13.3
Impact Factor
Theranostics 2023; 13(11):3568-3581. doi:10.7150/thno.82535 This issue Cite
Research Paper
The HDAC10 instructs macrophage M2 program via deacetylation of STAT3 and promotes allergic airway inflammation
1. Institute of Respiratory Diseases, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, China.
2. Department of Respiratory and Critical Care Medicine, The First Dongguan Affiliated Hospital, Guangdong Medical University, Dongguan 523121, China.
3. The Marine Biomedical Research Institute, Guangdong Medical University; The Marine Biomedical Research Institute of Guangdong Zhanjiang, China.
4. The Intensive Care Unit, The First Dongguan Affiliated Hospital, Guangdong Medical University, Dongguan 523121, China.
5. Department of Anesthesiology, Affiliated Hospital of Guangdong Medical University, Zhanjiang 524001, China.
*These authors contributed equally to this work.
Abstract
Background: Perturbation of macrophage homeostasis is one of the key mechanisms of airway inflammation in asthma. However, the exact mechanisms remain poorly understood.
Objectives: We sought to examine the role of histone deacetylase (HDAC) 10 as an epigenetic regulator that governs macrophage M2 program and promotes airway inflammation in asthma, and to elucidate the underlying mechanisms.
Methods: Peripheral blood and airway biopsies were obtained from healthy individuals and asthmatic patients. Asthma was induced by exposure to allergen in mice with myeloid-specific deletion of Hdac10 (Hdac10fl/fl-LysMCre) mice. HDAC10 inhibitor Salvianolic acid B (SAB), STAT3 selective agonist Colivelin, and the specific PI3K/Akt activator 1,3-Dicaffeoylquinic acid (DA) were also used in asthmatic mice. For cell studies, THP1 cells, primary mouse bone marrow derived macrophage (BMDMs) were used and related signaling pathways was investigated.
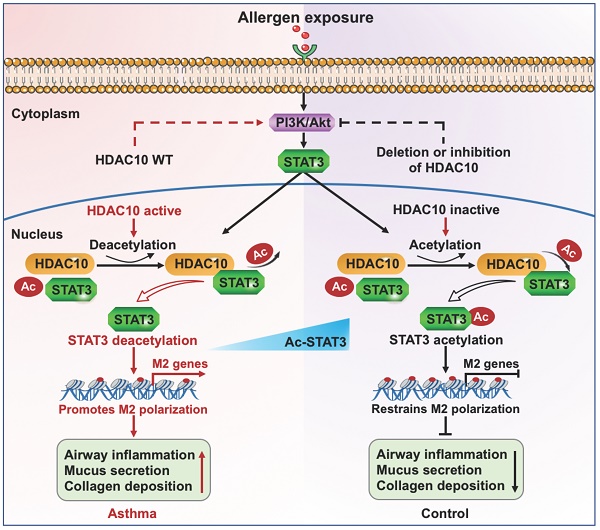
Results: HDAC10 expression was highly expressed by macrophages and promoted M2 macrophage activation and airway inflammation in asthmatic patients and mice. Hdac10fl/fl-LysMCre mice were protected from airway inflammation in experimental asthma model. Hdac10 deficiency significantly attenuated STAT3 expression and decreased M2 macrophage polarization following allergen exposure. Mechanistically, HDAC10 directly binds STAT3 for deacetylation in macrophages, by which it promotes STAT3 expression and activates the macrophage M2 program. Importantly, we identified SAB as a HDAC10 inhibitor that had protective effects against airway inflammation in mice.
Conclusions: Our results revealed that HDAC10-STAT3 interaction governs macrophage polarization to promote airway inflammation in asthma, implicating HDAC10 as a therapeutic target.
Keywords: HDAC10, STAT3, asthma, airway inflammation, macrophage.
 Global reach, higher impact
Global reach, higher impact