13.3
Impact Factor
Theranostics 2023; 13(6):1860-1875. doi:10.7150/thno.83353 This issue Cite
Research Paper
Histone H3K27 methyltransferase EZH2 regulates apoptotic and inflammatory responses in sepsis-induced AKI
1. Department of Urology, Renmin Hospital of Wuhan University, Wuhan, 430060, China
2. Department of Nephrology, Shanghai Changzheng Hospital, Naval Medical University, Shanghai, 200003, China
3. Department of Nephrology, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, China
#Bojun Li, Yuqi Xia and Shuqin Mei contributed equally to this work.
* Zhiguo Mao, Xiangjun Zhou and Fan Cheng contributed equally to this work.
Abstract
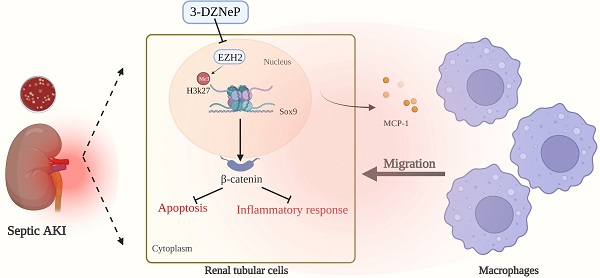
Rationale: The role of histone methylation modifications in renal disease, particularly in sepsis-induced acute kidney injury (AKI), remains unclear. This study aims to investigate the potential involvement of the histone methyltransferase zeste homolog 2 (EZH2) in sepsis-induced AKI and its impact on apoptosis and inflammation.
Methods: We first examined the expression of EZH2 in the kidney of sepsis-induced AKI (LPS injection) mice and LPS-stimulated tubular epithelial cells. We next constructed the EZH2 knockout mice to further confirm the effects of EZH2 on apoptosis and inflammatory response in AKI. And the inflammatory level of epithelial cells can be reflected by detecting chemokines and the chemotaxis of macrophages. Subsequently, we constructed the EZH2 knocked-down cells again and performed Chromatin Immunoprecipitation sequencing to screen out the target genes regulated by EZH2 and the enrichment pathway. Then we confirmed the EZH2 target gene and its regulatory pathway in vivo and in vitro experiments. Experimental results were finally confirmed using another in vivo model of sepsis-induced AKI (cecal perforation ligation).
Results: The study found that EZH2 was upregulated in sepsis-induced AKI and that silencing EZH2 could reduce renal tubular injury by decreasing apoptosis and inflammatory response of tubular epithelial cells. EZH2 knockout mice showed significantly reduced renal inflammation and macrophage infiltration. Chromatin immunoprecipitation sequencing and polymerase chain reaction identified Sox9 as a target of EZH2. EZH2 was found to be enriched on the promoter of Sox9. Silencing EZH2 resulted in a significant increase in the transcriptional level of Sox9 and activation of the Wnt/β-catenin signaling pathway. The study further reversed the effects of EZH2 silencing by silencing Sox9 or administering the Wnt/β-catenin inhibitor icg001. It was also found that Sox9 positively regulated the expression of β-catenin and its downstream pathway-related genes. Finally, the study showed that the EZH2 inhibitor 3-deazaneplanocin A significantly alleviated sepsis-induced AKI.
Conclusion: Our results indicate that silencing EZH2 can protect renal function by relieving transcriptional inhibition of Sox9, activating the Wnt/β-catenin pathway, and attenuating tubular epithelial apoptosis and inflammatory response of the renal interstitium. These results highlight the potential therapeutic value of targeting EZH2 in sepsis-induced AKI.
Keywords: EZH2, Acute kidney injury, Epigenetic, Inflammation, sepsis
 Global reach, higher impact
Global reach, higher impact