13.3
Impact Factor
Theranostics 2024; 14(2):547-570. doi:10.7150/thno.87193 This issue Cite
Review
Copper incorporated biomaterial-based technologies for multifunctional wound repair
1. Department of Orthopedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Avenue, Wuhan 430022, China.
2. Hubei Province Key Laboratory of Oral and Maxillofacial Development and Regeneration, Wuhan 430022, China.
3. Division of Plastic Surgery, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02152, USA.
4. Department of Hand, Plastic and Reconstructive Surgery, Microsurgery, Burn Center, BG Trauma Center Ludwigshafen, University of Heidelberg, Ludwig-Guttmann-Strasse 13, 67071 Ludwigshafen/Rhine, Germany.
5. Institute of Regenerative Biology and Medicine, Helmholtz Zentrum München, Max-Lebsche-Platz 31, 81377 Munich, Germany.
6. Department of Orthopaedics, Pingshan District People's Hospital of Shenzhen, Pingshan General Hospital of Southern Medical University, Shenzhen 518118, China.
7. Department of Biomedical Engineering, University Medical Center Groningen, University of Groningen, Antonius Deusinglaan 1, 9713 Groningen AV, The Netherlands.
8. W.J. Kolff Institute for Biomedical Engineering and Materials Science, University of Groningen, University Medical Center Groningen, Antonius Deusinglaan 1, 9713 Groningen AV, The Netherlands.
†Zhenhe Zhang, Hang Xue, Yuan Xiong and Yongtao Geng contributed equally to this work.
Received 2023-6-15; Accepted 2023-11-15; Published 2024-1-1
Abstract
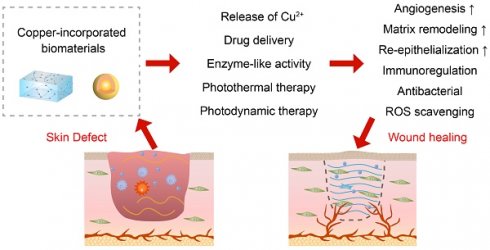
The treatment of wounds is a worldwide challenge, and wound infection can affect the effectiveness of wound treatment and further increase the disease burden. Copper is an essential trace element that has been shown to have broad-spectrum antibacterial effects and to be involved in the inflammation, proliferation, and remodeling stages of wound healing. Compared to treatments such as bioactive factors and skin grafts, copper has the advantage of being low-cost and easily available, and has received a lot of attention in wound healing. Recently, biomaterials made by incorporating copper into bioactive glasses, polymeric scaffolds and hydrogels have been used to promote wound healing by the release of copper ions. In addition, copper-incorporated biomaterials with catalytic, photothermal, and photosensitive properties can also accelerate wound healing through antibacterial and wound microenvironment regulation. This review summarizes the antibacterial mechanisms of copper- incorporated biomaterials and their roles in wound healing, and discusses the current challenges. A comprehensive understanding of the role of copper in wounds will help to facilitate new preclinical and clinical studies, thus leading to the development of novel therapeutic tools.
Keywords: Copper, Antibacterial, Nanozyme, Photothermal therapy, Photodynamic therapy
Introduction
The skin is an organ in direct contact with the external environment and its integrity is often compromised by trauma, burns and surgery [1]. Wound healing requires the concerted cooperation of multiple cells that undergo hemostasis, inflammation, proliferation, and remodeling phases to restore skin integrity [2]. However, diabetes and bacterial infections tend to alter the wound microenvironment, causing local hypoxia, accumulation of reactive oxygen species (ROS), and reduced expression of growth factors, leading to dysregulated inflammation, impaired angiogenesis, and reduced collagen production, resulting in non-healing wounds and, in severe cases, amputation or death [3]. It has been reported that the prevalence of chronic wounds is as high as 2%, requiring tens of billions of dollars to be spent on wound management each year [4]. Although autografts, allografts, xenografts, and bioengineered skin are potential treatments for skin defects, they have disadvantages such as disease transmission, biological rejection, and preparation difficulties [5]. With an aging population and an increasing number of people with diabetes, the incidence of chronic wounds will further increase, making it urgent to find more effective wound management methods [3].
Various metals, including copper, silver, gold, and zinc, have long been utilized in the treatment of infections. Among these antibacterial metals, copper stands out for its ability to achieve a balance between antibacterial efficacy and biocompatibility [6]. It not only demonstrates significantly greater antibacterial capacity than metals like zinc and gold but also boasts a higher cellular tolerance concentration compared to silver and zinc [6, 7]. In addition, compared to gold and silver, copper is more suitable for clinical translation due to its abundant natural content, ready availability and cost-effectiveness [8]. Especially in recent years, copper has been found to play a role in processes such as angiogenesis, collagen formation and matrix remodeling, further making it an ideal metal for treating wounds [9].
Despite the multifunctional properties of copper driving its application in wound care, its use in wound therapy has long been restricted to copper ions or copper oxides [10, 11]. This approach primarily relies on increasing the local concentration of copper ions to exert antibacterial and cellular function modulation effects. With the progress of nanomedicine, many copper-containing nanomaterials with high porosity, high specific area, and mimetic enzyme-like activity have been constructed for drug delivery, ROS scavenging and enhanced antibacterial efficacy, making them highly promising for wound treatment [12, 13]. In the past five years, the photothermal and photosensitive properties of copper-containing biomaterials have received widespread attention, leading to the proposal of wound treatment strategies based on bacterial clearance such as targeted photothermal antibacterial and low-temperature photothermal therapy, bringing wound treatment into the era of precision [14, 15].
Although copper-containing nanomaterials are multifunctional, the treatment of wounds requires specific media as wound dressings in order to exert wound protection and create a suitable environment for wound repair. Therefore, utilizing bioactive glass, polymer and hydrogel scaffolds for copper loading is considered an effective solution [16-18]. They can act as carriers for multifunctional copper nanomaterials, stabilizing the nanoparticles and sustaining the release of Cu2+, reducing the frequency of administration. In addition, these copper-containing composites are able to exhibit their own unique advantages in wound healing. For example, bioactive glass can release silicate ions and calcium ions, which together with copper ions modulate the function of cellular networks during wound healing [19]. Polymers can be prepared as nanofiber scaffolds to mimic the extracellular matrix to support cellular function [20]. And hydrogels can absorb exudate, moisturize, reduce wound temperature, relieve pain, and stop bleeding [21]. Overall, the introduction of these matrices has greatly enriched the functionality of copper-containing biomaterials, making them more compatible with the needs of wound therapy.
So far, various wound healing strategies based on copper-containing biomaterials have been developed, but a comprehensive summary of their application in wounds is still lacking. This review summarizes the antibacterial mechanisms of copper-containing biomaterials in wounds in the context of the microenvironment of wounds. Then, this review provides a comprehensive overview of the role played by copper in the different stages of wound healing. Subsequently, as mentioned above, since nanoparticles, bioactive glass, polymer scaffolds, and hydrogel scaffolds show different characteristics in wound treatment, this review summarizes the application of copper in wounds using this classification. In addition, since copper-containing photo-responsive materials have been extensively studied in recent years and have spawned a variety of therapeutic strategies, they are summarized in a separate section to allow for a better understanding of progress. Finally, this review discusses the biocompatibility issues and the construction directions of copper-containing biomaterials to provide guidance for subsequent research and clinical translation of copper-containing biomaterials.
Antibacterial effect of copper-incorporated biomaterials
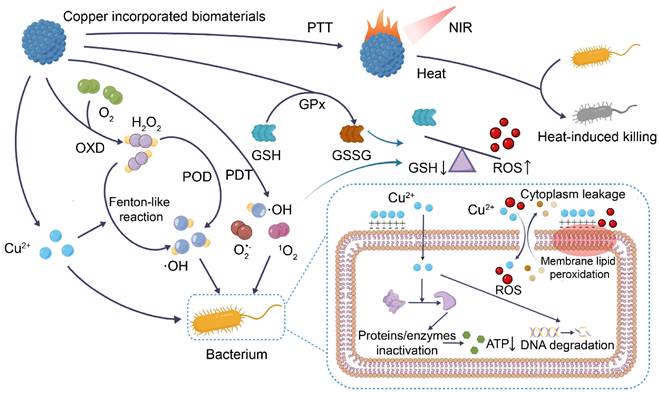
When the skin is damaged, bacteria tend to invade and proliferate, triggering a constant and high level of inflammation that prevents the wound from healing [2]. Copper has been used as an antibacterial agent since ancient times, not only to kill microorganisms including drug-resistant bacteria and fungi but also to inhibit biofilm formation [22]. Since human skin cells are less susceptible to copper than microorganisms, proper concentrations of copper can limit microbial growth without harming healthy cells [23]. In vivo studies have found a low risk of adverse reactions following direct skin contact with copper, additionally inferring that copper is safe for antibacterial use in wounds [24]. Here, the antibacterial mechanisms of copper-containing biomaterials are summarized (Figure 1).
Direct destruction of molecular and cellular structures
The cell membrane prevents extracellular substances from freely entering the cell and ensures the stability of the intracellular environment, which is the basis for cell survival [25]. Cu2+ released from copper-containing biomaterials can electrostatically bind to negatively charged bacterial cell membranes [23, 26]. The positive charge of Cu2+ changes the cell membrane potential and augments its permeability, thus, leading to structural damage of cell membranes and leakage of cellular contents [23].
Due to the increased permeability of the bacterial cell membrane and the small hydration radius of Cu2+ (87 × 10-3 nm), a large amount of Cu2+ can enter the cell [27]. In addition, the intracellular release of Cu2+ from copper-containing nanoparticles (NPs) that penetrate cell membranes or are swallowed by cells is also important for the accumulation of high levels of Cu2+ in bacterial cells [26, 28, 29]. High levels of intracellular Cu2+ can affect the biological activities of proteins by binding protein functional groups or by disrupting protein structure [30-32]. Cu2+ also has a high affinity for sulfur- and phosphorus-rich DNA, causing oxidative damage to DNA, destroying the supercoiled structure of DNA, and causing DNA breakage [26, 29, 33]. In addition, by replacing other metal ions in the ligand, Cu2+ also interferes with ATP synthesis-related enzyme activities, impeding ATP synthesis and ultimately inducing cell death [34].
It is worth noting that a new form of cell death dependent on copper has recently been discovered, known as cuproptosis [35]. Specifically, excess intracellular Cu2+ binds to lipoylated components in the tricarboxylic acid (TCA) cycle, leading to these protein aggregation and iron-sulfur clusters reduction, which induces proteotoxic stress and ultimately cell death. A recent study found that promoting bacterial metabolism can enhance the accumulation of copper-containing nanomaterials inside bacteria and cause copper overload, thereby inhibiting the bacterial TCA cycle, and ultimately leading to bacterial cuproptosis-like death [36]. Undoubtedly, studies on bacterial cuproptosis-like death will help to further understand the antibacterial mechanism of copper. In addition, it will promote the construction of copper-based biomaterials based on the "cuproptosis-like death" antibacterial strategy, and promote their antibacterial application in wounds.
Antibacterial mechanisms of copper-incorporated biomaterials. The biomaterials release Cu2+, which induces bacterial membrane lipid peroxidation and increases bacterial cell membrane permeability, leading to the efflux of cell contents. At the same time, Cu2+ and ROS enter the cell to inactivate proteins and enzymes, impair energy metabolism, and degrade DNA. Copper-incorporated biomaterials can also deplete bacterial glutathione (GSH), and generate hydroxyl radicals (·OH), superoxide anion radicals (O2•-) and singlet oxygen (1O2) via Fenton-like reaction, enzyme-like activity, and photodynamic therapy (PDT), leading to bacterial oxidative stress imbalance. Copper-incorporated photothermal materials also kill bacteria by raising the local temperature through photothermal conversion. (POD, peroxidase-like activity; OXD, oxidase-like activity; GPx, GSH peroxidase-like activity; PTT, photothermal therapy).
Fenton-like reaction
The toxicity of copper is also related to the ROS generated by copper alternating between dissimilar valence states [37]. Cu2+ released from copper-containing biomaterials can be converted to Cu+ by either endogenous or exogenous glutathione (GSH) [38]. Cu+ has shown an ability to undergo Fenton-like reactions with H2O2 to generate hydroxyl radicals (·OH) and Cu2+ [39]. In infected wounds, higher H2O2 levels contribute to Fenton-like reactions in copper-containing materials, generating more ·OH [26, 40]. ROS level that exceeds the scavenging capacity of bacteria induces bacterial membrane damage, DNA degradation, and membrane lipid peroxidation, leading to bacterial death [12, 41]. Of note, copper-based Fenton-like reaction systems catalyze the generation of ·OH at a faster rate and operate over a broader pH range compared to iron-based Fenton reaction systems [42]. Therefore, they hold a distinct advantage in bacterial eradication.
ROS production and GSH consumption by nanozymes
Nanozymes are nanomaterials with enzyme-mimetic activity [43]. Nanoparticles made of some metals and metal oxides have been shown to have peroxidase (POD), oxidase (OXD), catalase (CAT), and superoxide dismutase (SOD)-like activities for the regulation of ROS levels [44]. Currently, copper-containing nanozymes can attack bacteria by mimicking OXD, which converts O2 to H2O2, and POD, which converts H2O2 to more toxic ·OH [45]. The material releases copper ions, which induce bacterial membrane lipid peroxidation and increase bacterial cell membrane permeability, leading to the efflux of cell contents, while at the same time, copper ions and ROS enter the cell to inactivate proteins and enzymes, impair energy metabolism, and degrade DNA.
GSH, a tripeptide with antioxidant activity, accumulates substantially at the site of infection due to anaerobic glycolysis, thereby reducing the antibacterial effect of ROS [46, 47]. Copper-containing nanozymes with glutathione peroxidase (GPx)-like activity are able to deplete wound GSH, ensuring the antibacterial effect of copper-containing biomaterials [48, 49].
Photothermal and photodynamic therapy
Photothermal agents absorb light at specific wavelengths and convert light energy into heat [50]. This results in the thermal ablation of cells and is often used in photothermal therapy (PTT) for malignant tumors [51]. Photodynamic therapy (PDT) refers to the production of superoxide anion radicals (O2•-) by type I photodynamic reaction or singlet oxygen (1O2) by type II photodynamic reaction of photosensitizers under specific wavelength laser irradiation [52, 53]. In recent years, copper sulfide (CuS) has been used as a photothermal and photosensitizer for PTT and PDT at wounds due to its low toxicity, low cost, and stable photothermal and photodynamic properties [54]. After irradiation of the wound with a near-infrared (NIR) laser, CuS increases the local temperature of the wound, and generates O2•- and 1O2 with strong oxidizing properties to destroy the biofilm structure, and kill bacteria [53, 55]. Recently, a "hot ions effect" has also been identified, whereby the local increase in temperature caused by laser irradiation of copper-containing photothermal materials leads to a significant increase in the antibacterial capacity of Cu2+ [56]. The mechanism may be related to the increase in bacterial membrane permeability at elevated temperatures [56, 57].
Copper and wound healing
Mechanism of wound healing
Immediately after injury, damaged blood vessels constrict and platelets are activated and aggregated to form a blood clot consisting of platelets and fibrin to stop the wound from bleeding [58]. Inflammatory cells such as neutrophils and macrophages are recruited to the wound by chemokines to remove necrotic tissue and bacteria through phagocytosis, the release of inflammatory cytokines, and proteolytic enzymes [59]. In the subsequent proliferation phase, fibroblasts migrate to the wound, produce collagen, and some differentiate into myofibroblasts, which drive wound contraction [60]. Endothelial cells are stimulated by signals such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF)-2 to proliferate and form blood vessels in the wound to provide oxygen and nutrients to the wound [61]. The wound remodeling phase lasts for several years after the injury and is characterized by a progressive increase in type III collagen in the tissue, accompanied by a progressive decrease in type I collagen, during which matrix metalloproteinases (MMP) play a major regulatory role [62]. The remodeling of the wound makes the collagen fibers more closely arranged, the tissue strength increases, and the extracellular matrix structure gradually approaches the structure of normal skin tissue [63].
Under the influence of factors such as high glucose and infection, the wound healing cascade is disrupted, resulting in chronic wounds. Specifically, repeated tissue injury, microbes, and high levels of glucose overactivate inflammatory cells, leading to high interleukin (IL)-1β and tumor necrosis factor (TNF)-α expression and ROS accumulation [64]. Prolonged high levels of inflammation and oxidative stress at the wound site further lead to decreased proliferation and functionality of fibroblasts, keratinocytes, and endothelial cells, imbalance of MMP and tissue inhibitor of metalloproteinase (TIMP), degradation of growth factors and their receptors, resulting in impaired vascular formation, inadequate oxygen supply, disruption of extracellular matrix synthesis and remodeling, and compromised re-epithelialization [65].
In terms of treatment, although copper-containing biomaterials have achieved good results in the treatment of both acute and chronic wounds, their therapeutic mechanisms are different. It has long been recognized that acute wounds can heal without special treatment, but study has shown that the use of copper in acute wound accelerates wound healing [9]. This is because copper ions can promote the proliferation and migration of endothelial cells, fibroblasts and keratinocytes, accelerate angiogenesis, granulation tissue formation, collagen deposition and re-epithelialization in acute wounds, and ultimately make wounds heal in a shorter period of time. In chronic wounds, some copper-containing biomaterials are first considered to remove the cause. For example, copper-containing materials used in the treatment of infected wounds often give priority to the antibacterial properties of the material [66]. In addition, copper-containing materials would also focus on reversing unfavorable pathological features in chronic wounds, such as suppressing excessive inflammation by modulating immunity or scavenging excessive ROS by mimicking enzyme activity [67, 68]. The released copper ions are then used to promote angiogenesis, granulation tissue formation, collagen deposition and re-epithelialization, thus aiding in chronic wound healing.
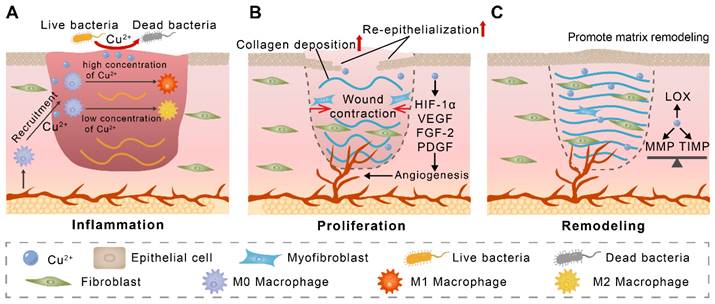
Given the versatility of copper in wound healing, we summarize the regulatory role of copper in wound healing in this section (Figure 2). The application of copper nanomaterials and copper-containing composite biomaterials in wounds will be discussed in subsequent sections.
The proposed mechanism of copper-mediated wound healing acceleration. (A) M0 macrophages are recruited to the wound by Cu2+ and subsequently polarized to M1 macrophages in response to high concentrations of Cu2+ or to M2 macrophages in response to low concentrations of Cu2+. (B) During the proliferation phase, copper promotes angiogenic factor expression and wound angiogenesis. Copper also accelerates wound contraction and re-epithelialization. (C) Copper maintains the balance of MMP and TIMP and acts as a cofactor for lysyl oxidase (LOX), which facilitates wound matrix remodeling.
Copper and inflammation
Inflammatory modulation is critical for tissue repair. Recently, the regulation of macrophages by copper ions has received much attention. Copper ions have been reported to promote the migration of Raw 264.7 macrophages [69]. Some in vivo studies have shown that placing copper-containing implants leads to more CD68+ macrophage infiltration in the surrounding soft tissue [70, 71]. These studies suggest that copper contributes to the recruitment of M0 macrophages to tissue injury. Currently, there are many studies pointing to the ability of copper ions to modulate macrophage polarization. However, the results of these studies are contradictory. For example, Xiao et al. found that wound tissue exhibited more M1 macrophages on the third day after treatment of wounds with copper-containing dressings [72]. In another study, Xu et al. found that copper decreased macrophage pro-inflammatory gene expression and increased anti-inflammatory gene expression [73]. While Jian et al. found that copper-containing hydrogel-treated macrophages exhibited more M1 polarization on day 3 and more M2 polarization on day 7 [74]. This inconsistency may be due to the fact that the regulation of macrophage polarization by copper ions is concentration-dependent, as a recent in vitro study found that treatment of the THP-1 monocyte cell line with Cu2+ at concentrations lower than 10 μM for 48 h promoted the expression of M2 macrophage-associated markers, whereas Cu2+ at concentrations higher than 100 μM increased M1 macrophage-associated marker expression [75]. Notably, polymorphonuclear granulocytes, MHC-II+ cells, and T lymphocytes may also be regulated by copper ions, although more studies are needed to confirm this [71, 76].
Copper and angiogenesis
Neovascularization transports oxygen and nutrients, removes metabolic waste, and promotes wound healing [77]. In vivo studies have shown that copper treatment promotes angiogenesis, which is related to the ability of copper to promote endothelial cell proliferation and migration [9, 11, 78, 79]. In one study, copper deficiency induced by tetrathiomolybdate significantly reduced the expression of pro-angiogenic factors and inhibited angiogenesis, further confirming the role of copper in promoting angiogenesis [80]. Copper promotes angiogenesis through several pathways, with copper-dependent VEGF expression being one of the important mechanisms. This can be achieved by copper stimulation of insulin-like growth factor-1 expression and upregulation of hypoxia-inducible factor (HIF)-1α expression [81, 82]. Copper-regulated VEGF is involved in several aspects of angiogenesis, including induction of migration and dynamic transformation of endothelial cells (tip cells and stalk cells), increase in vascular permeability and regulation of lumen diameter [83]. Moreover, it has been shown that placental growth factor levels can be upregulated by copper through activation of VEGF receptor-1 (VEGFR1) and synergistic enhancement of VEGF, thereby promoting angiogenesis [81, 84]. In addition to VEGF, copper modulates the expression and activity of several angiogenic factors, such as angiogenin (ANG), FGF, and platelet-derived growth factor, which collectively regulate angiogenic processes [11, 16]. For example, copper significantly upregulates the expression of ANG, which acts as an angiogenic agent by promoting basement membrane degradation, stimulating signaling, and exerting ribonucleolytic activity [85]. Copper acts as a transport cofactor for FGF-1 and promotes its release, thereby stimulating endothelial cell migration. Copper also plays a key role in angiogenesis by regulating the expression of endothelial nitric oxide synthase, which promotes the proliferation of endothelial cells, fibronectin, which promotes vessel elongation, and elastin, which forms and maintains the lumen [83].
Copper and wound contraction and re-epithelialization
Early contraction of the wound helps to reduce the area of exposed skin, thus reducing the risk of infection [86]. Copper treatment has been shown to accelerate centripetal contraction of the wound edges, thereby reducing wound healing time [10, 87]. Myofibroblasts are the main driver of wound contraction [88]. Although copper promotes the number of fibroblasts at the wound site, whether its promotion of wound contraction is related to the promotion of fibroblast to myofibroblast differentiation remains to be investigated [89].
The epidermis consists of multiple layers of keratinocytes [90]. In the process of wound healing, keratinocytes migrate to the surface of the wound and form a tight epidermal barrier through intercellular connections (re-epithelialization) [91]. Copper has been shown to upregulate the expression of integrin α2, integrin α6, and integrin β1, promoting keratinocytes migration and epidermal-dermal junction [92, 93]. In animal wound models, more epidermal cells and more intact epidermal structures are observed in copper-treated wounds, representing that copper promotes re-epithelialization of wounds [16, 94].
Copper and extracellular matrix deposition and remodeling
Wound remodeling involves the deposition and remodeling of the extracellular matrix, with fibroblasts dominating this process [95]. Copper has been shown to directly enhance fibroblast migration in vitro [93, 96]. In vivo studies have shown more fibroblasts in copper-treated wounds, which may be related to the fact that copper stimulates more MMP to degrade the matrix and further enhance the migration of fibroblasts [89, 97, 98]. In response to copper stimulation, fibroblasts increase the expression of extracellular matrix components such as type I collagen, dermatan sulfate, chondroitin sulfate, and decorin [99, 100]. Copper further promotes the expression of the TGF-β1, which acts on fibroblasts and increases the expression of collagen and fibronectin [101].
Degradation of the extracellular matrix by MMP is an important process in matrix remodeling [102]. Copper has been shown to increase the activity or expression levels of MMP1, MMP2, and MMP9 in fibroblasts [96, 103]. In contrast, TIMP has been shown to regulate the function of MMP and prevent excessive degradation of the matrix by MMP [104]. Copper was found to promote MMP expression while similarly increasing the secretion of TIMP-1 and TIMP-2 [97]. This plays an important role in the balance between TIMP and MMP [96, 97, 103]. In addition, copper acts as a cofactor for LOX and is involved in the regulation of collagen and elastin cross-linking [105]. Overall, in vivo studies have shown that copper-treated wounds have more collagen deposition, more ordered collagen fiber arrangement, and a similar structure to normal skin, further demonstrating that copper regulates extracellular matrix remodeling and aids in wound healing [16, 94].
Copper-incorporated nano-biomaterials
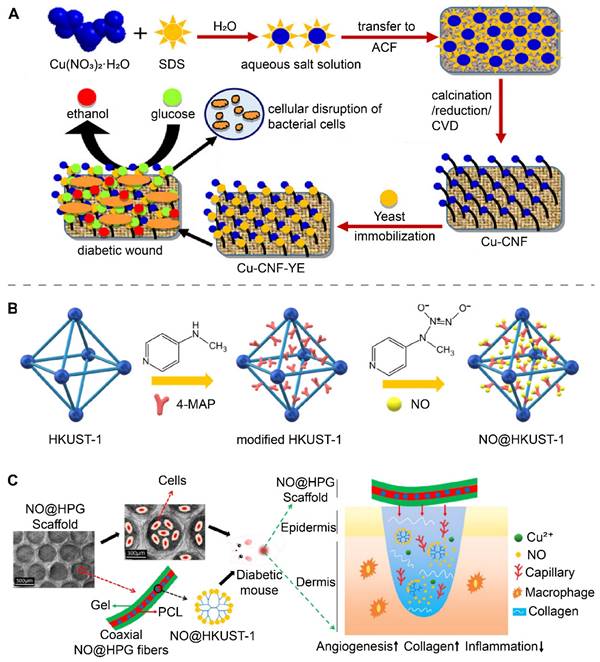
Cu and CuO nanoparticles, and materials based on these nanoparticles have been shown to exert antibacterial and accelerated wound-healing effects in vivo [9, 89]. The incorporation of other bioactive substances into copper-containing nanomaterials can provide additional strategies to treat wounds. Recently, Bhadauriya et al. immobilized yeast extract (YE) on Cu-dispersed carbon nanofibers (CNFs) to prepare Cu-CNF-YE (Figure 3A) [106]. YE was capable of converting glucose to ethanol in diabetic wounds, removing high levels of glucose from the wounds while also exerting antibacterial effects through ethanol, and acting synergistically with copper to promote diabetic wound healing.
Copper-containing nanomaterials doped with bioactive substances for wound treatment. (A) Synthesis and mechanism schematic illustration of the Cu-CNF-YE for diabetic wound healing. Reproduced with permission from [106]. Copyright 2018, American Chemical Society. (B) HKUST-1 modified with secondary amine groups for loading NO. (C) Schematic illustration of NO@HPG scaffolds for accelerating diabetic wound healing. Reproduced with permission from [13]. Copyright 2020, American Chemical Society.
Combining some bioactive substances to construct nano-biomaterials is a relatively simple method. In this section, we further focus on some nanomaterials such as drug delivery nano-systems, H2O2 self-supply systems, and nanozymes as they help to realize multifunctional wound treatment strategies.
Drug delivery nano-systems
Drug delivery nano-systems, which transport and release drugs to specific locations to function, have been used to load bioactive substances and gases to anti-infection and promote wound healing due to their advantages of crossing specific barriers, high loading capacity, slow drug release and high bioavailability [107]. Sun et al. developed cinnamaldehyde-loaded nanorods with NIR-mediated drug release properties using a chelate composed of Cu2+ and gallic acid (GA). The inherent antibacterial ability of cinnamaldehyde and the antioxidant capacity of GA enabled the nanorods to effectively accelerate the healing of infected wounds [108].
HKUST-1 is a metal-organic framework (MOF) formed by the coordination of 1,3,5 benzenetricarboxylate with copper ions and has a porous structure that has been shown to be useful for loading gas [109]. Recently, Zhang et al. prepared nitrogen monoxide (NO)-loaded secondary amine group modified HKUST-1 (NO@HKUST-1) (Figure 3B) [13]. NO@HPG was further prepared by blending NO@HKUST-1 particles into polycaprolactone (PCL) (Figure 3C). NO@HKUST-1 was able to release NO by displacing it with water. PCL effectively isolated water and prevented the rapid release of NO from NO@HKUST-1. With the slow degradation of PCL, NO@HKUST-1 came in contact with water and slowly released NO over 14 days. At the wound site, NO@HKUST-1 in NO@HPG released NO and Cu2+, both of which synergistically promote angiogenesis and accelerate diabetic wound healing. The Bohr effect refers to the fact that increasing the concentration of CO2 can lower the pH and reduce the affinity of hemoglobin for oxygen to release oxygen [110]. In a recent study, Li et al. prepared NIR-responsive PEGylated-h-CuS/bicarbonate [111]. When the temperature of the material increased, the bicarbonate decomposed to release CO2, which increased the local oxygen supply to the wound by lowering the local pH and promoting the release of oxygen from hemoglobin.
H2O2 self-supply and Fenton-like reaction system
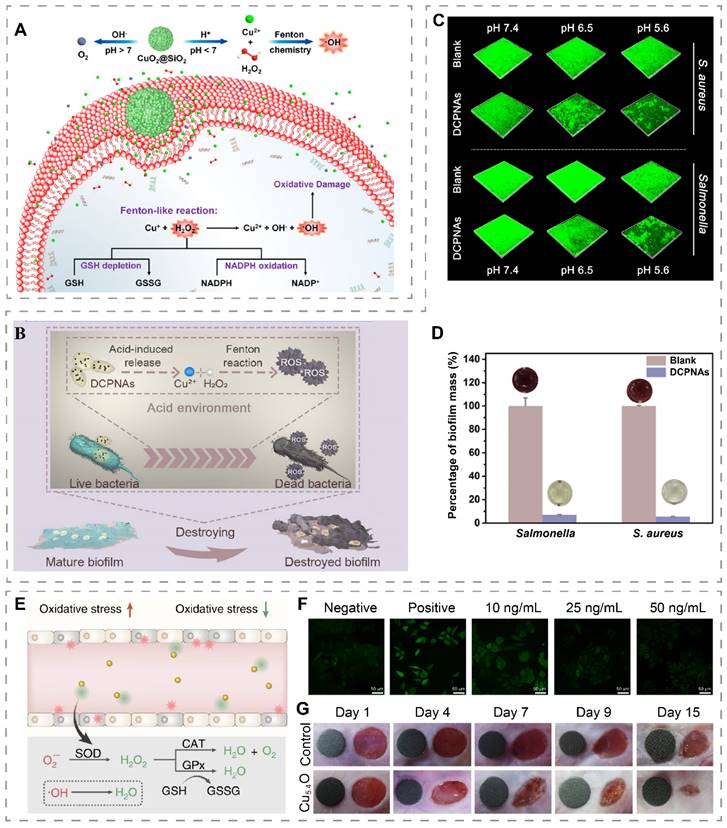
Although copper has a strong Fenton-like reaction capacity to catalyze the production of ·OH, ROS inherently has a short diffusion distance (~ 20 nm) and lifetime (< 200 ns), and thus antibacterial systems based on Fenton-like reactions should have sufficient H2O2 substrate to ensure sustained ·OH production [112]. Recently, it has been reported that CuO2 nanodots can decompose into Cu2+ and H2O2 under acidic conditions, and thus can be used to construct H2O2 self-supply systems [66]. Based on this, Zhang et al. synthesized CuO2 nanodots in the presence of PVP and NaOH [40]. The obtained CuO2 nanodots exhibited good dispersion in water due to their small size and PVP coating and were made into a spray for application on infected diabetic wounds. In the weakly acidic microenvironment of infection, CuO2 decomposed to Cu2+ and H2O2, which subsequently triggered Fenton-like reactions to produce the more ·OH, thus effectively killing Staphylococcus aureus (S. aureus), Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), and methicillin-resistant Staphylococcus aureus (MRSA). In addition, the released Cu2+ promotes migration and angiogenesis of human umbilical vein endothelial cells (HUVECs), therefore promoting the healing of infected wounds. In another study, CuO2 was encapsulated into mesoporous silica nanoshells to obtain pomegranate-like CuO2@SiO2 nanospheres for S. aureus-infected wound treatment (Figure 4A) [39]. The protection provided by the silica nanoshells slowed down the degradation of CuO2 under acidic and neutral conditions, allowing CuO2 to exert its functionality for a longer period of time. CuO2@SiO2 exhibited different reaction characteristics at different pH ranges. Specifically, at pH 7.4, CuO2@SiO2 can act as a powerful oxygen generator. Under acidic conditions, it generated ·OH and depleted bacterial endogenous antioxidants GSH and reduced β-nicotinamide adenine dinucleotide phosphate (NADPH) to assist in the clearance of wound bacteria.
Biofilms are microbial aggregates embedded in extracellular polymeric substances (EPS) matrix consisting of polysaccharides, lipids, proteins, and nucleic acids, which can resist the effects of antimicrobial agents by slowing down bacterial growth and utilizing the EPS barrier [114]. EPS matrix delays the penetration of ROS into the biofilm, resulting in insufficient ROS concentration in the biofilm and weakening its antibacterial effect. To address the low level of ROS within the biofilm, Li et al. designed dextran-coated CuO2 nanoaggregates (DCPNAs) [113]. The dextran on the surface enhanced the diffusion ability of DCPNAs in the biofilm and helped transport CuO2 into the biofilm for H2O2 self-supply and Fenton-like reactions within the biofilm (Figure 4B). DCPNAs showed pH-dependent capability for antibacterial and biofilm inhibition. After 24 hours of co-culture with bacteria, the mass of biofilm formed by DCPNAs-treated S. aureus and Salmonella at pH 5.6 was less than 10% of that of the PBS-treated group (Figure 4C-D). In S. aureus-infected wounds, DCPNAs treatment reduced the number of bacteria in the wound and accelerated wound healing.
H2O2 self-supply and Fenton-like reaction systems or nanozyme for wound treatment. (A) pH-responsive CuO2@SiO2 nanospheres for Fenton-like reaction and O2 generation. Reproduced with permission from [39]. Copyright 2021, American Chemical Society. (B) Schematic illustration of DCPNAs entering biofilm and antibacterial via acid-induced ROS production. (C) 3D CLSM images of biofilms showing the effect of DCPNAs in inhibiting biofilms under different pH conditions. (D) Effect of DCPNAs in disrupting biofilms (MTT assay). Reproduced with permission from [113]. Copyright 2021, American Chemical Society. (E) Mechanism of ROS scavenging by Cu5.4O USNPs. (F) ROS staining (green fluorescence) images of HEK293 cells treated with different concentrations of Cu5.4O USNPs. (G) Diabetic wounds treated with Cu5.4O USNPs at different time points (The diameter of the green disc is 6 mm). Reproduced with permission from [67]. Copyright 2020, Springer Nature.
Copper-containing nanozymes for wound healing.
| Nanozymes | Enzymatic activities | Performances | Applications | Ref |
|---|---|---|---|---|
| Cu-HCSs and CuO-HCSs | POD, CAT, SOD | Release of Cu2+, ROS production for antibacterial | S. aureus-infected wound | [12] |
| CuCo2S4 | POD | Used in cooperation with H2O2 to produce ROS for antibacterial and anti-biofilm formation | MRSA-infected burn wound | [118] |
| Cu5.4O USNPs | CAT, SOD, GPx | ROS scavenging | Diabetic wound | [67] |
| Cu2O/Pt nanocubes | OXD, POD, CAT | Co-used with H2O2 to produce ROS for antibacterial; released Cu2+ to promote wound healing | S. aureus-infected wound and diabetic wound | [120] |
| Cu-PBG | POD | ROS from POD-like activity of Cu-PBG for antibacterial and anti-biofilm effects | S. aureus-infected wound and E. coli-infected wound | [119] |
| CS-Cu-GA NCs | OXD, POD | Generation of ROS in synergy with chitosan, Cu NPs, and Cu2+ for antibacterial activity | S. aureus-infected wound | [45] |
| Cu-CDs | POD, CAT | ROS were generated for antibacterial and anti-biofilm | S. aureus-infected wound and mixed bacterial-infected wound | [121] |
| AuNPs/Cu-MOFNs | POD | Plasmonic nanozymes enhanced POD-like activity via LSPR to produce more ROS for antibacterial | S. aureus-infected wound | [122] |
Copper-incorporated nanozymes
Nanozymes are characterized by low cost, good stability, and adjustable enzyme-mimetic activity, and are widely used for antibacterial, biosensors and disease treatments [115-117]. The main strategy of treating wounds with copper-containing nanozymes is to generate ROS through POD and OXD-like activities, and use the generated ROS to promote wound healing via antibacterial and anti-biofilm formation [118, 119]. At present, copper-containing nanozymes have been applied to wound treatment (Table 1).
Killing bacteria by ROS production catalyzed by nanozymes is a commonly used therapeutic strategy. Recently, Li et al. synthesized a cuprous oxide/platinum (Cu2O/Pt) nanocubes with POD, CAT, and OXD-like activities for infected wound treatment [120]. By co-application with exogenous H2O2, H2O2 can be converted into ·OH and O2•-, thus exerting a potent antibacterial effect. The high levels of glucose in diabetic wounds induce high levels of ROS and reasons oxidative stress, which is an important cause of difficult wound healing in diabetic patients [123]. ROS scavenging by nanozymes is an effective strategy to improve the microenvironment of diabetic wounds. Recently, Liu et al. constructed ultra-small Cu5.4O NPs (Cu5.4O USNPs) with CAT, SOD, and GPx-like activities, whose SOD-like activity was about 21% of the natural SOD activity and can convert O2•- to H2O2, which was further scavenged by GPx-like activity or converted to O2 by CAT-like activity (Figure 4E) [67]. In vitro, Cu5.4O USNPs showed a dose-dependent ROS scavenging capacity (Figure 4F). In vivo study found faster wound healing in diabetic mice treated with Cu5.4O USNPs (Figure 4G).
Although local hypoxia in the wound is a contributing factor to angiogenesis, this is limited to acute hypoxia [124, 125]. On the contrary, chronic local hypoxia will affect the metabolic capacity of cells and hinder the generation of neovascularization and wound healing [124, 125]. It has been shown that oxygen pressure greater than 50 mmHg is beneficial for wound healing [126]. Recently, Xi et al. designed copper adhered carbon spheres (Cu-HCSs) with CAT, POD, and SOD-like activities and were able to catalyze H2O2 to provide O2 to the wound via CAT-like activity [12]. Co-application of Cu-HCSs with H2O2 to S. aureus-infected wounds resulted in significantly faster healing of the wounds. Liu et al. prepared copper-doped carbon dots (Cu-CDs) with POD and CAT-like activities [121]. 240 µg/mL Cu-CDs were able to catalyze the generation of more than 30 ppm O2 from 4 mM H2O2. Cu-CDs were used on wounds infected with S. aureus alone or with mixed bacteria and were found to reduce inflammatory exudation and significantly promote wound healing.
Copper-doped bioactive glasses
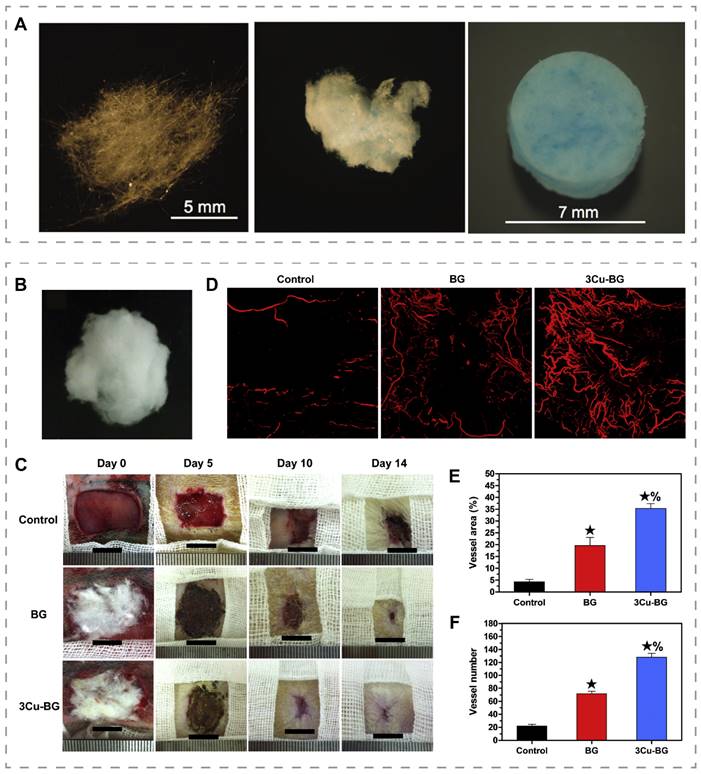
Bioactive glass was first manufactured in the late 1960s and is widely used in medical research [127]. Bioactive glasses have been found to inhibit inflammation and promote angiogenesis and re-epithelialization, which is related to the released calcium, silicon, and phosphorus ions [128-130]. By changing the composition of bioactive glass, it is possible to adjust its degradation rate and alter its bioactivity, allowing bioactive glass to be considered for soft tissue repair [131]. Recently, Lin et al. added 0.4 wt% CuO to 13-93B3 glass microfibers and obtained 13-93B3Cu glass microfibers with enhanced angiogenesis (Figure 5A) [132]. Zhao et al. prepared a cotton-like wound dressing consisting of borate bioactive glass microfibers with an average diameter of 0.85 µm by blowing high-pressure gas onto molten glass and then quenching the fibrous material (Figure 5B) [16]. The dressings were applied to full-thickness skin wounds of rats and found that the bioactive glass dressings containing 3.0 wt% CuO (3Cu-BG) treated wounds formed more blood vessels and closed faster than wounds treated with bioactive glass without copper (Figure 5C-F). To address the inflammation caused by the sharp morphology of glass particles, Li et al. constructed copper-containing bioactive glass coating on the surface of the natural eggshell membrane (ESM) by using pulsed laser deposition technique [133]. The coating on ESM showed enhanced angiogenesis and tissue repair.
Bioactive glasses can mainly be prepared by melting or sol-gel methods [134]. In 2004, Yan et al. prepared mesoporous bioactive glass (MBG) for the first time by using surfactant templating and sol-gel methods [135]. Recently, copper-doped MBG has demonstrated enhanced angiogenesis and extracellular matrix production [136]. With adjustable pore size, high pore volume and high specific surface area, MBG is expected to serve in the future as a delivery platform for copper, other metal ions, drugs or cytokines to enhance tissue repair [137].
Bioactive glass microfibers for promoting angiogenesis and wound healing. (A) Images of 13-93B3 bioglass (left), 13-93B3Cu bioglass fiber (middle) and mat of 13-93B3Cu bioglass fiber (right). Reproduced with permission from [132]. Copyright 2014, Wiley-VCH. (B) Photographs of the bioactive glass dressings containing 3.0 wt% CuO. (C) Representative images of wounds at different time points. (D) Micro-CT evaluation of blood vessels at the wound site. Quantitative analysis of vessel area (E) and vessel number (F). ★, compared to the control, p < 0.05; %, compared to BG, p < 0.05. Reproduced with permission from [16]. Copyright 2015, Elsevier.
Copper-incorporated polymer scaffolds
In recent years, polymers have been widely used in the study of wound healing due to their degradability, ability to absorb water and moisture, and appropriate mechanical properties, permeability, and porosity [138]. For some large and deep wounds, scaffolds can mimic the extracellular matrix structure, provide mechanical support for cells, and guide cell adhesion, proliferation, migration, and differentiation to help wound healing [139]. Polymer scaffolds have been criticized for their limited ability to absorb water. However, recent study has indicated that by adjusting the composition and structure of the polymer, polymer dressings with a wettability gradient enable unidirectional transport of fluids for effective removal of wound exudates [140]. Undoubtedly, this makes polymer dressings more suitable for the needs of wound treatment.
The addition of copper not only provides the polymeric scaffold with antibacterial ability, but also changes the mechanical properties of the polymeric scaffold [141]. Recently, Al-Saeedi prepared cellulose acetate (CA) fiber scaffolds containing CuO (CuO@CA) by electrospinning technology and found that the addition of CuO not only conferred the antibacterial ability to cellulose acetate but also dose-dependently increased hydrophilicity and tensile strength, making it more suitable for wound dressing [142]. Jaganathan et al. found that CuSO4 increased the hydrophilicity and tensile strength of polyurethane (PU) fibers [143]. While in another study, it was found that the moisture vapor transmission rate (MVTR) and air permeability (AP) of polyvinyl alcohol (PVA) scaffolds decreased with increasing CuO content, because CuO NPs blocked the pores between nanofibers [144].
Currently, a range of copper-doped polymer scaffolds have been developed for wound repair [10, 48]. Through the release of copper ions from polymer scaffolds, copper-containing polymer scaffolds have demonstrated more angiogenesis, matrix deposition and faster wound healing in animal studies [10, 48]. It is worth noting that the drug release from polymeric scaffolds is usually continuous and uncontrollable [142]. In contrast, wound microenvironment-responsive polymeric scaffolds can achieve selective drug release, improve drug utilization, and better promote wound healing [145]. Recently, Darder et al. prepared a blue cellulose/PAH-CuHARS foam scaffold, a cellulose scaffold containing copper-cystine high ratio structure (CuHARS) [17]. The CuHARS in the scaffold not only slowly degraded to release Cu2+ but also catalyzed the production of NO upon contact with the blood component S-nitrosocysteine (CysNO), which has the potential to exert antibacterial, anti-inflammatory, anticoagulant and wound healing promoting effects. Since HKUST-1 is prone to decomposition under acidic conditions, a recent study has prepared HKUST-1/chitosan (HKUST-1/CS) films [146]. The HKUST-1/CS films can release more Cu2+ to antibacterial under the stimulation of low pH and promote infected wound healing.
Copper-incorporated hydrogels
Hydrogels are cross-linking networks formed by hydrophilic polymers, which are characterized by their ability to absorb liquid several times their own volume [147]. Natural hydrophilic polymers used to synthesize hydrogels include hyaluronic acid (HA), alginate, chitosan, collagen, gelatin, starch, and other biodegradable natural polymers [148, 149]. In recent years, synthetic polymers such as polyacrylamide (PAM), polyethylene glycol (PEG), and polymethyl methacrylate (PMMA) have also been used to develop hydrogels due to their low immunogenicity and better mechanical properties [148]. With an appropriate swelling ratio and porous structure, hydrogel allows the exchange of nutrients and gases and plays the role of absorbing exudate and moisturizing in wounds [150]. In addition, through the modification of the molecular network of hydrogels or the modulation of the components, some hydrogels can also possess self-healing and adhesion properties, which can help to achieve physical hemostasis and provide an appropriate protective barrier for wounds [151]. Particularly after the introduction of copper, the antibacterial properties of hydrogels can be significantly enhanced. Therefore, copper- incorporated hydrogels have great potential for wound treatment.
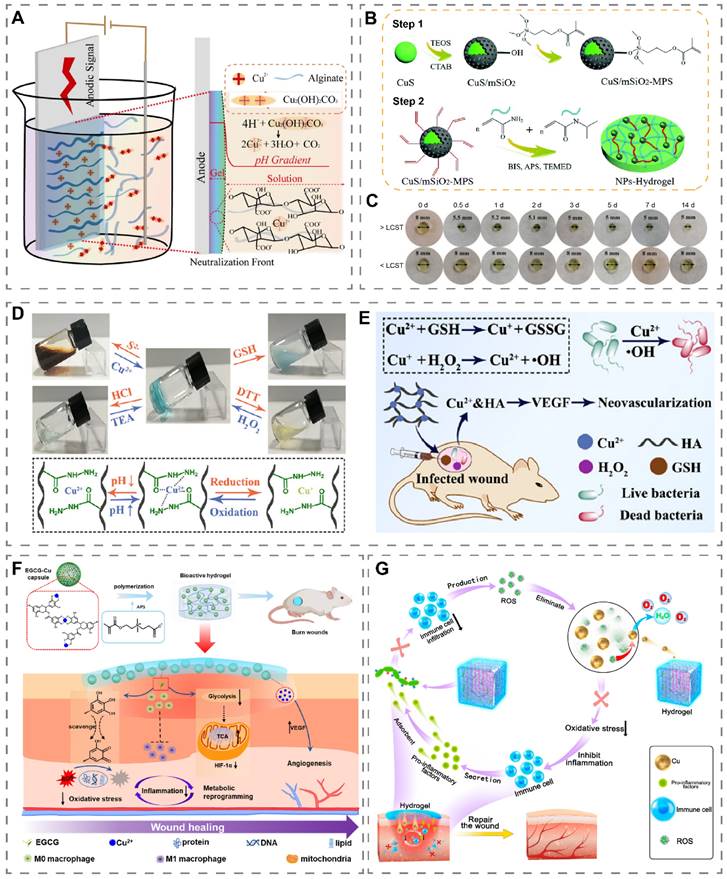
Generally, two main methods are used to prepare copper-containing hydrogels, one is to mix copper with the hydrogel prepolymer and then cross-link it, and the other is to dry the cross-linked hydrogel and then soak the copper-containing liquid to make copper enter the pores of the hydrogel [152, 153]. Recently, a Cu2+ cross-linked alginate hydrogel (Cu2+-Alg) was prepared by the electrodeposition method (Figure 6A) [18]. Specifically, a three-electrode system was placed in an alginate liquid containing the insoluble salt Cu2(OH)2CO3. After the application of a transient current, Cu2+ was released from Cu2(OH)2CO3 and cross-linked with alginate to obtain a Cu2+-Alg hydrogel on the anode surface. Compared with the conventional method, this method has the advantages of high porosity (more than 50%), fast preparation, uniform copper distribution and adjustable hydrogel thickness and copper content.
Preparation and application of copper-containing hydrogels. (A) Mechanism of preparing Cu2+-Alg hydrogels by electrodeposition. Reproduced with permission from [18]. Copyright 2021, Springer Nature. (B) Schematic diagram of the preparation of CuS/mSiO2-MPS/poly(NIAAm-co-AAm) hydrogel. (C) Temperature-induced volume change of hydrogel. >LCST (37 °C), <LCST (28 °C). Reproduced with permission from [154]. Copyright 2018, Royal Society of Chemistry. (D) Reversible sol-gel phase transition of HA-Cu hydrogel initiated by S2-/Cu2+, pH, and redox. (E) Schematic illustration of HA-Cu hydrogels for antibacterial and wound healing promotion. Reproduced with permission from [155]. Copyright 2022, American Chemical Society. (F) Schematic diagram of the preparation and wound healing mechanism of EGCG-Cu@CB hydrogel. Reproduced with permission from [156]. Copyright 2023, Elsevier. (G) Schematic diagram of Cu5.4O@Hep-PEG hydrogel accelerating wound healing through ROS clearance and pro-inflammatory factor capture. Reproduced with permission from [157]. Copyright 2021, Elsevier.
Stimuli-responsive hydrogels
For most hydrogels, slow release of Cu2+ to antibacterial, modulate inflammation, promote angiogenesis, collagen deposition, matrix remodeling and re-epithelialization are their main strategies to accelerate wound healing [158, 159]. In recent years, stimuli-responsive hydrogels have received widespread attention because of their ability to control the release of drugs in response to changes in the wound microenvironment [160]. Alginate hydrogels are bacterial and pH-responsive and have been used to prepare copper-containing hydrogels to accelerate tissue repair [161, 162]. Shahriari et al. prepared a pH-responsive Cu2+ cross-linked sodium alginate hydrogel [163]. Alginate hydrogel was hydrolyzed under acidic conditions in wounds, releasing more copper ions to resist infection. The bacteria-responsive property of alginate occurrs as a result of alginate lyase produced by bacteria which degrades alginate through the β-elimination of glycosidic bonds [164]. Chen et al. constructed alginate microcapsules containing both Cu-MOFs and Zn-MOFs using microfluidic electrospray method [165]. The microcapsules accelerated degradation with increasing E. coli concentration and released Cu2+ and Zn2+ to activate copper/zinc superoxide dismutase (Cu/Zn-SOD) to scavenge the accumulated ROS in the infected wound.
Temperature-responsive hydrogels undergo a sol-gel phase transition at the appropriate temperature and are suitable copper-loaded platforms [160]. Normally the human skin temperature is 26 °C-34 °C [166]. Wang et al. obtained PHN-Cu solutions with a lower critical solution temperature (LCST) of 29 °C by direct doping of Cu2+ in poly-(HEMA-co-NIPAM) solution [167]. PHN-Cu underwent a rapid phase change when applied to wounds, with the potential to stop bleeding by increasing wound pressure and to release Cu2+ to accelerate wound healing in diabetic rats. Recently, Tao et al. have demonstrated that the photothermal effect can elevate the temperature of copper-containing hydrogels, thereby accelerating the release of Cu2+ [168]. Li et al. added mesoporous silica (mSiO2) coated CuS NPs to hydrogels composed of acrylamide (AAm) and N-isopropylacrylamide (NIPAAm) to obtain CuS/mSiO2-MPS/poly(NIAAm-co-AAm) hydrogel (Figure 6B) [154]. At 37 °C (>LCST), the hydrogel diameter shrank from 8 mm at the beginning to 5 mm on the third day (Figure 6C). Based on this, NIR light irradiation can reduce the volume of the hydrogel by heating through the photothermal effect of CuS, thus releasing more Cu2+.
Recently, Qian et al. prepared an HA-based hydrogel capable of responding to balanced ion pairs (S2-/Cu2+), pH, and redox (Figure 6D) [155]. Notably, when Na2S was added to the HA-Cu hydrogel, Cu2+ in the hydrazide-Cu coordination unit combined with S2- to form photothermal agent CuS, which enabled the HA-Cu hydrogel to possess photothermal properties. Combining HA-Cu hydrogel with GSH effectively accelerated infected wound healing through ·OH and the release of Cu2+ (Figure 6E).
Hydrogels for modulating the wound microenvironment
Glucose oxidase (GOD) catalyzes the oxidation of glucose in the wound bed, decreasing the wound glucose concentration, and is thought to be useful in constructing cascade reaction systems. Recently, Wang et al. developed a hydrogel microparticle integrating GOD, CuO2, pH-responsive acrylic acid, and hyaluronic acid methacryloyl for wound healing and real-time monitoring of diabetic wounds [169]. GOD was able to convert glucose in the wound bed to H2O2 and gluconic acid, while lowering the pH of the wound and accelerating the breakdown of CuO2. Notably, both the glucose-catalyzed product and the CuO2 decomposition product contained H2O2, which could be used in copper-mediated Fenton-like reactions, resulting in a highly effective antibacterial effect. Moreover, the microparticle was pH-responsive, with a spectral blue-shift trend at decreasing pH, which could visualize the pH of the wound and be used to assess the glucose-catalyzed process and the wound microenvironment.
The immune microenvironment of wounds is susceptible to over-activation by interference from factors such as infection, which leads to a persistent inflammatory state and high oxidative stress levels at the wound site. The construction of copper-containing hydrogels with anti-inflammatory, antibacterial and antioxidant properties is expected to correct the pathologies that lead to delayed wound healing [65]. Yang et al. prepared a multifunctional hydrogel based on decellularized pomelo peel (DPP) coated with PVA-TSPBA hydrogel and doped with antibacterial gallic acid/copper MOFs [68]. DPP had good anti-inflammatory and antioxidant properties, and PVA-TSPBA hydrogel, as a ROS-sensitive hydrogel, could achieve ROS scavenging in wounds. The hydrogel stimulated M2 macrophage polarization and adjusted the wound microenvironment from a pro-inflammatory state to a pro-regenerative state, thereby effectively promoting the healing of infected wounds and reducing scar formation. It has been reported that excessive ROS in the wound bed might lead to mitochondrial dysfunction and enhanced glycolytic metabolism in macrophages, which induces macrophage M1-type polarization [170]. Enhanced glycolytic metabolism in M1-type macrophages leads to the accumulation of succinate by affecting mitochondrial oxidative phosphorylation and the tricarboxylic acid cycle, which in turn results in mitochondrial ROS production and initiates the uncontrolled inflammatory cycle. Li et al. prepared a hydrogel (EGCG-Cu@CB) encapsulating epigallocatechin gallate (EGCG) and Cu2+ (Figure 6F) [156]. EGCG not only scavenged excessive ROS in the wound bed but also metabolically reprogrammed macrophages by inhibiting glycolysis and normalizing the TCA cycle, which ultimately restored the redox homeostasis and blocked pro-inflammatory signaling. The accumulation of ROS and pro-inflammatory factors in wounds can further amplify local inflammation through inflammatory circulation. Current studies usually focus more on clearing ROS from wounds, ignoring the harm of pro-inflammatory factors already present in wounds [21, 67]. Recently, Peng et al. proposed a 'weeding and uprooting' treatment strategy for dual clearance of ROS and inflammatory factors from wounds (Figure 6G) [157]. In this study, heparin-PEG hydrogels loaded with Cu5.4O USNPs (Cu5.4O@Hep-PEG) were prepared. Wherein, the Cu5.4O nanozyme can clear excess ROS, and heparin-PEG hydrogels capture monocyte chemoattractant protein-1 (MCP-1) and IL-8 through electrostatic interaction, thus synergistically reducing macrophage and neutrophil infiltration, lowering inflammation level, and accelerating diabetic wound healing.
Copper-containing photothermal nanomaterials
PTT has received much attention in medical research as a non-contact, modifiable treatment modality [54]. The copper chalcogenide Cu2-xE (E = S, Te, Se, 0 ≤ x ≤ 1) based materials have good NIR-I and NIR-II absorption properties due to the localized surface plasmon resonance (LSPR) arising from copper defects, which results in the oscillating electromagnetic field of the driving light interacting with the Cu2-xE free carriers [171]. Among the many copper-based photothermal agents, CuS has been most widely used in wound repair due to its low cost, low toxicity, simple preparation, and excellent photothermal conversion efficiency [172]. Current applications of copper-based photothermal materials in wounds include antibacterial and promotion of wound healing. The antibacterial activity is mainly related to the heat-induced killing effect by PTT and the release of Cu2+ [54]. Notably, the photothermal effect can also be used to modulate drug release, which is non-contact and active [173]. It will contribute to on-demand drug delivery in wounds, which is highly promising in wound therapy. Recently, a series of targeted antibacterial strategies and synergistic therapies based on copper-containing photothermal materials have been proposed for wound treatment (Table 2).
Copper-based photothermal materials for wound healing.
| Photothermal materials | Performances | Laser | Applications | Ref |
|---|---|---|---|---|
| PATA3-C4@CuS | Targeting bacteria via electrostatic action; PTT, PDT | 980 nm, 1.5 W/cm2 | Infected wounds (bacterial type not reported) | [174] |
| GO-Tob@CuS | Targeting bacteria via electrostatic action; PTT, PDT | 980 nm, 1.5 W/cm2 | S. aureus and P. aeruginosa mixed infected wounds | [55] |
| CuS-PNIPAM | Adherence to bacteria by hydrophobic action of PNIPAM; PTT; controlled release of Cu2+ | 808 nm, 2.0 W/cm2 | S. aureus-infected wound | [15] |
| CSO@PM | PM-mediated targeting of bacteria and adsorbed toxins for anti-inflammation; PTT | 808 nm, 1.5 W/cm2 | lipopolysaccharides (LPS)-treated wound, P. aeruginosa-infected wound | [175] |
| G@CuS | Bacterially expressed gelatinases mediate Cu2+ release, PTT, and PDT | 808 nm, 1.8 W/cm2 | S. aureus-infected wound | [176] |
| BACA/CuNPs/GelMA | PTT, photothermal modulation of Cu2+ release | 808 nm, 1.2 W/cm2 | S. aureus-infected wound | [168] |
| CuS/mSiO2-MPS/poly(NIAAm-co-AAm) | PTT, PDT, photothermal modulation of Cu2+ release | 808 nm, 2.0 W/cm2 | S. aureus-infected wound | [154] |
| CuS NDs | PTT, PDT, photothermal modulation of Cu2+ release | 808 nm, 2.5 W/cm2 | MRSA-infected diabetic wound | [54] |
| AuAgCu2ONS | PTT, photothermal modulation of Cu2+ and Ag+ release | 808 nm, 1.5 W/cm2 | MRSA-infected wound | [177] |
| CuxO-PDA | Targeting bacteria via electrostatic action, PTT, and POD-like activity | 800 nm, 1.5 W/cm2 | S. aureus-infected wound | [178] |
| Cu SASs/NPC | PTT, POD-like activity, GSH consumption via GPx-like activity, | 808 nm, 1.0 W/cm2 | MRSA-infected wound | [49] |
| CuMnO2NFs | Targeting bacteria via electrostatic action; PTT, POD-like activity | 808 nm, 2.0 W/cm2 | S. aureus-infected wound | [179] |
| CuSNPs-HA-Fe3+-EDTA hydrogel | PTT, Fe2+ underwent Fenton reaction | 808 nm, 0.5 W/cm2 | S. aureus-infected wound | [180] |
| SiO2@CIN@CuS | Targeting bacteria via electrostatic action; antioxidant, PTT, photothermal regulation of CIN release | 980 nm, 1.0 W/cm2 | S. aureus-infected wound | [14] |
| Cu3SnS4 nanoflakes | Targeting bacteria via electrostatic action, antibacterial via release of Cu2+, PTT and PDT | 808 nm, 1.0 W/cm2 | MRSA-infected wound | [181] |
| CS-PLA/PCL | PTT, the release of Cu2+ | 808 nm, 0.4 W/cm2 | The wound of tumor-bearing mice, diabetic wound | [182] |
| BSA-BiZ/CuxS NC | PTT, PDT | 808 nm, 1.2 W/cm2 | MRSA-infected wound | [183] |
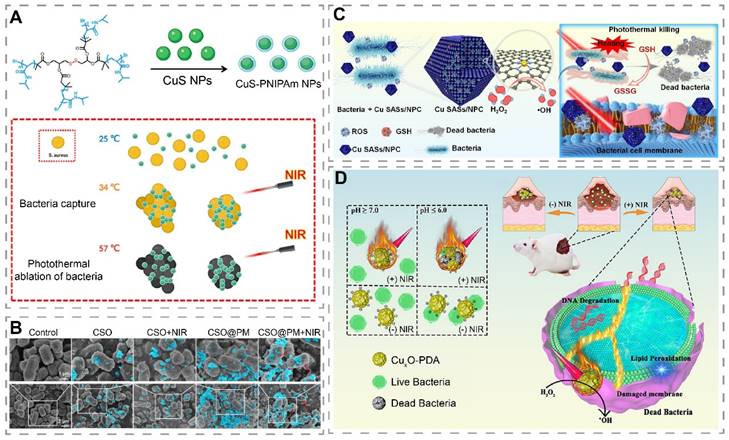
Targeted photothermal antibacterial materials and photothermal catalytic materials for wound treatment. (A) Schematic representation of the preparation and antibacterial mechanism of CuS-PNIPAm NPs. Reproduced with permission from [15]. Copyright 2022, Oxford University Press. (B) SEM images of P. aeruginosa treated with PBS, CSO, CSO@PM, CSO + NIR, CSO@PM + NIR. Blue indicates NPs. Scale bar: 1 µm (top), 2 µm (bottom). Reproduced with permission from [175]. Copyright 2021, Springer Nature. (C) Schematic diagram of Cu-SASs/NPC for the treatment of infected wounds through photothermal conversion and induction of bacterial oxidative stress imbalance. Reproduced with permission from [49]. Copyright 2021, Elsevier. (D) Schematic illustration of CuxO-PDA antibacterial and wound applications. Reproduced with permission from [178]. Copyright 2022, American Chemical Society.
Targeted photothermal antibacterial materials
Although photothermal materials can kill bacteria by increasing temperature, this excess in heat can potentiate damage to the normal surrounding tissues [184]. For wounds, after the normal skin cells around photothermal materials are killed by excessive temperature, wound healing can only be achieved by the migration of cells from more distant healthy tissue into the wound, which means that the wound healing may be delayed [182]. To enhance the targeting killing effect of photothermal materials on bacteria, Dai et al. constructed poly(5-(2-ethyl acrylate)-4-methylthiazole-g-butyl) (PATA-C4) modified CuS NPs (PATA-C4@CuS) for the precise killing of bacteria through electrostatic mediated bacterial membrane targeting [174]. PATA-C4@CuS was positively charged due to the presence of quaternary ammonium groups, and can bind to negatively charged bacterial membranes by electrostatic interaction to form a material-bacterial conjugate precipitate and kill bacteria via PTT and PDT. In contrast, PATA-C4@CuS combined with NIR showed good biocompatibility with fibroblasts, which further confirmed the bacterial targeting effect of PATA-C4@CuS. The difference in toxicity of PATA-C4@CuS to bacteria and fibroblasts could be attributed to the point that bacterial membranes hold more negative surface charges. In another study, Wang et al. increased the hydrophobicity of the four-armed poly(N-isopropylacrylamide) (PNIPAM) on the surface of CuS-PNIPAM NPs through photothermal effect, which enhanced the adhesion to bacterial membranes for bacterial capture and elimination (Figure 7A) [15]. Interestingly, Cu2+ released from the nanoparticles can subsequently chelate with the amide group of the PNIPAM shell, avoiding the local accumulation of Cu2+. Peng et al. prepared a platelet membrane (PM)-encapsulated mesoporous copper silicate microspheres (CSO) (CSO@PM) that targeted bacteria via Toll-like receptors, formyl peptide receptors, and chemokine receptors of PM (Figure 7B) [175]. In vivo studies have shown that CSO@PM + NIR can reduce wound inflammation and accelerate wound healing.
Photothermal catalytic materials
It has been shown that photothermal catalytic materials, which generated ·OH by POD-like activity, can exert stronger antibacterial effects in synergy with the heat-induced killing effect of PTT [185]. Wang et al. designed Cu single atomic site/N-doped porous carbon photothermal catalytic antibacterial nanoplatforms (Cu SASs/NPC) for the treatment of infected wounds (Figure 7C) [49]. Cu SASs/NPC had POD-like and GPx-like activities that catalyzed the production of ·OH and depletion of GSH, respectively. Meanwhile, Cu SASs/NPC showed a photothermal conversion efficiency of 82.78% under NIR light (808 nm, 1.0 W/cm2) irradiation. In vitro, Cu SASs/NPC+H2O2+NIR resulted in high ROS production in bacteria via POD-like activity and PTT. As GSH was depleted, ROS was able to exert better antibacterial effects, as shown by killing almost all E. coli and MRSA. In vivo, it showed the least number of residual bacteria, the least inflammatory response, and the fastest healing rate in MRSA-infected wounds. In another study, Bai et al. prepared an ultrathin two-dimensional N-doped porous carbon nanosheet supported Cu single atoms (CuNx-CNS, x = 2 or 4) with tunable N-coordination structures, which exhibited excellent POD, OXD, and CAT activities as well as good photothermal performances in the NIR-II window [186]. Compared with CuN2-CNS, CuN4-CNS possessed a stronger electron-pushing effect and oxygen adsorption ability, and thus exhibited stronger CAT and OXD activities, which significantly enhanced ROS generation. Notably, the photothermal effect under NIR-II irradiation could further promote ROS generation. The excellent enzyme mimetic activity synergized with the photothermal effect to effectively kill bacteria and eliminate biofilm. Due to the multiple facilitating effects of copper in wound healing, CuN4-CNS could reduce the inflammatory response of infected wounds and promote superficial wound healing. Encouragingly, CuN4-CNS also showed good therapeutic effects on deep implant-related infections, effectively reducing inflammation.
Considering the characteristics of short lifetimes and short diffusion distance of ROS, building a targeted bacterial system helps to produce toxic ·OH at a short distance, thereby improving the antibacterial efficacy [187]. He et al. modified CuxO with polydopamine (PDA) to construct a material with both photothermal and catalytic properties (CuxO-PDA), where PDA was used as a photothermal agent and a bacterial target binding site (electrostatic interaction) and CuxO was used to mimic POD activity (Figure 7D) [178]. In vitro study showed that the optimal temperature for POD-like activity of CuxO-PDA was 55 °C. Under NIR light irradiation, the elevated temperature helped CuxO-PDA to produce more ·OH. In vitro, CuxO-PDA+H2O2+NIR displayed a stronger bacterial killing effect than CuxO-PDA+H2O2 and CuxO-PDA+NIR, suggesting a potential synergistic antibacterial effect of PTT and POD-like activity of CuxO-PDA under NIR light irradiation.
Low-temperature PTT
Local temperatures above 45 °C can reason necrosis of normal skin cells and induce local inflammation [188]. The copper-containing photothermal materials currently applied to wounds can result in wounds above 50 °C, with the risk of skin burns [189]. Recently, low-temperature PTT has received a lot of attention in wound healing due to its mild thermal stimulation that can be antibacterial, promote angiogenesis, and has good biosafety [190, 191]. However, the antibacterial capacity of low-temperature PTT alone is limited, thus low-temperature PTT combined with PDT, antibacterial drugs, or gas therapy to enhance the antibacterial capacity, are promising strategies for the treatment of infected wounds [192].
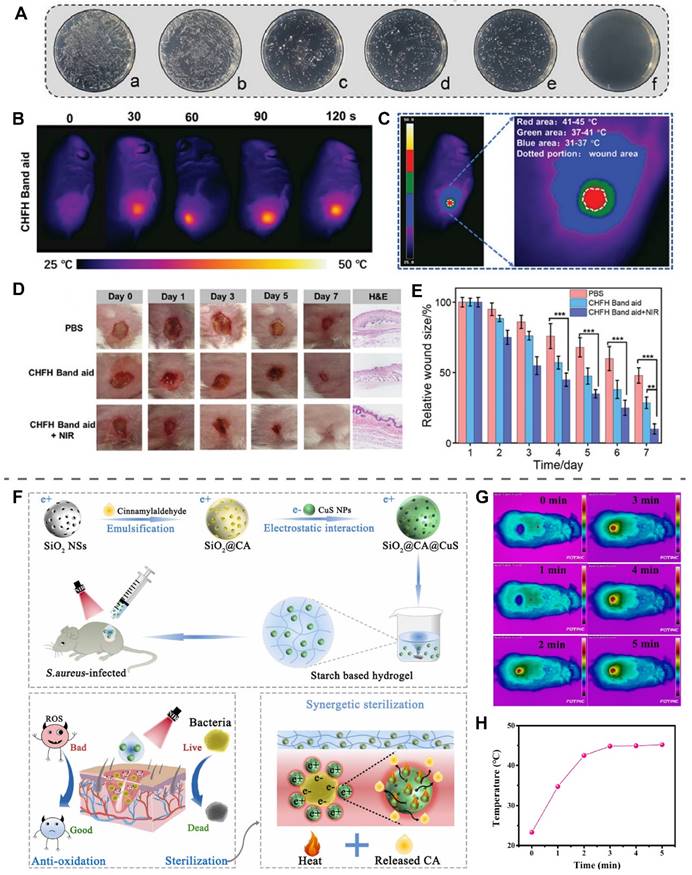
Lin et al. prepared an HA hydrogel loaded with CuS NPs using Fe3+-EDTA complexes as cross-linking agents (CHFH, CuSNPs-HA-Fe3+-EDTA hydrogel) [180]. NIR light irradiation of CHFH exerted a potent antibacterial effect through mild PTT (45 °C) of CuS in synergy with chemodynamic therapy of Fe3+ (Figure 8A). CHFH was prepared as a wound patch placed on the S. aureus-infected wounds, and NIR light (808 nm, 0.5 W/cm2) irradiation for 2 min was able to control the temperature of the wounds at 45 °C (Figure 8B-C). The wounds treated with CHFH+NIR light irradiation were completely healed on the seventh day, faster than the group using CHFH band aid without NIR light irradiation (Figure 8D-E). Further culture of the bacteria from the wounds found that CHFH+NIR showed the strongest antibacterial ability with an antibacterial efficiency of 98%.
Recently, Li et al. used SiO2 as a carrier loaded with cinnamaldehyde (CIN) and CuS NPs to obtain SiO2@CIN@CuS, which had an electrostatic binding effect on bacteria, for the treatment of S. aureus-infected wounds (Figure 8F) [14]. CuS exerted antioxidant effects and promoted the release of CIN by increasing the temperature of SiO2@CIN@CuS through photothermal conversion. Payable to the synergistic effect of CIN and PTT, SiO2@CIN@CuS exhibited more than 99% resistance to S. aureus and E. coli under NIR light irradiation at 43 °C in vitro. In the S. aureus-infected wound, starch hydrogel loaded with SiO2@CIN@CuS was irradiated with NIR light (980 nm, 1.0 W/cm2, 5 min), and the wound temperature reached a peak of 45 °C at 3 min and remained stable to ensure that no additional damage was instigated to the skin (Figure 8G-H). On day 7, Co-application of SiO2@CIN@CuS starch hydrogel and NIR light group exhibited more angiogenesis and faster wound healing than other groups, further demonstrating the effectiveness and usefulness of low-temperature PTT.
Low-Temperature PTT of copper-based photothermal materials. (A) Antibacterial effect of CHFH on S. aureus. (a: PBS, b: NIR, c: HFH, d: CHFH, e: CuSNPs + NIR, f: CHFH + NIR.) (B) Local temperature of the wound at different time points after NIR irradiation. (C) Temperature distribution of the wound after NIR irradiation. (D) Photographs of S. aureus-infected wounds at different time points after treatment. (E) Statistical analysis of relative wound size. Reproduced with permission from [180]. Copyright 2021, Wiley-VCH. (F) Schematic illustration of antibacterial mechanism of SiO2@CIN@CuS. (G) The photothermal effect of SiO2@CIN@CuS in S. aureus infected wounds under NIR irradiation (980 nm, 1 W/cm2, 5 min) (CIN in the text and CA in this figure both represent cinnamaldehyde). (H) The temperature of wounds treated with SiO2@CIN@CuS starch hydrogel after NIR irradiation. Reproduced with permission from [14]. Copyright 2022, Elsevier.
Conclusion and outlook
This review discussed the mechanism of copper involvement in wound healing, the antibacterial mechanism, and the application in wound healing of copper-containing biomaterials. In the last two decades, progress has been made in the antibacterial mechanism of copper and its modulation of inflammation, angiogenesis, collagen deposition, matrix remodeling, and re-epithelialization, laying an important foundation for the use of copper-doped biomaterials in wounds. Although copper-containing biomaterials have shown good promotion of wound healing in animal models, the following issues still need to be addressed before clinical application:
(1) Dosage and controlled release of copper: While high concentrations of copper enhance the antibacterial effect, there is a risk of inhibiting the proliferation of endothelial cells and fibroblasts. Based on existing studies, controlling local Cu2+ in the range of 40-220 μM would allow wounds to benefit from both antibacterial and cell proliferation promotion. Some copper-containing biomaterials use copper as a dopant in bioactive glasses, polymers, and hydrogel scaffolds to accelerate wound healing by releasing copper. Although these materials show great potential in wound healing, it should be noted that a large release of copper from the material in a short period would greatly reduce its biocompatibility. The amount of Cu2+ released from these copper-containing biomaterials is closely related to the amount of copper contained in the material, the type of copper contained, and the structure of bioactive glass, polymer, and hydrogel scaffolds. Reducing the amount of copper in the material, adding insoluble Cu or CuO instead of Cu2+, and adding copper to bioactive glasses that degrade slowly are simple and effective ways to control the release of copper. In addition, given that chronic wounds have microenvironmental characteristics of low pH, high glucose, high ROS and specific enzyme expression, constructing wound microenvironment-responsive copper-containing wound dressings would contribute to on-demand release of Cu2+, improved drug utilization and reduced toxic side effects. It is worth noting that, compared to the wound microenvironment stimuli-responsive drug delivery, photothermal-modulated drug delivery is an active and more controllable approach, which deserves more attention in subsequent studies.
(2) Biosafety: Current studies assessing the biosafety of copper-containing materials are based on organ pathology and blood tests after 2-3 weeks of treatment, and all have concluded that these copper-containing biomaterials have good biocompatibility. However, such assessment methods are inadequate. Before copper-containing biomaterials are introduced into the clinic, their long-term biosafety as well as their absorption, distribution and excretion characteristics in the body should be further explored. In addition, although PTT has been considered very promising in recent years, the cytotoxicity caused by the heat generated by PTT still needs to be considered by researchers. Selecting light with wavelengths located in bio-transparent windows, lowering the excitation power, shortening the irradiation time, and increasing material's photothermal conversion efficiency can help to avoid damage caused by phototherapy. Some studies have explored the conversion of exogenous H2O2 into highly toxic ·OH through Fenton-like reactions or POD-like activities to enhance antibacterial ability. Although these studies have demonstrated excellent antibacterial effects, there is a lack of in vitro research on the cytotoxicity of normal cells after simultaneous addition of copper-containing materials and exogenous H2O2. It is worth noting that copper is known for its efficient generation of H2O2, and this strategy of adding exogenous H2O2 may result in a large amount of ·OH within a short period of time. Therefore, it may be necessary to control and monitor the ·OH generated in these systems. Recently, the discovery of the mechanism of cuproptosis has also sparked discussions about the biosafety concerns associated with copper again. Whether the toxicity caused by excessive copper application in wounds is related to cuproptosis in wound healing-related cells, and whether copper deprivation strategies can be employed to mitigate copper overload in these cells and rescue them from improper copper application, will require further investigation in the future.
(3) Molecular mechanisms: Although copper has been shown to be involved in several aspects of wound healing, however, some specific processes remain unclear, such as how copper promotes wound contraction and how copper regulates macrophage M2 polarization. Furthermore, although some studies have demonstrated the role of copper in the regulation of certain key cellular signals by conventional protein expression analysis methods, for example, copper was shown to up-regulate HIF-1α expression to promote endothelial cell angiogenesis by western blot. However, this is not sufficient. In subsequent research, cutting-edge techniques such as single-cell transcriptomics, proteomics, and metabolomics should be employed to comprehensively uncover the molecular regulatory mechanisms of copper.
(4) Future directions and clinical translation: The efficacy of copper-containing biomaterials on chronic wounds is currently evaluated in mouse and rat models. However, there are differences between rodents and humans. For example, some diabetic wounds show a tendency to heal without intervention, so it is uncertain whether these biomaterials can achieve the same efficacy in humans. To obtain more accurate results, it may be necessary to seek animal models whose physiological and structural characteristics are closer to those of humans. In addition, some studies may adopt complex processes in the pursuit of multifunctionality, which can lead to difficulties for industrial mass production and high costs, as well as difficulties in ensuring consistency of efficacy from batch to batch. Some degradable nanomaterials have not been designed with sufficient consideration of clinical needs, and these nanomaterials are not suitable for prolonged preservation and transportation after production because of their instability, which greatly limits their clinical translation. Another thing that should not be overlooked is that the wound dressing obtained should be simple to use and avoid repeated manipulations, as this will help to minimize secondary injuries and improve patient compliance.
In conclusion, the advances made in wound therapy with copper-containing biomaterials are exciting. For copper-containing biomaterials aiming for clinical translation, more systematic and scientific approaches should be employed to assess efficacy and biocompatibility, while striving towards simplicity in preparation, ease of large-scale production, product stability, and economic feasibility. Only then will these materials be accepted by the market and ultimately benefit patients.
Abbreviations
ROS: reactive oxygen species; NPs: nanoparticles; GSH: glutathione; POD: peroxidase; OXD: oxidase; CAT: catalase; SOD: superoxide dismutase; GPx: glutathione peroxidase; PTT: photothermal therapy; PDT: photodynamic therapy; NIR: near-infrared; VEGF: vascular endothelial growth factor; FGF: fibroblast growth factor; MMP: matrix metalloproteinases; IL: interleukin; TNF: tumor necrosis factor; TIMP: tissue inhibitor of metalloproteinase; LOX: lysyl oxidase; HIF: hypoxia-inducible factor; ANG: angiogenin; MOF: metal-organic framework; NO: nitrogen monoxide; PCL: polycaprolactone; EPS: extracellular polymeric substances; MBG: mesoporous bioactive glass; CA: cellulose acetate; PVA: polyvinyl alcohol; HA: hyaluronic acid; PEG: polyethylene glycol; LCST: lower critical solution temperature; GOD: glucose oxidase; LSPR: localized surface plasmon resonance; PNIPAM: poly(N-isopropylacrylamide); PDA: polydopamine.
Acknowledgements
This work was supported by the Department of Science and Technology of Hubei Province (2020BCB004), the China Postdoctoral Science Foundation (2021M701333), the National Natural Science Foundation of China (82202676, 82272491, 82072444), and the People's Republic of China Ministry of Education, Science, and Technology Development Center (2021JH027). M.-A.S acknowledges Incentive Fund from the University of Groningen. Figure 1 was created by Figdraw (www.figdraw.com).
Author contributions
Zhenhe Zhang, Hang Xue, Yuan Xiong and Yongtao Geng conceived the conceptualization and wrote the manuscript. Adriana C. Panayi, Samuel Knoedler and Guandong Dai collated and produced relevant figures for the manuscript. Mohammad-Ali Shahbazi, Bobin Mi and Gouhui Liu revised the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chang K, Chou Y, Chen W, Chen C, Lin H. Mussel-Inspired Adhesive and Self-Healing Hydrogel as an Injectable Wound Dressing. Polymers (Basel). 2022;14:3346
2. Xiong Y, Mi B, Lin Z, Hu Y, Yu L, Zha K. et al. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity. Mil Med Res. 2022;9:65
3. Pastar I, Balukoff NC, Marjanovic J, Chen VY, Stone RC, Tomic-Canic M. Molecular Pathophysiology of Chronic Wounds: Current State and Future Directions. Cold Spring Harb Perspect Biol. 2023;15:a041243
4. Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haught R, Nusgart M. et al. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health. 2018;21:27-32
5. Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:Cd011255
6. Heidenau F, Mittelmeier W, Detsch R, Haenle M, Stenzel F, Ziegler G. et al. A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization. J Mater Sci Mater Med. 2005;16:883-8
7. Kawakami H, Yoshida K, Nishida Y, Kikuchi Y, Sato Y. Antibacterial Properties of Metallic Elements for Alloying Evaluated with Application of JIS Z 2801:2000. ISIJ Int. 2008;48:1299-304
8. Chandrakala V, Aruna V, Angajala G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Mater. 2022;5:1593-615
9. Alizadeh S, Seyedalipour B, Shafieyan S, Kheime A, Mohammadi P, Aghdami N. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem Biophys Res Commun. 2019;517:684-90
10. Sajjad W, Khan T, Ul-Islam M, Khan R, Hussain Z, Khalid A. et al. Development of modified montmorillonite-bacterial cellulose nanocomposites as a novel substitute for burn skin and tissue regeneration. Carbohydr Polym. 2019;206:548-56
11. Sen CK, Khanna S, Venojarvi M, Trikha P, Ellison EC, Hunt TK. et al. Copper-induced vascular endothelial growth factor expression and wound healing. Am J Physiol Heart Circ Physiol. 2002;282:H1821-7
12. Xi J, Wei G, An L, Xu Z, Xu Z, Fan L. et al. Copper/Carbon Hybrid Nanozyme: Tuning Catalytic Activity by the Copper State for Antibacterial Therapy. Nano Lett. 2019;19:7645-54
13. Zhang P, Li Y, Tang Y, Shen H, Li J, Yi Z. et al. Copper-Based Metal-Organic Framework as a Controllable Nitric Oxide-Releasing Vehicle for Enhanced Diabetic Wound Healing. ACS Appl Mater Interfaces. 2020;12:18319-31
14. Li L, Sun X, Dong M, Zhang H, Wang J, Bu T. et al. NIR-regulated dual-functional silica nanoplatform for infected-wound therapy via synergistic sterilization and anti-oxidation. Colloids Surf B Biointerfaces. 2022;213:112414
15. Wang Z, Hou Z, Wang P, Chen F, Luo X. CuS-PNIPAm nanoparticles with the ability to initiatively capture bacteria for photothermal treatment of infected skin. Regen Biomater. 2022;9:rbac026
16. Zhao S, Li L, Wang H, Zhang Y, Cheng X, Zhou N. et al. Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials. 2015;53:379-91
17. Darder M, Karan A, Real GD, DeCoster MA. Cellulose-based biomaterials integrated with copper-cystine hybrid structures as catalysts for nitric oxide generation. Mater Sci Eng C Mater Biol Appl. 2020;108:110369
18. Wang S, Liu X, Lei M, Sun J, Qu X, Liu C. Continuous and controllable electro-fabrication of antimicrobial copper-alginate dressing for infected wounds treatment. J Mater Sci Mater Med. 2021;32:143
19. Fan C, Xu Q, Hao R, Wang C, Que Y, Chen Y. et al. Multi-functional wound dressings based on silicate bioactive materials. Biomaterials. 2022;287:121652
20. Ali Zahid A, Chakraborty A, Shamiya Y, Ravi SP, Paul A. Leveraging the advancements in functional biomaterials and scaffold fabrication technologies for chronic wound healing applications. Mater Horiz. 2022;9:1850-65
21. Xiong Y, Chen L, Liu P, Yu T, Lin C, Yan C. et al. All-in-One: Multifunctional Hydrogel Accelerates Oxidative Diabetic Wound Healing through Timed-Release of Exosome and Fibroblast Growth Factor. Small. 2022;18:e2104229
22. Ahire JJ, Hattingh M, Neveling DP, Dicks LM. Copper-Containing Anti-Biofilm Nanofiber Scaffolds as a Wound Dressing Material. PLoS One. 2016;11:e0152755
23. Ninan N, Muthiah M, Bt Yahaya NA, Park IK, Elain A, Wong TW. et al. Antibacterial and wound healing analysis of gelatin/zeolite scaffolds. Colloids Surf B Biointerfaces. 2014;115:244-52
24. Gorter RW, Butorac M, Cobian EP. Examination of the cutaneous absorption of copper after the use of copper-containing ointments. Am J Ther. 2004;11:453-8
25. Togo T. Signaling pathways involved in adaptive responses to cell membrane disruption. Curr Top Membr. 2019;84:99-127
26. Lin Z, Liu L, Wang W, Jia L, Shen Y, Zhang X. et al. The role and mechanism of polydopamine and cuttlefish ink melanin carrying copper ion nanoparticles in antibacterial properties and promoting wound healing. Biomater Sci. 2021;9:5951-64
27. Klinkajon W, Supaphol P. Novel copper (II) alginate hydrogels and their potential for use as anti-bacterial wound dressings. Biomed Mater. 2014;9:045008
28. Raffi M, Mehrwan S, Bhatti TM, Akhter JI, Hameed A, Yawar W. et al. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann Microbiol. 2010;60:75-80
29. Huang X, Li T, Zhang X, Deng J, Yin X. Bimetallic palladium@copper nanoparticles: Lethal effect on the gram-negative bacterium Pseudomonas aeruginosa. Mater Sci Eng C Mater Biol Appl. 2021;129:112392
30. Liu Z, Song F, Ma ZL, Xiong Q, Wang J, Guo D. et al. Bivalent Copper Ions Promote Fibrillar Aggregation of KCTD1 and Induce Cytotoxicity. Sci Rep. 2016;6:32658
31. Babushkina IV, Mamontova IA, Gladkova EV. Metal nanoparticles reduce bacterial contamination of experimental purulent wounds. Bull Exp Biol Med. 2015;158:692-4
32. Mizrahi L, Achituv Y. Effect of heavy metals ions on enzyme activity in the Mediterranean mussel, Donax trunculus. Bull Environ Contam Toxicol. 1989;42:854-9
33. Venkataprasanna KS, Prakash J, Vignesh S, Bharath G, Venkatesan M, Banat F. et al. Fabrication of Chitosan/PVA/GO/CuO patch for potential wound healing application. Int J Biol Macromol. 2020;143:744-62
34. Luo F, Fu Z, Ren Y, Wang W, Huang Y, Shu X. Self-assembly CuO-loaded nanocomposite involving functionalized DNA with dihydromyricetin for water-based efficient and controllable antibacterial action. Biomater Adv. 2022;137:212847
35. Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M. et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254-61
36. Mei J, Xu D, Wang L, Kong L, Liu Q, Li Q. et al. Biofilm Microenvironment-Responsive Self-Assembly Nanoreactors for All-Stage Biofilm Associated Infection through Bacterial Cuproptosis-like Death and Macrophage Re-Rousing. Adv Mater. 2023;35:e2303432
37. Kar S, Bagchi B, Kundu B, Bhandary S, Basu R, Nandy P. et al. Synthesis and characterization of Cu/Ag nanoparticle loaded mullite nanocomposite system: A potential candidate for antimicrobial and therapeutic applications. Biochim Biophys Acta. 2014;1840:3264-76
38. Zhang G, Xie W, Xu Z, Si Y, Li Q, Qi X. et al. CuO dot-decorated Cu@Gd2O3 core-shell hierarchical structure for Cu(i) self-supplying chemodynamic therapy in combination with MRI-guided photothermal synergistic therapy. Mater Horiz. 2021;8:1017-28
39. Li X, Liang M, Jiang S, Cao S, Li S, Gao Y. et al. Pomegranate-Like CuO2@SiO2 Nanospheres as H2O2 Self-Supplying and Robust Oxygen Generators for Enhanced Antibacterial Activity. ACS Appl Mater Interfaces. 2021;13:22169-81
40. Zhang R, Jiang G, Gao Q, Wang X, Wang Y, Xu X. et al. Sprayed copper peroxide nanodots for accelerating wound healing in a multidrug-resistant bacteria infected diabetic ulcer. Nanoscale. 2021;13:15937-51
41. Yu M, Zhang G, Li P, Lu H, Tang W, Yang X. et al. Acid-activated ROS generator with folic acid targeting for bacterial biofilm elimination. Mater Sci Eng C Mater Biol Appl. 2021;127:112225
42. Cao W, Jin M, Yang K, Chen B, Xiong M, Li X. et al. Fenton/Fenton-like metal-based nanomaterials combine with oxidase for synergistic tumor therapy. J Nanobiotechnology. 2021;19:325
43. Cao H, Yang Y, Liang M, Ma Y, Sun N, Gao X. et al. Pt@polydopamine nanoparticles as nanozymes for enhanced photodynamic and photothermal therapy. Chem Commun (Camb). 2021;57:255-8
44. Wang H, Wan K, Shi X. Recent Advances in Nanozyme Research. Adv Mater. 2019;31:e1805368
45. Sun X, Dong M, Guo Z, Zhang H, Wang J, Jia P. et al. Multifunctional chitosan-copper-gallic acid based antibacterial nanocomposite wound dressing. Int J Biol Macromol. 2021;167:10-22
46. Klare W, Das T, Ibugo A, Buckle E, Manefield M, Manos J. Glutathione-Disrupted Biofilms of Clinical Pseudomonas aeruginosa Strains Exhibit an Enhanced Antibiotic Effect and a Novel Biofilm Transcriptome. Antimicrob Agents Chemother. 2016;60:4539-51
47. Wang X, Fan L, Cheng L, Sun Y, Wang X, Zhong X. et al. Biodegradable Nickel Disulfide Nanozymes with GSH-Depleting Function for High-Efficiency Photothermal-Catalytic Antibacterial Therapy. iScience. 2020;23:101281
48. Wang G, Ye J, Wang M, Qi Y, Zhang S, Shi L. et al. Copper boron-imidazolate framework incorporated chitosan membranes for bacterial-infected wound healing dressing. Carbohydr Polym. 2022;291:119588
49. Wang X, Shi Q, Zha Z, Zhu D, Zheng L, Shi L. et al. Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Bioact Mater. 2021;6:4389-401
50. Deng H, Yang Y, Zuo T, Fang T, Xu Y, Yang J. et al. Multifunctional ZnO@CuS nanoparticles cluster synergize chemotherapy and photothermal therapy for tumor metastasis. Nanomedicine. 2021;34:102399
51. Hussein EA, Zagho MM, Nasrallah GK, Elzatahry AA. Recent advances in functional nanostructures as cancer photothermal therapy. Int J Nanomedicine. 2018;13:2897-906
52. Zhi D, Yang T, O'Hagan J, Zhang S, Donnelly RF. Photothermal therapy. J Control Release. 2020;325:52-71
53. Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kedzierska E, Knap-Czop K. et al. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098-107
54. Qiao Y, Ping Y, Zhang H, Zhou B, Liu F, Yu Y. et al. Laser-Activatable CuS Nanodots to Treat Multidrug-Resistant Bacteria and Release Copper Ion to Accelerate Healing of Infected Chronic Nonhealing Wounds. ACS Appl Mater Interfaces. 2019;11:3809-22
55. Dai X, Zhao Y, Yu Y, Chen X, Wei X, Zhang X. et al. All-in-one NIR-activated nanoplatforms for enhanced bacterial biofilm eradication. Nanoscale. 2018;10:18520-30
56. Xu Q, Chang M, Zhang Y, Wang E, Xing M, Gao L. et al. PDA/Cu Bioactive Hydrogel with "Hot Ions Effect" for Inhibition of Drug-Resistant Bacteria and Enhancement of Infectious Skin Wound Healing. ACS Appl Mater Interfaces. 2020;12:31255-69
57. Tolner B, Poolman B, Konings WN. Adaptation of microorganisms and their transport systems to high temperatures. Comp Biochem Physiol A Physiol. 1997;118:423-8
58. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev. 2019;99:665-706
59. Han G, Ceilley R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv Ther. 2017;34:599-610
60. Yang F, Bai X, Dai X, Li Y. The biological processes during wound healing. Regen Med. 2021;16:373-90
61. Yamamoto N, Oyaizu T, Enomoto M, Horie M, Yuasa M, Okawa A. et al. VEGF and bFGF induction by nitric oxide is associated with hyperbaric oxygen-induced angiogenesis and muscle regeneration. Sci Rep. 2020;10:2744
62. Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015;44-46:113-21
63. Li W, Wang Y, Qi Y, Zhong D, Xie T, Yao K. et al. Cupriferous Silver Peroxysulfite Superpyramids as a Universal and Long-Lasting Agent to Eradicate Multidrug-Resistant Bacteria and Promote Wound Healing. ACS Appl Bio Mater. 2021;4:3729-38
64. Kim HS, Sun X, Lee JH, Kim HW, Fu X, Leong KW. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019;146:209-39
65. Chin JS, Madden L, Chew SY, Becker DL. Drug therapies and delivery mechanisms to treat perturbed skin wound healing. Adv Drug Deliv Rev. 2019;149-150:2-18
66. Lin LS, Huang T, Song J, Ou XY, Wang Z, Deng H. et al. Synthesis of Copper Peroxide Nanodots for H2O2 Self-Supplying Chemodynamic Therapy. J Am Chem Soc. 2019;141:9937-45
67. Liu T, Xiao B, Xiang F, Tan J, Chen Z, Zhang X. et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat Commun. 2020;11:2788
68. Yang J, Huang Z, Tan J, Pan J, Chen S, Wan W. Copper ion/gallic acid MOFs-laden adhesive pomelo peel sponge effectively treats biofilm-infected skin wounds and improves healing quality. Bioact Mater. 2024;32:260-76
69. Wang L, Ren L, Tang T, Dai K, Yang K, Hao Y. A novel nano-copper-bearing stainless steel with reduced Cu2+ release only inducing transient foreign body reaction via affecting the activity of NF-κB and Caspase 3. Int J Nanomedicine. 2015;10:6725-39
70. Hoene A, Prinz C, Walschus U, Lucke S, Patrzyk M, Wilhelm L. et al. In vivo evaluation of copper release and acute local tissue reactions after implantation of copper-coated titanium implants in rats. Biomed Mater. 2013;8:035009
71. Hoene A, Lucke S, Walschus U, Hackbarth C, Prinz C, Evert FK. et al. Effects of copper-impregnated collagen implants on local pro- and anti-inflammatory and regenerative tissue reactions following implantation in rats. J Biomed Mater Res A. 2020;108:871-81
72. Xiao T, Liu J, Li Y, Cai Y, Xing X, Shao M. et al. Microenvironment-responsive Cu-phenolic networks coated nanofibrous dressing with timely macrophage phenotype transition for chronic MRSA infected wound healing. Materials Today Bio. 2023;22:100788
73. Xu X, Lu Y, Li S, Guo S, He M, Luo K. et al. Copper-modified Ti6Al4V alloy fabricated by selective laser melting with pro-angiogenic and anti-inflammatory properties for potential guided bone regeneration applications. Mater Sci Eng C Mater Biol Appl. 2018;90:198-210
74. Jian G, Li D, Ying Q, Chen X, Zhai Q, Wang S. et al. Dual Photo-Enhanced Interpenetrating Network Hydrogel with Biophysical and Biochemical Signals for Infected Bone Defect Healing. Adv Healthc Mater. 2023;12:e2300469
75. Díez-Tercero L, Delgado LM, Bosch-Rué E, Perez RA. Evaluation of the immunomodulatory effects of cobalt, copper and magnesium ions in a pro inflammatory environment. Sci Rep. 2021;11:11707
76. Suska F, Gretzer C, Esposito M, Emanuelsson L, Wennerberg A, Tengvall P. et al. In vivo cytokine secretion and NF-kappaB activation around titanium and copper implants. Biomaterials. 2005;26:519-27
77. Blache U, Ehrbar M. Inspired by Nature: Hydrogels as Versatile Tools for Vascular Engineering. Adv Wound Care (New Rochelle). 2018;7:232-46
78. Barralet J, Gbureck U, Habibovic P, Vorndran E, Gerard C, Doillon CJ. Angiogenesis in calcium phosphate scaffolds by inorganic copper ion release. Tissue Eng Part A. 2009;15:1601-9
79. Das A, Sudhahar V, Chen GF, Kim HW, Youn SW, Finney L. et al. Endothelial Antioxidant-1: a Key Mediator of Copper-dependent Wound Healing in vivo. Sci Rep. 2016;6:33783
80. Pan Q, Kleer CG, van Golen KL, Irani J, Bottema KM, Bias C. et al. Copper Deficiency Induced by Tetrathiomolybdate Suppresses Tumor Growth and Angiogenesis1. Cancer Res. 2002;62:4854-9
81. Borkow G, Gabbay J, Dardik R, Eidelman AI, Lavie Y, Grunfeld Y. et al. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. Wound Repair Regen. 2010;18:266-75
82. Jiang Y, Reynolds C, Xiao C, Feng W, Zhou Z, Rodriguez W. et al. Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. J Exp Med. 2007;204:657-66
83. Zheng L, Han P, Liu J, Li R, Yin W, Wang T. et al. Role of copper in regression of cardiac hypertrophy. Pharmacol Ther. 2015;148:66-84
84. Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M. et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575-83
85. Kargozar S, Mozafari M, Ghodrat S, Fiume E, Baino F. Copper-containing bioactive glasses and glass-ceramics: From tissue regeneration to cancer therapeutic strategies. Mater Sci Eng C Mater Biol Appl. 2021;121:111741
86. Lombardi AA, Gibb AA, Arif E, Kolmetzky DW, Tomar D, Luongo TS. et al. Mitochondrial calcium exchange links metabolism with the epigenome to control cellular differentiation. Nat Commun. 2019;10:4509
87. Ninan N, Muthiah M, Park IK, Elain A, Wong TW, Thomas S. et al. In Vitro and In Vivo Evaluation of Pectin/Copper Exchanged Faujasite Composite Membranes. J Biomed Nanotechnol. 2015;11:1550-67
88. Li R, Liu K, Huang X, Li D, Ding J, Liu B. et al. Bioactive Materials Promote Wound Healing through Modulation of Cell Behaviors. Adv Sci (Weinh). 2022;9:e2105152
89. Sankar R, Baskaran A, Shivashangari KS, Ravikumar V. Inhibition of pathogenic bacterial growth on excision wound by green synthesized copper oxide nanoparticles leads to accelerated wound healing activity in Wistar Albino rats. J Mater Sci Mater Med. 2015;26:214
90. Talagas M, Lebonvallet N, Berthod F, Misery L. Lifting the veil on the keratinocyte contribution to cutaneous nociception. Protein Cell. 2020;11:239-50
91. Raja Sivamani K, Garcia MS Isseroff RR. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci. 2007;12:2849-68
92. Tenaud I, Leroy S, Chebassier N, Dreno B. Zinc, copper and manganese enhanced keratinocyte migration through a functional modulation of keratinocyte integrins. Exp Dermatol. 2000;9:407-16
93. Xiao J, Chen S, Yi J, Zhang H, Ameer GA. A Cooperative Copper Metal-Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv Funct Mater. 2017;27:1604872
94. Ogen-Shtern N, Chumin K, Silberstein E, Borkow G. Copper Ions Ameliorated Thermal Burn-Induced Damage in ex vivo Human Skin Organ Culture. Skin Pharmacol Physiol. 2021;34:317-27
95. Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10:200223
96. Philips N, Hwang H, Chauhan S, Leonardi D, Gonzalez S. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect Tissue Res. 2010;51:224-9
97. Siméon A, Emonard H, Hornebeck W, Maquart FX. The tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ stimulates matrix metalloproteinase-2 expression by fibroblast cultures. Life Sci. 2000;67:2257-65
98. Fujiwara M, Muragaki Y, Ooshima A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol. 2005;153:295-300
99. Huang P, Huang Y, Su M, Yang T, Huang J, Jiang C. In vitro observations on the influence of copper peptide aids for the LED photoirradiation of fibroblast collagen synthesis. Photomed Laser Surg. 2007;25:183-90
100. Siméon A, Wegrowski Y, Bontemps Y, Maquart FX. Expression of glycosaminoglycans and small proteoglycans in wounds: modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. J Invest Dermatol. 2000;115:962-8
101. Gopal A, Kant V, Gopalakrishnan A, Tandan SK, Kumar D. Chitosan-based copper nanocomposite accelerates healing in excision wound model in rats. Eur J Pharmacol. 2014;731:8-19
102. Caley MP, Martins VL, O'Toole EA. Metalloproteinases and Wound Healing. Adv Wound Care (New Rochelle). 2015;4:225-34
103. Siméon A, Monier F, Emonard H, Gillery P, Birembaut P, Hornebeck W. et al. Expression and activation of matrix metalloproteinases in wounds: modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. J Invest Dermatol. 1999;112:957-64
104. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562-73
105. Fogelgren B, Polgár N, Szauter KM, Ujfaludi Z, Laczkó R, Fong KS. et al. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J Biol Chem. 2005;280:24690-7
106. Bhadauriya P, Mamtani H, Ashfaq M, Raghav A, Teotia AK, Kumar A. et al. Synthesis of Yeast-Immobilized and Copper Nanoparticle-Dispersed Carbon Nanofiber-Based Diabetic Wound Dressing Material: Simultaneous Control of Glucose and Bacterial Infections. ACS Appl Bio Mater. 2018;1:246-58
107. Wang W, Lu K, Yu C, Huang Q, Du Y. Nano-drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnology. 2019;17:82
108. Sun X, Li L, Zhang H, Dong M, Wang J, Jia P. et al. Near-Infrared Light-Regulated Drug-Food Homologous Bioactive Molecules and Photothermal Collaborative Precise Antibacterial Therapy Nanoplatform with Controlled Release Property. Adv Healthc Mater. 2021;10:e2100546
109. Lin K, Adhikari AK, Ku C, Chiang C, Kuo H. Synthesis and characterization of porous HKUST-1 metal organic frameworks for hydrogen storage. Int J Hydrogen Energy. 2012;37:13865-71
110. Brandi C, D'Aniello C, Grimaldi L, Bosi B, Dei I, Lattarulo P. et al. Carbon dioxide therapy in the treatment of localized adiposities: clinical study and histopathological correlations. Aesthetic Plast Surg. 2001;25:170-4
111. Li W, Su C, Wang S, Tsai F, Chang C, Liao M. et al. CO2 Delivery To Accelerate Incisional Wound Healing Following Single Irradiation of Near-Infrared Lamp on the Coordinated Colloids. ACS Nano. 2017;11:5826-35
112. Jia C, Guo Y, Wu FG. Chemodynamic Therapy via Fenton and Fenton-Like Nanomaterials: Strategies and Recent Advances. Small. 2022;18:e2103868
113. Li M, Lan X, Han X, Shi S, Sun H, Kang Y. et al. Acid-Induced Self-Catalyzing Platform Based on Dextran-Coated Copper Peroxide Nanoaggregates for Biofilm Treatment. ACS Appl Mater Interfaces. 2021;13:29269-80
114. Ciofu O, Moser C, Jensen P, Høiby N. Tolerance and resistance of microbial biofilms. Nat Rev Microbiol. 2022 20; 621-635
115. Zandieh M, Liu J. Nanozyme Catalytic Turnover and Self-Limited Reactions. ACS Nano. 2021;15:15645-55
116. Cheng H, Zhang L, He J, Guo W, Zhou Z, Zhang X. et al. Integrated Nanozymes with Nanoscale Proximity for in Vivo Neurochemical Monitoring in Living Brains. Anal Chem. 2016;88:5489-97
117. Feng L, Dou C, Xia Y, Li B, Zhao M, Yu P. et al. Neutrophil-like Cell-Membrane-Coated Nanozyme Therapy for Ischemic Brain Damage and Long-Term Neurological Functional Recovery. ACS Nano. 2021;15:2263-80
118. Li D, Guo Q, Ding L, Zhang W, Cheng L, Wang Y. et al. Bimetallic CuCo2S4 Nanozymes with Enhanced Peroxidase Activity at Neutral pH for Combating Burn Infections. ChemBioChem. 2020;21:2620-7
119. Liu Y, Nie N, Tang H, Zhang C, Chen K, Wang W. et al. Effective Antibacterial Activity of Degradable Copper-Doped Phosphate-Based Glass Nanozymes. ACS Appl Mater Interfaces. 2021;13:11631-45
120. Deng C, Dong D, Wang T, Hu M, Sun L, Zhang X. et al. Promotion of diabetic wound healing using novel Cu2O/Pt nanocubes through bacterial killing and enhanced angiogenesis in rats. Biomater Adv. 2021;134:112552
121. Liu M, Huang L, Xu X, Wei X, Yang X, Li X. et al. Copper Doped Carbon Dots for Addressing Bacterial Biofilm Formation, Wound Infection, and Tooth Staining. ACS Nano. 2022;16:9479-9497
122. Liao X, Xu Q, Sun H, Liu W, Chen Y, Xia XH. et al. Plasmonic Nanozymes: Localized Surface Plasmonic Resonance Regulates Reaction Kinetics and Antibacterial Performance. J Phys Chem Lett. 2022;13:312-23
123. Deng L, Du C, Song P, Chen T, Rui S, Armstrong DG. et al. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid Med Cell Longev. 2021;2021:8852759
124. Howard MA, Asmis R, Evans KK, Mustoe TA. Oxygen and wound care: a review of current therapeutic modalities and future direction. Wound Repair Regen. 2013;21:503-11
125. Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163:257-68
126. Hopf HW, Gibson JJ, Angeles AP, Constant JS, Feng JJ, Rollins MD. et al. Hyperoxia and angiogenesis. Wound Repair Regen. 2005;13:558-64
127. Hench LL, Splinter RJ, Allen WC, Greenlee TK. Bonding mechanisms at the interface of ceramic prosthetic materials. 1971; 5: 117-41.
128. Xie W, Fu X, Tang F, Mo Y, Cheng J, Wang H. et al. Dose-dependent modulation effects of bioactive glass particles on macrophages and diabetic wound healing. J Mater Chem B. 2019;7:940-52
129. Tang F, Li J, Xie W, Mo Y, Ouyang L, Zhao F. et al. Bioactive glass promotes the barrier functional behaviors of keratinocytes and improves the Re-epithelialization in wound healing in diabetic rats. Bioact Mater. 2021;6:3496-506
130. Kaya S, Cresswell M, Boccaccini AR. Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater Sci Eng C Mater Biol Appl. 2018;83:99-107
131. Zheng K, Dai X, Lu M, Hüser N, Taccardi N, Boccaccini AR. Synthesis of copper-containing bioactive glass nanoparticles using a modified Stöber method for biomedical applications. Colloids Surf B Biointerfaces. 2017;150:159-67
132. Lin Y, Brown RF, Jung SB, Day DE. Angiogenic effects of borate glass microfibers in a rodent model. J Biomed Mater Res A. 2014;102:4491-9
133. Li J, Zhai D, Lv F, Yu Q, Ma H, Yin J. et al. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016;36:254-66
134. Migneco C, Fiume E, Verné E, Baino F. A Guided Walk through the World of Mesoporous Bioactive Glasses (MBGs): Fundamentals, Processing, and Applications. Nanomaterials (Basel). 2020;10:2571
135. Yan X, Yu C, Zhou X, Tang J, Zhao D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew Chem Int Ed Engl. 2004;43:5980-4
136. Wang X, Cheng F, Liu J, Smått JH, Gepperth D, Lastusaari M. et al. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: Biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016;46:286-98
137. Vallet-Regi M, Salinas AJ. Mesoporous bioactive glasses for regenerative medicine. Materials Today Bio. 2021;11:100121
138. Mogosanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463:127-36
139. Talikowska M, Fu X, Lisak G. Application of conducting polymers to wound care and skin tissue engineering: A review. Biosens Bioelectron. 2019;135:50-63
140. Liu L, Sun H, Zhang J, Xu B, Gao Y, Qi D. et al. Trilayered Fibrous Dressing with Wettability Gradient for Spontaneous and Directional Transport of Massive Exudate and Wound Healing Promotion. Adv Fiber Mater. 2023;5:574-87
141. Schuhladen K, Mukoo P, Liverani L, Neščáková Z, Boccaccini AR. Manuka honey and bioactive glass impart methylcellulose foams with antibacterial effects for wound-healing applications. Biomed Mater. 2020;15:065002
142. Al-Saeedi SI, Al-Kadhi NS, Al-Senani GM, Almaghrabi OA, Nafady A. Antibacterial potency, cell viability and morphological implications of copper oxide nanoparticles encapsulated into cellulose acetate nanofibrous scaffolds. Int J Biol Macromol. 2021;182:464-71
143. Jaganathan SK, Mani MP, Khudzari AZM. Electrospun Combination of Peppermint Oil and Copper Sulphate with Conducive Physico-Chemical properties for Wound Dressing Applications. Polymers (Basel). 2019;11:586
144. Sarwar MN, Ali HG, Ullah S, Yamashita K, Shahbaz A, Nisar U. et al. Electrospun PVA/CuONPs/Bitter Gourd Nanofibers with Improved Cytocompatibility and Antibacterial Properties: Application as Antibacterial Wound Dressing. Polymers (Basel). 2022;14:1361
145. Zhang S, Ge G, Qin Y, Li W, Dong J, Mei J. et al. Recent advances in responsive hydrogels for diabetic wound healing. Materials Today Bio. 2023;18:100508
146. Ren X, Yang C, Zhang L, Li S, Shi S, Wang R. et al. Copper metal-organic frameworks loaded on chitosan film for the efficient inhibition of bacteria and local infection therapy. Nanoscale. 2019;11:11830-8
147. Madzovska-Malagurski I, Vukasinovic-Sekulic M, Kostic D, Levic S. Towards antimicrobial yet bioactive Cu-alginate hydrogels. Biomed Mater. 2016;11:035015
148. Dimatteo R, Darling NJ, Segura T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv Drug Deliv Rev. 2018;127:167-84
149. Abdollahi Z, Zare EN, Salimi F, Goudarzi I, Tay FR, Makvandi P. Bioactive Carboxymethyl Starch-Based Hydrogels Decorated with CuO Nanoparticles: Antioxidant and Antimicrobial Properties and Accelerated Wound Healing In Vivo. Int J Mol Sci. 2021;22:2351
150. Liu X, Zhou S, Cai B, Wang Y, Deng D, Wang X. An injectable and self-healing hydrogel with antibacterial and angiogenic properties for diabetic wound healing. Biomater Sci. 2022;10:3480-3492
151. Chen J, He J, Yang Y, Qiao L, Hu J, Zhang J. et al. Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing. Acta Biomater. 2022;146:119-30
152. Barbucci R, Leone G, Magnani A, Montanaro L, Arciola CR, Peluso G. et al. Cu2+- and Ag+-complexes with a hyaluronane-based hydrogel. J Mater Chem. 2002;12:3084-92
153. A VT, Dinda AK, Koul V. Evaluation of nano hydrogel composite based on gelatin/HA/CS suffused with Asiatic acid/ZnO and CuO nanoparticles for second degree burns. Mater Sci Eng C Mater Biol Appl. 2018;89:378-86
154. Li M, Liu X, Tan L, Cui Z, Yang X, Li Z. et al. Noninvasive rapid bacteria-killing and acceleration of wound healing through photothermal/photodynamic/copper ion synergistic action of a hybrid hydrogel. Biomater Sci. 2018;6:2110-21
155. Qian J, Ji L, Xu W, Hou G, Wang J, Wang Y. et al. Copper-Hydrazide Coordinated Multifunctional Hyaluronan Hydrogels for Infected Wound Healing. ACS Appl Mater Interfaces. 2022;14:16018-31
156. Li Q, Song H, Li S, Hu P, Zhang C, Zhang J. et al. Macrophage metabolism reprogramming EGCG-Cu coordination capsules delivered in polyzwitterionic hydrogel for burn wound healing and regeneration. Bioact Mater. 2023;29:251-64
157. Peng Y, He D, Ge X, Lu Y, Chai Y, Zhang Y. et al. Construction of heparin-based hydrogel incorporated with Cu5.4O ultrasmall nanozymes for wound healing and inflammation inhibition. Bioact Mater. 2021;6:3109-24
158. Yang Y, Dong Z, Li M, Liu L, Luo H, Wang P. et al. Graphene Oxide/Copper Nanoderivatives-Modified Chitosan/Hyaluronic Acid Dressings for Facilitating Wound Healing in Infected Full-Thickness Skin Defects. Int J Nanomedicine. 2020;15:8231-47
159. Li S, Wang X, Chen J, Guo J, Yuan M, Wan G. et al. Calcium ion cross-linked sodium alginate hydrogels containing deferoxamine and copper nanoparticles for diabetic wound healing. Int J Biol Macromol. 2022;202:657-70
160. Zhang M, Hu W, Cai C, Wu Y, Li J, Dong S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Materials Today Bio. 2022;14:100223
161. Xu F, Cha Q, Zhang Y, Chen X. Degradation and Utilization of Alginate by Marine Pseudoalteromonas: a Review. Appl Environ Microbiol. 2021;87:e0036821
162. Severino P, da Silva CF, Andrade LN, de Lima Oliveira D, Campos J, Souto EB. Alginate Nanoparticles for Drug Delivery and Targeting. Curr Pharm Des. 2019;25:1312-34
163. Shahriari-Khalaji M, Hong S, Hu G, Ji Y, Hong FF. Bacterial Nanocellulose-Enhanced Alginate Double-Network Hydrogels Cross-Linked with Six Metal Cations for Antibacterial Wound Dressing. Polymers (Basel). 2020;12:2683
164. Wong TY, Preston LA, Schiller NL. Alginate Lyase: Review of Major Sources and Enzyme Characteristics, Structure-Function Analysis, Biological Roles, and Applications. Annu Rev Microbiol. 2000;54:289-340
165. Chen G, Yu Y, Wu X, Wang G, Gu G, Wang F. et al. Microfluidic Electrospray Niacin Metal-Organic Frameworks Encapsulated Microcapsules for Wound Healing. Research (Wash D C). 2019;2019:6175398
166. Suvorov NB, Belov AV, Kuliabin KG, Anisimov AA, Sergeev TV, Markelov OA. High Precision Human Skin Temperature Fluctuations Measuring Instrument. Sensors (Basel). 2021;21:4101
167. Wang Y, Chu X, Sun Y, Teng P, Xia T, Chen Y. A convenient approach by using poly-(HEMA-co-NIPAM)/Cu2+ solution sol-gel transition for wound protection and healing. J Biomed Mater Res B Appl Biomater. 2021;109:50-9
168. Tao B, Lin C, Deng Y, Yuan Z, Shen X, Chen M. et al. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J Mater Chem B. 2019;7:2534-48
169. Wang L, Chen G, Fan L, Chen H, Zhao Y, Lu L. et al. Biomimetic Enzyme Cascade Structural Color Hydrogel Microparticles for Diabetic Wound Healing Management. Adv Sci (Weinh). 2023;10:e2206900
170. Griffiths HR, Gao D, Pararasa C. Redox regulation in metabolic programming and inflammation. Redox Biol. 2017;12:50-7
171. Zhao Y, Chen B, Kankala RK, Wang S, Chen A. Recent Advances in Combination of Copper Chalcogenide-Based Photothermal and Reactive Oxygen Species-Related Therapies. ACS Biomater Sci Eng. 2020;6:4799-815
172. Cao J, Zhu W, Shen AG, Hu JM. Rational synthesis of Three-Layered plasmonic nanocomposites of copper Sulfide/Gold/Zinc-Doped Prussian blue analogues for improved photothermal disinfection and wound healing. J Colloid Interface Sci. 2022;610:621-33
173. Yao S, Wang Y, Chi J, Yu Y, Zhao Y, Luo Y. et al. Porous MOF Microneedle Array Patch with Photothermal Responsive Nitric Oxide Delivery for Wound Healing. Adv Sci (Weinh). 2022;9:e2103449
174. Dai X, Zhao Y, Yu Y, Chen X, Wei X, Zhang X. et al. Single Continuous Near-Infrared Laser-Triggered Photodynamic and Photothermal Ablation of Antibiotic-Resistant Bacteria Using Effective Targeted Copper Sulfide Nanoclusters. ACS Appl Mater Interfaces. 2017;9:30470-9
175. Peng Z, Zhang X, Yuan L, Li T, Chen Y, Tian H. et al. Integrated endotoxin-adsorption and antibacterial properties of platelet-membrane-coated copper silicate hollow microspheres for wound healing. J Nanobiotechnology. 2021;19:383
176. Han Q, Wang X, Qiu L, Zhou X, Hui Z, Ni X. et al. Gelatinase Responsive Nanogel for Antibacterial Phototherapy and Wound Healing. Gels. 2022;8:397
177. Qiao Y, He J, Chen W, Yu Y, Li W, Du Z. et al. Light-Activatable Synergistic Therapy of Drug-Resistant Bacteria-Infected Cutaneous Chronic Wounds and Nonhealing Keratitis by Cupriferous Hollow Nanoshells. ACS Nano. 2020;14:3299-315
178. He S, Feng Y, Sun Q, Xu Z, Zhang W. Charge-Switchable CuxO Nanozyme with Peroxidase and Near-Infrared Light Enhanced Photothermal Activity for Wound Antibacterial Application. ACS Appl Mater Interfaces. 2022;14:25042-9
179. Guo Z, Liu Y, Zhang Y, Sun X, Li F, Bu T. et al. A bifunctional nanoplatform based on copper manganate nanoflakes for bacterial elimination via a catalytic and photothermal synergistic effect. Biomater Sci. 2020;8:4266-74
180. Lin X, Fang Y, Hao Z, Wu H, Zhao M, Wang S. et al. Bacteria-Triggered Multifunctional Hydrogel for Localized Chemodynamic and Low-Temperature Photothermal Sterilization. Small. 2021;17:e2103303
181. Yang Y, Wang C, Wang N, Li J, Zhu Y, Zai J. et al. Photogenerated reactive oxygen species and hyperthermia by Cu3SnS4 nanoflakes for advanced photocatalytic and photothermal antibacterial therapy. J Nanobiotechnology. 2022;20:195
182. Wang X, Lv F, Li T, Han Y, Yi Z, Liu M. et al. Electrospun Micropatterned Nanocomposites Incorporated with Cu2S Nanoflowers for Skin Tumor Therapy and Wound Healing. ACS Nano. 2017;11:11337-49
183. Nain A, Huang HH, Chevrier DM, Tseng YT, Sangili A, Lin YF. et al. Catalytic and photoresponsive BiZ/CuxS heterojunctions with surface vacancies for the treatment of multidrug-resistant clinical biofilm-associated infections. Nanoscale. 2021;13:18632-46
184. Zhu X, Feng W, Chang J, Tan YW, Li J, Chen M. et al. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat Commun. 2016;7:10437
185. Nain A, Wei SC, Lin YF, Tseng YT, Mandal RP, Huang YF. et al. Copper Sulfide Nanoassemblies for Catalytic and Photoresponsive Eradication of Bacteria from Infected Wounds. ACS Appl Mater Interfaces. 2021;13:7865-78
186. Bai J, Feng Y, Li W, Cheng Z, Rosenholm JM, Yang H. et al. Alternative Copper-Based Single-Atom Nanozyme with Superior Multienzyme Activities and NIR-II Responsiveness to Fight against Deep Tissue Infections. Research (Wash D C). 2023;6:0031
187. Jin L, Cao F, Gao Y, Zhang C, Qian Z, Zhang J. et al. Microenvironment-Activated Nanozyme-Armed Bacteriophages Efficiently Combat Bacterial Infection. Adv Mater. 2023;35:e2301349
188. You C, Li Y, Dong Y, Ning L, Zhang Y, Yao L. et al. Low-Temperature Trigger Nitric Oxide Nanogenerators for Enhanced Mild Photothermal Therapy. ACS Biomater Sci Eng. 2020;6:1535-42
189. Zhou W, Zi L, Cen Y, You C, Tian M. Copper Sulfide Nanoparticles-Incorporated Hyaluronic Acid Injectable Hydrogel With Enhanced Angiogenesis to Promote Wound Healing. Front Bioeng Biotechnol. 2020;8:417
190. Sheng L, Zhang Z, Zhang Y, Wang E, Ma B, Xu Q. et al. A novel “hot spring”-mimetic hydrogel with excellent angiogenic properties for chronic wound healing. Biomaterials. 2021;264:120414
191. Xu X, Liu X, Tan L, Cui Z, Yang X, Zhu S. et al. Controlled-temperature photothermal and oxidative bacteria killing and acceleration of wound healing by polydopamine-assisted Au-hydroxyapatite nanorods. Acta Biomater. 2018;77:352-64
192. Yi X, Duan QY, Wu FG. Low-Temperature Photothermal Therapy: Strategies and Applications. Research (Wash D C). 2021;2021:9816594
Author contact
![]() Corresponding authors: Gouhui Liu; liuguohuiedu.cn. Bobin Mi; mibobinedu.cn. Mohammad-Ali Shahbazi; m.a.shahbazinl.
Corresponding authors: Gouhui Liu; liuguohuiedu.cn. Bobin Mi; mibobinedu.cn. Mohammad-Ali Shahbazi; m.a.shahbazinl.
 Global reach, higher impact
Global reach, higher impact