13.3
Impact Factor
Theranostics 2024; 14(2):608-621. doi:10.7150/thno.89520 This issue Cite
Research Paper
Inactivation of the NLRP3 inflammasome mediates exosome-based prevention of atrial fibrillation
1. University of Ottawa Heart Institute, Division of Cardiology, Department of Medicine, University of Ottawa, Ottawa, CANADA K1Y4W7.
2. Department of Cellular and Molecular Medicine, Faculty of Medicine, University of Ottawa, Ottawa, Canada, K1H8M5.
3. Ottawa Hospital Research Institute, Regenerative Medicine Program, Ottawa, Canada, K1H8L6.
Abstract
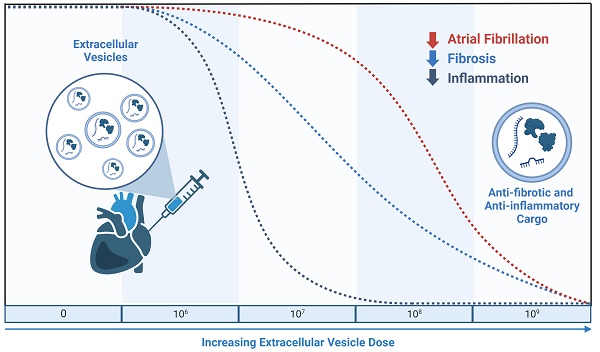
Rationale: Extracellular vesicles (EVs) from human explant-derived cells injected directly into the atria wall muscle at the time of open chest surgery reduce atrial fibrosis, atrial inflammation, and atrial fibrillation (AF) in a rat model of sterile pericarditis. Albeit a promising solution to prevent postoperative AF, the mechanism(s) underlying this effect are unknown and it is not clear if this benefit is dependent on EV dose.
Methods: To determine the dose-efficacy relationship of EVs from human explant-derived cells in a rat model of sterile pericarditis. Increasing doses of EVs (106, 107, 108 or 109) or vehicle control were injected into the atria of middle-age male Sprague-Dawley rats at the time of talc application. A sham control group was included to demonstrate background inducibility. Three days after surgery, all rats underwent invasive electrophysiological testing prior to sacrifice.
Results: Pericarditis increased the likelihood of inducing AF (p<0.05 vs. sham). All doses decreased the probability of inducing AF with maximal effects seen after treatment with the highest dose (109, p<0.05 vs. vehicle). Pericarditis increased atrial fibrosis while EV treatment limited the effect of pericarditis on atrial fibrosis with maximal effects seen after treatment with 108 or 109 EVs. Increasing EV dose was associated with progressive decreases in pro-inflammatory cytokine content, inflammatory cell infiltration, and oxidative stress. EVs decreased NLRP3 (NACHT, LRR, and PYD domains-containing protein-3) inflammasome activation though a direct effect on resident atrial fibroblasts and macrophages. This suppressive effect was exclusive to EVs produced by heart-derived cells as application of EVs from bone marrow or umbilical cords did not alter NLRP3 activity.
Conclusions: Intramyocardial injection of incremental doses of EVs at the time of open chest surgery led to progressive reductions in atrial fibrosis and inflammatory markers. These effects combined to render atria resistant to the pro-arrhythmic effects of pericarditis which is mechanistically related to suppression of the NLRP3 inflammasome.
Keywords: exosomes, extracellular vesicles, atrial fibrillation, postoperative, NLRP3
 Global reach, higher impact
Global reach, higher impact