13.3
Impact Factor
Theranostics 2023; 13(6):1949-1973. doi:10.7150/thno.78323 This issue Cite
Research Paper
Single-cell profiling of GP2-enriched pancreatic progenitors to simultaneously create acinar, ductal, and endocrine organoids
1. Institute of Molecular Oncology and Stem Cell Biology, Ulm University Hospital, Ulm, Germany
2. Department of Urology, Ulm University Hospital, Ulm, Germany
3. Helmholtz Pioneer Campus, Helmholtz Zentrum München, Neuherberg, Germany
4. Department of Internal Medicine I, Ulm University Hospital, Ulm, Germany
5. Central Unit Single Cell Sequencing, Medical Faculty, Ulm University, Ulm, Germany
6. Division of Interdisciplinary Pancreatology, Department of Internal Medicine I, Ulm University Hospital, Ulm, Germany
7. Core Facility Organoids, Ulm University, Ulm, Germany
* These authors jointly supervised this work and contributed equally
Abstract
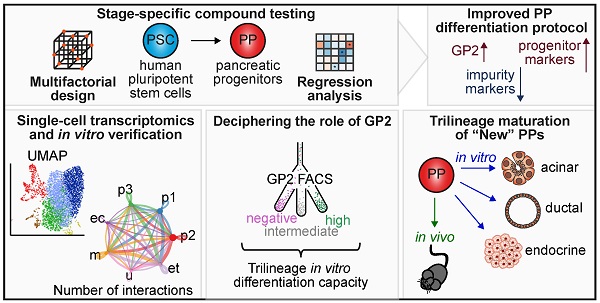
Rationale: Pancreatic lineage specification follows the formation of tripotent pancreatic progenitors (PPs). Current protocols rebuilding PPs in vitro have an endocrine lineage bias and are mostly based on PDX1/NKX6-1 coexpression neglecting other markers decisive for PP heterogeneity and lineage potential. However, true tripotent PPs are of utmost interest to study also exocrine disorders such as pancreatic cancer and to simultaneously generate all three pancreatic lineages from the same ancestor.
Methods: Here, we performed a comprehensive compound testing to advance the generation of multipotent progenitors, which were further characterized for their trilineage potential in vitro and in vivo. The heterogeneity and cell-cell communication across the PP subpopulations were analyzed via single-cell transcriptomics.
Results: We introduce a novel PP differentiation platform based on a comprehensive compound screening with an advanced design of experiments computing tool to reduce impurities and to increase Glycoprotein-2 expression and subsequent trilineage potential. Superior PP tripotency was proven in vitro by the generation of acinar, endocrine, and ductal cells as well as in vivo upon orthotopic transplantation revealing all three lineages at fetal maturation level. GP2 expression levels at PP stage ascribed varying pancreatic lineage potential. Intermediate and high GP2 levels were superior in generating endocrine and duct-like organoids (PDLO). FACS-based purification of the GP2high PPs allowed the generation of pancreatic acinar-like organoids (PALO) with proper morphology and expression of digestive enzymes. scRNA-seq confirmed multipotent identity, positioned the GP2/PDX1/NKX6-1high population next to human fetal tip and trunk progenitors and identified novel ligand-receptor (LR) interactions in distinct PP subpopulations. LR validation experiments licensed midkine and VEGF signaling to increase markers labelling the single cell clusters with high GP2 expression.
Conclusion: In this study, we guide human pluripotent stem cells into multipotent pancreatic progenitors. This common precursor population, which has the ability to mature into acinar, ductal and functional β-cells, serves as a basis for studying developmental processes and deciphering early cancer formation in a cell type-specific context. Using single-cell RNA sequencing and subsequent validation studies, we were able to dissect PP heterogeneity and specific cell-cell communication signals.
Keywords: Multipotent pancreatic progenitors, pancreatic organoids, in vitro differentiation, GP2, pancreatic acinar-like organoids
 Global reach, higher impact
Global reach, higher impact