colon | Clinical/patient samples | Bioinformatics analysis of PANoptosis-related genes and clinical data from TCGA and GEO databases | PANoptosis-related genes and gene clusters correlate with patient survival, immune system, and cancer-related biological processes and pathways | [7] |
| Cutaneous melanoma | Clinical/patient samples | Data collection and processing from TCGA | PANoptosis-related genes (ZBP1, MAP3K7, and RBCK1) could be used for risk assessment and OS prediction, reflect the immune landscape of patients and provide a novel reference for individualized tumor treatment | [115] |
| Breast | In vitro cell line and clinical data access. | Breast cancer and GSE176078 single-cell sequencing dataset from GEO and TCGA databases | PANoptosis pattern in a low-risk group correlates with better prognosis and higher levels of immune infiltration and immune checkpoint-related genes | [118] |
RT-PCR, real-time quantitative reverse transcription polymerase chain reaction; TCGA, the cancer genome atlas; GEO, gene expression omnibus; OS, overall survival; ZBP1, Z-DNA binding protein 1; MAP3K7, mitogen-activated protein kinase kinase kinase 7.
PANoptosis in immunity and its associated molecular mechanisms in cancer. PANoptosome complex consists of ZBP1 and NLRP3 as putative sensors, ASC and FADD as adaptors, and RIPK1, RIPK3, CASP1, and CASP8 as catalytic effectors. AIM2 regulates ZBP1 and pyrin to drive inflammatory signaling and PANoptosis. In other words, AIM2, ZBP1, and pyrin are members of a large multi-protein complex along with CASP1, CASP8, ASC, RIPK3, RIPK1, and FADD, that promote PANoptosis. PANoptosis drives innate immune responses and inflammation, influencing immune cells, cytokines, and chemokines. ZBP1, Z-DNA binding protein 1; AIM2, absent in melanoma 2; CASP, caspase; NLRP1, NLR family pyrin domain containing 1; FADD, Fas-associated death domain; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; RIPK, receptor interacting serine/threonine kinase; IL, interleukin; NK, natural killer, DCs, dendritic cells; JAK, Janus kinase; STAT, signal transducer and activator of transcription; IFN-γ, interferon-gamma.
Another study demonstrated that CASP6 mediates innate immunity, inflammasome activation, and activation of PANoptosis, partly by promoting the differentiation of alternatively activated macrophages (M2) [119]. These observations underscore the involvement of macrophages in the activation of PANoptosis and the formation of a PANoptosome complex.
6.2 Dendritic cells (DCs)
DCs are specialized antigen-presenting cells that play a crucial role in initiating adaptive immune responses. Although there is no direct evidence of the interaction between DCs and PANoptosis, they are believed to be involved in the induction and regulation of PANoptosis. A study found that the PANoptosis score significantly correlates with the infiltration levels of most immune cells such as DCs, as well as the tumor microenvironment and immune-related genes [6]. Some potential ways in which DCs can participate in PANoptosis include cytokine production, expression of death receptors, and formation of the PANoptosome. For instance, DCs are known to trigger the expression of cytosolic innate immune sensors and regulators such as ZBP1 [120], AIM2 [121], and RIPK1 [122], which promote the assembly of PANoptosomes to drive PANoptosis. Interestingly, the expression of ZBP1 is significantly elevated in not only DCs but also neutrophils, macrophages, natural killer (NK) cells, basophils, CD4+ T cells, and regulatory T (Treg) cells of infected patients compared with healthy controls [120]. Again, interferon-γ (IFN-γ), a key cytokine produced by DCs [123], promotes PANoptosis in mice. IFN-γ deficiency impaired the activation of PANoptosis-specific markers, including the CASP3, GSDMD, and MLKL, and reduced the expression of IL-1β [124]. In a similar study, TNF-α and IFN-γ associated PANoptosis triggered the activation of GSDMD, GSDME, CASP3, CASP7, CASP8, and MLKL, inducing cell death in 13 distinct human cancer cell lines derived from colon and lung cancer, melanoma, and leukemia [10]. This implies that PANoptosis caused by synergism of TNF-α and IFN-γ, among other immune cell cytokines, is an important mechanism to kill cancer cells and suppress tumor growth, presenting a potential therapeutic target. The participation of macrophages and DC in PANoptosis, as discussed above, is summarized in Figure 4.
The involvement of macrophage and DC in PANoptosis. CASP6 plays a key role in the innate immune system, inflammasome activation, and PANoptosis. It does this partly by promoting the differentiation of macrophages into M2 macrophages. CASP6 also plays an essential role in activating macrophages by cleaving IRAK-M, and exposure to IL-4 also enhances M2 activation, further increasing the expression of CASP6. DCs express cytosolic innate immune sensors and regulators such as ZBP1, AIM2, and RIPK1. These proteins help to assemble the PANoptosome, which triggers PANoptosis. IFN-γ produced by DCs promotes PANoptosis in mice. IFN-γ deficiency impairs the activation of PANoptosis-specific markers such as CASP3, GSDMD, and MLKL and reduces the expression of IL-1β. The combined effects of TNF-α and IFN-γ also drive PANoptosis. ZBP1, Z-DNA binding protein 1; AIM2, absent in melanoma 2; CASP, caspase; RIPK, receptor interacting serine/threonine kinase; MLKL, mixed lineage kinase domain like pseudokinase; IFN-γ, interferon-gamma; IL, interleukin; TNF-α, tumor necrosis factor alpha; DCs, dendritic cells; IRAK-M, interleukin-1 receptor-associated kinase-M; GSDMD, Gasdermin D.
6.3 Natural killer (NK) cells
NK cells are part of the innate immune system and play a key role in recognizing and killing cancerous and virus-infected cells. They have been shown to be involved in the induction of PANoptosis in some studies. For example, a study that analyzed the expression patterns, immunological role, and genetic alterations of PANoptosis genes in pan-cancer found that the PANoptosis pattern is associated with the infiltration of several immune cells, including NK cells [6]. Similarly, a single-cell analysis of PANoptosis-related gene and tumor microenvironment infiltration in hepatocellular carcinoma revealed a PANoptosis model associated with tumor immune cell infiltration, particularly NK cells [125]. In another study, the PANoptosis-associated genes, RING finger protein 34 (RNF34) was found to positively correlate with CD56dim NK cells and type 17 helper T cells. In contrast, nuclear factor-kappa-B inhibitor-alpha (NFKBIA) positively correlates with plasmacytoid DCs [126]. These observations, although few, highlight the involvement of immune cells in PANoptosis; thus, detailed mechanistic studies are required to explore their therapeutic and prognostic potentials in tumors.
6.4 Neutrophils
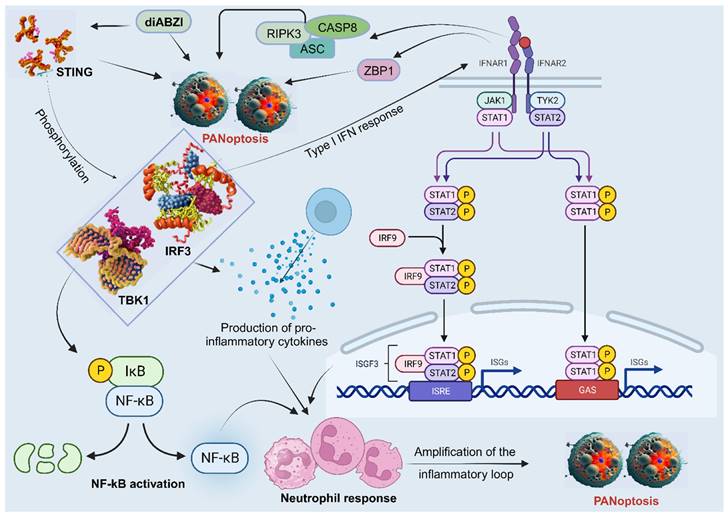
Neutrophils are the most abundant type of white blood cell and play a critical role in the first-line defense against invading pathogens. They have also been shown to be involved in the induction and regulation of PANoptosis in some studies, particularly in the context of bacterial infections. The stimulator of interferon genes (STING), a PANoptosis inducer, contributes to immune responses against tumors and may control viral infection [127]. A study showed that diamidobenzimidazole (diABZI), a STING agonist, induces neutrophilic response and PANoptosis. Mechanistically, the activation and dimerization of STING causes TBK1/IRF3 phosphorylation, leading to type I IFN response, NF-kB activation, and the production of pro-inflammatory cytokines TNFα and IL-6. Moreover, the activation of IFNAR1 and TNFR1 signaling pathways results in ZBP1 and RIPK3/ASC/CASP8 activation, leading to MLKL phosphorylation and necroptosis, CASP3 cleavage and apoptosis, as well as NLRP3 or AIM2 induced inflammasome formation and the subsequent release of mature IL-1β and pyroptosis [127]. This cascade of events, linked with the neutrophil response, amplifies the inflammatory loop and leads to upregulation of apoptosis, pyroptosis, and necroptosis, indicative of STING-dependent PANoptosis (Figure 5) [127]. However, more studies on the role of neutrophils in PANoptosis are needed.
The participation of neutrophils in PANoptosis. STING agonists trigger a series of events that lead to neutrophilic inflammation and PANoptosis. First, STING activation leads to the phosphorylation of TBK1/IRF3, which triggers the production of type I interferons and NF-κB activation. This results in the production of pro-inflammatory cytokines, such as TNFα and IL-6. Next, the activation of IFNAR1 and TNFR1 signaling pathways leads to the activation of ZBP1 and RIPK3/ASC/CASP8, all involved in PANoptosis. This cascade of events, linked with neutrophil recruitment, amplifies the inflammatory response and leads to STING-dependent PANoptosis. STING, stimulator of interferon genes; TBK1, TANK-binding kinase 1; IRF, interferon regulatory factor; NF-κB, nuclear factor kappa B; IL, interleukin; TNF-α, tumor necrosis factor alpha; IFNAR1, interferon alpha/beta receptor subunit 1; TNFR1, tumor necrosis factor receptor-1; ZBP1, Z-DNA binding protein 1; CASP, caspase; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; RIPK, receptor interacting serine/threonine kinase; JAK, Janus kinase; STAT, signal transducer and activator of transcription; IκB, IkappaB kinase; P, GAS, gamma-interferon-activated sequence; ISRE, interferon-stimulated response elements; ISGF, IFN-stimulated gene factor; ISG, IFN-stimulated gene; diABZI, diamidobenzimidazole; TYK2, tyrosine kinase 2.
6.5 Other immune cells/components
Several studies have demonstrated the involvement of other immune cells and components in PANoptosis, providing viable research areas in cancer therapy. A recent study that classified pancreatic cancer patients according to their PANoptosis-related patterns reported that patients with significantly increased infiltration of CD8+ T cells, monocytes, and naïve B cells had better prognosis compared to those with increased infiltration of macrophages, activated mast cells, and DCs [128]. However, the mechanisms of PANoptosis in cancer related to these cells are mainly lacking. In another study that investigated the participants of PANoptosis in regulating tumor progression, the findings suggested that immune-related signaling pathways potentially mediate the role of PANoptosis-related genes in tumorigenesis. This included the enrichment of lymphocyte-mediated immunity, activation of immune response, positive regulation of leukocyte activation, positive regulation of lymphocyte activation, T cell receptor complex, immunoglobulin complex, circulating immunoglobulin complex, and immunoglobulin receptor complex [115]. Similarly, it is reported that radixin (RDX), a PANoptosis prognosis-related gene, is widely involved in signaling pathways such as immune response and cell proliferation in breast cancer [118]. It is important to note that the involvement of these immune components and genes in PANoptosis is still an active area of research, and new findings may further expand our understanding of the specific cell types involved in this process.
7. Therapeutic implication of PANoptosis in cancer
7.1 Targeting key PANoptosis regulators
A number of studies have focused on the therapeutic target of the central modulators of PANoptosis, including ZBP1, RIPK3, MLKL, CASP8, and CASP6. ZBP1 is central in the assembly of the PANoptosome by recruiting RIPK3, which phosphorylates MLKL, leading to the translocation of MLKL to the plasma membrane to form pores that cause cell death [1,116,129]. Moreover, the homo-oligomerization of MLKL is required for its translocation. According to a study, MLKL oligomerization leads to the translocation of MLKL to lipid rafts of the plasma membrane, and the plasma membrane MLKL complex acts either by itself or via other proteins to increase the sodium influx, which increases osmotic pressure, eventually leading to membrane rupture [130]. CASP8 plays a role in PANoptosis by cleaving and activating CASP6, which cleaves MLKL, and leads to cell death. However, when CASP8 is inhibited, RIPK1 can form a complex with RIPK3 and FADD, which is responsible for initiating necroptosis and PANoptosis[2]. Due to the intricate role of these proteins in PCD, they form critical targets in cancer therapies that seek to regulate PANoptosis.
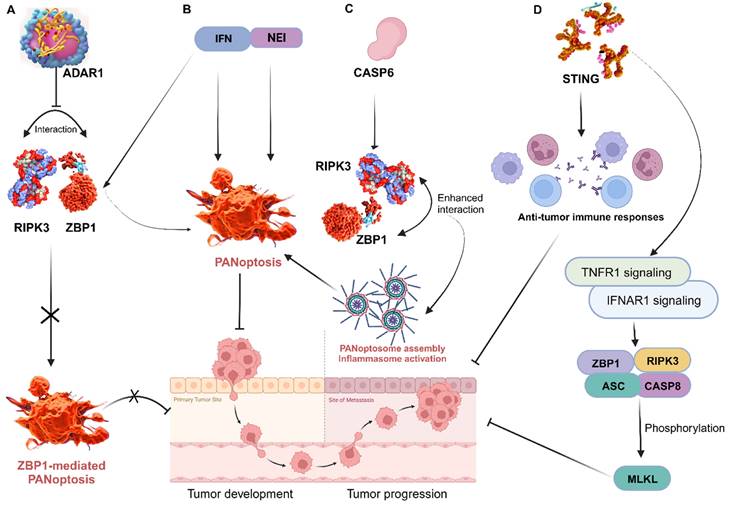
ZBP1 triggers inflammatory cell death and PANoptosis, whereas adenosine deaminase acting on RNA 1 (ADAR1) serves as an RNA editor that maintains homeostasis. A recent study identified and characterized ADAR1's interaction with ZBP1 to clarify its role in regulating cell death and tumorigenesis. The researchers found that ADAR1 restricts PANoptosis by interacting with the Zα2 domain of ZBP1 to suppress ZBP1 and RIPK3 interaction. Mice with a mutation in the ADAR1 gene (Adar1fl/flLysMcre) were resistant to developing colorectal cancer and melanoma, but ZBP1 Zα2 domain deletion restored tumorigenesis in these mice. Further analysis produced a conclusion that ADAR1 inhibits ZBP1-mediated PANoptosis, leading to the promotion of tumorigenesis [110]. This offers important information on the functions of ZBP1 and ADAR1, informing potential therapeutic strategies for cancer and other diseases. In other studies, treatment of murine bone marrow-derived macrophages (BMDMs) with nuclear export inhibitors (NEIs) increases the incidence of cell death by sequestering ADAR1 to the nucleus. Additionally, the use of interferons (IFNs) to induce ZBP1 also increases the incidence of cell death. Thus, emerging therapies are exploring the efficacy of the combination of NEI and IFN treatment to rapidly reduce tumor size and growth by inducing PANoptosis [105] (Figure 6). This is a highly viable area worth further exploration in cancer therapy.
Through its regulation of PANoptosis, CASP6 plays a vital role in tumorigenesis in humans and mediates anti-tumour immunity. Thus, a detailed exploration of CASP6 function in cancer via PANoptosis is important for preventing and treating tumors [108]. It is reported that CASP6 interacts with RIPK3 to enhance the interaction between RIPK3 and ZBP1, thus promoting PANoptosome assembly [131] (Figure 6). This can be targeted to improve cancer cell death in treatments. In another study, CASP6 facilitated ZBP1-mediated inflammasome activation, PANoptosis cell death, and host defense, opening additional avenues for treating cancer, infectious, and autoinflammatory diseases [119]. CASP 8 is another enzyme that plays a role in PCD and is activated by a variety of signals, including those from cytotoxic drugs. When CASP8 is activated, it triggers a cascade of events that leads to cell death [132]. It can mediate apoptosis, pyroptosis, and necroptosis; thus, the activation or deletion of CASP8 enzyme activity is closely related to PANoptosis [133]. Further exploration of the role of these caspases in tumors could lead to therapeutic and prognostic breakthroughs in cancer research. In addition, STING, a positive regulator of anti-tumor immune responses, is known to activate TNFR1 and IFNAR1 signaling pathways, resulting in ZBP1 and RIPK3/ASC/CASP8 activation and, consequently, MLKL phosphorylation and cell death [127] (Figure 5). These are viable therapeutic targets that aims at the core mechanisms associated with the execution of PANoptosis, thus require further exploration.
Targeting key PANoptosis regulators. A, ADAR1 impedes the interaction between ZBP1 and RIPK3, preventing ZBP1-mediated PANoptosis and promoting tumorigenesis; B, Treatment with NEI and IFN induce PANoptosis to impede tumor progression; C, The interaction of CASP6 with RIPK3 enhances that of RIPK3 and ZBP1, leading to PANoptosome assembly, which promotes ZBP1-mediated inflammasome activation and PANoptosis; D, STING enhances anti-tumor immune responses by activating TNFR1 and IFNAR1 signaling pathways, resulting in ZBP1 and RIPK3/ASC/CASP8 activation and consequently, MLKL phosphorylation and cell death. ZBP1, Z-DNA binding protein 1; ADAR1, adenosine deaminase acting on RNA 1; RIPK, receptor interacting serine/threonine kinase; NEI, nuclear export inhibitors; IFN, interferon; CASP, caspase; STING, stimulator of interferon genes; IFNAR1, interferon alpha/beta receptor subunit 1; TNFR1, tumor necrosis factor receptor-1; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; MLKL, mixed lineage kinase domain like pseudokinase.
7.2 Targeting metabolic pathways and enzymes
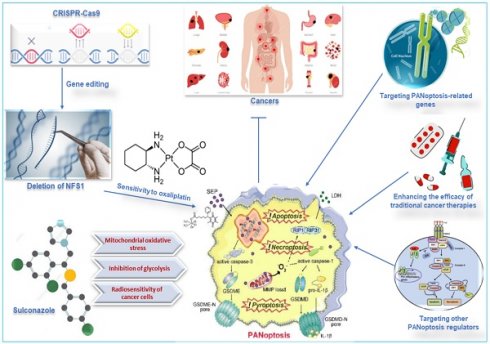
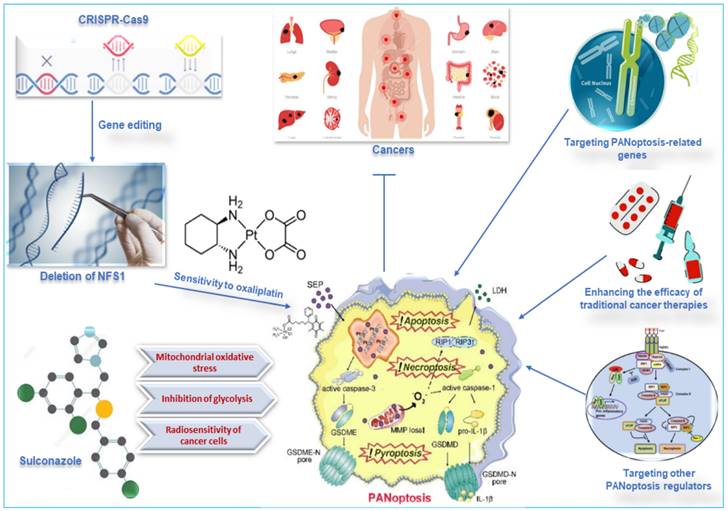
Metabolic enzymes are responsible for converting food into energy and other molecules that the body needs. Changes in the expression or activity of metabolic enzymes can affect how sensitive cells are to chemotherapy drugs. A study used CRISPR-Cas9 to delete the gene for cysteine desulfurase (NFS1), a metabolic enzyme involved in energy production, in colorectal cancer cells (CRC). The study found that deleting NFS1 made the CRC cells more sensitive to the chemotherapy drug oxaliplatin by enhancing PANoptosis. The study also found that NFS1 deficiency synergizes with oxaliplatin-triggered PANoptosis, by increasing the intracellular accumulation of ROS [12]. The findings of this study suggest that NFS1 may be a potential target for developing new cancer therapies via oxaliplatin-triggered PANoptosis. The Food and Drug Administration-approved drug, sulconazole, exhibits a spectrum of anticancer effects, including the induction of various types of PCD (apoptosis, necroptosis, pyroptosis, and ferroptosis), and inhibition of proliferation and migration of cancer cells, as well as glycolysis and its related pathways. A study found that sulconazole effectively inhibits cancer by inducing PANoptosis through the trigger of mitochondrial oxidative stress and inhibition of glycolysis, as it also increases the radiosensitivity of cancer cells [134]. These observations indicate that metabolic pathways such as glycolysis and metabolic enzymes such as NFS1 are crucial in not only triggering PANoptosis in cancer cells but increasing their susceptibility to radiotherapy and chemotherapy. This application, among other target routes of PANoptosis, is summarized in Figure 7.
Other therapeutic targets of PANoptosis in cancer. Deleting NFS1 using CRISPR-CASP9 makes CRC cells more sensitive to the chemotherapy drug oxaliplatin by enhancing PANoptosis. Thus, oxaliplatin-triggered PANoptosis serves as a viable therapeutic target in cancer. Sulconazole inhibits cancer by inducing PANoptosis through the trigger of mitochondrial oxidative stress, inhibition of glycolysis, and elevation of the radiosensitivity of cancer cells. Other targets of PANoptosis in cancer therapy include PANoptosis-related genes, other PANoptosis regulators, and inducing PANoptosis to improve the efficacy of traditional cancer therapies. CRISPR, clustered regularly interspaced short palindromic repeats; NFS1, a gene that encodes cysteine desulfurase; CASP, caspase; CRC, colorectal cancer.
7.3 Targeting PANoptosis-related genes
Resistance to PCD is a hallmark of cancer, thus, components of the cell death mechanism have long been investigated for their therapeutic, diagnostic, and prognostic potentials. A recent study that characterized PANoptosis, also a unique, innate immune-mediated inflammatory PCD pathway, reports the pan-cancer clinical significance of PANoptosis and identifies potential biomarkers. The authors found that the increased expression of PANoptosis genes ZBP1, ADAR, CASP2, CASP3, CASP4, CASP8, and GSDMD is detrimental in low-grade glioma across multiple survival models, while AIM2, CASP3, CASP4, and TNFRSF10 also negatively influenced kidney renal cell carcinoma. On the other hand, the elevated expression of PANoptosis genes ZBP1, NLRP1(NLR family pyrin domain containing 1), CASP8, and GSDMD was beneficial in skin cutaneous melanoma. Further therapeutic exploration indicated that treating melanoma cells via the activation of ZBP1-induced PANoptosis offers PANoptosis as a critical innate immune biomarker that can be targeted to improve patient outcomes in cancers [114]. As a relatively new area, the mechanistic involvement of these genes in PANoptosis should be explored in detail, particularly toward therapeutic targets in cancer and other diseases.
7.4 Targeting immune components
The effectiveness of immunotherapy relies on the cytokines and cytotoxic functions of effector immune cells to bypass the resistance to cell death and eliminate cancer cells. In the context of targeting immune cells in cancer treatment, PANoptosis is activated to induce inflammatory cell death, mediated by the synergism of cytokines, such as TNF-α and IFN-γ, which are produced by immune cells [10]. Mechanistically, TNF-α and IFN-γ induced PANoptosis cell death in cancer and suppress tumor growth by activating GSDMD, GSDME, CASP8, CASP3, CASP7, and MLKL [10]. Specific immune cells like macrophages can secrete TNF-α and IFN-γ [10], NK cells can directly kill cancer cells by secreting perforins and granzymes and through a variety of mechanisms including PANoptosis [135], and DCs can present cancer antigens to T cells and secrete cytokines that can induce PANoptosis [10,136]. These observations indicate the intricate relationship between PANoptosis and inflammasomes within innate immune cells, presenting a potential treatment strategy worth further exploration. By engineering extracellular vesicles which can induce tumor highly immunogenic PANoptosis, the researchers provided evidence that immunogenic PANoptotic cell death enables the reprogramming of immunosuppressive state and the enhancement of innate immune response by DAMPs release-promoted DC maturation and macrophage polarization (from M2 to M1 phenotype) [137]. Thus, targeting PANoptotic cell death provides not only an avenue against immune escape but also a positive feedback immune activation gateway for overcoming immune resistance to intractable cancers.
Moreover, constructing a PANoptosis signature plays a significant role in tumor immunity, promoting immune cell infiltration and enhancing the overall antitumor immune response against prostate adenocarcinoma. According to the authors, PANoptosis boosts tumor-specific immunity and positively correlates with infiltration of immune cells such as CD4, CD8, and NK cells in the TME. Immune checkpoints, including CCL2, CD274, CD4, CXCR4, and LAG3, positively correlate with the PANoptosis signature [138]. A robust innate immune response can significantly enhance the effectiveness of T cell-mediated anti-tumor immunity by priming, expanding, and promoting the infiltration of tumor-specific T cells into the tumor microenvironment [139]. Thus, PANoptosis is significantly involved in tumor immunity as it is linked with the promotion of immune cell infiltration, increases in the expression of immune checkpoint regulators, and rise of tumor immunogenicity. Further explorations of the correlation and mechanism of these immune variables and PANoptosis patterns may provide practical ways of enhancing tumor treatment. In addition, PANoptosis-related signature indicates gliomas' prognosis and immune infiltration features [140] and predicts patient survival and immune landscape in colon cancer [7]. The relationship between immune components and PANoptosis offers therapeutic and prognostic potential in cancer.
7.5 Enhancing the efficacy of traditional cancer therapies
In cancer, PANoptosis can be induced by a variety of factors, including chemotherapy, radiation therapy, and immunotherapy. PANoptosis can lead to the death of cancer cells, and it can also help to activate the immune system against cancer [129,141]. There is growing evidence that PANoptosis may be a promising target for cancer therapy. PANoptosis-inducing agents effectively kill cancer cells in vitro and in vivo [11,142]. Additionally, PANoptosis-inducing agents have been shown to enhance the efficacy of traditional cancer therapies, such as chemotherapy and radiation therapy, while some traditional cancer therapies could also induce PANoptosis. For example, a recent study identified FADD, an adaptor of PANoptosis and apoptosis, as a prominent risk factor in lung cancer, mainly localized in nucleoplasm and cytosol. Biological enrichment and immune infiltration analyses show that patients with a high expression of FADD respond better to chemotherapies such as 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR), bortezomib, docetaxel, and gemcitabine compared to immunotherapy [143]. However, in another study, the inhibition of FADD significantly reduced the ability of lung cancer cells to proliferate, and its knockdown promoted apoptosis and pyroptosis, presenting this PANoptosis-related gene as a potential treatment target in cancer [143]. Regardless, the effect of FADD may vary between cancer therapies and require in-depth exploration. A similar study also reported the significant value of the PANoptosis pattern in serving as a predictive indicator of immunotherapy response in patients with tumors [6].
PANoptosis has emerged as a promising strategy for enhancing immunotherapy efficacy. By inducing a combination of apoptosis, pyroptosis, and necroptosis, PANoptosis can effectively eliminate cancer cells while eliciting a robust anti-tumor immune response [144]. Several mechanisms contribute to the synergistic effects of PANoptosis and immunotherapy, including enhanced tumor antigen release, increased expression of immune checkpoint molecules, and reduced tumor immunosuppressive environment. For instance, PANoptosis has been shown to upregulate the expression of immune checkpoint molecules, such as PD-L1, on cancer cells [140]. These molecules can be targeted by immune checkpoint inhibitors (ICIs), which can further enhance the anti-tumor activity of T cells [140,144]. PANoptosis can also disrupt the immunosuppressive environment that often surrounds tumors by eliminating immunosuppressive cells, such as Tregs, and reducing the production of immunosuppressive factors, such as TGF-β [144]. Further research is needed to elucidate the mechanisms underlying these effects and to develop novel therapeutic strategies that combine PANoptosis-inducing agents with immunotherapy.
7.6 Targeting other PANoptosis regulators
By analyzing the adrenocortical carcinoma dataset in the cancer genome atlas (TCGA), a study found that the increased expression of cyclin-dependent kinase-1 (CDK1) is significantly associated with the adverse clinical outcomes of adrenocortical carcinoma. Further in vitro and in vivo experiments indicated that the overexpression of CDK1 promotes proliferation and epithelial-to-mesenchymal transition (EMT), partly by regulating PANoptosis of adrenocortical carcinoma cells through binding with the PANoptosome in a ZBP1-dependent way [142]. The subsequent target of CDK1 via inhibitors such as cucurbitacin E (CurE), with and without mitotane exhibited good safety and efficacy for the treatment of cancer [142]. NLRP12 initiates inflammasome and PANoptosome activation, resulting in cell death and inflammation upon exposure to heme/PAMPs or TNF. The induction of NLRP12 expression through TLR2/4-mediated signaling via IRF1 leads to inflammasome assembly, triggering the maturation of IL-1β and IL-18. This inflammasome, integral to a larger NLRP12-PANoptosome, orchestrates inflammatory cell death through the involvement of CASP8/RIPK3 [145]. This identifies NLRP12 as an essential cytosolic sensor for heme/PAMPs-mediated PANoptosis and inflammation, suggesting that NLRP12 and its pathway-associated molecules are potential drug targets in the induction of PANoptosis.
Interferon regulatory factor 1 (IRF1) is a protein that regulates many different biological processes, including the response to cancer. Mutations in the IRF1 gene or changes in its function have been linked to several types of cancer [146,147]. IRF1 plays a role in controlling cell growth, differentiation, and death. It also helps to regulate the immune system. When IRF1 is not working properly, it can lead to uncontrolled cell growth and division, which can eventually lead to cancer [146,148]. A study demonstrated that the lack of IRF1 results in hyper-susceptibility of colorectal tumorigenesis. Further analysis indicated that IRF1 protects against both AOM/DSS-induced and spontaneous colorectal cancer in mice, where the attenuated cell death in mice colon was due to defective PANoptosis (apoptosis, pyroptosis, and necroptosis) [13]. This identifies IRF1 as an upstream regulator of PANoptosis to induce cell death, serving as a potential therapeutic target in cancer. Table 2 summarizes targets of PANoptosis in recent studies in cancer.
7.7 The evolving application of PANoptosis in nanomedicine
In nanomedicine, nanoparticles such as extracellular vesicles, liposomes, dendrimers, and polymers are used to deliver drugs, genes, or other therapeutic agents to cells. Nanoparticles have been shown to be effective in inducing PANoptosis in cancer cells [150]. A recent study reports an ultrasound nanomedicine-triggered tumor immune-reediting therapeutic strategy that employs nano/genetically engineered extracellular vesicles. These vesicles have the potential to trigger a highly immunogenic form of PANoptosis cell death within tumors. This process iteratively initiates the activation of the cancer's innate immunity cycle by releasing DAMPs multiple times. Consequently, it primes many antigen-specific T cells and shapes a protective immune response by activating the cGAS-STING signaling pathways [137]. With the aid of immune checkpoint blockade, reprogramming the immune microenvironment further enhanced a prompt bridging of innate and adaptive immunity and significantly inhibited metastatic and rechallenged tumor growth. Moreover, intra-tumor levels of specific critical cytokines, including IL-2, TNF-α, IFN-γ, and IL-6, which are secreted by immune cells for promoting T-cell responses, were remarkably elevated along with PANoptosis-augmented recruitment of CD8+ and CD4+ T cells in tumor tissues [137]. These observations indicate that targeting PANoptosis cell death via nanoengineering may offer unique opportunities to boost anti-tumor immunological effects.
The potential targets of PANoptosis in cancer therapies
| Type of cancer | Study model | Therapy/target molecule | Key finding | Reference |
|---|---|---|---|---|
| Colorectal | Clinical samples, in vivo (ALB/c nude mice), and in vitro cells | Treatment with Oxaliplatin | NFS1 deficiency synergizing with oxaliplatin triggered PANoptosis | [12] |
| Colorectal | In vivo (Irf1-/- mice) of AOM/DSS-induced CRC, a spontaneous mouse model of CRC, and in vitro cells | Defective PANoptosis | IRF1 attenuates cell death in the colons of mice; lack of IRF1 induces hypersusceptible to colorectal cancer | [13][149] |
| Melanoma and colorectal | In vivo mice (Adar1fl/flLysMcreZbp1-/- and Adar1fl/flLysMcreZbp1ΔZα2), and in vitro cells | Targeting ADAR1, ZBP1 | ADAR1 suppresses ZBP1-mediated PANoptosis to promote tumorigenesis | [110] |
| Kidney renal cell carcinoma, low-grade glioma, and skin-cutaneous melanoma | Clinical sample data acquisition in databases, in vitro cells | Treatment with KPT-335 or LMB with or without IFN-γ/ Targeting PANoptosis genes, e.g., ZBP1 in melanoma cells | AIM2, CASP3, CASP4, and TNFRSF10 negatively affect renal cell carcinoma. ZBP1, ADAR, CASP2, CASP3, CASP4, CASP8, and GSDMD negatively affect low-grade glioma. ZBP1, NLRP1, CASP8, and GSDMD positively affect skin cutaneous melanoma | [114] |
| Adrenocortical carcinoma | In vitro (NCI-H295R and SW-13 cells), in vivo (female BALB/c-nu nude mice), and TCGA database | Treatment with cucurbitacin E with or without mitotane | Regulates EMT, the G2/M checkpoint, and PANoptosis. Exhibits good safety and efficacy in the treatment | [142] |
| Lung | Clinical samples data acquisition in databases (GEO and TCGA), in vitro (BEAS-2B, A549, H1299, and H441 cells) | Chemotherapies (AICAR, bortezomib, docetaxel, and gemcitabine) and immunotherapy/targeting FADD | Increased levels of FAAD positively correlate with response to the chemotherapies but negatively with immunotherapy. Inhibition of FADD prevents tumor cell proliferation, and knockdown promotes apoptosis and pyroptosis. | [143] |
| Esophageal | In vitro cell lines | Treatment with sulconazole | Induces PANoptosis by triggering oxidative stress and inhibiting glycolysis to increase radiosensitivity in cancer. Inhibits the proliferation and migration of cancer cells | [134] |
AIM2, absent in melanoma 2; CASP, caspase; NFS1, cysteine desulfurase; ZBP1, Z-DNA binding protein 1; ADAR1, adenosine deaminase acting on RNA 1; LMB, leptomycin B; TCGA, the cancer genome atlas; FADD, Fas-associated death domain; AICAR, 5-Aminoimidazole-4-carboxamide ribonucleotide; NLRP1, NLR family pyrin domain containing 1; GSDMD, Gasdermin D.
In addition, PANoptosis can be induced by various nanoparticles, including gold, silver, and carbon nanotubes by activating different signaling pathways, such as the RIPK1-RIPK3 axis, the CASP8-CASP3 axis, and the MLKL pathway, effectively eliminating cancer cells, suppressing tumor growth, and enhancing traditional cancer therapies [2,141]. Overall, PANoptosis is a promising new target in nanomedicine for cancer therapy, although it is still in its early stages of development. Further research is needed to develop more effective and safer nanoparticle-based treatments for cancer.
8. Conclusion and perspective
As a recently described form of cell death, PANoptosis is characterized by the simultaneous execution of apoptosis, necrosis, and pyroptosis. It is a complex process regulated by various signaling pathways, and it is implicated in a wide range of diseases, including cancer, infection, and inflammation. The available literature on PANoptosis is still in its early stages, but it is rapidly expanding with promising initial findings that suggest that it could be a valuable therapeutic target. The prospects of research on PANoptosis in cancer are up-and-coming, as PANoptosis has been shown to participate in the development and progression of cancer and is involved in resistance to cancer treatment. This indicates that PANoptosis could be a new and effective target for cancer therapy.
Some of the specific areas of future research on PANoptosis in cancer that have the potential to lead to new therapeutic advances include identifying the molecular mechanisms of PANoptosis in cancer. A better understanding of the molecular mechanisms of PANoptosis in cancer could lead to the development of new drugs that can specifically target this process. Moreover, developing new drugs to induce PANoptosis in cancer cells is a promising approach since such drugs could kill cancer cells and shrink tumors. On the other hand, drugs that inhibit PANoptosis in cancer cells could be used to overcome resistance to cancer treatment and improve the effectiveness of existing therapies. Furthermore, future research could also explore developing new drugs that target the tumor microenvironment to induce PANoptosis. Since the tumor microenvironment can protect cancer cells from PANoptosis, therapies that can target the tumor microenvironment to induce PANoptosis could be used to make cancer cells more susceptible to PANoptosis-inducing therapies. In addition to these specific areas of research, a number of general challenges need to be addressed to develop new PANoptosis-based cancer therapies. For example, developing drugs that can specifically target PANoptosis in cancer cells without harming healthy cells is important. It is also important to develop biomarkers that can be used to identify patients who are likely to respond to PANoptosis-based therapies.
In conclusion, the prospects of research on PANoptosis in cancer are very promising. PANoptosis is a new and exciting area of cancer research, and there is a great deal of potential to develop new and effective PANoptosis-based cancer therapies.
Acknowledgements
Funding
This study was sponsored by the National Natural Science Fund of China (Grant no. 82250410378), 2022 Jiangsu Excellent postdoctoral program (Grant no. 2022ZB634), Project of Zhenjiang key research and development plan (social development) (Grant No. SH2023050), and Project of Suzhou Science and Technology (Grant no. SKY2022027).
Author contributions
DKWO and FQ: conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing. PC, SO, and SA: conception and design, collection and assembly of data, data analysis and interpretation. FM and YQ: study design, data analysis and interpretation, manuscript writing, and final approval of manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Pandian N, Kanneganti T-D. PANoptosis: a unique innate immune inflammatory cell death modality. J Immunol. 2022;209:1625-33
2. Chen W, Gullett JM, Tweedell RE, Kanneganti T. Innate immune inflammatory cell death: panoptosis and panoptosomes in host defense and disease. Eur J Immunol. 2023;53:e2250235
3. Pan H, Pan J, Li P, Gao J. Characterization of panoptosis patterns predicts survival and immunotherapy response in gastric cancer. Clin Immunol. 2022;238:109019
4. González-Rodríguez P, Fernández-López A. PANoptosis: new insights in regulated cell death in ischemia/reperfusion models. Neural Regen Res. 2023;18:342
5. Ren H, Kang N, Yin S, Xu C, Qu T, Dai D. Characteristic of molecular subtypes based on panoptosis-related genes and experimental verification of hepatocellular carcinoma. Aging (Albany NY). 2023;15:4159-4181
6. Zhuang L, Sun Q, Huang S, Hu L, Chen Q. A comprehensive analysis of panoptosome to prognosis and immunotherapy response in pan-cancer. Sci Rep. 2023;13:3877
7. Wang X, Sun R, Chan S, Meng L, Xu Y, Zuo X. et al. PANoptosis-based molecular clustering and prognostic signature predicts patient survival and immune landscape in colon cancer. Front Genet. 2022;13:955355
8. Jiang W, Deng Z, Dai X, Zhao W. PANoptosis: a new insight into oral infectious diseases. Front Immunol. 2021;12:789610
9. Rajesh Y, Kanneganti T-D. Innate immune cell death in neuroinflammation and alzheimer's disease. Cells. 2022;11:1885
10. Malireddi RKS, Karki R, Sundaram B, Kancharana B, Lee S, Samir P. et al. Inflammatory cell death, panoptosis, mediated by cytokines in diverse cancer lineages inhibits tumor growth. ImmunoHorizons. 2021;5:568-80
11. Lee S, Karki R, Wang Y, Nguyen LN, Kalathur RC, Kanneganti T-D. AIM2 forms a complex with pyrin and ZBP1 to drive panoptosis and host defence. Nature. 2021;597:415-9
12. Lin J-F, Hu P-S, Wang Y-Y, Tan Y-T, Yu K, Liao K. et al. Phosphorylated NFS1 weakens oxaliplatin-based chemosensitivity of colorectal cancer by preventing panoptosis. Signal Transduct Target Ther. 2022;7:54
13. Karki R, Sharma BR, Lee E, Banoth B, Malireddi RKS, Samir P. et al. Interferon regulatory factor 1 regulates panoptosis to prevent colorectal cancer. JCI Insight. 2020;5:e136720
14. Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny M V. et al. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012;19:107-20
15. Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742-58
16. Tower J. Programmed cell death in aging. Ageing Res Rev. 2015;23:90-100
17. Nagata S, Tanaka M. Programmed cell death and the immune system. Nat Rev Immunol. 2017;17:333-40
18. Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol. 2020;21:678-95
19. Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175-93
20. Günther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062-71
21. D'Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43:582-92
22. US National Library of Medicine. Dose-escalation study of oral administration of s 55746 in patients with chronic lymphocytic leukaemia and b-cell non-hodgkin lymphoma. ClinicalTrials.gov. 2019 https://classic.clinicaltrials.gov/ct2/show/NCT02603445
23. US National Library of Medicine. Study of safety and efficacy of bcl201 and idelalisib in patients with fl and mcl. ClinicalTrials.gov. 2020 https://clinicaltrials.gov/study/NCT02603445
24. US National Library of Medicine. A study to evaluate the safety,pk and pd of apg-2575 in patients with hematologic malignancies. ClinicalTrials.gov. 2022 https://classic.clinicaltrials.gov/ct2/show/NCT03913949
25. US National Library of Medicine. A study with abbv-155 alone and in combination with taxane therapy in adults with relapsed and/or refractory solid tumors. ClinicalTrials.gov. 2023 https://classic.clinicaltrials.gov/ct2/show/NCT03595059
26. Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17:395-417
27. US National Library of Medicine. APG-1252 in patients with sclc or advanced solid tumors. ClinicalTrials.gov. 2021 https://classic.clinicaltrials.gov/ct2/show/NCT03387332
28. US National Library of Medicine. Testing the addition of navitoclax to the combination of dabrafenib and trametinib in people who have braf mutant melanoma. ClinicalTrials.gov. 2023 https://classic.clinicaltrials.gov/ct2/show/NCT01989585
29. US National Library of Medicine. Trametinib and navitoclax in treating patients with advanced or metastatic solid tumors. ClinicalTrials.gov. 2023 https://classic.clinicaltrials.gov/ct2/show/NCT02079740
30. US National Library of Medicine. A study of venetoclax and amg 176 in patients with relapsed/refractory hematologic malignancies. ClinicalTrials.gov. 2021 https://classic.clinicaltrials.gov/ct2/show/NCT03797261
31. US National Library of Medicine. Phase i study of mik665, a mcl-1 inhibitor, in patients with refractory or relapsed lymphoma or multiple myeloma. ClinicalTrials.gov. 2021 https://classic.clinicaltrials.gov/ct2/show/NCT02992483
32. US National Library of Medicine. Study of azd5991 alone or in combination with venetoclax in relapsed or refractory haematologic malignancies. ClinicalTrials.gov. 2022 https://classic.clinicaltrials.gov/ct2/show/NCT03218683
33. US National Library of Medicine. LCL161 plus topotecan for patients with relapsed/refractory small cell lung cancer and select gynecologic malignancies. ClinicalTrials.gov. 2022 https://classic.clinicaltrials.gov/ct2/show/NCT02649673
34. US National Library of Medicine. Dose-escalation study of birinapant and pembrolizumab in solid tumors. ClinicalTrials.gov. 2021 https://classic.clinicaltrials.gov/ct2/show/NCT02587962
35. Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7:a006080
36. Morrison JH. A mechanism emerges for the critical period hypothesis for estrogen treatment. Proc Natl Acad Sci. 2011;108:14375-6
37. Gong Y, Fan Z, Luo G, Yang C, Huang Q, Fan K. et al. The role of necroptosis in cancer biology and therapy. Mol Cancer. 2019;18:100
38. Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2019;26:99-114
39. Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18:1106-21
40. Qin X, Ma D, Tan Y-X, Wang H-Y, Cai Z. The role of necroptosis in cancer: a double-edged sword? Biochim Biophys acta Rev cancer. 2019;1871:259-66
41. Krysko O, Aaes TL, Kagan VE, D'Herde K, Bachert C, Leybaert L. et al. Necroptotic cell death in anti-cancer therapy. Immunol Rev. 2017;280:207-19
42. Yu X, Deng Q, Li W, Xiao L, Luo X, Liu X. et al. Neoalbaconol induces cell death through necroptosis by regulating ripk-dependent autocrine tnfα and ros production. Oncotarget. 2015;6:1995-2008
43. Xu B, Xu M, Tian Y, Yu Q, Zhao Y, Chen X. et al. Matrine induces rip3-dependent necroptosis in cholangiocarcinoma cells. Cell Death Discov. 2017;3:16096
44. Guo X, Li R, Cui J, Hu C, Yu H, Ren L. et al. Induction of ripk3/mlkl-mediated necroptosis by erigeron breviscapus injection exhibits potent antitumor effect. Front Pharmacol. 2023;14:1219362
45. Wang Y, Zhang Q, Wang B, Li P, Liu P. LiCl treatment induces programmed cell death of schwannoma cells through akt- and mtor-mediated necroptosis. Neurochem Res. 2017;42:2363-71
46. Xu Y, Ma H, Fang Y, Zhang Z, Shao J, Hong M. et al. Cisplatin-induced necroptosis in tnfα dependent and independent pathways. Cell Signal. 2017;31:112-23
47. Wu Y, Dong G, Sheng C. Targeting necroptosis in anticancer therapy: mechanisms and modulators. Acta Pharm Sin B. 2020;10:1601-18
48. Kesavardhana S, Malireddi RKS, Kanneganti T-D. Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol. 2020;38:567-95
49. Liu Y, Xue N, Zhang B, Lv H, Li S. Cold stress induced liver injury of mice through activated nlrp3/caspase-1/gsdmd pyroptosis signaling pathway. Biomolecules. 2022;12:927
50. Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao C. et al. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12:4310-29
51. Xia X, Wang X, Cheng Z, Qin W, Lei L, Jiang J. et al. The role of pyroptosis in cancer: pro-cancer or pro-“host”? Cell Death Dis. 2019;10:650
52. Gong W, Yang K, Zhao W, Zheng J, Yu J, Guo K. et al. Intestinal gasdermins for regulation of inflammation and tumorigenesis. Front Immunol. 2022;13:1052111
53. Zheng Y, Yuan D, Zhang F, Tang R. A systematic pan-cancer analysis of the gasdermin (gsdm) family of genes and their correlation with prognosis, the tumor microenvironment, and drug sensitivity. Front Genet. 2022;13:926796
54. Li L, Jiang M, Qi L, Wu Y, Song D, Gan J. et al. Pyroptosis, a new bridge to tumor immunity. Cancer Sci. 2021;112:3979-94
55. Du T, Gao J, Li P, Wang Y, Qi Q, Liu X. et al. Pyroptosis, metabolism, and tumor immune microenvironment. Clin Transl Med. 2021;11:e492
56. Hsu S-K, Li C-Y, Lin I-L, Syue W-J, Chen Y-F, Cheng K-C. et al. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics. 2021;11:8813-35
57. Tan J, Zhuo Z, Si Y. Application of pyroptosis in tumor research (review). Oncol Lett. 2023;26:376
58. Denton D, Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019;26:605-16
59. Yan X, Zhou R, Ma Z. Autophagy—cell survival and death. In: Advances in Experimental Medicine and Biology. 2019:667-96
60. Linder Kögel. Autophagy in cancer cell death. Biology (Basel). 2019;8:82
61. Yang G, Li Y, Zhao Y, Ouyang L, Chen Y, Liu B. et al. Targeting atg4b for cancer therapy: chemical mediators. Eur J Med Chem. 2021;209:112917
62. Patra S, Praharaj PP, Klionsky DJ, Bhutia SK. Vorinostat in autophagic cell death: a critical insight into autophagy-mediated, -associated and -dependent cell death for cancer prevention. Drug Discov Today. 2022;27:269-79
63. Dong Y, Wu Y, Zhao G-L, Ye Z-Y, Xing C-G, Yang X-D. Inhibition of autophagy by 3-ma promotes hypoxia-induced apoptosis in human colorectal cancer cells. Eur Rev Med Pharmacol Sci. 2019;23:1047-54
64. Ferreira PMP, Sousa RWR de, Ferreira JR de O, Militão GCG, Bezerra DP. Chloroquine and hydroxychloroquine in antitumor therapies based on autophagy-related mechanisms. Pharmacol Res. 2021;168:105582
65. Sun L, Xiong H, Chen L, Dai X, Yan X, Wu Y. et al. Deacetylation of atg4b promotes autophagy initiation under starvation. Sci Adv. 2022;8:eabo0412
66. Ocansey D, Yuan J, Wei Z, Mao F, Zhang Z. Role of ferroptosis in the pathogenesis and as a therapeutic target of inflammatory bowel disease (review). Int J Mol Med. 2023;51:53
67. Li J, Cao F, Yin H, Huang Z, Lin Z, Mao N. et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88
68. Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89-100
69. Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22:381-96
70. Wang H, Cheng Y, Mao C, Liu S, Xiao D, Huang J. et al. Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol Ther. 2021;29:2185-208 9
71. Yao X, Xie R, Cao Y, Tang J, Men Y, Peng H. et al. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J Nanobiotechnology. 2021;19:311
72. Kaiser AM, Attardi LD. Deconstructing networks of p53-mediated tumor suppression in vivo. Cell Death Differ. 2018;25:93-103
73. Yan H, Talty R, Jain A, Cai Y, Zheng J, Shen X. et al. Discovery of decreased ferroptosis in male colorectal cancer patients with kras mutations. Redox Biol. 2023;62:102699
74. Wang Q, Bin C, Xue Q, Gao Q, Huang A, Wang K. et al. GSTZ1 sensitizes hepatocellular carcinoma cells to sorafenib-induced ferroptosis via inhibition of nrf2/gpx4 axis. Cell Death Dis. 2021;12:426
75. Tang D, Kang R, Berghe T Vanden, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347-64
76. Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P. et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486-541
77. Lee J, Lee D, Lawler S, Kim Y. Role of neutrophil extracellular traps in regulation of lung cancer invasion and metastasis: structural insights from a computational model. Finley S, Ed. PLOS Comput Biol. 2021;17:e1008257
78. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18:134-47
79. Cahilog Z, Zhao H, Wu L, Alam A, Eguchi S, Weng H. et al. The role of neutrophil netosis in organ injury: novel inflammatory cell death mechanisms. Inflammation. 2020;43:2021-32
80. Zeng C, Zeng B, Dong C, Liu J, Xing F. Rho-rock signaling mediates entotic cell death in tumor. Cell Death Discov. 2020;6:4
81. Hamann JC, Kim SE, Overholtzer M. Methods for the study of entotic cell death. In: Methods in Molecular Biology. 2019:447-54
82. Mlynarczuk-Bialy I, Dziuba I, Sarnecka A, Platos E, Kowalczyk M, Pels KK. et al. Entosis: from cell biology to clinical cancer pathology. Cancers (Basel). 2020;12:2481
83. Kianfar M, Balcerak A, Chmielarczyk M, Tarnowski L, Grzybowska EA. Cell death by entosis: triggers, molecular mechanisms and clinical significance. Int J Mol Sci. 2022;23:4985
84. Robinson N, Ganesan R, Hegedűs C, Kovács K, Kufer TA, Virág L. Programmed necrotic cell death of macrophages: focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 2019;26:101239
85. Curtin NJ, Szabo C. Poly(adp-ribose) polymerase inhibition: past, present and future. Nat Rev Drug Discov. 2020;19:711-36
86. Zhou Y, Liu L, Tao S, Yao Y, Wang Y, Wei Q. et al. Parthanatos and its associated components: promising therapeutic targets for cancer. Pharmacol Res. 2021;163:105299
87. Zhang Y, Zhang C, Li J, Jiang M, Guo S, Yang G. et al. Inhibition of akt induces p53/sirt6/parp1-dependent parthanatos to suppress tumor growth. Cell Commun Signal. 2022;20:93
88. Martins I, Raza SQ, Voisin L, Dakhli H, Law F, De Jong D. et al. Entosis: the emerging face of non-cell-autonomous type iv programmed death. Biomed J. 2017;40:133-40
89. Wang L, Lankhorst L, Bernards R. Exploiting senescence for the treatment of cancer. Nat Rev Cancer. 2022;22:340-55
90. Wang B, Kohli J, Demaria M. Senescent cells in cancer therapy: friends or foes? Trends in Cancer. 2020;6:838-57
91. Bezu L, Kepp O, Kroemer G. Calreticulin exposure in mitotic catastrophe. Methods Mol Biol. 2021;2267:207-15
92. Yong KJ, Milenic DE, Baidoo KE, Brechbiel MW. 212Pb-radioimmunotherapy potentiates paclitaxel-induced cell killing efficacy by perturbing the mitotic spindle checkpoint. Br J Cancer. 2013;108:2013-20
93. Denisenko T V, Sorokina I V, Gogvadze V, Zhivotovsky B. Mitotic catastrophe and cancer drug resistance: a link that must to be broken. Drug Resist Updat. 2016;24:1-12
94. Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: present and emerging inducers. J Cell Mol Med. 2019;23:4854-65
95. Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J. et al. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821-33
96. Berg AL, Rowson-Hodel A, Wheeler MR, Hu M, Free SR, Carraway KL. Engaging the lysosome and lysosome-dependent cell death in cancer. In: Breast Cancer. Exon Publications. 2022:195-230
97. Song X, Zhu S, Xie Y, Liu J, Sun L, Zeng D. et al. JTC801 induces ph-dependent death specifically in cancer cells and slows growth of tumors in mice. Gastroenterology. 2018;154:1480-93
98. Pallichankandy S, Thayyullathil F, Cheratta AR, Subburayan K, Alakkal A, Sultana M. et al. Targeting oxeiptosis-mediated tumor suppression: a novel approach to treat colorectal cancers by sanguinarine. Cell Death Discov. 2023;9:94
99. Wang Y, Kanneganti T-D. From pyroptosis, apoptosis and necroptosis to panoptosis: a mechanistic compendium of programmed cell death pathways. Comput Struct Biotechnol J. 2021;19:4641-57
100. Christgen S, Tweedell RE, Kanneganti T-D. Programming inflammatory cell death for therapy. Pharmacol Ther. 2022;232:108010
101. Bellail AC, Tse MCL, Song JH, Phuphanich S, Olson JJ, Sun SY. et al. DR5-mediated disc controls caspase-8 cleavage and initiation of apoptosis in human glioblastomas. J Cell Mol Med. 2010;14:1303-17
102. Vanden Berghe T, Hassannia B, Vandenabeele P. An outline of necrosome triggers. Cell Mol Life Sci. 2016;73:2137-52
103. Zheng M, Kanneganti T. The regulation of the zbp1-nlrp3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (panoptosis). Immunol Rev. 2020;297:26-38
104. Gong L, Huang D, Shi Y, Liang Z, Bu H. Regulated cell death in cancer: from pathogenesis to treatment. Chin Med J (Engl). 2023;136:653-65
105. Camilli S, Lockey R, Kolliputi N. Nuclear export inhibitors selinexor (kpt-330) and eltanexor (kpt-8602) provide a novel therapy to reduce tumor growth by induction of panoptosis. Cell Biochem Biophys. 2023;81:421-426
106. Malireddi RKS, Kesavardhana S, Kanneganti T-D. ZBP1 and tak1: master regulators of nlrp3 inflammasome/pyroptosis, apoptosis, and necroptosis (pan-optosis). Front Cell Infect Microbiol. 2019;9:406
107. Christgen S, Zheng M, Kesavardhana S, Karki R, Malireddi RKS, Banoth B. et al. Identification of the panoptosome: a molecular platform triggering pyroptosis, apoptosis, and necroptosis (panoptosis). Front Cell Infect Microbiol. 2020;10:237
108. Qi L, Wang L, Jin M, Jiang M, Li L, Li Y. Caspase-6 is a key regulator of cross-talk signal way in panoptosis n cancer. Immunology. 2023;169:245-259
109. Banoth B, Tuladhar S, Karki R, Sharma BR, Briard B, Kesavardhana S. et al. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (panoptosis). J Biol Chem. 2020;295:18276-83
110. Karki R, Sundaram B, Sharma BR, Lee S, Malireddi RKS, Nguyen LN. et al. ADAR1 restricts zbp1-mediated immune response and panoptosis to promote tumorigenesis. Cell Rep. 2021;37:109858
111. Huang J, Jiang S, Liang L, He H, Liu Y, Cong L. et al. Analysis of panoptosis-related lncrna-mirna-mrna network reveals lncrna snhg7 involved in chemo-resistance in colon adenocarcinoma. Front Oncol. 2022;12:888105
112. Place DE, Lee S, Kanneganti T-D. PANoptosis in microbial infection. Curr Opin Microbiol. 2021;59:42-9
113. Abulaiti A, Maimaiti A, Yiming N, Fu Q, Li S, Li Y. et al. Molecular subtypes based on panoptosis-related genes and tumor microenvironment infiltration characteristics in lower-grade glioma. Funct Integr Genomics. 2023;23:84
114. Mall R, Bynigeri RR, Karki R, Malireddi RKS, Sharma BR, Kanneganti T-D. Pancancer transcriptomic profiling identifies key panoptosis markers as therapeutic targets for oncology. NAR Cancer. 2022;4:zcac033
115. Wang W, Zhou Q, Lan L, Xu X. PANoptosis-related prognostic signature predicts overall survival of cutaneous melanoma and provides insights into immune infiltration landscape. Sci Rep. 2023;13:8449
116. Samir P, Malireddi RKS, Kanneganti T-D. The panoptosome: a deadly protein complex driving pyroptosis, apoptosis, and necroptosis (panoptosis). Front Cell Infect Microbiol. 2020;10:238
117. Yan W-T, Yang Y-D, Hu X-M, Ning W-Y, Liao L-S, Lu S. et al. Do pyroptosis, apoptosis, and necroptosis (panoptosis) exist in cerebral ischemia? evidence from cell and rodent studies. Neural Regen Res. 2022;17:1761. 9
118. He P, Ma Y, Wu Y, Zhou Q, Du H. Exploring panoptosis in breast cancer based on scrna-seq and bulk-seq. Front Endocrinol (Lausanne). 2023;14:1164930
119. Zheng M, Karki R, Vogel P, Kanneganti T-D. Caspase-6 is a key regulator of innate immunity, inflammasome activation, and host defense. Cell. 2020;181:674-687.e13
120. Karki R, Lee S, Mall R, Pandian N, Wang Y, Sharma BR. et al. ZBP1-dependent inflammatory cell death, panoptosis, and cytokine storm disrupt ifn therapeutic efficacy during coronavirus infection. Sci Immunol. 2022;7:eabo6294
121. Fukuda K, Okamura K, Riding RL, Fan X, Afshari K, Haddadi N-S. et al. AIM2 regulates anti-tumor immunity and is a viable therapeutic target for melanoma. J Exp Med. 2021;218:e20200962
122. Varga Z, Rácz E, Mázló A, Korodi M, Szabó A, Molnár T. et al. Cytotoxic activity of human dendritic cells induces ripk1-dependent cell death. Immunobiology. 2021;226:152032
123. Detournay O, Mazouz N, Goldman M, Toungouz M. IL-6 produced by type i ifn dc controls ifn-γ production by regulating the suppressive effect of cd4+ cd25+ regulatory t cells. Hum Immunol. 2005;66:460-8
124. Xiao H, Zhao Q, Yuan J, Liang W, Wu R, Wen Y. et al. IFN-γ promotes panoptosis in pasteurella multocida toxin-induced pneumonia in mice. Vet Microbiol. 2023;285:109848
125. Shi X, Gao X, Liu W, Tang X, Liu J, Pan D. et al. Construction of the panoptosis-related gene model and characterization of tumor microenvironment infiltration in hepatocellular carcinoma. Oncol Res. 2023;31:569-90
126. Zhang Y-Y, Zhao H-S, Sun Y-F, Lu B-W, Sun L. Development and validation of biomarkers related to panoptosis in osteoarthritis. Eur Rev Med Pharmacol Sci. 2023;27:7444-58
127. Messaoud-Nacer Y, Culerier E, Rose S, Maillet I, Rouxel N, Briault S. et al. STING agonist diabzi induces panoptosis and dna mediated acute respiratory distress syndrome (ards). Cell Death Dis. 2022;13:269
128. Zhang B, Huang B, Zhang X, Li S, Zhu J, Chen X. et al. PANoptosis-related molecular subtype and prognostic model associated with the immune microenvironment and individualized therapy in pancreatic cancer. Front Oncol. 2023;13:1217654
129. Zhu P, Ke Z-R, Chen J-X, Li S-J, Ma T-L, Fan X-L. Advances in mechanism and regulation of panoptosis: prospects in disease treatment. Front Immunol. 2023;14:1120034
130. Chen X, Li W, Ren J, Huang D, He W, Song Y. et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105-21
131. Zheng M, Kanneganti T-D. Newly identified function of caspase-6 in zbp1-mediated innate immune responses, nlrp3 inflammasome activation, panoptosis, and host defense. J Cell Immunol. 2020;2:341-347
132. Fritsch M, Günther SD, Schwarzer R, Albert M-C, Schorn F, Werthenbach JP. et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575:683-7
133. Jiang M, Qi L, Li L, Wu Y, Song D, Li Y. Caspase-8: a key protein of cross-talk signal way in “ <scp>panoptosis</scp> ” in cancer. Int J Cancer. 2021;149:1408-20
134. Liu L-X, Heng J-H, Deng D-X, Zhao H, Zheng Z-Y, Liao L-D. et al. Sulconazole induces panoptosis by triggering oxidative stress and inhibiting glycolysis to increase radiosensitivity in esophageal cancer. Mol Cell Proteomics. 2023;22:100551
135. Yoon SR, Kim T-D, Choi I. Understanding of molecular mechanisms in natural killer cell therapy. Exp Mol Med. 2015;47:e141-e141
136. Wei L, Wang X, Zhou H. Interaction among inflammasome, panoptosise, and innate immune cells in infection of influenza virus: updated review. Immunity, Inflamm Dis. 2023;11:e997
137. Zhou L, Lyu J, Liu F, Su Y, Feng L, Zhang X. Immunogenic panoptosis-initiated cancer sono-immune reediting nanotherapy by iteratively boosting cancer immunity cycle. Adv Mater. 2023 e2305361
138. Yi X, Li J, Zheng X, Xu H, Liao D, Zhang T. et al. Construction of panoptosis signature: novel target discovery for prostate cancer immunotherapy. Mol Ther - Nucleic Acids. 2023;33:376-90
139. Raskov H, Orhan A, Christensen JP, Gögenur I. Cytotoxic cd8+ t cells in cancer and cancer immunotherapy. Br J Cancer. 2021;124:359-67
140. Song J, Xu Z, Fan Q, Sun Y, Lin X. The panoptosis-related signature indicates the prognosis and tumor immune infiltration features of gliomas. Front Mol Neurosci. 2023;16:1198713
141. Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B. et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15:174
142. Ren L, Yang Y, Li W, Zheng X, Liu J, Li S. et al. CDK1 serves as a therapeutic target of adrenocortical carcinoma via regulating epithelial-mesenchymal transition, g2/m phase transition, and panoptosis. J Transl Med. 2022;20:444
143. Wei S, Chen Z, Ling X, Zhang W, Jiang L. Comprehensive analysis illustrating the role of panoptosis-related genes in lung cancer based on bioinformatic algorithms and experiments. Front Pharmacol. 2023;14:1115221
144. Xiong Y. The emerging role of panoptosis in cancer treatment. Biomed Pharmacother. 2023;168:115696
145. Sundaram B, Pandian N, Mall R, Wang Y, Sarkar R, Kim HJ. et al. NLRP12-panoptosome activates panoptosis and pathology in response to heme and pamps. Cell. 2023;186:2783-2801.e20
146. Gao J, Senthil M, Ren B, Yan J, Xing Q, Yu J. et al. IRF-1 transcriptionally upregulates puma, which mediates the mitochondrial apoptotic pathway in irf-1-induced apoptosis in cancer cells. Cell Death Differ. 2010;17:699-709
147. Ogasawara S, Tamura G, Maesawa C, Suzuki Y, Ishida K, Satoh N. et al. Common deleted region on the long arm of chromosome 5 in esophageal carcinoma. Gastroenterology. 1996;110:52-7
148. Kim PKM, Armstrong M, Liu Y, Yan P, Bucher B, Zuckerbraun BS. et al. IRF-1 expression induces apoptosis and inhibits tumor growth in mouse mammary cancer cells in vitro and in vivo. Oncogene. 2004;23:1125-35
149. Jeyakumar T, Fodil N, Van Der Kraak L, Meunier C, Cayrol R, McGregor K. et al. Inactivation of interferon regulatory factor 1 causes susceptibility to colitis-associated colorectal cancer. Sci Rep. 2019;9:18897
150. Xia W, Tao Z, Zhu B, Zhang W, Liu C, Chen S. et al. Targeted delivery of drugs and genes using polymer nanocarriers for cancer therapy. Int J Mol Sci. 2021;22:9118
Author contact
![]() Corresponding authors: Yingchen Qian, M.D., Ph.D., Department of Pathology, Nanjing Jiangning Hospital, 168 Gushan Road, Dongshan Street, Jiangning District, Nanjing 211100, Jiangsu, P.R. China. Tel: +86 25-52087528, Fax: +86 25-52087528. E-mail address: qianyingchenwecom.work; Fei Mao, M.D., Ph.D., Department of Laboratory Medicine, Lianyungang Clinical College, Jiangsu University, Lianyungang 222006, Jiangsu, P.R. China. Tel: +86 518 8577 5003, Fax: +86 518 8577 5003. E-mail address: maofei2003edu.cn.
Corresponding authors: Yingchen Qian, M.D., Ph.D., Department of Pathology, Nanjing Jiangning Hospital, 168 Gushan Road, Dongshan Street, Jiangning District, Nanjing 211100, Jiangsu, P.R. China. Tel: +86 25-52087528, Fax: +86 25-52087528. E-mail address: qianyingchenwecom.work; Fei Mao, M.D., Ph.D., Department of Laboratory Medicine, Lianyungang Clinical College, Jiangsu University, Lianyungang 222006, Jiangsu, P.R. China. Tel: +86 518 8577 5003, Fax: +86 518 8577 5003. E-mail address: maofei2003edu.cn.
 Global reach, higher impact
Global reach, higher impact