13.3
Impact Factor
Theranostics 2024; 14(2):528-546. doi:10.7150/thno.87739 This issue Cite
Research Paper
Targeted modulation of intestinal epithelial regeneration and immune response in ulcerative colitis using dual-targeting bilirubin nanoparticles
1. Department of Gastroenterology, Guangdong Provincial People's Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou 510080, China.
2. The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China.
3. School of Medicine, South China University of Technology, Guangzhou 510006, China.
4. Department of Critical Care Medicine, The Fifth Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China.
5. Department of Gastroenterology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, 510630, China.
6. Shantou University Medical College, Shantou 515041, China.
7. Faculty of Synthetic Biology, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China.
8. Cord Blood Bank, Guangzhou Institute of Eugenics and Perinatology, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, China.
9. State Key Laboratory of Pharmaceutical Biotechnology, The University of Hong Kong, SAR, China.
10. Department of Pharmacology, School of Medicine, Southern University of Science and Technology, Shenzhen, Guangdong, 518055, China.
11. Botnar Research Centre, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford Old Road, B4495, Headington, Oxford OX3 7LD, UK.
†: These authors contributed equally to this work
Abstract
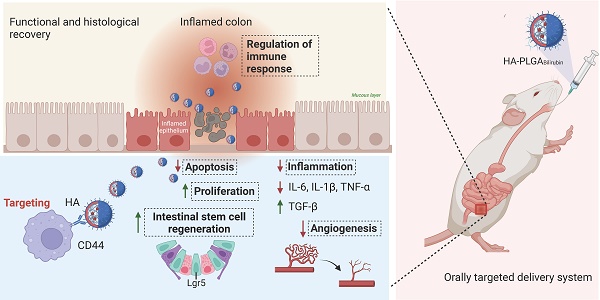
Rationale: The therapeutic benefits of bilirubin in the treatment of ulcerative colitis (UC) are considerable, whereas the underlying mechanism of bilirubin on UC remains unclear remains unexplored. In addition, the weak hydrophilicity and toxicity have limited its translational applications.
Methods: We have developed a colon dual-targeting nanoparticle, for orally delivering bilirubin through hydrogel encapsulation of hyaluronic acid (HA)-modified poly (lactic-co-glycolic acid) (PLGA) nanoparticles (HA-PLGABilirubin). Confocal microscopy and in vivo imaging were used to evaluate the uptake and the targeted property of HA-PLGABilirubin in UC. Immunohistochemistry, immunofluorescence, and transcriptomic analyses were applied to examine the therapeutic effect and potential mechanism of HA-PLGABilirubin in UC.
Results: Our results indicated that HA-PLGAbilirubin can significantly enhance the release of bilirubin at simulated intestinal pH and demonstrate higher cellular uptake in inflammatory macrophages. Moreover, in vivo biodistribution studies revealed high uptake and retention of HA-PLGAbilirubin in inflamed colon tissue of UC mouse model, resulting in effective recovery of intestinal morphology and barrier function. Importantly, HA-PLGAbilirubin exerted potent therapeutic efficacy against ulcerative colitis through modulating the intestinal epithelial/stem cells regeneration, and the improvement of angiogenesis and inflammation. Furthermore, genome-wide RNA-seq analysis revealed transcriptional reprogramming of immune response genes in colon tissue upon HA-PLGAbilirubin treatment in UC mouse model.
Conclusion: Overall, our work provides an efficient colon targeted drug delivery system to potentiate the treatment of ulcerative colitis via modulating intestinal epithelium regeneration and immune response in ulcerative colitis.
Keywords: ulcerative colitis, bilirubin, targeted therapy, intestinal stem cells, immune response
 Global reach, higher impact
Global reach, higher impact