13.3
Impact Factor
Theranostics 2023; 13(4):1217-1234. doi:10.7150/thno.77041 This issue Cite
Research Paper
First in vivo fluorine-19 magnetic resonance imaging of the multiple sclerosis drug siponimod
1. Max-Delbrück-Center for Molecular Medicine in the Helmholtz Association (MDC), Berlin Ultrahigh Field Facility, Berlin, Germany
2. Hasso Plattner Institute for Digital Engineering, University of Potsdam, Germany
3. Experimental and Clinical Research Center, a joint cooperation between the Charité Universitätsmedizin Berlin and the Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany
4. SRH Fernhochschule - The Mobile University, Riedlingen, Germany
5. MRI.TOOLS GmbH, Berlin, Germany
6. Medicinal Chemistry, Leibniz-Institut fϋr Molekulare Pharmakologie (FMP), Berlin, Germany
7. Charité - Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health (BIH), Berlin, Germany
Abstract
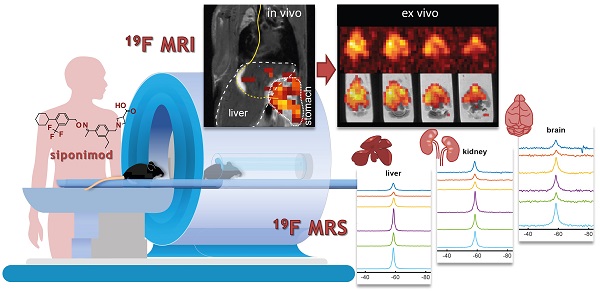
Theranostic imaging methods could greatly enhance our understanding of the distribution of CNS-acting drugs in individual patients. Fluorine-19 magnetic resonance imaging (19F MRI) offers the opportunity to localize and quantify fluorinated drugs non-invasively, without modifications and without the application of ionizing or other harmful radiation. Here we investigated siponimod, a sphingosine 1-phosphate (S1P) receptor antagonist indicated for secondary progressive multiple sclerosis (SPMS), to determine the feasibility of in vivo 19F MR imaging of a disease modifying drug.
Methods: The 19F MR properties of siponimod were characterized using spectroscopic techniques. Four MRI methods were investigated to determine which was the most sensitive for 19F MR imaging of siponimod under biological conditions. We subsequently administered siponimod orally to 6 mice and acquired 19F MR spectra and images in vivo directly after administration, and in ex vivo tissues.
Results: The 19F transverse relaxation time of siponimod was 381 ms when dissolved in dimethyl sulfoxide, and substantially reduced to 5 ms when combined with serum, and to 20 ms in ex vivo liver tissue. Ultrashort echo time (UTE) imaging was determined to be the most sensitive MRI technique for imaging siponimod in a biological context and was used to map the drug in vivo in the stomach and liver. Ex vivo images in the liver and brain showed an inhomogeneous distribution of siponimod in both organs. In the brain, siponimod accumulated predominantly in the cerebrum but not the cerebellum. No secondary 19F signals were detected from metabolites. From a translational perspective, we found that acquisitions done on a 3.0 T clinical MR scanner were 2.75 times more sensitive than acquisitions performed on a preclinical 9.4 T MR setup when taking changes in brain size across species into consideration and using equivalent relative spatial resolution.
Conclusion: Siponimod can be imaged non-invasively using 19F UTE MRI in the form administered to MS patients, without modification. This study lays the groundwork for more extensive preclinical and clinical investigations. With the necessary technical development, 19F MRI has the potential to become a powerful theranostic tool for studying the time-course and distribution of CNS-acting drugs within the brain, especially during pathology.
Keywords: Siponimod, Multiple Sclerosis, MRI, Fluorine, Molecular Imaging
 Global reach, higher impact
Global reach, higher impact